Translate this page into:
Design, synthesis, molecular properties and in vitro antioxidant and antibacterial potential of novel enantiopure isoxazolidine derivatives
⁎Corresponding authors at: Laboratory of Heterocyclic Chemistry, Natural Products and Reactivity, Faculty of Sciences of Monastir, University of Monastir, Avenue de l’Environnement, 5000 Monastir, Tunisia (K. Aouadi). kaiss_aouadi@hotmail.com (Kaïss Aouadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A novel series of enantiopure isoxazolidines have been synthesized in good yields and with high stereoselectivity by 1,3-dipolar cycloaddition between a (–)-menthone-derived nitrone as a glycine equivalent and various terminal alkenes. The structures and the stereochemistry of the obtained cycloadducts have been determined by spectroscopic methods. Almost all the compounds were predicted to exhibit various degrees of antioxidant and antibacterial potentiality. Finally, the drug likeness and bioactivity were calculated using Molinspiration software. The results indicated that all compounds are in accordance with Lipinski’s rule of five showing good drug likeness and bioactivity score for drug targets with no violations.
Keywords
(−)-Menthone
Chiral nitrone
Antioxidant capacity
Antibacterial potential
Molecular properties prediction
Drug likeness and bioactivity
Dedicated to the memory of Professor Abdelkader Ben Khemiss (1942–2016).
1 Introduction
Isoxazolidines are a class of powerful heterocycles, which readily undergo reductive cleavage of the N—O bond. They have been widely used as precursors of 1,3-amino-alcohols or a wide variety of natural products and derivatives (Berthet et al., 2016), especially alkaloids (Seerden et al., 1997), amino-acids (Aouadi et al., 2007, 2012a) and amino-sugars (Frederickson, 1997). Isoxazolidines themselves possess a variety of interesting biological activities such as antioxidant (Brahmi et al., 2016), antibacterial (Ghannay et al., 2017), antifungal (Kumar et al., 2003), antimycobacterial (Kumar et al., 2010) and antiretroviral activities (Loh et al., 2010). The 1,3-dipolar cycloaddition of nitrones and olefines is one of the most useful reactions for the synthesis of isoxazolidine heterocycles (Nguyen et al., 2012). These 1,3-dipolar cycloadditions have a high potential as they form cycloadducts with simultaneous creation of several chiral centers through highly stereocontrolled processes (Nguyen et al., 2010). In connection with our synthetic studies on the importance of chiral nitrones for the synthesis of potential biologically interesting molecules, we have developed approaches toward enantiopure amino-acids such as (2S,3R,4R)-(Aouadi et al., 2007) or (2S,3S,4R)-4-hydroxy-isoleucine (Aouadi et al., 2012a) and 4(S)-4-hydroxy-L-ornithine (Aouadi et al., 2012b) via 1,3-dipolar cycloaddition of olefins with a glycine–menthone-derived nitrone. We have also examined a new route toward 3-substituted 4-hydroxyproline derivatives (Praly et al., 2014; Cecioni et al., 2015) and enantiopure cycloalkylglycines (Abda et al., 2014). More recently, we have described the synthesis and investigated the antioxidant and antimicrobial properties of enantiopure N-substituted pyrrolidin-2,5-dione derivatives obtained by 1,3-dipolar cycloaddition between chiral nitrone and N-substituted maleimides (Ghannay et al., 2017).
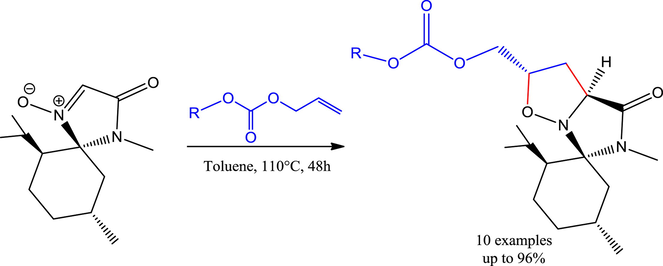
We are now reporting the synthesis of new enantiopure isoxazolidines derivatives by 1,3-dipolar cycloaddition of substituted aryl allyl carbonates with a glycine-menthone-derived nitrone and the assessment of their in vitro antioxidant and antibacterial activities.
2 Experimental
2.1 Materials and methods
TLC plates were inspected by UV light (λ = 254 nm). Silica gel column chromatography was performed with silica gel Si 60 (40–63 µm) purchased from Merck. Melting points were determined by a Barnstead Electrothermal 9100 melting. The NMR spectra were recorded on a Bruker instrument, operating at 400 or 500 MHz for 1H NMR and 100 or 125 MHz for 13C NMR and 376 MHz for 19F NMR. Optical rotations were determined by a Perkin Elmer polarimeter. High resolution mass spectrometry (HR-ESI-QToF) was determined by a Bruker MicroToF-Q II XL spectrometer.
2.2 General procedure (A) for the synthesis of allyl aryl carbonates
The alcohol (5 mmol, 1 eq) was dissolved, in the presence of a catalytic amount of TBAB, in dichloromethane (5 mL) and stirred with 4 M NaOH (2 mL) at 0 °C. Allyl chloroformate (1.13 eq) was slowly added and the reaction mixture was stirred for 1 h. The two layers were separated and the organic layer was washed with 2 M sodium hydroxide (5 mL), dried over Na2SO4 and evaporated under reduced pressure. The obtained oil was purified by flash column chromatography using petroleum ether and ethyl acetate (9:1) as an eluent.
2.3 General procedure (B) for the 1,3-DC of nitrone to various allyl aryl carbonates
To a solution of nitrone 1 (0.84 mmol, 200 mg) in toluene (15 mL) was added allyl aryl carbonates 2a–j and the mixture was refluxed for 48 h at 110 °C, under stirring. TLC showed the complete conversion of the nitrone 1. The solution was concentrated, and the residue was purified by flash chromatography (Cyclohexane/EtOAc, 7:3) to isolate cycloadducts 3a–j.
2.4 Allyl (6-bromonaphthalen-2-yl) carbonate (2f)
Obtained as a white solid (800 mg, 82%) following general procedure A:
Mp = 62–64 °C; 1H NMR (CDCl3, 400 MHz) δ 4.78 (dt, J = 1.6 Hz, J = 6.0 Hz, 2H); 5.35 (dq, J = 1.2 Hz, J = 8.4 Hz, 1H); 5.47 (dq, J = 1.2 Hz, J = 17.2 Hz, 1H); 6.02 (m, 1H); 7.35 (dd, J = 1.8 Hz, J = 9.2 Hz, 1H); 7.57 (dd, J = 2.0 Hz, J = 8.8 Hz, 1H); 7.63 (d, J = 2.4 Hz, 1H); 7.68 (d, J = 8.8 Hz, 1H); 7.77 (d, J = 8.8 Hz, 1H); 8.01 (d, J = 1.6 Hz, 1H). 13C NMR (CDCl3, 100 MHz) δ 69.3, 118.1, 119.7, 121.6, 128.6, 129.3, 129.8, 130.1, 131.0, 132.1, 132.5, 148.9, 153.4. HRMS, (ESI) calcd C14H11BrNaO3 [M+Na]+: 328.9784, found 328.9770.
2.5 Allyl 4-methoxybenzyl carbonate (2g)
Obtained as a colorless oil (920 mg, 84%) following general procedure A:
1H NMR (CDCl3, 400 MHz) δ 3.81 (s, 3H, OCH3); 4.62 (dt, J = 1.2 Hz, J = 6.0 Hz, 2H); 5.10 (s, 2H); 5.25 (dq, J = 1.2 Hz, J = 10.4 Hz, 1H); 5.35 (dq, J = 1.6 Hz, J = 17.2 Hz, 1H); 5.92 (m, 1H); 6.89 (dt, J = 2.4 Hz, J = 8.8 Hz, 2H); 7.33 (dt, J = 2.6 Hz, J = 8.8 Hz, 2H). 13C NMR (CDCl3, 100 MHz) δ 55.2, 68.4, 69.5, 113.9, 118.8, 127.3, 130.2, 131.5, 154.9, 159.8. HRMS, (ESI) calcd C12H14NaO4 [M+Na]+: 245.0784, found 245.0775.
2.6 Allyl 3-fluorobenzyl carbonate (2h)
Obtained as a colorless oil (1.2 g, 81%) following general procedure A:
1H NMR (CDCl3, 400 MHz) δ 4.65 (dt, J = 1.6 Hz, J = 6.0 Hz, 2H); 5.15 (s, 2H); 5.28 (dq, J = 1.2 Hz, J = 10.4 Hz, 1H); 5.37 (dq, J = 1.2 Hz, J = 17.2 Hz, 1H); 5.93 (m, 1H); 7.03 (m, 1H); 7.10 (dt, J = 1.6 Hz, J = 9.2 Hz, 1H); 7.15 (m, 1H); 7.33 (td, J = 5.6 Hz, J = 8.0 Hz, 2H). 13C NMR (CDCl3, 100 MHz) δ 68.7, 114.7, 115.1, 115.3, 115.5, 119.1, 123.5 (d, JC-F = 2.25 Hz), 130.1, 130.2, 131.4, 137.6 (d, JC-F = 5.55 Hz), 154.8, 161.6, 164.0. HRMS, (ESI) calcd C11H11FNaO3 [M+Na]+: 233.0584, found 233.0590.
2.7 Allyl 4-(trifluoromethyl)benzyl carbonate (2j)
Obtained as a colorless oil (1.8 g, 83%) following general procedure A:
1H NMR (CDCl3, 400 MHz) δ 4.65 (m, 1H); 5.15 (s, 2H); 5.22 (brs, 1H); 5.27 (m, 2H); 5.37 (dq, J = 1.2 Hz, J = 17.2 Hz, 1H); 5.94 (m, 1H); 7.50 (dt, J = 8.0 Hz, 1H); 7.63 (d, J = 8.4 Hz, 1H). 13C NMR (CDCl3, 100 MHz) δ 53.4, 68.8, 118.7, 119.2, 125.5 (q, JC-F = 2.85 Hz), 128.1, 131.3, 131.5, 139.2, 154.8. 19F NMR (CDCl3, 376 MHz) −62.71. HRMS, (ESI) calcd C12H11F3NaO3 [M+Na]+: 283.0552, found 283.0553.
2.8 ((1S,2S,2′S,3a′S,5R)-2-Isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl phenyl carbonate (3a)
Obtained as a colorless oil (309 mg, 83%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl phenyl carbonate (1.68 mmol, 300 mg). Rf = 0.37 (Cyclohexane/EtOAc 8/2); [α]D25 = −63.1 (c = 1, CH2Cl2); 1H NMR (CDCl3, 500 MHz) δ 0.82 (d, J = 6.5 Hz, 3H, CH3); 0.85 (d, J = 7.0 Hz, 3H, CH3); 0.90 (m, 1H); 0.93 (d, J = 6.5 Hz, 3H, CH3); 1.25 (t, J = 12.0 Hz, 1H); 1.37 (dd, J = 3.5 Hz, J = 12.5 Hz, 1H); 1.43 (m, 1H); 1.59 (m, 1H); 1.72 (qd, J = 3.5 Hz, J = 13.0 Hz, 1H); 1.81 (m, 1H); 2.05 (m, 1H); 2.31 (ddd, J = 7.0 Hz, J = 9.0 Hz, J 12.5 Hz,1H); 2.73 (ddd, 1H, J = 1.5 Hz, J = 6.0 Hz, J = 12.5 Hz); 2.74 (s, 3H, NCH3); 3.97 (d, J = 9.0 Hz, 1H); 4.19 (m, 1H); 4.24 (dd, J = 4.0 Hz, J = 12.0 Hz,1H); 4.36 (dd, J = 7.0 Hz, J = 12.0 Hz, 1H); 7.16 (d, 2H, J = 8.0 Hz); 7.24 (t, 1H, J = 7.5 Hz); 7.37 (t, J = 8.0 Hz, 2H). 13C NMR (125 MHz, CDCl3) δ 18.5, 22.3, 22.4, 24.1, 24.3, 26.0, 29.3, 34.5, 34.6, 40.7, 48.1, 65.2, 67.9, 73.7, 89.1, 120.9, 126.1, 129.4, 151.0, 153.4, 172.6. HRMS, (ESI) calcd C23H32N2NaO5 [M+Na]+: 439.22038, found 439.2203.
2.9 4-Ethylphenyl (((1S,2S,2′S,3a′S,5R)-2-isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl) carbonate (3b)
Obtained as a colorless oil (350 mg, 92%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl 4-ethylphenyl carbonate (2.52 mmol). Rf = 0.34 (Cyclohexane/EtOAc 6/4); [α]D25 = −55.6 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.81 (d, J = 6.8 Hz, 3H, CH3); 0.83 (d, J = 6.8 Hz, 3H, CH3); 0.81 (d, J = 6.8 Hz, 3H, CH3); 0.89 (m, 1H); 0.91 (d, J = 6.8 Hz, 3H, CH3); 1.20 (t, J = 7.6 Hz, 3H); 1.25 (d, J = 12.4 Hz, 1H); 1.35 (dd, J = 2.8 Hz, J = 12.0 Hz, 1H); 1.42 (m, 1H); 1.58 (m, 1H); 1.72 (qd, J = 3.6 Hz; J = 12.8 Hz, 1H); 1.80 (m, 1H); 2.04 (m, 2H); 2.28 (m, 1H); 2.62 (q, J = 7.6 Hz, 2H); 2.71 (ddd, J = 2.0 Hz, J = 6.4 Hz, J = 12.4 Hz, 1H); 2.72 (s, 3H, NCH3); 3.95 (d, J = 8.8 Hz, 1H); 4.20 (m, 2H); 4.34 (q, J = 6.8 Hz, 1H); 7.04 (d, J = 8.4 Hz, 2H); 7.17 (d, J = 8.4 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 15.4, 18.4, 22.2, 22.3, 24.0, 24.2, 25.9, 28.1, 29.2, 34.5, 34.6, 40.6, 48.0, 65.1, 67.7, 73.6, 89.0, 120.5, 128.6, 127.7, 141.9, 148.9, 153.5, 172.5. HRMS, (ESI) calcd C25H36N2NaO5 [M+Na]+: 467.2516, found 467.2505.
2.10 2,5-Dimethylphenyl (((1S,2S,2′S,3a′S,5R)-2-isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl) carbonate (3c)
Obtained as a colorless oil (260 mg, 76%) following general procedure B: nitrone (0.63 mmol, 150 mg), allyl 2,5-dimethylphenyl carbonate (1.89 mmol, 400 mg). Rf = 0.44 (Cyclohexane/EtOAc 6/4); [α]D25 = −49.1 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.81 (d, J = 6.8 Hz, 3H, CH3); 0.84 (d, J = 6.8 Hz, 3H, CH3); 0.89 (m, 1H); 0.92 (d, J = 6.8 Hz, 3H, CH3); 1.24 (t, J = 11.6 Hz, 1H); 1.36 (dd, J = 2.8 Hz, J = 12.0 Hz, 1H); 1.42 (m, 1H); 1.58 (m, 1H); 1.72 (qd, 1H, J = 3.6 Hz, J = 13.2 Hz); 1.81 (m, 1H); 2.05 (m, 2H); 2.15 (s, 3H, CH3); 2.28 (ddd, J = 6.8 Hz, J = 9.2 Hz, J = 16.4 Hz, 1H); 2.29 (s, 3H, CH3); 2.72 (ddd, J = 2.0 Hz, J = 6.0 Hz, J = 12.4 Hz, 1H); 2.73 (s, 3H, NCH3); 3.95 (d, J = 8.8 Hz, 1H); 4.21 (m, 2H); 4.36 (q, J = 8.0 Hz, 1H); 6.89 (s, 1H); 6.94 (d, J = 7.6 Hz, 1H); 7.08 (d, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 15.3, 18.4, 20.7, 22.2, 22.3, 24.0, 24.2, 25.9, 29.2, 34.5, 34.6, 40.6, 48.0, 65.1, 67.8, 73.6, 89.0, 121.7, 126.5, 127.0, 130.8, 136.9, 149.2, 153.3, 172.5. HRMS, (ESI) calcd C25H36N2NaO5 [M+Na]+: 467.2516, found 467.2509.
2.11 ((1S,2S,2′S,3a′S,5R)-2-Isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl (2-methoxyphenyl) carbonate (3d)
Obtained as a yellow oil (316 mg, 85%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl 2-methoxyphenyl carbonate (2.52 mmol, 524 mg). Rf = 0.22 (Cyclohexane/EtOAc 7/3); [α]D25 = −81.3 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.83 (d, J = 6.9 Hz, 3H, CH3); 0.86 (d, J = 6.8 Hz, 3H, CH3); 0.92 (m, 1H); 0.93 (d, J = 6.4 Hz, 3H, CH3); 1.25 (t, J = 11.6 Hz, 1H); 1.37 (dd, J = 3.2 Hz, J = 12.4 Hz, 1H); 1.45 (m, 1H); 1.60 (m, 1H); 1.73 (qd, J = 3.6 Hz, J = 12.8 Hz, 1H); 1.82 (m,1H); 2.05 (m, 2H); 2.31 (ddd, J = 7.2 Hz, J = 9.2 Hz, J = 12.4 Hz,1H); 2.74 (ddd, J = 1.6 Hz, J = 6.0 Hz, J = 12.8 Hz, 1H); 2.78 (s, 3H, NCH3); 3.80 (s, 3H, OCH3); 3.97 (d, J = 8.4 Hz, 1H); 4.20 (m,1H); 4.25 (dd, J = 4.0 Hz, J = 11.2 Hz, 1H); 4.36 (dd, J = 6.8 Hz, J = 11.2 Hz, 1H); 6.72 (m, 1H); 6.79 (m, 2H); 7.27 (t, J = 8.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 18.5, 22.3, 22.4, 24.1, 24.3, 26.0, 29.3, 34.6, 34.7, 40.7, 48.1, 55.4, 65.2, 67.9, 73.7, 89.1, 107.1, 111.9, 113.1, 129.8, 151.9, 153.3, 160.4, 172.6. HRMS, (ESI) calcd C24H34N2NaO6 [M+Na]+: 469.2309, found 469.2296.
2.12 2-Bromophenyl (((1S,2S,2′S,3a′S,5R)-2-isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl) carbonate (3e)
Obtained as a colorless oil (362 mg, 87%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl 2-bromophenyl carbonate (2.52 mmol, 680 mg). Rf = 0.54 (Cyclohexane/EtOAc 6/4); [α]D25 = −71.2 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.82 (d, J = 6.8 Hz, 3H, CH3); 0.85 (d, J = 6.8 Hz, 3H, CH3); 0.89 (m, 1H); 0.92 (d, J = 6.4 Hz, 3H, CH3); 1.25 (t, J = 12.0 Hz, 1H); 1.37 (dd, J = 3.2 Hz, J = 12.4 Hz, 1H); 1.44 (q, J = 6.8 Hz, 1H); 1.60 (m, 1H); 1.70 (m,1H); 1.81 (m,1H); 2.05 (m, 2H); 2.30 (m, 1H); 2.74 (ddd, J = 1.6 Hz, J = 6.0 Hz, J = 12.4 Hz, 1H); 2.75 (s, 3H, NCH3); 3.96 (d, J = 8.0 Hz, 1H); 4.25 (m, 2H); 4.40 (m, 1H); 7.14 (m, 1H); 7.21 (dd, J = 1.6 Hz, J = 8.0 Hz, 1H); 7.34 (m,1H); 7.60 (dd, J = 1.6 Hz, J = 8.0 Hz, 1H).13C NMR (100 MHz, CDCl3) δ 18.5. 22.3. 22.4. 24.1. 24.3. 26.0. 29.3. 34.5. 34.6. 40.7. 48.1. 65.1. 68.4. 73.6. 89.0. 115.9. 123.1. 127.7. 128.5. 133.5. 148.2. 152.5. 172.6. HRMS, (ESI) calcd C23H31BrN2NaO5 [M+Na]+: 517.1309, found 517.1299.
2.13 6-Bromonaphthalen-2-yl (((1S,2S,2′S,3a′S,5R)-2-isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl) carbonate (3f)
Obtained as a yellow oil (398 mg, 86%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl 6-bromonaphtyl carbonate (2.52 mmol, 770 mg). Rf = 0.42 (Cyclohexane/EtOAc 6/4); [α]D25 = −95.6 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.82 (d, J = 6.8 Hz, 3H, CH3); 0.84 (d, J = 6.8 Hz, 3H, CH3); 0.89 (m, 1H); 0.92 (d, J = 6.4 Hz, 3H, CH3); 1.25 (t, J = 12.0 Hz, 1H); 1.36 (dd, J = 2.8 Hz, J = 12.0 Hz, 1H,); 1.43 (m, 2H); 1.59 (m, 1H); 1.73 (qd, J = 3.6 Hz, J = 12.8 Hz, 1H); 1.81 (m, 1H); 2.05 (m, 2H); 2.31 (ddd, J = 6.8 Hz, J = 9.2 Hz, J = 12.4 Hz, 1H); 2.73 (ddd, J = 2.0 Hz, J = 6.0 Hz, J = 12.4 Hz, 1H); 2.74 (s, 3H, NCH3); 3.97 (d, J = 8.4 Hz, 1H); 4.20 (m,1H); 4.27 (dd, J = 3.6 Hz, J = 11.2 Hz, 1H); 4.39 (dd, J = 6.8 Hz, J = 11.2 Hz, 1H); 7.31(dd, J = 2.4 Hz, J = 8.8 Hz, 1H); 7.55 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H); 7.60 (d, J = 2.0 Hz, 1H); 7.65 (d, J = 8.8 Hz, 1H); 7.74 (d, J = 8.8 Hz, 1H); 7.98 (d, J = 1.2 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 18.4, 22.3, 24.1, 24.3, 25.9, 26.8, 29.2, 34.5, 34.6, 40.7, 48.0, 65.1, 68.0, 73.6, 89.0, 117.9, 119.7, 121.4, 128.6, 129.2, 129.7, 130.0, 132.0, 132.4, 148.8, 153.3, 172.5. HRMS, (ESI) calcd C27H33BrN2NaO5 [M+Na]+: 567.1465, found 567.1428.
2.14 ((1S,2S,2′S,3a′S,5R)-2-Isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl 4-methoxybenzyl carbonate (3g)
Obtained as a brown oil (369 mg, 96%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl 4-methoxybenzyl carbonate (2.52 mmol, 558 mg). Rf = 0.28 (Cyclohexane/EtOAc 7/3); [α]D25 = −76.8 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.80 (d, J = 6.4 Hz, 3H, CH3); 0.84 (d, J = 6.9 Hz, 3H, CH3); 0.85 (m, 1H); 0.89 (d, J = 6.6 Hz, 3H, CH3); 1.22 (m, 1H); 1.39 (m, 2H); 1.64 (m, 3H); 1.80 (m, 1H); 2.02 (m, 2H); 2.26 (m, 1H); 2.68 (ddd, J = 1.8 Hz, J = 6.0 Hz, J = 13.6 Hz, 1H); 2.72 (s, 3H, NCH3); 3.80 (s, 3H, OCH3); 3.93 (d, J = 9.0 Hz, 1H); 4.16 (m, 3H); 5.08 (s, 2H); 6.87 (d, J = 8.4 Hz, 2H); 7.31 (d, J = 8.4 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 18.4, 22.2, 22.3, 24.1, 24.3, 26.0, 29.2, 34.6, 34.7, 40.6, 48.1, 55.2, 65.2, 67.7, 73.8, 89.1, 113.9, 127.1, 130.3, 154.9, 159.8, 172.6. HRMS, (ESI) calcd C25H36N2NaO6 [M+Na]+: 483.2466, found 483.2462.
2.15 3-Fluorobenzyl (((1S,2S,2′S,3a′S,5R)-2-isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl) carbonate (3h)
Obtained as a colorless oil (320 mg, 86%) following general procedure B: nitrone (0.84 mmol, 200 mg), allyl 3-fluorobenzyl carbonate (2.52 mmol, 500 mg). Rf = 0.33 (Cyclohexane/EtOAc 7/3); [α]D25 = −66.4 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.80 (d, J = 6.8 Hz, 3H, CH3); 0.83 (d, J = 6.8 Hz, 3H, CH3); 0.87 (m, 1H); 0.88 (d, J = 6.4 Hz, 3H, CH3); 1.21 (t, J = 11.6 Hz, 1H); 1.34 (dd, J = 2.8 Hz, J = 12.0 Hz, 1H); 1.41 (q, J = 7.2 Hz, 1H); 1.57 (m, 1H); 1.69 (qd, J = 3.6 Hz, J = 13.2 Hz, 1H); 1.79 (m, 1H); 2.01 (m, 2H); 2.25 (ddd, J = 7.2 Hz, J = 9.2 Hz, J = 16.4 Hz, 1H); 2.68 (ddd, 1H, J = 2.0 Hz, J = 6.0 Hz, J = 12.8 Hz); 2.72 (s, 3H, NCH3); 3.93 (d, J = 8.8 Hz, 1H); 4.12 (m, 1H); 4.17 (dd, J = 4.4 Hz, J = 11.6 Hz, 1H); 4.24 (dd, J = 6.8 Hz, J = 11.2 Hz, 1H); 5.12 (s, 2H, CH2); 7.01 (m, 1H); 7.06 (dt, J = 1.6 Hz, J = 9.2 Hz, 1H); 7.12 (dd, J = 0.4 Hz, J = 7.6 Hz, 1H); 7.31 (td, J = 6.0 Hz, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 18.4, 22.2, 22.3, 24.1, 24.3, 25.9, 29.2, 34.6, 40.6, 48.0, 65.2, 67.6, 68.7,73.7, 89.0, 114.8, 115.0, 115.3, 115.5, 123.4, 123.5, 130.1, 130.2, 137.4, 137.5, 154.7, 161.6, 164.0, 172.6. HRMS, (ESI) calcd C24H33FN2NaO5 [M+Na]+: 471.2266, found 471.2253.
2.16 4-Fluorobenzyl (((1S,2S,2′S,3a′S,5R)-2-isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl) carbonate (3i)
Obtained as a colorless oil (334 mg, 89%) following general procedure B: nitrone (0.63 mmol, 150 mg), allyl 4-fluorobenzyl carbonate (1.89 mmol, 375 mg). Rf = 0.30 (Cyclohexane/EtOAc 7/3); [α]D25 = −61.8 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.78 (d, J = 7.0 Hz, 3H, CH3); 0.81 (d, J = 7.0 Hz, 3H, CH3); 0.85 (m, 1H); 0.86 (d, J = 6.5 Hz, 3H, CH3); 1.20 (t, J = 12.0 Hz, 1H); 1.33 (dd, J = 3.0 Hz, J = 12.0 Hz, 1H); 1.39 (quin, J = 7.0 Hz, 1H); 1.56 (m, 1H); 1.67 (qd, J = 3.5 Hz, J = 13.0 Hz, 1H); 1.77 (m, 1H); 1.96 (m, 2H); 2.23 (m, 1H); 2.65 (ddd, J = 1.5 Hz, J = 6.0 Hz; J = 12.5 Hz, 1H); 2.70 (s, 3H, NCH3); 3.91 (d, J = 9.0 Hz, 1H); 4.08 (m, 1H); 4.14 (dd, J = 4.0 Hz, J = 11.5 Hz, 1H); 4.21 (dd, J = 6.5 Hz, J = 11.5 Hz, 1H); 5.08 (s, 2H, CH2); 7.01 (t, J = 9.0 Hz, 2H); 7.32 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 18.3, 22.1, 22.2, 24.0, 24.2, 25.9, 26.8, 29.1, 34.5, 34.7, 40.6, 48.0, 65.1, 67.4, 86.9, 73.7, 89.0, 115.3, 115.5, 130.2, 130.3, 130.8, 130.9, 154.7, 161.7, 163.7, 172.5. HRMS, (ESI) calcd C24H33FN2NaO5 [M+Na]+: 471.2266, found 471.2261.
2.17 ((1S,2S,2′S,3a′S,5R)-2-Isopropyl-5,5′-dimethyl-4′-oxotetrahydro-2′H-spiro[cyclohexane-1,6′-imidazo[1,5-b]isoxazol]-2′-yl)methyl 4-(trifluoromethyl)benzyl carbonate (3j)
Obtained as a yellow oil (480 mg, 92%) following general procedure B: nitrone (1.05 mmol, 250 mg), allyl 4-trifluoromethylbenzyl carbonate (3.15 mmol). Rf = 0.34 (Cyclohexane/EtOAc 6/4); [α]D25 = −89.1 (c = 1, CH2Cl2); 1H NMR (CDCl3, 400 MHz) δ 0.79 (d, J = 6.4 Hz, 3H, CH3); 0.83 (d, J = 6.8 Hz, 3H, CH3); 0.84 (m, 1H); 0.87 (d, J = 6.4 Hz, 3H, CH3); 1.21(t, J = 12.0 Hz, 1H); 1.34 (dd, J = 3.2 Hz, J = 12.0 Hz, 1H); 1.40 (q, J = 6.8 Hz, 1H); 1.57 (m, 1H); 1.68 (qd, J = 3.2 Hz, J = 12.8 Hz, 1H); 1.78 (m, 1H); 1.98 (m, 2H); 2.24 (m, 1H); 2.67 (m, 1H); 2.71 (s, 3H, NCH3); 3.93 (d, J = 8.8 Hz, 1H); 4.10 (m, 1H); 4.17 (dd, J = 4.0 Hz, J = 11.2 Hz, 1H); 4.24 (dd, 1H, J = 6.4 Hz, J = 11.2 Hz); 5.18 (s, 2H, CH2); 7.46 (d, J = 8.0 Hz, 2H); 7.60 (d, J = 8.0 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 18.4, 22.2, 22.3, 24.0, 24.3, 25.9, 29.2, 34.6, 40.6, 48.0, 65.2, 67.6, 86.5, 73.7, 89.1, 118.9, 119.8, 122.5, 125.2, 125.5 (q, 1JC-F = 3.8 Hz), 127.9, 128.1, 130.1, 130.4, 130.7, 131.3, 139.0, 154.7, 172.5. HRMS, (ESI) calcd C25H33F3N2NaO5 [M+Na]+: 521.2234, found 521.2217.
2.18 Antioxidant activity
2.18.1 DPPH free radical scavenging activity
The antioxidant ability of samples to scavenge free radical was tested using a synthetic compound, 2,2 -diphenyl-1-picrylhydrazyl (DPPH) and was determined according to the same method reported by Ghannay et al. (2017). Ascorbic acid was served as standard for comparison and all measurements were done in triplicate.
2.18.2 Scavenging activity of ABTS•+ free radical
Radical scavenging activity of compounds was assessed by spectrophotometrically by [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] ABTS•+ cation decolorization test using the same method as described by Ghannay et al. (2017). Trolox was used as reference and all measurements were carried out on three identical samples.
2.18.3 Ferric-reducing/antioxidant power (FRAP) assay
The reducing power capacity of the tested compounds was carried out by potassium ferricyanide method according to the method described previously by Kadri et al. (2011). Ascorbic acid was used as standard controls and all tests were repeated three times.
2.19 Screening of antibacterial activity
The antibacterial activity was tested against six bacteria strains including four Gram-positive bacteria (Bacillus subtilis JN 934392, Bacillus cereus JN 934390, Staphylococcus aureus ATCC 6538, Micrococcusluteus), and two Gram-negative bacteria (Salmonella entericsero type Enteritidis ATCC43972 and Escherichia coli ATCC 25922). The growth conditions, the detection of the antibacterial activity by agar diffusion method, the minimum inhibitory concentration (MIC), and the minimum bactericidal concentration (MBC) were based on the same method as reported used by Ghannay et al. (2017).
3 Results and discussion
3.1 Synthesis
Substituted aryl allyl carbonates 2a–j have been synthesized according to general procedure from the corresponding alcohols and chloroformates (Breen, 2004) while the synthesis of dipolarophiles 2f, 2g, 2h and 2j have not been described in the literature. 1,3-Dipolar cycloaddition between the (–)-menthone-based nitrone as glycine equivalent 1 and substituted aryl allyl carbonates 2a–j was completed after 48 h in refluxing toluene to afford unprecedented isoxazolidine heterocycles 3a–j in a highly regio-, and stereo-selective fashion and with simultaneous creation of two stereogenic centers (Scheme 1) (see Table 1).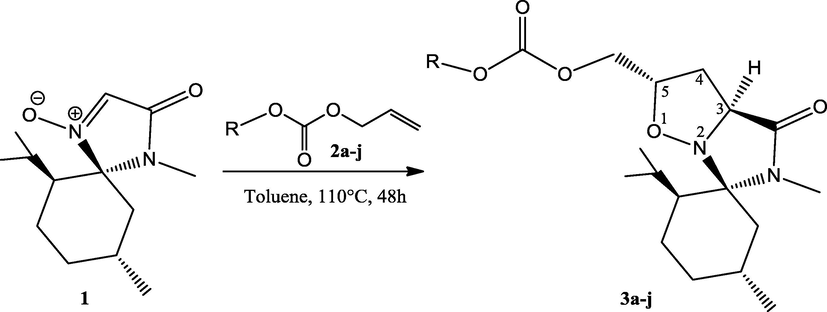
Synthesis of enantiopure isoxazolidines 3a–j.
Entry
Compounds
R
Aspect
Yieldsa (%)
1
3a
Phenyl
Colorless oil
83
2
3b
4-Et-Phenyl
Colorless oil
92
3
3c
2,5-diMe-Phenyl
Colorless oil
76
4
3d
2-OMe-Phenyl
Yellow oil
85
5
3e
2-Br-Phenyl
Colorless oil
87
6
3f
6-Br-Naphtyl
Yellow oil
86
7
3g
4-OMe-Benzyl
Brown oil
96
8
3h
3-F-Benzyl
Colorless oil
86
9
3i
4-F-Benzyl
Colorless oil
89
10
3j
4-CF3-Benzyl
Yellow oil
92
The structures of the newly synthesized compounds 3a–j were established by mono and two-dimensional NMR spectroscopies which provided data in accordance with literature (Aouadi et al., 2006, 2008). The stereoselectivity of isoxazolidines 3a–j results from the approach of substituted aryl allyl carbonates 2 on the opposite side to the isopropyl motif of the dipole 1. The reaction proceeds via a transition state positioning the substituted aryl allyl carbonates group away of the dipole corresponding to an exo-approach (Scheme 1).
2D NMR spectroscopy (COSY, HSQC) and HMBC experiments allowed the complete and unambiguous assignment of the chemical shifts of the carbons C-4 (δ = 34.5–34.6 ppm) and C-5 (δ = 73.6–73.8 ppm) and the regioselectivity of the compounds 3a–j. For example, the HMBC spectrum of compound 3a indicated that the carbonyl group of the imidazolidinone ring (δc = 172.6) correlated with H4 (δc = 2.31) and H4′ (δc = 2.73), C-6 (δc = 67.9) with H-4 (δc = 2.31) and H-4′ (δc = 2.73). Additionally, no HMBC correlation was observed between C-6 with H-3. The results were of particular significance to confirm the regioselectivity of the 1,3-dipolar cycloaddition of nitrone-derived (–)-menthone with alkenes 2a–j which more corroborate the literature data (Aouadi et al., 2006; Ghannay et al. 2016).
In 2D NOESY experiment we observed a weak NOE effects between protons H3–H4′ and H4–H5, medium effect between protons H5–H12′ and strong correlations between protons H3–H4, H4′–H5. Additional correlations were observed between H3 and both H13 and H14. This was also in accordance with the stereochemistry suggested for 3a (Fig. 1).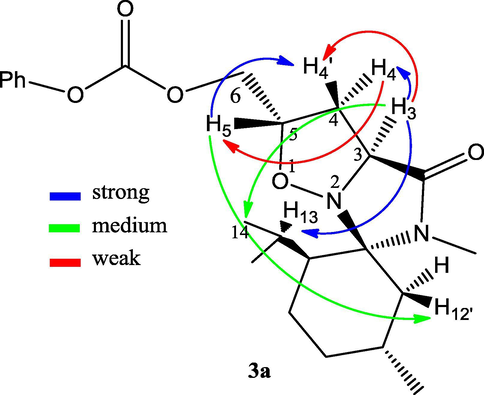
Characteristic NOESY correlations of compound 3a.
3.2 Antioxidant activity
In the present investigation, the antioxidant activity of the synthesized compounds was screened in vitro using the 1,1-diphenyl-2-picrylhydrazyl (DPPH); 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and ferric reducing antioxidant power (FRAP) assays. Samples were tested at different concentrations ranged from 1 to 10 mg/mL, then from the dose–response activities, the IC50 values were obtained and are presented in Table 2. IC50 (mg/mL): Values corresponding to the amount of extract required to scavenge 50% of radicals present in the reaction mixture.
Samples
C (mg/mL)
0
1
3
6
8
10
IC50
(3a)
0
5.98 ± 2.02
17.49 ± 2.15
35.78 ± 1.40
58.18 ± 1.80
80.00 ± 2.03
7.22 ± 1.98
(3b)
0
14.50 ± 1.09
21.92 ± 1.70
34.36 ± 1.33
47.65 ± 2.33
54.50 ± 1.50
8.63 ± 2.01
(3c)
0
10.66 ± 2.01
37.91 ± 1.66
60.82 ± 1.90
74.92 ± 1.44
79.91 ± 1.33
4.49 ± 1.66
(3d)
0
12.56 ± 1.09
28.93 ± 1.33
53.23 ± 2.15
80.00 ± 0.90
80.66 ± 1.09
5.60 ± 2.10
(3e)
0
7.86 ± 1.07
21.79 ± 2.08
34.21 ± 1.33
55.76 ± 1.07
77.33 ± 2.32
7.42 ± 2.01
(3f)
0
14.66 ± 1.9
44.30 ± 1.90
67.00 ± 1.05
71.60 ± 2.10
81.52 ± 2.01
3.67 ± 1.50
(3g)
0
16.69 ± 2.33
25.35 ± 2.05
32.99 ± 1.66
45.74 ± 1.07
70.90 ± 1.02
8.32 ± 1.20
(3h)
0
29.23 ± 1.02
57.93 ± 1.12
70.13 ± 1.99
81.44 ± 1.30
85.10 ± 1.98
2.42 ± 1.90
(3i)
0
8.66 ± 1.66
26.00 ± 1.33
37.53 ± 1.66
53.50 ± 1.09
58.60 ± 1.33
7.49 ± 1.45
(3j)
0
7.45 ± 1.09
21.79 ± 2.05
26.90 ± 2.00
38.31 ± 2.66
57.60 ± 2.66
9.20 ± 1.90
Ascorbic acid
0
59.89 ± 2.10
79.44 ± 1.50
78.89 ± 1.02
79.11 ± 1.33
79.11 ± 1.01
0.82 ± 0.66
3.2.1 Free radical-scavenging ability by the DPPH assay
DPPH radical scavenging activity results of tested compounds and ascorbic acid were recorded in terms of % inhibition of DPPH and IC50 (Concentration scavenging 50% of DPPH radical) are summarized in Table 2. They vary in a dose dependent manner with IC50 values for the different compounds ranged from 0.82 to 9.20 mg/mL. The stronger DPPH radical scavenging activity was allowed to compound 3h which is about 3 times lower than the than the specific inhibitor ascorbic acid (IC50 = 0.82 ± 0.66 mg/mL) followed by 3f and in lower degree 3c. All the others tested compounds showed weaker scavenging activity values than the positive control, ascorbic acid, specially the derivative 3j.
3.2.2 Free radical-scavenging ability by the ABTS assay
Free radical scavenging activity of tested compounds was determined according to ABTS radical assay based on electron and H atom transferas well as known to be attributable to their hydrogen-donating ability. The results were expressed in terms of % inhibition and IC50 values are displayed in Table 3. As shown, % inhibition of the ABTS radical activity followed similar pattern as for DPPH assay. Among all compounds, compounds 3c, 3f and 3h showed the potent antioxidant activity estimated by IC50 value which is about 2.8–3.3 times lower than the standard Trolox (0.78 ± 0.66 mg/mL). Compounds 3b and 3e exhibited poor activity while the other four compounds are inactive. IC50 (mg/mL): Values corresponding to the amount of extract required to scavenge 50% of radicals present in the reaction mixture. (–): Not detected.
Samples
C (mg/mL)
0
1
3
6
8
10
IC50
(3a)
0
7.14 ± 2.01
17.42 ± 1.66
21.71 ± 1.09
26.57 ± 1.32
35.85 ± 0.98
–
(3b)
0
12.57 ± 1.33
24.57 ± 1.09
31.57 ± 1.50
39.71 ± 0.70
54.57 ± 0.98
9.38 ± 0.56
(3c)
0
36.71 ± 1.51
52.42 ± 1.33
60.57 ± 0.98
67.57 ± 0.98
77.42 ± 1.01
2.62 ± 1.06
(3d)
0
5.28 ± 2.10
20.42 ± 2.09
39.57 ± 1.07
41.85 ± 1.09
49.85 ± 1.01
–
(3e)
0
14.28 ± 2.05
20.14 ± 2.01
36.42 ± 1.33
57.28 ± 0.70
69.71 ± 1.00
7.25 ± 0.99
(3f)
0
22.14 ± 2.02
51.14 ± 1.09
66.71 ± 1.10
76.28 ± 1.33
81.57 ± 0.98
2.85 ± 1.66
(3g)
0
23.28 ± 1.09
43.42 ± 1.05
53.14 ± 0.70
59.28 ± 1.50
69.14 ± 1.01
4.85 ± 0.98
(3h)
0
37.85 ± 1.15
56.85 ± 1.33
67.87 ± 1.10
73.42 ± 0.98
79.57 ± 1.09
2.23 ± 1.33
(3i)
0
17.85 ± 1.98
22.42 ± 2.00
27.42 ± 1.56
34.14 ± 1.33
45.42 ± 1.33
–
(3j)
0
5.57 ± 1.33
12.28 ± 1.98
26.71 ± 0.98
33.42 ± 0.66
41.14 ± 0.70
–
Trolox
0
66.57 ± 0.66
74.57 ± 1.50
83.42 ± 1.33
86.28 ± 0.98
88.83 ± 1.01
0.78 ± 0.66
3.2.3 FRAP assay
The values expressed from the FRAP assay represent the corresponding concentration of electron-donating antioxidants with the reduction in the ferric iron (Fe3+) to the ferrous ion (Fe2+). There was a significant difference among the FRAP values and they ranged between 0.69 and 9.60 mg/mL for the tested compounds (Table 4). The results showed that the potent antioxidant capacity was ascribed to compound 3h (EC50 = 0.69 ± 0.25 mg/mL) followed by 3c (EC50 = 0.93 ± 0.07 mg/mL) and 3f (EC50 = 0.95 ± 0.06 mg/mL) which were higher than the EC50 values of ascorbic acid (EC50 = 0.99 ± 0.07 mg/mL), used as positive control. As can be seen, all others compounds exhibited moderate activity. EC50 (mg/mL): Values corresponding to the amount of extract required to scavenge radicals present in the reaction mixture at 0.5 absorbance. (–): Not detected.
Samples
C (mg/mL)
0
1
3
6
8
10
EC50
(3a)
0
0.21 ± 0.05
0.33 ± 0.05
0.45 ± 0.18
0.58 ± 0.05
0.61 ± 0.09
6.65 ± 1.66
(3b)
0
0.22 ± 0.09
0.28 ± 0.90
0.32 ± 0.25
0.40 ± 0.08
0.53 ± 0.09
9.60 ± 1.50
(3c)
0
0.36 ± 0.25
0.65 ± 0.15
0.74 ± 0.09
0.83 ± 0.09
0.93 ± 0.50
0.93 ± 0.07
(3d)
0
0.14 ± 1.00
0.32 ± 0.02
0.50 ± 0.07
0.69 ± 0.09
0.88 ± 0.07
6.00 ± 1.33
(3e)
0
0.22 ± 1.50
0.34 ± 0.08
0.40 ± 0.06
0.58 ± 0.33
0.70 ± 0.10
7.02 ± 1.20
(3f)
0
0.63 ± 0.07
0.71 ± 0.09
0.81 ± 0.75
0.94 ± 0.98
1.04 ± 0.09
0.95 ± 0.06
(3g)
0
0.09 ± 0.08
0.20 ± 0.08
0.35 ± 0.07
0.49 ± 0.08
0.73 ± 0.09
8.00 ± 1.50
(3h)
0
0.73 ± 0.66
0.88 ± 0.66
0.96 ± 0.46
0.99 ± 0.15
1.14 ± 1.33
0.69 ± 0.25
(3i)
0
0.14 ± 0.05
0.22 ± 0.10
0.30 ± 0.03
0.39 ± 0.09
0.54 ± 0.25
9.40 ± 1.66
(3j)
0
0.10 ± 0.06
0.14 ± 0.06
0.22 ± 0.07
0.30 ± 0.10
0.39 ± 0.05
–
Ascorbic acid
0
0.50 ± 0.25
0.80 ± 0.08
0.90 ± 0.33
0.95 ± 0.08
0.98 ± 0.33
0.99 ± 0.07
3.3 Antibacterial activity
The antibacterial activity of the synthesized compounds were assessed qualitatively and quantitatively by the inhibition zones diameter (IZD), minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC), and was compared to that of the known antibacterial agent chloramphenicol. The results summarized in Table 5 revealed that all compounds exhibited various degrees of inhibition against the tested microorganisms with the potent antibacterial activity was displayed by the compound 3h with IZD = 24.00 ± 0.22 mm and 19.00 ± 0.88 mm against B. cereus and E. coli, respectively, which were very closed to the standard chloramphenicol, 26.00 ± 1.00 mm and 23.50 ± 0.00, respectively. Also, we note the moderate to poor activity of compounds 3a–c. However compounds 3e–g and 3i–j were totally lacked antibacterial activity. (–): Not detected.
Inhibition zones diameter (mm)a
Strains
(3a)
(3b)
(3c)
(3d)
(3e)
(3f)
(3g)
(3h)
(3i)
(3j)
Chloramb
Gram+
Bacillus subtilis
10.00 ± 0.22
9.00 ± 0.33
12.00 ± 0.33
10.00 ± 0.66
–
–
–
9.00 ± 0.00
9.00 ± 0.22
–
24.00 ± 0.00
Bacillus cereus
9.00 ± 0.22
12.00 ± 0.00
9.00 ± 0.88
11.00 ± 0.33
9.00 ± 0.22
–
12.00 ± 0.22
24.00 ± 0.22
10.00 ± 0.22
10.00 ± 0.22
26.00 ± 1.00
Micrococcus luteus
8.00 ± 0.00
8.00 ± 0.22
7.00 ± 0.22
–
–
–
–
9.00 ± 0.22
–
–
20.00 ± 2.00
Staphylococcus aureus
11.00 ± 0.33
9.00 ± 0.22
10.00 ± 0.22
–
–
–
–
10.00 ± 0.33
–
–
17.00 ± 1.00
Gram−
Escherichia coli
12.00 ± 0.66
13.00 ± 0.33
16.00 ± 0.22
12.00 ± 0.22
10.00 ± 0.22
–
10.00 ± 0.33
19.00 ± 0.88
9.00 ± 0.22
9.00 ± 0.33
23.50 ± 0.00
Salmonella enteritidis
9.00 ± 0.66
9.00 ± 0.00
10.00 ± 0.22
–
–
–
–
–
–
–
16.00 ± 0.00
Also, MIC and MBC parameters were screened for all compounds and the resulted listed in Table 6 were generally in accordance with those obtained for IZD measurement. As can be seen, the pronounced values obtained of antimicrobial activity for compound 3h with MIC = 1.56 mg/mL for both B. cereus and E. coli suggested that these compounds have hydrophobic character which increases easily the diffusion properties across the cell membranes and therefore facilitate the killing microorganisms by affecting the metabolic pathways or organelles of the pathogen. The good MIC value obtained for E. coli was related to its outer and inner membrane that facilitate the penetration of the compounds with the observation of many holes and gaps on the damaged cells, which led to killing them eventually. MIC: minimum inhibitory concentrations. MBC: minimum bactericidal concentrations. (–): Not detected.
Samples
Strains
Bacillus subtilis
Bacillus cereus
Micrococcus luteus
Staphylococcus aureus
Escherichia coli
Salmonella enteritidis
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
(3a)
6.25
50.00
6.25
25.00
12.50
100
6.25
50.00
3.12
12.50
6.25
25.00
(3b)
3.12
25.00
3.12
50.00
12.50
100
12.50
50.00
3.12
12.50
6.25
25.00
(3c)
6.25
12.50
3.12
50.00
12.50
100
3.12
12.50
1.56
6.25
3.12
12.50
(3d)
12.50
50.00
6.25
50.00
–
–
–
–
6.25
50.00
–
–
(3e)
–
–
3.12
50.00
–
–
–
–
6.25
50.00
–
–
(3f)
–
–
–
–
–
–
–
–
–
–
–
–
(3g)
–
–
3.12
12.50
–
–
–
–
6.25
25.00
–
–
(3h)
12.50
50.00
1.56
3.12
6.25
25.00
3.12
12.50
1.56
3.12
–
–
(3i)
6.25
12.50
3.12
12.50
–
–
–
–
12.50
50.00
–
–
(3j)
–
–
6.25
50.00
–
–
–
–
12.50
50.00
–
–
3.4 Bioactivity score of isoxazolidine derivatives
The bioactivity scores of title compounds for drug targets towards G protein–coupled receptors (GPCR) ligands, ion channel modulator, kinase inhibitors, nuclear receptor inhibitors and other enzyme targets based on Molinspiration software were also predicted by Molinspiration and are presented in Table 6. The scores allowed adequate identification of active, moderately active or inactive molecules. A molecule having bioactivity score more than 0.00 is most likely to exhibit considerable biological activities, while values −0.50 to 0.00 are expected to be moderately active and if score is less than −0.50 it is presumed to be inactive. The results depicted in Table 7 showed the following observations. GPCR ligand: Except for compound 3e, all other compounds were found to be considerably bioactive with bioactivity scores ranged from 0.00 to 0.21. Ion channel modulator and kinase inhibitors: all our compounds were found to be moderately active having bioactivity scores ranged from −0.39 to −0.06 and −0.49 to −0.22, respectively. Nuclear receptor inhibitors: compounds 3a, 3d, 3e and 3g were found to be inactive with the bioactivity scores (−0.58 to −0.52). However those of 3b–c, 3f and 3h–j were moderately bioactive. Protease inhibitor: all our compounds were found to be active with the bioactivity scores ranging from 0.01 to 0.25. Enzyme inhibitor: only compounds 3c, 3e and 3g were found to be moderately active with bioactive scores −0.03, but the others one are with better bioactivity having bioactivity scores between 0.01 and 0.12. Our study shows that compound 3h fulfill the highest bioactivity score as compared to others one.
Compd. No.
GPCR ligand
Ion channel modulator
Kinase inhibitor
Nuclearreceptor ligand
Proteaseinhibitor
Enzyme inhibitor
3a
0.05
−0.21
−0.45
−0.52
0.14
0.04
3b
0.06
−0.19
−0.47
−0.47
0.14
0.04
3c
0.00
−0.28
−0.47
−0.48
0.06
−0.03
3d
0.02
−0.24
−0.42
−0.55
0.08
0.01
3e
−0.04
−0.39
−0.49
−0.58
0.01
−0.03
3f
0.04
−0.28
−0.37
−0.41
0.20
0.09
3g
0.14
−0.17
−0.30
−0.58
0.01
−0.03
3h
0.19
−0.12
−0.26
−0.39
0.25
0.11
3i
0.19
−0.13
−0.26
−0.39
0.23
0.11
3j
0.21
−0.06
−0.22
−0.27
0.25
0.12
The results clearly reveal that the physiological actions of isoxazolidine derivatives might involve multiple mechanisms and could be due to the interactions with GPCR ligands, nuclear receptor ligands, and inhibit protease and other enzymes. The bioactivity score of compounds is suggestive of moderate interaction with all drug targets.
3.5 Drug likeness score of isoxazolidine derivatives
According to Lipinski’s rule of five (Ghannay et al., 2017), used as a filter for drug-like properties to predict oral bioavailability, we describe the molecular properties of a compound in order to estimate their pharmacokinetic parameters in the human body, including their absorption, distribution, metabolism and excretion. Most “drug-like” molecules have milogP ≤5 that means these shows good permeability across cell membrane, number of hydrogen bond acceptors ≤10 this measures molecular flexibility, molecular weight ≤500, polar surface area (TPSA) < 160 Ǻ2 which shown to be a very good descriptor characterizing drug absorption and number of hydrogen bond donor’s ≤5 is the sum of OHs and NHs. Molecules violating more than one of these rules decrease the activity and selectivity of a likely drug candidate and therefore make it unlikely orally active in humans. The results depicted in Table 8 evaluate the oral bioavailability of isoxazolidine derivatives. The miLogP valve of compounds 3b–c, 3e–f and 3j were found above five, suggesting that the molecules have poor permeability across the cell membrane however the others one, are below five justifying their good permeability and therefore are an indication for good lipid solubility that will help the drug to interact with the membranes and to be used for generation of bioactivity. Molecular weight is an important parameter in therapeutic drug action which acts by affecting the drug mechanisms when it increases beyond certain limit. Molecular weight of isoxazolidine derivatives was found to be less than 500, except for compound 3f indicating that these compounds are anticipated to be easily transported, diffuse and absorbed. Polar surface area (TPSA) values were ranged from 68.32 to 77.55 Ǻ2, which shown to be a very good descriptor characterizing drug absorption with increasing molecules flexibility and facilitating thereforetheir interaction with a particular binding pocket. Number of hydrogen bond acceptors (O and N atoms) and number of hydrogen bond donors (NH and OH) of all the tested compounds were found to be less than 10 and 5, respectively and were in accordance with the Lipinski’s rule of five. Interestingly, except for compound 3f, all others compounds exhibited number of violation (n violations) =1 or <0 meaning that are easily bind to receptor. As shown, the determined molecular properties of these compounds justify the uses of the tested molecules as drugs.
Compd. No.
milogPa
TPSAb
N atomsc
MWd
NONe
NOHNHf
Nviol.g
Nrotb.h
Voli
Rule
<5
<500
<10
<5
(<10)
3a
4.86
68.32
30
416.52
7
0
0
6
392.90
3b
5.78
68.32
32
444.57
7
0
1
7
426.26
3c
5.69
68.32
32
444.57
7
0
1
6
426.02
3d
4.48
77.55
32
446.54
8
0
0
7
418.45
3e
5.62
68.32
31
495.41
7
0
1
6
410.79
3f
6.78
68.32
35
545.47
7
0
2
6
454.78
3g
4.82
77.55
33
460.57
8
0
0
8
435.25
3h
4.90
68.32
32
448.54
7
0
0
7
414.63
3i
4.92
68.32
32
448.54
7
0
0
7
414.63
3j
5.66
68.32
35
498.54
7
0
1
8
441.00
3.6 Structure–activity relationship (SAR) analysis
The structure–activity relationship (SAR) analysis of the antioxidant and antibacterial activity of these compounds (3a–j) revealed that 3h is the powerful active compound of this series. The meta-fluoro substituted halogen (3h) (IC50 = 2.42 ± 1.90 mg/mL) act as electron withdrawing group with lower solvolysis rate which facilitates free radical scavenging activity. However, the para-fluoro substituted (3i) act as electron donating, exhibited poor DPPH radical inhibition effect (IC50 = 7.49 ± 1.45 mg/mL), which was confirmed by (Shakil et al., 2013). The enhanced scavenging activity for 3f is probably due to the steric effect of the substituted group. The weaker DPPH radical scavenging activity of 3b (IC50 = 8.63 ± 2.01 mg/mL) was due to the presence of para-substituted ethyl group which enhances the electronic density, as compared to 3a (IC50 = 7.22 ± 1.98 mg/mL), however the presence of ortho-methyl 3c enhances the DPPH scavenging activity (IC50 = 4.49 ± 1.66 mg/mL) due to the hyper-conjugation effect of H (from methyl group that is prominent only at o-position) (Ghannay et al., 2017). Generally, similar pattern of activity was also observed with ABTS, FRAP and antibacterial assays. Analyzing the obtained data, no linear correlations were observed between the biological activity and the lipophilicity of the synthesized compounds. Compound 3d with the lowest miLogP value (4.48) exhibited poor antioxidant and antibacterial activities. Compound 3f with the highest miLogP presented a potent antioxidant activity but is devoid of antimicrobial potential.
4 Conclusion
In summary, we have reported a rapid and efficient stereocontrolled synthesis of ten novel isoxazolidine heterocycles. The compounds were studied for their structure-activity relationship between the physicochemical properties and bioactivities observed. Compound 3h with the best antioxidant and antibacterial potential has the highest bioactivity score as compared to others one which showed moderate to poor drug likeness score. Compound 3h can therefore be considered as a hit for further investigations applications in medicinal chemistry.
Acknowledgment
The authors are grateful to the Ministry of Higher Education and Scientific Research of Tunisia for financial support.
References
- Stereoselective synthesis of enantiopure cycloalkylglycines by 1,3-dipolar cycloaddition of a chiral nitrone to cycloalkenes. Eur. J. Org. Chem. 2014:6017-6024.
- [Google Scholar]
- Cycloadditions of chiral nitrones to racemic 3-substituted butenes: a direct access with kinetic resolution to enantiopure dihydroxylated amino acids. Synlett 2006:3299-3303.
- [Google Scholar]
- New synthetic routes toward enantiopure (2S,3R,4R)-4-hydroxyisoleucine by 1,3-dipolar cycloaddition of a chiral nitrone to C4 alkenes. Synthesis 2007:3399-3405.
- [Google Scholar]
- Analogues of insulin secretagogue (2S,3R,4S)-4-hydroxyisoleucine: synthesis by 1,3-dipolar cycloaddition reactions of chiral nitrones to alkenes. Tetrahedron Asymmetry. 2008;19:1145-1152.
- [Google Scholar]
- 1,3-Dipolar cycloaddition of a chiral nitrone to (E)-1,4-dichloro-2-butene: a new efficient synthesis of (2S,3S,4R)-4-hydroxyisoleucine. Tetrahedron Lett.. 2012;53:2817-2821.
- [Google Scholar]
- Cycloaddition of a chiral nitrone to allylic motifs: an access to enantiopuresugar-based amino acids displaying a stable glycosidic bond and to 4(S)-4-hydroxy-L-ornithine. Tetrahedron. 2012;68:1762-1768.
- [Google Scholar]
- Unprecedented stereoselective synthesis of 3-methylisoxazolidine-5-aryl-1,2,4-oxadiazoles via 1,3-dipolar cycloaddition and study of their in vitro antioxidant activity. Synth. Commun.. 2016;46:2037-2044.
- [Google Scholar]
- Isoxazolidine: a privileged scaffold for organic and medicinal chemistry. Chem. Rev.. 2016;116:15235-15283.
- [Google Scholar]
- Enzymatic resolution of a secondary amine using novel acylating reagents. Tetrahedron Asymmetry. 2004;15:1427-1430.
- [Google Scholar]
- Novel routes to either racemic or enantiopure a-amino-(4-hydroxypyrrolidin-3-yl)acetic acid derivatives and biological evaluation of a new promising pharmacological scaffold. Eur. J. Med. Chem.. 2015;98:237-249.
- [Google Scholar]
- Optically active isoxazolidines via asymmetric cycloaddition reactions of nitrones with alkenes: applications in organic synthesis. Tetrahedron. 1997;53:403-425.
- [Google Scholar]
- Stereoselective synthesis of enantiopure N-substituted pyrrolidin-2,5-dione derivatives by 1,3-dipolar cycloaddition and assessment of their in vitro antioxidant and antibacterial activities. Bioorg. Med. Chem. Lett.. 2017;27:2302-2307.
- [Google Scholar]
- Crystal structure of (1S,2S,2’R,3a’S,5R)-2’-[(5-bromo-1H-indol-3-yl)methyl]-2-isopropyl-5,5’- dimethyldihydro-2’H-spiro[cyclohexane-1,6’-imidazo[1,5-b]isoxazol]-4’(5’H)-one. Acta Cryst.. 2016;E72:1081-1084.
- [Google Scholar]
- Chemical composition and in vitro antioxidant properties of essential oil of Ricinuscommunis L. J. Med. Plants Res.. 2011;5:1466-1470.
- [Google Scholar]
- Synthesis of novel isoxazolidine derivatives and their antifungal and antibacterial properties. Arch. Pharm. Pharm. Med. Chem.. 2003;336:159-164.
- [Google Scholar]
- 1,3-Dipolar cycloaddition of C-aryl-N-phenylnitrones to (R)-1-(1-phenylethyl)-3-[(E)-arylmethylidene] tetrahydro-4(1H)-pyridinones: synthesis and antimycobacterial evaluation of enantiomerically pure spiroisoxazolidines. Eur. J. Med. Chem.. 2010;45:124-133.
- [Google Scholar]
- Inhibition of HIV-1 replication by isoxazolidine and isoxazole sulfonamides. Chem. Biol. Drug Des.. 2010;75:461-474.
- [Google Scholar]
- 1,3-Dipolar cycloadditions of nitrones to heterosubstituted alkenes. Part 1: oxa and aza-substituted alkenes. Org. Prep. Proced. Int.. 2010;42:387-431.
- [Google Scholar]
- 1,3-Dipolar cycloadditions of nitrones to heterosubstituted alkenes Part 2: sila-, thia-, phospha- and halosubstituted alkenes. Org. Prep. Proced. Int.. 2012;44:1-81.
- [Google Scholar]
- Praly, J.-P., Aouadi, K., Cecioni, S., Denoroy, L., Parrot, S., 2014. FR2992645A1 and WO2014006307A1.
- Microwave synthesis, characterization and bio-efficacy evaluation of novel chalcone based 6-carbethoxy-2-cyclohexen-1-one and 2H-indazol-3-ol derivatives. J. Eur. Med. Chem.. 2013;59:120-131.
- [Google Scholar]
- 1,3-Dipolar cycloaddition reactions of nitrones with alkyl vinyl ethers catalyzed by chiral oxazaborolidines. Tetrahedron. 1997;53:11843-11852.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2018.03.013.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







