Translate this page into:
Characterization of secondary metabolites of leaf and stem essential oils of Achillea fragrantissima from central region of Saudi Arabia
⁎Corresponding author. mkhan3@ksu.edu.sa (Merajuddin Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this paper, a detailed study on chemical characterization of essential oils (EOs) constituents of leaves and stems of Achillea fragrantissima were carried out using GC-FID and GC–MS analysis employing two different stationary phase columns. In the studied plant which is collected from the central region of Saudi Arabia, trans-sabinyl acetate and trans-sabinol have been identified as the major components. To the best of our knowledge, these components are being reported for the first time as the major constituents in the EOs of A. fragrantissima. The results showed that chief chemical components of both (leaves and stems) oils were found to be almost same, however, their contents varied noticeably from each other. Among 108 identified components in the leaves oil, the major components were trans-sabinyl acetate (20.7 ± 0.00), trans-sabinol (14.9 ± 0.13), artemisia ketone (12.7 ± 0.46), santolina alcohol (10.1 ± 1.30), β-sesquiphellandrene (5.5 ± 0.01), β-thujone (5.1 ± 0.11). Whereas, in the stem oil 85 components were identified and trans-sabinyl acetate (24.0 ± 0.19), trans-sabinol (19.2 ± 0.01), artemisia ketone (16.3 ± 0.74), santolina alcohol (10.4 ± 1.50), and β-sesquiphellandrene (4.8 ± 0.01) were found to be the major components. Among the identified components form both oils, 23 components were specific to only leaves oil, whereas 85 components were found to be common in both oils.
Keywords
Essential oils
Achillea fragrantissima
GC–MS
trans-Sabinyl acetate
Asteracea
1 Introduction
Recently, due to the growing environmental concerns the interest of the scientific community in medicinal and other aromatic plants derived from the traditional sources of knowledge has been renewed (Martins and Brijesh, 2018; Petrovska, 2012). Achillea fragrantissima, which is traditionally used (in the form of tea, infusion, extracts and phytomolecules) for various medical purposes in the Arabian region against different types of diseases such as hepatobiliary disorders, inflammatory and spasmodic gastrointestinal complaints, skin inflammations and wound healing etc. (Bartolotti et al., 2018; Patocka and Navratilova, 2019). Besides, this plant is also known for its excellent anti-oxidative and anti-inflammatory properties, which are typically ascribed to its rich contents of polyphenols, flavonoids, terpenes and alkamides (Mudawi et al., 2017).
A. fragrantissima (Forssk.) Sch. Bip. (synonym Santolina fragrantissima Forssk.) belongs to the family Asteraceae and is locally known in Arabic as Qaysum, which is widely distributed in the North African, eastern mediterranean coastal and Middle Eastern regions (Barel et al., 1991). It is a desert flowering plant of the genus Achillea, which include more than 100 species and is chemically characterized by the accumulation of sesquiterpenic lactones and flavonoids (Hammad et al., 2014). So far, a variety of bioactive substances have been identified from the extracts of different parts of A. fragrantissima including different types of phenolic acids like protocatechuic, vanilic, chlorogenic etc., a variety of flavonoids like apigenin, apigenin-glycoside, luteolin, vitexin and so on. Besides various types of bioactive flavonoids other class of phytomolecules such as lignans (sesamin), terpenic lactones (achillolid A) and alkamides (pellitorin, 8,9-Z-dehydropellitorin, anacyclin) have also been extracted from A. fragrantissima (El-Ashmawy et al., 2016). However, the amount of these bioactive compounds present in A. fragrantissima varies widely depending upon the region where the plant grows.
Interestingly, A. fragrantissima has demonstrated enormous chemical diversity due to the presence of a variety of different chemotypes in the plants grown in different regions of the world. Although, few studies have been reported on the medicinal applications and isolation of bioactive phytochemicals of A. fragrantissima from Saudi Arabia. For instance, in a recent study, the phytochemical constituents of essential oils (EOs) extracted through a hydro-distillation process of dried aerial parts of A. fragrantissima cultivated in Egypt and Madinah Monawara, Saudi Arabia, were analyzed and compared using gas chromatography. Notably, the plant collected from Madinah contained α-thujone, whereas the plant from Sharkia (Egypt) has exhibited santolina alcohol as the major component (Farouk et al., 2019). However, to the best of our knowledge, detailed analysis of the chemical profile of the EOs of A. fragrantissima plant grown in the central region of Saudi Arabia has not been performed yet. Therefore, the study of the chemical constituents of A. fragrantissima cultivated in this region of Saudi Arabia is highly desirable. Herein, we study the chemical compositions of essential oils extracted from the leaves and stems of A. fragrantissima grown in the Riyadh region of Saudi Arabia. The chemical profiling of EOs was performed by GC-FID and GC–MS characterization techniques on two unlike stationary phase (polar and non-polar) columns.
2 Materials and methods
2.1 Plant material
Entire aerial parts of A. fragrantissima were collected from Rawdat Khuraim (Fig. 1) area of Riyadh, Saudi Arabia in February 2011. Identification of A. fragrantissima were authenticated by Dr. J. T. Pandalayil, a botanist at KSU. A specimen sample (AFR-21) of A. fragrantissima is retained in our research laboratory.
Geographic coordinate (via GPS) of the plant material collection location.
2.2 Essential oil extraction from the leaves and stems of A. fragrantissima
Firstly, the leaves and stems from the freshly collected aerial parts of A. fragrantissima were carefully separated from each other. The separated leaves (150 g) and stems (95 g) were chopped into small pieces (0.2–0.3 cm) and separately subjected to a Clevenger apparatus for hydro-distillation as described earlier (Khan et al., 2016b). After 3 h of distillation 2.5 g and 0.72 g yellow color oils were obtained from leaves and stems of A. fragrantissima, respectively. The yields of the oils from the leaves and stems of A. fragrantissima were 1.7% and 0.8% (w/w) on a fresh weight basis, respectively. The EOs obtained from leaves and stems of A. fragrantissima were dried over anhydrous Na2SO4 and stored at 4 °C until they were analyzed.
2.3 Chemicals
Analytical grade DEE (diethyl ether) from Sigma–Aldrich, Germany was used for the dilution of leaves and stems EOs of A. fragrantissima. Pure volatile constituents, e.g., α-pinene, α-terpinene, β-pinene, terpinen-4-ol, 1,8-cineole, eugenol, and α-bisabolol, along with volatile oils with high contents of limonene, sabinene, β-myrcene, β-phellandrene, α-terpinolene, germacrene D, bicyclogermacrene, caryophyllene oxide, α-thujene and α-terpinene were available with us and used for co-injection/comparative analysis.
2.4 GC and GC–MS analysis of A. fragrantissima EOs
Chemical analysis for the determination of A. fragrantissima leaves and stems EOs constituents were carried out by GC-FID and GC–MS analysis having two different stationary phase columns (HP-5MS and DB-Wax) applying the same method as described earlier (Khan et al., 2016a). Detailed methodology is provided in Supplementary materials (S1). The identified constituents of A. fragrantissima leaves and stems EOs and their relative percentages are provided in Table 1 and constituents are listed according to their elution order on the HP-5MS column. *Components are recorded as per their order of elution from a nonpolar column.
No.
Compound
LRILit
LRIExpa
LRIExpp
AFL (%)b
AFS (%)b
1
Isoamyl acetate
–
875
1123
t
t
2
Heptanal
–
901
1184
t
t
3
Santolina triene
906
907
1032
1.5 ± 0.41
1.2 ± 0.38
4
Artemisia triene
923
–
1067
t
t
5
Ethyl 3-Methyl-2-Butenoate
–
–
1226
t
t
6
α-Thujene
924
926
–
0.1
0.1
7
Ethyl tiglate
929
–
1238
t
t
8
α-Pinene
932
933
1019
0.2
0.2
9
Sabinene
969
973
1119
1.0
0.6
10
β-Pinene
974
976
1104
0.1
0.1
11
2-Pentyl furan
984
990
–
0.1
0.1
12
Myrcene
988
992
1164
0.6
0.1
13
Yomogi alcohol
999
999
1395
2.1 ± 0.57
2.4 ± 0.49
14
δ-3-Carene
1008
1012
–
0.1
0.1
15
α-Terpinene
1014
1016
1177
0.1
0.1
16
p-Cymene
1020
1024
1269
0.3
0.2
17
Limonene
1024
–
1196
0.1
0.1
18
β-Phellandrene
1025
1029
1205
0.2
–
19
1,8-Cineole
1026
1031
1208
0.4
0.3
20
(Z)-β-Ocimene
1032
1034
1238
0.1
0.1
21
Santolina alcohol
1034
1038
1409
10.1 ± 1.30
10.4 ± 1.50
22
γ-Terpinene
1054
1059
1245
0.2
–
23
(E)-2-Octenal
–
–
1431
t
t
24
Artemisia ketone
1056
1063
1352
12.7 ± 0.46
16.3 ± 0.74
25
cis-Sabinene hydrate
1065
1068
–
0.1
0.1
26
n-Octanol
1063
1070
1556
0.2
0.2
27
Artemisia alcohol
1080
1083
1511
0.8
0.9
28
α-Terpinolene
1086
1089
1282
t
–
29
Isobutyl tiglate
1088
1098
1361
t
0.1
30
Isopentyl 2-methylbutanoate
1100
1100
1280
0.1
0.1
31
Isopentyl isovalerate
1102
1104
1297
0.1
0.1
32
α-Thujone
1101
1107
1424
3.9 ± 0.03
3.6 ± 0.03
33
1-Octen-3-yl acetate
1110
1115
1378
0.1
0.1
34
β-Thujone
1112
1118
1445
5.1 ± 0.11
3.2 ± 0.08
35
trans-Sabinol
1137
1144
1710
14.9 ± 0.13
19.2 ± 0.01
36
trans-Verbenol
1140
1147
1687
0.1
t
37
Camphor
1141
1149
–
0.1
–
38
Sabina ketone
1154
1157
–
1.3 ± 0.11
0.3 ± 0.06
39
Isoborneol
1155
1160
–
0.1
0.1
40
Pinocarvone
1160
1165
1568
0.1
0.1
41
Lavandulol
1165
1167
1683
0.4
0.4
42
Artemisia acetate
1169
1172
–
0.3
0.3
43
Terpinen-4-ol
1174
1179
1608
0.5
0.3
44
Isoverbanol
–
1182
–
t
0.1
45
α-Thujenal
–
1186
1630
0.1
0.2
46
Cryptone
1183
1189
–
0.1
t
47
α-Terpineol
1186
1191
1705
0.1
–
48
Myrtenol
1193
1194
1800
0.1
0.1
49
Methyl chavicol
1195
1199
1673
0.4
0.3
50
(E)-Ocimenone
1235
1238
–
0.1
0.1
51
Cuminaldehyde
1238
1242
–
0.1
0.1
52
Ethyl phenyl acetate
–
1246
1789
t
–
53
Lavandulyl acetate
1288
–
1610
0.3
0.2
54
trans-Sabinyl acetate
1289
1297
1659
20.7 ± 0.00
24.0 ± 0.19
55
trans-Pinocarvyl acetate
1298
1302
–
t
–
56
Myrtenyl acetate
1324
1325
1693
0.1
–
57
p-Mentha-1,4-dien-7-ol
1325
–
2062
t
t
58
δ-Elemene
1335
1341
1472
0.6
0.3
59
Eugenol
1356
1359
2171
0.1
0.1
60
cis-Carvyl acetate
1365
1364
–
0.1
–
61
β-Elemene
1389
1395
1592
0.1
0.1
62
(Z)-Jasmone
1392
1401
1950
0.4
0.3
63
Methyl eugenol
1403
1404
2017
0.1
0.1
64
α-Gurjunene
1409
1417
–
0.1
0.1
65
Cuminyl acetate
–
–
1972
t
t
66
β-Caryophyllene
1417
1425
1599
0.2
0.1
67
β-Copaene
1430
1434
1594
0.1
0.1
68
trans-α-Bergamotene
1432
1438
1538
0.1
–
69
(E)-β-Farnesene
1454
1458
1669
0.9
0.1
70
β-Acoradiene
1469
1465
1665
0.1
0.1
71
Isoamyl phenylacetate
–
1481
2006
0.1
–
72
Germacrene D
1484
1488
1714
3.3 ± 0.33
1.8 ± 0.28
73
β-Selinene
1489
1493
1723
0.1
0.1
74
Bicyclosesquiphellandrene
–
–
1749
t
–
75
Bicyclogermacrene
1500
1503
1739
0.8
0.3
76
α-Muurolene
1500
1505
1729
0.2
0.2
77
trans-β-Guaiene
1502
1509
–
0.1
–
78
(E,E)-α-Farnesene
1505
–
1751
0.1
0.1
79
δ-Guaiene
–
–
1618
0.1
0.1
80
7-epi-α-Selinene
1520
1519
1764
0.1
–
81
β-Sesquiphellandrene
1521
1529
1775
5.5 ± 0.01
4.8 ± 0.01
82
δ-Cadinene
1522
–
1761
0.1
t
83
(Z)-Nerolidol
1531
1539
–
0.1
–
84
Elemol
1548
1554
2086
0.2
0.2
85
Germacrene D-4-ol
1574
1577
2056
0.1
–
86
Spathulenol
1577
1584
2131
0.3
0.1
87
Caryophyllene oxide
1582
1591
1990
0.1
t
88
Viridiflorol
1592
–
2092
0.1
t
89
Humulene epoxide II
1608
1606
2045
t
t
90
Isoeugenyl acetate
1614
1612
2404
0.1
–
91
1,10-di-epi-Cubenol
1618
1617
2067
0.4
0.3
92
1-epi-Cubenol
1627
1623
–
0.1
–
93
γ-Eudesmol
1630
1630
2178
0.2
0.2
94
α-Acorenol
1632
1635
2126
0.4
0.3
95
β-Eudesmol
1649
1658
2238
0.8
1.5
96
α-Cadinol
1652
1661
2243
0.1
–
97
7-epi-α-Eudesmol
1662
1666
–
0.1
0.1
98
β-Bisabolol
1674
1676
2152
0.2
0.1
99
epi-α-Bisabolol
1683
1688
–
0.3
0.1
100
α-Bisabolol
1685
1692
2222
0.2
–
101
(2Z,6Z)-Farnesol
1698
1694
2324
0.3
0.5
102
Tetradecanoic acid
–
1764
–
0.1
–
103
α-Costol
1773
1774
2590
0.1
0.1
104
Palmitic acid
1959
1958
–
0.1
0.2
105
(E)-Phytol
1942
2108
2618
0.3
0.1
106
n-Tricosane
2300
2300
2300
0.1
–
107
n-Pentacosane
2500
2500
2500
0.1
t
108
n-Heptacosane
2700
2700
2700
t
–
Monoterpene hydrocarbons
4.6
2.9
Oxygenated monoterpenes
75.8
83.5
Sesquiterpene hydrocarbons
12.6
8.3
Oxygenated sesquiterpenes
4.2
3.5
Aliphatic hydrocarbons
0.3
0.1
Oxygenated aliphatic hydrocarbons
0.8
0.9
Diterpenoid
0.3
0.1
Aromatics
0.1
0
Total identified
98.7
99.3
2.5 Calculation of linear retention indices (LRIs)
LRIs values of A. fragrantissima leaves and stems EOs constituents were determined following a previously reported method (Khan et al., 2016a), and these are listed in Table 1. Detailed methodology is provided in Supplementary materials (S2).
2.6 Identification of volatile components
Identification of the A. fragrantissima leaves and stems EOs constituents were carried out via analysis on DB-Wax and HP-5MS columns as described previously (Khan et al., 2016a). Detailed methodology is provided in Supplementary materials (S3). GC–FID chromatogram for the identified constituents of A. fragrantissima leaves and stems EOs on HP-5MS column is given in Fig. 1s and Fig. 2s, respectively (Supplementary materials).
3 Results and discussion
For the purpose of the detail analysis of the essential oil (EO) components of the aerial parts of A. fragrantissima. The EOs of leaves and stems of A. fragrantissima were extracted through a hydro-distillation process for three hours using a Clevenger-type apparatus (Khan et al., 2016b). The detail analysis of the as-obtained EOs was performed using a gas chromatography–mass spectrometry (GC–MS) and gas chromatography–flame ionization detector (GC–FID) using both polar and nonpolar columns. The analysis has revealed the presence of 108 compounds in the EO of leaves, whereas a total of 85 compounds were identified in the stems oil. Among the 108 compounds identified in both oils, 85 compounds were found to be present in both the oils. Whereas, 23 components were specifically present in the leaves oils. All the identified components and their respective amounts are provided in the Table 1 according to their elution order on a nonpolar (HP-5MS) column.
According to the results presented in the Table 1, oxygenated monoterpenes were found to be dominated in both oils. For example, the stems oil contained 83.5% of oxygenated monoterpenes, whereas, the leaves oil exhibited the presence of 75.8% of these components. Sesquiterpene hydrocarbons were present at distant second position in the studied oils, which were present in the amount of 8.3% in the stems oil and 12.6% in the leaves oil, respectively. After these two types of compounds which were mainly dominated, monoterpenes hydrocarbons (2.9% and 4.6%) and oxygenated sesquiterpenes (3.5% and 4.2%) were also present in appreciable amount in the stems and leaves oils of A. fragrantissima, respectively. Apart from these, some other classes of compounds were also found in negligible amount which include, aliphatic hydrocarbons, oxygenated aliphatic hydrocarbons, diterpenoids and aromatics etc. Notably, most of these compounds are present in both the studied oils, however their contents varied significantly.
Out of 85 compounds which were identified in the stems oil, most of the oil is constituted with only few compounds which include, trans-sabinyl acetate (24.0 ± 0.19), trans-sabinol (19.2 ± 0.01), artemisia ketone (16.3 ± 0.74), santolina alcohol (10.4 ± 1.50), and β-sesquiphellandrene (4.8 ± 0.01). Whereas the major chunk of the leaves oil is occupied by trans-sabinyl acetate (20.7 ± 0.00), trans-sabinol (14.9 ± 0.13), artemisia ketone (12.7 ± 0.46), santolina alcohol (10.1 ± 1.30), β-sesquiphellandrene (5.5 ± 0.01), β-thujone (5.1 ± 0.11). The results confirmed that chief chemical components of both (leaves and stems) oils were found to be almost same, however, their contents varied noticeably from each other (Fig. 2). Notably, the dominant volatile (more than 20% of the total oil) of the currently studied A. fragrantissima population seems to be trans-sabinyl acetate which belongs to a class of rare natural products (Radulović et al., 2015). In both stems and leaves oils, trans-sabinyl acetate is found to be the major component demonstrating a presence of 24 and 20% in the studied oils, respectively. According to a study published in 1964, sabinene, sabinol and sabinyl acetate are highly toxic metabolites (Casares, 1964).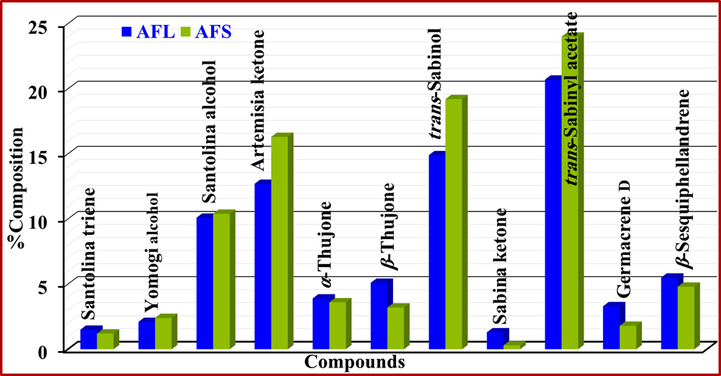
Comparison of major components in A. fragrantissima leaves and stems EOs.
However, an extensive literature survey about the phytochemical constituents of A. fragrantissima population belonging to the different regions of world has revealed that, none of the study published so far (to the best of our knowledge) has indicated towards the presence of trans-sabinyl acetate as the major component (Table 2).
Country
City
Chemotype
Major components (%)
Reference
Egypt
Sinai
α-Thujone
α-Thujone (29.5), santolina alcohol (18.3), artemisia ketone (15.2), β-thujone (10.8), trans-pinocarveol (6.8) and yomogi alcohol (4.4)
(El-Shazly et al., 2004)
Allamain
α-Thujone
α-Thujone (28.4), santolina alcohol (16.1), artemisia ketone (14.8), β-thujone (12.5), pinocarvone (4.7) and yomogi alcohol (3.2).
(Almadiy et al., 2016)
Sinai
Santolina alcohol
Santolina alcohol (18.3), artemisia ketone (15.2), α-thujone (28.4), β-thujone (12.5) and trans-pinocarveol (4.7)
(Nenaah, 2014; Nenaah et al., 2015)
Saint Catherine
α-Thujone
α-Thujone (34.0), trans-2,7-dimethyl-4,6-octadien-2-ol (24.4), 2,5,5-trimethyl-3,6-heptadien-2-ol (8.2), eucalyptol (8.2), 1,5-heptadien-4-one-3,3,6-trimethyl (7.7), artemisia alcohol (3.5)
(Zeedan et al., 2014)
Sharkia
Santolina alcohol
Santolina alcohol (27.2–30.8), α-thujone (11.8–18.9), artemisia ketone (11.8–14.5), lavandulol (0.33–12.5), β-thujone (7.2–8.6), 4(10)-thujen-3-ol (1.6–8.3) and trans-sabinyl acetate (4.7–8.3)
(Farouk et al., 2019)
Jordan
Mafraq
Artemisia ketone
Artemisia ketone (19.9), β-sesquiphellandrene (14.6), carvacrol (13.4), α-thujone (12.4) and artemisyl acetate (6.1)
(Alsohaili and Al-fawwaz, 2014)
Mafraq
β-Thujone
β-Thujone (11.3–22.1), trans-sabinyl acetate (0.8–10.2), α-terpineol (3.5–9.4), trans-menth-2-en-1-ol (6.5–13.3)
(Alsohaili, 2018)
Amman
α-Thujone
α-Thujone (13.8–33.8), β-thujone (11.9–24.1), artemisia ketone (3.0–22.0), santolina alcohol (3.5–18.3), santolina triene (1.8–7.3), yomogi alcohol (1.7–5.8) and trans-sabinyl acetate (1.2–5.2)
(Al-Jaber et al., 2018)
Saudi Arabia
Madinah
α-Thujone
α-Thujone (14.3–31.6), β-thujone (2.6–24.6), 4-terpineol (3.4–12.9), artemisia ketone (1.3–12.6), santolina alcohol (3.6–8.2) and trans-pinocarveol (0.0–6.5).
(Farouk et al., 2019)
Riyadh
trans-Sabinyl acetate
trans-Sabinyl acetate (20.7–24.0), trans-sabinol (14.9–19.2), artemisia ketone (12.7–16.3), santolina alcohol (10.1–10.4), β-thujone (3.2–5.1) and β-sesquiphellandrene (4.8–5.5)
Present study
Yemen
Dhamar province
Artemisia ketone
Artemisia ketone (49.5), camphor (14.7) and α-bisabolol (11.2)
(Mansi et al., 2019)
Since, A. fragrantissima plant is highly used in the Saudi Arabia for various medicinal purpose, the biological/toxicological profile of the phytochemical constituents of this plant may provide valuable information. Particularly, the evaluation of the in vitro and in silico toxicity of trans-sabinyl acetate which is rarely dominant in the A. fragrantissima population is highly required which we planned to perform in our future study. Moreover, the FDA (US Food and Drug administration) has included Juniperus sabina in the list of Poisonous Plant Database, due to the presence of trans-sabinol and its derivatives like sabinene, sabinol and sabinyl acetate as the major components (Asili et al., 2010; Severino, 2009). This toxic Juniper species, due to the presence of toxic sabinol derivatives causes congestion of the kidneys with hematuria, congestion of other abdominal viscera, menorrhagia and abortion (Craig et al., 2004; Pages et al., 1996). Besides, the Artemisia absinthium EO rich in trans-sabinyl acetate (45.2% of the total oil) has also shown the toxic effect (Judzentiene et al., 2012).
4 Conclusion
Herein, we have studied the phytochemical constituents of stems and leaves EOs of A. fragrantissima collected from central region of Saudi Arabia. The information gathered about the volatile constituents of studied plant is extensively compared with the EOs of A. fragrantissima collected from other regions of the world, including Egypt, Jordon, Yemen and Saudi Arabia. The EOs of aerial parts of A. fragrantissima have displayed considerable variation in their chemical compositions when compared to the plants collected from other regions. In this study, the investigated plant has exhibited trans-sabinyl acetate as major component, which is rarely obtained in such a large amount (∼24%) in A. fragrantissima collected from other parts of the world. According to FDA, the plants containing trans-sabinyl acetate have been classified as poisonous plants category; therefore, the application of this particular plant for any medicinal purpose may have adverse effect on health. Besides, the studied plant also contains trans-sabinol (14.9 ± 0.13), artemisia ketone (12.7 ± 0.46), santolina alcohol (10.1 ± 1.30), β-sesquiphellandrene (5.5 ± 0.01), β-thujone (5.1 ± 0.11) in significant amount which have several industrial applications.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group No (RG-1438-077).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams, R.P., 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Allured publishing corporation Carol Stream, IL, fourth ed.
- Essential Oil Composition and Anticholinesterase Activity Evaluation of Achillea fragrantissima Growing Wild in Jordan. J. Herbs Spices Med. Plants. 2018;24:272-281.
- [Google Scholar]
- Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT-Food Sci. Technol.. 2016;69:529-537.
- [Google Scholar]
- Seasonal variation in the chemical composition and antimicrobial activity of essential oil extracted from Achillea fragrantissima grown in Northern-Eastern Jordanian desert. J. Essent. Oil-Bear. Plants. 2018;21:139-145.
- [Google Scholar]
- Composition and antimicrobial activity of Achillea fragrantissima essential oil using food model media. Eur. Sci. J.. 2014;10:156-165.
- [Google Scholar]
- Chemical and antimicrobial studies of Juniperus sabina L. and Juniperus foetidissima Willd. essential oils. J. Essent. Oil-Bear. Plants. 2010;13:25-36.
- [Google Scholar]
- The antimicrobial activity of the essential oil from Achillea fragrantissima. J. Ethnopharmacol.. 1991;33:187-191.
- [Google Scholar]
- Phytochemicals from Achillea fragrantissima are modulators of AβPP metabolism. J. Alzheimer's Dis.. 2018;66:1425-1435.
- [Google Scholar]
- The chronic toxicity of naturally-occurring substances. Juniperus sabina. Food Cosmet. Toxicol.. 1964;2:680-681.
- [Google Scholar]
- Toxicity studies on western juniper oil (Juniperus occidentalis) and Port-Orford-cedar oil (Chamaecyparis lawsoniana) extracts utilizing local lymph node and acute dermal irritation assays. Toxicol. Lett.. 2004;154:217-224.
- [Google Scholar]
- Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pac. J. Trop. Med.. 2016;9:228-234.
- [Google Scholar]
- Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) from Egypt. Die Pharmazie-An Int. J. Pharmaceut. Sci.. 2004;59:226-230.
- [Google Scholar]
- Comparative study for the volatile constituents and the antioxidant activity of the essential oils of dried Achillea fragrantissima cultivated in Madinah Monawara, Saudi Arabia and Egypt. Int. J. Food Prop.. 2019;22:395-404.
- [Google Scholar]
- Biological activities of the hydro-alcoholic and aqueous extracts of Achillea fragrantissima (Forssk.) grown in Jordan. Nat. Sci.. 2014;6:23.
- [Google Scholar]
- Toxic activity and chemical composition of Lithuanian wormwood (Artemisia absinthium L.) essential oils. Records Naturals Products. 2012;6:180-183.
- [Google Scholar]
- A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc.. 2016;20:711-721.
- [Google Scholar]
- Characterization of leaves and flowers volatile constituents of Lantana camara growing in central region of Saudi Arabia. Arab. J. Chem.. 2016;9:764-774.
- [Google Scholar]
- Chemical Composition and Biological Activity of the Essential Oil Isolated from the Leaves of Achillea fragrantissima Growing Wild in Yemen. Pharmacognosy. J.. 2019;11
- [Google Scholar]
- Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother.. 2018;104:343-365.
- [Google Scholar]
- Evaluation of Anticonvulsant Activity and HPLC–DAD Profiling of Achillea fragrantissima (Gaisoom) Extracts Growing in Saudi Arabia. Asian J. Pharmaceut. Res. Health Care. 2017;9:92-100.
- [Google Scholar]
- Bioactivity of powders and essential oils of three Asteraceae plants as post-harvest grain protectants against three major coleopteran pests. J. Asia-Pacif. Entomol.. 2014;17:701-709.
- [Google Scholar]
- Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.) J. Stored Prod. Res.. 2015;61:9-16.
- [Google Scholar]
- Sabinyl acetate, the main component of Juniperus sabina L'Herit. essential oil, is responsible for antiimplantation effect. Phytother. Res.. 1996;10:438-440.
- [Google Scholar]
- Toxic essential oils. Part IV: The essential oil of Achillea falcata L. as a source of biologically/pharmacologically active trans-sabinyl esters. Food. Chem. Toxicol.. 2015;80:114-129.
- [Google Scholar]
- Antimicrobial, antiviral activity and GC–MS analysis of essential oil extracted from Achillea fragrantissima plant growing in Sinai Peninsula. Egypt. J. Microb. Biochem. Technol. S. 2014;8:6.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.03.004.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







