Translate this page into:
Comprehensive structural analysis of cis- and trans-tiliroside and quercetrin from Malvastrum coromandelianum and their antioxidant activities
⁎Corresponding author. kumarv@icfre.org (Vineet Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Malvastrum coromandelianum is widely used in traditional system of medicine for the cure of different ailments. In the present study, two compounds were isolated from the methanol extract and were characterized as tiliroside [kaempferol-3-O-β-d-(6″-E-p-coumaryl) glucopyranoside] and quercetrin (quercetin-3-O-α-l-rhamnopyranoside) based on comprehensive NMR, IR and mass spectral analysis. NMR also showed the presence of trans- and cis-isomers of tiliroside in 3.26:1.00 ratio. The change of NMR solvent from CH3OH-d4 to DMSO-d6 and resulting chemical shift values have been analyzed. The in vitro IC50 values against 1, 1-diphenyl-2-picryl-hydrazyl radical (DPPH) for tiliroside, quercetrin and extract was found to be 60.40 ± 0.24, 68.05 ± 0.36 and 71.98 ± 0.29 (in µg/mL ± s.d.), respectively. The ferric ion (Fe+3) reducing ability of ethyl acetate extract, tiliroside, quercetrin and standard ascorbic acid were 1.169 ± 0.006, 0.324 ± 0.012, 1.407 ± 0.008 and 1.167 ± 0.010 respectively with R2 > 0.990 at 50 µg/mL concentration. The results conclude that isolated compounds as well as extract have significant antioxidant activity and can be further developed as potential antioxidants.
Keywords
Malvastrum coromandelianum
Tiliroside
Quercetin-3-O-α-l-rhamnopyranoside
DPPH
Ferric reducing power
1 Introduction
Malvastrum coromandelianum L. (Garcke) synonym M. tricuspidatum A. (Gray) (Family: Malvaceae) is an invasive alien weed, native to North America and has naturalized all over India (Anonymous, 1962; Kirtikar and Basu, 1935). It is commonly known as ‘Kharenti’ or ‘false mallow’. The whole plant is considered as emollient, resolvent, bechic and its decoction is given in dysentery. The plant is being used in traditional system of medicine as an anti-inflammatory, analgesic and also for the cure of jaundice, ulcers (Sirkar, 1989), stomach ache (Ajibesin et al., 2008) etc. Its flowers are used for curing cough, chest and lung diseases (Srivastava et al., 1969). The different parts of the species are source to numerous herbal formulations for treating otitis (Jialin, 2014), tumor (Jiqiang, 2013), rheumatic arthralgia (Shenqing, 2012), gout (Jiaying, 2012), laying hen salpingitis (Xin and He, 2014), alongwith herbicidal composition (Hanting et al., 2013) and medicinal wine (Changding, 2013).
Pharmacological studies of the species confirmed broad range of biological activities. To elaborate, the aqueous extract of leaves showed significant antidiabetic and antihyperlipidemic activities (Deore et al., 2011; Sukanya et al., 2006). The water extract of whole plant inhibited inflammation induced by carrageenan as well as pain due to formalin stimulation (Khonsung et al., 2006). The crude water extract from aerial parts exhibited antibacterial activity against methicillin-resistant strains of Staphylococcus aureus (Chaiyasit et al., 2008; Jain et al., 2010). The chloroform and acetone extracts have significant antinociceptive activity (Reddy et al., 2001). The ethanolic extract from the species has wound healing (Gangrade et al., 2012) and immunomodulating activity (Balekar et al., 2013; Bhadoriya et al., 2012). The extract from roots, stem and leaves of plant showed hypotensive effect (Vermeersch et al., 1972). The leaf powder has larvicidal activity in dose dependent manner with LC50 value of 0.62 g/L in acetone and it delayed the growth of larvae of Aedes albopictus, a vector of dengue and chikungunya (Yadav et al., 2015). The essential oil from leaves showed fungitoxicity against the damping-off fungi with 92.31% inhibition for Pythium aphanidermatum, 82.22% for Pythium dedaryanum and 72.22% for Rhizoctonia solani (Wei and Zhenfeng, 2014). Interestingly, the extract rich in flavonoids was used for the treatment of prostate diseases, especially in the treatment of benign prostatic hyperplasia, prostate cancer and nonbacterial prostatitis (Pandey and Dubey, 1992).
Phytochemical screening revealed the presence of alkaloids, tannins, proteins, carbohydrates (Dhirendra et al., 2013) and vitamin-C in roots and leaves (Chauhan and Rawat, 2000). The phytoconstituents reported from aerial parts of the plant are β-phenylethylamine, dotriacontane, dotriacontanol, β-sitosterol, stigmasterol, campesterol, lutein, N-methyl-β-phenylethylamine, indole alkaloids (Prakash and Verma, 1983), and a steroidal saponin, 3-O-β-d-glucopyranosyl (1,2)-β-d-glucopyranosyl (1,4)-β-d-galactopyranoside 25R, 5α-spirostane-2α,3β-diol having antithyroidal activity (Panda and Kar, 2016). Further, a long alkyl side chain lactone, malvastrone has been isolated from the leaves (Alam et al., 1996). The seed oil from M. coromandelianum is a source of unusual cyclopropenoid fatty acids having palmitic acid (22.7%), palmitoleic acid (2.4%), stearic acid (2.7%), oleic acid (14.6%), linoleic acid (37.0%), malvalic acid (10.5%) and sterculic acid (10.1%) (Kallappa et al., 2004).
Despite the broad range of biological activities due to a number of interesting chemical constituents, the detailed chemical examination of the species has not been carried out. It is significant that chemical examination of the extract rich in flavonoids has not been reported earlier, despite having excellent anticancer activity (Pandey and Dubey, 1992). The previous studies have shown the efficacy of antioxidants, especially flavonoids and other polyphenols in preventing free radical damage to body and hence inhibit the activation process of various carcinogens and detoxification of activated carcinogens, thereby reducing the risk of cancer. Thus, in present investigation, two flavonoids (MM-E and MM-F) have been isolated from the aerial parts of the species and their structures have been characterized as tiliroside [kaempferol-3-O-β-d-(6″-E-p-coumaroyl) glucopyranoside; MM-E,] and quercetrin [quercetin-3-O-α-l-rhamnopyranoside, MM-F] based on spectroscopic analysis. Thorough NMR analysis of tiliroside revealed the presence of trans- and cis-isomers in 3.26:1.00 ratio. The chemical shift values of trans-tiliroside by changing from CH3OH-d4 to DMSO-d6 has also been investigated based on solute-solvent interactions. The ethyl acetate fraction and isolated compounds were also examined for in vitro free radical scavenging activity against diphenylpicrylhydrazyl (DPPH) and Fe3+ reducing ability. The laboratory analysis of isolated compounds and extract indicated significant antioxidant activity which is detailed in the following sections.
2 Exprimental
2.1 Materials
2.1.1 Plant material
The aerial parts of M. coromandelianum were collected from Forest Research Institute campus, Dehradun, India in June 2014. The plant material was authenticated by Dr. H. B. Naithani, Systematic Botanist, Botany Division, Forest Research Institute and a voucher specimen No. 170,580 was deposited at the herbarium of Systematic Botany Division, Forest Research Institute, Dehradun, India.
2.1.2 Chemicals and general experimental procedures
All organic solvents used for extraction of plant material were of laboratory grade (Merck). Solvents used for column chromatography, crystallization of compounds and chemicals used for determination of antioxidant property were of analytical grade. 1, 1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sisco Research Laboratories Pvt. Ltd., India. L-Ascorbic acid (standard) was purchased from Sigma Aldrich, St. Louis, MO, USA. Trichloroacetic acid, potassium ferrocyanide, ferric chloride, disodium hydrogen phosphate, sodium chloride and sodium hydroxide were purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India. The thin layer chromatography analyses were performed at room temperature using pre-coated plates (Merck, Silica gel 60 F254, 0.2 mm thickness). Detection of spots was done by viewing under UV light (254 and 366 nm) and spraying with 5% H2SO4 followed by heating in an oven at 100 °C for 5 min. Column chromatography was carried out using silica gel (100–200 mesh, Merck). IR spectra were recorded on a FT-IR Spectrophotometer Model RZX (Perkin Elmer). UV spectra were recorded on a Thermo Scientific Spectrascan UV 2700 spectrophotometer. NMR spectra were recorded using Bruker AV-II 400 MHz FT NMR with 5 mm multi-nuclear broad band inverse probe using TMS as an internal standard. Deuterated methanol (CH3OH-d4) was used as solvent for NMR recording. For identification and quantification of cis and trans-isomers, 1H NMR was recorded independently and raw data was processed using iNMR software. The spectra were also recorded in DMSO-d6 to study the effect of solvent on chemical shift values. ESI- MS spectrum was recorded in Thermo Fischer Scientific LCQ Advantage ion trap mass spectrometer in positive ion mode.
2.2 Extraction and isolation
Dried and powdered aerial parts (400 gm) of M. coromandelianum were extracted with solvents of elutropic series viz. petroleum ether (3 L), chloroform (3L) and methanol (3L) using soxhlet apparatus at the boiling temperature. The solvents were removed under vacuum using rotary evaporator. The above extraction process was repeated six times to obtain higher yield of extractives for isolation of compounds. The petroleum ether, chloroform and methanol extracts were obtained in 1.70% ± 0.21, 2.34% ± 0.41 and 10.35% ± 0.43 yields, respectively. The methanol extract (40 gm) was suspended in water (500 mL) and successively partitioned with chloroform (3 × 250 mL) and ethyl acetate (EtOAc; 3 × 250 mL) to yield chloroform and ethyl acetate fractions in 37.59% ± 1.01 and 0.25% ± 0.02 yields respectively. The fractionation was repeated multiple times to collect ethyl acetate fraction (5.19 gm) and subjected to column chromatography (column 132.00 cm × 2.54 cm) using silica gel (100–200 mesh, 101 gm). The retention volume was found to be 225 mL. The column was first eluted with pure chloroform (2 L), followed by increasing gradient of methanol in chloroform was increased in the ratio of 1:99, 3:97, 5:95, 7:93, 9:91, 10:90, 15:85, 20:80 and 30:70 respectively up to 30%. A total of 133 fractions of 250 mL each were collected. The fractions were examined using TLC and similar fractions were pooled together to get 9 major fractions from A to I. The fractions E (72 mg) and F (161 mg) were further purified by re-crystallization using pure methanol to obtain pure compounds MM-E (55 mg) and MM-F (147 mg) respectively.
2.2.1 Tiliroside (MM-E)
Bright yellow powder, Rf = 0.67 in MeOH: CHCl3 (21:79); IR: 3456, 1681, 1605, 1498, 1421, 1350, 1295, 1180 cm−1; UV/Vis λ-max (MeOH) nm: 211, 266, 308; ESI-MS (m/z)+: 595.1 Da [M+H]+, 617.1 Da [M+Na]+, 286.8 Da [M+H− glucose − (p-coumaryl group)]+, 309.0 Da [M+H− glucose− (p-coumaryl group) + Na]+; 1H and 13C NMR data are shown in Table 1.
→
MM-E
MM-F
Position ↓
δ H, J (Hz)
δCa
HMBC correlations
δ H, J (Hz)
δCa
HMBC correlations
2
–
157.92
–
–
157.11
–
3
–
133.83
–
–
134.84
–
4
–
177.97
–
–
178.24
–
5
–
161.50
–
–
161.78
–
6
6.13, d (2)
98.58
C-10, C-8
6.21, d (2.08)
98.42
C-8, C-10, C-5
7
–
164.48
–
–
164.44
–
8
6.30, d (2)
93.46
C-6, C-10, C-7, C-2
6.38, d (2.08)
93.33
C-10, C-6, C-7, C-9
9
–
156.95
–
–
157.90
–
10
–
104.19
–
–
104.51
–
1′
–
121.30
–
–
121.59
–
2′
8.00, d (8.8)
130.81
C-4′, C-2, C-6′
7.35, dd (2.08)
121.50
C-2, C-6′, C-4′
3′
6.83, d (8.8)
114.63
C-1′, C-4′, C-2′
–
144.99
–
4′
–
160.08
–
–
148.37
–
5′
6.83, d (8.8)
114.63
C-1′
6.93, d (8.32)
114.98
C-1′, C-3′, C-4′
6′
8.00, d (8.8)
130.81
C-4′, C-2
7.30, d (2.08, 8.32)
115.57
C-4′, C-3′, C-2′,C-2
1″
5.25, d (7.6)
102.63
C-3
5.37, d (1.42)
102.14
C-3, C-3″
2″
3.54b, m
74.38
C-4″, C-3″
4.25, quartet (1.72)
70.51
C-4″
3″
3.54b, m
76.61
C-5″, C-1″
3.78 (3.40)
70.62
C-5″
4″
3.37b, m
70.32
C-2″
3.38
71.88
C-6″
5″
3.51b, m
70.32
–
3.46
70.74
C-3″
6″a
4.34, dd (1.92, 11.88)
62.96
C-9″′
0.97, d (6.04)
16.25
C-4″, C-5″
6″b
4.23, dd (6.56, 11.88)
62.96
C-9″’
1″′
–
125.68
–
–
–
–
2″′
7.31, d (8.4)
129.76
C-4″′, C-2″′
–
–
–
3″′
6.70, d (8.4)
115.37
C-1″′
–
–
–
4″′
–
159.95
–
–
–
–
5″′
6.70, d (8.4)
115.37
C-1″′
–
–
–
6″′
7.31, d (8.4)
129.76
C- 4″′, C-2″′, C-7″′
–
–
–
7″′
7.43, d, (16)
145.14
C-9″′, C-2″′, C-8″′
–
–
–
8″′
6.10, d (16)
113.35
C-9″′, C-1″′
–
–
–
9″′
–
167.42
–
–
–
–
IR, UV-Vis and Mass spectra are given in complementary data.
2.2.2 Quercetin-3-O-α-L-rhamnopyranoside (MM-F)
Pale yellow solid, Rf = 0.45 in MeOH: CHCl3 (25: 75); IR: 3233, 1653, 1596, 1568, 1496, 1355, 1303, 1192, 1165, 1105 cm−1; UV/Vis λmax (MeOH) nm: 209, 256, 326; ESI- MS (m/z)+: 449 Da [M+H]+, 471 Da [M+Na]+, 302 Da [M+H-rhamnose]+; 1H and 13C NMR data is given in Table 1.
IR, UV-Vis and Mass spectra are given in the complementary data.
2.3 Anti-oxidant assays
2.3.1 DPPH (1,1-Diphenyl-2-picryl-hydrazyl radical) scavenging assay
DPPH radical scavenging activity was carried out with standard protocol (Rana et al., 2014; Kumara and Karunakaran, 2007). Briefly, 1 mL sample solutions of increasing concentration from 10 to 150 µg/mL (standard, pure compounds and ethyl acetate extract) was mixed with 1 mL methanol, vortexed and finally added to 1 mL of DPPH solution (0.1 mM in methanol). The reaction mixture was vortexed and incubated at room temperature in dark conditions for 30 min. Control consisted of 1 mL methanol with 1 mL DPPH solution. The absorbance of the resulting solution was measured at 517 nm. Free radical scavenging activity was calculated using the formula:
Scavenging activity (%) = (1 − AS517/AC517) × 100, where AS517 is the absorbance of the samples at different concentration and AC517 is the absorbance of control. IC50 is the minimum concentration (in µg/mL) at which 50% of DPPH (1,1-Diphenyl-2-picryl-hydrazyl) get scavenged.
2.3.2 Reducing power assay
Reducing power was determined using standard protocol (Rana et al., 2014). Briefly, 1 mL phosphate buffer (0.2 M with pH: 6.6) was added to 0.5 mL potassium ferricyanide (1%, w/v) and 1 mL of the sample (standard, pure compounds and ethyl acetate extract) of different concentrations (10–50 µg/mL in ethanol), was incubated at 50 °C in water bath for 20 min. The reaction mixture was cooled, followed by addition of 1 mL trichloroacetic acid (10%, w/v) and 0.2 mL freshly prepared ferric chloride solution (0.1%, w/v). The resulting reaction mixture was vortexed again and absorbance was measured at 700 nm. The reducing power was calculated using the formula:
Reducing power = [As700 – Ac700], where As700 is the absorbance of the sample and Ac700 is the absorbance of control in which FeCl3 solution has been replaced by water.
2.3.3 Statistical analysis
All the analyses were done in triplicate. The results were presented as the means ± standard deviation (s.d.). IC50 for DPPH scavenging activity was calculated by probit analysis using the IBM SPSS Statistic 20 package (IBM Corporation, United States) with significance level of p <0.105.
3 Results and discussion
3.1 Structural characterization of MM-E
MM-E (Fig. 1A) was obtained as a bright yellow solid. IR spectrum showed the hydroxyl absorption at 3456 cm−1 and a conjugated carbonyl absorption at 1681 cm−1 along with aromatic C⚌C absorption at 1605 cm−1. The positive ESI-MS mode showed molecular ion peak at [M+H]+ m/z: 595.1 Da with its sodium ion adduct at [M+Na]+ 617.1 Da respectively. Further, loss of sugar moiety and p-coumaryl group produced peak at [M+H-309]+ m/z: 286.8 Da and its sodium ion adduct at m/z: 309.0 Da. 1H and 13C NMR spectroscopic data (Table 1) based on HSQC spectra indicated that compound MM-E was a flavonol glycoside. The presence of the aromatic doublets at 6.13 ppm (d, 1H), and 6.30 ppm (d, 1H) having J = 2 Hz revealed meta coupling of 5,7-substituted ring-A protons and were assigned to protons H-6, H-8 respectively. The aromatic coupling system of protons at 8.00 ppm (d, J = 8.8 Hz, 2H) and 6.83 ppm (d, J = 8.8 Hz, 2H) were assigned to H-2′,6′ and H-3′,5′ of ring-B from 1H NMR spectrum. On the basis of spectral results, flavonol was assigned to be kaempferol moiety. The signals at 7.43 ppm (d, J = 16 Hz) and 6.10 ppm (d, J = 16 Hz) were assigned to two olefinic methine protons H-7′″, H-8′″ with trans coupling. The p-coumaryl group was characterized by the presence of A, A′, B, B′-type aromatic proton signals at 6.70 ppm (d, J = 8.4 Hz) for H-2′″, 6′″ and 7.31 ppm (d, J = 8.4 Hz) for H-3′′, 5′″. Multiple peaks between 3.54-3.37 ppm indicated glycosidic nature of the compound. The sugar moiety was identified as glucose based on doublet at 5.25 ppm integrating for anomeric proton with anomeric carbon signal at 102.63 ppm obtained from HSQC correlation. The other glycosidic carbon signals were found between 70 and 76 ppm in 13C NMR spectrum, while C-6′′ was observed at 62.96 ppm. The proton H-6″a and H-6″b was found at 4.34 ppm (dd, J = 1.92, 11.88 Hz) and 4.23 ppm (dd, J = 6.56, 11.88 Hz). The diaxial coupling (J = 7.44 Hz) between protons H-1″ and H-2″ of glucose suggested a β-configuration (Praveen and Khan, 1987; Agrawal, 1992) for the sugar residue. The 2D HMBC NMR spectrum confirmed the complete linkage of MM-E. The linkage of anomeric carbon C-1″of glucose with C-3 of kaempferol was deduced by cross peak correlation between H-1″ (5.25 ppm) and C-3 (133.83 ppm). The desheilding of C-6″a, 6″b signal of the glucose moiety compared with β-D-glucopyranoside (Agrawal, 1992) indicated that p-coumaroyl group might be attached to the C-6″ carbon of the glucose supporting an ester linkage. Further, the cross peak correlation of ester carbon C-9″′ (167.42 ppm) with both olefinic protons at H-7″′ (7.43 ppm) and H-8″′ (6.10 ppm) suggested the attachment of trans-double bond adjacent to ester group. The connection of 1, 4-disubstituted aromatic ring with trans-olefinic group was proposed by correlation of proton H-8″′ (6.10 ppm) with carbon C-1″′ (125.68 ppm). The substitution of coumaryl ring with hydroxyl group at C-4″′ was supported by correlation of protons H-2″′, H-6″′ (7.31 ppm) with carbon C-4″′ (159.95 ppm). With these HMBC correlations, the acyl unit was assigned to be trans-p-coumaric acid and point of attachment through glucose is C-6″ (methylene carbon of glucose) with ester carbon at C-9″′. The point of attachment of glucose with main flavonol moiety was found to be C-3 of ring-C on the basis of cross correlation of C-3 (133.83 ppm) with anomeric proton of glucose at H-1″ (5.25 ppm). The aromatic ring-B attached to ring-C at C-2 was inferred from correlation of C-2 (157.92 ppm) with H-2′, H-6′ (8.00 ppm). The other HMBC correlations of aromatic ring-B were found as H-3′/C-1′, H-3′/C-4′, H-3′/C-2′, H-2′/C-4′, H-2′/C-2, H-2′/C-6′ and H-6′/C-4′, H-6′/C-2. For aromatic ring-A, HMBC correlations were found to be H-6/C-10, H-6/C-8, H-8/C-6, H-8/C-10, H- 8/C-7 and H-8/C-2. Based on complete 1H, 13C, 2D HSQC, HMBC spectral analysis and Mass fragmentation study, compound MM-E was proposed to be tiliroside which was further confirmed with published data (Hua and Wang, 2004; Mekhelfi et al., 2014; Giang et al., 2004; Timmers and Urban, 2011; Matlawska et al., 1999). The detailed HSQC, HMBC spectra have been provided in complementary data as Figs. C3 and C6 respectively.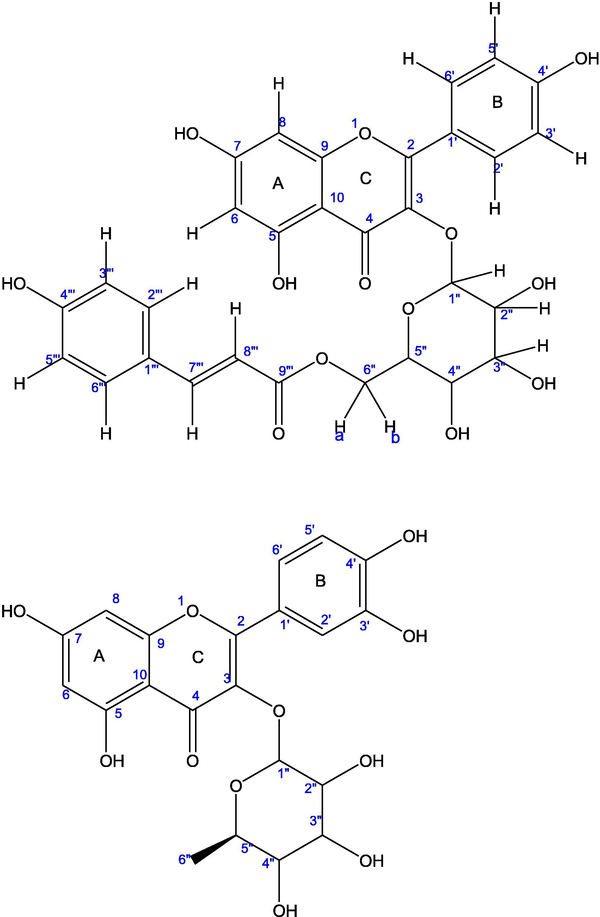
Structure of (A) tiliroside; (B) quercetrin.
3.1.1 Ratio of cis and trans isomeric forms of tiliroside (MM-E) and effect of solvent on chemical shift
While studying 1H NMR of compound MM-E (solvent-CH3OH-d4), some signals other than trans-tiliroside were also observed. In the quest for identification of these peaks, 1H NMR spectrum of MM-E was analyzed extensively. By processing the spectrum with iNMR software and close evaluation, MM-E was found to consist of both cis- and trans-isomeric form in mixture with predominance of trans isomer. Differentiation of cis and trans isomeric forms arises due to olefinic group at position H-7″′, 8″′. The olefinic proton H-7″′ was recognised with chemical shift at 7.32 ppm (J = 16 Hz) for trans isomer and 6.61 ppm (J = 12.8 Hz) for cis isomer. The olefinic proton H-8″′ was observed at 5.99 ppm (J = 16 Hz) for trans isomer and 5.43 ppm (J = 12.8 Hz) for cis isomer. The protons of trans-tiliroside is deshielded as compared to cis-form due to the fact that it is a type of α, β-unsaturated carbonyl system with planar geometry. The presence of polar carbonyl group adjacent to double bond and coplanarity reinforce the creation of partial polarization of carbonyl group which is further resonance stabilized by delocalization of +ve charge over the structures II a and III a (Fig. 2). The structure III a may further enter into aromatic delocalization due to planar geometry. Thus, resonance stabilization along with diamagnetic anisotropy of carbonyl group deshielded the protons (7″′, 8″′) of trans-tiliroside making signals downfield. Also in structure III a, β-position is carrying + ve charge causing larger deshielding of β-proton H-7″′ as compared to H-8″′. Additionally, 3JHH coupling value for trans-form (16 Hz) is larger because the H—C—C—H dihedral angle is 180° resulting in maximum backside overlap of p-orbitals according to Karplus and Conroy theory (Gunther, 1995).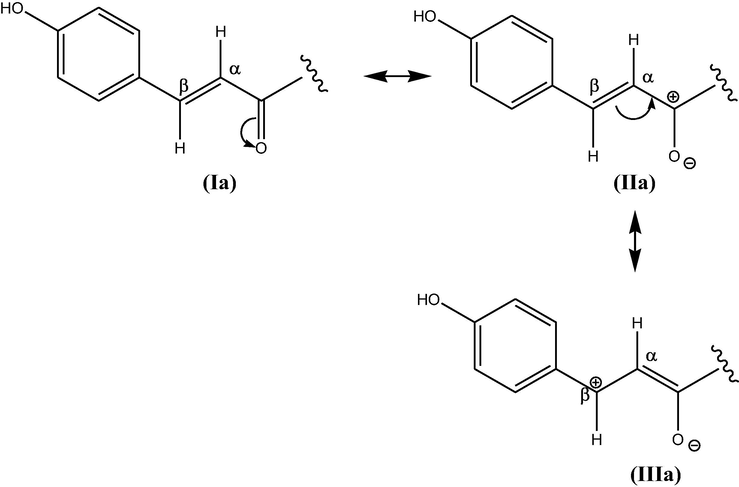
Resonance stabilisation in trans-isomer.
In contrast to trans- tiliroside, cis- form possessed non-planar geometry with distorted π- system. The charge created by partial polarization of carbonyl group in structure-II b (Fig. 3) is not further resonance stabilized because delocalization is not possible in non-planar structure which in turn reduces the deshielding caused by resonance stabilization as well as magnetic anisotropy of carbonyl group. Thus, protons (7″′, 8″′) of cis- tiliroside are upfield. Also, 3JHH coupling value for cis-form (12.8 Hz) is smaller as compared to trans- form as H—C—C—H dihedral angle is 60° in cis- form resulting in poor overlap of p-orbitals (Gunther, 1995). As cis and trans-tiliroside differed at olefinic position H-7″′ and H-8″′, so the ratio of two isomers were calculated using integration of proton from same position (7″′, 8″′) shown in Table 2. The calculation was done using formula:
where ITRANS = integration of (H-7″′ + H-8″′) of trans isomer, ICIS = integration of (H-7″′ + H-8″′) of cis isomer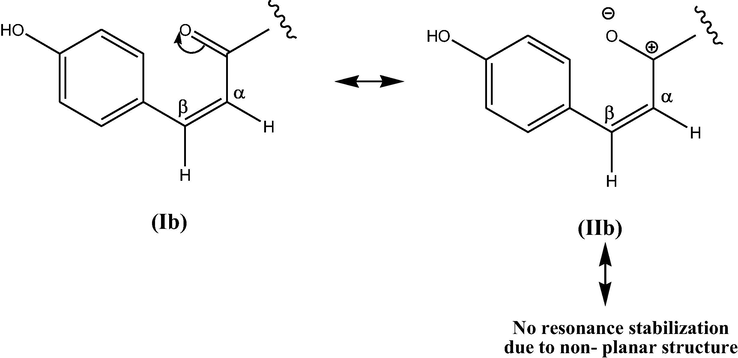
Resonance stabilisation in cis-isomer.
Isomers
H-7″′
H-8″′
Total
Cis
0.373
0.204
0.577
Trans
1.0
0.882
1.882
Total
–
–
2.459
The trans- and cis-tiliroside isolated from M. coromandelianum was calculated and found to be in the ratio of 3.26: 1 (trans: cis).
It is worth mentioning that cis- and trans-isomers in olefinic system are not interconvertible at room temperature because of restricted rotation around double bond. Trans-isomer is more stable than cis-isomer thermodynamically due to symmetry and steric effects. The interconversion could be achieved via breaking of π-bond and promoting electron from π-orbital (bonding) to π∗-orbital (antibonding) (Clayden et al., 2012). Thus, possibility of interconversion of cis- and trans- tiliroside during extraction from plant, isolation, purification and recrystallization process could be omitted. The excess of trans-isomer in tiliroside could be attributed to its greater stability as compared to cis-form.
The change of NMR solvent shows a significant effect on the chemical shift values of protons for flavonoids (Pauli, 2000). The 1H NMR of MM-E (Tiliroside) was also recorded in DMSO-d6. The whole spectrum moved downfield with deshielding effect. Anomeric protons of 3-O-glc moiety are the most influenced by change of solvent polarity with prominent shift of signal from 5.10 ppm (cis), 5.15 (trans) for (CH3OH-d4) to 5.42 ppm (cis) and 5.46 ppm (trans) for DMSO-d6. Increase in solvents dielectric constant tends to move the proton resonance towards downfield. Further, surrounding solvent environment partially polarize the electric dipole moment of polar solute, thereby, producing electric reaction field having linear relation with downfield shift of proton resonance (Becconsall and Hampson, 1965; Matsuo, 1967). The dielectric constant of DMSO is higher than methanol, thereby, making it more polar which in turn increases the extent of solute solvent interaction as well as the intermolecular hydrogen bonding. However, the signal dispersion is not appropriate in DMSO, making it less suitable solvent for NMR study of flavonoids (Pauli, 2000). Also in the present case, proton NMR signals are not properly dispersed and overlapping make the interpretation of spectra complicated. The chemical shift values for rest of protons from spectra in MeOH-d4 and DMSO-d6 are summarized in Table 3. The individual spectrum for MeOH-d4 and DMSO-d6 has been given in Figs. C11 and C14 respectively in complementary data.
Solvent
Proton position
CH3OH-d4
DMSO-d6
Cis-isomer
Trans-isomer
Cis-isomer
Trans-isomer
δ H, J (Hz)
6
6.10 (2)
6.04 (2)
6.21 (2)
6.16 (2)
8
6.11 (2)
6.22 (2)
6.36 (2)
6.39 (2)
2′
7.87 (8.8)
7.90 (8.8)
7.97 (8.8)
8.00 (8.8)
3′
6.85 (8.8)
6.73 (8.8)
6.93 (8.8)
6.87 (8.8)
5′
6.85 (8.8)
6.73 (8.8)
6.93 (8.8)
6.87 (8.8)
6′
7.87 (8.8)
7.90 (8.8)
7.97 (8.8)
8.00 (8.8)
1″
5.10 (7.6)
5.15 (7.6)
5.42 (8.8)
5.46
6″a
3.71
4.22 (2.4)
4.18 (2.4)
4.30 (2)
6″b
3.69
4.11 (6.8)
4.10
4.06 (6.4)
2″′
7.41 (8.4)
7.22 (8.4)
7.56 (8.8)
7.38 (8.8)
3″′
6.59 (8.4)
6.69 (8.4)
6.85
6.80 (8.8)
5″′
6.59 (8.4)
6.69 (8.4)
6.85
6.80 (8.8)
6″′
7.41 (8.4)
7.22 (8.4)
7.56 (8.8)
7.38 (8.8)
7″′
6.61 (12.8)
7.32 (16)
6.69 (12.8)
7.37 (15.6)
8″′
5.43 (12.8)
5.99 (16)
6.66 (12.8)
6.13 (15.6)
3.2 Structural characterization of compound MM-F
MM-F (Fig. 1B) was obtained as a pale yellow solid. IR spectrum showed the absorption bands at 3233 cm−1 and 1653 cm−1 representing hydroxyl and conjugated carbonyl groups alongwith aromatic stretching at 1596 cm−1. The positive ESI-MS analysis showed molecular ion peak [M+H]+ at m/z: 449.0 Da with sodium ion adduct of molecular ion [M+Na]+ at m/z: 471.0 Da respectively. The loss of rhamnose group produced peak [M+H-146]+ at m/z: 302.9 Da. 1H and 13C NMR spectroscopic data of MM-F (Table 1) based on HSQC spectra revealed signals for quercetin and a sugar moiety. In 1H NMR spectrum, coupling system with characteristic of a 3′, 4′-substituted ring-B between protons at 7.35 ppm (dd, J = 2.08 Hz, 1H), 6.93 ppm (d, J = 8.32 Hz, 1H) and 7.30 ppm (d, J = 2.08 Hz, 8.32 Hz, 1H) accounting to H-2′,5′,6′. The aromatic meta-coupled protons of a 5,7-substituted ring-A at 6.21 ppm (d, J = 2.08 Hz, 1H), and 6.38 ppm (d, J = 2.08 Hz, 1H) were assigned to H-6 and H-8 respectively confirming the identity of main flavonoid moiety as quercetin. The glycosidic nature was indicated by multiple peaks at 3.46–3.78 ppm in the 1H NMR spectrum. The sugar moiety was identified to be rhamnose from a doublet due to methyl group at 0.97 ppm with methyl carbon signal at 16.25 ppm. The other glycosidic carbon signals were found between 70 and 72 ppm in 13C NMR spectrum. The 2D HMBC NMR spectra was analyzed to carry out complete structural assignment of MM-F. The rhamnose unit was found to be monosubstituted and cross peak correlation of anomeric proton H-1″ at 5.37 ppm of rhamnose with carbon C-3 at 134.84 ppm of aromatic ring-C indicated point of attachment of sugar residue with ring-C at C-3. The aromatic ring-B was attached to ring-C at C-2 which was inferred from HMBC NMR correlation of C-2 (157.11 ppm) with H-2′ (7.35 ppm) and H-6′ (7.30 ppm). The other HMBC correlation of ring-B protons were found as H-2′/C-4′, H-2′/C-6′, H-5′/C-1′, H-5′/C-3′, H-6′/C-4′, H-6′/C-3′, H-6′/C-2′ respectively. For ring-A, HMBC correlations were observed at H-8/C-10, H-8/C-6, H-8/C-7, H-8/C-9 and H-6/C-8, H-6/C-10, H-6/C-5 which indicated the 5, 7-substitution of ring-A. HMBC correlation for sugar residues were found at H-2″/C-4″, H-3″/C-5″, H-4″/C-6′, H-6″/C-4″, H-6″/C-5″, H-5″/C-3″. Based on complete 1H, 13C, HSQC, HMBC spectral analysis and mass fragmentation study, compound MM-F was proposed to be quercetin-3-O-α-L-rhamnopyranoside which was further confirmed with published data (Bilia et al., 1996; Ishiguro et al., 1991; Markham and Temai, 1976; Aderogba et al., 2013). The HSQC and HMBC spectra have been provided in complementary data as Figs. C7 and C8 respectively.
3.3 Scavenging effect on DPPH radicals
DPPH is a stable purple coloured free radical showing maximum absorption at 517 nm and widely used to evaluate the antioxidant capacity of plant extracts as well as pure compounds. When an electron or hydrogen atom donating antioxidant is added to DPPH system, the stable diphenylpicrylhydrazine (DPPH-H, yellow colored) is formed (Rana et al., 2014). The number of DPPH radicals get reduced which is proportional to the number of available hydroxyl groups of antioxidants. DPPH scavenging activity of isolated compounds and ethyl acetate fraction revealed their strong antioxidant ability. IC50 values for DPPH free radical scavenging activity of MM-E, MM-F and ethyl acetate fraction and a comparison graph of free radical scavenging ability is shown in Fig. 4. The free radical scavenging activity of pure compounds as well as crude ethyl acetate extract increased significantly (p < .150) with increase in concentration of dose level from 10 µg/mL to 150 µg/mL. IC50 values of ethyl acetate extract, MM-E, MM-F and standard ascorbic acid were calculated as 71.98 ± 0.29, 60.40 ± 0.24, 68.05 ± 0.36, 64.27 ± 0.28 (in µg/mL ± s.d.). Free radical scavenging effect of isolated compounds and ethyl acetate extract were comparable to standard ascorbic acid.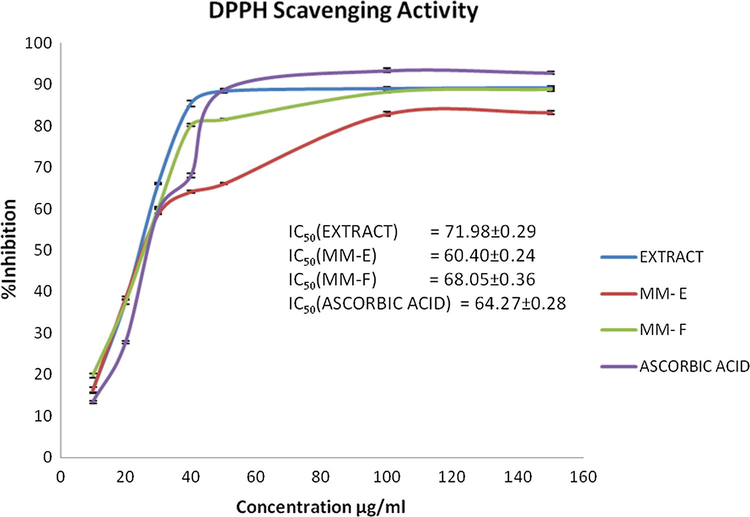
DPPH Scavenging ability.
3.4 Ferric (Fe3+) ion reducing power
The antioxidant power of a compound can also be calculated by reducing power assay, in which the presence of reductant in the test samples would reduce Fe3+ (Ferricyanide complex) to the Fe2+, with change in colour of the reaction mixture from yellow to variable shades from blue to green depending upon the reducing power of the compound. The reducing capacity of a compound is a significant indicator of its antioxidant ability (Rana et al., 2014; Yang et al., 2011). A reductant may also stop peroxide formation by carrying out reaction with certain precursors of peroxides. Higher value of absorbance at 700 nm indicates higher reducing power or stronger antioxidant activity. The reducing power of MM-E, MM-F and ethyl acetate fraction is shown in Fig. 5, alongwith standard ascorbic acid. The reducing power ability of crude extract was highest and least for MM-E among the compounds tested. At conc. of 50 µg/mL; the reducing power ability of ethyl acetate fraction, MM-E, MM-F and standard ascorbic acid were 1.169 ± 0.006 (R2 = 0.999), 0.324 ± 0.012 (R2 = 0.955), 1.407 ± 0.008 (R2 = 0.994), 1.167 ± 0.010 (R2 = 0.995) respectively. The order of reducing power ability was found in the order: ethyl acetate fraction> MM-F > standard > MM-E. The reducing power ability of extract and compounds was dose dependent and activity increases with the increase in concentration of test samples.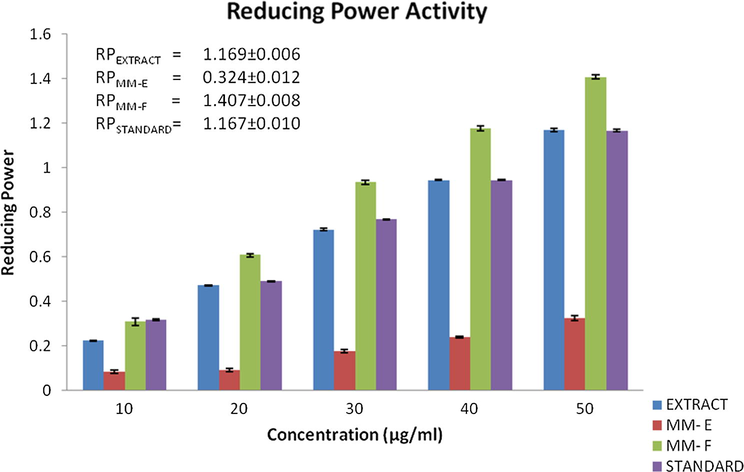
RP = Reducing ability at concentration of 50 µg/mL.
Free radicals cause oxidative cell damage to the tissues and leads to diseases like aging, diabetes, immunosuppression, neurodegeneration and cancer etc. The oxidative stress due to exogenous and endogenous sources is positively correlated to chronic and degenerative ailments. Free radicals act as strong carcinogens and damage DNA via altering the genes such as oncogenes, tumor suppressor, apoptosis-regulating, and DNA-repair genes that control cancer. The antioxidants especially polyphenols interrupt the intracellular signaling network of initiation and progression of cancer cells by modifying gene expression system, thereby modulating the apoptosis of cancer cells. Due to these bioactivities, polyphenols are useful in prevention as well as reduction of free radical damage which may lessen the risk of these diseases (Fresco et al., 2010; Link et al., 2010; Thomasset et al., 2006). Plant extracts and compounds from plant sources possess potential antioxidant ability thereby reducing free radical damage and hence preventing the diseases caused by these damages. In the present study, extract and isolated compounds were analyzed for antiradical activity (established by DPPH assay) and ferric reducing (Fe3+) ability. The results indicate significant antioxidant activity and these compounds can be further developed as potential antioxidants.
4 Conclusion
The present study resulted in isolation of two pure flavonol glycosides from ethyl acetate fraction from the aerial parts of M. coromandelianum. Based on extensive spectroscopic analysis, the compounds were identified as tiliroside [kaempferol-3-O-β-d-(6″-E-p-coumaryl)glucopyranoside; MM-E] and quercetrin [quercetin-3-O-α-l-rhamnopyranoside; MM-F]. NMR analysis showed that cis- and trans- tiliroside are in 1:3.26 ratio. These flavonoids have been reported for the first time from the species. The isolated compounds and ethyl acetate extract showed potent free radical scavenging activity and ferric ion reducing ability which is comparable to standard ascorbic acid. The anticancer activity of M. coromandelianum extract may also be due to strong antioxidant property. It signifies the importance of ethyl acetate extract as a rich source of powerful natural antioxidants which may also be helpful in preventing life threatening diseases like cancer and providing a rationale for the ethnomedicinal use of the plant species. The study may aid in utilization of the weed, abundantly available in waste and agricultural lands in India as a potential source of bioactive compounds.
Acknowledgement
The authors are grateful to the Director, Forest Research Institute (FRI), Dehradun for providing laboratory facilities. Thanks are also due to Dr. H.B. Naithani, Systematic Botanist, Forest Research Institute for authentication of the plant material.
References
- Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules. 2013;18:12633-12644.
- [Google Scholar]
- NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry. 1992;31:3307-3330.
- [Google Scholar]
- Ethnobotanical survey of AkwaIbom State of Nigeria Kola. J. Ethnopharmacol.. 2008;115:387-408.
- [Google Scholar]
- A new lactone from Malvastrum coromandelianums. Indian J. Chem Sect. B: Org. Chem.. 1996;12:1354-1355.
- [Google Scholar]
- The Wealth of India: a dictionary of Indian raw materials and industrial products-raw material series. New Delhi: Publications and Information Directorate, Council of Scientific & Industrial Research; 1962. p. :251.
- Immunopotentiating effect of ethanolic extract of Malvastrum tricuspidatum A Gray, whole plant. Indian J. Natural Prod. Res.. 2013;4:54-60.
- [Google Scholar]
- Solvent effects in proton and 13C NMR chemical shifts of polar compounds. Mol. Phys.: Int. J. Interface Chem. Phys.. 1965;1(10):21-32.
- [Google Scholar]
- Immunomodulatory effect of M. tricuspidatum. Int. J. Curr. Pharm. Res.. 2012;4(2):33-36.
- [Google Scholar]
- Phytochemical investigations of Licaniagenus. Flavonoids from Licania pyrifolia. Pharm. Acta Helvetiae. 1996;71:199-204.
- [Google Scholar]
- Antibacterial activity of Malvastrum coromandelianum garcke against methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus. Curr. Res. Bacteriol.. 2008;1:42-45.
- [Google Scholar]
- Changding P., 2013. Medicinal wine and its preparation method. CN 103301401 A 20130918.
- Chemical analysis of certain medicinal plants of Garhwal Himalayas. Asian J. Chem.. 2000;12(4):1339-1340.
- [Google Scholar]
- Clayden J., Greeves W., Warren S., 2012. Organic Chemistry, second ed. Oxford University Press (Chapter 7) 151–179.
- Antidiabetic and antihyperlipidemic activities of Malvastrum coromadeliaum Linn leaves in alloxan induced diabetic rats. Pharmacol. Online. 2011;3:147-154.
- [Google Scholar]
- Pharmacognostic and phytochemical investigation of the leaves of Malvastrum coromandelianum (L.) Garcke. Ancient Sci. Life. 2013;33(1):39-44.
- [Google Scholar]
- The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des.. 2010;16:114-134.
- [Google Scholar]
- Investigation of wound healing activity of Malvastrum tricuspidatum syn Malvastrum coromandelianum on experimental animals. Int. J. Pharm. Life Sci.. 2012;3(12):2259-2264.
- [Google Scholar]
- Flavonoid glucosides from the leaves of Croton tonkinensis Gagnep Euphorbiaceae. J. Chem.. 2004;42:125-128.
- [Google Scholar]
- Gunther H., 1995. NMR spectroscopy: Basic principles, concepts, and applications in chemistry, second ed. John Wiley & Sons (Chapter 4), 69–133.
- Hanting C., Zhiwei F., Yide S., Qiaoqiao H., Xiaoxia L., 2013.Herbicidal composition for Manihot esculenta field. CN 103392703 A 20131120.
- Chemical Components of Anaphalis sinica Hance. J. Chin. Chem. Soc.. 2004;51:409-415.
- [Google Scholar]
- Sarothralin G: a new antimicrobial compound from Hypericum japonicum. Part 6. A flavanonol rhamnoside from Hyperidcum japonicum. Phytochemistry. 1991;30:3152-3153.
- [Google Scholar]
- Antimicrobial activity and phytochemical screening of five wild plants against Escherichia coli, Bacillus subtilis and Staphylococcus aureus. J. Pharm. Res.. 2010;3(6):1260-1262.
- [Google Scholar]
- Jialin B., 2014. Chinese medicine for treatment of otitis media and its preparation method. CN 103656297 A 20140326.
- Jiaying M., 2012. Method for manufacturing traditional Chinese medicine preparation for treating gout. CN 102302706 A 20120104.
- Jiqiang N., 2013. Traditional Chinese medicine composition for treating tumor. CN 103393743 A 20131120.
- A moderate source of cyclopropenoid fatty acids in Malvastrum coromandelianum seed oil and its possible medicinal importance. J. Med. Aromat. Plant Sci.. 2004;26(2):315-317.
- [Google Scholar]
- Anti-inflammatory and Analgesic Activities of Water Extract of Malvastrum coromandelianum (L.) Garcke. Thai. J. Pharmacol.. 2006;28:3.
- [Google Scholar]
- Indian Medicinal Plants (2nd ed.). New Delhi: International Book Distributors; 1935. p. :305-306.
- Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chem.. 2007;100:356-361.
- [Google Scholar]
- Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol.. 2010;80:1771-1792.
- [Google Scholar]
- 13C NMR of Flavonoids. II. Flavonoids other than flavone and flavonol aglycones. Tetrahedron. 1976;32:2607-2611.
- [Google Scholar]
- Flavonoid compounds in Lavatera thuringiaca (Malvaceae) flowers. Acta Poloniae Pharm. – Drug Res.. 1999;56(6):453-458.
- [Google Scholar]
- Studies of the solvent effect on the chemical shifts in nmr spectroscopy. ii. solutions of succinic anhydride, maleic anhydride and the N-substituted imides. Can. J. Chem.. 1967;45:1829-1835.
- [Google Scholar]
- Phytochemical constituents of Thymelaea microphylla Coss. Et Dur. from Algeria. Der Pharmacia Lettre. 2014;6:152-156.
- [Google Scholar]
- Role of a gitogenin-type steroidal saponin (3-O-β-D-glucopyranosyl (1, 2)-β-D-glucopyranosyl (1, 4)-β-D-galactopyranoside-25R, 5α-spirostane-2α, 3β-diol), isolated from the leaves of Malvastrum coromandelianumin regulating thyrotoxicosis in rats. Bioorg. Med. Chem. Lett.. 2016;26:4804-4807.
- [Google Scholar]
- Effect of essential oils from some higher plants against fungi causing damping-off disease. Biologia Plantarum.. 1992;34(1–2):143-147.
- [Google Scholar]
- Higher order and substituent chemical shift effects in the proton NMR of glycosides. J. Nat. Prod.. 2000;63:834-838.
- [Google Scholar]
- Chemical constituents from the aerial portion of Malvastrum tricuspidatum A Gray. Indian J. Pharm. Sci.. 1983;45:102-103.
- [Google Scholar]
- Luteolin 7,4’-dimethyl ether 3’-glucoside from Gelonzum multiflorum. Phytochemistry. 1987;26:2130-2131.
- [Google Scholar]
- Multifunctional properties of polysaccharides from Dalbergia sissoo, Tectona grandis and Mimosa diplotricha. Carbohydr. Polym.. 2014;102:341-350.
- [Google Scholar]
- Antinociceptive activity of Malvastrum coromandelinum. Fitoterapia. 2001;72:278-280.
- [Google Scholar]
- Shenqing F.Z., 2012. Traditional Chinese medicine preparation for treating rheumatic arthralgia. CN 102366576 A 20120307.
- Pharmacological basis of Ayurvedic therapeutics. In: Atal C.K., Kapoor B.M., eds. Cultivation and Utilization of Medicinal Plants. Council of Scientific & Industrial Research: Publications and Information Directorate; 1989.
- [Google Scholar]
- Survey of Indian plants for saponins, alkaloids and flavonoid. Lloydia. 1969;32:297-304.
- [Google Scholar]
- Plant compositions having oral insulin-like hypoglycemic activity and hypolipidemic & antimicrobial activities. Eur. Pat. Appl. EP. 2006;1723960(A1):20061122.
- [Google Scholar]
- Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2006;120:451-458.
- [Google Scholar]
- On-line (HPLC- NMR) and off-line phytochemical profiling of the Australian plant Lasiopetalum macrophyllum. Nat. Prod. Commun.. 2011;7:551-560.
- [Google Scholar]
- Reportedly hypotensive Madagascan plant, Malvastrum coromandelianum. Bulletin de la Societe de Pharmacie de Lille. 1972;1:35-44.
- [Google Scholar]
- Wei X., Zhenfeng H., 2014. Malvastrum total flavonoids extract and its preparation method and uses. CN 103830288 A 20140604.
- Xin L., He H., 2014. Veterinary Chinese medicinal composition for treating laying hen salpingitis and its preparation method. CN 103735832 A 20140423.
- Screening of some weeds for larvicidal activity against Aedes albopictus, a vector of dengue and chikungunya. J Vector Borne Dis. 2015;52:88-94.
- [Google Scholar]
- In vitro antioxidant activities of sulfated polysaccharide fractions extracted from Corallina officinalis. Int. J. Biol. Macromol.. 2011;49:1031-1037.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2018.01.009.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







