Translate this page into:
Design and synthesis of Quinazolinone, Benzothiazole derivatives bearing guanidinopropanoic acid moiety and their Schiff bases as cytotoxic and antimicrobial agents
⁎Corresponding author. Tel.: +91 0422 2628645. venkateshom2001@gmail.com (Palani Venkatesh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Two series of Benzothiazole, Quinazolinone derivatives bearing guanidinopropanoic acid (38 compounds including 27 intermediates) and one series of Schiff base derivatives (14 compounds) were synthesized, characterized then evaluated for their cytotoxicity against human cervix cell line (HeLa) by MTT assay; antimicrobial activity against 11 pathogenic bacteria, 10 pathogenic fungus using standard of ciprofloxacin and Clotrimazole respectively. Compounds 13–18 showed significant activity against HeLa with IC50 range of 2–550 μM. Compound 3-(3-(6-hydroxybenzo[d]thiazol-2-yl)guanidino)propanoic acid (18) showed potent activity against human HeLa cell line with the half maximal inhibitory concentration (IC50) values of 1.8 μM which was close to the value of the positive control, doxorubicin. Antimicrobial result indicated that, compounds showed differential activity against the tested fungus and bacteria. Compounds 11, 14, 38 and 49 exhibited potent antibacterial and antifungal activity.
Keywords
Cytotoxicity
Benzothiazole
Quinazolinone
Guanidinopropanoic acid
Antimicrobial activity
1 Introduction
Cancer is continuing to be a major health problem in developing as well as underdeveloped countries. Surpassing heart diseases, it now become the number one killer disease in the world (Alaa and Abdel-Aziz, 2007; Medina et al., 1998, 1999; Hwang et al., 1999). Guanidine-containing molecules display a wide range of biologically important roles like influenza inhibitor zanamivir, antimicrobials, anticancer, antiviral, etc. (Sarah et al., 2006; Jiawang Liu et al., 2007; Nagwa et al., 2010). Mortimer et al. (2006) had reported Benzothiazole derivatives (Fig. 1) having significant anticancer activity and was selected for preclinical development then became the foundation for a second phase of analogue work. Abdulrahman et al. (2009) reported antitumor activity of novel derivatives of Quinazolinone (Fig. 2) against three (tumour cell lines human liver cell line (HEPG2), human breast cell line (MCF-7) and human cervix cell line (HeLa).![Benzo[d]thiazol-2-ylamino-2-thioxothiazolidin-4-one derivative.](/content/184/2016/9/1_suppl/img/10.1016_j.arabjc.2011.09.004-fig1.png)
Benzo[d]thiazol-2-ylamino-2-thioxothiazolidin-4-one derivative.
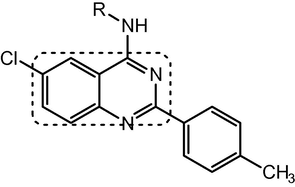
6-Chloro-2-p-tolylquinazolin-4-amine derivative.
Consequently, both Benzothiazole and Quinazolinone moiety separately substituted with Guanidine moiety (Figs 3 and 4) and the combination of Benzothiazole and Quinazolinone in one molecule through imine linkage (Fig. 5), can be considered as promising approach in drug-like molecules design.![3-(3-(6-Substituted Benzo[d]thiazol-2-yl) guanidino)propanoicacid derivative.](/content/184/2016/9/1_suppl/img/10.1016_j.arabjc.2011.09.004-fig3.png)
3-(3-(6-Substituted Benzo[d]thiazol-2-yl) guanidino)propanoicacid derivative.
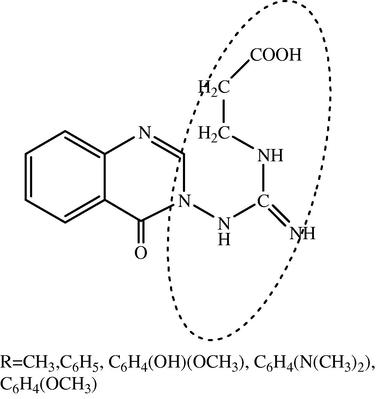
3-(3-(2-Substituted-4-oxoquinazolin-3(4H)-yl) guanidino)propanoicacid derivatives.
![2-(2,2-Disubstituted-1,2,3,8a-dihydroquinazolin-4(4aH)-ylideneamino)benzo[d]thiazole derivative.](/content/184/2016/9/1_suppl/img/10.1016_j.arabjc.2011.09.004-fig5.png)
2-(2,2-Disubstituted-1,2,3,8a-dihydroquinazolin-4(4aH)-ylideneamino)benzo[d]thiazole derivative.
Synthesised compounds (Figs. 3–5) were found in structural similarity with the earlier reported antimicrobial, anticancer (Fig. 6), and antiviral (influenza inhibitor zanamivir) (Fig. 7) compounds. So, we could expect that these compounds may also exhibit the above activities.![(Benzo[d]thiazol-2-yl)guanidine derivative.](/content/184/2016/9/1_suppl/img/10.1016_j.arabjc.2011.09.004-fig6.png)
(Benzo[d]thiazol-2-yl)guanidine derivative.
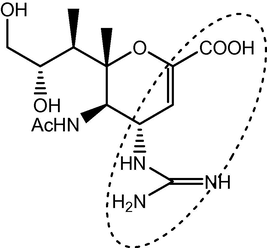
5-Acetamido-4-guanidino-6-methyl-4H-pyran-2-carboxylicacid derivative (influenza inhibitor zanamivir).
To confirm that, various Quinazolinones, Benzothiazole-2-amine derivatives and Schiff base derivatives (synthesized by condensation of Quinazolinone and Benzothiazole-2-amine derivatives) bearing guanidinopropanoic moiety were synthesised; evaluated for their cytotoxicity against human cervix cell line (HeLa) by MTT assay, antimicrobial activity against 11 pathogenic bacteria and 10 pathogenic fungus.
2 Experimental
2.1 Materials and methods
All the starting materials and solvents were purchased from commercially available sources. The melting points were determined on THERMONIC MODEL-C-LMP-1, Campbell melting point apparatus (uncorrected). 1H NMR were recorded on Brucker NRC-IISC 400/DRX-500/AV-III 500(S)/(L) AV-700 NMR spectrometer, ESI-MS were recorded with a Mariner System 5304 mass spectrometer and Elementary analysis was performed on a CHN-O-Rapid instrument within ±0.4% of the theoretical values at IIT Madras, Chennai. All the reactions were monitored using thin layer chromatography (TLC) using glass plate coated with silica gel G. TLC plates were developed in iodine and UV and chloroform: methanol (1:1) was taken as mobile phase.
2.2 Synthesis
2.2.1 Series I
Compounds 1–6, (6-substituted Benzo[d]thiazol-2-amine derivatives) and compounds 7–12, (1-(6-substituted Benzo[d]thiazol-2-yl)thiourea were prepared from the corresponding substituted anilines and from the compounds 1–6 respectively by using earlier reported methods (Pankaj et al., 2010) (Scheme 1).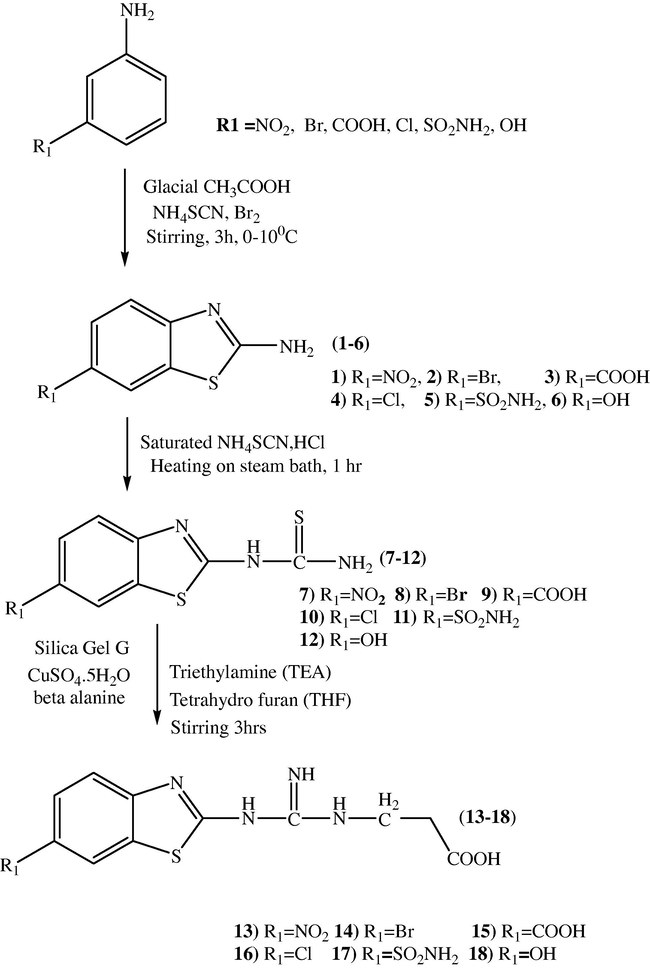
Synthesis of BTGP (13–18) from aryl amines (13–18).
2.2.1.1 Synthesis of 3-(3-(6-substituted Benzo[d]thiazol-2-yl)guanidino) propanoic acid derivatives (BTGP) (Scott et al., 2001) from 1-(6-substituted Benzo[d]thiazol-2-yl)thioureas (compounds 13–18, Scheme 1)
To the stirring mixture of compounds 7–12 containing Silica gel G (0.02 mol, 0.01g), Cupric sulphate pentahydrate (0.01 g), and Triethylamine (2 mL) in Tetrahydrofuran (25 mL), 1.78 g (0.02 mol) of β-alanine were added and stirring continued for 5–6 h, then kept at room temperature for 24 h. The crude product formed was recrystallised from methanol.
2.2.2 Series II
2.2.2.1 Synthesis of 2-substituted Quinazolin-4(3H)-one derivative from Anthranilamide (Anette Witt and Jan Bergman, 2000) (compounds 19–23, Scheme 2)
Anthranilamide (0.05 mol, 6.8 g) and aryl/heteroaryl aldehydes (0.05 mol) were refluxed in ethanol for 3 h at 70 °C, excess solvent was removed by rotary vacuum evaporator and solid residue (Schiff base) formed was refluxed with CuCl2 (0.05 mol, 6.72 g) in ethanol for further 3 h at 70 °C. Two-substituted Quinazolinones formed was recrystallised from ethyl acetate.
2.2.2.2 Synthesis of 3-amino-2-substituted Quinazolin-4(3H)-one derivatives from 2-substituted Quinazolin-4(3H)-one derivatives (Priyanka et al., 2010) (compounds 24–28, Scheme 2)
To the compounds 19–23 (0.2 mol), equal mixture of Conc. HCl and Hydrazine hydrate (6 mL) was added drop wise with stirring at 5–10 °C. Ethylene glycol (24 mL) and substituted Quinazolinones (19–23, 0.03 mol) were added to the above reaction mixture and refluxed for 3 h. On cooling solid was separated, filtered, washed with water and recrystallized from ethanol.
2.2.2.3 Synthesis of 1-(2-substituted-4-oxoquinazolin-3(4H)-yl) thioureas from 3-amino-2-substituted Quinazolin-4(3H)-one derivatives (compounds 29–33, scheme 2)
Thiourea analogues 29–33 were prepared from the compounds 24–28 by using earlier method (Scott et al., 2001).
2.2.2.4 Synthesis of 3-(3-(2-substituted-4-oxoquinazolin-3(4H)-yl) guanidino) propanoicacid derivatives (QGP) from 1-(2-substituted-4-Oxoquinazolin-3(4H)-yl) thioureas (Compounds 34–38, scheme 2)
Guanidino propanoic acid derivatives 34–38 were prepared from the compounds 29–33, method used was similar to the preparation of compounds 13–18.
2.2.3 Series III
2.2.3.1 Step I: Synthesis of 2-methyl-2-substituted-2,3-dihydroquinazolin-4(1H)-ones (compounds 39–40) from Anthranilamide (Al-Obaid et al., 2010)
To a stirring solution of Anthranilamide (110 mmol, 15 g) in Acetone (150 mL), p-Toluenesulfonic acid monohydrate (0.65 mmol, 0.125 g) was and the resulting homogeneous mixture was refluxed for 1 h. The solvent was subsequently cooled to ambient temperature and removed under reduced pressure. The resulting solid was partitioned between Ethyl acetate (250 mL) and saturated aqueous NaHCO3 (250 mL). The layers were shaken, and organic layer was separated and washed additionally with water (2 portions, 100 mL). The organic layer was dried over Na2SO4 and solvent evaporated to approximately one-half its volume, cooled and the solid obtained was recrystallized from the organic layer.
2.2.3.2 Step III: synthesis of Schiff base derivatives (compounds 41–54) from substituted 6-substituted benzo[d]thiazol-2-amine and 2-Methyl-2-substituted-2,3-dihydroquinazolin-4(1H)-ones (Jain et al., 2008)
Substituted Benzothiazole s (compounds 1–6, 0.02 mol) and Tetrahydroqunazolinones (compounds 39–40, 0.02 mol) were dissolved in 50 mL of ethanol and refluxed for 10–16 h. The reaction mixture was concentrated to approximately to 10 mL and allowed to cool. The resultant product was filtered out and recrystallized from ethanol.
2.2.4 Characterization
2.2.4.1 3-(3-(6-Nitrobenzo[d]thiazol-2-yl) guanidino) propanoic acid (Compound 13)
Pale yellow solid, Yield, 78%, m.p., 102–104 °C, (IR) υmax (KBr/cm−1), 3300–3235 (OH) 3128 (NH), 2354.66 (CH2), 1692 (C⚌O), 1640 (C⚌N), 1537.95 (NO2), 1339 (C–N), 1234 (C–S), 1H NMR (DMSO, δ ppm): 9.964 (s (br), 1H, COOH of CH2COOH), 7.66–7.65 (m, 2H, C5,7-H of Benzothiazole), 7.64–7.69 (s (br),1H, –C⚌NH of Guanidine), 7.23–7.11 (m, 1H, C4–H of Benzothiazole), 3.52 (s, 1H, NH) 3.23–3.31 (t, J = 7 Hz, 2H, CH2), 2.76–2.87 (t, J = 7 Hz, 2H, CH2). ESI–MS: 309.91 [M+]. Anal. Calcd for C11H11N5O4S (309.3): C, 62.20; H, 5.22; N, 20.72. Found: C, 62.22; H, 5.21; N, 20.61.
2.2.4.2 3-(3-(6-bromobenzo[d]thiazol-2-yl) guanidino) propanoic acid (Compound 14)
Greenish yellow solid, Yield, 89%, m.p., 152–154 °C, (IR) υmax (KBr/cm−1), 3288–3216 (OH) 3078 (NH), 2910 (CH2), 1611 (C⚌N), 1680 (C⚌O), 1304 (C–N), 735 (C–Br), 1265 (C–S), 1H NMR (DMSO, δ ppm): 9.87 (s, 1H, COOH of CH2COOH), 7.66–7.68 (m, 2H, C5,7-H of Benzothiazole), 7.73–7.86 (s, 1H, –C⚌NH of Guanidine), 7.63–7.71 (m, 1H C4–H of Benzothiazole), 3.72 (s, 1H, –NH), 3.33–3.55 (t, J = 9 Hz, 2H, CH2), 2.66–2.78 (t, J = 9 Hz, 2H, CH2). ESI-MS: 346 [M+]. Anal. Calcd for C11H11BrN4O2S (343.2): C, 38.50; H, 3.23; N, 16.32. Found: C, 38.27; H, 3.24; N, 16.58.
2.2.4.3 2-(3-(2-carboxyethyl) guanidino) benzo[d]thiazole-6-carboxylic acid (Compound 15)
Dark yellow solid, Yield, 78%, m.p., 147 °C, (IR) υmax (KBr/cm−1), 3286–3289 (OH) 3260 (NH), 2727 (CH2), 1678(C⚌O), 1474 (C⚌N), 1234 (C–S), 1H NMR (DMSO, δ ppm): 10.95 (s, 1H, (br), Ar-COOH), 9.88 (s, 1H, COOH of CH2COOH), 7.46–7.56 (m, 2H, C5,7–H of Benzothiazole), 7.87 (s (br), 1H, –C⚌NH of Guanidine), 7.53–7.423 (m, 1H, C4–H of Benzothiazole), 3.76 (s (br), 1H, –NH) 3.56–3.60 (t, J = 8 Hz, 2H, CH2), 2.56–2.48 (t, J = 8 Hz, 2H, CH2). ESI-MS: 308.98 [M+] Anal. Calcd for C12H12N4O4S (308.31):C, 46.75; H, 3.92; N, 18.17. Found: C, 46.64; H, 4.02; N, 18.47.
2.2.4.4 3-(3-(6-chlorobenzo[d]thiazol-2-yl) guanidino) propanoic acid (Compound 16)
Brown solid, Yield, 69%, m.p., 115–117 °C, (IR) υmax (KBr/cm−1), 3326–3287 (OH), 3126 (N–H), 2696 (CH2), 1624 (C⚌N), 1681(C⚌O), 1313 (C–N), 999 (C–Cl), 1245 (C–S), 1H NMR (DMSO, δ ppm): 9.99 (s, 1H, COOH of CH2COOH), 7.96–7.98 (m, 2H, C5,7-H of Benzothiazole), 7.47 (s, 1H, –C⚌NH of Guanidine), 7.53–7.41 (m, 1H C4-H of Benzothiazole), 3.62 (s, 1H, NH) 3.63–3.15 (t, J = 7 Hz, 2H, CH2), 2.31–2.24 (t, J = 7 Hz, 2H, CH2). ESI-MS: 300.78 [M+]. Anal. Calcd for C11H11ClN4O2S (298.75): C, 44.22; H, 3.71; Cl, 11.87; N, 18.75. Found: C, 44.11; H, 3.96; N, 18.45.
2.2.4.5 3-(3-(6-sulphanilamidobenzo[d]thiazol-2-yl)guanidino) propanoic acid (Compound 17)
Light green solid, Yield, 72%, m.p., 116–118 °C, (IR) υmax (KBr/cm−1), 3311–3286 (OH), 3132 (N–H str), 2926 (CH2), 1684 (C⚌O), 1434 (C⚌N), 1490 (SO2NH2), 1404, 641 (CH=CH aromatic), 1324 (C–N), 623 (C–S), 1H NMR (DMSO, δ ppm): 9.99 (s, 1H, COOH of CH2COOH), 7.96–7.99 (m, 2H, C5,7-H of Benzothiazole), 7.38 (s, 1H 1H, –C⚌NH of Guanidine), 7.43–7.11 (m, 1H C4-H of Benzothiazole), 3.77 (s (br), 1H, NH), 3.63–3.31 (t, J = 7 Hz, 2H, CH2), 2.42–2.12 (S (br), 2H, -SO2NH2), 2.86–2.68 (t, J = 7 Hz, 2H, CH2). ESI-MS: 343.35 [M+]. Anal. Calcd for C11H13N5O4S2 (343.38):C, 38.48; H, 3.82; N, 20.40. Found: C, 38.62; H, 3.89; N, 20.01.
2.2.4.6 3-(3-(6-hydroxybenzo[d]thiazol-2-yl) guanidino) propanoic acid (Compound 18)
Black solid, Yield, 59%, m.p., 146 °C, (IR) υmax (KBr/cm−1), 3392 (OH), 3130 (N–H str), 2359 (CH2), 1683 (C⚌O), 1548 (C⚌N), 1512, 1401 (C–N), 669 (C–S). 1H NMR (DMSO, δ ppm): 11.24 (s, 1H, Ar-OH), 9.89 (s, 1H, COOH of CH2COOH), 7.56–7.32 (m, 2H, C5,7-H of Benzothiazole), 7.17 (s, (br), –C⚌NH of Guanidine), 7.63–7.51 (m, 1H C4-H of Benzothiazole), 3.82 (s (br), 1H, NH) 3.33–3.51 (t, J = 7 Hz, 2H, CH2), 2.76–2.56 (t, J = 7 Hz, 2H, CH2). ESI-MS: 280.96 [M+]. Anal. Calcd for C11H12N4O3S (280.3): C, 47.13; H, 4.32; N, 19.99. Found: C, 47.18; H, 4.68; N, 19.89.
2.2.4.7 3-(3-(2-methyl-4-oxoquinazolin-3(4H)-yl)guanidino) propanoic acid (Compound 34)
Light brown solid, Yield, 63%, m.p., 133 °C, (IR) υmax (KBr/cm−1), 3319 (OH), 3129 (N–H str aliphatic), 2857 (CH2), 1679 (C⚌O), 1448 (C⚌N), 1360 (C–N), 1265 (C–CH3), 1H NMR (DMSO, δ ppm): 11.24 (s, (1H, COOH), 7.68–8.09 (m, 4H of, C5,6,7,8-H of Quinazolinone ring), 7.78 (s,(br),1H, s, 1H, –C⚌NH of Guanidine), 2.64–2.75 (s,1H, NH), 2.59–2.67 (t, J = 7.4 Hz, 2H, CH2), 1.85 (s, 3H, –CH3 attached C-2 of Quinazolinone ring), 1.35–1.75 (t, J = 7.4 Hz, 2H, CH2). ESI-MS: 289.89 [M+]. Anal. Calcd for C13H15N5O3 (289.29):C, 53.97; H, 5.23; N, 24.21. Found; C, 53.14; H, 5.38; N, 24.37.
2.2.4.8 3-(3-(4-oxo-2-phenylquinazolin-3(4H)-yl)guanidino) propanoic acid (Compound 35)
Pale green solid, Yield, 69%, m.p.,140 °C, (IR) υmax (KBr/cm−1), 3331 (OH), 3133 (N–H stretch, aliphatic), 2351 (CH2), 1684 (C⚌O), 1430 (C⚌N), 1492 (monsubstituted Benzene), 1393 (C–N). 1H NMR (DMSO, δ ppm): 11.48 (s, 1H, COOH), 7.59–8.10 (m, 4H of, C5,6,7,8-H of Quinazolinone ring), 7.83 (s, (br), 1H, –C⚌NH of Guanidine), 7.65–7.81 (d, J = 6 Hz, 2H, C2-phenyl-C2,6-H), 6.85–6.94 (m, 3H, C2-phenyl-C3,4,5-H), 2.68–2.71 (s, 1H, (br), NH), 2.31–2.39 (t, J = 7.4 Hz, 2H, CH2), 1.75–1.82 (t, J = 7.4 Hz, 2H, CH2). ESI-MS: 351.41 [M+]. Anal. Calcd for C18H17N5O3 (351.36):C, 61.53; H, 4.88; N, 19.93. Found: C, 61.65; H, 4.97; N, 19.34.
2.2.4.9 3-(3-(2-(4-hydroxy-3-methoxyphenyl)-4-oxoquinazolin-3(4H)-yl)guanidino) propanoic acid (Compound 36)
Yellowish brown solid, Yield, 66%, m.p., 215 °C, (IR) υmax (KBr/cm−1), 3367 (OH), 3127 (N–H str aliphatic), 3026 (CH2), 1676 (C⚌O), 1447 (C⚌N), 1360 (C–N), 1140 (O-CH3). 1H NMR (DMSO, δ ppm): 11.46 (s, 1H, Ar-OH), 9.91 (s, 1H, COOH of CH2COOH), 7.38–7.98 (m, 4H of, C5,6,7,8-H of Quinazolinone ring), 7.98 (s, (br), 1H, –C⚌NH of Guanidine), 7.75–7.85 (d, J = 6 Hz, 2H, C2-phenyl-C2,6-H), 6.57–6.52 (s, 1H, C2-phenyl-C3-H), 3.67 (s, 3H of –OCH3), 2.85–2.90 (s,1H,(br), NH), 2.59–2.60 (t, J = 7.4 Hz, 2H, CH2), 1.37–1.42 (t, J = 7.4 Hz, 2H, CH2). ESI-MS: 397.47 [M+]. Anal. Calcd for C18H17N5O3 (397.38): C, 61.53; H, 4.88; N, 19.93. Found; C, 61.62; H, 4.93; N, 19.73.
2.2.4.10 3-(3-(2-(4-(dimethylamino) phenyl)-4-oxoquinazolin-3(4H)yl) guanidino) propanoic acid (Compound 37)
Brown solid, Yield, 73%, m.p., 154 °C, (IR) υmax (KBr/cm−1), 3336 (OH), 3108 (N–H str), 2678.64 (CH2), 1687 (C⚌O), 1432 (C⚌N), 1187.04 (C–N), 813.813. 1H NMR (DMSO, δ ppm): 11.18 (s, (1H, COOH), 7.78–8.04 (m, 4H of, C5,6,7,8-H of Quinazolinone ring), 2.98–2.94 (s, 6H, N(CH3)2, 7.78 (s, (br), 1H, –C⚌NH of Guanidine), 7.55–7.55 (d, J = 6 Hz, 2H, C2-phenyl-C2,6-H), 6.67–6.67 (d, J = 2.4 Hz, 2H, C2-phenyl-C3,5-H), 2.94–2.98 (s,1H, NH), 2.495–2.485 (t, J = 7.4 Hz, 2H, CH2), 1.15–1.12 (t, J = 7.4 Hz, 2H, CH2). ESI-MS: 394.58 [M+]. Anal. Calcd for C20H22N6O3 (394.43):C, 60.90; H, 5.62; N, 21.31. Found: C, 60.56; H, 5.89; N, 21.29.
2.2.4.11 3-(3-(2-(4-Methoxyphenyl)-4-oxoquinazolin 3(4H) yl)guanidino) propanoic acid (Compound 38)
Yellowish green solid, Yield, 76%, m.p., 126 °C, (IR) υmax (KBr/cm−1), 3379 (OH), 3165 (N–H str), 1685 (C⚌O), 1493 (C⚌N), 1395 (C–N), 1253 (O-CH3). 1H NMR (DMSO, δ ppm): 11.56 (s, (1H, COOH), 7.85–8.10 (m, 4H of, C5,6,7,8-H of Quinazolinone ring), 7.78 (s,(br),1H, imine proton (C⚌NH)), 7.85–7.97 (d, J = 6 Hz, 2H, C2-phenyl-C2,6-H), 6.57–6.68 (d, J = 2.4 Hz, 2H, C2-phenyl-C3,5-H), 3.58–3.60 (s, 1H, -OCH3), 2.84–2.08 (s,1H,(br), NH), 2.53–2.73 (t, J = 7.4 Hz, 2H, CH2), 1.25–1.27 (t, J = 7.4 Hz, 2H). ESI-MS: 381.79 [M+]. Anal. Calcd for C19H19N5O4 (381.39); C, 59.84; H, 5.02; N, 18.36. Found; C, 59.34; H, 5.46; N, 18.15.
2.3 STEP III: 6-Chloro-N-(2,2-dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)benzo[d] thiazol-2-amine (Compound-41)
Black solid, Yield, 79%, m.p.,121 °C, (IR) υmax(KBr/cm−1), 3169 (N–H str), 1633 (C⚌N), 1519, 1399 (C–CH3), 1333 (C–N), 810 (C–Cl), 1298 (C–S), 1H NMR (δ-ppm): 7.87 (m, 2H, C6–7-H of Dihydroquinazolinone ring), 7.74–7.75 (m, 2H, C5,7-H of Benzothiazole ring), 7.55–7.57 (m, 1H, C4-H of Benzothiazole ring), 6.58–6.63 (m, 2H, C6–7-H of Dihydroquinazolinone ring), 2.48–2.49 (s, 6H, dimethyl). ESI-MS: 346.88 [M+].
Anal. Calcd for C17H17ClN4S (344.86); C, 59.21; H, 4.97; N, 16.25. Found: C, 59.65; H, 4.63; N, 16.35.
2.3.1 N-(2,2-Dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)-4-nitrobenzo[d] thiazol-2-amine (Compound-42)
Yellow solid, Yield, 80%, m.p., 116 °C, (IR) υmax (KBr/cm−1), 3161 (N–H stretch), 1617 (C⚌N), 1573 (NO2), 1509, 807 (CH⚌CH aromatic), 2845 (C–CH3), 1282 (C–S), 1H NMR (δ-ppm): 7.87 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 7.74–7.75 (m, 3H, C5,6,7-H of Benzothiazole ring), 6.58–6.64 (m, 4H, C5,6,7,8-H of Dihydroquinazolinone ring), 2.48–2.49 (s, 6H, dimethyl). ESI-MS: 355.91 [M+]. Anal. Calcd for C17H17N5O2S (355.41); C, 57.45; H, 4.82; N, 19.70. Found: C, 57.33; H, 4.96; N, 19.40
2.3.2 6-Bromo-N-(2,2-dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)benzo[d] thiazol-2-amine (Compound-43)
Black solid, Yield, 83%, m.p., 121 °C, (IR) υmax (KBr/cm−1), 3172 (N–H str), 1632 (C⚌N), 1485, 809 (CH⚌CH aromatic), 1332 (C–N), 751 (C–Br), 1276 (C–S), 1H NMR (δ-ppm): 7.85 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 7.74–7.75 (m, 2H, C5–7-H of Benzothiazole ring), 7.55–7.57 (m, 1H, C4-H of Benzothiazole ring), 6.58–6.63 (m, 4H, C5,6,7,8-H of Dihydroquinazolinone ring), 2.47–2.48 (s, 6H, dimethyl). ESI-MS: 391.33 [M+] Anal. Calcd for C17H17BrN4S (389.31); C, 52.45; H, 4.40; N, 14.39. Found: C, 52.76; H, 4.62; N, 14.46.
2.3.3 N-(2,2-Dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)-6-nitrobenzo[d] thiazol-2-amine (Compound-44)
Pale yellow solid, Yield, 76%, m.p., 120 °C, (IR) υmax (KBr/cm−1), 3168 (N–H), 1632 (C⚌N), 1560, 1482 (NO2), 1399 (C–N), 2177 (C–S), 1H NMR (δ-ppm): 7.87 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 7.74–7.75 (m, 2H, C5,7-H of Benzothiazole ring), 7.54–7.57 (m, 1H, C4-H of Benzothiazole ring), 6.58–6.63 (m,2H, C5,6–7,8-H of Dihydroquinazolinone ring), 2.47–2.49 (s, 6H, dimethyl). ESI-MS: 355.82 [M+]. Anal. Calcd for C17H17N5O2S (355.41); C, 57.45; H, 4.82; N, 19.70. Found: C, 57.24;, H, 4.63; N, 19.75.
2.3.4 2-(2,2-Dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH) ylideneamino)benzo[d] thiazole-6-carboxylic acid (Compound-45)
Light brown solid, Yield, 89%, m.p., 135 °C, (IR) υmax (KBr/cm−1), 3197–3135 (OH), 3173 (N–H str), 1692 (C⚌O), 1632 (C⚌N), 1484, 854 (CH⚌CH aromatic), 1332 (C–N), 1278 (C–S), 1H NMR (δ ppm): 11.15 (s, (br), Ar-COOH), 7.87 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 7.74–7.75 (m, 2H, C5,7-H of Benzothiazole ring), 7.56–7.59 (m, 1H, C4-H of Benzothiazole ring), 6.57–6.63 (m, 4H, C5,6–7,8-H of Dihydroquinazolinone ring), 2.46–2.44 (s, 6H, dimethyl). ESI-MS: 354.93 [M+]. Anal. Calcd for C18H18N4O2S (354.43); C, 61.00; H, 5.12; N, 15.81. Found: C, 61.27; H, 5.37; N, 15.56
2.3.5 2-(2,2-dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylideneamino)benzo[d] thiazole-6-sulfonamide (Compound-46)
Cream colour solid, Yield, 91%, m.p., 178 °C, (IR) υmax (KBr/cm−1), 3424 (N–H str), 1639 (C⚌N), 1484, 1396 (C–N), 1278 (SO2NH2), 1276 (C–S), 1H NMR (δ-ppm): 7.78 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 7.73–7.74 (m, 2H, C5,7-H of Benzothiazole ring), 7.52–7.57 (m, 1H, C4-H of Benzothiazole ring), 6.58–6.63 (m, 2H, C5,6,7,8-H of Dihydroquinazolinone ring), 2.846–2.821 (S, (br), 2H, –SO2NH2) 2.479–2.499 (s, 6H, dimethyl). ESI-MS: 390.71 [M+]. Anal. Calcd for C17H19N5O2S2 (389.5); C, 52.42; H, 4.92; N, 17.98. Found: C, 52.12; H, 4.99; N, 17.72.
2.3.6 2-(2,2-dimethyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylideneamino)benzo[d] thiazol-6-ol (Compound-47)
Brown solid, Yield, 93%, m.p., 180 °C, (IR) υmax (KBr/cm−1), 3855 (OH), 3225 (NH), 1640 (C⚌N), 1334 (C–N), 1271 (C–S), 1H NMR (δ-ppm): 10.24 (s, 1H, Ar-OH), 7.89 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 7.74–7.75 (m, 2H, C5–7-H of Benzothiazole ring), 7.55–7.57 (m, 1H, C4-H of Benzothiazole ring), 6.57–6.613 (m, 2H, C5,6,7,8-H of Dihydroquinazolinone ring), 2.481–2.490 (s, 6H, dimethyl). ESI-MS: 326.98 [M+].
Anal. Calcd for C17H18N4OS (326.42); C, 62.55; H, 5.56; N, 17.16. Found: C, 62.67; H, 5.87; N, 17.11.
2.3.7 6-chloro-N-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene) benzo [d] thiazol-2-amine (Compound-48)
Black solid, Yield, 69%, m.p., 123 °C, (IR) υmax (KBr/cm−1), 3405 (N–H str), 1666 (C⚌N), 1274 (C–S), 775 (C–Cl), 1H NMR (δ-ppm): 7.48–7.45 (m, 2H, C5,7-H of Benzothiazole ring), 7.29–7.12 (m, 1H, C4-H of Benzothiazole ring), 6.76–6.74 (m, 4H, C5,6,7,8-H of Dihydroquinazolinone ring), 6.54–6.58 (m, 5H, C2-phenyl of Dihydroquinazolinone ring), 3.30 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 2.31 (s, 3H, C2-CH3 of dihydro Quinazolinone). ESI-MS: 408.91 [M+]. Anal. Calcd for C22H19ClN4S (406.93); C, 64.93; H, 4.71; N, 13.77. Found: C, 64.81; H, 4.86; N, 13.45.
2.3.8 6-bromo-N-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)benzo [d] thiazol-2-amine (Compound-49)
Dark brown solid, Yield, 74%, m.p., 148 °C, (IR) υmax (KBr/cm−1), 3126 (N–H str), 1723, 1666 (C⚌N), 1272 (C–S), 745 (C–Br). 1H NMR (δ-ppm): 7.49–7.47 (m, 2H, C5–7-H of Benzothiazole ring), 7.29–7.13 (m, 1H, C4-H of Benzothiazole ring), 6.81–6.74 (m, 4H, C5,6,7,8-H of Dihydroquinazolinone ring), 6.61–6.48 (m, 5H, C2-phenyl of Dihydroquinazolinone ring), 3.318 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 2.34 (s, 3H, C2-CH3 of Dihydroquinazolinone). ESI-MS: 453.40 [M+] Anal. Calcd for C22H19BrN4S (451.38); C, 58.54; H, 4.24; N, 12.41. Found: C, 58.39; H, 4.38; N, 12.60.
2.3.9 N-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)-6-nitrobenzo[d] thiazol-2-amine (Compound-50)
Yellowish brown solid, Yield, 62%, m.p., 118 °C, (IR) υmax (KBr/cm−1), 3127 (N–H str), 1599 (C⚌N), 1481, 839 (CH⚌CH aromatic), 1443 (NO2), 1298 (C–N), 1276 (C–S), 1H NMR (δ-ppm): 7.49–7.46 (m, 2H, C5–7-H of Benzothiazole ring), 7.28–7.14 (m, 1H, C4-H of Benzothiazole ring), 6.73–6.76 (m, 4H, C5,6,7,8-H of Dihydroquinazolinone ring), 6.54–6.58 (m, 5H, C2-phenyl of Dihydroquinazolinone ring), 3.31 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 2.312 (s, 3H, C2-CH3 of Dihydroquinazolinone). ESI-MS: 418.50 [M+]. Anal. Calcd for C22H19N5O2S (417.48); C, 63.29; H, 4.59; N, 16.78. Found: C, 63.12; H, 4.76; N, 16.25.
2.3.9.1 N-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylidene)-4-nitrobenzo [d] thiazol-2-amine (Compound-51)
Yellow solid, Yield, 78%, m.p185 °C, (IR) υmax (KBr/cm−1), 3133 (N–H str), 1670 (C⚌N), 1576, 1558 (NO2), 1281 (C–S), 1H NMR (δ ppm): 7.49–7.46 (m, 3H, C5,6,7-H of Benzothiazole ring), 6.74–6.74 (m, 3H, C5,6,8-H of Dihydroquinazolinone ring), 6.72–6.78 (m, 5H, C2-phenyl of Dihydroquinazolinone ring), 6.54–6.58 (t, J = 3 Hz, 1H, C7-H of Dihydroquinazolinone ring), 3.32 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 2.32 (s, 3H, C2-CH3 of dihydro Quinazolinone). ESI-MS: 417.91 [M+]. Anal. Calcd for C22H19N5O2S (417.48); C, 63.29; H, 4.59; N, 16.78. Found: C, 63.20; H, 4.91; N, 16.55.
2.3.9.2 2-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)ylideneamino)benzo [d]thiazole-6-sulfonamide (Compound-52)
Cream colour solid, Yield, 69%, m.p., 125 °C, (IR) υmax (KBr/cm−1), 3121 (N–H str), 1645 (C⚌N), 1290 (SO2NH2), 1274 (C–S), 1H NMR (δ-ppm): 7.49–7.46 (m, 2H, C5–7-H of Benzothiazole ring), 7.29 –7.13 (m, 1H, C4-H of Benzothiazole ring), 6.76–6.74 (m, 3H, C5,6,8-H of Dihydroquinazolinone ring), 6.72–6.78 (t, J = 3 Hz, 1H, C7-H of Dihydroquinazolinone ring), 6.54–6.58 (m, 5H, C2-phenyl of Dihydroquinazolinone ring), 3.30 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 2.34 (s, 3H, C2-CH3 of dihydro Quinazolinone), 2.86–2.82 (s (br), 2H, 2H, –SO2NH2). ESI-MS: 451.58 [M+]. Anal. Calcd for C22H21N5O2S2 (451.56); C, 58.52; H, 4.69; N, 15.51. Found: C, 58.29; H, 6.21; N, 15.82.
2.3.9.3 2-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylideneamino)benzo[d] thiazole-6-carboxylic acid (Compound-53)
Brown solid, Yield, 68%, m.p., 123 °C, (IR) υmax (KBr/cm−1), 3414 (N–H str), 3367 (OH), 1628 (C⚌O), 1491 (C⚌N), 1276 (C–S). 1H NMR (δ-ppm): 11.71 (s, (br), Ar-COOH), 7.49–7.46 (m, 2H, C5,7-H of Benzothiazole ring), 7.29 –7.13 (m, 1H, C4-H of Benzothiazole ring), 6.76–6.74 (m, 3H, C5–6,8-H of Dihydroquinazolinone ring), 6.54–6.58 (t, J = 3 Hz, 1H, C7-H of Dihydroquinazolinone ring), 6.32–6.31(m, 5H, C2-phenyl of Dihydroquinazolinone ring), 3.30 (s, (br), 1H, –NH of Dihydroquinazolinone ring), 2.38 (s, 3H, C2-CH3 of dihydro Quinazolinone). ESI-MS: 416.92 [M+]. Anal. Calcd for C23H20N4O2S (416.5); C, 66.33; H, 4.84; N, 13.45. Found: C, 66.01; H, 4.89; N, 13.40.
2.3.9.4 2-(2-methyl-2-phenyl-1,2,3,8a-dihydroquinazolin-4(4aH)-ylideneamino)benzo [d]thiazol-6-ol (compound 54)
Black solid, Yield, 89%, m.p., 174 °C, (IR) υmax (KBr/cm−1), 3408 (NH str), 3132 (OH), 1657 (C⚌N), 1214 (C–S), 1H NMR (δ-ppm): 9.48 (s, (br), Ar -OH), 7.49–7.46 (m, 2H, C5,7-H of Benzothiazole ring), 7.29–7.13 (m, 1H, C4-H of Benzothiazole ring), 6.76–6.74 (m, 3H, C5,6,8-H of Dihydroquinazolinone ring), 6.54–6.58 (t, J = 3 Hz, 1H, C7-H of Dihydroquinazolinone ring), 3.30 (s, (br), 1H, NH of Dihydroquinazolinone ring), 6.34–6.35 (m, 5H, C2-phenyl of Dihydroquinazolinone ring), 2.49 (s, 3H, C2-CH3 of dihydro Quinazolinone). ESI-MS: 388.96 [M+]. Anal. Calcd for C22H20N4OS (388.49); C, 68.02; H, 5.19; N, 14.42. Found: C, 68.99; H, 5.27; N, 14.30.
3 Pharmacological evaluation
3.1 In vitro cytotoxicity
3.1.1 MTT assay method (Mosmann and Tim, 1983)
3.1.1.1 Procedure
The human cervical cancer cell line (HeLa), was obtained from National Centre for Cell Science (NCCS), Pune. The HeLa was grown in Eagles Minimum Essential Medium containing 10% fetal bovine serum (FBS). The cells were seeded into 96-well plates in 100 μl of respective medium containing 10% FBS, at plating density of 10,000 cells/well and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of compounds 13–18 and 34–38. The compounds were solubilised in Dimethylsulfoxide and diluted in respective serum free medium. The cells were exposed to various concentrations of the test drugs and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 48 h. Triplicate was maintained and the medium containing without drugs were served as a control. After 48 h, 15 μl of MTT (5 mg/mL) in phosphate buffered saline (PBS) was added to each well and incubated at 37 °C for 4 h. The medium with MTT was then flicked off and the formed formazan crystals were solubilised in 100 μl of DMSO and then measured the absorbance at 570 nm using micro plate reader. The IC50 value resulting from 50% inhibition of cell growth was calculated graphically as a comparison with the control doxorubicin.
3.2 Antimicrobial evaluation
3.2.1 In vitro antimicrobial activity (Sohail Saeed et al., 2010)
The micro dilution susceptibility test in Muller-Hinton Broth and Sabouraud Liquid Medium was used for the determination of antibacterial and antifungal activity, respectively. Standard sterilized filter paper disks (5 mm diameter) impregnated with a solution of the test compound in DMF (1 mg/mL) was placed on an agar plate seeded with the appropriate test organism showed in Tables 2 and 3 in triplicates. Agar-diffusion method was used for the determination of the preliminary antibacterial and antifungal activity. Ciprofloxacin (cp) and Clotrimazole were used as reference drugs antibacterial and antifungal agents, respectively. DMSO alone was used as control at the same above-mentioned concentration. The plates were incubated at 37 °C for 24 h for bacteria and for 48 days for fungi. Compounds that showed significant growth inhibition zones (>09 mm) using the twofold serial dilution technique, were further evaluated for their minimal inhibitory concentrations (MICs). The plates were incubated at 37 °C for 24 h. Compounds that showed significant growth inhibition zones (14 mm) using the twofold serial dilution technique, were further evaluated for their minimal inhibitory concentrations (MICs).
4 Results and discussion
4.1 Chemistry
Series 1 compounds 3-(3-(6-substitutedbenzo[d]thiazol-2-yl) guanidino) propanoic derivatives (13–18) were synthesized according to earlier reported method in the literature, shown in scheme 1. Compounds 13–18 prepared in 3 steps, the first step involved reaction of various substituted aromatic amines, ammoniumthiocyanate and bromine in glacial acetic acid under cold condition at 0–10 °C afforded 6-substituted benzo[d]thiazol-2-amine derivatives (1–6) which further treated with saturated ammoniumthiocynate and Conc. Hydrochloric acid (second step) to yield the corresponding thioureas (7–12). When the compounds 7–12, in third step treated with β-alanine dissolved in tetrahydro furan containing silica gel G, CuSO4.5H2O and triethyl amine (accelerates desulphuration) afforded the guanidinopropanoic acid derivatives 13–18 (Scheme 1) as a final compound of series one.
Series 2 Compounds 3-(3-(2-substituted-4-oxoquinazolin-3(4H)-yl) guanidino) propanoic acid derivatives (34–38) were prepared in 4 steps as shown in scheme 2. Initially, intermediate compounds 2-substituted quinazolin-4(3H)-one derivative (19–23) were prepared by refluxing with substituted aldehyde in ethanol for 3 h at 70 °C, when the formed non isolable intermediate Schiff bases were refluxed with CuCl2 in ethanol for 3 h at 70 °C produced the compounds 19–23. In compounds 19–23, amino group was introduced at N-3 of Quinazolinone by treating with hydrazine hydrate, ethylene glycol in acidic medium, refluxing the reaction mixture for 3 h to afford 3-amino-2-substituted quinazolin-4(3H)-one derivative (24–28). Compounds 19–23 do not form hydrazone when they are treated with hydrazine hydrate since the Quinazolinones 19–23 are cyclic amides (–NH–C⚌O), (Scheme 2). The method adopted for the prearation of Quinazolinone thioureas 29–33 and Quinazolinone guanidino propanoic acid derivatives 34–38 (scheme 2) were similar to the preparation 7–12 and 13–18 respectively.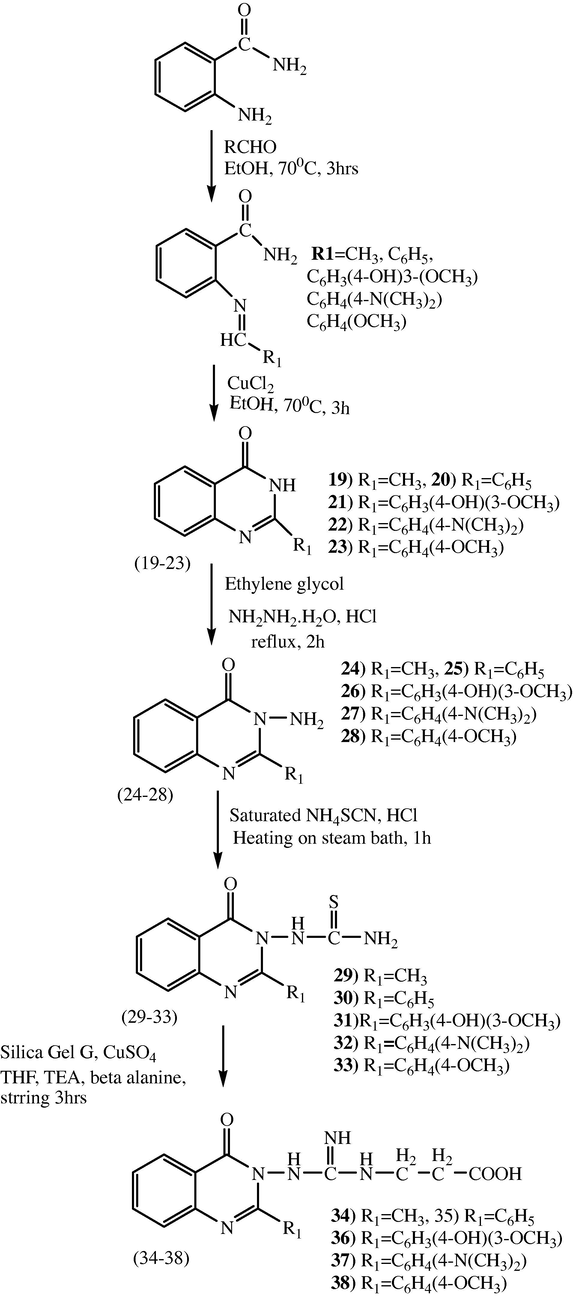
Synthesis of QGP derivatives (34–38) from anthranilamide.
Series 3 compounds Schiff bases (41–54) were prepared from substituted benzo[d]thiazol-2-amines (1–7) and 2-methyl-2-substituted-2,3-dihydroquinazolin-4(1H)-ones (39–40), shown in scheme 3. Intermediate compounds 39–40 were synthesised by treating anthranilamide with acetone and acetophenone (refluxed for 90 minutes) in presence of p-toluenesulfonic acid monohydrate as cyclising agent, Dihyhydroquinazolinones (39–40) formed (via non isolable imine formation and cyclization). Finally, Schiff base derivatives (41–54) were prepared by reacting the 2-substituted-2,3-dihydroquinazolin-4(1H)-ones (39–40) and substituted benzo[d]thiazol-2-amine(1–7) (Scheme 1, first step compounds) then refluxed for 10–16 h, the amino group of compounds 1–7 reacted with keto group of compounds 39–40 in ethanol to afford the Schiff base derivatives (41–54) (scheme 3).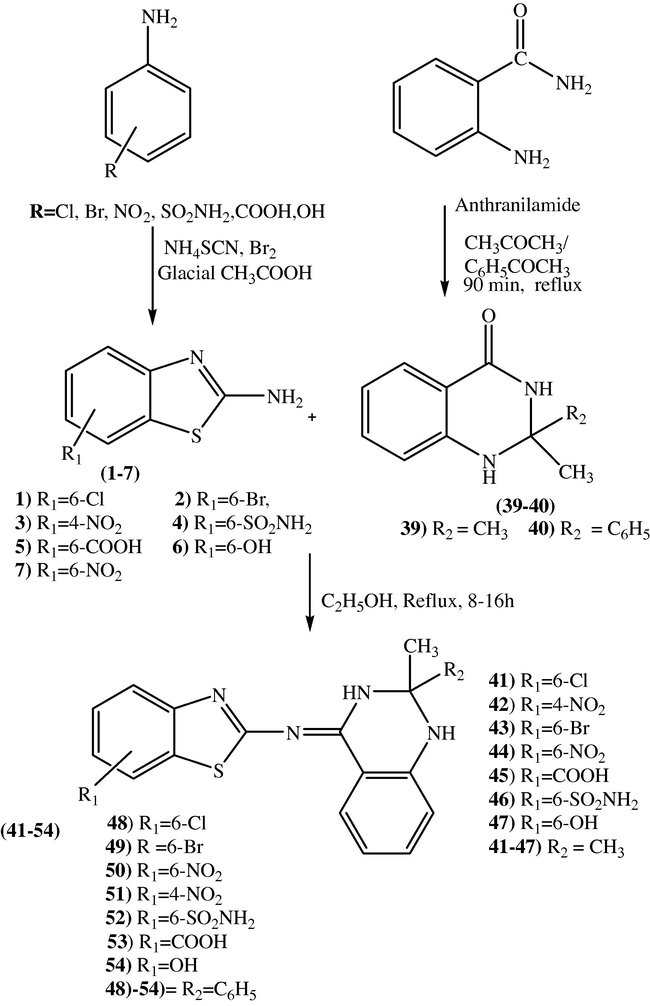
Synthesis of Schiff base derivatives (41–54) from Benzothiazole-2-amines (1–7) and 2,3-dihydroquinazolin-4(1H)-ones (39–40).
Infrared (IR) spectroscopy of the compounds 7–12 and 29–33, showed characteristic bands at 1051–1196 by the thiocarbonyl moiety C⚌S. Carboxylic acid group of the compounds 13–18, 34–38 showed characteristic broad bands at the range of about 3379–3216 cm−1 by the -OH stretching of the carboxylic acid, bands at about 1692–1679 cm−1 by the carboxylic C⚌O stretching. C⚌N absorption bands appeared in the region of at 1493–1430 cm−1.
Compounds 41–54 showed strong absorption bands in the region of 1390–1210 cm−1 by C⚌N, while the absorption bands for –NH2 and C⚌O were absent in compounds 41–54. The 1H NMR spectrum of the compounds 13–18 and 34–38 exhibited three broad singlets, one at about 9.575–11.187 ppm by the carboxylic acid proton, second at 7.585–7.788 by imine proton and third at 2.985–3.376 by –NH proton. Adjacent two methylene protons of the compounds 13–18 shown two triplets at 3.137–3.315 (J = 7.4 Hz) and 2.560–2.481 (J = 7.4 Hz). The compounds 34–38 were also shown two similar triplets at 2.495–2.485 (J = 7 Hz) and 1.156–1.120 (J = 7). Presence of two triplets with the same coupling constant in the compounds 13–18 and 34–38 confirmed presence of two adjacent methylene protons. In addition, methyl proton of compound 34 exhibited singlet at 1.452, phenolic OH proton and methoxy proton of compound 36 exhibited singlets at 6.387 and 3.946 respectively. Methoxy protons of compound 38 exhibited singlet at 3.489–3.498. Compound 41–54 were shown singlet at 7.859–7.888 ppm, which were assigned to third position –NH of dihydro Quinazolinone. Sulphanilamide group of compounds 17, 46 and 52 exhibited broad singlet at 2.121- 2.864 while carboxylic acid protons attached to Benzothiazole ring of compounds 15,45 and 53 exhibited one broad singlet at 10.955–11.718. The phenolic -OH group of compounds 45 and 54 were also shown broad singlet at about 9.486–11.243 ppm.
In mass spectrum, thiourea intermediates showed base peak due to cleavage of [C7H8N2]+• at m/z = 138 and another intense peak at about m/z 108 due to deamination from base peak with relative intensity of 60%. The obtained molecular ion m/z values of the final guanidinopropanoic acid derivatives were consistent with the molecular weight of compounds and the base peak was formed after the successive removal of propanoic acid moiety with one intense peak due to desulphuration from base peak with relative intensity of 62%.
4.2 In vitro cytotoxicity
The synthesized BTGP derivatives 13–18, QGP 34–38 and Schiff base derivatives 43–45, 48–53 (20 compounds) were evaluated for antiproliferative activities against human cancer cell line HeLa. The results were summarized in Table 1. All the compounds showed antiproliferative activities against the HeLa cell line with the half maximal inhibitory concentration (IC50) values of 2–550 μM (except Schiff base derivatives IC50 values of 743–875 μm) and compound 18 showed the most potent activity with a mean IC50 of 2 μM which was close to the value of the positive control, doxorubicin. Structure–activity relationship (SAR) analysis indicated that Benzothiazole bearing guanidinopropanoic acid 13–18 showed stronger activities than the Quinazolinone bearing guanidinopropanoic acid 34–38, with all the IC50 values below 100 μM. In the SAR further study of 13–18, the –OH, –COOH, and –SO2NH2 substituted at sixth position of Benzothiazole ring played an important role for the activity. Compounds 18 with –OH substitution at sixth showed the most potent activity against the HeLa cell lines, with the mean IC50 of 2 μM. From these results it is evident that hydrogen bonding substituent is necessary for the potent activity at the sixth position of Benzothiazole ring, further size of these groups also play important role in the activity, activity order is OH > COOH > SO2NH2. From the results it is clear that smaller hydrogen bonding group (donor Hydrogen containing) at sixth position of Benzothiazole ring necessary for the potent activity. In vitro cytotoxicity IC50 of doxorubicin is 1.8 μm.
S. no.
Compound no.
In vitro cytotoxicity (IC50 μm)
S. no.
Compound no.
In vitro cytotoxicity (IC50 μm)
(1)
13
67
(12)
43
865
(2)
14
64
(13)
44
743
(3)
15
37
(14)
45
867
(4)
16
32
(15)
48
750
(5)
17
59
(16)
49
782
(6)
18
2
(17)
50
875
(7)
34
527
(18)
51
855
(8)
35
443
(19)
52
795
(9)
36
550
(20)
53
810
(10)
37
524
(11)
38
534
4.3 In vitro antibacterial activity
Twenty-three compounds (7–17, 34–38, 44–46, 49–52 and 54) were evaluated for in vitro antimicrobial activity against 11 pathogenic bacteria and 10 pathogenic fungus. The results were recorded for each tested compound as the average diameter of inhibition zones (IZ) of bacterial growth around the disks in millimetre. The minimum inhibitory concentration (MIC) measurement was determined for all the compounds using the twofold serial dilution method. The MIC (μg/mL) values were recorded. Most of the tested exhibited differential activity against tested bacteria and fungi.
From the results of antibacterial activity it is evident that 6 compounds (11, 14, 34, 38, 44 and 46), 11 compounds (7, 10, 11, 12, 13, 14, 15, 11, 35, 36, 38 and 49), 4 compounds (10, 12, 17 and 49), 2 compounds (15 and 17), 3 compounds (34, 38, 44), 5 compounds (8, 16, 34, 36, 38) and 3 compounds (34, 38 and 49) were more active against Pseudomonas aeruginosa, Salmonella paratyphyi, Vibrio cholera, Bacillus lentus, Rhodosporium rubrum, Staphylococcus albus and Staphylococcus aureus, respectively than the reference drug ciprofloxacin and compounds 11, 14, 34, 38 and 49 showed the broad spectrum of activity (Table 2).
Organism
Compounds MIC (μg/ml)
7
8
10
11
12
13
14
15
16
17
34
35
36
37
38
44
45
46
49
51
52
54
Stda
Micrococcus luteus
25
12.5
6.25
12.5
6.25
12.5
12.5
25
25
12.5
25
12.5
6.25
50
12.5
12.5
12.5
25
12.5
12.5
12.5
25
<0.019
Escherichia coli
50
12.5
12.5
12.5
25
50
25
6.25
50
12.5
25
25
50
50
12.5
25
–
12.5
12.5
12.5
12.5
25
1.24
Pseudomonas aeruginosa
12.5
25
12.5
6.25
25
25
3.12
25
25
25
12.5
25
25
25
12.5
12.5
–
12.5
6.25
25
6.25
12.5
19.5
Bacillus subtilis
25
6.25
25
6.25
25
12.5
12.5
6.25
25
50
12.5
12.5
25
12.5
6.25
12.5
–
–
6.25
25
6.25
25
0.5
Klebsiella pneumonia
12.5
12.5
25
6.25
12.5
12.5
25
6.25
25
12.5
25
25
12.5
12.5
6.25
12.5
–
–
25
6.25
12.5
6.25
<0.019
Salmonella paratyphyi
25
50
12.5
6.25
12.5
25
25
12.5
12.5
25
25
6.25
6.25
12.5
6.25
12.5
–
–
3.125
12.5
12.5
12.5
43.4
Vibrio cholera
25
25
6.25
25
6.25
25
12.5
25
6.25
25
12.5
12.5
25
12.5
25
25
–
–
6.25
25
25
25
256
Bacillus lentus
12.5
12.5
12.5
12.5
12.5
25
12.5
6.25
50
6.25
50
25
50
25
12.5
6.25
12.5
25
6.25
50
25
12.5
0.781
Rhodosporium rubrum
50
12.5
12.5
12.5
25
12.5
12.5
50
50
12.5
6.25
50
50
12.5
6.25
6.25
–
25
12.5
12.5
25
12.5
32
Staphylococcus albus
12.5
6.25
35
25
12.5
25
25
50
6.25
12.5
6.25
50
6.25
12.5
6.25
12.5
6.25
50
25
12.5
12.5
12.5
0.5
Staphylococcus aureus
6.25
25
25
25
12.5
12.5
25
50
25
12.5
6.25
50
12.5
25
12.5
12.5
–
–
6.25
12.5
12.5
–
25
The results of antifungal activity indicated that, Compounds 34, 51, 45 and 37 were more potent against Monascus purpureus, Aspergillus fumigates, Aspergillus parasiticus and Microsporum gypseum than the reference drug Clotrimazole (Table 3).
Organism
Compounds MIC (μg/ml)
7
8
10
11
12
13
14
15
16
17
34
35
36
37
38
44
45
46
49
51
52
54
Stda
Monascus purpureus
25
25
12.5
50
12.5
12.5
12.5
12.5
12.5
25
3.12
12.5
12.5
50
12.5
12.5
–
–
6.25
12.5
6.25
12.5
30
Aspergillus niger
–
–
–
–
–
–
–
–
–
–
12.5
–
25
25
–
6.25
12.5
12.5
12.5
12.5
12.5
25
0.24–0.34
Candida albicans
12.5
12.5
50
–
6.25
12.5
6.25
12.5
25
25
25
12.5
6.25
12.5
25
–
–
–
6.25
–
–
12.5
0.25–2.10
Aspergillus fumigatus
25
–
–
–
–
25
25
25
12.5
12.5
12.5
25
12.5
25
25
12.5
25
12.5
6.25
6.25
12.5
25
12–5–25
Aspergillus parasiticus
–
–
–
–
–
–
–
–
–
–
12.5
–
–
25
25
12.5
6.25
12.5
12.5
6.25
12.5
25
>8
Monascus ruber
–
–
–
–
–
–
12.5
–
–
12.5
25
12.5
25
12.5
25
12.5
12.5
–
25
12.5
–
12.5
10
Streptomyces griseus
–
–
25
6.25
25
25
12.5
25
12.5
12.5
25
12.5
–
12.5
12.5
6.25
–
12.5
3.125
6.25
–
12.5
0.05
Microsporum gypseum
–
–
–
25
12.5
25
25
12.5
25
25
12.5
12.5
25
6.25
25
–
–
–
–
–
–
–
19.53
Trichophyton rubrum
–
–
–
25
12.5
25
25
12.5
12.5
12.5
12.5
25
25
12.5
12.5
–
–
–
–
–
–
–
0.4
Trichophyton mentagrophytes
–
–
–
50
25
50
12.5
12.5
12.5
25
–
3.12
12.5
25
6.25
12.5
25
6.25
25
12.5
12.5
12.5
0.0075
Collectively, compounds 11, 14, 38 and 49 are considered to be the most active antimicrobial members identified in this study with a broad spectrum of antibacterial activity against both Gram positive and Gram negative bacteria. Particularly, compounds 14 was superior over the ciprofloxacin against Pseudomonas aeruginosa (14, MIC 3.125 and Cp MIC 19.5 μg/mL) and compound 49 highly active against Salmonella paratyphyi (49, MIC 3.125 and Cp MIC 43.4 μg/mL). Compound 34 was more active against Monascus purpureus (34, MIC 3.125 and Clotrimazole MIC 30 μg/mL).
5 Conclusion
Compound 18 exhibited potent cytotoxicity against the human cervical cancer cell line (HeLa). Compounds 13–17 also showed the promising cytotoxicity. Compounds 11, 14, 38 and 49 are considered to be the most active antimicrobial members identified in this study with a broad spectrum of antibacterial activity against both Gram positive and Gram negative bacteria. Among the three series tested for cytotoxicity and antimicrobial activity, 3-(3-(6-substitutedbenzo[d]thiazol-2-yl) guanidino) propanoic derivatives (13–18) exhibited the promising cytotoxicity and antimicrobial activity.
Acknowledgements
The authors are thankful to Chairman Dr. Nalla G. Palaniswam & Dr. Thavamani D. Palanisami, Kovai Medical Center Research and Educational trust, Coimbatore, for providing facilities and laboratories.
References
- Eur. J. Med. Chem.. 2009;44:2379-2391.
- Eur. J. Med. Chem.. 2007;42:614-626.
- Eur. J. Med. Chem.. 2010;45:4188-4198.
- Tetrahedron. 2000;35(56):7245-7253.
- Anticancer. Res.. 1999;19:5087-5093.
- Jain, Devendra Kr., Bhawana Thadhaney., Ajit Joshi., Nasir Hussain., Ganpat, L., 2008. Indian J Chem B. 49B, 818-825.
- Bioorg. Med. Chem.. 2007;15(24):7773-7788.
- Bioorg. Med. Chem. Lett.. 1998;8:2653-2656.
- Bioorg. Med. Chem. Lett.. 1999;9:1843-1846.
- J. Med. Chem.. 2006;49:179-185.
- J. Immunol. Meth.. 1983;65(2):55-63.
- Eur. J. Med. Chem.. 2010;45:6058-6067.
- Asian J. Res. Chem.. 2010;3(1):42-47.
- Priyanka Yadav., Deepa Chauhan., Neeraj K. Sharma., Sachin Singhal., 2010. Int. J. Chem. Tech. Res. 2 (2), 1209–1213.
- Bioorg. Med. Chem.. 2006;14:8608-8621.
- J. Med. Chem.. 2001;44(8):1217-1230.
- Eur. J. Med. Chem.. 2010;45:1323-1331.
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.arabjc.2011.09.004.
Appendix A
Supplementary data
Supplementary data 1
Supplementary data 1







