Translate this page into:
Design, in-silico study and biological evaluation of newly synthesized 3-chlorobenzofuran congeners as antitubercular agents
⁎Corresponding authors at: Deptt. of Pharmacology, Faculty of Medicine, King Abdulaziz University, Jeddah 21589, Saudi Arabia (Shahid Karim). skaled@kau.edu.sa (Shahid Karim), shahid.karim@yahoo.co.in (Shahid Karim), yarmsy@rediffmail.com (M. Shahar Yar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Benzofuran is a heterocyclic scaffold present in various natural products and possess excellent pharmacological properties including anti-tubercular activity as well. A novel series 26 compound containing 3-chlorobenzofuran derivatives are designed, synthesized and spectroscopically characterized. In vitro screening of compounds was done against multidrug resistant Mycobacterium tuberculosis H37Rv strains. Out of these compounds 3a, 3b, 3c, 4b and 4c exhibited excellent inhibitory potency with IC 50 values in the range of 43–104 μM. Compound 3b was found to be the most potent with IC 50 value of 51.24 μM and IC 90 value of 88.04 μM. The compound may serve as lead for future development of potential and effective anti-tubercular agent.
Keywords
Benzofuran
Pyrazole
Anti-tubercular
Structure activity relationship
In-silico study
1 Introduction
Tuberculosis (TB) is a chronic communicable disease caused by Mycobacterium tuberculosis, majorly affecting lungs. TB is the 10th leading cause of death globally and every year millions of people get affected with it. According to 2018 global report of WHO, an estimated 1.3 million deaths were caused due to TB. In 2017, 10 million people developed TB globally of which India, China and Indonesia share maximum number of patients. Multi Drug Resistance- Tuberculosis (MDR-TB) continues to be a public health crisis. Over the decades, pathogens have become more resistant to the first and second line anti mycobacterial drugs such as isoniazid, rifampicin, ethambutol, streptomycin etc. In 2017, approximately half million people developed drug resistant TB worldwide. Drug resistance can further worsen the situations in future, so it is the critical need of the time to explore more in this field and look for novel, potent anti-TB molecules (Lienhardt et al., 2018; Floyd et al., 2018).
Benzofuran is a heterocyclic fused ring of benzene with furan possessing a number of pharmacologically properties such as antibacterial (Li et al., 2019), antifungal (Liang et al., 2016), anthelmintic (Kenchappa et al., 2017), anticancer (Abbas and El-Karim, 2019; El-Karim et al., 2015; Xie et al., 2015) anti-viral (Gouhar et al., 2018; He et al., 2015; Gao et al., 2019) and anti-tubercular (Zhang et al., 2017; Bodke et al., 2017). Benzofuran has shown important place in the drug development. Currently substituted Benzofurans are gaining interest as anti-mycobacterial agents, linking of pyrazine with benzofuran results into most active compound showing minimum inhibitory concentration (MIC) of 1.21 μg/mL. Exploitation of second and third position of benzofuran has been reported to give compounds with MIC value as low as 3.25 μg/mL (Telvekar et al., 2012; Nevagi et al., 2015).
Pyrazole is nitrogen containing heterocycle having an important role in medicinal chemistry. Pyrazole and its derivatives constitute a number of compounds exhibiting diverse pharmacological activities which includes analgesics, antimicrobial, antibacterial, antifungal, anticonvulsant, anticancer and antitubercular (Harikrishna et al., 2016; Nayak et al., 2016; Domiati et al., 2016; Muhammad et al., 2019; Chougala et al., 2017; El Shehry et al., 2018; Wu et al., 2018; Reddy et al., 2015; Zhang et al., 2019).
In recent years, a number of compounds having substituted benzofuran bearing pyrazole were reported, they exhibited broad-spectrum antimicrobial activities. (Chu et al., 2019; Manna and Agrawal, 2010). We have designed some novel benzofuran linked pyrazole derivatives to find better therapeutic agents for the treatment of tuberculosis. In present work, a series of 3-chlorobenzofuran 4,5-dihydropyrazole derivatives were synthesized and their antitubercular activity was evaluated against Mycobacterium tuberculosis H37RV.
2 Result and discussion
2.1 Chemistry
Derivatives of 3-Chlorobenzofuran were synthesized as showed in Scheme 1. 3-chloro benzofuran-2-carbaldehyde was taken as starting material and reacted with different aromatic aldehydes to give intermediates 2a-2n in excellent yield. Compounds 2a-2n were refluxed with benzohydrazide and hydrazine hydrate to finally yield compounds 3a-3e and 4a-4m respectively.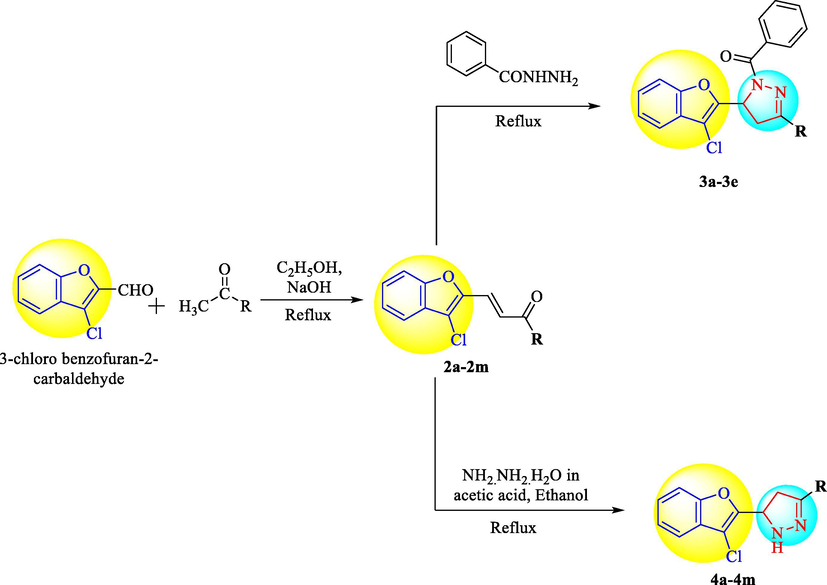
Synthesis of 3-Chlorobenzofuran derivatives.
The yield of the products varied with maximum yield of 98% with anisaldehyde. IR spectra of these chalcones revealed C = O, C = C and C-Cl stretching at 1685, 1605 and 750 cm−1. 1H NMR spectrum further confirmed the structure marked by the disappearance of aldehydic proton and appearance of two characteristic doublets one each for vinylic protons. Selected chalcones (2b, 2f, 2h, 2l & 2m) were reacted with benzoic acid hydrazide in glacial acetic acid to get the respective pyrazoline derivatives (3a-3e). All the compounds were purified by column chromatography and their spectral data was found satisfactory for the proposed structures. The chalcones (2a-2n) were reacted separately in ethanol with hydrazine hydrate by using molecular sieves in presence of glacial acetic acid. In few cases two products were obtained which were isolated by column chromatography, the minor product was identified as acetyl derivative. The pyrazolines (4a-4m) thus obtained (major product) were characterized on the basis of elemental analysis, IR, 1H NMR spectra. Physical constant of all the synthesized derivatives are presented in Table 1a.
Compound
R
Mol. Wt.
Mol. formulae
% Yield
Melting point (°C)
2a
H
282
C17H11ClO2
78
140–42
2b
4-Chloro
317
C17H10Cl2O2
87
148–50
2c
4-Methyl
296
C18H13ClO2
76
126–28
2d
4-Nitro
327
C17H10ClNO2
70
180–82
2e
4-Amine
297
C17H12ClNO2
84
184–86
2f
4-Hydroxy
298
C17H11ClO3
76
210–12
2 g
2-Hydroxy
298
C17H11ClO3
76
110–12
2 h
4-Methoxy
312
C18H13ClO3
98
106–08
2i
2,4-Dihydroxy
314
C17H11ClO4
80
100–02
2j
2,4-Dimethoxy
342
C19H15ClO4
84
138–40
2 k
3,4-Dimethoxy
342
C19H15ClO4
89
172–74
2l
4-Hydroxy 3-methyl
312
C18H13ClO3
76
202–04
2m
4-Hydroxy 2-methyl
312
C18H13ClO3
76
160–62
3a
4-Chloro
435
C24H16Cl2N2O2
62
150–52
3b
4-Hydroxy
416
C24H17ClN2O2
56
150–52
3c
4-Methoxy
430
C25H19ClN2O3
67
100–02
3d
4-Methoxy 3-methyl
430
C25H19ClN2O3
66
144–46
3e
4-Hyroxy 2-methyl
430
C25H19Cl2N2O3
70
148–50
4a
H
296
C17H13ClN2O
78
126–28
4b
4-Chloro
331
C17H12Cl2N2O
82
66–68
4c
4-Methyl
310
C18H15ClN2O
80
146–48
4d
4-Nitro
341
C17H12ClN3O3
74
126–28
4e
4-Amino
311
C17H14ClN3O
70
120–22
4f
4-Hydroxy
312
C17H13ClN2O2
78
268–70
4g
2-Hydroxy
312
C17H13ClN2O2
82
148–50
4h
4-Methoxy
326
C18H15ClN2O2,
87
182–84
2.2 Biological activity
2.2.1 Antitubercular
The newly synthesized compounds were evaluated in vitro for antitubercular activity against Mycobacterium tuberculosis H37Rv strain (ATCC27294) by Microplate Alamar Blue Assay (MABA) (Wahyuningrum et al., 2017; Xu et al., 2016; Bunalema et al., 2015; Nkenfou et al., 2015), at Tuberculosis Antimicrobial Acquisition and Coordination facility (TAACF). Isoniazid (INH) is reported to have MIC at 0.43 μM with selectivity index of > 1,250 by MABA (D Sriram, 2006). For each compound both IC50 and IC90 were determined, Table 1b represent anti-mycobacterial activity against H37Rv strain of mycobacterium tuberculosis of all synthesized inhibitors.
Compound
R
MABA: H37Rv data
IC50 (µM)
IC90 (µM)
2a
H
118.70
252.06
2b
4-Chloro
>400
>400
2c
4-Methyl
>400
>400
2d
4-Nitro
>400
>400
2e
4-Amine
>400
>400
2f
4-Hydroxy
113.72
>400
2g
2-Hydroxy
>400
>400
2h
4-Methoxy
>400
>400
2i
2,4-Dihydroxy
311.69
>400
2j
2,4-Dimethoxy
>400
>400
2k
3,4-Dimethoxy
>400
>400
2l
4-Hydroxy 3-methyl
>400
>400
2m
4-Hydroxy 2-methyl
<0.1950
>400
3a
4-Chloro
106.84
165.66
3b
4-Hydroxy
51.30
88.04
3c
4-Methoxy
89.36
143.82
3d
4-Methoxy 3-methyl
119.84
>400
3e
4-Hyroxy 2-methyl
178.37
277.27
4a
H
>400
>400
4b
4-Chloro
101.81
158.90
4c
4-Methyl
102.35
163.58
4d
4-Nitro
242.03
>400
4e
4-Amino
153.80
>400
4f
4-Hydroxy
316.81
>400
4g
2-Hydroxy
>400
>400
4h
4-Methoxy
92.68
178.64
Isoniazid
–
0.054
0.076
Selected compounds of the series (2a-4 m) were evaluated for in vitro antitubercular activity. Eight compounds of the series were found to be weakly active with MICs ranging from 88.04 to 178.58 µM while thirteen were found inactive, particularly chalcones (2a-2m), except 3-(3-Chlorobenzofuran-2-yl)-1-phenyl-2-propen-1-one (2a) that exhibited IC50 of 106.83 µM and IC90 of 252.06 µM. Derivatives 3a-3e showed good potency, 5-(3-Chlorobenzofuran-2-yl)-3-(4-hydroxyphenyl)-4,5-dihydro-1H-1-pyrazolyl-phenyl methanone (3b) is the most potent in all exhibited IC50 of 51.24 µM and IC90 of 87.98 µM. Pyrazoline derivatives (4a-4m) were active in comparison to parent chalcones (Fig. 2).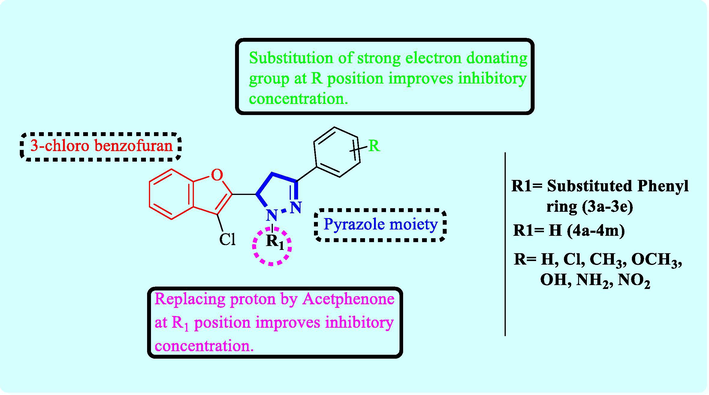
Structure activity relationship of.
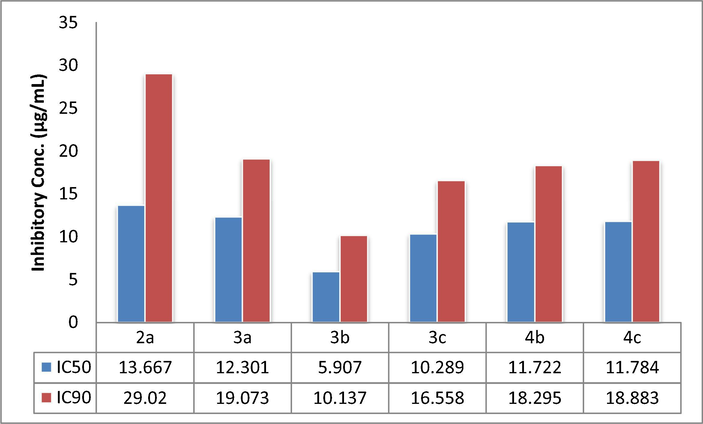
Graph representing inhibitory constants (IC50 & IC90) of potent compounds.
2.3 Molecular docking and ADME studies
Molecular docking was performed to understand the molecular interactions of the synthesized compounds at the binding site of the target protein. The target protein for anti-mycobacterial activity was obtained from Protein Data Bank (PDB ID: 5OIF). ADME calculation (Table 3) depicts that all compounds completely get absorbed when administered orally. Compounds 3b and 3c possessed two rotational bonds, 3a, has one rotational bond, while 4a and 4c have no rotational bonds. 3b, 4a and 4c, each has at least one hydrogen bond donor while all compounds have good number of h-bond acceptor. All compounds exhibited drug gable properties as per Lipinski’s rule. Molecular docking analysis suggests that all compounds were active as compared to isoniazid and pyrazinamide (Table 2). Compound 3b displayed maximum docking (−7.061 Kcal/mol) and glide score (−7.063 Kcal/mol) compared to isoniazid which has docking score of −5.696 Kcal/mol and glide score of −5.697 Kcal/mol. Binding site interactions reveal that 3b forms two strong hydrogen bonds with methionine (MET98- 1.95 Å) and threonine (THR196- 1.86 Å) residues while isoniazid and pyrazinamide forms four hydrogen bonds (GLY14, ALA22, SER94 and LYS165) and one hydrogen bond (ILE194) respectively at the binding site. Although the hydrogen bond forming residues are different when compared to isoniazid and pyrazinamide, they all share some common hydrophobic interactions suggesting binding in the similar region of the target. Images depicting two dimensional interactions and three dimensional binding poses of compound 3b and isoniazid have been shown in Fig. 3.
Compound
Docking score (Kcal/mol)
Glide score (Kcal/mol)
3a
−6.051
−6.051
3b
−7.061
−7.063
3c
−6.466
−6.466
4b
−6.324
−6.325
4c
−6.404
−6.405
Isoniazid
−5.696
−5.697
Pyrazinamide
−5.418
−5.418
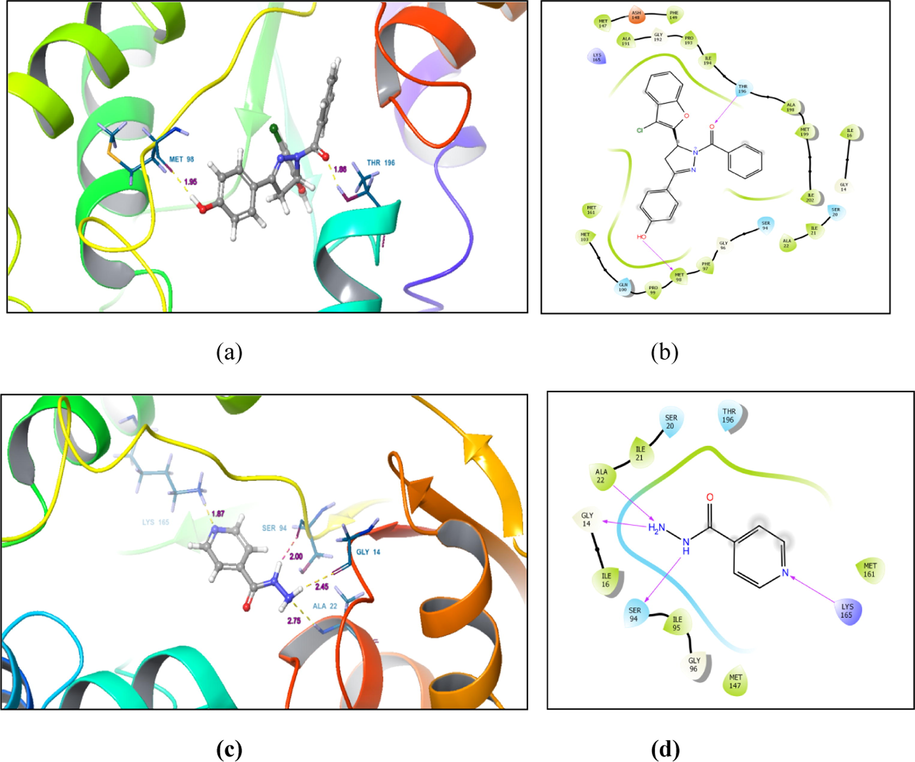
Molecular docking of compound 3b and isoniazid (a) 3D interaction pose of compound 3b at the binding site (b) 2D interaction of compound 3b with different residues at the binding site (c) 3D interaction pose of isoniazid at the binding site (d) 2D interaction of isoniazid with different residues at the binding site.
3 Conclusion
In this work, we have designed, synthesized and evaluated a novel series of chloro benzofuran derivatives for antitubercular activity. Most of the derivatives exhibited potent antimycobacterial property against Mycobacterium tuberculosis H37RV strain. Compound 3a, 3b, 3c, 4b and 4c showed good inhibitory potential. Structure activity relationship (Fig. 1) reveals in series 3a- 3e, compound 3a and 3c substituted with chloro and methoxy group at R1 position respectively have showed excellent inhibitory potency. Whereas compound from 4a to 4m series, 4b and 4c substituted with chloro and methyl group at R1 position has shown good inhibitory potency. Compound 3b is the representative compound which is substituted with hydroxyl group at R1 position is reported as most potent compound (IC50 = 51.24 and IC90 = 87.98 μM). The activity has been further supported by in silico study.
4 Experimental
4.1 General
All the chemicals used were of laboratory grade and were supplied by E. Merck, Germany and S.D. Fine Chemicals, India. The Melting points were determined by the open tube capillary method and was not corrected. Thin layer chromatography (TLC) plates prepared with silica gel G were used to monitor the reactions as well as to confirm the purity of the compounds synthesized and to check the purity of the commercial reagents. For this purpose, two distinct solvent systems; a) toluene: ethyl acetate: formic acid (5:4:1) and b) petroleum ether: toluene: acetic acid (5:4:1), have been used to run the TLC. The spots were viewed under iodine vapors/ultra violet light. Infrared spectral response was obtained on a Perkin-Elmer 1720 FT-IR spectrometer using KBr Pellets. 1H NMR spectra were recorded on Bruker AC 400 MHz in general (whereas in some cases, 300 MHz spectrometer was also used and sited accordingly) using TMS as internal standard in CDCl3 / DMSO‑d6. The FAB mass spectra were recorded on a JEOL SX 102/DA-6000 Mass Spectrometer.
4.2 Chemical synthesis
4.2.1 Synthesis of 3-chlorobenzofuran-2-carbaldehyde (1)
A freshly prepared solution of 2-(2-carboxyphenoxy)acetic acid (1 g) in 5 mL dimethyl formamide (DMF) was added drop wise with constant stirring to 7.8 mL of Vilsmeier reagent maintained at 0 °C. Once the addition was completed the reaction mixture was allowed to attain the room temperature and gradually temperature was increased till it reaches to 90 °C. The reaction was allowed to continue at 90 ± 2 °C for further 6 h. After completion of the reaction, the mixture was cooled and poured on to 250 mL crushed ice with constant stirring. The solid obtained was filtered, washed with plenty of water and recrystallized from ethanol. Percentage yield was found to be 15–20% and melting point recorded to be 74–76 °C.
FT-IR (KBr, cm−1): 2840 (Aldehydic C-H), 1679 (C = O) 758 (C-Cl); 1H NMR (CDCl3, δ, ppm): 7.39–7.43 (1H, m, Ar-H), 7.58 (2H, d, J = 3.6 Hz, Ar-H), 7.74 (1H, d, J = 8.0 Hz, Ar-H), 10.03 (1H, s, –CHO).
4.2.2 General method for compound synthesis (2a-2m)
To an ethanolic solution (25 mL) of 3-chlorobenzofuran-2-carbaldehyde (0.01 mol), an appropriately substituted acetophenone (0.01 mol) and 5 mL 10% aqueous solution of NaOH were added. The reaction mixture was stirred at 25 °C for 2–3 h. After the reaction was completed, the content was poured onto the crushed ice and neutralized with dilute HCl. The resulting precipitates were filtered, water washed, dried and re-crystallized from ethanol for the desired product (2a-2m).
4.2.2.1 3-(3-chlorobenzofuran-2-yl)-1-phenylprop-2-en-1-one (2a)
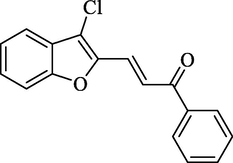
Molecular Formulae: C17H11ClO2, Molecular weight: 282, %Yield: 78, Melting Point: 140–42 °C FT-IR (KBr, cm−1): 1685 (C = O), 1606 (C = C), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 6.80 (1H, d, Hα, J = 8.4 Hz), 7.26–7.66 (9H, m, Ar-H), 7.68 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C17H11ClO2: C, 72.22; H, 3.92. Found: C, 72.06; H, 3.93%.
4.2.2.2 3-(3-Chlorobenzofuran-2-yl)-1-(4-chlorophenyl)-2-propen-1-one (2b)
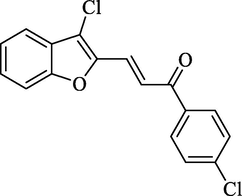
Molecular Formulae: C17H10Cl2O2, Molecular weight: 317, %Yield: 87, Melting Point: 148 °C FT-IR (KBr, cm−1):1682 (C = O), 1605 (C = C), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 6.86 (1H, d, Hα, J = 8.0 Hz), 6.90 (2H, d, Ar-H, J = 8 Hz), 7.26 (4H, m, Ar-H), 7.66 (2H, d, Ar-H, J = 8.8 Hz), 7.96 (1H, d, Hβ, J = 8.4 Hz). Anal. Calcd. for C17H10Cl2O2: C, 64.38; H, 3.18. Found: C, 64.16; H, 3.19%.
4.2.2.3 3-(3-Chlorobenzofuran-2-yl)-1-(4-methylphenyl)-2-propen-1-one (2c)
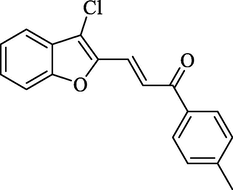
Molecular Formulae: C18H13ClO2, Molecular weight: 296, %Yield: 76, Melting Point: 126–128 °C FT-IR (KBr, cm−1): 1690 (C = O), 1601 (C = C), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 2.46 (3H, s, -CH3), 6.72 (1H, d, Hα, J = 8.0 Hz), 7.25–7.39 (5H, m, Ar-H), 7.48 (1H, d, Ar-H, J = 6.4 Hz), 7.66 (2H, d, Ar-H, J = 8.4 Hz), 7.86 (1H, d, Hβ, J = 8.0 Hz). Anal. Calcd. for C18H13ClO2: C, 72.85; H, 4.42. Found: C, 72.96; H, 4.41%.
4.2.2.4 3-(3-Chlorobenzofuran-2-yl)-1-(4-nitrophenyl)-2-propen-1-one (2d)
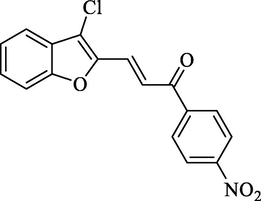
Molecular Formulae: C17H10ClNO2, Molecular weight: 327, %Yield: 70, Melting Point: 182–84 °C FT-IR (KBr, cm−1): 1680 (C = O), 1608 (C = C), 1523 & 1377 (NO2), 747 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 6.79 (1H, d, Hα, J = 8.8 Hz), 6.95(2H, d, Ar-H, J = 8.4 Hz) 7.32–7.56 (4H, m, Ar-H), 7.60 (2H, d, Ar-H, J = 8.4 Hz), 7.65 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C17H10ClNO4: C, 62.30; H, 3.08; N, 4.27. Found: C, 62.22; H, 3.08; N, 4.28%.
4.2.2.5 3-(3-Chlorobenzofuran-2-yl)- 1-(4-aminophenyl)-2-propen-1-one (2e)
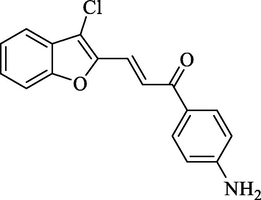
Molecular Formulae: C17H12ClNO2, Molecular weight: 297, %Yield: 84, Melting Point: 184–86 °C. FT-IR (KBr, cm−1): 1685 (C = O), 1606 (C = C), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.23 (2H, s, NH2), 6.86 (1H, d, Hα, J = 8.4 Hz), 7.26–7.48 (4H, m, Ar-H), 7.49 (1H, d, Ar-H, J = 6.4 Hz), 7.51 (1H, d, Ar-H, J = 6.4 Hz), 7.55 (2H, d, Ar-H, J = 8.4 Hz), 7.85 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C17H12ClNO2: C, 68.58; H, 4.06; N, 4.70. Found: C, 68.46; H, 4.08; N, 4.72%.
4.2.2.6 3-(3-Chlorobenzofuran-2-yl)-1-(4-hydroxyphenyl)-2-propen-1-one (2f)
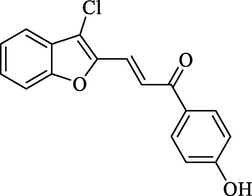
Molecular Formulae: C17H11ClO3, Molecular weight: 298, %Yield: 76, Melting Point: 210–212 °C FT-IR (KBr, cm−1): 3320 (OH), 1685 (C = O), 1606 (C = C), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 6.81 (1H, d, Hα, J = 8.4 Hz), 7.22–7.64 (8H, m, Ar-H), 7.87 (1H, d, Hβ, J = 8.8 Hz), 9.90 (1H, s, OH). Mass m/z: 299 (M+1). Anal. Calcd. for C17H11ClO3: C, 68.35; H, 3.71. Found: C, 68.38; H, 3.72%.
4.2.2.7 3-(3-Chlorobenzofuran-2-yl)-1-(2-hydroxyphenyl)-2-propen-1-one (2g)
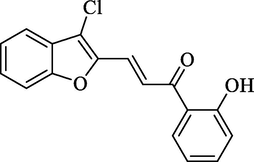
Molecular Formulae: C17H11ClO3, Molecular weight: 298, %Yield: 76, Melting Point: 110–12 °C FT-IR (KBr, cm−1): 3528 (OH), 1682 (C = O), 1614 (C = C), 743 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 6.94 (1H, d, Hα, J = 8.0 Hz), 7.23–7.81 (8H, m, Ar-H), 7.92 (1H, d, Hβ, J = 8.0 Hz), 10.11 (1H, s, OH). Anal. Calcd. for C17H11ClO3: C, 68.35; H, 3.71. Found: C, 68.28; H, 3.70%.
4.2.2.8 3-(3-Chlorobenzofuran-2-yl)-1-(4-methoxyphenyl)-2-propen-1-one (2h)
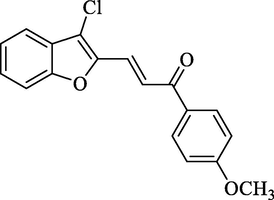
Molecular Formulae: C18H13ClO3, Molecular weight: 312, %Yield: 98, Melting Point: 106–08 °C FT-IR (KBr, cm−1): 1685 (C = O), 1645 (C = C), 750 (C–Cl).; 1H NMR (DMSO‑d6, δ, ppm): 3.52 (3H, s, OCH3), 6.82 (1H, d, Hα, J = 8.4 Hz), 6.91 (2H, d, Ar-H, J = 8 Hz), 6.99–7.67 (5H, m, Ar-H), 7.69 (1H, d, Ar-H, J = 6.8 Hz), 7.78 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19. Found: C, 69.26; H, 4.20%.
4.2.2.9 3-(3-Chlorobenzofuran-2-yl)-1-(2,4-dihydroxyphenyl)-2-propen-1-one (2i)
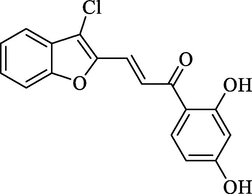
Molecular Formulae: C17H11ClO4, Molecular weight: 314, %Yield: 80, Melting Point: 98–100 °C FT-IR (KBr, cm−1): 3530 (OH), 1682 (C = O), 1606 (C = C), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 6.83 (1H, d, Hα, J = 8.8 Hz), 7.26–7.67 (7H, m, Ar-H), 7.72 (1H, d, Hβ, J = 8.8 Hz) 10.23 (2H, s, 2 × OH). Anal. Calcd. for C17H11ClO4: C, 64.88; H, 3.52. Found: C, 64.90; H, 3.53%.
4.2.2.10 (3-Chlorobenzofuran-2-yl)-1-(2,4-dimethoxyphenyl)-2-propen-1-one (2j)
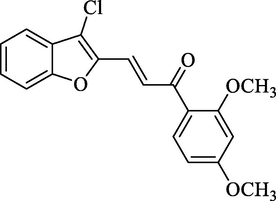
Molecular Formulae: C19H15ClO4, Molecular weight: 342, %Yield: 84, Melting Point: 138–40 °C FT-IR (KBr, cm−1): 1685 (C = O), 1653 (C = C), 735 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.66 (6H, s, 2 × OCH3), 6.92 (1H, d, Hα, J = 8.0 Hz), 7.24–7.84 (7H, m, Ar-H), 7.89 (1H, d, Hβ, J = 8.4 Hz). Anal. Calcd. for C19H15ClO4: C, 66.58; H, 4.41. Found: C, 66.66; H, 4.40%.
4.2.2.11 3-(3-Chlorobenzofuran-2-yl)-1-(3,4-dimethoxyphenyl)-2-propen-1-one (2k)
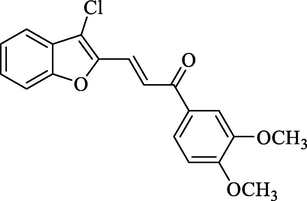
Molecular Formulae: C19H15ClO4, Molecular weight: 342, %Yield: 89, Melting Point: 172–74 °C FT-IR (KBr, cm−1): 1685 (C = O), 1602 (C = C), 765 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.68 (6H, s, 2 × OCH3), 6.82 (1H, d, Hα, J = 8.4 Hz), 6.89–7.68 (7H, m, Ar-H), 7.29 (1H, d, Hβ, J = 8.0 Hz). Anal. Calcd. for C19H15ClO4: C, 66.58; H, 4.41. Found: C, 66.48; H, 4.42%.
4.2.2.12 3-(3-Chlorobenzofuran-2-yl)-1-(4-hydroxy-3-methylphenyl)-2-propen-1-one (2l)
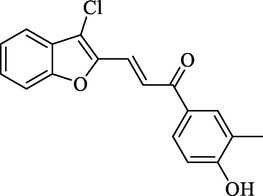
Molecular Formulae: C18H13ClO3, Molecular weight: 312, %Yield: 76, Melting Point: 202–04 °C FT-IR (KBr, cm−1): 3530 (OH), 1685 (C = O), 1606 (C = C), 756 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 2.26 (3H, s, CH3), 6.76 (1H, d, Hα, J = 8.8 Hz), 6.95–7.62 (7H, m, Ar-H), 7.68 (1H, d, Hβ, J = 8.8 Hz) 8.83 (1H, s, OH). Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19. Found: C, 69.26; H, 4.22%.
4.2.2.13 3-(3-Chlorobenzofuran-2-yl)-1-(4-hydroxy-2-methylphenyl)-2-propen-1-one (2m)
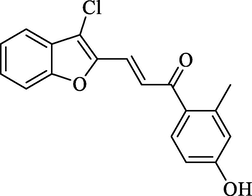
Molecular Formulae: C18H13ClO3, Molecular weight: 312, %Yield: 76, Melting Point: 160–62 °C FT-IR (KBr, cm−1): 3530 (OH), 1686 (C = O), 1606 (C = C), 754 (C-Cl).; 1H NMR (DMSO‑d6, δ, ppm): 2.42 (3H, s, CH3), 6.83 (1H, d, Hα, J = 8 Hz), 7.27–7.67 (7H, m, Ar-H), 7.68 (1H, d, Hβ, J = 8.4 Hz), 8.93 (1H, s, OH). Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19. Found: C, 69.12; H, 4.22%.
4.2.3 General procedure for synthesis of compounds (3a-3e)
An equimolar mixture of benzoic acid hydrazide (1-benzenecarbohydrazide) and appropriate chalcone (2b, 2f, 2h, 2l & 2m) were refluxed in glacial acetic acid for 10–12 h. The excess solvent removed under reduced pressure after completion of reaction and the viscous contents were poured on crushed ice and neutralized with diluted ammonia solution. The products (3a-3e) thus precipitated was filtered, washed with cold water and purified by column chromatography using (Petroleum: Hexane, 4:1).
4.2.3.1 Spectral Data of 5-(3-Chlorobenzofuran-2-yl)-3-(4-chlorophenyl)-4,5-dihydro-1H-1-pyrazolyl-phenyl methanone (3a)
Molecular Formulae: C24H16Cl2N2O2, Molecular weight: 435, %Yield: 62, Melting Point: 150–52 °C, FT-IR (KBr, cm−1): 1654 (C = O), 1583 (C = N). 1H NMR (CDCl3, δ, ppm): 3.21 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.84 (1H, dd, Hb, J = 12.4, 13.2 Hz), 5.29 (1H, dd, Hx, J = 5.6, 6.0 Hz), 7.35–7.82 (13H, m, Ar-H). Anal. Calcd. for C24H16Cl2N2O2: C, 66.22; H, 3.70; N, 6.44. Found: C, 66.35; H, 3.71; N, 6.46%.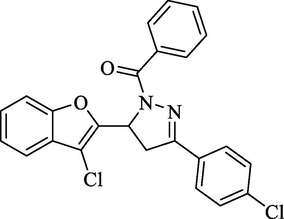
4.2.3.2 5-(3-Chlorobenzofuran-2-yl)-3-(4-hydroxyphenyl)-4,5-dihydro-1H-1-pyrazolyl-phenyl methanone (3b)
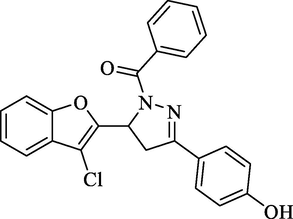
Molecular Formulae: C24H17ClN2O2, Molecular weight: 416, %Yield: 56, Melting Point: 150–52 °C FT-IR (KBr, cm−1): 3433 (OH), 1665 (C = O), 1592 (C = N). 1H NMR (CDCl3, δ, ppm): 3.34 (1H, dd, Ha, J = 5.2, 5.6 Hz), 3.64 (1H, dd, Hb, J = 12.4, 12.4 Hz), 5.29 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.11 (2H, d, Ar-H, J = 8 Hz), 7.15–7.99 (13H, m, Ar-H). Anal. Calcd. for C24H17ClN2O3: C, 69.15; H, 4.11; N, 6.72. Found: C, 68.97; H, 4.10; N, 6.71%.
4.2.3.3 5-(3-Chlorobenzofuran-2-yl)-3-(4-methoxyphenyl)-4,5-dihydro-1H-1-pyrazolyl-phenyl methanone (3c)
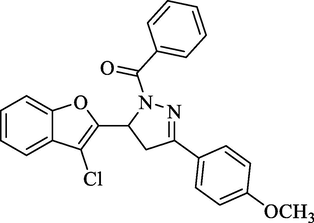
Molecular Formulae: C25H19ClN2O3, Molecular weight: 430, %Yield: 67, Melting Point: 100–02 °C, FT-IR (KBr, cm−1): 1655 (C = O), 1585 (C = N). 1H NMR (CDCl3, δ, ppm): 3.34 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.58 (1H, dd, Hb, J = 12.4, 12.4 Hz), 3.68 (3H, s, –OCH3), 5.38 (1H, dd, Hx, J = 6.0, 5.6 Hz), 7.14–7.78 (13H, m, Ar-H). Anal. Calcd. for C25H19ClN2O3: C, 69.69; H, 4.44; N, 6.50. Found: C, 69.81; H, 4.43; N, 6.49%.
4.2.3.4 5-(3-Chlorobenzofuran-2-yl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-phenylmethanone (3d)

Molecular Formulae: C25H19ClN2O3, Molecular weight: 430, %Yield: 66, Melting Point: 144–46 °C, FT-IR (KBr, cm−1): 1650 (C = O), 1598 (C = N). 1H NMR (CDCl3, δ, ppm): 2.45 (3H, s, -CH3), 3.34 (1H, dd, Ha, J = 6.0, 5.6 Hz), 3.46 (1H, dd, Hb, J = 12.0, 12.4 Hz), 5.57 (1H, dd, Hx, J = 6.0, 6.0 Hz), 7.13–7.56 (12H, m, Ar-H), 10.16 (1H, s, OH). Anal. Calcd. for C25H19ClN2O3: C, 69.69; H, 4.44; N, 6.50. Found: C, 69.74; H, 4.42; N, 6.52%.
4.2.3.5 5-(3-Chlorobenzofuran-2-yl)-3-(4-hydroxy-2-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-phenlmethanone (3e)
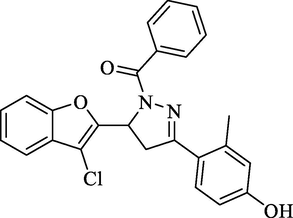
Molecular Formulae: C25H19Cl2N2O3, Molecular weight: 430, %Yield: 66, Melting Point: 144–46 °C, FT-IR (KBr, cm−1): 1655 (C = O), 1589 (C = N). 1H NMR (CDCl3, δ, ppm): 2.44 (3H, s, -CH3), 3.38 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.66 (1H, dd, Hb, J = 12.4, 12.4 Hz), 5.77 (1H, dd, Hx, J = 6.0, 6.0 Hz), 7.19 (1H, d, Ar-H, J = 8.4), 7.39–7.51 (2H, m, Ar-H), 7.57–7.61 (8H, m, Ar-H), 10.05 (1H, s, OH). Anal. Calcd. for C25H19ClN2O3: C, 69.69; H, 4.44; N, 6.50. Found: C, 69.56; H, 4.43; N, 6.48%.
4.2.4 General method for synthesis of compounds (4a-4m)
To an ethanolic solution of (2a-2m) (0.01 mol; 20 mL), a solution of 0.015 mol of hydrazine hydrate in 5 mL glacial acetic acid was added, this reaction mixture was allowed to reflux for 3–6 h in the presence of 100 mg of 5A × 1.5 mm molecular sieves. The contents were concentrated and poured onto 100 g of crushed ice after completion of the reaction, and neutralized with ammonia. To get the pure product, solid mass was filtered, washed with enough cold water and recrystallized with hydrated ethanol (4a-4m).
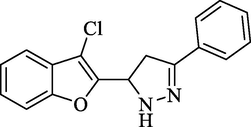
4.2.4.1 2-(3-Phenyl-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4a)
Molecular Formulae: C17H13ClN2O, Molecular weight: 296, %Yield: 78, Melting Point: 126–28 °C, FT-IR (KBr, cm−1): 3580 (N-H), 1596 (C = N), 1324 (C-N), 757 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.49 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.73 (1H, dd, Hb, J = 12.0, 12.4 Hz), 5.87 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.2–7.8 (9H, m, Ar-H). 8.5 (1H, s, NH). Anal. Calcd. for C17H13ClN2O: C, 68.81; H, 4.42; N, 9.44. Found: C, 68.72; H, 4.41; N, 9.45%.
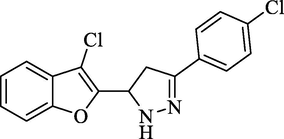
4.2.4.2 2-[3-(4-Chlorophenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (4b)
Molecular Formulae: C17H12Cl2N2O, Molecular weight: 331, %Yield: 82, Melting Point: 66–68 °C, FT-IR (KBr, cm−1): 3569 (N-H), 1596 (C = N), 1322 (C-N), 747 (C-Cl). 1H NMR (CDCl3, δ, ppm): 3.53 (1H, dd, Ha, J = 5.2, 5.6 Hz), 4.24 (1H, dd, Hb, J = 12, 12 Hz), 5.72 (1H, dd, Hx, J = 5.6, 5.6 Hz), 6.96 (1H, d, Ar-H, J = 6.4 Hz), 7.34 (2H, m, Ar-H and NH pyrazoline), 7.52 (1H, d, Ar-H, J = 6.4 Hz), 7.58 (2H, d, Ar-H, J = 7.6 Hz), 7.80 (2H, d, Ar-H, J = 8 Hz). Anal. Calcd. for C17H12Cl2N2O: C, 61.65; H, 3.65; N, 8.46. Found: C, 61.54; H, 3.64; N, 8.45%.
4.2.4.3 2-[3-(4-Methylphenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (4c)
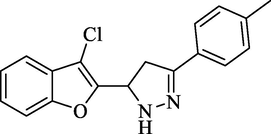
Molecular Formulae: C18H15ClN2O, Molecular weight: 310, %Yield: 80, Melting Point: 146–48 °C, FT-IR (KBr, cm−1): 3560 (N-H), 1590 (C = N), 1326 (C-N), 747 (C-Cl). 1H NMR (CDCl3, δ, ppm): 2.40 (3H, s, -CH3), 3.41 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.65 (1H, dd, Hb, J = 12, 12.4 Hz), 5.82 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.25 (2H, d, Ar-H, J = 8 Hz), 7.34 (1H, d, Ar-H, J = 7.6 Hz) 7.48 (2H, m, Ar-H and NH pyrazoline), 7.52 (1H, d, Ar-H, J = 6.8 Hz), 7.65 (2H, d, Ar-H, J = 8 Hz). Anal. Calcd. for C18H15ClN2O: C, 69.57; H, 4.86; N, 9.01. Found: C, 69.68; H, 4.85; N, 9.00%.
4.2.4.4 2-[3-(4-Nitrophenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (4d)
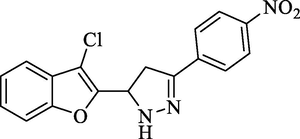
Molecular Formulae: C17H12ClN3O3, Molecular weight: 341, %Yield: 74, Melting Point: 126–28 °C, FT-IR (KBr, cm−1): 3569 (N-H), 1596 (C = N), 1322 (C-N), 747 (C-Cl).; 1H NMR (CDCl3, δ, ppm): 3.22 (1H, dd, Ha, J = 5.6, 5.6 Hz), 4.10 (1H, dd, Hb, J = 12.0, 12.0 Hz), 5.52 (1H, dd, Hx, J = 5.6, 5.2 Hz), 6.95(2H, d, Ar-H, J = 8.4 Hz), 7.32–7.43 (4H, m, Ar-H and pyrazoline), 7.90 (2H, d, Ar-H, J = 8.4 Hz). Anal. Calcd. for C17H12ClN3O3: C, 59.75; H, 3.54; N, 12.30. Found: C, 59.64; H, 3.56; N, 12.28%.
4.2.4.5 2-[3-(4-Aminophenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (4e)
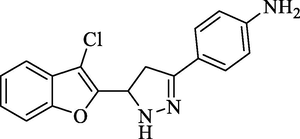
Molecular Formulae: C17H14ClN3O, Molecular weight: 311, %Yield: 70, Melting Point: 120–22 °C, FT-IR (KBr, cm−1): 3280 (N-H), 1596 (C = N), 1324 (C-N), 757 (C-Cl). 1H NMR (CDCl3, δ, ppm): 2.38 (2H, s, NH2), 3.40 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.64 (1H, dd, Hb, J = 12.0, 12.8 Hz), 5.82 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.26 (2H, m, Ar-H), 7.34 (1H, d, Ar-H, J = 6.8 Hz), 7.43 (1H, s, NH pyrazoline), 7.51 (1H, d, Ar-H, J = 6.8 Hz), 7.58 (2H, d, Ar-H, J = 7.6 Hz), 7.60 (2H, d, Ar-H, J = 8 Hz). Anal. Calcd. for C17H14ClN3O: C, 65.49; H, 4.53; N, 13.48. Found: C, 65.48; H, 4.52; N, 13.50%.
4.2.4.6 2-[3-(4-Hydroxyphenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (4f)
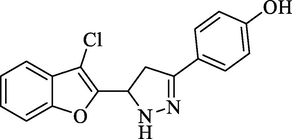
Molecular Formulae: C17H13ClN2O2, Molecular weight: 312, %Yield: 78, Melting Point: 268–70 °C, FT-IR (KBr, cm−1): 3532 (OH), 3285 (N-H), 1594 (C = N), 1324 (C-N), 756 (C-Cl).; 1H NMR (DMSO‑d6, δ, ppm): 3.79 (1H, dd, Ha, J = 5.2, 4.8 Hz), 4.07 (1H, dd, Hb, J = 12.4, 12.8 Hz), 6.07 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.27(2H, d, Ar-H, J = 8 Hz), 7.64–7.71 (3H, m, Ar-H and NH pyrazoline), 7.76(1H, d, Ar-H, J = 7.2 Hz), 8.00 (2H, d, Ar-H, J = 8 Hz), 9.91 (1H, s, OH). Mass m/z: 312 (M+). Anal. Calcd. for C17H13ClN2O2: C, 65.29; H, 4.19; N, 8.96. Found: C, 65.30; H, 4.20; N, 8.97%.
4.2.4.7 2-[3-(2-Hydroxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4g)
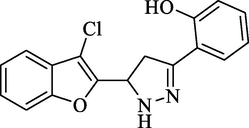
Molecular Formulae: C17H13ClN2O2, Molecular weight: 312, %Yield: 82, Melting Point: 148–50 °C FT-IR (KBr, cm−1): 3538 (OH), 3295 (N-H), 1596 (C = N), 1324 (C-N), 754 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.62 (1H, dd, Ha, J = 5.6, 5.6 Hz), 4.68 (1H, dd, Hb, J = 12.4, 12.4 Hz), 6.01 (1H, dd, Hx, J = 5.6, 5.2 Hz), 7.26–7.73 (6H, m, Ar-H and NH pyrazoline), 7.74 (1H, d, Ar-H, J = 6.8 Hz), 7.78 (1H, d, Ar-H, J = 6.8 Hz), 8.92 (1H, s, OH). Anal. Calcd. for C17H13ClN2O2: C, 65.29; H, 4.19; N, 8.96. Found: C, 65.38; H, 4.20; N, 8.95%.
4.2.4.8 2-[3-(4-Methoxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4h)
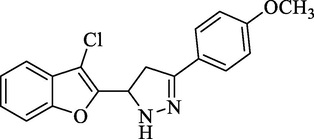
Molecular Formulae: C18H15ClN2O2, Molecular weight: 326, %Yield: 87, Melting Point: 182–84 °C FT-IR (KBr, cm−1): 3560 (N-H), 1590 (C = N), 1326 (C-N), 750 (C–Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.39 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.63 (1H, dd, Hb, J = 12.4, 12.0 Hz), 3.85 (3H, s, -OCH3), 5.81 (1H, dd, Hx, J = 5.6, 5.6 Hz), 6.94 (2H, d, Ar-H, J = 8.4 Hz), 7.26–7.32 (2H, m, Ar-H and NH pyrazoline), 7.34 (1H, d, Ar-H, J = 8.0 Hz), 7.51 (1H, d, Ar-H, J = 6.4 Hz), 7.70 (2H, d, Ar-H, J = 8.8 Hz). Anal. Calcd. for C18H15ClN2O2: C, 66.16; H, 4.63; N, 8.57. Found: C, 66.20; H, 4.64; N, 8.55%.
4.2.4.9 2-[3-(2,4-Dihydroxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4i)
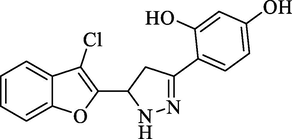
Molecular Formulae: C17H13ClN2O3, Molecular weight: 328, %Yield: 74, Melting Point: 140–42 °C FT-IR (KBr, cm−1): 3530 (OH), 3280 (N-H), 1596 (C = N), 1324 (C-N), 757 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 3.45 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.98 (1H, dd, Hb, J = 12, 12 Hz), 6.11 (1H, dd, Hx, J = 5.6, 5.6 Hz), 6.98–7.42 (3H, m, Ar-H), 7.45 (1H, d, Ar-H, J = 6.8 Hz), 7.61–7.72 (3H, m, Ar-H and NH pyrazoline), 10.2 (2H, s, 2 X OH). Anal. Calcd. for C17H13ClN2O3: C, 62.11; H, 3.99; N, 8.52. Found: C, 62.10; H, 4.00; N, 8.51%.
4.2.4.10 2-[3-(2,4-Dimethoxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4j)
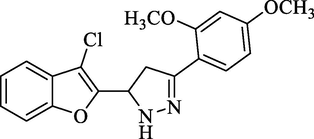
Molecular Formulae: C17H13ClN2O3, Molecular weight: 328, %Yield: 74, Melting Point: 140–42 °C FT-IR (KBr, cm−1): 3280 (N-H), 1590 (C = N), 1324 (C-N), 755 (C-Cl). 1H NMR (CDCl3, δ, ppm): 3.36 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.62 (1H, dd, Hb, J = 12, 12 Hz), 3.86 (6H, s, 2 X OCH3), 5.82 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.26–7.83 (7H, m, Ar-H and NH pyrazoline). Anal. Calcd. for C19H17ClN2O3: C, 63.96; H, 4.80; N, 7.85. Found: C, 64.05; H, 4.81; N, 7.84%.
4.2.4.11 2-[3-(3,4-Dimethoxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4k)
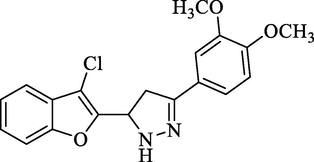
Molecular Formulae: C19H17ClN2O3, Molecular weight: 326, %Yield: 87, Melting Point: 268–70 °C FT-IR (KBr, cm−1): 3280 (N-H), 1596 (C = N), 1324 (C-N), 757 (C-Cl). 1H NMR (CDCl3, δ, ppm): 3.38 (1H, dd, Ha, J = 5.2, 5.2 Hz), 3.59 (1H, dd, Hb, J = 12.4, 12.4 Hz), 3.89 (6H, s, 2 X OCH3), 6.17 (1H, dd, Hx, J = 5.2, 5.2 Hz), 6.98 (1H, s, Ar-H), 7.06–7.13 (3H, m, Ar-H), 7.17 (1H, bs, NH), 7.50–7.58 (3H, m, Ar-H). Anal. Calcd. for C19H17ClN2O3: C, 63.96; H, 4.80; N, 7.85. Found: C, 64.08; H, 4.80; N, 7.84%.
4.2.4.12 2-[3-(4-Hydroxy-3-methylphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4l)
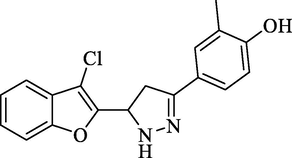
Molecular Formulae: C18H15ClN2O2, Molecular weight: 326, %Yield: 76, Melting Point: 150–70 °C FT-IR (KBr, cm−1): 3536 (OH), 3279 (N-H), 1590 (C = N), 1324 (C-N), 755 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 2.38 (2H, d, CH2 pyrazoline), 2.43 (3H, s, CH3), 4.52 (1H, t, CH), 7.23–7.72 (7H, m, Ar-H and NH), 9.92 (1H, s, OH). Anal. Calcd. for C18H15ClN2O2: C, 66.16; H, 4.63; N, 8.57. Found: C, 66.15; H, 4.62; N, 8.58%.
4.2.4.13 2-[3-(4-Hydroxy-2-methylphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (4m)
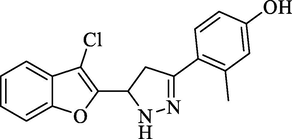
Molecular Formulae: C18H15ClN2O2, Molecular weight: 326, %Yield: 77, Melting Point: 232–34 °C FT-IR (KBr, cm−1): 3535 (OH), 3285(N-H), 1594 (C = N), 1327 (C-N), 754 (C-Cl). 1H NMR (DMSO‑d6, δ, ppm): 2.36 (2H, d, CH2 pyrazoline), 2.69 (3H, s, CH3), 4.52 (1H, t, CH), 7.32–7.82 (7H, m, Ar-H and NH), 10.21 (1H, s, OH). Anal. Calcd. for C18H15ClN2O2: C, 66.16; H, 4.63; N, 8.57. Found: C, 66.29; H, 4.64; N, 8.56%.
4.3 Antitubercular activity
The newly synthesized compounds were screened in vitro for activity against Mycobacterium tuberculosis H37Rv strain (ATCC27294) by Microplate Alamar Blue Assay (MABA) (Wahyuningrum et al., 2017; Xu et al., 2016; Bunalema et al., 2015; Nkenfou et al., 2015), at Tuberculosis Antimicrobial Acquisition and Coordination facility (TAACF).
4.4 In silico studies
Synthesized compounds were subjected to different in silico studies to predict their ADME properties, understand their interaction with target protein and their affinity towards at the binding site of the target. Schrodinger Maestro 11.4, on a dell desktop with i5 intel core processor, assembled with 16 GB RAM and 4 GB nividia graphics padwas used to carry out all in silico studies.
4.4.1 ADME properties
Chemical structures of all synthesized compounds were drawn on Chemdraw 12.0 as mol files and subjected to energy minimization using Ligprep module of Maestro. The conformers generated after ligand preparation were analysed for ADME properties using Quikprop. Some of the predicted properties include QP log Po/w, QP log BB, overall CNS activity, cell permeability, logKhsa for human serum albumin binding, the percentage of human oral absorption. The results obtained have been indicated in Table 3. Nrb = Number of Rotational Bond, HBD = Hydrogen Bond Donor, HBA = Hydrogen Bond Acceptor, QPlog Po/w = Predicted Octanol/water partition coefficient, %Abs = Percentage of Human Oral Absorption.
Compound
Nrb
HBD
HBA
QPlogPo/w
%Abs
Rule of five
Rule of three
3a
1
0
4
6.257
100
1
1
3b
2
1
4.75
5.112
100
1
1
3c
2
0
4.75
5.795
100
1
1
4b
0
1
2.5
4.875
100
0
1
4c
0
1
2.5
4.688
100
0
1
4.4.2 Molecular docking
All compounds which exhibited potent anti-tubercular activity were further studied for their binding with target protein. The co crystallized protein of Mycobacterium tuberculosis InhA for molecular docking study was obtained from Protein Data Bank (PDB ID- 5OIF) and prepared using protein preparation wizard module of Maestro. The protein was pre-processed to identify different chains, ligands as hetero atoms and water molecules. Relevant chain containing ligand for the study was retained and other parts were omitted. The protein was then optimised for hydrogen bond and finally subjected to energy minimization. The prepared protein was then treated for receptor grid generation. Once the grid was generated, docking of the prepared ligands along with established standard inhibitors like Isoniazid and Pyrazinamide was performed. The result obtained has been presented in Tables 2 and 4.
Compound
No. of H-bond
H-bond forming residue
H-bond length (Å)
Other residues with hydrophobic interaction
3a
1
SER20
2.4
ILE21, ALA22, MET147, ILE95, PHE97, MET199, ALA168,
3b
2
MET98
THR1961.95
1.86ILE16, ILE21, ALA22, PHE97, PRO99, MET103, ALA191, PRO193, ILE194, ALA198, MET199, ILE202
3c
1
THR196
1.88
ILE16, ILE21, ALA22, PHE97, MET98, PRO99, MET103, TYR158, PHE149, MET147, ALA191, PRO193, ILE194, ALA198, MET199, ILE202
4b
1
GLY14
2.15
ILE15, ILE16, ILE21, MET147, ILE95, PHE97
4c
2
GLY14
GLY961.98
2.341LE16, ILE21, PHE41, PHE97, ILE95, ALA191, ILE194
Isoniazid
4
GLY14
ALA22
SER94
LYS1652.45
2.75
2.00
1.87ILE16, ILE21, ILE95, MET147, MET161
Pyrazinamide
1
ILE194
1.97
ILE21, MET147, PHE149, ALA191, PRO193, MET199
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding the work through the research group project number (RG-1442-073).
One of the authors (KH) acknowledges the Indian council of medical research (ICMR), India, for the award of SRF [Ref No. 3/2/2/21/2019/NCD-III].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis and anticervical cancer activity of new benzofuran–pyrazol-hydrazono-thiazolidin-4-one hybrids as potential EGFR inhibitors and apoptosis inducing agents. Bioorg. Chem.. 2019;103035
- [Google Scholar]
- Synthesis, antibacterial and antitubercular activity of novel Schiff bases of 2-(1-benzofuran-2-yl) quinoline-4-carboxylic acid derivatives. Russ. J. Gen.. 2017;87(8):1843-1849.
- [Google Scholar]
- Antitubercular activity of Callistemon citrinus and Piptadenistrum africanum on resistant strains of Mycobacterium tuberculosis using Microplate alamar blue assay. Spatula DD. 2015;5(4):235-240.
- [Google Scholar]
- Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano [2, 3-c] pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur. J. Med. Chem.. 2017;125:101-116.
- [Google Scholar]
- Discovery of novel triazole-containing pyrazole ester derivatives as potential antibacterial agents. Molecules. 2019;24(7):1311.
- [Google Scholar]
- Evaluation of anti-inflammatory, analgesic activities, and side effects of some pyrazole derivatives. Inflammopharmacology. 2016;24(4):163-172.
- [Google Scholar]
- Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur. J. Med. Chem.. 2018;143:1463-1473.
- [Google Scholar]
- Design, synthesis, biological evaluation and molecular docking studies of novel benzofuran–pyrazole derivatives as anticancer agents. Bioorg. Chem.. 2015;63:1-12.
- [Google Scholar]
- The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir. Med.. 2018;6(4):299-314.
- [Google Scholar]
- Benzofuran-isatin-imine hybrids tethered via different length alkyl linkers: Design, synthesis and in vitro evaluation of anti-tubercular and anti-bacterial activities as well as cytotoxicity. Eur. J. Med. Chem.. 2019;165:323-331.
- [Google Scholar]
- Synthesis and utility of α, β-unsaturated ketone bearing naphthalene and benzofuran rings in the synthesis of some n-heterocycles with their antiviral and antitumor activity evaluation. J. Heterocycl. Chem.. 2018;55(10):2368-2380.
- [Google Scholar]
- Synthesis, and antitubercular and antimicrobial activity of 1′-(4-chlorophenyl) pyrazole containing 3, 5-disubstituted pyrazoline derivatives. New J. of Chem.. 2016;40(1):73-76.
- [Google Scholar]
- High-throughput screening, discovery, and optimization to develop a benzofuran class of hepatitis C virus inhibitors. ACS Comb. Sci.. 2015;17(10):641-652.
- [Google Scholar]
- Antifungal and anthelmintic activity of novel benzofuran derivatives containing thiazolo benzimidazole nucleus: an in vitro evaluation. J. Chem. Biol.. 2017;10(1):11-23.
- [Google Scholar]
- Domino cyclization/trifluoromethylation of 2-alknylphenols for the synthesis of 3-(trifluoromethyl) benzofurans and evaluation of their antibacterial and antifungal activities. Asian J. Org. Chem.. 2019;8(5):702-709.
- [Google Scholar]
- Design, synthesis and antifungal activity of novel benzofuran-triazole hybrids. Molecules. 2016;21(6):732.
- [Google Scholar]
- Tuberculosis research and development: seeding the future. Lancet Respir. Med.. 2018;6(4):242-244.
- [Google Scholar]
- Design, synthesis, and antitubercular evaluation of novel series of 3-benzofuran-5-aryl-1-pyrazolyl-pyridylmethanone and 3-benzofuran-5-aryl-1-pyrazolylcarbonyl-4-oxo-naphthyridin analogs. Eur. J. Med. Chem.. 2010;45(9):3831-3839.
- [Google Scholar]
- Muhammad, Z., Alshehrei, F., Zayed, M., Farghaly, T., Abdallah, M., 2019. Synthesis of novel bis-pyrazole derivatives as antimicrobial agents. Mini-Rev Med. Chem.
- Synthesis of new pyrazole-triazole hybrids by click reaction using a green solvent and evaluation of their antitubercular and antibacterial activity. Res. Chem. Intermediat.. 2016;42(4):3721-3741.
- [Google Scholar]
- Biological and medicinal significance of benzofuran. Eur. J. Med. chem.. 2015;97:561-581.
- [Google Scholar]
- In vitro antimycobacterial activity of six Cameroonian medicinal plants using microplate alamarBlue assay. Int. J Mycobacteriol.. 2015;4(4):306-311.
- [Google Scholar]
- Design, synthesis and biological evaluation of 1, 3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents. Eur. J. Med. Chem.. 2015;101:790-805.
- [Google Scholar]
- Novel N′-benzylidene benzofuran-3-carbohydrazide derivatives as antitubercular and antifungal agents. Bioorg. Med. Chem. Lett.. 2012;22(6):2343-2346.
- [Google Scholar]
- Antituberculosis activity of brotowali (Tinospora crispa) extract and fractions against mycobacterium tuberculosis using microplate alamar blue assay method. Majalah Obat Tradisional (Traditional Med. J.). 2017;22(2):124-130.
- [Google Scholar]
- Synthesis of 1, 3-diaryl pyrazole derivatives and evaluation of anticonvulsant and antimicrobial activities. Lat. Am. J. Pharm.. 2018;37(5):1017-1027.
- [Google Scholar]
- In vitro and in vivo characterization of a benzofuran derivative, a potential anticancer agent, as a novel Aurora B kinase inhibitor. Eur. J. Med. Chem.. 2015;89:310-319.
- [Google Scholar]
- In vitro interactions between R207910 and second-line anti-TB drugs or BTZ043 against mycobacterium tuberculosis by microplate alamar blue assay [Retraction] Int. J. Clin. Exp. Med.. 2016;9(3):6336-6341.
- [Google Scholar]
- Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem.. 2017;138:501-513.
- [Google Scholar]
- Synthesis of novel dihydrotriazine derivatives bearing 1, 3-diaryl pyrazole moieties as potential antibacterial agents. Bioorg. Med. Chem. Lett.. 2019;29(9):1079-1084.
- [Google Scholar]







