Translate this page into:
Bioassay-guided isolation of human carboxylesterase 2 inhibitory and antioxidant constituents from Laportea bulbifera: Inhibition interactions and molecular mechanism
⁎Corresponding authors. haoxj@mail.kib.ac.cn (Xiao-Jiang Hao), yuanchunmao01@126.com (Chun-Mao Yuan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Laportea bulbifera (Sieb. et. Zucc.) Wedd has long been utilized in Traditional Chinese Medicines (TCM) for the treatment of rheumatoid arthritis. However, the study of systematic anti-inflammatory chemical constituents in L. bulbifera has never been reported. Thus, bioassay-guided isolation for its roots part led to 46 compounds, including 38 phenolic derivatives. Their structures were determined on the basis of 1H and 13C NMR and MS spectra. All compounds were isolated from L. bulbifera for the first time except for 13 compounds. Most of the compounds showed good COX-2 inhibitory activity (IC50: 0.13–3.94 μM) and DPPH radical-scavenging activity (IC50: 1.57–9.55 μM). Four compounds (4, 17, 35, and 43) with different skeletons showed preferential COX-2 over COX-1 inhibition with selective indices ranging from 12 to 171. High content active compounds are important for elucidating the basis of the active substance of TCM. Compound 4 (COX-2, IC50 0.24 μM), a high content compound, represented one of the best selective COX-2 inhibitors. Another high content active compound (35) with a different skeleton might have different mechanism. Further study for the inhibition kinetics against COX-2 indicated compounds 4 and 35 were noncompetitive and competitive COX-2 inhibitors, respectively. Moreover, molecular docking and molecular dynamics simulation data further indicated that compound 4 could bind in the cavity of COX-2 and interacted with key residues VAL-538, PHE-142, and GLY-225 of COX-2 through hydrogen bonds. The results indicated that L. bulbifera roots could be applied as antioxidant and anti-inflammatory agents due to their potent selective COX-2 inhibitory and antioxidant activity of phenolic compounds.
Keywords
Laportea bulbifera (Sieb. et. Zucc.) Wedd
Bioassay-guided isolation
COX-2 selective inhibitors
Structure-activity relationships
Enzyme kinetics
Molecular simulation
1 Introduction
Inflammation is an innate immune response that protects against chemical irritants and foreign antigens, and many diseases can induce inflammatory responses in the human body (Cheung et al., 2002; Jang et al., 2017; Kim et al., 2014). Excessive responses can lead to multiple inflammatory diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and chronic granulomatous disease (Qin et al., 2020). There are multiple mediators of inflammation, and the arachidonic acid cascade in the cyclooxygenase (COX) pathway is one of the important processes. Cyclooxygenase enzymes (COXs) present in two isoforms (constitutive (COX-1) and inducible form (COX-2)) (Puig et al., 2000). COX-2 is a significant target for the discovery of many novel nonsteroidal anti-inflammatory drugs (NSAIDs) (Qin et al., 2020). In contrast, the inhibition for COX-1 could cause adverse gastrointestinal and renal effects (Sağlık, et al., 2021). Therefore, the studies for selective COX-2 inhibitors are important for new drug development. To date, several drugs targeting COX-2 are very popular on the market, such as nimesulide, meloxicam, and celecoxib.
The generation of oxygen free radicals is involved in the occurrence and development of inflammatory-related diseases, such as arthritic disorders and systemic inflammatory response syndrome (Closa and Folch-Puy, 2004; Comhair and Erzurum, 2002). Free radicals are both a cause and a result of inflammation (Chatterjee et al., 2016). Compounds with free radical scavenging activity could to some extent exhibit good anti-inflammatory activity.
Laportea bulbifera (Sieb. et. Zucc.) Wedd, named as Hong Hema, Hong Huoma, Rui Dagun or Wadou, is a perennial herb in the Urticaceae family. It is a folk medicine of Miao and Buyi ethnic minorities in Guizhou Province of China. This plant has long been utilized in traditional Chinese medicines for the treatment of rheumatoid arthritis and some other inflammatory diseases, such as skin itch and sports injury (Editorial Committee of Chinese Flora, 1995; Song et al., 2005). Its extract is usually made in ointments or capsules, such as Runzao Antipruritic Capsules (State Drug Administration, 2002a), Liuwei Shangfuning Ointments (State Drug Administration, 2002a), and Fufang Shangning Ointments (State Drug Administration, 2002b). In particular, Runzao Antipruritic Capsules are very popular on the market in China, and thus were included in the Report on the Scientific and Technological Competitiveness of Large Varieties of Traditional Chinese Medicine (TCM) (Yang et al., 2019). Moreover, its young leaves are edible, and the stem fibers are tough enough to be used in textiles (Editorial Committee of Chinese Flora, 1995). Modern pharmacological studies on this plant indicated that the crude extract exhibited many bioactivities, such as anti-inflammatory (Lu et al., 2012; Chen et al., 2019), immunosuppressive (Xiang et al., 2009), analgesic activities (Su et al., 2009), and anti-rheumatoid arthritis (Luo et al., 2011). Previous phytochemical investigation on L. bulbifera indicated the presence of flavonoids (Zhang et al., 2018; Yang et al., 2003), coumarins (Hou et al., 2010), steroids (Zhu et al., 2011), and phenolic acid (Zhu et al., 2011).
Although several scientific studies have indicated that crude extracts of L. bulbifera exhibit anti-inflammatory activities in vitro and in vivo (Wang et al., 2013; Chen et al., 2019), the study of the systematic chemical constituents on the anti-inflammatory effects has never been investigated. To investigate the potential anti-inflammatory compounds in L. bulbifera, bioactivity-guided identification of its COX-2 inhibitory and antioxidant compounds was performed. Consequently, bioassay-guided isolation of the ethyl acetate extract led to the isolation and identification of 46 compounds. Most of them exhibited good COX-2 inhibitory and DPPH radical-scavenging activity. Four compounds (4, 17, 35, and 43) with different skeletons showed preferential COX-2 over COX-1 inhibition with selective indices (SIs) ranging from 12 to 171. High content active compounds are important for the elucidating the basis of the active substance of TCM. Compound 4 (COX-2, IC50 0.24 μM), a high content compound, represented one of the best selective COX-2 inhibitors. Another high content active compound (35) with a different skeleton might have different mechanism. Therefore, these two COX-2 selective inhibitors were selected to study their COX-2 inhibitory mechanism, including their enzyme kinetics, molecular docking, and molecular dynamics.
2 Materials and methods
2.1 General information
Optical rotations were determined on a JASCOP-1020 polarimeter. 1H NMR and 13C NMR spectra were recorded on Bruker Avance NEO 600 spectrometer using TMS as an internal standard. ESI-MS analysis were carried out on Agilent 1100 instrument. Column chromatography was performed on silica gel (300–400 mesh; Qingdao Marine Chemical Co. Ltd., China), Sephadex LH-20 (40–70 µm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), and RP-C18 gel (40–63 µm, Merck, Darmstadt, Germany). Semi-preparative HPLC was performed on an instrument consisting of a Hanbon NP7005c controller, a Hanbon NP7005 pump, and a Hanbon NU3000c UV detector with a YMC-Triart-C18 column (250 × 10.0 mm, 5 μm). Fractions were monitored by TLC (GF 254, Qingdao Marine Chemical Co., Ltd.), and spots were visualized by heating silica gel plates immersed in 5 % H2SO4 in ethanol.
2.2 Plant material
The root parts of Laportea bulbifera (Sieb. et. Zucc.) Wedd were collected from Libo, Guizhou Province of China, in October of 2019. The identification of the plant was confirmed by Mr. Jun Zhang. A voucher specimen (H20191015) has been preserved in the key laboratory of chemistry for natural products of Guizhou Province and Chinese Academy of Sciences.
2.3 Extraction, isolation and identification of compounds
The air-dried powdered roots of L. bulbifera (49 kg) were extracted with 95 % ethanol (EtOH) under reflux for three times (each time for 3 h). The combined EtOH extracts were concentrated under vacuum to give a crude residue (2.0 kg), which was suspended in water. The water layer was successively partitioned with ethyl acetate (EtOAc) for three times. The EtOAc part (600 g) was separated by a silica gel column chromatography (CC) with CH2Cl2/MeOH (99:1, 95:5, 9:1, 8:2, 7:3, 6:4, 1:1, 4:6, 3:7, 2:8, 1:9, v/v) to afford eight fractions (Fr. 1–Fr. 8) after combing a lot of subfractions.
A RP-C18 column was used for fraction 3 (150 g), washed with gradient MeOH/H2O (50:50, 55:45, 65:35, 75:25, 85:15, 95:5, 100:0, v/v), to give twenty-five subfractions (Fr. 3A-Fr. 3Y). Subsequently, a silica gel column was used for fraction 3B (2.0 g), washed with gradient CH2Cl2/acetone (15:1, 10:1, 7:3, v/v) to afford two components (Fr. 3B1 and Fr. 3B2). Fraction 3B1 was subjected to the semi-preparative HPLC eluted with 28 % MeOH/H2O (2.0 mL/min) to get two compounds (1, 100 mg, tR = 13.3 min; 5, 80 mg, tR = 22.0 min). Fraction 3B2 was subjected to the semi-preparative HPLC eluted with 24 % MeOH/H2O (2.0 mL/min) to get two compounds (2, 83 mg, tR = 11.0 min; 6, 17 mg, tR = 19.4 min). Fraction 3 K was separated over Sephadex LH-20 (MeOH) and then chromatographed over a silica gel column eluted with gradient petroleum ether/acetone (20:1, 15:1, 9:1, 7:3, 1:1, v/v) to give five compounds (25, 15 mg; 26, 7 mg; 31, 6 mg; 27, 30 mg; 34, 8 mg). Fraction 3Q was applied to Sephadex LH-20 (MeOH) and chromatographed over silica gel column washed with gradient petroleum ether/EtOAc (40:1, 30:1, 20:1, 9:1, 7:3, 6:1, 1:1, v/v) to give six compounds (36, 5 mg; 37, 11 mg; 38, 14 mg; 43, 32 mg; 44, 23 mg; 46, 6 mg). Fraction 3Y was applied to a silica gel column eluted with gradient petroleum ether/CH2Cl2 (15:1, 10:1, 9:1, 8:2, 7:3, 1:9, v/v) to give five compounds (39, 26 mg; 40, 8 mg; 41, 13 mg; 42, 28 mg; 45, 6 mg).
A RP-C18 column was used for fraction 6 (79 g), washed with gradient MeOH/H2O (10:90, 20:80, 30:70, 40:60, 50:50, 70:30, 90:10, 100:0, v/v), to obtain ten subfractions (Fr. 6A- Fr. 6 J). Fraction 6A (5 g) was applied to a silica gel column eluted with CH2Cl2/MeOH (9:1) to get compound 4 (800 mg). Fraction 6B (4 g) was separated over Sephadex LH-20 (MeOH) to give six fractions (Fr. 6B1-Fr. 6B6). Fraction 6B1 (60 mg) was subjected to the semi-preparative HPLC eluted with 8 % MeOH/H2O (2.0 mL/min) to get two compounds (3, 15 mg, tR = 26.4 min; 7, 10 mg, tR = 52.6 min). Fraction 6B2 (700 mg) was chromatographed over a silica gel column (CH2Cl2/acetone, 25:1, 20:1, 15:1, 9:1, v/v) and then subjected to the semi-preparative HPLC eluted with 29 % MeOH/H2O (2.0 mL/min) to get compound 10 (13 mg, tR = 37.9 min). Fr. 6B3 (60 mg) was separated over Sephadex LH-20 (MeOH) to give 20 (45 mg). Fr. 6B3 (80 mg) was subjected to the semipreparative HPLC eluted with 50 % MeOH/H2O (2.0 mL/min) to get 8 (10 mg, tR = 27.5 min) and 9 (12 mg, tR = 45.0 min). Fr. 6B5 (10 g) was chromatographed over silica gel eluted with CH2Cl2/EtOAc (15:1, 9:1, 7:3, 1:1, v/v) to obtain three fractions (Fr. 6B5A-6B5C). Fr. 6B5C (500 mg) were separated over Sephadex LH-20 (MeOH) to give 32 (25 mg), 33 (13 mg), and 11 (13 mg). Fr. 6C (3.2 g) was separated over Sephadex LH-20 (MeOH) to give seven fractions (Fr. 6C1-Fr. 6C7). Fr. 6C3 (400 mg) was chromatographed over a silica gel column eluted with gradient CH2Cl2/MeOH (30:1, 20:1, 10:1, 9:1, 8:2, 1:1, v/v) to obtain four fractions (Fr. 6C3A-Fr. 6C3F). Fr. 6C3A (45 mg) was subjected to the semi-preparative HPLC eluted with 22 % MeOH/H2O (2.0 mL/min) to get two compounds (28, 14 mg, tR = 18.9 min; 12, 9 mg, tR = 25.8 min). Fr. 6C3D was subjected to the semi-preparative HPLC eluted with 18 % MeOH/H2O (2.0 mL/min) to get three compounds (13, 14 mg, tR = 18.9 min; 29, 9 mg, tR = 25.8 min; 21, 17 mg, tR = 40.3 min).
Fraction 7 (50 g) was applied to a RP-C18 chromatography column (CC) eluted with a MeOH/H2O gradient (10:90, 20:80, 30:70, 40:60, v/v) to yield five subfractions (7A–7E). Fraction 7A (5 g) was subjected on a silica gel column, eluted with CH2Cl2/MeOH (8:2, v/v) to give five subfractions (Fr. 7A1-Fr. 7A5). Subsequently, Fr. 7A1 (800 mg) was separated on Sephadex LH-20 column (MeOH) to get compound 22 (15 mg) and a major component, which was further purified by semi-preparative HPLC separation to get 14 (21 mg, tR = 15.6 min), 15 (9.6 mg, tR = 20.4 min), 23 (14.7 mg, tR = 23.6 min), 30 (18.9 mg, tR = 25.7 min), and 16 (13.9 mg, tR = 35.6 min). Fraction 7B (2.5 g) was applied to a silica gel column and then purified on Sephadex LH-20 (MeOH) to yield two compounds (35, 750 mg; 17, 80 mg). Fraction 7D (3 g) was purified on a silica gel column eluted with gradient CH2Cl2/acetone (15:1, 9:1, 7:3, 1:1, 1:9, v/v) to get three compounds (18, 12 mg; 19, 17 mg; 24, 20 mg).
1H (400 MHz) and 13C (100 MHz) NMR spectra of all isolates were acquired on a Bruker 400 MHz instrument. Compounds 1–46 were identified by comparison of the NMR data with those in the literatures.
2.4 In vitro COX-2 and COX-1 inhibitory assay
The inhibitory activities of the EtOAc and water parts, and all the isolated compounds toward COX-2 were evaluated using the COX-2 Inhibitor Screening Kit (Beyotime, Shanghai, China) (Chen et al., 2019; Jiao et al., 2019; Li et al., 2019). Stock solutions of test samples (the EtOAc and water parts, and all the isolated compounds) were prepared and diluted in DMSO. Celecoxib was used as positive control. According to the manufacturer's protocols, a recombinant human COX-2 enzyme in 96-well plates was incubated with the test samples at varying concentrations for 10 min at 37 ℃. After incubation, the COX-2 probe and substrate were added to each well and incubated for another 15 min at 37 ℃ in the dark. The intensity of the fluorescence was measured using a microplate reader (BioTek) with an excitation wavelength of 560 nm and an emission wavelength of 590 nm.
Inhibition of COX-1 was assessed using a COX fluorescent inhibitor screening assay kit from Cayman Chemical. Stock solutions of test samples were prepared and diluted in DMSO (Mohan et al., 2021; Sağlık, et al., 2021). The inhibition of COX-1 by compounds 4, 17, 35 and 43 was analyzed. All reactions were performed in a final volume of 100 μl in 96-well white opaque plate with flat bottom (Cayman) according to the manufacturer’s protocol. Briefly, 80 μl of reaction mix were added into each well (including 76 μl COX assay buffer; 1 μl COX probe; 2 μl diluted COX cofactor; 1 μl COX-1). Then, a multi-channel pipette was applied to add 10 μl of diluted arachidonic acid solution into each well to initiate all the reactions at the same time. Fluorescence values with an excitation wavelength of 535 nm and an emission wavelength of 587 nm were tested, using a microplate reader (BioTek) kinetically at 25 ℃ for 10 min.
The inhibitory ratio of COX-1 and COX-2 was calculated by the comparison of the sample treated incubations to control incubations. The IC50 values for COX-1 an COX-2 were calculated from the concentration–inhibition response curve.
2.5 Inhibitory kinetic analysis
Kinetic parameter and mechanism analyses of compounds 4 and 35 toward COX-2 were investigated by using Lineweaver-Burk analyses (Liu et al., 2019). The experiments were implemented at different concentrations of arachidonic acid in the absence and presence of compounds 4 (0.06–1.5 μM) and 35 (0.75–3.0 μM), and the data were fitted into Michaelis-Menten plots (Zhao et al., 2021). Moreover, their inhibition type was determined by Lineweaver-Burk analysis (Lin et al., 2019). The kinetic parameters (constant Ki) of compounds 4 and 35 were calculated according to the slope plots and inhibition kinetics model (He et al., 2020).
2.6 Molecular docking
The docking study was performed as described previously (Qin et al., 2020). Molecular docking analysis of COX-2 with compounds 1–46 was performed using AutoDock tools (version 1.1.2). The 3-dimensional structures of compounds were prepared using Chem 3D. The coordinates for COX-2 (PDB entry code: 5IKQ) enzyme was obtained from the Protein Data Bank (Qin et al., 2020). Water molecules were removed and polar hydrogens were added for the accurate calculation of partial charges. The grid box size was set at 122, 120, and 114 Å (x, y, and z) with center x = 30.4, y = 48.8, and z = 19.5 for the protein. For further docking, the edited COX-2 and ligand files were transcribed into PDBQT format files. All the parameters were the default. The best docked complex for COX-2 with compounds were selected on the basis of binding free energy value.
2.7 Molecular dynamic simulation
Molecular dynamics (MD) simulation was performed using the GROMACS package for the inhibitor-enzyme interaction mechanism. Compound 4 and 5IKQ were prepared separately according to the coordinates of the docked complex. The protein topology was optimized based on the amber99sb force field (Hornak et al., 2006; Sun et al., 2021). The partial charge of compound 4 was obtained by using the antechamber module of AmberTool. The topology parameters could be generated by the antechamber and ACPYPE programs. A rectangular box with a TIP3P water model was added around the complex with a solvation thickness of at least 1.2 nm. Na ions were added to ensure the neutrality. The production run was performed following proper minimization to fully relax the prepared system. A total 10 ns molecular dynamic trajectory was sampled under isothermal-sobaric condition at 300 K. All the simulations were executed under periodic boundary conditions (PBCs). The enzyme inhibitor interaction at the atomic level was retrieved from the MD trajectory with analytic tools integrated in GROMACS.
2.8 DPPH radical-scavenging assay
A 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay (Xu et al., 2021) was used to test the antioxidative activity. Five groups, a blank (MeOH), sample (mixed compound and DPPH solution), background (pure compound solution), negative (pure DPPH solution), and positive [mixed vitamin C (VC) and DPPH solution] controls could be applied for the test. Three tested groups, DPPH (0.15 mM), compounds (1–100 µM), and VC (1–100 µM), were dissolved in MeOH. Each 160 µl aliquot of MeOH was placed in the negative control and blank groups, while each 160 µl aliquot of the test compounds or VC was placed in the sample and background groups. MeOH (each 40 µl) was added to the blank and background groups, while 40 µl of DPPH was added to the negative, positive, and sample controls. Then, the absorbance of all tested samples was measured at 517 nm with a microplate reader (Multiskan Spectrum, Thermo Scientific Varioskan LUX) after 30 min of incubation at room temperature. The DPPH radical-scavenging rate was calculated as described in the literature (Xu et al., 2021). The IC50 values of the compounds and VC were calculated by SPSS (Statistical Package for the Social Sciences) software from the radical-scavenging rates at the final concentrations of 10, 5, 2.5, 1.25, 0.625, 0.3125 and 0.155 μg/mL.
2.9 Methods for HPLC
HPLC analysis was performed on a Agilent 1260 instrument using a X-bridge C18 column (5 μm, 4.5 × 250 mm) at 25 ℃ and a flow rate of 0.9 mL/min. The mobile phase components were solvent A (0.1 % phosphoric acid in H2O, v/v) and solvent B (acetonitrile) using the following gradient: 0–20 min (5–5 % B), 20–45 min (5–10 % B), 45–75 min (10–30 % B), 75–85 min (30–90 % B), 85–95 min (90–90 % B), 95–105 min (90–5 % B), with an injection volume of 10 μl. The ethyl acetate part and each isolate were applied to HPLC with the same condition.
2.10 Statistical Analysis.
All results of bioactivity test were expressed as the mean ± standard deviation (SD) (n = 3). Statistical comparisons were determined by Student t test. A P-value of < 0.05 was considered significant. A P-value of < 0.01 was considered highly statistically significant.
3 Results and discussion
3.1 Structure identification of isolated compounds
Using various chromatographic methods, 46 compounds were isolated from the active ethyl acetate (EtOAc) extract of L. bulbifera roots. Their structures were elucidated by their spectroscopic data analysis and comparisons with compounds previously reported in the literatures (Fig. 1). These 46 compounds were identified as (+)-catechin (1) (Hou et al., 2000), (-)-gallocatechin (2) (Foo et al., 2000), (-)-epigallocatechin 3-O-gallate (3) (Lee et al., 2000), (-)-epicatechin-3-O-gallate (4) (Lin et al., 2011), (+)-epicatechin (5) (Wei et al., 2013), (-)-epigallocatechin (6) (Foo et al., 2000), (-)-gallocatechin 3-O-gallate (7) (Choi et al., 2015), (+)-5,5-dimethyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromene-2,3,4,8,10-pentaol (8) (Hakamata et al., 2006), (-)-5,5-dimethyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromene-2,3,4,8,10-pentaol (9) (Hakamata et al., 2006), quercetin-3-O-rhamnoside (10) (Fossen et al., 1999), isorhamnetin (11) (Su et al., 2008), hyperoside (12) (Isaza et al., 2001), astragalin (13) (Xian et al., 2007), rutin (14) (Han et al., 2012), kaempferide (15) (Zhang et al., 2015), myricetin-3-O-α-L-rhamnopyranoside (16) (Souza et al., 2007), quercetin (17) (Guo et al., 2008), 4′-methoxyflavonol (18) (Babu et al., 2013), fisetin (19) (Mujwah et al., 2010), nobiletin (20) (Yue et al., 2013), luteoloside (21) (Ma et al., 2007), luteolin (22) (Chen et al., 2008), apigenin (23) (Tian et al., 2005), (+)-dihydromyricetin (24) (Jin et al., 2009), naringenin trimethyl ether (25) (Seidel et al., 2000), (+)-4′,5,7-trimethoxydihydroflavonol (26) (Zhang et al., 2012), 5-hydroxy-7,4′-dimethoxyflavone (27) (Righi et al., 2012), daidzin (28) (Kim et al., 2009), genistin (29) (Lee et al., 2002a), phloridzin (30) (Wang et al., 2008), flavokawain A (31) (Seidel et al., 2000), 7-methoxycoumarin (32) (Adekenov et al., 2017), scoparone (33) (Lee et al., 2002b), (+)-vibruresinol (34) (In et al., 2015), piceid (35) (Jeong et al., 2010), 2-hydroxy-3-(o-hydroxyphenyl) propanoic acid (36) (Wang et al., 1999), p-hydroxybenzoic acid (37) (Zou et al., 2006), vanillic acid (38) (Geng et al., 2017), (+)-cabralealactone (39) (Phongmaykin et al., 2008), β-sitosterol (40) (Chundattu et al., 2016), 7-keto-β-sitosterol (41) (Yu et al., 2007), methyl linoleate (42) (Huh et al., 2010), linoleic acid (43) (Marwah et al., 2007), methyl nonadecanoate (44) (Capon et al., 1998), methyl (9E,11E)-8-oxooctadeca-9,11-dienoate (45) (Thomas and Pryor, 1980), and squalene (46) (Chang et al., 2000). All compounds were isolated from L. bulbifera for the first time except for 13 compounds (1, 2, 5, 6, 20, 22, 33, 38, 40, 41, 42, 43, and 45). Compounds 1–38 are phenolic compounds. Relevant NMR data of all isolates are given in the Supplementary Information. The structures of all compounds are presented in Fig. 1.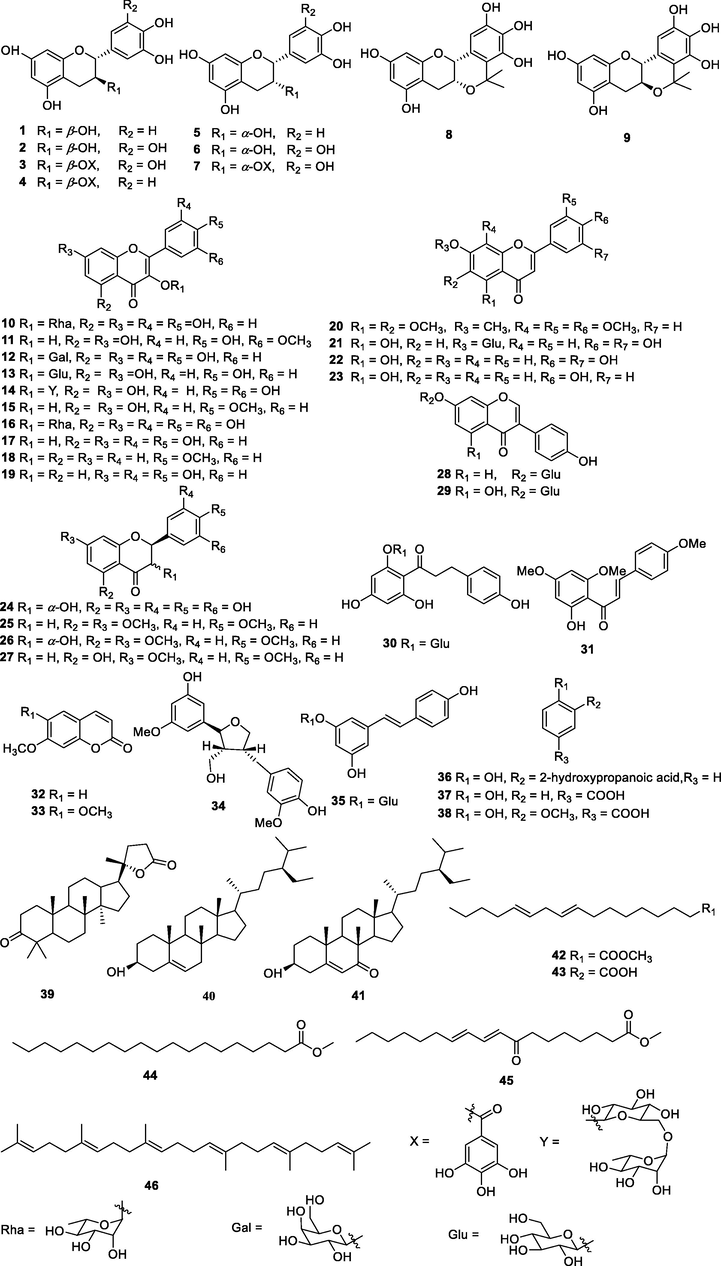
Chemical structures of isolated compounds.
3.2 COX-2 inhibitory effects of the extracts and isolates
The preliminary screening of COX-2 inhibitory activity implied that the EtOAc extract showed good COX-2 inhibitory activity with an inhibitory rate of 60.7% at a concentration of 100 µg/ml (Fig. 2A and Table 1). All compounds (1–46) isolated from the active EtOAc part were tested for COX-2 inhibitory activity at a concentration of 10 μM (Fig. 2B). 23 Compounds (1–9, 11, 12, 14–17, 19, 21, 22, 24, 34, 35, 38, and 43) showed good inhibitory rates, higher than 50%, at this concentration. Then, these active compounds were tested for their IC50, 13 of which showed strong inhibitory activity with IC50 values lower than 1 μM (Table 1). In special, compounds 4, 12, 14, 17, 19, and 22 exhibited optimal COX-2 inhibitory potency (IC50 values ranging from 0.13 to 0.24 μM). b The results are presented as the mean ± SD of three independent experiments: *P < 0.05, **p < 0.01 vs the blank control.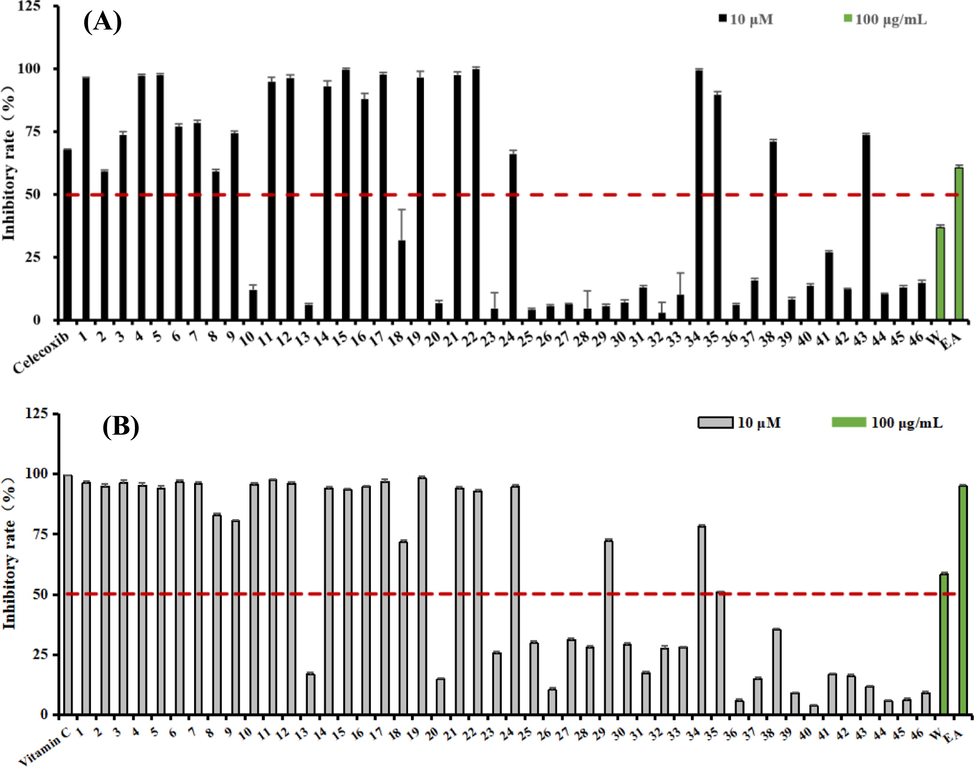
Effects of extraction and isolates for antioxidant and COX-2 inhibitory activity. (A). COX-2 inhibitory rate for the extraction and isolates; (B). DPPH radical-scavenging inhibitory rate for the extraction and isolates.
Compounds
COX-2
DPPH
Inhibitory rate (%) a
IC50 (μM)
Inhibitory rate (%) a
IC50 (μM)
1
96.34 ± 0.34**b
1.40 ± 0.05
96.18 ± 0.86**
2.10 ± 0.06
2
59.29 ± 0.58*
2.08 ± 0.06
94.75 ± 1.02**
2.61 ± 0.11
3
73.78 ± 1.20*
0.46 ± 0.03
96.32 ± 0.99**
1.80 ± 0.16
4
97.33 ± 0.47**
0.24 ± 0.06
95.26 ± 0.85**
1.68 ± 0.05
5
97.58 ± 0.53**
0.56 ± 0.04
94.09 ± 0.81**
2.04 ± 0.12
6
77.06 ± 1.11*
1.97 ± 0.61
96.49 ± 1.09**
1.73 ± 0.09
7
78.51 ± 0.97*
0.70 ± 0.01
95.72 ± 0.74**
1.80 ± 0.27
8
59.16 ± 0.84*
0.97 ± 0.38
82.69 ± 0.99**
3.21 ± 0.13
9
74.36 ± 0.83**
1.73 ± 0.33
80.42 ± 0.62**
2.63 ± 0.24
10
12.09 ± 1.97
–
95.58 ± 0.63**
1.57 ± 0.08
11
94.83 ± 1.92**
3.94 ± 1.14
97.60 ± 0.44**
1.66 ± 0.14
12
96.38 ± 1.34**
0.23 ± 0.01
95.82 ± 0.96**
1.72 ± 0.19
13
6.25 ± 1.45
–
16.77 ± 0.82
–
14
92.87 ± 2.36**
0.22 ± 0.06
94.05 ± 0.66**
1.78 ± 0.30
15
99.57 ± 0.36**
1.29 ± 0.02
93.45 ± 0.37**
1.74 ± 0.22
16
87.89 ± 2.40**
0.35 ± 0.07
94.66 ± 0.51**
1.63 ± 0.16
17
97.79 ± 0.76**
0.13 ± 0.02
96.81 ± 0.92**
2.22 ± 0.14
18
31.87 ± 12.20
–
71.57 ± 0.83*
9.51 ± 0.27
19
96.64 ± 2.51**
0.13 ± 0.01
98.12 ± 0.79**
1.67 ± 0.35
20
12.89 ± 1.13
–
14.74 ± 0.57
–
21
97.58 ± 1.19**
0.36 ± 0.07
93.98 ± 0.63**
1.85 ± 0.38
22
100.00 ± 0.78**
0.17 ± 0.03
92.87 ± 0.69**
3.01 ± 0.11
23
4.79 ± 2.31
–
25.56 ± 0.64
–
24
66.21 ± 1.34*
1.60 ± 0.37
94.73 ± 0.90**
1.69 ± 0.25
25
4.15 ± 0.59
–
29.67 ± 0.85
–
26
5.58 ± 0.56
–
10.31 ± 1.02
–
27
6.28 ± 0.52
–
31.22 ± 0.49
–
28
4.82 ± 1.23
–
27.79 ± 0.87
–
29
5.77 ± 0.79
–
71.99 ± 1.14*
6.23 ± 0.69
30
7.19 ± 0.95
–
29.01 ± 0.95
–
31
12.99 ± 0.78
–
17.17 ± 0.84
–
32
2.96 ± 2.01
–
27.61 ± 0.93
–
33
10.13 ± 2.10
–
27.82 ± 0.65
–
34
99.42 ± 0.49**
0.31 ± 0.02
77.94 ± 0.76*
5.89 ± 0.53
35
89.55 ± 1.49**
1.74 ± 0.03
50.93 ± 0.42*
9.55 ± 0.84
36
6.20 ± 0.51
–
5.90 ± 0.62
–
37
15.99 ± 0.65
–
14.88 ± 0.64
–
38
71.11 ± 0.91**
2.36 ± 0.12
35.34 ± 0.36
–
39
8.32 ± 0.85
–
8.89 ± 0.51
–
40
13.65 ± 0.89
–
3.68 ± 0.31
–
41
26.97 ± 0.85
–
16.75 ± 0.61
–
42
12.22 ± 0.55
–
15.90 ± 0.74
–
43
73.60 ± 0.61*
3.25 ± 0.85
11.63 ± 0.49
–
44
10.38 ± 1.03
–
5.67 ± 0.65
–
45
13.09 ± 0.86
–
6.21 ± 0.76
–
46
14.88 ± 1.03
–
9.04 ± 0.57
–
W c
36.92 ± 0.56
–
55.37 ± 0.29*
–
EtOAc c
60.67 ± 0.35*
–
92.68 ± 0.25**
–
Celecoxib d
67.77 ± 0.12**
0.02 ± 0.001
–
–
Vitamin C d
–
–
63.18 ± 0.11**
1.35 ± 0.005
3.3 COX-1 inhibitory effects of compounds with different skeletons
The inhibition of COX-1 led to peptic lesions in the gastrointestinal mucosa (Puig et al., 2000). Searching for the selective COX-2 inhibitors could decrease side effects over current novel nonsteroidal anti-inflammatory drugs. Similar skeleton compounds always have similar inhibitory mechanism. Therefore, four most active compounds in their different structural skeletons (4, 17, 35, and 43) were selected as representative compounds to test for the COX-1 inhibitory activities. The results indicated that those four compounds showed good COX-2 selectivity index ranging from 12 to 171 (Table 2).
Comp.
COX-1 IC50 (μM)a
COX-2 IC50 (μM)
Selectivity Indexb
4
8.77 ± 0.13
0.24 ± 0.06
37
17
22.22 ± 1.20
0.13 ± 0.02
171
35
21.62 ± 0.65
1.74 ± 0.03
12
43
>100
3.25 ± 0.85
>31
Celecoxib
21.52 ± 1.16
0.02 ± 0.001
1076
3.4 Inhibition kinetics of two representative compounds against COX-2
High content active compounds are important for the elucidating the basis of the active substance of Traditional Chinese Medicine. Compound 4 (COX-2, IC50 0.24 μM), a high content compound, represented one of the best COX-2 inhibitors among the isolates. Another high content active compound (35) with a different skeleton might have different mechanism. Therefore, those two compounds were selected for the the inhibition kinetics against COX-2. This experiment was determined by using Lineweaver-Burk plots to determine their inhibition type (Wang et al., 2018), and the results are shown in Figure 3 and Table 3. As shown in Figure 3, these two compounds (4 and 35) revealed COX-2 inhibitory activity in a dose-dependent manner, with IC50 values of 0.24 ± 0.06 and 1.74 ± 0.03 μM, respectively. As shown in Figure 3E, a series of lines of compound 4 intersected at a point on the negative X-axis in the Lineweaver-Burk plots, which indicated that the inhibition type of 4 was noncompetitive (He et al., 2020). As shown in Fig. 3F, compound 35 was assigned as a competitive inhibitor since its Lineweaver-Burk plot showed that a series of lines intersected at the Y axis. Thus, compound 4, a noncompetitive inhibitor, had an inhibition constant Ki value of 0.142 μM by Lineweaver-Burk and slope plots, and compound 35, a competitive inhibitor, revealed a Ki value of 3.175 μM (Table 3). The Ki values of the two compounds were in good agreement with their COX-2 inhibitory activity (Table 3). Vmax is the maximum rate at saturation of the substrate concentration. The unit for Vmax (nM/s/μg) means per μg protein in per second catalyzed substrate (nmol).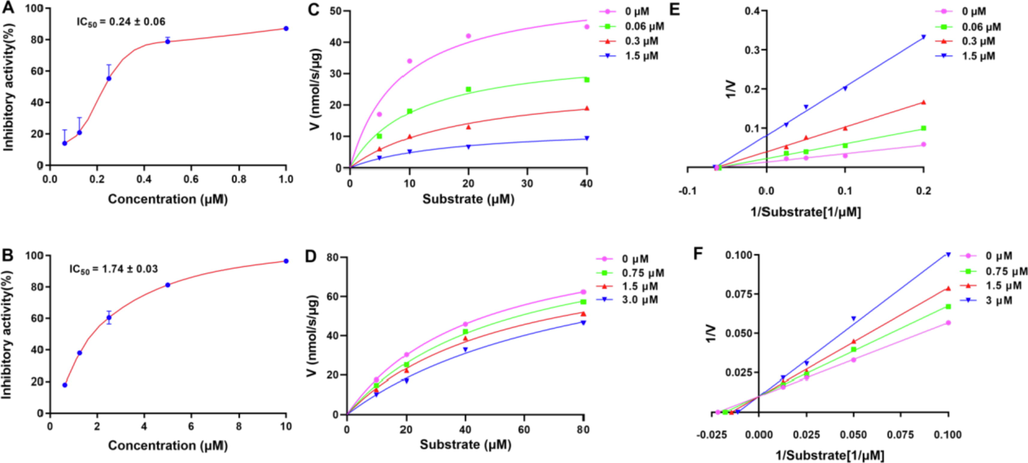
(A–B) Compounds 4 and 35 displayed concentration-dependent inhibitory activities against COX-2. (C–D) Michealis-Menten plots of compounds 4 and 35 against COX-2. (E-F) Lineweaver-Burk plots of compounds 4 and 35 against COX-2.
Compounds
Inhibition type
Vmax (nM/s/μg)
Km (μM)
Ki (μM)
4
Noncompetitive
57.25
9.73
0.142
35
Competitive
94.94
42.70
3.175
3.5 Molecular docking
Molecular docking was used to predict binding sites between the active compounds and COX-2 (Wang et al., 2010; Zhao et al., 2012). Those two compounds (4 and 35) used for the inhibition kinetics were also applied for in silico analysis with a human COX-2 protein model (PDB code: 5IKQ). As shown in Fig. 4A, compound 4 was packed well in the pocket formed by five amino acid residues of the protein, PHE-142, GLY-225, GLN-374, ASN-375, and VAL-538. In Fig. 4B, compound 35 also docked well with the protein, in which five hydrogen bonds were observed between this compound with ASP-347, GLN-192, HIS-90, PRO-514, and ASP-515 of the protein. As shown in Table 4, both compounds had lower bingd energy ranging from −7.5 to −7.8 kcal/mol, confirming their good docking results. Moreover, as shown in Fig. S96 (Supporting Information), arachidonic acid and compound 35 were packed in the same binding site with COX-2, different from that of 4. That means those two compounds might have different binding mechanism.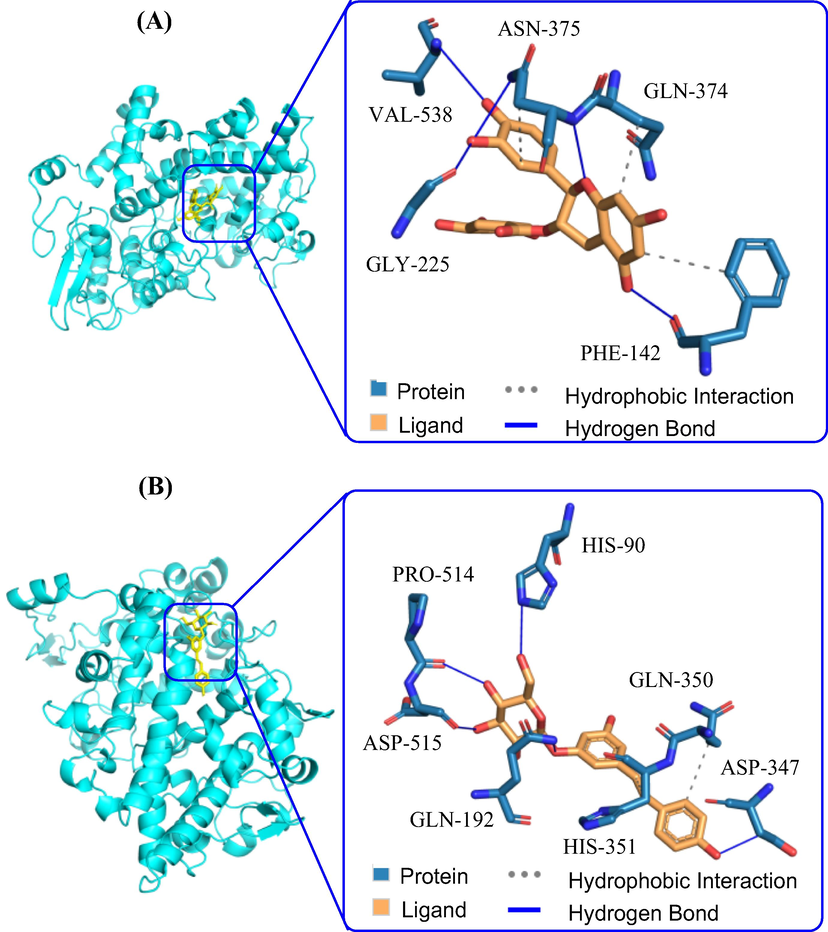
Binding pose of compounds 4 and 35 with COX-2. (A) The docking study between compound 4 and COX-2; (B) The docking study between compound 35 and COX-2.
Comp.
Interaction amino acids
Hydrogen bonds
Binding Energy(kcal/mol)
4
PHE-142, GLY-225, GLN-374, ASN-375, VAL-538
PHE-142, GLY-225, GLN-374, ASN-375, VAL-538
−7.8
35
PRO-514, ASP-515, GLN-192, HIS-351, ASP-347, GLN-350, HIS-90
PRO-514, ASP-515, GLN-192, ASP-347, HIS-90
−7.5
In addition, in order to discuss the relationship between the binding energies and the COX-2 inhibitory activities for all isolates, molecular docking was applied. All the docking results and binding affinity have been summarized in Fig. 5 and Table S1 (Supporting Information). Lower binding energy for docking, higher inhibitory activity for compounds (Rostamian et al., 2020; Rasouli et al., 2020). As shown in Figure 5, 13 compounds (3–5, 7–8, 12, 14, 16, 17, 19, 21, 22, and 34) in green (COX-2: IC50 lower 1 μM) had lowest binding energy (Binding affinity < -7 kcal/mol). As for the moderate COX-2 inhibitory active compounds in blue, the binding energies were ranged from −6 kcal/mol to −7 kcal/mol. 23 Compounds in organge in Fig. 5, had higher binding energies than −6 kcal/mol. Obviously, the docking energies are closely related to the COX-2 inhibitory activities.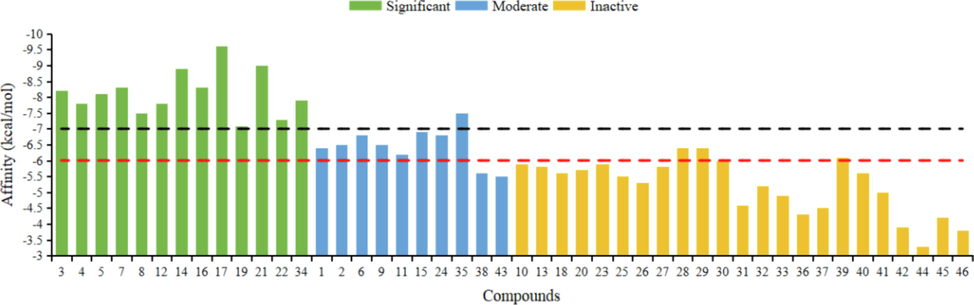
Binding affinity of compounds 1–46. Significant: compounds with IC50 lower 1 μM for COX-2 inhibitory activity. Moderate: compounds with IC50 ranging from 1 μM to 10 μM for COX-2 inhibitory activity. Inactive: compounds with IC50 higher than 10 μM for COX-2 inhibitory activity.
3.6 Molecular dynamics simulation
Molecular dynamics (MD) has become an important tool to resolve interactions involving enzyme-ligand complexes (Sun et al. 2021). Compound 4 (COX-2, IC50 0.24 μM), a high content compound, represented one of the best COX-2 inhibitors. Thus, this compound was selected for the dynamic interaction with COX-2. As shown in Fig. 6A, a stable complex of compound 4 and COX-2 could form, with the energy of the model system stabilized at approximately −6.7 × 105 kJ/mol. The stability of the simulated complex (compound 4 and COX-2) was screened via root mean square deviation (RMSD). The data indicated that the RMSD remained under 2.5 Å during 10 ns of MD simulation, implying a stable simulation run of the tested system (Fig. 6B). The regions with higher flexibility were verified by root mean square fluctuation (RMSF) for the 5IKQ residues (Fig. 6C). Hydrogen bonds between compound 4 and COX-2 were observed in Figure 4, and 9 key amino acid residues, including GLY-225, ASN-375, HIS-226, PHE-142, ASN-537, ARG-376, VAL-538, GLN-374, and GLY-553, were selected to emerge in this MD stimulation (Fig. 6E-F). Moreover, three amino acid residues (VAL-538, PHE-142, and GLY-225) kept close contact with COX-2 through hydrogen bond interactions due to the the calculated distances converging under 3.5 Å during the simulation process, as shown in Fig. 6G. Obviously, this interactions were stable and reliable. These results clarify the role of residues VAL-538, PHE-142, and GLY-225 in the inhibition of compound 4 against COX-2.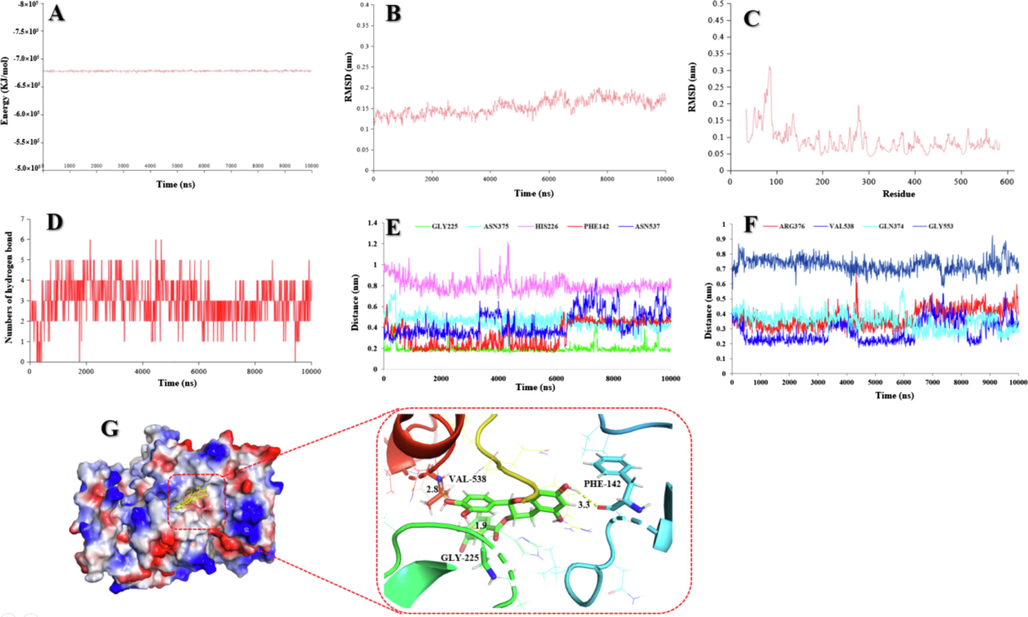
(A) The potential energy of compound 4 with COX-2 in 10 ns of MD simulation. (B) RMSD of compound 4 with COX-2 in 10 ns of MD simulation. (C) RMSF of compound 4 with COX-2 in 10 ns of MD simulation. (D) The numbers of hydrogen bond in 10 ns of MD simulation. (E) The distances compound 4 with GLY-225, ASN-375, HIS-226, PHE-142, and ASN-537 of COX-2. (F) The distances of compound 4 with ARG-376, VAL-538, GLN-374, and GLY-553 of COX-2. (G) 3D structure of the complex of compound 4 bounded COX-2 and their interaction form in the final frame of 10 ns MD simulation.
3.7 Antioxidant activity
The antioxidant activities of the EtOAc parts and compounds were evaluated using a DPPH radical-scavenging assay, as shown in Fig. 2B and Table 1 (Xu et al., 2021). The active EtOAc part was used for the further phytochemical study. In the DPPH assay, all isolates (1–46) were screened for the antioxidant activity at a concentration of 10 μM, as shown in Fig. 2B. Among them, 24 compounds (1–12, 14–19, 21, 22, 24, 29, 34, and 35) revealed great scavenging ability higher than 50%, and had the IC50 values lower than 10 μM (Table 1).
3.8 Structure-activity relationships
In the COX-2 inhibitory assay, 23 active compounds could be divided into six structural groups: flavanols (1–9), flavonoids (11, 12, 14–17, 19, 21, 22, and 24), lignan (34), stilbene (35), simple phenol derivatives (38), and a fatty acid (43). All the flavanols (1–9) showed good inhibitory activity, and flavanol derivatives with a galloyl group (3, 4, and 7) exhibited better activity than other compounds without this group (1, 2, and 6). For the flavonoid derivatives (10–23), the number of phenolic hydroxyl groups and their position played important roles in the COX-2 inhibitory activity. Although compounds 16 and 17 had the highest numbers of phenolic hydroxyl groups (five phenolic hydroxyl groups), compound 17 exhibited the best COX-2 inhibitory activity (IC50: 0.13 ± 0.02). The difference between these two compounds was only the position of a phenolic hydroxyl group, and therefore, a phenolic group at C-3 (17) could improve its activity. The same phenomenon could be observed for the compounds with four phenolic hydroxyl groups (10, 11, 12, 14, 19, and 22), in which compound 19 with a phenolic group at C-3 was also a best COX-2 inhibitor (IC50: 0.13 ± 0.01). Compounds with different skeletons having one or two phenolic groups (34, 35, and 38) also revealed good COX-2 inhibitory activity. Four fatty acid derivatives (42–45) were screened for the COX-2 inhibitory activity, and only compound 43 was active. Obviously, an acid group was important for improving its inhibitory activity among the fatty acid derivatives.
In the DPPH radical-scavenging assay, 24 compounds (1–12, 14–19, 21, 22, 24, 29, 34, and 35) with phenolic groups revealed significant activity with IC50 values lower than 10 μM. Thus, the antioxidant activity is closely related to the number of phenolic hydroxyl groups. These active compounds include six structural types of compounds: flavanols (1–9), flavonoids (10–12, 14–19, 21, and 22), flavanone (24), isoflavone (29), lignin (34), and stilbene (35). All flavanols (1–9) exhibited good antioxidant activity with IC50 values ranging from 1.68 to 3.21 μM, in which compound 4 with seven phenolic groups showed the best activity. Moreover, all the flavonoids expect for compounds 13, 20, and 23 with 0 to 3 phenolic groups exhibited good antioxidant activity, which supported the conclusion that the numbers and positions of the phenolic groups were responsible for their activity.
3.9 HPLC analysis
We conducted HPLC analyses of the active EtOAc extract and all the pure isolates (Yuan et al., 2013). The peaks of the EtOAc extract were assigned based on their retention times and UV spectra by comparison of the isolates with the EtOAc extract under the same chromatography conditions (Fig. 7). All the major and minor peaks of the EtOAc part were assigned, and overlapping compounds were also present in this spectrum. This study, to some extent, elucidated the basis of active substances for this plant.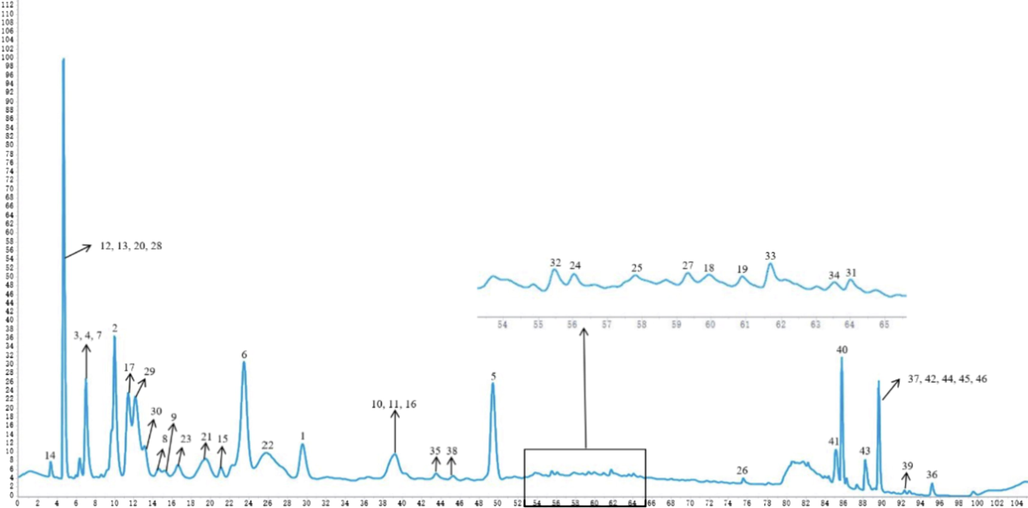
HPLC spectra of EA extraction (210 nm) and peak identification of all isolates.
4 Conclusion
In summary, bioactivity-guided isolation of the roots of Laportea bulbifera led to the isolation and identification of 46 compounds using NMR and MS techniques, among which 38 isolates were phenolic derivatives. All isolates were reported for the first time from this plant except for 13 compounds. Most of the compounds showed good COX-2 inhibitory activity (IC50: 0.13–3.94 μM) and DPPH radical-scavenging activity (IC50: 1.57–9.55 μM). Moreover, four compounds (4, 17, 35, and 43) with different skeletons showed preferential COX-2 over COX-1 inhibition with selective indices ranging from 12 to 171. Two high content compounds (4 and 35) with different skeletons were assigned as noncompetitive and competitive COX-2 inhibitors, respectively. Furthermore, molecular docking and molecular dynamics simulation for compound 4 clarify the role of residues VAL-538, PHE-142, and GLY-225 in the inhibition of compound 4 against COX-2. The chemical fingerprint and structure activity relationship were also investigated. Previous studies implied that crude extracts of L. bulbifera exhibit anti-inflammatory activities in vitro and in vivo (Wang et al., 2013). However, this study found a great deal of phenolic compounds with good COX-2 inhibitory and antioxidant activity. The results indicated that L. bulbifera roots could be applied as antioxidant and anti-inflammatory agents.
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (U1812403 ), the 13th Batch of Outstanding Young Scientific and Technological Talents in Guizhou Province (QKHPTRC [2021]5633), Natural Science and Technology Foundation of Guizhou Province (J[2019]1216), Guiyang Science and Technology Bureau ([2021]43-12), High-level Innovative Talents in Guizhou Province (Thousand Levels of Talent for Chunmao Yuan in 2018), and “Light of the West” Talent Cultivation Program of Chinese Academy of Sciences for Chunmao Yuan (RZ [2020]82).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Two new sesquiterpene lactones from Artemisia halophila. Chem. Nat. Compd.. 2017;53:284-289.
- [CrossRef] [Google Scholar]
- An expeditious synthesis of flavonols promoted by montmorillonite KSF clay and assisted by microwave irradiation under solvent-free conditions. Helv. Chim. Acta. 2013;96:1269-1272.
- [CrossRef] [Google Scholar]
- Marine nematocides: Tetrahydrofurans from a southern Australian brown alga, Notheia anomala. Tetrahedron. 1998;54:2227-2242.
- [CrossRef] [Google Scholar]
- The low polar constituents from Bidens pilosa L. var. Minor (Blume) Sherff. J. Chin. Chem. Soc-Taip.. 2000;47:1131-1136.
- [CrossRef] [Google Scholar]
- Chapter two - oxidative stress, inflammation and disease. In oxidative stress and biomaterials. In: Oxidative Stress and Biomaterials. Academic Press; 2016. p. :35-58.
- [Google Scholar]
- Chemical constituents in the leaves of Alsophila spinulosa. Acta Bot. Boreali-Occident. Sin.. 2008;28:1246-1249.
- [CrossRef] [Google Scholar]
- Spectrum–effect relationship of antioxidant and antiinflammatory activities of Laportea bulbifera based on multivariate statistical analysis. Biomed. Chromatogr.. 2019;34:1-16.
- [CrossRef] [Google Scholar]
- Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease. Lancet. 2002;360:1831-1837.
- [CrossRef] [Google Scholar]
- Separation of polyphenols and caffeine from the acetone extract of fermented tea leaves (Camellia sinensis) using high-performance countercurrent chromatography. Molecules. 2015;20:13216-13225.
- [CrossRef] [Google Scholar]
- Phytochemical investigation of Calotropis procera. Arab. J. Chem.. 2016;9:S230-S234.
- [CrossRef] [Google Scholar]
- Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56:185-191.
- [CrossRef] [Google Scholar]
- Antioxidant responses to oxidant-mediated lung diseases. Am. J. Physiol-Lung. C. 2002;283:L246-L255.
- [CrossRef] [Google Scholar]
- Flora of China. Beijing: Science Press; 1995. p. :32. 10.1371/journal.pone.0062794
- The phenols and prodelphinidins of white clover flowers. Phytochemistry. 2000;54:539-548.
- [CrossRef] [Google Scholar]
- Flavonoids from blue flowers of Nymphaèa caerulea. Phytochemistry. 1999;51:1133-1137.
- [CrossRef] [Google Scholar]
- Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine. 2017;26:55-61.
- [CrossRef] [Google Scholar]
- A new polyisoprenylated benzoylphloroglucinol derivative from Hypericum henryi subsp. uraloides (Guttiferae) Acta Bot. Yunnanica. 2008;30:515-518.
- [CrossRef] [Google Scholar]
- Planar catechin analogues with alkyl side chains: A potent antioxidant and an α-glucosidase inhibitor. J. Am. Chem. Soc.. 2006;128:6524-6525.
- [CrossRef] [Google Scholar]
- Vitexin and rutin isolation of hawthorn Leaves. Liaoning J. Tradit. Chin. Med.. 2012;39:2028-2030.
- [Google Scholar]
- Natural soluble epoxide hydrolase inhibitors from Inula helenium and their interactions with soluble epoxide hydrolase. Int. J. Biol. Macromol.. 2020;158:1362-1368.
- [CrossRef] [Google Scholar]
- Hydrolyzable tannins and related polyphenols from Eucalyptus globulus. J. Asian Nat. Prod. Res.. 2000;2:205-212.
- [CrossRef] [Google Scholar]
- Immunosuppressive constituents from Urtica dentata Hand. J. Asian Nat. Prod. Res.. 2010;12:707-713.
- [CrossRef] [Google Scholar]
- Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins: Struct. Funct. Bioinform.. 2006;65(3):712-725.
- [CrossRef] [Google Scholar]
- Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol. Pharm. Bull.. 2010;33:1242-1245.
- [CrossRef] [Google Scholar]
- Lignans and neolignans from the stems of Vibrunum erosum and their neuroprotective and anti-inflammatory activity. Arch. Pharm. Res.. 2015;38:26-34.
- [CrossRef] [Google Scholar]
- A flavonol glycoside-lignan ester and accompanying acylated glucosides from Monochaetum multiflorum. Phytochemistry. 2001;58:321-327.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of eudesmane-type sesquiterpenoids from Salvia plebeia. J. Nat. Prod.. 2017;80:2666-2676.
- [CrossRef] [Google Scholar]
- Inhibition of melanogenesis by piceid isolated from Polygonum cuspidatum. Arch. Pharm. Res.. 2010;33:1331-1338.
- [CrossRef] [Google Scholar]
- Screening cyclooxygenase-2 inhibitors from Andrographis paniculata to treat inflammation based on bio-affinity ultrafiltration coupled with UPLC-Q-TOF-MS. Fitoterapia. 2019;137:104259
- [CrossRef] [Google Scholar]
- Protective effect of daidzin against d-galactosamine and lipopolysaccharide-induced hepatic failure in mice. Phytother. Res.. 2009;23:701-706.
- [CrossRef] [Google Scholar]
- Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe. 2014;15:95-102.
- [Google Scholar]
- Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch. Pharm. Res.. 2002;25:842.
- [CrossRef] [Google Scholar]
- Phytochemical constituents from the fruits of Acanthopanax sessiliflorus. Arch. Pharm. Res.. 2002;25:280.
- [CrossRef] [Google Scholar]
- Antioxidative phenolic compounds from the roots of Rhodiola sachalinensis A. Bor. Arch. Pharm. Res.. 2000;23:455.
- [CrossRef] [Google Scholar]
- An experimental study of the anti-dysmenorrhea effect of Chinese herbal medicines used in Jin Gui Yao Lue. J. Ethnopharmacol.. 2019;245:112-181.
- [CrossRef] [Google Scholar]
- Evaluation of the potential hypoglycemic and beta-cell protective constituents isolated from Corni fructus to tackle insulin-dependent diabetes mellitus. J. Agr. Food Chem.. 2011;59:7743-7751.
- [CrossRef] [Google Scholar]
- Inhibitory kinetics and mechanism of rifampicin on α-glucosidase: Insights from spectroscopic and molecular docking analyses. Int. J. Biol. Macromol.. 2019;122:1244-1252.
- [CrossRef] [Google Scholar]
- Phytochemical constituents from Scutellaria baicalensis in soluble epoxide hydrolase inhibition: Kinetics and interaction mechanism merged with simulations. Int. J. Biol. Macromol.. 2019;133:1187-1193.
- [CrossRef] [Google Scholar]
- Study on effect of total coumarins from Urtica dentata on dextran sulfate sodium-induced colitis in mice. Chin. J. Chin. Mater. Med.. 2012;37:3316-3320.
- [CrossRef] [Google Scholar]
- Therapeutic effects of total coumarins from Urtica dentata Hand on collagen-induced arthritis in Balb/c mice. J. Ethnopharmacol.. 2011;138:523-529.
- [CrossRef] [Google Scholar]
- Musanahol: A new aureonitol-related metabolite from a Chaetomium sp. Tetrahedron. 2007;63:8174-8180.
- [CrossRef] [Google Scholar]
- Phenolic derivatives with free-radical-scavenging activities from Ixeridium gracile (DC.) Shih. Chem. Biodivers.. 2007;4:2172-2181.
- [CrossRef] [Google Scholar]
- Symmetrical and un-symmetrical curcumin analogues as selective COX-1 and COX-2 inhibitor. Eur. J. Pharm. Sci.. 2021;160:150743
- [CrossRef] [Google Scholar]
- First isolation of a flavonoid from Juniperus procera using ethyl acetate extract. Arab. J. Chem.. 2010;3:85-88.
- [CrossRef] [Google Scholar]
- A new sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus. Arch. Pharm. Res.. 2008;31:21-27.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of 3,4-diaryloxazolones: a new class of orally active cyclooxygenase-2 inhibitors. J. Med. Chem.. 2000;43:214-223.
- [CrossRef] [Google Scholar]
- Ganoderma cochlear metabolites as probes to identify a COX-2 active site and as in vitro and in vivo anti-inflammatory agents. Org. Lett.. 2020;22:2574-2578.
- [CrossRef] [Google Scholar]
- Deciphering inhibitory activity of flavonoids against tau protein kinases: a coupled molecular docking and quantum chemical study. J. Biomol. Struct. Dyn. 2020
- [CrossRef] [Google Scholar]
- Efficient synthesis of scutellarein. Nat. Prod. Res.. 2012;26:1278-1284.
- [CrossRef] [Google Scholar]
- Immunoinformatics and molecular dynamics studies to predict T-cell-specific epitopes of four Klebsiella pneumoniae fimbriae antigens. J. Biomol. Struct. Dyn.. 2020;14:1-11.
- [CrossRef] [Google Scholar]
- (Rel)-1β,2α-di-(2,4-dihydroxy-6-methoxybenzoyl)-3β, 4α-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii. Phytochemistry. 2000;55:439-446.
- [CrossRef] [Google Scholar]
- Chinese herbal medicine (Miao Yao Juan). Guizhou Science and Technology Publishing House; 2005.
- Identification and antioxidant activity of several flavonoids of Inga edulis leaves. J. Braz. Chem. Soc.. 2007;18:1276-1280.
- [CrossRef] [Google Scholar]
- Compendium of national standards for Chinese patent medicines division of orthopedics and traumatology. Beijing: State Drug Administration; 2002.
- Compilation of national standards for proprietary Chinese medicine. Beijing: State Drug Administration; 2002.
- Flavonoids and sterols from Alhagi sparsifolia. Chem. Nat. Compd.. 2008;44 365–365
- [CrossRef] [Google Scholar]
- Effects of analgesia, anti-inflammation and immunosuppression of acetic ether extract of Chinese medicine Honghuoma. Chin. Pharmacol. Bull.. 2009;25:559-560.
- [CrossRef] [Google Scholar]
- Inhibition of gut bacterial β-glucuronidase by chemical components from black tea: Inhibition interactions and molecular mechanism. Arab. J. Chem.. 2021;14:103457
- [CrossRef] [Google Scholar]
- Singlet oxygen oxidation of methyl linoleate: Isolation and characterization of the NaBH4-reduced products. Lipids. 1980;15:544-548.
- [CrossRef] [Google Scholar]
- Studies on the chemical constitutents in herb of Verbena officinalis. Chin. J. Chin. Mater. Med.. 2005;30:268-269.
- [Google Scholar]
- Novel antioxidant compounds from Tart cherries (Prunus cerasus) J. Nat. Prod.. 1999;62:86-88.
- [CrossRef] [Google Scholar]
- The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg. Med. Chem. Lett.. 2010;20:7159-7163.
- [Google Scholar]
- Total coumarins from Urtica dentata Hand prevent murine autoimmune diabetes via suppression of the TLR4-signaling pathways. J. Ethnopharmacol.. 2013;146:379-392.
- [CrossRef] [Google Scholar]
- Anti-HIV-1 activities of compounds isolated from the medicinal plant Rhus chinensis. J. Ethnopharmacol.. 2008;117:249-256.
- [CrossRef] [Google Scholar]
- Phytochemical constituents from Uncaria rhynchophylla in human carboxylesterase 2 inhibition: Kinetics and interaction mechanism merged with docking simulations. Phytomedicine. 2018;51:120-127.
- [CrossRef] [Google Scholar]
- Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules. 2013;18:1325-1336.
- [CrossRef] [Google Scholar]
- Evaluation of ferulic acid-biomembrane interaction by liposome electrokinetic chromatography. Chin. J. Anal. Chem.. 2007;35:1521-1524.
- [CrossRef] [Google Scholar]
- Immunosuppressive effects of an ethyl acetate extract from Urtica dentata Hand on skin allograft rejection. J. Ethnopharmacol.. 2009;126:57-63.
- [CrossRef] [Google Scholar]
- P-terphenyls from Aspergillus sp. GZWMJZ-055: Identification, derivation, antioxidant and α-glycosidase inhibitory activities. Front. Microbiol.. 2021;12:654963
- [CrossRef] [Google Scholar]
- Flavonoid constituents and their antioxidant activity of Laportea bulbifera Weddell. Kor. J. Pharmacogn.. 2003;34:18-24.
- [Google Scholar]
- Report on the Scientific and Technological Competitiveness of Large Varieties of Traditional Chinese Medicine. The People’s Medical Publishing House; 2019.
- Norsesquiterpene and steroid constituents of Humulus japonicus. Nat. Prod. Science. 2007;13:332-336.
- [Google Scholar]
- Pasteurized and sterilized maple sap as functional beverages: Chemical composition and antioxidant activities. J. Funct. Foods. 2013;5:1582-1590.
- [CrossRef] [Google Scholar]
- Chemical constituents of the root of Jasminum giraldii. Molecules. 2013;18:4766-4775.
- [CrossRef] [Google Scholar]
- Molluscicidal activity of Aglaia duperreana and the constituents of its twigs and leaves. Fitoterapia. 2012;83:1081-1086.
- [CrossRef] [Google Scholar]
- Chemical constituents in ethyl acetate extract from Physalis alkekengi var. franchetii. Chin. Tradit. Herbal. Drugs.. 2015;46:1120-1124.
- [CrossRef] [Google Scholar]
- Flavonoids from Laportea bulbifera and their anti-N1 neuraminidase activities. J. Shenyang Pharm. Univ.. 2018;35:931-935. +942
- [Google Scholar]
- Discovery of a novel chemotype of tyrosine kinase inhibitors by fragment-based docking and molecular dynamics. ACS Med. Chem. Lett.. 2012;2012(3):834-838.
- [Google Scholar]
- Natural soluble epoxide hydrolase inhibitors from Alisma orientale and their potential mechanism with soluble epoxide hydrolase. Int. J. Biol. Macromol.. 2021;183:811-817.
- [CrossRef] [Google Scholar]
- Studies on the chemical constituents of Laportea bulbifera. Chin. Med. Mat.. 2011;34:223-225.
- [CrossRef] [Google Scholar]
- Studies on chemical constituents of Paederia scandense. Chin. J. Chin. Mater. Med.. 2006;31:1436-1441.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103723.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







