Translate this page into:
Chemical profiling and evaluation of toxicological, antioxidant, anti-inflammatory, anti-nociceptive and tyrosinase inhibitory potential of Portulacaria afra using in-vitro, in-vivo and in-silico studies
⁎Corresponding authors. Sobiat350@gmail.com (Sobia Tabassum), kashifur.rahman@iub.edu.pk (Kashif ur Rehman Khan),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Portulacaria afra Jacq (Portulacaceae) is a medicinal plant used traditionally in the treatment of pain and inflammation. This study aimed to evaluate the phytochemical composition, toxicity, antinociceptive, and enzyme inhibition potential of P. afra. The methanol extract (PAME) was prepared through maceration and fractionated with ethanol to ethanol fraction of P. afra (ETPA). Both PAME and ETPA were found to be rich in total phenolic (TPC) and total flavonoid contents (TFC). Similarly, PAME showed the highest antioxidant potential through ABTS 93.16 ± 0.05 mg TE /g of dry extract (D.E.) while ETPA showed maximum results (462 ± 1.44 mg TE/g) in the CUPRAC method. The RP-UHPLC-MS analysis of PAME showed tentative identification of 101 compounds in the positive mode and 14 compounds in the negative mode of ionization while GC–MS profiling gave a total of 15 compounds. The acute oral toxicity study of PAME revealed the safety and biocompatibility of the extract up to a dose of 5000 mg/kg orally in rats. In-vitro data revealed that varying concentrations of P. afra significantly (p < 0.05) inhibited heat-induced BSA denaturation compared to indomethacin in a concentration-dependent manner. PAME suppressed carrageenan-induced paw edema at the 4th hr with maximum inhibition. The analgesic efficacy of PAME was determined by the hotplate, writhing method, and tail-flicking rat models. In the hot plate method, PAME treated groups showed a significant effect (p < 0.0001). While in the writhing model, the PAME treated groups showed a significant (p < 0.0001) anti-nociceptive effect at 200 mg/kg and 400 mg/kg compared to the control group. Similarly, PAME treated groups in the tail flicking model showed a significant (p < 0.001) anti-nociceptive effect at 200 mg/kg and 400 mg/kg. A dose-dependent increase in latency time (8.78 ± 0.090 at (30 min), 11.39 ± 0.005 at (60 min), 12.14 ± 0.01 at (90 min) 15.19 ± 0.03 at (120 min), p < 0.0001 was observed, compared to the control group. Similarly, the extract and fraction showed significant inhibitory potential against tyrosinase in in vitro and in-silico studies. Conclusively the current study unveiled P. afra as a novel non-toxic source with good total antioxidant-mediated anti-inflammatory and analgesic potential.
Keywords
Portulacaria afra
RP-UHPLC-MS
Acute oral toxicity
Anti-nociceptive
Anti-inflammatory
Molecular docking
1 Introduction
Medicinal plants containing biologically active compounds are an essential source of therapeutically active products (Altaf et al., 2019, Süntar 2020). The world health organization (WHO) stated in a report that 11 % of the 252 drugs are solely obtained from a plant source, while several synthetic drugs are derived from natural sources. Herbal medicines are preferred over synthetic drugs because they have fewer side effects (Khursheed et al., 2022). As per the WHO, the inflammatory process and its linked disorders pose the biggest threat to public health, with a continuous rise in the incidence of such diseases expected in the United States over the next 30 years (Ginwala et al., 2019).
Inflammation is a natural protective response to tissue damage resulting from physical trauma, toxic chemicals, microbial agents, or autoimmune disease. Leukocytes, particularly neutrophils, are attracted to the inflammatory center in response to damage (Dilshad et al., 2022). These cells produce pro-inflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and chemokines through the induction of the nuclear factor-kappa B (NF-KB) pathway. Prostaglandins are very effective vasodilators and induce an inflammatory response (Sajid-ur-Rehman et al., 2021a, 2021b) and a fever cytokines are endogenous pyrogens that cause fever and negatively affect the progression of metabolic dysfunction (Shi et al., 2019). The symptoms of inflammation are severe pain, swelling, and loss of function in the affected area (Saleem et al., 2020). Standard anti-inflammatory treatments, such as non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, treat moderate to severe pain and inflammation (Nguyen et al., 2020). However, long-term usage of the NSAIDs inhibits prostacyclin and prostaglandin E2, causing side effects such as nausea, vomiting, and peptic ulcer (Steiner and Higgins 2018, Jannat et al., 2022). Inflammation is one of the key contributors to worsening skin infections. Moreover, tanning and darkening are two prevalent undesirable skin conditions, and tyrosinase has been identified as the enzyme associated with these two conditions in humans (Khursheed et al., 2022). The drug candidates which are having anti-inflammatory effects, as well as tyrosinase inhibitory potential, seem to be a suitable choice for treating skin infections. Therefore, investigating natural sources particularly, plant source, is of prime importance. There is a boost increase observed in exploring plant species for their pharmacological potential. But still, many species with ethnomedicinal significance are unexplored. Our current investigations were fascinated by the ethnomedicinal significance of P. afra, which is traditionally used in skin disorders associated with some inflammation. P. afra (Portulacaceae) is a popular medicinal plant typically utilized in East South Africa for skin infections and other skin conditions such as ulcers, fungus, blisters, wounds, dermatitis, scars, lesions, tumors, cysts, and pimples (De Wet et al., 2013).(Olaokun et al., 2017, Dlova and Ollengo 2018, Nakamura et al., 2021). The family Portulacaceae comprises over 20 to 30 genera and about 450 species found chiefly in tropical and subtropical regions of the world (Augros et al., 2018). P. afra is an evergreen succulent plant with thick broad leaves that store water (Iranshahy et al., 2017). The Shrubs or tiny trees in the genus Portulacaria have sessile leaves, pale green, pedunculated, inconspicuous pink blooms, and pink fruit with a diameter of 2 to 6 mm. They also have a greatly ramified trunk. It is primarily found in Mozambique and the Republic of South Africa (Karimi et al., 2011). As previously stated, the genus Portulaca contains significant phytochemicals that have significant potential as therapeutic candidates for many diseases. Though there has not been much research into this plant's potential bioactivities, there's a gap in scientific knowledge about its complete potential. Therefore, this study aimed to evaluate phytochemical composition, toxicity, in-vitro and in-vivo anti-inflammatory, analgesic in a rat model, as well as enzyme inhibitory potential, and in-silico molecular docking studies of identified phytoconstituents to validate its folk use.
2 Materials and methods
2.1 Collection of plant material and preparation of extract
The whole plant was collected in April 2020 from the Baghdad-ul-Jadeed campus of The Islamia University of Bahawalpur, Pakistan. First, the plant was identified by taxonomist Ghulam Sarwar, Assistant professor in the department of Botany, The Islamia University of Bahawalpur, Pakistan, and voucher number 314 was allotted for future record and reference. Then, the plant was washed to remove dust and impurities, followed by shade drying, pulverized, and stored in a glass jar. Dried powder of a whole plant (3 kg) was soaked in a hydromethanolic solvent system comprising (methanol: water; 80%:20%) for 7 days with occasional stirring at room temperature (Dilshad et al., 2022). After 7 days, the plant material was filtered and evaporated under reduced pressure to get methanol extract of P. afra (PAME). The dried extract was treated with distilled water to obtain the suspension followed by solvent–solvent fractionation with ethanol which yielded an ethanol fraction of P. afra (ETPA). The ethanol fraction (ETPA) was dried using a rotary evaporator (Heidolph, Germany) at 35 °C. The properly dried PAME and ETPA were stored in an airtight container for further analysis.
2.2 Chemical composition
2.2.1 Determination of polyphenolic contents
2.2.1.1 Total phenolic contents (TPC)
The total phenolic contents in PAME and ETPA were determined using the Folin-Ciocalteu approach (Ahmad et al., 2022). The serial dilutions of gallic acid of 10 µg/mL, 20 µg/mL,40 µg/mL, 60 µg/mL, 80 µg/mL, 100 µg/mL, and 200 µg/Ml were prepared in methanol. In 1 ml of Eppendorf tube, 100 µL of plant extract solution was poured, followed by 100 µL of Folin-Ciocalteu reagent. The solution was mixed, and then 0.4 ml of 300 µM sodium carbonate (Na2CO3) solution was added and incubated for 2 h. 100 ml from every test mixture was added to a 96-microtiter plate, and the absorbance at (λ = 765 nm) was measured. TPC was measured in milligrams of gallic acid equivalent per gram of dry extract (mg GAE/g of dried extract).
2.2.1.2 Total flavonoid contents (TFC)
The total flavonoid contents in PAME and ETPA were determined using the aluminum chloride colorimetric method (Ahmad et al., 2022). The extract solution of each fraction (1 mg/ml) was prepared in methanol. Similarly, serial dilutions of 100 µg/mL, 200 µg/mL, 400 µg/mL, 600 µg/mL, 800 µg/mL, 1000 µg/mL, and 200 µg/mL, were prepared in methanol. 1 ml sample solution (1 mg/mL), 3 ml distilled water, 200 µL of 5% NaNO2 solution, and 200 µL (10% AlCl3) solution were poured into a test tube and an equal volume of 2 ml of (1 M) sodium hydroxide (NaOH) solution. After 5 min, 2.4 ml of distilled water was mixed. The solution's absorbance was taken at (λ = 510 nm) with an IRMECO U2020 UV–visible spectrophotometer. TFC was expressed in mg quercetin equivalent per gram of dry extract (mg QE/g dry extract).
2.2.2 Reversed phase-Ultra performance liquid chromatography-mass spectrometry (RP-UHPLC-MS) analysis
The secondary metabolites in the PAME were identified using conventional RP-UHPLC-MS analysis according to the previously established protocols (Arshad et al., 2021) with little modifications. The column parameter was Agilent Eclipse XDB-C18Narrow-bore,150 mm x2.1 mm, 3.4-µm (P/N 930991–903), column temperature: 25⁰C, autosampler temperature: 4⁰C, Flow rate: 0.5 ml/minutes, Solvents: A − 0.1% formic acid in the water, B-0.1% formic acid in acetonitrile, Injection volume: 1.0uL, sample analysis: U-ESI-E-XDB-C18-NB-MS-20(30) Positive Polarity U-ESI-E-XDB-C18-NB-MS-20(30) negative polarity, the total run time was 30 min. The mode of analysis of methanolic extract was positive and negative, Vcap 4000 V, and 3500 V respectively. The fragmented voltage, skimmer, OCT 1 RF Vpp, drying gas, gas temperature, Nebulizer, 125 V, 65 V, 750 V,10 L/min, 300C, and 45 psig, respectively. The range of mass to charge ratio (m/z) minimum was 100 to a maximum of 3200 in both modes. The reference ions for positive mode were used in the following range 121.0508, 922.0097 to negative mode 119.03632, 966.000725. The acquisition rate (spectra/s), acquisition time (ms/spectrum), and transients/spectrum were obtained at 1.03, 973, and 9632. The data was processed with Agilent mass hunter qualitative analysis B.07.00.
2.2.3 Gas chromatography-mass spectroscopy (GC–MS) analysis
The PAME was investigated by GC–MS, Agilent (6890 series and Hewlett Packard, 5973 ground sensor). Blown barriers were removed on an HP5MS column (30 m length & 250 µm diameter,0.25 µm film thickness). The apparatus includes an HP-5MS ultra-inert capillary non-polar column with dimensions of 30 mm 0.24 mm ID 0.24 µm film. Helium was employed as the carrier gas, with a flow rate of 1.0 ml/min. The injector was set to 255 °C, and the oven temperature was set to (50 °C) for 5 min, then gradually increased to (250 °C) at the rate (of 100 °C/min), and lastly to 3000 °C for 10 min at (70 °C/min). The constituents were identified using data from the NIST library, and the % composition was calculated using GC peak regions (Ahmed et al., 2022a, 2022b; Aziz et al., 2022).
2.3 In vitro biological profiling
2.3.1 In-vitro determination of antioxidant potential
The antioxidant potential of PAME and ETPA were evaluated by using DPPH (2,2 diphenyl-1-picrylhydrazyl), ABTS (2,2 azinobis 3-ethylbenothiazoline-6-sulfonic acid), ferric reducing antioxidant power (FRAP) and cupric reducing antioxidant capacity (CUPRAC) as per the standard protocols described by (Basit et al., 2022a, 2022b, 2022c). Results were expressed in milligrams of Trolox equivalent per gram of dry extract (mg TE/g DE).
2.3.2 In-vitro anti-inflammatory activity through inhibition of high-temperature bovine serum albumin denaturation test
The heat-induced bovine serum albumin (BSA) assay was employed to assess anti-inflammatory potential with minor modification (Naz et al., 2017). The reaction was completed comprising different concentrations (100, 200, 300, and 500 μg/mL) of plant extract and standard drug indomethacin (NSAID) 1% of the total w/v BSA, and phosphate-buffered saline (PBS, pH 6.4), with PBS serving as the control. The solution mixtures were incubated at 37 °C for 20 min before being raised to 70 for 5 min. After cooling, absorbance at 660 nm was measured with a UV–visible spectrophotometer (Shimadzu Double Beam UV-2600, Germany). The control indicates total protein denaturation (100%). The following % inhibition of BSA denatured proteins was calculated:
Whereas,
A1 = Control absorbance,
A2 = test sample absorbance.
Compounds reducing protein denaturation by more than 20% over a range of doses in the egg albumin protein denaturation experiment were deemed to have anti-inflammatory characteristics. They could be helpful in medication development.
2.3.3 Tyrosinase inhibition activity
The volume of 19 µL of 0.9 M potassium phosphate buffer having pH 6.7 and 39 µL extract/fraction solution (1 mg/mL) was mixed. Tyrosinase enzyme 38 µL (200 units/mL) was added and incubated for 15 min. The substrate L-DOPA 100 µL was added to the incubated mixture. This solution was further incubated for 20 min at room temperature. The absorbance was measured at 450 nm with a BioTek synergy HT microplate reader. The same procedure was adopted for negative control by adding 40 µL of buffer solution instead of extract/fraction. Kojic acid was used as the standard compound in tyrosinase inhibition activity (Ahmed et al., 2022a, 2022b).
2.4 In-vivo toxicological evaluation
2.4.1 Experimental animals
Wistar albino rats weighing (80–100 g) were kept in the Pharmacology & Physiology research laboratory animal house at IUB Punjab, Pakistan. All animals used in the study were housed in polycarbonate cages that measured (47 × 34 × 18 cm3). The typical conditions of temperature and humidity 25 ± 2 °C, 50–55%, respectively, and exposure to a 12-hour light/dark cycle, were kept throughout the study. The rats were fed conventional animal food and given free access to water. To reduce animal stress, acclimatized to the test environment for one week before the trial began. All methodologies were approved by the Pharmacy Animal Ethics Council in the Faculty of Pharmacy, IUB Punjab, Pakistan, with authorization number PAEC22/73. They complied with the UK Animals (Scientific Procedures) Act 1986s ethics and guidelines (Basit et al., 2022a, 2022b, 2022c).
2.4.2 Acute oral toxicity study
The safety profile of PAME was evaluated using OECD 425 criteria with minor modifications previously reported by (Yaseen et al., 2020). In brief, five healthy female albino rats (nulliparous and non-pregnant) were fasted overnight before receiving a single oral dose of PAME at a concentration of 5000 mg/kg orally. Initially, one rat was dosed and examined for up to four hours for any signs of toxicity, such as skin irritation, changes in general behavior, and eye lacrimation, before being observed again after 24 h. If there were no harmful symptoms, the same dose was administered to four more rats monitored once daily for 14 days (OECD 2008). Rats were examined on days 0, 7, and 14, and the percentage change in body mass was calculated. Animals were slaughtered at the end of the experiment, and blood was collected for biochemical and hematological analysis. On day 14, all the animals were executed with ketamine (350 mg/kg). All rats' body weight was measured, blood was obtained for biochemical and hematological analysis, and major organs were removed for histopathological investigation (Yaseen et al., 2020).
2.5 In vivo pharmacological profiling
2.5.1 Acute anti-inflammatory activity
The anti-inflammatory potential of methanol extract (PAME) of P. afra was assessed using carrageenan-induced paw edema which was modified somewhat from a previously published approach (Basit et al., 2022a, 2022b, 2022c). The animals were divided into five groups (n = 6) Group:1 (control group) was treated with distilled water (5 ml/kg distilled water, i.p). Group 2 (standard group) received indomethacin (15 mg/kg, i.p) half an hour earlier, groups 3, 4, and 5 received PAME extract at 100 mg/kg,200 mg/kg, and 400 mg/kg (orally) respectively. After 1 h of dosing, all groups were injected with a subcutaneous injection of carrageenan (0.1 ml, 1%) into the right hind paw. The paw diameter difference was measured using a digital Vernier caliper (Mitutoyo, Japan). The increase in paw diameter was considered a sign of inflammatory induction. Before carrageenan administration, the starting volume (V1) was assessed, and the following injection volume (V2) was measured at 0.5, 1, 2, and 4 h. The previously reported formula was used to compute the % inhibition of paw edema (Javed et al., 2020).
2.5.2 Anti-nociceptive potential of Portulacaria afra
The analgesic activity of PAME was measured by using various tests; tail immersion test, hot plate method, and acetic acid-induced writhing test.
2.5.2.1 Tail immersion method
The tail immersion (tail flick) experiment was carried out according to the methodology published by (Aziz et al., 2019). To assess the analgesic activity of PAME, a total of 36 rats (80–100 g) were divided into five groups. Group1 (control group) was treated with distilled water (5 ml/kg DW) while group 2 (standard group) received tramadol (30 mg/kg, p.o), groups 3, 4, and 5 received PAME (p.o) at 100 mg/kg,200 mg/kg, and 400 mg/kg respectively. The baseline reaction time of each group was recorded post-treatment by dipping the tail ends of the mice (last 1–2 cm) in hot water at (55 ± 1) °C. The flick reaction of rats was calculated, i.e., the time required (in seconds) to remove it from a hot water source, and the findings were compared with the control group. To protect the rats, a latency interval of 15 s was established as the cut-off point. The latent time of the tail-flick reflex was assessed before 30, 60, and 90 min of medication and extract delivery.
2.5.2.2 Hot plate method
The hot-plate test was performed with minor modifications as described by (Sajid-ur-Rehman et al., 2021a, 2021b). Before beginning the experiment, all (36) rats (80–100 g) were divided into five groups (n = 6). Group one (control group) was treated with distilled water (5 ml/kg DW per. oral), while group 2 (standard group) received tramadol (30 mg/kg per. oral). While group 3, 4, and 5 were treated with methanol extract (PAME) of (p.o) at 100 mg/kg, 200 mg/kg, and 400 mg/kg, delivered 30 min before the hot plate test, respectively. The medication and plant extract were dissolved in distilled water. The results were recorded after 30 min of dose administration to the individual groups. The reflexes of all groups, such as lapping, shaking, and leaping away from a hot surface (50 ± 2°Ċ), were recorded before and after the therapy at 30, 60, 90, and 120 min. To avoid paw injury, 25 s was set as the cut-off time.
2.5.2.3 Acetic acid-induced writhing method
The acetic acid-induced writhing experiment was carried out by the protocols published with minor modifications (Basit et al., 2022a, 2022b, 2022c). Before beginning the experiment, all the rats (80–100 g) were fasted for 30 min and divided into five groups (n = 6). Group 1 was treated with distilled water (DW) 5 ml/kg while group 2 received diclofenac sodium 15 mg/kg (p.o) group 3, 4, and 5 received methanol extract (PAME) of (p.o) at 100 mg/kg, 200 mg/kg, and 400 mg/kg respectively. The medication and plant extract were dissolved in distilled water. After 40 min of diclofenac and plant extract dosing,1% acetic acid (10 ml/kg, i.p.) was given to produce an abdomen constriction in all treated groups. The number of abdominal constrictions was recorded over 30 min after 5 min of acetic acid delivery and shown as an average mean.
2.6 Molecular docking analysis
The chemical compounds obtained from the results of GC–MS analysis were subjected to molecular docking with some anti-tyrosinase targets to validate the in-vitro and in-vivo results. The docking analysis used the crystal structures of the tyrosinase protein targets from the protein data bank (PDB). Polar hydrogens were inserted into the protein. Water, inhibitors, and extra chains were removed from the protein and then converted into the PDBQT format. To analyze their potential against the proteins, the ligands were taken from PubChem. The ligands were input into Open Babel and used the PyRx application to reduce their energy. The compounds were then transferred to PDBQT format for further investigation. The grid box is then constructed in the desired dimensions. Finally, the Discovery studio visualized the interactions.
2.7 Statistical analysis
Results were represented as mean ± SEM (n = 6). Two-way ANOVA followed by a Tukey's test was performed using Graph Pad Prism (San Diego, CA, USA) software. P-value < 0.05 was considered statistically significant.
3 Result and discussion
3.1 Phytochemical composition
The plant source has served as the front-line contributor in the provision of therapeutic alternatives to conventional therapeutic agents and lead compounds for the developement of novel drug candidates. Plants have a plethora of chemical constituents, which play an important role in their pharmacological effects. Therefore, investigating the phytochemical profile of plant species is of prime significance (Basit et al., 2023, Wairata et al., 2022). In the current investigations, the chemical composition of P. afra was evaluated through the determination of the phenolic and flavonoid contents, GC–MS, and LC-MS analysis. The selection of a suitable solvent is a key step in the extraction process of natural (Ahmad et al., 2019). In the current investigation, the hydromethanolic solvent system comprised of 80% methanol and 20% water was applied for the extraction. The extract obtained was fractionated with ethanol. The extended polarity index of the hydro-alcoholic solvent system makes it a suitable solvent for the extraction of natural products (Basit et al., 2022a, 2022b, 2022c). PAME showed a significant quantity of TPC (280 ± 1.5 mg GAE/g) and TFC (200 ± 1.12 mg QE/g), while ETPA showed a relatively lower quantity of TPC (163.25 ± 2.50 mg GAE/g) and TFC (159.21 ± 2.22 mg QE/g), respectively (Fig. 1A and 1B). The results of our study are in correlation with previous reports on the polyphenolic quantification of P. afra (Dlova and Ollengo 2018).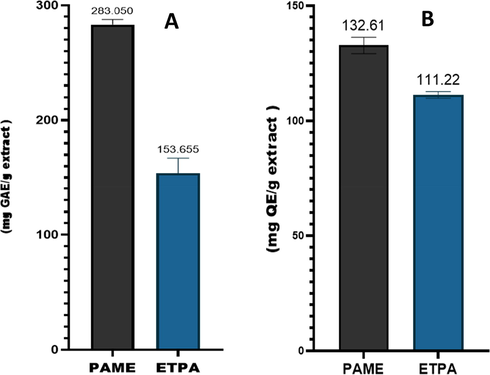
Polyphenolic contents in P. afra. (A): total phenolic contents (mg GAE/g extract), (B): total flavonoid contents (mg QE/g extract).
For a more in-depth evaluation of the chemical composition, PAME was subjected to RP-UHPLC-MS analysis using the positive and negative modes of ionization. A total of 101 chemical compounds were identified in the positive mode while 14 compounds were identified using the negative mode of ionization. The detailed tabulated results and chromatograms of RP-UHPLC-MS analysis are provided in the supporting information (Table 1S-2S and Fig. 1S-4S). Identification of the compounds was based on the default spectral library with the instrument and metlin database search (Arshad et al., 2021, Basit et al., 2022a, 2022b, 2022c).
The commonly used spectroscopic technique, GC–MS, is a reliable tool for investigating volatile components in plant extracts. For the first time, the PAME was examined by using Gas Chromatography-Mass Spectrometry (GC–MS). A total of 15 compounds were identified and results were summarized in (Table 1 & Fig. 2). The major compounds were benzene,1,3-dimethyl-7-propylidene (1.26%), ethylbenzene (5.99%), P-xylene (41.65%)-O-Xylene (17.92%), heneicosane (1.64%), phenol,2,4-bis (1,1-dimethyleth (2.42%), hexadecanoic acid methyl ester (2.74%),7,9-ditert-butyl-1-oxaspiro, deca-6,9-diene-2,8-dione (1.51%), methyloctadeca-9,12-dienoate (1.33%), 9,12,15-octadecatrienoicacid (1.67%), coprosten-3-onesemicarbazone (8.27%). Numerous publications about the antibacterial activity using various plant extracts that indicate the presence of heneicosane by GC–MS analysis have been reported. The 9, 12, 15-Octadecatrienoic acid was another anti-inflammatory component in a hydro-methanol extract of P.afra (Guerrero et al., 2017). The hexadecanoic acid methyl ester was a major compound in the hydro-methanol extract which has antioxidant, antidiabetic (Ahmadi et al., 2021), and anti-inflammatory properties, as well as the potential to lower blood cholesterol (Kaizal and Hussein 2019). The compound was also reported by (Belakhdar et al., 2015) (Asghar et al., 2011; Metwally et al., 2020). Our results are consistent with earlier findings on the same genus that were published by (Alu’datt et al., 2019). Phenol, 2, 4-bis (1, 1-dimethyl ethyl) have antimicrobial (Salini et al., 2014), antifungal (Rangel-Sánchez and Castro-Mercado, 2014), antioxidant (Ajayi et al., 2011), antitumor activity (Yogeswari et al., 2012) 7, 9-di-tert-butyl-1-oxaspiro (4,5) deca-6, 9-diene-2, 8-dione have also been reported to possess anti-mineralocorticoid activity which is used primarily as a diuretic and antihypertensive. This compound is also reported by (Rao et al., 2016). Heneicosane has shown antibacterial activity reported by (Vanitha et al., 2020). No reports on P. afra chemical profiling of hydro-methanol extract by GC–MS have been publicly disclosed. R.t: retention time.
Peak number
RT (min)
Area
Compounds
Molecular Formula
Similarity Index
Molecular mass
Chemical Class
Peak −1
3.07
5.99
Ethylbenzene
C8H10
97
106.16
Aromatic hydrocarbon
Peak −2
3.13
41.65
1,4-Dimethylbenzene
C8H10
97
106.16
Aromatic compounds
Peak −3
3.37
17.92
o-Xylene
C8H10
90
106.16
Alkanes
Peak −4
10.19
1.64
Heneicosane
C21H44
91
296.6
Fatty acids
Peak −5
10.43
2.42
Phenol,2,4-bis(1,1-dimethylethyl)-, phosphite
C17H30
90
278.5
Phenol
Peak −6
11.29
0.72
1-Hexadecene
C16H31Br
98
224.42
Phenol
Peak −7
12.63
1.46
Heptacosane
C27H56
98
380.7
Alkenes
Peak −8
13.50
0.74
Dichloroacetic acid
C17H34
98
128.94
Monocarboxylic acid
Peak −9
15.06
2.74
Hexadecanoicacid, methyl ester
C18H36O2
91
270.5
Ester
Peak −10
15.11
1.51
7,9-ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione
C17H24O3
99
276.4
Cyclic ketone
Peak −11
17.26
1.33
methyloctadeca-9,12-dienoate
C19H34O2
96
293.4
lineolic acids
Peak −12
17.35
1.67
9,12,15- Octadecatrienoic acid
C18H30O2
93
278.4
fatty acid
Peak −14
23.42
8.27
1-Coprosten-3-one semicarbazone
C28H47N3O
97
441.7
Imines derivatives
Peak −15
32.96
10.34
1,3-Bis(trimethylsilyl)benzene
C12H22Si2
95
222.47
Aromatic compound
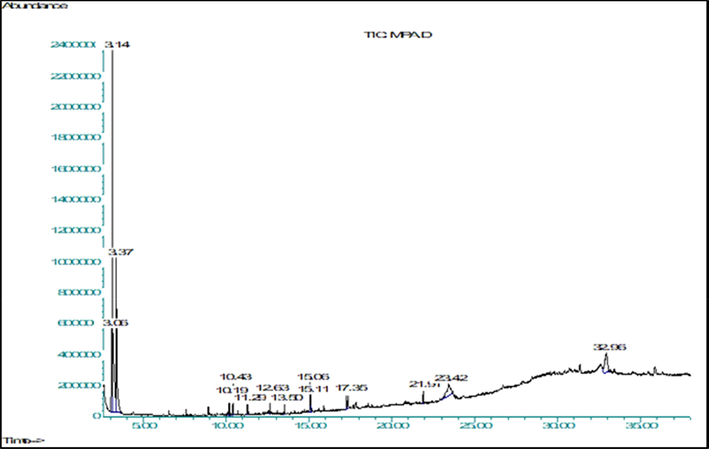
GC–MS chromatogram of PAME.
3.2 Antioxidant potential of Portulacaria afra
Antioxidant compounds obtained from phytoconstituents are acquiring acceptance as a substitute for synthetic antioxidants (Basit et al., 2022a, 2022b, 2022c). Because natural antioxidant compounds are generally safer than conventional antioxidants. Plants with potent antioxidant compounds may be evaluated for reducing hazardous oxidation in food ingredients or medications used to treat chronic diseases, i.e. cancer, diabetes, and myocardial infection (Salaj et al., 2021). That is why there is intense interest in exploring plant sources for their antioxidant potential. In this study, PAME and ETPA were evaluated for antioxidant potential using already established methods, namely DPPH, FRAP, ABTS, and CUPRIC. The PAME showed the highest antioxidant potential through ABTS and CUPRIC methods (93.16 ± 0.05, 90.88 ± 0.67 mg TE /g dry extract) followed by FRAP and DPPH methods (80.45 ± 0.20, 65.58 ± 1.3 mg TE/g dry extract respectively (Fig. 3). While ETPA also showed higher antioxidant potential through the CUPRIC and FRAP method (462 ± 1.44 mg TE/g, 420.00 ± 2.1 mg TE/g extract) followed by DPPH and ABTS method (312.40 ± 1.2 mg TE/g, 182.08 ± 1.9 mg TE/g). This plant's antioxidant capability rectify the polyphenolic quantification study, because the strong correlation between antioxidant power and polyphenolic compounds is well established (Lekouaghet et al., 2020, Ahmad et al., 2022). The findings of the current study are in correlation with previous reports on the antioxidant capacity determination of the plant, which have postulated low results in the DPPH method (Khanyile et al., 2021; Olaokun et al., 2017). Total antioxidant data showed that PAME and ETPA have a high potential for antioxidant action that might help to prevent inflammation and pain caused by free radicals.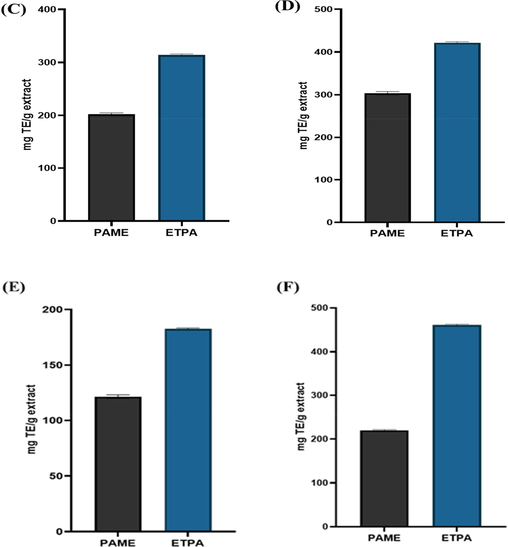
Antioxidant potential of PAME and ETPA using, (C): DPPH (D): FRAP, (F): ABTS (E), CUPRAC.
3.3 In-vitro enzyme inhibition
3.3.1 Tyrosinase inhibition assay
Tyrosinase is a therapeutically important enzyme involved in the melanogenesis process, and its inhibitors reduce skin hyperpigmentation, a common cause of the highly undesirable result of skin darkening (Ciganović et al., 2019). PAME and ETPA were tested using standard Kojic acid against the tyrosinase enzyme. The tyrosinase inhibition activity of PAME was found higher than ETPA as shown in Fig. 4. The species melanin-reducing ability also corrects the species' historic use in numerous skin conditions (De Wet et al., 2013). P. afra has positive antimicrobial effects, which validate its application to treat skin disorders. The plant leaves are the most frequently used to treat skin disorders (Nciki et al., 2016).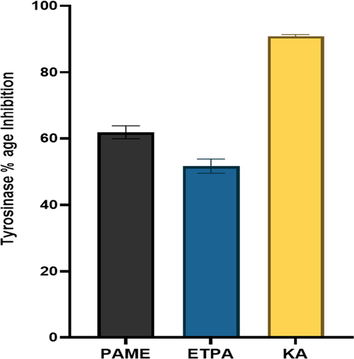
Tyrosinase inhibitory potential of P. afra. PAME: methanol extract of P.afra, ETPA: ethanol fraction of P. afra, KA: kojic acid.
3.4 Toxicity evaluation
The treatments with herbal drugs may cause serious concerns about their toxicity profiles in the absence of scientific data (Basit et al., 2022a, 2022b, 2022c). So, the toxicity of herbal drugs needs to be assessed in the animal model to assure their safety profile for human use. The acute toxicity was performed according to OECD guidelines on albino rats. On the oral administration of PAME at a dose of 5000 mg/kg to albino rats, no significant weight variation and changes in the behavioral pattern were observed in the body weight of animals when treated groups were compared with the control group of animals Table 3S and 4S. Similarly, no difference in the hematological parameters was observed in the animals of the treated group as shown in Table 2. The treated and control groups presented no significant changes in serum concentrations of bilirubin, urea, creatinine, alkaline phosphate, and protein at the dose of 5000 mg/kg orally. Moreover, no abnormality was observed in the histopathological examination of the vital organ after treatment with oral administration of PAME at 5000 mg/kg (Fig. 5 and Table 5S). The result of the study was mentioned in the following Table 3. These findings indicated that P. afra is non-toxic and biocompatible, making it suitable for usage as medicine. All values are expressed as mean ± SD., (n = 6). All values are expressed as mean ± SD., (n = 6).
Hematological parameters
Control group
Treated group 5000 mg/kg/2ml
WBC (µL)
6.61 ± 1.1
4.0 ± 0.19
RBC (µL)
5.47 ± 1.3
4.85 ± 3.1
HGB
11.3 ± 2.3
10.3 ± 0.8
HCT (%)
36.1 ± 2.1
36.1 ± 3.2
MCV (fl)
66.0 ± 1.3
62.1 ± 1.8
MCH (pg)
20 ± 2.7
21.2 ± 3.1
MCHC g/dl
31.3 ± 1.8
34.2 ± 1.1
PLT (µL)
926 ± 0.7
392 ± 3.4
LYM%
47.0 ± 2.2
12.2 ± 0.18
M*D%
15.2 ± 4.5
4.1 ± 2.1
NEUT%
37.8 ± 3.7
3.3 ± 2.3
LYM (µL)
3.1 ± 0.19
0.5 ± 0.21
MXD (µL)
1.0 ± 2.3
0.2 ± 0.43
NEUT (µL)
2.5 ± 2.7
40.5 ± 0.76
RDW-SD (fl)
41.7 ± 1.8
40.5 ± 0.68
RDW-CV %
18.5 ± 1.76
18.6 ± 1.3
PDW (fl)
14.4 ± 3.01
11.9 ± 1.2
MPV (fl)
10.4 ± 0.98
8.8 ± 0.15
P-LCR %
31.1 ± 0.64
19.4 ± 0.19
PCT %
0.96 ± 2.5
0.34 ± 0.64
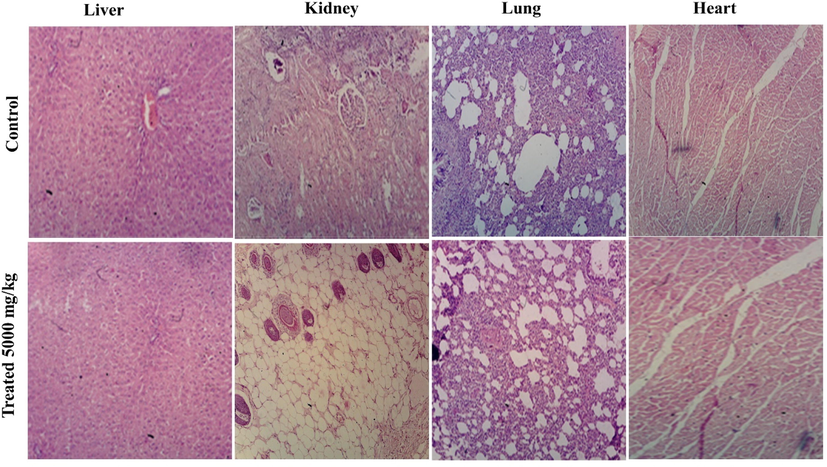
Micrographs of histopathological examination of vital organs of treated and control rats in oral toxicity evaluation of PAME.
Biochemical Parameters
Control group
Treated group 5000 mg/kg
Liver Test
ALT(U/L)
65 ± 0.14
70 ± 1.9
AST (U/L)
328 ± 0.19
332 ± 2.5
ALT(U/L)
336 ± 0.64
340 ± 1.3
Bilirubin
0.1 ± 0.18
0.2 ± 1.2
Albumin
4.3 ± 0.13
4.2 ± 0.15
Renal Function Test
Blood urea (mg/dl)
52 ± 1.7
55 ± 1.3
Serum Creatinine(mg/dl)
0.5 ± 0.45
0.6 ± 1.1
Serum Electrolyte
Sodium (Na)
138 ± 1.76
135 ± 0.18
Potassium (k)
11.9 ± 1.34
10.9 ± 1.9
Glucose Report
Blood glucose (Random)
44 ± 1.76
43 ± 1.3
3.5 Anti-denaturation effect of PAME
The protein denaturation test is an in vitro approach commonly applied for assessing the anti-inflammatory potential of natural products (Marrelli et al., 2022). In this, we evaluated PAME for the anti-denaturation effect on heat-induced denaturation of bovine serum albumin (BSA). The findings revealed that varying concentrations of PAME showed inhibition of heat-induced denaturation of BSA compared to standard indomethacin. As shown in Table 4. PAME and indomethacin inhibited heat-induced denaturation of BSA in a dose-dependent manner with an IC50 value of 475.23 ± 3.25 μg/mL for PAME (P < 0.05) and standard indomethacin showed inhibition with an IC50 value of 377.10 ± 3.11 μg/mL. These findings indicated that PAME extract has an anti-inflammatory property. All the values were expressed as mean ± SEM, IC50 50 % inhibitory concentration, and BSA bovine serum albumin.
Sample
Concentration (g/mL)
Inhibition (%)
IC50 (ug/mL)
MPA extract
100
2.21 ± 0.08
475.23 ± 3.25
200
24.75 ± 1.15
400
42.23 ± 2.51
600
55.96 ± 3.37
Indomethacin
100
10.7 ± 0.76
377 ± 3.11
200
30.57 ± 1.28
400
52.35 ± 2.01
600
78.71 ± 3.78
Compound name
Binding affinity
(KJ/mol)
Amino Acids
Types of Interaction
Standard
Kojic acid−6.4
SER:394
Conventional hydrogen bond
HIS:215, HIS:381
Pi-Pi Stacked
Phenol,2,4-bis(1,1-dimethylethyl)-, phosphite
−6.7
ARG:131, GLU:140, VAL:129
Conventional hydrogen bond
1 9,12,15- Octadecatrienoic
−5.1
ARG:131, GLU:140, VAL:129
Conventional hydrogen bond
LUU:136
Pi-Sigma
,9-ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione
−6.9
GLN:240
Conventional hydrogen bond
LYS:233
Carbon hydrogen bond
1-Coprosten-3-one semicarbazone
−7.9
SER:245, ARG:131, SER:243
Conventional hydrogen bond
3.6 Effect of PAME on carrageenan-induced paw edema
The carrageenan-induced paw edema model is a commonly used preclinical model for assessing the anti-inflammatory properties of plant extracts (Piva et al., 2021). Carrageenan is a potent molecule that promotes the production of pro-inflammatory and inflammatory mediators (leukotrienes, prostaglandins, bradykinin, histamine, and TNF (Posadas et al., 2004, Javed et al., 2020). Edema caused by carrageenan takes place in two stages. The first phase is primarily non-phagocytic edema lasting 1 to 1.5 h, preceded by a late stage lasting 2 to 6 h with enhanced edema induction. The first phase is marked by increased secretion of inflammatory mediators of inflammation and serotonin. In contrast, the second phase is defined by excessive production of cyclooxygenase, which causes an increase in the initiation of prostaglandins and reactive species, thereby activating inflammation through further overexpression of inflammatory cells such as interleukins and cytokines (Basit et al., 2022a, 2022b, 2022c). Prostaglandins are the primary cause of acute inflammation. Even though both the cyclooxygenase and lipoxygenase pathways are essential in the inflammatory process (Nunes et al., 2021). In the current investigation, the carrageenan (1%) was injected subcutaneously in the right hind paw of control, standard and drug-treated rats. The PAME presented an anti-inflammatory effect in a dose-dependent manner at 100, 200, and 400 mg/kg. The effect was assessed after 1 h, 2 h, 3 h, and 4 h. PAME, showed percentage inhibition of 11.87 %, 38.22%, and 42.53 % respectively. At 200 mg/kg, PAME unveiled an anti-inflammatory effect. After 1 h, the percentage inhibition was 14.56%, comparable to the standard indomethacin. In contrast, after the 2nd, 3rdand, 4th, hour PAME exhibited anti-inflammatory activity greater than standard, and PAME displayed the percentage inhibition extract was 14.56 %, 37.82%, 52.73%, 54.26% (p < 0.05), respectively. At 400 mg/kg, PAME showed anti-inflammatory effects 2nd and 3rd-hour. After the 2nd-hour, the percentage inhibition was 34.60% (p < 0.05), which is greater than the standard. At the 3rd, and 4th-hour, percentage inhibition was observed in the range of 51.63% to 57.16 (p < 0.05), which is also greater than standard (8) shows the results of the in-vivo anti-inflammatory activity (Fig. 6). PAME showed significant anti-inflammatory effects might be due to high antioxidant potential and phytoconstituents identified in PAME through GCMS, i.e. methyloctadeca-9,12-dienoat (Krishnamoorthy and Subramaniam 2014), 9,12,15- Octadecatrienoic acid (Kumar et al., 2010), 1,3-Bis (trimethylsilyl) benzene (Mou et al., 2013), Hexadecanoic acid methyl ester (Sympli 2021). Our results align with the previous findings (Petropoulos et al., 2016) on the genus of P. oleracea L.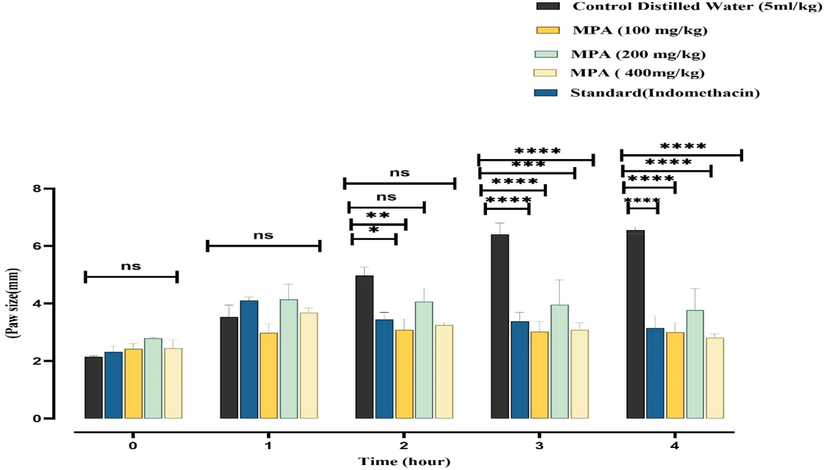
Anti-inflammatory potential of methanol extract of P. afra and data was mentioned as the Mean ± SEM of six animals in each group. The results were analyzed by Two-way ANOVA followed by the Tukey test), < 0.001 (***), and P < 0.05 (*) as compared to the control.
3.7 Anti-nociceptive potential of PAME
Plants are preferably regarded as rich sources that have assisted in the discovery of valuable analgesics such as morphine and aspirin. Existing pain medications, such as NSAIDs, can cause side effects. As a result, isolating novel anti-nociceptive chemicals, mainly from plants, can be advantageous in pain management and risk (Chandan et al., 2021). In the current study, the PAME was investigated for peripheral and central analgesic effects. The anti-nociceptive potential was evaluated by the hot plate, tail immersion, and acetic acid-induced writhing method in rats. The hot plate and tail immersion tests are sensitive acute pain tests used to determine analgesic responses. The abdominal writhing test is used to investigate peripheral action (Bahmani et al., 2014).
3.7.1 Hot-plate method
The hot plate methods could screen for centrally-acting analgesics (Rajamanickam and Rajamohan 2020). In the current study, the analgesic activity PAME of was determined using the hot plate method with modifications (Javed et al., 2020). The tramadol was used in the dose of (30 mg/kg) as the standard drug. Compared to the control group, the standard drug tramadol produced a significant (p < 0.0001) anti-nociceptive effect at all observational events, with a prominent effect at 90 min and 120 min of treatment. Furthermore, PAME after 30, 60, 90, and 120 min showed significantly increased time (p < 0.0001) at 200 mg/kg and 400 mg/kg after oral administration to the treated rats when compared to the control group, as shown in Fig. 7. Our results were supported by previous findings (Ghauri et al., 2021).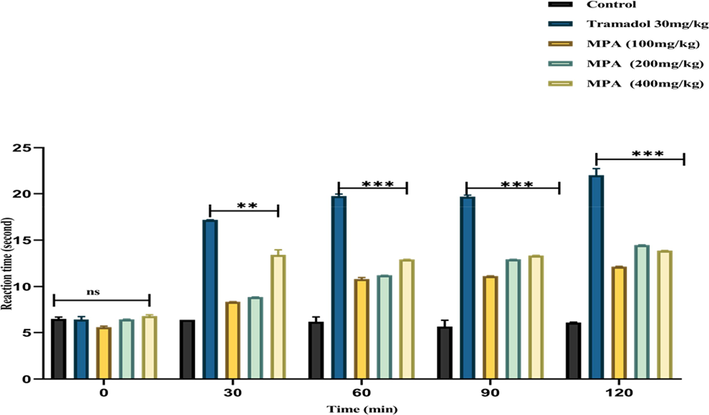
Analgesic Potential of P. afra using Hot Plate Method and the effect of (100 mg/kg, 200 mg/kg, 400 mg/kg) PAME and tramadol drug 30 mg/kg in the hot-plate test. The results were expressed as mean ± SEM (n = 6). Statistically significant value was determined by two-way ANOVA followed by Tukey's test hoc test. < 0.001 (****) and p < 0.05 (*) when treated groups were compared with the control group.
3.7.2 Acetic acid-induced writhing method
The acetic acid-induced writhing reflex methodology is a frequently established approach for assessing the peripheral analgesic effect of any plant sample, with acetic acid serving as the primary pain inducer in a mouse model (Bhuiyan et al., 2020). The reflex is thought to be triggered by abdominal mast cells and prostaglandin mechanisms (Ahmed et al., 2022a, 2022b). Acetic acid enhances the release of inflammatory markers and prostaglandins when administered intraperitoneally (i.p) (Shaheen et al., 2022). The release of these inflammatory mediators causes more abdominal tightness or pain (Krylova et al., 2019). In the current study, the analgesic activity of PAME was determined using the acetic acid-induced writhing model. The control and treated groups received orally 100 mg/kg, 200 mg/kg, and 400 mg/kg PAME before 1 h the injection of 1% acetic acid (10 ml/kg i.p). A significant nociceptive effect was observed in the control-treated group (distilled water 5 ml/kg). The number of writhing was (64.50 ± 1.5). While the standard group treated with diclofenac sodium (15 mg/kg) showed a marked decrease in abdominal constriction (21.5 ± 0.50). The PAME treated groups showed significant anti-nociceptive effect 200 mg/kg, 400 mg/kg (30.5 ± 0.01, p < 0.0001, 27.50 ± 1.5p < 0.0001) respectively when compared to control group (Fig. 8).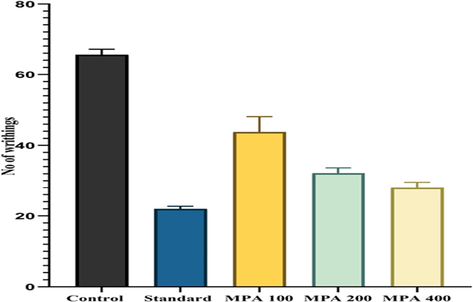
Analgesic Potential of P. afra using Acetic Acid-Induced Writhing Method The data was mentioned as Mean ± SEM of six animals in each group. The results were analyzed by Two-way ANOVA followed by the Tukey test), < 0.001 (***) and P < 0.05 (*) as compared to the control.
3.7.3 Tail immersion method
The tail immersion method was used for peripheral analgesic activity (Aziz et al., 2019). The approach was preferred because of its great selectivity for opioid-derived analgesics (Bhuiyan et al., 2020). This pain model can be produced by submerging the animals' tails in hot water or applying radiant heat to a tiny surface of the tail. Immersion of the tail in hot water leads to a rapid and linear rise in the temperature, which might cause a sudden tail twitch and pull back the entire body (Tekulu et al., 2020). The tail-flick model is noteworthy because the efficient analgesic produced in this model is highly linked to treating actual pain in the human body (Sharma et al., 2017).
In the current investigation, the analgesic activity of PAME was determined using the tail immersion method. In the current examination group 1 (Control group) was treated with (5 ml/kg, DW, p.o) while the group:2 was treated with tramadol (30 mg/kg p.o) group 3,4,5 received (100 mg/kg, 200 mg/kg, 400 mg/kg orally per body weight). The standard drug had a significant effect (p < 0.0001) during all time intervals (30,60,90,120 min) when compared to the control group. The treated groups showed significant anti-nociceptive effects at 200 mg/kg and 400 mg/kg (22.24 ± 0.090 ***p < 0.0001) (Fig. 9).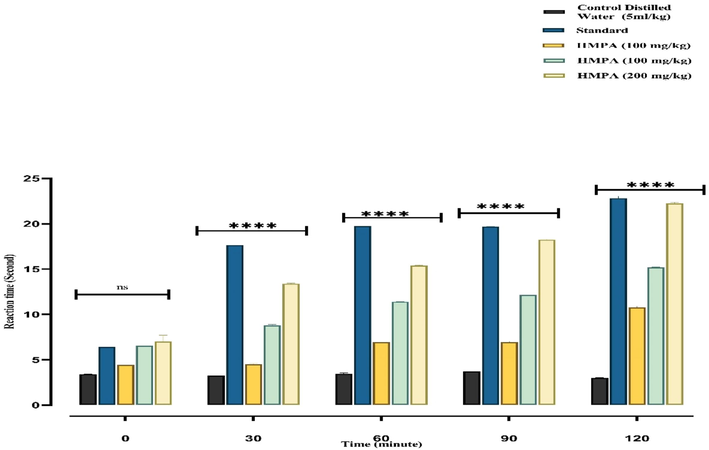
Analgesic Potential of P. afra using Tail Immersion method There was an increase in latency time with different doses of PAME extract and tramadol medication in the tail flicking test. All results are shown in mean ± SEM. n = 6P < 0.001 (***) and p < 0.05 (*) when compared to the control group.
A considerable dose-dependent increase in latency time (8.78 ± 0.090***at (30 min),11.39 ± 0.005***at (60 min), 12.14 ± 0.01 ***p at (90 min) 15.19 ± 0.03 ***p at (120 min) p < 0.0001 respectively when compared to the control group. While at 120 min, the given dose of 400 mg/kg per oral showed comparable effects to the standard latency time in the tail immersion method, suggesting that the extracts have a central anti-nociceptive effect. Our findings are consistent with previous findings on purslane reported by (Lobina et al., 2021). It is clear from the reported data that supraspinally mediated analgesic activity is mediated by μ, κ receptors, whereas spinal analgesic action is mediated by δ κ μ opioid receptors (Angst and Clark, 2006). The peripheral pain feeling is caused by a localized inflammatory response caused by the release of arachidonic acid from tissue phospholipids via the COX pathway (Sajid-ur-Rehman et al., 2021a, 2021b).
3.8 Molecular docking analysis
The computational technique of molecular docking is currently widely used in drug development. Docking gives the advantage of evaluating the binding affinity of protein binding complexes. In the present study, the chemical components from GCMS, as well as indicating the kind of interaction of the research compounds at the enzyme or receptor's location via particular critical interactions (Chandak et al., 2014). PyRx is a virtual screening tool that performs flexible multiple ligand docking analyses within protein binding sites (Borges et al., 2018). A total of fifteen compounds docked against tyrosinase (PDB: 4O1Z). The hydrogen bond is important in protein–ligand interactions, and other hydrophobic interactions, such as alkyl, and pi alkyl, provide persistent binding of ligands to proteins (Chen and Oezguen 2016). The other two compounds, Phenol, 2,2′-methylenebis, and 7,9-ditert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione have −7.7 and −7.1 binding affinities while Phenol, 2,2′-methylenebis has two hydrogen bonds with CYS A:41 and GLN A:42 while 7,9-ditert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione has hydrophobic interactions with different amino acids. Phenol, 2,2′-methylenebis, and 7,9-ditert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione have −7.2 and −6.6 binding affinities with COX-2 while Phenol, 2,2′-methylenebis showed hydrogen bond but 7,9-ditert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione showed alkyl interaction (Table 5 and Figs. 10-12).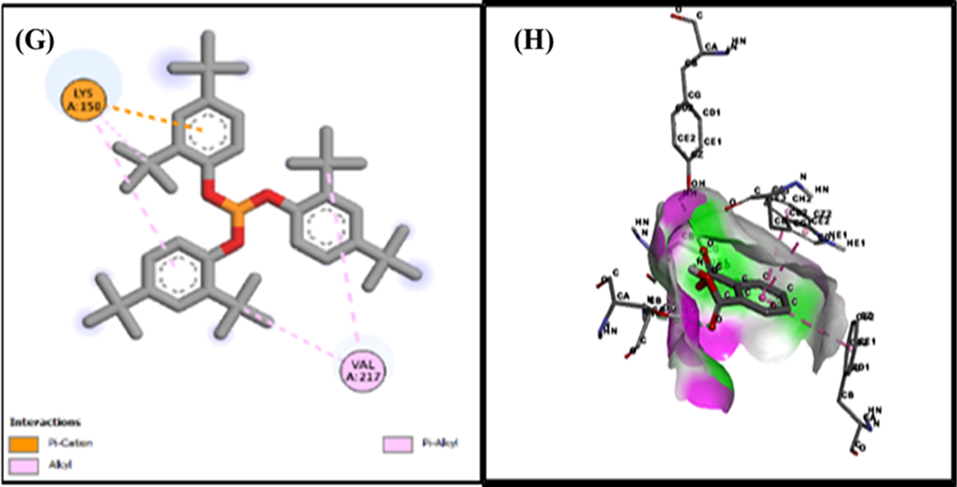
Docking of Phenol,2,4-bis (1,1-dimethylethyl)-Phosphite 2d (G), 3d (H) with tyrosinase.
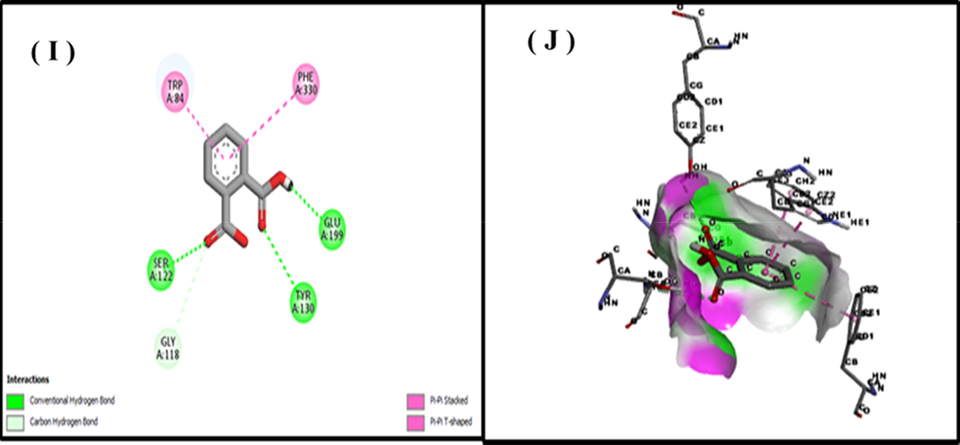
Docking of 9,12,15- Octadecatrienoic 2d (I), 3d (J) with tyrosinase.
![Docking of 7,9-ditert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione 2d, 3d 2d (K), 3d (L) with tyrosinase.](/content/184/2023/16/6/img/10.1016_j.arabjc.2023.104784-fig12.png)
Docking of 7,9-ditert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione 2d, 3d 2d (K), 3d (L) with tyrosinase.
4 Conclusion
The studies performed provides greater insight into the chemical composition and pharmacological potential of P. afra. The GC–MS and RP-UHPLC-MS analysis presented the tentative identification of functionally bioactive constituents in PAME. Both PAME and ETPA showed significant antioxidant potential. An acute oral toxicity study revealed the biocompatibility and safe nature of the extract. PAME showed dose-dependent inhibition of carrageenan-induced paw edema in the rat. Similarly, PAME also showed a significant antinociceptive effect in abdominal constriction assay, hot plate methods, and tail immersion methods. The in vitro evaluation of the tyrosinase inhibition potential of PAME and ETPA. The in vitro anti-tyrosinase results were rectified by in silico molecular docking studies of the selected compounds from the GC–MS profile. Overall, the findings encourage the use of P. afra as a therapeutic alternative in the traditional system of remedies and warrant further investigation on the isolation of bioactive constituents and their pharmacological profiling.
Funding
Not Applicable.
CRediT authorship contribution statement
Sobia Tabassum: Methodology, Formal analysis, Writing – original draft. Saeed Ahmad: Supervision, Methodology. Kashif ur Rehman Khan: Supervision, Methodology, Writing – original draft. Baber Ali: Formal analysis. Qaiser Jabeen: Methodology. M. Sajid-ur-Rehman: Data curation. Maqsood Ahmad: Formal analysis. Hafiz Muhammad Zubair: Software. Luay Alkazmi: Resources, Data curation. Gaber El-Saber Batiha: Resources, Data curation. Qamar-uz- Zaman: Software, Formal analysis. Abdul Basit: Methodology, Data curation, Software, Writing - original draft, Writing - review & editing.
Acknowledgements
The authors acknowledged Prof. Dr. Qaiser Jabeen, Head of the Department of Pharmacology, Faculty of Pharmacy, The Islamia University of Bahawalpur, Pakistan, for providing the lab facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- GC–MS profiling, phytochemical and biological investigation of aerial parts of Leucophyllum frutescens (Berl.) IM Johnst (Cenizo) S. Afr. J. Bot.. 2022;148:200-209.
- [Google Scholar]
- In vitro bioactivity of extracts from seeds of Cassia absus L. growing in Pakistan. J. Herb. Med.. 2019;16:1-5.
- [Google Scholar]
- The Effects of Solvent Polarity on Hypoglycemic and Hypolipidemic Activities of Portulaca Oleracea and Achillea Eriophora DC Extracts. Pharm. Chem. J.. 2021;54:1243-1254.
- [Google Scholar]
- Taurine loaded chitosan-pectin nanoparticle shows curative effect against acetic acid-induced colitis in rats. Chem. Biol. Interact.. 2022;351:109715
- [Google Scholar]
- Phytochemical, antioxidant, enzyme inhibitory, thrombolytic, antibacterial, antiviral and in silico studies of Acacia jacquemontii leaves. Arab. J. Chem.. 2022;15:104345
- [Google Scholar]
- Gas chromatography-mass spectrometry analysis and phytochemical screening of ethanolic root extract of Plumbago zeylanica. Linn.. 2011;5:1756-1761.
- [Google Scholar]
- Diversity of bioactive compounds and their therapeutic potential. New look to phytomedicine: Elsevier; 2019. p. :15-34.
- Herbal yield, nutritive composition, phenolic contents and antioxidant activity of purslane (Portulaca oleracea L.) grown in different soilless media in a closed system. Ind. Crop. Prod.. 2019;141
- [Google Scholar]
- RP-UHPLC-MS chemical profiling, biological and in silico docking studies to unravel the therapeutic potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules. 2021;11:53.
- [Google Scholar]
- Gas chromatography-mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bio-assays of crude extract of Iris germanica. Chem. J. Genet. Mol. Biol.. 2011;3:95-100.
- [Google Scholar]
- A new record of Portulaca (Portulacaceae) to La Réunion Island, Portulaca. cf. pilosa L.: native or recently (re-) introduced? Botany Letters.. 2018;165:193-199.
- [Google Scholar]
- In vivo and in silico evaluation of analgesic activity of Lippia alba. Clin. Phytosci.. 2019;5:1-9.
- [Google Scholar]
- Phytochemical, pharmacological, and In-silico molecular docking studies of Strobilanthes glutinosus Nees: An unexplored source of bioactive compounds. S. Afr. J. Bot.. 2022;147:618-627.
- [Google Scholar]
- A review study on analgesic applications of Iranian medicinal plants. Asian Pac. J. Trop. Med.. 2014;7:S43-S53.
- [Google Scholar]
- New mechanistic insights on Justicia vahlii Roth: UPLC-Q-TOF-MS and GC–MS based metabolomics, in-vivo, in-silico toxicological, antioxidant based anti-inflammatory and enzyme inhibition evaluation. Arab. J. Chem.. 2022;15:104135
- [Google Scholar]
- Chemical profiling of Justicia vahlii Roth. (Acanthaceae) using UPLC-QTOF-MS and GC-MS analysis and evaluation of acute oral toxicity, antineuropathic and antioxidant activities. J. Ethnopharmacol.. 2022;287
- [Google Scholar]
- New mechanistic insights on Justicia vahlii Roth: UPLC-Q-TOF-MS and GC-MS based metabolomics, in-vivo, in-silico toxicological, antioxidant based anti-inflammatory and enzyme inhibition evaluation. Arab. J. Chem.. 2022;104135
- [Google Scholar]
- Evaluation of anti-inflammatory, antioxidant and cytotoxic potential of Cardamine amara L. (Brassicaceae): A comprehensive biochemical, toxicological and in silico computational study. Frontiers Chemistry.. 2023;10:1577.
- [Google Scholar]
- Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci.. 2015;6:2778-2783.
- [Google Scholar]
- In vivo and in silico evaluation of antinociceptive activities of seed extract from the Holarrhena antidysenterica plant. Heliyon.. 2020;6:e03962.
- [Google Scholar]
- Anti-inflammatory and antialgic actions of a nanoemulsion of Rosmarinus officinalis L. essential oil and a molecular docking study of its major chemical constituents. Inflammopharmacology. 2018;26:183-195.
- [Google Scholar]
- Dual evaluation of some novel 2-amino-substituted coumarinylthiazoles as anti-inflammatory–antimicrobial agents and their docking studies with COX-1/COX-2 active sites. J. Enzyme Inhib. Med. Chem.. 2014;29:476-484.
- [Google Scholar]
- Evaluation of analgesic and anti-inflammatory activities and molecular docking analysis of steroidal lactones from Datura stramonium L. Phytomedicine. 2021;89:153621
- [Google Scholar]
- Regulation of protein-ligand binding affinity by hydrogen bond pairing. Sci. Adv.. 2016;2:e1501240.
- [Google Scholar]
- Glycerolic licorice extracts as active cosmeceutical ingredients: Extraction optimization, chemical characterization, and biological activity. Antioxidants.. 2019;8:445.
- [Google Scholar]
- Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J. Ethnobiol. Ethnomed.. 2013;9:1-10.
- [Google Scholar]
- Phytochemical profiling, in vitro biological activities, and in-silico molecular docking studies of Typha domingensis. Arab. J. Chem.. 2022;15:104133
- [Google Scholar]
- Traditional and ethnobotanical dermatology practices in Africa. Clin. Dermatol.. 2018;36:353-362.
- [Google Scholar]
- In vivo anti-inflammatory, antipyretic, analgesic activity and in vitro anti-proliferative activity of aqueous methanolic extract of Euphorbia granulata Forssk. Future J. Pharm. Sci.. 2021;7:1-10.
- [Google Scholar]
- Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants.. 2019;8:35.
- [Google Scholar]
- Chemical compounds and biological activity of an extract from bougainvillea x buttiana (var. rose) holttum and standl. Int. J. Pharm. Pharmac. Sci.. 2017;9:42-46.
- [Google Scholar]
- A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol.. 2017;205:158-172.
- [Google Scholar]
- Chemical and pharmacological profiling of Wrightia coccinea (roxb. Ex hornem.) sims focusing antioxidant, cytotoxic, antidiarrheal, hypoglycemic, and analgesic properties. Molecules. 2022;27:4024.
- [Google Scholar]
- Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea Burm. f. J. Ethnopharmacol.. 2020;259
- [Google Scholar]
- Detection of phytochemical compounds of dutch iris (iris hollandica) by using gas chromatography and mass spectrometry (gc-ms) technique. Plant Arch.. 2019;19:3904-3910.
- [Google Scholar]
- Protective effects of aqueous and ethanolic extracts of Nigella sativa L. and Portulaca oleracea L. on free radical induced hemolysis of RBCs. Daru. 2011;19:295.
- [Google Scholar]
- In vitro properties of Portulacaria afra leave extract. Biosci. Res.. 2021;18(1):455-463.
- [Google Scholar]
- Efficacy of Phytochemicals Derived from Roots of Rondeletia odorata as Antioxidant, Antiulcer, Diuretic, Skin Brightening and Hemolytic Agents—A Comprehensive Biochemical and In Silico Study. Molecules. 2022;27:4204.
- [Google Scholar]
- Phytochemical profiling of leaf, stem, and tuber parts of Solena amplexicaulis (Lam.) Gandhi using GC-MS. Int. Scholar. Res. Notices. 2014;2014
- [Google Scholar]
- Analgesic activity of hexaazaisowurtzitane derivatives. Bull. Exp. Biol. Med.. 2019;166:461-465.
- [Google Scholar]
- Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res.. 2010;4:191-195.
- [Google Scholar]
- In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from Leuzea conifera L. roots. S. Afr. J. Bot.. 2020;132:103-107.
- [Google Scholar]
- Analgesic properties of a food grade lecithin delivery system of Zingiber officinale and Acmella oleracea standardized extracts in rats. Nat. Prod. Res.. 2021;35:3078-3082.
- [Google Scholar]
- In vitro antioxidant and anti-denaturation effects of Buglossoides purpurocaerulea (L.) IM Johnst. fruit extract. Nat. Prod. Res. 2022:1-4.
- [Google Scholar]
- GC-MS analysis of bioactive components in six different crude extracts from the Soft Coral (Sinularia maxim) collected from Ras Mohamed, Aqaba Gulf, Red Sea. Egypt.. 2020;24:425-434.
- [Google Scholar]
- Antimicrobial and antioxidant activities and effect of 1-hexadecene addition on palmarumycin C2 and C3 yields in liquid culture of endophytic fungus Berkleasmium sp. Dzf12. Molecules. 2013;18:15587-15599.
- [Google Scholar]
- Early-life stress, depressive symptoms, and inflammation: the role of social factors. Aging Ment. Health 2021:1-9.
- [Google Scholar]
- Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab. Pakistan. BMC complementary and alternative medicine.. 2017;17:1-13.
- [Google Scholar]
- Plants used to treat skin diseases in northern Maputaland, South Africa: antimicrobial activity and in vitro permeability studies. Pharm. Biol.. 2016;54:2420-2436.
- [Google Scholar]
- Antipyretic, anti-inflammatory and analgesic activities of Periplaneta americana extract and underlying mechanisms. Biomed. Pharmacother.. 2020;123:109753
- [Google Scholar]
- Effect of the metanolic extract from the leaves of Garcinia humilis Vahl (Clusiaceae) on acute inflammation. Inflammopharmacology. 2021;29:423-438.
- [Google Scholar]
- Organisation for Economic Co-operation and Development. (2008). Test No. 425: acute oral toxicity: up-and-down procedure. OECD publishing, accessed on 01.08.2022.
- Phytochemical screening, antioxidant, anti-inflammatory and glucose utilization activities of three South African plants used traditionally to treat diseases. Biol. Med. (Aligarh). 2017;9:2.
- [Google Scholar]
- Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol.. 2016;55:1-10.
- [Google Scholar]
- Anti-inflammatory activity and chemical composition of aqueous extract and essential oil from leaves of Ocimum selloi Benth. J. Ethnopharmacol.. 2021;275:114136
- [Google Scholar]
- Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol.. 2004;142:331-338.
- [Google Scholar]
- Analgesic activity of flavonoids isolated from Persicaria glabra (wild) Advances in Traditional Medicine.. 2020;20:71-76.
- [Google Scholar]
- Avocado roots treated with salicylic acid produce phenol-2, 4-bis (1, 1-dimethylethyl), a compound with antifungal activity. J. Plant Physiol.. 2014;171:189-198.
- [Google Scholar]
- Antioxidant study and GC MS analysis of an ayurvedic medicine ‘Talisapatradi choornam’. Int. J. Pharm. Sci. Rev. Res.. 2016;36:158-166.
- [Google Scholar]
- Phytochemical profiling, in vitro and in vivo anti-inflammatory, analgesic and antipyretic potential of Sesuvium sesuvioides (Fenzl) Verdc. (Aizoaceae) Inflammopharmacology 2021:1-12.
- [Google Scholar]
- Phytochemical profiling, in vitro and in vivo anti-inflammatory, analgesic and antipyretic potential of Sesuvium sesuvioides (Fenzl) Verdc. (Aizoaceae) Inflammopharmacology. 2021;29:789-800.
- [Google Scholar]
- Traditional multi-herbal formula in diabetes therapy–Antihyperglycemic and antioxidant potential. Arab. J. Chem.. 2021;14:103347
- [Google Scholar]
- Polystichum braunii extracts inhibit Complete Freund’s adjuvant-induced arthritis via upregulation of I-κB, IL-4, and IL-10, downregulation of COX-2, PGE2, IL-1β, IL-6, NF-κB, and TNF-α, and subsiding oxidative stress. Inflammopharmacology. 2020;28:1633-1648.
- [Google Scholar]
- Antimicrobial and immunomodulatory potential of endophytic fungus Fusarium solani isolated from Withania somnifera. World J. Pharm. Res.. 2014;3:879-890.
- [Google Scholar]
- Anti-inflammatory and analgesic activities of black cumin (BC, Nigella sativa L.) extracts in in vivo model systems. Bulletin of the National Research Centre.. 2022;46:1-8.
- [Google Scholar]
- Anti-nociceptive and anti-inflammatory effects of the methanolic extract of Opuntia humifusa stem. Avicenna Journal of Phytomedicine.. 2017;7:366.
- [Google Scholar]
- Cytokines and abnormal glucose and lipid metabolism.. 2019;10:703.
- Anti-Fibrotic Therapies from Other Organs: What the Gut Can Learn from the Liver, Skin. Lung and Heart. Fibrostenotic Inflammatory Bowel Disease: Springer; 2018. p. :347-385.
- Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem. Rev.. 2020;19:1199-1209.
- [Google Scholar]
- Estimation of drug-likeness properties of GC–MS separated bioactive compounds in rare medicinal Pleione maculata using molecular docking technique and SwissADME in silico tools. Network Model. Anal. Health Informatics Bioinformat.. 2021;10:1-36.
- [Google Scholar]
- Anti-inflammatory and anti-nociceptive property of Capparis tomentosa Lam. root extracts. J. Ethnopharmacol.. 2020;253:112654
- [Google Scholar]
- Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crops Prod.. 2020;154:112748
- [Google Scholar]
- Total phenolic and flavonoid contents, antioxidant, antidiabetic and antiplasmodial activities of Garcinia forbesii King: A correlation study. Arab. J. Chem.. 2022;15:103541
- [Google Scholar]
- Methanolic extract of Ephedra ciliata promotes wound healing and arrests inflammatory cascade in vivo through downregulation of TNF-α. Inflammopharmacology. 2020;28:1691-1704.
- [Google Scholar]
- Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Global J. Pharmacol.. 2012;6:65-71.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104784.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







