Translate this page into:
Copper promoted desulfurization and C-N cross coupling reactions: Simple approach to the synthesis of substituted 2-aminobenzoxazoles and 2,5-disubstituted tetrazole amines
⁎Corresponding authors. dr.b.haribabu@gmail.com (Hari Babu Bollikolla), sfadil@ksu.edu.sa (Syed Farooq Adil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Copper-supported novel, facile and efficient methods for the synthesis of various 2-amino-benzoxazoles and 2,5-diphenyltetrazoleamines have been demonstrated. The reaction procedures are simple, with excellent substrate tolerance in good to high yields thus paving an excellent and useful way to establish a library of potentially active drug molecules. This methodology represents the first concept of copper-catalyst promoted domino C-N cross-coupling reaction towards the construction of 2-aminobenzoxazoles. In addition, we described report for the synthesis of 2,5-diaryltetrazoleamines using copper via inter molecular C-N cross-coupling reaction with aryl iodides. The proposed reaction mechanism involves copper based desulphurization/nucleophilic substitution and subsequent C-N cross-coupling reactions. We established numerous applications of this methodology for synthesizing diverse heterocyclic derivatives i.e. both electron rich and electron deficient systems.
Keywords
Desulfurization
Nucleophilic substitution
Copper catalyst
Mono/domino C-N cross-coupling reaction
Heterocyclic compounds
1 Introduction
Due to many fold applications of heterocycles, these are very important groups in the field of both synthetic chemistry as well as pharmaceutical chemistry. Mainly heterocyclic compounds like benzoxazole and tetrazoles, etc. have great importance in various drug molecules and biological sciences (Jeon and Park, 2004; Potashman et al., 2007; Ozden et al., 2008; Zhang et al., 2008) due to their extensive occurrence as a core moiety (Fig. 1). In addition, these compounds show anti-inflammatory, antimicrobial and antibacterial activities (Padalkar et al., 2016; Kaura et al., 2018; Chojnacka et al., 2019).
Some examples of biologically active molecules.
Due to the above mentioned biological importance many researchers have developed synthetic routes for the synthesis of heterocyclic compounds. In this connection, initially heterocycles such as 2-aminobenzoxazoles (Varma and Kumar, 1998; Chang et al., 2002; Pottorf et al., 2003) were prepared from o-aminophenol through traditional process, however, it needs high reaction temperature and longer reaction time. Hence, recently above said disadvantages has been overcome by researchers through more sustainable cross-coupling reactions using transition metals based catalytic systems. For example, benzoxazole moiety compounds were constructed via C-O cross-coupling reaction using transition metals like Cu (Evindar and Batey, 2006; Barbero et al., 2007; Karlsson et al., 2008; Ueda and Nagaswa, 2008), Fe (Bauer, 2008; Liu, 2010; Liu et al., 2012; Wu et al., 2012; Gu et al., 2013, 2014), Co (Saha et al., 2010; Cai and Xie, 2015; He et al., 2016), Ni (Phan et al., 2014; Jablonkai et al., 2015), Zn (Wu and Newmann, 2012; Banerjee et al., 2014, Sharma et al., 2014), Ti (Ladipo, 2006; Azizian et al., 2016), Pd (Ackermann et al., 2009; Huang et al., 2010; Kalkhambkar and Laali, 2012; Liu et al., 2013; Gao et al., 2014; Shen et al., 2014; Xie et al., 2014; Kumbhar and Salunkhe, 2015; Zhu et al., 2015), Pt (Yoo et al., 2011; Wang et al., 2015), Ru (Fan et al., 2011; Khalafi-Nezhad and Panahi, 2014; Molnar and Papp, 2016), Ir (Blacker et al., 2009; Scholten, 2013). However, as per our knowledge no report is available for the synthesis of 2-aminophenyl benzoxazole from thiourea. Therefore, in this context we would like to present the preparation of benzoxazoles through domino intra and inter molecular C-N cross-coupling reaction by employing copper salt as a catalyst under mild reaction conditions.
Similarly, tetrazoles another important organic compound, were synthesized using traditional processes like addition of NaNO2, NaN3 to carbodiimides or cyanamides (Finnegan et al., 1953; Gbrecht and Herbst, 1953; Marchalin and Martvon, 1980; Moderhack et al., 1990), nitriles (Lakshmikantam et al., 2005, 2006a, 2006b) and addition of TMSN3 to nitriles (Amantini et al., 2004; Tienan et al., 2008), nucelophilic substitution by N3- of (a) chlorine in α-chloroformamidines (Erle, 1982) and (b) sulfur from thioureas in presence of mercury (Batey and Powell, 2000; Yu et al., 2004) or lead salts (Finnegan et al., 1953) or iodine (Ramesh et al., 2011). They require high temperature, harsh reaction conditions and strong acids, they contain lack of regioselectivity and difficult to get starting precursors. In order to overcome the above mentioned drawbacks tetrazole synthesis were reported using transition metal (Su et al., 2006; Kundu et al., 2009; Nasrollahzadeh et al., 2009; Venkateshwarlu et al., 2009; Das et al., 2010; Ramana and Punniyamurthy, 2012, Mohan et al., 2018; S N Murthy et al., 2018a).
In continuous research on synthetic methodologies of heterocyclic compounds, recently, our group also developed efficient protocols for the synthesis of 1,5-disubstituted tetrazole (S N Murthy et al., 2018a) and substituted benzothiazoles (S N Murthy et al., 2018b) from thiourea through C-N cross-coupling reaction using Cu as catalyst under mild reaction conditions. Encouraged by the fruitful results of the mentioned protocols, in continuation of our studies towards the development of valuable synthetic methodologies for construction of diverse heterocyclic scaffolds, the authors wish to further investigate the utility of the above synthetic protocols towards the syntheses of 2,5-disustituted tetrazoles and substituted 2-amino-benzoxazoles via C-N cross-couplings using copper catalysis.
2 Experimental
2.1 Materials and methods
General information: Thiourea, copper sources like CuSO4·5H2O (98%), CuI (98%), CuBr (98%), Cu2O (97%), CuCl (99%) and Cu(OAc)2·H2O (98%) and the bases Et3N, Pyridine, K3PO4·3H2O, KOH, K2CO3, Cs2CO3 were purchased from Aldrich and utilized without further purification. The solvents were purchased and dried according to standard procedure prior to use (Furniss et al., 2004). 1H and 13C NMR spectra were recorded with a DRX-400 Varian spectrometer. A Perkin Elmer Spectrum one FT-IR spectrometer is utilized to record the Infrared (IR) spectra. During the experimental procedure for the synthesis of resulting compounds VKSI Medico Centrifuge machine was used.
2.2 General procedure for the synthesis of 2-iodoaryl isourea
To a stirred solution of solvent (2–3 mL), thiourea (1 mmol, 76 mg) was added slowly, followed by Et3N (1 mmol, 101 mg) and Copper source (50 mol %) at room temperature. The whole reaction mixture was stirred for one hour (until get the black colour) at room temperature. Later, to the previous solution, 2-iodophenol (2 mmol, 438 mg) was added. After completion of the reaction monitored by TLC, the reaction mixture was transferred into a centrifuged tube and the mixture was centrifuged for 10 min by using centrifugation machine. Black colour solid was removed from the centrifuged tubes. The clear solution was concentrated by using rotary evaporator and the crude mixture was purified by silica gel (60–120 mesh) column chromatography using ethylacetate in hexane as eluent system to obtain a 2-iodophenyl isourea as a solid substance.
2.3 General procedure for the synthesis of 2-(N-arylamino)benzoxazole
To a stirred mixture of N-2-iodoaryl isourea (1 mmol) in DMSO (2 mL), iodobenzene (1 mmol, 204 mg), Cs2CO3 (1 mmol, 325 mg), Cu(OAc)2·H2O (10 mol %, 20 mg) and 1,10-phenanthroline (20 mol%, 36 mg) were added consecutively in slowly for several minutes and the reaction mixture was stirred for 18 h at 110 °C. The reaction progress was monitored by thin layer chromatography (TLC) using ethyl acetate and hexane (1:4). The reaction mixture was cooled to room temperature after completion of the reaction (monitored by TLC). Then, the total mixture was washed with ethyl acetate (7 mL) and water (3 mL) for five times. Next, the organic layer was seperated and evoparated to collect the crude reaction mixture. The reaction mixture was purified by silica gel (60–120 mesh) column chromatography with eluent ethylacetate in hexane to obtain target product 2-(N-arylamino)benzoxazole which was characterized by NMR (1H and 13C), IR and elemental analysis.
2.3.1 N-Phenylbenzo[d]oxazol-2-amine (3a)
(Daswani et al., 2016, Yadav et al., 2018): White solid; yield 90%; mp 132–134 °C; 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 8 Hz, 1H), 7.46 (d, J = 8 Hz, 2H), 7.39–7.33 (m, 4H), 7.15 (t, J = 15.2 Hz, 2H), 6.9 (br s, 1H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.0, 138.4, 132.8, 131.6, 129.2, 128.5, 128.1, 127.8, 121.5, 120.9, 117.6; FT-IR (KBr) 3276 (—NH), 3076 (Sp2 C—H), 1614 (C⚌C), 1574, 1529, 1497, 1485, 1318, 1242, 1082 cm−1. Anal. Calcd. for C13H10N2O: C, 74.27; H, 4.79; N, 13.33; O, 7.61. Found: C, 74.39; H, 4.77; N, 13.28.
2.3.2 5-Methyl-N-o-tolylbenzo[d]oxazol-2-amine (3b)
White solid; yield 79%; mp 136–137 °C; 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H), 7.39–7.27 (m, 4H), 7.01 (d, J = 7.6 Hz, 2H), 6.72 (br s, 1H), 2.47 (s, 3H), 2.38 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 147.1, 137.0, 136.7, 136.2, 134.2, 132.0, 131.7, 130.5, 127.9, 127.1, 117.8, 115.3, 21.0, 20.2; FT-IR (KBr) 3175 (—NH), 3076 (Sp2 C—H), 2924 (Sp3 C—H), 2832, 1614 (C⚌C), 1574, 1529, 1497, 1485, 1318, 1242, 1082 cm−1. Anal. Calcd. for C15H14N2O: C, 75.61; H, 5.92; N, 11.76; O, 6.71. Found: C, 75.74; H, 5.90; N, 11.70.
2.3.3 N-(2-Isopropylphenyl)-5-methylbenzo[d]oxazol-2-amine (3c)
(Daswani et al., 2016): White solid; yield 76%; mp 140–141 °C; 1H NMR (400 MHz, CDCl3) δ 7.64–743 (m, 3H), 7.37–7.32 (m, 2H), 7.18 (d, J = 8.4 Hz, 2H), 5.99 (br s, 1H), 2.88–2.83 (m, 1H), 2.46 (s, 3H), 1.20 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 151.7, 141.6, 141.2, 135. 9, 132.5, 128.4, 128.2, 128.1, 125.0, 120.1, 117.2, 31.6, 22.6, 19.4; FT-IR (KBr) 3276 (—NH), 3178 (Sp2 C—H), 3076 (Sp2 C—H), 2965 (Sp3 C—H), 2852 (Sp3 C—H), 1674 (C⚌N), 1601 (C⚌C), 1546, 1519, 1457, 1401, 1358, 12632, 1071 cm−1. Anal. Calcd. for C17H18N2O: C, 76.66; H, 6.81; N, 10.52; O, 6.01. Found: C, 76.78; H, 6.78; N, 10.47.
2.3.4 5-Methyl-N-(2-nitrophenyl)benzo[d]oxazol-2-amine (3d)
White solid; yield 48%; mp 159–160 °C; 1H NMR (CDCl3, 400 MHz) 8.23–8.21 (m, 2H), 7.51–7.48 (m, 3H), 7.13 (d, J = 2 Hz, 1H), 7.04 (s, 1H), 2.33 (s, 3H); 13C NMR (CDCl3, 100 MHz) 162.8, 158.6, 147.1, 142.7, 135.8, 131.7, 129.0, 127.8, 126.9, 116.0, 112.7, 109.3, 23.4; FT-IR (KBr) 3178 (—NH), 3076 (Sp2 C—H), 2846 (Sp3 C—H), 1777 (C⚌N), 1574 (C⚌C), 1532 (NO2), 1497, 1485, 1357, 1262, 1121 cm−1. Anal. Calcd. for C14H11N3O3: C, 62.45; H, 4.12; N, 15.61; O, 17.83. Found: C, 62.56; H, 4.10; N, 15.56.
2.3.5 5-Methyl-N-m-tolylbenzo[d]oxazol-2-amine (3e)
White solid; yield 81%; mp 129–131 °C;1H NMR (CDCl3, 400 MHz) 8.25–8.21(m, 2H), 7.63–7.61 (d, J = 8 Hz, 1H), 7.51–7.48 (m, 2H), 7.37 (t, J = 0.8 Hz, 1H), 7.16–7.14 (dd, J = 8.4, 0.8 Hz, 1H), 2.49 (s, 3H), 2.14 (s, 3H); 13C NMR (CDCl3, 100 MHz) 162.7, 151.2, 140.06, 135.7, 131.4, 129.0, 127.6, 127.5, 126.0, 119.5, 114.9, 110.9, 24.5, 22.0; FT-IR (KBr) 3236 (—NH), 3123, 3076 (Sp2 C—H), 2924 (Sp3 C—H), 2845 (Sp3 C—H), 1614 (C⚌C), 1574, 1529, 1497, 1485, 1318, 1242, 1082 cm−1. Anal. Calcd. for C15H14N2O: C, 75.61; H, 5.92; N, 11.76; O, 6.71. Found: C, 75.74; H, 5.90; N, 11.70.
2.3.6 N-(3-Chlorophenyl)-5-methylbenzo[d]oxazol-2-amine (3f)
(Yadav et al., 2018): White solid; yield 77%; mp 145–146 °C; 1H NMR (CDCl3, 400 MHz) 8.18–8.16 (dd, J = 8 Hz, 1.2 Hz, 1H), 7.78–7.76 (m, 1H), 7.66–7.63 (m, 1H), 7.59–7.57 (m, 1H), 7.41–7.35 (m, 3H), 2.36 (s, 3H); 13C NMR (CDCl3, 100 MHz) 161.6, 150.9, 142.0, 134.6, 130.63, 129.2, 126.2, 125.7, 124.9, 123.27, 120.5, 110.9, 23.9; FT-IR (KBr) 3287 (—NH), 3182 (Sp2 C—H), 3054 (Sp2 C—H), 2943 (Sp3 C—H), 2832 (Sp3 C—H), 1643 (C⚌C), 1578, 1522, 1467, 1455, 1364, 1292, 1232, 1134, 1067 cm−1. Anal. Calcd. for C14H11ClN2O: C, 65.00; H, 4.29; Cl, 13.70; N, 10.83; O, 6.18. Found: C, 65.12; H, 4.28; N, 10.77.
2.3.7 5-Methyl-N-(3-nitrophenyl)benzo[d]oxazol-2-amine (3g)
White solid; yield 54%; mp 159–160 °C; 1H NMR (CDCl3, 400 MHz) 8.58–8.56 (dd, J = 7.6 Hz, 1.2 Hz, 1H), 8.38–8.36 (m, 1H), 7.81–7.79 (m, 1H), 7.74–7.70 (t, J = 8 Hz, 1H), 7.63–7.61 (m, 2H), 7.44–7.37 (m, 1H), 2.48 (s, 3H); 13C NMR (CDCl3, 100 MHz) 160.8, 151.1, 148.9, 141.9, 133.2, 130.3, 129.1, 126.3, 125.9, 125.3, 122.7, 120.7, 111.1, 21.9; FT-IR (KBr) 3297 (—NH), 3156, 3084 (Sp2 C—H), 2924 (Sp3 C—H), 2873 (Sp3 C—H), 1645 (C⚌C), 1545 (—NO2), 1501, 1457, 1389, 1334, 1265, 1156, 1082 cm−1. Anal. Calcd. for C14H11N3O3: C, 62.45; H, 4.12; N, 15.61; O, 17.83. Found: C, 62.69; H, 4.09; N, 15.55.
2.3.8 5-Methyl-N-p-tolylbenzo[d]oxazol-2-amine (3h)
White solid; yield 89%; mp 144–145 °C; 1H NMR (400 MHz, CDCl3) δ 7.63 (s, 1H), 7.45–7.32 (m, 4H), 7.12 (d, J = 8 Hz, 2H), 5.98 (br s, 1H), 2.46 (s, 3H), 2.29 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.3, 142.2, 135.9, 133.0, 130.8, 129.8, 128.7, 128.5, 123.2, 120.6, 117.8, 20.0, 19.6; FT-IR (KBr) 3256 (—NH), 3095 (Sp2 C—H), 2902 (Sp3 C—H), 2854 (Sp3 C—H), 1613 (C⚌C), 1574, 1514, 1315, 1234, 1120, 1094, 1017 cm−1. Anal. Calcd. for C15H14N2O: C, 75.61; H, 5.92; N, 11.76; O, 6.71. Found: C, 75.72; H, 5.90; N, 11.71.
2.3.9 N-(4-Methoxyphenyl)-5-methylbenzo[d]oxazol-2-amine (3i)
(Liu et al., 2013a,b): White solid; yield 91%; mp 131–132 °C; 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 8.4 Hz, 1H), 7.46–7.42 (m, 2H), 7.30 (s, 1H), 7.16–7.08 (m, 3H), 7.00 (br s, 1H), 3.87 (s, 3H), 2.26 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 154.7, 153.1, 142.9, 133.7, 132.6, 129.7, 129.5, 129.3, 126.1, 121.3, 114.9, 55.02, 20.56; FT-IR (KBr) 3345 (—NH), 3092 (Sp2 C—H), 2857 (Sp3 C—H), 1567 (C⚌C), 1535, 1506, 1321, 1271, 1235, 1182, 1123, 1074, 1033 cm−1. Anal. Calcd. for C15H14N2O2: C, 70.85; H, 5.55; N, 11.02; O, 12.58. Found: C, 70.97; H, 5.52; N, 10.96.
2.3.10 N-(4-Fluorophenyl)-5-methylbenzo[d]oxazol-2-amine (3j)
(Yadav et al., 2018): White solid; yield 70%; mp 147–148 °C; 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 1H), 7.49–7.34 (m, 4H), 7.00 (t, J = 8.4 Hz, 2H), 6.14 (br s, 1H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 158.2, 155.8, 152.1, 142.1, 134.7, 133.0, 128.5, 128.6, 128.4, 120.6, 119.2, 114.2, 114.0, 20.0; FT-IR (KBr) 3266 (—NH), 3105 (Sp2 C—H), 2904 (Sp3 C—H), 2812 (Sp3 C—H), 1622 (C⚌C), 1587, 1533, 1587, 1319, 1226, 1087, 1018 cm−1. Anal. Calcd. for C14H11FN2O: C, 69.41; H, 4.58; F, 7.84; N, 11.56; O, 6.60. Found: C, 69.55; H, 4.56; N, 11.50.
2.3.11 N-(4-Chlorophenyl)-5-methylbenzo[d]oxazol-2-amine (3k)
(Daswani et al., 2016): White solid; yield 71%; mp 137–138 °C; 1H NMR (400 MHz, CDCl3) δ 7.77 (s, 1H), 7.50 (t, J = 9.2 Hz, 2H), 7.26 (d, J = 7.6 Hz 2H), 7.16 (d, J = 8.8 Hz, 2H), 4.66 (br s, 1H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 151.1, 141.7, 136.9, 132.3, 128.3, 128.1, 127.7, 127.0, 124.7, 120.0, 118.1, 19.2; FT-IR (KBr) 3276 (—NH), 3076 (Sp2 C—H), 2958 (Sp3 C—H), 2150, 1637 (C⚌C), 1504, 1421, 1374, 1330, 1256, 1207, 1030 cm−1. Anal. Calcd. for C14H11ClN2O: C, 65.00; H, 4.29; Cl, 13.70; N, 10.83; O, 6.18. Found: C, 65.12; H, 4.28; N, 10.78.
2.3.12 4-(5-Methylbenzo[d]oxazol-2-ylamino)benzonitrile (3l)
White solid: yield 52%; mp 186–187 °C; 1H NMR (400 MHz, DMSO) δ 7.66 (d, J = 7.6 Hz, 2H), 7.46 (s, 1H), 7.39 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.8 Hz, 2H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.0, 143.5, 143.2, 133.8, 132.7, 129.4, 128.9, 121.2, 118.0, 117.4, 104.0, 20.7; FT-IR (KBr) 3269 (—NH), 3188 (Sp2 C—H), 2857 (Sp3 C—H), 2227 (—CN), 1602 (C⚌C), 1532, 1324, 1248, 1175, 1085 cm−1. Anal. Calcd. for C15H11N3O: C, 72.28; H, 4.45; N, 16.86; O, 6.42. Found: C, 72.41; H, 4.44; N, 16.81.
2.3.13 5-Methyl-N-(4-nitrophenyl)benzo[d]oxazol-2-amine (3m)
(Daswani et al., 2016): White solid: yield 50%; mp 186–187 °C; 1H NMR (400 MHz, DMSO) δ 8.92 (br s, 1H), 8.09–8.05 (m, 2H), 7.78–7.75 (m, 2H), 7.26 (s, 1H), 7.07–7.04 (m, 2H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.8, 146.3, 143.8, 141.8, 137.3, 129.6, 127.1, 125.6, 121.0, 120.7, 118.0, 21.5; FT-IR (KBr) 3314 (—NH), 3109 (Sp2 C—H), 2887 (Sp3 C—H), 1619 (C⚌C), 1509 (—NO2), 1330, 1250, 1112, 1088, 1025 cm−1. Anal. Calcd. for C14H11N3O3: C, 62.45; H, 4.12; N, 15.61; O, 17.83. Found: C, 62.57; H, 4.11; N, 15.54.
2.3.14 Methyl 4-(5-methylbenzo[d]oxazol-2-ylamino)benzoate (3n)
White solid; yield 52%; mp 159–160 °C; 1H NMR (400 MHz, CDCl3) δ 7.64 (s, 1H), 7.51 (d, J = 8.4 Hz, 1H), 6.82 (t, J = 6 Hz, 1H), 6.76–6.72 (m, 2H), 6.70 (d, J = 7.6 Hz, 1H), 6.61 (d, J = 8 Hz, 1H), 5.82 (br s,1H) 3.53 (s, 3H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ154.2, 145.8, 144.8, 141.4, 132.3, 131.2, 128.1, 119.9, 119.1, 106.5, 106.3, 99.3, 45.3, 19.2; FT-IR (KBr) 3360 (—NH), 3152 (Sp2 C—H), 2933 (Sp3 C—H), 2843 (Sp3 C—H), 1726 (C⚌O), 1622 (C⚌C), 1516, 1463, 1257, 1232, 1154, 1091, 1026 cm−1. Anal. Calcd. for C16H14N2O3: C, 68.07; H, 5.00; N, 9.92; O, 17.00. Found: C, 68.18; H, 4.99; N, 9.86.
2.3.15 N-(4-Ethylphenyl)-5-methylbenzo[d]oxazol-2-amine (3o)
White solid; yield 79%; mp 139–140 °C; 1H NMR (400 MHz, CDCl3) δ 7.63 (s, 1H), 7.42 (d, J = 8.4 Hz, 2H), 7.37–7.35 (m, 2H), 7.15 (d, J = 8.0 Hz, 2H), 6.00 (br s, 1H), 2.62–2.56 (q, 2H), 2.46 (s, 3H), 1.20 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 151.5, 141.5, 136.3, 135.8, 132.4, 128.3, 128.1, 126.4, 122.8, 120.0, 117.1, 26.3, 19.3, 14.3; FT-IR (KBr) 3250 (—NH), 3090 (Sp2 C—H), 2928 (Sp3 C—H), 1610 (C⚌C), 1574, 1496, 1449, 1309, 1246, 1125, 1096 cm−1. Anal. Calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10; O, 6.34. Found: C, 76.28; H, 6.38; N, 11.03.
2.3.16 N-(4-Isopropylphenyl)-5-methylbenzo[d]oxazol-2-amine (3p)
White solid; yield 76%; mp 144–145 °C; 1H NMR (400 MHz, CDCl3) δ 7.64 (s, 1H), 7.42 (d, J = 8.8 Hz, 2H), 7.37–7.32 (m, 2H), 7.18 (d, J = 8.4 Hz, 2H), 5.99 (br s, 1H), 2.88–2.83 (m, 1H), 2.46 (s, 3H), 1.20 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 151.7, 141.6, 141.2, 135. 9, 132.5, 128.4, 128.2, 128.1, 125.0, 120.1, 117.2, 31.6, 22.6, 19.4; FT-IR (KBr) 3251 (—NH), 3090 (Sp2 C—H), 2960 (Sp3 C—H), 2812 (Sp3 C—H), 1611 (C⚌C), 1574, 1496, 1447, 1307, 1243, 1126, 1097 cm−1. Anal. Calcd. for C17H18N2O: C, 76.66; H, 6.81; N, 10.52; O, 6.01. Found: C, 76.78; H, 6.80; N, 10.45.
2.3.17 5-Methyl-N-(2,4-dimethylphenyl)benzo[d]oxazol-2-amine (3q)
White solid; yield 84%; mp 138–139 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 6.4 Hz, 1H), 7.22 (s, 1H), 7.16 (d, J = 8 Hz, 1H), 7.05 (d, J = 8.0 Hz, 2H), 6.98 (s, 1H), 2.48 (s, 3H), 2.39 (s, 3H), 2.02 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 154.9, 143.4, 135.3, 134.8, 134.1, 133.2, 133.1, 131.4, 130.0, 129.9, 127.1, 125.5, 121.4, 21.0, 20.9, 18.1; FT-IR (KBr) 3276 (—NH), 3156 (Sp2 C—H), 2976 (Sp3 C—H), 2852 (Sp3 C—H), 2812, 1607 (C⚌C), 1573, 1495, 1447, 1244, 1122, 1088, 1070 cm−1. Anal. Calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10; O, 6.34. Found: C, 76.30; H, 6.36; N, 11.04.
2.3.18 5-Methyl-N-(2,5-dimethylphenyl)benzo[d]oxazol-2-amine (3r)
White solid; yield 81%; mp 120–121 °C; 1H NMR (400 MHz, CDCl3) δ 7.78 (s, 1H), 7.65 (s, 1H), 7.38–7.33 (m, 2H), 7.02 (d, J = 7.6 Hz, 1H), 6.84 (d, J = 7.2 Hz, 1H), 5.90 (br s, 1H), 2.47 (s, 3H), 2.34 (s, 3H), 2.11 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 154.2, 142.8, 136.7, 135.4, 133.7, 130.3, 129.6, 129.4, 129.3, 126.1, 125.2, 121.0, 20.6, 20.5, 17.4; FT-IR (KBr) 3282 (—NH), 3154 (Sp2 C—H), 2921 (Sp3 C—H), 2812 (Sp3 C—H), 1587 (C⚌C), 1527, 1492, 1462, 1381, 1306, 1263, 1088 cm−1. Anal. Calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10; O, 6.34. Found: C, 76.28; H, 6.37; N, 11.05.
2.3.19 5-Methyl-N-(2,6-dimethylphenyl)benzo[d]oxazol-2-amine (3s)
(Liu et al., 2013a,b): White solid; yield 77%; mp 147–148 °C; 1H NMR (400 MHz, CDCl3) δ 7.63 (s, 1H), 7.32 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 8.0 Hz, 1H), 5.96 (br s, 1H), 2.46 (s, 3H), 2.28 (s, 6H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 153.4, 152.3, 143.5, 139.8, 138.5, 134.4, 130.1, 129.9, 124.5, 122.1, 116.7, 21.8, 21.4; FT-IR (KBr) 3267 (—NH), 3104 (Sp2 C—H), 2920 (Sp3 C—H), 2842 (Sp3 C—H), 1624 (C⚌C), 1587, 1540, 1501, 1326, 1175, 1126, 1088 cm−1. Anal. Calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10; O, 6.34. Found: C, 76.29; H, 6.36; N, 11.04.
2.3.20 5-Methyl-N-(3,4-dimethylphenyl)benzo[d]oxazol-2-amine (3t)
White solid; yield 84%; mp 120–121 °C; 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 1H), 7.30–7.22 (m, 4H), 7.01 (d, J = 8.0 Hz, 1H), 6.59 (br s, 1H), 2.41 (s, 3H), 2.19 (s, 3H), 2.15 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 151.4, 141.5, 135.7, 134.8, 132.2, 128.3, 128.0, 127.8, 119.9, 118.0, 114.2, 19.0, 18.1, 17.1; FT-IR (KBr) 3275 (—NH), 3057 (Sp2 C—H), 2923 (Sp3 C—H), 2856 (Sp3 C—H), 1574 (C⚌C), 1533, 1498, 1455, 1375, 1315, 1254, 1218, 1168, 1115, 1092, 1020 cm−1. Anal. Calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10; O, 6.34. Found: C, 76.28; H, 6.38; N, 11.05.
2.3.21 5-Methyl-N-(3,5-dimethylphenyl)benzo[d]oxazol-2-amine (3u)
White solid; yield 89%; mp 132–133 °C; 1H NMR (400 MHz, CDCl3) δ 7.64 (s, 1H), 7.35 (d, J = 7.6 Hz, 2H), 7.15 (s, 2H), 6.70 (s, 1H), 5.93 (br s, 1H), 2.46 (s, 3H), 2.29 (s, 6H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.9, 142.9, 139.1, 138.0, 133.8, 129.5, 129.4, 129.3, 123.9, 121.4, 116.1, 21.2, 20.7; FT-IR (KBr) 3267 (—NH), 3104 (Sp2 C—H), 2922 (Sp3 C—H), 2853 (Sp3 C—H), 1624 (C⚌C), 1587, 1540, 1502, 1327, 1175, 1126, 1088 cm−1. Anal. Calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10; O, 6.34. Found: C, 76.27; H, 6.38; N, 11.06.
2.3.22 5-Methyl-N-phenylbenzo[d]oxazol-2-amine (3v)
(Yadav et. al., 2018): White solid; yield 88%; mp 146–147 °C; 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 1H), 7.52 (d, J = 8 Hz, 2H), 7.36–7.28 (m, 4H), 7.03 (t, J = 14.8 Hz, 1H), 6.55 (br s, 1H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.7,142.4, 138.7, 133.2, 128.8, 128.7, 128.0, 121.6, 120.8, 117.7, 20.2; FT-IR (KBr) 3285 (—NH), 3056 (Sp2 C—H), 2952 (Sp3 C—H), 2849 (Sp3 C—H), 1603 (C⚌C), 1574, 1534, 1497, 1456, 1321, 1234, 1121, 1085 cm−1. Anal. Calcd. for C14H12N2O: C, 74.98; H, 5.39; N, 12.49; O, 7.13. Found: C, 75.10; H, 5.37; N, 12.43.
2.3.23 5-Methoxy-N-phenylbenzo[d]oxazol-2-amine (3w)
White solid: yield 90%; mp 150–151 °C; 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 7.6 Hz, 1H), 7.54–7.47 (m, 3H), 7.40 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 6.02 (br s, 1H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 155.0, 153.0, 133.4, 132.1, 131.9, 129.7, 128.5, 121.7, 120.2, 120.4, 114.6, 113.6, 55.0; FT-IR (KBr) 3281 (—NH), 3057 (Sp2 C—H), 2961 (Sp3 C—H), 2835 (Sp3 C—H), 1616 (C⚌C), 1513, 1498, 1431, 1331, 1301, 1292, 1253, 1183, 1035 cm−1. Anal. Calcd. for C14H12N2O2: C, 69.99; H, 5.03; N, 11.66; O, 13.32. Found: C, 70.10; H, 5.01; N, 11.60.
2.3.24 5-Chloro-N-phenylbenzo[d]oxazol-2-amine (3x)
White solid; yield 81%; mp 171–172 °C; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 2.0 Hz, 1H), 7.47–7.44 (m, 2H), 7.40–7.37 (m, 2H), 7.10 (d, J = 9.2 Hz, 1H), 6.94 (br s, 1H), 6.81 (dd, J = 7.2, 2.4 Hz, 2H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 153.9, 152.1, 135.8, 131.9, 131.2, 130.1, 129.9, 127.9, 121.8, 119.2, 112.7; FT-IR (KBr) 3275 (—NH), 3085 (Sp2 C—H), 1607 (C⚌C), 1579, 1486, 1302, 1233, 1179, 1085, 1036 cm−1. Anal. Calcd. for C13H9ClN2O: C, 63.81; H, 3.71; Cl, 14.49; N, 11.45; O, 6.54. Found: C, 63.95; H, 3.68; N, 11.39.
2.3.25 5-Fluoro-N-phenylbenzo[d]oxazol-2-amine (3y)
Thick liquid; yield 70%; 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 4.8 Hz, 1H), 7.29–7.20 (m, 3H), 7.10 (s, 1H), 7.03 (d, J = 6.0 Hz, 3H), 5.80 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 160.8, 147.0, 136.4, 128.9, 127.1, 125.3, 123.7, 119.2, 116.1, 110.2; FT-IR (KBr) 3268 (—NH), 3157 (Sp2 C—H), 3072 (Sp2 C—H), 1633 (C⚌C), 1582, 1501, 1476, 1416, 1277, 1151, 1100, 1038 cm−1. Anal. Calcd. for C13H9FN2O: C, 68.42; H, 3.97; F, 8.32; N, 12.27; O, 7.01. Found: C, 68.55; H, 3.95; N, 12.22.
2.3.26 2-(Phenylamino)benzo[d]oxazole-5-carbonitrile (3z)
White solid; yield 59%; mp 129–130 °C; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.4 Hz, 2H), 7.25–7.22 (m, 2H), 6.98 (d, J = 8.0 Hz, 2H), 6.88 (s, 1H), 6.61 (br s, 1H) ; 13C NMR (100 MHz, CDCl3) δ 147.6, 139.8, 137.6, 135.0, 133.0, 128.0, 126.7, 124.1, 123.1, 119.3, 109.9; FT-IR (KBr) 3278 (—NH), 3177 (Sp2 C—H), 3058 (Sp2 C—H), 1633 (C⚌C), 1545, 1499, 1422, 1377, 1292, 1195, 1114, 1023 cm−1. Anal. Calcd. for C14H9N3O: C, 71.48; H, 3.86; N, 17.86; O, 6.80. Found: C, 71.60; H, 3.84; N, 17.80.
2.3.27 7-Nitro-N-phenylbenzo[d]oxazol-2-amine (3aa)
(Daswani et al., 2016): White solid; yield 55%; mp 171–172 °C; 1H NMR (CDCl3, 400 MHz) 8.26–8.24 (m, 3H), 7.56–7.49 (m, 5H); 13C NMR (CDCl3, 100 MHz) 164.3, 150.8, 140.8, 132.4, 131.2, 129.1, 128.3, 128.2, 126.3, 113.0, 110.7; FT-IR (KBr) 3275 (—NH), 3085 (Sp2 C—H), 1607 (C⚌C), 1579 (—NO2), 1486, 1302, 1233, 1179, 1085, 1036 cm−1. Anal. Calcd. for C13H9N3O3: C, 61.18; H, 3.55; N, 16.46; O, 18.81. Found: C, 61.30; H, 3.54; N, 16.41.
2.3.28 5,7-dimethyl-N-phenylbenzo[d]oxazol-2-amine (3ab)
White solid; yield 80%; mp 167–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.46–7.42 (m, 3H), 7.19 (s, 1H), 6.87 (d, J = 7.2 Hz, 2H), 5.8 (br s, 1H), 2.42(s, 3H), 2.10(s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 152.5, 152.4, 131.8, 130.7, 130.3, 127.6, 121.8, 119.9, 113.2, 20.2, 17.3; FT-IR (KBr) 3289 (—NH), 3076 (Sp2 C—H), 2957 (Sp3 C—H), 1607 (C⚌C), 1575, 1516, 1301, 1261, 1235, 1182, 1113, 1094, 1033 cm−1. Anal. Calcd. for C15H14N2O: C, 75.61; H, 5.92; N, 11.76; O, 6.71. Found: C, 75.74; H, 5.90; N, 11.70.
2.3.29 5,6-Dimethyl-N-phenylbenzo[d]oxazol-2-amine (3ac)
White solid; yield 82%; mp 148–149 °C; 1H NMR (400 MHz, CDCl3) δ 7.76 (s, 1H), 7.57 (s, 1H), 7.50 (d, J = 7.6 Hz, 2H), 6.85 (d, J = 8.8 Hz, 3H), 2.37 (s, 3H), 2.31 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO‑d6) δ 153.3, 151.6, 140.3, 136.3, 132.3, 131.2, 129.1, 127.8, 118.6, 116.6, 112.3, 17.6, 17.3; FT-IR (KBr) 3306 (—NH), 3122 (Sp2 C—H), 2920 (Sp3 C—H), 2847 (Sp3 C—H), 1603 (C⚌C), 1515, 1444, 1302, 1236, 1302, 1236, 1191, 1115, 1095, 1020 cm−1. Anal. Calcd. for C15H14N2O: C, 75.61; H, 5.92; N, 11.76; O, 6.71. Found: C, 75.75; H, 5.90; N, 11.70.
2.4 Experimental procedure for the synthesis of 2-phenyl-N-p-tolyl-2H-tetrazol-5-amine
At room temperature Et3N (1 mmol, 101 mg) and Cu(OAc)2·H2O (50 mol %, 119 mg) were added in slowly to a stirred solution of thiourea (1 mmol, 76 mg) in DMSO (2–3 mL). Then the entire reaction mixture was stirred at room temperature for one hour until get the black color and the reaction progress was monitored by thin layer chromatography. After confirming that the reaction was completed (monitored by TLC), to this, PhN3 (2 mmol, 238 mg) was added and the total reaction mixture was stirred for 2 h. Later, 4-iodotoulene (1 mmol, 218 mg), Cs2CO3 (1.5 mmol, 487.5 mg), Cu(OAc)2·H2O (10 mol %, 19.9 mg) and 1,10-phenanthroline (20 mol %, 36 mg) were added consecutively for several minutes and the reaction mixture was stirred for 14 h at 115 °C. The progress of the reaction was investigated by TLC (5% ethylacetate in hexane). After completion of the reaction, the reaction mixture was transferred into centrifuged tubes and the mixture was centrifuged for 10 min by using centrifugation machine. Black colour solid was settled in the bottom of centrifuged tubes. The clear solution was concentrated by using rotary evaporator and the crude mixture was purified by silica gel (60–120 mesh) column chromatography using 30% ethylacetate in hexane as eluent to obtain 2-phenyl-N-p-tolyl-2H-tetrazol-5-amine 4a as a white solid.
2.4.1 2-Phenyl-N-p-tolyl-2H-tetrazol-5-amine (4a)
White solid; yield 87%; mp 104–105 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.6 Hz, 2H), 7.54 (d, J = 7.2 Hz, 2H), 7.42 (d, J = 7.6 Hz, 2H), 6.75 (m, 3H), 5.34(br s, 1H),2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 146.8, 137.5, 135.6, 134.4, 132.8, 129.7, 128.8, 126.5, 124.5, 117.9, 110.1, 24.8; FT-IR (KBr) 3253 (—NH), 3153 (Sp2 C—H), 2919 (Sp3 C—H), 2852 (Sp3 C—H), 1572 (C⚌C), 1486, 1455, 1408, 1384, 1284, 1260, 1100, 1017 cm−1. Anal. Calcd. for C14H13N5: C, 66.92; H, 5.21; N, 27.87. Found: C, 67.00; H, 5.19; N, 27.81.
2.4.2 N-(4-Methoxyphenyl)-2-phenyl-2H-tetrazol-5-amine (4b)
White solid; yield 90%; mp 111–112 °C; 1H NMR (400 MHz, CDCl3) δ 7.25–7.22 (m, 4H), 6.98 (d, J = 8.0 Hz, 2H), 6.88–6.61 (m, 3H), 3.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 154.7, 142.9, 133.7, 132.6, 129.7, 129.5, 129.3, 126.1, 121.4, 55.0; FT-IR (KBr) 3154 (Sp2 C—H), 2922 (Sp3 C—H), 2862 (Sp3 C—H), 1607 (C⚌C), 1581, 1948, 1453, 1410, 1389, 1388, 1268, 1155, 1018 cm−1. Anal. Calcd. for C14H13N5O: C, 62.91; H, 4.90; N, 26.20; O, 5.99. Found: C, 63.04; H, 4.88; N, 26.15.
2.4.3 N-(4-Chlorophenyl)-2-phenyl-2H-tetrazol-5-amine (4c)
White solid; yield 78%; mp 96–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.77–7.48 (m, 4H), 7.25 (d, J = 6.8 Hz, 3H), 7.16 (d, J = 8.8 Hz, 2H), 5.96 (br s, 1NH); 13C NMR (100 MHz, CDCl3) δ 141.7, 137.0, 132.3, 128.3, 128.1, 127.8, 127.0, 124.7, 118.1; FT-IR (KBr) 3209 (—NH), 3089 (Sp2 C—H), 1666 (C⚌C), 1577, 1513, 1476, 1416, 1297, 1207, 1155, 1021 cm−1. Anal. Calcd. for C13H10ClN5: C, 57.47; H, 3.71; Cl, 13.05; N, 25.78. Found: C, 57.60; H, 3.69; N, 25.71.
2.4.4 N-(4-Fluorophenyl)-2-phenyl-2H-tetrazol-5-amine (4d)
White solid; yield 72%; mp 99–100 °C; 1H NMR (400 MHz, CDCl3) δ 7.64–7.33 (m, 7H), 7.12 (d, J = 8 Hz, 2H), 5.98 (br s, 1H, 1NH); 13C NMR (100 MHz, CDCl3) δ 152.0, 142.1, 134.7, 133.0, 128.6, 128.5, 128.4, 120.6, 119.3, 119.2, 114.3,114.0; FT-IR (KBr) 3278 (—NH), 3177 (Sp2 C—H), 3058 (Sp2 C—H), 1633 (C⚌C), 1545, 1499, 1422, 1377, 1292, 1195, 1114, 1023 cm−1. Anal. Calcd. for C13H10FN5: C, 61.17; H, 3.95; F, 7.44; N, 27.44. Found: C, 61.30; H, 3.93; N, 27.38.
2.4.5 4-(2-Phenyl-2H-tetrazol-5-ylamino)benzonitrile (4e)
White solid; yield 56%; mp 116–117 °C; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.4 Hz, 2H), 7.25–7.22 (m, 3H), 6.98 (d, J = 8.0 Hz, 2H), 6.88 (m, 2H), 6.61 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 147.6, 139.8, 137.6, 135.0, 133.0, 128.0, 126.7, 124.1, 123.1, 119.3, 109.9; FT-IR (KBr) 3278 (—NH), 3177 (Sp2 C—H), 3058 (Sp2 C—H), 1633 (C⚌C), 1545, 1499, 1422, 1377, 1292, 1195, 1114, 1023 cm−1. Anal. Calcd. for C14H10N6: C, 64.11; H, 3.84; N, 32.04. Found: C, 64.20; H, 3.81; N, 31.98.
2.4.6 N-(4-Nitrophenyl)-2-phenyl-2H-tetrazol-5-amine (4f)
White solid; yield 56%; mp 126–128 °C; 1H NMR (400 MHz, CDCl3) δ 8.09–8.05 (m, 2H), 7.78–7.76 (m, 2H), 7.74 (d, J = 8.0 Hz, 2H), 7.26–7.05 (m, 3H), 6.61 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 152.8, 146.3, 143.8, 141.8, 137.3, 127.1, 125.6, 121.0, 120.7; FT-IR (KBr) 3278 (—NH), 3177 (Sp2 C—H), 3058 (Sp2 C—H), 2958 (Sp3 C—H), 2863 (Sp3 C—H), 1633 (C⚌C), 1545, 1499, 1422, 1377, 1292, 1195, 1114, 1023 cm−1. Anal. Calcd. for C13H10N6O2: C, 55.32; H, 3.57; N, 29.77; O, 11.34. Found: C, 55.44; H, 3.55; N, 29.70.
2.4.7 N-(3,4-Dimethylphenyl)-2-phenyl-2H-tetrazol-5-amine (4g)
White solid; yield 82%; mp 95–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.4 Hz, 1H), 7.29 (d, J = 8 Hz, 2H), 7.15–7.07 (m, 4H), 6.96 (s, 1H), 2.40 (s, 3H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ146.9, 139.5, 138.3, 137.7, 135.8, 132.6, 131.9, 130.7, 127.4, 124.3, 123.8, 117.8, 110.1, 21.7, 20.0; FT-IR (KBr) 3253 (—NH), 3172 (Sp2 C—H), 2920 (Sp3 C—H), 2823 (Sp3 C—H), 1644 (C⚌C), 1436, 1407, 1384, 1261, 1206, 1172, 1012 cm−1. Anal. Calcd. for C15H15N5: C, 67.90; H, 5.70; N, 26.40. Found: C, 67.99; H, 5.68; N, 26.33.
2.4.8 N-(2,4-Dimethylphenyl)-2-phenyl-2H-tetrazol-5-amine (4h)
White solid; yield 74%; mp 96–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.68–7.27 (m, 5H), 7.09 (s, 1H), 6.84 (d, J = 7.2 Hz, 2H), 5.90 (br s, 1H, 1NH), 2.47 (s, 3H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 143.4, 135.3, 134.8, 134.1, 133.2, 131.4, 130.0, 129.9, 127.1, 125.5, 121.4, 21.0, 20.9; FT-IR (KBr) 3267 (—NH), 3098 (Sp2 C—H), 2912 (Sp3 C—H), 2842 (Sp3 C—H), 1651 (C⚌C), 1579, 1523, 1496, 1423, 1345, 1277, 1178, 1025 cm−1. Anal. Calcd. for C15H15N5: C, 67.90; H, 5.70; N, 26.40. Found: C, 67.98; H, 5.68; N, 26.34.
2.4.9 N-Phenyl-2-p-tolyl-2H-tetrazol-5-amine (4i)
White solid; yield 92%; mp 97–98 °C; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 8.4 Hz, 2H), 7.31–7.26 (m, 3H), 7.17-7-15 (m, 2H), 7.02–6.94 (m, 2H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 147.2, 139.7, 135.7, 134.9, 132.4, 129.0, 128.1, 126.9, 124.1, 118.0, 109.6, 21.9; FT-IR (KBr) 3294 (—NH), 3153 (Sp2 C—H), 2919 (Sp3 C—H), 1612 (C⚌C), 1572, 1486, 1455, 1408, 1384, 1284, 1260, 1100, 1017 cm−1. Anal. Calcd. for C14H13N5: C, 66.92; H, 5.21; N, 27.87. Found: C, 66.99; H, 5.19; N, 27.82.
2.4.10 2-(4-Methoxyphenyl)-N-phenyl-2H-tetrazol-5-amine (4j)
White solid; yield 95%; mp 101–102 °C; 1H NMR (400 MHz, CDCl3) δ 7.25–7.22 (m, 5H), 6.98 (d, J = 8.0 Hz, 2H), 6.88 (s, 1H), 6.61 (br s, 1H), 3.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.7, 147.6, 144.1, 139.3, 135.6, 127.9, 127.3, 122.4, 118.0, 114.5, 107.7, 55.9; FT-IR (KBr) 3283 (—NH), 3133 (Sp2 C—H), 2950 (Sp3 C—H), 2839 (Sp3 C—H), 1630 (C⚌C), 1516, 1484, 1422, 1408, 1329, 1276, 1243, 1204, 1147, 1025 cm−1. Anal. Calcd. for C14H13N5O: C, 62.91; H, 4.90; N, 26.20; O, 5.99. Found: C, 63.02; H, 4.88; N, 26.14.
2.4.11 2-(4-Chlorophenyl)-N-phenyl-2H-tetrazol-5-amine (4k)
White solid; yield 72%; mp 96–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 4.8 Hz, 2H), 7.29–7.10 (m, 5H), 7.03 (d, J = 6.0 Hz, 2H), 5.80 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 160.8, 147.0, 136.4, 128.9, 127.1, 125.3, 123.7, 119.2, 116.1; FT-IR (KBr) 3268 (—NH), 3157 (Sp2 C—H), 3072 (Sp3 C—H), 1633 (C⚌C), 1582, 1501, 1476, 1416, 1277, 1151, 1100, 1038 cm−1. Anal. Calcd. for C13H10ClN5: C, 57.47; H, 3.71; Cl, 13.05; N, 25.78. Found: C, 57.60; H, 3.69; N, 25.72.
3 Results and discussion
3.1 Synthesis of benzoxazoles
The total scheme utilized for the synthesis of 2-aminophenylbenzoxazole was shown in below Scheme 1. Initially, thiourea on copper promoted desulfurization and consecutive nucleophilic substitution with 2-iodophenol produced the intermediate 2-iodophenylisourea. Further, the obtained 2-iodophenylisourea undergo domino intra and inter molecular C-N cross coupling reactions respectively with iodobenzene in presence of copper catalyst to afford the target product 2-aminophenyl-benzoxazole (3a) under optimized reaction conditions.![Path way for the construction of N-phenylbenzo[d]oxazol-2-amine from thiourea.](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.09.001-fig2.png)
Path way for the construction of N-phenylbenzo[d]oxazol-2-amine from thiourea.
The authors initial efforts focused on finding the optimization conditions for the synthesis of 2-iodophenyl isourea based on thiourea and 2-iodophenol as a model substrates with various solvents and copper sources. As shown in Table 1, among the tested solvents, non-polar solvents like n-hexane and n-heptane couldn’t provide the target product (Table 1, entries 3–4). Other solvents such as EtOH, EtOAc, DMSO and DMF gave the final product in complete conversion (Table 1, entries 1–2 and 6–7). Very unfortunately, only a moderate yield of target product is obtained by performing the reaction in the presence of water (Table 1, entry 5). n.d. = not detected.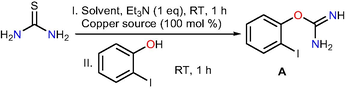
Entry
Solvent
Copper source
Conversion (%)b
A
1
EtOH
CuI
100
2
EtOAc
CuI
100
3
n-Hexane
CuI
n.d
4
n-Heptane
CuI
n.d
5
H2O
CuI
43
6
DMF
CuI
100
7
DMSO
CuI
100
8
DMSO
CuCl
100
9
DMSO
CuBr
100
10
DMSO
Cu2O
100
11
DMSO
CuSO4·5H2O
100
12
DMSO
Cu(OAc)2·H2O
100
13c
DMSO
Cu(OAc)2·H2O
100
14d
DMSO
Cu(OAc)2·H2O
54
15
DMSO
–
n.d
Various copper sources were tested and the results revealed that the reaction using both Cu (I) and Cu(II) sources can afford the product A in total conversion (Table 1, entries 8–12). The reaction by loading the lower quantity of catalyst 50 mol % and 25 mol % produced the final product in 100% and 54% conversion, respectively (Table 1, entries 13–14). Control experiment without loading the catalyst did not provide target product indicated that the use of metal source is essential (Table 1, entry 15).
Next, the authors performed the optimization reaction condition with diverse bases, copper sources and ligands towards the synthesis of 2-aminophenyl benzoxazole using 2-iodophenyl isourea and iodobenzene as model substrates. We were delighted to observe that the reaction may well provide the target product 3a in total conversion with 10 mol % copper catalyst, 20 mol % 1,10-phenanthroline (ligand) and 1.5 equiv. Cs2CO3(base) at 110 °C temperature in the presence of solvent DMSO (Table 2, entry 8). Firstly, the optimization was started with a set of ligands like L1-L5, among the used set of ligands, L4 (Table 2, entry 4) was found to be the most effective in comparison to L1-L3 and L5 (Table 2, entries 1–3 and 5). Of the bases tested, the reaction employing Cs2CO3 showed superior reactivity (Table 2, entry 8) compared to that of K3PO4·3H2O, K2CO3 and KOH. n.d. = not detected.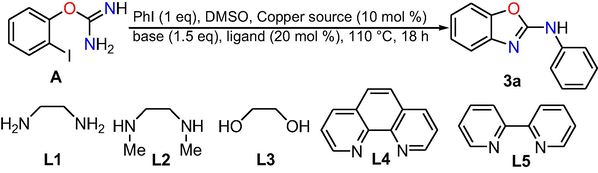
Entry
Copper source
Base
Ligand
Conversion(%)b
3a
1
CuI
K3PO4·3H2O
L1
57
2
CuI
K3PO4·3H2O
L2
60
3
CuI
K3PO4·3H2O
L3
52
4
CuI
K3PO4·3H2O
L4
78
5
CuI
K3PO4·3H2O
L5
73
6
CuI
KOH
L4
71
7
CuI
K2CO3
L4
61
8
CuI
Cs2CO3
L4
100
9
CuBr
Cs2CO3
L4
100
10
CuCl
Cs2CO3
L4
100
11
CuSO4·5H2O
Cs2CO3
L4
100
12
Cu(OAc)2·H2O
Cs2CO3
L4
100
13c
Cu(OAc)2·H2O
Cs2CO3
L4
45e
14d
Cu(OAc)2·H2O
Cs2CO3
L4
57e
15
Cu(OAc)2·H2O
Cs2CO3
–
16
16
–
Cs2CO3
–
n.d
17
CoCl2·H2O
Cs2CO3
L4
42
18
FeCl3·H2O
Cs2CO3
L4
35
19
NiCl2
Cs2CO3
L4
n.d
Further, various copper sources were examined and the results clearly suggested that the catalytic activity of both copper (I) and (II) sources (CuI, CuBr, CuCl, Cu(OAc)2·H2O and CuSO4·5H2O) was similar (Table 2, entries 8–12). Next, by lowering the amount of base (1.0 equiv) or the copper source (5 mol %) led to the N-arylation to afford target product in less conversion (Table 2, entries 13–14). In addition, the reaction provided target product in less conversion in the absence of ligand (Table 2, entry 15). Control experiments suggested that the use of metal source and ligand was essential to afford the final product. It is noteworthy that the formation of final product was not observed in reactions without loading the copper source and ligand (Table 2, entry 16). Furthermore, the reaction was examined in the presence of other metal sources; however, their catalytic activity is not more effective than copper sources (Table 2, entries 17–19).
Soon after having the optimized the reaction conditions, the authors further examined the scope and limits of the method with a series of substituted iodobenzenes (Table 3) and also with divergent 2-iodoaryl isoureas (Table 4).
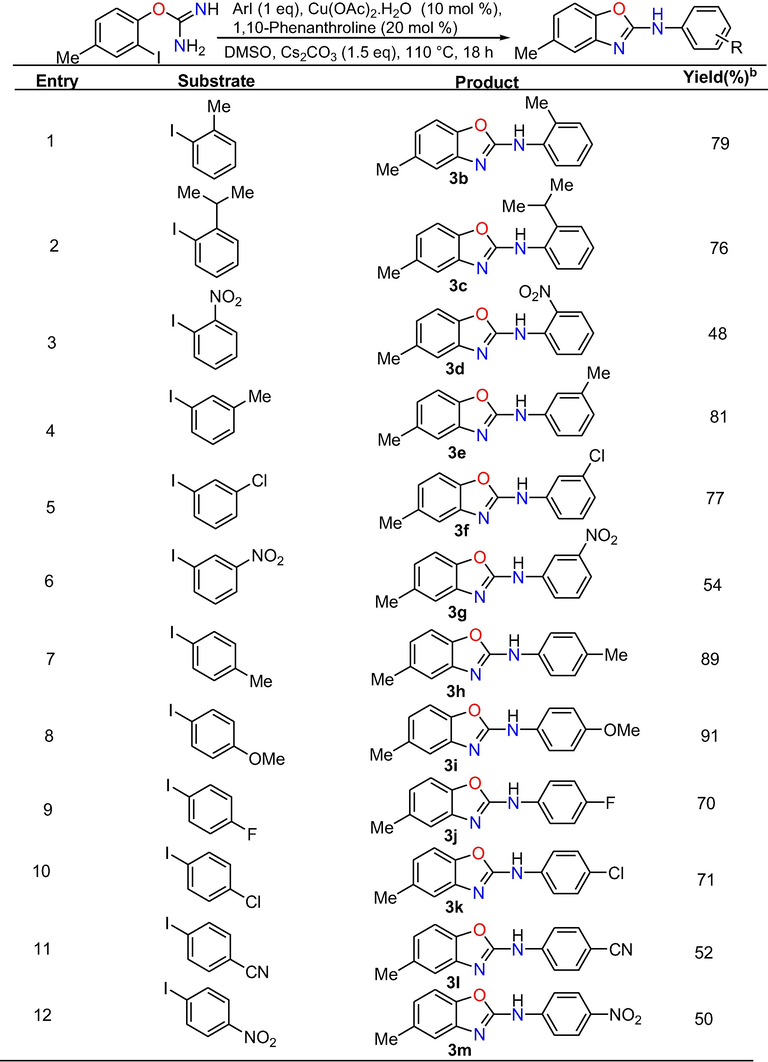
As summarized in below Table 3, 4-me-2-iodophenyl isourea readily underwent the reaction with various mono substituted phenyl iodides bearing substitutions like 2-Methyl, 2-isopropyl, 2-nitro, 3-Methyl, 3-chloro, 3-nitro, 4-methyl, 4-methoxy, 4-floro, 4-chloro, 4-cyano, 4-nitro, 4-COOCH3, 4-Ethyl and 4-isopropyl to obtain their corresponding target products 3b-p in 48–91% yields. Similarly, iodobenzene with disubstituted groups such as 2,4-dimethyl, 2,5-dimethyl, 2,6-dimethyl, 3,4-dimethyl and 3,5-dimethyl carried out the reaction with 4-methyl-2-iodophenyl isourea under optimized reaction conditions to afford their corresponding target products 3q-u in 84–89% yields.
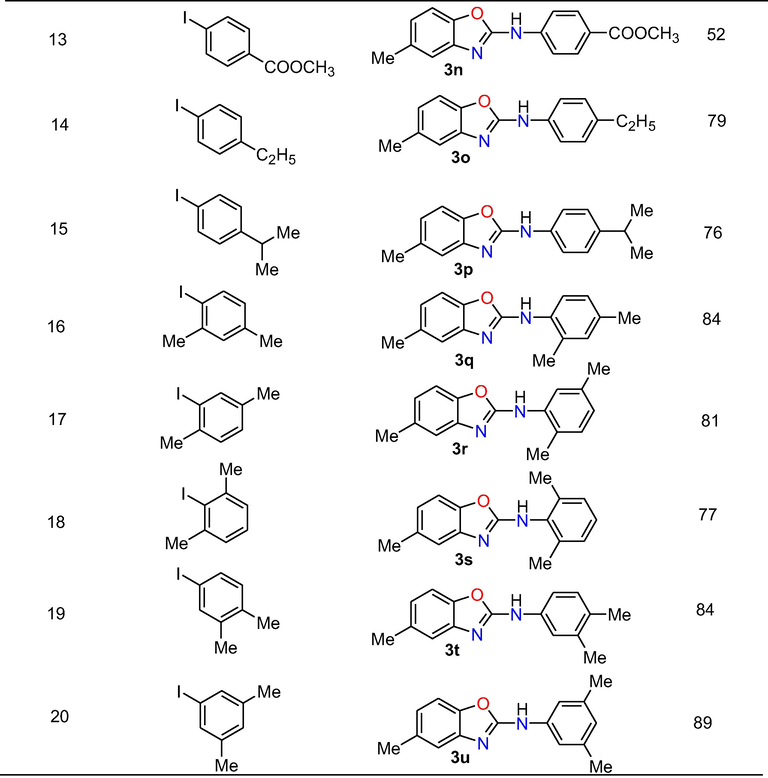
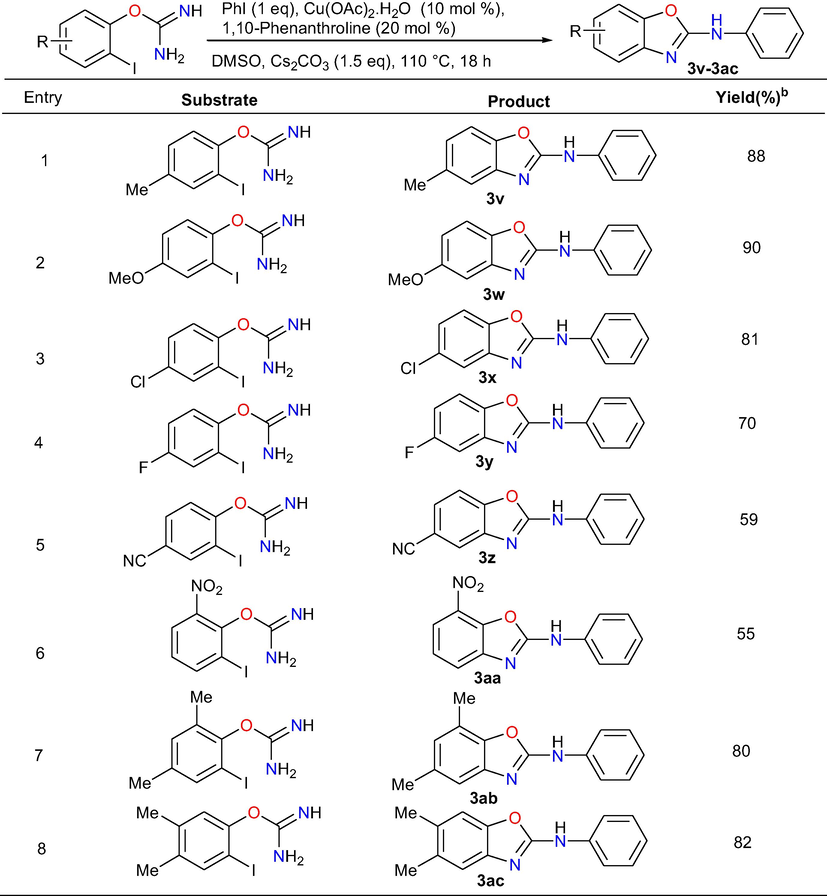
Moreover, we have also examined the applicability of the methodology by the reaction between various substituted iodophenyl isoureas and iodobenzene (Table 4). The reaction of iodophenyl isourea with electron donating groups such as 4-methyl and 4-methoxy with iodobenzene gave their respective cross-coupled products 3v and 3w in 88% and 90% yields, respectively. Similarly, 2-iodophenyl isourea holding electron withdrawing substituent’s like 4-chloro, 4-floro, 4-cyano and 2-nitro underwent the reaction with iodobenzene to produce their consecutive target products 3x, 3y, 3z and 3aa in 81%, 70%, 59% and 55% yields.
Phenyl ring having strong electron withdrawing substituent’s offered less reactivity compared to phenyl ring with weak electron withdrawing substituents. It might be occurred due to strong electron withdrawing capacity on reactive site. Moreover, final products 3ab and 3ac were obtained in 80% and 82% yield under standardized conditions by the domino cross-coupling reaction of disubstituted 2-iodophenyl isourea holding substitutions like 2,4-dimethyl and 3,4-dimethyl with iodobenzene.
In summary, by using this efficient method, a total of 29 2-arylaminobenzoxazoles in good yields were prepared and out of which nineteen compounds were new and the remaining known compounds were confirmed by comparision of their spectral data with literature data. Further, the outcomes of the aforementioned studies undoubtedly confirm that this method is well-suited for the substrates possessing both electron donating and withdrawing groups to provide the 2-aminobenzoxazoles derivatives in moderate to excellent yield.
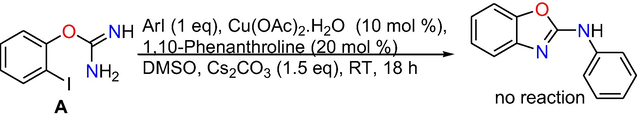
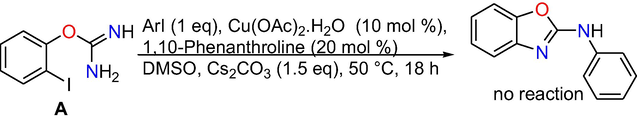
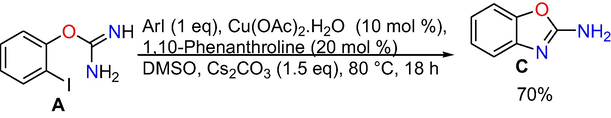
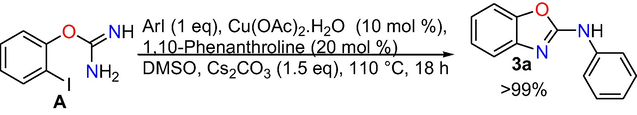
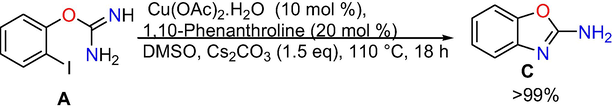
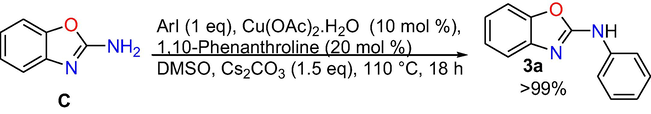
Control experiments: At the outset, control experiments were conducted to reveal the mechanism. When we have done the reaction at room temperature and at 50 °C, formation of target product is not observed (Eqs. (1) and (2)), however, the intra molecular C-N cyclized product 2-amino benzoxazole C was formed exclusively in 70% conversion performing the reaction at 80 °C (Eq. (3)), whereas when the reaction is done at 110 °C the target product 3a was produced in absolute conversion (Eq. (4)). In addition, the reaction was also performed in the absence of aryl iodide and it gave intra molecular C-N cyclised product 2-aminobenzoxazole C (Eq. (5)) under optimized reaction conditions. In addition, under optimized reaction conditions the reaction of 2-aminobenzoxazole with iodobenzene proceeded readily to afford target product 3a in whole conversion (Eq. (6)). The outcomes of the above control experiments obviously suggest that, 2-amino benzoxazole C was obtained first from 2-iodophenyl isourea A as intra molecular C-N cyclized product, next that may undergo inter molecular C-N cross-coupling reaction with iodo benzene to afford the final product 3a.
Based on the above observations and from previous literature reports, the possible mechanistic route towards the formation of substituted 2-aminophenyl benzoxazole from thiourea is shown in below Scheme 2. As we shown in mechanism path, intermediate R may be afforded from co-ordination of copper with thiourea and removal of proton using base.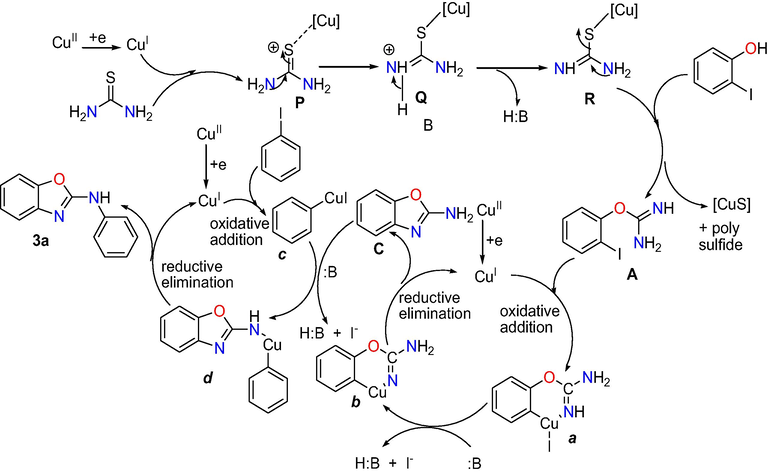
Proposed mechanism for the formation of 2-phenylamino benzoxazole.
During this way intermediates P and Q may be obtained. 2-Iodophenol reacts with intermediate R to provide 2-iodophenyl isourea A along with by-products CuS and poly sulphide (Ramana and Punniyamurthy, 2012; Usharani et al., 2017) via desulphurization/substitution (Ali et al., 2010; Sahoo et al., 2010; Guin et al., 2012a, 2012b; Mohan et al., 2016; S N Murthy et al., 2018c). According to literature reports (Bowmaker et al., 2009; Ramana et al., 2010), copper (II) species may reduce with isourea to give copper (I) species that may undergo oxidative addition with A to provide b via the formation copper (III) complex a using base. The copper (III) complex b may give intramolecular C-N cyclised product C via reductive elimination. On the other hand iodobenzene can undergo oxidative addition with copper (I) species to obtain intermediate c, that may react with intermediate product C to produce resulting inter molecular C-N cross-coupled (Deng et al., 2009; Lv and Bao, 2009; Cahiez and Moyeux, 2010; Chiba et al., 2010; Saha et al., 2010; Hu et al., 2011; Zhao et al., 2011; Wang et al., 2012; Tan and Teo, 2014) product 2- aminophenyl benzoxazole 3a via copper (III) complex d by reductive elimination.
3.2. Synthesis of 2,5-disubstituted tetrazoles: Inspired by the result of our reported protocol for regioselective synthesis of 1,5-disubstitutedtetrazoles (S N Murthy et al., 2018a), the authors attempted to extend the scope of the protocol towards the synthesis of 2,5-di-substituted tetrazoles. Target tetrazole was obtained in one pot reaction through in situ formation of phenyl tetrazole amine from thiourea in the presence of copper catalyst under mild reaction conditions (Scheme 3). Desulfurization of thiourea followed by consecutive nucleophilic substitution of phenyl azide and electrocyclization reactions provide 2-phenyl-2H-tetrazol-5-ylamine, which was treated with 4-iodotoulene in the presence of copper-ligand complex at 120 °C using Cs2CO3 base proceeded an inter molecular C-N cross-coupling reaction to obtain the target product 2-phenyl-N-p-tolyl-2H-tetrazol-5-amine (4a) in good yield.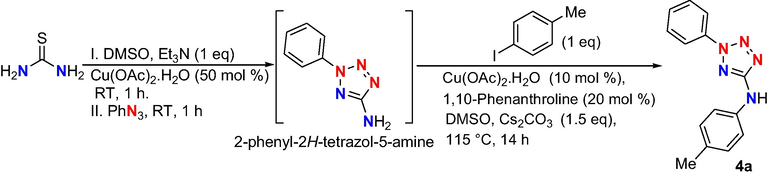
Synthesis of 2-phenyl-N-p-tolyl-2H-tetrazol-5-amine.
Next the scope of the protocol was extended to prepare 11 novel 2,5-di-phenyltetrazolamines (Scheme 4). Different substituted iodobenzenes were effectively participated in inter molecular C-N cross-coupling reaction with phenyl tetrazole amine to obtain corresponding target products 4a-4h in good to high yield. Similarly, various aryl azides effectively undergo the reaction to provide the intermediate aryltetrazole amines which were reacted with iodobenzene under optimized reaction conditions to afford the final products 4i-4k in good to excellent yield. In addition, the experimental results clearly suggesting that the aryl azides or aryl iodides with donating groups exhibited greater reactivity than those bearing withdrawing groups.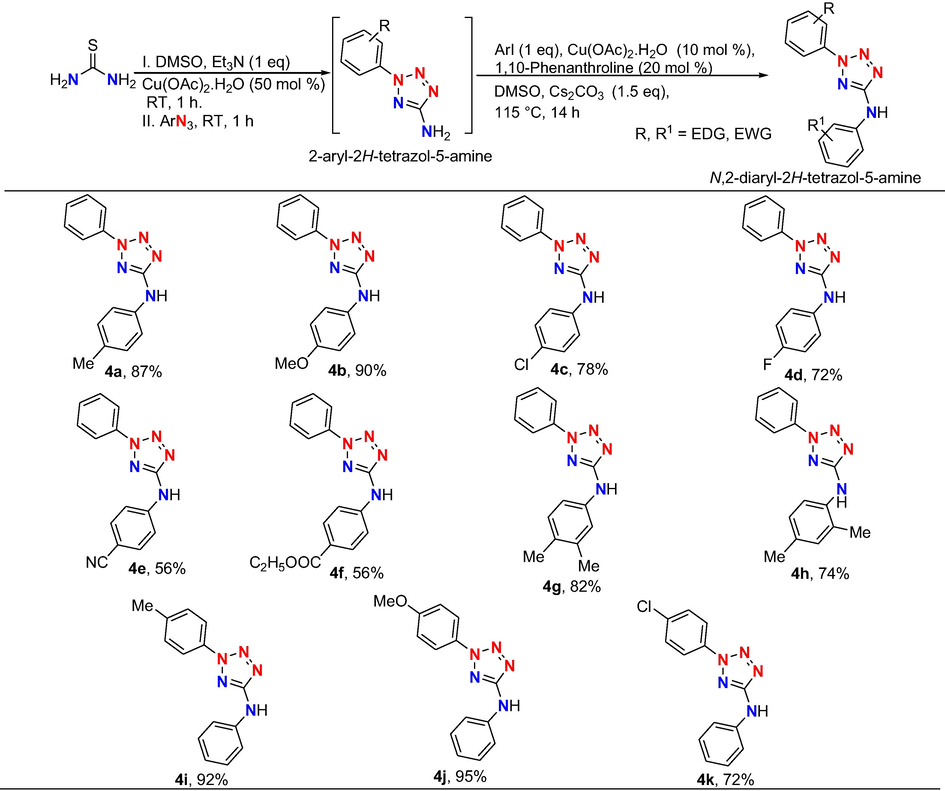
Synthesis of 2,5-disubstituted tetrazole aminesa.
Mechanism for the synthesis of 2,5-disubstituted tetrazole has been demonstrated in Scheme 5. Copper (I) species coordinate with thiourea to give complex X that may provide intermediate complex Z via intermediate Y using base. The intermediate Z reacts with Phenyl azide to get phenyl tetrazole amine D via substitution/electrocyclization (Ali et al., 2010; Sahoo et al., 2010; Guin et al., 2012a, 2012b; Mohan et al., 2016; S N Murthy et al., 2018c). According to literature reports (Bowmaker et al., 2009; Ramana et al., 2010) along with CuS and poly sulphide (Ramana and Punniyamurthy, 2012; Usharani et al., 2017) are obtained. Apart from this, aryl iodide and Cu(I) species which could be formed from Cu(II) species (Bowmaker et al., 2009; Ramana et al., 2010) undergo oxidative addition that lead to the formation of a that can further proceed an intermolecular C-N cross-coupling reaction (Deng et al., 2009; Lv and Bao, 2009; Cahiez and Moyeux, 2010; Chiba et al., 2010; Saha et al., 2010; Hu et al., 2011; Zhao et al., 2011; Wang et al., 2012; Tan and Teo, 2014) with tetrazole amine utilizing base to provide the intermediate b which can complete the catalytic cycle through reductive elimination to obtain target product 2,5-diphenyl tetrazole amine 4a.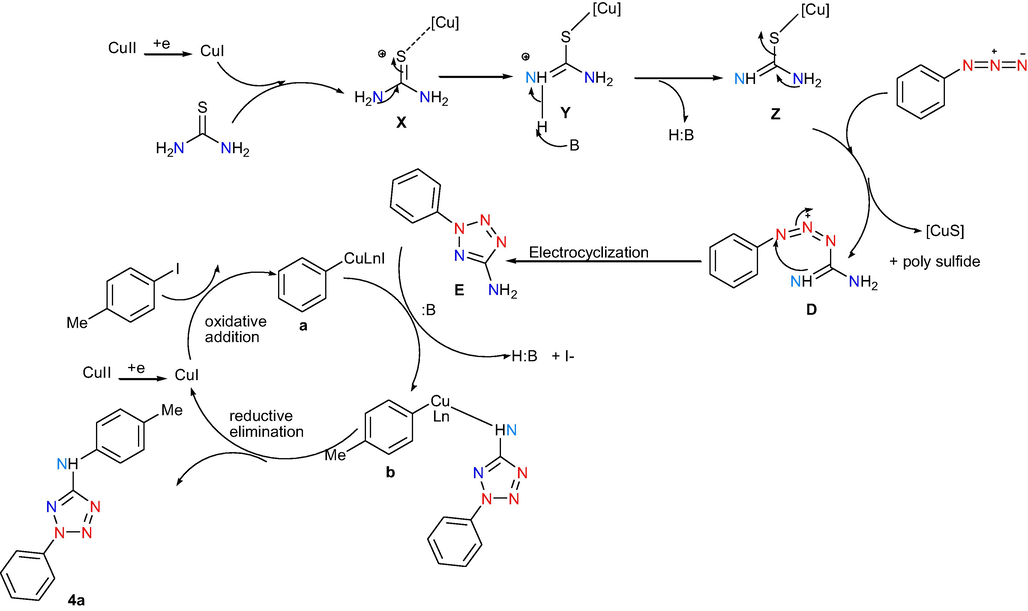
Plausible mechanism for the synthesis of 2,5-disubstituted tetrazole amine.
In order to show the present catalytic system is much better than the earlier reported methodologies, the efficiency of the present method is compared with other synthesis methods of title compounds in the below Tables 5–6.
S. No
Catalyst used
Temperature
Time
Remarks
Reference
1
FeCl3
120 °C
20 h
Expensive catalyst Hiher temperature and reaction time
Bonnamour et al., 2008
2
Ni2(BDC)2 (DABCO)
–
–
Expensive catalyst
Phan et al., 2014
3
Fe salt
dppf [1,10 -bis(diphenylphosphino)- ferrocene]150 °C
24 h
Expensive catalyst.
Higher temperature and reaction timeWu et al., 2012
4
TiCl3OTf
R T
70 min
Expensive catalyst
Azizian et al., 2016
5
([Pd(π-allyl)Cl]2
120 °C
12 h
Expensive catalyst and high temperature
Zhu et al., 2015
6
Pd(OAc)2 in imidazolium ionic liquids (bmim)BF4 and (bmim)PF6
60–75 °C
12 h
Expensive catalytic system
Kalkhambkar and Laali, 2012
7
PI/CB-Pt
Polymer‐Incarcerated Platinum Nanoclusters30 °C
20 h
Expensive catalyst and more reaction time
Yoo et al., 2011
8
RuCl3
80 °C
12 h
Expensive catalyst
Fan et al., 2011
9
[Cp*IrI2]2
80 °C
24 h
Expensive catalyst and more reaction time
Blacker et al., 2009
10
Cu(OAc)2·H2O
1 st step at R T
2nd step at 110 °C1st step – 1 h
2nd step-18 hIn expensive catalyst, lower reaction temperature and time
Present work
S. No
Reference
Catalyst used
Temperature
Time
Remarks
1
Lakshmikantham et al., 2006a
Zinc hydroxyapatite (ZnHAP)
120–130 °C
5–24 h
Expensive catalyst, Higher temperature and reaction time
2
Lakshmikantham et al., 2006b
Zn/Al hydrotalcite
120–130 °C
5–24 h
Expensive catalyst, Higher temperature and reaction time
3
Amantini et al., 2004
TMSN3·TBAF·3H2O
85–120 °C
1–48 h
Expensive catalyst, Higher reaction time
4
Tienan et al., 2008
Cu2O
80 °C
12–24 h
Expensive catalyst, Higher reaction time
5
Venkateshwarlu et al., 2009
Sb2O3
120–130 °C
8–10 h
Expensive catalyst, Higher temperature
6
Nasrollahzadeh et al., 2009
FeCl3·SiO2
120 °C
12 h
Expensive catalyst, Higher temperature
7
Present Work
Cu(OAc)2·H2O
1st step at R T
2nd step at 115 °C1st step – 1 h
2nd step-14 hIn expensive catalyst, lower reaction temperature and time
From the above tables, it can be concluded that in our present protocol we have developed the title compounds by using inexpensive, air stable, abundantly available copper source as catalyst and readily available thiourea as starting material under simple reaction conditions and also we observed target products with excellent yield. The present protocols involve lower reaction temperatures and times than most of the reported methods in literature. Based on these points we feel that our method is more efficient than other methods.
4 Conclusion
In summary, the facile and promising synthesis of 2-aminobenzoxazoles and 2,5-di-substituted tetrazole amines was established with the use of an inexpensive, air stable and readily available copper catalyst under mild reaction conditions. This methodology involves C-N cross-coupling reactions. Considering the cost effectiveness of catalytic system, simplicity, ecological adequacy and selectivity, these approaches would thus be extremely useful in construction of biologically potent 2-aminobenzoxazoles and tetrazole frameworks.
Acknowledgments
The first author thankful to Dr. A P J Abdul Kalam central Research Laboratory of Sir C R Reddy Colllege, Eluru, AP-India for financial support (ID: CRR/APJAK/PF/02/2019 dt. 19.01.2019) and CSIR, New Delhi, India for supporting chemicals (F.No.: EMR-02 (0198/2014. dt. 14.11.2019). The authors thankful to Department of Chemistry, Acharya Nagarjuna University, Nagarjuna Nagar, Guntur, AP-India for constant encouragement.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Palladium-catalyzed direct arylations of heteroarenes with tosylates and mesylates. Angew. Chem. Int. Ed.. 2009;48:201-204.
- [Google Scholar]
- A greener synthetic protocol for the preparation of carbodiimide. Tetrahedron Lett.. 2010;51:1019-1021.
- [Google Scholar]
- TBAF-catalyzed synthesis of 5-substituted 1H-tetrazoles under solventless conditions. J. Org. Chem.. 2004;69:2896-2898.
- [Google Scholar]
- Synthesis of benzimidazoles and benzoxazoles using TiCl3OTf in ethanol at room temperature. Tetrahedron Lett.. 2016;57:185-188.
- [Google Scholar]
- ZnO nanoparticles: a green efficient catalyst for the room temperature synthesis of biologically active 2-aryl-1,3-benzothiazole and 1,3-benzoxazole derivatives. Tetrahedron Lett.. 2014;55:5515-5520.
- [Google Scholar]
- Copper-catalysed intramolecular O-arylation of aryl chlorides and bromides: a straightforward approach to benzo[d]oxazoles in water. Tetrahedron. 2007;63:10425-10432.
- [Google Scholar]
- A general synthetic method for the formation of substituted 5-aminotetrazoles from thioureas: a strategy for diversity amplification. Org. Lett.. 2000;2:3237-3240.
- [Google Scholar]
- Recent advances in iron catalysis in organic synthesis. Curr. Org. Chem.. 2008;12:1341-1369.
- [Google Scholar]
- Synthesis of benzazoles by hydrogen-transfer catalysis. Org. Lett.. 2009;11:2039-2042.
- [Google Scholar]
- Iron-Catalyzed Intramolecular O-Arylation: Synthesis of 2-Aryl Benzoxazoles. Org. Lett.. 2008;10:2665-2667.
- [Google Scholar]
- Crystal structures and vibrational spectroscopy of copper(I) thiourea complexes. Inorg. Chem.. 2009;48:350-368.
- [Google Scholar]
- Cobalt-mediated direct carbon-hydrogen bond functionalizations. Curr. Org. Chem.. 2015;19:121-150.
- [Google Scholar]
- Synthesis of 2-arylbenzoxazoles via DDQ promoted oxidative cyclization of phenolic Schiff bases a solution-phase strategy for library synthesis. Tetrahedron Lett.. 2002;43:951-954.
- [Google Scholar]
- Generation of iminyl copper species from α-azido carbonyl compounds and their catalytic C−C bond cleavage under an oxygen atmosphere. Org. Lett.. 2010;12:2052-2055.
- [Google Scholar]
- Synthesis of tetrazole derivatives bearing pyrrolidine scaffold and evaluation of their antifungal activity against Candida albicans. Eur. J. Med. Chem.. 2019;164:106-120.
- [Google Scholar]
- A simple, advantageous synthesis of 5-substituted 1H-tetrazoles. Synlett 2010:391-394.
- [Google Scholar]
- A new NBS/oxone promoted one pot cascade synthesis of 2-aminobenzimidazoles/2-aminobenzoxazoles: a facile approach. New J. Chem.. 2016;4 0(9):8093-8099.
- [Google Scholar]
- CuI-catalyzed amination of arylhalides with guanidines or amidines: a facile synthesis of 1-H-2-substituted benzimidazoles. J. Org. Chem.. 2009;74:5742-5745.
- [Google Scholar]
- Reactions of N-acyl substituted α-chloroformamidines with nucleophilic reagents. Liebigs Ann. Chem.. 1982;1982(2):201-210.
- [Google Scholar]
- Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J. Org. Chem.. 2006;71:1802-1808.
- [Google Scholar]
- An efficient synthesis of 2-substituted benzoxazoles via RuCl3.3H2O catalyzed tandem reactions in ionic liquid. Chin. J. Chem.. 2011;29:773-777.
- [Google Scholar]
- Preparation and isomerization of 5-alkylaminotetrazoles. J. Org. Chem.. 1953;18:779-791.
- [Google Scholar]
- In Vogel’s Textbook of Practical Organic Chemistry (fifth ed.). Indian Branch, Delhi: Pearson Education Pte. Ltd.; 2004. p. :928-929.
- Room-temperature palladium-catalyzed direct 2-arylation of benzoxazoles with aryl and heteroaryl bromides. Chem. Commun.. 2014;50:10661-10664.
- [Google Scholar]
- The synthesis of certain 5-aminotetrazole derivatives. II. The action of hydrazoic acid on monoalkylcyanamides. J. Org. Chem... 1953;18:1014-1021.
- [Google Scholar]
- A novel strategy for the construction of substituted benzoxazoles via a tandem oxidative process. Chem. Commun.. 2013;49:10968-10970.
- [Google Scholar]
- Synthesis of 2-Aryl-benzoxazoles through Oxidation of C-H Bonds Adjacent to Oxygen Atoms. Eur. J. Org. Chem.. 2014;2014(2):319-322.
- [Google Scholar]
- Desulfurization strategy in the construction of azoles possessing additional nitrogen, oxygen or sulfur using a copper(I) catalyst. Adv. Synth. Catal.. 2012;354:2757-2770.
- [Google Scholar]
- Tandem synthesis of [1,2,4]-triazoles mediated by iodine-a regioselective approach. Tetrahedron. 2012;68:5066-5074.
- [Google Scholar]
- Sustainable synthesis of 2-arylbenzoxazoles over a cobalt-based nanocomposite catalyst. Org. Process Res. Dev.. 2016;20:1093-1096.
- [Google Scholar]
- A highly efficient palladium/copper cocatalytic system for direct arylation of heteroarenes: an unexpected effect of Cu(Xantphos)I. J. Am. Chem. Soc.. 2010;132:3674-3675.
- [Google Scholar]
- A general and efficient approach to 2H-indazoles and 1H-pyrazoles through copper-catalyzed intramolecular N-N bond formation under mild conditions. Chem. Commun.. 2011;47:10133-10135.
- [Google Scholar]
- A P-ligand-free nickel-catalyzed variation of the hirao reaction under microwave conditions. Curr. Org. Chem.. 2015;19:197-202.
- [Google Scholar]
- Synthesis and biological activity of Benzoxazole containing thiazolidinedione derivatives. Arch Pharm Res.. 2004;27:1099.
- [Google Scholar]
- Pd(OAc)2 catalyzed synthesis of 2-aryl- and 2-heteroaryl-benzoxazoles and benzothiazoles in imidazolium ionic liquids (ILs) without additives and with recycling/reuse of the IL. Tetrahedron Lett.. 2012;53:4212-4215.
- [Google Scholar]
- Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol.. 2008;21:1726-1732.
- [Google Scholar]
- Synthesis, biological evaluation and docking study of N-(2-(3,4,5-trimethoxybenzyl)benzoxazole-5-yl) benzamide derivatives as selective COX-2 inhibitor and anti-inflammatory agents. Bioorg. Chem.. 2018;81:191-202.
- [Google Scholar]
- Ruthenium-catalyzed synthesis of benzoxazoles using acceptorless dehydrogenative coupling reaction of primary alcohols with 2-aminophenol under heterogeneous conditions. Catal.. 2014;4:1686-1692.
- [Google Scholar]
- Recent advances in biopolymer supported palladium in organic synthesis. Curr. Org. Chem.. 2015;19:2075-2121.
- [Google Scholar]
- Indium triflate-catalyzed one-pot synthesis of 1-substituted-1H-1,2,3,4-tetrazoles under solvent-free conditions. Tetrahedron Lett.. 2009;50:2668-2670.
- [Google Scholar]
- Low-valent titanium-mediated reductive coupling of carbonyl compounds. Curr. Org. Chem.. 2006;10:965-980.
- [Google Scholar]
- Zinc hydroxyapatite-catalyzed efficient synthesis of 5-substituted 1H-tetrazoles. Synth. Commun.. 2006;36:1809-1814.
- [Google Scholar]
- An efficient synthesis of 5-substituted 1H-tetrazoles using Zn/Al hydrotalcite catalyst. J. Mol. Catal. A: Chem.. 2006;247:186-188.
- [Google Scholar]
- Nanocrystalline ZnO as an efficient heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles. Adv. Synth. Catal.. 2005;347:1212-1214.
- [Google Scholar]
- The palladium-catalyzed tandem decarboxylation, carbon–carbon triple bond oxidation and decarbonylative arylation of the benzoxazole C-H bond. RSC Adv.. 2013;3:9193-9196.
- [Google Scholar]
- Recent uses of iron catalysts in organic reactions. Curr. Org. Chem.. 2010;14:1099-1126.
- [Google Scholar]
- Iron-catalyzed arylation of benzoazoles with aromatic aldehydes using oxygen as oxidant. Green Chem.. 2012;14:1577-1580.
- [Google Scholar]
- Synthesis of 2-Aminobenzoxazoles and 3-Aminobenzoxazines via Palladium-Catalyzed Aerobic Oxidation of o-Aminophenols with Isocyanides. J. Org. Chem.. 2013;78(7):3009-3020.
- [Google Scholar]
- Copper-catalyzed cascade addition/cyclization: an efficient and versatile synthesis of N-substituted 2-heterobenzimidazoles. J. Org. Chem.. 2009;74:5618-5621.
- [Google Scholar]
- Reactions of pyridyl isothiocyanates with diazoalkanes and azoimide. Collect. Czeh Chem. Comm.. 1980;45:2329-2333.
- [Google Scholar]
- Dimroth rearrangement of imines derived from 1,5-diaminotetrazole. Ber.. 1990;123:1575-1578.
- [Google Scholar]
- An efficient methodology for the synthesis of thioureas from amine mediated by a cobalt source. Tetrahedron Lett.. 2016;57:5297-5300.
- [Google Scholar]
- Cobalt-promoted one-pot reaction of isothiocyanates toward the synthesis of aryl/alkylcyanamides and substituted tetrazoles. Chem. Heterocycl. Compd.. 2018;54:535-544.
- [Google Scholar]
- Ruthenium-catalyzed C-H activation and coupling reactions in organic synthesis. Curr. Org. Chem.. 2016;20:381-458.
- [Google Scholar]
- FeCl3–SiO2 as a reusable heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles via [2+3] cycloaddition of nitriles and sodium azide. Tetrahedron Lett.. 2009;50:4435-4438.
- [Google Scholar]
- Synthesis, potent anti-staphylococcal activity and QSARs of some novel 2-anilinobenzazoles. Eur. J. Med. Chem.. 2008;43:1390-1402.
- [Google Scholar]
- Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab J Chem.. 2016;9:S1125-S1130.
- [Google Scholar]
- Towards applications of metal–organic frameworks in catalysis: C-H direct activation of benzoxazole with aryl boronic acids using Ni2(BDC)2(DABCO) as an efficient heterogeneous catalyst. Catal. Sci. Technol.. 2014;4:369-377.
- [Google Scholar]
- Design, synthesis, and evaluation of orally active benzimidazoles and benzoxazoles as vascular endothelial growth factor-2 receptor tyrosine kinase inhibitors. J. Med. Chem.. 2007;50:4351-4373.
- [Google Scholar]
- Parallel synthesis of benzoxazoles via microwave-assisted dielectric heating. Tetrahedron Lett.. 2003;44:175-178.
- [Google Scholar]
- Preparation of 2-azido-1-substituted-1 H-benzo[d]imidazoles using a copper-promoted three-component reaction and their further conversion into 2-amino and 2-triazolyl derivatives. Chem. Eur. J.. 2012;18:13279-13283.
- [Google Scholar]
- Copper-catalyzed domino intra- and intermolecular C−S cross-coupling reactions: synthesis of 2-(arylthio)arylcyanamides. Org. Lett.. 2010;12:84-87.
- [Google Scholar]
- Tandem regioselective synthesis of tetrazoles and related heterocycles using iodine. Org. Biomol. Chem.. 2011;9:3235-3245.
- [Google Scholar]
- Cobalt-catalyzed intramolecular C-N and C–O cross-coupling reactions: synthesis of benzimidazoles and benzoxazoles. Org. Biomol. Chem.. 2010;8:5692-5699.
- [Google Scholar]
- Copper(I)-catalyzed cascade synthesis of 2-arylsulfanyl- arylcyanamides. Adv. Synth and Catal.. 2010;352:2538-2548.
- [Google Scholar]
- From soluble to supported iridium metal nanoparticles: active and recyclable catalysts for hydrogenation reactions. Curr. Org. Chem.. 2013;17(4):348-363.
- [Google Scholar]
- A ball-milling strategy for the synthesis of benzothiazole, benzimidazole and benzoxazole derivatives under solvent-free conditions. Green Chem.. 2014;16:4922-4930.
- [Google Scholar]
- Direct C-H bond arylation of (benzo)oxazoles with aryl chlorides catalyzed by N-heterocyclic carbene–palladium(II)–1-methylimidazole complex. Org. Lett.. 2014;6:1984-1987.
- [Google Scholar]
- Temperature dependent regioselective synthesis of aryl tetrazole amines using copper source. J. Organomet. Chem.. 2018;866:177-183.
- [Google Scholar]
- Copper-catalyzed synthesis of 2-aminophenyl benzothiazoles: a novel approach. Org. Biomol. Chem.. 2018;16:8267-8272.
- [Google Scholar]
- The synthesis of arylcyanamides: a copper-catalyzed consecutive desulfurization and C-N cross-coupling strategy. New. J. Chem.. 2018;42:918-922.
- [Google Scholar]
- A Facile Synthesis of 1-Substituted-1H-1,2,3,4-Tetrazoles Catalyzed by Ytterbium Triflate Hydrate. Eur. J. Org. Chem.. 2006;2006(12):2723-2726.
- [Google Scholar]
- Efficient cobalt-catalyzed C-N cross-coupling reaction between benzamide and aryl iodide in water. Org. Biomol. Chem.. 2014;12:7478-7481.
- [Google Scholar]
- Copper-catalyzed synthesis of 5-substituted 1H-tetrazoles via the [3+2] cycloaddition of nitriles and trimethylsilyl azide. Tetrahedron Lett.. 2008;49:2824-2827.
- [Google Scholar]
- Synthesis of 2-arylbenzoxazoles by copper-catalyzed intramolecular oxidative C-O coupling of benzanilides. Angew. Chem. Int. Ed.. 2008;47:6411-6413.
- [Google Scholar]
- Copper promoted desulfurization towards the synthesis of isothiocyanates. Tetrahedron Lett.. 2017;58:125-128.
- [Google Scholar]
- Manganese triacetate oxidation of phenolic schiffs bases: Synthesis of 2-arylbenzoxazoles. J. Heterocycl. Chem.. 1998;35:1539-1540.
- [Google Scholar]
- Antimony trioxide as an efficient lewis acid catalyst for the synthesis of 5-substituted 1H-tetrazoles. Synth. Commun.. 2009;39:426-432.
- [Google Scholar]
- Novel ultra-thin platinum nanowires and their catalytic applications. Curr. Org. Chem.. 2015;19:2142-2155.
- [Google Scholar]
- Copper-catalyzed synthesis of 4-aryl-1H-1,2,3-triazoles from 1,1-Dibromoalkenes and Sodium Azide. Eur. J. Org. Chem.. 2012;2012(2):424-428.
- [Google Scholar]
- Iron-Catalyzed 2-Arylbenzoxazole Formation from o-Nitrophenols and Benzylic Alcohols. Org. Lett.. 2012;14:2722-2725.
- [Google Scholar]
- Zinc-catalyzed organic synthesis: C-C, C-N, C-O bond formation reactions. Adv. Synth. Catal.. 2012;354:3141-3160.
- [Google Scholar]
- Palladium-catalyzed synthesis of benzoxazoles by the cleavage reaction of carbon–carbon triple bonds with o-aminophenol. Green Chem.. 2014;16:2132-2135.
- [Google Scholar]
- Iodide catalyzed synthesis of 2-aminobenzoxazoles via oxidative cyclodesulfurization of phenolic thioureas with hydrogen peroxide. Tetrahedron Lett.. 2018;59:252-255.
- [Google Scholar]
- Facile preparation of 2-substituted benzoxazoles and benzothiazoles via aerobic oxidation of phenolic and thiophenolic imines catalyzed by polymer-incarcerated platinum nanoclusters. Adv. Synth. Catal.. 2011;353:3085-3089.
- [Google Scholar]
- Discovery of pyrimidine benzimidazoles as Lck inhibitors: Part I. Bioorg. Med. Chem. Lett.. 2008;18:5618-5621.
- [Google Scholar]
- Regiospecific Synthesis of 1,2-Disubstituted (Hetero)aryl Fused Imidazoles with Tunable Fluorescent Emission. Org. Lett.. 2011;13:6516-6519.
- [Google Scholar]
- Palladium-Catalyzed C-H Arylation of (Benzo)oxazoles or (Benzo)thiazoles with Aryltrimethylammonium Triflates. Org. Lett.. 2015;17:4926-4929.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.09.001.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1