Translate this page into:
Chelate formation and stability analysis of cobalt, nickel and copper with lomatiol
⁎Corresponding author. mohdaminmir@gmail.com (Muhammad Waqar Ashraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The pH-metric studies on the interaction of divalent metal cations, Co (II), Ni (II) and Cu (II) with lomatiol have been performed under the thermodynamic conditions achievable at constant ionic strength and infinite dilution in which a mole of divalent metal, combined with 02 mol of lomatiol. Lomatiol being bidentate could replace two water molecules at a time. The values of stepwise formation constants showed no conspicuous difference at 25 °C and 35 °C, and the complexes, registered a decrease in their stability values suggesting the low temperature as a favorable condition under which the chelation of metal with lomatiol is feasible. The reaction takes place without the use of any specific catalyst although under alkaline conditions. The reactions of lomatiol with metal cations in solution had been adjudged as the spontaneous reaction on account of the negative ΔG value in all the metal lomatiol systems. Also the study showed the positive experimental values of entropy changes in all the systems and negative value of free energy. The continuous fall in the values of the stepwise formation constants (k1 > k2 > k3) in the divalent metal-lomatiol system has been assigned to the statistical factor.
Keywords
Lomatiol
Chelates
ΔG
Stability constant
Free energy change
Entropy change
1 Introduction
The recent times has seen importance and recognitions of coordination chemistry due to the recent advances on the instrumental automation with the target on the solution of the insoluble issues connected with the different disciplines of sciences, biosciences, medicine, industry etc, which remained unresolved in the absence of the instrumental advances as are seen today. The field which has been reasonably explored is the coordination chemistry, exploiting the functional positions on the different organics resulting in the formation of five or six membered rings showing the reasonable stability of the resultant complexes or chelates. The hydroxynaphthoquinone family has been found as bidentate ligands with capacity to chelate bivalent, trivalent and tetravalent metal cations spread our whole periodic table. In 1961, Bottei and Gerace (1961) prepared metal chelate polymers of 5,8- dihydroxy-1,4-nephthoquinone which were found as fine deliquescent powders, insoluble in a wide variety of polar & non polar solvents. Thermal & spectral study of some divalent metal chelates of 2, 5-dihydroxyp- benzoquinone, were carried out by Bottei and Fangman (1966). The properities were compared with those of naphthazarin chelates. Akiyama and Mizutani (1961) in 1969 reported on the electrical conductivity and energy gap of 5, 8-dihydroxy-1, 4-naphthoquinone and its metal polymers.
The thermal and spectral properties of chelates of lawsone and juglone with divalent copper, zinc. nickel and lead were investigated by Bottei and McEacheun (1970). The composition, structure and hydrogen bonding of the β-diketones. Naphthazarin (5,8-hydroxy-1,4-naphthozuinone), the basic unit of several tetracyclic antitumor, antibiotics, and its glutathione conjugate was reduced by the one-and two electron transfer flawproteins NADPH-cytochrome P450 reductase and DT- diaphoresis to their semi-and hydroquinone forms, respectively by Emsley et al. (2007). Den et al. (2011) studied reduction in cytotoxicity of the azo compound which was a complex of Co (II) with 2-hydroxyphenyl-azo- 2-napthol (HPAN) in comparison to the parent compound on A549- lung carcinoma and peripheral blood mononuclear cells, while Kang et al. (2006) studied the effects of the naphthoquinone analogue, Naphthazarin (Nap), and its derivative, methyl naphthazarin (MetNap), on vascular reactivity using isolated rat aortic rings and human umbilical vein endothelial cells (HUVECs).
Little reference has been seen in regard to the Interaction of metal cations with Lomatiol. (2-hydroxy-3(v-methyl-v-methylallyl)-1, 4 naphthoquinone). The present focus has been on the studies of the chelating properties of lomatiol with divalent metal cations under the applied thermodynamic conditions. Naphthoquinones constitute a significant class of natural and synthetic compounds extensively investigated due to their various pharmacological applications (Santos et al., 2004; Camara et al., 2008; Ferreira et al., 2006; Vargas et al., 2006). Also the most organometallic ligands of the reported copper, nickel and cadmium complexes are metallocenes, half-sandwich, carbene-, CO-, or π-ligands (Grasa et al., 2010; Amouri et al., 2010; Hearn et al., 2013; Liu and Sadler, 2014; Meggers, 2007; Liu et al., 2014; Li et al., 2015; Bach et al., 1996; Kastl et al., 2012). The metal chelate complexes have wide range of application in medical field, (Kontoghiorghes et al., 2020; Markowicz-Piasecka et al., 2019; Adlard and Bush, 2018). As per literature survey there is no report in the literature devoted to the synthesis of (Cobalt, Nickel and Copper with Lomatiol.
2 Materials and methods
All the chemicals and reagents used were of pure grade. The carbonate free distilled water was employed. The lomatiol was obtained by extracting the seeds of lomatia ilicifolia (Fig. 15) with boiling water acidified with acetic acid and allowing the filtered extract to cool, when the product crystallized out. It was recrystallized from the same solvent (m.p-128 °C). The purity of the lomatiol was analysed by 1HNMR Technique.
The pH–based protocol achieved under the thermodynamic conditions achievable applying constant ionic strength and infinite dilution in solution state is basic to the determination of the preceding terms essentially required to interpret the pH-database analysing the pH-curves at different temperature of the solutions.
where ΔNaOH is extent of complexation and chelation
Stepwise formation constants (k1, K2, k3……..) can be determined from the formation curves (pL versus ň) where ň at 0.5, 1.5, 2.5 yields corresponding pL value equivalent to log k1, log k2, logk3…….. Respectively with their validation when logk1/k2 ≥ 2.5, the basic condition for the application of the technique called Bjerrum technique (Wuenschell et al., 1991). The deviation, if any from this condition: logk1/k2 ≥ 2.5, demands the refinement of the k1, k2, k3…… values applying Irving and Rossetti method (equation (4)).
When n = 1, 2, 3… .… . . . . .
The equation (5), 6, 7 show resemblance to y = -mx + c, the intercept of which yields
Respectively
log where ň: formation function; pL: free-ligand exponent; pKH: Protonation constant; K: Dissociation Constant; βn: Over-all formation (stability) constant; k1, k2, k3….…: stepwise formation (stability) constant; ΔG: Free energy change; ΔH: enthalpy change; ΔS: entropy change
The ethanolic solution (2 × 10−3M) of lomatiol was prepared whereas the aqueous solutions of nitrates of metals under study such as Co (II), Ni (II), Cu (II), were prepared in carbonate-free distilled water.
The experimental protocol included the uses of the said solution of lomatiol and the metal nitrates in the formation of the following sets: A, B for each of the system consisting of lomatiol and a metal cation at constant Ionic strength (0.1 M KNO3) and infinite dilution to generate the thermodynamic conditions under which βc (conditional stability) becomes equivalent to βT (thermodynamic stability constant) as per the method of Wuenschell et al (Wuenschell et al., 1991).
A: 4 × 10-3M HNO3 + 1 × 10-3M lomatiol
B: 4 × 10-3M HNO3 + 1 × 10-3M lomatiol + 8x10-5M metal cations Set total volume: 50 ml in 50% EtOH in water by volume.
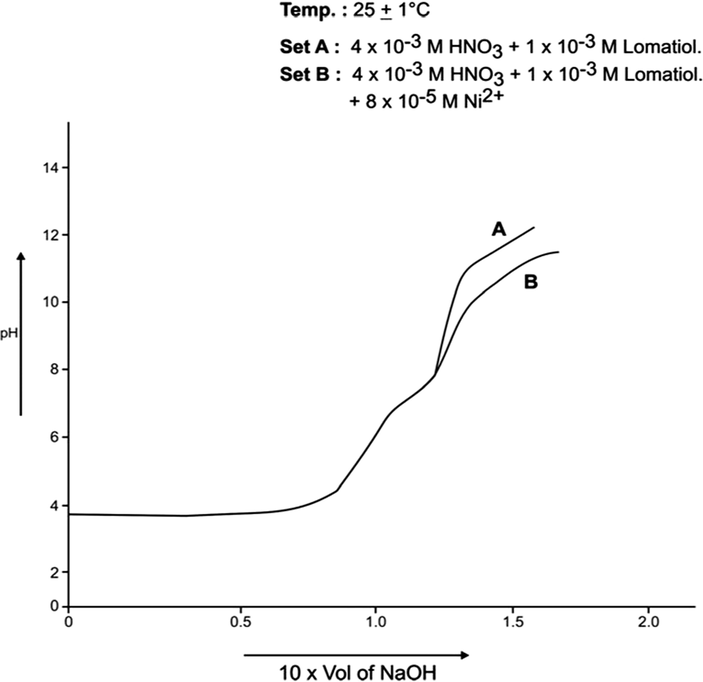
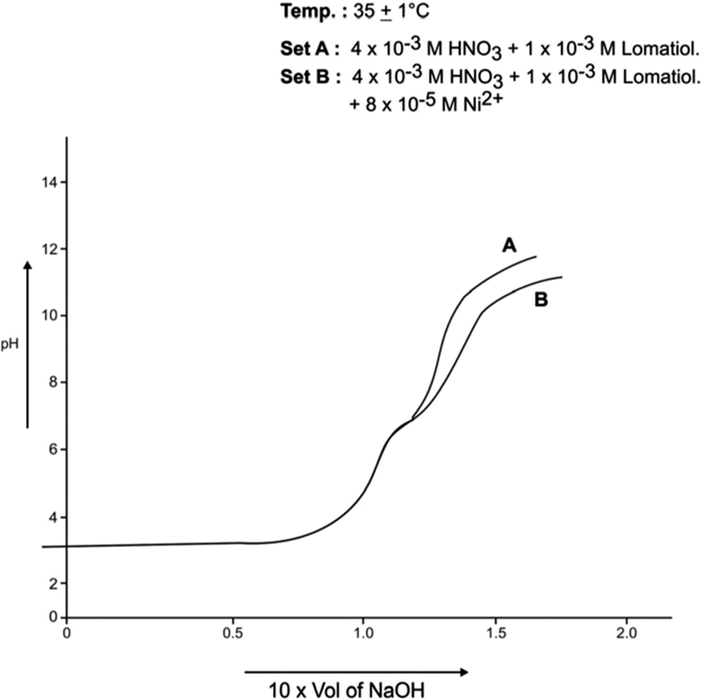
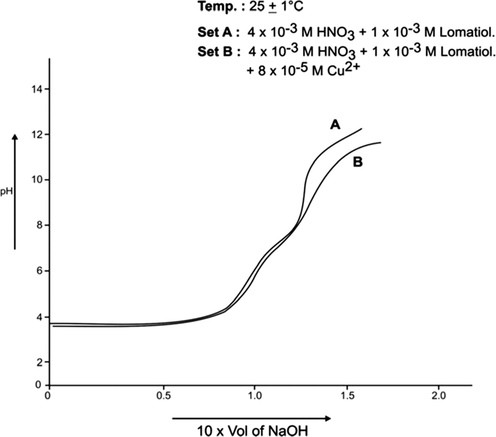
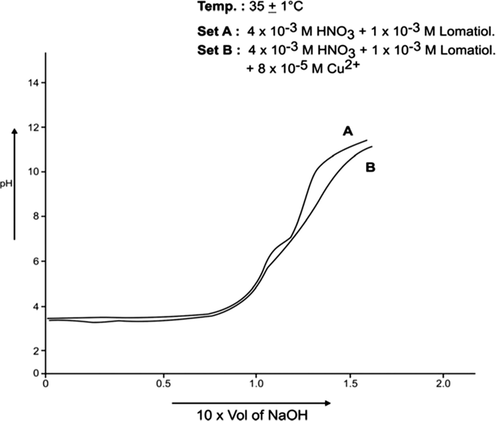
Each of A, B sets was pH- metrically titrated with the incremental addition of 1 × 10−1 NaOH (in 50% EtOH in water by volume) at 25 °C and 35 °C separately under the applied conditions. The pH data so obtained were used in plotting pH against the NaOH added (in ml). The plots so drawn for Co (II)-lomatiol, Ni (II) – Lomatiol, Cu (II)-Lomatiol, systems are given and shown as below.
The log pkH of lomatiol at 25 °C and 35 °C was calculated from Figs. 1, 2 respectively.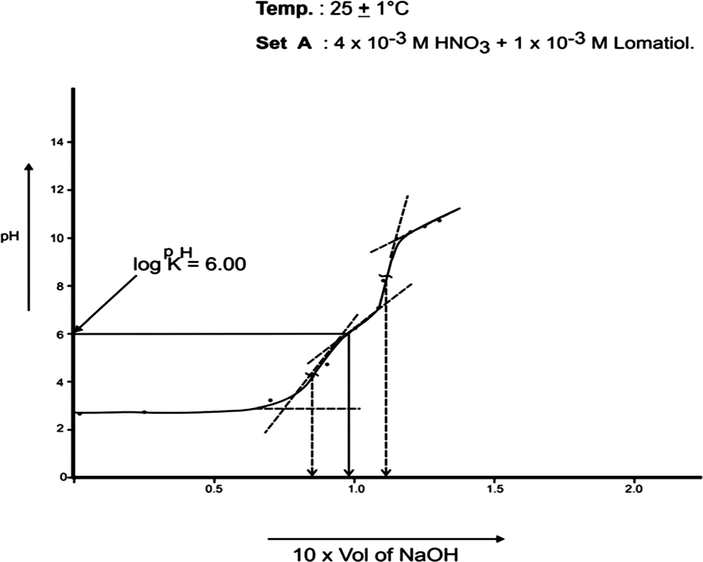
pH Titration of binary Mixture of HNO3 and Lomatiol with 1 × 10−1 M NaOH for the calculation of protonation constant (pKH).
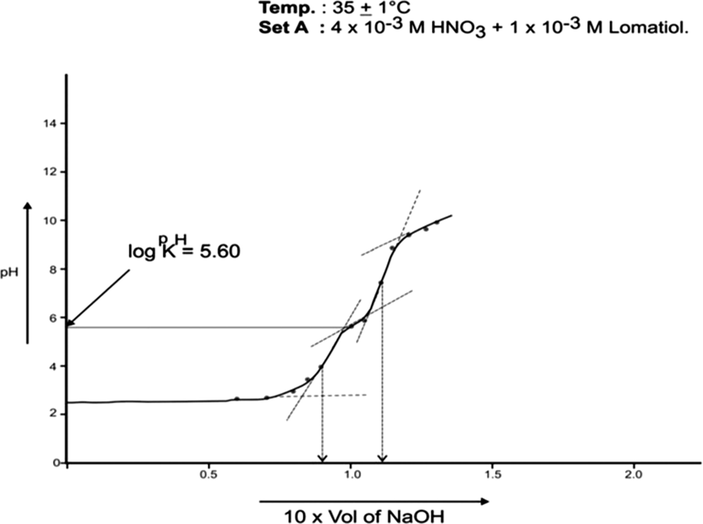
pH Titration of binary Mixture of HNO3 and Lomatiol with 1 × 10−1 M NaOH for the calculation of protonation constant (pKH).
3 Results and discussion
The pH studies were conducted under thermodynamic conditions achieved with the maintenance of constant strength and infinite dilution of the solutions (Table 1). About twelve fold concentration of lomatiol compared to the metal concentration in the solution was maintained to guard against the metal hydrolysis during the study time.
Temp (0C)
pK
PkH
25
6.00
106
35
5.60
105.6
The interpretation and the structural conclusions followed the estimation of the essential terms like , pL, PKH, etc to reach or jump to certain conclusions. The separation of the pH curves A, B, in each system indicated that B assumed higher acidity as compared to the earlier curve due to the furnishing of hydrogen ions with the dissociation of lomatiol and on the chelation of metal cation by lomatiol.
The curve A is a representation of titration of a binary mixture of strong acid (HNO3) and a weak acid (lomatiol). The corresponding pH value of the central point of the inflexion points seen on the curve exhibited the value of pH at half neutralization equivalent to pK value of lomatiol in line with Henderson and Hasselbatch equation (3). (Figs. 1, 2)
For
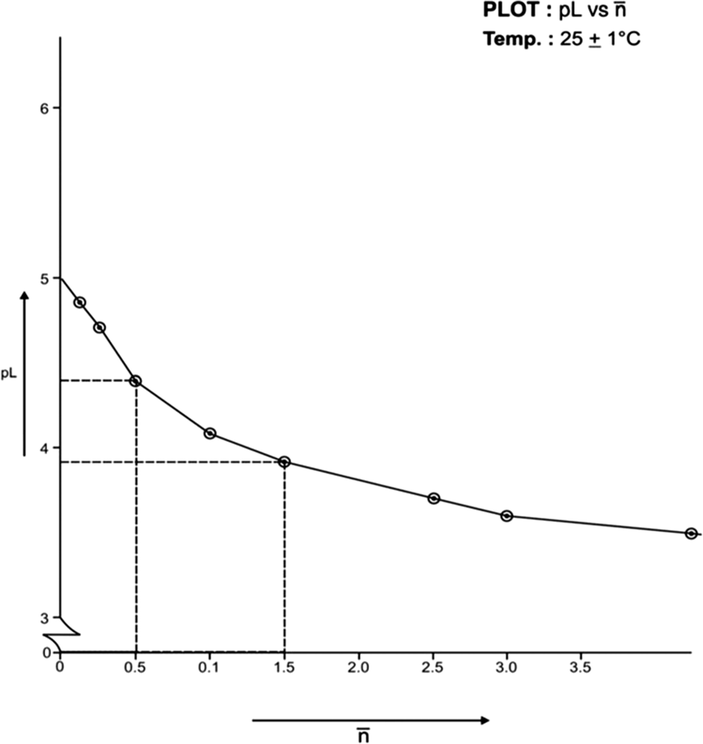
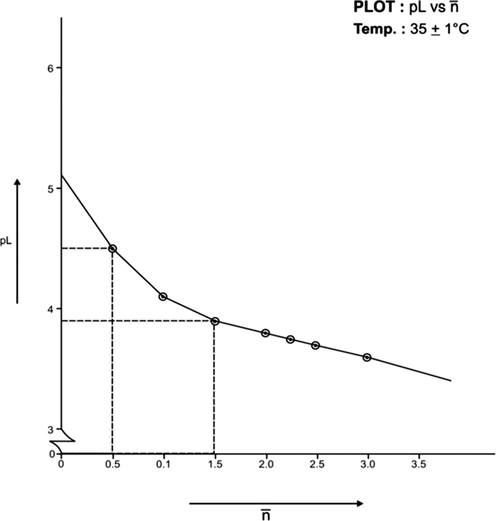
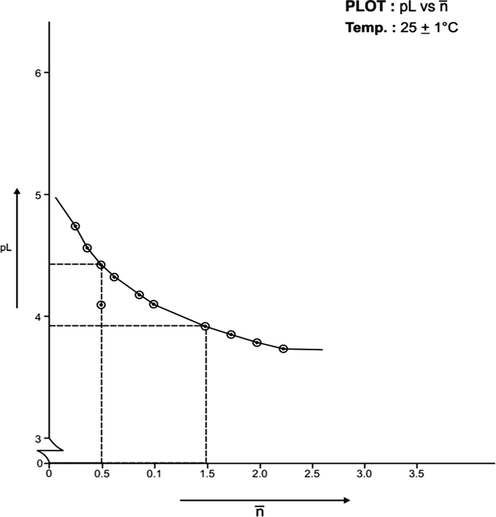
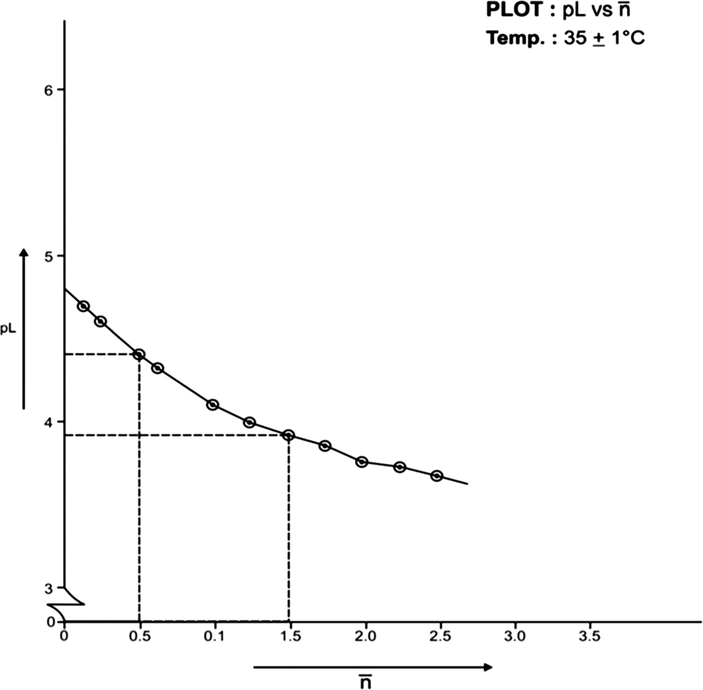
(formation function) of the system, the curves A and B in each system was based upon (Figs. 3, 5, 7, 9, 11, 13). The separation difference (ΔNaOH) at different pH values was noted and converted to equivalent hydrogen ion concentration (the extent of chelation), which on division by total metal concentration in the solution yielded
value. The pH, PkH and Δ NaOH values were further utilized to calculate pL (free ligand exponent) values. The set of pL and
values were plotted (pL versus
) to generate formation curves for each systems. The formation curve (at 25 °C and 35 °C) for Co (II), Ni (II), Cu (II), have been shown in Figs. 4, 6, 8, 10, 12, and 14 respectively.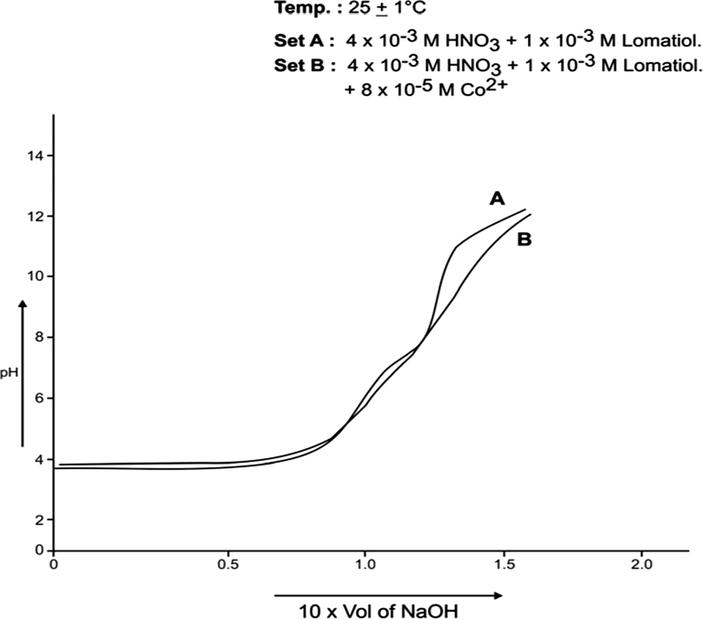
pH Metric Titration with 1 × 10−1 MNaOH.
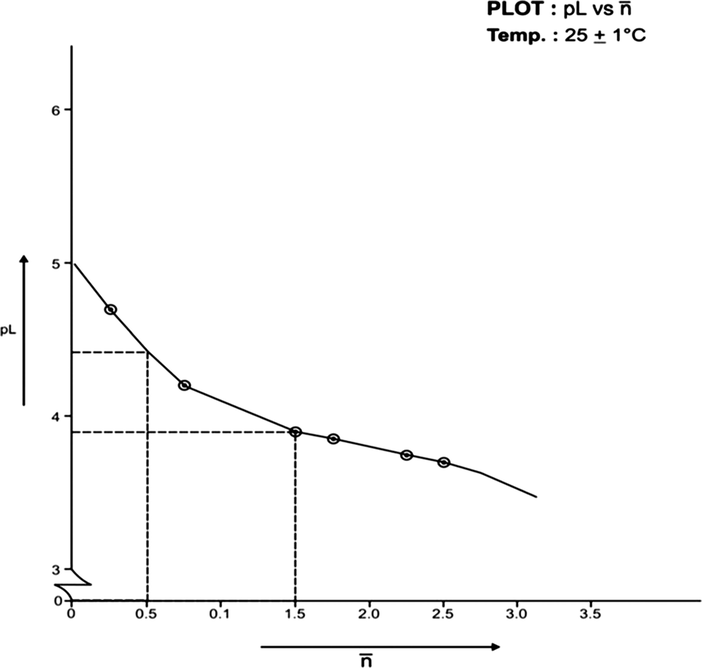
Formation Curve on Co (II)-Lomatiol System.
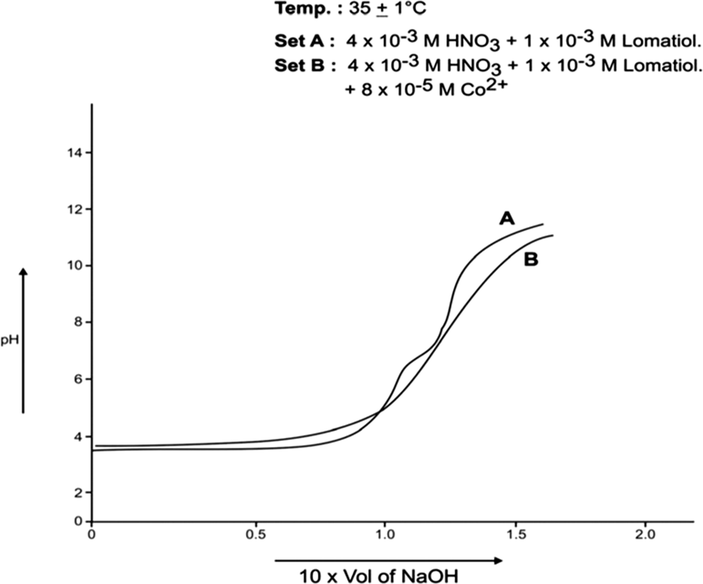
pH Metric Titration with 1 × 10−1 MNaOH.
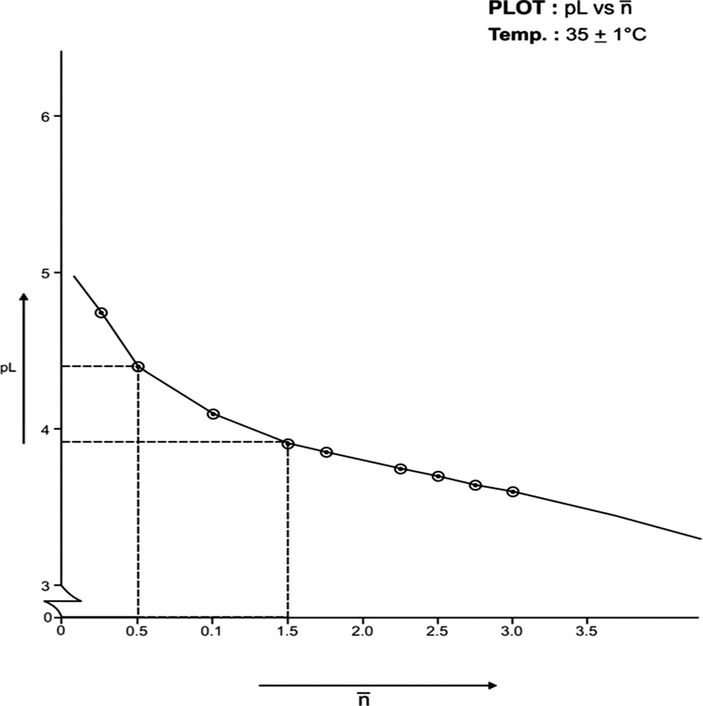
Formation Curve on Co (II)-Lomatiol System.
pH Metric Titration with 1 × 10−1 MNaOH.
Formation Curve on Ni (II)-Lomatiol System.
pH Metric Titration with 1 × 10−1 MNaOH.
Formation Curve on Ni (II)-Lomatiol System.
pH Metric Titration with 1 × 10−1 MNaOH.
Formation Curve on Cu (II)-Lomatiol System.
pH Metric Titration with 1 × 10−1 MNaOH.
Formation Curve on Cu (II)-Lomatiol System.
The limits of formation curves for bivalent and trivalent systems could spread the values ≥ 0.5 and ≥ 1.5 but ≤ 2.0 for bivalent metal - lomatiol systems, making possible to read the corresponding values of pL at = 0.5, 1.5, 2.5 (equivalent to log k1, log k2 and log k3 respectively) with leads on the further interpretation of the systems involved.
Analysis of the above figures clearly showed that the values on the systems involving bivalent metal cations approximated to 02 indicating the metal chelation with lomatiol in two steps.
Whereas the n¯ value neared to 03 for the systems where the trivalent metal was subjected to interaction with lomatiol. The steps involved are given below.
Lomatiol, being bidentate, has the capacity to replace two H2O molecule and may occupy the space vocated by 02 H2O molecule in the structural pattern of M (H2O)n at time in steps. The accompanying sequence has been cited as the sample representation.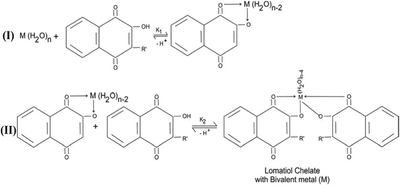

For systems involving trivalent metal, an additional insertion of a lomatiol molecule into the second step (II) scheme as below may occur with a lead to the metal ultimate structure: M (H2O) n-6 R3 (Scheme 1).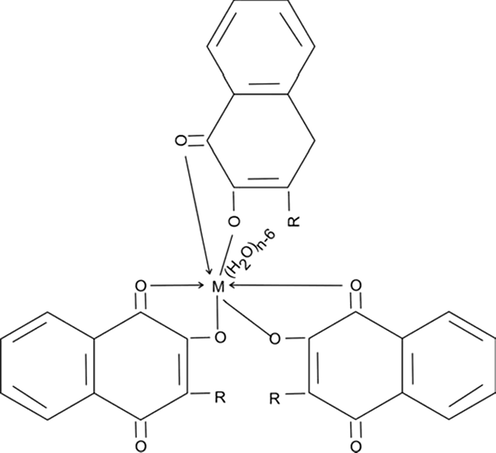
In Table 2 are given the log k1/k2 values of stepwise formation constants for bivalent metal cations with lomatiol systems calculated.
Systems
Temp.(°C)
log k1/k2
Co (II)- Lomatiol
25
0.52
35
0.48
Ni (II)- Lomatiol
25
0.48
35
0.60
Cu (II)-Lomatiol
25
0.48
35
0.46
On the examination of the Table 2, it was observed that the calculated values of k1 and k2 could not conform to the laid down conditions of Bjerrum technique (Pietrzk and Frank, 1979); that is, log k1/k2 ≥ 2.5, necessitating their refinement applying Irving and Rossotti procedure (Irving and Rossotti, 1954). The refined values of k1, k2 and k3 of the system under study are compiled in Table 2, 3.
Systems
Temp. (°C)
k1
k2
β2
ΔG° K. Cal mol−1
ΔH° K.Calmol−1
ΔS° Cal mol−1 deg−1
Co (II)-Lomatiol
25
2.75 × 104
7.59 × 103
2.09 × 108
−11.4199
+2.31
+46.07
35
2.88 × 104
8.22 × 103
2.37 × 108
−11.8811
Ni (II)-Lomatiol
25
2.75 × 104
8.70 × 103
2.39 × 108
−11.5022
+1.68
+44.22
35
3.16 × 104
8.31 × 103
2.63 × 108
−11.9450
Cu (II)-Lomatiol
25
3.16 × 104
8.71 × 103
2.75 × 108
−11.5846
−5.25
+21.23
35
2.51 × 104
8.22 × 103
2.06 × 108
−11.7960
The refined values of the stepwise formation constants followed the order: k1 > k2 > k3 indicating that the bond strength registered a decrease with the successive addition of lomatiol molecule to metals in reference- an attribution of steric effect showing coincidence with general observation.
For k2/ k1 = k3/ k2 = 0.33 value, the statistical factor is held responsible for the fall of values of stepwise formation constants with the addition of ligating molecule. The data on the present study showed deviation from 0.33 value, suggesting the involvement of other variables such as columbic forces in addition to the statistical factor, for the fall in the stepwise formation constants.
The metal cations are Lewis acid (electron deficient) with the capacity to accept electrons whereas the ligands, bidentate in nature like lomatiol are bases with the capacity to donate electrons. The lomatiol type ligand interacts with metal cations by way of donating an electron pair forming M—O bond, while —OH group in the ligand acts as a base by loss of proton suggesting the participation of anionic part of lomatiol (Scheme 2)
Lomatiol Chelate.
The temperature affects the stability of metal-ligand chelates. Examination of Table 2 the refined data on stability constants exhibited slight variation in the values of stability constants suggesting that the metal-ligand chelates of lomatiol showed no difference in the degree of dissociation with the rise of 10 °C (25°–35 °C). If the temperature is being raised up (above 45 °C) the chelate complex may get unstable, so the utmost condition for the reaction to complete is the maintenance of low temperature (Kalshetty et al., 2011) (Scheme 3).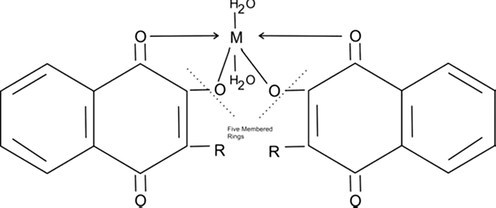
The free energy change (ΔG) assumed negative values for all the metal- lomatiol systems giving enough reasons to believe spontaneity of chelation processes. The entropy change being positive in all the systems led us to believe in the spontaneity of reaction. In Table 3 are given the ΔG, ΔH, ΔS values. The formation of the chelate complex being an autocatalytic phenomenon, so there is no requirement of any sort of catalyst, as the reaction proceeds by its own and occurs in a spontaneous manner.
In 1967 Shapet'Ko (Shapet'ko et al., 1967), demonstrated intramolecular hydrogen bonding in hydroxy naphthoquinones displaying five or six membered ring and thus the formation proton complexes involving primary and secondary bonds therein/thereon. The proton complexation gets replaced by metal complexation with the replacement of proton by metal cations on interaction with hydroxynaphthoquinone without disturbing other features and characteristics including primary and Secondary bonds formation.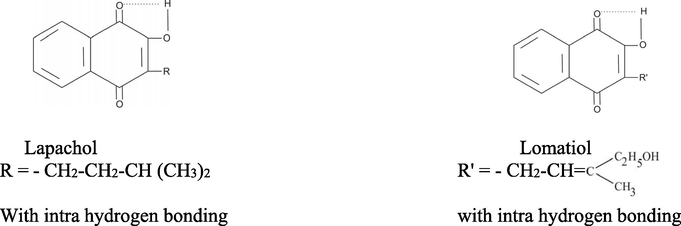
Basing upon the solution study data and the conclusion of Shapet' Ko, the tentative structures of the metal lomatiol chelation products are given below.
Metal chelates of lomatiol of divalent metal cations
Where M = Co (II), Ni (II), Cu (II)
Cobalt Ligancy: 06; Nickel Ligancy: 06; Copper Ligancy: 06 and 
4 1HNMR spectra of lomatiol
The 1HNMR spectra obtained from experimental and theoretical study were compared Fig. 15. Both spectra were combined on the same scale for an easier comparison. Experimental 1H NMR chemical shifts were, expressed in ppm, dispersed through the spectrum starting form tetramethylsilane as the internal standard. The theoretical calculations are used to confirm the data obtained by the experiments reported herein.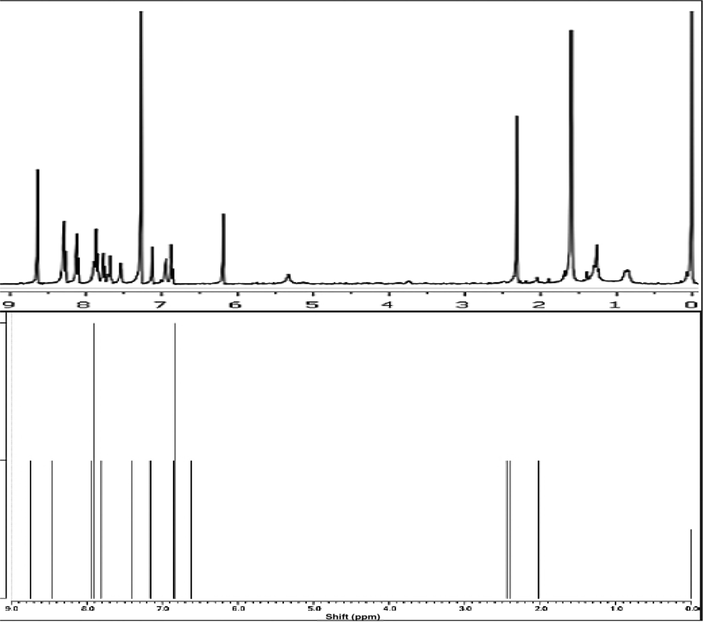
1HNMR Spectra of lomatiol using TMS as reference.
As seen from the Fig. 4, peaks belong to methyl hydrogens splitted into three between 2.02 and 2.45 ppm in the theoretical spectrum were seen as one peak in the experimental spectrum since the methyl protons have the same chemical environment leading their overlaps. The experimental methyl proton chemical shift values were stated as the average of three protons at 2.38 ppm. The peak seen at 6.19 ppm was assigned to deuterium exchangeable proton of the phenolic —OH group. The chemical shift value of this proton was 6.62 ppm in the calculated spectrum. The multiple peaks attributed to the aromatic protons between 6.94 and 8.64 ppm were calculated approximately at the same range between 6.84 and 8.74 ppm. The peaks ascribed to the protons numbered as 11 and 12 bound to aliphatic carbons were appeared at 7.54 and 6.84 ppm, respectively. These peaks of aliphatic protons were corresponded to the peaks at 7.80 and 6.84 ppm in the theoretical spectrum.
The 1H NMR spectra have no unidentified peaks other than the expected ones Fig. 15. These clear spectra also reveal that the molecule is highly pure.
5 Conclusion
From the obtained results it can be concluded that lomatiol as a ligand have enough donating electron sites to act as a good chelating ligand. The refined values of the stepwise formation constants followed the decreasing order indicating that the bond strength gets declined with the successive addition of lomatiol molecule to metals in reference- an attribution of steric effect showing coincidence with general observation. Also the free energy change (ΔG) shows negative values for all the metal- lomatiol systems suggests to believe spontaneity of chelation processes. Also the chelate formation reaction follows auto-catalytic nature, as the reaction takes place without the use of any catalyst, leading to its spontaneous nature. The entropy change being positive in all the systems led us to believe in the spontaneity of reaction. So in conclusion the lomatiol and the concerned metals form strong bonded complexes at normal conditions of temperature and pressure and without the use of any specific catalyst, could be used for many biological purposes.
Contribution by Authors: The idea development was by Dr. M. Amin Mir, the experimental setup was carried out by m. Amin Mir. The preparation of manuscript was carried out by Dr. M. Amin Mir and Dr. Waqar Ashraf.
Funding: There was no any funding agency for the concerned work, and all the expenditures were carried out by the authors itself, although we are thankful to PMU Saudi Arabia for allowing us to work on the project in reference.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Metals and Alzheimer’s disease: how far have we come in the clinic? J. Alzheimers Dis.. 2018;62:1369-1379.
- [Google Scholar]
- Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Phys. Soc.. 1961;26:1128-1130.
- [Google Scholar]
- Cyclometalated iridium(III) complexes as mitochondria-targeted anticancer agents. Chem. Int. Ed.. 2010;122:7692-7695.
- [Google Scholar]
- cis-{Pt(NH3)2(L)}2+/+ (L = Cl, H2O, NH3) Binding to Purines and CO: Does π-Back-Donation Play a Role? Aubke Angew. Chem., Int. Ed. Engl.. 1996;35:1974-1976.
- [Google Scholar]
- Thermal and spectral study of some divalent metal chemaltes of 2, 5- dihydroxy-p-benzoquinone.I. Inorg. Nucl. Chem.. 1966;28:1259-1264.
- [Google Scholar]
- Preparation and thermal stabilities of some metal chelate polymers of naphthazarin. J. Inorg. Nucl. Chem.. 1961;23:245-251.
- [Google Scholar]
- Thermal and spectral studies of some divalent metal chelates of lawsone and juglone. J. Inorg. Nucl. Chem.. 1970;32(8):2653-2663.
- [Google Scholar]
- Pinto, Molluscicidal activity of 2-hydroxy-[1, 4] naphthoquinone and derivatives. An. Acad. Bras. Cienc.. 2008;80:329-334.
- [Google Scholar]
- Electrochemical reduction of quinones in different media: a review. Chem.-Biol. Interact.. 2011;189(3):206-214.
- [Google Scholar]
- The composition, structure and hydrogen bonding of the diketones. Struct. Bond.. 2007;57:147-191.
- [Google Scholar]
- Synthesis and biological evaluation of β-lapachone and nor-β-lapachone complexes with 2-hydroxypropyl-β-cyclodextrin as trypanocidal agents. Bioorg. Med. Chem.. 2006;14:5459-5466.
- [Google Scholar]
- Gold(I) N-heterocyclic carbene complexes with an “activable” ester moiety: Possible biological applications. J. Organomet. Chem.. 2010;695:1119-1125.
- [Google Scholar]
- Organometallic Iridium(III) anticancer complexes with new mechanisms of action: NCI-60 screening, mitochondrial targeting, and apoptosis. ACS Chem. Biol.. 2013;8:1335-1343.
- [Google Scholar]
- The calculation of formation curves of metal complexes from pH titration curves in mixed solvents. J. Chem. Soc.. 1954;2904–2910
- [Google Scholar]
- Temperature effect on solution stability constants of the metal complexes with Schiff based derivative from 5-aldehydosalicyclic acid-aniline and its related compounds. Int. J. Appl. Biol. Pharm. Technol.. 2011;2(2):88-93.
- [Google Scholar]
- Naphthazarin and methylnaphthazarin cause vascular dysfunction by impairment of endothelium-derived nitric oxide and increased superoxide anion generation. Toxicol. in-vitro. 2006;20:43-51.
- [Google Scholar]
- Dual anticancer activity in a single compound: visible-light-induced apoptosis by an antiangiogenic iridium complex. Chem. Commun.. 2012;48:1863-1865.
- [Google Scholar]
- The history of deferiprone (L1) and the paradigm of the complete treatment of iron overload in thalassaemia. Mediterr. J. Hematol. Infect. Dis.. 2020;12:e2020011
- [Google Scholar]
- Phosphorescent iridium (III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials. 2015;39:95-104.
- [Google Scholar]
- The potent oxidant anticancer activity of organoiridium catalysts. Angew. Chem. Int. Ed.. 2014;53:3941-3946.
- [Google Scholar]
- Organoiridium complexes: anticancer agents and catalysts. Acc. Chem. Res.. 2014;47:1174-1185.
- [Google Scholar]
- Biocompatibility studies of gadolinium complexes with iminodiacetic acid derivatives. Biol. Trace Elem. Res.. 2019;189:426-436.
- [Google Scholar]
- Exploring biologically relevant chemical space with metal complexes. Curr. Opin. Chem. Biol.. 2007;11:287-292.
- [Google Scholar]
- Pietrzk, D.J., Frank, C.W., 1979. Analytical Chemistry. Academic Press, INC, New York, IInd Ed., 149.
- Strategies for the synthesis of bioactive pyran naphthoquinones. Med. Chem.. 2004;12:87-93.
- [Google Scholar]
- A quantitative study of dyeing with lawsone. J. Soc. Cosmet. Chem.. 1967;8(3):538-540.
- [Google Scholar]
- Synthesis of novel naphthoquinone-spermidine conjugates and their effects on DNA-topoisomerases I and II-a. J. Braz. Chem. Soc.. 2006;17:439-442.
- [Google Scholar]
- Chiral copperchelate complexes alter selectivities in metal affinity protein partitioning. J. Chromatogr.. 1991;543:345-354.
- [Google Scholar]







