Translate this page into:
Chemical change and mechanism of Millettia speciosa with sulfur fumigation by UHPLC–QTOF–MS/MS
⁎Corresponding author at: 15# Southwest Minzu University, No.16, South 4th Section 1st Ring Road, Chengdu 610041, Sichuan, China. zfzhang@swun.edu.cn (Zhifeng Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Millettia speciosa Champ. (MSCP), which is distributed and used in Southern China, is often sulfur-fumigated during postharvest handling to prevent insects and molds. In the present study, a comprehensive strategy was proposed by using ultrahigh-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry (UHPLC–QTOF–MS/MS) with multivariate analysis to rapidly identify potential chemical sulfur-fumigated MSCP (SF-MSCP) samples, discover chemical transformation mechanisms and control the quality of SF-MSCP. Eighty-six compounds were tentatively assigned, and ten compounds were screened out as potential markers. Moreover, the chemical transformation mechanism was deduced by hydrolysis, esterification and dehydration reactions that occurred during the sulfur-fumigated process. The developed method was successfully applied to predict 16 commercial MSCP samples, and 9 samples were found to contain sulfur compounds, ranging in sulfur residues from 100 to 310 mg/kg. These research outcomes can not only be used to distinguish the nonfumigated MSCP from SF-MSCP by chemical markers but also provide a chemical basis for further comprehensive investigating in-depth safety and functional evaluations of SF-MSCP.
Keywords
Millettia speciosa
UHPLC–QTOF–MS/MS
Multivariate analysis
Sulfur fumigation
Chemical transformation
1 Introduction
Millettia speciosa Champ. (MSCP) is called Niudali, a famous edible herb medicine that is widely distributed in Guangxi, Guangdong and Hainan Provinces of China (Wang et al., 2021). Phytochemical analysis has shown that MSCP mainly contains terpenoids, flavonoids, alkaloids, polysaccharides, phenylpropanoids and so on (Zhao et al., 2017; Wang et al., 2021). These constituents have been reported as the primary active ingredients associated with MSCP pharmacological activity, such as anti-fatigue (Zhao et al., 2015), enhancing immunomodulatory (Huang et al., 2020), antioxidative (Huang, Zhong, & Long, 2022), and anti-depressive activities (Chi et al., 2021). The root of MSCP has been used in Chinese medicine for the treatment of chronic bronchitis, coughing, rheumatic arthritis and kidney-deficiency syndrome (Zhao et al., 2017). In folk medicine, MSCP is commonly decocted with pig bone due to its health benefits, including boosting the immune system, reducing inflammation and recovering strength.
In southern China, the humid climate causes the medicine to easily mold, which may spoil medicinal materials during storage. Additionally, some medicinal materials may be damaged by insects during storage. To maintain a humid climate and better color, sulfur is often used to fumigate fresh MSCP during the postharvest handling process, which bleaches the color of materials and maintains a good appearance. In recent decades, sulfur fumigation with high efficiency, simple operation, reliability and low cost has been widely used for postharvest handling of some foods and medicinal herbs to guard against insects, maintain color and freshness, protect moisture and obtain a longer storage life (Jiang, Huang, Zheng, & Chen, 2013). However, the quality of the herbal medicine may change, and sulfur dioxide (SO2) can remain in it. Residual SO2 has been regarded as the major factor that affects the quality and safety of these sulfur-fumigated herbs (Wang et al., 2009). Recent studies have found that low sulfur dioxide residues do not affect the safety and efficacy of sulfurized herbs (Zhou et al., 2019). Nonetheless, many studies have shown that sulfur fumigation can cause chemical changes (He et al., 2018) affecting bioactivity (Yuan et al., 2019), respiratory system disorders (Wigenstam, Elfsmark, Bucht, & Jonasson, 2016), weakening immunomodulation(Ma et al., 2017), and even toxic effects on the liver and kidney (Jiang et al., 2020) in the long term. To date, there has been no report about the established analytical method for determining whether commercial MSCP samples undergo sulfur fumigation and whether sulfur fumigation alters the chemical composition of MSCP. Therefore, it is essential to explore a rapid, sensitive, reliable and confirmatory analytical-specific method to detect potential chemical changes and assess the quality of sulfur-fumigation MSCP.
Traditional phytochemical approaches have been used to isolate and identify individual components in herbs, but they are very difficult, time-consuming, costly and easily miss some potential small amounts of compounds (Li et al., 2012). HPLC-UV has often been applied to evaluate the quality of traditional Chinese medicine based on some known compounds, but it is difficult to apply this method to evaluate the unknown structures of new sulfur compounds (Zhang et al., 2021). These methods did not identify potential characteristic components to differentiate between the nonfumigation MSCP (NF-MSCP) and sulfur-fumigation MSCP (SF-MSCP) samples. Ultrahigh-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry (UHPLC–QTOF–MS/MS), a powerful, rapid, and sensitive analytical approach, is extensively applied to isolate and identify the complex components in medicinal herbs (Chen et al., 2018; Ma et al., 2020; Zhang et al., 2022). Additionally, multivariate statistical analysis, such as unsupervised principal component analysis (PCA) and supervised orthogonal partial least-squares discriminate analysis (OPLS-DA), was used to analyze the holistic discrepancy and discover the potential chemical markers that contribute to differentiating between the NF-MSCP and SF-MSCP samples. Thus, it may be an ideal method for evaluating MSCP samples.
The purpose of this research was to explore a novel approach by using UHPLC–QTOF–MS/MS and multivariate statistical analysis to identify the chemical components of both NF-MSCP and SF-MSCP samples. Furthermore, the established approach was applied to discover the potential characteristic markers that could rapidly differentiate between NF-MSCP and SF-MSCP samples and gain insights into the potential structural transformation mechanism.
2 Materials and methods
2.1 Chemical reagents and materials
Acetonitrile and methanol were LC-MS grade and purchased from Fisher Scientific Co. (Loughb Orough, UK). Ultra-pure water was produced by a Milli-Q water purification system (Millipore, Bedford, USA). Other chemicals were of analytical grade.
Four reference compounds including hypaphorine, isoliquiritigenin, formononetin and maackiain, were purchased from the National Institutes for Food and Drug Control (Beijing, China). The purity of the chemicals was determined to be more than 98 % by HPLC.
Ten batches of MSCP were obtained from different habitats of Guangxi Province. Commercial MSCP samples were obtained from different herbal markets in Yulin, Chengdu, Bozhou, Qinzhou and Nanning. The origins of all the samples were authenticated by Professor Zhifeng Zhang from the Institute of Qinghai-Tibetan Plateau, Southwest Minzu University, P. R. China, Sichuan, Chengdu. The details in this paper are shown in Table 1, and the samples were deposited in the medicine specimen room of Qin Zhou Provincial Health School (Qinzhou, China).
No. of sample
Description
Collecting location
The residual sulfur(mg/kg)
Characteristic markers (peak number)
NF-1
Non-fumigated sample
Qinzhou, Guangxi
–
SF-1
sulfur-fumigated sample
Qinzhou, Guangxi
245
1, 2, 12, 14, 63, 64, 65, 66
NF-2
Non-fumigated sample
Qinzhou, Guangxi
–
SF-2
sulfur-fumigated sample
Qinzhou, Guangxi
120
2, 11, 12, 38,63, 64, 65
NF-3
Non-fumigated sample
Yulin,Guangxi
–
SF-3
sulfur-fumigated sample
Yulin, Guangxi
330
1, 2, 11, 12, 14, 38,63, 64, 65, 66
NF-4
Non-fumigated sample
Yulin, Guangxi
–
SF-4
sulfur-fumigated sample
Yulin, Guangxi
342
2, 11, 12, 14, 38,63, 64, 65, 66
NF-5
Non-fumigated sample
Shangsi, Guangxi
–
SF-5
sulfur-fumigated sample
Shangsi, Guangxi
440
1, 2, 11, 12, 14, 38,63, 64, 65, 66
NF-6
Non-fumigated sample
Shangsi, Guangxi
–
SF-6
sulfur-fumigated sample
Shangsi, Guangxi
220
2, 12, 14, 38,63, 66
NF-7
Non-fumigated sample
Nanning, Guangxi
–
SF-7
sulfur-fumigated sample
Nanning, Guangxi
630
1, 2, 12, 14, 38,63, 64, 65, 66
NF-8
Non-fumigated sample
Nanning, Guangxi
–
SF-8
sulfur-fumigated sample
Nanning, Guangxi
540
2, 12, 14, 38,63, 64, 65, 66
NF-9
Non-fumigated sample
Qinzhou, Guangxi
–
SF-9
sulfur-fumigated sample
Qinzhou, Guangxi
420
1, 2, 11, 12, 38,63, 64, 65, 66
NF-10
Non-fumigated sample
Shangsi, Guangxi
–
S1
Commercial sample
Yulin, Guangxi
–
S2
Commercial sample
Yulin, Guangxi
–
S3
Commercial sample
Yulin, Guangxi
100
1, 14, 38,63, 64, 65
S4
Commercial sample
Yulin, Guangxi
138
2, 12, 14, 38,63, 64, 65, 66
S5
Commercial sample
Yulin, Guangxi
225
1,2, 11, 14, 38,63, 64, 65, 66
S6
Commercial sample
Hehuqchi, Chengdu
–
S7
Commercial sample
Hehuqchi, Chengdu
210
2, 12, 38,63, 64,
S8
Commercial sample
Hehuqchi, Chengdu
–
S9
Commercial sample
Hehuqchi, Chengdu
125
2, 12, 63, 64, 65, 66
S10
Commercial sample
Bozhou, Anhui
–
S11
Commercial sample
Bozhou, Anhui
–
S12
Commercial sample
Bozhou, Anhui
310
2, 11, 12, 38,63, 64, 65
S13
Commercial sample
Qinzhou, Guangxi
–
S14
Commercial sample
Qinzhou, Guangxi
140
2, 11, 38,63, 64, 66
S15
Commercial sample
Nanning, Guangxi
160
1, 12, 14, 63, 64, 65
S16
Commercial sample
Nanning, Guangxi
180
2, 11, 12, 63, 64, 65, 66
2.2 Instrumentation and chromatographic analytical conditions
The solutions were analyzed by a Waters ACQUITY UHPLC TM system (Waters Corporation, Milford, MA, USA) equipped with a binary solvent delivery manager, autosampler and PDA detector. The separation was conducted by an ACQUITY HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters, USA) at 35 °C, and a 1 μL aliquot of extracting solution was injected into the UHPLC at a flow rate of 0.4 mL/min. A mobile phase composed of 0.1 % aqueous formic acid (A) and acetonitrile (B) was applied as follows: 5–8 % B (0–1.5 min), 8–12 % B (1.5–3 min), 12–30 % B (3–4.5 min), 30–35 % B (4.5–10 min), 35–40 % B (10–12 min), 40–65 % B (12–15 min), 65–85 % B (15–18 min), 85–95 % B (18–21 min), 95–5 % B (21–21.1 min), and 5–5 % B (21.1–25 min).
Mass spectrometry was performed using a Waters definition accurate mass quadrupole time-of-flight (Q-TOF) Xevo G2-S mass spectrometer (Waters MS Technologies, Manchester, UK). The scan range was set as 100–1500 Da. The capillary voltage of the positive and negative modes was set at 2.8 kV. The source and desolvation temperatures were set at 100 and 400 °C, respectively. The cone and desolvation gas flow rates were set at 20 and 800 L/h, respectively. The collision-induced dissociation (CID) was set at 6 V for the precursor ion and 35–50 V for fragmentation information. The reference was leucine-enkephalin ([M−H]−=554.2615 and [M + H]+= 556.2771), which could accurately measure the molecular masses from the precursor ions and product ions. Finally, the data were processed and analyzed using MassLynx 4.1 software (Waters Co., Milford, CT, USA).
2.3 Preparation of samples
Ten batches of MSCP samples were obtained from Qinzhou, Nanning and Shangxi of Guangxi Province in China. Fresh MSCP samples were washed and cut into thin slices (1 cm) for each batch. Then, half of the fresh MSCP samples were dried in sunlight, and the other half was dried by sulfur fumigation in a desiccator. Sulfur-fumigation procedures were carried out in accordance with farmers. First, fresh MSCP slices were placed in the upper layer in the desiccator. Second, the sulfur powder was put and burned in the bottom layer. When the whole desiccator was full of SO2, the desiccator was then kept closed for 12 h. After fumigation, the MSCP slices were dried for 12 h at 40 °C.
2.4 Preparation of reference solutions
Reference stock solutions: Certain amounts of hypaphorine, isoliquiritigenin, formononetin and maackiain were dissolved in methanol/water (50 % v/v) to obtain four reference compound stock solutions, and each concentration was approximately equal to 1 mg/mL. A 0.1 mg/mL reference substance mixture solution was prepared by mixing and diluting the above four reference stock solutions. The reference substance mixed solution was further diluted and kept at 4 °C until further UHPLC–QTOF–MS/MS analysis.
2.5 Sample solution preparation
The dried sample was ground into powder, and 0.3 g of sample powder with 10 mL of methanol/water (50 % v/v) solution was added into a 50 mL volumetric flask, and ultrasonic extraction was performed (Kun Shan Ultrasonic Instruments Co., Ltd., Kun Shan, China) at 40 kHz for 30 min at 35℃. Then, the solution was filtered through a 0.22 μm millipore filter and analyzed by UHPLC–QTOF–MS/MS.
2.6 Data acquisition and analysis
Data acquisition was performed on a MassLynx 4.1 (Waters Corp., Milford, MA, USA). MarkerLynx XS software (Waters Corp., Milford, MA, USA) was used for processing and analysis of the data. The parameters were set as follows: initial retention time was set at 1 min, final retention time was set at 20 min, mass range was set at 100 to 1500 Da, mass tolerance was set at 0.01 Da, the mass window was set at 0.02 Da, and the retention time window was set at 0.02 min. Structural elucidation was analyzed with the Mass Fragment tool provided by MassLynx 4.1.
2.7 Determination of sulfur residues in MSCP samples
The sulfur residue content of MSCP was measured according to the Chinese Pharmacopoeia 2020 Edition (Volume Ⅳ) (Chinese Pharmacopoeia Commission, 2020). Ten grams of sample powder with 400 mL of distilled water and 10 mL of 6 mol/L HCl were added and distilled for 1.5 h in a sulfur dioxide assay apparatus, with flowing 0.2 L/min nitrogen. Then, 50 mL of 3 % hydrogen peroxide was mixed with 3 drops of 2.5 mg/mL methyl red ethanol indicator titrated by 0.01 mol/L NaOH to yellow in the conical flask, which was used to absorb the distillate. When the absorption liquid cooled to room temperature, it was titrated by 0.01 mol/L NaOH to yellow. Distilled water was used as the solvent for blank test correction. The result was calculated as shown in Eq. (1):
X is the total sulfur dioxide in the samples (in SO2), g/kg; V is the volume of NaOH standard solution used in the samples, mL; V0 is the volume of NaOH standard solution used in the blank, mL; 0.032 represents 1 mL of NaOH standard solution (C = 1.0 mol/L) equivalent to the mass of sulfur dioxide, g; C is the concentration of NaOH standard solution, mol/L; and m is the sample mass, g.
2.8 Statistical analysis
To distinguish between NF-MSCP and SF-MSCP samples, the mass spectrum information was imported into SIMCA-P14.1 software for multivariate statistical analysis, including PCA and OPLS-DA. The S-plot from OPLS-DA together with the importance in the projection (VIP) were employed to select the potentially characteristic compounds. Information on fragment ions, reported literature and the established database was used to confirm the molecular formula and identify the compound. The cleavage process of the compound was conducted by the MS data of precursor ions and product ions.
3 Results and discussion
3.1 Optimization of chromatography and MS conditions
At present, there are no reports about the separation of sulfur compounds from MSCP. Therefore, it is necessary to develop a new detection approach to isolate and identify sulfur compounds because commercial MSCP may be sulfur-fumigated. In our previous study (Zhang et al., 2022), an ACQUITY HSS T3 column (100 mm × 2.1 mm, 1.8 μm) was employed to isolate and analyze NF-MSCP due to its better peak patterns and stronger separation ability. Therefore, the HSS T3 column was chosen for analysis in this study.
To obtain the best sensitivity for separation, various kinds of mobile phases were optimized, including acetonitrile–water, methanol–water, acetonitrile-0.1 % formic acid in water, methanol-0.1 % formic acid in water. The results indicated that the mixture of acetonitrile-0.1 % aqueous formic acid was appropriate for the mobile phase due to its better separation ability and more satisfactory peak patterns of the sample. The mass spectrometric parameters were also optimized, including collision energy, cone voltage, and capillary voltage. Under the optimized chromatographic and mass spectrometry conditions, a total of 86 peaks were separated and detected in the MSCP samples in negative mode within 20 min (Fig. 1).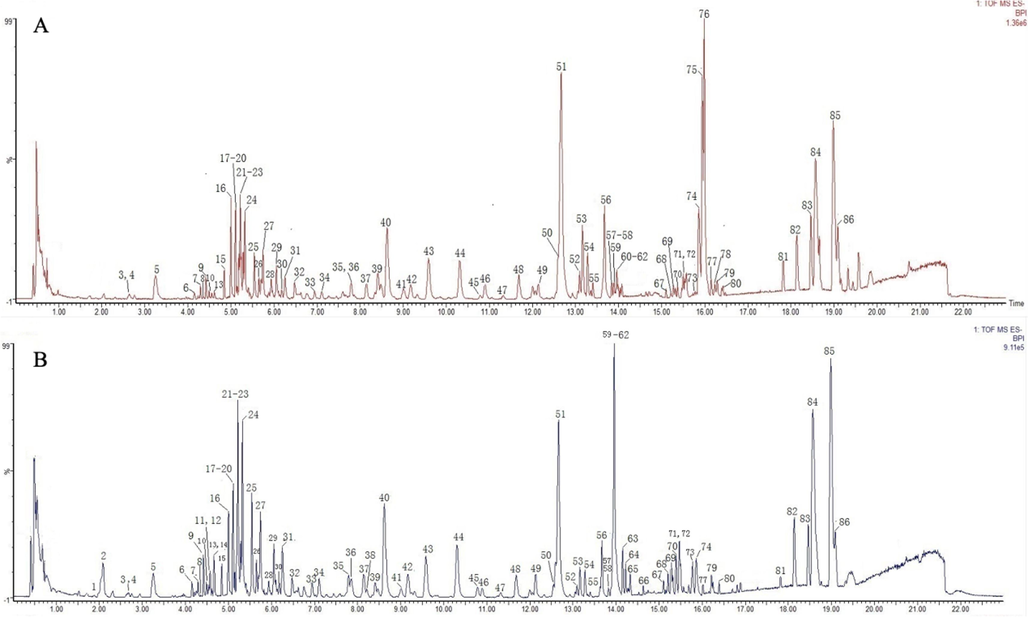
Representative BPI chromatograms of MSCP samples. A: NF-MSCP; B: SF-MSCP;
3.2 Comparison of detectable components in NF-MSCP and SF-MSCP samples
In total, 86 chemical constituents were detected using our newly developed analytical method, 76 of which were detected in NF-MSCP and 83 in SF-MSCP. As shown in Fig. 1, most peak heights at retention times from 1 to 14 min increased after sulfur fumigation, and the peak heights of 16 major peaks (peaks 9, 10, 13, 21, 23, 23, 24, 25, 27, 31, 40, 49, 59, 60, 62 and 84) found in NF-MSCP sharply increased in SF-MSCP. However, most peak heights at retention times from 14 to 16 min displayed a declining trend, and the peak heights of 10 major peaks (peaks 39, 51, 52, 53, 54, 55, 56, 75, 76 and 78) detected in NF-MSCP showed a significant decline in SF-MSCP, and three of them (peaks 75, 76 and 78) even disappeared after sulfur fumigation. It was also found that 10 peaks (peaks 1, 2, 11, 12, 14, 38, 63, 64, 65 and 66) were were newly generated as main peaks in SF-MSCP. These results indicated that sulfur fumigation induced the chemical transformation of the chemical constituents in MSCP. All the compounds identified in the NF-MSCP and SF-MSCP samples in the present work are shown in Table 2, including their retention time, molecular formula, and mass spectrometric information. Among them, 4 compounds were unambiguously confirmed by matching reference standards (peaks 5, 16, 41, and 69); however, the other compounds were tentatively identified by comparison with previously reported MS data and literature information.
NO.
Retention time(min)
ESI-MS (m/z)
Error(ppm)
MS/MS fragments ions
Formula
Identification
1
1.97
283.0369[M-H]-
0.2
203.0821,142.0654,116.0502
C11H12N2O5S
D-Tryptophan sulfate
2
2.09
325.0844[M-H]-
0.5
266.0110,222.0215,142.0647,116.0489
C14H18N2O5S
Hypaphorine sulfate
3
2.63
203.0821[M-H]-
1.2
142.0603,116.0743
C11H12N2O2
D-Tryptophan
4
2.67
216.0872[M-H]-
2.1
198.0769,172.0974,115.0029,100.0758,88.0394
C9H15NO5
L-Callipeltose
5
3.26
245.1290[M-H]-
1.3
187.0609,186.0587,142.0677
C14H18N2O2
Hypaphorine
6
4.15
581.1509[M-H]-
0.5
567.1358,419.1727,259.0613,241.0719
C26H30O15
Polygalaxanthone V
7
4.29
611.1612[M-H]-
0.9
341.0912,299.0587
C27H32O16
Unknown
8
4.35
505.1713[M-H]-
1.1
490.1404,475.1198,340.0756,328.0742
C25H30O11
Shomaside E
9
4.42
263.1383[M + H]+
1.2
ESI+204.1025,176.0602,158.0597
C14H18N2O3
physovenine
10
4.49
583.1660[M-H]-
−0.5
433.1331,301.0923,167.0343,152.0107,123.0443,108.0207
C26H32O15
seguinoside K
11
4.54
335.0279[M-H]-
−3.0
255.0653,135.0079,119.0509
C15H12O7S
Isoliquiritigenin sulfate
12
4.58
363.0221[M-H]-
1.4
283.0600,269.0432,255.0662,167.0330
C16H12O8S
Prunetin sulfate
13
4.62
453.3446[M + H]+
2.0
ESI+435.3313,407.3292,313.2197,285.1837,
C30H44O3
Unknown
14
4.68
351.0168[M-H]-
−2.8
230.9562,120.0801
C15H12O8S
Naringenin sulfate
15
4.84
564.4132[M-H]+
3.1
546.4023,337.2608,281.2479,241.1866,225.2604
C29H59NO9
Unknown
16
5.00
255.0663[M-H]-
2.4
135.0124,119.0522
C15H12O4
Isoliquiritigenin
17
5.06
283.0606[M-H]-
1.5
268.0379,254.1822,151.0072,118.0420
C16H12O5
Prunetin
18
5.11
790.5717[M-H]-
0.8
772.5717,451.3281,338.2433,225.1587,193.0880
C46H81NO9
Unknown
19
5.15
1089.5530[M-H]-
4.4
939.4581,909.3250,793.4388,778.2282,442.2473
C53H86O23
Soyasapogrnol B 3-O-α-L-arabinopyranosyl-(1 → 2)-β- D-galactopyranosyl-(1 → 2)-glucuronopyranosyl-22-O-β-D-glucopyranoside
20
5.19
1119.5635[M-H]-
4.3
939.4543,793.4285
C54H88O24
3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-L- rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 2)-glucopyranosyl-22-O-β-D-glucopyranoside
21
5.22
1119.5634[M-H]-
4.3
1101.5586,973.4937,911.5079,793.4413,749.4536,587.3992,205.0721,163.0608,
C54H88O24
23-hydroxyl pomalic acid 3-o-α-l-rhamnopyranosyl- (1 → 4)-β-d-glucopyranosyl-(1 → 6)-β-d-galactopyranosyl-28-o-β-d-glucopyranoside
22
5.24
1087.5355[M-H]-
2.8
941.4936,879.4833,473.3639,205.0746,179.0568,163.0615
C53H84O23
Oleanolic acid 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D- glucopyranosyl-(1 → 2)-β-D-galactopyranosyl-28-O-β-D-glucopyranoside
23
5.29
1117.5487[M-H]-
4.5
1099.5398,1055.5522,971.4926,953.4879,927.4984,909,5012,791.4277,773.4124,633.4033,205.0717,163.0604
C54H86O24
3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-L- rhamnopyranosyl-(1 → 2)-α-L-rhamnopyranosyl-(1 → 2)-β-D-glucuronopyranoyl-22-O-β-D-glucopyranoside
24
5.32
1117.5476[M-H]-
4.0
971.4926,955.4874,909,5012,791.4277,485.3267,205.0717
C54H86O24
3β-olean-12-en-28,29-dioic acid 3-O-α-L- rhamnopyranosyl-(1 → 2)-β-D-glucopyranosyl-(1 → 2)-β-D-galactopyranosyl-28-O-β- D-glucopyranoside
25
5.54
1103.5685[M-H]-
4.3
1085.5488,957.5008,941.4578,777.4440,485.3300,403.1258,205.0720
C54H88O23
Soyasapogrnol B 3-O-α-L-rhamnopyranosyl-(1 → 2)-β- D-galactopyranosyl-(1 → 2)-glucuronopyranosyl-22-O-β-D-glucopyranoside
26
5.65
1071.5372[M-H]-
2.2
939.4546,909.3124,867.4311,775.3973,764.4250,617.1602,455.3395
C53H84O22
Betulinic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L- rhamnopyranosyl-(1 → 2)-β-D-glucuronopyranosyl-28-O-β-D-glucopyranoside
27
5.74
1101.5529[M-H]-
4.3
955.4899,893.4901,775.4259,757.4174,205.0713,179.0555,163.0602,143.0347
C54H86O23
Oleanolic acid 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D- galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-D-glucopyranoside
28
5.93
1101.5524[M-H]-
3.8
955.4751,939.4596,793.3958,599.3540,455.1577
C54H86O23
Betulinic acid 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D- galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-D-glucopyranoside
29
6.06
1131.5275[M-H]-
4.6
1087.5253,985.4680,823.4686,205.0687,179.0540,163.0590
C54H84O25
3β-olean-12-en-28,29-dioic acid 3-O-α-L- rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-D-glucopyranoside
30
6.19
1073.5573[M + HCOO]-
2.8
455.1127,429.1175
C52H84O20
Oleanolic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L- rhamnopyranosyl-(1 → 2)-[α-L-arabinopyranosyl-(1 → 3)]-β-D-galactopyranoside
31
6.25
969.4728[M-H]-
3.4
951.4692,907.4745,823.4053,779.4261,761.4124,743.4004,643.3489,617.3655,599.3583,485.3264,467.3167,205.0695,163.0600
C48H74O20
millettiasaponin B
32
6.48
1071.5410[M-H]-
3.2
939.4578,909.3109,863.4765,793.3577,775.4262,617.1602,205.0711,179.0543,163.0592
C53H84O22
Oleanolic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L- rhamnopyranosyl-(1 → 2)-β-D-glucuronopyranosyl-28-O-β-D-glucopyranoside
33
6.94
1115.5316[M-H]-
3.8
969.4653,955.4852,807.4099,630.3679,545.2197,205.0694
C54H84O24
28-GlucosyloleanolicAcid3-[Rhamnosyl-(1 → 2)-Galactosyl-(1 → 3)-Glucuronide]
34
7.11
953.4763[M-H]-
1.8
925.4781,807.4175,645.1835,469.3312,205.0694,163.0599
C48H74O19
3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-L- rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 2)-glucuronopyranoside
35
7.79
969.4703
0.8
951.4592,823.4108,779.4122,761.4146,643.3506,601.3727, 485.3394,205.0721,163.0616
C48H74O20
Abrisaponin I
36
7.84
955.4916[M-H]-
1.4
937.4827,809.4239,630.3730,471.3493,205.0711,163.0611
C48H76O19
Saponin A
37
8.15
983.4875[M-H]-
2.3
965.4771,939.4827,921.4828,879.4709,837.4296,751.4257,733.4155,687.3705,645.3610,529.3487,487.3406,469.3267,205.0711,163.0608
C49H76O20
Wistaria-Saponin G
38
8.21
875.4055[M-H]-
−5.3
795.4521,537.3282,457.3607
C42H68O17S
Soyasaponin III sulfate
39
8.4
283.0613[M-H]-
0.4
268.0356,135.0073,
C16H12O5
5,4′-dihydroxy-3′-methoxyiso-flavone
40
8.62
1013.4966[M-H]-
0.9
909.4848,806.4464,763.4246,645.3632,601.3739,529.3506,487.3423,469.3312
C50H78O21
millettiasaponin A
41
9.00
267.0657[M-H]-
0.2
252.0425,223.0397,208.0503,195.0453,135.0103,132.0213
C16H12O4
Formononetin
42
9.16
791.4187[M-H]-
0.7
733.4240,645.3698,601.3768,441.3310,439.3253
C42H64O14
3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-L- rhamnopyranosyl-(1 → 2)-β-D-glucuronopyranoside
43
9.58
867.4397[M-H]-
2.2
705.3782,703.3315,687.3762,529.3535
C44H68O17
1β-[(2-O-α-L-rhamnopyranosyl-α-L-arabinopyranosyl)oxy]-26-(β-D-glucopyranosyloxy)furosta-5,20(22),25(27)-trien-3β-ol
44
10.3
997.5063[M-H]-
5.5
979.4883,935.5049,893.4923,851.4468,833.4354,789.4446,671.3807,653.3724,471.3477,205.0717,163.0613
C50H78O20
22β-acetyloxy-3β,24-dihydroxyolean-12-en-29-oic acid 3-α -L-rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl- (1 → 2)-glucuronopyranoside
45
10.78
999.5193[M-H]-
2.8
853.4432,673.3847,529.2606,471.1714
C50H80O20
Asiaticoside C
46
10.9
997.5044[M-H]-
3.6
979.4898,935.5024,893.4882,851.4393,833.4354,765.4454,747.4323,729.4205,529.2516,471.3477
C50H78O20
Unknown
47
11.32
645.3649[M-H]-
1.5
483.3116,465.3030,421.3070,327.2296
C36H54O10
abrusoside A
48
11.68
851.4454[M-H]-
2.9
765.4376,747.4301,671.3658,629.3654,603.3936,471.3475
C44H68O16
3-((3′-Malonyl)Xyl)-28-Glu-2-Hydroxy Oleanolic Acid
49
12.13
967.4920[M-H]-
1.8
949.4747,923.4655,905.4879,821.4285,803.4280,759.4307,615.3815,581.3735,553.3500,471.3467,205.0717,163.0612
C49H76O19
Dumortierinoside A Methyl Ester
50
12.54
997.5014[M-H]-
0.4
979.4917,925.5134,807.4156,629.3702,618.2419,205.0717,163.0604
C50H78O20
Unknown
51
12.68
941.5134[M-H]-
2.5
923.4998,795.4317,615.3881,597.3771,457.3674,205.0714,163.0608
C48H78O18
Soyasaponin I
52
13.09
795.4553[M-H]-
2.8
633.3791,615.3702,457.2651
C42H68O14
Soyasaponin III
53
13.16
911.5041[M-H]-
4.1
893.4884,765.4325,615.3879,457.3669,205.0710,163.0603,
C47H76O17
Soyasaponin II
54
13.28
925.5197[M-H]-
3.9
907.5051, 599.3842,509.4007, 457.3653
C48H78O17
Soyasapogrnol B 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L -rhamnopyranosyl-(1 → 2)-β-D-glucuronopyranoside
55
13.4
925.5185[M-H]-
2.6
907.5051,779.4551,763.4258,599.3937,441.3755
C48H78O17
Kaikasaponin III
56
13.67
939.4980[M-H]-
2.9
793.4335,775.4265,631.3806,455.3508,205.0709,163.0601
C48H76O18
Oleanolic acid 3-O- α-L-rhamnopyranosyl-(1 → 2)-β- D-galactopyranosyl-(1 → 2)-glucuronopyranoside
57
13.84
895.5074[M-H]-
2.0
599.3906,509.3978,439.3560,205.0710,163.0602
C47H76O16
Oleanolic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L- rhamnopyranosyl-(1 → 2)-β-D-galactopyranoside
58
13.84
1065.5299[M-H]-
2.7
1047.5105,1033.4703,902.4802,740,3681
C54H82O21
unknown
59
13.89
895.5071[M-H]-
1.8
565.3868,509.4007,439.3576,205.0710,163.0601
C47H76O16
Betulinic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L- rhamnopyranosyl-(1 → 2)-β-D-galactopyranoside
60
13.91
1037.5364[M-H]-
4.1
1019.5126,741.4143,583.3978,247.0903,205.0714,163.0589,157.0128,143.0354,131.0342,125.0236,113.0245
C53H82O20
Soyasaponin Betaa
61
13.91
1067.5472[M-H]-
4.2
1049.5487,921.4904,741.4242,583.3993,457.3721
C54H84O21
Soyasaponin VI
62
13.96
763.4257[M-H]-
0.8
617.4031,437.3429
C41H64O13
Oleanolic acid 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D- galactopyranoside
63
14.16
793.4381[M-H]-
−1.6
631.3919,437.3062,163.0617
C42H66O14
Ginsenoside Z-R1
64
14.22
921.4867 [M−H]-
1.8
777.4570,759.4265,597.3782, 437.3411, 163.0612,
C48H74O17
Soyasaponin Gammag
65
14.33
759.4333[M-H]-
1.2
C42H64O12
3-Glucuronyl-22-DDMP Soyasapogenol B
66
14.63
537.3287[M-H]-
6.9
457.3633
C30H50O6S
Soyasapogenol B sulfate
67
15.15
271.0603[M-H]-
0.9
151.0073,119.0472
C15H12O5
Naringenin
68
15.21
471.3461[M + HCOO]-
2.3
410.1615
C29H46O2
7-oxostigmasterol
69
15.28
283.0614[M-H]-
0.3
268.0379,239.0349
C16H12O5
Maackiain
70
15.4
313.2390([M−H]-
3.5
–
C18H33O4
Octadecanedioic acid
71
15.47
409.2383[M-H]-
1.0
391.2249,260.8799,241.0142
C26H34O4
Unknown
72
15.5
633.3798[M-H]-
1.1
589.3909,453.3358,179.0345,163.0409,135.0444
C39H54O7
Myriceric acid B
73
15.77
295.2298[M-H]-
7.5
277.2181
C18H32O3
9-hydroxy-10,12-octadecadienoic acid
74
15.86
540.3309[M + HCOO]-
4.3
255.2315,224.0683,168.0460
C27H47N3O8
unknown
75
15.94
617.3841[M-H]-
−3.1
573.3528,437.3425,179.0337,163.0414,134.0366
C39H54O6
Pyracrenic acid
76
15.99
617.3847[M-H]-
0.8
573.3528,437.3414,163.0146,145.02896
C39H54O6
3-O-Caffeoyloleanolic acid
77
16.09
633.3792 [M−H]-
0.8
589.3902,473.2837,179.0361,163.0410,135.0459
C39H54O7
2-O-Caffeoyl Maslinic Acid
78
16.16
633.3798[M-H]-
1.1
589.3906,453.3407,179.0342,163.0402,135.0447
C39H54O7
2α-Hydroxypyracrenic acid
79
16.25
647.3947[M-H]-
−0.2
633.3776,615.3176,409.2353,179.0344,161.0225
C40H56O7
Eucalyptolic acid
80
16.39
795.4116[M-H]-
1.0
633.3787,615.3682,571.3779,543.3683,179.0346,161.0236,135.0438
C48H60O10
Myriceric acid C
81
17.82
617.3851[M-H]-
1.5
471.3428,453.3366,147.0441
C39H54O6
Isoneriucoumaric acid
82
18.14
617.3856[M-H]-
2.3
471.3388,453.3426,146.9675
C39H54O6
27-p-Coumaroyloxyursolic acid
83
18.46
455.3527[M-H]-
0.4
439.3582
C30H48O3
Betulinic acid
84
18.57
455.3522[M-H]-
−0.7
439.3544,231.1741,173.1318
C30H48O3
Oleanoic acid
85
18.99
603.4058[M-H]-
1.5
179.0341,161.0240,134.0367
C39H56O5
Betulin-3-caffeate
86
19.09
603.405[M-H]-
0.3
179.0333,161.0240,134.0364
C39H56O5
Uvaol-3-caffeate
3.3 Identification of the chemical compounds of NF-MSCP
A total of 76 chemical constituents were detected in NF-MSCP, and 67 of them were identified or tentatively assigned, including 4 alkaloids, 6 flavanones, 51 terpenoids and 6 others. As shown in Table 2, compound 3 produced an [M−H]- ion at m/z 203.0821 with the molecular formula C11H12N2O2, and it further yielded fragment ions at m/z 142.0603 [M−H−CH2NO2]- and 116.0743[M-H-C3H6NO2]-. Compound 3 was tentatively assigned as D-tryptophan. Compound 4 yielded an [M−H]- ion at m/z 216.0872. Compound 4 further produced fragment ions at m/z 198.0769, 2.0974, 115.0029, 100.0758, and 88.0394, and it was tentatively confirmed as L-callipeltose. Compound 5 displayed an [M−H]- ion at m/z 245.1290, and it further yielded fragment ions at m/z 186.0587 [M−H−N(CH3)3]- and 142.0677 [M−H−N(CH3)3-CO2]-. It was definitely identified as hypaphorine using the corresponding reference standard. Compound 9 showed an [M + H]+ ion at m/z 263.1383, and it further generated fragment ions at m/z 204.1025, 176.0602, and 158.0597, and it was tentatively assigned as physovenine (Zhang, Cui, Lin, & Cai, 2021).
A total of 6 flavanones were tentatively assigned in NF-MSCP samples in this study. Compound 16 generated an [M−H]- ion at m/z 255.0663 with the formula C15H12O4, which yielded fragment ions at 135.0124 and 119.0522. Compound 16 was unambiguously assigned as isoliquiritigenin by comparison with the corresponding reference standards. Similarly, compounds 41 and 69 were unambiguously confirmed as formononetin and maackiain, respectively, compared with the corresponding standard substances. Compounds 17 and 39 exhibited an [M−H]- ion at m/z 283.06 with the same formula C16H12O5. Compound 17 further generated fragment ions at m/z 268.0379 and 151.0072, and it was tentatively confirmed as prunetin (Wang et al., 2021). Compound 39 further produced fragment ions at m/z 268.0356 and 135.0073, and it was tentatively identified as 5,4′-dihydroxy-3′-methoxyisoflavone (Wang et al., 2021). Compound 67 showed an [M−H]- ion at m/z 271.0603 with product ions at 151.0073 and 119.0472, and it was tentatively assigned as naringenin (Fu et al., 2016).
As shown in Table 2, 51 terpenoids were tentatively confirmed in NF-MSCP samples in this study. Compound 40 was used as a detailed example to demonstrate the fragmentation patterns of terpenoids. Compound 40 yielded an [M−H]- ion at m/z 1013.4966 with the formula C50H78O21, which further yielded fragment ions at m/z 909.4848 [M−H−C3H4O4]-, 763.4246 [M−H−C3H4O4- Rha]-, 687.3744 [M−H−Rha−Gal−H2O]-, 645.3632 [M−H−Rha−Gal−H2O−C2H2O]-, 601.3739 [M−H- C3H4O4-Rha-Gal]-, 529.3506 [M−H−Rha−Gal−GluA]-, 487.3423 [M−H−Rha−Gal−GluA−C2H2O]-, and 469.3312 [M−H−Rha−Gal−GluA−C2H2O−H2O]-. Therefore, compound 40 was tentatively assigned as millettiasaponin A, according to a previous report in MSCP (Uchiyama et al., 2003). The mass spectra in negative mode and the hypothesized fragmentation pathway of compound 40 are shown in Fig. 2. By a similar pathway, compounds 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 42, 44, 47, 51, 52, 53, 54, 56, 57, 59 and 62 were identified as terpenoid glycosides according to the fragmentation and the Clog P values, which had been previously reported in the literature (Zhao et al., 2017; Wang et al., 2021; Zhang, Cui, Lin, & Cai, 2021). Compounds 35, 36, 37, 55, 60, 61, 83 and 84 have previously been reported in the same species (Chiang & Chang, 1982; Massiot, Lavaud, Benkhaled, & Men-Olivier, 2004; Shi, Wen, & Tu, 2006; Ha et al., 2014) and have been identified in MSCP. In a similar manner, compounds 43, 45, 48, 49, 72, 75, 76, 77, 78, 79, 80, 81, 82, 85 and 86 were tentatively assigned as terpenoid glycosides by comparison with their fragment ions.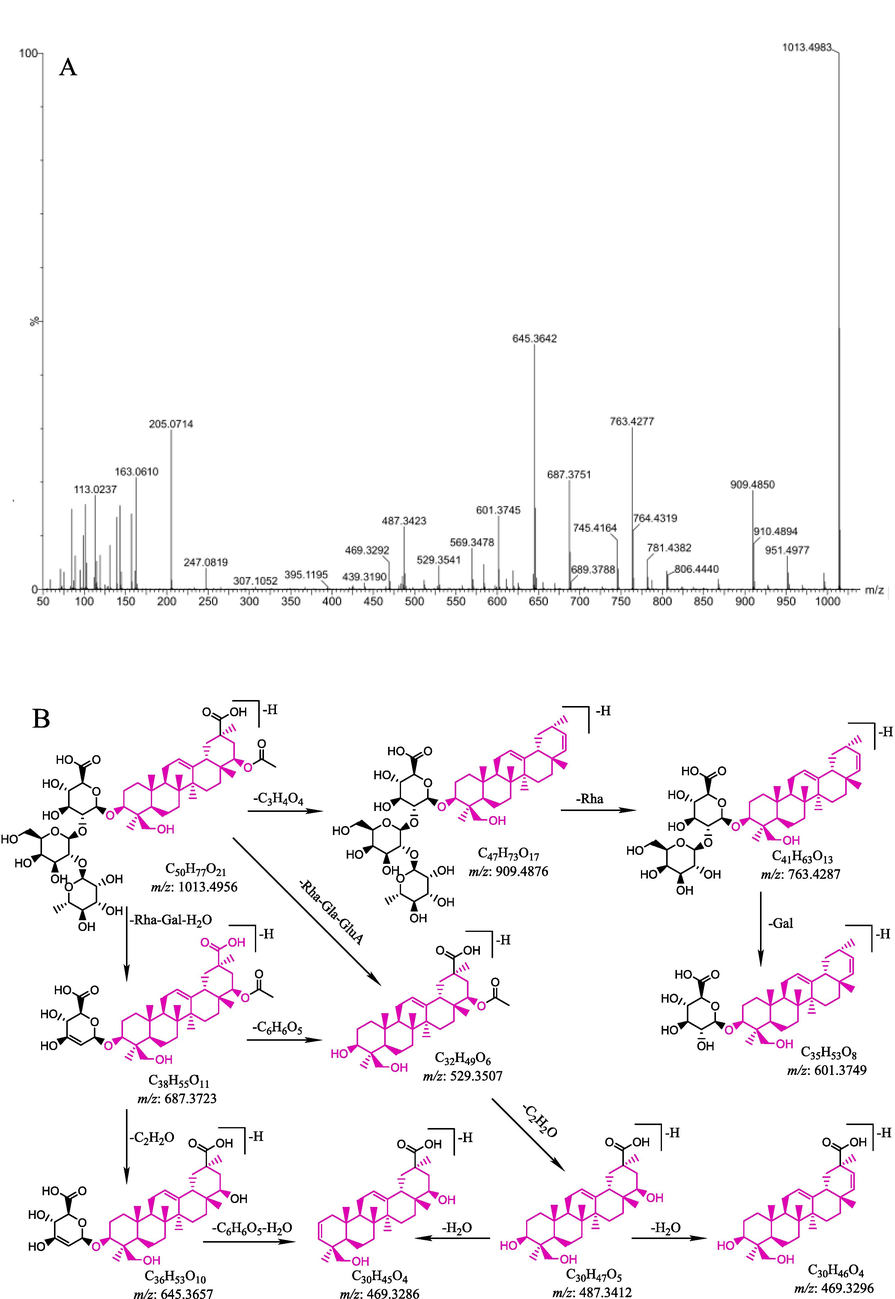
A: The MS/MS spectrum in negative mode of compound 40; B: hypothesized fragmentation pathway of compound 40;
In this study, six constituents attributed to fatty acids and other compounds were identified. Compound 6 possessed an [M−H]- ion at m/z 581.1509, and it further generated fragment ions at m/z 567.1358[M-CH3]-, 419.1727[M-H-CH3-Rha]-, 259.0613[M-H-CH3-Rha-Glu]-, and 241.0719[M-H-CH3-Rha-Glu-H2O]-. Therefore, it was tentatively identified as polygalaxanthone V by comparison with the related literature (Zhang, Cui, Lin, & Cai, 2021). According to the fragmentation ions and related literature, compounds 8, 10, 68, 70 and 73 were tentatively deduced as shomaside E, seguinoside K (Yin et al., 2008), 7-oxostigmasterol, octadecanedioic acid (Wang et al., 2021), and 9-hydroxy-10,12-octadecadienoic acid, respectively.
3.4 Elucidating the identification of sulfate compounds of SF-MSCP
Ten markers (peaks 1, 2, 11, 12, 14, 38, 63, 64, 65 and 66) were detected in the 70 % methanol extract of the SF-MSCP sample. Among them, compounds 63, 64 and 65 were regarded as terpenoid metabolites, and the other 7 compounds (peaks 1, 2, 11, 12, 14, 38 and 66) were identified as sulfate derivatives according to the confirmed molecular and fragment ions. Compound 63 gave an [M−H]- ion at m/z 793.4381, and it further generated fragment ions at m/z 731.4019 [M−H−Gal]- and 437.3062 [M−H−Gal−GluA]-. Thus, compound 63 was tentatively identified as zingibroside R1. In the same manner, compounds 64 and 65 were tentatively recognized as soyasaponin gammag and 3-glucuronyl-22-DDMP soyasapogenol B.
Seven sulfate derivatives were identified in the present SF-MSCP samples. Two alkaloid sulfate compounds (1 and 2) were tentatively identified. Compound 2 generated an [M−H]- ion at m/z 325.0858, 80 Da more than that of hypaphorine (5) (C14H18N2O2, m/z 245.1290). Its empirical molecular formula C14H18N2O5S indicated an exact “SO3” addition to hypaphorine, and it further produced 266.0110, 222.0215, 142.0647 and 116.00489 corresponding to the successive loss of N(CH3)3, CO2 and SO3. The mass spectra in negative mode and hypothesized fragmentation pathways of compound 2 are shown in Fig. 3A-B. Therefore, compound 2 was tentatively identified as hypaphorine sulfate. Similarly, compound 1 was tentatively assigned as D-tryptophan sulfate. It showed an [M−H]- ion at m/z 283.0369, 80 Da more than that of D-tryptophan (3) (C11H12N2O2, m/z 203.0821). Its empirical molecular formula C11H12N2O5S suggested an exact “SO3” addition to D-tryptophan. The characteristic fragment ions at m/z 203.0821, 142.0654 and 116.0502 were regarded as successive losses of SO3, HNCOOH and C2H2, respectively.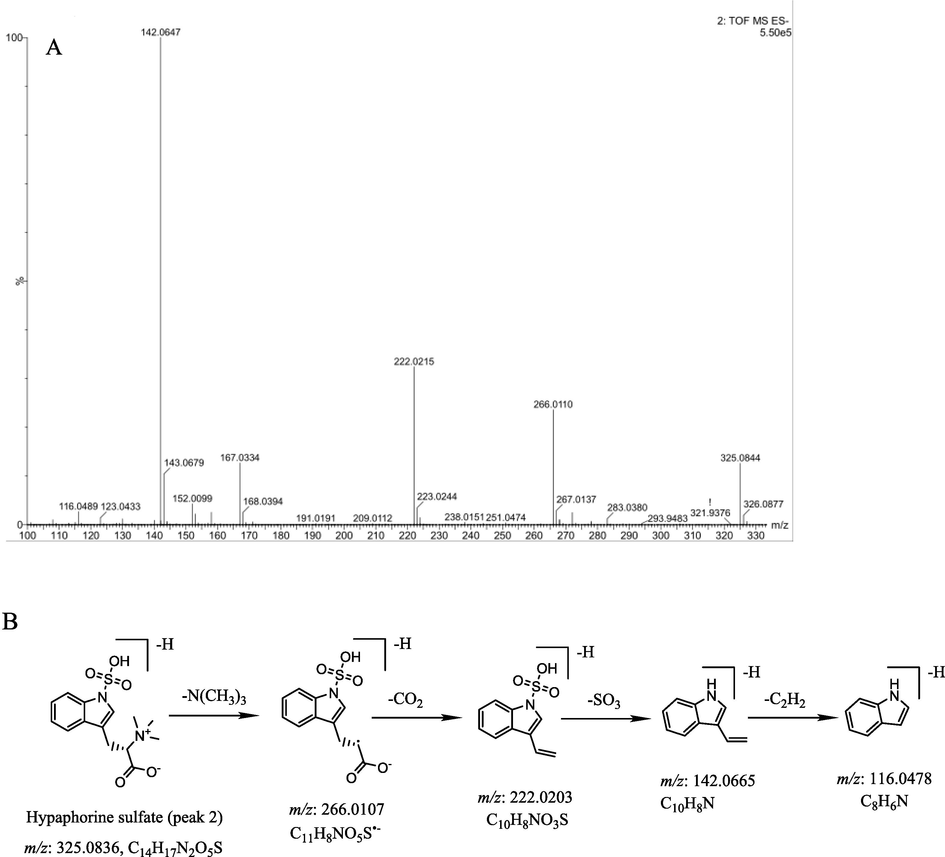
A: The MS/MS spectrum in negative mode of compound 2; B: The hypothesized fragmentation pathway of compound 2;
Similarly, three flavanone sulfate compounds (11, 12 and 14) were tentatively identified. Compound 11 yielded an [M−H]- ion at m/z 335.0279 with the molecular formula C15H12O7S, indicating an exact “SO3” addition to isoliquiritigenin (16) (C15H12O4, m/z 255.0663), and it further generated 255.0653, 135.0079 and 119.0509. A fragment ion at m/z 255.0653 corresponded to the loss of SO3. Compound 11 was tentatively assigned as isoliquiritigenin sulfate. Compound 12 had an accurate mass at m/z 363.0221, 80 Da more than that of prunetin (17) (C16H12O5, m/z 283.0606). Its empirical molecular formula C16H12O8S indicated an exact “SO3” addition to prunetin. Fragment ions at m/z 283.0600, 269.0432, 255.0662 and 167.0330 were observed, and compound 12 was tentatively deduced to be prunetin sulfate. Compound 14 showed an [M−H]- ion at m/z 351.0528 with the formula C15H12O8S, which was 80 Da more than that of naringenin (67) (C15H12O5, m/z 271.0603), showing an exact “SO3” addition to naringenin. Compound 14 also displayed an [M + H]+ ion at 353.0378 in positive ion mode. The fragment ions at m/z 230.9562 and 120.0801 were observed in positive ion mode, indicating that “SO3” was added to the hydroxy group of ring A. Compound 14 was tentatively deduced as naringenin sulfate. The hypothesized fragmentation pathways of compound 14 is presented in Supplementary Figure S1.
Two terpenoid sulfate compounds (38 and 66) were tentatively identified. Compound 38 had an accurate mass [M−H]- ion at m/z 875.4055 with the formula C42H68O17S, which was 80 Da more than that of soyasaponin III (52) (C42H68O14, m/z 795.4553), indicating an exact “SO3” addition to soyasaponin III. It further produced 795.4521, 537.3282 and 457.3607, corresponding to the successive or simultaneous loss of SO3, a galactosyl moiety and a glucopyranosiduronic acid moiety, respectively. Therefore, Compound 38 was tentatively identified as soyasaponin III sulfate. Compound 66 produced an [M−H]- ion at m/z 537.3287 with the formula C30H50O6S, and it further generated an ion at m/z 457.3633 consistent with soyasaponin I (51), soyasaponin III (52), soyasaponin II (53) and so on. Sulfate derivatives connected with feature compounds primarily come from esterification and dissociation of soyasapogenol B saponin. Therefore, Compound 66 was tentatively assigned as soyasapogenol B sulfate. The hypothesized fragmentation pathways of compounds 38 and 66 are shown in Supplementary Figure S2.
3.5 Possible transformation mechanism of components induced by sulfur fumigation
The transformation mechanism of terpenoid saponins was promoted by a hydrolysis reaction caused by SO2. According to the structural skeleton, the potential transformation pathways of terpenoid saponins could be classified into 3 types (oleanoic acid, betulinic acid and soyasapogenol B), as shown in Fig. 4. For the oleanoic acid-type terpenoid saponins, oleanolic acid3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-D-glucopyranoside (27) was first hydrolyzed with the cleavage of glycosidic bonds and transformed into oleanolic acid 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 2)- glucuronopyranoside (56) under the promotion of SO2. Then, it was converted into ginsenoside Z-R1 (63, metabolite) and oleanoic acid (84) by successive hydrolysis reactions. Similarly, oleanolic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L-rhamnopyranosyl-(1 → 2)-β-D- galactopyranoside (57) was transformed into oleanolic acid 3-O-α-L-rhamnopyranosyl- (1 → 2)-β-D-galactopyranoside (62) and oleanoic acid (84) by successive hydrolysis reactions under the promotion of SO2. 3-O-Caffeoyloleanolic acid (76) was hydrolyzed to produce oleanolic acid (84). This could illustrate the phenomenon that peak 76 disappeared completely and peaks 62 and 84 increased significantly. In the case of the betulinic acid type, betulinic acid 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-D glucopyranoside (28), betulinic acid 3-O-α-L-arabinopyranosyl-(1 → 2)-α-L-rhamnopyranosyl- (1 → 2)-β-D-galactopyranoside (59) and pyracrenic acid (75) were hydrolyzed to generate betulinic acid (83). It could also explain the phenomenon that peak 75 nearly disappeared. The result of the soyasapogenol B type indicated that soyasapogrnol B 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D- galactopyranosyl-(1 → 2)-glucuronopyranosyl-22-O-β-D-glucopyranoside (25) was deglycosylated to generate soyasaponin I (51); then, soyasaponin I could be converted to soyasaponin III (52). It was also found that soyasaponin VI (61) was transformed into soyasaponin I (51) or soyasaponin gammag (64, metabolite) by hydrolysis. Similarly, soyasaponin gammag (64, metabolite) was transformed into soyasaponin III (52) or 3-glucuronyl-22-DDMP soyasapogenol B (65, metabolite) under the promotion of SO2. Taken together, terpenoid saponins could be converted into a large number of intermediate terpenoid saponins and their metabolites by hydrolysis reactions under the influence of the sulfur-fumigation process.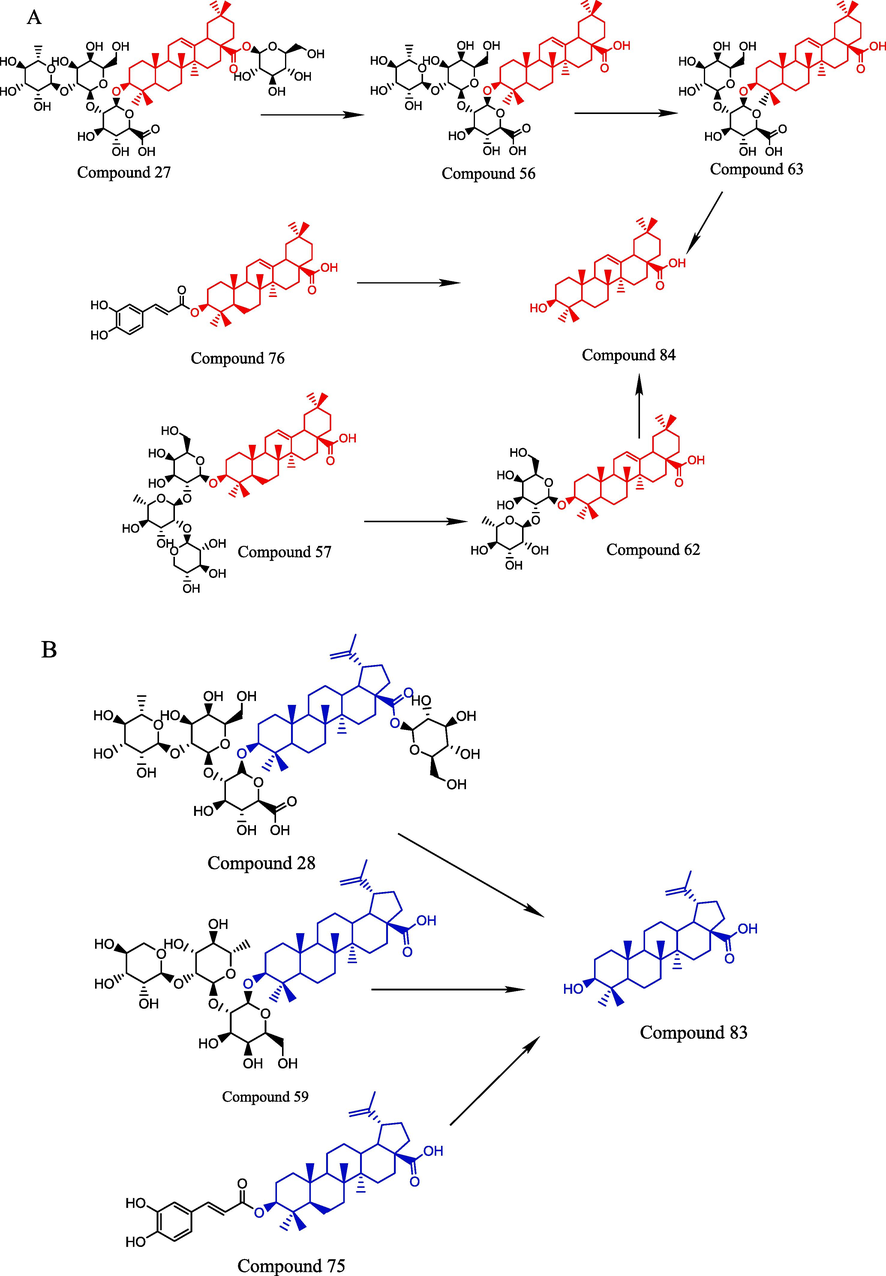
A: Possible transformation mechanism of Oleanoic acid type; B: Possible transformation mechanism of Betulinic acid type; C: Possible transformation mechanism of Soyasapogenol B type.
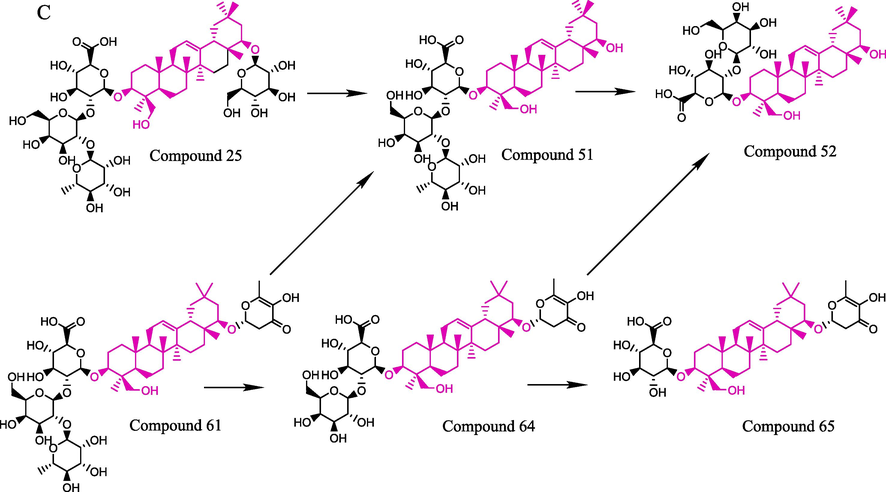
A: Possible transformation mechanism of Oleanoic acid type; B: Possible transformation mechanism of Betulinic acid type; C: Possible transformation mechanism of Soyasapogenol B type.
It was also necessary to explain the chemical transformation mechanism of sulfate compounds that may be potentially harmful. From the present study, it was found that D-tryptophan (3) could be converted into D-tryptophan sulfate (1) during sulfur fumigation processing. Hypaphorine (5) is the characteristic precursor of hypaphorine sulfate (2). The findings agreed with the study that alkaloids were converted into their sulfate derivatives during the sulfur-fumigation process (Ma et al., 2014). The same change occurred for isoliquiritigenin (16), prunetin (17) and naringenin (67). The newly produced peaks appearing as isoliquiritigenin sulfate (11), prunetin sulfate (12) and naringenin sulfate (14) were generated by the addition of “SO3” to different hydroxyl moieties in the flavonoid (He et al., 2018), while each sulfate derivative presents a single peak in the mass spectrum due to the isomers. In previous research, sulfur fumigation led to triterpene saponins transforming into their sulfate derivatives (Li et al., 2012; Kang et al., 2018; Zhang et al., 2021). In our work, we also found that sulfate derivatives of triterpene saponins could be generated during the sulfur fumigation process. Soyasaponin III (52) was partially converted to soyasaponin III sulfate (38) under sulfur fumigation, which caused a decrease in content. Soyasaponin III sulfate (38) may have two configurations, which are formed from the hydroxyl groups at position C-22 or C-24 of soyasaponin III (52). On the other hand, soyasaponin III sulfate lost sugar groups under acidic conditions and produced soyasapogenol B sulfate (66) during sulfur fumigation. These newly produced peaks indicated that sulfur fumigation can induce the chemical transformation of MSCP. The possible mechanisms deduced in the transformation mechanism of sulfate compounds are shown in Fig. 5.
A: The possible transformation mechanism of compounds 1 and 2; B: The possible transformation mechanism of compounds 11, 12 and 14; C: The possible transformation mechanism of compounds 38 and 66;
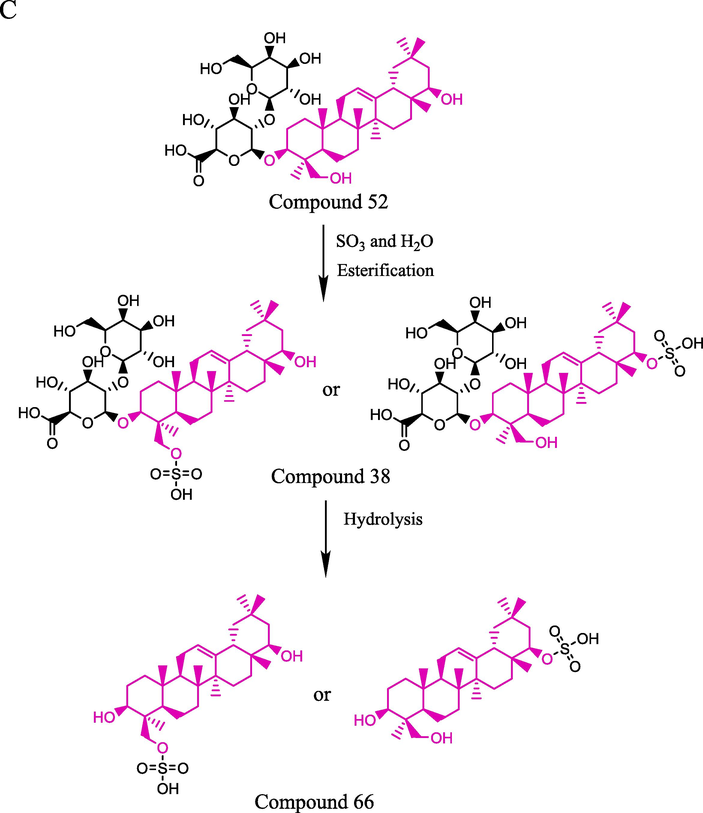
A: The possible transformation mechanism of compounds 1 and 2; B: The possible transformation mechanism of compounds 11, 12 and 14; C: The possible transformation mechanism of compounds 38 and 66;
3.6 Exploring potential chemical markers based on multivariate statistical analysis
To distinguish the chemical profile differences between the NF-MSCP and SF-MSCP samples, unsupervised PCA was performed by SIMCA-P14.1. As shown in Fig. 6A, the result of the PCA score scatter plot showed that all samples were nearly classified into two groups. Except for sample SF-2, the other eight SF-MSCP samples (SF-1, SF-3 ∼ 9) were gathered into the right of the t [1] plot, while all the NF-MSCP samples (NF-1 ∼ 10) were gathered to the left, illustrating that the chemical constituents and/or contents of components in MSCP remarkably changed during sulfur-fumigated processing.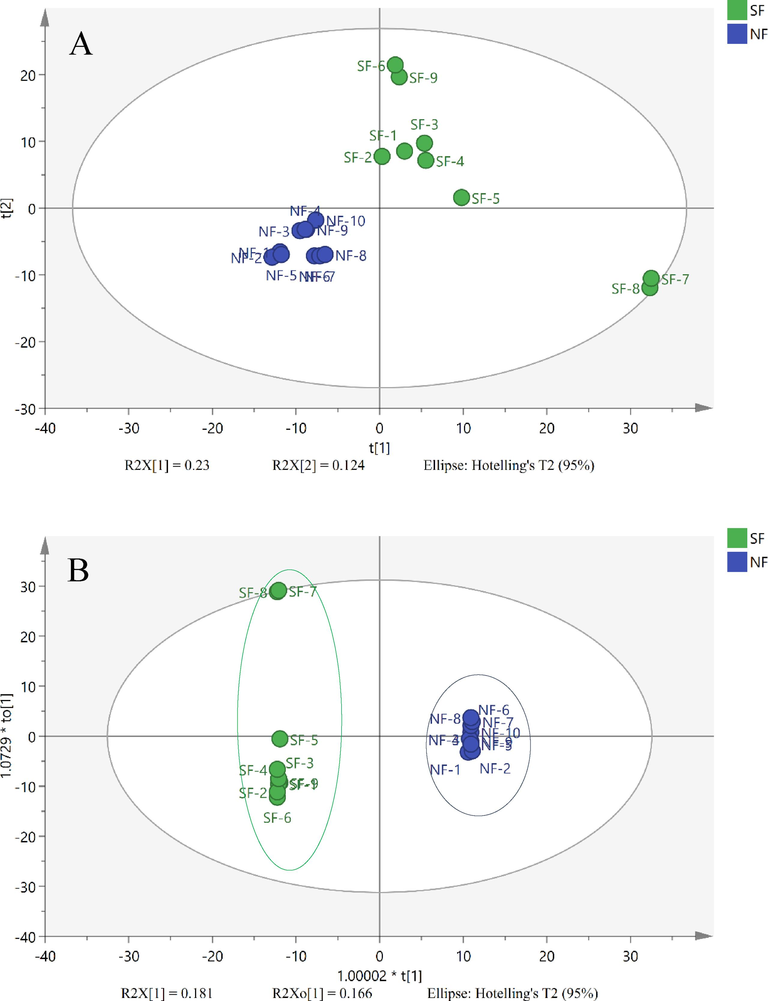
A: PCA Scores plot of NF-MSCP and SF-MSCP samples; B: OPLS-DA Scores plot of NF-MSCP and SF-MSCP samples; C: S-Plot of NF-MSCP and SF-MSCP samples;
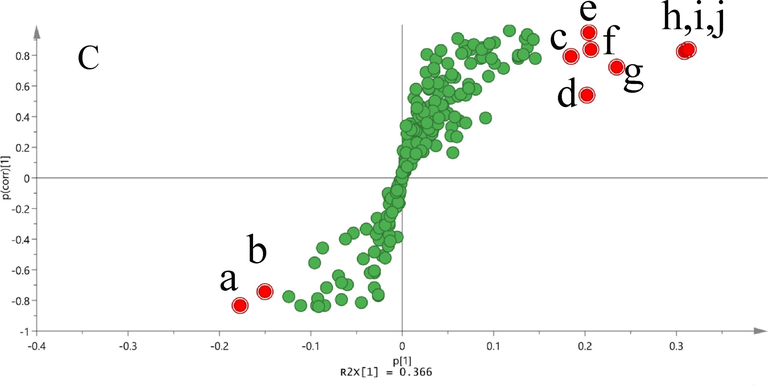
A: PCA Scores plot of NF-MSCP and SF-MSCP samples; B: OPLS-DA Scores plot of NF-MSCP and SF-MSCP samples; C: S-Plot of NF-MSCP and SF-MSCP samples;
Supervised OPLS-DA was further used to discriminate between NF-MSCP and SF-MSCP samples. The analysis indicated that the NF-MSCP and SF-MSCP samples were obviously divided into two groups in OPLS-DA plots (Fig. 6B). An S-plot from OPLS-DA was further used to discover the potential chemical markers, and the points at either end of the S-plot together with the Variables of Importance in Projection (VIP) value (VIP > 2.0) generated from OPLS-DA were screened out as significant markers concerned with the difference between NF-MSCP and SF-MSCP samples(Kang et al., 2018; Zhang et al., 2021). From Fig. 6C, two points (a and b) on the lower left side and eight points (c, d, e, f, g, h, i and j) on the upper right of the S-type were considered important chemical markers. According to the base peak ion chromatogram, ten compounds were newly generated from sulfur-fumigated samples (peak 1: D-tryptophan sulfate; peak 2: hypaphorine sulfate; peak 11: isoliquiritigenin sulfate; peak 12: prunetin sulfate; peak 14: naringenin sulfate; peak 38: soyasaponin III sulfate; peak 63: ginsenoside Z-R1; peak 64: soyasaponin gamma; peak 65: lucuronyl-22-DDMP soyasapogenol B; peak 66: soyasapogenol B sulfate). Therefore, these ten compounds could be selected as potential markers to differentiate between the NF-MSCP and SF-MSCP samples.
3.7 Inspection of commercial MSCP samples
Ten markers were discovered distinguishing the NF-MSCP and SF-MSCP samples according to the above mechanism. The newly developed approach was used to inspect a total of 16 commercial MSCP samples obtained from the Yulin, Hehuachi, Qinzhou and Nanning markets in China. The results indicate that sulfur-containing compounds were found in 9 samples (Table 1), meaning that more than 50 % of commercial MSCP samples contained sulfur. It was also found that metabolites 63, 64 and 65 were detected in the above 9 sulfur-fumigated samples, but the metabolites were not detected in other commercial samples. It is obvious that the chemical constituents of MSCP can be transformed during the sulfur fumigation process.
3.8 Determination of sulfur residues
The sulfur residues in the samples are listed in Table 1. The sulfur contents of the sulfur-fumigated MSCP samples (SF-1 ∼ 9) ranged from 120 to 630 mg/kg. Sulfur residues were also found in nine commercial MSCP samples, and the sulfur contents of the samples (S3, S4, S5, S7, S9, S12, S14, S15 and S16) were in the range of 100–310 mg/kg. It is important to note that there was much residual sulfur on the sample after sulfur fumigation, which might increase the potential incidence of toxicity. However, the bioactivities and toxicity of sulfur-fumigated MSCP need to be further studied.
4 Conclusions
Sulfur fumigation has been reported to cause chemical transformation and alter the original bioactive ingredients in herbs or their extracts, which could lead to changes in bioactivity and pharmacokinetics and even toxicity (Wigenstam, Elfsmark, Bucht, & Jonasson, 2016; Yuan et al., 2019; Shen et al., 2020). In this study, a novel UHPLC–QTOF–MS/MS approach based on chemical profiling and multivariate statistical analysis was established to discover chemical markers in SF-MSCP samples. Ten potential chemical markers was found in sulfur-fumigated samples, including 7 sulfur-containing compounds and 3 terpenoid metabolites.The new sulfated derivative was generated through esterification and/or addition of the original compound with SO3 during the sulfur-fumigation process. The newly developed approach was successfully used to analyze sixteen commercial MSCP samples, nine of them which contained sulfur-containing compounds. These sulfur residue contents of the SF-MSCP sample ranged from 100 to 310 mg/kg.
These research outcomes not only can be used to distinguish the NF-MSCP from SF-MSCP by chemical markers but also provide a chemical basis for further comprehensive investigating in-depth safety and functional evaluations of SF-MSCP.
Author contributions
This study was conceived by Zhifeng Zhang. Jianguang Zhang drafted the manuscript. Lili He conducted the UHPLC–QTOF–MS/MS analysis. Junjun Wang and Li Yang identified all the chemical compounds. Ming Chen determined the sulfur residues. Zichang Liang conducted the statistical analysis.
Acknowledgment
This work was supported by the Sichuan Provincial Regional Innovation Cooperation Project, China (2023YFQ0084), the Fundamental Research Funds for the Central Universities, Southwest Minzu University, China (ZYN2023099) and the Technology Research and Development Project of Qinzhou Science and Technology Bureau, China (20198509).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
References
- Rapid evaluation of chemical consistency of artificially induced and natural Resina Draconis using ultra-performance liquid chromatography quadrupole-time-of-flight mass spectrometry-based chemical profiling. Molecules. 2018;23(8):1850-1855.
- [CrossRef] [Google Scholar]
- Urinary metabonomics study of anti-depressive mechanisms of Millettia speciosa Champ on rats with chronic unpredictable mild stress-induced depression. J. Pharm. Biomed. Anal.. 2021;205(25):114338
- [CrossRef] [Google Scholar]
- Isolation and structural elucidation of some sapogenols from Abrus cantoniensis. Planta Med.. 1982;46(9):52-55.
- [CrossRef] [Google Scholar]
- Chinese Pharmacopoeia Commission. (2020). Chinese Pharmacopoeia 2020 (Chinese Edition). China: Beijing.
- Chemical constituents from roots of Millettia speciosa. Chinese Herb. Med.. 2016;8(4):385-389.
- [CrossRef] [Google Scholar]
- Rapid characterisation and comparison of saponin profiles in the seeds of Korean Leguminous species using ultra performance liquid chromatography with photodiode array detector and electrospray ionisation/mass spectrometry (UPLC–PDA–ESI/MS) analysis. Food Chem.. 2014;146(1):270-277.
- [CrossRef] [Google Scholar]
- Rapid discrimination of raw and sulfur-fumigated Smilax glabra based on chemical profiles by UHPLC-QTOF-MS/MS coupled with multivariate statistical analysis. Food Res. Int.. 2018;108:226-236.
- [CrossRef] [Google Scholar]
- A novel polysaccharide from the roots of Millettia Speciosa Champ: preparation, structural characterization and immunomodulatory activity. Int. J. Biol. Macromol.. 2020;145:547-557.
- [CrossRef] [Google Scholar]
- Effect of acetylation modification on the emulsifying and antioxidant properties of polysaccharide from Millettia speciosa Champ. Food Hydrocoll.. 2022;124:107217
- [CrossRef] [Google Scholar]
- Sulfur fumigation, a better or worse choice in preservation of Traditional Chinese Medicine. Phytomedicine. 2013;20(2):97-105.
- [CrossRef] [Google Scholar]
- The effects of sulfur fumigation processing on Panacis Quinquefolii Radix in chemical profile, immunoregulation and liver and kidney injury. J. Ethnopharmacol.. 2020;249:112377
- [CrossRef] [Google Scholar]
- Elucidation of characteristic sulfur-fumigated markers and chemical transformation mechanism for quality control of Achyranthes bidentate blume using metabolome and sulfur dioxide residue analysis. Front. Plant Sci.. 2018;9:790.
- [CrossRef] [Google Scholar]
- Ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry based chemical profiling approach to rapidly reveal chemical transformation of sulfur-fumigated medicinal herbs, a case study on white ginseng. J. Chromatogr. A. 2012;1231:31-45.
- [CrossRef] [Google Scholar]
- Sulfur fumigation reducing systemic exposure of ginsenosides and weakening immunomodulatory activity of ginseng. J. Ethnopharmacol.. 2017;195:222-230.
- [CrossRef] [Google Scholar]
- UHPLC UHD Q-TOF MS/MS analysis of the impact of sulfur fumigation on the chemical profile of Codonopsis Radix (Dangshen) Analyst. 2014;139(2):505-516.
- [CrossRef] [Google Scholar]
- Rapid discrimination of Notopterygium incisum and Notopterygium franchetii based on characteristic compound profiles detected by UHPLC-QTOF-MS/MS coupled with multivariate analysis. Phytochem. Anal.. 2020;31(3):355-365.
- [CrossRef] [Google Scholar]
- Soyasaponin VI, A new maltol conjugate from alfalfa and soybean. J. Nat. Prod.. 2004;55(9):1339-1342.
- [CrossRef] [Google Scholar]
- Effect of sulfur-fumigation process on ginseng: Metabolism and absorption evidences. J. Ethnopharmacol.. 2020;256:112799
- [CrossRef] [Google Scholar]
- Chemical constituents of Abrus cantoniensis. Chinese Trad. Herb. Med.. 2006;37(11):1610-1613.
- [CrossRef] [Google Scholar]
- New olenae-type triterpene saponins from Millettia speciosa. Heterocycles. 2003;60(3):655-661.
- [Google Scholar]
- Study of the destructive effect to inherent quality of Angelicae dahuricae radix (Baizhi) by sulfur-fumigated process using chromatographic fingerprinting analysis. J. Pharm. Biomed. Anal.. 2009;49(5):1221-1225.
- [CrossRef] [Google Scholar]
- Metabolomic profiling of M. speciosa champ at different growth stages. Food Chem.. 2021;376:131941
- [CrossRef] [Google Scholar]
- Inhaled sulfur dioxide causes pulmonary and systemic inflammation leading to fibrotic respiratory disease in a rat model of chemical-induced lung injury. Toxicology 2016:368-369.
- [CrossRef] [Google Scholar]
- Three new phenolic glycosides from the caulis of Millettia speciosa. Magn. Reson. Chem.. 2008;46(4):387-391.
- [CrossRef] [Google Scholar]
- Chemical profiles, antioxidant activity and acute toxicity of raw and sulfur-fumigated Smilacis Glabrae Rhizoma. J. Ethnopharmacol.. 2019;234:76-84.
- [CrossRef] [Google Scholar]
- Ameliorating effect on glycolipid metabolism and chemical profile of Millettia speciosa champ. extract. J. Ethnopharmacol.. 2021;279(114360)
- [CrossRef] [Google Scholar]
- A comprehensive evaluation protocol for sulfur fumigation of ginseng using UPLC-Q-TOF-MS/MS and multivariate statistical analysis. Lwt-Food Sci. Technol.. 2021;145:111293
- [CrossRef] [Google Scholar]
- Phytochemistry and antioxidant activities of the rhizome and radix of Millettia speciosa based on UHPLC-Q-exactive orbitrap-MS. Molecules. 2022;27(21):7398.
- [CrossRef] [Google Scholar]
- Anti-fatigue and antioxidant activity of the polysaccharides isolated from Millettiae speciosae Champ. Leguminosae. Nutrients. 2015;7(10):8657-8669.
- [CrossRef] [Google Scholar]
- Botanical characteristics, chemical and nutritional composition and pharmacological and toxicological effects of medicinal and edible plant Millettia speciosa Champ. Food Sci.. 2017;38(9):293-306.
- [Google Scholar]
- Less SO 2 residue may not indicate higher quality, better efficacy and weaker toxicity of sulfur-fumigated herbs: Ginseng, a pilot study. J. Hazard. Mater.. 2019;364:376-387.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105509.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







