Translate this page into:
Design, characterization and enhanced bioavailability of hydroxypropylcellulose-naproxen conjugates
⁎Corresponding author. majaz172@yahoo.com (Muhammad Ajaz Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Polysaccharides are beneficially used as drug carriers via prodrug formation and offer a mechanism for better effectiveness and delivery of the drug. The unique geometry of hydroxypropylcellulose (HPC), a polysaccharide, allows the attachment of drug molecules with a higher degree of substitution because the hydroxyls groups are projected outside the HPC chains. Therefore HPC-Naproxen conjugates, i.e., macromolecular prodrugs, were synthesized using a powerful acylation reagent carbonyldiimadazole (CDI) in N,N' dimethylacetamide (DMAc) solvent. The reactions were carried out at 80 °C under stirring for 24 h and inert environment. This reaction strategy appeared efficient to obtain a high degree of drug substitution (DS = 0.88–1.40) on the polymer parent chain as calculated by UV–visible spectrophotometry after hydrolysis of the samples. The method provides high efficacy as product yields were high (77–81%). Macromolecular prodrugs (MPDs) with different DS of naproxen designed were found soluble in organic solvents.
Keywords
Esterification
Hydroxypropylcellulose
Naproxen
Pharmacokinetics
Prodrug
1 Introduction
Naproxen (6-methoxy-α-methyl-2-naphthaleneacetic acid) is a propionic acid derivative and a non-steroidal anti-inflammatory drug used to treat pain (Todd and Clissold, 1990); (Shah et al., 2017), rheumatic and inflammatory diseases. Although naproxen is a powerful pain killer, there are several side effects associated with it which include; GIT irritation (Blower and Armstrong, 1987), hyperacidity, nausea, heartburn, bruising, ulcerative properties, headache, hemorrhage (Shanbhag et al., 1992), itching skin, etc. To prevent these adverse side effects, it is desirable to form prodrugs of naproxen (Zawilska et al., 2013; Mahmoud and Mohammad, 2010).
Different prodrugs of naproxen have been synthesized to get better dermal permeability by masking its COOH group. Esterification of naproxen with N-hydroxymethylsuccinimide, N-hydroxymethyl isatin and acyloxyalkyl and aminoacyloxyalkyl esters results in reduction of its gastrointestinal toxicity with improving bioavailability and enhance skin permeation (Mahfouz et al., 1999; Rautio et al., 2000). Esterification of naproxen with thio, alkyl esters, and ethylpiperazine resulted in prodrugs formation which retained the anti-inflammatory activity with reduced gastrointestinal irritation (Venuti et al., 1989; Calvi et al., 1985).
Besides these small molecular mass esters, only a polysaccharide (dextran) has also been reported for the formation of macromolecular prodrugs (MPDs) of naproxen (Cordeiro et al., 2020). MPDs of naproxen were made using dextran polymer with its low degree of substitution (DS) (Harboe et al., 1988). Likewise, dextran, HPC, a cellulose ether has attracted the attention of researchers for the development of NSAIDs and antibiotic prodrugs (Hussain et al., 2013). HPC is very compatible with biological system, NSAIDs and antibiotics (Sharma and Sharma, 2008) and it is acid resistance (El Seoud and Heinze, 2005; Hussain et al., 2017) therefore, it is being widely used in matrix tablets, ophthalmic solutions, and micro-bead formulations, etc (Picker-Freyer and Dürig, 2007; Abraham et al., 2009; Karewicz et al., 2010). Recently, salicylic acid and aspirin prodrugs based on HPC (Hussain et al., 2015) have also been reported where useful results (e.g., high DS) were obtained.
The aforesaid studies led us to develop naproxen’s MPDs based on HPC to get its high loading/DS which otherwise shows very low DS, e.g., in case of MPDS on to dextran. Secondly, keeping in mind the biodegradability, biocompatibility, and hydrophilicity of HPC as a novel drug carrier, we are interested to produce the MPDs of naproxen on to this versatile polymer. The resultant conjugates are expected to afford sustained release of naproxen owing to enzymatic hydrolysis in vivo and in vivo, so pharmacokinetic studies of the HPC-naproxen (HPC-NAP) conjugates were carried out in the rabbit model.
2 Materials and methods
2.1 Materials
HPC (DS 3.00, MS 3.46, Mw 1.16 × 105) was procured from Nanjing Yeshun Industry and International Trading Co. Ltd, Jiangsu, China and dried at 110 °C for 5 h before use. Analytical grade methanol, ethanol, propanol, and hexane (Sigma Aldrich) were used. N,N-dimethlyacetamide (DMAc), diethylether and 1,1′-carbonyldiimidazole (CDI) were used as received from Sigma Aldrich. HCl and NaOH (Merck) were used for hydrolysis of drug and preparation of standard solutions for UV/Vis spectroscopy, respectively.
2.2 Measurements
FT-IR spectra of the conjugates were recorded on an IR-prestige-21 (Shimadzu, Japan) spectrometer. KBr pellet method was used to record the spectra of samples. Structures of the conjugates were determined using 1H NMR (400 MHz = 16–64 scans) in DMSO‑d6. To get a pharmacokinetic profile of the prodrugs, the HPLC method was developed. The HPLC system (Agilent Technologies 1200 Series, Germany) was set up with a UV–Vis detector (G1315B DAD), pump (G1311A) and degasser (G1322A). Shim-Pack columns (ODS 5 μm, 4.6 × 250 mm) were used for elution.
2.3 Synthesis of HPC-NAP conjugate 1, a typical example
Naproxen (0.63 g, 2.75 mmol) was dissolved in DMAc (20 mL) and stirred (24 h, room temperature) then CDI (0.44 g, 2.75 mmol) was added. HPC (1 g, 2.75 mmol) was dissolved in DMAc (20 mL) and kept under stirring (1 h, 80 °C) to get the optical clear solution. Both reaction mixtures, i.e., NAP /CDI and HPC were then mixed and the reaction was then carried out with continuous stirring under nitrogen environment at 80 °C for 24 h. The HPC-NAP conjugate synthesized in this way was precipitated out by adding the reaction mixture dropwise to the diethyl ether (250 mL). Washing of the precipitates was also carried out with diethyl ether (50 mL) followed by drying it in a vacuum oven at 50 °C for 24 h.
Analytical data HPC-NAP conjugate 1. Yield: 74%; DS: 0.25 by UV/Vis spectrophotometer; FTIR (KBr): 3129 (OH), 1711 (CO Ester), 1449 (CH2) cm−1.
Analytical data HPC-NAP conjugate 2. Yield: 78%; DS: 0.60 by UV/Vis spectrophotometer; FTIR (KBr): 3132 (OH), 1724 (CO Ester), 1447 (CH2) cm−1.
Analytical data HPC-NAP conjugate 3. Yield: 84%; DS: 1.13 by UV/Vis spectrophotometer; FTIR (KBr): 3393 (OH), 1724 (CO Ester), 1435 (CH2) cm−1; 1H NMR (DMSO‑d6): δ (ppm) = 2.02 (H-10), 3.26–3.83 (other AGU-H-1–8), 1.01 (H-9), 7.11–7.65 (aromatic-Hs).
2.4 Determination of degree of substitution by UV/Vis spectrophotometric analysis
UV/Vis spectrophotometry was used for the determination of the DS of HPC-NAP conjugates 1–3. Aq. solution of NaOH (0.1 N) was used to prepare a standard solution of naproxen and further dilutions (2–20 ppm) were made from this solution. Using UV/Vis spectrophotometer, the calibration curve was drawn. HPC-NAP conjugate (10 mg each) was added in aq. solution of NaOH (0.1 M). to prepare the sample solution. The solution was stirred (80 °C, 3 h) for hydrolysis of the conjugate to yield free drug. After hydrolysis, the volume of each solution was made up to 10 mL. The amount of drug (mg of drug/ 100 mg of conjugate) was determined with the help of the calibration curve obtained from the standard solution of the drug and results were used to calculate the degree of substitution (DS).
2.5 Pharmacokinetic analysis
Pharmacokinetics profile of naproxen prodrugs, efficient and rapid reverse phase (RP) HPLC/UV method were developed and validated to calculate the drug contents. An Agilent 1200 series HPLC instrument was used. This instrument was equipped with a Degasser (G1322A), a DAD variable wavelength UV detector (G1315B) and a quaternary pump (G1311A). For naproxen prodrugs, the mobile phase was acetonitrile:water (42:58 v/v) (pH-3) and λmax 254 nm was used. 1.0 mL min−1 was the flow rate of each mobile phase with an injection volume of 10 μL in each run.
Drug and prodrug (100 mg) were dissolved in 100 mL mobile phase for the preparation of 1000 ppm stock solution of each drug. This stock solution was diluted to get further desired concentrations. Before packing into HPLC vials all standard solutions of drugs and prodrugs were filtered using nylon syringe filter (0.45 µ) and sonicated before analysis. An isocratic mode was used for all chromatographic separations.
2.5.1 RP HPLC/UV method validation
For the validation of HPLC/UV methods ICH guidelines were followed before studying the pharmacokinetic profile of the prodrugs. HPLC of NAP in plasma and NAP from HPC-NAP conjugate after its absorption/hydrolysis in vivo in plasma is given in supplementary data (Fig. S1). Different validation parameters that were studied are being summarized below.
2.5.1.1 Linearity, precision, and accuracy
The linearity of analytical response in all methods was confirmed using a range of standard solutions (5–50 μg/mL) of prodrug HPC-NAP conjugate. Calibration curves were obtained after injecting 10 μL of every sample from chromatograms and the r2 value was determined. For each designed method precision was calculated through many runs (six) of standard solution (10 μg/mL) of the prodrug. The relative standard deviations (RSD) were determined and Intra-day precision and inter-day precisions were also determined to check the variability of each method. The accuracy of the method was calculated in terms of percentage recovery. For determination of accuracy different samples were spiked with known amounts of prodrug solutions. The amounts of samples recovered demonstrated the percentage recovery.
2.5.1.2 Specificity and robustness
The specificity of each method is determined using selected chromatographic conditions for a blank and a standard solution (10 μg/mL) of each drug. The specificity of the method was confirmed because chromatograms demonstrated no obstruction by any impurity. Different parameters (temperature of column, composition, pH and flow rate of mobile phase) were changed for the establishment of the robustness of each method. No important change in chromatograms was observed by these experiments.
2.5.1.3 Limit of detection (LOD), limit of quantification (LOQ) and stability study
Dilute solutions of known concentrations were used for calculation of LOD & LOQ of each method to a final response equal to three times the signal-to-noise ratio and as ten times of signal-to-noise ratio respectively. The stability study of the sample in the mobile phase was carried out before analysis to assess any degradation of the sample. The stability study was carried out for 72 h in this work.
2.5.2 Pharmacokinetic studies
2.5.2.1 Participants and study design
For pharmacokinetic studies of each drug and prodrug, six healthy male albino rabbits (≈ 1.5 kg each) were chosen. All the rabbits were kept under 12 h light and dark periods. Before the administration of either with pure drugs or conjugates, both groups of rabbits were kept under fast overnight (10 h) with ad libitum access to water. HPC-naproxen conjugate (110 mg conjugate = 44 mg drug) was administered orally by gavage tube to animal models. All the animals were kept under observation. They were given water 1 h after drug administration and standard green food after 5 h. Study procedures where animal models are used has been approved by the Institutional Research Ethics Committee of The University of Lahore, Lahore vide letter no. IAEC-2016-15A. The studies on animal models were in accord with the directions of the National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.5.2.2 Specimen collection and storage
After oral administration of drugs and prodrugs, blood samples (3–5 mL) were collected from the jugular vein of each rabbit. The heparinized disposable syringes were used at prescribed time intervals. Then these blood samples were centrifuged at 3500 × g for 20 min to separate plasma. Plasma samples were stored in a freezer at −10 °C for further studies.
2.5.2.3 Plasma sample preparation
Plasma samples were treated with acetonitrile: methanol (1.0 mL each) plasma protein precipitation and stored for about 15 min. The precipitates were then separated by centrifugation 3000 × g and the clear supernatant was taken by a syringe and filtered with 0.45 μ nylon syringe filters before HPLC/UV analysis.
2.5.2.4 Bioequivalence of drug and prodrug
Plasma concentration vs. time curves for each drug and prodrug were constructed using Microsoft Excel® 2010. The linear trapezoidal method was used to calculate several pharmacokinetic parameters i.e. area under the curve from time zero to time t (AUC0-t), area under the curve from time zero to time infinity (AUC0-∞), peak drug concentration (Cmax), the time to peak drug concentration (tmax), clearance (Cl), the half-life of the drug (t½) and volume of distribution (Vd).
3 Results and discussions
3.1 Synthesis of HPC-NAP conjugates as macromolecular prodrugs
The HPC-NAP conjugates were synthesized using homogenous methodology widely accepted for the esterification reaction of cellulose and related polysaccharide materials (Heinze et al., 2003; Hussain et al., 2013). In the first step, naproxen was activated using a powerful and fascinating acylating reagent CDI just by mixing them in DMAc solvent and stirring for 24 h. As revealed from literature, CDI efficiently reacts with carboxylic acid-containing molecules to make their highly reactive derivatives, i.e., imidazolides (Hussain et al., 2004). Therefore, naproxen imidazolides were efficiently synthesized which then reacts with hydroxyl groups on to HPC polymer to form prodrug/conjugates of naproxen under homogenous reaction conditions. The reaction is shown in Fig. 1.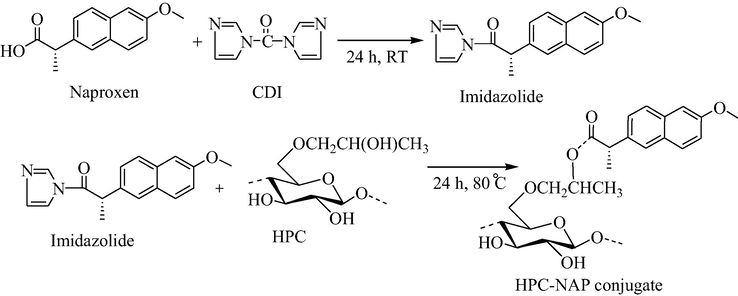
Synthesis of HPC-NAP conjugates applying in situ activation of NAP with CDI.
The products obtained were soluble in different solvents like dimethylformamide (DMF), dimethylsulfoxide (DMSO) and DMAc. Moreover, all the samples of HPC-NAP conjugates 1–3 were found insoluble in water due to high drug attachment to the polymer. Different reaction conditions and results are summarized in Table 1. All the impurities and un-reacted drug were soluble in diethyl ether; therefore, all macromolecular prodrugs were thoroughly washed with diethyl ether thrice. The samples were characterized by different spectroscopic techniques.
Sample
Molar ratioa
DC
DSb
Yield
Solubility
1
1:1:1
13
0.25
74%
DMAc, DMF, DMSO
2
1:2:2
26
0.60
78%
DMAc, DMF, DMSO
3
1:3:3
40
1.13
84%
DMAc, DMF, DMSO
The degree of substitution of HPC-NAP conjugates was determined using UV/Vis spectroscopic technique. The amount of drug-loaded per HPC repeating unit was calculated in terms of mg of drug per 100 mg of conjugate and DS was expressed as substituted drug moieties per repeating unit of the polymer. Conjugates 1–3 showed DS in the range of 0.25–1.13 indicating the significant potential of synthesized prodrugs in terms of the amount of drug-loaded covalently to the HPC.
FTIR spectra of HPC-NAP conjugates 1–3 showed absorbance of ester moiety (COEster) at 1724–1737 cm1, aromatic C-H group was detected at 2837–3132 cm−1 and free hydroxyl groups (–OH) were observed at 3200–3500 cm−1. The polymer C-O-C peaks were detectable in the range of 1040–1065 cm−1. The FTIR spectrum showed the success of the reaction as the presence of new ester signals was observed. All of the different signals of CH2 groups appeared at 1445–1452 cm−1 for conjugates. The typical overlay spectra of HPC-NAP conjugates 3, naproxen and HPC are shown in Fig. 2. The FTIR spectrum of HPC-NAP conjugates 3 showed ester absorption at 1724 cm1 confirming the covalent loading of the drug to HPC. The aromatic C-H groups are detectable at 2837 cm−1 and remaining free hydroxyl groups (–OH) appeared at 3393 cm−1. The polymer C-O-C peak is also evident in the spectrum at 1192 cm−1.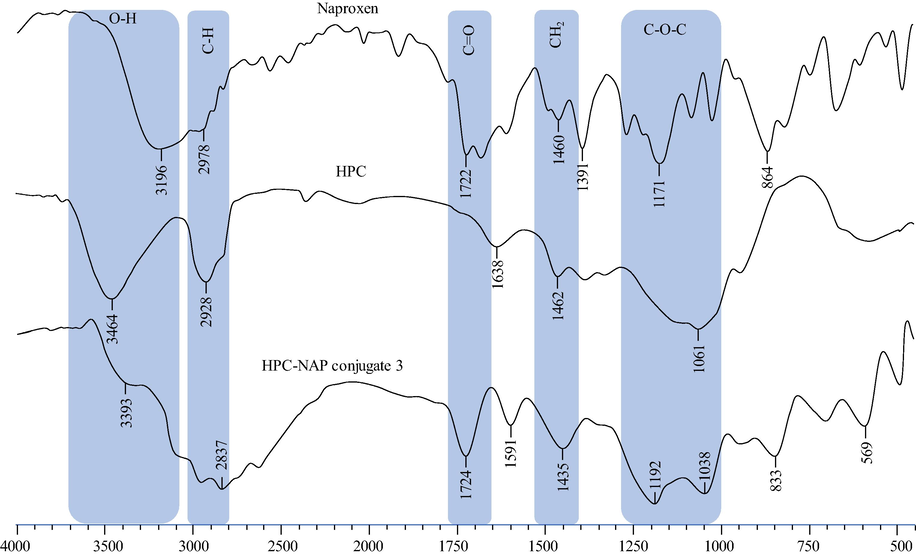
FTIR spectra of naproxen, HPC and HPC-NAP conjugate 3.
1H NMR (400 MHz, DMSO‑d6) spectrum of HPC-NAP conjugate 3 (Fig. 3) showed the presence of an aromatic ring attached to HPC at δ 7.11–7.65 ppm (H-11-H-15). The protons of the HPC polymer backbone/anhydroglucose unit (AGU) were detectable at δ 3.26 to 3.83 ppm. The protons of methyl at hydroxypropyl moiety of HPC polymer were detected at δ 1.01 ppm. The signals of –CH2 and –CH of hydroxypropyl moiety are overlapped with AGU signals. See supplementary data for 1H NMR spectra of NAP (Fig. 2S) and HPC (Fig. 3S).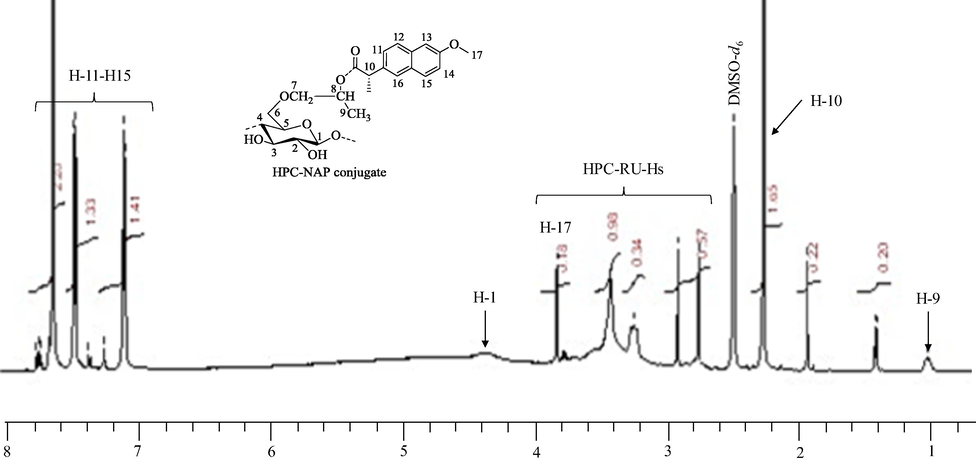
1HNMR spectrum of HPC-NAP conjugate 3.
3.2 Pharmacokinetic studies of HPC-NAP conjugate 3
Table 2 consists of various method validation parameters for HPC-NAP conjugate. The overlay plot of plasma concentration vs. time for HPC-NAP conjugate is shown in Fig. 4, while results are summarized in Table 3.
No.
Parameter
HPC-naproxen conjugate
(Mean)
1
Concentration range
1–20 μg mL−1
2
LOD
50 ng mL−1
3
LOQ
150 ng mL−1
4
Linear regression co-efficient (r2)
0.9989
5
Precision Intra-day (RSD)*
0.543%
6
Precision Inter-day (RSD)*
0.231%
7
Accuracy (% recovery)*
99.08%
8
Precision (RSD)*
0.256%
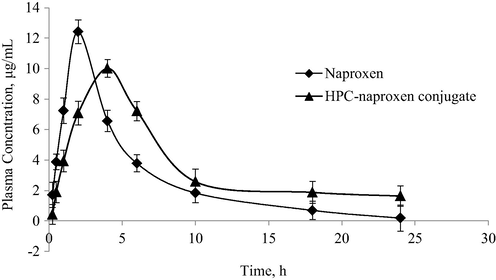
Overlay plot of mean plasma concentration vs. time after oral administration of 110 mg of HPC-naproxen conjugate 3 (equal to 44 mg naproxen) to rabbits.
Parameter
.
HPC-naproxen conjugate
tmax
2.0 h
4.0 h
Cmax
12.43 ± 1.23 μg mL−1
10.01 ± 0.13 μg mL−1
t1/2
5.81 ± 0.74 h
10.15 ± 1.37 h
AUC0-∞h
68.05 ± 5.54 h μg mL−1
113.28 ± 6.73 h μg mL−1
Vd
0.73 ± 0.07 L kg−1
0.74 ± 0.67 L kg−1
Cl
0.09 ± 0.005 L h−1
0.05 ± 0.002 L h−1
The graph for plasma concentration vs. time curve of HPC-NAP conjugate 3 shows the enhanced bioavailability of naproxen from HPC-NAP conjugate as compared with the drug, in the rabbit model. The pharmacokinetic parameters such as tmax and t1/2 have higher values for HPC-NAP conjugate, i.e., 4.0 h and 10.15 h then naproxen itself, i.e., 2 h and 5.81 h, respectively. The longer t1/2 value of naproxen after oral administration of conjugate strongly supports HPC-NAP conjugate as sustained-release formulation. AUC0-∞ of HPC-NAP conjugate is also larger than that of the drug which is proof of enhanced bioavailability of naproxen and could be of great therapeutic value like lower cost, lower variability between patients and between administration times, and reduced side effects. Maximum plasma concentration mean values (Cmax) from conjugate and drug are 12.43 and 10.01 μg mL−1, respectively (Table 3). Comparable Cmax value for HPC-NAP conjugate provides the benefit of lower dosage for the same therapeutic levels as are offered by naproxen. The longer half-life may offer the MIC levels for a longer period.
4 Conclusions
NAP conjugates based on HPC were synthesized successfully using efficient reaction methodology and comprehensively characterized. Pharmacokinetic studies showed that bioavailability of naproxen was enhanced after attachment on to the HPC backbone and the conjugate showed sustained release characteristics. HPC-NAP conjugates were noticed to be effective anti-inflammatory agents with noticeable immunomodulatory properties. By forming such prodrugs of different NSAIDs, modeling this study, one may alleviate NSAID associated gastric irritancy and get the improved pharmacokinetic profile.
Acknowledgement
We are thankful to the Higher Education Commission of Pakistan for financial support under the scheme National Research Programme for Universities and project No. 20-3651. We are thankful to Candid Pharmaceuticals, Sialkot, Pakistan for the generous gift of naproxen (USP). We are also thankful to Dr. Kamran, College of Pharmacy, UOS and Dr. M. Zaman, College of Pharmacy, UOL for helpful discussions regarding pharmaceutical analyses. Animal houses of The University of Lahore and University of Sargodha were utilized for animal hosting and caring; hence we are deeply indebted to both of them.
References
- Sustained ophthalmic delivery of ofloxacin from an ion-activated in situ gelling system. Pak. J. Pharm. Sci.. 2009;22:175-179.
- [Google Scholar]
- Synthesis and pharmacologic study of new esters of naproxen with derivatives of N-oxyethylpiperazine. Il Farmaco; edizione scientifica. 1985;40:334-346.
- [Google Scholar]
- Biocompatible locust bean gum as mesoporous carriers for naproxen delivery. Mater. Chem. Phys.. 2020;239:121973-121987.
- [Google Scholar]
- Organic esters of cellulose: new perspectives for old polymers. In: Polysaccharides I. Berlin, Heidelberg: Springer; 2005. p. :103-149.
- [Google Scholar]
- Macromolecular prodrugs. VI. Coupling of the highly lipophilic agent naproxen to dextrans and in vitro characterization of the conjugates. Farmaci. Sci. Ed. 1988;16:73-85.
- [Google Scholar]
- Unconventional cellulose esters: synthesis, characterization and structure–property relations. Cellulose. 2003;10:283-296.
- [Google Scholar]
- Acylation of cellulose with N, N′- carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol. Rapid Commun.. 2004;25:916-920.
- [Google Scholar]
- Hydroxypropylcellulose-aceclofenac conjugates: high covalent loading design, structure characterization, nano-assemblies and thermal kinetics. Cellulose. 2013;20:717-725.
- [Google Scholar]
- Novel high-loaded, nanoparticulate and thermally stable macromolecular prodrug design of NSAIDs based on hydroxypropylcellulose. Cellulose. 2015;22:461-471.
- [Google Scholar]
- Polysaccharide-based materials in macromolecular prodrug design and development. Int. Mater. Rev.. 2017;62:78-98.
- [Google Scholar]
- “Smart” alginate-hydroxypropylcellulose microbeads for controlled release of heparin. Int. J. Pharm.. 2010;385:163-169.
- [Google Scholar]
- Cyclic amide derivatives as potential prodrugs II: N-hydroxymethylsuccinimide-/isatin esters of some NSAIDs as prodrugs with an improved therapeutic index. Eur. J. Med. Chem.. 1999;34:551-562.
- [Google Scholar]
- Brain-specific delivery of naproxen using different carrier systems. Arch. Pharm. Pharm. Med. Chem.. 2010;343:639-647.
- [Google Scholar]
- Physical mechanical and tablet formation properties of hydroxypropylcellulose: in pure form and in mixtures. AAPS PharmSciTech. 2007;8:82.
- [Google Scholar]
- Piperazinylalkyl prodrugs of naproxen improve in vitro skin permeation. Eur. J. Pharm. Sci.. 2000;11:157-163.
- [Google Scholar]
- Ester and amide prodrugs of ibuprofen and naproxen: synthesis, anti-inflammatory activity, and gastrointestinal toxicity. J. Pharm. Sci.. 1992;81:149-154.
- [Google Scholar]
- Macromolecular drugs: novel strategy in target specific drug delivery. J. Clin. Diagnos. Res.. 2008;2:1020-1023.
- [Google Scholar]
- Naproxen. Drugs. 1990;40:91-137.
- Synthesis and biological evaluation of Ω-(N, N, N-trialkylammonium) alkyl esters and thioesters of carboxylic acid nonsteroidal antiinflammatory agents. Pharm. Res.. 1989;6:867-873.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.04.010.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







