Translate this page into:
Development of a novel electrocoagulation anode for real urban wastewater treatment: Experimental and modeling study to optimize operative conditions
⁎Corresponding authors. elazzouzi89@gmail.com (M. Elazzouzi), achraf@iet.cn (A. El Kasmi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Electrocoagulation (EC) is one of the most cost-effective techniques for eliminating pollutants from wastewater. The objective of the present work was to develop a novel low-cost EC anode-based aluminum scrap shape by valorizing used aluminum cans, in attempts to increase the contact rate between the pollutant molecules and EC anode leading to enhance the pollutants removal efficiency from urban wastewater. Moreover, to optimize the EC operative conditions with reducing the number of required experiments, modeling by Box-Behnken design (BBD) in response surface methodology was performed to investigate the effects of many operating parameters: initial pH, applied current, operating time and packed density, on the removal efficiency of chemical oxygen demand (COD), biochemical oxygen demand (BOD), phosphorous (P) and fecal coliforms (FC). Considering the treatment energy consumption, the results led to two important operative conditions for cost-effective EC treatment: economic and optimal conditions for experimental and modeled removals. The predicted results from BBD modeling regarding economic operative conditions were found to be COD (Y1) 80%, BOD (Y2) 84%, P (Y3) 96%, and FC (Y4) 99%, with an energy consumption (Y5) of 3.50 kWh m−3. Accordingly, experimentally validation using economic operative conditions resulted in removal efficiencies of COD (78.5%), BOD (83%), P (94.7%), and FC (99%) with a lower energy consumption of 2.52 kWh m−3. Hence, the predicted results revealed a good agreement with the experimental data. These findings could pave the way to reduce significantly the number of required experiments for other research works, and make aluminum based-electrode as a promising candidate for development and scale-up a low-cost EC electrode.
Keywords
Low-cost aluminum scrap anode
Real urban wastewater treatment
Box-Behnken design (BBD)
Response surface methodology (RSM)
Economic and optimal operative conditions
Treatment energy consumption.
1 Introduction
The urbanization expansion and population growth in recent decades were accompanied by a rapid increase in urban wastewater production. As known, untreated urban wastewater poses dangers to the environment and human health, due to the existence of numerous toxic substances such as viruses, heavy metal, pathogens and other inorganic and organic pollutants in wastewater. Thusly, the management and treatment of urban wastewater present a serious concern in many countries all over the world. Many treatment technologies have been evolved to control the urban wastewater, including: adsorption (Margot et al., 2013), activated sludge (Ni et al., 2009), chemical coagulation (De Feo et al., 2008), advanced oxidation process (Zhou et al., 2011), and reverse osmosis (Dialynas and Diamadopoulos, 2009). Although these methods can remove most of the organic pollutants, they are costly and involve several operational difficulties that could limit their use at the large scale. Therefore, the development of efficient, facile and inexpensive methods to treat urban wastewater is still needed.
Recently, electrocoagulation (EC) treatment technique has been considered as an alternative method over the aforementioned techniques, due to its advantages that include compact treatment facility, less sludge production, small equipment space and relatively low cost. Accordingly, the material type of the electrode is regarded among the main factors affecting the performance of EC process (Chou et al., 2009). Thus, in attempts to select an appropriate electrode material for treatment of a wastewater, different parameters should be considered and mainly material cost, oxidation potential, and targeted pollutant. As reported, different metallic electrodes have been widely used for wastewater treatment, like boron-doped diamond (Ruiz et al., 2011), copper (Prajapati et al., 2016) and zinc (Hussin et al., 2017); while, aluminum is rarely investigated that revealed a good coagulation performance (Elazzouzi et al., 2018; Omwene and Kobya, 2018). Moreover, it was reported that anode metal plate is an energy-consuming process, which recently increased the interest to reduce the cost of conventional electrocoagulation process through the development of low-cost electrode.
For instance, in a study conducted by Ardhan et al. (Ardhan et al., 2014), a low-cost anode made from iron scrap was employed to achieve high color and chemical oxygen demand (COD) removals from synthetic wastewater, which indicated that the process can effectively remove 98% and 93% of color and COD, respectively. Un et al. investigated the treatability of COD that originated from textile wastewater using EC process with a packed bed anode made from wrapping iron wire (Tezcan Un and Aytac, 2013). A good COD removal efficiency of 98.46% and energy consumption of 0.093kWh mg−1 were obtained at 60 min of retention time and 50 mA cm−2 of current density with an initial pH of 9. However, conventional anodes are associated with high fabrication costs, making EC process economically unviable. The shape of the electrodes may also affect the performance of EC process. On the other hand, a huge amount of waste-metal scraps (metal chips, filings, shavings, etc) as by-products is generated by metalworking shops. Accumulation of these kind of scraps leads to an environmental threat, which renders its removal tedious. In the context of waste valorization, the use of waste-aluminum scraps as an anode in EC process would be advantageous in terms of waste management and feasibility. Moreover, the scrap shape anodes have a larger electrode contact surface area than the other shape of the anodes, thereby providing a high contact area between the electrodes and pollutants in the EC reactor, leading to overall increase in EC process efficiency. Therefore, the shape and cost-effectiveness of aluminum scrap based-electrode could result in promised candidate for development and scale-up the low-cost electrode.
It is of interest here to point out that in conventional optimization approaches, a large experiments number is required leading to increasing the use of material, treatment time and supplied power. Hence, the application of a theoretical prediction approach can permit to reduce the number of needed experiments. Recently, Response Surface Methodology (RSM) has been performed for optimization of numerous processes and particularly for wastewater treatment of dye (Dastkhoon et al., 2017) and paper industry (Dil et al., 2015). Thus, Box-Behnken Design (BBD) in RSM is an important design tool that successfully used for the process optimization, and provided comprehensive conclusions and detailed information even for a small given number of experiments and of interactive effects between operating parameters. Recently, BBD has been successfully tested to optimize the efficiency of EC processes for several wastewaters (Garg and Prasad, 2016; Tak et al., 2015). Isa et al. used BBD to optimize the experimental conditions for boron removal from wastewater using EC process (Isa et al., 2014). At optimal operating conditions, 98% of boron was removed from synthetic wastewater. However, the optimization process of real urban wastewater treatment using RSM is still scarce.
In the present work, a novel low-cost EC process using an anode made from aluminum-waste scrap was used for the treatment of real urban wastewater. Then, BDD was also investigated to achieve the optimum conditions for high removal of chemical oxygen demand (COD), biological oxygen demand (BOD), phosphorous (P) and fecal Coliforms (FC) with a minimum of energy consumption. The results obtained from this study could be a reference study for real urban wastewater treatment research works with reduced number of required experiments for the optimum conditions of removal of pollutants from urban wastewater using low-cost EC process.
2 Materials and methods
2.1 Wastewater characteristics
The used samples were collected near the entrance of the urban wastewater treatment plant located in Al-Hoceima city (Northeast of Morocco). The collected wastewater was analyzed (as described in Table 1) and directly used in the experiments without any pretreatment. Monomeric method (respirometer system OxyTop, Fisher scientific, UK) was performed to analyze BOD. UV–vis spectrophotometer device (Spectrocoquant PHAR 300 MERK, MERCK, Japan) was used to analyze the COD and P. Conductivity and pH measurements were performed using (pH/ion/Cond 750 WTW Inolab WTW, Germany). While, the aluminum metal-scrap was made from aluminum chips (length: 0.9–13 mm; width: 0.3–2.0 mm) that collected from a metal machining shop in Al-Hoceima city (Morocco). All analyses were performed according to standard analyses (APHA, 2005).
Parameters
Units
Values
COD
mg L−1
1100
BOD
mg L−1
560
TSS
mg L−1
450
P
mg L−1
12
FC
NPP/100 ml
4.5 × 107
Conductivity
µs cm−1
3240
Turbidity
NTU
350
Initial pH
pHi
7.5
2.2 Electrocoagulation system
Fig. 1 shows the set-up of a novel EC used in this present study. A Plexiglas cylindrical packed bed EC batch was equipped with cylindrical perforated Al cathode, and the anode was comprised of Al scraps packed within a PVC perforated tube centrally placed in the cathode. The PVC perforated tube has a length of 10 cm, diameter of 3 cm and thickness of 0.1 cm, which contains small holes of 0.3 cm distributed regularly on its wall surface to permit a homogeneous transport of metal ions from the electrode. The electrodes were connected in the monopolar mode and immersed up to 6 cm in depth. The total surface area of cylindrical electrode was 0.012 m2 and the inter-electrode distance between the cathode and anode was maintained at 1 cm. The Al scrap aluminum was made from the used aluminum cans, which was cut into small pieces of dimension of 5 mm*5 mm then used as anode after being carefully washed.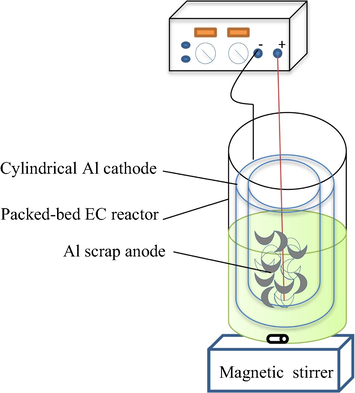
Schematic diagram of the EC experimental setup: (1) DC power supply, (2) Perforated PVC containing scrap aluminum as the anode, (3) Aluminum plate cathode, (4) Circulation pump.
A digital DC power supply (galvanostatic mode, ALR3002M model) was used in the range current of 0–2.5 A and range voltage of 0–30 V and connected to the system. The agitation of the solution was performed using a magnetic stirrer at a constant agitation speed of 200 rpm to assure uniform bulk distribution of generated ions in the solution. The pH of urban wastewater was adjusted using aqueous HCl 0.1 M or NaOH 0.1 M. Aluminum scraps were rinsed for a while using a diluted HCl solution in attempts to eliminate the dust and organic matter that could be adhered on the surface of used aluminum cans, before being washed with distilled water. Then after, they were dried at 100 °C for 12 h in a vacuum drying oven (NBF-6050, China). The complexes formed during wastewater treatment are easily removed from the sample by flotation. All experiments were carried out at room temperature and the samples were taken directly without filtration during the predetermined reaction time.
2.3 Experimental design
The experimental design and mathematical modeling were performed using Design expert 10.0 software (Stat-ease, US), ANOVA analysis and Box-Behnken design were chosen for the optimization. The design includes 29 EC experiments sets, and the main operational variables are applied current (X1), electrolysis time (X2), initial pH (X3) and packed density (X4). While, the corresponding responses are COD (Y1), BOD (Y2), P (Y3), FC (Y4) and energy consumption (Y5). The levels and range factors chosen are illustrated in Table 2, which have been selected based on the preliminary experiment-trials and previous experimental research works (Elazzouzi et al., 2017, 2018). The obtained experimental data were analyzed by RSM to fit the following polynomial model of second order:
Parameter (range)
X1
X2
X3
X4
Levels
I (A)
time (min)
pH
Packed density (g cm−3)
(0.5–1.5)
(2–10)
(5–9)
(0.4–0.8)
−1
0.5
2
5
0.4
0
1
6
7
0.6
+1
1.5
10
9
0.8
The experimental data were fitted to different models (linear, quadratic, third-order polynomials, and two factor interactions (2FI)). They were then statistically analyzed to select the appropriate model, which reflects no significant lack of fit and it has the highest value of , , and low-predicted residual error sum of squares. In addition, the models’ factors were evaluated at a confidence level of 95%. In order to validate the results of the model under optimum conditions, the lab experiments were performed and compared to the predicted results.
3 Results and discussions
3.1 ANOVA analysis
RSM was used to study the optimization and the effect of process parameters, and to design the experiments. The Box Behnken-design of 29 experiments with four factors of applied current, initial pH, packed density and EC time with three levels is presented in Table 3. BBD suggested a second order polynomial model, which represents the relation between operating variables and all responses. The response functions with the determined coefficients for COD, BOD, P and FC removals as well as the energy consumption (EC) are presented by Eqs. (2), (3), (4), (5) and (6) as follows:
Independent variables
Response (Expérimental values)
Run
X1:I (A)
X2:time (min)
X3:pH
X4:Packed density (g cm−3)
Y1 (COD %)
Y2 (BOD %)
Y3 (P %)
Y4 (FC %)
Y5 (EC kWh m−3)
1
0.5
2
7
0.6
47
57
86
92
0.3
2
1
2
9
0.6
53
66
90
94
0.6
3
1
2
5
0.6
52
63
88
93
0.6
4
1
2
7
0.8
55
68
92
95
0.6
5
1
2
7
0.4
51
65
89
94
0.8
6
0.5
6
5
0.6
56
70
85
93
0.9
7
0.5
6
9
0.6
57
72
87
95
0.9
8
1.5
2
7
0.6
60
75
90
96
0.9
9
0.5
6
7
0.8
60
76
90
94
1
10
0.5
6
7
0.4
52
74
87
95
1.2
11
0.5
10
7
0.6
63
77
89
96
1.5
12
1
6
7
0.6
68
78
93
99
1.8
13
1
6
7
0.6
68
78
93
99
1.8
14
1
6
7
0.6
68
78
93
99
1.8
15
1
6
7
0.6
68
78
93
99
1.8
16
1
6
7
0.6
68
78
93
99
1.8
17
1
6
9
0.8
69
74
96
98
2
18
1
6
5
0.8
67
70
93
98
2
19
1
6
9
0.4
65
73
94
99
2.4
20
1
6
5
0.4
63
68
92
99
2.4
21
1.5
6
5
0.6
83
82
97
98
2.7
22
1.5
6
9
0.6
84
84
97
99
2.7
23
1.5
6
7
0.8
85
84
97
99
3
24
1
10
9
0.6
78
75
95
99
3
25
1
10
5
0.6
76
74
96
97
3
26
1
10
7
0.8
76
78
95
99
3.3
27
1.5
6
7
0.4
82
82
96
99
3.6
28
1
10
7
0.4
74
77
93
99
4
29
1.5
10
7
0.6
80
79
98
99
4.5
In attempts to test the validity of the models developed in this study, a statistical analysis of variance was performed. Table 4 shows that the model validity can be verified by these parameters of regression coefficient R2, adequacy of precision, F-values and P-values. It was reported that the value of R2 should be at least 0.8 for good fitting of a model (Bashir et al., 2017). In the present study, the values of R2 for removal of COD, BOD, P, and FC are respectively 0.9501, 0.9516, 0.9483 and 0.9538. The obtained R2 values indicate that the model matches well with the experimental data, and all obtained values met good adequacy and satisfactory level. The predicted R2 is also in reasonable agreement with the adjusted R2 and the values are given in Table 4.
Response
Model
STD. Dev.
R2
R2adj
R2Pred
PRESS
CV%
Adeq. Precision
Mean
Remarks
COD removal
Linear
3.77
0.8995
0.8827
0.8441
529,56
5.24
17.840
66.48
Suggested
2FI
4.28
0.9028
0.8489
0.6851
1069,87
Quadratic
3.48
0.9501
0.9001
0.7123
977,28
Suggested
Cubic
2.72
0.9870
0.9391
−0.8792
6384,00
Aliased
BOD removal
Linear
4.27
0.6141
0.5498
0.4260
651,66
2.67
17.405
74.24
2FI
4.55
0.6724
0.4904
0.0524
1075,84
Quadratic
1.98
0.9516
0.9033
0.7214
316,32
Suggested
Cubic
2.60
0.9643
0.8332
−4.1475
5844,00
Aliased
P removal
Linear
1.47
0.8579
0.8342
0.7805
80,37
1.26
16.348
92.31
Suggested
2FI
1.51
0.8879
0.8257
0.6426
130,90
Quadratic
1.16
0.9483
0.8967
0.7025
108,96
Suggested
Cubic
0.42
0.9970
0.9862
0.5740
156,00
Aliased
FC removal
Linear
1.44
0.6849
0.6323
0.5670
67,96
0.74
16.504
97.03
2FI
1.64
0.6928
0.5222
0.2568
116,66
Quadratic
0.72
0.9538
0.9076
0.7340
41,76
Suggested
Cubic
0.74
0.9793
0.9034
−1.9815
468,00
Aliased
Energy consumption
Linear
0.35
0.9141
0.8998
0.8673
4,59
7.40
43.11
1.97
2FI
0.28
0.9589
0.9361
0.8725
4.41
Quadratic
0.15
0.9914
0.9829
0.9507
1.71
Suggested
Cubic
0.000
1.0000
1.0000
Aliased
The values of adequate precision are 17.84 for COD, 17.40 for BOD, 16.34 for P, 16.50 for FC and 43.11 for energy consumption, which indicates an adequate signal taking into account that the ratio higher than 4 is desirable. Hence, this model can be applied to navigate the design space. F-values explain the distribution of experimental values around the fitted model, and the F-values obtained in this study are all significant. The other criterion used for evaluating the model was P-values that define the significance of the model terms. P-values lower than 5% can confirm that the regression model is significant. In the present study, we can mention that all P-values of responses are less than 5%, which indicates a higher significance of the model terms. Furthermore, the obtained data shows that the model Y1 terms X1, X2, X1X2, and
have significant effects on COD removal, the model Y2 terms X1, X2, X3, X4, X1X2 and X12 have significant effects on BOD removal, the model Y3 terms X1, X2, X3, X4, X1X2, X3X4, X32 and X42 have significant effects on P removal, and the model Y4 terms X1, X2, X3, X1X4 and X2X3 have significant effects on FC removal, whereas the model Y5 terms X1, X2, X3, X1X2, X1X4, X42 have significant effects on energy consumption. The quadratic model for COD, BOD, P and FC present the highest R2, adjusted R2, predicted R2, F-values, and lowest P-values when compared to other models; therefore, it was selected for further analysis to optimize the EC process. In addition, Fig. 2 shows the plot of predicted values versus actual ones and indicates an adequate agreement between the applied model data and the experimental data.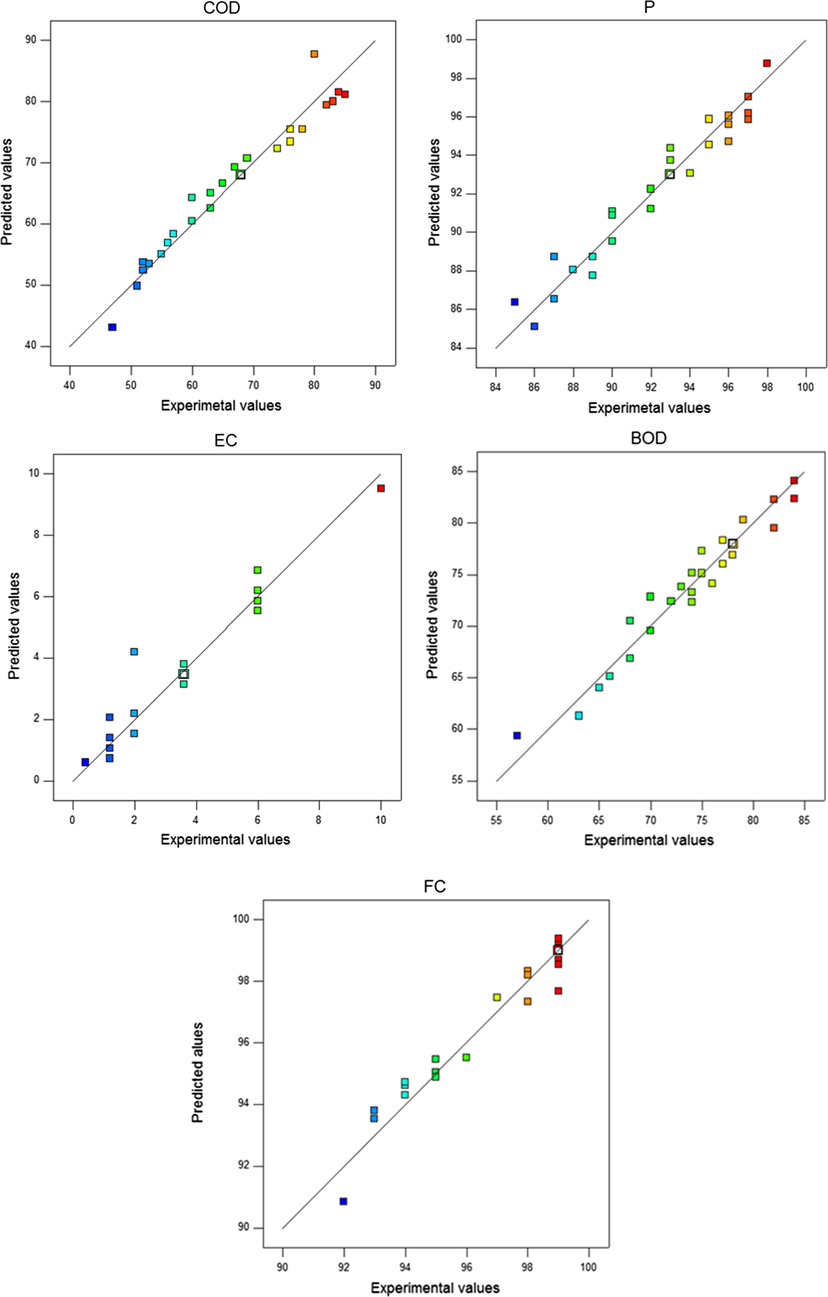
Regression plot of the experimental data versus the predicted values from the RSM.
3.2 Effect of variables on COD, BOD, P, and FC removal efficiency
3.2.1 Interaction effect of initial pH and applied current
Fig. 3 shows the 2D plot that describes the effect of initial pH and applied current on COD, BOD, P, and FC removals efficiency. While keeping electrolysis time and packed density at the center point (6 min and 0.6 g cm−3), as initial pH increased from 5 to 9 the removal efficiencies of COD, BOD, P and FC increased from 60 to 80%, 70 to 85%, 88 to 96%, 94 to 99%, respectively. Fig. 3 represented that the highest treatment rate can be achieved when initial pH is around 7, which is in accordance with reported findings (Jiang et al., 2002). Thus, it is also reported that amorphous Al(OH)3 has the minimum solubility within the pH range of 6.5–7.8 (Akbal and Camcı, 2011), owing to the availability of enough coagulant in this pH medium. At low pH 5, several monomeric and polymeric species, like
,
,
,
,
transform into insoluble Al(OH)3(s) flocs through polymerization and precipitation mechanism. While, at high pH > 9, the monomeric
concentration increases, which leads to decrease the insoluble amorphous Al(OH)3(s) flocs (El-Naas et al., 2009). It could be concluded that pH within the range of 5–9 is suitable to achieve good removal of pollutants from wastewater by EC process.![Effect of initial pH (X3) and current (X1) on COD (Y1), BOD (Y2), P (Y3) and FC (Y4) removal efficiencies; at [NaCl]initial = 2 g/L and time = 6 min.](/content/184/2021/14/1/img/10.1016_j.arabjc.2020.11.018-fig3.png)
Effect of initial pH (X3) and current (X1) on COD (Y1), BOD (Y2), P (Y3) and FC (Y4) removal efficiencies; at [NaCl]initial = 2 g/L and time = 6 min.
3.2.2 Interaction effect of operating time and applied current
Fig. 4 presents the contour plot for the effects of applied current and operating time on the COD, BOD, P and FC removal efficiencies. As previously expected, the removal efficiency increased with the increase of operating time and applied current. As an example, increasing operating time from 2 to 6 min (the center point), and applied current from 0.5 to 1.5 A, the removal efficiency of COD, BOD, P and FC increased from 50 to 80%, 60 to 86%, 86 to 98% and from 92 to 100%, respectively. This behavior can be attributed to the increase in applied current and operating time leading, in turn, to increase Al3+ ions released by the anode and increase the bubble generating rate at the cathode. By consequent, it results in an important amount of precipitate for a faster and a more efficient removal of pollutants, which is in accordance with reported results (Adhoum and Monser, 2004; Holt et al., 2002).![Effect of time (X2) and current (X1) on COD (Y1), BOD (Y2), P (Y3) and FC (Y4) removal efficiencies; at pH = 7 and [NaCl]initial = 2 g/L.](/content/184/2021/14/1/img/10.1016_j.arabjc.2020.11.018-fig4.png)
Effect of time (X2) and current (X1) on COD (Y1), BOD (Y2), P (Y3) and FC (Y4) removal efficiencies; at pH = 7 and [NaCl]initial = 2 g/L.
3.2.3 Interaction effect of packed density and applied current
Fig. 5 shows the contour plot for the effect of packed density and applied current on the removal efficiencies of BOD, COD, P, and FC; while keeping initial pH at 7 and electrolysis time at 6 min. As the packed density (X4) increased from 0.4 to 0.8 g cm−3 and applied current (X1) from 0.5 to 1.5 A, the removal efficiencies of COD, BOD, P and FC increased from 74 to 84%, 52 to 80%, 88 to 96% and 95 to 99%, respectively.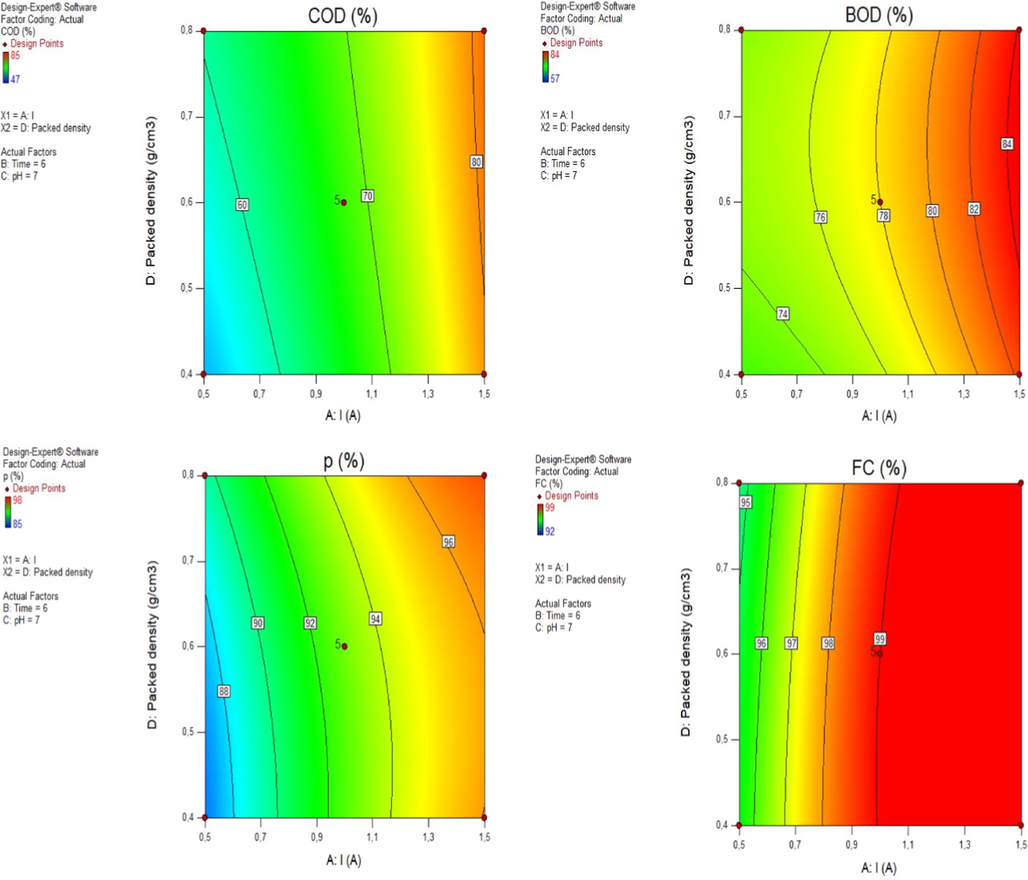
Effect of packed density (X4) and current (X1) on COD (Y1), BOD (Y2), P (Y3) and FC (Y4) removal efficiencies; at time = 6 min and pH = 7.
Generally, the number of microscopic galvanic cells in the packed-bed EC reactor is positively proportional to the dosage of Al scraps. Therefore, it is reasonable that the COD, BOD, P and FC removal rates were enhanced by increasing the aluminum scrap dosage in the reactor. According to these results, the decline in removal performances with a decrease in packed bed density could be explained by the fact that loose metal scraps cause a reduction in contact of anode material, which raises the external resistance from the solution, hence lowering EC performance (Ye et al., 2016).
3.3 Effect of current and packed density on energy consumption
In the EC treatment process, energy consumption is one of the important economic parameters. The energy consumption of a treated unit volume of urban wastewater can be calculated using the following equation:
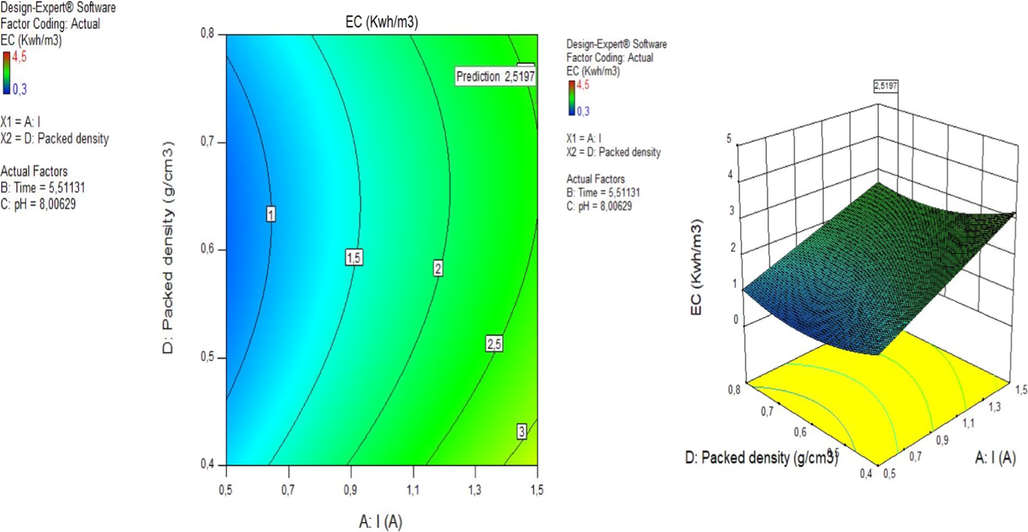
Effect of current (X2) and packed density (X4) on energy consumption.
3.4 Optimization using BDD
The option of point prediction was used in order to optimize the process parameters. There are five response options for goal fields: none, minimum, target, within range and maximum. In order to determine the optimum process parameters for the maximum COD, BOD, P, and FC removal efficiencies, two methodologies of optimization were used in this present study. The first methodology was performed to maximize the removal efficiencies, while the energy consumption was set within the range. The second one was used to maximize BOD, COD, FC and P removal efficiencies, whereas the energy consumption was minimized. According to the BBD results, the optimal operating conditions for the maximum COD, BOD, FC and P removals were found to be 1.5 A, 7.65 min, 7.48 and 0.75 g cm−3 for current (X1), electrolysis time (X2), pHi (X3) and packed density (X4), respectively. Under these conditions, the predicted removal efficiencies of COD, BOD, P and FC are found to be 85.13%, 84%, 98% and 99.7%, respectively. Then, the experiments were performed using the optimized conditions and led to the removal efficiency values of 82%, 83.2%, 97.5% and 99% for COD, BOD, P and FC, respectively. The obtained experimental results for all response parameters match well with the predicted results, which reflects a good suitability of the model to optimize the EC treatment process. Moreover, the economic conditions for the maximum removal of pollutants are the applied current (X1) of 1.5 A, electrolysis time (X2) of 5.52 min, initial pH (X3) of 8.00 and packed density (X4) of 0.74 g cm−3. Under these conditions, the predicted removal efficiencies of COD, BOD, P and FC reached 80, 84, 96 and 99%, respectively. Similarly, the experimental results were 78.5, 83, 94.7 and 99% for COD, BOD, P and FC, respectively. According to these results, an initial pH of 7.5 that corresponds to the original pH of the studied urban wastewater can be beneficial for achieving the highest COD, BOD, P and FC removal efficiencies. Thus, it can be concluded here that the EC applied conditions can be performed without any change of the initial pH. From Table 5, when the packed density increased from 0.74 to 0.75 g cm−3 and operating time decreased from 7.65 to 5.52 min, the difference in BOD and FC removal efficiencies between the optimum and economic conditions were quite negligible; while the COD and P removal efficiencies increased significantly from 78.5% to 82.0% and from 94.7 to 97.5%, respectively. In accordance with that, the specific energy consumption decreased from 3.63 to 2.52 kWh m−3. Therefore, the very good correlation between the predicted and the experimental results verified the model validity. This indicated that the optimal values of the individual factors were determined using RSM as a powerful tool.
Independent variables
Response
Condition
CD
time
pH
Packed density
COD (%)
BOD (%)
P (%)
FC (%)
Energy consumption (kWh.m−3)
X1 (A)
X2 (min)
X3 (pH)
X4(g.cm−3)
Actual
Predicted
Actual
Predicted
Actual
Predicted
Actual
Predicted
Actual
Predicted
Optimum
1.50
7.65
7.48
0.75
82
85.13
83.2
84
97.5
98
99
99.7
3.63
3.50
Economic
1.50
5.52
8.00
0.74
78.5
80
83
84
94.7
96
99
99
2.62
2.52
4 Comparison with results in the literature
The effectiveness of the EC using scrap aluminum in this study was compared with the literature findings that treat different types of wastewater employing different kinds of electrode material (Table 6). From the displayed data, it can be seen that COD and P removals from wastewater solutions varied from 52 to 95.48% and from 90 to 100%, respectively. For these results, the optimum conditions for COD and P removals were obtained as pHi of 3–6.2 and 3–4, current efficiency of 6–631 and 10–20 A/m2, and EC time of 10–30 and 20–100 min, respectively. At these conditions, energy consumption varied from 0.50 to 39.7 KWh m−3 and from 4 to 14.86 KWh m−3, respectively. In this study, the energy consumption needed to achieve 82% of COD and 97.5% of P was found to be 3.5 KWh m−3, which is sufficiently low as compared to values mentioned in Table 6. Therefore, it can be concluded that the optimized process is economically feasible.
Electrode type
Wastewater type and Operating conditions
Optimum value
Re (%)
Energy consumption
Ref.
Aluminum Ball
Synthetic Humic acid (HA) wastewater: pH 4–8, CD = 2–10 mA cm−2, tEC = 5–25 min, [HA]10–50 mg L−1
pHi = 4, CD = 6 A.m−2,
tEC = 25 min, [HA] = 30 mg L−1
Re,COD = 90.8%,
EC = 1 kWh m−3
(Kac et al. (2017); Khandegar and Saroha (2013))
Iron scrap
Synthetic color wastewater (Reactive blue 21): Current = 0.6–1.2, tEC = 0–26 min
Current = 0.9 A, tEC = 10 min
Re,COD = 93%
EC = 0.50 kWh m−3
(Ardhan et al., 2014)
Iron plate
Domestic wastewater:
[PO4] = 5–50 mg L−1
CD = 10–40 A m−2, tEC = 0–100 min, pHi = 4–7[PO4] = 52 mg L−1, pHi = 4, CD = 20 A m−2, tEC = 100 min
Re,PO4 = 99%
EC = 4.17 kWh m−3
(Omwene and Kobya, 2018)
Aluminum plate
Leachate wastewater: tEC = 0–30 min, CD = 348–631 A m−2,
tEC = 30 min, CD = 631 A m−2
Re,COD = 56%
EC = 39.7 kWh m−3
(Ilhan et al., 2008)
Aluminum plate
Synthetic Phosphate wastewater: j = 20–180A m−2, tEC = 0–140 min, [P] = 100 mg/L, pHi = 2–11
pHi = 3
Re,P = 100%
EC = 4 kWh m−3
(Attour et al., 2016)
Aluminum plate
BR, j = 2.5–10A m−2, tEC = 20 min, [P] = 100 mg/L, pHi = 3
pHi = 3
Re,P = 90%
EC = 14.86 kWh m−3
(İrdemez et al., 2006)
Aluminum plate
Olive oil mill wastewater: CD = 10–40 mA cm−2, pHi = 4–9, tEC = 2–30 min
CD = 20 min, pHi = 6.2, tEC = 30 min
Re,COD = 52%
EC = 2 kWh m−3
(Inan et al., 2004)
Aluminum plate + H2O2 + PAC
Poultry slaughtering wastewater: pHi = 2–10, tEC = 0–100 min, [H2O2] = 0–0.25 mg L−1 , [PAC] 0–0.6 mg L−1, CD = 30–50 mA cm−2
pHi = 3, tEC = 40 min, [H2O2] = 0.2 mg L−1 , [PAC] = 0.5 mg L−1 , CD = 50 mA cm−2
Re,COD = 95.48%
EC = 103.89 kWh m−3
(Eryuruk et al., 2018)
Aluminum scrap
Real urban wastewater: pHi 5–9, Current = 0.5–1.5 A, tEC = 0–10 min, [NaCl] = 1–3 mg L−1
pHi7.85, Current = 1.5 A, tEC = 5.84 min, [NaCl] = 3 mg L−1
Re,COD = 81%
Re,BOD = 84%
Re,P = 97%
Re,FC = 99%EC = 3.55 kWh m−3
This work
5 Conclusion
A novel developed EC system with an electrode (anode) composed from used aluminum scrap that is packed in a perforated cylinder of PVC, was developed to optimize the BOD, COD, P and FC removals from real urban wastewater. In addition, modeling by Box-Behnken design (BBD) was investigated to predict the optimal and economic conditions for the efficient removal of pollutants. The model suggested by the BBD for the maximum removals of COD, BOD, P, FC, and minimum energy consumption exhibited that the obtained values are in the desirable range and matched in good adequacy with the model. Thus, the proposed second-order polynomial model was acceptable owing to the high accuracy obtained with R2 values of COD (0.950), BOD (0.951), P (0.948), FC (0.953) through ANOVA analysis; therefore, quadratic models were selected for predicting the responses. Hence, the optimum experimental conditions resulted in removal efficiency of 82.0% COD, 83.2% BOD, 97.5% P and 99.0% FC, with an energy consumption of 3.50 kWh m−3. Accordingly, after the set of the economic conditions, the treatment efficiency became 78.5% for COD, 83% for BOD, 94.7% for P and 99% for FC with a lower energy consumption of 2.52 kWh m−3. The results reflected clearly the predicted values agreed well with the obtained experimental values of COD, BOD, P and FC. Thus, the findings here showed that using scrap aluminum as low-cost anode of EC process for real urban wastewater treatment is more efficient than plate electrode, which can be considered as first reported results for such kinds of real urban wastewater treatment with BBD modeling, and suggested to be very useful for further research works regarding either other wastewaters or investigating other types of scrap anodes.
Acknowledgment
The authors would like to express their gratitude to Dr. Aouaaram from ONEE (Al Hoceima) where the urban wastewater samples were taken from. Dr. El Kasmi would kindly thank the support of Chinese Academy of Sciences for senior international scientists (Grant No. 2017PE009) and CAS project (Grant No. 2018/43).
References
- Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process. Process Intensif.. 2004;43:1281-1287.
- [Google Scholar]
- APHA. 2005. American Public Health Association. (2013). Standard Methods for the Examination of Water and Wastewater. 21st ed. American Public Health Association, Washington DC, 1220p.
- Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination. 2011;269:214-222.
- [Google Scholar]
- Novel anode made of iron scrap for a reduced-cost electrocoagulator. Chem. Eng. J.. 2014;253:448-455.
- [Google Scholar]
- Intensification of phosphate removal using electrocoagulation treatment by continuous pH adjustment and optimal electrode connection mode. Desalin. Water Treat.. 2016;57:13255-13262.
- [Google Scholar]
- Electro persulphate oxidation for polishing of biologically treated palm oil mill effluent (POME) J. Environ. Manage.. 2017;193:458-469.
- [Google Scholar]
- Effect of operating parameters on indium (III) ion removal by iron electrocoagulation and evaluation of specific energy consumption. J. Hazard. Mater.. 2009;167:467-474.
- [Google Scholar]
- Improved adsorption performance of nanostructured composite by ultrasonic wave: optimization through response surface methodology, isotherm and kinetic studies. Ultrason. Sonochem.. 2017;37:94-105.
- [Google Scholar]
- Definition of a practical multi-criteria procedure for selecting the best coagulant in a chemically assisted primary sedimentation process for the treatment of urban wastewater. Desalination. 2008;230(1–3):229-238.
- [Google Scholar]
- Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater. Desalination. 2009;238:302-311.
- [Google Scholar]
- Synthesis and characterization of ZnO-nanorods loaded onto activated carbon and its application for efficient solid phase extraction and determination of BG from water samples by micro-volume spectrophotometry. New J. Chem.. 2015;39:9407-9414.
- [Google Scholar]
- A novel electrocoagulation process using insulated edges of Al electrodes for enhancement of urban wastewater treatment: Techno-economic study. Process Saf. Environ. Prot.. 2018;116:506-515.
- [Google Scholar]
- Electrocoagulation flocculation as a low-cost process for pollutants removal from urban wastewater. Chem. Eng. Res. Des.. 2017;117:614-626.
- [Google Scholar]
- Assessment of electrocoagulation for the treatment of petroleum refinery wastewater. J. Environ. Manage.. 2009;91:180-185.
- [Google Scholar]
- Electrochemical treatment of wastewaters from poultry slaughtering and processing by using iron electrodes. J. Clean. Prod.. 2018;172:1089-1095.
- [Google Scholar]
- Development of Box Behnken design for treatment of terephthalic acid wastewater by electrocoagulation process: optimization of process and analysis of sludge. J. Environ. Chem. Eng.. 2016;4:178-190.
- [Google Scholar]
- A quantitative comparison between chemical dosing and electrocoagulation. Colloids Surf., A. 2002;211:233-248.
- [Google Scholar]
- Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Cleaner Prod.. 2017;147:206-216.
- [Google Scholar]
- Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J. Hazard. Mater.. 2008;154:381-389.
- [Google Scholar]
- Olive oil mill wastewater treatment by means of electro-coagulation. Sep. Purif. Technol.. 2004;36:23-31.
- [Google Scholar]
- The effects of current density and phosphate concentration on phosphate removal from wastewater by electrocoagulation using aluminum and iron plate electrodes. Sep. Purif. Technol.. 2006;52:218-223.
- [Google Scholar]
- Laboratory study of electro-coagulation–flotation for water treatment. Water Res.. 2002;36:4064-4078.
- [Google Scholar]
- Removal of humic acid by fixed-bed electrocoagulation reactor: studies on modelling, adsorption kinetics and HPSEC analyses. J. Electroanal. Chem.. 2017;804:199-211.
- [Google Scholar]
- Electrocoagulation for the treatment of textile industry effluent–a review. J. Environ. Manage.. 2013;128:949-963.
- [Google Scholar]
- Treatment of micropollutants in municipal wastewater: ozone or powdered activated carbon? Sci. Total Environ.. 2013;461:480-498.
- [Google Scholar]
- Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater. Water Res.. 2009;43:751-761.
- [Google Scholar]
- Treatment of domestic wastewater phosphate by electrocoagulation using Fe and Al electrodes: a comparative study. Process Saf. Environ. Prot.. 2018;116:34-51.
- [Google Scholar]
- Electrocoagulation treatment of rice grain based distillery effluent using copper electrode. J. Water Process Eng.. 2016;11:1-7.
- [Google Scholar]
- Mineralization of Acid Yellow 36 azo dye by electro-Fenton and solar photoelectro-Fenton processes with a boron-doped diamond anode. Chemosphere. 2011;82:495-501.
- [Google Scholar]
- Optimization of color and COD removal from livestock wastewater by electrocoagulation process: application of Box-Behnken design (BBD) J. Ind. Eng. Chem.. 2015;28:307-315.
- [Google Scholar]
- Electrocoagulation in a packed bed reactor-complete treatment of color and cod from real textile wastewater. J. Environ. Manage.. 2013;123:113-119.
- [Google Scholar]
- Treatment of Ni-EDTA containing wastewater by electrocoagulation using iron scraps packed-bed anode. Chemosphere. 2016;164:304-313.
- [Google Scholar]
- Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: feasibility test of advanced oxidation processes with/without pretreatment. Chem. Eng. J.. 2011;166:932-939.
- [Google Scholar]







