Translate this page into:
Biodesalination performance of Phormidium keutzingianum concentrated using two methods (immobilization and centrifugation)
⁎Corresponding author. alyhassan@uaeu.ac.ae (Ashraf Aly Hassan) hosniaa@mail.uc.edu (Ashraf Aly Hassan) alyhassan@uaeu.ac.ae (Ashraf Aly Hassan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Comparison between two treatment methods for Phormidium keutzingianum was studied. Chloride ion measurements affirmed biodesalination process using P. keutzingianum. Biosorption and bioaccumulation process was debriefed with salt uptake mechanism. Osmoregulation experiment confirmed that P. keutzingianum can survive extreme salt.
Abstract
The biodesalination performance of the cyanophycean Phormidium keutzingianum concentrated using two methods (centrifugation and immobilization) was evaluated for the first time at NaCl concentrations of 10, 30, 50, and 70 g/L. In addition, the osmoregulation ability of P. keutzingianum was assessed at high salinities (50, 70, 85, and 100 g/L) over a relatively short time period. The pH, electrical conductivity, and chloride, nitrate, and phycocyanin concentrations were measured during all experiments. The immobilization method of concentration resulted in a chloride removal rate of up to 44 % during the osmoregulation experiment at a salinity of 100 g/L. For the centrifugation method, the chloride removal rate was 26 % on the 20th day of observation for 10 g/L. Thus, the immobilization method resulted in faster chloride ion removal than the centrifugation method. The findings demonstrate that biodesalination efficiency can be enhanced by the use of different P. keutzingianum preparation methods.
Keywords
Biodesalination
Phormidium keutzingianum
Biosorption
Bioaccumulation
Algal growth
Osmoregulation
1 Introduction
The primary applications of algae/cyanobacteria include biofuels, food processing, animal feed, fertilizers, therapeutic supplements, and cosmetics (Barsanti and Gualtieri, 2014). Recently, cyanobacteria cultivation has been suggested to treat wastewater because cyanobacteria consume nitrate and phosphate ions for growth (Ahmad, 2021; El-Sheekh et al., 2022). Several recent studies have focused on the use of algae and cyanobacteria to treat saline water (Zafar et al., 2021). This procedure, which is commonly termed biodesalination, is a potential inexpensive desalination solution in places that lack sources of freshwater. In biodesalination, algae/cyanobacteria consume the ions that negatively affect the water quality as a source of nutrients for growth. As a result, the water can be treated, and the biomass can be further utilized for other commercial products. Furthermore, biodesalination is expected to result in less brine being returned to the sea, resulting in reduced marine toxicity.
Cyanobacteria consume ions and nutrients through different metabolic processes, including biosorption and bioaccumulation (Wei et al., 2020). In biosorption, ions attach to the outer cell walls of living organisms due to the presence of different charges, extracellular polymeric substances (EPS), and functional groups (Sahle-Demessie et al., 2019; Wei et al., 2020). In bioaccumulation, cyanobacteria consume ions through diffusion and ionic transport, and the ions accumulate inside the cells (Zafar et al., 2021). Therefore, the efficiency of the cyanobacteria is independent of the cultivation method used to remove salt from the water. Different growth factors can be considered to increase the efficiency of algae/cyanobacteria for biodesalination, including the selection of species, light intensity, nutrient media, air, and carbon dioxide (Gao et al., 2021; Singh and Singh, 2015; Zafar et al., 2021).
In biodesalination studies, the main goal of algae/cyanobacteria cultivation is to achieve the maximum salt removal from brackish or saline water. Barahoei et al. (2021) used Chlorella vulgaris (C. vulgaris) for brackish water desalination and obtained a maximum salt removal rate of ∼80 % in water with a low salinity of 1 g/L. C. vulgaris is a freshwater species that is adaptable to saline water. Therefore, some of these cyanobacteria and algae are found in halophilic environments such as salty marshes and salt lakes. Phormidium keutzingianum is a terrestrial cyanobacteria with a filamentous morphology. While P. keutzingianum is known to be halotolerant (Kirkwood et al., 2008), it has not been investigated in different treatment methods. In studies on salt removal with algae and cyanobacteria biomass is often directly inoculated in water (suspension) (Figler et al., 2021, 2019; Lutzu and Dunford, 2019; Sahle-Demessie et al., 2019). Such studies on salt removal from algae/cyanobacteria suspensions have obtained salt removal rates reaching 70–80 % in different experimental setups. In another study, algae and wastewater activated sludge consortia was tested on produced wastewater removing 25 % of chloride ions using the strain in suspension (Nadersha and Aly Hassan, 2022). Table 1 summarizes the findings of studies on salt removal using several strains of algae and cyanobacteria based on the factors of treatment method, salt removal efficiency, treatment time, nutrient media, and light intensity.
Species/Taxa
TM a
Salinity (g/L)
RE b
TT c (days)
NM d
LI e (Lux)
Reference
Phormidium keutzingianum (Cyanobacteria)
SS1
30
50
7046 %
44 %
29 %68
BG-11+
1 % NaCl2
2885
Zafar et al. (2022b)
Phormidium keutzingianum (Cyanobacteria)
SS
10
40 %
80
BG-11+
1 % NaCl2885
Zafar et al. (2022a)
Chlorella vulgaris (Algae)
SS
1
80 %
15
M.BG-11
1700
Barahoei et al. (2021)
Coelastrum morus (Algae)
SS
10
70 %
14
BBM
1320
Figler et al. (2021)
Scenedesmus obliquus (Algae)
SS
8.8
20 %
0.02
BG-11
1650
Wei et al. (2020)
Scenedesmus obliquus (Algae)
C.3
8.8
15 %
0.02
BG-11
1650
Wei et al. (2020)
Chlorella vulgaris (Algae)
SS
20
27 %
14
BBM
1320
Figler et al. (2019)
Scenedesmus obliquus (Algae)
SS
4
30 %
40
f/2 medium4
1320
Sahle-Demessie et al. (2019)
Scenedesmus obliquus (Algae)
SS
5
39 %
14
BBM
1320
Figler et al. (2019)
Phormidium keutzingianum (Cyanobacteria)
SS
13.5
64 %
30
BG-11+
1 % NaCl1320
Lutzu and Dunford (2019)
Geitlerinema amphibium (Cyanobacteria)
SS
13.5
33 %
30
A+5
1320
Lutzu and Dunford (2019)
Geitlerinema carotinosum (Cyanobacteria)
SS
13.5
35 %
30
A+
1320
Lutzu and Dunford (2019)
Aphanothece sp. (Cyanobacteria)
SS
13.5
28 %
30
BG-11
1320
Lutzu and Dunford (2019)
Nannochloropsis oculate (Algae)
SS
2.8
10 %
10
f/2 medium
1900
Shirazi et al. (2018)
Scenedesmus obliquus (Algae)
SS
4.8
80 %
16
BG-11
1650
Gan et al. (2016)
As shown in Table 1, most studies on biodesalination by algae/cyanobacteria have focused on suspended algae/cyanobacteria. Wei et al. (2020) applied the concentrating method on S. obliquus by centrifuging and freeze-drying the sample at −60 °C for 12 h. However, in their study, salinity was reduced by 15 % using concentrated S. obliquus, which was less than the treatment used in suspension, i.e., 20 %. Technically dense biomasses should have more tendency to consume ions based on species taxonomy and their natural habitat, which is hypothesized with the same argument on study of ions uptake (Zafar et al., 2021). Zafar et al. (2022a) used P. keutzingianum strain in suspension and achieved around 40 % removal efficiency of chloride ion in 10 g/L salinity. Their study was not performing any further treatment on the strain P. keutzingianum. Another significant factor in biodesalination studies is the salinity ranges, which were mostly in the ranges of brackish water, i.e., between 1 and 20 g/L; see Table 1 for reference.
Furthermore, P. keutzingianum is one of the halophytic strains that have the capacity to survive even at harsh salinity of 70 g/L (Zafar et al., 2022b). Another observation found in Table 1 is that the studies conducted at lower brackish salinities have significant salt removal efficiencies. Therefore, in this study, we evaluated the biodesalination performance of P. keutzingianum concentrated using two approaches (immobilization and centrifugation) in water from salinity levels (10, 30, 50, and 70 g/L NaCl).
The critical factors related to growth and higher cell density are mainly supplemental media and light intensity. Nutrient media serves as a food supplement for the growth of algae or cyanobacteria cells containing essential ions such as sodium, calcium, potassium, magnesium, iron, nitrate, nitrite, phosphate, sulfate, and chloride ions. The light intensity is necessary for the cultivation of algae/cyanobacteria due to the presence of chlorophyll-a, b pigment that executes the photosynthesis process (Ho et al., 2012; Singh and Singh, 2015). Algae consume ions and light for photosynthesis, in which natural sugars are produced along with adenosine triphosphate (ATP) for energy and growth (Gao et al., 2021). In most nutrient media, the salts are similar; however, only the final concentration of ions varies in different recipes. Moreover, the target is to grow a higher number of cells with the nutrient media.
Moreover, the durations of past studies on algae/cyanobacteria in suspension were relatively long. Therefore, we also attempted to reduce the time needed to treat saline water. Finally, we conducted an osmoregulation experiment using only the immobilization method of concentration to evaluate the tolerance of P. keutzingianum to extreme salinities (50, 70, 85, and 100 g/L NaCl) over short retention times. The objectives were as follows: 1) estimate the salt tolerance of P. keutzingianum; 2) evaluate the ion/salt removal rate of P. keutzingianum by measuring the electrical conductivity (EC) and Cl− ion content; 3) examine the biosorption and bioaccumulation of P. keutzingianum concentrated by centrifugation and immobilization in water with different salinities and reveal the possible mechanism; and 4) evaluate the osmoregulation effect of P. keutzingianum concentrated by immobilization to reveal the rate of Cl− ion removal over short durations.
2 Materials and methods
2.1 Materials
2.1.1 Cyanobacterial culture inoculation
P. keutzingianum was collected from the culture collection center at the University of Texas, Austin. The stock of cyanobacteria was inoculated into a 1000-mL Erlenmeyer flask at 23 °C and cultivated at an irradiance of 2885 lx continuously.
The volume of P. keutzingianum solution was scaled up to 5000 mL in a Schott bottle. Air was supplied continuously to increase the stock of cyanobacteria. The Schott bottle was agitated at 2000 rpm on a magnetic stirrer to uniformly mix the cyanobacteria.
2.1.2 Reagents and nutrient media preparation
The following analytical grade reagents in the experiments were used containing sodium nitrate NaNO3 (98.5 %), dipotassium hydrogen phosphate K2HPO4 (98.5 %), magnesium sulfate heptahydrate MgSO4·7H2O (99 %), calcium chloride dihydrate CaCl2·2H2O (99 %), citric acid monohydrate C6H8O7·H2O (99.5 %), disodium EDTA dihydrate Na2EDTA·2H2O, and anhydrous sodium carbonate Na2CO3 (99.8 %). For preparing trace metal solution following analytical grade reagents used containing anhydrous boric acid H3BO3 (99.5 %), manganese chloride tetrahydrate MnCl2·4H2O (99.5 %), zinc sulfate heptahydrate ZnSO4·7H2O (99 %), sodium molybdate dihydrate Na2MoO4·2H2O (99.5 %), copper sulfate pentahydrate CuSO4·5H2O, cobalt (II) nitrate hexahydrate Co(NO3)2·6H2O The BG-11 medium contained all these reagents in 10 mL volume per liter. The trace metal solution was spiked with a 1 mL of volume per liter. P. keutzingianum was grown in BG-11 + 10 g/L NaCl. All these reagents were purchased from the Scientific Progress L.L.C., AlAin, UAE. Distilled water was used to prepare the stock solutions of these reagents and their final concentrations are presented in (Supplementary Data-Table S1).
2.1.3 Saline medium preparation
For saline water experiments, synthetic saltwater was prepared in the laboratory using NaCl. The salt was added to the growth medium BG-11, which was the source of nutrition for the cyanobacteria. The salt was added to obtain NaCl concentrations of 10, 30, 50, and 70 g/L in the BG-11 medium (BG-11 + 10 g/L NaCl, BG-11 + 30 g/L NaCl, BG-11 + 50 g/L NaCl, and BG-11 + 70 g/L NaCl), respectively; Supplementary Data Table S2). For the osmoregulation experiment, salt was added to the BG-11 medium to obtain final concentrations of BG-11 + 50 g/L NaCl, BG-11 + 70 g/L NaCl, BG-11 + 85 g/L NaCl, and BG-11 + 100 g/L NaCl. The term “salinity” used in this article refers to the NaCl salt concentrations and its reduction measured by electrical conductivity and ion chromatography.
2.1.4 Negative control preparation
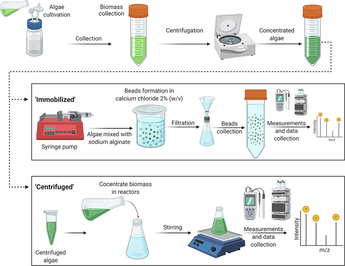
A negative control was prepared for the centrifugation experiments by adding NaCl salt to BG-11 medium to obtain salt concentrations of 10, 30, 50, and 70 g/L (Supplementary Data-Table S2). The composition of BG-11 was the same as for the growth of cyanobacteria. The cyanobacteria could not grow in the negative control and were replaced instantly if cyanobacteria grew inside the reactors. The negative control for the immobilization experiments was prepared by adding alginate beads into solutions with different salt concentrations. A 5 % sodium alginate (SA) solution was prepared at 5000–7000 rpm on a magnetic stirrer. The mixed SA solution was placed on a syringe pump, and beads were prepared by dropping the SA into a 2 % (w/v) calcium chloride solution at a rate of 5 mL/min. Each reactor was poured with 12.5 % of beads volume in 200 mL of final volume.
2.2 Cyanobacteria treatment methods
2.2.1 Cyanobacteria concentration via centrifugation
The cyanobacteria were collected from the stock in 50-mL centrifuge tubes. The tubes were centrifuged (Thermo Scientific-SL8, Waltham, Massachusetts, U.S.) at 6000 rpm for 10 min. The supernatant was discarded, and the cyanobacteria were washed twice with deionized (DI) water. The centrifuged cyanobacteria were resuspended to a volume of 10 mL with DI water in a graduated cylinder. The cyanobacteria concentrate was kept in a refrigerator at 4 °C overnight.
Batch experiments using the centrifuged cyanobacteria were conducted using Schott bottles (250 mL) as reactors. The salt, including the nutrient source, was added to a volume of 240 mL. The solution was mixed by agitating at 5000 rpm on a magnetic stirrer. The stirred solution was placed in the refrigerator overnight at 4 °C. The remaining 10 mL of centrifuged cyanobacteria was then added to the reactor to reach the final volume of 250 mL. The reactors were placed on a 10-plate magnetic stirrer (Fine PCR-ST10, South Korea) at 200 rpm for experimentation.
2.2.2 Cyanobacteria concentration via immobilization
Immobilized beads were formed as follows. First, immobilized algal beads were prepared by collecting cyanobacteria from the stock in 50-mL falcon tubes (approximately 24–30 tubes in total) to reach the desired volume. The cyanobacteria-filled tubes were centrifuged at 7500 rpm for 15 min, and the supernatant was discarded. The biomass was resuspended using a vortex machine set at 2000 rpm. The concentrated biomass was collected in a graduated cylinder up to 50 mL. To prepare the algal beads, SA 5 % (w/v) solution was prepared in a 500-mL beaker and stirred on a magnetic stirrer at 7000–8000 rpm for complete mixing. To determine the optimal ratio of SA to blue-green algae, a preliminary experiment was performed to check the efficacy of the algal beads with mixing ratios of 1:1, 2:1, and 3:1 (SA: blue-green algae, v/v) were tested. A 4 % (w/v) calcium chloride solution was prepared to stabilize the spherical shape of SA beads. Finally, the SA and blue-green algae mixture were loaded into a syringe pump, and the beads were formed in the calcium chloride solution, which was placed in beakers below the syringe pump (flow rate = 5 mL/min). The beads were left overnight to settle and form circular shapes. On the next day, the beads were washed with DI water. The average bead size was approximately 2.3 ± 0.5 mm. Based on the preliminary experiment, a 2:1 ratio of SA to blue-green algae (v/v) was optimal. This ratio was used in all immobilized cyanobacteria experiments, including the osmoregulation experiment. Finally, the beads were placed in the BG-11 + NaCl reactors. Four different concentrations of NaCl were evaluated: 10, 30, 50, and 70 g/L. Three replicate experiments were conducted for each concentration to ensure precision and accuracy in the results. Each reactor contained 200 mL of BG-11 + NaCl and 25 mL of beads. A mesh was added to displace the beads toward the wall of the beaker to receive sufficient light for growth.
2.2.3 Phycocyanin (PC) experiment
After some time, a blue pigment was observed in the reactors. The pigment released by the cyanobacteria was determined to be PC, a primary blue phycobiliprotein pigment found in cyanobacteria that has many applications in the food, cosmetics, and biotechnology industries (Wood et al., 2015). To verify the presence of PC, we conducted a series of experiments with salinities of 10, 30, 50, and 70 g/L in 200-mL reactors with immobilized cyanobacteria. The cyanobacteria were prepared as described in Section 2.2.2, and the experiments were performed in triplicate. The PC concentration was determined using the following equation based on the optical densities at 615 and 652 nm (OD615 and OD652, respectively), which were measured using a spectrophotometer (Hach DR-3900, Germany) (Arashiro et al., 2020; Beer and Eshel, 1985):
2.2.4 Osmoregulation experiment
To verify the ability of the algal beads to uptake ions under extreme salinity, an osmoregulation experiment was conducted using immobilized cyanobacteria, as described in Section 2.2.2. The reactor volume was 250 mL. The difference between this experiment and the immobilization experiment described in Section 2.2.2 was the salt concentration in the reactor and the experimental duration. The salinities used in this experiment were 50, 70, 85, and 100 g/L. Water samples were collected from the reactors before adding the cyanobacteria and at different time points after cyanobacteria inoculation (15, 30, 60, 120, 240, and 360 min). The Cl− and NO3– concentrations were monitored using ion chromatography. Table 2 summarizes all experiments conducted during this study. Note: * denotes that the experiment was conducted in triplicate.
Treatment method
Salinity (g/L)*
Temperature (°C)
Light Intensity (Lux)
Volume of reactor (mL)
Duration of experiment
Centrifugation
10
23–25
2885
250
60 d
30
50
70
Immobilization
10
23–25
2885
200
14 d
30
50
70
Immobilization (Phycocyanin)
10
23–25
2885
200
14 d
30
50
70
Immobilization (Osmoregulation)
50
23–25
2885
200
360 min
70
85
100
2.3 Statistical analysis
All reactors were run in triplicate, and the parametric results are reported as mean ± standard deviation. Differences between means were evaluated by one-way analysis of variance (ANOVA), and the significance of differences were assessed by Tukey’s test using OriginPro 2020 SR1 Version 9.7 (OriginLab, Northampton, MA, USA). The ANOVA analysis compared the two cyanobacteria concentration methods (centrifugation and immobilization) at a given salinity along with different salinities. Tukey’s t-test results are shown in the (Supplementary data – Fig. S2) as a box plot results.
2.4 Parametric and analytical measurements
The pH was continuously monitored using a pH probe (Extech-407228, China). Salinity was measured based on the EC (Horiba-LAQUA EC210, Japan). The anion concentration was measured by ion chromatography (Thermo Scientific-Dionex Aquion AS-DV, Waltham, Massachusetts, U.S.). NO3– and Cl− were the most abundant ions measured by ion chromatography. A dilution factor of 1000× to 2000× was used when sampling saline water for ion chromatography. For the osmoregulation experiment, a higher dilution factor of 2500× (85–100 g/L) was used due to the higher instrument sensitivity. The diluted samples were filtered by a 0.22-µm nylon membrane to avoid contamination of the ion chromatography column.
The optical density at 620 nm was measured by a spectrophotometer (HACH-DR3900, Germany) to estimate the growth of centrifuged P. keutzingianum in 10-mL aliquots collected from the reactors (Suryata et al., 2010; Zafar et al., 2022a). The corresponding data can be found in Section 1 (Supplementary Data – Fig. S1).
3 Results and discussion
3.1 Changes in pH
The pH is an essential parameter that provides information about the redox reaction and metabolic reactions that lead to the exchange of electrons/ions during photosynthesis in algae/cyanobacteria (Gao et al., 2021). Fig. 1a illustrates the change in pH in solutions of centrifuged cyanobacteria during cultivation from day 0 to day 70. In the negative control, the pH remained in the range of 7.5–8.0 for all tested salinities. In contrast, the pH values measured on day 0 in the reactors containing centrifuged cyanobacteria were 8.8, 8.9, 9.0, and 9.2 for salinities of 10, 30, 50, and 70 g/L, respectively. The maximum pH (11.9) was observed on day 42 at the salinity of 10 g/L. Similar patterns were observed at salinities of 30, 50, and 70 g/L, with maximum pH values of 11.4, 11, and 10.9, respectively. At the end of the experiment (70 days), the pH values at salinities of 10, 30, 50, and 70 g/L had decreased to 10.8, 9.8, 9.1, and 9.4, respectively.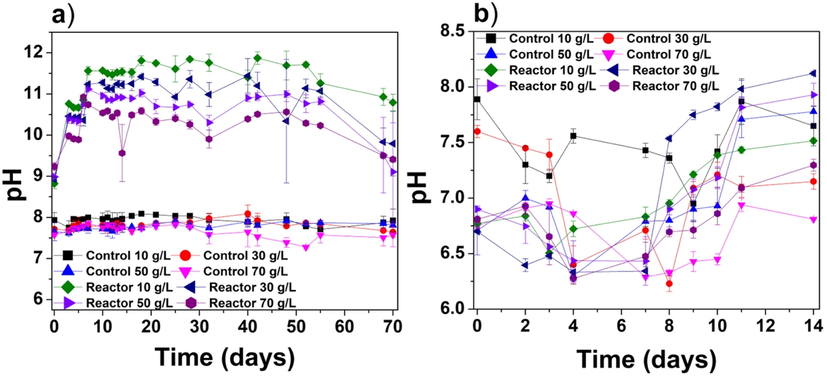
The pH as a function of time for the (a) centrifuged cyanobacteria experiment and (b) immobilized cyanobacteria experiments.
The photosynthetic activity of algae/cyanobacteria usually increases the pH due to the transfer of electrons from photosystem II (PSII) to photosystem (PSI). However, other factors also contribute to the increase in pH. During photosynthesis, the cyanobacteria experience electron exchange, ionic exchange, and carbon fixation. The Na+/H+ antiporters, which are key channels involved in Na+ and H+ circulation in eukaryotes and prokaryotes, play essential roles in Na+ homeostasis and affect cell pH (Padan and Schuldiner, 1993). Since the current experiment was conducted in hypersaline water, significant Na+ ions were available for the Na+/H+ antiporters. The optimum pH range for cyanobacteria is between 7.5 and 10 (Singh et al., 2016). However, in this study, we observed higher pH values in the centrifuged cyanobacteria experiments at all tested salinities. Two factors might have contributed to this high pH. First, at high salinity, the Na+/H+ antiporters may activate at an elevated Na+ concentration (Blumwald et al., 1984; Nitschmann and Packer, 1992). However, cyanobacteria can neutralize the pH in the cell cytoplasm (Elanskaya et al., 2002). A sodium cycle involving Na+/H+ antiporters maintains pH homeostasis in cyanobacteria growing at high pH (i.e., 12–14), similar to heterotrophic bacteria (Padan and Schuldiner, 1993). Second, after adding cyanobacteria, the pH can increase as the carbonate/bicarbonate in the culture medium is dissolved, producing carbon dioxide; this process generates OH− ions during the cultivation of cyanobacteria (Richmond and Grobbelaar, 1986). Both these phenomena can occur simultaneously and explain the increasing pH during the centrifuged cyanobacteria experiments.
Fig. 1b shows the change in pH during the immobilized cyanobacteria experiments over 14 d. The pH in these experiments remained in relatively close to neutral (6.25–8.25); thus, the change in pH was not as significant as in the centrifuged cyanobacteria experiments. In both the negative control and reactors with immobilized cyanobacteria, the pH fluctuated throughout the experiment. On day 0, the pH values of the experimental reactors were 6.7, 6.7, 6.9, and 6.8 for salinities of 10, 30, 50, and 70 g/L, respectively; the corresponding pH values on day 14 were 7.5, 8.1, 7.9, and 7.3, respectively. The lack of any distinct trend in pH may be due to the overlapping of the SA beads. However, the transport of ions through immobilized surfaces is not well understood at present. A similar fluctuation in pH without any clear trend was found in another study of immobilized algae (Chlorella sorokiniana) (Mollamohammada et al., 2020). The pH trend in the immobilized cyanobacteria experiments remains unknown because immobilization could have growth inhibition that may affect the transport of ions from the inside to the outside surface of the beads. However, comparing Fig. 1a & b reveals a significant difference between the pH values in the centrifuged and immobilized cyanobacteria experiments with an F-value of ∼183. There is a significant difference between the pH means of two treatment methods. Understanding this large difference in pH requires further in-depth investigation at nanoscale in future.
3.2 EC in the centrifuged and immobilized cyanobacteria experiments
The EC reduction in the centrifuged and immobilized cyanobacteria experiments are shown in Fig. 2a and 2b, respectively. Several studies have measured EC to estimate the desalination rate using different algal/cyanobacteria species (Figler et al., 2019; Gan et al., 2016; Sahle-Demessie et al., 2019; Shirazi et al., 2018; Wei et al., 2020). However, cyanobacteria are involved in photosynthesis and carbon fixation, which generate oxygen (Moroney and Somanchi, 1999) and may release bicarbonate and carbonate ions back to the water. Since bicarbonate/carbonate ions contribute to overall salinity (Williams and Sherwood, 1994), these ions may affect the EC readings. As shown in Fig. 2a, the EC decreased gradually during the 70-day centrifuged cyanobacteria experiment under all tested salinities. In contrast, no significant reduction in electrical conductivity was observed in the negative control. In the centrifuged cyanobacteria reactor with a salinity of 30 g/L, the maximum reduction in EC reached 11.5 %. For centrifuged cyanobacteria experiments, the final EC reductions for salinities of 10, 50, and 70 g/L were 8.1 %, 8.4 %, and 9.1 %, respectively, while for 30 g/L salinity the reduction was 10.8 %.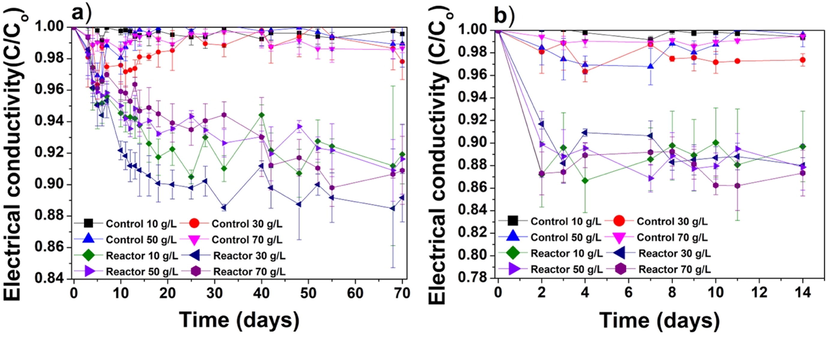
Reduction in electrical conductivity (C/Co) monitored over the durations of the (a) centrifuged cyanobacteria and (b) immobilized cyanobacteria experiments.
Fig. 2b shows EC values during the 14-day immobilized cyanobacteria experiments. The maximum EC reduction (13.3 %) was observed on the 4th day in the reactor with a salinity of 10 g/L. The EC reductions obtained at the salinities of 10, 30, and 50 g/L were 10.3 %, 12.0 %, and 12.1 %, respectively. For the salinity of 70 g/L, the EC decreased significantly within 2 days of inoculation with the immobilized cyanobacteria, and the EC reduction reached 12.6 %. At the end of the experiment (day 14), the maximum EC reduction (12.7 %) was observed for the salinity of 70 g/L. EC reduction trend was similar to those reported by (Zafar et al., 2022a), where the same strain was studied in suspension. In their study, the EC reduction was affected by the cyanobacteria carbon fixation cycle, which were hindering the results obtained from ion chromatography. Since the current study was tested on same cyanobacteria therefore similar EC reduction pattern was observed in both centrifuged and immobilized cyanobacteria experiments. It was anticipated that EC values might show some difference in immobilized experiments, however no significant reduction was observed. ANOVA results showed significant statistical differences in EC results between the centrifuged and immobilized cyanobacteria experiments with an F-value of ∼8.4.
3.3 Chloride ion concentration in the centrifuged and immobilized cyanobacteria experiments
In desalination studies, removing Cl− ions is essential because these ions significantly contribute to salinity, in addition to Na+, SO42−, K+, Ca2+, and Mg2+. In seawater with a salinity of 35 g/L, most of the major ions are present in the form of Ca2+, Mg2+, K+, Na+, SO42−, Cl−, and HCO3− at concentrations of 10.2, 53.2, 10.2, 468.0, 28.2, 545, and 2.4 mmol/L, respectively (Bąbel and Schreiber, 2014). Therefore, in this study, Cl− ion removal was considered to be the best indicator of biodesalination potential. Fig. 3a illustrates the reduction in Cl− ions to assess the removal efficiency of centrifuged cyanobacteria. The maximum removal rate of Cl− ions differed based on the salinity of the reactor. For instance, for the salinity of 10 g/L, the maximum Cl− ion removal of 26.2 % was observed on the 19th day after inoculation with P. keutzingianum. For the salinities of 30, 50, and 70 g/L, the maximum chloride ion removal rates (18.4 %, 24.2 %, and 21.7 %, respectively) were observed on the 14th, 39th, and 5th days, respectively. Therefore, in the centrifuged cyanobacteria experiments, the maximum Cl− ion uptake by P. keutzingianum was observed at the salinity of 10 g/L.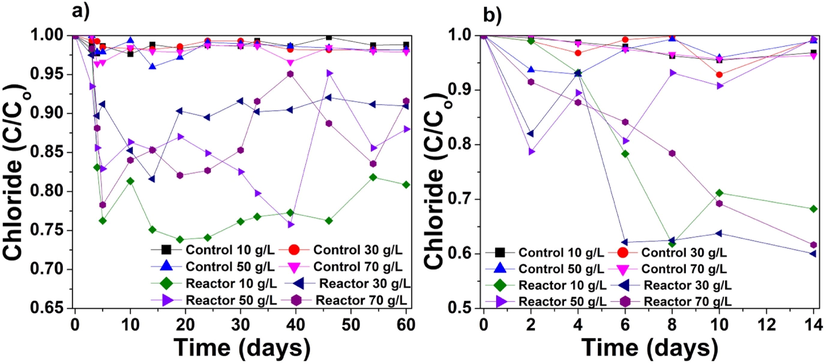
Reduction in Cl− ion concentration (C/Co) in the (a) centrifuged cyanobacteria and (b) immobilized cyanobacteria experiments.
We found remarkably more Cl− ion uptake by the algal beads compared to the centrifuged cyanobacteria. Fig. 3b shows the reduction in Cl− ion concentration during the immobilized cyanobacteria experiments. The maximum Cl− ion reduction rates obtained in the reactors with salinities of 10, 30, 50, and 70 g/L were 38.1 %, 39.9 %, 21.3 %, and 38.3 % on day 8, 14, 2, and 14, respectively. Thus, the maximum Cl− ion removal by immobilized cyanobacteria was obtained under a salinity of 30 g/L. The difference in Cl− ion reduction between the centrifuged and immobilized cyanobacteria experiments was statistically significant for all the salinities with an F-value of ∼3.2 (Supplementary data – Table S-3).
To understand the mechanism of Cl− ion removal by P. keutzingianum, many factors affecting chloride ion uptake should be considered. Fig. 4 shows a general schematic of the biosorption and bioaccumulation processes; the explanation of other phenomena that might occur is explained in detail below.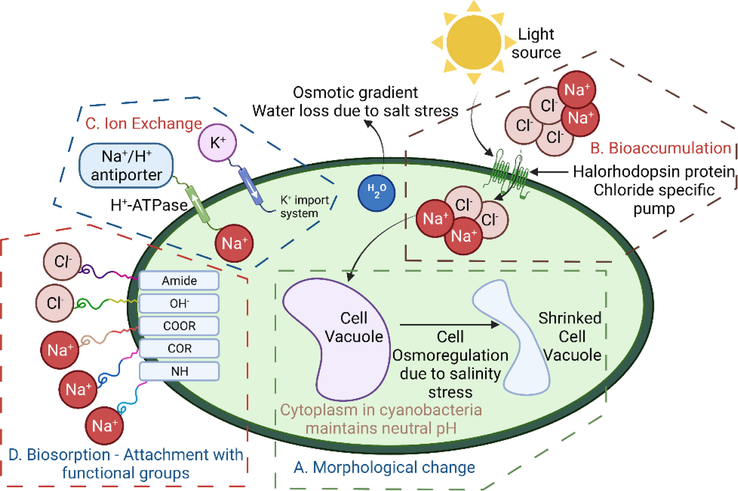
A mechanism of salt removal by P. keutzingianum: A) morphological changes; B) bioaccumulation process; C) ion exchange; D) biosorption of salt and binding with functional groups.
3.3.1 Modification in cell morphology after salinity exposure
Na+ ion is essential for nutrient transport (e.g., Na+-dependent HCO3− transport) in unicellular organisms (Amezaga et al., 2014; Apte and Thomas, 1983; Espie et al., 1988). Na+ ion is also essential for cell subdivision in heterotrophic cyanobacteria and pH homeostasis under alkaline environments (Elanskaya et al., 2002; Maeso et al., 1987; Padan and Schuldiner, 1993). When the Na+ ion concentration in the cell increases to a toxic level, the fatty acids in the cell membrane destabilize, the electron transport between H2O and PSII is inhibited, and photoautotrophic growth stops (Allakhverdiev and Murata, 2008; Bhargava et al., 2003; Huflejt et al., 1990). Successful salt acclimation depends upon the adaptation of cyanobacterial cells to the environment and the length of salt exposure (Amezaga et al., 2014; Hagemann, 2011). The adjustment of cell turgor pressure is one of the earliest responses to salt stress (Blumwald et al., 1984). A halophytic alga, Pheridia tenuis, reported storing salt in the cell vacuoles (Khan and Weber, 2006; Yensen, 2006). However, the following changes occur when cyanobacteria are exposed to salt: (1) restoration, unbalancing of the cell turgor (walled cell) pressure, and cell volume change (wall-less cell) (Gao et al., 2021); (2) changes in cell membrane porosity (Minas et al., 2015); (3) adjustment between K+ uptake and Na+ discharge from the cell; (4) enzyme deactivation; (5) increased production of reactive oxygen species (Fal et al., 2022); (6) decreased growth rate; and (7) cellular ionic disproportion and significant cell dehydration, which affects the cell morphology (Fig. 4a) (Mahajan and Tuteja, 2005; Tiflickjian and Raybum, 1986). When Na+ and Cl− ions move into the cell vacuole, they cause cell dehydration. K+ ions and other organic solutes, such as proline and glycine, are released into the cell cytoplasm from the vacuole. The algal cytoplasm balances the osmotic pressure, as confirmed by a study on Chlorella autotrophica (Ahmad and Hellebust, (1984). An osmotic gradient is formed when cyanobacteria are introduced into extreme salinities; this phenomenon is termed “salt shock” (Kirst, 1990).
3.3.2 Bioaccumulation
Bioaccumulation is another process that may occur during the uptake of salt by cyanobacteria/cyanobacteria (Fig. 4b). Bioaccumulation is an energy-dependent biological process in living cells in which salt and other ions accumulate inside the biomass (Chojnacka, 2010; Fal et al., 2022). Bioaccumulation is a slow process because salt ingestion takes place until the cells are in living condition. It takes place until the salt deposits inside the cell through diffusion or ion-exchange process. Due to the concentration gradient, salt diffuses steadily into the cell with the help of ATPase enzymes or ATP as an energy source (Timková et al., 2018). Usually, ATP is considered an external energy source in cells. However, ATP-independent light-activated biological batteries called “halorhodopsin” proteins have been identified in cyanobacteria (Amezaga et al., 2014; Sahle-Demessie et al., 2019). Halorhodopsin occurs naturally in the salt-tolerant haloarchaea species and is known as a Cl−-specific pump (Klare et al., 2011; Schobert and Lanyi, 1982). Halorhodopsin is considered to remove chloride ions from the surrounding brackish or hypersaline water.
3.3.3 Ion exchange
K+ ions are essential ions associated with the cytoplasm and are vital for algal growth (Iyer et al., 2015). The exchange of Na+ with ions such as K+ and H+ allows cyanobacteria to actively transport intracellular Na+ out of the cytoplasm against the electrochemical Na+ gradient (Gao et al., 2021; Hagemann, 2011; Rai and Tripathi, 2009), as shown in Fig. 4c. Initially, Na+ competes with K+ for uptake (Gao et al., 2021), and the ionic exchange leads to a deficiency in K+ in the cell cytosol (Affenzeller et al., 2009). Homeostasis between K+ and Na+ is maintained by the intake of K+ into the cell through the K+ import system, and loss of Na+ occurs (Chen et al., 2019; Hagemann, 2011; Matsuda et al., 2004; Rai et al., 2015).
3.3.4 Biosorption
Fig. 4d schematically shows the process of biosorption, a fast ionic removal process in which metals ions bind to the outer surfaces of the cell membranes of inactivated algal cells (Ahalya and Ramachandra, 2003; Ahluwalia and Goyal, 2007; Goyal et al., 2003; Gupta and Rastogi, 2008; Velásquez and Dussan, 2009; Wang et al., 2019). Functional groups on the outer cell surface can also attach Na+ and Cl− ions to contribute to biosorption (Ivánová et al., 2012). Wei et al. (2020) reported that —OH and amide groups bind with Cl− ions due to their positive charges, while —COR, —COOR, and —NH attach to Na+ ions due to the negative charge groups. Sahle-Demessie et al. (2019) reported that bioaccumulation and biosorption occur simultaneously in all living organisms.
Some channeling has taken the Cl− by centrifuged and immobilized P. keutzingianum, but both biosorption and bioaccumulation may have occurred in both experiments. However, the immobilized cyanobacteria may have been encapsulated, resulting in more Cl− uptake due to the denser algal culture. The centrifuged cyanobacteria cells were in suspension, and both biosorption and bioaccumulation may have occurred. Similarly, centrifuged cyanobacteria results are very low compared to studies conducted in suspension (Table 1). However, for the immobilized cyanobacteria, we cannot assume that the cells were active and played a role in ionic movement because of the fact that the cyanobacteria is tightly compacted with SA inside the beads.
3.4 Competition for nutrients in centrifuged and immobilized cyanobacteria
Nitrate (NO3–) ion is a significant source of nutrition for the growth of cyanobacteria. NO3– ions are assimilated into cells, where they are converted into nitrite ions (NO2–) for other metabolic processes through nitrite reductase enzymes (Larsdotter et al., 2007; Sommer, 1983; Zafar et al., 2021). Fig. 5a shows the concentrations of NO3– ion during the centrifuged cyanobacteria experiments, including the negative controls. The cyanobacteria consumed NO3– ions for their growth as a nitrogen source. However, some NO3– ions were released back into the water, showing a high nitrate concentration. The initial nitrate ions in the reactors were high (1711.2, 1728.2, 1705.8, and 1672.2 ppm for salinities of 10, 30, 50, and 70 g/L, respectively). The NO3– uptake remained inconsistent throughout the experiment; the final NO3– concentrations were 1293.8, 1211.6, 1771.4, and 1932.2 ppm for the salinities of 10, 30, 50, and 70 g/L, respectively. Thus, for the higher salinities (50 and 70 g/L), NO3– ions leached from the cells into the water in response to salt stress, resulting in a loss of NO3– storage. In cyanobacteria, nitrate ions provide the nitrogen source as a food supplement against high salinities (Rai and Abraham, 1995). Mohapatra et al. (1998) studied Scenedesmus bijugatus in high salt concentrations with an observation of higher growth in nitrogen and phosphorus-rich conditions. The results from the Fig. 5a suggest that the centrifuged cyanobacteria could not accumulate NO3– ions at higher salinities for a longer time duration. This could happen when extreme osmotic stress events might have occurred due to extreme salinities (Fal et al., 2022). However, during the commencement of experiments, the trend for nitrate ions was unconventional in the centrifuged cyanobacteria. Another reason could be the centrifuged cells that start increasing and consume NO3– ions during the lag phase and release NO3– ions back during the death phase. These findings are similar to another study performed on P. keutzingianum (Zafar et al., 2022b).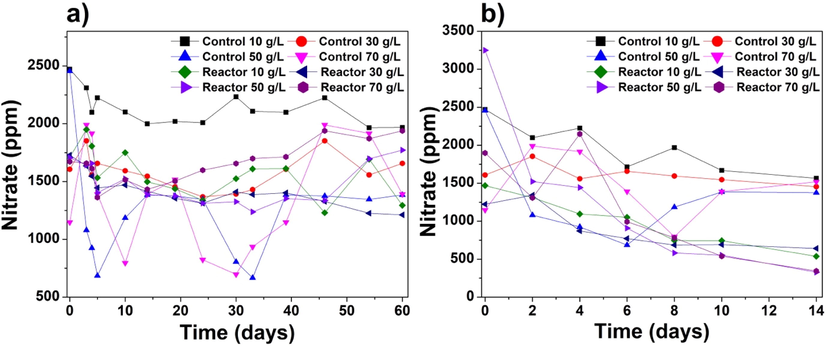
Concentrations of NO3– ion (ppm) during the (a) centrifuged cyanobacteria and (b) immobilized cyanobacteria experiments.
Fig. 5b shows the NO3– ion concentrations during the immobilized cyanobacteria experiments. The initial (day 0) nitrate concentrations were 1469.1, 1223.9, 3249, and 1897.4 ppm in the reactors with salinities of 10, 30, 50, and 70 g/L, respectively. The NO3– removal by the immobilized cyanobacteria might have occurred via the biosorption process. NO3– ions are the most common product of nitrogen fixation in both centrifuged and immobilized cyanobacteria. The pattern of NO3– uptake was more complex for the centrifuged cyanobacteria than for the immobilized cyanobacteria. Similarly, in Fig. 5b, it was found that the immobilized cyanobacteria showed a reduction of NO3– ions. The final NO3– ion concentrations at the end of the experiment were decreased, corresponding to NO3– removal rates of 63.8 %, 47.6 %, 89.9 %, and 81.9 % for salinities of 10, 30, 50, and 70 g/L, respectively. The argument is comparable to another study on nitrate reduction using immobilized S. sp. and C. sorokiniana, where 90 % of nitrate was reduced from groundwater within 9 to 12 sequential days (Mollamohammada et al., 2020). The differences in NO3– uptake between the centrifuged and immobilized cyanobacteria were significant for all tested salinities, with an F-value of 4.718 (Supplementary data – Table S-3).
3.5 PC release during the cultivation of immobilized cyanobacteria
When the immobilized cyanobacteria were placed in beakers for experimentation, a blue pigment (PC) appeared. PC is a light-harvesting pigment found in prokaryotic cyanobacteria that can store nitrogen proteins (Querques et al., 2015). However, in our experiments, PC was first detected based on the red fluorescence that appeared when the graduated cylinder was viewed under a flashlight (Fig. 6a). Copper sulfate (0.01 g) was added to confirm the presence of PC based on the interaction between Cu2+ on phycocyanobilin protein. The red fluorescence disappeared upon the addition of copper sulfate, confirming that P. keutzingianum released PC during the experiments (Fig. 6b).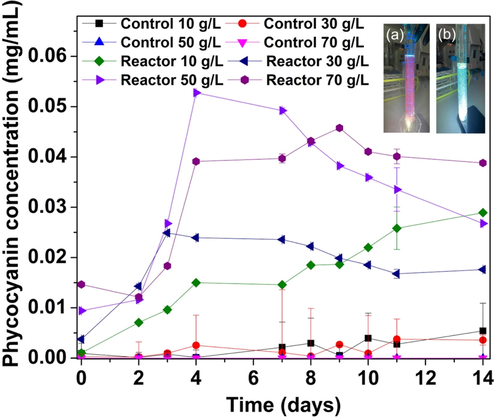
A concentration of phycocyanin production from immobilized bead reactors; (a) Red fluorescence appeared due to the release of PC pigment by P. keutzingianum during the immobilized cyanobacteria experiment (top right corner). (b) After adding 0.01 g copper sulfate, the red fluorescence disappeared due to the interaction between Cu2+, which replaces the bond —COOH of the protein phycocyanin.
Fig. 6 shows the PC concentration during the immobilized cyanobacteria experiments. PC was primarily released on days 2–4 of the experiment. For the salinity of 10 g/L, the PC concentration increased more slowly over time compared to the other salinities. On day 0, the maximum PC concentration (0.015 mg/mL) was observed in the reactor with a salinity of 70 g/L because the high salinity caused the release of PC via osmotic pressure. In the reactors with salinities of 10, 30, and 50 g/L, the initial PC concentrations were 0.001, 0.003, and 0.009 mg/mL, respectively. Overall, the maximum PC concentration (0.052 mg/mL) was observed on day 4 in the reactor with a salinity of 50 g/L. After day 4, the PC concentration in all reactors decreased except for the reactor with the salinity of 10 g/L, in which PC release was observed after day 4. While all the controls were free from cyanobacteria, significantly less amounts of PC were detected spectrophotometrically, potentially due to the turbidity of the solution. The cyanobacteria may have re-absorbed the PC due to the temperature effect or cell cracks. In a study on Nostoc muscorum, Arnon et al. (1974) found that after PC was released, the PSI and PSII systems were retained in the cells that carried out the photosynthetic process. The release of PC did not disturb the electron transport.
3.6 Osmoregulation experiment with immobilized cyanobacteria
Despite the significant growth of P. keutzingianum at the high salinity of 70 g/L, an extreme salinity test was performed to evaluate the resistance of the cyanobacteria to salt stress under extreme salinity (50, 70, 85, and 100 g/L) for short durations (0–360 min). Only Cl− and NO3– ions were detected, and some ions appeared at irregular intervals; therefore, inconsistent ions were neglected in the analysis. Fig. 7a shows the trend in Cl− ion removal (C/Co). The maximum removal rates obtained for the salinities of 50, 70, 85, and 100 g/L were displayed in Table 3. Thus, from these results, it can be concluded that the Cl– ion uptake increased as the salinity increased; however, the sampling time varies between different salinities.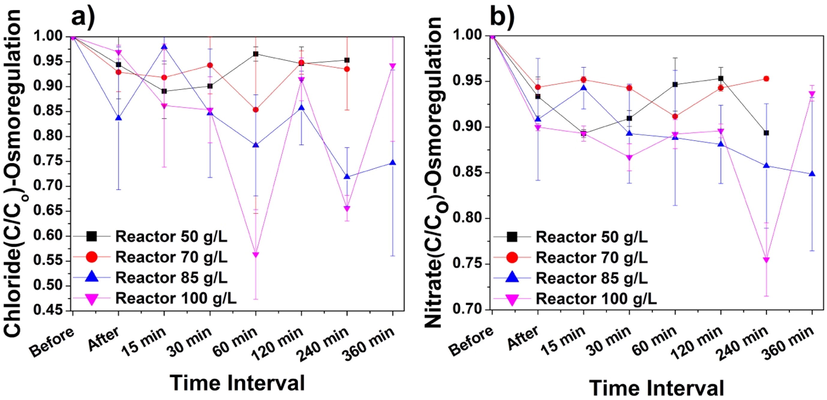
Reductions in (a) Cl− ions and (b) NO3– ions during the 6-h osmoregulation experiment performed on immobilized P. keutzingianum at salinities of 50, 70, 85, and 100 g/L. “Before” means before inoculation with cyanobacteria. “After” means after inoculation with cyanobacteria.
Sr. No.
Salinity (g/L)
Max. Cl− ion R.E. (%)
Time (min) when max Cl− R.E. was achieved
Max. NO3– ion R.E. (%)
Time (min) when max NO3– R.E. was achieved
1
50
11
15
10.7
15
2
70
14.6
120
8.8
60
3
85
28.1
240
15.1
360
4
100
43.7
60
24.5
240
Fig. 7b illustrates the reduction in NO3– ions (C/Co) during the osmoregulation experiment. At salinities of 50, 70, 85, and 100 g/L, the maximum NO3– removal rates were shown in Table 3. It is worth noting that more nitrate is consumed at higher salinities because the NO3– acts as a nitrogen source, which protects against highly toxic salt concentrations (Rai and Abraham, 1995). The argument corroborates our results obtained in the current study for the osmoregulation experiment. Zafar et al. (2022b) observed in salinity of 70 g/L that, nitrate ion concentration increased at the end of the treatment cycle. In this study, the argument is correct for only one reactor, i.e., 100 g/L. The reactor with 100 g/L salinity caused much osmotic stress on the cell, and due to this, a major variation in Cl− and NO3– ions has been observed. In the end, the “salt shock” condition must have prevailed for the salinity of 100 g/L. The salt shock condition has already been discussed in Section 3.3.1. The uptake of Cl− and NO3– in such short time periods does not provide in-depth information about biosorption or bioaccumulation processes. However, the osmoregulation results indicate that ion exchange occurred continuously between the water and the cyanobacteria cells from the beginning of the experiment (Fig. 7a and 7b).
4 Limitations, prospects, and recommendations
The biodesalination process is less energy-consuming; however, the preparation of immobilized and centrifuged cyanobacteria can consume more electricity. The regeneration of sodium alginate beads and reusing biomass is possible but challenging. The immobilizing process of algae and cyanobacteria is time-consuming. Including all the limitations, the exact time at which algae or cyanobacteria can consume maximum salt concentration should be monitored continuously. pH difference was significant in two treatment methods and can be further investigated about the interaction and ion-exchange at nanoscale. Studies on immobilized beads should be conducted in continuously flow stirred tank reactors at a pilot scale level.
Some more studies should be conducted on various hazardous industrial wastewater for strains like P. keutzingianum, which is highly resistant to toxicity. The production of biofuels, lipids and fatty acids can be evaluated for strain P. keutzingianum. Genetic modification of the studied strain is recommended as the scope of future work for greater salt removal efficiencies for the biodesalination process. According to the observed salt tolerance capacity of the strain P. keutzingianum, it is suggested that the brine treatment from reverse osmosis plants can be useful for biodesalination exploration. Studies on CO2 capturing using P. keutzingianum should be conducted as checking the capability of the strain towards acidic scale.
5 Conclusions
In our experiments, P. keutzingianum showed remarkable salt tolerance regardless of the method used for concentration (centrifugation or immobilization). In the centrifuged cyanobacteria experiments, the EC showed a maximum reduction of 11.5 % at the salinity of 30 g/L. Similarly, in the immobilized cyanobacteria experiments, the maximum EC reduction (13.3 %) was achieved at the salinity of 10 g/L. However, the maximum Cl− removal rates for the centrifuged and immobilized cyanobacteria were 26.2 % at the salinity of 10 g/L and 39.9 % at the salinity of 30 g/L, respectively. Thus, ion chromatography indicated a higher desalination than EC measurements, similar to another finding (Zafar et al., 2022a). Also, in the chloride estimations, the decrease in removal efficiency could happen due to the swollen cell or cell death phase. The cell death phase could happen only for centrifuged cyanobacteria due to cell rupture and high osmotic pressure inside the cell resulting in the loss of chloride ions back into the water. While in immobilized cyanobacteria, this phenomenon was not observed, and chloride was continuously reduced until the end of experimentation. One reason could be that the SA encapsulation prevented direct exposure to osmotic conditions and transfused chloride ions essential for growth. Moreover, the other reason could be biosorption that happens fast due to extra covering of SA on the outer surface of cells, increasing the surface area.
The findings of this study indicate that salt accumulation and nutrient uptake by P. keutzingianum occurred through both processes of biosorption and bioaccumulation, which eventually happens in all living organisms. Compared to the centrifuged cyanobacteria, the immobilized cyanobacteria achieved better removal efficiency in a shorter treatment time. In the osmoregulation experiment, no apparent metabolic inhibition was observed in the cyanobacteria, and a Cl− ion removal rate of 43.7 % was obtained at 60 min after inoculation in shorter intervals. Overall, the salinity was reduced in all the tested conditions on P. keutzingianum; therefore, biodesalination performance increased when the biomass was concentrated. Similarly, the performance in this study was better than the literature, as shown in Table 1. The release of phycocyanin pigment indicated a good sign of the P. keutzingianum strain’s adaptability to the saline environment and future scope that can be utilized as a commercial value product. Another conclusion is that the cell activities continued even at extreme salinities throughout the experiment.
The immobilization method has the advantage of relatively easy biomass harvesting compared to cells cultivated in suspension. In the immobilization method, only refreshing the SA material is required to prolong the encapsulation of the cyanobacteria inside the beads. Thus, if appropriately modified and monitored, the immobilized cyanobacteria can be used in biodesalination water treatment plants as part of an efficient and energy-saving biodesalination strategy with minimal release of living cells into the effluent.
Funding
This work was supported by the National Water Center and United Arab Emirates University [Grant No G00003297 and G00003661].
CRediT authorship contribution statement
Salma Shaikhoun: Software, Data curation, Writing – original draft. Abdul Mannan Zafar: Conceptualization, Methodology, Data curation, Software, Formal analysis, Visualization, Investigation, Validation, Writing – original draft. Yin-Hu Wu: Supervision, Validation, Funding acquisition. Ashraf Aly Hassan: Methodology, Supervision, Validation, Funding acquisition, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. J. Exp. Bot.. 2009;60:939-954.
- [Google Scholar]
- Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol.. 2007;98:2243-2257.
- [CrossRef] [Google Scholar]
- The usage of Cyanobacteria in wastewater treatment: prospects and limitations. Lett. Appl. Microbiol. 2021
- [Google Scholar]
- Osmoregulation in the extremely euryhaline marine micro-alga Chlorella autotrophica. Plant Physiol.. 1984;74:1010-1015.
- [Google Scholar]
- Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth. Res.. 2008;98:529-539.
- [Google Scholar]
- Biodesalination: A Case Study for Applications of Photosynthetic Bacteria in Water Treatment1[C] Plant Physiol.. 2014;164:1661-1676.
- [CrossRef] [Google Scholar]
- Sodium transport in filamentous nitrogen fixing cyanobacteria. J. Biosci.. 1983;5:225-233.
- [Google Scholar]
- Natural pigments from microalgae grown in industrial wastewater. Bioresour. Technol.. 2020;303:122894
- [CrossRef] [Google Scholar]
- Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1974;357:231-245.
- [Google Scholar]
- Bąbel, M., Schreiber, B.C., 2014. 9.17 – Geochemistry of Evaporites and Evolution of Seawater, in: Holland, H.D., Turekian, K.K.B.T.-T. on G. (Second E. (Eds.), Elsevier, Oxford, pp. 483–560. https://doi.org/10.1016/B978-0-08-095975-7.00718-X.
- Direct brackish water desalination using Chlorella vulgaris microalgae. Process Saf. Environ. Prot.. 2021;148:237-248.
- [CrossRef] [Google Scholar]
- Algae: Anatomy, Biochemistry, and Biotechnology. CRC Press; 2014.
- Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res.. 1985;36:785-792.
- [Google Scholar]
- Mutational engineering of the cyanobacterium Nostoc muscorum for resistance to growth-inhibitory action of LiCl and NaCl. Curr. Microbiol.. 2003;47:5-11.
- [Google Scholar]
- Na+ H+ exchange in the cyanobacterium Synechococcus 6311. Biochem. Biophys. Res. Commun.. 1984;122:452-459.
- [Google Scholar]
- K + and Na + transport contribute to K + /Na + homeostasis in Pyropia haitanensis under hypersaline stress. Algal Res.. 2019;40:101526
- [CrossRef] [Google Scholar]
- Biosorption and bioaccumulation–the prospects for practical applications. Environ. Int.. 2010;36:299-307.
- [Google Scholar]
- Functional analysis of the Na+/H+ antiporter encoding genes of the cyanobacterium Synechocystis PCC 6803. Biochemistry (Moscow). 2002;67:432-440.
- [Google Scholar]
- Role of microalgae and cyanobacteria in wastewater treatment: genetic engineering and omics approaches. Int. J. Environ. Sci. Technol.. 2022;19:2173-2194.
- [CrossRef] [Google Scholar]
- Characterization of the Na+-requirement in cyanobacterial photosynthesis. Plant Physiol.. 1988;88:757-763.
- [Google Scholar]
- Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon 2022:e08811.
- [Google Scholar]
- Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale. Energies 2021
- [CrossRef] [Google Scholar]
- Salt Tolerance and Desalination Abilities of Nine Common Green Microalgae Isolates. Water (Basel). 2019;11:2527.
- [CrossRef] [Google Scholar]
- Simultaneous biological desalination and lipid production by Scenedesmus obliquus cultured with brackish water. Desalination. 2016;400:1-6.
- [CrossRef] [Google Scholar]
- Algae-Based Approach for Desalination: An Emerging Energy-Passive and Environmentally Friendly Desalination Technology. ACS Sustain. Chem. Eng. 2021
- [CrossRef] [Google Scholar]
- Comparative studies on the microbial adsorption of heavy metals. Adv. Environ. Res.. 2003;7:311-319.
- [CrossRef] [Google Scholar]
- Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—A comparative study. Colloids Surf. B Biointerfaces. 2008;64:170-178.
- [CrossRef] [Google Scholar]
- Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol. Rev. 2011
- [CrossRef] [Google Scholar]
- Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol.. 2012;113:244-252.
- [CrossRef] [Google Scholar]
- Changes in membrane lipid composition during saline growth of the fresh water cyanobacterium Synechococcus 6311. Plant Physiol.. 1990;94:1512-1521.
- [Google Scholar]
- Determination of the functional groups in algae parachlorella kessleri by potentiometric titrations. Nova Biotechnologica et Chimica. 2012;11:93-99.
- [Google Scholar]
- Uptake of potassium by algae and potential use as biofertilizer. Indian J. Plant Physiol.. 2015;20:285-288.
- [Google Scholar]
- Ecophysiology of High Salinity Tolerant Plants. Springer Science & Business Media; 2006.
- Cyanobacterial diversity and halotolerance in a variable hypersaline environment. Microb. Ecol.. 2008;55:453-465.
- [CrossRef] [Google Scholar]
- Salinity Tolerance of Eukaryotic Marine Algae. Annu. Rev. Plant Physiol. Plant Mol. Biol.. 1990;41:21-53.
- [CrossRef] [Google Scholar]
- Transmembrane signal transduction in archaeal phototaxis: the sensory rhodopsin II-transducer complex studied by electron paramagnetic resonance spectroscopy. Eur. J. Cell Biol.. 2011;90:731-739.
- [Google Scholar]
- Biologically mediated phosphorus precipitation in wastewater treatment with microalgae. Environ. Technol.. 2007;28:953-960.
- [CrossRef] [Google Scholar]
- Growing Algae in Produced Water Generated During Oil and Gas Production Using Hydraulic Fracturing Technique. Chem. Eng. Trans.. 2019;74:1261-1266.
- [CrossRef] [Google Scholar]
- Sodium requirement for photosynthesis and its relationship with dinitrogen fixation and the external CO2 concentration in cyanobacteria. Plant Physiol.. 1987;85:585-587.
- [Google Scholar]
- Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys.. 2005;444:139-158.
- [CrossRef] [Google Scholar]
- Na+-dependent K+ uptake Ktr system from the cyanobacterium Synechocystis sp. PCC 6803 and its role in the early phases of cell adaptation to hyperosmotic shock. J. Biol. Chem.. 2004;279:54952-54962.
- [Google Scholar]
- Biodesalination: an emerging technology for targeted removal of Na+ and Cl− from seawater by cyanobacteria. Desalinat. Water Treat. 2015;55:2647-2668.
- [CrossRef] [Google Scholar]
- Effects of Nutrients at Different Salinities on Growth of the Freshwater Green Alga Scenedesmus bijugatus in Water of Narendra Pond, Puri, Orissa. Int. Rev. Hydrobiol.. 1998;83:297-304.
- [CrossRef] [Google Scholar]
- Nitrate removal from groundwater using immobilized heterotrophic algae. Water Air Soil Pollut.. 2020;231:1-13.
- [Google Scholar]
- How Do Algae Concentrate CO2 to Increase the Efficiency of Photosynthetic Carbon Fixation? Plant Physiol.. 1999;119:9-16.
- [CrossRef] [Google Scholar]
- Biodesalination and treatment of raw hypersaline produced water samples using indigenous wastewater algal consortia. Desalination. 2022;528:115638
- [Google Scholar]
- NMR studies on Na+ transport in Synechococcus PCC 6311. Arch. Biochem. Biophys.. 1992;294:347-352.
- [Google Scholar]
- Na+/H+ antiporters, molecular devices that couple the Na+ and H+ circulation in cells. J. Bioenerg. Biomembr.. 1993;25:647-669.
- [Google Scholar]
- Microalgal phycocyanin productivity: strategies for phyco-valorization. J. Chem. Technol. Biotechnol.. 2015;90:1968-1982.
- [CrossRef] [Google Scholar]
- Relationship of combined nitrogen sources to salt tolerance in freshwater cyanobacterium Anabaena doliolum. J. Appl. Bacteriol.. 1995;78:501-506.
- [Google Scholar]
- Effect of Salinity, pH, Light Intensity on Growth and Lipid Production of Microalgae for Bioenergy Application. J. Biol. Sci.. 2015;15:260-267.
- [CrossRef] [Google Scholar]
- Comparative assessment of Azolla pinnata and Vallisneria spiralis in Hg removal from G.B. Pant Sagar of Singrauli Industrial region, India. Environ. Monit. Assess. Dordrecht. 2009;148:75-84.
- [CrossRef] [Google Scholar]
- Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass. 1986;10:253-264.
- [Google Scholar]
- Bio-desalination of brackish and seawater using halophytic algae. Desalination. 2019;465:104-113.
- [CrossRef] [Google Scholar]
- Halorhodopsin is a light-driven chloride pump. J. Biol. Chem.. 1982;257:10306-10313.
- [Google Scholar]
- Simultaneous biomass production and water desalination concentrate treatment by using microalgae. Desalinat. Water Treat. 2018;135:101-107.
- [Google Scholar]
- Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016
- [Google Scholar]
- Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev.. 2015;50:431-444.
- [CrossRef] [Google Scholar]
- Nutrient competition between phytoplankton species in multispecies chemostat experiments. Arch. Hydrobiol.. 1983;96:399-416.
- [Google Scholar]
- Geothermal CO2 bio-mitigation techniques by utilizing microalgae at the Blue Lagoon, Iceland. In: Proceedings, 34th Workshop on Geothermal Reservoir Engineering. Stanford: Stanford University; 2010.
- [Google Scholar]
- Nutritional Requirements for Sexual Reproduction in Mesotaenium Kramstai (chlorophyta) 1. J. Phycol.. 1986;22:1-8.
- [CrossRef] [Google Scholar]
- Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations. 2018;5:54.
- [Google Scholar]
- Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Hazard. Mater.. 2009;167:713-716.
- [CrossRef] [Google Scholar]
- Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci. Rep.. 2019;9:5277.
- [CrossRef] [Google Scholar]
- The role of adsorption in microalgae biological desalination: Salt removal from brackish water using Scenedesmus obliquus. Desalination. 2020;493:114616
- [CrossRef] [Google Scholar]
- Definition and measurement of salinity in salt lakes. Int. J. Salt Lake Res.. 1994;3:53-63.
- [Google Scholar]
- Biomass and phycocyanin production from cyanobacteria dominated biofilm reactors cultured using oilfield and natural gas extraction produced water. Algal Res.. 2015;11:165-168.
- [CrossRef] [Google Scholar]
- Yensen, N.P., 2006. Halophyte Uses For The Twenty-First Century, in: Khan, M.A., Weber, D.J. (Eds.), Ecophysiology of High Salinity Tolerant Plants. Springer Netherlands, pp. 367–396. https://doi.org/10.1007/1-4020-4018-0_23.
- Recent updates on ions and nutrients uptake by halotolerant freshwater and marine microalgae in conditions of high salinity. J. Water Process Eng.. 2021;44:102382
- [Google Scholar]
- Unprecedented biodesalination rates–Shortcomings of electrical conductivity measurements in determining salt removal by algae and cyanobacteria. J. Environ. Manage.. 2022;302:113947
- [CrossRef] [Google Scholar]
- Biodesalination using halophytic cyanobacterium Phormidium keutzingianum from brackish to the hypersaline water. Chemosphere. 2022;307:136082
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104282.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







