Translate this page into:
Coumarin derivatives as promising antibacterial agent(s)
⁎Corresponding authors. rnpadhy54@gmail.com (Rabindra Nath Padhy), sairampaidesetty@gmail.com (Sudhir Kumar Paidesetty)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This review aims to outline the antibacterial activity of coumarin derivatives, and discuss their SARs to pave the way for the further rational development.
Abstract
Exploration of coumarin nucleus as potent antibacterial agent(s). Recent progression of synthetic approaches of coumarin derivatives. Structural-activity-relationship studies on coumarin derivatives.
Abstract
Nowadays, bacterial infections epitomize significant health threats globally with an increased morbidity and mortality. Most contemporary antibacterial agents are resisted by pathogenic bacteria - the multidrug resistant (MDR) bacterial strains arising from cross resistances operative in natural bacterial consortia inside human body and in environments. Consequently, the development of newer potential drug candidate(s) is required against the broad spectrum of MDR bacteria. Indeed, the phytochemical coumarin and its derivatives had been reported with broad biological inhibitory properties, including antibacterial activities. In this review, several methods of synthetic strategies of coumarin derivatives as antibacterials were considered with individual schematic compounds by structure-activity relationship (SAR) studies as essential corollaries. Overall, substituents at positions C-3 and C-4 of coumarin are coveted for the development of newer antibacterial agents.
Keywords
Coumarin
Semi-synthesis
Antibacterial
Structural-activity-relationship
1 Introduction
Today resistance patterns of pathogenic bacteria to frequently used drugs and antibiotics have become the commonplace incidence, creating annoys in clinics and havocs in public health worldwide. The problem is graver than that can be imagined because of continual genetic improvements of pathogenic bacterial strains by achieving resistance to drug/antibiotics that were never treated often; unfortunately, with any new generation of antibiotics or newly introduced drugs newer resistant bacteria evolve persistently. The inherent/intrinsic bacterial genetic exchange mechanisms facilitate bacterial consortia for gaining characters from genetically similar as well as, distant bacteria. Approximately 17 million people are dying each year from infectious diseases, and approximately, 50,000 people in all age group are affected (Holmes et al., 2017; Laxminarayan et al., 2006). Thus, the use of antibiotics rapidly loose effectiveness; thereby a vacuum is created in need/source of coveted antibacterial. Consequently, the development of new antibacterial drug candidate(s) remains as the call of the day. Increasing incidences of microbial infection by the development of microbial resistance of the most antibiotics through either genome of microbial mutations or an evolved the mechanism of resistance of action which is major health problem.
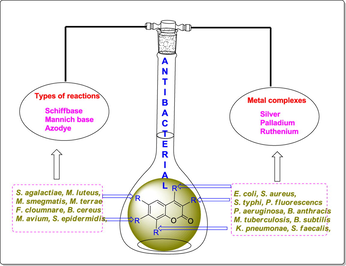
The molecular manipulations of promising two lead compounds with same biological actions are a rational approach to design and develop newer drug(s); it involves an effort to combine two different pharmacophore groups of similar activity into a hybrid lead candidate. Natural products particularly phytochemicals such as, vanillin, thymol, eugenol, menthol, umbelliferon, carvacrol, curcumin, and a few more are being used for several therapeutic purposes namely, antibacterial, antifungal and anticancer agents through main stream medicinal chemistry approaches (Baral et al., 2019; Sahoo et al., 2020a, 2020b). Moreover, coumarin (a phytochemical) is chemically the benz[α]pyroneand freely occurring as constituents or could be condensed with carbohydrate said to be glycosides (Fig. 1). It is a fused ring system between benzene and lactone known as ‘pyrone’ and structurally resembles to chromone; but the difference in both the positions of carbonyl or ketone system present in individual structures (Jain and Joshi, 2012). The carboxamide coumarin derivative i.e., ‘Novobiocin’ (Kasperkiewicz et al., 2020) and Chlorobiocin, Aminocoumarin and a few more that are commercially recommended antibiotics. Consequently, these derivatives are being reported asanti-microbial, anti-oxidant, anti-cancer, anti-HIV, anti-diabetic and anti-viralagents (Detsi et al., 2017). Herein, on basis of rationalisation of synthetic strategies of coumarin derivatives and their candidate’s potency against several pathogenic bacterial strains were studied with structure–activity relationship (SAR) studies. Moreover, the number of significant antibacterial coumarin candidates had been developed by attaching several substituents as functionality group, incorporation of new scaffolds and coordinate of metal ion complexes in different positions of coumarin. Similarly, norharmane, a natural bioactive compound and nostocine, a phyco-constituent had leant themselves to structural modifications at several positions with hopes to develop newer anticancer drug candidates (Sahoo et al., 2019a, 2019b).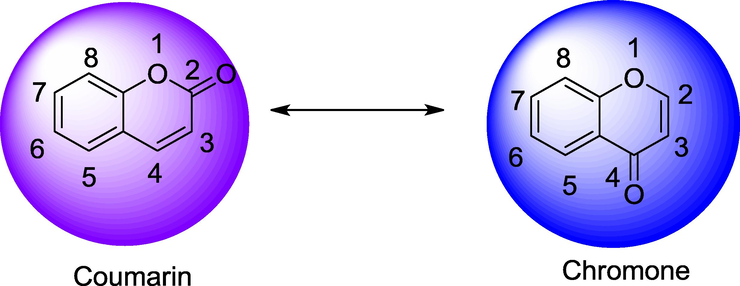
Structure of chromene nucleus.
This aim of this review is to have a concise account and detailed highlights of structural derivatives of coumarin with individually associated schematic strategies; by the by, to locate candidate(s) with significant antibacterial potency (Fig. 2). This would be the countenance to diverse groups of chemists, biologists and drug developers, to distinguish and to identity promising structures to be judged for further promotion in the development of newer therapeutic or antibacterial agent(s). Indeed, to locate a novel/curious molecule and its derivatives for treating various malicious infectious diseases remain as the obsessive quest in pharmacology. The involvement of principles of medicinal chemistry in modifying a phytochemical would be an eclectic approach for possible use as future antibacterials, in the face of MDR bacteria is a step against the general trend of floccinaucinihilipilification against phytocompounds as drug(s) (see Schemes 1–80).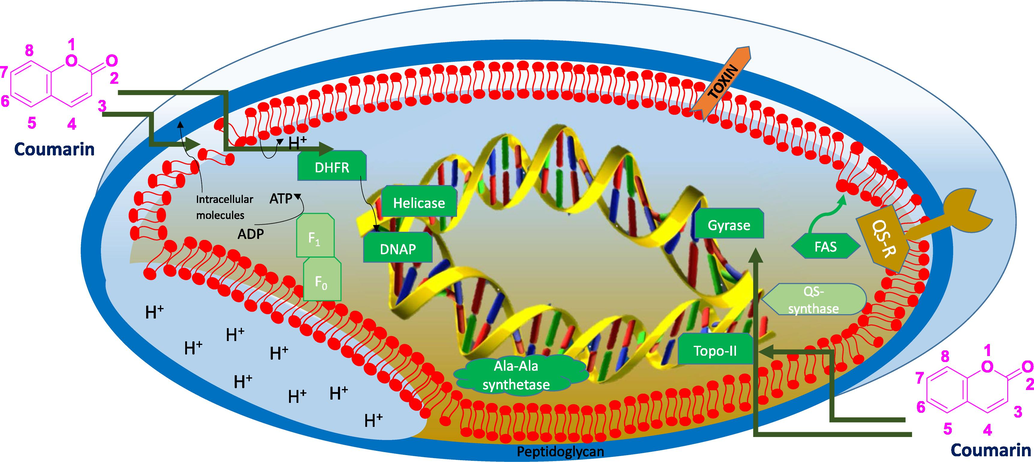
Schematic representation of bacterial cell inhibitory actions of coumarin derivatives.
2-Quinoxalone-coumarin hydrazone derivatives.
4-Azidomethyl-7-methyl-coumarin bearing sulfonamide.
Coumarin chalcone derivatives.
Metal complexes of 3-Acetylcoumarin-INH hybrid.
Coumarin fused with tetrahydroisoquinoline derivatives.
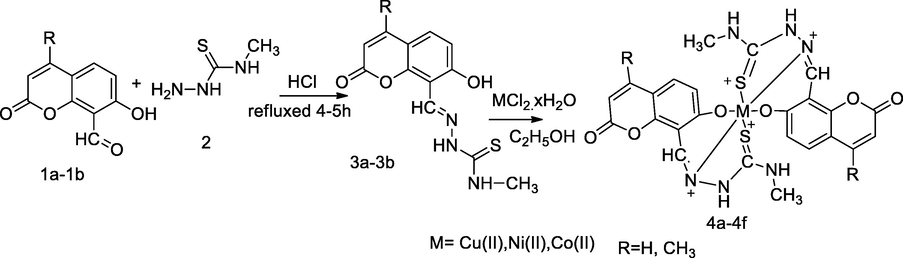
Metal complexes with 4-Methyl 7-hydroxy coumarin thiosemicarbazone.
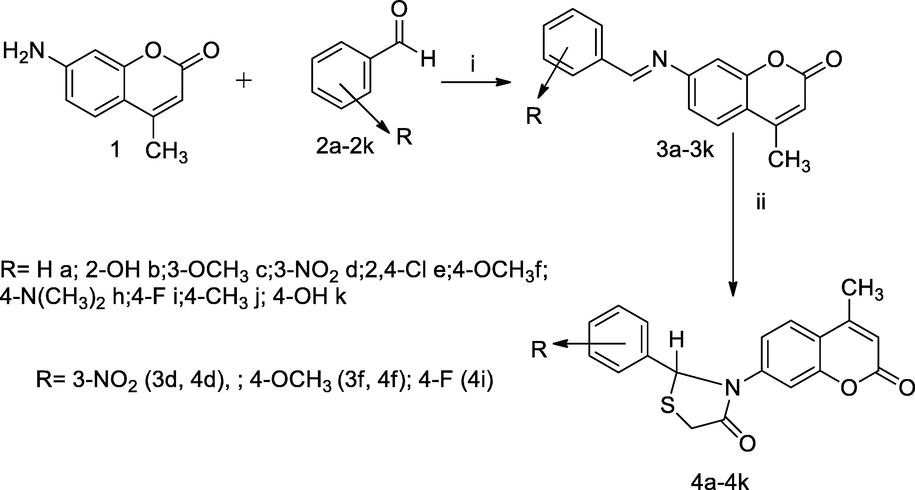
4-Methyl coumarin bearing thiazolidinone moiety.
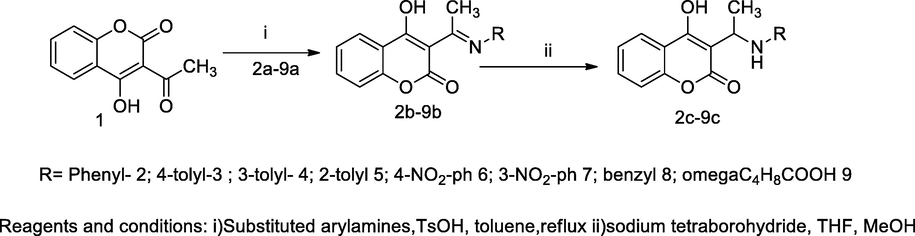
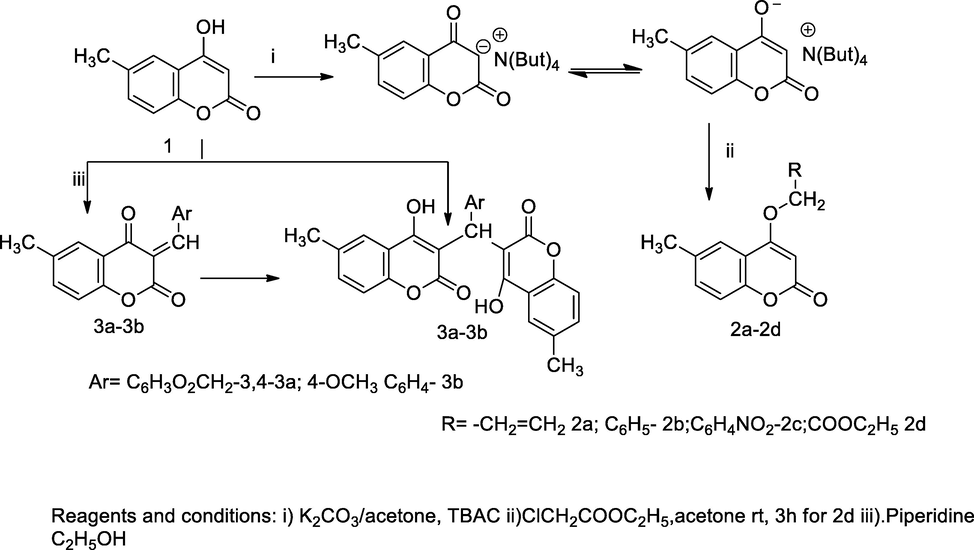
3-Aminoalkyl-4-hydroxy coumarin derivatives.
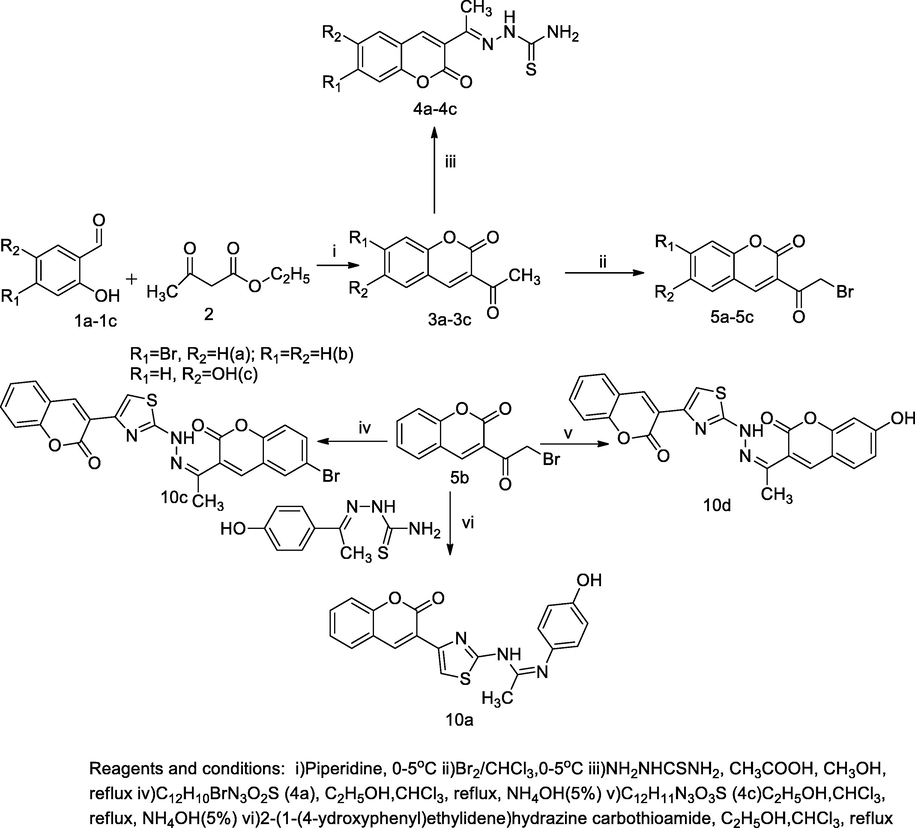
Hydrazonyl thiazolyl substituted coumarin derivatives.
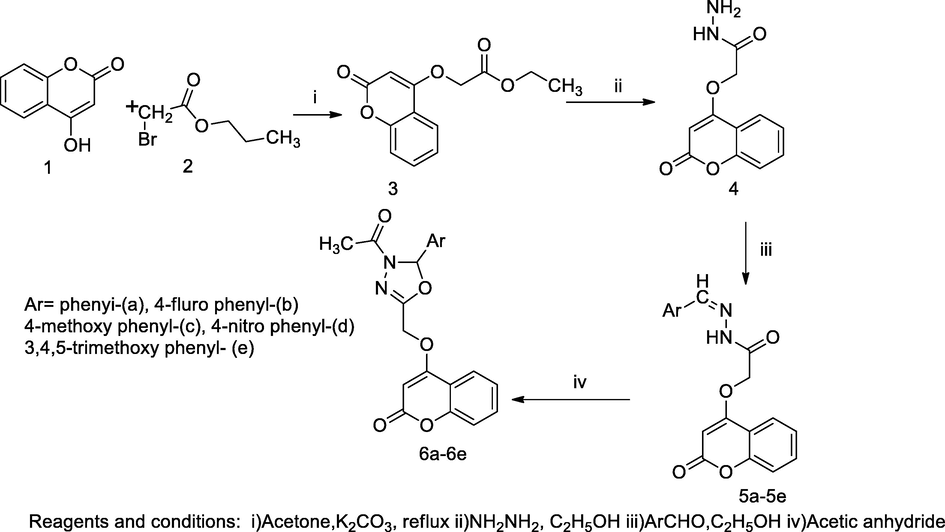
4-Hydroxycoumarin bearing oxadiazolone derivatives.
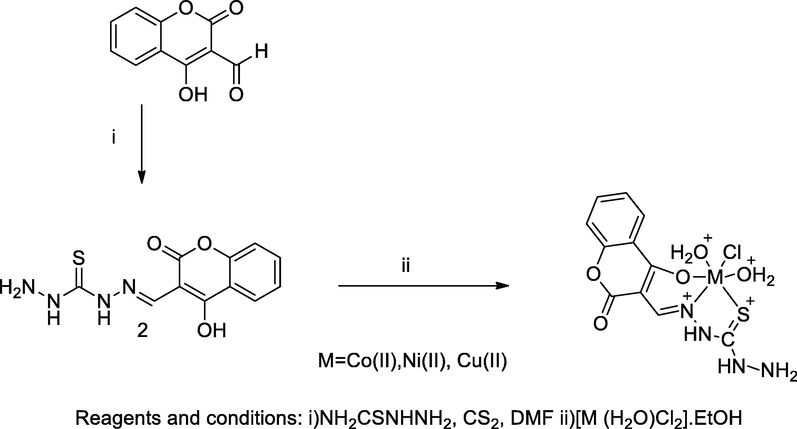
Metal complexes-semithiocarbazone of 4-Hydroxy coumarin.
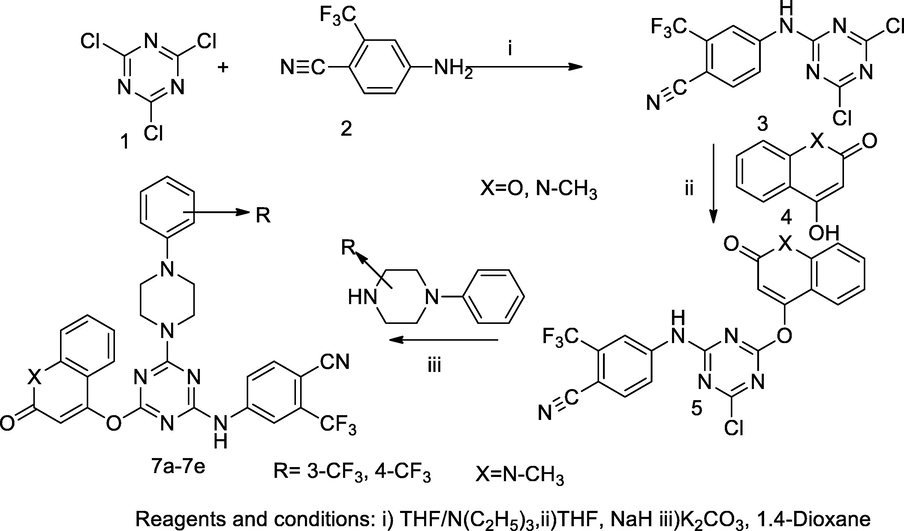
Coumarin bearing triazines derivatives.
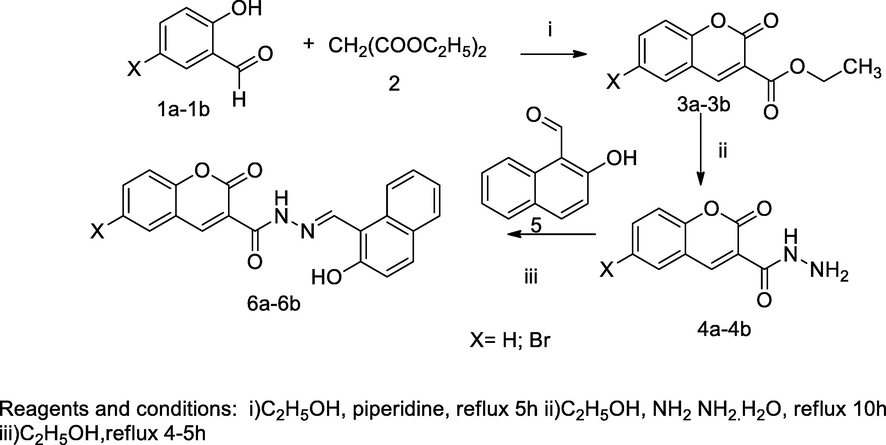
Metal complexes-Coumarin schiff base derivatives.
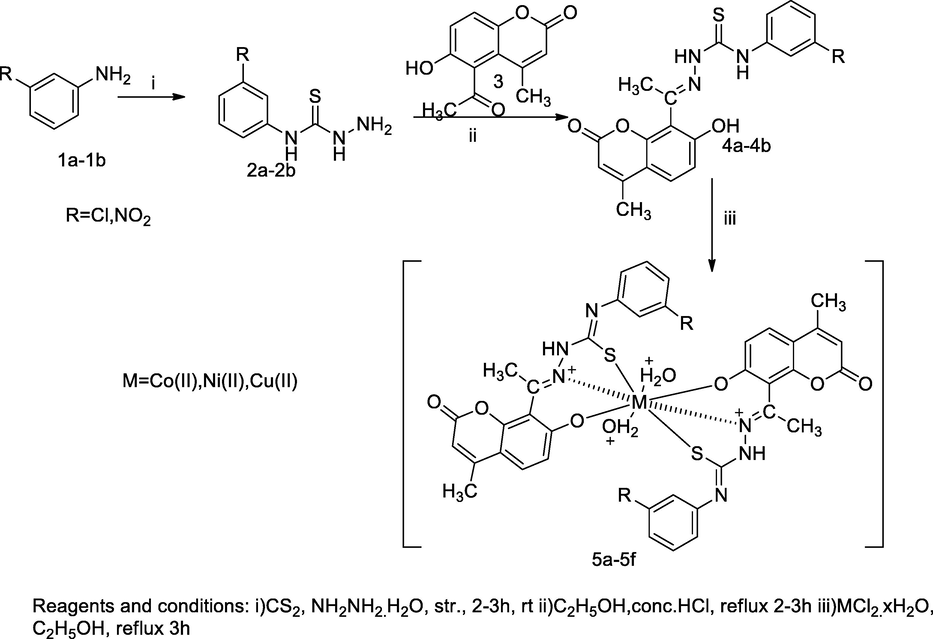
Metal complexes of Coumarin-thiosemicarbazones derivatives.
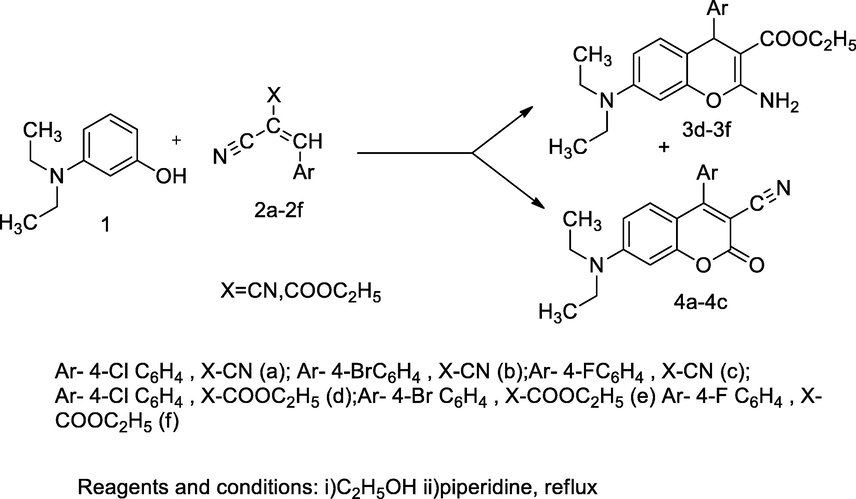
3,4,7-Tri substituted coumarin derivatives.
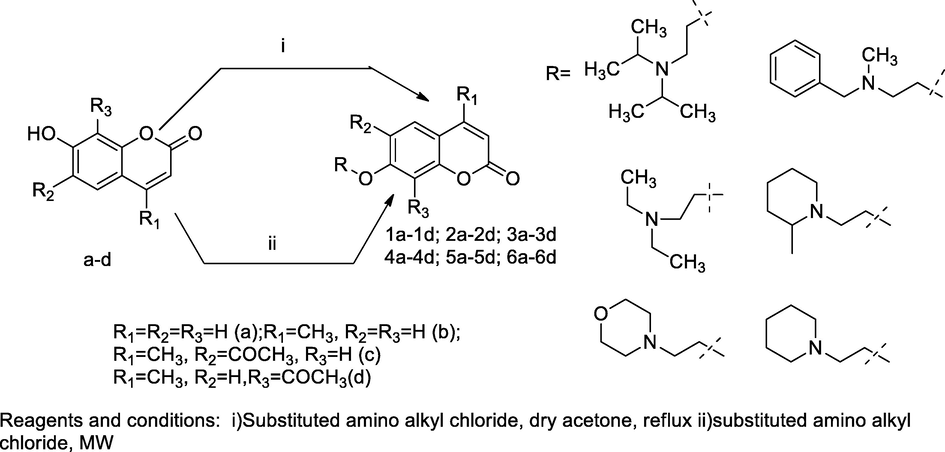
2-Amino alkylated coumarin derivatives.
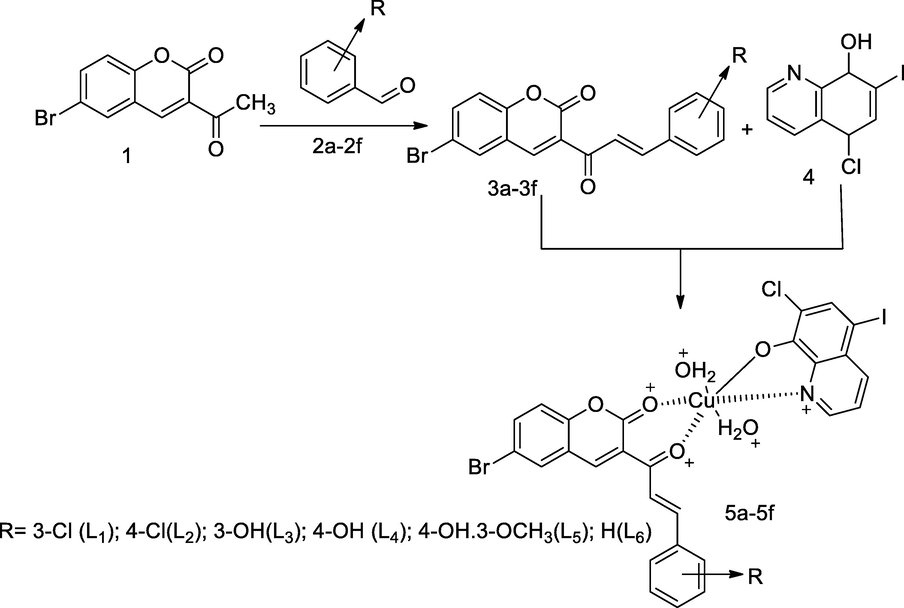
Metal complexes with Coumarin chalcones-clioquinol.
3,4,7,8-Tetra substituted coumarin derivatives.
Thiazolyl- pyrazoline coumarin derivatives.
4-Aryloxymethyl substituted coumarin derivatives.
Metal complexes of 4,4′-Bis-hydroxy coumarin with phenanthroline.
Bis-4-Hydroxy coumarin derivative.
![Chromeno[2,3-d]pyrimidinone with coumarin derivatives.](/content/184/2021/14/2/img/10.1016_j.arabjc.2020.102922-fig26.png)
Chromeno[2,3-d]pyrimidinone with coumarin derivatives.
Silver salt of benzimidazolinium with coumarin derivatives.
Coumarinyl piperazine bearing propanol derivatives.
Dicoumarol derivatives.
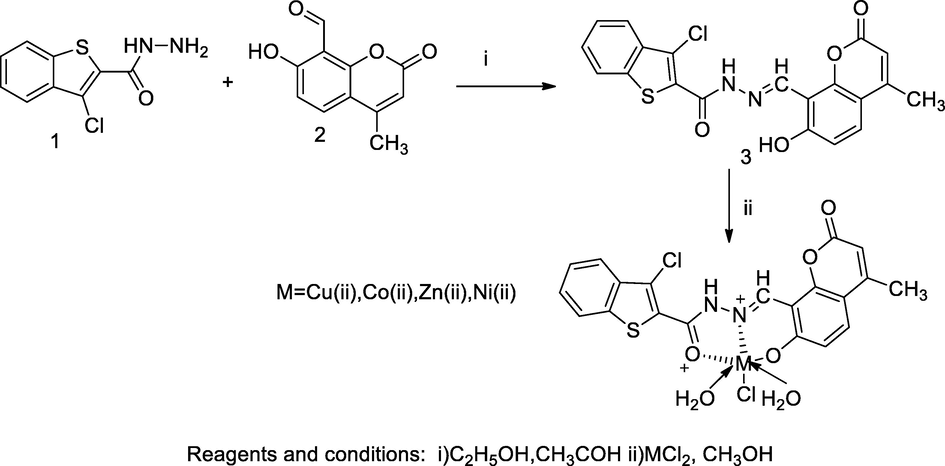
Metal complexes Thiophene hydrazine-coumarin.
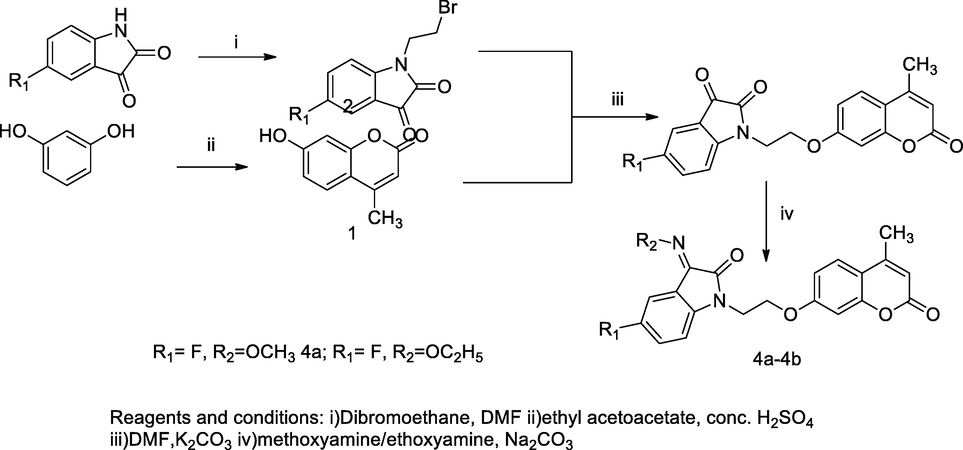
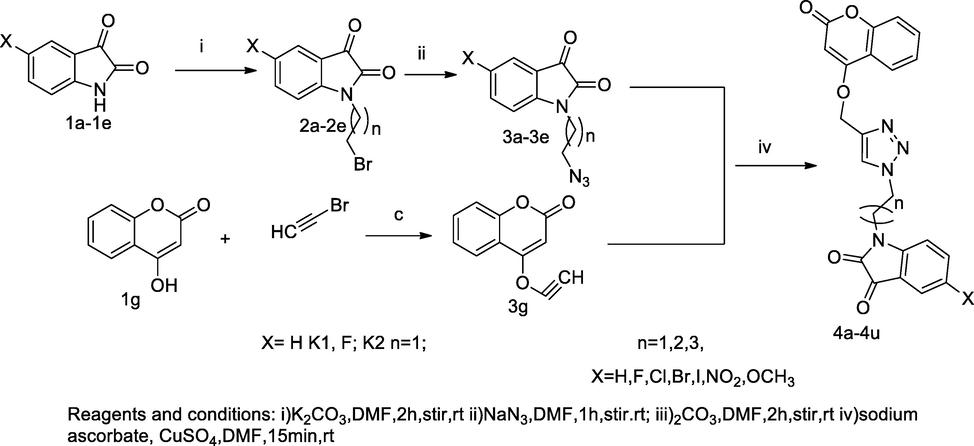
Indolin- 2,3-dione-coumarin derivatives.
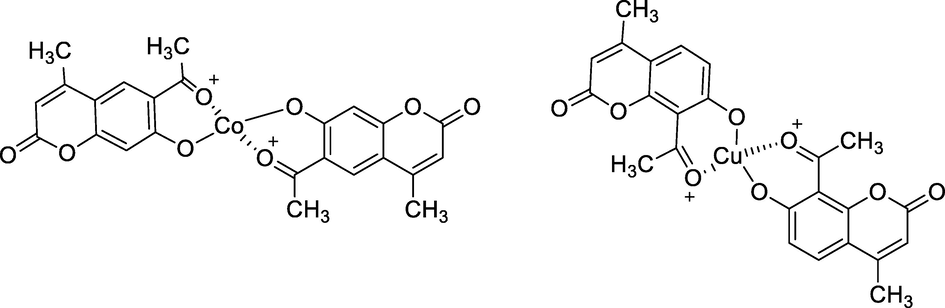
Metal complexes of Acetyl coumarin derivatives.
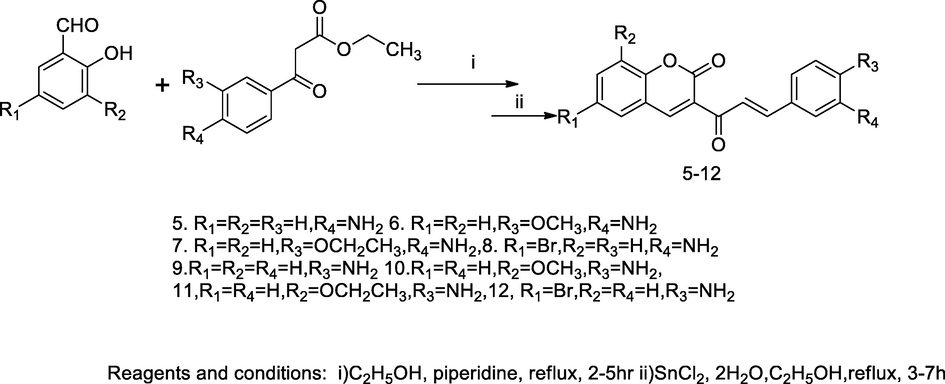
Arylchalcone of coumarin derivatives.
Metal chelate of Schiff base with coumarin derivatives.
Transitional metal complexes with coumarin derivatives.
3-Arylazo-4-hydroxy coumarin derivatives.
Antipyrinyl azo-coumarin derivatives.
1,2,3-Triazolyl substituted coumarin derivatives.
Coumarin bearing triazole derivatives.
Silver complexes with Heterocyclic substituted coumarin derivatives.
Silver complexes with coumarinyloxy phenoxy acetic acid derivatives.
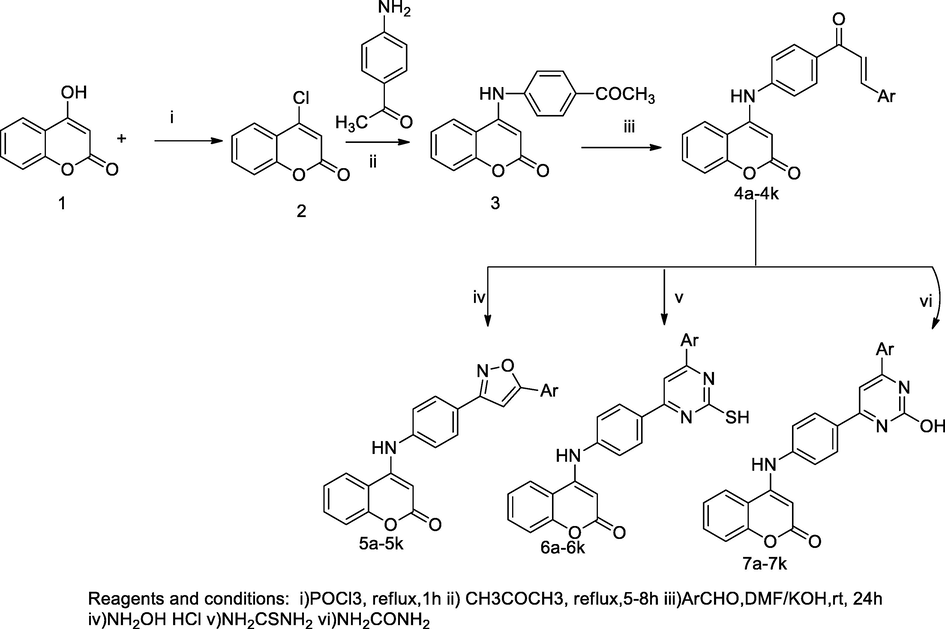
4-Amino coumarin derivatives.
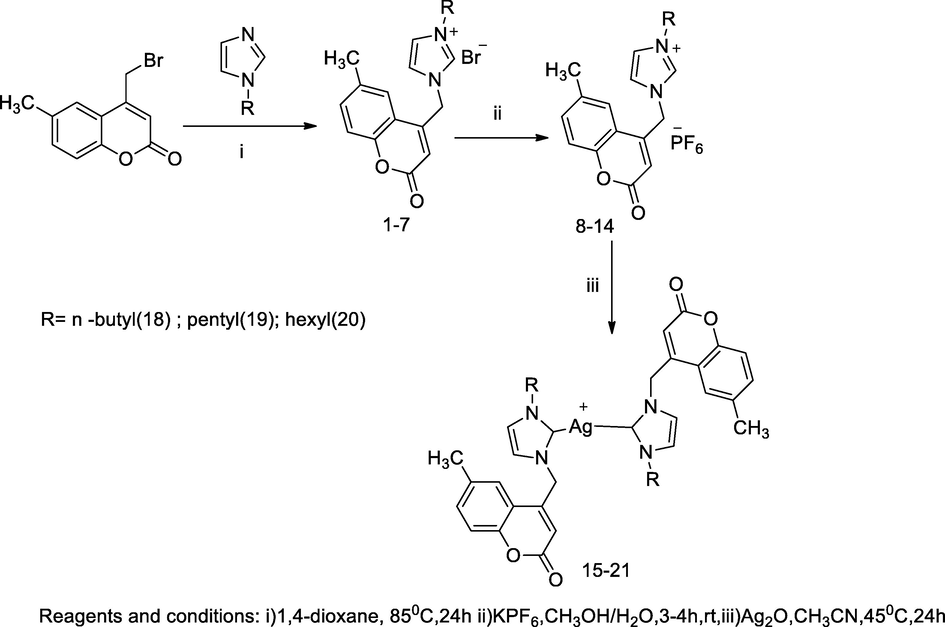
Imidazolinium methyl coumarin silver complexes.
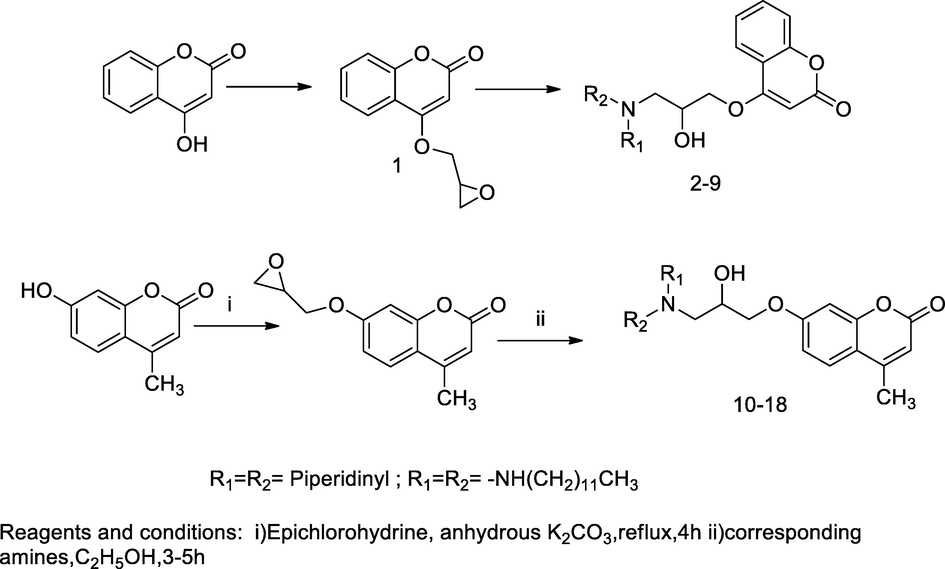
7-Coumarinyloxy amino propanol derivatives.
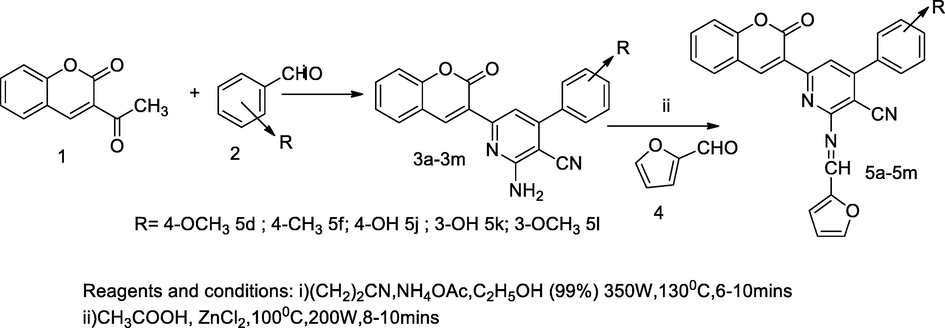
3-Heteroaryl substituted coumarin derivatives.
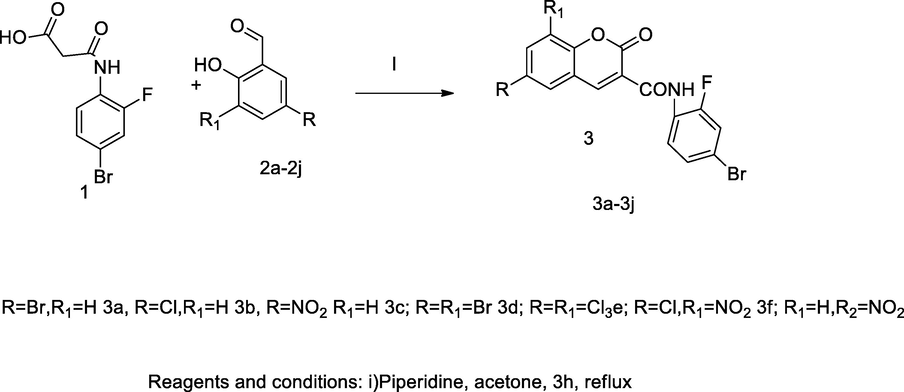
3-Chromenyl carboxamide of coumarin derivatives.
![Coumarin based pyano[2,3-c]pyrazole derivatives.](/content/184/2021/14/2/img/10.1016_j.arabjc.2020.102922-fig47.png)
Coumarin based pyano[2,3-c]pyrazole derivatives.
Silver complexes of bis benzimidazolinium methyl with coumarin.
Indolidenyl of coumarin derivatives.
Pyrimidinone bearing coumarin derivatives.
Indole carbaxahydrazide Schiff base coumarin derivatives.
Metal complexes 3-Arylazo 4-hydroxy coumarin.
Coumarinyl- 3-semithiocarbazone congeners.
Coumarin fused furan derivatives.
Bis 3,3′- Coumarin derivatives.
Coumarinyloxy bearing oxazoline derivatives.
Silver complexes with imidazolinum methyl coumarin derivatives.
7- Coumarinyl methyl theophylline derivatives.
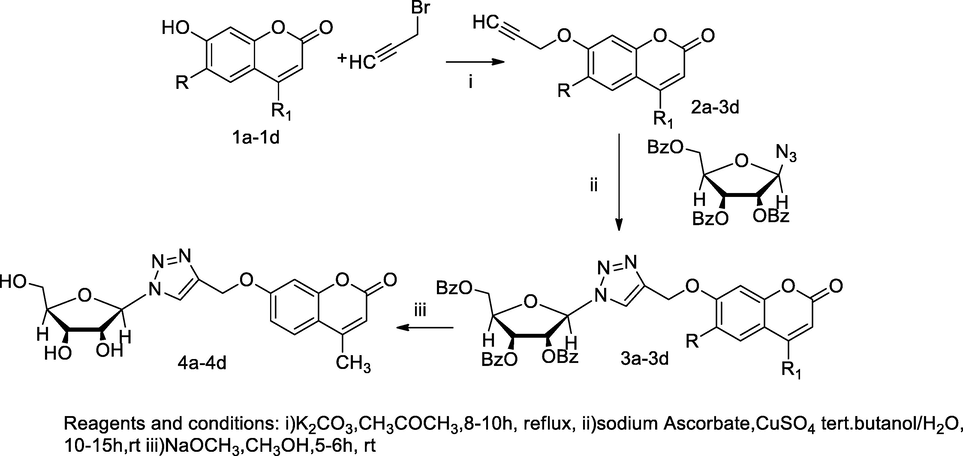
Ribofuranosyl-coumarinyloxy bearing 1,2,3- triazole derivatives.
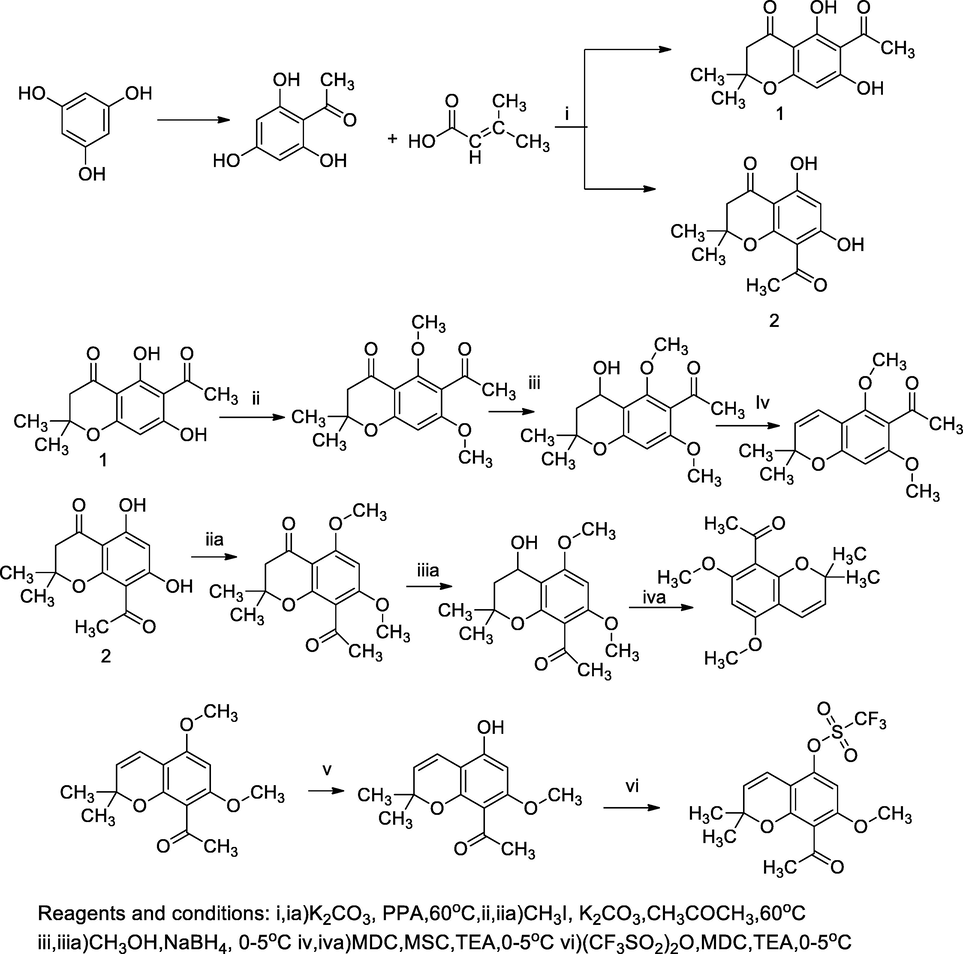
Disubstituted of chromane derivatives.
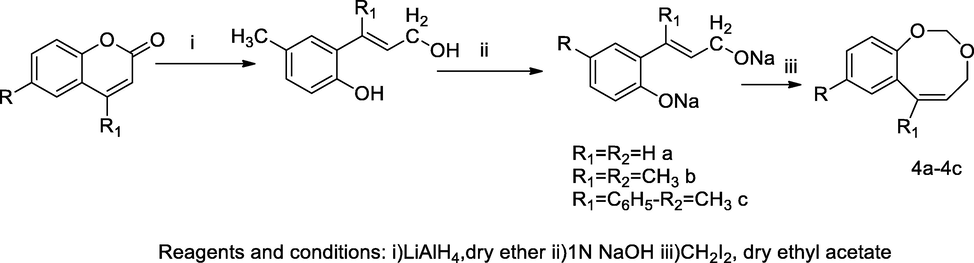
Coumacine derivatives.
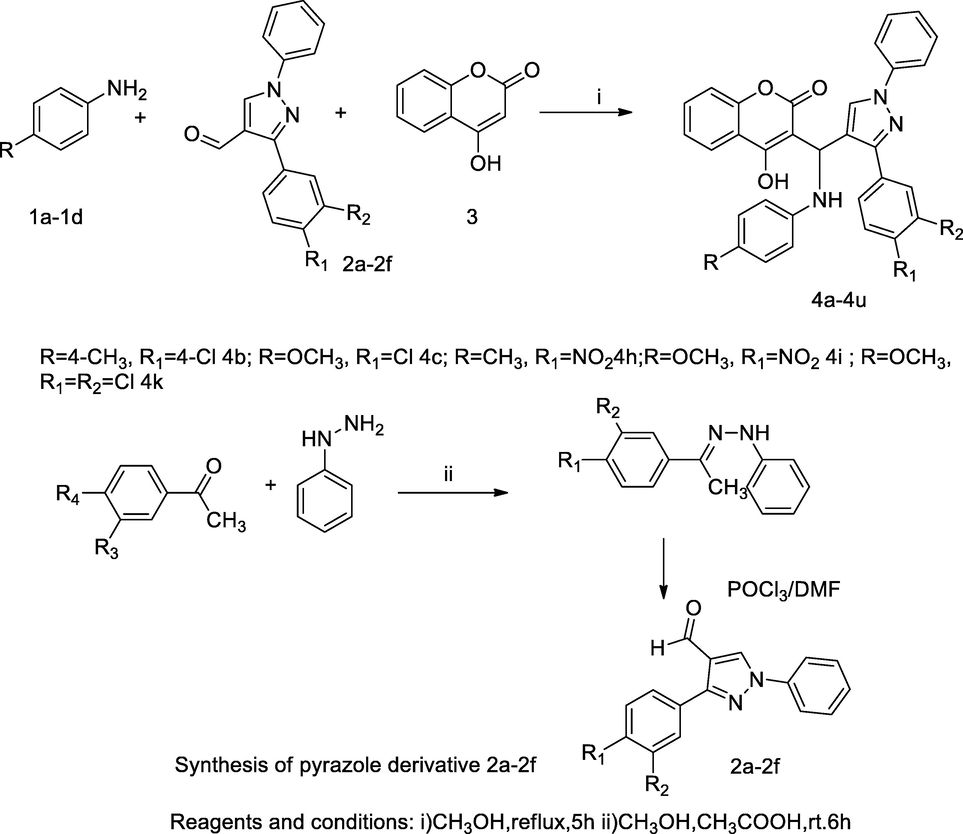
Pyrazole-anilino connected coumarin derivatives.
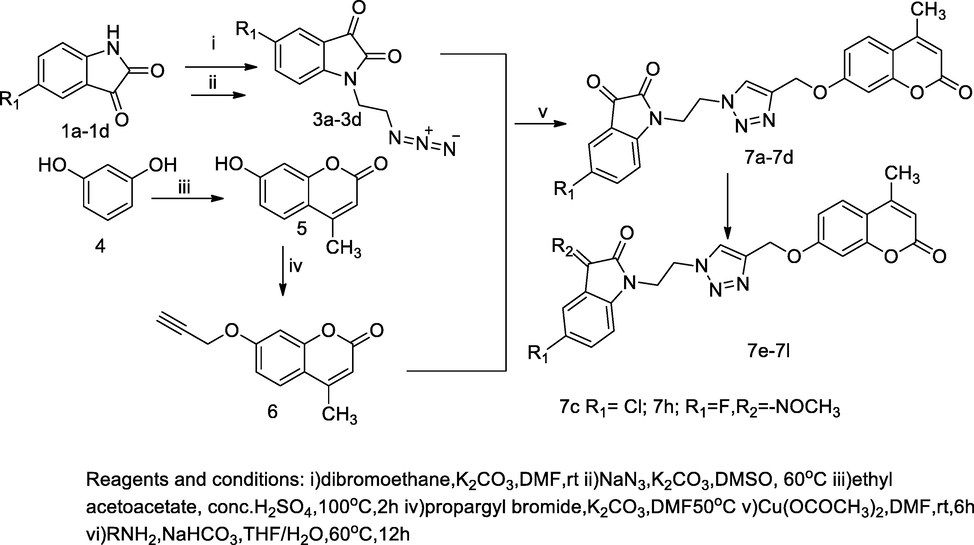
Isatin- linked 1, 2, 3-triazole with coumarin.
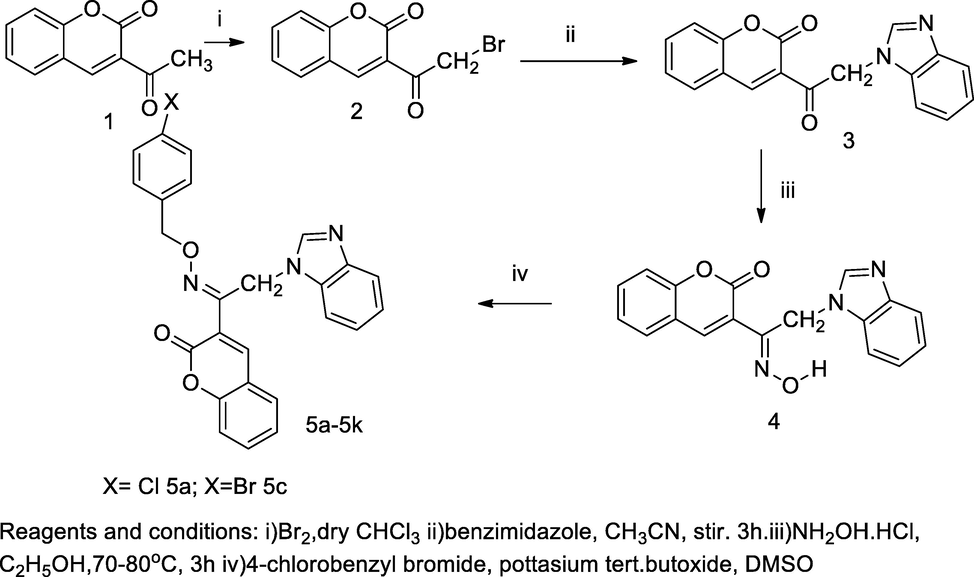
N- Benzoimidazolyl oxime of acetyl coumarin derivatives.
![Coumarin based pyrano[3,2-c] coumarin derivatives.](/content/184/2021/14/2/img/10.1016_j.arabjc.2020.102922-fig65.png)
Coumarin based pyrano[3,2-c] coumarin derivatives.
3-Thiazolylamino coumarin derivatives.
N- Pyrazolyl coumarin- 3-carboxamide derivatives.
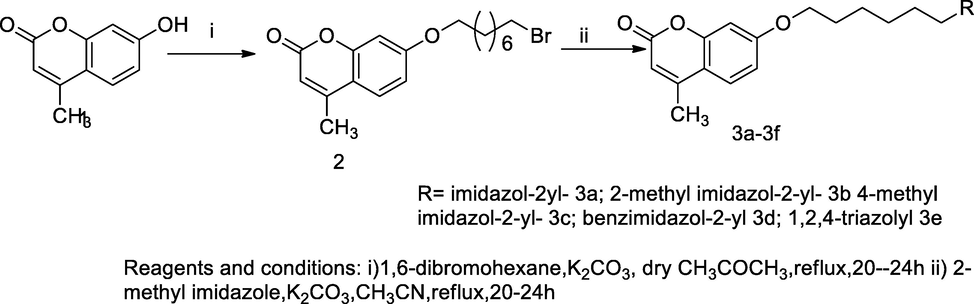
1-Alkyloxy imidazolyl coumarin derivatives.
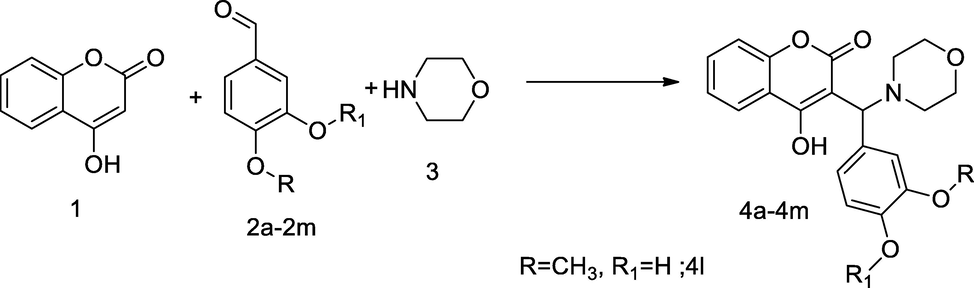
Coumarin mannich based derivatives.
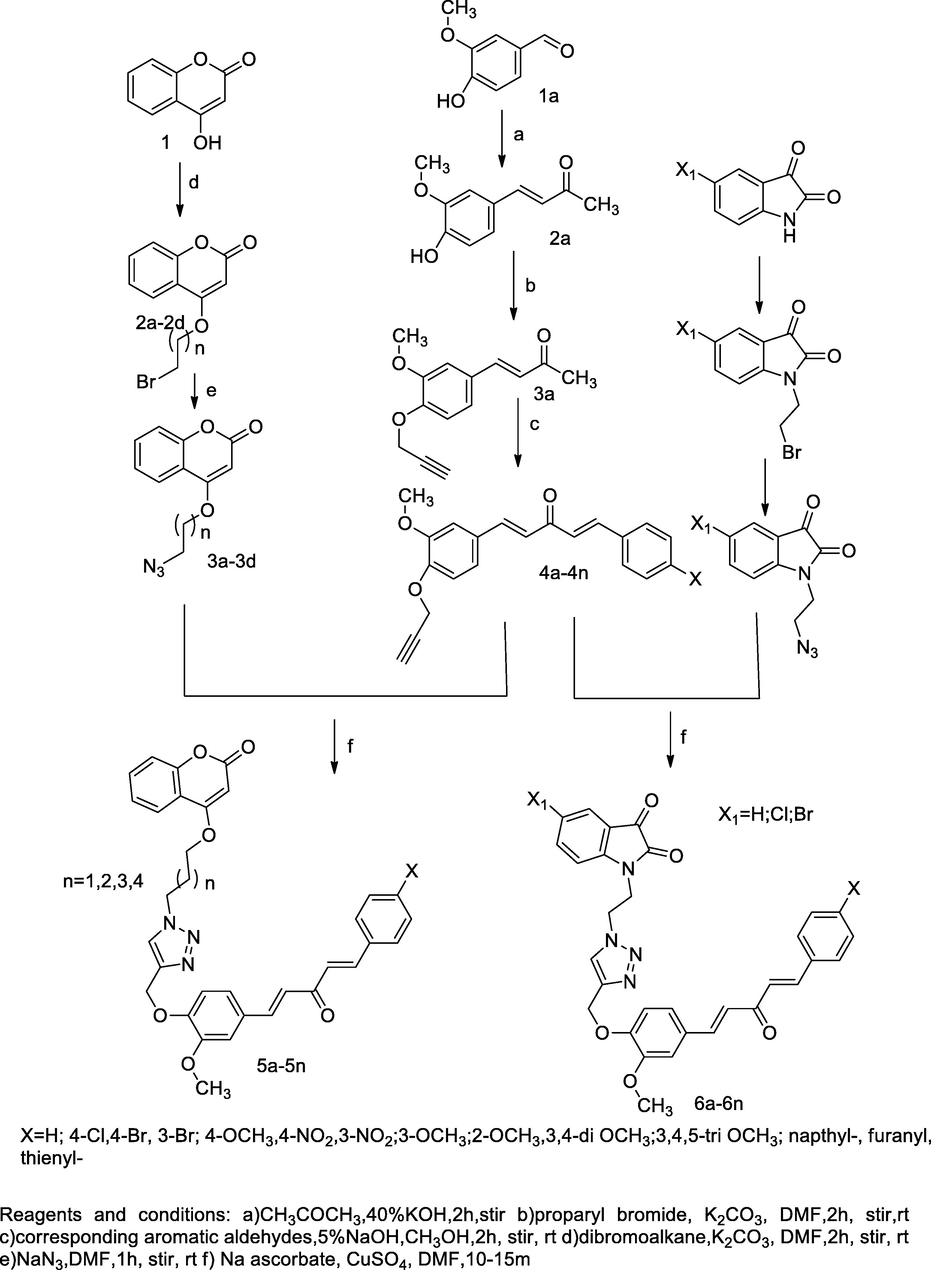
Curcumin and isatin linked coumarin derivatives.
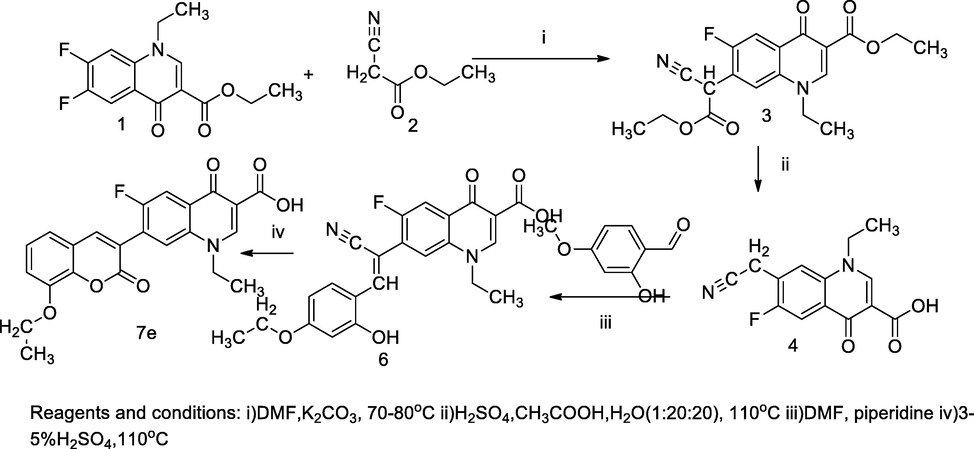
Fluroquinolone based coumarin derivatives.
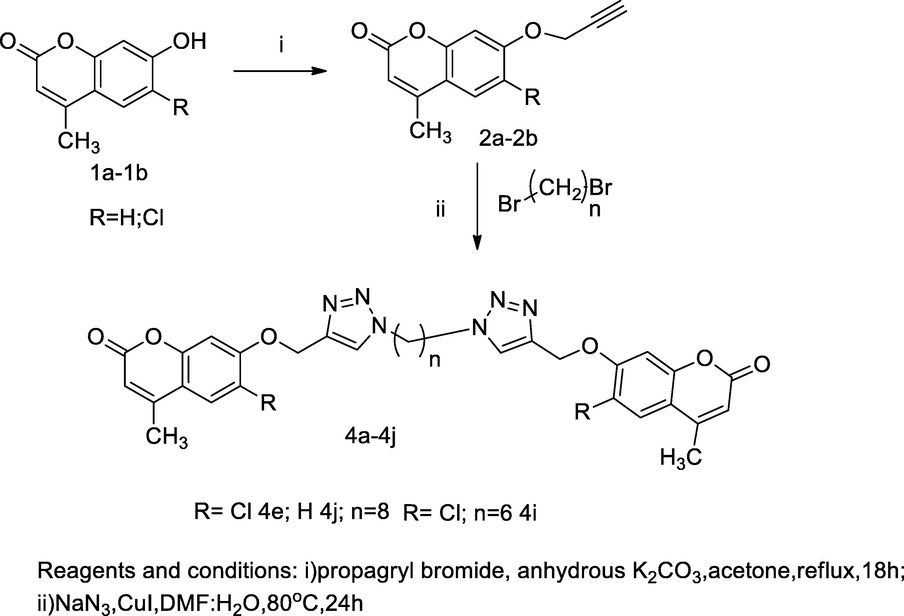
Dimer of Triazole-coumarin hybrids derivatives.
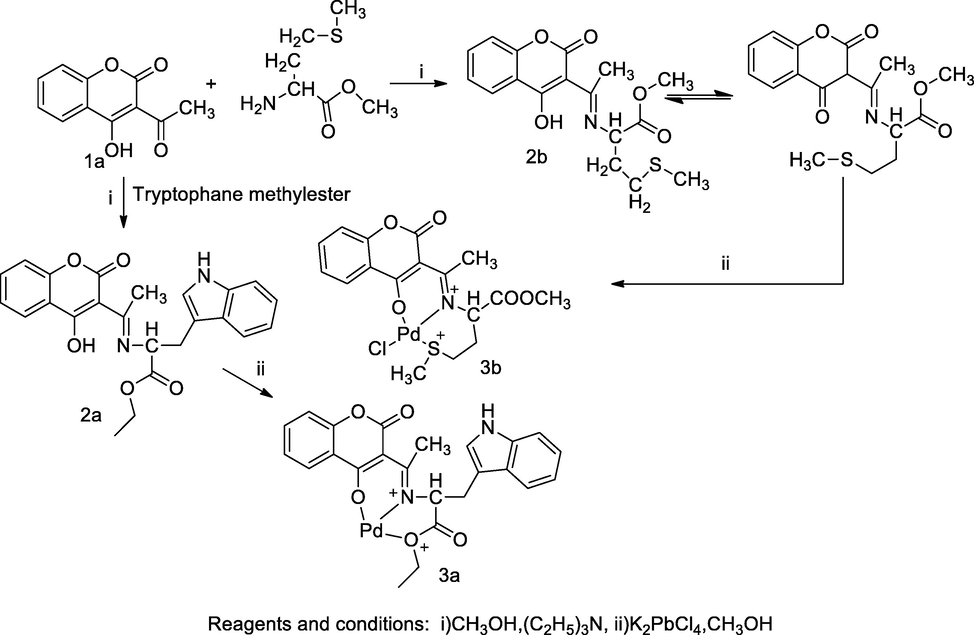
Palladium complexes coumarin derivatives.
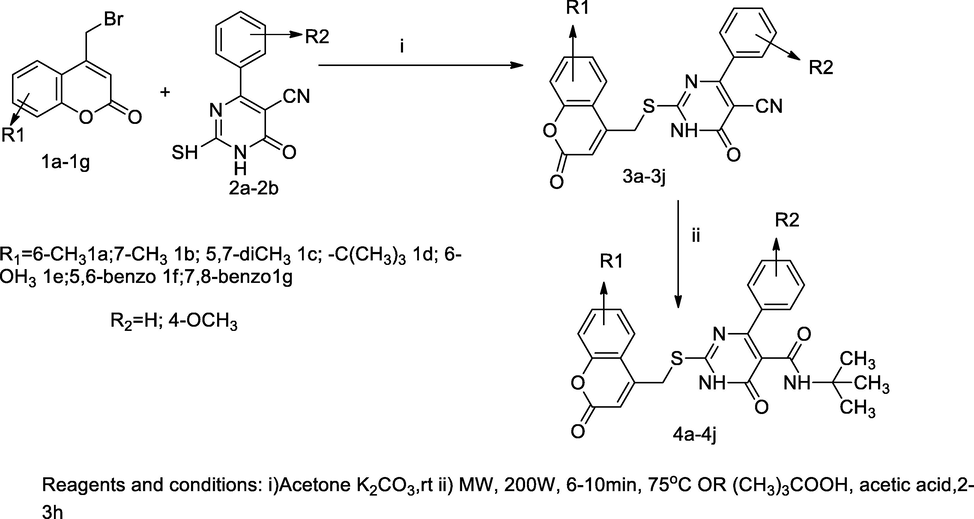
Coumarinyl pyrimidinone derivatives.
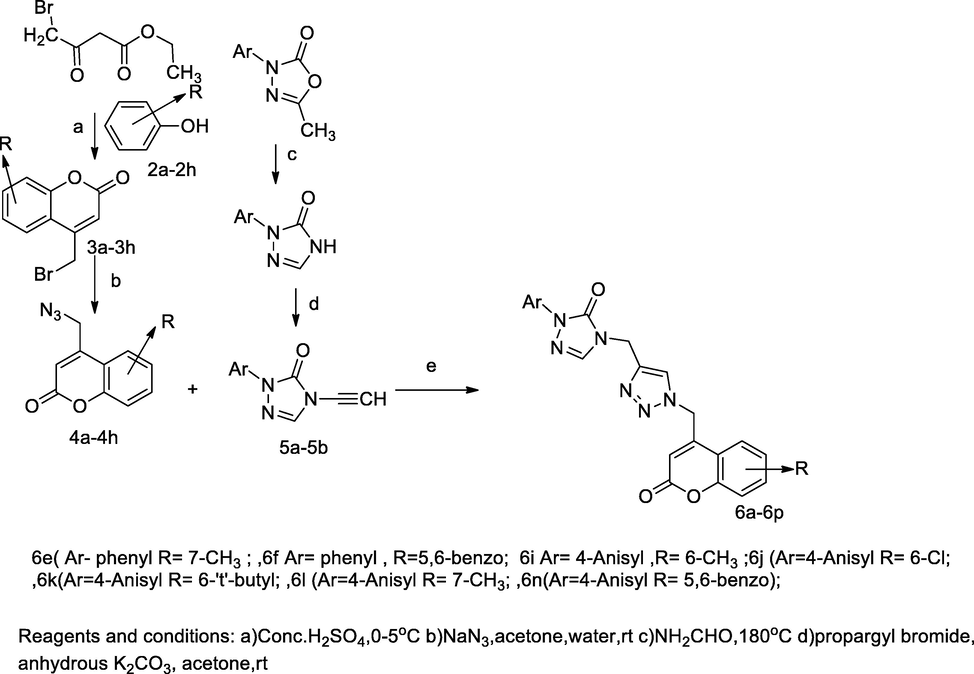
1,2,3-Triazole substituted coumarin derivatives.
Coumarinyloxy derivatives.
Isatin-triazolyl coumarin derivatives.
Coumarin 3-carboxamide derivatives.
3-Aroyl substituted coumarin derivatives.
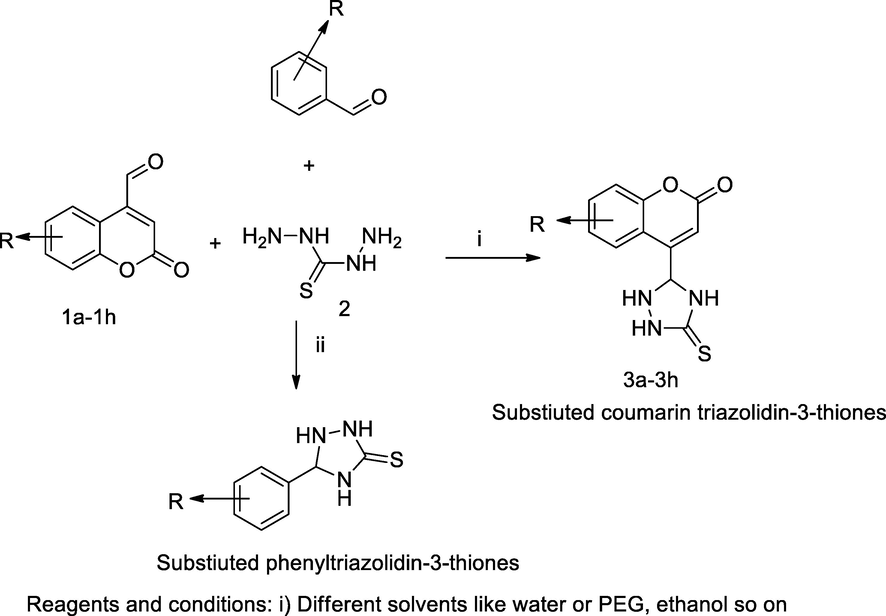
4-Triazolidin-thione coumarin derivatives.
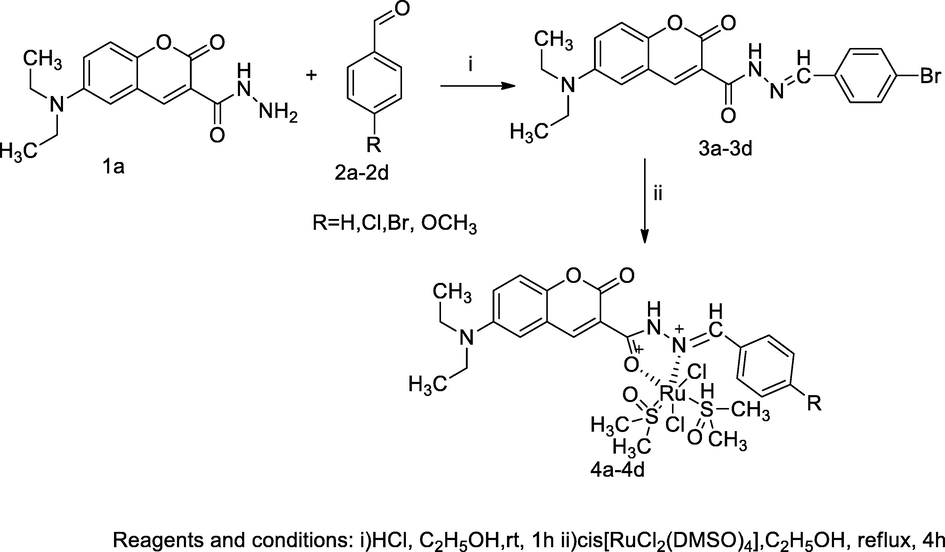
Ruthenium complexes of 3-acetohydrazone coumarin.
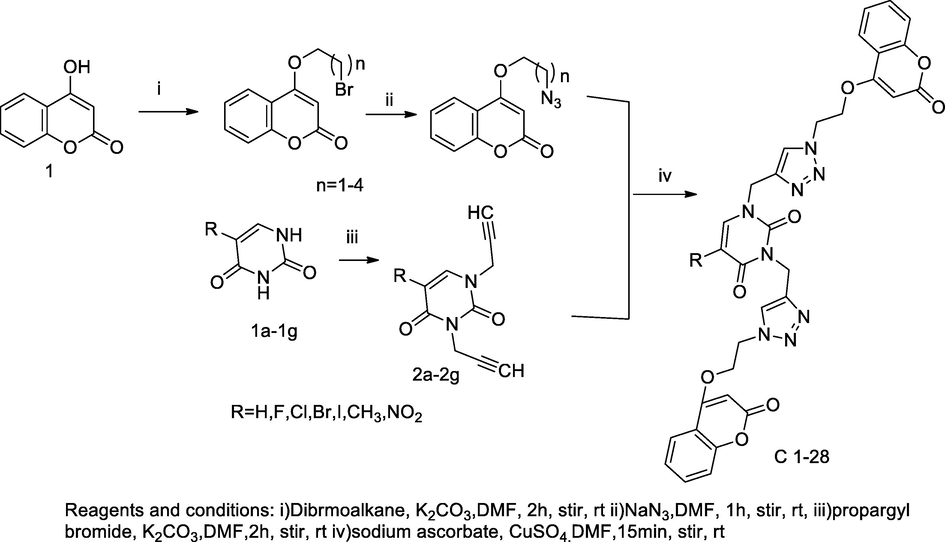
Bis-Triazole uracil based coumarin derivatives.
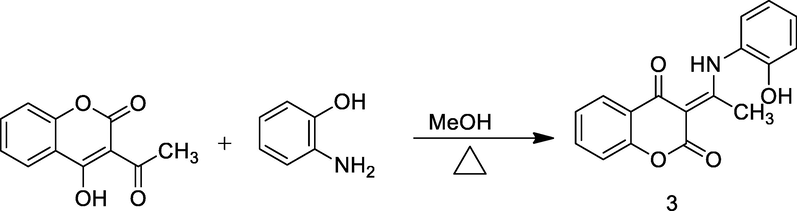
4-Hydroxyl- 3-acetyl coumarin derivatives.
1.1 Sources of coumarin
Coumarin was first isolated from a higher flowering plant, Dipteryx odorata (known as tonka beans) in 1820 and seeds of this plant contain aromatic organic bioactive compound coumarin. Concomitantly, other several sources have been located in (Apiaceae, Asteraceae, Apocynaceae, Ruteceae, Calophyllaceae, Fabaceae and a few more- family) (Table 1).
Sl. No.
Plant name
General name
Family
Reference
1.
Agathosma betulina
Boegoe
Rutaceae
(Moolla and Viljoen, 2008)
2.
Ammi majus
Bishop’s weed
Apiaceae
(Bartnik and Mazurek, 2016)
3.
Amyris elemifera
Torchwood
Rutaceae
(Burke and Parkins, 1979)
4.
Angelica archangelica
Garden angelica
Apiaceae
(Kumar et al., 2013)
5.
Apium graveolens
Leaf celery
Apiaceae
(Ramezani et al., 2009)
6.
Artemisia tridentata
Sagebrush
Asteraceae
(Barua et al., 1980)
7.
Asclepiascurassavica
Scarlet milkweed
Apocynaceae
(Reddy, 2012)
8.
Calophyllum brasiliense
Árbol de Santa Maria
Calophyllaceae
(Ruiz-Marcial et al., 2007)
9.
Calophylum dispar
Laurelwood
Calophyllaceae
(Guilet et al., 2001)
10.
Cinnamomum cassia
Chinese cassia
Lauraceae
(Woehrlin et al., 2010)
11.
Cinnamomum verum
Ceylon cinnamon
Lauraceae
(Ballin and Sørensen, 2014)
12.
Citrus reticulata
Citrus plant
Rutaceae
(Takemura et al., 1993)
13.
Citrus hassaku
Jagada
Rutaceae
(Nakatani et al., 1987)
14.
Cleome hasslerian
Spider flower
Capparaceae
(Kumar et al., 1988)
15.
Coleonema album
Aasbossie
Rutaceae
(Gray, 1981)
16.
Daucus carota
Carrot
Apiaceae
(Gilani et al., 2000)
17.
Daucus carota
Wild carrot
Apiaceae
(Abenavoli et al., 2003)
18.
Dipteryx odorata
Tonka bean
Fabaceae
(Gleye et al., 2003)
19.
Eriostemon brucei
Philotheca
Rutaceae
(Jefferies and Worth, 1973)
20.
Eryngium campestre
Field eryngo
Apiaceae
(Erdelmeier and Sticher, 1985)
21.
Ferula asafetida
Asafetida
Apiaceae
(Iranshahy and Iranshahi, 2011)
22.
Ferula communis
Gaint fennel
Apiaceae
(Appendino et al., 1988)
23.
Galium odoratum
Sweet woodruff
Rubiaceae
(Martin and Bodson, 2010)
24.
Gerbera jamesoni
Transvaal daisy
Asteraceae
(Inoue et al., 1989)
25.
Helianthus annuus
Sunflower
Asteraceae
(Serghini et al., 2001)
26.
Heracelum thomsoni
Cowparsnip
Apiaceae
(Patnaik et al., 1987)
27.
Hierochloe odorata
Vanilla grass
Poaceae
(Sinha et al., 2008)
28.
Hierochloe odorata
Sweet grass
Poaceae
(Brown et al., 1960)
29.
Kielmeyera elata
White gul mohur
Calophyllaceae
(Gramacho et al., 1999)
30.
Melilotus officinalis
Sweet clover
Fabaceae
(Brown et al., 1960)
31.
Micromelum minutum
Lime berry
Rutaceae
(Rahmani et al., 1994)
32.
Murraya exotica
Orange jessamine
Rutaceae
(Negi et al., 2005)
33.
Notopterygium forbesii
Notopterygium Root
Apiaceae
(Ma et al., 2008)
34.
Paramignya trimera
Xao tam phan
Rutaceae
(Dang et al., 2017)
35.
Pelargonium sidoides
South African geranium
Geraniaceae
(Krone et al., 2001)
36.
Pelea barbigera
Rutaceae
(Higa and Scheuer, 1974)
37.
Peucedanum formosanum
Milk parsley
Apiaceae
(Chen et al., 2008)
38.
Peucedanum mogoltavicum
Milk parsley
Apiaceae
(Nikonov, 1972)
39.
Peucedanum ostruthium
Milk parsley
Apiaceae
(Urbain et al., 2005)
40.
Phebalium stenophyllum
Narroe leafed phebalium
Rutaceae
(Bevalot et al., 1988)
41.
Polygala paniculata
Milkwort
Polygalaceae
(Hamburger et al., 1985)
42.
Prangos tschimganica
Prangos
Apiaceae
(Shikishima et al., 2001)
43.
Prunus armeniaca
Siberian apricots
Rosaceae
(Kayano et al., 2004)
44.
Prunus avium
Cherries
Rosaceae
(Santamour and Riedel, 1994)
45.
Ribes nigrum
Black currants
Grossulariaceae
(Knox et al., 2003)
46.
Setaria italicia
Foxtail
Poaceae
(Jain et al., 1991)
47.
Toddalia asiatica
Orange climber
Rutaceae
(Oketch-Rabah et al., 2000)
48.
Verbascum thapsus
Mullein
Scrophulariaceae
(Pardo et al., 1998)
49.
Zanthoxylum dipetalum
Kawa’u,Hea’e
Rutaceae
(Fish et al., 1976)
50.
Zanthoxylum suberosum
Fagara
Rutaceae
(Krajniak et al., 1973)
2 Synthesis and antibacterial activities of coumarin derivatives
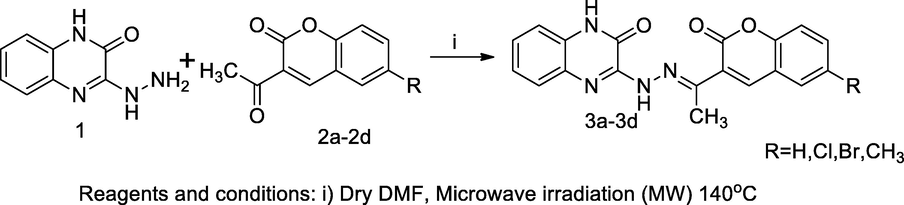
2.1 Synthesis of 2-quinoxalone-coumarin hydrazone derivatives
A series of quinoxalone coumarin hydrazone derivatives 3a-3d were prepared by the microwave synthetic procedure and the obtained congeners had been evaluated for antimicrobial actions. These compounds were prepared by the condensation of equimolar concentration of precursor, 3-hydrazonquinoxalone 1 with substituted 3-acetylcoumarins, 2a-2d in dry DMF, microwave-oven at 400 MW. The developed product was recrystallized by ethanol. The starting hydrazine quinoxalone was prepared by the cyclisation reaction of ortho phenylenediamine (OPD) with oxalic acid in the presence of a mineral acid, which further was reacted with hydrated hydrazine. Overall, SAR studies of these derivatives indicated that the attachment of halogen group to the phenyl ring of coumarin nucleus might have been reported for notable effect on antibacterial actions; the chloro substituted coumarin compound (E)-3-(2-(1-(6-chloro-2-oxo-2H-chromen-3-yl)ethylidene)hydrazinyl)quinoxalin-2(1H)-one 3 had shown inhibition with E. coli and S. aureus at 30 and 32 mm as zone of inhibition (ZOI) in comparison to Streptomycin (Ajani et al., 2010).
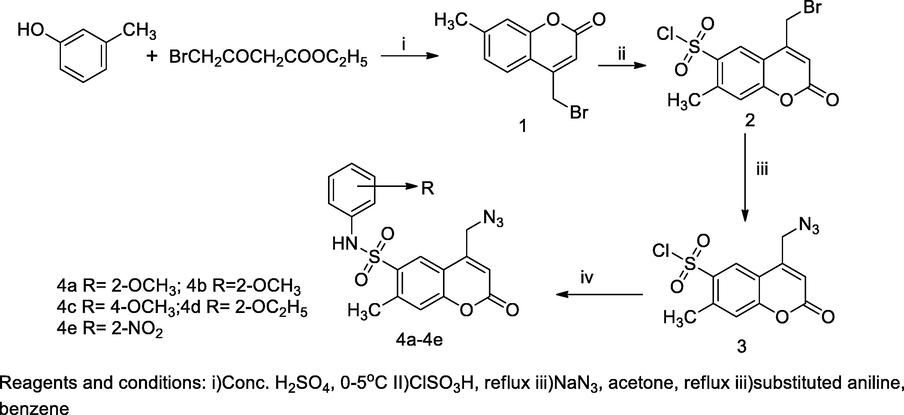
2.2 Synthesis of 4-azidomethyl-7-methyl-coumarin bearing sulfonamide
A series of 4-azidomethyl coumarin bearing sulfonamide derivatives 4a-4e had been synthesized with four different steps. Initially, the cresol reacted with ethyl acetoacetate in cyclizing agent to produce 7-methyl-4-bromomethyl coumarin 1, and the obtained compound 1 had undergone chlorosulfonation, yielding the corresponding product, 7-methyl-4-bromomethyl coumarinyl 6-sulfonyl chloride 2, which was reacted with sodium azide in acetone yielding 4-azidomethyl coumarin 3; and finally these intermediates in amination with substituted anilinesproduce 4-(azidomethyl)-7-methyl-2-oxo-N-subst.phenyl-2H-chromene-6-sulfonamide (4a-4e). From SAR studies of these compounds it was clear that the presence of methoxy and nitro-group in benzene sulfonamide residue attached at C-7 position of 4-azidomethyl coumarin, which may produce more significant antibacterial actions. These compounds with methoxy substituents as attached at in either ortho or para of phenyl ring had exhibited control of Enterococcus faecalis at the MIC value 1 µg/mL in comparison to Ciprofloxacin. The potent compounds indicated that the compounds with chloro, nitro, methoxy and bromo substiuent attached in phenyl ring presence may be in association with increased antibacterial activity (Basanagouda et al., 2010).
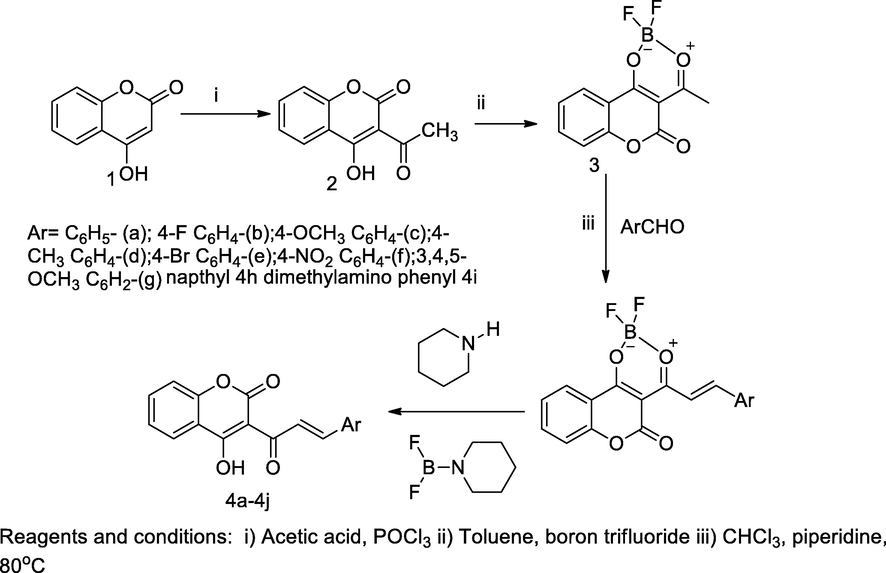
2.3 Synthesis of coumarin chalcone derivatives
A series of coumarin chalcones had been synthesised and these derivatives 4a-4i were screened for the antibacterial activities against several bacterial strains. Initially, acetylated coumarin 2 was obtained by acetylation of 4-hydroxy coumarin 1, in the presence of acetic acid and phosphorus oxy trichloride, which further on heating toluene solution the compound 2 in boron trifluoride etherate the corresponding boron compound 3 was obtained. Finally, the corresponding chalcones of coumarin derivatives were prepared by the condensation of 3-acetyl-difloroboronyloxycoumarin 3 with different aryl aldehyde in chloroform solution and a mixed small amount piperidine was added and the mixture was refluxed at 80 °C. The compound coumarin bearing napthyl containing chalcone analogue, 4h was obtained and coumarin derived 4-dimethyl amino phenyl chalcone 4i had shown a good antibacterial activity against E. coli with 31 and 32 mm as ZOI, compared to standard Gentamicin (Hamdi et al., 2010).
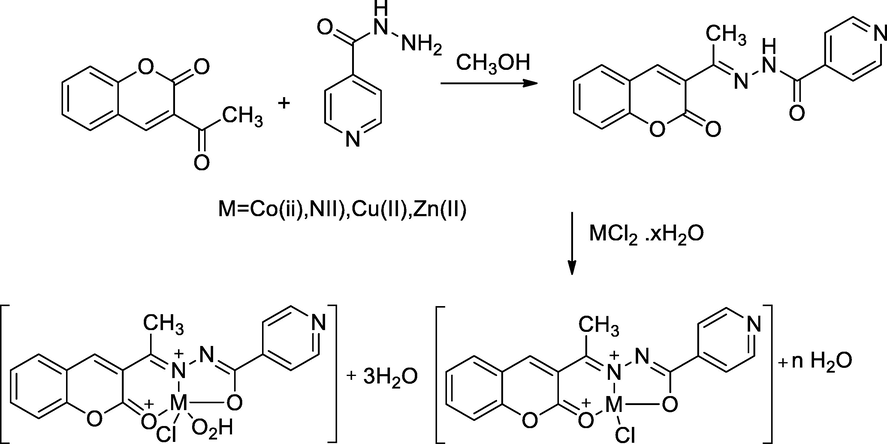
2.4 Synthesis of metal complexes of 3-acetylcoumarin-INH hybrid
Four transitional metal M(II) complexes of Schiff base of isoniazid 4a-4d were synthesized by reaction of two potent ligands such as, isoniazid 2 with 3-acetyl coumarin 1 in methanol on mild condition; by the by, the results of antibacterial action against some pathogenic bacterial strains were notable. In this reaction, ligands can chelate to metal ions in the carboimidol form of INH hydrazone via deprotonation, coumarin carbonyl and azomethine also involved the same. Antitubercular activity results showed that compounds schiff base INH–coumarin has been less effective than corresponding metal complexes. Among all the complexes, the Cu(II) complexes had notable antibacterial activity against pathogenic S. aureus, S. faecalis and E. coli at 25, 6.25, 50 µg/mL, whereas Co(II) and Ni(II) had been noted anti-tubercular activity at each MIC value 25 µg/mL in compare to Ciprofloxacin and Isoniazid as the standard, respectively (Hunoor et al., 2010).
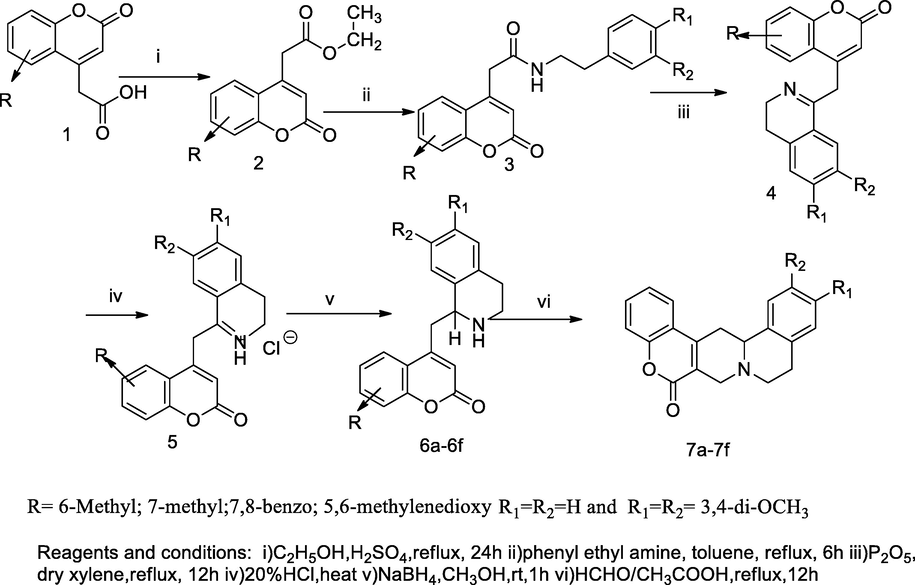
2.5 Synthesis of coumarin fused with tetrahydroisoquinoline derivatives
On the basis of Bischler-napieralsky's protocols, two series of coumarin congeners of tetrahydroisoquinoline 6a-6f and protoberiberine 7a-7f were synthesised, and the obtained congeners were tested for DNA cleaving activity against infection bacterial strains. From the precursor, coumarin 4-carboxylic acid 1 was esterified with ethanol to obtain the corresponding ethyl ester 2, which further on reaction with disubstituted aryl ethyl amine in toluene solution, yielded 2-(2-oxo-2H-chromen-4-yl)-N-disubstituted phenethyl acetamide 3. Then, the correspondingly obtained acetamide 3 was readily cyclized in the presence of phosphorous pentoxide to produce the compound 4 containing methylated disubstituted, 3,4-dihydroisoquinolinyl linked to C-4 position coumarin nucleus. Furthermore, the liberated compound 1,2,3,4-tetrahydroquinoline 5 formed by the reduction of the product 4 in sodium tera borohydride (NaBH4), and finally the fragmented coumarin ring was fused with tetrahydroquinoline; the compound 5 was synthesized by reflux condensation of the desired tetrahydroisoquinoline 6a-6f with methanol in acetic acid. These intermediate candidates further by cyclisation and condensation with formaldehyde in acetic acid produced another series of coumarin congeners7a-7f, which were recognised chemically as protoberberine. Among all the desired compounds 6f, 7e and 7f, was fused with 3,4-dimethoxy or 7,8-benzo system of coumarin moieties and those had been reported as good inhibitory actions against bacterial DNA cleavage of S. aureus (Jadhav et al., 2010).
2.6 Synthesis of metal complexes with 4-methyl 7-hydroxy coumarin thiosemicarbazone
A series of Ni(II), Co(II) and Cu(II) complexes had been synthesized from two coumarin thiosemicarbazone 3a-3b derivatives, and the obtained analogues and metal complexes 4a-4f were evaluated for antibacterial activity. An individual compound such as, 7-formyl-6-hydroxy 4-methyl coumarin 1a and 8-formyl-7-hydroxy coumarin 1b was reacted with N-methyl thiosemicarbazide 2 to produce the corresponding N-methyl thiosemicarbazone-coumarin 3a-3b (ligands). The desired ligands act as tridentate, which coordinated to metal ions through azomethine group, thione of sulfur and phenolic hydroxyl group via deprotonation. The Co(II) and Ni(II) complexes of 4-methyl 7-hydroxy coumarin thiosemicarbazone 3a had been found notableinhibitory activities against E. coli, P. aeruginosa and S. typhi at each MIC value 10 µg/mL in comparison to Gentamicin (Patil et al., 2010).
2.7 Synthesis of 4-methyl coumarin bearing thiazolidinone moiety
In this scheme, thiazolidinonyl bearing coumarin analogues had been synthesised by the reaction of 7-amino 4-methyl coumarin 1 with appropriate substituted aryl aldehyde 2a-2k to produce several Schiff base derivatives 3a-3k, which on further treatment with thioglycolic acid in the presence of anhydrous zinc chloride yielded cyclised corresponding title analogues 4a-4k. Furthermore, SAR studies of these derivatives attributed that the compounds with para-substituents of phenyl of thiazolidinonyl ring induce their antibacterial potencies. Preliminary, in-vitro antibacterial activity of these Schiff base compounds had been less active than its corresponding thiazolidinonyl analogue. The obtained result indicated that the compounds with nitro, methoxy and fluro substituents in phenyl ring of title analogues such as 3d, 3f, 4d, 4f and 4i exhibited good antibacterial agent at MIC doses 100–10 µg/mL in comparison to Ciprofloxacin (Ronad et al., 2010).
2.8 Synthesis of 3-aminoalkyl-4-hydroxy coumarin derivatives
A series 3-amino alkylated-4-hydroxy coumarin derivatives was synthesized by both conventional and microwave assisted methods. Then, the liberated products were examined for antioxidant and antibacterial activities. These analogues had been synthesized by the reaction of 3-acetyl 4-hydroxy coumarin 1 with substituted aryl amines in the presence of catalyst toluene sulfonic acid in anhydrous toluene to produce the respective Schiff based coumarin derivative 2, which further was reduced by an individual analogue with sodium borohydride in tetrahydrofuran to yield monoalkylated 4-hydroxy coumarin 3; whereas yields of these analogues had been comparatively more in using microwave assisted methods than the conventional synthetic method, particularly for the analogue namely, nitro substituents anilino derivatives. The antibacterial results of the compounds with unsubstituted phenyl, tolyl residue at anilino of the title compounds namely, 9c, 2c and 3c were of notable inhibition growth against S. aureus, E. coli and P. fluorescence at ranges of zones of inhibition (ZOI) 3.9–15.6 µg/mL in comparison to Streptomycin as the standard (Vukovic et al., 2010).
2.9 Synthesis of thiazolyl hydrazonyl substituted coumarin derivatives
Twelve derivatives bearing hydrazonyl thiazolyl substituted coumarin were synthesised by the reflux condensation of 3-bromoacetyl coumarin 5b, and substituted phenyl/substituted 3-acetylcoumarin thiosemicarbazone 4a-4c in chloroform and ethanol (2:1) yield thiazolyl linked coumarin analogues. By the principle of Hanztsch’s reaction, the formation of thiazole ring, which was incorporated in structure between bromoacetyl group and the corresponding thiosemicarbazone congener, in the presence of mixed solvents ethanol and chloroform. The insertion of hydroxyl group and bromo substituents of benzylidene imine residue had resulted in significant antimycobacterial activity. The compound 6-bromo-3-(1-(2-(4-(2-oxo-2H-chromen-3-yl)thiazol-2-yl)hydrazono)ethyl)–2H-chromen-2-one 10c was reported as a good antimycobacterial agent with 15 µM as the MIC value in comparison to Isoniazid (INH) (Arshad et al., 2011).
2.10 Synthesis of 4-hydroxy coumarin bearing oxadiazolone derivatives
A novel series of 4-hydroxycoumarin derivatives bearing oxadiazolinyl, was prepared by an intermediate compound ethyl 2-((2-oxo-2H-chromen-4-yl)oxy)acetate 3; this underwent hydrazinolysis in hydrated hydrazine to produce the corresponding hydrazide 4, which further was used to synthesize by the corresponding Schiffbase with the condensation with different aryl aldehyde in glacial acetic acid for getting N'- substituted benzylidene-2-((2-oxo-2H-chromen-4-yl)oxy)acetohydrazide 5. Finally these obtained compounds individually reacted and cyclised with acetic anhydride to produce the corresponding 4-((4-acetyl-5-subst.phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy)-2H-chromen-2-one 6. Introduction of 4-fluoro phenyl substituted in the oxadiazolinyl nucleus in compounds 5b and 6b had a notable antibacterial activity against S. aureus, E. coli and P. aeruginosa with ZOI as 26–34 mm in comparison to Gentamicin. In these prepared compounds, fluro substituted phenyl in either hyrazone Schiffbase structure or attached with one of substituent in oxadiazinyl ring had been verified for antibacterial activity (Hamdi et al., 2011).
2.11 Synthesis of metal complexes-semithiocarbazone of hydroxy coumarin
Several transitional metal complexes of 4-hydroxy-3-thiocarbohydrazone 2 were formed by the reaction with chloride, acetate and nitrate salts of the following metals, Co(II), Cu(II), Ni(II) and Cr(III) in ethanol. The desired ligand was prepared by the reaction of 3-formyl 4-hydroxy coumarin with a mixture of carbon disulfide and thiosemicarbazide in the presence of dimethylformamide. In these complexation reactions, the ligand acted as monobasic tridentate ONS electron donors in all metal complexes. Furthermore, the obtained ligand with complexes structurally interpreted by thermal gravimetrical analysis. Among all the metal complexes the compounds in which, the one with cobalt coordinated with ligand 2 had good antibacterial activity against E. coli at the MIC value, 100 µg/mL (Mosa et al., 2011).
2.12 Synthesis of coumarin bearing triazine derivatives
Two series of quinolonyl/ coumarinyl triazine derivatives 7a-7e and 8a-8d were synthesized. In this synthesis, an intermediate subtract, 4-((4,6-dichloro-1,3,5-triazin-2-yl)amino)-2-(trifluoromethyl)benzonitrile 3 was synthesized by the reaction mixture of 4-amino- 2-trifluoromethyl benzonitrile 1 and trichloro- 1,3,5-triazine 2 in the presence of triethylamine by nucleophilic displacement of chlorine atom from triazine nucleus. The obtained product further reacted with either 4-hydroxy coumarin or 1-methyl quinolone in the presence of sodium hydride in THF to produce another precursor of title compound 5. Finally, the desired compounds corresponding 4-((4-chloro-6-((1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)oxy)-1,3,5-triazin-2-yl)amino)-2-(trifluoromethyl)benzonitrile 7 and 4-((4-((2-oxo-2H-chromen-4-yl)oxy)-6-(4-phenylpiperazin-1-yl)-1,3,5-triazin-2-yl)amino)-2-(trifluoromethyl)benzonitrile 8 were prepared by nucleophilic displacement of another chlorine atom of product 5 with 4- substituted aryl piperazinyl 6 in the presence of 1,4-dioxane and potassium carbonate. The compounds bearing quinolone as 7c and 7d had shown good antibacterial activity against S. aureus as 27 mm ZOI with the MIC value 6.25 µg/mL in comparison to the standard Ciprofloxacin. Moreover, these derivative 8d contain coumarin ring in structure had shown good antibacterial action against E. coli at the MIC value 12.5 µg/mL (Patel et al., 2011).
2.13 Synthesis of metal complexes-coumarin Schiff base derivatives
In this scheme, Co(II), Cu(II) and Ni(II) complexes of substituted (E)-N'-((2-hydroxynaphthalen-1-yl)methylene)-2-oxo-2H-chromene-3-carbohydrazide 6a-6b have been synthesized with respective Schiff base derived from 2-hydroxy naphthaldehyde 5 and substituted coumarin 3-carbohydrazide 4a-4b. The desired the Schiff base had been acting as tetradentate ligand, which metal ions coordinated to azomethine nitrogen, lactone carbonyl oxygen, hydrazide of nitrogen and naphthyl hydroxyl group and complexation through deprotonation of napthanol. Then, the obtained Schiff base analogues 6a-6b were liberated by the condensation of respective coumarin 3-carbohydrazide 4a-4b with 2-hydroxy naphthaldehyde 5 in ethanol. Consequently, the formation of respective complexes, 7a-7f by treating the desired Schiffbase condenses with the corresponding hydrated metal chloride in ethanol and add few quantity of sodium acetate. The desired Schiff base and its complexes have been evaluated for in vitro antibacterial activity against several pathogenic bacterial strains by bacterial DNA cleaving method. Among all the complexes the compounds 7a and 7b had shown as good antibacterial activity against E. coli and MIC value at 10 µg/mL in comparison to Gentamicin (Patil et al., 2011a).
2.14 Synthesis of metal complexes of coumarin-thiosemicarbazones derivatives
A series of transitional metal complexes of Schiff base derived from 3-substituted aryl thiosemicarbazides 4a-4b and 8-acetyl- 7-hydroxy 4-methylcoumarin 3 had been synthesized. The acetylated compound 3 was prepared by the equimolar quantity of 7-hydroxy-4-methyl-coumarin and acetic anhydride in the presence of anhydrous aluminium chloride in the oil bath at 160–165 °C for 4 h. The desired Schiff base was prepared by the condensation of 8-acetyl 7-hydroxy coumarin 3 and 3-substituted aryl thiosemicarbazides 4a-4b in ethanol with few drops of hydrochloric acid and followed respective metal complexes were formed by heating with the reaction mixture of equimolar concenteration alcholic solution metal chlorides and corresponding thiosemicarbazones in ethanol. During reaction, the metal ions were coordinated to azomethine group, thione-thiols and phenolic hydroxyl group of the structures through deprotonation, which indicate that the desired Schiff base act as tridentate ligand. The cobalt complexes of (E)-N-(3-chlorophenyl)-2-(1-(7-hydroxy-4-methyl-2-oxo-2H-chromen-8-yl)ethylidene)hydrazinecarbothioamide 5a had shown antibacterial activity against S. typhi, P. aeruginosa and E. coli at MIC value 10 µg/mL in comparison to Gentamicin as the standard (Patil et al., 2011b).
2.15 Synthesis of 3,4,7-tri substituted coumarin derivatives
A series of tri substituted coumarin analogues had been synthesized by heated with the reaction mixture of 3-diethylamino phenol 1 and various substituted α-cyano cinnamonitriles 2a-2f in ethanol and base piperidine yields ethyl 2-amino-7-(diethylamino)-4-phenyl-4H-chromene-3-carboxylate 3a-3f and 7-(diethylamino)-2-oxo-4-substituted phenyl-2H-chromene-3-carbonitrile 4a-4c. The SARs of 4-substituted aryl coumarin analogues indicate that the substitution of chloro or fluoro at the para position of phenyl ring and attachment of cyano at C-3 position in the coumarin moiety, which may increase the inhibitory activity. The compounds bearing 4-substituted phenyl in either 4H-chromene 3-carboxylate or 2H-chromene 3-nitrile such as, 3a, 3f and 4c were found notable antibacterial activity against S. aureus and S. epidermidis at 14 and 15 mm of ZOI diameter in comparison to Ampicillin (Sabry et al., 2011).
2.16 Synthesis of 4-amino alkylated coumarin derivatives
A series of compounds O-aminoalkyl substituted 7-hydroxy coumarin from substituted 7-hydroxy coumarin a-d were evaluated antibacterial activity with several bacterial strains. These, compounds were obtained from 7-hydroxy, C4, C6 or C8 substituted coumarin, in the initial step- the respective O-alkyl amino coumarin derivatives synthesized by alkylation of phenol moiety of derivatives of coumarin with suitable chloro ethyl substituted amines in dry acetone as well as, potassium carbonate and the final derivatives were converted into hydrochloride salts. Furthermore, the antibacterial activity of the compound alkylamino substituted phenolic OH at C-7 position and 6-acetyl-4-methyl-7-(2-morpholinoethoxy)-2H-chromen-2-one had been reported with notable inhibitory activity against Bacillus strains (Trykowska Konc et al., 2011).
2.17 Synthesis of metal complexes with coumarin chalcones-clioquinol
A series of metal complexes of coumarin compounds were derived from corresponding chalocones 3a-3f, which had been prepared by the condensation of 6-bromo-3-acetyl coumarin 1 with the appropriate substituted aryl aldehyde 2a-2f in piperidine base and ethanol. Cu(II) complexes had been synthesized by the heating the mixture compounds of cupric nitrate with corresponding chalcones 3a-3f and Cliquinol 4. The newly formed cupper complexes as octahydral structure were evaluated in vitro antimycobacterial and antibacterial activities. Among all the compounds, copper complexes of ligands 6-bromo-3-(3-(3-hydroxyphenyl)acryloyl)-2H-chromen-2-one 5c and 6-bromo-3-(3-(4-hydroxyphenyl)acryloyl)-2H-chromen-2-one5d with Cliquinol had found good antibacterial and antimycobacterial agent at MIC value 25 µg/mL each in compare to Isoniazid and Ethambutol (Patel et al., 2012).
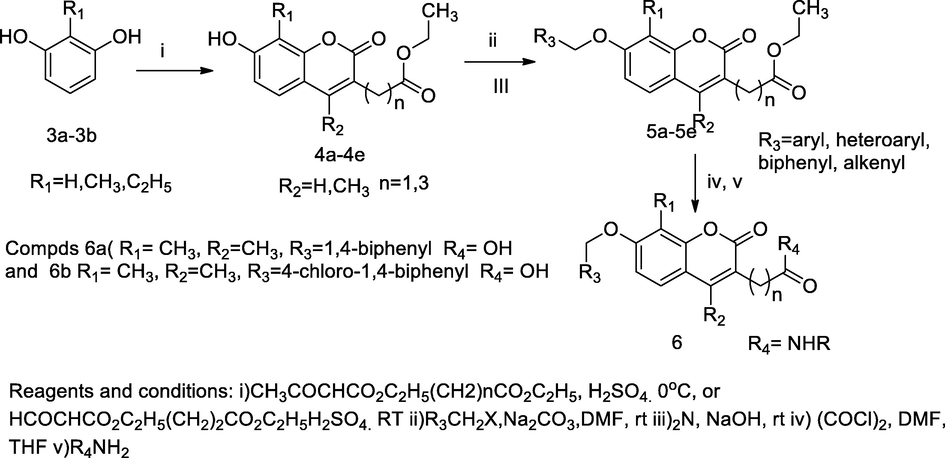
2.18 Synthesis of 3,4,7,8-tetra substituted coumarin derivatives
A series of biphenyl coumarin based bacterial helicase inhibitors of B. anthracis and S. aureus were designed and synthesized from substituted resorcinol. The title coumarin had been synthesized in three step reaction; in which initially 2-methyl/ethyl resorcinol was reacted with several β-ketoester provided 7-hydroxy coumarin carboxylate as an intermediate, which further underwent the hydrolysis of the desired ester, which provided the corresponding coumarin 3-carboxylic acid. The corresponding acids undergo alkylation by the treatment with either alkyl, aryl and alkenyl, or biphenyl halides in sodium carbonate and dimethyl formamide. These were further converted to respective amides by treatment with several amines. The desired amides were prepared from coumarin 3-carboxylic acid compounds. The compound 2-(7-([1,1′-biphenyl]-4-yloxy)-4,8-dimethyl-2-oxo-2H-chromen-3-yl)acetic acid had exhibited more significant activity against B. anthracis and S. aureus at MIC value 1.25 and 2.00 µM, respectively. SARs of these compounds indicate that the compounds having biphenyloxy with different substitution such as, floro, chloro, cyano and trifluoromethyl groups on the distal end phenyl ring would be responsible for antibacterial efficacy compared to unsubstituted phenyl ring (Li et al., 2012).
2.19 Synthesis of thiazolyl-pyrazoline coumarin derivatives
A novel series of coumarin compounds bearing thiazolyl and pyrazolone linked derivatives were synthesized by the reflux condensation of alcoholic solution of 6-bromo 3-bromoacetyl coumarin 1 with another intermediate reactant 5-hydroxy-3,5-bis(trifluoromethyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 2. The intermediate trifluoromethyl pyrazolone carboxamide 2 was synthesized by the reaction of 2,2,2-trifluoroethyl 4,4,4-trifluoro-3-oxobutanoate with thiosemicarbazide. Moreover, the compound 6c had notable activity against B. subtilis and S. epidermidis at 25 and 20 mm as ZOI in comparison to Cefixime (Aggarwal et al., 2013).
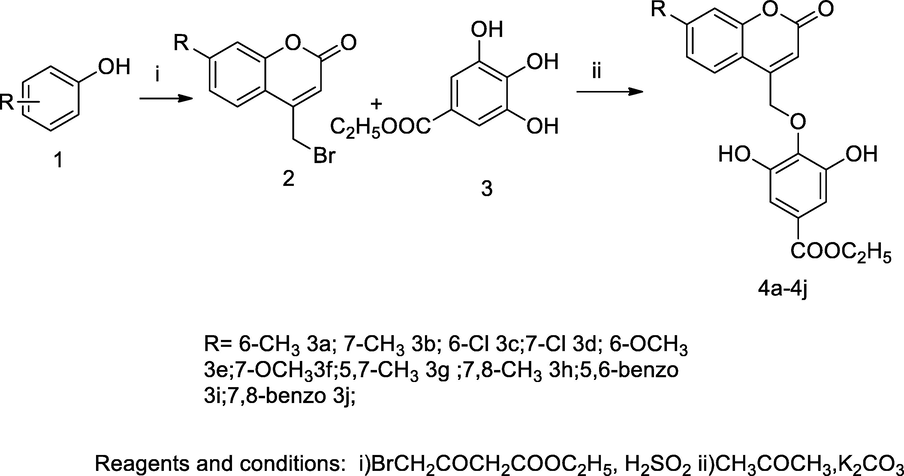
2.20 Synthesis of 4-aryloxymethyl coumarin derivatives
A series of potent 4-acyloxymethyl substituted coumarin derivatives were derived from the reaction 4-bromomethyl coumarin with methyl gallate, and the obtained hybrized products had good antibacterial activity. An organic synthon 4-bromomethyl coumarin was obtained from brominated ethyl acetoacetate with various substituted phenols under cyclisation in the presence of condensing agent sulfuric acid, then it was reacted with methyl gallate in dry acetone and potassium carbonate to yield the desired gallate ether by involving nucleophilic displacement reactions. Thereafter, a monitored antibacterial activity result revealed that the compounds 3a-3j were more active against E. faecalis and S. aureus. The compound ethyl 4-((7,8-dimethyl-2-oxo-2H-chromen-4-yl)methoxy)-3,5-dihydroxybenzoate3h was an effective congener against E. faecalis at MIC value 0.2 µg/mL in comparison to Ciprofloxacin (Revankar et al., 2013).
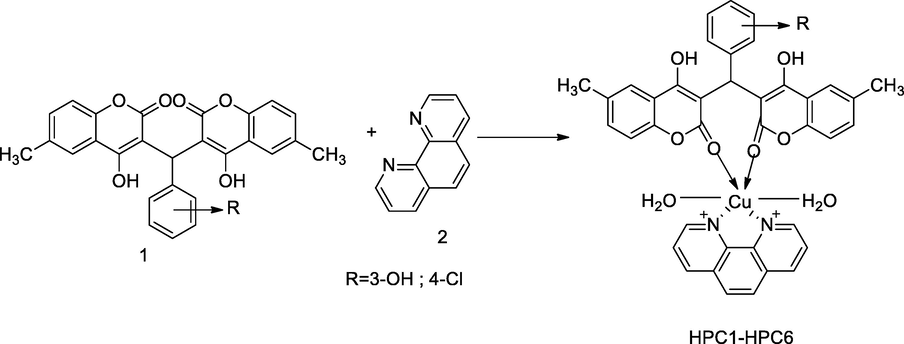
2.21 Synthesis of metal complexes of bis hydroxy coumarin with phenanthroline
A series cupric complexes containing bis-hydroxy substituted coumarin and 1,10-phenanthroline have been synthesized. The reactant ligand 1 was prepared from 4-hydroxy coumarin by treatment with a substituted aryl aldehyde in ethanol with the addition of sulfuric acid as catalyst. The desired copper complexes HPC1-HPC6 were prepared by the mixing of bishydroxy substituted coumarin and 1,10-phenanthroline with cupric nitrate in ethanol. The compounds HPC2 and HPC3 had shown a comparatively good in vitro controlling activity against S. pyogenesis. Concomitantly, the compound HPC2 had remarkable inhibitory action against M. tuberculosis at the MIC value, 3.125 µg/mL in comparison to Ciprofloxacin, Streptomycin, Isoniazid and Ethambutol as standards (Dholariya et al., 2013).
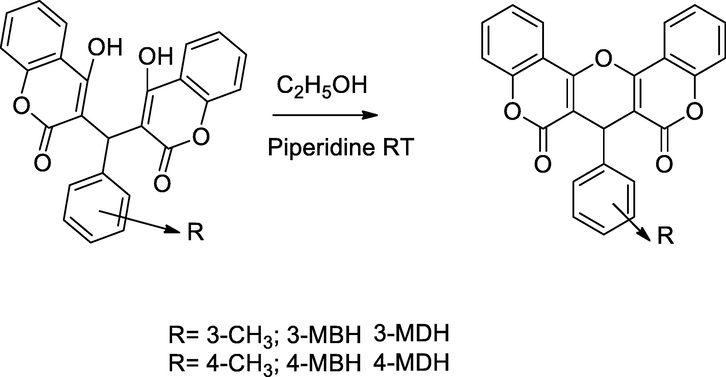
2.22 Synthesis ofbis-4-hydroxy coumarin derivatives
The bis 3,3′-4-hydroxy coumarin moiety was prepared for the Bis-4-hydroxy coumarin derivative which was screened for antibacterial activity against methicillin sensitive S. aureus and MRSA. The compound 3,3′-(m-tolyl methylene)bis(4-hydroxy-2H-chromen-2-one)3-MBH had a notable bactericidal action against S. aureus at the MIC value 64 µg/mL. The compound 3-MBH was synthesized by the condensation of 4-hydroxy coumarin with 3-methyl benzaldehyde(m-tolualdehyde in ethanol, then those compounds converted to 3-MDH by cyclization under the basic piperidine in ethanol (Li et al., 2013).
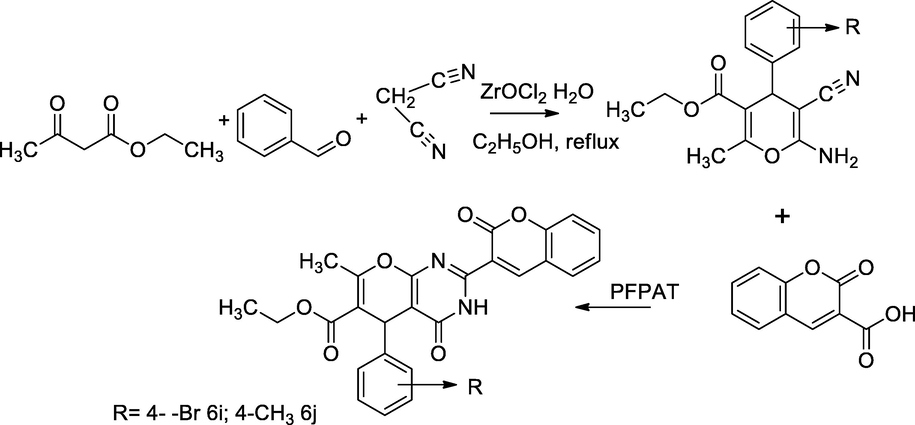
2.23 Synthesis of chromeno[2,3-d]pyrimidinone with coumarin derivatives
In this scheme, the coumarin-linked scaffold, chromeno[2,3-d]pyrimidinone 6a-6j had been synthesized by catalytic condensation of ethyl 6-amino-5-cyano-2-methyl-4-subst.phenyl-4H-pyran-3-carboxylate with coumarin 3-carboxylic acid 5, in the presence of organocatalyst 5 mol of pentafluoro ammonium triflate (PFPAT). In this reaction, the intermediate reactant 1 was synthesized by the reflux condensation of ethyl acetoacetate with substituted aryl aldehyde 2a-2j and malonic dinitrile in ethanolic of ZrOCl2 known as Bignelli reactions. The compound ethyl 5-(4-bromophenyl)-7-methyl-4-oxo-2-(2-oxo-2H-chromen-3-yl)-4,5-dihydro-3H-pyrano[2,3-d]pyrimidine-6-carboxylate 6i had been reported against E. coli at the MIC value 6.25 µg/mL with the inhibitory zone 18 to 20 mm; concomitantly, ethyl 7-methyl-4-oxo-2-(2-oxo-2H-chromen-3-yl)-5-(p-tolyl)-4,5-dihydro-3H-pyrano[2,3-d]pyrimidine-6-carboxylate 6j had shown control against both E. coli and S. aureus at MIC value 6.25 and in comparison to Ciprofloxacin as the standard (Ghashang et al., 2014).
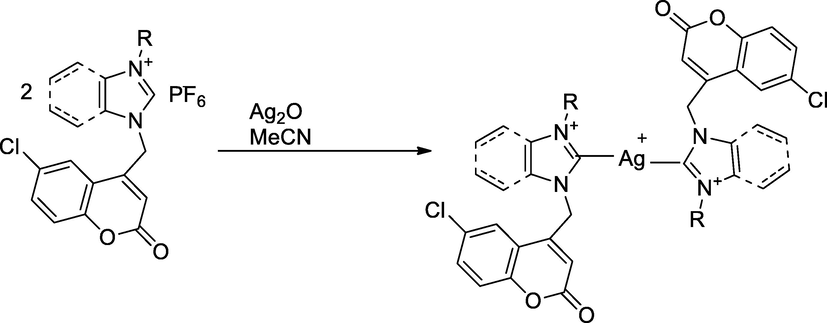
2.24 Synthesis of silver salt of benzimidazolinium with coumarin derivatives
A series of steric modulate chlorocoumarin derivative containing imidazolium/benzimidazolium salts and their bis-silver complexes were synthesized. Initially, the reaction of N-alkyl benz/imidazolium with 4-bromomethyl-6-chlorocoumarin in 1,4-dioxane solvent, which further liberated bromide salt; this was reacted with posphorous hexaflurophosphate to get the corresponding 1-alkyl-3-(6′-chloro-4′-methylenecoumarin) imidazolium hexaflurohexaphosphate 2. Thereafter, the silver(I) complexes were prepared by the reaction (benz)imidazoium salt with moist silver oxide in acetonitrile solution, stirred at 45 °C at 24 h. The liberated silver(I) complexes NHC were recrystalised from acetonitrile. these complexes with mixture of salts were evaluated antibacterial activity. The silver(I) complex bearing n-butyl (benz)imidazolium chloro coumarin had shown a good antibacterial agent against E. coli and P. aeruginosa at MIC value 4 µg ml-1individually in comparison to standard drug Ampicillin (Achar et al., 2017a).
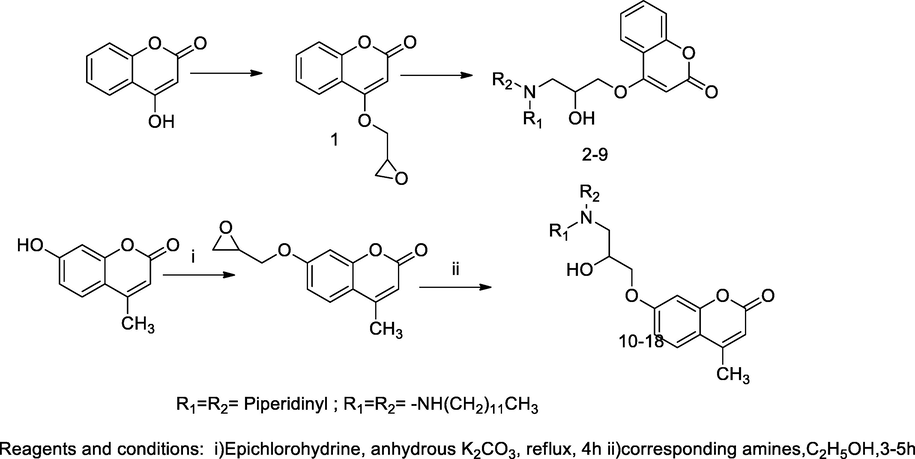
2.25 Synthesis of coumarinyl piperazine bearing propanol derivatives
A series of coumarin bearing piperazinyl derivatives 4a-4n were synthesized. The obtained compounds were tested for antibacterial activity against both Gram ÷ and Gram- bacteria, S. aureus, B. subtilis, E. coli and P. Aeruginosa and those were potent antibacterial agents. Initially, the 4-hydroxycoumarin was reacted with epichlorohydrin in dry acetone and potassium carbonate yield 4-(oxiran-2-ylmethoxy)-2H-chromen-2-one 1, which further reacted with 1-(4-substituted phenyl)piperazine in dimethyl formamide and potassium carbonate to produce the desired compounds. The compound 4-(2-hydroxy-3-(4-(4-methoxyphenyl)piperazin-1-yl)propoxy)-2H-chromen-2-one 4g had shown a good antibacterial activity against S. aureus and B. subtilis at the MIC value 0.23 and 0.35 µg/mL in comparison to Penicillin G as the standard (Wang et al., 2014).
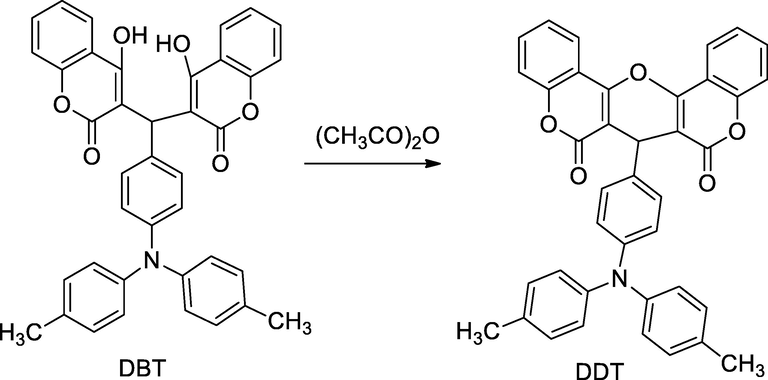
2.26 Synthesis of dicoumarol derivatives
Dichromene derivatives were synthesized by the intermediate 3,3′-((4-(di-p-tolylamino)phenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) DBT and the condensation of equimolar of ethanolic solution of 4-hydroxy coumarin; 4-(di-4-tolyl-amino) benzaldehyde in presence of piperidine, which further undergoes cyclisation in acetic anhydride yield 7-(4-(di-p-tolylamino)phenyl)-6H-pyrano[3,2-c:5,6-c′]dichromene-6,8(7H)-dione DDT. The synthesized two bis hydroxy coumarin derivatives were evaluated against S. aureus and MRSA with subsequent MIC values 32 and 64 µg/mL, in comparison to Levofloxacin, Ceftazidime, Cetriaxone and Gentamicin as the standard(Li et al., 2014).
2.27 Synthesis of metal complexes thiophene hydrazone - coumarin Schiff base
A series of transitional divalent metal ion complexes were synthesized from Schiff base ligand named as 3-chloro-N'-((7-hydroxy-4-methyl-2-oxo-2H-chromen-8-yl)methylene)benzo[b]thiophene-2-carbohydrazide 3, which was synthesized by equimolar mixture of methanolic solution of 8-formyl-7-hydroxy-4-methyl coumarin 2 and 3-chloro-benz[b]thiophene 2-carbohydrazide 1 with a few drops of glacial acetic acid. Then, finally metal complexes were obtained by the mixture of hot alcoholic solution of intermediate ligand 3 with respective metals chlorides. The obtained complexes were characterised by different spectral studies. Based spectral studies, the chelating ability of the ligand had been confirmed in complexation with Cu(II), Ni(II), Co(II) and Zn(II) ions whereas, ligands acted as ONO electron donor tridentate chelate to metal ions. During the complexation reactions, deprotonation was carried out at phenolic hydroxyl group of the ligand structure (Mahendra Raj et al., 2014).
2.28 Synthesis of indolin-2,3-dione –coumarin derivatives
Linked through ethylene, a series of Isatin-coumarin compounds, 4a-4b were synthesized and screened their antimycobacterial action against M. tuberculosis and multidrug resistant tuberculosis. The targeted compounds 3a-3b were synthesized from two intermediate reactant named as 7-hydroxy-4-methyl coumarin 1 and 1-bromomethyl 5-substituted isatin 2, which were mutually reacted in the presence of dimethyl formamide (DMF) and potassium carbonate. From the converted to 3-(alkoxyimino)-1-(2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)ethyl)indolin-2-one 4a-4b by treated with methoxyamine or ethoxyamine hydrochloride in sodium carbonate solution, respectively. The reactant 7-hydroxy-4-methyl coumarin was prepared by the Pechmann condensation of resorcinol and ethyl acetoacetate using polyphosphoric acid, whereas another product was alkylated of substituted isatin with dibromoethane in DMF. These tethered isatin-coumarin compounds were evaluated for their antibacterial actions. The compounds 4a and 4b were reported as good antibacterial agent against M. tuberculosis at MIC 50 µg/mL in comparison to standard drugs Isoniazid and Rifampicin, respectively (Gao et al., 2018).
2.29 Synthesis of metal complexes of acetyl coumarin derivatives
The new Cu(II) complexes with 6-acetyl-7-hydroxy 4-methylcoumarin and 8-acetyl-7-hydroxy 4-methylcoumarin had been prepared by electrochemical method. During the reaction, the two bidentate coumarin ligands bind to Cu(II) through the acetyl group and deprotonation of phenolic hydroxy group. Among all the tested compounds, the complex with acetyl at C6 coumarin 2 had been reported as a good antibacterial agent against Micrococcus luteus at MIC value 0.017 mg/mL (Klepka et al., 2015).
2.30 Synthesis of arylchalcone of coumarin derivatives
A series of aryl chalcone hybrids derived from coumarin (5–12) had been synthesized efficiently with moderate yield through the principle of knowingly reaction treating the appropriate salicylaldehyde and respective ethyl substituted benzoyl acetate with piperidine in ethanol under reflux for 2–5 h, which further were synthesized nitro substituted of benzoyl coumarin derivative from intermediate precursor undergone nitration and subsequently reduction of these derivatives with stannous chloride yield corresponding amino substituted coumarin chalcones. All these compounds had been tested for their antibacterial activity against seventeen marine gram negative bacterial strains including different species of Tenacibaculum. The compounds with amino substituted coumarin chalcones such as, (E)-3-(3-(3-amino-4-methoxyphenyl)acryloyl)-2H-chromen-2-one 6, (E)-3-(3-(3-amino-4-ethoxyphenyl)acryloyl)-2H-chromen-2-one 7, (E)-3-(3-(4-aminophenyl)acryloyl)-8-methoxy-2H-chromen-2-one 10 and (E)-3-(3-(4-aminophenyl)acryloyl)-8-ethoxy-2H-chromen-2. This compound 11 had been reported as antibacterial agent against Tenacibaculum maritimum in comparison to Oxolinic A, Enrofloxacin and Ampicillin as standards (Vazquez-Rodriguez et al., 2015).
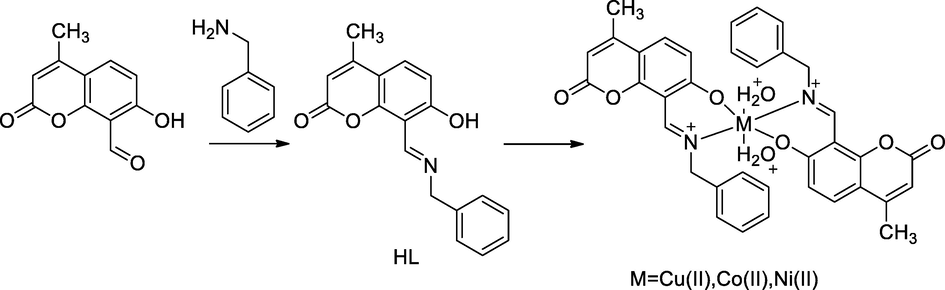
2.31 Synthesis of a metal chelate of Schiff base with coumarin derivatives
A new series of Cu(II), Ni(II) and Co(II) complexes had been synthesized with Schiff base (HL), which was derived from 8-formyl-7-hydroxy −4-methyl coumarin with benzylamine. The coordination of metal ions to the ligand through phenolic hydroxy group of coumarin and nitrogen of azomethine groups were prepared by the principle of Schiff bases. The obtained compound 3 had been reported as having good antibacterial control against E. coli, P. aureginosa, K. pneumonia and S. aureus (Prabhakara et al., 2015).
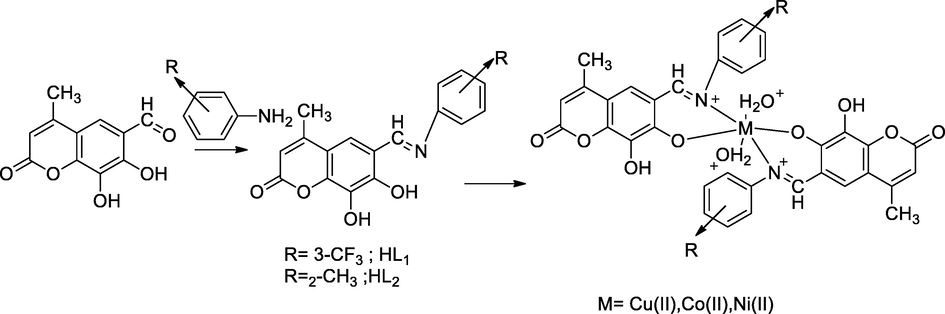
2.32 Synthesis of transitional metal complexes with coumarin derivatives
A series of Cu(II), Ni(II) and Co(II) complexes of Sciffbase coumarin derivatives had been synthesized from 6-formyl,7,8-dihydroxy coumarin with either 3-trifluoromethyl aniline or o-toluidine. The Schiff base ligands had been reported as an intermediate of 7,8-dihydroxy-4-methyl-6-(((3-(trifluoromethyl)phenyl)imino)methyl)-2H-chromen-2-one HL1 and 7,8-dihydroxy-4-methyl-6-((o-tolylimino)methyl)-2H-chromen-2-one HL2. The compound with copper metal ion complexed of 7,8-dihydroxy-4-methyl-6-(((3-(trifluoromethyl)phenyl)imino)methyl)-2H-chromen-2-one had shown as good antibacterial agent against Klebsiella sp. at 12 mm and MIC value 100 µg/mL in comparison to Gentamicin as the standard (Patil et al., 2015).
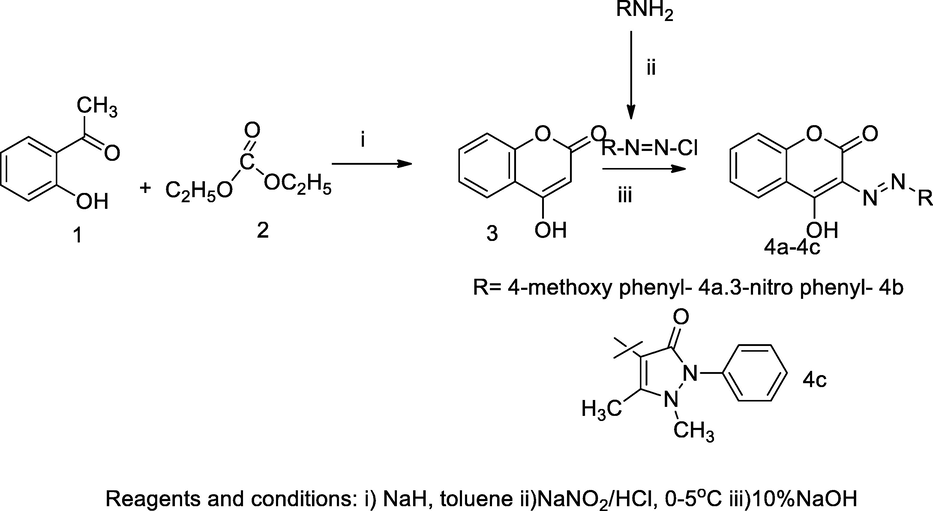
2.33 Synthesis of 3-arylazo 4-hydroxy coumarin derivatives
A series of 3-arylazo 4-hydroxy coumarin derivatives were obtained by azo coupling reaction of substituted aryl diazonium salt including antipyrine with 4-hydroxy coumarin. The hydroxy coumarin analogues, 4-hydroxy-3-((4-methoxyphenyl)diazenyl)-2H-chromen-2-one 4a, 4-hydroxy-3-((3-nitrophenyl)diazenyl)-2H-chromen-2-one 4b had exhibited potential antibacterial activities against bacterial strains with MIC 31.25 μg/mL whereas the compound (4-((4-hydroxy-2-oxo-2H-chromen-3-yl)diazenyl)-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one 4c had as good antibacterial agent against K. pneumoniae at 26.50 mm inhibitory zone (Sahoo and Sudhir Kumar, 2015).
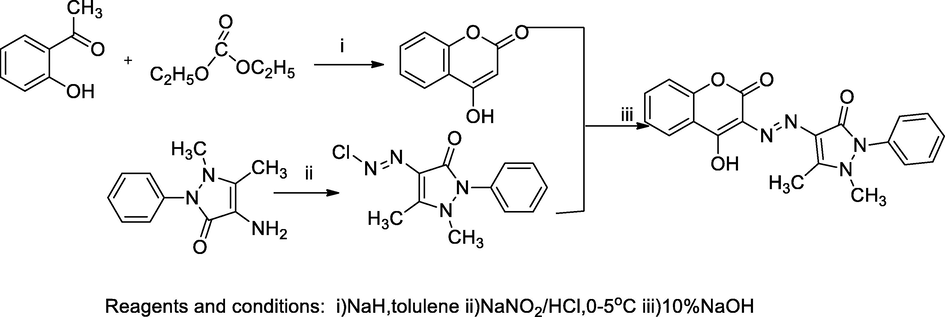
2.34 Synthesis of antipyrinyl azo-coumarin derivatives
A series of 3-hetrarylazo coumarin was prepared from 4-hydroxycoumarin by azo coupling reaction by several heteroaryldiazonium salts reacted with the coupling component 4-hydroxy coumarin in NaOH solution, the obtained azo coumarin analogues were evaluated in vitro for antibacterial studies by agar diffusion method. The compound (4-((4-hydroxy-2-oxo-2H-chromen-3-yl)diazenyl)-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one 4e had a good antibacterial activity against S. aureus as the ZOI, 25 mm with the MIC value 31.25 µg/mL in comparison to Ampicillin (Sahoo et al., 2015).
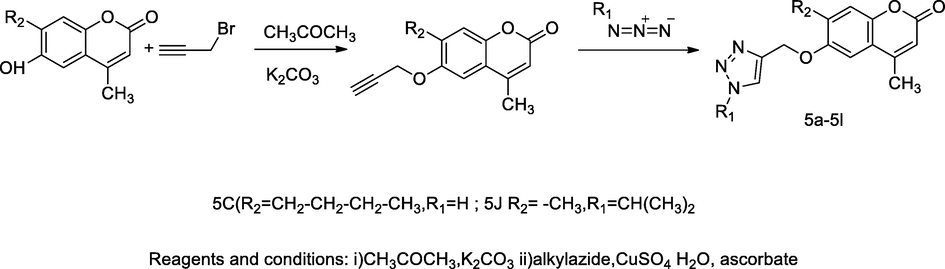
2.35 Synthesis of 1,2,3-triazolyl substituted coumarin derivatives
A series of twelve triazolylmethyloxy substituted alkyl coumarin analogues, 5a-5 l were synthesized by reacting with 4-methyl-6-(prop-2-ynyloxy)-2H-chromen-2-one and substituted alkyl azide in the principle, ‘click reaction’, and the obtained compound 4-methyl-6-((1-subst. alkyl-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one yielded 5a-5 l. The compound 5c having n-butyl substituted coumarin linked with triazolyloxymethyl at C-6 position and isopropyl substituted coumarin linked with triazolyloxymethyl 5j had been reported as a good in vitro antibacterial agent against E. coli and S. aureus at MIC values 8 and 7 µg/mL, respectively (Kolichala et al., 2018).
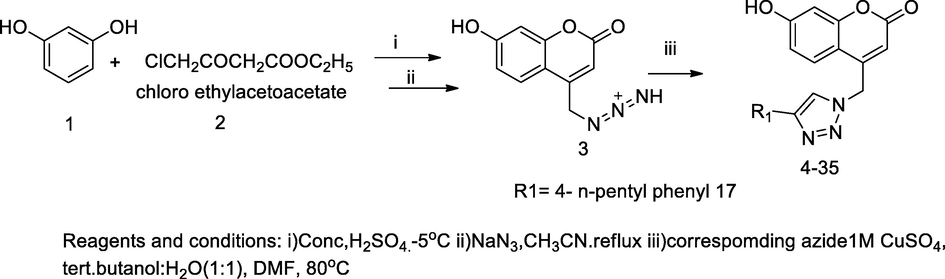
2.36 Synthesis of coumarin bearing triazole derivatives
In this scheme, coumarin derivatives having substituted triazole ring attached were designed and synthesized using copper(I) catalysed by ‘Huisgen 1,3-dipolar’ reaction of terminal alkyne with treatment azide. In the scheme, intermediate 4-azidomethyl coumarin derivatives were liberated as sodium azide with 4-chloromethyl- 7-hydroxy coumarin and 4-chloromethyl- 7-methyl coumarin, then these were prepared by the reaction of 3-hydroxy phenol and 3-methyl phenol with chloro ethyl acetoacetate under cyclisation in the presence of dehydrating agents concentrated sulfuric acid. Furthermore, these 1,2,3-triazole-coumarin hybrids were obtained by ‘click chemistry’ of 4-azidomethyl coumarin derivative and substituted alkynes in the presence of catalyst Cu(I), which generally was prepared in situ by copper sulfate and metallic copper. Moreover, the synthesis of 1,2,3-triazolyl substituted aryl sulfonamide of coumarin azide and the corresponding N-propargylated aryl sulfonamides were prepared. The compound 7-hydroxy-4-((4-(4-pentylphenyl)-1H-1,2,3-triazol-1-yl)methyl)-2H-chromen-2-one17 had shown as a good antibacterial agent against E. faecalis at MIC value 8 µg/mL (Kraljević et al., 2016).
2.37 Synthesis of silver complexes with heterocyclic substituted coumarin derivatives
A series of coumarin silver(I) metal complexes bearing N-heterocyclic carabene (NHC) were synthesized by interaction of the corresponding imidazolinium or benz[d] imidazolinium chloride and silver oxide in dichloromethane at room temperature; and the obtained corresponding metal complexes were characterised by elemental analysis, 13C/1HNMR and mass spectral studies. These silver NHC complexes were prepared in the reactions consisting of three diverse steps, initially with coumarin ligand 4-(chloromethyl)-6,8-dimethyl-2H-chromen-2-one 1. precursor prepared from chloroethyl acetoacetate, which was condensed with 2,4-dimethylphenol in the presence of concentrated sulphuric acid; thereafter, silver NHC complexes were prepared by the treatment of N-substituted imidazolinium or N-substituted benzimidazolinium chloride with equivalent silver oxide in DCM after 24 h. Among all complexes, the compound with coumarin bearing N-naphyl benzimidazolinium in structural frame 5e was the good antibacterial agent against S. aureus and E. faecalis at MC value 25 µg/mL, individually in comparison to Ampicillin and Ciprofloxacin (Karataş et al., 2016).
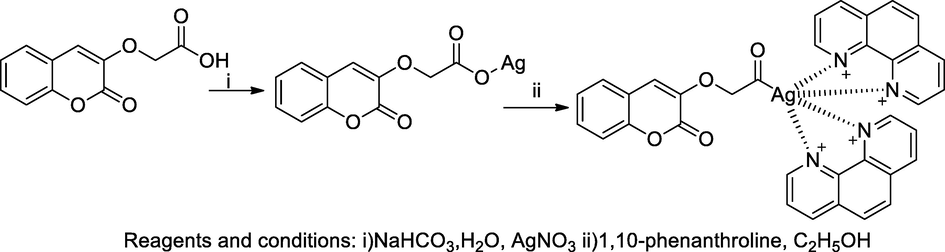
2.38 Synthesis of silver complexes with coumarinyloxyphenoxy acetic acid derivatives
Silver metal complexes bearing coumarin with phenathroline adducts were prepared by the ligand 2-((2-oxo-2H-substituted chromen-3-yl)oxy)acetic by condensation of an appropriate substituted 3-hydroxy coumarin with bromoethyl acetate with further hydrolysis of the obtained ester on in acetone: water mixture. The silver(I) complexes were prepared by deprotonation of substituted coumarinyl phenoxy acetic acid ligands using stoichiometrically equal amount of sodium bicarbonate and silver nitrate. Furthermore, silver (I)-phenathroline adduct were obtained by mixing of the silver substituted coumarinyl phenoxy acetate with phenathroline in ethanol. The compound having silver metal complex containing phenathrolinehad was a good antibacterial agent against enterococci with the MIC50 value 16 µM in compare to Vancomycin as the standard (Mujahid et al., 2016).
2.39 Synthesis of 4-amino coumarin derivatives
Three series of 4-amino substituted were designed and synthesized from intermediate corresponding chalcones yielded 4-amino coumarin derivatives. Phenyl substituted isoxazole, pyrimidinthione and pyrimidin-2-one moiety were connected coumarin at C-4 position of 4-amino coumarin. These three series compounds were synthesised by cyclisation of coumarin derived chalcones treated with hydroxylamine, thiourea and urea. In this synthesis, the starting material 4-chloro coumarin 2 was prepared by heating of the mixture of 4-hydroxy coumarin 1 with phosphorous oxytrichloride, which further yielded the corresponding 4-((4-acetylphenyl)amino)-2H-chromen-2-one 3 form an intermediate by reacting with 4-amino acetophenone in the presence of sodium carbonate. A series of α,β- unsaturated carbonyl substituted 4-amino coumarin (4a-4k) was prepared by mixing of the 4-((4-acetylphenyl)amino)-2H-chromen-2-one 2 in DMF solution with an appropriate aryl aldehyde in the presence of potassium hydroxide at room temperature. Thereafter, an individual chalcone derivative in alcoholic solution was mixed with hydroxylamine hydrochloride to produce the corresponding 4-((4-(5-subst.phenylisoxazol-3-yl)phenyl)amino)-2H-chromen-2-one 5a-5k by the cyclisation; whereas, other derivatives such as, 4-((4-(6-(subst.phenyl)-2-hydroxypyrimidin-4-yl)phenyl)amino)-2H-chromen-2-one 7a-7k, 4-((4-(6-(subst.phenyl)-2-mercaptopyrimidin-4-yl)phenyl)amino)-2H-chromen-2-one analogues 6a-6k were prepared by cycliation of chalcones with urea and thiourea, respectively. These derivatives had been monitored for antibacterial activities against eight bacterial strains. The compound 4-((4-(6-(3,4-dimethoxyphenyl)-2-mercaptopyrimidin-4-yl)phenyl)amino)-2H-chromen-2-one 7j was reported as having good antimycobacterial activity at MIC value 25 µg/mL. Furthermore, compounds 4-((4-(6-(3,4-dihydroxyphenyl)-2-mercaptopyrimidin-4-yl)phenyl)amino)-2H-chromen-2-one 7i and 4-((4-(6-(4-methoxyphenyl)-2-mercaptopyrimidin-4-yl)phenyl)amino)-2H-chromen-2-one 7 k had been reported as being as good antibacterial agents against S. aureus, B. cereus and E. coli at 28 mm ZOI and with the MIC value 3.12 µg/mL (Patel et al., 2017).
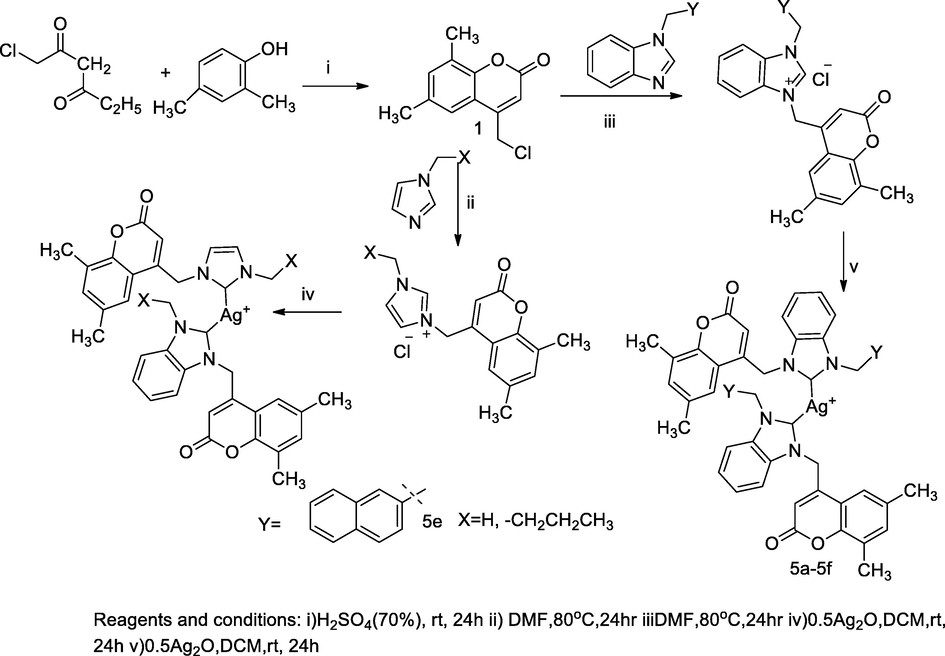
2.40 Synthesis of imidazoliniummethyl coumarin silver complexes
A series of coumarin tethered N-heterocyclic carbene (NHC) silver (I) complexes (5–21) was designed and synthesized. NHC ligands bearing N-substituted imidazolium and benzimidazolium had been developed by integument of transition metal complexes and those had been suitable for biological uses for treating several bacterial infections. Thecoumarin tethered bis imidazolinium salt had been developed through two steps alkylation reactions, initially alkylation of either imidazole or benzimidazole to obtain 1-alkyl imidazole or 1-alkyl benzimidazole, respectively, which further the formation respective imidazolium bromide salt had been accomplished in the successive N-alkylation through addition of equimolar concentration of N-alkylated with 4-bromomethyl- 6- methylcoumarin in 1,4-dioxane solution at 85 °C for 24 h. Furthermore, the imidazolium bromide salts with potassium hexaflurophosphate in methanol and water (9:1) produced the desired sterically tethered coumarin- imidazolium hexafluropentaphosphate in high yields and these compounds acted as NHC ligands upon in situ reaction deprotonation at C-2. Finally, the targeted silver- bis NHC complexes had been synthesized by reactions of the corresponding imidazolium salts with silver oxide (Ag2O) in acetonitrile at 45 °C under dark condition. Among all the tested compounds, silver salts of bis butyl, pentyl and hexyl substituted imidazolium of 6-methyl coumarin had shown good antibacterial action against E. coli at MIC value 8 µg/mL. Moreover, the compound 18, 19 and 20 were reported as having good antibacterial activity against E. coli at MIC value 8 µg/mL. Similarly, compounds 15 and 18 were reported active against P. aeruginosa at MIC value 8 µg/mL in comparison to Ciprofloxacin as the standard (Achar et al., 2017b).
2.41 Synthesis of 7-coumarinyloxy amino propanol derivatives
In the scheme, 7/4-coumarinyloxy propanol substituted amine were synthesised from starting material either 7-hydroxy 4-methyl coumarin or 4-hydroxycoumarin. The synthesis of the desired compounds comprised two step-reactions among which, 7-hydroxy-4-methyl coumarin/4-hydroxy coumarin was treated with epichlorohydrin in the presence of anhydrous potassium carbonate to produce the corresponding oxirane derivative with a high yield; thereafter, those intermediates reacted with various primary or secondary cyclic amines in ethanol at room temperature yielded coumarinyl oxy propanol amines analogues. During the reaction, nucleophilic oxirane ring had opened by the treatment with amines to produce coumarinyl amino alcohol derivatives. SAR analyses of these compounds revealed that compounds with 7-substituted amino propanol of 4-methyl coumarin derivatives had greater (stronger) antibacterial action compared to 4-substituted amino propanol coumarin derivatives, due the methyl group which might have induced the hydrophobic interaction leading to a better penetration of the compounds through bacterial cell membranes, Furthermore, the compounds with long alkyl chain substituent on side of amino group had been shown active than short alkyl chain amine substituent compounds. Among all the tested compounds, long alkyl chain contains twelve carbons had promising bioactive compound due to high lipophilicity effects. The antibacterial action results indicated that the compound 7-(3-(di(piperidin-1-yl)amino)-2-hydroxypropoxy)-4-methyl-2H-chromen-2-one17 and compound bearing dodecyl amine substituent at C-7 of 4-methyl coumarin 18 was reported as good antibacterial agent against K. pneumoniae at MIC value 6.25 µg/mL, in comparison to Novobiocin and Vancomycin. The compound with oleyl group substiuent at amine of 4-methyl coumarin 16 had shown a good antibacterial activity against four bacterial strains namely, S. aureus, E. coli, K. Pneumoniae and P. aeruginosa with MIC value ranging 6.25–25 µg/mL (Priyanka et al., 2017).
2.42 Synthesis of 3-heteroaryl substituted coumarin derivatives
In this scheme, a series of 2-((furan-2-ylmethylene)amino)-6-(2-oxo-2H-chromen-3-yl)-4-substituted phenylnicotinonitrile derivatives 5a-5m were designed and synthesized through the microwave irradiation of the reaction mixture of 2-amino-6-(2-oxo-2H-chromen-3-yl)-4-substituted phenylnicotinonitrile 3a-3 m and furan 2-carboxaldehyde 4 in glacial acetic acid to which with zinc chloride was added at 300 W. Initially, 3-acetyl coumarin 1 was reacted with different aryl aldehyde 2 malononitrile and ammonium acetate in microwave irradiation at 130 °C for 6–10 min, and the liberated 2-amino-6-(2-oxo-2H-chromen-3-yl)-4-substituted phenylnicotinonitrile. Then the compounds 5d, 5f, 5j, 5k and 5l with 4-methoxy, 4-methyl, 4-hydroxy, 3-hydroxy and 3-methoxy respectively; those were attached to phenyl substituent of niocotinyl nitrile of coumarin at C-3 position; it had shown as good antibacterial activities against E. coli and P. aeruginosa in comparison to Ampicillin. SAR analyses of these nicotinyl nitrile derivatives indicated that the compound having electron donating capability such as, methoxy, hydroxy and methyl substituent in structure might have contributed to the leading antimicrobial actions (Desai et al., 2017).
2.43 Synthesis of 3-chromenyl carboxamide of coumarin derivatives
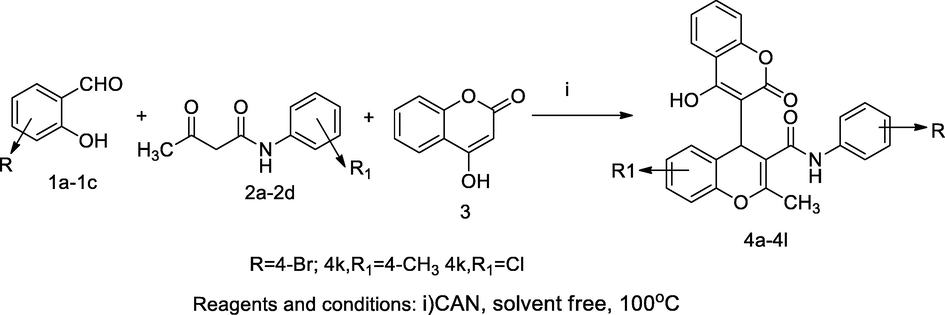
A series compounds of chromenyl carboxamide 4a-4l was synthesized from multi reactant reaction between substituted salicylaldehyde 1a-1c and derivatives acetoacetanilides 2a-2d with 4-hydroxy coumarin 1 in the presence of catalyst ceric ammonium nitratee (CAN) under solvent-free condition by the principle‘Knovengal-michael’ reaction. The reactant acetoacetanilide with both electron donating and electron withdrawing substituents attached to phenyl terminal of the structure reacted with salicylaldehyde and 4-hydroxycoumarins in CAN to yield the respective chromenyl carboxamide derivatives. In this synthesis, a lot of catalysts was used in during reactions, but among all CAN had efficient for cyclisation that afforded good yield under solvent free condition. The solvatochromic properties of the compounds were analysed by using solvents of the increasing order of polarity. The compound 6′-bromo-4-hydroxy-2′-methyl-2-oxo-N-(p-tolyl)-2H,4′H-[3,4′-bichromene]-3′-carboxamide4k and 6′-bromo-4-hydroxy-2′-methyl-2-oxo-N-(4-chlorophenyl)-2H,4′H-[3,4′-bichromene]-3′-carboxamide 4 l had shown good antibacterial actions against S. aureus and B. subtilis at MIC value 9.3 µg/mL in comparison to Ampicillin (Chitreddy and Shanmugam, 2017).
2.44 Synthesis of pyrazole bearing coumarin derivatives
A series of coumarin based pyrano[2,3-c]pyrazole derivatives 3a-3e had been synthesized by multiple component reaction steps. In the synthesis, initially the mixture of hydrate hydrazine and alcoholic solution of ethyl acetoacetate was stirred for few minutes then added with substituted coumarin 4-carboxaldehyde, malononitrile or ethyl cyanoacetate with base and stirred for 2 h to produce an intermediate ethyl 2-cyano-3-(2-oxo-2H-chromen-4-yl)acrylate 1, which further reacted with pyrazolin-5-one 2 by ‘Michael addition and intramolecular cyclisation’ to produce coumarin bearing pyrano[2,3-c]pyrazole derivatives 3a-3e. Moreover, these derivatives were the substituted coumarin based pyrazolyl propanoic acid 4a-4e in the presence of various acidifying agents for yielding, whereas the mechanism of organic reaction involveds open pyran ring system under an acidic condition as nitrile hydrolysis and decoxylation one of the carboxyl groups. The obtained coumarin bearing pyrano[2,3-c]pyrazole derivatives and the corresponding coumarin based pyrazolyl propanoic acid were monitored for in vitro antibacterial activity against some bacterial strains. Concomitantly, molecular docking studies had been performed with bacterial dihydropteroate synthetase (DHPS). These tested compounds were subjected to in vitro antibacterial activities, which revealed that the compounds 4b and 4c had good antibacterial activities against E. coli at 0.78, 1.56 µg/mL, respectively. Similarly, the compound 4b was had in vitro control against E. faecalis at MIC value 1.56 µg/mL in comparison to Ciprofloxacin (Chougala et al., 2017).
2.45 Synthesis of silver complexes of bis benzimidazolinium methyl with coumarin
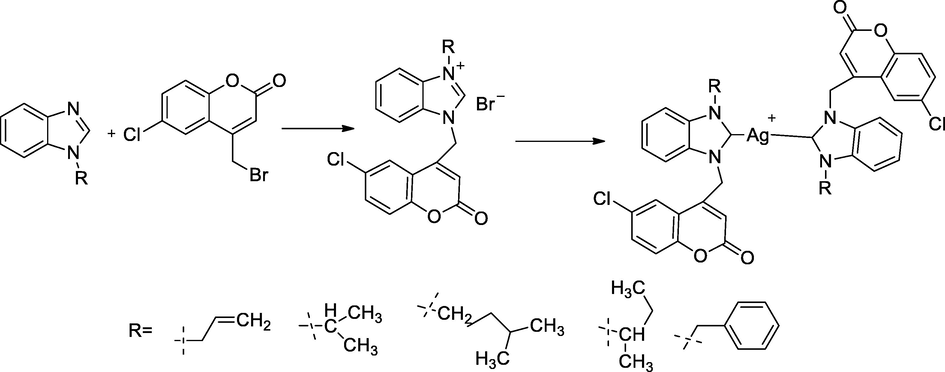
In this scheme, a series of silver complexes bearing N-heterocyclic carbenes (NHC) 5a-5e had been synthesized and were explored for biological actions. In the synthesis of complexes, initially benzimidazole underwent alkylated with different bromides such as, allyl, isopropyl, benzyl, isopentyl and 2-methylpropyl, to produce N-alkyl/allyl benzimidazole 1a-1e, which products were reacted with 4-bromomethyl-6-chloro coumarin 2 in 1,4-dioxane at room temperature for 10 min and those were the refluxing mixture for 24 h to afford off-white solid product of the corresponding 1,3-disubstituted benzimidazolium bromide salts 3a-3e; and further those were recrystallized from acetonitrile and were made to reacte with hexaflurophosphate form as respective bromides salts into their 1,3-disubst.benzimidazolium hexaflorophosphate 4a-4e products through metathesis reaction and the obtained salts were recystalised from acetonitrile:diethyl ether mixtures. In the coumarin tethered silver salts bis-NHC 5a-5e were prepared by the corresponding hexaflourophosphate that was stirred with silver oxide (Ag2O) in acetonitrile at 45 °C for 24 h. The antibacterial activity of these compounds was tested, which indicated the compounds 5a, 5b, 5c, 5d and 5e had been good antibacterial agents against P. aeruginosa at MIC value 8 µg/ mL in comparison to Ampicillin (Achar et al., 2017c).
2.46 Synthesis of indolidenyl of coumarin derivatives
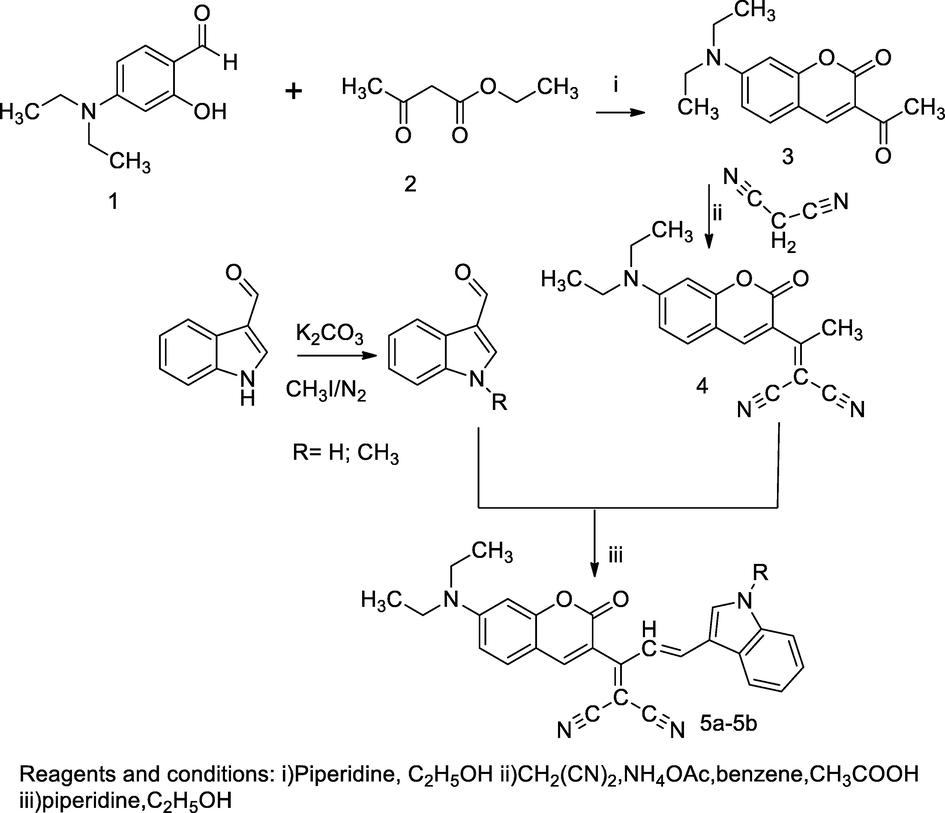
In the scheme, an intermediate 2-(1-(6-(diethylamino)-2-oxo-2H-chromen-3-yl)ethylidene)malononitrile 4 was prepared by the condensation of malonyl dinitrile and 3-acetyl-7-(diethylamino)-2H-chromen-2-one 3 in the presence of ammonium acetate and acetic acid using benzene as the solvent. An intermediate 3-acetyl-7-(diethylamino)-2H-chromen-2-one 3 was prepared by the reaction of diethylamino salicylaldehyde and ethyl acetoacetate in a few drops of piperidine. Furthermore, the reaction of 2-(1-(6-(diethylamino)-2-oxo-2H-chromen-3-yl)ethylidene)malononitrile with and N-subst. indole-3-carboxaldehyde in piperidine solution afforded the title target compound 2-(1-(6-(diethylamino)-2-oxo-2H-chromen-3-yl)-3-(subst.1H-indol-3-yl)allylidene) malononitrile 5. The compound 5a had been reported as an agent against E. coli at 10 mm ZOI in agar well diffusion plates (Aksungur et al., 2017).
2.47 Synthesis of pyrimidinone bearing coumarin derivatives d as good antibacterial
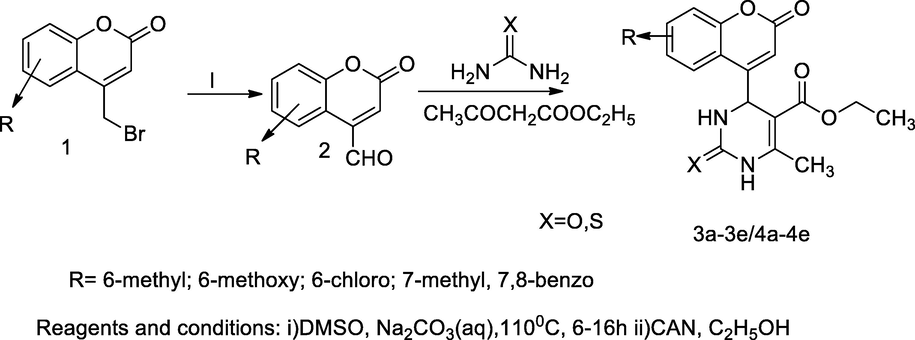
A series of compounds, dihydro pyrimidin 2-one/thione containing coumarin derivatives 3a-3e/4a-4e had been synthesized from 4-formyl substituted coumarin and ethyl acetoacetate using thiourea and urea in the presence of an effective catalyst CAN. Those condensations had involved by the principle of ‘Biginelli’ reactions. During the synthesis, the mechanism with CAN was catalysed in the Biginelli reaction indicated that the reaction would proceed through the formation of acylamino in intermediate ion, which is formed in situ by the reaction of formyl coumarin with urea/thiourea. All these synthesized were evaluated for the inherent antibacterial action against S. aureus. Among all the tested compounds, the compound ethyl 6-methyl-4-(7-methyl-2-oxo-2H-chromen-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate3d had been reported having good antibacterial activity against S. aureus at the MIC value, 0.2 µg/mL (Sunagar et al., 2017).
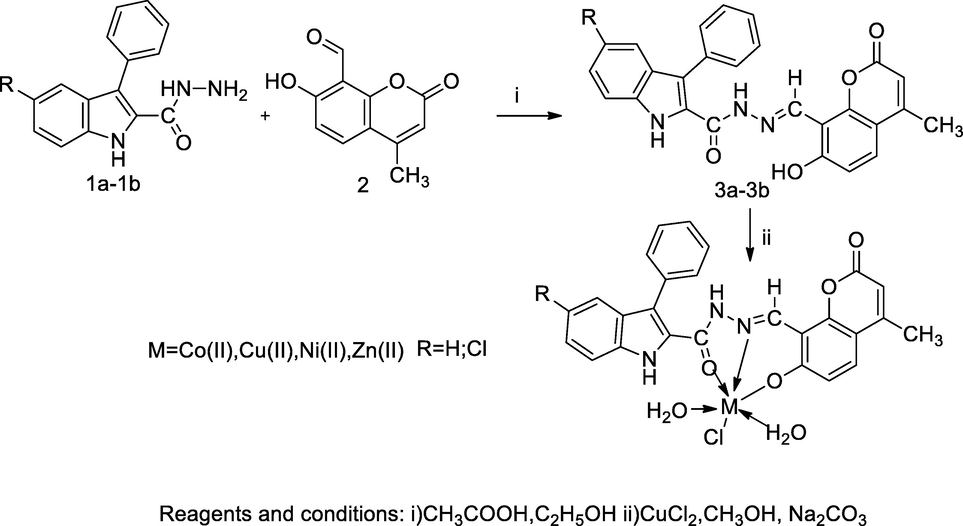
2.48 Synthesis of indole carbaxahydrazide Schiff base coumarin derivatives
In the scheme, a series of transitional metal ions Cu(II), Co(II), Ni(II) and Zn(II) complexes of the Schiff base 5-substituted N'-((7-hydroxy-4-methyl-2-oxo-2H-chromen-8-yl)methylene)-3-phenyl-1H-indole-2-carbohydrazide ligand 3a-3b were synthesized. An intermediate ligand was prepared by the reaction of methanolic solution 5-substituted-3-phenyl-1H-indole-2-carbohydrazide 1a-1b with 4-methyl-7-chloro 8-formyl coumarin 2 in presence glacial acetic acid then Schiff base ligand reacted with respective metal chlorides in methanolic solution to obtain the corresponding metal complexes of indole carmax hydrazide coumarin derivatives. Metal ions had been coordinated with oxygen atom of carbonyl, nitrogen of azomethine and functional group of phenolic hydroxyl through deprotonation and possess octahedral geometries. The cupper complex of ligand 3a had been reported as a good antibacterial agent against B. subtilis, E. coli and S. aureus at the MIC value 12.50 µg/mL individually (Mahendra raj and Mruthyunjayaswamy, 2017).
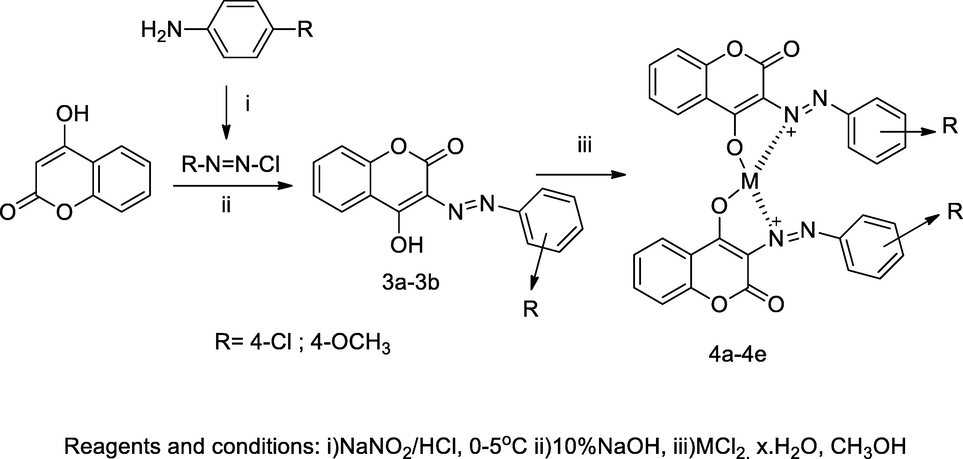
2.49 Synthesis of metal complexes 3-arylazo 4-hydroxy coumarin
In the scheme, a series metal ion complexes of 3-arylazo-4-hydroxy coumarin 4a-4h were synthesized. The metal complexes bearing ligands 3-aryazo 4-hydroxy coumarin 3a-3b were prepared by the coupling of reaction of respective aryl diazonium salt with 4-hydroxy coumarin in sodium hydroxide at mild condition which further reacted with various metal chloride salt in hot methanol. Furthermore, two moles of ligands were joined together with an individual metal ions like Cu(II), Co(II), Ni(II) and Zn(II) through deprotonation of enolic hydroxyl group of coumarin. The antibacterial activity of ligands and the individual complexes were monitored for in vitro antibacterial activities by agar well diffusion method. Among all the tested compounds, the compound with cobalt complexes of (E)-3-((4-chlorophenyl)diazenyl)-4-hydroxy-2H-chromen-2-one and (E)-3-((4-methoxyphenyl)diazenyl)-4-hydroxy-2H-chromen-2-one had excellent antibacterial activities against E. coli (Sahoo and Paidesetty, 2017).
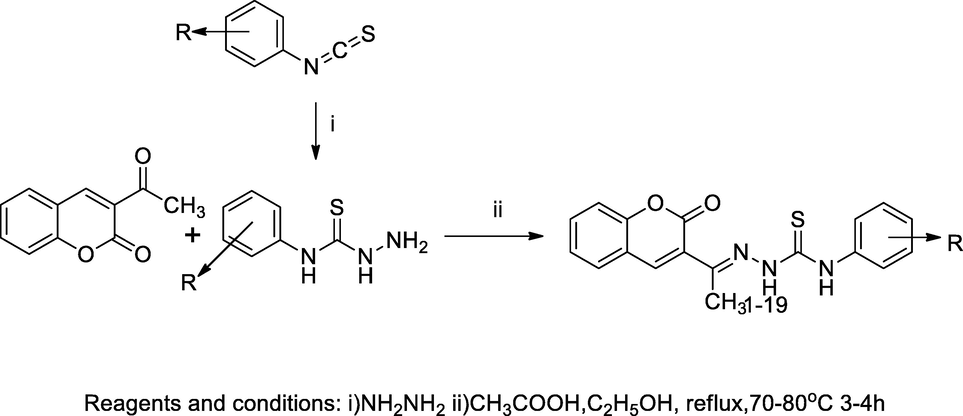
2.50 Synthesis of coumarin 3-semi thiocarbazone
In this scheme, (E)-2-(1-(2-oxo-2H-chromen-3-yl)ethylidene)hydrazine carbothioamide derivatives had been synthesised by the reaction of 3-acetyl coumarin, hydrated hydrazine and substituted phenyl isothiocyanate in presence of a small amount catalyst glacial acetic acid in refluxing ethanol and a good yield was liberated. An intermediate 3-acetyl coumarin was prepared ecofriendly by using catalyst starch sulfuric acid (SSA) or cellulose sulfuric acid (CSA) in Pechmann condensation reaction from which, salicylaldehyde was condensed with ethyl acetoacetate. All the liberated products were used for assessing antibacterial activities against E. coli. S. aureus, P. aeruginosa and S. pyogenes. Thereafter, the tested compound 15 containing an electron deactivating group fluoro substituted at the ortho and para position of phenyl ring and had good antibacterial activities against E. coli and S. aureus at MIC value 50 µg/mL each (Vekariya et al., 2017).
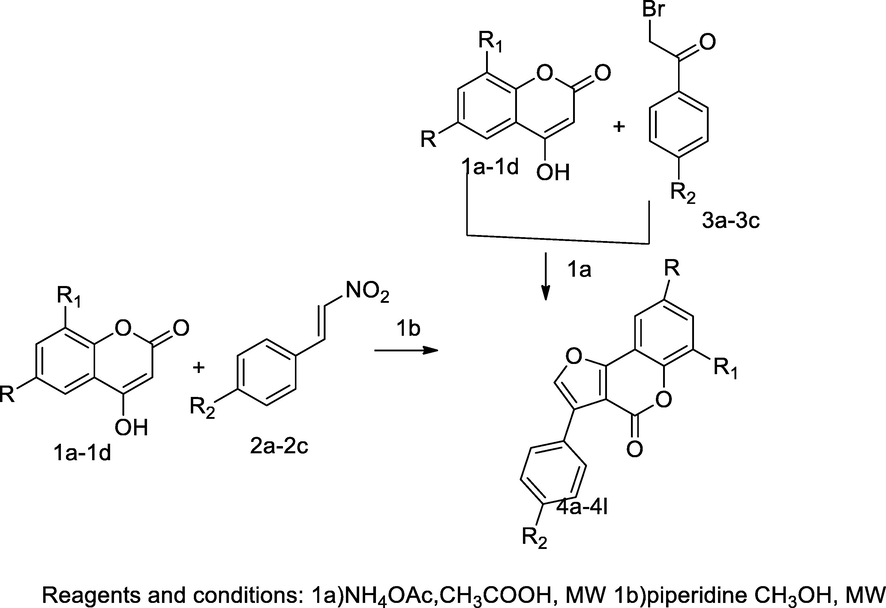
2.51 Synthesis of coumarin fused furan derivatives
Two series of the 3-aryl- furo[3,2-c] coumarins derivatives 4a-4l were prepared by microwave assisted synthetic method with two different conditions; in the first method, the synthesis of these derivatives were carried out by the reaction of substituted 4- hydroxycoumarin 1a-1d with an appropriate 2-aryl-1-nitro ethylene 2a-2c in methanol by the principle of Nef reaction whereas other methods. The reaction of substituted 4- hydroxycoumarin 1a-1d with an appropriate 2-aryl methyl bromide 3a-3c in acetic acid and ammonium acetate under the Feist-Benary reaction condition. Among these two methods, in method of feist benary condition, furancoumarin derived products had good yields. SARs of these derivatives attributed that the compounds with fused furo[3,2-c] coumarin skeleton had inherent antibacterial action in addition to substituted electron donating groups like methoxy; methyl was attached to phenyl at para position that might have increased the potency of inhibition growth of bacteria. The compound 8-chloro-3-(p-tolyl)-4H-furo[3,2-c]chromen-4-one 4 h was reported having a good antibacterial agent action E. coli at MIC value 25 mm (Kaneria et al., 2017).
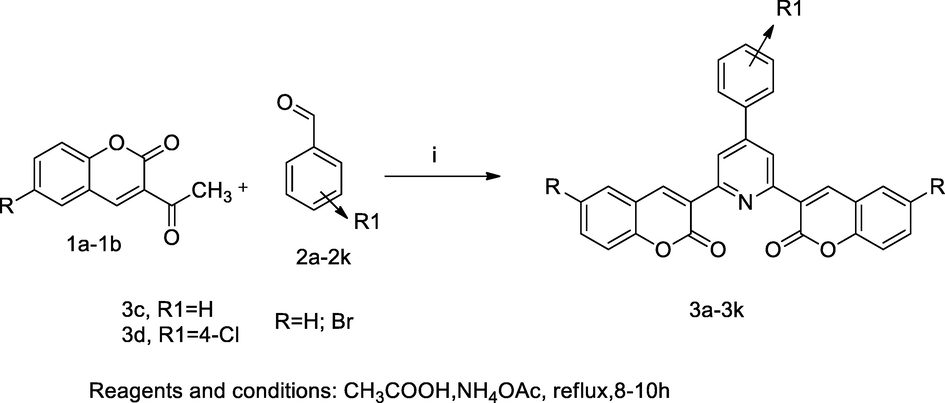
2.52 Synthesis of bis 3,3-coumarin derivatives
In the scheme, a series of bis coumarin derivatives 3a-3k were synthesized by the reaction of one pot convenient with multiple component through chichibabin reaction. These derivatives were synthesized by the reaction of 3-acetyl coumarin or bromo substituted 3-acetyl coumarin 1a-1b with substituted aryl aldehyde 2a-2k and ammonium acetate under acidic conditions. During this synthesis initially involves the formation of imine by 3-acetyl coumarin is treated with ammonia. Then condensation was performed in between substituted aryl aldehyde and enolic state of acetyl coumarin and finally coumarin chalcones and imines cyclisation to yield 3,3′-(4-subst. phenylpyridine-2,6-diyl)substituted bis(2H-chromen-2-one). The antibacterial activity revealed that the compounds 3c and 3d were reported as having good antibacterial activities against P. aeruginosa, B. subtilis, E. coli at MIC values 15 µg/mL in comparison to Amoxicillin and Gentamicin as standards (Kenchappa et al., 2017).
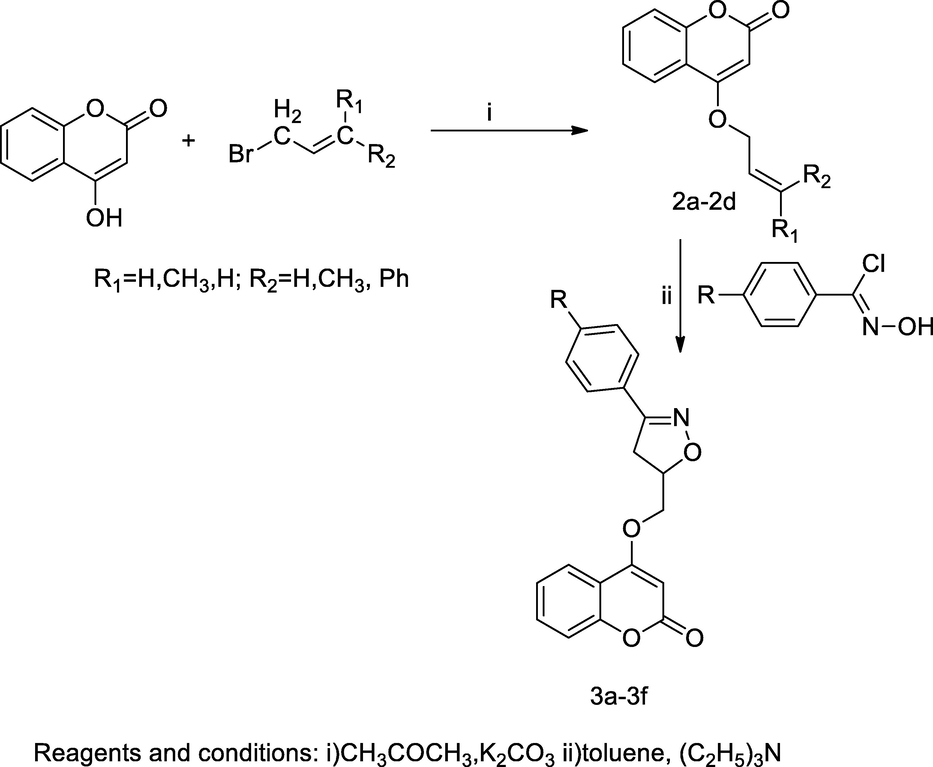
2.53 Synthesis of coumarinyloxy bearing oxazoline derivatives
In this scheme, a series of coumarinyloxy bearing 3-substituted phenyl/pyrroyl oxazoline 3a-3f compounds were synthesized from the starting precursor 4-hydroxycoumarin, then 4-hydroxy coumarin was converted into 4-allyloxy coumarin derivatives 2a-2d by the condensation with appropriate allylic halides in the presence of anhydrous acetone and potassium carbonate for 20 h. Furthermore, the obtained intermediate 4-allyloxy coumarin was reacted with (Z)-N-hydroxybenzimidoyl chloride in the presence of anhydrous toluene by 1,3-dipolar cycloaddition, which yielded coumarinyloxy bearing isoxazolone derivatives. Introduction of the substituted phenyl or pyrrole ring at C-3 position of desired target were obtained as the isoxazolone derivative. Among all the tested compounds the analogue 4-((3-(1H-pyrrol-2-yl)-4,5-dihydroisoxazole-5-yl)methoxy)-2H-chromen-2-one 3f had shown good antibacterial action against E. faecalis at the MIC value 0.31 mg/mL in comparison to Gentamicin as the standard (Zghab et al., 2017).
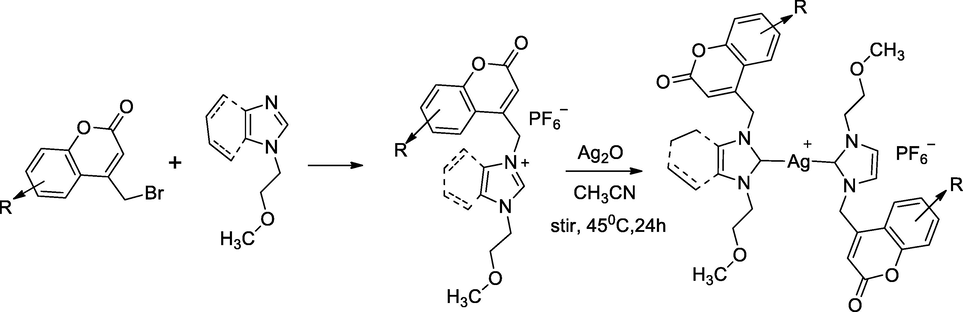
2.54 Synthesis of silver complexes with imidazolinum methyl coumarin derivatives
In this scheme, a series of silver complexes of substituted coumarin tethered NHC carbene ligands were synthesized and their biological actions were explored. N,N’- Dialkylated (imidazole or benzimidazole) referred as azolium salts as NHC precursors, initially in an electrophilic substitution reaction between imidazole/benzimidazole and n-bromo alkyl ether in the presence of dimethyl sulfoxide and excess potassium hydroxide to gave n-alkyl imidazole/benzimidazole, which further with ether functionalized derivatives undergone alkylation by treated with 4-bromomethyl −6-substituted coumarin in 1,4-dioxane at 24 h refluxing liberated corresponding N-alkoxy-N’-substituted coumarin azolium bromide salts. These bromides salt derivatives had been converted into the corresponding hexaflurophasphate salts by the treatment with methanol and water (1:9v/v) solution of hexaflurophosphate. Finally, the ether substituted azolium hexaflourophosphate salt was treated with silver oxide in the presence of acetonitrile at 45 °C for 24 h under the absence of light and the obtained desired silver complex substituted coumarin tethered bis azolium hexaflurophosphate salts and this reaction was performed by in situ deprotonation of azolium salt. The silver complex of 6-methyl coumarin bearing bis imidazolium hexaflourophosphate salt 9 had reported as good antibacterial agent against E. coli at MIC at 08 µg/mL in comparison to Ampicillin as the standard (Achar et al., 2018).
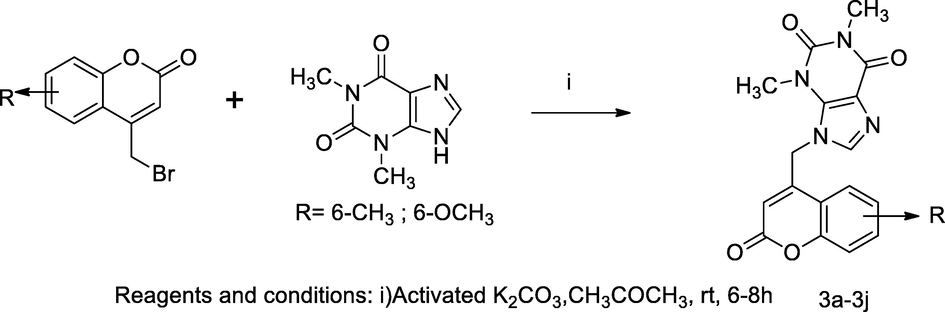
2.55 Synthesis of 7-coumarinyl methyl theophylline derivatives
A new series of hybrid compounds containing xanthine derivative like theophylline 3a-3j with substituted 4-bromomethyl coumarin were synthesized. The derivatives of 1,3-dimethyl-9-((substituted 2-oxo-2H-chromen-4-yl)methyl)-1H-purine-2,6(3H,9H)-dione 3a-3j were synthesized by the mixture of theophylline and anhydrous potassium carbonate with substituted 4-bromomethyl coumarin in acetone for 6–8 h. The SARs of antibacterial action of coumarin-theophylline hybrid congeners revealed that the derivative, which haved electron donating substitutions at coumarin nucleus possesses a good inhibition of growth and 6-methyl coumarin-theophylline congener had the maximum inhibitory action with MIC at 3.9 µg/mL against Gram + bacterium S. aureus, where as another derivative 6-methoxy coumarin-theophylline hybrid had MIC at 7.8 µg/mL. The compounds bearing electron donating substituent at C-6 position decrease order of efficacy methyl > t.butyl > methoxy>,5,6-benzo for different bacterial strains. Moreover, the compound 1,3-dimethyl-9-((6-methyl-2-oxo-2H-chromen-4-yl)methyl)-1H-purine-2,6 (3H,9H)-dione 3a was reported as having good antibacterial action against S. aureus and E. coli at MIC 3.9 µg/mL. Similarly, the compound 3f was recorded as good antibacterial agent against S. aureus and E. coli, S. typhi at MIC 7.8 µg/mL in comparison to Tetracycline as the standard (Mangasuli et al., 2018).
2.56 Synthesis of ribofuranosyl –coumarinyloxy bearing 1,2,3-triazole derivatives
A series of substituted ribofuranosyl coumarinyl 1,2,3-triazole 4a-4d had been synthesized by cycloaddition reaction between azido sugar and 7-alkynated 4-methyl coumarin 2a-2d in presence of Cu(I) with good yields. During synthesis of these compounds, initially with an intermediate 7-hydroxy substituted coumarin 1a-1d were treated with propargyl bromide in the presence of potassium carbonate to produce corresponding 7-propargyloxy substituted coumarin in an 85% yield. Then after, 7-propargyloxy coumarin was reacted with the corresponding 2-azido-2,3,5-tribenzoyl-β- D-ribofuranose in the presence of ascorbate-CuSO4 in THF through Cu(I) mediated cycloaddition reaction to afford resultant N’-2,3,5-tribenzoyloxy β- D-ribofuranosyl-4-coumarinyl-7-oxymethyl-1,2,3-triazole in 70% yield. Then debenzoylation of the resulted targeted ribofuranosyl coumarinyl 1,2,3-triazole derivatives. The compound N’-2,3,5-tribenzoyloxy β- D-ribofuranosyl-4-methylcoumarinyl-7-oxymethyl-1,2,3-triazole and N’-2,3,5-tribenzoyloxy β- D-ribofuranosyl-4-coumarinyl-7-oxymethyl-1,2,3-triazole had been reported having a good inhibitory action against M. tuberculosis at MIC 5.1 µM (Srivastava et al., 2018).
2.57 Synthesis of disubstituted of chromane derivatives
A series of disubstituted chromane derivatives were designed and synthesized from an intermediate either with 6-acetyl-5,7-dihydroxy-2,2-dimethylchroman-4-one 1 or 8-acetyl-5,7-dihydroxy-2,2-dimethylchroman-4-on 1a. These intermediates underwent methylation with methyl iodide to obtain corresponding 6-acetyl-5,7-dimethoxy-2,2-dimethylchroman-4-one 2 and 8-acetyl-5,7-dimethoxy-2,2-dimethylchroman-4-one 2a, respectively. Then, reduction of carbonyl of pyrone system of 2 and 2a in the presence of sodium tetrabrohydride and methanol in mild condition afforded 1-(4-hydroxy-5,7-dimethoxy-2,2-dimethylchroman-6-yl)ethanone 3 and 1-(4-hydroxy-5,7-dimethoxy-2,2-dimethylchroman-8-yl)ethanone 3a, respectively. Finally the desired compounds 1-(5,7-dimethoxy-2,2-dimethyl-2H-chromen-6-yl)ethanone 4 and 1-(5,7-dimethoxy-2,2-dimethyl-2H-chromen-8-yl)ethanone 4a were obtained by dehydration of immediate precorsor 3 and 3a, respectively using MDS/TEA/MSC. The compound 8-acetyl-7-methoxy-2,2-dimethyl-2H-chromen-5-yl trifluoromethanesulfonate was reported as a good antibacterial agent against K. pneumoniae, E. coli, P. aeruginosa and S. aureus at MIC 0.64, 0.64, 0.84, 0.02 µgml−1, respectively in comparison to Novobiocin as the standard (Ponnusamy et al., 2018).
2.58 Synthesis of coumacine derivatives
A group of bicyclo twelve-membered heterocyclic coumacine rings 4a-4c derived from substituted coumarin 1a-1c was synthesized by convenient methods. Initially, substituted coumarin 1a-1c were prepared through Pechmann condensation between the phenolic derivative and ethyl acetoacetate in the presence of concentrated sulfuric acid, then after reduction of corresponding substituted coumarin in the presence of Lithium aluminum tetrahydride (LAH) in ether afforded (E)-2-(4-hydroxybut-2-en-2-yl)-substituted phenol 2a-2c by opened coumarin ring system. The diol derivatives were converted to their disodium salts having reacted with sodium hydroxide, which further on treatment with diiodomethane (CH2I2) in anhydrous condition coumacine derivatives were obtained. Finally, the reaction was performed by the reactant methylene diiodide and nucleophilicity of phenoxide with unsaturated alcoholic group. The coumacine-1 had notable action against E. coli, K. pneumoniae, P. aeruginosa at MIC 5 µg/mL individually, in comparison to standard drug Ciprofloxacin (Mustafa, 2018).
2.59 Synthesis of pyrazole-anilino connected coumarin derivatives
A series of compounds with bearing triazolyl methyl aniline-linked 4-hydroxycoumarin 4a-4u were synthesized by the reaction of corresponding alcoholic solution of pyrazole carboxaldehyde 2a-2f and substituted aniline and 4-hydroxycoumarin 3. Then the obtain desired 3-((1,3-diphenyl-1H-pyrazol-4-yl)(phenylamino)methyl)-4-hydroxy-2H-chromen-2-one derivatives 4a-4u. An intermediate pyrazole carboxaldehyde were synthesized by the reaction of alcoholic solution with the substituted acetophenone and phenylhydrazine with added of phosphorous oxy trichloride. In the synthesis free solvent and catalyst chosen during the reaction. The plausible mechanism of synthetic series of coumarin pyrazolyl linked aniline derivatives was initially aniline subtract and pyrazole carbaldehyde condensedand resulting in the formation of imine. In SARs reveal that compounds 4b, 4c, 4h, 4i and 4k with electron donating substituent methoxy and methyl, which attached at anilino side chain in structure have been evident antibacterial activity with compared to electron withdrawing substituent chloro, nitro so on in aniline ring. The compounds 4b, 4c, 4h, 4i and 4k had been reported as good antibacterial agent against, M. luteus, S. aureus, P. aeruginosa at MIC values ranging between 1.9 and 7.8 µg/mL in comparison to the standard drug Novobiocin (Kovvuri et al., 2018).
2.60 Synthesis of isatin- linked 1,2,3-triazole with coumarin
A series of newly synthesized compounds 1, 2, 3-triazole tethered indolinone-coumarin hybrids 7a-7l from two reactant substrates such as, 4-methyl 7-(prop-2-ynyloxy) coumarin 6 and azidoethyl substituted isatin 3a-3d. In the synthesis the corresponding hybrids, initially 7-hydroxy 4-methyl coumarin 5 was prepared by Pechmann condensation of ethyl acetoacetate and resorcinol 4 in acidifying agent; and the compound 4, being heated with 7-hydroxy 4-methyl coumarin and propargyl bromide in the presence of potassium carbonate at 50 °C. Another intermediate 1-(2-azidoethyl)indoline-2,3-dione was prepared by the reaction between isatin and 1,2-dibromoethane in the presence of potassium carbonate yield N-(2-bromoethyl isatin). Consequently, this product reacted with sodium azide at 60 0C to afforded 1-(2-azidoethyl)indoline-2,3-dione. These two precursors were further used for the synthesis of the triazole tethered coumarin isatin hybrids through cupper(I)-promoted alkyne-azide cyclo addition in the presence of DMF and copper acetate. Finally the desired products were condensed with appropriate amine hydrochloride to obtain respective (Z)-3-(methoxyimino)-1-(2-(4-(((4-methyl-2-oxo-2H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl)indolin-2-one derivatives 7e-7l. The compound 5-chloro-1-(2-(4-(((4-methyl-2-oxo-2H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl)indoline-2,3-dione 7c and (Z)-3-(methoxyimino)-1-(2-(4-(((4-methyl-2-oxo-2H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl)indolin-2-one 7h had exhibited as a good antibacterial agent against M. smegmatis at MIC value 50 µg/mL in comparison to standard drugs, Rifampicin and Isoniazid (Liu et al., 2018a, 2018b).
2.61 Synthesis of N- benzoimidazolyl oxime of acetyl coumarin derivatives
A series of N- benzimidazolyl acetyl coumarin oxime derivatives 5a-5k were designed and synthesized from precursor 3-acetyl coumarin 1. Initially 3-acetyl coumarin 1 was brominated in dry chloroform afforded 3-bromomethyl coumarin 2. Furthermore, nucleophilic substitution on compound 2 reacted with benzimidazole in acetonitrile obtained 3-(2-(1H-benzo[d]imidazol-1-yl)acetyl)-2H-chromen-2-one 3. Consequently, condensation of hydroxylamine hydrochloride in ethanol on the compound 3 produced the corresponding oxime, 4. Finally, the desired oxime ethereal derivatives 5a-5k were synthesised by the esterification on compound 4 with various substituted benzyl bromide in DMSO and tertiary-butoxide. The compound (Z)-3-(2-(1H-benzo[d]imidazol-1-yl)-1-(((4-chlorobenzyl)oxy)imino)ethyl)-2H-chromen-2-one 5a and (Z)-3-(2-(1H-benzo[d]imidazol-1-yl)-1-(((4-bromobenzyl)oxy)imino)ethyl)-2H-chromen-2-one 5c had exhibited as good antibacterial agent against B. subtilis at the value, MIC 0.95 and 6.25 µg mL−1 (Singh et al., 2017).
2.62 Synthesis of 3,4-benzocoumarin linked coumarin derivatives
A series of pyrano[3,2-c] coumarin derivatives 5a-5c had been synthesized by using multiple component reactions. Initially, the precursor 2-((2-oxo-2H-chromen-4-yl)methylene)malononitrile was achieved by multiple condensations of 4-formyl coumarin 1, substituted malonyl nitrile and 4-hydroxy coumarin in one-step component reactions. In the reaction, the numbers of catalysts, sodium carbonate, potassium carbonate, sodium benzoate, sodium bisulfite and some amino acids, as L-proline, cysteine used in multiple component reaction approaches for enhancing productivity of the desired compounds. As L-proline being reported as a notable organic green catalyst due to duality nature, as proton -donating and –accepting, the mechanism of synthetic reaction was involved the principle of knoevenagel condensation of substituted 4-formyl coumarin and ethyl cyanoacetate/malononitrile to form corresponding intermediate alkene, which further underwent michael nucleophilic addition of 4-hydroxycoumarin, followed by intramolecular cyclisation to liberate the corresponding 2-amino-5-oxo-4-(2-oxo-2H-chromen-4-yl)-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives 2a-2c. The compound 2-amino-4-(6-chloro-2-oxo-2H-chromen-4-yl)-5-oxo-4,5-dihydropyrano [3,2-c]chromene-3-carbonitrile 2c, 4-(6-chloro-2-oxo-2H-chromen-4-yl)-3,4-dihydropyrano[3,2-c]chromene-2,5-dione 5c and 4-(7-methyl-2-oxo-2H-chromen-4-yl)-3,4-dihydropyrano[3,2-c]chromene-2,5-dione 5dwere had shown good antibacterial activity against E. faecalis at MIC 3.25 µg/mL, individually in comparison to the standard drug Ciprofloxacin (Chougala et al., 2018).
2.63 Synthesis of 3-thiazolylamino coumarin derivatives
A series of 3-thiazolyl amino derivatives 2a-2o was synthesised from substituted 3-acetylcoumarin 1a-1b, initially acetyl coumarin was converted into 3-bromoacetyl coumarin by bromination on starting 3-acetyl coumarin in the presence of bromine in chloroform. Then after, by the principle ‘Hanstzch’s condensation’, the substituted 3-bromoacetyl coumarin with either N-substituted or N,N’-disubstituted urea through cyclisation to yielded target 3-thiazolyl amino derivatives 2a-2o. Among all the test compounds, the 3-(2-((3,4-dichlorophenyl)amino)thiazol-4-yl)-8-methoxy-coumarin 2 g had been a good antibacterial agent against S. pneumonia at MIC 75 µM. Similarly, the compound 3-(2-((4-bromophenyl)amino)thiazol-4-yl)-8-methoxy-coumarin 2 h too exhibited a good antibacterial activity against E. coli, P. aerogenes, S. typhi, S. aureus at MIC 73 µM, individually in comparison to standard drugs Streptomycin, Kanamycin and Vancomycin (Osman et al., 2018).
2.64 Synthesis of N- pyrazolyl coumarin-3-carboxamide derivatives
In this synthesis, coumarinyl linked pyrazole carbaxamide derivatives 7a-7g were synthesised from an intermediate 5-(2-hydroxybenzylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione. The substituted compounds 5a-5f were synthesised by the reaction of substituted salicylaldehyde with dimedone in the presence of piperidine acetate in ethanol. The obtained products were recrystallized in ethanol, then substituted coumarin 3-carboxylic acid 5a-5f were liberated. Concomitantly, another intermediate ethyl 5-amino-1-substituted phenyl-1H-pyrazole-4-carboxylate derivatives 6a-6c were prepared by the mixture containing 4-substituted phenyl hydrazine hydrochloride and ethyl 2-cyano ethoxyacrylate/ ethoxymethylenemalonylnitrile in ethanol. Finally, the derivatives of compounds 6a-6c were converted into the corresponding pyrazole bearing carboxamides in the presence of phosphorus oxytrichloride and pyridine solution in mild condition. The obtained target compounds ethyl 5-(2-oxo-2H-chromene-3-carboxamido)-1-phenyl-1H-pyrazole-4-carboxylate derivatives had been recrystallized. Moreover, the compounds 7b 5-(6-chlorocoumarin-3-yl-carboxamido)-1-(4-chlorophenyl)-1H-pyrazole-4-carboxylic acid and ethyl 1-(4-chlorophenyl)-5-(7-(diethylamino)-coumarin-3-yl-carboxamido)-1H-pyrazole-4-carboxylate 7e had shown antibacterial action compared to ciprofloxacin against E. coli, and Salmonella sp. at MIC value 0.25 mg/L, individually in comparison to standard drugs and further exhibited potent inhibition with Topo II and Topo IV with IC50 values, 9.4–25 mg/L. SAR studies of these derivatives indicated that compounds having pyrazole carboxamide moiety linked with p-chloro substituted coumarin in structural frame, which might be responsible for inhibitory action of both topoisomerase II and IV of E. coli and Salmonella sp. (Liu et al., 2018a, 2018b).
2.65 Synthesis of 1- alkyloxy imidazolyl coumarin derivatives
Thirty-nine derivatives of coumarin linked with imidazole and alkyloxy group had been synthesized. These derivatives were synthesized from the starting material 7-hydroxy coumarin and 7-hydroxy 4-methyl coumarin 1a, which was prepared by heating of the solution 2,4-dihydroxy benzaldehyde and chloroethyl acetate with phosphonium ethyl acetate in ethanol, whereas another coumarin derivative 1b was prepared by the principle of Pechmann condensation reaction. Then, resorcinol was reacted with ethyl acetoacetate in sulfuric acid. Then compound 1 was reacted with the corresponding α,w-dibromo alkanes and triethylamine in anhydrous acetone produced the corresponding 7-(3-bromo alkyloxy)-2H-chromen-2-one derivatives 2. The compound 2was treated with the substituted heterocyclic amines namely, triazole, imidazole, 2-methyl imidazole, piperazine, piperidine and pyrazole, and a few more in the presences of anhydrous acetone and acetonitrile and the obtained compound 7-(3-(1H-heteroaryl-)alkyloxy)-2H-coumarin derivative 3. In SAR study indicated that the long alkyl chain linker and substituted imidazole group would be responsible for antibacterial action and inhibitory activity against Fabl and Fabk. Among all these compounds, 7-((6-(2-methyl-1H-imidazol-1-yl)hexyl)oxy)-2H-chromen-2-one derivatives 3b were reported as having good antibacterial activity against F. cloumnare, S. agalactiae and S. aureus at 8, 8, 64 µM in comparison to standard drugs Enrofloxacin and Norfloxacin (Hu et al., 2018).
2.66 Synthesis of coumarin mannich based derivatives
A series of 3-methylated amino 4-hydroxy coumarin derivatives 4a-4m was designed virtually and these derivatives were validated through computational tools. These derivatives were synthesized by the reflux condensation of 4-hydroxycoumarin, substituted aldehyde 2a-2m and secondary amines such as, morpholine, piperidine, pyrrolidine, piperazine so on with the presence of DCM by the principle of Mannich condensation reaction. The compound 4-hydroxy-3-((4-hydroxy-3-methoxyphenyl)(morpholino)methyl)-coumarin 4 l had shown as a good antibacterial agent against S. aureus at MIC 12.50 µg/mL (Sahoo et al., 2019c).
2.67 Synthesis of curcumin and isatin linked coumarin derivatives
Two series of compounds of triazole tethered mono carbonyl curcumin-coumarin 5a-5n and isatin-coumarin 6a-6n hybrids had been synthesised. Initially vanillin was reacted with acetone in the presence of base potassium at 25 °C and the obtained compound was (Z)-4-(4-hydroxy-3-methoxyphenyl)but-3-en-2-one 2a. Furthermore, these obtained products were reacted with propargyl bromide to produce (Z)-4-(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)but-3-en-2-one 3a. Then the substituted arylaldehyde was reacted with 3a in the presence of dilute NaOH in methanol, which yielded monocarbonyl of curcumin 4a-4n. In another intermediate reactant, by the treatment of various appropriate 1,2- dibromo alkanes with 4-hydroxy coumarin 1 in the presence of potassium carbonate in DMF could produce 4-(2-bromoalkyloxy)-2H-chromen-2-one 2a-2d, which further treated with sodium azide at 25 °C in DMF to yield 4-(2-azidoalkoxy)-2H-chromen-2-one 3a-3d. Simultaneously, isatin derivatives were subjected to treatment with 1,2-dibromoethane in the presence of potassium carbonate at 25 °C in DMF to obtain product; which on further treatment with sodium azide produceN-azidoethyl indolin-2,3-dione yielded the intermediate reactants such as, 4-(2-azidoethoxy)-coumarin 3a-3d and N-azidoethyl isatin. These were reacted with various proparylated analogues of curcumin 4a-4n in the presence of copper sulfate and sodium ascorbate in DMF and the obtained desired target triazole was linked monocarnoyl curcumin-coumarin 5a-5n and isatin-coumarin hybrid molecules. The SAR studies revealed that substitution bromo at C-5 position of isatin and 4-chloro substitution as isatin-curcumin of phenyl ring and 4-methoxy as coumarin-curcumin hybrids would suitable for inhibition of antibacterial action. The compound 5-bromo isatin linked with mono carbonyl curcumin through 1,2,3-triazole was reported as a good antibacterial agent against E. coli at MIC 6.25 µg/mL (Singh et al., 2019).
2.68 Synthesis of fluro-quinolone based coumarin derivatives
A series of fluoroquinolone derivatives bearing substituted bicyclo or tricyclic ring at C-7 position was synthesized from an intermediate ethyl 6,7-difluoro-N-substituted −4-oxo-1,4-dihydroquinoline-3-carboxylate 1 through several step reactions. In the synthesis initially, the compound 1 was reacted with ethyl cyanoacetate or t-butyl cyanoacetate in the presence of potassium carbonate at 70 °C in DMF yield2. By the ester hydrolysis in acidic medium yield the corresponding fluoroquinolone such as, ethyl 7-(cyanomethyl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid derivatives 3. The cyanomethyl was introduced at C-7 position of quinolone ring, then the condensed compounds 3 with 2-hydroxy benzaldehyde derivatives in the presence of catalytic piperidine and in DMF and produced (Z)-7-(1-cyano-2-(2-hydroxy-4-methoxyphenyl)vinyl)-N-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4. Then the compound 4 was treated 3–5% sulfuric acid at 110 °C and the obtained desired compound was 1-ethyl-6-fluoro-7-(substituted-with2-oxo-2H-chromen-3-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid. In nucleophilic substitution of fluoro atom of fluoroquinolone to cyanomethyl at C-7 position acted as the source of nucleophiles and was formed as bicyclic ring system (coumarin or 1,4-dihydro benzoxepine ring) through intramolecular cyclisation. The obtained quinolone derivatives were evaluated against several strains of Mycobacterium sp. The compound 1-ethyl-6-fluoro-7-(8-ethoxy-2-oxo-2H-chromen-3-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 7e had promising antimycobacterial action against M. tuberculosis (H37Rv), M. terrae and M. avium at MIC doses, 0.7 and 1.5 µg/mL respectively(Charushin et al., 2018).
2.69 Synthesis of bis 1,2,3-triazolyl methyloxy coumarin derivatives
A series of dimer compounds containing bis 1,2,3-triazolyl methoxy linked with 4-methyl- 7-hydroxy coumarin derivatives under microwave irradiation methods was synthesized and the obtained products had antimycobacterial and antibacterial activities. Initially an intermediate 4-methyl-7-(prop-2-yn-1-yloxy)-2H-chromen-2-one 2 was prepared by a two step reaction, firstly 4-methyl 7-hydroxy coumarin derivative 1 was prepared by the Pechmann condensation of substituted resorcinol and ethyl acetoacetate in the presences of acidifying agent, then after the compound 1 was reacted with propargyl bromide in the presence of dry acetone and potassium carbonate for yielding 2. In the synthesis of dimer of triazole-coumarin derivatives initially, nucleophilic substitution of dibromoalkane with sodium azide liberated azidoalkane and coupled with 4-methyl-7-(prop-2-yn-1-yloxy)-2H-chromen-2-one in the presence of cupper catalysed by 1,3-cycloaddition via azido-alkyne reactions. The title compounds were optimised by CuI in DMF:H2O (1:3) under microwave irradiation at 180 W for 10 min. All the desired target molecules were screened for their antimycobacterial action using resazurin microtiter assay (REMA) in comparison to standard Rifampin and isoniazid (INH). Moreover, the compound 7,7′-(((1,1′-octane 1,8-diyl) bis(1H-1,2,3-triazole-4,1-diyl))bis(methylene)) bis(oxy))bis(6-chloro-4-methyl-2H-chromen-2-one) 6j had shown good antibacterial action against B. subtilis, S. aureus, E. coli at MIC doses 3.125 µg/mL for each; and the compound 7,7′-(((1,1′-methylenebis(1H-1,2,3-triazole-4,1-diyl))bis(methylene))bis(oxy))bis(4-methyl-2H-chromen-2-one) 6e had in vitro control over B. subtilis, S. aureus and P. vulgaris at MIC doses 6.25 µg/mL. Consequently, compounds 6i and 6j had excellent antimycobacterial action with MIC 1,56 µg/mL. In SAR studies, it was known that electron-negative chlorine substituted coumarin at C-6 position and longer lipophilic alkyl chain linker between two ring systems play an important role for significant antibacterial action (Ashok et al., 2018).
2.70 Synthesis of palladium complexes coumarin derivatives
A series of palladium (Pd) complexes coumarin based tryptophan 3a and methionine 3b were synthesised from 4-hydroxy-3-acetyl coumarin. In the reaction, initially prepared corresponding enamine ligands (E)-methyl 2-((1-(4-hydroxy-2-oxo-2H-chromen-3-yl)ethylidene)amino)-4-(methylthio)butanoate, (E)-ethyl 2-((1-(4-hydroxy-2-oxo-2H-chromen-3-yl)ethylidene)amino)-3-(1H-indol-3-yl)propanoate by the reaction of 4-hydroxy-3-acetyl coumarin with methionine methyl ester hydrochloride and tryptophan methyl ester in the presence of triethylamine and methanol solution respectively. Further these Pd complexes were processed by the reaction of aqueous solution two ligands 3 and 3a with potassium –tetrachloride palladate in methanol and the mixture was stirred for 3hr. The ligand acted as a tridentate co-ordinated by one hydroxyl oxygen atom and the carbonyl oxygen of ester coumarin nucleus and nitrogen of enamine 3b. The compound 3b was reported as good antibacterial activity against S. aureus, P. aeruginosa, E. faecalis at MIC 208, 166, 208 µg/mL respectively in comparison to standard drug Ceftriaxone, Vancomycin (Stojković et al., 2018).
2.71 Synthesis of coumarinyl pyrimidinone derivatives
A series of coumarinyl linked with 1,6-dihydro pyrimidine carboxamide derivatives had been synthesized as intermediate reactants substituted 4-bromomethyl coumarin 1a-1g and 2-mercapto-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile 2a-2b. The intermediate compounds 1a-1 g were prepared by Pechmann cyclisation of phenol and bromoethyl acetoacetate, whereas two compound 2a-2b were prepared by a mixture of equimolar quantities of ethyl cyanoacetate, thiourea and aromatic aldehyde. Thenafter, condensation of substituted 4-bromomethyl coumarin and 2-mercapto-6-oxo-4-substituted phenyl-1,6-dihydropyrimidine-5-carbonitrile in anhydrous pottasium carbonate using as acetone solvent to obtain 6-oxo-2-(((2-oxo-2H-chromen-4-yl)methyl)thio)-4-substituted phenyl-1,6-dihydropyrimidine-5-carbonitrile derivatives 3a-3j. Finally, these dihydropyrimidine carbonitrile derivatives were converted the corresponding N-(tert-butyl)-6-oxo-2-(((2-oxo-2H-chromen-4-yl)methyl)thio)-4-phenyl-1,6-dihydropyrimidine-5-carboxamide 4a-4j by the compounds 3a-3j, which reacted with terta-butyl acetate in the presence of sulfuric acid and acetic acid. Then, the desired coumarinyl-pyrimidine carboxamide was carried out using both conventional and microwave irradiation yield 55% and 82 to 90% respectively. The compound 4f had shown good antibacterial agent against S. aureus at MIC 2.5 µg/mL and comparison to standard drug as Ciprofloxacin. In SARs revealed that the electron donating substituents in coumarin ring had been reported as positive effect to antibacterial activity efficiency order of substitutions methyl > tri-butyl > methoxy, 5,6-benzo 4f attached at C-6 coumarin had a good inhibitory action against S. aureus (Chavan et al., 2018).
2.72 Synthesis of triazole substituted coumarin derivatives
1,2,3-triazole derivatives were prepared by the Cu(I)ions catalysed [2 + 3]cycloaddition reaction between organic azides and terminal alkynes at an ambient temperature. In this synthesis, a series of triazolyl bearing coumarin derivatives 6a-6p were performed via., azide-alkyne cycloaddition reaction. The substituted 4-azidomethyl coumarins were synthesized by two step reaction, substituted 4-bromomethyl coumarin were prepared by Pechmann cyclisation of bromoethyl acetoacetate and the substituted phenol in acidifying agent. Then the obtained products were reacted with sodium azide in aqueous. Additionally,an intermediate compounds 4-ethynyl-1-substituted phenyl-1H-1,2,4-triazol-5(4H)-one 5a-5b were prepared by the reaction of 1-substituted phenyl-1H-1,2,4-triazol-5(4H)-one with prop-1-yne using potassium carbonate in anhydrous acetone solution. This reaction was followed by azide-alkyne cycloaddition of ethynyl-1-substituted phenyl-1H-1,2,4-triazol-5(4H)-one and substituted 4-ethyl azido coumarin 4a-4h in presence of copper ascorbate in THF/water 1:1 for yield of 4-((1-((2-oxo-2H-chromen-4-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-1-substituted phenyl-1H-1,2,4-triazol-5(4H)-one 6a-6p (coumarinyl-1,2,3-triazolyl-1,2,4-triazolone) and recrystallized from suitable solvents. SARs of these derivatives indicated that the presence of electron donating groups in coumarin ring and phenyl attached 1,2,4-triazole compounds were enhanced the antimycobacterial action against M. tuberculosis. The compounds 4-((1-((6-methyl-2-oxo-2H-chromen-4-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-1-phenyl-1H-1,2,4-triazol-5(4H)-one 6e was recorded as a good antimycobacterial agent against M. tuberculosis at MIC value 1.60 µg/ml individually in comparison to standard drug Pyrazinamide (Somagond et al., 2019).
2.73 Synthesis of coumarinyloxy derivatives
In this scheme, coumarinyloxy derivatives 2a-2d were synthesized by undertaking O alkylation of 6-methyl-4-hydroxy coumarin 1 under phase transverse catalyse (PTC) reaction. PTC coumarin undergone alkylation by the treatment with alkyl halides (allyl bromide, benzyl chloride,4-nitrobenzyl chloride, ethyl chloro acetate) using base medium as potassium carbonate and tertiary butyl ammonium chloride afforded corresponding oxygen alkylated at C-4 position through nucleophilic displacement. Likewise, PTC reaction of coumarin derivatives with phenyl isothiocyanate in equimolar quantity through nucleophile addition on the carbon nitrogen double of phenyl isothiocyanate to produce3-(N-phenyl) thiocarbamide coumarin. Similarly, coumarin was reacted with aromatic aldehyde like pipernal, anisaldehyde in equal amount in presence of piperidine as base, afforded corresponding 3-arylidene 6-methyl-4-hydroxy coumarin 3a-3b. The compound 2d was reported as a good antibacterial agent against E. coli at the MIC value 32 µg/mL (Regal et al., 2020).
2.74 Synthesis of isatin-triazolyl coumarin derivatives
A series of triazolyl linker isatin-coumarin hybrid molecules were synthesized. In this reaction, the substituted isatin 1a-1e reacted with 1,2-dibromo alkanes using potassium carbonate as base DMF solvent then after resultant intermediate 2a-2e was react with sodium azide in DMF produce 1-(4-azidoalkyl)-substituted isatin 3a-3e. Another reactant 4-(prop-2ynyloxy)-coumarin 3g was further reacted with various derivatives of 1-(4-azidoalkyl)-indolin-2,3-dione in presence of copper sulfate pentahydrate with sodium ascorbate in DMF solution to yield desired target candidates 1-(2-(4-(((2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl) substituted indoline-2,3-dione hybrids 4a-4u. SARs of these desired derivatives indicate that electron density of the fifth position of isatin remarkable influence of antibacterial action and activity is directly proportional to increase the electronegativity on same position of isatin so order of potency substitution fluro > chloro > bromo > iodo > nitro > methoxy > hydrogen and concerned for linker space carbon length n = 1 > 2 > 3. All the synthesized products were evaluated for their antibacterial potential against bacterial strains E. coli, S. enteric, S. aureus. The compound 1-(2-(4-(((2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl) indoline-2,3-dione 4a and 1-(2-(4-(((2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl) 5-fluoro- indoline-2,3-dione 4b had shown as good antibacterial action against S. aureus at the MIC value 30 and 312 µg/mL (Bhagat et al., 2019).
2.75 Synthesis of coumarin 3-carboxamide derivatives
A series of substituted coumarin 3-carboxamide derivatives 3a-3j were synthesized by facile green synthetic methods. In this synthesis, the precursor 3-((4-bromo-2-fluorophenyl)amino)-3-oxopropanoic acid 1 was prepared by the condensation reaction of 4-bromo-2-fluoro aniline and diethyl malonate in presence ethanol and sodium carbonate. The obtained product 1 was mixed with substituted salicylaldehyde by addition of a few drops of piperidine and finally the reaction mixture was heated at 105 °C for 4 h. The obtained precipitate N-(4-bromo-2-fluorophenyl)-substituted-2-oxo-2H-chromene-3-carboxamide was recrystallize with acetone. The compounds N-(4-bromo-2-fluorophenyl)-6-nitro-2-oxo-2H-chromene-3-carboxamide 3c, 6,8-dibromo-N-(4-bromo-2-fluorophenyl)-2-oxo-2H-chromene-3-carboxamide 3d, 6,8-dichloro-N-(4-bromo-2-fluorophenyl)-2-oxo-2H-chromene-3-carboxamide 3e, 6-chloro-N-(4-bromo-2-fluorophenyl)-8-nitro-2-oxo-2H-chromene-3-carboxamide 3f and N-(4-bromo-2-fluorophenyl)-8-nitro-2-oxo-2H-chromene-3-carboxamide 3j had been shown growth inhibits against human pathogenic fungal species, Candida albicans at MIC 1000 µg/ml and comparison to standards Fluconazole and Ciprofloxacin (Khan et al., 2019).
2.76 Synthesis of 3-Aroyl substituted coumarin derivatives
Two series of Co(II), Cu(II), Zn(II) complexes of 3-(2-hydroxybenzoyl)-2H-chromen-2-one, 3-(3-hydroxy-2-naphthoyl)-2H-chromen-2-one were synthesized. These ligands were prepared by the reaction of equimolar alcoholic solution of 4-hydroxy coumarin and respective aldehyde such as, salicylaldehyde and 2-hydroxy naphthaldehyde in presence of triethylamine as catalyst quantity and refluxed for 1.5 h. The obtained desired ligands were recrystallized by methanol. Thereafter, these alcoholic solution ligands were reacted with corresponding metal chloride hydrated MCl2 H2O in presence of ammonium hydroxide to produce corresponding complexes. ATB: Cefoxitin complex from ligand L1 was effective toward S. aureus, E. coli, P. aeruginosa at MIC value 30 µg/mL in comparison to the standard drug Cefoxitin (Belkhir-Talbi et al., 2019).
2.77 Synthesis of 4-triazolidin-thione coumarin derivatives
Two series of substituted coumarin containing 1,2,4-triazolidin −3-thione derivatives 3a-3j were synthesised, by the formation of respective semithiocarbazone in nucleophilic addition reaction of semithiocarbazide to electron deficient carbon atom of carbonyl compound of substituted 4- formyl coumarin/benzaldehyde and followed by intramolecular nucleophilic attack of amine (NH2) of thiosemicarbazone to azomethine to liberate the desired target molecules substituted coumarin and phenyl triazolidin-2-thiones. SAR studies of these compounds revealed that substituted phenyl ring replaced by substituted coumarin triazolidin thiones enhanced the antitubercular activity, where as various mono substituted electron donating group like methoxy, methyl, attached either phenyl triazolidine thione or respective coumarin triazolidine thione derivatives had been reported as more potent than disubstituted system. Mono substitution of phenyl ring and coumarin bearing triazolidine thion had been shown a good antimycobacterial action, as the substituted coumarin triazolidin thione methyl, methoxy, 5,6-benzo and 7,8 benzo moderate increases the antibacterial action whereas in phenyl triazolothiones the substituted hydroxy, 5,6-benzo in the phenyl ring enhanced the significant antibacterial action. The compound 7-methoxy-4-(5-thioxo-1,2,4-triazolidin-3-yl)–2H-chromen-2-one 3d was reported as good antibacterial agent against B. subtilis at MIC 0.8 µg/mL in comparison to the standard drug ciprofloxacin (Shaikh et al., 2019).
2.78 Synthesis of ruthenium complexes of 3-acetohydrazone coumarin
Coumarin hydrazide/hyrazone hybrids had been shown having potent anticancer and antibacterial actions. A new series Ruthenium(II)-DMSO complexes of substituted coumarin 3-acylhydrazone 4a-4d were synthesised. In these complexes, the four novel substituted coumarin 3-acyl hydrazones derivatives ligands 3a-3d had been synthesized by the reaction involving condensation the compound 6-diethyl amino-coumarin 3-carbohydrazide 1 with substituted benzaldehyde 2a-2d in the presence of hydrochloric acid and ethanol. Thereafter these obtained ligands were further had been reacted with complexes cis[RuCl2(DMSO)4] in presence of ethanol and the mixture was refluxed for 4 h and finally the obtained yellow precipitate complexes washed several times in cold ethanol. Among all the desired hydrazone ligand the compound, Ruthenium (II) (E)-N'-(4-bromo benzylidene)-6-(diethylamino)-2-oxo-2H-chromene-3-carbohydrazide 3c in DMSO complex had been seen with notable antibacterial activity against S. aureus at MIC 40.5 µM (de Almeida et al., 2019).
2.79 Synthesis of bis triazole uracil based coumarin derivatives
Incorporation of 1,2,3-triazole ring in several drug designing strategies due to its better aromatic stabilization, good binding affinity, isostere of carboxylic group and resistant towards both oxidation and reduction in acidic and alkaline medium. 1,2,3- triazole and its derivatives had been shown with a wide range pharmacological actions. Thus, researchers had more attentions as 1,2,3-triazole ring is tethering agent in drug design. A series of compounds of bis coumarinyl alkyloxy 1,2,3-triazole linker with uracil hybrids C1-28 were designed and synthesized. Antibacterial potentials of these obtained analogues were studied. These compounds were synthesized from 4-(2-azidoethoxy)–2H-chromen-2-one 1. Initially the 4-hydroxy coumarin was dissolved DMSO and followed by addition of dibromoethane in presence of potassium carbonate to obtain alkylated coumarin, which further react with sodium azide in DMSO solution to give 4-(2-azidoethoxy)–2H-chromen-2-one 1. Another reactant, 5-substituted-1,3-di(prop-2-yn-1-yl)pyrimidine-2,4(1H,3H)-dione 2a-2g was prepared by the reaction of substituted uracil 1a-1g with propargyl bromide in the presence of DMF solution as solvent and was used as potassium carbonate at a room temperature. Finally, 3-azidoalkylated coumarin were treated with propargylated uracil in the presence of copper sulfate and sodium ascorbate in DMF solution at room temperature to get the desired target analogues triazole tethered coumarin-uracil hybrids C 1–28. SAR studies of these compounds indicated that the analogues containing substituted uracil were more potent with antibacterial actions than compounds without non-substituted uracil. Thus, compound poses electron withdrawing substituent had shown more inhibitory action, whereas potency decreased with increasing chain carbon length in between two nuclei. Among all the tested candidates, the compound bearing chloro uracil substituted triazolyl ethoxy coumarin C-3 had reported as good antibacterial agent(s) against E. faecalis, S. aureus, P. aeruginosa and E. coli at MIC values, 7.23 µg/ml in comparison to the standard drug Levofloxacin (Sanduja et al., 2020).
2.80 Synthesis of schiffbase of 4-hydroxyl-3-acetyl coumarin derivatives
In this scheme, 4-hydroxy coumarin compound with the Schiff base enamine aminophenol 3 was synthesized by the reaction of 3-acetyl-4-hydroxy coumarin 1 with aminophenol 2 in methanol. The compound (E)-3-(1-((2-hydroxyphenyl)amino)ethylidene)chroman-2,4-dione 3 had been shown good antibacterial activities against S. aureus, B. cereus, E. coli, K. pneumonia at MIC doses, 39, 78, 78, 78 mg dm−3, respectively in comparison to the standard drug Chloramphenicol (Avdović et al., 2019).
3 SARs of coumarin derivatives as antibacterial agents
The exploration of synthetic and semisynthetic coumarin derivatives against inhibitory actions of notorious Gram positive, negative and acid-fast mycobacteria are emphasised here. Evidently, more than twenty-five percent of developed molecules had been seen upstanding antibacterial action(s) and a few more had moderate to less efficacy. In the principle of medicinal chemistry synthetic strategies, molecular hybridization is an established etiquette for development of novel compounds. Indeed, the phytochemical coumarin is natural heterocyclic ring with various biological actions among all; the antibacterial action(s) is more predominant by the intermixing of various components (Fig. 3).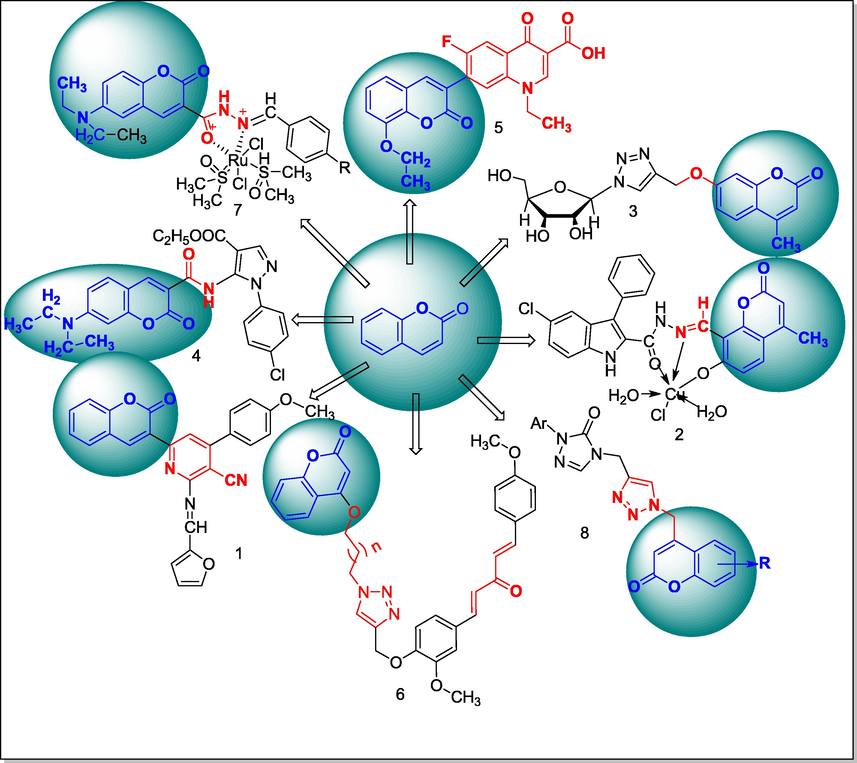
Structural-activity-relationships of coumarin derivatives.
In this SAR study of coumarin had briefly emphasized on integument of active sites of the congener for properties of inhibitory actions. 2-(furan-2-ylmethyleneamino)-6-coumarinyl-4-substituted nicotinic nitriles 1 had been notable antibacterial inhibitory properties, due to the presence of electron donating substituents, –OCH3, –CH3 of phenyl ring and electron withdrawing NO2, halogen groups respectively. Similarly, metal complexes bearing coumarinyl carbohydrazide with indole Schiffbase derivatives 2 had exhibited remarkable antibacterial activity due to the presence of withdrawing chloro substituents incomplexes, which have better zone of inhibition than methylated ligand. Indeed, the increases antibacterial efficacy is directly proportionate to lipophilic character of metal chelate ions, which could favour to permeation by lipid layer of bacterial cell membrane. Moreover, substituted ribofuranosyl coumarinyl 1,2,3-triazole derivatives 3 had been reported as potent candidates against clinical isolates of MDR human pathogenic bacterial strains. The structure bearing ribosylfuranosyl 1,2,3-triazole nucleus connected to 4-methyl-7-hydroxycoumarin at C-7 position through oxymethylene (–OCH2) linker. Concomitantly, the coumarinyl linked pyrazole carbaxamide derivatives 4 had been reported as good antibacterial bacterial agent as inhibitors of Topoisomerase II and Topoisomerase IV. On the N-(4-chloro phenyl) pyrazole 5-carboxamide 4 structure of coumarin at C-3 position, an attachment of diethyl amino or bromo may lead inhibitory effect on bacterial growth.
Furthermore, monocarbonylcurcumin-coumarin ring linker with 1,2,3-triazole nucleus through two carbon chain compounds due to the presence of 4-methoxy substitution at curcumin-coumarin hybrids 5 may have showed good antibacterial actions. Similarly, coumarin flouroquinolone hybrids 6 were reported as having good antimycobacterial actions due to flouro quinolone ring, which is essential for any antibacterial action. Thus, the developed molecules may have greater degrees of inhibitory actions on bacterial DNA gyrase or topoisomerase. Ruthenium(II)-DMSO complexes of substituted coumarin 3-acylhydrazone 7 had been reported from 7-diethylamino- coumarin hydrazide. These complexes had shown greater inhibitory action due to the presence of hydrazide group and metal ion Ruthenium(II) in structural frame. On the structure of compound 8, where the presence of 1,2,3-triazolyl substituted coumarin ring which makes the molecules had exhibited significant antibacterial actions.
4 Conclusion
This phyto-compound coumarin, with its congeners would provide a frame for pharmacophore-based drug discovery against bacterial diseases. Herein, a comprehensive review of the various reaction strategies such as, Schiffbase, Azo-dye, Mannich-base, transitional metal complexes, Pechmann condensation and a few more synthetic principles for antibacterial activities are described. These are expected to be beneficial to control MDR bacterial pathogens in the rising demands of antibacterial candidates, from clinicians today. Indeed, these synthetic/semi-synthetic approaches of additions of newer phyto-based modified chemical entities with in vitro inhibitory actions against pathogenic microorganisms; particularly, against MRSA, mycobacteria and several other ghoulish infectious bacteria. The evolving of MDR bacterial strains have spiraled to unbridled notorious standards, due to the accumulation of multidrug resistance in them; surprisingly, one would hardly find a more vivid illustration of any commensal like, the Gram-positive Staphylococcus aureus, which is now the methicillin-resistant S. aureus (MRSA), transforming into a perilous MDR-MRSA with an armamentarium of multidrug resistance, Today ‘MDR-MRSA’ is regarded as the ghoulish superbug of the health domain! Thus, the necessity of some newer antibacterial agents to overcome the grievous resistance pattern of MRSA and other bacterial infective agent(s). Additionally, the SAR studies are the coveted corollary, as highlighted in detail. Further work is necessary to understand the various signalling unknown mechanisms with mode of administration and pharmacokinetics and dynamic properties in drug development cascades.
Acknowledgements
Authors gratefully acknowledge the financial support from Siksha O Anusandhan Deemed to be University for PhD research grant (Redg. No. 1781611002/2017) of C. R. Sahoo, and Deans of SPS and IMS & SUM Hospital of SOA University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coumarin inhibits the growth of carrot (Daucus carota L. cv. Saint Valery) cells in suspension culture. J. Plant Physiol.. 2003;160:227-237.
- [CrossRef] [Google Scholar]
- Ether and coumarin–functionalized (benz)imidazolium salts and their silver(I)–N–heterocyclic carbene complexes: Synthesis, characterization, crystal structures and antimicrobial studies. J. Organomet. Chem.. 2018;854:64-75.
- [CrossRef] [Google Scholar]
- Coumarin-tethered (benz)imidazolium salts and their silver(I) N-heterocyclic carbene complexes: Synthesis, characterization, crystal structure and antibacterial studies. Appl. Organomet. Chem.. 2017;31:e3770
- [CrossRef] [Google Scholar]
- Synthesis, structural characterization, crystal structures and antibacterial potentials of coumarin–tethered N–heterocyclic carbene silver(I) complexes. J. Organomet. Chem.. 2017;833:28-42.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, crystal structure and biological studies of silver(I) complexes derived from coumarin-tethered N-heterocyclic carbene ligands. Polyhedron. 2017;123:470-479.
- [CrossRef] [Google Scholar]
- Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5- trifluoromethyl-4,5-dihydropyrazol-1-yl)-4-(coumarin-3-yl)thiazoles. Eur. J. Med. Chem.. 2013;62:508-514.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioorgan. Med. Chem.. 2010;18:214-221.
- [CrossRef] [Google Scholar]
- Aksungur, T., Aydıner, B., Seferoğlu, N., Özkütük, M., Arslan, L., Reis, Y., Açık, L., Seferoğlu, Z., 2017. Coumarin-indole conjugate donor-acceptor system: Synthesis, photophysical properties, anion sensing ability, theoretical and biological activity studies of two coumarin-indole based push-pull dyes. J. Mol. Struct., 1147, 364-379.https://doi.org/10.1016/j.molstruc.2017.06.079.
- Ferprenin, a prenylated coumarin from Ferula communis. Phytochemistry. 1988;27:944-946.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur. J. Med. Chem.. 2011;46:3788-3794.
- [CrossRef] [Google Scholar]
- Ashok, D., Gundu, S., Aamate, V.K., Devulapally, M.G., Bathini, R., Manga, V., 2018. Dimers of coumarin-1,2,3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking and evaluation as antimycobacterial and antimicrobial agents. J. Mol. Struct. 1157, 312-321.https://doi.org/10.1016/j.molstruc.2017.12.080.
- Spectroscopic and theoretical investigation of the potential anti-tumor and anti-microbial agent, 3-(1-((2-hydroxyphenyl)amino)ethylidene)chroman-2,4-dione. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2019;206:421-429.
- [CrossRef] [Google Scholar]
- Coumarin content in cinnamon containing food products on the Danish market. Food Control. 2014;38:198-203.
- [CrossRef] [Google Scholar]
- Microwave-Assisted Rapid and Efficient Synthesis of New Series of Chromene-Based 1,2,4-Oxadiazole Derivatives and Evaluation of Antibacterial Activity with Molecular Docking Investigation. J. Heterocycl. Chem.. 2019;56:552-565.
- [CrossRef] [Google Scholar]
- Isolation of methoxyfuranocoumarins from ammi majus by centrifugal partition chromatography. J. Chromatogr. Sci.. 2016;54:10-16.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur. J. Med. Chem.. 2010;45:1151-1157.
- [CrossRef] [Google Scholar]
- Belkhir-Talbi, D., Makhloufi-Chebli, M., Terrachet-Bouaziz, S., Hikem-Oukacha, D., Ghemmit, N., Ismaili, L., M.S Silva, A., Hamdi, M., 2019. Synthesis, characterization, theoretical studies, ADMET and drug-Likeness analysis: Electrochemical and biological activities of metal complexes of 3-(2-hydroxybenzoyl)-2H-chromen-2-one. J. Mol. Struct. 1179, 495-505.https://doi.org/10.1016/j.molstruc.2018.11.035.
- Coumarins from Three Phebalium species. Biochem. Syst. Ecol.. 1988;16:631-633.
- [CrossRef] [Google Scholar]
- Design, synthesis, antimicrobial evaluation, and molecular modeling studies of novel indolinedione-coumarin molecular hybrids. ACS. Omega.. 2019;4:8720-8730.
- [CrossRef] [Google Scholar]
- Brown, S.A., Towers, G.H., Wright, D., 1960. Biosynthesis of the coumarins. Tracer studies on coumarin formation in Hierochloe odorata and Melilotus officinalis. Can. J. Biochem. Physiol., 38, 143-156. https://doi.org/10.1139/o60-016.
- C. Barua, N., P. Sharma, R., Madhusudanan, K.P., Thyagarajan, G., Herzt, W., 1980. Coumarins in artemisia caruifolia. Phytochemistry. 19, 2217-2218.https://doi.org/10.1016/S0031-9422(00)82232-3.
- Synthesis and antimycobacterial evaluation of new (2-oxo-2H-chromen-3-yl) substituted fluoroquinolones. J. Fluor. Chem.. 2018;208:15-23.
- [CrossRef] [Google Scholar]
- Molecular docking studies and facile synthesis of most potent biologically active N-tert-butyl-4-(4-substituted phenyl)-2-((substituted-2-oxo-2H-chromen-4-yl)methylthio)-6-oxo-1,6-dihydropyrimidine-5-carboxamide hybrids: An approach for microwave-assisted. Bioorg. Chem.. 2018;78:185-194.
- [CrossRef] [Google Scholar]
- Chemical constituents and anti-platelet aggregation activity from the root of Peucedanum formosanum. J. Food Drug Anal.. 2008;16:15-25.
- [Google Scholar]
- Solvent free-synthesis of highly functionalized 4H-chromene-3-carboxamide derivatives using cerium ammonium nitrate and their antioxidant, antibacterial and solvatochromism studies. J. Mol. Liq.. 2017;243:494-502.
- [CrossRef] [Google Scholar]
- Green, unexpected synthesis of bis-coumarin derivatives as potent anti-bacterial and anti-inflammatory agents. Eur. J. Med. Chem.. 2018;143:1744-1756.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano[2,3-c]pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur. J. Med. Chem.. 2017;125:101-116.
- [CrossRef] [Google Scholar]
- Two acridones and two coumarins from the roots of Paramignya trimera. Tetrahedron Lett.. 2017;58:1553-1557.
- [CrossRef] [Google Scholar]
- New Ru(II)–DMSO complexes containing coumarin-N-acylhydrazone hybrids: Synthesis, X-ray structures, cytotoxicity and antimicrobial activities. Polyhedron. 2019;171:20-31.
- [CrossRef] [Google Scholar]
- A microwave-assisted facile synthesis of novel coumarin derivatives containing cyanopyridine and furan as antimicrobial agents. J. Saudi Chem. Soc.. 2017;21:S153-S162.
- [CrossRef] [Google Scholar]
- Coumarin derivatives: an updated patent review (2015–2016) Exp. Opin. Ther. Pat.. 2017;27:1201-1226.
- [CrossRef] [Google Scholar]
- Dicoumarol complexes of Cu(II) based on 1,10-phenanthroline: Synthesis, X-ray diffraction studies, thermal behavior and biological evaluation. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2013;108:319-328.
- [CrossRef] [Google Scholar]
- Coumarin derivatives from Eryngium campestre. Planta Med.. 1985;51:407-409.
- [CrossRef] [Google Scholar]
- Detalolactone: A novel pyranocoumarin from the root bark of Zanthoxylum dipetalum. Phytochemistry. 1976;15:313-316.
- [CrossRef] [Google Scholar]
- Synthesis of Ethylene Tethered Isatin-Coumarin Hybrids and Evaluation of Their in vitro Antimycobacterial Activities. J. Heterocycl. Chem.. 2018;55:1484-1488.
- [CrossRef] [Google Scholar]
- Pentafluorophenylammonium triflate (PFPAT) catalyzed facile construction of substituted chromeno[2,3-d]pyrimidinone derivatives and their antimicrobial activity. J. Adv. Res.. 2014;5:209-218.
- [CrossRef] [Google Scholar]
- Gilani, A.H., Shaheen, F., Saeed, S.A., Bibi, S., Irfanullah, Sadiq, M., Faizi, S., 2000. Hypotensive action of coumarin glycosides from Daucus carota. Phytomedicine.7, 423-426. https://doi.org/10.1016/S0944-7113(00)80064-1.
- Acaricidal activity of tonka bean extracts. Synthesis and structure-activity relationships of bioactive derivatives. J. Nat. Prod.. 2003;66:690-692.
- [CrossRef] [Google Scholar]
- Cytotoxic coumarins from Calophyllum dispar. Phytochemistry. 2001;58:571-575.
- [CrossRef] [Google Scholar]
- Expedious synthesis for α, β-unsaturated coumarin derivatives using boran chelates: A novel class of potential antibacterial and antioxidant agents. Comptes Rendus Chim.. 2010;13:1261-1268.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopy and electrochemistry of new 4-(4-acetyl-5- substituted-4, 5-dihydro-1, 3,4-oxodiazol-2-yl)methoxy)-2H-chromen-2-ones as a novel class of potential antibacterial and antioxidant derivatives. Comptes Rendus Chim.. 2011;14:548-555.
- [CrossRef] [Google Scholar]
- ChemInform Abstract: Hawaiian plant studies part 16, coumarins and flavones from Pelea barbigera(gray) hillebrand (rutaceae) Chem. Informationsd.. 1974;1:1350-1352.
- [CrossRef] [Google Scholar]
- Holmes, K.K., Bertozzi, S., Bloom, B.R., Jha, P., Gelband, H., DeMaria, L.M., Horton, S., 2017. Major Infectious Diseases: Key Messages from Disease Control Priorities, Third Edition, in: Disease Control Priorities, Third Edition (Volume 6): Major Infectious Diseases. https://doi.org/10.1596/978-1-4648-0524-0_ch1.
- Synthesis and biological evaluation of coumarin derivatives containing imidazole skeleton as potential antibacterial agents. Eur. J. Med. Chem.. 2018;143:958-969.
- [CrossRef] [Google Scholar]
- Spectroscopic, magnetic and thermal studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of 3-acetylcoumarin-isonicotinoylhydrazone and their antimicrobial and anti-tubercular activity evaluation. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2010;77:838-844.
- [CrossRef] [Google Scholar]
- Biosynthesis of 4-hydroxy-5-methylcoumarin in a Gerbera jamesonii hybrid. Phytochemistry. 1989;28:2329-2330.
- [CrossRef] [Google Scholar]
- Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin) - A review. J. Ethnopharmacol.. 2011;134:1-10.
- [CrossRef] [Google Scholar]
- Synthesis, structure and DNA cleavage studies of coumarin analogues of tetrahydroisoquinoline and protoberberine alkaloids. Eur. J. Med. Chem.. 2010;45:3575-3580.
- [CrossRef] [Google Scholar]
- Coumarin: Chemical and pharmacological profile. J. Appl Pharm. Sci.. 2012;2:236-240.
- [CrossRef] [Google Scholar]
- The chemistry of the Western Australian rutaceae—VI. Tetrahedron. 1973;29:903-908.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis and biological activity of 3-aryl-furo[3,2-c]coumarins. Arab. J. Chem.. 2017;10:S1100-S1104.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antimicrobial activities of novel silver(I) complexes with coumarin substituted N-heterocyclic carbene ligands. Bioorganic Med. Chem.. 2016;24:643-650.
- [CrossRef] [Google Scholar]
- Antagonists of Vitamin K-popular coumarin drugs and new synthetic and natural coumarin derivatives. Molecules. 2020;25:1465.
- [CrossRef] [Google Scholar]
- Antioxidant properties of prunes (Prunus domestica L.) and their constituents. BioFactors. 2004;21:309-313.
- [CrossRef] [Google Scholar]
- Synthesis of some 2, 6-bis (1-coumarin-2-yl)-4-(4-substituted phenyl) pyridine derivatives as potent biological agents. Arab. J. Chem.. 2017;10:S1336-S1344.
- [CrossRef] [Google Scholar]
- Design, synthesis and validation of anti-microbial coumarin derivatives: An efficient green approach. Heliyon.. 2019;5:e02615
- [CrossRef] [Google Scholar]
- Synthesis, structural studies and biological activity of new Cu(II) complexes with acetyl derivatives of 7-hydroxy-4-methylcoumarin. J. Inorg. Biochem.. 2015;145:94-100.
- [CrossRef] [Google Scholar]
- Anti-influenza virus activity of crude extract of Ribes nigrum L. Phyther. Res.. 2003;17:120-122.
- [CrossRef] [Google Scholar]
- Regioselective Synthesis and Antibacterial Activity Studies of 1,2,3-Triazol-4-YL]-4-methyl-2H-chromen-2-ones. J. Heterocycl. Chem.. 2018;6:1398-1402.
- [CrossRef] [Google Scholar]
- Catalyst-free synthesis of pyrazole-aniline linked coumarin derivatives and their antimicrobial evaluation. J. Saudi Chem. Soc.. 2018;22:665-677.
- [CrossRef] [Google Scholar]
- The chemical constituents of Australian zanthoxylum species: VI. A further examination of the constituents of the bark of zanthoxylum conspersipunctatum (rutaceae) Aust. J. Chem.. 1973;26:687-689.
- [CrossRef] [Google Scholar]
- Synthesis, in vitro anticancer and antibacterial activities and in silico studies of new 4-substituted 1,2,3-triazole–coumarin hybrids. Eur. J. Med. Chem.. 2016;124:794-808.
- [CrossRef] [Google Scholar]
- Immunomodulatory principles of Pelargonium sidoides. Phyther. Res.. 2001;15:122-126.
- [CrossRef] [Google Scholar]
- Coumarins from Angelica archangelica Linn. and their effects on anxiety-like behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry.. 2013;40:180-186.
- [CrossRef] [Google Scholar]
- Cleomiscosin D, a coumarino-lignan from seeds of Cleome viscosa. Phytochemistry. 1988;27:636-638.
- [CrossRef] [Google Scholar]
- Advancement of global health: key messages from the Disease Control Priorities Project. Lancet. 2006;367:1193-1208.
- [CrossRef] [Google Scholar]
- Coumarin-based inhibitors of bacillus anthracis and staphylococcus aureus replicative DNA helicase: Chemical optimization, biological evaluation, and antibacterial activities. J. Med. Chem.. 2012;5:10896-10908.
- [CrossRef] [Google Scholar]
- Li, J., Hou, Z., Li, F., Zhang, Z.D., Zhou, Y., Luo, X.X., Li, M.K., 2014. Synthesis, photoluminescent, antibacterial activities and theoretical studies of 4-hydroxycoumarin derivatives. J. Mol. Struct. 1075, 509-514. https://doi.org/10.1016/j.molstruc.2014.07.010.
- Synthesis, crystal structures, and anti-drug-resistant Staphylococcus aureus activities of novel 4-hydroxycoumarin derivatives. Eur. J. Pharmacol.. 2013;721:151-157.
- [CrossRef] [Google Scholar]
- 1H–1,2,3-Triazole-tethered Isatin–coumarin Hybrids: Design, Synthesis and In Vitro Anti-mycobacterial Evaluation. J. Heterocycl. Chem.. 2018;55:775-780.
- [CrossRef] [Google Scholar]
- Novel coumarin-pyrazole carboxamide derivatives as potential topoisomerase II inhibitors: Design, synthesis and antibacterial activity. Eur. J. Med. Chem.. 2018;157:81-87.
- [CrossRef] [Google Scholar]
- Novel coumarin glycoside and phenethyl vanillate from Notopterygium forbesii and their binding affinities for opioid and dopamine receptors. Bioorgan. Med. Chem.. 2008;16:3218-3223.
- [CrossRef] [Google Scholar]
- Mahendra raj, K., Mruthyunjayaswamy, B.H.M., 2017. Synthesis, spectroscopic characterization, electrochemistry and biological activity evaluation of some metal (II) complexes with ONO donor ligands containing indole and coumarin moieties. J. Saudi Chem. Soc.1074, 572-582. https://doi.org/10.1016/j.jscs.2014.01.001.
- Mahendra Raj, K., Vivekanand, B., Nagesh, G.Y., Mruthyunjayaswamy, B.H.M., 2014. Synthesis, spectroscopic characterization, electrochemistry and biological evaluation of some binuclear transition metal complexes of bicompartmental ONO donor ligands containing benzo[b]thiophene moiety. J. Mol. Struct. 1059, 280-293.https://doi.org/10.1016/j.molstruc.2013.12.010.
- Synthesis of coumarin-theophylline hybrids as a new class of anti-tubercular and anti-microbial agents. Eur. J. Med. Chem.. 2018;146:747-756.
- [CrossRef] [Google Scholar]
- Investigation on the morphological and chemical variability of sweet woodruff (Galium odoratum (L.) Scop.) in Southern Belgium. Acta Hortic.. 2010;860:61-66.
- [CrossRef] [Google Scholar]
- “Buchu” - Agathosma betulina and Agathosma crenulata (Rutaceae): A review. J. Ethnopharmacol.. 2008;119(3):413-419.
- [CrossRef] [Google Scholar]
- Novel transition metal complexes of 4-hydroxy-coumarin-3- thiocarbohydrazone: Pharmacodynamic of Co(III) on rats and antimicrobial activity. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2011;81:35-43.
- [CrossRef] [Google Scholar]
- Novel silver(I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J. Inorg. Biochem.. 2016;163:53-67.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antibacterial activity of novel heterocycle, coumacine, and two of its derivatives. Saudi Pharm. J.. 2018;26:870-875.
- [CrossRef] [Google Scholar]
- 7-Geranyloxycoumarin from Juice Oil of Hassaku (Citrus hassaku) and Antimicrobial Effects of Related Coumarins. Agric. Biol. Chem.. 1987;51:419-423.
- [CrossRef] [Google Scholar]
- Two new dimeric coumarins isolated from Murraya exotica. Chem. Pharm. Bull.. 2005;53:1180-1182.
- [CrossRef] [Google Scholar]
- The structure of mogoltavin, a coumarin from the roots of Peucedanum mogoltavicum. Chem. Nat. Compd.. 1972;8:37-38.
- [CrossRef] [Google Scholar]
- A new antiplasmodial coumarin from Toddalia asiatica roots. Fitoterapia. 2000;71:636-640.
- [CrossRef] [Google Scholar]
- Osman, H., Yusufzai, S.K., Khan, M.S., Abd Razik, B.M., Sulaiman, O., Mohamad, S., Gansau, J.A., Ezzat, M.O., Parumasivam, T., Hassan, M.Z., 2018. New thiazolyl-coumarin hybrids: Design, synthesis, characterization, X-ray crystal structure, antibacterial and antiviral evaluation. J. Mol. Struct. 1166, 147-154.https://doi.org/10.1016/j.molstruc.2018.04.031.
- Phytotoxic iridoid glucosides from the roots of Verbascum thapsus. J. Chem. Ecol.. 1998;24:645-653.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of coumarin based isoxazoles, pyrimidinthiones and pyrimidin-2-ones. Arab. J. Chem.. 2017;10:S3990-S4001.
- [CrossRef] [Google Scholar]
- Multiple heating rate kinetic parameters, thermal, X-ray diffraction studies of newly synthesized octahedral copper complexes based on bromo-coumarins along with their antioxidant, anti-tubercular and antimicrobial activity evaluation. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2012;96:468-479.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and pharmacological activities of 2-[4-cyano-(3-trifluoromethyl)phenyl amino)]-4-(4-quinoline/coumarin-4-yloxy)-6- (fluoropiperazinyl)-s-triazines. J. Fluor. Chem.. 2011;132:617-627.
- [CrossRef] [Google Scholar]
- DNA cleavage, antimicrobial, spectroscopic and fluorescence studies of Co(II), Ni(II) and Cu(II) complexes with SNO donor coumarin Schiff bases. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2010;75:347-354.
- [CrossRef] [Google Scholar]
- DNA cleavage, antibacterial, antifungal and anthelmintic studies of Co(II), Ni(II) and Cu(II) complexes of coumarin Schiff bases: Synthesis and spectral approach. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2015;137:641-651.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, in vitro antimicrobial and DNA cleavage studies of Co(II), Ni(II) and Cu(II) complexes with ONOO donor coumarin Schiff bases. J. Mol. Struct.. 2011;985:330-338.
- [CrossRef] [Google Scholar]
- Co(II), Ni(II) and Cu(II) complexes with coumarin-8-yl Schiff-bases: Spectroscopic, in vitro antimicrobial, DNA cleavage and fluorescence studies. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2011;79:1128-1136.
- [CrossRef] [Google Scholar]
- Spasmolytic activity of angelicin: A coumarin from Heracleum thomsoni. Planta Med.. 1987;53:517-520.
- [CrossRef] [Google Scholar]
- Drug efficacy of novel 3-O-methoxy-4-halo disubstituted 5,7-dimethoxy chromans; evaluated via DNA gyrase inhibition, bacterial cell wall lesion and antibacterial prospective. Bioorgan. Med. Chem.. 2018;26:3438-3452.
- [CrossRef] [Google Scholar]
- Synthesis, spectral, thermal, fluorescence, antimicrobial, anthelmintic and DNA cleavage studies of mononuclear metal chelates of bi-dentate 2H-chromene-2-one Schiff base. J. Photochem. Photobiol. B Biol.. 2015;148:322-332.
- [CrossRef] [Google Scholar]
- Priyanka, Singh, V., Ekta, Katiyar, D., 2017. Synthesis, antimicrobial, cytotoxic and E. coli DNA gyrase inhibitory activities of coumarinyl amino alcohols. Bioorg. Chem. 71, 120-127.https://doi.org/10.1016/j.bioorg.2017.01.019
- New coumarin and dihydrocinnamic acid derivatives from two malaysian populations of Micromelum minutum. Phytochemistry. 1994;37:561-564.
- [CrossRef] [Google Scholar]
- Antinociceptive and anti-inflammatory effects of isolated fractions from Apium graveolens seeds in mice. Pharm. Biol.. 2009;47:740-743.
- [CrossRef] [Google Scholar]
- Reddy, S.H., 2012. Phytochemical Screening and Antibacterial Studies on Leaf and Root Extracts of Asclepias Curassavica (L). IOSR J. Pharm. Biol. Sci. 39-44.https://doi.org/10.9790/3008-0213944.
- Synthesis and antimicrobial activity of some new coumarin and dicoumarol derivatives. J. Heterocycl. Chem.. 2020;57:1368-1378.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and docking studies of 4-aryloxymethyl coumarins derived from substructures and degradation products of vancomycin. Eur. J. Med. Chem.. 2013;70:750-757.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of 7-(2-substituted phenylthiazolidinyl)-benzopyran-2-one derivatives. Eur. J. Med. Chem.. 2010;45:85-89.
- [CrossRef] [Google Scholar]
- Antiproliferative, cytotoxic and antitumour activity of coumarins isolated from Calophyllum brasiliense. J. Pharm. Pharmacol.. 2007;59:719-725.
- [CrossRef] [Google Scholar]
- Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem.. 2011;46:765-772.
- [CrossRef] [Google Scholar]
- Molecular dynamics and computational study of Mannich-based coumarin derivatives: potent tyrosine kinase inhibitor. J. Biomol. Struct. Dyn.. 2020;38(18):5419-5428.
- [CrossRef] [Google Scholar]
- Nostocine A derivatives as human DNA topoisomerase II-alpha inhibitor. Indian J. Pharmaceut. Educ. Res.. 2019;54(3):669-675.
- [CrossRef] [Google Scholar]
- Nornostocine congeners as potential anticancer drugs: An overview. Drug Dev. Res.. 2019;80:878-892.
- [CrossRef] [Google Scholar]
- Norharmane as a potential chemical entity for development of anticancer drugs. Eur. J. Med. Chem.. 2019;162:752-764.
- [CrossRef] [Google Scholar]
- Design, molecular docking of synthesized schiff-based thiazole/pyridine derivatives as potent antibacterial inhibitor. Indian Drugs. 2019;56(11):20-25.
- [Google Scholar]
- Synthesis, spectral characterization of some new 3-heteroaryl azo 4-hydroxy coumarin derivatives and their antimicrobial evaluation. J. Taibah Univ. Sci.. 2015;9:187-195.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of novel synthesized coumarin based transitional metal complexes. J. Taibah Univ. Med. Sci.. 2017;12:115-124.
- [CrossRef] [Google Scholar]
- Biological evaluation and spectral characterization of 4-hydroxy coumarin analogues. J. Taibah Univ Med. Sci.. 2015;10:306-319.
- [CrossRef] [Google Scholar]
- Uracil-coumarin based hybrid molecules as potent anti-cancer and anti-bacterial agents. J. Saudi Chem. Soc.. 2020;24:251-266.
- [CrossRef] [Google Scholar]
- Distribution and inheritance of scopolin and herniarin in some Prunus species. Biochem. Syst. Ecol.. 1994;22:197-201.
- [CrossRef] [Google Scholar]
- Sunflower (Helianthus annuus L.) response to broomrape (Orobanche cernua Loefl.) parasitism: Induced synthesis and excretion of 7-hydroxylated simple coumarins. J. Exp. Bot.. 2001;52:2227-2234.
- [CrossRef] [Google Scholar]
- Synthesis, Antitubercular and Antimicrobial Activity of 1,2,4-Triazolidine-3-thione Functionalized Coumarin and Phenyl Derivatives and Molecular Docking Studies. ChemistrySelect.. 2019;4:105-115.
- [CrossRef] [Google Scholar]
- Chemical constituents of Prangos tschimganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem. Pharm. Bull.. 2001;49:877-880.
- [CrossRef] [Google Scholar]
- Monocarbonyl curcumin based molecular hybrids as potent antibacterial agents. ACS Omega. 2019;4:11673-11684.
- [CrossRef] [Google Scholar]
- Coumarin-benzimidazole hybrids as a potent antimicrobial agent: Synthesis and biological elevation. J. Antibiot. (Tokyo).. 2017;70:954-961.
- [CrossRef] [Google Scholar]
- A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr.. 2008;59:299-326.
- [CrossRef] [Google Scholar]
- Click chemistry based regioselective one-pot synthesis of coumarin-3-yl-methyl-1,2,3-triazolyl-1,2,4-triazol-3(4H)-ones as newer potent antitubercular agents. Arch. Pharm. Weinheim. 2019;352:1900013.
- [CrossRef] [Google Scholar]
- Synthesis and antimycobacterial activity of 1-(β-D-Ribofuranosyl)-4-coumarinyloxymethyl- / -coumarinyl-1,2,3-triazole. Eur. J. Med. Chem.. 2018;150:268-281.
- [CrossRef] [Google Scholar]
- Stojković, D.L., Jevtić, V. V., Vuković, N., Vukić, M., Čanović, P., Zarić, M.M., Mišić, M.M., Radovanović, D.M., Baskić, D., Trifunović, S.R., 2018. Synthesis, characterization, antimicrobial and antitumor reactivity of new palladium(II) complexes with methionine and tryptophane coumarine derivatives. J. Mol. Struct. 1157, 425-433.https://doi.org/10.1016/j.molstruc.2017.12.095.
- 3,4-Dihydropyrimidinone-coumarin analogues as a new class of selective agent against S. aureus: Synthesis, biological evaluation and molecular modelling study. Bioorgan. Med. Chem.. 2017;25:1413-1422.
- [CrossRef] [Google Scholar]
- Structural Elucidation of Citrumarins: Four Novel Binary Coumarins Isolated from Citrus Plants. Chem. Pharm. Bull.. 1993;41:73-76.
- [CrossRef] [Google Scholar]
- Synthesis and pharmacological activity of O-aminoalkyl derivatives of 7-hydroxycoumarin. Eur. J. Med. Chem.. 2011;46:2252-2263.
- [CrossRef] [Google Scholar]
- Coumarins from Peucedanum ostruthium as inhibitors of acetylcholinesterase. Pharm. Biol.. 2005;43:647-650.
- [CrossRef] [Google Scholar]
- Design, synthesis and antibacterial study of new potent and selective coumarin-chalcone derivatives for the treatment of tenacibaculosis. Bioorgan. Med. Chem.. 2015;23:7045-7052.
- [CrossRef] [Google Scholar]
- A one pot, three component synthesis of coumarin hybrid thiosemicarbazone derivatives and their antimicrobial evolution. J. Assoc. Arab Univ. Basic Appl. Sci.. 2017;23:10-19.
- [CrossRef] [Google Scholar]
- Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: Synthesis and in vitro assessments. Food Chem.. 2010;120:1011-1018.
- [CrossRef] [Google Scholar]
- Synthesis, molecular docking and biological evaluation of coumarin derivatives containing piperazine skeleton as potential antibacterial agents. Bioorganic. Med. Chem.. 2014;22:5727-5737.
- [CrossRef] [Google Scholar]
- Quantification of flavoring constituents in cinnamon: High variation of coumarin in cassia bark from the German retail market and in authentic samples from Indonesia. J. Agric. Food Chem.. 2010;58:10568-10575.
- [CrossRef] [Google Scholar]
- Regiospecific synthesis, antibacterial and anticoagulant activities of novel isoxazoline chromene derivatives. Arab. J. Chem.. 2017;10:S2651-S2658.
- [CrossRef] [Google Scholar]







