Translate this page into:
Cu(II)/polyimide linked COF: An effective mesoporous catalyst for solvent-free 1,5-benzodiazepine synthesis
⁎Corresponding author at: Department of Chemistry, Bandar Abbas Branch, Islamic Azad University, Bandar Abbas 7915893144, Iran. fmoeinpour@iauba.ac.ir (Farid Moeinpour)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Copper(II)@polyimide linked covalent organic frameworks (Cu@PI-COF) under solvent-free and microwave assisted conditions have been used in an efficient one-pot protocol for the preparation of 1,5-benzodiazepines via 1,2-diaminobenzene, aromatic aldehydes and dimedone. By applying solvent-free conditions and microwave irradiation, three-component condensation provides safe operations, low pollution, quick access to products, and an easy set-up. As a result of its reusability, the catalyst can also be reutilized many runs without missing any activity.
Keywords
1,5-benzodiazepines
Covalent organic framework
Solvent-free
Microwave
1 Introduction
The application of COFs as a heterogeneous ligand for immobilizing transition metal ions to support a variety of organic reactions has been shown to be promising (Chen, Chen et al. 2021, Zhang, Lu et al. 2021, Chen, Zhang et al. 2022, Chen, Zhang et al. 2022). Lately, PI-COFs (covalent organic polyimide frameworks) with high thermal resistance, extraordinary mechanical quality, big pore sizes and predominant chemical stability have been created and prepared by incorporating linear and tetrahedral building blocks through the imidization response. While many studies have demonstrated the potential of PI-COFs to be excellent functional materials for drug delivery (Fang, Wang et al. 2015), chemo and biosensors (Wang, Guo et al. 2022) and organic dye depollution (Wang, Xue et al. 2017), the study of PI-COFs as transition metal carriers has received relatively little attention.
Among the heterocyclic compounds that are widely used in medicinal chemistry, benzodiazepines are one of the compounds that have been studied; On the other hand, the synthesis of these compounds has been noticed by organic chemists in recent years through their many biological and medicinal characteristics and industrial uses (Mishra, Sharma et al. 2022). Benzodiazepines are one of the most important sedative-hypnotic drugs. Due to the double effectiveness of these compounds in the treatment of convulsions, anxiety, and insomnia, they have been successful in therapeutic issues and have been able to take the place of barbiturates, and at the same time, they are more reliable. The most famous drugs in this category are alprazolam, diazepam, clonazepam, oxazepam, lorazepam and chlordiazepoxide (Sanabria, Cuenca et al. 2021).
Many methods of synthesizing 1,5-benzodiazepines have one or more restrictions, such as prolonged reaction times, incidence of side reactions, harsh reaction conditions, low yields, use of corruptive chemicals (viz. trifluoroacetic acid, gaseous hydrogen chloride) and hazardous reagents (viz. chloroform, piperidine, pyridine), high-boiling-point solvent (viz. dimethylformamide), and cumbersome processing (Nasir, Ali et al. 2017). Catalysts such as boron trifluoride etherate (Herbert and Suschitzky 1974), sodium borohydride (Morales, Bulbarela et al. 1986), ceric ammonium nitrate (Varala, Enugala et al. 2006), acetic acid under microwave conditions (Pozarentzi, Stephanidou-Stephanatou et al. 2002), magnesium oxide/phosphoryl chloride (Balakrishna and Kaboudin 2001), ytterbium(III) trifluoromethanesulfonate (Curini, Epifano et al. 2003), ferrocene anchored activated carbon (Kusuma, Patil et al. 2022), cerium-enriched magnetic nano-catalyst (Wen, Wang et al. 2022), MIL-101 metal–organic framework (Sarkar, Gupta et al. 2021) and Cu(II)-clay nano-catalyst (Shaikh, Baseer et al. 2020) were used to improve the efficiency of the reaction. The most impediments of these catalysts are that they are misplaced within the work-up strategy, cannot be recuperated or reutilized, and are exceedingly poisonous and costly (Singh, Sharda et al. 2020). Hence, an improved catalyst for the synthesis of 1,5-benzadiazepines is required to attain gentle reaction conditions, simple operation, economy, and selectivity.
As part of our investigation plan in the context of nano-catalysts (Moeinpour and Khojastehnezhad 2012, Moeinpour, Dorostkar-Ahmadi et al. 2014, Khojastehnezhad, Moeinpou et al. 2015, Rahimizadeh, Seyedi et al. 2015, Khodsetan and Moeinpour 2020, Monajjemifar, Moeinpour et al. 2021, Moeinpour, Khalifeh et al. 2022) we report herein polyimide linked covalent organic frameworks (PI-COFs) used as nano-catalysts for the preparation of benzodiazepine derivatives. The generic procedure is given in Scheme 1.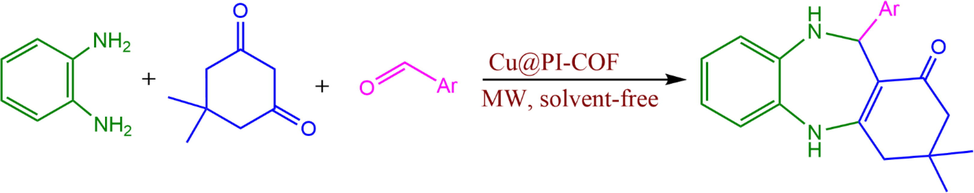
Cu@PI-COF catalyst-mediated synthesis of 1,5-benzadiazepines.
2 Experimental
2.1 Synthesis of PI-COF
PI-COF was synthesized by a reaction in which MEL (melamine) condenses with BTCD (benzene-1,2:4,5-tetracarboxylic dianhydride) (Scheme 1), as reported previously by Han et al. (Han, Zhang et al. 2018). For further details please see Electronic Supporting Material (ESM).
2.2 1,5-Benzodiazepines generic preparation method
In a Pyrex test tube, a mixture of 1,2-diaminobenzene (0.108 g,1 mmol), aromatic aldehydes 3a-o (1 mmol), dimedone or 5,5-dimethyl-1,3-cyclohexanedione (0.140 g, 1 mmol) and 0.2 g catalyst was subjected to microwave radiation with a power of 180 W (MicroSynth) under solvent free conditions for convenient reaction time (5 min). It was monitored using TLC to determine how the reaction was progressing (normal hexane: ethyl acetate, 7:3). After termination of the reaction, the temperature of the reaction mixture was lowered to ambient temperature and 15 mL of hot ethanol was added. Then, to prepare for the next run, the Cu@PI-COF was removed from the cooled mixture by filtration, washed with a solution of acetone and then dried overnight. It was then transferred into a beaker the catalyst-free reaction mixture that had been prepared. The colloidal solution obtained from the separation was crystallized with hot ethanol to obtain the pure product. The 1,5-benzodiazepine products were completely purified by recrystallization and a yellow precipitate was successfully obtained. It has been established that the structures of the products can be assigned via 1H NMR, and their melting points have been compared with those found earlier in the literature.
2.3 Selected spectral data
2.3.1 11-(4-Methoxyphenyl)-3,3-dimethyl-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e] [1,4]diazepin-1-one (Table 2, entry 4)
1H NMR (400 MHz, DMSO, ppm): δ: 1.05 (s, 3H, –CH3), 1.09 (s, 3H, –CH3), 2.04 (ABq, 2H, J = 15.8 Hz, –CH2), 2.62 (s, 2H, –CH2-C⚌O), 3.33 (s, 3H, O-CH3), 5.27 (brs, 1H, N—H), 5.82 (s, 1H, –CH), 6.14 (d, 1H, J = 1.3 Hz, Ar-H), 6.42–6.44 (m, 2H, Ar-H), 6.48 (d, 1H, J = 8.4 Hz, Ar-H), 6.56–6.59 (m, 2H, Ar-H), 6.91–6.93 (m, 1H, Ar-H), 8.79 (s, 1H, N—H).
2.3.2 3,3-dimethyl-11-(3-nitrophenyl)-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e][1,4]diazepin-1-one (Table 2, entry 9)
1H NMR (400 MHz, DMSO, ppm): δ:1.06 (s, 3H, –CH3), 1.11 (s, 3H, –CH3), 2.08 (ABq, 2H, J = 16.0 Hz, –CH2), 2.63 (s, 2H, –CH2-C⚌O), 5.79 (brs, 1H, N—H), 6.40 (s, 1H, –CH), 6.54 (d, 1H, J = 7.2 Hz, Ar-H), 6.57–6.67 (m, 2H, Ar-H), 6.95 (d, 1H, J = 1.5 Hz, Ar-H), 7.41 (t, 1H, J = 7.9 Hz, Ar-H), 7.52 (d, 1H, J = 7.7 Hz, Ar-H), 7.88 (d, 1H, J = 8.1 Hz, Ar-H), 7.97 (s, 1H, Ar-H), 8.95 (s, 1H, N—H).
2.3.3 11-(2-Chlorophenyl)-3,3-dimethyl-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e][1,4] diazepin-1-one (Table 2, entry 11)
1H NMR (400 MHz, DMSO, ppm): δ: 1.04 (s, 3H, –CH3), 1.09 (s, 3H, –CH3), 2.05 (ABq, 2H, J = 16.0 Hz, –CH2), 2.67 (s, 2H, –CH2-C⚌O), 5.57 (brs, 1H, N—H), 5.93 (s, 1H, –CH), 6.46 (d, 1H, J = 8.0 Hz, Ar-H), 6.63–6.68 (m, 2H, Ar-H), 6.75 (d,1H, J = 8.4 Hz, Ar-H), 7.02 (d, 1H, J = 1.5 Hz, Ar-H), 7.07 (d, 2H, J = 2.2 Hz, Ar-H), 7.51 (d, 1H, J = 2.1 Hz, Ar-H), 9.02 (s, 1H, N—H).
2.3.4 11-(2,4-Dichlorophenyl)-3,3-dimethyl-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e] [1,4]diazepin-1-one (Table 2, entry 12)
1H NMR (400 MHz, DMSO, ppm): δ: 1.04 (s, 3H, –CH3), 1.07 (s, 3H, –CH3), 2.06 (ABq, 2H, J = 16.0 Hz, –CH2), 2.63 (s, 2H, –CH2-C⚌O), 5.67 (brs, 1H, N—H), 5.95 (s, 1H, –CH), 6.49 (d, 1H, J = 1.5 Hz, Ar-H), 6.61–6.67 (m, 2H, Ar-H), 6.75 (d, 1H, J = 8.4 Hz, Ar-H), 7.05 (dd, 1H, 1J = 7.7, 2J = 1.5 Hz, Ar-H), 7.08 (d, 1H, 1J = 8.4, 2J = 2.2 Hz, Ar-H), 7.51 (d, 1H, J = 2.1 Hz, Ar-H), 9.05 (s, 1H, N—H).
2.4 Characterizations
Shimadzu spectrometer (8400 s, Kyoto, Japan) were used to register FT-IR spectra with KBr pellets in the 400–4000 cm−1 range. X-ray diffractometers (Philips) were used to determine crystallographic structure using Cu Kα radiation (λ = 1.54 Å). The morphology of nano powders was characterized by using field emission scanning electron microscopy (FESEM, MIRA III, TESCAN) and transmission electron microscopy (TEM, Zeiss, LEO 912AB (120 kV), Germany). In 77 K, the Autosorb-1 Quantachrome Sorptometer (USA) was used to analyze Brunauer-Emmett-Teller porosity and surface area. A 400 MHz spectrometer was used to capture 1H NMR spectra at ambient temperature in DMSO‑d6. ICP-AES (inductively coupled plasma atomic emission spectroscopy) was performed on Varian VISTA-PRO instrument. Thermo gravimetric analyses (TGA) were performed on a thermogravimetric/differential thermal analyzer (Netzsch- TGA 209 F1) by heating at 10 °C min−1 to 800 °C.
3 Results and discussion
The preparation route of Cu@PI-COF is shown in Schemes 2 and 3.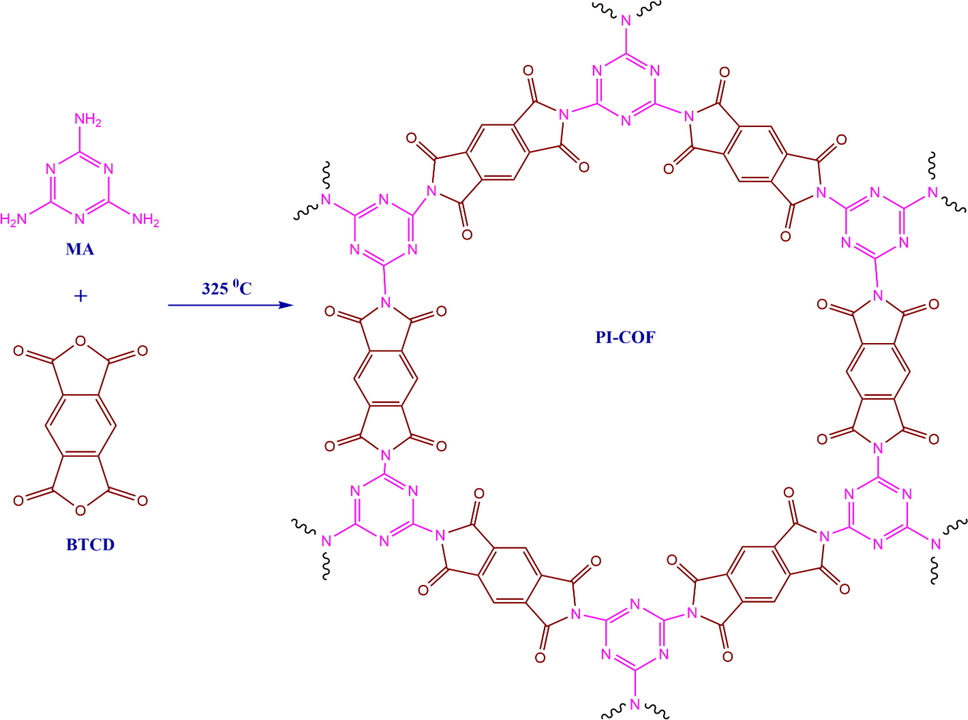
Synthesis of PI-COF.
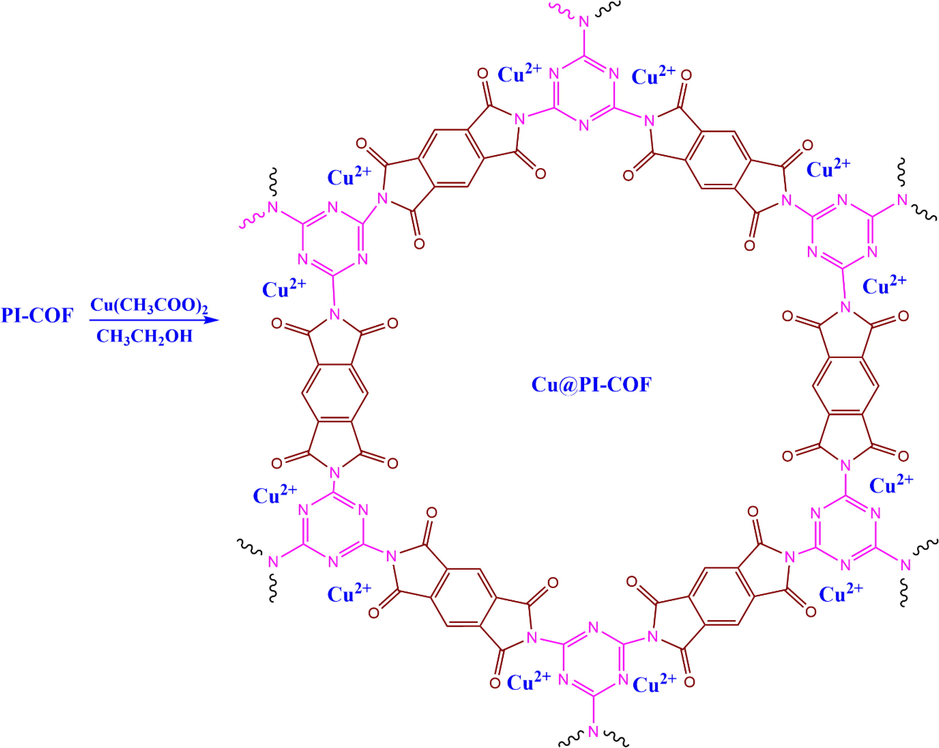
Cu@PI-COF catalysis platform illustration.
Characterization of the PI-COF structure has been done by FT-IR, XRD, and FESEM and TEM techniques. Figures and related interpretations are given in the Electronic Supporting Material (ESM), Figs S1-S3.
The stabilization of the catalyst is a significant factor for its practicable applications. TGA showed that Cu@PI-COF was intact up to 380 °C, implying good thermostability (Fig. 1).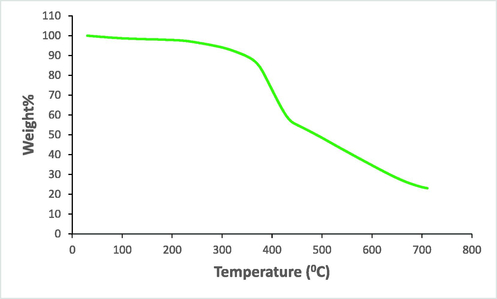
TGA plot of Cu@PI-COF.
The Fig. S4 illustrates N2 adsorption and desorption isotherms for the PI-COF and Cu@PI-COF composites. Pure PI-COFs have a specific surface area (SSA) of 43.90 m2/g and pore volumes of 0.035 cm3/g. Adsorption quantities of nitrogen gradually decrease after loading the active ingredient. Cu@PI-COF has SSA and pore volume of 30.73 m2/g and 0.029 cm3/g, respectively. Moreover, the pore sizes measured for PI-COF and Cu@PI-COF were 3.7 nm and 3.3 nm respectively (Fig. S5). Therefore, based on the above, a pore-blocking effect may occur because Cu(II) occupies the PI-COF pores (Xu and Prodanović 2018, Moeinpour, Soofivand et al. 2019).
After the characterization of Cu@PI-COF, its catalytic function in the multicomponent preparation of 1,5-benzodiazepines was evaluated. Optimizing the reaction conditions was the next step in synthesis of 1,5-benzodiazepines. Therefore, the catalytic efficiency was examined during the 1,5-benzodiazepines synthesis as model under different reaction conditions (microwave strength, catalyst dosage and time). To achieve this goal, reaction with three components in one pot consisting of 1,2-diaminobenzene (1 mmol,0.108 g), dimedone (1 mmol, 0.140 g) and benzaldehyde (1 mmol, 0.106 g) was chosen as the sample under solvent-free conditions (Scheme 1). The findings were tabulated in Table 1. The findings indicated that without the Cu@PI-COF the reaction did not go forward even after 30 min (Table 1, entry 1). The results showed that the optimal conditions were reached when the reaction was accomplished in the presence of 0.2 g Cu@PI-COF under solvent-free conditions and under microwave irradiation leading to corresponding benzodiazepine (3,3-dimethyl-11-phenyl-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e][1,4]diazepin-1-one) in 5 min and 96 % yield (Table 1, entry 5). Remarkably, the yield of the reaction did not show a significant change with respect to conversion by increasing the Cu@PI-COF dosage up to 0.5 g (Table 1, entries 6–7). If we reduce the Cu@PI-COF dose to 0.01 g, the yield also decreases (Table 1, entry 8). To show the performance of Cu(II) during the reaction, the performance of PI-COF in the reaction under optimal conditions was also studied. The findings represented that when PI-COF was used, no progression in the sample reaction was observed due to the absence of Cu(II), affirming that its presence is required for the catalysis of the reaction (Table 1, entry 12). Additionally, the sample reaction was performed with Cu(CH3COO)2 for 5 min under optimal conditions, yielding corresponding benzodiazepine (3,3-dimethyl-11-phenyl-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e][1,4]diazepin-1-one) with 33 % yield (Table 1, entry 13). The results demonstrate that Cu(CH3COO)2 exhibits negligible performance compared to Cu@PI-COF under optimized conditions. Solvents are not considered here due to the green chemistry design.
Entry
Catalyst (g)
Power (W)
Time (min.)
Yield (%)
1
–
180
30
0
2
Cu@PI-COF (0.2)
180
10
98
3
Cu@PI-COF (0.2)
300
10
88
4
Cu@PI-COF (0.2)
100
10
85
5
Cu@PI-COF (0.2)
180
5
96
6
Cu@PI-COF (0.3)
180
5
97
7
Cu@PI-COF (0.5)
180
5
97
8
Cu@PI-COF (0.01)
180
5
25
9
Cu@PI-COF (0.02)
180
5
38
10
Cu@PI-COF (0.05)
180
5
55
11
Cu@PI-COF (0.1)
180
5
78
12
PI-COF (0.2 g)
180
5
0
13
Cu(CH3COO)2 (0.2 g)
180
5
33
Entry
Ar
Product
Yield (%)a
Mp (°C)
Observed
Literature
1
C6H5
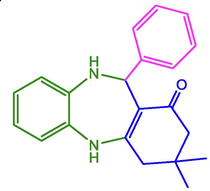
96
246–248
250–252 (Naeimi and Foroughi 2016)
2
4-Cl-C6H4
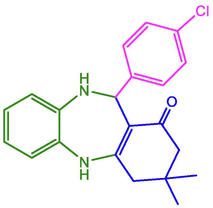
98
232–234
235–237 (Nasir, Ali et al. 2017)
3
4-NO2-C6H4
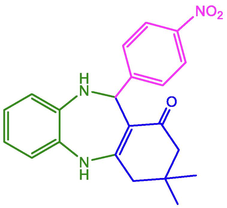
96
273–274
274–275 (Naeimi and Foroughi 2016)
4
4-OCH3-C6H4
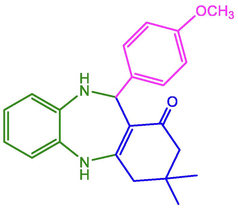
97
225–227
229–231 (Nasir, Ali et al. 2017)
5
4-CH3-C6H4
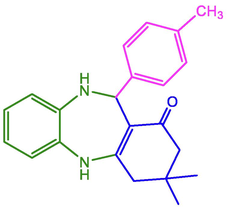
98
227–229
224–226 (Nasir, Ali et al. 2017)
6
2-OH-C6H4
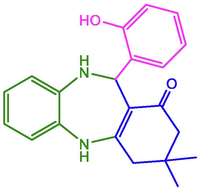
96
197–199
201–202 (Nasir, Ali et al. 2017)
7
2-OH-3-OCH3-C6H4
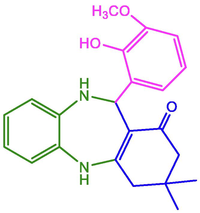
93
271–273
269–270 (Nasir, Ali et al. 2017)
8
2-Thienyl
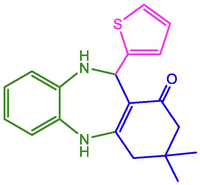
96
226–227
227–229 (Naeimi and Foroughi 2016)
9
3-NO2-C6H4
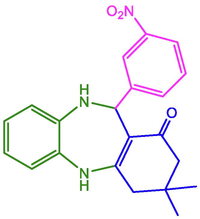
93
145–146
144–146 (Maleki and Kamalzare 2014)
10
2-NO2-C6H4
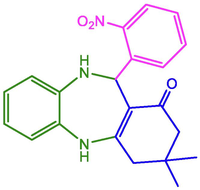
95
230–232
230–232 (Naeimi and Foroughi 2015)
11
2-Cl-C6H4
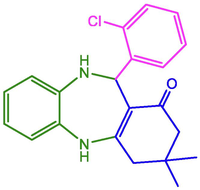
96
232–234
233–235 (Maleki and Kamalzare 2014)
12
2,4-Dichloro-C6H3
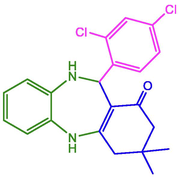
98
251–252
250–252 (Maleki and Kamalzare 2014)
13
4-OH-C6H4
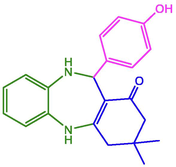
98
225–226
225–226 (Maleki and Kamalzare 2014)
14
4-Dimethylamino-C6H4
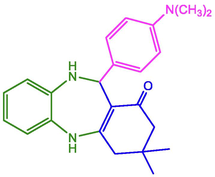
98
230–231
228–230 (Kolos, Yurchenko et al. 2004)
15
2-Furyl
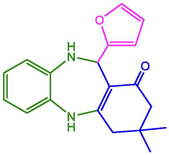
94
216–217
216–218 (Maleki and Kamalzare 2014)
16
C6H5
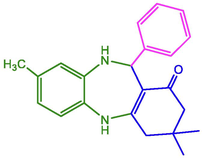
95
268–270
267–269 (Indalkar, Patil et al. 2017)
17
C6H5
94
270–272
270–272 (Esfandiari, Kareem Abbas et al. 2022)
After identifying the most suitable reaction conditions, we needed to assess the scope and efficacy of the reaction. In this respect, 1,2-diaminobenzene, dimedone and aromatic aldehydes were selected to carry out the reaction to give the related benzodiazepine derivatives and the outcomes are depicted in Table 2. Regarding the aromatic aldehydes comprising both electron-withdrawing and electron-donating substituents, they can be effectively transformed to benzodiazepines in high yields, as indicated in Table 2. Further, the optimized reaction protocol was also extendable to substituted o-phenylenediamines (Table 2, entries 16 and 17), resulted in good yields of the corresponding 4,7-disubstituted-1,5-benzodiazepines. As can be seen from the data in Table 2, this procedure can be carried out for all aromatic aldehydes. It can be stated that the reaction time was significantly shortened by microwave irradiation and the products were synthesized with the best efficiency, without producing by-products and requiring purification by column or flash chromatography. By comparing melting points and 1H NMR spectra with authentic samples, the products were identified.
A possible mechanism for the reaction is outlined in Scheme 4. The reaction undergoes a primary nucleophilic addition followed by water elimination to give intermediate A. The intermediate A is then condensed with aromatic aldehydes to yield B, which is then transformed into the benzodiazepine 4 by intramolecular [6 + 1] cyclization (Bennamane, Kaoua et al. 2008, Zohreh, Alizadeh et al. 2010).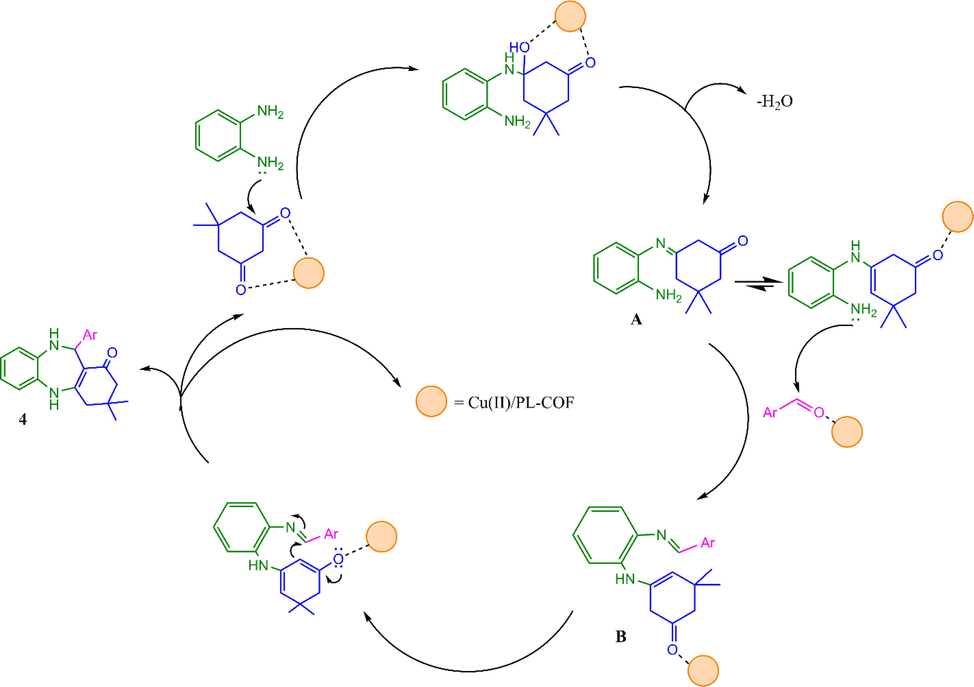
Plausible mechanism for Cu@PI-COF catalyzed synthesis of benzodiazepines.
The stability and recyclability of the Cu@PI-COF was confirmed in the sample reaction for the production of benzodiazepine 3,3-dimethyl-11-phenyl-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e][1,4]diazepin-1-one. After the reaction was complete, ethanol was added and the Cu@PI-COF was isolated by filtration, washed with acetone, then desiccated and reused for the next experiment without appreciable loss of its catalytic performance. As shown in Fig. 2, the catalyst can still be used after 5 cycles of continuous power. ICP-AES analysis indicated that the extent of Cu(II) leached into the reaction media is very small (0.5 ppm).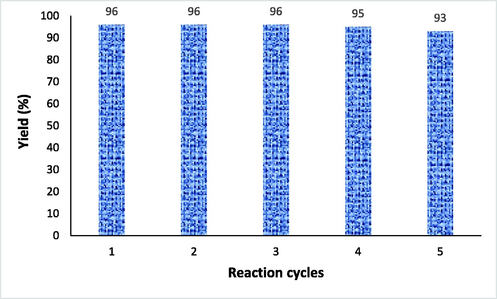
Recyclability of Cu@PI-COF in the sample reaction.
The TEM and SEM pictures of the fresh and reused catalysts showed that only minor morphological changes happened (Fig. 3a and 3b).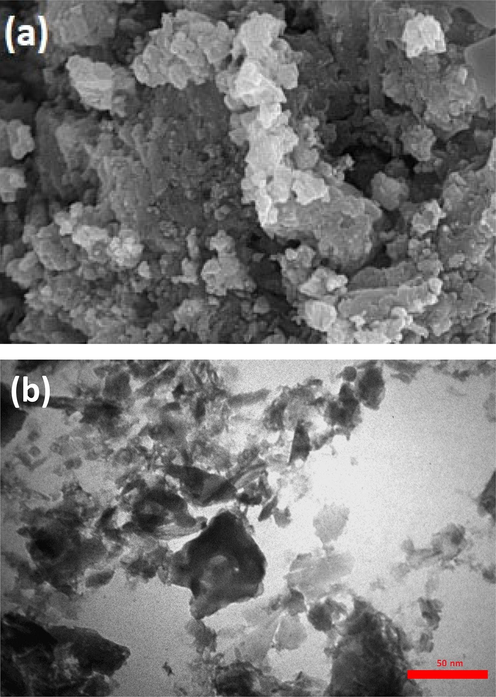
SEM (a) and TEM (b) images of recycled Cu@PI-COF catalyst.
Table 3 compares the efficiency of the present method for the production 1,5-benzodiazepine derivatives is compared with other published papers. As illustrated in Table 3, the Cu@PI-COF catalyst has the best efficiency in the shortest time compared to the reported processes in the presence of different catalysts.
Entry
Catalyst
Time (min)
Yield (%)a
Ref.
1
ZnS
19
85
(Naeimi and Foroughi 2015)
2
Fe3O4@chitosan
120
91
(Maleki and Kamalzare 2014)
3
p-toluenesulfonic acid
180
70
(Tonkikh, Strakovs et al. 2004)
4
CeO2/CuO@N-GQDs@NH2 nanocomposite
30
94
(Esfandiari, Kareem Abbas et al. 2022)
5
Fe(III)-nicotine-based organocatalyst
30
95
(Nasr-Esfahani, Mohammadpoor-Baltork et al. 2019)
6
Silica supported NiO
10
98
(Nasir, Ali et al. 2017)
7
Cu@PI-COF
5
98
This study
4 Conclusion
Briefly, the use of a Cu@PI-COF catalyst under microwave irradiation facilitates the preparation of 1,5-benzodiazepines through condensation between 1,2-diaminobenzene, dimedone, and various aromatic aldehydes. It should be noted that this method is very simple and effective and is used to synthesize derivatives of this group of compounds. This new and effective one-pot three-component procedure not only features the use of microwaves and a substantial product yield, but also offers mild reaction conditions, high purity, shorter reaction times, ease of operation, reutilization of heterogeneous nano catalysts, easy workup and high atom economy. The association of microwave irradiation and heterogeneous catalysis has enabled the future improvement of effective, rapid, and eco-friendly synthetic methods. The reutilization of Cu@PI-COF was high and it could be reutilized 5 times without significantly decreasing its first activity. We expect that this synthetic manner will provide better scope for the preparation of benzodiazepine analogs and will be a more viable replacement for the other available protocols.
Acknowledgement
This study would not have been possible without the financial support of the Islamic Azad University Bandar Abbas Branch.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A simple and new method for the synthesis of 1, 5-benzodiazepine derivatives on a solid surface. Tetrahedron Lett.. 2001;42:1127-1129.
- [Google Scholar]
- Synthesis of new amino-1, 5-benzodiazepine and benzotriazole derivatives from dimedone. Org. Commun.. 2008;1:62-68.
- [Google Scholar]
- Application of covalent organic framework materials as heterogeneous ligands in organic synthesis. Chin. J. Org. Chem.. 2021;41:3826.
- [Google Scholar]
- Copper-decorated covalent organic framework as a heterogeneous photocatalyst for phosphorylation of terminal alkynes. Green Chem.. 2022;24:4071-4081.
- [Google Scholar]
- Palladium anchored on a covalent organic framework as a heterogeneous catalyst for phosphorylation of aryl bromides. Applied Organomet. Chem.. 2022;36:e6480.
- [Google Scholar]
- Ytterbium triflate promoted coupling reaction between aryl alkynes and aldehydes. Synlett. 2003;2003:0552-0554.
- [Google Scholar]
- Synthesis of benzodiazepines promoted by CeO2/CuO@ nitrogen graphene quantum dots@NH2 nanocomposite. Polycycl. Aromat. Compd.. 2022;42:1235-1248.
- [Google Scholar]
- 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc.. 2015;137:8352-8355.
- [Google Scholar]
- Copper immobilized at a covalent organic framework: an efficient and recyclable heterogeneous catalyst for the Chan-Lam coupling reaction of aryl boronic acids and amines. Green Chem.. 2018;20:4891-4900.
- [Google Scholar]
- Syntheses of heterocyclic compounds. part XXIX. substituted 2, 3-dihydro-1 H-1, 5-benzodiazepines. J. Chem. Soc. Perkin Trans.. 1974;I:2657-2661.
- [Google Scholar]
- An efficient, environmentally benign, and solvent-free protocol for the synthesis of 4-substituted 1, 5-benzodiazepines catalyzed by reusable sulfated polyborate. Tetrahedron Lett.. 2017;58:4496-4502.
- [Google Scholar]
- Cu(II)-containing nano-silica triazine based dendrimer: a green and proficient catalyst for the synthesis of propargylamines. Polycycl. Aromat. Compd.. 2020;42:3142-3156.
- [Google Scholar]
- Molybdenum oxide supported on silica (MoO3/SiO2): an efficient and reusable catalyst for the synthesis of 1, 8-dioxodecahydroacridines under solvent-free conditions. J. Mex. Chem. Soc.. 2015;59:29-35.
- [Google Scholar]
- Investigation of the products of interaction of cyclic diketones with nitrogen-containing 1, 4-binucleophiles. Chem. Heterocycl. Compd.. 2004;40:1550-1559.
- [Google Scholar]
- Ferrocene anchored activated carbon as a versatile catalyst for the synthesis of 1, 5-benzodiazepines via one-pot environmentally benign conditions. RSC Adv.. 2022;12:14740-14756.
- [Google Scholar]
- An efficient synthesis of benzodiazepine derivatives via a one-pot, three-component reaction accelerated by a chitosan-supported superparamagnetic iron oxide nanocomposite. Tetrahedron Lett.. 2014;55:6931-6934.
- [Google Scholar]
- Solid support based synthesis of 1, 5-benzodiazepines: a mini review. Synth. Commun.. 2022;52:481-503.
- [Google Scholar]
- Multicomponent preparation of 1-amidoalkyl-2-naphthols using silica-supported molybdenum oxide (MoO3/SiO2) as a mild and recyclable catalyst. Res. Chem. Intermed.. 2014;40:3145-3152.
- [Google Scholar]
- An efficient one-pot synthesis of 1, 8-dioxodecahydroacridines using silica-supported polyphosphoric acid (PPA-SiO2) under solvent-free conditions. E-J. Chem.. 2012;9:504-509.
- [Google Scholar]
- Controlled release of losartan from acid-and heat-treated halloysite nanotubes. Med. Chem. Res.. 2019;28:160-168.
- [Google Scholar]
- Cu (II)/triazine-based dendrimer as an efficacious recoverable nano-catalyst for CO2 fixation under solvent-free conditions. Catal. Lett.. 2022;152:3679-3690.
- [Google Scholar]
- Investigation into the catalytic performance of Cu(II) supported graphene quantum dots modified NiFe2O4 as a proficient nano-catalyst in the synthesis of propargylasmines. Catal. Lett.. 2021;151:1444-1455.
- [Google Scholar]
- New synthesis of dihydro-and tetrahydro-1, 5-benzodiazepines by reductive condensation of o-phenylenediamine and ketones in the presence of sodium borohydride. Heterocycles (Sendai). 1986;24:135-139.
- [Google Scholar]
- ZnS nanoparticles as an efficient recyclable heterogeneous catalyst for one-pot synthesis of 4-substituted-1, 5-benzodiazepines. New J. Chem.. 2015;39:1228-1236.
- [Google Scholar]
- Facile three-component preparation of benzodiazepine derivatives catalyzed by zinc sulfide nanoparticles via grinding method. Res. Chem. Intermed.. 2016;42:3999-4020.
- [Google Scholar]
- Silica-supported NiO nanocomposites prepared via a sol–gel technique and their excellent catalytic performance for one-pot multicomponent synthesis of benzodiazepine derivatives under microwave irradiation. New J. Chem.. 2017;41:5893-5903.
- [Google Scholar]
- Preparation and application of a new supported nicotine-based organocatalyst for synthesis of various 1, 5-benzodiazepines. Catal. Lett.. 2019;149:1057-1066.
- [Google Scholar]
- An efficient method for the synthesis of 1, 5-benzodiazepine derivatives under microwave irradiation without solvent. Tetrahedron Lett.. 2002;43:1755-1758.
- [Google Scholar]
- Nanomagnetically modified ferric hydrogen sulfate (NiFe2O4@SiO2-FHS): a reusable green catalyst for the synthesis of highly functionalized piperidine derivatives. J. Iran. Chem. Soc.. 2015;12:839-844.
- [Google Scholar]
- Benzodiazepines: their use either as essential medicines or as toxics substances. Toxics. 2021;9:25.
- [Google Scholar]
- A green and sustainable approach for the synthesis of 1, 5-benzodiazepines and spirooxindoles in one-pot using a MIL-101 (Cr) metal–organic framework as a reusable catalyst. New J. Chem.. 2021;45:19553-19564.
- [Google Scholar]
- Microwave-assisted green synthesis of 1, 5 benzodiazepines using Cu(II)-clay nanocatalyst. J. King Saud Univ. Sci.. 2020;32:979-985.
- [Google Scholar]
- Multicomponent catalytic synthesis of 1, 5-benzodiazepines: an update. Mini. Rev. Org. Chem.. 2020;17:465-484.
- [Google Scholar]
- 11-Aryl-3, 3-dimethyl-7-and 7, 8-Substituted 1, 2, 3, 4, 10, 11-Hexahydro-5H-dibenzo [b, e]-1, 4-diazepin-1-ones. Chem. Heterocycl. Compd.. 2004;40:949-955.
- [Google Scholar]
- Ceric ammonium nitrate (CAN) promoted efficient synthesis of 1, 5-benzodiazepine derivatives. Synlett. 2006;2006:1009-1014.
- [Google Scholar]
- Self-exfoliating double-emission N-doped carbon dots in covalent organic frameworks for ratiometric fluorescence “Off–On” Cu2+ detection. ACS Appl. Nano Mater.. 2022;5:1339-1347.
- [Google Scholar]
- Preparation of two new polyimide bond linked porous covalent organic frameworks and their fluorescence sensing application for sensitive and selective determination of Fe 3+. New J. Chem.. 2017;41:14272-14278.
- [Google Scholar]
- Cerium-enriched magnetic/recyclable dual-acid nanocatalyst for one-pot three-component synthesis of functionalized 1,5-benzodiazepines. Mater. Chem. Phys.. 2022;275:125242
- [Google Scholar]
- Effect of pore geometry on nitrogen sorption isotherms interpretation: a pore network modeling study. Fuel. 2018;225:243-255.
- [Google Scholar]
- Covalent organic framework stabilized CdS nanoparticles as efficient visible-light-driven photocatalysts for selective oxidation of aromatic alcohols. Chin. Chem. Lett.. 2021;32:2207-2211.
- [Google Scholar]
- Novel approach to 1, 5-benzodiazepine-2-ones containing peptoid backbone via one-pot diketene-based Ugi-4CR. J. Comb. Chem.. 2010;12:497-502.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104694.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







