Determination of the solubility of rivaroxaban (anticoagulant drug, for the treatment and prevention of blood clotting) in supercritical carbon dioxide: Experimental data and correlations
⁎Corresponding author. sodeifian@kashanu.ac.ir (Gholamhossein Sodeifian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Supercritical processes are utilized in pharmaceutical, chemical, agricultural and food industries. To design the micro or nanoparticles formation processes via a supercritical fluid (SCF), it is necessary to have available the solubility data of a solid solute in a solvent such as supercritical carbon dioxide (SC-
Keywords
Supercritical carbon dioxide
Rivaroxaban
Solubility
Peng-Robinson
Sodeifian model
Soave-Redlich-Kwong
Nomenclature
- a0-a7
-
Parameters for density-based models
- a, b
-
EoS constants
-
-
Percent of average absolute relative deviation
-
-
Concentration of drug inside collection vial (µg/mL)
- CVD
-
Covolume dependent mixing rule
-
-
Rivaroxaban solvation heat in kJ/mol
-
-
Rivaroxaban vaporization heat in kJ/mol
-
-
Total enthalpy of rivaroxaban in kJ/mol
-
-
Interaction parameters of Eqs. (19) and (20)
- Mij
-
Interaction parameters of Eqs. (27) and (28)
-
-
Molar mass of CO2, and drug in g/mol
-
-
Mean square regression
-
-
Mean square residual
-
-
Mole of CO2
-
-
Mole of rivaroxaban drug
- N
-
The number of experimental data
- NIST
-
National Institute of Standards and Technology
- P
-
Pressure (bar)
- Pc
-
Critical pressure (bar)
- Pref
-
Reference pressure (0.1 MPa)
-
-
Pure solid sublimation pressure (Pa)
- PR
-
Peng − Robinson
- Q
-
Number of independent variables in each equation
- R
-
Universal gas constant in J/(mol
-
-
Correlation coefficient
-
-
Solubility of rivaroxaban in equilibrium state (g/L)
- SRK
-
Soave-Redlich-Kwong
-
-
Sum of squares error
-
-
Regression sum of squares
-
-
Total sum of squares
- SCF
-
Supercritical fluid
- SC-CO2
-
Supercritical carbon dioxide
- T
-
Temperature (K)
-
-
Boiling temperature (K)
-
-
Reduced temperature
-
-
Critical temperature (K)
- U
-
Expanded uncertainty
-
-
Molar volume of rivaroxaban drug (cm3/mol)
-
-
Volumes of the collection vial
-
-
Volume of the sampling loop
- vdW2
-
Twin-parametric van der Waals mixing rule
- y2
-
Equilibrium molar fraction
-
-
Calculated values of the solute solubility
-
-
Experimental value of the solute solubility
-
-
Density of SC-CO2 (kg/m3)
-
-
Acentric factor
Greek Symbols
1 Introduction
Rivaroxaban is an oral, direct Factor Xa inhibitor, which is at an advanced stage of clinical development for prevention and treatment of thromboembolic disorders (Mueck et al., 2011). In fact, this anticoagulant drug can be used for the treatment and prevention of blood clotting. It was also evaluated for auxiliary anticipation after intense coronary disorder (Mega et al., 2012) and for thromboprophylaxis in intensely sick therapeutic patients (Cohen et al., 2011; Douxfils et al., 2012). The bioavailability of drugs after consumption depend on their solubility and solubility rate in an aqueous medium. For improvement these essential properties, the particle size reduction is an important tool (Sodeifian et al., 2020). Traditional methods for reducing particle size in the pharmaceutical industry include milling, evaporation, recrystallization, etc. (Sodeifian et al., 2017; Adachi and Lu, 1983). For this purpose, a supercritical fluid (SCF) process is useful in the pharmaceutical and biochemical industries compared to conventional methods. SCFs have properties between gas and liquid, such as viscosity, density and diffusivity, providing a wide application range for energy, solubility, polymers, extraction and etc. (Brunner, 2015; Sim Yeoh et al., 2013; Kiran, 2016; Sodeifian et al., 2020).
In general, using SCF technology, morphology, shape, and size of particles can be changed, which increases the efficiency and performance of the drug (Sodeifian et al., 2021). The common methods used in SCF technology can be mentioned RESS, SAS, GAS, RESOLVE ASES, SEDS, SFEE and PGSS (Chen et al., 2018; Montes et al., 2017; Jafari et al., 2015; Ciou et al., 2018; Lee et al., 2020; Kodama et al., 2018; Lee et al., 2018; Banchero, 2021; Aredo et al., 2021; Tokunaga et al., 2021; Razmimanesh et al., 2021). This is attributed to the supercritical conditions, as stated before, where properties of CO2 are between gas and liquid. This provides a suitable situation for CO2 solvent to achieve particle formation. Supercritical carbon dioxide (SC-
Due to the experimental limitations like time consuming and high cost of experiments in various temperatures and pressures to measure the solubility of a drug in SC-
To the best of knowledge, there is no yet any report on the measurement and modeling of rivaroxaban drug in SC-
2 Experimental
2.1 Materials
Methanol was purchased from Merck company (Germany). Pure CO2 (99.99 %) was provided by Fadak company (Kashan, Iran). Rivaroxaban with formulation
| Compound | Structure | MW (g/mol) | CAS number | Melting point | Purity (mass%) and purification method by supplier |
|---|---|---|---|---|---|
| Rivaroxaban |
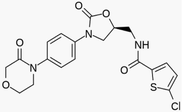
|
435.90 | 366789–02-8 | 230 °C | ≤99.99, HPLC |
| Carbon dioxide |

|
44.01 | 124–38-9 | ≥99.99, GC | |
| Methanol |
|
32.04 | 67–56-1 | 99.98, GC |
2.2 Experimental details
Experimental set up for measuring the solubility of rivaroxaban is shown in Fig. 1 (Sodeifian et al., 2019). Previous articles can be used to know more details (Sodeifian et al., 2021; Sodeifian et al., 2020).
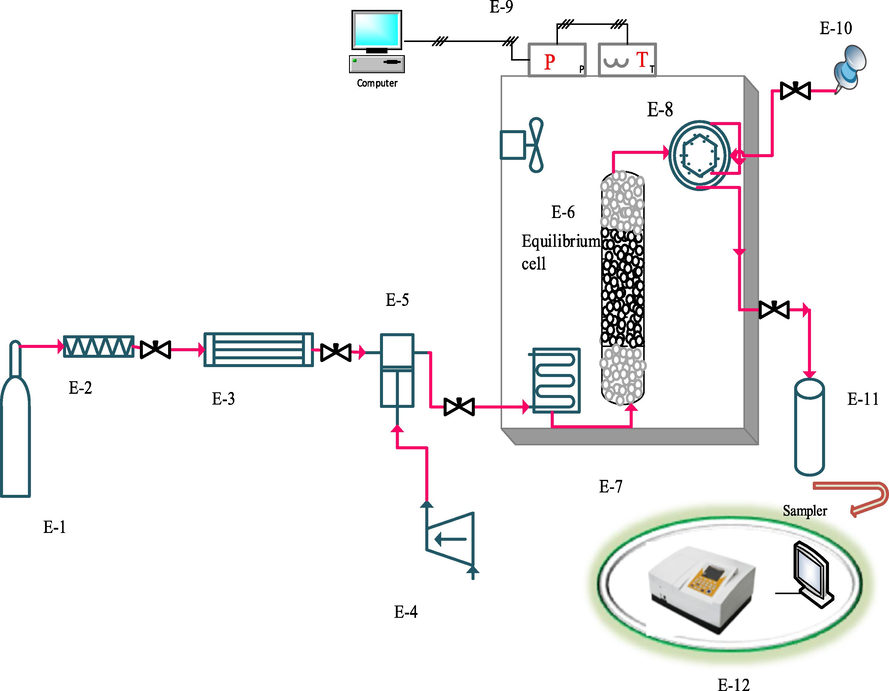
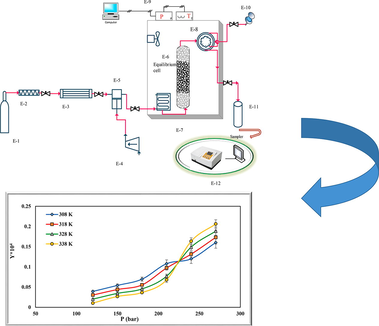
- Schematic of experimental process. E-1 CO2 cylinder; E-2, filter; E-3, refrigerator unit; E-4, air compressor; E-5, high pressure pump (Haskel model, MS-110, 0.33HP, air-driven liquid pump, USA); E-6, equilibrium cell; E-7, oven; E-8, six-port, two position valve (injection ring); E-9, control panel; E-10, syringe; E-11, collection vial; E-12, spectrophotometer UV–vis.
Components used include a CO2 storage cylinder, a refrigerator, a filter, a solubility column, a sample collector, and temperature and pressure measuring equipment. First, the refrigerator turned on and the temperature was set at 253 K. After the refrigerator temperature was stabilized, the CO2 in the tank was directed to the refrigerator. CO2 gas liquefied at 253 K. Liquid CO2 entered the reciprocating pump (Haskel model, MS-110, 0.33HP, air-driven liquid pump, USA) with the pressure in the tank (about 60 bar) to provide the desired pressure. Then fixed with a barometer up to
Two parameters
And
3 Thermodynamic framework
To investigate the correlation of solubility of rivaroxaban in SC-CO2, the two EoSs (PR and SRK) with vdW2 and CVD mixing rules and six semi-empirical models based on density were used.
3.1 Semi-empirical models
In this paper, six semi-empirical were used to investigate the correlation of rivaroxaban solubility in SC-CO2. Two models of Chrastil and Belghait, relating a solute solubility to density and temperature of SCF, and other models including Sodeifian, Jouyban, Bartle and Jafari Nejad, relating solubility to temperature, pressure and density of SCF, were used. Table 2 shows the equations of these models.
| Model | Equation | Number of parameters | References |
|---|---|---|---|
| Chrastil |
|
3 | (Sridar et al., 2013) |
| Bartle |
|
3 | (Reddy and Garlapati, 2019) |
| Jafari Nejad |
|
4 | (Jafari Nejad et al., 2010) |
| Jouyban |
|
6 | (Si-Moussa et al., 2017) |
| Sodeifian |
|
6 | (Sodeifian et al., 2019) |
| Belghait |
|
8 | (Belghait et al., 2018) |
| Temperature (K)a | Pressure (bar)a |
Density of SC- |
|
S (Equilibrium solubility) (g/L) | Experimental standard deviation, S(ÿ) × (105) | Expanded uncertainty of mole fraction (105 U) |
|---|---|---|---|---|---|---|
| 308 | 120 150 180 210 240 270 |
769 817 849 875 896 914 |
0.393 0.542 0.697 1.079 1.200 1.603 |
0.0607 0.0889 0.1189 0.1895 0.2158 0.2942 |
0.011 0.014 0.020 0.041 0.051 0.061 |
0.027 0.037 0.051 0.095 0.115 0.141 |
| 318 | 120 150 180 210 240 270 |
661 744 791 824 851 872 |
0.304 0.440 0.554 0.973 1.316 1.731 |
0.0403 0.0657 0.0879 0.1610 0.2248 0.3031 |
0.012 0.021 0.012 0.015 0.010 0.073 |
0.026 0.046 0.034 0.052 0.063 0.165 |
| 328 | 120 150 180 210 240 270 |
509 656 725 769 802 829 |
0.203 0.345 0.455 0.770 1.490 1.886 |
0.0208 0.0455 0.0663 0.1189 0.2400 0.3138 |
0.010 0.011 0.013 0.019 0.021 0.032 |
0.022 0.025 0.031 0.051 0.078 0.104 |
| 338 | 120 150 180 210 240 270 |
388 557 652 710 751 783 |
0.104 0.268 0.365 0.679 1.635 2.062 |
0.0081 0.0300 0.0478 0.0969 0.2466 0.3242 |
0.001 0.002 0.011 0.028 0.021 0.025 |
0.005 0.013 0.028 0.064 0.084 0.104 |
3.2 Equation of state (EoS) models and mixing rules
The following formula is used to calculate the solubility of rivaroxaban in SC-CO2, assuming that SC-CO2 and rivaroxaban are compound 1 and 2, respectively:
Various methods can be used to estimate the physicochemical properties of drug compounds such as
Where P, T, V and R are pressure, temperature, molar volume and gas constant, respectively. The parameters a and b calculated as follows:
Soave-Redlich-Kwong (SRK) EoS is written as follows:
vdW2 and CVD mixing rules are explained as below:
A: vdW2 mixing rule:
Where lij and Kij are interaction parameters and given:
B: CVD mixing rule:
Where
Where
4 Results and discussion
4.1 Experimental data
In this study, the solubility of rivaroxaban in SC-CO2 was investigated at various temperatures (308–338 K) and pressures (120–270 bar). Drug solubility in SC-CO2 is given in Table 3. All experiments were repeated triple to increase measurement accuracy. Using Spane-Wagner EoS (Span and Wagner, 1996), the density of
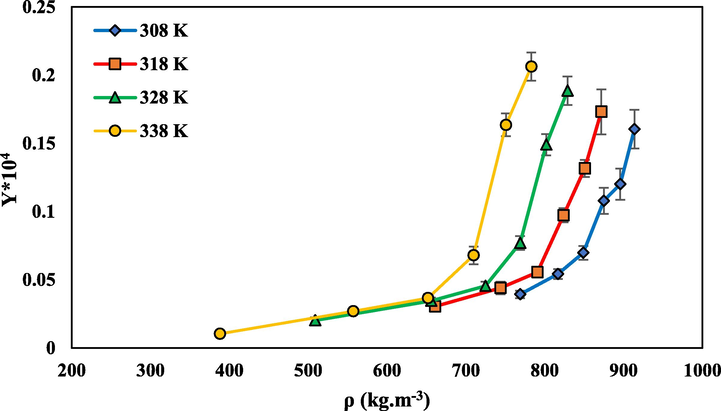
- The solubility of rivaroxaban in SC-CO2 vs density of SC-CO2 at different temperatures.
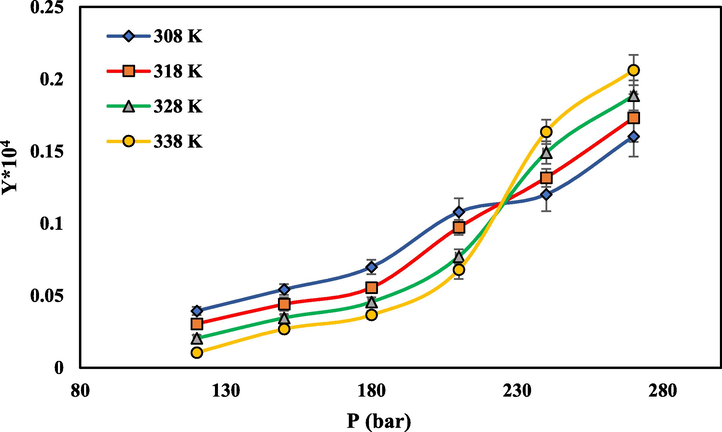
- The solubility of rivaroxaban in SC-CO2 vs pressure at different temperatures.
4.2 Semi-empirical models
As stated before, six semi-empirical models were used to correlate solubility data of rivaroxaban in SC-CO2. Average absolute relative deviation (AARD), adjusted correlation coefficient (
| Equation | Parameters | References |
|---|---|---|
|
|
|
(Esmaeili et al., 2019) |
|
|
|
(Sodeifian and Sajadian, 2019) |
|
|
|
(Ardestani et al., 2020) |
| Model | Chrastil | Bartle | Jafari Nejad | Jouyban | Sodeifian | Belghait |
|---|---|---|---|---|---|---|
| parameter | ||||||
|
|
9.508 | 15.912 | −24.973 | −13.986 | −20.982 | −168.862 |
|
|
−5246.281 | −7612.323 | 0.002 | −1.104 | 1.137 | −0.265 |
|
|
−49.845 | 0.015 | 1.583E-6 | 1.182E-5 | 1.106 | 3.969e-5 |
|
|
— | — | 1.812 | 3.232E-5 | 7.596e-4 | 6.705e-4 |
|
|
— | — | — | 0.701 | −0.017 | 0.068 |
|
|
— | — | — | 2.962 | −389.092 | −8.536e-5 |
|
|
— | — | — | — | — | 0.357 |
|
|
— | — | — | — | — | 50314.666 |
| AARD | 25.201 | 24.828 | 13.788 | 12.463 | 14.297 | 19.188 |
| Radj | 0.959 | 0.960 | 0.921 | 0.969 | 0.923 | 0.936 |
| F-value | 90.285 | 91.603 | 44.410 | 90.54 | 45.266 | — |
As shown in Table 5, considering the values of AARD for each model (Chrasil (AARD = 25.201); Bartle (AARD = 24.828), Jafari Nejad (AARD = 13.788), Jouyban (AARD = 12.463), Sodeifian (AARD = 14.297), and Belghait (AARD = 19.188)), Jouyban model has the lowest value of AARD and Chrastil model has the highest value of AARD. Therefore, the Jouyban model shows a better correlation with solubility data. Fig. 4 demonstrates the solubility correlation obtained from six models.
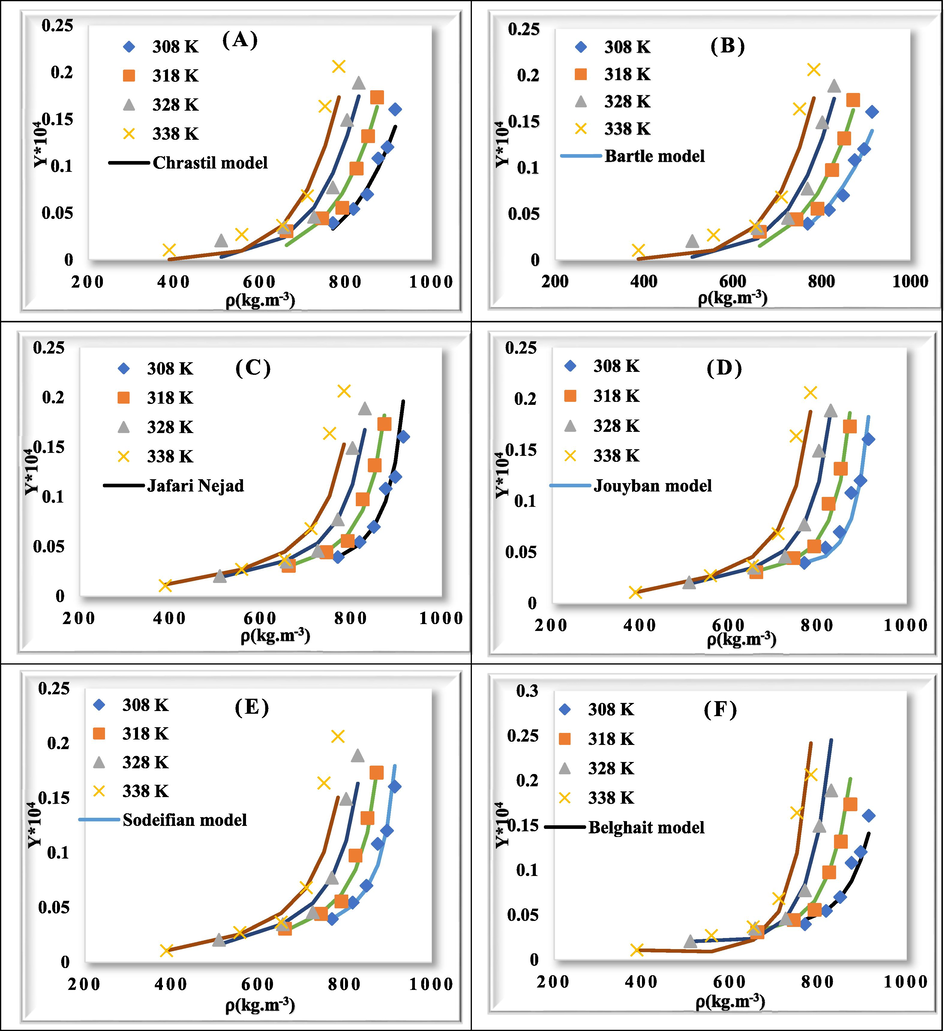
- Comparison of experimental (point) and calculated (line) values of rivaroxaban solubility.
In terms of the number of adjustable parameters, the rivaroxaban solubility could be poorly correlated using models with three adjustable parameters, as compared to those with four, six and seven parameters, as shown in Table 5. In addition to AARD, Radj was calculated to compare models of different numbers of independent variables. A model with more adjustable parameters can be considered superior to other models with less parameters that, in addition to AARD decreasement, Radj also increases. This can be seen for Jouyban model.
Elaborating on the results presented in this research, it can be reasoned that the energy term (coefficient of the temperature term (a1 = ΔH/R)) in the Chrastil and Bartle et al. models eased the determination of vaporization heat (ΔHvap), total reaction heat (ΔHtotal), and solvation heat (ΔHsol) of the considered drug-CO2 systems based on the regressed energy parameters. Indeed, the solvation heat (ΔHsol) was defined as the difference between ΔHvap (Bartle et al. model) and ΔHtotal (Chrastil’s model). According to Table 6, wherein enthalpy of dissolution of rivaroxaban in SC-CO2 is presented, the corresponding values of ΔHtotal and
| Compound |
|
|
|
|---|---|---|---|
| Rivaroxaban | 43.617 | 63.288 | −19.671 |
aObtained from the Chrastil model.
bObtained from the Bartle model.
c
4.3 Solubility correlation by EoS based model
Two mixing rules (vdW2 and CVD) were used to estimate the parameters of PR and SRK EoSs. The physicochemical properties of rivaroxaban were calculated with Ambrose, Joback, Marrero and Pardillo, Grigoras, Fedors and Lee-Kesler methods and shown in Table 7.
| Component |
|
|
|
|
|
|
|||
|---|---|---|---|---|---|---|---|---|---|
| 308 | 318 | 328 | 338 | ||||||
|
|
|||||||||
| Rivaroxaban |
|
|
|
|
|
0.0047 | 0.0169 | 0.0557 | 0.1685 |
|
|
— | 304.2 | 73.8 | 0.228 | — | — | |||
aEstimated by Marrero and Pardillo (Poling et al., 2001).
bEstimated by Joback (Poling et al., 2001).
cEstimated by Grigoras method (Poling et al., 2001).
dEstimated by Ambrose-Walton (Poling et al., 2001).
eEstimated by Fedors (Fedors, 1974).
fEstimated by lee-Kesler (Lee and Kesler, 1975).
Table 8 shows the results two EoSs (PR and SRK) with two mixing rules (vdW2 and CVD). Parameters
| Model | Temperature | Mij | kij | lij | Radj | F-value | AARD% |
|---|---|---|---|---|---|---|---|
| PR EoS + vdW2 | 308 | — | 0.441 | 0.343 | 0.986 | 90.18 | 5.104 |
| 318 | — | 0.504 | 0.443 | 0.989 | 1.141 | 8.012 | |
| 328 | — | 0.571 | 0.557 | 0.974 | 47.713 | 15.53 | |
| 338 | — | 0.650 | 0.701 | 0.966 | 35.528 | 14.732 | |
| SRK EoS + vdW2 | 308 | — | 0.466 | 0.369 | 0.986 | 88.662 | 5.314 |
| 318 | — | 0.522 | 0.462 | 0.989 | 110.17 | 8.091 | |
| 328 | — | 0.527 | 0.410 | 0.755 | 4.317 | 15.117 | |
| 338 | — | 0.701 | 0.787 | 0.873 | 9.033 | 20.634 | |
| PR EoS + CVD | 308 | 1.030 | — | — | 0.926 | 16.048 | 7.364 |
| 318 | 1.092 | — | — | 0.8 | 5.447 | 15.826 | |
| 328 | 1.183 | — | — | 0.37 | 1.396 | 22.286 | |
| 338 | 1.339 | — | — | 0.842 | −0.038 | 34.938 | |
| SRK EoS + CVD | 308 | 1.040 | — | — | 0.825 | 6.308 | 9.729 |
| 318 | 1.100 | — | — | 0.687 | 3.231 | 17.577 | |
| 328 | 1.207 | — | — | 0.575 | 0.378 | 31.97 | |
| 338 | 1.982 | — | — | 1.649 | −0.828 | 71.861 |
Parameters
The values A, B, C, and D for
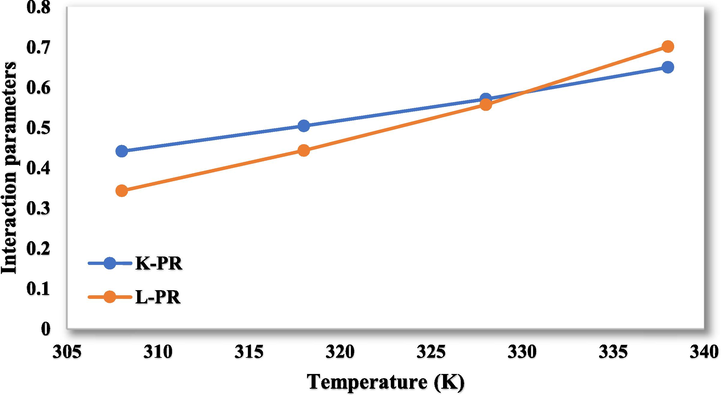
- The interaction parameters for the values of
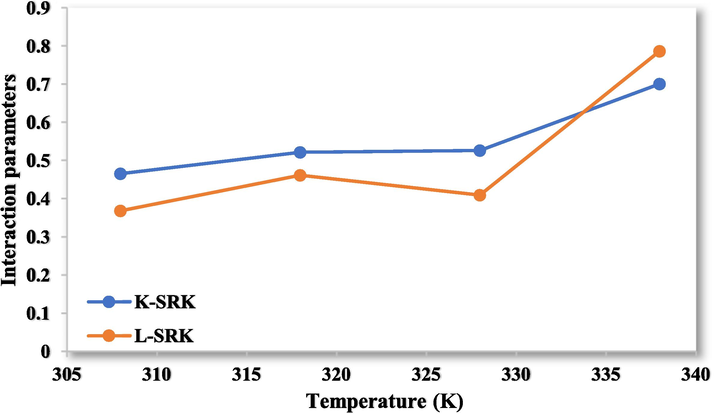
- The interaction parameters for the values of
| Model | Interaction parameters | |||
|---|---|---|---|---|
|
|
|
|||
| A | B | C | D | |
| PR-vdW2 | 0.0069 | −1.7001 | 0.0119 | −3.3262 |
| SRK-vdW2 | 0.0071 | −1.7393 | 0.012 | −3.3755 |
Fig. 7 shows plots of experimental data (point) and calculated values (line) for rivaroxaban drug with PR-vdW2 (a & b), SRK-vdW2 (c & d), PR-CVD (e & f), and SRK-CVD (g & h) at various temperatures relative to density and pressure, respectively. The correlation between experimental data and calculated values in different modes is well shown.
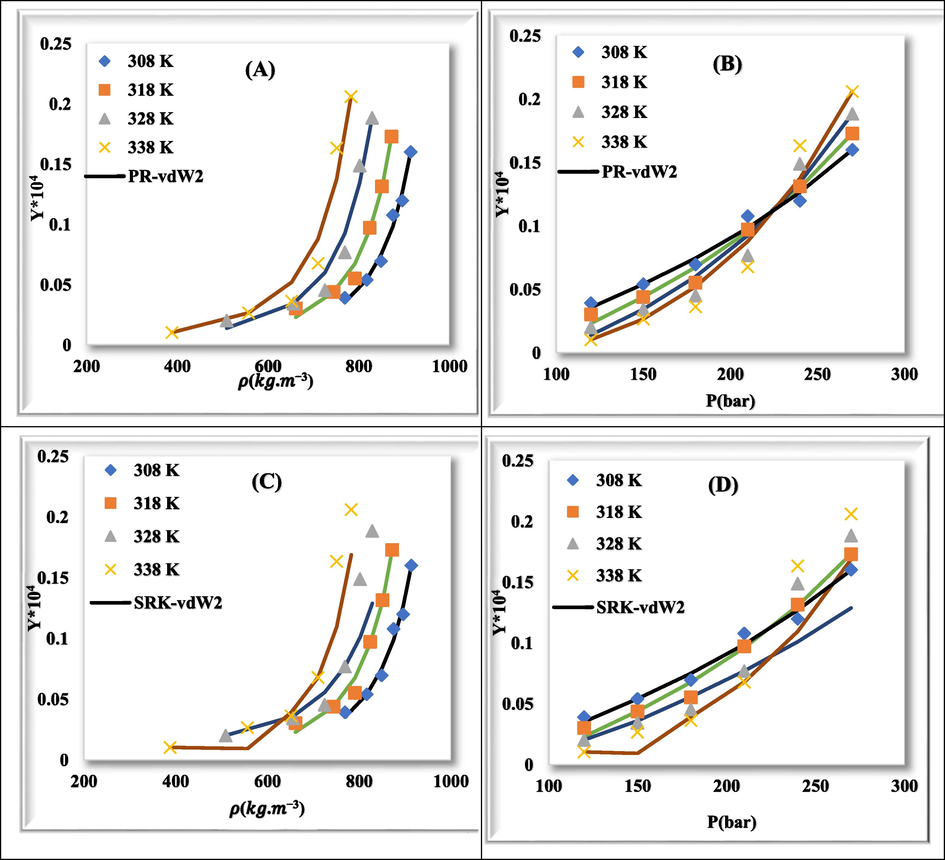
- Comparison of experimental (point) and calculated (line) solubility of rivaroxaban at various temperatures. (a) & (b): PR-vdW2, (c) & (d): SRK-vdW2, (e) & (f): PR-CVD, and (g) & (h): SRK-CVD.
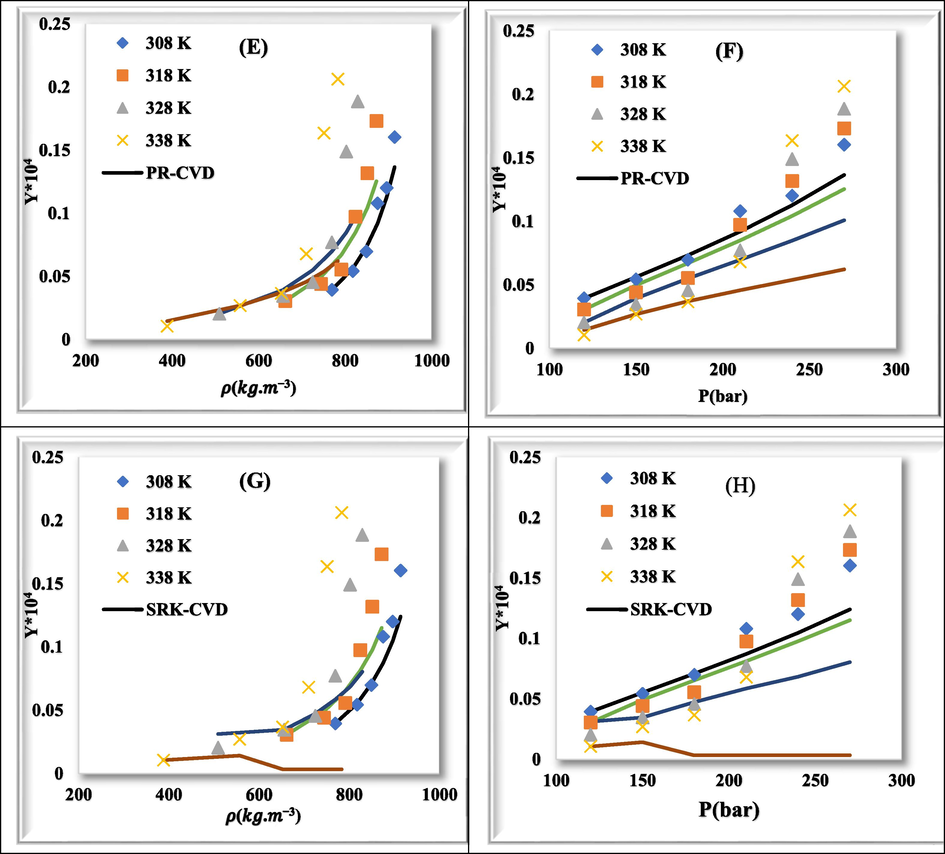
- Comparison of experimental (point) and calculated (line) solubility of rivaroxaban at various temperatures. (a) & (b): PR-vdW2, (c) & (d): SRK-vdW2, (e) & (f): PR-CVD, and (g) & (h): SRK-CVD.
5 Conclusion
The solubility of rivaroxaban was determined at various temperatures and pressures in SC-
Acknowledgement
The authors would like to thank of the deputy of research, University of Kashan, under Grant Number (#Pajoohaneh 1401/19) for supporting this valuable project. Also, researchers would like to thank Parsian drug Company (Tehran), especially, Drs. Shojaei and Yosoufian, for providing the required drug API.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilib.. 1983;14:147-156.
- [CrossRef] [Google Scholar]
- Experimental measurement and thermodynamic modeling of capecitabine (an anticancer drug) solubility in supercritical carbon dioxide in a ternary system: effect of different cosolvents. J. Chem. Eng. Data. 2020;65:4762-4779.
- [CrossRef] [Google Scholar]
- Formation of edible oil-loaded beeswax microparticles using PGSS – Particles from Gas-Saturated Solutions. J. Supercritical Fluids. 2021;169:105106
- [CrossRef] [Google Scholar]
- Representing solute solubility in supercritical carbon dioxide: a novel empirical model. Chem. Eng. Res. Des.. 2015;93:355-365.
- [CrossRef] [Google Scholar]
- Supercritical carbon dioxide as a green alternative to achieve drug complexation with cyclodextrins. Pharmaceuticals. 2021;14:562.
- [CrossRef] [Google Scholar]
- Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data. 1991;20:713-756.
- [CrossRef] [Google Scholar]
- Semi-empirical correlation of solid solute solubility in supercritical carbon dioxide: comparative study and proposition of a novel density-based model. C. R. Chim.. 2018;21:494-513.
- [CrossRef] [Google Scholar]
- A combined model for the solubility of different compounds in supercritical carbon dioxide. Chem. Eng. Res. Des.. 2015;104:416-428.
- [CrossRef] [Google Scholar]
- A five-parameter empirical model for correlating the solubility of solid compounds in supercritical carbon dioxide. Fluid Phase Equilib.. 2016;411:74-80.
- [CrossRef] [Google Scholar]
- Supercritical process technology related to energy and future directions – An introduction. J. Supercritical Fluids. 2015;96:11-20.
- [CrossRef] [Google Scholar]
- Continuous nanonization of lonidamine by modified-rapid expansion of supercritical solution process. J. Supercritical Fluids. 2018;133:486-493.
- [CrossRef] [Google Scholar]
- Experimental investigation for the solubility and micronization of pyridin-4-amine in supercritical carbon dioxide. J. CO2 Util.. 2017;18:173-180.
- [CrossRef] [Google Scholar]
- Measurement of solid solubility of warfarin in supercritical carbon dioxide and recrystallization study using supercritical antisolvent process. Adv. Powder Technol.. 2018;29:479-487.
- [CrossRef] [Google Scholar]
- Semi-empirical models and a cubic equation of state for correlation of solids solubility in scCO2: dyes and calix[4]arenes as illustrative examples. Fluid Phase Equilib.. 2016;426:37-46.
- [CrossRef] [Google Scholar]
- Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J. Thromb. Thrombolysis. 2011;31:407-416.
- [CrossRef] [Google Scholar]
- Cubic equation-of-state correlation of the solubility of some anti-inflammatory drugs in supercritical carbon dioxide. Fluid Phase Equilib.. 2006;239:188-199.
- [CrossRef] [Google Scholar]
- Assessment of the impact of rivaroxaban on coagulation assays: Laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb. Res.. 2012;130:956-966.
- [CrossRef] [Google Scholar]
- A data-driven model for predicting the effect of temperature on oil-water relative permeability. Fuel. 2019;236:264-277.
- [CrossRef] [Google Scholar]
- Calculation of the solid solubilities in supercritical carbon dioxide using a new Gex mixing rule. J. Supercritical Fluids. 2009;51:148-158.
- [CrossRef] [Google Scholar]
- A three-parameter cubic equation of state for prediction of thermodynamic properties of fluids. J. Chem. Thermodyn.. 2008;40:84-95.
- [CrossRef] [Google Scholar]
- A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci.. 1974;14:147-154.
- [CrossRef] [Google Scholar]
- Significance of the crossover pressure in solid-supercritical fluid phase equilibria. Ind. Eng. Chem. Res.. 1991;30:1955-1964.
- [CrossRef] [Google Scholar]
- Solubilities of solids in supercritical fluids using dimensionally consistent modified solvate complex models. Fluid Phase Equilib.. 2009;283:97-101.
- [CrossRef] [Google Scholar]
- Measurement and thermodynamic modeling of solubility of Tamsulosin drug (anti cancer and anti-prostatic tumor activity) in supercritical carbon dioxide. J. Supercritical Fluids. 2020;163:104875
- [CrossRef] [Google Scholar]
- Correlation of solute solubility in supercritical carbon dioxide using a new empirical equation. Chem. Eng. Res. Des.. 2014;92:2734-2739.
- [CrossRef] [Google Scholar]
- Correlation of solid solubilities of pharmaceutical compounds in supercritical carbon dioxide with solution model approach. J. Taiwan Inst. Chem. Eng.. 2013;44:349-358.
- [CrossRef] [Google Scholar]
- Prediction of solute solubility in supercritical carbon dioxide: a novel semi-empirical model. Chem. Eng. Res. Des.. 2010;88:893-898.
- [CrossRef] [Google Scholar]
- Gas-Antisolvent (GAS) crystallization of aspirin using supercritical carbon dioxide: experimental study and characterization. Ind. Eng. Chem. Res.. 2015;54:3685-3696.
- [CrossRef] [Google Scholar]
- Relationship between the binary interaction parameters (kij) of the Peng-Robinson and those of the Soave–Redlich–Kwong equations of state: Application to the definition of the PR2SRK model. Fluid Phase Equilib.. 2010;295:26-37.
- [CrossRef] [Google Scholar]
- Estimation of pure-component properties from group-contributions. Chem. Eng. Commun.. 1987;57:233-243.
- [CrossRef] [Google Scholar]
- Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions. J. Supercritical Fluids. 2002;24:19-35.
- [CrossRef] [Google Scholar]
- Jouyban, A., Khoubnasabjafari, M., Acree, W.E., 2005. Mathematical representation of solute solubility in binary mixture of super- critical fluids by the Jouyban-Acree model. 3.
- Modeling the entrainer effects on solubility of solutes in supercritical carbon dioxide. Chem. Pharm. Bull.. 2005;53:290-295.
- [CrossRef] [Google Scholar]
- Khimeche, K., Alessi, P., Kikic, I., Dahmani, A., 2007. Solubility of diamines in supercritical carbon dioxide Experimental determination and correlation. 10.
- Supercritical fluids and polymers – The year in review – 2014. J. Supercritical Fluids. 2016;110:126-153.
- [CrossRef] [Google Scholar]
- Effect of the Z-isomer content on nanoparticle production of lycopene using solution-enhanced dispersion by supercritical fluids (SEDS) J. Supercritical Fluids. 2018;133:291-296.
- [CrossRef] [Google Scholar]
- A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J.. 1975;21:510-527.
- [CrossRef] [Google Scholar]
- Microencapsulation of red palm oil as an oil-in-water emulsion with supercritical carbon dioxide solution-enhanced dispersion. J. Food Eng.. 2018;222:100-109.
- [CrossRef] [Google Scholar]
- Storage stability and degradation kinetics of bioactive compounds in red palm oil microcapsules produced with solution-enhanced dispersion by supercritical carbon dioxide: a comparison with the spray-drying method. Food Chem.. 2020;304:125427
- [CrossRef] [Google Scholar]
- Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med.. 2012;366:9-19.
- [CrossRef] [Google Scholar]
- Micronization of vanillin by rapid expansion of supercritical solutions process. J. CO2 Util.. 2017;21:169-176.
- [CrossRef] [Google Scholar]
- Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet.. 2011;50:675-686.
- [CrossRef] [Google Scholar]
- Modified Wilson’s model for correlating solubilities in supercritical fluids of some polycyclic aromatic solutes. Polycyclic Aromat. Compd.. 2018;38:244-256.
- [CrossRef] [Google Scholar]
- Solubility of 9-tetrahydrocannabinol in supercritical carbon dioxide. Experiments Modeling. 2010;5
- [Google Scholar]
- The properties of gases and liquids (5th ed.). New York: McGraw-Hill; 2001.
- An investigation into Sunitinib malate nanoparticle production by US- RESOLV method: effect of type of polymer on dissolution rate and particle size distribution. J. Supercritical Fluids. 2021;170:105163
- [CrossRef] [Google Scholar]
- Dimensionless empirical model to correlate pharmaceutical compound solubility in supercritical carbon dioxide. Chem. Eng. Technol.. 2019;42:2621-2630.
- [CrossRef] [Google Scholar]
- Comparison and modelling of rutin solubility in supercritical carbon dioxide and subcritical 1,1,1,2-tetrafluoroethane. J. CO2 Util.. 2017;21:1-8.
- [CrossRef] [Google Scholar]
- Shamsipur M, Karami AR, Yamini Y, Sharghi H (2004) Solubilities of some 1-hydroxy-9,10-anthraquinone derivatives in supercritical carbon dioxide. 7.
- Modeling of ionic liquid+polar solvent mixture molar volumes using a generalized volume translation on the Peng-Robinson equation of state. Fluid Phase Equilib.. 2015;395:51-57.
- [CrossRef] [Google Scholar]
- Solubility measurement method and mathematical modeling in supercritical fluids. EJ. 2013;17:67-78.
- [CrossRef] [Google Scholar]
- Novel density-based model for the correlation of solid drugs solubility in supercritical carbon dioxide. C. R. Chim.. 2017;20:559-572.
- [CrossRef] [Google Scholar]
- Determination of solubility of Aprepitant (an antiemetic drug for chemotherapy) in supercritical carbon dioxide: empirical and thermodynamic models. J. Supercritical Fluids. 2017;128:102-111.
- [CrossRef] [Google Scholar]
- Solubility measurement of an antihistamine drug (Loratadine) in supercritical carbon dioxide: assessment of qCPA and PCP-SAFT equations of state. Fluid Phase Equilib.. 2018;472:147-159.
- [CrossRef] [Google Scholar]
- Solubility measurement of a pigment (Phthalocyanine green) in supercritical carbon dioxide: Experimental correlations and thermodynamic modeling. Fluid Phase Equilib.. 2019;494:61-73.
- [CrossRef] [Google Scholar]
- Experimental data and thermodynamic modeling of solubility of Sorafenib tosylate, as an anti-cancer drug, in supercritical carbon dioxide: Evaluation of Wong-Sandler mixing rule. J. Chem. Thermodyn.. 2020;142:105998
- [CrossRef] [Google Scholar]
- Experimental measurement of solubilities of sertraline hydrochloride in supercriticalcarbon dioxide with/without menthol: data correlation. J. Supercritical Fluids. 2019;149:79-87.
- [CrossRef] [Google Scholar]
- Evaluation of the response surface and hybrid artificial neural network-genetic algorithm methodologies to determine extraction yield of Ferulago angulata through supercritical fluid. J. Taiwan Inst. Chem. Eng.. 2016;60:165-173.
- [CrossRef] [Google Scholar]
- Mathematical modelling for extraction of oil from Dracocephalum kotschyi seeds in supercritical carbon dioxide. Nat. Prod. Res.. 2018;32:795-803.
- [CrossRef] [Google Scholar]
- Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: assessment of new empirical model. J. Supercritical Fluids. 2019;146:89-99.
- [CrossRef] [Google Scholar]
- Prediction of solubility of sodium valproate in supercritical carbon dioxide: experimental study and thermodynamic modeling. J. Chem. Eng. Data. 2020;65:1747-1760.
- [CrossRef] [Google Scholar]
- Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC–CO2): experimental correlations and thermodynamic modeling. J. Mol. Liq.. 2020;297:111740
- [CrossRef] [Google Scholar]
- Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: application of SAFT-VR EoS. Fluid Phase Equilib.. 2020;507:112422
- [CrossRef] [Google Scholar]
- Measuring and modeling the solubility of an antihypertensive drug (losartan potassium, Cozaar) in supercritical carbon dioxide. J. Mol. Liq.. 2021;331:115745
- [CrossRef] [Google Scholar]
- The solubility of Sulfabenzamide (an antibacterial drug) in supercritical carbon dioxide: evaluation of a new thermodynamic model. J. Mol. Liq.. 2021;335:116446
- [CrossRef] [Google Scholar]
- Solubility of Amlodipine Besylate (Calcium Channel Blocker Drug) in supercritical carbon dioxide: measurement and correlations. J. Chem. Eng. Data. 2021;66:1119-1131.
- [CrossRef] [Google Scholar]
- Investigation of essential oil extraction and antioxidant activity of Echinophora platyloba DC. using supercritical carbon dioxide. J. Supercritical Fluids. 2017;121:52-62.
- [CrossRef] [Google Scholar]
- Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liq.. 2020;299:112179
- [CrossRef] [Google Scholar]
- A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data. 1996;25:1509-1596.
- [CrossRef] [Google Scholar]
- Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci.. 2008;63:4292-4301.
- [CrossRef] [Google Scholar]
- A new model for the solubility of dye compounds in supercritical carbon dioxide. Thermochim Acta. 2013;561:91-97.
- [CrossRef] [Google Scholar]
- Supercritical fluids for pharmaceutical particle engineering: methods, basic fundamentals and modelling. Chem. Eng. Process. Process Intensif.. 2012;60:9-25.
- [CrossRef] [Google Scholar]
- Microencapsulation of drug with enteric polymer Eudragit L100 for controlled release using the particles from gas saturated solutions (PGSS) process. J. Supercritical Fluids. 2021;167:105044
- [CrossRef] [Google Scholar]
- Thermodynamic modeling of solubilities of various solid compounds in supercritical carbon dioxide: effects of equations of state and mixing rules. J. Supercritical Fluids. 2011;55:861-875.
- [CrossRef] [Google Scholar]
- Applications of cubic equations of state for determination of the solubilities of industrial solid compounds in supercritical carbon dioxide: a comparative study. Chem. Eng. Sci.. 2012;71:283-299.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104421.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







