Translate this page into:
Comprehensive chemical profiling and quantification of Shexiang Xintongning tablets by integrating liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry
⁎Corresponding authors. liping2004@126.com (Ping Li), yanghuacpu@126.com (Hua Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
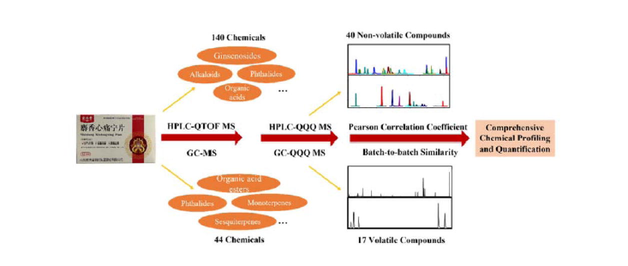
Shexiang Xintongning tablet (SXXTN) is a traditional Chinese medicine (TCM) preparation for the treatment of coronary heart disease (CHD) angina pectoris. However, due to the complexity of the compounds in SXXTN, the active chemical components responsible for the therapeutic effect are still ambiguous. The purpose of our study was to characterize the chemical profile of SXXTN and quantify the representative chemicals. The high-performance liquid chromatography coupled with time-of-flight mass spectrometry (HPLC-QTOF MS) method and gas chromatograph coupled with mass spectrometry (GC–MS) method were utilized to identify the chemical constituents of SXXTN. A total of 140 compounds including alkaloids, ginsenosides, organic acids, esters, triterpenes, phthalides and amino acid were identified in accordance with their retention times, accurate masses and characteristic MS/MS fragment patterns. Forty-four volatile components were characterized by GC–MS through NIST database matching. In the further research of quantitative analysis, 40 non-volatile compounds and 17 volatile compounds were determined and successfully applied for detecting in 7 batches of SXXTN samples by high performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry (HPLC-QQQ MS) and gas chromatograph coupled with triple-quadrupole tandem mass spectrometry (GC-QQQ MS) in multiple reaction monitoring (MRM) mode, respectively. The quantitative methods were verified in linearity, precision, repeatability stability and recovery. The above results indicated that the established method was practical and reliable for synthetical quality evaluation of SXXTN. In addition, our study might supplement the chemical evidence for disclosing the material basis of its therapeutic effects.
Keywords
Shexiang Xintongning tablet
traditional Chinese medicine prescription
Material basis
Multi-component content determination
GC–MS
HPLC-MS
- CHD
-
coronary heart disease
- ESI
-
electrospray ionization
- GC–MS
-
gas chromatograph coupled with mass spectrometry
- GC-QQQ MS
-
gas chromatograph coupled with triple-quadrupole tandem mass spectrometry
- HPLC
-
high-performance liquid chromatography
- HRMS
-
high resolution mass spectrometry
- HPLC-QTOF MS
-
high-performance liquid chromatography coupled with time-of-flight mass spectrometry
- HPLC-QQQ MS
-
high performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry
- LOD
-
limit of detection
- LOQ
-
limit of quantitation
- MRM
-
multiple reaction monitoring
- OA
-
oleanane
- PPD
-
20(S)-protopanaxadiol
- PPT
-
20(S)-protopanaxatriol
- RDA
-
Retro Diels-Alder
- RSD
-
relative standard deviation
- SXXTN
-
Shexiang Xintongning tablet
- TCM
-
traditional Chinese medicine
- TLC
-
thin-layer chromatography
- TICs
-
typical total ion chromatograms
Abbreviations
1 Introduction
Preparations of TCM formulae have been extensively utilized for clinical medication owing to their therapeutic effects on various diseases and relatively low side effects (Sun et al., 2017). Shexiang Xintongning tablet (SXXTN), a newly hospital preparation which has got a wide application in China to treat coronary heart disease angina pectoris (qi stagnation and blood stasis syndrome) and reportorial clinical studies have shown its efficacy (Shen and Lu, 2005). SXXTN comprised of Artificial Musk, Corydalis Rhizoma (Corydalis yanhusuo W. T. Wang.), Ginseng Radix et Rhizoma (Panax ginseng C. A. Mey.), Chuanxiong Rhizoma (Ligusticum chuanxiong Hort.), Styrax (Liquidambar orientalis Mill.) and Borneolum Syntheticum. All of the above crude drugs have been reported to be associated with the effect of SXXTN in the treatment of CHD. Here, Musk and Corydalis Rhizoma are reported to reduce infarct size and improve cardiac function (Li et al., 2008; Ling et al., 2010). The mechanisms of Ginseng Radix et Rhizoma in preventing coronary artery disease, myocardial hypertrophy, heart failure and arrhythmia are gradually being revealed (Zheng et al., 2012). Chuanxiong Rhizoma, Styrax and Musk have been proved to have the role of anti-myocardial ischemia (Liu et al., 2016; Wang et al., 2019; Wu et al., 2011). Besides, Borneolum Syntheticum as an adjuvant has been reported to provide new possibilities for the treatment of atherosclerosis (Zhang et al., 2017). Muscone, tetrahydropalmatine, ginsenoside, tetramethylpyrazine, cinnamic acid and borneol have been reported as important bioactive components relevant to treatment of CHD. Recently, SXXTN was revealed have the function of reducing oxidative stress-mediated damage and enhancing angiogenesis, and might play an important role in the treatment of myocardial infarction (Li et al., 2020). Obviously, the identification and detection of the main components in SXXTN is the premise and key to reveal its active ingredients. However, the chemical composition of SXXTN is complicated, having both volatile small molecules and non-volatile components such as alkaloids, organic acids and ginsenosides. In previous studies, the chemical constituents of each crude drugs in SXXTN have been reported (He et al., 2018; Zheng et al., 2018; Yang et al., 2021; Gurbuz et al., 2013; Ding et al., 2022; Sun et al., 2014), but little attention was paid to the integral chemical composition of SXXTN. Thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) have made powerful contributions for quality control of SXXTN (Zhang et al., 2016). Nevertheless, they were preferred to assay the limited components in SXXTN with difficult access to comprehensive chemical information. Thus, new methods for chemical profiling and quantification of SXXTN are necessary to solve the limitations of the previous techniques.
Comprehensive profiling of chemical constituents in TCM preparations is still facing great challenges on separation, detection and identification due to their structural complexities and diversities. Nowadays, various chromatographic coupled with mass spectrometry techniques, such as GC–MS (Su et al., 2008) and LC-MS (Xu et al., 2015; Luo et al., 2019), are widely used in the study of TCM preparations due to their combined superiorities of high-efficient separation and high-sensitive detection for multi-components in complex samples. On one hand, HPLC-QTOF MS and GC–MS could provide molecular weights and abundant fragment information for structure identification of compounds in TCM preparations. On the other hand, tandem mass spectrometers coupled to LC or GC systems were powerful tools for high-throughput quantitative analysis of TCM preparations owing to their high-selective simultaneous detection of multiple compounds with MRM modes. Therefore, the integration of LC-MS and GC–MS was a potentially effective approach for in-depth chemical profiling and quality control of SXXTN.
In this paper, HPLC-QTOF MS and GC–MS analysis methods were established for the global characterizations of complicated non-volatile and volatile ingredients in SXXTN. Besides, considering the representative components of all relevant single drugs in SXXTN, the abundance and activity of chemicals and the availability of reference substances, 57 compounds were further quantitatively analyzed by HPLC-QQQ MS and GC-QQQ MS method. The aims of our study were comprehensively qualitative and quantitative profiling the chemical basis of SXXTN, which were expected to improve the quality control, promote the pharmacological researches and expand the clinical applications.
2 Materials and methods
2.1 Reagents and materials
Seven batches of SXXTN were generously provided by Shandong Hongjitang Pharmaceutical Group Co., ltd. (Shandong, China) and listed in Table S1. A total of 101 reference standards and 3 internal standards were presented in Table S2. All standards were≥98 % by HPLC and 1H NMR analyses.
Ultrapure water (18.2 MΩ cm) for analysis was prepared by a Milli-Q water purification system (Millipore, Bedford, MA, USA). Methanol and acetonitrile (HPLC grade) were provided by Merck (Darmstadt, Germany), and formic acid (HPLC grade) were purchased from ROE (Newark, New Castle, DE, USA). Ethyl alcohol (HPLC grade) was offered by Sichuan Ruijingte Technology Co., ltd. (Sichuan, China).
2.2 Standard solution and samples preparations
The reference standards were solubilized by 75 % methanol-aqueous solution (v/v) to obtain 1.00 mg/mL reserve solution and diluted with appropriate solvent to a range of proper concentrations.
In qualitative analysis, for LC-MS, the SXXTN was ground into powder. SXXTN powder (0.3003 g) was accurately weighed and ultrasonic extracted (40 kHz, 500 W) with 5 mL 75 % methanol-aqueous solution (v/v) for 30 min. The extracts were centrifuged (13,000 rpm, 10 min, 4℃) before LC-MS analysis. For GC–MS, the powder (0.3000 g) was accurately weighed, then sonicated for 30 min at 40 kHz with 5 mL of ethanol. The filtrate was filtered by 0.45 μm filter membrane and centrifuged before sampling.
For quantitative analysis, to determine the non-volatile constituents, each batch of SXXTN powder (0.3 g) was weighed in three parallel times, then ultrasonic extracted (40 kHz, 500 W) with 5 mL 75 % methanol for 30 min. The filtrate was filtered by 0.45 μm filter membrane and centrifuged (13000 rpm, 10 min, 4℃). For alkaloids quantification (group A, 24 alkaloids), the supernatant was diluted after adding proper nitidine chloride (IS1, 1.11 μg/mL) as internal standard. For ginsenosides and acids quantification (group B, 14 ginsenosides, cinnamic acid and phenylalanine), the supernatant was diluted after proper saikosaponin C (IS2, 0.985 μg/mL) adding. To determine the volatile constituents, about 0.3 g the powder of SXXTN was extracted with 10 mL ethanol under ultrasonic conditions in ice-water bath for 20 min. The extraction was filtered through syringe filter (0.45 μm) and centrifuged. Isoborneol, borneol, 3-phenylpropyl cinnamate and cinnamyl cinnamate possessed significantly higher abundances in SXXTN comparing with other volatile components, indicating the large differences of contents among various compounds. Therefore, the supernatant was diluted 10 times before injecting to GC–MS for quantitative analysis of high-abundant volatile compounds (Group D), whereas directly injected for others with relatively low-abundances (Group C). And a certain amount of naphthalene (IS3, 23.2 μg/mL) was added to the supernatants as internal standard.
2.3 HPLC-QTOF MS analysis conditions
Agilent 1290 HPLC system (Agilent corporation, USA) was used to determine the non-volatile components of SXXTN. A ZORBAX Eclipse Plus C18 column (150 × 2.1 mm, 1.8 μm, Agilent Technologies, Santa Clara, USA) was used for sample separation. The mobile phase consisted of 0.1 % (v/v) formic acid in water (A) and acetonitrile (B) with the gradient elution set as follows: 0–3 min, 10 %-12 % B; 3–8 min, 12 %-17 % B; 8–20 min, 17 %–22 % B; 20–30 min, 22 %-35 % B; 30–45 min, 35 %-42 % B; 45–50 min, 42 %-60 % B; 50–57 min, 60 % B; 57–60 min, 60 %-80 % B; 60–68 min, 80 %-100 %. The flow rate was set at 0.4 mL /min, and the column temperature was maintained at 30 ℃. Sample volume was 1 μL for injection.
The Q-TOF mass spectrometer equipped with electrospray ionization (ESI) source was used to acquire data in positive and negative ion modes. The operation conditions were as below: drying gas (N2) temperature, 300 ℃; drying gas flow, 8.0 L/min; nebulizer gas (N2) pressure, 35 psig; sheath gas (N2) temperature, 350 ℃; sheath gas flow, 11.0 L/min; capillary voltage positive ion mode, 4000 V; negative ion mode, 3500 V; fragmentor voltage, 120 V; skimmer voltage, 65 V. Full-scan MS and MS/MS data was collected over the m/z range of 50–1500 using extended dynamic range. Collision energy of secondary mass spectrometry was set as 15 eV, 30 eV and 45 eV.
2.4 GC–MS analysis conditions
Compound identification was performed by Agilent 7890B GC system combined with Agilent 5977 Mass Selection Detector. Samples were separated by Agilent HP-5MS (30 m × 0.25 mm, 0.25 μm) column. The carrier gas was high purity helium, and the flow rate was 1 mL/min. Initial column temperature was 60 ℃, and programmed to rise at 20 °C/min to 85 °C (1 min held), 5 ℃/min to 100 ℃ (5 min held), 15 ℃/min to 150 ℃ (6 min held), 5 ℃/min to 200 ℃ (4 min held), rising at 5 ℃/min to 280 ℃ (5 min held). The injection volume was 1 μL and the splitting ratio was 30:1. The temperature of injector and aux heaters was controlled at 250℃ and 280℃, respectively. MS quadrupole and ion source temperature were maintained at 150℃ and 230℃, severally. MS data were recorded at 70 eV and acquired in full scan mode over the range of m/z 40–600.
2.5 HPLC-QQQ MS analysis conditions
The quantitative analysis was performed on the Shimadzu LCMS-8050 triple quadrupole tandem mass spectrometry detector (Shimadzu, Kyoto, Japan) with an Agilent Zorbax Eclipse Plus C18 column (2.1 × 150 mm, 1.8 µm, Agilent Technologies, Santa Clara, USA). For group A, 0.1 % (v/v) formic acid water (A) and acetonitrile (B) were used as mobile phases, and the gradient elution procedure was as follows: 0–12 min, 19 %-20 % B; 12–14 min, 20 %-35 % B; 14–16 min, 35 %-90 % B; 16–19 min, 90 %-100 % B. For group B, the mobile phase was water (A) and acetonitrile (B), with the following gradient elution: 0–3 min, 10 %-12 % B; 3–6 min, 12 %-35 % B; 6–14 min, 35 %-36.5 % B 14–15 min, 36.5 %-90 % B; 15–19 min, 90 %-100 % B. The flow rate was maintained at 0.4 mL/min, with the injection volume 2 μL for all samples. The MS conditions were as below: capillary voltage, 4000 V; drying gas temperature, 300 °C. The flow rate of drying gas (N2) and nebulizer gas (N2) was 10.0 L/min and 3.0 L/min, severally. Analytes were determined in MRM modes, and the optimized parameters were shown in Table S3 and S4.
2.6 GC-QQQ MS analysis conditions
The quantitative analysis of volatile components was operated on an Agilent 7890B gas chromatography coupling to Agilent 5977A mass spectrometry (Agilent, Santa Clara, CA, USA). For group C, the initial column temperature was 60 ℃, and programmed to rise at 20 °C/min to 85 °C, 5 ℃/min to 100 ℃ (5 min held), 15 ℃/min to 150 ℃, 5 ℃/min to 180 ℃ (1 min held), finally rising at 15 ℃/min to 280 ℃ (2 min held). For group D, the initial column temperature was set at 100 ℃, and programmed to rise at 10 °C/min to 110 °C, 3 ℃/min to 120 ℃, 55 ℃/min to 265 ℃, finally rising at 18 ℃/min to 280 ℃ (2 min held). The injection volume was 1 μL and the splitting ratio was 10:1. The MRM parameters for all analytes are presented in Table S5 and S6. Other analytical conditions refer to Section 2.4.
3 Results and discussion
3.1 Qualitative analysis of SXXTN based on diagnostic ion strategy by HPLC-QTOF MS
The HPLC-QTOF MS conditions of the mobile phase systems (methanol-aqueous, acetonitrile-aqueous, and acetonitrile-aqueous with 0.1 % formic acid), gradient program, column temperature (25 °C, 30 °C, and 35 °C) and the flow rate (0.2, 0.3 and 0.4 mL/min) were optimized in order to obtain overall constituents of SXXTN with good resolution within a short analysis. The total peak area was adopted as a criterion for optimization. Ultimately, the optimum conditions mentioned in Section 2.3 were preferred.
Diagnostic ion strategy is regarded as a powerful approach for rapid characterization of chemicals in TCMs based on the principle that similar chemical constituents have similar cleavage rules and the fragmentation information, which is applicable for the identification of structural analogues in complex TCMs and formulae (Wang et al., 2017). In our study, by comparing with the reference standards, the known compounds were marked. On the basis of MS/MS analysis of authentic compounds, the characteristic fragmentation pathways of compounds with the same carbon skeleton were presented, the obtained rules were further applied to the structural characterization of its derivatives. For other unknown compounds, identification based on MS/MS spectra and relevant literature or online databases, including PubChem search (
https://pubchem.ncbi.nlm.nih.gov/) and the Human metabolome database (
https://www.hmdb.ca/). The typical total ion chromatograms (TICs) of SXXTN by HPLC-QTOF MS in both of positive and negative ion modes were displayed in Fig. 1. Totally, 140 compounds were identified based on diagnostic ion strategy, including 60 alkaloids, 34 ginsenosides, 21 organic acids, 12 phthalides, 10 triterpenes, 2 esters and 1 amino acid. The chemical structures and detailed information of compounds could be viewed in Fig. 2 and Table 1, respectively. The MS/MS spectra and fragmentation pathways of the representative chemicals were shown in Fig S1 and Fig S2.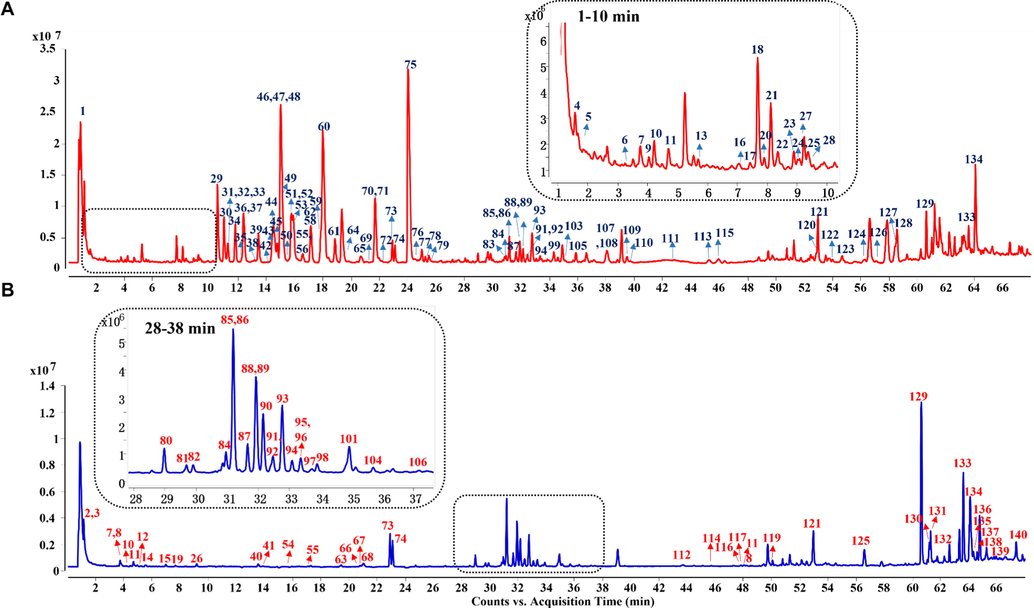
Total ion current chromatograms of SXXTN in positive ion mode (A) and negative ion mode (B) by HPLC-QTOF MS.
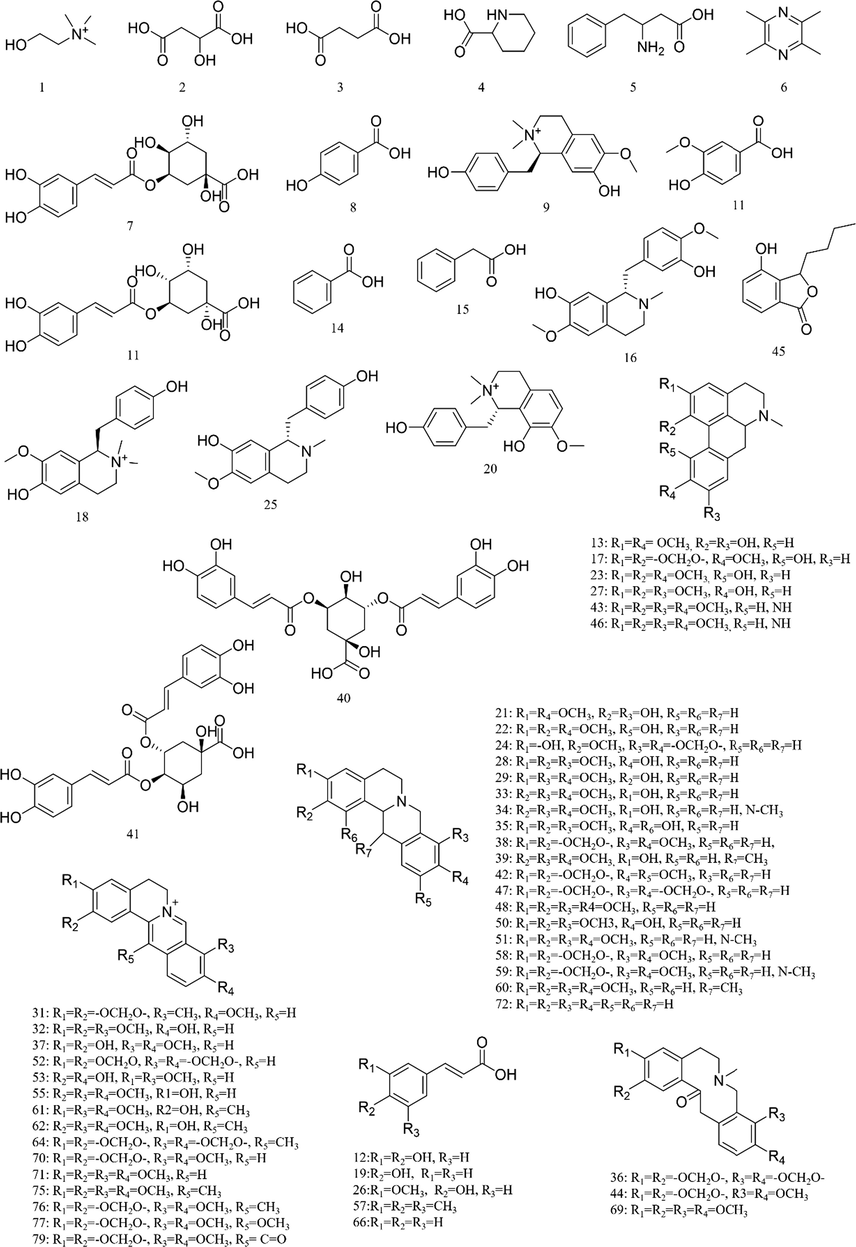
Structures of chemical constituents from SXXTN.
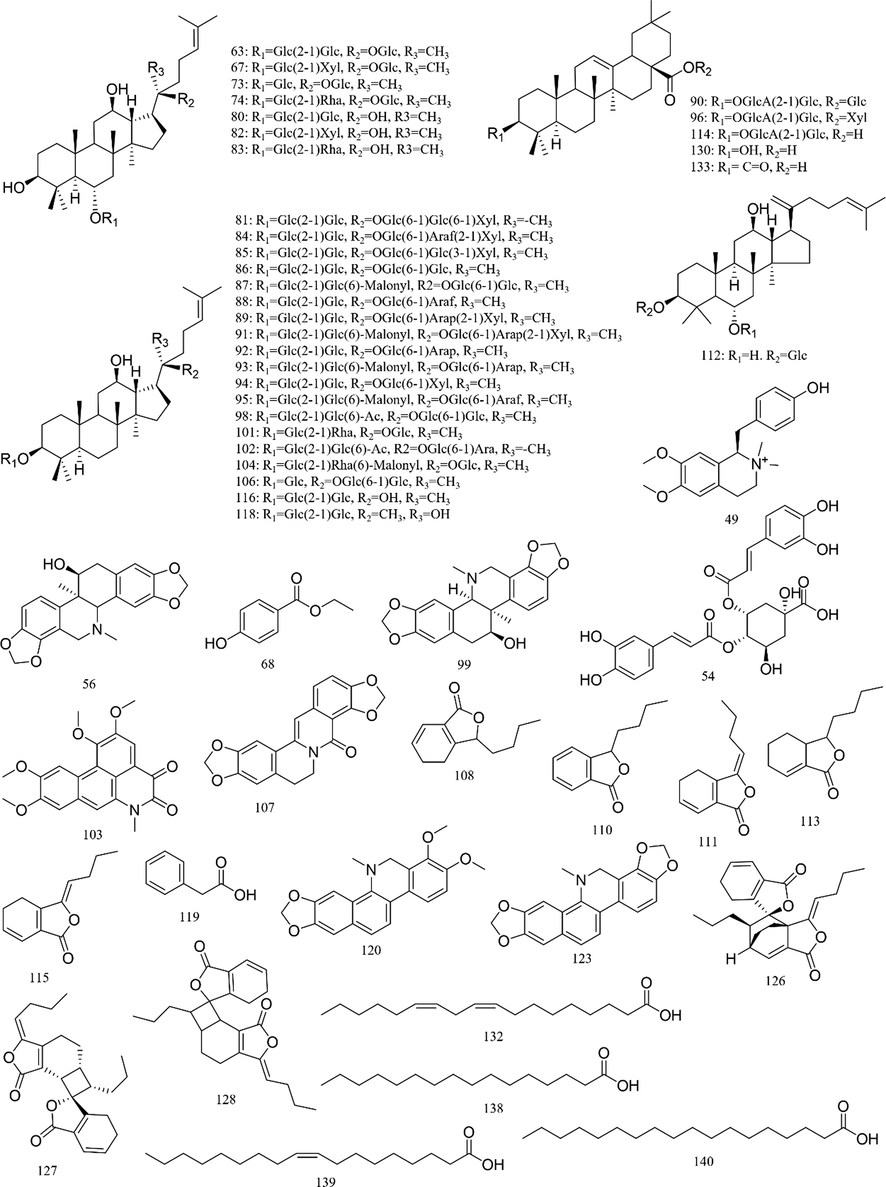
Structures of chemical constituents from SXXTN.
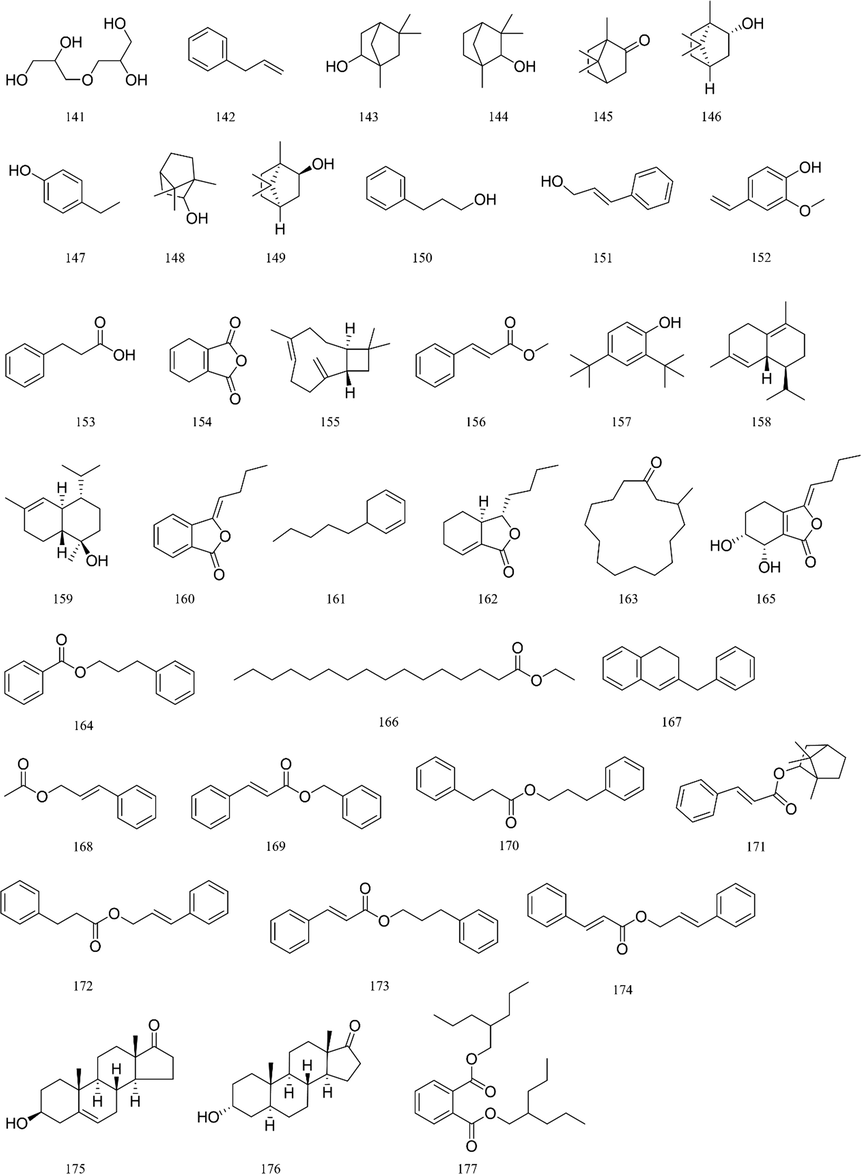
Structures of chemical constituents from SXXTN.
No.
tR
(min)
Formula
Precursorions (m/z)
Diff (ppm)
Fragment ions
(m/z)
Identification
Structural Types
1
0.88
C5H14NO+
104.1069 [M + H]+
−0.87
58.0658,60.0813
Choline
Alkaloid
2*
1.06
C4H6O5
133.0143 [M−H]-
0.40
115.0036,89.0252,71.0148
Malic acid
Organic acid
3
1.33
C4H6O4
117.0192 [M−H]-
−1.13
99.9252,73.0303
Succinic acid
Organic acid
4*
1.46
C6H11NO2
130.0867 [M + H]+
3.42
70.0653,84.0814,56.0510
Dl-pipecolinic acid
Organic acid
5*
1.56
C9H11NO2
166.0864 [M + H]+
0.87
120.0806,103.0547
Phenylalanine
Amino acid
6*
3.28
C8H12N2
137.1067 [M + H]+
−4.50
55.0550,80.0475
Tetramethylpyrazine
Alkaloid
7
3.74
C16H18O9
353.087 [M−H]-
−2.28
191.0562,179.0339
Neochlorogenic acid
Organic acid
8*
3.89
C7H6O3
137.024 [M−H]-
−3.05
93.0348,65.0413
4-Hydroxybenzoic acid
Organic acid
9
4.07
C19H24NO3
314.1755 [M]+
1.37
269.1173,175.0748,107.0491
Magnocurarine
Alkaloid
10*
4.22
C16H18O9
353.0901 [M−H]-
6.50
191.0563,179.0364,173.0456
Chlorogenic acid
Organic acid
11*
4.75
C8H8O4
167.0341 [M−H]-
−5.28
152.3304,123.0429,108.0130
Vanillic acid
Organic acid
12*
5.04
C9H8O4
179.0352 [M−H]-
1.22
135.0458
Caffeic acid
Organic acid
13
5.69
C19H21NO4
328.1547 [M + H]+
1.11
265.0853,297.1108,282.0882,165.0713
Isoboldine
Alkaloid
14
5.71
C7H6O2
121.0291 [M−H]-
−3.33
92.0281,76.9491
Benzoic acid
Organic acid
15
7.04
C8H8O2
135.0456 [M−H]-
3.31
120.0225,92.0278
Phenylacetic acid
Organic acid
16
7.07
C19H24NO4
330.1702 [M]+
0.65
299.1269,192.1032,175.0186,
143.0016Reticuline
Alkaloid
17
7.44
C19H19NO4
326.1386 [M + H]+
−0.26
295.0969,263.0696,235.0752
Bulbocapnine
Alkaloid
18
7.66
C19H24NO3
314.1759 [M]+
2.64
269.1173,237.0907,175.0745,
107.0490Lotusine
Alkaloid
19
7.71
C9H8O3
163.0402 [M−H]-
0.81
119.0497
4-Hydroxycinnamic acid
Organic acid
20
7.92
C19H24NO3
314.1743 [M]+
−2.45
237.0885,209.0961,107.0488
Oblongine
Alkaloid
21*
8.13
C19H21NO4
328.1549 [M + H]+
1.72
178.0862,163.0627,151.0755
Scoulerine
Alkaloid
22
8.39
C20H23NO4
342.1697 [M + H]+
−0.83
178.0856,326.1402
Corytenchine
Alkaloid
23*
8.83
C20H23NO4
342.1699 [M + H]+
−0.25
279.1015,311.1278,342.1699
Isocorydine
Alkaloid
24
9.06
C19H19NO4
326.1393 [M + H]+
1.89
178.0854,151.0730
Cheilanthifoline
Alkaloid
25
9.10
C18H21NO3
300.1596 [M + H]+
0.60
269.1175,237.0921,192.1025
N-Methylcoclaurine
Alkaloid
26*
9.19
C10H10O4
193.0505 [M−H]-
−0.69
178.0277,149.0594,134.0374
Ferulic acid
Organic acid
27
9.21
C20H23NO4
342.1698 [M + H]+
−0.54
192.1020,148.0753
Lirioferine
Alkaloid
28*
9.36
C20H23NO4
342.1700 [M + H]+
−0.25
327.1472,165.0909,192.1016
Corydalmine
Alkaloid
29*
10.57
C20H23NO4
342.1707 [M + H]+
2.09
327.1472,326.1414,178.0866
Tetrahydrocolumbamine
Alkaloid
30
11.02
C21H25NO4
356.1856 [M + H]+
−0.10
341.1609,326.1389,308.1276,
192.1020,177.0783
N-Methyltetrahydropalmatrubie
Alkaloid
31
11.24
C19H16NO4
322.1074 [M + H]+
−2.57
307.0839,294.2059,279.0888
Berberrubine
Alkaloid
32
11.29
C21H25NO4
356.1857 [M + H]+
0.18
341.1617,326.1390,192.1018,
165.0909,150.0672
N-Methylcorydalmine
Alkaloid
33*
11.35
C20H23NO4
342.1695 [M + H]+
−1.42
326.1387,178.0860,163.0629,
151.0725,119.0489Corypalmine
Alkaloid
34
11.83
C21H25NO4
356.1860 [M + H]+
1.03
341.1590,326.1380,308.1283,192.1020
N-Methylcorypalmine
Alkaloid
35
12.26
C20H23NO5
358.1652 [M]+
0.84
356.1856,340.1516
Capaurimine
Alkaloid
36*
12.41
C20H19NO5
354.1343 [M + H]+
1.98
336.1229,206.0812,189.0777,275.0705
Corydinine
Alkaloid
37*
12.45
C19H18NO4+
324.1228 [M]+
−0.72
307.9500,280.0005,309.0006
Demethyleneberberine
Alkaloid
38
13.17
C20H21NO4
340.1538 [M + H]+
−1.57
324.1229,309.1100,296.1274
Sinactine
Alkaloid
39
13.41
C21H25NO4
356.1855 [M + H]+
−0.38
341.1632,340.1554,326.1415
Corybulbine
Alkaloid
40
13.58
C25H24O12
515.118 [M−H]-
−2.91
353.0853,191.0556
Isochlorogenic acid A
Organic acid
41
13.84
C25H24O12
515.1182 [M−H] -
−6.14
353.0867,191.0541
Isochlorogenic acid B
Organic acid
42
14.20
C20H20NO4+
338.1390 [M]+
0.93
322.1055,380.0926,294.1122,280.0937
Tetrahydrocorysamine
Alkaloid
43*
14.40
C20H23NO4
342.1679 [M + H]+
−0.25
325.1432,294.1250,279.1035,251.1113
Norglaucine
Alkaloid
44*
14.54
C21H23NO5
370.1659 [M + H]+
2.70
188.0706,290.0939,321.1141,352.1548
Allocryptopine
Alkaloid
45
14.79
C12H14O3
207.1016 [M + H]+
0.14
189.0893,175.0196,123.0433,67.0544
4-Hydroxy-3-butylphthalide
Phthalide
46*
14.93
C21H25NO4
356.1846 [M + H]+
−2.91
294.1254,310.1206,325.1436
Glaucine
Alkaloid
47*
14.96
C19H17NO4
324.1243 [M + H]+
2.05
176.0713,294.1251,149.0579,
Tetrahydrocoptisine
Alkaloid
48*
15.04
C21H25NO4
356.1879 [M + H]+
2.71
192.1032,165.0918,194.1271,326.1479
Tetrahydropalmatine
Alkaloid
49
15.09
C20H25NO3+
328.1916 [M + H]+
2.68
283.1348,251.1070,236.0850
6-O-methylotusine
Alkaloid
50*
15.36
C21H25NO4
356.1856 [M + H]+
−0.38
354.1478,325.1338,194.2615
Yuanhunine
Alkaloid
51
15.75
C22H27NO4
370.2008 [M + H]+
−1.31
354.1693,206.1174,190.0871,165.0900
N-Methyltetrahydropalmatine
Alkaloid
52*
15.79
C19H14NO4+
320.0926 [M]+
2.70
292.0972,262.0880,234.0919
Coptisin
Alkaloid
53*
15.90
C20H20NO4+
338.1395 [M]+
2.41
323.1142,322.1015
Columbamine
Alkaloid
54
16.36
C25H24O12
515.1167 [M−H]-
−5.44
353.0848,191.0513
Isochlorogenic acid C
Organic acid
55*
16.58
C20H20NO4+
338.1390 [M]+
0.93
323.1167,294.1143,322.1093
Jatrorrhizine
Alkaloid
56
16.70
C12H14O3
207.1015 [M + H]+
−0.34
189.0818,161.0967
Senkyunolide F
Phthalide
57
17.10
C12H14O5
237.0753 [M−H]-
−6.53
193.0849,108,0193
Trimethoxycinnamic acid
Organic acid
58*
17.16
C20H21NO4
340.1556 [M + H]+
3.72
176.0716,149.0608
Canadine
Alkaloid
59
17.93
C21H24NO4+
354.1708 [M]+
2.30
165.0906,190.0876
N-Methylcanadine
Alkaloid
60*
18.03
C22H27NO4
370.2023 [M + H]+
2.74
355.1790,192.1032,176.0731,165.0912
Corydaline
Alkaloid
61
18.86
C21H22NO4+
352.1543 [M]+
−0.10
337.1308,322.1064,309.1345,293.1041
13-Methylcolumbamine
Alkaloid
62
19.38
C21H22NO4+
352.1550 [M]+
1.89
337.1322,322.1101,336.1254
Dehydrocorybulbine
Alkaloid
63*
19.45
C48H82O19
1007.538[M + COOH]-
−5.16
961.5223,799.4737,637.4235,475.3723
20-O-glucoginsenoside Rf
Ginsenoside
64*
19.58
C20H16NO4+
334.1069 [M]+
0.35
291.0887,261.0785,147.0680
Worenine
Alkaloid
65
20.73
C20H22NO5+
356.1503 [M]+
2.95
338.1383,322.1085,308.1241,192.0662,
164.0828,149.0594Pseudotetrahydropalmatine
Alkaloid
66*
20.73
C9H8O2
147.0447 [M−H]-
−3.08
119.0485,117.0334,103.0543
Cinnamic acid
Organic acid
67*
20.78
C47H80O18
977.5267 [M + COOH]-
−6.11
931.5112,799.4755,637.4223,475.3682
Notoginsenoside R1
Ginsenoside
68*
21.28
C9H10O3
165.0562 [M−H]-
2.92
137.0213,92.0268
Ethylparaben
Ester
69
21.37
C22H27NO5
386.1955 [M + H]+
−1.81
368.1833,190.0847,178.0980
Muramine
Alkaloid
70*
21.58
C20H18NO4
336.1237 [M]+
1.98
321.1008,306.0783,320.0931
Berberine
Alkaloid
71*
21.96
C21H22NO4+
352.1551 [M]+
2.17
337.1324,322.1101,308.1302,294.1139,
279.0938Palmatine
Alkaloid
72
22.23
C22H26NO4+
368.1836 [M]+
−5.35
352.1524,338.1259,192.0987
Tetrahydroprotoberberine
Alkaloid
73*
22.90
C42H72O14
845.4884 [M + COOH]-
−2.38
799.4690,637.4205,475.3705,161.0433
Ginsenoside Rg1
Ginsenoside
74*
23.09
C48H82O18
991.545 [M + COOH]-
−3.35
945.5269,783.4777,637.4213,475.3705
Ginsenoside Re
Ginsenoside
75*
24.01
C22H24NO4
366.1705 [M]+
1.41
351.1488,350.1417,336.1260
Dehydrocorydaline
Alkaloid
76*
24.47
C21H20NO4+
350.1379 [M]+
−2.24
334.1062,306.1124,320.0961
13-Methylberberine
Alkaloid
77
25.01
C22H24NO4+
366.17 [M]+
−0.91
336.1226,351.1454
13-Methoxyberberine
Alkaloid
78
25.24
C19H14NO4+
320.0922 [M]+
1.45
292.0947,262.0843,234.0893
Coptisin isomer
Alkaloid
79
25.51
C20H18NO5+
352.1197 [M]+
4.97
336.0877,322.0695,306.0756,292.0591
13-Oxoberberine
Alkaloid
80*
28.91
C42H72O14
845.4891 [M + COOH]-
−1.55
799.4723,637.4220,475.3721,161.0441
Ginsenoside Rf
Ginsenoside
81*
29.69
C59H100O27
1239.6357 [M−H]-
−1.79
1107.5968,1077.5859
Notoginsenoside R4
Ginsenoside
82*
29.90
C41H70O13
815.4785 [M + COOH]-
−1.65
769.4610,637.4229,475.3727,
161.0449,391.2853Notoginsenoside R2
Ginsenoside
83*
30.84
C42H72O13
829.4945 [M + COOH]-
−1.20
783.4794,637.4245,475.3739,
391.2800,161.043920(S)-Ginsenoside Rg2
Ginsenoside
84*
30.93
C58H98O26
1245.6046 [M + Cl]-
0.45
1209.6246,1077.5795
Ginsenoside Ra2
Ginsenoside
85*
31.18
C59H100O27
1239.6358 [M−H]-
−1.17
1107.8589,864.3445,783.5100
Ginsenoside Ra3
Ginsenoside
86*
31.19
C54H92O23
1107.5962 [M−H]-
0.49
945.5333,783.4762,179.0540
Ginsenoside Rb1
Ginsenoside
87
31.66
C57H94O26
1193.594 [M−H]-
−1.72
1159.5908,1107.5793,1089.5701,
945.5294Malonylginsenoside Rb1
Ginsenoside
88*
31.92
C53H90O22
1123.5884 [M + COOH]-
−1.94
1077.5685,945.5308,784.4864
Ginsenoside Rc
Ginsenoside
89*
31.92
C58H98O26
1245.6046 [M + Cl]-
0.45
1209.6278,945.5503
Ginsenoside Ra1
Ginsenoside
90*
32.14
C48H76O19
955.4849 [M−H]-
−6.18
793.4271,631.3664,523.3719,455.3456
Ginsenoside Ro
Ginsenoside
91
32.39
C61H100O29
1295.6251 [M−H]-
−2.05
1251.6282,1209.6131,1191.6035,
1059.5610Malonylginsenoside Ra1/Ra2
Ginsenoside
92
32.47
C56H92O25
1163.5837 [M−H]-
−1.54
1119.5849,1077.5763,1059.5647,
927.5217Malonylginsenoside Rb2
Ginsenoside
93*
32.77
C53H90O22
1123.5874 [M + COOH]-
−2.83
783.4804,945.5378,149.0458
Ginsenoside Rb2
Ginsenoside
94*
33.09
C53H90O22
1113.5633 [M + Cl]-
1.37
1077.4828,945.5401,783.4882,
621.4349Ginsenoside Rb3
Ginsenoside
95
33.37
C56H92O25
1163.5826 [M−H]-
−2.56
1119.5877,1077.5761,1059.5664
Malonylginsenoside Rc
Ginsenoside
96*
33.50
C47H74O18
925.4793 [M−H]-
−1.01
763.4258,569.3849
Pseudoginsenoside RT1
Ginsenoside
97
33.72
C56H92O25
1163.5808 [M−H]-
−4.03
1119.5819,1077.5723,1059.5612,
927.5204Malonylginsenoside Rb2/Rc isomer
Ginsenoside
98
33.90
C56H94O24
1185.5829 [M + Cl]-
0.00
1149.6073,1107.5942,1089.5846
Quinquenoside R1
Ginsenoside
99
34.23
C21H22NO5+
368.1492 [M]+
−4.21
353.1250,338.1017,336.1226
Corynoline
Alkaloid
100
34.84
C56H92O25
1163.5859 [M−H]-
0.35
1119.5911,1077.5854,783.4934
Malonylginsenoside Rb2/Rc isomer
Ginsenoside
101*
34.94
C48H82O18
991.5454 [M + COOH]-
−3.35
945.5265,783.4762,621.4260,459.3774
Ginsenoside Rd
Ginsenoside
102
35.12
C55H92O23
1119.5956 [M−H]-
−0.06
1077.5746,1059.5686,937.1230
Ginsenoside RS2
Ginsenoside
103
35.17
C21H19NO6
382.1282 [M + H]+
−0.82
336.0869,308.0974,265.0691
Pontevedrine
Alkaloid
104
35.65
C51H84O21
1031.5382 [M−H]-
−4.88
987.5375,945.5286,927.5203,
783.4779,765.4668Malonyl Ginsenoside Rd
Ginsenoside
105
35.86
C22H24NO4+
366.1687 [M]+
−3.51
350.1374,336.1240,322.1413,308.1290
Dehydrocorydaline isomer
Alkaloid
106*
37.22
C48H82O18
991.547 [M + COOH]-
−1.33
945.5397
Gypenoside XVII
Ginsenoside
107*
37.95
C19H13NO5
336.0869 [M + H]+
0.75
308.0913,293.0668,250.0864
8-Oxycoptisine
Alkaloid
108*
38.05
C12H16O2
193.1228 [M + H]+
2.56
105.0706,137.0609,147.1169
Senkyunolide A
Phthalide
109
39.48
C22H24NO4+
366.1695 [M]+
−1.32
350.1388,336.1237,322.1447,308.1251
Dehydrocorydaline isomer
Alkaloid
110*
39.70
C12H14O2
191.1066 [M + H]+
−0.29
135.0455,145.1003
Butylphthalide
Phthalide
111*
42.85
C12H14O2
191.1059 [M + H]+
−3.96
145.1010,173.0957,117.0695
(E)-Ligustilide
Phthalide
112
43.70
C36H60O8
665.4265 [M + COOH]-
−0.78
655.3976,569.2387,327.1338
Ginsenoside Rk3/Rh4
Ginsenoside
113*
45.28
C12H18O2
195.1382 [M + H]+
1.25
177.1269,149.1309,125.0595
Sedanolide
Phthalide
114*
45.71
C42H66O14
793.4335 [M−H]-
−5.65
613.3633,523.3703,455.3451
Zingibroside R1
Ginsenoside
115*
45.95
C12H14O2
191.1073 [M + H]+
−1.34
145.1008,173.0960,112.9674,117.0694
(Z)-Ligustilide
Phthalide
116*
47.90
C42H72O13
829.4918 [M + COOH]-
−4.45
783.4861,621.4345,113.0268, 459.3739,161.0423
20(S)-Ginsenoside Rg3
Ginsenoside
117
48.02
C42H66O14
793.4377 [M−H]-
−0.35
613.3632,569.3760,455.3473
Zingibroside R1 isomer
Ginsenoside
118*
48.22
C42H72O13
819.4670 [M + Cl]-
0.37
783.4903,621.4341,459.3756
20(R)-Ginsenoside Rg3
Ginsenoside
119
50.05
C18H18O3
281.118 [M−H]-
1.71
163.0350.145.0262,117.0316
Isoeugenyl phenylacetate
Ester
120*
52.84
C21H19NO4
350.1385 [M + H]+
−0.53
335.1146,319.1191,334.1083
Dihydrochelerythrine
Alkaloid
121
52.94
C30H47O4-
471.3487 [M]-
1.52
393.3162,71.0506
2-Hydroxyoleanolate or isomer
Triterpene
122
53.96
C24H30O4
383.2227 [M + H]+
2.65
191.1063,149.0599
Senkyunolide P or isomer
Phthalide
123*
54.68
C20H15NO4
334.1097 [M + H]+
0.65
319.0841,304.0967,279.1013
Dihydrosanguinarine
Alkaloid
124
56.19
C24H30O4
383.2211 [M + H]+
−1.53
191.1063,149.0597
Senkyunolide P or isomer
Phthalide
125
56.57
C30H47O4-
471.3480 [M]-
0.03
359.2921,162.8327
2-Hydroxyoleanolate or isomer
Triterpene
126*
57.08
C24H28O4
381.2059 [M + H]+
−0.36
191.1075,173.0972,279.1508
Tokinolide B
Phthalide
127*
58.38
C24H28O4
381.2070 [M + H]+
2.53
191.107,267.1386,141.1136
Riligustilide
Phthalide
128*
58.55
C24H28O4
381.2069 [M + H]+
2.27
191.107,141.1136
Angelicide
Phthalide
129
60.63
C30H46O4
469.3316 [M−H]-
−1.56
305.1903,164.8363
Glycyrrhetic acid or isomer
Triterpene
130*
61.18
C30H48O3
455.3528 [M−H]-
−0.59
410.3532
Oleanolic acid
Triterpene
131
61.28
C30H46O4
469.3314 [M−H]-
−1.99
423.3197,211.1525
Glycyrrhetic acid or isomer
Triterpene
132*
63.34
C18H32O2
279.2327 [M−H]-
−0.91
261.2193,59.0146
Linoleic acid
Organic acid
133
63.59
C30H46O3
453.3376 [M−H]-
0.40
407.3308,325.2544,100.9336
Oleanonic acid or isomer
Triterpene
134
64.04
C30H46O3
453.3376 [M−H]-
0.40
407.3316,97.0653
Oleanonic acid or isomer
Triterpene
135
64.08
C30H46O3
453.3374 [M−H]-
−0.04
407.3316,97.0661
Oleanonic acid or isomer
Triterpene
136
64.31
C30H46O3
453.3380 [M−H]-
1.28
407.3244,97.0693
Oleanonic acid or isomer
Triterpene
137
64.58
C30H46O4
469.3319 [M−H]-
−0.92
336.1460,141.8654
Glycyrrhetic acid or isomer
Triterpene
138*
64.76
C16H32O2
255.2329 [M−H]-
−0.21
237.2247,116.9283
Palmitic acid
Organic acid
139*
65.26
C18H34O2
281.2490 [M−H]-
1.41
116.9279
Oleic acid
Organic acid
140*
67.38
C18H36O2
283.2622 [M−H]-
−7.25
265.2464,211.6753,141.7728
Octadecanoic acid
Organic acid
3.1.1 Identification of alkaloids in SXXTN
Sixty alkaloids in SXXTN demonstrated quasi-molecular ions [M + H]+ or [M]+ in positive ion mode and listed in Table 1, mostly originated from Corydalis Rhizoma and identified as four main types, including tetrahydroproberberines, berberines, protopines and aporphines.
A total of 19 tetrahydroprotoberberine-type (21, 22, 24, 28, 29, 33, 34, 35, 38, 39, 42, 47, 48, 50, 51, 58, 59, 60, 72) and 3 protopine-type alkaloids (36, 44, 69) were tentatively identified or unambiguously characterized with the characteristic cleavage pathway of Retro Diels-Alder (RDA) reaction, which can be used to distinguish them from other types of alkaloids (Yuan et al., 2016). In MS/MS of tetrahydropalmatine (48) shown as Fig. S1A, the fragment ion with the strongest intensity was located at m/z 192.1019 [M + H-C10H12O2]+, and it was found that the complementary fragment ion m/z 165.0909 [M + H-C11H13NO2]+ was corresponding to the RDA reaction of C ring. The detailed fragmentation pathways of tetrahydropalmatine (48) were displayed in Fig. S2A. For protopine-type alkaloids, C-14 position is linked to oxygen to form carbonyl, which is easy to dehydrate and forms stable fragment ions, thus distinguishing it from tetrahydroberberberine-type alkaloids (Yuan et al., 2016). Taking protopine (36) as an example (Fig. S1B), the product ions at m/z 336.1232 [M + H-H2O]+and m/z 188.0709 [M + H-C9H8O2-H2O]+ may be formed by neutral losses of H2O from molecular ions and m/z 206.0813 (Fig. S2B).
Fifteen protoberberine-type (31, 32, 37, 52, 53, 55, 61, 62, 64, 70, 71, 75, 76, 77, 79) and six aporphine-type alkaloids (13, 17, 23, 27, 43, 46) were identified in SXXTN with the cleavage pathway based on the fragmentation of substituents (Yuan et al., 2016) as displayed in Fig. S2C. For protoberberine-type alkaloids, usually losing 15 Da (–CH3) substituent as see in MS/MS spectrum (Fig. S1C) of berberine (70), the main product ions appeared at m/z 320.0919 [M−CH4]+ and m/z 321.0978 [M−CH3]+. In addition, the successive losses of CH3 and CO were the characteristic cleavage pathway of this alkaloid. For aporphine-type alkaloids, the fragment ions with the highest relative abundance usually appear when the methoxy group at 31 Da is lost. For example, fragment ion m/z 297.1108 [M + H-OCH3]+ was found in isoboldine (13), showing a loss of 31 Da (–OCH3). Due to the loss of NH2CH3 (Fig. S2D), a crucial characteristic ion at m/z 325.1445 was obtained in glaucine (46) (Fig. S1D).
3.1.2 Identification of ginsenosides in SXXTN
A total of 34 ginsenosides in SXXTN were displayed in Table 1, mostly from Ginseng Radix et Rhizoma and demonstrated quasi-molecular ions [M−H]- or [M + COOH]- in negative ion mode due to formic acid in the mobile phase. Based on their aglycone, ginsenosides can be classified into three main categories: 20(S)-protopanaxadiol (PPD), 20(S)-protopanaxatriol (PPT) and oleanane type (OA) saponin.
19 PPD-type ginsenosides (81, 84, 85, 86, 87, 88, 89, 91, 92, 93, 94, 95, 98, 101, 102, 104, 106, 116, 118) were characterized and prone to produce [20(S)-protopanaxadiol-H]- (C30H51O3) characteristic aglycone fragment ions at m/z 459.38 (Yang et al., 2021). For example, in MS/MS spectrometry (Fig. S1E), Compound 101 gave abundant ion at m/z 783.4762 ([M−H−Glc]-), m/z 621.4260 ([M−H−2Glc]-) and m/z 459.3774 ([20(S)-protopanaxadiol-H]-, C30H51O3), resulting from sequential eliminations of sugar residues (Fig. S2E). A total of 7 PPT-type ginsenosides (63, 67, 73, 74, 80, 82, 83) were tentatively identified and clearly marked with characteristic ions at m/z 475.37 ([20(S)-protopanaxatriol-H]-, C30H51O4) (Yang et al., 2021). As presented in Fig. S1F and Fig. S2F, ginsenoside Rg1 (73) produced abundant characteristic ions including m/z 673.4209 ([M−H−Glc]-) and m/z 475.3713 ([(20(S)-protopanaxatriol-H]-). Three OA-type ginsenosides (90, 96, 114) were unambiguously elucidated as ginsenoside Ro, pseudoginsenoside RT1 and zingibroside R1 by comparison with reference standards with characteristic ions at m/z 455.35 ([Oleanolicacid-H]-, C30H47O3) (Yang et al., 2021) as shown in MS/MS spectrum (Fig. S1G) of ginsenoside Ro (90). The fragmentation pathways were shown in Fig. S2G.
Aglycones can be identified by discovering diagnostic ions and neutral loss can be observed to determine the number and type of glycosidic bond cleavage of ginsenosides. As shown in Fig. S1H, ginsenoside Rb2 (93) appeared m/z 945.5378 ([M−H−Ara]-) and m/z 783.4803 ([M−H−Ara−Glc]-) in MS/MS spectrum.
3.1.3 Identification of organic acids in SXXTN
In total, 21 organic acids (2, 3, 4, 7, 8, 10, 11, 12, 14, 15, 19, 26, 40, 41, 54, 57, 66, 132, 138, 139, 140) were identified from SXXTN and shown in Table 1. Organic acids were easy to generate fragment ions in MS/MS with losing CO, CO2, –COOH, H2O, etc. (Yan, Wang, 2014). For example, fragment ions m/z 178.0273 [M−H−CH3]-, m/z 149.0561 [M−H−CO2]-, m/z 134.0371 [M−H−CH3−CO2]- and m/z 160.8423 [M−H−CH3−H2O]- were observed in secondary mass spectrometry of ferulic acid (26) (Fig. S1I) following specific cleavage pathways (Fig. S2H). In the MS/MS spectrometry of cinnamic acid (66) (Fig. S1J), the fragment ions with the highest abundance were observed to be m/z 103.0548 [M−H−CO2]-, which conformed to the cleavage characteristics of organic acids.
3.1.4 Identification of phthalides in SXXTN
Totally, 12 phthalides were tentatively identified or unambiguously authenticated, including 9 monomeric phthalides (45, 56, 108, 110, 111, 113, 122, 124) and 3 phthalide dimers (126, 127, 128). Monomeric phthalide compounds with a phthalide structure unit as the core, are prone to neutral loss of H2O, CO, CO2 and alkyl radicals or alkyl chains (CH3, C2H4, C3H6, C4H8, etc.) (Yan et al, 2022) as shown in Fig. S2I. In the MS/MS spectrometry of senkyunolide A (1 0 8), the product ion m/z 175.1123 was produced by the precursor ion loss of H2O. On this basis, the characteristic fragment m/z 147.1167 was produced by the successive loss of CO and m/z 137.0595 was the production of alkyl radical C4H8 lost by precursor ions (Fig. S1K). The phthalide dimer compounds are formed by the polymerization of two phthalide monomers. They are induced to dissociate into monomeric phthalide in MS/MS, and the highest intensity ions at m/z 191.11 are often produced (Zhang et al., 2018). Take Angelicide (1 2 8) as example, the fragment ion with highest abundance was observed at m/z 191.1066 in MS/MS spectrometry (Fig. S1L). On this basis, the cleavages of phthalide skeletons could also be observed in the MS/MS spectra of phthalide dimers, which were similar to monomeric phthalides.
3.2 GC–MS qualitative analysis of SXXTN
In preceding reports, the volatile components in SXXTN such as Artificial Musk, Chuanxiong Rhizoma, Styrax and Borneolum Syntheticum have been revealed (Ding et al., 2022; He et al., 2018; Gurbuz et al., 2013; Sun et al., 2014), whereas little attention was paid to the volatile components in the intact SXXTN prescription. In this work, we supplemented the information of volatile chemicals and improved the global characterizations of complicated ingredients in SXXTN.
The GC–MS conditions of the temperature program, splitting ratio (10:1, 30:1 and 50:1) and the injector temperature (250℃, 280℃ and 300℃) were optimized in the direction of analyzing comprehensive volatile constituents of SXXTN with well separation performance in a short analysis. The total peak area was calculated as a criterion for optimization. The final conditions were described in Section 2.4.
The TICs of SXXTN by GC–MS can be viewed in Fig. 3. There were 44 volatile compounds tentatively identified from SXXTN, based on the mass spectrometric data of reference standards, the mass spectral library (NIST17) and the literature, including 12 organic acid esters, 6 monoterpenes, 6 phthalides, 3 organic acids, 3 sesquiterpenes, 3 alcohols, 3 alkanes, 3 hydrocarbons, 2 steroids, 1 macrocyclic ketone, 1 alkaloid and 1 anhydride (Table 2). The chemical structures were illustrated in Fig. 2. Among them, seven compounds (66, 68, 110, 108, 111, 138, 46) have been identified in the previous LC-MS analysis. According to the comparison with authentic standards, 25 components were clearly marked. Isoborneol (1 4 6), borneol (1 4 8), cinnamyl alcohol (1 5 1), (E)-ligustilide (1 1 1), muscone (1 6 3), 3-phenylpropyl cinnamate (1 7 3) and cinnamyl cinnamate (1 7 4) exhibited relatively high abundances during GC–MS analysis of SXXTN.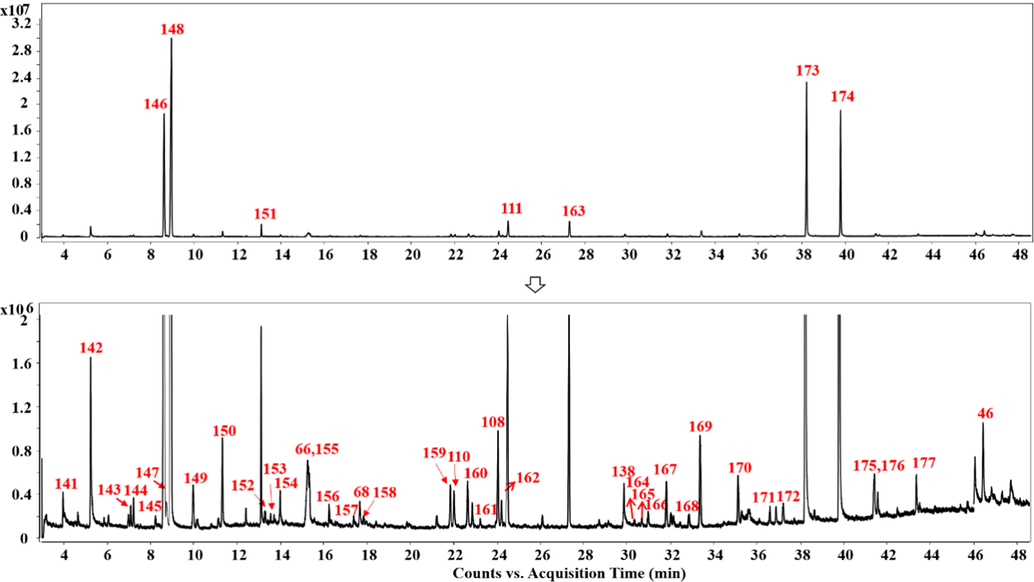
Total ion current chromatograms of SXXTN by GC–MS.
No.
Rt (min)
Identification
Match
Formula
Structural Types
141
4.03
Glycerin
91.1
C3H8O3
Alcohol
142
5.26
Allylbenzene
82.7
C9H10
Hydrocarbon
143
7.06
Bicyclo [2,2,1] heptan-2-ol.1,5,5-trimethyl
85.2
C10H18O
Monoterpenoid
144*
7.19
Fenchol
93.6
C10H18O
Monoterpenoid
145*
8.19
Camphor
90.3
C10H16O
Monoterpenoid
146*
8.58
Isoborneol
98.2
C10H18O
Monoterpenoid
147*
8.71
Phenol, 4-ethyl-
90.7
C8H10O
Phenol
148*
8.92
Borneol
97.4
C10H18O
Monoterpenoid
149
9.95
Bicyclo [2.2.1] heptan-2-ol, 1,7,7-trimethyl-, (1S-endo)-
88.3
C10H18O
Monoterpenoid
150*
11.31
3-Phenylpropanol
96.5
C9H12O
Alcohol
151*
13.11
Cinnamyl alcohol
98.5
C9H10O
Alcohol
152*
13.28
2-Methoxy-4-vinylphenol
91.4
C9H10O2
Phenol
153*
13.57
Hydrocinnamic acid
96.1
C9H10O2
Organic acid
154
13.96
1,4-Cyclohexadiene-1,2-dicarboxylic anhydride
86.5
C8H6O3
Anhydride
66*
15.27
Cinnamic acid
72.1
C9H8O2
Organic acid
155*
15.34
Caryophyllene
88.7
C15H24
Sesquiterpene
156*
16.25
Ethyl cinnamate
77.7
C11H12O2
Organic acid ester
157*
17.37
2,4-Di-tert-butylphenol
84.6
C14H22O
Phenol
68*
17.66
Ethylparaben
95.1
C9H10O3
Organic acid ester
158
17.84
Δ-Cadinene
80.7
C15H24
Sesquiterpene
159*
21.84
Cadinol
91.7
C15H26O
Sesquiterpene
110*
22.02
Butylphthalide
93.1
C12H14O2
Phthalide
160*
22.66
Z-Butylidenephthalide
92.4
C12H12O2
Phthalide
161
23.23
5-Pentylcyclohexa-1,3-diene
87.4
C11H18
Hydrocarbon
108*
24.06
Senkyunolide A
89.2
C12H16O2
Phthalide
162*
24.22
Neocnidilide
92.0
C12H18O2
Phthalide
111*
24.46
(E)-Ligustilide
94.9
C12H14O2
Phthalide
163*
27.32
Muscone
95.7
C16H30O
Cyclic ketone
138
29.92
Palmitic acid
82.1
C16H32O2
Organic acid
164
30.36
3-Phenylpropyl benzoate
92.6
C16H22O4
Organic acid ester
165
30.69
Hexadecanoic acid, ethyl ester
88.4
C18H36O2
Organic acid ester
166
31.00
Senkyunolide H
84.1
C12H16O4
Phthalide
167
31.83
3-benzyl-1,2-dihydronaphthalene
70.3
C17H17
Hydrocarbon
168
32.86
(Z)-Cinnamyl benzoate
96.0
C16H14O2
Organic acid ester
169*
33.39
Benzyl cinnamate
95.7
C16H14O2
Organic acid ester
170
35.15
Benzenepropanoic acid, 3-phenylpropyl ester
94.3
C18H20O2
Organic acid ester
171
36.62
Borny cinnamate
91.5
C19H24O2
Organic acid ester
172
36.89
Benzenepropanoic acid, 3-phenyl-2-propenyl ester
94.8
C18H18O2
Organic acid ester
173*
38.26
3-Phenylpropyl cinnamate, (E)-
97.4
C18H18O2
Organic acid ester
174*
39.86
Cinnamyl cinnamate
97.4
C18H16O2
Organic acid ester
175*
41.40
Prasterone
76.3
C19H28O2
Steroid
176*
41.43
Androsterone
73.3
C19H30O2
Steroid
177
41.58
Phthalic acid, di(2-propylpentyl) ester
87.0
C24H38O4
Organic acid ester
46
46.06
Glaucine
89.7
C21H25NO4
Alkaloid
3.3 Quantification of 40 non-volatile compounds in SXXTN by HPLC-QQQ MS
Forty confirmed non-volatile chemicals were further quantified by the optimized HPLC-QQQ MS method (Table S3-S4) to evaluate the quality of SXXTN. According to the difference in the response of the components in positive and negative ion modes, two MRM methods with different polarity were established for Quantification. The typical MRM chromatograms of analytes were illustrated in Fig S3.
Nice linearity with coefficients of determination (R2 > 0.9900) were obtained for the 40 analytes. Limit of detection (LOD) and limit of quantitation (LOQ) tests were performed and listed in Table S7. As exhibited in Table S8, relative standard deviations (RSD) of repeatability, intra- and inter-day precision were 1.27 % − 4.79 %, 1.07 % − 5.41 % and 1.18 % − 9.43 %, respectively. Besides, all analytes could remain stable within 24 h under 4℃, with the RSD ranging 0.60 % − 6.14 % and recoveries of 40 compounds were ranged from 80.36 % − 117.13 % with the RSD ranging 2.38 % − 12.61 %. Consequently, the established HPLC-QQQ MS approach was proved as a sensitive, repeatable and accurate tool for the quantification of non-volatile compounds in SXXTN.
According to the established HPLC-QQQ MS quantitative analysis method, the content of 40 compounds in 7 batches of SXXTN provided by the enterprise was determined as shown in Table 3 and Fig. 4. The total content of 40 analytes in each batch was 2.03 % − 2.30 %. Among them, the components with higher content (>1.00 mg/g) were norglaucine (43), glaucine (46), dehydrocorydaline (75) and ginsenoside Rb1 (86). In the previous reported, aforementioned compounds presented promising effects for myocardial protection (Wen et al., 2022; Han et al., 2012; Zheng et al., 2017; Kong et al., 2018). For example, studies have found that intraperitoneal injection of dehydrocorydine in ApoE-/- mice can not only inhibit the development of atherosclerosis, but also improve aortic compliance and plaque stability (Wen et al., 2022). Tetrahydropalmatine can activate PI3K/Akt/eNOS/NO pathway, increase the expression of HIF-1a and VEGF, and inhibit iNOS-derived NO production in myocardium. This effect may reduce the accumulation of inflammatory factors (including TNF-a and MPO) and reduce the degree of apoptosis (Han et al., 2012). It has also been reported that ginsenoside Rb1 can improve heart failure, which may be achieved by regulating the mitochondrial membrane in cardiomyocytes (Kong et al., 2018).
No.
Components
B1
B2
B3
B4
B5
B6
B7
non-volatile components
5
Phenylalanine
86.83 ± 2.32
86.5 ± 3.25
64.25 ± 6.71
61.17 ± 5.4
29.33 ± 2.88
20.92 ± 1.01
85.92 ± 7.67
6
Tetramethylpyrazine
1.36 ± 0.05
1.47 ± 0.13
2.28 ± 0.05
1.5 ± 0.08
2.64 ± 0.05
3.14 ± 0.05
3.19 ± 0.05
21
Scoulerine
65.31 ± 6.33
71.92 ± 2.5
82.53 ± 2.59
74.67 ± 1.67
103.25 ± 5.29
107.08 ± 6.98
107.58 ± 2.62
23
Isocorydine
17.97 ± 0.65
19.44 ± 0.46
20.53 ± 0.67
19.72 ± 0.61
28.89 ± 0.68
28.97 ± 0.47
29.72 ± 0.57
28
Corydalmine
47.47 ± 1.46
50.17 ± 1.52
56.75 ± 1.18
51.81 ± 0.77
51.25 ± 4.1
51.67 ± 2.82
51.53 ± 1.5
29
Tetrahydrocolumbamie
631.94 ± 12.95
621.94 ± 38.75
710.56 ± 17
602.22 ± 9.18
988.89 ± 26.79
1043.61 ± 2.55
1040.56 ± 44.92
33
Corypalmine
68.64 ± 0.6
73.22 ± 2.53
78.36 ± 1.64
75.42 ± 2.3
127.64 ± 1.77
137.22 ± 5.23
133.61 ± 3.72
36
Protopine
849.11 ± 12.48
869.92 ± 34.43
935.56 ± 35.06
860.64 ± 15.65
1062.14 ± 19.22
1125.22 ± 42.62
1125.03 ± 27.57
37
Demethyleneberberine
8.14 ± 0.05
8.5 ± 0.36
9.22 ± 0.21
8.58 ± 0.17
7.81 ± 0.13
8.25 ± 0.17
8.11 ± 0.34
43
Norglaucine
10063.33±
154.529121.39±
263.539767.78±
337.788266.94±
90.497842.22±
509.167953.89±
104.368121.94±
194.84
44
Allocryptopine
544.53 ± 27.26
534.5 ± 14.24
575.03 ± 20.11
530.03 ± 13.11
631.28 ± 27.8
672.31 ± 28.3
684.06 ± 22.77
46
Glaucine
910 ± 5.46
955.56 ± 31.9
1068.33 ± 48.18
929.44 ± 9.66
1009.17 ± 88.93
1036.11 ± 14.82
1061.11 ± 29.27
48
Tetrahydropalmatine
432.22 ± 14.2
422.78 ± 18.95
486.94 ± 22.12
418.89 ± 8.67
646.39 ± 57.4
674.17 ± 11.67
683.61 ± 12.48
52
Coptisin
513.14 ± 18.5
533.58 ± 19.6
631.47 ± 17.92
528.25 ± 20.18
683.81 ± 46.08
770.03 ± 47.76
768.31 ± 12.25
53
Columbamine
414.25 ± 8.33
426.61 ± 12.44
466.33 ± 14.99
422.61 ± 18.19
385.47 ± 25.7
417.39 ± 23.39
418.83 ± 8.24
55
Jatrorrhizine
24.75 ± 2.32
24.64 ± 3.56
28.42 ± 1.98
26.58 ± 1.69
28.64 ± 2.79
31.25 ± 2.35
32.92 ± 1.08
58
Canadine
103.61 ± 3.37
103.33 ± 3.63
120.28 ± 2.41
104.72 ± 0.48
171.39 ± 12.14
183.89 ± 2.55
183.89 ± 2.55
60
Corydaline
445.28 ± 11.71
455.83 ± 15.83
537.22 ± 17.02
443.89 ± 5.55
839.17 ± 50.26
885 ± 23.11
897.78 ± 7.74
66
Cinnamic acid
482.5 ± 15.52
529.5 ± 20.11
505 ± 31.83
515.33 ± 16.33
477.33 ± 14.1
515.25 ± 17.37
308.08 ± 18.32
67
Notoginsenoside R1
50.25 ± 0.25
53.58 ± 1.28
50.83 ± 4.94
49.25 ± 2.7
24.75 ± 2.14
26.25 ± 1.39
23.08 ± 1.01
70
Berberine
171.39 ± 4.86
176.39 ± 2.51
190.11 ± 4.03
170.92 ± 0.75
197.5 ± 4.01
203.06 ± 4.76
201.83 ± 2.35
71
Palmatine
588.44 ± 12.35
603.11 ± 6.88
638.22 ± 13.26
581.14 ± 4.43
540.64 ± 9.63
560.03 ± 13.97
555.5 ± 2.28
73
Ginsenoside Rg1
803.67 ± 14.68
831.42 ± 17.47
777.58 ± 34.06
731.5 ± 37.15
929.33 ± 27.19
915.58 ± 18.06
867.67 ± 54.56
74
Ginsenoside Re
565.17 ± 19.12
579.67 ± 36.71
547.42 ± 43.84
521.58 ± 32.77
559.42 ± 23.51
562.67 ± 11.2
826.83 ± 81.78
75
Dehydrocorydaline
1666.11 ± 18.15
1677.22 ± 49.14
1840.28 ± 40.79
1677.22 ± 25.69
1548.06 ± 73.42
1607.5 ± 28.83
1554.44 ± 17.8
76
13-Methylberberine
11.92 ± 0.17
11.92 ± 0.17
12.89 ± 0.24
11.81 ± 0.05
8.58 ± 0.14
8.86 ± 0.1
9 ± 0.08
80
Ginsenoside Rf
102 ± 3.36
111 ± 7.15
102.83 ± 10.26
90.5 ± 6.43
135.25 ± 3.5
135.83 ± 7.52
108.08 ± 6.79
83
Ginsenoside Rg2
92.83 ± 2.74
98.42 ± 5.84
96.58 ± 7.38
90 ± 2.61
77.25 ± 1.32
79 ± 2.84
60.92 ± 3.17
84
Ginsenoside Ra2
71.83 ± 1.91
74.25 ± 2.82
76.33 ± 3.4
72 ± 2.14
83.67 ± 0.88
84.67 ± 1.91
80.17 ± 1.89
85
Ginsenoside Ra3
211 ± 0.43
199.25 ± 16.69
190.67 ± 3.39
188.33 ± 9.7
203.92 ± 3.69
214.67 ± 6.57
290.83 ± 27.19
86
Ginsenoside Rb1
1025.25 ± 6.51
1045.08 ± 54.55
1030.92 ± 80.79
960.92 ± 29.16
708.08 ± 22.64
716.33 ± 15.95
571.33 ± 22.92
88
Ginsenoside Rc
281.42 ± 4.23
294.17 ± 8.08
288.5 ± 13.56
267.58 ± 8.38
227.75 ± 0.9
226.5 ± 0.66
221.58 ± 10.04
89
Ginsenoside Ra1
155.58 ± 3.83
167.67 ± 9.87
169.25 ± 10.4
148.33 ± 3.22
184.5 ± 9.79
187.33 ± 7.04
180.58 ± 5.11
93
Ginsenoside Rb2
379.33 ± 7.69
398.17 ± 20.89
394.67 ± 31.29
357.75 ± 12.89
282.5 ± 12.67
281.42 ± 5.58
225.75 ± 5.91
94
Ginsenoside Rb3
37.75 ± 1
39.17 ± 2.01
41.75 ± 3.12
34.33 ± 2.7
24.5 ± 2.41
24.42 ± 0.95
227.75 ± 6.71
101
Ginsenoside Rd
248.33 ± 10.47
261.92 ± 8.61
261.25 ± 20.12
236.75 ± 9.85
178.5 ± 7.7
180.17 ± 4.69
142.25 ± 6.29
107
8-Oxycoptisine
27.08 ± 3.18
25.36 ± 0.67
27.75 ± 0.38
25.06 ± 0.38
27.81 ± 0.42
28.86 ± 0.27
28 ± 0.38
116
20(S)-Ginsenoside Rg3
57.67 ± 2.36
65.42 ± 3.15
64.58 ± 1.42
60.08 ± 2.47
15.83 ± 0.14
15.33 ± 0.38
14.5 ± 0.5
120
Dihydrochelerythrine
36.69 ± 4.46
34.36 ± 0.87
36.33 ± 0.22
35.44 ± 0.59
49.53 ± 0.63
49.08 ± 0.33
49.64 ± 0.79
123
Dihydrosanguinarine
31.5 ± 2.58
32 ± 0.58
34.17 ± 0.46
32.89 ± 0.42
45.86 ± 0.21
46.67 ± 0.38
45.5 ± 0.87
volatile components
144
Fenchol
31.79 ± 0.72
24.14 ± 0.74
31.36 ± 0.23
28.89 ± 1.00
132.64 ± 1.08
233.88 ± 1.23
212.26 ± 0.61
145
Camphor
122.36 ± 0.58
99.94 ± 1.17
130.70 ± 0.35
115.32 ± 0.86
40.82 ± 0.14
78.08 ± 1.08
67.93 ± 0.08
146
Isoborneol
10746.58 ± 56.15
8297.41 ± 30.66
10784.14 ± 10.58
11041.49 ± 41.12
6992.38 ± 22.46
10192.18 ± 55.59
10537.26 ± 85.96
147
4-Ethylphenol
111.73 ± 2.12
101.78 ± 2.15
110.57 ± 0.64
107.77 ± 1.56
92.31 ± 5.60
122.53 ± 3.56
120.49 ± 0.66
148
Borneol
16154.89 ± 17.28
12716.32 ± 71.79
16158.12 ± 26.98
16557.61 ± 73.28
11800.29 ± 15.21
15303.33 ± 71.80
16110.40 ± 39.41
150
3-Phenylpropanol
287.24 ± 5.17
269.94 ± 5.95
295.45 ± 2.43
282.39 ± 4.51
323.49 ± 4.19
342.09 ± 10.25
332.53 ± 1.81
151
Cinnamyl alcohol
677.68 ± 11.97
659.66 ± 7.87
687.77 ± 9.42
676.31 ± 1.38
775.47 ± 15.66
782.07 ± 17.59
775.85 ± 9.96
155
Caryophyllene
99.41 ± 0.63
88.88 ± 0.23
101.31 ± 0.57
96.25 ± 1.05
68.95 ± 0.09
108.27 ± 0.63
102.55 ± 0.62
110
Butylphthalide
120.17 ± 0.72
119.28 ± 0.8
120.54 ± 0.52
118.96 ± 0.03
175.46 ± 0.95
166.65 ± 0.62
166.47 ± 0.78
160
Z-Buthlidenephthalide
470.46 ± 8.24
461.27 ± 6.60
469.83 ± 4.71
462.35 ± 5.09
918.91 ± 4.81
838.41 ± 5.49
834.35 ± 3.20
108
Senkyunolide A
445.75 ± 1.01
445.75 ± 5.70
453.88 ± 4.66
436.02 ± 2.77
1001.42 ± 2.58
963.05 ± 5.76
953.08 ± 6.39
162
Neocnidilide
114.94 ± 0.39
111.39 ± 1.46
112.35 ± 0.51
111.00 ± 0.5
209.89 ± 0.45
195.90 ± 0.17
196.40 ± 1.01
111
(E)-Ligustilide
708.93 ± 4.13
683.43 ± 5.57
698.54 ± 2.74
689.34 ± 2.44
1470.36 ± 15.50
1400.27 ± 10.26
1388.91 ± 5.15
163
Muscone
1185.77 ± 14.67
1092.55 ± 4.44
1174.19 ± 3.1
1145.85 ± 16.89
1258.91 ± 21.03
1206.96 ± 16.67
1173.77 ± 4.76
169
Benzyl cinnamate
663.55 ± 3.06
634.83 ± 5.35
664.60 ± 2.63
652.38 ± 1.63
694.71 ± 2.69
689.84 ± 4.29
665.64 ± 1.52
173
3-Phenylpropyl Cinnamate
3532.42 ± 24.74
3194.93 ± 115.13
3503.38 ± 37.00
3493.68 ± 142.94
3855.59 ± 47.58
3730.66 ± 119.27
3828.99 ± 29.46
174
Cinnamyl cinnamate
4781.76 ± 48.54
4442.54 ± 117.11
4790.28 ± 38.97
4753.69 ± 164.10
5193.83 ± 65.96
4995.83 ± 171.5
5131.81 ± 42.73
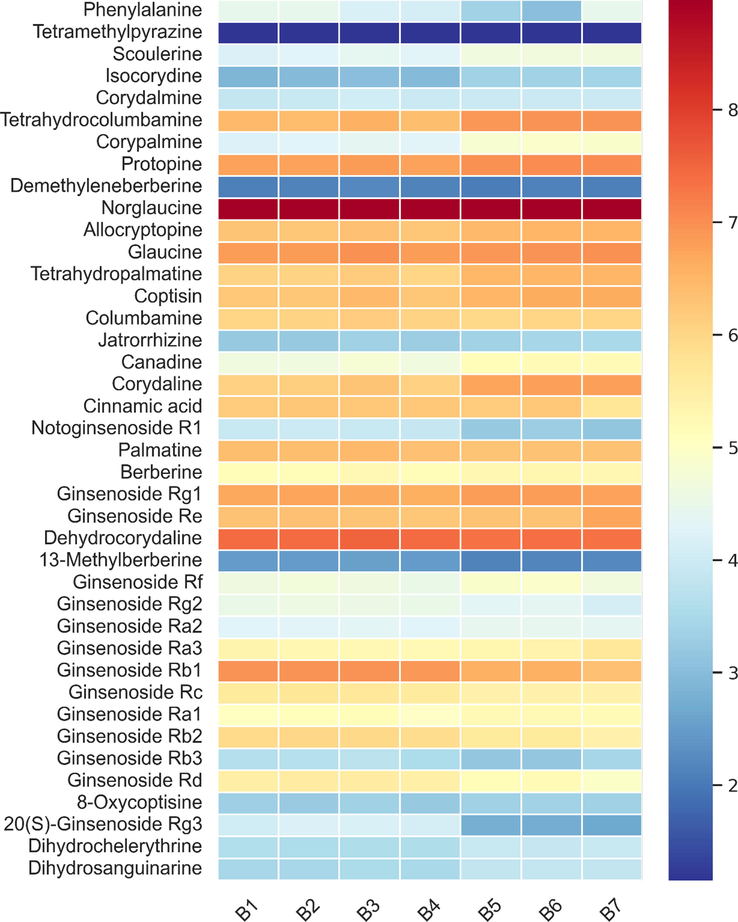
The content distribution heatmap of 40 non-volatile compounds in 7 batches of SXXTN.
3.4 Quantification of 17 volatile compounds in SXXTN by GC-QQQ MS
We established rapid and accurate quantitative methods for detecting the contents of the major volatile compounds in SXXTN. Seventeen confirmed compounds including 5 phthalides, 3 organic acid esters, 3 alcohols, 3 monoterpenes, 1 sesquiterpene, 1 phenol and 1 macrocyclic ketone were determined by GC-QQQ MS with naphthalene (IS3) as internal standards. The optimized conditions were shown in Table S5-S6 and typical MRM chromatograms of 17 analytes were illustrated in Fig S4.
The optimized GC-QQQ MS method was validated in the aspect of linearity, LODs, LOQs, precision, repeatability, stability and recovery and the results were presented in Table S9 and Table S10. Reasonable correlation coefficient values (R2 > 0.9904) indicated good correlations between investigated standards concentrations and their peak areas within the ranges tested. The ranges of LODs and LOQs for all the analytes were 0.002 - 2.642 μg/mL, and 0.013 – 6.653 μg/mL, respectively. The RSDs of repeatability, intra- and inter-day precision were 1.63% - 9.66%, 0.44% – 9.06%, 0.80% – 8.06%, respectively. All analytes could remain stable within 24 h under 4℃, with the RSD ranging 1.14% - 7.97%. The developed method had good accuracy with the recoveries were between 90.20% and 123.51%. These results provided that the established method was accurate, reproducible, and reliable for assessing the quality of volatile compounds in SXXTN.
According to the established GC-QQQ MS quantitative analysis method, the contents of main volatile components in 7 batches of SXXTN provided by the enterprise were determined as displayed in Table 3 and Fig. 5. The total content of analytes in each batch was 3.34 %-4.26 % in SXXTN. Borneol (1 4 6), isoborneol (1 4 8), cinnamyl cinnamate (1 7 4), 3-phenylpropyl cinnamate (1 7 3) and muscone (1 6 3) were the predominant components and were closely related to the anti-coronary heart disease and angina pectoris effect of SXXTN (Liu et al., 2017; Wu et al., 2011; Wang et al., 2020).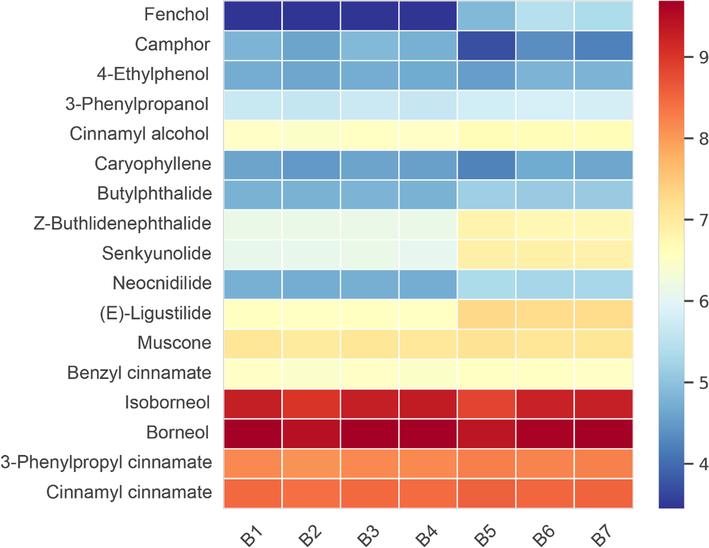
The content distribution heatmap of 17 volatile compounds in 7 batches of SXXTN.
Combined with the quantitative analysis of non-volatile components, the total contents of 57 main components in 7 batches of SXXTN were 5.50 % − 6.49 %. The percentages of different structural types of chemicals in the total 57 analytes were as follows: monoterpenes and sesquiterpenes accounted for the largest proportion (42 %), followed by alkaloids (26 %), organic acids and esters (18 %), ginsenosides (6 %) and phthalide (5 %). Both volatile and non-volatile components should be taken into consideration for quality evaluation of SXXTN. More batches of samples are more conducive to assessing the consistency and stability of SXXTN.
4 Conclusion
In view of the current deficiencies in the constituent research and quality control of SXXTN, efficient, stable and reliable LC-MS and GC–MS methods were established in our study. A total of 177 chemical components were identified from SXXTN and content of 57 components in 7 batches of SXXTN was further determined. To the best of our knowledge, this is the initial report on the comprehensive profiling of chemical constituents in SXXTN by LC-MS and GC–MS. The evaluation approach provided much more qualitative and quantitative information of multi-components in SXXTN than other single-marker quality assessments. In all, this study provided comprehensive material basis of SXXTN, which could be beneficial to improve the quality control. Furthermore, it could facilitate the pharmacological research and clinical application of SXXTN in some degree.
Acknowledgements
The authors sincerely thank Hui-Ying Wang (State Key Laboratory of Natural Medicines, China) for the technical assistance.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81730104).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- From non-targeted to targeted GC-MS metabolomics strategy for identification of TCM preparations containing natural and artificial musk. Chin. Med.. 2022;17:41.
- [Google Scholar]
- Characterization of volatiles and anti-ulcerogenic effect of Turkish sweetgum balsam (Styrax liquidus) J. Ethnopharmacol.. 2013;148:332-336.
- [Google Scholar]
- l-Tetrahydropalmatine, an Active Component of Corydalis yanhusuo W.T. Wang, Protects against Myocardial Ischaemia-Reperfusion Injury in Rats. PLoS. One.. 2012;7:e38627.
- [Google Scholar]
- A modified multiscale peak alignment method combined with trilinear decomposition to study the volatile/heat-labile components in Ligusticum chuanxiong Hort - Cyperus rotundus rhizomes by HS-SPME-GC/MS. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci.. 2018;1079:41-50.
- [Google Scholar]
- The effects of ginsenoside Rb1 on fatty acid β-oxidation, mediated by AMPK, in the failing heart. Iran. J. Basic. Med. Sci.. 2018;21:731-737.
- [Google Scholar]
- Chinese Medicine She-Xiang-Xin-Tong-Ning, Containing Moschus, Corydalis and Ginseng, Protects from Myocardial Ischemia Injury via Angiogenesis. Am. J. Chin. Med.. 2020;48:107-126.
- [Google Scholar]
- Comparison of the therapeutic effects of different compositions of muskone in the treatment of experimental myocardial infarct in rats and analgesia in mice. Phytother. Res.. 2008;22:1219-1223.
- [Google Scholar]
- Corydalis yanhusuo rhizoma extract reduces infarct size and improves heart function during myocardial ischemia/reperfusion by inhibiting apoptosis in rats. Phytother. Res.. 2010;20:448-453.
- [Google Scholar]
- Four Main Active Ingredients Derived from a Traditional Chinese Medicine Guanxin Shutong Capsule Cause Cardioprotection during Myocardial Ischemia Injury Calcium Overload Suppression. Phytother. Res.. 2017;31(3):507-515.
- [Google Scholar]
- Screening of Bioactive Ingredients in Ligusticum Chuanxiong Hort for Protection against Myocardial Ischemia. Cell. Physiol. Biochem.. 2016;40:770-780.
- [Google Scholar]
- Rapid identification and analysis of the active components of traditional Chinese medicine Xiaoxuming decoction for ischemic stroke treatment by integrating HPLC-Q-TOF/MS and RRLC-QTRAP MSn method. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci.. 2019;1124:313-322.
- [Google Scholar]
- Observation of the Blood Rheology and Clinical Effects of Shexiangxintongning tablet on 60 Cases with Coronary Heart Disease Combined with Angina Pectoris. Chin. J. Nat. Med.. 2005;7:114-117.
- [Google Scholar]
- Hypothesis of active components in volatile oil from a Chinese herb formulation, Shao-Fu-Zhu-Yu decoction, using GC-MS and chemometrics. J. Sep. Sci.. 2008;31(6–7):1085-1091.
- [Google Scholar]
- Simultaneous determination of borneol and its metabolite in rat plasma by GC-MS and its application to pharmacokinetic study. J. Pharm. Anal.. 2014;4(5):345-350.
- [Google Scholar]
- Discussion on efficacy evaluation thought and method for innovation medicine of Chinese herbal compound formula based on clinical application characteristics. Zhongguo Zhongyao Zazhi.. 2017;42(5):852-855.
- [Google Scholar]
- Relationship between dose and effects of Suhexiang against myocardial ischemia of model rats. Acta Chin. Med.. 2019;34:2157-2163.
- [Google Scholar]
- Isoborneol Attenuates Low-Density Lipoprotein Accumulation and Foam Cell Formation in Macrophages. Drug Des. Dev. Ther.. 2020;14:167-173.
- [Google Scholar]
- Rapid analysis on phenolic compounds in Rheum palmatum based on HPLC-Q-TOF/MSE combined with diagnostic ions filter. China J. Chin. Mater. Med.. 2017;42(10):1922-1931.
- [Google Scholar]
- Antiatherosclerotic effect of dehydrocorydaline on ApoE(-/-) mice: inhibition of macrophage inflammation. Acta. Pharmacol. Sin.. 2022;6:1408-1418.
- [Google Scholar]
- Protective effects of muscone on ischemia-reperfusion injury in cardiac myocytes. J. Ethnopharmacol.. 2011;138(1):34-39.
- [Google Scholar]
- Chemical profiling and quantification of Gua-Lou-Gui-Zhi decoction by high performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. J. Ethnopharmacol.. 2015;138(1):34-39.
- [Google Scholar]
- Analysis on chemical constituents in Danggui-Shaoyao-San by LC-Q-TOF-MS and LC-IT-MSn. Chin. Tradit. Herb. Drugs. 2014;45(8):1056-1062.
- [Google Scholar]
- A comprehensive investigation on the chemical diversity and efficacy of different parts of Ligusticum chuanxiong. Food. Funct.. 2022;13:1092-1107.
- [Google Scholar]
- Localization of constituents for determining the age and parts of ginseng through ultraperfomance liquid chromatography quadrupole/time of flight-mass spectrometry combined with desorption electrospray ionization mass spectrometry imaging. J. Pharm. Biomed. Anal.. 2021;193:113722
- [Google Scholar]
- The classification and identification of complex chemical compositions based on UPLC-Q-TOF/MS using yanhusuo herb as an example. Anal. Methods.. 2016;8(10):2274-2281.
- [Google Scholar]
- A strategy to improve the identification reliability of the chemical constituents by high-resolution mass spectrometry-based isomer structure prediction combined with a quantitative structure retention relationship analysis: Phthalide compounds in Chuanxiong as a test case. J. Chromatogr. A.. 2018;1552:17-28.
- [Google Scholar]
- (+)-Borneol improves the efficacy of edaravone against DSS-induced colitis by promoting M2 macrophages polarization via JAK2-STAT3 signaling pathway. Int. Immunopharmacol.. 2017;53:1-10.
- [Google Scholar]
- Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp. Anim.. 2017;66(3):217-228.
- [Google Scholar]
- Roles and mechanisms of ginseng in protecting heart. Chin. J. Integr. Med.. 2012;18:548-555.
- [Google Scholar]
- Study on the discrimination between Corydalis Rhizoma and its adulterants based on HPLC-DAD-Q-TOF-MS associated with chemometric analysis. J. Chromatogr. B.. 2018;1090:110-121.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104527.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







