Translate this page into:
Construction of a new 2D coral-like covalent organic framework as CuI nanoparticles carrier for the preparation of diverse triazoles
⁎Corresponding authors. zolfi@basu.ac.ir (Mohammad Ali Zolfigol), mzolfigol@yahoo.com (Mohammad Ali Zolfigol), myarie.5266@gmail.com (Meysam Yarie)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
General experimental route for preparation of triazoles by using PDC-COF@CuI as catalyst.
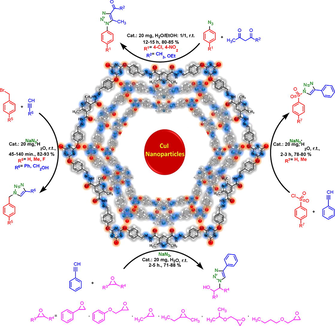
Abstract
The new 2D coral-like covalent organic framework (COF) with hydrazone linkages containing pyridine dicarbohydrazide moieties was constructed. Different parameters of COF such as chemical composition, morphology, porous structure, specific surface area and thermal stability were investigated. Catalytic application of desired COF as CuI nanoparticles carrier was investigated for the preparation of diverse triazoles.
Abstract
In this work, a novel two-dimensional coral-like covalent organic framework (COF) with hydrazone linkages containing pyridine dicarbohydrazide moieties namely PDC-COF was designed and developed through a condensation reaction between the tri(4-formyl phenoxy) cyanurate (TFPC) and 2,6-dimethylpyridine-3,5-dicarbohydrazide (DMPDC) without applying inert atmosphere and using of any acidic catalyst. PDC-COF applied as carrier for CuI nanoparticles for construction of a robust heterogeneous catalyst (PDC-COF/CuI). This catalyst shows high thermal stability (up to 500 °C), good porosity (≃50 m2 g−1), acceptable crystalline structure with coral-like morphology. Accordingly, the obtained data revealed that the synthesized PDC-COF/CuI is highly active heterogeneous catalyst for the preparation of diverse triazoles by using different starting materials under green and mild reaction conditions. In addition, in comparison with the reported data in the literature, all of the prepared compounds have good yields (71–93%) and relatively short reaction times (45 min–15 h). Moreover, PDC-COF/CuI shows excellent stability and reusability (up to 6 times) without any significant decreases in its catalytic performance.
Keywords
Covalent organic framework
Hydrazone linkage
Triazole
Heterogeneous catalyst
1 Introduction
In few last years, covalent organic frameworks (COFs) as versatile, reticular and irreplaceable class of porous organic polymers with inherent porosity and excellent adjustable functionality have attracted considerable attention (Geng et al., 2020; Lee and Cooper, 2020; Dey et al., 2021; Cusin et al., 2021). Advanced features such as high specific surface area, tunability, sustainability, low density, high thermal and chemical stability and possibility of post-synthetic modification have led to a promising outlook towards these compounds (Pachfule et al., 2018; Altaf et al., 2021; Li et al., 2020). Unique and tremendous utilities have been devoted to these pre-designable polymers which include catalytic applications, gas storage and separation, energy storage, mass transport, sensor, drug delivery and etc. (Yang et al., 2016; Schneemann et al., 2021; Yang et al., 2020; Xu et al., 2021; Babu et al., 2019; Scicluna and Vella-Zarb, 2020; Liu et al., 2019; Gao et al., 2018; Lyu et al., 2019). In general, different features of COFs such as the chemical stability, catalytic activity and adsorption behavior are in turn affected by essence of linkages. Hydrazone linkages due to their irreversible tautomerization of enol to keto and intramolecular hydrogen bonding interactions between N—H and C⚌O groups can be enhanced the stability and crystallinity of polymer geometry (Li et al., 2019; Uribe-Romo et al., 2011; Mitra et al., 2017; Qian et al., 2020). In addition, hydrazone linkages are privileged active sites for confining metals and this is a developing trend in relevant areas (Zhuang et al., 2021; Li et al., 2015; Yang et al., 2016). These architectures have well-defined durable skeletons and coherent pores which their morphology can be predicted already and also, they can be active catalytic precursors for many organic transformations (Liu et al., 2020; Krishnaraj et al., 2021; Cheng and Wang, 2021; Puthiaraj et al., 2016). Catalytic utilities of COFs are well known in the domain of photocatalysis, metal-based catalysis, asymmetric catalysis and electrocatalysis (Liu et al., 2019; Xu et al., 2020; Rogge et al., 2017; Zhang et al., 2021; Li et al., 2020). Hereupon, there are intensifying efforts for post-synthetic metalation of these versatile systems which leads to a revolutionary prospect in heterogeneous catalysis (Dong et al., 2021; Ma et al., 2020).
The catalytic applications of CuI nanoparticles due to their high natural abundance, selectivity, high activity and low cost have achieved a great deal of interest such as electrocatalysis, organometallic catalysis, photocatalysis, and gas-phase catalysis (Kolb et al., 2001; Rostovtsev et al., 2002; Wu et al., 2014; Gawande et al., 2016). In this regard, CuI nanoparticles as catalyst represent a paradigm example of click chemistry and is regarded as one of the most critical synthetic trends for the preparation of triazoles (Qin et al., 2010). However, the CuI-based catalyst alone has limitations and was applied only once and coordination of copper residues with the constructed triazole ring prevents its easy separation. However, CuI supported porous polymers such as CuI supported COFs as a heterogeneous catalyst can easily separate and reuse several times (Qin et al., 2007; Chen et al., 2022).
Recently, catalytic performance of copper decorated COFs as heterogeneous catalytic platforms have been drawn great attentions, in several reactions such as Henry, carbon–carbon coupling, carbon–nitrogen (Chan-Lam) coupling, Huisgen reactions and photocatalysis processes (Romero-Muñiz et al., 2021; Han et al., 2018; Han et al., 2018; Chen et al., 2022). Due to their high atom economy, efficiency, regioselectivity, and functionality tolerance, Cu (I)-coordinated COFs are integral parts of click chemistry domain. Formation of triazole families through COF/Cu(I)-catalyzed cycloaddition reactions are indisputable synthetic routes which represents several benefits such as mild reaction conditions, simple recovering and reusing method of catalyst and easy workup procedure (Romero-Muñiz et al., 2021; Haque et al., 2020; Bu et al., 2021; Jin et al., 2019). Surveying of literatures of recent years, reveals several reports for applying copper decorated COFs in Huisgen reaction (Muller and Bräse, 2011; Vilé et al., 2022).
Over the last few decades, five-membered N-heterocycle compounds exhibit comprehensive versatility and utility. Synthesis of 1,2,3-triazoles as a major family of five-membered N-heterocycles, have been emerged as a prominent academic and industrial research field (Song and Deng, 2018; Feng et al., 2020; Deng et al., 2019). These special fragments are associated with a wide range of biological and chemical compounds (Zeng et al., 2020; Schulze and Schubert, 2014; Xu et al., 2020). Also, 1,2,3-triazole moiety is necessary for the construction of many energetic materials, pharmacological compounds, luminescent systems, dyes and chemo-sensors and have been flourished by their extensive biological activities such as anti-bacterial, anti-cancer, anti-microbial, anti-malarial, c-Met kinase and xanthine oxidase inhibitors (Barve et al., 2017; Grob et al., 2021; Tasca et al., 2015; Sajja et al., 2017). Copper(I)-catalyzed transformation is the most useful tool for the synthesis of 1,3,5-triazoles and this is a paradigm of click chemistry (Castillo et al., 2020; Liang and Astruc, 2011).
In this investigation, a new 2D covalent organic framework with hydrazone linkages as CuI nanoparticles carrier was synthesized via a straightforward procedure through a condensation reaction between the tri(4-formyl phenoxy) cyanurate (TFPC) and 2,6-dimethylpyridine-3,5-dicarbohydrazide (DMPDC) without applying inert atmosphere and using of any acidic catalyst and its catalytic application was investigated for the preparation of diverse triazoles by using different starting materials (Schemes 1 and 2).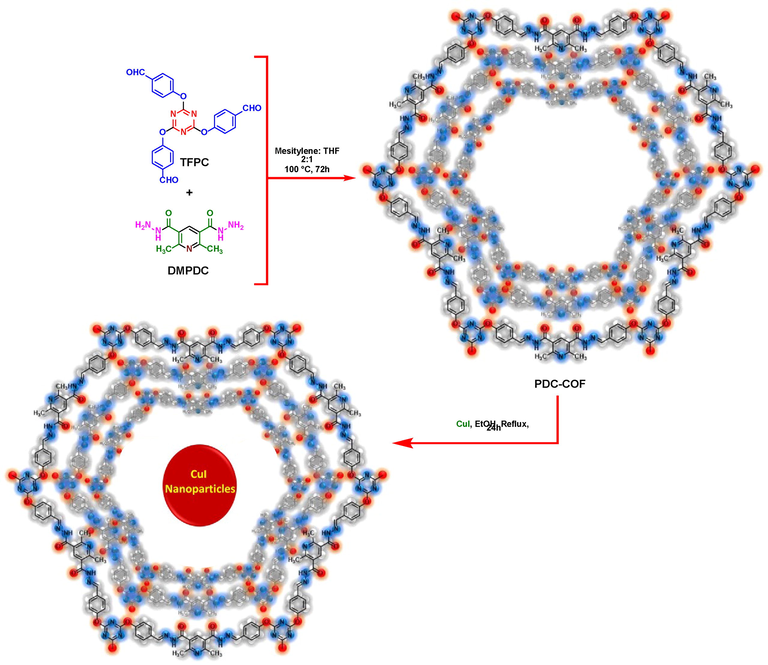
Representation of experimental procedure for the synthesis of PDC-COF/CuI.
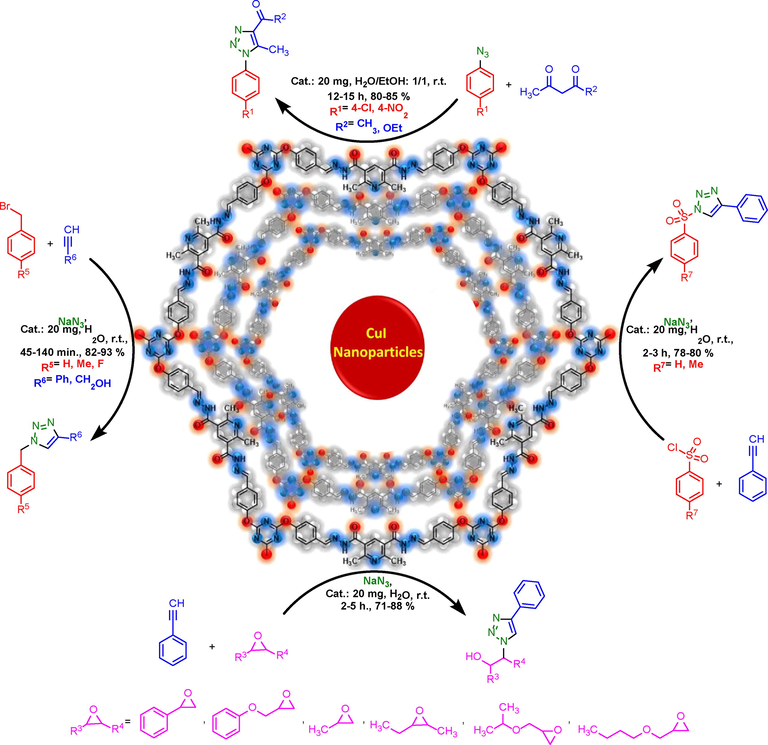
General experimental route for preparation of triazoles by using PDC-COF/CuI as catalyst.
2 Results and discussion
2.1 Spectroscopic characterization of PDC-COF and PDC-COF/CuI
The formation of PDC-COF/CuI was confirmed by several techniques. Different parameters such as chemical composition, morphology, porous structure, specific surface area and thermal stability of the PDC-COF/CuI were investigated with more details in below.
At first, by applying FT-IR, analysis the main functional groups and chemical structure of PDC-COF/CuI, PDC-COF, DMPDC and TFPC were investigated via a comparative manner. According to FT-IR spectrum of TFPC monomer, the clear bands of C⚌C, C⚌O and CHO appeared at 1538, 1696 and 2830 cm−1. Also, in FT-IR spectrum of DMPDC monomer, the typical vibrational modes of N—H, NH2, amide type C⚌O and C⚌N groups are shown respectively at 3304, 3201, 1672 and 1637 cm−1. In FT-IR spectrum of PDC-COF, the appearance of a new peak in 1658 cm−1 is a definitive sign for the formation of imine linkages. Furthermore, deletion of stretch vibration band of NH2 (in DMPDC monomer) and manifestation of a broad peak at about 3200–3600 cm−1 verified the successful construction of PDC-COF. In addition, there are a little difference between PDC-COF and PDC-COF/CuI. This is a good indication that CuI is confined in the PDC-COF networks and yet no change in its structure has taken place (Fig. 1). Also, the chemical structure of DMPDC and TFPC and was verified by 1H NMR and 13C NMR analysis (see ESI).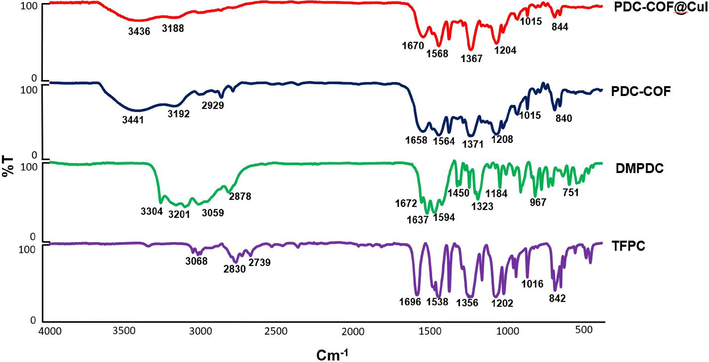
Comparison of FT-IR spectra of PDC-COF/CuI, PDC-COF and DMPDC and TFPC as two key monomers.
2.2 Investigation of morphology of PDC-COF and PDC-COF/CuI
The morphologies of PDC-COF and PDC-COF/CuI were investigated by FESEM and TEM analysis (Figs. 2 and 3). Interestingly, FESEM images showed that PDC-COF possessed a steady filamentous and coral-like morphology with an average size in the range of about 26–53 nm. Furthermore, the network-like structure and high porosity for both of the PDC-COF and PDC-COF/CuI are clearly recognizable in achieved FESEM images. Also, this network structure does not contain small polymer fragments, which confirms the removal of unwanted small oligomeric fragments and starting materials. In addition, FESEM images of PDC-COF/CuI confirmed that the morphology remained almost unchanged after adding CuI. On the other, this ordered filamentous and coral-like morphology has become an ideal candidate for fixing of CuI nanoparticles on its surface and/or cavities. Also, the flake and layered morphology of PDC-COF verified by TEM images. Furthermore, the aggregation observed in TEM images can be attributed to the hydrogen bonding and π–π stacking interactions between layers.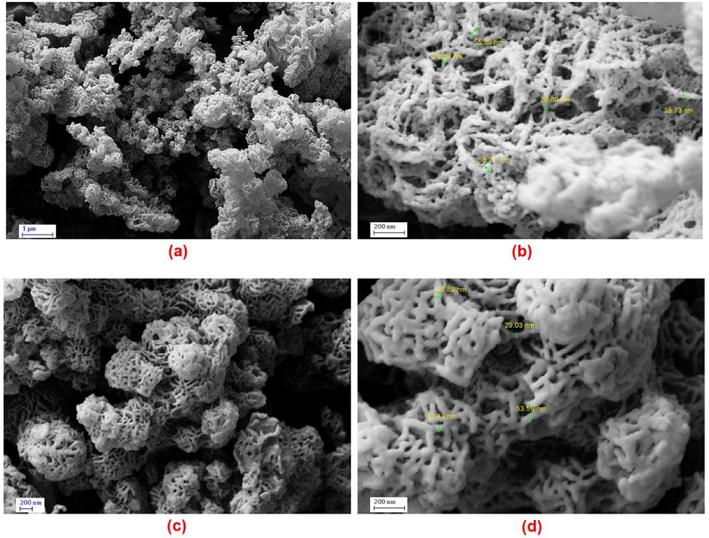
FESEM images of PDC-COF (a–b) and PDC-COF/CuI (c–d).
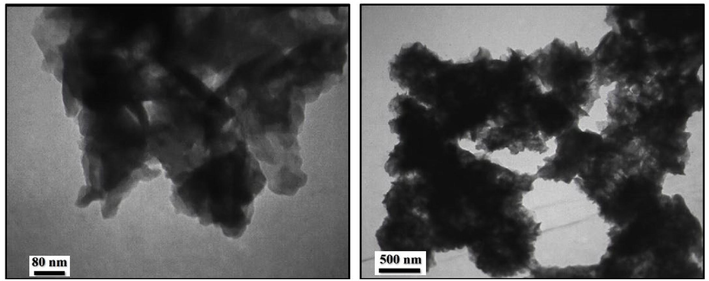
TEM images of PDC-COF.
2.3 Porosity of PDC-COF and PDC-COF/CuI
Nitrogen adsorption/desorption isotherms of PDC-COF and PDC-COF/CuI at 77 K were shown in Fig. 4 which the presence of hysteresis loops confirms their mesoporous structure. As expected, after adding CuI to PDC-COF, a decrease in the BET surface area of PDC-COF/CuI (50.3 m2g−1) and its total pore volume is 1.63 cm3.g−1 compared to the pristine PDC-COF (60.7 m2g−1) and its total pore volume is 0.44 cm3.g−1 are observed. These results are in agreement with confinement of a significant amount of CuI nanoparticles on surface and/or cavities. In addition, the general reduction in specific surface area is due to the presence of flexible hydrazone linkages (Li et al., 2019).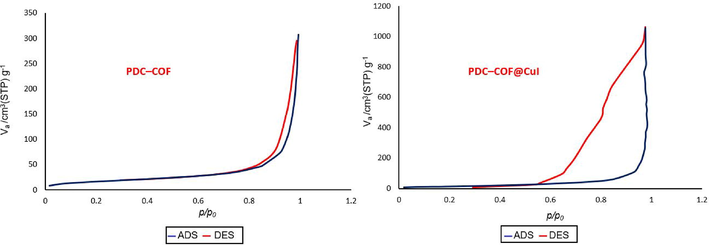
Nitrogen adsorption/desorption isotherms of PDC-COF and PDC-COF/CuI.
2.4 Confirmation of presence of desired elements
The EDX analysis of PDC-COF and PDC-COF/CuI are shown in Fig. 5 which confirms that C, N and O elements are present in both structures. Also, in addition to mentioned elements, the EDX analysis of PDC-COF/CuI clearly reveals the presence of Cu and I elements within the structure (Fig. 4). Moreover, the obtained results of elemental mapping analysis form PDC-COF and PDC-COF/CuI confirm the above points and also, illustrate uniform distribution of predicted elements (Figs. 6 and 7).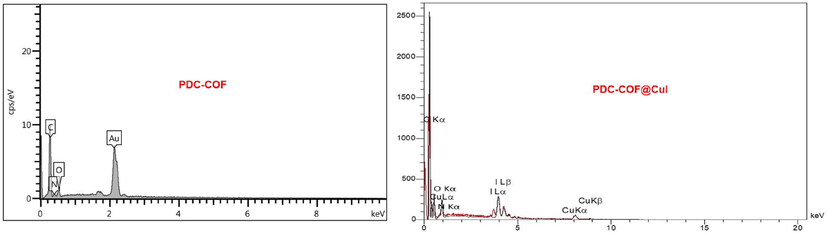
EDX analysis of PDC-COF and PDC-COF/CuI.
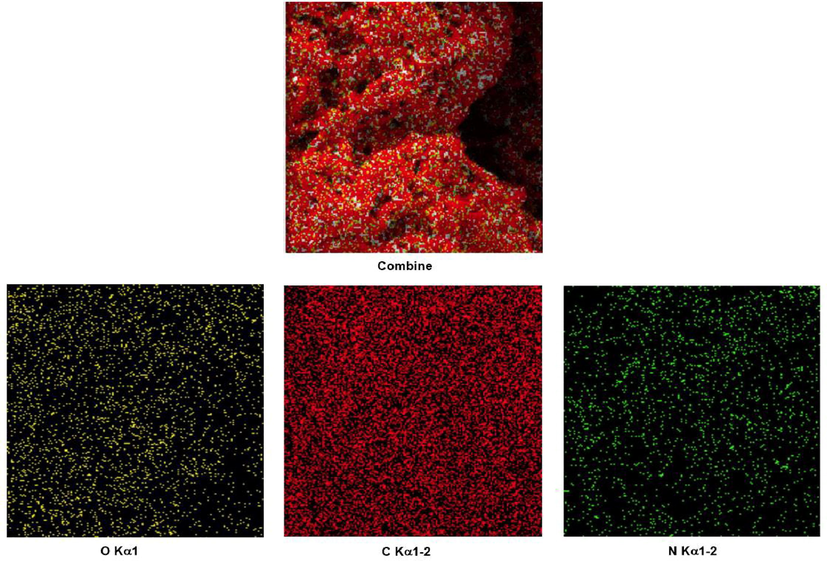
Elemental mapping analysis of PDC-COF.
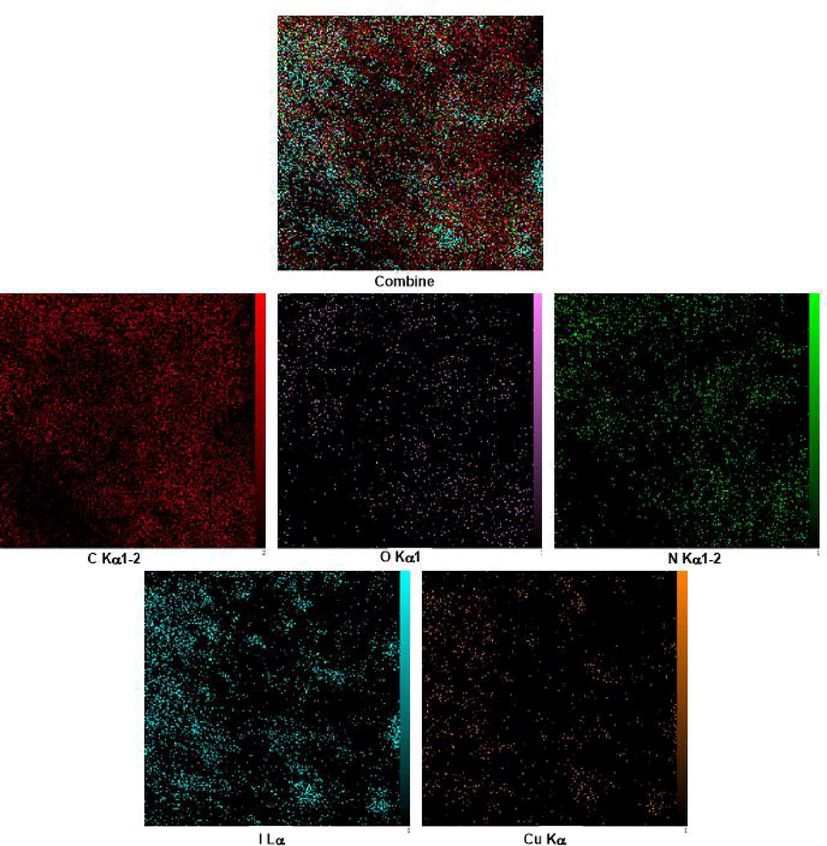
Elemental mapping analysis of PDC-COF/CuI.
2.5 Crystalline structure
XRD as a robust technique can be provide a useful information for exploring crystallographic structures of COFs. According to the previously relevant reports, there are varied XRD patterns from COFs and other porous organic polymers that have amorphous phases, while metal decorated COFs have distinctive and sharp peaks (Sebati and Ray, 2018; Luykx et al., 2008). Also, the XRD pattern depends on the amount of trapped metal (Mondal et al., 2016). As depicted in Fig. 8, the XRD pattern of PDC-COF represents the presence of amorphous phases (Fig. 8a), while CuI adding changes the XRD pattern and leads to appearance of sharp peaks which verified the presence of crystalline CuI nanoparticles within the structure of PDC-COF/CuI (Fig. 8a-b). Interestingly, in this case when 1 g of PDC-COF and 0.3 g of CuI were used, its associated XRD pattern is almost identical to the XRD pattern of CuI (Fig. 8b, see: ICDD Card # 01-0581). But, when 1 g of PDC-COF and 0.1 g of CuI were used, the PDC-COF XRD pattern is poorly observed (Fig. 8 c).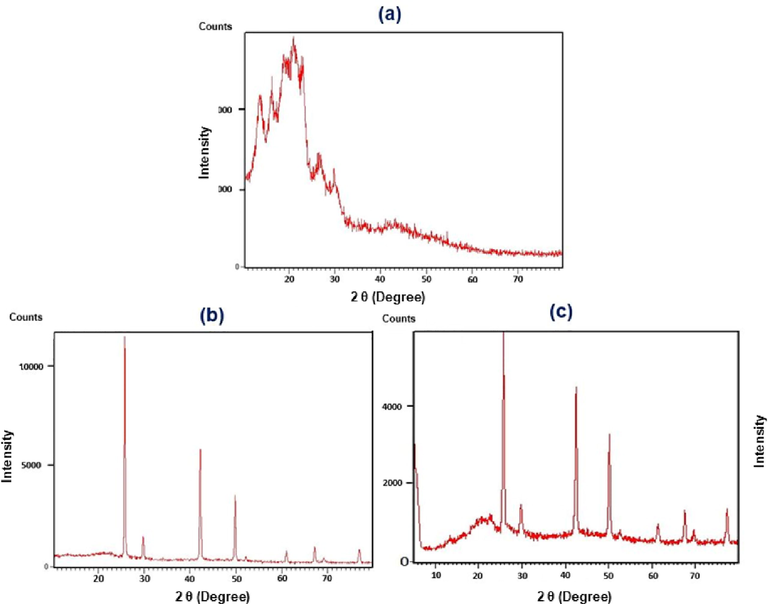
XRD patterns of PDC-COF (a), PDC-COF/CuI when 1 g of PDC-COF and 0.3 g of CuI were used (b) and PDC-COF/CuI when 1 g of PDC-COF and 0.1 g of CuI were used.
2.6 Thermal stability
The thermal stability is one of the most important parameters of practical applications of COFs. The TGA/DTG analysis was performed for PDC-COF and PDC-COF/CuI and fortunately the results show excellent thermal stability with thermal decomposition temperature about 500 °C for both PDC-COF and PDC-COF/CuI. It goes without saying that there is very little weight loss before 500 °C that is related to trapped solvents (Fig. 9).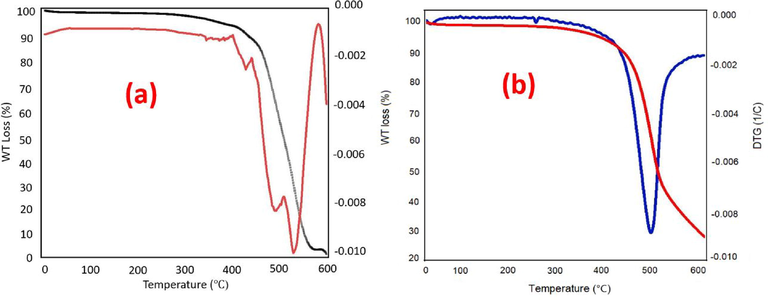
TGA/DTG curves of PDC-COF (a) and PDC-COF/CuI (b).
2.7 Catalytic performance studies
According to revealed results from precise characterization of PDC-COF/CuI, it is a suitable candidate as a heterogeneous catalyst for organic transformations. So, we conducted the triazole synthesis reactions by using PDC-COF/CuI as an efficient catalyst. At first, benzyl bromide, phenyl acetylene and sodium azide were selected as model substrates for determination of optimal reaction conditions. Gratifyingly, all of reactions were performred at room temperature that is in accordance with the green chemistry principles. The parameters of solvent and amount of catalyst loading were investigated and the model reaction was tested in different protic, aprotic and equal mixture of H2O-EtOH that the model reaction has a good yield in most of solvents. However, because of its compatibility with the environment and the principles of green chemistry, water was selected as the best solvent. After that, different amount of catalyst including 30, 20, 10 mg were used which the obtained data reveald that the 20 mg of catalyst is the best amount. Moreover, it is worthy to mention that in the absence of the catalyst no product was produced (Table 1).
Entry
Solvent
Catalyst loading (mg)
Time (min.)
Yield (%)b
1
–
20
45
70
2c
H2O
20
45
93
3
EtOH
20
45
90
4
EtOH–H2O (1:1)
20
45
92
5
EtOAc
20
90
55
6
CH3CN
20
90
78
7
CH2Cl2
20
45
85
8
THF
20
60
82
9
H2O
30
45
93
11
H2O
10
45
76
12
H2O
–
120
–
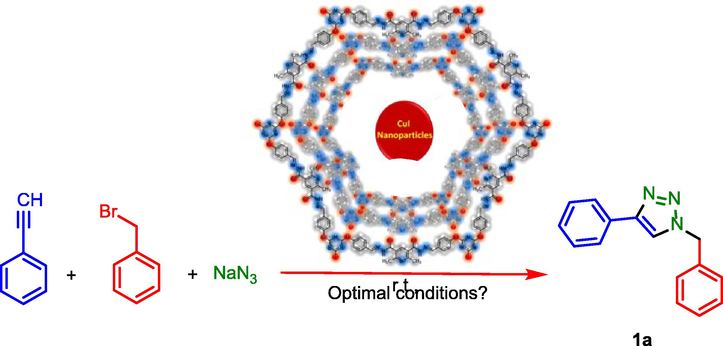
After optimization of the reaction conditions, the substrate scope of PDC-COF/CuI catalyzed click reactions for the preparation of triazoles by applying different starting materials were explored. In the first path, phenyl acetylene or prop-2-yn-1-ol, sodium azide and different benzyl bromide derivatives or sulfonyl chloride derivatives were applied for the preparation of triazoles. Then, after preparation of 1-azido-4-chlorobenzene and 1-azido-4-nitrobenzene, triazole systems were synthesized by the reaction of these aryl azides and acetyl acetone or ethyl acetoacetate. In the third series, different epoxide derivatives, sodium azide and phenyl acetylene were used that leads to formation of another series of triazoles (Table 2). All the products were obtained with good to high yeilds.
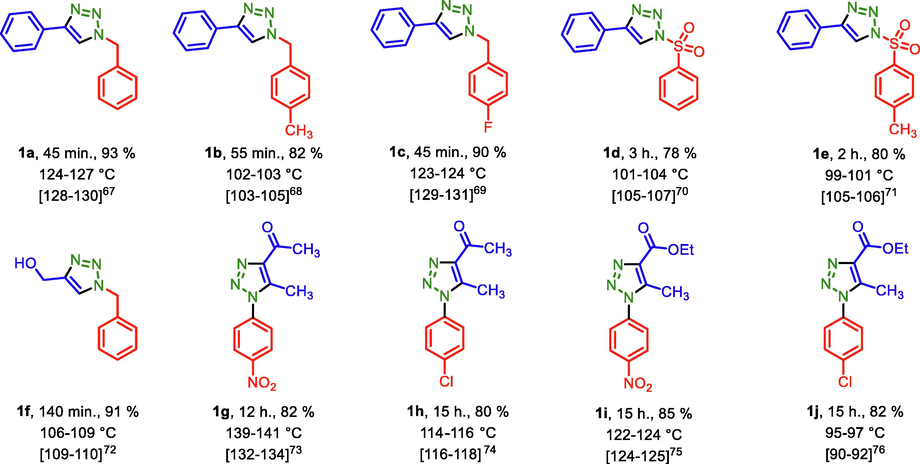
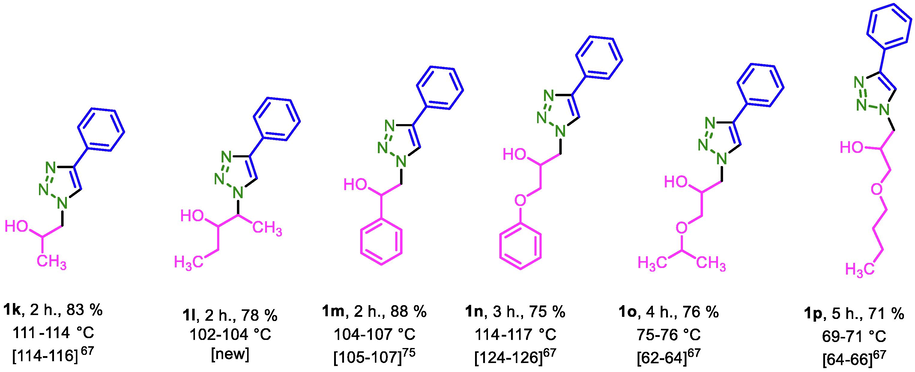
In another investigation, recycling and reusing capability of PDC-COF/CuI as a significant parameter was evaluated by its reusing up to six times. For this purpose, the model reaction (synthesis of triazole1a) was performed under optimal reaction conditions. After completing of each of reactions, the reaction mixture was dissolved in EtOH for separating of insoluble catalyst via centrifuging method. However, slight reduction has occurred in the reaction yield of product (Scheme 3).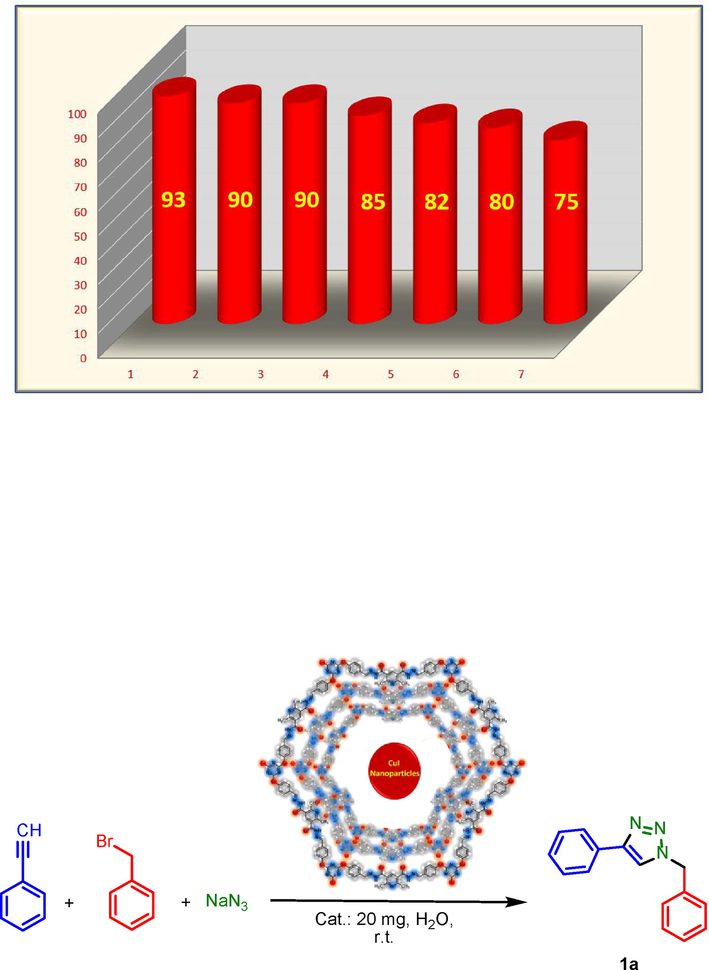
Recycling test of PDC-COF/CuI in the preparation of 1a.
In separate study, to confirm the SN2 type reaction for ring opening of epoxides, 1m as model compound was oxidized by Jones reagent (Scheme 4). According to revealed result, oxidation of hydroxy functional group in 1m yielded ketone that shown SN2 type reaction for ring opening of epoxides and synthesis of desired triazoles (Zarei et al., 2019).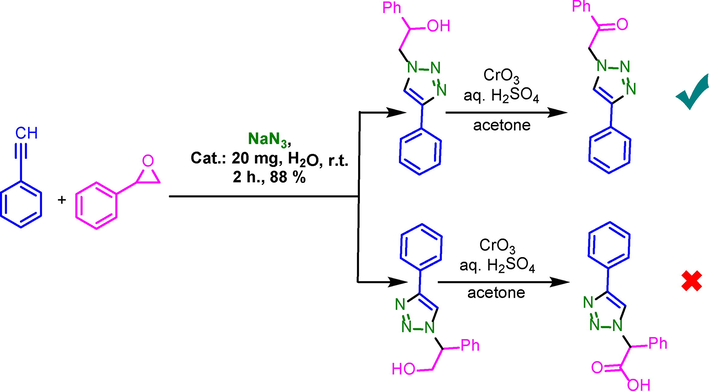
General procedure for oxidation of hydroxy functional group of 1m to confirm the SN2 type reaction.
In another study, we investigated the facility and efficiency of PDC-COF/CuI with some of the previously reported works for the preparation of 1a molecule. Accordingly, the presented catalyst shows a good catalytic performance with a shorter reaction time and a high yield of product (Table 3).
Entry
Catalyst
Time
Yield (%)
1
PDC-COF/CuI (20 mg), H2O, r.t.
45 min.
93
2
D-sorbitol/urea/NH4Cl/CuI (5 mol%), 85 °C (Ilgen and König, 2009)
5 h
91
3
2-(2′-hydroxyphenyl)-2-oxazoline-Cu(OAc)2, (1 mol%), H2O, 70 °C (Bagherzadeh et al., 2017)
12 h
87
4
CuAl2O4 (5 mg), H2O, 80 °C (Khalili et al., 2019)
45 min.
97
5
[Cu(CPA)(BDC)]n, (10 mol%), H2O/MeOH, 70 °C (Naskar et al., 2021)
60 min.
93
6
RuH2CO(PPh3)3, (2 mol%), H2O, 80 °C (Siyang et al., 2015)
2 h
89
7
Si- 1,5,7-triazabicyclo[4.4.0]dec-5-ene /Cu, 3 mol%, DMF, 40 °C (Coelho et al., 2010)
3 h
83
8
SBA-15-Tz-RuIJII) TPP (15 mg), H2O, 90 °C (Du et al., 2018)
12 h
88
9
NHC–copper complexe, (5 mol%), MeOH, r.t. (Szadkowska et al., 2017)
24 h
73
3 Experimental section
3.1 Experimental procedure for the synthesis of PDC-COF
Initially, DMPDC and TFPC were synthesized according to the previous reports (Torabi et al., 2021; Dinari and Hatami, 2019). Then, a mixture of TFPC (0.456 mmol, 0.2 g), DMPDC (0.676 mmol, 0.15 g) and mixture of mesitylene/THF (6 mL, 2:1) was added into a 10 mL test tube and sonicated at room temperature for 15 min. In the next step, the mixture of reaction was sealed in a 25 mL Teflon lined stainless steel vessel and heated at 100 °C for 72 h. After completing the reaction, the obtained yellow precipitate was washed several times with MeOH and CHCl3. Finally, the remained solid was dried in a vacuum oven for 12 h at 70 °C.
3.2 Synthesis of PDC-COF/CuI
1 g of the synthesized PDC-COF, 0.1 g of CuI and 40 mL dry EtOH were added into a 10 mL round-bottomed flask and stirred at room temperature for 12 h, following which the solid was washed with dry EtOH and then was activated by vacuum at 70 °C.
3.3 General procedure for the preparation of triazole derivatives (1a-1f) by using PDC-COF/CuI as catalyst
Benzyl bromide derivatives (1 mmol) or sulfonyl chloride derivatives (1 mmol), phenyl acetylene (1 mmol, 0.102 g) or prop-2-yn-1-ol (1 mmol, 0.056 g), sodium azide (1 mmol, 0.065 g) and PDC-COF/CuI (0.02 g) were added to the 20 mL of H2O and stirred at 25 °C for appropriate time. After completing each of reaction which were confirmed by TLC techniques (n-hexane/ethyl acetate, 7/3), the remained solid was extracted with ethyl acetate and H2O. Then, organic phase was separated and its solvent was evaporated to give pure product.
3.4 Preparation of triazole derivatives (1 g-1j) in the presence of PDC-COF/CuI
1-Azido-4-nitrobenzene (1 mmol, 0.164 g) or 1-azido-4-chlorobenzene (1 mmol, 0.153) and acetyl acetone (1.1 mmol, 0.11 g) or ethyl acetoacetate (1.1 mmol, 0.143 g) and PDC-COF/CuI (0.2 g) were added to the mixture of 20 mL H2O/EtOH (1:1) and vigorously stirred at room temperature. The progress of reaction was tested by TLC (n-hexane/ethyl acetate, 7/3). After completing of each reaction, the desired product was extracted with ethyl acetate and H2O. Then, organic phase was separated and its solvent was evaporated. Finally, the remained solid was washed with H2O to give pure solid.
3.5 Preparation of triazole derivatives (1 k-1p) in the presence of PDC-COF/CuI
Epoxide derivatives (1 mmol), phenyl acetylene (1 mmol, 0.102 g), sodium azide (1 mmol, 0.065 g), PDC-COF/CuI (0.2 g) and 20 mL of H2O as solvent were added into a 50 mL round-bottomed flask and stirred at room temperature. The progress of reaction was tested by TLC (n-hexane/ethyl acetate, 7/3). After completing each of reactions, 100 mL ethyl acetate was added to the mixture of reaction and extracted. Then, organic phase was separated and purified by TLC plate techniques with n-hexane/ethyl acetate as eluent.
4 Conclusion
In summary, a novel two-dimensional coral-like covalent organic framework (COF) with hydrazone linkages containing pyridine dicarbohydrazide moieties CuI nanoparticles carrier namely PDC-COF/CuI was designed and synthesized via a green and simple route and in a precise study was fully characterized. Furthermore, the network-like structure and good porosity for both of the PDC-COF and PDC-COF/CuI well verified by FESEM images and BET data. According to TGA/DTG analysis, PDC-COF/CuI has excellent thermal stability up to 500 °C. Also, the prepared PDC-COF/CuI show high activity as a heterogeneous and recoverable catalyst for the preparation of diverse triazoles by using different starting materials under green and mild reaction conditions.
Acknowledgments
We thank the Bu-Ali Sina University for financial support to our research group.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Covalent organic frameworks: Advances in synthesis and applications. Mater. Today Commun.. 2021;28:102612
- [Google Scholar]
- Functional π-conjugated two-dimensional covalent organic frameworks. ACS Appl. Mater. Interfaces. 2019;11:11029.
- [Google Scholar]
- Two new copper (II) complexes with chelating N, O-type bidentate ligands: synthesis, characterization, crystal structure and catalytic activity in azide–alkyne cycloaddition reaction. Inorg. Chim. Acta. 2017;466:398.
- [Google Scholar]
- Silver (I)-catalyzed regioselective synthesis of triazole fused-1,5-benzoxazocinones. Org. Lett.. 2017;19:2370.
- [Google Scholar]
- Rhodium-catalyzed denitrogenative transannulation of N-sulfonyl-1, 2, 3-triazoles with glycals giving pyrroline-fused N-glycosides. Org. Lett.. 2021;23:6357.
- [Google Scholar]
- Copper ferrite nanoparticle modified starch as a highly recoverable catalyst for room temperature click chemistry: Multicomponent synthesis of 1, 2, 3-triazoles in water. New J. Chem.. 2018;42:3078.
- [Google Scholar]
- Copper (I)-modified covalent organic framework for CO2 insertion to terminal alkynes. Mol. Catal.. 2021;499:111319
- [Google Scholar]
- Water-compatible synthesis of 1, 2, 3-triazoles under ultrasonic conditions by a Cu (I) complex-mediated click reaction. ACS Omega. 2020;5:30148.
- [Google Scholar]
- Copper-decorated covalent organic framework as a heterogeneous photocatalyst for phosphorylation of terminal alkynes. Green Chem.. 2022;24:4071.
- [Google Scholar]
- Covalent organic frameworks in catalytic organic synthesis. Adv. Synth. Catal.. 2021;363:144.
- [Google Scholar]
- Polymer-supported 1,5,7-triazabicyclo [4.4.0] dec-5-ene as polyvalent ligands in the copper-catalyzed Huisgen 1,3-dipolar cycloaddition. Adv. Synth. Catal.. 2010;352:1179.
- [Google Scholar]
- Chemical conversion and locking of the imine linkage: enhancing the functionality of covalent organic frameworks. Angew. Chem.. 2021;133:14356.
- [Google Scholar]
- In-water synthesis of 5-thiolated 1, 2, 3-triazoles from β-thioenaminones by diazo transfer reaction. J. Org. Chem.. 2019;84:14179.
- [Google Scholar]
- Covalent organic frameworks and supramolecular nano-synthesis. ACS Nano. 2021;15:12723.
- [Google Scholar]
- Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal. J. Environ. Chem. Eng.. 2019;7:102907
- [Google Scholar]
- Platinum single atoms anchored on a covalent organic framework: boosting active sites for photocatalytic hydrogen evolution. ACS Catal.. 2021;11:13266.
- [Google Scholar]
- Catal. Sci. Technol.. 2018;8:3238.
- General synthesis of tri-carbo-substituted N-2-Aryl-1, 2, 3-triazoles via Cu-catalyzed annulation of azirines with aryldiazonium salts. J. Org. Chem.. 2020;85:10872.
- [Google Scholar]
- Enantiomorphic perovskite ferroelectrics with circularly polarized luminescence. ACS Appl. Nano Mat.. 2018;1:4756.
- [Google Scholar]
- Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev.. 2016;116:3722.
- [Google Scholar]
- Covalent organic frameworks: design, synthesis, and functions. Chem. Rev.. 2020;120:8814.
- [Google Scholar]
- 1, 5-disubstituted 1, 2, 3-triazoles as amide bond isosteres yield novel tumor-targeting minigastrin analogs. ACS Med. Chem. Lett.. 2021;12:585.
- [Google Scholar]
- Copper immobilized at a covalent organic framework: an efficient and recyclable heterogeneous catalyst for the Chan-Lam coupling reaction of aryl boronic acids and amines. Green Chem.. 2018;20:4891.
- [Google Scholar]
- Triazinetriamine-derived porous organic polymer-supported copper nanoparticles (Cu-NPs@ TzTa-POP): an efficient catalyst for the synthesis of N-methylated products via CO2 fixation and primary carbamates from alcohols and urea. New J. Chem.. 2020;44:15446.
- [Google Scholar]
- Organic reactions in low melting mixtures based on carbohydrates and L-carnitine-a comparison. Green Chem.. 2009;11:848.
- [Google Scholar]
- Construction and catalytic applications of an amino-functionalized covalent organic framework. Transit. Met. Chem.. 2019;44:689-697.
- [Google Scholar]
- A one-pot, copper-catalyzed azidation/click reaction of aryl and heteroaryl bromides in an environmentally friendly deep eutectic solvent. Tetrahedron. 2017;73:7024.
- [Google Scholar]
- Copper aluminate spinel in click chemistry: An efficient heterogeneous nanocatalyst for the highly regioselective synthesis of triazoles in water. Synlett. 2019;30:2136.
- [Google Scholar]
- Anchoring Cu nanoparticles on functionalized multi-walled carbon nanotube for regioselective synthesis of 1, 2, 3-triazoles via click reaction. Appl. Organometal. Chem.. 2021;35:e6281.
- [Google Scholar]
- Three-component synthesis of 1, 4-disubstituted 1, 2, 3-triazoles using a novel and efficient nano alumina based Cu (II) catalyst. Org. Prep. Proced. Int.. 2021;53:509.
- [Google Scholar]
- Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Edit.. 2001;40:2004.
- [Google Scholar]
- Upconverting Er3+–Yb3+ inorganic/covalent organic framework core-shell nanoplatforms for simultaneous catalysis and nanothermometry. ACS Appl. Mater. Interfaces. 2021;13:47010.
- [Google Scholar]
- Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions. ACS Appl. Mater. Interfaces. 2019;11:11706.
- [Google Scholar]
- New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev.. 2020;49:2852.
- [Google Scholar]
- An azine-linked covalent organic framework: synthesis, characterization and efficient gas storage. Chem. Eur. J.. 2015;21:12079.
- [Google Scholar]
- Construction of covalent organic frameworks via three-component one-pot Strecker and Povarov reactions. J. Am. Chem. Soc.. 2020;142:6521.
- [Google Scholar]
- The copper (I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev.. 2011;255:2933.
- [Google Scholar]
- Recent advances in covalent organic frameworks (COFs) as a smart sensing material. Chem. Soc. Rev.. 2019;48:5266.
- [Google Scholar]
- Structural engineering of two-dimensional covalent organic frameworks for visible-light-driven organic transformations. ACS Appl. Mater. Interfaces. 2020;12:20354.
- [Google Scholar]
- Visible-light-induced tandem radical addition–cyclization of 2-aryl phenyl isocyanides catalyzed by recyclable covalent organic frameworks. Green Chem.. 2019;21:2905.
- [Google Scholar]
- A review of analytical methods for the identification and characterization of nano delivery systems in food. J. Agric. Food Chem.. 2008;56:8231.
- [Google Scholar]
- Porous crystalline olefin-linked covalent organic frameworks. J. Am. Chem. Soc.. 2019;141:6848.
- [Google Scholar]
- Covalent triazine framework confined copper catalysts for selective electrochemical CO2 reduction: operando diagnosis of active sites. ACS Catal.. 2020;10:4534.
- [Google Scholar]
- Targeted drug delivery in covalent organic nanosheets (CONs) via sequential postsynthetic modification. J. Am. Chem. Soc.. 2017;139:4513.
- [Google Scholar]
- Size-dependent catalytic activity of palladium nanoparticles fabricated in porous organic polymers for alkene hydrogenation at room temperature. ACS Appl. Mater. Interfaces. 2016;8:15307.
- [Google Scholar]
- Click chemistry finds its way into covalent porous organic materials. Angew. Chem. Int. Ed.. 2011;50:11844.
- [Google Scholar]
- A reusable efficient green catalyst of 2D Cu-MOF for the click and knoevenagel reaction. Molecules. 2021;26:5296.
- [Google Scholar]
- Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J. Am. Chem. Soc.. 2018;140:1423.
- [Google Scholar]
- Triazine-based covalent organic polymers: design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A. 2016;4:16288.
- [Google Scholar]
- Linkage engineering by harnessing supramolecular interactions to fabricate 2D hydrazone-linked covalent organic framework platforms toward advanced catalysis. J. Am. Chem. Soc.. 2020;142:18138.
- [Google Scholar]
- Click polymerization: facile synthesis of functional poly (aroyltriazole) s by metal-free, regioselective 1, 3-dipolar polycycloaddition. Macromolecules. 2007;40:2308.
- [Google Scholar]
- Visible-light activated metal catalyst-free vicinal diazidation of olefins with sulfonium iodate (I) species. Chemcomm. 2019;55:2833.
- [Google Scholar]
- Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev.. 2017;46:3134.
- [Google Scholar]
- Layered copper-metallated covalent organic frameworks for Huisgen reactions. ACS Appl. Mater. Interfaces. 2021;13:54106.
- [Google Scholar]
- A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective ‘‘ligation’’ of azides and terminal alkynes. Angew. Chem. Int. Edit.. 2002;41:2596.
- [Google Scholar]
- Design, synthesis and in vitro anti-tuberculosis activity of benzo [6, 7] cyclohepta [1, 2-b] pyridine-1, 2, 3-triazole derivatives. Bioorg. Med. Chem.. 2017;27:5119.
- [Google Scholar]
- Beyond click chemistry–supramolecular interactions of 1, 2, 3-triazoles. Chem. Soc. Rev.. 2014;43:2522.
- [Google Scholar]
- Evolution of nanocarrier drug-delivery systems and recent advancements in covalent organic framework-drug systems. ACS Appl. Nano Mat.. 2020;3:3097.
- [Google Scholar]
- Advances in nanostructured metal-encapsulated porous organic-polymer composites for catalyzed organic chemical synthesis. Catalysts. 2018;8:492.
- [Google Scholar]
- Efficient, green and regioselective synthesis of 1, 4, 5-trisubstituted-1, 2, 3-triazoles in ionic liquid [bmim] BF4 and in task-specific basic ionic liquid [bmim] OH. J. Iran. Chem. Soc.. 2013;10:883.
- [Google Scholar]
- Highly efficient click reaction on water catalyzed by a ruthenium complex. RSC Adv.. 2015;5:4693.
- [Google Scholar]
- Recent developments on triazole nucleus in anticonvulsant compounds: a review. J. Enzyme Inhib. Med. Chem.. 2018;33:453.
- [Google Scholar]
- Synthesis, structural characterization and catalytic activities of sulfur-functionalized NHC–copper (I) complexes. Eur. J. Org. Chem.. 2017;2017:4074.
- [Google Scholar]
- Micellar promoted multi-component synthesis of 1, 2, 3-triazoles in water at room temperature. Green Chem.. 2015;17:1414.
- [Google Scholar]
- Catalytic applications of porous organic polymers. Iran. J. Catal.. 2021;11:417-424.
- [Google Scholar]
- Synthesis of triarylpyridines with sulfonate and sulfonamide moieties via a cooperative vinylogous anomeric-based oxidation. Sci. Rep.. 2021;11:16846.
- [Google Scholar]
- Magnetic porous organic polymer: catalytic application in the synthesis of hybrid pyridines with indole, triazole and sulfonamide moieties. RSC Adv.. 2022;12:8804.
- [Google Scholar]
- Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc.. 2011;133:11478.
- [Google Scholar]
- Azide-alkyne click chemistry over a heterogeneous copper-based single-atom catalyst. ACS Catal.. 2022;12:2947.
- [Google Scholar]
- Copper-catalyzed reaction cascade: direct conversion of alkynes into N-sulfonylazetidin-2-imines. Angew. Chem. Int. Ed.. 2006;45:3157.
- [Google Scholar]
- A recyclable and reusable supported Cu (I) catalyzed azide-alkyne click polymerization. Sci. Rep.. 2014;4:5107.
- [Google Scholar]
- by Amine-functionalized PN. covalent organic frameworks for benzyl alcohol oxidation. ACS Appl. Nano Mater.. 2020;3:6416.
- [Google Scholar]
- Vinylene-linked two-dimensional covalent organic frameworks: synthesis and functions. Acc. Chem. Res.. 2021;2:252.
- [Google Scholar]
- Ytterbium-catalyzed intramolecular [3+ 2] cycloaddition based on furan dearomatization to construct fused triazoles. Org. Lett.. 2020;22:5176.
- [Google Scholar]
- Covalent organic frameworks for photocatalytic applications. Appl. Catal.. 2020;276:119174
- [Google Scholar]
- Structure-modulated crystalline covalent organic frameworks as high-rate cathodes for Li-ion batteries. J. Mat. Chem. A. 2016;4:18621.
- [Google Scholar]
- Regioselective synthesis of novel α-ariloxy alcohols over silica-bonded 1, 4-diaza-bicyclo [2.2. 2] octane-acetic acid bromide as new catalyst. J. Mol. Struct.. 2019;1175:428.
- [Google Scholar]
- Directing-group-enabled cycloaddition of azides and alkynes toward functionalized triazoles. Org. Lett.. 2020;22:2220.
- [Google Scholar]
- Advances in chiral metal-organic and covalent organic frameworks for asymmetric catalysis. Small. 2021;17:2005686.
- [Google Scholar]
- High-performance adsorption of chromate by hydrazone-linked guanidinium-based ionic covalent organic frameworks: Selective ion exchange. Sep. Purif. Technol.. 2021;274:118993
- [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105090.
Appendix A
Supplementary material
The following are the Supplementary material to this article:Supplementary data 1
Supplementary data 1







