Translate this page into:
Cupressus arizonica fruit essential oil: A novel green inhibitor for acid corrosion of carbon steel
⁎Corresponding authors. ercleehs@hanyang.ac.kr (Han-Seung Lee), hlgaz@hanyang.ac.kr (Hassane Lgaz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Natural-based corrosion inhibitors have gained great research interest thanks to their low cost and higher performance. The Cupressus arizonica fruit essential oil (CAFEO) has a higher extraction yield than leaves; however, it has less antibacterial and antifungal activities. The three main components in the CAFEO were α-pinene (51.07%), myrcene (17.92%), and limonene (9.66%). Essential oils with a higher percentage of α-pinene were found to have outstanding corrosion inhibition properties. Therefore, herein, the CAFEO was investigated as a green corrosion inhibitor for carbon steel (CS) in 1.0 mol/L HCl using electrochemical, i.e., potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS), and scanning electron microscope (SEM) techniques. The experimental results revealed that CAFEO successfully inhibited the carbon steel corrosion in 1.0 mol/L HCl solution. Results from PDP indicated that the inhibitor had a mixed-type effect with a predominance cathodic character. EIS data showed that the charge transfer resistance of the CS electrode increased from 20.9 Ω cm2 in blank solution to 294.5 Ω cm2 in HCl solution inhibited with 0.5 g/L of CAFEO at 298 K, leading to a significant decrease in the double layer capacitance values and an inhibition efficiency (η%) of 93%. The high temperatures showed a negative effect on the corrosion inhibition efficiency of the tested inhibitor. At 323 K, the η% of CAFEO decreased to 77%. Besides, SEM images showed that the inhibitor formed a protective barrier against acid attack, preventing carbon steel from corrosion. Theoretical calculations by Density Functional Theory (DFT) were performed to investigate the reactivity of the three main components of CAFEO.
Keywords
Corrosion inhibitor
Green inhibitor
Cupressus arizonica
Essential oil
DFT
SEM
1 Introduction
The acidic solutions used in several industrial applications cause damage to metallic materials by electrochemical and chemical reactions (Bhrara et al., 2008; Chami et al., 2015; Dehghani et al., 2019). They are the widely used minerals for acid treatment (acidizing) in oil and gas production to improve wells' productivity (stimulation). Their use results in a corrosion phenomenon, which leads to a high economic loss. Numerous techniques are available for protecting metals from corrosion ranging from material selection and coatings to galvanization and corrosion inhibitors (Cherrad et al., 2020). The use of inhibitors is one of the most cost-effective and practical choices to prevent the degradation of metal surfaces in an acidic medium (Benhiba et al., 2021; Benzbiria et al., 2021; Berrissoul et al., 2020a; Bouoidina et al., 2019). Corrosion inhibitors are chemical substances that minimize the dissolution of the metal surfaces when present in a corrosive medium at optimized concentrations.
Most of the inorganic inhibitors and part of synthetic inhibitors, acting as good corrosion inhibitors, are vastly toxic to the environment (Jamil et al., 2018; Manssouri et al., 2020a). Thus, their use is now restricted in most applications. This fact challenges researchers to search for green and eco-friendly alternatives. Among these alternatives, plant extracts and oils are considered a winning card thanks to their green nature, low cost, availability, and most importantly, their excellent anticorrosion proprieties (Abdel-Gaber et al., 2020; Pal et al., 2019; Subekti et al., 2020). For instance, most plant essential oils possess several biological activities such as antioxidant, anti-inflammatory, and antibacterial (Abdallah et al., 2021; Berrissoul et al., 2020b; Manssouri et al., 2020b). Another factor that attracts the attention of corrosion researchers to plant-based inhibitors is the presence of complex organic species based on nitrogen, oxygen, and sulfur atoms in their composition. These elements are electro-active groups representing major adsorption centers, which mimic the most effective synthetic corrosion inhibitors (Cherrad et al., 2020). In most cases, the presence of several components that possess heteroatoms and several reactive sites in a plant extract composition suggests its potential application as a corrosion inhibitor (Ashassi-Sorkhabi et al., 2005).
Significant scientific studies have shown that essential oils can serve as good corrosion inhibitors in acidic environments. In our recent study, we have reported the essential oil of Cupressus arizonica (CAEO) leaves as an efficient corrosion inhibitor for carbon steel in acidic solutions (1.0 mol/L HCl) (Cherrad et al., 2020). The results of the polarization curves have shown that this oil is a mixed-type inhibitor. Its inhibitory efficiency reached 95% at 500 ppm because of the inhibitive layer formation on the carbon steel surface. In the same context, Manssouri et al. (Manssouri et al., 2020a), studied Santolina pectinata oil as an effective corrosion inhibitor in 1.0 mol/L HCl. They found that the oil can effectively protect the carbon steel corrosion via inhibiting the charge transfer, leading to an inhibition efficiency of 85% at a concentration of 3 g/L. The polarization curves showed a mixed-type effect. In addition, Nutmeg oil has been confirmed as an effective inhibitor for carbon steel corrosion in an acid medium (1.0 mol/L HCl) through electrochemical studies. Results were reported by Abdellah et al. (Abdallah et al., 2021). They approved that the increasing of concentration up to 500 ppm raises the inhibition efficiency to 94.73%. The polarization measurements suggested that nutmeg oil behaved as a mixed-type inhibitor.
There are a wide variety of phytochemicals identified in plant extracts, and the common point between those chemicals in the presence of several reactive sites in the form of heteroatoms, double bonds, long carbon chains, and aromatic rings (Bhardwaj et al., 2021; Bilgiç, 2021; Verma et al., 2018). This complex mixture of molecules constitutes a corrosion inhibitor formulation with outstanding anti-corrosion properties. A recent review by Hossain et al. (Hossain et al., 2020) nicely addressed the remarkable advantages of essential oils as powerful corrosion and biocorrosion inhibitors.
On the other hand, it is of paramount importance for life on Earth to preserve plants given their actual or potential usefulness as renewable natural resources. Many aspects of human activity are dependent on plants. According to World Health Organization (WHO), millions of people depend on plants for their traditional medicine, especially in developing countries (Haq, 2004). However, ecologists and conservationists believe that hundreds of wild species are disappearing at an alarming rate (Zahran, 2010). Therefore, preserving plants, especially endemic species, is a worldwide responsibility. One of the main ways to preserve endemic species is by integrating them into the economic cycle.
The Cupressus arizonica, which belongs to the Cupressus family, is a versatile, fast-growing evergreen tree. It is a North American native ornamental plant. Several efforts have been made as a contribution to the valorization of this plant (Cesur et al., 2021; Chéraif et al., 2007; Chichiriccò and Pacini, 2008; Shahali et al., 2007). The antibacterial and antifungal activities of Moroccan Cupressus Arizonica Greene essential oils have been reported by Bouksaim et al. (Bouksaim et al., 2018). Authors found that Cupressus arizonica fruits essential oil did not affect mold growth. However, Cupressus arizonica fruits essential oil extraction yield is significantly higher than that of leaves. Studies in our laboratory showed that CAFEO has 1.29% extraction yield compared to 0.85% of that from leaves. Moreover, as it is shown in the present work, the essential oil from the fruit has a high level of α-Pinene (51.07%) compared to its small amount in fruits EO (9.39%). Considering these facts and the lack of a previous study on its corrosion inhibition properties, it would be important to evaluate the potential application of CAFEO in the corrosion protection of metals as a phase of the valorization cycle of the whole plant.
Considering the facts mentioned above and the encouraging results from Cupressus arizonica leaves, herein, we report the corrosion inhibition effect of Cupressus arizonica fruit essential oil (CAFEO) on carbon steel in 1.0 mol/L HCl using potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS) measurements along with surface characterization using SEM. The effect of temperatures has also been studied. Although a concentrated HCl solution (15–20 wt%) is used in the acidizing process, a low concentration of 1.0 mol/L is selected in the present wok to assess the effectiveness of tested corrosion inhibitors. This HCl concentration is widely used as a reference for multiple academic researches prior any field tests (Haque et al., 2018; Kowsari et al., 2016; Sanaei et al., 2019). Furthermore, DFT calculations were carried out to investigate the chemical reactivity of the three main components of CAFEO.
2 Materials and methods
2.1 Plant collection and essential oil extraction
The Cupressus arizonica fruits were collected in March 2017 from the middle Atlas of Morocco, Azrou city. There are several techniques for extracting essential oils. The choice of the technique is made according to the physico-chemical characteristics of the oil to be extracted and the nature of the plant material. The hydrodistillation of the fresh or dry plant is one of the most used techniques (Pharmacognosie, 1999). This method is generally suitable for essential oils with heat-resistant chemical constituents. In the present work, 200 g of plant material was immersed in 1 L of distilled water and subject to a hydrodistillation for about 4 h using a Clevenger type apparatus in accordance with the recommendations of the European Pharmacopoeia 7.0 (Elena, 2012). The obtained essential oil was dried over anhydrous sodium sulfate and then stored in the refrigerator at 4 °C in dark glass bottles until use. The yield of the essential oil (%, v/w) was determined and expressed on a dry weight basis using the following formula:
Components’ identification was carried out following the procedure described in our recent work (Cherrad et al., 2020). Table 1 lists the name and percentage of CAFEO components, while Fig. 1 represents the molecules of major components.
KI (Kováts indices)
Compounds
Content (%)
935
α-Pinene
51.07
950
Camphene
0.61
977
β-pinene
4.92
989
Myrcene
17.92
1008
δ-3-carene
2.02
1021
P-cymene
0.47
1028
Limonene
9.66
1041
(E)-β-Ocimene
1.31
1054
γ-Terpinene
0.29
1084
Terpinolene
2.6
1091
p-cymenene
0.33
1107
Cis-camphenol
0.33
1139
Cis-hydrate pinene
0.58
1144
Camphor
0.64
1159
Trans- β- terpineol
0.41
1176
Terpinen-4-ol
1.37
1190
α-terpineol
2.38
1219
cis-Sabinene hydrate acetate
1.25
1237
E-ocimenone
1.4
1347
α-cubeben
0.34
The molecular structure of major components presents in CAFEO.
2.2 Materials and solutions
The chemical composition (in wt. %) of carbon steel samples used in this work is given in Table 2. The carbon steel samples were polished with abrasive paper of grain size from #400 to #1600, followed by rinsing with distilled water, then degreasing with ethanol and rinsing with distilled water, finally drying under airflow. An exposed area of 1 cm2 was allowed to interact with aggressive solutions.
Elements
C
Si
Mn
Cr
Mo
Ni
Al
Cu
Co
V
W
Fe
wt. %
0.11
0.24
0.47
0.12
0.02
0.1
0.03
0.14
< 0.0012
< 0.003
0.06
Balance
The test solution (1.0 mol/L HCl) was made by the dilution of 37% analytical grade HCl (Sigma Aldrich, France) using distilled water. The essential oil was dissolved in pure ethanol; adding the oil directly to the acid solution by gentle mechanical agitation for 1 h. Different concentrations of CAFEO were used for testing its corrosion protection abilities. The final concentration range were: 0.0125 g/L to 0.5 g/L.
2.3 Weight loss measurements
Carbon steel samples were prepared according to ASTM G1-03 standard procedures [35] for weight loss tests. The prepared and weighted carbon steel specimens were suspended and dipped in acidic solution (1.0 M HCl) with several concentrations of CAFEO for 24 h at 298 K. After the required time, the samples were taken, washed using bidistilled water and acetone, and reweighted. The obtained average weight loss (ΔW) values were used to calculate the corrosion rate (CR, in mg cm-2h−1) and inhibition efficiencies (ηW %) using the following formulas [36, 37]:
2.4 Electrochemical measurements
Electrochemical experiments were performed using a potentiostat PGZ301 (Radiometer Analytical, Lyon, France) piloted via VoltaMaster Ver. 4. This potentiostat was equipped with a pyrex cell connected to the thermostats with a double wall. The working, counter, and reference electrodes were carbon steel, standard calomel, and platinum, respectively. The potential was stabilized at open circuit potential (OCP) for 30 min before the measurements. PDP curves were plotted at 1 mV/s of scan rate from −700 to −300 mV/SCE. EIS tests were carried out using a 10 mV of amplitude AC signal, over a frequency domain from 100 kHz to 10 mHz. The impedance diagrams were fitted using EC-Lab software.
2.5 SEM analysis
Carbon steel samples used for surface characterization were treated as described in Section 2.2. Samples were exposed to inhibited and uninhibited solutions for 24 h immersion time at 298 K. SEM analysis was performed using JEOL-Model JSM-IT 100 with the acceleration energy of 20 kV.
2.6 Theoretical calculations
The geometry optimization and frontier molecular orbitals of the main components of CAFEO were determined using Dmol3 model implemented in Material Studio software. The Generalized Gradient Approximation (GGA) with double numerical basis sets plus polarization (DNP) (Perdew et al., 1996) and COSMO solvation model (Klamt and Schüürmann, 1993) were used for all calculations. All other parameters correspond to “fine” quality in Dmol3 code.
3 Results and discussion
3.1 Weight loss measurements
Weight loss is a commonly used chemical method to evaluate the corrosion rate of metals and corrosion inhibition performance of inhibitors. Weight loss tests are carried out on carbon steel samples in the absence and presence of investigated essential oil. Weight loss data are listed in Table 3. By inspecting the results in Table 3, we can notice a significant decrease in the corrosion rate of carbon steel samples exposed to inhibited HCl solutions compared to blank tests. It highlights the concentration-dependence of inhibitor’s effect on carbon steel corrosion process and the mitigating abilities of tested CAFEO. At higher concentration of 0.5 g/L, the corrosion rate significantly decreased to 0.037 mg cm-2h−1 compared to 0.625 mg cm-2h−1 in blank conditions. At 0.5 g/L, the inhibition efficiency reaches 94%, while it decreases to 83% at 0.025 g/L of CAFEO. Results show that inhibiting HCl solutions, even with low concentrations of CAFEO, induces a lower corrosion rate. This is mainly due to the effective adsorption of CAFEO’s components on steel surface, creating a protective barrier that prevents corrosion initiation.
[CAFEO](g/L)
CR
(mg cm-2h−1)
(%)
Blank
0.625 ± 0.008
–
0.5
0.037 ± 0.003
94
0.1
0.056 ± 0.005
91
0.05
0.080 ± 0.004
87
0.025
0.105 ± 0.007
83
3.2 Potentiodynamic polarization curves
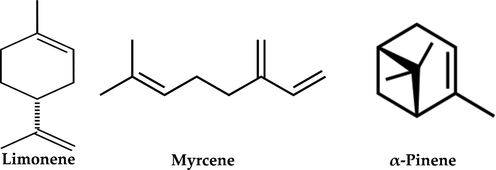
The OCP curves of the carbon steel samples in 1.0 mol/L HCl solutions in the absence and presence of 0.5, 0.05, and 0.125 g/L of CAFEO are shown in Fig. 2(a). With the immersion time gone on, all OCP curves became stable. The addition of CAFEO to HCl solutions shifts the potential slightly to the cathodic region while promoting a better stabilization of the OCP over time. It might indicate that investigated inhibitor has a more cathodic effect on the corrosion process.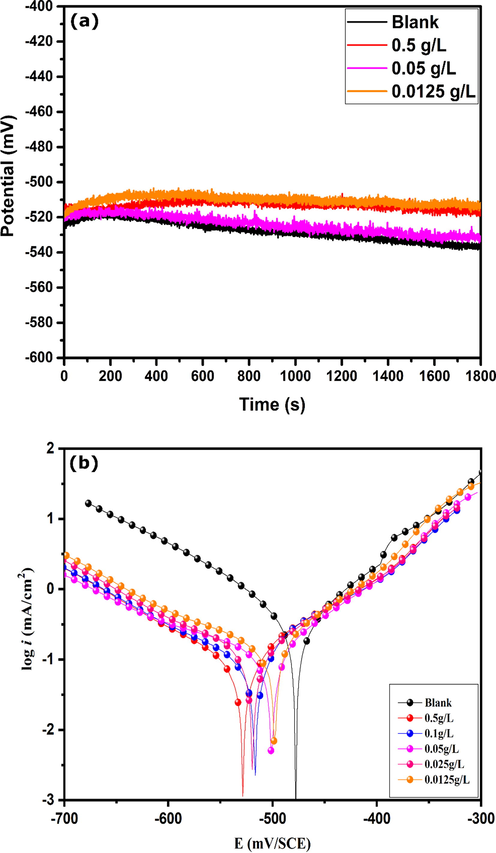
Open circuit potential curves (a) and Tafel polarization curves (b) of carbon steel without and with various concentrations of CAFEO in 1.0 mol/L HCl.
The polarization curves of carbon steel in 1.0 mol/L HCl solution in the absence and presence of various concentrations of CAFEO are shown in Fig. 2(b). The corrosion current density (icorr), corrosion potential (Ecorr), cathodic (βc) and anodic (βa) Tafel line slopes are obtained from the Tafel plots. Table 4 shows all corrosion kinetic parameters, including η% of the CAFEO inhibitor calculated from PDP measurements.
[CAFEO]
(g/L)
-Ecorra
(mV/SCE)
icorrb
(µA/cm2)
-βcc (mV/dec)
βad
(mV/dec)
η(%)
Blank
474.83
996
112.3
83.9
0.5
527.17
76
120.3
95.8
92
0.1
514.56
95
141.1
91.7
90
0.05
499.19
104
190.4
90.7
89
0.025
516.37
136
144.8
108.8
86
0.0125
494.82
145
162.7
80.7
85
From the analysis of PDP curves, it can be observed that both corrosion reactions, i.e., anodic and cathodic, are changed after adding the inhibitor to HCl medium. However, the cathodic branch is greatly affected by inhibitor addition. Shifting the potentiodynamic polarization curves to the E = 0 can allow a better comparison and a more distinguishable anodic and cathodic behavior. The curves shifted to E = 0 are shown in Figure S1 (Supplementary material). It can be noticed that the addition of different concentrations of tested oil decreases the current densities of both anodic and cathodic curves compared to that of blank solution. However, the inhibitor's addition to the blank solution has a more pronounced effect on the cathodic branches. It can be concluded that the presence of inhibitor has a mixed-type impact, decreasing the corrosion rate of both anodic and cathodic corrosion reactions, but with a predominance cathodic effect (Shahini et al., 2021). Besides, cathodic polarization curves indicate a wide range of linearity, suggesting that Tafel's law is well supported in the cathodic region, and the mechanism of hydrogen reduction is controlled by the kinetics of pure activation (charge transfer) (Bammou et al., 2011). On the other hand, the shape of polarization curves is similar in the absence and presence of inhibitor, suggesting that the tested oil inhibited the steel corrosion by first adsorbing on the metal surface and then blocking its reaction sites without affecting the mechanism of anodic and cathodic reactions.
Results in Table 4 show that in the presence of different concentrations of CAFEO, the values of anodic (βa) and cathodic (βc) Tafel slopes are slightly changed, suggesting that the inhibitory action of CAFEO occurred by blocking the cathode sites present on the surface of the metal and decrease the rate of steel dissolution (Rekkab et al., 2012).
The corrosion inhibition performance of tested oil can be determined from PDP curves using the following equation (Znini et al., 2011):
As shown in Table 4, the increasing inhibitor concentration causes a reduction in the icorr values, consequently increasing the inhibitory efficiency. The latter reaches a value of 92%, for 0.5 g/L concentration. The inhibitor shows good corrosion protection even at a low concentration of 0.0125 g/L (η(%) = 85%). This inhibitory action is due primarily to the adsorption of several organic molecules present in the oil composition (Abdullah Dar, 2011).
3.3 EIS measurements
3.3.1 Effect of inhibitor concentration
Electrochemical impedance spectroscopy is a useful non-destructive technique to characterize the corrosion inhibition properties of an inhibitive system (Chugh et al., 2020; Kumar et al., 2017). Nyquist diagrams of carbon steel in 1.0 mol/L HCl in the absence and presence of different concentrations of CAFEO are shown in Fig. 3. Nyquist diagrams of carbon steel in 1.0 mol/L HCl show a single semicircle; its diameter increases with increasing concentrations of the inhibitor. The single semicircle indicates that the process at the electrode surface has one relaxation time constant (Aslam et al., 2019). It is also clear that these semicircles are imperfect due to the frequency dispersion effect in solid electrodes as a result of the heterogeneous nature of the electrode surface (Erami et al., 2019; Liao et al., 2020; Orazem and Tribollet, 2008).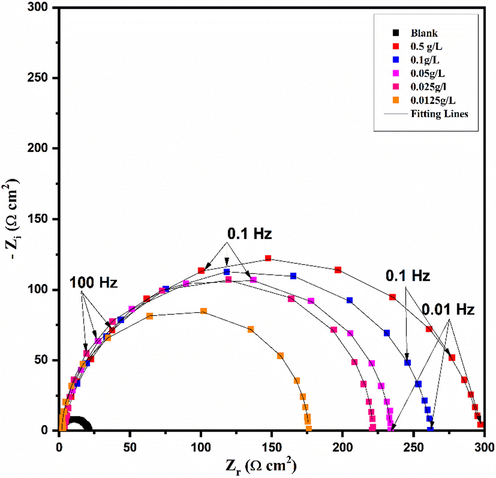
EIS plots of carbon steel in absence and presence of different concentrations of CAFEO at 298 K.
The equivalent electrical circuit used to simulate and fit the experimental EIS results is shown in Fig. 4. It consists of solution resistance (Rs), polarization resistance (Rp), and constant phase element (CPE), which is used instead of the pure capacitor to account for non-ideality of the interface. There is a relationship that correlates CPE with impedance by the following equation (Macdonald and Barsoukov, 2005):
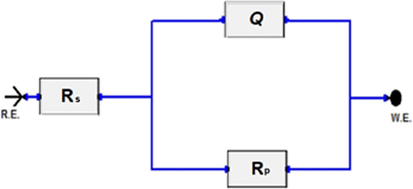
The electrical equivalent circuit used to fit EIS data.
The Ceff’ dl effective capacity can be calculated from the CPE parameters when the time-constant dispersion is induced by local in homogeneities along the interface using the following equation (Brug et al., 1984):
The protective ability of CAFEO inhibitor in term of the inhibition efficiency is calculated using Rp as follows:
The electrochemical parameters extracted from EIS fitting are represented in Table 5 and the corresponding inhibition efficiency. Analysis of Table 5 shows that the values of Rp increase by the addition of inhibitor, which is further raised by raising the inhibitor concentrations. This notices an increased resistance against charge transfer, thus reducing the corrosion rate. Alternatively, the values of Ceff, dl exhibited a declining trend with an increase in the inhibitor concentrations. This decrease suggests the modification of the double-layer behavior. It is reported that a lower effective capacitance value indicates a higher thickness of the barrier film formed on the steel surface (Hirschorn et al., 2010). Therefore, it can be concluded from the results in Table 5 that, with the increase in inhibitor's concentration, the thickness of the inhibitor film formed by adsorption of oil’s molecules on the steel surface becomes higher, which signifies higher corrosion protection. The inhibitor performance can be further measured by determining the inhibition efficiency, as represented in Table 5. The inhibitory efficiency rises by raising the inhibitor concentrations. An excellent inhibition efficiency is obtained at 0.5 g/L, which equals 93%. The inhibitory efficiencies obtained by the two electrochemical methods are in good agreement.
[CAFEO]
(g/L)
Rsa
(Ohm cm2)
Rpb
(Ohm cm2)
nc
Qd 10-4
(Sn Ohm−1 cm−2)
Ceff, dl
(µF cm−2)
η(%)
Blank
0.11
20.9
0.848
5.17
89.5
0.5
0.632
294.5
0.878
0.60
14.5
93
0.1
0.867
258.4
0.918
0.64
26.6
92
0.05
0.894
230.3
0.959
0.70
46.2
91
0.025
0.769
218
0.989
0.72
64.5
90
0.0125
0.469
173.9
0.986
0.83
71.8
88
3.3.2 Effect of temperature
EIS curves for carbon steel in inhibited and uninhibited solutions at different temperatures are exposed in Fig. 5. The impedance data from the EIS experiments performed at different temperatures are given in Table 6. By inspecting results in Fig. 6(a), a drastic decrease can be seen in the corrosion resistance of steel exposed to HCl solution with the increase of temperature. The same behavior can be observed after adding inhibitor to HCl medium (Fig. 6(b)), due to the increase of corrosion rate in both cases. However, results show that the EIS behavior is like that obtained from the effect of inhibitor concentration. Besides, the variation in the corrosion protection at different temperatures can be observed from the EIS parameters listed in Table 6.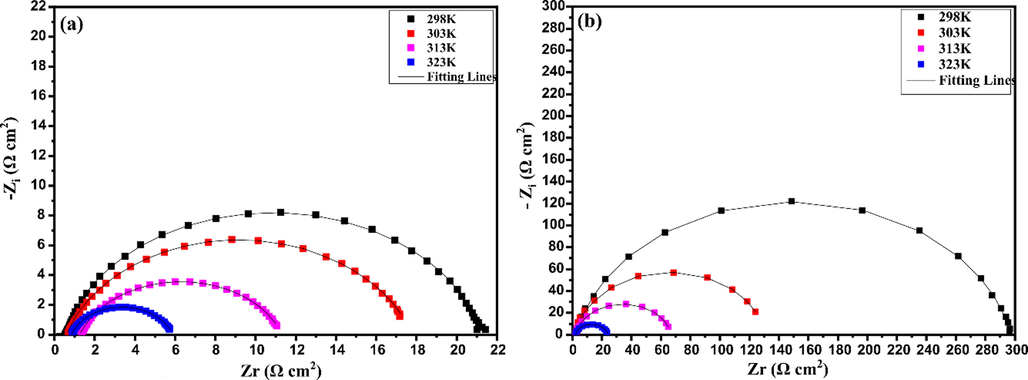
Nyquist diagrams of carbon steel in (a) 1.0 mol/L HCl solution and (b) the presence of 0.5 g / L of inhibitor at various temperatures.
Inhibition system
Temperature
(K)Rs
(Ohm cm2)Rp
(Ohm cm2)n
Q 10-4
(Sn Ohm−1 cm−2)Ceff, dl
(µF cm−2)% IE
298
0.440
20.85
0.848
5.16 8
114
–
1.0 mol/L HCl
303
0.593
17.18
0.812
6.87 4
111
–
313
1.257
10.07
0.783
9.82
148
–
323
0.846
5.04
0.806
17.26
345
–
298
0.632
294.5
0.878
0.60
14
93
HCl + 0.5 g/L CAFEO
303
1.882
128.5
0.925
0.689
33
87
313
2.181
64.63
0.909
0.954
40
84
323
2.327
21.57
0.920
1.759
88
77
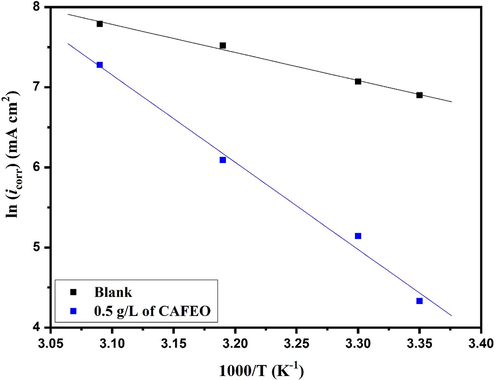
Arrhenius plots of ln icorr vs. 1/T for carbon steel in 1.0 mol/L HCl and in the presence of CAFEO at 0.5 g/L.
From Table 6, it is clear that the presence of CAFEO causes an increase in the diameter of the semicircles. The value of Rp in the presence of inhibitor is more significant than that obtained in the blank solution. The inhibited solution decreases from 294.5 to 21.57 Ohm cm2 as the temperature increases from 298 K to 323 K, respectively. This behavior causes a decrease in the value of the inhibitory efficiency from 92% to 77% for the same temperatures. These results indicate that the inhibitory effect of tested oil on the corrosion behavior of carbon steel in 1.0 mol/L HCl medium decreases with increasing temperature. The formation of an inhibitor barrier on the surface of steel becomes less stable at higher temperatures. This is frequently ascribed to the desorption of adsorbed molecules at higher temperatures.
3.4 Activation energy
The corrosion inhibition data obtained from temperature effect tests can be used to calculate the activation kinetic parameters, namely the activation energy (Ea). The apparent activation energy of the corrosion process in inhibited and uninhibited systems are determined using the Arrhenius-type plot by employing the following equation (Akinbulumo et al., 2020; Oshomogho et al., 2020):
Where R, A, and T denote gas constant, Arrhenius factor, and absolute temperature, respectively. The icorr values were extracted from PDP curves of carbon steel in inhibited and uninhibited solutions at different temperatures. Data are represented in the supplementary material (Figure S2, Table S1). Fig. 6 shows the variation of the logarithms of icorr as a function of the temperature inverse in 1.0 mol/L HCl with 0.5 g/L concentration of CAFEO.
The activation energy values determined from Arrhenius plots in the absence and presence of CAFEO are 29.11 and 90.48 kJ/mol, respectively. The Ea value of 1.0 mol/L HCl containing CAFEO is significantly higher than that obtained in the blank solution. These results can be ascribed to an elevation of the energy barrier of corrosion reaction. Szauer et al. (Szauer and Brandt, 1981) suggested that the rise in activation energy can be attributed to a significant reduction in the adsorption of inhibitor molecules at the metal surface with a temperature rise. In addition, some authors (Bentiss et al., 2005; Martinez and Stern, 2002; Mora et al., 2004) have explained the increase in activation energy to the contribution of physical interactions to the adsorption process.
3.5 Density functional Theory calculations
Quantum chemical parameters are useful theoretical tools to evaluate an inhibitor’s reactivity and its most atomic reactive sites (Damej et al., 2021; Serdaroğlu et al., 2020; Singh et al., 2020). In particular, frontier molecular orbitals can provide a valuable prediction of the inhibitor’s ability to donate or accept electrons, which is beneficial information in understanding the corrosion inhibition mechanism. A molecule can have more ability to donate electrons when its Highest Occupied Molecular Orbital (HOMO) energy is high, while its ability to accept electrons is associated with a low value of the Lowest Unoccupied Molecular Orbital (LUMO) energy (Olasunkanmi and Ebenso, 2020). The three components of CAFEO having higher composition percentage are investigated by DFT calculations. Fig. 7 shows the optimized structures, HOMO and LUMO densities of α-pinene, myrcene, and limonene, along with HOMO and LUMO energies and energy gap values. It can be noticed from Fig. 7 that α-pinene, which is the major molecule of CAFEO, has well-distributed HOMO/LUMO densities, particularly around its double bond. The same can be said for the other two molecules, where densities are concentrated around double bonds. It signifies that these molecules can participate in donor–acceptor interactions with steel surface by sharing their π-electrons with vacant d-orbitals of iron. Simultaneously, their vacant antibonding π-orbitals accept electrons from filled metal orbitals (retro-donation) (Haque et al., 2018).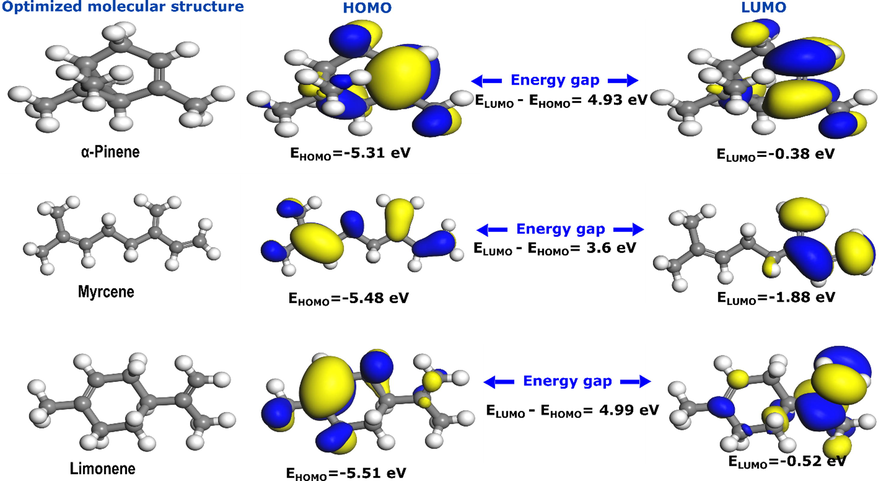
Optimized molecular structures and frontier molecular orbitals of α-Pinene, Myrcene, and Limonene. Numerical values of HOMO, LUMO and energy gap are given in eV.
In terms of individual reactivity, α-pinene has the strong electron-donating power with HOMO energy of −5.31 eV, followed by myrcene (-5.48 eV) and limonene (-5.51 eV). However, in the case of electron-accepting power, the myrcene shows the highest electron-accepting characteristic with a LUMO value of −1.88 eV, followed by limonene (-0.52 eV) and α-pinene (-0.38 eV). Considering both HOMO and LUMO energies, the energy gap, which indicates a molecule’s stability (high energy gap) or reactivity (low energy gap), can be determined. Results show that myrcene has the lowest energy gap of 3.6 eV, followed by α-pinene (4.93 eV), and then limonene with 4.99 eV. It signifies that myrcene has the highest reactivity among the three molecules, followed by α-pinene and limonene. These three components are expected to have the most substantial contribution to the corrosion inhibition effect of CAFEO. However, the inhibition action is mainly due to the synergistic effect of all compositions, including those with a minor percentage.
3.6 Scanning electron microscope (SEM)
The corrosion protection ability of the investigated oil can also be evaluated using a surface characterization technique. To this end, SEM images were determined for carbon steel exposed to HCl solution without and with inhibitor, as represented in Fig. 8. The SEM image of the steel exposed to HCl alone (Fig. 8(a)) shows a severe corrosive attack. It can be seen that a thick and cracked layer of corrosion products, mainly oxides and hydroxides, covered the entire electrode surface (Abd El-Lateef, 2015). In contrast, Fig. 8(b) shows a steel surface with a minimized destructive attack. The protected steel surface is mainly due to the adsorption of CAFEO's molecules on the steel surface, forming a protective barrier that prevents corrosion attacks.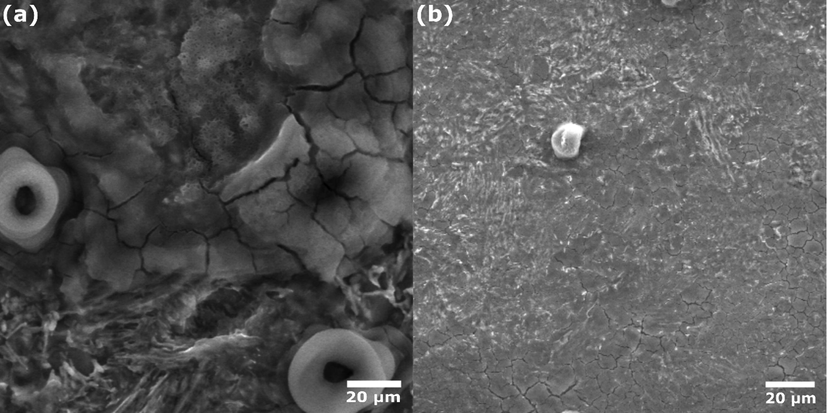
SEM images of carbon steel after 24 h immersion in 1.0 mol/L HCl without (a) and with (b) 0.5 g/L of CAFEO at 298 K.
3.7 Comparison with other green inhibitors
The application of green corrosion inhibitors to protect different metals and alloys is a growing research subject (Chevalier et al., 2014; Martins et al., 2021; Oguzie, 2008; Ramezanzadeh et al., 2018; Yang, 2021). The main advantage of these materials is the low-cost as compared to synthetic corrosion inhibitors. The composition of natural-based corrosion inhibitors consists of plenty of moderate to complex molecules with several functional groups, heteroatoms, and aromatic rings. These features make them resemble those of traditional organic corrosion inhibitors. In the literature, there are many natural-based corrosion inhibitors in plant extracts. Table 7 lists some natural-based materials used as corrosion inhibitors in different experimental conditions. Compared to other inhibitors, results show that the CAFEO tested in the present study shows excellent corrosion inhibition properties. An inhibition efficiency higher than 90% by a natural-based inhibitor at low concentration is a very promising result. It further confirms the high potential of this kind of material for the adequate protection of metals and alloys in highly aggressive conditions.
Inhibitor
Optimum conc./Material/Solution
Highest inhibition efficiency(%)
Reference
Artemisia Mesatlantica essential oil
3000 mg/L/Carbon steel/1.0 M HCl
92.0
(Boumhara et al., 2015)
Eleusine aegyptiaca leaf
2400 mg/L/Mild steel/1.0 M HCl
91.3
(Rajeswari et al., 2014)
Juniperus Phoenicea Essential Oil
250 mg/L/Mild steel/1.0 M HCl
88.3
(Tiskar et al., 2016)
Musa acuminate Peel
500 mg/L/Mild steel/0.5 M H2SO4
85.6
(Saxena et al., 2020)
Opuntia elatior fruit
500 mg/L/Mild steel/1.0 M HCl
79.7
(Loganayagi et al., 2014)
Acid garlic essential oil
2500 mg/L/Carbon steel/1.0 M HCl
95.8
Mangifera indica leaf
1000 mg/L/Mild steel/1.0 M HCl
92.0
(Ramezanzadeh et al., 2019)
Cupressus arizonica fruit essential oil
500 mg/L/Carbon steel/1.0 M HCl
92.0
This work
4 Conclusions
The Cupressus arizonica fruit essential oil (CAFEO) has a chemical composition rich in α-Pinene (51.07%), a higher extraction yield than that from leaves; however, it showed fewer biological activities. The present work investigated the corrosion inhibition characteristics of Cupressus arizonica fruit essential oil for carbon steel in HCl medium using electrochemical techniques and scanning electron microscope (SEM). The results showed that tested oil could be used as an effective corrosion inhibitor for carbon steel in HCl medium. Its performance increased with increasing concentration and reached an inhibition efficiency of 92% at 0.5 g/L. EIS measurements showed that the presence of CAFEO decreased carbon steel corrosion via the adsorption of oil’s molecules on its surface. Potentiodynamic polarization curves suggested that the CAFEO had a mixed-type effect but more pronounced on the cathodic corrosion reaction. The temperature greatly influenced the inhibition efficiency of the CAFEO, which decreased to a 77% value at 323 K. Furthermore, quantum chemical calculations by DFT showed that the three main components of CAFEO can participate in Donor-Acceptor interactions through their bonding and antibonding π-electrons, with myrcene showing the highest reactivity. A comparison with similar materials showed that tested oil could be a promising corrosion inhibitor for carbon steel in HCl medium.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al- Qura University, Saudi Arabia, for supporting this work by Grant Code. 19-SCI-1-01-0033. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIT) (No. NRF-2018R1A5A1025137).”
References
- Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions. Corros. Sci.. 2015;92:104-117.
- [CrossRef] [Google Scholar]
- Natural nutmeg oil as a green corrosion inhibitor for carbon steel in 1.0 M HCl solution: Chemical, electrochemical, and computational methods. J. Mol. Liq.. 2021;323:115036
- [CrossRef] [Google Scholar]
- Eucalyptus leaf extract as a eco-friendly corrosion inhibitor for mild steel in sulfuric and phosphoric acid solutions. Int. J. Ind. Chem.. 2020;11:123-132.
- [CrossRef] [Google Scholar]
- A review: Plant extracts and oils as corrosion inhibitors in aggressive media. Ind. Lubrication Tribol.. 2011;63:227-233.
- [CrossRef] [Google Scholar]
- Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results in Materials. 2020;5:100074
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl. Surf. Sci.. 2005;239:154-164.
- [CrossRef] [Google Scholar]
- Inhibitory effect of sodium carboxymethylcellulose and synergistic biodegradable gemini surfactants as effective inhibitors for MS corrosion in 1 M HCl. J. Mater. Res. Technol.. 2019;8:4521-4533.
- [CrossRef] [Google Scholar]
- Inhibition effect of natural Artemisia oils towards tinplate corrosion in HCl solution: Chemical characterization and electrochemical study. Int. J. Electrochem. Sci.. 2011;6:1454-1467.
- [Google Scholar]
- Corrosion inhibition performance of 4-(prop-2-ynyl)- [1,4]-benzothiazin-3-one against mild steel in 1 M HCl solution: Experimental and theoretical studies. International Journal of Hydrogen Energy, XIX Mexican Hydrogen Society Congress Special Issue. 2021;46:25800-25818.
- [CrossRef] [Google Scholar]
- Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/ hydrochloric acid system. Corros. Sci.. 2005;47:2915-2931.
- [CrossRef] [Google Scholar]
- Benzbiria, N., Echihi, S., Belghiti, M.E., Thoume, A., Elmakssoudi, A., Zarrouk, A., Zertoubi, M., Azzi, M., 2021. Novel synthetized benzodiazepine as efficient corrosion inhibitor for copper in 3.5% NaCl solution. Materials Today: Proceedings, The International Conference on Water Depollution and Green Energy 2019 37, 3932–3939. 10.1016/j.matpr.2020.09.030
- Anticorrosion effect of a green sustainable inhibitor on mild steel in hydrochloric acid. J. Colloid Interface Sci.. 2020;580:740-752.
- [CrossRef] [Google Scholar]
- Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution. Experimental and theoretical approach. J. Mol. Liq.. 2020;313:113493
- [CrossRef] [Google Scholar]
- Phytochemicals as steel corrosion inhibitor: an insight into mechanism. Corros. Rev.. 2021;39:27-41.
- [CrossRef] [Google Scholar]
- Inhibiting effects of butyl triphenyl phosphonium bromide on corrosion of mild steel in 0.5 M sulphuric acid solution and its adsorption characteristics. Corros. Sci.. 2008;50:2747-2754.
- [Google Scholar]
- Bilgiç, S., 2021. Plant Extracts as Corrosion Inhibitors for Mild Steel in HCl Media - Review I. Int. J. Corros. Scale Inhibition 10. 10.17675/2305-6894-2021-10-1-9.
- Chemical Composition and Antibacterial and Antifungal Activities of Moroccan Cupressus Arizonica Greene Essential Oils. Eur. J. Sci. Res.. 2018;149:401-409.
- [Google Scholar]
- Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1M HCl solution: Electrochemical and XPS investigations. J. Ind. Eng. Chem.. 2015;29:146-155.
- [CrossRef] [Google Scholar]
- Towards Understanding the Anticorrosive Mechanism of Novel Surfactant Based on Mentha pulegium Oil as Eco-friendly Bio-source of Mild Steel in Acid Medium: a Combined DFT and Molecular Dynamics Investigation. Chem. Res. Chin. Univ.. 2019;35:85-100.
- [CrossRef] [Google Scholar]
- The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem.. 1984;176:275-295.
- [Google Scholar]
- The usability of Cupressus arizonica annual rings in monitoring the changes in heavy metal concentration in air. Environ. Sci. Pollut. Res. 2021:1-7.
- [Google Scholar]
- Inhibitive effect of ester-quats surfactants in the series of (alcanoyloxy)propyl n-alkyl dimethyl ammonium bromide on the corrosion of iron in acid medium. Colloids Surf., A. 2015;480:468-476.
- [CrossRef] [Google Scholar]
- Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene. Biochem. Syst. Ecol.. 2007;35:813-820.
- [CrossRef] [Google Scholar]
- Unveiling corrosion inhibition properties of the cupressus arizonica leaves essential oil for carbon steel in 1.0 m hcl. Int. J. Corros. Scale Inhibition. 2020;9:607-622.
- [CrossRef] [Google Scholar]
- Enhanced corrosion resistance of mild steel in 1 M hydrochloric acid solution by alkaloids extract from Aniba rosaeodora plant: Electrochemical, phytochemical and XPS studies. Electrochim. Acta. 2014;131:96-105.
- [Google Scholar]
- Cupressus arizonica pollen wall zonation and in vitro hydration. Plant Syst. Evol.. 2008;270:231-242.
- [Google Scholar]
- Comparative Investigation of Corrosion-Mitigating Behavior of Thiadiazole-Derived Bis-Schiff Bases for Mild Steel in Acid Medium: Experimental, Theoretical, and Surface Study. ACS Omega. 2020;5:13503-13520.
- [CrossRef] [Google Scholar]
- Experimental and theoretical explorations of S-alkylated mercaptobenzimidazole derivatives for use as corrosion inhibitors for carbon steel in HCl. J. Mol. Liq.. 2021;331:115708
- [CrossRef] [Google Scholar]
- A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq.. 2019;282:366-384.
- [CrossRef] [Google Scholar]
- Pharmacognostic methods for analysis of herbal drugs, According to European Pharmacopoeia. Edited by Purusotam Basnet. 2012;37
- [Google Scholar]
- Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros. Sci.. 2019;151:190-197.
- [Google Scholar]
- Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1 M hydrochloric acid. Results Phys.. 2018;9:1481-1493.
- [CrossRef] [Google Scholar]
- Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta. 2010;55:6218-6227.
- [Google Scholar]
- Application of Essential Oils as Green Corrosion Inhibitors. Arab J Sci Eng. 2020;45:7137-7159.
- [CrossRef] [Google Scholar]
- Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem. Cent. J.. 2018;12:7.
- [CrossRef] [Google Scholar]
- COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans.. 1993;2:799-805.
- [Google Scholar]
- In situ synthesis, electrochemical and quantum chemical analysis of an amino acid-derived ionic liquid inhibitor for corrosion protection of mild steel in 1M HCl solution. Corros. Sci.. 2016;112:73-85.
- [Google Scholar]
- Corrosion inhibition performance of chromone-3-acrylic acid derivatives for low alloy steel with theoretical modeling and experimental aspects. J. Mol. Liq.. 2017;243:439-450.
- [CrossRef] [Google Scholar]
- Physical properties obtained from measurement model analysis of impedance measurements. Electrochim. Acta. 2020;354:136747
- [Google Scholar]
- Opuntiol: An active principle of Opuntia elatior as an eco-friendly inhibitor of corrosion of mild steel in acid medium. ACS Sustain. Chem. Eng.. 2014;2:606-613.
- [Google Scholar]
- Impedance spectroscopy: theory, experiment, and applications. History. 2005;1:1-13.
- [Google Scholar]
- Effect of Santolina pectinata (Lag.) Essential Oil to protect against the corrosion of Mild steel in 1.0 M HCl: Experimental and quantum chemical studies. Mediterranean. J. Chem.. 2020;10:253-268.
- [CrossRef] [Google Scholar]
- Experimental and computational studies of perillaldehyde isolated from Ammodaucus leucotrichus essential oil as a green corrosion inhibitor for mild steel in 1.0 M HCl. Chem. Pap.. 2020;75:1103-1114.
- [CrossRef] [Google Scholar]
- Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl. Surf. Sci.. 2002;199:83-89.
- [CrossRef] [Google Scholar]
- Martins, G.R., Guedes, D., Marques de Paula, U.L., de Oliveira, M. do S.P., Lutterbach, M.T.S., Reznik, L.Y., Sérvulo, E.F.C., Alviano, C.S., Ribeiro da Silva, A.J., Alviano, D.S., 2021. Açaí (Euterpe oleracea Mart.) Seed Extracts from Different Varieties: A Source of Proanthocyanidins and Eco-Friendly Corrosion Inhibition Activity. Molecules 26, 3433. 10.3390/molecules26113433.
- Corrosion protection properties of cerium layers formed on tinplate. Corros. Sci.. 2004;46:563-578.
- [CrossRef] [Google Scholar]
- Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros. Sci.. 2008;50:2993-2998.
- [CrossRef] [Google Scholar]
- Experimental and computational studies onpropanone derivatives of quinoxalin-6-yl-4,5-dihydropyrazole as inhibitors of mild steel corrosion in hydrochloric acid. J. Colloid Interface Sci.. 2020;561:104-116.
- [CrossRef] [Google Scholar]
- Green corrosion inhibition of mild steel using Prunus Dulcis seeds extract in an acidic medium. Global J. Pure Appl. Sci.. 2020;26:171-178.
- [CrossRef] [Google Scholar]
- Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peels as green corrosion inhibitors of mild steel in aggressive chloride media. J. Mol. Liq.. 2019;276:347-361.
- [CrossRef] [Google Scholar]
- Pharmacognosie, B.J., 1999. phytochimie, plantes médicinales. Revue et Augmentée, Tec & Doc, Paris.
- Corrosion inhibition of Eleusine aegyptiaca and Croton rottleri leaf extracts on cast iron surface in 1M HCl medium. Appl. Surf. Sci.. 2014;314:537-545.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: Electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl. Surf. Sci.. 2019;463:1058-1077.
- [CrossRef] [Google Scholar]
- Studying the Urtica dioica leaves extract inhibition effect on the mild steel corrosion in 1 M HCl solution: Complementary experimental, ab initio quantum mechanics, Monte Carlo and molecular dynamics studies. J. Mol. Liq.. 2018;272:120-136.
- [CrossRef] [Google Scholar]
- Green corrosion inhibitor from essential oil of eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J. Mater. Environm. Sci.. 2012;3:613-627.
- [Google Scholar]
- Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem.. 2019;69:18-31.
- [CrossRef] [Google Scholar]
- Electrochemical studies and surface examination of low carbon steel by applying the extract of Musa acuminata. Surf. Interfaces. 2020;18:100436
- [CrossRef] [Google Scholar]
- Eco-friendly sodium gluconate and trisodium citrate inhibitors for low carbon steel in simulated cooling water system: Theoretical study and molecular dynamic simulations. J. Mol. Liq.. 2020;319:114108
- [CrossRef] [Google Scholar]
- Shahali, Y., Majd, A., TAJADDOD, G., POURPAK, Z., Haftlang, M., MOEIN, M., 2007. Comparative study of the pollen protein contents in two major varieties of Cupressus arizonica planted in Tehran.
- Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: Theoretical and electrochemical studies. J. Mol. Liq.. 2021;332:115876
- [Google Scholar]
- Aminoantipyrine derivatives as a novel eco-friendly corrosion inhibitors for P110 steel in simulating acidizing environment: Experimental and computational studies. J. Nat. Gas Sci. Eng.. 2020;83:103547
- [CrossRef] [Google Scholar]
- Development of environmental friendly corrosion inhibitor from the extract of areca flower for mild steel in acidic media. Eastern-Eur. J. Enterprise Technol.. 2020;2:34-45.
- [CrossRef] [Google Scholar]
- Adsorption of oleates of various amines on iron in acidic solution. Electrochim. Acta. 1981;26:1253-1256.
- [CrossRef] [Google Scholar]
- Juniperus Phoenicea essential oil as green corrosion inhibitor for mild steel in molar hydrochloric acid. Matériaux & Techniques. 2016;104:609.
- [CrossRef] [Google Scholar]
- An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq.. 2018;266:577-590.
- [CrossRef] [Google Scholar]
- Role of Organic and Eco-Friendly Inhibitors on the Corrosion Mitigation of Steel in Acidic Environments—A State-of-Art Review. Molecules. 2021;26:3473.
- [CrossRef] [Google Scholar]
- Zahran, M.A., 2010. Climate–vegetation and human welfare in the coastal deserts, in: Climate-Vegetation. Springer, pp. 249–295.
- Chemical composition and inhibitory effect of Mentha spicata essential oil on the corrosion of steel in molar hydrochloric acid. Int. J. Electrochem. Sci.. 2011;6:691-704.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103849.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







