Translate this page into:
Electrochemical behavior of antioxidants: Part 3. Electrochemical studies of caffeic Acid–DNA interaction and DNA/carbon nanotube biosensor for DNA damage and protection
⁎Corresponding author. abdelhamid_refat@yahoo.com (Refat Abdel-Hamid),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Multi-walled carbon nanotubes-modified glassy carbon electrode biosensor was used for electrochemical studies of caffeic acid–dsDNA interaction in phosphate buffer solution at pH 2.12. Caffeic acid, CAF, shows a well-defined cyclic voltammetric wave. Its anodic peak current decreases and the peak potential shifts positively on the addition of dsDNA. This behavior was ascribed to an interaction of CAF with dsDNA giving CAF–dsDNA complex by intercalative binding mode. The apparent binding constant of CAF–dsDNA complex was determined using amperometric titrations. The oxidative damage caused to DNA was detected using the biosensor. The damage caused by the reactive oxygen species, hydroxyl radical (•OH) generated by the Fenton system on the DNA-biosensor was detected. It was found that CAF has the capability of scavenging the hydroxide radical and protecting the DNA immobilized on the GCE surface.
Keywords
Caffeic acid
dsDNA
Biosensor
Damage
Protection
1 Introduction
The interaction of small organic molecules with DNA is of interest for both therapeutic and scientific reasons. Such interactions may be used for conformational recognition to find new structures of DNA and sequence-specific differences along the helix of a DNA molecule.
Reactive oxygen species (ROS)-induce oxidative DNA damage. One of the most reactive radical species that induces lesions in DNA is the hydroxyl radical (•OH). This species cause cell injury when they are generated in excess or the cellular antioxidant defense is impaired. It attacks both the deoxyribose sugar and the purine and pyrimidine bases resulting in intermediate radicals, which are the immediate precursors for DNA base damage (Jaruga and Dizdaroglu, 1996). The hydroxyl radical by the Fenton system is important because it has been implicated as an important mediator of oxidative damage in vivo. It is a strong oxidative system in terms of reducing the possibility of mutation and consequently cancer (Burkitt, 2003). In contrast cells develop a complex defense system, which acts through the enzymatic activities and protection by the low molecular weight antioxidants. Another form of protection is the use of synthetic and natural origin compounds that show antioxidant effect on the cell. Antioxidants act as reducing agents (free radical terminators), metal chelating and singlet oxygen quenchers (Vertuani et al., 2004). Much interest has been developed in recent years in order to find and to characterize natural antioxidant. This is due to worldwide growing trend toward the usage of natural antioxidants in food industry and also their health benefits.
In the last years, conventional electrochemical sensors modified by DNA were utilized as simple devices for the sensitive determination of electroactive and non-electroactive compounds interacting with DNA (Labuda et al., 2000; Mascini et al., 2001; Erdem and Ozsoz, 2002; Oliveira-Brett et al., 2002). The strategy is based on employing DNA itself as a biosensor for DNA damage detection.
Lodovici, et al. examined the antioxidant activity of some natural phenolic compounds present in food including CAF (Lodovici et al., 2001). They concluded that these compounds have interesting protective activity against DNA oxidative damage in vitro. A protective effect of CAF and its related catechols against hydroxyl radical formation in vitro has been reported (Iwahashi et al., 1990). Capacity of some antioxidants, including caffeic acid, against oxidative damage of DNA was evaluated (Barroso et al., 2011). A biosensor guanine and adenine based on a GCE was used for the evaluation of the antioxidant capacity of CAF (Barroso et al., 2011). It was found that CAF has the ability to scavenge the superoxide radical and therefore protect the DNA base. Biosensor consisted of a guanine or adenine base electro-immobilized on a GCE was used to study the purine base damage induced by the hydroxyl radical generated by the Fenton-type reaction (Barroso et al., 2012). Five antioxidants including CAF were applied to counteract the deleterious effects of the hydroxyl radical. These antioxidants have the ability to scavenge the hydroxyl radical. Adenine-rich oligonucleotide adsorbed on carbon paste electrode for the assessment of the antioxidant capacity is proposed (Barroso et al., 2011). The method was based on the partial damage of a DNA layer adsorbed on the electrode surface by •OH radicals. The subsequent electrochemical oxidation of the intact adenine bases is to generate an oxidation product that was able to catalyze the oxidation of NADH. The presence of antioxidant compounds, including CAF, scavenges hydroxyl radicals leaving more adenines unoxidized and thus decreasing the electrocatalytic current of NADH.
Caffeic acid is a naturally occurring compound that is found in all plants as a key intermediate in the biosynthesis of lignin (Larson, 1988). Owing to the highly antioxidant activities (Rice-Evans et al., 1997; Friedman, 1997), the oxidative behavior of CAF was the subject of intensive investigations in the literature. Recently, the mechanism of electrochemical oxidation of caffeic acid was studied in aqueous phosphate solutions with different pHs (Abdel-hamid et al., 2013). The study was performed using either a bare glassy carbon (GC) or GC-modified with multi-walled carbon nanotubes (MWCNTs) electrode. It was found that CAF electrochemically oxidizes following an ECEC, radical–radical mechanism.
In continuation of our work on electrochemical behaviour of antioxidants (Abdel-Hamid et al., 2013a), the aim of the present work is to study the interaction of caffeic acid with dsDNA using electrochemical methods. Cyclic voltammetry and double potential step chronocoulometry are used to trace the interaction of CAF with dsDNA on glassy carbon/multi-walled carbon nanotubes modified electrode. The study is extended to investigate the damage of dsDNA by •OH radicals and its protection by caffeic acid using a glassy carbon-based dsDNA biosensor. The biosensor consists of a dsDNA layer immobilized on GCE as an oxidation target. A Fenton-type reaction is used as the method of inducing damage by generating •OH radicals. The antioxidant properties of CAF are evaluated on tracing the changes of DNA layer by using the square wave voltammetric DNA oxidation peak.
2 Material and methods
Caffeic acid (P99%) was purchased from Sigma–Aldrich. The multi-walled carbon nanotubes (purity: >90%; carbon basis, D × L 110–170 nm × 5–9 μm) were from Aldrich. All solutions were prepared from BDH analytical grade chemicals. Double stranded calf thymus, dsDNA, (sodium salt, type I) was purchased from Sigma and used without further purification. Stock solutions of dsDNA were prepared by dissolving the appropriate amount of DNA in deionized water and were stored at 4 °C and used after 5 days.
An EG&G Princeton Applied Research Model 273A potentiostat/Galvanostat controlled by a computer was used for voltammetric measurements. The electrochemical experiments were controlled with an EG&G Princeton Applied Research Model M 270 software. Background data were stored and subtracted from the experimental data set, minimizing side effects such as double layer charging current. Electrochemical experiments were carried out by immersing the desired electrode in a 0.2 M phosphate buffer solution. A conventional three-electrode electrolytic cell was employed. Saturated calomel electrode, SCE, and platinum electrode were used as reference and counter electrodes, respectively. Glassy carbon/multi-walled carbon nanotubes modified electrode, MWCNTs/GCE and glassy carbon/dsDNA were employed as working electrodes.
Clean GCE was obtained by polishing it with 0.5 μm alumina powder on a polishing cloth. After rinsing the surface with deionized water, the polished GCE was sonicated for 5 min in water/ethanol to get rid of trace amounts of alumina powder from the surface and rinsed again with deionized water. 1.0 mg mL−1 suspension of multi-walled carbon nanotubes, MWCNTs, was prepared on dispersing 5 mg of MWCNTs in 5.0 ml dimethylformamide. The mixed solution was sonicated for 6 h. The MWCNTs/GCE electrode was prepared by dropping 1.0 ml of MWCNTs suspension onto the clean surface of GCE. Then the solvent was evaporated overnight and the surface was fully dried at atmospheric condition with a stream of purified nitrogen.
The biosensor, dsDNA/glassy carbon modified electrode, dsDNA/GCE, was prepared by dropping 10.0 μL of dsDNA solution (5.0 mg mL−1) onto the clean surface of GCE and the solvent was evaporated at 4 °C. Finally, in order to remove the excess of DNA, the dsDNA/GCE was immersed in a blank 0.2 M phosphate buffer solution for 15 min, and then gently rinsed with deionized water.
Hydroxyl radical (Fenton solution) was generated by mixing Fe2+: EDTA: H2O2 (1 μmol L−1:2 μmol L−1:40 μmol L−1) in the molar ratio of 1:2:40. Mello reported that when an excess of hydrogen peroxide is added in the reaction a high DNA damage is obtained (Mello et al., 2006). EDTA was added for solubility reasons. All solutions were prepared with deionized water. Reduced transition metal ion such as Fe2+ reacts with hydrogen peroxide in a one-electron redox (Fenton) reaction producing hydroxyl radical and hydroxide anion:
3 Results and discussion
3.1 Cyclic voltammetry
The cyclic voltammograms, cv, of 0.2 M phosphate buffer solution (pH = 2.12) containing 98.0 μM caffeic acid at a bare and at MWCNTs/GCE at scan rate of 50 mV s−1 are shown in Fig. 1. The cyclic voltammograms exhibit an anodic peak current potential at ≈ 0.50 V with cathodic peak on reversing scan, indicating that the cv involves a reversible redox process. It can be seen that the CAF oxidation peak potential,
, at the bare GCE is weak and broad while the response is considerably improved at the MWCNTs/GCE. At the modified electrode, the oxidation peak current,
, is considerably enhanced. This was attributed to the electro-catalytic effect caused by MWCNTs (Britto et al., 1999), meanwhile the MWCNTs increase the effective area of the electrode.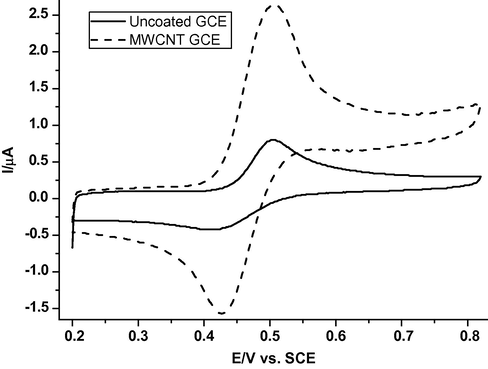
Cyclic voltammograms of 98.0 μM CAF on bare GCE (solid line) and MWCNTs/GCE modified electrode (dotted line) in 0.2 M phosphate buffer solution (pH 2.21) at a scan rate of 50 mV s−1.
Cyclic voltammograms of 20.1 μM CAF in the absence and presence of different amounts of dsDNA in 0.2 M phosphate buffer solution (pH 2.21) at a scan rate of 20 mV s−1 at MWCNTs/GCE electrode are shown in Fig. 2. Curve 1 is the voltammogram of the CAF solution; it has a well-defined cyclic voltammetric wave at 0.478 V (versus SCE), which belongs to electrode oxidation of the two catechol hydroxyl groups of CAF (Carter et al., 1989). Curves 2–6 are voltammograms of a mixture of CAF with different amounts of dsDNA. No new redox peaks appear with the addition of dsDNA, while the peak current,
, decreases obviously and the peak potential,
, of CAF shifts positively. The dependence of the anodic peak current on the concentration of DNA is shown in Fig. 3. This strongly demonstrated that CAF interacts with dsDNA. The decrease of peak current is explained with a decrease of free CAF concentration in solution after the addition of dsDNA. It was well demonstrated by Bard et al. (Bard and Xu, 1995) that the binding mode between dsDNA and small molecules occurred with an electrostatic interaction if the formal potential shifted negatively after the addition of dsDNA, whereas the intercalated interaction dominates for the positively shifted formal potential. So the binding mode between CAF and dsDNA could be simply attributed to the major intercalated interaction.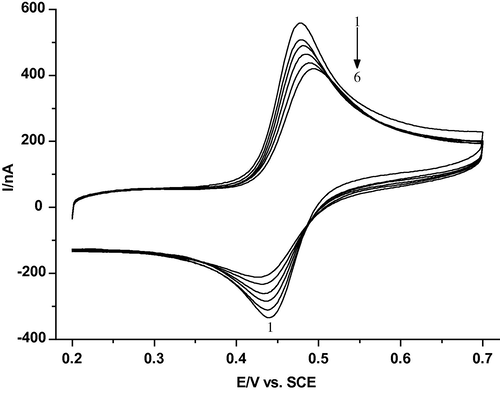
Cyclic voltammograms of 20.1 μM CAF at MWCNTs/GCE in the absence and presence of DNA: (1) 0; (2) 0.0661; (3) 0.0982; (4) 0.128; (5) 0.155 and (6) 0.2 (mg ml−1) in 0.2 M phosphate buffer solution (pH 2.21) at a scan rate of 20 mV s−1.
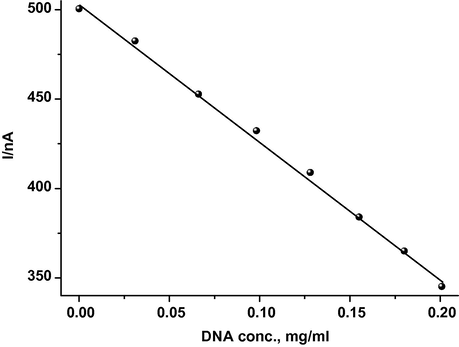
Dependence of the anodic peak current of CAF, 20.10 μM, on the concentration of dsDNA at a scan rate of 20 mV s−1.
To show that the decrease in the peak current is due to the diffusion of CAF–dsDNA adduct, voltammograms are recorded for K4[Fe(CN)6] solution in the presence and absence of DNA, which cannot interact with DNA. The peak potential of K4[Fe(CN)6] is not affected by the addition of DNA and the peak current only decreases by about 1.5% in the presence of DNA. Thus, the change in peak current and peak potential of CAF is caused by the CAF binding to the dsDNA.
3.2 Double potential step chronocoulometry
Double potential step chronocoulometric experiments are carried out to determine the diffusion coefficients, D of CAF and CAF–dsDNA complex and deduce the current nature. According to the following equation (Bard and Faulkner, 2001)
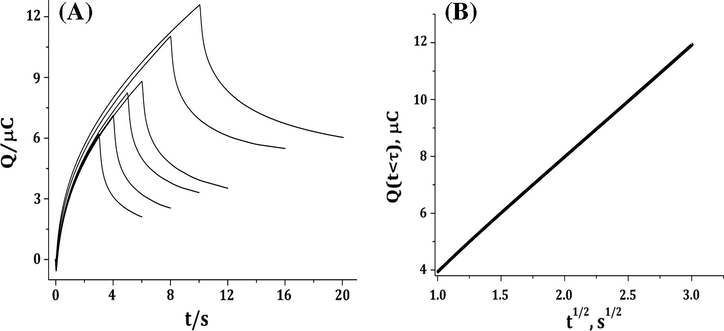
Chronocoulograms of CAF, (A) and Q(t < τ) versus t1/2 (B) in 0.2 M phosphate buffer solution (pH 2.21) at different duration times.
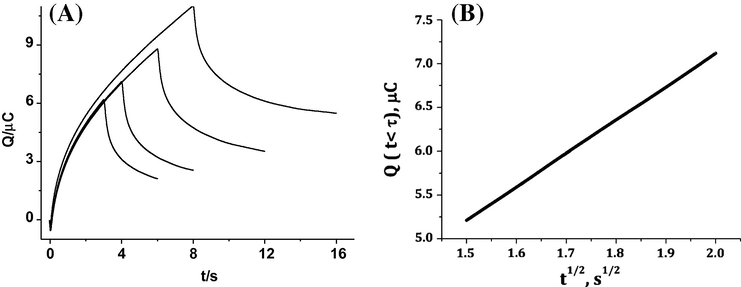
Chronocoulograms of CAF-DNA, (A) and Q(t < τ) versus t1/2 (B) in 0.2 M phosphate buffer solution (pH 2.21) at different duration times.
To investigate the binding mode of caffeic acid with dsDNA, the binding constant, β, is determined using amperometric titrations. Current titrations are performed by keeping concentration of caffeic acid constant while varying the concentrations of dsDNA using the cyclic voltammetric responses at pH 2.12. The interaction of CAF with dsDNA can be described using the following equation:
The equation for amperometric titration is described as follows (Ibrahim, 2001):
![log (1/[DNA]) against log(IH−G/(IG − IH−G)) relationship of caffeic acid; [CAF] = 20.1 μM, scan rate of 20 mV s−1.](/content/184/2016/9/3/img/10.1016_j.arabjc.2013.02.008-fig6.png)
log (1/[DNA]) against log(IH−G/(IG − IH−G)) relationship of caffeic acid; [CAF] = 20.1 μM, scan rate of 20 mV s−1.
In order to verify that •OH radicals generated by the Fenton solution are able to damage dsDNA, the dsDNA-GCE biosensor is placed in a freshly prepared Fenton solution of pH 4.35 for 10 s. Next, the biosensor is rinsed with water and square wave voltammograms are recorded. Fig. 7 shows the square wave voltammograms, SWV, of the dsDNA biosensor before and after the interaction with the Fenton solution, in the absence and presence of antioxidant. The dsDNA biosensor gives an anodic SWV wave at 224 mV versus SCE due to the oxidation of the immobilised dsDNA on the glassy carbon electrode, curve 1, the blank signal (Palecek and Jelek, 2002). On immersing the biosensor in the Fenton solution, curve 2, a decrease of the anodic peak current is observed lower than that of the blank signal. This decrease is used to infer damage to the dsDNA after being oxidized by •OH radicals. On the addition of antioxidant, caffeic acid, to the solution of •OH radical, as shown by curve 3, the peak current, ip, increases compared to curve 2. Indeed, this is indicative that the dsDNA is protected by the antioxidant present in the solution. Antioxidants are well-known to exhibit a protective effect with a scavenging effect of ROS preventing dsDNA damage. Consequently, the number of lesions diminishes, yielding a larger number of dsDNA bases for electrochemical oxidation (Fojta, 2002). It is well known that, caffeic acid is considered to be a good scavenger of free radicals produced during the metabolic pathways of detoxification. Furthermore, CAF has strongly polar hydroxyl groups, and is possibly related to the antioxidant action by chelating trace elements such as iron.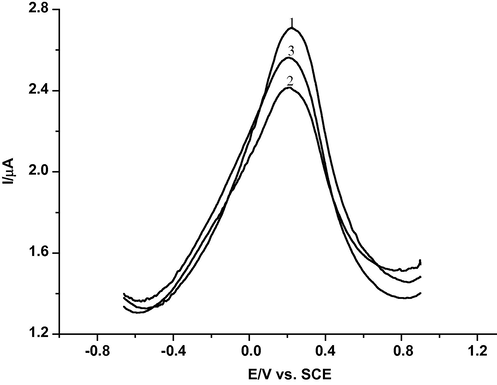
SWV of dsDNA-biosensor in 0.2 M phosphate buffer solution (pH 3.45), frequency = 50 Hz: (1) total oxidation of dsDNA-biosensor signal (maximum peak current), (2) immersion of the dsDNA-biosensor in a Fenton solution and (3) immersion of the dsDNA-biosensor in a Fenton solution with caffeic acid.
4 Conclusion
Electrochemical studies of the interaction of caffeic acid, CAF, with dsDNA were carried out using a multi-walled carbon nanotubes/glassy carbon electrode biosensor. The anodic peak current of CAF decreases and the peak potential shifts positively on the addition of dsDNA. This was ascribed to an interaction of CAF with dsDNA giving CAF–dsDNA complex by intercalative binding mode. The apparent binding constant of CAF–dsDNA complex was determined using amperometric titrations. The oxidative damage caused to DNA was detected using the biosensor. The DNA damage caused by the reactive oxygen species, hydroxyl radical (•OH) was detected. It was found that CAF has the capability of scavenging the hydroxide radical and protecting the DNA immobilized on the GCE surface.
References
- Arabian J. Chem. 2013 accepted for publication
- J. Indian Chem. Soc. 2013 accepted for publication
- Electrochemical Methods, Fundamentals and Applications (2nd ed.). New York: Wiley; 2001.
- J. Am. Chem. Soc.. 1995;117:2627-2631.
- Biosens. Bioelectron.. 2011;30:1.
- Biosens. Bioelectron.. 2011;26:3748.
- Biosens. Bioelectron.. 2011;26:2396.
- Food Chem.. 2012;132:1055.
- Adv. Mater.. 1999;11:154.
- Prog. React. Kinet. Mech.. 2003;28:75.
- J. Am. Chem. Soc.. 1989;111:8901.
- Electroanalysis. 2002;14:965.
- Electroanalysis. 2002;14:1449.
- J. Agric. Food Chem.. 1997;45:1523.
- Anal. Chim. Acta. 2001;443:63.
- Arch. Biochem. Biophys.. 1990;276:242.
- Nucleic Acids Res.. 1996;24:1389.
- J. Anal. Chem.. 2000;367:364.
- Phytochemistry. 1988;27:969.
- Food Chem. Toxicol.. 2001;39:1205.
- Fresenius J. Anal. Chem.. 2001;369:15.
- Biosens. Bioelectron.. 2006;21:1374.
- Talanta. 2002;56:959.
- Crit. Rev. Anal. Chem.. 2002;32:261.
- Trends Plant Sci.. 1997;2:152.
- Curr. Pharm. Des.. 2004;10:1677.







