Translate this page into:
Contents of flavonoid compounds in Dendrobium officinale Kimura et Migo determined by QuEChERS-HPLC-MS/MS: Method validation and influencing factors
⁎Corresponding authors at: Institute of Agro-product Safety and Nutrition, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, Zhejiang Province, China. csheng866@126.com (Qing Sheng), zhaoxueping@tom.com (Xueping Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dendrobium officinale Kimura et Migo (D. officinale), a dual-use plant with both botanical medicine and food applications, has gained global popularity. Flavonoids are important bioactive compounds of D. officinale; nevertheless, the quantitative analysis method for flavonoid components is lack, restricting an in-depth understanding of their functions. A QuEChERS method coupled with HPLC-MS/MS was developed to detect 17 flavonoid components of D. officinale. Octadecylsilane was selected as the clean-up sorbent, which exhibited a relatively weak affinity for flavonoids yet an effective removal of pigments from extracts. Method validation showed that limits of detection (LODs) and limits of quantification (LOQs) were 2.1–49.5 and 7.1–165.2 μg/kg, respectively. The recoveries ranged from 73 % to 112 %, with inter-day and intra-day RSDs below 20 %. Among the 17 flavonoids measured, naringenin, rutin and schaftoside were the predominant compounds, with the highest values of 57.18, 563.17 and 67.73 mg/kg, respectively. These three flavonoids accounted for over 80 % of the total content of 17 flavonoids. Moreover, the Multiple Factor Analysis indicated that the origin and cultivation method exerted great influence on the flavonoid concentrations in D. officinale. The results have important implications for further research and utilization of flavonoids in D. officinale.
Keywords
Dendrobium officinale Kimura et Migo
QuEChERS
Flavonoid components
The origin
Cultivation method
- D. officinale
-
Dendrobium officinale Kimura et Migo
- LOD
-
Limit of detection
- LOQ
-
Limit of quantification
- QuEChERS
-
Quick, Easy, Cheap, Effective, Rugged, Safe
- C18
-
Octadecylsilane
- PSA
-
Primary secondary amine
- g-MWCNTs
-
Graphitized multi-wall carbon nanotubes
- MWCNTs-OH
-
Hydroxylated multi-wall carbon nanotubes
- MWCNTs-COOH
-
Carboxylated multi-wall carbon nanotubes
- MFA
-
Multiple Factor Analysis
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- Trolox
-
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylicacid
- ABTS
-
2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate)
- ESI
-
Electrospray ionization
- MRM
-
Multiple reaction monitoring
- Ct
-
Total content of flavonoid
- CE
-
Collision energy
- Rf
-
Concentration ratio of flavonoid component in the extract before and after purification
Abbreviations
1 Introduction
Dendrobium officinale Kimura et Migo (D. officinale) has been traditionally applied in folk medicine in China and Southeast Asian countries for more than 2000 years (Guoet al., 2021). It shows antioxidant, anti-tumor and immune-enhancing effects, and is increasingly used in pharmaceutical, health food and cosmetic industries (Huanget al., 2016; Luoet al., 2016; Chanet al., 2018; Meng et al., 2019). In 2019, D. officinale was listed as a dual-use plant for botanical medicine and food applications by the National Health Commission of the People’s Republic of China (National Health Commission of the People’s Republic of China, 2020). D. officinale has become widely popular not only in the Chinese market, but also spread to many other countries, such as Australia, Japan and the United States (Xianget al., 2013). In the first half of 2020, 13 tons of D. officinale were exported with a total value of 12.08 million US dollars (Fu et al., 2021).
The antioxidant activity is one of the most significant biological functions of D. officinale (Yuanet al., 2020), which is closely related to contents and types of flavonoids (Yuanet al., 2020; Wanet al., 2021; Maet al., 2023). The determination of concentrations and compositions of flavonoids is fundamental to clarify their mechanism of action. The total content of flavonoids in foods and herbs is generally determined by the sodium nitrite-aluminum nitrate spectrophotometry method (Xuet al., 2013; Zhang et al., 2017). Though this method is simple and convenient, it cannot provide information on the compositions of flavonoids, thus restricting research on their functions. Recently, some studies have focused on determination of flavonoid compositions and concentrations by using LC-MS (Yeet al., 2017; Zhou et al., 2018; Kimet al., 2023). In these works, D. officinale samples were extracted by methanol and then directly measured on LC-MS without sample clean-up. Nevertheless, D. officinale is a complex matrix, containing numerous substances such as pigments, polysaccharides, bibenzyls and alkaloids (Tang et al., 2017). These co-extracts would reduce the column efficiency and cause deterioration of chromatographic peak shape. Hence, the purification of extracts is essential to measure flavonoid compounds in D. officinale by using LC-MS. The QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) method is a widely used sample extraction and purification technique in the analysis of pesticide residues in agricultural products (Garcia-Vara et al., 2022). It shows advantages of simplicity, speed, cost-effectiveness and time-saving compared to traditional extraction techniques, such as matrix solid-phase dispersion or solid-phase extraction (SPE). The procedure includes sample homogenisation, solvent extraction/partitioning (with a salting-out process), clean-up through a dispersive SPE (dSPE) by using combinations of sorbents and salts, and analysis (Garcia-Vara et al., 2022). Potentially, the QuEChERS method could be used in the detection of bioactive compounds in foods. Recently, it has been modified for the determination of phenolic compounds in fruits and vegetables (Aguiaret al., 2020; Nicácioet al., 2020). However, the application of the QuEChERS method to detect flavonoid compounds of D. officinale has not been reported.
The accumulation of bioactive compounds is influenced by geographical location, cultivation environment and growth stage (Zhouet al., 2018; Gaiet al., 2019). D. officinale is mainly distributed in Zhejiang, Yunnan, Guangdong, Anhui and Guangxi Provinces (Zhouet al., 2018). Flavonol contents of D. officinale grown in Guangdong Province were reported to be higher than those grown in Zhejiang and Guangxi Provinces (Lanet al., 2022). The cultivation methods of D. officinale include facility-aided cultivation and imitative wild cultivation, and the available products in the market are derived from various processing techniques, including Fengdou and dry stems. However, it remains unclear how cultivation method, growth years, collecting time and processing techniques impact the content and composition of flavonoid compounds.
In this study, the QuEChERS method was applied in quantitative analysis of flavonoid components in D. officinale. The goals of the current study were: i) to develop a QuEChERS method in combination with HPLC-MS/MS for determining 17 flavonoid components in D. officinale. The efficacy of five sorbents for sample clean-up including octadecylsilane (C18), primary secondary amine (PSA), graphitized multi-wall carbon nanotubes (g-MWCNTs), hydroxylated multi-wall carbon nanotubes (MWCNTs-OH), carboxylated multi-wall carbon nanotubes (MWCNTs-COOH)) were explored and compared; ii) to determine the levels of 17 flavonoid components in 47 D. officinale samples by using the validated method. Furthermore, the Multiple Factor Analysis (MFA) was performed to scrutinize the relationship between flavonoid contents and factors such as origin, cultivation method, growth years, collecting time and processing; iii) finally, the antioxidant activities of the extracts were examined by the DPPH radical scavenging activity assay, ABTS radical scavenging activity assay and FRAP assay.
2 Materials and methods
2.1 Chemicals and reagents
HPLC-grade of methanol, acetonitrile, ammonium acetate and formic acid were obtained from Merck (Darmstadt, Germany). Analytical grade of NaNO2, Al(NO3)3·9H2O and NaOH were purchased from Macklin (Shanghai, China). Analytical grade of acetic acid, potassium persulfate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylicacid (Trolox), 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) and ferric chloride sodium acetate were supplied by J&K (Beijing, China). Sorbents PSA and C18 were provided by Agilent Technologies (Tianjin, China). g-MWCNTs (outer diameter: 10–20 nm, length: 5–30 μm), MWCNTs-COOH (outer diameter: 10–20 nm, length: 10–30 μm) and MWCNTs-OH (outer diameter: 10–20 nm, length: 5–30 μm) were acquired from XFNANO (Nanjing, China). Flavonoid standards including naringenin, apigenin, cynaroside, quercetin, eriodictyol, isorhamnetin, quercitrin, rutin, hesperetin, isoquercitrin, kaempferol, apiin, hyperoside, schaftoside, chrysoeriol, taxifolin and naringin were purchased from Anpu (Shanghai, China), with purities ranging from 97.1 % to 99.9 %. The structure of flavonoid compounds was listed in Table S1. Standard stock solutions of all flavonoid compounds (100 mg/L) were prepared by using methanol and stored at 4 °C until use.
2.2 Sample collection and pretreatment
Forty-seven samples of D. officinale were collected from production bases and markets. Detailed sample information including origin, cultivation method, growth years, collecting time, and processing was recorded in Table S2. Samples were cultivated under facility-aided cultivation mode or imitative wild cultivation mode. Fresh stems were processed into Fengdou, dry stems and slices. Generally, D. officinale of two-year-old was suggested to be harvested and sold in the market, and D. officinale of more than two-year-old were also collected to explore the effect of growth years on flavonoid contents. D. officinale was normally harvested during November to May of next year and D. officinale was also collected in non-harvest period to investigate the effect of collecting time on flavonoid contents. When fresh stems were collected, they were cut into small sections and then roasted using a DHG-9070A blast dryer (Shanghai Jinghong Experimental Equipment Co. LTD, China) for 24 h at 80 °C, following the same baking conditions utilized in production bases. The dry stems or slices were grounded into powders and stored at −20 °C until analysis.
2.3 Sample extraction
0.2 g of D. officinale sample was weighed and transferred into a 15 mL centrifuge tube. Subsequently, 8 mL of extraction solution (acetonitrile/water, volume ratio of 3:1) was added into the tube, and the mixture was vortexed for 5 min, followed by sonication for 30 min at room temperature. After sonication, the mixture was centrifuged at 4000 g for 5 min, and the supernatant was collected for the determination of flavonoid concentrations and the analysis of antioxidant activity (Fig. S1).
2.4 Determination of 17 flavonoid compounds by QuEChERS-HPLC-MS/MS
2.4.1 Sample clean-up
1.6 mL of the sample extract was transferred to a centrifuge tube which contained 50 mg of C18. The mixture was vortexed and then centrifuged at 5018 g for 5 min. Afterwards, the supernatant was collected and analyzed using HPLC-MS/MS (Fig. S1). For analysis of flavonoids with a high content level, such as naringin, rutin and schaftoside, samples were diluted by a factor of 100 using acetonitrile/water (3:1, v/v) and then filtered through a 0.22 μm filter. On the other hand, samples were directly filtered through a 0.22 μm filter for analysis of flavonoid components with a relative low content
2.4.2 HPLC-MS/MS conditions
The flavonoid components were measured using HPLC-MS/MS (LCMS 8050, Shimadzu, Japan). Chromatographic separation was achieved on an ACQUITY UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm) at 40 °C, and the eluent was 0.1 % formic acid in water with 2 mmol/L ammonium acetate (A) and methanol (B) at a flow rate of 0.25 mL/min. The injection volume was 2 μL. The gradient elution was performed as shown: 0–0.5 min 0–10 % B, 0.5–3 min 10 %-85 % B, 3–5 min 85 %-95 % B, 5–9 min 95 %-95 % B, 9–9.01 min 95 %-10 % B.
The mass system was equipped with an electrospray ionization (ESI) source operating in the negative mode. The nebulizer and drying gases were 99.95 % nitrogen, and their flow rates were 3.0 and 10.0 L/min, respectively. The heating gas was 99.95 % air with a flow rate of 10.0 L/min. Argon (99.99 %) was utilized as the collision gas at a pressure of 270 kPa. Other parameters were as follows: interface voltage of 4.0 kV, interface temperature of 300 °C, desolvation line temperature of 250 °C, heat block temperature of 400 °C, and detector voltage of 1.82 kV. Data was collected using the multiple reaction monitoring (MRM) mode. The MS/MS transition for quantification and confirmation and the retention time for each target chemical were summarized in Table S3. The MRM chromatograms of 17 components were presented in Fig. S2.
2.5 Method validation and uncertainty evaluation
The method used for analysis of 17 flavonoid compounds in D. officinale was validated according to the guidelines presented by European Commission (2021), and the International Union of Pure and Applied Chemistry (IUPAC, 2002). The linearity, sensitivity, accuracy and precision of the method were evaluated.
The linearity was evaluated by building external calibration curves for each compound. Each calibration curve was plotted with a minimum of five suitable concentrations within the range of 1 to 200 μg/L. The sensitivity of the method was determined by the limit of detection (LOD) and the limit of quantification (LOQ), which corresponded to the concentration of each analyte at a signal-to-noise ratio of 3 and 10, respectively. The accuracy was assessed by recoveries. Mixed standards containing 17 flavonoid components were spiked at three concentration levels, namely low, medium and high (80 %, 100 % and 120 % of the original amount) into the sample, and the recovery was calculated using the formula: Recovery (%) = (total mass in spiked sample − mass in non-spiked sample)/mass spiked × 100 (Xin et al., 2023). Both fortified and unfortified samples were treated identically. The precision of the method was evaluated by intra-day and inter-day variations.
Uncertainty of the measurement was assessed by the combination of individual uncertainty including purity of the standards, balance weighing (weighing of samples and standards), volume measuring by using a pipette (measuring of standard solutions and solvents), volumetric flask used for preparation of standard solution, and method repeatability obtained by the RSD of the measurements (ISO, 1993). The combined relative standard uncertainty (u) was calculated by Eq. (1). The expanded relative uncertainties (U) with a coverage factor of 2 (k = 2) was estimated by Eq. (2).
urel,p, urel,w, urel,m, urel,v and urel,rep indicated the relative uncertainty of purity of standards, balance weighing, volume measuring, volumetric flask in preparation of standard solution and the method repeatability, respectively.
2.6 Total contents of flavonoid determined by NaNO2-Al(NO3)3 colorimetry
The total contents of flavonoid in D. officinale (Ct) were determined according to the method of Zhishen et al. (1999) with minor modifications. 2 mL of the sample extract was first mixed with 0.3 mL of 5 % NaNO2 (w/w). Afterwards, 0.3 mL of 10 % Al(NO3)3 (w/w) and 4 mL of 4 % NaOH (w/w) were added and the sample was then thoroughly mixed. The absorbance was measured at 510 nm. Rutin was used as a standard, and the calculation of Ct was based on rutin equivalents (mg RE/g).
2.7 Antioxidant assays
The antioxidant activity of D. officinale was evaluated by using the DPPH radical scavenging capacity, ABTS assay and FRAP total antioxidant capacity according to available method (Jin et al., 2016; Zhang et al., 2017; Meng et al., 2019). Additionally, three specific methods for the determination of antioxidant activities were presented in Supplemental Materials.
2.8 Calculations and statistical analysis
To test the significance of the difference in Ct under different extraction conditions, t-test was performed using IBM SPSS Statistics 25 with a statistical significance level of 0.05. Spearman correlation was applied to determine the relationship between the antioxidant activity of 47 D. officinale samples and the total content of flavonoid (Ct) and the individual flavonoid concentration. Moreover, the relationship between flavonoid contents and factors including origin, cultivation method and growth years was explored by the Multiple Factor Analysis (MFA) performed on XLSTAT 2018 (Addinsoft, USA). In MFA analysis, flavonoid contents and antioxidant activity values were standardized by Z-score. Considering that D. officinale slices were obtained by cutting the dry stem into slices, and the mechanic cutting would not cause variations in flavonoid contents, the factor ‘processing’ was sorted as dry stem and Fengdou, and slices were merged with dry stem.
3 Results and discussion
3.1 Method development for determination of 17 flavonoid compounds using QuEChERS-HPLC-MS/MS
3.1.1 Optimization of HPLC-MS/MS conditions
To achieve a satisfactory resolution, sensitivity and short sample running time, various mobile phases were tested. The pKa for flavonoid compounds is from 5.7 to 7.52, and LogP values vary from −3.06 to 3.19 (Table S4). Considering the acid-base property and wide polarity of flavonoid compounds, four mobile phases were examined. These were A (water − methanol), B (0.1 % formic acid − methanol), C (2 mmol/L ammonium acetate containing 0.1 % formic acid − acetonitrile) and D (2 mmol/L ammonium acetate containing 0.1 % formic acid water − methanol). A mixed standard solution of 17 flavonoid components with a concentration of 0.1 mg/L was injected, and the peak shape and peak area under the four mobile phases were compared. The peak shapes were comparable under all four mobile phases, appearing sharp and symmetrical. The peak areas of the 17 flavonoids under the four mobile phase conditions were shown in Fig. 1. Under each mobile phase, the intensity of chrysoeriol was the highest, which was nearly 64 times that of kaempferol. Meanwhile, the peak area of kaempferol was the highest under the mobile phase D. Herein, mobile phase D (2 mmol/L ammonium acetate containing 0.1 % formic acid water − methanol) was chosen for the analysis of flavonoid compounds. Furthermore, optimization of MS parameters is another essential step for obtaining the highest sensitivity. The precursor ion was determined in full scan with an m/z range of 100–1000 followed by the optimization of MRM transitions and the corresponding collision energy (CE). The optimized MRM transitions and CE for 17 flavonoid components were listed in Table S3.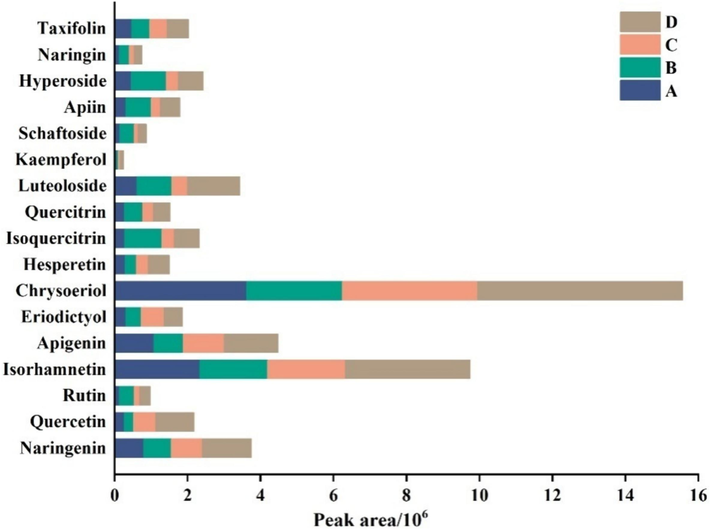
Peak areas of flavonoid compounds under the four mobile phases. A (water − methanol), B (0.1 % formic acid − methanol), C (2 mmol/L ammonium acetate containing 0.1 % formic acid − acetonitrile) and D (2 mmol/L ammonium acetate containing 0.1 % formic acid water − methanol).
3.1.2 Optimization of extraction conditions
Efficient and reproducible sample extraction is critical to the chemical analysis. Currently, flavonoids in herbs and foods are extracted using methods including hot water bath, Soxhlet extraction, ultrasonic assisted-extraction and microwave-assisted extraction (Søltoftet al., 2009; Wong et al., 2017; Moldovanet al., 2019; Manganget al., 2020). Ultrasound assisted-extraction shows advantages of simple operation, low cost and high yield since ultrasonic treatment could induce cavitation effects and destroy cell walls efficiently to promote solute transfer from the solid phase to the liquid phase (Cui et al., 2022; Chakraborty et al., 2020). Herein, the ultrasound-assisted extraction technology was selected to extract flavonoids from D. officinale. Further, the extraction procedure was optimized by investigating the influence of various factors such as types of organic solvent, water content, solid–liquid ratio and extraction time. The results were shown in Fig. 2.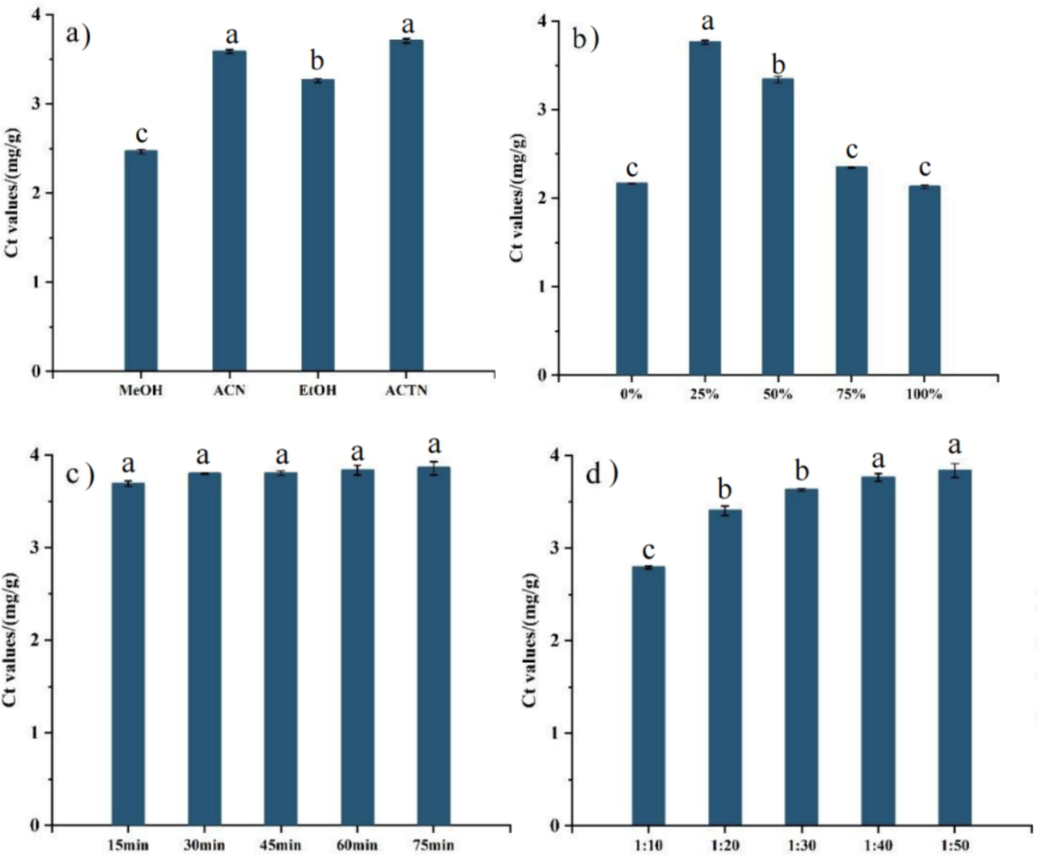
Effects of extraction conditions on total content of flavonoid (Ct): (a) extraction solvents: MeOH (methanol), ACN (acetonitrile), ACTN (acetone) and EtOH (ethanol), (b) water content, (c) extraction time, and (d) solid–liquid ratio. The different letters in each subfigure indicated the significant difference among Ct values under various extraction conditions (p < 0.05).
The extraction efficiencies of four organic solvents (methanol, acetonitrile, acetone and ethanol) were compared under constant parameters (water content of 25 %, solid–liquid ratio of 1:40 (g/mL) and an extraction time of 30 min). Acetone resulted in the highest Ct of 3.71 mg/g, followed by acetonitrile with a Ct of 3.60 mg/g (Fig. 2a). It was indicated that there was no significant differences in Ct values under these two extraction solvents (t-test, p > 0.05). Considering the volatility and toxicity of acetone, acetonitrile was selected as the extraction solvent. Subsequently, the effect of water content in the extraction solvent on extraction efficiency was investigated when acetonitrile was used as extraction solvent with a solid–liquid ratio of 1:40 (g/mL) and an extraction time of 30 min. The highest Ct of 3.77 mg/g was achieved when the water content was 25 % (Fig. 2b). Therefore, it was concluded that the optimal extractant was consisted of acetonitrile/H2O (75/25, v/v). Extraction time was critical in achieving optimal ultrasound-assisted extraction efficiency. The Ct was measured at intervals between 15 and 75 min while keeping other parameters constant (acetonitrile/H2O of 75/25, solid–liquid ratio of 1:40 (g/mL)). The flavonoid content increased initially and then stabilized after 30 min (Fig. 2c). It was appropriate to select 30 min as ultrasonic time. Finally, the effect of solid–liquid ratio on the Ct was assessed using the optimal conditions obtained above. When the solid–liquid ratio was 1:10 (g/mL), the sample matrix was not completely immersed by the extraction solvent, resulting in incomplete extraction and low flavonoid extraction efficiency. As shown in Fig. 2d, there was no significant difference (t-test, p > 0.05) between Ct values at ratios of 1:40 (g/mL) and 1:50 (g/mL). Therefore, a solid–liquid-ratio of 1:40 (g/mL) was selected for sample extraction to reduce solvent usage.
3.1.3 Optimization of the QuEChERS method
D. officinale is a complex matrix containing polysaccharides, proteins and pigments (Chen et al. 2021), and the purification of the extract is vital for the analysis of flavonoid components. Herein, we developed a QuEChERS method for analyzing flavonoid compounds in D. officinale. The effectiveness of the sorbent depends on selective adsorption capacity, physicochemical properties, for instance functional groups and polarity, and the characteristics of the sample extract, such as pH and ionic strength (Dąbrowski et al. 2005). Five types of adsorbents (PSA, C18, g-MWCNTs, MWCNTs-OH, MWCNTs-COOH) were tested and the dosage was optimized. The amount of sorbent tested in this study was determined based on the optimized dosage reported in previous studies (Li et al., 2020; Xuet al., 2021; Garcia-Varaet al., 2022; Shinet al., 2022). The effect of sorbents on purification efficiency was evaluated by examining the variations in flavonoid contents and color of extracts before and after purification. The concentration ratio of flavonoid component in the extract before and after purification (Rf) was presented in Table 1, implying the adsorption capacity of the adsorbent. It was indicated that PSA and C18 had a minimal adsorption capacity for flavonoids, while g-MWCNTs showed adsorption of isorhamine, apigenin, kaempferol and apiin, with Rf values ranging from 56 % to 68 %. MWCNTs-COOH and MWCNTs-OH could adsorb 76.5 % and 64.7 % of flavonoid components, respectively, showing relative strong adsorption of flavonoids in comparison to g-MWCNTs. This may be explained by that flavonoid components have a planar structure, and that the highly ordered graphite structure of g-MWCNT reduces the interaction between adsorbents and flavonoid components (Zhu et al., 2019; Li et al., 2020). Moreover, PSA removed less pigment than C18 indicated by the negligible variation in the color of sample extract treated by PSA (Fig. S3). Correspondingly, C18 was chosed as the purification sorbent. When considering the dosage of C18, the sample extract had comparable light yellow in color when C18 dosage was changed from 50 to 75 mg. Consequently, 50 mg of C18 was applied in the sample clean-up.
Flavonoids
PSA
C18
g-MWCNTs
MWCNTs-COOH
MWCNTs-OH
75 mg
50 mg
25 mg
75 mg
50 mg
25 mg
5 mg
2.5 mg
5 mg
2.5 mg
5 mg
2.5 mg
Naringenin
106
83
81
88
81
91
81
92
72
79
79
93
Quercetin
91
83
87
91
74
86
74
82
52
61
51
58
Rutin
77
78
80
93
83
89
81
81
14
39
31
63
Isorhamnetin
99
90
82
88
79
77
60
60
26
39
27
47
Apigenin
107
99
89
96
85
79
62
69
25
48
30
53
Eriodictyol
97
99
94
92
100
81
90
86
67
79
91
106
Chrysoeriol
78
85
85
85
86
80
73
86
37
59
37
54
Hesperetin
101
109
101
102
114
106
103
110
101
108
98
99
Isoquercitrin
84
78
83
87
87
73
73
73
20
41
41
64
Quercitrin
81
78
74
93
84
73
87
70
20
36
38
58
Luteoloside
85
79
103
89
98
78
77
85
10
37
70
41
Kaempferol
76
75
82
76
90
80
56
39
50
31
62
45
Schaftoside
73
80
77
96
88
93
83
91
58
74
73
81
Apiin
89
96
96
97
97
99
68
89
19
44
32
58
Hyperoside
75
82
89
91
92
87
75
79
25
44
34
53
Naringin
79
92
116
102
95
108
117
103
70
88
81
84
Taxifolin
85
93
93
98
101
100
95
102
82
102
88
87
3.2 Method validation and comparison
The QuEChERS-HPLC-MS/MS method was validated to verify that its performance was qualified for the routine analysis of flavonoid components in D. officinale. Performance parameters including linearity, sensitivity, accuracy and precision were assessed.
All flavonoids showed a good linearity in the range of 1–200 μg/L, and the correlation coefficient was greater than 0.99 (Table 2). The LOD of 17 flavonoid components ranged from 2.1 to 49.55 μg/kg, and the LOQ ranged from 7.12 to 165.2 μg/kg (Table 2). The recoveries were examined at three levels, providing recoveries of 73 % to 112 %. The intra-day precision and inter-day precision were varied from 1.9 % to 13.3 % and 3.7 % to 15.1 %, respectively. Therefore, the validation results met the criteria of SANTE/11312/2021 (European Commission, 2021), demonstrating that the QuEChERS-HPLC-MS/MS method can provide good linearity, sensitivity, accuracy as well as precision for simultaneous analysis of 17 flavonoid components in D. officinale. Additionally, the relative uncertainties of 17 flavonoid components were in the range of 2.4 % to 15.4 % and the expanded relative uncertainties were in the range of 4.8 % to 30.8 % (Table S5).
No.
Flavonoids
Calibration curve (1–200 μg/L)
r2
LOD (μg/kg)
LOQ (μg/kg)
Recovery (%)
Intra-day RSD (%)
Inter-day RSD (%)
Low
Medium
High
1
Naringenin
y = 10770x + 2940.5
0.9997
4.3
14.3
83
78
97
3.0
9.9
2
Quercetin
y = 3749.5x − 9400.3
0.9979
31.4
104.8
85
83
78
2.3
6.7
3
Rutin
y = 1851.1x − 2159
0.9997
4.6
15.2
83
80
89
6.9
13.8
4
Isorhamnetin
y = 19393x - 10,138
0.9962
5.3
17.8
103
112
106
1.9
3.7
5
Apigenin
y = 11375x − 2195.9
0.9995
2.3
7.8
73
84
78
6.0
12.2
6
Eriodictyol
y = 4030.4x − 7641.3
0.9992
49.5
165.2
107
107
100
7.1
8.1
7
Chrysoeriol
y = 40809x − 9176.6
1.000
6.2
20.6
87
85
80
3.1
7.8
8
Hesperetin
y = 5106.5x − 669.98
0.9999
7.4
24.5
98
95
88
2.3
6.4
9
Isoquercitrin
y = 4800.4x − 4315
0.9997
4.1
13.5
92
91
85
8.0
9.1
10
Quercitrin
y = 2994.4x − 3977
0.9997
2.1
7.1
95
97
98
13.3
9.9
11
Luteoloside
y = 9930.4x − 5001.7
0.9999
11.3
37.8
86
82
86
6.5
13.2
12
Kaempferol
y = 706.74x − 1781.6
0.9974
11.2
37.2
91
90
94
6.4
8.5
13
Schaftoside
y = 1659x − 805.88
0.9998
15.0
50.1
87
88
96
11.2
12.5
14
Apiin
y = 4543.7x − 5884
0.9996
16.7
55.6
101
95
95
5.4
10.4
15
Hyperoside
y = 4222.4x − 5855.4
0.9995
6.1
20.2
111
100
106
5.5
7.4
16
Naringin
y = 1680.3x + 967.08
0.9999
17.2
58.0
92
93
91
3.5
15.1
17
Taxifolin
y = 3314.4x − 4672.6
0.9996
48.8
162.4
102
111
101
11.8
7.8
The quantitative analysis of 17 flavonoid components in this work applied QuEChERS method in sample preparation to reduce the interference in sample extracts. The study on the quantitative analysis of flavonoid components in D. officinale was relative rare and only one relevant publication was available. The method developed in this work was compared with those reported on D. catenatum flowers, D. chrysotoxum flowers and D. officinale, and sample extraction and instrumental analysis was summarized in Table S6. The method in this work cost less amount of solvent and less sample preparation time, and afforded higher sensitivity when compared with Zhang et al. (2019) and Hu et al. (2023). Meanwhile, the method in this work showed comparable sensitivity with that of Lv et al. (2017).
3.3 Total content of flavonoid in D. officinal
The total content of flavonoid (Ct) ranged from 1.57 to 9.36 mg/g, with an average value of 4.21 mg/g. The highest Ct of 9.36 mg/g was observed for sample 4 collected from Panan, Zhejiang Province, while the lowest Ct of 1.57 mg/g was measured in sample 44 taken from Taizhou, Zhejiang Province. Samples (1–4) were obtained from the same production base located in Jinhua, Zhejiang Province, being grown under imitative wild cultivation for 2, 3, 4 and 5 years, respectively. The Ct value of samples 1–4 indicated that the total content of flavonoid was enhanced with the increase of growth year (Fig. 3). The finding was similar with the study of Xin et al. (2015) who observed that flavonoid contents in Astragali Radix was increased with the extended growth time.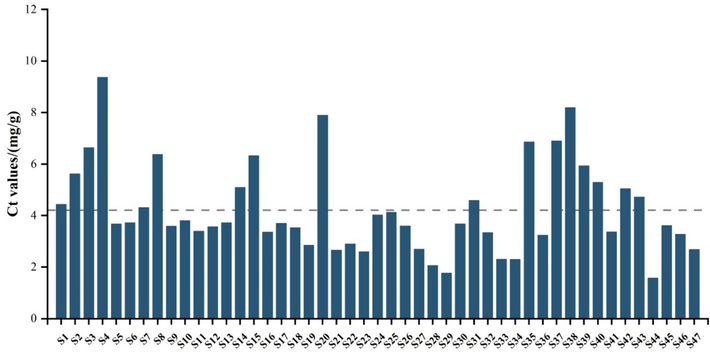
Total contents of flavonoid (Ct) in 47 of D. officinale samples.
3.4 Contents of 17 flavonoid components
Flavonoids belong to a large group of secondary metabolites in D. officinal. The basic skeletons of flavonoids are apigenin, vitexin, quercetin and kaempferol. In this study, we employed the validated method to determine the content of 17 flavonoid components with available standards in D. officinal samples from Zhejiang, Yunnan, Anhui and Guangxi Provinces. The total concentration of 17 flavonoid components is denoted as C17, ranging from 33.09 to 655.44 mg/kg. It was observed that naringenin, rutin and schaftoside were the three predominant flavonoid components in D. officinal (Fig. 4a), accounting for more than 80 % of the C17. The concentration of naringenin, rutin and schaftoside in D. officinal were 5.19–57.18, 2.01–563.17 and 4.43–67.73 mg/kg, respectively. The highest concentration of naringin, rutin and schaftoside were observed in samples 4, 43 and 35, respectively. The cultivation methods for these three samples were imitative wild cultivation, while their origins were Jinhua, Quzhou and Taizhou, Zhejiang Province, respectively. The concentration of the remaining 14 flavonoid components in D. officinal was relatively low, accounting for only 1.05 %-16.83 % of the C17 measured. The variation in individual flavonoid concentrations with extended growth years was examined in samples 1–4, which were grown under the same cultivation method yet for different years. It was noted that the variation of flavonoid components with growth years were compound-dependent. For example, the contents of naringenin, isorhamnetin, eriodictyol, kaempferol and taxifolin increased with the extension of growth year, while the contents of chrysoeriol, quercetin, and apiin remained relatively stable for 2–4 years before reaching their highest levels in the fifth year. This might be due to the different functions of flavonoids.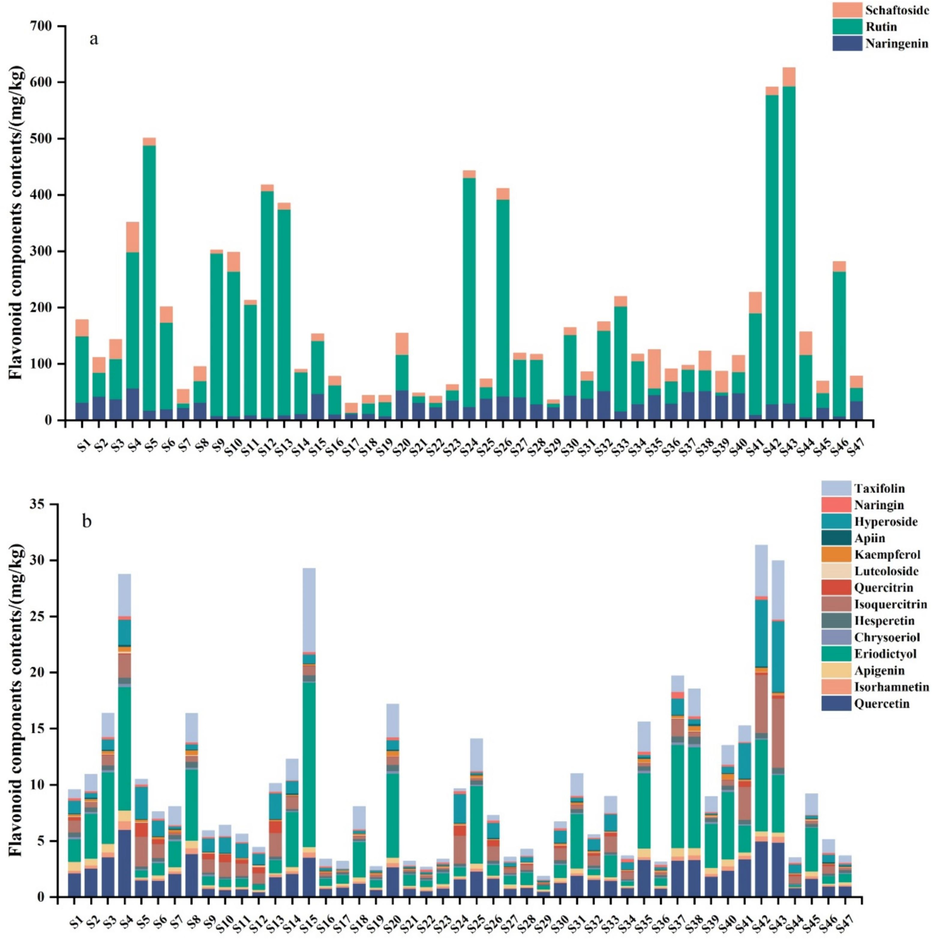
17 flavonoid components in 47 of D. officinale samples. (a) Contents of naringenin, rutin and schaftoside, and (b) Contents of the remaining 14 flavonoid components.
3.5 Antioxidant capacity of D. officinale
D. officinale sample extracts were screened for antioxidant capacity by using DPPH, ABTS, and FRAP assays (Zhaoet al., 2021). DPPH radical scavenging capacity in D. officinale sample extracts ranged from 35.62 % to 89.64 %, a 2.5-fold variations (Table S7). ABTS free radical scavenging rate varied from 16.77 % to 96.02 %, a 5.7-fold differences (Table S7). FRAP values ranged from 277.51 to 1523.41, a 5.5-fold variations (Table S7). Sample 29 from Mangshi, Yunnan Province showed the lowest antioxidant capacity indicated by three antioxidant capacity assays, while sample 15 from Lishui, Zhejiang Province had the highest DPPH radical scavenging ability. Sample 4 from Jinhua, Zhejiang Province exhibited the highest ABTS and FRAP antioxidant activities. Correlation analysis was performed to investigate the relationship between the flavonoid concentrations and antioxidant activities of D. officinale sample extracts (Table S8). The correlation coefficients between Ct and DPPH radical scavenge, ABTS radical scavenge and total antioxidant capacity of FRAP exceeded 0.7, highlighting the important role of flavonoids in the antioxidant ability of D. officinale. As regard to flavonoid compounds, 12 out of 17 compounds, including naringenin, quercetin, apigenin and isorhamnetin, were significantly positively correlated with the antioxidant activity determined by the three assays. The results were comparable with Huang et al. (2014), who found the correlation between the content of flavonoids and the antioxidant activity of D. officinale extracts. Furthermore, quercetin, one of most well-known flavonoids, has observed to be a powerful scavenger of reactive oxygen species (Wang et al., 2016).
3.6 Relationship among flavonoid components, antioxidant activity and influencing factors
Flavonoid contents in D. officinale may be influenced by various factors such as origin, cultivation method, growth years, collecting time and processing techniques. MFA was applied to study the relationship among the origin, processing, growth years, collection period, cultivation method, total flavonoid content (Ct), 17 flavonoid component contents and antioxidant activity of D. officinale (Fig. 5). The first two factors exhibited a variability of 26.20 % and 14.05 %, respectively. 12 out of 17 flavonoid compounds, including quercetin, isorhamnetin, eriodictyol, schaftoside, luteoloside, apiin, hesperetin, taxifolin, apigenin, kaempferol, hyperoside and isoquercitrin were clustered together in the first quadrant. On the other hand, rutin, naringin, and quercetin were separated from these 12 flavonoid compounds. The Ct and antioxidant activity were found to be closely related to the 12 flavonoid components since they were clustered in the near proximity, and it suggested that those 12 flavonoids contributed significantly to the antioxidant activity. This observation was consistent with the results obtained from correlation analysis. Eight different origins of D. officinale were distributed across four quadrants. Contents of 12 flavonoid components were relatively high in D. officinale sampled from Quzhou, Guangxi and Anhui compared to samples from Yunnan, Wenzhou and Taizhou, and it was consistent with the fact that samples obtained from Quzhou, Guangxi and Anhui were found to be closely associated with the 12 flavonoid components. A positive correlation was found between the D. officinale collected from Taizhou and quercetin. Accordingly, the contents of 12 flavonoid components in samples grown under imitative wild cultivation were observed to be relatively high compared to those cultivated in green-house. Moreover, long-growth-year (more than 2 years) was favored for accumulating these 12 flavonoid components. Fig. 5b plotted the coordination of all classes and indicated that the origin and cultivation methods contributed more to the flavonoid content and antioxidant activity because they have a more proximate relationship. By differentiating the color of sample dot according to cultivation method, D. officinale samples cultivated under facility-aided and imitative wild methods were mainly scattered in two separate areas on MFA score plot (Fig. 5c). Nevertheless, when the colour of sample dots was sorted according to the origin, samples from Jinhua, Quzhou and Lishui had large overlapping, suggesting that multiple factors other than the origin account for the accumulation of flavonoid in D. officinale.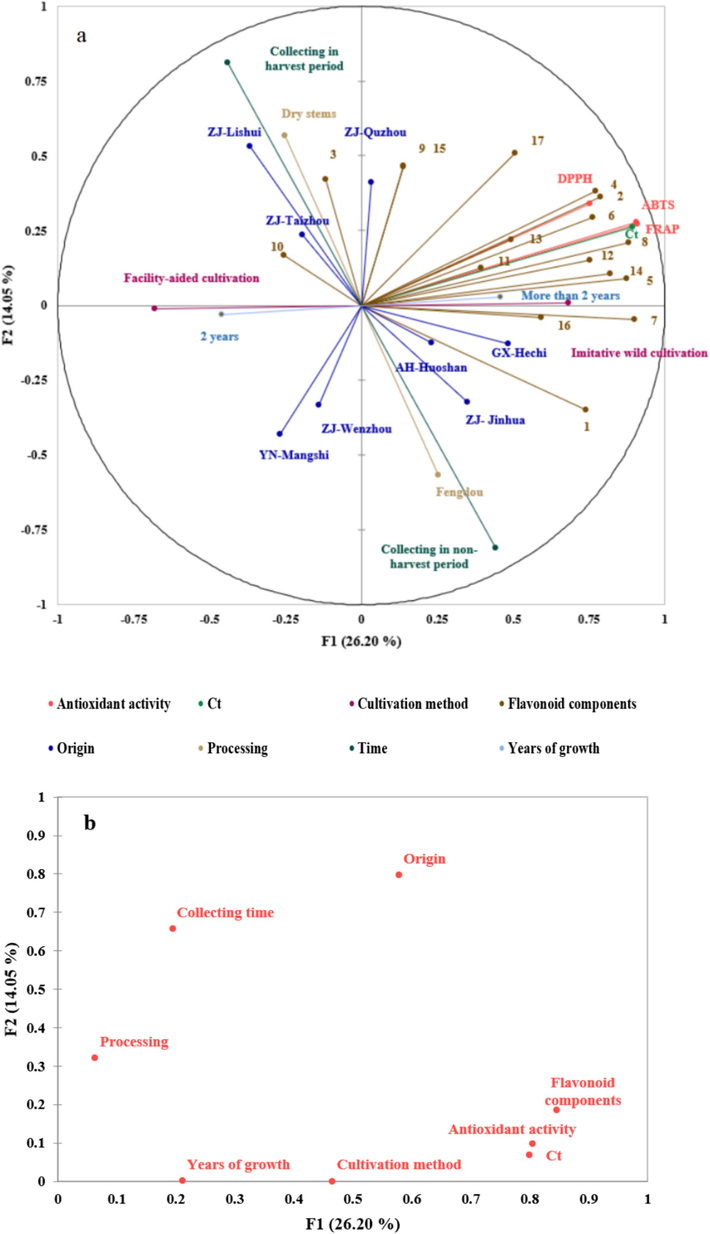
The results of MFA analysis (1-Naringenin, 2-Quercetin, 3-Rutin, 4-Isorhamnetin, 5-Apigenin, 6-Eriodictyol, 7-Chrysoeriol, 8-Hesperetin, 9-Isoquercitrin, 10-Quercitrin, 11-Luteoloside, 12-Kaempferol, 13-Schaftoside, 14-Apiin, 15-Hyperoside, 16-Naringin and 17-Taxifolin). (a) Correlation map of all variables, (b) coordinates of the eight variable classes, (c) observation scores plot of MFA (cultivation method) and (d) observation scores plot of MFA (origin).
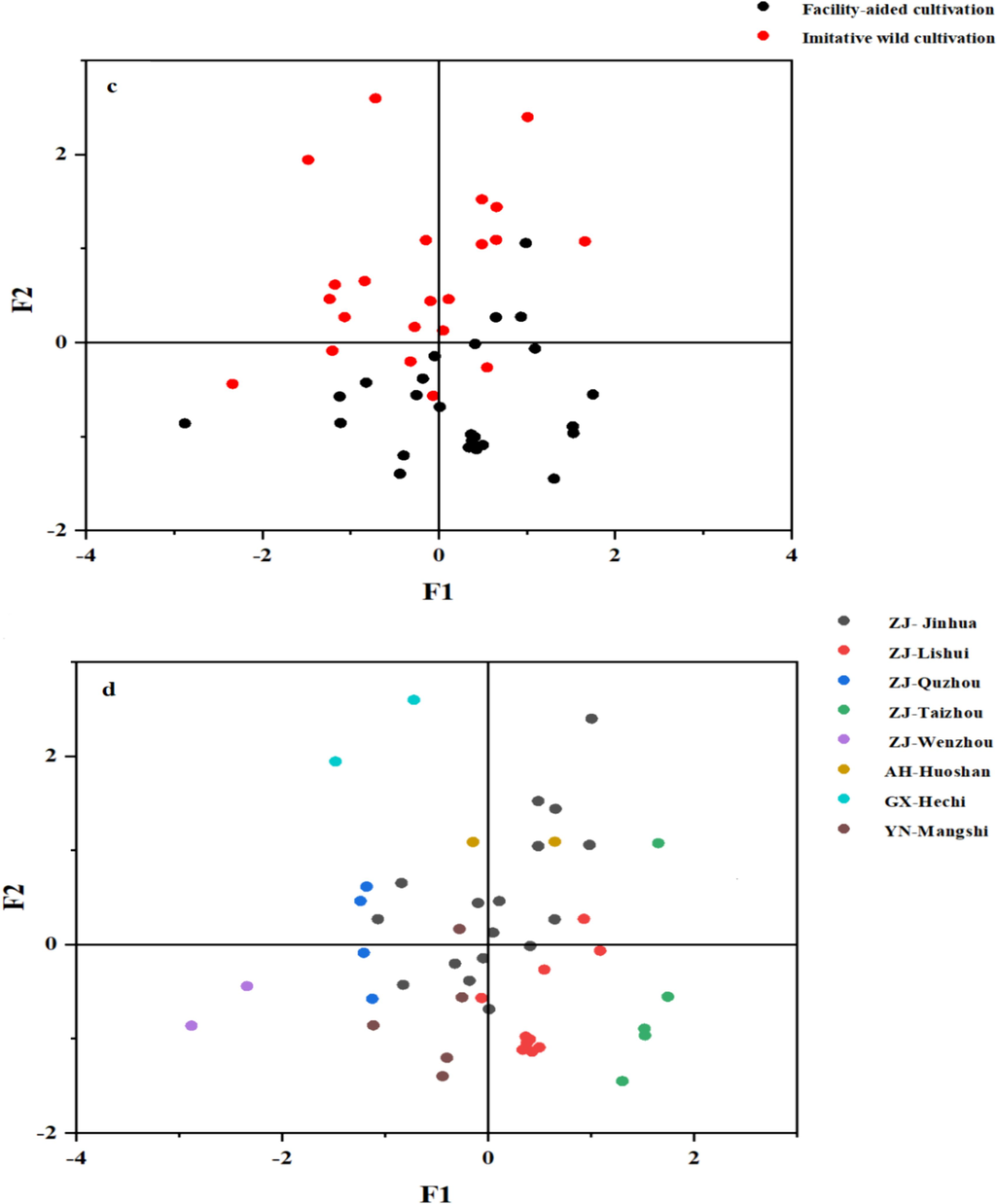
The results of MFA analysis (1-Naringenin, 2-Quercetin, 3-Rutin, 4-Isorhamnetin, 5-Apigenin, 6-Eriodictyol, 7-Chrysoeriol, 8-Hesperetin, 9-Isoquercitrin, 10-Quercitrin, 11-Luteoloside, 12-Kaempferol, 13-Schaftoside, 14-Apiin, 15-Hyperoside, 16-Naringin and 17-Taxifolin). (a) Correlation map of all variables, (b) coordinates of the eight variable classes, (c) observation scores plot of MFA (cultivation method) and (d) observation scores plot of MFA (origin).
4 Conclusion
A reliable and sensitive QuEChERS-HPLC-MS/MS method was established to simultaneously determine 17 flavonoid compounds in D. officinale. The sample was ultrasonic-extracted with 75 % acetonitrile followed by purification using C18. The analytical performance was satisfactory and was successfully applied to 47 of D. officinale samples. Naringenin, rutin and schaftoside were the three predominant components in D. officinal, accounting for over 80 % of the total concentrations of 17 flavonoids. MFA analysis showed that the origin and cultivation method had a great influence on the flavonoid content in D. officinale. Correlation analysis demonstrated a positive correlation between flavonoid contents and antioxidant activity. This study provided a simple and efficient method for the measurement of 17 flavonoid components in D. officinale, which can aid in the function research of D. officinale. This work can provide guidelines for the cultivation of D. officinale, thus helping to further utilize and research flavonoids in D. officinale. In future, if more flavonoid standards are available, the method could be validated for these flavonoids and made minor modificaitons such as change the amount of adsorbent if necessary.
CRediT authorship contribution statement
Fengting Sun: Writing – original draft. Zhenlan Xu: Conceptualization, Writing – review & editing. Xiaoyan Xu: Methodology. Yan Gao: Formal analysis. Zuoyi Zhu: Formal analysis. Xinyu Han: Methodology. Chunrong Zhang: Validation. Tao Tang: Methodology. Qiang Wang: Conceptualization. Qing Sheng: Project administration. Xueping Zhao: Project administration.
Acknowledgements
We thank National Natural Science Foundation of China (Grant number 21806143) and Yi Pin Yi Ce Project (Grant number ZJNY2016001-03) for financial support. The authors also thank the anonymous reviewers for their valuable comments and suggestions on this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical fingerprint of free polyphenols and antioxidant activity in dietary fruits and vegetables using a non-targeted approach based on QuEChERS ultrasound-assisted extraction combined with UHPLC-PDA. Antioxidants. 2020;9(4):305.
- [CrossRef] [Google Scholar]
- Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from Bitter Gourd using Response Surface Methodology (RSM) Food Biopro. Process.. 2020;20:114-122.
- [CrossRef] [Google Scholar]
- Antioxidation and melanogenesis inhibition of various Dendrobium tosaense extracts. Molecules. 2018;23(7):1810.
- [CrossRef] [Google Scholar]
- Traditional uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol.. 2021;12:726528
- [CrossRef] [Google Scholar]
- Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochem.. 2022;90:106190
- [CrossRef] [Google Scholar]
- Adsorption of phenolic compounds by activated carbon - A critical review. Chemosphere. 2005;58(8):1049-1070.
- [CrossRef] [Google Scholar]
- European Commission (2021). Guidance document on analytical quality control and method validation procedures for pesticide residues analysis in food and feed, SANTE/11312/2021.
- Dissipation, occurrence, and risk assessment of 12 pesticides in Dendrobium officinale Kimura et Migo. Ecotoxicol. Environ. Saf.. 2021;222:112487
- [CrossRef] [Google Scholar]
- Chitosan promoting formononetin and calycosin accumulation in Astragalus membranaceus hairy root cultures via mitogen-activated protein kinase signaling cascades. Sci. Rep.. 2019;9(1):10367.
- [CrossRef] [Google Scholar]
- QuEChERS-based analytical methods developed for LC-MS/MS multiresidue determination of pesticides in representative crop fatty matrices: Olives and sunflower seeds. Food Chem.. 2022;386:132558
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted extraction of polysaccharide from Dendrobium officinale: Kinetics, thermodynamics and optimization. Food Chem.. 2021;177:108227
- [CrossRef] [Google Scholar]
- Comparative analysis of flavonoids extracted from Dendrobium chrysotoxum flowers by supercritical fluid extraction and ultrasonic cold extraction. Sustain. Chem. Pharm.. 2023;36:101267
- [Google Scholar]
- Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules. 2016;21(6):701.
- [CrossRef] [Google Scholar]
- Correlation of the antioxidant property with the total phenolic content and total flavonoids of different Dendrobium officinale extracts. Chin. J. Appl. Environ. Biol.. 2014;20(3):438-442.
- [Google Scholar]
- ISO (1993). Guide to the Expression of Uncertainty in Measurement, International Organization for Standardization, Geneva, Switzerland, 1st ed.
- IUPAC (2002). Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC technical report). Pure and Appl. Chem. 74, 835-855.
- Antioxidant properties and color parameters of herbal teas in China. Ind. Crop Prod.. 2016;87:198-209.
- [CrossRef] [Google Scholar]
- Flavonoids in different parts of common buckwheat (Fagopyrum esculentum) and Tartary buckwheat (F. tataricum) during growth. J. Food Compos. Anal.. 2023;120:105362
- [CrossRef] [Google Scholar]
- Does the metabolome of wild-like Dendrobium officinale of different origins have regional differences? Molecules. 2022;27(20):7024.
- [CrossRef] [Google Scholar]
- High adsorption of benzoic acid on single walled carbon nanotube bundles. Sci. Rep.. 2020;10:10013.
- [CrossRef] [Google Scholar]
- Screening of pesticide residues in traditional Chinese medicines using modified QuEChERS sample preparation procedure and LC-MS/MS analysis. J. Chromatogr. B. 2020;1152:122224
- [CrossRef] [Google Scholar]
- Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J Biol. Macromol.. 2016;89:219-227.
- [CrossRef] [Google Scholar]
- Qualitative and quantitative analysis of the components in flowers of Hemerocallis citrina Baroni by UHPLC-Q-TOF-MS/MS and UHPLC-QQQ-MS/MS and evaluation of their antioxidant activities. J. Food Compo. Anal.. 2023;120:105329
- [CrossRef] [Google Scholar]
- Optimized microwave-assisted extraction of bioflavonoids from Albizia myriophylla bark using response surface methodology. J. Food Sci. Tech.. 2020;57:2107-2117.
- [CrossRef] [Google Scholar]
- Structural characterization and immunomodulating activities of polysaccharides from a newly collected wild Morchella sextelata. Int. J. Biol. Macromol.. 2019;129:608-614.
- [CrossRef] [Google Scholar]
- Superfine grinding of Dendrobium officinale: the finer the better ? Int. J. Food Sci. Tech.. 2019;54(6):2199-2208.
- [CrossRef] [Google Scholar]
- A design of experiments strategy to enhance the recovery of polyphenolic compounds from Vitis vinifera by-products through heat reflux extraction. Biomolecules. 2019;9(10):529.
- [CrossRef] [Google Scholar]
- National Health Commission of the People’s Republic of China (2020). Nine substances as dual-use plants with both botanical medicine and food applications.
- Nicácio, A.E., Rodrigues, C.A., Jardim, I.C.S.F., Visentainer, J.V., Maldaner, L., 2020. Modified QuEChERS method for phenolic compounds determination in mustard greens (Brassica juncea) using UHPLC-MS/MS. Arab. J. Chem.13(3), 4681-4690. http://creativecommons.org/licenses/ by-nc-nd/4.0/.
- Comparison of QuEChERS and liquid-liquid extraction methods for the simultaneous analysis of pesticide residues using LC-MS/MS. Food Control. 2022;141:109202
- [CrossRef] [Google Scholar]
- Pressurised liquid extraction of flavonoids in onions. Method development and validation. Talanta. 2009;80(1):269-278.
- [CrossRef] [Google Scholar]
- Tang, H., Zhao, T., Sheng, Y., Zheng, T., Fu, L., Zhang, Y., 2017. Dendrobium officinale Kimura et Migo: a review on its ethnopharmacology, phytochemistry, pharmacology, and pndustrialization. Evid. Based Compl. Alt. Med. 7436259. DOI: 10.1155/2017/7436259.
- Comparative analysis of chemical constituents by HPLC-ESI-MSn and antioxidant activities of Dendrobium huoshanense and Dendrobium officinale. Biomed. Chromatogr.. 2021;36(1):5250.
- [CrossRef] [Google Scholar]
- The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Tech.. 2016;56:21-38.
- [Google Scholar]
- Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem.. 2017;231:231-237.
- [CrossRef] [Google Scholar]
- Molecular systematics of Dendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences. Mol. Phylogenet. Evol.. 2013;69(3):950-960.
- [CrossRef] [Google Scholar]
- Development and validation of a HILIC-MS/MS method for simultaneous quantitative of taste-active compounds in foods. J. Food Compo. Anal.. 2023;120:105302
- [CrossRef] [Google Scholar]
- Influence of different growing years on accumulation of flavonoids and saponins in Astragali Radix. J. Chinese Med. Mater.. 2015;38(7):1366-1369.
- [CrossRef] [Google Scholar]
- Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev.. 2013;12(2):341-367.
- [CrossRef] [Google Scholar]
- Pesticide multi-residues in Dendrobium officinale Kimura et Migo: Method validation, residue levels and dietary exposure risk assessment. Food Chem.. 2021;343:128490
- [CrossRef] [Google Scholar]
- Chemical differentiation of Dendrobium officinale and Dendrobium devonianum by using HPLC fingerprints, HPLC-ESI-MS, and HPTLC analyses. Evid. Based Compl. Alter. Med.. 2017;2017:8647212.
- [CrossRef] [Google Scholar]
- Tissue-specific transcriptome for Dendrobium officinale reveals genes involved in flavonoid biosynthesis. Genomics. 2020;112(2):1781-1794.
- [CrossRef] [Google Scholar]
- Dendrobium officinale leaves as a new antioxidant source. J. Func. Foods.. 2017;37:400-415.
- [CrossRef] [Google Scholar]
- Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of Dendrobium catenatum flowers and selection of quality control herb-markers. Food Res. Int.. 2019;123:732-745.
- [Google Scholar]
- Phytochemical profiles of edible flowers of medicinal plants of Dendrobium officinale and Dendrobium devonianum. Food Sci. Nutr.. 2021;9:6575-6586.
- [CrossRef] [Google Scholar]
- Simultaneous identification and determination of flavonoids in Dendrobium officinale. Chem. Cent. J.. 2018;12(1):40.
- [CrossRef] [Google Scholar]
- Simultaneous determination of 131 pesticides in tea by on-line GPC-GC-MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem.. 2019;276:202-208.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105911.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







