Efficiency of a novel nitrogen-doped Fe3O4 impregnated biochar (N/Fe3O4@BC) for arsenic (III and V) removal from aqueous solution: Insight into mechanistic understanding and reusability potential
⁎Corresponding authors. wubo@fzu.edu.cn (Bo. Wu), Dr.AQ.Geographer@gmail.com (Abdul Qadeer), zeeshan@nwpu.edu.cn (Zeeshan Ajmal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Worldwide, arsenic contamination has become a matter of extreme importance owing to its potential toxic, carcinogenic and mutagenic impact on human health and the environment. The magnetite-loaded biochar has received increasing attention for the removal of arsenic (As) in contaminated water and soil. The present study reports a facile synthesis, characterization and adsorption characteristics of a novel magnetite impregnated nitrogen-doped hybrid biochar (N/Fe3O4@BC) for efficient arsenate, As(V) and arsenite, As(III) removal from aqueous environment. The as-synthesized material (N/Fe3O4@BC) characterization via XRD, BET, FTIR, SEM/EDS clearly revealed magnetite (Fe3O4) impregnation onto biochar matrix. Furthermore, the adsorbent (N/Fe3O4@BC) selectivity results showed that such a combination plays an important role in targeted molecule removal from aqueous environments and compensates for the reduced surface area. The maximum monolayer adsorption (Qmax) of developed adsorbent (N/Fe3O4@BC) (18.15 mg/g and 9.87 mg/g) was significantly higher than that of pristine biochar (BC) (9.89 & 8.12 mg/g) and magnetite nano-particles (MNPs) [7.38 & 8.56 mg/g] for both As(III) and As(V), respectively. Isotherm and kinetic data were well fitted by Langmuir (R2 = 0.993) and Pseudo first order model (R2 = 0.992) thereby indicating physico-chemical sorption as a rate-limiting step. The co-anions (PO43-) effect was more significant for both As(III) and As (V) removal owing to similar outer electronic structure. Mechanistic insights (pH and FTIR spectra) further demonstrated the remarkable contribution of surface groups (OH–, –NH2 and –COOH), electrostatic attraction (via H- bonds), surface complexation and ion exchange followed by external mass transfer diffusion and As(III) oxidation into As(V) by (N/Fe3O4@BC) reactive oxygen species. Moreover, successful desorption was achieved at varying rates up to 7th regeneration cycle thereby showing (N/Fe3O4@BC) potential practical application. Thus, this work provides a novel insight for the fabrication of novel magnetic biochar for As removal from contaminated water in natural, engineering and environmental settings.
Keywords
Adsorption
Arsenic
Engineered adsorbents
Biochar
Desorption
Water contamination
1 Introduction
Toxic compounds such as heavy metal (loids) especially, arsenic (As) adversely affect to the human health and the natural environment mainly associated with its high toxicity, mutagenic and carcinogenic characteristics (Chakraborti et al., 2016; Qadeer et al., 2020; Essekri et al., 2022). Mineral weathering, geochemical reactions, mining, erosion, oil exploitation and As-bearing pesticides application have been reported as major source of groundwater As contamination (Gong et al., 2011). Depending upon pH and the redox potential, As contamination in water and soil can exist in different inorganic species such as; arsenite, As(III) and arsenate, As(V) and in organic form as; methylated As arsenocholine, and/or arsenobetaine (Urík et al., 2009; Zhang et al., 2017). In oxygen-rich environment (250–750 mV), the As (V) can exist in the form of H2AsO4-, H3AsO4, HAsO42- and AsO43-. Whereas, under oxygen-deficient environments (-250 to +250 mV), As(III) species are mainly dominant in the form of Arsenious acid (H3AsO3) at pH 1–9, which is in turn transformed into H2AsO3- as the solution pH approaches 12 at − 250 to − 500 mV redox (Kumarathilaka et al., 2018). To minimize its threshold level in an aquatic environment, many regulation authorities set an optimum threshold contaminant level of about 10 μg L−1 in drinking water (Fisher et al., 2015; Qadeer et al., 2022; Qadeer et al., 2022). In order to achieve low contamination level in water, several treatment strategies are employed including; coagulation, flocculation, photodegradation, ion exchange, chemical precipitation membrane filtration, and solvent extraction and adsorption (Yn et al., n.d.; Ff and Qi, 2011; Chen et al., 2019; Zhang and Jiang, 2019; Meng et al., 2020; Zhang et al., 2021; Ajmal et al., 2021; Hayat et al., 2022). These techniques are, however, associated with low adsorption/desorption capacities with high operational costs (Ajmal et al., 2020; Hayat et al., 2022; Brini et al., 2021). Notably, a positive bias towards adsorption technique is gaining more attention due to its high flexibility in design, ease in operation, and no sludge production and cost-effectiveness (Usman et al., 2018; He et al., 2016). To this end, various adsorbents including magnetite nano-particles (Wang et al., 2010), surface modified biochar’s (Ali et al., 2020; Ying et al., 2013), and granular activated carbon (Adeo et al., 2011) have been tested for the treatment of As contaminated water. Although the application of adsorption is somehow efficient, but the associated high cost most often limits their large-scale application (Ahmed et al., 2018; Khorram et al., 2016; Malik et al., 2021; Li et al., 2022). To minimize their high operational costs, the search for low cost, but highly efficient alternative sorbents is ongoing (Kizito et al., 2017; Wu et al., 2017; Ahmed et al., 2021; Hayat et al., 2022). The use of engineered surface adsorbents from agricultural-biomass has occupied a center stage by providing a low-cost alternative. Biochar (BC) is regarded as a potent raw material for making engineered adsorbent for aqueous separation processes (Hao et al., 2000; Kosik et al., 2020; Qadeer et al., 175 (2021)). In pristine form, the use of BC in aqueous separation is limited to its surface area, low contaminant specificity, and low end-process recovery (Naiya et al., 2009). After sorption, filtration and centrifugation steps are required for its efficient separation and irreversible sorption means that there are limited sorption sites to affect the second cycle contaminant removal. This phenomenon in turn limits the industrial scale BC application. However, to improve BC separation and species specificity during adsorption and regeneration (free of active sites) process, it’s a prerequisite to modify and activate BC before being used as an effective adsorbent material (Bakouri et al., 2010; Pan et al., 2022).
For the case of As sorption, BC pretreatment or modification is an important step to increase sorption effectiveness and efficiency. In particular As(III) and As(V) adsorption capacities on pristine BC is generally known to be low owing to its more negative charge on its surface (Mudhoo et al., 2011). In literature, various BC modification techniques have already been employed to improve its porosity, functional groups, surface area and point of zero charge (pHpzc) (Jin et al., 2014). These strategies include, activation by alkaline/acidic solutions, impregnation with nanoparticles of iron (Fe) and nano zero valent metals and conjugation with functional groups such as amides and thiols (Tan et al., 2015). Most recently, modification of biochar with Magnetite (Fe3O4) is receiving incredible research attention owing to its structural FeII presence, high stability, more reactive surface sites, and good surface chemistry (Usman et al., 2013; Duan et al., 2018; Xi et al., 2021; Zhang et al., 2021). Furthermore, magnetic surface modification has been envisaged to offer a promising solution in terms of fast pollutant recovery associated with its high separation characteristics by applying external magnetic field. The use of magnetic biochar was encouraged to recover metal ions from contaminated water (Baokang et al., 2018). Moreover, for enhanced metal (loids) adsorption, nitrogen (N) doping has also been considered as a valuable aspect in sorption studies, mainly due to attracting anionic species (arsenic), but depending upon solution pH. The analysis of N doping could provide a highly positive charged sorption sites and ultimately enhance material adsorption characteristics towards anionic species (Yoo et al., 2018). For instance, the N-doped aquatic animal waste BC has already been reported for enhanced nickel (Ni) ions removal from contaminated water (Yin et al., 2019). The N-doping effect on corn straw derived BC for the adsorption of copper (Cu) and cadmium (Cd) was observed to be more significant (Usman et al., 2012). To the best of our knowledge, there exist enormous studies on N-doped BC to evaluate its effectiveness for the elimination of metal (loids) for aqueous solution (Chen et al., 2022; Wan et al., 2019; Wang et al., 2021).
However, there is no information available in order to draw a solid conclusion towards As(III) and As(V) removal from aqueous solution by using a Fe3O4 impregnated N-doped biochar. Thus, the main objective of the present study is: (i) to carry out the facile synthesis and characterization of magnetic (N/Fe3O4@BC) in order, (ii) to evaluate the sorption capacity (including the underlying mechanisms) for As(III) and As(V) from aqueous solutions, (iii) and evaluate its separation, regeneration and reuse in continuous sorption process. Furthermore, the mechanistic evaluation was also investigated by using different models and integration techniques. It is expected that this study would prove a milestone for the ongoing research efforts for water purification from both engineering aspect and scientific approach under natural and environmental settings.
2 Experimental section
2.1 Material preparation and characterization
The preparation of magnetite nano-particles (MNPs) was carried out according to the procedure reported previously (Usman et al., 2012). The biochar preparation was carried out via slightly modified the method described earlier (Harikishore Kumar Reddy and Lee, 2014). Briefly, the wood pellets were cut into small pieces, and then transformed into powder form followed by washing and drying at 105 °C for 24 h. The magnetic biochar synthesis was carried out by immersing the as-obtained wood powder about 50 g by vigorous stirred (30 min) 500 mL solution of 9.25 g Ferric sulfate (Fe2(SO4)3⋅9H2O) and 10 g of Iron(II) sulfate (FeSO4). The solution pH was maintained in the range of (10–11) using 10 M sodium hydroxide (NaOH). Thereafter, the resultant mixture was dried at 65 °C followed by calcination at 550 °C for 150 min under nitrogen environment (Harikishore Kumar Reddy and Lee, 2014). A range of characterization techniques were used to determine the physico-chemical characteristics of the developed adsorbent. The Brunauer–Emmett–Teller (BET) surface area properties were investigated by using BELSORP MAX (BEL, Japan. Inc). The X-ray spectra by using Cu Kα radiation source were recorded on X-ray diffractometer (SHIMADZU XRD-6000). The scanning electron microscopic images were captured over (SEM, ZEISS SUPRA 55). The Fourier-transform infrared spectroscopy (FTIR) spectra were recorded within an intensity range of (400–4000 cm−1) on FTIR measure equipment (FT-IR, Bruker Vector 22).
2.2 Batch experiments
Adsorption of As(III) and As(V) was evaluated by batch adsorption trials. Synthetic As(V) and As(III) stock solutions (1000 mg/L) were prepared through disodium hydrogen arsenate (Na2HAsO4·7H2O Fluka, > 98 %) and sodium arsenite (Na3AsO4 Fluka, > 98 %). From the stock concentration, several dilutions (1–50 mg/L) were made. The solution pH was adjusted between 2 and 11 by using 0.1 M NaOH or HCl. The effect of different parameters on As(III & V) sorption was investigated by varying several parameters such as; reaction time was varied from 5 min to 24 h, the initial As concentration was varied from (1–35 mg/L), the phosphate concentration was varied from (1–6 mg/L), pH (2–11), and temperature (15–55 °C). The sorption vials were placed in water bath shaker at a constant agitation speed 150 rpm. At the end of each reaction time, about 5 mL of supernatant was collected and filtered through 0.45 µm syringe filter. The optimum adsorption capacity (mg/g) and removal (%) of As(V) was calculated after measuring residual adsorbate concentration of filtrate sample via molybdenum blue method as described by (Spectro molybdate method) at 880 nm (Dhar et al., 2004), via UV–vis spectrophotometer, and then, adsorption capacity on the basis of residual concentration was calculated through Eqs.1 and 2...
2.3 Desorption and regeneration tests
To investigate saturated particle regeneration potential for the developed adsorbent, the recovered particles were subject to several desorption/re-sorption trials using same pollutant concentrations and under similar conditions as those used with fresh particles. Desorption experiments were carried out by using deionized water and alkaline solutions. The adsorbate-loaded samples were centrifuged and the supernatant was decanted and discarded. Desorption was initiated by adding 10 mL of 0.1 M NaOH solution and agitated for 24 h. After agitation, the supernatant was filtered through 0.45-µm propylene syringe. Total desorbed concentration was measured through previously described adsorption estimation method. Prior to next adsorption experiments, the material was separated out, and oven dried (at 105 °C for 24 h). The dried particles were then used for subsequent adsorption/desorption/regeneration analysis. The percentage (%) desorption performance was calculated through Eq. (3):
Where; C denotes desorbed metal ions concentration (mg/L), x is adsorbed capacity of metal ions prior to desorption (mg/g), m represents the total mass of particle, which is used in desorption analysis (g), while V denotes the desorption solution volume (L).
3 Results and discussion
3.1 Materials characterization
Characterization results showed that the surface area properties, like pristine BC surface area characteristics were found to be superior than magnetite modified biochar Fe3O4@N/BC as shown in Table 1. Conversely, the lower porosity characteristics after magnetic modification are closely associated with the blockage of fine pores from iron species, which in turn led to the loss of micro-porosity (Kumar et al., 2014). Moreover, the decrease in surface area properties are due to the presence of higher proportion of iron layer over pristine BC surface sites, and ultimately led to decline in surface area (Chen et al., 2011).
| Adsorbent Sample | BET SSA (m2/g) | Pore volume cm3/g | Pore diameter (nm) | PZC |
|---|---|---|---|---|
| Magnetic biochar (N/Fe3O4@BC) | 109.40 | 0.447 | 16.3 | 8.2 |
| Biochar (BC) | 123.07 | 0.442 | 38.8 | 7.7 |
| Magnetite nano-particles (MNPS) | 72.20 | 0.411 | 23.0 | 7.1 |
SSA; specific surface area, PZC; point of zero charge.
The variations in BC and (N/Fe3O4@BC) crystallographic structure were characterized by X-ray Powder Diffraction (XRD) Fig. 1a. A clear difference in XRD diffractograms of pristine BC and (N/Fe3O4@BC) was observed. A clear peak, especially at 30.19 Ao was observed for (N/Fe3O4@BC). According to Wang et al., (Wang et al., 2015), these new peaks onto N/Fe3O4@BC were closely associated with the presence of Fe3O4. Moreover, the peaks at 42.13 Ao are an indication of cubic iron oxides particles over (N/Fe3O4@BC). The corresponding peaks at 57.43 Ao for (N/Fe3O4@BC), further indicates the occurrence of Fe3O4 (di-iron oxide, magnetite). The FTIR spectra were also recorded for MNPs, BC and Fig. 1b–d. Some new additional bands specifically at 501–585 cm−1 were observed for MNPs and BC, thereby indicating the presence of Fe–O surface functional group (Baig et al., 2014). Moreover, the basic proportion of K and Ca content, and Mg were considerably higher in pristine BC as compared to MNPs and (N/Fe3O4@BC). However, according to EDS spectra the contents of Fe were found higher in MNPs followed by (N/Fe3O4@BC) and BC.
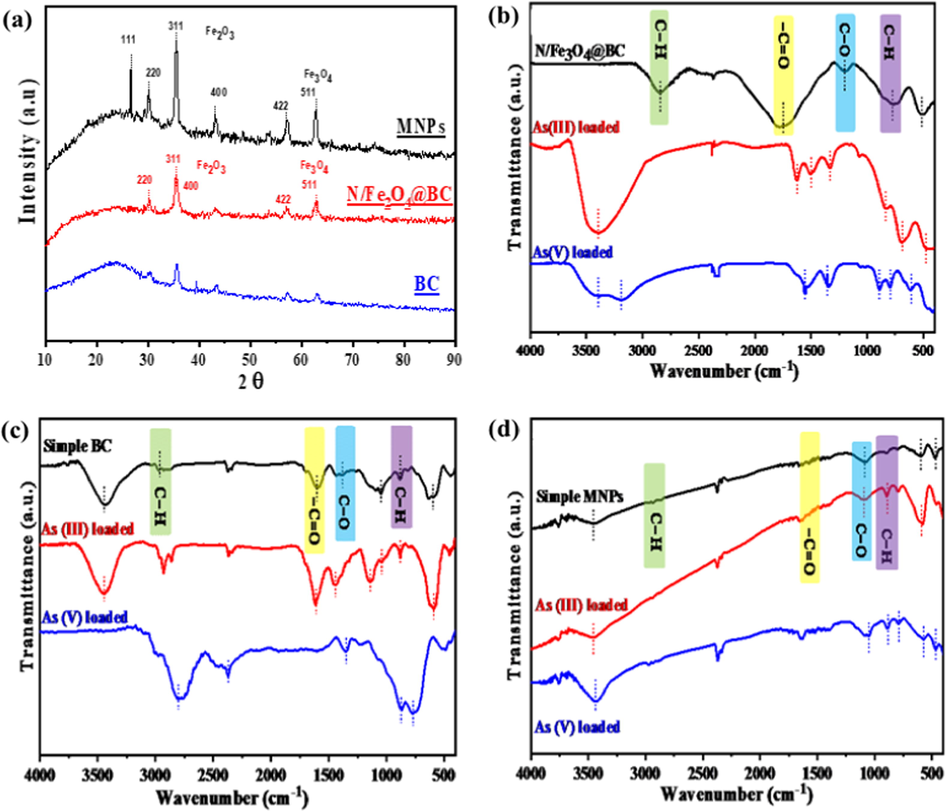
-
(a) XRD diffractogram and, (b-d) FTIR spectrum of all tested magnetite (N/Fe3O4@BC, BC, MNPs) before and after arsenic As(II) and As(V) adsorption.
The EDS spectra of N/Fe3O4@BC also indicated a sharp line showing the existence of Fe impregnation into N/Fe3O4@BC matrix shown in Fig. 2. Overall, all tested adsorbent surface sites were uniform in content, and containing a lot of irregular shaped material, as well as a wide range of undesirable particle aggregation Figure. 1Sƚ. In comparison, (N/Fe3O4@BC)exhibited lower carbon content, which obviously indicated that magnetic modification process results into the loss of organic matter contents Fig. 2 (Perlman et al., 267 (1992)).
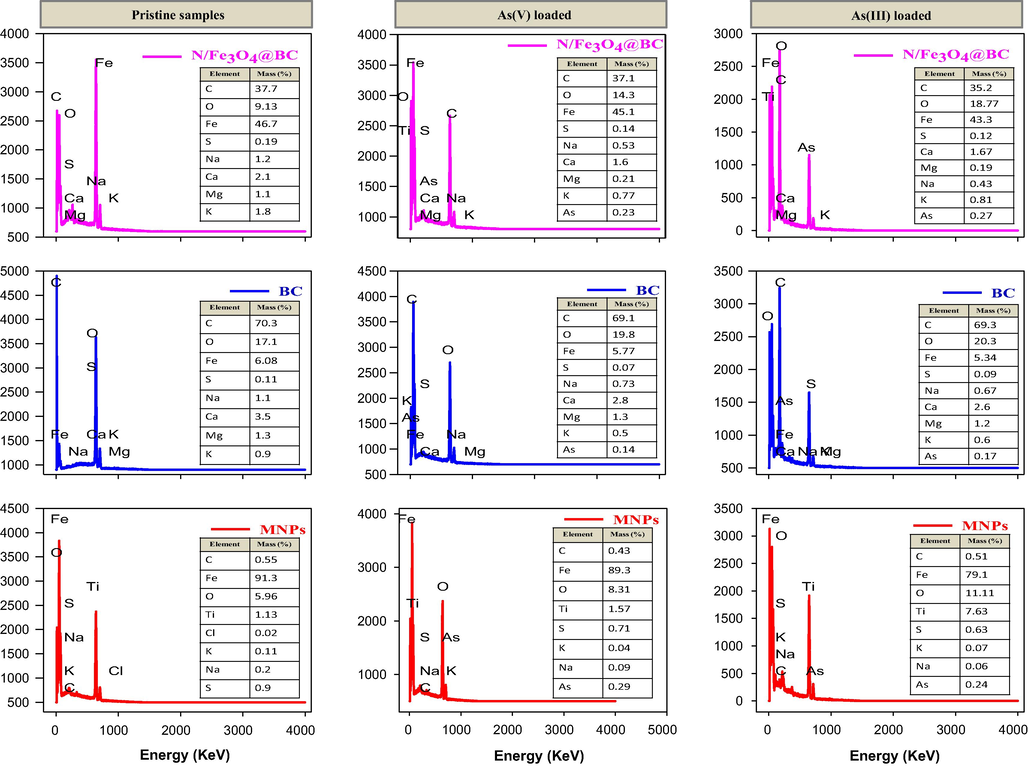
- EDS spectra of all tested adsorbent before and after As(III & V) adsorption.
3.2 Adsorption characteristics
3.2.1 Effect of as initial concentration
Optimum adsorption performance of tested materials is utterly associated with initial solution concentration since, it drives mass transfer rate underneath elevated concentration ranges between bulk liquid to solid interfaces (Auta and Hameed, 2013). The results of Fig. 3a & b, show the variation in solution concentration for As adsorption towards tested material surface sites within examined concentrations ranges (1–5 mg/L). The adsorption capacity (mg/g) and adsorption efficiency (%) indicated an entirely reverse order against elevated As solution concentration. From the Fig. 3a & b, it is clearly apparent that As(III, V) adsorption was increased by increasing initial solution concentration (1–35 mg/L) (Co–Ce), while that of % removal was decreased. The maximum adsorption rate at highest concentration (35 mg/L) was observed to be 43.74 mg/g for As(III) and 23.69 mg/g for As(V) in (N/Fe3O4@BC)followed by 23.94 mg/g for As(III) and 12.45 mg/g for (As(V) in BC and 18.10 mg/g for As(III) and 16.30 mg/g for As(V) in MNPs. On the other hand, declining in % removal could be a better elaboration of higher ionic gradient built up against fixed low adsorbent dosage (fixed number of active surface sites) (Bellacosa et al., 1998). The increase in adsorption capacity by increasing adsorbate concentration could be better elaborated via Eq. (2). The plausible explanation could be that the N/Fe3O4@BC might have more surface interaction sites after N doping and magnetic core shell structure depending upon adsorbate species interaction and the adsorbent interstitial space, those are responsible for adsorption process (Yu et al., n.d.).
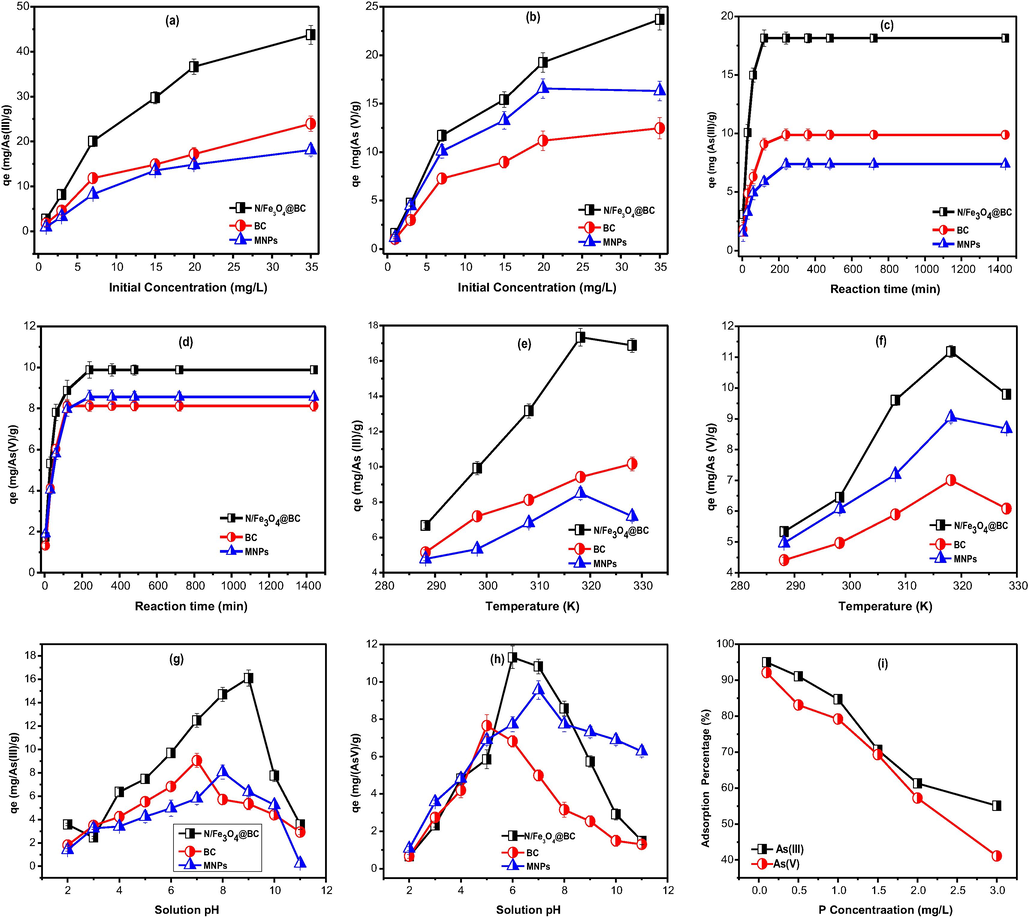
- Adsoprtion of Arsenic As (III & V) as a function of (a, b) initial concentration (c, d) reaction time (e, f) Temperature (g, h) Solution pH (i) Phosphate concentration.
3.2.2 Effect of contact time
Contact time is considered as an important parameter in adsorption studies for target pollutant elimination (Ajmal et al., 2018). The present study reports As(III & V) adsorption as a function of reaction time by determining the relationship between adsorption capacity and material surface characteristics. It was found that initial rapid As uptake rate was more obvious within a shorter period of time of 15, 30 and 60 min for (N/Fe3O4@BC), BC and MNPs respectively. Thereafter, a declining pattern was started, where adsorption became slower until attained equilibrium (Fig. 3c & d). It can be seen that the surface adsorption was quick towards all tested materials surface sites in the order of (N/Fe3O4@BC) > BC > MNPs), respectively. All these characteristics clearly indicate the availability of more surface-active sites by producing external adsorption phenomenon towards nano-porous surface sites of tested adsorbents at the early stages of reaction, while in case of equilibrium point, porous and highly active surface adsorption site comparatively required more time to reach at equilibrium. This trend could be attributed to higher surface area and well porosity characteristics of the developed adsorbents, which should be further explored for potential realistic implication of tested materials. Conversely, a rapid uptake rate could be better explained in terms of larger driving forces existence at initial phases and therefore, initial rapid and highest adsorption capacity was more obvious (vertical curve established) for all tested material onto their porous surface sites (Nassar, 2010). The observed highest uptake capacity for As(III) and As(V) were (18.14, 9.87 mg/g) for (N/Fe3O4@BC), (9.89, 8.12) for BC and (7.38, 8.56 mg/g) for MNPs. Therefore, it is clearly understood that the magnetic modification of (N/Fe3O4@BC) was effectively involved in As sorption from bulk liquid to solid interface during adsorption process.
3.2.3 Effect of reaction temperature
The adsorption of arsenic species as a function of temperature was evaluated within a temperature range from 288 to 328 K. The results Fig. 3e & f, demonstrated that adsorption capacity and % adsorption was enhanced with increasing temperature ranges from 288.15 K to 318.15 K, by a maximum adsorption capacity of 17.34 & 9.37 mg/g, for As(III) and (8.49 & 9.04) for As(V) onto (N/Fe3O4@BC), and MNPs, while for BC, the maximum adsorption of As(III) and As(V) was 10.16 ± 0.35 mg/g and 7.0 ± 0.14 mg/g) at 318.18 K and 328.15 K. The findings suggest that As removal is an endothermic process, and thereby temperature above ambient would be relatively suitable approach for higher adsorption for all tested adsorbents. The enhance in sorption capacity with increasing temperature could be due to decrease in solution viscosity and high free energy, which in turn promotes As diffusivity from external laminar layer to adsorbent pores (Nassar, 2010). However, a slightly decreasing pattern for adsorption capacity, and % removal was observed at 318 K for (N/Fe3O4@BC)and MNPs, and at 338 K for BC. It further suggests, that exothermic process was started thereafter. Therefore, it was implied that endothermic interactions mainly govern the As adsorption process between 318.15 and 328.15 K and afterward exothermic interactions might be possible at higher temperature (338.15 K) leading decreased sorption.
3.2.4 Effect of solution pH
The solution pH is considered as a main influential parameter during the adsorption process (Ajmal et al., 2020). The adsorption of As(V) and As(III) by all three tested adsorbents was examined over a wide range of pH from 3 to 11 as shown in Fig. 3g & h. The maximum As(V) adsorption of 11.31 mg/g for (N/Fe3O4@BC), 7.64 mg/g for BC and 9.56 mg/g for MNPs was observed at pH 6, pH 5 and pH 7 respectively, while pH 9, pH 7 and pH 8 were found to be the optimum pH values for As(III) adsorption at a rate of 16.10 mg/g, 9.04 mg/g and 8.05 mg/g for N/Fe3O4@BC, BC and MNPs respectively. It was found that alkaline conditions are unsuitable for both As(V) and As(III) adsorption onto MNPs, BC and (N/Fe3O4@BC). For example, the results of BC revealed that As adsorption increased, when pH increased from 3 to 5 for As (V) and 3 to 7 for As(III) and then declined on elevated pH, which indicated that As(III & V) adsorption onto BC surface sites was might be attributed to the Van der Waals interaction between BC surface and neutral H3AsO3 and H2AsO4 species. It is reported that dominant As(V) species are present as H2AsO-4, HAsO42- and AsO43- within the pH range between 2 and 14 respectively (Wei et al., 2016). Therefore, ability of tested adsorbent to adsorb metal ions decreases significantly by increasing pH. As we know that, lower pH is responsible for adsorbent surfaces sites protonation thus, higher adsorption is closely associated with higher electrostatic attraction as a result of increased protonation of material surface sites, which in turn enhanced aqueous arsenate anions, thereby boosting As(V) adsorption. With the rise of initial solution pH, the positively charged tested adsorbent surface sites gradually decreases, thus resulted an increase in electrostatic repulsion between liquid and solid interface hence, decreasing adsorption. Similar finding has been reported for As(V) adsorption onto iron oxides and iron-containing oxides and biochar surfaces (Min et al., 2006).
On the other hand, As(III) species such as H3AsO3- and H2AsO3- are mainly found at pH lower than 9.2, while that of AsO2 exists at pH higher than 9.2 (Zhang et al., 2016). Adsorption primarily occurred through the electrostatic attractions between negative As species and positively charged tested adsorbent surface sites. It means that the solution pH not only have significant impact onto material surface charge but also greatly influenced the speciation of As species in solution. More obviously, the MNPs could also play an important role to oxidize As(III) into As(V) based on solution pH. Therefore, higher adsorption of As(III) under near neutral pH (8) conditions could be due to higher As(III) oxidation into As(V), mainly, because of dissolved oxygen as well as oxidizing intermediate at neutral pH than that of higher or lower pH condition (Zhang et al., 2016; Luo et al., 2012). Moreover, the decrease of adsorption efficiency for all tested adsorbent at alkaline pH is attributed to the more electrostatic repulsion between and negatively charged tested magnetite surface sites and anionic As species (AsO2, HAsO42) (Luo et al., 2012). Because surplus OH– groups exist at that stage and adsorption primarily might occurred via hydrogen bonding between adsorbate and adsorbent surface sites. Another possible reason could be higher competition between anionic (AsO2, HAsO42-) species and hydroxyl groups (OH–) at higher pH condition (Shakoor et al., 2016). All tested adsorbents shown their point of zero charge about 8.2, 7.7 and 7.1. However, they can give rise to attraction below than these values or repulsion above these values depending upon adsorbate species charge and the adsorbent surface characteristics.
However, these results clearly indicate that the arsenic adsorption towards tested material surface sites was mainly controlled via two important phenomena: (i) electrostatic interaction (ii) As(III) oxidation between bulk liquid to solid interface (Shakoor et al., 2016). As(III) oxidation mainly occurred via contact with O2 and the oxic conditions that leads to Fe(III) drying. As, anoxic conditions mostly responsible for magnetite transformation into maghemite that could also drives the oxidation process (Navarathna et al., 2019). Overall, the higher (Qmax) of (N/Fe3O4@BC)compared to pristine MNPs and BC indicates As (III &V) adsorption (N/Fe3O4@BC)was due to surface complexation pathway (Luo et al., 2017).
3.2.5 Effect of phosphate on as adsorption
The result regarding the effect of phosphate onto As adsorption is displayed in Fig. 3i. In practical, ionic competition especially in real wastewater is more common due to multiple contaminant diversifications. It can be seen that as the concentration of phosphate was increased, then a significant reduction of both As(III) and As(V) was observed in (N/Fe3O4@BC)and MNPs samples. It means that the competing ions especially the phosphate have their significant impact over As species in real wastewater. It could be better explained in terms of similar charge properties as well as structural characteristics of phosphorus (P) in the form of PO43-, which could be strongly bounded over positively charged (N/Fe3O4@BC)and MNPs surface sites than that by As species mainly depending upon solution pH and redox potential (Vatutsina et al., 2007). The previous results also corroborate our investigation, where the presence of P showed a strong binding interaction with MNPs. Tuutijarvi et al. (2012) also reported that P ions have enough capability to influence the adsorption of As(V) over iron based maghemite particles surface sites at pH 7 with an initial concentration > 3 mg/L (Tuutijärvi et al., 2012). In case of pristine BC, the P as competing ions didn’t significantly affect As adsorption. It’s because of negative BC surface sites, which in turn created an electrostatic repulsion phenomenon. Furthermore, the higher concentration ranges of both competing ions should also be investigated for their potential practical application.
3.3 Adsorption data modeling
3.3.1 Adsorption isotherm
In general, adsorption isotherm studies are critical to determine dominant adsorption phase existence between bulk liquid to solid interface for engineered adsorption systems designing (Safa and Bhatti, 2011). Thus, equilibrium adsorption data was evaluated by using two commonly employed Langmuir (Langmuir, 1918) and Freundlich models (Freundlich, 1906). The mathematical expressions are given below, while the graphical representation is presented in Fig. 4a & b.
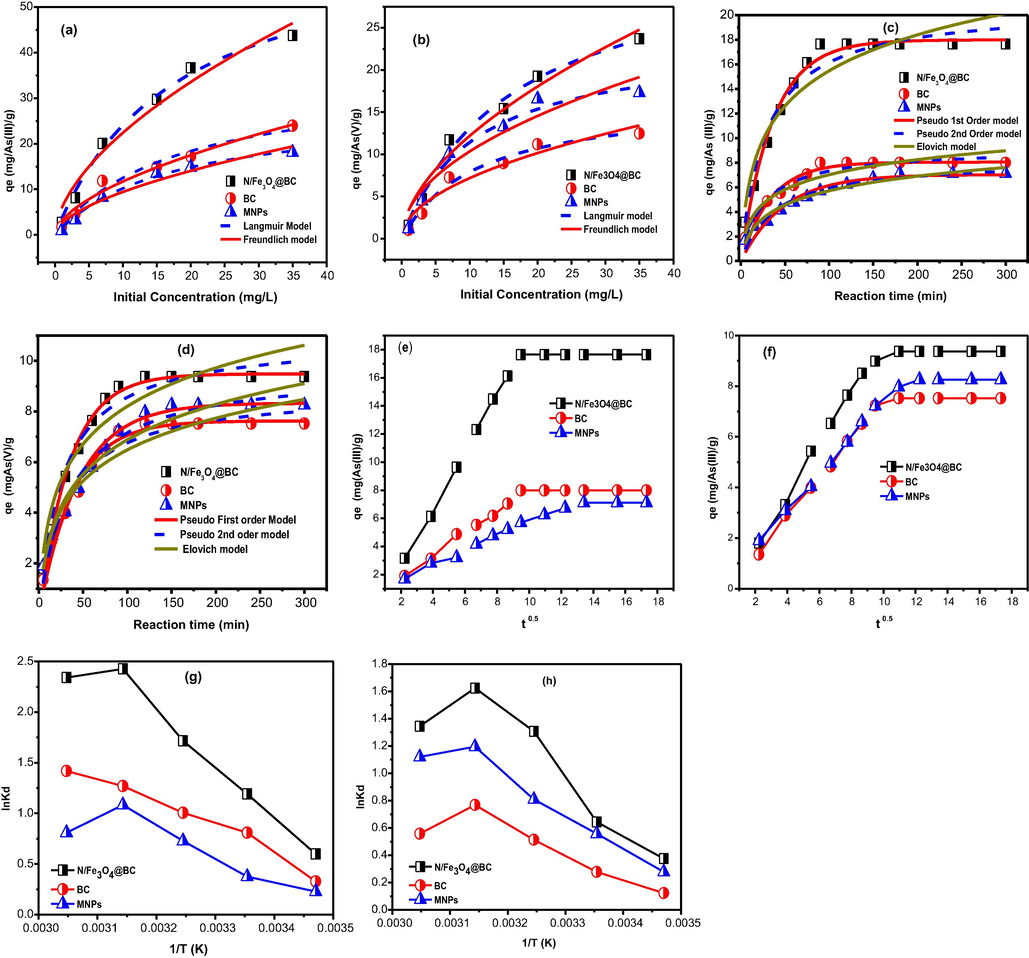
- The equilibrium and kinetic adsorption data modeling (a, b) As(III & V) data mdeling via Langmuir and Freundlich model (c,d) As(III & V) data interpretation via Pseudo 1st, 2nd and Elovich model (e, f) As(III & V) data interpretation via Intraparticle model.
| Material | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Q (mg.g−1) | K(L/mg) | RL2 | KF (Ln/(mg(n-1).g) | n | RF2 | |
| As(III)- N/Fe3O4@BC | 66.80 | 0.056 | 0.993 | 5.86 | 1.73 | 0.966 |
| As(III)- BC | 34.78 | 0.055 | 0.971 | 3.08 | 1.75 | 0.940 |
| As(III)- MNPs | 28.25 | 0.059 | 0.990 | 2.49 | 1.72 | 0.946 |
| As(V)- N/Fe3O4@BC | 34.54 | 0.061 | 0.987 | 3.30 | 1.78 | 0.962 |
| As(V)- BC | 16.56 | 0.091 | 0.978 | 2.27 | 2.04 | 0.905 |
| As(V)- MNPs | 23.41 | 0.095 | 0.976 | 3.32 | 1.96 | 0.942 |
It represents the adsorption process is favorable, when 0 < RL < 1, unfavorable RL > 1, linear adsorption RL = 1 and RL = 0, irreversible adsorption (Ngah et al., 2002). In the present study, the separation factor values were 0 < RL < 1, which further indicated that As adsorption as more favorable process through monolayer surface adsorption sites with optimum monolayer adsorption capacity of 66.80 mg g−1, 34.28 mg g−1 and 28.25 mg g−1 for As(III) and 34.54 mg g−1, 16.56 mg g−1 and 23.41 mg g−1 for As(V) in the order (N/Fe3O4@BC) > BC > MNPs, respectively. Conversely, the successful applicability of Freundlich model (R2 > 0.95), indicated the favourability of As adsorption onto multilayer surface adsorption sites of tested adsorbents. Multilayer adsorption phase further indicated that adsorption was more suitable onto adsorbent for maximum pollutant removal in aqueous environments. On the other hand, the higher Kf values indicates easy removal of As(III) and As(V) ions from contaminated water to adsorbent surface sites. The Freundlich R2 values for both As(III) and As(V) are presented in Table 2. In addition, adsorption intensity (1/n) is considered as important factor in adsorption process, which represents the adsorption from bulk liquid to solid interface. If the values of adsorption intensity n are < 1, then the adsorption is considered poor, while that in between 1 and 2 indicates the moderately difficult adsorption, on the other hand, if n lies in between 2 and 10, the adsorption is considered excellent. Even though, all the Freundlich R2 values were > 0.95 indicating physical adsorption up to some extent, the values of 1/n, clearly indicate that high intensity of sorption towards all tested adsorbents (Tofan et al., 2016). The calculated 1/n values are listed in Table 2, and were found higher than 1, indicating more than one mechanism involved in As adsorption towards all tested-adsorbents.
3.3.2 Adsorption kinetics
Adsorption kinetic data was evaluated by using Pseudo first order, pseudo second order, Elovich (Gerente et al., 2007) and intraparticle models (Choy et al., 2004). Mathematical expressions are given below, while the graphical representation is presented in Fig. 4c & d.
| Material | Pseudo 1st order | Pseudo 2nd order | Elovich | Intraparticle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qexp | qe (mg/g) | K1 (1/h) | R2 | qe (mg/g) | K1 (g/mg/h) | R2 | α (mg/g) | β (g/mg) | R2 | Kd (mg/g/min−1) | C (mg/g) | R2 | |
| N/Fe3O4@BC | 17.6 | 17.9 | 0.029 | 0.988 | 20.7 | 0.0017 | 0.951 | 1.52 | 0.23 | 0.897 | 0.20 | 1.1 | 0.739 |
| BC | 7.99 | 8.04 | 0.027 | 0.968 | 9.14 | 0.0043 | 0.943 | 0.87 | 0.55 | 0.914 | 0.39 | 2.5 | 0.765 |
| MNPs | 7.12 | 7.29 | 0.026 | 0.955 | 8.22 | 0.0031 | 0.941 | 0.56 | 0.43 | 0.941 | 0.66 | 2.6 | 0.907 |
| As (V) | |||||||||||||
| N/Fe3O4@BC | 9.37 | 9.48 | 0.028 | 0.992 | 10.89 | 0.0037 | 0.953 | 0.86 | 0.55 | 0.909 | 0.37 | 2.0 | 0.93 |
| BC | 7.52 | 7.62 | 0.025 | 0.982 | 8.81 | 0.0033 | 0.958 | 0.60 | 0.54 | 0.920 | 1.93 | 2.2 | 0.94 |
| MNPs | 8.26 | 8.33 | 0.022 | 0.962 | 9.71 | 0.0028 | 0.946 | 0.55 | 0.49 | 0.948 | 0.50 | 2.4 | 0.89 |
The fitted curves for all models are presented in Fig. 4c & d. Firstly, the assumption was made for intraparticle model Fig. 3e & f, where R2 values were ≤ 0.95 and straight lines were not passing through the origin and bilinear trend was more obvious (C ≠ 0), then the intraparticle diffusion as rate limiting step was rejected for all the tested adsorbents and the initial linear trend always indicates the involvement of film diffusion. Hence, the larger values of intercept clearly indicate the involvement of mutually external forces via (film and intra-particle), which are in counter play for As(III, V) adsorption onto tested adsorbent surfaces sites, and intraparticle is not rate limiting step (Tofan et al., 2016). Secondly, it was noted that higher correlation coefficient values of pseudo first order (0.99–0.98) rather than that of pseudo second (0.95–0.90) and Elovich (0.88–0.79) indicate the successful suitability for both As(III) and As(V), and the calculated (qecal) and the experimental values (qecal) were also found to be closer with each other. Thus, higher pseudo first order model correlation coefficient values than pseudo second order, thus implying the physio-sorption mechanism as more effective. Thus, our results totally contradict with certain previous investigation where As(III &V) adsorption over magnetite nano-particles, biochar and magnetic biochar were primarily governed by chemisorption mechanism owing to best fitting of experimental data to pseudo second order model (Jiaming et al., 2018). On the other hand, the sorption rate constant values of N/Fe3O4@/BC particles is higher than BC particles, thus these findings further suggest that As(III, V) sorption is not solely depends upon material superior surface characteristics, because if it is the cause then BC and MNPs must have larger adsorption rate constant. It further indicates that sorption via surface functional group might be more dominant over (N/Fe3O4@BC) particle than that of other tested adsorbents. In reality, by seeing BET surface area characteristics, one would expect MNPs and BC to be evidence for superior adsorption rather than (N/Fe3O4@BC) in aqueous solution.
3.3.3 Adsorption thermodynamics
Adsorption thermodynamics is an important parameter in adsorption studies. Variations in temperature plays an important role to evaluate material adsorption performance. The adsorption of As(III) and As(V) to materials surface sites was performed by varying the temperature from 288.15 K to 328.15 K. Vent Hoff equation was used for data fitting as manifested in Fig. 4e & f.
The Ce and Co (mg/L) stands for final and initial metal ions concentrations. The Gibb’s free energy change (ΔG°), was calculated through Kd by Eq. (11) and Eq. (12).
The R is the ideal gas constant with value 8.314 J·mol−1·K−1 and T is the temperature (K). Thus, a graph between lnKd v/s 1/T gives the values of enthalpy (ΔH) and entropy (ΔS) change through its slope and intercepts values. All the calculated modeled parameters i.e., ΔH°, ΔG°, ΔS° and Kd with R2 for all temperatures to all tested adsorbents are listed in Table 4. From the concern Table, it can be seen that Kd values were enhancing by increasing temperature from (288.15 – 328.15 K). Thus, increasing Kc and positive ΔH° values suggest that adsorption is endothermic in nature. On the other hand, negative Gibb’s free energy ΔG° values suggest adsorption is mainly physical, spontaneous and more favorable by increasing temperature from 288.15 K to 328.15 K. The entropy changes values (ΔS° values for all tested adsorbents were in positives range). These positive entropies change values, further indicates the higher randomness with quick interaction between adsorbate/adsorbent interfaces by increasing thermostat temperature. The increase of sorption with temperature could be due to rapid reaction between As(III) and As(V) ions and adsorbent surface functional groups at higher temperature due to decreasing solution viscosity and high free energy, which in turn promotes the As diffusivity from external laminar layer to adsorbent fine pores (Xiong et al., 2015). Furthermore, it has been shown in literature, that at ΔH° < 25 kJ mol−1, the acting forces during adsorbate/adsorbent interaction would be Van der Waals forces and might be ascribed to physisorption but if ΔH° values are in between 40 kJ mol−1 and 200 kJ mol−1, then the main dominant forces would be the chemical bonding, thereby indicating the chemical adsorption phenomena (Xiong et al., 2015).
| Temperature (K) | Kd | -ΔGo (J/mol) | ΔHo(kj·mol−1) | ΔSo (j·mol K−1) | R2 |
|---|---|---|---|---|---|
| N/Fe3O4@BC As(III) | |||||
| 288.15 | 1.82 | 1434.1 | 37.4 | 135.3 | 0.9412 |
| 298.15 | 3.29 | 2950.8 | |||
| 308.15 | 5.56 | 4397.0 | |||
| 318.15 | 11.3 | 6419.9 | |||
| 328.15 | 10.4 | 6387.1 | |||
| BC | |||||
| 288.15 | 1.38 | 784.6 | 20.9 | 76.1 | 0.968 |
| 298.15 | 2.24 | 2006.2 | |||
| 308.15 | 2.73 | 2577.1 | |||
| 318.15 | 3.56 | 3359.7 | |||
| 328.15 | 4.13 | 3870.8 | |||
| MNPs | |||||
| 288.15 | 1.26 | 546.2 | 14.9 | 53.8 | 0.760 |
| 298.15 | 1.46 | 930.8 | |||
| 308.15 | 2.07 | 1864.5 | |||
| 318.15 | 2.95 | 2862.9 | |||
| 328.15 | 1.75 | 2208.0 | |||
| N/Fe3O4@BC | As(V) | ||||
| 288.15 | 1.46 | 899.5 | 23.3 | 84.5 | 0.796 |
| 298.15 | 1.90 | 1596.4 | |||
| 308.15 | 3.70 | 3349.4 | |||
| 318.15 | 5.07 | 4295.8 | |||
| 328.15 | 3.84 | 3668.2 | |||
| BC | |||||
| 288.15 | 1.13 | 294.8 | 10.9 | 39.0 | 0.752 |
| 298.15 | 1.32 | 690.1 | |||
| 308.15 | 1.67 | 1315.4 | |||
| 318.15 | 2.16 | 2033.5 | |||
| 328.15 | 1.39 | 1521.6 | |||
| MNPs | |||||
| 288.15 | 1.32 | 666.9 | 18.4 | 66.3 | 0.927 |
| 298.15 | 1.75 | 1382.5 | |||
| 308.15 | 2.25 | 2073.4 | |||
| 318.15 | 3.31 | 3162.1 | |||
| 328.15 | 3.07 | 3056.3 | |||
3.4 Potential adsorption mechanism
Proposed adsorption mechanism was investigated using a series of parameters and FTIR spectra. The FTIR spectra of all tested adsorbents (N/Fe3O4@BC, BC and MNPs) before and after As adsorption are provided in Fig. 1b–d. As it can be seen from the virgin spectra of BC and MNPs, that all tested materials were found to have almost five distinct bands at 581, 579, 563 and 937, 937 and 933, 1143, 1141 and 1129, 1635, 1631 and 1634, 3440, 3431 and 3435 cm− 1 frequencies. These bands indicate Fe-OH stretching and bending vibrations hydroxyl groups, which are converted from the iron oxide in transient complex species form such as Fe-OH,-Fe(OH)2, or FeO(OH) onto tested (N/Fe3O4@BC), BC and MNPs surface sites (Tang et al., 2011). The band that shifts from 711 to 790 and 810–899 cm− 1 as well as 937, 937 and 933 cm− 1 in to 909, 904 and 915 cm− 1 and 901, 922 and 917 cm− 1, indicates As adsorption is might be due to As-O stretching band vibration in a partial As3 +→ Fe substitution (Baig et al., 2014). In addition, peaks attributable to C—H alkene (801–899 cm−1), C—O aloxy (1221–1238 cm−1), –C⚌O stretch (1508–1539 cm−1), C—H stretch (2876–2980 cm−1) were also observed for (N/Fe3O4@BC), BC and MNPs. After As(III) adsorption these band were shifted to 895, 899 and 889 cm−1, for (N/Fe3O4@BC), BC and MNPs. Similarly, the shift in band with corresponding peaks 1219.4, 1222.2 and 1242.3 was also observed after As(V) adsorption over, BC and MNPs (Alam et al., 2018). Moreover, after As(III) adsorption some peaks (583, 585 and 587) were shifted to 576, 580 and 579 for (N/Fe3O4@BC), BC and MNPs. In addition, some new peaks appeared at 683, 677, 763, 644 and 681 cm−1, which indicate some inorganic As compound adsorption. Intensities of some corresponding peaks at 1635, 1631, 1634 cm−1 and ∼ 3440, 3431, 3435 cm−1 were seems to be decreased, which clearly indicates surface OH groups involvement, which might be replaced by As(III) and As(V) after adsorption to form Fe-O As(III)/As(V) complex. However, broad band at 3440, 3450, 3460 cm−1 corresponding to OH stretching model shows hydrogen bonding and physisorbed water molecules (Chen et al., 2011). The peak originally at 3440 cm−1 which is commonly associated with As(III) and As(V) was shifted to 3387 cm−1 and 3464 cm−1 suggesting involvement of N—H amino (stretch) group. At the same time, the peak at 1587 cm−1 was shifted to 1595 cm−1 indicating involvement of N—H amino (bending) group. This is a clear indication of the successful adsorption of As(III) and As(V) over (N/Fe3O4@BC), BC and MNPs nanoparticles via interaction with OH– groups. Moreover, higher adsorption of As(V) than that As(III) on MNPs, could be clearly seen as the absorption peak in the range of 810–899 cm−1 is more intense than that of 711–790–792 cm−1 over MNPs surface sites (Zhu et al., 2009). Overall FTIR spectra from 2800 to 3400 cm−1 and 650–1750 cm−1 indicate As species binding with adsorbent. Hence, all FTIR peaks variation after arsenic As adsorption indicated the involvement of surface, Fe-O, carbonyl group and specifically hydroxyl groups (Shakoor et al., 2016). The results of pH analysis as earlier discussed are consistent, where hydroxyl plays an important role with FTIR assumption during adsorption process.
However, the pH results clearly indicate that the As adsorption towards tested adsorbents surface sites was mainly controlled via two important phenomenon: (i) electrostatic interaction and (ii) Oxidation of As(III) into As(V) between bulk liquid to solid interface (Shakoor et al., 2016). Moreover, another interesting phenomenon was noticed whenever initial metal concentration was increased, then the final pH was found to be decreased. This indicates that the adsorption is dominated by an ion-exchange mechanism by BC and (N/Fe3O4@BC)surface sites substantial oxygen content including hydroxyl and carboxyl groups, and could be considered as active center for ion exchange phenomenon (Klimmek et al., 2001). Data better fitted to Langmuir model clearly indicates the dominance of monolayer adsorption via surface complex pathway, as the affinity of As to Fe/BC, and As–Fe complexation on (N/Fe3O4@BC) after H+ ions exchange in aqueous solution. Thus, our finding clearly demonstrates adsorption via electrostatic attraction, ion exchange and surface complexation pathways. Therefore, ion exchange mechanism could not be ignored during whole adsorption process. All in all, the deeper insights and multiple potential adsorption pathways further required a wide range of high level various spectroscopic techniques analysis.
3.5 Desorption characteristics
Recycling process of tested material after As ions removal is an important parameter for reducing the pollutant load in the environment (Naciri et al., 2019). Many studies have evaluated the desorption and spent adsorbents regeneration to determine their potential continuous application in pollutant removal. Desorption of As loaded adsorbent was determined by using distilled water, NaOH and H2SO4 solutions (Fig. 5a & b). Distilled water resulted into low desorption, which was found to be nearly 26.3, 23.6 and 24.1 % for As(III) in the order of, BC > MNPs > (N/Fe3O4@BC). The As(V) desorption was 27.5, 24.5 and 25.1 % in distilled water in the order of (N/Fe3O4@BC), BC and MNPs Fig. 5a & b. Maximum As(III) desorption up to 87.1, 91.3 and 81.1 % was observed in alkaline environment from (N/Fe3O4@BC), BC and MNPs. On the other hand, As(V) desorption from (N/Fe3O4@BC), BC and MNPs was 96.3 %, 94.2 % and 95.1 %. Herein, also the low desorption of As(III) compared to As(V) was also observed and that might be As(III) oxidation into As(V), which clearly proved in pH investigation of As(III) into As(V). Even though, the low concentration of As(V) was also observed in As(III) desorption solution. But the surprising fact is the As(III) concentration was also observed in As(V) desorption solution in the order of MNPs > (N/Fe3O4@BC) > BC. So, the As(V) reduction phenomenon up to some extent could not be ignored during whole adsorption process. The obtained data indicates that spent adsorbents could be used up to 5th regeneration cycles without substantial loss of adsorption capacity during consecutive adsorption/desorption cycles. The (N/Fe3O4@BC) particle showed better desorption as well as higher adsorption performance after 5 regeneration cycles owing to its good magnetic properties and easy separation Fig. 5c & d. After consecutive adsorption desorption cycles the higher adsorption performance of (N/Fe3O4@BC) and MNPs particle might be due to the breakdown of large bulks into small one which resulted into exposure of new surface adsorption sites for maximum pollutant adsorption. On the other hand, a complete desorption was not achieved throughout the experimentation, which can be better explained in terms of adsorbent surface sites passivation, strong chemical binding between adsorbent and adsorbate as well as oxidation/reduction reaction during consecutive adsorption/ desorption cycles (H.J. A et al.,2015). Decrease in adsorption of all tested adsorbents with the passage of consecutive regeneration cycles is due to loss of adsorption surface sites. Similar results of decrease in adsorption have been reported in literature (Kizito et al., 2017). Therefore, these materials could be used as cost-effective alternative over other adsorbents with remarkable regeneration potential to remediate As contaminated water and side remediation system.
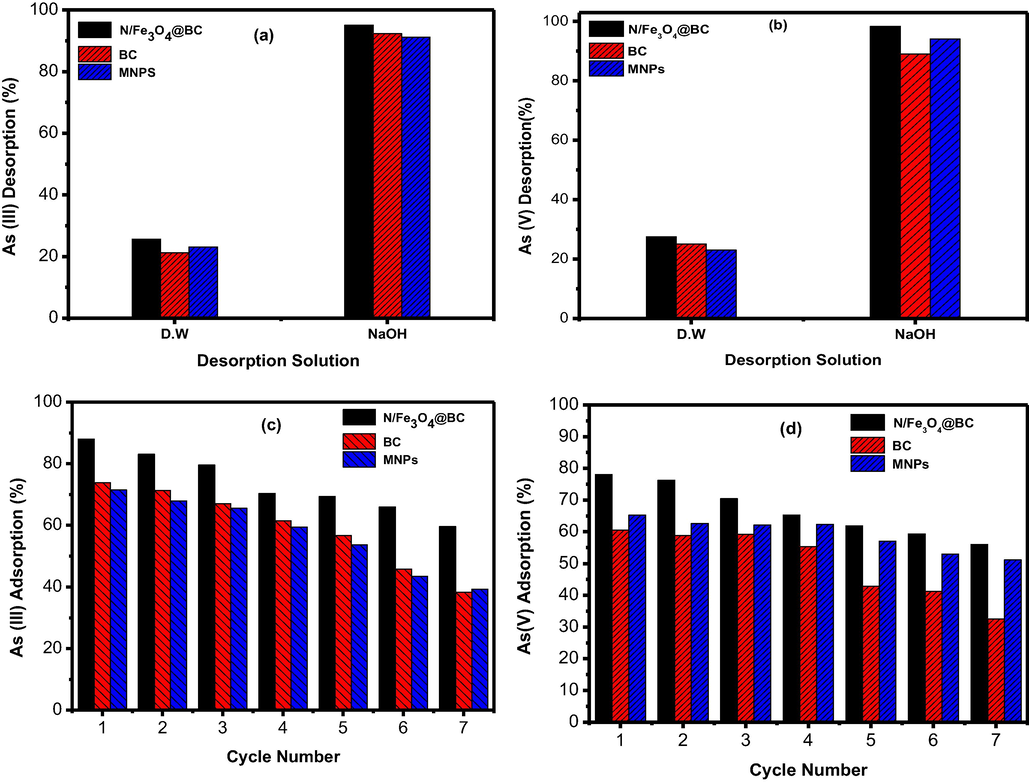
- Desorption performance of all tested adsorbents for As(III) and As(V) (a,b) as well as subsequesnt adsorption/desorption cycles (c, d) by using distilled water, alkaline eluents.
4 Conclusions
In this study, the application of nitrogen doped magnetic biochar (N/Fe3O4@BC) as potential adsorbent for As removal from aqueous solution was evaluated. The obtained results revealed that As adsorption was strongly affected by variation in material structural characteristics and solution pH. Higher adsorption performance by (N/Fe3O4@BC) was observed compared to pristine BC and MNPs. Overall, As(III) adsorption was found to be in the order of (N/Fe3O4@BC) > BC > MNPs, while in case of As(V) the MNPs exhibited higher adsorption than BC, and a reverse order was observed (N/Fe3O4@BC) > MNPs > BC. Kinetic and isotherm data were satisfactorily tailored by Pseudo first order and Langmuir model thereby suggesting the physio-chemical process was the rate-limiting step. The kinetic results further indicated that the external mass transfer and physisorption affect arsenic adsorption more significantly. An alkaline eluent was a better desorption strategy allowing material regeneration up to 5 adsorption cycles without significant loss in adsorption capacity. Based on the monolayer sorption maximum and the potential to regenerate/recover sorption capacity the developed magnetically modified and N-doped biochar could prove an effective adsorbent for the treatment of low-to moderately As contaminated water.
Acknowledgment
This work is financially supported by the National Natural Science Foundation of China (50971043, 51171046, 21973012), Key Research and Development Program of China (2017YFB0701700), Natural Science Foundation of Fujian Province (2021 J01590, 2014 J01176, 2018 J01754, 2020 J01474), National Key Laboratory of Eco-materials Advanced Technology (Fuzhou University), Fujian Province University (STHJ-KF1708), Student Research and Training Program (SRTP) of Fuzhou University (27297), State Administration for Market Regulation (2021MK050) and Fujian Provincial Department of Science & Technology (2021H6011).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Equilibrium, kinetics and thermodynamic studies of the biosorption of Mn (II) ions from aqueous solution by raw and acid-treated corncob biomass. BioResources. 2011;6:4117-4134.
- [Google Scholar]
- Carbon fiber paper@MgO films: in situ fabrication and high-performance removal capacity for phosphate anions. Environ. Sci. Pollut. Res.. 2018;25:34788-34792.
- [Google Scholar]
- Solvent assisted synthesis of hierarchical magnesium oxide flowers for adsorption of phosphate and methyl orange: kinetic, isotherm, thermodynamic and removal mechanism. Surf. Interf.. 2021;23:100953
- [Google Scholar]
- Z. Ajmal, A. Muhmood, M. Usman, S. Kizito, J. Lu, R. Dong, S. Wu, Phosphate removal from aqueous solution using iron oxides: Adsorption, desorption and regeneration characteristics, Journal of Colloid & Interface Science, (2018) S0021979718306039
- Use of nano-/micro-magnetite for abatement of cadmium and lead contamination. J. Environ. Manage.. 2020;264:110477
- [Google Scholar]
- Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal. J. Environ. Manage.. 2020;253:109730
- [Google Scholar]
- C.L. Bianchi, M. Laabd, A. Albourine, R. Dong, Prospects of Photocatalysis in the Management of Nitrate Contamination in Potable Water. In: Oladoja N.A., Unuabonah E.I., eds. Progress and Prospects in the Management of Oxyanion Polluted Aqua Systems. Cham: Springer International Publishing; 2021. p. :185-217.
- [Google Scholar]
- Adsorption of As (III) and As (V) from aqueous solution by modified Cassia fistula (golden shower) biochar. Appl. Water Sci.. 2018;8
- [Google Scholar]
- High sorption efficiency for As(III) and As(V) from aqueous solutions using novel almond shell biochar. Chemosphere. 2020;243
- [Google Scholar]
- Acid modified local clay beads as effective low-cost adsorbent for dynamic adsorption of methylene blue. J. Ind. Eng. Chem.. 2013;19:1153-1161.
- [Google Scholar]
- Effect of synthesis methods on magnetic Kans grass biochar for enhanced As(III, V) adsorption from aqueous solutions. Biomass Bioenergy. 2014;71:299-310.
- [Google Scholar]
- Effectiveness of acid-treated agricultural stones used in biopurification systems to avoid pesticide contamination of water resources caused by direct losses: part I. equilibrium experiments and kinetics. Bioresour. Technol.. 2010;101:5084-5091.
- [Google Scholar]
- Preparation of high mechanical performance nano-Fe3O4/wood fiber binderless composite boards for electromagnetic absorption via a facile and green method. Nanomaterials. 2018;8:52.
- [Google Scholar]
- Akt activation by growth factors is a multiple-step process: the role of the PH domain. Cornell University Press. 1998;17:313-325.
- [Google Scholar]
- Synthesis and characterisation of PANI- coated Heliotrope Leaves (PANI@HL) with high clean-up capacity for Orange G dye from aqueous media. Int. J. Environ. Anal. Chem.. 2021;1–17
- [Google Scholar]
- Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere. 2016;152:520-529.
- [Google Scholar]
- A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol.. 2011;102:716-723.
- [Google Scholar]
- Enhanced sorption of trivalent antimony by chitosan-loaded biochar in aqueous solutions: characterization, performance and mechanisms. J. Hazard. Mater.. 2022;425:127971
- [Google Scholar]
- 20 W all fiber supercontinuum generation from picosecond MOPA pumped photonic crystal fiber. Laser Phys.. 2011;21:519-521.
- [Google Scholar]
- Intraparticle diffusion in single and multicomponent acid dye adsorption from wastewater onto carbon. Chem. Eng. J.. 2004;103:133-145.
- [Google Scholar]
- A rapid colorimetric method for measuring arsenic concentrations in groundwater. Anal. Chim. Acta. 2004;526:203-209.
- [Google Scholar]
- Magnetically recyclable nanocatalyst with synergetic catalytic effect and its application for 4-nitrophenol reduction and Suzuki coupling reactions. Chem. Eng. Sci.. 2018;130:806-813.
- [Google Scholar]
- Enhanced adsorptive removal of crystal violet dye from aqueous media using citric acid modified red-seaweed: experimental study combined with RSM process optimization. J. Dispersion Sci. Technol.. 2022;43:1359-1372.
- [Google Scholar]
- Removal of heavy metal ions from wastewaters: a review-ScienceDirect. J. Environ. Manage.. 2011;92:407-418.
- [Google Scholar]
- Microbiological and chemical quality of packaged sachet water and household stored drinking water in freetown, Sierra Leone. PLoS ONE. 2015;10
- [Google Scholar]
- Application of chitosan for the removal of metals from wastewaters by adsorption mechanisms and models review. Crit. Rev. Environ. Sci. Technol.. 2007;37:41-127.
- [Google Scholar]
- Heat flow density in bohai bay basin: data set compilation and interpretation. Procedia Earth Planet. Sci.. 2011;2:212-216.
- [Google Scholar]
- H.J. A, F.G. B, H.A.T. C, S.M.H.A. D, Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies, Arabian Journal of Chemistry, 8 (2015) 837-849.
- Decolorization of wastewater. Sci: Crit. Rev. Environ; 2000.
- D. Harikishore Kumar Reddy, S.-M. Lee, Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 454 (2014) 96-103.
- A. Hayat, M. Sohail, T.A. Taha, S. Kumar Baburao Mane, A.G. Al-Sehemi, A.A. Al-Ghamdi, W.I. Nawawi, A. Palamanit, M.A. Amin, A.M. Fallatah, Z. Ajmal, H. Ali, W. Ullah Khan, M. Wajid Shah, J. Khan, S. Wageh, Synergetic effect of bismuth vanadate over copolymerized carbon nitride composites for highly efficient photocatalytic H2 and O2 generation, J. Colloid. Interf. Sci., 627 (2022) 621-629.
- A. Hayat, M. Sohail, W. Iqbal, T.A. Taha, A.M. Alenad, A.G. Al-Sehemi, S. Ullah, N.A. Alghamdi, A. Alhadhrami, Z. Ajmal, A. Palamanit, W.I. Nawawi, H.S. AlSalem, H. Ali, A. Zada, M.A. Amin, Molecular engineering optimized carbon nitride photocatalyst for CO2 reduction to solar fuels, (2022) 100483.
- Enhanced photocatalytic overall water splitting from an assembly of donor-π-acceptor conjugated polymeric carbon nitride. J. Colloid Interface Sci.. 2022;624:411-422.
- [Google Scholar]
- Treatment of alkaline stripped effluent in aerated constructed wetlands: feasibility evaluation and performance enhancement. Water. 2016;8:386.
- [Google Scholar]
- Enhanced As (V) removal from aqueous solution by biochar prepared from iron-impregnated corn straw. J. Chem.. 2018;2018:1-8.
- [Google Scholar]
- Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: adsorption property and its improvement with KOH activation. Bioresour. Technol.. 2014;169:622-629.
- [Google Scholar]
- Dissipation of fomesafen in biochar-amended soil and its availability to corn (Zea mays L.) and earthworm (Eisenia fetida) J. Soils Sediments. 2016;16:2439-2448.
- [Google Scholar]
- Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration. J. Environ. Manage.. 2017;201:260-267.
- [Google Scholar]
- Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: Role of media and tidal operation. Sci. Total. Environ. 2017;592:197-205.
- [Google Scholar]
- Comparative analysis of the biosorption of cadmium, lead, nickel, and zinc by algae. Environ. Sci. Technol.. 2001;35:4283-4288.
- [Google Scholar]
- Engineered biochar - a sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2020
- [Google Scholar]
- Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014
- [Google Scholar]
- Arsenic speciation dynamics in paddy rice soil-water environment: sources, physico-chemical, and biological factors-a review. Water Res.. 2018;140
- [Google Scholar]
- The adsorption of Gases and liquid on plane surfaces of glass, mice and platinum. J. Am. Chem. Soc.. 1918;40:1361-1403.
- [Google Scholar]
- Recent progress in g–C3N4–based materials for remarkable photocatalytic sustainable energy. Int. J. Hydrogen Energy. 2022;47:21067-21118.
- [Google Scholar]
- Research process in removing magnesium and manganese from huangmailing phosphorite mine. China Non-Metal. Miner. Industry 2017
- [Google Scholar]
- Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide-MnO2 nanocomposites. Chem. Eng. J. -LAUSANNE-. 2012;187:45-52.
- [Google Scholar]
- Investigation of textile dyeing effluent using activated sludge system to assess the removal efficiency. Water Environ. Res.. 2021;93:2931-2940.
- [Google Scholar]
- The role of transparent exopolymer particles (TEP) in membrane fouling: a critical review. Water. Res.. 2020;181:115930
- [Google Scholar]
- Removal of arsenite and arsenate using hydrous ferric oxide incorporated into naturally occurring porous diatomite. Environ. Sci. Technol.. 2006;40:1636.
- [Google Scholar]
- Arsenic: an overview of applications, health, and environmental concerns and removal processes. Crit. Rev. Environ. Sci. Technol.. 2011;41
- [Google Scholar]
- Preparation, characterization and photocatalytic degradation of Rhodamine B dye over a novel Zn3(PO4)2/BiPO4 catalyst, Journal of Environmental. Chem. Eng. 2019
- [Google Scholar]
- Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J. Colloid Interface Sci.. 2009;333:14-26.
- [Google Scholar]
- Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater.. 2010;184:538-546.
- [Google Scholar]
- Removal of Arsenic(III) from water using magnetite precipitated onto Douglas fir biochar. J. Environ. Manage.. 2019;250:109429
- [Google Scholar]
- Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym.. 2002;50:181-190.
- [Google Scholar]
- A facile molecular aggregation of isoquinoline based g-C3N4 for high photocatalytic performance under visible light illumination. Mater. Res. Bull.. 2022;152:111865
- [Google Scholar]
- Thyrotropin-releasing hormone binding to the mouse pituitary receptor does not involve ionic interactions. a model for neutral peptide binding to G protein-coupled receptors. J. Biol. Chem.. 1992;267
- [Google Scholar]
- Concentrations, pollution indices and health risk assessment of heavy metals in road dust from two urbanized cities of Pakistan: comparing two sampling methods for heavy metals concentration. Sustain. Cities. Soc.. 2020;53:101959
- [Google Scholar]
- Agricultural plastic mulching as a potential key source of microplastic pollution in the terrestrial ecosystem and consequences. Resour., Conserv. Recycl.. 2021;175:105855
- [Google Scholar]
- Rebuttal to comment on “alternative plasticizers as emerging global environmental and health threat: another regrettable substitution?” focus on DINCH as an example. Environ. Sci. Technol.. 2022;56:5294-5297.
- [Google Scholar]
- Alternative plasticizers as emerging global environmental and health threat: another regrettable substitution? Environ. Sci. Technol.. 2022;56:1482-1488.
- [Google Scholar]
- Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination. 2011;272:313-322.
- [Google Scholar]
- Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol.. 2016;46
- [Google Scholar]
- Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere. 2015;125:70-85.
- [Google Scholar]
- High-efficiency diode-pumped acousto-optically Q-switched 1123 nm ceramic Nd:YAG laser. Laser Phys.. 2011;21:695-699.
- [Google Scholar]
- Natural and waste materials as green sorbents for cd(ii) removal from aqueous effluents. Environ. Eng. Manage. J.. 2016;15:1049-1058.
- [Google Scholar]
- Effect of competing anions on arsenate adsorption onto maghemite nanoparticle. Chin. J. Chem. Eng. 2012
- [Google Scholar]
- Removal of arsenic (V) from aqueous solutions using chemically modified sawdust of spruce (Picea abies): Kinetics and isotherm studies. Int. J. Environ. Sci. Technol.. 2009;6
- [Google Scholar]
- FeII induced mineralogical transformations of ferric oxyhydroxides into magnetite of variable stoichiometry and morphology. J. Solid State Chem.. 2012;194:328-335.
- [Google Scholar]
- Treatment of hydrocarbon contamination under flow through conditions by using magnetite catalyzed chemical oxidation. Environ. Sci. Pollut. Res.. 2013;20:22-30.
- [Google Scholar]
- Magnetite and green rust: synthesis, properties, and environmental applications of mixed-valent iron minerals. Chem. Rev.. 2018;118:3251-3304.
- [Google Scholar]
- A new hybrid (polymer/inorganic) fibrous sorbent for arsenic removal from drinking water. React. Funct. Polym.. 2007;67:184-201.
- [Google Scholar]
- Concurrent adsorption and micro-electrolysis of Cr (VI) by nanoscale zerovalent iron/biochar/Ca-alginate composite. Environ. Pollut.. 2019;247:410-420.
- [Google Scholar]
- Highly adsorptive pristine and magnetic biochars prepared from crayfish shell for removal of Cu (II) and Pb (II) J. Taiwan Inst. Chem. Eng.. 2021;127:175-185.
- [Google Scholar]
- Characterization of Epstein-Barr virus BGLF4 kinase expression control at the transcriptional and translational levels. J. Gen. Virol.. 2010;91:2186-2196.
- [Google Scholar]
- Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3), and phosphate (PO43) Chemosphere. 2015;119:646-653.
- [Google Scholar]
- Extremely high arsenic removal capacity for mesoporous aluminium magnesium oxide composites. Environ. Sci. Nano. 2016;3
- [Google Scholar]
- Treatment of anaerobic digestate supernatant in microbial fuel cell coupled constructed wetlands: Evaluation of nitrogen removal, electricity generation, and bacterial community response. Sci. Total Environ.. 2017;580:339-346.
- [Google Scholar]
- Continuous flow reduction of organic dyes over Pd-Fe alloy based fibrous catalyst in a fixed-bed system. Chem. Eng. Sci.. 2021;231:116303
- [Google Scholar]
- Investigation on the efficiency and mechanism of Cd(II) and Pb(II) removal from aqueous solutions using MgO nanoparticles. J. Hazard. Mater.. 2015;299:664-674.
- [Google Scholar]
- Evaluation of Removal Efficiency of Ni(II) and 2,4-DCP Using in Situ Nitrogen-Doped Biochar Modified with Aquatic Animal Waste. XXXX: ACS Omega; 2019.
- An environmentally benign protocol: catalyst-free Michael addition of aromatic amines to α, β-unsaturated ketones in glycerol. Res. Chem. Intermed.. 2013;39:517-525.
- [Google Scholar]
- A. Yn, A. Ah, B. Za, C. Aba, A. Bb, D. Jan, A. Aa, C. Jcv, A. Me, A. Ab, Influence of Sr-doping on structural, optical and photocatalytic properties of synthesized Ca3(PO4)2, Journal of colloid and interface science, 572 269-280.
- Adsorption of nitrate onto nitrogen-doped activated carbon fibers prepared by chemical vapor deposition. Korean J. Chem. Eng.. 2018;35:2468-2473.
- [Google Scholar]
- W. Yu, F. Lian, G. Cui, Z. Liu, accepted manuscript n-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution n-doping effectively enhances the adsorption capacity of biochar for heavy metal 1 ions from aqueous solution 2 3.
- Membrane fouling in aerobic granular sludge (AGS)-membrane bioreactor (MBR): effect of AGS size. Water Res.. 2019;157:445-453.
- [Google Scholar]
- Aerobic granular sludge (AGS) scouring to mitigate membrane fouling: performance, hydrodynamic mechanism and contribution quantification model. Water Res.. 2021;188:116518
- [Google Scholar]
- Substrate-assisted encapsulation of Pd-Fe bimetal nanoparticles on functionalized silica nanotubes for catalytic hydrogenation of nitroarenes and azo dyes. ACS Appl. Nano Mater.. 2021;4:5854-5863.
- [Google Scholar]
- Simultaneous oxidation and adsorption of As(III) from water by cerium modified chitosan ultrafine nanobiosorbent. J. Hazard. Mater.. 2016;308:1-10.
- [Google Scholar]
- Zhang, Liankai, Yang, Hui, Tang, Jiansheng, Qin, Xiaoqun, Liu, Wen, Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China, Applied Geochemistry: Journal of the International Association of Geochemistry and Cosmochemistry, 77 (2017) 80-88
- Self-assembled 3D flower-like hierarchical β-Ni(OH)2 hollow architectures and their in situ thermal conversion to NiO. Nanoscale Res. Lett. 2009
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104209.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







