Translate this page into:
Chemical characterization, antioxidant properties and enzyme inhibition of Rutabaga root’s pulp and peel (Brassica napus L.)
⁎Corresponding author. a.mollica@unich.it (Adriano Mollica)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Rutabaga (Brassica napus L.) belonging to Brassicaceae family, is a rich source of polyphenols and glucosinolates. Its consumption in human diet is highly appreciated for its nutritional contribution and health benefits. Brassica napus L. is recognized as the world's most widely grown temperate oilseed crop containing erucic acid for industrial applications, plants germination, animal feed and fuel. In this work we prepared two different extracts of Rutabaga root’s pulp and peel, e.g. ultrasound assisted extract (UAE) and homogenizer assisted extract (HAE). The four extracts have been analyzed by HPLC-MS to assess the phytochemical characterization and tested by antioxidant and enzyme inhibitor assays. Rutabaga pulp and peel extracts possess tyrosinase and glucosidase inhibitory activities together with a moderate antioxidant ability. Our results show a high level of glucosinolates, in particular neoglucobrassicin in the peel extract, which let us suppose a potential application as crop in industry and as supplement in human diet.
Keywords
Rutabaga
Antioxidants
Tyrosinase
Glucosinolates
Peel
Pulp
1 Introduction
Nowadays researchers are focusing their attention on the discovery and evaluation of plant-based products as a natural source of compounds useful in medicinal chemistry as nutraceuticals, in order to avoid the numerous side effects of synthetic compounds or in association with drugs for the treatment of several diseases. In particular phenolic compounds provided by the secondary metabolism of plants, protect multiple organs from oxidation and are considered natural antioxidants.
Antioxidants are distinguished on the base of function (e.g. free-radical scavengers, scavengers of non-radical oxidizing agents, compounds that inhibit the generation of oxidants, transition metal chelating agents, compounds able to stimulate the production of endogenous antioxidant compounds), polarity (water-soluble and liposoluble), source (exogenous or endogenous), mechanism (e.g. hydrogen atom transfer (HAT), single electron transfer (SET), and the ability to chelate transition metals) (Granato et al., 2018).
Flavonoids and the other phenolic compounds are plant secondary metabolites characterized by an aromatic ring bearing at least one hydroxyl group. More than 8000 phenolic compounds as naturally occurring substances from plants have been reported and half of these phenolic compounds are flavonoids presenting glycosides as aglycone, and methylated derivatives. These phytochemical substances are interesting candidates for pharmaceutical and medical applications as effective antioxidants, anticancer, antibacteria, cardioprotective agents, anti-inflammation, immune system promoting, skin protection from UV radiation (Tungmunnithum et al., 2018). Since a few decades ago, the research studies focusing on flavonoids and the other phenolic compounds from medicinal plant species have increased considerably, because of their versatile benefits for human health.
In the last decades, several attempts have been performed to control global health problems including Alzheimer’s disease (Cacciatore et al., 2012) and diabetes mellitus (Llorent-Martínez et al., 2018). The prevalence of these diseases is dramatically increasing by day, thus we need urgent precautions to manage them. Enzymes are considered as effective targets for therapeutic strategies; For example, acetylcholinesterase hydrolyzes acetylcholine in the synaptic gap thus blocking neurotransmission. Several studies indicated that the level of acetylcholine is very low in Alzheimer’s patients, thus the inhibition of acetylcholinesterase could improve the level of acetylcholine to enhance memory functions (De Simone et al., 2020; Dorababu, 2019). In addition, diabetes mellitus could be controlled by the inhibition of carbohydrate hydrolyzing enzymes, such as α-amylase and α-glucosidase, which could slow down blood glucose level (Costante et al., 2015; Chinsembu, 2019; Santos et al., 2018). Tyrosinase is also the main target for controlling hyperpigmentation problems, which catalyzes the synthesis of melanin (Mukherjee et al., 2018). Some compounds have been developed as enzyme inhibitors to control the above-mentioned diseases, despite their undesirable side effects. In this sense, seeking novel enzyme inhibitors from natural sources is a critical point in the scientific scenario (Zengin et al., 2018).
Brassica vegetables contain high levels of vitamins (Heimler et al., 2006), tocopherols (mainly α-tocopherol and γ-tocopherol) (Goffman and Möllers, 2000), and folic acid; the latter is a coenzyme involved in the single carbon transfer of DNA, RNA and protein components synthesis, reducing the risk of vascular diseases and cancer (Devi et al., 2008). They also contain carotenoids, among them lutein and β-carotene are the most abundant and able to prevent oxidative damage (Eberhardt et al., 2005), and minerals such as calcium and iron, phosphorus, sulphur, chlorine and potassium (Tıraşoğlu et al., 2005). In particular Brassica oleracea L. and Brassica napus L. belonging to Brassicaceae family, are used as phytoremediation in soils detoxification due to their capability to remove toxic metals from contaminated soils (Salt et al., 1995).
Glucosinolates (GSLs) are sulfur-containing phytochemicals present in Brassicaceae (Ahuja et al., 2011). The most known GSLs in Brassicaceae’s vegetable are neoglucobrassicin, glucobrassicanapin and glucobrassicin (Vallejo et al., 2004); these bioactive compounds contain a cyano group and a sulphate group which confer them the protective role against plant’s pathogen and insect attacks together with myrosinase (Wittstock et al., 2016; Zrybko et al., 1997).
Myrosinase is capable to react with GSLs during a plant’s tissue damage event, converting GSLs into isothiocyanates (ITCs) (Zrybko et al., 1997) and indoles as decomposition products. They possess chemo-preventive properties against different types of tumours such as pancreas, liver and colon tumours, however a toxic effect at high dosage has been also documented in literature (Wu et al., 2005).
Rutabaga (Brassica napus L.) belongs to Brassicaceae family which is composed of more than 3500 species (Jahangir et al., 2009; Sasaki and Takahashi, 2002). It is inexpensive and the root is widely used as food and spice in northern Europe, northern America (Pasko et al., 2013), China, Japan and India whereas the seeds are a source of vegetable oil (Kusznierewicz et al., 2008). The root pulp has a color ranging from white to orange-yellow, with sweet and slightly spicy taste. It can be cooked in the oven, boiled or in foil under the embers.
In this work we analysed the chemical composition of ultrasound assisted extracts (UAE) and homogenizer-assisted extracts (HAE) of Rutabaga pulp and peel in terms of total phenolic and flavonoid contents, focusing our attention on glucosinolates (GSLs) composition using analytical methods, antioxidant properties and enzymatic inhibitory capability. The aim of this work is to define the phytochemical profile and biological activities of Rutabaga plant’s part extracts in order to delineate a possible use of the waste product Rutabaga peel, in crop industry and animal consumption and to define its possible role as nutraceutical by a potential incorporation in human diet as food supplement.
2 Materials and methods
2.1 Plants material
Rutabaga (Brassica napus L.) was purchased in November 2018 in Gdansk (Poland) (Fig. 1). The peel and the pulp were frozen in liquid nitrogen separately then lyophilized in order to dry both completely. The dried material was grounded to fine powder and stored in the dark at 4 °C pending extraction.
Rutabaga root vegetable from Brassica napus L.
2.2 Reagents, chemicals and standard
The stock standard solution for HPLC analysis was prepared by dissolving 10 mg of analyte in 10 mL of methanol and stored in a glass-stopped bottle at 4 °C in the dark. Standard solutions at diverse concentrations were prepared daily diluting aliquotes of stock solutions in water. HPLC-grade methanol and acetonitrile were from Sigma-Aldrich (Milan, Italy).
The chemicals were purchased from Sigma-Aldrich (Darmstadt, Germany). They were: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), gallic acid, rutin, caffeic acid, electric eel acetylcholinesterase (AChE) (type-VI-S, EC 3.1.1.7), horse serum butyrylcholinesterase (BChE) (EC 3.1.1.8), galantamine, acetylthiocholine iodide (ATChI), butyrylthiocholine chloride (BTChI) 5,5-dithio-bis(2-nitrobenzoic) acid (DTNB), tyrosinase (EC1.14.18.1, mushroom), α-glucosidase (EC. 3.2.1.20, from Saccharomyces cerevisiae), α-amylase (EC. 3.2.1.1, from porcine pancreas), sodium molybdate, sodium nitrate, sodium carbonate, Folin-Ciocalteu reagent, hydrochloric acid, sodium hydroxide, trolox, ethylenediaminetetraacetate (EDTA), neocuproine, cupric chloride, ammonium acetate, ferric chloride, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), ammonium molybdate, ferrozine, ferrous sulphate hexahydrate, kojic acid and acarbose. All chemicals were of analytical grade.
Chromatographic analysis was performed using deionized water ≥18 MΩ·cm resistivity purified with a Milli-Q system (Millipore, Bedford, USA) and HPLC-grade acetonitrile (Sigma-Aldrich; Madrid, Spain). All solvents and solutions were filtered through a 0.45-µm polytetrafluoroethylene (PTFE) filter from Supelco (Bellefonte, PA, USA) before use.
2.3 Extraction
In the present paper two non-conventional extraction methods, namely homogenizer-assisted extraction (HAE) and ultrasonication-assisted extraction (UAE) were used with methanol as solvent. In HAE, the plant material (2 g) was extracted with 20 mL of methanol by using one ultra-turrax (IKA, T25, digital ultra-turrax, Staufen, Germany, 6000g) for 5 min. In UAE, the plant material (2 g) was mixed with methanol (20 mL) for 30 min in an ultrasonic bath (ultrasonic frequency of 30 KHz, Daihan, WUC-D10H, Wonju-si, Korea). Then the mixture was filtered and the solvent was evaporated by rotary-evaporator. All extracts were stored +4 °C.
2.4 Chemical profiles
Total phenolic and flavonoid contents of the extracts were determined by colorimetric assays (Zengin and Aktumsek, 2014). Gallic acid (GAE) and rutin (RE) were chosen as standards for phenols and flavonoids respectively.
For chromatographic analysis, 5 mg of dried extract was re-dissolved in 1 mL of methanol and filtered through 0.45 µm PTFE membrane filters (Llorent-Martínez et al., 2018). The HPLC system is equipped as follows: Agilent Series 1100 with a G1315B diode array detector (Agilent Technologies, Santa Clara, CA, USA); Luna Omega Polar C18 analytical column of 150 × 3.0 mm and 5 µm particle size (Phenomenex, Torrance, CA, USA), Polar C18 Security Guard cartridge (Phenomenex) of 4 × 3.0 mm; mobile phases were water + formic acid 0.1% v/v (eluent A) and acetonitrile (eluent B), gradient elution of 10–25% B in 0–25 min, 25–100% B in 25–30 min, eluent B was returned to 10% with a 7 min stabilization time; flow rate of 0.4 mL min−1 and injection volume of 10 µL.
The HPLC system is joined to an ion trap mass spectrometer (Esquire 6000, Bruker Daltonics, Billerica, MA, USA) with an electrospray interface operating in negative mode; scan range at m/z 100–1200 with a speed of 13,000 Da/s; drying gas (N2) flow rate and temperature, 10 L/min and 365 °C; nebulizer gas (N2) pressure, 50 psi; capillary voltage, 4500 V; capillary exit voltage, −117.3 V; auto MSn mode for data acquisition, with isolation width of 4.0 m/z and fragmentation amplitude of 0.6 V (MSn up to MS4).
2.5 Antioxidant properties
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing power (FRAP), phosphomolybdenum, cupric reducing antioxidant capacity (CUPRAC) and the metal chelating activities were performed following Grochowski et al. (2017) methods, considering standard equivalents to evaluate antioxidant properties (trolox and EDTA). The enzymatic inhibitory activities of the extracts against acetylcholinesterase (AChE), butyrylcholinesterase (BChE) (by Ellman’s method), α-amylase, α-glucosidase, and tyrosinase were detected using standard in vitro bioassays. All assays were performed considering three technical replications. The enzyme inhibition abilities were explained as standard equivalent and IC50 values (mg /mL).
2.6 Statistical analysis
All experimental results were expressed as mean ± SD standard deviation. Normality test i.e. Shapiro-wilk and Anderson-Darling were submitted to data; depending on the results, Kruskal wallis or One-way analysis of variance were performed to check the significant differences among samples. Means of samples were compared using Dunn or Turkey’s post-hoc at the 5% confidence level, when significant differences were found.
Hierarchical clustering analysis was performed with the aim to group samples according to their similarity, by using average Euclidean distances and Ward method. Then PLS-DA and Heatmap were achieved to identify the most discriminant biological activities and to characterize the clusters obtained from HCA analysis. All analysis were performed under R v 3.6.1.
3 Results and discussion
3.1 Phytochemical analysis
This is the first report on the glucosinolates composition of Brassica napus var. napobrassica. Nine compounds were identified in the analyzed extracts by HPLC-ESI-MSn: a disaccharide, seven glucosinolates, and sinapic acid. Results are summarized in Table 1. The identification of glucosinolates was performed according to bibliographic data (Gao et al., 2014; Velasco et al., 2011). Compounds 2, 4, 5, and 8 have been previously reported in B. napus pabularia (Velasco et al., 2011). Some of the glucosinolates reported in leaves of B. napus pabularia have not been characterized in B. napus var. napobrassica. In addition, compounds 3, 6, and 7 were not found in B. napus pabularia. These differences are due not only to the different varieties of B. napus analyzed, but also to the different morphological parts: leaves in B. napus pabularia, whereas pulp and peel in napobrassica variety. However, the prevalence of glucosinolates is similar in both studies.
Pulp
Peel
Ref.
No.
tR (min)
[M−H]−m/z
m/z (% base peak)
Assigned identification
HAE
UAE
HAE
UAE
1
1.7
341
MS2 [341]: 179 (100), 161 (67), 149 (11), 143 (24), 119 (36), 113 (34)
Disaccharide
+
+
+
+
Verardo et al. (2009)
2
2.6
388
MS2 [388]: 332 (81), 308 (11), 301 (18), 275 (24), 259 (100), 210 (61), 195 (23), 136 (50)
Progoitrin
+
–
+
–
Velasco et al. (2011)
3
4.5
420
MS2 [420]: 340 (26), 275 (60), 259 (100)
Glucoerucin
+
+
–
–
Gao et al. (2014)
4
6.2
447
MS2 [447]: 367 (83), 291 (96), 275 (53), 259 (100), 205 (33), 195 (71)
Glucobrassicin
+
+
–
–
Gao et al. (2014) and Velasco et al. (2011)
5
8.0
422
MS2 [422]: 342 (11), 275 (13), 259 (100), 244 (41), 229 (8), 195 (16), 180 (21), 163 (7)
Gluconasturtiin
+
+
+
+
Velasco et al. (2011)
6
8.0
434
MS2 [434]: 354 (11), 275 (82), 259 (100), 192 (39)
Glucoraphenin
+
+
+
+
Gao et al. (2014)
7
9.7
477
MS2 [477]: 447 (26), 446 (100), 259 (22)
Neoglucobrassicin isomer
+
+
+
+
Velasco et al. (2011)
8
14.2
477
MS2 [477]: 447 (60), 446 (100), 259 (8)
Neoglucobrassicin
+
+
+
+
Velasco et al. (2011)
9
20.0
223
MS2 [223]: 208 (52), 164 (100)
MS3 [223 → 164]: 149 (100)Sinapic acid
+
+
+
+
Velasco et al. (2011)
Compounds 1 and 5–9 were detected in all extracts, however, compounds 3 and 4 (glucoerucin and glucobrassicin) were only in pulp extracts. Two extraction methods were tested, homogenized-assisted extraction and ultrasound-assisted extraction, observing that the first sample treatment provided the best extraction of compounds (approximately 30–60% improvement considering the chromatographic peak areas of all the compounds). In fact, compound 2 (progoitrin) was not extracted with the ultrasound procedure.
A relative quantification was performed by measuring peak areas of each compound in MS mode using the Extracted Ion Chromatograms, with precursor ion [M−H]−. The relative percentage of each compound was calculated and shown in Fig. 2, in which the heat map highlights the most abundant compounds (the darker the color, the higher the concentration). It can be observed that, in addition to glucosinolates, only a disaccharide formed by two glucoses (compound 1) provided a significant contribution to the profiles observed. However, glucosinolates represented approximately 70–80% of the extracts. The most abundant glucosinolate was neoglucobrassicin, which accounted for approximately 25 and 50% of all compounds in pulp and peel extracts, respectively. Gluconasturtiin and glucoraphenin were also important contributors to the composition of pulp extracts, whereas only gluconasturtiin was significant in peel extracts. A higher concentration of glucosinolates was observed in peel extracts compared to pulp extracts. A visual comparison can be observed in Fig. 2; the big difference was due to compound 8 (neoglucobrassicin), which is present at high concentration in peel extracts.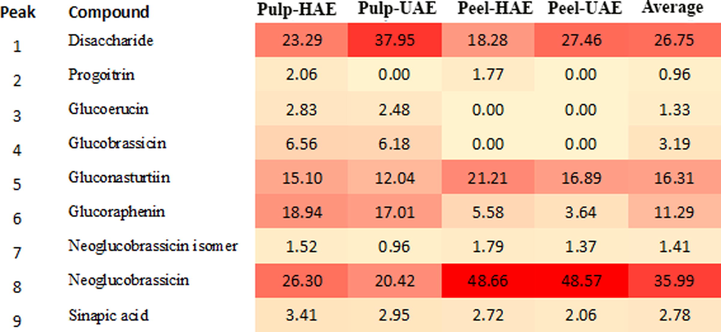
Relative peak areas and heat map obtained by HPLC-ESI-MS analysis of Rutabaga pulp and peel extracts.
3.2 Antioxidant properties
The total phenolic, flavonoid composition, antioxidant activity and metal chelating of Rutabaga root pulp-HAE (Homogenated assisted extract) and Rutabaga-UAE (ultrasound assisted extract) were evaluated using four different methods, including free radical scavenging (DPPH and ABTS) and reducing power (CUPRAC and FRAP) assays. The results are reported in Table 2. Regarding the total phenolic composition, results show that Rutabaga peel extracts possess higher values than Rutabaga pulp-extracts. The best value is reported for Rutabaga peel UAE with 18.14 ± 0.09 mg GAE/g, probably due to the extraction technique ability. Rutabaga peel presents a major content in terms of flavonoids in comparison to the pulp, with no relevant differences between the different extraction methods.
Samples/Methods
Total phenolic content (mg GAE/g)a
Total flavonoid content (mg RE/g)a
DPPH (mg TE/g)a
ABTS (mg TE/g)a
CUPRAC (mg TE/g)a
FRAP (mg TE/g)a
Metal chelating (mg EDTAE/g)a
PPBD (mmol TE/g)b
Pulp-HAE
10.76 ± 0.10c
0.51 ± 0.02c
na
19.07 ± 2.18b
32.27 ± 0.56b
22.70 ± 0.37c
na
0.64 ± 0.05c
Pulp-UAE
11.57 ± 0.05bc
0.55 ± 0.02bc
na
15.93 ± 3.13b
32.81 ± 0.57ab
24.19 ± 0.06bc
na
0.87 ± 0.04b
Peel-HAE
16.55 ± 0.14ab
1.09 ± 0.01a
5.22 ± 1.05ab
44.71 ± 1.82ab
41.06 ± 1.57a
30.60 ± 0.42ab
9.42 ± 0.83ab
1.07 ± 0.05a
Peel-UAE
18.14 ± 0.09a
1.04 ± 0.02ab
8.11 ± 0.81a
47.37 ± 3.79a
45.62 ± 1.00a
34.14 ± 0.90a
4.79 ± 0.43b
1.11 ± 0.13a
Regarding DPPH assay, no activity for Rutabaga root pulp was detected, while 5.22 ± 1.05 mg TE/g and 8.11 ± 0.81 mg TE/g were found for Rutabaga peel-HAE and Rutabaga peel-UAE respectively, suggesting different radical scavenging ability for each extraction method in combination to the plant’s parts. In addition, ABTS value resulted to be higher in Rutabaga peel than the Rutabaga pulp (47.37 ± 3.79 mg TE/g for UAE-extract).
The reducing power capacity determined by the CUPRAC and FRAP assays resulted to be higher in Rutabaga-peel-UAE, with 45.62 ± 1.00 mg TE/g for CUPRAC and 34.14 ± 0.90 mg TE/g for FRAP respectively; for both of them UAE reports the best results. Rutabaga pulp is not able to exert metal chelating activity, while Rutabaga-peel HAE shows a value of 9.42 ± 0.83 mg EDTAE/g, keeping good results also in PPBD assay (1.11 ± 0.13 mmol TE/g for Rutabaga peel HAE).
The observed data could be explained by the presence of glucosinolate in the Rutabaga extracts. In earlier studies some glucosinolate-rich plants exhibited significant antioxidant properties. For example, Chang et al. (2019) investigated antioxidant capacity and phytochemical composition of six kale varieties, observing a positive correlation between FRAP results and glucosinolate content, based on glucoraphasatin (Montaut et al., 2010), glucobrassin (Sun et al., 2018), sinigrin and gluconapin (Oh et al., 2017). Taken together, the glucosinolates have been suggested as health-promoting phytochemicals and in this sense, Rutabaga is considered as an important source for supporting healthy life.
3.3 Enzyme inhibition evaluation
Enzyme inhibitory activity of Rutabaga root peel and pulp extracts towards AChE, BChE, tyrosinase, α-amylase, and α-glucosidase, was evaluated in comparison with specific standard drugs (galantamine, kojic acid, and acarbose). The results are reported in Table 3. The AChE inhibition activity of Rutabaga pulp and peel extracts are comparable but the best value was found in Rutabaga pulp-HAE with 4.86 ± 0.15 mg GALAE/g (IC50: 0.61 mg/mL), the weakest value was found in Rutabaga peel-HAE 3.60 ± 0.36 mg GALAE/g (IC50: 0.83 mg/mL) that was lower than Rutabaga peel-UAE (4.41 ± 0.23 mg GALAE/g). The extracts also exhibited good BChE inhibitor activity with the best value for Rutabaga Pulp-UAE (7.09 ± 0.37 mg GALAE/g against 4.53 ± 0.34 mg GALAE/g for Rutabaga Pulp-HAE). UAE was the best extraction technique and the pulp results were high in BChE inhibition activity content. Regarding the tyrosinase inhibition activity, good values were found for both peel and pulp; Rutabaga pulp-HAE showed 101.65 ± 0.34 mg KAE/g (IC50: 0.79 mg/mL) suggesting an improved selectivity toward tyrosinase. This is an interesting point since in our previous paper on Brassica Oleracea L. we also detected a significant tyrosinase inhibitory effect for soxhlet extract of Cavolo Nero (Mollica et al., 2018). Our extracts also possess α-amylase inhibition activity in particular for Rutabaga peel, with the best value showed by Rutabaga peel UAE (0.71 ± 0.01 mmol ACAE/g). In α-glucosidase inhibition assay, Rutabaga pulp-HAE extract showed the best result (35.31 ± 0.37 mmol ACAE and IC50: 0.72 mg/mL). In this paper, glucosinolates were determined as main compounds in the tested extracts. Some authors reported that the high levels of glucosinolates in plants exhibited significant enzyme inhibitory properties (Abbas et al., 2017; Hichri et al., 2019; Marrelli et al., 2018). However the qualification of compounds in the extracts are not enough to fully understand enzyme inhibition properties, especially on the basis of the structure–activity relation. Thus the isolation and quantification of other secondary metabolites such as alkaloids and terpenoids, are strongly requested in future studies. Values are reported as mean ± SD. of three parallel experiments. GALAE: Galatamine equivalents; KAE: Kojic acid equivalents; ACAE: Acarbose equivalent; HAE: Homogenizer assisted extraction; UAE: Ultrasound assisted extraction. Different superscripts (a, b, c and d) indicate significant differences in the extracts (p < 0.05). aStatistical evaluation was done by Kruskal-Wallis test. bStatistical evaluation was done by ANOVA test.
AChE inhibitionb
BChE inhibitionb
Tyrosinase inhibitiona
α-Amylase inhibitionb
α-Glucosidase inhibition a
Samples/Methods
(mg GALAE/g)
IC50 (mg/mL)
(mg GALAE/g)
IC50 (mg/mL)
(mg KAE/g)
IC50 (mg/mL)
(mmol ACAE/g)
IC50 (mg/mL)
(mmol ACAE)
IC50 (mg/mL)
Pulp-HAE
4.86 ± 0.15a
0.61 ± 0.02b
4.53 ± 0.31b
1.15 ± 0.08b
101.65 ± 0.34a
0.79 ± 0.01bc
0.66 ± 0.01a
2.20 ± 0.05a
35.31 ± 0.37a
0.72 ± 0.01c
Pulp-UAE
4.18 ± 0.08bc
0.71 ± 0.02b
7.09 ± 0.37a
0.74 ± 0.04c
100.63 ± 1.20ab
0.80 ± 0.01abc
0.68 ± 0.01a
2.14 ± 0.04a
18.66 ± 0.52ab
1.04 ± 0.03bc
Peel-HAE
3.60 ± 0.36c
0.83 ± 0.09a
4.10 ± 0.76b
1.28 ± 0.24a
100.60 ± 0.98ab
0.80 ± 0.01ab
0.70 ± 0.04a
2.07 ± 0.12a
13.92 ± 0.78c
1.40 ± 0.08ab
Peel-UAE
4.41 ± 0.23ab
0.68 ± 0.03b
6.02 ± 0.65a
0.86 ± 0.10c
98.32 ± 1.70b
0.82 ± 0.01a
0.71 ± 0.01a
2.03 ± 0.04a
16.21 ± 0.36bc
1.20 ± 0.03abc
Galatamine
–
0.003 ± 0.001c
–
0.005 ± 0.001d
–
nt
–
nt
–
nt
Kojic acid
–
nt
–
nt
–
0.08 ± 0.01c
–
nt
–
nt
Acarbose
–
nt
–
nt
–
nt
–
0.88 ± 0.01b
–
1.60 ± 0.01a
3.4 Multivariate analysis
Hierarchical clustering analysis (HCA) was submitted to the biological activities dataset of Rutabaga with the aim to find relatively homogeneous groups of samples. The result obtained was depicted in Fig. 3; samples were assigned to two main clusters according with the organs which were themselves divided into two sub-clusters respectively, in terms of extraction techniques. According to HCA, among both factors “organs” and “extraction techniques”, the most important factor responsible for the earlier observed difference in biological activities of Rutabaga samples was “organs”. HCA is an unsupervised technique, which allows obtaining an overview of the structuring of data, however no information regarding the biological activities responsible for this clustering and those characterizing each of the obtained clusters were provided. Instead of answering a supervised method, Partial least squares discriminant analysis (PLS-DA) was achieved using “organs” as class membership.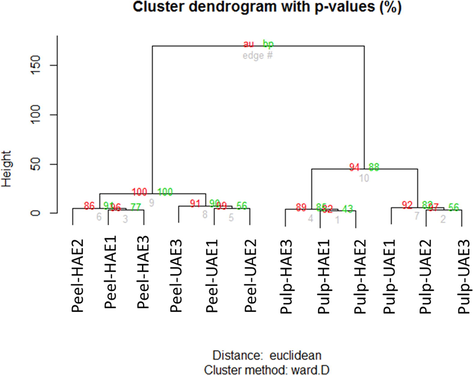
Hierarchical clustering analysis.
PLS-DA is a supervised algorithm used to improve partition between different clusters of samples, which is done by associating two data matrices, e.g. a raw data (X) and a corresponding dependent class membership (Y). As presented in Fig. 4A the two organs were successfully separated, suggesting that certain biological activities of pulp and peel were significantly different. To identify those biological activities significantly different in the two Rutabaga parts, the main variable involved in projection (VIP) score was calculated. As shown in Fig. 4B, DPPH, ABTS, CUPRAC, FRAP, MCA, PPBD showing a VIP larger than 1 were found to be the most discriminant biological activities. Using Student’s t-test or Wilcoxon, it appears clearly that peel was most active against DPPH, ABTS, CUPRAC, FRAP, MCA, PPBD than pulp (Fig. 4C). The performance of the Model recorded by estimating the classification error rate and AUC values was found to be excellent; the balance error rate (BER) and AUC value for the first component were 0 and 1 respectively (Fig. 4D and E).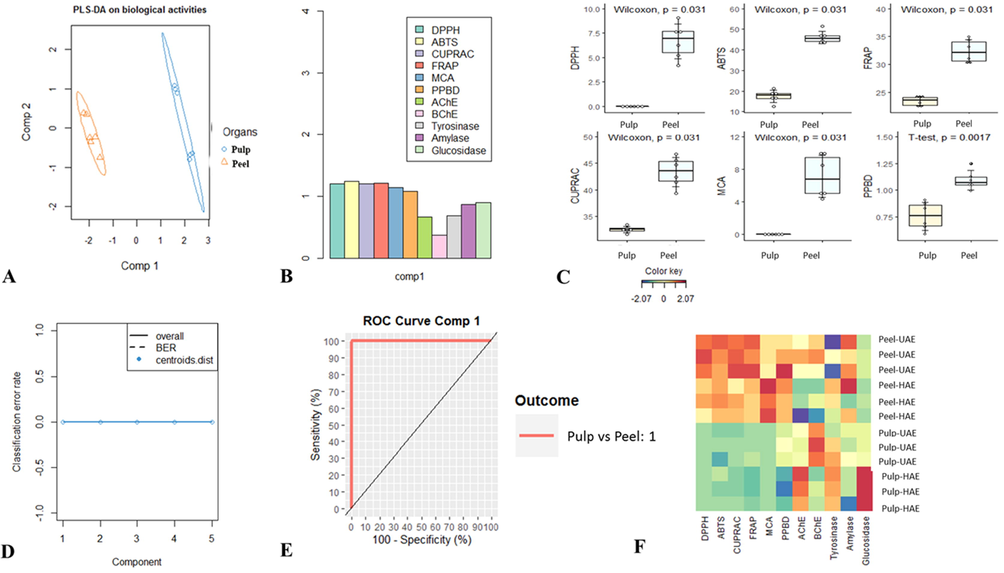
Supervised Partial Least Squares Discriminant Analysis. A: Samples plot. B: The most discriminant biological activities identifying though VIP score calculation. C: Characterization of pulp and skin samples taking account of the identified most discriminant biological activities. D: The model goodness per component for centroids Distance using 5-fold CV repeated 10 times. E: AUC (Area Under the Curve average) and ROC (Receiver Operating Characteristic) Curve using one-vs-all comparisons. F: Heatmap showing variation of biological activities between pulp and peel extracts. red color indicates high activity. Blue color indicates low activity.
After the PLS-DA analysis, which helped the discrimination of Rutabaga parts, it was necessary to assess the impact of the second factor e.g. extraction techniques on the biological activities of each organ. Consequently a heatmap was generated, by visual examination of the corresponding results reported in Fig. 4F; the influence of extraction technique was more effective in pulp than in the peel particles. The effect of pulp was remarkable in AChE, BChE and α-glucosidase inhibition assays, in particular, extract obtained using HAE showed good inhibition ability against AChE and α-glucosidase, while UAE extract was most effective against BChE. Regarding the peel, the effect was observed in both cholinesterase inhibition assays, but UAE product was found to be most active. According to these observations, the choice of technique for phytochemical compounds extraction must be based upon pharmacological activity evaluation. In fact although there are several extraction techniques, there is no single appropriate technique for extraction, purification and isolation of molecules from herbals. Thus the choice of one technique must depend on its intrinsic advantages and disadvantages.
4 Conclusion
Over the years, ultrasonic-assisted extraction (UAE) technique has emerged as an alternative technique for bioactive compounds recovery from various herbals matrices. UAE is a green extraction procedure, in which cavitation phenomena causes high shear forces in plant matrices resulting in cell walls breakdown. However it can lead to a significant degradation of active compounds at high temperature (Alupului et al., 2009). In addition comparing with Homogenization-assisted extraction (HAE) technique, UAE equipment turn out to be more expensive and it is hardly available in industrial scale-up production because of its low throughput of materials (Jiao et al., 2014). In fact HAE is characterized by a low consumption of solvent and time, no heating and mechanical stresses are required; the latter induce the rupture of plant tissues and cell walls for better release of plant molecules, through a continuous stream with shear, turbulence and friction. As a result, HAE was an advisable and effective technique for the extraction of bioactive compounds from A. rutabaga organs; nonetheless optimization of certain extraction parameters is required.
Brassica napus L. represents the main source of widely grown temperate oilseed crop in the world. Brassica napus L. seeds are rich in erucic acid-containing lipids, germplasm and gene involved in the codification of enzymes regulating the triacylglycerols (TAGs) pathways for industrial applications. In fact cold-pressed rapeseed oil promotes health benefits through its fatty acid profile and bioactive compounds (e.g. tocopherols, phytosterols, and carotenoids) that influence the regulation of blood lipid, glycemic contents and insulin sensitivity, providing for antioxidant and cytotoxic activities.
Our work represents the first exhaustive analysis of the total flavonoid/phenolic contents, antioxidant and enzyme inhibition properties of two different Rutabaga (Brassica napus L.) pulp and peel extracts obtained through two diverse extraction techniques, HAE (homogenizer-assisted extraction) and UAE (ultrasound-assisted extraction). We found high levels of glucosinolates approximately 70–80% of the extracts, in particular neoglucobrassicin is present in the peel extract. To better explain the healthy properties of Rutabaga, we tested all extracts in different bioassays, proving their good antioxidant properties and the significative enzymatic inhibitor ability on tyrosinase. Further in vivo studies are required to determine the possible impact on agriculture practice, plant organs protection and the general benefits of Rutabaga on the human diet.
CRediT authorship contribution statement
Azzurra Stefanucci: Investigation, Writing - review & editing. Gokhan Zengin: Data curation, Software. Eulogio J. Llorent-Martinez: Data curation. Marilisa Pia Dimmito: Formal analysis. Alice Della Valle: Formal analysis. Stefano Pieretti: Methodology. Gunes Ak: Methodology, Validation. Kouadio Ibrahime Sinan: Methodology, Validation. Adriano Mollica: Conceptualization, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro, in vivo and in silico anti-hyperglycemic inhibition by sinigrin. Asian Pac. J. Trop. Med.. 2017;10:372-379.
- [Google Scholar]
- Defence mechanisms of Brassicaceae: implications for plant-insect interactions and potential for integrated pest management. In: Sustainable Agriculture. Vol Volume 2. Springer; 2011. p. :623-670.
- [Google Scholar]
- Ultrasonic vs. microwave extraction intensification of active principles from medicinal plants. AIDIC Conf. Ser. 2009:1-8.
- [Google Scholar]
- GPE and GPE analogues as promising neuroprotective agents. Mini-Rev. Med. Chem.. 2012;12:13-23.
- [Google Scholar]
- Health-promoting phytochemicals and antioxidant capacity in different organs from six varieties of Chinese kale. Sci. Rep.. 2019;9:1-10.
- [Google Scholar]
- Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Herbal Med.. 2019;15:100230.
- [Google Scholar]
- DPP-4 inhibitors: A patent review (2012–2014) Expert Opin. Therapeut. Patents. 2015;25:209-236.
- [Google Scholar]
- Advanced analytical methodologies in Alzheimer’s disease drug discovery. J. Pharm. Biomed. Anal.. 2020;178:112899.
- [Google Scholar]
- Folate contents of some selected Fijian foods using tri-enzyme extraction method. Food Chem.. 2008;106:1100-1104.
- [Google Scholar]
- Critical evaluation of current Alzheimer’s drug discovery (2018–19) & futuristic Alzheimer drug model approach. Bioorg. Chem.. 2019;93:103299.
- [Google Scholar]
- Correlation analyses of phytochemical composition, chemical, and cellular measures of antioxidant activity of broccoli (Brassica oleracea L. var. italica) J. Agric. Food Chem.. 2005;53:7421-7431.
- [Google Scholar]
- Removal of off-flavours from radish (Raphanus sativus L.) anthocyanin-rich pigments using chitosan and its mechanism (s) Food Chem.. 2014;146:423-428.
- [Google Scholar]
- Changes in tocopherol and plastochromanol-8 contents in seeds and oil of oilseed rape (Brassica n apus L.) during storage as influenced by temperature and air oxygen. J. Agric. Food Chem.. 2000;48:1605-1609.
- [Google Scholar]
- Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem.. 2018;264:471-475.
- [Google Scholar]
- In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett.. 2017;20:365-372.
- [Google Scholar]
- Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem.. 2006;99:464-469.
- [Google Scholar]
- Alpha-glucosidase and amylase inhibitory effects of Eruca vesicaria subsp. longirostris essential oils: synthesis of new 1, 2, 4-triazole-thiol derivatives and 1, 3, 4-thiadiazole with potential inhibitory activity. Pharm. Biol.. 2019;57:564-570.
- [Google Scholar]
- Health-affecting compounds in Brassicaceae. Comprehens. Rev. Food Sci. Food Saf.. 2009;8:31-43.
- [Google Scholar]
- A pilot-scale homogenization-assisted negative pressure cavitation extraction of Astragalus polysaccharides. Int. J. Biol. Macromol.. 2014;67:189-194.
- [Google Scholar]
- Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT – Food Sci. Technol.. 2008;41:1-9.
- [Google Scholar]
- Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem.. 2018;42:5204-5214.
- [Google Scholar]
- Phytochemical and biological profile of Moricandia arvensis (L.) DC.: an inhibitor of pancreatic lipase. Molecules. 2018;23:2829.
- [Google Scholar]
- Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother.. 2018;107:129-138.
- [Google Scholar]
- Glucoraphasatin: Chemistry, occurrence, and biological properties. Phytochemistry. 2010;71:6-12.
- [Google Scholar]
- Validation of medicinal herbs for anti-tyrosinase potential. J. Herbal Med.. 2018;14:1-16.
- [Google Scholar]
- Investigation of glucosinolates, and the antioxidant activity of Dolsan leaf mustard kimchi extract using HPLC and LC-PDA-MS/MS. J. Food Biochem.. 2017;41:e12366.
- [Google Scholar]
- Rutabaga (Brassica napus L. var. napobrassica) seeds, roots, and sprouts: a novel kind of food with antioxidant properties and proapoptotic potential in Hep G2 hepatoma cell line. J. Med. Food. 2013;16:749-759.
- [Google Scholar]
- Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology (N. Y.). 1995;13:468-474.
- [Google Scholar]
- A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem.. 2018;157:1460-1479.
- [Google Scholar]
- A flavonoid from Brassica rapa flower as the UV-absorbing nectar guide. Phytochemistry. 2002;61:339-343.
- [Google Scholar]
- Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera) RSC Adv.. 2018;8:33845-33854.
- [Google Scholar]
- Determination of trace elements in cole (Brassica oleraceae var. acephale) at Trabzon region in Turkey. J. Quant. Spectrosc. Radiat. Transfer. 2005;94:181-187.
- [Google Scholar]
- Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Molecules. 2018;5:93-109.
- [Google Scholar]
- In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem.. 2004;52:135-138.
- [Google Scholar]
- Phytochemical fingerprinting of vegetable Brassica oleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochem. Anal.. 2011;22:144-152.
- [Google Scholar]
- Analysis of underivatized oligosaccharides by liquid chromatography/electrospray ionization tandem mass spectrometry with post-column addition of formic acid. Rapid Commun. Mass Spectromet.: Int. J. Devoted Rapid Dissemin. Up-to-the-Minute Res. Mass Spectromet.. 2009;23:1607-1618.
- [Google Scholar]
- Glucosinolate Breakdown. Advances in Botanical Research. Academic Press; 2016. p. :125-169.
- Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat. Res./Rev. Mutat. Res.. 2005;589:81-102.
- [Google Scholar]
- Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: an endemic plant to Turkey. Afr. J. Tradit. Complement. Alternat. Med.. 2014;11:481-488.
- [Google Scholar]
- Combination of phenolic profiles, pharmacological properties and in silico studies to provide new insights on Silene salsuginea from Turkey. Comput. Biol. Chem.. 2018;77:178-186.
- [Google Scholar]
- Determination of glucosinolates in domestic and wild mustard by high-performance liquid chromatography with confirmation by electrospray mass spectrometry and photodiode-array detection. J. Chromatogr.. 1997;767:43-52.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.07.013.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







