Translate this page into:
Electrocatalytic decomposition mechanism of hydroxyamine nitrate on the Cu(111) surface
*Corresponding authors: E-mail addresses: ysql123@yeah.net (T. Li), deco168@qq.com (Y. Huang)
-
Received: ,
Accepted: ,
Abstract
Hydroxylamine nitrate (HAN)-based electrically controlled solid propellants are currently the mainstream electrically controlled solid propellants. To gain a deeper understanding of their electrically controlled combustion mechanisms, this study employed quantum chemical simulations to systematically explore the electrocatalytic decomposition of HAN on a Cu(111) surface. The findings indicate that the electrocatalytic decomposition of NO3- ions on the anode surface is the dominant reaction for O2 generation, with a reaction barrier significantly lower than that of water electrolysis, demonstrating a pronounced reaction advantage. By optimizing the configurations of adsorbed molecules during the reaction and calculating the barriers for each reaction step, we have detailed the mechanism of HAN’s electrocatalytic decomposition on the Cu(111) surface. Additionally, the reaction on a cathode surface is relatively facile, with the key step being the adsorption and decomposition of NH3OH+ ions on the cathode surface. The generation of hydrogen ions in the anode reaction and their consumption in the cathode reaction lead to the cathode solution becoming alkaline and the anode solution becoming acidic after electrolysis. These findings provide important theoretical insights into the electrically controlled combustion mechanisms of HAN-based electrically controlled solid propellants.
Keywords
Electrocatalytic decomposition
Electrode surface
Hydroxylamine nitrate
Quantum chemistry calculations
Reaction barrier
1. Introduction
Electrically controlled solid propellants, a new variant that can be ignited, burned, and extinguished using electrical energy control, are attracting widespread attention in the controllable propulsion field [1]. At present, most electrically controlled solid propellants are based on hydroxylamine nitrate (HAN) [2], a high-energy oxidant with a high specific impulse and low toxicity [3].
Research on HAN has mainly focused on the mechanism of thermal decomposition. In separate studies using mass spectrometry, Izato et al. [4] and Souagh et al. [5] commonly identified HNO3, N2O, and H2O as thermal decomposition partial products of HAN, but Izato et al. [4] believed that the products also include N2 and NH3, and Souagh et al. [5] considered the products also include NO and NO2. Using an infrared spectrometer, Cheng et al. [6] identified N2O, NO, H2O, and NO2 as the thermal decomposition products of HAN. Meanwhile, the reaction pathway of HAN thermal decomposition has been investigated through quantum chemical simulations. Early studies reported various formation pathways of N2O. For example, Oxley et al. [7] believed that N2O is produced by the reaction of intermediate products HNO2 and NH2OH. In recent years, Izato et al. [4], Taylor et al. [8], and Zhang et al. [9] discovered that N2O is produced by the reaction of intermediate products HNO and HNO. However, elucidating the electrocatalytic decomposition mechanism of HAN on electrode surfaces is essential for advancing the electrically controlled combustion mechanism of solid propellants. At present, this topic has been scantily researched and usually focuses on electrolytic effects or product analysis. For example, Koh et al. [10] studied the effects of power supply and electrode materials on the electrocatalytic decomposition of HAN. They demonstrated higher performance with copper or aluminum electrodes than with carbon electrodes and reported that a power supply exceeding 100 W can effectively trigger decomposition. Sun et al. [11] studied the effects of electrolysis voltage and electrode surface area on the electrolysis of HAN solution. They reported an optimal voltage for electrolysis under fixed conditions. This is caused by an inhibition phenomenon at the anode, which can be alleviated by increasing the surface area of the anode electrode. Haung et al. [12] experimentally studied electrolysis in the HAN solution using a U-shaped bottle as the electrolytic cell container, which effectively separates the products of anode and cathode electrolysis. After electrolysis, the cathode and anode solutions became alkaline and acidic, respectively, producing NH3 on the cathode and O2 on the anode. Other scholars have studied the catalytic decomposition mechanisms of nitrate on electrode surfaces through quantum chemical simulations, providing useful references for studying the electrocatalytic decomposition mechanism of HAN on electrode surfaces. For example, Zhou et al. [13] studied the catalytic decomposition mechanism of HAN on electrode surfaces in depth. They optimized the adsorption configuration and catalytic decomposition pathway of HAN on Ir(110) surfaces. Calle-Vallejo et al. [14] calculated the variation trend of adsorption energy of nitrate on 20 surfaces of an Ag/Au bimetallic system. They showed that defective Ag/Au surfaces can enhance the catalytic activity of nitrate reduction. Several researchers have also conducted computational studies on the electrocatalytic decomposition of water using quantum chemical simulations, which offer valuable insights. For instance, Ren et al. [15] analyzed the hydrogen evolution performance of a novel electrocatalyst, FeCoCuSx/CFF. Their results indicated that the Gibbs free energy of hydrogen adsorption at the Co sites within the CuS-Co9S8 heterostructure is close to zero. This finding suggests that the catalyst exhibits excellent hydrogen evolution performance.
To further elucidate the electrocatalytic decomposition mechanism of HAN on the electrode surface and explain the relevant experimental phenomena, this study builds upon the electrolysis experiments of HAN solution in a U-tube reported by Huang et al. [12]. We aim to explore the reaction pathways for the generation of NH₃ and O₂ during the electrocatalytic decomposition of HAN on the Cu (111) surface using quantum chemical simulations.
2. Materials and Methods
Small-molecule calculations were performed using the Gaussian 16 software [16]. The geometric optimization and vibration analysis of some small molecules were calculated using the B3LYP functional at the 6-31G(d) basis-set level. The B3LYP functional is commonly used and provides fast convergence with low dependence on integration lattice points. At the 6-31G(d) basis-set level, the B3LYP functional is sufficiently accurate for geometric optimization and vibration analysis [17]. The free energies of some small molecules were calculated using the coupled-cluster method with single, double, and perturbative triple excitations (CCSD(T)) method at the def2-QZVPP basis-set level. The CCSD (T) method is a high-precision quantum chemistry calculation method with sufficient accuracy at the def2-QZVPP basis-set level [18,19]. In this article, the adsorption structure of molecules on the Cu(111) surface was simulated using the CP2K software [20], which quickly calculates the periodic crystal structure of Cu metal using the density functional theory. Figure 1 is a unit cell model diagram of the Cu(111) surface, a common stable crystal plane. Panels (a) and (b) of this figure present a front and top view of the model, respectively. The adsorption structure was calculated under the Perdew–Burke–Ernzerhof functional with the DZVP–GTH pseudopotential basis set. The calculation speed was improved using the Gaussian plane wave method. The vacuum layer was set to 15 Å to avoid interactions between adjacent unit cell molecules, the plane wave cutoff energy was 600 Ry, the energy and intermolecular force convergence standards were set to 10-6 Hartree and 4.5 × 10-4 Hartree/Bohr, respectively, and the k-point setting was 3 × 3 × 1. The Van der Waals correction was performed using the Grimme method [21]. The free energy was calculated as
where and represent the changes in the single-point and zero-point energies, respectively, represents the enthalpy change during a temperature change from 0 K to , and represents the entropy change.
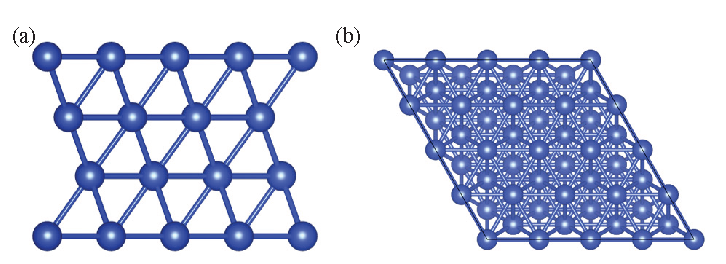
- Unit cell model diagram of the Cu (111) surface: (a) front view; (b) top view.
2.1. Reaction path of electrocatalytic decomposition
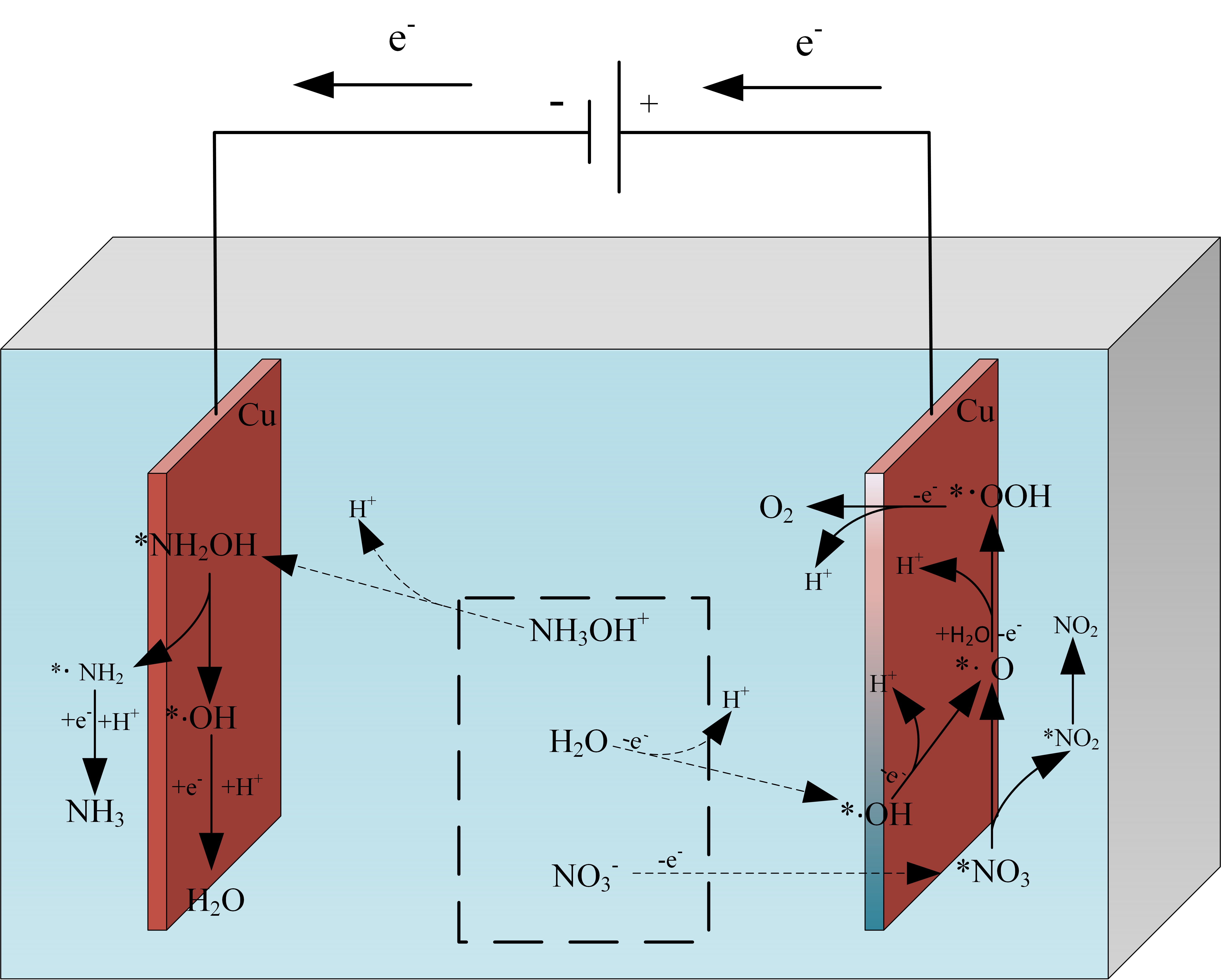
Under water-solvent conditions, HAN decomposes into NH3OH+ and NO3– ions [22]. The aqueous solvent condition is simulated using the implicit solvent SMD (Solvent Model based on Density) model. The configuration changes of HAN under vacuum and water-solvent conditions have been shown in Figure 2, and the energies were calculated using Gaussian 16 software [16]. Under vacuum and water-solvent conditions, the HAN molecule is configured as NH2OH·HNO3 and NH3OH+·NO3–, respectively. As NH3OH+·NO3– has a lower free energy of configuration than NH2OH·HNO3, HAN easily decomposes into NH3OH+ and NO3– ions under water-solvent conditions. The electrocatalytic decomposition mechanism of HAN has been shown in Figure 3, where ‘*’ represents adsorbed molecules, and radicals are identified with ‘·’ (for example, ‘·OH’ denotes OH radicals). Under a potential difference, anions and cations will move toward the anode (which tends to lose electrons) and cathode (which tends to gain electrons), respectively. As the NO3– ions move toward the anode, they lose one electron and adsorb as *NO3 on the anode surface Eq. (1). Figure 4(a) shows the configuration of *NO3. The two O atoms of NO3 are adsorbed on adjacent Cu atoms at the anode surface with different bond lengths (2.040 Å and 2.045 Å) and a bond angle of 119.337° between the adsorbed O and N atoms. *NO3 dissociates into *NO2 and *·O on the anode surface Eq.(2). *NO2 is configured as shown in Figure 4(b). The two O atoms of NO2 are adsorbed on adjacent Cu atoms on the anode surface with the same bond lengths as *NO3 (2.040 Å and 2.045 Å). The bond angle between the adsorbed O and N atoms is slightly smaller in *NO2 than the bond angle between the adsorbed O and N atoms in *NO3. Figure 4(c) shows the configuration of *·O. The bond lengths between the O and adjacent Cu atoms on the anode surface are 1.906 Å and 1.907 Å, with a bond angle of 88.604°. *NO2 can desorb from the anode surface as NO2 see Eq. (3):
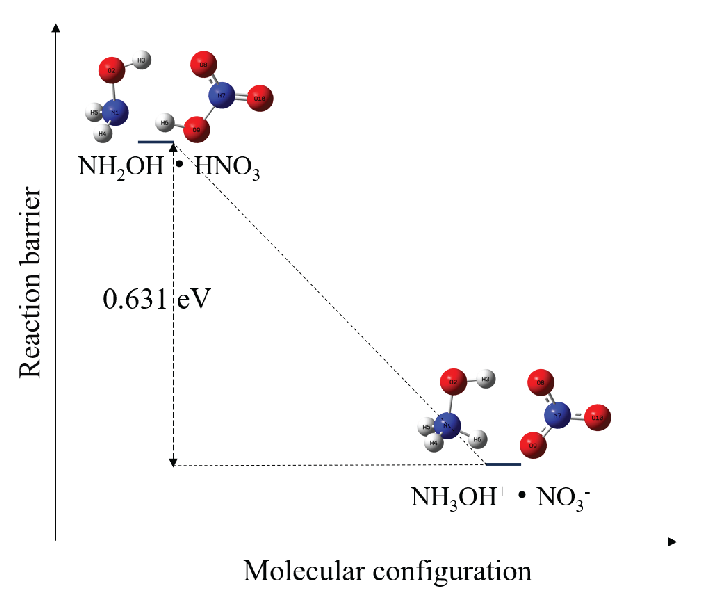
- Configurational changes of HAN under vacuum and water-solvent conditions.
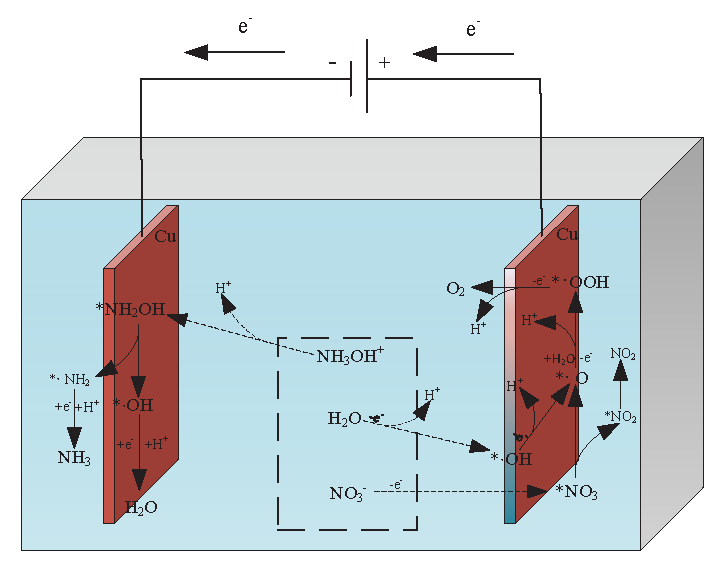
- Diagram showing the electrocatalytic decomposition mechanism of HAN.
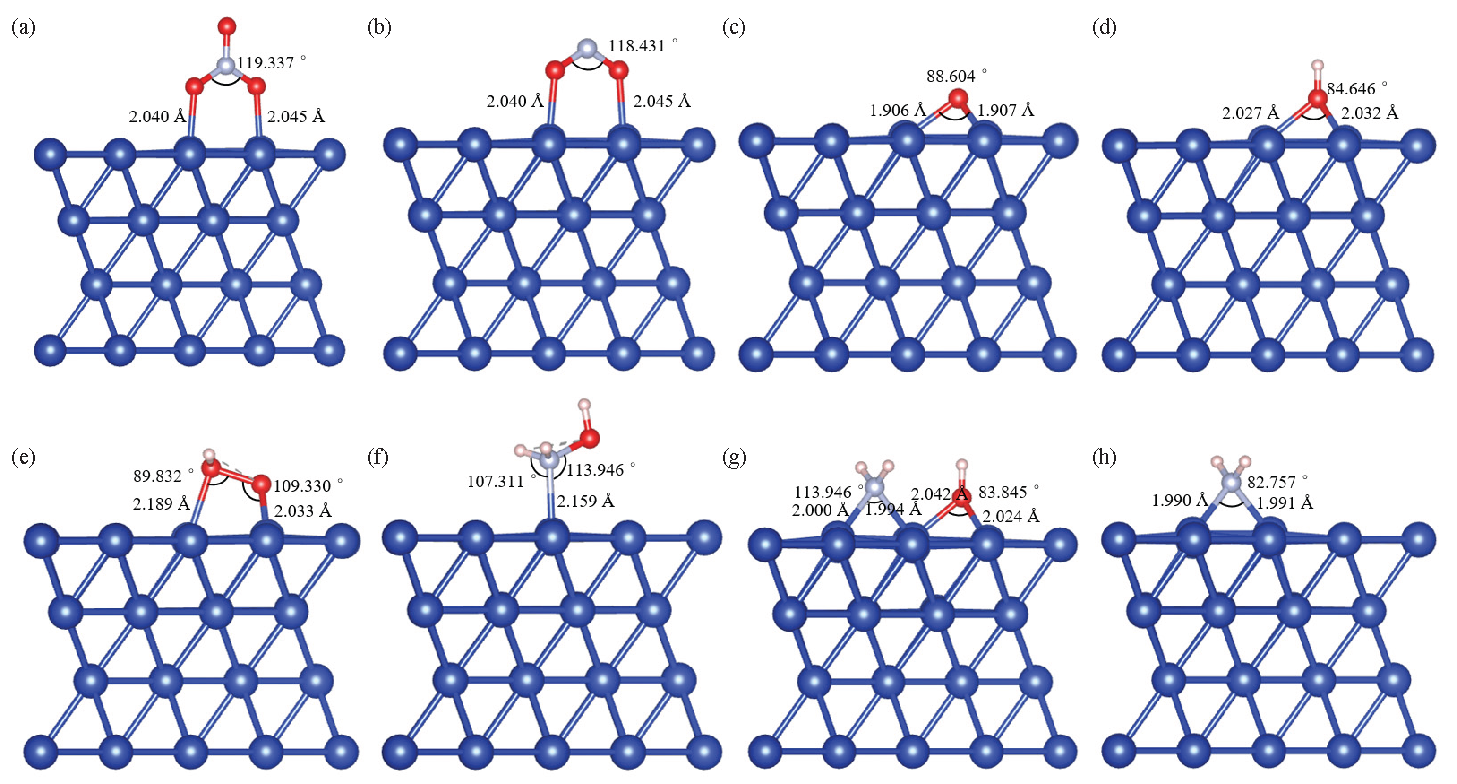
- Configurations of the adsorbed molecules involved in electrocatalytic decomposition: (a) *NO3; (b) *NO2; (c) *·O; (d) *·OH; (e) *·OOH; (f) *NH2OH; (g) *(·NH2 + ·OH); (h) *·NH2.
H2O molecules lose one electron, decomposing into *·OH and H+ ions on the anode surface Eq. (4). Figure 4(d) shows the configuration of *·OH. The bond lengths between the O atom and adjacent Cu atoms on the anode surface are 2.027 Å and 2.032 Å, with a bond angle of 84.646°. *·OH loses one electron on the anode surface, forming *·O and H+ Eq. (5). The *·O radicals react with H2O molecules, losing one electron to form H+ and *·OOH Eq. (6). Figure 4(e) shows the configuration of *·OOH. The two O atoms of ·OOH are adsorbed on adjacent Cu atoms on the anode surface with bond lengths of 2.189 Å and 2.033 Å and bond angles of 89.832° and 109.330°, respectively. *·OOH loses one electron on the anode surface, forming H+ and O2 Eq. (7). The reaction equations are as follows:
Under the action of the potential difference, NH3OH+ ions move toward the cathode, where they dissociate into *NH2OH and H+ ions on the cathode surface Eq. (8). Figure 4(f) shows the configuration of *NH2OH. The N atom of NH2OH adsorbs with a bond length of 2.159 Å near the Cu atom on the cathode surface. The bond angles are 107.311° between the Cu, N, and H atoms and 113.946° between the Cu, N, and O atoms. *NH2OH dissociates into *(·NH2 + OH) Eq. (9) with the configuration shown in Figure 4(g). The bond lengths between the N atom of ·NH2 and the two adjacent Cu atoms on the cathode surface are 2.000 Å and 1.994 Å with a bond angle of 113.946°. Meanwhile, the bond lengths between the O atom of · OH and the two adjacent Cu atoms on the cathode surface are 2.042 Å and 2.024 Å, with a bond angle of 83.845°. *·NH2 obtains an electron at the cathode and combines with H+ ions to form NH3 Eq. (10). Figure 4(h) shows the configuration of NH2. The N atom of ·NH2 bonds with two adjacent Cu atoms on the cathode surface with bond lengths of 1.990 Å and 1.991 Å and a bond angle of 82.757°. *·OH obtains an electron at the cathode and combines with H+ ions to form H2O Eq. (11). The reaction equations are as follows:
3. Results and Discussion
3.1. Anodic reaction mechanism
Table 1 lists the thermodynamic parameters of the relevant molecules during the anodic reaction process, calculated using CP2K software. As analyzed in the previous section, NO3– ions near the anode first lose one electron and adsorb to the anode surface. To avoid the CP2K calculation of the free energy of NO3– ions, the formation free energy of Eq. (1) was obtained by summing the formation free energies of Eqs. (12), (13), and (14); that is, , where represents the formation free energy and the subscript denotes the number of the reaction equation. In this expression, , where G represents the free energy and H+ + e– is replaced by half the free energy of H2. According to Calle-Vallejo et al. [14], and . As only the vibrations of adsorbed molecules are relevant, the surface free energy of the anode is replaced by the single-point energy [23]. From the data in Table 1, . Eqs. (12)–(14) are computed as
| Molecular formula | Eele (Hartree) | T (K) | ZPE (Hartree) | (Hartree) | G (Hartree) |
|---|---|---|---|---|---|
| * | –3080.168 | —— | —— | —— | —— |
| *NO3 | –3138.089 | 298.15 | 0.016 | 0.012 | –3138.077 |
| HNO3(g) | –58.494 | 298.15 | 0.025 | 0.017 | –58.477 |
| H2(g) | –1.162 | 298.15 | 0.012 | –0.002 | –1.164 |
| *NO2 | –3122.099 | 298.15 | 0.011 | 0.008 | –3122.091 |
| *·O | –3096.186 | 298.15 | 0.003 | 0.003 | –3096.183 |
| NO2(g) | –41.881 | 298.15 | 0.009 | –0.017 | –41.897 |
| O2(g) | –31.928 | 298.15 | 0.004 | –0.002 | –31.931 |
| H2O(l) | –17.220 | 298.15 | —— | 0.000 | –17.220 |
| *·OH | –3096.806 | 298.15 | 0.013 | 0.012 | –3096.794 |
| *·OOH | –3112.733 | 298.15 | 0.017 | 0.015 | –3112.718 |
Note: *: anodic surface; Eele: Single-point energy; T: Temperature; ZPE: Zero-point energy; ΔG: Free energy correction: G: Represents free energy.
The formation free energy of *NO3 dissociation into *NO2 and *·O on the anode surface can be expressed as
. From the data in Table 1, . Given the formation free energy of NO2 on the anode surface and the data in Table 1, was obtained . The formation free energy of H2O molecules decomposing into *·OH and H+ ions on the anode surface can be expressed as , giving based on the data in Table 1. The free energy of *·O and H+ formation on the anode surface after *·OH adsorption is computed as from the data in Table 1. The formation free energy of H+ and *·OOH formed by the reaction between *·O and H2O molecules is . From the data in Table 1, . Finally, the formation free energy of H+ and O2 on the anode surface of *·OOH, given by , was obtained as using the data of Table 1.
From the above analysis, it was concluded that O2 on the anode surface can be formed through two pathways: electrocatalytic decomposition of NO3– ions and electrocatalytic decomposition of H2O molecules. Figure 5 shows the potential barrier diagram of NO3– ion decomposition on the anode surface. The potential barrier of electron adsorption of NO3– ions is almost 0, and that of *NO3 decomposition into *NO2 and *·O is negative, indicating that the process can easily spontaneously occur. Meanwhile, NO2 desorption, *·O to *·OOH conversion, and *·OOH to O2 conversion must cross potential barriers of 1.125, 2.799, and 1.028 eV, respectively. Figure 6 shows the potential barrier diagram of H2O decomposition on the anode surface. The conversion of adsorbed H2O molecules to *·OH, conversion of *·OH to *·O, and subsequent conversion to *·OOH and O2, like NO3– ions, must cross potential barriers of 0.310 eV, 0.782 eV. As NO3– conversion to O2 has a lower potential barrier than H2O conversion to O2 on the anode surface, the decomposition rate of NO3– exceeds that of H2O, and NO3– decomposition is the main pathway of O2 generation at the anode.
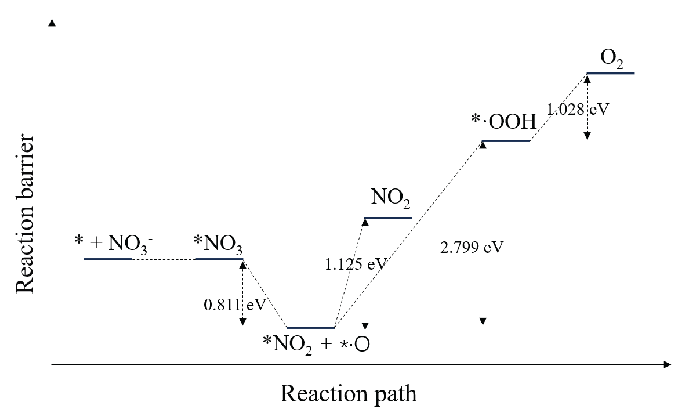
- Reaction barrier diagram of NO3– ion decomposition on the anode surface.
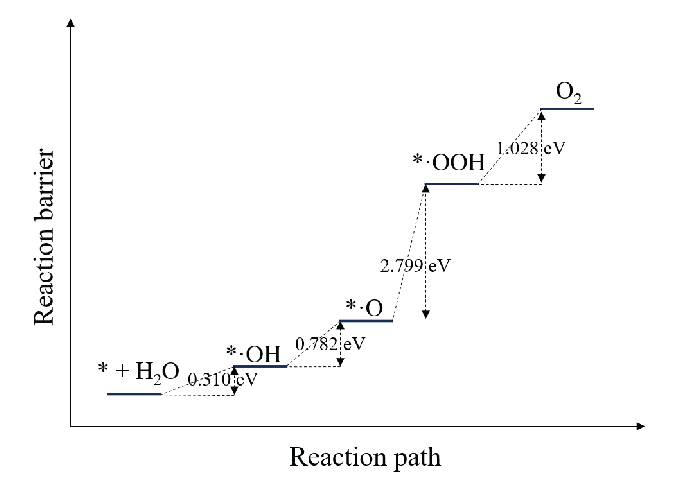
- Reaction barrier diagram of H2O molecule decomposition on the anode surface.
3.2. Cathodic reaction mechanism
Table 2 lists the thermodynamic parameters of the molecules involved in the cathodic reaction process, calculated using CP2K software. As analyzed in the previous subsection, NH3OH+ ions first dissociate into *NH2OH and H+ ions on the cathode surface. To avoid the CP2K calculation of the free energy of NH3OH+ ions, the formation free energy of the acid-dissociation reaction of NH3OH+ ions Eq. (15) was first calculated using Gaussian software. Next, the formation free energy of Eq. (16) was calculated in CP2K software to obtain the formation free energy of Eq. (8), namely, , where the formation free energy of Eq. (15) was calculated as and that of Eq. (16) was obtained as using the data in Table 2. Therefore, was obtained as . The formation free energy of NH2OH dissociation into *(·NH2 + ·OH) is . From the data in Table 2, was obtained as . The reaction equations are given by
| Molecular formula | Eele (Hartree) | T (K) | ZPE (Hartree) | (Hartree) | G (Hartree) |
|---|---|---|---|---|---|
| * | –3080.168 | —— | —— | —— | —— |
| *NH2OH | –3107.887 | 298.15 | 0.041 | 0.039 | –3107.848 |
| NH2OH | –27.692 | 298.15 | 0.039 | 0.014 | –27.678 |
| H2(g) | –1.162 | 298.15 | 0.012 | –0.002 | –1.164 |
| *(·NH2 + ·OH) | –3107.956 | 298.15 | 0.038 | 0.036 | –3107.920 |
| *·NH2 | –3091.318 | 298.15 | 0.025 | 0.023 | –3091.295 |
| H2O(l) | –17.220 | 298.15 | —— | 0.000 | –17.220 |
| NH3 | –11.738 | 298.15 | 0.034 | 0.014 | –11.723 |
| *·OH | –3096.806 | 298.15 | 0.013 | 0.012 | –3096.794 |
| *·OOH | –3112.733 | 298.15 | 0.017 | 0.015 | –3112.718 |
Note: *: cathode surface; Eele: Single-point energy; T: Temperature; ZPE: Zero-point energy; ΔG: Free energy correction: G: Represents free energy
*(·NH2 + ·OH) reacts with H+ ions to remove an H2O molecule Eq. (17). Next, *·NH2 and H+ ions combine to form NH3 Eq. (17) with a formation free energy of . From the data in Table 2, . Meanwhile, the formation free energy of Eq. (10) is using the data in Table 2. *(·NH2 + ·OH) reacts with H+ ions to remove an NH3 molecule Eq. (18). The *·OH and H+ ions then combine to form H2O with a formation free energy of . From the data in Table 2, . The formation free energy of Eq. (11) is . From the data in Table 2, . The reaction equations are as follows:
As shown in the above analysis, NH3OH+ ions gradually decompose on the cathode surface. Figure 7 shows the reaction potential-barrier diagram of NH3OH+ decomposition on the cathode surface. The potential barrier of electron adsorption by NH3OH+ ions and subsequent *NH2OH decomposition is 0.279 eV. As the energy increases during conversion, the reaction cannot easily proceed spontaneously. However, when *NH2OH decomposes into *(NH2 + OH), the energy barrier is lowered and this reaction can spontaneously proceed. The energy also decreases when *(·NH2 + ·OH) converts to *NH2 and *·OH, but the larger decrease in energy during conversion to *·OH indicates a faster reaction rate of conversion to *·OH than of conversion to *NH2. Finally, the desorption of adsorbed molecules ·NH2 and ·OH from the cathode surface can also spontaneously proceed. Therefore, the decisive step in NH3OH+ decomposition is electron adsorption and decomposition of NH3OH+ ions to *NH2OH. All subsequent decomposition processes can spontaneously occur.

- Reaction barrier diagram of NH3OH+ ion decomposition on the cathode surface.
4. Conclusions
The main conclusions of the study are summarized below.
-
(1)
Under water-solvent conditions, HAN easily decomposes into NH3OH+ and NO3– ions. Under the action of a potential difference, NH3OH+ ions and NO3– ions move toward the cathode and anode, respectively, where they undergo electrocatalytic decomposition reactions. Specifically, NH3OH+ ions decompose into NH3 and H2O on the cathode surface, and NO3– ions decompose into NO2 and O2 on the anode surface.
-
(2)
O2 at the anode surface can be generated via two pathways: the electrocatalytic decomposition of NO3– ions and the electrocatalytic decomposition of H2O molecules on the anode surface. The key difference between these two pathways lies in the formation of the *·O intermediate. For NO₃⁻ ions, the energy decreases during the formation of *·O, with a reaction barrier of -0.811 eV, indicating that the reaction is highly spontaneous. In contrast, for H₂O molecules, the energy increases during the formation of *·O, with a reaction barrier of +0.782 eV. This suggests that the formation of *·O from H₂O is more challenging. Therefore, the electrocatalytic decomposition of NO₃⁻ ions on the anode surface are the dominant pathway for O₂ generation at the anode.
-
(3)
The cathodic surface reaction easily occurs, and its rate-determining step is the electron adsorption and decomposition of NH3OH+ ions to form *NH2OH. The energies of all subsequent decomposition processes decrease, indicating that the reaction occurs spontaneously. On the cathodic surface, *(·NH2 +· OH) conversion to *·NH2 competes with *(NH2 + ·OH) conversion to *·OH. The greater energy decrease during conversion to *·OH indicates a higher reaction rate of *·OH formation than of *·NH2 formation from *(NH2 +· OH).
-
(4)
During the anodic reaction, several processes generate hydrogen ions (H⁺), while at the cathode, multiple reactions consume hydrogen ions. This leads to the cathodic solution becoming alkaline and the anodic solution becoming acidic after the electrocatalytic decomposition of the HAN solution.
-
(5)
The electrocatalytic decomposition mechanism of HAN on the Cu(111) surface is highly complex. This study primarily analyzes the main pathways for the formation of O₂ and NH₃. Future research could utilize quantum chemical simulations to predict potential by-products, thereby guiding experimental investigations. Additionally, deeper insights into the reaction mechanism could be revealed through atomic charge calculations and orbital composition analysis.
Acknowledgement
The authors would like to thank Shiyanjialab (www.shiyanjia.com) for the language editing service.
CRediT authorship contribution statement
Men Li and Tianpeng Li: Conceptualization, Men Li: Methodology, Men Li, Tianpeng Li and Xinbao Gao: Validation, Men Li: Data Curation, Men Li: Writing – Original Draft Preparation, Men Li, Tianpeng Li, Yin Huang and Xinbao Gao: Writing – Review & Editing.
Declaration of competing interest
All authors disclose no conflicts of interest.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Research progress of new electrically controlled solid propellants for smart ammunition. Chinese Journal of Explosives & Propellants. 2024;47:287-304. https://doi.org/10.14077/j.issn.1007-7812.202306019.
- [Google Scholar]
- Research progress in ECSP preparation and electronically controlled combustion technology. Journal of Solid Rocket Technology. 2024;47:155-163. https://doi.org/10.7673/j.issn.1006-2793.2024.02.002.
- [Google Scholar]
- A review on hydroxylammonium nitrate (HAN) decomposition techniques for propulsion application. Acta Astronautica. 2022;196:194-214. https://doi.org/10.1016/j.actaastro.2022.04.011
- [Google Scholar]
- Decomposition pathways for aqueous hydroxylammonium nitrate solutions: A DFT study. Central European Journal of Energetic Materials. 2017;14:888-916. https://doi.org/10.22211/cejem/71193
- [Google Scholar]
- Comparative study of the thermal decomposition of hydroxylammonium nitrate green energetic compound: Combination between experimental and DFT calculation. International Journal of Energetic Materials and Chemical Propulsion. 2022;21:31-8. https://doi.org/10.1615/intjenergeticmaterialschemprop.2022044056
- [Google Scholar]
- Study on thermal decomposition characteristics of hydroxylammonium nitrate and its stabilization technology. Chemical Propellants & Polymeric Materials. 2018;16:80-5. https://doi.org/10.16572/j.issn1672-2191.201809027.
- [Google Scholar]
- Thermal decomposition of hydroxylamine nitrate. Propulsion. 1988;872:63-70. https://doi.org/10.1117/12.943754.
- [Google Scholar]
- On the formation of ammonia from the thermal decomposition of hydroxylammonium nitrate vapor. Journal of Ionic Liquids. 2024;4:100083. https://doi.org/10.1016/j.jil.2024.100083
- [Google Scholar]
- Thermal decomposition mechanism of aqueous hydroxylammonium nitrate (HAN): Molecular simulation and kinetic modeling. The Journal of Physical Chemistry. A. 2018;122:8086-8100. https://doi.org/10.1021/acs.jpca.8b05351
- [Google Scholar]
- Role of electrodes in ambient electrolytic decomposition of hydroxylammonium nitrate (HAN) solutions. Propulsion and Power Research. 2013;2:194-200. https://doi.org/10.1016/j.jppr.2013.07.002
- [Google Scholar]
- Experimental studies on parametric effects and reaction mechanisms in electrolytic decomposition and ignition of HAN solutions. ACS Omega. 2022;7:18521-18530. https://doi.org/10.1021/acsomega.2c01183
- [Google Scholar]
- Research progress on new intelligent electronic control solid propellant technology. In: The 40th Technical Exchange Conference and the 4th Aerospace Power Joint Conference of China Aerospace Third Professional Information Network. 2024.
- [Google Scholar]
- A theoretical investigation of the catalytic decomposition of hydroxylamine nitrate on ir(1 1 0) surface. Computational and Theoretical Chemistry. 2023;1225:114141. https://doi.org/10.1016/j.comptc.2023.114141
- [Google Scholar]
- Theoretical design and experimental implementation of Ag/Au electrodes for the electrochemical reduction of nitrate. Physical Chemistry Chemical Physics: PCCP. 2013;15:3196-3202. https://doi.org/10.1039/c2cp44620k
- [Google Scholar]
- In situ growth of feCo sulfides on cobalt iron foam for efficient high-current-density hydrogen evolution reaction electrocatalysis. Journal of Colloid and Interface Science. 2025;682:288-297. https://doi.org/10.1016/j.jcis.2024.11.251
- [Google Scholar]
- Gaussian 16, Revision A.03. Wallingford CT: Gaussian, Inc.; 2016.
- Study of lubrication adsorption behavior based on molecular dynamics simulation. Equipment Environmental Engineering. 2024;2024:28-36. https://doi.org/10.2139/ssrn.4879964.
- [Google Scholar]
- Quantum chemical benchmark databases of gold-standard dimer interaction energies. Scientific Data. 2021;8:55. https://doi.org/10.1038/s41597-021-00833-x
- [Google Scholar]
- The mechanism and kinetics of the atmospheric oxidation of CF3(CF2)2CH = CH2 (HFC-1447fz) by hydroxyl radicals: ab initio investigation. Physical Chemistry Chemical Physics. 2024;26:10989-10997. https://doi.org/10.1039/d3cp06149c
- [Google Scholar]
- CP2K: An electronic structure and molecular dynamics software package - quickstep: Efficient and accurate electronic structure calculations. The Journal of Chemical Physics. 2020;152:194103. https://doi.org/10.1063/5.0007045
- [Google Scholar]
- A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-d) for the 94 elements H-Pu. The Journal of Chemical Physics. 2010;132:154104. https://doi.org/10.1063/1.3382344
- [Google Scholar]
- Effect of metal sequestrants on the decomposition of hydroxylammonium nitrate. Catalysts. 2021;11:1488. https://doi.org/10.3390/catal11121488
- [Google Scholar]
- Origin of the overpotential for oxygen reduction at a fuel-cell cathode. The Journal of Physical Chemistry. B. 2004;108:17886-17892. https://doi.org/10.1021/jp047349j
- [Google Scholar]







