Translate this page into:
Creatinine Imprinted Photonic Crystals Hydrogel Sensor
⁎Corresponding authors. murtazascholar@bit.edu.cn (Ghulam Murtaza), minxue@bit.edu.cn (Min Xue), mengzh@bit.edu.cn (Zihui Meng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
There is a demand for simple, selective, and efficient assays for the determination of clinically important metabolites such as creatinine for healthcare. Creatinine is the by-product of muscle energy metabolism and is excreted by the kidneys. To measure creatinine in the human serum, a creatinine imprinted photonic crystal hydrogel (CIPC hydrogel) for naked-eye detection is developed. CIPC hydrogel utilizes polystyrene-based two-dimensional (2D) photonic crystal colloidal arrays (PCs-array) embedded in the polyacrylamide hydrogel containing methacrylic acid which imprinted the creatinine template. The nanocavities in the hydrogel produced after the removal of the template bind to and recognize creatinine in the serum samples. The binding is selective and specific for creatinine. The binding is observed as shrinkage of the hydrogel volume and a decrease in the particle spacing which is monitored through changes in the Debye diffraction ring diameter and a visible blue-green to blue color shift. The binding event and the mechanism are investigated by molecular dockings. The CIPC sensor demonstrates a limit of detection (LOD) of 2.45 ± 1.6 µM, a linear detection range (25–500 µM), and recovery from 85.6 % to 99.9 % in the serum samples. CIPC hydrogel is available for the rapid and quantitative onsite detection of creatinine in the human serum sample.
Keywords
Creatinine
Photonic crystal
Hydrogel
Sensor, molecular docking
Molecular imprinting
1 Introduction
Creatinine is present in human blood and urine as the by-product of creatine phosphate metabolism in muscles and provides energy to muscle tissues (Pundir et al., 2019). Creatinine is present in the blood and its levels depend on age, race, gender, and body type (Stevens et al., 2010). The determination of creatinine in biological fluids is significant for the evaluation of renal dysfunction, thyroid disease, and muscle damage (Yadav et al., 2012). and could also provide a rapid and simple diagnosis of acute myocardial infarction (Sibilitz et al., 2014).
Renal dysfunction is a common cause of mortality and morbidity. The incidence of renal failure has almost doubled in the last fifteen years. The glomerular filtration is reduced to less than 15 mL min−1 in renal failure, which results in serum electrolyte disturbance and accumulation of creatinine and urea (Tamadon and Mousavi, 2015). The normal level of creatinine in the serum and its excretion through urine in healthy individuals is 45–140 μM and 0.8–2.0 g/ 24 h respectively (Kumar et al., 2017). Kidney dysfunction can be determined through creatinine levels above 140 μM in human serum and less than 40 μM shows decreased muscle mass, which is a biomarker for the muscular disorder (Pundir et al., 2019).
The conventional techniques for creatinine determination in clinical samples include colorimetric assays, spectrometry (del Campo et al., 1995), high-pressure liquid chromatography (HPLC) (Yang Wang et al., 2019), mass spectroscopy, (Schwedhelm et al., 2000) Infrared (IR) spectroscopy (Pezzaniti et al., 2001), capillary zone electrophoresis (CZE) (Clark et al., 2001), flow injection analysis systems with electrochemical and spectrometric detection, and nuclear magnetic resonance (NMR) (Sung et al., 2002). These analytical techniques, although accurate, are not suitable in onsite testing settings to provide point-of-care testing (POCT) for patient care. Alternatively, Biosensors have enabled POCT settings for the detection of creatinine in the whole blood, and rapid and reliable test results for the patients (Skurup et al., 2008).
Creatinine sensors include potentiometric and amperometric sensors (Killard and Smyth, 2000; Nontawong et al., 2019), Fe3O4 nanoparticles-based sensors (Hassanzadeh and Ghaemy, 2017), and colorimetric sensors based on poly-lactic-co-glycolic acid (PLGA) and 1-butyl-3-methylimidazolium (BMIM) chloride (Press, 2015). These sensors either are based on color reactions involving multiple chemical reactions or the enzymes which have the limitation of storage conditions being sensitive to temperatures and pH. One way of avoiding many of the drawbacks of either method is to measure creatinine concentration using molecularly imprinted polymers (MIPs) as the recognition element (Canfarotta et al., 2018). MIP is a crosslinked polymer that is synthesized in the presence of a target template where monomers of the polymer can form non-covalent interactions with the template (Belbruno, 2019). Later, when the template is washed out, it leaves nanocavities where the target molecule can rebind and the binding is as effective and selective as that of antibodies, though much more stable and in-expensive (Williams et al., 2022). This technology has been used for imprinting DNA, proteins, and whole cells (Chen et al., 2016; Liu et al., 2013). This technology has been used for imprinting DNA, proteins, and whole cells (Chen et al., 2016; Liu et al., 2013). Williams et al proposed the use of nano-molecularly imprinted polymers (nMIPs) in conjunction with the novel Heat Transfer Method (HTM) to sense creatinine from blood samples. They performed glutaraldehyde based crosslinking of creatinine to the glass beads (Williams et al., 2022). The method presented good selectivity; however, it involved multiple heat transfer measurements through electrodes and did not show its potential as a rapid and convenient method. Contrarily, hydrogels have been used effectively for (MIPs) since they provide restricted diffusion and can retain large amounts of water without shape change (Batista et al., 2021).
Recently, molecularly imprinted photonic crystals (MIPCs) biosensors have attracted substantial interest due to their great potential for label-free detection of biomolecules (Chen et al., 2016). MIPCs have been used because of their visual diffraction signals and excellent response to external stimuli (Fenzl et al., 2015; Muscatello and Asher, n.d.). The hydrogels may swell or contract in an aqueous solution in response to environmental changes, leading to changes in Braggsˊ diffraction (von Freymann et al., 2013). Our research group has reported numerous PCs sensors for the detection of glucose, antibiotics (Qi et al., 2019; Yifei Wang et al., 2019), G-series nerve agents, and the cancer biomarker Kynurenine (Rizvi et al., 2020a). For creatinine detection, Sharma et al. developed a creatinine deiminase (CD) functionalized PCs-based polyacrylamide hydrogel sensor which could respond to serum creatinine with a limit of detection (LOD) of 6 µM (Sharma et al., 2004). The measurements rely on the enzymatic reaction and utilized a spectrometer., There is still a need for a rapid, sensitive, and instrument-free method to meet the POCT needs.
In this context, we further advanced the detection of creatinine and report creatinine imprinted two-dimensional (2D) PCs hydrogel (CIPC) sensor for the detection of creatinine in human serum without complex pre-treatment of the sample and any complex instrumentation. The CIPC hydrogel involves imprinting of creatinine template in the hydrogel embedded with 2D PCs with the generation of cavities specific for creatinine. The binding of creatinine in the sample to these cavities results in the shrinking of the hydrogel and a decrease in particle spacing within a short time. The change in hydrogel volume and particle spacing can be measured with a laser pointer and a scale. The binding also results in a visible blue-green to-blue color shift. CIPC hydrogel is rapid, convenient, and produces a visual response to the target binding visible to the naked eye. The sensing principle of the hydrogel was explained by molecular docking and it is compared with other related detection methods.
2 Experimental section
2.1 Materials/Chemicals
Creatinine, creatine, pyrrolidinone, Bovine serum albumin (BSA), and N-hydroxysuccinimide (NHS) were purchased from Aladdin Co, Ltd. Styrene was procured from Sigma Aldrich. Acrylic acid (AA), 1-propanol, 2-(cyclohexylamino) ethanesulfonic acid (CHES), L-arginine from Shanghai biological science and technology Co. Ltd, glycine from Beijing solar bioscience and technology, and L-tyrosine were obtained from Shanghai Titan scientific. Acrylamide, potassium persulfate (KPS), N,N0-methylenebisacrylamide (BIS), 2,2-diethoxyacetophenone(DEAP), dimethyl sulfoxide (DMSO), acetic acid, and sodium dodecyl sulfate (SDS) were purchased from Acros Organics. The virus-free human blood samples were obtained from China Pharmaceutical and Biological Products Testing Institute, Beijing China.
2.2 Preparation of samples
Creatinine stock solutions (2 mM) were prepared in distilled water and dilutions were prepared in 10 mM CHES buffer (pH 10.0). Blood samples were centrifuged at 2500 rpm at 4 °C for 15 min to collect serum. The serum samples were diluted ten times with the 10 mM PBS pH 7.4 buffer before analysis by the hydrogel as described in our previous study (Rizvi et al., 2020b). The serum was spiked with creatinine (100 µM, 200 µM, 500 µM prepared in CHES buffer (pH 10).
2.3 Fabrication of photonic crystals array (PC-array) and creatinine imprinted photonic crystals (CIPC) hydrogel
The polystyrene (PS) colloidal nanoparticles were synthesized by emulsifier-free emulsion polymerization described by Reese et al. (Reese and Asher, 2002). According to the method, 48.4 g of styrene, 1.2 g KPS and 480 mL water were poured into a 1000 mL flask. N2 was passed through the mixture for 40 min to remove O2. Then the temperature was increased to 70 °C. 1.2 g KPS prepared in 80 mL water was added to the reaction mixture for polymerization at 70 °C overnight in the presence of N2. After repeated centrifugation and washing in deionized water, 600 nm monodispersed PS colloidal particles were obtained. The PS colloidal suspensions (25 % wt) and 1-propanol were mixed at a ratio of 1: 1 (v/v). 20 mL of this suspension was gradually layered onto the top of a water surface in a 25 cm diameter glass container to form a hexagonal close-packed 2D photonic crystals array (PCs-array). The PCs-array was picked up on a glass slide (Scheme 1). After the solvent evaporated, the sample was treated in an oven at 80 °C for 2 h.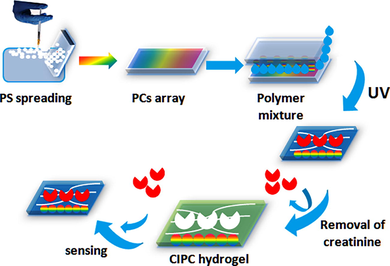
Schematic of the CIPC hydrogel fabrication.
A creatinine imprinted 2D PCs (CIPC) hydrogel film was polymerized over the 2D PCs-array on the glass slides using DEAP as a photoinitiator according to our previously developed method with some modification (Xue et al., 2014). The recipe of the pre-polymer mixture contained 0.36 g of AM, 38.5 mL of MAA, 10 mg of BIS, creatinine (0.1 g), and 2 mL of deionized water. Nitrogen gas was passed through this mixture for 10 min to remove oxygen. Then, 28 µL of a 10 % solution of DEAP in DMSO (13.6 mmol of DEAP) was added to the AM-BIS-MAA suspension. This mixture was layered onto a PCs-array on a glass slide. A glass slide (60 mm × 24 mm × 0.12 mm) was placed over the solution covering the PCs-array (Scheme 1). The polymerization was performed at 365 nm in a UV incubator for 2 h.
The 2D particles were embedded within the CIPC hydrogel and the CIPC hydrogel film was peeled off the glass slide and washed with 10 % acetic acid containing 10 % SDS by incubating it on the bath oscillator for 2 h to remove the creatinine template and create nanocavities specific for creatinine. The PCs were still part of the hydrogel. The hydrogel was cut into small pieces, each (2 cm × 2 cm). A non-imprinted photonic crystal (NIPC) hydrogel without creatinine was also prepared and used as a control.
2.4 Analysis and detection procedure
The CIPC hydrogel films were incubated in a 2 mL solution of creatinine samples (250 nM −1000 µM) or serum samples for 5–15 min and observed under a laser pointer, Debye diffraction ring diameters were measured and particle spacing was found. The particle spacing was calculated by the formula d = 2λ/3sinα given by Zhang et al. (Zhang et al., 2013); where α is the Debye diffraction’s diffraction angle (α = tan−1(r/h)), λ is the laser light wavelength, d is the particle spacing, h is the distance between CIPC hydrogel and the screen, and r is the radius of Debye diffraction ring (Fig. 1). The Debye diffraction ring was utilized to measure the particle spacing of the CIPC hydrogel. A violet laser of wavelength 405 nm was pointed on the hydrogel at 90° for 10 min. The diffracted light on the screen perpendicular to the laser reflects the 2D particle spacing and order by showing a round ring.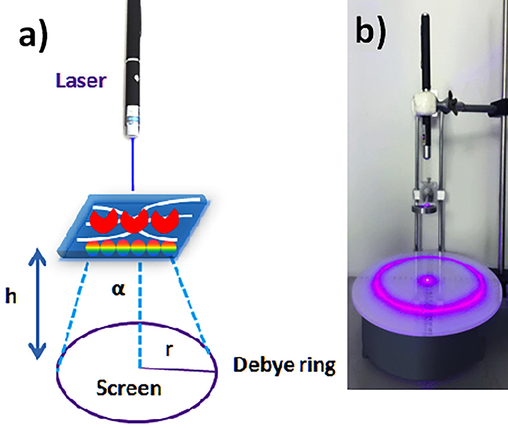
Detection procedure. (a) CIPC hydrogel with PCs-array and diffraction of laser light. (b) Detection device.
2.5 Characterization of CIPC hydrogel
The size of polystyrene (PS) nanoparticles was measured by dynamic light scattering (DLS) on the Zeta sizer (Malvern Nano Zetasizer) and the structures of the PCs-array and the CIPC hydrogel were analyzed by SEM Quanta FEG 250, FEI. The reflectance spectra were measured with a fiber optic UV/VIS/NIR-spectrometer (Avaspec-2048TEC, Avantes) equipped with an FC-UV600-S-SR fiber optic reflection probe and an Avalight-DH-S-BAL light source and the FC-UV600-S-SR fiber optic reflection probe was utilized to record the diffraction spectrum from the CIPC.
The photograph of the hydrogel sensors was taken using a digital camera (Sony, Cyber-shot DSC-N2) and the hydrogel sensors were placed on a silver mirror.
FTIR was used to see the attached functional monomers (MAA) and template (creatinine) in the hydrogel.
For the effect of pH changes, the hydrogel sensors were immersed in a 2 mL buffer solution with a pH range from 3 to 10.5 at room temperature. After 5 min, the Debye diffraction rings were measured.
The adsorption efficiency of the CIPC hydrogel was analyzed by capillary zone electrophoresis (CZE) (Kubáň et al., 2019), on Agilent 7100 equipped with a diode array UV (DAD-UV) detector. Briefly, small pieces of the CIPC hydrogels each weighing (2.6 mg) were incubated in the 2 mL of creatinine solutions (25–1000 µM) prepared in 10 mM CHES buffer at pH 10.0 for 2 min at room temperature and taken out. The supernatants were analyzed by CZE-UV at the wavelength of 200 nm for the presence of creatinine. Peak areas were calculated for the measurement of creatinine concentrations in solution and supernatant solutions.
3 Docking simulations
Molecular dockings were performed to understand molecular interactions of creatinine with the CIPC hydrogel network components, i.e. AM, BIS, and MAA. as described by Aysha et al (Rizvi et al., 2020b). The simulations were considered a better method to visualize the imprinting, washing, and application steps of the designed sensor. Furthermore, it can also explain the binding mechanism briefly. Before exploring creatinine interaction with hydrogel components, we designed their 3D structures. The structures were built using the Marvin JS web server by using the given information. The 3D structure of creatinine was retrieved from the Chemspider database. The 3D coordinates of both models were rebuilt to fix missing atoms using the MDWeb version 1.0 webserver, which employed the AMBERTools version 1.2 engine. All prepared structures were visualized by UCSF Chimera. FlexAID (Flexible Artificial Intelligence Docking) (Gaudreault and Najmanovich, 2015), embedded in NRGSuite, a small molecule docking program was utilized that accounts for target side-chain flexibility and utilizes a soft scoring function.
4 Results and discussion
4.1 Characterization of PCs-array and CIPC hydrogel
4.1.1 Scanning electron microscopy
The average size of PS nanoparticles was optimized to obtain the Debye diffraction ring and was found to be 630 nm measured by DLS as shown in Fig. 2a. Fig. 2b shows PCs-array with compact closely packed PS nanoparticles and CIPC hydrogel embedded with PCs.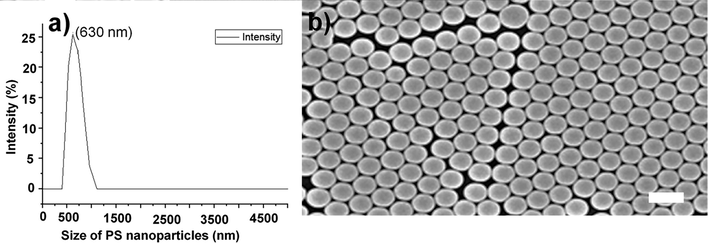
Morphology of PS-nanoparticles. (a) DLS size measurement of PS-nanoparticles. (b) CIPC hydrogel embedded with PCs-array showing PS nanoparticles dispersed evenly. The scale bar is 650 nm which demonstrates the size of PS nanoparticles.
4.1.2 Fourier Transform Infrared Resonance (FTIR) and Nuclear Magnetic Resonance (NMR)
FTIR analysis of CIPC hydrogel (Fig. 3a) shows a prominent deep stretch of the peak at 3226 cm−1. Creatinine was successfully incorporated into the CIPC hydrogel. The peak shows the absorption by the O–H hydrogen bond stretch between carbonyl groups of MAA and the amine group of creatinine, O= of BIS and the H of the amine of creatinine, O= of AM, and the H of creatinine or the H from the NH2 group of AMs and the O= of creatinine (Coates, 2004). Other peaks at 2960 represent sp3 C-H, 1505 represent amide, and 924 represent phosphate. There were no differences found between NIPC and CIPC hydrogels in from 2500 cm−1 to 400 cm−1 ranges.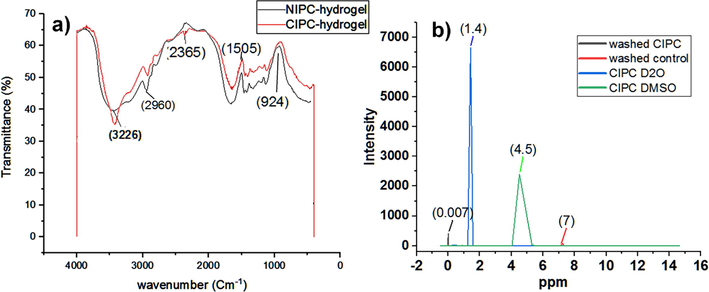
FTIR and NMR spectra. (a) FTIR analysis of CIPC hydrogels compared with NIPC hydrogel. (b) 1H NMR spectra. Creatinine gave rise to two signals at 3 ppm and 4.5 ppm (ppm: parts per million). Control represents NIPC hydrogel. All the spectra have been analyzed in origin 2019. Washed means washing with acetic acid and SDS solution.
NMR of CIPC hydrogel was taken after polymerization with the dried form of the gels with the PCs array intact inside. The samples were analyzed in duplicate by 1H NMR at 400 MHz (Avance, Bruker, Karlsruhe, Germany). A standard Bruker double-resonance probe, supporting rotors of 2.5 mm outer diameter, was used. All samples were spun at a magic angle spinning (MAS) frequency of 30 kHz. The 908 radio-frequency pulse lengths were set to 3 ms, and the recycle delay between consecutive transients of the experiments was 3 s. DQ MAS experiments were performed using two rotor periods of the back-to-back (BABA) recoupling sequence. Deuterium oxide (D2O) and DMSO were used as solvents for creatinine to compare the absorption in both solvents.NMR spectra of CIPC hydrogel and NIPC hydrogel were taken before washing and after washing with acetic acid and SDS. The results showed that after washing both of the hydrogels had almost the same spectra intensity (Fig. 3b) demonstrating that CIPC hydrogel was properly washed and nanocavities were produced after washing off creatinine. So there was no chemical shift or new spectra in CIPC hydrogel after washing and no adsorption and both NIPC and CIPC NMR results were the same.
However, CIPC produced two characteristic NMR signals with creatinine in D2O and DMSO at 1.4 ppm and 4.5 ppm respectively which corresponded to (Hβ, M, 2H/ -CH3) and (Hα, S, 1H/ –CH2) respectively (Arakali et al., 1997; Foxall et al., 1993) (Fig. 3b). These results confirmed the successful imprinting of creatinine in the hydrogel.
4.2 Optimization of buffer pH, creatinine, and monomers concentration
The effect of pH on the particle spacing change was also investigated. The hydrogels were kept in a 2 mL buffer solution with different pH from 3 to 10.5 at room temperature. Debye diffraction rings diameter was measured after 5 min. For this study, the CIPC hydrogels were immersed in different pH solutions of phosphate buffer, CHES buffer, and Citrate buffers. It can be seen that in Figure S1a of supplementary material, as the pH of citrate buffer decreased to 3, the hydrogel shrank as particle spacing decreased from 620 to 590 nm with a net difference of 30 nm.
Increasing creatinine concentration swells the hydrogel and particle spacing increases to 610 nm, so there was only a change of 10 nm in the case of citrate buffer. In the case of phosphate buffer pH 7.4, there was also a change in particle spacing of 10 nm due to hydrogel shrinkage from 530 nm to 520 nm. In the case of CHES buffer pH 9, particle spacing decreased from 590 nm to 560 nm due to hydrogel shrinkage then by further increasing creatinine concentration to 200 µM particle spacing decreased from 470 nm with a net change of 120 nm. Therefore, considering the maximum shrinking response, CHES buffer pH 9.0 was used in further sensing experiments.
The concentrations of creatinine and MAA were optimized by using their different ratios as shown in Figure S1b. Creatinine and MAA were prepared in different ratios 1:2, 1:4, 1;6. Then these hydrogel filmsˊ diffraction rings were measured. CIPC hydrogel with 1:2 of creatinine and MAA showed particle spacing change greater than 650 nM to 770 nM, whereas, with the ratios, i.e. 1:4 and 1:6, particle spacing was below 500 nM. Therefore, 1:2 was selected for further experiments after comparison with other ratios 1:4 and 1:6.
4.3 Determination of creatinine
Creatinine was determined by the CIPC hydrogel. The binding of CIPC-hydrogel to its target, i.e. creatinine was measured in terms of diffraction ring diameter change and the resulting change in particle spacing. CIPC hydrogel responded to a wide concentration range of creatinine (25 nM-1000 µM as shown in Fig. 4a. The particle spacing decreased linearly from 589 nm to 369 nm with creatinine in the concentration range 25 µM-500 µM (Y = = −0.451x + 585.8, R2 = 0.962). The net change of 220 nm particle spacing (Fig. 4b) resulted in hydrogel shrinking along with the visible color change from blue-green to blue in a short time of 2 min. The intensity of color change increased with increasing concentration of creatinine, which is the direct way the determination of creatinine (Fig. 4c). NIPC hydrogel underwent only 5 nm changes in particle spacing with up to 1000 µM of creatinine (Fig. 4a) and showed no noticeable color change.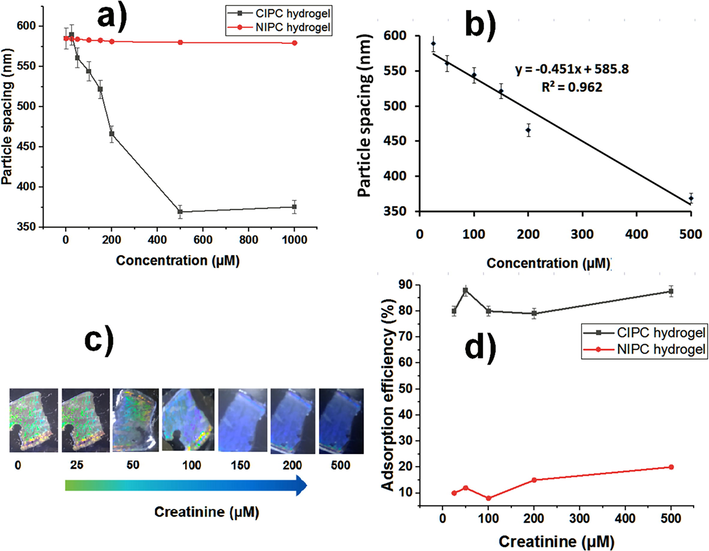
CIPC hydrogel usage for creatinine measurement. (a) Creatinine detection (250 nM-1000 µM). (b) Linear detection range (25 µM-500 µM). (c) The color change of CIPC hydrogel is monitored in visible light and corresponds to the relevant creatinine concentration. (d) Adsorption efficiency of CIPC and NIPC hydrogels.
The Debye diffraction ring measurement is an effective way for quantitative determinations, and it is more convenient, simple, cost-effective, and point-of-care. The limit of detection (LOD) was calculated by the analytical methods committee (Committee, 1987) and was found to be 2.45 ± 1.60 µM (LOD = 3.3 × Sb/sensitivity; where Sb is the standard deviation of the blank solution measurements (n = 10) without creatinine and sensitivity is the ratio of change in response to change in concentration of creatinine measured with CIPC hydrogel. The limit of quantification (LOQ) was determined to be 7.45 µM (LOQ = 10 × Sb/sensitivity). The LOD found is twice lower as the LOD found by Sharma et al. (Sharma et al., 2004), and our imprinting technique is much easier, more specific, and cheaper as compared with the photonic crystals functionalized with a creatinine deiminase (CD) enzyme.
Adsorption efficiency (%) of the CIPC and NIPC hydrogels at five creatinine concentrations (25 µM, 50 µM, 100 µM, 200 µM, and 500 µM) were measured (Fig. 4d), a maximum up adsorption efficiency was found to be 87.6 % at 500 µM in the CIPC hydrogel (Table 1). Non-imprinted hydrogel did not show a significant change in the adsorption. CE experimental conditions: 25.0 mM borax-borate buffer (pH 8.0), λ = 200 nm, 25 kV, 25 °C, injection: 50 mbar, 5 s. Adsorption efficiency = (Ci-Cf)/Ci × 100 %, where Ci is the initial concentration of creatinine before adsorption and Cf is the concentration of creatinine found in the supernatants. mAU is the mili absorbance unit. RSD is the relative standard deviation (n = 3).
Creatinine initial (Ci) conc. (µM)
Peak Area (mAu)
Creatinine conc. in supernatant Cf (µM)
Peak Area (mAU)
Adsorption efficiency %±RSD
25
7.2
5
1.4
80 ± 1.17
50
10.1
6
1.2
88 ± 0.15
100
12.2
20
2.4
80 ± 1.00
200
14.3
50
3.5
79 ± 0.39
500
17.6
62
2.1
87 ± 0.77
4.4 Validation of results by UV/VIS/NIR spectrometry
The response of CIPC hydrogel to creatinine was also measured by a UV/VIS/NIR spectrometer in the wavelength range (200–1200 nm). With an increase in the concentration of creatinine from 1 µM to 500 µM, CIPC-hydrogel demonstrated an overall 3800 a.u change in the intensity from 3677 a.u to 7477 a.u (Fig. 5a), compared with a relatively negligible intensity change of 56 a.u by NIPC hydrogel with the same concentrations of creatinine (Fig. 5b).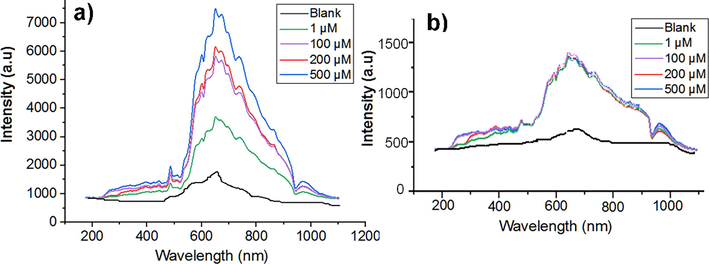
Measurements of creatinine with a UV/VIS/NIR-spectrometer. (a) CIPC hydrogel. (b) NIPC hydrogel. Blank is the buffer solution without creatinine.
4.5 Reversibility
CIPC hydrogel also demonstrated excellent reusability and reversibility which was found by measuring changes in the Debye diffraction ring diameter after incubation in 500 µM of creatinine. Then creatinine was washed off the CIPC with methanol/acetic acid (9:1 v/v) while keeping on the oscillator for 10 min after use. The CIPC demonstrated reusability five times. The same hydrogel was also tested multiple times with different concentrations of creatinine and demonstrated reusability (Figure S2 of supplementary material).
4.6 Selectivity/Interference studies
One of the hurdles in the detection of creatinine in the sensorsˊ application is the possible matrix effect offered by the complex composition of the serum samples. However, our sensor did not need any pre-treatment; instead, the serum samples were only diluted with buffer and used directly. The selectivity of CIPC hydrogel was evaluated by testing against similar amino acids and analogs of creatinine. The serum was spiked with amino acids (Figure S3a of supplementary material) or similar analogs of creatinine (Figure S3b).
Figure S3 shows that there was a very visible change in color and Debye diffraction ring diameter with creatinine. Particle spacing decreased from 650 nm to 420 nm with a change of 230 nm whereas only a change of 20 nm from 430 to 410 nm in the case of other amino acids and no color change was observed.
The selectivity of CIPC hydrogel was also evaluated in presence of analogs of creatinine such as pyrrolidinone, and N-hydroxysuccinamide. CIPC hydrogel demonstrated a clear response with creatinine as compared with other analogs (Figure S3b). A particle spacing change of 650 nm to 400 nm with a difference of 250 nm was observed with creatinine, however, only a change of 20 nm from 430 nm to 410 nm was observed with other analogs of creatinine, without any significant color change.
4.7 Molecular docking for sensing mechanism
The imprinting, washing, and application of hydrogel for the detection of creatinine was simulated with help of NRGSuite plugin for PyMol. It includes GetCleft function to detect the cavity in/on the receptor for binding of the ligand, and binding features of FlexAID, which utilizes the complementarity function (CF) among contact surfaces. The top results were arranged according to the binding energy values and RMSD. The hydrogel was used as a target and creatinine as a ligand. The docking complex was used to explain the imprinting step by visualizing surface occupancy Fig. 6. After washing off the ligand, a cavity is produced, which was visualized in the simulation by removing the ligand from the resultant complex.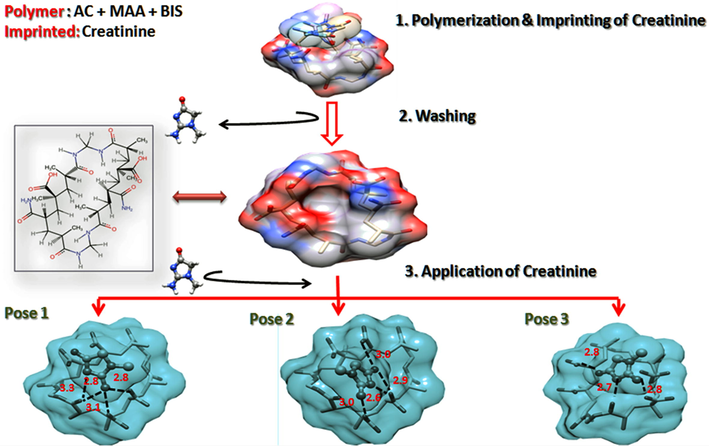
Molecular docking-based sensing mechanism. (a) Fabrication of CIPC-hydrogel. (b) Removal of creatinine. (c) Binding and sensing application.
The hydrogel is specific for the detection of creatinine, verified after application, and occupies the same cavity as creatinine. This shows a strong hydrogen bonding between creatinine and the gel monomers, including AM and MAA. Three H-bonds were predicted in pose 1 and pose 2, while 4 H-bonds were predicted in pose 3. Other interactions included overlaps between COOH, C=O, and NH2 of both molecules.
5 Application in serum samples
The analysis of serum samples revealed that the creatinine found in the healthy subjects was in the range of 20.6 ± 0.1 µM to 45.1 ± 0.5 µM (Table 2). The recoveries with 100 µM, 200 µM, and 500 µM of creatinine spiked in five serum samples were also calculated. The average recoveries found for creatinine in serum were from 85.6 % to 99.9 % (Table 2), which demonstrated that there was no significant interference of the sample matrix in the detection of creatinine. SD is the standard deviation of n = 3.
Sample
Creatinine added (µM)
Creatinine found (µM) ± SD
Recovery (%)
1
0
20.7 ± 0.3
100
119.2 ± 0.2
98.7
200
215.3 ± 0.1
97.5
500
520 ± 0.09
99.9
2
0
20.6 ± 0.1
100
113.2 ± 0.2
93.8
200
214.3 ± 0.2
97.1
500
515 ± 0.1
98.9
3
0
30.7 ± 0.1
100
128.2 ± 0.1
98.0
200
210.2 ± 0.1
91.1
500
515 ± 0.05
97.1
4
0
40.3 ± 0.2
100
120.2 ± 0.2
85.6
200
210.2 ± 0.1
87.3
500
532 ± 0.2
98.5
5
0
45.1 ± 0.5
100
130.6 ± 1.2
90.0
200
240.5 ± 1.2
98.1
500
540 ± 0.7
99.0
6 Comparison of methods for creatinine detection
CIPC hydrogel demonstrates convenient synthesis, usage, and portable sensing platform, without compromising sensitivity, reproducibility and selectivity, and rapid response in 2 min. This gives it extra features compared with the traditional FIA analysis, GC–MS, amperometric detection, and electrochemical biosensor based on creatininase methods, CIPC hydrogel does not require any sample pre-treatment steps. It gives sensing visible to the naked eye with only a laser pointer and a ruler, but it is also compatible with a UV/VIS/NIR spectrometer which gives it superior properties compared with the three-dimensional PCs sensor (Sharma et al., 2004). A comparison of creatinine detection methods is presented in Table 3. The LOD is comparable to the contemporary methods within the healthy standard range in serum, but the CIPC hydrogel sensor is more convenient and is reproducible/reusable and selective for creatinine.
No.
Method
LOD
Linear range
Reference
1
Luminol chemiluminescence/FIA analysis
1.5 mM
3–150 mM
(Kubo and Toriba, 1997)
2
Amperometric detection based on creatinine amidohydrolase/creatine amidinohydrolase/sarcosine
oxidase
0.5 µM
10–650 μM
(Yadav et al., 2011)
3
Disposable non-enzymatic electrochemical creatinine sensor
0.0746 μM
6–378 μΜ
(Raveendran et al., 2017)
4
Amperometric creatinine biosensor
0.01 μΜ
0.01 μΜ to 12 μΜ
(Kumar et al., 2019)
5
molecularly-imprinted polymer-coated copper oxide nanoparticle-modified carbon-paste-electrode
0.083 μΜ
0.5 to 200 μΜ
(Nontawong et al., 2019)
6
Colorimetric poly-lactic-co-glycolic acid (PLGA) and 1-butyl-3-methylimidazolium (BMIM) chloride-based polymer
5 μΜ
Not reported
(Press, 2015)
7
Photonic crystal hydrogel sensor
6 μM
10–700 µM
(Sharma et al., 2004)
8
CIPC hydrogel
Uses a laser pointer and ruler and also compatible with UV/VIS/NIR-spectrometer
2.45 µM
25–500 µM
Present study
7 Conclusion
A novel CIPC hydrogel is presented for highly selective, cheaper, and instrument-free detection of creatinine without compromising sensitivity. This method relies on alteration in diffraction ring diameter at different concentrations of creatinine added to the buffer and serum samples with the visible color changes from blue-green to blue within a short time of 2 min. This proposed sensor is highly specific and selective for creatinine. The advantages of the CIPC hydrogel ensure its promising use as a POC sensor for the quantitative determination of creatinine in clinical samples.
CRediT authorship contribution statement
Bushra Rafique: Conceptualization, Methodology, Visualization, Data curation, Software, Investigation, Writing – original draft, Writing – review & editing. Rizwan Ullah Khan: Writing – review & editing. Aysha Sarfraz Rizvi: Writing – review & editing. Muhammad Irfan: Methodology. Ghulam Murtaza: Writing – review & editing. Lili Qiu: Supervision, Conceptualization, Writing – review & editing. Min Xue: Supervision, Conceptualization, Writing – review & editing. Zihui Meng: Supervision, Conceptualization, Writing – review & editing.
Acknowledgments
National Natural Science Foundation of China [U1530141, 21804009, 21874009].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Study of creatinine and its 5-alkoxy analogs: Structure and conformational studies in the solid and solution states by x-ray crystallography, NMR, UV and mass spectrometry. Nucleosides Nucleotides. 1997;16:2193-2218.
- [CrossRef] [Google Scholar]
- Molecularly imprinted materials for biomedical sensing. Med. Devices Sensors. 2021;4:e10166.
- [CrossRef] [Google Scholar]
- Canfarotta, F., Cecchini, A., Piletsky, S., 2018. CHAPTER 1: Nano-sized Molecularly Imprinted Polymers as Artificial Antibodies. In: RSC Polymer Chemistry Series. Royal Society of Chemistry, pp. 1–27. https://doi.org/10.1039/9781788010474-00001.
- Molecular imprinted photonic crystal for sensing of biomolecules. Mol. Imprinting. 2016;4:1-12.
- [CrossRef] [Google Scholar]
- High-throughput multi-analyte screening for renal disease using capillary electrophoresis. J. Pharm. Biomed. Anal.. 2001;25:795-801.
- [CrossRef] [Google Scholar]
- Coates, J., 2004. Encyclopedia of Analytical Chemistry -Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem.
- Committee, A.M., 1987. Recommendations for the Definition, Estimation and Use of the Detection Limit 112, 199–204.
- Spectrophotometric simultaneous determination of creatinine and creatine by flow injection with reagent injection. Fresenius’ J. Anal. Chem.. 1995;352:557-561.
- [CrossRef] [Google Scholar]
- A photonic crystal based sensing scheme for acetylcholine and acetylcholinesterase inhibitors. J. Mater. Chem. B. 2015;3:2089-2095.
- [CrossRef] [Google Scholar]
- NMR spectroscopy as a novel approach to the monitoring of renal transplant function. Kidney Int.. 1993;43:234-245.
- [CrossRef] [Google Scholar]
- FlexAID: Revisiting Docking on Non-Native-Complex Structures. J. Chem. Inf. Model.. 2015;55:1323-1336.
- [CrossRef] [Google Scholar]
- An effective approach for the laboratory measurement and detection of creatinine by magnetic molecularly imprinted polymer nanoparticles. New J. Chem.. 2017;41:2277-2286.
- [CrossRef] [Google Scholar]
- Creatinine biosensors: principles and designs. Trends Biotechnol.. 2000;18:433-437.
- [CrossRef] [Google Scholar]
- Capillary electrophoresis of small ions and molecules in less conventional human body fluid samples: A review. Anal. Chim. Acta. 2019;1075:1-26.
- [CrossRef] [Google Scholar]
- Chemiluminescence flow injection analysis of reducing agents based on the luminol reaction. Anal. Chim. Acta. 1997;353:345-349.
- [CrossRef] [Google Scholar]
- An improved amperometric creatinine biosensor based on nanoparticles of creatininase, creatinase and sarcosine oxidase. Anal. Biochem.. 2017;537:41-49.
- [CrossRef] [Google Scholar]
- Fabrication of an improved amperometric creatinine biosensor based on enzymes nanoparticles bound to Au electrode. Biomarkers. 2019;24:739-749.
- [CrossRef] [Google Scholar]
- Molecularly imprinted photonic polymer based on β-cyclodextrin for amino acid sensing. Talanta. 2013;116:283-289.
- [CrossRef] [Google Scholar]
- Muscatello, M.M.W., Asher, S.A., n.d. Poly(vinyl alcohol) Rehydratable Photonic Crystal Sensor Materials**. https://doi.org/10.1002/adfm.200701210.
- Novel amperometric flow-injection analysis of creatinine using a molecularly-imprinted polymer coated copper oxide nanoparticle-modified carbon-paste-electrode. J. Pharm. Biomed. Anal.. 2019;175
- [CrossRef] [Google Scholar]
- Preliminary investigation of near-infrared spectroscopic measurements of urea, creatinine, glucose, protein, and ketone in urine. Clin. Biochem.. 2001;34:239-246.
- [CrossRef] [Google Scholar]
- Measurement of creatinine in human plasma using a functional porous polymer structure sensing motif. Int. J. Nanomed.. 2015;10:93-99.
- [CrossRef] [Google Scholar]
- Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron.. 2019;126:707-724.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase-functionalized two-dimensional photonic crystal for the sensing of G-series nerve agents. Anal. Bioanal. Chem.. 2019;411:2577-2585.
- [CrossRef] [Google Scholar]
- Fabrication of a disposable non-enzymatic electrochemical creatinine sensor. Sens. Actuators B Chem.. 2017;243:589-595.
- [CrossRef] [Google Scholar]
- Emulsifier-free emulsion polymerization produces highly charged, monodisperse particles for near infrared photonic crystals. J. Colloid Interface Sci.. 2002;248:41-46.
- [CrossRef] [Google Scholar]
- Determination of Kynurenine Enantiomers by Alpha-Cyclodextrin, Cationic-βeta-Cyclodextrin and Their Synergy Complemented with Stacking Enrichment in Capillary Electrophoresis. J. Chromatogr. A 2020:461128.
- [CrossRef] [Google Scholar]
- Development of Molecularly Imprinted 2D Photonic Crystal Hydrogel Sensor for Detection of L-Kynurenine in Human Serum. Talanta. 2020;208:120403
- [CrossRef] [Google Scholar]
- Tandem mass spectrometric quantification of 8-iso-prostaglandin F(2α) and its metabolite 2,3-dinor-5,6-dihydro-8-iso-prostaglandin F(2α) in human urine. J. Chromatogr. B. 2000;744:99.
- [CrossRef] [Google Scholar]
- A General Photonic Crystal Sensing Motif: Creatinine in Bodily Fluids. J. Am. Chem. Soc.. 2004;126:2971-2977.
- [CrossRef] [Google Scholar]
- Creatinine, eGFR and association with myocardial infarction, ischemic heart disease and early death in the general population. Atherosclerosis. 2014;237:67-75.
- [CrossRef] [Google Scholar]
- New creatinine sensor for point-of-care testing of creatinine meets the National Kidney Disease Education Program guidelines. Clin. Chem. Lab. Med.. 2008;46:3-8.
- [CrossRef] [Google Scholar]
- Amperometric biosensors employing an insoluble oxidant as an interference-removing agent. Anal. Chim. Acta. 2002;461:251-260.
- [CrossRef] [Google Scholar]
- Stability of renal function in spite of low glomerular filtration rate: a case report. Iran. Red Crescent Med. J.. 2015;17 e21604–e21604
- [CrossRef] [Google Scholar]
- Bottom-up assembly of photonic crystals. Chem. Soc. Rev.. 2013;42:2528-2554.
- [CrossRef] [Google Scholar]
- Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron.. 2019;136:84-90.
- [CrossRef] [Google Scholar]
- Fast screening of antibiotics in milk using a molecularly imprinted two-dimensional photonic crystal hydrogel sensor. Anal. Chim. Acta. 2019;1070:97-103.
- [CrossRef] [Google Scholar]
- Nano-molecularly imprinted polymers for serum creatinine sensing using the heat transfer method. Talanta Open. 2022;5:100087
- [CrossRef] [Google Scholar]
- A 2-D photonic crystal hydrogel for selective sensing of glucose. J. Mater. Chem.. 2014;2:9559-9565.
- [CrossRef] [Google Scholar]
- Amperometric creatinine biosensor based on covalently coimmobilized enzymes onto carboxylated multiwalled carbon nanotubes/polyaniline composite film. Anal. Biochem.. 2011;419:277-283.
- [CrossRef] [Google Scholar]
- Immobilization of creatininase, creatinase and sarcosine oxidase on iron oxide nanoparticles/chitosan-g-polyaniline modified Pt electrode for detection of creatinine. Enzyme Microb. Technol.. 2012;50:247-254.
- [CrossRef] [Google Scholar]
- Two-dimensional array Debye ring diffraction protein recognition sensing. Chem. Commun.. 2013;49:6337-6339.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
The additional related contents are given in supplementary material. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104684.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







