Translate this page into:
Comparison of the nutritional and phytochemical composition and antioxidant activities of Aralia elata (Miq.) Seem fruits in Northeast China
⁎Corresponding author. zhangxiuling1968@126.com (Xiuling Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Aralia elata (Miq.) Seem is a wild medicinal and culinary plant. The newly grown shoots are edible, and the stem and root bark are traditional materials used for medicinal extraction. However, few studies have examined the quality and biological activity of the fruit. In this study, we compared the nutritional and phytochemical composition and antioxidant activity of A. elata fruit (AF) picked from five regions (Harbin, Benxi, Raohe, Jiaohe, and Mudanjiang) in Northeast China. AF picked from Harbin had the highest saponin, anthocyanin, and flavonoid contents. UHPLC-MS/MS analyses showed that araloside A was one of the main saponin monomers in AF. The highest arloside A concentration was detected in fruits picked from Harbin and the lowest concentration was detected in fruits picked from Benxi. The relative reactive oxygen species production in cells after pretreatment with AFs from Harbin (50 μg/mL) was 0.78 ± 0.01, second only to those from Raohe. AFs (50 μg/mL) from Harbin had the highest catalase activity (43.61 ± 0.44 U/mg protein), followed by that of the AF collected from Jiaohe, Benxi, Mudanjiang and Raohe. The malondialdehyde levels of the AFs (50 μg/mL) obtained from Benxi, Raohe, Jiaohe, and Mudanjiang were significantly higher than those of the AF obtained from Harbin (2.67 ± 0.15 nmol/mg protein). Compared with AFs from Benxi, Raohe, Jiaohe, and Mudanjiang, the strongest H2O2 scavenging ability (144.95 ± 2.89 mmol TE/100 g dm) was observed for AF from Harbin. The ferric reducing antioxidant power of AFs ranged from 7.70 to 4.52 mmol TE/100 g dm in the order of Harbin > Mudanjiang > Raohe > Jiaohe > Benxi. The results provided support for the selection of the best quality AF and the expanded cultivation of A. elata.
Keywords
Aralia elata (Miq.) Seem fruits
Saponin
Amino acids
Cellular antioxidant
Radical scavenging activity
1 Introduction
The contents of amino acids, vitamins, microelements, and cellulose in wild vegetables are higher than those in ordinary vegetables. Wild vegetables improve human immunity, resist infection by viruses and bacteria, and prevent cancer and diabetes. Aralia elata (Miq.) Seem is a woody wild vegetable that grows mainly in the colder regions of Russia, Japan, South Korea and China (Wei et al., 2020; Shikov et al.,2014; Mitsuhashi, 1976). Fruits are reddish-brown spherical small granules with diameters of approximately 3 to 5 mm. Fruit contains 4–6 seeds accounting for three-quarters of the total fruit volume. Generally, fruits mature between August and September. The stem bark, root bark and leaves of A. elata are rich in a variety of nutritional and phytochemical components, including saponins, flavonoids, sugars, amino acids, proteins, and inorganic compounds (Tokiko et al., 2012; Han et al., 2021). Araloside is the most important and the main active ingredient in A. elata. Aralosides are generally pentacyclic triterpene saponins, and the glycosides are located at positions 3 and 28. Shading is an important environmental condition for the accumulation of saponins in A. elata (Cheng et al., 2021). Kochetkov et al. (1962) isolated three saponins from the root bark of A. elata and named them aralosides A and B. Han et al. (2021) identified 111 saponin monomers from the leaves and buds of A. elata using ultra-performance liquid chromatography and time of flight mass spectrometry. However, few studies have examined the chemical composition of A. elata fruits. Based on previous research results on the chemical composition of the roots, stems and leaves of A. elata, we determined the saponin, flavonoid, anthocyanin, and amino acid contents in the fruits to supplement this deficiency. A. elata possesses various biological activities, such as antioxidant activities (Wang et al., 2018; Zhang et al., 2013). A. elata has been widely used in food and medicine in Asian regions, such as China and Japan. Stem and root bark of A. elata are used as traditional Chinese medicines to treat diabetes, gastric ulcers, hepatitis and other diseases. The newly grown shoots of A. elata have entered the Japanese diet as a green, organic, and nutritious wild vegetable. A. elata fruits (AFs) are mostly soaked in water and wine to prepare functional drinks that are used to prevent colds in the folk traditions of China and Japan.
As a skiophilous tree species, A. elata benefits from a cold and humid climate (Chen et al., 2008). A. elata populations are likely distributed in korean pine and spruce forests, which are mainly located on the shady slopes of great mountains (Wei et al., 2020). Northeast China includes Heilongjiang, Liaoning and Jilin Provinces. The average temperature in winter is < -15 °C, and the temperature difference is large throughout the year. Based on these geographical features, Northeast China is one of the main growing regions of A. elata. Our laboratory conducted field research in Heilongjiang, Liaoning, and Jilin Provinces from May 17 to May 27, May 29 to June 7, June 20 to June 28, 2019, to obtain detailed information on the growth and distribution of A. elata. Field research results showed that Harbin (the capital city of Heilongjiang Province, China), Mudanjiang (prefecture-level city of Heilongjiang Province, China), Raohe (Shuangyashan city of Heilongjiang Province, China), Jiaohe (Jilin city of Jilin Province, China) and Benxi (prefecture-level city of Liaoning Province, China) are the main production areas of A. elata in the three provinces of Heilongjiang, Jilin, and Liaoning, respectively. These regions have vast mountain areas and low temperatures throughout the year. The local people have a long history of using A. elata, and artificial planting has been fully promoted. Therefore, AFs from Harbin, Mudanjiang, Raohe, Jiaohe and Benxi were collected as raw materials for this experiment.
AFs exert a certain therapeutic effect on pharyngitis, nephritis, appetite disorder, and fatigue according to Chinese folk medicine. Regular drinking of AF water extracts effectively relieves gastric ulcers, rheumatic joint pain, and diabetes. Because gastric ulcers, diabetes, and inflammation are all related to oxidative stress-induced damage (Salami et al., 2021; Li et al., 2021; Reuter et al., 2010), we suspected that A. elata is the plant people use to obtain extracts from fruits that regulate redox homeostasis. The original simple term of oxidative stress describes a series of biological reactions of redox imbalance caused by the production and accumulation of reactive oxygen species (ROS) in an organism (Sies, 2015; Sies, 2018). However, with increasing numbers of studies, this definition has gradually changed. The latest version of this definition is the imbalance between oxidants and antioxidants, leading to a disruption of redox signalling (Sies, 2018). Excessive oxidative stress damages biological macromolecules such as DNA and protein and causes cell dysfunction and even cell apoptosis. Oxidative stress is proposed to trigger and exacerbate cancer and neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease (Caliri, 2021; Bhatia and Sharm, 2021). Therefore, more suitable antioxidants must be identified to inhibit oxidative stress damage. In addition to endogenous antioxidants, cells also must use exogenous antioxidants to regulate redox homeostasis. Compared with synthetic antioxidants, most people prefer to use nontoxic and natural antioxidants as food additives and drugs for disease prevention.
In this study, we compared the phytochemicals (flavonoids, anthocyanins, total saponins, monomer saponins, and free amino acids), free radical scavenging abilities (2,2-diphenyl-1-picrylhydrazyl, DPPH.; 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), ABTS.+; ferric reducing antioxidant power, FRAP; H2O2; hydroxyl radicals; superoxide anion; and ferrous ion-chelating ability) and cellular antioxidant activity of AFs grown in five different regions. The regions where AFs were collected were classified through principal component analysis to identify the regions with the best fruit quality. The relationship between the phytochemical composition and antioxidant activity of AF was analysed by calculating the correlation coefficient to obtain new information on the raw material resources of natural antioxidants and lay a foundation for broadening the application of AF in the food and pharmaceutical fields.
2 Materials and methods
2.1 Chemicals and abbreviations
Minimal essential medium (MEM), penicillin–streptomycin (PSS) solution, foetal bovine serum (FBS) and the fluorescent probe (DCFH-DA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Cell Counting Kit-8 (CCK-8) assays were purchased from Beyotime (Shanghai, China). Kits used to assay malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) activities and total antioxidant capacity (T-AOC) were purchased from Nanjing Jiancheng Bioengineering Co. Ltd. (Nanjing, Jiangsu, China).
2.2 Raw materials
AFs obtained from Harbin, Benxi, Raohe, Jiaohe, and Mudangjiang were hand-collected from August 25 to September 10, 2019. We selected the shady slopes of the mixed forests of korean pine and A. elata as the sample collection sites in five regions of Harbin, Benxi, Raohe, Jiaohe, and Mudanjiang. Each sampling site was established with three standard areas of 10 m × 10 m. Ten A. elata plants were randomly selected to collect 20 kg of fruits (including rachises) in each standard area. The collected fruits were all reddish-brown, fully ripened fruits to ensure similar states of the AFs. AFs were placed in a fresh-keeping box containing ice packs and transported back to the laboratory as soon as possible. The location and geographical information of the five zones (Harbin, Benxi, Raohe, Jiaohe, and Mudangjiang) is shown in Fig. 1 and Table 1. The prefrozen AF samples were freeze-dried with a vacuum freeze dryer (LGJ-1A-50, Beijing, China) and then crushed with a disintegrator (JP1000B, Zhejiang, China). The powder was stored at −20 °C until use.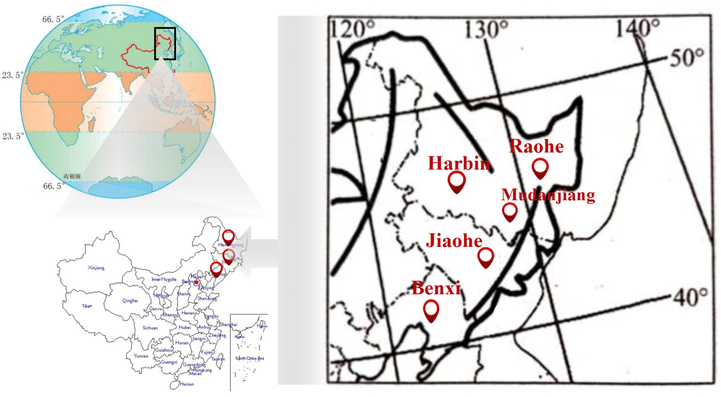
Map of the location of harvest of Aralia elata (Miq.) Seem fruits.
Jiaohe
Harbin
Raohe
Benxi
Mudanjiang
Province
Jilin
Heilongjiang
Heilongjiang
Liaoning
Heilongjiang
Latitude (s)
127.34
126.64
134.02
123.77
129.62
Longitude (w)
43.72
45.76
46.80
41.29
44.58
Altitude (m.a.s.L)
196
127
545
253
230
Minimum temperature (℃)
−10
−30
−17
−14.3
−15
Precipitation (mm)
709
753
553
750
535
2.3 Extraction procedures
AF extracts (AFEs) were obtained using the method described by Tian et al. (2020). Compared with organic solvents such as methanol and ether, ethanol is nontoxic and safe. Therefore, ethanol was chosen as the extraction solvent in this study. AF powders (500 g per region) from Harbin, Benxi, Raohe, Jiaohe, and Mudangjiang were soaked in an ethanol–water solution (80%) for 30 min and extracted with a MARS-II (1000 W, 2450 MHz) microwave-accelerated reaction system. The extraction parameters were as follows: sample–solvent ratio, 1:30; microwave power, 530 W; microwave time, 40 s; and number of extraction cycles, 3. AFEs were concentrated with a rotary evaporator (RE-5203, Shanghai, China) and freeze-dried with a vacuum freeze dryer. AFEs used in all assays were liquid.
2.4 Analysis of the flavonoid content
The flavonoid content was determined using the method reported by Miliauskas et al. (2004), with some modifications. Five hundred microlitres of AFE, 4.5 mL of distilled water, 3 mL of a 5% NaNO2 solution and 0.3 mL of a 10% AlCl3 solution were mixed and reacted for 12 min at room temperature. Then, 2 mL of NaOH were added, and the absorbance was measured at 510 nm using an ultraviolet–visible spectrophotometer (Cary 50 Bio, Victoria, Australia). Rutin (0.2 mg/mL) was used as the standard solution.
2.5 Analysis of the total saponin content
The method described by Hiai et al. (1976) was used to determine the total saponin content. Five hundred microlitres of AFE were evaporated in a water bath at 45 °C. Then, a 5% vanillin–glacial acetic acid solution (0.2 mL) and perchloric acid (0.8 mL) were added. The mixtures were heated in a constant temperature water bath at 60 °C for 15 min. The samples were transferred to an ice bath. After 5 min, 5 mL of glacial acetic acid were added, and the absorbance was measured at 560 nm. Oleanolic acid (1 mg/mL) was used as the standard solution.
2.6 Analysis of the total anthocyanin content
The total anthocyanin content of AFEs was determined using the pH-differential method (Lee et al., 2005). Two millilitres of AFE were diluted 10 times with pH 1 and pH 4.5 buffer and then incubated at room temperature for 60 min. The absorbance was measured at 520 nm and 700 nm, respectively. The total anthocyanin content was calculated using the method described by Lee et al. (2005) with the following equation:
where A= (A520 nm – A700 nm)pH 1.0 – (A520 nm – A700 nm)pH 4.5; MW = 449.2 g/mol for the molecular weight of cyanidin-3-glucoside; DF = fold dilution; l = path length in cm; V = sample volume; and ε = 26900 L.mol/cm for the molar extinction coefficient of cyanidin-3-glucoside.
2.7 Uhplc-MS/MS analysis
The AFEs were passed through a macroporous resin column and sequentially eluted with deionized water and 20% and 80% ethanol. The solutions eluted with the 80% ethanol solution were collected, concentrated with a rotary evaporator, and freeze-dried to obtain total saponins. The total saponins were dissolved in methanol (1 mg/mL) and filtered with a 0.22 μm filter membrane.
The chromatographic analysis was conducted using an ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) at 40 °C. A volume of 5 μL was injected at a flow rate of 0.4 mL/min. The mobile phase consisted of 0.1% formic acid (A) and pure acetonitrile (B). The elution programme was as follows: 0–3 min, 5–15% B; 3–3.5 min, 15% B; 3.5–6 min, 15–30% B; 6–6.5 min, 30% B; 6.5–12 min, 30–70% B; 12–12.5 min, 70% B; 12.5–18 min, 70–100% B; and 18–20 min, 100% B.
Mass spectrometry was carried out in negative ion detection mode, and the scanning ranges of the primary and secondary mass spectra were 100–1200 m/z and 50–1000 m/z, respectively. Each gas path used nitrogen. The specific parameters were as follows: ion spray floating voltage, 5500 V; source gas 1, 50 psi; source gas 2, 50 psi; temperature, 550 °C; declustering potential, 90 V; and collision energy, 10 V.
2.8 Free amino acid analysis
The method described by Yoo and Chang (2016) was modified slightly to analyse the content of free amino acids. AF powders (50 mg per region) from Harbin, Benxi, Raohe, Jiaohe, and Mudangjiang were hydrolysed with 6 mol/L HCl for 22 h at 110 °C. The suspension was filtered into a 50 mL volumetric flask. One millilitre of the filtrate was dried under a vacuum at 45 °C, dissolved by adding 2 mL of sodium citrate buffer solution, and filtered with a 0.22 μm filter membrane. The amount of amino acids was determined using an amino acid analyser (L-3000, Jiangsu, China). A mixed amino acid standard working solution (100 nmol/mL) was used as the standard solution.
2.9 Cell culture
HEK293 cells were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in MEM supplemented with 1% PSS and 10% FBS at 37 °C in an incubator with 5% CO2. HEK293 cells (5–6 × 103 cells/mL) were seeded into flat-bottomed plates and cultured for 24 h in a CO2 incubator.
2.10 Analysis of cytotoxicity/proliferation and protective effects
Once all the cells grew to approximately 70–80% confluence, the medium was removed, and the cells were pretreated with AFE (1–200 μg/mL) for 24 h. Normal cells without treatment were regarded as the control group. Cell viability was measured using a CCK-8 assay to evaluate the cytotoxicity or proliferation effect of the AFEs. The cells were pretreated with AFE (1–50 μg/mL) for 24 h. Then, the medium was gently removed, and the cells were exposed to 400 μM H2O2 for 4 h. HEK293 cells incubated with H2O2 (400 μM) served as the control group, and the protection potential of the control group was designated as 0.0%. Cell viability was measured using a CCK-8 assay to evaluate the protective effects of AFE on H2O2-induced oxidative stress.
2.11 Cck-8 assay
Ten microlitres of CCK-8 solution were added to the cells and incubated at 37 °C for 3 h. The 96-well plate was subsequently placed in a multimode microplate reader (Tecan, Infinite M200 Pro) to measure the absorbance at 450 nm. Six parallel experiments with cells in each treatment group were performed, and all the assays were repeated three times. Cell viability was calculated using the following equation:
2.12 Measurement of intracellular ROS production
A DCFH-DA fluorescent probe was used to determine intracellular ROS production. Normal cells without treatment were used as the control group, and H2O2-treated cells served as the injury group. Incomplete medium containing DCFH-DA (10 μM) was added to the cells, and the cells were then incubated at 37 °C for 20 min. The DCFH-DA fluorescent probe was removed, and the cells were washed three times with phosphate-buffered saline (PBS). The green fluorescence in HEK293 cells was analysed using a fluorescence microscope (BX43 upright microscope, Olympus, Long Island, New York, USA). The fluorescence intensity was immediately detected using a multimode microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
2.13 Measurement of MDA levels, antioxidant enzyme activities, and T-AOC
Normal cells without treatment were used as the control group, and H2O2-treated cells served as the injury group. Cells digested with trypsin were washed twice with PBS. The remaining cell pellet was lysed in ice-cold RIPA lysis buffer containing 1 mM PMSF for 40 min. The SOD, GSH-Px, and CAT activities and T-AOC and MDA contents were determined according to the instructions provided with the corresponding assay kits. All assays were repeated at least three times.
2.14 Radical scavenging activity measurement
The radical scavenging activity of AFEs (1 g/mL) was determined. The ABTS.+, DPPH., FRAP, hydroxyl radical, superoxide anion, H2O2 and ferrous ion-chelating abilities were determined using the methods described by Re et al. (1999), Yen and Chen (1995), Benzie and Strain (1996), Halliwell et al. (1987), Chan and Bielski (1974), Jayaprakah et al. (2001), and Singh and Rajini (2004), respectively. The results are presented as mmol of Trolox per kg d.m. All assays were repeated at least three times.
2.15 Statistical analysis
All assays were conducted three times independently, and the data are reported as the mean ± standard deviation. The data were subjected to one-way analysis of variance (ANOVA) using SPSS 20.0 software to analyse the statistical significance. Significance was established at a level of P < 0.05. Principal component analysis (PCA) was performed using SPSS 20.0 software to assess similarity and for the classification of data.
3 Results
3.1 Contents of flavonoids, anthocyanins and saponins in AFEs from five regions
Fig. 2 presents the variations in flavonoid, anthocyanin and saponin contents in AFEs from five different regions. Significant differences were observed in the AFEs from the five regions (p < 0.05). The flavonoid, anthocyanin, and saponin contents in the AFE from Harbin were higher than those in AFEs from the other four regions. The anthocyanin contents in the AFEs from Jiaohe and Benxi were significantly lower than those in the AFEs from Harbin, Raohe and Mudanjiang. The lowest saponin content was detected in the AFE from Raohe among the five regions (211.20 ± 36.64 mg OA/g). The flavonoid, anthocyanin and saponin contents in the AFE from Mudanjiang were at intermediate levels among the AFEs from five regions.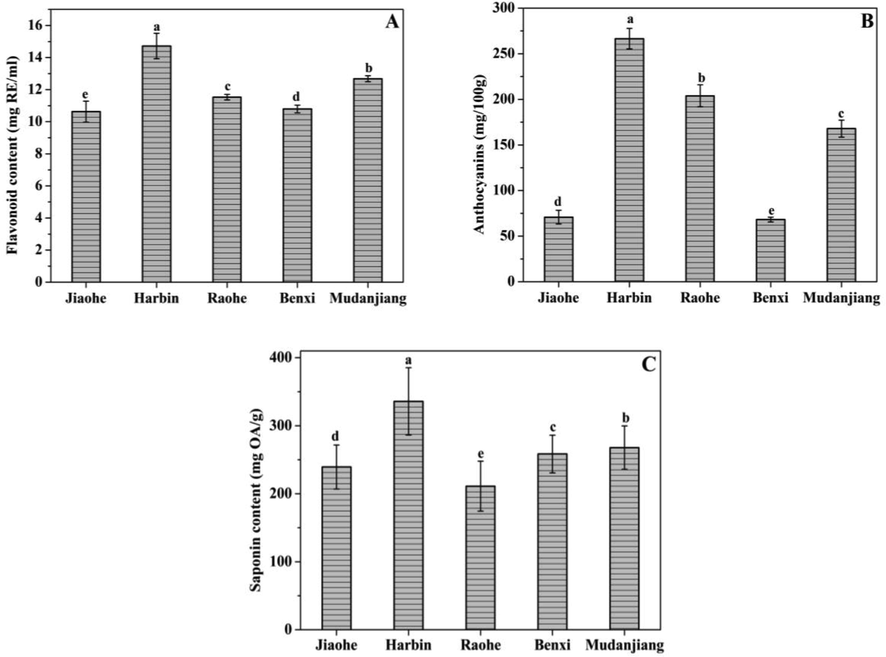
Flavonoid (A), Anthocyanins (B), and Saponin (C) content of Aralia elata (Miq.) Seem fruits. Vertical bars indicate mean values ± SD. The different letters within each column indicate significant differences at p < 0.05.
3.2 Identification of saponins
Among the complex and diverse active ingredients of A. elata, saponins are the main effective ingredient. Saponin components are important for the functional properties of A. elata. Research scholars also have used araloside as their main research object (Han et al., 2021). The saponins in the AFEs were analysed qualitatively in this experiment to assess the quality of AFs at the monomer compound level. As shown in Fig. 3, approximately 11 visible peaks were determined, 6 of these peaks were identified (Table 2). AFEs presented similar qualitative components in fruits from different regions. Among the components that were identified, araloside A was present at the highest concentration in AFs from all regions except Benxi. The highest araloside A content was detected in the AFE from Harbin. Chikusetsusaponin IV was the second most abundant compound in the AFEs from the five regions among all the identified components. Congmuyenoside X was present at the highest concentration among known ingredients in the AFE from Benxi.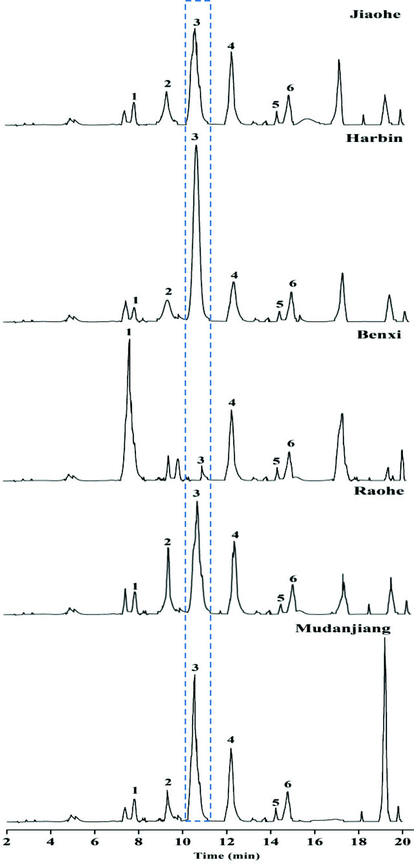
Major components of Aralia elata (Miq.) Seem fruits determined using UHPLC-MS/MS.
Peak
Compound Name
tR (min)
[M + Na]+
1
Congmuyenoside X
7.79
1127.5609
2
Aralosdie C
9.28
1111.5283
3
Araloside A
10.56
949.4743
4
Chikusetsusaponin IV
12.18
949.4813
5
Acutoside A
14.22
803.4506
6
Momordin Ic
14.76
764.4346
3.3 Amino acid contents
The contents of 17 amino acids in AFs obtained from the five origins were analysed, including 8 essential amino acids and 9 nonessential amino acids (Table 3). Significant differences in the contents of amino acids were observed in AFs from the five different regions (p < 0.05). The sum of the contents of nonessential amino acids and essential amino acids in AFs ranged from 2.22 ± 0.25% to 3.60 ± 0.32% and 3.68 ± 0.27% to 5.41 ± 0.41%, respectively. Total amino acid contents of 7.75 ± 0.34%, 6.93 ± 0.21%, 5.89 ± 0.44%, 8.17 ± 0.29%, and 9.01 ± 0.36% were observed in the AFEs from Harbin, Jiaohe, Raohe, Mudanjiang, and Benxi, respectively. According to the experimental results, L-glutamic acid was dominant in the AFs, while L-methionine and L-tyrosine were the least abundant amino acids. Mean ± standard deviation (n = 3). The mean values followed by different superscripts in the same row are significantly different (p < 0.05).
Amino acid
Aralia elata(Miq.) seem Friuit
Harbin
Jiaohe
Raohe
Mudanjiang
Benxi
Essential amino acid
L-Threonine
0.33 ± 0.021c
0.31 ± 0.058d
0.27 ± 0.022e
0.35 ± 0.052b
0.42 ± 0.051a
L-Valine
0.39 ± 0.014c
0.33 ± 0.074d
0.31 ± 0.023e
0.41 ± 0.078b
0.44 ± 0.050a
L-Methionine
0.06 ± 0.005c
0.07 ± 0.003b
0.07 ± 0.002b
0.07 ± 0.007b
0.08 ± 0.009a
L-Isoleucine
0.37 ± 0.058c
0.33 ± 0.059d
0.29 ± 0.032e
0.40 ± 0.056b
0.46 ± 0.022a
L-Leucine
0.71 ± 0.067c
0.64 ± 0.026d
0.53 ± 0.034e
0.75 ± 0.065b
0.85 ± 0.075a
L-Phenylalanine
0.39 ± 0.074c
0.34 ± 0.037d
0.29 ± 0.012e
0.40 ± 0.033b
0.46 ± 0.016a
L-Lysine
0.56 ± 0.057c
0.48 ± 0.061d
0.33 ± 0.043e
0.60 ± 0.076b
0.66 ± 0.064a
L-Histidine
0.20 ± 0.013c
0.18 ± 0.028d
0.13 ± 0.009e
0.21 ± 0.012b
0.23 ± 0.035a
Total essential amino acid
3.01
2.68
2.22
3.19
3.60
Non-essential amino acid
L-Aspartic acid
0.85 ± 0.084c
0.71 ± 0.063d
0.61 ± 0.054e
0.89 ± 0.067b
0.98 ± 0.076a
L-Glutamic acid
1.50 ± 0.194c
1.35 ± 0.196d
1.19 ± 0.128e
1.57 ± 0.117a
1.55 ± 0.098b
L-Gycine
0.42 ± 0.047c
0.37 ± 0.051d
0.33 ± 0.034e
0.45 ± 0.045b
0.52 ± 0.019a
β-Alanine
0.43 ± 0.012c
0.41 ± 0.065d
0.34 ± 0.049e
0.44 ± 0.069b
0.53 ± 0.043a
L-Cystine
0.09 ± 0.001e
0.12 ± 0.011b
0.11 ± 0.013c
0.10 ± 0.005d
0.13 ± 0.012a
L-Tyrosine
0.06 ± 0.003c
0.05 ± 0.003d
0.03 ± 0.003e
0.07 ± 0.001b
0.10 ± 0.013a
L-Arginine
0.53 ± 0.032c
0.49 ± 0.048d
0.35 ± 0.043e
0.58 ± 0.054b
0.64 ± 0.045a
Proline
0.41 ± 0.047b
0.34 ± 0.042e
0.39 ± 0.024d
0.40 ± 0.023c
0.45 ± 0.023a
L-Serine
0.45 ± 0.068c
0.41 ± 0.086d
0.33 ± 0.058e
0.47 ± 0.045b
0.51 ± 0.054a
Total non-essential amino acid
4.74
4.25
3.68
4.98
5.41
Total free amino acid
7.75
6.93
5.89
8.17
9.01
3.4 Cytotoxicity/proliferation impact of AFEs in HEK293 cells
The cytotoxicity and effect of AFEs from different origins on the proliferation of HEK293 cells were tested using a CCK-8 assay (Fig. 4A). In this study, cell viability between 95% and 105% was considered to indicate no cytotoxicity or effect on proliferation. Experimental data showed that the cell viability presented concentration-dependent increases after an incubation with increasing concentrations of AFEs from Jiaohe, Harbin and Benxi. Concentration-dependent decreases in viability were observed after an incubation with increasing concentrations of AFEs from Raohe and Mudanjiang. In particular, the cell viability reached 116.76 ± 1.83% when the concentration of AFE from Harbin was 200 μg/mL. Moreover, when the concentration exceeded 50 μg/mL, AFEs from Jiaohe, Harbin and Benxi altered the proliferation of HEK293 cells, while AFEs from Benxi and Raohe exerted cytotoxic effects.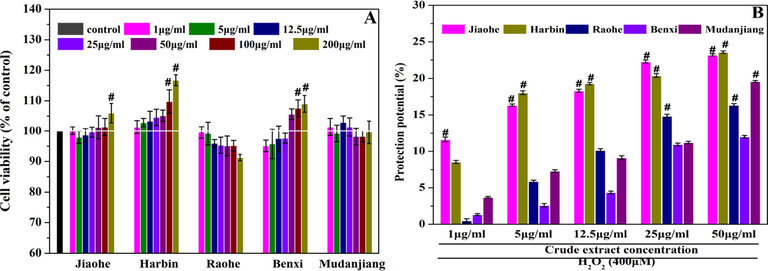
The effect of Aralia elata (Miq.) Seem fruit extract on HEK293 cells. (A) Cytotoxicity effect of Aralia elata (Miq.) Seem fruit extract on HEK293 cells. Normal cells without treatment were regarded as the control group. (B) Protective effect of Aralia elata (Miq.) Seem fruit extract on H2O2-induced oxidative damage in HEK293 cells. HEK293 cells incubated with H2O2 (400 μM) were used as the control group, and the protection potential of the control group was designated as 0.0%. Vertical bars indicate mean values ± SD. # statistically significant difference versus control group (p < 0.001).
3.5 Protective effects of AFEs on H2O2-induced oxidative stress
An oxidative stress model was established by incubating cells with 400 μM H2O2 to evaluate the protective effects of AFE on oxidative injury. A concentration-dependent increase in the potential protective effect of AFEs from the five regions on oxidative stress-induced damage was observed (Fig. 4B). The protective potentials of the AFEs from Jiaohe and Harbin were 18.31 ± 0.20% and 19.28 ± 0.15% (12.5 μg/mL), respectively, which were significantly higher than those of Raohe (10.15 ± 0.20%), Benxi (4.37 ± 0.18%), and Mudanjiang (9.14 ± 0.27%). The protective effects of AFEs from Raohe and Harbin were 23.3 and 17.1 times higher than those of AFEs from Jiaohe (1 μg/mL), respectively. Based on the results, AFEs inhibited H2O2-induced oxidative stress injury, but the degree of inhibition of AFEs from different regions was significantly different (p < 0.05).
3.6 Effects of AFEs on ROS generation and MDA levels
Fig. 5 reflects the relative ROS production in HEK293 cells and the green fluorescence intensity. The relative ROS production increased rapidly after HEK293 cells were incubated with H2O2, while AFEs reversed this increase. When the concentration of an AFE from any of the five studied regions exceeded 25 μg/mL, it effectively reduced the relative intracellular ROS production compared with that of the injured group to a level that was even lower than the ROS level of normal cells. When the concentration was 12.5 μg/mL, AFEs from Harbin, Raohe and Jiaohe significantly reduced the ROS levels induced by H2O2. The AFE obtained from Raohe significantly inhibited ROS production at any concentration (1–50 μg/mL). The fluorescence intensity in photos of cells treated with 50 μg/mL extracts were obtained using laser scanning confocal microscopy. The trend for the fluorescence intensity was consistent with that of relative ROS production. MDA is one of the end products of lipid peroxidation induced by ROS. The MDA level in the injured cells increased substantially compared with that in the control cells. However, the MDA level in the cells pretreated with increasing concentrations of an AFE gradually decreased (Fig. 6F). Overall, the AFE from Harbin reduced the MDA levels to the greatest extent and the AFE from Mudanjiang reduced the levels to the lowest extent among AFEs from the five regions. When the AFE concentration was 25 μg/mL, the order of reduction in MDA levels was Harbin > Benxi > Raohe > Jiaohe > Mudanjiang. Therefore, AFEs resulted in increased resistance to H2O2-induced oxidative stress, mainly by reducing intracellular ROS production and MDA levels.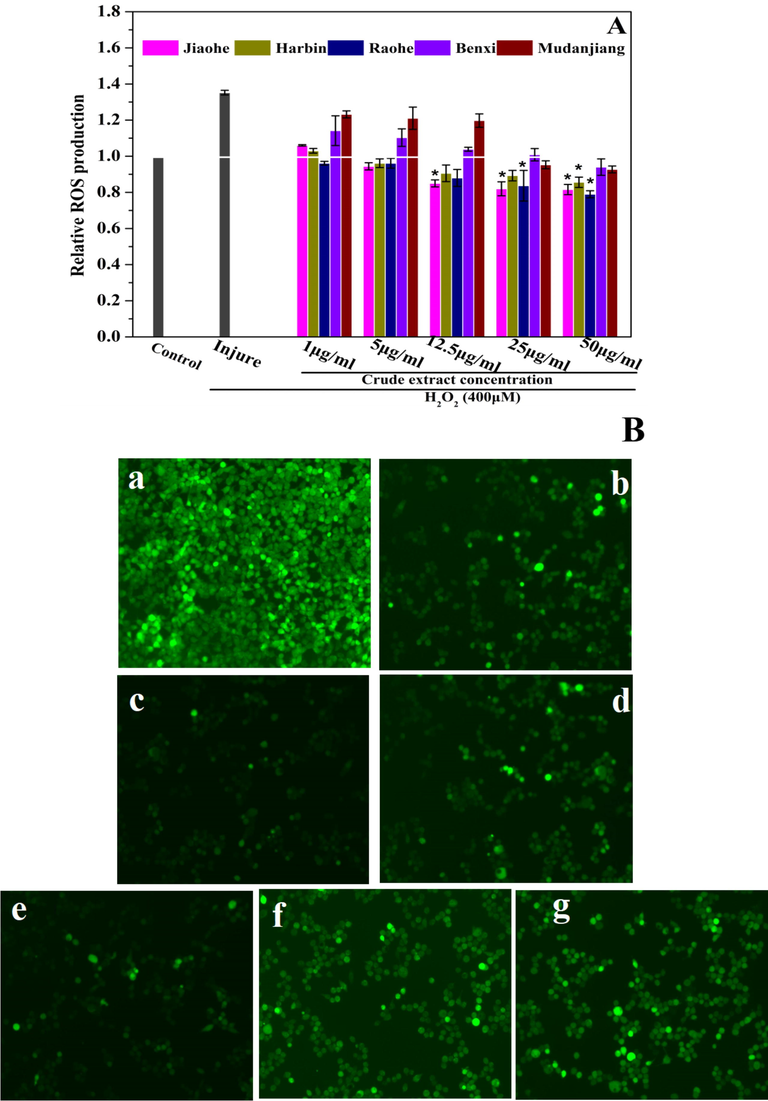
The effect of Aralia elata (Miq.) Seem. fruit extract on reactive oxygen species (ROS) generation. (A) Production of intracellular ROS was measured using the fluorescence probe DCFH-DA. (B) Fluorescence images in HEK293 cells were collected by laser scanning confocal microscopy (200 × ), 12.5 μg/mL Aralia elata (Miq.) Seem. fruit extract + 400 mM H2O2. (a) control group; (b) injure group; (c) Jiaohe; (d) Harbin; (e) Raohe; (f) Mudanjiang; (g) Benxi. Vertical bars indicate mean values ± SD. *p < 0.001 versus the injury group.
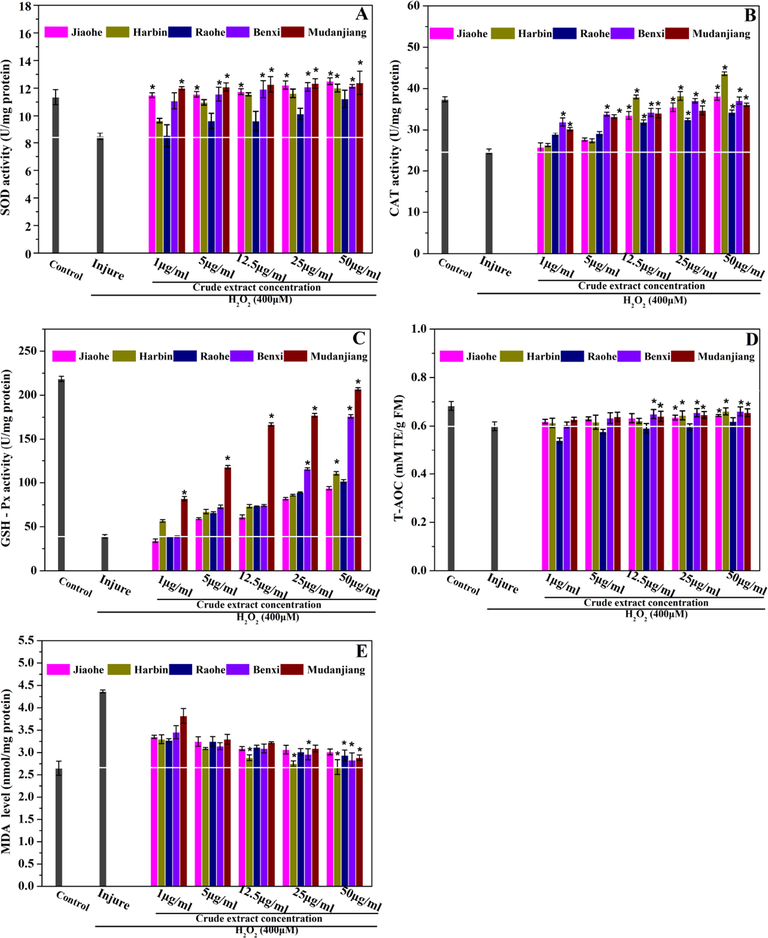
The effect of Aralia elata (Miq.) Seem. fruit extract on antioxidant status of H2O2-exposed HEK293 cells. (A) Superoxide dismutase (SOD) activity; (B) Catalase (CAT) activity; (C) Glutathione peroxidase (GSH-Px) activity; (D) Total antioxidant capacity (T-AOC) activity; (E) Malondialdehyde (MDA) level. Vertical bars indicate mean values ± SD. * statistically significant differerces versus injure group (p < 0.001).
3.7 Regulatory effects of AFEs on antioxidant enzymes and T-AOC
Relevant kits were used to determine the antioxidant enzyme and total antioxidant activities in HEK293 cells. The results are shown in Fig. 6. CAT activity, SOD activity, GSH-Px activity, and T-AOC were significantly decreased in HEK293 cells damaged by H2O2 compared with those in the control group. Nevertheless, CAT activity, SOD activity, GSH-Px activity, and T-AOC were significantly increased in HEK293 cells pretreated with AFE. Moreover, the antioxidant enzyme activity and T-AOC levels remained similar. The SOD activity and T-AOC of the cells treated with the AFE obtained from Raohe were 12.38 ± 0.84 U/mg protein and 0.65 ± 0.01 mM TE/g FM (50 μg/mL), respectively, which were significantly lower than those of cells treated with the AFEs obtained from the other four regions. The highest CAT activity was observed in cells treated with the AFE (50 μg/mL) obtained from Harbin, followed by the AFEs obtained from Jiaohe, Benxi, Mudanjiang and Raohe. The GSH-Px activity in cells treated with the AFEs obtained from the five regions was in the following order: Mudanjiang > Benxi > Harbin > Raohe > Jiaohe (50 μg/mL). Notably, the T-AOCs of cells treated with AFEs obtained from all regions except Raohe were not significantly different, but the differences were significant compared with the injury group.
3.8 Effects of AFEs on the free radical scavenging capacity
Seven assays were used as supplementary methods to evaluate the chemical antioxidant potential of AFEs (Fig. 7). Significant differences were observed in the antioxidant activities of AFEs from different regions. Based on descriptive statistics, the ferrous ion-chelating ability and hydroxyl radical and superoxide anion scavenging activities were normally distributed, and the values of the remaining indicators were positively skewed. The highest DPPH. free radical scavenging ability was observed for the AFE from Raohe among the AFEs, followed by those from Harbin, Mudanjiang, Benxi, and Jiaohe. The ABTS.+ radical scavenging activity of the AFEs ranged from 1.69 to 1.86 mmol TE/100 g dm, among which the AFE from Raohe showed the highest activity. The FRAP of AFEs ranged from 7.70 to 4.52 mmol TE/100 g dm in the order of Harbin > Mudanjiang > Raohe > Jiaohe > Benxi. The AFE from Jiaohe had the strongest ability to remove hydroxyl radicals among the AFEs from the five regions. Meanwhile, the AFE from Raohe had the strongest ability to remove superoxide anions. The ferrous ion-chelating ability of the AFEs from the five regions was significantly different, with the largest value observed for the Jiaohe AFE and the smallest value observed for the Harbin AFE. The H2O2 scavenging ability of the Harbin extracts was the strongest, and the difference between the AFEs of the remaining four regions was not significant.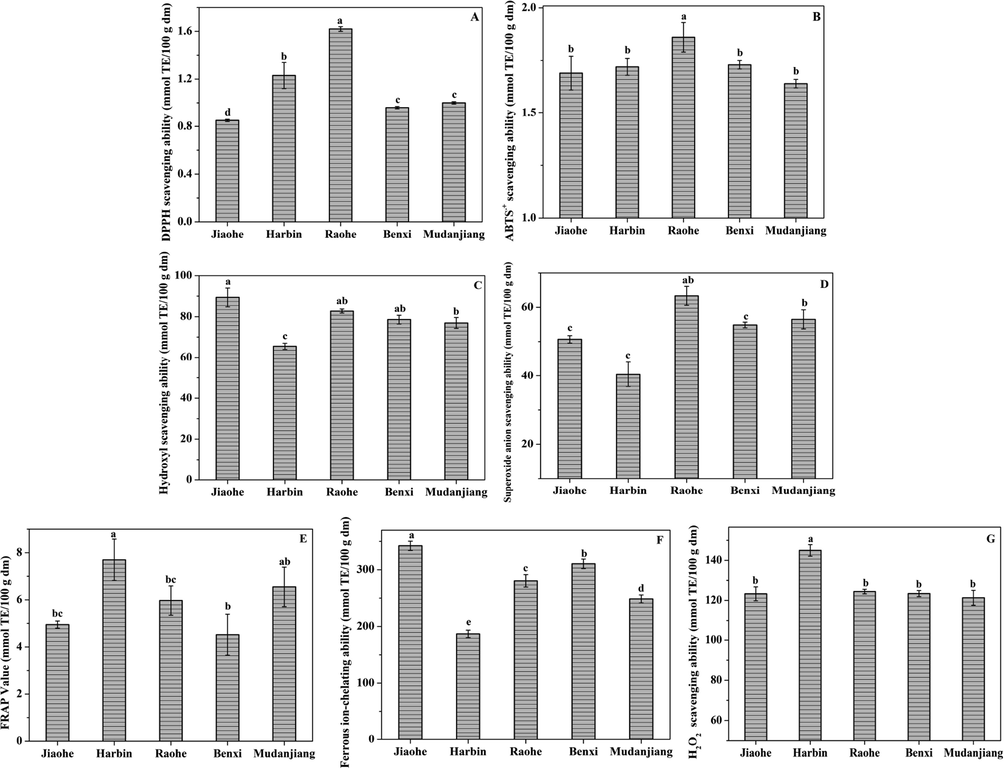
Analysis of the radical scavenging activity of the extracts of Aralia elata (Miq.) Seem fruits. (A) DPPH. free radical scavenging ability; (B) ABTS.+ radical scavenging activity; (C) Hydroxyl radicals scavenging ability; (D) superoxide anionradical scavenging ability; (E) Ferric reducing antioxidant power; (F) Ferrous ion-chelating ability; (G) H2O2 levels.
3.9 Correlation coefficient
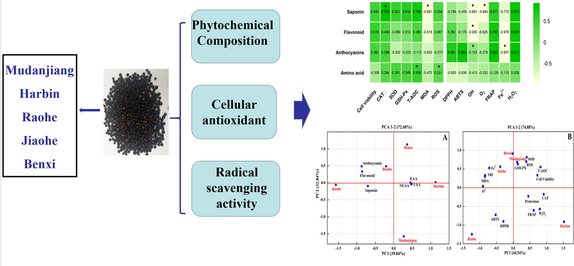
The correlation coefficient was calculated to clarify the relationship between the phytochemical components and the antioxidant activity in AFs. A positive and significant correlation was observed between saponin contents and cellular antioxidant activity (r = 0.908, CAT; r = 0.768, T-AOC; r = 0.521, SOD; r = 0.618, GSH-Px). In addition, the data showed a clear linear relationship between anthocyanin contents and the free radical scavenging capacity (r = 0.681, DPPH; r = 0.865, ABTS; r = 0.942, FRAP; r = 0.917, H2O2). However, the contents of flavonoids and amino acids exerted a minor effect on the cellular antioxidant capacity and the free radical scavenging capacity (Fig. 8). Thus, saponins had a substantial contribution to the cellular antioxidant capacity, and anthocyanins were important compounds affecting the free radical scavenging capacity.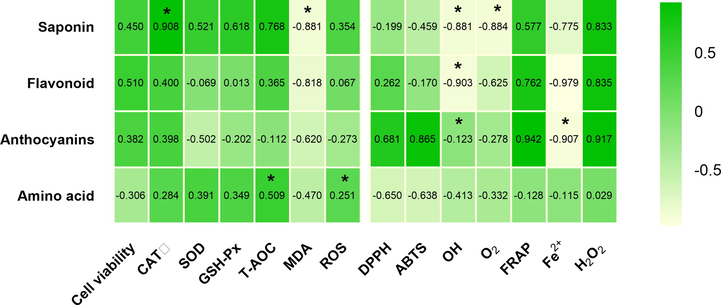
Correlation coefficients of phytochemical compounds and antioxidant-related index. CAT: the activity of catalase; SOD: the activity of superoxide dismutase; GSH-Px: the activity of glutathione peroxidase; T-AOC: the total antioxidant capacity; MDA: the malondialdehyde levels; ROS: the production of reactive oxygen species; DPPH: the DPPH. free radical scavenging ability; ABTS: the ABTS.+ radical scavenging activity; FRAP: the ferric reducing antioxidant power; Fe2+ : the ferrous ion-chelating ability; H2O2: the H2O2 levels. * The correlation is significant at the 0.05 level.
3.10 Principal component analysis
PCA was used as an unsupervised method to examine the similarity among AFs from Harbin, Benxi, Raohe, Jiaohe, and Mudangjiang. The phytochemical compounds and antioxidant capacities of AFEs from five regions were analysed using PCA. As shown in Fig. 9A, principal component 1 (PC1), the first component of phytochemical compounds, accounted for 39.84% of the total variance, and PC2 represented 32.84% of the total variance. Harbin data were near the X-axis and separated by essential amino acids, nonessential amino acids, total free amino acids and P. The Jiaohe data were also close to the X-axis but had a large negative score on the PC1 axis. Benxi and Raohe data had large positive scores on PC2 and were clearly separated from other regions. They were mainly affected by anthocyanins and flavonoids. Mudanjiang data had a negative score on the PC2 axis, which was opposite to the Benxi and Raohe data. The antioxidant capacity was evaluated using PCA, and the classification of regions was different from the classification obtained from the phytochemical compound PCA. PC1 and PC2 accounted for 74.88% of the total variance, of which PC1 explained 44.54% and PC2 explained 30.34% (Fig. 9B). Benxi, Mudanjiang and Jiaohe data were grouped into one cluster. Harbin and Raohe data were located at relatively opposite ends of the PC1 axis and had large negative scores on PC2. Raohe data were mainly affected by the ABTS.+ and DPPH. radical scavenging activities. Meanwhile, Harbin data were separated according to the FRAP assay, CAT activity, protective effect on oxidative stress, and H2O2 scavenging ability.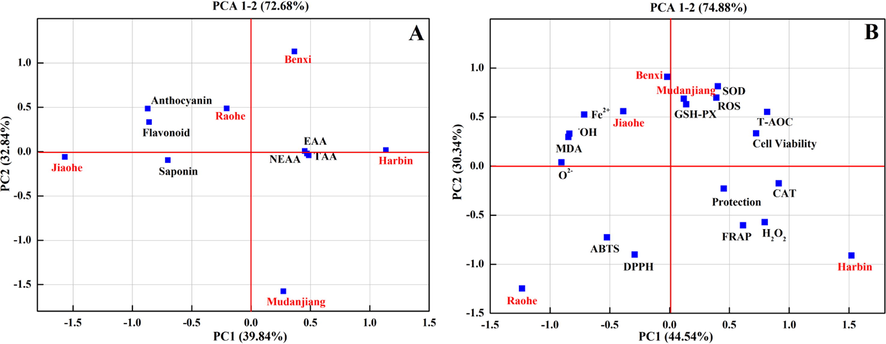
Principal component analysis characterization (PC1 vs PC2) of Aralia elata (Miq.) Seem. fruit origin using the phytochemical compounds (A, 16 variables) and antioxidant-related index (B, 15 variables). EAA: Essential amino acids; NEAA: Non-essential amino acid ; TAA: Total free amino acids; Cell Viability: Cytotoxicity of AE fruits extracts in HEK293 cells; Protection: Protection against H2O2-oxidative stress; CAT: the activity of catalase; SOD: the activity of superoxide dismutase; GSH-Px: the activity of glutathione peroxidase; T-AOC: the total antioxidant capacity; MDA: the malondialdehyde levels; ROS: the production of reactive oxygen species; DPPH: the DPPH. free radical scavenging ability; ABTS: the ABTS.+ radical scavenging activity; FRAP: the ferric reducing antioxidant power; Fe2+ : the ferrous ion-chelating ability; H2O2: the H2O2 levels.
4 Discussion
The stem bark, root bark and leaves of A. elata contain many active ingredients, mainly saponins, flavonoids, fats, sugars, anthocyanins, aromatic oils, volatile oils, amino acids, proteins, and inorganic compounds (Hwang et al., 2017; Liu et al., 2016). We hypothesized that AFs also contained similar active ingredients. Therefore, flavonoid, saponin, anthocyanin, and amino acid contents in AFs from the five regions were determined in this study. Anthocyanins repair genes and maintain DNA integrity (Trivedi et al., 2016). A large number of studies have shown that anthocyanins from vegetables and fruits, especially berries, are beneficial for reducing age-related oxidative stress. For example, black currants rich in anthocyanins reduce the oxidative stress-induced damage to blood vessels in aged rats (Chaker et al., 2020). Anthocyanins in blueberries ameliorate CCl4-induced liver fibrosis by regulating oxidative stress and inflammatory factors (Sun et al., 2018). A positive and significant correlation was found between anthocyanin contents and chemical antioxidant activity in some small berries, such as raspberries, sweet potatoes and cranberries (Nile et al., 2015). This finding suggested that anthocyanins might determine the antioxidant capacity of these berries to a certain extent. The free radical scavenging activity of AFEs also showed a significant positive correlation with the anthocyanin contents, consistent with the results reported by Nile et al. (2015). Flavonoids are beneficial to human health because they possess anti-inflammatory, antioxidant, and immunoregulatory activities (Bayatet al., 2021). Flavonoids extracted from the leaves of Murraya paniculata (L.) Jack increase the expression levels of the Nrf2 and HO-1 genes and inhibit oxidative stress, inflammation and apoptosis (Zou et al., 2021). Flavonoids in fruits, vegetables, and herbs are also the main factors contributing to the antioxidant activity. For example, flavonoids and polyphenols in peanut skin are significantly correlated with antioxidant activity, while the correlation between anthocyanins and antioxidant activity was not significant. The antioxidant activity of peanut skin is not directly related to the colour intensity, and the presence of colourless flavonoids might explain the higher antioxidant activity of peanut skin (Shem-Tov et al., 2012). As shown in the present study, AF contained a certain amount of flavonoids, but their effect on the antioxidant activity of AF was not significant. A mass of saponins usually has high surface activity and forms complexes with sterols on the plasma membrane of fungi, plants and animals. In addition, the integrity of the plasma membrane is also affected by saponins. Previous studies have shown that saponins promote the absorption of certain molecules. For example, Quil-A saponins stimulate the absorption of human gamma globulin in Oreochromis mossambicus (Jenkins et al., 1991). The saponin contents in AF were determined in this study, and saponins were an important contributor to the cellular antioxidant activity of AFs. However, the reasons why saponins may be an important component of AFEs that inhibit oxidative stress rather than anthocyanins, flavonoids and amino acids remain unclear and require exploration in future studies. The contents of flavonoids, saponins, and anthocyanins in AFs from five different regions were significantly different, and the AFE from Harbin had the highest contents (Fig. 2). The presence of these components provided a basis for AFEs to inhibit H2O2-induced oxidative stress damage. Harbin data were also separately divided into a cluster according to the PCA results (Fig. 9A). Many factors might have caused this phenomenon, including different growth environments, including latitude, longitude, altitude, and temperature differences. Environmental stimulation contributes to the accumulation of antioxidants in plants, thereby improving fruit quality in terms of the antioxidant content and health benefits. The contents of amino acids are very important for evaluating the quality and nutritional value of AFs. Different types of amino acids obtained from the daily diet play different roles in the body. AFs from the five zones were enriched in L-glutamic acid (Table 2). L-Glutamic acid is beneficial for the growth and development of human skeletal muscles, regulation of gene expression and reducing the accumulation of excess fat (Wu, 2009).
Hydrogen peroxide is an important metabolite in the redox process, and it is also a central signalling molecule that regulates redox and signalling pathways in physiological oxidative stress. When the cellular H2O2 concentration is 1–10 nM, the cell is in a redox steady-state. High H2O2 concentrations trigger the oxidative stress response by activating Nrf2 and NF-KB. An H2O2 concentration greater than 100 nM will cause oxidative damage, leading to DNA and protein damage, destroying the redox signalling pathway and eventually leading to growth arrest and cell death (Sies, 2017). A large gradient in the H2O2 concentration has been observed inside and outside the cell, and the extracellular concentration exceeds the intracellular concentration by more than 100 times (Sies, 2017). We chose 400 μM H2O2 to establish a model of oxidative stress-induced damage, which is the same as the oxidative stress model used by Liu et al. (400 μM H2O2) (Liu et al., 2016a,b).
Redox homeostasis plays a fundamental and essential role in regulating basal metabolism and maintaining the normal functions of an organism (Mendez-Pfeiffer et al., 2020; Bimbirait-Survilien et al., 2021). High intracellular ROS accumulation prevents the self-generated redox defence system from removing excess ROS, and redox homeostasis will be replaced by oxidative stress (Sies, 2015). Excess ROS damage biological molecules and lead to the development and progression of diseases (Halliwell, and Gutteridge, 1985). The AFEs from five different regions effectively eliminated the accumulation of ROS in HEK293 cells (Fig. 5). Therefore, we suspected that AFE has potential in preventing diseases caused by oxidative stress. Previous studies have also reported that many natural ingredients, such as polyphenolic compounds in strawberries and honey, also exert the same effect (Battino et al., 2021). By calculating the correlation coefficient, we found that the saponins in AFEs had a substantial contribution to the cellular antioxidant activity of the extract (Fig. 8). Therefore, saponins may be one of the key components of AFE involved in eliminating intracellular ROS. MDA is an important product of lipid peroxidation induced by ROS and is often used as a biomarker of oxidative stress levels. The AFEs also reduced MDA levels, with the AFE from Harbin exerting the best effect (Fig. 6F). Many natural active substances also reduce MDA levels. For example, L-ascorbic acid protects against cellular oxidative stress damage by reducing the cellular MDA content (Zhang et al., 2018). The antioxidant defence systems of organisms (including enzymatic defences and nonenzymatic defences) are activated under oxidative stress conditions (Regoli et al., 2014). Antioxidant enzymes and nonenzymatic antioxidants work together in complex antioxidant defence networks to remove excess ROS, thereby protecting organisms from oxidative damage. AFEs effectively inhibited H2O2-induced damage by increasing the activity of antioxidant enzymes and the antioxidant defence system (Fig. 6). These results were similar to those reported by Chang et al. (2018). Antioxidant enzymes (SOD, GSH-Px, and CAT) and antioxidant proteins play important roles in the process of ROS clearance. Therefore, increasing the activity of antioxidant enzymes and antioxidant defence systems are effective strategies for inhibiting oxidative stress-induced damage. Nrf2 is a key transcription factor that is involved in cellular pathways related to oxidative stress (Xue et al., 2021). Under normal physiological conditions, Nrf2 and Keap1 form a complex in the cytoplasm. When cells are damaged by oxidative stress, Nrf2 is decoupled from Keap1. Free Nrf2 enters the cell nucleus and binds to antioxidant response elements. The expression of antioxidant enzyme-related genes is activated, and the activities of antioxidant enzymes such as SOD, CAT, and GSH-PX are increased. AFEs played an important role in maintaining the redox homeostasis of HEK293 cells by reducing ROS accumulation and increasing the activity of antioxidant enzymes. The protective effect of AFEs may be achieved by regulating the Nrf2 signalling pathway and then inducing the expression of antioxidant enzymes. This hypothesis must be verified in future research.
Generally, known natural phytochemicals have complex chemical properties, and thus we cannot use a single method to evaluate the antioxidant activity of certain plant extracts. Therefore, we used a series of detection methods to comprehensively evaluate the antioxidant activity of AFEs in this experiment (Fig. 7). The Benxi AFE was characterized by relatively low ABTS.+ and DPPH. radicals scavenging activities and phenolic contents (anthocyanins and flavonoids), and these results were further verified by the PCA results. These results were similar to those reported by Wojdyło et al. (2013), in which the polyphenol content affected the antioxidant properties of the extracts. The FRAP assay and H2O2 scavenging ability of the AFE from Harbin were higher, which might be related to the higher contents of saponins, anthocyanins and flavonoids. Previous studies have proven that polyphenols, especially anthocyanins, contribute substantially to the antioxidant activity of plant extracts (Wu et al., 2004). In the present study, we observed a significant correlation between anthocyanin content and the free radical scavenging activity (Fig. 8). Hydroxyl radicals are some of the strongest oxidative radicals among ROS and react with biological macromolecules to induce cytotoxicity (Ji et al., 2020). The AFEs from Mudanjiang and Benxi had high hydroxyl radical scavenging activities, consistent with the results shown in Fig. 5. Mudanjiang and Benxi were also divided into a cluster based on the PCA results.
5 Conclusions
In this study, the chemical composition, cellular antioxidant activity, and free radical scavenging ability of AFEs from five regions in Northeast China were evaluated. The experimental results showed that the region exerts substantial effects on the phytochemical composition and antioxidant properties of AFEs. According to the experimental results, the highest contents of saponins, flavonoids and anthocyanins were observed in fruits from Harbin among all regions. In the study of individual saponins, the highest concentration of arloside A was detected in the fruit from Harbin, and the highest concentration of congmuyenoside X was detected in the fruit from Benxi. In terms of quantity, the content of L-glutamic acid was the highest in fruits from all regions. The AFEs from Jiaohe and Harbin exerted the most significant protective effects on H2O2-induced oxidative damage. HEK293 cells treated with the AFE from Harbin showed the highest CAT enzyme activity, the lowest MDA level, and significantly reduced ROS production. In the test of free radical scavenging activity, the FRAP and H2O2 scavenging activities of the AFE from Harbin were the strongest. The AFE from Raohe had the strongest ability to remove DPPH., ABTS.+ and superoxide anions. Meanwhile, the Jiaohe extract had the strongest ferrous ion-chelating activity and hydroxyl radical scavenging activity. Saponins and anthocyanins were important contributors to the T-AOC of AFE. The evaluation of the free radical scavenging and cellular antioxidant activities of these AFEs provides insight into obtaining more effective antioxidants from medicinal and culinary plants. In addition, different cultivation regions may modulate the concentration of phytochemicals, which in turn affects the antioxidant activity. However, the AFE from Harbin was rich in a variety of active ingredients and had significant antioxidant activity, and thus it is worthy of further investigation. This study is very useful for the expansion of AF cultivation in China.
Funding
This work was supported by the National Key R&D Program of the 13th Five-Year Project of China: Research, Development, and Demonstration of the Technology for Ecological Sustainability of Typical Mountain Vegetable Resources in Forests of Northeast China (2016YFC0500307) and the First-class Discipline Team of Northeast Agricultural University, “Storage, Transportation and Processing of Forest Fruits and Vegetables”.







