Translate this page into:
Dual recognition strategy for ultra-sensitive fluorescent detection of Hg2+ at femto-molar level based on aptamer functionalized sulfur quantum dots
⁎Corresponding authors. guodawei0298@163.com (Wei Guo), xmzhuang@iccas.ac.cn (Xuming Zhuang), zhaoljytu@ytu.edu.cn (Lijun Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study, a dual recognition strategy for ultrasensitive detection of Hg2+ was successfully developed for the first time based on aptamer functionalized sulfur quantum dots (Apt-SQDs). The developed Apt-SQDs not only retained the good fluorescence properties of quantum dots but also overcame the problem of poor selectivity of SQDs for heavy metal ions. This system used the dual recognition strategy, including the combination of Sx2− and Hg2+ and T-Hg2+-T structures to excellently identify and capture Hg2+, and an ultrahigh sensitivity fluorescent aptasensor was fabricated. The fluorescent aptasensor had a good response to Hg2+ at concentrations ranging of 10−15 to 10−7 M with an ultralow limit of detection of 0.3 fM, and the response to other metal ions was far less than that to Hg2+. It was successfully applied to detect Hg2+ in nearby environmental water samples (tap water, lake water and river water) with a good recovery rate. Moreover, portable test papers that would be useful for Hg2+ monitoring in environmental water were designed. The dual recognition strategy not only achieves ultrasensitive fluorescent detection of Hg2+ but also provides a new insight into the further expansion of the application of SQDs.
Keywords
Dual recognition strategy
Sulfur quantum dots
Aptamer
Mercury ions
Ultrasensitive
1 Introduction
Heavy metal contamination has always been one of the inevitable problems of human society. Although some heavy metal elements (Cu2+, Zn2+, etc.) (Takeda et al., 2014; Kukic et al., 2014; Han et al., 2017) are essential trace elements for biological activities, most of the heavy metal elements (Morales et al., 2015) cause irreversible damage to organisms, especially the human body (Abraham et al., 2021; Hino et al., 2010; Li et al., 2020a,b). Mercury (Hg2+) is one of the most harmful heavy metal elements. It has been proven to not only display a strong affinity for sulfhydryl groups in the body, allowing it to interact with most sulfhydryl-containing substances, such as proteins and important enzymes involved in the metabolism of substances in the body (cytochrome oxidase (Sugio et al., 2010), succinate dehydrogenase (Tamás and Zelinová, 2017) and lactate dehydrogenase (Ozkan, 2011), and subsequently inactivate the enzyme and harm human health but also cause kidney failure and a variety of neurological symptoms (movement disorders (Taylor et al., 2018), Minamata disease (Eto et al., 2010), etc.). Once Hg2+ enters the human body, it is difficult to remove and causes serious damage to the human body. Therefore, timely monitoring of ultratrace Hg2+ concentrations in the environment might effectively prevent bioenrichment and avoid eventual entry into the human body. Therefore, the ultrasensitive detection of Hg2+ is very important. At present, approaches based on various materials have been developed to detect Hg2+, including electrochemistry (Yerga et al., 2013), electrochemiluminescence (Zhao and Zhou, 2012), surface-enhanced Raman spectroscopy (Li et al., 2018) and fluorescence (Lu et al., 2015) methods, such as Yuan and his coworkers summarized some small molecular fluorescent probes for detection of Hg2+ developed in recent years (Yuan et al., 2022). Shellaiah and Sun reviewed metal–organic frameworks used for detecting and removing elemental mercury and Hg2+ (Shellaiah and Sun, 2021). Amico and his group studied some research progress using micro extraction techniques in determination of environmental Hg2+ (Amico et al., 2022). Daniel and his colleges reported many electrochemical methods for mercury analysis in various samples (Daniel et al., 2013). In particular, the fluorescence method has attracted much attention because of its advantages of high sensitivity, simple operation and a fast detection time. Liu and colleagues designed and developed a highly sensitive fluorescence probe based on anthraquinone derivatives for the detection of Hg2+ (Liu et al., 2021a,b,c). Ma and his colleagues efficiently synthesized a novel tetraphenylene-based fluorescence probe based on aggregation-induced emission for the sensitive detection of Hg2+ (Ma et al., 2018). In recent years, a fluorescent aptasensor technology developed based on the fluorescence method has gradually attracted the attention of the larger public. Although many sensors based on aptamers have been developed (Tabaraki and Rahmatinya, 2021; Peng et al., 2018a,b), significant challenges remain in the detection of ultratrace Hg2+ concentrations.
Fluorescent aptasensors that combine the high specificity of aptamers with the good sensitivity of the fluorescence method exhibit good analytical performance (Zhou et al., 2019). With aptamers as recognition units and new fluorescent dyes as conversion labeling materials, the sensor further develops in the direction of less sample consumption, high specificity and sensitivity, a fast response speed and short reaction time, playing an increasingly important role in life science detection (Hassan and Derosa, 2020), food safety (Guo et al., 2014) and environmental monitoring (Peng et al., 2018a,b). Chiu and Huang reviewed the recent development of biosensors with functional adaptors and different types of nanomaterials (Chiu and Huang, 2009). Shi and coworkers constructed a fluorescent aptasensor based on aptamer-modified copper@gold nanoclusters to detect Hg2+ (Shi et al., 2021). Hu and colleagues constructed an ultrasensitive homogeneous fluorometric assay for Hg2+ based on gold nanoparticles and CdTe quantum dots (Hu et al., 2016). Although the performance of these fluorescent aptasensors is good, the difficulties in preparation, significant biotoxicity and high cost of these materials obviously limit their applications. A cheap, easy-to-prepare, low-toxicity and environmentally friendly nanomaterial is needed to construct fluorescent aptasensors.
In recent years, sulfur quantum dots (SQDs) have become a new pure element of quantum dot nanomaterials. SQDs not only retain the good optical properties of traditional quantum dots but also overcome potential problems, such as heavy metal pollution, poor biocompatibility and harsh synthetic conditions. Bao and colleagues reported a simple method for the detection of cobalt ions based on SQDs fluorescence quenching (Bao et al., 2019). Chen and colleagues developed a highly sensitive and selective fluorescence sensor for the detection of silver ions (Ag+) using SQDs (Chen et al., 2016). Fu et al. established an electrochemical sensor for Ag+ determination based on an SQDs-modified Au electrode (Fu et al., 2020). However, the postsynthetic modification of SQDs synthesized with polyethylene glycol-400 (PEG-400) as a passivator is difficult, and some heavy metal elements easily combine with sulfur ions, which results in limited selectivity of SQDs in heavy metal ion detection. Therefore, using SQDs as a fluorescence probe to detect heavy metal ions has certain limitations, which has hindered the development of Hg2+ detection strategies. As a method to solve this problem, an efficient enrichment method for Hg2+ based on the thymine-Hg2+-thymine structure (T-Hg2+-T) has attracted widespread attention and become an effective method for the detection of Hg2+. Zhang and his colleagues designed a fluorescence detection method for Hg2+ in aqueous solution based on n-methyl porphyrin IX/G-quadruple DNA using T-Hg2+-T (Zhang et al., 2017). Shi and his coworkers designed a photochemical method for Hg2+ determination based on T-Hg2+-T with double signal amplification (Shi et al., 2017). Xu and colleagues developed a molecular imprinting technology strategy based on T-Hg2+-T to enrich Hg2+ in water samples (Xu et al., 2012). Inspired by the studies described above, we intend to combine these two technologies and utilize their synergistic effects to achieve ultrasensitive detection of Hg2+.
In the present study, we improved the synthesis method through the successful postsynthetic modification of SQDs with amino groups (NH2-SQDs) and combined these materials with the aptamer of modified carboxyl groups (—COOH) to form the desired fluorescence probe (Apt-SQDs). Combined with the capacity of SQDs to interact with Hg2+ and the enrichment function of the T-Hg2+-T structure, an ultrasensitive fluorescent aptasensor was established to detect Hg2+ through a dual recognition strategy. The mechanism of the fluorescent aptasensor is shown in Scheme 1. On the one hand, the mechanism is attributed to the strong capacity of Sx2− to bind Hg2+. On the other hand, it is attributed to the effective enrichment of Hg2+ by the aptamer. The synergism of the dual recognition strategy may substantially improve the sensitivity of Hg2+ detection and achieve the ultrasensitive detection of Hg2+. Benefiting from the selectivity and sensitivity of the developed strategies, the fluorescent aptasensor provided a useful tool for evaluating Hg2+ contamination in water samples.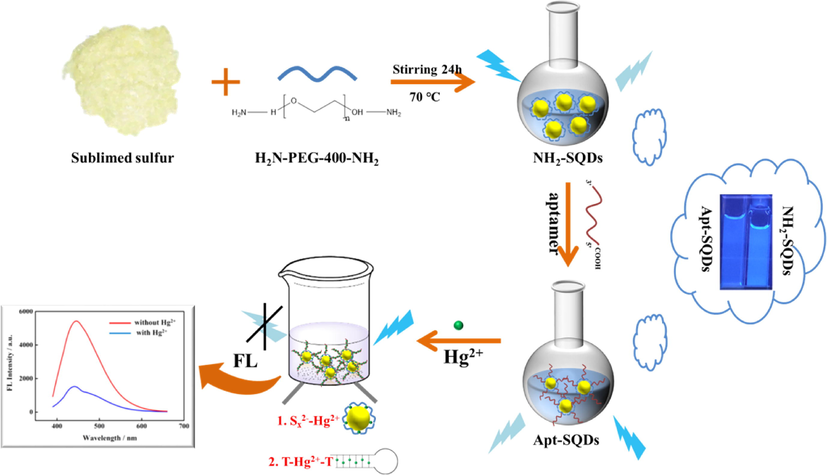
Mechanism of the fluorescent aptasensor based on Apt-SQDs.
2 Experimental
2.1 Reagents and apparatus
Sublimated sulfur powder, NaOH, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N-hydroxy succinimide (NHS) and Hg2+ were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Hydrogen peroxide (H2O2, V/V 30%) and other metal ions (Ni2+, Mn2+, Fe3+, Zn2+, Co2+, Cd2+, Pb2+, Cr3+, Cu2+, Al3+, Mg2+ and Fe2+) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Tianjin, China). NH2-polyethylene glycol-400-NH2 (NH2-PEG-400-NH2) was synthesized by Huawei Ruike Chemical Co., Ltd. (Beijing, China). Dialysis membranes (1000 Da) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All chemicals were of analytical reagent grade. All the solutions used in this experiment were prepared with ultrapure water (18.2 MΩ cm). The aptamer used in this study was obtained from Sangon Biotechnology Co., Ltd. (Shanghai, China). The base sequence (5́-3́) of the oligonucleotide was 5́–HOOC-(CH2)6-TTTTTTTTTTTT-3́.
High-resolution transmission electron microscopy (HR-TEM) images were captured using a JEM-2100F transmission electron microscope at an accelerating voltage of 200 kV (JEOL Ltd, Japan). A Nicolet 5700 Fourier transform infrared (FT-IR) spectrometer (Thermo Electron Corporation, USA) was used to obtain FT-IR spectra. Ultraviolet–visible absorption spectra (UV–vis) were recorded using an A560 ultraviolet–visible spectrometer (AOE Instruments, Shanghai) at a wavelength interval of 2 nm. X-ray photoelectron spectra (XPS) were measured using a Thermo ESCALAB-250 instrument (Thermo Fisher Scientific, USA). The fluorescence emission spectrum was obtained using an F-4700 fluorescence spectrophotometer (HITACHI, Japan). X-ray diffraction spectra (XRD) were measured using a SmartLab3 instrument (Rigaku Corporation, Japan).
2.2 Synthesis of NH2-SQDs
The NH2-SQDs was prepared according to a previous report (Wang et al., 2019). Briefly, 3 mL of NH2-PEG-400-NH2 and 50 mL of the prepared NaOH solution (0.08 g mL−1) were added to 1.4 g of sublimated sulfur powder and stirred in an oil bath at 70 °C for 24 h. During this period, the color of the solution gradually changed from dark yellow to light yellow, and then 3 mL of H2O2 were added to it for etching to prepare NH2-SQDs with good fluorescence performance. NH2-SQDs were poured into dialysis membranes (with a molecular weight of 1000 Da) to remove unreacted small molecules, and dialysis was performed for 72 h with a water change every 12 h. Afterward, a light yellow solid was obtained by freeze-drying at −20 °C for 24 h. The synthesized solid NH2-SQDs were stored at 4 °C until use in subsequent experiments.
2.3 Synthesis of Apt-SQDs
The Apt-SQDs was accomplished using a previously published method (Pasanphan and Chirachanchai, 2008). First, aptamers modified with —COOH (Apt-COOH, 10 mL) were activated by EDC (250 mg) and NHS (120 mg) with stirring for 1.5 h at room temperature to promote a better interaction with NH2-SQDs. NH2-SQDs was added and allowed to fully react with the activated —COOH for 2 h at room temperature to obtain Apt-SQDs, which were stored at 4 °C until use in subsequent experiments.
2.4 Fabrication of ultrasensitive fluorescent aptasensors
A series of experiments was designed to explore the sensitivity of the fluorescence sensing platform based on NH2-SQDs. First, the fluorescence properties of Apt-SQDs were fully measured and compared with those of pure SQDs under the same conditions. Next, different concentrations of Hg2+ solutions (10−15 to 10−7 M) were prepared. Dilute 10 µL of the original Hg2+ solution (10−3 M) to 100 times (10−5 M) by adding 990 µL of ultrapure water. According to these steps, the different concentrations of Hg2+ that we want could be obtained. But we should ensure that the number of dilutions should be as small as possible to ensure the accuracy of the result. Then, different concentrations of Hg2+ solutions were added to the Apt-SQDs in a cuvette, and a fluorescence spectrophotometer was used to record the fluorescence quenching of Apt-SQDs by Hg2+.
2.5 Quantum yield (QY) measurement
The QY of SQDs, NH2-SQDs and Apt-SQDs were determined with reference to quinine sulfate (in 0.1 M H2SO4). The calculation of QY is according to the following equation: where ‘Q’ refers to the quantum yield, ‘I’ and ‘A’ stand for emission intensity and absorbance, respectively. ‘η’ is the refractive index of the solvent. The ‘R’ subscripts represent reference standard (QR = 0.54).
2.6 Stern-Volmer plot
For fluorescence quenching analysis, a Stern–Volmer plot (F0/F and [Q] relationship) was used according to the following equation: where F0 and F are the fluorescence intensities in the absence and presence of quencher, respectively, and [Q] is the quencher concentration. The Stern–Volmer constant, KSV, provides a direct measure of the quenching efficiencies and is determined from the linear portion of the Stern–Volmer plot.
2.7 Determination of Hg2+ in real samples
Water samples were collected from three different areas in Yantai City to evaluate the performance of the proposed fluorescence sensing platform. The collected samples were incubated for 2 h and then filtered through a 0.45 μm membrane to remove large particles of impurities and suspended solids. After treatment, the samples were stored at 4 °C to prevent bacterial growth. After the aforementioned treatment, Hg2+ was detected using the ultrasensitive fluorescence sensing platform, and different concentrations of Hg2+ were added to the real sample to evaluate the detectability of the fluorescence sensing platform in real samples.
2.8 Preparation of test papers
Because Hg2+ exhibits a high level of biological toxicity, an effective and convenient method for monitoring Hg2+ concentrations in environmental water samples must be developed. The test paper based on the Apt-SQDs quickly detected trace Hg2+ in environmental water samples through colorimetry. According to previous reports (Guo et al., 2020), the filter paper was cut into 1 × 1.5 cm pieces, which were immersed into the Apt-SQD solution. Then, the filter papers were dried at room temperature. Finally, filter papers were treated with different concentrations of Hg2+ and reacted for 5 min. The color changes were recorded by irradiation with a 365 nm UV lamp.
3 Results and discussion
3.1 Characterization of NH2-SQDs and Apt-SQDs
The morphology and particle size distribution of the prepared SQDs, NH2-SQDs and Apt-SQDs were characterized using HR-TEM. Fig. 1A-C and the insets show the TEM and HR-TEM images of SQDs, NH2-SQDs and Apt-SQDs, respectively. The morphologies of the three preparations were highly similar and all of them were spherical with good dispersion, suggesting that the original structure of SQDs was not damaged in the process of functionalization. However, in Fig. 1D-F, SQDs had the smallest particle size (∼2.5 nm), followed by NH2-SQDs (∼4 nm), and Apt-SQDs had the largest particle size (∼6 nm), which may be caused by the gradual increase in SQDs functionalization. Fig. S1 showed the dynamic light scattering (DLS) spectra of SQDs, indicating that the average particle size of prepared SQDs was about 4 nm.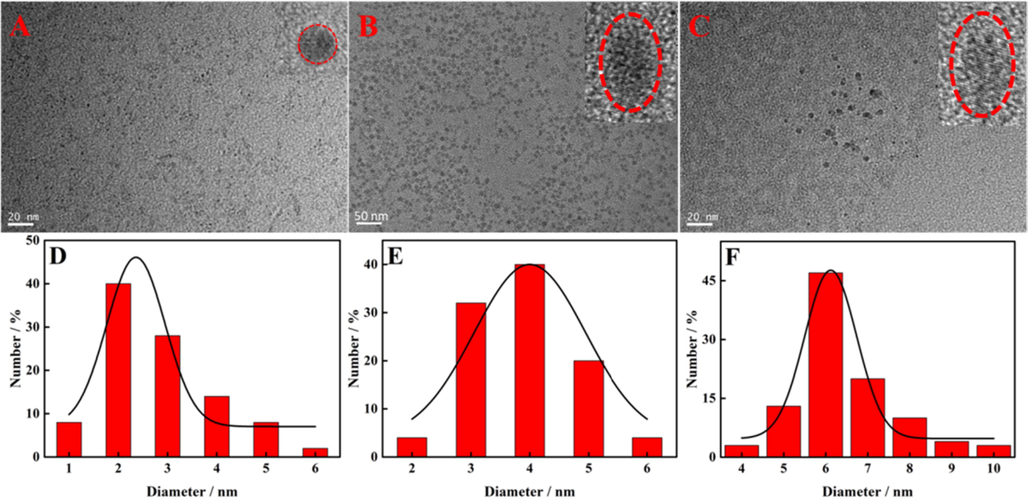
TEM images of (A) SQDs, (B) NH2-SQDs and (C) Apt-SQDs. Insets show HR-TEM images of SQDs, NH2-SQDs and Apt-SQDs at 5 nm. Histogram showing the size distribution of (D) SQDs, (E) NH2-SQDs and (F) Apt-SQDs.
Fig. 2A shows the UV–Vis spectra of SQDs (black curve), NH2-SQDs (red curve) and Apt-SQDs (blue curve). At 303 nm, all three nanomaterials produced obvious absorption peaks, potentially due to the adsorption of S22− on the surface of the SQDs 33. The spectrum (red curve) of NH2-SQDs was similar to that of SQDs (black curve). However, at approximately 350 nm, NH2-SQDs produced a small and not obvious absorption peak, which may indicate that other Sx2− molecules were adsorbed on the surface of SQDs during functionalization, similar to the situation reported in the literature 33. However, Apt-SQDs (blue curve) generated a weak absorption peak at 370 nm due to the weak absorption of the carbonyl group of the amide bond and the conjugation of N, and thus we estimated that aptamer had successfully combined with SQDs to generate Apt-SQDs. And the UV–Vis spectra of aptamer was shown in Fig. S2. An obvious absorption peak was observed at 260 nm, which was characteristic of aptamer. In addition, FT-IR spectra of NH2-SQDs (black curve) and Apt-SQDs (red curve) were recorded to further confirm the successful preparation of Apt-SQDs, as shown in Fig. 2B. Both NH2-SQDs and Apt-SQDs produced characteristic peaks at 3400 and 2870 cm−1 of SQDs, similar to previous reports (Wang et al., 2019). However, the two nanomaterials showed N-H stretching vibration absorption peaks and N-H bending vibration peaks at 3300 and 1500 cm−1, respectively, while Apt-SQDs had C⚌O stretching vibration peaks at 1700 cm−1. Therefore, Apt-SQDs was successfully synthesized and were applied in the next experiment. FT-IR spectra were analyzed to further verify our conclusions, as shown in Fig. S3. Compared with Fig. 2B, characteristic peaks were located at 3300, 2870, 1680 and 1500 cm−1, and characteristic peaks of thymine were located at 3509, 1870, 1196 and 549 cm−1, which was also consistent with a previous report (Bader, 1991), proving that the binding mode of Apt-SQDs to Hg2+ was mainly attributed to the T-Hg2+-T structure. Fig. 2C and 2D further reveal the composition of Apt-SQDs analyzed using the XPS approach. Fig. 2C shows the presence of mainly four different peaks in the spectrum, indicating that Apt-SQDs mainly contain C, O, N and S. The S peak was selected for further study, and the amplified peak is shown in Fig. 2D. As shown in the figure, the S peak was subdivided into five peaks in the high-resolution XPS spectrum at 161.72, 162.86, 166.86, 168.01 and 169.06 eV. The peaks at 161.72 and 162.86 eV were attributed to S, while the peaks at 166.86 and 168.01 eV were attributed to SO32− (2p2/3), and the peak at 169.06 eV was attributed to SO32− (2p1/2). Therefore, abundant groups were present on the surface of Apt-SQDs (Wang et al., 2019). The XRD pattern of SQDs presented many sharp peaks, as is shown in Fig. S4, indicating its better crystalline properties, which is similar to previous reports (Chen et al., 2016). The QY of SQDs and Apt-SQDs were 5.3% and 3.4%, respectively, which proves that functionalization has no great impact on SQDs performance. When Hg2+ was present, QY of SQDs and Apt-SQDs decreased to 1.4% and 1.1%, respectively, indicating that the prepared material is responsive to Hg2+. The fluorescence lifetime of SQDs and Apt-SQDs were shown in Fig. S5, exhibiting fluorescence lifetime with value of 6.4 ns, which was superior to existing organic fluorophores (generally less than 5 ns). Zeta potential of SQDs, NH2-SQDs and Apt-SQDs were shown in Fig. S6. It can be seen that the surface of SQDs have many negative groups. Due to –NH2 is the electron-donating group, NH2-SQDs has a more negative charge on its surface, so the zeta potential of NH2-SQDs was more negative than SQDs. As for Apt-SQDs, the aptamer carries more negative charge, which is consistent with the data obtained in Fig. S5 (white column). However, after the addition of Hg2+, the HgS and T-Hg2+-T structure reduces the negative charge on the surface of SQDs, NH2-SQDs and Apt-SQDs, so the Zeta potential increases (gray column).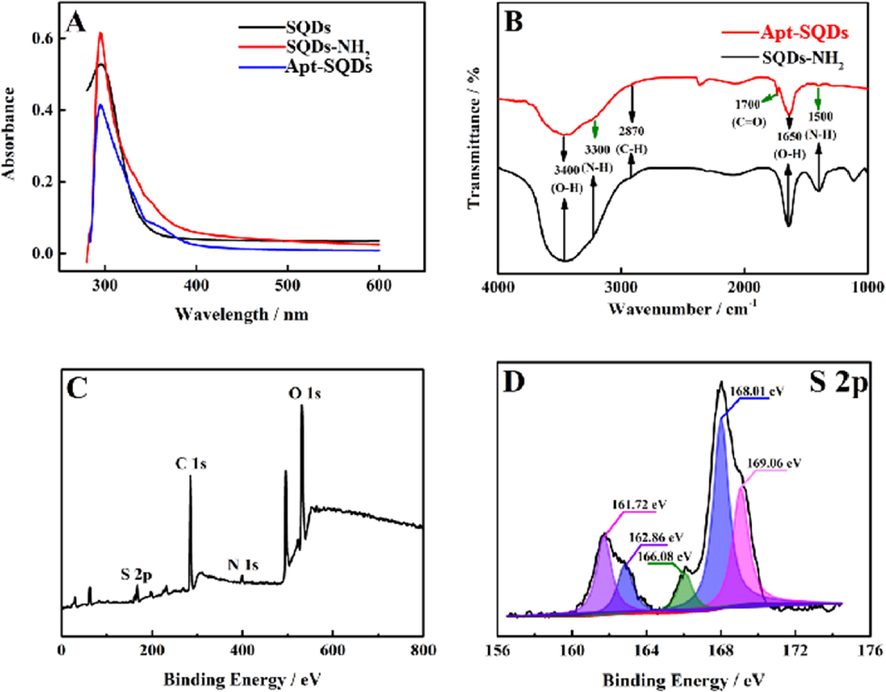
(A) UV–Vis spectra of SQDs (black curve), NH2-SQDs (red curve) and Apt-SQDs (blue curve). (B) FT-IR spectra of NH2-SQDs (black curve) and Apt-SQDs (red curve). (C) XPS survey spectrum and (D) high-resolution S 2p XPS spectrum of NH2-SQDs.
3.2 Quenching mechanism of dual recognition strategy for Hg2+
XPS spectra were recorded to further study the modes of NH2-SQDs and Apt-SQDs binding to Hg2+, as shown in Fig. 3. As shown in Fig. 3A-H, the binding modes of NH2-SQDs and Apt-SQDs with Hg2+ were thoroughly studied. The elemental analysis is shown in Fig. 3A and E, and the SQDs contained Hg, S, C, N and O elements, as expected. Fig. 3B-D shows Hg 4f, S 2p and N 1s spectra, respectively. Fig. 3B shows the XPS spectrum of Hg 4f electrons to reveal the binding modes of NH2-SQDs with Hg2+. The peak at 100.9 eV was attributed to HgS. This result indicated that HgS was the main form of Hg binding to NH2-SQDs. Fig. 3C shows the spectrum of S 2p electrons. The peak at 161.5 eV was also attributed to HgS, which also proved the conclusion listed above that Hg and NH2-SQDs interacted through HgS. Fig. 3F shows the XPS spectrum of Hg 4f electrons to reveal the binding modes of Apt-SQDs with Hg2+. The peak at 100.9 eV in Fig. 3F was much smaller than that in Fig. 3B, which proved that the binding mode of HgS still existed in the interaction of Apt-SQDs and Hg2+, but it was not the main mode. A similar conclusion was drawn from the comparison between the data presented in Fig. 3G and Fig. 3C. The comparison between Fig. 3H and Fig. 3D shows that the peak at 403.4 eV shifted to 401.9 eV, suggesting that the binding mode of Apt-SQDs and Hg2+ was indeed different from that of NH2-SQDs and Hg2+, which may be caused by the T-Hg2+-T structure.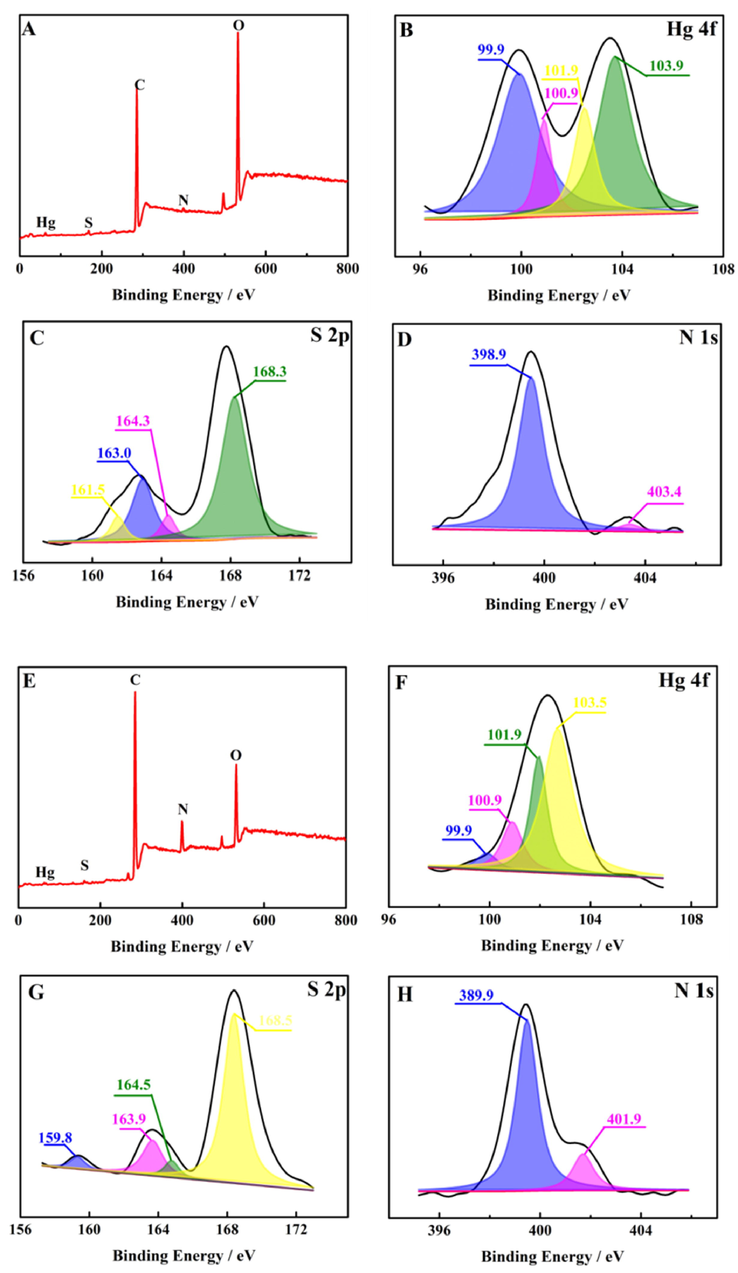
XPS results for (A) NH2-SQDs + Hg2+ and high-resolution (B) Hg 4f, (C) S 2p, (D) N 1 s spectra. XPS results for (E) Apt-SQDs + Hg2+ and high-resolution (F) Hg 4f, (G) S 2p, (H) N 1 s spectra.
In order to further proving the quenching mechanism of dual recognition strategy for Hg2+, the FT-IR of aptamer was measured in Fig. S7. Comparing with Fig. S3, it can be seen clearly that it also included N-H stretching vibration absorption peaks, N-H bending vibration peaks and C⚌O stretching vibration peaks, indicating the adaptor structure was not destroyed during the process of functionalization. However, when the solution exists Hg2+, characteristic peak of aptamer would produce blue shift, attributed to T-Hg2+-T structure.
3.3 Optimizations of conditions
Some optimization experiments were performed to explore the optimal experimental conditions. As shown in Fig. S8A, when Hg2+ was added, the fluorescence intensity of Apt-SQDs decreased rapidly, and did not change further after 1 min. Based on this result, Apt-SQDs was useful for Hg2+ detection, their response speed to Hg2+ detection was extremely fast, and the detection was basically completed within 1 min. The inset showed the quenching of fluorescence intensity in the presence of Hg2+. Fig. S8B shows the results for the detection of Hg2+ with Apt-SQDs at different pH values. The fluorescence of Apt-SQDs showed excellent pH stability, and the fluorescence intensity changed by only 5% in the range of pH 4.0–12.0. When Hg2+ was added, SQDs showed a good response in the range of pH 6.0–8.0, and 7.0 was selected as the final pH. We speculate that the mechanism of high pH affecting Apt-SQDs fluorescence may be: when pH is too high, Hg2+ generates Hg(OH)2 under alkaline conditions. Due to its low solubility, there is not enough Hg2+ in the solution to react with Apt-SQDs. Fig. S8C shows the fluorescence spectra of SQDs with different concentration (10−1, 1, 2, 4, 6, 8 and 10 mg mL−1). It can be seen that with the increase of SQDs concentration, fluorescence intensity increased first and then decreased, and reached the maximum at 1 mg mL−1. The decrease of fluorescence intensity of SQDs at high concentration may be attributed to the fluorescence quenching caused by particle aggregation. The influence of ion strength on SQDs fluorescence intensity was explored, and the results were shown in Fig. S8D. Different concentrations of NaCl (10−4, 10−3, 10−2 and 10−1 M) was selected as testing reagent. The result shows that within a certain range of ionic strength, the fluorescence intensity of SQDs almost unchanged.
3.4 Performance of Apt-SQDs for Hg2+ detection
Fig. 4A shows the fluorescence spectra recorded after adding Hg2+ solutions with different concentrations (10−15 to 10−7 M) to Apt-SQDs (1 mg mL−1, pH = 7.4 in PBS). The fluorescence signal of Apt-SQDs decreased gradually with the addition of increasing Hg2+ concentrations. The quantitative curves for different concentrations of Hg2+ were obtained under certain conditions. Corresponding standard curves were prepared according to the degree of fluorescence quenching, as shown in Fig. 4B. When the Hg2+ concentration ranged from 10−15 to 10−7 M, the linear equation was ΔI = 419.8 lgc(Hg2+) + 7268 (R2 = 0.9956) with a low limit of detection (LOD) of 0.3 fM, indicating the effective detection of Hg2+ at ultralow concentrations (ΔI = F0 - F, where F0 represents the initial fluorescence intensity and F represents the fluorescence intensity after adding Hg2+). In order to further prove the sensitivity of Apt-SQDs to Hg2+, the fluorescence spectra for NH2-SQDs detecting various concentrations of Hg2+ was supplemented, as shown in Fig. S9. By comparison with Fig. 4, it can be clearly seen that the sensitivity of Apt-SQDs is better than NH2-SQDs. Meanwhile, we speculate that Apt-SQDs has better selectivity to Hg2+ than NH2-SQDs. In addition, Table S1 summarizes the performance of several existing Hg2+ sensors and reported detection methods in terms of the LOD and detection range. Compared with other methods, the sensor platform had a lower LOD and a wider detection range, which met the increasingly rigorous requirements for real sample detection. Table S2 summarizes the detection methods of Hg2+ based on CQDs. It can be seen from the table that our material sensitivity and detection range are far superior to previous reports. To get insight into the fluorescence quenching mechanism involved, the fluorescence quenching data were analyzed by the Stern-Volmer equation and KSV is the Stern-Volmer constant. As shown in Fig. S10, the Stern-Volmer plot does not fit a conventional linear Stern-Volmer equation, indicating both dynamic and static quenching processes occur in this sensor system.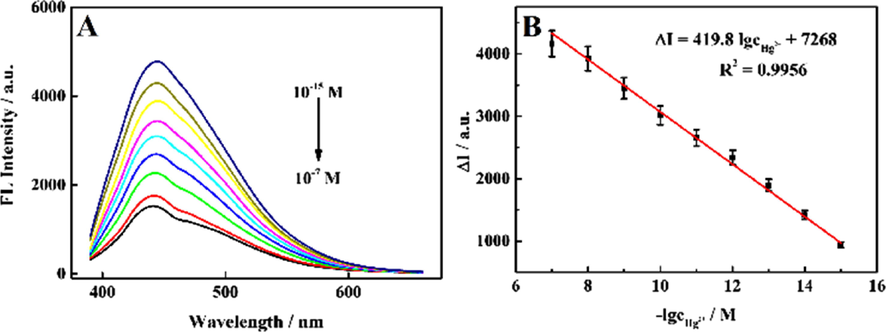
(A) The signal response of the proposed fluorescent aptasensor for detecting various concentrations of Hg2+ and the corresponding concentrations of Hg2+ (10−15, 10−14, 10−13, 10−12, 10-11, 10−10, 10−9, 10−8 and 10−7 M, from top to bottom, n = 8). (B) Linear relationship between the fluorescence intensity and the logarithm of Hg2+ concentrations.
The good selectivity of the sensor platform based on Apt-SQDs was a prerequisite for practical application. The interference of other ions must be eliminated to accurately determine the content of Hg2+ in real samples. Fig. 5A shows the response of the sensing platform to Hg2+ and other heavy metal ions under the same conditions, and the degree of fluorescence quenching was recorded. With the exception of Hg2+, other heavy metal ions had little effect on the fluorescence intensity of the sensing platform, which was mainly attributed to the high selectivity of the T-Hg2+-T structure for Hg2+. Common anions were further tested for interference with the probe, as shown in Fig. S11, indicating that the prepared probe has good selectivity. Furthermore, reproducibility of the sensing platform was also important. Five groups of Apt-SQDs were prepared under the same conditions to construct the fluorescence sensing platform and test fluorescence performance (Fig. 5B). The fluorescence performance of the fluorescence sensing platform based on the five groups of Apt-SQDs was almost identical, and thus the reproducibility of the sensor platform was good. In addition, good stability was one of the important factors determining the feasibility of this sensing platform. Therefore, within two weeks, the same batch of sensing platforms constructed from Apt-SQDs was tested to determine the fluorescence stability under the same experimental conditions, and the results are shown in Fig. 5C. The experimental results indicated that the performance of the constructed sensing platform was very stable. Even after two weeks of placement, the fluorescence emission intensity of the sensor platform was approximately the same as that recorded at the beginning of the experiment. Without special instructions, all the above experiments were repeated eight times to obtain accurate results, that means, n = 8.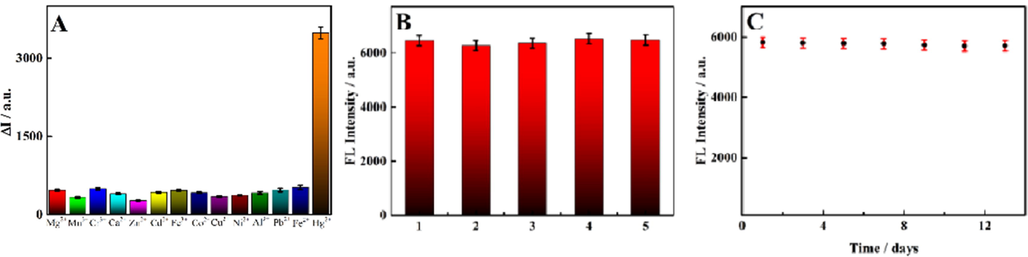
(A) Selectivity toward other interfering substances in the presence 1.0 × 10−8 M Hg2+: Ca2+, Mg2+, Mn2+, Fe3+, Zn2+, Co2+, Cd2+, Pb2+, Cr3+, Cu2+, Al3+, Ni2+ and Fe2+ (the concentration of each of the aforementioned interferents was 1.0 × 10−7 M). (B) Reproducibility of the five groups of Apt-SQDs. (C) The fluorescence stability of Apt-SQDs within two weeks (n = 8).
Although our study can achieve sensitive detection of Hg2+, there are still many methods superior to our work due to the limitation of fluorescence detection technology and material (Zhang et al., 2020; Rathnakumar et al., 2021., Liu et al., 2021a,b,c; Hasanjani and Zarei, 2018; Babamiri et al., 2018; Zhang et al., 2015).
3.5 Detection of Hg2+ concentrations in real samples
With the development of society, the problem of heavy metal pollution has gradually attracted the attention of the public, especially the prblem of heavy metal pollution in water. Hg2+ was well recognized by the sensing platform. We randomly tested the local water source (Yantai City) as real samples. The concentration of Hg2+ was calculated from the standard curve, as shown in Table 1. The recovery rate of Hg2+ was 87.21–114.1%, and the relative standard deviation (RSD) was 1.1–3.8%. Therefore, the sensing platform had high accuracy, accuracy and selectivity and was useful for the detection of Hg2+ in water samples.
Samples
Added / M
Found / M
Recovery / %
RSD / %
Tap water
0
0
–
–
10−7
0.9835. × 10−7
98.35
2.1
10−12
1.073 × 10−12
107.3
1.5
10−15
1.141 × 10−15
114.1
3.8
Lake water
0
0
–
–
10−7
0.9748 × 10−7
97.48
3.6
10−12
1.038 × 10−12
103.8
1.1
10−15
0.8721 × 10−15
87.21
2.9
River water
0
0
–
–
10−7
1.044 × 10−7
104.4
1.7
10−12
1.102 × 10−12
110.2
3.4
10−15
0.8937 × 10−15
89.37
2.6
3.6 Portable test papers for Hg2+ detection
If Hg2+ enters the water environment, it will eventually endanger human health. Therefore, a portable and fast method to monitor Hg2+ in environmental water samples must be developed. Fig. S12 clearly shows the quenching of the fluorescence of the test papers to different degrees within 1 min in the presence of different concentrations of Hg2+. After an incubation with increasing Hg2+ concentrations, the degree of fluorescence quenching gradually increased, consistent with the result of Hg2+ detection by the fluorescent aptasensor. Based on this result, the test paper had ultrahigh sensitivity for Hg2+ detection, which may be a good qualitative method for Hg2+ and had potential application in the safety monitoring of Hg2+ in environmental water samples.
4 Conclusions
In this paper, an ultrasensitive dual recognition strategy for detecting Hg2+ was successfully developed based on Apt-SQDs. Functionalized SQDs were combined with a variety of groups, which not only ensured the fluorescence performance of functionalized SQDs but also substantially expanded the potential applications of SQDs. The synthesized Apt-SQDs were stable for more than ten days and characterized using many kinds of techniques. Based on the strong binding of Sx2− to Hg2+ and the efficient enrichment of T-Hg2+-T, a novel fluorescence sensor for the rapid and ultrasensitive detection of Hg2+ was constructed. Apt-SQDs had the advantages of a quick response, high selectivity, low LOD and wide linear range for Hg2+ detection. The dual recognition strategy was applied to the detection of Hg2+ concentrations in real environmental water samples (tap water, lake and river), with good results and recoveries of 97.39–106.9%. Moreover, the fluorescence quenching effect was obviously observed with the naked eye when trace Hg2+ concentrations were detected with portable test papers based on Apt-SQDs. The good performance of Hg2+ detection indicated that the method had potential application value in the field monitoring of Hg2+ concentrations in environmental water samples.
Acknowledgement
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (21778047), and Natural Science Foundation of Shandong Province (grant no. ZR2021MB024).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alterations in gene expression due to chronic lead exposure induce behavioral changes. Neurosci. Biobehav. Rev.. 2021;126:361-367.
- [CrossRef] [Google Scholar]
- Recent applications and novel strategies for mercury determination in environmental samples using microextraction-based approaches: A review. J. Hazard. Mater.. 2022;433:128823
- [CrossRef] [Google Scholar]
- Switchable electrochemiluminescence aptasensor coupled with resonance energy transfer for selective attomolar detection of Hg2+ via CdTe@CdS/dendrimer probe and Au nanoparticle quencher. Biosens. Bioelectron.. 2018;102:328-335.
- [CrossRef] [Google Scholar]
- A quantum theory of molecular structure and its applications. Chem. Rev.. 1991;91:893-901.
- [CrossRef] [Google Scholar]
- A novel sulfur quantum dot for the detection of cobalt ions and norfloxacin as a fluorescent “switch”. Dalton Trans.. 2019;48:8288-8296.
- [CrossRef] [Google Scholar]
- Water soluble sulphur quantum dots for selective Ag+ sensing based on the ion aggregation-induced photoluminescence enhancement. Anal. Methods. 2016;8:632-636.
- [CrossRef] [Google Scholar]
- Electrochemical determination of mercury: A review. Talanta. 2013;116:1091-1104.
- [CrossRef] [Google Scholar]
- The pathology of methylmercury poisoning (Minamata disease) Neuropathology. 2010;30:471-479.
- [CrossRef] [Google Scholar]
- Electrochemical detection of silver ions by using sulfur quantum dots modified gold electrode. Sensors and Actuators B-Chemical. 2020;304:127390
- [CrossRef] [Google Scholar]
- Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens. Bioelectron.. 2014;56:340-344.
- [CrossRef] [Google Scholar]
- Fast and selective detection of mercury ions in environmental water by paper-based fluorescent sensor using boronic acid functionalized MoS2 quantum dots. J. Hazard. Mater.. 2020;381:120969
- [CrossRef] [Google Scholar]
- Highly efficient cofactors of Cu2+-dependent deoxyribozymes. Chemistryselect. 2017;2:3925-3931.
- [CrossRef] [Google Scholar]
- Recent advances in cancer early detection and diagnosis: role of nucleic acid based aptasensors. TrAC-Trends Anal. Chem.. 2020;124:115806
- [CrossRef] [Google Scholar]
- An electrochemical sensor for attomolar determination of mercury(II) using DNA/poly-L-methionine-gold nanoparticles/pencil graphite electrode. Biosens. Bioelectron.. 2018;128:1-8.
- [CrossRef] [Google Scholar]
- Heavy ion irradiation induces autophagy in irradiated C2C12 myoblasts and their bystander cells. J. Electron Microsc.. 2010;59:495-501.
- [CrossRef] [Google Scholar]
- Aptamer-based aggregation assay for mercury(II) using gold nanoparticles and fluorescent CdTe quantum dots. Microchim. Acta. 2016;183:2131-2137.
- [CrossRef] [Google Scholar]
- Zn2+ efflux through lysosomal exocytosis prevents Zn2+-induced toxicity. J. Cell Sci.. 2014;127:3094-3103.
- [CrossRef] [Google Scholar]
- AuNS@Ag core-shell nanocubes grafted with rhodamine for concurrent metal-enhanced fluorescence and surfaced enhanced Raman determination of mercury ions. Anal. Chim. Acta. 2018;1018:94-103.
- [CrossRef] [Google Scholar]
- Copper and iron ions accelerate the prion-like propagation of α-synuclein: A vicious cycle in Parkinson's disease. Int. J. Biol. Macromol.. 2020;163:562-573.
- [CrossRef] [Google Scholar]
- Rapid and ultrasensitive detection of mercury ion (II) by colorimetric and SERS method based on silver nanocrystals. Microchem. J.. 2020;161:105790
- [CrossRef] [Google Scholar]
- Recent advances in field-effect transistor sensing strategies for fast and highly efficient analysis of heavy metal ions. Electrochem. Sci. Adv. 2021 e2100137
- [CrossRef] [Google Scholar]
- An anthraquinone-based “turn-on” fluorescence probe for Hg2+ detection and its application in cell imaging. Inorg. Chem. Commun.. 2021;130:108753
- [CrossRef] [Google Scholar]
- Grain-boundary-rich polycrystalline monolayer WS2 film for attomolar-level Hg2+ sensors. Nat. Commun.. 2021;12:3870.
- [CrossRef] [Google Scholar]
- Determination of mercury(II) by fluorescence using deoxyribonucleic acid stabilized silver nanoclusters. Anal. Lett.. 2015;48:281-290.
- [CrossRef] [Google Scholar]
- A novel tetraphenylethene-based fluorescence probe based on the Hg2+-promoted deprotection of thioacetal. Heterocycles. 2018;96:850-857.
- [CrossRef] [Google Scholar]
- Altering genomic integrity: heavy metal exposure promotes transposable element-mediated damage. Biol. Trace Elem. Res.. 2015;166:24-33.
- [CrossRef] [Google Scholar]
- Determination of mercury and nickel by amperometric biosensor prepared with thermostable lactate dehydrogenase. Trans. Nonferrous Metals Soc. China. 2011;21:2332-2338.
- [CrossRef] [Google Scholar]
- Conjugation of gallic acid onto chitosan: An approach for green and water-based antioxidant. Carbohydr. Polym.. 2008;72:169-177.
- [CrossRef] [Google Scholar]
- Current progress in aptasensors for heavy metal ions based on photoelectrochemical method: a review. Curr. Anal. Chem.. 2018;14:4-12.
- [CrossRef] [Google Scholar]
- Preparation of protein hybrid fluorescence nanoclusters for rapid detection of mercury ion. Chin. J. Anal. Chem.. 2018;46:373-378.
- [CrossRef] [Google Scholar]
- Plasmon-coupled silver nanoparticles for mobile phone-based attomolar sensing of mercury ions. ACS Applied Nano Materials. 2021;4:8066-8080.
- [CrossRef] [Google Scholar]
- Progress in metal-organic frameworks facilitated mercury detection and removal. Chemosensors. 2021;9:101.
- [CrossRef] [Google Scholar]
- Sensing of mercury ions in Porphyra by Copper@Gold nanoclusters based ratiometric fluorescent aptasensor. Food Chemistry. 2021;344:128694.
- [CrossRef] [Google Scholar]
- Photoelectrochemical determination of Hg(II) via dual signal amplification involving SPR enhancement and a folding-based DNA probe. Microchim. Acta. 2017;184:1379-1387.
- [CrossRef] [Google Scholar]
- Volatilization of Metal Mercury from Organomercurials by Highly Mercury-Resistant Acidithiobacillus ferrooxidans MON-1. Biosci. Biotechnol. Biochem.. 2010;74:1007-1012.
- [CrossRef] [Google Scholar]
- Bifunctional nitrogen and fluorine Co-doped carbon dots as fluorescence probe for silicon and mercury by pH switching. Journal of Fluorescence. 2021;31:881-887.
- [CrossRef] [Google Scholar]
- Intracellular Zn2+ signaling in cognition. J. Neurosci. Res.. 2014;92:819-824.
- [CrossRef] [Google Scholar]
- Mitochondrial complex II-derived superoxide is the primary source of mercury toxicity in barley root tip. J. Plant Physiol.. 2017;209:68-75.
- [CrossRef] [Google Scholar]
- Prenatal lead, cadmium and mercury exposure and associations with motor skills at age 7years in a UK observational birth cohort. Environ. Int.. 2018;117:40-47.
- [CrossRef] [Google Scholar]
- Hydrogen Peroxide Assisted Synthesis of Highly Luminescent Sulfur Quantum Dots. Angew. Chem.-Int. Ed.. 2019;58:7040-7044.
- [CrossRef] [Google Scholar]
- Novel Hg2+-imprinted polymers based on thymine–Hg2+–thymine interaction for highly selective preconcentration of Hg2+ in water samples. J. Hazard. Mater.. 2012;237:347-354.
- [CrossRef] [Google Scholar]
- Electrochemical determination of mercury: A review. Talanta. 2013;116:1091-1104.
- [CrossRef] [Google Scholar]
- Recent progress in small-molecule fluorescent probes for detecting mercury ions. Crit. Rev. Anal. Chem.. 2022;52:250-274.
- [CrossRef] [Google Scholar]
- Electrochemical DNA sensor for inorganic mercury(II) ion at attomolar level in dairy product using Cu(II)-anchored metal-organic framework as mimetic catalyst. Chem. Eng. J.. 2020;383:123182
- [CrossRef] [Google Scholar]
- A label-free “turn-on” fluorescence method for detecting mercury ion in aqueous solution based on n-methyl mesoporphyrin IX (NMM)/G-quadruplex DNA. Anal. Sci.. 2017;33:165-169.
- [CrossRef] [Google Scholar]
- Electrochemical sensor based on electrodeposited graphene-Au modified electrode and nanoAu carrier amplified signal strategy for attomolar mercury detection. Anal. Chem.. 2015;87:989-996.
- [CrossRef] [Google Scholar]
- Ultrasensitive electrochemiluminescence detection of mercury ions based on DNA oligonucleotides and cysteamine modified gold nanoparticles probes. Sensors Actuators B-Chem.. 2012;171:860-865.
- [CrossRef] [Google Scholar]
- Recent developments in fluorescent aptasensors for the detection of antibiotics. Current Opin. Biomed. Eng.. 2019;13:16-24.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104080.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







