Translate this page into:
Change in chemical composition and enhancement of intestinal microflora of acid hydrolyzed polysaccharides from Zizyphus jujube and Sterculia lychnophora
⁎Corresponding author. pohsien0105@pu.edu.tw (Po-Hsien Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Water extraction combined with acid hydrolysis modified the polysaccharides. The viscosity of polysaccharides can change with temperature, with the potential for food applications. Polysaccharides greatly impact microbial proliferation (in vitro).
Abstract
The homology between food and medicine has been a successful and well-accepted concept in traditional medicine, where people believe that beneficial naturally available substances can be replenished via diet to promote health. In this study, the polysaccharides in jujube fruit (Ziziphus jujuba Mill.) and dried boat-fruited Sterculia seeds (Sterculia lychnophora) were extracted using different methods (water, acid, and alkaline extraction), and their physicochemical properties and compositions were investigated. Additionally, the effects of polysaccharides on the growth of known intestinal bacteria were investigated using in vitro incubation. The results showed that the acid and alkaline extraction methods resulted in the highest yields of polysaccharides with a high uronic acid content and satisfactory antioxidant activity. The water-extracted polysaccharides exhibited a suitable viscosity. Moreover, the water-extracted polysaccharides were acid-hydrolyzed for 2–6 h, followed by gel permeation chromatography (GPC) to obtain acidic hydrolyzed polysaccharides with a wide range of molecular weights and high carbohydrate contents. Subsequently, five known strains of intestinal bacteria cultured with the acidic hydrolyzed polysaccharides from jujube fruits and pandahai (10 mg/mL) were found to proliferate in Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus delbrueckii, and Propionibacterium freudenreichir in 0–48 h cultures. However, further evidence is required to validate these results, either in gastrointestinal simulations or animal models.
Keywords
Polysaccharides
Jujube fruits
Pandahai
Intestinal bacteria
Acid-hydrolyzed
Traditional medicine
1 Introduction
Jujube fruits (Ziziphus jujuba Mill.) are used in traditional medicine because of their health-promoting properties and enrichment of various biological components such as phenolics, triterpenoids, flavonoids, amino acids, organic acids, trace minerals, and polysaccharides (Agrawal et al., 2023; Chang et al., 2010; Ji et al., 2020a; Ji et al., 2020b). Jujube fruit is consumed either partially fresh by consumers, dried as the homology of food and medicinal ingredients, added to dishes (or simmered in soups), or decocted with various herbs (Agrawal et al., 2023; Xu et al., 2022; Yang et al., 2020).
Dried boat-fruited Sterculia seeds (Sterculia lychnophora Hance, also called pandahai or pàng dàhǎi) are primarily found in Vietnam, Cambodia, Laos, Thailand, Malaysia, Indonesia, India, and South China, and are commonly used in traditional medicine to soothe the throat of upper respiratory sounds and treat digestive disorders as a cooling agent. The primary biological activities stem from polysaccharides, alkaloids, phenolics, glycosides, cerebrosides, peptides, fatty acids, and steroids (Li et al., 2011; Lv et al., 2022; Oppong et al., 2018; Oppong et al., 2020; Soejarto et al., 2023; Wang et al., 2017; Wu et al., 2007a).
Polysaccharides are polymers and among the four most significant molecular biomolecules, constituting > 10 monosaccharides linked by glycosidic bonds. Polysaccharides contain lipids, proteins, and nucleic acids, while the molecular weights widely range from thousands to millions of Daltons (Da), depending on the molecular weight of the chain, which the variable structures and various bioactivities (Gao et al., 2023; Li et al., 2023; Liu et al., 2018; Muthusamy et al., 2021; Wen et al., 2023; Zhang et al., 2023a; Zhang et al., 2023b; Zhao et al., 2021).
The biological activities of polysaccharides extracted from fruits, vegetables, herbs, and microbes have been validated and have received widespread attention in the market and academia (Gao et al., 2023; Song et al., 2023; Sun et al., 2023; Xu et al., 2023). Chou et al., (2023b) commercialized Huáng qí (root of Astragalus membranaceus) polysaccharides in the market, which has positively affected exercise performance and provided potential anti-aging properties in aging mice. In addition, polysaccharides, such as pectin, mannan (Man), arabinose (Ara), glycosides, and non-starch polysaccharides, including lipopolysaccharides and peptidoglycan, are fermented and degraded by intestinal microorganisms to release short-chain fatty acids involved in various biological actions, such as immunomodulatory, anti-inflammatory, antioxidant, and antimicrobial effects (Bagchi & Jayaram Kumar, 2016; Ebrahimzadeh Leylabadlo et al., 2020; Koh et al., 2016; Li et al., 2023; Patra et al., 2023; Xie et al., 2023; Xu et al., 2023; Zhao et al., 2021). Interestingly, owing to their wide range of biological activities, polysaccharides have been developed as carriers to encapsulate drugs with gel nano-encapsulation (Rosales & Fabi, 2023) and have been applied in advanced wound dressings in combination with the mechanical properties of hydrogels (Shi et al., 2022).
Therefore, the primary purpose of this study was to investigate the effects of different extraction methods on the physicochemical properties of jujube fruit and pandahai polysaccharides and evaluate their effects on the growth of beneficial bacteria. The second aim was to facilitate the development and application of herbal polysaccharides and provide researchers with a more comprehensive understanding of the field.
2 Materials and methods
2.1 Materials
Dried jujube fruits were purchased from the Gongguan Farmers’ Association (Miaoli County, Taiwan). Dried boat-fruited Sterculia seeds (pandahai) were purchased from a local market (Taichung City, Taiwan, origin, Vietnam). Both the samples were cleaned with distilled water and dried at 45 °C for 24 h. The samples were sealed in an aluminum foil bag (PET/AL/PET/PE, 35 × 23 cm, 0.14 mm thick) and stored at 4 °C. All the chemicals were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Clostridiun perfringens (ATCC 3626) and Bifidobacterium longum (ATCC 15708) were purchased from the American Type Culture Collection (ATCC; Manassas, Virginia, USA). Bifidobacterium bifidum (BCRC 11844), Propionibacterium freudenreichii (BCRC 11076), and Lactobacillus delbrueckii subsp. Bulgaricus (BCRC14008) were purchased from the Bioresource Collection and Research Center (BCRC; Hsinchu, Taiwan).
2.2 Polysaccharide extraction and acid-hydrolysis
Polysaccharides extraction and acid hydrolysis were performed according to method described by Wang et al. (2022), with slight modifications. The dried fruits were pressed, crushed, and immersed in 25 folds (by dry weight) distilled water, acid solution (HCl, 0.0001 M, pH 4), and alkaline solution (NaOH, 0.0001 M, pH 10) for 24 h. Next, homogenization (10,000 rpm, 5 min and every 30-sec interval with a 5-sec pause) was performed with a homogenizer and stirred overnight before passing through a 70-mesh sieve (mesh size as standard of 0.21 mm) to collect the filtrate, and the residue was replicated twice in the above operation. Next, 95 % alcohol (4-fold volume of the concentrate) was added to the concentrate and allowed to settle overnight at 4 °C. The precipitates were centrifuged again (10,000 × g, 4 °C) for 15 min to collect the deposits, followed by freeze-drying at −80 °C for 48 h using a freeze-dryer (FD-5 N, Eyela). All dried samples were ground into polysaccharides, packed into aluminum foil bags, and preserved in a desiccator for future use.
Subsequently, the polysaccharides were hydrolyzed with 2 M HCl (2, 4, and 6 h), filtered with a 0.45 µm PVDF membrane, and then analyzed by GPC (Sephacryl S-200 high resolution for jujube fruits and Sephacryl S-400 high resolution for pandahai, General Electric Healthcare Co., Chicago, USA), with column size of L × I.D. 60 cm × 16 mm, 50 μm average part size, and mobile phase with a deionized water flow rate of 0.50 mL/min. The collection volume was 2.5 mL/tube, with a total of 95 tubes. The molecular weights of the monosaccharides were calculated by interpolating the molecular weights and retention times of the standards [8 molecular weights of pullulan (Shodex P-82), Showa Denko Co., Ltd., Tokyo, Japan]. Followed by dialysis to remove the salts < 0.5 kDa, while the dialyzed monosaccharides solutions of different molecular weights were freeze-dried and ground into powder before storage in stock for future use.
2.3 Composition analysis of samples
Compositional analysis of jujube fruits and pandahai was performed using the AACC (2023) standard methods. The analysis included moisture (44–11.01), crude protein (46–10.01), crude fat (30–10.01), crude fiber (32–05.01), and ash (08–01.01), whereas 100 % minus the above crude protein, fat, ash, and fiber contents as the nitrogen-free extract (NFE) content.
2.4 Physicochemical properties of polysaccharides
2.4.1 Total sugar contents analysis
Total sugar content was determined according to the method described by Nielsen (2017). Briefly, 1 mL of sample (100 µg/mL) was added to 1 mL of 5 % phenol in a tube. Subsequently, 5 mL 98 % sulfuric acid was added rapidly (within 30 s), shaken uniformly, and cooled in a water bath. Finally, the absorbance of the samples was measured at 490 nm using a spectrophotometer (Model U-2001, Hitachi Ltd., Tokyo, Japan). The standard curve prepared for glucose ranged from 0 to 100 µg/mL, and the sugar content of the samples was determined through interpolation.
2.4.2 Protein content determination
The protein content of the samples was determined using a BCA protein assay kit (71285 Millipore, Merck KGaA) according to the method described by Cortés-Ríos et al. (2020) and standard operating procedures provided by the manufacturer.
2.4.3 Uronic acid content determination
The uronic acid content of the samples was determined as described by Huang et al. (2011), with some modifications. The sample (100 µg/mL) of 0.2 mL was mixed with 1.2 mL of 0.0125 M sulfuric acid/sodium tetraborate solution, shocked, and cooled at 4 °C for 5 min. Subsequently, the samples were placed in a boiling water bath at 100 °C for 5 min and transferred to an ice bath (4 °C) for 5 min. Next, 20 µL of 0.15 % meta-hydroxy diphenyl solution (prepared in 0.5 % NaOH solution) was added with shaking and mixing, allowed to stand for 5 min, and absorbance was measured at 520 nm. A standard curve was prepared with 0––100 µg/mL galactoglycolic acid, whereby the uronic acid content in the sample was calculated by interpolation.
2.4.4 Monosaccharide composition determination
Monosaccharide composition was determined based on the approach described by Ji et al., (2020a), with some modifications. A 50 mg sample was acid-hydrolyzed with 50 mL of 2 M HCl at 85 °C for 16 h. Next, it was concentrated, dried, re-dissolved in 10 mL of deionized water, and purified using an ion-exchange resin to neutralize the solution before filtering with 0.22 µm membranes. Subsequently, the acid hydrolysis solution was mixed with ABEE Derivatization Marker Reagent [165 mg of benzocaine (C9H11NO2), 35 mg sodium cyanoborohydride (CH3BNNa), 40 µL of glacial acetic acid, and 0.35 mL of boiling methanol (heated in a boiling water bath)] in a ratio of 2:8. The solution was incubated in an 80 °C water bath for 1 h, and then, deionized water (0.4 mL) was added to end the reaction. Finally, 0.4 mL of chloroform was added, and the supernatant was extracted by centrifugation (1780 × g, 5 min, 25 °C) before analysis by HPLC filtered through a 0.22 µm filter.
Finally, 0.4 mL of chloroform was added (removing extra derivatization marker reagent), and the supernatant was extracted by centrifugation (1780 × g, 5 min, 25 °C) before analysis by HPLC filtered through a 0.22 µm filter. The detailed analytical conditions were YMC-Triart C18 column (USP L1, ID 3 mm, length 100 mm, particle size 3 μm, YMC America Inc., Devens, MA, USA) (column temperature 50 °C), an L-7100 pump (Hitachi), and a UV-detector L-7400 (Hitachi) with a detection wavelength of 308 nm. The mobile phase was performed using 0.04 M potassium borate buffer (pH 8.9): 100 % acetonitrile = 99:1 at a 0.5 mL/min flow rate. In addition, the standards were prepared with 0–5 mg/mL of galactose (Gal), glucose (Glu), rhamnose (Rha), Ara, Man, and xylose (Xyl), and the composition and contents of the samples were calculated according to the different standards with interpolation.
2.5 Antioxidant capacity measurement
2.5.1 1, 1-diphenyL-2-picrylhydrazyl (DPPH) free radical scavenging ability
The DPPH radical-scavenging ability of the samples was determined as described by Wang et al., (2019a) and Zhao et al. (2023) with minor modifications. Briefly, 100 µL of different concentrations (0––50 mg/mL) and 95 % ethanol solution of butylated hydroxytoluene (BHT) as the control group (0––50 mg/mL) were sequentially added to 400 µL of 100 mM Tris-HCl buffer solution (pH 7.4) and 500 µL of 250 µM DPPH ethanol solution, followed by shaking for 20 s to mix and avoid a light reaction for 20 min to determine the absorbance at 517 nm. The following formula was used to calculate the DPPH radical scavenging ability (%), and the half-effective concentration (EC50) was calculated by linear regression analysis, that is, the concentration required for the sample to reach a 50 % effect. A smaller the value indicates better the antioxidant ability of the sample.
2.5.2 Ferric reducing antioxidant power (FRAP)
The FRAP of the samples was determined as described by Li et al. (2020) with some modifications. The sample and EDTA were mixed with 250 µL of different concentrations (0–50 mg/mL) in sequence with 925 µL phosphate buffer (pH 7.4) and 25 µL of FeCl2, left standing for 30 s, 50 µL of ferrozine was added, and the reaction was allowed to proceed for 10 min. Finally, the absorbance was measured at 562 nm, where a low value indicates better chelating ability of the sample.
2.5.3 Antioxidant reaction rate coefficient (k)
In addition, the antioxidant ability of the samples showed a dose-dependent dependence on their concentration, whereas all the antioxidant abilities above the samples tended toward a plateau after reaching a particular concentration. Therefore, the following equation was used to calculate the sample reaction rate coefficient (k): a higher value indicates a better antioxidant ability of the sample (Constales et al., 2017; Parsons, 2021).
I0 indicates the antioxidant ability of the samples at different concentrations.
I Indicate the equilibrium maximum antioxidant ability reached greater than a particular concentration
C is the sample concentration.
k is the reaction rate coefficient, which indicates that the greater the value, the more rapid the antioxidant ability of the sample.
2.6 Viscosity properties determination
Viscosity analysis in this study was performed as described by Huang et al. (2023) and Wang et al., (2019b), with slight modifications. Both samples, jujube (4, 8, 12, 16, and 20 mg/mL) and pandahai (0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL) polysaccharides, were analyzed using a viscometer (model DV-II, AMETEK Brookfield Inc., Middleboro, MA, USA) with a small sample adapter, probe spindle 31, and rotational speed of 12 rpm at various temperatures (50, 60, 70, 80, and 90 °C) to determine the viscosity of all samples (centipoises, cps).
2.7 Determination compositions of the functional groups
The functional groups in the samples were determined according to the method described by Chou et al., (2023a). The dried sample (2 mg) was mixed with 120 mg of potassium bromide (KBr), followed by the addition of 30 mg, and pressed into a 13 mm film tablet using a tablet press. Subsequently, a Fourier-transform infrared spectrometer (FT-IR, Satellite 5000, Mattson Technology, Fremont, CA, USA) was used to scan the 400–4000 cm−1 range.
2.8 Proliferation of intestinal bacteria
The cryopreservation tube (containing 1 mL of bacterial broth) was removed from the −80 °C refrigerator and quickly thawed in the anaerobic workstation (AW400SG, Munro Instruments Limited, Essex, UK), then mixed with 10 mL medium [consisting of 7.5 mL of sterile water and 2.5 mL of 4-fold Reinforced Clostridial medium broth (RCM, 1054110500, Merck KGaA) or De Man, Rogosa and Sharpe agar (MRS) (69966, Merck KGaA) medium] poured into a triangular tapered flask, which was cultured for 48 h.
Next, 200 µL of the activated bacterial broth (diluted with phosphate buffer solution to 0.35 absorbance at 600 nm, with bacterial counts of about 1 × 107-–108) was taken, then 200 µL medium (4-fold RCM or MRS) and 400 µL samples (20 and 50 mg/mL) were added to the microcentrifuge tubes, which were co-culture in an anaerobic workstation for 0, 24, 48, and 72 h. Afterward, 0.1 mL of the bacterial broth was placed in a sterile microcentrifuge tube and added with 0.9 mL of phosphate buffer solution and mixed evenly, followed by serial dilution (101-–109 fold). Next, 0.1 mL of the bacterial broth with different dilutions was spread onto the plate (containing RCM or MRS solid medium) and incubated for 48 h to observe colony formation and then counted [effective range between 25 and 250 Colony Forming Units (CFU)].
2.9 Statistical analysis
All analyses in this study were performed in triplicate, and the values were expressed as the mean ± standard deviation (SD) unless mentioned otherwise. All data are analyzed using SAS package statistical software (version 9.4, SAS Institute, Cary, NC, USA). One-way ANOVA was used to analyze each trial group. Duncan's multi-variable analysis was used to analyze the groups' differences, while p < 0.05 was considered a statistically significant difference.
3 Results and discussion
3.1 Compositional analysis of jujube fruit and pandahai samples
The general compositional analysis of jujube fruit and pandahai showed that the moisture content of jujube was ∼13.15 %, whereas the other constituents of dry matter weight were 5.01 % crude protein, 0.47 % crude fat, 3.28 % crude fiber, 1.88 % ash, and 89.36 % NEF (Table A1). The moisture content of pandahai was 11.18 %; other components contributing to the dry weight were 4.12 % crude protein, 5.29 % crude fat, 3.61 % crude fiber, 1.99 % ash, and 84.99 % NEF.
3.2 Polysaccharide extraction yield, composition, and various monosaccharides content
The polysaccharides extracted by different extraction methods ranged from 6 % to 8 % and 28 % to 37 % for jujube fruits and pandahai, respectively (Table 1), and the extraction yields were significantly higher using the acid or alkaline solutions than with water extraction (p < 0.05). Wu et al. (2019) reported an actual yield of 1.97 % for water extraction of jujube fruit polysaccharides using improved optimization conditions, and Li and Huang (2021) reported alkaline extraction yields of 3.3 %–5.09 % and hypothesized that the differences were associated with variety, origin, and extraction conditions compared with this study. Moreover, Wu et al., (2007b) reported that pandahai sourced from Southeast Asia exhibits a higher polysaccharide content than China. This difference is likely attributable to the tropical climate of this region. In addition, acidic or alkaline environments could break the hydrogen bonds between cellulose and hemicellulose, allowing more insoluble polysaccharides to be effectively released from the cell wall and converted to soluble polysaccharides, thereby increasing the yield. In contrast, water extraction yields free polysaccharides and has a low yield (Kou et al., 2022). Traditional herbal medicines are typically boiled in water, which dissolves many components at high temperatures, where some active ingredients deteriorate and reduce the efficacy of the polysaccharides (Liu et al., 2018). Lowercase letters with different superscripts in same column indicate significant differences (p < 0.05).
Sample/Extracted method
Extraction yield (%)
Totalcarbohydrate (%)
Protein(%)
Uronic acid(mg/g)
Galactose(Gal)
Mannose(Man)
Glucose(Glu)
Aarabinose(Ara)
Xylose(Xyl)
Rhamnose(Rha)
Jujube fruits
Water extraction
6.11 ± 0.01f
60.53 ± 2.86e
5.90 ± 0.10e
26.71 ± 1.81 h
11.0.41 ± 0.62a
23.37 ± 0.83a
39.69 ± 1.87c
20.18 ± 1.49b
2.00 ± 0.04a
3.35 ± 0.04b
Water-extracted followed by 2 M HCl hydrolyzed
2 h
–
95.34 ± 4.67a
1.74 ± 0.07 g
26.41 ± 0.12 g
29.13 ± 1.87a
20.30 ± 0.54d
8.24 ± 0.12b
16.64 ± 0.04c
15.69 ± 0.96a
10.00 ± 0.54a
4 h
–
94.78 ± 4.57a
0.83 ± 0.03 h
23.17 ± 2.01i
3.05 ± 0.57d
81.74 ± 4.21a
–
10.41 ± 0.39e
–
4.81 ± 0.18b
6 h
–
93.68 ± 5.86a
0.61 ± 0.06i
18.71 ± 2.65 k
7.47 ± 0.06c
77.00 ± 2.48b
–
11.51 ± 0.06d
–
–
Acidic
8.47 ± 0.02d
69.16 ± 6.06d
9.59 ± 0.21b
31.56 ± 0.95f
7.442 ± 0.09b
18.16 ± 0.32b
51.32 ± 0.40b
19.82 ± 1.04b
–
2.56 ± 0.07a
Alkaline
8.33 ± 0.05e
61.33 ± 4.31e
8.08 ± 0.33c
40.21 ± 1.97b
–
5.95 ± 0.06c
66.29 ± 0.83a
27.76 ± 2.30a
–
–
Pandahai
Water
28.49 ± 0.09c
77.75 ± 1.21c
4.61 ± 0.11f
35.47 ± 2.86d
7.01 ± 0.04c
46.49 ± 0.70a
–
–
46.49 ± 2.94a
–
Water-extracted followed by 2 M HCl hydrolyzed
2 h
–
95.67 ± 2.84a
1.74 ± 0.04 g
33.87 ± 0.23e
21.24 ± 0.59b
31.83 ± 0.85c
21.70 ± 0.17b
–
–
23.17 ± 1.31a
4 h
–
94.38 ± 4.31a
0.83 ± 0.03 h
29.34 ± 1.02 g
–
59.63 ± 1.96a
20.64 ± 0.21c
–
–
19.73 ± 0.43b
6 h
–
95.74 ± 1.32a
0.61 ± 0.05i
21.59 ± 1.65j
15.47 ± 2.34c
15.72 ± 1.84d
7.85 ± 0.08a
19.70 ± 0.10a
28.69 ± 0.96b
12.56 ± 0.05c
Acidic
37.11 ± 0.02a
83.33 ± 1.84b
7.77 ± 0.07d
38.75 ± 2.57c
14.86 ± 0.88b
48.31 ± 2.64a
17.58 ± 0.50b
–
–
17.14 ± 1.41a
Alkaline
36.88 ± 0.17b
74.58 ± 1.97d
12.85 ± 0.13a
46.73 ± 1.15a
17.19 ± 1.30a
32.64 ± 1.64b
32.14 ± 1.30a
–
–
18.03 ± 2.03a
The total carbohydrate content of the polysaccharides extracted using the acid method was significantly higher than that of the water and alkaline extracts (p < 0.05) (Table 1). Jujube fruit, extracted with the acidic solution, exhibited the highest total carbohydrate content of 69.16 %, whereas hot water and alkaline extracts were similar at 60 %- 61 %. The total carbohydrate content of the pandahai polysaccharides followed a similar trend, as the acidic extract had a high total carbohydrate content of 83.33 %, followed by the water extract at 77.75 % and the alkaline extract at 74.58 %. These results are similar to those reported by Ai et al. (2012). Sandarani and Kulathunga (2017) reported the availability of carbohydrates with few impurities when produced with citric acid.
The protein contents of polysaccharides in acidic and alkaline extracts of jujube fruits were 9.59 % and 8.08 %, respectively, which were higher than water extracts (5.90 %) (p < 0.05) (Table 1). However, similar results were obtained for pandahai, with acidic and alkaline extracts of 7.77 % and 12.85 %, which were higher than water extract of 4.61 % (p < 0.05). However, Braspaiboon et al. (2020) reported that water extracts more proteins from plants than acid and alkaline, which was contrary to the results of the present study.
The uronic acid content of jujube fruit polysaccharides was 31.56 % and 40.21 % for acid and alkaline extraction, respectively, which was higher than 26.71 % for water extraction (p < 0.05) (Table 1). Similar results were obtained for uronic acid content of the pandahai polysaccharides, with 38.57 % and 46.73 % for acidic and alkaline extracts higher than water-extracted 35.47 % (p < 0.05). However, the uronic acid content of the pandahai polysaccharides contradicted the findings of a previous study (Ai et al., 2012). The results showed that different extraction methods (pH) significantly affected uronic acid content, while the acid extraction method contributed to the cleavage of polysaccharide glycosidic bonds (Devasvaran et al., 2023; Kou et al., 2022; Yao et al., 2017). Specifically, acids and alkalis degrade plant cell walls, disrupting the ester and hydrogen bonds between polysaccharides, and facilitating the release and solubilization of polysaccharides during extraction (Chen et al., 2020). In addition, the extraction of polysaccharides (temperature and acidity values) was positively correlated with the degradation rate, while the monosaccharide composition varied (Chen et al., 2020) and the ratio of neutral sugars to uronic acid was higher (Lu, 2023). Notably, different extraction techniques can lead to different polysaccharide compositions, providing various biological activities (such as antioxidant activity) (Zhou et al., 2008).
Similarly, several studies have reported that manipulating polysaccharide extraction methods and decolorization and decontamination process affect the biological activities that alter the structural properties (viscosity, chemical composition, molecular weight distribution, molecular conformation, and morphology) (Gao et al., 2023; Ji et al., 2020a; Kou et al., 2022; Leong et al., 2021; Rosales & Fabi, 2023; Song et al., 2023; Tao et al., 2023; Wang et al., 2021; Wu et al., 2007b; Wu et al., 2012; Xu et al., 2022; Zhang et al., 2023a; Zhang et al., 2023b; Zhao et al., 2021). This study analyzed the molar ratios of monosaccharide components in polysaccharides and showed that water-extracted polysaccharides extracted from jujube fruit were Gal, Man, Glu, Ara, Xyl, and Rha = 11.41: 23.37: 39.69: 20.18: 2.00: 3.35 (p < 0.05) (Table 1). Notably, some monosaccharides (such as Gal, Xyl, and Rha) were not detected with either acidic or alkaline extraction, indicating that the monosaccharide components of the polysaccharide extracts of jujube fruits differed between the extraction methods. In contrast, the components of pandahai water-extracted polysaccharides were 7.01 Gal, 46.49 Man, and 17.58 Xyl (p < 0.05) (Table 1), whereas acidic- and alkaline-extracted components revealed more Xyl and less Glu and Rha (negligible), while Ara was not detected in any of the extraction methods. However, the components differed slightly from those reported by Chang et al. (2010) and Wu et al. (2012), and were presumably influenced by differences in origin or extraction conditions. According to Ji et al., (2020a) and Ji et al., (2020b), polysaccharides derived from jujube were separated into three distinct monosaccharide components of varying molar ratios. Notably, these components were comparable to those observed in the present study, which was attributed to differences in the cultivar, cultivation region, and extraction method. Notably, the level of polysaccharides remains far from optimum for regular extraction processes, with subsequent purification and acidic hydrolysis being adopted to obtain a fine structure (Zhang et al., 2023a). Subsequently, jujube fruit and pandahai polysaccharides had total carbohydrate contents close to 95 % which were higher than the original polysaccharides in this study after purifying water-extracted polysaccharides by GPC and acid hydrolysis for 2–6 h (Table 1). However, the protein content of all groups decreased and was negatively correlated with the acid hydrolysis time (p < 0.05). Therefore, GPC purification and acid hydrolysis effectively remove proteins or complexes covalently bound to polysaccharides to obtain biologically active glycoproteins or peptide polysaccharides (Zhang et al., 2007). Similar decreases were observed in uronic acid content. We hypothesized that acid hydrolysis may reduce the carboxyl group of uronic acid (COO–) by combining hydrogen ions (H+) with uncharged –COOH under acidic conditions. It is possible that the acidic conditions led to the breakage of the polysaccharide glycosidic bonds, which were removed during dialysis via the diffusion effect. In addition, the monosaccharide components of jujube fruits and pandahai polysaccharides changed significantly after acid hydrolysis compared to the original polysaccharides. The monosaccharide composition of jujube fruits showed an increase in Man content depending on the hydrolysis time, whereas Gal, Xyl, and Rha contents increased after 2 h of acid hydrolysis but decreased inexplicably and significantly after 4–6 h (Table 1). However, Glu and Ara contents decreased and were negatively correlated with the hydrolysis time. In contrast, the monosaccharide components of pandahai polysaccharides, such as Gal, Man, Glu, and Rha, increased, followed by a decrease with hydrolysis time, while Ara was positively correlated with hydrolysis time, which was opposite to Xyl (Table 1). Therefore, 6 h of acidic hydrolysis increased the Gal, Glu, Ara, and Rha content.
Overall, the pandahai polysaccharide components are similar to those reported by Ai et al. (2012) and Oppong et al. (2018). Moreover, Li et al. (2011) showed that the uronic acid content of water-soluble polysaccharides from jujube also decreased after hydrolysis with TCA. Notably, large molecules are not available for microbial utilization and metabolism, whereas small molecules (monosaccharides or oligosaccharides) facilitate the proliferation of Bifidobacteria (Gibson, 1999).
3.3 Antioxidant capacity evaluation of polysaccharides with different extractions
The DPPH radical scavenging capacity has been frequently used to evaluate polysaccharide antioxidants in vitro (Ji et al., 2020a). Chang et al. (2010) reported that the galacturonic acid content in polysaccharides exhibits a dose-dependent relationship with DPPH free radical scavenging capacity. However, molecular weight is reportedly negatively correlated with antioxidant capacity; in particular, small oligogalacturonide molecules (1 kDa) performed satisfactorily (Huang et al., 2011). It is widely accepted that low-molecular-weight compounds exhibit greater efficacy in their interactions with free radicals (Zhang et al., 2023b).
In this study, EC50 of DPPH free radical scavenging ability showed that the polysaccharides of jujube fruits in water, acidic, and alkaline extracts were 2.06, 0.82, and 0.85 mg/mL (p < 0.05), respectively (Table 2), which were consistent with previously published studies (Ji et al., 2020a; Wu et al., 2007b). EC50 of pandahai polysaccharides were 5.66, 2.46, and 2.78 mg/mL (p < 0.05) (Table 2). This suggests that acidic and alkaline-extracted jujube fruits and pandahai polysaccharides were approximately two-fold better than water extracted, but lower than BHT (control group). Therefore, we predicted that the reaction rate coefficient (k) of the DPPH free radical scavenging ability of jujube fruits and pandahai polysaccharides was significantly weaker than that of BHT (p < 0.05), with no significant difference between the extraction methods. Moreover, the jujube fruit and pandahai polysaccharides served as suitable electron donors, which would reduce free radicals and achieve the chain reaction of the termination of free radicals. Lowercase letters with different superscripts in same column indicate significant differences (p < 0.05).
DPPH radical scavenging power
Ferric reducing antioxidant power (FRAP)
EC50 (mg/mL)
k
EC50 (mg/mL)
k
Jujube fruits
Water
2.06 ± 0.15d
19.36c
5.15 ± 0.02c
96.37b
Acidic
0.82 ± 0.04e
20.49b
3.63 ± 0.06e
95.60c
Alkaline
0.85 ± 0.05e
20.47b
1.09 ± 0.02f
96.04b
Pandahai
Water
5.66 ± 0.77a
11.64d
8.93 ± 0.17a
31.34d
Acidic
2.46 ± 0.01c
11.66d
5.65 ± 0.14b
31.40d
Alkaline
2.78 ± 0.22b
11.45d
4.71 ± 0.02d
31.41d
BHT
0.25 ± 0.00f
182.64a
–
–
EDTA
–
–
0.26 ± 0.00 g
163.14a
Alkaline-extracted polysaccharides of jujube fruits (EC50 of 1.09 mg/mL, and k of 96.04) from FRAP assay were similar to those as reported by Chang et al. (2010) and Ji et al., (2020a), while pandahai (EC50 of 4.71 mg/mL and k of 31.41) performed substantially better than others (p < 0.05) yet inferior to the control group (EDTA; EC50 of 0.26 mg/mL and k of 163.14). Although a positive correlation has been reported between uronic acid content and FRAP (Chang et al., 2010), this was not observed in the present study. Overall, the antioxidant capacity of polysaccharides from jujube fruits was superior to that of pandahai fruits, albeit lower than that of BHT and EDTA. In addition, acidic- and alkaline-extracted polysaccharides may have their different active groups, contributing to different antioxidant capacities.
3.4 Effects of various temperatures and concentrations on the viscosity of polysaccharides
The viscosity of the polysaccharide extracted from jujube fruit water was not significantly different from 5,100–5,500 cps at 50–80 °C, but viscosity significantly decreased to 4,000 cps at 90 °C (p < 0.05) (Fig. 1). Additionally, 16 % at 50 °C–70 °C and 20 % at 50 °C–80 °C showed the highest viscosities outside the measurable range while acid-extracted polysaccharides showed the same 16 % and 20 % concentration viscosities at 50 °C and 50 °C–60 °C; alkaline-extracted polysaccharides revealed no such phenomenon. Interestingly, the viscosities of the pandahai polysaccharide extracts followed similar trends. However, the viscosities of the jujube fruit and pandahai polysaccharides were dose-dependent on the concentration of polysaccharides, ranging from 60 to 5,500 cps. The viscosities of the polysaccharides were negatively correlated with temperature. This may be attributed to the swelling state that seems to be exhibited in these polysaccharide solutions at high temperatures, and the results agreed with those of Ai et al. (2012). In addition, the viscosities were in the following order: water > acidic > alkaline extraction; these results were consistent with previous findings (Ai et al., 2012). In this study, jujube fruits and pandahai polysaccharides exhibited Newtonian fluid properties, which were hypothesized to be related to their molecular weights. The higher the molecular weight, the more flexible is the chain, leading to a higher degree of pseudo-plasticity (Wang et al., 2021). Additionally, the branching of polysaccharides is critical for enhancing solution viscosity (Bai et al., 2017).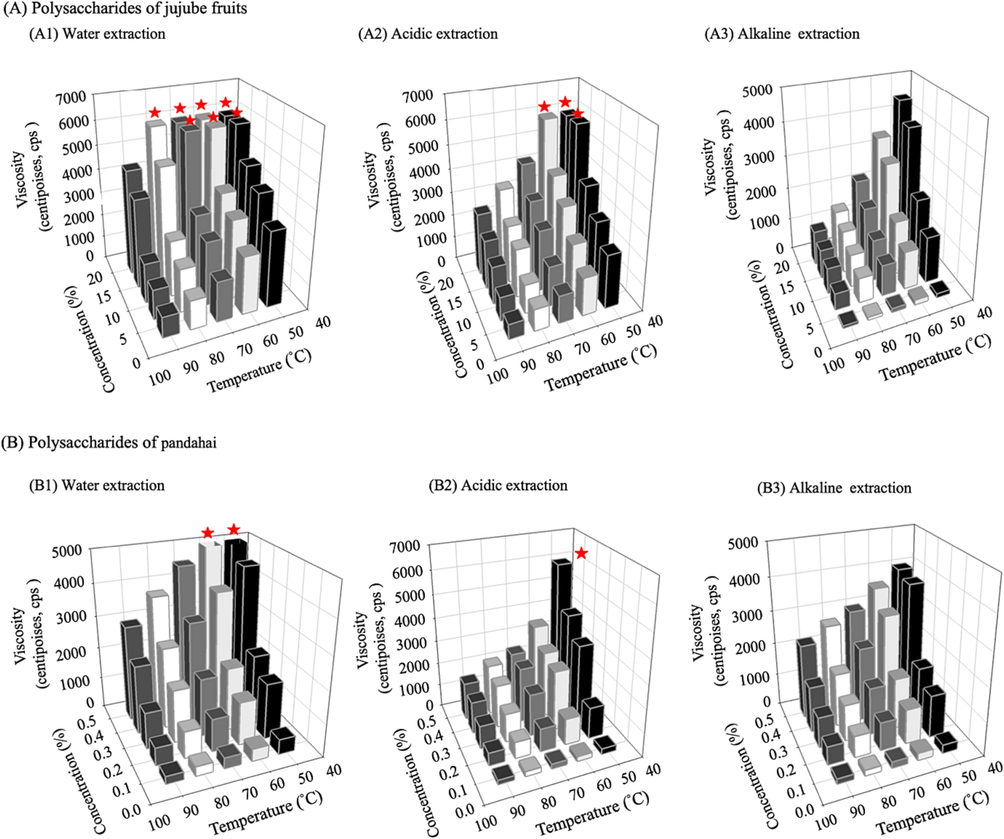
Effects of temperature and concentration on viscosity of jujube (Zizyphus jujuba Mill.) and pandahai (Sterculia lychnophora Hance) crude extracted polysaccharides using different treatments.
3.5 Molecular weight distribution of polysaccharides
The molecular weight of a polysaccharide is a critical factor affecting its biological activities and is essential for structural analyses (Zhang et al., 2023a). The molecular weight distribution of polysaccharides extracted from jujube fruits and pandahai hydrolyzed with 2 M HCl for 2, 4, and 6 h showed that the initial jujube molecular weights (Fig. 2A1) were in the estimated range of 22,800––112,000 Da, similar to previous studies (Chang et al., 2010). The molecular weight range of pandahai is 788,000–2,000,000 Da (Fig. 2B1). As expected, both polysaccharides had smaller molecular weights and broader distributions after acidic hydrolysis (Fig. 2A2–A4 and B2–B4). In contrast, the small molecule distribution increased with the acid hydrolysis time. Chen et al. (2020) reported that smaller polysaccharide chains are more likely to pass through biofilms and can be more effective without provoking an immune stress response.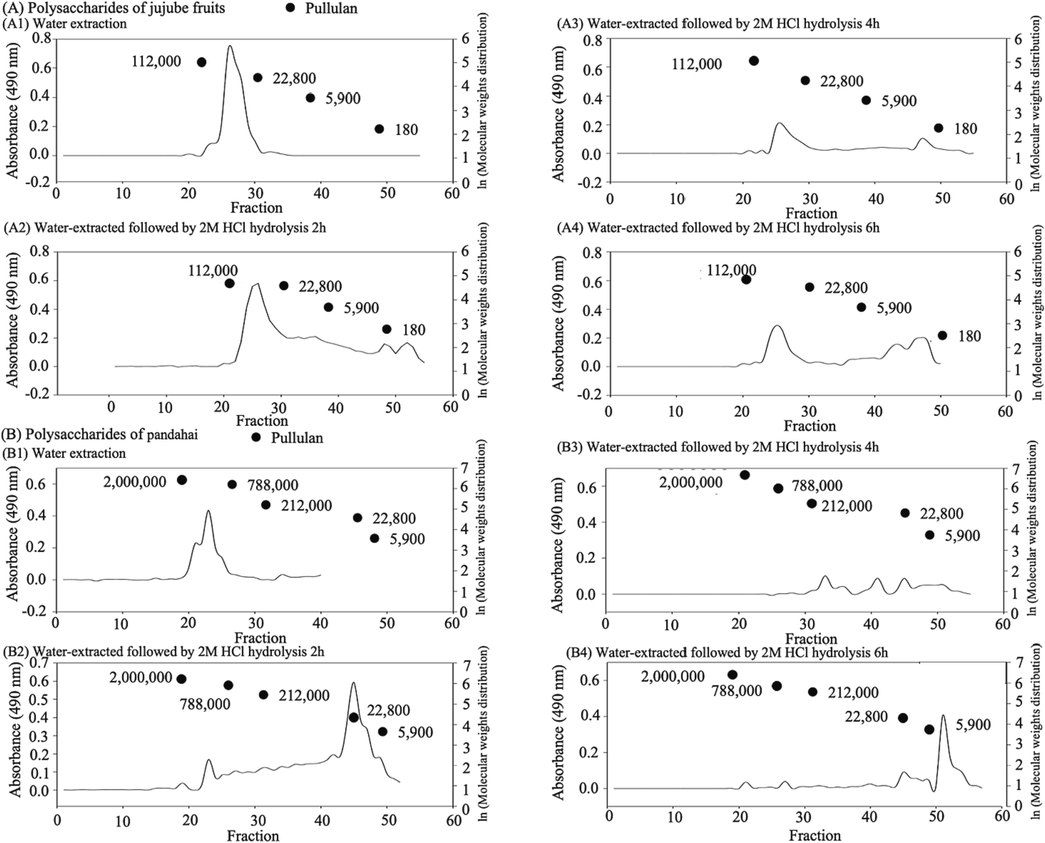
Molecular weight distribution of jujube (Zizyphus jujuba Mill.) and pandahai (Sterculia lychnophora Hance) water-extracted crude polysaccharides after acid hydrolysis.
3.6 Polysaccharide structures analyzed by FTIR
Four samples of jujube fruit polysaccharides and acidic hydrolyzed polysaccharides were analyzed using FTIR. The results showed that all groups exhibited an absorption peak at 3,200–3,600 cm−1 wavelength, corresponding to a hydroxyl group. The peak was most significant after 2 h of acidic hydrolysis and decreased with increasing hydrolysis time (Fig. 3A). All groups exhibited C—H functional groups with aldehyde stretching and vibration at 2,850–2,950 cm−1, similar to those reported by Xie et al. (2023) and Xu et al. (2022). In addition, polysaccharides from jujube fruit and the acidic hydrolyzed 2 h groups revealed an aromatic nitrile (R—C≡N; Csp3—H) functional group at 2,300–2,400 cm−1 (Patra et al., 2023; Zhang et al., 2023b), while the 4 h and 6 h acidic hydrolyzed groups lacked this group. Therefore, R-C≡N disappeared with hydrolysis time. Polysaccharides of jujube fruits show the stretching vibration peak of C⚌O at 1,730–1,760 cm−1 wavelength. The peaks of esterified/reduced carboxyl groups (COOCH3) and free carboxyl groups (COO—) were detected at 1,600–1,630 cm−1 (Ji et al., 2020b; Kou et al., 2022; Xie et al., 2023; Zeng et al., 2015; Zhang et al., 2023b), whereas only reduced/free carboxyl groups were detected in the three groups at 2–6 h of acidic hydrolysis. The absorption peaks were detected in all groups at 1,400–1,600 cm−1, which were for C—O stretching vibrations, and the significant absorption peaks between 1,010–1,095 cm−1 indicate the possible presence of pyranose rings (Chang et al., 2010; Ji et al., 2020b; Nie et al., 2018; Xie et al., 2023; Xu et al., 2022; Zhang et al., 2023b), with the maximum peak at 2 h of acidic hydrolysis. The results of this study indicated that acid hydrolysis induced the conversion of reduced carboxyl groups to free groups, whereas acidic hydrolysis increased the reduced/free carboxyl group peaks in all three groups from 2 to 6 h. However, the number of free carboxyl groups decreased with increasing hydrolysis time. These results agree with the changes in uronic acid content. We hypothesized that the reduced free carboxyl groups may have caused the formation of free carboxyl groups by the hydroxyl ions.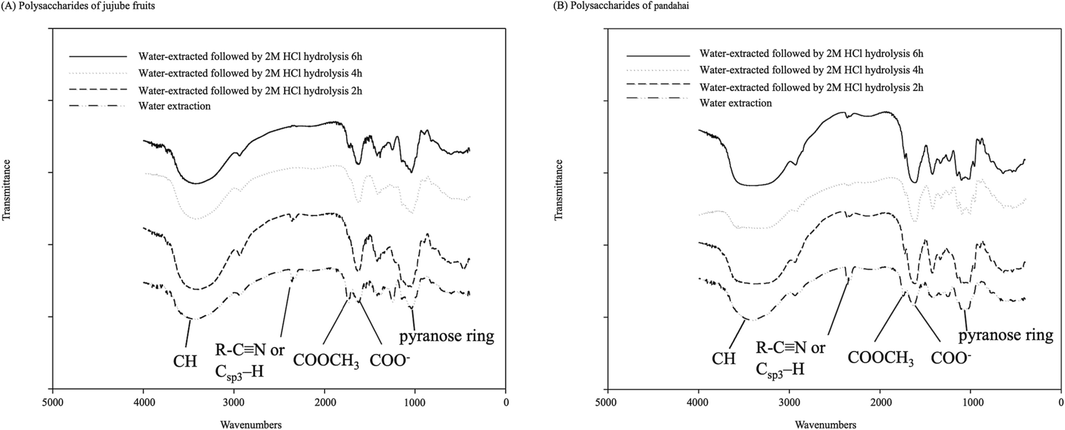
Fourier-transform infrared spectra of jujube fruit (Zizyphus jujuba Mill.) and pandahai (Sterculia lychnophora Hance) water-extracted crude polysaccharides and acid-hydrolyzed polysaccharides.
Moreover, FTIR results of pandahai and its acidic hydrolyzed polysaccharides exhibited the same trend. The absorption peaks of the hydroxyl groups of each group appeared at 3,200–3,600 cm−1 wavelength, whereas the C—H functional group of the aldehyde stretching vibration appeared at 2,850–2,950 cm−1 (Fig. 3B). However, peaks of the R—C≡N functional group at 2,300–2,400 cm−1 decreased significantly during acidic hydrolysis in all three acid-hydrolyzed groups compared to the polysaccharide group. Similarly, the esterified carboxyl groups (1,600–1,630 cm−1 wavelength) of pandahai polysaccharide increased with acidic hydrolysis, whereas C⚌O (1,730–1,760 cm−1 wavelengths) decreased with acidic hydrolysis. The pyranose rings (1,010–1,095 cm−1) peaked at 2 h of acidic hydrolysis, all consistent with the above jujube fruit measurements.
3.7 In vitro effects on proliferation of intestinal bacteria
The gut microbiota has multiple physiological roles; beneficial bacteria (Lactobacillus and Bifidobacterium) facilitate gastrointestinal health, prevent infections, and treat diarrhea (Chen et al., 2023; Tsai et al., 2023; Xu et al., 2023). Several reports have been published concerning the crucial role of the gut-brain axis in the health of the host, which affects the physiological functions of the brain and nervous system, such as immune and endocrine functions (Bostick et al., 2022; Chen et al., 2023; Tsai et al., 2023; Xu et al., 2023). Therefore, the gut microbial ecosystem is a complex network of bacteria that positively and negatively affects human health (Huang & Yeh, 2022).
In this study, the effects of the molecular weight of polysaccharides on the growth of five known strains of intestinal bacteria (four strains of probiotic bacteria, Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus delbrueckii, and Propionibacterium freudenreichir; one strain of the harmful bacterium Clostridium perfringens) were evaluated using extracted and acid-hydrolyzed polysaccharides (10 mg/mL) from jujube fruits and pandahai. These results showed that the three groups of jujube fruit increased the proliferation of B. bifidum and P. freudenreichir by ∼1–4 log CFU/mL compared to the control group (extracted polysaccharides) during 0–72 h of incubation (p < 0.05) (Table 3). Acidic hydrolysis for 2–4 h during the 24 h incubation increased the proliferation of these two probiotic bacteria up to 10 log CFU/mL compared to the control group and those acidic-hydrolyzed for 6 h (p < 0.05). However, after incubation with Bifidobacterium longum and Lactobacillus delbrueckii, the highest proliferation capacity was achieved at 48 h (Table 4). Unfortunately, the four kinds of jujube fruit polysaccharides in this study exhibited no inhibitory effect against Clostridium perfringens, while the group acid-hydrolyzed for 2 h experienced the lowest bacterial count at 0 h (6.90 ± 0.18 log CFU/mL). It is plausible that this outcome is affected by sampling error. Lowercase letters with different superscripts in same column indicate significant differences (p < 0.05). Superscripted capital letters indicate significant differences between the groups (p < 0.05). Lowercase letters with different superscripts in the same column indicate significant differences (p < 0.05). Superscripted capital letters indicate significant differences between the groups (p < 0.05).
Sample
Strain
Treatments
Co-culture time (h)/Unit: log CFU/mL
0
12
24
48
72
Jujube fruits
Bifidobacterium bifidum
Control
6.27 ± 0.07jJ
7.17 ± 0.16hI
8.78 ± 0.02dE
8.67 ± 0.08dE
7.63 ± 0.16 g
Water-extracted followed by 2 M HCl hydrolysis at
2 h
6.03 ± 0.04jK
8.08 ± 0.07eF
10.07 ± 0.02bB
9.79 ± 0.01cC
8.62 ± 0.10dE
4 h
6.29 ± 0.03 kJ
8.06 ± 0.17eF
10.20 ± 0.08aB
9.70 ± 0.13cC
8.64 ± 0.24dE
6 h
6.42 ± 0.01iJ
7.84 ± 0.45efFG
9.31 ± 0.05cC
9.50 ± 0.17cC
8.15 ± 0.26fF
Bifidobacterium longum
Control
6.51 ± 0.19gJ
7.17 ± 0.20eI
8.89 ± 0.02aE
8.52 ± 0.05bE
7.89 ± 0.14dG
Water-extracted followed by 2 M HCl hydrolysis at
2 h
6.31 ± 0.14hJ
6.97 ± 0.17fI
8.64 ± 0.04bE
8.74 ± 0.12aE
8.31 ± 0.01cE
4 h
6.27 ± 0.24hJ
7.02 ± 0.13eI
8.73 ± 0.05aE
8.84 ± 0.26aE
8.22 ± 0.19cEF
6 h
6.74 ± 0.06gI
7.08 ± 0.07eI
8.52 ± 0.07bE
8.57 ± 0.21bE
8.47 ± 0.08bE
Lactobacillus delbrueckii
Control
7.31 ± 0.48fH
8.17 ± 0.10eF
8.62 ± 0.04cE
8.94 ± 0.14bD
8.68 ± 0.06cE
Water-extracted followed by 2 M HCl hydrolysis at
2 h
7.48 ± 0.10fH
8.21 ± 0.11eF
8.61 ± 0.07cE
9.42 ± 0.12aCD
8.77 ± 0.08cE
4 h
7.31 ± 0.15fH
8.20 ± 0.17eF
8.52 ± 0.12cdE
9.03 ± 0.11bD
8.42 ± 0.10dEF
6 h
7.26 ± 0.09gH
8.22 ± 0.12eF
8.62 ± 0.05cE
9.36 ± 0.08aC
8.69 ± 0.05cE
Propionibacterium freudenreichir
Control
5.58 ± 0.34hL
6.83 ± 0.13gI
8.72 ± 0.11dE
8.55 ± 0.13eE
7.43 ± 0.38fgH
Water-extracted followed by 2 M HCl hydrolysis at
2 h
5.66 ± 0.22hL
7.85 ± 0.11fG
9.84 ± 0.06bC
8.77 ± 0.07dE
7.92 ± 0.03fG
4 h
5.63 ± 0.01hL
7.83 + 0.12fG
10.09 ± 0.12aB
9.26 ± 0.24cD
7.80 ± 0.18fG
6 h
5.42 ± 0.01hL
7.84 ± 0.45fG
9.31 ± 0.05cC
8.79 ± 0.17dE
8.15 ± 0.26eF
Clostridium perfringens
Control
7.27 ± 0.34eHI
8.28 ± 0.14cF
9.29 ± 0.19bD
8.14 ± 0.21cF
7.29 ± 0.14eH
Water-extracted followed by 2 M HCl hydrolysis at
2 h
6.90 ± 0.18gI
8.30 ± 0.03cF
9.52 ± 0.32aC
7.87 ± 0.18dH
7.36 ± 0.08eH
4 h
7.21 ± 0.06efI
8.23 ± 0.20cF
9.59 ± 0.03aD
7.26 ± 0.24eHI
7.10 ± 0.17eI
6 h
7.14 ± 0.18fHI
8.17 ± 0.36cF
9.43 ± 0.23aD
7.78 ± 0.14dH
7.15 ± 0.14eHI
Sample
Strain
Treatments
Co-culture time (h)/Unit: log CFU/mL
0
12
24
48
72
Pandahai
Bifidobacterium bifidum
Control
6.13 ± 0.08jK
7.28 ± 0.17hI
9.03 ± 0.15dE
8.54 ± 0.14eF
7.52 ± 0.27ghH
Water-extracted followed by 2 M HCl hydrolysis at
2 h
6.18 ± 0.07jK
8.07 ± 0.07fG
11.17 ± 0.28aA
9.89 ± 0.27cC
8.51 ± 0.21eF
4 h
6.26 ± 0.05iJ
7.96 ± 0.27fG
11.27 ± 0.34aA
9.67 ± 0.16cC
8.53 ± 0.35eF
6 h
6.34 ± 0.04iK
8.03 ± 0.18fFG
10.11 ± 0.18bB
9.30 ± 0.23dCD
8.26 ± 0.37efF
Bifidobacterium longum
Control
6.13 ± 0.08iJ
6.80 ± 0.04gJ
8.35 ± 0.04cF
9.24 ± 0.04aCD
7.73 ± 0.09eH
Water-extracted followed by 2 M HCl hydrolysis at
2 h
6.02 ± 0.11iK
6.52 ± 0.05hJ
8.41 ± 0.03cF
9.27 ± 0.17aC
8.45 ± 0.08cF
4 h
6.29 ± 0.29hiJK
6.76 ± 0.04gJ
8.38 ± 0.15cF
9.34 ± 0.24aC
8.18 ± 0.14cdFG
6 h
6.17 ± 0.06iK
7.07 ± 0.40fI
8.09 ± 0.12dFG
8.96 ± 0.15bE
7.80 ± 0.07deH
Lactobacillus delbrueckii
Control
7.13 ± 0.48eI
8.71 ± 0.13bcF
9.02 ± 0.41abE
9.24 ± 0.41aC
8.86 ± 0.12bcE
Water-extracted followed by 2 M HCl hydrolysis at
2 h
7.28 ± 0.12eI
8.84 ± 0.17bcF
9.01 ± 0.73abE
9.42 ± 0.21aC
8.64 ± 0.16cF
4 h
7.43 ± 0.15eI
8.40 ± 0.71bcdF
8.92 ± 0.21bE
9.36 ± 0.33aC
8.24 ± 0.20dFG
6 h
7.37 ± 0.08eI
8.55 ± 0.21cdF
9.12 ± 0.52abE
9.48 ± 0.84aC
8.96 ± 0.10bE
Propionibacterium freudenreichir
Control
6.78 ± 0.34hI
7.93 ± 0.13fG
9.72 ± 0.11aC
7.86 ± 0.09fG
7.30 ± 0.24gHI
Water-extracted followed by 2 M HCl hydrolysis at
2 h
6.56 ± 0.22hiIJ
7.85 ± 0.11fG
9.84 ± 0.06aC
8.77 ± 0.07dE
7.92 ± 0.03fG
4 h
6.73 ± 0.01hI
7.73 ± 0.12fgG
9.09 ± 0.12cD
8.25 ± 0.10eF
7.43 ± 0.14gH
6 h
6.62 ± 0.01iIJ
7.64 ± 0.45fgGI
9.31 ± 0.05bC
7.71 ± 0.12fgC
7.49 ± 0.27gH
Clostridium perfringens
Control
7.85 ± 0.13fG
8.05 ± 0.12eFG
9.07 ± 0.07cD
7.36 ± 0.06hC
7.20 ± 0.24hiI
Water-extracted followed by 2 M HCl hydrolysis at
2 h
7.96 ± 0.02fG
8.25 ± 0.22deF
9.46 ± 0.24bC
7.57 ± 0.11gC
7.12 ± 0.27hiI
4 h
8.12 ± 0.16eFG
8.57 ± 0.14dF
9.79 ± 0.13aC
7.52 ± 0.10gC
7.23 ± 0.12hiI
6 h
8.04 ± 0.21deFG
8.59 ± 0.32dF
9.76 ± 0.21aC
7.26 ± 0.22ghI
7.13 ± 0.15iI
Moreover, in the four groups of pandahai polysaccharides, the maximum bacterial counts were achieved after 24 h of incubation with B. bifidum and P. freudenreichir, among which the three groups of acidic hydrolyzed pandahai polysaccharides showed the best performance, with maximum bacterial counts ranging from 10.11 to 11.27 log CFU / mL (p < 0.05) (Table 4). We hypothesized that the molecular weight, structure, and primary branched-chain monosaccharide composition may be related to their suitability for the growth and utilization of B. bifidum and P. freudenreichir. In addition, the maximum bacterial counts of 8.96–9.48 log CFU/mL (p < 0.05) were achieved by all groups of B. longum and L. delbrueckii cultivated for 48 h. Notably, pandahai polysaccharides exhibited similar results as jujube fruit polysaccharides in the proliferation of the four strains of probiotic bacteria, whereas no inhibitory effect was observed on the harmful bacterium C. perfringens. Therefore, according to these results, 2–4 h of acid hydrolysis of polysaccharides by jujube fruits and pandahai used in the present study resulted in better performance and beneficial effects of the beneficial bacterial substances to promote the proliferation of B. bifidum, B. longum, L. delbrueckii, and P. freudenreichir in cultures from 0 to 72 h. However, the molecular weights and compositions of the main branched polysaccharides chains were correlated. Notably, Yang et al. (2023) reported that the addition of 0.05 %–0.2 % Potentilla anserine polysaccharide during yogurt fermentation favors the maintenance of viable counts of beneficial bacteria in laboratory-scale yogurt; however, the difference may not be industrially significant when the fermentation results are studied in detail. This phenomenon may be associated with lactic acid bacteria obtaining a carbon source from lactose or glucose more readily than from polysaccharides (Aljewicz et al., 2020). Overall, this could be attributed to the acid-hydrolyzed polysaccharides from jujube fruits and pandahai being close to oligosaccharides in molecular weight distribution, facilitating the proliferation of Bifidobacteria spp. Hence, the probiotic properties of jujube fruits and pandahai require further confirmation through gastrointestinal simulations or animal studies.
4 Conclusions
According to the results of this study, the extraction of polysaccharides from jujube fruit and pandahai using acidic and alkaline methods produced a higher polysaccharide yield than water extraction, increasing the uronic acid and protein contents while improving antioxidant capacity, including DPPH radical scavenging ability and FRAP. The viscosities of the two polysaccharides showed the same trend and were dose-dependent, with viscous properties observed at 50–80 °C. The water-extracted polysaccharides from jujube fruit and pandahai have broader thermal processing conditions. In addition, the monosaccharide composition of jujube fruits and pandahai polysaccharides among the three extraction methods included Gal, Man, Glu, Ara, Xyl, and Rha. However, the ratio of polysaccharides to acid-extracted polysaccharides differed significantly (p < 0.05). The jujube fruit and pandahai-acid-hydrolyzed polysaccharides contained smaller molecules and more carbohydrates, thus improving their biological activities. FTIR spectroscopy confirmed that the functional group composition of polysaccharides and acid-hydrolyzed jujube fruits and pandahai changed significantly. Finally, in vitro, the proliferative effects of adding 10 mg/mL jujube fruit and pandahai acid-hydrolyzed polysaccharides for 2–4 h on four known strains of beneficial bacteria were demonstrated. Therefore, this study suggests the development of functional foods, contributing to the expansion of functional food ingredients and providing a polysaccharide extraction method for jujube fruit and pandahai.
Acknowledgments
This research was financially supported by Taichung Veterans General Hospital/ Providence University Joint Research Program, (TCVGH-PU-1138102), Taiwan. This research was also supported by the National Science and Technology Council (NSTC) under Project 112-2622-B-126-001.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AACC, 2023. Approved methods of analysis, 11th Ed. Method 44-11.01, 46-10.01, 30-10.01, 32-05.01, and 08-01.01. In. St. Paul, MN, USA: Cereals & Grains Association.
- An updated review of Ziziphus jujube: major focus on its phytochemicals and pharmacological properties. Pharmacol. Res. - Modern Chin. Med.. 2023;8:100297
- [CrossRef] [Google Scholar]
- Extraction, partial characterization and bioactivity of polysaccharides from boat-fruited sterculia seeds. Int. J. Biol. Macromol.. 2012;51(5):815-818.
- [CrossRef] [Google Scholar]
- A Comprehensive study of the impacts of oat β-glucan and bacterial curdlan on the activity of commercial starter culture in yogurt. Molecules. 2020;25(22):5411.
- [CrossRef] [Google Scholar]
- Studies on water soluble polysaccharides from Pithecellobium dulce (Roxb.) Benth. seeds. Carbohydr. Polym.. 2016;138:215-221.
- [CrossRef] [Google Scholar]
- Impact of polysaccharide molecular characteristics on viscosity enhancement and depletion flocculation. J. Food Eng.. 2017;207:35-45.
- [CrossRef] [Google Scholar]
- Gut microbiome-mediated regulation of neuroinflammation. Curr. Opin. Immunol.. 2022;76:102177
- [CrossRef] [Google Scholar]
- Comparison of the effectiveness of alkaline and enzymatic extraction and the solubility of proteins extracted from carbohydrate-digested rice. Heliyon. 2020;6(11):e05403.
- [Google Scholar]
- Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol.. 2010;47(4):445-453.
- [CrossRef] [Google Scholar]
- A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol.. 2020;151:635-649.
- [CrossRef] [Google Scholar]
- A three-arm, randomized, double-blind, placebo-controlled study to evaluate the safety of Lactobacillus salivarius AP-32 and Bifidobacterium animalis CP-9 used individually in healthy infants. Nutrients. 2023;15(15):3426.
- [CrossRef] [Google Scholar]
- Characterization and antibacterial properties of fish skin gelatin/guava leaf extract bio-composited films incorporated with catechin. LWT. 2023;178:114568
- [CrossRef] [Google Scholar]
- Potential antidepressant effects of a dietary supplement from Huáng qí and its complex in aged senescence-accelerated mouse prone-8 mice. Front. Nutr.. 2023;10
- [CrossRef] [Google Scholar]
- Constales, D., Yablonsky, G.S., D’hooge, D.R., Thybaut, J.W., Marin, G.B., 2017. Chapter 9 - Experimental data analysis: Data processing and regression. In D. Constales, G. S. Yablonsky, D. R. D’hooge, J. W. Thybaut, & G. B. Marin (Eds.), Advanced Data Analysis & Modelling in Chemical Engineering. Elsevier. pp. 285-306. https://doi.org/10.1016/B978-0-444-59485-3.00009-6.
- Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal. Biochem.. 2020;608:113904
- [CrossRef] [Google Scholar]
- Optimisation of the extraction of crude polysaccharides from Clinacanthus nutans leaves for antioxidant applications: Content analysis, chemometrics and metabolomics analysis. Ind. Crop. Prod.. 2023;202:117086
- [CrossRef] [Google Scholar]
- Non-alcoholic fatty liver diseases: from role of gut microbiota to microbial-based therapies. Eur. J. Clin. Microbiol. Infect. Dis.. 2020;39(4):613-627.
- [CrossRef] [Google Scholar]
- Preparation, structure, and biological activities of the polysaccharides from fruits and vegetables: a review. Food Biosci.. 2023;54:102909
- [CrossRef] [Google Scholar]
- Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J. Nutr.. 1999;129(7):1438S-1441S.
- [CrossRef] [Google Scholar]
- Antioxidant activity and emulsion-stabilizing effect of pectic enzyme treated pectin in soy protein isolate-stabilized oil/water emulsion. J. Agric. Food Chem.. 2011;59(17):9623-9628.
- [CrossRef] [Google Scholar]
- Application of egg white hydrolysate (EWH) to improve frothing functionality of pasteurized liquid egg in large quantity production. Heliyon. 2023;9(1):e12697.
- [Google Scholar]
- Gut microbiota in Kawasaki disease. In: Kuo H.-.-C., ed. Kawasaki Disease. Springer Nature Singapore; 2022. p. :181-195.
- [CrossRef] [Google Scholar]
- Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol.. 2020;149:1008-1018.
- [CrossRef] [Google Scholar]
- Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol.. 2020;147:844-852.
- [CrossRef] [Google Scholar]
- From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332-1345.
- [CrossRef] [Google Scholar]
- Structural properties and hypoglycaemic activity of polysaccharides extracted from the fruits of Lycium barbarum L. using various extraction media. Ind. Crop. Prod.. 2022;188:115725
- [CrossRef] [Google Scholar]
- Extraction of polysaccharides from edible mushrooms: emerging technologies and recent advances. Carbohydr. Polym.. 2021;251:117006
- [CrossRef] [Google Scholar]
- Bioresource utilization of djulis (Chenopodium formosanum) biomass as natural antioxidants. Sustainability. 2020;12(15):5926.
- [CrossRef] [Google Scholar]
- Isolation, purification and structure of a new water-soluble polysaccharide from Zizyphus jujuba cv. Jinsixiaozao. Carbohydr. Polym.. 2011;83(2):477-482.
- [CrossRef] [Google Scholar]
- Extraction, purification, separation, structure, derivatization and activities of polysaccharide from Chinese date. Process Biochem.. 2021;110:231-242.
- [CrossRef] [Google Scholar]
- Polysaccharides from Tumorous stem mustard prevented high fructose diet-induced non-alcoholic fatty liver disease by regulating gut microbiota, hepatic lipid metabolism, and the AKT/FOXO1/MAPK signaling pathway. J. Funct. Foods. 2023;102:105448
- [CrossRef] [Google Scholar]
- Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol.. 2018;111:780-786.
- [CrossRef] [Google Scholar]
- Changes in the structure of polysaccharides under different extraction methods. eFood. 2023;4(2):e82.
- [Google Scholar]
- Study on the anti-hyperuricemic bioactivity and chemical components of Sterculiae lychnophorae Semen. J. Funct. Foods. 2022;95:105173
- [CrossRef] [Google Scholar]
- Recent advances in the extraction and characterization of seed polysaccharides, and their bioactivities: a review. Bioact. Carbohydr. Diet. Fibre. 2021;26:100276
- [CrossRef] [Google Scholar]
- Chapter 2 - Methodologies for Studying Bioactive Polysaccharides. In: Nie S., Cui S.W., Xie M., eds. Bioactive Polysaccharides. Academic Press; 2018. p. :51-97.
- [CrossRef] [Google Scholar]
- Total carbohydrate by phenol-sulfuric acid method. In: Nielsen S.S., ed. Food Analysis Laboratory Manual. Springer International Publishing; 2017. p. :137-141.
- [CrossRef] [Google Scholar]
- Ethnopharmacology, phytochemistry, and pharmacology of Sterculia lychnophora Hance (Pangdahai) Chin. J. Nat. Med.. 2018;16(10):721-731.
- [CrossRef] [Google Scholar]
- Secondary metabolites from Sterculia lychnophora Hance (Pangdahai) Biochem. Syst. Ecol.. 2020;92:104125
- [CrossRef] [Google Scholar]
- Kinetic simulations of the effect of antioxidants on the metmyoglobin reactions with hydrogen peroxide and their relevance and application to the Trolox equivalent equivalent antioxidant assay. Int. J. Chem. Kinet.. 2021;53(9):999-1013.
- [CrossRef] [Google Scholar]
- Structural and chemical insights into the prebiotic property of hemicellulosic polysaccharide from Santalum album L. Carbohydr. Polym.. 2023;321:121291
- [CrossRef] [Google Scholar]
- Pectin-based nanoencapsulation strategy to improve the bioavailability of bioactive compounds. Int. J. Biol. Macromol.. 2023;229:11-21.
- [CrossRef] [Google Scholar]
- A review: different extraction techniques of pectin. J. Pharmacognosy Natural Products 2017
- [CrossRef] [Google Scholar]
- Hydrogel loading 2D montmorillonite exfoliated by anti-inflammatory Lycium barbarum L. polysaccharides for advanced wound dressing. Int. J. Biol. Macromol.. 2022;209:50-58.
- [CrossRef] [Google Scholar]
- Soejarto, D., Sydara, K., Elkington, B., Douangdeuane, B., Souliya, O., Xayvue, M., 2023. Chapter 4 Conservation of Medicinal Plants of Laos, pp. 55–84. https://doi.org/10.1201/9781003216636-4.
- Biopharmaceutical applications of microbial polysaccharides as materials: a review. Int. J. Biol. Macromol.. 2023;239:124259
- [CrossRef] [Google Scholar]
- Plant polysaccharides utilized by gut microbiota: new players in ameliorating cognitive impairment. J. Tradit. Complement. Med.. 2023;13(2):128-134.
- [CrossRef] [Google Scholar]
- Polysaccharide decolorization: Methods, principles of action, structural and functional characterization, and limitations of current research. Trends Food Sci. Technol.. 2023;138:284-296.
- [CrossRef] [Google Scholar]
- Suppressive effects of Lactobacillus on depression through regulating the gut microbiota and metabolites in C57BL/6J Mice Induced by Ampicillin. Biomedicines. 2023;11(4):1068.
- [CrossRef] [Google Scholar]
- Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop.. 2019;22(1):230-238.
- [CrossRef] [Google Scholar]
- Chinese quince seed gum: Flow behaviour, thixotropy and viscoelasticity. Carbohydr. Polym.. 2019;209:230-238.
- [CrossRef] [Google Scholar]
- N-Doped hierarchical porous carbon from waste boat-fruited sterculia seed for high performance supercapacitors. RSC Adv.. 2017;7(27):16678-16687.
- [CrossRef] [Google Scholar]
- Physicochemical and rheological properties of crude polysaccharides extracted from Tremella fuciformis with different methods. CyTA – J. Food. 2021;19(1):247-256.
- [CrossRef] [Google Scholar]
- Pectin polysaccharide from Flos Magnoliae (Xin Yi, Magnolia biondii Pamp. flower buds): hot-compressed water extraction, purification and partial structural characterization. Food Hydrocoll.. 2022;122:107061
- [CrossRef] [Google Scholar]
- Structural characterizations and α-glucosidase inhibitory activities of four Lepidium meyenii polysaccharides with different molecular weights. Natural Products Bioprospecting. 2023;13(1):18.
- [CrossRef] [Google Scholar]
- Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem.. 2007;105(4):1599-1605.
- [CrossRef] [Google Scholar]
- Preparation, partial characterization and bioactivity of water-soluble polysaccharides from boat-fruited sterculia seeds. Carbohydr. Polym.. 2007;70(4):437-443.
- [CrossRef] [Google Scholar]
- Structure characteristics and rheological properties of acidic polysaccharide from boat-fruited sterculia seeds. Carbohydr. Polym.. 2012;88(3):926-930.
- [CrossRef] [Google Scholar]
- Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol.. 2019;135:1151-1161.
- [CrossRef] [Google Scholar]
- Characterization of sea buckthorn polysaccharides and the analysis of its regulatory effect on the gut microbiota imbalance induced by cefixime in mice. J. Funct. Foods. 2023;104:105511
- [CrossRef] [Google Scholar]
- Inhibitory effects of a water-soluble jujube polysaccharide against biofilm-forming oral pathogenic bacteria. Int. J. Biol. Macromol.. 2022;208:1046-1062.
- [CrossRef] [Google Scholar]
- Effect of Dendrobium officinale polysaccharides on central nervous system disease: based on gut microbiota. Int. J. Biol. Macromol.. 2023;240:124440
- [CrossRef] [Google Scholar]
- Impact of Potentilla anserine polysaccharide on storage properties of probiotic yak yoghurt. Int. Dairy J.. 2023;141:105585
- [CrossRef] [Google Scholar]
- Advances in understanding of health-promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics. Food Front.. 2020;1(4):398-419.
- [CrossRef] [Google Scholar]
- Hypolipidaemic and antioxidant capacities of polysaccharides obtained from Laminaria japonica by different extraction media in diet-induced mouse model. Int. J. Food Sci. Technol.. 2017;52(10):2274-2281.
- [CrossRef] [Google Scholar]
- Molecular structural characteristics of polysaccharide fractions from Canarium album (Lour.) raeusch and their antioxidant activities. J. Food Sci.. 2015;80(11):H2585-H2596.
- [CrossRef] [Google Scholar]
- Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol.. 2007;18(1):4-19.
- [CrossRef] [Google Scholar]
- Sulfated modification, basic characterization, antioxidant and anticoagulant potentials of polysaccharide from Sagittaria trifolia. Arab. J. Chem.. 2023;16(7):104812
- [CrossRef] [Google Scholar]
- Advance in Morchella sp. polysaccharides: Isolation, structural characterization and structure-activity relationship: a review. Int. J. Biol. Macromol.. 2023;247:125819
- [CrossRef] [Google Scholar]
- Physicochemical properties and biological activities of polysaccharides from the peel of Dioscorea opposita Thunb. extracted by four different methods. Food Sci. Human Wellness. 2023;12(1):130-139.
- [CrossRef] [Google Scholar]
- Comparative study of structural properties and biological activities of polysaccharides extracted from Chroogomphus rutilus by four different approaches. Int. J. Biol. Macromol.. 2021;188:215-225.
- [CrossRef] [Google Scholar]
- Preliminary studies on the chemical characterization and antioxidant properties of acidic polysaccharides from Sargassum fusiforme. J. Zhejiang Univ. Sci. B. 2008;9(9):721-727.
- [CrossRef] [Google Scholar]
Appendix A
See Table A1. Sample compositions expressed as percentages on weight basis (n = 3); *NEF = 100 % - (Crude protein + Crude fat + Crude fiber + Ash); lowercase letters with different superscripts in the same column indicate significant differences (p < 0.05).
Items (%)
Jujube fruits
Pandahai
Moisture
13.15 ± 1.02a
11.18 ± 0.78b
Crude protein
5.01 ± 0.08a
4.12 ± 0.19b
Crude fat
0.47 ± 0.14b
5.29 ± 0.15a
Crude fiber
3.28 ± 0.19b
3.61 ± 0.11a
Ash
1.88 ± 0.01a
1.99 ± 0.02a
Nitrogen-free extract (NFE)*
89.36 ± 0.36a
84.99 ± 0.21b







