Translate this page into:
Deciphering the healing power of Swertia Chirayita: A potential treatment for acute liver injury
⁎Corresponding author. zhangyi@cdutcm.edu.cn (Yi Zhang),
⁎⁎Corresponding author at: State Key Laboratory of Southwestern Chinese Medicine Resources, School of Ethnic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. winter9091@163.com (Shaohui Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute Liver Injury (ALI), a highly prevalent liver disease in the last two decades, but there is still no effective treatment for it. As a member of Tibetan medicine, Swertia chirayita is commonly used for treating liver-related diseases, while the behavior of S. chirayita in CCl4-induced ALI is deficient. In this work, the chemical constituents of S. chirayita were identified using a UPLC-Q-Exactive Orbitrap MS method. And the potential targets of the active compound of S. chirayita in CCl4-induced ALI have been explored on the basis of bioinformatics analysis and ALI model. The findings revealed that a total of 49 compounds have been identified in S. chirayita, and 10 have been absorbed into blood as prototypes. Network pharmacology and molecular docking studies have shown that S. chirayita has a multi-component, multi-target profile for the treatment of CCl4-induced ALI. Biochemical indicators testing demonstrated that Swertiamarine (STW) improved the levels of AST, ALT, MDA, SOD, TNF-α and IL-6 in CCl4-induced ALI mouse model. Additionally, H&E staining indicated that STW significantly improved cellular necrosis, inflammatory infiltration, and other forms of pathological liver damage in mice. Meanwhile, quantitative reverse transcription PCR (qRT-PCR) showed that STW could ameliorate CCl4-induced ALI by regulate the expression of EGFR, CASP3, STAT3 and other targets. In conclusion, as one of the active compounds of S. chirayita, STW could alleviate ALI by modulating the expression of biochemical indicators, liver pathological indicators and critical targets. This will provide a theoretical basis for the further research and development of the S. chirayita.
Keywords
Swertia chirayita
UPLC-Q-Exactive Orbitrap MS
Network pharmacology
Molecular docking
Acute liver injury
- ALI
-
Acute liver injury
- ALT
-
Alanine aminotransferase
- AMPK
-
Adenosine 5‘-monophosphate (AMP)-activated protein kinase
- AST
-
Aspartate aminotransferase
- GO
-
Gene ontology
- H&E
-
Hematoxylin and eosin
- IL-6
-
Interleukin- 6
- KEGG
-
Kyoto encyclopedia of genes and genomes
- MDA
-
Malondialdehyde
- qRT-PCR
-
Quantitative reverse transcription PCR
- SOD
-
Superoxide Dismutase
- STW
-
Swertimarin
- TNF-α
-
Tumor necrosis factor-alpha
- VEGF
-
Vascular endothelial growth factors
Abbreviations
1 Introduction
Acute liver injury (ALI) is a common liver condition that is often caused by drug use, chemical exposure, or alcohol consumption. The main factors contributing to ALI are oxidative stress, inflammatory responses, necrosis, and apoptosis (Wu et al., 2021, Xin et al., 2021). Notably, carbon tetrachloride (CCl4) is a well-known hepatotoxic substance frequently used in experimental models to induce both acute and chronic liver injury (Xiong et al., 2020). When CCl4 is metabolized in a biological system, it produces harmful compounds like trichloromethyl radicals and peroxymethyl radicals, leading to an excess of superoxide dismutase and ultimately causing cellular necrosis, apoptosis, and liver damage (Di Paola et al., 2022, Wang et al., 2022a,b). Currently, synthetic drugs such as polyenyl phosphatidylcholine and dicyclomine are commonly used to treat liver damage, but they can have unwanted side effects like gastrointestinal irritation and weight gain (Gawrieh and Chalasani 2015). Therefore, it is important to investigate natural products that may provide effective and less toxic alternatives to traditional treatments.
The study and utilization of natural products and biomolecules in promoting human health have been fundamental in medical and therapeutic sciences for centuries. These compounds, derived from a wide range of botanical, zoological, and microbiological sources, form the basis of traditional medicine (Hamza et al., 2020, Wang et al., 2022a,b). Modern pharmacognosy has brought scientific rigor to this field, revealing the intricate mechanisms through which these substances interact with biological pathways to provide health benefits (Xing et al., 2023). Biomolecules like phenolic compounds, alkaloids, terpenoids, and peptides are extracted from plants, animals, and microorganisms, demonstrating various pharmacological activities, including antioxidant, anti-inflammatory, antimicrobial, and anticancer effects (Amin 2008, Abdu et al., 2022, Bouabdallah et al., 2023, Du et al., 2023). Hence, natural products and biomolecules remain vital for the discovery and development of new health-promoting agents, emphasizing the ongoing pursuit of sustainable and effective medical treatments.
S. chirayita, a member of the Swertia L. of the gentianidae family, is widely used in Tibetan medicine and is mentioned in classic Tibetan medical texts such as Jing Zhu Ben Cao and Si Bu Yi Dian (He et al., 2022). Indigenous people utilized this medicinally important plant in a variety of ways. The whole plant directly or in decoction is traditionally used to cure inflammation, liver diseases, and digestive disorders. Juices made from different parts of this plant are used to treat malarial fevers, liver diseases and to stimulate the digestive tract (Swati et al., 2023). In addition, as a famous Tibetan medicinal product, Tibetan medicine considers S. chirayita to be a natural product with a bitter taste and cold nature, and prescription drugs containing S. chirayita, including ShiWei DiDa Capsule, ShiWuWei SaiErDou Pill and WuWei ZhangYaCai TangSan, are commonly used clinically for the treatment of hepatitis, cirrhosis, hepatic fibrosis, and cholecystitis (Guo et al., 2023). Moreover, it contains various chemical constituents, including terpenoids, flavonoids, and organic acids, which contribute to its hepatoprotective effects. S. chirayita's active ingredients, such as swertiamarin (STW), sweroside, and mangiferin, are commonly used in the treatment of liver diseases such as liver fibrosis, acute and chronic hepatitis, and hepatocellular carcinoma (Jaishree and Badami 2010). However, the specific role of S. chirayita in relation to CCl4-induced ALI has not been extensively studied.
In 2007, Andrew L. Hopkins introduced the concept of network pharmacology, which aims to systematically understand the beneficial pathways and underlying mechanisms of biological agents and traditional Chinese medicines. This approach provides a framework to comprehend the complex interactions between multiple targets and diseases (Guo et al., 2021). Molecular docking, a technique used in computer-aided drug design, is also employed to validate network pharmacology predictions and has become an efficient research method in structural molecular biology (Mao et al., 2022). Both network pharmacology and molecular docking align with the holistic and evidence-based treatment principles of Chinese medicine, making them increasingly important in contemporary Chinese medicine research (Tang et al., 2016, Xie et al., 2021, Peng et al., 2023, Yang et al., 2023). However, there have been limited investigations applying these techniques to explore the role of S. chirayita in CCl4-induced ALI.
In the present study, the main objectives were to identify the compounds present in S. chirayita and those absorbed into the bloodstream. And this was achieved this by using UPLC-Q-Exactive Orbitrap MS. Additionally, network pharmacology and molecular docking techniques was employed to identify the main targets and active compounds of S. chirayita in ALI. And the hepatoprotective effect of STW was also validated the in an ALI model. Finally, the key targets were confirmed using quantitative real-time polymerase chain reaction (qRT-PCR). Overall, these findings provide a solid foundation for further research on the enhancement and utilization of S. chirayita. A comprehensive diagram of our research methods is provided in Fig. 1.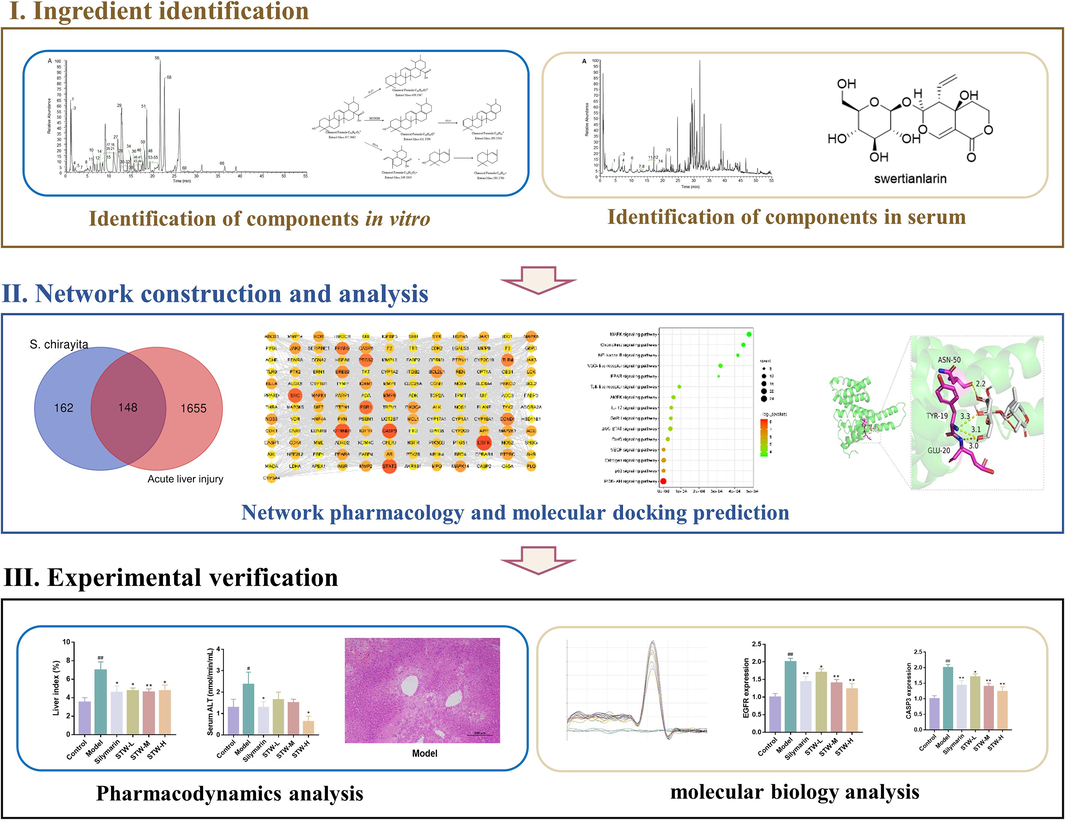
The flowchart of this paper.
2 Material and method
2.1 Chemicals and reagents
S. chirayita was sourced from Qinghai Jiumei Tibetan Medicine Pharmaceutical Co., Ltd (Qinghai, China) in 2022. Swertiamarin (STW), silymarin, loganic acid, chlorogenic acid, sweroside, isoorientin, and ursolic acid, all with a purity exceeding 98 %, were acquired from Lemeitian Pharmaceutical Technology Co., Ltd (Chengdu, China). CCl4 was provided by Shanghai Yien Chemical Technology Co., Ltd (Shanghai, China), while isopropanol, chloroform and anhydrous ethanol were obtained from Chengdu Kelong Chemical Co., Ltd (Chengdu, China). Diagnostic tools included aspartate aminotransferase (AST) and alanine aminotransferase (ALT) kits from Jiangsu Edison Biotechnology Co., Ltd (Yancheng, China), a Superoxide Dismutase (SOD) kit courtesy of Beijing Box Biotechnology Co., Ltd (Chengdu, China), and a Malondialdehyde (MDA) kit procured from Biyuntian Biotechnology Co., Ltd (Shanghai, China). Tumor necrosis factor-alpha (TNF-α) and Interleukin-6 (IL-6) ELISA kits were obtained from Jiangsu Enzyme-free Biotechnology Co., Ltd (Yancheng, China). The Trizol reagent was purchased from Thermo Fisher ScientificThermo Fisher Scientific Co., Ltd (MA, US), and both the ABScript III RT Master Mix and qPCR Genious 2X SYBR Green Fast qPCR Mix were sourced from Wuhan Aibotech Biotechnology Co., Ltd (Wuhan, China). The names of the plants (S. chirayita) mentioned in the text have been verified through “World Flora Online” (http:/www.worldfloraonline.org) or MPNS (https://mpns.kew.org).
2.2 Analysis of the chemical composition of S. Chirayita
2.2.1 Preparation of S. chirayita extract
To extract the active compounds, 250 g of S. chirayita was subjected to two rounds of extraction with 2000 mL of 80 % ethanol under reflux for 90 min each. The resulting extracts were then combined, concentrated, and freeze-dried to yield the final S. chirayita extract.
2.2.2 Analysis of serum pharmacochemistry of S. chirayita
Sprague-Dawley rats (SCXK (Xiang) 2019–0004) were obtained from Hunan Slaughterhouse Laboratory Animal Co., Ltd (Changsha, China) and were housed at the Level III Laboratory of Pharmacology Research in Chinese Medicine (TCM-09–315) at Chengdu University of Traditional Chinese Medicine. The facilities were managed by the State Administration of Traditional Chinese Medicine and maintained a 12-hour day/night cycle with a consistent temperature of 24 ± 2 °C. The rats were fed standard chow (provided by Hunan Laboratory Animal Co., Ltd., Hunan, China) throughout the study.
Based on the clinical dosage of S. chirayita in humans (6–9 g/70 kg) (Swati et al., 2023), we calculated a dosage of 156 mg/kg for rats considering the acquisition rate of 80 % ethanolic extract from S. chirayita (23.07 %). To enhance the presence of chemical components, we increased the dosage to 1.56 g/kg for the test. The experimental procedure was as follows: after a seven-day adjustment period, the rats were randomly divided into two groups: a control group (treated with saline, 10 mL/kg) and a S. chirayita test group (administered an 80 % ethanolic extract of S. chirayita, 1.56 g/kg). The treatment was continued for seven more days through gavage. Prior to experimental blood sampling, the rats underwent a standard fasting period of 12 h with access to water. Blood samples were collected at specific time intervals after feeding: 5, 10, 15, 30, 45, 60, 90, 120, 240, and 480 min. These samples were then left at room temperature for one hour and centrifuged at 3500 r/min for 15 min at 4 °C. The clear top layer was stored at −80 °C. Following the completion of the treatment and blood sampling process, all Sprague-Dawley rats were humanely euthanized in accordance with the guidelines provided by the American Veterinary Medical Association (AVMA) for the Euthanasia of Animals. The euthanasia protocol was part of our experimental design approved by the Animal Care and Use Committee at Chengdu University of Traditional Chinese Medicine, with the ethics approval number 2018–15, demonstrating our commitment to the ethics of animal research and the principles of 3Rs (Replacement, Reduction, and Refinement).
2.2.3 Preparation of UPLC-Q-Exactive Orbitrap MS analysis
First, the extract samples of S. chirayita was prepared for the study of chemical composition of it based on the method of UPLC-Q-Exactive Orbitrap MS. Briefly, to obtain a preparative concentration of 20 mg/mL of solubles, 20 mg of S. chirayita extract was added to a chromatographic grade methanol solution. Next, the serum samples (blank and S. chirayita groups) of SD rat were explored based on the means of serum pharmaceutical chemistry. Specifically, the blank serum or drug-containing serum sample were added to a 1:5 (v/v) acetonitrile solution for chromatography and obtained the supernatant through centrifugation. Next, 300 μL of chromatographic grade methanol was added to dissolve the serum sample. Finally, all samples were be filtered through a 0.22 μm membrane. Finally, the samples were analyzed using UPLC on a Vanquish model coupled to a Q-Exactive Orbitrap MS (Thermofisher, Waltham, MA, USA).
2.2.4 Chromatographic conditions
Sample separation was performed using a Waters XB-C18 column (100 mm × 2.1 mm, 1.7 μm) at a flow rate of 0.3 mL/min. The column temperature was maintained at 40 °C, and an injection volume of 2 μL was used. The mobile phase consisted of a 0.1 % aqueous formic acid solution (solvent A) and acetonitrile (solvent B). The elution gradient was programmed as follows: the proportion of solvent B was increased from 2 % to 10 % over 0–5 min, held at 10 % B from 5 to 10 min, gradually increased from 10 % to 65 % B between 10 and 30 min, further increased from 65 % to 95 % B during 30–45 min, and finally decreased from 95 % to 2 % B between 45 and 55 min.
2.2.5 Mass spectrometry conditions
A Q-Exactive Orbitrap mass spectrometer (MS) equipped with an electrospray ionization (ESI) feature was used to operate in both positive and negative ion modes. The specific parameters for data acquisition were set as follows: the spray voltage was maintained at 3500 V for both positive and negative ion modes; the ion transfer tube temperature was set at 320 °C, and the extra air heating temperature was set at 350 °C. The full MS resolution was set to 35,000, while the secondary mass spectral resolution was set to 17,500. The scan mass ranged from 100 to 1500 Da, and the collision energy gradient was set at 20, 40, and 60 Ev.
2.3 Network pharmacological analysis
2.3.1 Potential targets prediction
Using network pharmacological analysis, this study aimed to investigate the interaction between S. chirayita and ALI. Initially, the SwissTargetPrediction database (http:/www.swisstargetprediction.ch) was utilized to identify potential targets of the prototype blood components. Subsequently, ALI-related targets were gathered from the GeneCards (https://www.genecards.org/) and OMIM (https://omim.org/) databases. These targets were then standardized using the UniProt database (https: liww.uniprot.org/). Finally, Venn analysis (http://bioinfogp.cnb.csis.es/tools/venny/index.html) was performed to identify potential S. chirayita targets for ALI intervention.
2.3.2 Enrichment analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analytical tools were utilized to identify potential pathways associated with these common targets. Additionally, a protein–protein interaction (PPI) network was created using the STRING database (http://string-db.org/) by inputting the relevant target genes.
2.4 Molecular docking analysis
ChemDraw 3D 20.0 software was used to illustrate the three-dimensional structures of the blood-absorbed compounds. The PDF files for the ten main targets were obtained from the PDB database (https://www.rcsb.org). The binding affinity of the prototype blood components to the top 10 key targets was then analyzed and visualized using AutoDock Vina software.
2.5 Analysis of the intervention of the STW in CCl4-induced ALI
2.5.1 Animals and experimental design
Kunming mice were obtained from Beijing SPIFU Laboratory Animal Co., Ltd. (Beijing, China) and housed at the Level III Laboratory of Pharmacology Research in Chinese Medicine (TCM-09-315) at Chengdu University of Traditional Chinese Medicine, managed by the State Administration of Traditional Chinese Medicine. The mice were kept under a 12 h day/night cycle with a constant temperature of 24 ± 2 °C. After acclimating for 7 days, 54 mice were divided into six groups. The dosage for each group was determined based on references (Tian et al., 2014, Zhang et al., 2015) as follows: the Control group (received saline, 10 mL/kg daily), the Model group (also given saline, 10 mL/kg daily), the Silymarin group (received Silymarin, 0.2 g/kg daily), and low-, medium-, and high-dose STW groups (STW-L, 0.07 g/kg daily, STW-M, 0.14 g/kg daily and STW-H group, 0.28 g/kg daily respectively). These dosages were administered for 14 consecutive days. Two hours after the final dose, the Control group received an intraperitoneal injection of a peanut oil solution (10 mL/kg), while the rest of the groups were given intraperitoneal injections of 0.5 % CCl4 mixed with the peanut oil solution (10 mL/kg). After 20 h, the blood samples were collected through the ophthalmic venous plexus. Additionally, the liver of each mouse was divided into two parts and stored in 4 % paraformaldehyde and −80 °C, respectively. The euthanasia protocol for mice followed the same procedure as described in section 2.2.2. The Chengdu University of Traditional Chinese Medicine Animal Use Committee approved all procedures, which were conducted in accordance with their guidelines.
2.5.2 Liver index analysis
The liver index is a commonly used metric in the field of toxicology, which measures the weight of an animal's liver relative to its total body weight. It is used to assess the severity of liver enlargement. The liver index is calculated using the formula: Liver index (%) = [Liver weight (g) / Mouse weight (g)] × 100. A higher liver index indicates a more severe case of hepatomegaly.
2.5.3 Biochemical analysis of the serum
The mice blood samples were collected into centrifuge tubes without heparin and sodium and allowed to coagulate at room temperature for an hour. Afterward, the samples were centrifuged at 3500 r/min for 15 min at 4 °C. The resulting serum samples were utilized to evaluate the concentrations of AST, ALT, TNF-α, and IL-6, following established protocols.
2.5.4 Biochemical analysis of the liver
Liver tissues from mice weighing 200 mg were homogenized with 1 mL of PBS buffer in a 1.5 mL centrifuge tube using a homogenizer set at 60 Hz for 90 s at 4 °C. This homogenization procedure was repeated four times. The homogenates were then centrifuged at 3500 r/min for 15 min at 4 °C. The supernatant was collected and used as the liver tissue homogenate sample. Subsequently, the levels of AST, ALT, MDA, and SOD in the liver tissue homogenates were measured following the manufacturer's instructions.
2.5.5 Histological analysis of the liver
For histopathological analysis, liver tissues were fixed in 4 % neutral formaldehyde and then stained with hematoxylin and eosin (H&E). The tissue samples were subjected to a series of steps, including gradient dehydration with ethanol, clarification, and embedding, before being sectioned. Microscopic images of the sections were captured using an Olympus microscope (Tokyo, Japan) after H&E staining.
2.5.6 Analysis of qRT-PCR
For the analysis of gene expression, total RNA was extracted from approximately 60 mg of liver tissue using Trizol reagent. The concentration and purity of the total RNA were measured with the Nanodrop Micro Nucleic Acid Analyzer (Thermo Fisher Scientific, USA). Subsequently, cDNA was synthesized from 1000 ng of total RNA, and a qRT-PCR reaction was performed on the Quantitative Real-Time Fluorescence PCR Detection System (Rocgene, Beijing, China). GAPDH was used as the reference gene to normalize RNA content. The primer sequences used for this process are provided in Table 1. The qPCR analysis parameters were set as follows: initial denaturation at 95 °C for 3 min, followed by 45 amplification cycles involving denaturation at 95 °C for 5 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 60 s. The relative expression levels of the target genes were then calculated using the 2^−Δ(ΔCt) method.
Genes
Primer
Sequences 5′–3′
EGFR
Forward primer
TTC AGC AAC AAC CCC ATC CTC TG
Reverse primer
GCT TGG ATC ACA TTT GGG GCA AC
CASP3
Forward primer
TCA CTC GCG TTA ACA GGA AGG TG
Reverse primer
TGA GCA TGG ACA CAA TAC ACG GG
CTNNB1
Forward primer
GGG TCC TCT GTG AAC TTG CTC AG
Reverse primer
GGC ACC AAT GTC CAG TCC AAG AT
STAT3
Forward primer
GTC AGC AAT GGA GTA CGT GCA GA
Reverse primer
GTT GGC GGG TCT GAA GTT GAG AT
SRC
Forward primer
TCC AGG CTG AGG AGT GGT ACT TT
Reverse primer
CCA GCT TGC GGA TCT TGT AGT GT
ESR1
Forward primer
ATG CAC CAT TGA CAA GAA CCG GA
Reverse primer
ACC CAT TTC ATT TCG GCC TTC CA
PTGS2
Forward primer
TCA GGT CAT TGG TGG AGA GGT GT
Reverse primer
TCC TGC TTG AGT ATG TCG CAC AC
PPARG
Forward primer
TGC CAG TTT CGA TCC GTA GAA GO
Reverse primer
CTC CCT GGT CAT GAA TCC TTG GC
TLR4
Forward primer
TCA TGG CAC TGT TCT TCT CCT GC
Reverse primer
TAA GCC ATG CCA TGC CTT GTC TT
MMP9
Forward primer
AAA ACC TCC AAC CTC ACG GAC AC
Reverse primer
CGC GGC AAG TCT TCA GAG TAG TT
GAPDH
Forward primer
GCC CAG AAC ATC ATC CCT GCAT
Reverse primer
GCC TGC TTC ACC ACC TTC TTGA
2.5.7 Analysis of molecular dynamics
To further identify the key targets of STW for intervention in ALI, Molecular dynamics simulations including RMSD, RMSF, SARA, etc. of STW in complex with critical was performed using Gromacs2022 software. Specifically, Amber14sb was selected as the protein force field and Gaff2 was selected as the ligand force field.
2.5.8 Analysis of immunohistochemistry
Liver tissue sections were dewaxed and hydrated. Antigen repair was then performed in heated sodium citrate buffer. Closure was performed after removal of endogenous peroxidase. Finally, primary and secondary antibodies were incubated.
2.6 Statistical analysis
In this study, all data were analyzed using the GraphPad Prism 7.0 software through one-way analysis of variance (ANOVA). The values were reported as mean ± standard deviation. A p-value less than 0.05 was considered statistically significant.
3 Result
3.1 Analysis of chemical compounds of S. chirayita
S. chirayita has significant medicinal and developmental value. However, the specific medicinal substances it contains are not well understood. To address this, a comprehensive analysis of the S. chirayita's phytochemical constituents was conducted using the UPLC-Q-Exactive Orbitrap MS technique. The result revealed the presence of 49 chemical constituents in the 80 % ethanol extract of S. chirayita, including 15 xanthones, 12 organic acids, 8 flavonoids, 6 iridoids, 4 phenylacetones, 3 terpenoids, and 1 fatty acids (Fig. 2, Supplementary Fig. S1, Supplementary Fig. S2 and Table 2). Additionally, the cleavage patterns of mangiferin, 1-Hydroxy-2,3,4,5-tetramethoxyxanthone, caffeic acid, isoorientin, and ursolic acid were elucidated, and described the identification process. Through this method, a total of 49 chemical components in S. chirayita was successfully identified. * standard substances.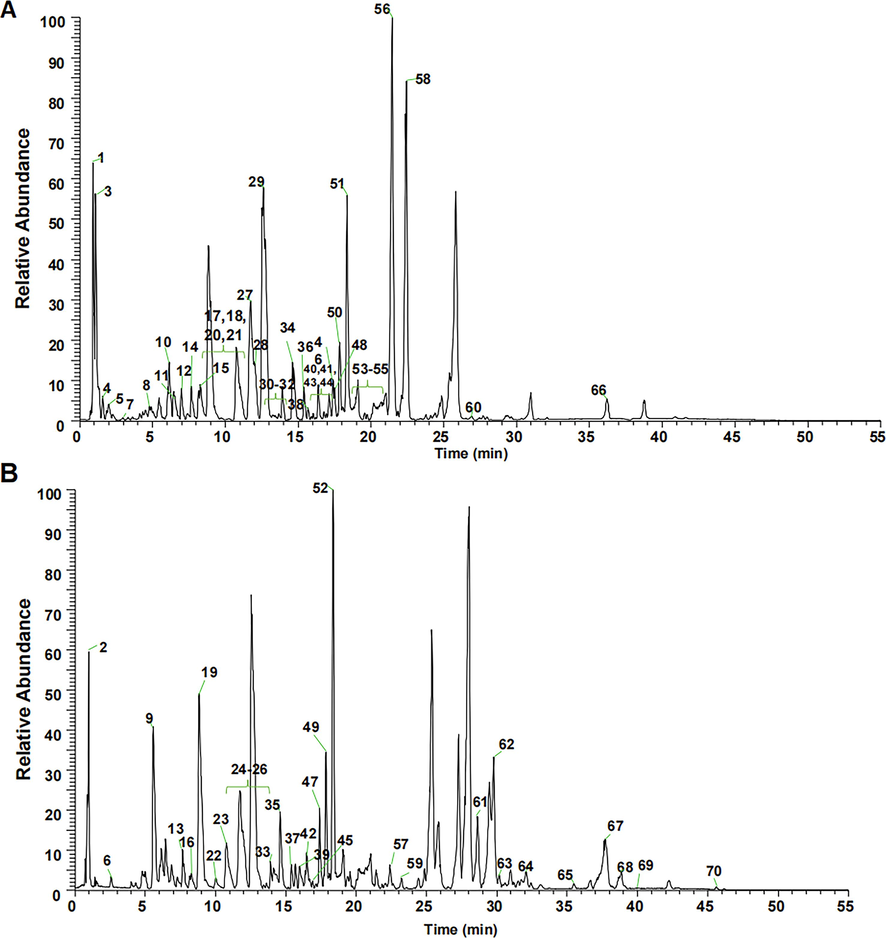
TIC of 80 % ethanolic extract of S. chirayita in the negative (A) and positive (B) ion modes.
No
tR/
minFormula
Ion mode
Found at mass
Extraction mass
δ/ppm
Fragment ions
Compounds
Type
Ref
1
3.08
C7H6O5
[M−H]-
169.0142
169.0137
−2.96
125.0237 107.0132 97.0286 81.0336 69.0371
Gallic acid
Organic acids
(Zhao et al., 2018)
2
4.40
C16H24O10
[M−H]-
375.1297
375.1298
0.27
213.0766 151.0756 125.0595
8-Epiloganic acid
Iridoids
(Kucharska et al., 2017)
3
5.58
C10H12O2
[M+H]+
165.0910
165.0913
1.82
147.0807 137.0806 95.0498 91.0549 67.0550 55.0552
4-Phenylbutyric acid
Organic acids
(Kim et al., 2023)
4
6.69
C7H6O3
[M−H]-
137.0244
137.0237
−5.11
118.7628 109.0286 93.0337
3-Hydroxybenzoic acid
Organic acids
(Wang et al., 2015)
*5
7.04
C16H24O10
[M−H]-
375.1297
375.1298
0.27
213.0766 179.0558 169.0865 151.0756 125.0595 119.0340 113.0236
Loganic acid
Iridoids
(Tao et al., 2017)
6
7.33
C7H6O2
[M+H]+
123.0441
123.0443
1.63
95.0498 77.4372
p-Hydroxybenzaldehyde
Organic acids
(Wang et al., 2016)
7
7.55
C8H8O4
[M−H]-
167.0350
167.0345
−2.99
152.0110 123.0805 108.0211 95.0494 61.9312
Vanillic acid
Organic acids
(Liu et al., 2019)
*8
8.14
C16H18O9
[M−H]-
353.0878
353.0887
2.53
191.0559 161.0240 127.0394
Chlorogenic acid
Phenylpropanoids
(Deng et al., 2022)
*9
8.27
C16H22O10
[M+H]+
375.1286
375.1287
0.27
279.1593 213.0759 195.0656 177.0549 167.0704 149.0598
Swertiamarin
Iridoids
(Swati et al., 2023)
10
8.78
C7H6O4
[M−H]-
153.0193
153.0187
−3.92
125.0603 109.0653 108.9588 91.0549 81.0332
Protocatechuic acid
Organic acids
(Liu et al., 2019)
11
8.84
C10H8O3
[M+H]+
177.0546
177.0548
1.13
149.0601 121.0652 105.0704 103.0548
6-Hydroxy-4-methylycoumarin
Phenylpropanoids
(Kim et al., 2023)
12
8.85
C9H6O4
[M−H]-
177.0193
177.0188
−2.82
177.0189 149.027 133.0288 121.0289 105.0338 93.0336 81.0336
6,7-Dihydroxycoumarin
Phenylpropanoids
(Huang et al., 2021)
13
8.87
C7H6O4
[M−H]-
153.0193
153.0187
−3.92
109.0287 108.0209 91.0179 81.0336
Protocatechuate
Organic acids
(Kim et al., 2023)
14
10.20
C9H8O2
[M+H]+
149.0597
149.0600
2.01
131.0853 121.0653 107.0496 103.0548
Cinnamic acid
Organic acids
(Guan et al., 2019)
15
10.77
C16H20O9
[M−H]-
357.1180
357.1185
1.40
195.0656 177.0549 149.0600 121.0652
Gentiopicroside
Iridoids
(Zhang 2021)
*16
11.75
C16H22O9
[M−H]-
357.1191
357.1199
2.24
328.3954 257.5395 195.0661 177.0554 149.0237 125.0236
Sweroside
Iridoids
(Zhang 2021)
17
12.71
C19H18O11
[M−H]-
421.0776
421.0782
1.42
331.0463 301.0357 271.0251
Mangiferin
Xanthones
(Xu et al., 2008)
18
13.26
C8H8O5
[M−H]-
183.0299
183.0296
−1.64
168.0061 124.0159
Methyl gallate
Organic acids
(Gong et al., 2020)
19
14.36
C15H10O6
[M+H]+
287.0550
287.0554
1.39
287.0554 165.0188 153.0185 121.0289
Kaempferol
Flavonoids
(Liu et al., 2019)
*20
14.58
C21H20O11
[M−H]-
447.0932
447.0937
1.12
429.0831 411.0738 357.0702 369.0601 357.0623 327.0515 299.0563 285.0411
Isoorientin
Flavonoids
(Liu et al., 2019)
21
14.77
C20H20O11
[M+H]+
437.1078
437.1082
0.92
341.0661 317.0660 287.0555 272.0317
Irisxanthone
Xanthones
(Kim et al., 2023)
22
15.49
C27H30O16
[M−H]-
609.1461
609.1467
0.98
301.0358 300.0279 271.0253 151.0032
Rutin
Flavonoids
(Terpinc et al., 2016)
23
15.67
C21H20O10
[M+H]+
433.1129
433.1133
0.92
311.0202 283.0242
Isovitexin
Flavonoids
(Liu et al., 2019)
24
16.20
C10H8O4
[M−H]-
191.0350
191.0348
−1.05
176.0111 148.0160 104.0258
Scopoletin
Phenylpropanoids
(Kim et al., 2023)
25
16.38
C21H20O12
[M−H]-
463.0882
463.0886
0.86
343.0445 301.0357 300.0279 271.0253 255.0302 243.0298 178.9980 151.0030
Quercetin-5-O-beta-D-glucopyranoside
Flavonoids
(Liu et al., 2019)
26
16.63
C9H8O4
[M−H]-
179.0350
179.0358
4.47
135.0445 134.0365 107.0495 89.0235
Caffeic acid
Organic acids
(Liu et al., 2019)
27
17.02
C25H28O15
[M−H]-
567.1355
567.1352
−0.53
273.0407 258.0171 230.0219
1,5-Dihydroxy-3-methoxy-6-O-primeverosyl xanthone
Xanthones
(Wani et al., 2013)
28
17.06
C11H12O4
[M+H]+
209.0808
209.081
0.96
191.0703 163.0754 119.0494 91.0547
3,4-Dimethoxycinnamic acid
Organic acids
(Kim et al., 2023)
29
17.39
C14H10O6
[M+H]+
275.0550
275.0552
0.73
257.0447 232.0368 229.0499 201.0549
2,3,6,8-Tetrahydroxy-1-methylxanthone
Xanthones
(Kim et al., 2023)
30
17.86
C29H30O14
[M−H]-
601.1563
601.1568
0.83
245.0459 227.0351 201.0556 141.0188
Amaroswerin
Iridoids
(Kim et al., 2023)
31
17.90
C17H16O7
[M+H]+
333.0969
333.0970
0.30
318.0736 303.0501 318.0736 285.0390 275.0550
1-Hydroxy-2,3,4,5-tetramethoxyxanthone
Xanthones
(Mou et al., 2020)
32
18.14
C27H30O15
[M−H]-
593.1512
593.1523
1.85
473.1093 431.0991 311.2930
Glucosylvitexin
Flavonoids
(Huang et al., 2021)
33
19.46
C15H10O6
[M−H]-
285.0405
285.0410
1.75
269.0451 253.7174 241.0512 213.0553 153.0185 135.0443 117.0341
Luteolin
Flavonoids
(Liu et al., 2019)
34
19.61
C15H10O7
[M−H]-
301.0354
301.0358
1.33
273.0045 257.0458 239.0348 107.0129
Quercetin
Flavonoids
(Liu et al., 2019)
35
20.48
C14H10O6
[M−H]-
273.0405
273.0408
1.10
258.0172 230.0221
Bellidifolin
Xanthones
(Zhang et al., 2021)
36
21.44
C13H8O6
[M−H]-
259.0248
259.0250
0.77
215.0348 187.0397 171.0447 159.0444
Demethylbellidifolin
Xanthones
(Swati et al., 2023)
37
22.43
C13H8O6
[M−H]-
259.0248
259.0250
0.77
215.0348 187.0396 171.0450 159.0442
Norswertianin
Xanthones
(Zheng et al., 2014)
38
24.86
C14H10O6
[M−H]-
273.0405
273.0407
0.73
258.0171 230.0219
Swertianin
Xanthones
(Mahendran et al., 2014)
39
25.84
C14H10O6
[M−H]-
273.0405
273.0407
0.73
258.0171 230.0220
Isobellidifolin
Xanthones
(Swati et al., 2023)
40
27.28
C17H16O7
[M+H]+
333.0969
333.0971
0.60
318.0736 303.0501 318.0736 285.0390 275.0551
1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Xanthones
(Mou et al., 2020)
41
27.92
C16H14O6
[M+H]+
303.0863
303.0864
0.33
288.0630 273.0395 270.0524 259.0604 245.0446
8-Hydroxy-1,3,5-trimethoxyxanthone
Xanthones
(Jia et al., 2011)
42
28.66
C15H12O5
[M+H]+
273.0758
273.0758
0.00
258.0525
1-Hydroxy-3,5-dimethoxyxanthone
Xanthones
(Swati et al., 2023)
43
29.45
C15H12O6
[M+H]+
289.0707
289.0709
0.69
274.0474 256.0367 246.0525
1,8-Dihydroxy-3,5-dimethoxyxanthone
Xanthones
(Swati et al., 2023)
44
29.76
C15H12O6
[M+H]+
289.0707
289.0709
0.69
274.0474 256.0367 246.0492 228.0420
1,8-Dihydroxy-3,7-dimethoxyxanthone
Xanthones
(Swati et al., 2023)
45
31.49
C30H46O4
[M+H]+
471.3469
471.3474
1.06
453.3367 189.1641
Glycyrrhetinic acid
Organic acids
(Zheng et al., 2022)
46
34.56
C30H48O4
[M+H]+
473.3625
473.3633
1.69
427.3678 409.3469 255.2125 207.1747 203.1796 201.1641 189.1639 135.1170 121.1017 107.0860
Maslinic acid
Terpenes
(Kim et al., 2023)
47
37.73
C30H48O3
[M+H]+
457.3676
457.3682
1.31
439.3578 411.3625 393.3507 249.1844 191.1795
Oleanolic acid
Terpenes
(Chen et al., 2011)
*48
38.22
C30H48O3
[M+H]+
457.3676
457.3697
4.59
439.3596 411.3626 393.3474 249.1849 191.1794
Ursolic Acid
Terpenes
(Wang et al., 2016)
49
38.98
C18H30O2
[M+H]+
279.2319
279.2331
4.30
261.2215 243.2114 223.1699 137.1327 123.1172 109.1017 95.0862 81.0706
Linolenic acid
Fatty acids
(Kapoor and Huang 2006)
3.1.1 Mangiferin
The compound under investigation, known as Mangiferin, exhibited a retention time of 12.71 min. Its molecular formula is C19H18O11, and the mass spectral signal for its quasi-molecular ion peaks [M−H]- was detected at m/z 421.0782, with a negligible error of 1.42 ppm. The compound's secondary mass spectra revealed characteristic fragment ions at m/z 331.0463, m/z 301.0357, and m/z 271.0251. Notably, the ions observed at m/z 331.0471 and m/z 301.0375 correspond to [M−H−C3H6O3]- and [M−H−C4H8O4]- mass spectrometric fragments, respectively, resulting from internal sugar breakage. These findings, along with a reference to previous work (Xu et al., 2008), confirm the identification of the compound as Mangiferin. The cleavage pattern of Mangiferin is depicted in Fig. 3A.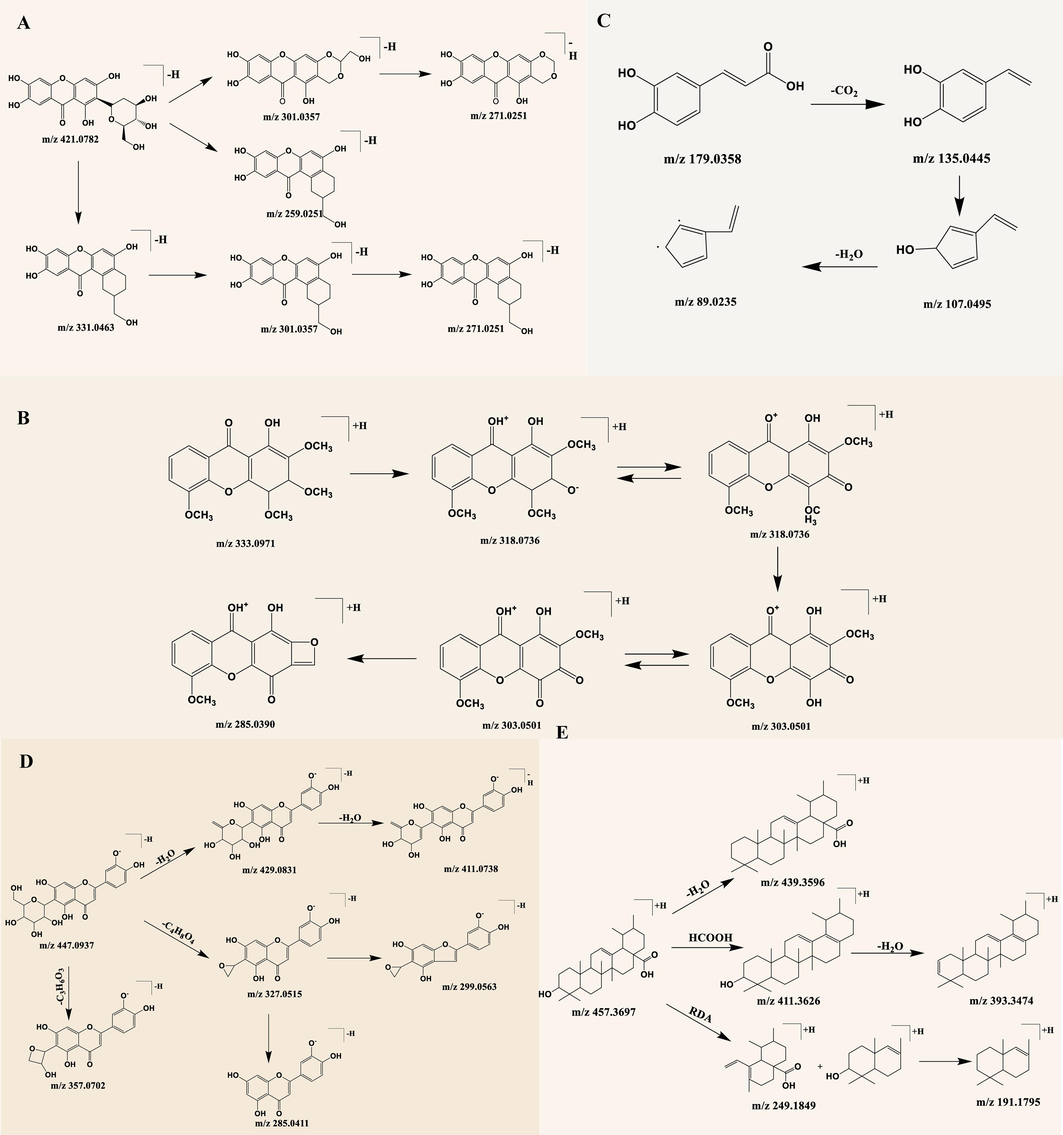
Diagram of cleavage pattern of chemical composition of 80% ethanolic extract of S. chirayita.
3.1.2 1-Hydroxy-2,3,4,5-tetramethoxyxanthone
The compound of interest exhibited a retention time of 17.9 min. Its molecular formula was determined as C17H16O7 and the mass spectral signal of its quasi-molecular ion peaks [M+H]+ was recorded at m/z 333.0970, with a deviation of 0.30 ppm. The secondary mass spectra displayed characteristic fragment ions at m/z 318.0736, m/z 303.0501, m/z 318.0736, m/z 285.0390, and m/z 275.0550. The ion m/z 318.0739 represents the fragment [M+H-CH3], which is formed by the removal of one methyl molecule from the precursor ion [M+H]+. Meanwhile, m/z 303.0515 corresponds to the fragment [M+H]+ formed by the simultaneous loss of two methyl group molecules [M+H-2CH3]+. Further, fragments at m/z 285.0385 and 275.0589 are likely the ions [M+H-2CH3-H2O]+ and [M+H-2CH3-CO]+, respectively, resulting from neutral losses of H2O (18 Da) and CO (28 Da) at m/z 303.0515. Hence, based on this analysis, the compound has been identified as 1-Hydroxy-2,3,4,5-tetramethoxyxanthone (Mou et al., 2020). The cleavage pattern of 1-Hydroxy-2,3,4,5-tetramethoxyxanthone is presented in Fig. 3B.
3.1.3 Caffeic acid
The compound under analysis had a retention time of 16.63 min. Its molecular formula was determined as C9H8O4 and the mass spectral signal of its quasi-molecular ion peaks [M−H]− was recorded at m/z 179.0358, with a deviation of 4.47 ppm. The secondary mass spectra exhibited characteristic fragment ions at m/z 135.0445, m/z 107.0495, and m/z 89.0235. Based on these findings and referencing to previous work (Xiao et al., 2022), the compound was identified as caffeic acid. The cleavage pattern of caffeic acid is illustrated in Fig. 3C.
3.1.4 Isoorientin
The compound of interest exhibited a retention time of 14.58 min and was identified as C21H20O11 based on the inferred molecular formula. Mass spectral analysis showed a quasi-molecular ion peak [M−H]- at m/z 447.0937, with a minimal error of 1.12 ppm. The secondary mass spectra of the compound displayed characteristic fragment ions at m/z 429.0831, m/z 411.0738, m/z 357.0702, m/z 327.0515, m/z 299.0563, and m/z 285.0411. Notably, the ions observed at m/z 357.0617 and m/z 327.0514 could indicate neutral losses of 90 Da and 120 Da, respectively, which are common features in C-glycosides. These findings, along with support from existing literature (Liu et al., 2019), confirm the identification of the compound as isoorientin. The fragmentation pathway of isoorientin is depicted in Fig. 3D.
3.1.5 Ursolic acid
The compound exhibited a retention time of 37.73 min. Its molecular formula was determined to be C30H48O, and the mass spectral signal showed quasi-molecular ion peaks [M+H]+ at m/z 457.3697, with a minimal error of 4.59 ppm. Secondary mass spectra exhibited characteristic fragment ions at m/z 439.3596, m/z 411.3626, m/z 249.1849, and m/z 191.1795. The losses of neutral molecules H2O (18 Da) and HCOOH (46 Da), as well as the retro-Diels-Alder (RDA) fragmentation of the C ring, were identified based on previous literature (Wang et al., 2015). Therefore, this compound can be identified as ursolic acid. The fragmentation pattern is illustrated in Fig. 3E.
3.2 Analysis of serum pharmacochemistry of S. chirayita
Due to the complex chemical composition of TCM/ethnomedicines, it is more effective to analyze the process of how they enter the body and identify their blood-entry components when applying network pharmacology to the study of TCM/ethnomedicines. Blood-entry components are considered as potential bioactive components of TCM/ethnomedicines that exert pharmacological effects. Therefore, an examination of the blood entry prototype components of S. chirayita in rats was conducted using the UPLC-Q-Exactive Orbitrap MS technique. As a result, a total of 10 blood-entry prototype components was successfully identified, including swertianlarin, sweroside, mangiferin, bellidifolin, and isobellidifolin, among others (Figs. 4, 5, and Table 3). In conclusion, these findings confirm that these ten blood-entry prototype components may be the main components of S. chirayita responsible for its medicinal effects.
TIC of drug-containing plasma in the negative (A) and positive (B) ion modes.
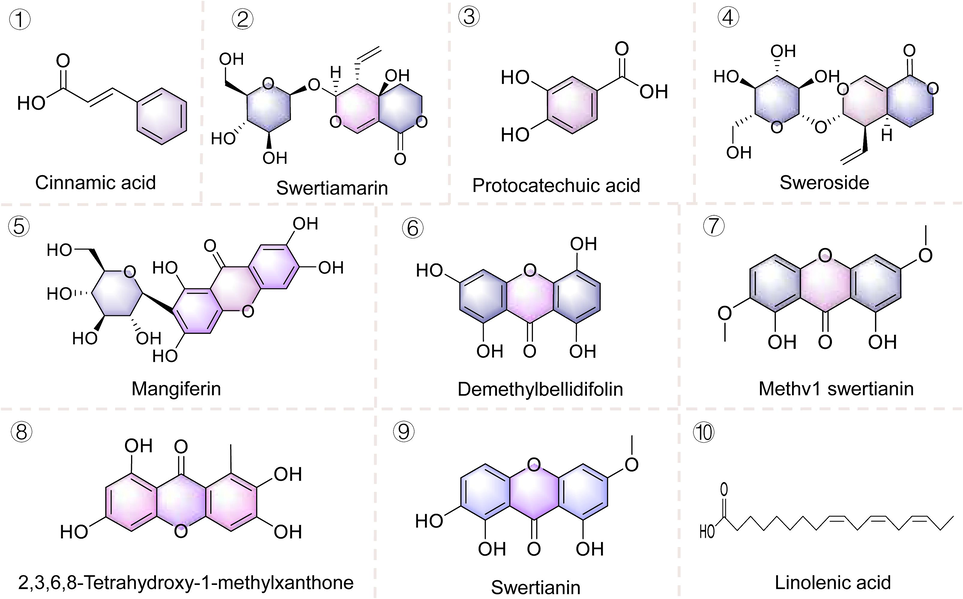
The structure of prototypic components of 80 % ethanolic extract of S. chirayita in the serum of SD rats.
No
tR/
minFormula
Ion mode
Found at mass
Extraction mass
δ/ppm
Fragment ions
Compounds
Type
1
3.62
C9H8O2
[M+H]+
149.0597
149.0601
2.68
131.0495 121.0652 107.0497 79.0549
Cinnamic acid
Organic acids
2
6.19
C16H22O10
[M−H]-
373.1140
373.1143
0.80
193.0352 149.5000 113.0237 89.0234 85.0286 71.0129 59.0128
Swertiamarin
Iridoids
3
7.18
C7H6O4
[M−H]-
153.0193
153.0188
−3.28
125.0236 109.0288 108.0210
Protocatechuic acid
Organic acids
4
11.72
C16H22O9
[M+H]+
359.1337
359.1346
2.51
197.0815 154.9906 110.0207
Sweroside
Iridoids
5
12.57
C19H18O11
[M−H]-
421.0776
421.0784
1.90
331.0464 301.0358 271.0258 259.0245
Mangifera
Xanthones
6
18.75
C13H8O6
[M−H]-
259.0248
259.0251
1.16
215.0343 187.0394
Demethylbellidifolin
Xanthones
7
19.95
C13H8O6
[M−H]-
259.0248
259.0252
1.54
215.0352 187.0395
Methv1swertianin
Xanthones
8
20.42
C14H10O6
[M+H]+
275.055
275.0554
1.45
232.0371 229.0503 201.0551
2,3,6,8-Tetrahydroxy-1-methylxanthone
Xanthones
9
24.57
C14H10O6
[M−H]-
273.0405
273.041
1.83
258.0173 230.0221
Swertianin
Xanthones
10
36.53
C18H30O2
[M+H]+
279.2319
279.2326
2.51
261.2224 243.2121 223.1703 149.0237 137.1328 123.1173 109.1018 95.0862 81.0707
Linolenic acid
Others
3.3 Network pharmacology analysis
3.3.1 Potential targets prediction
To clarify the potential targets of S. chirayita against ALI, we used the SwissTargetPrediction database to predict the potential targets of 10 blood-entry prototype components of S. chirayita. And 310 relevant targets were obtained. Additionally, 1,803 ALI-associated targets we identified based on GeneCards and OMIM databases. By using the Venny online tool, 148 potential common targets were obtained (Fig. 6A). These 148 targets are considered as potential targets for S. chirayita to exert its anti-ALI effects.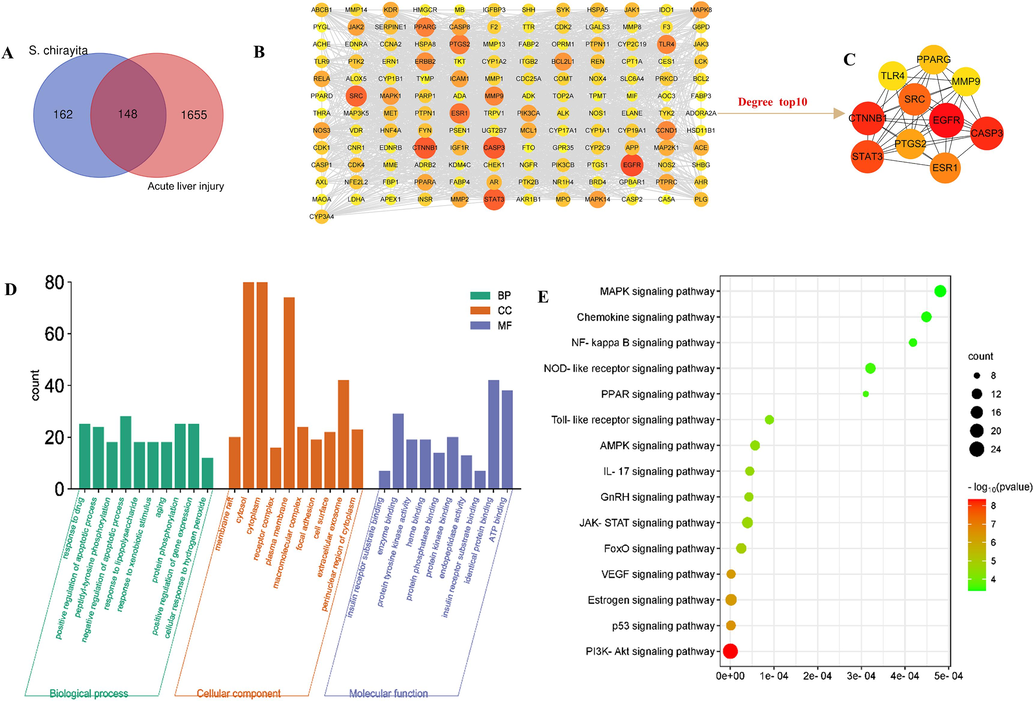
Hub genes of the chemical components of 80 % ethanolic extract of S. chirayita absorbed in blood and acute liver injury. (A) Venn diagram and candidate targets of the chemical components of 80 % ethanolic extract of S. chirayita absorbed in blood and acute liver injury, (B) Protein-protein interaction (PPI) network, (C) KEGG enrichment diagram, (D) GO including CC, MF, and BP analysis diagram, (E) Molecular docking heat map of 10 core targets with 10 critical compounds. The color represents the binding ability. The greener the color, the lower the binding ability and the higher the affinity between the receptor and the ligand.
3.3.2 PPI network analysis and enrichment analysis
To investigate the potential function and mechanism of action of S. chirayita on ALI, we conducted PPI network analysis and enrichment analysis on the 148 cross-targets mentioned earlier. Firstly, the STRING database was utilized to construct a PPI network comprising these 148 common targets (Fig. 6B). This network was crucial in understanding how S. chirayita intervenes in ALI, as all of these genes were included. Subsequently, the cytoHubba plug-in in Cytoscape 3.7.1 were employed to filter the top targets based on their degree value, resulting in the identification of the top 10 targets, which included EGFR, CASP3, CTNNB1, STAT3, SRC, ESR1, PTGS2, PPARG, TLR4 and MMP9 (Fig. 6C). Taken together, these 10 targets were deemed as the core targets responsible for S. chirayita's anti-ALI effects. GO analysis revealed that these ten typical blood components could potentially regulate ALI through biological processes such as drug response and positive regulation of apoptosis (Fig. 6D). Furthermore, KEGG analysis suggested that S. chirayita might govern ALI through various signaling pathways, including the PI3K/Akt pathway, HIF-1 pathway, MAPK pathway, VEGF pathway, AMPK signaling pathway, FoxO signaling pathway, JAK/STAT signaling pathway, NF-κB signaling pathway, among others (Fig. 6E and Table 4).
No
Signaling pathway
FDR
PValue
Count
1
HIF-1 signaling pathway
3.85E-10
3.01E-11
17
2
PI3K-Akt signaling pathway
2.72E-08
4.5E-09
25
3
p53 signaling pathway
1.23E-06
3.52E-07
11
4
Estrogen signaling pathway
1.71E-06
5.22E-07
14
5
VEGF signaling pathway
1.73E-06
5.43E-07
10
6
FoxO signaling pathway
2.51E-05
1.24E-05
12
7
JAK-STAT signaling pathway
3.93E-05
2.28E-05
13
8
GnRH signaling pathway
4.26E-05
2.55E-05
10
9
IL-17 signaling pathway
4.45E-05
2.78E-05
10
10
AMPK signaling pathway
5.67E-05
3.6E-05
11
11
Toll-like receptor signaling pathway
8.96E-05
6.23E-05
10
12
PPAR signaling pathway
0.00031
0.000262
8
13
NOD-like receptor signaling pathway
0.000321
0.000276
12
14
NF-kappa B signaling pathway
0.000418
0.000364
9
15
Chemokine signaling pathway
0.000449
0.000398
12
16
MAPK signaling pathway
0.000481
0.000439
15
17
Adipocytokine signaling pathway
0.001118
0.001059
7
3.4 Molecular docking analysis
Molecular docking simulations were used to investigate the interaction dynamics between the ten blood-entry prototype components of S. chirayita and key protein targets associated with ALI. The top ten targets, including EGFR, CASP3, CTNNB1, STAT3, SRC, ESR1, PTGS2, PPARG, TLR4, and MMP9 (Fig. 6C and Table 5). These targets exhibited strong binding potential with S. chirayita constituents, as indicated by docking scores surpassing the threshold of −6 (Fig. 7 and Table 6). These findings suggest that the constituents of S. chirayita, particularly STW, may play a significant role in the herb's therapeutic efficacy in ALI.
No
Targets
Name of targets
Uniprot ID
Degree
1
EGFR
Epidermal growth factor receptor
P00533
86.0
2
CASP3
Caspase-3
P42574
80.0
3
CTNNB1
Catenin beta-1
P35222
80.0
4
STAT3
Signal transducer and activator of transcription 3
P40763
78.0
5
SRC
Proto-oncogene tyrosine-protein kinase Src
P12931
75.0
6
ESR1
Estrogen receptor
P03372
69.0
7
PTGS2
Prostaglandin G/H synthase 2
P35354
66.0
8
PPARG
Peroxisome proliferator-activated receptor gamma
P37231
63.0
9
TLR4
Toll-like receptor 4
O00206
61.0
10
MMP9
Matrix metalloproteinase-9
P14780
61.0
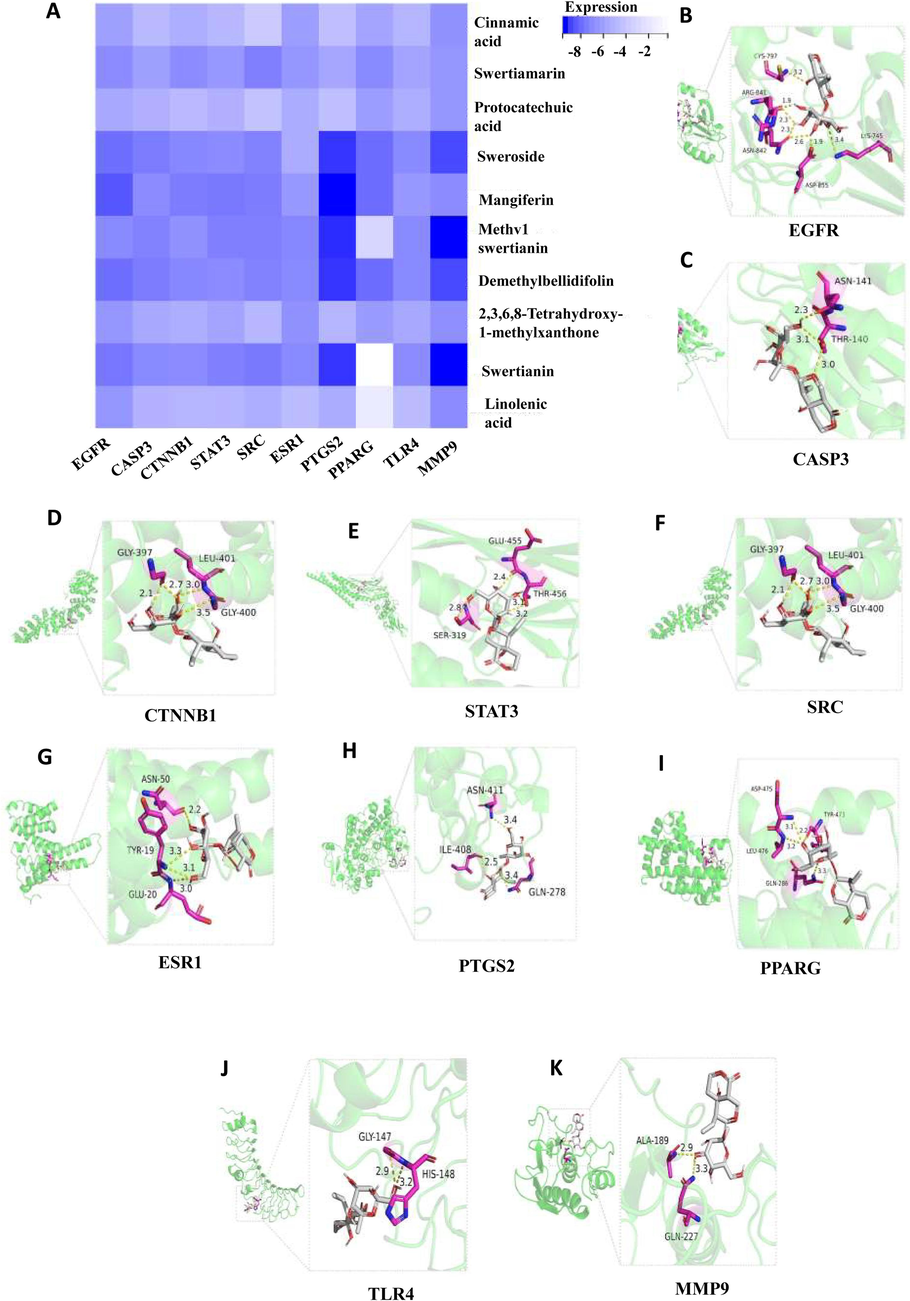
Docking patterns of 10 targets with STW with the lower binding ability. (A) EGFR&STW, (B) CASP3&STW, (C) CTNNB1&STW, (D) STAT3&STW, (E) SRC&STW, (F) ESR1&STW, (G) PTGS2&STW, (H) PPARG&STW, (I) TLR4&STW, (J) MMP9&STW.
Compounds
EGFR
CASP3
CTNNB1
STAT3
SRC
ESR1
PTGS2
PPARG
TLR4
MMP9
3w2s
1nme
6 m90
6njs
3vro
7baa
5f1a
3sz1
3ul7
6esm
Cinnamic acid
−6.3
−5
−5.6
−5.1
−4
−6.4
−4.7
−6.1
−5.2
−6.9
Swertianmarin
−7.1
−6.3
−7.1
−6.6
−7.6
−6.7
−6
−7.2
−6.1
−6.7
Protocatechuic acid
−5.9
−5.5
−5
−5.5
−4.5
−5.7
−4.7
−6.4
−5.6
−6.7
Sweroside
−8.1
−7.7
−7.3
−7.1
−7.5
−5.7
−9.3
−8.2
−7.2
−9.0
Mangiferin
−8.7
−7.1
−7.7
−7.8
−7.7
−6.6
−9.6
−8.2
−6.6
−7.1
Methv1swertianin
−7.2
−7.5
−6.9
−7.6
−7.6
−7.3
−9.4
−3.3
−7.2
−9.6
Demethylbellidifolin
−8.1
−7.7
−7.3
−7.1
−7.5
−7.2
−9.3
−8.2
−7.2
−9.0
2,3,6,8-Tetrahydroxy-1-methylxanthone
−6.7
−5.9
−5.6
−6.1
−5.1
−6.9
−5.2
−6.5
−5.9
−7.0
Swertianin
−7.9
−7.2
−7.1
−7.6
−7.8
−7.2
−9.3
−0.3
−7.1
−9.6
Linolenic acid
−6.7
−5.4
−5.2
−5.3
−5.6
−4.9
−5.7
−1.8
−4.9
−7.1
3.5 Network analysis of STW in ALI
Swertiamarin has been traditionally used in the treatment of liver diseases and has shown promising hepatoprotective effects in preliminary studies. Based on the results of blood component analysis and molecular docking, STW was hypothesized is the crucial chemical component for S. chirayita in its anti-ALI effects. All in all, this historical and preliminary evidence provided a strong foundation for its selection as a focus for our detailed investigation. To further investigate, a network of potential targets involved in the anti-ALI properties of STW was constructed. The result revealed a total of 139 targets associated with the anti-ALI effects of STW (Supplementary Fig. S3A). Among these targets, EGFR, CASP3, CTNNB1, STAT3, ESR1, PTGS2, PPARG, TLR4, and MMP9 were identified as key targets in the anti-ALI intervention of S. chirayita (Supplementary Fig. S3B). Notably, CTNNB1, STAT3, ESR1, PTGS2, PPARG, TLR4, and MMP9 were also found to be key targets in the ALI intervention of STW. This finding further supports our molecular docking results. In summary, STW was conclude that is the key pharmacodynamic substance responsible for the anti-ALI effects of S. chirayita. Therefore, the anti-ALI pharmacodynamic effects of STW was subsequently verified.
3.6 Analysis of the intervention of the STW in CCl4-induced ALI
3.6.1 Analysis of liver index
An evaluation of the liver index following STW administration offers insights into the therapeutic impact of S. chirayita on liver pathology. In mouse models subjected to CCl4-induced hepatic injury, treatment with STW significantly decreased the liver index, serving as an indicator of reduced organ swelling and damage. Compared to the blank group, those in the model group exhibited a statistically significant increase in liver index (p < 0.01). In contrast, administration of STW at varying doses, as well as silymarin, distinctly attenuated the liver index, suggesting a dose-responsive protective effect against the hepatic injury (p < 0.05) (Fig. 8A). These findings underscore the potential of STW as a mitigating agent in CCl4-induced liver damage.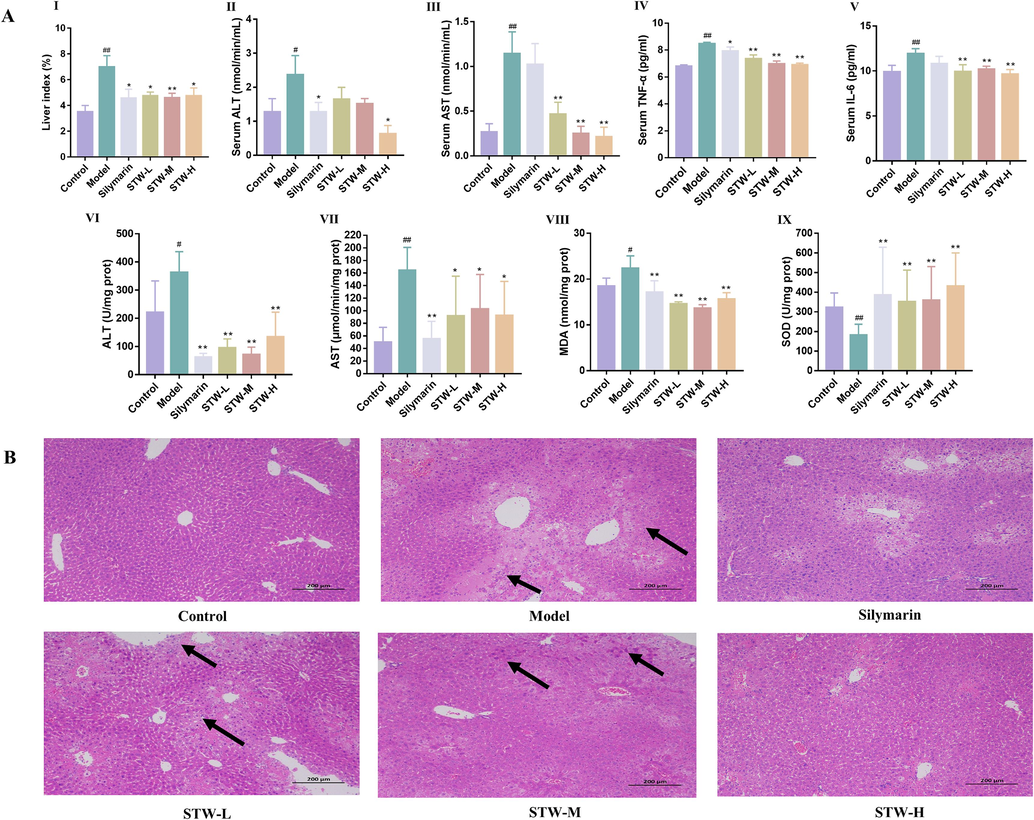
(A) Effect of the liver index, the biochemistry of serum and liver tissue in CCl4-induced acute liver injury model. (I) liver index, (II) concentration of ALT in serum, (III) concentration of AST in serum, (IV) concentration of TNF-α in serum, (V) concentration of IL-6 in serum, (VI) concentration of ALT in liver, (VII) concentration of AST in liver, (VIII) concentration of MDA in liver, (IX) concentration of SOD in liver. (B) STW ameliorates induced histopathological changes in the liver tissues of CCl4-induced acute liver injury model. #p < 0.05, ##p < 0.01, vs. the control group; *p < 0.05, **p < 0.01, vs. the model group.
3.6.2 Biochemical analysis of the serum
When exposed to high quantities of CCl4, the liver is attacked, resulting in an excessive production of free radicals. This, in turn, increases the permeability of cell membranes, leading to the infiltration of inflammatory neutrophils and a significant influx of ALT, AST, TNF-α, and IL-6 into the bloodstream. These factors contribute to the elevated serum levels (Masuda 2006, Niu et al., 2017). Our findings demonstrate a statistically significant reduction in ALT levels among subjects treated with silymarin or STW-H groups compared to the model group, indicating a protective effect on the liver (p < 0.05). Similarly, AST, TNF-α, and IL-6 levels were significantly lower in all STW dosed groups (low, medium, and high), as well as the silymarin-treated group, in comparison to the model group. This highlights the potential of the treatment to alleviate the inflammatory response associated with hepatic injury (Fig. 8A). These differences emphasize the therapeutic potential of STW in mitigating CCl4-induced hepatic damage.
3.6.3 Biochemical analysis of the liver
The therapeutic efficacy of STW in acute CCl4-induced hepatic injury was evaluated by examining liver biochemical parameters. Since the liver is the primary site of acute CCl4-induced ALI (Dong et al., 2013), it was important to assess the effects of STW. Compared to the control group, all STW treatment protocols (low, medium, and high doses) as well as silymarin administration showed significant improvements in hepatic ALT, AST, MDA, and SOD levels (p < 0.01). These findings not only support the serum biochemical results but also provide additional evidence that STW has a hepatoprotective effect against CCl4-induced damage. This reinforces the potential use of STW for intervention in CCl4-induced ALI (Fig. 8A).
3.6.4 Histological analysis of the liver
Histopathological examinations were conducted using H&E staining to provide a detailed understanding of the hepatoprotective impact of STW. In the control group, liver sections exhibited well-preserved lobular architecture, orderly arranged hepatocytes, and intact liver capsule structures without any swelling or inflammatory infiltration. In contrast, liver samples from the CCl4-induced model group showed extensive inflammatory infiltrate surrounding vascular structures, along with significant hepatocellular degeneration and necrosis. Treatment with different dosages of STW (low, medium, and high) and silymarin resulted in significant improvements in these pathological changes. The extent of improvement in inflammatory infiltration, edema, degeneration, and necrosis of hepatocytes varied depending on the treatment concentration, but was observed in all treated groups (Fig. 8B). Notably, the group receiving the highest dosage of STW (STW-H) exhibited the most substantial amelioration of CCl4-induced liver damage, highlighting the potential of STW in counteracting hepatic insult.
3.6.5 Analysis of qRT-PCR
To substantiate the interactions proposed by our network pharmacology framework, we used qRT-PCR to evaluate the gene expression of pivotal targets. The results showed that STW administration significantly downregulated the mRNA levels of EGFR (Fig. 9A), CASP3 (Fig. 9B), CTNNB1 (Fig. 9C), STAT3 (Fig. 9D), ESR1 (Fig. 9F), PTGS2 (Fig. 9G), PPARG (Fig. 9H), TLR4 (Fig. 9I) and MMP9 (Fig. 9J). Interestingly, SRC expression was found to be upregulated upon STW treatment (Fig. 9E). These expression patterns not only confirmed the network pharmacology predictions but also validated the target-related pathways involved in the therapeutic action of STW. The alignment of these qRT-PCR findings with the in-silico data provides strong evidence for the regulatory role of STW on these key genetic targets, strengthening the credibility of the computational strategies used to understand the molecular basis of S. chirayita's pharmacological effects.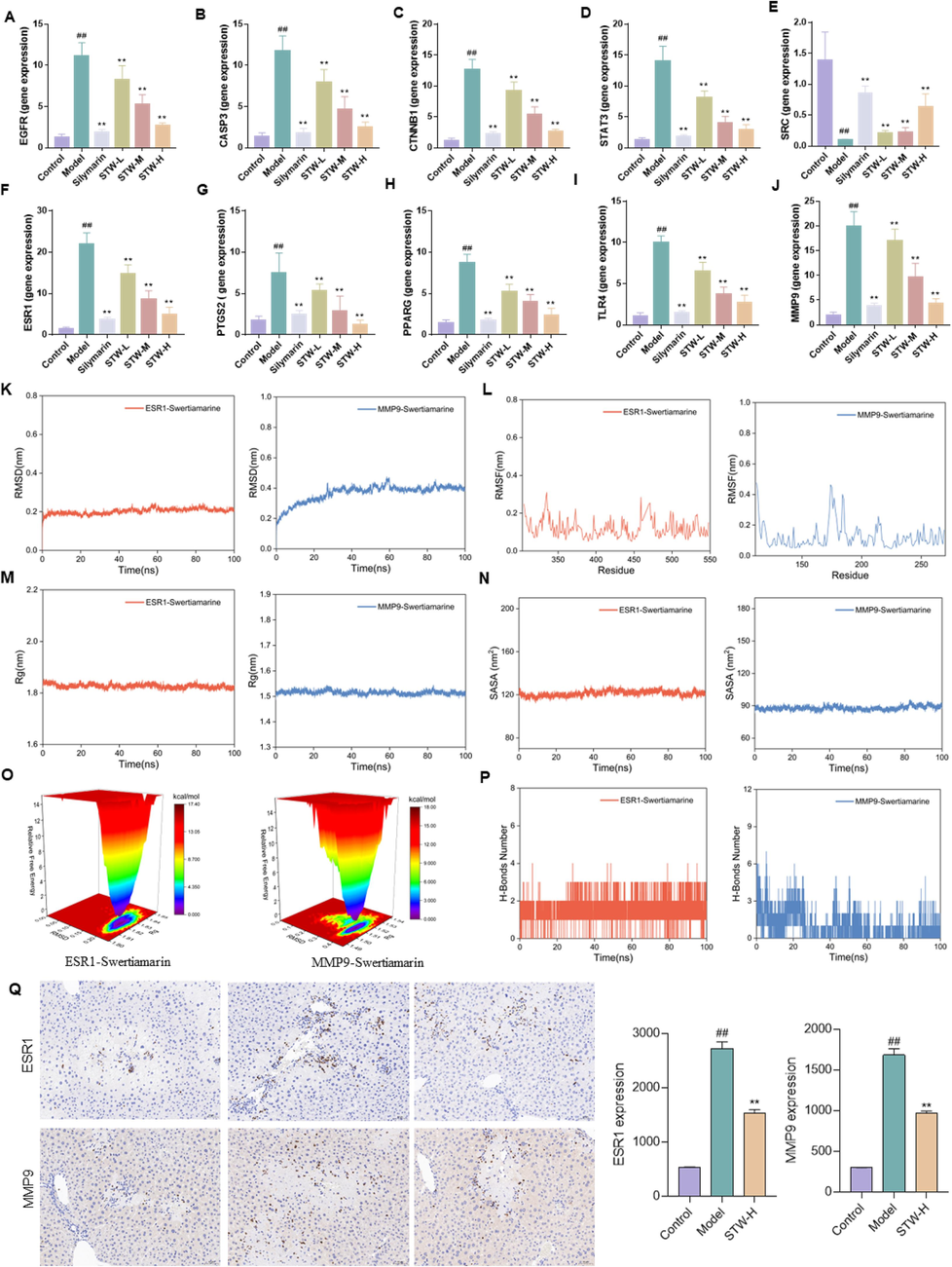
Potential targets for STW intervention in a CCl4-induced acute liver injury model. (A) mRNA level of EGFR, (B) mRNA level of CASP3, (C) mRNA level of CTNNB1, (D) mRNA level of STAT3, (E) mRNA level of SRC, (F) mRNA level of ESR1, (G) mRNA level of PTGS2, (H) mRNA level of PPARG, (I) mRNA level of TLR4, (J) mRNA level of MMP9. (K) RMSD analysis of ESR1 and MMP9 with STW, (L) RMSF analysis of ESR1 and MMP9 with STW, (M) Rg analysis of ESR1 and MMP9 with STW, (N) SARA analysis of ESR1 and MMP9 with STW, (O) Free energy landscape map analysis of ESR1 and MMP9 with STW, (P) H-Bounds number analysis of ESR1 and MMP9 with STW, (Q) IHC analysis of ESR1 and MMP9 in liver. #p < 0.05, ##p < 0.01, vs. the control group; *p < 0.05, **p < 0.01, vs. the model group.
3.6.6 Analysis of molecular dynamics and immunohistochemistry
To enhance the validation of molecular docking individual qRT-PCR results, we combined both expression levels and selected the target with an expression level greater than 15 and a molecular docking score lower than −6.5 kcal·mol−1 for further validation. The molecular dynamics findings indicated that STW effectively bound to both ESR1 and MMP9. Specifically, the RSMD analysis revealed that the RMSD curves of ESR1 and MMP9 proteins in complex with STW stabilized after initial fluctuations, with fluctuations remaining within 0.5 nm, indicating a stable binding formation between them and STW (Fig. 9K). The RMSF curves for ESR1 and MMP9 proteins in complex with STW did not fluctuate beyond 1 nm, with no amino acid residues showing significant fluctuations, suggesting that STW did not affect the overall structural stability of the proteins (Fig. 9L). Rg (Fig. 9M) and SASA (Fig. 9N) results confirmed that the addition of STW did not notably impact the overall structural stability of the protein. The free energy landscape map displayed a single minimum energy cluster for both ESR1 and MMP9 in association with STW, indicating a high stability of the complex formed by them with STW (Fig. 9O). The results from the analysis of hydrogen bond quantity revealed that ESR1, MMP9, and STW all formed stable hydrogen bonding interactions (Fig. 9P). Additionally, to further elucidate the protein levels of ESR1 and MMP9, an immunohistochemistry assay was conducted. The findings demonstrated that STW notably enhanced the protein expression levels of ESR1 and MMP9 (Fig. 9Q), providing further evidence that these targets could be crucial for the intervention of swertiamarin in acute liver injury. The consistency between the results of immunohistochemistry, molecular dynamics, and PCR underscored the promising potential of bioinformatics analysis in drug research.
4 Discussion
Our integrative analysis of S. chirayita through UPLC-Q-Exactive Orbitrap MS has successfully demystified the plant's pharmacologically active constituents, identifying 49 chemical entities with potential medicinal relevance. These entities span a diverse array of phytochemical classes, including xanthones, organic acids, flavonoids, and terpenoids, which may collectively contribute to the observed medicinal properties of the herb. Intriguingly, these chemical investigations align with traditional claims of S. chirayita's therapeutic versatility and offer a foundation for understanding the molecular basis of its efficacy. Furthermore, the precise fragmentation patterns elucidated for compounds like mangiferin and 1-Hydroxy-2,3,4,5-tetramethoxyxanthone enable accurate identification and deepen our understanding of their intrinsic pharmacodynamic profiles.
Pharmacokinetic studies have demonstrated that the absorption of a drug is the primary stage in the therapeutic mechanism of a medication (Lin et al., 2022). Presently, oral administration is the most common route for traditional Chinese medicines, with their active constituents primarily transported to target sites through the bloodstream to achieve an optimal blood concentration (Tan et al., 2023). The components present in the blood are deemed vital for eliciting therapeutic effects. Consequently, extending beyond compound characterization, our serum pharmacochemical analysis pinpointed ten blood-entry components, including STW, sweroside, and others, that may play a cardinal role in mediating S. chirayita's therapeutic effects in vivo. This vital insight bridges the traditional usage of the herb with a contemporary understanding of its bioactive constituents.
The network pharmacology approach further refined our exploration using the ten blood-entry components, pinpointing potential molecular targets and signaling pathways that S. chirayita might modulate. Key amongst these, EGFR, CASP3, and STAT3 were identified as central nodes within a complex therapeutic network, potentially orchestrating the plant's protective effects against ALI. The KEGG analysis revealed that S. chirayita may impact ALI through various signaling pathways, such as PI3K/Akt, HIF-1, MAPK, FoxO, JAK/STAT, and NF-κB, among others. Previous studies have suggested that oxidative stress and inflammation play a role in the pathogenesis and progression of CCl4-induced ALI (Zhan et al., 2021, Bekkouch et al., 2022). The NF-κB signaling pathway is well-known for its association with inflammation. Previous studies have shown that cyanidin effectively reduces the symptoms of CCl4-induced ALI by suppressing targets related to the NF-κB signaling pathway (Wang et al., 2022a,b). Similarly, evidence suggests that the JianPi-QingHua formula significantly improves the dysregulation of inflammatory factors in a nonalcoholic steatohepatitis model by modulating critical elements within the NF-κB pathway, thereby providing hepatoprotective effects (Tian et al., 2023). The PI3K/AKT signaling pathway acts as a negative feedback mechanism for Toll-like receptor signaling, limiting the pro-inflammatory response in LPS-stimulated macrophages and promoting an anti-inflammatory response by regulating the expression of miRNAs for TLR4 and SOCS111 (Liu et al., 2023). Another study reveals that fraxin exerts hepatoprotective effects by inhibiting MAPK and NF-κB activation. This is achieved by reducing the expression of MAPKs, NF-κB, and COX-2 proteins in the liver tissue of mice with CCl4-induced liver injury (Niu et al., 2017). The HIF-1 is a heterodimer composed of both HIF1-α and HIF1-β subunits. Under hypoxic conditions, HIF1-α upregulates the expression of p53 protein, leading to its accumulation. In the same conditions, p53 can directly interact with HIF1-α to prevent the expression of HIF1-α induced by hypoxia. This interaction promotes MDM2-mediated ubiquitination and proteasomal degradation (Chu et al., 2022). Previous studies have shown the involvement of HIF-1 in various liver diseases, including acetaminophen (APAP)-induced liver injury, fatty liver, alcoholic hepatitis, nonalcoholic steatohepatitis (Mesarwi et al., 2021), liver fibrosis (Du et al., 2022) and liver cancer (Chen et al., 2023, Kou et al., 2023). Nevertheless, there is a limited number of reports investigating the regulatory role of these pathways in CCl4-induced ALI. Further molecular docking and network analyses revealed that STW may play an important role in S. chirayita resistance to ALI. Particularly noteworthy, STW, is unique to the Swertia L. genus, which includes S. chirayita and S. mussotii (Guo et al., 2023). However, it has been relatively understudied in the context of CCl4-induced ALI. Therefore, as a signature component of Swertia L., we have carried out further studies to highlight the important research value of STW.
A considerable amount of research has emphasized the role of inflammatory cytokines in the development of liver injury. It is possible that reducing these cytokines could potentially decrease the occurrence of diseases. Recent evidence suggests that TNF-α and IL-6 are particularly involved in experimental models of CCl4-induced ALI (Zhang et al., 2016, Öztürk Akcora et al., 2017). Moreover, when the liver is exposed to high levels of CCl4, the abnormal release of free radicals increases the permeability of cell membranes, leading to the infiltration of inflammatory neutrophils and the significant release of AST, ALT, IL-6, and TNF-α into the bloodstream, resulting in elevated serum levels. Consequently, the overexpression of ALT, AST, TNF-α, and IL-6 in the serum is considered a crucial indicator of ALI. The liver, which acts as the body's detoxifying organ, contains antioxidants such as SOD, MDA, glutathione peroxidase, and catalase. These antioxidants play a vital role in eliminating ROS and protecting the liver during alcohol metabolism (Dai et al., 2023). In this experiment, a 20-hour exposure to CCl4 resulted in abnormal liver and serum expression of liver function markers (ALT and AST), inflammatory factors (IL-6 and TNF-α), and oxidative factors (MDA and SOD) in the model group. However, intervention with STW significantly reversed these abnormalities. Histopathological examination of the liver revealed a significant and dose-dependent improvement in cellular necrosis, inflammatory infiltration, and other liver tissue lesions after the STW intervention. It is important to note that the mechanism of these identified targets in CCl4-induced ALI is not fully understood and requires further investigation. Previous studies have shown that targets such as EGFR, CASP3, and STAT3 are closely associated with diseases such as liver fibrosis (Wu et al., 2022), NASH (Peng et al., 2023), and ALI (Dai et al., 2023). However, the specific mechanisms of these targets in CCl4-induced ALI are still unclear. The qRT-PCR results showed that STW significantly decreased the mRNA expression of key targets such as EGFR, CASP3, and STAT3, thereby relieving CCl4-induced ALI. And molecular dynamics and immunohistochemistry further demonstrated the potential mechanism maybe related to ESR1 and MMP9. These findings corroborate the outcomes of our network pharmacological and molecular docking analyses, strengthening the hypothesis that STW plays a central role in S. chirayita’s medicinal effect on liver pathology.
However, our study has certain limitations and areas for improvement. Firstly, we focused only on the in vitro and blood components of the 80 % ethanol extract of S. chirayita. To provide more comprehensive experimental data, it would have been beneficial to conduct a comparative analysis of the chemical composition of ethanolic extracts at various concentrations. Secondly, although we used the UPLC-Q-Exactive Orbitrap MS technique for ex vivo compositional studies, it may have missed some trace components. Therefore, further investigations on the metabolites of S. chirayita are necessary. Additionally, our study only detected 10 prototypical blood components of S. chirayita using UPLC-Q-Exactive Orbitrap MS and did not consider the in vivo metabolism of these compounds. Thirdly, we only tested the compound STW, which had the highest docking score, against 10 core targets. To enhance the comprehensiveness of our study, it is important to conduct further in-depth research involving additional compounds and explore their protective activity in models. Finally, future investigations should focus on elucidating the mechanism of action of STW in intervening in CCl4-induced ALI. This can be achieved through methodologies such as intestinal flora assessment, metabolomics, transcriptomics, plasmon resonance technology, and other tools, combined with in vivo validation in core gene-deficient mice.
While S. Chirayita and its key ingredient Swertiamarin have been traditionally used for treating liver diseases, our study offers several novel contributions to clinical practice. This includes the innovative application of systems biology approaches to systematically analyze the potential mechanisms of Swertiamarin in ALI, and the in-depth exploration of molecular mechanisms through network pharmacology and molecular docking. Furthermore, by providing experimental validation in the CCl4-induced ALI rat model, our study supports the clinical translation of Swertiamarin from a traditional remedy to a modern therapeutic agent. This highlights the multi-target nature of Swertiamarin, aligning with personalized medicine principles and suggesting potential for developing individualized treatment strategies for liver diseases.
5 Conclusions
In summary, UPLC-Q-Exactive Orbitrap MS, network pharmacology, and molecular docking techniques were utilized to analyze the bioactive compounds in S. chirayita. Additionally, in vivo experiments were conducted to investigate the mechanism of the active ingredient, STW, in ameliorating CCl4-induced ALI. These results suggested that STW from S. chirayita may be the most crucial active substance for improving ALI. Furthermore, several key targets that play an important role in this process were identified, including EGFR, CASP3, CTNNB1, STAT3, SRC, ESR1, PTGS2, PPARG, TLR4, and MMP9, especially ESR1 and MMP9, and in addition, the PI3K/Akt, JAK/STAT, and HIF-1 pathways were considered as key pathways for its function. This research provides a solid theoretical framework for further exploration and development of S. chirayita and STW, paving the way for potential therapeutic applications.
6 Availability of data and materials
All data generated or analyzed during this study are included in this article.
CRediT authorship contribution statement
Sa Guo: Investigation, Methodology, Visualization, Writing – original draft. Cen Wu: Investigation, Methodology. Xinwei Liu: Investigation, Methodology. Xianli Meng: Writing – review & editing. Yi Zhang: Supervision, Writing – review & editing. Shaohui Wang: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (81973573).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of sorafenib and quercetin alone or in combination in treating hepatocellular carcinoma: in vitro and in vivo approaches. Molecules. 2022;27:1-22.
- [CrossRef] [Google Scholar]
- Ketoconazole-induced testicular damage in rats reduced by gentiana extract. Exp. Toxicol. Pathol.. 2008;59:377-384.
- [CrossRef] [Google Scholar]
- Ginger (Zingiber officinale Roscoe), Lemon (Citrus limon L.) Juices as preventive agents from chronic liver damage induced by CCl(4): a biochemical and histological study. Antioxidants (Basel). 2022;11:1-15.
- [CrossRef] [Google Scholar]
- Steroidal saponins: naturally occurring compounds as inhibitors of the hallmarks of cancer. Cancers. 2023;15:3900.
- [CrossRef] [Google Scholar]
- HIF-1α-activated TMEM237 promotes hepatocellular carcinoma progression via the NPHP1/Pyk2/ERK pathway. Cell Mol. Life Sci.. 2023;80:120.
- [CrossRef] [Google Scholar]
- Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography-ion trap mass spectrometry. Biomed. Chromatogr.. 2011;25:1381-1388.
- [CrossRef] [Google Scholar]
- Regulatory mechanism of HIF-1α and its role in liver diseases: a narrative review. Ann. Transl. Med.. 2022;10:109.
- [CrossRef] [Google Scholar]
- Nootkatone supplementation ameliorates carbon tetrachloride-induced acute liver injury via the inhibition of oxidative stress, NF-κB pathways, and the activation of Nrf2/HO-1 pathway. Antioxidants. 2023;12:194.
- [CrossRef] [Google Scholar]
- Identification of metabolites of isochlorogenic acid A in rats based on UHPLC-Q-Exactive Orbitrap MS. Shangdong Sci.. 2022;35:1002-4026.
- [CrossRef] [Google Scholar]
- S-acetyl-glutathione attenuates carbon tetrachloride-induced liver injury by modulating oxidative imbalance and inflammation. Int. J. Mol. Sci.. 2022;23:4429.
- [CrossRef] [Google Scholar]
- Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol.. 2013;62:120-130.
- [CrossRef] [Google Scholar]
- Integrated chemical interpretation and network pharmacology analysis to reveal the anti-liver fibrosis effect of penthorum chinense. Front. Pharmacol.. 2022;13:788388
- [CrossRef] [Google Scholar]
- Cycas revoluta leaves: as a potential flavonoids source for targeted regulation of immune-related markers in lung adenocarcinoma. Ind. Crops Prod.. 2023;202:116967
- [CrossRef] [Google Scholar]
- Pharmacotherapy for nonalcoholic fatty liver disease. Semin. Liver Dis.. 2015;35:338-348.
- [CrossRef] [Google Scholar]
- Analysis on chemical constituents of turkish galls by HPLC-ESI-IT-TOF- MS/MS. Tradit. Chin. Drug Res. Clin. Pharmacol.. 2020;31:1227-1232.
- [CrossRef] [Google Scholar]
- Simultaneous determination of calycosin-7-O-β-D-glucoside, cinnamic acid, paeoniflorin and albiflorin in rat plasma by UHPLC-MS/MS and its application to a pharmacokinetic study of Huangqi Guizhi Wuwu Decoction. J. Pharm. Biomed. Anal.. 2019;170:1-7.
- [CrossRef] [Google Scholar]
- Exploring the protective effect of Gynura procumbens against type 2 diabetes mellitus by network pharmacology and validation in C57BL/KsJ db/db mice. Food Funct.. 2021;12:1732-1744.
- [CrossRef] [Google Scholar]
- Swertia L.: a comprehensive review of its genetic relationship, chemical compositions, pharmacological effects, toxicities, and applications. Phytother. Res.. 2023;37:2605-2643.
- [CrossRef] [Google Scholar]
- Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool.. 2020;81:43.
- [CrossRef] [Google Scholar]
- Progress in the chemical composition and pharmacological effects of chinese Swertia L. plants. Ginseng Res.. 2022;34:45-53.
- [CrossRef] [Google Scholar]
- Analysis of chemical constituents in odontosoria chinensis based on UPLC-Q-TOF-MS. Prac. Clin. J. Integr. Tradit. Chin. West Med.. 2021;21:155-159.
- [CrossRef] [Google Scholar]
- Antioxidant and hepatoprotective effect of swertiamarin from enicostemma axillare against D-galactosamine induced acute liver damage in rats. J Ethnopharmacol.. 2010;130:103-106.
- [CrossRef] [Google Scholar]
- Anti-tumour activities and a high-performance liquid chromatography mass spectrometric method for analysis of the constituents of Lomatogonium carinthiacum. Nat. Prod. Res.. 2011;25:100-107.
- [CrossRef] [Google Scholar]
- Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol.. 2006;7:531-534.
- [CrossRef] [Google Scholar]
- Hypoxia-inducible factor 1α/IL-6 axis in activated hepatic stellate cells aggravates liver fibrosis. Biochem. Biophys. Res. Commun.. 2023;653:21-30.
- [CrossRef] [Google Scholar]
- Iridoids, phenolic compounds and antioxidant activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.) Molecules. 2017;22:405.
- [CrossRef] [Google Scholar]
- Integration of In vitro and in vivo models to predict cellular and tissue dosimetry of nanomaterials using physiologically based pharmacokinetic modeling. ACS Nano.. 2022;16:19722-19754.
- [CrossRef] [Google Scholar]
- Strategy for rapid screening of antioxidant and anti-inflammatory active ingredients in Gynura procumbens (Lour.) Merr. based on UHPLC-Q-TOF-MS/MS and characteristic ion filtration. Biomed. Chromatogr.. 2019;33:e4635.
- [Google Scholar]
- A systematic pharmacology-based in vivo study to reveal the effective mechanism of Yupingfeng in asthma treatment. Phytomedicine. 2023;114:154783
- [CrossRef] [Google Scholar]
- Retracted: anti-diabetic activity of Swertia corymbosa (Griseb.) Wight ex C.B. Clarke aerial parts extract in streptozotocin induced diabetic rats. J. Ethnopharmacol.. 2014;151:1175-1183.
- [CrossRef] [Google Scholar]
- San Pian decoction can treat nitroglycerin-induced migraine in rats by inhibiting the PI3K/AKT and MAPK signaling pathways. J. Ethnopharmacol.. 2022;296:115470
- [CrossRef] [Google Scholar]
- Learning toxicology from carbon tetrachloride-induced hepatotoxicity. Yakugaku Zasshi.. 2006;126:885-899.
- [CrossRef] [Google Scholar]
- Hepatocyte HIF-1 and intermittent hypoxia independently impact liver fibrosis in murine nonalcoholic fatty liver disease. Am J Respir Cell Mol Biol.. 2021;65:390-402.
- [CrossRef] [Google Scholar]
- Cleavage patterns of oxygen-substituted Xanthone analogues by electrospray mass spectrometry. Central South Pharmacy.. 2020;18:953-958.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of fraxin against carbon tetrachloride-induced hepatotoxicity in vitro and in vivo through regulating hepatic antioxidant, inflammation response and the MAPK-NF-κB signaling pathway. Biomed Pharmacother.. 2017;95:1091-1102.
- [CrossRef] [Google Scholar]
- Tyrosine kinase inhibitor BIBF1120 ameliorates inflammation, angiogenesis and fibrosis in CCl(4)-induced liver fibrogenesis mouse model. Sci Rep.. 2017;7:44545.
- [CrossRef] [Google Scholar]
- In silico and in vivo demonstration of the regulatory mechanism of Qi-Ge decoction in treating NAFLD. Ann. Med.. 2023;55:2200258.
- [CrossRef] [Google Scholar]
- Swertia chirayita: a comprehensive review on traditional uses, phytochemistry, quality assessment and pharmacology. J. Ethnopharmacol.. 2023;300:115714
- [CrossRef] [Google Scholar]
- Integrated serum pharmacochemistry, 16S rRNA sequencing and metabolomics to reveal the material basis and mechanism of Yinzhihuang granule against non-alcoholic fatty liver disease. J. Ethnopharmacol.. 2023;310:116418
- [CrossRef] [Google Scholar]
- A network pharmacology approach to uncover the pharmacological mechanism of XuanHuSuo powder on osteoarthritis. Evid. Based Complement. Alternat. Med.. 2016;2016:3246946.
- [CrossRef] [Google Scholar]
- UHPLC-MS/MS quantification combined with chemometrics for the comparative analysis of different batches of raw and wine-processed Dipsacus asper. J. Sep. Sci.. 2017;40:1686-1693.
- [CrossRef] [Google Scholar]
- LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chem.. 2016;210:9-17.
- [CrossRef] [Google Scholar]
- JianPi-QingHua formula attenuates nonalcoholic fatty liver disease by regulating the AMPK/SIRT1/NF-κB pathway in high-fat-diet-fed C57BL/6 mice. Pharm Biol.. 2023;61:647-656.
- [CrossRef] [Google Scholar]
- The hepatoprotective effect and chemical constituents of total iridoids and xanthones extracted from Swertia mussotii Franch. J. Ethnopharmacol.. 2014;154:259-266.
- [CrossRef] [Google Scholar]
- LC-ESI-MS/MS analysis of chemical constituents in Yinhuang Qingfei capsule. China Meas. Test.. 2016;42:36-40.
- [CrossRef] [Google Scholar]
- Cyanidin alleviated CCl4-induced acute liver injury by regulating the Nrf2 and NF-κB signaling pathways. Antioxidants. 2022;11:2383.
- [Google Scholar]
- Investigation of the mechanism of Isobavachalcone in treating rheumatoid arthritis through a combination strategy of network pharmacology and experimental verification. J. Ethnopharmacol.. 2022;294:115342
- [CrossRef] [Google Scholar]
- Study of the chemical composition of the white hairy silver dewberry based on the UPLC-Q-Orbitrap HRMS technique. J. Chin. Med. Mater.. 2015;43:1901-1906.
- [CrossRef] [Google Scholar]
- Induction of apoptosis in human pancreatic MiaPaCa-2 cells through the loss of mitochondrial membrane potential (ΔΨm) by Gentiana kurroo root extract and LC-ESI-MS analysis of its principal constituents. Phytomedicine. 2013;20:723-733.
- [CrossRef] [Google Scholar]
- Preventive effect of flavonoid extract from the peel of Gonggan (Citrus reticulata Blanco Var. Gonggan) on CCl(4)-induced acute liver injury in mice. J. Inflamm. Res.. 2021;14:5111-5121.
- [CrossRef] [Google Scholar]
- Yao medicine Amydrium hainanense suppresses hepatic fibrosis by repressing hepatic stellate cell activation via STAT3 signaling. Front. Pharmacol.. 2022;13:1043022.
- [CrossRef] [Google Scholar]
- Identification of chemical constituents in Xiao’er Qingyan granules by UPLC-Q-TOF-MS/MS. Chin. J. Mod. Appl. Pharm.. 2022;39:1627-1636.
- [CrossRef] [Google Scholar]
- Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv.. 2021;28:2187-2197.
- [CrossRef] [Google Scholar]
- The novel nanocomplexes containing deoxycholic acid-grafted chitosan and oleanolic acid displays the hepatoprotective effect against CCl(4)-induced liver injury in vivo. Int. J. Biol. Macromol.. 2021;185:338-349.
- [CrossRef] [Google Scholar]
- Integrating UPLC-Q-Exactive Orbitrap/MS, network pharmacology and experimental validation to reveal the potential mechanism of Tibetan medicine Rhodiola granules in improving myocardial ischemia-reperfusion injury. J. Ethnopharmacol.. 2023;314:116572
- [CrossRef] [Google Scholar]
- Aloperine attenuates carbon tetrachloride-induced mouse hepatic injury via Nrf2/HO-1 pathway. Trop. J. Pharm. Res.. 2020;19:983-988.
- [CrossRef] [Google Scholar]
- Application of organic mass spectrometry to the structural identification of trace impurities in mangosteen APIs. J. Mass Spectrom.. 2008;29:218-225. 1004-2997(2008)04-218-08
- [Google Scholar]
- Exploring the pharmacological mechanisms of Shuanghuanglian against T-cell acute lymphoblastic leukaemia through network pharmacology combined with molecular docking and experimental validation. Pharm. Biol.. 2023;61:259-270.
- [CrossRef] [Google Scholar]
- Efficient preparation of black tea extract (BTE) with the high content of theaflavin mono- and digallates and the protective effects of BTE on CCl(4)-induced rat liver and renal injury. J. Agric. Food Chem.. 2021;69:5938-5947.
- [CrossRef] [Google Scholar]
- Systematic characterization and quality evaluation of polybasic proto-gentian cyclic enol ether terpene components. Changchun: Changchun University of Chinese Medicine; 2021. 10.26980/d.cnki.gcczc.2021.000010
- Swertianlarin, an herbal agent derived from Swertia mussotii Franch, attenuates liver injury, inflammation, and cholestasis in common bile duct-ligated rats. Evid. Based Complement. Alternat. Med.. 2015;2015:948376
- [CrossRef] [Google Scholar]
- ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radic. Biol. Med.. 2016;101:272-283.
- [CrossRef] [Google Scholar]
- Chemical profile of Swertia mussotii Franch and its potential targets against liver fibrosis revealed by cross-platform metabolomics. J. Ethnopharmacol.. 2021;274:114051
- [CrossRef] [Google Scholar]
- Analysis of chemical constituents of different polarity fractions from Liuwei Dihuang pills by HPLC. Tradit. Chin. Drug Res. Clin. Pharmacol.. 2018;29:489-496.
- [CrossRef] [Google Scholar]
- Xanthones from Swertia mussotii as multitarget-directed antidiabetic agents. ChemMedChem.. 2014;9:1374-1377.
- [CrossRef] [Google Scholar]
- Analysis of chemical constituents and blood-entry components of Hanxia Baijutsu Tianma Tang based on UPLC-Q-Orbitrap-HRMS technique. Chin. J. Hosp. Pharm.. 2022;42:2331-2339.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105930.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







