Translate this page into:
Correlation between chemical composition and radical scavenging activity of 10 commercial essential oils: Impact of microencapsulation on functional properties of essential oils
⁎Corresponding author. mohamadnrc@yahoo.com (Mohamad Yehia Sayed Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Correlation between antioxidant activity and composition of ten essential oils were evaluated. The radical scavenging activity was correlated to presence of phenolic compounds in the EOs. Presence of antioxidant compounds in EOs free from phenolic compounds resulted in high activity. Sodium alginate showed high retention and encapsulation efficiency of clove EO.
Abstract
A comparative study was carried out on the essential oils of 10 aromatic plants that are extensively used in Egypt for their distinctive aroma and functional properties. Each essential oil (EO) was characterized by means of gas chromatography-mass spectrometry (GC–MS) analysis and evaluated for its radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azinobis (2-ethyl-benzolhiaxoline-6-sulfonic acid)(ABTS) assays. The phenolic content of the 10 EOs was in the descending order: clove > thyme > majoram > basil > anise > chamomile > cinnamon > dill > ginger > rosemary. The radical scavenging activity of the EOs was correlated to the presence of phenolic compounds, such as eugenol, thymol, carvacrol and trans-anethol, or the synergism between the antioxidant activity of nonphenolic compounds such as terpinene-4-ol, α-terpinene, curcumene and chamazulene. Clove essential oil exhibited the highest oil content and radical scavenging activity so it was encapsulated, separately, in three coating materials. Sodium alginate showed the highest retention, encapsulation efficiency and loading capacity of clove EO. Microencapsulation in sodium alginate and chitosan improved the antioxidant activity and phenolic content of the encapsulated clove EO compared with carboxymethyl cellulose. The results support the possibility of using the encapsulated EOs as natural and easy handle antioxidants.
Keywords
Scavenging radical activity
Essential oil
Encapsulation
Phenolic content
Emulsion extrusion
1 Introduction
Since ancient times herbs and spices have been used for their flavouring qualities as well as their medicinal and preservative properties. Extensive studies have been carried out to evaluate their antioxidant activities and beneficial effect on human health (Yashin et al., 2017). Lipid oxidation is the major cause of free radical generation, which are the main cause of carcinogenesis, mutagenesis, inflammation, DNA changes, aging and cardiovascular diseases (Iqbal et al., 2008). Formation of free radicals by lipid oxidation during food processing and storage is the major cause for their quality loss (Akoh and Min, 1998). Addition of antioxidants to fatty foods can control rancidity development, retard the formation of toxic oxidation products, maintain nutritional quality and extend the shelf life of food products (Maisuthisakul et al., 2007). However, several studies have linked some synthetic antioxidants such as butylated hydroxyl toluene (BHT) and butylated hydroxyl anisole (BHA) to carcinogenesis and hepatic damage (Haumann, 1990). The use of antioxidants from natural sources has become more and more popular as a mean of improving the stability of fats and oils as well as a treatment of some human diseases (Yashin et al., 2017). Essential oils (EOs) from aromatic plants are gaining increasing interest because of their relatively safe status, their wide acceptance by consumers and exploitation for potential multipurpose functional use as antioxidant and radical scavenging agents (Sacchetti et al., 2005; Zengin et al., 2018). Essential oils contain a variety of functional bioactive compounds, which have possible applications in the field of prevention or treatment of some diseases (Taghipour et al., 2019). However, the activity and sensory quality of the EOs can be lost by volatilization of their active compounds, their degradation, oxidation and chemical interaction (Ayala-Zavala et al., 2008). Microencapsulation of EOs is used in flavour industry to minimize these negative processes by their entrapping in protective layers of appropriate coating materials (Gouin, 2004). Emulsion extrusion is one of the most common approaches of microencapsulation especially for emulsifying or dispersing the hydrophobic components in an aqueous solution (Yuliani et al., 2006). Microencapsulation should retain and protect the encapsulated EOs from their loss and chemical damage during industrial processing and consumption. In addition, microencapsulation increases the solubility of oils in water and makes them easier to handle (Liolios et al., 2009). Selection of encapsulating materials is very important to obtain an effective system tailored to final application. Sodium alginate (sod-Alg), chitosan (Ch) and carboxymethyl cellulose (CMC) are commonly used as natural wall materials in food, biochemical and environmental fields because of their structure function (Nitta and Numata, 2013).
Several studies have been conducted on the volatile composition and antioxidant activity of the essential oils of various aromatic plants. However, most of these studies were carried out on individual or limited number of EOs. Therefore, in the present work a comparative study was carried out on the essential oils of 10 aromatic plants that are extensively used in Egypt for their distinctive aroma. The link between the composition of each EO, its free radical scavenging activity and phenolic content was investigated. The EO that showed the highest free radical scavenging activity was encapsulated in different coating materials (sod- Alg, Ch and CMC) by employing the emulsion extrusion technology. The efficiency of each coating material of the produced beads was evaluated in terms of retention of the main volatile compounds, the free radical scavenging activity and phenolic content of the entrapped EO.
2 Material and methods
2.1 Aromatic plants
The aromatic plants (Table 1) were purchased from Ferrous Company, Giza, Egypt. All plants are grown in Egypt except for clove and cinnamon were imported from Indonesia and India. The plants were sun dried (average temperature 33.0 ± 1.0 oC, relative humidity 69.5 ± 2.5%) and crushed into 30 mesh particle size.
No
Common name
Scientific name***
Test parts
Oil yield mL/100 g dw**
1
Rosemary
Rosmarinus officinalis L.
Leave
2.13*±0.12b
2
Thyme
Thymus vulgaris L.
Leave
1.90 ± 0.10c
3
Anise
Illicium verum
Seed
0.60 ± 0.06ef
4
Cinnamon
Cinnamon cassia
Bark
0.67 ± 0.08ef
5
Dill
Anethum graveolens L.
Seed
1.17 ± 0.15d
6
Marjoram
Origanum majorana L
Leave
0.30 ± 0.04f
7
Basil
Ocimum basilicum L.
Leave
0.75 ± 0.08e
8
Clove
Syzygium aromaticum
Bud
3.86 ± 0.31a
9
Ginger
Zingiber officinale Rosc
Root
0.53 ± 0.07ef
10
Chamomile
Matricaria recutita L.
Leave
0.50 ± 0.05f
2.2 Chemicals
Authentic volatile compounds and standard n-paraffin (C8-C22), sodium alginate (sod-Alg), chitosan (Ch) low molecular weight (MW), carboxymethyl cellulose (CMC), trisodium polyphosphate (TPP), ferric chloride (FeCl3), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid (ABTS) and Folin- Ciocalteu reagents were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA) and Merck (Darmstadt, Germany). All other chemicals were of analytical grade and the solvents (diethyl ether, methanol and ethanol) were purified and distilled before use.
2.3 Extraction of essential oils
Each crushed aromatic plant (100 g) was subjected to hydro-distillation in a Clevenger apparatus for 3 h for extraction of its essential oil. The obtained EO was dried over anhydrous sodium sulphate and immediately kept in a dark sealed glass vial at 4 °C until analysis.
2.4 Phenolic content
Total phenolic content of each hydrodistilled essential oil was determined by the Folin-Ciocalteu method (Singleton et al., 1999). Briefly, 500 µL of each EO were mixed with 250 µL of Folin-Ciocalteau reagent in a test tube. After 5 min, the mixture was neutralized with 1.25 mL of 20% aqueous Na2CO3 solution and stands in the dark for 40 min. The absorbance of the developed colour was measured at 725 nm against the solvent blank (methanol), using a UV/Vis-spectrophotometer (Model UV-1601, SHIMADZU, Kyoto, Japan). The total phenolic content was determined by means of a calibration curve prepared by using different concentrations of gallic acid and expressed as mg of gallic acid equivalent (GAE) per mL of each EO. The phenolic content in the encapsulated EO was determined as mentioned above.
2.5 Radical scavenging activity
The antioxidant activity of the ten investigated EOs was assessed on the basis of the scavenging activity of the stable radicals 2, 2-diphenyl-1-picrylhydrazyl and 2, 2-azino (2-ethyl-benzolhiaxoline-6-sulfonic acid) (DPPH and ABTS, respectively). The DPPH assay was carried out according to Yen and Chen (1995). Each essential oil (200 μL) was added to 4 mL of 0.1 mM DPPH solution in methanol. The mixture was shaken vigorously and allowed to stand for 30 min at room temperature. The decrease in free radical concentration was monitored by reading absorbance at 517 nm using a UV/Vis-spectrophotometer (Model UV-1601, SHIMADZU, Kyoto, Japan). The standard curve was prepared by using ascorbic acid; the results were corrected for dilution and expressed as mg ascorbic acid equivalents per mL of essential oil (mg AAE / mL EO). All determinations were performed in triplicate.
The ABTS assay was carried out according to Re et al. (1999) with some modifications. Stock solution was prepared by dissolving ABTS in water to a 70 mM concentration. The ABTS radical cation (ABTS.+) was prepared by reacting the stock solution with 2045 mM potassium persulfate and allowing the mixture to react for 12–16 h before use. The prepared ABTS.+ was then diluted with ethanol to obtain an absorbance of 0.7 (±0.02) units at 734 nm and equilibrated at 30 °C. Each EO (0.1 mL) was mixed with 2.9 mL of diluted ABTS.+ solution and allowed to react for 20 min at ambient temperature before reading the absorbance at 734 nm (Arnao, 2000). The trolox calibration curve was plotted, and the results were corrected for dilution and expressed as mmole Trolox equivalents per mL of EO (mmole TE/ mL EO). All determinations were performed in triplicate.
2.6 Gas chromatography–mass spectrometry (GC–MS) analysis
Analysis of the hydrodistilled EOs was conducted by using a gas chromatography (Hewlett–Packard model 5890), coupled to a mass spectrometer (Hewlett–Packard-MS model 5970) and equipped with a DB5 fused silica capillary column (60 m, 0.32 mm i.d., 0.25 µm film thickness). The oven temperature was maintained initially at 50 °C for 5 min, and then programmed from 50 to 250 °C at a rate of 4 °C/min. Helium was used as the carrier gas, at flow rate of 1.1 mL/ min. The essential oil was dissolved in diethyl ether (30 µL essential oil / mL diethyl ether), and then 2 µL of this solution were injected in the GC with a split ratio 1:10. The temperature of injection was 220 °C. Mass spectra in the electron impact mode (EI) were obtained at 70 eV and scan m/z range from 39 to 400 amu. The retention indices (Kovats index) of the separated volatile compounds were calculated with reference to the retention time of a series of n-alkanes (C8–C22) as external standard, run at the same conditions. The isolated peaks were identified by matching them with data from the library of mass spectra (National Institute of Standard and Technology, NIST) and comparing with those of authentic compounds and published data (Adams, 2001). The percentage composition of each oil was computed by the normalization method from the GC peak area, calculated by mean of three injections.
2.7 Encapsulation of essential oil
Microencapsulation of the EO, which showed the highest oil content and radical scavenging activity, was conducted according to Chan (2011). Sodium alginate, chitosan and carboxymethyl cellulose (CMC) were used as encapsulating materials. Each encapsulating material was dissolved in distilled water to produce a polymer solution with a concentration of 2% (w/v) and left standing for 3 h to disengage bubble before use. Each solution was homogenized into a 100 mL beaker with stirring at a speed of 300 rpm for 10 h by a magnetic stirrer. The EO was gradually added to the polymer solution during mixing until the desired oil loading was obtained. For alginate beads, 50 mL of alginate-oil emulsion were sprayed into a collecting water bath containing calcium chloride solution 2% (w/v) using an Inotech Encapsulator IER-50 (Switzerland) with a 500- µm nozzle, and the resulting microcapsules were allowed to harden in the CaCl2 solution for 3 h. For chitosan and CMC beads, calcium chloride solution was replaced by TPP and ferric chloride solution, respectively. Finally, the microbeads were rinsed twice with distilled water; tissue paper was used to absorb the surface excessive water and oil onto the wet microcapsules.
The entrapped EOs were extracted and quantified as described in previous study (Soliman et al., 2013). The loaded oil was extracted from the oil-loaded beads (0.5 g) with 5 mL sodium citrate (0.055 M) and 5 mL hexane. The absorbance of the extracted oil was measured at 280 nm by using spectrophotometer (Model UV-1601, SHIMADZU, Kyoto, Japan). The amount of each extracted EO was calculated from the standard curve constructed at 280 nm for clove EO with usage of dissolved microbeads with no EO as control. Loading capacity (%) was calculated from the following equation:
Where
W0 = Quantity of loaded EO
WMs = Quantity of microspheres (MSs).
Encapsulation efficiency (EE %) was calculated from the following equation:
W0 = Quantity of loaded EO
W1 = Initial quantity of EO.
The loading capacity and EE were determined in triplicate for each bead.
2.8 Scanning electron microscopic analysis
The clove EO microbeads were observed visually and analyzed using high-resolution scanning electron microscope (SEM) with suitable accelerating voltage and magnifications. SEM analysis of microbeads was performed by using Tescan SEM (Tescan vega 3 SBU, Czech Republic). Samples were mounted on aluminum microscopy stubs using carbon tap, then coated with gold (5 nm) for 90 s using Quorum techniques Ltd, sputter coater (Q 150 t, England).
2.9 Statistical analysis
Analysis was performed in triplicate for each sample for all the tests. Data were analyzed using one-way analysis of variance (ANOVA) by the Statgraphics package (Statistical Graphics Corporation, 1993; Manugistics Inc., USA) and least significant difference (LSD) was performed to determine any significant difference amongst various treatments that were used to compare the means. Differences were considered to be significant at p < 0.05.
3 Result and discussion
3.1 Oil yield and chemical composition of essential oils
The total yields of the essential oils were in the descending order: clove > rosemary > thyme > dill seeds > basil > cinnamon > anise seeds > ginger > chamomile > marjoram (Table 1). Clove comprised the highest yield (3.86 mL/100 g dw) compared with the other investigated samples. In contrast, marjoram showed the lowest yield of essential oil (0.30 mL/100 g dw). In a previous study by Gharib and Teixeira da Silva (2012), the air dried marjoram recorded a higher yield of essential oil (1.625 mL/100 g dw), whereas, rosemary recorded a lower content (0.802 mL/100 g dw) than that found in the present study (2.13 mL/100 g dw). This difference may be due to environmental and climatic factors as well as the drying methods of the aromatic plants (Martos et al., 2007; Fadel and El-Massry, 2000).
Typical gas chromatograms of the ten investigated EOs are shown in Fig. 1S. The main constituents, percent composition and Kovat indices of each essential oil are cited in Table 2. It is obvious that the ten essential oils were greatly varied in composition. Some compounds were identified in several oils whereas others were present only in one oil. Fig. 2S presenting the major compound identified in each essential oil. The qualitative analysis of the essential oil (EO) of anise seeds revealed the presence of 11 compounds representing 99.0% of the total oil. trans-Anethol, the antioxidant compound (Cu, 1986), was the predominant identified compound (88.7%) followed by anisaldehyde (4.0%), methyl chavicol (1.4%) and germacrene D (0.6%). Anethol was the major identified compound in all previous studies (Sahab et al., 2018). However, there were minor differences in composition of the other compounds in reported literature (Jamshidzadeh et al., 2015). trans-Anethol content varied between 78% and 95% in the essential oils of twenty-nine anise seed samples collected from different locations in Turkey (Aslan et al., 2004). In the same study, α-terpineol, methyl chavicol and linalool were reported as relatively important compounds. Monoterpene hydrocarbons: compounds Nos. 1–10, 12 and 13; oxygenated monoterpenes: compounds Nos. 11, 14–46,48,49,55 and 71; sesquiterpene hydrocarbons: compounds Nos. 47, 50–54, 56–70, 72, 75 and 88.; oxygenated sesquiterpenes: compounds Nos. 73, 74, 76–79, 80–87 and 89;spiroether: compound No. 90.
No
RIa
RI Litb
Volatiles compounds
Relative area %c
Anise
Cinnamon
Marjoram
Clove
Rosemary
Ginger
Chamomile
Dill
Thyme
Basil
1
928
927
α-Thujene
–
–
–
–
–
–
–
–
0.2 ± 0.01
–
2
936
936
α-Pinene
–
–
–
–
6.2 ± 0.31
0.7 ± 0.04
–
–
0.3 ± 0.02
–
3
952
950
Camphene
–
–
–
–
2.0 ± 0.10
3.3 ± 0.17
–
–
0.1 ± 0.01
–
4
974
973
Sabinene
–
–
0.5 ± 0.03
–
–
–
–
–
–
–
5
988
989
β-Myrcene
–
–
0.1 ± 0.01
–
1.8 ± 0.09
1.0 ± 0.05
–
–
0.6 ± 0.03
–
6
1006
1004
α-Phellandrene
–
–
0.9 ± 0.04
–
0.1 ± 0.01
0.2 ± 0.01
–
0.2 ± 0.01
–
0.1 ± 0.01
7
1018
1017
α-Terpinene
–
–
0.9 ± 0.05
–
0.2 ± 0.01
0.5 ± 0.02
–
–
–
–
8
1027
1022
p-Cymene
–
–
1.9 ± 0.09
–
---
---
–
–
11.5 ± 0.59
–
9
1031
1030
β-Phellandrene
–
–
–
–
1.6 ± 0.08
4.0 ± 0.20
–
–
–
–
10
1032
1029
Limonene
–
–
–
–
–
–
9.4 ± 0.50
–
–
11
1035
1031
1,8-Cineol
–
–
–
–
50.6 ± 2.50
–
–
–
–
1.9 ± 0.10
12
1047
1047
trans- Ocimene
–
–
–
–
---
–
–
–
–
0.3 ± 0.02
13
1059
1059
γ-Terpinene
–
–
–
–
0.1 ± 0.01
–
0.1 ± 0.01
–
2.1 ± 0.12
–
14
1077
1083
trans-Linalool oxide
–
–
–
–
–
–
–
–
0.1 ± 0.01
15
1081
1086
Terpinolene
–
–
2.0 ± 0.10
0.1 ± 0.01
0.2 ± 0.01
–
–
–
0.1 ± 0.01
0.1 ± 0.01
16
1082
1086
Camphenilone
–
–
---
–
---
–
–
–
0.1 ± 0.01
–
17
1104
1099
Linalool
0.1 ± 0.01
–
1.6 ± 0.08
–
1.3 ± 0.06
1.7 ± 0.09
0.1 ± 0.01
–
0.9 ± 0.04
19.6 ± 1.00
18
1135
1123
cis-p-Menth-2-en-1-ol
–
–
1.9 ± 0.10
–
–
–
–
–
–
–
19
1153
1143
Camphor
–
–
1.6 ± 0.08
–
18.0 ± 0.91
0.1 ± 0.01
–
0.2 ± 0.01
0.1 ± 0.01
–
20
1163
1153
Citronellal
–
–
–
–
–
0.3 ± 0.02
–
–
–
–
21
1173
1166
Borneol
–
–
–
0.1 ± 0.01
2.4 ± 0.12
–
–
–
–
0.3 ± 0.01
22
1181
1177
Terpinen-4-ol
–
–
31.6 ± 1.59
–
–
0.4 ± 0.02
–
–
0.6 ± 0.03
–
23
1189
1185
Dill ether
–
–
–
–
–
–
–
0.2 ± 0.01
–
–
24
1199
1195
Methyl chavicol
1.4 ± 0.07
–
–
–
–
–
–
–
–
34.3 ± 1.72
25
1201
1190
α-Terpineol
–
–
11.4 ± 0.57
–
6.8 ± 0.34
1.3 ± 0.07
0.1 ± 0.01
–
0.3 ± 0.02
–
26
1203
1201
cis-Dihydrocarvone
–
–
–
–
–
–
–
5.3 ± 0.27
–
–
27
1211
1204
trans-Dihydrocarvone
–
–
–
–
–
–
–
21.2 ± 1.07
–
–
28
1224
1225
trans-Cinnamaldehyde
–
1.0 ± 0.05
–
–
–
–
–
–
–
–
29
1233
1234
Thymol methyl ether
–
–
–
–
–
–
–
–
0.5 ± 0.03
–
30
1241
1243
Carvacrol methyl ether
–
–
–
–
–
–
–
–
0.6 ± 0.03
–
31
1243
1242
Neral
–
–
–
–
–
2.9 ± 0.15
–
–
–
0.9 ± 0.05
32
1246
1245
Carvotane acetone
0.4 ± 0.02
–
–
–
–
–
–
–
–
–
33
1251
1242
Carvone
–
–
2.1 ± 0.10
–
–
–
–
60.2 ± 3.03
–
–
34
1258
1255
Linalyl acetate
–
–
0.3 ± 0.01
–
–
–
–
–
0.2 ± 0.01
–
35
1263
1251
Anisaldehyde
4.0 ± 0.20
–
–
–
–
–
–
–
0.1 ± 0.01
–
36
1272
1270
Geranial
–
–
–
–
–
4.7 ± 0.24
–
–
–
–
37
1286
1283
Bornyl acetate
–
–
3.3 ± 0.17
–
5.3 ± 0.27
1.0 ± 0.05
–
–
0.3 ± 0.02
–
38
1289
1279
cis-Cinnamaldehyde
–
92.7 ± 4.70
–
–
–
–
–
–
–
–
39
1297
1286
trans- Anethole
88.7 ± 4.46
–
–
–
–
–
–
–
–
40
1302
1290
Thymol
–
–
1.1 ± 0.06
–
0.4 ± 0.02
–
–
–
57.2 ± 2.91
–
41
1320
1300
Carvacrol
–
–
0.5 ± 0.03
–
–
–
–
–
11.2 ± 0.58
–
42
1339
1347
Terpinyl acetate
–
–
11.5 ± 0.58
–
–
–
–
–
0.6 ± 0.03
0.3 ± 0.01
43
1348
1356
Thymol acetate
–
–
0.9 ± 0.05
–
–
–
–
–
–
–
44
1357
1354
Citronellyl acetate
–
–
–
–
–
0.4 ± 0.02
–
–
–
–
45
1362
1366
Piperitenone oxide
–
–
–
–
0.1 ± 0.01
–
–
–
–
–
46
1365
1357
Eugenol
–
0.8 ± 0.04
–
89.9 ± 4.52
–
–
–
0.6 ± 0.03
–
4.6 ± 0.23
47
1375
1376
α-Copaene
–
0.6 ± 0.03
–
–
–
0.7 ± 0.03
–
–
–
–
48
1380
1373
Carvacrol acetate
–
–
–
–
–
–
–
–
1.1 ± 0.05
–
49
1383
1385
Geranyl acetate
–
–
–
–
–
0.3 ± 0.02
–
–
–
–
50
1387
1389
α-Cubebene
0.4 ± 0.02
–
–
–
–
–
–
1.0 ± 0.05
–
–
51
1396
1390
β-Elemene
–
–
–
–
–
0.2 ± 0.01
0.2 ± 0.01
–
–
4.5 ± 0.22
52
1425
1420
β-Caryophyllene
–
0.1 ± 0.01
8.7 ± 0.44
1.4 ± 0.07
0.5 ± 0.02
–
0.1 ± 0.01
–
2.1 ± 0.10
0.6 ± 0.03
53
1428
1436
γ-Elemene
–
–
–
–
–
1.0 ± 0.05
–
–
–
–
54
1438
1434
trans-α-Bergamotene
–
–
–
–
–
–
–
–
–
8.0 ± 0.40
55
1445
1430
trans-Cinnamyl acetate
–
1.4 ± 0.07
–
–
–
–
–
–
–
–
56
1450
1453
α-Humulene
1.6 ± 0.08
–
1.5 ± 0.08
0.4 ± 0.02
0.2 ± 0.01
–
–
–
0.1 ± 0.01
1.2 ± 0.06
57
1454
1445
cis-β-Farnesene
–
–
–
–
–
–
5.7 ± 0.29
–
–
–
58
1460
1459
allo-Aromadendrene
–
–
1.1 ± 0.05
–
–
–
–
0.7 ± 0.03
–
59
1464
1458
β-Santalene
–
–
–
–
–
–
–
–
–
1.2 ± 0.06
60
1479
1476
γ-Muurolene
–
–
–
–
–
–
–
–
0.3 ± 0.02
–
61
1481
1480
Germacrene D
0.6 ± 0.03
0.3 ± 0.01
0.8 ± 0.04
–
–
–
0.8 ± 0.04
–
0.1 ± 0.01
0.4 ± 0.02
62
1486
1474
α-Curcumene
–
–
–
–
–
15.8 ± 0.79
–
–
63
1488
1482
α-Amorphene
–
0.1 ± 0.01
–
–
–
–
–
–
1.8 ± 0.09
64
1493
1494
Bicyclogermacrene
–
–
2.6 ± 0.13
–
–
–
1.6 ± 0.08
–
–
–
65
1499
1495
Zingiberene
–
–
–
–
–
31.2 ± 1.57
–
–
–
66
1502
1498
α-Muurolene
–
–
5.4 ± 0.27
–
–
–
–
–
–
67
1503
1504
α-Farnesene
–
–
–
0.1 ± 0.01
0.7 ± 0.04
1.8 ± 0.09
0.2 ± 0.01
–
–
–
68
1506
1508
β-Bisabolene
–
0.5 ± 0.03
–
–
–
–
–
–
0.4 ± 0.02
1.3 ± 0.06
69
1517
1513
γ-Cadinene
–
–
0.2 ± 0.01
–
–
–
–
0.6 ± 0.03
0.4 ± 0.02
0.9 ± 0.05
70
1524
1523
δ-Cadinene
–
1.5 ± 0.07
–
–
–
–
–
–
0.5 ± 0.03
5.1 ± 0.26
71
1525
1523
Eugenyl acetate
–
–
–
7.9 ± 0.40
–
–
–
–
–
–
72
1529
1524
β-Sesquiphellandrene
1.1 ± 0.05
–
–
–
–
15.0 ± 0.57
–
–
–
–
73
1541
1547
Elemol
–
–
–
–
–
–
–
–
0.6 ± 0.03
0.7 ± 0.03
74
1561
1564
transNerolidol
–
–
–
–
–
–
1.4 ± 0.07
–
–
–
75
1564
1550
Germacrene B
–
–
–
–
–
1.5 ± 0.08
–
–
–
–
76
1571
1567
Cis-Nerolidol
–
–
–
–
–
2.3 ± 0.11
–
–
0.1 ± 0.01
–
77
1575
1576
Spathulenol
–
0.1 ± 0.01
0.2 ± 0.01
–
–
–
–
–
–
–
78
1589
1580
Caryophyllene oxide
0.4 ± 0.02
–
0.3 ± 0.01
–
–
0.2 ± 0.01
0.2 ± 0.01
–
3.0 ± 0.15
–
79
1600
1588
Epiglobulol
–
–
–
–
–
0.2 ± 0.01
–
–
–
–
80
1623
1621
Dill apiole
0.3 ± 0.01
–
–
–
–
–
–
–
–
–
81
1628
1630
γ-Eudesmol
–
–
–
–
–
2.3 ± 0.12
–
–
0.4 ± 0.02
1.6 ± 0.08
82
1639
1651
α-Cadinol
–
–
1.2 ± 0.06
–
–
0.4 ± 0.02
–
–
1.0 ± 0.05
9.4 ± 0.47
83
1659
1672
β-Bisabolol
–
–
2.4 ± 0.12
–
–
–
–
–
0.7 ± 0.04
–
84
1669
1663
α-Bisabolol oxide B
–
–
–
–
–
–
14.7 ± 0.75
–
–
–
85
1680
1682
α-Bisabolol
–
–
–
–
–
2.8 ± 0.14
–
–
–
–
86
1693
1692
α-Bisabolone oxide A
–
–
–
–
–
–
12.9 ± 0.65
–
–
–
87
1741
1743
Fernesol
–
–
–
–
–
–
–
0.3 ± 0.02
–
88
1745
1740
Chamazulene
–
–
–
–
–
–
3.2 ± 0.16
–
–
–
89
1757
1763
α-Bisabolol oxide A
–
–
–
–
–
–
45.5 ± 2.29
–
–
–
90
1888
1888
En-in-dicycloether
–
–
–
–
–
–
9.2 ± 0.40
–
–
–
99.0 ± 4.97
99.1 ± 4.98
98.5 ± 4.95
99.9 ± 5.02
98.5 ± 4.96
98.2 ± 4.93
96.0 ± 5.00
98.1 ± 4.98
99.4 ± 4.97
99.2 ± 4.99
Eleven volatile compounds were identified in the hydrodistilled EO of cinnamon bark (Table 2), representing 99.1% of the total oil. cis- Cinnamaldehyde, the major identified compound (92.7%), in addition to trans-cinnamyl acetate (1.4%), trans-cinnamaldehyde (1.0%) and eugenol (0.8%) were the identified oxygenated compounds whereas the other seven compounds were sesquiterpenes. Cinnameldehyde has many biological and pharmacological significance (Ashakirin et al., 2017). cis – Cinnamaldehyde, β-caryophyllene and eugenol have been reported as the predominant compounds in previous studies concerned with chemical analysis of cinnamon bark EO (Kim et al., 2015).
The GC- MS analysis of marjoram EO revealed the presence of 29 volatile compounds, representing 98.5% of the total oil (Table 2). The major constituents were terpinen-4-ol, α-terpineol, α-terpinyl acetate, β-caryophyllene, camphor, α-phellandrene, terpinolene, α-muurolene and cis-p-menth-2-en-1-ol. Several studies were reported on the chemical composition of marjoram EO; most of them indicated terpinene-4-ol as the main constituent (Baratta et al., 1998; Gharib and Teixeira da silva, 2012). However, there were great variations among the other identified compounds. Analysis of marjoram EO collected from Yemen showed remarkable variations being trans-sabinene, cis-sabinenehydrate, γ-terpinene and α-terpinyl acetate the major identified compounds (Al-Fatimi, 2018).
The seven volatile compounds identified in the hydrodistilled oil of clove buds were representing 99.9% of the total oil. Eugenol was the major compound (89.9%) followed by eugenyl acetate (7.9%), β-caryophyllene (1.4%) and α-humulene (0.4%). These results are in agreement with those of Lee and Shibamoto (2001), who reported eugenol and eugenyl acetate as the major compounds in the essential oil of clove buds. Eugenol, β-caryophyllene, α-caryophyllene and carvacrol were the major identified compounds in the EO of clove from Indonesia and India (Hossain et al., 2012). Eugenol has been shown to be effective for treatment of different diseases including cancer (Raja et al., 2015).
Analysis of the EO of rosemary dry leaves revealed presence of nineteen compounds representing 98.5% of the total oil. 1, 8-Cineol was the predominant compound followed by camphor, α-terpineol, α-pinene, bornyl acetate and borneol (Table 2). The present results were consistent with those of previous studies concerned with composition of rosemary EO. Although the relative quantities of the individual compounds showed some variations (Hendel et al., 2019; Ayoob et al., 2018). The chemical composition of the EO of rosemary leaves collected from Sinai and Giza in Egypt were studied early (Soliman et al., 1994). The results revealed that verbenone, camphor, bornyl acetate and limonene being the major compounds in sample from Sinai, whereas camphor, α-pinene and 1, 8-cineol were the main compounds in sample from Giza. 1,8-cineol has antifungal and antiaflatoxigenic activity (Kim et al., 2018).
Thirty volatile compounds were identified in the essential oil of ginger representing 98.2% of the total oil (Table 2). Zingiberene was the principal compound. It comprised 31.2% of the total oil followed by α-curcumene, β- sesquiphellandrene, geranial, β-phellandrene, camphene, neral, α-bisabolole and γ-eudesmol. Nampoothiri et al. (2012) carried a comparative study between the compositions of three ginger cultivars from Sub Himalayan region. The three samples showed some deviations and similarities in composition of their volatile oils. Zingiberene and β- sesquiphellandrene were the major compounds in the first sample, zingiberene and γ-curcumene were the main compounds in the second whereas geranial and neral were the predominant compounds in the third sample. The essential oil composition of ginger cultivated in Ecuador’s Amazonia region showed high amounts of zingiberene (23.9%), β-bisabolene (11.4%) and β-sesquiphellandrene (10.9%). Similar results were found for ginger oil from Italy (Sacchetti et al., 2005). Zingiberene was the most abundant compound identified in the EOs of ginger from Guinea (West Africa) and China (Toure and Xiaoming, 2007).
GC - MS analysis of chamomile essential oil revealed the presence of sixteen compounds representing 96.0% of the total oil. α- Bisabolol oxide A (45.5%), α- bisabolol oxide B (14.7%), α- bisabolone oxide A (12.9%), En-in-dicycloether (9.2%), cis-β-farnesene (5.7%) and chamazulene (3.2%) were the major identified compounds. These results are in agreement with those of Goger et al. (2018). Fırat et al. (2018) detected six volatile compounds in chamomile essential oil. Among them α-bisabolol oxide A comprised 47.7% followed by cis-β-farnesene 21.05%. α-Bisabolol oxide A in EO of chamomile from Egypt was more than three folds that from Brazil (Presibella et al., 2006). The major volatile compounds in EO of chamomile grown in Iran were in descending order: α-bisabolol oxide A > chamazulene > α-bisabolone oxide A > α-bisabolol oxide B (Amiri and Sharafzadeh, 2014). α-Bisabolol exhibits several pharmacological properties such as antibiotic and anticancer (Kamatou and Viljoen, 2010).
Ten volatile compounds were identified in the EO of dill seeds, representing 98.1% of the total oil (Table 2). The major compounds were carvone 60.2%, trans-dihydrocarvone 21.2%, limonene 9.4% and cis-dihydrocarvone 5.3%. These results agreed with some previous studies (Radulescu et al., 2010). Carvone was the major identified compound in Estonian dill seed oil followed by limonene and cis-dihydrocarvone (Vokk et al., 2011), whereas trans-dihydrocarvone was found in a very low concentration. The composition of dill seeds EO reported by Singh (2012) showed remarkable differences being limonene the major compound followed by grandisol and carvone. The odour was characterized by strong lemon odour and considered as a rich source of limonene.
The chemical composition of the hydrodistilled thyme oil is cited in Table 2. Thirty six volatile compounds were identified representing 99.4% of the total oil. The main compound was thymol followed by p-cymene, carvacrol, caryophyllene oxide, γ-terpinene and β-caryophyllene. These results are partially confirming those of previous studies (Tomaino et al., 2005; Mokhtarzadeh et al., 2018). The high percentage of thymol in the present study revealed that the thyme under investigation could be perceived as thymol chemotype. No significant differences were found between the composition of Iranian and British thyme EOs being thymol the predominant compound followed by γ-terpinene, p-cymene and carvacrol (Alizadeh, 2013). The authors proposed the biosynthesis pathways of thymol and carvacrol from γ-terpinene and p-cymene.
Twenty four volatile compounds were identified in the EO of basil leaves, representing 99.2% of the total detected constituents. Among these compounds, methyl chavicol was the major one followed by linalool, α-cadinol, trans-α-bergamotene, δ-cadinene, eugenol, β-elemene and 1, 8-cineol. The GC- MS analysis of the hydrodistilled EO from Egyptian sweet basil leaves revealed that linalool was the major compound followed by methyl chavicol and 1, 8-cineole (Chenni et al., 2016). Ismail (2006) reported different composition of the Egyptian basil EO, where linalool, 1,8-cineol, eugenol and methyl cinnamate were the predominant compounds. GC- MS analysis of the hydrodistilled oil of two basil samples from Turkey (Ocimunbasilium and Ocimum minimum) revealed that methyl eugenol, α-cubebene and nerol, were the major compounds in the first, whereas geranyl acetate and terpinen-4-ol were the predominant compounds in the second. Semeniuc et al. (2018) found that methyl chavicol, 1,8- cineol and linalool acetate were the major identified compounds in basil EO from Romania.
3.2 Total phenolic and radical scavenging activity
The phenolic compounds are known to have antioxidant activity; it is likely that the activity of most EOs is due to these compounds (Rozanida et al., 2006). As shown in Table 3, the EO of clove buds comprised the highest phenolic content (456.83 mg GAE/mL EO), whereas, rosemary essential oil showed the lowest phenolic content (2.68 mg GAE/EO). Previous study by Gharib and Teixeira da Silva (2012) showed an opposite trend. Different letters among the values in each column mean significant difference at p < 0.05.
Aromatic plants
TP (mg GAE a/mL EO)
DPPH (mg AAE b/mL EO)
ABTS (mmol TE c/mL EO)
Thyme
185.58b ± 1.33
6.81c ± 1.09
234.73c ± 2.13
Ginger
4.92f ± 0.021
2.13e ± 0.13
20.97f ± 0.29
Clove
456.83a ± 6.85
12.53a ± 1.03
636.77a ± 3.25
Basil
32.67d ± 1.27
9.43b ± 1.02
177.93d ± 1.62
Rosemary
2.68f ± 0.57
1.14f ± 0.019
26.97f ± 0.42
Dill
6.43ef ± 0.13
0.77f ± 0.02
8.86f ± 0.13
Marjoram
148.33c ± 3.22
12.01a ± 0.02
451.46b ± 2.65
Cinnamon
7.16ef ± 1.19
5.48d ± 0.035
166.94d ± 1.33
Anise
10.56e ± 0.16
4.73d ± 0.02
169.66d ± 1.42
Chamomile
8.38ef ± 0.27
1.64ef ± 0.019
57.85e ± 0.55
In present study, DPPH radical and ABTS radical assays were used for the evaluation of free radical scavenging properties of the ten investigated essential oils (Table 3). Both methods revealed similar decreasing order of radical scavenging activity for the EOs that exhibited the highest activities. Whereas, there were some variations among the EOs that showed moderate or low activity (Table 3).
As shown in Table 3, clove buds EO provided the highest DPPH and ABTS radical-scavenging activity. This finding is mainly attributed to the synergistic effect between the phenolic compounds even at low concentrations (Radünz et al., 2019). The high phenolic content in clove buds EO (Table 3) confirms this result. The distilled aroma compounds of clove buds at concentration from 50 to 500 µg/mL inhibited hexanal oxidation by 100% for 30 days (Lee and Shibamoto, 2001). The authors attributed this result to the high content of the phenolic compound, eugenol.
Marjoram showed the second high scavenging ability on DPPH and ABTS radicals (Table 3). In comparison with basil and rosemary, marjoram EO exhibited the highest antioxidant power (Baratta et al., 1998). Al-Fatimi (2018) correlated the potent antioxidant activity (AOA) of marjoram EO to the presence of alcohol terpenes, mainly terpinen-4-ol, the major identified compound in the present study (Table 2). Synergistic interactions among the antioxidant compounds identified in marjoram essential oil such as α-terpinene, p-cymene, carvone, thymol, carvacrol and germacrene D may have role to play. Hajlaoui et al. (2016) correlated the high antioxidant activity of marjoram EO to the high content of terpinene −4-ol. As shown in Table 3, the basil EO tested in the DPPH and ABTS assays exhibited high antioxidant properties (9.43 mg AAE/ mL EO and 177.93 mmol TE/mL EO, respectively). This finding is mainly attributed to the presence of eugenol in considerable concentration (4.64%) (Table 3). The EO of basil from Thailand (linalool/eugenol chemotype) presented a high (antioxidant) AO power (Pripdeevech et al., 2010). On the contrary, the basil essential oil (linalool chemotype) from Algeria, free from eugenol, showed AOA lower than α-tocopherol (Hadj-Khelifa et al., 2016). Dabire et al. (2011) reported that the decrease in eugenol content in the EO, in presence of linalool, gave rise to a significant decrease in its antioxidant power.
The thyme EO tested in the DPPH and ABTS assays showed intermediate scavenging radical activity compared with the other nine tested EOs (Table 3). The two phenolic compounds, thymol and its isomer carvacrol, are responsible for this finding in addition to other antioxidant compounds such as p-cymene and γ-terpinene (Kulisic et al., 2004). The present results are in agreement with those of a previous study, which reported that the antioxidant efficiency of some EOs was in the descending order: clove > basil > thyme (Tomaino et al., 2005). A comparative study between the EOs of Iranian and British thyme revealed a positive correlation between the concentration of thymol, phenolic content and antioxidant activity (Alizadeh, 2013). Sacchetti et al. (2005) assessed the antioxidant activity of 11 essential oils compared to that of thyme EO. All the essential oils notably reduced the concentration of DPPH free radical with efficiency lower than that of thyme EO, which showed 75.6% inhibition.
The DPPH and ABTS radical scavenging activity of cinnamon bark EO was lower than that of thyme (Table 3). The presence of eugenol (0.8%) in the cinnamon bark EO (Table 2) may enhance its scavenging radical ability. Tomaino et al. (2005) correlated the high activity of clove and cinnamon EOs to the presence of eugenol. This finding was in agreement with the concept that the presence of a phenolic group containing an electron repelling group in ortho position to the phenolic group is required to achieve the strong radical scavenging effect.
In the present study anise EO showed a relatively low free radical scavenging activity in the DPPH and ABTS assays (Table 3). trans-Anethol, the major identified compound, might be responsible for the observed antioxidant power (Burits and Bucar, 2003).
The free radical scavenging activity of ginger EO may be due to the presence of some antioxidant compounds such as curcumins, neral and geranial (Yashin et al., 2017). Choi et al. (2000) confirmed the citral isomers (neral and geranial) radical scavenging activity towards DPPH free radical. Ginger EO reduced the concentration of DPPH free radical with efficiency lower than that of thyme (Sacchetti et al., 2005).
As shown in Table 3 chamomile EC showed also a relatively low free radical scavenging activity, compared with the other investigated essential oils. Chamomile EO has been reported as a natural antioxidant, its AOA may be correlated to the presence of chamazulen (Table 2), which is considered as an important antioxidant compound (Buckle, 2015). The antioxidant capacity of chamomile EO as well as its major constituents was evaluated using DPPH assay (Fırat et al., 2018). The free radical inhibitory activity was in the descending order chamazulene ˃ α-bisabolol oxide A ˃ chamomile EO ˃ cis-β-farnesene ˃ α-bisabolol.
Rosemary EO, which contained the lowest phenolic content (Table 3), exhibited a very low antioxidant activity. This finding may be correlated to the method of drying and/or the chemotype of the investigated rosemary leaves (Sacchetti et al., 2005). The DPPH scavenging activity of rosemary EO isolated from fresh leaves was proved to be higher than that of dried leaves (Gharib and Teixeira da silva, 2012). The antioxidant activity of rosemary EO rich in verbenone (21.8%) and borneol (10.4%) was relatively high compared with other 11 essential oils (Sacchetti et al., 2005). The performance of this rosemary EO was found to be better than that of rosemary of α-pinene/1,8-cineol/camphor chemotype (Baratta et al., 1998). The results in Table 2 indicated that EO of rosemary dry leaves investigated in the present study was from a α-pinene/1,8-cineol/camphor chemotype. Dill EO showed the lowest DPPH and ABTS free radical scavenging activity (Table 3) compared with the other nine investigated EOs. Its activity may be attributed to the presence of limonene and carvone at high concentrations (Yashin et al., 2017).
3.3 Microencapsulation of clove EO
As have been shown in the aforementioned results the EO of clove buds exhibited the highest EO content, phenolic yield and antioxidant activity. So, it was selected to evaluate the efficiency of different commonly used encapsulating materials (sod-Alg, Ch and CMC).
3.3.1 Scanning electron microscopy (SEM)
The morphologies of clove essential oil (CE) encapsulated in sodium alginate (CE-Alg), chitosan (CE-Ch) and CMC (CE-CMC) microbeads are shown in Fig. 1. The particle sizes of microbeads were 0.24 mm, 0.29 mm and 0.32 mm for CE-Alg., CE-Ch and CE-CMC, respectively. Varona et al. (2013) stated that small particle sizes give rise to a broad size distribution and consequently increase the loaded essential oil. The majority of the CE-Alg and CE-Ch microbeads were spherical with aggregation and some deformation. The microbeads CE-CMC showed spherical beads with less aggregation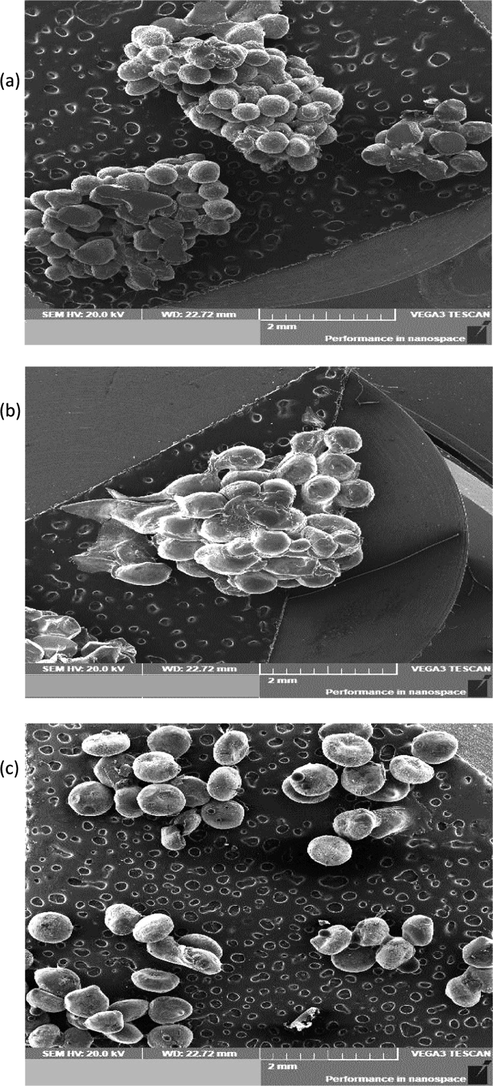
Scanning Electron Microscopy (SEM) analysis. SEM graphs of CE-Alg (a), CE-Ch (b) and CE-CMC (c).
3.3.2 Effect of microencapsulation on the main compounds in clove EO
The GC–MS analysis revealed a significant increase in eugenol content (from 89.9 to 96.90 and 96.00%) in clove essential oil (CE) encapsulated in sodium alginate (CE-Alg) and chitosan (CE-Ch), respectively (Table 1S). Whereas, no variation was found in eugenol content in oil encapsulated in CMC (CE – CMC). β - Caryophyllene showed a significant decrease in all samples. Eugenyl acetate exhibited a significant (p < 0.05) decrease in the clove EO extracted from CE-Alg and CE-Ch beads.
3.3.3 Loading capacity and encapsulation efficiency of coating materials
As shown in Fig. 2, CE-Alg beads showed the highest loading capacity (26.31%) followed by CE-Ch (26.0%), whereas CE-CMC exhibited the lowest value (22.46%). The same trend was found for the encapsulation efficiency (EE %) where, CE-Alg beads showed the highest value (96.3%) followed by CE-Ch (94.1%) and CE-CMC (89.2%) (Fig. 1). These results are comparable to those achieved with clove essential oil encapsulated in alginate beads (Soliman et al., 2013). The authors reported that the formulation including sodium alginate concentration 2% w/v, calcium chloride concentration 0.5% w/v and cross linking time 20 min gave maximum loading capacity (23.5%) and encapsulation efficiency (94%) of clove oil. The difference in the encapsulation efficiency among the investigated samples (CE-Alg, CE-Ch and CE-CMC) may be attributed to the changes in the essential oil physicochemical properties, which are determined by its composition (Table 2) (Arana-Sanchez et al., 2010).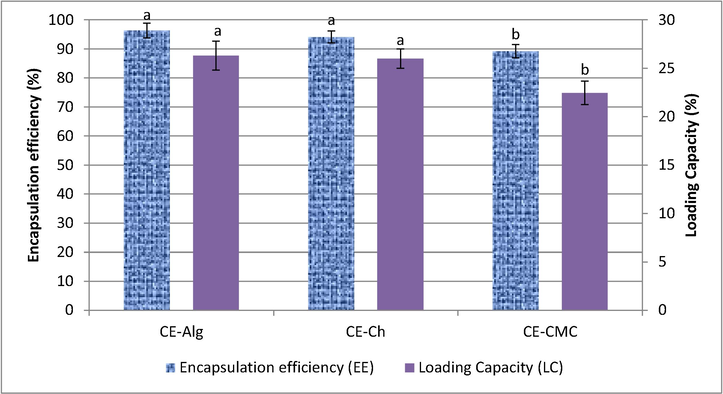
Encapsulation efficiency and loading capacity of different microcapsule materials. CE-Alg: Clove essential oil encapsulated in alginate, CE-CH: Clove essential oil encapsulated in chitosan, CE-CMC: Clove essential oil encapsulated in Carboxy methylcellulose. Different letters among the EE or LC mean significant difference at p < 0.05.
3.3.4 Effect of coating materials on phenolic content and antioxidant activity
As shown in Fig. 3 CE-Alg exhibited the highest phenolic content (134.83 mg GAE/mL EO) followed by CE-Ch (119.22 mg GAE/mL EO) and CE-CMC (90.51 mg GAE/mL EO). As mentioned before, (Table 2), the GC - MS analysis revealed that the concentration of eugenol was in the order: CE-Alg ˃ CE-Ch ˃ CE-CMC in the encapsulated clove EO. The radical scavenging activity showed similar trend. The radical scavenging effect of the encapsulated clove EO in sample CE-Alg revealed the highest scavenging effect on DPPH radical (Fig. 2) followed by CE-Ch. This difference in the antioxidant activity could be attributed to the changes in composition of the entrapped oil (Table 1S) and the antioxidant activity of the coating materials (Rajalakshmi et al., 2013). Arana-Sanchez et al. (2010) studied the changes in the composition of oregano essential oil occurred after microencapsulation. They correlated the increase in its antioxidant activity to the increase of the two volatile phenolic compounds thymol and carvacrol.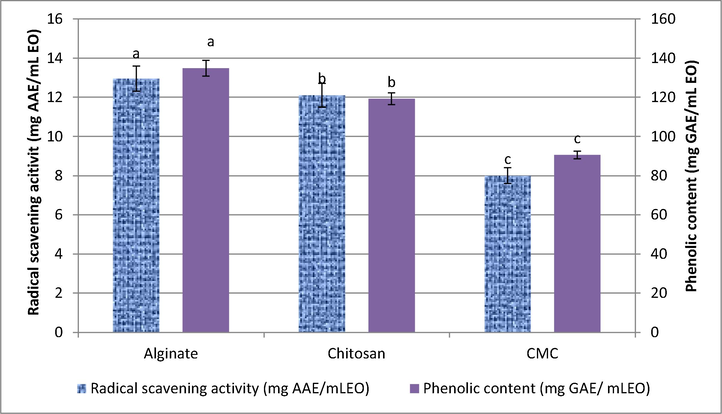
Radical scavenging activity and Phenolic content of the encapsulated clove EO. Different letters among the DPPH scavenging activity or phenolic content of the encapsulated EO mean significant difference at p < 0.05.
4 Conclusions
Currently there is an increasing interest in using the essential oils of the aromatic plants as natural antioxidants in both food and pharmaceutical industries. Thus, it is very important to conduct studies linking the chemical composition, phenolic content and antioxidant function of the essential oils of ten aromatic plants extensively consumed in Egypt in food and drinks. Their antioxidant ability was in a descending order: clove > marjoram > thyme > basil > anise > cinnamon > ginger > chamomile > rosemary > dill. The DPPH radical scavenging activity of clove, thyme and anise EOs was mainly attributed to the high content of the phenolic compounds: eugenol, thymol and trans-anethole, respectively. Whereas, the scavenging radical activity of the other EOs was correlated to the synergism between the nonphenolic antioxidant compounds such as terpinene-4-ol, α-terpinene, curcumene and chamazulene. Based on the obtained results clove EO showed the highest oil content and radical scavenging activity; thus, it was subjected to microencapsulation in three separate coating materials. The improvement in free radical scavenging activity of the EO entrapped in sodium alginate and chitosan compared with CMC may be attributed to their high content of the phenolic compound, eugenol, and the antioxidant activity of these coating materials. The results indicated that encapsulation of the EOs by the emulsion extrusion process can be considered as an efficient technique for the production of natural, easily handle antioxidants that are convenient to be used in food and pharmaceutical industries
Acknowledgement
This work was supported by Ministry of Higher Education and Scientific Research, Egypt (grant number: 33 – 13 – A2).
Declaration of Competing Interest
There are no conflicts to declare.
References
- Identification of essential oil components by gas chromatography/quadruple mass spectroscopy. Allured: Publishing Corporation, Carol Stream llinois; 2001.
- Food Lipids: Chemistry, Nutrition, and Biotechnology. New York.: Marced Dekker Inc; 1998. p. :840.
- Volatile Constituents, Antimicrobial and AntioxidantActivities of the Aerial Parts of Origanum majorana L. from Yemen. J. Pharma. Res. Int.. 2018;23:1-10.
- [CrossRef] [Google Scholar]
- Essential oil constituents, phenolic content and antioxidant activity in Iranian and British Thymus vulgaris L. Int. J. Agric. Crop Sci.. 2013;6:213-218.
- [Google Scholar]
- Essential oil components of German chamomile cultivated in Firoozabad, Iran. Orient. J. Chem.. 2014;30:365-367.
- [Google Scholar]
- Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippiagraveolens H. B. K.) with different composition when microencapsulated in b-cyclodextrin. Lett. Appl. Micro.. 2010;50:585-590.
- [CrossRef] [Google Scholar]
- Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci. Technol.. 2000;11:419-421.
- [CrossRef] [Google Scholar]
- Chemistry and bioactivity of cinnamaldehyde: a natural molecule of medicinal importance. Int. J. Pharm. Sci. Res.. 2017;8:2333-2340.
- [Google Scholar]
- Variation in essential oil content and composition in Turkish anise (Pimpinella anisum L.) populations. Turk. J. Agric. Forest.. 2004;28:173-177.
- [Google Scholar]
- Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in -cyclodextrin. J. Incl Phenom. Macro.. 2008;60:359-368.
- [Google Scholar]
- Essential oil composition of Rosmarinus officinalis L. from Kashmir (India) EC Microboil. 2018;14:29-32.
- [Google Scholar]
- Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data.. 2011;40 043101–1 - 043101–47
- [Google Scholar]
- Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Frag. J.. 1998;13:235-244. https:// doi: 10.1002/(SICI)1099-1026(1998070)13:4<235::AID-FFJ733>3.0.CO;2-T
- [Google Scholar]
- Basic plant taxonomy, basic essential oil chemistry, extraction, biosynthesis, and analysis. In: Barlow J., ed. Clinical aromatherapy. St. Louis: Churchill Livingstone; 2015. p. :37-72.
- [Google Scholar]
- Antioxidant activity of Nigella sativa essential oil. Phytother. Res.. 2003;4:323-328.
- [CrossRef] [Google Scholar]
- Preparation of ca-alginate beads containing high oil content: influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym.. 2011;84:1267-1275.
- [CrossRef] [Google Scholar]
- Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydrodistillation and solvent free microwave extraction. Molecules. 2016;21:E113-E128.
- [CrossRef] [Google Scholar]
- Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-Diphenyl-2-picrylhydrazyl. J. Agric. Food Chem.. 2000;48:4156-4161.
- [CrossRef] [Google Scholar]
- Cu, J.Q., 1986. The kingdom of essential oil. In: Lawrence B.M., Mookherjee B.D., Willi, B.J. (Eds.), Flavour and Fragrance: a World Perspective, Proceedings of the 10th International Congress of Essential oils. Washington, DC, USA.
- Effect of drying the plant material on the chemical composition of the essential oil and antioxidant activity of extracts of Ocimum basilicum L. Int. J. Biol. Chem. Sci.. 2011;5:1082-1095.
- [Google Scholar]
- Rosmarinus officinalis L: effect of drying on the volatile oil of fresh leaves and antioxidant activity of their extracts. J. Essent. Bear. Pl.. 2000;3:5-19.
- [Google Scholar]
- Antioxidant activity of chamomile essential oil and main components. Nat. Volatiles Essent. Oils. 2018;5:11-16.
- [Google Scholar]
- Gharib, F.A.E., Teixeira da silva, J.A., 2012. Composition of total phenolic content and antioxidant activity of the essential oil of four Lamiaceae herbs. Med. Aromat. Plant Sci. Biotechnol. 7: 19-27.
- Antimicrobial and toxicity profiles evaluation of the chamomile (Matricariarecutita L.) essential oil combination with standard antimicrobial agents. Ind. Crops Prod.. 2018;120:279-285.
- [CrossRef] [Google Scholar]
- Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci. Technol.. 2004;15:330-347.
- [CrossRef] [Google Scholar]
- Chemical composition and antioxidant activity of essential oil of Ocimum basilicum leaves from the northern region of Algeria. Topcl. J. Herb. Med.. 2016;1:25-30.
- [Google Scholar]
- Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and antiacetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog.. 2016;95:86-94.
- [CrossRef] [Google Scholar]
- Antioxidants: firms seeking products they can label as 'natural' AOCS. Inform. 1990;1:1002-1013.
- [Google Scholar]
- Essential oil from aerial parts of wild Algerian rosemary: screening of chemical composition, antimicrobial and antioxidant activities. J. Essent. Bear. Pl. 2019
- [CrossRef] [Google Scholar]
- Hossain, M.A., Al-Hashmi, R.A., Weli, A.M., Al-Riyami, Q. , Al-Sabahib, J.N., 2012. Constituents of the essential oil from different brands of Syzigium caryophyllatum L by gas chromatography-mass spectrometry. Asian Pacific J. Trop. Biomed. S1446-S1449. https:// doi:10.1016/S2221-1691(12)60435-3.
- Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Res. Int.. 2008;41:194-200.
- [CrossRef] [Google Scholar]
- Central properties and chemical composition of Ocimum basilicum essential oil. Pharm. Biol.. 2006;44:619-626.
- [CrossRef] [Google Scholar]
- An in vivo and in vitro investigation on hepatoprotective effects of Pimpinella anisum seed essential oil and extracts against carbon tetrachloride induced toxicity. Iran. J. Basic Med. Sci.. 2015;18:205-211.
- [Google Scholar]
- A Review of the Application and Pharmacological Properties of a-Bisabolol and a-Bisabolol-Rich Oils. J. Am. Oil. Chem. Soc.. 2010;87:1-7.
- [CrossRef] [Google Scholar]
- Antifungal and Antiaflatoxigenic Activities of 1,8-Cineole and t-Cinnamaldehyde on Aspergillus flavus. Appl. Sci.. 2018;8:1655-1664.
- [CrossRef] [Google Scholar]
- Cinnamon bark oil and its components inhibit biofilm formation and toxinproduction. Int. J. Food Micro.. 2015;195:30-39.
- [CrossRef] [Google Scholar]
- Use of different methods for testing activity of oregano essential oil. Food Chem.. 2004;85:633-640.
- [CrossRef] [Google Scholar]
- Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum(L.) Merr. et Perry] Food Chem.. 2001;74:443-448.
- [CrossRef] [Google Scholar]
- Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem.. 2009;112:77-83.
- [CrossRef] [Google Scholar]
- Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem.. 2007;100:1409-1418.
- [CrossRef] [Google Scholar]
- In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control.. 2007;22:1715-1722.
- [CrossRef] [Google Scholar]
- Determination of volatile components in Thymus vulgaris L. under in vitro conditions. J. Essent. Bear. Pl.. 2018;21:277-281.
- [CrossRef] [Google Scholar]
- Comparison of essential oil composition of three ginger cultivars from sub Himalayan region. Asian Pac, J. Trop. Biomed 2012:S1347-S1350.
- [Google Scholar]
- Biopolymer-based nanoparticles for drug/ gene delivery and tissue engineering. Int. J. Mol. Sci.. 2013;14:1629-1654.
- [CrossRef] [Google Scholar]
- Comparison of chemical constituents of Chamomill arecutita L. rauschert essential oil and its antichemotactic activity. Braz. Arch. Biol. Technol.. 2006;49:717-724.
- [CrossRef] [Google Scholar]
- The chemical composition and antioxidant activities of basil from Thailand using retention indices and comprehensive two dimensional gas chromatography. J. Serb. Chem. Soc.. 2010;75:1503-1513.
- [CrossRef] [Google Scholar]
- Chemical composition of the volatile oil from different plant parts of Anethum graveolens L. (Umbelliferae) cultivated in Romania. Farmacia. 2010;58:594-600.
- [Google Scholar]
- Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem.. 2019;15:180-186.
- [CrossRef] [Google Scholar]
- Versatile and synergistic potential of eugenol. A Review. Pharm. Anal. Acta. 2015;6:367-372.
- [CrossRef] [Google Scholar]
- Rajalakshmi, A., Krithiga, N., Jayachitra, A.A., 2013. Antioxidant Activity of the chitosan extracted from shrimp exoskeleton. Middle-East J. Sci. Res. 16, 1446-1451. https://doi: 10.5829/idosi.mejsr.2013.16.10.12033
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical. Bio. Med.. 1999;26:1231-1237.
- [CrossRef] [Google Scholar]
- Rozanida, A.R., Nurul, N.I., Helme, M.N.H., Zanariah, H.A., 2006. Cosmeceutical product from species in the family Zingiberaceae, In: Mazura, M.P. (Ed.), Harnessing Cures from Nature: Trends and Prospects. Forest Research Institute: Kepong, Selangor, Malaysia, pp. 31–36.
- Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem.. 2005;91:621-632.
- [CrossRef] [Google Scholar]
- Application of anise and rocket essential oils in preservation of old manuscripts against fungal deterioration. Int. J. Conserv. Sci.. 2018;9:235-244.
- [Google Scholar]
- Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules. 2018;23:2261-2273.
- [CrossRef] [Google Scholar]
- Chemical constituents of essential oil from anethum sowa kurz. seed. J. Chem. Pharm. Res.. 2012;4:4156-4160.
- [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enz.. 1999;299:152-178.
- [CrossRef] [Google Scholar]
- Microencapsulation of essential oils within alginate: formulation and in vitro evaluation of antifungal activity. J. Encap. Adsor. Sci.. 2013;3:48-55.
- [CrossRef] [Google Scholar]
- Analysis and Biological Activity of the essential oil of Rosmarinus officinalis from Egypt. Flavour Frag. J.. 1994;9:29-33.
- [CrossRef] [Google Scholar]
- Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed.. 2019;14:5303-5321.
- [CrossRef] [Google Scholar]
- Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem.. 2005;89:549-554.
- [CrossRef] [Google Scholar]
- Gas chromatographic analysis of volatile components of Guinean and Chinese gingers oils (Zingiber Officinal) extraction by steam distillation. J. Agron.. 2007;6:350-355.
- [CrossRef] [Google Scholar]
- Encapsulation of lavandin essential oil in poly-(∊-caprolactones) by PGSS process. Chem. Eng. Technol. 2013
- [CrossRef] [Google Scholar]
- Dill (Anethum graveolens L.) and parsley (Petroselinum crispum(Mill.)Fuss) from Estonia: seasonal differences in essential oil composition. Agron. Res.. 2011;9:515-520.
- [Google Scholar]
- Yashin, A., Yashin, Y., Xia, X., Nemzer, B., 2017. Antioxidant activity of spices and their impact on human health. Antioxidants (Basel). 6, 1-18. https://doi: 10.3390/antiox6030070.
- Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem.. 1995;43:27-32.
- [Google Scholar]
- Extrusion of mixtures of starch and d-limonene encapsulated with b-cyclodextrin: flavour retention and physical properties. Food Res. Int.. 2006;39:318-331.
- [CrossRef] [Google Scholar]
- Novel thymol bearing oxypropanolamine derivatives as potent some metabolic enzyme inhibitors – Their antidiabetic, anticholinergic and antibacterial potentials. Bioorg. Chem.. 2018;119–126
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.06.034.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







