Translate this page into:
Development of effervescent tablet formulation for rapid control of mosquito problem in early stages from different breeding sites
⁎Corresponding author at: C-251 Shaheen Bagh, Jamia Nagar, Okhla, New Delhi-25, India nusratsiddiqa20@gmail.com (Nusrat Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Development of Neem formulation for solving mosquito problem in water bodies. Physic-chem. analysis was done in tablet formulation. Bio-efficacy evaluation was checked against larval stages of mosquito. Water quality was determined after application of tablet in water bodies.
Abstract
Mosquito problem remains a major problem due to unavailability of effective formulation for controlling mosquito at the starting stages. The aim of this study was to develop a neem based effervescent tablet for immediate and fast destruction of mosquito larvae in any unrecognized ecosystems like stagnant water after rain or water blockage or for household applications. Effervescent tablet was prepared by the 30% neem oil absorbed over silica and then combine acids/acid salts and bicarbonates in definite proportion (citric acid, tartaric acid, sodium bicarbonate and sodium carbonate). The powdered mixer was then compressed in tablet machine. The tablet shows bubbling reaction by releasing CO2 when dispersed in water. The tablet was evaluated for hardness, thickness, disintegration, weight variation, Ph, content % and accelerated storage test. The tablet shows all the physicochemical parameters within the limits. Bio-efficacy evaluation was tested against larvae. It shows 75–90% mortality for Aedes agyptii larvae.
Keywords
Effervescent tablet
Acids
Acid salts
Disintegration
Larval mortality
1 Introduction
Mosquitoes are the main vector for transmission of many dangerous diseases like malaria, chikangunia, dengue etc. There are many mosquito control products are available but most of the product are effective only for adult mosquito control and very less for larval control. These available formulations are only limiting to mosquito control in houses not for outdoor mosquitoes (Yakob and Yan (2009)). So, mosquito problem remain constantly a major problem Larval control is the very effective strategy for termination of mosquito problem before the instance that it would become the reason of people death (Gu et al. (2006)). In Recent years a very effective way of mosquito control by spraying of stagnant water around residential areas are by pesticides like organophosphorous, pyrithroids for destruction of larval stages. This type of spraying may terminate the larval stages but main drawback is that by using these spraying pesticide, environment and non-target organisms negatively affected. Spraying pesticide may go into the air or water bodies cause bioaccumulation as well as air and soil pollution due to long residual effect of pesticides (Gayan et al., 2013). In humans it causes some acute as well as chronic defects. So, it should be replaced to some other control strategies.
Larvicides are of mainly synthetic but many botanical pesticides can be used for larval control. Neem is the most important and widely used botanical which has antifeedent, anti ovipositional and growth regulating properties (Dua et al. 2009; Egho and Ilondu (2012)). Including these properties neem shows repellency and kill mosquito in any stage of their life cycle. So, neem can effectively applied as larvicide and adult mosquito control. Neem is specifically effective against the mosquito population only and left no harmful residue after usage. Many neem formulations are available for adult mosquito control like repellent cream, coil, aerosol sprays. But no neem formulation is available for larval control. This study aims to develop effervescence tablet formulation, a very fast and efficient formulation for larval control with high and uniform dispersion of neem. These tablets were developed by compression of neem oil with inert constituents and dispersed in water quickly by releasing carbon dioxide.
2 Materials and method
2.1 Neem oil- active principle
Neem oil was supplied by Theni, Gujarat, India. The percentage of constituents like triterpenoid compounds like Azadirachtin A + B (0.03%) and Nimbin (2.6%) along with fatty acids, specifically Omega-6 (5–15%), Omega-9 (22–25%), Stearic acid (9–24%) and Palmitic acid (12–15%). Specific gravity, saponification value and impurities & moisture content of neem oil are 0.921 g.ml-1at 280.15 K, 203 mgKOHg−1, and 0.17 g.g−1 w/w respectively.
2.2 Disintegrating agent
D + Glucose purchased from sigma Aldrich of purity >99.5%.
2.3 Dispersing agent
Sodium dodecyl sulfate of purity 98% purchased from Sigma Aldrich.
2.4 Effervesence agents
Sodium bicarbonate anhydrous, free flowing with water solubility 100 mg/ml purchased from sigma Aldrich, Citric acid anhydrous (99.5%) , white crystalline powder, free flowing procured from Merck.,Tartaric acid purchased from sigma Aldrich.
2.5 Binder
Magnesium stearate technical grade purchased from sigma Aldrich.
2.6 Filler
Silica (Grade –Supersil 100) purchased from supersil chemical Pvt. Ltd. It is obtained in pure white amorpous powder, particle size 18–22 µm, Surface Area (110–125 M2 / gm), Moisture content at 105 °C for 1 hr is 5%,Bulk density- (0.08–0.11 gm/cc), (Oil absorption 240–270%)
All materials were used as purchased no further purification was required.
The bioefficacy evauation was done in Aedes agyptii in Institute of Pesticide Formulation Technology, Gurugram.
3 Method
3.1 Preparation of tablet in three steps
3.1.1 Wet granulation
Weighed 30 g of neem oil and transfer the weighed neem oil in the full cone nozzle sprayer. After that 15 g silica was weighed in 500 ml beaker. Then neem oil was sprayed over silica and mix simultaneously by glass rod. The 30 g Neem oil was the optimized weight of neem oil for 15 g silica. Beyond 30 g neem oil addition silica particles will cause aggregration of particles and flowability will also affected. After neem oil impregnation over silica inert ingredients were added in the sequence – first sodium lauryl sulphate as dispersing agent, followed by glucose as disintegrating agent, and finally silica as filler. All the inert agents were mixed in the homogenizer (Kitchen blender) (Sujata-Dynomax) which was set by speed regulator at speed of 200 rpm for 10 mintes. After uniform mixing then effervessence agents citric acid, tartaric acid and sodium bicarbonate were added.
3.1.2 Lubrication
After homogeneous mixing of all the ingredients magnesium stearate as lubricating agent was added and mixed uniformly by grinder at speed 100–200 rpm for 5 min.
3.1.3 Compression
All the homogeneously blended powdered composition was granulated by adding 1–2 g methanol in the homogenizer at speed of 200 rpm for 5 min. After granulation then granulated powdered material was transferred to tablet machine (AJANTA, Model Number/Name: Aei-tm-sh-13) funnel and compressed by tablet punch machine with 17.8 mm punch set (Fig. 1).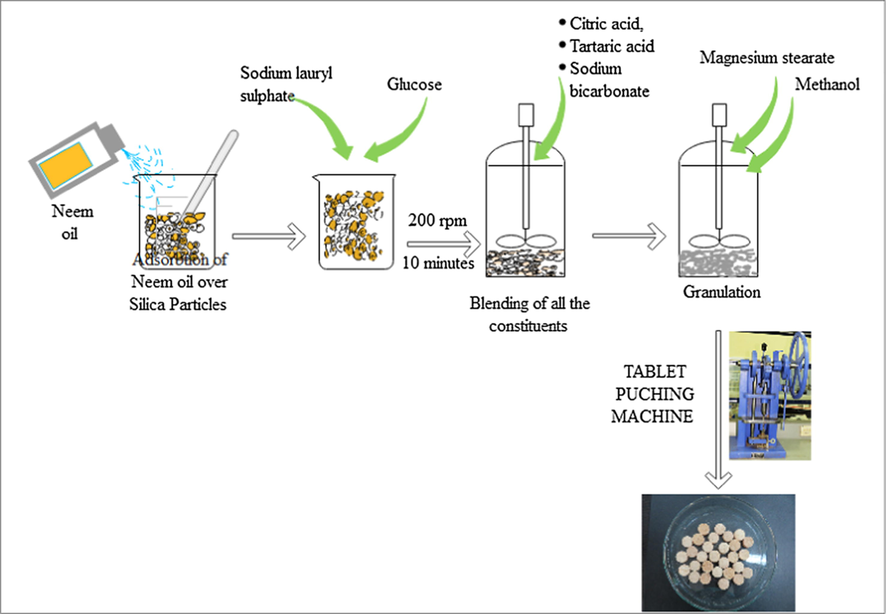
Flow diagram showing the sequence of tablet processing to make effervescence tablet.
3.2 Precompression test
3.2.1 Particle size determination
The particle size was measured by particle size analyzer (Malvern Instrument model system 2000) equipped with a He-Ne laser light source (Malvern series 2000 software) .Distilled water was used as dispersant.
3.2.2 Flowability
Flowability was determined as per Collaborative International Pesticides Analytical Council (CIPAC MT-193). In this method powdered mixer with all active and inert ingredients were poured through the funnel. The mixed powder was poured through the funnel until it touches the tip of funnel. R is the radius of the conical pile and H is the hight of powdered cone as shown in Fig. 2. Flow ability was calculated by the formula described in eq.1 as given below:-
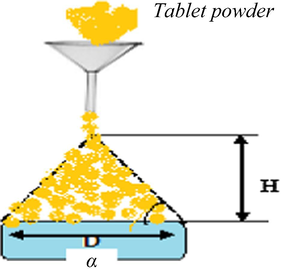
Diagramatic representation of flowability measurement.
3.3 Apparent bulk density
Apparent bulk density was measured by volume and weight measurement of the tablet powdered mixer. For apparent bulk density measurement, tablet mixer was weighed first and then pours in the measuring cylinder and packed without any tapping. The volume of the known amount of weight tablet mixer was noted from the graduated cylinder. Apparent density was calculated by following formula as described in eq (2):-
m = weight of powder (gms)
v = volume of tablet mixed (without tapping)
3.4 Packed bulk density
Same procedure was repeated as in apparent bulk density measurement. The only difference is that cylinder should be tapped till no change was seen in the volume mark of cylinder after putting powered content. Packed bulk density was calculated by following formula as described in Eq. (3):
m = weight of tablet mixer in gms for tight paking of cylinder
vb = volume of packed mixer (final tapped volume)
3.5 Compressibility percent (%)
Compressibility % was calculated by the putting obtained values of apparent bulk density and packed bulk density measurement as given in equation (4). The values were interpreted by the carr’s grading index as in Table 1.
Compressibility %
flow
5–15
excellent
12–16
good
18–21
fair
23–35
poor
33–38
Very poor
Less than 40
Not eligible
3.6 Post compression analysis of tablet
3.6.1 Hardness of tablet
Tablet hardness was checked by hardness tester (Monsanto tablet hardness tester). In this tester, 10 tablets was placed on the plunger while putting tester should show zero reading of lower side and then with known force upside plunger was placed on tablets. Records the force by which tablets will break these tablets, the force was recorded in newton as the hardness of the tablet (Masareddy et al. (2010)).
3.6.2 Tablet dimensions
Tablet thickness was measured by vernier caliper. Thickness measurement was done randomly selected 10 tablets. Diameter was calculated by scale of 15 cm length.
3.6.3 Friability
Friability was determined by Roche friabilator. In this measurement tablets was dropped from a 5 in. height and then revolving under abrasive and shocking moments in a friabilator at 25 rpm for 5 min. After these revolutions tablets was de-dusted by soft cloth and weighed again. The friability was checked in three replicates. The tablet friability percentage was calculated as:
3.6.4 Weight variation test
In this test 20 tablets were selected arbitrarily and weighed separately. Then average weight of 20 tablets was calculated. After that % weight variation was calculated as:
Weight vatriation test as per u.s.p only be considered pass, when not more than 2 tablets deviated from the limit of ± 5% from the average tablet weight (Lachman et al. (1991)).
3.6.5 pH measurement
Dissolve one tablets in 200 ml water and then p H of water was measured by the pH meter. The Ph measurement was done in triplicates (Aslani and Jahangiri (2013)).
3.7 Rate of disintegration
Disintergration is measured as per CIPAC method MT 197. In this method one effervessence tablet was put in 250 ml distilled water and mixed by glass rod by gentle stirring for specific time period. The absence of no residue left on sieve indicate complete disintergration of tablet in water. This test was repeated three times.
3.8 Moisture content
Ten tablets were weight and then dried in desicator with calcium sulphate for 4–6 hrs. Then the moisture content was calculated as follows:-
3.9 Content analysis by UV– visible and HPLC
For content analysis twenty tablets were weighed and fine powder was made by grinding. An equivalent amount of 300 mg of neem oil dispersed silica was weighed and kept in 10 ml volumetric flask. The tablet powder was sonicated for 5 min and stir over magnetic stirrer for 10 min. After complete mixing then the mixer was filtered through Whatman cellulose filter paper and further aqeous dilutions were made. Finally, absorbance was measured at 208 nm by UV –visible spectroscopy(Perkin Elmer) against blank. Same filtrate was analyzed by HPLC (Shimadzu -(LC-2010AHT) by taking Azadirachtin as standard in following conditions:-
Stationary Phase – C-18 Column,
Mobile Phase –Acetonitrile/ Water (35:65).
Run Time-60 Minutes,
Flow Rate 1.2 ml/Min,
Injection Volume-10 µl,
Detector Wavelength −214
Volume of Injection-10 µl.FTIR
FT-IR was performed by Thermo fisher scientific ATR-FTIR(Model:Nicolet™) spectrophotometer. The FT-IR characterization was performed in crused powdered materials of tablet after post compression. The infrared (IR) Spectra were recorded and values are expressed as λ max cm−1.
3.10 Accelerated stability test (ATS)
In ATS studies as per CIPAC method MT 46.3 in this method test tablet was stored in triplicates at 54 °C and room temperature for 15 days. After 15 days all the parameters were studied again for assessing the stability of effervescence tablet.
3.11 Bio-efficacy assay of neem effervescence tablet on Aedes aegypti
-
Maintenance of the mosquito larvae of Aedes aegypti
Bioassay of Aedes aegypti was maintained in Institute of Pesticide Formulation Technology,Gurugram,Haryana in standard conditions i.e 25 ± 5 ͦ C temperature and 70 ± 2% RH. The larvae were kept in water basins along with starch and cellulose as larval feed.
-
Larval bioefficacy
After assessment of physicochemical parameters effervessence tablet biefficacy was checked against larvae of mosquito species Aedes aegypti. The bioefficacy of tablet was measured in mortality percentage of larvae after application of tablet in 7 replicates. Larvicidal mortality test of the tablet was evaluated on 3rd instar larvae of Aedes aegypti as per WHO guidelines. Ten larvae was collected by the dropper and transferred into 250 ml containg dispersed tablet in water. Bio-assay was done in 7 replicates.
The experiment was carried out in seven different beakers as 7 replicates. Ten larvae was kept as control in separate beaker without tablet. Control and treated set was kept undisturbed at 25 ± 5 °C and 70 ± 8 RH. Mortality % was checked after 24 hr and 48 hr in replicates and control (Schnaubelt (1999) and then % mortality was calculated by abborts formula (Abbott 1925).
% Test mortality -% Control mortality × 100
% Mortality =
100 - % Control mortality
-
Ovipositional deterrent activity bioassay
The Ovipositional bioassay was carried out according to the method mentioned in (Xue et al. (2003)) with small modification. Twenty gravid females of Ae. aegypti female mosquitoes were released in iron framed mosquito cage having dimensions 30 × 30 × 25 cm. After 24hr the eggs were counted and ovipositional deterrent was calculated as ovipositional detterent percentage (%) calculation as shown below:-
Ovipositional Detterent Percentage (%) = EC – ET / EC
EC = No. of eggs laid in Control
ET = No. of eggs laid in test (with dispersed tablet)
-
Ovicidal activity
Ovicidal activity was checked by mentioned method with slight modification (Su and mulla, 1998). The ovicidal test was carried out in 28 ± 1ͦC and 35–45% RH. In ovicidal test 25 gravid females were placed in the mosquito cage. Five bowls filled with 100 ml distilled water and one tablet was dispersed in each bowl. One bowl was filled with water and left without dispersing the tablet and treated as control. The hatching of eggs was checked after 40 hrs of oviposition and calculates the hatching rate.
% of egg mortality = Total no. of hatched larvae/total no. of eggs X 100
4 Statistical analysis (DMRT) of bio-efficacy results
The results calculated here are means of replicates. The standard deviation was calculated. The data was analysed by one-way analysis of variance (ANOVA) and significant effects were noted using SAS 9.4 software. Duncan’s multiple range test (DMRT) 5% levels was also employed to test for significant differences between treatments.
5 Quantification of dissolved oxygen in applied water bodies
Dissolved oxygen analysis of tablet applied water was done according to the wrinkler’s method. One tablet of 0.1 cm diameter was dispersed in 100 ml water and kept it for 5 days at 20 ± 5 ͦ C. In the given sample 1 ml of alkali azide and then 1 ml manganous sulfate solution was added and mixed vigorously. After that it was titrated with sodium thiosulphate (0.025 N) till color changes from dark yellow to light yellow. Then few drops of starch indicator was added and continue to titrate till the solution becomes colorless as original sample. Record the volume of 0.025 N Sodium thiosulphate consumed. 1 ml of 0.025 N sodium thiosulfate is equivalent to 1 mg/L dissolved oxygen.
6 Results and discussion
6.1 Optimized composition of effervescence tablet
All the inert ingredients and effervescence combination amount were optimized in 30% Neem oil along with wetting and dispersing agents. The effervescence combination was optimized separately. In effervescence tablets both the combinations were mixed homogenously and followed by compression in Table 2.
Active ingredient base stock
Effervescence combination
Ingredients
Wt %
Ingredients
Wt %
Neem oil
30
Citric acid
8
silica
15
Tartaric acid
10
Wetting and dispersing agent
Lubricant
Effervescence
combination10
10
40Sodium carbonate
22
6.2 Mechanism of fast dispersion in water
Effervescence liberate CO2 when dispersed in water by following chemical reaction (H. Stahl 2003):
-
Chemical reaction of citric acid with sodium bicarbonate is defined as:
C6H8O7 (aq) + 3NaHCO3 (aq) → Na3C6H5O7 (aq) + 3H2O + 3CO2 (g) ↑
Citric acid + Sodium bicarbonate → Sodium citrate + Water + Carbon dioxide
-
Similarly Chemical reaction of tartaric acid with sodium bicarbonate is defined as:
H2C4H4O6(aq) + 2NaHCO3 (aq) → Na2C4H4O6 (aq) + 2H2O + 2CO2 (g) ↑
Tartaric acid + Sodium bicarbonate → Sodium tartarate + Water + Carbon dioxide
6.3 Precompression test
6.3.1 Particle size determination
Particle size were in the range of d50 value was 4.06 ± 3 µm and d90 value was 18.01 ± 3 by particle size analyzer. All particles are of uniform size mostly in the range of 11–20 µm .It shows that particles were fine and easily bind after compaction and disperse uniformly in water (Shanmugam, 2015).
6.3.2 Flowability
Flowability was calculated by the angle of repose calculation. The compaction and good flowability reduce the handling hazards from dust formation and regulate the dissolution (Kristensen & Hansen, 1995). Angle of repose was measured 26.38 ± 6 that showed that tablet mixer was free flowing and no agglomeration will occur in between particles. So, the fined tablet mixer is appropriate for making effervescence tablets.
6.3.3 Compressibility percent (%)
Compressibility % was in between 14 and 15 % which is excellent as per carr’s index. Compressibility is depends upon the density of the powdered mixed. More the density of powdered mixer more will be the compressibility and results into less dissolution. Here bulk density and apparent density of powdered mixed is 0.546 and 0.565 respectively. These lesser values of density indicate that the tablet powder is porous, fluffy and fine in size. This results into excellent compression % which will more suitable for uniform and fast dispersion of effervescence tablet.
6.4 Post compression analysis of tablet
6.4.1 Hardness of tablet
Hardness of the effervessence tablet was less as compared to normal tablet because of porous structure inside the tablets. The hardness of neem efferevessence tablet was 5.6 kg/cm2.
6.4.2 Tablet diamensions
Thickness was calculated in between 5.6 and 5.8 mm and diameter 17.8 mm. The measured size is suitable to prevent the mechanical resistance and increase the tensile strength of tablet (Tissen et al., 2011).
6.4.3 Friability
Friability is the test for measuring toughness and physical strength of the uncoated tablets by exposing them to mechanical stress conditions. This test is very important to identify the tablet stability during transport and packing processes. Friability percentage should be less than 1% otherwise the tablet is unstable and failed the friability test (Masareddy et al. (2010)). Friability of the neem effervescence tablet was 0.8–0.9%.
6.4.4 Weight variation test
Weight variation is the significant measurement to ensure that each tablet have uniform quantity of active ingredient. The weight variation test has passed by the effervescence tablet as per U.S.P limits i.e below 5%.Weight of all the tablets were uniform and very low S.D (standard deviation values). The weight variation was in between 1.06 ± 0.003 to 1.09 ± 0.004.
6.4.5 pH measurement
pH should be less than 6 i.e. slightly acidic which is very suitable for effervescence reactions. The effervescence tablet pH values were 5.3–6.7.
6.4.6 Rate of disintegration
Disintegration of tablet is used for measuring the compactness of tablet and identification of time required for complete dissolution of tablet in water. The disintegration time was only 60 sec. After 60 sec the water is transparent no particles were seen except some foaming over the top of beaker.
6.4.7 Moisture content
For increasing shelf life of effervescent tablet moisture content should be less than 0.3%. Water content was low 0.20 ± 0.03 to 0.30 ± 0.04%. The effervescent tablet should be stored in air tight container to prevent moisture absorption.
6.4.8 Content analysis by UV– visible and HPLC
UV –Visible shows 98.06 ± 0.348% recovery. It shows that inert ingredient not interfering after tablet formulation. Tablet filterate shows 250 ppm azadirachtin content and during storage of ATR only 2% was degraded. Content analysis shows that after tablet formation neem ingredient remain same and not undergoing any degradation or decomposition loss.
6.4.9 Ftir
Neem oil effervesence tablet shows absorbance band at 3255.17 cm−1 (Pyrollidine N-H Stretching) in PVP K-30 which act as dispersing agent, methy ester bands at (2952 cm−1), C = O carboxylic acid of fatty acids (1744 cm−1) in neem oil, C = C of aromatic ring at (1433 cm-1) in active ingredients as shown in Fig. 3. All the red arrows shows bands of neem oil and green arrows are corresponds to inert ingredients structural groups. FT-IR of effervessence tablet showed separate peaks for inert and active ingredients. Hence, the FT-IR data illustrates that tablet inert ingredient are nonreactive to neem oil and maintain the active constituents same as in neem oil in the tablet formulation.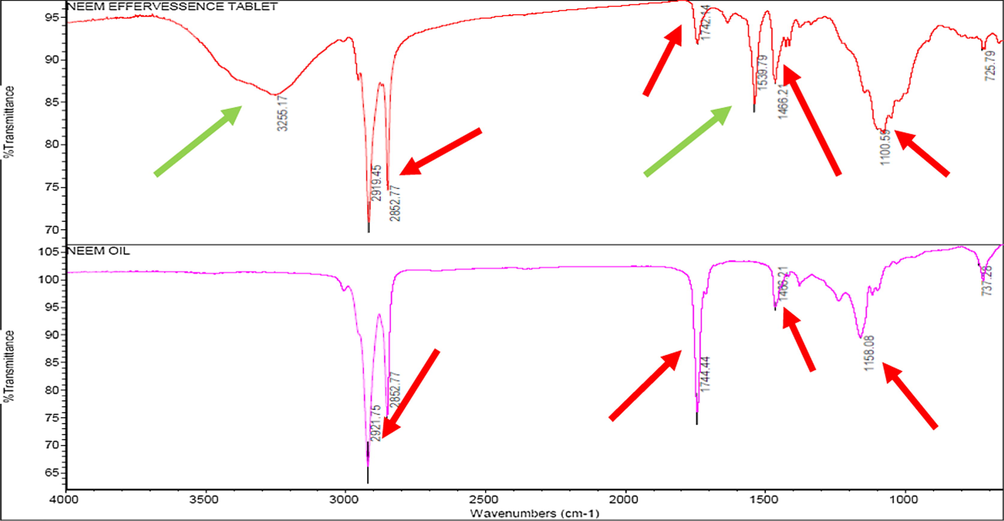
FTIR analysis of effervescence tablet.
6.5 Accelerated stability test
Accelerated stability testing is the validated method for predicting the stability of product for long period of time under different conditions. This test has significant role in setting shelf life of the any formulated product as Table 3.
Physiochemical evaluation
Initial
After 15 days
25 °C
54 °C
Hardness (kg/cm2)
5.6 ± 0.07
5.7 ± 0.06
5.7 ± 0.05
Tablet dimensions (c.m)
0.5
0.5
0.5
Friability (%)
0.8 ± 0.34
0.83 ± 0.32
0.85 ± 0.24
Weight variation test
1.09 ± 0.004
1.08 ± 0.002
1.06 ± 0.005
Ph
5.72 ± 0.020
5.73 ± 0.015
5.81 ± 0.023
Rate of disintegration (sec)
60
60
50
Moisture content %
0.30 ± 0.04
0.35 ± 0.06
0.25 ± 0.03
Content uniformity
99.6 ± 4.30
98.6 ± 2.50
95.6 ± 5.30
The results of table 3 illustrated that after storing at various conditions tablet physicochemical evaluation data was slightly changed which was remain in the limits. This shows that tablet is stable during extended period of time at various conditions with same efficiency as freshly prepared tablet. It confirmed that no degradation and change was occurred during storage
6.6 Larval bioefficacy
There are many problems are associated with the use of synthetic pesticide for combating the mosquito problem like pesticide resistance, toxicity and many other harmful effects towards non-targets. Hence, there is urgent need for safe alternates like plant derived products which are safe and effective against mosquito control. The present study of Neem effervescence tablet gave on an average 85% larval mortality after dispersion in water and effective up to 1 week after application. Table shows that decrease in mortality percent was slightly varied from the initial this shows that the formulation has residual effect for long period of time. Neem has antifeeding property by altering the insects chemo receptors for food (Mordue (Luntz) AJ, Blackwell A, (1993)). Mosquito larvae when ingest the dispersed neem content which results larval mortality. Neem has growth inhibitor activity which will stop the larval growth and results into larval mortality(Nathan et al., 2005).The bio-activity was good due to their uniform dispersion in water and effectively control the mosquito larvae on breeding places.
Effervescence tablet gave on an average 85–90% larval mortality after 24 hrs and remain effective for 1 week with on an average 75% larval mortality after dispersion of four tablet in 250 ml water and effective up to 1 week after application. The neem tablet was compared with commercial grade larvicidal product. The larval mortality % was found more in developed formulation than commercial grade. The Table 4 shows, reduction in mortality percent was slightly varied from the initial. This tablet effectively controls the mosquito larvae on breeding places. The tablet is very effective for Instantaneous mosquito control problem. (See Table 5.)
No. of Effervescence Tablets
Replicates
% Conc.
of Azadirachtn% Mortality
% Mortality
% Mortality
(EFT)
(After 24 h)
(After 48 h)
(After 1 week)
1
2
3
4
EFT-I
0.6
79.66 ± 3.01
66.00 ± 6.53
57.33 ± 1.53
1.2
86.55 ± 2.99
76.65 ± 5.45
63.45 ± 1.67
1.8
90.34 ± 2.45
86.34 ± 4.59
75.34 ± 1.45
2.4
95.29 ± 2.56
89.87 ± 3.56
80.23 ± 1.56
1
0.6
76.66 ± 3.41
66.00 ± 5.53
47.33 ± 1.53
2
1.2
80.45 ± 3.79
79.55 ± 5.35
63.45 ± 1.67
3
EFT-II
1.8
88.34 ± 3.45
87.34 ± 4.59
78.34 ± 1.56
4
2.4
96.39 ± 2.46
90.67 ± 4.56
79.33 ± 1.77
1
0.6
74.56 ± 3.33
63.00 ± 5.43
53.23 ± 1.43
2
1.2
83.80 ± 3.99
73.35 ± 5.68
68.65 ± 1.70
3
EFT-III
1.8
91.28 ± 2.75
89.44 ± 4.60
73.24 ± 1.76
4
2.4
99.39 ± 2.58
89.99 ± 4.78
80.43 ± 1.59
Bti (Bacillus thuringenisis)- tablet
EFT-C
Control
Commercial
Product
0
72.34 ± 3.450
59.34 ± 5.340
50.54 ± 1.45
Effervescence tablet(EFT)
in three replicatesTotal no. of eggs
% of egg hatching
% of egg mortality
EFT-1
EFT-2
EFT-3
CONTROL20
20
19
2010
5
5
190
95
95
1
6.7 Ovicidal activity
Ovicidal activity was found in neem effervescence tablets after 24 hrs of application 90–95% eggs were died and eggs were not hatched. Left 5–10% eggs hatched into feeble larval stages and not capable to set out pupae stage this indicate the growth inhibitory activity of the neem constituents in neem oil tablet. The ovicidal activity of neem effervescence tablet mainly due to the presence of bioactive constituent azadirachtin (A. indica) in the mosquito
eggs (Su and mulla, 1998). This means only one application is enough for termination of all early stages of mosquito as shown in table V in three replicates.
6.8 Ovipositional detterent
The effervescence tablet containing neem oil shows ovipositional detterent activity shown in table 6. The active ingredient of neem oil is azadirachtin content, many research studies identified it as ovicidal detterent in coleoptra, dipteral and Lepidoptera (Schmutterer, 1988).
Mean no. eggs/week in effervescence tablet container 500 ml (%)
Effervescence tablet
(EFT)in three replicates
1 Tablet
2 Tablet
3 Tablet
Control
EFT-1
15 ± 8
7 ± 12
5 ± 4
21 ± 20
EFT-2
16 ± 7
9 ± 13
3 ± 6
19 ± 10
EFT-3
18 ± 9
8 ± 10
4 ± 8
20 ± 13
The neem containing effervescence tablet gives maximum deterrent action in three dispersed tablet in 500 ml i.e. 80–90% ovipositional deterrent activity. Remaining laid eggs were not viable to hatch due to presence of azadirachtin content in water and results into egg mortality. The effectiveness of efferevesence tablet is upto 15 days. Reduction in egg laying over water surface will terminate the mosquito problem by targeted destruction of early cause of mosquito problem from the root level (Halder et al. (2011)).
6.9 Dissolved oxygen content of applied effervescence tablet aqueous medium
Dissolved oxygen is the main water component which regulate the survival of aquatic organisms in water (Alabaster, 1982). In respiration, an aquatic organism takes water oxygen by gills and some by skin and diffuses throughout the bloodstreams. Any declination in external dissolved oxygen causes several alterations in physiological as well as behavioral responses and ultimately results into death of fishes or some other permanent defects. Dissolved oxygen content of Neem effervesce tablet dispersed water was checked in lab conditions and local pond and compared with pure water dissolved oxygen content. Dissolved oxygen of original water was7.5 mg/l after dispersing effervescence tablet it was slightly reduced to 6.9 mg/l but within the limits of range of tolerance i.e 1–20 mg/l. This study indicates that after dispersion of effervesce tablet in aquatic bodies survival of non target organisms remain unaffected and water quality in terms of dissolved oxygen maintained.
7 Conclusion
Effervescent tablet is the new formulation and proposed a considerable significance in controlling mosquito problem from the generation point i.e. early stages of mosquito .Another most important aspect of this invention is that neem oil is absorbed easily in tablet and have fast bioavailability by immediate release by using different inert ingredients. Effervescent tablet physicochemical analysis and stability data shows that tablet is passing all the evaluations and have extended period of stability during storage. This tablet is very effective for larval control and gives 85–90% mortality immediately after application. The tablet can be used in stagnant water around the residential areas after rainy season or some drainage problems which will serve as mosquito breeding place. So, effervescent tablet can be used a very good formulation for fast and immediate mosquito control.
Acknowledgement
We thanks to ministry of chemicals & fertilizers, Govt. of India for financial support in accomplishment of this research study.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- A method of computing the effectiveness of an insecticides. J. Econ. Entomol.. 1925;18:265-267.
- [Google Scholar]
- Water Quality Criteria for Freshwater Fish (Second edition). 1982.
- Formulation, characterization and physicochemical evaluation of ranitidine effervescent tablets. Adv. Pharm. Bull.. 2013;3(2):315-322.
- [CrossRef] [Google Scholar]
- Larvicidal activity of neem oil (Azadirachta indica) formulation against mosquitoes. Malar J. 2009;8:124-130.
- [Google Scholar]
- Seeds of Neem Tree (Azadirachta indica A. Juss). Promising Biopesticide in the Management of Cowpea Insect Pests and Grain Yield in the Early Cropping Season at Asaba and Abraka, Delta State. Nigeria J. Agricultural Science. 2012;4:81-83.
- [Google Scholar]
- Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol. Control. 2013;67(3):469-474.
- [Google Scholar]
- Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. Proc. Natl. Acad. Sci. USA. 2006;103:17560-17563.
- [Google Scholar]
- Evaluation of target specific larvicidal activity of the leaf extract of Typhonium trilobatum against Culex quinquefasciatus Say. Asian. Pac. J Trop. Biomed.. 2011;1:119-203.
- [Google Scholar]
- Decay of plant detritus in organic-poor marine sediment: Production rates and stoichiometry of dissolved C and N compounds. J. Mar. Res.. 1995;53:675-702.
- [Google Scholar]
- The Theory and Practice of Industrial Pharmacy (3rd ed.). Mumbai: Vargheese Publishing House; 1991.
- Development and evaluation of floating matrix tablets of riboflavin. Int. J. PharmTech Res.. 2010;2:1439-1445.
- [Google Scholar]
- Mordue (Luntz) AJ, Blackwell A (1993) Azadirachtin: an Update. J Insect Physiol., 39: 903-924.
- Potential of azadirachtin-containing pesticides for integrated pest control in developing and industrialized countries. J. Ins. Phys.. 1988;34:713-719.
- [Google Scholar]
- Medical Aromatherapy Healing with Essential Oils. Berkeley, California: Frog Ltd.; 1999.
- Granulation techniques and technologies: recent progresses. Bioimpacts.. 2015;5(1):55-63.
- [CrossRef] [Google Scholar]
- Ovicidal activity of neem products (Azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae) J. Am. Mosq. Cont. Assoc.. 1998;14:204-209.
- [Google Scholar]
- Development of mini-tablets with 1mm and 2mm diameter. Int. J. Pharma.. 2011;416:164-170.
- [Google Scholar]
- Laboratory evaluation of 18 repellent compounds as oviposition deterrents of Ae. albopictus and as larvicides of Ae. aegypti, Anopheles quadrimaculatus, and Culex quinquefasciatus. J. Am. Mosq. Control Assoc.. 2003;19:397-403.
- [Google Scholar]
- Modeling the effects of integrating larval habitat source reduction and insecticide treated nets for malaria control. PLoS ONE. 2009;4:6921-6929.
- [Google Scholar]







