Translate this page into:
Copolymerization of ethylene and isoprene initiated by metallocene catalyst
⁎Corresponding authors. jamie@ujs.edu.cn (Jamile Mohammadi Moradian), liguo@ujs.edu.cn (Li Guo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, we explore the dynamic nature of metallocene catalysts during ethylene/isoprene E/IP) copolymerization, with a focus on the influence of various reaction parameters. Challenges, why do olefinic catalysts show lower activity and molecular weight (Mw) at higher temperatures and low activity with higher diene content? Firstly, we investigate the observed phenomena of decreased catalyst activity and Mw at elevated temperatures using 1.25 μmole of the metallocene. Notably, a substantial increase in active sites from 30 °C to 40 °C, reaching a peak of 89 %. Surprisingly, further temperature increments lead to a noticeable decline in active sites. At 30 °C, the active site primarily engages in the insertion of IP through 3,4 connections, with no detectable cis-1,4 or trans-1,4 connections, which suggests a lack of stereoselectivity at this temperature. At 40–50°C, cis/trans-1,4 connections and active sites are approaching a higher level. This implies a reduction in chain transfer reactions, making catalytic active sites more favorable to cis/trans-1,4 connections. Secondly, we observe a remarkable impact of IP concentration in the E/IP copolymers, active centers, and activity show stability when the amount of IP was 0.116–0.48 mol/L and then started to decline when the amount of IP 0.96 mol/L, indicating a threshold beyond which deactivation occurs. Increasing IP concentration not only reduces activity and active sites but also fails to reactivate dormant polyethylene sites. The Mw and active centers of copolymers progressively decrease with elevated IP concentration, suggesting a faster chain transfer reaction with IP compared to E.
Keywords
Metallocene
Olefin
Catalyst
Cocatalyst
Isoprene
Ethylene
1 Introduction
Starting in the early 1950s, Giulio Natta and Karl Ziegler made significant progress in the field of polyolefin production (Claverie and Schaper, 2013; Khan et al., 2018; Mülhaupt, 2003). However, compared to homogeneous single-site catalysts (Hlatky, 2000; Delferro and Marks, 2011; Jiang et al., 2020), the catalytically active transition metal of the Z-N catalyst is consistently found at the end of the polyolefin chain (Chung and Rhubright, 1993; Kaminsky, 2004). Similarly, olefinic palladium, and diamine nickel catalysts, “Brookhart catalysts”, the catalytically active transition metal, move along the polymer chain during the polymerization process (Kaminsky, 2004; Bolig and Brookhart, 2007). In these circumstances, the content of toxic transition metals falls in the residues in the ppm range, and significantly reducing the content of transition metals in the polyolefin materials required additional purification processes. On the other hand, the realm of homogeneous single-site catalysts has undergone remarkable progress in the last twenty years (Zaera, 2022; Ali et al., 2020; Ali et al., 2020). Contemporary group IV, homogeneous catalysts for olefin polymerization, are extremely efficient and yield precisely tailored polyolefin with targeted molecular weights (Mw), tacticities, and comonomer incorporation levels. Innovations like chain-shuttling polymerization and multisite functionalities have further broadened the versatility of metallocene catalysts (Stürzel et al., 2016; Zanchin and Leone, 2021; Du et al., 2015; Hu et al., 2017). The journey towards an enhanced understanding of polymerization mechanisms since the 1960s has led to the development of highly active and stereospecific catalysts, revolutionizing and simplifying polyolefin production (Hu et al., 2017; Lamberti et al., 2009; Brintzinger et al., 1995; Ewen, 1984). Among these olefinic catalysts families, the most influential pioneer in the field has been the discovery of metallocene catalysts technology for P and E and for their copolymers, especially when modified with their bridged and ligand that boast activities and exceeding 106 g polyolefin/g (Ali et al., 2021; Ali et al., 2021; Ali et al., 2021). The precise control of polymer properties, encompassing molar mass, dispersity index (Đ), and microstructure, is achievable through modification of bridged and ligand of metallocene catalysts compared to Z-N (Akram et al., 2021). However, modification of ligand and bridged moieties in metallocene catalysts brings some complicated changes in molecular weight distribution, microstructure, molecular weight, and activities as well. Some metallocene catalysts were kinetically addressed for these issues, but only five points were explored such as Eric S. Cueny et al. explored the living coordinative polymerization of olefins, whereas propagation (chain growth) proceeds in the absence of irreversible chain termination and demonstrated that it was a new opportunity for researchers to design and explore new fundamental types of polyolefins (Cueny et al., 2020; Cueny, 2022). Clark R. Landis et al., investigated metallocene with the combination of borate catalyzed α-olefin polymerization involves four crucial steps, initiation into a metal–alkyl bond, propagation through subsequent insertions, and chain transfer following either a 1,2- or 2,1-α-olefin insertion (Moscato et al., 2012). However, Additional steps, such as catalyst dormancy, death, and other rare events, may also occur during the polymerization under different temperatures with changes in monomers or their concentration. Motivated by these interesting explorations, we are seeking to determine the chain transfer reaction of E and IP in the E/ IP copolymerizations, whether it takes place by β-Hydrogen transfer or inserting by 3,4 OR 1,4 insertions. Currently facing a challenge regarding why catalysts show lower activity at low temperatures and low activity with higher isoprene content. This study also illustrates the intricate nature of the mechanisms involved, such as the presence of catalyst dormancy under certain conditions and distinct variations in reactivity between catalysts that have not yet been activated, species that are actively propagating, and the products containing β-hydride groups resulting from chain transfer. However, Additional steps, such as catalyst dormancy, death, and other rare events, may also occur during the polymerization under different temperatures and monomer concentrations. In the literature, some reports assumed that 100 % of the metallocene catalyst added is significantly active throughout the polymerization process and recommended that the catalyst exhibit 100 % active sites. However, several research groups' comprehensive kinetic investigations have found that this does not happen in all cases (Liu et al., 2001; Han et al., 1996; Ali et al., 2022). The active-site count of the same metallocene catalyst might fluctuate due to factors such as monomers, activators, temperature, and other additions. The modern research concerning the kinetics and mechanisms of chemical transformations benefits from experimental methods that provide detailed information under actual reaction conditions. Amongst the most critical transformations in chemistry are those that use comonomers with different concentrations and polymerization ratios under different temperatures.
2 Materials and method
Polymerization solvent toluene with commercial grade was bought from the Albemarle Co. and further purified through refluxed over sodium metal and distilled under nitrogen, To obtain accurate results for active sites of metallocene catalyst, the E was bought from Zhejiang Gas Mixing Co with commercial grade. Before polymerizations, E was subjected to additional purification using specialized columns containing a deoxygenating agent and 4 Å molecular sieves. The IP was purchased from Aldrich, distilled over sodium, and kept under a nitrogen atmosphere and low temperature. TPCC and TIBA were purchased from Albemarle Co., prepared in heptane, and stored in the same environment as isoprene. Cocatalyst borate [(Ph3C) B(C6F5)4] was prepared according to literature, and metallocene rac-Me2Si(2-Me-4-Ph-Ind)2ZrCl2 was kindly donated by Shanghai Research Institute, Shanghai, China. All moisture- and oxygen-sensitive operations were conducted significantly, employing Schlenk techniques and glove box.
2.1 Polymerization
The polymerization reactions were conducted in a 100 mL Schlenk flask under an environment devoid of reactive gases. Furthermore, the reactor underwent a series of nitrogen purges and vacuum evacuations. The specified quantity of solvent, E (1 MPa), isoprene, and TIBA, were constantly injected and operated for a duration of 10 min. The copolymerization reaction was initiated by the addition of metallocene and borate, which had a considerable impact. Following the specified duration, the copolymerization reaction was terminated using TPCC and allowed to proceed for 5 min. Ultimately, the copolymers were precipitated in acidic ethanol and then subjected to filtration and numerous washes until all unreacted TPCC was eliminated. For accurate results regarding active sites, a limited quantity of copolymers was subjected to additional purification based on our previous research (Ali et al., 2022; Ali et al., 2021). Subsequently, the copolymers were thoroughly dried in a vacuum oven before conducting the sulfur content test.
2.2 Characterization
The copolymers were characterized using high-temperature GPC and NMR spectroscopy. The molecular structure and composition of the copolymers were analyzed using Varian Mercury plus 300 spectrometers (Varian Corporation) operating at a frequency of 75 MHz and a temperature of 120 °C. The copolymer samples were dissolved in o-dichlorobenzene-d4 at a concentration of 10 wt%. Hexamethyldisiloxane was employed as an internal chemical shift reference. A total of 200 scans were collected to provide the appropriate spectra. In order to determine the MW and MWD of the copolymer, a higher-temperature gel permeation chromatography (GPC) technique was employed. This involved using PL-gel MIXED-B columns as the stationary phase and 1,2,4-trichlorobenzene as the eluent, with a flow rate of 1.0 mL/min. Polystyrene with a narrow MWD was utilized as a universal calibration standard prior to conducting the test. The copolymer samples were analyzed using YHTS-2000 ultraviolet fluorescence to determine the S content. Similarly, samples of blank and TPPC-quenched copolymers were examined, (See Scheme 1), in comparison to the TPPC quenched copolymer, the blank sample exhibited a sulfur concentration of 0 ppm.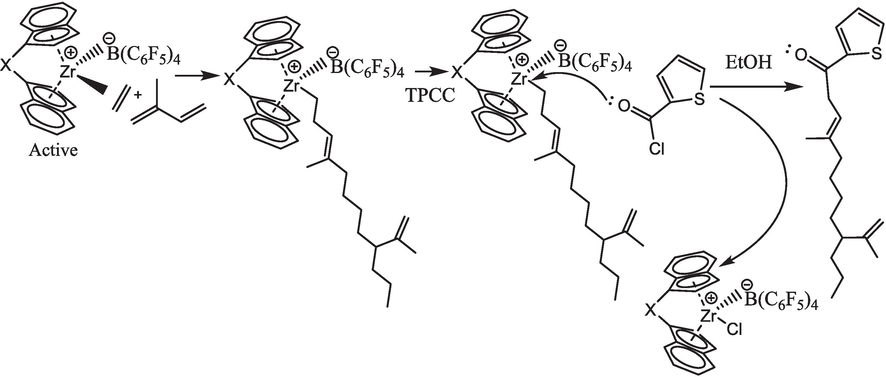
Schematic representation of metallocene-catalyzed E/IP copolymerization quenching through TPCC.
3 Results and discussion
Nina V. Semikolenova et al. investigated the effect of polymerization temperature on the efficiency of non-symmetrical metallocene catalysts (Semikolenova et al., 2017). Their experimental results showed that the reaction rate decreased slowly at 40 °C, increasing by about twofold within 20 min. As the polymerization temperature increased from 40 to 60 °C, the initial catalytic activity increased but then dropped sharply. In our study, by using symmetrical metallocene catalysts in the copolymerization of E/IP, we noticed that from 30 °C to 50 °C, activity significantly increased but further increased in the temperature noticeably decreased. Its mean, compared to non-symmetrical metallocene catalysts, symmetrical metallocene catalyst active sites are activated at low temperatures. Similarly, reaction temperature significantly influenced the MW and MWD of the resulting E/IP copolymers, for example, a higher Mw was obtained at 30 °C, but further increased in temperature, showing a decreasing trend in Mw. In contrast, at 60 °C, the E/ IP copolymer exhibited a broad but bimodal MWD, meaning that some chain transfer reaction occurs in the copolymerization process at higher temperatures. Compared to PE, E/ IP copolymers show a narrow MWD, meaning that IP as a comonomer overcomes those chain transfer reactions that were occurring with PE (see Supporting information Fig. S3) and works as a reactivated species. In the case of non-symmetrical metallocene catalysts at higher temperatures, PE showed a bimodal MWD type (Ali et al., 2020). According to Semikolenova et al, the theoretical splitting of bimodal MWD of PE which exhibited a low-molecular-weight fraction of 24 % and higher 76 %, mean chain transfer reactions are increased with increasing temperature, and recommended the two types of hypotheses (1) two different chain-terminations and (2) the existence of two types of active centers (Stürzel et al., 2016; Semikolenova et al., 2017). In the case of metallocene, In Table 1, the MWD is bordered at 40 °C and 60 °C, meaning that metallocene shows two types of active centers and chain terminations at these temperatures that will be discussed in the second part of this work.
Run
tp
yield
(g)IP c
mol%
cis-1,4- mol%
trans-1,4- mol%
3,4- mol%
cis/trans-1,4- mol%
Mw b
(kg/mol)
Ɖ b
[C*]/[Zr]
(%)
kp
(L/mol·s)
kp IP
(L/mol·s)
1.1
30
1.0
1.12
nd1
nd1
1.03
0.11
863
2.2
69.4
388
0.80
1.2
40
1.29
1.26
0.02
0.05
1.19
0.07
821
3.5
89.6
387
0.91
1.3
50
1.04
0.97
0.03
0.02
0.92
0.05
334
2.3
72.3
389
0.69
1.4
60
0.79
1.18
nd1
nd1
0.50
0.69
255
4.0
60.2
354
0.77
By the quantitative analysis of ultraviolet fluorescence, the content of active sites was calculated using Eq. (1): kp = kp [C*]/[M]. M represents the equilibrium concentration of monomers. The starting concentration of [E] is 0.085 mol/L at a pressure of 0.1 MPa and a temperature of 50 °C in toluene (Ali et al., 2020). The average Rp value was calculated based on the catalyst quantity and polymer yield after 20 min. The kp was determined using the mean value obtained after 20 min. As shown in Table 1, the active site was significantly increased when temperature increased from 30 °C to 40 °C and reached the maximum level of 89 %.
However, further increases in the temperature noticeably decreased the active sites (see Fig. 1). This means that the metallocene catalyst did not require a higher temperature for activation. The catalyst's optimal performance at a relatively lower temperature enhances its efficiency and offers a sustainable and resource-efficient approach to catalysis. This exploration urges us to reconsider the temperature dynamics traditionally associated with metallocene activation (See Fig. 2 and Fig. 3).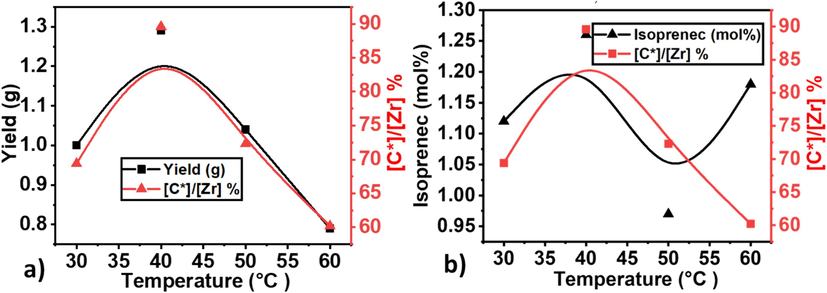
Metallocene catalyzed E/IP copolymerization under different temperatures a) comparison of yield and active center and b) comparison of active center % and isoprene mole%.
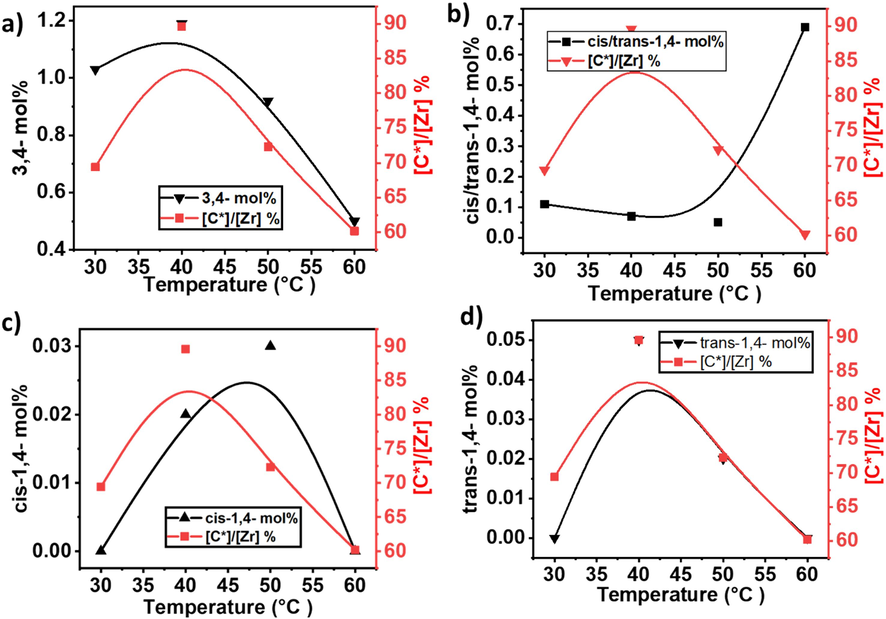
Comparison of active sites and isoprene insertion in the ethylene/isoprene copolymer growing polymer chain. a) comparison of active centers % and 3,4 insertion of isoprene b) active centers % and cis/trans-1,4 insertion of isoprene c) cis-1,4 insertion and active centers and d) comparison of trans-1,4 insertion of isoprene and active centers%.
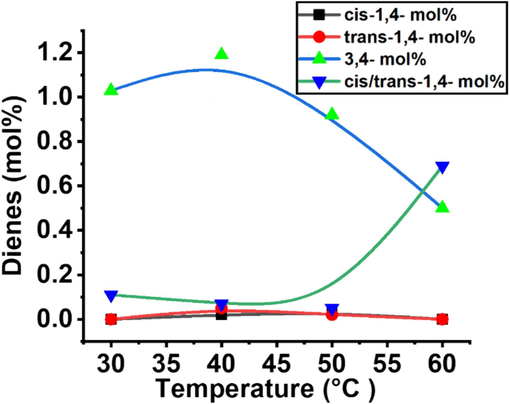
The insertion of isoprene through 3,4 and cis/trans-1,4 connections and their mol% comparison in the E/IP copolymers.
The propagation rate constant (kp) is also a critical parameter in understanding the kinetics of copolymerization reactions. The investigation into the kp at different temperatures (30 °C to 60 °C) has revealed intriguing insights into the thermal dynamics of the copolymerization process. As shown in Table 1 and Fig. 4, a relatively stable behavior in the kp of PE within the temperature range of 30 °C to 50 °C. The values drift between 387–389 L/mol·s during this calculation, suggesting a robust and consistent kp for PE. This stability implies that the copolymerization reaction maintains a steady pace, and the temperature increase within this range doesn't significantly impact the propagation kinetics of PE. At temperatures between 30 °C and 50 °C, catalysts may exhibit an optimal balance between activity and stability. The controlled environment within this temperature range likely provides favorable conditions for maintaining a stable kp. According to the literature, metallocene catalysts are sensitive to temperature variations, and the balance between the activation energy and deactivation processes often influences the catalytic activity (Dornik et al., 2004; Xu et al., 2001; Chakravarti and Ray, 2001; Wester et al., 1998). In the specified temperature range, the metallocene catalyst may strike a balance, allowing for efficient activation of monomers and stable propagation without significant side reactions or catalyst deactivation. However, a notable deviation is observed when the temperature is 60 °C. On this occasion, a sharp decrease in the kp of PE is witnessed (see Fig. 4a). This sudden decline indicates a temperature sensitivity threshold beyond which the efficiency of the copolymerization reaction reduces. At 60 °C, the lower kp could be attributed to the fundamental kinetics of the copolymerization reaction. For example, at this temperature, E and IP incorporation into the copolymer may be well-matched, leading to a drop in the consistent propagation rate value. The intricate balance between the chemical kinetics and thermal dynamics at this higher temperature merits further investigation to comprehend the underlying mechanisms responsible for this observed decrease.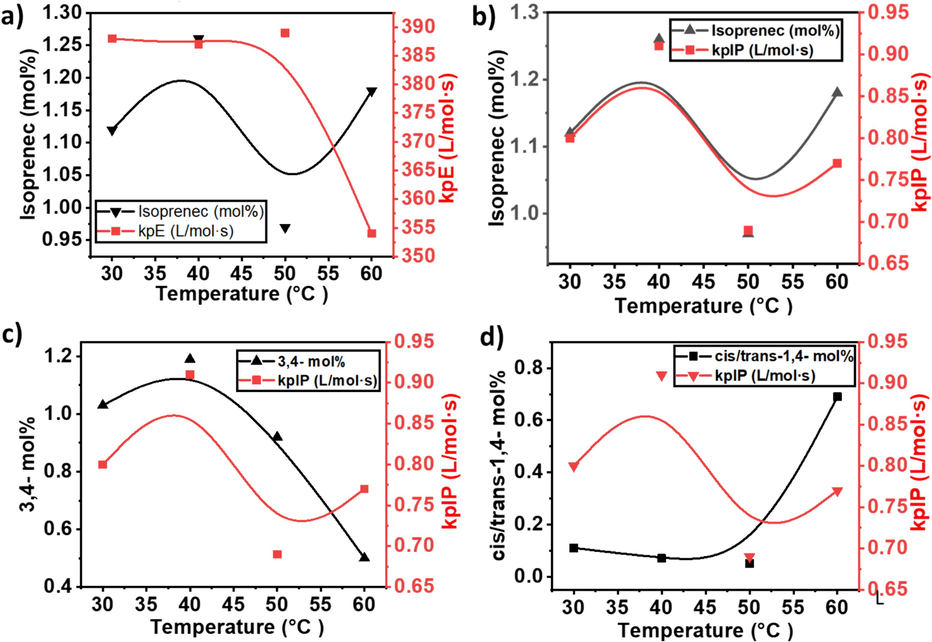
Metallocene catalyzed ethylene/isoprene copolymerization propagation rate constant at different temperatures and their comparison with isoprene mol% a) comparison of isoprene mol% and ethylene kpE b) isoprene mol% and isoprene kpIP c) 3,4 isoprene mol% and isoprene kpIP and d) cis/trans-1,4 isoprene mol% and isoprene kpIP.
At temperatures ranging from 30 to 40 °C, the catalyst exhibits higher activity, resulting in a greater incorporation of isoprene see Fig. 4d. Nevertheless, as the temperature rises from 40 °C to 50 °C, the elevated temperature might accelerate chain transfer events or deactivation activities, resulting in a transient decrease in isoprene concentration. At temperatures over 50 °C, the system will probably regain stability, facilitating the more effective integration of isoprene into the copolymer.
Under temperatures ranging from 30 to 40 °C, the kpE value is significantly elevated, which promotes the effective integration of isoprene into the polymer chain. Consequently, the amount of isoprene rises due to the improved growth of ethylene and isoprene. At a temperature of 50 °C, the kpE decreases, most likely because of increased chain transfer or deactivation processes. This results in a decrease in the amount of isoprene incorporated. Interestingly, as the temperature approaches 60 °C, the system appears to reach a stable state, and the kpE may once again rise as the catalyst adjusts to the elevated temperature, facilitating improved coordination and integration of monomers. This leads to the isoprene concentration reverting to values comparable to those found at 30 °C and 40 °C.
In contrast, the propagation rate constant (kpIP) of isoprene, consistently lower than that of PE, exhibits a distinctive response to temperature variations (see Fig. 5). Notably, IP shows a maximum kp at 40 °C, highlighting an optimal temperature for its copolymerization. However, as the temperature rises further to 60 °C, a sharp decline in the kp is observed, dropping to a lower level of 0.77 L/mol·s. This behavior reflects the sensitivity of IP copolymerization to higher temperatures, emphasizing the need for a nuanced understanding of the interplay between temperature and the kinetics of copolymer formation. The contrasting responses of E and IP emphasize the complexity inherent in E/IP copolymerization reactions and pave the way for further exploration into optimizing reaction conditions for enhanced polymerization efficiency. This study deepens our understanding of copolymerization kinetics and offers valuable insights for tailoring copolymer properties based on temperature modulation.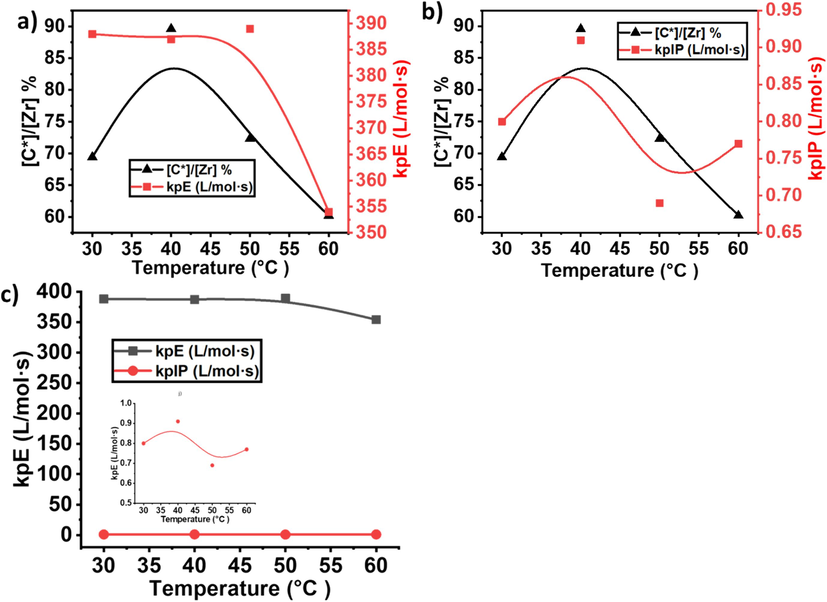
Comparison of active sites and propagation rate constant at different temperatures in the ethylene/isoprene copolymer growing polymer chain. a) A comparison of active centers % and kpE, b) active centers % and kpIP, and c) a comparison of kpE and kpIP.
Chain termination via β-H elimination in metallocene at higher temperatures is also a possible factor for lower kpE and kpIP. At 60 °C, the chain termination process through β-H elimination becomes more pronounced, influencing the overall polymerization kinetics. Chain termination via β-H elimination involves the removal of a hydrogen atom from the polymer chain, resulting in the formation of a double bond. This process can be facilitated by the presence of β-hydrogen atoms on the growing polymer chain. The elevated temperature enhances the probability of β-H elimination events, leading to premature chain termination. In addition, as the temperature increases, the kinetic energy of the polymerization system rises, affecting the reactivity of the metallocene catalyst. At 60 °C, the system is more energetic, leading to increased molecular movements and collisions.
3.1 Effect of isoprene concentration on the active sites and propagation rate constant
To elucidate the metallocene kinetically and mechanically in the E/IP copolymers, we conducted the E/IP copolymer with different isoprene concentrations under the same reaction condition. In Table 2, with 0.06 mol/L of isoprene, the activity of the copolymer is very high, nearly double that which was obtained with 0.12 mol/L. In addition, when isoprene concentration increased to 4 times in the feed, a small decline in activity was observed (see Fig. 6a). However, at 1.44 mol/l of isoprene, copolymer activity reached a lower level and showed a sharp decrease in the yield. On the other side, the active center fraction of the system displays the same trend-like activity of the catalysts.
Run
Conc
IP
Yield (g)
IPc
mol%
cis-1,4- mol%
trans-1,4- mol%
3,4- mol%
cis/trans-1,4- mol%
Mw b
(kg/mol)
Ɖ b
[C*]/[Zr]
(%)
kp
(L/mol·s)
kpIP
(L/mol·s)
2.1
0.06
2.25
0.41
nd1
nd1
0.35
0.06
973
2.7
98.9
624
4.13
2.2
0.12
1.62
0.63
nd1
nd1
0.54
0.09
953
4.0
92.9
477
2.31
2.3
0.48
1.04
0.97
0.03
0.02
0.92
0.05
334
2.3
72.3
377
1.66
2.4
0.96
0.45
3.62
0.37
0.09
3.16
0.46
268
3.6
32.3
360
1.23
2.5
1.44
0.33
3.68
0.39
0.11
3.18
0.50
201
3.7
21.6
404
0.60
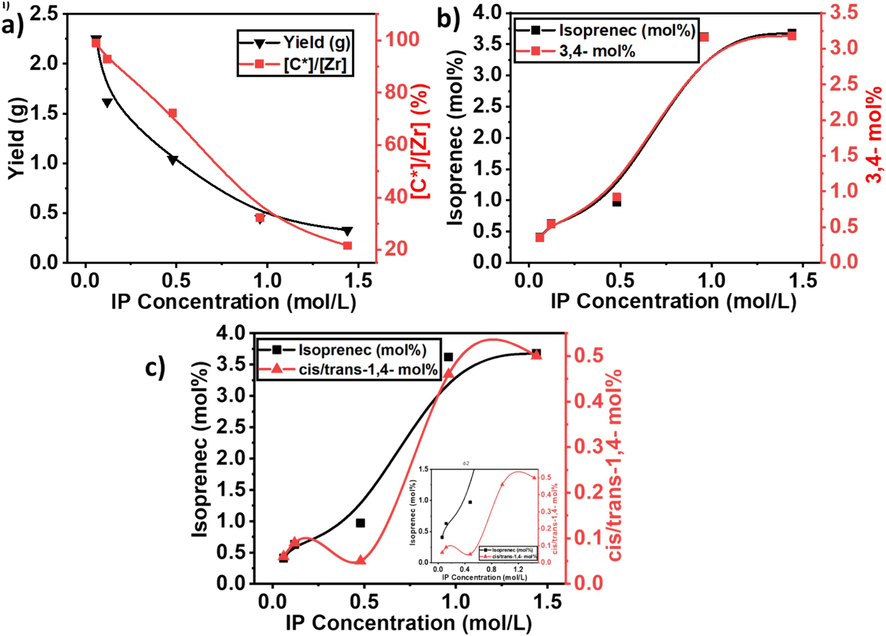
a) The comparison of yield and active center, b) comparison of total inserted isoprene and 3,4%, and c) comparison of total inserted isoprene and cis/trans-1,4 connections in the E/IP copolymers under different isoprene concentrations.
The isoprene as comonomers can suppress the active sites of the catalyst during the polymerization process. According to the literature reports, by increasing the amount of IP in the feed, the linear or cyclic diene significantly decreased the activity of catalysts (Ali et al., 2022; Ali et al., 2022; Ali et al., 2022). The E/ip copolymer's active centers and activity show stability when the amount of IP was 0.116 to 0.48 mol/L, and then start to decline when the amount of isoprene 0.96 mol/L, recommending that under the lower concentration of IP, catalyst did not show deactivation. However, the activity of the catalyst is higher than PE, meaning that IP as a comonomer, activated the active site that was dormant during the E polymerization. Though, under the higher concentration of IP, active sites and activity were significantly reduced and lower than previously reported PE (Ali et al., 2020), while insertion of isoprene is higher (see Fig. 6a and b), which is recommended that higher insertion and coordination of isoprene in the copolymerization system significantly slowed down the chain propagation. We noticed that increasing the IP concentration not only decreased the activity and active sites it also failed to reactivate the dormant site of PE. Furthermore, the copolymers' Mw exhibited a gradual decrease with an increasing concentration of Ip. This observation suggests that the rate of chain transfer reactions with isoprene is significantly faster than compared to E.
The MWD shown in Supporting information (See Supporting information Fig. S2) demonstrates that all E/IP copolymers exhibited a monomodal even with a higher concentration (1.44 mol/L) of IP. This means that E/IP copolymers exhibited an active site with a similar nature and exposed the single-site nature of the metallocene. In contrast, Song et al. investigated the E/IP copolymerization catalyzed by the Ziegler-Natta heterogeneous catalyst under different IP concentrations; when the IP concentration was increased in the feed, the low Mw in the MWD curve appeared, they recommended that the appeared component in the MWD curve belonged to the homopolymer of isoprene (Song et al., 2018; Ali et al., 2024). This means that compared to the Ziegler-Natta catalyst, the metallocene catalyst completely copolymerized the IP with E and showed the minimum chain termination reactions and the active site of the metallocene catalyst under the higher isoprene concentration. Furthermore, the MWD of E is slightly broader with higher isoprene concentration, ranging from 3 to 4. This indicates that the catalyst shows multiple active sites during the E/IP copolymerization. The higher amount of IP co-monomers increased the MWD and indicated that chain transfer reactions significantly increased with increased IP concentration.
As illustrated in Figs. 7, 8, and Table 2, the IP insertion is increased with the increase of IP concentration, but the active sites and kp are significantly decreased. At 0.06 mol/L of IP, the maximum level of active sites and kp was achieved, mean under the lower concentration, the active site of the catalyst is more involved in the E monomer insertion. In contrast, the maximum insertion of IP (3.68 %) was obtained with a feed concentration of 1.44 m/L, but the active site reached to lower level of 21.6 % (see Fig. 7a), which is lower than the homopolymerization of ethylene (Ali et al., 2020). It's suggested that the higher concentrations of IP show a deactivation effect on metallocene catalysts. In addition, the higher concentration of IP significantly converts the active site catalyst into a dormant site that was initially active for E monomers.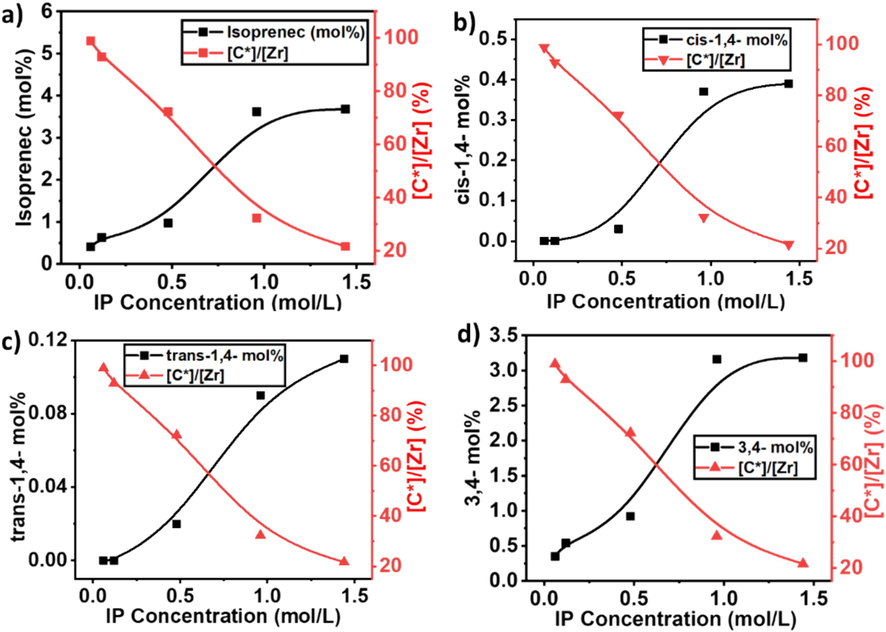
a) The comparison of isoprene mol% in the polymer chain and active center, b) showing the 1,4 connection of isoprene and active centers c) comparison of trans-1,4 connections and active centers and d) showing a comparison of 3,4 connections and active centers in E/IP copolymers under different isoprene concentrations.
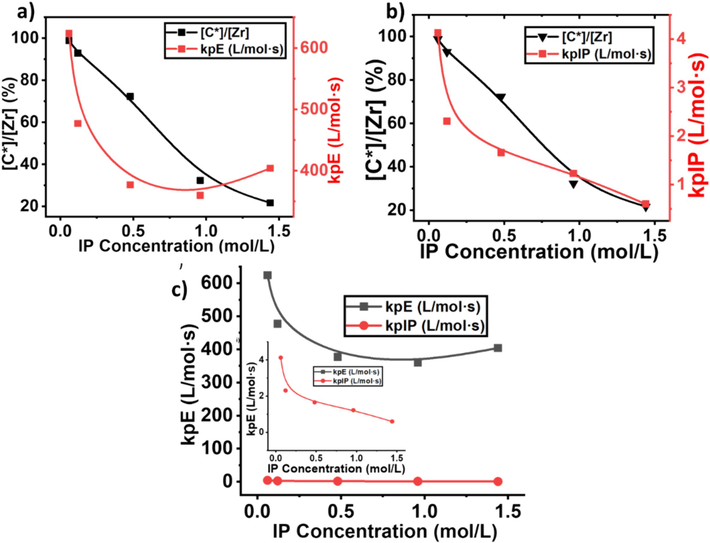
a) And b) show a comparison of active center and propagation constant for ethylene and isoprene, while c) gives a comparison of kpE and kpIP.
The insertion of isoprene with 3,4 and cis/trans-1,4 connections progressively increased with increasing of IP concentration. But compared to cis/trans-1,4 connections of isoprene, 3,4 connections is higher and reached to the maximum level (see Fig. 7b, c, and d). These results indicate that compared to heterogeneous zeigler-natta catalyst, metallocene exhibited a stable and controlled polymerization process.
As illustrated in Table 2 and (see Supporting information Fig. S1) at a lower concentration of IP, like between 0.06 and 0.12 mol/L, the insertion of cis-1,4 connections and trans-1,4 connections of IP did not show the insertion in the polymer chain. But further increased in the IP concentration, progressively increased the insertion of cis/trans-1,4 connection. Compared to trans-1,4 connections of IP, cis-1,4 connections of IP are higher but still lower than the 3,4 connections. These consequences indicate that the catalytic active site of the metallocene catalyst with a lower concentration of IP could not insert the IP monomers with cis/trans-1,4 connections. However, when the concentration was 0.48 mol/L, cis/trans-1,4 connections of IP started the insertion. This means that with a lower concentration of IP, metallocene did not show its stereoselectivity. For the insertion of cis/trans-1,4 connections minimum of 0.48 mol/L isoprene is required in the feed. We can also assume that at lower concentrations of IP, metallocene catalyst active sites first coordinated with isoprene through 3,4 connections, which aggregated around the active site of the catalyst and significantly reduced the insertion of cis/trans-1,4 connections isoprene.
The results of the kp for E and IP in the copolymers under different isoprene concentrations are shown in Table 2. The kpIP is much lower than kpE, at the concentration of 0.06 mol/L, the kpE is 624 L/mol·s and kpIP 4.13 L/mol·s individually, recommended the lower diffusion barriers of the system, which are near to the actual propagation content. One time increase in the isoprene concentration in the feed, minor changes in terms of decline in the kpE and kpIP were observed (see Fig. 8). A little increase in the concentrations of IP in the feed may be increased competition for adsorption on the active sites of the metallocene catalyst, and it might be IP has a higher affinity for the active sites compared to E, leading to a reduction in the kp for E. In contrast, under the same concentration, in the E/ENB copolymerization kpE and kpENB were 310 L/mol·s and 0.987 L/mol·s, respectively. However, the active center of E/IP copolymers is 0.92 %, while in the E/ENB copolymers 79 %, meaning that metallocene catalyst is more active with IP comonomer than ENB (Ali et al., 2022). Similarly, a further increase in the IP concentration from 0. 48 mol/L to 0.69 mol/L, the kp and active sites of the system did not change in large difference, as mentioned in Table 2 run no 2.3 and 2.4, the microstructure of the E/IP copolymer showing the insertion of cis/trans-1,4 connections which is showing the leading reactivity effect of the active sites and maintain the propagation rate constant (see Fig. 8a, b and c). Similarly, a further increase in the isoprene concentration noticeably decreased the kp of E and IP and reached to lower level of 404 L/mol·s and 0.60 L/mol·s.
According to the literature, the presence of a higher concentration of IP may influence the stability of the metallocene catalyst or lead to catalyst poisoning, reducing its activity and active site concentration (Ali et al., 2021; Barsan et al., 1998). In addition, IP, being a larger molecule than ethylene, may introduce steric hindrance effects during the propagation step see Fig. 9a and 9b. This steric hindrance can slow down the addition of monomers to the growing polymer chain, resulting in a decrease in the kp. According to Fan et al., a higher concentration of IP could lead to a saturation of active sites with IP, limiting the available sites for E and reducing the overall kp (Song et al., 2018). Compared to cyclic dienes, at 1.44 mil/L of isoprene in the feed, kpE 404 L/mol·s and 0.60 L/mol·s, which is still higher than the kpE of the E/ENB copolymerization, which means if increased the isoprene concentration 10 times of ENB, still metallocene showing a higher kp (Ali et al., 2023; Ali et al., 2022). In addition, compared to ENB, IP exhibits less bulky molecules, and both π bonds are available for coordination, which reduces the chain transfer reactions that involve the transfer of a growing polymer chain to another molecule and declines the active sites (Li et al., 2008). In other words, the active centers in the E/IP copolymers at lower concentration of IP that produces copolymers may be made up of contact ion pairs with large kp values.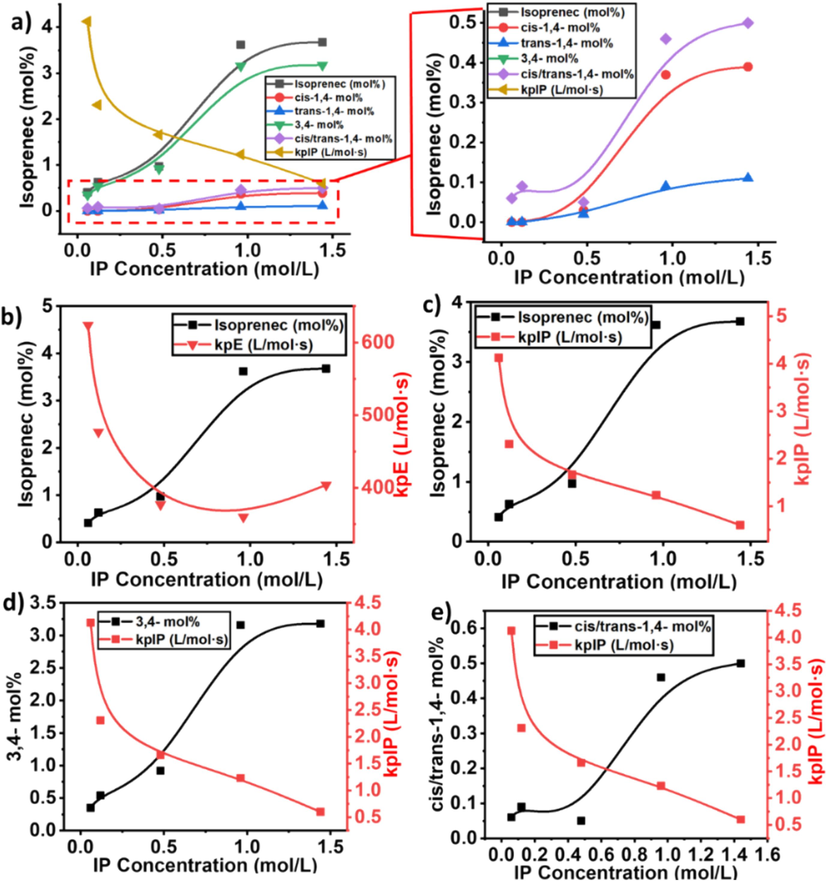
a) Showing an isoprene mole % in the obtained copolymers, b) the comparison of isoprene mol% and propagation constant of ethylene, c) the comparison of isoprene mol% and propagation constant of isoprene, d) comparison of 3,4 connection and isoprene propagation constant of isoprene, and e) comparison of cis/trans-1,4 connections and propagation constant of isoprene in the E/IP copolymers.
Generally, active sites with contact ion pairs demonstrate the higher Mw of the polymer, which can be seen in Table 2. While, whereas [C*]/[Zr] in the E/IP copolymers at higher concentrations could be made up of a large number of loosely associated ion pairs with reduced kp values. The moderate decline of kpE and kpIP with isoprene concentration (see Fig. 9b, c, d) could also be described by the lower diffusion barrier in the E/IP copolymer. However, the effect of the active center ratio on the kp should also be considered. According to Guo et al., the active center fraction in homopolymerization is lower than in E/ENB copolymerization, particularly in the later stages (Ali et al., 2021).
Similarly, the active centers generated with lower concentrations of IP have higher kp than those formed generated with higher concentrations of IP. As seen in Table 2, proton spectra showed that the 3.4 connection of IP takes place first with higher insertion, and it exhibits a bulkier structure compared to the cis/trans-1,4 isoprene connection. A part of a metallocene catalyst in the form of contact ion pairs may be incapable of accepting cis/trans-1,4 isoprene for further coordination, resulting in behaving as dormant or inactive in the E/IP copolymers.
The greater the concentration of IP in the E/IP copolymerization, the copolymers formed tend to have lower crystallinity. As a result, the presence of dissolved copolymers contributes to an increased diffusion barrier within the system. The impact of this increased diffusion barrier is reflected in the reaction kinetics. Specifically, with a higher concentration of isoprene, E/IP copolymers exhibit a lower kpE and kpIP, because E/IP copolymers look like a lower crystalline and might be part of the copolymers dissolved in the reaction solvent (toluene) and increased diffusion barrier in the system and lead to lower kpE and kpIP. In conclusion, changes in the thermodynamics of the polymerization reaction due to the increased concentration of isoprene may influence the overall kinetics, including the propagation rate constant and active site concentrations.
In Table 1, Fig. 1b, and Supporting information, the regio- and stereoselectivities of IP insertions exhibit a continuous improvement as the reaction temperature escalates from 30 to 40 °C. Notably, active sites responsible for generating copolymer with lower IP content, Mw, and appear to exhibit diminished regio- and stereospecificities. At lower temperatures, the active sites are evidently more prone to producing copolymer chains characterized by lower regio- and stereoselectivities, resulting in a mixture with varied insertion patterns of IP. However, as the reaction temperature increases, these active sites become progressively deactivated or suppressed. Consequently, their influence on the overall copolymerization product diminishes.
The observed temperature-dependent variation in the formation of 3,4-isoprene (3,4-IP) units within the copolymerization process can be elucidated through the lens of a specialized coordination and insertion mechanism. As shown in Scheme 3, 3,4-double of IP monomer first coordinates to the active site of the catalyst and then inserted into the propagation chain (Zr-P). In addition, the insertion of the 1,2-double bond significantly takes place immediately in the growing polymer chain after 3,4-double insertion. According to Song et al., and Zhaomin Hou et al., the insertion of IP comonomer through a 1,2-double connection into a growing polymer chain significantly requires higher activation energy compared to a 3,4-connection (Song et al., 2018; Li et al., 2009). In our case, at 60 °C, metallocene-catalyzed E/IP copolymers showed a higher 1,4 connection. Therefore, the correlation between temperature and regio- and stereoselectivities may serve as a crucial indicator in comprehending the copolymerization mechanism.
In our previous work, we reported the homopolymerization of ethylene with a similar catalyst and explored the broader molecular weight distribution of PE because of chain transfer reactions (Ali et al., 2020). Later on, Song et al. reported the E/IP copolymerization with Z-N catalyst and found the E/IP copolymers with dimolecular weight distribution when isoprene concentration was 2 mol/L (Song et al., 2018). In the case of E/IP copolymers catalyzed with metallocene, increasing isoprene concentration in the feed leads to an increase in the incorporation rate of IP, and produces narrow MWD. Especially when IP was 1.44 mol/L, the content of IP was a maximum of 3.68 mol%, while IP insertion through cis-1,4- 0.49 mol%, which is lower than 3,4-connection. This means that excess of IP in the feed increased the 3,4-connection of IP, which hinder E from coordination on the active centers leading to retarded E insertion in the copolymer chains. The catalyst's copolymerization properties changed continually with the IP concentration due to the complex variations in copolymer microstructure (including the regio and stereoselectivities of IP insertions). In addition, the Mw of the E/IP copolymers slowly declines with an increase of IP concentration in the feed, recommending that chain transfer reactions significantly increase (See Scheme 2). However, it is slower with E than IP, especially when the complexes have larger steric hindrances around the metal center. The larger steric catalyst offers 3,4 insertion as a major component for growing polymer chain and does not leave much space for β-hydrogen elimination. Due to this less space, the resulting olefin/hydride species could not undergo ligand exchange with an incoming IP monomer.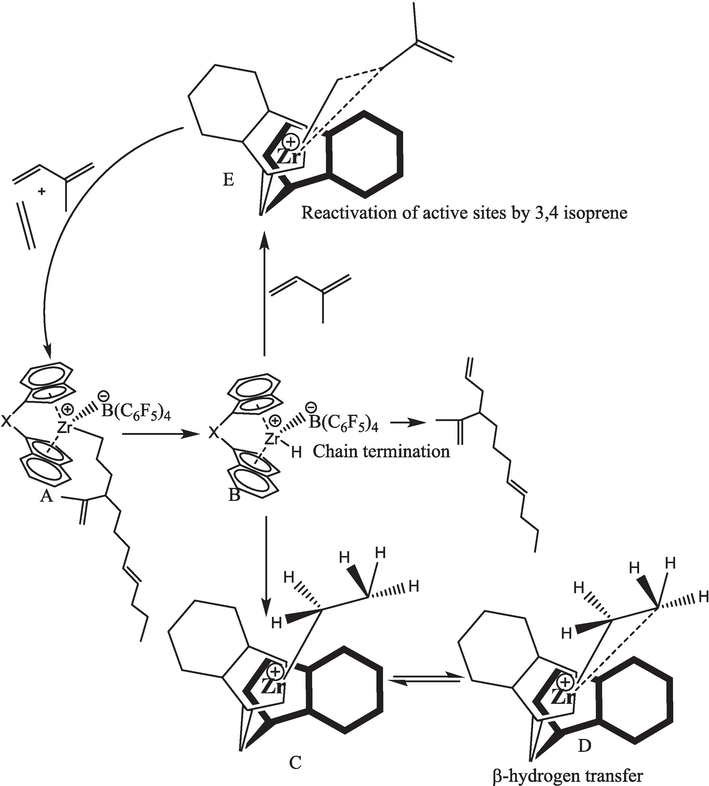
A possible chain transfer reaction in the metallocene-catalyzed the E/ IP copolymer under the different reaction temperatures and reactivated the dormant site by isoprene.
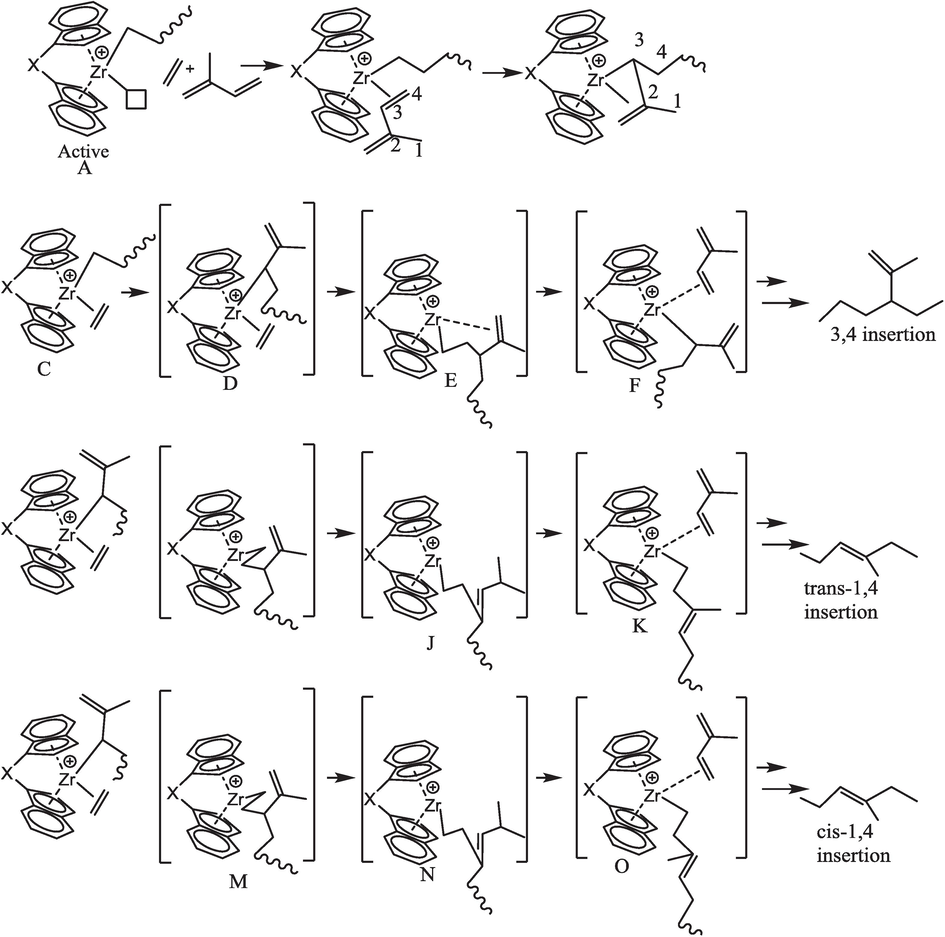
Proposed mechanism of copolymerization between ethylene and isoprene catalyzed by a metallocene catalyst.
In Scheme 2, the catalytic sites denoted as L2Zr—CH2-CH2-IP, carrying a propagation chain (A), may potentially undergo a β-H transfer reaction. This process gives rise to species (B) featuring a Metal-hydrogen bond, leading to the formation of L2Zr—H. Subsequently, this species transforms into L2Zr—CH2H3 (C) by incorporating E into the L2Zr—H bond. Within the Zr center and methyl hydrogen, the resulting Zr–CH2CH3 (D) species becomes inaccessible to incoming IP monomers, thereby reducing overall system activity. Various reports in the literature discuss strategies to reactivate such dormant or inactive species. Notably, Kissin Y.V and colleagues explored the activation mechanism of co-monomers in heterogeneous Z–N catalyst systems through their model. They investigated in the copolymers how comonomers were activating the dormant sites of the catalyst; later on, Fan et al. followed the same model for the metallocene catalyst in the polymerization of E/P copolymers. They significantly analyzed the propylene comonomer in the (Zr—H) can bypass the reaction and increase the [Zr]/[C*] in the system.
Regarding these possible chain termination and chain transfer reactions, the silicon bridge exhibits a greater steric hindrance due to its bulky Cp ligands that might prevent the β-hydrogen elimination. Therefore, we were expecting this catalyst system chain transfer reaction to be significantly difficult and show a living feature in the E-IP copolymerization. In the case of Z-N catalyzed E/IP copolymers, 1,4-isoprene insertion is a major component for chain transfer reactions exhibiting enough space for β-hydrogen elimination (Song et al., 2018).
In the copolymerization of E/IP, our investigation focuses on determining whether polymerization begins with E molecules or isoprene molecules. Xiaofang et al. investigated the E/IP polymerization mechanism by using DFT calculations and indicated that the E/IP unsymmetrical metallocene-catalyzed copolymerization initiation needs to start with an allyl-type intermediate, which is comparable to the intermediate generated during the homo polymerization of isoprene (Ali et al., 2020; Ali et al., 2022; Li et al., 2009; Wang et al., 2020). Through the utilization of literature knowledge of DFT and other computational studies and analyses involving MWD, active sites, and nuclear magnetic resonance (NMR), we have acquired ample information to describe the E/IP copolymers and their mechanism accurately.
As illustrated in Scheme 3, in the E/IP symmetrical metallocene-catalyzed copolymerization (A), the insertion of an IP comonomer in a growing polymer chain took place through 3,4 connections (B), which was significantly increased with increased IP concentration in the feed see Table 2 and Scheme 1. While the insertion of IP through the 1,4 connection in the propagation chain is less, even though cis-1,4- and trans-1,4- are 0. Notably, active sites responsible for generating copolymers with lower IP content 1,4 connection (cis-1,4- and trans-1,4-) and higher molecular weight (MW) appear to exhibit reduced stereospecificities. In addition, at lower IP concentration, these active sites are evidently more prone to producing copolymer chains characterized by lower regio- and stereoselectivities, resulting in a mixture with varied insertion patterns of IP that was not obtained. This means that for enough regio- and stereoselectivities of E/IP copolymers, a minimum of isoprene should be more than 0.06 mol/L. In addition, at lower concentrations of IP, the metallocene active site could not assist the 1,4 insertions due to its higher steric hindrance of 3,4 connections in the propagation chain. When the concentration of IP is increased in the feed, we noticed that coordination of IP 3,4 connections and 1,4 connections (cis-1,4- and trans-1,4-) also appeared, but the 3,4 connections were still higher. This means that the coordination of IP through 3,4 connections to the catalyst transition metal is preferable, and it is dominant in the chain initiation during the metallocene catalyst system. The resulting 3,4- IP insertion into the Zr-alkyl bond might be leading to the formation of (C).
The higher steric hindrance of silicon bridge metallocene, compared to isoprene E is a smaller molecule and coordinates to the metal center (C) easily, before the isomerization of (C to D) or possibly (I) that leads to producing the copolymers with E/3–4-isoprene units. According to the reports of Xiaofang et al., substituted cyclopentadienyl favors the insertion of the E and produces an EE sequence in the obtained polymer (G and H) (Li et al., 2009). Notably, these catalysts exhibited a penchant for favoring ethylene insertion and yielding a copolymer with a predominant EE (ethylene-ethylene) sequence, as evidenced in the resultant polymer (G and H). The mechanistic insight into this phenomenon involves the coordination of ethylene (E) at the Zr metal in the π-allyl intermediate, followed by the subsequent insertion of isoprene (IP) to generate an E-1,4-isoprene fashion, as illustrated in Scheme 2 (depicted by J, K, and L), as well as in (M) and (N). Drawing from established literature, it is well-known that in the realm of polyisoprene or ethylene/isoprene copolymers, catalysts encounter challenges in the insertion of trans-1,4-isoprene, particularly if they possess less steric features. In our specific metallocene catalyst system, the copolymerization process yielded cis-1,4-isoprene as a minor product and trans-1,4-isoprene as the major product. It is noteworthy, however, that both of these isomeric products were obtained in lesser quantities compared to the copolymer with 3,4-isoprene insertion. This observation underscores the distinctive behavior of our catalyst, providing valuable insights into the regioselective and stereoselective aspects of the copolymerization reaction.
4 Conclusion
Our comprehensive investigation into metallocene-catalyzed E/IP copolymerization has revealed a complex interplay of factors influencing the active-site count. Examining temperature as a critical parameter, we observed a noteworthy increase in active sites, reaching 89 % as temperatures rose from 30 °C to 40 °C. Surprisingly, further temperature increments resulted in a decline in active sites, challenging the conventional conception that higher temperatures generally enhance metallocene activation. At 30 °C, the active site predominantly facilitated the insertion of IP through 3,4 connections, indicating a lack of stereoselectivity at this temperature. Contrastingly, at 40–50 °C, cis/trans-1,4 connections and active sites approached a higher level of 89 %, revealing of reduced chain transfer reactions and making catalytic active sites more favorable to cis/trans-1,4 connections. Secondly, examining isoprene concentration, we found stability in active centers and activity within the range of 0.116 to 0.48 mol/L, followed by a decline at 0.96 mol/L, suggesting deactivation under higher IP concentrations. Importantly, increasing IP concentration decreased activity and active sites and failed to reactivate dormant polyethylene sites. The observed progressive decrease in copolymer Mw and active centers with increased IP concentration underscores the accelerated chain transfer reactions with IP compared to E. In addition, as IP concentration increases, slight changes in rate constants (kpE and kpIP) take place, indicating an aggressive competition for attachment to metallocene catalyst sites. This competition, driven by isoprene's stronger attraction than ethylene, leads to a reduction in the rate of E propagation.
CRediT authorship contribution statement
Amjad Ali: Writing – review & editing, Writing – original draft, Methodology. Muhammad Nadeem: Formal analysis, Data curation, Conceptualization. Ahmad Naveed: Software, Investigation, Data curation. Jamile Mohammadi Moradian: Visualization, Validation, Formal analysis, Data curation. Syed Najeeb-Uz-Zaman Haider: Software, Formal analysis, Conceptualization. Shahid Khan: Validation, Data curation, Conceptualization. Adnan Murad Bhayo: Visualization, Validation, Investigation. Jianwei Lu: Visualization, Validation, Software, Methodology. Rai Nauman Ali: Visualization, Formal analysis, Data curation. Naushad Ahmad: Visualization, Validation, Funding acquisition, Formal analysis. Fan Zhiqiang: Writing – review & editing, Supervision, Project administration, Data curation. Li Guo: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition, Data curation.
Acknowledgments
The authors would like to thank the Researchers Supporting Project Number (RSPD2024R668), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of alkylaluminum cocatalyst on ethylene/1-hexene copolymerization and active center distribution of MgCl2-supported Ziegler-Natta catalyst. J. Macromol. Sci. A. 2021;58(8):539-549.
- [Google Scholar]
- Kinetics and mechanism of ethylene and propylene polymerizations catalyzed with ansa-zirconocene activated by borate/TIBA. J. Organomet. Chem.. 2020;922:121366
- [Google Scholar]
- Ethylene–propylene copolymerization and their terpolymerization with dienes using ansa-Zirconocene catalysts activated by borate/alkylaluminum. J. Macromol. Sci. A. 2020;57(2):156-164.
- [Google Scholar]
- Comparative analysis of ethylene/diene copolymerization and ethylene/propylene/diene terpolymerization using ansa-zirconocene catalyst with alkylaluminum/borate activator: the effect of conjugated and nonconjugated dienes on catalytic behavior and polymer microstructure. Molecules. 2021;26(7):2037
- [Google Scholar]
- Kinetic and thermal study of ethylene and propylene homo polymerization catalyzed by ansa-zirconocene activated with alkylaluminum/borate: Effects of alkylaluminum on polymerization kinetics and polymer structure. Polymers. 2021;13(2):268
- [Google Scholar]
- Kinetic and thermal study of ethylene-propylene copolymerization catalyzed by ansa-zirconocene activated with Alkylaluminium/borate: effects of linear and branched alkylaluminium compounds as cocatalyst. J. Polym. Res.. 2021;28(5):186
- [Google Scholar]
- Rapid kinetic evaluation of homogeneous single-site metallocene catalysts and cyclic diene: how do the catalytic activity, molecular weight, and diene incorporation rate of olefins affect each other? RSC Adv.. 2021;11(50):31817-31826.
- [Google Scholar]
- Methods for predicting ethylene/cyclic olefin copolymerization rates promoted by single-site metallocene: kinetics is the key. Polymers. 2022;14(3):459.
- [Google Scholar]
- Polymerization kinetics of bicyclic olefins and mechanism with symmetrical ansa-metallocene catalysts associated with active center count: Relationship between their activities and structure and activation path. RSC Adv.. 2022;12(24):15284-15295.
- [Google Scholar]
- Evaluation of the dielectric and insulating properties of newly synthesized ethylene/1-hexene/4-vinylcyclohexene terpolymers. ACS Omega. 2022;7(35):31509-31519.
- [Google Scholar]
- Progress toward polymerization reaction monitoring with different dienes: how small amounts of dienes affect ansa-zirconocenes/borate/triisobutylaluminium catalyst systems. Polymers. 2022;14(16):3239.
- [Google Scholar]
- Ansa-metallocene catalyst based on 3-phenyl and 4-methyl substituted: catalyst evaluation in conjugated and non-conjugated diene polymerization. J. Polym. Res.. 2023;30(8):318.
- [Google Scholar]
- Copolymerization of ethylene and isoprene via silicon bridge metallocene [rac-Me2Si (2-Me-4-Ph-Ind) 2ZrCl2] catalyst: A new way to control the composition and microstructure of copolymers. Chemosphere. 2024;347:140700
- [Google Scholar]
- Polymerization of isobutylene and the copolymerization of isobutylene and isoprene initiated by the metallocene derivative Cp* TiMe2 (μ-Me) B (C6F5) 3. Macromolecules. 1998;31(24):8439-8447.
- [Google Scholar]
- Activation of sp3 C–H bonds with cobalt (I): Catalytic synthesis of enamines. J. Am. Chem. Soc.. 2007;129(47):14544-14545.
- [Google Scholar]
- Stereospecific olefin polymerization with chiral metallocene catalysts. Angew. Chem. Int. Ed. Eng.. 1995;34(11):1143-1170.
- [Google Scholar]
- Kinetic study of olefin polymerization with a supported metallocene catalyst. II. Ethylene/1-hexene copolymerization in gas phase. J. Appl. Polym. Sci.. 2001;80(8) 1096-119
- [Google Scholar]
- Kinetic aspects of the copolymerization between Alpha-olefins and borane monomer in Ziegler-Natta catalyst. Macromolecules. 1993;26(12):3019-3025.
- [Google Scholar]
- Ziegler-Natta catalysis: 50 years after the Nobel Prize. MRS Bull.. 2013;38(3):213-218.
- [Google Scholar]
- Chromophore quench labeling: simulated snapshots of molar mass distributions for the rapid mechanistic analysis of catalytic alkene polymerization. ACS Catal.. 2022;12(2):1117-1127.
- [Google Scholar]
- Quantitative validation of the living coordinative chain-transfer polymerization of 1-Hexene using chromophore quench labeling. Macromolecules. 2020;53(14):5816-5825.
- [Google Scholar]
- Deactivation kinetics of metallocene-catalyzed ethene polymerization at high temperature and elevated pressure. Macromol. Mater. Eng.. 2004;289(5):475-479.
- [Google Scholar]
- Copolymerization of isoprene with ethylene catalyzed by cationic half-sandwich fluorenyl scandium catalysts. J. Polym. Sci. A Polym. Chem.. 2015;53(24):2898-2907.
- [Google Scholar]
- Mechanisms of stereochemical control in propylene polymerizations with soluble Group 4B metallocene/methylalumoxane catalysts. J. Am. Chem. Soc.. 1984;106(21):6355-6364.
- [Google Scholar]
- Determination of the number of active sites for olefin polymerization catalyzed over metallocene/MAO using the CO inhibition method. Macromolecules. 1996;29(23):7305-7309.
- [Google Scholar]
- Heterogeneous single-site catalysts for olefin polymerization. Chem. Rev.. 2000;100(4):1347-1376.
- [Google Scholar]
- DFT studies on isoprene/ethylene copolymerization catalyzed by cationic scandium complexes bearing different ancillary ligands. Chin. J. Chem.. 2017;35(5):723-732.
- [Google Scholar]
- Effects of titanium dispersion state on distribution and reactivity of active centers in propylene polymerization with MgCl2-supported Ziegler-Natta catalysts: A kinetic study based on active center counting. ChemCatChem. 2020;12(20):5140-5148.
- [Google Scholar]
- The discovery of metallocene catalysts and their present state of the art. J. Polym. Sci. A Polym. Chem.. 2004;42(16):3911-3921.
- [Google Scholar]
- Kinetics of short-duration ethylene–propylene copolymerization with MgCl2-supported Ziegler-Natta catalyst: differentiation of active centers on the external and internal surfaces of the catalyst particles. J. Appl. Polym. Sci.. 2018;135(12):46030.
- [Google Scholar]
- Mechanism of stereospecific polymerization of α-olefins by late-transition metal and octahedral group 4 metal catalysts. Coord. Chem. Rev.. 2009;253(15–16):2082-2097.
- [Google Scholar]
- Homo-and copolymerization of 5-ethylidene-2-norbornene with ethylene by [2-C5Me4-4, 6-tBu2C6H2O] TiCl2/AliBu3/Ph3CB (C6F5) 4 catalyst system and epoxidation of the resulting copolymer. Polymer. 2008;49(12):2839-2844.
- [Google Scholar]
- Alternating and random copolymerization of isoprene and ethylene catalyzed by cationic half-sandwich scandium alkyls. J. Am. Chem. Soc.. 2009;131(38):13870-13882.
- [Google Scholar]
- A 2H-labeling scheme for active-site counts in metallocene-catalyzed alkene polymerization. J. Am. Chem. Soc.. 2001;123(12):2915-2916.
- [Google Scholar]
- Mechanistic investigations into the behavior of a labeled zirconocene polymerization catalyst. Organometallics. 2012;31(5):2097-2107.
- [Google Scholar]
- Catalytic polymerization and post polymerization catalysis fifty years after the discovery of Ziegler's catalysts. Macromol. Chem. Phys.. 2003;204(2):289-327.
- [Google Scholar]
- Origin of “multisite-like” ethylene polymerization behavior of the single-site nonsymmetrical bis (imino) pyridine iron (II) complex in the presence of modified methylaluminoxane. ACS Catal.. 2017;7(4):2868-2877.
- [Google Scholar]
- Trans-1, 4-stereospecific copolymerization of ethylene and isoprene catalyzed by MgCl2-supported Ziegler-Natta catalyst. J. Polym. Sci. A Polym. Chem.. 2018;56(24):2715-2722.
- [Google Scholar]
- From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev.. 2016;116(3):1398-1433.
- [Google Scholar]
- A computational study of isoprene polymerization catalyzed by iminopyridine-supported iron complexes: ligand-controlled selectivity. Chem. Phys. Lett.. 2020;755:137811
- [Google Scholar]
- The effect of temperature on the polymerization of propene with dimethylsilylbis (1-indenyl) zirconium dichloride/methylaluminoxane and dimethylsilylbis (2-methyl-1-indenyl) zirconium dichloride/methylaluminoxane, Modeling of kinetics. Macromol. Chem. Phys.. 1998;199(9):1989-2004.
- [Google Scholar]
- Kinetic study of olefin polymerization with a supported metallocene catalyst. I. Ethylene/propylene copolymerization in gas phase. J. Appl. Polym. Sci.. 2001;80(1):81-114.
- [Google Scholar]
- Designing sites in heterogeneous catalysis: are we reaching selectivities competitive with those of homogeneous catalysts? Chem. Rev.. 2022;122(9):8594-8757.
- [Google Scholar]
- Polyolefin thermoplastic elastomers from polymerization catalysis: Advantages, pitfalls and future challenges. Prog. Polym. Sci.. 2021;113:101342
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105989.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







