Translate this page into:
Cassia fistula leaves extract profiling and its emphasis on induced ulcerative colitis in male rats through inhibition of caspase 3 and cyclooxygenase-2
⁎Corresponding author. gehan_ibrahim@pharm.suez.edu.eg (Jihan M. Badr)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The objective of the study was to determine the total phenolic and total flavonoid contents (TPC and TFC, respectively), as well as the solvent-partitioned fractions, of the leaf extract of Cassia fistula. Using DPPH, TAC, and FRAP tests, the in vitro antioxidant properties of crude extract and its ethyl acetate fraction were investigated. Additionally, C. fistula chemical profiling was accomplished using LC-ESI-MS/MS. Along with that, the effectiveness of crude extract (200 and 400 mg/kg) in treating male rats with ulcerative colitis (UC) induced by acetic acid was assessed. According to the findings, the ethyl acetate fraction had the highest concentrations of TPC (12.36 1.46 mg GAE/100 mg) and TFC (5.12 0.64 mg QE/100 mg), as well as impressive in vitro antioxidant activity with IC50 values of DPPH (12.7 g/mL), FRAP (4.87 0.71 mM Fe+2/g), and TAC (55. Fifty-five chemicals, predominantly phenolics (catechins, flavonoids, anthraquinones, chalcones, and phenolic acids), were detected by the LC/MS/MS. The anti-UC effect of C. fistula was dose-dependent. The hematological parameters, liver biomarkers, oxidative stress, histopathology, and immunohistochemistry revealed that prophylactic administration of crude extract to colitis rats ameliorated all the previous biomarkers. However, when the crude extract was administered on established colitis, it facilitated the recovery of the inflamed mucosa and showed better results than the protection mode. The phytoconstituents of C. fistula that were discovered here by LC/MS/MS were examined by molecular docking studies for their binding affinities towards COX-2 and caspase-3 proteins to highlight the anti-inflammatory and antiapoptotic activities of the plant. The Emodin compound displayed the highest binding affinity for COX-2 proteins, while isorhamnetin was discovered to have the best binding associations with the essential amino acids of caspase-3. Procyanidin B2 interestingly shows potent interactions with important amino acids of two targets. This study has made it possible to utilize C. fistula in the treatment of UC and has clarified its mechanism of action.
Keywords
C. fistula
Antioxidant
TFC
TPC
LC/MS/MS
ulcerative colitis
COX-2
caspase-3
in silico
1 Introduction
Inflammation is a natural physiological process that occurs in response to tissue injury, infection, and a variety of other conditions that contribute to pathological changes (Kwon et al., 2005). Inflammatory bowel diseases (IBD) are divided into two types: UC and Crohn's disease, both of which are marked by epithelial ulceration. It can lower the quality of life of patients and increase the risk of colon cancer (Abraham et al., 2017). Inflammatory Bowel Disease (IBD) is a condition with an unknown definitive cause, but it is widely accepted that various factors such as environmental, microbial, genetic, and immunogenic elements interact to trigger the mucosal T-cell immune response. This immune response leads to the release of inflammatory mediators, including reactive nitrogen species (RNS) and reactive oxygen species (ROS). The consequences of this immune activation include cell infiltrations, a breakdown of the mucosal barrier, a decrease in antioxidant enzymes in the colonic mucosa, the production of anti-inflammatory cytokines, and the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Ultimately, these processes contribute to apoptotic injuries in the colon (Shahid et al., 2022).
Most patients with IBD who require ongoing treatment to keep the disease under control find that the existing medications are far from satisfactory. Furthermore, many of the currently synthetic drugs, such as aminosalicylates, corticosteroids, immune modulators, and biological treatments, have a range of adverse effects with varying reactions that can deteriorate over time as well as limit their clinical efficacy. Nearly 50% of IBD patients are dissatisfied with the current IBD treatments, which is why plant-based drugs are growing in popularity. This is due to their pharmacological traits, which also aid in addressing nutritional shortages, decreasing oxidative stress and inflammation, and preventing unforeseen negative effects (Bastaki et al., 2022). Chromatography, microscopy, and other research techniques, as well as phytochemical, pharmacological, and allied approaches, are the principal techniques utilized to evaluate these drugs (Sharma, 2021).
Nearly all medicinal plants are composed of phenolic compounds, particularly flavonoids, which are powerful bioactive substances. Plants from the Acacia species (Fabaceae Family) are abundant in phenolic compounds, flavonoids, and tannins and can be used in various medical conditions (Abdallah et al., 2020). Cassia fistula (C. fistula) Linn is a particular plant belonging to the family Fabaceae that has great ethnomedicinal significance and is widely used in Unani and Ayurvedic medicines and holds a prominent place in traditional medicine. Renowned for its efficacy against various ailments, including skin diseases, liver issues, tuberculous glands, and more, the plant has been suggested for treating conditions like haematemesis, pruritus, leucoderma, and diabetes. Traditional methods of administration include infusions, decoctions, or powders. The plant's versatile applications highlight its significance in traditional healing practices (Ali, 2014). It originated in India and Sri Lanka and has since expanded to several locations, including Mexico, China, East and South Africa (Sanoria et al., 2020). C. fistula confirmed the therapeutic effects and its role in the management of health via modification of biological processes due to its high antioxidant content. Some outcomes based on animal models have shown the safety and effectiveness of medications, opening up new avenues for the treatment of human illness and assisting in disease prevention (Rahmani, 2015). The generation of ulcerative colitis (UC) through acetic acid (AA) has led to extensive exploration of chemical-induced animal models of colonic inflammation. These models aim to comprehend the underlying pathophysiological mechanisms of UC and evaluate the effectiveness of treatment medications (Dothel et al., 2013, Zabihi et al., 2024).
The antioxidant properties of C. fistula leaves have been reported (Khan et al., 2012; Kaur et al., 2020). The plant has been found to possess antioxidant, antimicrobial, anti-inflammatory, antidiabetic, antitumor, and hepatoprotective activities, among others. Pharmacological reviews on medicinal plants highlight the significance of C. fistula in providing valuable bioactive natural products. The exploration of these properties opens avenues for developing novel pharmaceutical products with potential therapeutic benefits. C. fistula's multifaceted pharmacological profile underscores its importance in the search for new and effective medicinal compounds (Mwangi et al., 2021). The current study focused on investigating the antioxidant potential of C. fistula leaves, considering their traditional significance. Additionally, the study explored the total phenolic and flavonoid content in different extracts of C. fistula leaves. The research also evaluated the leaves' potential protective and treatment effects against ulcerative colitis induced by acetic acid. Furthermore, the study involved molecular docking of the identified compounds against protein structures of caspase-3 and cyclooxygenase-2 (COX-2) using Auto Dock Vina software.
2 Materials and Methods
2.1 Chemicals and reagents
Chloroform (99.50%), ethyl acetate (99.90%), butanol (99.7%), methanol (99.8%), n. hexane (99.0%), silica gel, TLC plates, and acetic acid (AA, 99.8%) were obtained from Alpha Chemika (India). Sulfasalazine was brought from KAHIRA PHARM & CHEM (Cairo, Egypt). The highest purity and analytical grade were used for all other chemicals. Spectrophotometric of colonic tissue for the determination of malondialdehyde (MDA), catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), reduced glutathione (GSH), cyclooxygenase-2 (COX-2) and caspase-3 were purchased from Biodiagnostic Co. (Giza, Egypt).
2.2 Plant material and sample preparation
Fresh leaves of C. fistula, Fabaceae (Leguminosae) were collected in March from Suez Canal University, Ismailia, Egypt. Dr. Yasmen Mohamed Hassan of the Department of Botany at Suez Canal University's Faculty of Science verified the authenticity of the plant. For future use, a specimen voucher was kept in the lab [202005 M1].
For seven days, the plant material was allowed to air dry. Using a heavy-duty blender, seven kilograms of the dried material were ground into a fine powder, yielding (3.5 kg). A greenish-black residue weighing 660 g was obtained by macerating fine powder with 3 × 8 L of methanol, filtering, and concentrating the solution in a rotary evaporator at 40 °C. After allowing the residue to dry, fractionation of the crude extract involved suspending 290 g in 200 mL of distilled water and partitioning with n-hexane (Hex). The resulting aqueous layer was concentrated and sequentially partitioned with chloroform (CHCl3), ethyl acetate (EtOAc), and n-butanol (n-BuOH) (Khurm et al., 2021). Vacuum concentration of these solvent extracts yielded fractions of n-hexane, CHCl3, EtOAc, and n-butanol, weighing 128.4 g, 10.18 g, 63.24 g, and 79.59 g, respectively.
2.3 Assessment of total phenolic and flavonoids
The total phenolic content (TPC) of both crude and fractional extracts of C. fistula was determined using spectrophotometry, following the protocol outlined by Singleton and Rossi (1965). The Folin-Ciocalteau method was employed for the TPC assay, and the results were quantified and presented as mg gallic acid equivalents (GAE) per gram of extract. Additionally, the total flavonoid content (TFC) was assessed using the method described by Djeridane et al. (2020), with TFC results expressed as mg quercetin equivalents (QE) per gram of extract.
2.4 In vitro antioxidant activity of crude extract and its EtOAc fraction
The DPPH free radical scavenging, FRAP, and TAC tests were employed in triplicate by the Regional Centre for Mycology and Biotechnology (RCMB) at Al-Azhar University to assess the antioxidant properties of crude extract and its EtOAc fraction.
2.4.1 DPPH free radical scavenging activity
Measurements were also made of the absorbance of the reference compound ascorbic acid and the DPPH radical without antioxidant (control) (Oboh, 2005). Every calculation was made in three duplicates and averaged. The following formula was used to determine the DPPH radical's percentage inhibition (PI):
The study utilized the 50 % inhibitory concentration (IC50) and graphical representations of the dose–response curve to determine the concentration required to inhibit the (DPPH) radical by 50 %. This approach allowed for the assessment of the effectiveness of the tested compounds in scavenging the DPPH radical.
2.4.2 Ferric reducing antioxidant power (FRAP)
The formula for the (FRAP) of the methanol extract was derived following the method outlined by Nsimba et al. (2008). The reaction progress was tracked by measuring the absorbance change at 593 nm using the Milton Roy Spectronic 1201 spectrophotometer in Houston, Texas, USA. This approach allowed for the quantification of the antioxidant power of the methanol extract.
2.4.3 Total antioxidant capacity (TAC)
The extract TAC was assessed utilizing a phospho-molybdenum test and spectrophotometric analysis. With a few adjustments, the technique was carried out as Saeed et al. (2012) had previously reported. Milton Roy, Spectronic 1201, Houston, TX, USA was used to measure the absorbance using a UV–visible spectrophotometer at 695 nm against a blank sample. Gallic acid equivalents were calculated from the TAC values (mg/g of dry material). The reference substance was butylated hydroxytoluene (BHT; Sigma-Aldrich, St. Louis, MO, USA).
2.5 LC-ESI-TOF-MS/MS analysis of C. fistula crude extract
LC-ESI-TOF-MS/MS (SCIEX, Santa Clara, CA, USA) was used to investigate the nature and diversity of C. fistula phytochemicals. The samples were prepared as described before (Eltamany et al., 2020; Eltamany et al., 2022; Goda et al., 2022; Anlas et al., 2023), deionized H2O, CH3CN, and MeOH were combined in the mobile phase working solution in a 50/25/25 ratio. One mL of MP-WS was mixed with 50 mg of weighted dry extract before being vortexed for two min. Following that, 10 min of ultrasonication and 10 min of 10,000 rpm centrifugation were completed. The produced solution was withdrawn, and a further 50 L aliquot was diluted with the reconstitution solvent. A 2.5 g/L solution was prepared and injected in both negative and positive modes using 25 mL. A blank sample with 25 L of mobile phase working solution was also injected. In positive TOF MS mode, (A) 5 mM NH4COOH buffer (pH = 3) with 1 % MeOH was used; in negative TOF MS mode, (B) 5 mM NH4COOH (pH = 8) with 1 % methanol was used; and for both modes, (C) mobile phase consisting of 100 % CH3CN was used. The pre-column had in-line filter discs (Phenomenex, 0.5 m X 3.0 mm), and the column was an X-select HSS T3 (Waters, 2.5 m, 2.1 X 150 mm), with a flow rate of 0.3 mL/min. The column operated at a temperature of 40 °C, with an injection volume of 10 µL.
Data processing utilized MS-DIAL3.52ur and Master View software, with peak extraction criteria: Sample-to-blank feature intensities greater than 5 and a Signal-to-Noise ratio of at least 5 for non-targeted analysis. Compound identification relied on precise mass measurements (m/z), MS/MS data, spectrum library exploration, and comparisons with public repositories (MassBank NORMAN, MassBank MoNA, PubChem, xxxx) and literature retention periods. (Hu et al., 2023)
2.6 In vivo study
2.6.1 Experimental and Animal Ethics
Three hundred and fifty male albino rats weighing between one hundred and two hundred grams were acquired at the age of ten weeks from the Egyptian Organisation for Biological Products and Vaccines located in Cairo, Egypt. The rats were acclimatized for ten days under controlled conditions of 25 ± 3 °C, with a regular light/dark cycle, and were provided with unrestricted access to water and laboratory meal pellets during this period. All experimental procedures were approved and performed in compliance with the guide lines of the Research Ethical Committee of the Faculty of Pharmacy, Suez Canal University (Approval No. 202005 M1).
2.6.2 Induction of Ulcerative Colitis and Treated Groups
After a 24-hour fast with unrestricted access to water, the animals' stomach contents were emptied. The procedure that Hagar et al. (2007) had previously outlined was used to produce colitis. An intraperitoneal injection of KX rat cocktail (0.1 mL/100 g rat weight) was used to sedate each rat. includes 9.1 mg/kg of xylazine and 91 mg/kg of ketamine. A 2 mm diameter polyurethane enteral feeding tube was placed to a depth of 4.5 cm, and 2 mL of 3 % AA was injected into the rectum through it. The rats were kept in the Trendelenburg position during reinstallations and for a minute after stellations to stop solution leakage. The rats' diarrhea or rectal bleeding after 24 h suggested that colitis had been induced (Alsharif et al., 2022) C. fistula extract of doses (200 and 400 mg/kg) (Bhakta et al., 1999) were dissolved in 0.1 % DMSO and administered orally to the rats as indicated in the following groups.
Seven groups of five rats each were used in this experimental study, and they were split randomly as follows:
Group I: For 17 days, 0.1 % dimethyl sulfoxide (DMSO) was administered transrectally to the control group.
Group II: Colitis group, in which 14 days of 0.1 % DMSO transrectally were administered after 3 days of no therapy to produce ulcerative colitis (UC).
Group III: After transrectally administering AA for three days straight to induce colitis, 500 mg/kg (Owusu et al., 2020) of sulfasalazine medication for body weight was given for 14 days.
Group IV: Following UC induction, MLE 200 mg/kg of body weight was administered for 14 days.
Group V: Following UC induction, MLE 400 mg/kg of body weight was administered for 14 days.
Group VI: UC was induced after 14 days of MLE 200 mg/kg of body weight being given as a protective dose.
Group VII: UC was produced after 14 days of MLE 400 mg/kg of body weight being given as a protective dose.
2.6.3 Determination of body weight, and macroscopic scores
The body weight of each animal was measured at the start of the experiment and again after 21 days for every group. The colon's weight after the trial was assessed, as was the colon's weight-to-length ratio.
Macroscopic inflammation scores were assigned based on clinical features of the colon using an arbitrary scale ranging from 0 to 4 as follows: 0 (no macroscopic changes), 1 (mucosal erythema only), 2 (mild mucosal edema, slight bleeding or small erosions), 3 (moderate edema, slight bleeding ulcers or erosions), 4 (severe ulceration, edema, and tissue necrosis) (Salama et al., 2020).
Stool consistency was assessed with the following scoring criteria: 0 for normal, 1 and 2 for loose stool, and 3 and 4 for diarrhea.
2.6.4 Collection of blood and tissue
Blood samples were swiftly collected from the medial retro-orbital venous plexus of starved rats under anesthesia, using capillary tubes (Micro Hematocrit Capillaries, Mucaps) (El-Shenawy et al., 2006). The collected blood was processed to extract serum, which was then centrifuged for 15 min at 4000 rpm to assess liver biomarkers. Additional blood collected in tubes containing EDTA was reserved for hematological analysis.
A suitable colonic slice was completed after adipose tissue was removed and a cool, normal saline rinse was administered. Colon homogenates were extracted from the remaining sections and stored for biochemical research at 80 0C. Colonic segments including the third section were preserved in 10 % neutral buffered formalin for further histological and immunohistochemical examination.
2.6.5 Hematological evaluation
Cell counts were conducted using the Humalog System Analyzer, providing data on white blood cells (WBCs), red blood cells (RBCs), platelets count, hemoglobin (HGB), hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC). The measurement included the number of WBCs per cubic milliliter of blood, and WBC differentiation was also assessed.
2.6.6 Liver biomarker evaluation
Serum activities of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) were assessed using kits from Sentinel Diagnostics (Milan, Italy). Additionally, total bilirubin and protein levels were determined through spectrophotometry with commercial kits from Boehringer Mannheim GmbH (Mannheim, Germany). In the protein assay, the reaction with copper in the biuret reagent led to an increase in absorbance at an alkaline pH. The resulting rise in absorbance at 550 nm, attributed to the formation of a colored complex, was directly proportional to the concentration of protein in the reaction (Gornall, 1949).
2.6.7 Assessment of oxidative stress and antioxidants
Malondialdehyde (MDA) a marker of lipid peroxidation (LPO) in mucosal tissue induced by reactive oxygen species was measured in serum by the thiobarbituric acid assay (TBA) as previously described by Ahmed and Zaki (2009).
In addition, serum antioxidant defense enzymes that counteract the oxidative stress in colonic tissues were estimated. Superoxide dismutase (SOD) was determined according to Nishikimi et al. (1972). Catalase (CAT) was responsible for the conversion of H2O2 into H2O and was measured as outlined in Aebi (1984). Glutathione peroxidase (GPx) was determined as previously mentioned by Paglia and Valentine (1967). Reduced glutathione (GSH) levels were estimated based on the method of Beutler et al. (1969), who reported that the 5,5′-dithiobis- (2-nitrobenzoic acid) is reduced by the SH group to form 1 mol of 2-nitro-5-mercaptobenzoic acid per mole of SH. Finally, serum total antioxidant capacity was evaluated following the method described (Han et al., 2012).
2.6.8 Histopathological study
The collected colon tissues were preserved for 24 h in 10 % neutral buffered formalin, followed by a tap water wash, dehydration, and xylene clearance. The samples were then regularly prepared to yield paraffin-embedded slices that were 5–7 µm thick, and they were then stained with H&E stain for light microscopy analysis (Nagahashi et al., 2017).
2.6.9 Immunohistochemical (IHC) evaluation of cyclooxygenase-2 (COX-2) and caspase-3
In colonic tissues, the activity of COX-2 (an inflammatory marker) and Caspase-3 (a marker for apoptosis) was determined by IHC. Sections from colon tissues were prepared and stained with anti-caspase 3 (Catalog number GTX30246, GeneTex, Irvine, CA, USA) and anti-COX-2 (Catalog number ab16701, Abcam, Cambridge, UK.) and after preparation according to the methods mentioned (Chang et al., 2019; Eltamany et al., 2021), respectively. The immunoreaction was visualized using 3,3-diaminobenzidine (Power-Stain™ 1.0 Poly HRP DAB Kit for Mouse + Rabbit, GENEMED, South San Francisco, CA, USA) as a chromogen, with Mayer’s hematoxylin used as a counterstain. Selected portions of the colon were captured with a digital camera (Olympus Dp25). Quantification of COX-2 and Caspase-3 reactivity was performed through image analysis software (ImageJ version 1.53 h). The results were expressed as the mean area of immunopositive cells (IHC) per mm2, calculated using a specific equation:
2.7 Molecular docking
Molecular modeling investigations were conducted using Chimera-UCSF and AutoDock Vina on Linux-based systems. Binding sites within proteins were identified by measuring the sizes of grid boxes covering the co-crystallized ligands after their structures had been generated and optimized in Maestro. Following standard procedures (Nafie et al., 2019; Kishk et al., 2020; Kishk et al., 2022), the studied compounds were docked against the Caspase 3 (PDB = 6 CKZ) and COX-2 (PDB = 5 W58) protein structures using AutoDock Vina software. The results of molecular docking, interpreting binding activities in terms of binding energy and ligand-receptor interactions, were analyzed.
2.8 Statistical analysis
SPSS version 20 was employed to conduct the statistical analysis. The data were presented as means ± S.E. via Duncan's new multiple range test (MRT) and One-Way Analysis of Variance (ANOVA). P values ≤ 0.05 were considered statistically significant.
3 Results and Discussion
3.1 Total phenolic content (TPC) and flavonoid content(TFC)
As displayed in Table 1, the solvents used for extraction and fractionation influenced the estimated TPC and TFC. Among the tested samples, the EtOAc fraction of C. fistula showed the highest TPC (12.36 ± 1.46 mg GAE/g) and TFC (5.12 ± 0.64 mg. QE/100 mg) followed by n. butanol fraction of C. fistula with TFC of 5.03 ± 0.94 mg QE/100 mg) and TPC of 11.47 ± 1.29 mg GAE/ 100 mg then the crude extract with TFC and TPC of 4.25 ± 0.71 mg QE/ 100 mg and 10.56 ± 1.32 mg GAE/100 mg, respectively. The CHCl3 exhibited notable TPC and TFC while n. hexane fraction recorded the least TFC and TPC. TPC is expressed as mg gallic acid equivalents (GAE)/g extract and TFC is expressed as mg quercetin equivalents (QE)/g extract. Data are presented as mean ± S.D (n = 5).
Sample
Total Flavonoids (mg QE)/100 mg)
Total Phenolic (mg GAE)/100 mg)
C. fistula crude extract
4.25 ± 0.71
10.56 ± 1.32
n-Hexane fraction
1.04 ± 0.32
3.22 ± 0.94
CHCl3 fraction
3.89 ± 0.63
9.48 ± 1.72
EtOAc fraction
5.12 ± 0.64
12.36 ± 1.46
n- BuOH fraction
5.03 ± 0.94
11.47 ± 1.29
High flavonoid and phenolic contents in medicinal plants are linked to antioxidant activity, which plays a vital role in the prevention and treatment of several chronic conditions caused by oxidative stress (Keshavarzi et al., 2019). The hexane fraction had the lowest TPC and TFC amounts of the crude extract and fractions, whereas the ethyl acetate (EtOAc) fraction had the greatest contents of total phenolic and flavonoids. These results agreed with earlier investigations that compared the influence of different solvents on the derivation of plant polyphenols and indicated that EtOAc is selectively the best solvent for extracting phenolic compounds, especially flavonoids (Das et al., 2014; Pintać et al., 2018; Autor et al., 2022).
3.2 Antioxidant Activity of crude extract and its EtOAc fraction
Three distinct antioxidant tests (TAC, FRAP, and DPPH) were used to assess the in vitro antioxidant activity of crude extract and EtOAc fraction. In Table 2, both the EtOAc fraction and the crude extract exhibited notable TAC with 65.19 ± 5.33 mg GAE/g and 48.37 ± 3.19 mg GAE/g respectively compared to that of BHT (the positive control; 76.43 ± 3.89 mg GAE/g). Data are presented as mean ± S.D (n = 3). TAC: total antioxidant capacity, FRAP: ferric antioxidant power, and BHT: butylated hydroxytoluene
Samples
TAC (mg GAE/g)
FRAP (mMol Fe+2/g)
DPPH IC50(µg/mL)
Crude extract
48.37 ± 3.19
2.76 ± 0.38
60.6
EtOAc fraction
65.19 ± 5.33
4.87 ± 0.71
12.7
BHT
76.43 ± 3.89
6.98 ± 0.76
–
Ascorbic acid
–
–
10.6
In comparison to the positive control (BHT with 6.98 mMol Fe+2 /g), the FRAP results showed that crude extract and its EtOAc fraction demonstrated promising ferric reduction ability with 2.76 and 4.87 mMol Fe+2 /g, respectively (Table 2). The process involves observing a shift in absorbance, where a higher absorbance indicates increased reduction capacity in the tested samples. This method allows measurement and assessment of the compounds' ability to undergo reduction reactions (Nishikimi et al., 1972).
The crude extract as well as its EtOAc fraction had definite and remarkable quenching activity on DPPH free radicle exhibiting a dose-dependent scavenging rate (Figs. 1 and 2). The calculated IC50 of EtOAc fraction was 12.7 ± 0.94 µg/mL while that of the crude extract was 60.6 ± 3.46 µg/mL compared to the positive control (Ascorbic acid IC50 = 10.6 ± 0.8 µg/mL) (Table 2).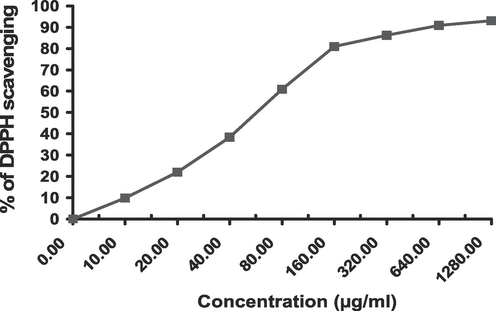
Dose-dependent DPPH free radicle scavenging of crude extract.
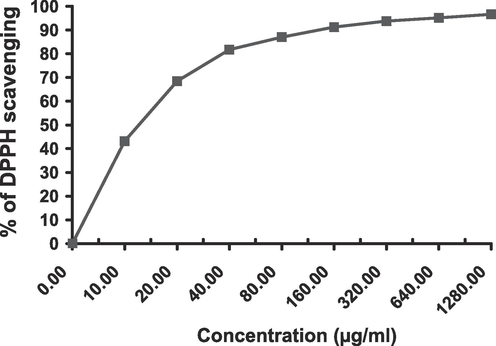
Dose-dependent DPPH free radicle scavenging of ethyl acetate fraction.
3.3 Metabolite profiling of C. fistula leaf methanol extract
The Phyto-metabolomics of C. fistula was scrutinized by the hyphenated analysis; LC-ESI-TOF-MS/MS (Figures S1- S4). C. fistula metabolites were tentatively recognized by comparing the m/z values of the precursor ions, MS2 fragmentation pattern, and retention times compared to those documented in the literature and mass spectrum data. Herein, 55 natural products were determined in extract, of which 50 compounds were phenolics. The dominant phenolic class was flavonoids (Table 3, Fig. 3).
Compound No.
Rt (min)
Proposed compound
Molecular formula
Precursor type
Obs. m/z for
Precursor
Calcd. m/z for
Precursor
Mass error
MS/MS
Ref.
Catechins and procyanidins
1.
4.645
Procyanidin B2
C30H26O12
[M−H]-
577.1353
577.1346
1.2129
577,451,425,407,289
(Kusumaningtyas, 2020; Elmongy et al., 2022)
2.
4.919
Procyanidin B1
C30H26O12
[M−H]-
577.1381
577.1346
6.0644
577,425,289
(Elmongy et al., 2022)
3.
5.478
Epicatechin
C15H14O6
[M−H]-
289.0703
289.0712
−3.1134
289,123,161,203,245
(Goufo et al., 2020; Abdelhameed et al., 2021)
Flavones, Flavonols and flavanones
4.
2.741
Eriodictyol-7-O-glucoside
C21H22O11
[M−H]-
449.1100
449.1084
3.5626
449,287,269,259
(Negm et al., 2022b)
5.
4.932
Luteolin-3′, 7-di-O-glucoside
C27H30O16
[M−H]-
609.1421
609.1456
−5.7458
609,563,447,285
(Abbas et al., 2022)
6.
5.071
Quercetin-3,4′-O-di-β-glucopyranoside
C27H30O17
[M−H]-
625.143
625.1405
3.9991
625,463,301
(Attallah et al., 2021)
7.
5.557
Kaempferol-7-neohesperidoside
C27H30O15
[M−H]-
593.1591
593.1506
14.3303
593,285
(Abdelhameed et al., 2021; Abbas et al., 2022)
8.
5.795
Kaempferol-3-O-robinoside-7-O-rhamnoside
C33H40O19
[M−H]-
739.2134
739.2086
6.4934
739,575,285,284,179
(Abd Ghafar et al., 2018; Thabit et al., 2018)
9.
5.821
Rutin
C27H30O16
[M + H]+
611.1655
611.1612
7.0358
611,465,303
(Attallah et al., 2021)
10.
6.713
Vitexin
C21H20O10
[M + H]+
433.1151
433.1135
3.6942
433,397,313,283,271
(Abbas et al., 2022)
11.
6.784
Taxifolin
C15H12O7
[M−H]-
303.0512
303.0505
2.3098
303,285,151
(Sun et al., 2007; Chen et al., 2020)
12.
6.9785
Quercitrin
C21H20O11
[M−H]-
447.0938
447.0927
2.4603
447,284,301
(Wang et al., 2013; Eltamany et al., 2022)
13.
7.408
Isorhamnetin-3-O-glucoside
C22H22O12
[M−H]-
477.1045
477.1033
2.5152
447,315,300
(Brito et al., 2014; Abbas et al., 2022)
14.
7.492
Naringenin-7-O-glucoside
C21H22O10
[M−H]-
433.1139
433.1135
0.9235
433,271,256
(Attallah et al., 2021; Elmongy et al., 2022; Negm et al., 2022b)
15.
7.617
Myricetin
C15H10O8
[M−H]-
317.03
317.0297
0.9463
137, 151,179, 289, 317
(Pereira et al., 2017)
16.
7.691
Diosmin
C28H32O15
[M + H]+
609.1779
609.1819
−6.5662
609,301,286
(Chou et al., 2021; Abbas et al., 2022)
17.
7.781
Kaempferol-3-O -rhamnoside
C21H20O10
[M−H]-
431.0995
431.0978
3.9434
431,285,255,151
(Abbas et al., 2022), Abo-Elghiet et al., 2022)
18.
8.369
Eriodictyol
C15H12O6
[M−H]-
287.0564
287.0556
2.7869
135,151,287
(Brito et al., 2014; Abdelhameed et al., 2021)
19.
9.007
Luteolin
C15H10O6
[M−H]-
285.0406
285.0399
2.4558
285,133
(Elhady et al., 2022; Goda et al., 2022)
20.
9.271
Quercetin
C15H10O7
[M−H]-
301.0354
301.0348
1.9931
301,283,151
(Brito et al., 2014; Chen et al., 2020)
21.
9.631
Isorhamnetin
C16H12O7
[M−H]-
315.0515
315.0505
3.1741
317,151,243,271,300
(Lin et al., 2013; Goda et al., 2022)
22.
9.733
Kaempferol
C15H10O6
[M + H]+
287.0572
287.0556
5.57
278, 269, 258,165
(Satheeshkumar et al., 2014; MassBank. 2023)
23.
10.418
Apigenin
C15H10O5
[M−H]-
269.0461
269.0450
4.0885
269,225,151,117
(Abdelhameed et al., 2021; Abbas et al., 2022)
24.
10.712
Diosmetin
C16H12O6
[M−H]-
299.0561
299.0556
1.6719
299
(Elmongy et al., 2022)
25.
10.771
Naringenin
C15H12O5
[M−H]-
271.06119
271.0606
2.1766
271,151
(Elmongy et al., 2022; Chen et al., 2020)
26.
12.609
Hesperetin
C16H14O6
[M + H]+
301.0876
303.0869
4.9822
153, 303
(Tong et al., 2012; MassBank. 2023)
27.
15.810
Acacetin
C16H12O5
[M−H]-
283.0605
283.0606
−0.3533
268, 283
(Chen et al., 2020)
Anthocyanins
28.
4.213
Cyanidin-3-O-glucoside
C21H21O11
[M−2H]-
447.0944
447.0922
4.9207
447,284
(Elhady et al., 2022; MassBank, 2023)
29.
5.620
Delphinidin 3-O-rutinoside
C27H31O16
[M−2H]-
609.1458
609.1450
1.3133
609
(Hegazy et al., 2022)
30.
7.804
Petunidin-3-O-β-glucopyranoside
C22H23O12
[M]+
479.1224
479.1184
8.3487
479,317,302,285
(Abdel Maboud, 2021; Negm et al., 2022a)
Chalcones
31.
3.681
Okanin-4′-O-glucoside
C21H22O11
[M−H]-
449.1064
449.1084
−4.4533
449, 431
(Abbas et al., 2022)
32.
7.630
Phlorizin
C21H24O10
[M−H]-
435.1293
435.1291
0.4596
435,433,389,273,167
(El-Hawary et al., 2021; Negm et al., 2022a)
33.
8.295
4-deoxy phloridzin
C21H24O9
[M−H]-
419.1357
419.1342
3.5788
419,257
(Tsugawa et al., 2019; Abdelhameed et al., 2021)
Anthraquinones
34.
2.152
Sennoside A
C42H38O20
[M−H]-
861.1897
861.1897
0.0000
341,681,699, 861
(Ye et al., 2007; Zibaee et al., 2023)
35.
7.781
Rhein
C15H8O6
[M−H]-
283.0245
283.0243
0.70
239,257, 283
(Ye et al., 2007; Fu et al., 2015)
36.
13.612
Emodin
C15H10O5
[M−H]-
269.0450
269.0450
–
225,241, 269
(MassBank, 2023,81,83,84]
37.
18.951
Chrysophanol
C15H10O4
[M−H]-
253.0515
253.0501
5.5325
225,253
(Ye et al., 2007; Fu et al., 2015)
Coumarins
38.
4.227
Daphnetin
C9H6O4
[M−H]-
177.0198
177.0188
5.6491
177,133
(Tong et al., 2012; Abbas et al., 2022)
39.
4.278
Esculin
C15H16O9
[M−H]-
339.0735
339.0716
5.6035
339,177
(Elhady et al., 2022)
40.
7.283
Scopoletin
C10H8O4
[M + H]+
193.0514
193.0501
6.7340
193,133
(Abbas et al., 2022)
Phenolic acids and their derivatives
41.
1.312
Gentisic acid
C7H6O4
[M−H]-
153.0187
153.0188
−0.6535
153,108
(Wang et al., 2013; Abdelhameed et al., 2021)
42.
1.874
p-Coumaric acid
C9H8O3
[M−H]-
163.0387
163.0395
−4.9068
163,119
(Chou et al., 2021)
43.
2.086
Ferulic acid
C10H10O4
[M−H]-
193.0510
193.0501
4.6620
193,178,134
(Zhong et al., 2020; Abdelhameed et al., 2021)
44.
2.205
Sinapic acid
C11H12O5
[M−H]-
223.0608
223.0606
0.8966
223,208,149
(Abbas et al., 2022)
45.
3.228
p-Salicylic acid
C7H6O3
[M−H]-
137.0247
137.0239
5.8384
137,93
(Abbas et al., 2022)
46.
6.424
5-Methoxysalicylic acid
C8H8O4
[M−H]-
167.0354
167.0344
5.9868
167,152
(Elmongy et al., 2022)
47.
9.941
Syringaldehyde
C9H10O4
[M−H]-
181.0494
181.0501
−3.8663
181
(Paganelli et al., 2020)
Stilbenoids
48.
5.807
Astringin
C20H22O9
[M−H]-
405.1196
405.1186
2.4684
405,243
(Elmongy et al., 2022); Attallah et al., 2021)
49.
9.109
Resveratrol
C14H12O3
[M−H]-
227.0728
227.0708
8.8078
227, 185
(Sun et al., 2007)
Miscellaneous
50.
5.449
Vitamin B2
C17H20N4O6
[M + H]+
377.1434
377.1461
−7.1590
377,243,198,172
(Abbas et al., 2022)
51.
3.004
Mandelic acid
C8H8O3
[M−H]-
151.0403
151.0395
5.2966
151,125
(Zhong et al., 2020)
52.
1.209
Quinic acid
C7H12O6
[M−H]-
191.0567
191.0556
5.7575
191,173,111,85
(Karar and Kuhnert 2015; Fan et al., 2017)
53.
1.338
Tagatose
C6H12O6
[M−H]-
179.057
179.0556
7.8188
179,89
(Abbas et al., 2022)
54.
14.982
Glycyrrhizate
C42H62O16
[M−H]-
821.3917
821.3960
−5.2350
821,775
(Elmongy et al., 2022)
55.
20.040
γ-Linolenic acid
C18H30O2
[M−H]-
277.2176
277.2168
2.8858
277,259,233,59
(Abbas et al., 2022)
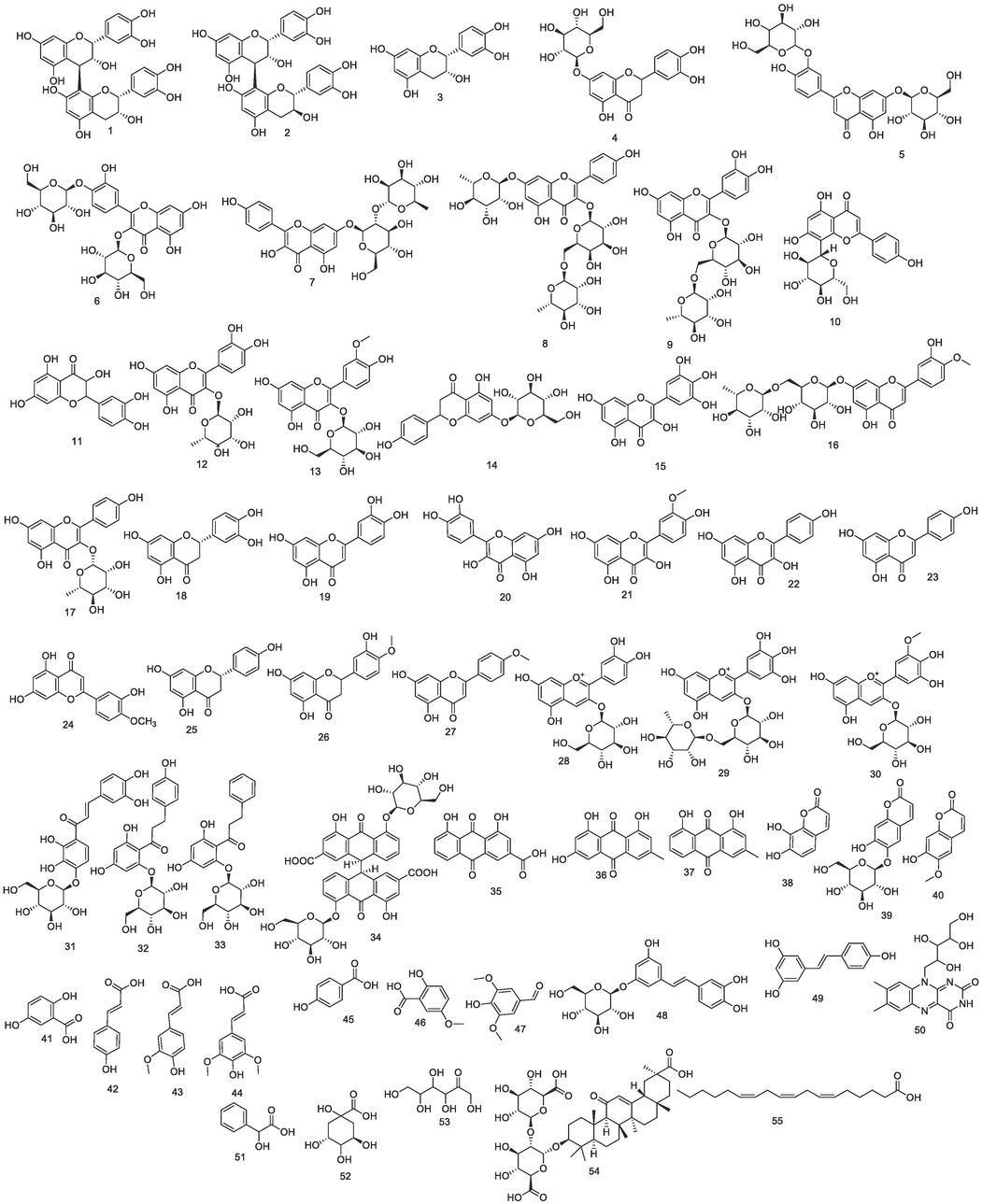
Chemical structures identified by LC-MS/MS.
This passage highlights the identification of various flavan-3-ols, flavones, flavonols, and flavanones in C. fistula. While epicatechin and procyanidin B2 were previously reported in C. fistula, procyanidin A2 and epiafzelechin were newly discovered, differing from the previously recorded procyanidin B1 (Tan et al., 2018; Kaur et al., 2020; Aabideen et al., 2021; Sharma et al., 2021; Kanwal et al., 2022; Omer et al., 2022). Eriodictyol-7-O-glucoside, kaempferol-3-O-robinoside-7-O-rhamnoside, vitexin, taxifolin, hesperetin, naringenin-7-O-glucoside, quercetin-3,4′-O-di-β-glucopyranoside, and diosmin were found in the Cassia genus for the first time. Additionally, kaempferol-3-O-rhamnoside (Costa Silva et al., 2019) and diosmetin (Alhawarri et al., 2023) were recognized for the first time in this specific plant species. Notably, eriodictyol-7-O-neohesperidoside (Thabit et al., 2018; Kanwal et al., 2022), previously identified in C. fistula, was not detected; instead, its aglycone was present.
Three anthocyanins were recognized; Cyanidin-3-O-glucoside and petunidin-3-O-β-glucopyranoside were recorded in C. fistula before (Omer et al., 2022) while delphinidin 3-O-rutinoside was identified in the plant for the first time. Three chalcones were observed of which phlorizin was reported previously in C. fistula (Omer et al., 2022) however the others; okanin-4′-O-glucoside and 4-deoxy phloridzin have not been reported before in the plant.
All the detected anthraquinones in the present study (rhein, emodin, chrysophanol, and sennoside A) were identified before in the plan. However, other anthraquinones such as sennoside B, physcion, chrysophanol 1-O-glucoside (chrysophanein) 1-O-methyl chrysophanol, and the glucosides of both emodin and rhein reported earlier in C. fistula were not found (Sharma, 2021; Aabideen et al., 2021; Sharma et al., 2021; Kanwal et al., 2022).
Esculin, daphnetin, and scopoletin are coumarin compounds recorded in our extract among which scopoletin was detected before in C. fistula (Sharma, 2021; Kanwal et al., 2022). However, esculin and daphnetin were detected for the first time. Nevertheless, several reported coumarins such as isoscopoletin, esculetin, 2,5‐dimethyl‐7 hydroxy chromone, and umbelliferone identified earlier in the plant (Kanwal et al., 2022;48] were not recognized.
Our extract is rich in phenolic acids and their derivatives. Among the detected phenolic acids, p-coumaric, ferulic (Kanwal et al., 2022; Omer et al., 2022), and sinapic acid (Kaur et al., 2020) were reported before in the plant (Tan et al., 2018; Kanwal et al., 2022). While gentisic, p-salicylic, and 5-Methoxysalicylic acids were recognized for the first time in C. fistula. An earlier study has reported the presence of syringic acid in the plant (Omer et al., 2022) however, it was not identified in the current study. Instead, we pick up its aldehyde derivative (syringaldehyde). On the other hand, other reported phenolic acids (Laxmi et al., 2015; Kanwal et al., 2022; Omer et al., 2022) such as gallic, ellagic, vanillic chlorogenic, and caffeic cinnamic acids were not recorded.
Astringin and resveratrol are naturally occurring stilbenes. Astringin was determined in the genus Cassia (family: Fabaceae). Meanwhile, resveratrol was found in C. fistula (Kusumaningtyas et al., 2020).
Besides the detected phenolics, other non-phenolic compounds were observed belonging to various phytochemical classes including vitamins (riboflavin), organic acids (mandelic and quinic acids), sugars (tagatose), fatty acids (γ-linolenic acid) and triterpenes (glycyrrhizate). All these compounds are identified for the first time in C. fistula except γ-linolenic acid (Kanwal et al., 2022). These findings highlight that the antioxidant activity exhibited by the extract is attributed to phenolics rather than other compounds since the former chemical class preponderates in C. fistula. Therefore, this justifies why the EtOAc displayed higher antioxidant activity compared to crude extract.
3.4 In vivo study
3.4.1 Rat body weight and colon weight-To-length ratio in AA-induced colitis
Acetic acid (AA) led to a notable decrease in rat body weight within the positive control group. Simultaneously, in the UC (ulcerative colitis) group, AA significantly increased the ratio of wet weight to length, a marker comparable to that of the negative control rats. When compared to the UC group, treatment with sulfasalazine demonstrated a significant decrease in the wet weight/length ratio and showed a notable increase in the percentage change in rat body weight, particularly when administered alongside prevention and treatment involving C. fistula at doses of 200 mg/kg/day and 400 mg/kg (Table 4). The disease activity index of cores of colitis is mentioned in Table 4.
Groups
% change in body weight
Colon weight (g)
weight/length ratio (g/cm)
Microscopic scores
I
19.45 ± 1.29
2.42 ± 0.063
0.13 ± 0.003
normal stool consistency (0)
II
-15.89 ± 2.65a
3.91 ± 0.14
0.21 ± 0.01 a
Water diarrhea (4)
III
31.16 ± 1.71b
3.46 ± 0.32
0.18 ± 0.02b
Loose stool (2)
IV
35.29 ± 2.00b
2.89 ± 0.14
0.15 ± 0.01b
Loose stools (3)
V
44.05 ± 2.58b
2.85 ± 0.16
0.15 ± 0.01b
(1)
VI
35.08 ± 2.53b
2.67 ± 0.09
0.14 ± 0.00b
(2)
VII
36.30 ± 0.60b,e
3.10 ± 0.12
0.16 ± 0.01b,d
(1)
Data presented as mean ± S.E (n = 5). Colon length = 19 cm. I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose (200 mg/kg), V; protection with a high dose (400 mg/kg), VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05). Macroscopic inflammation scores were assigned based on clinical features of the colon using an arbitrary scale ranging from 0 to 4 as follows: 0 (no macroscopic changes), 1 (mucosal erythema only), 2 (mild mucosal edema, slight bleeding or small erosions), 3 (moderate edema, slight bleeding ulcers or erosions), 4 (severe ulceration, edema, and tissue necrosis)
The phenolic bioactive chemicals, which are present in variable amounts in different extracts of C. fistula can operate either individually or synergistically to give rise to their antioxidant capabilities and pharmacological effects. Motivated by the aforementioned considerations, we have evaluated the therapeutic potential (as a prophylaxis and treatment) of C. fistula against acetic acid-induced UC in rats. In the current study, AA was administered intra-rectally causing a drastic reduction in the body weight of the rats. Weight loss in colitis is brought on by dietary deficiencies due to decreased appetite, food aversion or malabsorption, and rapid loss of bodily fluids owing to diarrhea and colon hemorrhage. These observations are in parallel with a study described by Owusu et al. (2020). The body weight is significantly affected by the standard drug and extract. The protection with the extract is more effective in the body weight than the treatment, especially when using a high dose.
3.4.2 Macroscopic alterations in AA-induced UC
After being longitudinally opened and sanitized with ordinary saline, colons were examined (Fig. 4A-4G). When the colons of the control group were inspected, it was found that the mucosa and serosa were both intact and there were no signs of tissue loss or bleeding (Fig. 4A). Investigation revealed considerable bleeding, extensive ulceration, and tissue edema over a sizable surface area in the acetic acid-induced group (Fig. 4B). Erosions and damage to the mucosal lining were seen (green arrows). Sulfasalazine and treated groups showed the best level of protection against mucosal injury and tissue necrosis, both at high and low dosages, with no obvious bleeding (Fig. 4C, 4E, and 4G). Pretreated groups did not have severe damage to the rat colons or tissue erosions, despite the slight bleeding (Fig. 4D).
Macroscopic imaging: A) Shows the colonic section of the control group (Group I), B) The colonic section of acetic-acid-induced colitis (Group II). C) The colonic section of the sulfasalazine-treated group (Group III). D) The colonic section of the pretreated group with a low dose (Group IV). E) The colonic section of the pretreated group with a high dose (Group V). F) The colonic section of the treated group with a low dose (Group VI). G) The colonic section of the treated group with a high dose (Group VII).
A considerable rise in the rats' colon weight/length ratio was seen in the AA group. This is due to goblet cell hyperplasia, necrosis, severe tissue edema, and inflammatory cell infiltration (El-Abhar et al., 2008). When rats were treated with methanol-leaf extract (MLE), fecal pellets free of obvious blood or mucus stains were observed. This could be a result of the mucus layer not being damaged and the prevention of severe blood loss, both of which are indications that prospective anti-ulcerative medications were successful in preventing the condition (Al-Rejaie et al., 2012; Owusu et al., 2020). It is well known that the mucus layer helps to speed up the healing of chemically caused epithelium injury and also reduces diarrhea and blood loss through feces (Awaad et al., 2017). Therefore, it should come as no surprise that MLE preservation of the mucus layer improved colonic ulceration. Our findings validate that administering MLE to rats before and after AA administration has a significant impact on UC. This is evident through the reduction in the macroscopic examination score, preservation of the intestinal mucosa, and a conspicuous decrease in inflammation of the colon tissue.
3.4.3 Hematological parameters and leukocytes
After acetic acid-induced colitis, the levels of RBCs, HGB, HCT, MCV, and MCH dramatically decreased as compared to the control group, However, the level of PLT significantly increased (Table 5). Sulfasalazine-treated rats had significantly reduced PLT counts and significantly increased RBCS, HGB, and HCT levels when compared to positive control animals. Rats prophylactically administered low and high dosages of crude extract had considerably more RBCs, HGB, HCT, and PLT than UC animals. RBC and HCT levels considerably rose in the low and high treatment groups in comparison to the UC group. The number of RBCs in the low-dosage treatment and MCH and MCHC in the low-dose preventative groups both increased significantly as compared to the sulfasalazine-treated group. Data presented as mean ± S.E (n = 5). RBCs; red blood cells, HGB; hemoglobin, HCT; hematocrit, MCV; mean corpuscular volume, MCH; mean corpuscular hemoglobin, MCHC; mean corpuscular hemoglobin concentration, and PLT; platelet. I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose (200 mg/kg), V; protection with a high dose (400 mg/kg), VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose of leaf crude extract, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05).
Groups
RBCs
HGB
HCT
MCV
MCH
MCHC
PLT
I
6.83 ± 0.18
13.85 ± 0.12
42.5 ± 0.41
94.5 ± 4.49
30 ± 1.63
32.5 ± 0.41
524.5 ± 26.54
II
5.33 ± 0.10a
11.6 ± 0.40 a
36 ± 1.32 a
61.33 ± 0.76 a
19.33 ± 0.58 a
31.67 ± 0.29
902.33 ± 26.57a
III
7.87 ± 0.38b
14.87 ± 0.35b
46.13 ± 1.91b
58.63 ± 1.48
18.93 ± 0.62
32.3 ± 0.58
709.67 ± 12.20b
IV
7.77 ± 0.18b
16.83 ± 0.62b
43.40 ± 1.75b
57.8 ± 1.21
22.27 ± 0.52b,e
38.43 ± 1.52b,e
691.67 ± 42.18b
V
7.23 ± 0.32b
14.73 ± 0.24b,c
43.63 ± 1.71b
59.9 ± 0.68
20.3 ± 0.68
33.83 ± 0.87
612 ± 36.69b
VI
6.67 ± 0.18b,e
12.93 ± 0.55b
39.37 ± 1.16b
57.93 ± 0.95
19.03 ± 0.52
32.87 ± 1.21
674 ± 36.69b
VII
7.2 ± 0.35b
12.17 ± 1.35
45 ± 0.58b,d
63.47 ± 2.59
19.47 ± 1.3
32.57 ± 1.87
819.33 ± 61.33
In AA-induced colitis, the WBC count significantly increased while the eosinophil level significantly dropped in comparison to the control group (Table 6). Both the sulfasalazine-treated group and the ones treated with low and high dosages of extract showed a significant decrease in neutrophils and monocytes, and a significant rise in lymphocytes and eosinophils. The number of WBCs and neutrophils significantly dropped in a low dose (200 mg/kg) prophylactic group of C. fistula. The number of RBCs in the low-dosage treatment group and MCH and MCHC in the low-dose preventative group both significantly increased in contrast to the sulfasalazine-treated group.
Groups
WBCs
Band/Staff
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
I
6.35 ± 0.12
1.5 ± 0.41
61.5 ± 2.04
30.5 ± 2.04
5.50 ± 0.41
3.50 ± 0.41
–
II
18.85 ± 1.67a
2.33 ± 0.29
60.33 ± 2.93
30.33 ± 1.53
6.33 ± 0.76
2.00 ± 0.00a
–
III
13.7 ± 0.86
1.33 ± 0.33
5.67 ± 0.33b
75.33 ± 0.88b
3.33 ± 0.33b
14.67 ± 1.20b
–
IV
11.3 ± 1.66b
1.67 ± 0.33
9.67 ± 1.76b
35.33 ± 2.67e
4.67 ± 0.67
3.33 ± 0.33b,e
–
V
14.3 ± 1.46
2.00 ± 0.58
14.00 ± 2.08b,e
77.33 ± 2.19b,c
5.67 ± 1.20
5.33 ± 1.45e
–
VI
15.4 ± 1.91
0.33 ± 0.33b
5.00 ± 0.58b
75.67 ± 0.88b
3.33 ± 0.33b
16.33 ± 1.45b
–
VII
20.7 ± 1.75e
0.67 ± 0.33b
5.00 ± 0.58b
73.67 ± 1.76b
4.67 ± 0.33d,e
17.67 ± 1.45b
–
The WBC count significantly increased in acetic acid-induced colitis (Table 6). In the sulfasalazine-treated group, as well as in the low and high doses of C. fistula-treated groups, neutrophils, and monocytes were dramatically decreased, whereas lymphocytes and eosinophils were significantly increased. When the extract was administered prophylactically at 200 mg/kg, the number of WBCs and neutrophils drastically decreased.
Data presented as mean ± S.E (n = 5). WBCs are white blood cells. I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose, V; protection with a high dose, VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05).
In current results, different outcomes were found for MLE therapy or protection on the hematological parameters. Reduced RBC characteristics have been associated with anemia brought by the AA treatment as previously described (Alia et al., 2019). The WBCs elevated in the AA group as compared to control animals. According to a report, an increase or reduction in granulocyte cells corresponds to an increase or decrease in inflammatory activity (Alia et al., 2019). Sulfasalazine did not cause any significant change in differential leukocyte parameters that were assessed, these results are in agreement with Owusu et al. (2020).
3.4.4 Effect of C. fistula on liver functions in AA-induced colitis
All liver functions in the acetic acid-induced group showed significant changes in which ALT, AST, total bilirubin, and ALP increased significantly while total protein decreased significantly as compared to control animals (Table 7). ALT, AST, total bilirubin, and ALP were markedly decreased in the sulfasalazine-treated group when compared to the UC group. ALT and AST activities significantly decreased in low and high-dose preventative groups and high-dose treatment animals compared to the sulfasalazine-treated and UC rats. Data presented as mean ± S.E (n = 5). ALT; alanine aminotransferase, AST; aspartate aminotransferase, and ALP; Alkaline phosphatase I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose, V; protection with a high dose, VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05).
Groups
ALT (U/L)
AST (U/L)
Total Bilirubin (mg/dl)
ALP (U/L)
Total protein (g/dl)
I
29.5 ± 0.87
54.33 ± 2.85
0.62 ± 0.02
556 ± 3.06
9.1 ± 0.31
II
64 ± 3.61a
135.67 ± 0.88a
0.8 ± 0.06a
2011.67 ± 65.90a
6.03 ± 0.15a
III
45 ± 0.58b
100.6 ± 1.55b
0.3 ± 0.06b
199.63 ± 6.12b
7.07 ± 0.55
IV
24.67 ± 2.96b,e
82.33 ± 2.19b,e
1.0 ± 0.06e
200.33 ± 15.94b
7.1 ± 0.26b
V
30 ± 1.53b,e
73.67 ± 2.19b,c,e
0.97 ± 0.15e
412.73 ± 3.82b,c,e
9.3 ± 0.6b,c
VI
51.33 ± 3.48
93.6 ± 5.98b
0.37 ± 0.03b
314.47 ± 18.01b,e
5.8 ± 0.47
VII
51.43 ± 1.29b,e
39.07 ± 2.76b,d,e
0.37 ± 0.03b
412.73 ± 11.11b,d,e
7.77 ± 0.49b,d
The ALP activity decreased significantly in all prevention and treatment groups as compared to the UC rats (Table 7). The total protein increased by 1.17-, 1.54-, and 1.28-fold in low and high prevention groups as well as the high treated group as compared to UC animals, respectively (Table 7).
The administration of MLE considerably reduced the rise of ALP in groups treated for colitis; this effect may have been caused by the extract's possible anti-inflammatory properties. The outcomes concur with those of other authors (Sarkar et al., 2015; Hasona and Hussien, 2017) who reported a notable increase in ALP activity in the serum of rats with AA-induced colitis.
3.4.5 Effect of C. fistula on antioxidant and oxidative stress in aa-induced colitis
Examination of all rats' colons revealed the presence of MDA, a marker for LPO. Comparing the UC (ulcerative colitis) group to the control group, a significant elevation in colon MDA levels was observed (Table 8). This is indicated by tissue damage (Vishwakarma et al., 2015). However, rats receiving a high dose for prevention or treatment exhibited a noteworthy decrease in colon MDA levels compared to the UC group. Additionally, the UC group displayed significantly reduced activity of SOD and CAT compared to the negative control group. Conversely, the activity of GPx in UC rats was notably increased compared to the control animals. Furthermore, levels of GSH and TAC declined significantly in the UC group compared to the negative control rats. Data presented as mean ± S.E (n = 5). MDA; malondialdehyde, SOD; superoxide dismutase, CAT; catalase, GPx; glutathione peroxidase, GSH; reduced glutathione, TAC; total antioxidant capacity. I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose, V; protection with a high dose, VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05).
Groups
MDA
SOD
CAT
GPx
GSH
TAC
I
503.33 ± 1.76
941.67 ± 4.17
13.87 ± 0.49
18.48 ± 0.72
42.33 ± 3.33
0.64 ± 0.03
II
857 ± 11.53a
870.17 ± 17.63 a
4.87 ± 0.05 a
52.42 ± 2.97 a
12.33 ± 0.88 a
0.43 ± 0.03 a
III
870 ± 3.51
3249.99 ± 1.16b
14.03 ± 1.16b
18.74 ± 0.43b
37.00 ± 1.15b
0.47 ± 0.03
IV
955.67 ± 24.18b,e
924.33 ± 7.25b,e
14.82 ± 1.38b
131.78 ± 4.39b,e
16.67 ± 1.2b,e
0.37 ± 0.09
V
577.67 ± 8.09b,c,e
941.67 ± 4.17b,e
12.15 ± 0.47b
94.81 ± 2.45b,c,e
16.33 ± 1.76 e
0.17 ± 0.03b,e
VI
1015 ± 1.73b,e
1895.83 ± 41.67b,e
15.86 ± 1.08b
18.91 ± 0.31b
16.33 ± 0.33b,e
0.47 ± 0.03
VII
562.67 ± 11.89b,d,e
3770.83 ± 3.19b,d,e
8.77 ± 0.41b,d,e
55.29 ± 3.19 d,e
27.00 ± 0.58b,d,e
0.33 ± 0.07
Treatment and protection of rats with a high dose (400 mg/kg/day) produced a marked significant decrease in LPO concentration that reached the control value (562.67 ± 11.89 μmol/g and 577.67 ± 8.09, respectively). Interestingly, the activity of SOD and CAT along with the content of GSH showed significant restoration in the groups treated with sulfasalazine, both in the pretreatment and treated groups, compared to the UC (ulcerative colitis) animals (Table 8).
The existence of enzymatic and non-enzymatic antioxidant systems to defend tissues from pro-oxidants is well recognized (Thomas, 1995). GSH is non-enzymatic, whereas SOD, CAT, and GPx are enzymatic endogenous antioxidants (Flora et al., 2012). Another significant conclusion of the current study was that AA therapy causes a drop in the tissue levels of SOD, CAT, and GPx. These results are consistent with the previous study that showed AA-induced colitis to have impaired antioxidant mechanisms and enhanced LPO (Nieto et al., 2000). The TAC level was decreased in AA, while the protection with a high dose has increased significantly, this is consistent with the results of the previous studies (Ermis et al., 2013; Cagin et al., 2016). The results of the current investigation showed that administering the extract orally to rats with AA-induced colitis might lessen the severity of the colonic mucosal injury, stop the rise in MDA levels, and replenish depleted levels of antioxidant enzyme activities. Therefore, the extract is more efficient when given after colitis has been induced in a dose-dependent manner. The findings revealed that extract significantly and dose-dependently reduced both carrageenan-induced hind paw edema and cotton-pellet granuloma (Gobianand et al., 2010).
Numerous plant species, including, Moringa oleifera (Minaiyan et al., 2014), Coriandrum sativum (Heidari et al., 2016), and Helichrysum oligocephalum (Popov et al., 2006), have had protective effects on colonic tissues against UC examined. However, there aren’t many publications examining the impact of C. fistula. The pathological analysis supported the biochemical changes. The detected histological alternation, such as clogged blood arteries, leukocytic infiltration, and various degrees of cell degeneration, were consistent with prior studies of UC by other authors (Tanide et al., 2014; 2016).
The present investigation is in parallel with the study of Zabihi et al. (2024) that revealed the extract from the C. fistula plant has beneficial effects on the healing process of ulcerative colitis, demonstrating a reduction in colitis symptoms. Particularly, the composition of the aqueous extract proved effective in diminishing the activity of the myeloperoxidase (MPO) enzyme when compared to the control group. These findings suggest a potential therapeutic role for C. fistula in managing ulcerative colitis by mitigating inflammation and influencing specific enzyme activity involved in the condition as well as controlling the oxidative/antioxidant balance. Zabihi et al. (2024) used high doses with short time; 600 and 800 mg/kg once daily for 5 days.
3.4.6 Histological evaluation
Colonic mucosa from the control group was shown by H&E-stained sections to be made up of densely aggregated simple tubular glands (Lieberkuhn crypts), which extended down to the muscularis mucosa and were embedded in the lamina propria. The crypts' lining was made up mostly of numerous goblet cells and surface epithelial columnar absorptive cells with an apical brush border and basal oval nuclei. Basal nuclei and vacuolated cytoplasm were visible in goblet cells. A small number of intraepithelial lymphocytes were also visible. Undifferentiated cells with basal oval vesicular nuclei were present in the basal regions of the crypts (Fig. 5A). In the positive control group, staining revealed focal areas of severe mucosal deformation, exfoliation, and ulceration with loss of the whole crypt lining in the acetic acid-induced UC group. Some crypts displayed dilatation along with a notable reduction in goblet cells or their disappearance. Transmural necrosis, edoema, and diffuse inflammatory cell infiltration in the mucosa were histological features of untreated rats. Desquamated regions, loss of the epithelium, and focal ulceration of the colonic mucosa that penetrated the muscularis mucosa were present. Hyperplasia of the crypt cells and shorter crypt cell lengths are signs of malabsorption. Tubular adenomas are present along with hyperplastic colorectal polyps (hyperplastic polyposis) in (Fig. 5B).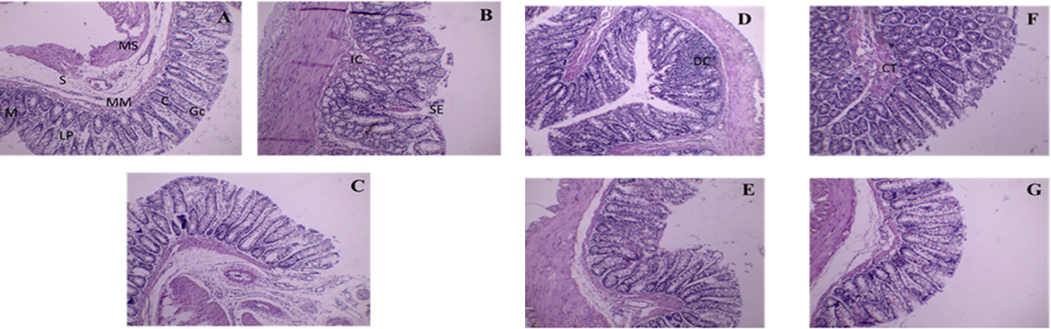
(A-G) Light micrographs of colonic mucosa. A) Control rats showing, normal architecture, and arrangement of the lining layers; mucosa (M), muscularis mucosa (MM), submucosa (S), and musculosa (MS). Apical parts of the mucosa; absorptive crypt cells I, numerous Goblet cells (GC), and obvious lamina propria (LPB). B) colonic mucosa was observed 17 days after induction of colitis and oral treatment with saline, with some generation in the acetic acid group. Showing, IC: invasion of some inflammatory cells, separation of the epithelium (SE), and damaging crypts (DC). C) Colonic mucosa 17 days after induction of colitis and oral treatment with sulfasalazine. Showing, maintained the regular arrangement of cell structure, epithelium remained intact and Goblet cells and crypts were both normally distributed. D) Colonic mucosa observed 17 days after pretreatment with a low dose extract to induce colitis; showing alteration in mucosal, submucosal, and musculosal layers, with damaged crypts. E) colonic mucosa observed 17 days after pretreatment with a high dose to induced colitis: minimal inflammatory reaction in the mucosa and submucosa without any feature of tissue destruction. Note also, that Crypts had a normal architecture with intact vacuolated goblet cells. F) colonic mucosa observed 17 days after induction of colitis and oral treatment with a low dose; more connective tissue cells (CT) and increased number of crypts. G) Colonic mucosa 17 days after induction of colitis and oral treatment with a high dose; the treatment ameliorates.
The degree and severity of the histological signs of cell damage were considerably decreased in the sulfasalazine-treated group. The lamina propria had a few mildly inflammatory cells, but the epithelium was undamaged. However, sulfasalazine reduced microscopic harm and preserved the normal alignment of cell structure. Lieberkühn's goblet cells and crypts were both discernible and well-spaced. Reduced leukocytosis was seen (Fig. 5C). The prophylactic low dose (200 mg/kg/day) group's histological characteristics included necrosis of a few crypts and a moderate inflammatory response in the mucosa and submucosa (Fig. 5D). Fig. 5E showed pseudo-Peyer's patches without any discernible polyps. The mucosa deteriorated and infiltrated with fewer inflammatory cells in the preventative group, which received a high dose (400 mg/kg/day), than in the protection group which received a low dose. With the growth of multiple goblet cells, crypt cells grew larger. Polyps cannot be seen. little inflammation without any signs of tissue damage in the mucosa and submucosa. Additionally, crypts displayed a typical architectural design with complete vacuolated goblet cells.
The treatment groups showed desquamated regions and epithelium loss after receiving a lower dose (200 mg/kg/day) of C. fistula. With many increases in the quantity and total degeneration of goblet cells, the crypt architecture was also altered. Inflammatory cells had invaded the lamina propia with no evidence of polyps (Fig. 5F). However, higher-dose therapy (400 mg/kg/day) improved microscopic damage and preserved the regular organization of cell structure. Lieberkühn's goblet cells and crypts were both distinct and evenly spaced. Infiltration and necrosis were diminished (Fig. 5G).
Rats given a high dose of MLE showed normal epithelium on their surface and only a small amount of inflammatory cells were infused. Animals administered the extract as treatment were protected against the AA's effects of inflammation, congestion, ulceration, erosion, necrosis, and hyperplasia. The complete colonic mucosa's cytoarchitecture was also preserved. This demonstrates the extract's capacity to safeguard the animals and slow the spread of the illness. The treated animals had better healing than the protective male rats.
3.4.7 Immunohistochemically evaluation
As seen in the figures, several cells in the negative control group had a mildly positive cytoplasmic COX2 immuno-histochemical reaction, which was manifested as a brownish color (Fig. 6A). However, sections taken from group II showed many cells with a high positive cytoplasmic COX2 reaction (Fig. 6B). The section from group III, on the other hand, had several cells that had a positive cytoplasmic COX2 reaction (Fig. 6C). Sections from groups IV and VI, however, revealed a few cells with a slightly positive COX2 reaction in the cytoplasm (Fig. 6D and 6E). Sections from groups V and VII displayed a COX2 reaction that was almost normal (Fig. 6F and 6G). Meanwhile, immuno-stained sections from the control group showed a few cells with a weakly positive cytoplasmic caspase-3 immuno-histochemical reaction, shown as a brownish coloring (Fig. 7A). However, the group II section showed several cells with a markedly positive caspase-3 reaction (Fig. 7B). Additionally, a few cells in the group III section had a weakly positive caspase-3 reaction (Fig. 7C). A mildly positive caspase-3 reaction was seen in several cells in sections from groups IV and VI (Fig. 7D and 7E). Sections from groups V and VII, on the other hand, displayed a nearly typical caspase-3 response (Fig. 7F and 7G).![COX-2 immunostaining. A) Shows control COX-2 expression (Group I), brown color indicates specific immunostaining of cleaved COX-2 and light blue color indicates nuclear hematoxylin staining. B) The colonic section of acetic-acid-induced colitis (Group II) depicts numerous cells with a strong positive mainly cytoplasmic COX-2 reaction. C) The colonic section of the sulfasalazine-treated group (Group III) shows a near-control COX-2 expression. D) The colonic section of the pretreated group with a low dose (Group IV); shows multiple cells with a moderately positive cytoplasmic COX-2. E) The colonic section of the pretreated group with a high dose (Group V); reaction reveals few cells with a weak positive cytoplasmic COX-2 reaction. F) The colonic section of the treated group with a low dose (Group VI); shows multiple cells with a moderately positive cytoplasmic COX-2. G) The colonic section of the treated group with a high dose (Group VII); shows a near-control COX-2 expression. [Original magnification: 40x].](/content/184/2024/17/4/img/10.1016_j.arabjc.2024.105672-fig6.png)
COX-2 immunostaining. A) Shows control COX-2 expression (Group I), brown color indicates specific immunostaining of cleaved COX-2 and light blue color indicates nuclear hematoxylin staining. B) The colonic section of acetic-acid-induced colitis (Group II) depicts numerous cells with a strong positive mainly cytoplasmic COX-2 reaction. C) The colonic section of the sulfasalazine-treated group (Group III) shows a near-control COX-2 expression. D) The colonic section of the pretreated group with a low dose (Group IV); shows multiple cells with a moderately positive cytoplasmic COX-2. E) The colonic section of the pretreated group with a high dose (Group V); reaction reveals few cells with a weak positive cytoplasmic COX-2 reaction. F) The colonic section of the treated group with a low dose (Group VI); shows multiple cells with a moderately positive cytoplasmic COX-2. G) The colonic section of the treated group with a high dose (Group VII); shows a near-control COX-2 expression. [Original magnification: 40x].
![Caspase-3 immunostaining. A) Shows control caspase-3 expression (Group I), brown color indicates specific immunostaining of cleaved caspase-3 and light blue color indicates nuclear hematoxylin staining. B) The colonic section of acetic-acid-induced colitis (Group II) depicts numerous cells with a strong positive mainly nuclear caspase-3 reaction. C) The colonic section of the sulfasalazine-treated group (Group III) shows a near-control caspase-3 expression. D) The colonic section of the pretreated group with a low dose (Group IV); shows multiple cells with a moderately positive cytoplasmic caspase-3. E) The colonic section of the pretreated group with a high dose (Group V); reaction reveals few cells with a weak positive cytoplasmic caspase-3 reaction. F) The colonic section of the treated group with a low dose (Group VI); shows multiple cells with a moderately positive cytoplasmic caspase-3. G) The colonic section of the treated group with a high dose (Group VII); shows a near-control caspase-3 expression.[Original magnification: 40x].](/content/184/2024/17/4/img/10.1016_j.arabjc.2024.105672-fig7.png)
Caspase-3 immunostaining. A) Shows control caspase-3 expression (Group I), brown color indicates specific immunostaining of cleaved caspase-3 and light blue color indicates nuclear hematoxylin staining. B) The colonic section of acetic-acid-induced colitis (Group II) depicts numerous cells with a strong positive mainly nuclear caspase-3 reaction. C) The colonic section of the sulfasalazine-treated group (Group III) shows a near-control caspase-3 expression. D) The colonic section of the pretreated group with a low dose (Group IV); shows multiple cells with a moderately positive cytoplasmic caspase-3. E) The colonic section of the pretreated group with a high dose (Group V); reaction reveals few cells with a weak positive cytoplasmic caspase-3 reaction. F) The colonic section of the treated group with a low dose (Group VI); shows multiple cells with a moderately positive cytoplasmic caspase-3. G) The colonic section of the treated group with a high dose (Group VII); shows a near-control caspase-3 expression.[Original magnification: 40x].
Image J software (version 1.53 h) was used for morphometric analysis to semi-quantify COX-2 and Caspase −3 reactivity in the analyzed tissues. Following Fig. 8, when compared to the control group, group II showed a highly significant rise in the mean percentage area of positive COX-2 reactivity. In contrast to the AA-induced colitis, sulfasalazine, and low C. fistula-treated groups, group VII showed a considerable decline. When compared to the AA-induced group, a substantial reduction was also seen in groups III, IV, V, and VI. On the other hand, group II showed a highly significant increase in the mean percentage of positive caspase-3 reaction when compared to the control group. Group VII among the II, III, and VI groups showed a notable improvement in the mean percentage area. Comparing Groups III, IV, and V to the AA-induced UC group, there was a noticeable decline. Group VI and the AA-induced UC rats, however, showed no statistically significant difference (Fig. 9).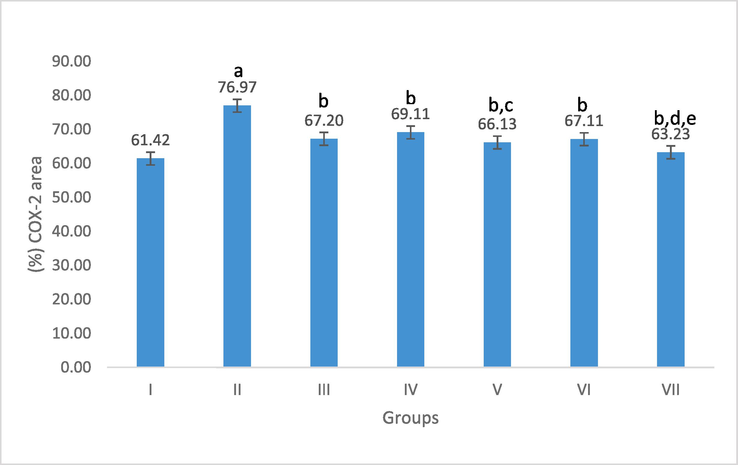
Effect of C. fistula on COX-2 positively stained area percentage in colon rats. Data presented as mean ± S.E (n = 5). I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose, V; protection with a high dose, VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05).
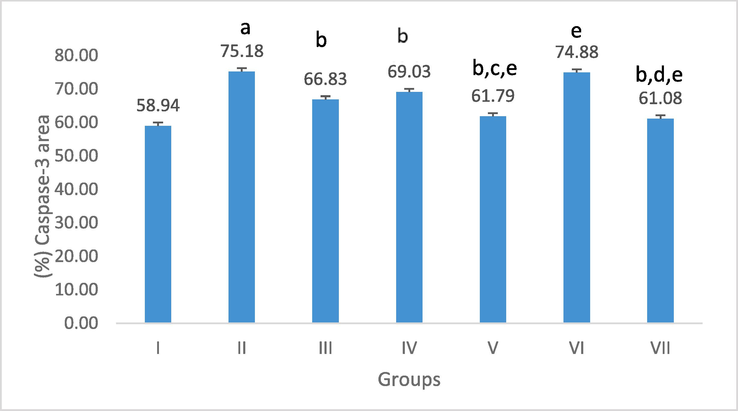
Effect of C. fistula on caspase-3 positively stained area percentage in colon rats. Data presented as mean ± S.E (n = 5). I; control, II; acetic acid, III; treated with sulfasalazine drug, IV; protection with a low dose, V; protection with a high dose, VI; treatment with a low dose and VII; treatment with a high dose. a significant difference as compared to control, b Significant difference as compared to acetic acid, c significant difference as compared to protection with a low dose, d significant difference as compared to treatment with a low dose, and e significant difference as compared to treatment with sulfasalazine drug (p ≤ 0.05).
The colonic caspase-3 and COX-2 activity of the AA group was higher than that of the control group concerning the inflammatory response. A highly substantial rise in group AA when compared to the control group was revealed by morphometrical analysis of the mean percentage of positive caspase-3 and COX-2 reaction, which confirmed this. Improvement in the mean percentage area was remarkable in the treated group with a high dose of MLE amongst the AA, sulfasalazine-treated, and treated group with a low dose.
3.5 Molecular docking
Using molecular docking simulation, the chemicals found by LC/MS/MS in this investigation were tested against the COX-2 and caspase-3 proteins. As can be observed in Tables 9 and 10, the majority of the compounds under investigation demonstrated strong binding affinities to the two proteins, with binding energies of −3.4 to −15.26 Kcal/mol and −5.8 to −16.25 Kcal/mol, respectively, towards the COX-2 and caspase-3 proteins. In addition, these compounds had noticeable molecular interactions with the key amino acids; His 121 and Arg 207 inside caspase-3, as well as Tyr 355 and Arg 120 inside COX-2 protein. Isorhamnetin exhibited the highest affinity, indicated by its low docking score value (-19.4Kcal/mol) and the best ligand-receptor interaction to caspase-3 proteins (1 Arene-arene with His 121 and 2 HB; one with His 121 and the other one with Arg 207)
Compound
Binding energy (Kcal/mol)
Caspase-3
PDB = 6 CKZ
COX-2
PDB = 5 W58
Procyanidin B2
−16.25
−15.26
Procyanidin B1
−9.6
–
Epicatechin
−10.36
−13.2
Eriodictyol-7-O-glucoside
−11.2
–
Luteolin-3′, 7-di-O-glucoside
−16.8
−18.6
Quercetin-3,4′-O-di-β-glucopyranoside
−9.5
−10.3
Kaempferol-7-neohesperidoside
−10.3
−9.8
Kaempferol-3-O-robinoside-7-O-rhamnoside
−12.3
−8.9
Rutin
−11.36
−9.8
Vitexin
−7.36
−10.36
Taxifolin
−8.59
−8.9
Quercitrin
−9.58
−8.5
Hesperetin
−16.8
−9.5
Isorhamnetin-3-O-glucoside
−6.8
–
Naringenin-7-O-glucoside
−9.68
−8.9
Myricetin
−5.8
−12.3
Quercetin
−11.2
−8.9
Diosmin
−8.68
−11.2
Kaempferol-3-O-α-rhamnoside
−6.58
−13.2
Eriodictyol
−8.5
−10.2
Luteolin
−8.6
−13.2
Isorhamnetin
−19.4
−11.2
Apigenin
−5.26
−10.2
Diosmetin
−9.2
−11.1
Naringenin
−11.2
−10.1
Kaempferol
−16.2
−8.5
Acacetin
−10.2
−10.2
Cyanidin-3-O-galactoside
−16.9
−15.8
Delphinidin 3-O-rutinoside
–
−9.8
Petunidin-3-O-β-glucopyranoside
−13.1
−9.2
Okanin-4′-O-glucoside
−16.2
−15.6
Phlorizin
−13.2
−11.0
4-deoxy phloridzin
−13.6
−8.9
Sennoside A
–
–
Rhein
–
−11.2
Emodin
–
−13.2
Chrysophanol
–
−13.1
Esculin
−11.3
−5.6
Daphnetin
−10.3
−12.3
Scopoletin
−10.3
−12.1
Gentisic acid
–
−6.3
p-Coumaric acid
−11.1
−4.5
Ferulic acid
−13.6
−5.6
Sinapic acid
−8.6
−5.60
p-Salicylic acid
–
–
5-Methoxysalicylic acid
–
–
Syringaldehyde
–
−5.7
Astringin
−12.3
−6.48
Resveratrol
−9.68
−3.4
Vitamin B2
−9.8
−4.5
Mandelic acid
–
–
Quinic acid
–
–
Tagatose
–
–
Glycyrrhizate
−10.3
–
γ-Linolenic acid
–
–
Caspase-3
PDB = 6 CKZCOX-2
PDB = 5 W58
Co-crystallized ligand
2H-bond with His 121 Lipophilic interactions Arg 207
2H-bonds with Tyr 355 and Arg 120
Procyanidin B2
1HB with Arg 207
1HB with His 121
1 Arene-cation His 1211 HB with Ile 12
1 HB with Asp 15
1 Arene-cation His 13
Procyanidin B1
1HB with Arg 207
1 Arene-cation His 13
Epicatechin
1HB with Arg 207
1HB with His 1212H-bonds with Tyr 355 and Arg 120
Eriodictyol-7-O-glucoside
2HB with Arg 207
1 Arene-cation His 13
Luteolin-3′, 7-di-O-glucoside
1 Arene-cation His 121
1 HB with Asp 15
1 Arene-cation His 13
Quercetin-3,4′-O-di-β-glucopyranoside
1 Arene-cation His 121
1HB with Arg 2071 HB with Ile 12
1 Arene-cation His 13
Kaempferol-7-neohesperidoside
1 Arene-cation His 121
1HB with His 1211 HB with Ile 12
1 Arene-cation His 13
Kaempferol-3-O-robinoside-7-O-rhamnoside
1 Arene-cation His 121
1HB with His 1211 HB with Asp 15
1 Arene-cation His 13
Rutin
1HB with His 121
1 Arene-cation His 1211 HB with Ile 12
1 HB with Asp 15
Vitexin
1 HB with His 121
I HB with Arg 2072H-bonds with Tyr 355 and Tyr 385
Taxifolin
1HB with Arg 207
1HB with His 1211H-bond with Tyr 355 and Tyr 385
Quercitrin
1HB with Arg 207
1HB with His 1211 HB with Ile 12
1 HB with Asp 15
Hesperetin
1 HB with Arg 64
1 HB with Arg 207
1 Arene-cation Arg 2071 HB with Ile 12
1 HB with Asp 15
Isorhamnetin-3-O-glucoside
1HB with Arg 207
1 Arene-cation His 13
Naringenin-7-O-glucoside
1HB with Arg 207
1HB with His 1211 HB with Ile 12
1 HB with Asp 15
Myricetin
1HB with Arg 207
2H-bonds with Tyr 355
Quercetin
1HB with Arg 207
1HB with His 1211 HB with Ile 12
1 HB with Asp 15
Diosmin
1 Arene-cation His 121
1HB with Arg 2072 HB with Ile 12
Kaempferol-3-O-α-rhamnoside
1HB with Arg 207
1HB with His 1211 HB with Ile 12
1 HB with Asp 15
Eriodictyol
1HB with Arg 207
2 HB with Asp 15
Luteolin
1HB with His 121
2H-bonds with Tyr 355 and Arg 120
Isorhamnetin
1 HB with His 121
1 HB with Arg 207
1 Arene-arene His 1211 HB with Ile 12
1 HB with Asp 15
Apigenin
1 HB with His 121
2H-bonds with Tyr 355 and Arg 120
Diosmetin
1 HB with Arg 207
1 HB with Ile 12
1 HB with Asp 15
Naringenin
1 HB with His 121
1 Arene-arene His 1212 HB with Ile 12
Kaempferol
1 HB with Arg 207
1 Arene-arene Arg 2071 HB with Ile 12
1 HB with Asp 15
Acacetin
2 HB with Arg 207
2 HB with Asp 15
Cyanidin-3-O-galactoside
1 Arene-cation His 121
1HB with Arg 2071 HB with Ile 12
1 Arene-cation His 13
Delphinidin 3-O-rutinoside
1 Arene-arene His 121
1 HB with Ile 12
1 Arene-cation His 13
Petunidin-3-O-β-glucopyranoside
1 HB with His 121
1 Arene-arene Arg 2071 Arene-cation His 13
1 HB with Asp 15
Okanin-4′-O-glucoside
1 HB with His 121
1 Arene-arene His 1211 Arene-cation His 13
1 HB with Ile 12
1 HB with Asp 15
Phlorizin
2 HB with Arg 207
1 Arene-cation His 13
1 HB with Ile 12
4-deoxy phloridzin
2 HB with His 121
1 Arene-cation His 13
1 HB with Asp 15
Sennoside A
–
1 Arene-cation His 13
Rhein
–
2H-bonds with Tyr 355, Ser 530
Emodin
–
2 HB with Ile 12
1 HB with Asp 15
Chrysophanol
–
1 HB with Ile 12
1 HB with Asp 15
Esculin
1 HB with His 121
1 HB with Arg 2071 Arene-cation His 13
1 HB with Ile 12
Daphnetin
1 HB with His 121
2 HB with Ile 12
1 HB with Asp 15
Scopoletin
1 HB with Arg 207
1 HB with Ile 12
1 HB with Asp 15
Gentisic acid
–
1 HB with Ile 12
p-Coumaric acid
1 HB with His 121
1 HB with Ile 12
Ferulic acid
1 Arene-arene His 121
1 HB with His 1211 HB with Ile 12
1 HB with Tyr 355
Sinapic acid
1 HB with His 121
1 HB with Ile 12
1 HB with Arg 120
p-Salicylic acid
–
–
5-Methoxysalicylic acid
–
–
Syringaldehyde
–
1 HB with Arg 120
Astringin
1 HB with His 121
1 HB with His 1211 HB with Ile 12
1 HB with Asp 15
Resveratrol
1 HB with His 121
1 HB with Arg 120
Vitamin B2
1 HB with His 121
1 HB with Arg 2071H-bonds with Tyr 355
Mandelic acid
–
–
Quinic acid
–
–
Tagatose
–
–
Glycyrrhizate
1 HB with Arg 207
–
γ-Linolenic acid
–
–
On the other hand, emodin displayed the best interaction with COX-2. Since its docking score value (-13.2Kcal/mol) and the best ligand-receptor interaction to COX-2 protein (it formed 3 HB; 2 HB with Ile 12 and 1HB with Asp 15)
Procyanidin B2, one of the top interacting compounds, demonstrated significance towards both proteins. It exhibited a docking score value of −16.25Kcal/mol and formed 1HB with Arg 207, 1HB with His 121, and an Arene-cation His 121 the key amino acids in the active site of caspase-3 protein. It exhibited binding energy of −15.26 Kcal/mol and formed 1 HB with Ile 12, 1 HB with Asp 15, and 1 Arene-cation His 13, the crucial amino acids in the target protein that represent the essential residues in the binding of isorhamnetin, procyanidin B2 towards caspase-3 protein and emodin, procyanidin B2 towards COX-2 protein were highlighted in Figs. 10 and 11, respectively.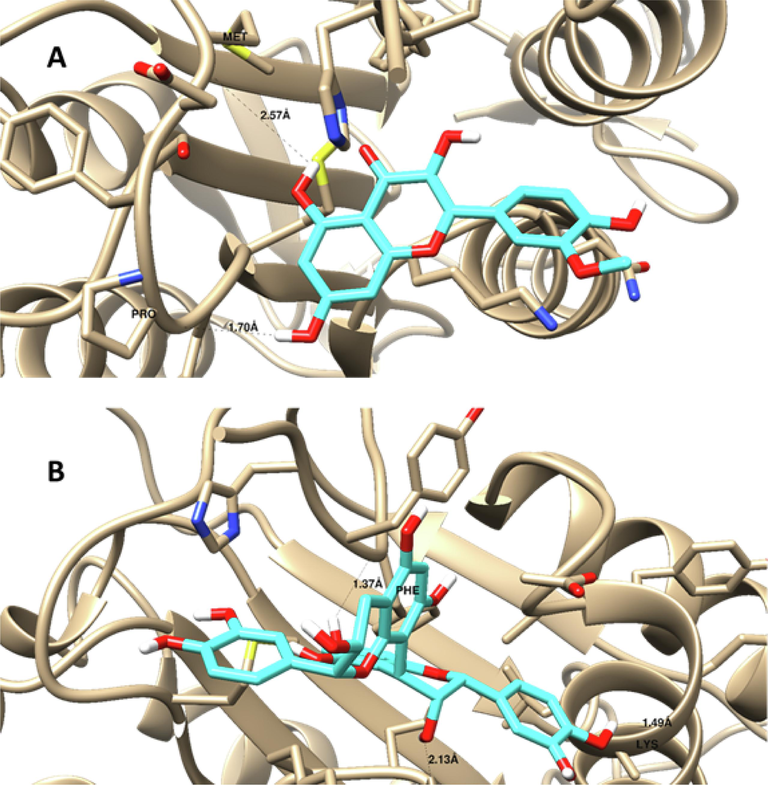
Ligand-disposition and receptor interactions of A) Isorhamnetin and B) Procyanidin B2 inside Caspase-3 protein demonstrating a surface representation. 3D images were made by Chimera software.
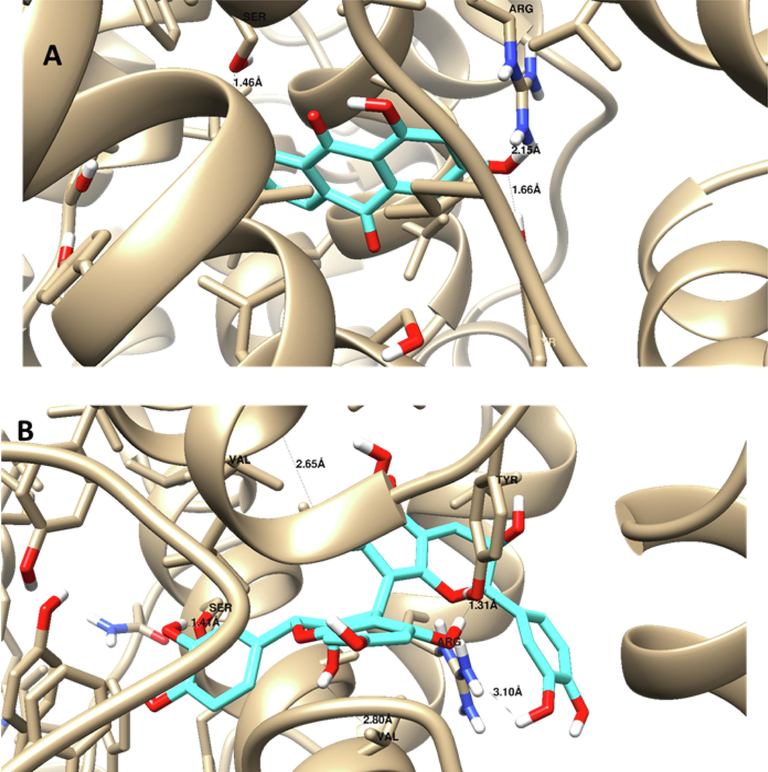
Ligand disposition and receptor interactions of A) Emodin and B) Procyanidin B2 inside COX-2 protein demonstrating a surface representation. 3D images were made by Chimera software.
Docking studies revealed that most of the identified compounds in C. fistula displayed good binding affinities towards the target proteins; caspase-3 and COX-2 where isorhamnetin was the most interactive compound with caspase-3. Emodin was the best compound to bind with COX-2 and procyanidin B2 which had strong dual affinity to both investigated proteins. These results supported the experimental ones given the antiapoptotic and anti-inflammatory effects of C. fistula that were mediated by caspase-3 and COX-2 inhibition, respectively. Furthermore, our docking finding matched with the literature regarding the antiapoptotic effects of isorhamnetin, since it reversed H2O2-induced apoptotic damage in H9c2 cardiomyocytes. It also reduced atherosclerosis in animals and shielded human brain microvascular endothelial cells from methylglyoxal- and oxygen-glucose-induced cytotoxicity. These protective actions were mediated by caspase-3 inactivation (Sun et al., 2012; Luo et al., 2015; Li et al., 2016). On the other side, the COX-2 inhibition effect of emodin was reported in earlier studies. Emodin attenuated neuropathy induced by oxaliplatin through the inhibition of spinal inflammation mediated by COX2/NF-κB (Yang et al., 2023) Also, this anthraquinone suppressed inflammation and development and progression of cancer in intestinal carcinogenesis associated with AOM/DSS-triggered colitis (Zhang et al., 2021) Furthermore, emodin reduced rheumatoid arthritis procession by the reduction of plasma levels of TNF-ɑ and IL-6 as well as downregulation of COX-2 protein expression in synovial tissue resulting in decreased production of PGE (2) (Stompor-Gorący, 2021; Cheng et al., 2022). Besides, the COX-2 and caspase-3 inhibition activities of procyanidin B2 were evidenced (Yang et al., 2015; Song et al., 2021; Cai et al., 2022).
4 Conclusion
This comprehensive investigation aimed to shed light on the antioxidant properties and potential therapeutic effects of C. fistula leaves. The study findings highlight the richness of C. fistula leaves in phenolic compounds, particularly flavonoids, as indicated by total phenolic content (TPC), total flavonoid content (TFC), and LC/MS/MS analysis. The research underscores the in vitro antioxidant potential of C. fistula, demonstrated through free radical quenching, Fe3+ ions reduction, and pho-molybdenum assay. Moreover, the study evaluates the therapeutic potential of C. fistula ((either as treatment or protectant) against acetic acid-induced ulcerative colitis, showing that the plant extract, especially in crude form, mitigates injuries and restores hematological and biochemical parameters, especially when used as treatment rather than as a protectant. It ameliorates histopathological lesions, prevents reactive oxygen species (ROS) overproduction, and downregulates inflammatory and apoptosis markers (COX-2 and caspase-3). The research suggests the need for further investigations to develop pharmaceutical formulations of C. fistula and conduct clinical trials to assess its therapeutic potential in ulcerative colitis patients.
Funding
This research received no external funding
Institutional Review Board Statement:
The study was conducted by the Declaration of Helsinki and approved by the Ethics Committee of FACULTY OF PHARMACY, SUEZ CANAL UNIVERSITY (NO. 202005 M1).
Acknowledgments
The authors would like to acknowledge Dr. Heba Nageh Gad El-Hak, Assistant Professor of Histology, Zoology Department, Faculty of Science, Suez Canal University, for histochemical and immune-histochemical results interpretation.
References
- Cassia fistula Leaves, UHPLC-QTOF-MS/MS based metabolite profiling, and molecular docking insights to explore bioactives role towards inhibition of pancreatic Lipase. Plants. 2021;10(7):1334.
- [CrossRef] [Google Scholar]
- Novel neuroprotective potential of Bunchosia armeniaca (Cav.) DC against lipopolysaccharide induced Alzheimer’s disease in mice. Plants. 2022;11(14):1792.
- [CrossRef] [Google Scholar]
- Antioxidant, α-glucosidase, and nitric oxide inhibitory activities of Phyllanthus acids and LC–MS/MS profile of the active extract. Food Biosci.. 2018;25:134-140.
- [CrossRef] [Google Scholar]
- Protective mechanism of acacia saligna butanol extract and its nano-formulations against ulcerative colitis in rats as revealed via biochemical and metabolomic assays. Biology. 2020;9(8):195.
- [CrossRef] [Google Scholar]
- Cytotoxic potentials and phytoconstituents profiling of Blepharis edulis (Forssk.) Pers. Using Uhplc/q-Tof-Ms-Ms. Al-Azhar J. Pharm. Sci.. 2021;63:37-56.
- [CrossRef] [Google Scholar]
- Thonningia sanguinea extract: antioxidant and cytotoxic activities supported by chemical composition and molecular docking simulations. Plants. 2021;10(10):2156.
- [CrossRef] [Google Scholar]
- LC/MS analysis of Viscum cruciatum Sieber ex Boiss. extract with antiproliferative activity against MCF-7 cell line via G0/G1 cell cycle arrest: An in-silico and in-vitro study. J. Ethnopharmacol.. 2022;295:115439
- [CrossRef] [Google Scholar]
- Inflammatory bowel disease: pathophysiology and current therapeutic approaches. Handb. Exp. Pharmacol.. 2017;239:115-146.
- [Google Scholar]
- Assessment the ameliorative effect of pomegranate and rutin on chlorpyrifos-ethyl-induced oxidative stress in rats. Nature Sci.. 2009;7:49-61.
- [Google Scholar]
- Potential anti-cholinesterase activity of bioactive compounds extracted from Cassia grandis L.f. and Cassia timoriensis DC. Plants. 2023;12(2):344.
- [CrossRef] [Google Scholar]
- Cassia fistula Linn: a review of phytochemical and pharmacological studies. Int J Pharm Sci Res. 2014;5(6):2125-2130.
- [Google Scholar]
- The hematological profiles of high-fat diet mice model with moringa oleifera leaves ethanol extract treatment. Biomed. Pharmacol. J.. 2019;12:2143-2149.
- [CrossRef] [Google Scholar]
- Possible biochemical effects following inhibition of ethanol-induced gastric mucosa damage by Gymnema sylvestre in male Wistar albino rats. Pharm. Biol. (abingdon, U. K.). 2012;50:1542-1550.
- [CrossRef] [Google Scholar]
- Miconazole Mitigates acetic acid-induced experimental colitis in rats: insight into inflammation, oxidative stress, and keap1/nrf-2 signaling crosstalk. Biology. 2022;11(2):303.
- [CrossRef] [Google Scholar]
- In vitro biological activities and preliminary phytochemical screening of different extracts from Achillea sintenisii Hub-Mor. Arabian J. Chem.. 2023;16(1):104426
- [CrossRef] [Google Scholar]
- Promising antiviral activity of agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study. Pharmaceu.. 2021;14(12):1313.
- [CrossRef] [Google Scholar]
- Extraction of phenolic compounds from Populus Salicaceae bark. Biomolecules. 2022;12(4):539.
- [CrossRef] [Google Scholar]
- Anti-ulcerogenic and anti-ulcerative colitis (UC) activities of seven amines derivatives. Saudi Pharm. J.. 2017;25:1125-1129.
- [CrossRef] [Google Scholar]
- Lycopodium mitigates oxidative stress and inflammation in the colonic mucosa of acetic acid-induced colitis in rats. Molecules. 2022;27(9):2774.
- [CrossRef] [Google Scholar]
- Evaluation of anti-inflammatory effects of Cassia fistula (Leguminosae) leaf extracts on rats. J Herbs Spices Med Plants.. 1999;6:67-72.
- [CrossRef] [Google Scholar]
- HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules. 2014;19(11):17400-17421.
- [CrossRef] [Google Scholar]
- Effects of dexpanthenol on acetic acid-induced colitis in rats. Exp. Ther. Med.. 2016;12:2958-2964.
- [CrossRef] [Google Scholar]
- Procyanidin B2 ameliorates the progression of osteoarthritis: An in vitro and in vivo study. Int. Immunopharmacol.. 2022;113:109336
- [CrossRef] [Google Scholar]
- Targeting ROS and cPLA2/COX2 expressions ameliorated renal damage in obese mice with endotoxemia. Int. J. Mol. Sci.. 2019;20(18):4393.
- [CrossRef] [Google Scholar]
- Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals. 2020;13(4):55.
- [CrossRef] [Google Scholar]
- Mechanism of emodin in the treatment of rheumatoid arthritis. Evid. Based Complement. Alternat. Med. 2022 ID 9482570
- [Google Scholar]
- LC-ESI-QTOF-MS/MS characterisation of phenolics in herbal tea infusion and their antioxidant potential. Fermentation.. 2021;7(2):73.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant activity, and inhibitory capacity of α-amylase, α-glucosidase, lipase, and non-enzymatic glycation, in vitro, of the leaves of Cassia bakeriana Craib. Ind. Crops Prod.. 2019;140:111641
- [CrossRef] [Google Scholar]
- Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds. BMC Complementary Altern. Med.. 2014;14:45.
- [Google Scholar]
- Antioxidant activity of some Algerian medicinal plants extracts containing Phenolic compounds. Food Chem.. 2020;97(4):654-660.
- [CrossRef] [Google Scholar]
- Animal models of chemically induced intestinal inflammation: predictivity and ethical issues. Pharmacol. Ther.. 2013;139:71-86.
- [CrossRef] [Google Scholar]
- Modulating effect of ginger extract on rats with ulcerative colitis. J. Ethnopharmacol.. 2008;118:367-372.
- [CrossRef] [Google Scholar]
- Metabolic profiling, chemical composition, antioxidant capacity, and in vivo hepato- and nephroprotective effects of sonchus cornutus in mice exposed to cisplatin. Antioxidants.. 2022;11(5):819.
- [CrossRef] [Google Scholar]
- Apple leaves and their major secondary metabolite phlorizin exhibit distinct neuroprotective activities: Evidence from in vivo and in silico studies. Arabian J. Chem.. 2021;14(6):103188
- [CrossRef] [Google Scholar]
- Antidiarrheal and antibacterial activities of Monterey cypress phytochemicals: in vivo and in vitro approach. Molecules. 2022;27(2):346.
- [CrossRef] [Google Scholar]
- Hypoglycemic effect of Cleome droserifolia ethanolic leaf extract in experimental diabetes, and on non-enzymatic antioxidant, glycogen, thyroid hormone, and insulin levels. Diabetol. Croat.. 2006;28:35-41.
- [Google Scholar]
- Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of Carrichtera annua DC. (cruciferae). Antioxidants.. 2020;9(12):1286.
- [CrossRef] [Google Scholar]
- The Antioxidant Carrichtera annua DC. ethanolic extract counteracts cisplatin-triggered hepatic and renal toxicities. Antioxidants.. 2021;10(6):825.
- [CrossRef] [Google Scholar]
- Comparative assessment of the antioxidant and anticancer activities of Plicosepalus acacia and Plicosepalus curviflorus: Metabolomic profiling and in silico Studies. Antioxidants.. 2022;11(7):1249.
- [CrossRef] [Google Scholar]
- Protective effect of dexpanthenol on bleomycin-induced pulmonary fibrosis in rats. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2013;386:1103-1110.
- [Google Scholar]
- Identification of the chemical constituents in Simiao wan and rat plasma after oral administration by GC-MS and LC-MS. Evidence-Based Complem. Altern. Med. 2017
- [Google Scholar]
- Toxicity of lead: a review with recent updates. Interdiscip. Toxicol.. 2012;5:47.
- [CrossRef] [Google Scholar]
- A practical method for the rapid detection and structural characterization of major constituents from traditional Chinese medical formulas: analysis of multiple constituents in Yinchenhao decoction. Anal. Methods.. 2015;7(11):4678-4690.
- [Google Scholar]
- Anti-inflammatory and Antipyretic Activities of Indian Medicinal Plant Cassia fistula Linn. (Golden Shower) in Wistar Albino Rats. Int. J. Pharmacol.. 2010;6:719-725.
- [Google Scholar]
- In vitro and in vivo studies of anti-lung cancer activity of Artemesia judaica L. crude extract combined with LC-MS/MS metabolic profiling, docking simulation, and HPLC-DAD quantification. Antioxidants. 2022;11(1):17.
- [CrossRef] [Google Scholar]
- Determination of serum proteins by means of the biuret reactions. J. Biol. Chem.. 1949;177:51.
- [Google Scholar]
- A Reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants.. 2020;9(5):398.
- [CrossRef] [Google Scholar]
- Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur. J. Pharmacol.. 2007;554:69-77.
- [Google Scholar]
- Biochemical, histopathological, and histochemical effects of Vitis vinifera L extract on acetic acid-induced colitis. J. Complementary Med. Res.. 2017;6:351.
- [Google Scholar]
- Antitrypanosomal, anti topoisomerase-i, and cytotoxic biological evaluation of some African plants belonging to Crassulaceae, chemical profiling of extract using UHPLC/QTOF-MS/MS. Molecules. 2022;27(24):8809.
- [CrossRef] [Google Scholar]
- Effect of Coriandrum sativum hydroalcoholic extract and its essential oil on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed.. 2016;6:205-214.
- [Google Scholar]
- Unveiling the anticancer, antimicrobial, antioxidative properties, and UPLC-ESI-QTOF-MS/GC–MS metabolite profile of the lipophilic extract of siam weed (Chromolaena odorata) Arabian J. Chem.. 2023;16(7):104834
- [CrossRef] [Google Scholar]
- molecular mechanisms of Cassia fistula against epithelial ovarian cancer using network pharmacology and molecular docking approaches. Pharmaceutics.. 2022;14(9):1970.
- [CrossRef] [Google Scholar]
- UPLC-ESI-Q-TOF-MS/MS characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits, and their herbal derived drops (Crataegutt Tropfen) J. Chem. Biol. Therap.. 2015;1:102.
- [CrossRef] [Google Scholar]
- Antioxidant, antiproliferative, and apoptosis-inducing efficacy of fractions from Cassia fistula L. leaves. Antioxidants.. 2020;9(2):173.
- [CrossRef] [Google Scholar]
- Protective effects of walnut extract against oxidative damage in acetic acid-induced experimental colitis rats. Physiol. Pharmacol.. 2019;23:51-58.
- [Google Scholar]
- Investigation of the effects of extraction solvent/technique on the antioxidant activity of Cassia fistula L. J. Medic. Plants Res.. 2012;6(3):500-503.
- [CrossRef] [Google Scholar]
- The genus Cassia L.: Ethnopharmacological and phytochemical overview. Phytotherapy Res.. 2021;35(5):2336-2385.
- [CrossRef] [Google Scholar]
- Molecular insights into human transmembrane protease serine-2 (TMPS2) inhibitors against SARS-CoV2: Homology modeling, molecular dynamics, and docking studies. Molecules. 2020;25(21):5007.
- [CrossRef] [Google Scholar]
- Design and synthesis of coumarin derivatives as cytotoxic agents through PI3K/AKT Signaling pathway inhibition in HL60 and HepG2 cancer cells. Molecules. 2022;27(19):6709.
- [CrossRef] [Google Scholar]
- Two stilbenes from Indonesian Cassia grandis and their antibacterial activities. Res. J. Chem. Environ.. 2020;24:61-63.
- [Google Scholar]
- Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. (Amsterdam, Neth.).. 2005;69:395-406.
- [CrossRef] [Google Scholar]
- Phytochemicals screening and analysis using HPLC to determine the antimicrobial efficacy of Cassia fistula extract. Adv. Bio Res.. 2015;2015(6):1-7.
- [Google Scholar]
- The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen-glucose deprivation. J. Neurochem.. 2016;136:651-659.
- [CrossRef] [Google Scholar]
- Simultaneous determination of formononetin, calycosin, and isorhamnetin from Astragalus mongholicus in rat plasma by LC-MS/MS and application to pharmacokinetic study. Zhong Yao Cai. 2013;36:589-593.
- [Google Scholar]
- Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS One. 2015;10:e0120259.
- [Google Scholar]
- MassBank. Available online: https://massbank.eu/MassBank// (accessed on 12 April 2023).
- Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed.. 2014;4:127-136.
- [Google Scholar]
- The medicinal properties of Cassia fistula L: A review. Biomed. Pharmacol.. 2021;144:112240
- [CrossRef] [Google Scholar]
- Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids. 2019;152:108485
- [CrossRef] [Google Scholar]
- Formalin-fixed paraffin-embedded sample conditions for deep next-generation sequencing. J. Surg. Res.. 2017;220:125-132.
- [CrossRef] [Google Scholar]
- Promising antifungal activity of Encephalartos laurentianus de Wild against Candida albicans clinical isolates: in vitro and in vivo effects on renal cortex of adult albino rats. J. Fungi.. 2022;8(5):426.
- [Google Scholar]
- Wound-healing potential of rhoifolin-rich fraction isolated from Sanguisorba officinalis roots supported by enhancing re-epithelization, angiogenesis, anti-inflammatory, and antimicrobial effects. Pharmaceuticals.. 2022;15(2):178.
- [CrossRef] [Google Scholar]
- Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig. Dis. Sci.. 2000;45:1820-1827.
- [Google Scholar]
- Measurement of superoxide dismutase. Biochem. Biophys. Res. Commun.. 1972;46:849-854.
- [Google Scholar]
- Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem.. 2008;106(2):760-766.
- [CrossRef] [Google Scholar]
- Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT–Food Sci. Technol.. 2005;38:513-517.
- [Google Scholar]
- phenolic profile, antioxidant and enzyme inhibitory activities of leaves from two Cassia and two Senna species. Molecules. 2022;27(17):5590.
- [CrossRef] [Google Scholar]
- Acetic acid-induced ulcerative colitis in Sprague Dawley rats is suppressed by hydroethanolic extract of Cordia vignei leaves through reduced serum levels of TNF-α and IL-6. Intern. J Chronic Dis.. 2020;6:2020.
- [Google Scholar]
- Quantitative analysis of phenolic compounds in crude extracts of Myrcia splendens leaves by HPLC-ESI-MS/MS. Rodriguésia.. 2020;71:e00552019.
- [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med.. 1967;70:158-169.
- [Google Scholar]
- Antioxidant capacity and identification of bioactive compounds of Myrtus communis L. extract obtained by ultrasound-assisted extraction. J. Food Technol.. 2017;54:4362-4369.
- [Google Scholar]
- Pintać, D., Majkić, T., Torović, L., Orčić, D., Beara, I., Simin, N., Mimica-Dukic, N., Lesjak, M., 2018. Solvent selection for efficient extraction of bioactive compounds from grape pomace. 111Prod. 111, 379-390.
- Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice. World J. Gastroenterol.. 2006;12:6646-6651.
- [Google Scholar]
- Cassia fistula Linn: Potential candidate in health management. Pharmacol. Res.. 2015;7:217-224.
- [CrossRef] [Google Scholar]
- Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complementary Altern. Med.. 2012;12:221.
- [Google Scholar]
- Salama, R.M., Darwish, S.F., Shaffei, I.E., Elmongy, N.F., Afifi, M.S. and Abdel-Latif, G.A., 2020. Protective effect of Morus macroura Miq. fruit extract against acetic acid-induced ulcerative colitis in rats: Involvement of miRNA-223 and TNFα/NFκB/NLRP3 inflammatory pathway. bioRxiv, pp.2020-12. 10.1101/2020.12.22.423927.
- Cassia fistula: Botany, phytochemistry and pharmacological leverages-a review. Int J Pharm Pharm Sci.. 2020;12:90-93.
- [Google Scholar]
- Antiulcer potential of morin in acetic acid-induced gastric ulcer via modulation of endogenous biomarkers in laboratory animals. Pharmacologia.. 2015;6:273-281.
- [Google Scholar]
- Identification and quantification of aldose reductase inhibitory flavonoids in herbal formulation and extract of Gymnema sylvestre using HPLC-PDA and LC-MS/MS. Chromatogr. Res. Int.. 2014;2014
- [Google Scholar]
- Sinapic acid ameliorates acetic acid-induced ulcerative colitis in rats by suppressing inflammation, oxidative stress, and apoptosis. Molecules. 2022;27(13):4139.
- [CrossRef] [Google Scholar]
- Pharmacological and chemical potential of Cassia fistula L- a critical review. J. Herbal Medic.. 2021;26:100407
- [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic.. 1965;16:144-158.
- [Google Scholar]
- Procyanidin B2 inhibits lipopolysaccharide-induced apoptosis by suppressing the Bcl 2/Bax and NF κB signaling pathways in human umbilical vein endothelial cells. Mol. Med. Rep.. 2021;23:267.
- [Google Scholar]
- The transport of oxidized glutathione from human erythrocytes. J. Biol. Chem.. 1969;244:9-16.
- [Google Scholar]
- The health benefits of Emodin, a Natural anthraquinone derived from Rhubarb—A Summary Update. Int. J. Mol. Sci.. 2021;22:9522.
- [Google Scholar]
- Screening Non-colored Phenolics in Red Wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules. 2007;12(3):679-693.
- [CrossRef] [Google Scholar]
- Isorhamnetin inhibits H₂O₂-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J. Cell. Biochem.. 2012;113:473-485.
- [Google Scholar]
- Chemical profiling and screening of the marker components in the fruit of Cassia fistula by HPLC and UHPLC/LTQ-Orbitrap MSn with chemometrics. Molecules. 2018;23(7):1501.
- [CrossRef] [Google Scholar]
- The healing effect of strawberry extract on acetic acid-induced ulcerative colitis in rat. World Appl. Sci. J.. 2014;31:281-288.
- [CrossRef] [Google Scholar]
- Healing acceleration of acetic acid-induced colitis by marigold (Calendula officinalis) in male rats. Saudi J Gastroenterol.. 2016;22:50.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and neuroprotective activities of Cassia fistula (L.) using the Caenorhabditis elegans model. PeerJ. 2018;6:5159.
- [CrossRef] [Google Scholar]
- The role of free radicals and antioxidants: how do we know that they are working? Critical Rev, Food Sci. Nutrit.. 1995;35:21-39.
- [CrossRef] [Google Scholar]
- Simultaneous determination of naringin, hesperidin, neohesperidin, naringenin, and hesperetin of Fractus aurantii extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal.. 2012;58:58-64.
- [Google Scholar]
- A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods. 2019;16:295-298.
- [Google Scholar]
- Mesalazine–probiotics beads for acetic acid experimental colitis: formulation and characterization of a promising new therapeutic strategy for ulcerative colitis. Drug Deliv.. 2015;22:94-99.
- [Google Scholar]
- Simultaneous determination of gallic acid and gentisic acid in organic anion transporter expressing cells by liquid hromatography–tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci.. 2013;937:91-96.
- [Google Scholar]
- Emodin suppresses oxaliplatin-induced neuropathic pain by inhibiting COX2/NF-κB mediated spinal inflammation. J. Biochem. Mol. Toxicol.. 2023;37:e23229.
- [Google Scholar]
- Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules. 2015;20(7):12250-12265.
- [CrossRef] [Google Scholar]
- Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom.. 2007;18:82-91.
- [Google Scholar]
- Scavenging effect of methanolic extracts of Peanut Hulls on free-radical and active-oxygen species. J. Agric. Food Chem.. 1994;42:629-632.
- [Google Scholar]
- Anti-inflammatory effect of aqueous extract of Cassia fistula in acetic acid model of colitis in rats. J. Medicinal Plants By-product.. 2024;2024 Jan 4
- [Google Scholar]
- Emodin inhibits inflammation, carcinogenesis, and cancer progression in the AOM/DSS model of colitis-associated intestinal tumorigenesis. Front. Oncol.. 2021;10:564674
- [Google Scholar]
- LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs.. 2020;18(6):331.
- [CrossRef] [Google Scholar]
- Comparative LC-ESIMS-Based metabolite profiling of Senna italica with Senna alexandrina and evaluating their hepatotoxicity. Metabolites. 2023;13(4):559.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105672.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







