Translate this page into:
Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone
⁎Corresponding author. wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
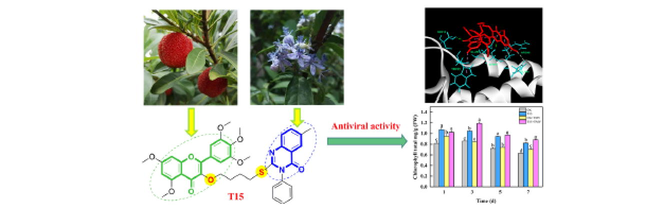
Plant bacteria and viruses have a huge negative impact on food crops in the world. Therefore, it is important to create new and efficient green pesticides. In this paper, a series of myricetin derivatives containing quinazolinone sulfide were introduced. Good antibacterial and antiviral activities of the drug molecules 2-((3-((5,7-dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propyl)thio)-6-fluoro-3-phenylquinazolin-4(3H)-one (T5) and 2-((4-((5,7-dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butyl)thio)-6-methyl-3-phenylquinazolin-4(3H)-one (T15) respectively were found by biological activity screening. The value of dissociation constant (Kd) of compound T15 to TMV CP was 0.024 ± 0.006 μM, determined by Microscale thermophoresis (MST), which was far less than the value of 8.491 ± 2.027 μM of commercial drug ningnanmycin (NNM). The interaction between compound T15 and TMV CP was further verified by molecular docking. Compound T15 formed strong hydrogen bonds with residues SER:49 and SER:15 (1.92 Å, 2.20 Å, respectively), which were superior to the traditional hydrogen bonds formed by NNM with residue SER:215 (3.64 Å). In addition, the effects of compound T15 on the contents of chlorophyll and peroxidase (POD) in tobacco were studied, and the results indicated that compound T15 could enhance the disease resistance of tobacco plants to a certain extent.
Keywords
Myricetin
Quinazolinone
Antibacterial
Antiviral
TMV CP
Chlorophyll content
Defensive enzyme activities
- TMV CP
-
Tobacco mosaic virus coat protein
- MST
-
Microscale thermophoresis
- NNM
-
Ningnanmycin
- POD
-
peroxidase
- Xac
-
Xanthomonas axonopodis pv. citri
- Xoo
-
Xanthomonas oryzae pv.oryzae
- BT
-
Bismerthiazol
- TC
-
Thiodiazole-copper
- EC50
-
Median effective concentration
- CK
-
control check
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- HRMS
-
high-resolution mass spectrometry
Abbreviations
1 Introduction
Bacteria and viruses are the two major pathogens of plant, which cause huge losses in crop yields worldwide due to their extremely high infectivity and wide host-range (Li et al., 2016; Kashyap et al., 2017; Shasmita et al., 2019; Cai et al., 2020; Abdullahi et al., 2020). However, conventional chemicals used to control plant bacteria and viruses, such as bismerthiazol (BT), thiodiazole-copper (TC), ningnanmycin (NNM), have led to increasing resistance of plant pathogens due to large-scale single use. Moreover, traditional chemicals are being eliminated from the market gradually, due to their high phytotoxicity, great environmental damage, excessive pesticide residues, and difficulty in degradation (Buttimer, et al., 2017; Ren, et al., 2020; Wu, et al., 2021; Singh, et al.,2021). In recent years, with the transition of pesticide development to the green and safe direction, plant-derived pesticides became one of the third generational pesticide sources that are highly valued at home and abroad because of their advantages.
Myricetin is a polyhydroxy flavonol, extracted from the bark of myricaceae (Myricaceae) plant, also exists in various edible plants such as grapes, rattan tea, and buckwheat (Miean, et al., 2001; Kalinova, et al., 2009). A number of research results have shown that myricetin has various pharmacological activities such as antioxidant, anti-tumor, and anti-inflammatory, is a very important natural lead compound in the development of drugs and cosmetics (Mendes, et al., 2018; Mendes, et al., 2019; Sun, et al., 2019; Chobot, et al., 2020; Akhtar, et al., 2021). In recent years, with the aggravation of plant diseases, more and more pesticide researchers have turned their attention to the direction of natural products. Existing research results show that myricetin as a lead compound after structural modification have various biological activities such as antiviral and antibacterial, which opens up a new research direction to find more efficient and environmentally friendly botanical pesticides (Jiang, et al., 2020a; He, et al., 2021; Liu, et al., 2021; Peng, et al., 2021). The structures of these compounds are shown in Fig. 1.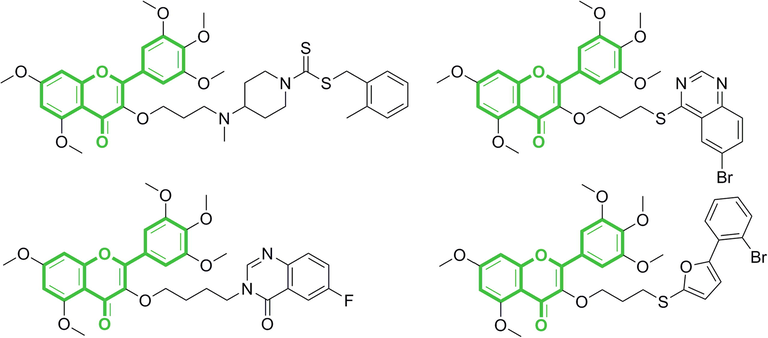
Structures of myricetin derivatives previously reported by our group.
Quinazolin-4(3H)-one, also known as 4-hydroxyquinazoline, is a series of nitrogen-containing heterocyclic compounds, exists widely in natural alkaloids (Al-Amiery, et al., 2014; Mahdavi, et al., 2016). In early studies, quinazolin-4(3H)-one and its derivatives exhibited various pharmacological activities such as antioxidant, antibacterial, and antitumor, and were the main active groups in various drug structures (Nanthakumar, et al., 2014; Rakesh, et al., 2019; Soliman, et al., 2020; Huang, et al., 2020). Recently, quinazolin-4(3H)-one and its derivatives have shown good antibacterial and antiviral activities in the research reports of pesticide creation for many times, so they have attracted the attention of scholars (Zu, et al., 2020; Hao, et al., 2020; Liu, et al., 2021; Peng, et al., 2021). The structures of these compounds are shown in Fig. 2.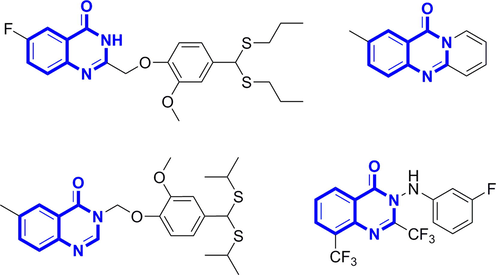
Structures of quinazolinone derivatives with antiviral and antibacterial activities.
In this article, we introduced quinazolinone, as an active group existing in natural product Febrifugine and antibacterial agent Proquinazid, into the structure of myricetin, and synthesized a series of myricetin derivatives containing thioether quinazolinone. The design idea of the target compounds is shown in Fig. 3. The target compounds with better activity were screened out through the tests of antibacterial and antiviral activities. Experiments, such as scanning electron microscope, microscale thermophoresis, molecular docking, and determination of chlorophyll and defense enzyme content, were used to preliminarily explore the mechanism of action of the compounds, in order to find highly active antibacterial and antiviral drugs.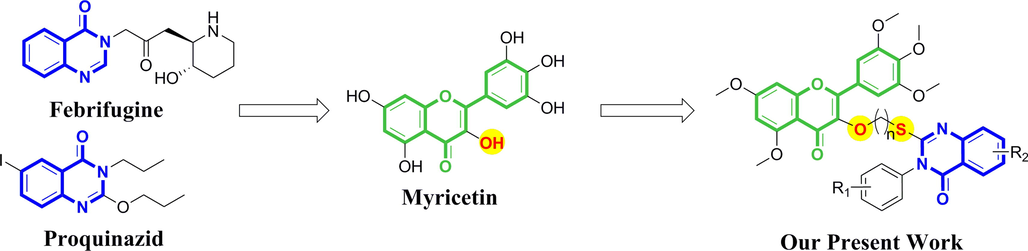
Design idea for target compounds (Febrifugine and Proquinazid are two commercial antibacterial agents that contain quinazolinone groups).
2 Materials and methods
2.1 Instruments and chemicals
The target compounds were characterized, by using JEOL-ECX500 nuclear magnetic resonance spectrometer (Tokyo, Japan) and Thermo Scientic Q Exactive mass spectrometer (Missour, America). The Kd values of compounds to TMV CP were determined by using NanoTemper Monolith NT.115 microscale thermophoresis instrument (München, Germany). All solvents were purchased from Tianjin Zhi Yuan Regent Co., Ltd. (Tianjin, China). All reagents were purchased from Shanghai Titan chemical Co., Ltd. (Shanghai, China) and Adamas Reagent, Ltd. (Shanghai, China). Enzyme activity kits were purchased from Suzhou Keming Biotechnology Co., Ltd (Jiangsu, China).
2.2 Synthesis
2.2.1 General synthesis procedure for intermediates 1 and 2
Intermediates 1 and 2 were synthesized with the method described in the references (Xue et al., 2015; Jiang, et al., 2020b; Li, et al; 2019).
2.2.2 General synthesis procedure for intermediate 3
Intermediate 3 was synthesized according to previous literature (Ran, et al., 2020). Substituted benzoic acid (13.23 mmol), absolute ethanol (20 mL), substituted phenyl isothiocyanate (13.23 mmol) and triethylamine (14.55 mmol) were added to the reaction flask in sequence. After heating and stirring to reflux for 4–6 h, a white solid was precipitated. The reaction system was filtered under reduced pressure, and the filter cake was washed with a small amount of ethanol to obtain the crude product of Intermediate 3, which was directly put into the next step after drying without purification.
2.2.3 General synthesis of target compounds T1 − T25
As shown in Scheme 1, the target compounds T1 − T25 were synthesized with the method, which had been modified by us later, from the literature (Hagar, et al., 2016). Intermediate 2 (1.96 mmol), intermediate 3 (2.36 mmol), and K2CO3 (3.93 mmol) were added to 20 mL of DMF, reacted at 90 ℃ for 6 h. The reaction system was poured into 500 mL of ice water, and a white solid was precipitated. After being held stationarily for 2 h, it was filtered under reduced pressure and dried to obtain a crude product, which was separated and purified by column chromatography later (ethyl acetate: petroleum ether = 2:1, v/v).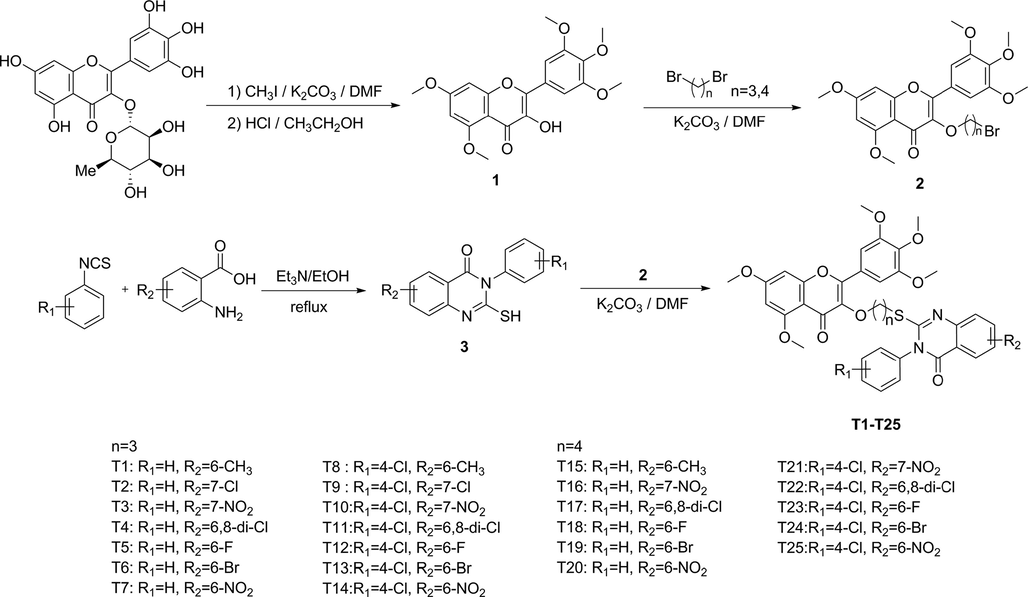
Synthetic route of target compounds T1 − T25.
2.3 Biological assays
2.3.1 Evaluation of antibacterial activity in vitro
According to the reports in the literature, the antibacterial activity of the compounds T1 − T25 were tested by the turbidimetric assays. (Xiang, et al., 2020). The bacteria used were Xanthomonas axonopodis pv. citri (Xac) and Xanthomonas oryzae pv.oryzae (Xoo). Commercially available BT and TC were used as control agents. Methylated myricetin was used as the lead compound for the comparison of antibacterial activity. The corresponding concentration of DMSO solution served as a negative control. Three parallel experiments were performed for each sample.
2.3.2 Evaluation of antiviral activity in vivo
The antiviral activity of compounds T1 − T25 were tested with the half-leaf blight spot methods. (Chen, et al., 2019; Zhang, et al., 2021). During the experiment, DMSO was used as the solvent, and Tween-20 was used as the co-solvent. NNM was used as the control drug. Methylated myricetin was used as the lead compound for activity comparison. Three parallel experiments were performed for each sample.
2.4 Microscale thermophoresis experiment
The Kd values of some target compounds and NNM to TMV CP were determined with the method reported in the literature. (Li et al., 2019; Wu, et al., 2021). DMSO-dissolved drug was formulated into 16 concentration gradients at room temperature. Compounds were tested for Kd values on NanoTemper Monolith NT.115 after adding equal amounts of TMV CP to groups of each concentration.
2.5 Molecular docking
Molecular docking is a process in which receptors and substrates recognize each other through energy matching and geometric matching. Molecular docking is very important in both supramolecular architecture and drug design (José, et al., 2020; Gabriel, et al., 2021). The molecular docking of compound T15 and NNM to TMV CP were carried out with the method reported in the literature (Chen, et al., 2021). Molecular docking of the TMV CP (PDB code: 1EI7) with the structure of the selected compound was performed on Discovery Studio.
2.6 Determination of chlorophyll content
According to the method in the literature, the compound T15 with better protective activity was selected for the determination of the change of chlorophyll content (Gan, et al., 2017). The virus-inoculated tobacco leaves were ground in liquid nitrogen to pulverize. A mixed solution of ethanol and acetone was added and allowed to be held stationarily for 1 h in the dark. The absorbance at 645 nm and 663 nm were measured separately. The chlorophyll content was calculated according to the following formula.
2.7 Determination of defense enzyme activity
Peroxidases (POD) are involved in plant development, stress responses and hormone signaling. It is closely related to the photosynthesis and respiration of plants (Wu, et al., 2019). The compound T15 was tested for the activity of the defense enzyme according to the method in the literature (Zhou, et al., 2021). The virus-inoculated tobacco leaves were added to liquid nitrogen and ground to pulverize. The POD content of the leaves was determined with the method of enclosed instructions of testing kit.
3 Results and discussion
3.1 Structural characterization of some target compounds
2-((3-((5,7-dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)propyl)thio)-6-fluoro-3-phenylquinazolin-4(3H)-one (T5). White solid, m.p. 185.6–187.3 °C, yield, 27%; 1H NMR (500 MHz, Chloroform-d) δ 7.83 (dd, J = 8.1, 2.9 Hz, 1H, Ph − H), 7.55 – 7.53 (m, 1H, Ph − H), 7.53 – 7.51 (m, 3H, Ph − H), 7.41 (td, J = 8.3, 2.9 Hz, 1H, Ph − H), 7.28 (s, 2H, Ph − H), 7.28 – 7.25 (m, 2H, Ph − H), 6.48 (d, J = 2.2 Hz, 1H, Ph − H), 6.34 (d, J = 2.3 Hz, 1H, Ph − H), 4.11 (t, J = 6.2 Hz, 2H, −O − CH2CH2CH2 − S − ), 3.95 (s, 3H, Ph − OCH3), 3.89 (s, 3H, Ph − OCH3), 3.88 (s, 3H, Ph − OCH3), 3.87 (s, 6H, Ph − OCH3), 3.26 – 3.22 (m, 2H, −O − CH2CH2CH2 − S − ), 2.11 (p, J = 6.3 Hz, 2H, −O − CH2CH2CH2 − S − ); 13C NMR (126 MHz, Chloroform-d) δ 174.02, 167.85, 164.11, 161.07, 158.87, 156.67, 153.04, 152.77, 144.65, 140.48, 139.92, 135.78, 126.05, 123.24, 123.05, 120.97 (d, J = 8.4 Hz), 112.17, 111.99, 109.44, 105.85, 95.94, 92.48, 71.09, 61.11, 56.54, 56.39, 55.94, 29.73, 29.43; 19F NMR (471 MHz, Chloroform-d) δ −114.11; HRMS (ESI) calcd for C37H34O9N2FS [M + H]+: 701.19636, found 701.19629.
2-((4-((5,7-dimethoxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy)butyl)thio)-6-methyl-3-phenylquinazolin-4(3H)-one (T15). White solid, m.p. 196.9–198.2 °C, yield, 56%; 1H NMR (500 MHz, Chloroform-d) δ 7.98 (s, 1H, Ph − H), 7.53 – 7.48 (m, 4H, Ph − H), 7.45 (d, J = 8.3 Hz, 1H, Ph − H), 7.31 (s, 2H, Ph − H), 7.29 – 7.26 (m, 2H, Ph − H), 6.47 (d, J = 2.3 Hz, 1H, Ph − H), 6.33 (d, J = 2.3 Hz, 1H, Ph − H), 4.04 – 4.00 (m, 2H, −O − CH2CH2CH2CH2 − S − ), 3.94 (s, 3H, Ph − OCH3), 3.90 – 3.87 (m, 12H, Ph − OCH3), 3.19 – 3.14 (m, 2H, −O − CH2CH2CH2CH2 − S − ), 2.44 (s, 3H, Ph − CH3), 1.83 (p, J = 3.0, 2.6 Hz, 4H, −O − CH2CH2CH2CH2 − S − ); 13C NMR (126 MHz, Chloroform-d) δ 174.07, 164.04, 162.06, 161.09, 158.85, 156.30, 153.00, 152.60, 145.98, 140.68, 139.88, 136.21, 136.07, 135.80, 129.90, 129.71, 129.27, 126.63, 126.15, 119.58, 109.47, 105.84, 95.87, 92.46, 71.82, 61.12, 56.52, 56.38, 55.92, 32.26, 29.54, 25.43, 21.35; HRMS (ESI) calcd for C39H39O9N2S [M + H]+: 711.23708, found 711.23706.
3.2 Antibacterial activity of the target compounds T1 − T25
The results of Table 1 show that most of the compounds had a certain inhibitory effect on Xac and Xoo when the concentration of compound was 100 μg/mL. In particular, compounds T1 and T5 exhibited good inhibitory activity against Xac, with the inhibition rates of 87.8 and 89.1% respectively, which were superior than those of commercial drugs BC (60.2 %) and TC (53.6 %). The inhibitory activity against Xoo of compounds T2, T5, T15 and T16, with inhibition rates of 79.6, 82.1, 89.1 and 77.7% respectively, had better advantages than those of BT (54.0%) and TC (54.2%).
Compd.
Xac
Xoo
100 μg/mL
50 μg/mL
100 μg/mL
50 μg/mL
T1
87.8 ± 2.5
64.4 ± 1.2
40.4 ± 0.9
28.0 ± 2.3
T2
75.0 ± 3.7
52.1 ± 1.8
79.6 ± 1.9
58.6 ± 0.6
T3
59.3 ± 1.4
34.5 ± 4.0
40.6 ± 4.1
32.3 ± 6.7
T4
39.0 ± 2.2
28.9 ± 6.2
17.2 ± 3.0
14.2 ± 5.3
T5
89.1 ± 0.8
64.8 ± 1.7
82.1 ± 0.9
67.5 ± 3.2
T6
59.2 ± 2.0
40.7 ± 3.6
49.6 ± 6.8
30.2 ± 7.9
T7
31.0 ± 6.9
14.6 ± 1.4
19.5 ± 0.3
17.0 ± 4.5
T8
58.5 ± 2.3
49.2 ± 2.1
27.7 ± 1.2
18.3 ± 3.2
T9
40.6 ± 5.6
16.5 ± 4.1
24.0 ± 2.2
19.2 ± 7.2
T10
32.8 ± 1.6
18.9 ± 3.1
37.0 ± 1.9
22.0 ± 1.7
T11
47.9 ± 9.9
23.0 ± 1.7
28.5 ± 1.9
15.2 ± 3.5
T12
61.5 ± 4.8
51.7 ± 4.1
30.6 ± 4.8
16.3 ± 7.3
T13
45.2 ± 6.0
29.5 ± 3.4
28.4 ± 1.1
17.7 ± 3.2
T14
17.9 ± 8.8
9.9 ± 7.1
22.8 ± 2.1
14.0 ± 2.5
T15
68.3 ± 1.3
42.7 ± 4.6
89.1 ± 0.6
64.3 ± 0.8
T16
52.4 ± 6.0
34.8 ± 3.7
77.7 ± 1.4
64.8 ± 1.7
T17
61.2 ± 2.7
28.9 ± 5.6
27.1 ± 2.0
10.5 ± 1.7
T18
58.2 ± 0.8
36.8 ± 4.5
44.2 ± 5.8
30.3 ± 2.3
T19
68.2 ± 6.4
46.9 ± 2.7
36.9 ± 0.4
14.3 ± 4.2
T20
63.9 ± 0.7
26.7 ± 1.6
29.4 ± 3.4
15.0 ± 3.4
T21
68.6 ± 3.7
41.8 ± 1.6
24.1 ± 3.3
15.7 ± 3.2
T22
64.6 ± 1.1
53.1 ± 1.3
44.7 ± 3.1
24.3 ± 2.4
T23
56.8 ± 2.6
37.6 ± 2.8
25.2 ± 2.7
19.3 ± 0.9
T24
47.4 ± 2.6
27.2 ± 2.4
30.2 ± 2.6
17.1 ± 1.3
T25
19.9 ± 9.5
4.6 ± 1.3
17.8 ± 2.9
12.9 ± 3.6
myricetin
43.5 ± 2.0
30.3 ± 0.8
55.2 ± 1.3
37.4 ± 2.2
BT
60.2 ± 1.3
44.6 ± 1.8
54.0 ± 1.2
42.9 ± 1.1
TC
53.6 ± 0.4
36.8 ± 1.2
54.2 ± 0.7
38.0 ± 0.8
Based on the activity screening results, the EC50 values of some target compounds were tested, whose results are displayed in Table 2. The EC50 values of compounds T1 and T5 against Xac were 23.6 and 22.4 μg/mL respectively, which were less than the control drugs BT (62.2 μg/mL) and TC (97.5 μg/mL). The EC50 values of compounds T5 and T15 against Xoo were 18.5 and 21.6 μg/mL respectively, which were much better than BT (63.7 μg/mL) and TC (86.1 μg/mL). In particular, compound T5 has good inhibitory activity against both plant bacteria.
Bacteria
Compd.
n
R1
R2
Toxic Regression equation
r2
EC50 (μg/mL)
Xac
T1
3
H
6 − CH3
y = 1.5892x + 2.8183
0.969
23.6 ± 2.9
T5
3
H
6 − F
y = 1.6060x + 2.8316
0.963
22.4 ± 2.1
myricetin
−
−
−
y = 0.9290x + 2.9584
0.992
135.6 ± 2.4
BT
−
−
−
y = 1.0215x + 3.1677
0.989
62.2 ± 2.5
TC
−
−
−
y = 1.0219x + 2.9673
0.978
97.5 ± 1.4
Xoo
T5
3
H
6 − F
y = 1.1404x + 3.5541
0.974
18.5 ± 2.6
T15
4
H
6 − CH3
y = 1.5790x + 2.8935
0.958
21.6 ± 1.8
myricetin
−
−
−
y = 0.7711x + 3.4838
0.949
92.5 ± 2.2
BT
−
−
−
y = 0.7396x + 3.6657
0.959
63.7 ± 1.4
TC
−
−
−
y = 1.0206x + 3.0250
0.991
86.1 ± 3.7
3.3 Antiviral activity of the target compounds T1 − T25
The test results of antiviral activity of the target compounds T1 − T25 are shown in Table 3. When the compound concentration was 500 μg/mL, compounds T2 and T15 had good curative activity against TMV, and the inhibition rates were 68.1 and 67.3% respectively, which were higher than the commercial drug NNM (54.1%). The protective activity against TMV of compounds T2, T11 and T15, with the inhibition rates of 64.3, 68.2 and 64.3 % respectively, were better than the commercial drug NNM (57.1%). Fig. 4 reveals the morphology of compound T15 against TMV in vivo.
Compd.
n
R1
R2
Curative (%)
Protective (%)
Inactivating (%)
T1
3
H
6 − CH3
42.9 ± 2.7
29.6 ± 2.5
47.1 ± 6.7
T2
3
H
7 − Cl
68.1 ± 4.2
64.3 ± 2.1
61.3 ± 3.9
T3
3
H
7 − NO2
40.9 ± 2.6
46.3 ± 2.7
59.4 ± 2.0
T4
3
H
6,8 − di − Cl
40.8 ± 3.4
57.1 ± 4.6
46.1 ± 5.7
T5
3
H
6 − F
46.5 ± 3.4
57.6 ± 4.1
63.8 ± 3.0
T6
3
H
6 − Br
55.8 ± 6.8
44.8 ± 5.1
48.6 ± 7.6
T7
3
H
6 − NO2
50.7 ± 1.9
44.4 ± 6.1
60.4 ± 7.1
T8
3
4 − Cl
6 − CH3
53.7 ± 2.9
48.9 ± 4.9
43.1 ± 3.0
T9
3
4 − Cl
7 − Cl
51.2 ± 3.5
31.1 ± 4.1
53.5 ± 7.2
T10
3
4 − Cl
7 − NO2
49.9 ± 2.6
48.3 ± 2.4
43.2 ± 3.6
T11
3
4 − Cl
6,8 − di − Cl
59.4 ± 7.1
68.2 ± 4.7
68.8 ± 9.0
T12
3
4 − Cl
6 − F
28.1 ± 3.0
44.5 ± 5.2
56.0 ± 3.3
T13
3
4 − Cl
6 − Br
44.1 ± 2.1
47.3 ± 2.8
52.9 ± 5.7
T14
3
4 − Cl
6 − NO2
49.3 ± 4.5
48.5 ± 3.5
35.6 ± 2.2
T15
4
H
6 − CH3
67.3 ± 2.8
64.3 ± 4.3
64.6 ± 6.7
T16
4
H
7 − NO2
25.4 ± 8.9
39.2 ± 5.6
32.4 ± 6.7
T17
4
H
6,8 − di − Cl
44.4 ± 3.3
58.9 ± 2.3
54.2 ± 6.4
T18
4
H
6 − F
42.4 ± 1.9
28.1 ± 4.1
57.8 ± 2.9
T19
4
H
6 − Br
47.1 ± 4.6
49.2 ± 5.4
66.7 ± 7.8
T20
4
H
6 − NO2
57.3 ± 5.8
43.8 ± 3.1
41.8 ± 1.8
T21
4
4 − Cl
7 − NO2
43.1 ± 5.8
48.4 ± 2.9
41.3 ± 2.4
T22
4
4 − Cl
6,8 − di − Cl
55.6 ± 2.4
37.6 ± 2.2
58.2 ± 1.8
T23
4
4 − Cl
6 − F
30.6 ± 3.1
51.7 ± 3.4
22.4 ± 3.1
T24
4
4 − Cl
6 − Br
52.3 ± 5.6
43.0 ± 1.9
48.5 ± 4.5
T25
4
4 − Cl
6 − NO2
51.8 ± 4.6
49.6 ± 1.7
64.3 ± 4.1
myricetin
−
−
−
36.7 ± 2.6
45.6 ± 3.9
43.4 ± 2.3
NNM
−
−
−
54.1 ± 2.1
57.1 ± 1.9
85.3 ± 3.8
Tobacco leaf morphology effects of T15 and NNM against TMV in vivo. The left side of each leaf was drug-treated, the right side of each leaf was untreated with drug.
The EC50 values of compounds T2, T11, T15 and NNM are summarized in Table 4. Compounds T2, T11 and T15 have good curative activity against TMV, with EC50 values of 190.0, 227.3 and 166.9 μg/mL respectively, which were lower than the commercial drug NNM of 319.7 μg/mL. In terms of protective activity, the EC50 values of compounds T2, T11 and T15 against TMV were 228.7, 178.9 and 130.5 μg/mL respectively, which were lower than NNM (341.3 μg/mL).
Compd.
n
R1
R2
Regression equation
r2
EC50 (μg/mL)
Curative
T2
3
H
7 − Cl
y = 1.0787x + 2.5418
0.998
190.0 ± 3.4
T11
3
4 − Cl
6,8 − di − Cl
y = 0.7626x + 3.2028
0.982
227.3 ± 2.7
T15
4
H
6 − CH3
y = 0.8419x + 3.1289
0.989
166.9 ± 2.1
NNM
−
−
−
y = 0.6087x + 3.4754
0.998
319.7 ± 4.7
Protective
T2
3
H
7 − Cl
y = 1.2654x + 2.0146
0.962
228.7 ± 2.3
T11
3
4 − Cl
6,8 − di − Cl
y = 0.9593x + 2.8390
0.942
178.9 ± 3.2
T15
4
H
6 − CH3
y = 0.7412x + 3.4319
0.966
130.5 ± 4.8
NNM
−
−
−
y = 0.9198x + 2.6743
0.962
341.3 ± 3.9
3.4 Structure-activity relationship
Referring to the previous research reports on quinazolinones, most of the changes of substituents are carried out at the 6-position. In addition, electron withdrawing groups were often used as substituents. (Zu, et al., 2021). From the results of antibacterial activity, the compounds with − CH3 and − F at the 6th substitution position have better antibacterial activity. For example, compounds T1, T5 and T15 exhibited inhibitory activities more than 80% on Xac and Xoo at the tested concentration of 100 μg/mL. The results of antiviral activity showed that some compounds containing − Cl and − CH3 had better inhibitory activity on TMV. Compared with the previously reported compounds (Liu, et al., 2021), the antibacterial activity of some of the compounds with n = 3 was improved after the introduction of thioether, and the anti-TMV activity was also generally improved.
3.5 Microscale thermophoresis
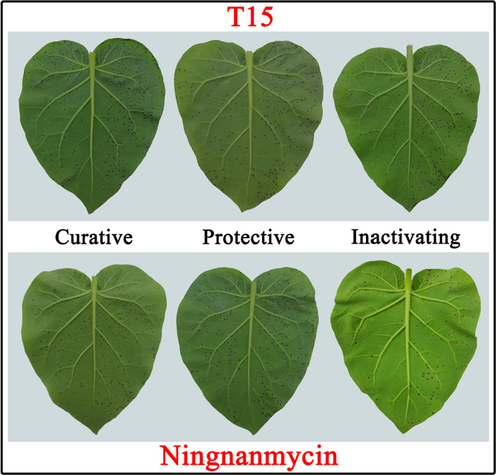
The Kd value shows the affinity of the interaction between two molecules. The smaller the value is, the stronger the affinity of the two molecules is (Wang, et al., 2020). Fig. 5 shows that the binding constants (Kd) of compounds T2, T11, T15 and NNM to TMV CP. Among them, the Kd value of compound T15 to TMV CP was 0.024 ± 0.006 μM, which was smaller than that of compounds T2 (0.052 ± 0.016 μM) and T11 (0.054 ± 0.023 μM). However, the above data are extremely small compared with NNM (8.491 ± 2.027 μM). It shows that the binding abilities of compounds T2, T11 and T15 to TMV CP are much greater than that of the control drug NNM. These results were positively correlated with EC50 values.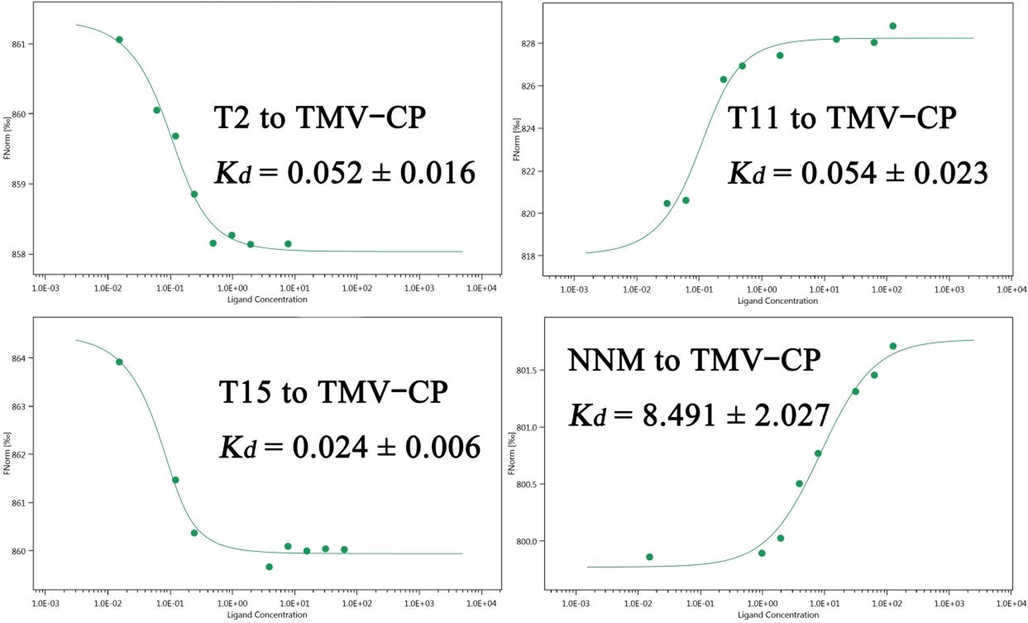
The Kd values of componds T2, T11, T15 and NNM to TMV CP.
3.6 Molecular docking
The length of hydrogen bonds is negatively correlated with the binding force, and the number of hydrogen bonds is positively correlated with the binding force (Wang, et al., 2021).The pictures in Fig. 6 validate the binding ability of the compound T15 and NNM to TMV CP. Compound T15 (A, a) interacted with surrounding residues SER A:49, SER A:15, ARG A:46, GLN A:45, GLU A:50 and TRP A:52 in the TMV CP active pocket via conventional hydrogen bond, carbon hydrogen interacts with π-alkyl. Compound T15 formed two strong hydrogen bonds with residues SER A:49 (1.92 Å) and SER A:15 (2.20 Å). The commercial drug NNM (B, b), interacted with surrounding residues THR B:237, ALA B:240 and GLN B:234 in the active pocket of TMV CP via π-donor hydrogen bond, π-alkyl and conventional hydrogen bond. The naked amino group in the NNM structure formed a traditional hydrogen bond (2.30 Å) with residue SER B:215. Based on the above analysis, it was found that compound T15 was superior to commercial drug NNM in terms of the number of action sites and hydrogen bond length. Compared with NNM, T15 has a stronger binding ability than that of TMV CP, which has the potential for being a more effective antiviral agent.
Molecular docking of compound T15 (A, a) and NNM (B, b) to TMV CP.
3.7 Effects of compound T15 on chlorophyll content in tobacco
Chlorophyll is an important substance for plants to carry out photosynthesis. It is reported that plant diseases induce a decrease in chlorophyll content in plants, resulting in reduced crop yields (Mandal, et al., 2009). The following conclusions could be drawn from Fig. 7: firstly, the chlorophyll content in CK group increased slightly on the third day, but decreased gradually in the later period as time went by. Secondly, CK + TMV group showed a slight increase in chlorophyll content after the TMV inoculation on first day, followed by a decreasing trend. Thirdly, the chlorophyll content in T15 group increased significantly on the first day after TMV inoculation, and decreased in the later period. Finally, the overall change trend of the T15 + TMV group was similar to that of the CK group, but its chlorophyll content increased significantly on the third day, which showed that the compound T15 caused the chlorophyll content in the plant to reach its peak at this time, and the chlorophyll content gradually decreased with plant aging at a later stage.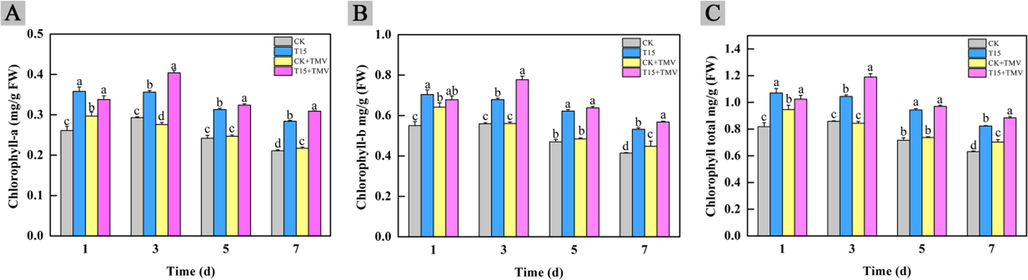
Changes of chlorophyll content in tobacco after compound T15 treatment.
3.8 Effects of compound T15 on peroxidase (POD) content in tobacco
The elevated POD content helps to scavenge free radicals in plants to improve disease resistance (Gan, et al., 2021). The changes in peroxidase (POD) content in tobacco after treatment with compound T15 are displayed in Fig. 8. The POD content of each group gradually increased over time during the 7 d of TMV inoculation. In general, POD content in CK + TMV and T15 + TMV groups increased significantly on the fifth and seventh days compared with CK and T15 groups. In addition, POD contents of T15 group and T15 + TMV group were higher than that of group without compound T15 treatment. The above analysis showed that compound T15 could make peroxidase increase to a certain extent and enhance plant disease resistance.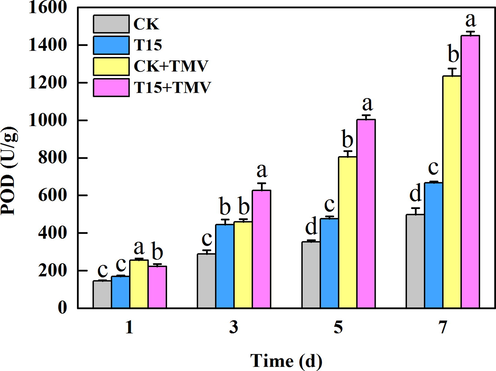
Effects of compound T15 on POD content in tobacco.
4 Conclusions
In summary, myricetin derivatives containing thioether quinazolinone have antibacterial and anti-TMV activity. Compounds T2, T11 and T15 even surpassed the commercial drug NNM in terms of curative and protective activity, which was also confirmed by their Kd values. Molecular docking proved that compound T15 has strong binding ability to TMV CP further. Meanwhile, the results of chlorophyll and peroxidase content measurements indicated that compound T15 enhances the disease resistance of tobacco plants, and was a potential antiviral drug molecule.
Acknowledgements
This research was completed with the support of the National Natural Science Foundation of China (No. 21867003), the Science Foundation of Guizhou Province (No. 20192452), Natural Science research project of Guizhou Education Department (No. 2018009), Science and Technology Program of Guizhou Province (No. 20191139), the Young Science and Technology Talents Development Program of Education Department of Guizhou Province (No. 2018251).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem.. 2020;13:8012-8025.
- [Google Scholar]
- Anticancer potential of myricetin bulk and nano forms in vitro in lymphocytes from myeloma patients. Arch. Toxicol.. 2021;95:337-343.
- [Google Scholar]
- Antioxidant and antimicrobial activities of novel quinazolinones. Med. Chem. Res.. 2014;23:236-242.
- [Google Scholar]
- Plant-derived compounds: A potential source of drugs against Tobacco mosaic virus. Pestic. Biochem. Physiol.. 2020;169:104589
- [Google Scholar]
- Antibacterial and antiviral activities and action mechanism of flavonoid derivatives with a benzimidazole moiety. J. Saudi Chem. Soc.. 2021;25:101194
- [Google Scholar]
- Synthesis, antiviral activity, and 3D-QSAR study of novel chalcone derivatives containing malonate and pyridine moieties. Arab. J. Chem.. 2019;12:2685-2696.
- [Google Scholar]
- In vitro evaluation of pro- and antioxidant effects of flavonoid tricetin in comparison to Myricetin. Molecules. 2020;25:5850.
- [Google Scholar]
- Benchmarking antioxidant-related properties for gallic acid through the use of DFT, MP2, CCSD, and CCSD(T) approaches. J. Phys. Chem. A. 2021;125:198-208.
- [Google Scholar]
- Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. J. Agric. Food Chem.. 2017;65:4367-4377.
- [Google Scholar]
- Synthesis of novel antiviral ferulic acid−eugenol and isoeugenol hybrids using various link reactions. J. Agric. Food Chem.. 2021;69:13724-13733.
- [Google Scholar]
- Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4(3H)-one derivatives; an account on the N- versus S-Alkylation. J. Mol. Struct.. 2016;1108:667-679.
- [Google Scholar]
- Luotonin A and its derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food Chem.. 2020;68:8764-8773.
- [Google Scholar]
- Design, synthesis and biological activities of myricetin derivatives containing quinazoline thioether moiety. Chin. J. Org. Chem.. 2021;41:708-718.
- [Google Scholar]
- Pyridazino[1,6-b]quinazolinones as new anticancer scaffold: Synthesis, DNA intercalation, topoisomerase I inhibition and antitumor evaluation in vitro and in vivo. Bioorg. Chem.. 2020;99:103814
- [Google Scholar]
- Antibacterial activities of novel dithiocarbamate-containing 4H-chromen-4-one derivatives. J. Agric. Food Chem.. 2020;68:5641-5647.
- [Google Scholar]
- Design, synthesis and antibacterial activities against Xanthomonas oryzae pv. oryzae, Xanthomonas axonopodis pv. citri and Ralstonia solanacearum of novel myricetin derivatives containing sulfonamide moiety. Pest Manag. Sci.. 2020;76:853-860.
- [Google Scholar]
- Probing structural properties and antioxidant activity mechanisms for eleocarpanthraquinone. J. Mol. Model.. 2020;26:233.
- [Google Scholar]
- Level of Catechin, Myricetin, Quercetin and Isoquercitrin in buckwheat (fagopyrum esculentum moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem.. 2009;57:2719-2725.
- [Google Scholar]
- Synthesis and biological activities of myricetin derivatives containing quinoxaline. Chem. J. Chin. Univ.. 2019;40:909-917.
- [Google Scholar]
- New strategies and methods to study interactions between tobacco mosaic virus coat protein and its inhibitors. Int. J. Mol. Sci.. 2016;17:252.
- [Google Scholar]
- Cucumber mosaic virus coat protein: The potential target of 1, 4-pentadien-3-one derivatives. Pestic. Biochem. Physiol.. 2019;155:45-50.
- [Google Scholar]
- Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega. 2021;6:30826-30833.
- [Google Scholar]
- First report on anti-TSWV activities of quinazolinone derivatives containing a dithioacetal moiety. J. Agric. Food Chem.. 2021;69:12135-12142.
- [Google Scholar]
- Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. J. Plant Dis. Protect.. 2009;116:164-168.
- [Google Scholar]
- A computational investigation on the antioxidant potential of myricetin 3,4’-di-O-α-L-rhamnopyranoside. J. Mol. Model.. 2018;24:133.
- [Google Scholar]
- Evaluation of the antioxidant potential of myricetin 3-O-α-L-rhamnopyranoside and myricetin 4’-O-α-L-rhamnopyranoside through a computational study. J. Mol. Model.. 2019;25:89.
- [Google Scholar]
- Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) content of edible tropical plants. J. Agric. Food Chem.. 2001;49:3106-3112.
- [Google Scholar]
- Anti-inflammatory and antibacterial activity study of some novel quinazolinones. Arab. J. Chem.. 2014;7:1049-1054.
- [Google Scholar]
- Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H -chromen-4-one derivatives. J. Agric. Food Chem.. 2021;69:11085-11094.
- [Google Scholar]
- Design, synthesis, and structure-activity relationship of quinazolinone derivatives as potential fungicides. J. Agric. Food Chem.. 2021;69:4604-4614.
- [Google Scholar]
- Anticancer and DNA binding studies of potential amino acids based quinazolinone analogs: synthesis, SAR and molecular docking. Bioorg. Chem.. 2019;87:252-264.
- [Google Scholar]
- Discovery of potent and novel quinazolinone sulfide inhibitors with anti-ToCV activit. J. Agric. Food Chem.. 2020;68:5302-5308.
- [Google Scholar]
- Design, synthesis, antiviral bioactivity, and mechanism of the ferulic acid ester-containing sulfonamide moiety. ACS Omega. 2020;5:19721-19726.
- [Google Scholar]
- Priming with salicylic acid induces defense against bacterial blight disease by modulating rice plant photosystem II and antioxidant enzymes activity. Pestic. Biochem. Physiol. Physiol. Mol. Plant Pathol.. 2019;108:101427
- [Google Scholar]
- Antioxidant and antimicrobial study of Schefflera vinosa leaves crude extracts against rice pathogens. Arab. J. Chem.. 2021;14:103243
- [Google Scholar]
- Antioxidant activity of novel quinazolinones bearing sulfonamide: potential radiomodulatory effects on liver tissues via NF-kB/ PON1 pathway. Eur. J. Med. Chem.. 2020;197:112333
- [Google Scholar]
- New micelle myricetin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Drug Deliv.. 2019;26:575-585.
- [Google Scholar]
- Novel pyrazole-4-acetohydrazide derivatives potentially targeting fungal succinate dehydrogenase: design, synthesis, three-dimensional quantitative structure−activity relationship, and molecular docking. J. Agric. Food Chem.. 2021;69:9557-9570.
- [Google Scholar]
- Design, synthesis, anti-TMV activity, and preliminary mechanism of cinnamic acid derivatives containing dithioacetal moiety. Pestic. Biochem. Physiol.. 2020;164:115-121.
- [Google Scholar]
- The class III peroxidase (POD) gene family in cassava: identification, phylogeny, duplication, and expression. Int. J. Mol. Sci.. 2019;20:2730.
- [Google Scholar]
- Synthesis, antibacterial activity, and mechanisms of novel 6-sulfonyl-1,2,4-triazolo[3,4-b] [1,3,4] thiadiazole derivatives. J. Agric. Food Chem.. 2021;69:4645-4654.
- [Google Scholar]
- In vivo antiviral activity and disassembly mechanism of novel 1-phenyl-5-amine-4-pyrazole thioether derivatives against tobacco mosaic virus. Pestic. Biochem. Physiol.. 2021;173:104771
- [Google Scholar]
- Design and synthesis of novel 1,3,4-oxadiazole sulfone compounds containing 3,4-dichloroisothiazolylamide moiety and evaluation of rice bacterial activity. Pestic. Biochem. Physiol.. 2020;170:104695
- [Google Scholar]
- Novel myricetin derivatives: design, synthesis and anticancer activity. Eur. J. Med. Chem.. 2015;97:155-163.
- [Google Scholar]
- Purine nucleoside derivatives containing a sulfa ethylamine moiety: design, synthesis, antiviral activity, and mechanism. J. Agric. Food Chem.. 2021;69:5575-5582.
- [Google Scholar]
- Design, synthesis and anti-TMV activity of novel α-aminophosphonate derivatives containing a chalcone moiety that induce resistance against plant disease and target the TMV coat protein. Pestic. Biochem. Physiol.. 2021;172:104749
- [Google Scholar]
- Design, synthesis, and anti-ToCV activity of novel 4(3H)-quinazolinone derivatives bearing dithioacetal moiety. J. Agric. Food Chem.. 2020;68:5539-5544.
- [Google Scholar]
- Synthesis, anti-tomato spotted wilt virus activities, and interaction mechanisms of novel dithioacetal derivatives containing a 4(3H)-quinazolinone pyrimidine ring. J. Agric. Food Chem.. 2021;69:14459-14466.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104019.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







