Translate this page into:
Characterization of chemical compositions by a GC–MS/MS approach and evaluation of antioxidant activities of essential oils from Cinnamomum reticulatum Hay, Leptospermum petersonii Bailey, and Juniperus formosana Hayata
⁎Corresponding authors. yfding2008@163.com (Youfang Ding), yjliubio@bjfu.edu.cn (Yujun Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
GC–MS/MS was used to characterize chemical components of three rare plant essential oils. Three volatiles were newly identified and bio-functions of four were yet reported previously. DPPH and ABTS antioxidant capacities of the three rare plant essential oils were evaluated. CREO had the most moderate antioxidant capacities due to its more esters and less aromatics.
Abstract
Essential oils (EOs) are one type of the most significant plant metabolites. Limited works have been conducted on EOs extracted from Cinnamomum reticulatum Hay (CREO), Leptospermum petersonii Bailey (LPEO), and Juniperus formosana Hayata (JFEO), which belong to the Lauraceae, Cupressaceae, and Myrtaceae families, respectively. The present work aimed to characterize and compare EOs chemical compositions of the three aromatic plant species and simultaneously evaluate their antioxidant activities. Using GC–MS/MS techniques, totally 135 volatile organic compounds (VOCs) belonging to nine chemical classes were detected, with 93, 102 and 116 VOCs from and 7, 8 and 16 VOCs unique to CREO, LPEO and JFEO, respecively, and 72 VOCs common to all the three EOs. The main compound identified both in LPEO and JFEO was (−)-bornyl acetate (20.23% and 28.40%, respectively), and the dominated compounds in CREO were L-α-terpineol (16.21%) and 1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl)naphthalene (11.68%), all accounting for more than 20% of their total contents. An in-depth dissection of major chemical compositions of the three EOs found that three VOCs were newly identified and biological functions of four VOCs were not yet reported previously. In addition, higher antioxidant capacities, measured with DPPH and ABTS assays, were exhibited in JFEO (IC50 8.37 ± 2.98 and 0.53 ± 3.80 mg/ml, respectively) and in LPEO (IC50 13.93 ± 2.11 and 1.32 ± 0.97 mg/ml) than in CREO (IC50 250.58 ± 1.48 and 4.81 ± 3.23 mg/ml), which may be due to CREO contained more esters and less aromatics than the other two EOs.
Keywords
Cinnamomum reticulatum Hay
Leptospermum petersonii Bailey
Juniperus formosana Hayata
Essential oil (EO)
Chemical composition
Antioxidant activity
1 Introduction
In recent decades, medicinal properties of phytochemicals have gained a great interest due to their high pharmacological activities, low toxicity, and other health benefits (Auddy et al., 2003). Essential oils (EOs), a class of phytochemicals derived primarily from aromatic and medicinal plants, are attracting more and more attention because of their various unique biological and pharmacological activities, such as antioxidant, anti-inflammatory, antimicrobial, and anticancer properties (de Sousa Barros et al., 2015).
Chemical compositions of plant EOs are complex, mostly containing terpenoids and aromatic, aliphatic, and sulfur compounds as identified by GC–MS and/or HPLC-MS/MS (Oliveira et al., 2013; Bouchekrit et al., 2016; Benbelaïd et al., 2017; Monzote et al., 2017). Moreover, the chemical composition of EOs of a plant species is affected by its geographical location, the season of harvest (Zhang et al., 2019a,b), as well as the extraction techniques (van Vuuren et al., 2014; Caputo et al., 2020). Although there are many similarities in the chemical composition of different types of EOs from different plant species, recent studies have demonstrated that only the similarity in chemical compositions does not essentially convey equivalent biological efficacy, and the relative content of each compound in each EO plays a more important role in determining its main efficacy (Shawky et al., 2018). Therefore, researchers began to pay more attention to the comparison of individual chemical composition among different EOs under the same extraction and/or analysis conditions.
Plants EOs have been studied rather extensively, and Lauraceae, Cupressaceae, and Myrtaceae are the most prominent plant families that can synthesize EOs in large quantities. Cinnamomum reticulatum Hay (Lauraceae), also known as black camphor, is a rare evergreen broad-leaved tree endemic to Taiwan (Fujia, 1972). To date, a number of researches have focused on the compositions of its roots, stems, and leaves (Lin et al., 2010) with only Fujia (1972) reporting the composition of C. reticulatum EO (CREO) some 50 years ago, of which only <34 volatile organic compounds (VOCs) were identified. As for the biological and pharmacological activities of CREO, there are no further studies and reports.
Juniperus formosana Hayata (Cupressaceae), an evergreen shrub or tree, is endemic to China and is widely distributed in 14 provinces at low latitudes. J. formosana is generally considered as a medicinal plant and its roots are used in traditional Chinese medicine to treat inflammation (Zheng et al., 2020). Besides, its fruits and fresh branches with leaves are used in traditional Tibetan medicine for treatment of pruritus cutanea, hemorrhoids, deep-rooted boils, and anthrax (Lee et al., 2010). Guo et al. (2016) reported that J. formosana EO (JFEO) is mainly composed of α-pinene, 4-terpineol, limonene, and β-phellandrene, and its chemical composition changes with season. JFEO possesses natural insecticidal and repellent properties, antibacterial and antioxidant activities, and excellent acetylcholinesterase inhibition effects (Kim and Park, 2012; Guo et al., 2016; Zhang et al., 2020).
Leptospermum petersonii (Myrtaceae), commonly known as Australian Rose (lemon-scented tea-tree), is a rare small tree that grows naturally in lowland or floodplain of northern New South Wales and is traditionally used as a tea or medicine (Saifullah et al., 2020). The odor of common L. petersonii is described as “extremely pleasant and lemony”, and its leaves currently used predominantly to produce EO (van Vuuren et al., 2014). Previous reports have proposed two chemical varieties of this species based on the compositions of their EOs (Brophy et al., 2000). Van Vuuren et al. (2014) identified 40 VOCs from L. petersonii EO (LPEO) with pleasant smell and claimed that its major compositions were citronellal, citronellol, neral, and geranial, whereas Caputo et al. (2020) reported 64 VOCs from LPEO with a rose-like odor which contained geraniol and geranyl acetates as its main constituents. LPEO was found to be a potent biological agent in inhibiting growths of Aspergillosis spp and Candida albicans (Kim and Park, 2012; Afolabi et al., 2020).
DPPH is a relatively stable aliphatic radical with a free electron on its N atom, and its ethanol solution is purple and has the largest absorption peak at 517 nm. After adding antioxidant, DPPH captures an electron from the antioxidant and the purple color fades and becomes colorless. The principle agent of ABTS•+ antioxidant assay is K2S2O8 and it directly generates stable cationic radical ABTS•+ to make the solution purple. Then the reaction of antioxidant substances with the ABTS•+ can make the purple color fade. Both the DPPH and ABTS scavenging capacity assays are considered to be valid and convenient colorimetric methods to examine both hydrophilic and lipophilic antioxidants. The former is successfully utilized for investigating antioxidant properties of wheat grain and bran, vegetables, conjugated linoleic acids, herbs, edible seed oils, and flours in several different solvent systems (Prior et al., 2005), and the latter is widely applied to measurement of the antioxidant activity of various substances, including pure compounds and plant extracts (Erel 2004; Re 1999).
Although the chemical compositions of EOs of the above three aromatic plants have been studied to a certain extent, there are few in-depth studies. Particularly, EO composition of C. reticulatum is still poorly understood. In addition, due to differences in extraction technology, EOs compositions identified from the same plant (e.g., J. formosana or L. petersonii) in different reports are also very different. Thus, in this study, compositions of the three EOs were identified at the same time by the same advanced GC–MS/MS techniques for the first time, and evaluation of their antioxidant capacities were simultaneously conducted, aiming at comparison of the similarities and differences of these three well-known EOs extracted and analyzed under the same conditions.
2 Materials and methods
2.1 Preparation of EOs
Cinnamomum reticulatum Hay (Lauraceae), Juniperus formosana Hayata (Cupressaceae), and Leptospermum petersonii Bailey (Myrtaceae) were introduced from Taiwan by Baicaoji Horticultural Farm, Haicang District, Xiamen city, China, and were identified according to the herbaria of Institute of Botany, Chinese Academy of Sciences (voucher No.01846108), Northwest University of Agriculture and Forestry Science and Technology (voucher No. 0194220), and Xiamen Botanical Garden (voucher is XMBG-0002329), respectively. Extractions by steam distillation and volatile oil extractor (XH-JYCL, Shanghai Xinhu Experimental Equipment Co., Ltd) of the three EOs from the fresh branches and leaves of six-year-old seedlings of the three aromatic tree species were conducted according to a method reported by Mohamadi et al. (2013) with slight modification. Briefly, 100 g of crushed leaves were added to 400 mL of distilled water and an appropriate amount of sodium chloride in a flask, then the flask was heated in an enclosed electric furnace and kept boiling slightly for 5 h. When the EO content in the extractor did not increase, stop heating. The individual EOs were collected in brown reagent bottles and placed in a −80 °C refrigerator until analysis.
2.2 Chemical composition analysis of EOs by GC–MS/MS
Individual frozen EOs (1 g) were ground into powder under liquid nitrogen and transferred to vials for GC–MS/MS analysis (Agilent, Palo Alto, CA, USA). This operation can well stabilize and preserve the volatile compounds in each of the EOs. Before the vials were sealed, 10 μL hexane was added as an internal standard to correct the integral area of each VOC to obtain its accurate relative content. The vials were placed in the tray of a solid-phase microextraction autosampler, coupled to an Agilent 7890B gas chromatograph apparatus and an Agilent 7000D mass spectrometer (Stevens Creek Blvd, Santa Clara, CA, USA). VOCs of individual EOs were separated on a DB-5MS (5% phenyl-polymethylsiloxane) capillary column (30 m × 0.25 mm × 1.0 μm). Flow rate of the carrier gas helium was set at 1.0 mL/min. The temperature program started at 40 °C, increased at 6 °C/min to 280 °C, and then kept for 5 min. The column effluent was ionized by electron ionization at an energy of 70 eV with a transfer temperature 280 °C and an ion source temperature 230 °C. Mass spectra was scanned in the m/z range of 30–350 amu at one-second intervals. VOCs were identified by comparing their electron ionization mass spectra with the MWGC 1.0 Mass Spectral Library established independently by Maiwei (Jiaxing Maiwei metabolism Biotechnology Co., Ltd, Hubei, China), the NIST and Wiley databases, as well as the retention time of authentic standards.
2.3 Assays of antioxidant activities
DPPH• scavenging activity of each EO was determined as reported by Brahmi et al. (2020) with slight modifications. In brief, 1 mL EO methanol solution (mG/V) at different concentrations (i.e., 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mg/ml) was added to 1 mL of 0.2 mmol/L DPPH• methanol solution (mM/V). After mixing, it was left for 30 min at room temperature and then measured at 517 nm using a microplate reader (Bio-Rad xMarkTM Microplate Absorbance Spectrophotometer, USA).
Antioxidant activity of EOs was also estimated by the ABTS assay (Zakia et al., 2016;Farhadi et al., 2020; Wu et al., 2020). Briefly, 7.0 mM ABTS was mixed with 2.45 mM potassium persulfate solution (v/v = 1:1), and the formed mixture was reacted at 25 °C for 30 min in the dark to generate the radical cation (ABTS•+). Then, ABTS•+ solution was diluted with methanol until the absorbance reached 0.7 ± 0.02 at 734 nm. Subsequently, different concentrations (i.e., 1, 2, 3, 4, and 5 mg/ml) of EOs were allowed to react with the fresh ABTS•+ solution at 25 °C for 6 min in the dark. Absorbance was taken at 734 nm with the microplate reader described above.
Ascorbic acid (Vc) was employed as the reference for measurements of both DPPH and ABTS antioxidant activities. The inhibition rate in percent (I%) was calculated according to following formula: I% = [1 − (Atest − Asample) / Acontrol] × 100, where Acontrol is the absorbance of the control reaction (containing all reagents except the tested EO), Atest is the absorbance of the reaction mixtures, and Asample is the absorbance of the tested EO. All determinations were performed in triplicate, and data were presented as means ± SD. Microsoft Excel Software was used for the calculation of the values.
2.4 Data processing and statistical analysis
After obtaining the GC–MS/MS fingerprints, the original data were exported by the Qualitative Analysis Workflows B.08.00 software, then peak extraction was carried out by Mass Hunter software for the integration and correction of chromatographic peaks to enhance the accuracy of quantification. In addition, relative content of a volatile substance in percentage was obtained with the area of each peak divided by the area of the total peaks. Finally, each sample or reference standard was represented by a GC–MS/MS total ion current (TIC) diagram. All of the detected peaks were identified by comparing both the MS spectra and the retention index with those available in the MWGC 1.0 database. The mass spectra obtained were investigated carefully, and only those molecules with matching probability greater than 80% were considered. Within each sample, the retention time (RT) and m/z data pairs were used as the identifier for each peak, and the ion intensities for each peak detected were then normalized to the sum of the peak intensities in that sample. To account for any difference in concentration between samples, all data were normalized to a total value of 100.
Each analysis was replicated biologically three times. For each VOC, the relative area was normalized respect to that of the internal standard. To explore differences in VOC compositions of the three EOs, data were statistically processed using the SPSS software (version 26.0, Statistical Package for the Social Sciences Inc., Chicago, USA).
3 Results
3.1 Overall characterization of VOCs from the three EOs
Total ion chromatograms (TICs; Fig. S1A) of the QC sample which was a mixture of all the three EOs (i.e., CREO, LPEO and JFEO) for ensuring precision of the assays, and the combined TICs performed at the beginning, the middle and the end of the measurement period of the QC (Fig. S1B) suggest that the relative standard deviations of the retention time of the characteristic peaks of metabolites, the accurate mass number, and the ion abundance be <2%, 1 ppm, and 10%, respectively, demonstrating that the GC–MS/MS system was stable and the collected data was reliable during the process of data acquisition.
Fig. 1 shows the TICs of metabolites in CREO (Fig. 1A), LPEO (Fig. 1B), and JFEO (Fig. 1C), demonstrating that they were obviously different in their chemical compositions. Combined with the molecular feature extraction function of Mass Hunter analysis software and then based on the MWGC 1.0 database, these metabolites were analyzed qualitatively and quantitatively. As listed in Table S1, a total of 135 VOCs were detected, with 93, 102, and 116 from CREO, LPEO, and JFEO in respective. In addition, 72 VOCs were common in all the three EOs, accounting for 84.09%, 87.95%, and 85.82% in relative contents in CREO, LPEO, and JFEO, respectively.
Total ion chromatograms (TICs) for gas chromatography-mass spectrometry analyses of three individual EOs. A, B, and C, TICs for CREO, LPEO, and JFEO, respectively. EO, essential oil; CREO, LPEO, and JFEO, EOs extracted from leaves of Cinnamomum reticulatum, Leptospermum petersonii, and Juniperus formosana, respectively.
3.2 Characterization of nine chemical classes of the three EOs
As shown in Fig. 2, 135 volatile substances identified in one, two or three EOs are divided into 9 categories, namely terpenes, esters, aromatics, ketones, aldehydes, heterocyclics, alcohols, acids and others (Fig. 2A). Obviously, among the three EOs, the numbers of chemical substances of individual chemical classes were basically similar. With respect to the relative contents, they were obviously different (P < 0.05), except terpenoids and alcohols (Fig. 2A and B).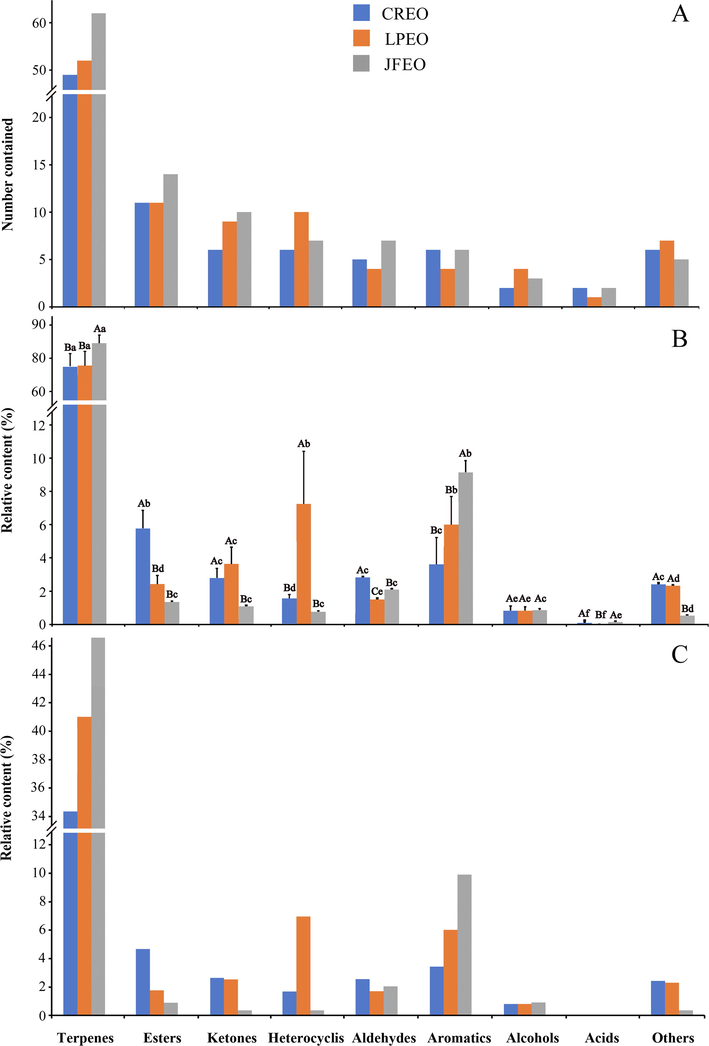
Numbers, relative contents and top3 relative contents of VOCs of nine individual chemical classes in three EOs. Values for relative contents are means ± standard deviation (SD) of three replicates (n = 3). Different letters A, B, and C indicate significant difference (P < 0.05) in relative contents of individual chemical classes among the three EOs, and different letters a, b, c, d, e, and f indicate significant difference (P < 0.05) in relative contents of individual chemical classes among the nine chemical classes. VOC, volatile organic compound; EO, essential oil; CREO, LPEO, and JFEO, EOs extracted from leaves of Cinnamomum reticulatum, Leptospermum petersonii, and Juniperus formosana, respectively.
Sixty-seven terpenes: As showed in Fig. 2B, although terpenes in relative contents appeared comparable in the three EOs, there were no significant differences between CREO and LPEO but they were both significantly different from JFEO (P < 0.05). The relative contents of the highest three (top3) terpenes were 16.21%, 11.68% and 6.30% and their total relative content was 34.19% among the 49 terpenes detected in CREO; 20.23%, 13.68% and 7.15%, totally 41.06%, among the 52 terpenes in LPEO; and 28.40%, 11.78% and 6.56%, totally 46.74%, among the 62 terpenes in JFEO. Among the three EOs, the proportion of the top3 terpenes in the relative content of total terpenes in CREO was the lowest (Table S1 and Fig. 2C).
Seven aromatics: Total aromatics were significantly higher in JFEO than in both CREO and LPEO, with the later two showing no significant difference (Fig. 2B; P < 0.05). Relative contents of its top3, both so close to their totals, were 2.47%, 0.56% and 0.41%, totally 3.44%, among the 6 aromatics in CREO; 4.56%, 1.43% and 0.20%, totally 6.19%, among the 4 aromatics in LPEO; and 9.13%, 0.73% and 0.04%, totally 9.9%, among the 6 aromatics in JFEO. (Table S1 and Fig. 2C).
Eleven heterocyclics: Heterocyclics were significantly higher in LPEO than in CREO and JFEO, with the later two showing no significant difference (Fig. 2B; P < 0.05). Relative contents of its top3 were 1.09%, 0.53% and 0.07%, totally 1.69%, among the 6 heterocyclics in CREO; 6.44%, 0.38% and 0.13%, totally 6.95%, among the 10 heterocyclics in LPEO; and 0.24%, 0.07% and 0.04%, totally 0.35%, among the 7 heterocyclics in JFEO. Heterocyclics were obviously higher in LPEO than in CREO and JFEO both in numbers and relative content, although all the three top3 heterocyclics were so close to their totals (Table S1 and Fig. 2C).
Fifteen esters: Esters in relative contents were significantly higher in CREO than in LPEO and JFEO, with the later two showing no significant difference (Fig. 2B; P < 0.05). Relative contents of its top3 were 2.75%, 1.58% and 0.34%, totally 4.67%, among the 11 esters in CREO; 1.23%, 0.28% and 0.27%, totally 1.78%, among the 11 esters in LPEO; and 0.42%, 0.33% and 0.15%, totally 0.90% in JFEO. All the three top3 esters accounted for most of their totals (Table S1 and Fig. 2C).
Twelve ketones: Ketones in CREO and LPEO were significantly higher that those in JFEO, with the former two showing no significant difference (Fig. 2B; P < 0.05). Relative contents of its top3 were 2.28%, 0.23% and 0.14%, totally 2.65%, among the 6 ketones in CREO; 1.05%, 0.93% and 0.55%, totally 2.53%, among the 9 ketones in LPEO; and 0.17%, 0.10% ant 0.09%, totally 0.36%, among the 10 ketones in JFEO. The sum of the top3 was close to its total ketones in CREO in relative content, but those of the other two top3 were much lower (Table S1 and Fig. 2C).
Seven aldehydes: Both the total and the top3 of aldehydes in the three EOss were comparable, and significant differences among their totals were observed (Fig. 2B; P < 0.05). Relative contents of its top3 were 2.07%, 0.25% and 0.24%, totally 2.56% among the 5 aldehydes in CREO; 0.95%, 0.68% and 0.08%, totally 1.71%, among the four aldehydes in LPEO; and 1.02%, 0.95% and 0.07%, totally 2.04%, among the 7 aldehydes in JFEO. Aldehydes of both total and top3 were so close in relative contents in the three EOs (Table S1 and Fig. 2C).
Four alcohols: Total alcohols were extremely low and showed no significant differences in the three EOs (P < 0.05) and the relative contents of top3 were nearly the same as those of their totals (Fig. 2B). Only two (0.78% and 0.02%) and three (0.85%, 0.06% and 0.001%) alcohols were detected in CREO and JFEO, respectively. The top3 alcohols were 0.36%, 0.25% and 0.19% among the 4 alcohols in LPEO (Table S1 and Fig. 2C).
Three acids: Total acids were also extremely low in the three EOs, especially in LPEO from which only one acid was detected. Relative content of this sole acid was significantly lower than those of CREO and JFEO (P < 0.05), both of which contained two acids (Table S1 and Fig. 2C).
Nine ‘others’: All the three EOs contained <3% in relative content of the so-called ‘others’ VOCs class. Total ‘others’ were significantly lower in JFEO than in CREO and LPEO, with the later two showing no significant difference (Fig. 2B; P < 0.05). Top3 of the ‘others’, both comparable to their totals, were 1.68%, 0.48% and 0.28%, totally 2.44%, among the 6 ‘others’ in CREO; 1.38%, 0.82% and 0.11%, totally 2.31%, among the 7 ‘others’ in LPEO); and 0.16%, 0.13% and 0.07%, totally 0.36% among the 5 ‘others’ in JFEO (Table S1 and Fig. 2C).
3.3 Specific substances in EOs of the three individual plants
As shown in Table S2 and Table 1, seven VOCs, accounting for 3.18%, were unique in CREO, including ‘2-methyl-2-phenylethyl-butyrate’ (2.75 ± 8.48%), ‘2-isopropyl-5-methyl-9-methylene-bicyclo[4.4.0]dec-1-ene’ (0.24 ± 7.77%) and ‘tert-butylbenzene’ (0.11 ± 4.00%), with the other four being <0.1% in relative content. Eight VOCs, accounting for 2.35%, unique in LPEO, including ‘1-methyl-4-(2-methylepoxyethyl)-7-oxabicyclic [4.1.0] heptane’ (0.61 ± 10.45%), ‘4-hydroxy-2-methylacetopheno’ (0.49 ± 2.81%), ‘α-methyl-α-(1-methyl-2-propenyl)-benzenemethanol’ (0.36 ± 3.43%) and ‘4-(2,6,6- trimethyl-cyclohexa-1,3-dienyl)-pent-3-en-2-one’ (0.20 ± 22.11%), with the other four being <0.1%. Sixteen VOCs, accounting for 0.59%, were unqiue in JFEO, including ‘bicyclosesquiphellandrene’ (0.22 ± 3.29%), ‘4′,6′-dihydroxy-2′,3′-dimethylacetophenone’ (0.06 ± 0.75%), ‘salvial-4(14)-en-1-one’ (0.05 ± 3.02%) and ‘(4aS-trans)-1,2,3,4,4a,9,10,10a-octahydro-1,1,4a-trimethyl-7-(1-methylethyl)-phenanthrene’ (0.05 ± 3.53%), with the other twelve being <0.05%. These specific compounds might be markers to distinguish the three EOs. It should be pointed out that compared to LPEO and JFEO, CREO contained the least kinds of unique VOCs, but the highest proportion in the total relative content (Table 1).
Unique VOCs
Relative contents (%)
Total (%)*
CREO*
1
2-Methyl-2-phenylethyl-butyrate
2.75 ± 8.48a*
3.18
2
2-Isopropyl-5-methyl-9-methylene-bicyclo[4.4.0]dec-1-ene
0.24 ± 7.77a
3
tert-Butylbenzene
0.11 ± 4.00a
4
Pinocarvone
0.06 ± 4.43a
5
Methacrylamide
0.01a
6
Carvenone
0.01 ± 7.97a
7
(1R-trans)-2,2-Dimethyl-3-(2-methyl-1-propenyl)-cyclopropanecarboxylic acid
0.001 ± 8.76a
LPEO
1
1-Methyl-4-(2-methylepoxyethyl)-7-oxabicyclic [4.1.0] heptane
0.61 ± 10.45a
2.35
2
4-Hydroxy-2-methylacetopheno
0.49 ± 2.81a
3
α-Methyl-α-(1-methyl-2-propenyl)-benzenemethanol
0.36 ± 3.43a
4
4-(2,6,6-Trimethyl-cyclohexa-1,3-dienyl)-pent-3-en-2-one
0.20 ± 22.11a
5
2-((3,3-Dimethyloxiran-2-yl)methyl)-3-methylfuran
0.05 ± 2.62a
6
3,7,7-Trimethyl-1-penta-1,3-dienyl-2-oxabicyclo[3.2.0]hept-3-ene
0.03 ± 9.94a
7
3-Methyl-4-isopropylpheno
0.01a
8
trans-1-Methyl-4-(1-methylethyl)-2-cyclohexene-1-ol
0.01 ± 0.44a
JFEO
1
Bicyclosesquiphellandrene
0.22 ± 3.29a
0.59
2
4′,6′-Dihydroxy-2′,3′-dimethylacetophenone
0.06 ± 0.75a
3
Salvial-4(14)-en-1-one
0.05 ± 3.02a
4
(4aS-trans)-1,2,3,4,4a,9,10,10a-Octahydro-1,1,4a-trimethyl-7-(1-methylethyl)-phenanthrene
0.05 ± 3.53a
5
[4aS-(4a. α.,5. α.,8a.β.)]- Decahydro-1,1,4a-trimethyl-6-methylene-5-(3-methylene-4-pentenyl)-1H-Naphtho[2,1-b]pyran
0.03 ± 1.16a
6
3,6-Dimethyl-4H-furo[3,2-c]pyran-4-one
0.03 ± 3.49a
7
α-Orocalene
0.03 ± 1.16a
8
5-Isopropenyl-2-methylcyclopent-1-enecarboxaldehyde
0.02 ± 9.57a
9
1-Methoxy-4-methyl-2-(1-methylethyl)-benzene
0.02 ± 2.33a
10
(4aS,4bR,10aS)-7-Isopropyl-1,1,4a-trimethyl-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthrene
0.01 ± 2.36a
11
[3S-(3. α.,4a. α.,6a.β.,10a. α.,10b.β.)] − 3-Ethenyldodecahydro-3,4a, 7,7,10a-pentamethyl-1H-naphtho [2,1-b] pyran
0.01 ± 4.17a
12
1,7,7-Trimethylbicyclo[2.2.1]hept-5-en-2-ol
0.01 ± 6.29a
13
Palmitoleic acid
0.01 ± 1.95a
14
Benzyl benzoate
0.006a
15
2,6,10-Trimethyltridecane
0.003a
16
6,10,14-Trimethyl-2-pentadecanone
0.002 ± 1.19a
3.4 Comparison of two antioxidant activities of the three EOs
For a plant sample, due to the different preparation conditions of the extracts or EOs, the species and concentrations of the extracted phytochemicals will be different, so will their antioxidant capacities (Saifullah et al., 2020). Antioxidant property of an individual sample may also vary according to the assay methods used since the reactions of individual phytochemicals with chemicals in various assays are principally different (Thaipong et al., 2006). Antioxidant capacities of the three individual EOs obtained under the same extraction conditions were then evaluated by both the DPPH and ABTS assays with ascorbic acid (Vc) as a positive control. It is obvious that the antioxidant activities of the positive control (Fig. 3A and B) had already reached 100% at their lowest doses tested (IC50 value: 3.86 ± 6.24 mg/mL). As shown in Fig. 3A, LPEO and JFEO exhibited similar DPPH• scavenging activities (IC50 values: 13.93 ± 2.11 and 8.37 ± 2.98 mg/mL, respectively), and they were dramatically higher than those of CREO (IC50 value: 250.58 ± 1.48 mg/mL). In addition, the antioxidant activities of LPEO and JFEO at their highest doses were close to 100% free radical scavenging rates. The ABTS•+ scavenging activities of JFEO (Fig. 3B) were first close and then equal to those of the positive control (IC50 values: 0.53 ± 3.80 and 0.32 ± 12.69 mg/mL, respectively), and those of LPEO (IC50 value: 1.32 ± 0.97 mg/mL) and CREO (IC50 value: 4.81 ± 3.23 mg/mL) increased at a dose-dependent manner, with the latter being roughly half of the former at every individual doses tested.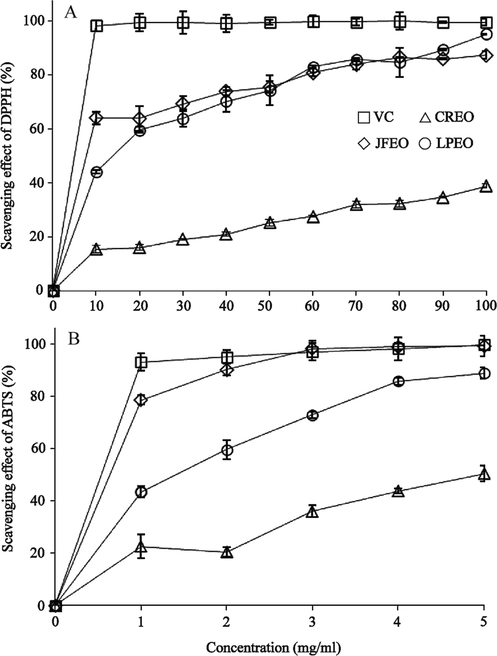
Scavenging effect on CREO, LPEO and JFEO in different concentrations. (A) DPPH: Dilute pure essential oil with methanol to obtain 10–100 mg/ml of essential oil methanol solution; (B) ABTS•+: Dilute pure essential oil with methanol to obtain 1–5 mg/ml of essential oil methanol solution. CREO, Cinnamomum reticulatum essential oil; LPEO, Leptospermum petersonii essential oil; JFEO, Juniperus formosana essential oil; VC, ascorbic acid.
4 Discussion
CREO was characterized by a high content of terpenes, accounting for 74.29% in relative content (Fig. 2B), in addition to 11 esters (5.68%), 6 aromatics (3.61%), 6 ketones (2.84%), 5 aldehydes (2.70%), 6 heterocyclics (1.77%), 2 alcohols (0.8%), 2 acids (0.07%), and 6 others (2.50%) (Fig. 2A and B). Thus far, an article by Fujia (1972) is the only report on composition of CREO, and it identified only 34 VOCs, 97.6% of which being composed of three substances, namely, linalool (96.8%), β-pinene (0.5%), and α-terpineol (0.3%). In the present study, as many as 93 VOCs were detected from CREO, however, no linalool was identified. The reason for such a dramatic difference might be the differences in extraction methods, detection techniques, and/or the picking time of CREO. In the current study, α-terpineol, only accounting for 16.21% (Table2), was the highest, which widely exists in turpentine and EOs from tea tree, lemon, and camphor, possessing pharmacological activities such as antibacterial, antispasmodic, anti-inflammatory, and anti-analgesic effects (Hammer et al., 2003; de Sousa Barros et al., 2015). The second highest VOCs was ‘1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl) naphthalene’ (11.68%) (Table 2), which was firstly reported by the present study thus needs to be further characterized. The third one, ‘(+)-γ-cadinene’ (6.31%) (Table 2), has good analgesic, anti-inflammatory, diuretic, and other functions, and those EOs containing it as the main composition showed obvious anti-inflammatory effect on croton oil-induced ear inflammation, although the inhibition rate of edema was only 40% (Babarinde et al., 2021; Marinas et al., 2021). As the fourth highest, ‘eucalyptol’ (5.29%) (Table 2), a cyclic monoterpenoid ether, is the dominant portion of eucalyptus oil, an EO of Eucalyptus globulus and accessible by steam distillation of its leaves. Some herbs and spices such as basil and cardamom also contain it as characterizing flavour compound, which is used in flavorings, fragrances, confectionery, cosmetics, cough suppressants, and insect repellents, and at the same time, it also has high anti-inflammatory and pain effects (Zeller et al., 2015; de Melo et al., 2017). (1S-cis)-1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene (4.93%) was the fifth highest one (Table 2), and only a small number of studies have discussed this substance, which is mainly the main component of extracts from Cunninghamia lanceolata or Cinnamomum camphora (Peng et al., 2010; Liu and Peng 2011).
Top5 VOCs*
Relative contents (%)
Total (%)†
CREO*
1
L-α-Terpineol
16.21 ± 3.24a*
44.41
2
1,2,3,4-Tetrahydro-1,6-dimethyl-4-(1-methylethyl)naphthalene
11.68 ± 7.47a
3
(+)-γ-Cadinene
6.30 ± 1.61a
4
Eucalyptol
5.29 ± 14.04a
5
(1S-cis)-1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene
4.93 ± 2.16b
LPEO
1
(−)-Bornyl acetate
20.23 ± 4.57b
53.68
2
3,6,6-Trimethyl-bicyclo[3.1.1]hept-2-ene
13.68 ± 13.62a
3
2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene
7.15 ± 0.02a
4
2,2′-Isopropylidenebis(5-methylfuran)
6.44 ± 6.75a
5
[1R-(1. α.,7.β.,8a.α.)]-1,2,3,5,6,7,8,8a- Octahydro-1,8a-dimethyl-7-(1-methylethenyl)-naphthalene
6.18 ± 5.86a
JFEO
1
(−)-Bornyl acetate
28.40 ± 3.42a
61.41
2
β-Oplopenone
11.78 ± 2.36a
3
1-Methyl-3 − (1-methylethyl) − benzene
9.13 ± 6.41a
4
(1S-cis)-1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene
6.56 ± 2.79a
5
D-Limonene
5.54 ± 9.23a
Similarly, LPEO composed of 52 terpenes (75.55%), 10 heterocyclics (7.32%), 4 aromatics (6.19%), 9 ketones (3.54%), 11 esters (2.44%), 4 aldehydes (1.78 %), 4 alcohols (0.85%), 1 acids (0.02%), and 7 others (2.49%) (Fig. 2A and B). Based on differences of chemical composition, LPEO can be classified into two types. The most common type is type-A, which has a pleasant odor and is mainly composed of monoterpenes (γ-terpinene, α-pinene, and β-pinene). Type-B has a rose-like odor and is very rare, and its main VOCs are geranyl acetate, geraniol, and other esters (Brophy et al., 2000). Caputo et al. (2020) identified only 64 VOCs from LPEO based also on GC–MS technology, which is far less than the 102 VOCs detected by this study. The reason for this difference may be that GC–MS/MS was used in this study, and tandem mass spectrometry could better identify and quantitatively analyze trace metabolites (Lee and Lau, 2011). The main composition of LPEO in this study was a kind of jasmine rose scented ‘(−)-bornyl acetate’ (Table 2), and the geranyl acetate identified was also higher than 1% (1.23%). Besides, biological functions of ‘3,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene’ (13.68%), ‘2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene’ (7.15%), and ‘2,2′-isopropylidenebis(5-methylfuran)’ (6.44%) have not been reported previously, and ‘3,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene’ and ‘2,2′-isopropylidenebis(5-methylfuran) ’ are newly identified VOCs in this study (Table 2).
JFEO was characterized by the highest percentage of (−)-bornyl acetate (28.39%), followed by β-oplopenone (11.78%), 1-methyl-3-(1-methylethyl)-benzene (9.13%), (1S-cis)-1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene (6.56%) and D-limonene (5.54%) (Table 2). Of them, (−)-bornyl acetate has the jasmine rose fragrance, and it can enhance the sweetness of honey, and β-opraxone with the second highest relative content has not been explored previously thus needs to be further studied. 1-Methyl-3-(1-methylethyl)- benzene, which ranks third (Table 2), to which only a few reports have discussed its function, showing that it has the ability to resist the invasion of termites (Rasib and Aihetasham, 2016). (1S-cis)-1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene, the fourth highest (Table 2), has been identified in the extracts from Cunninghamia lanceolata or Cinnamomum camphora (Peng et al., 2010; Liu and Peng 2011). The most widely studied D-limonene (Table 2), which ranks the fifth, has a wide range of physiological functions, including antimicrobial, anti-corrosion, antioxidant, and antidepressant functions (Zhang et al., 2019a,b). Adams et al. (1995) reported that JFEO from Gansu Province is mainly composed of α-pinene (47.7%), myrcene (11.2%), and limonene (4.0%), and that from Anguo of Hebei Province is primarily composed of α-pinene (21.66%), L-α-terpineol (11.25%), and limonene (11.00%) (Guo et al., 2016), but JFEO from Kunming were consisted of α-pinene (9.56%), (−)-geranyl acetate (5.17%), and limonene (4.26%), which are similar to a certain extent to those of the present study from Xiamen of Fujian Province. It can be seen that different growth environments (including latitude, altitude, solar radiation) and physiological conditions of the individual plants of J. formosana at the time of sample collection greatly affected the chemical composition of JFEO.
Among natural products, EOs from aromatic and medicinal plants exhibit biological activities and are of particular concern because of their free radical scavenging properties (de Sousa Barros et al., 2015). Numerous studies have looked at EOs as environmentally friendly alternatives to artificially synthetic antioxidants (El-Gawad et al., 2019). To our knowledge, characteristics of CREO’s antioxidant activities has not been reported previously. From this study, it can be seen that CREO had only moderate antioxidant capacity in general and it was the lowest among the three EOs. On the contrary, LPEO showed quite good antioxidant capacity which is similar to that reported by Zhao et al. (2008). Zhang et al. (2020) demonstrated that JFEO possesses only moderate antioxidant capacity, inconsistent with our finding that JFEO showed the strongest antioxidant activity of the three EOs (Fig. 3). This inconsistency is most likely due to differences in the chemical composition of EOs obtained by different extraction methods. And compared CREO with the other two EOs (LPEO and JFEO), the poor antioxidant capacity of CREO may be related to the following characteristics, that is, CREO contained more esters and less aromatics than those of the other two EOs (Fig. 2B) and the proportion of its top five VOCs was the lowest, whereas JFEO with the strongest antioxidant activity exhibited the highest proportion of its top5 VOCs (Table 2).
5 Conclusions
The present study first performed the overall characterization of VOCs from the three EOs. A total of 135 VOCs belonging to nine chemical classes were detected, with 93, 102, and 116 from CREO, LPEO, and JFEO in respective. In addition, 72 VOCs were common to all the three EOs, accounting for 84.09%, 87.95%, and 85.82% in relative contents in CREO, LPEO, and JFEO, respectively. It is also found that seven VOCs, accounting for 3.18%, were unique to CREO; eight VOCs, accounting for 2.35%, unique to LPEO; and sixteen, accounting for 0.59%, were unqiue to JFEO. These specific compounds might be markers to distinguish the three EOs. It should be pointed out that compared to LPEO and JFEO, CREO contained the least kinds of unique VOCs, but the highest proportion in the total relative content. Furthermore, it is found that three VOCs, namely, ‘1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl)naphthalene’ from CREO ‘3,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene’ and ‘2,2′-isopropylidenebis(5-methylfuran)’ from LPEO, were newly identified, and biological functions of four VOCs, namely, ‘3,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene’, ‘2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene’, and ‘2,2′-isopropylidenebis(5-methylfuran) ’ from LPEO and β-opraxone from JFEO, were not yet reported previously. Finally, the three EOs possessed high antioxidant capacities, with those of JFEO the highest and CREO the most moderate which may be due to CREO contained more esters and less aromatics than the other two EOs.
CRediT authorship contribution statement
Yuanhang Li: Conceptualization, Investigation, Writing – original draft. Xinxin Cao: Formal analysis, Investigation, Validation. Jing Sun: Formal analysis, Investigation, Validation. Wanqi Zhang: Formal analysis, Investigation, Validation. Jin Zhang: Data curation. Youfang Ding: Conceptualization, Visualization, Funding acquisition. Yujun Liu: Conceptualization, Supervision.
Acknowledgements
This work was supported by the Xiamen Bureau of Science and Technology planning project (Grant No. 3502Z20199005), Xiamen Bureau of Science and Technology, China.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Essential oil of Juniperus formosana Hayata leaves from China. J. Essent. Oil Res.. 1995;7(6):687-689.
- [CrossRef] [Google Scholar]
- Leptospermum petersonii as a potential natural food preservative. Molecules. 2020;25(23):5487.
- [CrossRef] [Google Scholar]
- Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol.. 2003;84(2–3):131-138.
- [CrossRef] [Google Scholar]
- Chemical composition and repellent potentials of two essential oils against larger grain borer, Prostephanus truncatus (Horn.) (Coleoptera: Bostrichidae) Biocatal. Agric. Biotechnol.. 2021;32(4)
- [CrossRef] [Google Scholar]
- Composition and antimicrobial activity of Cistus munbyi essential oil: an endemic plant from Algeria. J. Forestry. Res.. 2017;28(6):1129-1134.
- [CrossRef] [Google Scholar]
- Essential oils from Elaeoselinum asclepium: chemical composition, antimicrobial and antioxidant properties. Asian. Pac. J. Trop. Bio.. 2016;6(10):851-857.
- [CrossRef] [Google Scholar]
- Comparison of chemical composition and biological activities of algerian seed oils of pistacia lentiscus L. opuntia ficus indica (L.) mill. and argania spinosa L. skeels. Ind. Crop. Prod.. 2020;151:112456.
- [CrossRef] [Google Scholar]
- Leaf essential oils of the genus Leptospermum (Myrtaceae) in eastern Australia. Part 7. Leptospermum petersonii, L. liversidgei and allies. Flavour. Frag. J.. 2000;15(5):342-345.
- [CrossRef] [Google Scholar]
- Chemical composition and biological activities of the essential oils of Leptospermum petersonii and Eucalyptus gunnii. Front. Microbiol.. 2020;11:409.
- [CrossRef] [Google Scholar]
- Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod.. 2015;76:557-564.
- [CrossRef] [Google Scholar]
- Acute and neuropathic orofacial antinociceptive effect of eucalyptol. Inflammopharmacology. 2017;25:247-254.
- [CrossRef] [Google Scholar]
- A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable abts radical cation. Clin. Biochem.. 2004;37(4):277-285.
- [CrossRef] [Google Scholar]
- Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of lactuca serriola l. revealed antioxidant and allelopathic activity. Chem. Biodivers.. 2019;16(8)
- [CrossRef] [Google Scholar]
- Fujia, Y.F.a.S.-i., 1972. Biogenesis of thr Essential oils in Camphor Trees. XXX. On the Components of the Essential oil of Cinnamomum reticulatum Hay. In: Taiwan. B. Chem. Soc. Jpn. vol. 45, no. 4, pp. 1242–1243. https://doi.org/10.1246/bcsj.45.1242.
- Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of achillea millefolium at different growth stages. Ind. Crops Prod.. 2020;152:112570.
- [CrossRef] [Google Scholar]
- Contact and repellent activities of the essential oil from Juniperus formosana against two stored product insects. Molecules. 2016;21(4):504.
- [CrossRef] [Google Scholar]
- Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol.. 2003;95(4):853-860.
- [CrossRef] [Google Scholar]
- Fumigant antifungal activity of Myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Molecules. 2012;17(9):10459-10469.
- [CrossRef] [Google Scholar]
- Anti-lipase and lipolytic activities of etoh extract from juniperus rigida. Korean. J. Radiol.. 2010;41(3):216-220.
- [CrossRef] [Google Scholar]
- Effects of Panax ginseng on tumor necrosis factor-alpha-mediated inflammation: a mini-review. Molecules. 2011;16(4):2802-2816.
- [CrossRef] [Google Scholar]
- Isolation of new esters from the stems of Cinnamomum reticulatum Hay. Nat. Prod. Res.. 2010;24(8):775-780.
- [CrossRef] [Google Scholar]
- 80°C-based td-gc/ms analysis of chemical components from branches of Cinnamomum camphora. Key Eng. Mater.. 2011;480–481:466-471.
- [CrossRef] [Google Scholar]
- Chemical composition, antipathogenic and cytotoxic activity of the essential oil extracted from Amorpha fruticosa fruits. Molecules. 2021;26(11)
- [CrossRef] [Google Scholar]
- Essential oil from piper aduncum: chemical analysis, antimicrobial assessment, and literature review. Medicines (Basel). 2017;4(3):49.
- [CrossRef] [Google Scholar]
- Comparison of microwave-assisted distillation and conventional hydrodistillation in the essential oil extraction of flowers rosa damascena mill. J. Essent. Oil Res.. 2013;25(1):55-61.
- [CrossRef] [Google Scholar]
- Chemical study and larvicidal activity against Aedes aegypti of essential oil of Piper aduncum L. (Piperaceae) An. Acad. Bras. Cienc.. 2013;85(4):1227-1234.
- [CrossRef] [Google Scholar]
- Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem.. 2005;53(10):4290-4302.
- [CrossRef] [Google Scholar]
- Analysis on nano biomedical components of acetic ether extractives of cunninghamia lanceolata Biology by Py-GC/MS. INEC 2010
- [CrossRef] [Google Scholar]
- Constituents and termiticide potential of some wood extracts against Coptotermes heimi (Wasmann) (Isoptera: Rhinotermitidae) Turk. J. Chemt.. 2016;40(2)
- [CrossRef] [Google Scholar]
- Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26
- [CrossRef] [Google Scholar]
- Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon. 2020;6(4):e03666.
- [CrossRef] [Google Scholar]
- Fingerprint profile and efficacy-associated markers of Nigella sativa oil for geographical origin determination using targeted and untargeted HPTLC-multivariate analysis. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci.. 2018;1087–1088:108-117.
- [CrossRef] [Google Scholar]
- Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal.. 2006;19(6–7):669-675.
- [CrossRef] [Google Scholar]
- Essential oil composition and antimicrobial interactions of understudied tea tree species. S. Afr. J. Bot.. 2014;92:7-14.
- [CrossRef] [Google Scholar]
- Salt intervention for the diversities of essential oil composition, aroma and antioxidant activities of Kushui rose (R. setate×R. rugosa) Ind. Crops Prod.. 2020;150
- [CrossRef] [Google Scholar]
- Essential oils composition, antibacterial and antioxidant activities of hydrodistillated extract of eucalyptus globulus fruits. Ind. Crops Prod.. 2016;89:167-175.
- [CrossRef] [Google Scholar]
- Two new troponoides with anti-inflammatory activity from the stems of Juniperus formosana hayata. Nat. Prod. Res. 2020
- [CrossRef] [Google Scholar]
- Antidepressant-like effect of Citrus sinensis (L.) osbeck essential oil and its main component limonene on mice. J. Agric. Food Chem.. 2019;67(50):13817-13828.
- [CrossRef] [Google Scholar]
- Effects of harvest season and storage time on the essential oil of the linalool chemotype of Cinnamomum camphora. J. Essent. Oil. Bear. P. L.. 2019;22(5):1379-1385.
- [CrossRef] [Google Scholar]
- Antioxidant activities of eleven australian essential oils. Nat. Prod. Communn.. 2008;3(5):837-842.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant, antibacterial and cholinesterase inhibitory activities of three Juniperus species. Nat. Prod. Res.. 2020;34(24):3531-3535.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103609.
Appendix A
Supplementary material
The following are the Supplementary data to this article:
Total ion chromatograms (TICs) for the gas chromatography-mass spectrometry analyses of the QC sample. The QC sample was a mixture of CREO, LPEO, and JFEO. A, total TICs for gas chromatography-mass spectrometry analysis of the QC sample; B, the combined TICs for gas chromatography-mass spectrometry analysis of the substances of the QC sample performed at the beginning, the middle and the end of the measurement period of the QC sample. QC, quality control; EO, essential oil; CREO, LPEO, and JFEO, EOs extracted from leaves of Cinnamomum reticulatum, Leptospermum petersonii, and Juniperus formosana, respectively.
Supplementary data 1
Supplementary data 1







