Translate this page into:
Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste
⁎Corresponding author. askal_m@yahoo.com (Askal Maimulyanti)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Natural deep eutectic solvent (NADES) has been successfully used as a green alternative for the extraction of polyphenolic compounds (phenolic) from coffee husk waste. The NADES was produced by combining b the choline chloride compound with glycerol, glucose, citric acid, and proline. Furthermore, it was characterized using FTIR with the appearance of a widening hydroxyl peak at a wave number of 3277–3364 cm−1, indicating the presence of hydrogen bond interactions. The results showed that the best composition of NADES solvent was choline chloride and proline ratio of 1:1, providing an extraction yield of 5.88 mg GAE g−1 with a polyphenol concentration in NADES of 294.02 mg/L. Optimum extraction conditions were carried out with the addition of 50 % water, extraction time of 30 min, and the ratio of sample weight to solvent volume (1:10), obtaining a yield of 6.16 mg GAE g−1 and the concentration of polyphenols in NADES of 307.81 mg/L. The effect of temperature on the extraction process can increase the extract yield under conditions at 80 °C with a yield of 10.07 mg GAE g−1 and a polyphenol concentration of 671.4 mg/L. The chlorogenic acid group of polyphenolic compounds was identified using HPLC at a retention time of 3.454 min with a concentration of 63 mg/L. Based on the results, NADES can be use as a green solvent for extracting active compounds from coffee husk waste. These extract can safely be applied to various medicinal and food products.
Keywords
Green extraction
NADES
Phenolic
Coffee husk
1 Introduction
Green technology is one way to find new solvents to replace organic solvents with high volatility and are generally toxic (Abbott et al., 2003). Various research has been conducted to support the green industry by developing green solvents. Conventional extraction methods for compounds from natural materials can be done by maceration method using organic solvents such as acetone, ethanol, ethyl acetate, methanol, hexane, and other organic solvents. This process takes a long time, uses a large amount of solvent, and harms the environment and human health. In addition, the extraction technique also requires solvent removal and purification processes to obtain solvent-free extracts. Currently, the development of innovation is urgently needed to develop eco-friendly solvents in the extraction of active ingredients. This is conducted to obtain natural extracts with residual solvents that do not pollute the environment.
Green solvents can be developed using eutectic solvents, or natural deep eutectic solvents (NADES) derived from natural ingredients. NADES can be made with the composition of compounds including hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD). The most common NADES are based on choline chloride (ChCl) compounds and other hydrogen bond donors such as sugars, amino acids, succinic acid, glycerol, and citric acid. NADES is easy to produce, non-toxic, biodegradable, and highly viscous (Owczark et al., 2016; Paiva et al., 2014). NADES able to extract the polar and non-polar compounds from natural resources. In addition, NADES can also dissolve macromolecular compounds (Francisco et al., 2012). Several studies on NADES have been developed to determine of the constituent compounds, physicochemical properties, and solvent stability such phenyletanes and phenyl propanoids from R. rosea (Shikov, et al., 2020). The NADES has been developed to extract compounds from roots of G. glabra with acid-based NADES (Shikov, et al., 2022) However, the use of NADES solvent, which is applied to separating active substances from natural ingredients, is still limited. Several studies have been conducted using NADES as a solvent for the separation of compounds applied to the extraction of flavonoids (Ali et al., 2019), alkaloids, saponin acids (Duan et al., 2016), and phenolic compounds (Wei et al, 2015; Alanon et al., 2018). So far, the application of NADES for the extraction of natural products is still limited to the efficiency of NADES for extraction. In fact, the characteristics of NADES solvent as a safe solvent have the potential to be applied in the extraction process of solid waste produced from an industrial process, one of which is coffee skin waste.
Coffee husk is the outer skin of coffee with a hard surface that has been separated from the husk (silver skin). If managed with biotechnology, residues from the coffee industrial process can be used as flavors, energy drinks, soft drinks, emulsions, cosmetics, and biosorbents (Murthy & Naidu, 2012). Coffee husk has the potential to be used because it contains caffeine, tannins, polyphenols, pectin, monosaccharides, and disaccharides compounds (Padmapriya, et al, 2013; Silva, et al., 2021) which can be used in food products. In addition, it also contains protein, fat, crude fiber, caffeine, and tannins (Janissen et al., 2018). Previous research showed that the nutritional composition contained in coffee husk can be applied to beverages that can treat various diseases.
Various extraction techniques have been used to separate active compounds from coffee husk waste, such as phenolic extraction using water, methanol (Silva et al., 2021), ethanol (Prihadi et al., 2020), and supercritical fluid extraction techniques using CO2 (Andrade et al., 2012). If managed properly and sustainably, waste generated by an industrial process can increase the added value of the waste so that it can develop new innovative products. Exploration for added value in terms of products produced by coffee husk waste can be managed and applied to food industry products (Machado et al, 2012). By looking at the potential contained in coffee husks and managing waste properly to produce products that can be used in industry, it is necessary to extract the active ingredients from coffee husks by developing a separation technique using green solvent using NADES solvent. The NADES used is safer, easily degraded by the environment, and can be safely applied to food products. This research was to formulate NADES as a green solvent for handling coffee husk waste to produce active compounds and its was applied to industrial products.
2 Material and methods
2.1 Materials and instruments
The material coffee husk get from the farmer of west Java, Indonesia. The reagent used in this research were choline chloride p.a (MERCK), glycerol p.a (MERCK), glucose p.a (MERCK), citric acid p.a (MERCK), proline p.a (MERCK), folin–ciocalteu p.a (MERCK), sodium carbonate p.a (MERCK), gallic acid p.a (MERCK), 1–1-diphenyl-2-picrylhydrazyl (DPPH) p.a (MERCK), methanol p.a (MERCK), acetic acid p.a (MERCK), acetonitrile p.a (MERCK). The instrument were FTIR (Alpha Bruker-IR), Spectrophotometri UV–vis (SHIMADZU EUROPA GmbH, UV-1800), HPLC (Agilent 1200).
2.2 Preparation of sample
The sample in this research was dried (<1%) coffee husk that was grinded and sieved through a 20-mesh filter. The sample was then stored in a plastic container to prevent sun exposure and avoid more humidity.
2.3 Preparation of NADES
NADES was prepared with a certain mole comparison between HBA (pa, Merck) and HBD (pa, Merck). HBA compound used in this research was from choline chloride with a variation of HBD compound, i.e., a mixture of glucose, organic acid, and amino acid. HBD utilized in this research was composed of glucose, citric acid, glycerol, and proline. The NADES was synthesized by heating, consisting of mixtures of solid–liquid and solid–solid at the temperature of 50-90℃ with constant stirring. The stirring was done in a 30–120 min range until a clear solution was formed. The yielded mixture was then cooled to room temperature and proceeded to characterization.
2.4 Characterization of NADES using FT-IR
Spectroscopy analysis using FT-IR was utilized to determine the interaction between choline chloride as HBA and a mixture of glucose, carboxylic acid, and amino acid as HBD. Analysis using FT-IR was conducted at a wavenumber of 4000–400 cm−1.
2.5 Extraction of active substance from coffee husk employing NADES
Extraction of the active substance from coffee husk employing NADES was carried out by mixing 0.5 g dried sample with 10 mL NADES in beaker glass followed by stirring at 120 rpm min−1 at room temperature for 30 min. The mixture was then filtered to get the supernatant and analyzed using a spectrophotometer.
2.6 Optimization of extraction parameter
The extraction process was conducted to pick the optimum experimental condition to separate polyphenol compounds in the coffee husk. The factors that influenced the separation were components of NADES, the water content in NADES, extraction time, sample amount ratio to NADES solvent ratio, and temperature. The effect of water addition was observed by varying the water contents, namely: 20 %, 30 %, 40 %, 50 %, and 60 %. The comparison of the ratio of sample amount to NADES was done in a variation of 1:10, 1:15, 1:20, 1:25, and 1:30. The extraction was also diversified in a range of 30 – 150 min with an interval of 30 min. The temperature of extraction was varied at 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C.
2.7 Determination of the phenolic content
The total polyphenol of coffee husk extract was determined by the Folin-Ciocalteu method. The extract was initially dissolved with a comparison of 1:10. A 500 µL extract was mixed with 2.5 mL Folin-Ciocalteu reagent (1:10), 2 mL Na2CO3 7,5% (w/v). The solution was then incubated for 2 h in a dark room at ambient temperature. The solution was measured at 760 nm with a UV–vis spectrophotometer. The result exhibited gallic acid equivalent (GAE)/g dried weight. The standard solution was used gallic acid with the concentration of 0–50 mg/L (Costa, et al., 2014).
2.8 Determination of antioxidant activity
The determination of antioxidants used 1–1-diphenyl-2-picrylhydrazyl (DPPH) in reaction tubes containing the test solution. The solution was left for 30 min at room temperature. The absorbances of test and control solutions were measured using a UV–vis spectrophotometer. 1 mL of 0.5 mM DPPH in methanol solvent was piped to several vial bottles and mixed with test solution in varying concentrations of 0,10, 20, 30, 40, and 50 ppm. The solution was then diluted to 5 mL. Afterward, the absorbance was measured after 30 min at the wavelength of 517 nm (Molyneux, 2004).
Percentage of free radical scavenging is examined by the following equation:
Description:
A0 = Absorbance of test solution.
AT = Absorbance of control solution.
2.9 Investigation of chlorogenic acid using HPLC
Examining the chlorogenic acid content in coffee husk extract was conducted regarding the procedure reported by Narita and Inouye (2012) with some modifications. The analysis used a reverse phase HPLC column. The mobile employed in this research consisted of solvent A (50 mM acetic acid in distillate water) and solvent B (50 mM acetic acid in acetonitrile) using the gradient elution technique. The injection volume of coffee husk extract was 10 µL, and the flow rate was 1.0 mL min−1. The content of chlorogenic acid was investigated by weighing 30–40 mg of the sample into a 100 mL volumetric flask. This sample was later diluted using a mixture of acetone–water (1:2 v/v). Subsequently, the solution was sonicated until perfectly dissolved (15 min). The solution was then filtered using an econofilter (0.45 µm). The step was continued by injecting 30 µL of the filtrate to HPLC, applying the “Chlorogenic Acid” method. The set of this method was: column (250 mm, 5 µm, 4.6 mm), temperature (30 °C), flow rate (0.7 mL min−1), flow system (isocratic), wavelength (327 nm), time (10 min). The standard chlorogenic acid solution was arranged in concentrations of 0, 10, 20, 50, 80, 100, 130, 150, and 200 ppm. The validation of method with linearity (0.99), RSD (0.15), and recovery (99.74 %).
3 Result and discussions
3.1 Synthesis of NADES
NADES was synthesized by combining BHA compounds from choline chloride and BHD compounds from glycerol, glucose, and proline groups. The result of the combination of NADES synthesis can be seen on Table 1 and Fig. 1.
Solvent
Component 1
Structure
Component 2
Structure
Mole Ratio
Water
NADES1
Choline Chloride
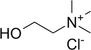
Glycerol
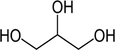
1:2
–
NADES 2
Glucose
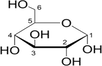
1:1
–
NADES 3
Citric acid
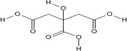
1:1
20 %
NADES 4
Proline
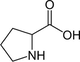
1:1
20 %

NADES synthesis based on choline chloride.
From the experiment, NADES was made by variation ratio mole to obtain clear solvent used for extraction. The addition of 20 % of water made the cloudy solvent become a clear solvent of NADES and reduce its viscosity. The type of NADES used is very important to extract target compounds from the sample matrix (Ali et al., 2019). NADES is a eutectic mixture of a quaternary ammonium salt and hydrogen donor compounds. This study used four different types of NADES to extract polyphenol compounds from coffee husks. Generally, the extraction process is strongly influenced by NADES affinity with the compound target (Bonacci et al., 2020). NADES were made by heating the crystal at 100 °C and stirring at 300 rpm until the crystal melted and formed a liquid with a certain mole ratio. The solvent obtained is a transparent solution, colorless, and has a low viscosity at room temperature. In extreme cases, molten salt at room temperature is formed by mixing quaternary ammonium salts with metal salts (Abbott et al., 2003). The solvent can be prepared with mixture of pure salts and unreactive with the water. The eutectic salt mixtures can be derived from amides, acids, and amines (Abbott et al., 2004). These synthesized NADES were used to extract polyphenol compounds from coffee husk waste.
3.2 Characterization of NADES
The functional groups of characterization of synthesized NADES were conducted using FTIR as shown in Fig. 2.
FTIR spectra of synthesized NADES.
The infra-red spectra in Fig. 2 shows the characteristic peaks of the functional groups of the NADES. The synthesized NADES showed that the melted mixture formed a homogeneous solvent and the resulting hydrogen bond as indicated by the broad peak at 3500 cm−1. The hydroxides group shows that the synthesized NADES form hydrogen bonds with other compounds so it can extract the active substances from natural materials. The peaks with detected wavenumber in NADES can be seen in Table 2.
Solvent
Wavenumber (cm−1)
NADES 1
3288, 2927, 2876, 1478,1039, 953, 864, 608, 584
NADES 2
3277, 2919, 1479, 1023, 953, 768, 704, 554
NADES 3
3364, 1714, 1639, 1478, 1205, 1081, 1006, 790, 593
NADES 4
3355, 1615, 1480, 1406, 1335, 1034, 1050, 1007, 669
In the FTIR spectra of NADES, it is observed that there are hydrogen bonds formed between choline chloride and glycerol, glucose, citrid acid, and proline compounds. The bond was formed due to the interaction of a hydrogen atom and an electronegative atom of HBD compounds. The broad peak indicates the hydrogen bond appears in the wavenumber at 3000–3500 cm−1, corresponding to stretching of the free –OH groups of the molecule. The O—H and N—H stretching groups usually appear at 3000–3700 cm-1 (Dutta, 2017). The main peak that appears is the alkyl compound, C—H at 600–3300 cm−1 (Dutta, 2017).
NADES 1 is a combination of choline chloride and glycerol, indicating the peak at 3288 cm−1 and corresponding to hydroxil group (–OH). The peak at 2927 cm−1 is associated with the alkyl group, i.e. C—H stretching. The strong vibration at 1478 cm−1 is attributed to vibration of –CH2. Meanwhile, the peak at 1039 cm−1 corresponds to symetric stretching of the C—N+ group (Dutta, 2017). In addition, a peak appears at 864–953 cm−1, corresponding to C—O and stretching of C—C group. The functional group as chloride is presented by a band at 584 cm−1. NADES 2 with the choline chloride and glucose composition showed the broad peak at 3227 cm-1, corresponding to hydroxil group and indicating the presence of hydrogen bonds. Other peaks that appear similar to NADES 1 are at 1479, 1023, and 953 cm−1, indicating the vibration of C-CH2, C⚌N+, and stretching of C—O (Mohammed et al., 2017). The functional group of C—O—C stretching appears at 764 cm−1. The peak at 554 cm−1 indicates the presence of compounds containing chloride. NADES 3 with the choline chloride and citric acid composition shows a peak for the OH group at 3364 cm−1. Furthermore, other peaks at 1478, 1081, and 790 cm−1 show the vibration of C-CH2, C⚌N+ and C—O stretching, respectively. The chloride group is presented by absorption band at 593 cm−1. The peak at wavenumber of 1081 cm−1 indicates the C—N stretching group, which usually appears in the range of 1090–1020 cm−1 (Coates, 2006). Furthermore, the carboxylate group appears at 1639–1478 cm−1 as carboxylate salts (Coates, 2006). The peak at 1714 cm−1 is characteristic of carboxylic acid which usually appears at 1700–1725 cm−1 (Coates, 2006). NADES 4 with the choline chloride and proline composition indicates a hydroxil group peak at 3355 cm−1. The strong peak at 1615 cm−1 is attributed to carboxylate group. Other peaks that appear at 1406 cm−1 and 1034 cm−1 indicate the presence of CH2 and C⚌N+ vibration, respectively. The wavenumber at 669 cm−1 is attributed to the chloride group, while the peak at 1050 cm−1 indicated the presence of C—N stretching, which usually appears at 1090–1020 cm−1 (Coates, 2006).
3.3 Polyphenol extraction with NADES
NADES made previously was used for the polyphenol extraction from coffee husk waste. Extraction results were analyzed for polyphenol content using gallic acid standard. Polyphenol extraction yield in coffee husks extracted in NADES is presented in Fig. 3.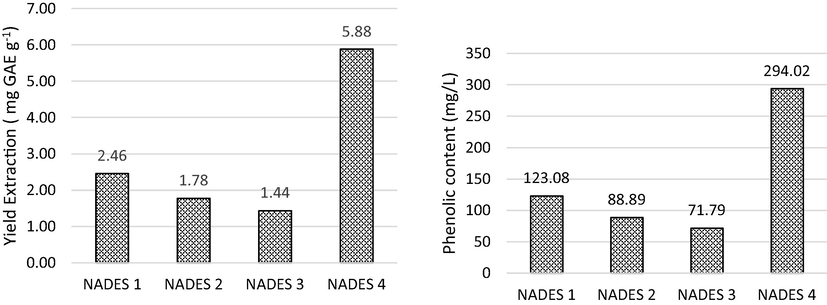
Polyphenol extraction results from coffee husk using NADES.
Fig. 3 shows that the content of polyphenolic compounds extracted using NADES can be referred to as the extraction yield. The extraction use sample dan solvent (1:10) for 30 min. Choline chloride-based NADES with HBD group of glycerol, glucose, citric acid, and proline compounds were 2.46, 1.178, 1.14, and 5.88 mg/g, respectively. The Optimum composition for the extraction using choline chloride and proline with 20 % of water. Extraction efficiency was obtained successively with the composition of choline chloride with proline > glycerol > glucose > citric acid. Duan (2006) has reported that proline-based NADES can extract phenolic from Chinese herbal medicine compared to ethanol. This indicates that this type of NADES is more suitable for polar bioactive compounds (Ali et al., 2019). The concentration of polyphenols extracted in NADES using choline chloride and BHD glycerol, glucose, citric acid, and proline were obtained respectively at 23.08, 88.89, 71.79, and 294.02 mg/L. The choline chloride with proline can form hydrogen bonds to extract the polar compounds. The type of NADES is very important for extracting the target compound in the sample matrix to increase efficiency (Ali et al., 2019). The Choline chloride-proline-based NADES 4 is the best for polyphenols extraction in coffee husk waste.
3.4 Antioxidant activity of extraction yield
Polyphenol compounds have strong activity as antioxidants. Compounds that have a high concentration of polyphenols can act as antioxidants. The antioxidant test of coffee husk extract using NADES was carried out using the radical scavenging method using DPPH. The results obtained are presented in Fig. 4.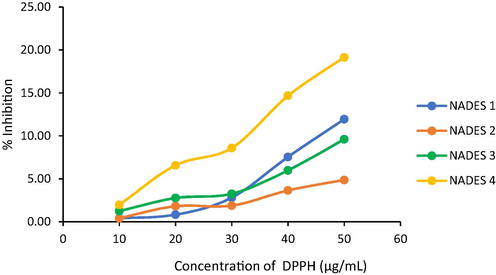
The relationship between DPPH concentration and % inhibition.
Fig. 4 shows the results of the antioxidant activity test of NADES with the various compositions.The greatest % inhibition was shown in NADES 4. It has the highest antioxidant activity and able to inhibit the radicals. The ability of antioxidant activity of coffee husk extract in NADES 4 > NADES 1 > NADES 3 > NADES 2. The IC50 values of the extracts are shown in Fig. 5.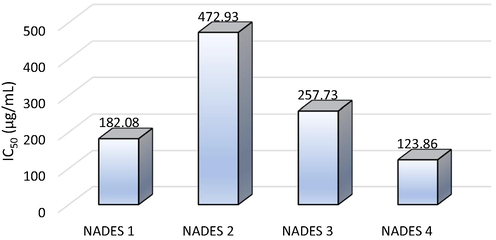
The IC50 values from coffee husk extract.
Fig. 5 shows the IC50 values of coffee husk extractions in NADES. The IC50 value indicates the ability of antioxidants to inhibit radical activity by 50 %. The lower the IC50 value, the stronger the antioxidant activity ability. The IC50 is obtained by plotting Y values of 50 % in the linear regression y = a + bx. Based on the experimental results, the antioxidant activity values of NADES 1, 2, 3, and 4, respectively, were 182.08, 472.93, 257.73, and 126.86 g/mL. Therefore, the greatest antioxidant activity was the choline chloride-proline-based NADES 4. The next research is extraction using NADES 4 (choline chloride-proline) for extraction optimization.
3.5 Polyphenols extraction parameters based on choline Chloride-Proline from coffee husk waste
Optimization of polyphenols extraction from coffee husk waste has been observed by varying the water content, extraction time, ratio weight/volume, and temperature. The parameter can be used to determined the yield extraction (mg GAE/g). It can be seen in Fig. 6.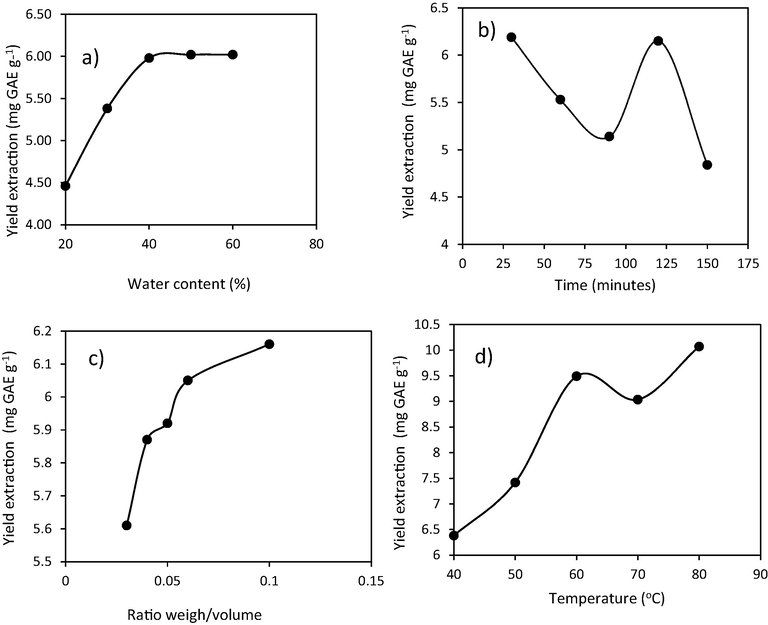
Effect of extraction parameters on the amount of polyphenol extracted by NADES.
The effect of water content variation on polyphenols extraction can be seen in Fig. 6a. The research was conducted by varying the water content from 20 % to 60 %. The addition of 20 % water content resulted in the number of polyphenols extracted as gallic acid obtained at 4.46 mg GAE (gallic acid equivalent)/g. The yield is smaller because the formation of hydrogen bonds is not yet complete. The addition of water content to 50 % increased the extraction efficiency to 6.02 mg GAE/g. After 60 % water content was added, the amount of polyphenols extracted became stable, so the optimum extraction conditions were obtained with the addition of 50 % water. The effect of water addition to amino acid-based NADES resulted in increasing solvent polarity (Ali, et al., 2019). The addition of water has been done to reduce the viscosity and to maintain the extraction efficiency. In addition, the presence of water in the composition of NADES could change the polarity and solubility of phenolics in the solvent (Barbieri et al., 2020). Higher water concentration would increase the risk of disrupting the hydrogen bonds of NADES and could change its molecular structure. Thus, it is necessary to limit the interaction of hydrogen bonds between bioactive compounds and NADES components (Ali et al., 2019). If the extraction using the water only, so the water must be evaporated to prevent the microorganisms. The extration using the water must be in maceration technique for several days.
The effect of extraction time variation on polyphenols extraction can be seen in Fig. 6b. Extraction time variations were carried out for 30, 60, 90, 120, and 150 min. The experiment results show that 30 min of extraction obtained a yield of 6.19 mg GAE/g. The longer the extraction time, the lower the extraction efficiency. The experiment results show that after 90 min of extraction, the yield decreased to 5.14 mg TAE/g. Generally, long-term extraction is unsuitable for bioactive compounds because it can change the chemical structure of the target compound (Ali et al., 2019). The transformation can be occur the hydrolysis or moleculer reaction between target compounds and solvent. At 120 min of extraction, there was increasing in efficiency due to the desorption of the target compound in the solvent. It caused increasing extraction efficiency from 90 to 120 min extraction time (range of 30 min). The extraction yield obtained was 6.15 mg GAE/g. After 150 min of extraction, the extraction efficiency decreased to 4.84 mg GAE/g. The varying effect of extraction time showed a fluctuating pattern in polyphenol extraction in NADES. This result is due to the extraction and adsorption processes. This is an equilibrium reaction in a solution system. The amount of extract decreased with extraction time from 30 to 90 min. The efficiency increased at 120 min and down again after 150 min. Thirty minutes extraction time is sufficient to obtain optimum results of phenolic extracts. In this study, the extraction time was relatively short, which helped stabilize the NADES for effective extraction. Increasing extraction time can make polyphenol compounds released into the extraction medium.
The effect of ratio weight/volume extraction variation to polyphenols extraction can be seen in Fig. 6c. It shows the effect of the ratio of solute to solvent increasing from 1:10, 1:15, 1:20, 1:25, to 1:30. The results show that the larger sample/solvent ratio causes the extraction efficiency to increase. Based on the experimental results, the optimum ratio of sample and solvent was obtained at a composition of 1:10 or 0.1 g/mL with a yield of 6.16 mg GAE g−1. Compared to sample to solvent ratio (1:30) yielded 5.61 mg GAE g−1. The solid/liquid ratio plays an important role in the perfection of the extraction because to determine the ability of NADES to dissolve the target compound, it is mainly dominated by the ability to dissolve the solvent. (Ali et al, 2019).
The effect of temperature variation to polyphenols extraction can be seen in Fig. 6d. The study results show that the higher the extraction temperature, the greater the amount of polyphenol extracted. In general, the solubility will increase at high temperatures due to a decrease in diffusivity and viscosity. This is very influential for thick solvents such as NADES (Bonacci, et al., 2020). Heating the solution to a temperature of 40–80 °C obtained 6.38 to 10.07 mg GAE g−1 extraction yield. Changes in temperature from 60 °C to 70 °C resulted in a reduced amount of polyphenols extracted. This is due to the high-temperature heating solution will cause instability of natural material compounds in the sample matrix so that the extraction results obtained are unstable. After 70 °C, the extraction yield increased again. The maximum extraction yield was 10.07 mg GAE g−1 on heating at 80 °C. Extraction is not carried out at higher temperatures because heating at high temperatures causes the structure of bioactive compounds to decompose (Ali et al., 2019). Increasing temperature cause a decrease in surface tension and interaction between the active compound with the sample matrix, which can lead to increased desorption and breakdown of compound by the solvent (Bonacci et al., 2020). The effect of the extraction parameters on the levels of soluble polyphenols in NADES can be seen in Fig. 7.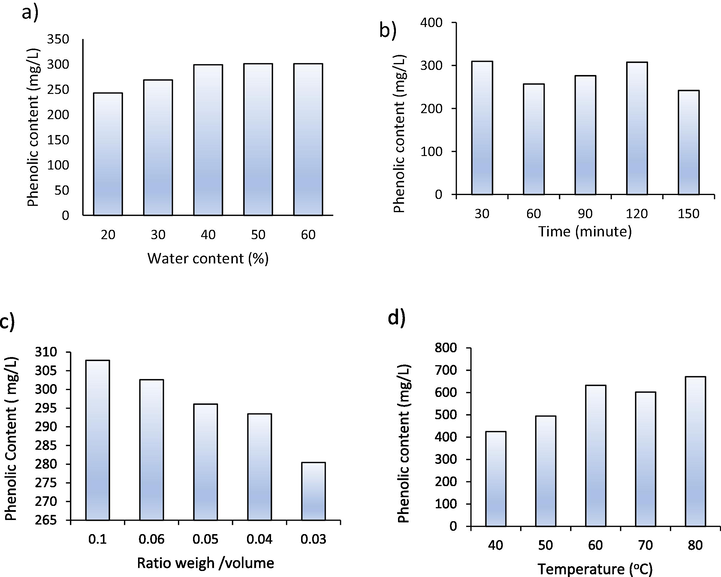
Extracted phenolic content.
Fig. 7 shows the concentration of polyphenols as gallic acid extracted in NADES with different extraction parameters. The effect of water concentration causes an increase in the concentration of polyphenols produced. Polyphenol content began to stabilize with the addition of 50 % water. The concentration of polyphenols obtained was 301.08 mg/L. The optimum extraction time was 30 min with a concentration of 309.70 mg/L. The ratio of sample weight to solvent was obtained at 1:10 with a concentration of 307.81 mg/L. The effect of temperature causes an increasing concentration of polyphenols extracted. Heating the solution to 80 °C obtained polyphenol content of 671, 34 mg/L.
3.6 Analysis of phenolic compounds as chlorogenic acid (CGA) in NADES
Identification of chlorogenic acid phenolic compounds in coffee skin extract with NADES using HPLC. The HPLC analysis results were compared with the chlorogenic acid standard. The chromatogram of HPLC obtained can be seen in Fig. 8.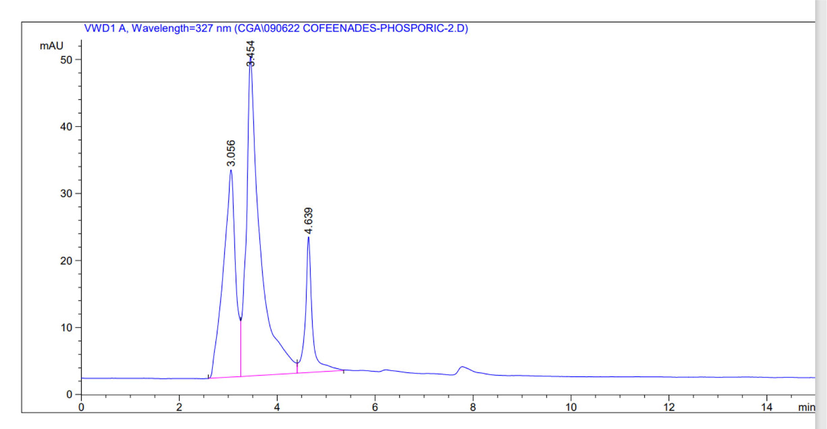
HPLC identification result of chlorogenic acid from coffee husk waste with NADES solvent.
The chromatogram showed that chlorogenic acid compounds’ peak was at a retention time of 3.545 min. The identification results were carried out using chlorogenic standards and obtained chlorogenic acid levels of 63 mg/L. The yield of chlorogenic acid was 0,13 % in coffee husk waste. Based on these results, chlorogenic acid compounds in coffee husk waste can be extracted using choline chloride-proline based NADES. Thus, green solvent-based extracts can handle waste, producing active compounds as added value for a product. The previous research showed the use of proline-malic acid-based NADES to extract chlorogenic acid from the herbal plant Artimiciae scopariae with a yield of 3.77 mg/g (Yue, et al., 2020). These developed NADES solvents are safer, and direct extraction can be applied without solvent removal. Chlorogenic acid (CGA) is a class of phenolic acid compounds, natural polyphenol compounds found in coffee skin extract. CGA is a natural and biologically active polyphenol with therapeutic roles such as anti-inflammatory, antioxidant, cardioprotective, and antiviral. CGA can also be used as a food additive that has a therapeutic effect that improves health (Naveed et al., 2018).
4 Conclusion
This study resulted a combination of green solvent (NADES to extract the active compounds from coffee husk. The functional groups involved in hydrogen bonding are the hydroxyl, carboxylic groups, and amide present in NADES. The interaction of hydrogen bonds and phenolic compounds is responsible for their extraction ability. NADES based on choline chloride and proline proved effective in the extraction of polyphenolic compounds from coffee husk waste. Polyphenol compounds have high antioxidant activity. Extraction was influenced by the water content in NADES, extraction time, ratio of sample weight to solvent and temperature. The results of the identification of polyphenolic compounds obtained chlorogenic acid which was analyzed using HPLC. The cholorogenic acid was found 63 mg/L in extract of coffee husk. NADES as a green solvent has been succeeded to extract the active compounds from coffee husk and it safe to be applied to food industry.
Acknowledgements
We thanks to Politeknik AKA Bogor for the financial of the research with SK Direktur No 60 tahun 2022.
References
- Novel solvent properties of choline chloride/urea mixture. Chem. Commun. 2003:70-71.
- [CrossRef] [Google Scholar]
- Deep eutectic solvents formed between choline chloride and carboxylic acid: versatile alternative to ionic liquids. J. Am. Chem. Soc.. 2004;126:9142-9147.
- [CrossRef] [Google Scholar]
- Choline chloride derivative-bases deep eutectic liquids as a novel green alternative solvent for extraction of phenolic compounds from olive leaf. Arabian J. Chem.. 2018;13(1):1685-1701.
- [CrossRef] [Google Scholar]
- Effective extraction of flavonoids from Lyceum barbarum L. fruits by deep eutectic solvents based ultra sound-assisted extraction. Talanta. 2019;203:16-22.
- [CrossRef] [Google Scholar]
- Supercritical fluida extraction from spent coffee ground and coffee husk: antioxidant and effect of operational variables on extract composition. Talanta. 2012;88:544-1522.
- [CrossRef] [Google Scholar]
- Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus Officinalis L) phenolic compounds. Ind. Crop. Prod.. 2020;114:1-8.
- [CrossRef] [Google Scholar]
- Natural deep eutectic solvent as extraction media for the main phenolic compounds from olive oil processing water. Antioxidants. 2020;9(513):1-14.
- [CrossRef] [Google Scholar]
- Interpretation of infra red spectra : A practical approach, Encyclopedia of Analytical Chemistry. Jhon Willey & Sons Ltd; 2006. p. :1-23.
- Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng.. 2016;4(4):2405-2411.
- [CrossRef] [Google Scholar]
- Furrier transform matrix infra red spectroscopy. Spectroscopy Methods Nanomater. Characterization 2017:73-94.
- [Google Scholar]
- New natural and renewable low transition temperature mixture (LLTMs) sreening as solvent for lognocelluloic bimass processing. Green Chem.. 2012;14:2153-2157.
- [CrossRef] [Google Scholar]
- Chemical composition and value-adding application of coffee industry by product A review. Resour. Conserv. Recycl.. 2018;128:110-117.
- [CrossRef] [Google Scholar]
- Growth of fungal strains on coffee industry residues with removal of pholyphenolic compounds. Biochem. Eng. J.. 2012;60:87-90.
- [CrossRef] [Google Scholar]
- Furier transform infra red (FTIR) spectroscopy. Membrane Characterization. 2017;3–29
- [CrossRef] [Google Scholar]
- The Use of Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol.. 2004;26(2):211-219.
- [Google Scholar]
- Sustainable management of coffee industry by product and value addition A review. Resour. Conserv. Recycl.. 2012;66:45-58.
- [CrossRef] [Google Scholar]
- High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem.. 2012;135:943-949.
- [CrossRef] [Google Scholar]
- Chlorogenic acid (CGA), A pharmacological review and call for further research. Biomed. Pharmacother.. 2018;9(218):67-74.
- [CrossRef] [Google Scholar]
- Natural deep eutectic solvents in extraction process. Ch&ChT. 2016;10(4):601-606.
- [CrossRef] [Google Scholar]
- Natural deep eutectic solvent-solvent for the 21st century. Sustain. Chem. Eng.. 2014;2(5):1063-1071.
- [CrossRef] [Google Scholar]
- Antioxidant fiber from coffee husk extrack and potential for nutraceutical. Rasayan J. Chem.. 2020;13(2):955-959.
- [CrossRef] [Google Scholar]
- Natural deep eutectic solvents for the extraction of phenyletanes and phenylpropanoids of Rhodiola rosea L. Molecules. 2020;25(1825):1-11.
- [CrossRef] [Google Scholar]
- The ability of acid-based natural deep eutectic solvents to co-extracts elements from the roots of Glycyrrhiza glabra L, and assosiated health risk. Molecules. 2022;27(7690):1-12.
- [CrossRef] [Google Scholar]
- Obtaining bioactive compounds from the coffee husk (Coffea arabica L) using different extraction methods. Molecules. 2021;26(46):1-13.
- [CrossRef] [Google Scholar]
- Application of natural deep eutectic solvent for extraction and determinantion of phenolic in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol.. 2015;149:237-244.
- [CrossRef] [Google Scholar]
- A quick selection of natural deep eutectic solvents for the extraction of chlorogenic acid from herba Artemisiae scopariae. RSC Adv.. 2020;10:23405-23409.
- [CrossRef] [Google Scholar]







