Biosynthesis optimization of silver nanoparticles (AgNPs) using Trichoderma longibranchiatum and biosafety assessment with silkworm (Bombyx mori)
⁎Corresponding author. liuxian7007@syau.edu.cn (Xian Liu), qinli1963@163.com (Li Qin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Silver nanoparticles (AgNPs) have raised public concern due to their widespread application in the field of agriculture, medicine, and environment and their potential toxic effects on humans and the environments. In this study, biosynthesis of nanosilver particles mediated by Trichoderma longibranchiatum using orthogonal experimental design (OED) was optimized. Silkworm larvae were exposed via the mulberry leaves to AgNPs to evaluate their toxic effects. The results showed that 2 mmol/L silver nitrate and 55 °C of reaction temperature at pH 7.0 for 24 h were the optimum values for AgNPs biosynthesis with the synthesis amount and antifungal activity of AgNPs as the indices. The characterization of the biosynthesized AgNPs was conducted using electron microscopy, energy dispersive X-ray analysis (EDS), UV/visible spectrophotometry, Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD). The crystalline structured nanoparticles were spherical or polyhedral with a mean size ranging about 5–50 nm. FTIR showed that many functional group moieties (–OH, –CH3, –C–O, etc.) involved as a capping and reducing agent in AgNPs biosynthesis. After the larvae were fed with 50 mg/mL AgNPs, there were no obvious adverse effects on the growth of larvae and cocoon quality. Further supplement of AgNPs-B could promote the weight of larvae and the cocoon shell ratio. The data presented herein provided valuable information on a reliable eco-friendly, simple, low-cost biosynthesis of AgNPs and its biosafety evaluation which may contribute to its increased application in the future.
Keywords
Nanosilver
Biosynthesis optimization
Orthogonal Experimental Design
Trichoderma longibranchiatum
Biosafety assessment
1 Introduction
In recent years, all kinds of nanomaterials have been widely used in the fields of agriculture, medicine and environment, etc. (Loo et al., 2015; Al Abboud, 2018; Naganthran et al., 2022). However, nanomaterials have received widespread concerns due to their extremely small sizes and large surface to volume ratios which lead to both chemical and physical differences in their properties. As a member of this family, Silver nanoparticles (AgNPs) is an important and widely used nanomaterial in the world. AgNPs synthesis is emerging as one of the fastest-growing field for a wide-ranging application (Rai et al., 2011). The synthesis strategies of AgNPs, including physical, chemical and biological methods, have been developed during the last few years. However, physical and chemical methods are time-consuming, expensive or posing threat to environment. Compared with the traditional synthetic methods, biological strategies as a green synthetic approach provide a novel idea for nanoparticles formation, using bacteria, fungi and plants (Rauwel et al., 2015).
Biosynthesis of nanoparticles using filamentous fungi is advantageous as compared to plants and bacteria because fungi produce more proteins which provide more residues to stable nanoparticles and greater biocompatibility in the uses of nanoparticles (Gholami-Shabani et al., 2014) and extracellular synthesis of nanoparticles makes processing and biomass handling easier (Al Abboud, 2018). Trichoderma asperellum (Mukherjee et al., 2008), T. reesei (Gemishev et al., 2019), T. harzianum (Al Abboud, 2018), T. hamatum(Abdel-Kareem and Zohri, 2018)and T. koningii (Tripathi et al., 2013) have been used for synthesis of metal nanoparticles. Trichoderma spp. has a lower cost in large-scale production for their lower cultivation requirements and higher growth rates in both industrial and laboratory scales than others (Lόpez-Bucio et al., 2015). There are many kinds of literatures about biosynthesis of AgNPs and the optimization of the process parameters mainly to improve the stability of the product and to increase yield (Guilger-Casagrande and de Lima, 2019). Studies have found that changes in reaction temperature, the concentration of the metal, pH value, light, and agitation affect the physicochemical characteristics of nanoparticles (Rajput et al., 2016; Saxena et al., 2016; Ma et al., 2017). Insufficient study on mechanisms of nanoparticles biosynthesis results in an empirical selection of conditions for a high yield (Voeikova et al., 2018). The optimization of nanoparticles biosynthesis can increase the nanoparticle yield and change their physicochemical properties, which may result in a change of the biological activity of nanoparticles (Rauwel et al., 2015). Absorbance, size and morphology of nanoparticles are the indicators to determine the nanoparticle biosynthesis conditions (Chan and Don, 2013b; Bonnia et al., 2020). However, these indicators are indirectly measure the properties and cannot accurately determine the yield and biological activity of nanoparticles, which are the key parameters for the application of nanoparticles.
With excessive usage of nanomaterials in agricultural, industrial and medical processes, safety concerns over environmental and human health are of increasing importance (Areecheewakul et al., 2020). These nanoparticles escaping into the environment during their fabrication, usage and disposal may interact with the biotic and abiotic species (Sharma et al., 2019). Previous studies have shown that exposure to AgNPs can induce developmental defects of zebrafish embryos (AshaRani et al. 2008), damage the tissue of midgut and induce reactive oxygen species (ROS) production in silkworms (Chen et al., 2019), cause DNA damage and cell cycle arrest in human cells (AshaRani et al. 2009), and significantly reduce the germination rate of plant seeds (Yin et al., 2012). Risk assessment of nanomaterials has been debated for human health concerns with a focus on new approaches and on refining the existing methodologies to make the use of nanomaterials safe and sustainable (Krug, 2019; Johnston et al., 2020). The growth of nanotechnology in combination with the digitization era needs more safety-related data of AgNPs to organisms (Furxhi, 2022).
Insects, accounting for approximately two thirds of all animal species, are widely distributed in nature and play an important role in maintaining the natural ecological balance (Chen et al., 2019). Bombyx mori is a Lepidopteran model insect with poor heavy metals and disease resistance making a suitable for monitoring environmental poisons (Nouara et al., 2018). In recent years, there are many kinds of literatures focusing on nanotoxicity study using silkworm as test animal to evaluate the toxicity and biological effects of nanomaterials (Ma et al., 2019; Ni et al., 2015; Xing et al., 2016). Most of these research projects have proven the correlation between results in mammals and silkworms (Renwick and Kavanagh, 2007; Sekimizu et al., 2012; Meng et al., 2017). AgNPs can improve the growth of silkworms, but high concentrations of AgNPs ( ≧ 800 mg/L) resulted in silkworm death (Meng et al., 2017). Cheng et al. found that AgNPs had slightly negative influences on the growth of the silkworm larvae (Cheng et al., 2017). Silkworm exposure to Boron nitride nanosheets (BN NSs) caused no obvious adverse effects on the growth, silk properties or tissues of silkworms, but the expressions of genes in midgut related to some specific functions and pathways were significantly changed, indicating that BN NSs might have potential to gene dysfunction (Ma et al., 2020).
The objectives of this study are optimization of silver nanoparticle synthesis using T. longibranchiatum at different AgNO3 concentrations, pH values and reaction temperature according to the synthesized amount and antifungal activity of AgNPs. We evaluated toxicity of these AgNPs using silkworms to provide a reference for assessing the nanotoxicity hazards in the environment.
2 Materials and methods
2.1 Organism and reagents
Trichoderma longibranchiatum was originally isolated from the roots of oak trees and spores had been maintained at 4 °C on silica gel pellets at the culture collection of Sericultural lab, Shenyang Agricultural University, China. Then, it has been re-cultivated before use in silver nanoparticles biosynthesis.
Fusarium oxysporum was originally isolated from the melon root with the typical symptoms of melon fusarium wilt disease and was kept at 4 °C in Sericulture lab (Zhang et al., 2013).
The silkworm (Bombyx mori) used in this experiment is Huakang No.3.
AgNO3 (AR Grade) was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Commercial nano-silver (AgNPs - C) was purchased from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China), its purity is 99.5 % and diameter range at 60 nm – 120 nm.
2.2 Supernatant preparation and AgNPs biosynthesis
For getting the T. longibranchiatum supernatant, the fungus was grown on potato dextrose broth (PDB) contained (g/L): 200.0, fresh potato cubes, and 20.0, dextrose, at 28 °C and 100 rpm for 72 h in an oscillator. After that, the culture was filtered via Whatman filter paper No.1 and the filtrate was used for the extracellular formation of silver nanoparticles. Silver nitrate (AgNO3) was dissolved in distilled water and used as a silver source for AgNPs biosynthesis (AgNPs-B). The synthesis of AgNPs was carried out in an Erlenmeyer flask containing 100 mL of T. longibranchiatum cell-free filtrate mixed with a certain volume of aqueous silver nitrate solution. The pH values (5, 7 and 9) and the concentration of silver nitrate (1.0, 2.0 and 3.0 mmol/L) and incubation temperatures (35, 45 and 55 °C) were adjusted according to the Orthogonal Experimental Design (OED) described in the next section. Reaction mixtures were incubated without agitation under light conditions for 24 hrs. All experiments were done in triplicates and the mean values were presented. The biosynthesized amount of AgNPs - B and their inhibition ratio to F. oxysporum were measured.
2.3 Experimental design and optimization by orthogonal experimental design
An orthogonal design table L9(34) was designed to optimize the levels of the most effective variables in AgNPs biosynthesis and to analyze their relationships. Based on the one-factor experimental results (Yao et al., 2020), three critical variables selected were silver nitrate concentration, reaction incubation time and reaction pH value, which were labelled as A, B and C respectively. Every parameter was studied at three different levels (Table 1). The biosynthesized amount and anti-fungal activity of AgNPs - B as well as their comprehensive index (50 % biosynthesized amount + 50 % antifungal activity) were used to analyze the biosynthesized conditions.
| Levels | Factors | ||
|---|---|---|---|
| A: AgNO3 (mmol L−1) | B: Reaction temperature (℃) | C: pH | |
| 1 | 1 | 35 | 5 |
| 2 | 2 | 45 | 7 |
| 3 | 3 | 55 | 9 |
2.4 Characterization of synthesized AgNPs - B
The spectra of AgNPs - B were measured in the wavelength ranging between 300 and 800 nm, with the resolution of 1 nm using UV/Vis spectrophotometer (UV – Vis U − 3010, Japan). X-ray diffraction pattern of AgNPs - B was analyzed by a diffractometer (BRUKER D8-Focus, Germany) operating with Cu Kα1 radiation generated at an accelerating voltage of 40 kV and 30 mA with a scan rate of 3°/min for 2θ values between 30 − 85°. AgNPs solution was centrifuged for 20 min at 10,000 rpm and drop coated on a carbon-coated copper grid and dried. AgNPs - B shape and size were obtained from a transmission electron microscope (JEOL JEM-2100F, Japan) operating at 40 kV, where a drop of aqueous AgNPs was loaded on a carbon-coated copper grid, and allowed to dry at room temperature (Qu et al., 2021). The diameter of AgNPs was analyzed and calculated with Nano Measuer1.2. Elemental analysis of the biosynthesized AgNPs-B was studied using TEM (JEOL JEM-2100F, Japan) operated at 40 kV and coupled with energy dispersive X-ray analysis (EDX) for compositional analysis and the conformation of the presence of elemental silver (Lotfy et al., 2021). FTIR spectrum was determined by spectroscopy (Nicolet IS50, USA) in the wavelength range 400–4,000 cm−1 at a resolution of 4 cm−1 to analyze the functional groups using the powder of AgNPs in KBr pellets.
2.5 Antifungal activity of biosynthesized AgNPs - B silver nanoparticles
The antifungal activity of synthesized particles was assayed by inhibiting mycelial growth of F. oxysporum. The mycelium disc (d = 1 cm) from the fringe of 4 day - grown fungal colony was transferred to potato dextrose agar medium containing AgNPs - B (200 mg/L), in triplicate. Controls were prepared using potato dextrose agar only. Then the plates were incubated for 4 days at 25 °C and the diameter of colonies was measured to calculate the inhibition ratio of AgNPs - B to F. oxysporum.
2.6 Biosafety test of AgNPs with silkworms
Mulberry leaves were soaked in 50 mg/L AgNPs or AgNO3 solution. The soaked leaves were dried naturally at room temperature for half an hour, and silkworm larvae (Huakang No.3) were fed with these soaked leaves from the second day of the 2nd instar to the 5th instar once a day, and the control groups of larvae were fed with the fresh mulberry leaves treated with ddH2O without AgNPs or AgNO3. All the larvae were maintained at 25 ± 0.5 °C and relative humidity of 70 – 75 % with a 12 - h light/12 - h dark cycle. Each treatment was replicated three times with 30 larvae in each replicate. The weight of mulberry leaves was measured daily to calculate the feeding amounts. The molting larvae were counted every 2 h to calculate the percent of the molting silkworms. In every instar stage, 80 % of larvae entering molting stage indicated as the end of this instar to determine the larval duration. The weight of 5 randomly selected 3rd – molting, 4th-molting and matured larvae were measured. The cocoon was removed from the cocooning frame, the number of 5th instars survival larvae were observed and recorded. The average single weight of the cocoon, the average single cocoon shell, and cocoon shell ratio were recorded and calculated. Cocoon shell ratio (%) = (the average single cocoon shell weight (g)) × 100/ (the average single cocoon weight (g)). All dead larvae and pupae of B. mori were removed from the pots and numbered. Observations on the survival rate of silkworm larvae were recorded at the beginning of the experiment.
2.7 Statistical analyses
Statistical analyses were conducted using SPSS for Windows, Version 22.0 and Graphpad Prism 6.01 (GraphPad, San Diego, USA). Data were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was conducted to compare the differences of the means among multi-group data. Dunnett’s test was performed when each dataset was compared with the control data. Statistical significance for all tests was judged at a probability level of 0.05 (P < 0.05) or 0.01(P < 0.01).
3 Results
3.1 Optimization conditions of AgNPs - B biosynthesis
The amount of biosynthesis, antifungal activity of AgNPs - B and their comprehensive index were the evaluation indices of the orthogonal experiment design for the preparation of AgNPs - B. Table 2 presents the results obtained for the different experimental factors, and showed the influence of the AgNO3 concentration, reaction temperature, and pH value on the biosynthesized amount and antifungal activity of nanoparticles. The results showed that the treatment A2B3C1 (2 mmol/L AgNO3, 55 °C and pH = 5) was the optimal biosynthesized condition according to any of three evaluation indices. The biosynthesized amount reached to 17.63 mg and the inhibition ratio to 56.11 %.
| No. | Treatments | AgNO3 concentration (mmol L−1)(A) | Temperature (℃)(B) | pH (C) | Biosynthesized amount(mg) | Inhibition ratio(%) | Compound index |
|---|---|---|---|---|---|---|---|
| 1 | A1B1C1 | 1 | 35 | 5 | 45.30 ± 1.30b | 55.19 ± 6.57 a | 85.56 ± 5.38 ab |
| 2 | A1B2C2 | 1 | 45 | 7 | 42.53 ± 4.82b | 53.26 ± 2.87 a | 81.50 ± 3.35 bc |
| 3 | A1B3C3 | 1 | 55 | 9 | 28.40 ± 3.48c | 11.27 ± 3.22f | 34.72 ± 2.26 e |
| 4 | A2B1C2 | 2 | 35 | 7 | 22.5 ± 9.13 cd | 36.04 ± 6.88b | 49.64 ± 13.39 d |
| 5 | A2B2C3 | 2 | 45 | 9 | 25.13 ± 1.19c | 17.48 ± 5.19 de | 36.81 ± 4.73 e |
| 6 | A2B3C1 | 2 | 55 | 5 | 54.13 ± 1.65 a | 56.11 ± 5.45 a | 94.26 ± 3.50 a |
| 7 | A3B1C3 | 3 | 35 | 9 | 17.63 ± 0.93 d | 23.18 ± 4.69 cd | 34.68 ± 3.02 e |
| 8 | A3B2C1 | 3 | 45 | 5 | 52.67 ± 2.40 a | 54.99 ± 3.86 a | 92.03 ± 3.05 a |
| 9 | A3B3C2 | 3 | 55 | 7 | 53.73 ± 1.70 a | 31.38 ± 6.07 bc | 73.85 ± 3.39c |
Different letter(s) in the same column differ significantly according to Duncan’s test (p < 0.05).
In this work, Table 2 and Table 3 were obtained through the range analysis. Table 3 was the range analysis of three evaluation indexes. The optimal level of factors for AgNPs - B biosynthesis was determined through the comparison between the different K values (Table 3). Further, R-value represented the difference between the maximum and the minimum K values. High yield and high biological activity of AgNPs - B were the production goals, consequently, our data indicated that K value should be big as possible. As shown in Table 3, pH value played an important role in the biosynthesis of AgNPs - B, followed by the reaction temperature and the concentration of AgNO3, respectively. Moreover, the optimal parameters to obtain high quality AgNPs - B was A3 (3.0 mmol/L), B3 (55 °C) and C1 (5) using AgNPs-B yield as evaluation index and A1 (1.0 mmol/L), B2 (45 °C) and C1 (5) using biological activity or both compound index as evaluation indexes respectively, based on the orthogonal experimental designed.
| Orthogonal indices | Factors | |||
|---|---|---|---|---|
| A | B | C | ||
| K1 | 348.70 | 256.30 | 456.30 | |
| AgNPs amount | K2 | 305.30 | 361.00 | 356.30 |
| (mg) | K3 | 372.10 | 408.80 | 213.50 |
| R | 22.30 | 50.80 | 80.90 | |
| Order of importance | C > B > A | |||
| Optimal level | A3B3C1 | |||
| K1 | 1.1876 | 1.1407 | 1.6563 | |
| Inhibition ratio | K2 | 1.1094 | 1.2657 | 1.2032 |
| (%) | K3 | 1.0938 | 0.9844 | 1.2032 |
| R | 0.0938 | 0.2813 | 1.1250 | |
| Order of importance | C > B > A | |||
| Optimal level | A1B2C1 | |||
| K1 | 201.78 | 169.88 | 271.85 | |
| Compound index | K2 | 180.71 | 210.34 | 192.15 |
| K3 | 200.55 | 202.82 | 119.04 | |
| R | 21.07 | 40.46 | 152.81 | |
| Order of importance | C > B > A | |||
| Optimal level | A1B2C1 | |||
In the above formula, F stands for A, B and C, and l stands for levels 1–3.
Fig. 1 shows the antifungal activity of AgNPs - B obtained at different reaction conditions (Fig. 1). AgNPs - B with different antifungal activity were obtained for the different samples. It was also observed that pH-value had an important influence on the antifungal activity, in which AgNPs - B synthesized at low pH had a higher antifungal activity. The colonies’ diameters of F. oxysporum were smaller at pH = 5 than those of others (Fig. 1 A, F, and H).
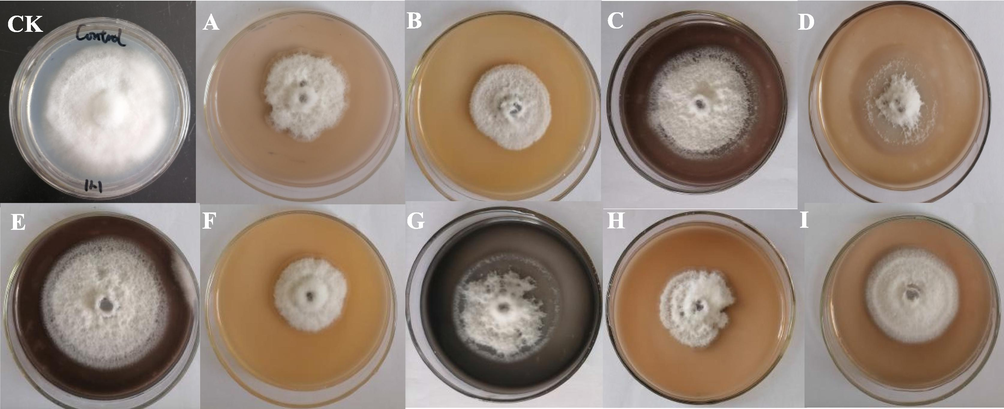
- Inhibition effect of different AgNPs-B to Fusarium oxysporum.
3.2 Characterization of silver nanoparticles
The color of the mycelial filtrate turned from light yellow to dark brown after incubation with AgNO3 for 24 hrs (Fig. 2). The appearance of dark brown color was an indication of AgNPs - B biosynthesis. The specific absorption peak of different AgNPs - B occurred at 420 nm − 580 nm (Fig. S1), which further confirmed the biosynthesis of AgNPs - B and the presence of silver element.
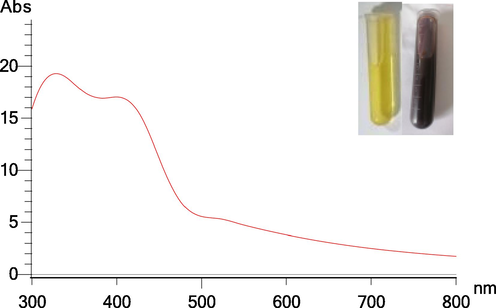
- UV–Vis spectrum of AgNPs - B synthesized obtained at an optimized method. The inset shows the color change of reaction solution at 24 h.
The AgNPs - B with the highest antifungal activity was used to study the characterization. The TEM images and nanoparticle diameter distribution of the AgNPs - B were shown in Fig. 3. The picture shows the individual silver nanoparticles and their aggregates. The morphology of AgNPs - B was roughly spherical or polyhedral and their size distribution ranged from 5 nm to 50 nm with the majority ranged 20–35 nm.
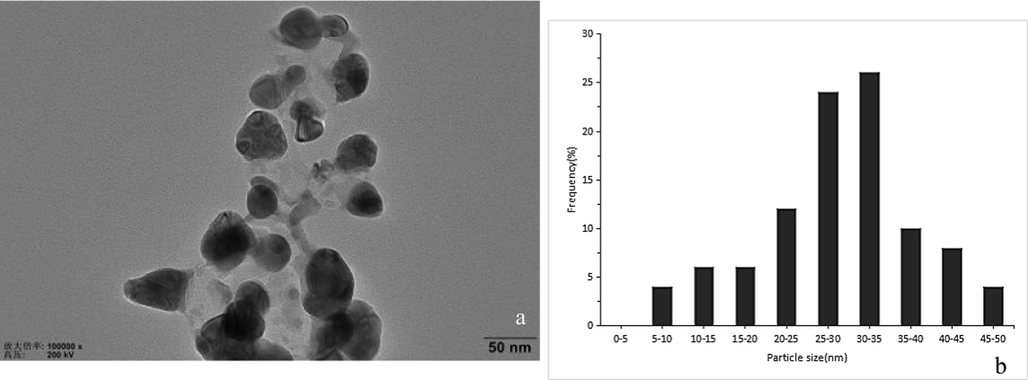
- Transmission electron micrographs of AgNPs - B (a) and particle diameter distribution of AgNPs - B (b).
The EDX spectrum (Fig. 4) exhibited a strong peak at 3 keV that was attributed to the SPR of Ag nanocrystals (68.6 %) and some other weaker peaks from copper and carbon for the copper grid used in TEM, which could be found in the TEM graph (Fig. 4).
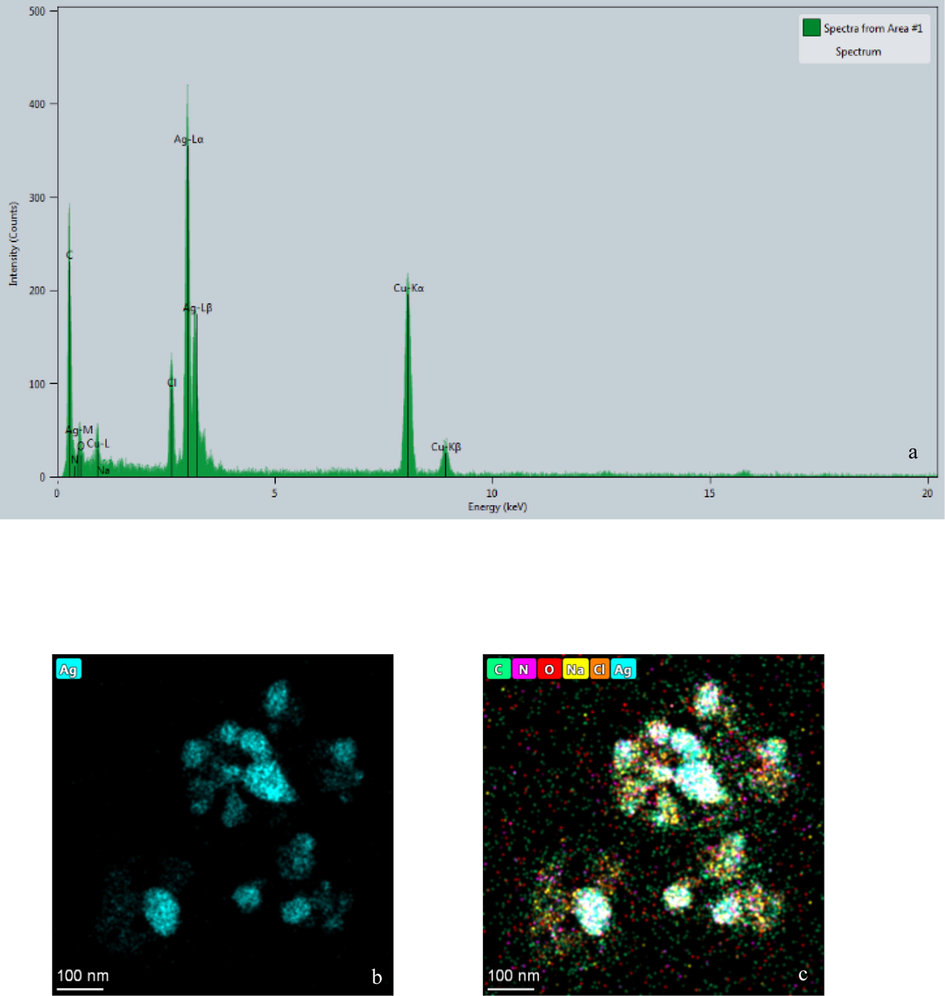
- EDX spectrum of synthesized AgNPs - B (a) and distribution of silver (b) and other elements (c) in elemental mapping.
on peaks at 2θ value of 38.06°, 44.39°, 64.4° and 77.29° corresponding to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) lattice plane values, respectively (Fig. 5), which showed the face-centered cubic (FCC) structure of metallic silver (International Center for Diffraction Data, ICDD, silver files No. 00–001-1164 and No. 04–0783).
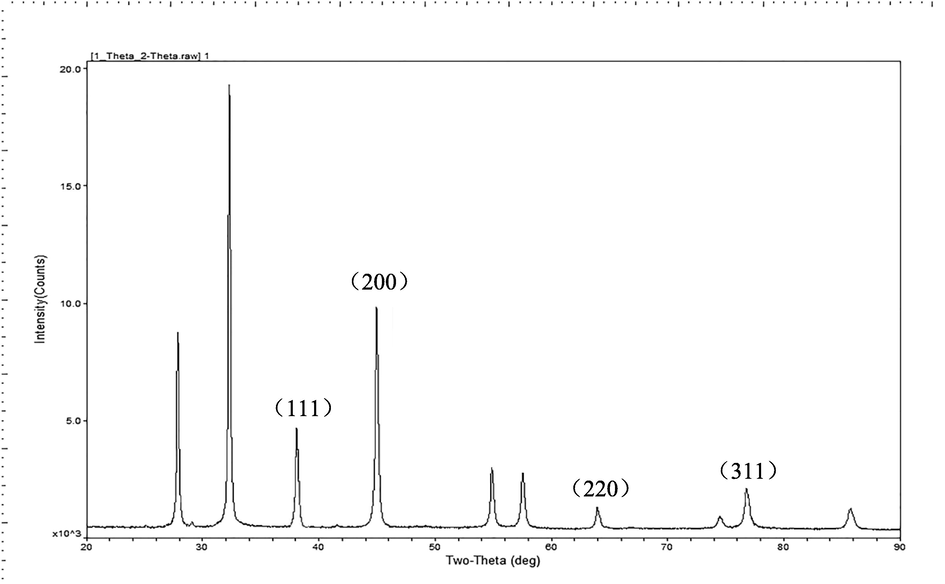
- XRD diffractogram of AgNPs – B.
FTIR is a powerful tool for identifying the functional groups involved in the coating of nanoparticles by producing an infrared absorption spectrum. The wavelength of light absorbed is characteristic of the chemical bond as can be seen in this annotated spectrum. The FTIR measurement showed that AgNPs - B exhibited 5 peaks at 3621.66, 2356.59, 1681.62, 1380.78 and 1176.36 cm−1 (Fig. 6), which corresponded to the –OH group of phenols, C≡C stretching modes of vibration in alkyne groups, bending N-H stretching vibration of amine groups (Huq, 2020), –CH3 symmetric deformation in aromatic and aliphatic compounds and –C–O stretch of alcohols, carboxylic acids and esters respectively (Qu et al., 2021).
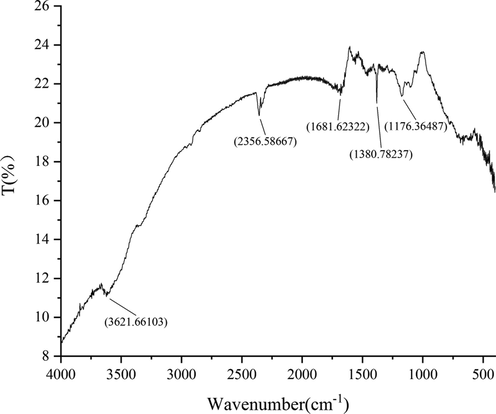
- Fourier Infrared Absorption Spectra of AgNPs - B.
3.3 Effects of AgNPs on the growth and development of silkworm
Several development indexes such as survival rate, larvae weight, molting through the development stages, were used to measure AgNPs effects on silkworms. AgNPs were fed to silkworm larvae from 2nd instar to 5th instar. After feed supplementation of AgNPs, silkworm larval period was not statistically significantly different between the control and other treatments. However, AgNPs and AgNO3 could slightly extend the development time of larvae (Fig. 7 a). Total feeding leaves per larva treated with AgNPs and AgNO3 were mildly lower than that of CK (Fig. 7 - c). Bodyweight of the larvae was weighed in 3rd – molting period, 4th - molting period and matured silkworm; compared to the control, the larvae’s body weight treated with AgNPs - B and AgNO3 increased slightly, but AgNPs - C did not affect the larval weight (Fig. 7 - b). No difference in the survival rate of larvae were observed, which was close to 100 % in all treatments (Fig. 7 - d). These development indexes of larvae showed that the AgNPs and AgNO3 did not affect the silkworm growth and development, indicating the low concentration of AgNPs was safety to the larvae.
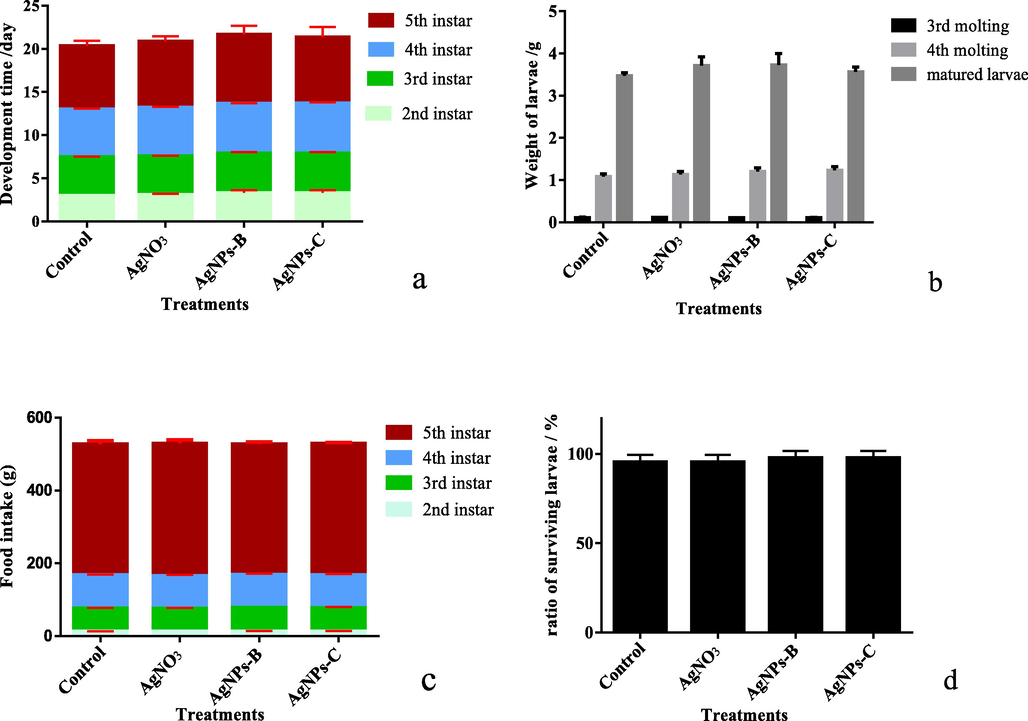
- Effect of AgNPs on growth and development of silkworm larvae a: the development time of silkworm larvae from 2nd instar to 5th instar; b: the weight of 5 larvae at 3rd molting, 4th molting stage, and matured stage; c: the feeding amounts of larvae; d: the ratio of survival larvae; AgNPs - B is biosynthetic nano - silver; AgNPs - C is commercial nano - silver.
3.4 Effect of AgNPs on the silkworsm cocoon yield and quality
The effect of AgNPs on silkworm cocoon yield and quality was also evaluated by measuring the rate of cocoon, total cocoon weight, cocoon shell weight, cocoon shell ratio, cocoon shape (cocoon length, and cocoon width) (Table 4). The results showed that there was no significant difference between the control and other treatments for B. mori in the aforementioned evaluation indexes. The average weight of the cocoon in the control and AgNPs-C group had the smallest value of 1.68 g, whereas the average weight of the cocoon was 1.72 g for AgNO3 group and 1.69 g for the AgNPs-B group; the cocoon shell ratio from the AgNPs-B group exhibited the largest value of 20.16 %, whereas the cocoon shell ratio was 19.59 % for the control group.
| Treatments | Rate of cocoon (%) | Cocoon weight (g) | Cocoon shell weight (g) | Cocoon shell ratio (%) | Cocoon length (mm) | Cocoon width (mm) |
|---|---|---|---|---|---|---|
| Control | 95.56 ± 5.09 a | 1.68 ± 0.02 a | 0.33 ± 0.01 a | 19.59 ± 0.92 a | 32.89 ± 0.37 a | 19.05 ± 0.06 a |
| AgNO3 | 91.11 ± 10.18 a | 1.72 ± 0.09 a | 0.35 ± 0.01 a | 20.11 ± 0.79 a | 32.26 ± 1.17 a | 19.07 ± 0.34 a |
| AgNPs-B | 93.33 ± 6.67 a | 1.69 ± 0.12 a | 0.34 ± 0.15 a | 20.16 ± 0.76 a | 32.30 ± 0.58 a | 18.75 ± 0.03 a |
| AgNPs-C | 93.33 ± 6.67 a | 1.68 ± 0.04 a | 0.34 ± 0.11 a | 19.91 ± 0.41 a | 33.18 ± 0.36 a | 18.96 ± 0.44 a |
AgNPs - B is biosynthetic nano - silver; AgNPs - C is commercial nano – silver.
4 Discussion
Microorganisms, including bacteria (such as actinomycetes), fungi, and yeasts, have been widely used for producing metal nanoparticles (Alghuthaymi et al., 2015; Soni and Prakash, 2015; Waghmare et al., 2015) because they are eco-friendly and cost-effective methods, while avoiding toxic, harsh chemicals and the high energy demand, which are required for physiochemical synthesis. Although the exact mechanism underlying biological synthesis of nanoparticles is not yet fully elucidated (Singh et al., 2016), the possible mechanism that may involve the reduction of silver ions by various reducing agents (metal-resistant genes, proteins, peptides, enzymes, etc.) is the electron shuttle enzymatic metal reduction process. Furthermore, these organic materials help in providing natural capping to synthesize nanoparticles, thereby preventing the aggregation of nanoparticles and helping them to remain stable for a long time (Li et al., 2011; Singh et al., 2016). Many recent literatures have been published mainly discussing a variety of parameters affecting biosynthesis of nanoparticles to rationally probe into an ideal production system (Wisnuwardhani et al., 2019; Bonnia et al., 2020). Biosynthesis parameters of metal nanoparticles, including pH, reaction temperature, metal salt concentration, aeration, irradiation, reaction time and bioresources (Chan and Don, 2013b; Ma et al., 2017) have been considered by researchers to optimize synthesis condition. Filamentous fungi have an excellent potential to produce around 6,400 bioactive substances (Bérdy, 2005), which can be widely used as reducing and stabilizing agents for the fast and sustainable synthesis of nanoparticles (Alghuthaymi et al., 2015; Khan et al., 2017). Furthermore, fungi, such as Trichoderma spp. can be easily cultivated on a large scale (“nanofactories”) with a lower cost for their lower cultivation requirements and higher growth rates (Lόpez-Bucio et al., 2015; Qu et al., 2021). In the present study, the color of the reaction solution changed from yellow to dark brown (Fig. 2 inset), which reflected a primary indication for AgNPs - B formation (Chan and Don, 2013a). The specific absorption peak of AgNPs - B occurred in the visible range of 420–580 nm (Fig.S1), possibly due to their sizes, shapes and particle interactions with the medium such as agglomeration (Elgorban et al., 2015). Our analysis with Orthogonal Experimental design showed that optimal biosynthesized condition of AgNPs - B was 2 mmol/L AgNO3, 55 °C and pH = 5. Orthogonal Experimental Design (OED) is use to investigate the impacts of various factors on synthesis of AgNPs for reducing the number of cases which is not used in published papers and the results can meet the requirements of comprehensive test. Through the range analysis, pH value played an important role in the biosynthesis of AgNPs - B, followed by the reaction temperature and the concentration of AgNO3, respectively. The findings of existing literature suggested that there should be a limit to the concentration of AgNO3 used in order to obtain nanoparticles with ideal physicochemical characteristics (Guilger-Casagrande and de Lima, 2019). The addition of excess amounts of metal ions resulted in the size and morphology changes of nanoparticles (Phanjom and Ahmed, 2017). In addition, a higher concentration of AgNO3 may lead to greater toxicity to organisms in the application (Balakumaran et al., 2015). Saxena et al., found that an increase in concentration of AgNO3 up to 2 mmol/L led to a complete reduction of Ag+ (Saxena et al., 2016), which correlates with our results. The temperature used in the biosynthesis of AgNPs employing fungi could affect the speed of the synthesis and was attributed to denaturation or inactivation of enzymes and other molecules, which lead to changes on the size, stability and quality of the nanoparticles. Saxena et al. (2016) reported that 80◦C was the optimum temperature for the production of high stable silver nanoparticles using Sclerotinia sclerotiorum MTCC 8785. Costa et al. (2017) found that the optimum synthesis temperature was 60 °C using Duddingtonia flagans. Fayaz et al. (2009) indicated that increase in reaction temperature from 20 °C to 40 °C led to a decrease in size of silver nanoparticles and increase in monodispersity. In our study, 55 °C was the optimal reaction temperature, which is closed to that of the above study. pH is also one of the most important factors that influence the size and shape of nanoparticles by changing the electrical charges of biomolecules, which might affect the reducing and stabilizing ability. In our study, pH was the most important factor. Acid pH (pH = 5) favored the synthesis of silver nanoparticle using T. longibranchiatum. The synthesized amount and antifungal activity of AgNPs were higher than other microorganisms. Using other fungi such as Verticillium luteoalbum, Fusarium acuminatum, Penicillium fellitanum for the biosynthesis of metal nanoparticles, acid pH is required for stable nanosilver (Gericke and Pinches, 2006; Nguyen et al., 2022).
Toxicity assays of nanosilver are necessary to monitor ecotoxicity to some sensitive organisms in the event of an environmental release including the health risk to human. Silkworms, Bombyx mori, are a promising model animal in health safety and environmental pollution assessment due to their sensitivity to chemical compounds like pesticides, drugs, and heavy metals (Nouara et al., 2018). Earlier studies have found that AgNPs had different levels of toxic effects on plants, microorganisms and animals (Afrasiabi et al., 2016; Rajeshkumar et al., 2016; Zuverza-Mena et al., 2016). Chen et al. (2019) found that AgNPs at 100 mg/L could promote the growth of silkworms, and upregulated 39 genes and downregulated 4 genes, whereas at 400 mg/L AgNPs downregulated some digestive enzymes, damage the tissue of midgut in silkworm, meantime induced the accumulation of reactive oxygen species which may cause oxidative stress. Incorporation of AgNPs (100 μg/mL) in supplementary diets increased significantly the larval weight, but decreased the relative growth rate at the high concentration (1000 μg/mL). In the meantime, exposure to the high concentration of AgNPs also altered hemocytes (Gad, 2020). AgNPs could improve the survival rate of the infected silkworms with nuclear polyhydrosis virus (BmNPV), but caused clumping of hemocytes in vivo and this was associated with slight weight loss and reduced cocoon size (Zou et al., 2012). Ke et al. (2019) found that with the increase of the AgNPs concentration, the weight of silkworm cocoons slightly decreased. AgNPs had no obvious influence on the growth of larvae at 1 % and the silkworm cocoons in color, size, and other appearances, which suggests that the addition of AgNPs had no influence on the formation of cocoons. Whereas some studies showed that a low concentration group of AgNPs promoted growth of larvae and larval silk glands (SGs), indicating that the addition of AgNPs could potentially increase silk protein synthesis (Prabu et al., 2011; Zhang et al., 2019). Xue et al. (2018) had also reported similar results that dietary TiO2NPs promoted silk protein synthesis in silkworms by influencing Akt/Tor signaling in silk glands. Our results showed that silkworm larvae from 2nd instar to 5th instar fed on mulberry leaves with AgNPs at concentrations 50 mg/L did not affect the silkworm larval growth or larvae weight, indicating that low concentration of AgNPs was not toxic to silkworm larvae. AgNPs did not affect the rate of the cocoon, total cocoon weight, cocoon shape, which indicated that larvae treated with AgNPs could normally secret silk for the cocoon. These results agreed with Ke et al. (2020) that AgNPs had no influence on the size of the cocoon. The results of the present study were also consistent with those reported by Prabu et al. (2011) & Zhang et al. (2019) that AgNPs could potentially increase silk protein synthesis attributing a higher cocoon shell ratio in group treated with AgNPs.
5 Conclusions
Biosynthesis of AgNPs - B using T. longibrachaitum may be considered as an eco-friendly, cost-effective and efficient approach among various nanosilver production methods. Biosynthesized AgNPs can act as excellent antimicrobial agents against fungal plant pathogens. The present study showed the optimum conditions of AgNPs synthesis were as follow: silver nitrate (2 mmol/L), reaction temperature (55 °C), and pH value (7.0). The prepared AgNPs showed the spherical shape of cubic crystal nanoparticles with size ranges from 5 to 50 nm and many functional group moieties. Supplement of AgNPs-B had no adverse effects on the development of silkworm larvae and cocoon quality, which indicated that AgNPs-B was not toxic to silkworm at 50 mg/L. The results will supply useful safety information for the broad application of silver nanoparticles. However, effect of biosynthesized AgNPs on the tissue structures and physiological and biochemical characteristics of larvae needs to be further studied in detail.
CRediT authorship contribution statement
Xiaohui Cui: Conceptualization, Methodology, Writing – original draft. Zhen Zhong: Writing – original draft, Formal analysis. Runxi Xia: Writing – review & editing, Data curation. Xian Liu: Supervision, Writing – review & editing. Li Qin: Writing – review & editing, Funding acquisition.
Acknowledgement
This research was financially supported by China Agriculture Research System of MOF and MARA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdel-Kareem, M.M., Zohri, A.A., 20and18. Extracellular mycosynthesis of gold nanoparticles using Trichoderma hamatum: optimization, characterization and antimicrobial activity. Letters in Applied Microbiology 67(5). DOI:10.1111/lam.13055.
- Dietary silver nanoparticles reduce fitness in a beneficial, but not pest, insect species. Arch. Insect Biochem. Physiol.. 2016;93:190.
- [CrossRef] [Google Scholar]
- Fungal biosynthesis of silver nanoparticles and their role in control of Fusarium wilt of sweet pepper and soil-borne fungi in vitro. Int. J. Pharmacol.. 2018;14:773-780.
- [CrossRef] [Google Scholar]
- Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip.. 2015;29:221-236.
- [CrossRef] [Google Scholar]
- Toxicity assessment of metal oxide nanomaterials using in vitro screening and murine acute inhalation studies. NanoImpact. 2020;18:100214
- [CrossRef] [Google Scholar]
- Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19:255102
- [CrossRef] [Google Scholar]
- Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279-290.
- [CrossRef] [Google Scholar]
- Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res.. 2015;178:9-17.
- [CrossRef] [Google Scholar]
- Study on parameters optimization of silver nanoparticles biosynthesized using aqueous extract of Imperata cylindrica. Desalin. Water Treat.. 2020;174:186-195.
- [CrossRef] [Google Scholar]
- Biosynthesis and structural characterization of Ag nanoparticles from white rot fungi. Mat. Sci. Eng. C. 2013;33:282-288.
- [CrossRef] [Google Scholar]
- Optimization of process variables for the synthesis of silver nanoparticles by Pycnoporus sanguineus using statistical experimental design. J. Korean Soc. Appl. Biol. Chem.. 2013;56(1):11-20.
- [CrossRef] [Google Scholar]
- Silver nanoparticle toxicity in silkworms: omics technologies for a mechanistic understanding. Ecotoxicol. Environ. Saf.. 2019;172:388-395.
- [Google Scholar]
- Characterization of silkworm larvae growth and properties of silk fibers after direct feeding of copper or silver nanoparticles. Mater. Design. 2017;129:125-134.
- [CrossRef] [Google Scholar]
- Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagus fungus Duddingtonia flagans. Int. J. Nanomed.. 2017;12:6373-6381.
- [CrossRef] [Google Scholar]
- Antifungal silver nanoparticles: synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2015:1-7.
- [Google Scholar]
- Fungal based synthesis of silver nanoparticles - an effect of temperature on the size of particles. Colloids Surf. B Biointerfaces. 2009;74:123-126.
- [CrossRef] [Google Scholar]
- Health and environmental safety of nanomaterials: O data, where art thou? NanoImpact. 2022;25:100378
- [CrossRef] [Google Scholar]
- Toxicity effect of silver nanoparticles to the haemocytes and antioxidant activity of silkworm Bombyx mori. Physiol. Entomol.. 2020;45:154-160.
- [CrossRef] [Google Scholar]
- A green approach for silver nanoparticles preparation by cell-free extract from Trichoderma reesei fungi and their characterization. Mater. Res. Express. 2019;6:095040
- [CrossRef] [Google Scholar]
- Biological synthesis of metal nanoparticles. Hydrometallurgy. 2006;83:132-140.
- [CrossRef] [Google Scholar]
- Antimicrobial activity and physical characterization of silver nanoparticles green synthesized using nitrate reductase from Fusarium oxysporum. Appl. Biochem. Biotechnol.. 2014;172:4084-4098.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles mediated by fungi: a review. Front. Bioeng. Biotechnol.. 2019;7:287.
- [CrossRef] [Google Scholar]
- Huq, M.l., 2020. Biogenic Silver Nanoparticles Synthesized by Lysinibacillus xylanilyticus MAHUQ-40 to Control Antibiotic-Resistant Human Pathogens Vibrio parahaemolyticus and Salmonella Typhimurium. Frontiers in Bioengineering and Biotechnology 8, 597502. DOI: 10.3389/fbioe.2020.597502.
- Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact. 2020;18:100219
- [CrossRef] [Google Scholar]
- Size-dependent uptake and distribution of AgNPs by silkworms. ACS Sustain. Chem. Eng.. 2019;8(1):460-468.
- [CrossRef] [Google Scholar]
- An overview: biological organisms that serves as nanofactories for metallic nanoparticles synthesis and fungi being the most appropriate. Bioceram Dev. Appl.. 2017;7:101.
- [CrossRef] [Google Scholar]
- New tools in risk assessment of nanomaterials. NanoImpact. 2019;16:100189
- [CrossRef] [Google Scholar]
- Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater.. 2011;8:270974
- [CrossRef] [Google Scholar]
- Bactericidal mechanisms revealed for rapid water disinfection by superabsorbent cryogels decorated with silver nanoparticles. Environ. Sci. Technol.. 2015;49:2310-2318.
- [Google Scholar]
- Biosynthesis of silver nanoparticles by Aspergillus terreus: characterization, optimization, and biological activities. Front. Bioeng. Biotechnol.. 2021;9:633468
- [CrossRef] [Google Scholar]
- Trichoderma as biostimulant: exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic.. 2015;196:109-123.
- [CrossRef] [Google Scholar]
- Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater. Sci. Eng. C. 2017;77:963-971.
- [CrossRef] [Google Scholar]
- Intrinsically reinforced silks obtained by incorporation of graphene quantum dots into silkworms. Sci. China Mater.. 2019;62:245-255.
- [CrossRef] [Google Scholar]
- In vivo toxicity evaluation of boron nitride nanosheets in Bombyx mori silkworm model. Chemosphere. 2020;247:125877
- [CrossRef] [Google Scholar]
- Silkworm: a promising model organism in life science. J. Insect Sci.. 2017;17(5):1-6.
- [CrossRef] [Google Scholar]
- Effects of Ag nanoparticles on growth and fat body proteins in silkworms (Bombyx mori) Biol. Trace Elem. Res.. 2017;180:327-337.
- [CrossRef] [Google Scholar]
- Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology. 2008;19(7):075103
- [CrossRef] [Google Scholar]
- Synthesis, characterization and biomedical application ofsilver nanoparticles. Materials. 2022;15:427.
- [CrossRef] [Google Scholar]
- Antibacterial properties of silver nanoparticles greenly synthesized using guava fruit extract as a reducing agent and stabilizer. Appl. Nanosci. 2022
- [CrossRef] [Google Scholar]
- Effect of TiO2 nanoparticles on the reproduction of silkworm. Biol. Trace Elem. Res.. 2015;164:106-113.
- [CrossRef] [Google Scholar]
- Silkworm, Bombyx mori, as an alternative model organism in toxicological research. Environ. Sci. Pollut. Res.. 2018;25:35048-35054.
- [CrossRef] [Google Scholar]
- Effect of different physicochemical conditions on the synthesis of silver nanoparticles using fungal cell filtrate of Aspergillus oryzae (MTCC No. 1846) and their antibacterial effects. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2017;8:1-13.
- [CrossRef] [Google Scholar]
- Studies on the growth rate of silkworm bombyx mori (L.) (lepidoptera: bombycidae) fed with control and silver nanoparticles (AgNps) treated MR2 mulberry leaves. Int. J. Indus. Entomol.. 2011;22(2):39-44.
- [Google Scholar]
- Biosynthesis of silver nanoparticles (AgNPs) employing Trichoderma strains to control empty-gut disease of oak silkworm (Antheraea pernyi) Mater. Today Commun.. 2021;28:102619
- [CrossRef] [Google Scholar]
- Biogenic nanoparticles: an introduction to what they are, how they are synthesized and their applications. In: Rai M., Duran N., eds. Metal Nanoparticles in Microbiology. Berlin/Heidelberg: Springer; 2011. p. :1-16.
- [Google Scholar]
- Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct.. 2016;1116:165-173.
- [Google Scholar]
- Fungal isolate optimized for biogenesis of silver nanoparticles with enhanced colloidal stability. Langmuir. 2016;32:8688-8697.
- [CrossRef] [Google Scholar]
- A Review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mat. Sci. Eng.. 2015;2015:682749
- [CrossRef] [Google Scholar]
- Renwick, J., and Kavanagh, K., 2007. Insects as models for studying the virulence of fungal pathogens of humans, pp. 45–67. New insights in medical mycology. Springer, Dordrecht, the Netherlands.
- Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. Springerplus. 2016;5(1):861.
- [CrossRef] [Google Scholar]
- Animal welfare and use of silkworm as a model animal. Drug Discov. Therap.. 2012;6(4):226-229.
- [CrossRef] [Google Scholar]
- Assessment of biotic and abiotic behaviour of engineered SiO2 nanoparticles for predicting its environmental providence. NanoImpact. 2019;17:100200
- [CrossRef] [Google Scholar]
- Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol.. 2016;588–599
- [CrossRef] [Google Scholar]
- Antimicrobial and mosquitocidal activity of microbial synthesised silver nanoparticles. Parasitol. Res.. 2015;114:1023-1030.
- [CrossRef] [Google Scholar]
- Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv. Nat. Sci.: Nanosci. Nanotechnol.. 2013;4:035005
- [CrossRef] [Google Scholar]
- Optimization of microbial synthesis of silver sulfide nanoparticles. Appl. Biochem. Microbiol.. 2018;54(8):800-807.
- [CrossRef] [Google Scholar]
- Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3. Biotech. 2015;5:33-38.
- [Google Scholar]
- Optimization of Silver Nanoparticles Synthesis using Kawista (Limonia Acidissima Groff.) leaves ethanol extract. In: Annual Conference of Science and Technology 1375. 2019.
- [CrossRef] [Google Scholar]
- Impact of fluorescent silicon nanoparticles on circulating hemolymph and hematopoiesis in an invertebrate model organism. Chemosphere. 2016;159:628-637.
- [CrossRef] [Google Scholar]
- Titanium nanoparticles influence the akt/tor signal pathway in the silkworm, bombyx mori, silk gland. Arch. Insect Biochem. Physiol.. 2018;99(12):e21470
- [CrossRef] [Google Scholar]
- Optimization of synthesizing silver nanoparticles from Trichoderma strains for inhibition of Fusarium oxysporum. Chin. J. Biotechnol.. 2020;36(9):1859-1868. In Chinese
- [Google Scholar]
- Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE. 2012;7(10):e47674
- [CrossRef] [Google Scholar]
- Impact of adding glucose-coated water-soluble silver nanoparticles to the silkworm larval diet on silk protein synthesis and related properties. J. Biomater. Sci. Polym. Ed. 2019
- [CrossRef] [Google Scholar]
- DNA sequencing and UP-PCR characterization of Fusarium oxysporum isolates from three Cucurbit species. Plant Pathol. J.. 2013;12(2):78-84.
- [CrossRef] [Google Scholar]
- Reducing the pathogenicity of BmNPV in silkworms using silver nanoparticles. Entomol. Exp. Appl.. 2012;144:301-310.
- [Google Scholar]
- Effects of silver nanoparticles on radish sprouts: root growth reduction and modifications in the nutritional value. Front. Plant Sci.. 2016;7:90.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104142.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







