Translate this page into:
Efficient and sustainable bleaching of cotton/spandex integrating UVA radiation and peroxide activator: A study focus on the degradation mechanism of natural pigments in fibres
⁎Corresponding authors. huiyujiang@wtu.edu.cn (Huiyu Jiang), zhouyuyang_suda@hotmail.com (Yuyang Zhou) yuyangzhou@suda.edu.cn (Yuyang Zhou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Establishing an energy/water-saving bleaching process is highly expected by textile plants to reduce industrial carbon emission, wastewater discharge and general cost. Therefore, an efficient UVA-assisted N-[4-(triethylammoniomethyl)-benzoyl]- caprolactam-chloride (TBCC) -activated hydrogen peroxide (H2O2) bleaching strategy at ambient temperature for cotton/spandex (C/S) fabric was developed in this study. Results reveal that 7.4 g/L of TBCC-activated H2O2 (30%) achieves a slightly higher whiteness index (WI) on C/S fabrics than those only treated with 60 g/L H2O2 (30%). The treated C/S fabric displays a good dyeability using direct dyes due to the qualified wettability. The mild pH process protects C/S fabrics from damage with a low tensile loss (less than 10%) in warp and weft directions. The WI of C/S fabric mainly depends on the concentration of TBCC/H2O2 couple and radiation time. Moreover, the degradation mechanism of cotton pigment in UVA/TBCC/H2O2 bleaching system was implemented including the identification of effective reactive oxygen species (ROS), tracking of the concentration variation of hydroxyl radical (HO•) under UVA irradiation, and the exploration of degrading kinetics and pathway of morin as the model of pigment impurity. Indicated by the degradation rate constant, both TBCC and UVA plays an accelerating role in enhancing the degradation efficiency. In the radical scavenging experiment, HO• and superoxide radical (O2–•) are two major ROS participating in the degradation. And therein, HO• makes larger contribution than O2–•. HO• concentration has a high positive correlation with radiation duration and TBCC dosage based on the fluorescence labelling method. Confirmed by LC-MS/MS analyses, 2,4,6-trihydroxybenzoic acid (MW: 170.12) and 2,4,-dihydroxybenzoic acid (MW: 154.12) are two major segments degraded from the oxidation and ring-opening of C-ring in morin. Finally, considering energy, water and chemical conservations, the condition i.e. 7.4 g/L of H2O2 (30%) and 20 g/L of TBCC under 30 W UVA radiation for 1 h, is recommended, which significantly increases the WI by 46.5%, and saves about 90% energy and 50% water consumption compared with the conventional waterbath bleaching at 95 °C for 45 min when similar WI enhancement was achieved.
Keywords
Peroxide activator
UV radiation
Degradation mechanism
Cotton fibre
Energy conservation
1 Introduction
Conventional textile industry has long been criticized for its environmental endangerment due to the large water/energy consumptions, and the environmentally- and economically- burdened effluent discharges, which is in desperate need of transformation and upgrading towards advanced and sustainable industry (Shahid and Mohammad 2013, Shahid and Mohammad 2013, Ahmad et al., 2015). Cotton plays a significant role in the family of textile substrates for its hygroscopicity and wearing comfortableness, which takes up over 80 % out of 32 million mts of natural fibre production according to a report in 2018 (Ahmed and Mondal 2021). Bleaching is an indispensable step that aims to achieving required whiteness by removing the intrinsic colored impurities from cotton fibres available for the following processes such as coloration, printing and functionalization. Conventionally, cotton fabric is bleached in a large-bath-ratio waterbath containing high concentration of hydrogen peroxide (H2O2), along with alkaline agent, chelating agent, surfactants, etc., at high temperature up to 95 °C for around 60 min. Such caustic condition not only causes fibre strength declination rising from the breakage of cellulosic macromolecule, but also requires a vast amount of energy for heating that contributes to the global industrial carbon emission (Xu et al., 2011, Abdel-Halim 2012, Tissera et al., 2016). Furthermore, the chemical residuals such as oxidants, silicates, surfactants adversely impact on water quality and lay textile plants with additional burden on wastewater treatment (Vandevivere et al., 1998, Harane and Adivarekar 2016, Shi et al., 2023). Thus, the development of water/energy conservative bleaching strategy for cotton fabric is in great demand to tackle with two current intractable issues, i.e., freshwater crisis and carbon emission.
Cold pad-batch bleaching with H2O2 is one appealing approach running merely at ambient temperature and using very low amount of bleaching aqueous bath, however which usually requires over 24 hrs’ processing time due to the slow decomposition rate of H2O2 without heating, leading to a low processing efficiency during mass production. To date, several H2O2 activators have been developed to realize the low-temperature but efficient bleaching process (Xu et al., 2011, Zeng and Tang 2014, Liu et al., 2018, Li et al., 2020, Zhou et al., 2021). The principle is as follows: H2O2 activators play the role of peroxy acid (PA) precursors by perhydrolysis reaction to generate PA which exhibits lower O-O bonding energy than that in H2O2 (Kim and Huang 2020). Thus at ambient temperature, PA shows higher decomposition propensity than H2O2 to produce reactive oxygen species (ROS) to degrade the colour impurity. And therefore, the issues of low efficiency and long processing hours are mitigated by combining activators and H2O2. Tetra-acetylethylenediamine (TAED) is a widespread H2O2 activator showing the commercial-available and environmentally-benign advantage, but its undesirable solubility especially at ambient temperature makes it incompatible with cold pad-batch process. Our previous study also discovered the activating function of TAED was suppressed at low temperature (e.g. 50 °C) owing to its low solubility (Li et al., 2020). Although sodium 4-(nonanoyloxy)benzene sulphonate (NOBS) displays good solubility due to its sulfonic group, the byproduct generated from it during bleaching is toxic and odorous (Cai et al., 2001). Fortunately, a water soluble activator namely N-[4-(triethylammoniomethyl)-benzoyl]-caprolactam-chloride (TBCC) has been developed and readily applied for cotton bleaching recently without toxic and odorous byproducts. Equivalent bleaching effectiveness of using TBCC/H2O2 at 50 °C to that only using H2O2 at 95 °C is observed (Cui et al., 2016), thanks to the much higher redox potential of PA than H2O2. Moreover, pad-steaming for four minutes using TBCC/H2O2 generates a similar bleaching effect to the treatment only with H2O2 for one hour (Peng et al., 2018). These two examples imply that both temperature and processing time are reduced by involving TBCC/H2O2 system. It is noteworthy that TBCC is a cationic activator distinguishing it from non-ionic TAED and anionic NOBS. In view of this, TBCC shows high affinity towards anionic surface of cotton fibre, which promotes the ‘in-situ’ generation of PA on fibre surface, rather than the formation of PA in solution and transfer to fibre surface – a two-step procedure (Xu et al., 2010). This decreases the invalid decomposition of PA before reaching fibre surface, facilitates better contact of PA with impurities, and drives complete degradation of impurities. In all, the good solubility and ‘in-situ’ bleaching mode of TBCC/H2O2 system is expected to display higher activating efficiency at low temperature. Therefore, TBCC/H2O2 system is a potentially suitable candidate for cold pad-batch bleaching process.
In addition to bleaching activators, sustainable techniques such as ozone, sonication, microwave, plasma, etc. have also been adopted to upgrade the processing efficiency(Bhikari Charan Panda et al., 2021). Recently, ultraviolet (UV)-assisted bleaching, i.e., to expose the padded fabric to UV light instead of conventional batch or steaming process, has gradually gained popularity due to its high efficiency, energy/water saving, and easy manipulation(Bhikari Charan Panda et al., 2021, Li et al., 2022). Under the catalysis of UV, large quantity of hydroxyl radicals (HO•) produced from H2O2 due to the O–O bond hemolysis (Ghodbane and Hamdaoui 2010, Zhang and Huang 2020), attacks the chromophore of colour impurities on cotton to achieve the purpose of bleaching (de Oliveira et al., 2017, Wang et al., 2020). UV band includes UVC (200 ∼ 280 nm), UVB (280 ∼ 320 nm), and UVA (320 ∼ 400 nm). Due to the high energy, UVC radiation reduces the mechanical strength (ca. 50% off) of cotton fibre, thus is not preferable for textile processing (Eren 2018, Wang et al., 2020). Although UVB is milder than UVC, and has been proofed effective in H2O2 bleaching of cotton (Tang and Sun 2017), the high purchase and maintenance cost of UVB lamp, makes it less competitive for textile mass production. Thus, the low-energy and cost-effective UVA radiation turns to be the suitable UV band for cotton bleaching. Upon the successfulness achieved in the degradation of dye effluent based on photocatalytic function of UVA/H2O2 system (Nie et al., 2008, Kattel et al., 2017, Nie et al., 2021), it is interesting to explore the possibility of integrating UVA radiation with TBCC/H2O2 system, to establish an efficient and energy/water saving bleaching strategy for cotton textile. Further, the bleaching mechanism of UVA assisted TBCC/H2O2 system, and factorial manipulation against bleaching effectiveness, are still pending for in-depth exploration, which finally paves the way from laboratory research towards mass production.
Cotton/spandex (C/S) fabric is one of the most favorable textile materials that combines the advantages of cotton (e.g. hygroscopicity and comfortableness) and spandex (e.g stretchability), which has been widely used to produce jeans, apparel, hosiery, etc. (Tezel and Kavuşturan 2008, Van Amber 2017). Only a small quantity (∼3%) of spandex significantly enhances the stretchability of fabric. The specific challenge in the bleaching of C/S fabric over pure cotton is the vulnerability of spandex to high temperature, high pH and condensed oxidants. In other words, conventional cotton bleaching process is impropriate to directly apply to C/S fabric. With these regards, a UVA-assisted TBCC-activated bleaching strategy is customized for C/S fabric, aiming at simultaneously reducing the energy, water and time consumption during bleaching. The bleaching efficacy was quantified by whiteness index (WI) comparing with steaming or batch bleaching. The scouring effect reflected by water absorbance was also evaluated to demonstrate the realization of developing one-step scouring/bleaching based on UV-assisted TBCC/H2O2 system. To evaluate whether the pre-treated fabric meets the demand for subsequent dyeing and finishing, the colouration and mechanical properties of fabrics were examined. Further, the impact of four significant factors (concentration of TBCC/H2O2 couple, UV band, UV Power, radiation duration) on the WI was revealed. In terms of bleaching mechanism of UVA-assisted TBCC/H2O2 system, the degradation kinetic of pigment impurity model - morin was explored via Langmuir-Hinshelwood first-order kinetics model. Importantly, an exclusive in-depth study was implemented by integrating continuous identifying and tracking of effective bleaching species using radical scavenging experiment and fluorescence (FL) labelling method, and the liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analyses for degraded morin segments, to speculate the reasonable degradation pathway. At last, the water and energy conservation during the UVA-assisted bleaching process based on TBCC/H2O2 system were estimated.
2 Material and method
2.1 Materials
Desized, unscoured and unbleached cotton/spandex (C/S) fabric (Specification: 97% cotton/3% spandex; density 290 g/m2), and 100% cotton (C) fabric (Density: 148 g/m2), were obtained from Zhejiang Tengma Textile Co., Ltd., China. TBCC (purity>90%) was prepared through a condensation and substitution reaction based on a previous report (Cui et al., 2016). The structure of TBCC was confirmed by LC-MS and hydrogen nuclear magnetic resonance spectroscopy (1H NMR) (Figure S1). Non-ionic penetrating agent (Industrial grade) was provided by Jiangsu Haian Petroleum Chemical Plant, China. Hydrogen peroxide (purity 30%), ethylenediamine tetraacetic acid disodium (EDTA-2Na) and sodium salts were in analytical grade. Morin (purity>90%), nitrotetrazolium blue chloride (NBT, purity 98%), dimethyl sulphoxide (DMSO, purity ≥ 99.5%), benzoquinone (BQ, purity ≥ 99%) and sodium azide (SA, purity ≥ 99.5%), sodium oxalate (SO, purity ≥ 99.8%) were purchased from Aladdin. Benzenepentacarboxylic acid (BA, purity>98%) was bought from TCI Chemical Industry Co. Ltd., China. Three direct dyes Direct Red 4BS, Direct Blue 15, Direct Yellow 12 with three representative colour hues (See chemical structures in Figure S2), were used for the dyeing of C/S fabric. Industrial neutral detergent for fabric soaping was obtained from Ningbo Runhe High-Tech Material Co., Ltd., China. Deionized water was used. All H2O2 mentioned refers to pure H2O2.
2.2 Fabric treatment
Fabrics were subject to padding (i), bleaching (ii) and washing & drying (iii) processes. For comparative study, four bleaching processes were formulated, i.e., UV-radiating bleaching (UV-Bl, a), steaming bleaching (St-Bl, b), cold pad-batch bleaching (CP-Bl, c), and conventional waterbath bleaching (Conv-Bl, d). Conv-Bl was performed by directly immersing fabric in bleaching bath without padding process. The processing parameters of Conv-Bl are listed in Table S1. UV-Bl is the major process explored in this study, while St-Bl, Conv-Bl and CP-Bl are only used to compare with UV-Bl. Ten gram of fabric was used for each process. Detailed processes are displayed in Fig. 1. Besides, two bleaching systems, i.e., NaOH/H2O2 (Table 1a) and TBCC/H2O2 (Table 1b) are incorporated to UV-Bl process. UV-Bl(ii) was carried out in a customized lamp-box installed with 6 UV lamps (See Figure S3). The power of each lamp was 15 W. The total radiation power can be adjusted to 30 W, 60 W or 90 W by a switch. Two kinds of lamps, i.e., UVA (TLD 15 W/03, Philips Co., Ltd.) and UVC (G15T8, SANKYO DENKI Co., Ltd.) were prepared in this research. Note: The molar ratio of TBCC/H2O2/NaHCO3 in formulation B were set as 1/1.2/1.5 following a previous study (Peng et al., 2018). The reaction of molar ratio of TBCC/H2O2 is 1/1. A slightly excessive H2O2 is conducive to the complete perhydrolysis. NaHCO3 maintains a suitable pH to avoid side reactions. * pH stands for the actual pH value of the bleaching solution which was prepared according to a liquor ratio of 25: 1.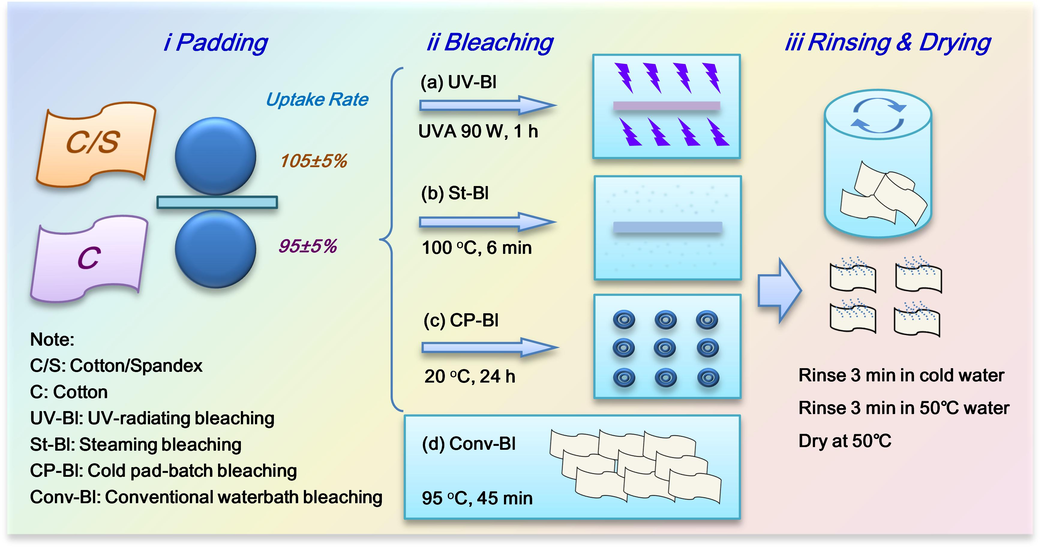
Four bleaching processes for the comparative study.
Formulation
30% H2O2
(g/L)TBCC
(g/L)NaOH
(g/L)NaHCO3
(g/L)EDTA-2Na
(g/L)Penetrating
Agent (g/L)pH*
A-1
3.7
–
0.35
–
0.35
3
10.56
A-2
7.4
–
0.7
–
0.7
3
10.70
A-3
10
–
1
–
1
3
10.77
A-4
30
–
3
–
2
3
10.83
A-5
60
–
6
–
3
3
10.88
B-1
0.93
2.5
–
0.85
0.25
3
7.41
B-2
1.9
5
–
1.7
0.5
3
7.39
B-3
3.7
10
–
3.4
1
3
7.37
B-4
5.6
15
–
5.1
1.5
3
7.36
B-5
7.4
20
–
6.8
2
3
7.35
B-6
11.1
30
–
10.3
3
3
7.21
B-7
14.8
40
–
13.6
4
3
7.15
B-8
18.5
50
–
17
5
3
6.94
2.3 Single factor test
Concentration of oxidants: The relationship between the concentration of oxidants and WI was explored using recipes of Group B in Table 1. C/S fabrics were bleached under 90 W UVA radiation for 1 h following procedure (a) in Fig. 1.
UV wavelength: Fabrics were first padded with bleaching solution of Group B-3,5,6, and then irradiated under UVA (365 nm) or UVC (254 nm) at power of 90 W for 1 h.
Power of UV lamp: Fabrics were first padded with bleaching solution of Group B-3,5,6, and then irradiated under UVA at power of 30 ∼ 90 W for 1 h.
UV exposure time: Fabrics were first padded with bleaching solution of Group B-5, and then irradiated under UVA at power of 90 W for 5 ∼ 120 min.
2.4 Dyeing experiment
The dyeing property of the bleached fabric was evaluated through dyeing with direct dyes with three-primary colours. Fabrics were immersed in the dyeing solution containing 0.5% owf dyes and 5 g/L Na2SO4 with a mass ratio (solution to fabric) of 25:1. The temperature of dyeing process started at 40 °C, raised up at a speed of 3 °C/min, and kept constant temperature of 90 °C for 45 min. Then the dyed fabrics were washed at 50 °C for 2 min followed by tap water for 2 min, and finally dried naturally.
2.5 Mechanism study
Degradation of morin in H2O2/TBCC system: A volume of 500 mL Morin solution (240 μmol/L) was prepared with the addition of NaOH (2 mmol/L) for dissolution. Then six identical mixtures containing 50 mL Morin solution, 50 mL TBCC solution (16 mmol/L), and 1 mL H2O2 solution (1 mo1/L) were prepared. Then, petri dishes holding as-prepared mixtures were loaded in the lamp-box under 90 W UVA or UVC radiation for different duration (0–30 min). In parallel, a series of conical flask holding the as-prepared mixtures were kept at constant temperatures without radiation for different duration. The Abs intensities of these mixtures were continuously detected by TU-1950 UV–Vis spectrophotometer (Beijing Persee General Instrument Co., Ltd.). To explore the role of TBCC in the morin degradation process, another set of experiment was carried out without TBCC for comparison.
Radical scavenging test: To explore valid radicals in degrading morin within TBCC/H2O2 solution, four kinds of radical scavengers, i.e., DMSO, BQ, SA and SO were used as the scavengers of HO•, O2–•, 1O2 and h+, respectively. (Cheng et al., 2022, Kaur et al., 2022). NBT was also included as the radicals’ probe (Dong et al., 2021). To verity the impact of radicals on WI improvement, DMSO and BQ were added into the actual bleaching solution to capture the radicals, respectively (Li et al., 2020, Wang et al., 2020). The fabric was immersed in a mixture of 1 g/L penetrating agent, 12.6 g/L TBCC, 4 g/L 30%H2O2, 3 g/L NaHCO3 and 0–60 mL/L DMSO or 0–2.5 g/L BQ and bleached under UVA radiation for 45 min. The weight ratio of fabric to solution is 1:50.
LC-MS and MS: To demonstrate the completion of degradation reaction, LC-MS (Shimadzu, Japan) was used to identify the structural change of morin before and after degradation. The mobile phase consisted of 0.025% ammonia water (phase A) and acetonitrile (phase B) (A/B = 95%/5%∼5%/95%). Morin (6 mmol/L) was degraded in a homogeneous mixture containing 50 mmol/L TBCC, 60 mmol/L H2O2 and 2 mmol/L NaOH, followed by exposing the solution at UVA irradiation for 1 h. The degraded reaction solution was immediately freeze-dried at the end of radiation at −60◦C for 16 h to obtain the powder-like product. The degradation product was purified by column chromatography with the eluent consisting of methanol and methylene chloride. The molecular weight of the purified degraded products were detected by MS (Shimadzu, Japan) to speculate their chemical structures.
UV–Vis absorption spectroscopy: The concentration of morin was also monitored by UV–Vis absorption spectroscopy. The characteristic absorption peaks of morin existed at 275 nm, 317 nm and 410 nm, respectively (Sen and Yildiz 2017). The quantitative relationship between the absorbance at 410 nm and the concentration of morin solution was obtained in our previous study (Li et al., 2022). Based on this, the morin concentration at the initial time point (C0) and certain time point (C) during the degradation process can be calculated. Furthermore, the degradation rate of morin was explored based on degradation kinetics calculated via the simplified Langmuir-Hinshelwood first-order kinetics model (See Eq. (1) (Cao et al., 2022)).
where K (min−1) and t (min) are the rate constant and the reaction time, respectively.
Concentration of HO•: The HO• concentration was measured by FL labeling method according to a recent report (Si et al., 2014, Li et al., 2022). Benzenepentacarboxylic acid (BA) as a sensitive fluorescent probe was used to capture HO•. The measurement was carried out on F-2500 FL spectrophotometer (Hitachi, Japan). The parameters are as follows: band pass slit: 10.0 nm; excitation voltage of 700 V; Scan rate: 300 nm/min; EX wavelength: 307 nm).
To explore the effect of UVA radiation and bleaching time on the HO• concentration, a simulated bleaching solution containing 400 μmol/L BA, 200 μmol/L H2O2 and 200 μmol/L TBCC at a pH value of 10.5 adjusted by NaOH was prepared and subjected to the UVA irradiation (90 W) in the lamp-box for different duration (0–120 min). The FL intensity was taken by the end of treatment. Considering the test accuracy, fabric and surfactant were not used. As reference, another set of experiment went aside using the same condition in the absence of UVA radiation.
To further examine the influence of TBCC/H2O2 on the HO• concentration, a batch of simulated bleaching solutions at different TBCC/H2O2 concentrations (i.e., 100–800 μmol/L) were made with addition of 400 μmol/L BA. Then, above bleaching solution was subject to 90 W UVA radiation for 15 min. It is worth noting that the concentration of TBCC and H2O2 increased simultaneously according to 1:1 as the reaction molar ratio between TBCC and H2O2.
2.6 Measurement of fabric
Colour feature: CIE (L*, a*, b*), tristimulus values (X, Y and Z), colour depth (K/S) and whiteness index (WI) of fabric, were obtained from 110 reflectance spectrophotometer (Datacolor, USA) using D65 illuminant at 10◦ standard observer. The yellowness index (YI) was calculated using Eq. (3),
Tensile strength: The YG028 universal material testing machine (Wenzhou Fangyuan, China) was adopted to evaluate the tensile strength of untreated (A-5) and bleached (B-5) fabrics based on ISO 2062: 2009. The two clamps held the fabric sample (50 × 300 mm2) and pulled it at a speed of 20 cm/min. The average results of each sample from five parallel tests in the both directions were reported.
ATR FT-IR: The ATR-FTIR spectra of untreated and bleached fabrics were recorded on a Nicolet iS50 ATR-FTIR spectrometer (Thermo Fisher Scientific Inc, USA) in the wavenumber range of 4000–500 cm−1 at a resolution of 16 cm−1.
XRD: The X-ray diffraction spectra of untreated and bleached fabrics were obtained from Ultima IV X-ray diffractometer (Rigaku Analytical Devices, Inc. Japan) at scattering angles ranging from 10° to 80°. The voltage and currency were 40 kV and 40 mA, respectively.
3 Results and discussion
3.1 General evaluation of UVA-assisted bleaching based on TBCC/H2O2 system
3.1.1 Whiteness and wetting property
The bleaching effectiveness quantified by WI exhibits great impact on the subsequent dyeing and finishing processes, thus prior to systematic and in-depth investigation, the UVA-assisted bleaching processes using conventional NaOH/H2O2 system (Formulation A in Table 1) and activated TBCC/H2O2 system (Formulation B in Table 1) were carried out and compared (see Fig. 1a). As seen in Table 2, 7.4 g/L of H2O2 (30%) enhanced the WI of C/S fabric from 51.8 to 63.8, and further upgraded to 76.3 when using 60 g/L of H2O2 (30%). The appearance of C/S fabric changed evidently from light yellow to white by visual inspection, indicating that such process achieved a medium-to-high bleaching level. With the participation of TBCC as H2O2 activator, only 7.4 g/L of H2O2 (30%) (B-5) enabled a slightly higher WI than 60 g/L of H2O2 (30%) (A-5). Similar phenomenon was also observed on C fabric. A higher WI was achieved in TBCC/H2O2 system, even though which contains much less H2O2 than that in NaOH/H2O2 system. Such result proves the evident efficiency enhancement by TBCC-activated system. The reason is as follows: 4-(triethylammoniomethyl) -perbenzoic acid (TPA) was in-situ generated from TBCC via the perhydrolysis, and was able to powerfully oxidize the yellowish impurities only using a low dosage owing to the much higher redox potential of TPA than H2O2. Simultaneously, TPA itself was easy to decompose due to its lower peroxy bond energy than H2O2. The decomposition product ROS enabled the colour pigments to be degraded thoroughly with the generation of 4-(triethylammoniomethyl) benzoic acid (TBA) (See Figure S4). Further, cold pad-batch (CP-Bl) and steaming (St-Bl) bleaching process was performed to analyze the effect of different processes on the efficiency of TBCC/H2O2. As shown in Table 3, both C/S and C fabrics obtained a higher WI after the UVA-assisted bleaching (UV-Bl), meaning that TBCC/H2O2 system functions more completely during UV-Bl compared with CP-Bl and St-Bl. Compared with St-Bl, the processing temperature of UV-Bl decreased from 100 °C to 30 °C, which is conducive to the inhibition of side-reactions during bleaching, such as the hydrolysis of TBCC, bimolecular decomposition of TPA, and the excessive oxidation of fibers (Lee et al., 2010, Wang et al., 2014), thereby improving the utilization efficiency of TPA and ROS. Compared with CP-Bl, UV light itself probably led to the chromophore structural breakages of colour impurities in cotton fibres. Meanwhile, the perhydrolysis of TBCC and the degradation of cotton pigments could be promoted with the aid of photocatalytic function of UV exposure. Therefore, the efficiency and feasibility of the designed UVA/TBCC/H2O2 bleaching strategy is confirmed.
The wetting property reflected by capillary effect and spreading time is another significant indicator to demonstrate the scouring effectiveness. As displayed in Table 2, the capillary effect of the fabric treated in formulations A-5 and B-5 both exceeded 8 cm/30 min, demonstrating that those two formulations imparted fabric with qualified wetting property that meet the requirement for subsequent dyeing and finishing (Li et al., 2022). It also needs to be emphasized that the pH of the treatment solution using formulation B-5 was 7.35 which is much milder than that of formulation A-5 (pH 10.88). Therefore, the mild pH is another advantage of this process design. In other words, less acid is required to neutralize the resultant treatment solution before discharge. And also much less water is needed to rinse the bleached fabric. Result also proves that UV-assisted TBCC/H2O2 system enhances the wetting property of C/S and C under near-neutral conditions. This is probably because of the breakage of continuously distributed hydrophobic aliphatic monohydric alcohol wax on cotton surface attacked by TPA under UVA radiation. Besides, the wetting property of C fabric was better than that of C/S fabric indicated by spreading time, though the C fabric is undesized. This is due to a lower density of C fabric which is conducive to the penetration of treatment solution. In all, the results above confirm that the UV-assisted TBCC/H2O2 system integrates scouring and bleaching processes into one step. Note: The formulations for fabric treatment are displayed in Table 1. Note: The parameters of these three processes are displayed in Fig. 1.
Sample information
WI
Capillary effect (cm/30 min)
Spreading time (s)
C/S
C
C/S
C
C/S
C
Untreated
51.8 ± 1.7
38.6 ± 1.2
0
0
>60
>60
Formulation A-2
63.8 ± 0.7 (23.2%)
63.2 ± 1.1 (63.7%)
7.6 ± 0.3
5.9 ± 0.1
40 ± 9
45 ± 8
Formulation A-5
76.3 ± 1.8 (47.3%)
73.7 ± 1.6 (90.9%)
9.8 ± 0.3
9.3 ± 0.3
23 ± 2
10 ± 3
Formulation B-5
78.0 ± 0.7 (50.8%)
74.6 ± 1.0 (83.3%)
9.6 ± 0.4
9.1 ± 0.3
25 ± 1
24 ± 4
Sample information
C/S
C
UV-Bl
CP-Bl
St-Bl
UV-Bl
CP-Bl
St-Bl
Formulation B-3
70.72 ± 1.0
68.71 ± 1.2
70.02 ± 0.8
68.50 ± 0.8
62.62 ± 1.3
64.20 ± 0.7
Formulation B-5
78.0 ± 0.7
72.67 ± 1.1
71.07 ± 0.7
74.6 ± 1.0
70.57 ± 1.3
69.51 ± 0.8
Formulation B-6
76.76 ± 0.8
74.36 ± 1.2
73.06 ± 0.6
76.07 ± 1.1
73.54 ± 1.1
71.48 ± 0.7
By manipulating the compositions in the bleaching solution and UVA radiation (Table S2), WI of C/S fabric was further improved after introducing UVA at the usage of equivalent bleaching compositions and conditions. The effectiveness of TBCC and UVA radiation to WI enhancement is also confirmed, and therein the contribution of TBCC is more evident than that of UVA radiation. In general, above results demonstrate that the advantages of the UVA-assisted process using TBCC/H2O2 system include: 1) the significant reduction of H2O2 (30%) usage from 60 g/L to 7.4 g/L, 2) the effective enhancement in the wetting property (integrating the scouring with bleaching), and 3) the reduction of acid, water, and energy consumption for post pH neutralization. These three aspects are all essential benefits to establishing the sustainable bleaching process. As sample treated by formulations A-5 and B-5 show similar WI, thus further comparisons in terms of colour feature, dyeability and tensile property, are made between these two samples in the following sections.
3.1.2 Dyeability
Table S3 shows the lightness L*, a*[redness (+) and greenness (-)] and b* [yellowness (+) and blueness (-)] coordinates of untreated and bleached C/S fabrics. Untreated C/S fabric has small L* and large a*/b* values, and shows faint grey-yellowish appearance due to the abundantly existing impurities such as wax, natural pigments, seed shells, ash, etc. After UVA-assisted H2O2 bleaching, C/S fabric turns much whiter with an obvious increase in L* and decrease in a*/b* values. Further decrease in a*/b* values and less seed shells are witnessed on C/S fabric after UVA-assisted TBCC/H2O2 bleaching (See inserted photo). These colour features are consistent with the result in Table 2.
In the case of dyeing with three representative direct dyes, both blue and red dyes display higher saturation (C) and colour depth (K/S) on bleached than untreated C/S fabrics (Fig. 2), mainly due to the improved water absorbing capability of the fabric that facilitates a higher dye exhaustion. Although higher C of Direct Yellow 12 is occurred on bleached than untreated C/S fabrics, the K/S is conversely. The L* increased instead after dyed with yellow dyes. This may due to the high brightness of Direct Yellow 12 that could hardly cover the background grey-yellowish colour of the original fabric. In all, the coloration assessment above confirms the necessity for thorough UVA-assisted scouring/bleaching process using TBCC/H2O2 before the dyeing procedure.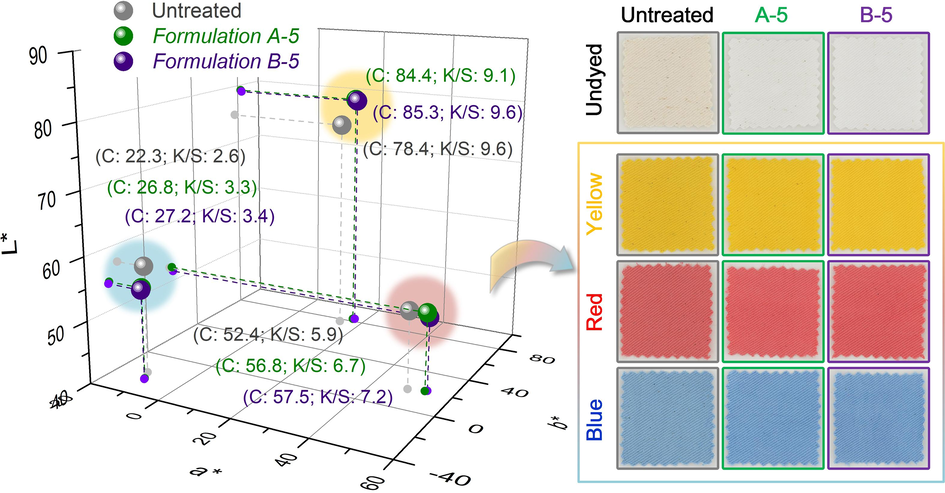
Colour coordinates of dyed fabrics in 3D colour space and the corresponding fabric samples.
3.1.3 Tensile property
The great tensile loss of bleached cotton fabric especially for C/S fabric containing oxidant-sensitive polyurethane, is one major concern during conventional bleaching treatment, which reduces the resistance against the external force and shortens the lifespan of final textile products. The harsh conventional bleaching procedure usually taken place at pH around 12 at high temperature over 95 °C for 60 min, is the major reason for tensile strength loss of fabric. Using UVA-assisted bleaching process at ambient temperature in this research, the tensile loss was reduced by c.a. 12.4% in warp direction and 3.1% in weft direction (Table 4). Further mitigation (less than 10%) was made by introducing TBCC as H2O2 activator that enables bleaching at neutral pH. C/S Fabric has larger elongation in weft direction than in warp direction due to the existence of stretchable spandex fibre. The elongation of C/S fabric after UVA-assisted bleaching increased, which is consistent with our previous research (Li et al., 2022). As there are negligible changes both in functional groups indicated by ATR-FTIR (Figure S5a) and crystalline region reflected by XRD (Figure S5b), the decrease in tensile force and increase in elongation is predicted due to the breakage of non-covalent bonds between the macromolecules in cellulose. Note: The percentage in the bracket is the variation of each data compared with untreated fabric.
Sample information
Tensile force (N) at break
Elongation (%) at break
Warp
Weft
Warp
Weft
Untreated
767 ± 41
489 ± 47
3.21 ± 0.19
10.22 ± 0.35
Formulation A-5
672 ± 11 (-12.4%)
474 ± 13 (-3.1%)
3.95 ± 0.11 (+23.1%)
10.81 ± 0.21 (+5.8%)
Formulation B-5
690 ± 31 (-10%)
488 ± 26 (-0.2%)
3.96 ± 0.17 (+23.4%)
11.35 ± 0.47 (+11.1%)
3.2 Process factor analyses
It is confirmed that C/S fabric treated by UVA-assisted process using TBCC/H2O2 had qualified dyeability and tensile performance. This section attempts to further explore the impact of four significant factors (Concentration of 30%H2O2, UV band, UV Power, and radiation duration) on the bleaching effectiveness. With the increase of H2O2 concentration (Concentration of TBCC is determined according to Table 1), the WI displays a linear increase and reaches a maximum WI of 78 at 7.4 g/L (Fig. 3a). This indicates the pigment impurity was thoroughly reacted with the oxidants. However, to further increase the concentration of H2O2 and TBCC, the WI conversely decreased and the YI increased. A reason for this fact is as follows, the high concentration of TBCC was adsorbed on the fiber surface, which may generate steric hindrance hindering the penetration of TPA into the fiber interior (Chen et al., 2016). Thus, 7.4 g/L of H2O2 (30%) and 20 g/L of TBCC are recommended.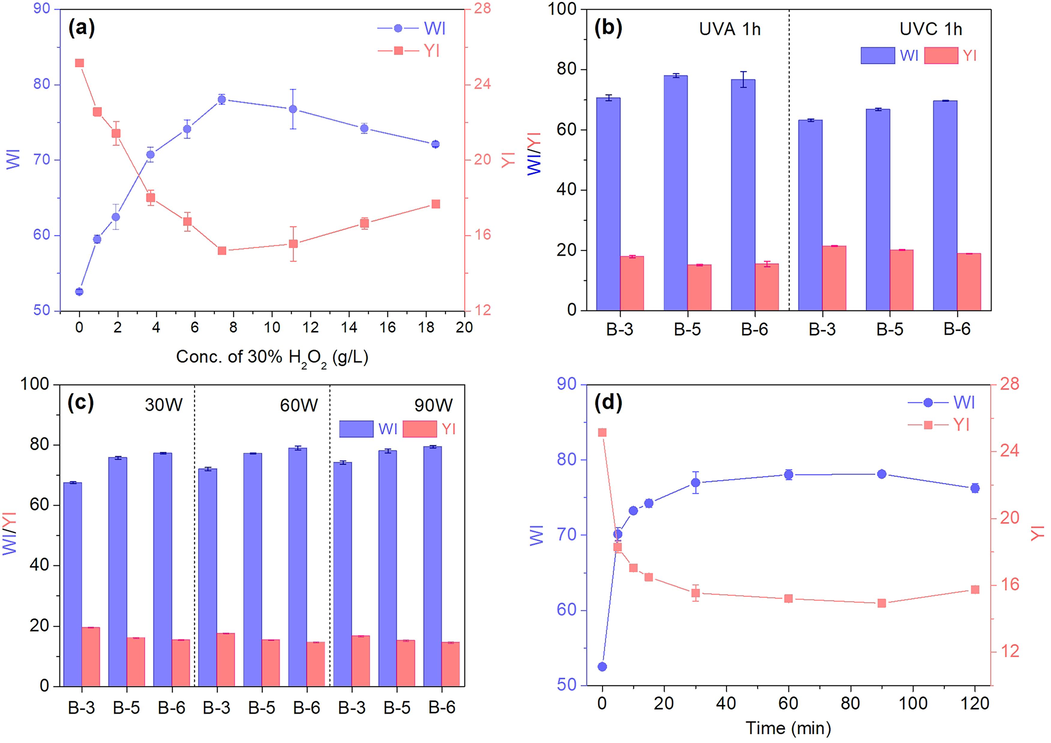
Single-factor analysis on the WI of C/S fabric: (a) Concentration of 30% H2O2, (b) UV band, (c) UV Power, and (d) radiation duration.
As depicted in Fig. 3b, UVA enabled higher WI and lower YI of fabric compared with UVC after one hour radiation. Under UVC radiation, the WI of fabric continuously increased when higher concentration of oxidants was used, indicating that the pigment impurity on cotton still requires further oxidation. The optimal WI under UVA was obtained on sample B-5. There is a slight increase of WI at higher power (30 ∼ 90 W) of UVA radiation (Fig. 3c). Standing from the energy-saving perspective, the UVA power around 30 ∼ 60 W is able to achieve desirable WI on C/S fabric. Fast decolouration took place in the first 30 min (Fig. 3d), during which, the colour impurities especially those located on the surface or embedded in the shallow layer of cotton fibre are destroyed. During the UVA radiation from 30 to 90 min, WI and YI were stabilised around 78. The oxidants were further penetrated into fibre interior to further react with the rest of impurities. Obviously, time exceeding 90 min is too much for bleaching resulting in the increase of YI instead. This is due to the over-oxidation of the hydroxyl groups occurred on the cellulosic macromolecular of cotton fibre rising from condensed oxidant, thus generating significant numbers of carbonyl and carboxyl groups. These groups act as the precursors for the formation of chromophores which have strong and broadband optical absorption in the visible spectrum regions (Milanovic et al., 2020). In general, the concentration of oxidative couples and the processing time are two significant factors influencing the quality of fabric during UVA-assisted bleaching and should be paid attention to during practical processing.
3.3 Degradation mechanism study
3.3.1 Degradation rate of morin by altering temperature and UV wavelength
Contributed by the activated effect of TBCC, the degradation of morin almost reached equilibrium by the first 10 min (Fig. 4a). Thus, it is reasonable to apply non-linear fitting to the first-order kinetics model up to 10 min. The high R2 close to 1 indicates the good fitness to the model. The larger K value means the faster degradation of morin. In conventional waterbath treatment, morin molecules degraded faster at higher temperature (indicated by K value) due to the accelerated physical and chemical reactions such as the perhydrolysis of TBCC for high concentration of TPA and its decomposition products ROS, the contact probability among morin molecules, TPA and ROS, and the oxidative degradation of morin by TPA and ROS. With the participation of UV light at ambient temperature (25 °C), the K value is much higher than that merely in waterbath. Such result demonstrates the accelerating function of UV on TBCC/H2O2 bleaching. It is also worth noting that UVC displayed lower efficiency than UVA, which is consistent with the effect of UVC on WI (see Fig. 3b). Additionally, the K value under UVA at 25 °C is close to that of 50 °C merely in waterbath treatment, in other words, to achieve a similar bleaching effect, the energy in heating up the waterbath and fabric are economized by introducing UVA radiation. For comparison, similar treatment processes without TBCC were carried out. Results are displayed in Fig. 4b. Obviously, the K value without TBCC is much smaller than that with TBCC. Thus combining the results in Fig. 4a and b, it proofs the rationality of the research design that integrates the TBCC and UVA irradiation into H2O2 bleaching process.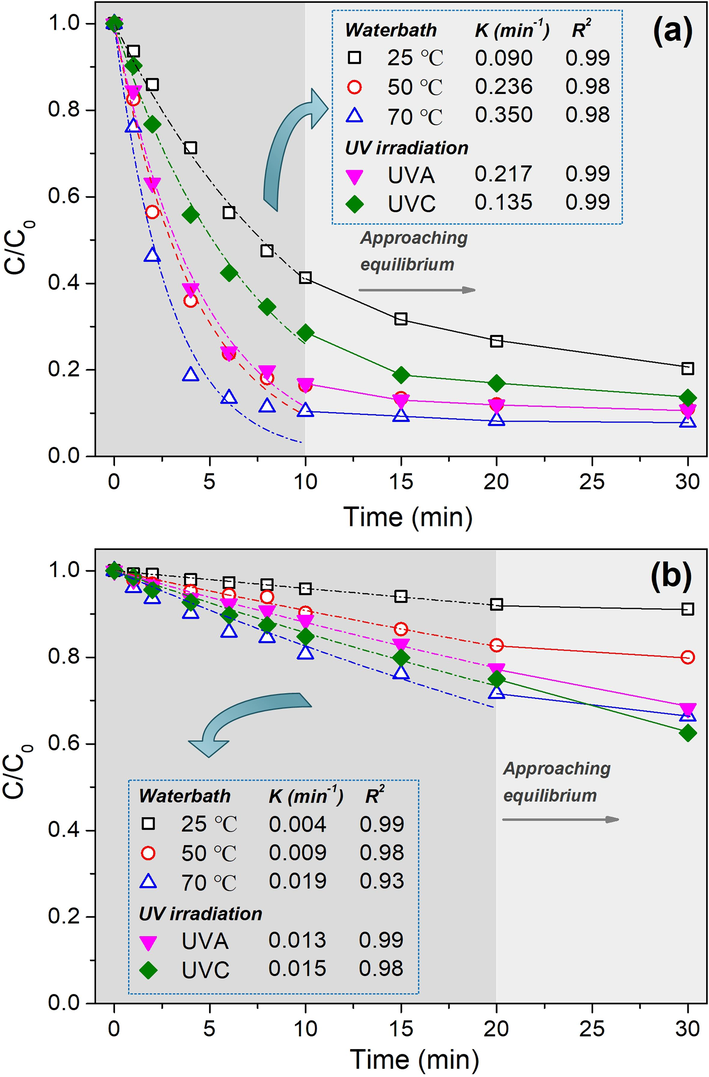
Degradation rate of morin in H2O2 oxidative system (a) with TBCC and (b) without TBCC.
3.3.2 Determination of effective ROS for morin degradation
To further explore that bleaching mechanism, the role of different ROS in TBCC/H2O2 bleaching system and their contributions to the morin degradation, were explored through radical scavenging experiment. Four radical scavengers, i.e., dimethyl sulphoxide (DMSO), benzoquinone (BQ), sodium azide (SA) and sodium oxalate (SO) were used for HO•, O2–•, 1O2 and h+, respectively according to previous reports (Si et al., 2014, Cheng et al., 2022, Kaur et al., 2022). Upon the addition of these scavengers to four identical simulated solutions, the larger impact on degradation indicates the major role of the corresponding free radicals in the bleaching process. As seen in Fig. 5a, the addition of SO and SA show negligible impact on the degradation process compared with control sample. DMSO largely slowed down the degradation process followed by BQ. Such result demonstrates the HO• is the major free radical accounting for morin degradation followed by O2–•. There is almost no bleaching function of 1O2 and h+ in the solution. Similar conclusion was also drawn in the previous studies (Liu et al., 2018, Li et al., 2022).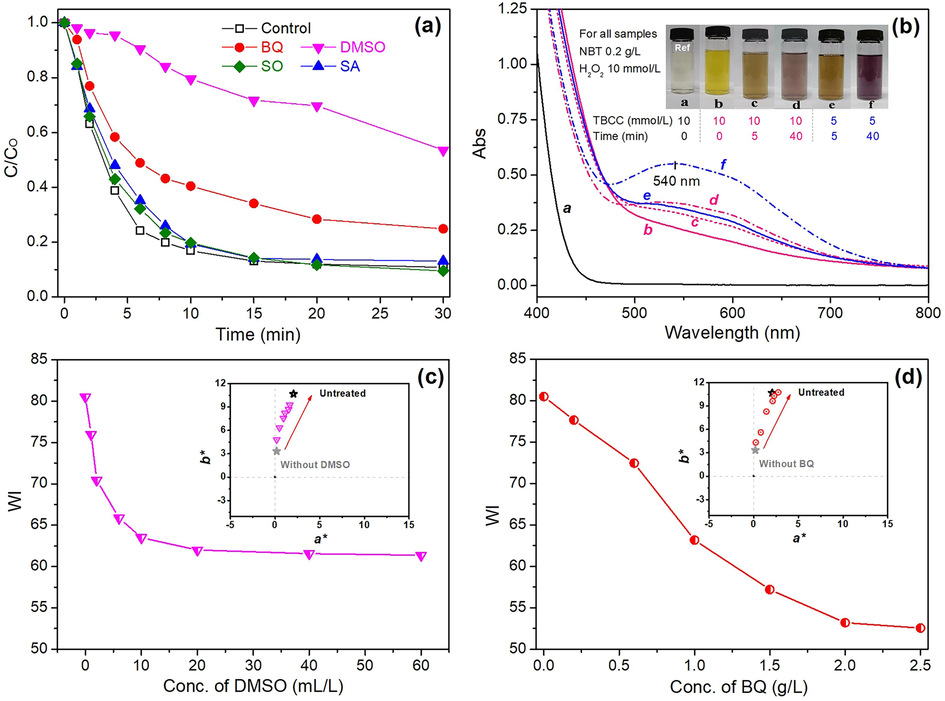
(a) Impact of radical scavengers on morin degradation in UVA-assisted TBCC/H2O2 bleaching system; (b) Colouration reaction between NBT and O2–•; and impact of the concentration of (c) DMSO and (d) BQ on the WI, a* and b* of bleached fabrics.
It has been confirmed that HO• is mainly from the decomposition of peroxy acid (Li et al., 2020). To further verify the source of O2–• in bleaching, NBT was added to the solution which enables colouration reaction with O2–• (Fig. 5b) (Dong et al., 2021). Without morin (Reference sample a), solution displayed colorless and transparent appearance compared with yellowish morin solution (Sample b)(See inserted photo). When the quantity of TBCC/H2O2 was set as 1:1, the depth of purple colour turned darker when UVA radiation prolonged to 40 min (Sample b,c,d). When 1/2 ratio of TBCC/H2O2 was used, the solution turned even darker than the corresponding solutions using 1/1 ratio of TBCC/H2O2, which indicates more O2–• was generated in the former case. Such phenomenon indicates that O2–• mainly originates from the decomposition of H2O2. Similar findings were also reported by other researchers (see reaction equation i,ii,iii) (Dannacber and Schlenker 1996, Gierer 1997, Wenhu et al., 2017). The function of HO• and O2–• in bleaching was further confirmed in practical bleaching process (Fig. 5c,d), in which the WI greatly reduced along with increasing reddish (a*) and yellowish (b*) intensity with the addition of their respective scavengers (DMSO and BQ). It is also found that the WI decreased drastically after the addition of DMSO due to the high oxidizing efficiency of HO•. However in the case of BQ, the WI decreasing pace is slower and the WI decreasing range is larger. This is probably because that O2–• owns lower reactivity and longer lifespan, and diffuses to a longer distance, than HO• (Nordberg and Arner 2001). In general, the analysis above confirms the UVA-assisted TBCC/H2O2 bleaching effect is dependable on HO• and O2–• radicals, and therein, HO• makes larger contribution than O2–• radicals.
3.3.3 HO• concentration
Upon the confirmation of the HO• as the major bleaching ROS, the concentration of HO• under different irradiation time and using different concentration of TBCC was explored in this section. As seen in Fig. 6a and b, the FL intensity representing the concentration of HO• continuously increases towards 8 k units under UVA radiation as the time approaches to 120 min, which is around 8 times over that without UVA radiation. This reveals the function of UVA to initiate the generation of HO• in TBCC/H2O2 bleaching system. By using higher concentration of TBCC, the concentration of HO• displays an increase almost along a linearship relation (Fig. 6c). The results above demonstrate that the UVA irradiation and participation of TBCC as the precursor of TPA are two significant factors to generate HO•.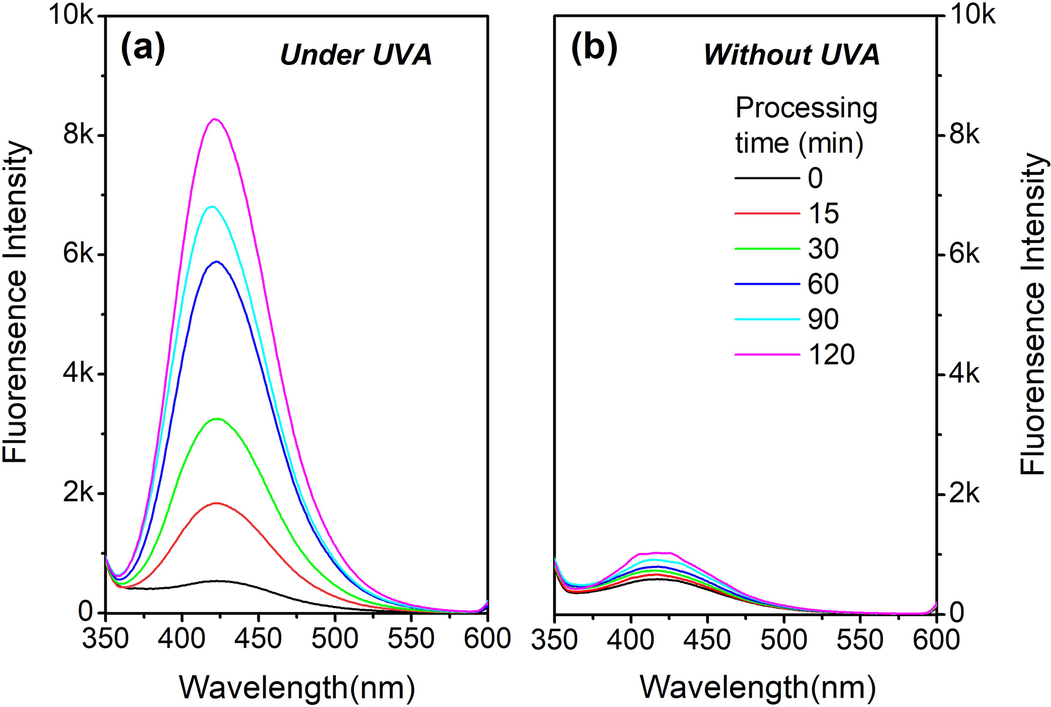
Concentration of HO• in TBCC/H2O2 system (a) with and (b) without UVA radiation, and (c) using different TBCC concentrations.
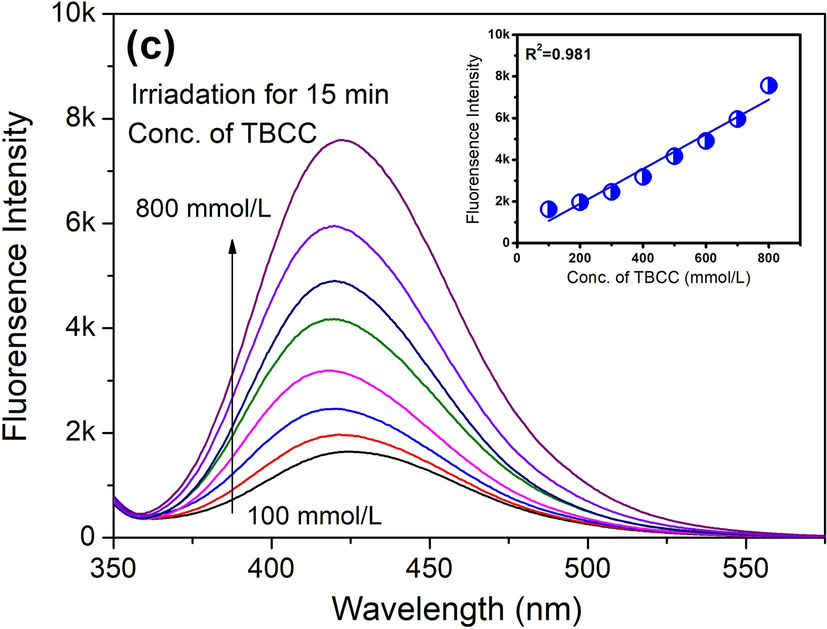
Concentration of HO• in TBCC/H2O2 system (a) with and (b) without UVA radiation, and (c) using different TBCC concentrations.
3.3.4 LC-MS and MS analyses
To gain further understanding of degradation mechanism, the degrading pathway of morin in UVA-assisted TBCC/H2O2 system was explored by LC-MS and MS. In Figure S6, the molecular ion peak at m/z 303.0 (M + H) was identified as the evidence of undegraded morin by LC-MS method at a retention time of 0.901 min. In terms of the morin after radiation treatment (Figure S7), the characteristic peak at m/z 303.0 disappeared, demonstrating that the morin was completely degraded. The retention times are 0.222 min and 0.784 min, representing the molecular ion peak of perhydrolyzed side-products of TBCC (caprolactam, m/z 114.0 and 4-(triethylammoniomethyl) benzoic acid, m/z 236.1), and unreacted TBCC (m/z 331.1), respectively. In other words, unreacted TBCC and its two perhydrolyzed side-products in the bleaching solution are detected by LC-MS. However, there is not sufficient information about the degraded morin segments, probability due to the much stronger signal intensity of TBCC and its perhydrolyzed side-products over that of the degraded segments of morin. Thus, column chromatography was adopted for precise isolation of each product in the mixture before MS analysis.
As depicted in Fig. 7, two major residual segments from morin after isolation were detected by MS, which are 2,4,6-trihydroxybenzoic acid (MW: 170.12) and 2,4,-dihydroxybenzoic acid (MW: 154.12). Thus, the degrading routine of morin under UVA-assisted TBCC/H2O2 system is proposed as follows (See the inserted reaction formulas). First, HO• radicals generated by TPA and UVA irradiation attack the C-ring of morin through 1,4-conjugate addition reaction to generate semi-acetal intermediate (1). Second, the instable semi-acetal intermediate turns into tricarbonyl intermediate through ring-open reaction (2). Finally, the electron-deficient carbonyl is attacked by HO• radicals through nuclear addition, hydrolysis and decarboxylation reactions (3) (Ilunga and Meijboom 2016). Thus, P1 and P2 are generated (Bingwa et al., 2018, Ilunga et al., 2018).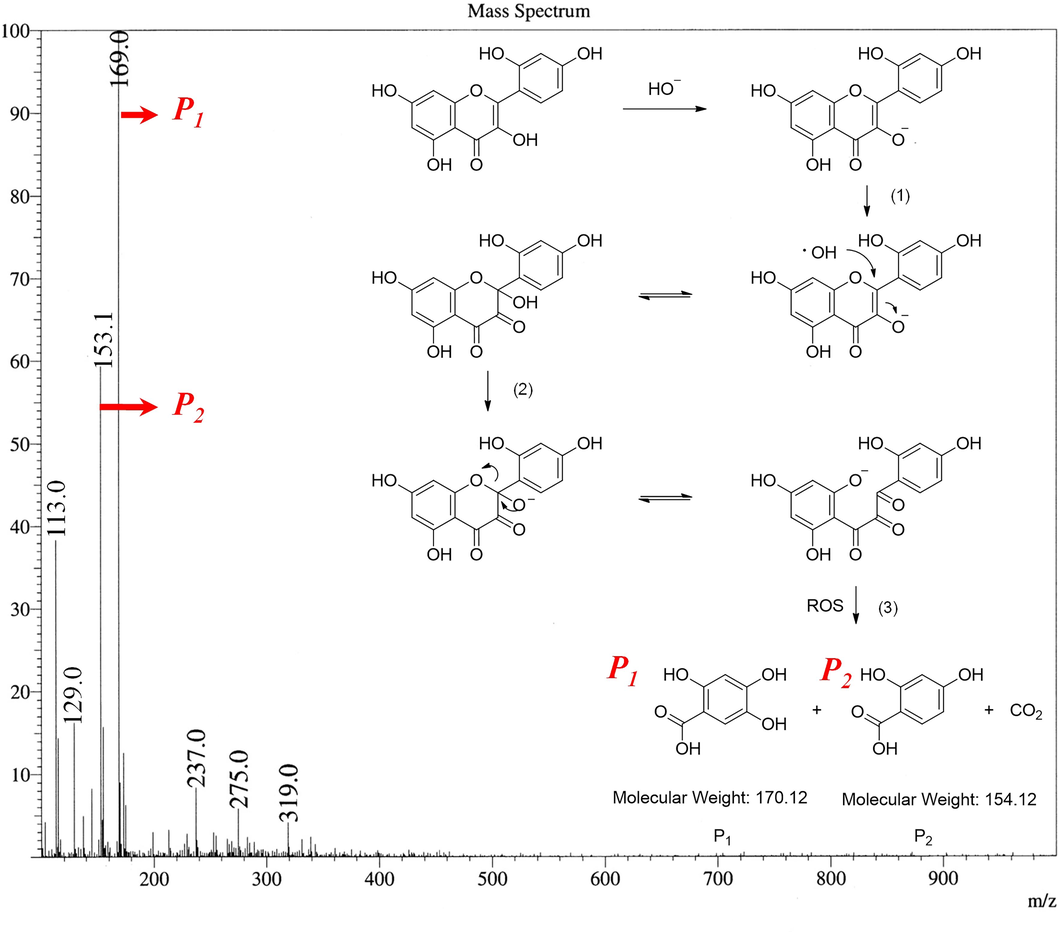
MS (positive ion mode) of degradation product and speculated degradation pathway of the morin.
3.4 Sustainability evaluation
The advantage of UVA-assisted bleaching process using TBCC/H2O2 in sustainable textile industry is evaluated with respect of water, energy and time consumptions referencing to the WI enhancement of fabric. Four processes including UV-Bl, CP-Bl, St-Bl and Conv-Bl (Fig. 1) were involved and compared. A recipe matrix accompanied with corresponding energy/water consumption and resultant WI enhancement, are displayed in Table 5. The detailed calculation methods of energy and water consumption are stated in Supporting Information.
Process
Temp
(oC)
Power
(w)
Time
(h)
30%H2O2
(g/L)
TBCC
(g/L)
pH
ΔWI
(%)
Energy
(KJ)
Water
(kg)
UV-Bl-1
30
90
1
60
0
10.88
47.3
2.7 × 104
1650
UV-Bl-2
30
90
1
7.4
20
7.35
50.8
2.7 × 104
970
UV-Bl-3
30
30
1
7.4
20
7.35
46.5
0.9 × 104
970
UV-Bl-4
30
90
0.25
7.4
20
7.35
43.4
0.675 × 104
970
St-Bl-1
100
0
0.1
7.4
20
7.35
37.3
6.006 × 104
970
St-Bl-2
100
0
0.1
60
0
12.5
39.2
6.006 × 104
1650
Conv-Bl-1
50
0
0.75
4
12.6
6.85
50.3
8.125 × 104
1260
Conv-Bl-2
95
0
0.75
6
0
11.5
47.0
2.067 × 105
1940
CP-Bl
25
0
24
7.4
20
7.35
40.4
0
970
Following the treatment recipes in the matrix, an evident trend for WI improvement is discovered. UV-Bl process exerts equivalent whiteness upgrade on the fabrics to those treated by Conv-Bl, and also displays superior WI increment over St-Bl and CP-Bl recipes, which verifies the application prospect of UV-Bl as an efficient bleaching process. An obvious energy reduction occurs on UV-Bl recipes compared with St-Bl and Conv-Bl processes when similar WI enhancement is achieved. A general comparison of the energy consumptions in different recipes is described in the pie chart (Figure S8). The main energy consumptions for St-Bl and Conv-Bl processes rise from the evaporation of the water on fabric (taking up 54.5% of the total) and the heating of the bleaching bath (taking up 89.6% of the total), respectively. In terms of UV-Bl, energy is mainly consumes by UV lamps rather than those used for phase change in St-Bl and heating bath in Conv-Bl processes, thus displaying the energy-saving advantages.
Approximate WI enhancement is obtained from UV-Bl-3 and Conv-Bl-2 processes, however 95.6% energy is saved in the front procedure. Compared the UV-Bl-2 and UV-Bl-3 processes, a significant reduction in energy consumption from 2.7 × 104 KJ to 0.9 × 104 KJ takes place when 30 W UVA power is applied instead of 90 W, however resulting in only 4.3% decline in ΔWI. In other words, UV-Bl-3 is preferable standing from the energy-saving perspective if there is no strong requirement on the whiteness. Considering the bleaching effectiveness, 46.5% WI enhancement is achieved using 30 W UVA radiation and TBCC/H2O2 system (UV-Bl-3), which is similar to that (47.3%) using 90 W UVA radiation and NaOH/H2O2 system (UV-Bl-1). Excitedly, the energy consumption in the frontier process is only 1/3 of that in the latter process, which confirms the significant energy-saving merit rising from the activation of TBCC for H2O2. Theoretically, there is no energy consumption during CP-Bl process as it is an ambient-temperature procedure. Although 40.4% WI enhancement is achieved in CP-Bl process slightly lower than UV-Bl-4 process, the time required in CP-Bl is 96 times over that of UV-Bl-4, resulting in a low processing efficiency during the continuous mass production. The pH of the process is highly related to the water consumption due to the pH neutralization, in other words, the near-neutral process benefits the water saving. Within the design matrix, UV-Bl-3 enables large WI enhancement with less energy, water and time consumption.
4 Conclusion
In this research, an UVA-assisted scouring/bleaching one-step strategy based on TBCC/H2O2 system is customised for C/S fabric. Above strategy can be efficiently achieved at room-temperature (30 °C) in a mild medium (pH 7.35). When similar bleaching effect is achieved, the UVA-assisted process saves about 90% energy and 50% water consumptions compared with the conventional waterbath process which performed at 95 °C with pH of 11.5. In terms of the bleaching mechanism of UVA/TBCC/H2O2 system, the ROS which plays a key role in decoloration, and the degradation path of morin as the model of cotton pigment, were comprehensively analysed. Results show that 7.4 g/L of H2O2 (30%) activated by TBCC displays better whiteness on C/S fabric over 60 g/L of H2O2 (30%) without TBCC. The TBCC/H2O2 concentration and radiation duration display significant impacts on the bleaching efficiency, followed by UV band and UV power. Thanks to the mild bleaching process, an acceptable tensile loss of C/S fabric of 10% in warp direction and 0.2% in weft direction is obtained. The scoured and bleached C/S fabric shows a satisfied dyeing property due to its qualified water absorbency. The bleaching condition, i.e., 7.4 g/L of H2O2(30%), 20 g/L TBCC, 30 W power of UVA, and 1 h, is preferred considering the bleaching efficacy, energy and water conservations. Implied by the degradation rate constant, the accelerating function of UVA irradiation on the degradation of morin, and the higher catalytic efficiency of UVA than UVC, are confirmed. HO• and O2–• are two major ROS taking apart in the morin degradation, therein the front one is more significant than the latter. MS detects two major degradation segments i.e., 2,4,6-trihydroxybenzoic acid and 2,4,-dihydroxybenzoic acid which originate from the oxidation and ring-opening of C-ring in morin. The research result of bleaching mechanism provides a theoretical basis for the future application of UVA/TBCC/H2O2 system.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (52303062), Opening Project of Hubei Key Laboratory of Biomass Fibers and Eco-Dyeing & Finishing (STRZ202303), Opening Project of the Opening Project of China National Textile and Apparel Council Key Laboratory of Natural Dyes, Soochow University (SDHY2217).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Simple and economic bleaching process for cotton fabric. Carbohydr. Polym.. 2012;88:1233-1238.
- [CrossRef] [Google Scholar]
- Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv.. 2015;5:30801-30818.
- [CrossRef] [Google Scholar]
- Ahmed, F. and M. I. H. Mondal, 2021. Introduction to natural fibres and textiles. Fundamentals of Natural Fibres and Textiles, Elsevier: 1-32.
- Effect of alkali and alkaline earth metal dopants on catalytic activity of mesoporous cobalt oxide evaluated using a model reaction. Appl. Catal. A. 2018;555:189-195.
- [CrossRef] [Google Scholar]
- Bleaching of Natural Fibers with TAED and NOBS Activated Peroxide Systems. AATCC Review.. 2001;1:31-34.
- [Google Scholar]
- Strong tribocatalysis of strontium titanate nanofibers through harvesting friction energy for dye decomposition. Ceram. Int.. 2022;48:9651-9657.
- [CrossRef] [Google Scholar]
- Recognizing a limitation of the TBLC-activated peroxide system on low-temperature cotton bleaching. Carbohydr. Polym.. 2016;140:1-5.
- [CrossRef] [Google Scholar]
- Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis. J. Clean. Prod.. 2022;348:131301
- [CrossRef] [Google Scholar]
- Synthesis of peroxide activator for low-temperature bleaching of cotton fabric. Journal of Textile Research.. 2016;37:88-92.
- [Google Scholar]
- Colorimetric Analysis of Cotton Textile Bleaching through H2O2 Activated by UV Light. Journal of the Brazilian Chemical Society. https:// 2017
- [CrossRef] [Google Scholar]
- Bicarbonate activated hydrogen peroxide with cobalt nanoparticles embedded in nitrogen-doped carbon nanotubes for highly efficient organic dye degradation. Colloids Surf A Physicochem Eng Asp. 2021;630:127645
- [CrossRef] [Google Scholar]
- Photocatalytic hydrogen peroxide bleaching of cotton. Cellul.. 2018;25:3679-3689.
- [CrossRef] [Google Scholar]
- Decolorization of antraquinonic dye, C.I. Acid Blue 25, in aqueous solution by direct UV irradiation, UV/H2O2 and UV/Fe(II) processes. Chem. Eng. J.. 2010;160:226-231.
- [CrossRef] [Google Scholar]
- Formation and Involvement of Superoxide (O2-/HO2·) and Hydroxyl (OH·) Radicals in TCF Bleaching Processes: A. Review. 1997;51:34-46.
- [CrossRef] [Google Scholar]
- Sustainable processes for pre-treatment of cotton fabric. Textiles and Clothing Sustainability.. 2016;2
- [CrossRef] [Google Scholar]
- Isothermic adsorption of morin onto the reducible mesoporous manganese oxide materials surface. Appl Catal B. 2018;224:928-939.
- [CrossRef] [Google Scholar]
- Synthesis of narrowly dispersed silver and gold nanoparticles and their catalytic evaluation for morin oxidation. Appl. Catal. A. 2016;509:17-29.
- [CrossRef] [Google Scholar]
- Oxidative degradation of emerging micropollutant acesulfame in aqueous matrices by UVA-induced H2O2/Fe2+ and S2O82-/Fe2+ processes. Chemosphere. 2017;171:528-536.
- [CrossRef] [Google Scholar]
- Time-dependent mechanistic insight into photo-degradation of mixed hydrophobic disperse dyes by magnetically separable nitrogen iron codoped titania under visible light using process variable optimization. J. Clean. Prod.. 2022;342:130940
- [CrossRef] [Google Scholar]
- Reactivity of Peracetic Acid with Organic Compounds: A Critical Review. ACS ES&T Water.. 2020;1:15-33.
- [CrossRef] [Google Scholar]
- Hydrolytic stability of a series of lactam-based cationic bleach activators and their impact on cellulose peroxide bleaching. Cellul.. 2010;17:671-678.
- [CrossRef] [Google Scholar]
- Establishing an ultrasound-assisted activated peroxide system for efficient and sustainable scouring-bleaching of cotton/spandex fabric. Ultrason. Sonochem.. 2020;68:105220
- [CrossRef] [Google Scholar]
- Energy-Saving One-Step Pre-Treatment Using an Activated Sodium Percarbonate System and Its Bleaching Mechanism for Cotton Fabric. Materials.. 2022;15
- [CrossRef] [Google Scholar]
- Bleaching of cotton fabric with tetraacetylhydrazine as bleach activator for H2O2. Carbohydrate Polymer. 2018;188:221-227.
- [CrossRef] [Google Scholar]
- Development of o-phthalic anhydride as a low-temperature activator in H2O2 bleaching system for cotton fabric. Cellul.. 2018;25:859-867.
- [Google Scholar]
- Stability of TEMPO-oxidized cotton fibers during natural aging. Carbohydr. Polym.. 2020;230:115587
- [CrossRef] [Google Scholar]
- Efficient photodegradation of Acid Red B by immobilized ferrocene in the presence of UVA and H2O2. J. Hazard. Mater.. 2008;154:146-152.
- [CrossRef] [Google Scholar]
- Natural alumina/silica suspended particles in water to enhance ofloxacin degradation with UVA-H2O2 driven by surface chemistry. J. Hazard. Mater.. 2021;412:125259
- [CrossRef] [Google Scholar]
- Reactive Oxygen Species, Antioxidants and the Mammalian Thioredoxin System. Free Radic. Biol. Med.. 2001;31:1287-1312.
- [CrossRef] [Google Scholar]
- Bhikari Charan Panda, S. K., K. Sen and S. Mukhopadhyay, 2021. Sustainable pretreatments in textile wet processing. J. Clean. Prod. 129725. https://doi.org/10.1016/j.jclepro.2021.129725.
- Establishing a rapid pad-steam process for bleaching of cotton fabric with an activated peroxide system. ACS Sustain. Chem. Eng.. 2018;6:8599-8603.
- [CrossRef] [Google Scholar]
- The investigation of oxidative bleaching performance of peripherally Schiff base substituted tri-nuclear cobalt-phthalocyanine complexes. Inorg. Chim. Acta. 2017;462:30-39.
- [CrossRef] [Google Scholar]
- Perspectives for natural product based agents derived from industrial plants in textile applications–a review. J. Clean. Prod.. 2013;57:2-18.
- [CrossRef] [Google Scholar]
- Recent advancements in natural dye applications: a review. J. Clean. Prod.. 2013;53:310-331.
- [CrossRef] [Google Scholar]
- Production of higher toxic intermediates of organic pollutants during chemical oxidation processes: A review. Arab. J. Chem.. 2023;16:104856
- [CrossRef] [Google Scholar]
- Study on H2O2/TAED and H2O2/TBCC bleaching mechanism related to hydroxyl radical with a fluorescent probe. Carbohydr. Polym.. 2014;103:581-586.
- [CrossRef] [Google Scholar]
- Generation of hydroxyl radicals and effective whitening of cotton fabrics by H2O2 under UVB irradiation. Carbohydrater Polymer. 2017;160:153-162.
- [CrossRef] [Google Scholar]
- Experimental Investigation of Effects of Spandex Brand and Tightness Factor on Dimensional and Physical Properties of Cotton/Spandex Single Jersey Fabrics. Text. Res. J.. 2008;78:966-976.
- [CrossRef] [Google Scholar]
- Ultrasound energy to accelerate dye uptake and dye–fiber interaction of reactive dye on knitted cotton fabric at low temperatures. Ultrason. Sonochem.. 2016;29:270-278.
- [CrossRef] [Google Scholar]
- Chapter Two - Apparel and Household Textiles and Their Role in Forensics. In: Carr D., ed. Forensic Textile Science. Woodhead Publishing; 2017. p. :15-26.
- [Google Scholar]
- Treatment and Reuse of Wastewater from the Textile Wet-Processing Industry Review of Emerging Technologies. J. Chem. Technol. Biotechnol.. 1998;72:289-302.
- [CrossRef] [Google Scholar]
- X-ray photoelectron spectroscopy analysis of cotton treated with the TBCC/H2O2/NaHCO3 system. Text. Res. J.. 2014;84:2149-2156.
- [CrossRef] [Google Scholar]
- An environmentally friendly bleaching process for cotton fabrics: mechanism and application of UV/H2O2 system. Cellul.. 2020;27:1071-1083.
- [CrossRef] [Google Scholar]
- Studies on affecting factors and mechanism of oily wastewater by wet hydrogen peroxide oxidation. Arab. J. Chem.. 2017;10:S2402-S2405.
- [CrossRef] [Google Scholar]
- Bleaching cellulosic fibers via pre-sorption of N-[4-(triethylammoniomethyl)-benzoyl]-butyrolactam chloride. Cellul.. 2010;17:849-857.
- [CrossRef] [Google Scholar]
- Review of Bleach Activators for Environmentally Efficient Bleaching of Textiles. J. Fiber Bioeng. Inf.. 2011;4:209-219.
- [CrossRef] [Google Scholar]
- Application of a novel bleach activator to low temperature bleaching of raw cotton fabrics. The Journal of The Textile Institute.. 2014;106:807-813.
- [CrossRef] [Google Scholar]
- Modeling the kinetics of UV/peracetic acid advanced oxidation process. Environ. Sci. Tech.. 2020;54:7579-7590.
- [CrossRef] [Google Scholar]
- An eco-friendly approach to low-temperature and near-neutral bleaching of cotton knitted fabrics using glycerol triacetate as an activator. Cellul.. 2021;28:8129-8138.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105348.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







