Translate this page into:
Controlled synthesis of 3D marigold-like ZnIn2S4/Ti3C2 for rapid and efficient removal of antibiotics
⁎Corresponding authors. pingmao@hyit.edu.cn (Ping Mao), sunaiwu@hyit.edu.cn (Aiwu Sun), yychem@njust.edu.cn (Yong Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Developing of a rapid and efficient photocatalyst for the removal of antibiotics with high concentration in wastewater remediation is of great importance. 3D marigold-like ZnIn2S4/Ti3C2 was successfully fabricated by coupling 2D Ti3C2 nanosheets with hierarchical 3D ZnIn2S4 using a hydrothermal method. The degradation efficiency of ZnIn2S4/Ti3C2 composite for tetracycline (50 mg/L) and 7-Aminocephalosporanic acid (25 mg/L) within 120 min and 90 min can reach up to 91% and 95%, respectively. The introduction of Ti3C2 modulates the 3D marigold-like architecture of ZnIn2S4, which not only boosted its photon capture performance and adsorption capacity caused by the increased specific surface area, but also effectively separated photo-generated electrons and holes via the well-defined 2D/2D interface between ZnIn2S4 and Ti3C2. The synergistic effect of physical adsorption and photocatalytic degradation contributes to the high degradation efficiency and fast degradation performance. ZnIn2S4/Ti3C2 still exhibits high photocatalytic activity and excellent physicochemical stability after many cycles.
Keywords
Antibiotics
3D marigold-like
ZnIn2S4/Ti3C2
Photocatalysis
Synergistic effect
1 Introduction
Since the discovery of antibiotics, they have been widely used because of their excellent bacteriostatic and bactericidal effects. However, with the mass production and abuse of antibiotics, especially after the prevalence of COVID-19, its pollution to the environment is increasingly serious, thus antibiotics have become a new pollutant (Ahmed et al., 2015). In order to solve this problem, a large number of scholars have explored various methods to remove antibiotics, such as adsorption (Gao et al., 2012), biological degradation (Micoine et al., 2013), and chemical oxidation (Yan et al., 2017), et al. However, the problems of high energy consumption and high cost of these traditional methods restrict their application seriously (Deng 2020). Moreover, with the rapid development of photocatalytic technology, photocatalysis exhibits great potential in the degradation of antibiotics (Liang et al., 2021, Huang et al., 2016a, Huang et al., 2016b).
Photocatalytic technology is based on the oxidation and reductive of photo-generated electrons and holes generated by semiconductor materials under incident light irradiation (Abdul Mubarak et al., 2022, Huang et al., 2014, Huang et al., 2019). It can excite O2 and H2O into •O2− and •OH, which have a strong redox ability to degrade antibiotics. Photocatalysts, including TiO2 (Meng et al., 2019) and ZnO (Kassem et al., 2021) have been widely used to degrade organic pollutants. However, due to its inherent defects, such as poor excitation ability of visible light, poor stability and insufficient exposure of active sites, the photodegradation efficiency of antibiotics is still not satisfactory (Qu et al., 2020). Ternary sulfide ZnIn2S4 is a typical visible light-driven photocatalyst with an adjustable band gap (2.06–2.85 eV). Its layered structure exhibits good photocatalytic activity and stability (Kulkarni et al., 2017, Peng et al., 2022). It can be used in various fields such as hydrogen evolution (Jing et al., 2010, Chen et al., 2014, Yuan et al., 2et al., 2et al., 2et al., 2et al., 2et al., 2et al., 2016, Peng et al., 2021), photocatalytic CO2 reduction (Ye et al., 2019), organic pollutants degradation (Lu et al., 2019, Hao et al., 2021) and environmental remediation (Gao et al., 2017, Li et al., 2014). In addition, compared with binary sulfides (CdS (Guo et al., 2022a), Sb2S3 (Wang et al., 2016a)), ZnIn2S4 has similar optical properties but does not contain toxic metal ions. Compared with other ternary sulfides CuGaS2 (Leach and Macdonald 2016) and Zn3In2S6 (Wu et al., 2018), ZnIn2S4 has the advantages of easy preparation and relative stability in the photocatalytic process. However, some disadvantages, including poor light absorption ability, poor photo-generated carrier migration ability, and fast photo-generated electron-hole recombination, have hindered its further application. Modifying semiconductor photocatalytic materials with other materials, such as Ag3PO4@ZnIn2S4, ZnIn2S4@MoO3 (Ouyang et al., 2021, Zhang et al., 2021), and p-ZnIn2S4/RGO/n-g-C3N4 (Yu et al., 2020), effectively improve the activity of ZnIn2S4. Therefore, functional modifications are desired for ZnIn2S4-based photocatalysts(Raja et al., 2021).
Compared with traditional cocatalyst, the 2D materials with controllable interfaces and a relatively high specific surface area can offer a larger number of advantages for the migration ability of photogenerated charge carriers (Guo et al., 2022b). MXenes (Ti3C2) is a new type of transition metal carbides, which can be obtained by selective etching of the A-element from the MAX phases (Qiu et al., 2022, Wang et al., 2023). It has become a significant and increasingly popular class of post-graphene 2D nanomaterial, since the discovery of Ti3C2 in 2011 (Wang et al., 2018). Owing to its unique physical and chemical properties, such as adjustable band gap, hydrophobicity, anisotropic behavior of carrier mobility, metallic conductivity, favorable optical and mechanical properties (Hong Ng et al., 2017), it has shown stupendous potential in electrochemical devices, membrane separation, catalysis, sensor, adsorption and cell imaging (Ling et al., 2014, Peng et al., 2014, Ding et al., 2017, Li et al., 2020). MXenes have excellent electronic conductivity, convenient for the separation and transfer of photo-generated electrons. In addition, owing to its adjustable work function (1.6–6.0 eV), which relies on the difference of surface functional groups, the interface Schottky junction between Ti3C2 and most photocatalysts can be constructed as an electron sink to inhibit the recombination of photoexcited electrons and holes (Cai et al., 2018, Yang et al., 2019a, Liu et al., 2019a). Cao et al. prepared 2D/2D Heterojunction of ultrathin MXene/Bi2WO6 nanosheets and showed high photocatalytic activity for CO2 reduction (Cao et al., 2018). Cheng et al. synthesized the composite of MXene/CdLa2S4 via a facile hydrothermal method. The composite exhibited enhanced photocatalytic activity for the production of hydrogen (Cheng et al., 2020). Furthermore, the Cui group reported 2D layered CdS@Ti3C2@TiO2 composites with enhanced visible-light-driven photocatalytic activity for organic pollutants degradation (Liu et al., 2019b). Therefore, introducing Ti3C2 into ZnIn2S4 is expected to solve the above disadvantages.
Herein, inspired by this, a 3D marigold-like ZnIn2S4/Ti3C2 hetero-system coordinating with 2D/2D interface was successfully fabricated by a simple hydrothermal method (Scheme 1). The photocatalytic activities were assessed by the degradation of tetracycline (TC) and 7-Aminocephalosporanic acid (7-ACA). Compared with the pristine ZnIn2S4, the intense interfacial contact between ZnIn2S4 and Ti3C2 significantly increased the specific surface area and reactive site of all ZnIn2S4/Ti3C2 heterojunction photocatalysts, thus their photocatalytic activities were improved. The degradation mechanism was studied by characterization analysis and photoelectrochemical tests, and the enhanced photocatalytic activity was explained. Therefore, this study is expected to provide a new strategy for the efficient removal of antibiotics.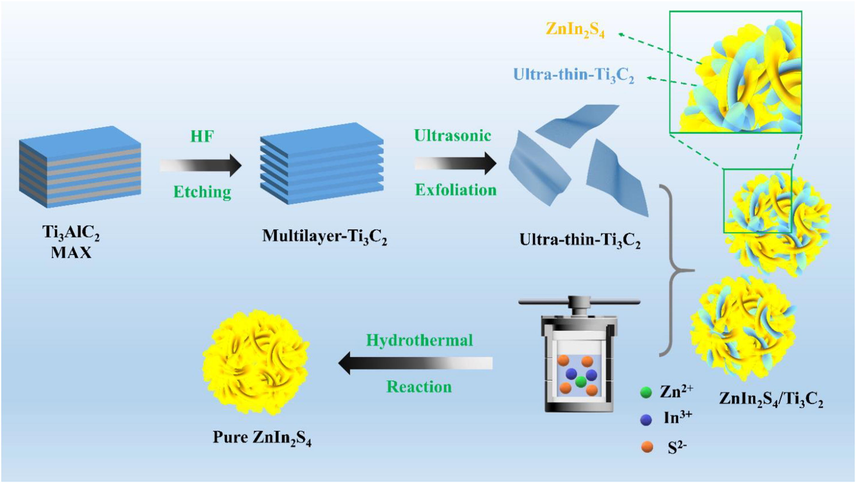
Schematic illustration of the fabrication process of pristine ZnIn2S4 and 3D marigold-like ZnIn2S4/Ti3C2.
2 Experimental
2.1 Preparation of Ti3C2 ultrathin nanosheets
Similar to the previous study (Cao et al., 2018), Ti3C2 ultrathin nanosheets were synthetized by selective etching the MAX phase, and then ultrasonic stripping of multilayer Ti3C2. In a typical procedure, 300 mg of Ti3AlC2 powder was slowly added to a plastic container containing 20 mL of HF solution (content ≥ 40%), sealed and stirred magnetically for 72 h at room temperature. The suspension was then filtrated and filtration residue was washed with DI water several times until pH ≥ 6. The resulting precipitate was dried overnight at 60℃ in a vacuum. The obtained dried solids were re-dispersed and vigorous stirred in 20 mL of DMSO for 12 h. Afterwards, the suspension was centrifuged and washed multiple times with DI water remove the remaining DMSO. The collected precipitate was re-dispersed to 20 mL of DI water, and an ultrasound was performed in the ice bath and Ar for 120 min. Finally, the suspension was centrifuged at 5000 rpm for 10 min to remove the multilayer Ti3C2. The concentration of Ti3C2 suspension was about 1 mg/mL.
2.2 Preparation of Ti3C2/ZnIn2S4 (MZ)
Various amounts of Ti3C2 (3 mg, 6 mg, and 9 mg) were dispersed in 50 mL of DI water to form suspensions by ultrasound, respectively. And then ZnCl2 (0.5 mmol), InCl3·4H2O (1 mmol), and excess L-Cysteine were added into the above suspensions. After stirred magnetically for 3 h, 2 mmol of thioacetamide (TAA) was added. Afterwards, the resulting solutions were transferred to 100 mL Teflon-lined autoclaves and heated at 160 °C for 12 h. After cooling, light yellow-green precipitates were separated by centrifugation at 10000 rpm for 10 min and washed with DI water and ethanol several times. The resulting precipitates were dried in a vacuum oven at 60 °C for 12 h. MZ composites prepared with 3 mg, 6 mg, and 9 mg of Ti3C2 were named as MZ-3, MZ-6, and MZ-9, respectively. As a comparison, pure ZnIn2S4 was also prepared by the above method without Ti3C2.
2.3 Evaluation of photocatalytic activity
Firstly, 20 mg of the prepared sample was added into 100 mL of 50 mg/L tetracycline (TC) solution and 25 mg/L 7-Aminocephalosporanic acid (7-ACA) solution, respectively. The adsorption–desorption equilibrium was then obtained by stirring in the dark for 30 min. And then, the photodegradation reactions were performed by the visible light (300 W Xe lamp with a cut-off filter, λ > 420 nm). After a certain irradiation time, 5 mL of the suspension was taken out, and the photocatalyst was then removed with a 0.22 μm filter before measurement. The absorbance of suspensions at 356 nm was measured by a Shimadzu UV2600 spectrophotometer at a certain interval to monitor the change of concentration of TC. The concentration of 7-ACA was determined by high-performance liquid chromatography (HPLC, APS80-16PLUS), which used an ultraviolet detector and an RP18 column (dimension 4.6 × 250 mm, 5.0 μm), with sodium acetate-acetic acid buffer solution and acetonitrile (90:10, v/v) as effluent at a flow rate of 1.0 mL/min. The injection volume was 10 μL. The degradation efficiency is calculated by Eq. (1).
To assess the stability of photocatalytic activity of the photocatalyst, the sample was collected by centrifugation after one test, washed with DI water several times and dried for the next cycle test.
3 Results and discussion
3.1 Characterization of all samples
The crystal structure of the Ti3C2, ZnIn2S4, and MZ composites was investigated by XRD analysis. As shown in Fig. S1, the most intense diffraction peak at 38.7° (2θ) in the Ti3AlC2 pattern disappeared, and 9.6° allotted to the (0 0 2) plane of Ti3C2 moved to lower angles, indicating that Ti3AlC2 was successfully converted to Ti3C2 after etching treatment by HF (Alhabeb et al., 2017). XRD patterns of other samples are shown in Fig. 1a, the decreased peak intensity of the synthesized Ti3C2 indicated that the MAX powder lost its crystallinity after Al etching (Tariq et al., 2018). There are three strongly characteristic diffraction peaks at 21.6°, 27.7°, and 47.2°, corresponding to (0 0 6), (1 0 2), and (1 1 0) lattice planes of hexagonal ZnIn2S4 (JCPDS No.72–0773). After combination, the typical peaks of MZ composites are almost unchanged compared with that of the pristine ZnIn2S4, suggesting the excellent crystal structure of ZnIn2S4 is still maintained in MZ composites. However, no obvious Ti3C2 peaks can be found in the patterns of MZ composites owing to its low content (Cao et al., 2018).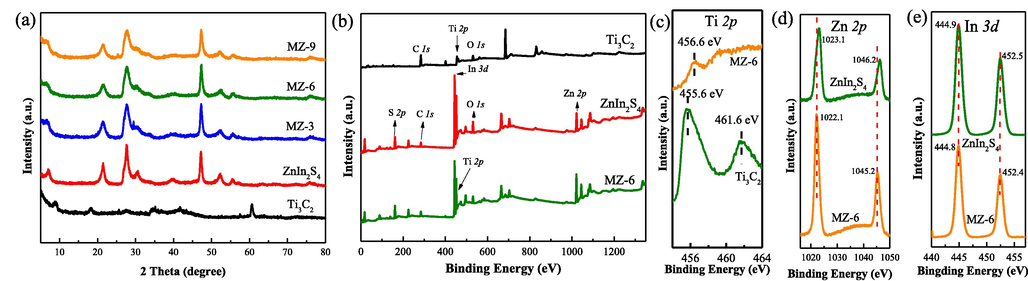
(a) XRD patterns of Ti3C2, ZnIn2S4, MZ-3, MZ-6, MZ-9. (b) XPS survey spectra of Ti3C2, ZnIn2S4 and MZ-6. (c) Ti 2p, (d) Zn 2p, (e) In 3d core-level spectra of MZ-6, respectively.
To further study the recombination between ZnIn2S4 and Ti3C2, the surface element components and charge transfer in Ti3C2, ZnIn2S4, and MZ-6 were studied by XPS analyses. From the survey scan of the MZ-6 (Fig. 1b), the concomitant C, O, S, Ti, Zn, and In elements were detected. An obvious Ti peak could be seen through the high-resolution map of Ti 2p of MZ-6 (Fig. 1c), indicating the successful synthesis of ZnIn2S4/Ti3C2. And the peak showed a shift in relation to that in the pristine Ti3C2. Noticeable shift of bind energy was observed in the high-resolution XPS spectra of Zn 2p (Fig. 1d), suggesting that the electrons transfer from ZnIn2S4 to Ti3C2 in the Schottky junction, which indicates the close and robust interaction between Ti3C2 and ZnIn2S4. The Schottky junction can significantly improve the carrier separation rate and photocatalytic activity of sample (Ou et al., 2021).
FESEM and EDX measurements were used to analyze the morphology and composition of Ti3AlC2, multilayer Ti3C2, ultra-thin Ti3C2, pristine ZnIn2S4, and MZ-6 composite, respectively. The results are shown in Fig. 2, the original Ti3AlC2 is a bulk layered ternary carbide (Fig. 2a). After HF etching, accordion-like Ti3C2 is obtained (Fig. 2b). The few-layer ultrathin Ti3C2 nanosheets were then obtained by DMSO-intercalation assisted ultrasonic exfoliation (Fig. 2c). As shown in Fig. 2d-f, pristine ZnIn2S4 is a 3D marigold-like hierarchical architecture assembled from a large number of nanosheets. The diameters of these assembled 3D ZnIn2S4 vary from 6 to 10 μm. Further observation shows that the nanosheets on the surface of these microspheres are incomplete and irregular. When Ti3C2 is introduced, 3D marigold-like hierarchical architecture does not change due to the small amount of Ti3C2. Interestingly, the diameter of the composite decreases significantly, but the nanosheets on the surface become more regular (Fig. 2g-i). Meanwhile, the elemental mapping of MZ-6 shown in Fig. 2j demonstrates that Zn, In, S, Ti, and C are uniformly distributed in the sample. The 2D nanosheets of Ti3C2 self-assemble and modify the hierarchical architecture of ZnIn2S4, which can give the sample a scattering cross section, and make it have good capability of antibiotics capture and photon trapping(Wang et al., 2021).
FE-SEM images of (a) Ti3AlC2, (b) multilayer Ti3C2, (c) ultra-thin Ti3C2, (d-f) pristine ZnIn2S4 and (g-i) MZ-6. (j) EDX elemental mapping of MZ-6.
TEM characterization was then used to further discuss the morphology and internal structure of MZ-6. As well as SEM results, 3D structure with severe agglomeration can also be found in Fig. 3a, and the diameter of MZ-6 is around 2 μm. HRTEM image shown in Fig. 2b also displays the spiny exterior of MZ-6. The clear lattice fringes with a spacing of 0.32, 0.22, and 0.26 nm belonged to ZnIn2S4 (1 0 2), ZnIn2S4 (1 0 8), and Ti3C2 (0 1 1 0) can be observed from the magnified interface (Fig. 3c), successful verifying the 2D/2D interface formed between ZnIn2S4 and Ti3C2, which can effectively facilitate the charge separation during photocatalysis. In view of the above characterizations, the possible construction mechanism of 3D marigold-like ZnIn2S4/Ti3C2 can be proposed. Zn2+ and In3+ in the solution were adsorbed on the negatively charged surface of Ti3C2. Then, in-situ hybridization of ZnIn2S4 on Ti3C2 nanosheets was simultaneously carried out by hydrothermal crystallization process, and the strong 2D/2D interface interaction between ZnIn2S4 and Ti3C2 was produced (Yang et al., 2019b). Afterwards, the 2D ZnIn2S4/Ti3C2 subunits were assembled to construct the well-defined 3D marigold-like hierarchical architecture (Wang et al., 2021).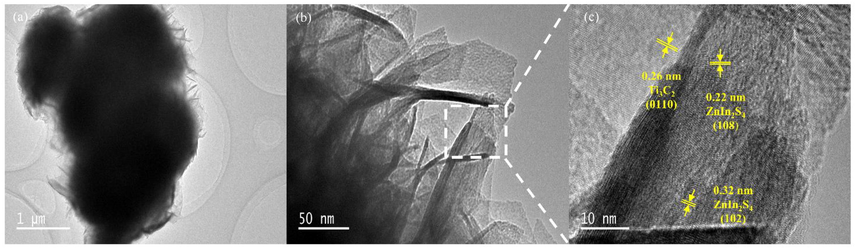
TEM (a, b) images of the MZ-6 and HRTEM of the enlarged area of the block (c).
To understand the light-harvesting capability of Ti3C2, ZnIn2S4, and MZ composites, ultraviolet–visible diffuse reflectance spectra were carried out. As shown in Fig. 4a, Ti3C2 exhibits a high absorption band in the visible region due to its dark color, the same as the previously reported work (Lukatskaya Maria et al., 2013). However, ZnIn2S4 gets a great improvement on the light absorption due to the introduction of Ti3C2. All MZ composites showed an obvious improvement of light absorption in the range of 450–800 nm, and with the increase of Ti3C2 content, the light absorption intensity of MZ composites increased slightly, suggesting the effective improvement of ZnIn2S4 light absorption through the Ti3C2 loading. The band gap values of ZnIn2S4, MZ-3 and MZ-6 can be calculated by the Tauc equation (αhν)n = A (hν - Eg). The band gap value of ZnIn2S4 is estimated to be 2.36 eV (Fig. 4b). The band gap becomes narrower after the addition of Ti3C2. The values of the band gap energy of MZ-3 and MZ-6 were 2.33 eV and 2.27 eV, respectively. Moreover, the exact conductive band (CB) potential of MZ-6 was calculated to be –0.90 V (ENHE = EAg/AgCl + 0.197 (V)) by Mott-Schottky measurement. Thus, the valence band (VB) of ZnIn2S4 is 1.37 V.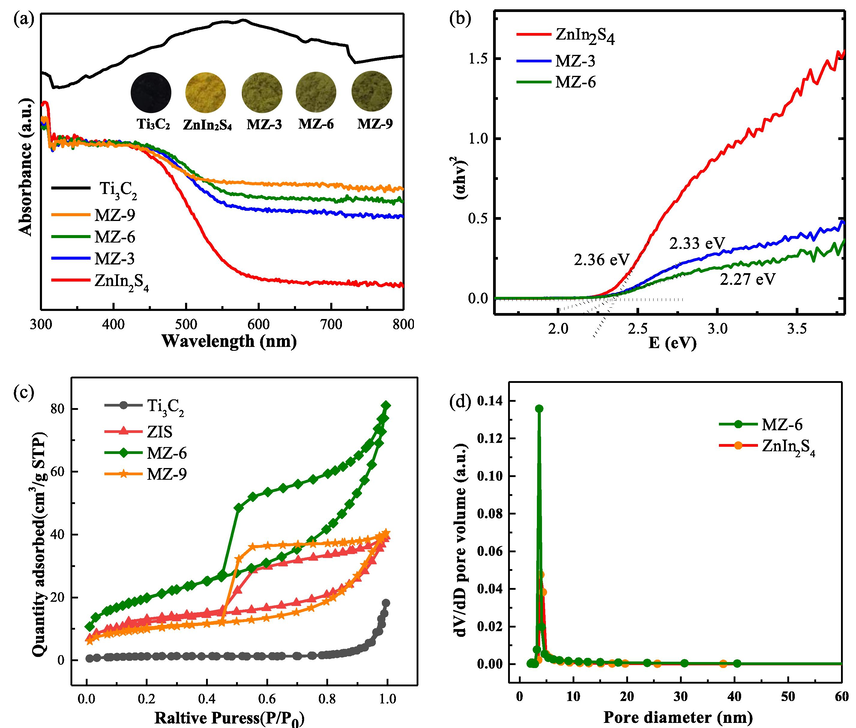
(a) UV–vis diffuses reflectance spectra (DRS) of Ti3C2, ZnIn2S4 and MZ composites (digital pictures). (b) The band gap of ZnIn2S4. (c) N2 adsorption–desorption isotherms and (d) pore size distribution of Ti3C2, ZnIn2S4 and MZ-6 at 77K.
The surface-active site is an essential factor affecting photocatalytic degradation efficiency. Therefore, the specific surface areas and pore sizes of Ti3C2, ZnIn2S4, and MZ composites were determined. As shown in Fig. 4c. Ti3C2 has a typically reversible microporous adsorption curve (type I curves), indicating that the material is mainly composed of micropores (Sun et al., 2020). Both ZnIn2S4 and MZ composites exhibit Ⅳ-type adsorption with the H-3 type hysteresis loops, which implied that mesoporous structures are occur in these samples. Meanwhile, the specific surface area of Ti3C2, ZnIn2S4, MZ-6, and MZ-9 was 4.67, 43.27, 71.46, and 34.9 m2/g, respectively. MZ-6 has the highest specific surface area due to the well-defined 3D hierarchical architecture. However, excess of Ti3C2 in MZ-9 leads to the agglomeration of the components, thus the specific surface area of MZ-9 is unambiguous decreased. Higher specific surface area is conductive to the formation of more surface active sites, and strengthens the photon capture, which can enhance photocatalytic activity (Xiao et al., 2018). Fig. 4d shows the pore size distribution curves of the samples. The distribution of pore size for ZnIn2S4 and MZ-6 is mainly below 5 nm. Combined with the previous SEM results, the well-defined 3D hierarchical architecture initiated by introduction of Ti3C2 is helpful for the formation of mesoporous and the increase in specific surface areas. Thus, MZ-6 is expected to be the most promising photocatalyst.
3.2 Photocatalytic measurement
To investigate the photocatalytic activities of Ti3C2, ZnIn2S4, and MZ composites on antibiotics under visible light irradiation, the photocatalytic degradation of TC and 7-ACA was assessed. In order to test the stability of all samples, blank tests were used to check for metal leakage from the samples and the content of related metals was detected by ICP. No Zn, Ti and other metals were detected, indicating good stability of all samples. The degradation efficiencies of all samples for TC are shown in Fig. 5a. The concentration of TC is not varied in the blank experiment, implying that TC possess high stability. In addition, Ti3C2 barely exhibits a degradation effect on TC. However, the photocatalytic degradation efficiency of pristine ZnIn2S4 is about 70.2%. Moreover, the photocatalytic efficiency of MZ composites is significantly improved after incorporation with Ti3C2. MZ-3 exhibits a degradation efficiency of approximately 86%. MZ-6 shows the optimum photocatalytic degradation, its degradation efficiency for TC can reach up to 91% after 2 h of visible-light irradiation. Unfortunately, the photocatalytic degradation efficiency of MZ-9 only has 82%. Clearly, the introduction of Ti3C2 can effectively improve the photocatalytic activity of ZnIn2S4, but there is an optimal ratio between ZnIn2S4 and Ti3C2. Besides TC, 7-ACA was also degraded under visible light. The result is shown in Fig. 5b, 7-ACA also possess high stability under visible light irradiation. As was the case with the TC, all MZ composites are more efficient than pristine ZnIn2S4. Similarly, after 90 min of visible light irradiation, MZ-6 shows the best degradation efficiency on 7-ACA, which can reach up to 95%.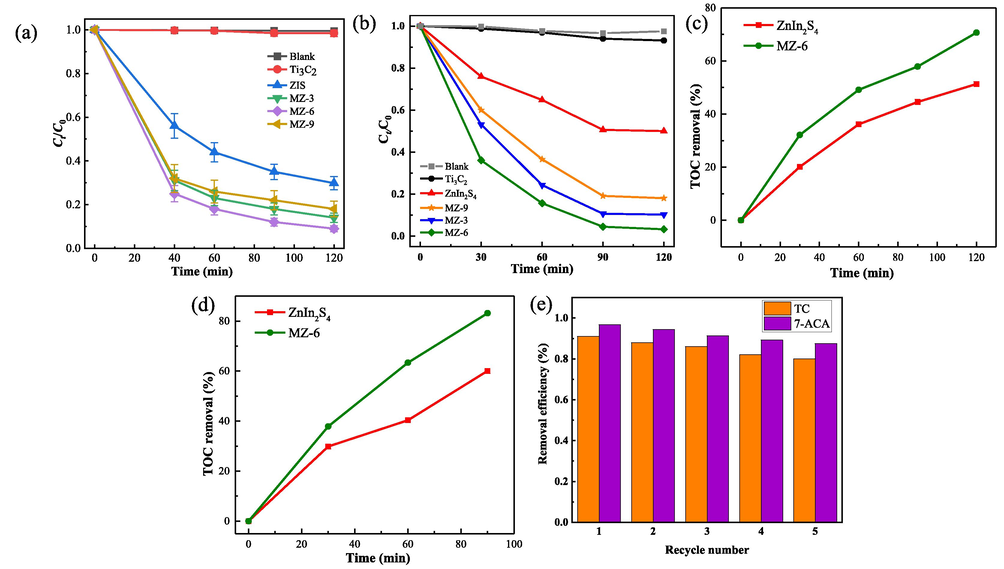
Photocatalytic degradation of TC (a) and 7-ACA (b) by samples under visible light irradiation. TOC removal curve of TC (c) and 7-ACA (d) in the presence of pristine ZnIn2S4 and MZ-6 under visible light irradiation. (e) Photocatalytic degradation of TC and 7-ACA in solution for 5 cycles using MZ-6 under visible light irradiation.
Furthermore, the experiments were quantitatively explained by the pseudo-first-order reaction kinetics equation. The results are shown in Fig. S4a and Fig. S4b, MZ-6 shows the highest photocatalytic activity for TC (k = 0.01947 min−1) and 7-ACA (k = 0.02999 min−1). The above results indicate that MZ-6 is an effective photocatalyst for the degradation of different antibiotics.
Besides photocatalytic activities, the adsorption activities of all samples were also investigated. As shown in Fig. S3, the adsorption–desorption equilibrium of all samples for TC and 7-ACA can be reached within 30 min. Meanwhile, among all the samples, MZ-6 exhibits the best adsorption capacity for TC and 7-ACA, which can achieve 29.4% and 14.5%, respectively. Ti3C2 has barely adsorption capacity, and ZnIn2S4 only reached 11.7% and 8.4%. Similarly, the trend of the adsorption activities of all samples are in consist with their BET specific surface areas, indicating that the BET specific surface areas and the well-defined pores distribution play fundamental roles in their adsorption performance. Consequently, the reason why MZ-6 has the superior degradation performance: MZ-6 possesses higher specific surface area, which not only provides more transmission paths and improves the absorption and utilization of visible light, but also enriched the surrounding concentration of antibiotics. Afterwards, the instruction of Ti3C2 has a positive promoting effect for the photocatalytic degradation of ZnIn2S4. Unfortunately, the further increase of Ti3C2 results in severe agglomeration in MZ-9, which significantly decrease its exposure of active sites and photodegradation performances.
To further analyze the advantages of photocatalytic performance of MZ-6, the photocatalytic degradation of MZ-6 for TC and 7-ACA was compared with other similar photocatalysts reported in the literatures. As shown in Table 1. MZ-6 still has good 91% and 95% degradation efficiency for 50 mg/L of TC and 25-mg/L of 7-ACA, and the degradation velocity reaches 113.8 mg/g·h and 59.4 mg/g·h, which is 1.5 and 14.8 times higher than other photocatalysts, respectively. It is obvious that MZ-6 exhibits a highest photocatalytic degradation performance. This result further indicated that MZ-6 could be considered as a promising photocatalyst for the degradation of antibiotics.
photocatalyst
Dosage (mg)
Antibiotics (mg/L)
Volume (mL)
Solid-to-liquid Ratio (g/L)
Photocatalytic Efficiency/Time
Degradation Velocity (mg/g·h)
Ref
TiO2/ZnIn2S4
10
TC (20)
50
0.20
95.1%/120 min
47.6
(Liu et al., 2021)
CeO2/ZnIn2S4
10
TC (30)
50
0.20
91%/120 min
68.3
(Hao et al., 2021)
ZnIn2S4/BiPO
15
TC (40)
50
0.30
84%/90 min
74.7
(Lu et al., 2019)
ZnIn2S4/Ta3N5
20
TC (15)
60
0.33
89.95%/180 min
13.5
(Xiao et al., 2020)
ZnIn2S4/(CQDs)
20
TC (10)
80
0.25
85.07%/240 min
8.5
(Xu et al., 2018)
MZ-6
20
TC (50)
100
0.10
91%/120 min
113.8
This work
Ag-CsPbBr3/CN
100
7-ACA (10)
100
1.00
92.79%/140 min
4.0
(Zhao et al., 2019)
MZ-6
20
7-ACA (25)
100
0.20
95%/120 min
59.4
This work
To understand whether the antibiotics were removed in the form of mineralization or conversion to other organic compounds during photocatalysis, the total organic carbon (TOC) of degradation products was detected. The results of the TOC removal rates of TC and 7-ACA by photocatalysts are shown in Fig. 5c and d. As it can be seen, the TOC removal rates were lower than the degradation efficiencies during photocatalysis. But the trend of the TOC removal rate is similar to that of the photocatalytic degradation curve. This is because there are still small quantities of intermediates in the solution that have not been wholly converted to inorganic substances (Lu et al., 2019). Nevertheless, compared with the pristine ZnIn2S4, the mineralization capacity of MZ-6 is demonstrated to be significantly improved.
It’s very important to study the reusability of the photocatalyst in practical application. To this end, the stability and reusability experiments were investigated. The photocatalytic activity of MZ-6 only declines about 14.4% (for TC) and 11.2% (for 7-ACA) after 5 cycles (Fig. 5e), indicating that MZ-6 possess an outstanding stability through the whole photocatalytic degradation process. The XRD patterns of MZ-6 before and after the photocatalytic reaction are shown in Fig. S5, no obvious change in crystal structure was observed. SEM and TEM images (Fig. S6) of MZ-6 after the photo degradation showed that the 3d marigold like structure did not change greatly, but there were some larger holes in some spheres, which might be caused by the loss of some crystals because of the repeated use, and this was the main reason for the slight decline in photocatalytic performance of MZ-6. The unchanged structure of the photocatalyst further proves that the photocatalyst has good chemical stability. In conclusion, MZ-6 exhibits well-maintained degradation efficiency, excellent stability and high practical application potential.
The effect of different pH values on the degradation were explored. The result was clearly seen from Fig. 6a, the adsorption efficiency of MZ-6 on TC reached 36% at pH = 3.5, and decreased with the increase of pH value, indicating that MZ-6 showed excellent adsorption properties under acidic conditions. The change of TC overall removal by MZ-6 was also same, which may be caused by the electrostatic interaction between TC and MZ-6. As we know, TC is an amphoteric compound with multiple ionizing functional groups, and behaves in different forms under different pH conditions: positive ions (TCH3+) (acidity), zwitterions (TCH20) (neutral), and negative ions (TCH−/TC2−) (alkalinity)(Xiang et al., 2020). Meanwhile, the surface of ZIS was positively charged when pH<2.4 and negatively charged when pH>2.4 (Luo et al., 2022). Therefore, a stronger electrostatic attraction between positively charged TC and negatively charged MZ-6 under acidic condition, which promoted the adsorption of TC. And the electrostatic repulsion was produced under alkaline condition. However, the better stability and the smaller particle size of the prepared samples also accelerated the separation of photogenerated electron-hole pairs, resulting in more active sites of contact between the catalyst and TC, thus the superior removal efficiency of TC in wide pH range could be achieved by MZ-6. In addition, the effect of pH on 7-ACA was clearly seen from Fig. 6b, the enhanced removal of 7-ACA was achieved both acidic and alkaline conditions. This phenomenon could be caused by the decomposition of 7-ACA (Sahoo et al. 1996).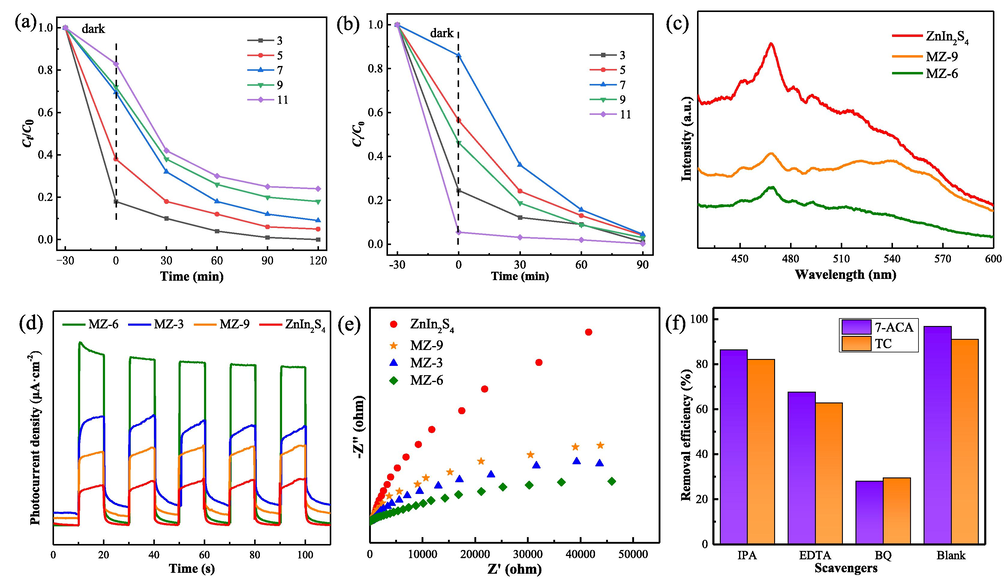
Photocatalytic degradation of TC (a) and 7-ACA (b) over different pH. (c) PL spectra of ZnIn2S4, MZ-6 and MZ-9. Transient photocurrent responses (d) and EIS (e) of ZnIn2S4 and MZ composites. (f) Trapping experiment of active species during the photodegradation of TC and 7-ACA.
3.3 Mechanism considerations
The recombination process of the photoexcited electron-hole pairs is usually illustrated by the photoluminescence (PL) spectroscopy (Chen et al., 2017). Photocatalysts produce electron-hole pairs under light irradiation. And then, the photoinduced carrier migrates to the surface of the photocatalyst for a redox reaction. The combination of electrons and holes produces a fluorescence signal that can be observed by photoluminescence spectra. In general, the higher photoluminescence intensity is, the more severe recombination of photo-generated electron-hole pairs is (Wang et al., 2016b). Herein, the fluorescence spectra of pristine ZnIn2S4, MZ-3, and MZ-6 series composites were determined by PL. As shown in Fig. 6c, owing to the band gap recombination of photo-generated electrons and holes, a broad peak or pristine ZnIn2S4 is observed near 468 nm. The fluorescence intensity of all MZ composites is lower than that of ZnIn2S4, suggesting that the hybridization of Ti3C2 nanosheets with ZnIn2S4 can significantly reduce the electron-hole pairs recombination, which further indicates that the photocatalytic activities of MZ composites are improved.
To further investigate the spatial separation and transfer of the charge carriers in photocatalysis, photoelectrochemical tests were carried out (Tan et al., 2019). The electronic conductivity and the charge carrier transfer capacity of photocatalysis can be evaluated by the transient photocurrent response curves and electrochemical impedance spectra (EIS) (Yang et al., 2018). The results of transient photocurrent response of ZnIn2S4 and MZ composites under intermittent solar simulator irradiation are shown in Fig. 6d. The positive photocurrent curve of ZnIn2S4 shows that it is an n-type semiconductor. The photocurrent density increases rapidly and reaches saturation when the light is bright, but as soon as the light is turned off, it immediately drops to nearly zero. Moreover, the photocurrent intensity of ZnIn2S4 increased after the modification of Ti3C2, but there is an optimized ratio between ZnIn2S4 and Ti3C2, and MZ-6 shows the highest photocurrent response. The rise of photocurrent confirmed that MZ-6 Schottky junction can promote the separation of photocarriers, prolong the life of photocarriers, improve the absorption of visible light, and then promote the enhancement of photocatalytic activity. However, excessive Ti3C2 in MZ-9 results in a decrease in photocurrent intensity, which may be caused by strong light absorption of Ti3C2 and agglomeration of components. In general, the smaller the semicircle diameter of the Nyquist diagram is, the smaller the charge transfer resistance of photocatalyst is. An arc radius per sample can be observed in Fig. 6e, suggesting that only surface charge-transfer mode occurred during photocatalysis. The smaller the semicircle diameter of the Nyquist diagram of MZ-6 confirms the contribution of a 2D/2D interface to conductivity. In comparison to ZnIn2S4, it is demonstrated that the electronic conductivity of MZ-6 is higher due to electron transfer between ZnIn2S4 and Ti3C2. The lowest charge transfer resistance and highest electronic conductivity facilitate the most efficient charge separation for MZ-6. This is consistent with its significantly enhanced photocatalytic activity.
Generally, hydroxyl radical (•OH), superoxide radical (•O2−), and hole (h+) are three typical active substances for pollutant degradation. To study the role of each active substance during photocatalysis of MZ composites, corresponding masking agent was introduced to investigate the change of degradation activity of MZ-6 for TC and 7-ACA under visible light. In these experiments, the masking agents used to eliminate •OH, •O2−, and h+ were isopropanol (IPA), benzoquinone (BQ), and ethylenediaminetetraacetic acid (EDTA), respectively. The result is shown in Fig. 6f, the degradation efficiency of MZ-6 for TC (7-ACA) decreased from 91% (96.75%) to 82.12% (86.34%), 29.52% (28.06%), and 62.82% (67.57%) when IPA, BQ and EDTA were added, respectively. It indicates that •O2− and h+ are the major active substances in the degradation of TC. Moreover, the pH values during the trapping agent experiment were detected. The result is shown in Fig. S7, the initial pH of the solution was different due to the masking agent. The pH of the solution containing IPA and EDTA decreased gradually with the increase of light time, but the solution containing BQ was the opposite. Combined their degradation efficiencies’ showed that this is mainly caused by the degradation and intermediates product (CO2).
To better understand the photocatalytic degradation of MZ composites for 7-ACA and TC, the intermediates were identified by LC-MS and HPLC-MS experiments. Similar to previous studies (Zhao et al., 2019), three typical species with the value of m/z at 231, 229, and 146 can be observed in Fig. S8. As illustrated in Fig. S9, we propose two different pathways to degrade 7-ACA according to these typical species. In the first reaction pathway, since β-lactam antibiotics are prone to ring-opening reactions (Lima et al., 2020), the structure A (7-ACA, m/z = 272.05) can be degraded to the structure B (m/z = 231.04) with the ring-opening reactions of β-lactam. As the degradation continues, structure C (m/z = 145.04) will be degraded from structure B. And then, structure C converts to CO2 and H2O. In another reaction pathway, the –COOH group falls off from structure A (7-ACA, m/z = 272.05) to generate the intermediate D (m/z = 229.06). And with prolonged illumination, all intermediates tend to convert to CO2 and H2O. As shown in Fig. S10 and Fig. S11, The m/z of TC is 445, two possible TC degradation pathways were analyzed. In pathway I, TC firstly produce structure B (m/z = 417) through N-dealkylation reaction. Then h+ attacked electron-rich C1 = C18, resulting in structure C (m/z = 393), structure B and structure C could further transform during photocatalytic oxidation to form structure D (m/z = 349). Subsequently, structure E (m/z = 279) was generated by decarboxylation and ring-opening reaction. In pathway II, TC was demethylated to form structure F (m/z = 431). Afterwards, structure F was further converted to structure G (m/z = 398) by losing amino groups and H2O. Then, structure H (m/z = 284) was generated after losing aldehyde groups and benzene ring-opening reaction. Ultimately, these intermediates could be further decomposed into smaller non-polar molecular materials, even CO2 and H2O (Li et al., 2022).
On this basis, the possible photodegradation mechanism of MZ composites was proposed. Firstly, ZnIn2S4/Ti3C2, which has a large specific surface area of 3D marigold-like structure, enrich antibiotics in its surrounding area via physical adsorption, so that the concentration of antibiotics around it is relatively high. Secondly, under the irradiation of visible light, the high specific surface area provides more transmission paths for visible light. As shown in Fig. 7, photogenerated electrons are excited from VB of ZnIn2S4 to CB. Since the CB potential of ZnIn2S4 is lower than the Fermi level of Ti3C2, photogenerated electrons will be transferred to Ti3C2 through the 2D/2D interface between ZnIn2S4 and Ti3C2. Meanwhile, the in-situ ZnIn2S4/Ti3C2 composite facilitates the close contact with each other, greatly improves the interface charge migration and photoinduced electron-hole pair separation, promotes the electron capture of Ti3C2, and significantly reduces the electron-hole pair recombination. In addition, the Fermi energy levels of ZnIn2S4 and Ti3C2 are lower than the potential of O2/−O2−, the electrons that accumulate on Ti3C2 can be rapidly transferred to the surface to participate in the formation of •O2−, giving it strong oxidation properties. Finally, TC and 7-ACA can be degraded by•O2− and h+. The reactions were described as follows:
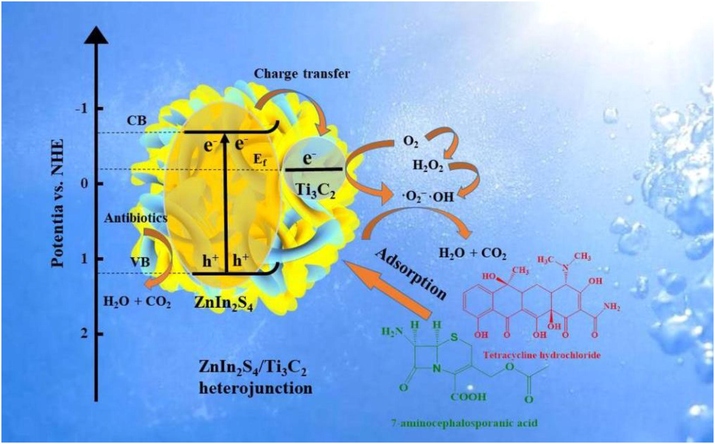
The mechanism of photodegradation and photoreduction of MZ-6.
4 Conclusion
In summary, well-defined 3D marigold-like MZ composites had been successfully prepared by a simple hydrothermal method. The introduction of Ti3C2 ameliorates the disadvantage of sulfides being easy to photocorroded by light to some extent. These composites exhibit outstanding photocatalytic activity, The optimized MZ-6 composite possess the best photocatalytic degradation and adsorption activity for antibiotics, and the removal efficiency of TC and 7-ACA exceeded 90% in a short time. Importantly, MZ still has excellent physical and chemical stability and high photocatalytic activity after multiple cycles. In addition, •O2− and h+ play a major role in the degradation of antibiotics. This study further confirms that Ti3C2 can be used as a co-catalyst to broaden its application in rapid and efficient photocatalysis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 51908240), the Natural Science Foundation of Jiangsu Province (No. BK20181064), the Natural Science Research of Jiangsu Higher Education Institutions of China (No. 18KJB610003), Qing Lan Project, the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX21_1499), the Foundation of Jiangsu Provincial Key Laboratory of Palygorskite Science and Applied Technology (No. HPK201905).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The chemistry of MIL-125 based materials: Structure, synthesis, modification strategies and photocatalytic applications. J. Environ. Chem. Eng.. 2022;10(1):106883
- [Google Scholar]
- Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ.. 2015;532:112-126.
- [Google Scholar]
- Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene) Chem. Mater.. 2017;29(18):7633-7644.
- [Google Scholar]
- Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B. 2018;239:545-554.
- [Google Scholar]
- 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater.. 2018;28(21):1800136.
- [Google Scholar]
- Enhancement of photocatalytic H2 evolution on ZnIn2S4 loaded with in-situ photo-deposited MoS2 under visible light irradiation. Appl. Catal. B. 2014;160–161:614-620.
- [Google Scholar]
- Photogenerated electron modulation to dominantly induce efficient 2,4-dichlorophenol degradation on BiOBr nanoplates with different phosphate modification. Appl. Catal. B. 2017;209:320-328.
- [Google Scholar]
- Boosting the photocatalytic activity of CdLa2S4 for hydrogen production using Ti3C2 MXene as a co-catalyst. Appl. Catal. B. 2020;267:118379.
- [Google Scholar]
- Low-cost adsorbents for urban stormwater pollution control. Front. Environ. Sci. Eng.. 2020;14(5):83.
- [Google Scholar]
- A Two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. Int. Ed.. 2017;56(7):1825-1829.
- [Google Scholar]
- Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci.. 2012;368(1):540-546.
- [Google Scholar]
- Modification of ZnIn2S4 by anthraquinone-2-sulfonate doped polypyrrole as acceptor-donor system for enhanced photocatalytic degradation of tetracycline. J. Photochem. Photobiol. A Chem.. 2017;348:150-160.
- [Google Scholar]
- Layered and poriferous (Al, C)-Ta2O5 mesocrystals supported CdS quantum dots for high-efficiency photodegradation of organic contaminants. Sep. Purif. Technol.. 2022;284:120297.
- [Google Scholar]
- Synergistic modulation on atomic-level 2D/2D Ti3C2/Svac-ZnIn2S4 heterojunction for photocatalytic H2 production. Colloids Surf. A: Physicochem. Eng. Aspects. 2022;648
- [Google Scholar]
- Facile solvothermal synthesis of a Z-Scheme 0D/3D CeO2/ZnIn2S4 heterojunction with enhanced photocatalytic performance under visible light irradiation. Chem. Eng. J.. 2021;409:128168.
- [Google Scholar]
- Correction: Recent progress in layered transition metal carbides and/or nitrides (MXenes) and their composites: synthesis and applications. J. Mater. Chem. A. 2017;5(18):8769.
- [Google Scholar]
- Oxygen vacancy induced bismuth oxyiodide with remarkably increased visible-light absorption and superior photocatalytic performance. ACS Appl. Mater Interfaces. 2014;6(24):22920-22927.
- [Google Scholar]
- Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B. 2016;181:779-787.
- [Google Scholar]
- Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B. 2016;185:68-76.
- [Google Scholar]
- Heterojunction architecture of N-doped WO3 nanobundles with Ce2S3 nanodots hybridized on a carbon textile enables a highly efficient flexible photocatalyst. Adv. Funct. Mater.. 2019;29(45):1903490.
- [Google Scholar]
- Enhanced hydrogen production from water over Ni doped ZnIn2S4 microsphere photocatalysts. Catal. Lett.. 2010;140(3):167-171.
- [Google Scholar]
- Design of mesoporous ZnO @ silica fume-derived SiO2 nanocomposite as photocatalyst for efficient crystal violet removal: Effective route to recycle industrial waste. J. Clean. Prod.. 2021;326:129416.
- [Google Scholar]
- Nanostructured binary and ternary metal sulfides: synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A. 2017;5(42):22040-22094.
- [Google Scholar]
- Optoelectronic properties of CuInS2 nanocrystals and their origin. J. Phys. Chem. Lett.. 2016;7(3):572-583.
- [Google Scholar]
- In situ construction of a C3N5 nanosheet/Bi2WO6 nanodot S-scheme heterojunction with enhanced structural defects for the efficient photocatalytic removal of tetracycline and Cr(vi) Inorg. Chem. Front.. 2022;9(11):2479-2497.
- [Google Scholar]
- Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants. Environ. Res.. 2020;185:109409.
- [Google Scholar]
- Fabrication of graphene wrapped ZnIn2S4 microspheres heterojunction with enhanced interfacial contact and its improved photocatalytic performance. Dalton Trans.. 2014;43(7):2888-2894.
- [Google Scholar]
- A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci. Total Environ.. 2021;774:145762.
- [Google Scholar]
- β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem.. 2020;208
- [Google Scholar]
- Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Nat. Acad. Sci.. 2014;111(47):16676-16681.
- [Google Scholar]
- MXene as a non-metal charge mediator in 2D layered CdS@Ti3C2@TiO2 composites with superior Z-scheme visible light-driven photocatalytic activity. Environ. Sci. Nano. 2019;6(10):3158-3169.
- [Google Scholar]
- The enhanced performance of Cr(VI) photoreduction and antibiotic removal on 2D/3D TiO2/ZnIn2S4 nanostructures. Ceram. Int.. 2021;47(12):17015-17022.
- [Google Scholar]
- Ti3C2/BiVO4 Schottky junction as a signal indicator for ultrasensitive photoelectrochemical detection of VEGF165. Chem. Commun.. 2019;55(91):13729-13732.
- [Google Scholar]
- Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloy. Compd.. 2019;811:151976.
- [Google Scholar]
- Lukatskaya Maria, R., Mashtalir, O., Ren Chang, E., Dall’Agnese, Y., Rozier, P., Taberna Pierre, L., Naguib, M., Simon, P., Barsoum Michel, W., Gogotsi, Y., 2013. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341(6153), 1502–1505.
- Construction of MgIn2S4/ZnIn2S4 micro-flowers: Efficient degradation of tetracycline hydrochloride over a wide pH range. Appl. Surf. Sci.. 2022;581:152417
- [Google Scholar]
- Total syntheses and biological reassessment of lactimidomycin, isomigrastatin and congener glutarimide antibiotics. Chem. – A Eur. J.. 2013;19(23):7370-7383.
- [Google Scholar]
- Formation of noble-metal-free 2D/2D ZnmIn2Sm+3 (m = 1, 2, 3)/MXene Schottky heterojunction as an efficient photocatalyst for hydrogen evolution. Chem. Eng. J.. 2021;424:130170.
- [Google Scholar]
- Direct Z-scheme ZnIn2S4@MoO3 heterojunction for efficient photodegradation of tetracycline hydrochloride under visible light irradiation. Chem. Eng. J.. 2021;424:130510.
- [Google Scholar]
- Peng, Y., Geng, M., Yu, J., Zhang, Y., Tian, F., Guo, Y.n., Zhang, D., Yang, X., Li, Z., Li, Z. and Zhang, S., 2021. Vacancy-induced 2H@1T MoS2 phase-incorporation on ZnIn2S4 for boosting photocatalytic hydrogen evolution. Appl. Catal. B: Environ. 298, 120570.
- Peng, Y., Guo, X., Xu, S., Guo, Y.n., Zhang, D., Wang, M., Wei, G., Yang, X., Li, Z., Zhang, Y., Tian, F., 2022. Surface modulation of MoS2/O-ZnIn2S4 to boost photocatalytic H2 evolution. J. Energy Chem. 75, 276–284.
- Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc.. 2014;136(11):4113-4116.
- [Google Scholar]
- MXenes nanocomposites for energy storage and conversion. Rare Met.. 2022;41(4):1101-1128.
- [Google Scholar]
- Enhanced photocatalytic degradation of antibiotics in water over functionalized N, S-doped carbon quantum dots embedded ZnO nanoflowers under sunlight irradiation. Chem. Eng. J.. 2020;382:123016.
- [Google Scholar]
- Facile synthesis of sphere-like structured ZnIn2S4-rGO-CuInS2 ternary heterojunction catalyst for efficient visible-active photocatalytic hydrogen evolution. J. Colloid Interface Sci.. 2021;602:669-679.
- [Google Scholar]
- Facilitated transport of 7-aminocephalosporanic acid in a bulk liquid membrane. J. Membr. Sci.. 1996;112(2):147-154.
- [Google Scholar]
- Surface functionalization of single-layered Ti3C2Tx mXene and its application in multilevel resistive memory. ACS Appl. Mater Interfaces. 2020;12(8):9865-9871.
- [Google Scholar]
- Heterogeneous photocatalysts: an overview of classic and modern approaches for optical, electronic, and charge dynamics evaluation. Chem. Soc. Rev.. 2019;48(5):1255-1271.
- [Google Scholar]
- Efficient visible-light photocatalysis of 2D-MXene nanohybrids with Gd3+- and Sn4+-codoped bismuth ferrite. ACS Omega. 2018;3(10):13828-13836.
- [Google Scholar]
- Titania composites with 2 D transition metal carbides as photocatalysts for hydrogen production under visible-light irradiation. ChemSusChem. 2016;9(12):1490-1497.
- [Google Scholar]
- Multi-ion intercalated Ti3C2Tx MXene and the mutual modulation within interlayer. Particuology. 2023;72:10-16.
- [Google Scholar]
- Clay-inspired MXene-based electrochemical devices and photo-electrocatalyst: State-of-the-art progresses and challenges. Adv. Mater.. 2018;30(12):1704561.
- [Google Scholar]
- In-situ construction of 3D marigold-like CoAl-LDH/Ti3C2 heterosystem collaborating with 2D/2D interface for efficient photodegradation of multiple antibiotics. Appl. Surf. Sci.. 2021;569:151084.
- [Google Scholar]
- Facile synthesis of Sb2S3/ultrathin g-C3N4 sheets heterostructures embedded with g-C3N4 quantum dots with enhanced NIR-light photocatalytic performance. Appl. Catal. B. 2016;193:36-46.
- [Google Scholar]
- Construction of hierarchical 2D–2D Zn3In2S6/fluorinated polymeric carbon nitride nanosheets photocatalyst for boosting photocatalytic degradation and hydrogen production performance. Appl. Catal. B. 2018;233:58-69.
- [Google Scholar]
- Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere. 2020;255:127057.
- [Google Scholar]
- In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B. 2018;220:417-428.
- [Google Scholar]
- Eco-friendly synthesis of core/shell ZnIn2S4/Ta3N5 heterojunction for strengthened dual-functional photocatalytic performance. Int. J. Hydrogen Energy. 2020;45(55):30341-30356.
- [Google Scholar]
- Fabricating carbon quantum dots doped ZnIn2S4 nanoflower composites with broad spectrum and enhanced photocatalytic Tetracycline hydrochloride degradation. Mater. Res. Bull.. 2018;97:158-168.
- [Google Scholar]
- Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B. 2017;202:518-527.
- [Google Scholar]
- Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight. Chem. Eng. J.. 2018;349:808-821.
- [Google Scholar]
- Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal. B. 2019;258:117956
- [Google Scholar]
- Urchin-like hierarchical CoZnAl-LDH/RGO/g-C3N4 hybrid as a Z-scheme photocatalyst for efficient and selective CO2 reduction. Appl. Catal. B. 2019;255:117771
- [Google Scholar]
- Nickel-loaded black TiO2 with inverse opal structure for photocatalytic reduction of CO2 under visible light. Sep. Purif. Technol.. 2019;220:8-15.
- [Google Scholar]
- Novel ternary p-ZnIn2S4/rGO/n-g-C3N4 Z-scheme nanocatalyst with enhanced antibiotic degradation in a dark self-biased fuel cell. Ceram. Int.. 2020;46(7):9567-9574.
- [Google Scholar]
- MoS2-graphene/ZnIn2S4 hierarchical microarchitectures with an electron transport bridge between light-harvesting semiconductor and cocatalyst: A highly efficient photocatalyst for solar hydrogen generation. Appl. Catal. B. 2016;188:13-22.
- [Google Scholar]
- Hierarchical Ag3PO4@ZnIn2S4 nanoscoparium: An innovative Z-scheme photocatalyst for highly efficient and predictable tetracycline degradation. J. Colloid Interface Sci.. 2021;586:708-718.
- [Google Scholar]
- Enhanced photocatalytic activity of Ag-CsPbBr 3/CN composite for broad spectrum photocatalytic degradation of cephalosporin antibiotics 7-ACA. Appl. Catal. B. 2019;247:57-69.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104883.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







