Translate this page into:
Co-biomass degradation of fluoranthene by marine-derived fungi; Aspergillus aculeatus and Mucor irregularis: Comprehensive process optimization, enzyme induction and metabolic analyses
⁎Corresponding author at: Department of Pure and Applied Botany, College of Biosciences, Federal University of Agriculture, P.M.B. 2240, Abeokuta, Ogun State, Nigeria. bankolepo@funaab.edu.ng (Paul Olusegun Bankole),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The application and relevance of marine-derived fungi in the mycoremediation of environment polluted with polycyclic aromatic hydrocarbons (PAHs) is promising whilst limiting environmental hazards. The present study investigated the fluoranthene degradation efficiency of marine-derived fungal co-culture, Aspergillus aculeatus (AA) and Mucor irregularis (MI) in batch processes (Plackett-Burman experiments) enhanced with the addition of surfactants and solid-state substrates. Further optimization studies done through fractional factorial design revealed that the co-culture exhibited 98.4% fluoranthene degradation capacity after 7 days of incubation. The role played by enzymes was revealed with 93, 85 and 71% induction of laccase, lignin peroxidase and manganese peroxidase respectively during fluoranthene degradation. The Gas Chromatography-Mass Spectrometry analysis revealed the formation of five metabolites; 1,2- dihydroxyfluoranthene, 9H-fluorene-1,9-dicarboxylic acid, benzene-1,2,4-tricarboxylic acid, benzene-1,3-dicarboxylic acid and benzoic acid after fluoranthene degradation by AA + MI co-culture which was used in predicting a metabolic pathway. The findings of this study elucidated the promising potentials of marine-derived fungal co-biomass in the eco-friendly remediation of polycyclic aromatic hydrocarbons thus promoting green technology.
Keywords
Aspergillus aculeatus
Mucor irregularis
Co-biomass
Fluoranthene
Biodegradation
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds characterized by the presence of two or more fused aromatic rings. Their ubiquitous abundance in nature is attributable to indiscriminate oil exploration activities, vandalization, fossil fuel combustion and oil spillages (Bacosa and Inoue, 2015), especially in developing countries. PAH pollution is of great environmental and health concern because of its stable, persistent and recalcitrant features (Vaidya et al., 2018). They have been found over the years to be highly toxic. Fluoranthene is a non-alternant, three-member ring high molecular weight PAH originally isolated from coal tar and abundantly found in the environment (Kweon et al. 2007). It is ubiquitously present in petroleum sludge and combustion products. Fluoranthene is one of 16 United States Environmental Protection Agency (USEPA) priority pollutants PAHs (Abdel-Shafy and Mansour, 2016). Fluoranthene is on the priority list because of its teratogenic, genotoxic, mutagenic (Hentati et al. 2016) and carcinogenic properties (classified as a group-3 carcinogen by the International Agency for Research on Cancer) (IARC 2010).
The major challenge and bottleneck faced in the removal of most low molecular weight PAHs like fluoranthene from the environment are (i) low vapour pressure and aqueous solubility (ii) great recalcitrance to microbial attack owing to its high hydrophobicity (Okere and Semple, 2012; Luning Prak and Pritchard, 2002). Researchers have reported microbial degradation of PAHs in aqueous environment with the addition of surfactants under enzymatic conditions (Arun and Eyini, 2011; Hadibarata and The, 2014). Therefore, in tackling this challenge during bioremediation process, surfactants are introduced to enhance the solubility of fluoranthene in aqueous systems for increased bioavailability (Li and Chen, 2009). The potency of surfactants to solubilize most organic pollutants has over the years encouraged several researchers in embracing surfactant-mediated biotransformation technology (Hickey et al., 2007).
Researchers have deployed conventional physicochemical methods (photolysis, oxidation and coagulation) in the removal of PAHs albeit with criticisms of sludge formation and high cost. Nowadays, the paradigm shift is largely on exploring microbes as a promising and pragmatic technique for PAH removal due to their cheap and eco-friendly nature (Birolli et al., 2018; Kumari et al., 2018). In doing so, solid-state fermentation technology is usually introduced in the degradation of pollutants to enhance the proliferation of microbes and improve the enzyme hydrolytic process (Bankole et al., 2018). It has become increasingly important for researchers to fashion out a multi-scale eco-friendly, and efficient technique for removing PAHs including fluoranthene. Several researchers have reported complete degradation and biotransformation of fluoranthene by different classes of bacteria and fungi. Vasconcelos et al. (2019) reported the efficiency of Xylaria sp. and Tolypocladium sp. (derived from marine origin) in the removal of both pyrene and benzo[a]pyrene. Similarly, the potency of Marasmiellus sp. in the complete degradation of pyrene was reported by Vieira et al (2018). However, it is desirable to discover new marine-derived fungal strains with the capacity to degrade PAHs. In addition, the application of fungal co-culture derived from marine origin has been largely less explored and under-reported. To ensure enhanced degradation of PAHs, the application of fungal co-culture has been deployed and promoted by several researchers as one of the most favourable bioremediation approaches as they induce multiple non-selective extracellular and intracellular enzymes. The application of fungal co-culture in PAH degradation process has the edge over single cultures in terms of efficient, effective, coordinated and synergistic co-metabolic mineralization activities (Kumari et al. 2018).
Complex degradation processes involving fungi cultures, PAHs and surfactants could be time-consuming. Also, laboratory microcosm study of interaction effects of different parameters under specified experimental conditions may be quite arduous to achieve. Hence, it is highly recommended to conduct biodegradation experiments involving high molecular weight PAHs and different assays in batches (Patowary et al., 2015). Therefore, there is a need to deploy response surface methodology in the optimisation of different variables involved in the batch PAH degradation process to save cost, energy and time. To the best of our knowledge, this is the first report on mathematical and statistical modelling of surfactant-enhanced degradation of fluoranthene by the marine-derived fungal co-culture in a solid-state fermentative medium.
Hence, the present study was conducted to (1) achieve enhanced fluoranthene degradation using marine-derived fungal co-culture, Aspergillus aculeatus (AA) and Mucor irregularis (MI) in a fermentative medium supplemented with surfactants (2) to investigate the enzymes produced by the fungi during fluoranthene degradation and (3) determine the mechanism involved in the fluoranthene degradation process.
2 Materials and methods
2.1 Chemicals
Fluoranthene (>98% GC purity) was selected as the PAH contaminant and procured from Sigma-Aldrich, UK. Stock solutions of fluoranthene were prepared at 500 mg L−1 in acetone and were diluted into different experimental concentrations. Sodium dodecyl benzene sulfonate (SDBS) (>97% GC purity), an anionic surfactant, was obtained from Acros Organics, USA and used without further purification. Tween 80 (purity > 99%), a non-ionic surfactant, was purchased from Sigma-Aldrich, United Kingdom. Other chemicals and reagents used were of high purity and analytical grade.
2.2 Microorganism
AA and MI were previously isolated from the shorelines of the Atlantic Ocean in Lagos State, Nigeria and reported for their PAH’s biodegradation efficiency (Bankole et al., 2020a; 2021). Both filamentous fungi showed great degradation ability on common low and high molecular weight PAHs. Potato dextrose agar was used in maintaining the fungi (AA and MI) and kept in the refrigerator at 4 °C for further experimental use.
2.3 Fungi growth and initial screening
aculeatus and M. irregularis were sub-cultured and maintained on 4% (w/v) potato dextrose agar slants and stored at 4 °C for 7 days. Solid-State Fermentation (SSF) medium was developed with 50 g common bean husks (Bankole et al., 2018). Sterilization of the SSF medium was performed by autoclaving at 121 °C for 30 min after 80% wet weight adjustment of the substrate humidity. The set-up was kept in 500 mL Erlenmeyer flasks in static conditions at 30 °C for 7 days. The marine-fungal co-culture was developed by mixing equal proportions of the SSF medium having 5% (w/w) of the mycelia of each fungal strain (Bankole et al. 2018). The degradation medium {CaCl2·2H2O-0.003 g, Na2HPO4·12H2O-13.0 g, KH2PO4-1.80 g, MnSO4·H2O-0.002 g, (NH4)2SO4-0.8 g, FeSO4·7H2O-0.002 g and MgSO4-0.1 g and 1000 mL of sterile distilled water at pH 7.0} was inoculated with 5% wet weight of each fungus mycelium, fluoranthene (150 mg L−1) and the SSF medium (Bankole et al. 2020b). Erlenmeyer flasks containing the same components with no fungal strains were set as controls. The controls were made of heat-treated dead fungi mycelia. The flasks were incubated at 28 °C for 7 days and reduction of fluoranthene was determined on 1, 5 and 7 days. All cultures were made in triplicates and incubated in dark conditions.
2.4 Experimental design
The response surface methodology (RSM) involves the synergistic application of mathematical and statistical methods in studying the variations in parameters. It also provides the opportunity of proposing a classical second-order model to achieve a response in the interaction between the target compound and other parameters. Factors affecting degradation of PAHs in aqueous batch culture process are enormous. However, two surfactants (SDBS and Tween 80) were added to the medium to enhance the solubility and bioavailability of fluoranthene during biodegradation. Among these factors, the significant ones such as surfactants, temperature, concentration of PAH and dry weight were selected to reduce experimental costs and time. Plackett–Burman design was used to determine the main (positive) effect of independent process parameters on the degradation of fluoranthene by the marine-derived fungal co-culture (AA + MI). The eleven (11) components in the medium were screened at two levels (−1 and +1) representing minimum and maximum values of each variable (Vieira et al., 2018). The variables optimized were temperature (20–40 °C), pH (6–7), Dry weight of co-culture (1–3 g), salinity (25–35 ppm), malt extract (1–2 g L−1), yeast extract (1–2 g L−1), peptone (0.5–1 g L−1), glucose (g L−1), fluoranthene concentration (50–150 mg L−1), SDBS (100–500 mg L−1) and Tween 80 (300–500 mg L−1) in a 12 assay experiments. Actual and coded values of the independent variables are presented in Table 3. The experimental design was statistically analyzed with Design-expert software v. 12.0.1.0 (Stat-Ease, Inc., Minneapolis, USA). The variable optimized were pH (6–7), dry weight of co-culture (1–3 g), yeast extract (g L−1), fluoranthene concentration (50–150 mg L−1), SDBS (100–500 mg L−1) and Tween 80 (300–500 mg L−1) in a 12-assay experiment. The surfactants (SDBS and Tween 80) were added to enhance the production of enzymes and fluoranthene degradation. pH 6 and 7 were maintained in the fermentative medium to support optimal production of fungal hyphae. Furthermore, a fractional factorial design (FFD) (24) was deployed to evaluate the effects of 4 factors that showed greater influence on fluoranthene degradation in the second Plackett – Burman assay experiments. The independent parameters include pH (6–7), dry weight of co-culture (1–3 g), fluoranthene concentration (50–150 mg L−1), and Tween 80 (mg L−1) while the dependent variable is the removal efficiency of fluoranthene (%) from the fermentative medium. The optimization of the favourable and highly influential parameters was achieved in 16 assay experiments with the coded values of each parameter rated – 1 for low and 1 for high. At all levels of optimization for fluoranthene degradation, the influence of each parameter was deduced from the Pareto charts. Confirmatory experiments for validation of the results were conducted in triplicate, and the values observed by the optimized condition were set as control.
2.5 Validation of optimized conditions during fluoranthene degradation through growth-linked degradation experiments
The most optimum conditions for fluoranthene degradation were used in the design of the validation experiments (Bankole et al., 2020a).
2.6 Characterization of fluoranthene metabolites after degradation
2.6.1 Extraction of metabolites and Gas Chromatography-Mass Spectrometry (GC–MS) analysis
The extraction of fluoranthene and its metabolites in the SSF medium was done with the addition of 50 mL ethyl acetate. The ethyl acetate played a pivotal role in the removal of adsorbed pollutants in the mycelia cells as well as their breakdown. Ethyl acetate (50 mL) was added to each Erlenmeyer flask containing the fungal co-culture grown in the SSF medium. Under optimized conditions, the media containing the fluoranthene and fungal co-culture were extracted with 50 mL ethyl acetate under shaking conditions (250 rpm) for 15 min. The mixture was centrifuged for 3 min at 10,000 rpm. The component was thereafter filtered, transferred to a 250 mL separating funnel and shaken vigorously for 1 min. The organic phase was dried over sodium tetraoxosulphate VI salt while the aqueous phase was re-suspended and re-extracted with ethyl acetate. An aliquot (1 mL) was used as standard solution for the GC–MS analysis. Determination of metabolites obtained after degradation was done using the method Radzi & Abu (2015). The analysis was performed with the use of GC–MS QP2010 Plus (Shimadzu, Japan) equipped with RTX-5MS capillary column (C18) of diameter (30 m × 0.25 mm i.d., 0.25 µm film thickness) where the analytes were separated. The splitless injection mode was employed and helium (>99% purity) was used as a carrier gas at a flow velocity rate of 40 cm s−1. The initial temperature in the GC was set at 60 °C for 2 min and thereafter raised to 280 °C at 6 °C min−1 rates. The thermal conditions in the injection were however maintained for 2 min at 290 °C. At 1.5 s per scan, the ions were characterized within a scanning range of 50–550 amu and 70 eV electron mode. Luan et al. (2006) method was used for the identification of the metabolites obtained through comparison of the mass spectra with the data available in the NIST library. Furthermore, the prominent metabolites were analysed in the SIM mode in comparison with spectra reported by previous researchers.
2.6.2 High-Performance Liquid Chromatography (HPLC) analysis
Fluoranthene degradation profile and its metabolites were analyzed using HPLC (Shimadzu, Japan) following the method of Villemain et al. (2006). The procedure was repeated at 180 rpm for 30 min. Thereafter, the mycelia pellets were filtered off with Whatman paper. The aqueous phase was allowed to settle in static conditions and separate from the organic phase. The organic phase content was dried over an anhydrous sodium tetraoxosulphate VI and evaporated to dryness under a vacuum. The dried pellet (residue) was immediately resuspended in 1.5 mL acetonitrile followed by slight vortexing for 5 min. An aliquot (20 µL) was injected into the HPLC column port for further analysis. The HPLC system is equipped with an automatic injector, chromatographic pump and fluorescence detector. The separation column is made of Supelcosil TM (Supelco Inc., Bellefonte, PA) with a diameter of 4.6 mm × 150 mm and film thickness of 5 μm. The mobile phase was acetonitrile: water (70:30, v:v). The flow rate was 40 cm s−1 and detection was at 450 nm (λ emission).
2.7 Enzyme analyses
The induction of lignin peroxidase, manganese peroxidase and laccase by the individual fungus and co-culture (AI + MI) in the SSF medium was determined with Shimadzu QP 2010 GC–MS equipment (Shimadzu, Japan). The supernatant was obtained after centrifugation at 10,000 rpm for 10 min at 4 °C. Laccase activity was estimated by monitoring the oxidation of 1 mM ABTS in sodium acetate buffer (100 mM, pH 4.9) at 420 nm (Eggert et al. 1996). Lignin peroxidase activity was determined by monitoring the oxidation of n-propanol with the formation of propanaldehyde at 300 nm (Hatvani and Mecs 2001). MnP induction was based on phenol red oxidation (Tien and Kirk, 1988). All experiments were performed in triplicates. The quantification was expressed as 1 unit per liter (U L−1).
2.8 Statistical data analysis
Normality of data on the initial screening of fluoranthene degradation, enzyme analyses and validation experiments were affirmed through Levene and Shapiro-Wilk tests to correct outliers and random errors (Field, 2013). Furthermore, the means of fluoranthene degradation rate and growth profile of fungal co-culture during validation experiment were separated using Analysis of Variance-Duncan’s Multiple Range Test (p ≤ 0.05). Graph Pad Prism (V7) software was used in plotting the graphs.
3 Results
3.1 Fluoranthene degradation by marine-derived fungi
In the preliminary screening experiment done in potato dextrose broth, the fungi tested showed an average of 70% capacity to reduce fluoranthene after 15 days of incubation (Fig. 1). The results showed 71%, 73% and 76% degradation of fluoranthene from the fermentative medium by the mycelia of AA, MI and AA + MI co-culture at the end of the 15-day experimental period (Fig. 1). Similarly, the significant growth of the fungi in the medium was evident with relative increase in dry weight. At the end of the growth studies, the dry weight of A. aculeatus, M. irregualris and AA + MI were 2.21, 2.68 and 2.78 g which represent 150%, 146%, and 168% increase in dry weight respectively from day 5 (Fig. 1a–c). No significant reduction in fluoranthene was observed in the control experiments.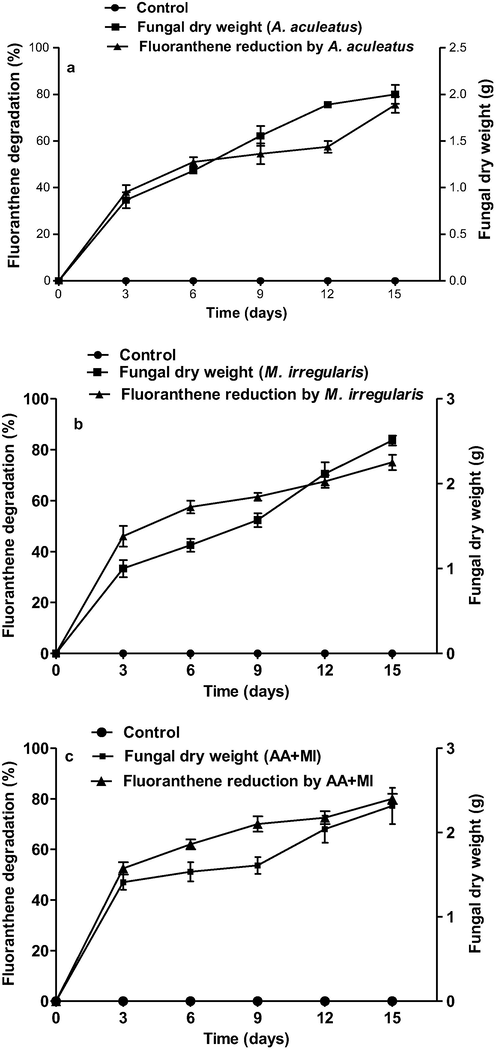
Growth profiles and fluoranthene degradation rate in fermentative medium having (a) A. aculeatus (b) M. irregularis (c) AA + MI at 30 °C and pH 7.
3.2 Fluoranthene degradation by AA + MI co-culture
The Plackett – Burman design involved eleven variables and twelve assays having two centre points (+1-upper and −1-lower). The fluoranthene degradation ranged from 39.4 to 70.7%. Highest degradation rates were observed on assays 7 and 8 (Table 1). A new Plackett-Burman design was developed to further optimize fluoranthene degradation based on the degradation results obtained in the initial optimization screening and statistical analysis done with the physicochemical variables. Table 1 showed that the maximum fluoranthene degradation (78.2%) was observed at assay 11 (Yeast-0.3 g/mL, SDBS-100 mg/L, Tween 80–300 mg/L, fluoranthene concentration-50 mg/L, pH 7 and dry weight-3 g). The least fluoranthene degradation efficiency (42.0%) was observed at assay 8 (Yeast-0.3 g/mL, SDBS-100 mg/L, Tween 80–300 mg/L, fluoranthene concentration-150 mg/L, pH 6, and dry weight-1 g) Table 2. In the initial screening, six of the eleven variables showed significantly (p ≤ 0.05) and positive effects except for salinity, malt extract, peptone, glucose and temperature (Fig. SM2a) Thereafter, salinity and other variables such as temperature, peptone, glucose, and malt extract which had negative and non-significant effects on fluoranthene degradation were excluded from subsequent screening experiments. In the second screening experiment, there was a significant improvement in fluoranthene degradation. The experiments revealed that four of six parameters showed a positive influence (p ≤ 0.05) on the degradation of fluoranthene (Fig. SM2b).
Assays
Temperature (°C)
pH
Dry weight (co-culture) (g)
Salinity (ppm)
Malt extract (g 100 mL−1)
Yeast extract (g 100 mL−1)
Peptone (g 100 mL−1)
Glucose (g 100 mL−1)
Fluoranthene concentration (mg L−1)
SDBS (mg L−1)
Tween 80 (mg L−1)
Fluoranthene degradation (%)
1
25 (−1)
7 (+1)
3 (+1)
35 (+1)
0.3 (−1)
0.1 (−1)
0.2 (−1)
0.5 (+1)
50 (−1)
500 (+1)
500 (+1)
52.3
2
30 (+1)
7 (+1)
1 (−1)
25 (−1)
0.3 (−1)
0.3 (+1)
0.2 (−1)
0.5 (+1)
150 (+1)
100 (−1)
500 (+1)
59.6
3
25 (−1)
7 (+1)
1 (−1)
35 (+1)
0.5 (+1)
0.1 (−1)
0.4 (+1)
0.5 (+1)
150 (+1)
100 (−1)
300 (−1)
56.5
4
30 (+1)
7 (+1)
1 (−1)
35 (+1)
0.5 (+1)
0.3 (+1)
0.2 (−1)
0.3 (−1)
50 (−1)
500 (+1)
300 (−1)
66.0
5
25 (−1)
6 (−1)
1 (−1)
35 (+1)
0.3 (−1)
0.3 (+1)
0.4 (+1)
0.3 (−1)
150 (+1)
500 (+1)
500 (+1)
58.4
6
25 (−1)
6 (−1)
3 (+1)
25 (−1)
0.5 (+1)
0.3 (+1)
0.2 (−1)
0.5 (+1)
150 (+1)
500 (+1)
300 (−1)
55.4
7
30 (+1)
6 (−1)
3 (+1)
35 (+1)
0.3 (−1)
0.3 (+1)
0.4 (+1)
0.5 (+1)
50 (−1)
100 (−1)
300 (−1)
67.9
8
30 (+1)
7 (+1)
3 (+1)
25 (−1)
0.5 (+1)
0.3 (+1)
0.4 (+1)
0.3 (−1)
50 (−1)
100 (−1)
500 (+1)
70.7
9
30 (+1)
6 (−1)
1 (−1)
25 (−1)
0.5 (+1)
0.1 (−1)
0.4 (+1)
0.5 (+1)
50 (−1)
500 (+1)
500 (+1)
66.8
10
30 (+1)
7 (+1)
3 (+1)
25 (−1)
0.3 (−1)
0.1 (−1)
0.4 (+1)
0.3 (−1)
150 (+1)
500 (+1)
300 (−1)
55.6
11
30 (+1)
6 (−1)
3 (+1)
35 (+1)
0.5 (+1)
0.1 (−1)
0.2 (−1)
0.3 (−1)
150 (+1)
100 (−1)
500 (+1)
59.1
12
25 (−1)
6 (−1)
1 (−1)
25 (−1)
0.3 (−1)
0.1 (−1)
0.2 (−1)
0.3 (−1)
50 (−1)
100 (−1)
300 (−1)
39.4
Run
Yeast extract
SDBS
Tween 80
pH
Fluoranthene concentration
Dry weight (co-culture)
Fluoranthene degradation (%)
(g 100 mL−1)
(mg L−1)
(mg L−1)
(mg L−1)
(g)
1
0.1 (−1)
100 (−1)
300 (−1)
7 (+1)
50 (−1)
3 (+1)
57.8
2
0.1 (−1)
500 (+1)
300 (−1)
7 (+1)
150 (+1)
1 (−1)
52.2
3
0.1 (−1)
100 (−1)
500 (+1)
6 (−1)
150 (+1)
3 (+1)
47.2
4
0.1 (−1)
500 (+1)
500 (+1)
6 (−1)
150 (+1)
3 (+1)
60.7
5
0.3 (+1)
100 (−1)
500 (+1)
7 (+1)
150 (+1)
1 (−1)
59.0
6
0.1 (−1)
500 (+1)
500 (+1)
7 (+1)
50 (−1)
1 (−1)
73.3
7
0.3 (+1)
500 (+1)
300 (−1)
6 (−1)
50 (−1)
3 (+1)
74.7
8
0.3 (+1)
100 (−1)
300 (−1)
6 (−1)
150 (+1)
1 (−1)
42.0
9
0.3 (+1)
500 (+1)
300 (−1)
7 (+1)
150 (+1)
3 (+1)
70.5
10
0.3 (+1)
500 (+1)
500 (+1)
6 (−1)
50 (−1)
1 (−1)
76.7
11
0.3 (+1)
100 (−1)
500 (+1)
7 (+1)
50 (−1)
3 (+1)
78.2
12
0.1 (−1)
100 (−1)
300 (−1)
6 (−1)
50 (−1)
1 (−1)
42.8
All the physicochemical parameters showed positive effects on fluoranthene degradation except for SDBS and yeast extract. Generally, from the screening results obtained from Plackett-Burman experiments 1 and 2, it is very clear that Tween 80, fluoranthene concentration and fungal co-culture dry weight exerted a positive influence on the removal of fluoranthene from the fermentative medium. Furthermore, based on the results obtained from the Plackett-Burman 1 and 2 experiments, a two-level fractional factorial (FFD 24) design was deployed to optimize fluoranthene degradation using four physicochemical variables (pH, Tween 80, fluoranthene concentration and fungal co-culture dry weight). The maximum fluoranthene degradation obtained was (98.4%) (Table 3). All the tested variables showed a positive effect on fluoranthene degradation except for pH which exerted little effect on the fermentative degradation system (Fig. SM2c). Multiple statistical analyses deployed in this study led to the success achieved during fluoranthene degradation since the highest initial removal efficiency was 70.73%.
Run
Tween 80
Fluoranthene concentration
Dry weight (co-culture)
pH
Fluoranthene degradation (%)
(mg L−1)
(mg L−1)
(g)
1
500 (+1)
50 (−1)
1 (−1)
7 (+1)
82.3
2
500 (+1)
50 (−1)
3 (+1)
7 (+1)
98.4
3
500 (+1)
150 (+1)
1 (−1)
7 (+1)
64.3
4
300 (−1)
50 (−1)
1 (−1)
6 (−1)
56.8
5
500 (+1)
150 (+1)
1 (−1)
6 (−1)
52.6
6
300 (−1)
150 (+1)
1 (−1)
6 (−1)
38.8
7
500 (+1)
150 (+1)
3 (+1)
6 (−1)
63.3
8
300 (−1)
150 (+1)
1 (−1)
7 (+1)
50.5
9
500 (+1)
50 (−1)
1 (−1)
6 (−1)
70.5
10
300 (−1)
150 (+1)
3 (+1)
7 (+1)
61.3
11
300 (−1)
50 (−1)
3 (+1)
7 (+1)
79.3
12
300 (−1)
50 (−1)
3 (+1)
6 (−1)
67.5
13
300 (−1)
150 (+1)
3 (+1)
6 (−1)
49.5
14
500 (+1)
50 (−1)
3 (+1)
6 (−1)
81.3
15
300 (−1)
50 (−1)
1 (−1)
7 (+1)
68.5
16
500 (+1)
150 (+1)
3 (+1)
7 (+1)
94.2
3.2.1 Validation experiment
The growth-linked experiment conducted in optimized conditions-assay 2 (Tween 80–500 mg L−1, dry weight-3 g, pH 7 and fluoranthene concentration-50 mg L−1) (Table 1) validated the appropriateness of the fractional factorial design used in the present study. In the validation experiment, fluoranthene degradation recorded was 94.5% after 7 days of cultivation which was very close to assay 16 from the FFD (94.2%) (Table 3). The result affirmed that the culture conditions were one the best to achieve maximum degradation of fluoranthene by the marine-derived fungal co-culture, AA + MI. However, enhanced degradation (99.5 ± 0.5%) and growth rate (2.9 ± 0.2 g) of the AA + MI co-culture were recorded in an experiment conducted under optimized variables (Fig. SM3). Taken together, it was observed that the degradation efficiency recorded during the validation experiment (99.5%) was a little variance from degradation percentage (98.4%) obtained during process optimization using Plackett-Burman design. These findings further elucidated the correlation between the optimization of process variables and validation experiments. Hence, we concluded that the Plackett-Burman design-fractional factorial experiments used in this study were sufficient and appropriate for improved and enhanced degradation of fluoranthene by the fungal co-culture, AA + MI.
3.3 Induction of enzymes during fluoranthene degradation
3.3.1 Laccase (lac) induction during fluoranthene degradation
The time course induction of laccase observed in agitated and static conditions showed a significant increase within 10 days experimental period. Production of laccase in agitated conditions was significantly higher than in static conditions. Induction of laccase by the co-culture AA + MI in static and agitated conditions increased significantly by 89% and 93% respectively. A steady increase in the induction of laccase on day 1 in static and agitation conditions ranged from 229.1 U L−1 and 235.78 U L−1 to 976 U L−1 and 988 U L−1 on day 7 respectively (Fig. 2).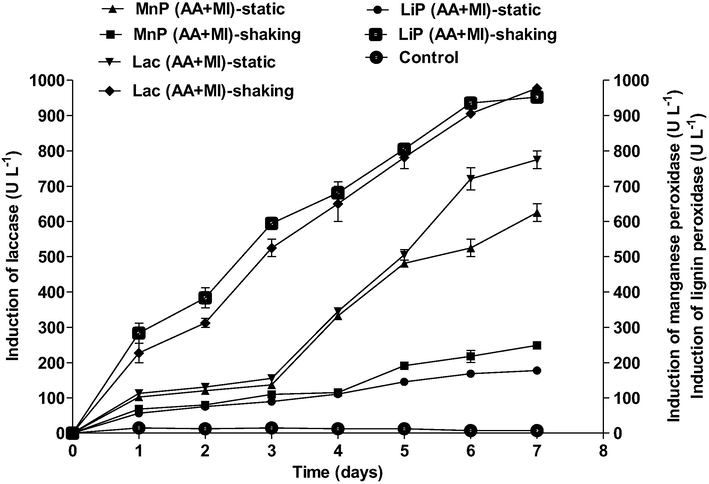
Enzyme induction during fluoranthene degradation by AA, MI and AA + MI in static and shaking conditions (pH 7, temperature 30 °C).
3.3.2 Lignin peroxidase (LiP) induction during fluoranthene degradation
The enzyme assay analysis revealed a steady increase in the induction of LiP from 24 to 240 h in both static and aerophilic conditions under SSF. The findings in this study revealed 70%, and 85% increase in the production of LiP by AA + MI under static and agitated conditions respectively (Fig. 2).
3.3.3 Manganese peroxidase (MnP) induction during fluoranthene degradation
Significant increase was observed in the induction of MnP by the co-biomass (AA + MI) during the degradation of fluoranthene. The results revealed 66 and 71% surge in MnP induction under static and shaking conditions respectively (Fig. 2).
3.4 Characterization of fluoranthene metabolites
3.4.1 GCMS analysis
A putative pathway was predicted using the metabolites obtained after fluoranthene degradation. The GC–MS analysis result revealed the formation of new metabolites; 1,2-dihydroacenaphthylene, 9H-fluoren-9-one and benzoic acid after complete degradation of fluoranthene by the microbial co-culture. Initial cleavage of fluoranthene ring led to the formation of 2-formylacenaphthylene-1-carboxylic acid (aI) (MW: 224.21, m/z: 225) Fig. 3a, Table SM1. Further breakdown of 2-formylacenaphthylene-1-carboxylic acid into 1,2-dihydroacenaphthylen-1-ol, (aII) (MW: 182.22, m/z: 182) and 1,2-dihydroacenaphthylen-1-ol (aIII) (MW: 170.21, m/z: 171) was caused by hydroxylating dioxygenase reactions instigated by A. aculeatus strain bpo 2 (Fig. 3a). The rupture of the extradiol ring in 1,2-dihydroacenaphthylen-1-ol (aIII) resulted in the formation of 1,2-dihydroacenaphthylene (aIV) (MW: 154.21, m/z: 155) Fig. 3b. Interestingly, we observed that the degradation of fluoranthene by M. irregularis coincidentally led to the formation of final metabolite, 1,2- dihydroacenaphthylene obtained after degradation by A. aculeatus. Further breakdown of the mixture led to the formation of 1,2-dihydroacenaphthylene (bI) into 1,2-dihydroacenaphthylene-1,2-diol (bII) (MW: 186.32, m/z: 186). 1,2-dihydroacenaphthylene-1,2-diol (bII) might have undergo further dehydrogenation leading to the formation of 9H-fluoren-9-one (bIII) (MW: 180.55, m/z: 181) Fig. 3b, Table 2. For AA + MI co-culture, the asymmetric cleavage and degradation of fluoranthene caused by actions of lignin peroxidase, laccase and manganese peroxidase resulted in five metabolites (cI-V). The initial cleavage of aromatic ring in fluoranthene led to the formation of 1,2- dihydroxyfluoranthene (cI) (MW: 238.28, m/z: 237) (Fig. 3c, Table SM1). Further rupture in the ring cleavage of 1,2- dihydroxyfluoranthene by the action of the peroxidase enzymes led to the formation of 9H-fluorene-1,9-dicarboxylic acid (cII) (MW: 254.23, m/z: 256)., and ciii-benzene-1,2,4-tricarboxylic acid (cIV) (MW: 210.14, m/z: 211). The decarboxylation and dehydrogenation of benzene-1,2,4-tricarboxylic acid resulted in the formation of a less toxic metabolite, benzoic acid (cV) (MW: 122.12, m/z: 121). Fragmentograms of the compounds involved in the degradation of fluoranthene are presented in Table SM1. In addition, we deduced that the co-culture (AA + MI) was able to reduce fluoranthene to a less toxic substance (benzoic acid) better than MI (9H-fluoren-9-one) and AA (1,2-dihydroacenaphthylene). The metabolites obtained in this study suggest the sole electron oxidative pathway involved in the degradation mechanism by the fungi either singly or in co-culture leading to the production of varying intermediate products. The metabolites obtained after degradation of fluoranthene using solid-state fermentation technology were very different from the ones previously reported by several researchers. This suggests that the technology might be responsible for the outright breakdown into the simplest, lowest molecular weight metabolite after 10 days of cultivation. The action of enzymes and the culture conditions could be possibly implicated given the varying metabolites obtained in this present study. It is worthy of note that most PAHs are exposed to further attack by microbes after the initial cleavage of their asymmetric ring. The prominent conjugates obtained in this present study were dihydrophenols, acenaphthylene and fluorenone.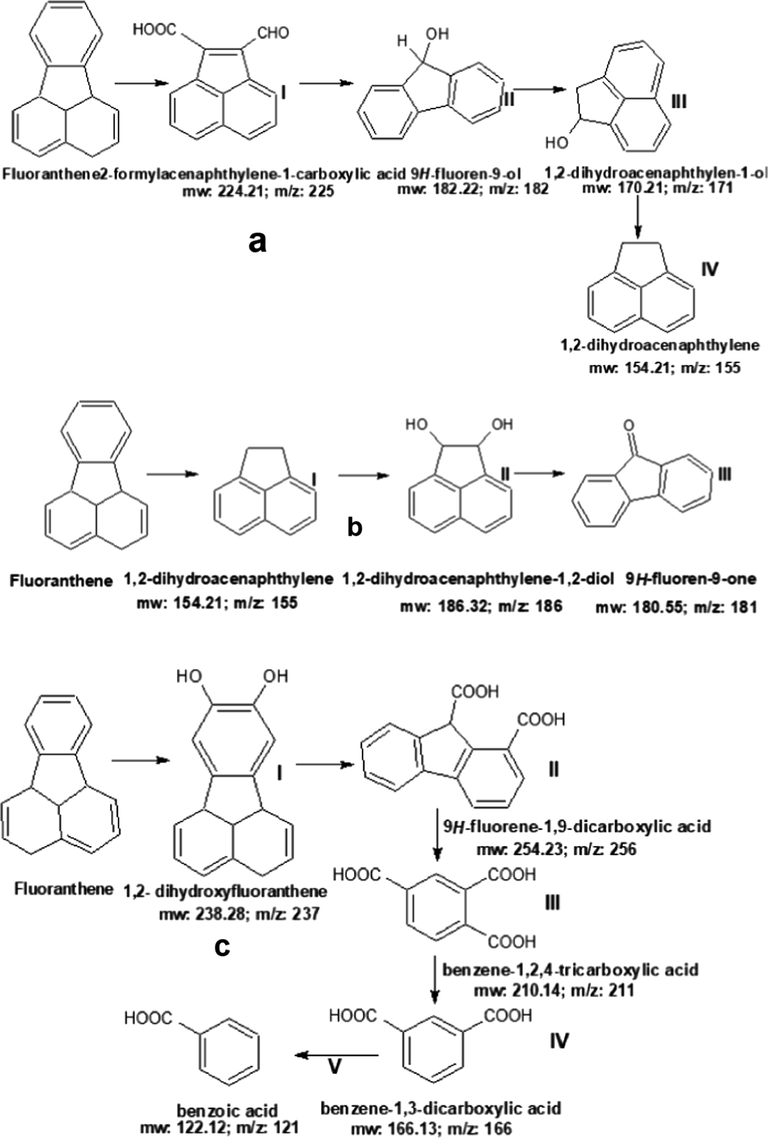
Prediction of the putative metabolic pathway of fluoranthene degradation by (a) AA (b) MI and (c) AA + MI.
3.4.2 HPLC analysis
This analysis was carried out to further support metabolites obtained after GC–MS analyses. HPLC chromatogram of untreated fluoranthene showed peaks at retention times 2.413, 4.718 and 5.013 min (Fig. SM4a). The metabolites obtained fluoranthene degradation by AA revealed peaks with retention times (Rt) 2.999, 3.019, 3.178, and 5.574 min (Fig. SM4b). Fluoranthene degradation by MI showed peaks at higher retention times 3.256, 3.489, 3.783 and 6.927 min (Fig. SM4c) than the ones obtained during degradation by AA. Fluoranthene degradation by AA + MI co-culture showed more peaks at different retention times 2.455, 3.516, 3.782, 5.316 and 5.724 (Fig. SM4d) than chromatogram profiles obtained after degradation by AA and MI singly. However, we can postulate that the least metabolite of fluoranthene was obtained at peak retention time (Rt-2.455 min). The variations in the retention times of untreated fluoranthene and metabolites obtained after degradation by AA, MI and AA + MI co-culture affirmed the eco-friendly removal of fluoranthene metabolites.
4 Discussion
The sequence data of A. aculeatus and M. irregularis were thereafter deposited at the GenBank with accession numbers MT492456 (Bankole et al., 2020b) and MK373020 (Bankole et al., 2020a) with designated names; A. aculeatus strain bpo2 and M. irregularis bpo1. The evolutionary relationships inferred through the neighbour-joining tree method involving bootstrap consensus of 500 replicates with 517 positions in the final dataset revealed the close identities with other 31 fungi strain nucleotide sequences submitted earlier in the GenBank (Fig. SM1). The performance of both fungi as co-culture was further explored for optimal degradation of fluoranthene in subsequent batch experiments to save time and cost. The marine environment which is constantly being subjected to pollution has the capacity of yielding microbial communities with autochthonous organic pollutant degrading capacity (Kastner et al., 1994). Fungi isolated from such marine environments show great dexterity in utilizing hydrocarbons as energy and carbon sources (Vieira et al., 2018). Hence, the application of fungi derived from the marine environment impacted by PAHs could be a promising technology in the remediation, reclamation and eco-friendly development of contaminated sites (Vasconcelos et al 2019). Additionally, performing PAH’s degradation experimental runs via Plackett-Burman designs is economical, time and energy saving (Vieira et al., 2018). The influence of yeast in improved degradation of fluoranthene observed in this study corroborated the reports of Arulazhagan and Vasudevan (2011). Similarly, the impact of surfactants on PAH degradation has been reported by several researchers. Increased enzyme induction was reported by Gopinath et al. (2013) during the application of surfactants in PAHs degradation. Balaji et al. (2014) also emphasized the importance of surfactants in improved production of fungal extracellular enzymes which in turn enhanced the degradation of PAHs. (Hickey et al., 2007) reported that the addition of biosurfactant showed a similar effect as phenanthrene in the enhanced removal of fluoranthene in the culture system. Couto and Sanroman (2005) reported that of all factors important for optimised microbial growth and enhanced induction of enzymes in solid-state fermentation. This accounted for the fluoranthene degradation efficiency by the fungal co-culture. These findings corroborated previous reports of Chi et al. (2007) who reported higher induction of laccase in co-cultures of two white-rot fungi, Pleurotus ostreatus and Ceriporiopsis subvermispora than their respective monocultures. Chi et al. (2007) reported that oxidative stress is promoted by co-biomass which also instigated the switch to secondary metabolism by fungal partners thus encouraging higher induction of enzymes. The efficient degradation of fluoranthene after the initial cleavage of the asymmetric ring is due to secondary metabolism by lignin peroxidase (Ghevariya et al., 2011). The selection of a suitable growth substrate for the co-biomass culture is greatly responsible for the high induction of manganese peroxidase which in turn enhanced fluoranthene degradation in the fermentative medium (Elisashvili et al., 2008). Overall, the mineralization of PAHs by fungi follows the mechanism: induction of enzymes; MnP, LiP and Lac (Kadri et al. 2017).
The findings of this study revealed that laccase and lignin peroxidase played vital role in the metabolism of fluoranthene than manganese peroxidase (Vieira et al. 2018). The high induction of enzymes is attributed to faster agitation of the culture medium which in turn led to a higher fluoranthene degradation efficiency. Furthermore, the shaking conditions were responsible for the adequate supply of oxygen and dissolution of fluoranthene intermediates in the medium which were metabolized by the fungal strains and their co-culture (Ye et al., 2011). The induction of enzymes and their activities might have improved the availability of oxygen which is germane in PAH’s degradation process (Puglisi et al., 2007). These results indicate that the microbial co-culture exhibited an enzymatic system as the major mechanism during fluoranthene degradation. Vieira et al. (2018) reported that fungi strains are mostly implicated in the breakdown of PAHs into metabolites which could be further mineralised. In addition, these conjugates are most times water-soluble, and easily get further detoxified and excreted from human body systems. Taken together, the different mechanisms and metabolites produced during fluoranthene degradation further highlight different characteristics the pollutant possesses (Shetty et al., 2015). Based on these GC–MS results, a metabolic pathway of fluoranthene biodegradation by AA, MI and AA + MI co-culture was proposed (Fig. 2). These results were in agreement with the reports of Reddy et al. (2017) and He et al. (2018). Overall, fluoranthene degradation mechanism and process are well elucidated with the HPLC chromatograms indicating the formations of multiple peaks (Vaidya et al., 2018). In all the HPLC chromatograms, it is noteworthy to state that peaks depicting phthalic acid and salicylic acid were not observed which suggests their complete mineralization by the fungi strains and their co-culture (Patel et al., 2019). Therefore, we could deduce that increased fluoranthene degradation in the absence of phthalic acid and salicylic acid could be attributed to a lack of feedback inhibition of enzymes involved in secondary mineralization (Patel et al., 2019). Conversely, it is equally possible that phthalic acid and salicylic acid may undergo decarboxylation to form benzoic acid (final metabolite in Fig. 3c) (Reddy et al., 2017).
5 Conclusion
The fungal co-culture (AA + MI) exhibited 98.4% fluoranthene degradation efficiency after 5 days in the fermentation medium. Enzyme analyses revealed the role played by laccase and lignin peroxidase which further suggested that the degradation followed the system and mechanism. More importantly, this current study further buttressed the crucial role that fungi isolated in saline conditions in the mineralization of organic pollutants which could be biologically explored in eco-friendly remediation of the environment.
CRediT authorship contribution statement
Paul Olusegun Bankole: Conceptualization, Methodology. Victor Taghoghor Omoni: . Sikandar Imamsab Mulla: . Seun Owolabi Adebajo: . Adedotun Adeyinka Adekunle: .
Acknowledgements
Paul Olusegun Bankole express special thanks to the Association of Commonwealth Universities for the Blue Charter Fellowship Award
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egyptian J. Petrol.. 2016;25(1):107-123.
- [Google Scholar]
- Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar. Pollut. Bull.. 2011;62(2):388-394.
- [CrossRef] [Google Scholar]
- Comparative studies on lignin and polycyclic aromatic hydrocarbons degradation by basidiomycetes fungi. Bioresour. Tech.. 2011;102:8063-8070.
- [Google Scholar]
- Polycyclic aromatic hydrocarbons (PAHs) biodegradation potential and diversity of microbial consortia enriched from tsunami sediments in Miyagi. Japan. J. Hazard. Mater.. 2015;283:689-697.
- [Google Scholar]
- Enzymatic bioremediation of polyaromatic hydrocarbons by fungal consortia enriched from petroleum contaminated soil and oil seeds. J. Environ. Biol.. 2014;35(3):521-529.
- [Google Scholar]
- Biodegradation of a monochlorotriazine dye, cibacron brilliant Red 3B-A in solid-state fermentation by wood-rot fungal consortium, Daldinia concentrica and Xylaria polymorpha. Int. J. Biol. Macromol.. 2018;120:19-27.
- [CrossRef] [Google Scholar]
- Biodegradation of fluorene by the newly isolated marine-derived fungus, Mucor irregularis strain bpo1 using response surface methodology. Ecotox. Environ. Safe.. 2020;208:111619
- [CrossRef] [Google Scholar]
- Enhanced enzymatic removal of anthracene by the mangrove-soil derived fungus, Aspergillus sydowii BPO1. Frontiers Environ. Sci. Eng.. 2020;14(6):113.
- [CrossRef] [Google Scholar]
- Impact of redox-mediator in the degradation of olsalazine by marine-derived fungus, Aspergillus aculeatus strain bpo2: response surface methodology, laccase stability and kinetics. Ecotox. Environ. Safe.. 2021;208:111742.
- [CrossRef] [Google Scholar]
- Biodegradation of anthracene and several PAHs by the marine derived fungus Cladosporium sp. CBMAI 1237. Mar. Poll. Bull.. 2018;129:525-533.
- [Google Scholar]
- Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes. Int. Biodeterior. Biodegrad.. 2007;59:32-39.
- [Google Scholar]
- Application of solid-state fermentation to ligninolytic enzyme production. Biochem. Engin. J.. 2005;22:211-219.
- [Google Scholar]
- A fungal metabolic mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett.. 1996;391:144-148.
- [Google Scholar]
- Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol.. 2008;99:457-462.
- [Google Scholar]
- Field, 2013. Discovery statistics using IBM SPSS Statistics Fourth ed. Thousand Oaks, CA: Sage Publications, London. pp. 213-664.
- Enhanced chrysene degradation by halotolerant Achromobacter xylosoxidans using Response Surface Methodology. Bioresour. Technol.. 2011;102:9668-9674.
- [Google Scholar]
- Strategies to characterize fungal lipases for applications in medicine and dairy industry. BioMed. Res. Int.. 2013;2013:154549
- [CrossRef] [Google Scholar]
- Optimization of pyrene degradation by white-rot fungus Pleurotus pulmonarius F043 and characterization of its metabolites. Bioprocess Biosyst. Eng.. 2014;37(8):1679-1684.
- [Google Scholar]
- Production of laccase and manganese peroxidase by Lentinus edodes on malt-containing by-product of the brewing process. Process Biochem.. 2001;37:491-496.
- [CrossRef] [Google Scholar]
- Genome Sequence and Metabolic Analysis of a Fluoranthene-Degrading Strain Pseudomonas aeruginosa DN1. Front. Microbiol.. 2018;9:2595.
- [CrossRef] [Google Scholar]
- Biodegradation of fluoranthene by a newly isolated strain of Bacillus stratosphericus from Mediterranean seawater of the Sfax fishing harbour. Tunisia. Environ. Sci. Pollut. Res.. 2016;23:15088-15100.
- [Google Scholar]
- Effect of surfactants on fluoranthene degradation by Pseudomonas alcaligenes PA-10. Appl. Microbiol. Biotechnol.. 2007;74:851-856.
- [Google Scholar]
- Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: a review. J. Environ. Sci.. 2017;51:52-74.
- [Google Scholar]
- Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons. Appl. Microbiol. Biotechnol.. 1994;41:267-273.
- [Google Scholar]
- Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol.. 2018;254:174-179.
- [Google Scholar]
- A polyomic approach to elucidate the fluoranthene-degradative pathway in Mycobacterium vanbaalenii PYR-1. J. Bacteriol.. 2007;189:4635-4647.
- [Google Scholar]
- Effect of non-ionic surfactants on biodegradation of phenanthrene by a marine-bacteria of Neptunomonas naphthovorans. J. Hazard. Mat.. 2009;162:66-73.
- [Google Scholar]
- Study of metabolites from the degradation of polycyclic aromatic hydrocarbons (PAHs) by bacterial consortium enriched from mangrove sediments. Chemosphere. 2006;65:2289-2296.
- [CrossRef] [Google Scholar]
- Solubilization of polycyclic aromatic hydrocarbon mixtures in micellar non-ionic surfactant solution. Water Res.. 2002;36:3463-3472.
- [Google Scholar]
- Biodegradation of PAHs in ‘pristine’soils from different climatic regions. J Bioremed Biodegrad S. 2012;1(2):006.
- [Google Scholar]
- Synergistic biodegradation of phenanthrene and fluoranthene by mixed bacterial cultures. Bioresour. Tech.. 2019;284:115-120.
- [CrossRef] [Google Scholar]
- Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) Employing Biosurfactant Producing Pseudomonas aeruginosa KS3, Indian. J. Biotechnol.. 2015;14:208-215.
- [Google Scholar]
- Bioavailability and degradation of phenanthrene in compost amended soils. Chemosphere. 2007;67(3):548556
- [CrossRef] [Google Scholar]
- Degradation of polycyclic aromatic hydrocarbons (pyrene and fluoranthene) by bacterial consortium isolated from contaminated road side soil and soil termite fungal comb. Environ. Earth Sci.. 2015;74(6):5383-5391.
- [CrossRef] [Google Scholar]
- Biodegradation of fluoranthene by Paenibacillus sp. strain PRNK-6: a pathway for complete mineralization. Arch. Microbiol. 2017
- [CrossRef] [Google Scholar]
- Complete genome sequence of the phenanthrene-degrading soil bacterium Delftia acidovorans Cs1-4. Stand. Genomic Sci.. 2015;10:1-10.
- [CrossRef] [Google Scholar]
- Lignin peroxidase of Phanerochaete chrysosporium. In: Wood W.A., Klogg S.T., eds. Methods in Enzymology-Biomass, Part B, Lignin, Pectin, and Chitin. Vol vol. 161. San Diego: Academic Press Inc; 1988. p. :238-249.
- [Google Scholar]
- Degradation of Chrysene by Enriched Bacterial Consortium. Front. Microbiol.. 2018;9:1333.
- [CrossRef] [Google Scholar]
- Pyrene degradation by marine-derived ascomycete: process optimization, toxicity, and metabolic analyses. Environ. Sci. Pollut. Res. 2019
- [CrossRef] [Google Scholar]
- Polycyclic aromatic hydrocarbons degradation by marine-derived basidiomycetes: optimization of the degradation process. Brazilian J. Microbiol.. 2018;49(4):749-756.
- [Google Scholar]
- Biotransformation of anthracene and fluoranthene by Absidia fusca Linnemann. Electron. J. Biotechnol.. 2006;9
- [Google Scholar]
- Biodegradation of anthracene by Aspergillus fumigatus. J. Hazard. Mater.. 2011;185(1):174-181.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104036.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







