Translate this page into:
Design, synthesis, and evaluation of novel 3-(piperazin-1-yl)propan-2-ol-modified carbazole derivatives targeting the bacterial membrane
⁎Corresponding authors. xiangzhou@gzu.edu.cn (Xiang Zhou), syang@gzu.edu.cn (Song Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
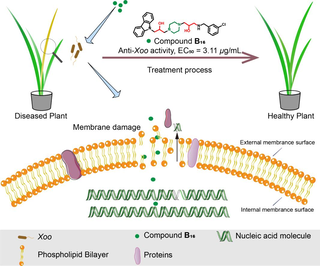
Grain of high yield and quality is needed worldwide due to the needs of a rapidly increasing human population. However, diseases caused by some stubborn types of phytopathogenic bacteria can limit the health and yields of crops. Even worse, conventional commercial bactericides have limited efficacy against such diseases. Therefore, exploring some efficacious bactericidal alternatives is urgently needed. In this work, a new type of 3-(piperazin-1-yl)propan-2-ol modified carbazole derivatives was synthesized and assessed for their bactericidal activity. Among them, compound B16 was the optimal active molecule, giving the EC50 values of 3.11 (Xanthomonas oryzae pv. oryzae), 3.20 (Xanthomonas axonopodis pv. citri) and 3.54 μg/mL (Pseudomonas syringae pv. actinidiae). Pot experiments revealed compound B16 to be able to control rice bacterial leaf blight. Some biochemical assays illustrated that our designed compounds could destroy the integrality of bacterial cell membranes and thereby leading to leaking the intracellular protein. These findings may be regard as a reference for the design of novel membrane-targeting antimicrobial agents for managing stubborn plant bacterial diseases.
Keywords
Carbazole derivatives
Antibacterial activity
Cell membrane
Plant bacterial diseases
1 Introduction
Grain of high yield and quality is needed worldwide due to the needs of a rapidly increasing human population (Zeng et al., 2017; Benchlih et al., 2023; Xiang et al., 2023). However, diseases caused by some stubborn types of phytopathogenic bacteria can limit the health and yields of crops (Yang et al., 2023; Ali et al., 2022; Ma et al., 2022). For instance, Xanthomonas oryzae pv. oryzae (Xoo), Xanthomonas axonopodis pv. citri (Xac), and Pseudomonas syringae pv. actinidiae (Psa) can destroy the plants and products of rice, citrus fruits, and kiwifruit (Qi et al., 2023a; Lu et al., 2023). The application of agrochemicals is considered to be the most effective measure to manage these bacterial diseases in plants (Xiao et al., 2023).
However, long-term use of traditional bactericidal drugs has led grievous resistance, for example, streptomycin sulfate (Lyu et al., 2019). The emergence of resistant phytopathogens means that existing products must be used more effectively and new products must be developed. Notably, targeting the cell membrane of bacteria has become an attractive strategy to kill bacteria (Jin et al., 2020; Naclerio and Sintim, 2020; Cauz et al., 2019).
Products derived from natural sources have been widely used to develop new pesticides and medicines (Ran et al., 2023; Ji et al., 2022). Their diverse structure, abundance resources, and environmental friendliness has enabled the development of pesticides for controlling insects, weeds, and microbes (Hasheminejad et al., 2019; Fang et al., 2022).
An increasing number of naturally available carbazole derivatives (Fig. 1) has been shown to have antibacterial (Ashok et al., 2016), antifungal (Merzouki et al., 2023), antiviral (Yang et al., 2019), and anticancer properties (Issa et al., 2019) in human medicine. However, use of simple carbazole-based derivatives to counteract phytopathogenic bacteria in farming is rare. Numerous studies have revealed the importance of carbazoles targeting the cell membrane, which have promising prospects for the discovery of new antibacterial agents for managing plant diseases caused by bacterial infection (Hurley et al., 2015; Lin et al., 2020). Some carbazole derivatives reported by our research team have been shown to have promising skeletons for the discovery of novel agents to manage diverse bacterial diseases in plants (Huang et al., 2021; Zhao et al., 2019a). Advanced literature and our previous work showed that carbazole core had the ability to target bacterial cell membranes. Hence, carbazoles have attracted researchers’ attention for the discovery of new cell membrane-targeting bactericides. (Lin et al., 2020, Xue et al., 2021; Gerits et al., 2016) Meanwhile, Shaquiquzzaman and colleagues (Shaquiquzzaman et al., 2015) and Vignaroli (Vignaroli et al., 2017) stated that piperazine and isopropanolamine moieties displayed antibacterial (Zeng et al., 2023a), antifungal (Chen et al., 2020), anticancer (Keskin et al., 2016), anti-inflammatory (Jain et al., 2020), and antiviral activities (Aggarwal et al., 2017), as well as being soluble in water. In addition, the piperazine and isopropanolamine moieties were further discovered to potentially disrupt membrane integrity and function by forming a strong binding affinity toward bacterial membranes (Zhou et al., 2023; Zeng et al., 2023a). Thus, engineering new derivatives of carbazoles by introducing piperazine and isopropanolamine moieties may provide new perspectives for discovering novel antibacterial agents.Fig. 2..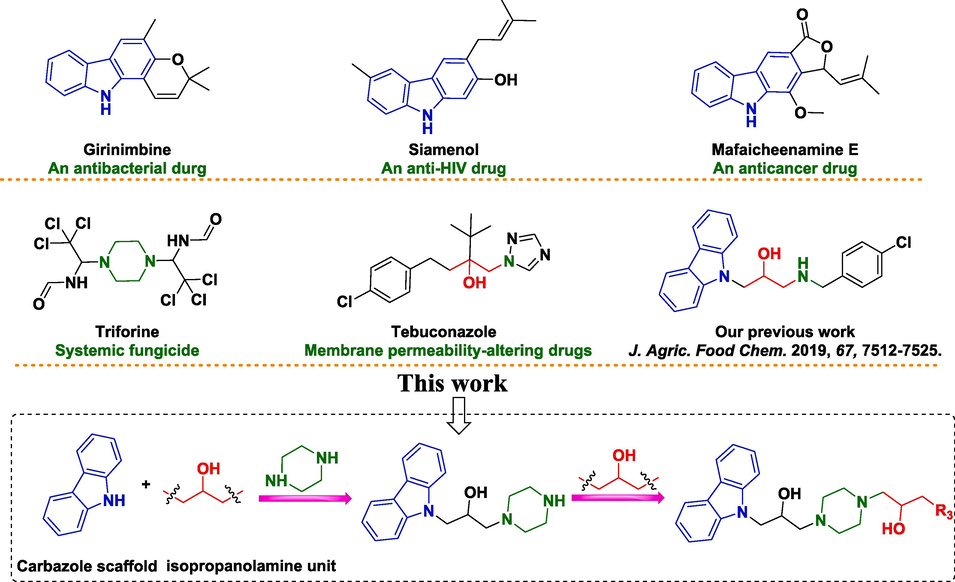
Some carbazole, piperazine, and isopropanolamine structures and the design strategy of title molecules.
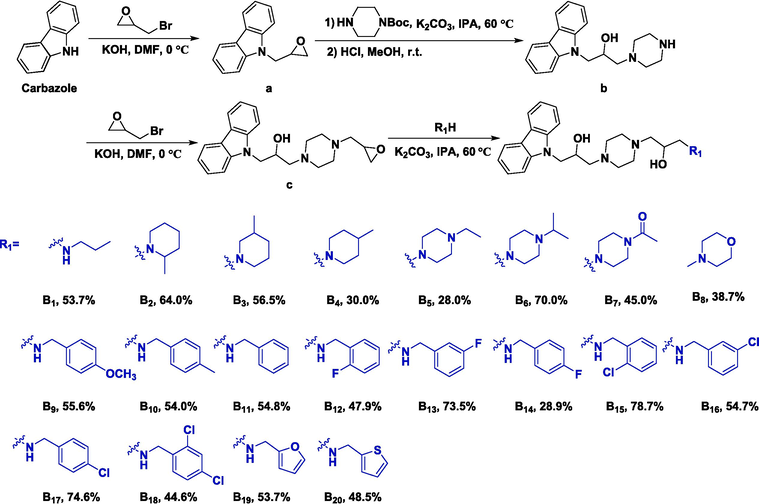
Synthesis procedure for title molecules B1–B20.
In this work, in order to probe more efficacious antibacterial active molecules, novel types of 3-(piperazin-1-yl)propan-2-ol-based carbazoles were elaborately prepared. Furthermore, the in vitro and in vivo antibacterial potency of all title molecules against three intractable pathogenic strains (Xoo, Xac, and Psa) were evaluated via turbidimetric tests and pot experiments. The potential antibacterial mechanisms of designed molecules were studied by using scanning electron microscopy (SEM), electrical conductivity, and fluorescence staining.
2 Materials and methods
2.1 Instruments and chemicals
Nuclear magnetic resonance (NMR) spectroscopy of intermediates and target compounds was done using an ECX 500 system or Biospin AG-400 setup. High-resolution mass spectrometry (HRMS) was done using a Q-Exactive Orbitrap system. Methanol was used as the solvent for liquid chromatography-mass spectrometry.
TEM images were imaged using an FEI Talos F200C electron microscope (FEI) with a voltage of 200 kV. The molecular ζ-potential was assayed by the DelsaNanoC analyzer. The in vitro antibacterial evaluation was expressed as OD595 value, and monitored by using Cytation™ 5 multi-mode readers. Finally, cell membrane permeability experiment was conducted by a DDS-307 electrical conductivity analyzer.
2.2 General experimental procedure
In vitro and in vivo bioassays towards the pathogenic bacteria, as well as cell membrane-permeability studies, and morphological study, were conducted according to methods described in our previous works (Li et al., 2023; Qi et al., 2023b; Shao et al., 2023; Chu et al., 2023; Wang et al., 2022). The wild-type (WT) Xanthomonas oryzae pv. oryzae (Xoo) strain ZJ173 comes from Nanjing Agricultural University which was kindly provided by Ming-Guo Zhou.
2.3 Propidium iodide (PI) staining assay
We wished to evaluate the permeability and integrity of Xoo cells stimulated by our designed molecules (Perrine-Walker and Le, 2020; Rego et al., 2022). PI staining was carried out and compound B16 (half-maximal effective concentration (EC50) = 3.11 μg/mL against Xoo cells) was selected as the treatment molecule. In detail, Xoo cells were cultured at logarithmic phase [optical density at 595 nm (OD595) = 0.6–0.8] and adjusted to 0.2 (OD595). Then, Xoo cells were treated with molecule B16 (0, 6.22, 12.44, or 24.88 μg/mL) for 14 h in a shaker (28 °C, 180 rpm). Furthermore, Xoo cells were collected by centrifugation (8000 × g, 2 min, 4 °C) and resuspended in 10 mM of phosphate-buffered saline (pH = 7.2). Finally, 100 μL of the treated Xoo cultures was stained by PI solution (20 μg/mL, 10 μL) for 20 min, and then the images were collected on a fluorescence spectrophotometer with Olympus BX53 microscope (Olympus, Tokyo, Japan).
2.4 Detection of intracellular protein in Xoo cells
The protein concentration within Xoo cells was verified by SDS-PAGE (Yang et al., 2021). Briefly, samples were treated with compound B16 (0, 0.78, 1.56, 3.11, and 6.22 μg/mL) as described in section 2.3. After incubation for 14 h, treated Xoo cells were collected and lysed via sonication (power: 35 %; ultrasound: 5 s; interval: 5 s) for 30 min on an ice bath. Total intracellular proteins were obtained by centrifugation (10000 rpm, 10 min, room temperature) to remove Xoo cell debris. Furthermore, the protein concentration was measured by the Bradford assay and diluted to 20 times with PBS. Finally, the protein content of Xoo cells in the supernatant of each sample was analyzed by SDS-PAGE.
2.5 Compound B16 interacts with the DNA of Xoo
According to the previously experimental method (Zhao et al., 2019b). DNA of Xoo a was extracted using the TIANamp Bacteria DNA Kit, and the concentration was further measured by a UV–1900 spectrophotometer (Shimadzu Co., Japan) to be a value of 9.38 × 10 -8 mol/L (ξ260 = 6600 L mol−1 cm−1). Furthermore, compound B16 diluted in Tris-HCl buffer solution (50 mM, pH 7.4) to yield a final concentration of 1 × 10-8 mol/L. Following the addition of the different dosages of DNA at room temperature for 20 min. The fluorescence spectra of mixture were recorded using an PTI QuantaMaster 8000 fluorescence spectrophotometer (Horiba Scientific, Canada), equipped with a 1.0 cm quartz cell. Both the excitation and emission slit widths were set to 5 nm, with an excitation wavelength of 363 nm.
2.6 Pathogenicity assay
According to our previous work (Zeng et al., 2023b; Jin et al., 2020), Xoo cells (OD595 = 0.1) were treated with compound B16 (0.78, 1.56, 3.11, and 6.22 μg/mL) for 21 h; an equal volume of DMSO was used as the negative control. All treated Xoo cells were centrifuged and resuspended using sterile water to ensure OD595 = 0.5. Thereafter, the resuspended Xoo solutions were treated on rice leaves using the leaf-cutting method. All treatments were repeated thrice followed by cultivation in a greenhouse. Finally, the lesion length on a rice leaf was measured 14 days after inoculation.
2.7 Phytotoxicity test on rice plant
According to the methods we reported previously (Ding et al., 2023; Su et al., 2023), adult rice leaves were sprayed with a solution of compound B16 (200 or 500 μg/mL) or an equivalent volume of dimethyl sulfoxide. Then, treated rice plants were cultivated in a greenhouse, and photographed 7 days later.
2.8 Statistical analysis.
Each assay was conducted at least three times. Growth curves and bacterial reduction of extracellular results were processed using Origin 2021 ( www.originlab.com/2021). Statistical analyses were conducted using SPSS 20.0 (IBM, Armonk, NY, USA) by one-way ANOVA. Data are the mean ± SD.
2.9 Preparation process of intermediates and target molecules
2.9.1 Synthesis of intermediate a
9H-carbazole (5.00 g, 29.90 mmol) and KOH (2.01 g, 35.88 mmol) were dissolved in 15 mL of anhydrous dimethylformamide (DMF). Then, 2-(bromomethyl)oxirane (4.92 g, 35.88 mmol) was supplemented, and the mixture then allowed to react for 6 h at 0 °C. Upon complete consumption of 9H-carbazole, the mixture was quenched with saturated 40 mL of NH4Cl solution and extracted by ethyl acetate (3 × 50 mL). The organic layer was washed by saturated NH4Cl solution, dried with anhydrous Na2SO4, and concentrated under a vacuum. Finally, intermediate a was further purified by column chromatography on silica gel using petroleum ether and ethyl acetate (180:1, v/v) as the eluent.
2.9.2 Synthesis of intermediate b
Briefly, intermediate a (22.39 mmol), and 22.39 mmol of K2CO3 were dissolved with 30 mL of isopropanol in a 100 mL round-bottomed flask. Then, 22.39 mmol of tert-butyl piperazine-1-carboxylate was supplemented, and the mixture then reacted for 6 h at 60 °C. After intermediate a had been completely consumed, the solvent was evaporated under reduced pressure. Then, the crude residue was dissolved by CH2Cl2, washed with water, dried by anhydrous Na2SO4, and evaporated under a vacuum. Furthermore, the residue was redissolved in 15 mL of MeOH, acidified using concentrated 2 mL of HCl, and stirred at room temperature for an additional 6 h. Upon full conversion, the solvent was removed and the aqueous phase washed by EtOAc, and NaOH solution (3 mol/L) added dropwise until pH 9 − 10 was reached. Finally, the desired crude product was filtered to obtain intermediate b as a yellow oil (4.03 g, yield = 44.0 %).
2.9.3 Synthesis of intermediate c
To a mixture of intermediate b (2.00 g, 6.46 mmol) and KOH (0.36 g, 6.46 mmol) in anhydrous DMF (15 mL) was supplemented with 2-(bromomethyl)oxirane (1.33 g, 9.70 mmol) slowly followed by stirring for 6 h at 0 °C. After that, the mixture was quenched by saturated NH4Cl aqueous solution (15 mL), extracted with EtOAc (3 × 30 mL), dried by anhydrous Na2SO4, and evaporated under reduced pressure. The crude residue was then purified further by column chromatography on silica gel using CH2Cl2 and CH3OH (200:1, v/v) as the eluent to obtain intermediate c as a white solid (1.77 g, yield = 56.4 %).
2.9.4 General synthesis for target compounds B1–B20
Intermediate c (0.30 g, 0.8 mmol), the corresponding amine (1.6 mmol), K2CO3 (0.11 g, 0.8 mmol), and isopropanol (10.0 mL) were supplemented to a 50 mL round-bottomed flask, then stirred at 60 °C until intermediate c was completely consumed. After that, the solvent was evaporated under reduced pressure, the residue was re-dissolved by water, extracted using EtOAc, dried with anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was further purified by column chromatography on silica gel using CH2Cl2 and CH3OH (8:1, v/v) as the eluent to afford the expectant title compounds B1–B20.
1-(4-(3-(9H-carbazol-9-yl)-2-hydroxypropyl)piperazin-1-yl)-3-(propylamino)propan-2-ol (B1) was a yellow oil (187.17 mg; yield = 53.70 %).
The characterization data (NMR and HRMS) for title molecules B2–B20 are described in Supporting Information.
2.9.5 Synthesis of target compounds C1–C7
Intermediate b (0.23 g, 0.7 mmol), an epoxy intermediate (1.6 mmol), K2CO3 (0.15 g, 1.1 mmol), and isopropanol (10.0 mL) were supplemented to a 50 mL oven-dried vial containing a magnetic stirring bar, and stirred for 8 h at 60 °C. When the reaction completed, the mixture was concentrated and further purified by flash chromatography on silica gel (CH2Cl2:CH3OH, 60:1) to afford the target compound C1 (yellow oil, yield = 70.3 %).
The characterization data (NMR and HRMS) for the other intermediates and target compounds are described in Supporting Information.
3 Results and discussion
3.1 In vitro bioassay of target compounds and the structure–activity relationship (SAR) analyses
Two series of carbazoles were evaluated for promising antibacterial activity with the turbidimeter test (Table 1). Positive agents were bismerthiazol (BT) and thiodiazole copper (TC) (Qi et al., 2023a; Chu et al., 2023).
Compd.
Xoo
Xac
Psa
regression equation
EC50 (μg/mL)a
regression equation
EC50 (μg/mL)
regression equation
EC50 (μg/mL)
B1
y = 6.4684x − 1.2716
9.07 ± 0.22
y = 5.1174x – 1.9327
3.98 ± 0.11
y = 3.3593x + 1.0391
15.10 ± 0.34
B2
y = 5.1792x − 0.1507
9.87 ± 0.11
y = 2.6532x + 2.4468
9.17 ± 0.23
y = 3.7600x − 0.9196
37.53 ± 1.16
B3
y = 4.3257x + 1.1101
7.93 ± 0.47
y = 2.791x + 3.1034
4.78 ± 0.33
>100
B4
y = 5.2382x − 0.5245
11.34 ± 0.32
y = 4.5397x + 0.9996
7.61 ± 0.63
>100
B5
y = 5.708x − 2.6925
22.26 ± 0.72
y = 2.199x + 3.2149
6.48 ± 0.52
y = 7.0387x − 6.6301
44.95 ± 2.81
B6
y = 5.7740x + 3.016
24.45 ± 0.97
y = 2.4149x + 3.0267
6.56 ± 0.89
y = 12.229x + 16.841
61.09 ± 2.37
B7
y = 11.373x − 12.83
36.96 ± 0.36
y = 2.0158x + 2.1358
26.36 ± 1.38
> 100
B8
> 100
y = 2.0639x + 2.5883
14.74 ± 2.11
> 100
B9
y = 4.930x + 1.9856
4.09 ± 0.06
y = 6.5838x + 0.5134
4.80 ± 0.17
> 100
B10
y = 10.070x − 0.9312
3.88 ± 0.01
y = 4.8744x − 3.0000
2.57 ± 0.14
y = 4.0071x + 1.7434
6.50 ± 0.36
B11
y = 11.878x − 2.2635
4.09 ± 0.10
y = 3.0314x + 3.5464
3.02 ± 0.50
y = 0.6553x + 4.5236
5.33 ± 0.59
B12
y = 5.2708x − 1.7229
4.19 ± 0.08
y = 2.5331x + 3.4275
4.18 ± 0.11
y = 3.1672x + 2.4055
6.59 ± 0.06
B13
y = 13.461x − 4.0312
4.69 ± 0.04
y = 3.3087x + 2.9040
4.30 ± 0.31
y = 5.3444x − 0.4114
10.29 ± 0.08
B14
y = 4.6096x + 0.5946
9.03 ± 0.66
y = 2.1058x + 3.0381
8.54 ± 1.88
y = 11.611x − 8.7659
15.33 ± 3.12
B15
y = 15.213x − 7.2434
6.38 ± 0.12
y = 2.9666x + 3.1965
4.05 ± 0.22
y = 5.0128x + 0.0757
9.60 ± 1.07
B16
y = 7.934x + 1.0892
3.11 ± 0.03
y = 2.9281x − 3.5221
3.20 ± 0.08
y = 4.1671x + 2.7119
3.54 ± 0.13
B17
y = 4.3875x + 2.7148
3.32 ± 0.10
y = 5.1203x + 1.459
4.92 ± 0.34
y = 10.202x − 2.6750
5.65 ± 0.84
B18
y = 5.7740x + 3.016
24.45 ± 0.97
y = 4.8225x + 0.3799
9.08 ± 0.55
> 100
B19
y = 11.747x − 1.7541
3.73 ± 0.10
y = 5.3088x + 2.0127
3.65 ± 0.35
y = 1.1340x + 4.1045
6.16 ± 0.70
B20
y = 4.5137x + 2.032
4.55 ± 0.05
y = 4.2307x + 1.8757
5.48 ± 0.19
y = 2.6709x + 2.2109
11.07 ± 0.89
BTb
y = 3.6885x − 0.6944
34.98 ± 1.46
y = 3.302x − 1.1895
74.90 ± 3.21
> 100
TCb
y = 3.6545x − 1.9426
79.39 ± 3.36
y = 2.822x + 0.0152
58.40 ± 4.8
> 100
Antibacterial tests revealed the excellent activity of compounds B1–B20 against all tested bacterial strains. Interestingly, compound B16 had a prominent effect against Xoo, Xac, and Psa, with EC50 values of 3.11, 3.20, and 3.54 μg/mL, respectively. By contrast, intermediate c (with substituted piperidine) had different bioactivity depending on the substituent. If the substituent − CH3 was located on the piperidine ring, the activity of compounds B2–B4 against Xoo was in the order 3 − CH3 (7.93 μg/mL) > 2 − CH3 (9.87 μg/mL) > 4 − CH3 (11.34 μg/mL). If the substituents − CH2CH3, −CH(CH3)2, or − COCH3 were located on the piperazine ring, the anti-Xoo ability of molecules B5–B7 decreased significantly compared with that of compound B16, with EC50 values ranging from 22.26 μg/mL to 36.96 μg/mL. However, compound B8 with the substituent morpholine had no activity against Xoo. Target compounds with benzylamines substituents displayed excellent activity against Xoo, with EC50 values ranging from 3.11 μg/mL to 24.45 μg/mL. Among them, the substituents containing halogenated atoms had prominent activity against Xoo. In particular, compounds substituted with chlorine atoms (as well as their substituted positions and number) had prominent activity against Xoo in the order compound B16 (3 − Cl, 3.11 μg/mL) > B17 (4 − Cl, 3.32 μg/mL) > B15 (2 − Cl, 6.38 μg/mL). The activity of molecule B18 (EC50 = 24.45 μg/mL) toward Xoo was slightly higher than that of BT (EC50 = 34.98 μg/mL). For molecules substituted with fluorine atoms, the activity against Xoo was in the order B12 (2 − F, 4.19 μg/mL) > B13 (3 − F, 4.69 μg/mL) > B14 (4 − F, 9.03 μg/mL). For activity toward Xoo, a similar tendency was discovered to that towards Xac and Psa in vitro. With respect to activity against Xac, compared with control BT (74.90 μg/mL) and TC (58.40 μg/mL), molecules B1–B20 possessed higher antibacterial ability, with EC50 values ranging from 2.57 μg/mL to 26.36 μg/mL. Compared with molecule B16, the 4-OCH3-benzyl moiety-containing compound B10 and benzyl moiety-containing molecule B11 given EC50 values of 2.57 μg/mL and 3.02 μg/mL against Xac, and exhibited excellent activity. With regard to Psa, the antibacterial activity of target compounds was laudable, and was superior to controls TC (>100 μg/mL) and BT (>100 μg/mL).
Molecule B1 (with substituted propylamino group) was the optimal active molecule against the three types of pathogenic bacteria. For compounds B19 and B20, similar antibacterial ability against Xoo, Xac, and Psa was observed. Analyses of the bioactivity results of target compounds B1–B20 against the three types of pathogenic bacteria revealed three main observations. First, the contribution of substituted piperazine and piperidine towards bioactivity was inferior to that of benzylated compounds. Second, if a benzylamine moiety was introduced into the carbazole containing-isopropanolamine, then the antibacterial activity of the title molecules towards the three types of pathogenic bacteria was increased. Third, compounds B19 and B20 (which contained thiophene or furan substituents) had strong activity against Xoo (EC50 = 3.73 and 4.55 μg/mL), Xac (EC50 = 3.65 and 5.48 μg/mL) and Psa (EC50 = 6.16 and 11.07 μg/mL). All title molecules had significant inhibitory effects towards Xoo and Xac. Compound B16 was most active compound against Xoo (EC50 = 3.11 μg/mL) and Psa (EC50 = 3.54 μg/mL). Compound B10 (4-CH3-benzyl) was the most active compound in terms of anti-Xac activity.
In order to probe the effect of benzylamine groups on antibacterial ability, a new series of carbazole derivatives decorated with 3-phenoxypropan-2-ol (C1–C7) was designed and synthesized (Fig. 3). The in vitro bioassay (Table 2) displayed that the phenoxy-substituted compounds C1–C7 exhibited comparative activity against Xoo and Xac to that of the benzylamine moiety-containing compounds B9–B20 to afford EC50 values of 3–20 μg/mL, but showed reduced potency against Psa. Compound C7 provided optimal EC50 values against Xoo (EC50 = 3.79 μg/mL), and compound C4 afforded optimal EC50 values toward Xac (EC50 = 4.59 μg/mL), respectively. The position of the substituent on the benzene ring had a significant distinction on antibacterial activity. For instance, the anti-Xoo potency of Br atom-containing molecules was in the order para- (C7: 4-bromophenoxy, 3.79 μg/mL) > meta- (C6: 3-bromophenoxy, 4.58 μg/mL) > ortho- (C5: 2-bromophenoxy, 8.17 μg/mL), and had the same trend as that for activity against Xac. Taken together, these findings suggested that a 3-phenoxypropan-2-ol moiety could exert antibacterial activity (comparable activity towards Xoo and Xac, but lower activity toward Psa) when compared with that of benzylamine moiety-modified compounds. A benzylamine moiety was vital for exerting broad-spectrum antibacterial activity.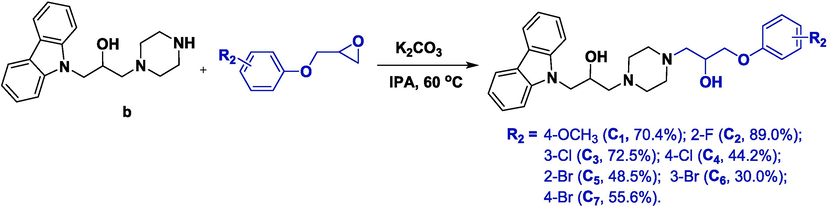
Synthetic procedure for compounds C1–C7.
Compd.
Xoo
Xac
Psa
regression equation
EC50 (μg/mL)a
regression equation
EC50 (μg/mL)
regression equation
EC50 (μg/mL)
C1
y = 30.069x − 34.855
21.16 ± 0.80
y = 2.852x + 2.1975
9.61 ± 0.51
y = 1.3476x + 3.3969
15.47 ± 2.69
C2
y = 4.6781x + 0.4882
9.21 ± 0.37
y = 3.3399x + 2.1751
7.01 ± 1.62
y = 4.3552x − 0.5733
19.04 ± 0.45
C3
y = 10.637x − 6.7433
12.71 ± 0.32
y = 3.2233x + 2.4078
6.37 ± 0.52
> 100
C4
y = 5.1188x + 1.6498
4.51 ± 0.43
y = 4.6822x + 1.9018
4.59 ± 0.28
y = 4.1861x − 0.2995
18.45 ± 0.81
C5
y = 2.7181x + 2.5200
8.17 ± 0.29
y = 1.2677x + 3.2564
23.74 ± 3.05
y = 1.4877x + 3.0753
19.67 ± 5.16
C6
y = 4.2917x + 2.1641
4.58 ± 0.12
y = 3.9341x + 1.6069
7.29 ± 0.82
> 100
C7
y = 5.0397x + 2.0861
3.79 ± 0.17
y = 3.8456x + 1.7646
6.94 ± 0.43
y = 2.8317x − 0.3232
75.33 ± 10.48
BTb
y = 3.6885x − 0.6944
34.98 ± 1.46
y = 3.302x − 1.1895
74.90 ± 3.21
> 100
TCb
y = 3.6545x − 1.9426
79.39 ± 3.36
y = 2.822x + 0.0152
58.40 ± 4.8
> 100
Giving the above-mentioned results, the SAR study was investigated systematically. First, when R1 was N-containing heterocyclic (such as piperazine) or benzylamine groups, the contribution of substituted benzylamine towards bioactivity was superior to substituted piperazine and piperidine moieties. Furthermore, introduction of halogen atom on the benzylamine groups could slightly increase the anti-Xoo activity: compound B16 (3 − Cl, 3.11 μg/mL), B17 (4 − Cl, 3.32 μg/mL) > B11 (H, 4.09 μg/mL) and B9 (4-OCH3, 4.09 μg/mL). Secondly, when R1 was replaced as phenols groups, their bioactivities towards Xoo and Xac were comparable to the corresponding benzylamine-substituted derivatives, but their anti-Psa activity was dramatically decreased. By the way, the electron-withdrawing substituent on the benzene ring had a significant enhancement on antibacterial activity. For instance, the anti-Xoo potency of molecules was in the order (C4: 4-chlorine, 4.51 μg/mL), (C6: 3-bromo, 4.58 μg/mL) and (C7: 3-bromo, 3.79 μg/mL) > (C1: 2-bromophenoxy, 21.16 μg/mL). Overall, compound B16 exhibited the optimal bioactivities, especially against Xoo (EC50 = 3.11 μg/mL), and Psa (EC50 = 3.54 μg/mL), and was thus selected to evaluate its in vivo bioactivity.
3.2 In vivo effectiveness against rice bacterial leaf blight and kiwifruit bacterial canker
Taking account of the results of in vitro bioassays, compound B16 (with the lowest EC50 values of 3.11 and 3.54 μg/mL against Xoo and Psa, respectively) was chosen to assess the inhibitory effect for managing RBLB and BCK (Zhang et al., 2023).
Bioassay results (Table 3, Table 4, and Fig. 4) showed that compound B16 had activity against rice BLB at 200 μg/mL. It could protect 35.12 % of samples and cure 38.56 % of samples, and was more efficacious than the commercial TC (19.3 % and 24.56 %, respectively). Compound B16 possessed excellent protective activity against rice BCK at 200 μg/mL (Fig. 5). It could protect 67.61 % of samples [superior to that of TC (42.53 %)] and could cure 45.54 % of samples [superior to that of TC (48.32 %)]. These results suggested that compound B16 could be used to manage bacterial diseases of plants. aStatistical analysis was conducted by ANOVA under the condition of equal variances assumed (P > 0.05) and equal variances not assumed (P < 0.05). Different uppercase letters indicated the control efficiencies with a significant difference among different treatment groups at P < 0.05.
Treatment
Curative activity
Protective activity
Morbidity (%)
Disease index (%)
Control efficiency (%)b
Morbidity (%)
Disease index (%)
Control efficiency (%)b
B16
100
51.85
38.56 a
100
53.33
35.12 a
TC
100
68.15
19.30c
100
63.70
24.56b
CKa
100
84.40
100
84.40
Treatment
Curative efficiency (%)
Protective efficiency (%)
B16
45.54 ± 6.35 a
67.61 ± 3.73 a
TC
48.32 ± 1.83 a
42.53 ± 4.37 a
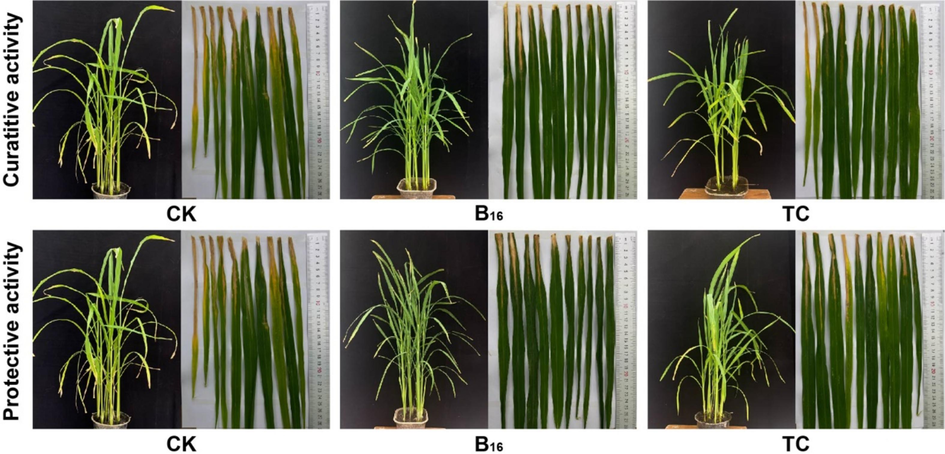
In vivo bioassay of compound B16 towards rice bacterial leaf blight at 200 μg/mL.
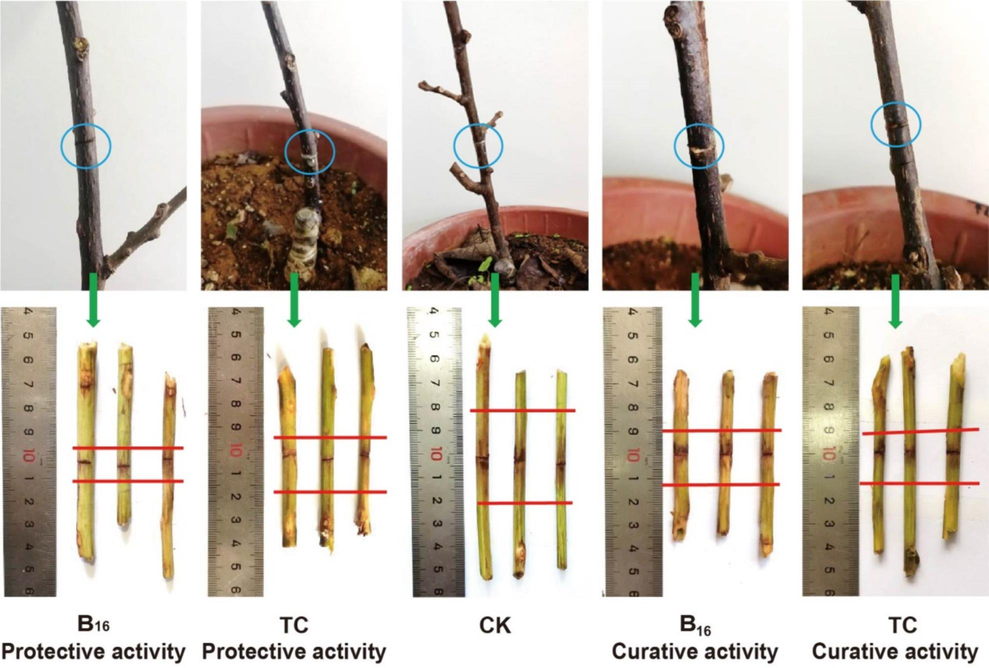
The inhibitory effect of compound B16 and TC toward kiwifruit bacterial canker under greenhouse conditions at 200 μg/mL.
3.3 Antibacterial mechanism study
3.3.1 Bacterial growth affected by compound B16
We wished to explore the mechanism of action of molecule B16 against Xoo cells. Growth curves were created using compound B16 at 0, 0.78, 1.56, 3.11, 6.22, and 12.44 μg/mL. The growth rate was influenced slightly by compound B16 at doses of 0.78 and 1.56 μg/mL when compared with that of the control sample (Fig. 6). Notably, the growth of Xoo cells was obviously inhibited under a compound-B16 concentration of 3.11 μg/mL. In particular, the growth of Xoo cells was inhibited completely upon incubation with compound B16 at doses of 6.22 and 12.44 μg/mL. These outcomes suggested that compound B16 possessed a potent inhibitory effect on the growth of Xoo cells.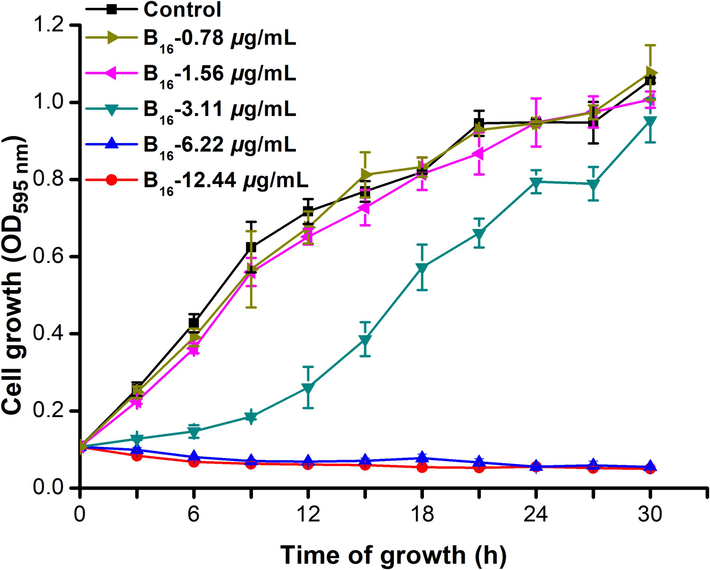
Growth curve of Xoo treated with molecule B16 at 0, 0.78, 1.56, 3.11, 6.22 and 12.44 μg/mL, respectively.
3.3.2 Morphological study
The morphologic changes of Xoo cells elicited by treatment with molecule B16 was studied further by SEM using a Nova NanoSEM 450 system (FEI, Hillsboro, OR, USA) (Xie et al., 2023). The surface of control Xoo cells possessed an integrated bacterial surface (Fig. 7). Some Xoo cells treated with compound B16 (6.22 μg/mL) appeared to have a slightly shrunken and distorted cell surface. However, more serious damage to Xoo cells was observed at a dose of 12.44 μg/mL (Fig. 7c): malformed, distorted, cracked, and leaking holes appeared on the surface of most Xoo cells. Hence, compound B16 had a negative impact on the morphology of Xoo cell membranes.
SEM results of Xoo cells after incubating with molecule B16 at 0 μg/mL (a), 6.22 μg/mL (b) and 12.44 μg/mL (c), respectively. Scale bars: 2 μm.
3.3.3 Membrane permeability assay
A membrane-permeability assay was undertaken according to our previously reported methods to verify the functions of Xoo cell membranes (Zeng et al., 2023b; Kong et al., 2008; Zhang et al., 2020). Conductivity is increased with changes in the membrane permeability of bacteria. The relative conductivity of a Xoo-cell suspension (Fig. 8) increased rapidly after incubation with compound B16 (6.22 and 12.44 μg/mL), especially 1 h after treatment, when the relative conductivity reached 30.82 % and 59.78 %, respectively, much higher than that of the control (20.55 %). The relative conductivity increased persistently during the next 5 h at a compound-B16 concentration of 12.44 μg/mL. These data indicated that molecule B16 could affect the permeability of the Xoo’s membranes significantly, which was in accordance with the morphology data.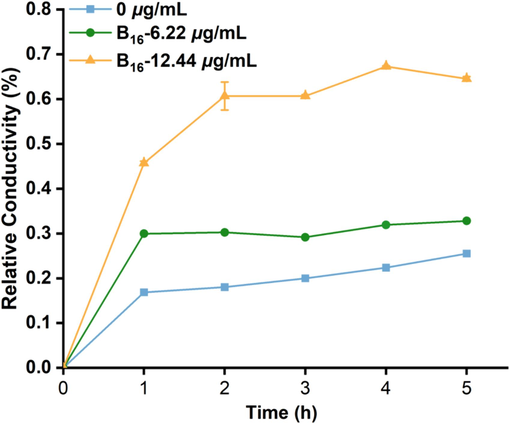
Relative conductivity of Xoo treated with B16 at different concentrations of 0, 6.22 and 12.44 μg/mL, respectively.
3.3.4 PI staining experiment
The dye PI can emit red fluorescence from intercalation with DNA in membrane-damaged cells. PI is used widely to detect cell-membrane permeability and cell viability (Gan et al., 2020; Bownik et al., 2022). The effect on the permeability of Xoo cell membranes was observed through measurement of fluorescence intensity with different doses of compound B16 (Fig. 9). Compared with healthy cells (Fig. 9a), gradually enhanced red fluorescence (Fig. 9b–d) was observed when cells were treated with compound B16. In particular, cells emitted low fluorescence intensity at compound-B16 doses of 12.44 and 24.88 μg/mL. These data demonstrated that target compound B16 could negatively impact the membrane permeability of, and lead to metabolic dysfunction in, Xoo cells. These could be key factors for designing molecules with outstanding antibacterial potency.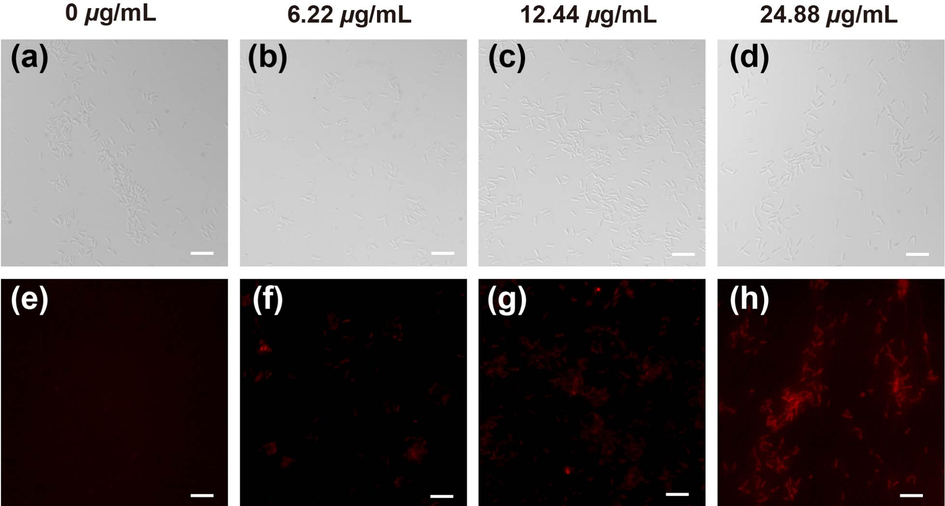
Morphology of Xoo cells staining by PI after incubated with B16 at the dosages of 0, 6.22, 12.44 and 24.88 μg/mL, respectively.
3.4 Determination of intracellular protein content
The total protein concentration in Xoo cells was measured by the Bradford assay to evaluate the damage wrought by compound B16 on bacterial cell membranes (Zhang et al., 2022; kalaivani et al., 2021). The intracellular protein content of Xoo cells decreased in a dose-dependent manner with increasing doses of compound B16 (Fig. 10). In particular, the protein content in Xoo cell was reduced significantly at a compound-B16 dose of 3.11 μg/mL; the protein content was < 500 μg/mL, which was much lower than that in control cells (>7000 μg/mL). To further investigate the effect of compound B16 on bacterial DNA, the fluorescence titration experiment (Deng et al., 2021; Zhao et al., 2019b) was carried out. The potential interaction between total DNA extracted from Xoo and compound B16 was displayed in Fig. S1. The results indicated that the fluorescence intensity of compound B16 significantly decreased after the addition of DNA in a dosage-dependent manner. Suggesting that the structure of B16 may be encapsulated in the grooves of the DNA helix, thereby reducing the fluorescence intensity of the carbazole structure.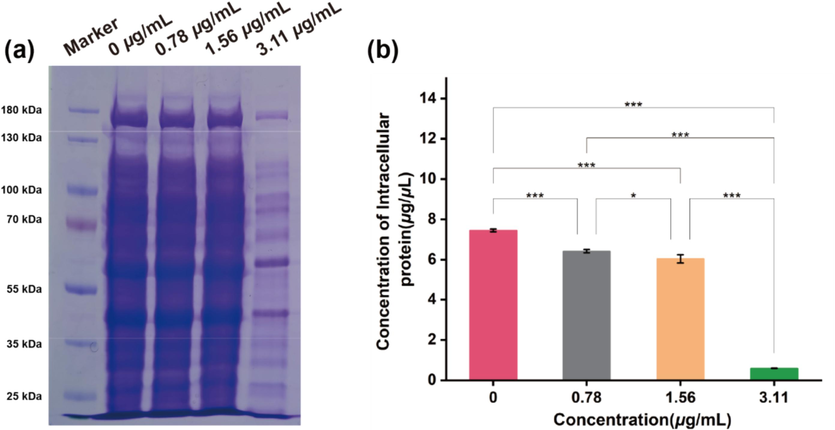
The intracellular protein content of Xoo after incubated with B16 for 14 h at the dosages of 0, 0.78, 1.56 and 3.11 μg/mL, respectively. a) Intracellular proteins content in Xoo cell via SDS-PAGE method staining by coomassie blue. b) Corresponding intracellular protein concentration in Xoo cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The above-mentioned results indicated that our designed compound B16 affected the cell membrane function by increasing the permeability of bacterial cell membranes as well as bounding to DNA and further reducing the protein expression. These findings further support the potential of B16 as a promising lead compound for the development of new bactericides.
3.5 Pathogenicity assays of Xoo on rice
A pathogenicity test was carried out to ascertain the effect of compound B16 on the pathogenicity of Xoo against rice plants (Zeng et al., 2023a; Zhang et al., 2023). The lesion length on rice leaves of the control sample was 10.12 cm at 14 days after inoculation (Fig. 11a). Obvious suppression of leaf lesions was observed upon treatment with compound B16 at > 1.56 μg/mL. Hence, compound B16 could suppress the pathogenicity of phytopathogenic bacteria.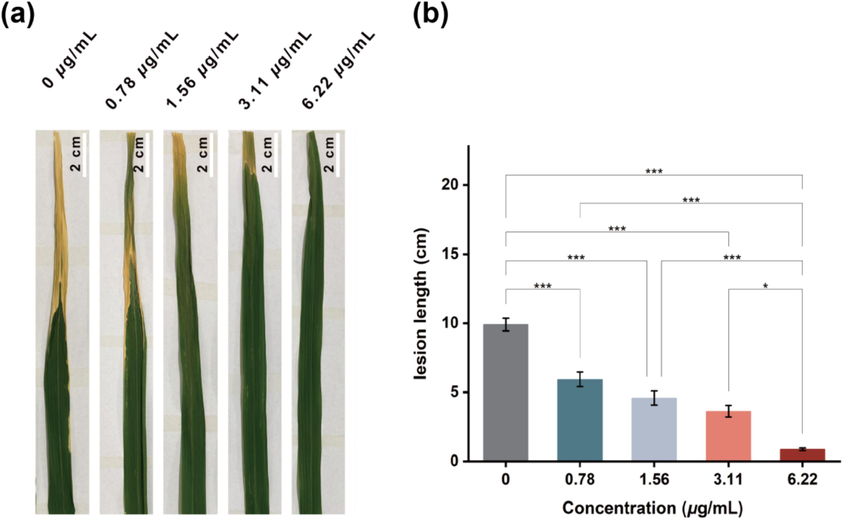
Pathogenicity analysis triggered by B16 at the dosages of 0, 0.78, 1.56, 3.11 and 6.22 μg/mL on rice plants for 14 days.
3.6 The phytotoxicity and in silico ‘drug-likeness’ evaluation
Finally, the pharmaceutical development of the title compound was assessed, which is the most important step for drug development (Zhou et al., 2022). Toxicity and pharmacokinetic data were used to evaluate if compound B16 could be a lead compound. Phytotoxicity was evaluated on rice plants: after 7 days of incubation: compound B16 did not show toxicity towards rice plants (Fig. S2). Furthermore, ADMETlab 2.0 (https://admetmesh.scbdd.com/) was employed to ascertain the absorption, distribution, metabolism, excretion, and toxicity (ADMET) and drug-like properties of compound B16 (Xiong et al., 2021). The physicochemical, ADMET, and drug-like properties of compound B16 are displayed in Fig. 12 and Table S2. Interestingly, molecule B16 showed acceptable physicochemical, ADMET, and pharmaceutical properties. Especially, compound B16 had great potential drug-likeness properties, including the Lipinski Rule and Golden Triangle Rule. In summary, our results suggested the excellent pharmacokinetic characteristics and low phytotoxicity of compound B16.Fig. 13..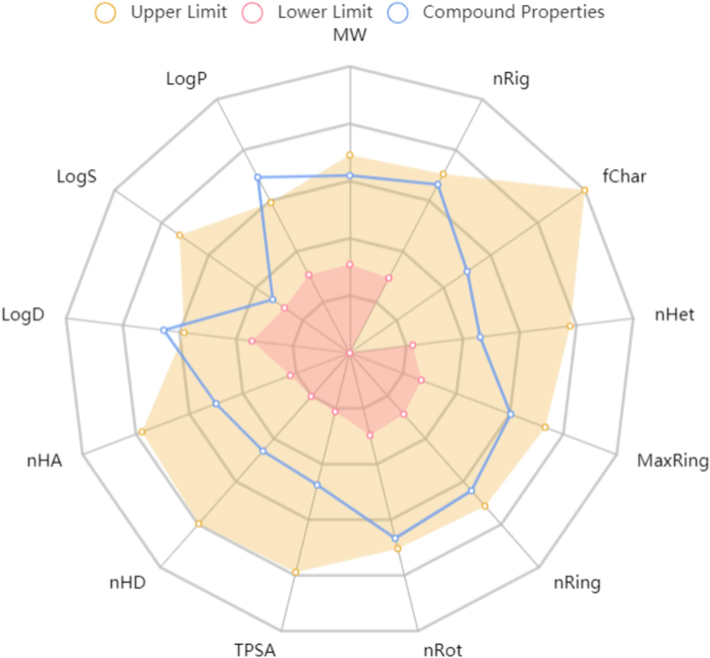
Physicochemical properties of compound B16.
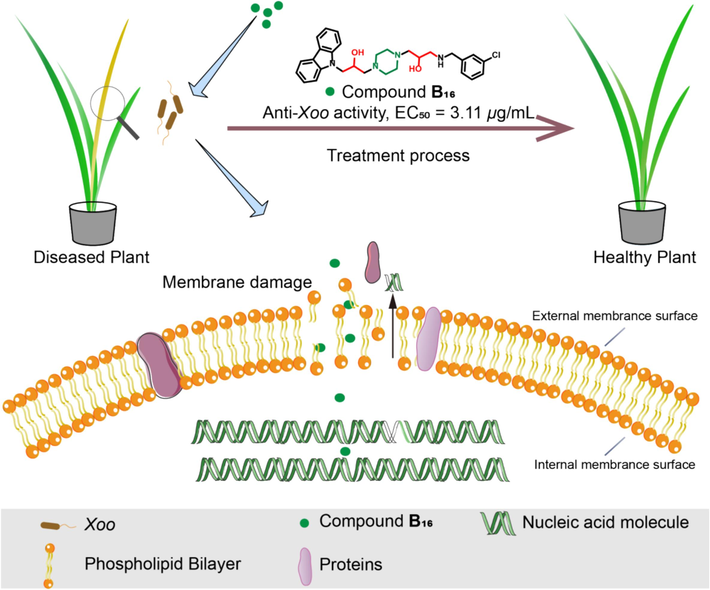
The hypothesized mechanism of action of compound B16.
4 Conclusion
A series of 3-(piperazin-1-yl)propan-2-ol-decorated carbazole analogs was synthesized and their antimicrobial ability were evaluated. Bioassay results suggested that the majority of title molecules showed outstanding bactericidal ability towards Xoo, Xac, and Psa, with the optimal EC50 values of 3.11 (B16), 2.57 (B10), and 3.54 (B16) μg/mL, respectively, which were superior to commercial BT (30.43, 79.36, and > 100 μg/mL) and TC (82.64, 62.71, and > 100 μg/mL). Furthermore, the SAR analyses indicated that electron-withdrawing group-substituted benzyl amine moieties and the amide groups of compounds B1–B20 were the crucial active cores for their outstanding antibacterial effects. In vivo bioassays suggested that compound B16 could control RBLB and protect against BCK. SEM as well as experiments on PI staining and the permeability of cell membranes indicated that compound B16 had a negative impact on the physiological function and morphology of bacterial cell membranes, changed their permeability, and led to the leakage of intracellular substances, which may be key factors for bacterial death. Additionally, a binding experiment demonstrated that compound B16 could bind to bacterial DNA, suggesting a potential mechanism by which the compound might inhibit protein synthesis.
This evidence collectively supports the conclusion that compound B16 could be regarded as a promising lead compound for discovering new bactericides because it targets bacterial cell membranes and potentially interferes with protein synthesis to treat phytopathogenic bacterial infections.
CRediT authorship contribution statement
Si-Yue Ma: Formal analysis, Investigation, Methodology, Writing – original draft. Ying-Guo Ding: Formal analysis, Investigation, Methodology. Xin-Xin Tuo: Formal analysis, Investigation. Guo-Qing Wang: Investigation. Hong-Wu Liu: Formal analysis, Investigation. Jiao Meng: Formal analysis, Investigation. Tai-Hong Zhang: Investigation, Validation. Li-Wei Liu: Formal analysis, Investigation. Pu-Ying Qi: Investigation. Xiang Zhou: Funding acquisition, Investigation, Writing – review & editing. Song Yang: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (32372610, U23A20201, 32160661, 32202359), National Key Research and Development Program of China (2022YFD1700300), and GZU (Guizhou University) Found for Newly Enrolled Talent (No. 202229).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of antiviral activity of piperazine against chikungunya virus targeting hydrophobic pocket of alphavirus capsid protein. Antivir. Res.. 2017;146:102-111.
- [CrossRef] [Google Scholar]
- Advances, limitations, and prospects of biosensing technology for detecting phytopathogenic bacteria. Chemosphere. 2022;296:133773
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis and biological evaluation of carbazole-based Chalcones, Aurones and Flavones. Med. Chem. Res.. 2016;25:909-922.
- [CrossRef] [Google Scholar]
- Modes of action of biocontrol agents and elicitors for sustainable protection against Bacterial Canker of tomato. Microorganisms. 2023;11:726.
- [CrossRef] [Google Scholar]
- Behavioral disturbances induced by cyanobacterial oligopeptides microginin-FR1, anabaenopeptin-A and microcystin-LR are associated with neuromotoric and cytotoxic changes in Brachionus Calyciflorus. J. Hazard. Mater.. 2022;438:129472
- [CrossRef] [Google Scholar]
- Violacein targets the cytoplasmic membrane of bacteria. Acs. Infect. Dis.. 2019;5:539-549.
- [CrossRef] [Google Scholar]
- Pharmacokinetics and pharmacodynamics of posaconazole. Drugs. 2020;80:671-695.
- [CrossRef] [Google Scholar]
- Novel benzothiazole derivatives as potential anti-quorum sensing agents for managing plant bacterial diseases: Synthesis, antibacterial activity assessment, and SAR Study. J. Agr. Food. Chem.. 2023;71:6525-6540.
- [CrossRef] [Google Scholar]
- Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT. 2021;150:112018
- [CrossRef] [Google Scholar]
- Discovery of novel 3-(piperazin-1-yl)propan-2-ol decorated carbazole derivatives as new membrane-targeting antibacterial agents. Arab. J. Chem.. 2023;16:104991
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of amide derivatives derived from natural product Waltherione f. Med. Chem. Res.. 2022;31:485-496.
- [CrossRef] [Google Scholar]
- Synergistic effect of propidium iodide and small molecule antibiotics with the antimicrobial peptide dendrimer G3KL against gram-negative bacteria. Molecules. 2020;25:5643.
- [CrossRef] [Google Scholar]
- Elucidation of the Mode of Action of a New Antibacterial Compound Active against Staphylococcus aureus and Pseudomonas aeruginosa. PLOS ONE. 2016;11(5):e0155139.
- [Google Scholar]
- Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food. Chem.. 2019;275:113-122.
- [CrossRef] [Google Scholar]
- Rational optimization of 1,2,3-triazole-tailored carbazoles as prospective antibacterial alternatives with significant in vivo control efficiency and unique mode of action. J. Agr. Food. Chem.. 2021;69:4615-4627.
- [CrossRef] [Google Scholar]
- Membrane-targeting DCAP analogues with broad-spectrum antibiotic activity against pathogenic bacteria. Acs. Med. Chem. Lett.. 2015;6:466-471.
- [CrossRef] [Google Scholar]
- Carbazole scaffolds in cancer therapy: A review from 2012 to 2018. J. Enzym. Inhib. Med. Ch.. 2019;34:1321-1346.
- [CrossRef] [Google Scholar]
- Piperazine: A promising scaffold with analgesic and anti-inflammatory potential. Drug. Res.. 2020;71:62-72.
- [CrossRef] [Google Scholar]
- 1,3,4-oxadiazole derivatives as plant activators for controlling plant viral diseases: Preparation and assessment of the effect of Auxiliaries. J. Agr. Food. Chem.. 2022;70:7929-7940.
- [CrossRef] [Google Scholar]
- Antibacterial activity and rice-induced resistance, mediated by C15surfactin A, in controlling rice disease caused by Xanthomonas oryzae pv. oryzae. Pestic. Biochem. Phys.. 2020;169:104669
- [CrossRef] [Google Scholar]
- Seed treatment and foliar application of methyl salicylate (MeSA) as a defense mechanism in rice plants against the pathogenic bacterium, Xanthomonas oryzae pv. oryzae. Pestic. Biochem. Phys.. 2021;171:104718
- [CrossRef] [Google Scholar]
- Dasatinib for the treatment of chronic myeloid leukemia: Patient selection and special considerations. Drug. Des. Dev. Ther.. 2016;10:3355-3361.
- [CrossRef] [Google Scholar]
- Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. Coli. Colloid. Surface. b.. 2008;65:197-202.
- [CrossRef] [Google Scholar]
- Insights into a class of natural eugenol and its optimized derivatives as potential tobacco mosaic virus helicase inhibitors by structure-based Virtual Screening. Int. J. Biol. Macromol.. 2023;248:125892
- [CrossRef] [Google Scholar]
- Development of highly potent carbazole amphiphiles as membrane-targeting antimicrobials for treating gram-positive bacterial infections. J. Med. Chem.. 2020;63:9284-9299.
- [CrossRef] [Google Scholar]
- Immune Mechanism of Ethylicin-Induced Resistance to Xanthomonas oryzae pv. oryzae in Rice. J. Agr. Food. Chem.. 2023;71:288-299.
- [CrossRef] [Google Scholar]
- Variation in Streptomycin Resistance Mechanisms in Clavibacter michiganensis. Phytopathology. 2019;109:1849-1858.
- [CrossRef] [Google Scholar]
- Drug repurposing strategy part 1: From approved drugs to agri-bactericides leads. J. Antibiot.. 2022;76:27-51.
- [CrossRef] [Google Scholar]
- Eco-friendly synthesis, characterization, in-silico ADMET and molecular docking analysis of novel carbazole derivatives as antibacterial and antifungal agents. J. Mol. Struct.. 2023;1271:133966
- [CrossRef] [Google Scholar]
- Multiple ways to kill bacteria via inhibiting novel cell wall or membrane targets. Future. Med. Chem.. 2020;12:1253-1279.
- [CrossRef] [Google Scholar]
- Propidium iodide enabled live imaging of pasteuria sp.-pratylenchus zeae infection studies under fluorescence microscopy. Protoplasma. 2020;258:279-287.
- [CrossRef] [Google Scholar]
- Anti-virulence strategy of novel dehydroabietic acid derivatives: Design, synthesis, and antibacterial evaluation. Int. J. Mol. Sci.. 2023;24:2897.
- [CrossRef] [Google Scholar]
- Natural products-based botanical bactericides discovery: Novel abietic acid derivatives as anti-virulence agents for plant disease management. J. Agric. Food. Chem.. 2023;71:5463-5475.
- [CrossRef] [Google Scholar]
- Geranylation of chalcones by a fungal aromatic prenyltransferase. J. Agric. Food. Chem. 2023;71:4675-4682.
- [CrossRef] [Google Scholar]
- Monitoring yeast regulated cell death: Trespassing the point of no return to loss of plasma membrane integrity. Apoptosis. 2022;27:778-786.
- [CrossRef] [Google Scholar]
- Design, synthesis, antibacterial evaluation, three-dimensional quantitative structure–activity relationship, and mechanism of novel Quinazolinone derivatives. J. Agric. Food. Chem.. 2023;71:3939-3949.
- [CrossRef] [Google Scholar]
- Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem.. 2015;102:487-529.
- [CrossRef] [Google Scholar]
- Discovery and structure–activity relationship studies of novel tetrahydro-β-carboline derivatives as apoptosis initiators for treating bacterial infections1. J. Integr. Agric. 2023
- [CrossRef] [Google Scholar]
- Prodrugs of Pyrazolo[3,4-d]pyrimidines: From Library Synthesis to Evaluation as Potential Anticancer Agents in an Orthotopic Glioblastoma Model. J. Med. Chem.. 2017;60:6305-6320.
- [CrossRef] [Google Scholar]
- Discovery of novel rost-4-ene derivatives as potential plant activators for preventing phytopathogenic bacterial infection: Design, synthesis and biological studies. Pest. Manag. Sci.. 2022;78:3404-3415.
- [CrossRef] [Google Scholar]
- Plant protein-based self-assembling core–shell nanocarrier for effectively controlling plant viruses: Evidence for nanoparticle delivery behavior, Plant Growth Promotion, and plant resistance induction. Chem. Eng. J.. 2023;464:142432
- [CrossRef] [Google Scholar]
- Exploiting natural maltol for synthesis of novel Hydroxypyridone derivatives as promising anti-virulence agents in bactericides discovery. J. Agr. Food. Chem.. 2023;71:6603-6616.
- [CrossRef] [Google Scholar]
- Novel sulfonamide derivatives containing a piperidine moiety as new bactericide leads for managing plant bacterial diseases. Int. J. Mol. Sci.. 2023;24:5861.
- [CrossRef] [Google Scholar]
- ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res.. 2021;49
- [CrossRef] [Google Scholar]
- Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. Journal of Enzyme Inhibition and Medicinal Chemistry. 2021;36(1):296-307.
- [CrossRef] [Google Scholar]
- Carbazole alkaloids from Clausena anisum-olens: Isolation, characterization, and Anti-HIV Evaluation. Molecules. 2019;25:99.
- [CrossRef] [Google Scholar]
- Photo-stimuli smart supramolecular self-assembly of azobenzene/β-cyclodextrin inclusion complex for controlling plant bacterial diseases. Adv. Funct. Mater.. 2023;33
- [CrossRef] [Google Scholar]
- Dysregulation of CLPP by small-molecule activators used against Xanthomonas oryzae pv. oryzae infections. J. Agr. Food. Chem.. 2021;69:7545-7553.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of piperazine hybridized coumarin indolylcyanoenones with antibacterial potential. Molecules. 2023;28:2511.
- [CrossRef] [Google Scholar]
- New inspiration of 1,3,4-oxadiazole agrochemical candidates: Manipulation of a type III secretion system-induced bacterial starvation mechanism to prevent plant bacterial diseases. J. Agr. Food. Chem.. 2023;71:2804-2816.
- [CrossRef] [Google Scholar]
- Rational design of high-yield and superior-quality rice. Nat. Plants. 2017;3
- [CrossRef] [Google Scholar]
- Resistance-driven innovations in the discovery of bactericides: Novel triclosan derivatives decorating isopropanolamine moiety as promising anti-biofilm agents against destructive plant bacterial diseases. Pest Manag. Sci.. 2023;79:2443-2455.
- [CrossRef] [Google Scholar]
- Physicochemical properties and antibacterial mechanism of TP microcapsules/LZM-PVA gradual sustained-release composite coatings. Prog. Org. Coat.. 2020;146:105740
- [CrossRef] [Google Scholar]
- Synthesis and Antibacterial Activities of 2-Oxo-N-phenylacetamide Derivatives Containing a Dissulfone Moiety Target on Clp. J. Agr. Food. Chem.. 2022;70:9356-9366.
- [CrossRef] [Google Scholar]
- Zhao, Y. L., Huang, X., Liu, L. W., Wang, P. Y., Long, Q. S., Tao, Q. Q., Li, Z., & Yang, S., 2019. Identification of Racemic and Chiral Carbazole Derivatives Containing an Isopropanolamine Linker as Prospective Surrogates against Plant Pathogenic Bacteria: In Vitro and In Vivo Assays and Quantitative Proteomics. J. Agric. Food Chem., 67(26), 7512–7525. doi: 10.1021/acs.jafc.9b02036.
- Identification of racemic and chiral carbazole derivatives containing an isopropanolamine linker as prospective surrogates against plant pathogenic bacteria: in vitro and in vivo assays and quantitative proteomics. J. Agr. Food. Chem.. 2019;67:7512-7525.
- [CrossRef] [Google Scholar]
- Discovery of simple diacylhydrazine-functionalized cinnamic acid derivatives as potential microtubule stabilizers. Int. J. Mol. Sci.. 2022;23:12365.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of piperazine derivatives involved in the 5-HT 1A R/BDNF/PKA pathway. Journal of Enzyme Inhibition and Medicinal Chemistry. 2023;39(1)
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105850.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







