Translate this page into:
Diagnostic product ions-based chemical characterization and antioxidative activity evaluation of solid fermentation for Astragali radix produced by Paecilomyces cicadae
⁎Corresponding authors. myweixia@126.com (Xia Wei), zhangjiayu0615@163.com (Jiayu Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Studies on herbal medicines and fermentation products have become increasingly essential with the development of modern industry and technology. In order to verify that fermentation can bring about changes, Paecilomyces cicadae [Paecilomyces cicadae (Miquel.) Samson] was used to ferment Astragali radix [Astragalus membranaceus (Fisch.) Bge. var. mongho-licus (Bge.) Hsiao]. After solid fermentation for Astragali radix produced by Paecilomyces cicadae (SF-AP) was established, an efficient strategy based on ultra-high performance liquid chromatography-linear ion trap-Orbitrap mass spectrometry (UHPLC-LTQ-Orbitrap MS) was developed to screen and identify the chemical transformations in SF-AP and Astragali radix according to the acquired diagnostic product ions (DPIs). As a result, 114 compounds including 45 saponins and 69 flavonoids were finally identified and validated. Moreover, two kinds of antioxidative tests corresponding to the scavenging of DPPH· and ABTS·+ were applied to evaluate the antioxidative activity of Astragali radix before and after fermentation. The results demonstrated that some significant chemical transformations such as relative content fluctuations and structural isomerism owing to the occurrence of hydrolysis and conversion reactions and the antioxidative activity of SF-AP was much higher than that of the Astragali radix. This study could provide a new method for the utilization of Astragali radix and constructive guidance for the further research of fermented herbal medicines.
Keywords
Solid fermentation for Astragali radix produced by Paecilomyces cicadae (SF-AP)
Chemical transformation
Diagnostic product ions (DPIs)
Antioxidative activity
Ultra-high performance liquid chromatography-linear ion trap-Orbitrap mass spectrometry (UHPLC-LTQ-Orbitrap MS)
1 Introduction
Microbial fermentation has already been applied in the processing of herbal medicines and functional food for thousands of years, such as Banxiaqu (pinellia ternata fermented mass) and Dandouchi (sojae semen praeparatum). Previous studies have demonstrated that fermentation plays an important role in toxicity reducing and efficacy enhancing (Ming et al., 2017). The prime reason was that microorganisms could generate sorts of important secondary metabolic products, and the macromolecular constituents could be decomposed into small molecules during the process (Hussain et al., 2016; Stanton et al., 2005; Xu et al., 2015).
As annual or perennial herb or shrub that is prevalently distributed in temperate and arid areas, Astragali radix [Astragalus membranaceus (Fisch.) Bge. var. mongho-licus (Bge.) Hsiao] belonging to the popular genera of plants in Leguminosae, has been widely used in herbal medicines for over 2,000 years. It contains saponins, flavonoids, etc, which are known for their anti-inflammatory, anti-oxidant, and other pharmacological effects (Fu et al., 2015). It is commonly used as food and beverage additive and nutritional dietary supplement to enhance the body's resistance against various diseases in numerous Asian countries. In terms of Paecilomyces cicadae [Paecilomyces cicadae (Miquel.) Samson], one kind of fungus owing high nutritional and medical value is formed by paecilomyces parasitizing the nymphs of cicadas. Modern pharmacological studies have showed that it had similar clinical effects to Cordyceps just like regulating immunity, improving the kidney function, strengthening with tonics, anti-oxidation, anti-tumor and anti-virus, etc (Zhao et al., 2018a,2018b; Zhang et al., 2017).
In the preliminary report, and as part of our long-term investigation for fermentation, we have described the chemical constituent profiling and lowering uric acid activity of Paecilomyces cicadae liquid fermentation for Astragli Radix (Wang et al., 2019a, Wang et al., 2019b). Compared with the previous study of Paecilomyces cicadae liquid fermentation for Astragli Radix, we found that the distinguishment between solid fermentation and liquid fermentation lies in the difference of medium state. The concept of solid fermentation covers a wide range, including the fermentation mode of suspending insoluble solid substances in liquid (also known as carrier culture) and cultivating microorganisms on wet solid materials with almost no flowable water. There are a great many advantages of solid fermentation, such as simple operation, low energy consumption, easy domination, less pollution, and so on. Nowadays, modern fermentation technology has been gradually changed from traditional natural fermentation that relying on production experience to pure strain fermentation, which represents fermentation technology and system are becoming increasingly mature (Martins et al., 2011; Singhania et al., 2009; Wang et al., 2016; Liu et al., 2004).
In order to prove that the fermentation process can some cause favorable chemical changes, an ultra-high performance liquid chromatography-linear ion trap-Orbitrap mass spectrometry (UHPLC-LTQ-Orbitrap MS) method coupled with the assistance of diagnostic product ions (DPIs) analysis was developed to characterize the chemical transformation and further obtain a comprehensive knowledge about constituents in the established SF-AP system. Meanwhile, two antioxidative tests including DPPH· scavenging activity and ABTS·+ scavenging activity were utilized to evaluate the antioxidative effects of Astragali radix before and after solid fermentation.
2 Experimental
2.1 Chemicals and materials
The identity of Astragali radix was authenticated by histological and morphological methods according to monograph of Chinese Pharmacopoeia (version 2015) by Prof. Long Dai in BIN ZHOU Medical University (Yanai city, Shandong). Paecilomyces cicadae (Miquel) Samson (No. cfcc81169) was provided by China Forestry Culture Collection Center (Beijing, China). A total of thirteen reference substances including six triterpene saponins, i.e. β-D-Glucopyranoside,(3β, 6α, 16β, 20R, 24S)-3-[(3, 4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20, 24-epoxy-16, 25-dihydroxy-9, 19-cyclolanostan-6-yl, Astragaloside I, Astragaloside II, Astragaloside IV, Isoastragaloside I, Isoastragaloside II and seven flavonoids, i.e. Calycosin, Genistin, Complanaruside, Formononetin, Ononin, Astraisoflavan-7-O-β-D-glucoside and Isoquercitrin, were all purchased from Chengdu Must Biotechnology Co. Ltd. (Sichuan, China). The structures were fully elucidated by comparing the ESI-MS, 1H NMR and 13C NMR spectra data with the published literature. All of their purities were acceptable (≥98%) according to HPLC-UV analysis.
Acetonitrile, methanol and formic acid of LC-MS grade were all purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA). 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1, 1-diphenyl-2-picrylhydrazyl (DPPH), Potassium persulfate (K2S2O4) were obtained from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). All the other chemicals of analytical grade were provided by Beijing Chemical Works (Beijing, China). Deionized water used throughout the experiment was purified by Milli-Q Gradient Å 10 System (Millipore, Billerica, MA, USA). Grace PureTM SPE C18-Low solid-phase extraction cartridges (200 mg/3 mL, 59 μm, 70 Å) were purchased from Grace Davison Discovery Science (Deerfield, IL, USA).
2.2 The preparation of SF-AP system
2.2.1 Fungus activation and liquid culture
Paecilomyces cicadae was inoculated on potato liquid medium in 500 mL conical flask, and then it was cultured in an incubator with constant temperature and humidity to activate it by setting parameters at 27℃ and relative humidity of 80% for 5 days. Activated Paecilomyces cicadae were selected by inoculation ring and cultured in potato liquid medium at 25℃ and 140 r/min for 7 days.
2.2.2 Solid-state fermentation
The powder (5 g) of Astragali radix was placed in 250 mL conical bottle and then soaked with 6 mL distilled water. After that, the conical bottle loaded with wet medicinal powder was sterilized at 121 ℃ for 30 min. 3 mL liquid spawn of activated Paecilomyces cicadae was inoculated and cultured in a solid fermentation flask with constant temperature of 26 ℃ and humidity of 90%. Astragali radix were ground into powder passing with 100 mesh sieve and cultured for 14 days at 26 ℃ under aerobic conditions.
2.3 Analytical sample preparation
SF-AP samples were taken on the 14th day for the subsequent analyses. Then they were ground into powder passing 100 mesh sieve. Furthermore, the above two powder samples were respectively dissolved in methanol at a concentration of 100 mg/mL. Samples were ultrasonic extracted for 35 min and then the solutions were evaporated. After concentration, the initial mobile phase was used to resolve these two samples.
SF-AP (1 mL) and Astragali radix (1 mL) solution was respectively added into the SPE cartridges, which were orderly pretreated with 5 mL methanol and 5 mL deionized water. Afterwards, the SPE cartridges were successively washed with 3 mL deionized water and 3 mL methanol. The methanol eluate was evaporated to dryness by water bath. Then the residue was redissolved in 200 µL methanol solution and centrifuged for 30 min (13,500 rpm, 4 ℃). The supernatant was finally used for the subsequent analysis.
2.4 Instrument and conditions
UHPLC analysis was performed on DIONEX Ultimate 3000 UHPLC system (Thermo Fisher Scientific, MA, USA), which was equipped with a binary pump, an auto-sampler and a column compartment. The chromatographic separation was carried out at 40 ℃ using Waters ACQUITY HSS T3 column (2.1 × 100 mm i.d., 1.8 μm; Waters Corporation, Milford, MA, USA). The mobile phase consisted of 0.1% formic acid aqueous solution (A) and acetonitrile (B) at a flow rate of 0.2 mL/min. The linear gradient procedure was described as follows: 0–6 min, 8%–25% B; 6–13 min, 25%–32% B; 13–20.5 min, 32%–48% B; 20.5–26 min, 38%–44% B; 26–30 min, 44%–92% B. The injection volume was 3 μL.
HRMS spectral analysis was executed on LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific, MA, USA). The optimized operating parameters in negative and positive ion modes were set as follows: sheath gas flow rate of 40 arb, auxiliary gas flow rate of 20 arb, capillary voltage of ± 25 V, electrospray voltage of 3.0 kV, tube lens of ± 110 V, and capillary temperature of 350 °C. The components were detected using full-scan MS analysis from m/z 100–1,200 with a resolution of 30,000 in both positive and negative ion modes. The collision energy for collision induced dissociation (CID) was set to 40%.
2.5 Peak selections and data processing
Thermo Xcalibur 2.1 workstation (Thermo Fisher Scientific, MA, USA) was used for data acquisition and processing. In order to acquire as many fragment ions as possible, this method targeted the peaks with intensity over 10,000 for the subsequent structural identification. The predicted atoms for chemical formulas of all the deprotonated molecular ions were set as follows: C [0–50], H [0–100], O [0–30], N [0–1] and Ring Double Bond (RDB) equivalent value [0–15]. The maximum mass errors between the measured and calculated values were fixed within ± 5 ppm.
2.6 Determination of antioxidative capacity in vitro
2.6.1 Sample preparation
The two powder samples (Astragali radix and SF-AP), each weighting 3 g, were respectively suspended in 50 mL of 80% methanol solution. Each sample was sonicated for 45 min and centrifuged at 5,000 rpm for 10 min. Then the supernatant fraction was filtrated to obtain extraction solution. Subsequently, the two kinds of extraction solutions were diluted to different concentrations for the determination of antioxidative activity in vitro.
2.6.2 DPPH· scavenging activity assay
DPPH· solution (0.5 mmol/L) was prepared and then 1 mL was respectively added into various Astragali radix and SF-AP concentrations (1 mL). The mixed solutions were incubated at 25 °C for 30 min and protected from light. Finally, the absorbances of these sample solutions were measured at 517 nm (Zeng et al., 2012). DPPH· scavenging activity was calculated as shown in formula (1).
(A0 was the absorbance of DPPH and methanol solution; A1 was the absorbance of DPPH and sample solution)
2.6.3 ABTS·+ scavenging activity assay
An ABTS·+ stock solution was prepared by mixing 7 mmol/L ABTS with 2.45 mmol/L K2S2O4 in water, which was placed in the dark at room temperature for 16 h to obtain a dark blue solution (Hsu et al., 2011). The ABTS·+ stock solution should be diluted with absolute ethanol before the experiment.
Two kinds of sample solutions were diluted in different concentrations, and then 1 mL of these samples were respectively taken into the ABTS·+ solution (2 mL). After the reaction lasted for 6 min at room temperature, the absorbances of these sample solutions were determined at 734 nm. ABTS·+ scavenging activity was calculated as shown in formula (2).
(A0 was the absorbance of ABTS and methanol solution; A1 was the absorbance of ABTS and sample solution.)
3 Results and discussion
3.1 The establishment of analytical strategy
In the present study, the analytical strategy we established included five steps. The first step was to establish the solid-state fermentation system by activating fungus and culturing liquid. Secondly, the samples of SF-AP and Astragali radix were respectively prepared into two solutions and then pretreated by SPE cartridge for the subsequent analytical experiments. Thirdly, a sensitive and validated method based on UHPLC-LTQ-Orbitrap mass spectrometer was developed for the comprehensive analysis of chemical constituents in SF-AP and Astragali radix. The structures of the representative constituents were elucidated according to the accurate mass measurement, fragmentation patterns, DPIs and literature reports. Fourthly, the chemical transformations and relative content fluctuations were compared with each other to clarify the material basis transformations brought about by solid-state fermentation. Finally, based on the summarized chemical transformations, the antioxidant activities of SF-AP and Astragali radix were also evaluated. The general procedures of the strategy were summarized into a diagram in Fig. 1.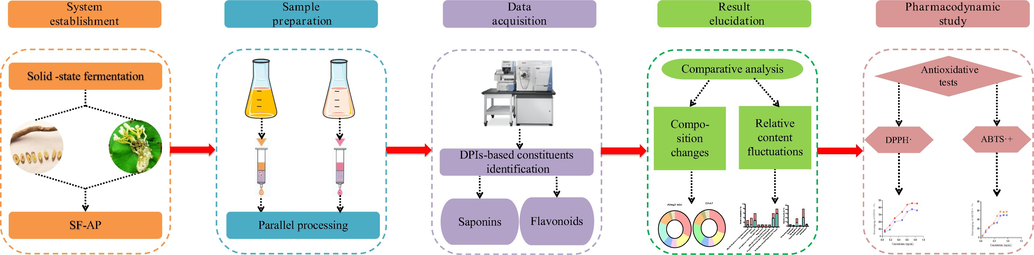
The summary diagram of analytical strategy and methodology.
3.2 The characterization of chemical constituents
Structural elucidation was performed on the basis of chromatographic retention behaviors, accurate mass measurements, mass fragmentation patterns, DPIs and previous relevant literature. It should be noted that DPIs were significant to rapidly perform the structural elucidation, which were produced in by the comparable fragmentation patterns of the constituents with similar backbone (Zhao et al., 2018a,2018b). Finally, a total of 114 chemical constituents including 45 triterpene saponins (Table 1) and 69 flavonoids (Table 2) were accurately or tentatively characterized. #: Unambiguously identification by comparing with the reference substances; *: Structural validation by using the reference substances. +: detected; -: undetected; A: Astragli radix; S: SF-AP. #: Unambiguously identification by comparing with the reference substances; *: Structural validation by using the reference substances; +: detected; -: undetected; A: Astragli radix; S: SF-AP.
Peak
tR/min
Ion mode
Formula
Theoretical Mass m/z
Experimental Mass m/z
Error (ppm)
MS/MS fragment ions
Identification
A
S
A1
7.57
P
C48H79O18
943.52664
943.52582
−0.288
MS2[9 4 3]:925(1 0 0),927(76),1399(37),486(30),859(13),927(13),845(10),827(6)
Soyasaponin I/isomer
+
+
A2
9.32
P
C43H71O15
827.47875
827.47443
−4.218
MS2[8 2 7]:709(1 0 0),809(10),691(9),768(4),737(2),695(2),577(2),335(2),467(1)
Astragaloside II isomer
+
+
A3
9.80
P
C38H63O11
695.43704
695.43274
−4.391
MS2[6 9 5]:577(1 0 0),499(35),677(25),514(10),559(7),605(4),532(4),199(2)
Mongholicoside II isomer
+
+
A4
10.38
P
C38H63O11
695.43704
695.43274
−4.391
MS2[6 9 5]:577(1 0 0),677(12),519(9),499(6),559(6),636(4),578(4),605(3)
Mongholicoside II
+
+
A5*
10.75
N
C47H77O19
945.50700
945.50916
3.023
MS2[9 4 5]:783(1 0 0),489(3),621(2),765(1),651(1)
Astragaloside V
+
+
P
C47H79O19
947.52155
947.52026
−0.788
MS2[9 4 7]:437(1 0 0),455(58),419(38),587(21),785(16),473(14),599(11),535(11),738(10),472(10),277(10)
A6
11.51
P
C49H81O18
957.54229
957.54187
0.134
MS2[9 5 7]:776(1 0 0),777(22),794(19),644(12),335(9),795(4),336(3),643(2),645(2),353(1)
Azukisaponin V methyl ester
–
+
A7
11.61
N
C41H69O14
785.46983
785.47198
4.834
MS2[7 8 5]:491(1 0 0),623(26),415(16),740(13),767(11),367(10),489(8)
Cyclocanthoside E/isomer
+
+
A8
11.77
N
C41H69O14
785.46983
785.46277
−4.892
MS2[7 8 5]:491(1 0 0),829(55),830(26),767(24),653(21)
Cyclocanthoside E/isomer
+
+
A9*
11.79
N
C41H67O14
783.45363
783.45612
1.578
MS2[7 8 3]:489(1 0 0),621(46),651(36),383(15),737(12),453(11),646(11),515(8),471(6)
Isoastragaloside IV
+
+
A10
12.38
N
C42H69O15
813.46474
813.46729
4.154
MS2[8 1 3]:767(1 0 0),745(78),652(47),651(30),489(30),633(27),795(26)
Astramembranoside A
+
+
P
C42H71O15
815.47930
815.47729
−1.788
MS2[8 1 5]:554(1 0 0),711(98),276(97),252(96),250(94),505(93),315(92),806(89)
A11
12.46
N
C41H69O14
785.46983
785.47180
4.605
MS2[7 8 5]:491(1 0 0),623(24),767(13),741(4),653(4),701(3)
Cyclocanthoside E/isomer
+
+
A12
12.51
P
C49H81O20
989.53212
989.53296
1.404
MS2[9 8 9]:503(1 0 0),827(99),599(96),483(95),330(94),584(94),344(93),452(93),603(92)
Agroastragaloside IV
+
+
A13
12.59
N
C43H69O15
825.46419
825.46735
4.151
MS2[8 2 5]:765(1 0 0),783(45),757(17),787(12),779(11),788(5),673(5),401(4)
Astragaloside II isomer
+
+
A14
13.19
N
C36H61O11
669.42248
669.42383
4.468
MS2[6 6 9]:601(1 0 0),632(62),654(55),623(50),436(42),541(41),651(21)
Mongholicoside A
+
+
A15
13.23
P
C40H65O12
737.44760
737.44507
−2.690
MS2[7 3 7]:557(1 0 0),691(23),689(20),511(19),577(18),509(12),673(12),493(11),571(8),605(7),677(7),529(6),475(4)
Huangqiyenin F
–
+
A16
13.79
N
C43H71O15
827.48039
827.48138
3.181
MS2[8 2 7]:759(1 0 0),767(39),783(36),757(34),781(33),809(24),785(22),770(20)
Agroastragaloside II isomer
+
+
A17#
13.95
P
C43H71O15
827.47875
827.47742
−1.605
MS2[8 2 7]:639(1 0 0),558(98),232(94),443(94),640(91),294(90),579(90),231(89),295(83),371(83)
Isoastragaloside II
+
+
N
C43H69O15
825.46419
825.46710
2.849
MS2[8 2 5]:765(1 0 0),783(63),644(31),762(19)
A18#
14.31
P
C41H69O14
785.46818
785.46722
−1.226
MS2[7 8 5]:782(1 0 0),720(78),237(74),575(74),526(73),434(72),480(72),248(70)
Astragaloside IV
+
+
N
C41H67O14
783.45363
783.45813
2.144
MS2[7 8 3]:621(1 0 0),489(50),490(31),651(23),708(23),553(18),700(17),471(14),
A19
14.56
N
C41H67O14
783.45363
783.45654
4.115
MS2[7 8 3]:489(1 0 0),383(13),651(12),453(4),401(2),471(2),381(2),760(2)
Astragaloside III
+
+
A20
14.56
N
C41H69O14
785.46983
785.46246
−4.286
MS2[7 8 5]:491(1 0 0),489(53),385(15),383(11),491(11),622(11),718(5)
Isoastragaloside IV isomer
+
+
A21
16.01
P
C36H59O10
651.41082
651.40857
−2.616
MS2[6 5 1]:177(1 0 0),199(62),269(44),234(42),180(38),574(37),663(37),379(36),229(35),300(32)
Huangqiyenin A
–
+
A22
16.10
N
C51H81O21
1029.52758
1029.52173
−4.619
MS2[1029]:985(1 0 0),984(18),967(2)
Agroastragaloside III
+
+
P
C51H83O21
1031.54214
1031.54199
−0.141
MS2[1031]:984(1 0 0),494(57),558(52),331(50),667(49),936(48),323(47),482(46),300(45)
A23
16.23
N
C47H73O17
909.48532
909.48804
4.192
MS2[9 0 9]:891(1 0 0),613(99),523(80),453(76),849(61),569(58),435(36),746(25),495(18)
Acetylastragaloside I/isomer
+
+
A24
16.29
N
C48H77O18
941.51209
941.50549
−4.259
MS2[9 4 1]:922(1 0 0),524(56),873(36),923(32),615(27),523(26),879(20),456(18),
Soyasaponin I/isomer
+
+
A25
16.36
P
C42H67O14
795.45308
795.45203
−0.632
MS2[7 9 5]:421(1 0 0),597(86),214(81),295(74),429(74),512(72),233(72),625(71)
Huangqiyenin E
–
+
A26
16.71
N
C43H69O15
825.46419
825.46796
4.890
MS2[8 2 5]:765(1 0 0),633(30),744(18),634(17),736(11),717(9),536(8),703(7)
Astragaloside II isomer
+
+
P
C43H71O15
827.47875
827.47729
−1.762
MS2[8 2 7]:269(1 0 0),592(67),629(66),351(64),296(63),632(60),709(60),247(59),277(57)
A27
16.74
N
C36H59O11
667.40683
667.40820
4.512
MS2[6 6 7]:649(1 0 0),449(82),623(81),299(80),450(74),485(54)
Mongholicoside B
+
+
A28
16.84
N
C48H77O18
941.51209
941.51392
3.694
MS2[9 4 1]:923(1 0 0),525(73),615(51),744(49),879(41),457(40),795(37),437(35),597(16)
Soyasaponin I/isomer
+
+
P
C48H79O18
943.52664
943.52496
−1.200
MS2[9 4 3]:599(1 0 0),797(88),441(79),423(48),617(28),581(23),520(10),269(8),454(8),867(8),448(8)
A29#
16.91
N
C43H69O15
825.46419
825.46631
3.892
MS2[8 2 5]:783(1 0 0),765(49),633(24),795(10),697(9),758(7)
Astragaloside II
+
+
A30
17.25
N
C43H71O15
827.48039
827.48267
4.740
MS2[8 2 7]:809(1 0 0),757(69),781(40),758(38),769(25),783(20),767(19)
Agroastragaloside II
+
+
A31
17.53
N
C42H69O15
813.46474
813.46686
4.625
MS2[8 1 3]:725(1 0 0),455(43),633(30),651(29),767(28),523(25),407(22),795(21)
Astramembranoside A
+
+
A32
18.80
N
C42H65O14
793.43853
793.44080
4.937
MS2[7 9 3]:631(1 0 0),775(24),663(8),724(7),747(5),718(5),697(4)
Huangqiyenin E/isomer
+
+
A33
18.83
N
C42H69O15
813.46474
813.46692
4.699
MS2[8 1 3]:745(1 0 0),767(36),489(20),729(18),726(15),651(14),305(9)
Astramembranoside A
+
+
A34#
18.94
N
C45H71O16
867.47476
867.47809
3.104
MS2[8 6 7]:807(1 0 0),821(63),765(53),783(22),849(21),687(17)
Isoastragaloside I
+
+
A35
19.16
N
C47H73O17
909.48532
909.48846
4.654
MS2[9 0 9]:891(1 0 0),849(48),763(47),453(46),569(29),523(27),613(19),407(16)
Acetylastragaloside I/isomer
+
+
A36
19.21
N
C48H77O18
941.51209
941.50427
−4.555
MS2[9 4 1]:922(1 0 0),524(44),879(37),614(36),523(36),613(32),732(31)
Soyasaponin I
+
+
A37
19.27
P
C42H67O14
795.45308
795.45209
−0.557
MS2[7 9 5]:439(1 0 0),597(89),421(44),600(43),528(36),253(35),299(33),245(31)
Huangqiyenin E
+
+
A38
19.37
N
C45H71O16
867.47476
867.47766
4.608
MS2[8 6 7]:821(1 0 0),799(34),731(23),717(16),343(15),787(11),831(8)
Astragaloside I isomer
+
+
A39
20.15
N
C42H65O14
793.43853
793.44073
4.849
MS2[7 9 3]:725(1 0 0),455(43),631(30),663(29),747(28),775(21),689(20),279(19),636(14),588(13),753(11)
Huangqiyenin E/isomer
+
+
A40
20.20
P
C36H63O11
671.43704
671.43341
−4.586
MS2[6 7 1]:479(1 0 0),461(33),478(8),443(8),611(8),653(6),177(4),417(3),460(3),199(2)
Mongholicoside A
+
+
A41#
20.37
N
C45H71O16
867.47476
867.47662
2.410
MS2[8 6 7]:849(1 0 0),799(85),783(81),821(77),747(39),687(33)
Astragaloside I
+
+
A42#
20.92
N
C45H71O16
867.47476
867.47943
3.684
MS2[8 6 7]:703(1 0 0),747(80),599(73),821(53),783(44),799(35),807(32),687(24)
β-D-Glucopyranoside,(3β,6α,16β,20R,24 s)-3-[(3,4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20,24-epoxy-16,25-dihydroxy-9,19-cyclolanostan-6-yl
+
+
A43
22.12
N
C45H73O16
869.49096
869.49335
4.644
MS2[8 6 9]:823(1 0 0),851(46),599(18),767(15),536(11),809(10),749(8),705(8)
Agroastragaloside I
+
+
A44
22.76
N
C47H73O17
909.48532
909.48846
4.654
MS2[9 0 9]:849(1 0 0),867(27),711(10),453(8),891(7),803(2)
Acetylastragaloside I/isomer
+
+
A45
22.77
P
C48H75O19
955.48853
955.48853
−1.231
MS2[9 5 5]:742(1 0 0),1884(96),478(96),406(95),864(95),561(94),701(94),567(94),919(91)
Malonylastragaloside I
+
+
N
C48H73O19
953.47570
953.47968
4.898
MS2[9 5 3]:935(1 0 0),5379(67),627(40),891(23),469(22),907(15),849(3),807(14)
Peak
tR/min
Ion mode
Formula
Theoretical Mass m/z
Experimental Mass m/z
Error (ppm)
MS/MS fragment ions
Identification
A
S
B1
1.24
P
C23H27O10
463.16042
463.15952
−0.763
MS2[4 6 3]:268(1 0 0),330(13),398(8),365(3),398(3),453(3),136(3)
Astraisoflavan-7-O-β-D-glucoside/isomer
–
+
B2#
1.54
P
C21H21O12
465.10330
465.09921
−4.617
MS2[4 6 5]:429(1 0 0),303(59),398(23),314(23),285(21),363(18),199(17),366(17)
Isoquercitrin
+
+
B3
3.71
N
C23H23O11
475.12513
475.12048
−4.331
MS2[4 7 5]:257(1 0 0),275(92),437(65),179(46),180(45),276(42),419(39),438(38),457(26),283(16)
Odoratin-7-O-β-D-glucoside/isomer
+
+
B4
3.98
P
C23H27O10
463.16042
463.15796
−4.131
MS2[4 6 3]:205(1 0 0),415(93),266(68),267(36),378(33),433(32),301(27)
Astraisoflavan-7-O-β-D-glucoside/isomer
+
+
B5
4.37
N
C29H37O16
641.20926
641.21063
4.708
MS2[6 4 1]:479(1 0 0),317(75),595(35),611(30),623(26),379(24),610(22)
5′-hydroxy isomucronulatol 2′,5′-di-O-glucoside
+
+
B6
4.37
P
C24H25O12
505.13460
505.13318
−1.727
MS2[5 0 5]:333(1 0 0),335(41),306(33),373(26),438(21),281(21),343(13),282(11),317(9),181(7),487(6)
Neocomplanoside/isomer
+
+
B7
4.76
N
C28H31O16
623.16231
623.16388
4.165
MS2[6 2 3]:299(1 0 0),284(31),604(7),283(6),461(6),605(5),415(5),577(4)
Complanatuside isomer
+
+
B8
4.86
P
C23H29O10
465.17607
465.17184
−4.919
MS2[4 6 5]:303(1 0 0)446(6),429(6),432(5),302(2),346(1),301(1)
Astraisoflavan-7-O-β-D-glucoside/isomer
+
+
B9#
5.23
N
C28H31O16
623.16231
623.16364
3.780
MS2[6 2 3]:299(1 0 0),284(32),461(10),240(4),461(3),577(2),605(2),211(2),239(2)
Complanatuside
+
+
B10
5.37
N
C22H21O11
461.10948
461.11050
4.773
MS2[4 6 1]:299(1 0 0),284(9)
Kaempferol- 4′- methylether-3-D-glucoside
+
+
P
C22H23O11
463.12404
463.12265
−1.809
MS2[4 6 3]:445(1 0 0),371(29),253(19),285(19),344(4),401(3),301(3)
B11
5.53
P
C16H17O5
289.10760
289.10645
−2.076
MS2[2 8 9]:271(1 0 0),205(91),270(68),233(41),207(16),261(15),231(13),247(10),163(8),219(7),184(7),177(6),229(5),213(5)
(3R)-7,2′,3′-Trihydroxy-4′-methoxy isoflavonone/isomer
+
–
B12
5.92
P
C16H13O5
285.07630
285.07529
−1.614
MS2[2 8 5]:270(1 0 0),253(43),225(19),137(8),229(7),257(3),181(2),271(1)
Calycosin isomer
+
-+
N
C16H11O5
283.06175
283.06198
4.642
MS2[2 8 3]:268(1 0 0),269(3),255(1)
B13
6.19
N
C22H21O12
477.10440
477.10532
4.382
MS2[4 7 7]:315(1 0 0),301(18),300(14),347(13),431(11),459(5),297(4)
Isorhamnetin-3-O-β-D-glucoside
+
+
B14
6.19
P
C22H23O10
447.12912
447.12695
−3.630
MS2[4 4 7]:300(1 0 0),283(19),255(7),167(5),301(5),259(4),138(3),269(3),168(2),297(1)
Calycosin-7-O-β-D-glucoside isomer
B15
6.19
N
C24H23O12
503.12005
503.12112
4.401
MS2[5 0 3]:299(1 0 0),284(23),443(4),467(2),488(1),240(1)
Neocomplanoside/isomer
+
+
B16
6.21
P
C16H13O5
285.07630
285.07526
−1.719
MS2[2 8 5]:270(1 0 0),253(42),225(18),137(8),229(6),271(4),257(3),181(2)
Calycosin isomer
+
+
N
C16H11O5
283.06175
283.06180
4.006
MS2[2 8 3]:268(1 0 0),269(5),239(2),265(1),255(1)
B17
6.34
P
C17H15O6
315.08686
315.08603
−0.903
MS2[3 1 5]:300(1 0 0),283(20),255(8),167(5),259(4),301(2),287(2),175(2)
7,3′-dihydroxy-8,4-dimethoxyisoflavone isomer
+
+
B18
6.34
P
C23H25O11
477.13969
477.13779
−2.825
MS2[4 7 7]:458(1 0 0),356(98),398(41),361(26),305(14),459(11),289(8),357(7),445(7),333(7),287(6),272(5),169(5)
Odoratin-7-O-β-D-glucoside isomer
+
+
B19
6.35
N
C17H13O6
313.07231
313.07236
4.415
MS2[3 1 3]:298(1 0 0),285(2),295(1),269(1),283(1)
7,3′-Dihydroxy-8,4-dimethoxyisoflavone/isomer
+
+
B20
6.72
N
C15H9O5
269.04610
269.04605
4.947
MS2[2 6 9]:225(1 0 0),241(36),197(23),181(22),236(16),226(11),183(11),251(9),213(9),201(8),254(8)
5,7,4′-trihydroxy- isoflavonone/isomer
+
+
B21
7.00
N
C16H11O5
283.06175
283.06192
4.430
MS2[2 8 3]:268(1 0 0),269(3),265(1),239(1)
Calycosin isomer
+
+
P
C16H13O5
285.07630
285.07529
−1.614
MS2[2 8 5]:270(1 0 0),253(43),225(20),285(17),137(9),229(7),286(4),257(3),181(2)
B22
7.01
P
C16H17O5
289.10760
289.10651
−1.868
MS2[2 8 9]:270(1 0 0),271(22),184(8),252(8),166(7),205(4),182(2)
(3R)-7,2′,3′-trihydroxy-4′-methoxy isoflavonone/isomer
+
–
B23*
7.10
N
C22H21O10
445.11457
445.11575
3.351
MS2[4 4 5]:283(1 0 0),268(17),255(9)
Calycosin-7-O-β-D-glucoside
+
+
B24
7.19
N
C17H13O6
313.07231
313.07230
4.224
MS2[3 1 3]:298(1 0 0),181(17),245(8),137(6),295(6),269(5),285(5),194(3)
7,3′-dihydroxy-8,4-dimethoxyisoflavone/isomer
+
+
B25#
7.25
N
C21H19O10
431.09892
431.09961
2.421
MS2[4 3 1]:268(1 0 0),269(48),311(8),162(6),
Genistin
+
+
B26
7.30
N
C24H23O11
487.12513
487.12631
4.793
MS2[4 8 7]:193(1 0 0),178(15),161(13),179(11),323(10),163(8),355(5),203(5),293(4)
Calycosin-7-O-β-D-glucoside-6″-O-acetate/isomer
+
+
B27
7.35
N
C16H11O5
283.06175
283.06189
4.324
MS2[2 8 3]:268(1 0 0),269(1)
Calycosin isomer
+
+
B28
7.37
P
C16H13O5
285.07630
285.07571
−0.140
MS2[2 8 5]:270(1 0 0),253(43),225(20),255(14),137(8),229(7),268(5),257(3),181(2),197(1)
Calycosin isomer
B29
7.39
P
C17H15O6
315.08686
315.08575
−1.792
MS2[3 1 5]:300(1 0 0),283(19),255(9),269(8),297(5),167(5),259(4),138(3)
Kumatakenin
+
+
B30
7.52
N
C23H27O10
463.16152
463.16220
4.023
MS2[4 6 3]:301(1 0 0),283(40),273(37),191(36),341(11),176(9),268(3)
Astraisoflavan-7-O-β-D-glucoside/isomer
+
+
B31
7.69
P
C15H11O5
271.06065
271.05978
−1.180
MS2[2 7 1]:151(1 0 0),250(78),251(12),66(8),252(7),215(7),153(6),243(5),256(5),137(4),253(4)
5,7,4′-trihydroxy- isoflavonone/isomer
+
–
B32
7.70
N
C24H23O11
487.12513
487.12631
4.793
MS2[4 8 7]:283(1 0 0),268(50),427(14),193(11),419(10),253(3)
Calycosin-7-O-β-D-glucoside-6″-O-acetate
+
+
B33
7.88
N
C16H11O4
267.06683
267.06693
4.533
MS2[2 6 7]:252(1 0 0),253(5),249(2)
Formononetin isomer
+
+
P
C16H13O4
269.08138
269.08051
−1.209
MS2[2 6 9]:254(1 0 0),237(51),213(35),253(13),107(9),118(6),241(6),136(5)
B34
7.89
N
C15H9O5
269.04610
269.04617
4.393
MS2[2 6 9]:225(1 0 0),254(88),241(78),201(64),181(53),197(43),180(38),223(30)
5,7,4′-trihydroxy- isoflavonone/isomer
+
+
P
C15H11O5
271.06065
271.05972
−1.402
MS2[2 7 1]:243(1 0 0),153(87),215(85),239(50),66(41),149(36),253(34),211(30),221(25),159(16),199(14)
B35
7.93
N
C29H37O15
625.21434
625.21527
4.116
MS2[6 2 5]:301(1 0 0),463(9),286(4),445(3),607(2),271(2),473(1)
Isomucronulatol-7,2′-di-O-glucoside
+
+
B36
7.99
P
C17H17O5
301.10760
301.10669
−1.196
MS2[3 0 1]:167(1 0 0),284(66),269(54),241(19),191(19),147(17),267(10),163(9),245(9)
3,9-dimethoxy-10-hydroxypterocarpan/isomer
+
+
B37
8.12
N
C16H11O5
283.06175
283.06168
4.582
MS2[2 8 3]:268(1 0 0),269(3),255(1)
Calycosin isomer
+
+
B38
8.15
P
C24H25O11
489.13969
489.13794
−2.449
MS2[4 8 9]:285(1 0 0),177(5),471(5),387(4),470(4),471(3),294(3),443(2),371(2)
Calycosin-7-O-β-D-glucoside-6″-O-acetate/isomer
+
+
B39
8.24
N
C16H11O4
267.06683
267.06702
4.870
MS2[2 6 7]:252(1 0 0),253(1)
Formononetin isomer
+
+
B40
8.24
N
C23H23O11
475.12513
475.12625
4.813
MS2[4 7 5]:267(1 0 0),456(1),252(1)
Odoratin-7-O-β-D-glucoside/isomer
+
+
B41#
8.26
P
C22H23O9
431.13421
431.13263
−2.386
MS2[4 3 1]:269(1 0 0),343(0.3),413(0.2)
Ononin
+
+
B42
8.27
P
C16H13O4
269.08138
269.08038
−1.692
MS2[2 6 9]:254(1 0 0),237(51),213(40),241(17),66(14),252(12)
Formononetin isomer
+
+
B43
8.27
P
C26H27O11
515.15534
515.15076
−4.819
MS2[5 1 5]:339(1 0 0),321(3),497(2),199(1)
Calycosin-7-O-β-D-glucoside-6″-O-butylene ester/isomer
+
–
B44
8.43
N
C17H15O5
299.09305
299.09293
4.115
MS2[2 9 9]:284(1 0 0),269(1),255(1)
3,9-dimethoxy-10-hydroxypterocarpan/isomer
+
+
P
C17H17O5
301.10760
301.10641
−2.126
MS2[3 0 1]:167(1 0 0),269(26),191(21),147(19),163(12),273(11),207(9),286(6),241(6),270(3)
B45
8.49
N
C16H15O5
287.09305
287.09317
4.165
MS2[2 8 7]:135(1 0 0),272(91),165(46),177(29),121(22),147(19)
(3R)-7,2′,3′-trihydroxy-4′-methoxy isoflavonone
+
+
B46
8.49
N
C29H37O15
625.21434
625.21716
4.139
MS2[6 2 5]:323(1 0 0),367(71),324(70),343(48),325(36),445(26),547(24),366(17)
Isomucronulatol-7,2′-di-O-glucoside/isomer
+
+
B47
8.70
N
C29H37O15
625.21434
625.21558
−4.782
MS2[6 2 5]:323(1 0 0),301(30),245(5),263(3),268(3),283(3),341(2),607(2)
Isomucronulatol-7,2′-di-O-glucoside/isomer
+
+
B48
8.78
N
C16H11O4
267.06683
267.06683
4.158
MS2[2 6 7]:252(1 0 0),253(3),249(2),223(1)
Formononetin isomer
+
+
B49
8.92
N
C17H15O5
299.09305
299.09314
4.817
MS2[2 9 9]:284(1 0 0),269(4)
3,9-dimethoxy-10-hydroxypterocarpan/isomer
+
+
P
C17H17O5
301.10760
301.10641
−2.126
MS2[3 0 1]:167(1 0 0),269(22),191(20),147(15),163(10),273(9),207(7),241(6),286(2),270(2)
B50
9.02
N
C17H17O5
301.10870
301.10870
4.479
MS2[3 0 1]:286(1 0 0),109(14),135(12),147(10),283(8),271(6),179(3)
(3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan/isomer
+
+
P
C17H19O5
303.12325
303.12225
−1.485
MS2[3 0 3]:167(1 0 0),149(32),123(19),284(16),181(14),168(7),219(6),270(5),193(5)
B51
9.13
P
C16H13O4
269.08138
269.08041
−1.581
MS2[2 6 9]:269(1 0 0),252(51),237(28),213(22),270(21),253(7)
Formononetin isomer
+
+
B52
9.19
N
C16H11O4
267.06683
267.06680
4.046
MS2[2 6 7]:252(1 0 0),253(5)
Formononetin isomer
+
+
P
C16H13O4
269.08138
269.08023
−2.250
MS2[2 6 9]:254(1 0 0),237(52),269(51),213(40),253(15),270(13),107(10),136(6)
B53
9.23
N
C17H17O5
301.10870
301.10880
4.811
MS2[3 0 1]:286(1 0 0),135(19),109(15),147(10),121(8),283(6),271(6),179(6)
(3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan/isomer
+
+
P
C17H19O5
303.12325
303.12219
−1.683
MS2[3 0 3]:167(1 0 0),149(29),123(22),181(16),193(6),285(2),219(1),168(1)
B54#
9.23
N
C23H27O10
463.16152
463.16254
2.757
MS2[4 6 3]:301(1 0 0),286(5),299(1)
Astraisoflavan-7-O-β-D-glucoside
+
+
B55
9.37
N
C17 H13O5
297.07740
297.07748
4.823
MS2[2 9 7]:282(1 0 0),283(4),279(3),267(2),253(2),254(1),167(1)
Afromosin
+
+
P
C17 H15O5
299.09195
299.09119
−0.702
MS2[2 9 9]:284(1 0 0),166(23),243(21),239(11),267(11),285(10),137(4)
B56
9.44
P
C16H13O4
269.08138
269.08035
−1.804
MS2[2 6 9]:269(1 0 0),254(75),237(39),213(31),270(17),252(11)
Formononetin isomer
+
+
N
C16H11O4
267.06683
267.06699
4.757
MS2[2 6 7]:252(1 0 0),253(1)
B57#
9.60
N
C16H11O5
283.06175
283.06183
2.112
MS2[2 8 3]:268(1 0 0),255(5)
Calycosin
+
+
P
C16H13O5
285.07630
285.07520
−1.929
MS2[2 8 5]:270(1 0 0),253(43),225(20),137(9),229(7),257(3),181(2),175(1)
B58
9.80
P
C17H19O5
303.12325
303.12247
−0.759
MS2[3 0 3]:167(1 0 0),149(30),123(28),181(19),193(6)
(3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan/isomer
+
+
N
C17H17O5
301.10870
301.10886
4.011
MS2[3 0 1]:286(1 0 0),109(17),135(12),147(8),271(7),283(7),259(3),121(3)
B59
9.98
N
C17 H15O5
299.09305
299.09329
4.319
MS2[2 9 9]:284(1 0 0),269(4)
3,9-dimethoxy-10-hydroxypterocarpan
+
+
P
C17H17O5
301.10760
301.10657
−1.594
MS2[3 0 1]:167(1 0 0),269(22),191(20),147(16),163(10),273(10),207(7),241(6),270(3)
B60
10.00
P
C17H15O6
315.08686
315.08588
−1.379
MS2[3 1 5]:300(1 0 0),283(19),138(11),255(5),186(7),168(7),294(5),167(5),259(4),296(3)
Kumatakenin
+
+
B61
10.01
N
C15H9O5
269.04610
269.04617
4.393
MS2[2 6 9]:241(1 0 0),225(20),213(17),123(9),251(8),145(6),197(5)
5,7,4′-trihydroxy- isoflavonone
+
+
B62
10.25
N
C17H15O5
299.09305
299.09323
4.118
MS2[2 9 9]:284(1 0 0),269(6),267(6),165(4),271(4),281(2)
3,9-dimethoxy-10-hydroxypterocarpan/isomer
+
+
B63
10.34
P
C26H27O11
515.15534
515.15393
−1.666
MS2[5 1 5]:411(1 0 0),353(19),497(13),455(13),393(10),369(10),597(10),337(9),335(8),395(7),167(6)
Calycosin-7-O-β-D-glucoside-6″-O-butylene ester/isomer
+
+
B64
10.38
N
C17H17O5
301.10870
301.10886
4.011
MS2[3 0 1]:286(1 0 0),135(38),121(17),109(13),147(10),283(8),179(7),271(6)
(3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan/isomer
+
+
P
C17H19O5
303.12325
303.12247
−0.759
MS2[3 0 3]:167(1 0 0),149(29),123(23),181(16),193(7),285(2),261(1),167(1)
B65
10.99
N
C16H11O4
267.06683
267.06693
4.533
MS2[2 6 7]:252(1 0 0),253(5),249(2)
Formononetin isomer
+
+
B66
11.75
P
C17H17O5
301.10760
301.10690
−0.499
MS2[3 0 1]:167(1 0 0),269(22),191(20),147(16),163(10),281(10),207(7),241(6),267(4)
3,9-dimethoxy-10-hydroxypterocarpan/isomer
+
+
B67#
14.00
N
C16H11O4
267.06683
267.06699
2.757
MS2[2 6 7]:252(1 0 0),253(3)
Formononetin
+
+
P
C17H13O5
269.08138
269.08041
−1.581
MS2[2 6 9]:254(1 0 0),237(45),251(36),213(26),253(14),107(10),118(5)
B68
14.69
P
C17H17O5
301.10760
301.10666
−1.296
MS2[3 0 1]:167(1 0 0),269(81),147(46),191(45),163(28),273(24),267(20),241(18),281(7),270(6),284(4)
3,9-dimethoxy-10-hydroxypterocarpan/isomer
+
+
B69
15.28
P
C17H19O5
303.12325
303.12262
−0.264
MS2[3 0 3]:167(1 0 0),149(33),123(22),181(15),193(7)280(7),199(2)
(3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan
+
+
3.2.1 Structural identification of triterpenoid saponins in SF-AP and Astragali radix
Saponins are important effective components existing in Astragali radix, most of which are tetracyclic triterpenoids. Based on the retention time, ESI-MS and ESI-MS/MS data, a total of 45 constituents attributed to triterpene saponins were screened and identified from SF-AP sample and Astragali radix. These constituents mostly belong to cycloartane-type triterpenoids, which were mainly derivatives of 9, 19-cyclolanostane cycloastragenol or 9, 19-cyclolanostane cyclocanthogenin, 9, 10-secocycloartane and oleanane-type triterpenoid saponins (Chu et al., 2010).
In addition, Astragaloside I and Astragaloside IV were selected as subjects to determine their DPIs for the subsequent structral identification. Both reference standards possessed the same backbone structure while the quantity of acetyl groups connected to xylose were different. There are two acetyl groups at 2 and 3 position of xylose in Astragaloside I, while zero acetyl group in Astragaloside IV. Owing to the special structure of acetyl group (Ac), Astragaloside I could generate some characteristic fragment ions by loss of Ac (42 Da), Ac + H2O (60 Da) and 2Ac (84 Da). Moreover, by comparing with the characteristic dissociation pathways in the MS/MS spectra of the other reference standards, some DPIs of triterpene saponins could be summarized in Fig. 2, which provided a basis for further characterization of the others candidates. Taking negative ion mode as an example, the mass spectrometry cleavage of triterpene saponins usually lose glucose (Glc, 162 Da), xylose (Xyl, 132 Da), H2O (18 Da), CO2 (44 Da), malonyl (Ma, 86 Da) and acetyl group (Ac, 42 Da) to generate the corresponding DPIs.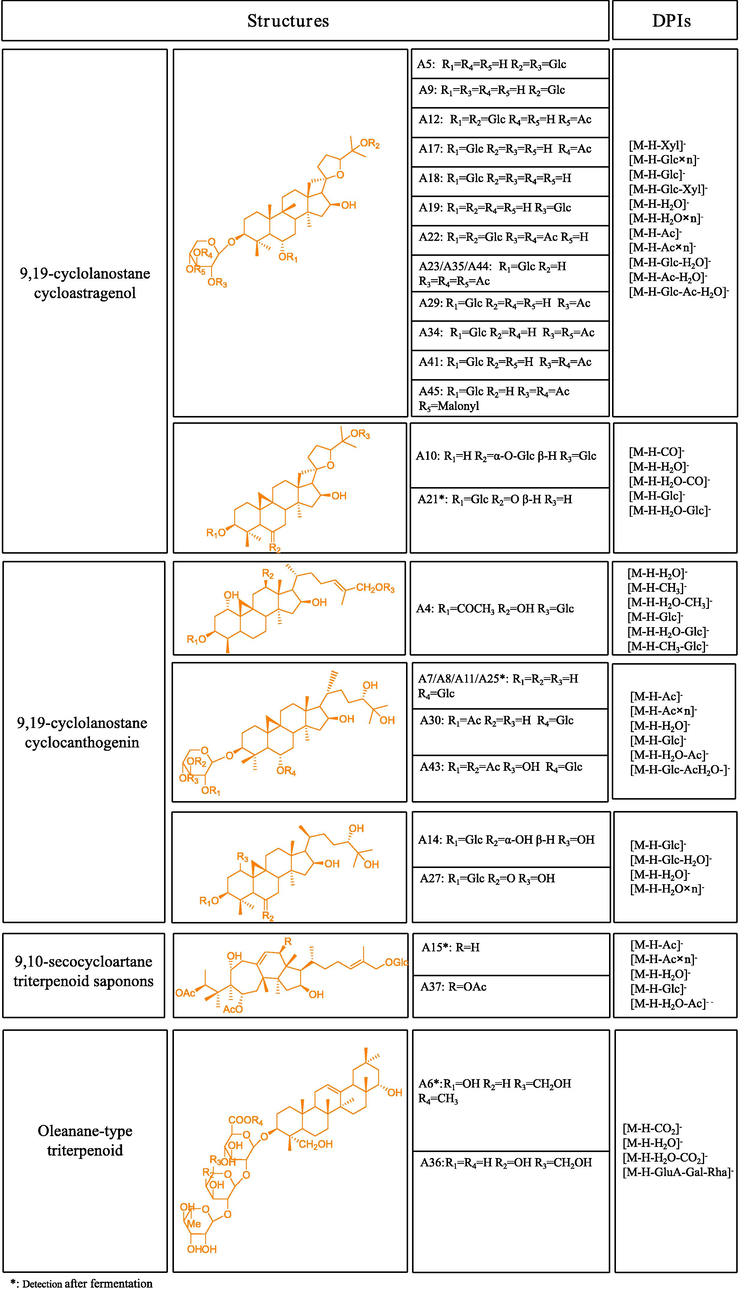
The summary structures and DPIs for the triterpenoid saponins in SF-AP and Astragali radix.
A7, A8 and A11 all possessed the [M−H]- ions at m/z 785.46983 (C41H69O14, mass error within ± 5 ppm). In their ESI-MS2 spectra, they could yield a wide range of DPIs just like [M−H−Glc−Xyl]- ion at m/z 491, [M−H−Glc]- ion at m/z 623, [M−H−Xyl]- ion at m/z 653, [M−H−H2O]- ion at m/z 767, and [M−H−CO2]- ion at m/z 741. Hence, A7, A8 and A11 were tentatively judged as Cyclocanthoside E or its isomers.
A10, A31 and A33 showed the identical [M−H]- ions at m/z 813.46474 (C42 H69 O15, mass error within ± 5 ppm). In the ESI-MS2 spectra, a number of DPIs such as m/z 651 [M−H−Glc]-, m/z 633 [M−H−Glc−H2O]-, m/z 795 [M−H−H2O]- and m/z 767 [M−H−H2O−CO]- were all observed. Meanwhile, combined with the bibliography data and fragmentation pathways, A10, A31 and A33 were tentatively characterized as Astramembranoside A or its isomers.
A13, A17, A26 and A29 all possessed the [M−H]- ions at m/z 825.46419 (C43H69O15, mass error within ± 5 ppm). Owing to the successive loss of acetyl, acetyl + H2O and xylose + acetyl + H2O, the [M−H]- ion generated a serial of DPIs at m/z 783, m/z 765 and m/z 633 in the ESI-MS2 spectra. Based upon the comparison of ESI-MS/MS spectra and retention time with the corresponding reference standards, A29 was positively identified as Astragaloside II, while A17 was unambiguously characterized as Isoastragaloside II. The accurate mass weight and major product ions of A13 and A26 were broadly similar to those of A29 and A17, which indicated that A13 and A26 could be deduced as the isomers of Astragaloside II or Isoastragaloside II.
A14 afforded [M−H]- ion at m/z 669.42248 (C36H61O11) with mass error of 4.47 ppm. Due to the loss of H2O (18 Da), 2H2O (36 Da) and glucose (162 Da), the product ions in its ESI-MS2 spectrum at m/z 651, m/z 633 and m/z 507 were respectively yielded. Therefore, according to the fragmentation pathways and literature data (Wang et al., 2019a), A14 could be deduced as Mongholicoside A.
A23, A35 and A44 all afforded the [M−H]- ions at m/z 909.48532 (C47H73O17, mass error within ± 5 ppm). In the MS/MS spectra, there were some product ions such as [M−H−H2O]- at m/z 891, [M−H−Ac−H2O]- at m/z 849 and m/z 453 [M−H−3Ac−Xyl−Glc−2H2O]- at m/z 453. And thus, A23, A35 and A44 were tentatively characterized as Acetylastragaloside I or its isomers.
Both A32 and A39 provided the deprotonated [M−H]- ions at m/z 793.43853 (C42H65O14, mass error within ± 5 ppm). The characteristic product ions such as [M−H−Glc]- ion at m/z 631, [M−H−H2O]- ion at m/z 775 and [M−H−2Ac−H2O−CO]- ion at m/z 663 were all illustrated in the ESI-MS2 spectra. Hence, A32 and A39 were deduced to be Huangqiyenin E or its isomer.
A34, A38, A41 and A42 gave the identical [M−H]- ions at m/z 867.47476 (C45H71O16, mass error within ± 5 ppm). Based upon the obtained high-resolution mass spectrometry data, they yielded the DPIs at m/z 849, m/z 807, m/z 783, m/z 747 and m/z 687 by the respective loss of H2O (18 Da), acetyl + H2O (60 Da), 2acetyl (84 Da), 2acetyl + 2H2O (120 Da) and glucose + H2O (180 Da) in the ESI-MS2 spectra. Compared with the standard substances, A41 was identified as Astragaloside I and A34 was characterized as Isoastragaloside I, while A38 was characterized as Isoastragaloside I isomer. Moreover, A42 was deduced as β-D-Glucopyranoside-(3β, 6α, 16β, 20R, 24 s)-3-[(3, 4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20, 24-epoxy-16, 25-dihydroxy-9, 19-cyclolanostan-6-yl.
A43 possessed the [M−H]- ion at m/z 869.49096 (C45H73O16, mass error of 4.644 ppm). In the ESI-MS2 spectrum, it yielded some DPIs at m/z 851, m/z 809, m/z 767 and m/z 749 through the successive loss of H2O, acetyl + H2O, 2acetyl + H2O and 2acetyl + 2H2O, respectively. Hence, A43 was tentatively interpreted as Agroastragaloside I.
A45 generated its [M−H]- ion at m/z 953.47570 (C48H73O19) with mass error of 4.898 ppm. It further produced a series of fragment ions at m/z 935 [M−H−H2O]-, m/z 627 [M−H−Glc−Ma−Ac−2H2O]-, m/z 891 [M−H−CO2−H2O]-, and m/z 807 [M−H−Ma−Ac−H2O]- in its ESI-MS2 spectrum. Therefore, A45 was tentatively interpreted as Malonylastragaloside I. In addition, the ESI-MS2 spectra of A9, A17, A44 and A45 were illustrated in Fig. 3.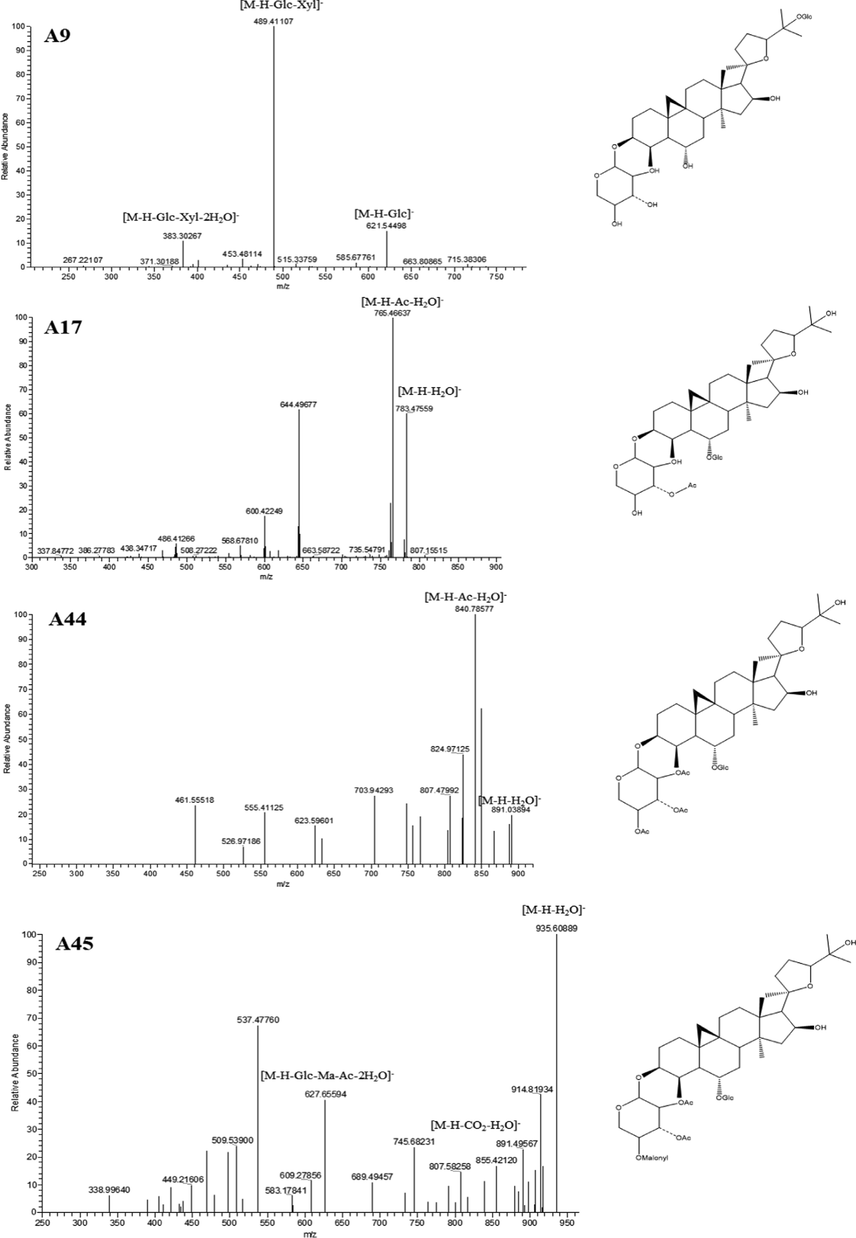
The ESI-MS2 spectra and chemical structures of A9, A17, A44 and A45.
In order to verify the fragmentation regularities, the other two reference substances were conducted. Take Astragaloside V and Isoastragaloside IV as examples, which would make structural validation clear. Based on the obtained high-resolution mass spectrometry data, it could be seen that Astragaloside V (A5*) produced the [M−H]- ion at m/z 945.50700 (C47H77O19). Then the [M−H]- ion generated a series of characteristic product ions at m/z 783 [M−H−Glc]-, m/z 651 [M−H−Glc−Xyl]-, m/z 621 [M−H−2Glc]- and m/z 489 [M−H−Xyl−2Glc]- in its ESI-MS2 spectrum. Isoastragaloside IV (A9*) gave rise to [M−H]- ion at m/z 783.45363 (C41H67O14). In its ESI-MS2 spectrum, the [M−H]- ion at m/z 783 further generated several product ions at m/z 489, m/z 651, m/z 621, m/z 471, and m/z 453 by the subsequent losing xylose + glucose, xylose moiety, glucose moiety, xylose + glucose + H2O, and xylose + glucose + 2H2O. By referring to the cracking mode of these two reference substances, some similar rules in cracking could be found. For the special structures of flavonoid glycoside, the ions of [M−H−162]- and [M−H−132]- were usually produced via the loss of glucose moiety and xylose moiety in their ESI-MS2 spectra.
3.2.2 Structural identification of flavonoids in SF-AP and Astragali radix
Flavonoids are the other major category of components existing in Astragali radix. The DPIs for flavonoids have previously summarized on the basis of high-resolution MS data acquired in Fig. 4. The characteristic DPIs just like [M−H−CH3]- (15 Da), [M−H−H2O]- (18 Da), [M−H−CO2]- (44 Da) and [M−H−CO]- (28 Da) were obviously observed according to the ESI-MS/MS data of the obtained reference standards. Meanwhile, flavonoid glycosides usually first split up the glycosidic bond, and then produce the corresponding aglycone ions (Ren et al., 2007; Es-Safi et al., 2007). Generally speaking, the glucose group is usually replaced at the C-7 or C-3 position of the ligand ketone. Subsequently, the aglycone ions would be cleaved to form a series of fragment ion. In addition, they will ordinarily lose Glc + H2O + CO (208 Da) and Glc + CO + H2O + CH3 (223 Da). For polymethoxylated flavones, the fragment ions produced by loss of one or more methyl radicals from the protonated molecule ions are usually detected, which could be regarded as their DPIs (Zhang et al., 2011; Shang et al., 2017). Finally, 64 flavonoids including 33 isoflavnones, 15 isoflavans, 7 pterocarpans and 9 flavnones were identified from SF-AP while 69 flavonoids including 35 isoflavnones, 18 isoflavans, 7 pterocarpans and 9 flavnones were characterized from Astragali radix.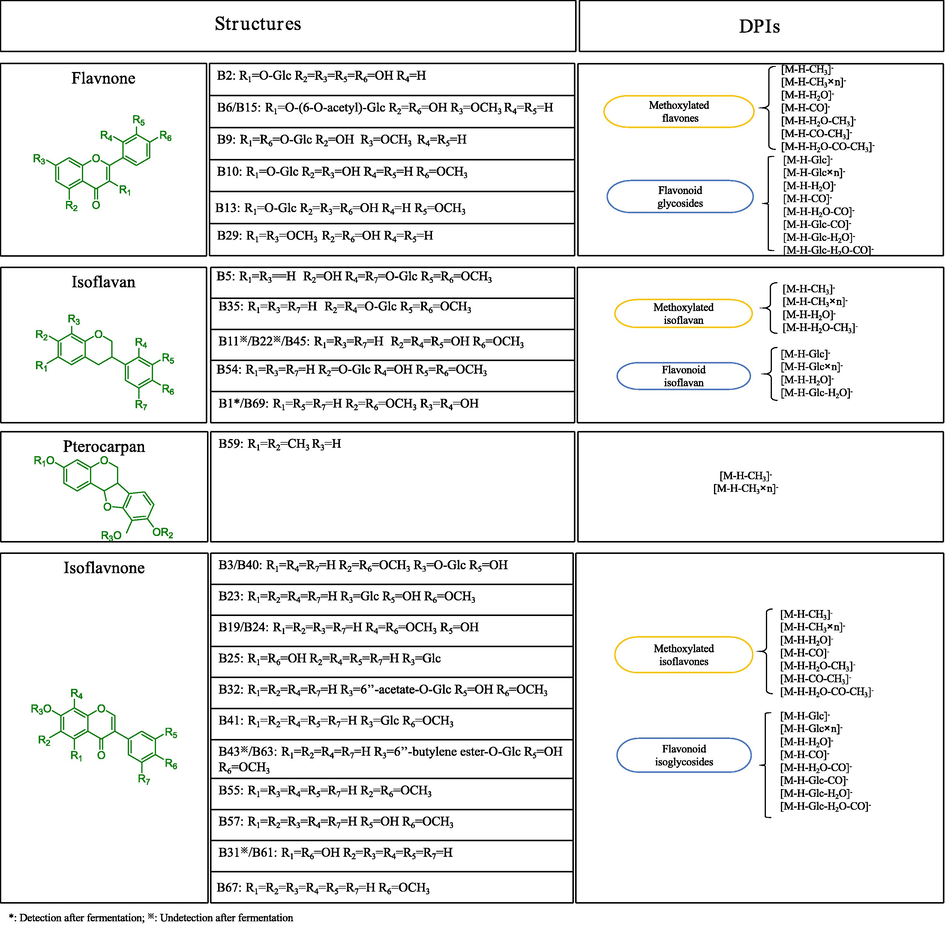
The summary structures and DPIs for the flavonoids in SF-AP and Astragali radix.
Both B3 and B40 yielded [M−H]- ions at m/z 475.12513 (C23H23O11, mass error within ± 5 ppm). In the ESI-MS2 spectra, a number of DPIs were observed such as m/z 267 [M−H−Glc−H2O−CO]-, m/z 252 [M−H−Glc−CO−H2O−CH3]- and m/z 429 [M−H−H2O−CO]-. Based on this, B3 and B40 were tentatively characterized as Odoratin-7-O-β-D-glucoside or its isomer.
B5 possessed the [M−H]- ion at m/z 641.20926 (C29H37O16) with mass error of 4.708 ppm. Due to the presence of ESI-MS2 product ions at m/z 479, m/z 317, m/z 433 and m/z 611 by losing glucose, 2glucose, CO + H2O and 2CH3, B5 was finally characterized as 5′-hydroxy-isomucronulatol-2′, 5′-di-O-glucoside.
Both B7 and B9 generated the same [M−H]- ions at m/z 623.16231 (C28H31O16, mass error within ± 5 ppm). Moreover, a range of characteristic DPIs at m/z 415 [M−H−Glc−CO−H2O]-, m/z 461 [M−H−Glc]-, m/z 299 [M−H−2Glc]- and m/z 577 [M−H−CO−H2O]- were detected in the ESI-MS/MS spectra. As a result, B9 was tentatively characterized as Complanatuside, while B7 was judged as Complanatuside isomer.
B10 gave rise to the [M−H]- ion at m/z 461.10948 (C22H21O11) with mass error of 4.773 ppm. Based on the obtained high-resolution mass spectrometry data, it yielded respective base peak ions at m/z 446 [M−H−CH3]-, m/z 299 [M−H−Glc]-, m/z 267 [M−H−Glc−CH2−H2O]- and m/z 271 [M−H−Glc−CO]- in the ESI-MS2 spectra. Therefore, according to the fragmentation pathways, B10 was characterized as Kaempferol-4′-methylether-3-D-glucoside.
Six isomeric constituents, B12, B16, B21, B27, B37 and B57, afforded the same theoretical [M−H]- ions at m/z 283.06175 (C16H11O5, mass error within ± 5 ppm), respectively. In the ESI-MS spectra, they showed the characteristic ESI-MS2 product ions at m/z 268 and m/z 255 by lossing of CH3 (15 Da) and CO (28 Da). Combined with the standard substance, B57 was unambiguously identified as Calycosin. Meanwhile, the other five constituents including B12, B16, B21, B27 and B37 were tentatively characterized as Calycosin isomers.
B13 possessed the [M−H]- ion at m/z 477.10440 (C22H21O12) with mass error of 4.382 ppm. In the ESI-MS2 spectrum, it produced many DPIs just like [M−H−Glc]- ion at m/z 315, [M−H−H2O−CO]- ion at m/z 431, [M−H−Glc−H2O]- ion at m/z 297, [M−H−Glc−CH3]- ion at m/z 300 and [M−H−H2O]- ion at m/z 459. Combined with the standard substance, B13 was positively identified as Isorhamnetin-3-D-glucoside.
B15 afforded the [M−H]- ion at m/z 503.12005 (C24H23O12, mass error of 4.401 ppm). In the ESI-MS/MS spectrum, it showed the product ions at m/z 299 [M−H−acetyl−Glc]-, m/z 488 [M−H−CH3]- and m/z 467 [M−H−2H2O]-. And thus, it indicated that B15 could be characterized as Neocomplanoside or its isomer.
B19 and B24 afforded the same [M−H]- ions at m/z 313.07231 (C17H13O6, mass error within ± 5 ppm). They further produced DPIs at m/z 298 ([M−H−CH3]-), m/z 295 ([M−H−H2O]-), m/z 285 ([M−H−CO]-) and m/z 269 ([M−H−CO2]-) in the ESI-MS/MS spectra. Hence, according to the proposed fragmentation patterns, B19 and B24 could be assumed as 7,3′-dihydroxy-8,4-dimethoxyisoflavone or its isomer.
B20, B34 and B61 afforded the same [M−H]- ions at m/z 269.04610 (C15H9O5, mass error within ± 5 ppm), respectively. There were a battery of DPIs at m/z 254 ([M−H−CH3]-), m/z 241 ([M−H−Glc−CO]-), m/z 225 ([M−H−CO2]-), m/z 197 ([M−H−CO−CO2]-) and m/z 181 ([M−H−2CO2]-) in the ESI-MS/MS spectra. Therefore, B20, B34 and B61 could be characterized as 5,7,4′-trihydroxy- isoflavonone or its isomers.
B25 generated the [M−H]- ion at m/z 431.09892 (C21H19O10) with mass error of 2.421 ppm. In the ESI-MS2 spectrum, it further possessed the product ions at m/z 269 by the loss of 162 Da (glucose). By Combing with the standard substance, B25 was positively characterized as Genistin.
Seven constituents containing B33, B39, B48, B52, B56, B65 and B67, which respectively afforded the same identical [M−H]- ions at m/z 267.06683 (C16H11O4, mass error within ± 5 ppm). In the ESI-MS/MS spectra, they further yielded the product ion at m/z 252 by the loss of CH3 radical. Combined with the standard substance, B67 was unambiguously identified as Formononetin, while B33, B39, B48, B52, B56 and B65 could be deduced as Formononetin isomers.
B45 afforded the [M−H]- ion at m/z 287.09305 (C16H15O5, mass error of 4.165 ppm). In the ESI-MS/MS spectrum, it further generated the product ions at m/z 272 [M−H−CH3]-, m/z 269 [M−H−H2O]- and m/z 255 [M−H−CH2−H2O]-. Based upon this, B45 was deduced to be (3R)-7,2′,3′-trihydroxy-4′-methoxy isoflavonone.
B50, B53, B58 and B64 all gave rise to the same [M−H]- ions at m/z 301.10870 (C17H17O5, mass error within ± 5 ppm), respectively. In the ESI-MS/MS spectra, the DPIs at m/z 286 ([M−H−CH3]-), m/z 283 ([M−H−H2O]-)and m/z 271 ([M−H−2CH3]-) were all observed. Combined with the obtained fragmentation pathways, B50, B53, B58 and B64 were characterized as (3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan or its isomers.
Five isomeric constituents, including B44, B49, B59, B62 and B72, produced the same [M−H]- ions at m/z 299.09305 (C17H15O5, mass error within ± 5 ppm), respectively. There were a series of DPIs at m/z 284 [M−H−CH3]-, m/z 269 [M−H−2CH3]-, m/z 267 [M−H−CH2−H2O]- and m/z 281 [M−H−H2O]- in the ESI-MS2 spectra. And thus, B44, B49, B59, B62 and B72 were tentatively characterized as 3,9-dimethoxy-10-hydroxypterocarpan or its isomers.
B54 gave rise to the identical [M−H]- ion at m/z 463.16152 (C23H27O10, mass error 2.757 ppm). In the ESI-MS/MS spectrum, it showed the characteristic product ions at m/z 301 and m/z 286 by the loss of 162 Da (glucose) and 177 Da (glucose + CH3). Combined with the corresponding standard substance, B54 was identified as Astraisoflavan-7-O-β-D-glucoside.
B55 afforded the [M−H]- ion at m/z 297.07740 (C17 H13O5) with mass error of 4.823 ppm. In its ESI-MS2 spectrum, it generated some characteristic product ions such as m/z 282, m/z 253, m/z 267 and m/z 279 through the successive loss of CH3, CO2, 2CH3, H2O, orderly. Based upon this, B55 was concluded to be Afromosin isomer. In addition, the ESI-MSn spectra of B9, B23, B57 and B61 were all illustrated in Fig. 5.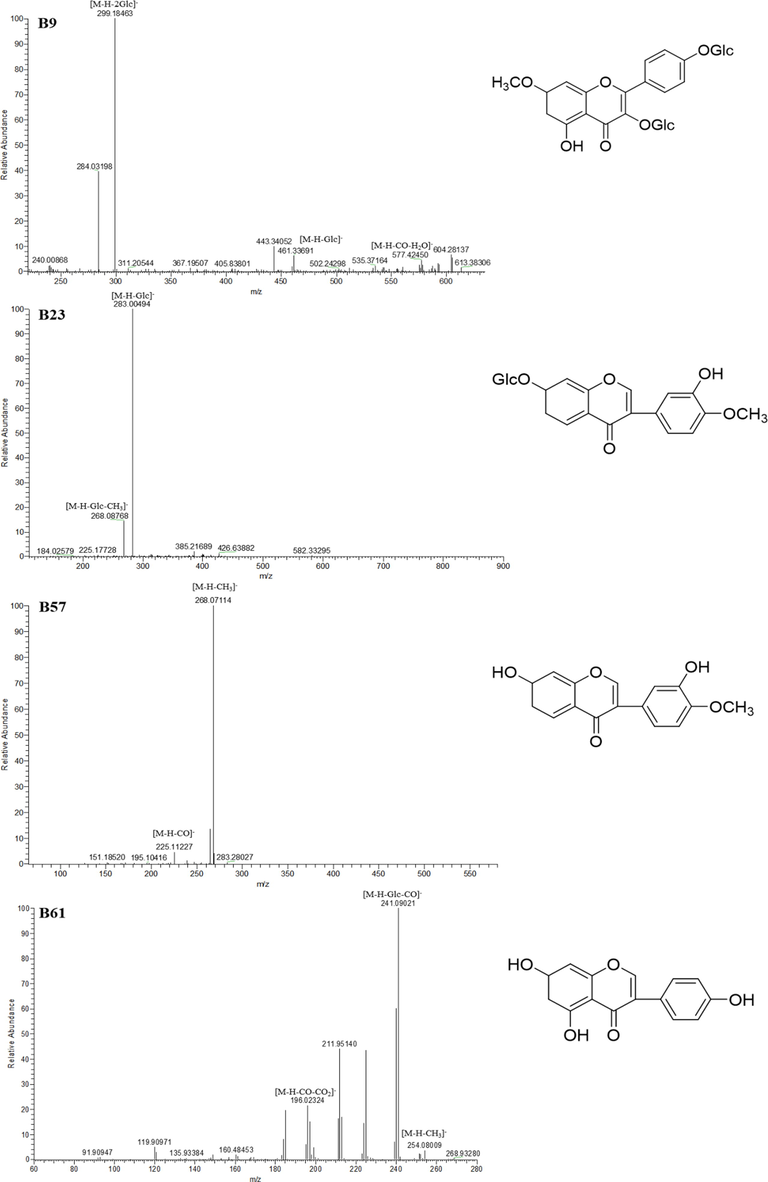
The ESI-MS2 spectra and chemical structures of B9, B23, B57 and B61.
For the reference substance with flavonoid structure, Calycosin-7-O-β-D- glucoside (B23*) yielded the [M−H]- ion at m/z 445.11457 (C22H21O10) with mass error of 3.351 ppm. In the ESI-MS2 spectra, a series of DPIs such as m/z 283, m/z 268 and m/z 255 were observed in negative mode. The existence of these molecular weights verified the loss of glucose (162 Da), glucose + CH3 (177 Da) and glucose + CO (190 Da), orderly. Its characteristic ions just like [M−H−Glu]-, [M−H−Glu−CH3]- and [M−H−Glu−CO]- could also be verified from the DPIs for flavonoids which have previously summarized. Therefore, DPIs mentioned above could be summarized the fragmentation regularities of group compositions and utilized for deducting the structures of related compounds from abundant complex constituents.
3.3 Comparative analysis of the main constituents existing in Astragali radix and SF-AP
Our previous study of liquid fermentation found that 42 constituents were attributed to saponins while the remaining 65 were identified as flavonoids [7]. However, in this report, coupled with the high-resolution mass data, obtained DPIs, retention time, standard references and related literatures, a total of 110 chemical constituents including 45 triterpene saponins and 65 flavonoids, while 109 components containing 41 triterpene saponins and 68 flavonoids were screened and identified from SF-AP and Astragali radix, respectively (Fig. 6). After comparing the results from SF-AP and Astragali radix, it could be found that the newly generated constituents after fermentation could be attributed to Azukisaponin V methyl ester, Huangqiyenin F, Huangqiyenin A, Huangqiyenin E and Astraisoflavan-7-O-β-D-glucoside. In the meantime, Astragalus flavonoids such as Calycosin-7-O-β-D-glucoside-6″-O-butylene ester, 5,7,4′-trihydroxy-isoflavonone, (3R)-7,2′,3′-trihydroxy-4′-methoxy isoflavonone and Calycosin were undetected after fermentation. Movever, by comparing these two fermentation methods, it was illustrated that many more isomeric constituents could be generated from solid fermentation, while some constituents such as Pratensein and Calycosin-7-O-β-D-glucoside-6″-O-butylene ester were only observed in liquid fermentation.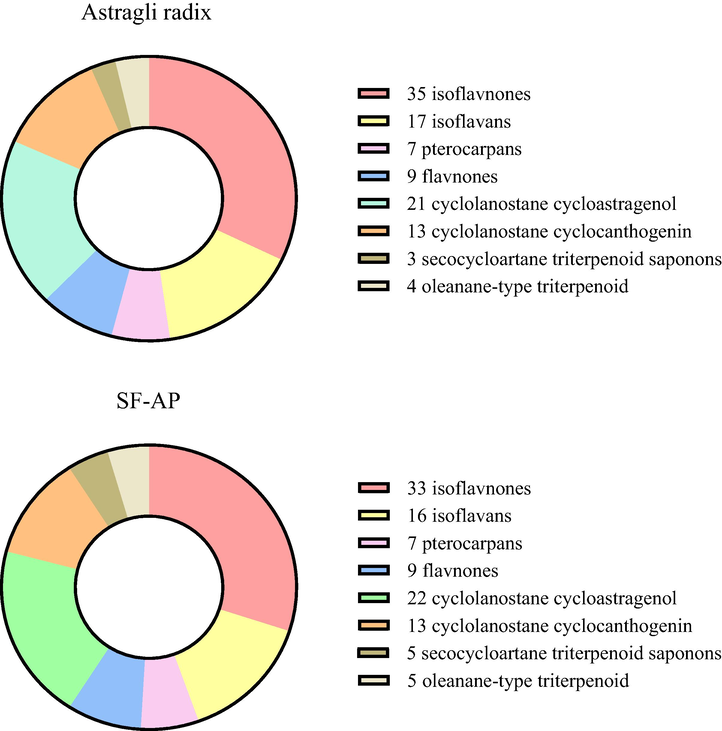
The composition of constituents existing in Astragli radix and SF-AP.
It is worth noting that the most obvious change during the fermentation transversion is the relative content of some representative components. According to the experimental results (Fig. 7), it could be seen that the relative content of some constituents were increased, such as Malonylastragaloside I, Soyasaponin I, Astragaloside IV, (3R)-8,2′-dihydroxy-7,4′-dimethoxy-isoflavan, Cyclocanthoside E, Astraisoflavan-7-O-β-D-glucoside, Odoratin-7-O-β-D-glucoside, Isoquercitrin, 5′-hydroxy isomucronulatol 2′,5′-di-O-glucoside, while some constituents including Astragaloside II, Astragaloside V, Formononetin, 3,9-dimethoxy-10- hydroxypterocarpan were observed with decreased relative content after the process of fermentation. Compared with the previous study of liquid fermentation, there were similar changes about the increase content of Astragaloside IV, but some other components like Cyclocanthoside E/isomers have no significant same change trend in fact.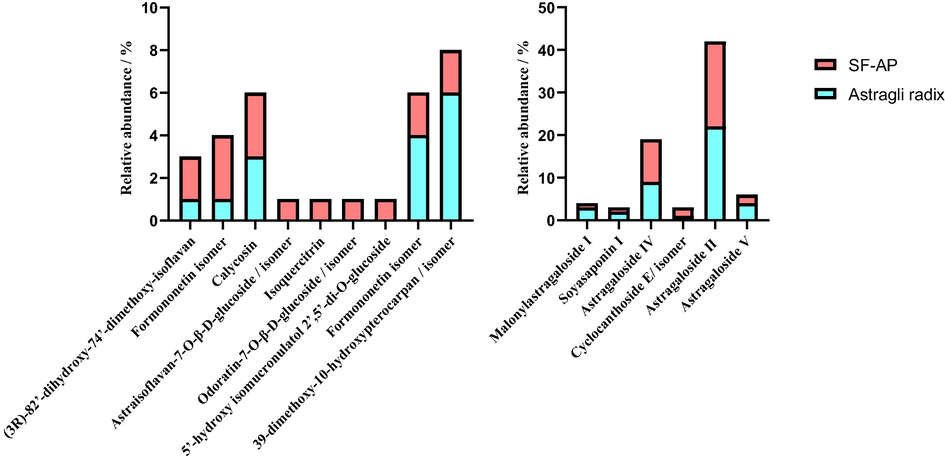
The changes of representative constituents including flavonoids and triterpene saponins before and after fermentation.
Among them, Astragaloside IV has various pharmacological activities especially in cardiovascular diseases, digestive diseases, cancer and the other modern high incidence, high-risk diseases (Ren et al., 2013; Zhang et al., 2006). Meanwhile, Astragaloside IV is officially used as a quality-marker for Astragali Radix in Chinese Pharmacopoeia (2015 version). The increased content of Astragaloside IV may be due to the loss of acetyl group or glucose moiety in the fermentation transversion of Astragaloside II and Astragaloside V and the other components (Fig. 8). In this sense, it could be deduced that the transformation during the fermentation process was more conducive to playing a therapeutic effect in clinical application.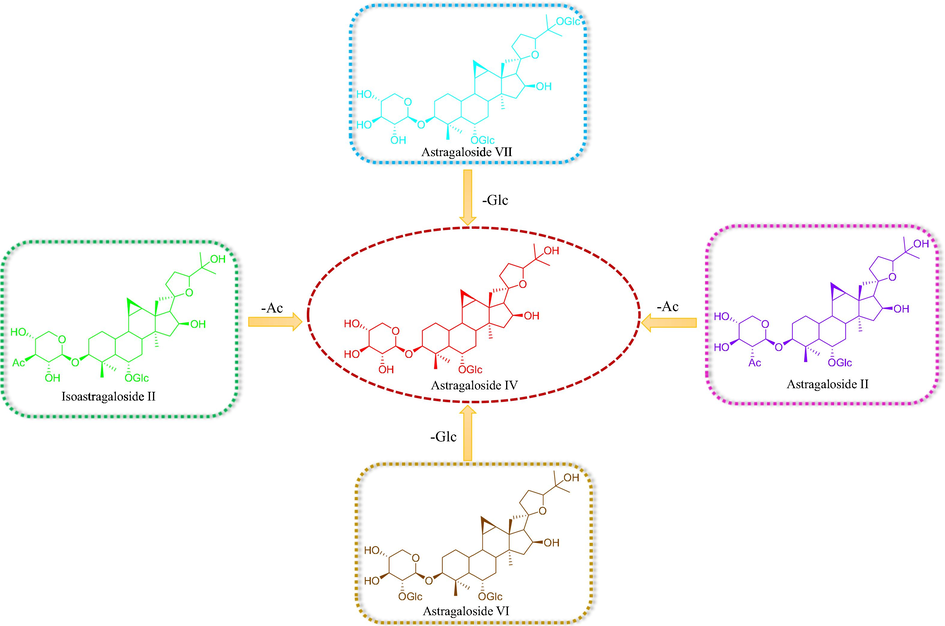
The proposed transformations after fermentation.
3.4 The antioxidative activity of Astragali radix and SF-AP
For further study, two kinds of antioxidative tests were chosen to evaluate the antioxidative activity of Astragali radix before and after solid fermentation. According to the results, the scavenging ability of the SF-AP to DPPH· was significantly improved by comparing with Astragali radix. By selecting the mass concentration range of the samples as 0.06 ∼ 0.84 mg/mL, the scavenging activity of Astragali radix increased from 6.21% to 36.02% while that of the SF-AP increased from 8.23% to 45.65%. In addition, with the increase of samples’ mass concentration, the scavenging ability of Astragali radix and SF-AP to ABTS·+ were enhanced. When the mass concentration was between 0.06 and 0.96 mg/mL, it could be seen that the scavenging ability of ABTS·+ of SF-AP was much higher than Astragali radix. While the mass concentration reached 0.96 mg/mL, the scavenging ability of Astragali radix to ABTS·+ was 49.5% while the scavenging ability of SF-AP was 57.4% (shown in Fig. 9).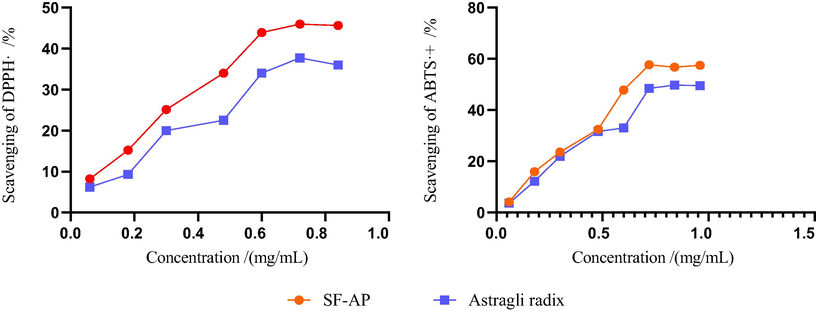
The evaluation of antioxidative activity in vitro.
The improvement of antioxidative activity of SF-AP is complex and a great many factors are attributed to it. What’s more, some physical and chemical changes during the fermentation process played the decisive part on the antioxidative activity of Astragali radix. For instance, a varieties of secondary metabolites produced after fermentation: some methoxylated flavones lost methoxy(s) and then produced a great deal of OH-flavones; some flavonoid glycosides were hydrolyzed into aglycones, which could also increase the antioxidative activity.
4 Conclusion
In this study, UHPLC-LTQ-Orbitrap MS was used to acquire chemical profiles of Astragali radix and SF-AP. Combining with the fragmentation rules, chromatographic behavior, DPIs and related literature data, 114 compounds including 45 saponins and 69 flavonoids were finally identified in both positive and negative ion modes. By comparison with Astragali radix, some components contained in SF-AP had significant chemical changes such as content fluctuation and isomerism owing to the occurrence of hydrolysis and other conversion reactions. Moreover, two kinds of antioxidative tests were applied to evaluate the antioxidative activity of Astragali radix before and after fermentation. The antioxidative activity of SF-AP in two kinds of antioxidative tests corresponding to the scavenging of DPPH· and ABTS·+ were both significantly higher than that of Astragali radix.
Based on the comparison of the above two aspects, fermentation can improve the internal conversion efficiency and the content of compounds, so as to improve the therapeutic effect. Although the specific chemical transformation mechanism during the fermentation process still needs further exploration, this study set a good example for the comprehensive chemical identification and much more in-depth pharmacodynamics study of the fermentation system between microbiota and Chinese herbal medicines.
Acknowledgments
This work has been financially supported by Young and Creative Team for Talent Introduction of Shandong Province, Binzhou Medical University Scientific Research Fund for High-level Talents (2019KYQD06), Locality-University Cooperation Project of Yantai City (2019XDRHXMPT18), and Independent Topic Selection of Beijing University of Chinese Medicine (2019-JYB-XSCXCY-06).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Radix Astragali (Astragalus): Latest Advancements and Trends in Chemistry, Analysis. Pharmacol. Pharmacokinet. [J] Curr. Org. Chem.. 2010;14:1792-1807.
- [Google Scholar]
- Fragmentation study of iridoid glucosides through positive and negative electrospray ionization, collision-induced dissociation and tandem mass spectrometry[J] Rapid Commun. Mass SP.. 2007;21:1165-1175.
- [Google Scholar]
- Review of the Botanical Characteristics, Phytochemistry, and Pharmacology of Astragalus membranaceus (Huangqi)[J] Phytother. Res. Ptr.. 2015;28:1275-1283.
- [Google Scholar]
- ABTS•+ scavenging activity of polypyrrole, polyaniline and poly(3,4-ethylenedioxythiophene)[J] Polym. Int.. 2011;60:69-77.
- [Google Scholar]
- Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines[J] Food Res. Int.. 2016;81:1-16.
- [Google Scholar]
- Chinese Vinegar and its Solid-State Fermentation Process[J] Food Rev. Int.. 2004;20:407-424.
- [Google Scholar]
- Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review[J] Biotechnol. Adv.. 2011;29:365-373.
- [Google Scholar]
- Balancing Herbal Medicine and Functional Food for Prevention and Treatment of Cardiometabolic Diseases through Modulating Gut Microbiota[J] Front. Microbiol.. 2017;8:2146.
- [Google Scholar]
- Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry[J] Rapid Commun. Mass SP.. 2007;21:3039-3050.
- [Google Scholar]
- Pharmacological effects of Astragaloside IV: a literature review[J] J. Tradit. Chin. Med.. 2013;33:413-416.
- [Google Scholar]
- An integrated strategy for rapid discovery and identification of the sequential piperine metabolites in rats using ultra high-performance liquid chromatography/high resolution mass spectrometery[J] J. Pharmaceut. Biomed.. 2017;146:387-401.
- [Google Scholar]
- Fermented functional foods based on probiotics and their biogenic metabolites[J] Curr. Opin. Biotechnol.. 2005;16:198-203.
- [Google Scholar]
- Chemical Constituent Profifiling of Paecilomyces cicadae Liquid Fermentation for Astragli Radix. [J] Molecules. 2019;24:2948-2968.
- [Google Scholar]
- Effect of Bidirectional Fermentation System of Paecilomyces cicadae /Astragalus Membranaceus of in Hyperuricemia Models and Study on Its Components[J] Modern Chin. Med.. 2019;11:012.
- [Google Scholar]
- Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar[J] Sci. Rep.-UK. 2016;6:26818.
- [Google Scholar]
- A systematic, comparative study on the beneficial health components and antioxidative activities of commercially fermented soy products marketed in China[J] Food Chem.. 2015;174:202-213.
- [Google Scholar]
- Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry[J] J. Pharmaceut. Biomed.. 2011;56:950-961.
- [Google Scholar]
- Effect of the Polysaccharides of Fermentation of Paecilomyces Cicadae for Glycyrrhiza Residue on Immune Activity[J] Pharm. Biotechnol.. 2017;2:129-132.
- [Google Scholar]
- Establishment of Bidirectional Fermentation System of Paecilomyces cicadae/Astragalus Membranaceus and Study on Its Components[J] World Chin. Med.. 2018;13:270-273.
- [Google Scholar]
- Astragaloside IV from Astragalus membranaceus Shows Cardioprotection during Myocardial Ischemia in vivo and in vitro[J] Planta Med.. 2006;72:4-8.
- [Google Scholar]
- Rapid Screening and Identification of Daidzein Metabolites in Rats Based on UHPLC-LTQ-Orbitrap Mass Spectrometry Coupled with Data-Mining Technologies. [J] Molecules.. 2018;23:151.
- [Google Scholar]
- Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction[J] Carbohyd. Polym.. 2012;89:694-700.
- [Google Scholar]







