Translate this page into:
Design, synthesis and biological evaluation of tetracyclic azafluorenone derivatives with topoisomerase I inhibitory properties as potential anticancer agents
⁎Corresponding authors at: Graduate Institute of Cancer Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei 110, Taiwan. Fax: +886 2 66387537 (H.-S. Huang). Fax: +886 2 2391 5295 (J.-J. Lin). jingjerlin@ntu.edu.tw (Jing-Jer Lin), huanghs99@tmu.edu.tw (Hsu-Shan Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Several 9-chloro-11H-indeno[1,2-c]quinolin-11-one derivatives have been designed which is replacing side chains with different groups containing oxygen, nitrogen or sulfur atoms. Substitution of C-6 on the starting structure, 6,9-dichloro-11H-indeno[1,2-c]quinolin-11-one, using apposite nucleophilic group with a suitable base or acid could be obtained 28 novel tetracyclic azafluorenone derivatives. The cytotoxic activity of these analogues was examined in cancer cell lines by MTT assay and compounds 4, 5, 13, and 26 were selected to evaluate in topoisomerase I drug screening assay, respectively. At the same time, 17 compounds were selected for NCI-60 anticancer drug screen to prevent the narrower concept of an in vitro screening model. Its worth to find that 9-chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (12) showed greater cytotoxicity than another azafluorenone derivatives with an average GI50 of 10.498 μM over 60 cell lines. We also found that another analogue, 9-chloro-6-(2-methylpiperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (13), exhibited preferential growth inhibition effect toward cancer cell lines and showed a significant inhibitory effect on topoisomerase I.
Keywords
Indenoquinolinone
Topoisomerase I
Azafluorenone
Cytotoxicity
NCI-60 anticancer drug screen
1 Introduction
Camptothecin (CPT) is a cytotoxic quinoline alkaloid, as well as TAS-103 is a quinoline derivative which has been reported to be potent topoisomerase (topo) I and/or topo II poison (Aoyagi et al., 2000; Fujimoto, 2007; Yoshida et al., 2008; Shah et al., 2010). Two clinically anticancer drugs, irinotecan and topotecan, are derivatives of the CPT family that are used in colorectal cancer, either as single agents or in combination with radiotherapy and/or other chemotherapy drugs (Kehrer et al., 2001; Alagoz et al., 2012). Despite clinical success, they are the only Topo I-targeted anticancer drugs which still have several problems with CPT-derived anticancer agents (Teicher, 2008). Ongoing research aims to the lactone and A-D planar ring in CPT and its analogues and derivatives which suffer from major limitation and application drawback of non-mechanism related toxicity and poor solubility profile (Ulukan and Swaan, 2002; Kiselev et al., 2012; Perzyna et al., 2003). Because the direct target of above small molecules remained unclear, we tried structural hybridization of some preclinical and clinical anticancer drugs to design tetracyclic azafluorenone-derived small molecules as potential anticancer drugs (Fig. 1).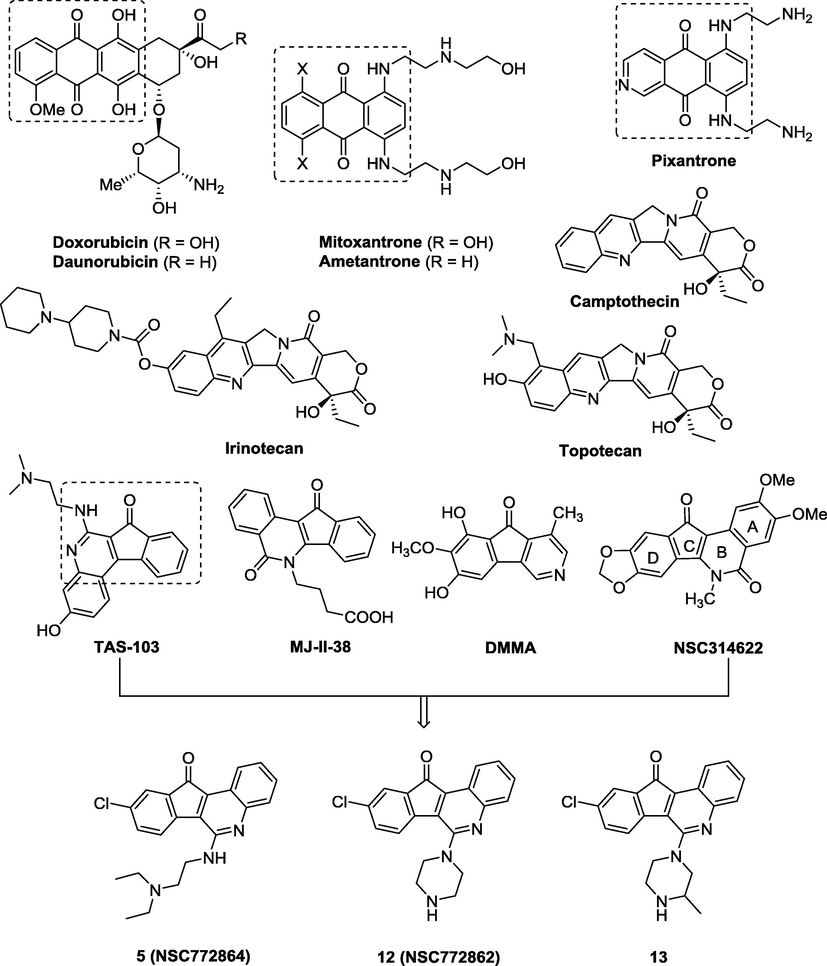
Structural hybridization design of tetracyclic quinoline derivatives.
The anthracycline antibiotics (e.g. doxorubicin, daunorubicin, mitoxantrone and ametantrone) have been shown to provide significant antiproliferative (or cytostatic) properties (Chen et al., 2013, 2015) and inhibit topoisomerase II (Suzuki et al., 1995) as well as intercalate into the minor groove of double-strand DNA base pairs via the core planar pharmacophore group (Wang et al., 1994). In addition, DMMA (6,8-dihydroxy-7-methoxy-1-methyl-3-azafluorenone), an active compound of the azafluorenone, was isolated from Polyalthia cerasoides in 2010 (Pumsalid et al., 2010) which exhibits cytotoxic to various cancer cell lines (Banjerdpongchai et al., 2013). Moreover, recent studies illustrated related indenoquinoline skeleton incorporate ring system and various substituents had been found as potential dual topo I/II inhibitors and anticancer candidates (Tseng et al., 2013, 2010, 2009, 2008). There are also several studies have found that azafluorenone-derived compounds had potent anticancer (Coothankandaswamy et al., 2010; McLaughlin, 2008; Pan et al., 2011), cytotoxic (Pumsalid et al., 2010), antimicrobial (Koyama et al., 2005; Addla et al., 2012), antitubercular (Yempala et al., 2012), anti-inflammatory (Chuang et al., 2008; Rojano et al., 2007) and antiprotozoal activities (Waechter et al., 1999).
Based on above considerations, azafluorenones can be considered as an attractive scaffold for the development of anticancer reagents. In the past, several studies had found that DNA top IB can regulate DNA topological structures by sequential breakage and is involved in DNA transcription, replication, and recombination (Shen et al., 2010; Stewart et al., 1998). Toward supporting the aforementioned hypothesis, we recently disclosed evidence as well as an in-depth continuous investigation toward synthesis of fluorenone analogs (Lee et al., 2013) led to discovery of azafluorenone-derived scaffold small molecules for the polypharmacology. With this motivation, we herein also describe efficient synthetic procedures for the preparation of a series of non-nucleoside fused tetracyclic compounds comprising benzene moiety and an azafluorenone backbone. We attempted to identify additional potent enzyme inhibitors and work toward some azafluorenone-derived structural modifications in order to develop some potential anticancer structures leads which could be compared to parent molecules in cancer chemotherapy.
2 Chemistry
2.1 Materials and instruments
The Pfitzinger synthetic reaction of isatin and 4-chlorophenylacetic acid was stirred at 200 °C with sodium acetate (NaOAc) as a basic catalyst for 3 h to obtain compound 1 (52%) as a key intermediate. An isatin was reacted with NaOAc as base by the amide bond hydrolysis to obtain 2-(2-aminophenyl)-2-oxoacetic acid. A reactant with a ketone group reacted with the aniline to give the imine and the enamine. The enamine cyclized and dehydrated to give the desired quinoline-4-carboxylic acids (1). Treatment of 1 with phosphoryl trichloride (POCl3) afforded 6,9-dichloro-11H-indeno[1,2-c]quinolin-11-one (2). A series of 6-substituted 9-chloro-11H-indeno[1,2-c]quinolin-11-one homologues can be synthesized and the preparation involved various synthetic routes with approximate yields (overall 17–95%) in all steps: (i) reaction of isatin with 4-chlorophenylacetic acid and NaOAc; (ii) reaction of 1 with POCl3; and (iii) reaction of compound 2 with a series of apposite primary or secondary amines, 2-mercaptoethanol, conc. hydrochloride (HCl), or sodium methoxide yielded the corresponding side chain compounds 3–29, respectively (Scheme 1). The quantity of the isolated products was dependent on substrate and reaction condition. All the crude mixtures were purified through tedious recrystallization from ethyl acetate/n-hexane and/or ethanol. The molecular weight of all synthetic compounds was determined by HRMS. The protons and carbons from tetracyclic azafluorenone structures were also obvious from the 1H NMR and 13C NMR spectra.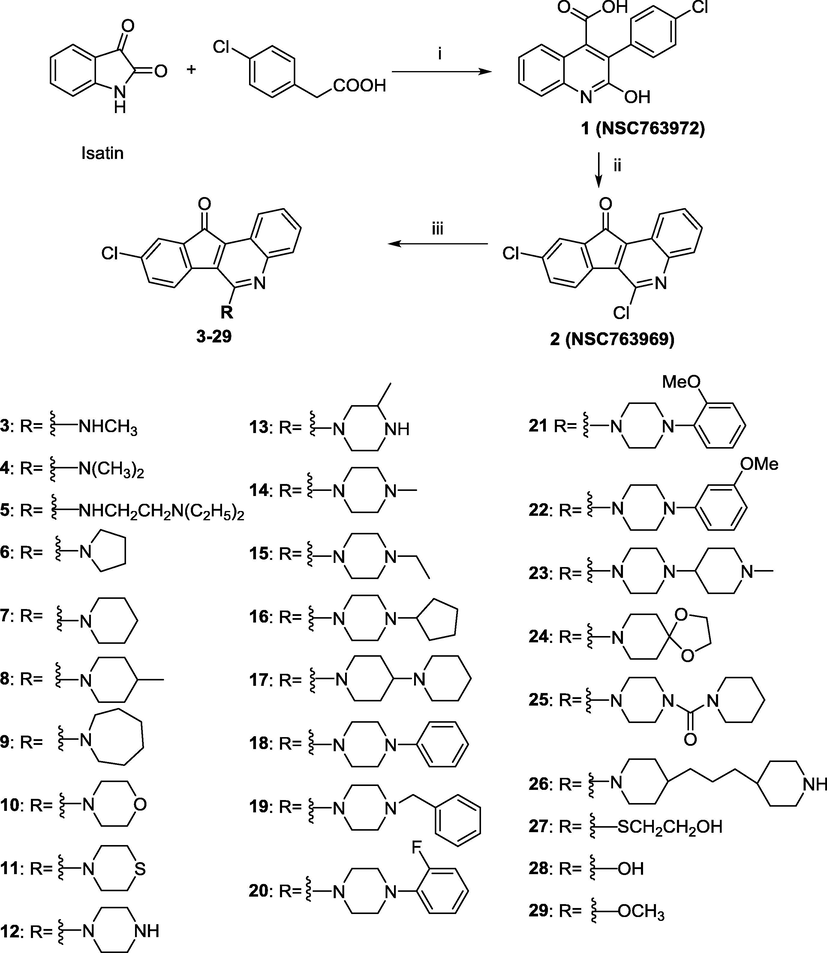
Overall synthetic routes of the tetracyclic azafluorenone derivatives. Reagents and conditions: (i) 1: NaOAc, 200 °C, 2 h; (ii) 2: POCl3, 150 °C, reflux, 48 h; (iii) 3–26: appropriate amines, DMF, pyridine, miniclave, 150 °C, 2 h; 27: mercaptoethanol, K2CO3; 28: DMF, conc. HCl, reflux, 24 h; 29: NaOMe, MeOH, reflux, 16 h.
2.2 Synthesis of target compounds 1–29
The synthetic methods and physical data of compounds 1–29 were described and all compounds were tested and compared for their growth inhibition, cytotoxicity and topoisomerase activities. In this study, we synthesized and dedicated on the role of our systematic, tetracyclic, and heterocyclic pharmacophore by introducing a series of side chains linked to the 9-chloro-11H-indeno[1,2-c]quinolin-11-one moiety.
All reactions were monitored through a TLC (silica gel 60 F254) plate with a 254-nm UV lamp. 1H NMR and 13C NMR were measured on Varian GEMINI-300 (300 MHz) or Agilent 400 MR DD2 (400 MHz); δ values are in ppm relative to TMS (tetramethylsilane) as an internal standard. Multiplicities are recorded as s (singlet), d (doublet), t (triplet), q (quartet), quin (quintuplet), dd (doublet of doublets), dt (triplet of doublets), td (doublet of triplets), m (multiplet), and br (broadened). Mass spectra: High resolution electrospray ionization (HRESI): Finnigan MAT 95S (Instrumentation Center, National Taiwan University, Taipei, Taiwan) and High resolution electron impact ionization (HREI): Finnigan MAT MAT-95XL (Instrumentation Center, National Tsing Hua University, Hsinchu, Taiwan). Melting points of synthetic compounds were determined with a Büchi B-545 melting point apparatus. Typical experiments illustrating the synthetic procedures for the preparation of the tetracyclic small molecules are described below. These compounds were synthesized, starting from isatin and 4-chloro-phenylacetic acid. All the reagents and solvents required for synthesis were purchased from either Merck Chemical Company or Sigma–Aldrich Chemical Company without further purification.
2.2.1 Synthetic procedure i: preparation of compound 1
A mixture of isatin (3.14 g, 21 mmol), 4-chloro-phenylacetic acid (3.41 g, 20 mmol), and sodium acetate (1.00 g) was heated at 200 °C for 2 h (TLC monitoring). After cooling, 100 mL acetic acid was added to the mixture. The precipitate was filtrated and washed with acetic acid and n-hexane, and then collected the obtained orange compound.
2.2.2 Synthetic procedure ii: preparation of compound 2
A suspension of compound 1 (3.03 g, 10.1 mmol) and POCl3 (20 mL) was stirred and heated at 150 °C for 48 h. After cooling, the mixture was poured into ice-water (300 mL) at 0 °C cautiously. The resulting precipitate that separated was collected by filtration. The filtered cake was suspended in 10% NaHCO3 solution (300 mL) with vigorous stirring for 1 h. The resulting precipitate was collected and washed with H2O. The crude was recrystallized from dichloromethane to give an orange product.
2.2.3 General procedure iii: preparation of compounds 3–26
Compound 2, primary or secondary amine (10 mmol) and N,N-diisopropylethylamine (DIPEA) (2 mmol) were dissolved in miniclave containing DMF (10 mL) and stirred at 150 °C for 4 h. The reaction was poured into ice-water (100 mL). The resulting precipitate was collected by filtration and purified by crystallization from ethanol to afford desired compound.
2.2.4 Synthetic procedure iv: preparation of compound 27
Compound 2, 2-mercaptoethanol (10 mmol) and N,N-diisopropylethylamine (DIPEA) (2 mmol) were dissolved in miniclave containing DMF (10 mL) and stirred at room 150 °C for 4 h. The reaction was poured into ice-water (100 mL). The resulting precipitate was collected by filtration and purified by crystallization from ethanol to afford desired compounds.
2.2.5 Synthetic procedure v: preparation of compound 28
A mixture of compound 2, conc. HCl (2 mL) and DMF (10 mL) was refluxed at 120 °C. After 4 h, the conc. HCl was added into the reaction again (TLC monitored). The mixture was evaporated in vacuum or dean-stark trap, treated with H2O (20 mL), and filtered. The crude solid was washed with EtOH to give 28 as a red solid.
2.2.6 Synthetic procedure vi: preparation of compound 29
A methanol solution (20 mL) containing sodium methoxide (1.08 g, 20 mmol) was slowly added into the suspension of compound 2 in MeOH (10 mL) for 10 min. The reaction was refluxed at 100 °C for 16 h (TLC monitored). After cooled, the solvent was removed by rotary evaporator vacuum, filtrated and washed with ethanol and n-hexane to collect an orange solid.
2.3 Physical data
2.3.1 3-(4-Chlorophenyl)-2-hydroxyquinoline-4-carboxylic acid (1)
The pure compound was obtained as an orange solid (yield 80%). Mp 310–311 °C (EtOH). FT-IR (KBr; ν cm−1): 3234 (NH), 1637 (CO). 1H NMR (300 MHz, CDCl3): δ (ppm) 6.60 (s, 1H, —OH), 6.91 (td, J = 7.2 Hz, 0.6 Hz, 1H, Ar—H), 7.12 (d, J = 8.4 Hz, 1H, Ar—H), 7.51 (d, J = 8.7 Hz, 2H, Ar—H), 7.53–7.59 (m, 2H, Ar—H), 7.74 (d, J = 8.7 Hz, 2H, Ar—H), 9.83 (s, 1H, —COOH). 13C NMR (75 MHz, CDCl3): δ (ppm) 117.12, 120.66, 121.83, 122.20, 126.31, 128.86, 129.15, 132.67, 134.22, 135.21, 136.23, 139.59, 182.29 (CO). HRMS (ESI) m/z calcd for C16H10NO3Cl+ [M]+ 299.0349, found [M+H]+: 300.0424, [M−H]−: 298.0238.
2.3.2 6,9-Dichloro-11H-indeno[1,2-c]quinolin-11-one (2)
Product 2 was cyclized from compound 1 using POCl3 at 150 °C for 48 h. The red solid material was isolated in 30% yield. (Rf = 0.70 at CH2Cl2). Mp 241–243 °C. FT-IR (KBr; ν cm−1): 1719 (C⚌O). 1H NMR (300 MHz, CDCl3) δ (ppm): 7.52 (dd, J = 8.25, 1.8 Hz, 1H, Ar—H), 7.62–7.68 (m, 2H, Ar—H), 7.70–7.76 (m, 1H, Ar—H), 7.97–8.01 (dt, J = 7.5, 0.6 Hz, 1H, Ar—H), 8.10 (d, J = 7.8 Hz, 1H, Ar—H), 8.77–8.80 (m, 1H, Ar—H). 13C NMR (75 MHz, CDCl3) δ (ppm): 122.97, 124.59, 125.30, 125.69, 129.13, 130.32, 131.70, 135.06, 135.14, 136.19, 136.74, 136.80, 140.15, 145.25, 150.48, 192.80 (CO). HRMS (ESI) m/z calcd for C16H7NOCl2+, [M]+: 298.9905, found [M+H]+: 299.9965 (100), 301.9947 (65), 303.9917 (10).
2.3.3 9-Chloro-6-(methylamino)-11H-indeno[1,2-c]quinolin-11-one (3)
Product 3 was prepared from compound 2 and methylamine. The red solid material was isolated in 75% yield (Rf = 0.51 at CH2Cl2: n-hexane = 2:1). Mp 189–191 °C (EtOH). FT-IR (KBr; ν cm−1): 1716 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.01 (s, 3H, N—CH3), 7.41–7.47 (m, 2H, Ar—H), 7.57–7.62 (m, 3H, Ar—H), 7.84 (d, J = 8.4 Hz, 1H, Ar—H), 8.68 (d, J = 8.1 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 42.22, 120.81, 124.29, 124.93, 124.99, 127.02, 128.13, 130.44, 131.60, 134.33, 135.16, 135.24, 136.59, 141.85, 149.69, 158.14, 194.48 (CO). HRMS (ESI) m/z calcd for C17H11N2OCl+ [M]+: 294.0560, found [M+H]+: 295.0634.
2.3.4 9-Chloro-6-(dimethylamino)-11H-indeno[1,2-c]quinolin-11-one (4)
Product 4 was prepared from compound 2 and dimethylamine. The red solid material was isolated in 74.5% yield (Rf = 0.51 at CH2Cl2: n-hexane = 1:1). Mp 193–195 °C (EtOH). FT-IR (KBr; ν cm−1): 3407 (NH), 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.04 (s, 6H, —N—(CH3)2), 7.41–7.48 (m, 2H, Ar—H), 7.57–7.63 (m, 3H, Ar—H), 7.86 (d, J = 8.7 Hz, 1H, Ar—H), 8.68–8.71 (m, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 42.19, 120.80, 124.27, 124.91, 124.96, 126.99, 128.13, 130.40, 131.57, 134.30, 135.14, 135.22, 136.54, 141.84, 149.70, 158.13, 194.45 (CO). HRMS (EI) m/z calcd for C18H13N2OCl+ [M]+: 308.0716, found 308.0708.
2.3.5 6-(2-(Diethylamino)ethylamino)-9-chloro-11H-indeno[1,2-c]quinolin-11-one (5)
Product 5 was prepared from compound 2 and N1,N1-diethylethane-1,2-diamine. The red solid material was isolated in 17.5% yield (Rf = 0.46 at CH2Cl2: n-hexane = 2:1). Mp 160–161 °C (EtOH). FT-IR (KBr; ν cm−1): 3317 (NH), 1712 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.11 (t, J = 7.2 Hz, 6H, —CH3), 2.66 (q, J = 7.1 Hz, 4H, —NCH2—), 2.84 (t, J = 5.7 Hz, 2H, —CH2N—), 3.71–3.73 (m, 2H, —NHCH2—), 6.14 (br, 1H, NH), 7.28–7.33 (m, 1H, Ar—H), 7.41–7.55 (m, 3H, Ar—H), 7.60 (d, J = 1.5 Hz, 1H, Ar—H), 7.70 (d, J = 8.7 Hz, 1H, Ar—H), 8.60 (m, J = 8.1 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 12.23, 38.81, 46.92, 51.64, 119.00, 122.48, 124.42, 124.95, 125.40, 126.94, 127.86, 130.53, 134.07, 134.90, 135.08, 135.45, 141.24, 150.68, 152.96, 194.65 (CO). HRMS (ESI) m/z calcd for C22H22N3OCl+ [M]+: 379.1451, found[M+H]+: 380.1510.
2.3.6 9-Chloro-6-(pyrrolidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (6)
Product 6 was prepared from compound 2 and pyrrolidine. The red solid material was isolated in 43.6% yield (Rf = 0.51 at CH2Cl2: n-hexane = 2:1). Mp 149–150 °C (EtOH). FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.98 (quin, J = 3.6 Hz, 4H, pyrrolidine-H), 3.56 (t, J = 6.6 Hz, 4H, pyrrolidine-H), 7.33–7.42 (m, 3H, Ar—H), 7.54 (td, J = 7.5, 1.5 Hz, 2H, Ar—H), 7.76 (d, J = 8.4 Hz, 1H, Ar—H), 8.63 (dd, J = 8.4, 0.9 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 25.07, 50.37, 120.39, 124.25, 124.80, 124.96, 126.29, 127.78, 130.33, 130.40, 134.10, 134.83, 135.27, 136.46, 142.50, 149.78, 155.73, 194.65 (CO). HRMS (ESI) m/z calcd for C20H15N2OCl+ [M]+: 334.0873, found [M+H]+: 335.0952.
2.3.7 9-Chloro-6-(piperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (7)
Product 7 was prepared from compound 2 and piperidine. The red solid material was isolated in 43% yield (Rf = 0.63 at CH2Cl2: n-hexane = 2:1). Mp 191–192 °C (EtOH). FT-IR (KBr; ν cm−1): 1717 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.71 (br, 2H, piperidine-H), 1.83–1.86 (m, 4H, piperidine-H), 3.33 (br, 4H, piperidine-H), 7.43–7.48 (m, 2H, Ar—H), 7.58–7.66 (m, 3H, Ar—H), 7.90 (d, J = 8.1 Hz, 1H, Ar—H), 8.70 (d, J = 8.7 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 224.37, 26.01, 51.30, 120.81, 124.22, 124.41, 124.83, 127.09, 128.29, 130.24, 131.88, 134.24, 135.01, 135.17, 136.35, 142.05, 149.79, 158.35, 194.38 (CO). HRMS (ESI) m/z calcd for C21H17N2OCl + [M]+: 348.1029, found [M+H]+: 349.1106.
2.3.8 9-Chloro-6-(4-methylpiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (8)
Product 8 was prepared from compound 2 and 4-methylpiperidine. The brown solid material was isolated in 25.4% yield (Rf = 0.61 at CH2Cl2: n-hexane = 2:1). Mp 190–192 °C (EtOH). FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.07 (d, J = 6 Hz, 3H, —CH3), 1.46–1.60 (m, 3H, —CH2—, —CH—), 1.84–1.87 (m, 2H, —CH2—), 2.96 (t, J = 11.6 Hz, 2H, —NCH2—), 3.67–3.71 (m, 2H, —NCH2—), 7.44–7.46 (m, 2H, Ar—H), 7.58 (m, 3H, Ar—H), 7.82–7.85 (m, 1H, Ar—H), 8.66–8.69 (m, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 22.07, 30.87, 34.43, 50.69, 120.88, 124.27, 124.46, 124.91, 127.15, 128.31, 130.32, 131.98, 134.33, 135.07, 135.23, 136.44, 142.13, 149.83, 158.25, 194.52 (CO). HRMS (ESI) m/z calcd for C22H19N2OCl+ [M]+: 362.1186, found [M+H]+: 363.1260.
2.3.9 6-(Azepan-1-yl)-9-chloro-11H-indeno[1,2-c]quinolin-11-one (9)
Product 9 was prepared from compound 2 and azepane. The red solid material was isolated in 36% yield (Rf = 0.69 at CH2Cl2: n-hexane = 2:1). Mp 146–147 °C (EtOH). FT-IR (KBr; ν cm−1): 1712 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.72–1.75 (m, 4H, —CH2—), 1.86 (br, 4H, —CH2—), 3.64 (t, J = 5.6 Hz, 4H, —NCH2—), 7.40–7.46 (m, 2H, Ar—H), 7.54–7.60 (m, 3H, Ar—H), 7.78–7.80 (m, 1H, Ar—H), 8.68 (dd, J = 8.4, 0.6 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 27.96, 28.40, 52.91, 120.48, 124.24, 124.76, 124.89, 126.60, 127.98, 130.30, 131.09, 134.13, 134.97, 135.15, 136.84, 142.54, 149.74, 157.83, 194.62 (CO). HRMS (ESI) m/z calcd for C22H19N2OCl+ [M]+: 362.1186, found [M+H]+: 363.2000.
2.3.10 9-Chloro-6-morpholino-11H-indeno[1,2-c]quinolin-11-one (10)
Product 10 was prepared from compound 2 and morpholine. The red solid material was isolated in 47% yield (Rf = 0.54 at CH2Cl2: n-hexane = 2:1). Mp 207–208 °C (EtOH). FT-IR (KBr; ν cm−1): 1712 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.41 (t, J = 4.5 Hz, 4H, —CH2—), 3.98 (t, J = 4.5 Hz, 4H, —CH2—), 7.48 (td, J = 8.1, 2.1 Hz, 2H, Ar—H), 7.59–7.65 (m, 3H, Ar—H), 7.87 (d, J = 8.7 Hz, 1H, Ar—H), 8.69–8.72 (dt, J = 8.1, 0.9 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 50.56, 66.98, 121.18, 124.35, 124.43, 125.23, 127.65, 128.51, 130.59, 131.46, 134.42, 135.21, 135.55, 136.80, 141.66, 149.81, 157.31, 194.14 (CO). HRMS (ESI) m/z calcd for C20H15N2O2Cl+ [M]+: 350.0822, found [M+H]+: 351.0898.
2.3.11 9-Chloro-6-thiomorpholino-11H-indeno[1,2-c]quinolin-11-one (11)
Product 11 was prepared from compound 2 and thiomorpholine. The orange solid material was isolated in 74% yield (Rf = 0.33 at CH2Cl2: n-hexane = 2:1). Mp 228–230 °C (EtOH). FT-IR (KBr; ν cm−1): 1711 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 2.91 (t, J = 5.1 Hz, 4H, —CH2—), 2.69–2.73 (br, 4H, —CH2—), 7.45–7.50 (td, J = 7.8, 1.8 Hz, 2H, Ar—H), 7.57–7.64 (m, 3H, Ar—H), 7.85 (d, J = 9.0 Hz, 1H, Ar—H), 8.68–8.71 (dd, J = 8.25, 1.2 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 27.38, 52.36, 121.07, 124.29, 125.19, 127.66, 128.48, 129.44, 130.57, 131.59, 134.40, 135.10, 135.54, 136.87, 141.70, 149.70, 157.66, 194.17 (CO). HRMS (ESI) m/z calcd for C20H15N2OSCl + [M]+: 366.0594, found [M+H]+: 367.0664.
2.3.12 9-Chloro-6-(piperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (12)
Product 12 was prepared from compound 2 and piperazine. The red solid material was isolated in 48% yield (Rf = 0.43 at CH2Cl2: n-hexane = 2:1). Mp 180–181 °C. FT-IR (KBr; ν cm−1): 3341 (NH), 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.16 (t, J = 4.8 Hz, 4H, —CH2—), 3.36 (br, 4H, —CH2—), 7.46–7.49 (m, 2H, Ar—H), 7.62–7.66 (m, 3H, Ar—H), 7.87 (d, J = 8.7 Hz, 1H, Ar—H), 8.71 (d, J = 8.7 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 46.17, 51.51, 121.05, 124.31, 124.54, 125.11, 127.45, 128.46, 130.48, 131.69, 134.39, 135.16, 135.40, 136.68, 141.88, 149.84, 157.83, 194.37 (CO). HRMS (ESI) m/z calcd for C20H16N3OCl+ [M]+: 349.0982, found [M+H]+: 350.1063.
2.3.13 9-Chloro-6-(2-methylpiperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (13)
Product 13 was prepared from compound 2 and 3-methylpiperazine. The red solid material was isolated in 18% yield (Rf = 0.49 at CH2Cl2: n-hexane = 4:1). Mp 199–200 °C (EtOH). FT-IR (KBr; ν cm−1): 3222 (NH), 1719 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.17 (d, J = 6.3 Hz, 3H, —CH3), 2.70 (t, 1H, —CH2—), 3.03–3.07 (m, 1H, —NCH—), 3.15–3.19 (m, 3H, —CH2—, —CH2NH—), 3.60–3.65 (d, J = 12.6 Hz, 2H, —NHCH2—), 7.44–7.48 (m, 2H, Ar—H), 7.58–7.62 (m, 3H, Ar—H), 7.86 (d, J = 8.4 Hz, 1H, Ar—H), 8.69 (d, J = 7.8 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 19.96, 45.98, 50.63, 50.75, 57.67, 120.98, 124.29, 124.47, 125.09, 127.39, 128.41, 130.47, 131.63, 134.37, 135.15, 135.36, 136.65, 141.89, 149.82, 157.56, 194.36 (CO). HRMS (ESI) m/z calcd for C21H18N3OCl+ [M]+: 363.1138, found [M+H]+: 364.1201.
2.3.14 9-Chloro-6-(4-methylpiperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (14)
Product 14 was prepared from compound 2 and 1-methylpiperazine. The red solid material was isolated in 51% yield (Rf = 0.4 at CH2Cl2: n-hexane = 2:1). Mp 205–207 °C (EtOH). FT-IR (KBr; ν cm−1): 3462 (NH), 1720 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 2.42 (s, 3H, N-CH3), 2.70 (br, 4H, —CH2—), 3.43 (br, 4H, N—CH2—), 7.44–7.47 (m, 2H, Ar—H), 7.58–7.61 (m, 3H, Ar—H), 7.84 (d, J = 8.4 Hz, 1H, Ar—H), 8.67 (d, J = 8.1 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 46.38, 49.97, 55.17, 121.00, 124.28, 124.54, 125.07, 127.38, 128.46, 130.45, 131.51, 134.35, 135.13, 135.38, 136.63, 141.84, 149.80, 157.37, 194.29 (CO). HRMS (ESI) m/z calcd for C21H18N3OCl+ [M]+: 363.1138, found [M+H]+: 364.1222.
2.3.15 9-Chloro-6-(4-ethylpiperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (15)
Product 15 was prepared from compound 2 and 1-ethylpiperazine. The red solid material was isolated in 20% yield (Rf = 0.43 at CH2Cl2: n-hexane: MeOH = 2:1: 0.5). Mp 182–184 °C (EtOH). FT-IR (KBr; ν cm−1): 1710 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.19 (t, 3H, J = 7.2 Hz, —CH3), 2.57 (q, 2H, J = 7.4 Hz, —NCH2—), 3.03 (br, 4H, —CH2—), 3.46 (br, 4H, —CH2—), 7.43–7.48 (m, 2H, Ar—H), 7.57–7.60 (m, 3H, Ar—H), 7.85 (d, J = 8.4 Hz, 1H, Ar—H), 8.69 (dd, J = 8.25, 0.9 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 12.06, 49.99, 52.69, 52.85, 121.02, 124.30, 124.59, 125.07, 127.36, 128.49, 130.43, 131.53, 134.36, 135.19, 135.38, 136.64, 141.89, 149.84, 15,740, 194.32 (CO). HRMS (ESI) m/z calcd for C22H20N3OCl+ [M]+: 377.1295, found [M+H]+: 378.1380.
2.3.16 9-Chloro-6-(4-cyclopentylpiperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (16)
Product 16 was prepared from compound 2 and 1-cyclopentylpiperazine. The red solid material was isolated in 37% yield (Rf = 0.46 at CH2Cl2: n-hexane: MeOH = 2:1: 0.5). Mp 183–184 oC (EtOH). FT-IR (KBr; ν cm−1): 1716 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.19 (t, 3H, J = 7.2 Hz, —CH3), 2.57 (q, 2H, J = 7.4 Hz, —NCH2—), 3.03 (br, 4H, —CH2—), 3.46 (br, 4H, —CH2—), 7.43–7.48 (m, 2H, Ar—H), 7.57–7.63 (m, 3H, Ar—H), 7.85 (d, J = 8.7 Hz, 1H, Ar—H), 8.69 (d, J = 8.1 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 24.32, 30.67, 50.12, 52.36, 67.87, 120.99, 124.28, 124.61, 125.05, 127.31, 128.50, 130.40, 131.56, 134.36, 135.19, 135.32, 136.58, 141.94, 149.85, 157.48, 194.40 (CO). HRMS (ESI) m/z calcd for C25H24N3OCl+ [M]+: 417.1608, found [M+H]+: 418.1689.
2.3.17 9-Chloro-6-(4-(piperidin-1-yl)piperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (17)
Product 17 was prepared from compound 2 and 1-(piperidin-4-yl)piperidine. The red solid material was isolated in 57% yield. (Rf = 0.51 at CH2Cl2: n-hexane: MeOH = 2:1: 0.5). Mp 174–175 °C. FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.48–1.50 (m, 2H, —CH2—), 1.63–1.65 (m, 2H, —CH2—), 1.72–1.85 (m, 4H, —CH2—), 2.08 (d, J = 11.4 Hz, 2H, —CH2—), 2.38–2.46 (m, 1H, —CH2—), 2.60 (s, 4H, —CH—), 2.91–3.02 (m, 2H, —CH2—), 3.76 (d, J = 12.3 Hz, 2H, —CH2—), 7.41–7.45 (m, 2H, Ar—H), 7.55–7.61 (m, 3H, Ar—H), 7.82 (d, J = 8.4 Hz, 1H, Ar—H), 8.66 (d, J = 7.8 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 24.99, 26.61, 28.77, 50.17, 50.85, 62.51, 120.98, 124.27, 124.42, 124.96, 127.26, 128.36, 130.34, 131.86, 134.37, 135.08, 135.32, 136.45, 141.99, 149.80, 157.82, 194.39 (CO). HRMS (ESI) m/z calcd for C26H26ClN3O+ [M]+: 431.1764, found [M+H]+: 432.1822.
2.3.18 9-Chloro-6-(4-phenylpiperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (18)
Product 18 was prepared from compound 2 and 1-phenylpiperazine. The red solid material was isolated in 52% yield (Rf = 0.91 at CH2Cl2: n-hexane: MeOH = 3:1:0.5). Mp 193–194 °C (EtOH). FT-IR (KBr; ν cm−1): 1714 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.47 (br, 4H, —CH2—), 3.57 (br, 4H, —CH2—), 6.93 (t, J = 7.2 Hz, 1H, Ar—H), 7.04 (d, J = 7.8 Hz, 2H, Ar—H), 7.30–7.36 (m, 2H, Ar—H), 7.45–7.51 (m, 2H, Ar—H), 7.58–7.70 (m, 3H, Ar—H), 7.88 (d, J = 7.8 Hz, 1H, Ar—H), 8.72 (d, J = 8.1 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 49.44, 50.16, 116.66, 120.53, 121.14, 124.32, 124.52, 125.21, 127.60, 128.49, 129.57, 130.56, 131.60, 134.43, 135.16, 135.49, 136.68, 141.74, 149.78, 151.65, 157.41, 194.30 (CO). HRMS (ESI) m/z calcd for C26H20N3OCl+ [M]+: 425.1295, found [M+H]+: 426.1370.
2.3.19 6-(4-Benzylpiperazin-1-yl)-9-chloro-11H-indeno[1,2-c]quinolin-11-one (19)
Product 19 was prepared from compound 2 and 1-benzylpiperazine. The red solid material was isolated in 41% yield (Rf = 0.37 at CH2Cl2: n-hexane = 2:1). Mp 178–180 °C (EtOH). FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 2.73 (br, 4H, —CH2—), 3.41 (br, 4H, —CH2—), 3.65 (s, 2H, —CH2—) 7.28–7.47 (m, 7H, Ar—H8,10, Ar—H), 7.57–7.63 (m, 3H, Ar—H2,3,4), 7.84 (d, J = 8.7 Hz, 1H, Ar—H7), 8.68 (d, J = 7.05 Hz,1H, Ar—H1). 13C NMR (75 MHz, CDCl3): δ (ppm) 50.11, 53.21, 63.34, 120.97, 124.26, 124.60, 125.04, 127.34, 127.50, 128.42, 128.64, 129.40, 130.42, 131.61, 134.35, 135.11, 135.30, 136.53, 138.46, 141.83, 149.78, 157.55, 194.38 (CO). HRMS (ESI) m/z calcd for C27H22N3OCl+ [M]+: 439.1451, found [M+H]+: 440.1503.
2.3.20 9-Chloro-6-(4-(2-fluorophenyl)piperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (20)
Product 20 was prepared from compound 2 and 1-(2-fluorophenyl)piperazine. The red solid material was isolated in 41% yield (Rf = 0.46 at CH2Cl2: n-hexane = 2:1). Mp 182–183 °C (EtOH). FT-IR (KBr; ν cm−1): 1715 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.37 (br, 4H, —CH2—), 3.59 (br, 4H, —CH2—), 7.00–7.12 (m, 4H, Ar—H), 7.44–7.50 (m, 2H, Ar—H), 7.59–7.68 (m, 3H, Ar—H), 7.88 (d, J = 8.1 Hz, 1H, Ar—H), 8.71 (d, J = 8.1 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 50.27, 50.63, 116.47, 116.75, 119.48, 121.09, 123.06, 123.17, 124.31, 124.53, 124.80, 124.86, 125.17, 127.54, 128.49, 130.54, 131.55, 134.40, 135.16, 135.45, 141.76, 149.78, 157.37, 194.29 (CO). HRMS (ESI) m/z calcd for C26H19ClN3O+ [M]+: 443.1201, found [M+H]+: 444.1269.
2.3.21 9-Chloro-6-(4-(2-methoxyphenyl)piperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (21)
Product 21 was prepared from compound 2 and 1-(2-methoxyphenyl)piperazine. The red solid material was isolated in 37% yield (Rf = 0.38 at CH2Cl2: n-hexane = 2:1). Mp 129–131 °C (EtOH). FT-IR (KBr; ν cm−1): 1714 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.36 (br, 4H, —CH2—), 3.60 (br, 4H, —CH2—), 3.90 (s, 3H, —OCH3), 6.91–7.07 (m, 4H, Ar—H), 7.47 (t, J = 7.5 Hz, 2H, Ar—H), 7.59–7.63 (m, 2H, Ar—H), 7.68 (d, J = 7.8 Hz, 1H, Ar—H), 7.87 (d, J = 8.1 Hz, 1H, Ar—H), 8.70 (d, J = 8.4 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 50.39, 50.75, 55.75, 112.15, 118.68, 121.03, 121.49, 123.55, 124.28, 124.61, 125.09, 127.40, 128.47, 130.46, 131.58, 134.36, 135.15, 135.36, 136.61, 141.66, 141.85, 149.81, 152.86, 157.53, 194.38 (CO). HRMS (ESI) m/z calcd for C27H22ClN3O2+ [M]+: 455.1401, found [M+H]+: 456.1473.
2.3.22 9-Chloro-6-(4-(3-methoxyphenyl)piperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (22)
Product 22 was prepared from compound 2 and 1-(3-methoxyphenyl)piperazine. The red solid material was isolated in 86% yield (Rf = 0.43 at CH2Cl2: n-hexane = 2:1). Mp 189–191 °C (EtOH). FT-IR (KBr; ν cm−1): 1723 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.46 (br, 4H, —CH2—), 3.55 (br, 4H, —CH2—), 3.83 (s, 3H, —OCH3), 6.50 (m, J = 8.1 Hz, 1H, Ar—H), 6.57 (s, 1H, Ar—H), 6.65 (d, J = 8.4 Hz, 1H, Ar—H), 7.22 (d, J = 8.1 Hz, 1H, Ar—H), 7.44–7.51 (m, 2H, Ar—H), 7.60–7.68 (m, 3H, Ar—H), 7.88 (d, J = 8.1 Hz, 1H, Ar—H), 8.71 (d, J = 7.2 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 49.36, 50.08, 55.46, 103.25, 105.29, 109.41, 121.14, 124.31, 124.50, 125.18, 127.60, 128.49, 130.25, 130.56, 131.56, 134.42, 135.15, 135.49, 136.68, 141.70, 149.77, 153.00, 157.35, 161.17, 194.23 (CO). HRMS (ESI) m/z calcd for C27H22ClN3O2+ [M]+: 455.1401, found [M+H]+: 456.1464.
2.3.23 9-Chloro-6-(4-(1-methylpiperidin-4-yl)piperazin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (23)
Product 23 was prepared from compound 2 and 1-(1-methylpiperidin-4-yl)piperazine. The red solid material was isolated in 31% yield (Rf = 0.90 at CH2Cl2: n-hexane: MeOH = 2:1: 0.5). Mp 208–209 °C (EtOH). FT-IR (KBr; ν cm−1): 1710 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.64–1.72 (m, 2H, —CH2—), 1.88 (d, J = 10.5 Hz, 1H, —CH2—), 1.95–2.03 (m, 2H, —CH2—), 2.29 (s, 4H, —CH—, —CH3), 2.82 (br, 4H, —CH2—), 2.95 (d, J = 9.6 Hz, 1H, Ar—H), 3.40 (br, 4H, —CH2—), 6.47–6.50 (m, 1H, Ar—H), 6.57 (s, 1H, Ar—H), 6.65 (d, J = 8.4 Hz, 1H, Ar—H), 7.22 (d, J = 8.1 Hz, 1H, Ar—H), 7.42–7.47 (m, 2H, Ar—H), 7.57–7.61 (m, 3H, Ar—H), 7.84 (d, J = 8.4 Hz, 1H, Ar—H), 8.68 (d, J = 7.8 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 28.54, 46.34, 49.24, 50.49, 55.62, 61.83, 120.96, 124.27, 124.61, 125.04, 127.31, 128.45, 130.40, 131.54, 134.33, 135.15, 135.31, 136.55, 141.89, 149.81, 157.49, 194.38 (CO). HRMS (ESI) m/z calcd for C26H27ClN4O + [M]+: 446.1873, found, [M+H]+: 447.1944.
2.3.24 9-Chloro-6-(4-(1,4-dioxa-8-azaspiro[4,5]dec-8-yl)-11H-indeno[1,2-c]quinolin-11-one (24)
Product 24 was prepared from compound 2 and 1,4-dioxa-8-azaspiro[4,5]dec-8-yl. The red solid material was isolated in 56% yield (Rf = 0.34 at CH2Cl2: n-hexane = 2:1). Mp 218–219 °C (EtOH). FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.98 (t, J = 5.7 Hz, 4H, —CH2—), 3.51 (br, 4H, —NCH2—), 4.04 (s, 4H, —OCH2—), 7.46 (td, J = 8.7, 2.1 Hz, 2H, Ar—H), 7.57–7.62 (m, 3H, Ar—H), 7.83 (d, J = 8.7 Hz, 1H, Ar—H), 8.69 (dd, J = 8.4, 0.9 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 35.00, 48.35, 64.66, 107.31, 120.98, 124.25, 125.06, 127.32, 128.41, 130.42, 131.65, 134.38, 135.09, 135.36, 136.57, 141.97, 149.74, 157.34, 194.43 (CO). HRMS (ESI) m/z calcd for C23H19ClN2O3 + [M]+: 406.1084, found [M+H]+: 407.1154.
2.3.25 9-Chloro-6-(4-((piperazin-1-yl)(piperidin-1-yl)methanone)-11H-indeno[1,2-c]quinolin-11-one (25)
Product 25 was prepared from compound 2 and (piperazin-1-yl)(piperidin-1-yl)methanone. The red solid material was isolated in 47% yield (Rf = 0.17 at CH2Cl2: n-hexane = 2:1). Mp 266–267 °C (EtOH). FT-IR (KBr; ν cm−1): 1647, 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.63 (s, 2H, —CH2—), 3.28–3.47 (m, 16H, —CH2—), 7.42–7.49 (m, 2H, Ar—H), 7.57–7.61 (m, 3H, Ar—H), 7.82 (d, J = 8.4 Hz, 1H, Ar—H), 8.69 (d, J = 7.8, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 24.86, 25.97, 47.12, 47.99, 49.99, 121.16, 124.32, 124.47, 125.20, 127.65, 128.39, 130.58, 131.62, 134.49, 135.09, 135.54, 136.68, 141.56, 149.69, 157.36, 164.84 (CO), 194.20 (CO). HRMS (ESI) m/z calcd for C23H19ClN2O3 + [M]+: 460.1666, found [M+H]+: 461.1739.
2.3.26 9-Chloro-6-(4-(3-(piperidin-4-yl)propyl)piperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (26)
Compound 26 was prepared from compound 2 and 4-(3-(piperidin-4-yl)propyl)piperidine. The pure product was obtained as red powder (yield 8.4%) (Rf = 0.41 at CH2Cl2: n-hexane = 2:1). Mp 149–151 °C (EtOH). FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 1.07–1.46 (m, 12H, —CH2—, —CH—), 1.66–1.91 (m, 4H, —CH2—), 2.54–2.62 (m, 2H, —NCH2(axial)—), 2.90–2.94 (m, 2H, —NCH2(axial)—), 3.06 (d, J = 12 Hz, 2H, —NCH2(equatorial)—), 3.70 (d, J = 12.3 Hz, 2H, —NCH2(equatorial)—), 7.41–7.47 (m, 2H, Ar—H), 7.56–7.61 (m, 3H, Ar—H), 7.84 (d, J = 8.4 Hz, 1H, Ar—H), 8.68 (d, J = 8.1, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 23.81, 32.58, 33.95, 35.88, 36.49, 37.04, 37.65, 47.07, 50.75, 120.89, 124.27, 124.44, 124.92, 127.15, 128.31, 130.32, 131.96, 134.33, 135.08, 135.23, 136.43, 142.12, 149.83, 158.22, 194.52 (CO). HRMS (ESI) m/z calcd for C29H32ClN3O + [M]+: 473.2234, found [M+H]+: 474.2318.
2.3.27 6-(2-Hydroxyethylthio)-9-chloro-11H-indeno[1,2-c]quinolin-11-one (27)
Compound 27 was prepared from compound 2 and 2-mercaptoethanol. The pure product was obtained as red powder (yield 95%) (Rf = 0.23 at CH2Cl2: n-hexane = 2:1). Mp 169–170 °C (EtOH). FT-IR (KBr; ν cm−1): 1718 (C⚌O). 1H NMR (300 MHz, CDCl3): δ (ppm) 3.66 (t, J = 5.4 Hz, 2H, —SCH2—), 4.11 (t, J = 5.25 Hz, 2H, —CH2OH), 4.34 (br, 1H, —OH), 7.47 (dd, J = 8.4, 2.1 Hz, 1H, Ar—H), 7.53 (td, J = 8.4, 1.5 Hz, 1H, Ar—H), 7.61 (d, J = 2.1 Hz, 1H, Ar—H), 7.65 (td, J = 8.4, 1.5 Hz, 1H, Ar—H), 7.87 (d, J = 8.1 Hz, 1H, Ar—H), 7.92 (d, J = 8.1 Hz, 1H, Ar—H), 8.72 (d, J = 8.4 Hz, 1H, Ar—H). 13C NMR (75 MHz, CDCl3): δ (ppm) 34.05, 63.23, 121.54, 124.64, 125.36, 125.83, 127.90, 128.79, 131.14, 134.11, 134.61, 135.02, 136.08, 136.70, 140.76, 149.91, 155.11, 193.79 (CO). HRMS (EI) m/z calcd for C18H12ClNO2S +[M]+: 341.0277, found 341.0287.
2.3.28 6-Hydroxy-9-chloro-11H-indeno[1,2-c]quinolin-11-one (28)
The pure product was obtained as red solid (yield 57%) (Rf = 0.24 at ethyl acetate: n-hexane = 3:2). Mp 384 °C (dec.). 1H NMR (400 MHz, DMSO-d6): δ (ppm) 7.30 (t, J = 7.6 Hz, 1H, Ar—H), 7.40 (d, J = 8.0 Hz, 1H, Ar—H), 7.56 (t, J = 7.6 Hz, 1H, Ar—H), 7.61 (s, 1H, Ar—H), 7.63 (d, J = 6.8 Hz, 1H, Ar—H), 7.93 (d, J = 7.6 Hz, 1H, Ar—H), 7.37 (d, J = 8.4 Hz, 1H, Ar—H), 12.42 (br, 1H, —OH). 13C NMR (100 MHz, CDCl3): δ (ppm) 115.12, 116.39, 123.91, 124.41, 125.02, 131.62, 133.69, 134.33, 134.82, 135.68, 136.68, 140.78, 141.10, 159.20, 194.44 (C⚌O). HRMS (ESI) calcd for C16H8NO2Cl [M]+ 281.0244; found [M+H]+ 282.0322, [M−H]− 280.0178.
2.3.29 6-Methoxy-9-chloro-11H-indeno[1,2-c]quinolin-11-one (29)
The pure product was obtained as orange powder (yield 60%) (Rf = 0.52 at CH2Cl2: n-hexane = 1:1). Mp 259–261 °C (EtOH). 1H NMR (400 MHz, CDCl3): δ (ppm) 4.24 (3H, s, —OCH3), 7.44 (1H, dd, J = 8.0 Hz, 2.0 Hz, Ar—H), 7.47 (1H, td, J = 7.6 Hz, 1.2 Hz, Ar—H), 7.59 (1H, d, J = 2.0 Hz, Ar—H), 7.62 (1H, td, J = 8.0 Hz, 1.6 Hz, Ar—H), 7.75 (1H, d, J = 7.6 Hz, Ar—H), 7.85 (1H, d, J = 8.4 Hz, Ar—H), 8.67 (1H, dd, J = 8.0 Hz, 1.2 Hz, Ar—H). 13C NMR (100 MHz, CDCl3): δ (ppm) 53.92, 120.66, 124.09, 124.86, 125.03, 126.68, 127.48, 129.08, 130.25, 134.31, 134.51, 135.16, 136.14, 140.25, 148.79, 158.13, 193.88 (C⚌O). HRMS (ESI) calcd for C17H10NO2Cl [M]+ 295.0400; found [M+H]+ 296.0482.
2.4 Cell culture and MTT assay
All of the synthesized compounds (1–29) were tested against renal CAKI-1 cell line by MTT assay. Moreover, we selected four compounds 4, 5, 13, and 26 to evaluate the topo I inhibitory activity. Simultaneously, seventeen of our structures (1, 2, 3, 4, 5, 8, 10, 11, 12, 14, 16, 18, 22, 25, 27, 28 and 29) were selected by NCI and were investigated against a panel of 60 human tumor cell lines. According to the primary screening, compounds 1, 5 and 12 were chosen for further cell growth inhibition screening for the GI50 (50% growth inhibitory concentration), TGI (the total growth inhibition), and LC50 (the 50% lethal concentration) by NCI.
A 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) (Sigma, USA) assay was performed to determine the cell viability and IC50 values of our synthetic compounds against the MCF-7 (Mosmann, 1983; Denizot and Lang, 1986) and CAKI-1 cell lines. MCF-7 (3000 cells/well) and CAKI-1 (5000 cells/well) cells were seeded in 96-well microplates with RPMI-1641 supplemented with 10% FBS and treated with various concentrations of compounds for 72 h. After treatment, each well was washed with phosphate-buffered saline (PBS) three times, 100 μL of the MTT solution (0.5 mg/mL final concentration in the medium) was added to each well, and cells were incubated at 37 °C for 1 h. MTT is converted to blue formazan crystals by mitochondrial succinate dehydrogenase. The plates were then washed with PBS and solubilized in 100 μL of dimethyl sulfoxide (DMSO) per well. The absorbances at 540 nm were determined using an enzyme-linked immunosorbent assay (ELISA) microplate reader. Effects of our synthetic compounds on cell viability were demonstrated as the relative activity (relative to the DMSO control group).
2.5 DNA topoisomerase I assay
The topoisomerase I assay kit (TopoGEN, Inc., USA) was performed as described previously with modifications (Shchekotikhin et al., 2011). The activity of DNA topoisomerase I was determined by evaluating the relaxation of supercoiled DNA pHOT. The selected synthetic compounds and camptothecin were dissolved in DMSO at 10 mM as stock solution. A mixture containing 0.25 μg of the plasmid PHOT DNA and 1–2 units of recombinant human DNA topoisomerase I (TopoGEN INC., USA) was incubated with the prepared 0.5% DMSO (negative control), camptothecin 100 μM (positive control) or compounds in the buffer (10 mM Tris-HCl, pH 7.9; 1 mM EDTA; 0.15 M NaCI; 0.1% BSA; 0.1 mM Spermidine; 5% glycerol) at 37 °C for 45 min. The reaction was quenched by the addition of sodium dodecyl sulfate (final 1% concentration) and proteinase K (final 50 μg/mL concentration) at 37 °C for 15 min. To the reaction mixtures, the loading buffer containing 0.25% bromophenol blue and 50% glycerol was added 0.1 volume in reactions mixtures. These samples were electrophoresed on 1% agarose gel at 60 V for 1.5 h with TAE (Tris-acetate-EDTA) as the buffer. The gels were stained with ethidium bromide for 10 min and destained with water for 20 min after electrophoresis.
2.6 NCI in vitro 60-cell drug screening experiments
Eight of our synthesized compounds were selected by the NCI, and their anticancer activities at a single dose of 10 μM were determined by a sulforhodamine B (SRB) colorimetric assay according to previous protocols (Sikic, 1991; Monks et al., 1997; Kandeel et al., 2015). Cells (3000–5000 per well) were seeded into 96-well microtiter plates for 24 h at 37 °C, with 5% CO2, 95% air, and 100% relative humidity. Two plates of each cell line were fixed with trichloroacetic acid (TCA) as a control of the cell population for each cell line at the time of drug exposure (T0). After additional incubation with the vehicle (DMSO) or the test compounds for 48 h, cells were fixed with cold 50% (w/v) TCA (final concentration, 10% TCA) and then incubated for 60 min at 4 °C. Plates were then washed with tap water, and cells were treated with 100 μL of the SRB solution at 0.4% (w/v) in 1% acetic acid for 10 min at room temperature. After staining, the plates were washed with 1% acetic acid to remove any unbound dye, and SRB-bound cells were solubilized with 0.01 M Trizma base. The absorbance was measured using a spectrophotometer at a wavelength of 515 nm. Using the absorbance measurements, including time zero (T0), control growth (C), and test growth in the presence of a drug (TX), the percentage growth was calculated for each compound as follows: 100 − [(TX − T0)/(C − T0)] × 100 for which concentrations in which TX ⩾ T0.
3 Results and discussion
The synthetic methods of 6-substituted 9-chloro-11H-indeno[1,2-c]quinolin-11-ones are depicted in Scheme 1. The intermediate 3-(4-Chlorophenyl)-2-hydroxyquinoline-4-carboxylic acid (1) was obtained from the reaction of isatin with 4-chlorophenylacetic acid via the Pfitzinger reaction (Pfitzinger, 1886, 1888). It is a common reaction to provide quinoline-4-carboxylic acids under basic condition. The following is the starting material compound 2, 6,9-dichloro-11H-indeno[1,2-c]quinolin-11-one, and it was produced from intermediate (1) in POCl3 and carried out in an open system round-bottle flask with a condenser. The synthetic strategy of compounds 3–26 was accomplished via the reaction of an appropriate amine with compound 2 in DMF, and then treatment with pyridine to afford the desired compounds, which were purified by recrystallization. It was also worthy to note that compound 2 could react with HCl to obtain 28 or with sodium methoxide (NaOMe) to afford 29, respectively. All the synthetic methods are illustrated in the procedure Scheme 1.
The effects of the synthesized indenoquinolone derivatives 2–29 on cell viability of the MCF-7 breast cancer cell line and CAKI-1 renal cancer cell line were experimentally assessed by performing a MTT assay. Results are summarized in Table 1 and are expressed as IC50 (μM) values. From the obtained results, we observed that some indenoquinolones exhibited interesting activities on the tested cancer cell lines. We found that the most potent compounds against CAKI-1 cells were 12, 13, 23 and 27 with IC50 values less than 2 μM. In addition, the potent compounds against MCF-7 cells were 5 (2.13 ± 0.57), 12 (2.20 ± 0.33), 13 (2.40 ± 0.43), and 23 (2.31 ± 0.35) which showed low IC50 values of cell viability. Of the compounds analyzed, we observed that introduction of substituted piperazinyl groups at the 6-position of the indenoquinolone scaffold could modulate the inhibition of cell viability of MCF-7 and CAKI-1 compared to the indenoquinolone 2 which possess two chloro atoms. However, the IC50 values of compounds 14–22 containing a N-substituted piperazinyl group decreased potency significantly. Further, the micromolar IC50 values of some potent compounds are presented in Table 1.
Compound
IC50 (μM)a ± SDb
Compound
IC50 (μM) ± SD
CAKI-1
MCF-7
CAKI-1
MCF-7
1
>30
>30
16
19.66 ± 4.75
>30
2
>30
>30
17
5.83 ± 0.37
3.07 ± 0.36
3
23.26 ± 5.74
3.28 ± 0.88
18
3.59 ± 1.40
3.51 ± 1.4
4
13.52 ± 2.79
3.06 ± 0.77
19
>30
>30
5
3.85 ± 0.56
2.13 ± 0.57
20
10.80 ± 2.91
>30
6
>30
>30
21
>30
>30
7
>30
>30
22
12.96 ± 3.16
>30
8
>30
>30
23
1.81 ± 1.10
2.31 ± 0.35
9
29.64 ± 2.55
>30
24
5.95 ± 0.03
>30
10
7.19 ± 0.35
>30
25
>30
>30
11
9.07 ± 3.86
4.0 ± 0.55
26
21.83 ± 2.74
>30
12
1.52 ± 0.21
2.2 ± 0.33
27
1.99 ± 0.03
>30
13
1.66 ± 0.11
2.4 ± 0.433
28
>30
>30
14
8.18 ± 0.69
>30
29
>30
>30
15
9.66 ± 1.91
3.24 ± 0.66
CPTc
–d
1.24 ± 0.72
According to the antiproliferative activities and the similar side chains, compounds 4, 5, 13, and 26 were selected to evaluate the topoisomerase I inhibition. In Fig. 2, the selected compounds showed various inhibitory effects against topoisomerase I at 25 and 100 μM. Among them, the synthetic compound 13 not only exhibited more potent inhibitory activity than compounds 4, 5, 26, and CPT, but also completely blocked topoisomerase I-mediated DNA relaxation at 25 μM. Compound 7 was chosen for further testing against topoisomerase I in a concentration-dependent manner doses at 3.125, 6.25, 12.5, and 25 μM (Fig. 3).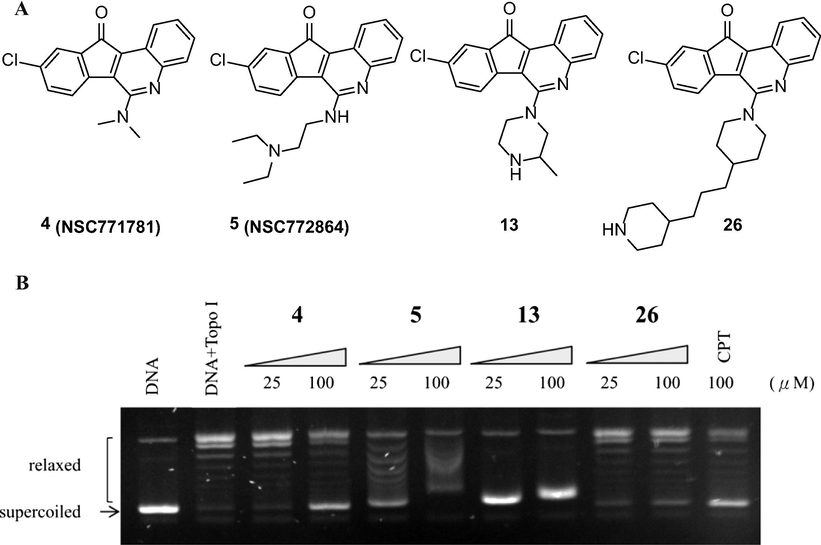
Structures and the inhibition of topoisomerase I relaxation activities of compounds 4, 5, 13, and 26. (A) Structure of compounds 4, 5, 13, and 26. (B) Effect of compounds 4, 5, 13, and 26 on topoisomerase I mediated supercoiled pHOT DNA relaxation. Lane 1: untreated supercoiled pHOT DNA. Lane 2: the pHOT DNA treated with topoisomerase I in the absence of drugs. Lanes 3–4 (compound 4), 5–6 (compound 5), 7–8 (compound 13) and 9–10 (compound 26) are the pHOT DNA treated with topoisomerase I in the present of drugs at 25 or 100 μM, respectively. Lane 11 is the pHOT DNA treated with topoisomerase I in the presence of CPT at 100 μM.
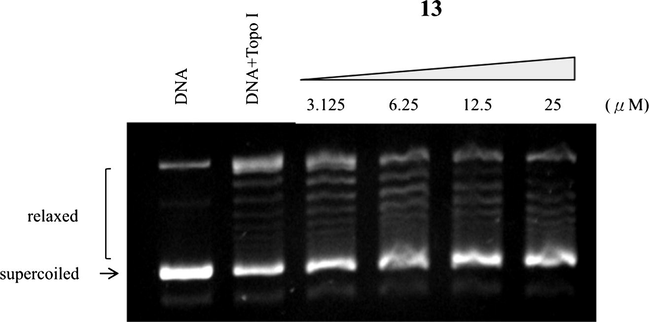
Effect of compound 13 on topoisomerase I mediated supercoiled pHOT DNA relaxation. Lanes 1: untreated supercoiled pHOT DNA. Lanes 2: the pHOT DNA treated with topoisomerase I in the absence of drugs. Lanes 3–6 are the pHOT DNA treated with topoisomerase I in the presence of compound 13 at 3.125, 6.25, 12.5 and 25 μM.
The effects of sixteen compounds 1 (NSC763972), 2 (NSC763969), 3 (NSC772856), 4 (NSC771781), 5 (NSC772864), 8 (NSC772860), 10 (NSC771782), 12 (NSC772862), 14 (NSC771783), 16 (NSC772859), 18 (NSC772861), 22 (NSC772863), 25 (NSC772858), 27 (NSC763971), 28 (NSC763970), and 29 (NSC765596) on cell viability were evaluated against the NCI-60 human tumor cell lines at 10 μM in vitro using the SRB protein-binding dye (Sikic, 1991; Monks et al., 1997). As shown in Table 2, this class of compounds bearing ethanolthiol group (27), hydroxyl group (28), methoxy group (29), and several N-substituted piperazinyl groups revealed no significant activities for all the cancer cell lines which respond to the previous in vitro consequences. Three synthetic compounds 1, 5, and 12 were selected for an advanced 60-cell panel assay at five logarithm concentrations (10−2, 10−1, 100, 101 and 102 μM) and the results were indicated with GI50, TGI and LC50, because of their significant cytotoxicity when tested using MTT viability assay.
Panel/cell line
Compounds/growth percenta
Compd. No.
1
2
3
4
5
8
10
11
12
14
16
18
22
25
27
28
29
NCI No.
NSC763972
NSC763969
NSC772856
NSC771781
NSC772864
NSC772860
NSC771782
NSC772857
NSC772862
NSC771783
NSC772859
NSC772861
NSC772863
NSC772858
NSC763971
NSC763970
NSC765596
Leukemia
CCRF-CEM
22.62
64.86
101.50
92.13
42.00
104.33
88.85
105.04
−35.45
92.44
95.17
93.52
93.78
109.87
71.56
105.78
89.25
HL-60(TB)
3.18
102.00
102.75
95.49
94.33
98.84
97.09
94.50
−38.36
101.15
103.04
105.45
99.09
109.78
91.75
102.31
108.77
K-562
12.16
102.16
90.99
88.81
45.29
103.57
89.01
100.89
−65.71
92.13
99.26
95.17
94.25
95.70
63.81
97.48
108.53
MOLT-4
43.74
70.83
93.26
88.92
34.63
94.41
88.74
93.29
−43.22
92.90
98.39
98.13
95.46
104.77
59.49
95.10
110.33
RPMI-8226
20.68
92.83
91.82
88.05
81.94
97.99
83.40
95.20
−43.57
85.92
94.89
95.33
93.63
104.31
62.28
90.60
90.30
SR
29.86
65.85
91.67
86.12
46.92
98.51
85.94
99.53
−55.19
75.13
97.09
95.93
95.05
105.15
61.70
77.02
N.T.
Non-small cell lung cancer
A549/ATCC
11.94
108.98
96.83
98.85
51.79
101.69
101.47
104.35
18.70
96.43
101.56
90.89
101.22
99.12
76.42
104.88
99.70
EKVX
N.T.b
79.27
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
30.15
72.14
N.T.
HOP-62
29.61
94.89
87.10
78.02
24.86
64.18
78.71
78.17
−61.80
61.42
108.83
N.T.
90.22
96.39
91.46
91.26
96.01
HOP-92
N.T.
71.79
N.T.
44.26
N.T.
N.T.
66.08
N.T.
N.T.
38.50
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
77.75
NCI-H226
66.09
91.86
92.19
84.79
80.86
94.59
87.21
94.22
49.94
86.17
92.80
79.43
89.19
96.06
80.52
85.66
89.22
NCI-H23
58.72
79.30
86.83
82.62
80.63
90.42
90.40
94.33
49.19
86.27
92.80
83.34
87.22
95.41
66.22
81.56
93.26
NCI-H322M
89.67
95.38
80.85
78.83
69.83
107.08
91.28
92.29
35.89
87.41
98.50
98.63
91.93
94.59
73.47
90.72
99.80
NCI-H460
79.73
98.68
84.38
94.17
25.15
101.21
102.14
93.32
−66.36
91.83
101.46
97.04
101.76
98.85
79.22
83.32
98.27
NCI-H522
1.78
89.37
90.50
88.97
77.39
98.45
91.11
92.76
18.08
96.62
98.72
80.10
79.75
99.28
68.89
92.22
95.89
Colon cancer
COLO 205
14.63
99.18
66.47
100.97
45.69
104.79
100.71
96.30
−81.49
93.65
104.54
102.19
106.63
98.52
87.39
98.25
108.91
HCC-2998
88.12
98.52
57.51
81.50
88.65
102.76
107.23
103.16
−55.83
103.21
106.04
106.23
105.79
106.23
96.27
102.24
104.46
HCT-116
35.26
105.87
88.31
98.57
36.96
102.51
93.64
89.08
−83.19
92.78
91.34
78.18
93.02
101.67
77.94
103.43
88.60
HCT-15
21.44
114.88
66.20
80.49
50.38
97.78
80.29
97.88
−78.92
92.83
89.64
80.71
100.39
99.35
80.10
108.54
94.98
HT29
81.42
104.27
99.44
94.89
9.75
104.84
91.34
95.52
−80.89
93.76
93.22
82.40
95.93
108.65
84.03
102.38
111.60
KM12
79.30
111.92
66.82
94.44
53.42
94.80
109.99
97.97
−47.09
99.06
99.33
98.34
105.95
101.58
84.62
102.73
98.35
SW-620
66.13
98.29
96.25
96.35
43.72
88.89
103.71
91.88
−73.67
92.76
93.06
93.14
91.16
92.51
86.99
95.26
98.89
CNS cancer
SF-268
84.54
117.27
97.27
100.26
65.88
96.49
103.58
96.13
34.33
92.35
96.39
85.45
83.89
107.51
96.53
106.64
113.64
SF-295
N.T.
92.80
95.71
93.22
N.T.
96.18
102.43
93.33
23.75
90.01
96.87
60.91
90.05
97.83
73.28
92.48
N.T.
SF-539
104.63
110.08
92.08
87.80
85.04
90.22
96.24
92.93
36.91
95.23
101.34
83.17
83.97
92.98
100.05
105.79
91.49
SNB-19
93.45
101.30
95.95
104.19
94.02
103.87
100.01
113.43
67.25
85.41
95.06
88.40
105.27
108.66
89.19
99.28
96.35
SNB-75
74.33
82.91
79.70
69.12
71.52
84.42
81.55
72.13
22.34
70.00
86.36
33.20
37.76
80.84
66.06
80.33
59.75
U251
20.22
100.63
96.03
98.98
N.T.
N.T.
95.72
111.96
N.T.
85.86
N.T.
N.T.
N.T.
98.15
78.04
96.46
97.67
Melanoma
LOX IMVI
33.38
84.23
93.40
85.45
N.T.
N.T.
91.20
100.04
N.T.
87.17
N.T.
N.T.
N.T.
103.19
68.41
86.91
116.91
MALME-3M
73.87
102.06.
114.83
111.50
52.55
98.31
136.93
101.05
−32.28
106.59
92.48
100.05
92.79
102.62
107.23
103.37
107.54
M14
72.55
109.79
112.68
113.63
76.44
96.60
106.99
101.69
−56.24
106.31
105.34
97.52
105.07
101.18
98.89
108.50
96.50
MDA-MB-435
N.T.
100.46
107.24
97.33
92.72
104.53
106.62
107.70
−6.57
98.43
99.93
99.55
100.75
102.57
95.15
100.35
100.31
SK-MEL-2
110.64
117.11
N.T.
95.09
N.T.
N.T.
104.29
N.T.
N.T.
104.36
N.T.
N.T.
N.T.
N.T.
95.85
117.12
109.44
SK-MEL-28
73.75
90.08
114.89
113.43
87.40
105.21
113.81
110.84
−91.38
110.81
107.58
104.82
103.93
107.03
86.90
100.02
106.24
SK-MEL-5
66.21
86.30
80.96
94.89
82.51
98.52
99.19
89.52
59.18
97.67
97.48
99.23
96.87
92.50
68.56
84.87
98.51
UACC-257
38.15
127.99
107.69
101.11
92.18
106.47
103.31
107.44
−39.24
95.34
98.37
98.40
93.79
104.23
106.91
106.31
98.20
UACC-62
86.86
94.45
77.41
78.16
99.56
93.36
88.33
94.90
−81.71
96.67
95.06
87.17
86.44
93.92
84.47
95.17
74.08
Ovarian cancer
IGROV1
87.42
92.82
54.41
83.38
43.43
94.36
86.47
72.45
3.23
75.03
85.84
65.13
80.87
81.63
69.73
91.28
96.92
OVCAR-3
82.98
119.93
84.91
103.48
73.13
97.59
102.34
96.58
22.86
93.41
99.54
98.07
104.27
103.33
89.42
117.80
111.89
OVCAR-4
40.23
94.13
72.83
84.28
52.50
97.11
96.67
98.19
35.59
94.70
94.20
90.32
87.08
98.31
73.19
80.74
N.T.
OVCAR-5
104.97
137.00
81.54
89.14
89.34
104.92
90.30
94.37
39.14
99.31
106.41
88.56
98.22
102.33
112.54
126.77
96.81
OVCAR-8
16.01
101.08
94.33
84.55
61.36
96.38
95.25
96.11
−46.09
87.51
96.55
75.82
83.98
104.73
90.21
107.63
98.75
NCI/ADR-RES
43.83
96.78
92.22
92.57
69.55
90.28
94.32
102.85
−4.00
88.19
100.39
97.88
92.82
107.13
73.58
97.00
94.29
SK-OV-3
N.T.
N.T.
92.62
99.12
84.20
99.78
107.26
84.60
57.81
87.98
96.85
78.58
86.02
95.59
N.T.
N.T.
119.79
Renal cancer
786-0
103.15
112.40
103.34
108.01
74.13
102.73
103.71
99.53
25.31
89.51
97.90
81.41
81.67
105.05
100.58
114.55
103.51
A498
N.T.
N.T.
82.03
96.00
88.98
87.55
93.88
99.15
59.79
72.81
88.64
97.48
104.53
104.44
N.T.
N.T.
93.51
ACHN
51.33
111.67
88.46
87.55
55.86
94.75
81.66
86.42
−83.29
88.10
100.03
68.58
93.51
95.59
88.12
104.75
90.83
CAKI-1
N.T.
75.67
73.84
84.65
64.35
83.89
94.99
82.68
17.84
83.81
93.83
80.23
86.59
90.50
41.86
71.62
90.65
RXF 393
82.62
112.80
97.15
101.32
51.90
97.51
114.61
106.09
20.51
94.00
95.60
72.95
78.38
107.22
79.39
99.93
114.49
SN12C
48.72
88.03
94.82
95.38
61.60
99.08
96.95
93.10
15.89
83.57
99.08
87.90
88.17
101.12
84.58
96.69
89.48
TK-10
94.62
138.45
65.31
91.84
95.55
134.79
101.98
121.79
47.07
99.56
N.T.
115.61
133.34
131.97
99.82
126.63
N.T.
UO-31
15.98
80.62
56.91
48.63
25.86
76.25
45.74
65.33
−40.61
47.60
72.57
65.02
86.64
82.74
56.23
70.50
55.69
Prostate cancer
PC-3
32.02
92.73
89.44
82.55
41.01
90.64
79.64
87.77
−58.51
82.93
86.19
79.79
72.83
93.07
58.35
93.44
74.44
DU-145
89.22
113.91
106.88
106.50
65.48
103.47
119.02
106.04
−41.83
102.37
110.73
102.96
98.98
114.04
92.27
111.80
121.06
Breast cancer
MCF7
50.00
74.25
5.24
23.99
12.70
64.65
52.31
49.61
−100.00
68.30
75.18
53.31
77.46
97.24
67.82
68.09
71.98
MDA-MB-231/ATCC
41.97
87.66
83.87
76.68
25.16
90.96
79.28
93.08
−10.45
62.53
82.96
73.80
79.81
93.54
72.56
86.98
74.61
HS 578T
84.98
91.01
85.28
72.84
84.70
98.38
88.93
87.68
60.88
70.47
99.40
N.T.
71.75
102.06
58.28
105.98
92.74
BT-549
91.18
119.62
101.88
102.55
87.48
95.52
93.95
92.91
58.35
82.89
102.74
89.83
101.57
105.34
92.88
120.08
92.95
T-47D
−30.98
86.97
23.53
44.88
43.28
67.68
88.81
83.86
−31.54
76.27
92.20
75.93
86.68
95.99
63.89
67.23
78.97
MDA-MB-468
45.23
107.92
−31.61
0.60
33.81
97.12
59.47
97.71
−35.66
95.71
103.99
95.37
101.04
106.42
77.36
80.08
99.51
Mean
55.93
98.17
84.15
87.15
62.40
96.20
93.66
94.75
−14.37
88.15
96.53
87.29
92.15
100.53
79.52
96.60
96.23
Delta
86.91
33.31
115.76
86.55
52.65
32.02
47.92
45.14
85.63
49.65
23.96
54.09
54.39
19.69
49.37
29.37
40.54
Range
141.62
73.59
146.50
113.03
89.81
70.61
91.19
72.18
167.25
72.31
38.16
82.41
95.58
51.13
82.39
59.54
65.37
Compound 1, the average concentration required to inhibit GI50 was 8.982 μM with a range of 0.238 μM (non-small cell lung cancer: NCI-H522) to >39.0 μM (colon cancer: HT-29). With 5, the average concentration required to inhibit GI50 was 14.36 μM with a range of 0.44 μM (breast cancer: MCF-7) to >100 μM (colon cancer: HCC-2998). Furthermore, the average GI50 concentration of 12 was 10.498 μM, with a range of 2.52 μM (ovarian cancer: IGROV1) to >100 μM (melanoma: SK-MEL-28). Compounds 1, 5, and 12 exhibited dose-dependent inhibition of proliferation in all 60 cancer cell lines. Compound 1 is active against most of the cancer cell lines with GI50 values <1 μM for 12% (7/58) of the cell lines. With initial assessment at relative doses, 12 is more potent than 5. Compound 12 exhibited highly inhibitory effect against most of the cancer cell lines with GI50 values <5 mM for 36.8% of the 57 cell lines. The GI50, TGI and LC50 values of the active compounds within each series are given in Table 3. MIDa = Average sensitivity of all cell lines in μM. MIDb = Average sensitivity of all cell lines of a particular subpanel in μM. Selectivity ratio = MIDa:MIDb. N.T. = no test.
Panel/cell line (μM)
1 (NSC763972)
5 (NSC772864)
12 (NSC772862)
GI50
TGI
LC50
GI50
TGI
LC50
GI50
TGI
LC50
Subpanel MIDb
Selectivity ratio
Subpanel MIDb
Selectivity ratio
Subpanel MIDb
Selectivity ratio
Leukemia
CCRF-CEM
1.42
1.114
8.063
15.5
>100
8.24
9.696
1.475
3.50
7.80
6.32
11.000
0.954
>100
>100
HL-60(TB)
1.06
4.28
>100
N.T.
N.T.
N.T.
36.9
>100
>100
K-562
0.315
>100
>100
8.75
3.61
7.46
7.80
>100
>100
MOLT-4
1.97
15.3
>100
6.24
3.43
7.91
4.41
>100
>100
RPMI-8226
0.549
>100
>100
20.20
4.31
9.73
7.48
>100
>100
SR
1.37
43.4
>100
5.05
4.06
11.60
3.09
>100
>100
Non-small cell lung cancer
A549/ATCC
2.95
5.840
1.538
>100
>100
15.20
11.819
1.210
3.51
6.72
4.87
6.477
1.621
>100
>100
EKVX
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
N.T.
HOP-62
3.84
>100
>100
4.51
2.83
5.74
12.3
>100
>100
HOP-92
1.21
13.4
>100
3.08
5.80
25.10
N.T.
N.T.
N.T.
NCI-H226
11.6
>100
>100
1.88
–
–
7.77
>100
>100
NCI-H23
3.44
63.6
>100
2.63
4.00
8.35
6.65
>100
>100
NCI-H322M
16
92.3
>100
34.50
13.10
36.20
5.62
>100
>100
NCI-460
7.44
86.3
>100
5.15
3.20
6.74
4.15
>100
>100
NCI-H522
0.238
0.644
3.57
27.60
3.80
7.23
3.98
>100
>100
Colon cancer
COLO 205
3.77
9.560
0.939
15.8
39.8
2.41
18.400
0.778
–
–
12.2
9.763
1.075
>100
>100
HCC-2998
11.0
>100
>100
>100
4.08
7.94
30.9
>100
>100
HCT-116
0.807
>100
>100
6.19
3.19
6.06
5.42
>100
>100
HCT-15
0.716
>100
>100
4.37
3.51
7.50
4.34
>100
>100
HT29
39.0
>100
>100
4.23
3.16
6.28
7.84
>100
>100
KM12
4.62
>100
>100
6.48
3.11
5.96
2.50
>100
>100
SW-620
7.01
>100
>100
5.12
3.06
6.67
5.14
>100
>100
CNS cancer
SF-268
4.28
14.364
0.625
>100
>100
17.70
14.875
0.962
3.27
6.52
8.71
8.053
1.304
>100
>100
SF-295
10.8
87.9
>100
17.80
4.60
9.85
7.79
>100
>100
SF-539
23.1
64.2
>100
20.90
3.15
5.86
14.2
>100
>100
SNB-19
30.9
>100
>100
18.20
3.42
–
8.27
>100
>100
SNB-75
N.T.
N.T.
>100
3.25
2.35
4.86
4.53
>100
>100
U251
2.74
>100
>100
11.40
3.06
5.88
4.82
>100
>100
Melanoma
LOX IMV1
0.64
10.637
0.844
57.00
>100
6.04
15.654
0.914
3.04
6.02
4.74
19.894
0.528
>100
>100
MALME-3M
8.32
42.20
>100
17.30
3.83
7.46
8.87
>100
>100
M14
3.75
>100
>100
16.50
5.48
19.30
7.49
>100
>100
MDA-MB-435
3.97
>100
>100
20.20
3.86
7.62
7.01
>100
>100
SK-MEL-2
17.10
50.20
>100
37.70
4.73
10.40
7.36
>100
>100
SK-MEL-28
33.00
>100
>100
6.00
3.21
5.83
>100
>100
>100
SK-MEL-5
7.36
22.50
56.5
1.15
2.61
–
4.10
>100
>100
UACC-257
6.39
65.10
>100
21.20
3.39
6.37
35.1
>100
>100
UACC-62
15.20
88.40
>100
14.80
3.31
6.68
4.38
>100
>100
Ovarian cancer
IGROV1
14.3
13.649
0.658
>100
>100
11.20
17.448
0.820
3.62
7.82
5.94
13.591
0.772
>100
>100
OVCAR-3
4.46
95.1
>100
N.T.
N.T.
N.T.
12.8
>100
>100
OVCAR-4
8.83
21.9
49.30
2.99
2.79
5.68
6.39
>100
>100
OVCAR-5
16.1
66.1
>100
21.50
3.32
6.52
34.5
>100
>100
OVCAR-8
1.49
>100
>100
13.80
3.63
7.76
8.66
>100
>100
NCI/ADR-RES
2.26
25.2
>100
19.30
3.67
7.41
22.5
>100
>100
SK-OV-3
48.1
>100
>100
35.90
5.57
17.20
4.35
>100
>100
Renal cancer
786-O
23.4
10.988
0.817
>100
>100
26.80
12.733
1.124
3.29
6.01
23.5
6.370
1.648
>100
>100
A498
6.75
71.7
>100
8.76
1.78
50.80
2.63
>100
>100
ACHN
4.2
>100
>100
6.44
3.04
5.89
3.14
>100
>100
CAKI-1
7.92
>100
>100
14.80
3.26
6.80
2.52
>100
>100
RXF 393
17.9
63.6
>100
N.T.
N.T.
N.T.
4.55
>100
>100
SN12C
3.24
>100
>100
4.12
2.87
6.16
5.42
>100
>100
TK-10
22.7
68.9
>100
24.60
3.70
6.38
N.T.
N.T.
N.T.
UO-31
1.79
35.3
>100
3.61
2.88
6.48
2.83
>100
>100
Prostate cancer
PC-3
0.606
2.968
3.026
>100
>100
12.50
15.650
0.914
3.82
8.98
5.93
5.360
1.959
>100
>100
DU-145
5.33
>100
>100
18.80
2.94
5.43
4.79
>100
>100
Breast cancer
MCF7
7.73
7.280
1.234
>100
>100
0.44
12.345
1.159
2.60
5.54
5.42
6.813
1.541
>100
>100
MDA-MB-231/ATCC
2.44
13.8
75.1
2.32
2.71
5.70
7.23
>100
>100
HS 578T
20.3
>100
>100
19.30
10.00
46.10
4.77
>100
>100
BT-549
7.55
>100
>100
32.50
9.50
45.50
10.6
>100
>100
T-47D
0.84
2.86
8.96
17.80
3.56
7.19
8.44
>100
>100
MDA-MB-468
4.82
29.4
>100
1.71
3.07
–
4.42
>100
>100
MIDa
8.982
14.306
10.498
4 Conclusion
Because of the anticancer potential demonstrated by the indenoquinolone scaffold, an approach for synthesizing 6-substituted-9-chloro-indenoquinolones was developed, and the inhibition activities of the synthetic compounds on cell viability were evaluated. Based on our biological results, it was envisioned that introduction of piperazinyl groups with a 4-substituted side chain at the 6-position of the indenoquinolone scaffold decreased the cell viability of the breast cancer cell line MCF-7 and renal cancer cell line CAKI-1. Among the synthesized compounds, 12 (with a piperazinyl group) and 13 (with a 2-methylpiperazinyl group) were the most-active compound exhibiting potent inhibitory activity on the cell viability of MCF-7 and CAKI-1 cells. Through a series of promising in vitro experiments, we found that 6-substituted-9-chloro-indenoquinolone derivatives, especially compound 13, not only exhibited preferential growth inhibition effects toward cancer cell lines but also showed the inhibitory effect on topoisomerase I. Based on our results and structure–activity relationships (SARs) studies, compounds 12 and 13 could be potent antibreast cancer candidates and promising lead compounds that warrants further structure optimization.
Acknowledgments
The present study was supported by Ministry of Science and Technology, Taiwan (MOST104-2113-M-038-001), Taipei Medical University (TMUTOP103003-1) and National Defense Medical Center (TMU-NDMC-104-02), respectively. We are grateful to thank NIH-NCI for their supports.
References
- Design, synthesis and antimicrobial evaluation of novel 1-benzyl 2-butyl-4-chloroimidazole embodied 4-azafluorenones via molecular hybridization approach. Rev. Bioorg. Med. Chem. Lett.. 2012;22(24):7475-7480.
- [CrossRef] [Google Scholar]
- DNA repair and resistance to topoisomerase I inhibitors: mechanisms, biomarkers and therapeutic targets. Rev. Curr. Med. Chem.. 2012;19(23):3874-3885.
- [Google Scholar]
- Establishment and characterization of 6-[[2-(dimethylamino)ethyl]amino]-3-hydroxy-7H-indeno[2,1-c]quinolin-7-one dihydrochloride (TAS-103)-resistant cell lines. Rev. Jpn. J. Cancer Res.. 2000;91(5):543-550.
- [Google Scholar]
- 6,8-Dihydroxy-7-methoxy-1-methyl-azafluorenone induces caspase-8- and -9-mediated apoptosis in human cancer cells. Rev. Asian Pac. J. Cancer Prev.. 2013;14(4):2637-2641.
- [Google Scholar]
- Structure-based hybridization, synthesis and biological evaluation of novel tetracyclic heterocyclic azathioxanthone analogues as potential antitumor agents. Rev. Eur. J. Med. Chem.. 2015;103:615-627.
- [CrossRef] [Google Scholar]
- Structure-based design, synthesis and biological evaluation of novel anthra[1,2-d]imidazole-6,11-dione homologues as potential antitumor agents. Rev. Eur. J. Med. Chem.. 2013;69C:278-293.
- [CrossRef] [Google Scholar]
- Cyclopeptides with anti-inflammatory activity from seeds of Annona montana. Rev. J. Nat. Prod.. 2008;71(8):1365-1370.
- [CrossRef] [Google Scholar]
- The alternative medicine pawpaw and its acetogenin constituents suppress tumor angiogenesis via the HIF-1/VEGF pathway. Rev. J. Nat. Prod.. 2010;73(5):956-961.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Rev. J. Immunol. Methods. 1986;89(2):271-277.
- [Google Scholar]
- Promising antitumor activity of a novel quinoline derivative, TAS-103, against fresh clinical specimens of eight types of tumors measured by flow cytometric DNA analysis. Rev. Biol. Pharm. Bull.. 2007;30(10):1923-1929.
- [Google Scholar]
- Synthesis, anticancer activity and effects on cell cycle profile and apoptosis of novel thieno[2,3-d]pyrimidine and thieno[3,2-e] triazolo[4,3-c]pyrimidine derivatives. Rev. Eur. J. Med. Chem.. 2015;90:620-632.
- [CrossRef] [Google Scholar]
- Modulation of camptothecin analogs in the treatment of cancer: a review. Rev. Anticancer Drugs. 2001;12(2):89-105.
- [Google Scholar]
- Azaindenoisoquinolines as topoisomerase I inhibitors and potential anticancer agents: a systematic study of structure-activity relationships. Rev. J. Med. Chem.. 2012;55(4):1682-1697.
- [CrossRef] [Google Scholar]
- Structure-activity relations of azafluorenone and azaanthraquinone as antimicrobial compounds. Rev. Bioorg. Med. Chem. Lett.. 2005;15(4):1079-1082.
- [CrossRef] [Google Scholar]
- Design, synthesis and antiproliferative evaluation of fluorenone analogs with DNA topoisomerase I inhibitory properties. Rev. Bioorg. Med. Chem.. 2013;21:7125-7133.
- [CrossRef] [Google Scholar]
- Paw paw and cancer: annonaceous acetogenins from discovery to commercial products. Rev. J. Nat. Prod.. 2008;71(7):1311-1321.
- [CrossRef] [Google Scholar]
- The NCI anti-cancer drug screen: a smart screen to identify effectors of novel targets. Rev. Anti-Cancer Drug Des.. 1997;12(7):533-541.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Rev. J. Immunol. Methods. 1983;65(1–2):55-63.
- [Google Scholar]
- Isolation and synthesis of antiproliferative eupolauridine alkaloids of Ambavia gerrardii from the Madagascar Dry Forest. Rev. J. Nat. Prod.. 2011;74(5):1169-1174.
- [CrossRef] [Google Scholar]
- Indolizino[1,2-b]quinolines derived from A-D rings of camptothecin: synthesis and DNA interaction. Rev. J. Enzyme Inhib. Med. Chem.. 2003;18(2):101-109.
- [CrossRef] [Google Scholar]
- Chinolinderivate aus Isatinsäure. Review of Journal für Praktische Chemie. 1886;33(1):100.
- [CrossRef] [Google Scholar]
- Chinolinderivate aus Isatinsäure. Review of Journal für Praktische Chemie. 1888;38(1):582-584.
- [CrossRef] [Google Scholar]
- A new azafluorenone from the roots of Polyalthia cerasoides and its biological activity. Rev. Nat. Prod. Commun.. 2010;5(12):1931-1934.
- [Google Scholar]
- Constituents of Oxandra cf. xylopioides with anti-inflammatory activity. Rev. J. Nat. Prod.. 2007;70(5):835-838.
- [CrossRef] [Google Scholar]
- Electrochemical reduction mechanism of camptothecin at glassy carbon electrode. Rev. Bioelectrochem.. 2010;79(2):173-178.
- [CrossRef] [Google Scholar]
- The first series of 4,11-bis[(2-aminoethyl)amino]anthra[2,3-b]furan-5,10-diones: synthesis and anti-proliferative characteristics. Rev. Eur. J. Med. Chem.. 2011;46(1):423-428.
- [CrossRef] [Google Scholar]
- Synthesis and antiproliferative activity of indolizinophthalazine-5,12-dione derivatives, DNA topoisomerase IB inhibitors. Rev. Eur. J. Med. Chem.. 2010;45(9):3938-3942.
- [CrossRef] [Google Scholar]
- A model for the mechanism of human topoisomerase I. Rev. Sci.. 1998;279(5356):1534-1541.
- [Google Scholar]
- Efficient induction of chromosome-type aberrations by topoisomerase II inhibitors closely associated with stabilization of the cleavable complex in cultured fibroblastic cells. Rev. Mutat. Res.. 1995;328(2):151-161. (pii: 0027510795000054)
- [Google Scholar]
- Next generation topoisomerase I inhibitors: rationale and biomarker strategies. Rev. Biochem. Pharmacol.. 2008;75(6):1262-1271.
- [CrossRef] [Google Scholar]
- Synthesis and antiproliferative evaluation of 6-arylindeno[1,2-c]quinoline derivatives. Rev. Bioorg. Med. Chem.. 2009;17(21):7465-7476.
- [CrossRef] [Google Scholar]
- Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. Rev. Bioorg. Med. Chem.. 2008;16(6):3153-3162.
- [CrossRef] [Google Scholar]
- Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. Part 2. Rev. J. Med. Chem.. 2010;53(16):6164-6179.
- [CrossRef] [Google Scholar]
- Discovery of indeno[1,2-c] quinoline derivatives as dual topoisomerases I/II inhibitors: Part 3. Rev. Mol. Divers.. 2013;17(4):781-799.
- [CrossRef] [Google Scholar]
- Camptothecins: a review of their chemotherapeutic potential. Rev. Drugs. 2002;62(14):2039-2057.
- [Google Scholar]
- Antiprotozoal activity of aporphine alkaloids isolated from Unonopsis buchtienii (Annonaceae) Rev. Phytother. Res.. 1999;13(2):175-177.
- [CrossRef] [Google Scholar]
- Doxorubicin and DNA minor groove-binding oligopeptide conjugates as anticancer agents. Rev. Gene. 1994;149(1):63-67.
- [Google Scholar]
- Molecular hybridization of bioactives: synthesis and antitubercular evaluation of novel dibenzofuran embodied homoisoflavonoids via Baylis–Hillman reaction. Rev. Bioorg. Med. Chem. Lett.. 2012;22(24):7426-7430.
- [CrossRef] [Google Scholar]
- A new mechanism of 6-((2-(dimethylamino)ethyl)amino)-3-hydroxy-7H-indeno(2,1-c)quinolin-7-one dihydrochloride (TAS-103) action discovered by target screening with drug-immobilized affinity beads. Rev. Mol. Pharmacol.. 2008;73(3):987-994.
- [CrossRef] [Google Scholar]







