Translate this page into:
Chlorophyll triggered one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones via photo induced electron transfer reaction
⁎Corresponding author. rupesh.manak@gmail.com (Rupesh Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The photocatalytic potential of chlorophyll has been investigated for the facile synthesis of dihydropyrimidinones utilizing concentrated solar irradiation towards sustainable energy solutions. This, one-pot, multicomponent Biginelli reaction, which involves a photoinduced electron transfer (PET) mechanism, affords a green and efficient approach for the transformation of the commercial aldehydes, β-keto ester and urea into valuable 3,4-dihydropyrimidin-2(1H)-ones with wide substrate scope and diversity. These improved reaction conditions allow the formation of a variety of substituted dihydropyrimidinones with high yields and purity in a short duration of time and mild reaction conditions.
Keywords
Chlorophyll
3,4-dihydropyrimidin-2(1H)-ones
Photochemical reaction
Biginelli reaction
Concentrated solar radiation
Photo-induced electron transfer (PET)
1 Introduction
Chlorophyll is a complex, natural and abundant organic pigment that acts as photocatalyst in green plants. It behaves as the principal photoreceptor in the green plants that transfers energy to the reaction centres to form bigger sugar molecules from simple carbon dioxide and water through the initiation of photoinduced electron transfer (PET) (Bassham et al., 1950). Illuminating photochemical synthesis is greatly motivated by photosynthesis, one of the most captivating natural phenomena (Gust et al., 2009; Kim et al., 2015). The perfect harvesting of the solar light and photoelectron tranfer through a series of steps to the redox centres is the theme of the photosynthesis (Deisenhofer and Michel, 1989; Fleming et al., 1988). Imitating of the photosynthesis in the simulating environment is the key idea behind the use of solar energy for chemical research in the photocatalysis (Shaw et al., 2016; Xie and Schlücker, 2015; Pitzer et al., 2018). Some of the organic chemists used photoredox catalysis in visible light for organic transformations(McClelland and Weiss, 2019; Thomas et al., 2018; Chakraborty et al., 2019; Prier et al., 2013; Nicewicz and MacMillan, 2008; Trucker and Stephenson, 2012). Metal complexes and some of the organic dyes are extensively used to initiate and execute the photoexcitation with visible light through a single electron transfer process. Although these catalysts are immensely efficient in conducting photoinduced electron transfer processes for organic transformations, but they show some disadvantages pertaining to its inclusion of rare earth metals like ruthenium (Ru) and iridium (Ir) which are expensive, toxic and found in trace amounts in earth crusts. Hence, the development of renewable photocatalyst is highly desirable. Rarely the chlorophyll (Fig. 1) has been applied in mimicking photosynthesis for photosensitized organic transformations (Guo et al., 2017) though it is capable of harvesting solar energy and transferring its energy for the conversion of chemical entities into the desired products. The idea of solar induced photosensitized reactions becomes even more evident being a long standing demand of society to harness the solar energy for its sustainable development. It has also improved the latitude of light promoted transformations.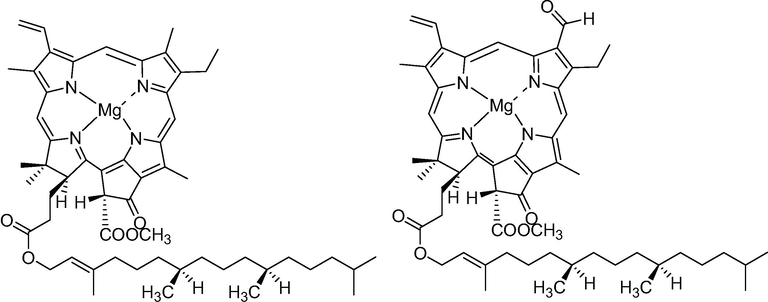
Structure of chlorophyll a & chlorophyll b.
Dihydropyrimidinones (DHPMs) and their derivatives are exceedingly important heterocycles because of their important therapeutic and medicinal properties. The medicinal importance of DHPMS exhibit antiviral, antibacterial, antitumor and anti-inflammatory properties (Atwal et al., 1991). The dihydropyrimidinones have some other interesting pharmacological properties of being calcium channel modulators, anti-HIV in some natural products containing the DHPM skeleton and anti-cancer by inhibiting kinesin motor protein (Kappe, 2000). Nifedipine and Monastrol are two examples used in the treatment of cardiovascular diseases and as kinesin-5 inhibitors respectively (Fig. 2) (Jains et al., 1987).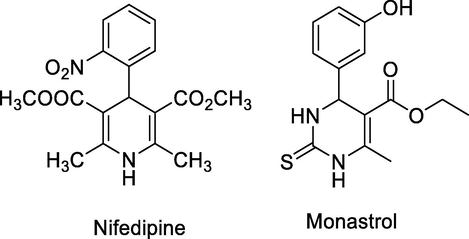
Structure of kinesin-5 inhibitors.
The vast applications of DHPMs have inspired the development of novel synthetic methodologies for their preparation and chemical transformations. DHPMS have been prepared using one of the different type of catalysts such as ionic liquids (Alvim et al., 2013), different sulfonic acids (Gong et al., 2015), and transition metal catalysts (Wang et al., 2010; Sharma et al., 2012). DHPMs have also been synthesized under other varied conditions such as solvent free (Khademinia et al., 2015), ultrasonication synthesis (Li et al., 2003) and microwave synthesis (Pasunooti et al., 2011). These previously reported methods consist of the expensive reagents, long reaction times, harsh conditions and high temperature result in either environmental pollution or unsatisfactory yields (Marcantoni and Petrini, 2016; Fedorova et al., 2016; Hang et al., 2016). The development of energy efficient one pot chemical transformations in single step avoiding toxic reagents, environment unfriendly solvents and costly purification techniques is a basic goal to achieve. Unlike conventional methodologies, photochemical processes enable the generation of various reactive species under mild conditions (Lewis, 2007; Morton, 2006; Albini and Fagnoni, 2004, 2008; Roth, 1989; Brimioulle et al., 2015; Hoffmann, 2008, 2015; Protti and Fagnoni, 2009; Ravelli et al., 2016). Manipulation of these reactive entities can provide many synthetically useful products even selectively. Our group recently (Harsh et al. 2018) reported the photochemical synthesis of functionalized benzimidazoles using the concentrated solar radiations as the sustainable energy source under environment friendly conditions.
To achieve the maximum solar energy harvesting, the selection of the photoreceptor is the most important part. Lower the energy required to stimulate the sensitizer, larger the fraction of the solar spectrum can be engrossed to match the absorption maximum. In this preview, the use of chlorophyll as photosensitizer is advantageous because of it’s mid to low energy absorption (λmax = 465 nm & 665 nm) and proves to be very effective in the solar spectrum (400–700 nm).
We rationalized the use of chlorophyll for the efficient intake of concentrated solar radiation (CSR) in the illuminated synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs). We sought a novel, innovative way to harvest and transport the photons for initiation of chemical reaction.
2 Experimental
2.1 Materials and methods
Oxygen- and moisture-sensitive reactions were carried out under nitrogen atmosphere. Solvents were purified and dried by standard methods prior to use. All commercially available reagents and solvents are purchased common commercial suppliers (like Aldrich, Merck, Spectrochem, Acros etc.) and used without purification unless otherwise required. Analytical thin layer chromatography (TLC) was conducted on Merck Kieselgel 60F254. Column chromatography was performed on silica gel (100–200 mesh). Melting points were determined in open glass capillary tubes using a Mel-Temp apparatus and are uncorrected. 1H NMR spectra were obtained with CDCl3/DMSO at 400 MHz, using Bruker spectrometers (residual chloroform referenced to 7.26 ppm) or DMSO‑d6 (residual DMSO referenced to 2.50 ppm and residual water in DMSO‑d6 appearing at 3.34 ppm). Chemical shift values are expressed as parts per million downfield from TMS and J values are in hertz. Splitting patterns are indicated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double of a doublet, ddd: doublet of a doublet of a doublet, and br: broad peak. 13C NMR spectra were recorded with CDCl3 at 75 MHz, using Bruker spectrometers (residual chloroform referenced to 77.0 ppm) or DMSO‑d6 (residual DMSO referenced to 39.5 ppm). HRMS were recorded on Bruker high resolution spectrometer.
2.2 General procedure for extraction of chlorophyll
Leaves of fresh spinach (Spinacia oleracea) were purchased from the local market. Extraction was made by grinding up 10 g of spinach in 30 mL of acetone as per the reported methods (Khalyfa et al., 1992; Johnston et al., 2013; Abraham and Rowan, 1991). The extracted clear suspension containing crude chlorophylls was further subjected to purification according to the method described by Iriyama et al using dioxane/water (Iriyama et al., 1974). Crude chlorophyll was also treated with hexane/water, chlorophyll dissolve in hexane leaving almost all the other things in water. Resultant green coloured clear solution is used as it is in the reaction. Absorption spectra of the extraction further confirms the presence of chlorophyll. For the confirmation of chlorophyll, the UV–Visible spectra of the extracted chlorophyll from spinach is recorded and compared to the reported data (Welburn, 1994; Smith et al., 1984).
2.3 General procedure of the synthesis of 3,4-dihydropyrimidin-2(1H)-ones
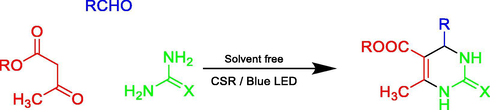
In a typical experiment, a mixture of aromatic aldehyde (1 mmol), alkyl acetoacetate (1 mmol) and urea (1.2 mmol) was added in a test tube. The reaction mixture was stirred on a magnetic stirrer and irradiated in concentrated solar radiation (CSR) for the appropriate time (Tables 1 and 2) using fresnel lens as the concentrator. The progress of the reaction was monitored by TLC. After completion of the reaction, the crude product, so obtained, was recrystallized with ethanol to get pure 3,4-dihydropyrimidin-2(1H)-ones in excellent yields (upto 92%).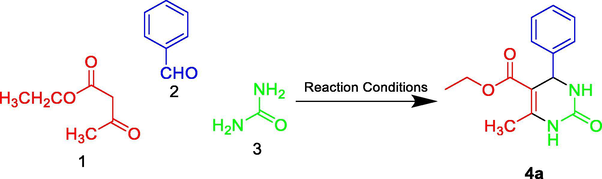
S. No.
Reaction Conditions
Photocatalyst
Solvent
Time (h)
Yield (%)
1
Broad Sunlight
–
–
18 h
nd
2
Blue light
–
–
30 h
trace
3
Concentrated solar radiation
–
–
40 min
8
4
Broad Sunlight
Chlorophyll
–
18 h
25
5
Blue light
Chlorophyll
–
30 h
30
6
Blue light
Chlorophyll
Ethanol
30 h
15
7
Blue light
Chlorophyll
Acetone
30 h
22
8
Concentrated solar radiation
Chlorophyll
–
30 min
85
9
Concentrated solar radiation
Chlorophyll
–
40 min
92
10
Concentrated solar radiation (With coolant)
Chlorophyll
–
40 min
50
11
Concentrated solar radiation (With coolant)
Chlorophyll
Ethanol
40 min
30
12
Concentrated solar radiation (With coolant)
Chlorophyll
Methanol
40 min
25
S. No.
Ester
Aldehyde
X
Product
Yield (CSR,%)
1
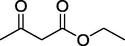
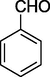
O
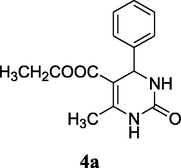
92%
2.
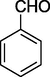
O
85%
3.
O
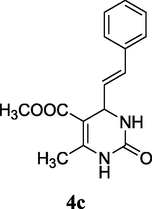
82%
4.
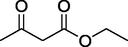
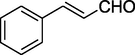
O
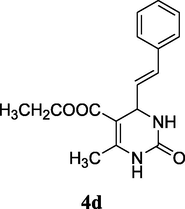
86%
5.
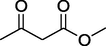
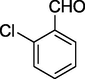
O
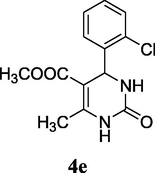
81%
6.
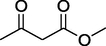

O
77%
7.
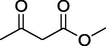
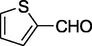
O
88%
8.
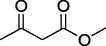
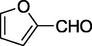
O
72%
9.
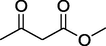
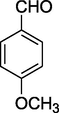
O
80%
10.
O
84%
11.
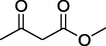
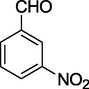
O
52%
12.
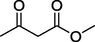
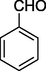
S
70%
13.
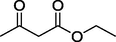
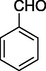
S

76%
14.
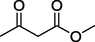
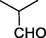
O
45%
15.
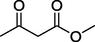

O
0%
2.4 5-Ethoxycarbonyl-4-phenyl-6-methyl-3,4-dihydropyridin-2(1H)-one (4a)
Yield 92%; white solid, mp 202–203 °C; 1H NMR (400 MHz, DMSO‑d6) δ 9.20 (1H, s, NH), 7.74 (1H, s, NH), 7.29 (5H, m, ArH), 5.14 (1H, d, J = 4.0 Hz, CH), 3.99 (2H, q, J = 16.0, 8.0 Hz, CH2), 2.25 (3H, s, CH3), 1.09 (3H, t, J = 8.0 Hz, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 165.8, 152.6, 148.8, 145.3, 128.8, 127.7, 126.7, 99.7, 59.6, 54.4, 18.2, 14.5; IR (KBr) cm−1: 3315 (NH), 3110 (NH), 1712 (CO), 1662 (CO); MS m/z 261 (M+1); Anal. Calc. for C14H16N2O3: C, 64.60; H, 6.20; N, 10.76; found: C, 64.63; H, 6.21; N, 10.70.
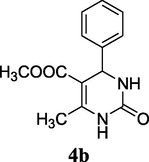
2.5 5-Methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (4b)
Yield 85%; White solid; mp 208–210 °C; 1HNMR (400 MHz, DMSO‑d6) δ 9.12 (1H, s, NH), 7.73 (1H, s, NH), 7.32 (5H, m, ArH), 5.12 (1H, d, J = 4.0 Hz, CH), 3.71 (s, OCH3), 2.28 (3H, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 163.2, 152.1, 148.9, 145.3, 128.5, 127.9, 127.0, 100.5, 55.5, 54.1, 15.2; IR (KBr) cm−1: 3328 (NH), 3215 (NH), 1708 (CO), 1658 (CO); MS m/z 245 (M+1); Anal. Calc. for C13H14N2O3: C, 63.40; H, 5.73; N, 11.38; found: C, 63.47; H, 5.72; N, 11.32.
2.6 Methyl (E)-6-methyl-2-oxo-4-styryl-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4c)
Yield 82%; white solid; mp 191–192 °C; 1H NMR (400 MHz, DMSO‑d6) δ 9.20 (1H, s, NH), 7.58 (1H, s, NH), 7.32 (5H, m, ArH), 6.36 (1H, d, J = 16.0 Hz, CH⚌CH), 6.20 (1H, dd, J = 16.0, 4.0 Hz, CH⚌CH), 4.73 (1H, s, CH), 3.63 (3H, s, OCH3), 2.20 (3H, s, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 166.1, 153.0, 149.2, 136.7, 130.5, 129.1, 128.4, 128.0, 126.8, 98.1, 52.1, 51.4, 18.3; IR (KBr) cm−1: 3233 (NH), 3109 (NH), 1709 (CO), 1680, 1645 (CO); MS m/z 273 (M+1); Anal. Calc. for C15H16N2O3: C, 66.16; H, 5.92; N, 10.29; found: C, 66.17; H, 5.96; N, 10.20.
2.7 Ethyl (E)-6-methyl-2-oxo-4-styryl-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4d)
Yield 86%; white solid; mp 225–227 °C; 1H NMR (400 MHz, DMSO‑d6) δ 9.20 (1H, s, NH), 7.54 (1H, s, NH), 7.39 (5H, m, ArH), 6.37 (1H, d, J = 16.0 Hz, CH⚌CH), 6.21 (1H, dd, J = 16.0, 8.0 Hz, CH⚌CH), 4.73 (1H, s, CH), 4.12 (2H, m, CH2), 2.20 (3H, s, CH3), 1.12 (3H, t, J = 8.0 Hz); 13C NMR (75 MHz, DMSO‑d6) δ 165.6, 152.8, 148.8, 136.5, 129.7, 128.6, 128.0, 127.5, 126.2, 97.5, 59.1, 51.7, 18.2, 14.4; IR (KBr) cm−1: 3225 (NH), 3112 (NH), 1703 (CO), 1675, 1640 (CO); MS m/z 287 (M+1); Anal. Calc. for C16H18N2O3: C, 67.12; H, 6.34; N, 9.78; found: C, 67.20; H, 6.36; N, 9.79.
2.8 5-Methoxycarbonyl-4-(2-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4e)
Yield 81%; white solid; mp 226–228 °C; 1H NMR (400 MHz, DMSO‑d6) δ 9.32 (1H, s, NH), 7.73 (1H, s, NH), 7.51 (3H, m, ArH), 6.80 (1H, m, ArH), 4.41 (1H, s, CH), 3.44 (3H, s, OCH3), 2.30 (3H, s, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 166.0, 151.9, 149.9, 132.1, 130.2, 129.9, 129.6, 129.1, 128.2, 98.2, 51.8, 51.2, 18.2; IR (KBr) cm−1: 3315 (NH), 3114 (NH), 1696 (CO), 1643 (CO); MS m/z 282 (M+2); Anal. Calc. for C13H13ClN2O3: C, 55.62; H, 4.67; N, 9.98; found: C, 55.64; H, 4.70; N, 9.94.
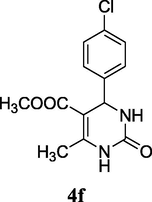
2.9 Methyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4f)
Yield 77%; white solid; mp 179–181 °C; 1H NMR (400 MHz DMSO‑d6) δ 9.29 (s,1H, NH), 7.73 (s, 1H, NH), 7.42 (m, 4H, ArH), 5.08 (s, 1H, CH), 3.60 (s, 3H, OCH3), 2.27 (s, 3H, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 166.0, 152.3, 149.7, 132.7, 132.0, 129.0, 128.6, 128.1, 127.9, 98.2, 51.7, 51.4, 18.6; IR (KBr) cm−1: 3338 (NH), 3220 (NH), 1721 (CO), 1639 (CO); MS m/z 282 (M+2); Anal. Calc. for C13H13ClN2O3: C, 55.62; H, 4.67; N, 9.98; found: C, 55.68; H, 4.70; N, 9.95.
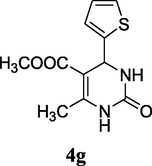
2.10 6-Methyl-2-oxo-4-thiophen-2-yl-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid methtl ester (4g)
Yield 88%; white solid; mp 157–158 °C; 1H NMR (400 MHz, DMSO‑d6) δ 9.36 (s, 1H, NH), 7.94 (s, 1H, NH), 7.36 (d, 1H, J = 8.0 Hz, thiophene-SCH), 6.92 (m, 2H, thiophene-CH), 5.41 (s, 1H, CH), 3.60 (s, 3H, OCH3), 2.23 (s, 3H, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 165.4, 152.5, 150.3, 141.4, 127.0, 126.0, 124.6, 110.6, 58.5, 52.0, 19.3; IR (KBr) cm−1: 3311 (NH), 3120 (NH), 1705 (CO), 1651 (CO); MS m/z 253 (M+1); Anal. Calc. for C11H12N2O3S: C, 52.37; H, 4.79; N, 11.10; found: C, 52.35; H, 4.80; N, 11.00.
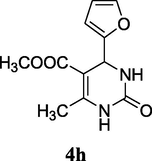
2.11 Methyl 4-(furan-2-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4h)
Yield 72%; white solid; 1H NMR (400 MHz DMSO‑d6) δ 9.42 (s, 1H, NH), 7.56 (s, 1H, NH), 7.37 (d, 1H, J = 8.0 Hz, furan-OCH), 6.34 (m, 2H, furan-CH), 5.40 (s, 1H, CH), 3.56 (s, 3H, OCH3), 2.21 (s, 3H, CH3); 13C NMR (75 MHz, DMSO‑d6) δ 165.1, 152.4, 151.5, 150.9, 142.4, 110.5, 107.9, 106.2, 58.1, 51.9, 19.2; IR (KBr) cm−1: 3278 (NH), 3128 (NH), 1702 (CO), 1652 (CO); MS m/z 237 (M+1); Anal. Calc. for C11H12N2O4: C, 55.93; H, 5.12; N, 11.86; found: C, 55.95; H, 5.19; N, 11.78.
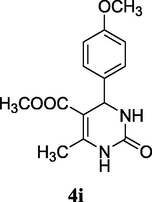
2.12 Methyl 4-(4-methoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4i)
Yield 80%; light brown solid; m.p.: 190–192 °C; 1H NMR (400 MHz, DMSO‑d6) δ 10.22 (s, 1H, NH), 8.23 (s, 1H, NH), 7.25 (m, 2H, ArH), 6.88 (m, 2H, ArH), 5.27 (s, 1H, CH), 3.82 (s, 3H, Ar—OCH3), 3.50 (s, 3H, OCH3), 2.38 (s, 3H, CH3). 13C NMR (75 MHz, DMSO‑d6) δ 165.5, 160.6, 153.4, 134.8, 127.3, 113.8, 106.2, 55.7, 52.8, 52.2, 19.0; IR (KBr) cm−1: 3259 (NH), 3118 (NH), 1711 (CO), 1642 (CO); MS m/z 277 (M+1); Anal. Calc. for C14H16N2O4: C, 60.86; H, 5.84; N, 10.14; found: C, 60.92; H, 5.79; N, 10.05.
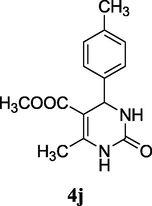
2.13 Methyl 6-methyl-2-oxo-4-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4j)
Yield 84%; light brown solid; m.p.: 213–215 °C; 1H NMR (400 MHz, DMSO‑d6,) δ 10.29 (s, 1H, NH), 8.35 (s, 1H, NH), 7.04 (m, 2H, ArH), 6.75 (m, 2H, ArH), 5.27 (s, 1H, CH), 3.54 (s, 3H, OCH3), 2.28 (s, 3H, CH3), 2.24 (s, 3H, Ar-CH3); 13C NMR (75 MHz, DMSO‑d6) δ 165.0, 152.5, 151.2, 139.5, 134.8, 129.4, 128.1, 107.5, 53.1, 51.8, 21.5, 19.8; IR (KBr) cm−1: 3260 (NH), 3113 (NH), 1704 (CO), 1649 (CO); MS m/z 261 (M+1); Anal. Calc. for C14H16N2O3: C, 64.60; H, 6.20; N, 10.76; found: C, 64.54; H, 6.22; N, 10.61.
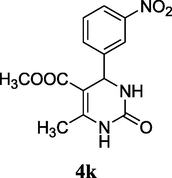
2.14 Methyl 6-methyl-4-(3-nitrophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4k)
Yield 52%; yellow solid; m.p.: 271–273 °C; 1H NMR (400 MHz, DMSO‑d6) δ 10.44 (s, 1H, NH), 8.61 (s, 1H, NH), 7.95 (m, 4H, ArH), 5.25 (s, 1H, CH), 3.58 (s, 3H, OCH3), 2.35 (s, 3H, CH3). 13C NMR (75 MHz, DMSO‑d6) δ 166.1, 155.1, 147.9, 139.7, 128.9, 126.5, 123.9, 108.0, 53.5, 51.0, 20.3; IR (KBr) cm−1: 3239 (NH), 3119 (NH), 1716 (CO), 1660 (CO); MS m/z 292 (M+1); Anal. Calc. for C13H13N3O5: C, 53.61; H, 4.50; N, 14.43; found: C, 53.69; H, 4.54; N, 14.37.
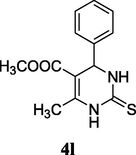
2.15 Methyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4l)
Yield 70%; light brown solid; m.p.: 220–222 °C; 1H NMR (400 MHz, DMSO‑d6) δ 10.40 (s, 1H, NH) 9.71 (s, 1H, NH), 7.28 (m, 5H, ArH), 5.20 (s, 1H, CH), 3.56 (s, 3H, OCH3), 2.32 (s, 3H, CH3). 13C NMR (75 MHz, DMSO‑d6) δ 175.2, 166.0, 145.7, 134.1, 129.0, 127.1, 126.7, 105.8, 54.3, 51.5, 18.6; IR (KBr) cm−1: 3313 (NH), 3140 (NH), 1667 (CO), 1182 (CS); MS m/z 263 (M+1); Anal. Calc. for C13H14N2O2S: C, 59.52; H, 5.38; N, 10.68; found: C, 59.58; H, 5.45; N, 10.66.
2.16 Ethyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4m)
Yield 76%; white solid; m.p. 211 °C; 1H NMR (400 MHz, DMSO‑d6): δ 10.27 (s, 1H, NH), 9.58 (s, 1H, NH), 7.20 (m, 3H, ArH), 6.92 (m, 2H, ArH), 5.13 (s, 1H, CH), 3.99 (m, 2H, OCH2), 2.27 (s, 3H, CH3), 1.08 (m, 3H, CH3); 13C NMR (75 MHz, DMSO‑d6): δ 173.94, 165.09, 158.66, 144.68, 135.64, 127.55, 113.79, 100.88, 59.47, 55.01, 17.07, 13.96; IR (KBr): 3328, 3174, 1670, 1573 cm−1; IR (KBr) cm−1: 3298 (NH), 3123 (NH), 1661 (CO), 1186 (CS); MS m/z 277 (M+1); Anal. Calc. for C14H16N2O2S: C, 60.85; H, 5.84; N, 10.14; found: C, 60.90; H, 5.83; N, 10.08.
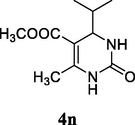
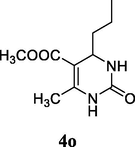
2.17 Methyl 4-isopropyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4n)
Yield 45%; White solid; 1HNMR (400 MHz, DMSO‑d6) δ 9.12 (1H, s, NH), 7.73 (1H, s, NH), 4.28 (1H, d, J = 4.0 Hz, CH), 3.70 (s, OCH3), 2.30 (3H, CH3), 2.00 (1H, m, CH), 1.25 (d, J = 7.0 Hz, 6H, —CH3); 13C NMR (75 MHz, DMSO‑d6) δ 165.1, 152.4, 150.9, 106.5, 58.1, 51.9, 30.7, 19.2, 18.4; IR (KBr) cm−1: 3240 (NH), 3115 (NH), 1706 (CO), 1652 (CO); MS m/z 213 (M+1); Anal. Calc. for C10H16N2O3: C, 56.59; H, 7.60; N, 13.20; found: C, 56.61; H, 7.70; N, 13.17.
3 Results and discussion
Initially, the cyclization reaction of ethyl acetoacetate (1 equiv.) (1), benzaldehyde (1.0 equiv.) (2) and urea (1.2 equiv.) (3) under an air atmosphere was chosen as the prototype reaction system. First of all, the optimization experiments were executed to establish that both chlorophyll and concentrated solar radiation are necessary for the reaction (Table 1). In diffused sunlight without using any catalyst no reaction was observed (Entry 1, Table 1). The reaction was also carried out under irradiation with 8 W blue LED strip and only a trace amount of the desired product was obtained while under CSR only 8% of the product was reported (Entries 2 & 3, Table 1). Pleasantly, when the prototype reaction was performed with chlorophyll under diffused sunlight, the desired product was obtained with 25% yield after 18 h (Entry 4, Table 1). The similar reaction under irradiation with blue light provided 30% yield of the product after 30 h (Entry 5, Table 1) and with 15% and 18% yield when the reaction was performed in ethanol and methanol as solvent respectively (Entries 6 & 7, Table 1).
The results indicated that chlorophyll is necessary for the model reaction, but the low yield and long reaction time made us disappointed. The diffused and uneven solar intensity during the longer exposure may be the reasons for the poor results. These reasons make the protocol impractical and further prompted us to explore the convenient and practical procedure. The concentrated solar radiation (CSR) was used for the purpose by employing the fresnel lens as the concentrator and excellent results were obtained (Fig. 3).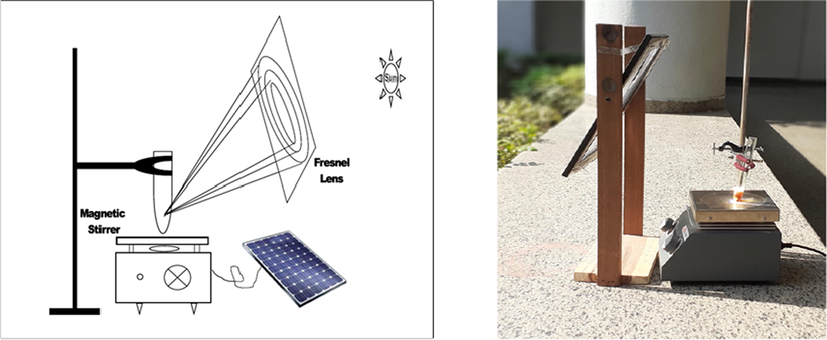
(a) Schematic representation of the experimental CSR Photochemical setup.a,b (b) Actual experimental setup of the CSR mediated synthesis. (aAll the experiments were conducted under similar conditions at Kapurthala, Punjab, India 30°19′51.71″N, 75°29′24.97″E/30.33103N, 75.490268E, The reactions were performed during 12.00 noon −2.30 pm when the solar radiations are reportedly minimally inclined with maximum intensity.)
The natural pigment chlorophyll was extracted from spinach leaves as per reported procedure and used as it is (Khalyfa et al., 1992; Iriyama et al., 1974; Johnston et al., 2013; Welburn, 1994; Smith et al., 1984; Abraham and Rowan, 1991). The prototype reaction was exposed under concentrated solar radiation using chlorophyll as photosensitizer with no solvent and gave the desired product with 85% yield only after 30 min (Entry 8, Table 1). Further, 10 min increase in the reaction time also afforded the enhanced yield of 92% in 40 min (Entry 9, Table 1) and the additional change in the reaction timings did not show any significant improvement in the product yield. Concentrated solar radiation is also well known to show thermal effect in a very short span of time due to its highly concentrated nature. In order to verify the thermal effect of resultant CSR, an experiment was also carried out under CSR using water as a coolant with chlorophyll. It presented the formation of DHPM with 50% yield only (Entry 10, Table 1) which clearly show the combined photo-thermal effect of CSR with chlorophyll on the product formation. The reaction was also executed in solvents like ethanol and methanol, but desirable yields could not be obtained (Entries 11 & 12, Table 1).
After getting the optimized conditions in hand, the substrate scope of this photosensitized reaction was investigated (Table 2). A series of aromatic aldehydes 2 with alkyl acetoacetate 1 (ethyl/methyl) and urea/thiourea 3 were examined. CSR in the presence of chlorophyll excellently represented its photo-thermal effect. It can be observed that both ethyl acetoacetate and methyl acetoacetate reacted well with aromatic aldehydes either with electron donating groups (Entries 1–4, 9 & 10,) or electron withdrawing groups (Entries 5 and 6,) to give corresponding products in good to excellent yields (82–92% & 77–81%) respectively. Wherein the 3-nitrobenzaldehyde was used, the desired product was obtained in a relatively low yield (52%, Entry 11,). Heteroaromatic aldehydes were also examined (Entries 7 & 8,) and provided the products 4g and 4h with 88% and 72% yield respectively. Further, to extend the scope, the reaction was also carried out using thiourea instead of urea which also responded very well to provide the products with good yields (70–76%, Entries 12 and 13,). The highest yield of 92% was achieved (Entry 1). The reaction of isobutyraldehyde afforded 4n in 45% yield, while desired product was not isolated with butyraldehyde.
Reactions were also tried for its reproducibility under the similar set of conditions and provided similar results. The use of Fresnel lens allowed the solar energy to wield its photo-thermal effect for the promotion of chemical transformations under the imitated photo-catalytic environment. In prospect studies, both the electron withdrawing and electron donating components carrying aromatic aldehydes are well endured. Strategic compared to the earlier reported methods (Ma et al. 2016, Achary et al. 2018, Kassaee et al. 2010), this reveals the application of chlorophyll and concentrated light as the protocol provides a highly energy efficient, sustainable and atom economical method (Harsh et al., 2018).
To insight into the pathway, the reaction for the synthesis of 4a was investigated using as representative examples (Scheme 1). The ESI-MS analyses of the reaction mixture of 1, 2 and 3 after 15 min shown the presence of major species A, B and 4a (Fig. 4).
Gram Scale Synthesis.
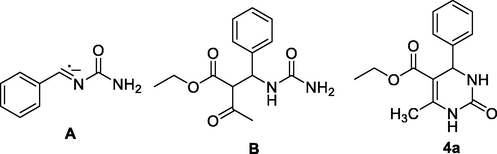
Major species identified using ESI-MS of reaction mixture after 15 min (see Supporting Information).
The radical scavenger experiment using TEMPO exhibited the formation of a trace amount of the desired product, this revealed that the reaction comprises a radical intermediate (Scheme 2).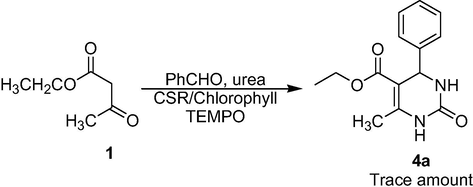
Radical Scavenger Experiment.
A plausible reaction pathway for this photosensitized cyclization reaction using chlorophyll is proposed in Scheme 3 on the basis of performed experiments and previous work (Bassham et al., 1950; Zubay and Geoffrey, 1993; Borg et al., 1970; Dolphin and Felton, 1974; Shanmugam et al., 2015). The absorption of a photon excites the chlorophyll from its ground state to the excited state. This absorption of solar radiation is converted into electronic excitation energy that initiates photoinduced electron transfer (PET) (Bassham et al., 1950). It is presumed that the aromatic ring is activated by the solar irradiation through chlorophyll. The aromatic aldehyde 2 is facilitated by chlorophyll through photoinduced electron transfer generating a radical anion intermediate 4. This radical anion undergoes nucleophillic addition with urea 3 giving a reactive iminium intermediate 5. The excited state chlorophyll oxidize enol form of ethylacetoacetate 6 into corresponding cation radical 7. The cation radical 7 attack the iminium intermediate 5 to get the cyclized dehydrated 3,4-dihydropyrimidinone derivative 4a (Scheme 3).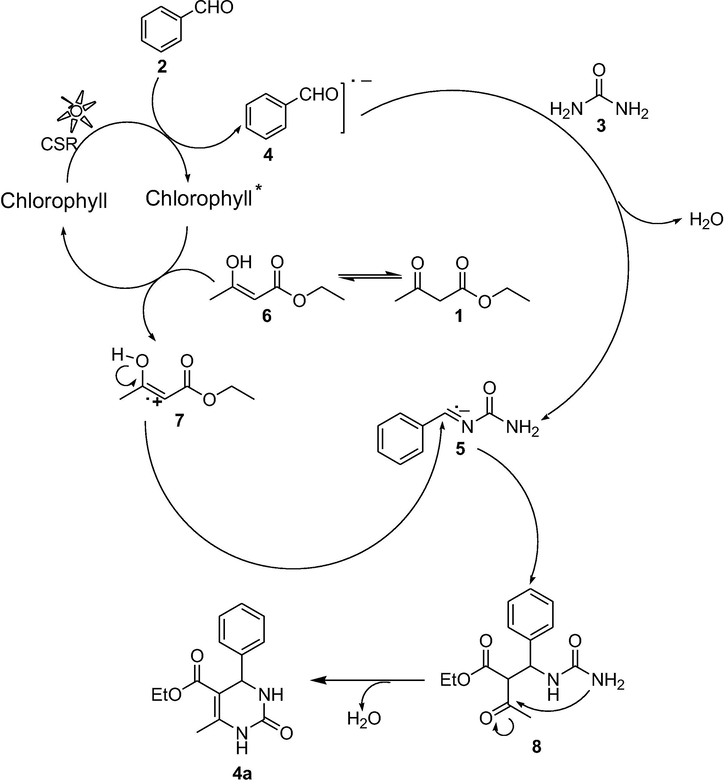
Proposed mechanism for the Chlorophyll catalyzed Photosynthesis of Pyrimidinones.
Chlorophyll behaves as a strong reducing agent (Zubay and Geoffrey, 1993) because of its reported half-wave reduction potential of −1.1 V and able in transferring an electron to the oxidant producing a π-radical cation. Consequently, it is assumed that Chlorophyll underwent one electron redox reaction (Borg et al., 1970; Dolphin and Felton, 1974; Shanmugam et al., 2015). As aryl aldehyde, due to high electron withdrawing capability shows high electron affinity and assumed to be in the close proximity of the chlorophyll molecule, the excited electron can be transferred from the initial chlorophyll molecule to the aryl aldehyde. It generates positive charge on the chlorophyll molecule and negative charge on the aryl aldehyde (Photoinduced charge separation) (El-Khouly et al., 2001).
4 Conclusions
This unprecedented chlorophyll sensitized method presents a convenient, energy efficient, sustainable, atom economical and ecological method for the synthesis of dihydropyrimidinones. This method also achieved scalability and reproducibility. A wide range of aldehydes were studied to this new synthetic imitation to give functionalized dihydropyrimidinones with good efficiencies. We also presented the concentrated solar radiation (CSR) as an energy efficient protocol in this multicomponent click reaction (MCR-Click) which might have prospective solicitations in other areas, such as pharmaceutical, material science and polymer chemistry except for long standing organic chemistry. Photoelectron excitation using chlorophyll could effectively play its role in the accomplishment of the desired products.
Declaration of Competing Interest
There is no conflict of interest.
References
- Chlorophylls. Boca Raton, FL: CRC Press; 1991. p. :797-834.
- Phosphate functionalized graphene oxide with enhanced catalytic activity for Biginelli type reaction under microwave condition. Chem. Engg. J.. 2018;331:300-310.
- [Google Scholar]
- Green chemistry and photochemistry were born at the same time. Green Chem.. 2004;6:1-6.
- [Google Scholar]
- 1908: Giacomo Ciamician and the concept of green chemistry. ChemSusChem. 2008;1:63-66.
- [Google Scholar]
- Ionic liquid effect over the biginelli reaction under homogeneous and heterogeneous catalysis. ACS Catal.. 2013;3:1420-1430.
- [Google Scholar]
- Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem.. 1991;34:806-811.
- [Google Scholar]
- The path of carbon in photosynthesis: viii. The rôle of malic acid. J. Biol. Chem.. 1950;185:781-787.
- [Google Scholar]
- The π-cation radical of chlorophyll-a. Proc. Natl. Acad. Sci. U.S.A.. 1970;67:813-820.
- [Google Scholar]
- Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed.. 2015;54:3872-3890.
- [Google Scholar]
- InP/ZnS quantum dots as efficient visible-light photocatalysts for redox and carbon−carbon coupling reactions. Chem. Mater.. 2019;31:2258-2262.
- [Google Scholar]
- The Photosynthetic reaction center from the purple bacterium rhodopseudomonas viridis (Nobel Lecture. Angew. Chem. Int. Ed. Engl.. 1989;28:829-847.
- [Google Scholar]
- Biochemical significance of porphyrin.pi. cation radicals. Acc. Chem. Res.. 1974;7:26-32.
- [Google Scholar]
- Photoinduced electron transfer between chlorophylls (a/b) and fullerenes (C60/C70) studied by laser flash photolysis. Photochem. Photobiol.. 2001;74:22-30.
- [Google Scholar]
- Asymmetric biginelli reaction catalyzed by silicon, titanium and aluminum oxides. Catal. Lett.. 2016;146:493-498.
- [Google Scholar]
- Rates of primary electron transfer in photosynthetic reaction centres and their mechanistic implications. Nature. 1988;333:190-192.
- [Google Scholar]
- β-Cyclodextrin-propyl sulfonic acid: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3,4-dihydropyrimidones via Biginelli reaction. Tetrahedron. 2015;71:4830-4834.
- [Google Scholar]
- Chlorophyll-catalyzed visible-light-mediated synthesis of tetrahydroquinolines from n, n-dimethylanilines and maleimides. J. Org. Chem.. 2017;82:1888-1894.
- [Google Scholar]
- A highly enantioselective Biginelli reaction using self-assembled methanoproline–thiourea organocatalysts: asymmetric synthesis of 6-isopropyl-3,4-dihydropyrimidines. Chem. Commun.. 2016;52:80-83.
- [Google Scholar]
- Concentrated solar radiation promoted unconventional greener approach: solvent-free benign synthesis of functionalized benzimidazoles. ARKIVOC VII 2018:119-130.
- [Google Scholar]
- Photochemical reactions as key steps in organic synthesis. Chem. Rev.. 2008;108:1052-1103.
- [Google Scholar]
- Electron and hydrogen transfer in organic photochemical reactions. J. Phys. Org. Chem.. 2015;28:121-136.
- [Google Scholar]
- A simple method for extraction and partial purification of chlorophyll from plant material, using dioxane. J. Biochem.. 1974;76:901-904.
- [Google Scholar]
- A Green approach to separate spinach pigments by column chromatography. J. Chem. Educ.. 2013;90:796-798.
- [Google Scholar]
- Biologically active dihydropyrimidones of the Biginelli-type–a literature survey. Eur. J. Med. Chem.. 2000;35:1043-1052.
- [Google Scholar]
- Synthesis of 3,4-dihydropyrimidin-2(1H)-ones using TiO2 as a heterogeneous catalyst and conventional heating. Helvetica Chimica Acta. 2010;93(2):261-264.
- [Google Scholar]
- Solid state synthesis, characterization, optical properties and cooperative catalytic performance of bismuth vanadate nanocatalyst for Biginelli reactions. RSC Adv.. 2015;5:24313-24318.
- [Google Scholar]
- Extraction, purification, and characterization of chlorophylls from spinach leaves. J. Agric. Food Chem.. 1992;40:215-220.
- [Google Scholar]
- Artificial photosynthesis for sustainable fuel and chemical production. Angew. Chem. Int. Ed.. 2015;54:3259-3266.
- [Google Scholar]
- An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by NH2SO3H under ultrasound irradiation. Ultrason. Sonochem.. 2003;10:119-122.
- [Google Scholar]
- d-Xylonic acid: a solvent and an effective biocatalyst for a three-component reaction. Green Chem.. 2016;18(6):1738-1750.
- [Google Scholar]
- Recent developments in the stereoselective synthesis of nitrogen-containing heterocycles using N-acylimines as reactive substrates. Adv. Synth. Catal.. 2016;358:3657-3682.
- [Google Scholar]
- Selective photocatalytic oxidation of benzyl alcohol to benzaldehyde or C-C coupled products by visible-light-absorbing quantum dots. ACS Appl. Energy Mater.. 2019;2:92-96.
- [Google Scholar]
- Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science. 2008;322:77-80.
- [Google Scholar]
- A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett.. 2011;52:80-84.
- [Google Scholar]
- Carbonyl olefin cross-metathesis through a visible-light-induced 1,3-diol formation and fragmentation sequence. Angew. Chem. Int. Ed.. 2018;57:16219-16223.
- [Google Scholar]
- Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev.. 2013;113:5322-5363.
- [Google Scholar]
- The sunny side of chemistry: green synthesis by solar light. Photochem. Photobiol. Sci.. 2009;8:1499-1516.
- [Google Scholar]
- Application of visible and solar light in organic synthesis. In: Bergamini G., Silvi S., eds. Applied photochemistry when light meets molecules. Switzerland: Springer Int. Publ.; 2016. p. :281-342. Ch. 6
- [Google Scholar]
- The beginnings of organic photochemistry. Angew. Chem. Int. Ed.. 1989;28:1193-1207.
- [Google Scholar]
- Utilizing the electron transfer mechanism of chlorophyll a under light for controlled radical polymerization. Chem. Sci.. 2015;6:1341-1349.
- [Google Scholar]
- Green and recyclable glycine nitrate (GlyNO3) ionic liquid triggered multicomponent Biginelli reaction for the efficient synthesis of dihydropyrimidinones. RSC Adv.. 2012;2:10648-10651.
- [Google Scholar]
- The NMR spectra of porphyrins. 27*—proton NMR spectra of chlorophyll-a and pheophytin-a. J. Org. Magn. Reson.. 1984;22:779-783.
- [Google Scholar]
- How trap states affect charge carrier dynamics of CdSe and InP quantum dots: visualization through complexation with viologen. ACS Energy Lett.. 2018;3:2368-2375.
- [Google Scholar]
- Shining light on photoredox catalysis: theory and synthetic applications. J. Org. Chem.. 2012;77:1617-1622.
- [Google Scholar]
- Efficient, green, solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones via biginelli reaction catalyzed by Cu(NO3)2·3H2O. Synth. Commun.. 2010;40:1115-1122.
- [Google Scholar]
- The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol.. 1994;144:307-313.
- [Google Scholar]
- Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat. Commun.. 2015;6:7570.
- [Google Scholar]
- Biochemistry (third ed.). Dubuque, Iowa: W.M.C. Brown Publishers; 1993.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.11.002.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







