Translate this page into:
Enhanced superhydrophobicity of electrospun carbon nanofiber membranes by hydrothermal growth of ZnO nanorods for oil–water separation
⁎Corresponding authors. haiyan9943@163.com (Jiao Li), gxl_ouc@126.com (Xueli Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Development of electrospun nanofiber membranes with the selective wettability characteristics for effectively separating oil–water mixtures is an extremely advisable strategy. In this study, a superhydrophobic electrospinning carbon nanofiber (F/ZnO/CNF) membrane was successfully prepared by electrospinning and in-situ growth of ZnO, and subsequent fluorination reaction with 1H, 1H, 2H, 2H-perfluorooctyltriethoxysilane (POTS). Benefiting from the influence of needle-like nanostructure and low surface energy, the as-prepared F/ZnO/CNF membrane shows excellent superhydrophobicity. When the growth duration of ZnO is 3 h, the obtained F/ZnO/CNF-3 membrane possesses outstanding water contact angle (WCA, 159.7°) and splendid oil–water separation efficiency (>99 %). Meanwhile, due to its the superior environmental stability the obtained F/ZnO/CNF-3 membrane exhibits excellent low and high temperature resistance, and enhanced resistance to various organic solvents in the face of a series of harsh environments.
Keywords
Superhydrophobicity
Carbon nanofiber membrane
Electrospinning
Hydrothermal
Oil-water separation
1 Introduction
With the continuous progress of urbanization and industrialization, a large amount of oily sewage has been discharged from light and heavy industries, such as food, petrochemical and textile (Chen and Xu, 2013; Rasouli et al., 2021). This situation has not only caused waste of resources, but also seriously affected the ecological environment and the sustainable and healthy development of human society (Joye Samantha, 2015; Ma et al., 2016). At present, there are many traditional treatment methods for oily wastewater, such as gravity sedimentation, coagulation and flocculation, adsorption, gas flotation, and electric field (Saththasivam et al., 2016; Kukkar et al., 2020; Wei et al., 2018; Kwon et al., 2010; Duman et al., 2021; Duman et al., 2022a). However, expensive operation cost, poor separation efficiency, large footprint and secondary environment contamination are the main disadvantages of the above conventional oil–water separation methods (Chu et al., 2015; Wang et al., 2015).
Fortunately, membrane separation technique is employed as a facile method to practicably treat oily wastewater, which could effectively address the issues of traditional separation methods (Padaki et al., 2015). Lipophilic-hydrophobic and/or hydrophilic-oleophobic membranes were successfully developed according to their selective (Si et al., 2018; Wei et al., 2018). At present, various hydrophobic membrane materials have successfully been used to construct membranes with different requirements for oil–water separation (Zhang et al., 2019; Lin et al., 2022; Raman et al., 2021; Deng et al., 2019). For example, Zhao et al. (2020) introduced polymethylsiloxane (POMS) onto the surface of wood to prepare superhydrophobic materials with a water contact angle (WCA) of 153°, however, the separation performance was not described in detail. Duman et al. (2022b) reported a highly hydrophobic electrospun PVA (WCA: 148.1°) and agar/PVA (WCA: 150.1°) membrane, indicating that the cross-linking of glutaraldehyde and the coating of methyltrichlorosilane (MTCS) could improve the hydrophobicity of these materials. Xu et al. (2022) prepared superhydrophobic nickel-coated stainless steel meshes via magnetic field-assisted jet electrodeposition, and the as-prepared sample shows superhydrophobic property (WCA: 154°) and high separation flux (>97 %). Especially, the electrospinning nanofiber membranes were widely used for separation of the oil–water mixtures (Liao et al., 2018; Wang et al., 2022; Huang et al., 2022; Wang et al., 2021) due to the easy preparation, controllable structure, high porosity of membranes. However, the hydrophobicity reported in the above related studies still needs to be enhanced to meet the increasingly high requirements. Consequently, the exploration and discovery of advanced method to further improve the hydrophobicity and separation performance has been a key issue in our study.
Inspired by the superhydrophobic surfaces, such as lotus leaves and mosquito eyes in nature, the hydrophobicity of the electrospinning fiber membrane can be enhanced by constructing micro-/nanostructures on the fiber to improve the surface roughness and decrease the surface free energy (Xu et al., 2017; Liu et al., 2012; Yan et al., 2021). Up to now, various superhydrophobic electrospun fiber membranes were prepared by doping nanomaterials (silicon oxide, titanium oxide, carbon nanotubes) or post-processing, including heat treatment, dip coating, hydrothermal, chemical vapor deposition (CVD) (Xiong et al., 2020; He et al., 2019; Zhao et al., 2022; Ma et al., 2005; Ma et al., 2018; Shami et al., 2016; Ge et al., 2018). However, the above electrospun polymer fiber membranes could not maintain performance stability under some circumstances, such as low or high temperature and various organic solvents, leading to deteriorated hydrophobic performance and low oil–water separation efficiency. Fortunately, carbon nanofiber (CNF) membranes could perfectly overcome the above problems due to their chemical stability, thermal stability, and inherent hydrophobicity. Liu et al. (2014) reported a flexible electrospun CNF membrane with a porous structure. The hydrophobicity and porosity of the as-prepared membranes are improved due to the sublimation of terephthalic acid (PTA), and the membrane exhibits high oil absorption and good recyclability in organic solvents. Tai et al. (2014) obtained a superhydrophobic carbon–silica nanofiber membrane with excellent chemical, thermal stability and high permeate flux (∼3032.4 L/(m2·h)) via electrospinning and heat treatment, which was used for rapidly separation of oily wastewaters. Feng et al. (2019) investigated superhydrophobic electrospun CNF membrane with core–shell structure based on electrospinning and CVD method. The results show that the superhydrophobic CNF membrane could maintain superhydrophobicity in various harsh environments and still keep excellent separation efficiency (>98 %) after multiple cycles. Nowadays, the CNF membrane has been developed in the field of oil–water separation (Al-anzi and Siang, 2017), however, the routine hydrophobicity and complex preparation process still hinder its application in the field of oil–water separation. Therefore, a simple hydrothermal method is used to construct micro-nano structures on the surface of carbon fiber to improve its roughness. And also, the ZnO micro-nano structures, especially for nanorods and nanowires, could be formed on different substrates by hydrothermal method to obtain rough surfaces due to the advantages of safety, low cost and special polar surface (Du et al., 2015; Yang et al., 2019). In addition, it was particularly important strategy to enhance the hydrophobicity of CNF membranes by reduce the surface energy. Fluorinated compounds containing strong electronegative fluorine could be grafted onto solid surfaces to form waterproof coatings to reduce the surface energy (Chen et al., 2022). In summary, increasing the surface roughness and reducing the surface energy could significantly improve the superhydrophobicity of the CNF.
Based on the above advantages, herein, a superhydrophobic electrospun carbon nanofiber membrane (F/ZnO/CNF) with needle-like nanostructure was successfully obtained by electrospinning, in-situ growth of ZnO, and subsequent fluorination reaction. The oil and water remediation performance, environmental stability, and solvent resistance of F/ZnO/CNF membrane were systematically investigated.
2 Experiment
2.1 Materials
Polyacrylonitrile (PAN, Mw = 150 000) was supplied by Sigma-Aldrich Co., ltd. Zinc acetate dihydrate (Zn(Ac)2·2H2O) was obtained from Shanghai Macklin Biochemical Co., ltd., China. 1H, 1H, 2H, 2H-perfluorooctyltriethoxysilane (POTS), Oil red O and methylene blue were purchased from Shanghai Aladdin Chemical Co., ltd., China. Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), hexamethylenetetramine (HMTA) and Span-80 were obtained from Sinopharm Chemical Reagent Co., ltd., China. N,N-Dimethylformamide (DMF), ethanol, n-butanol, carbon tetrachloride, dichloromethane, toluene, ethyl acetate, petroleum ether, cyclohexane, acetone, HCl and NaOH were provided by Far East Fine Chemicals Co., ltd., China.
2.2 Preparation of F/ZnO/CNF membrane
Fig. 1 demonstrates the detailed fabrication process of the superhydrophobic F/ZnO/CNF membrane. First step was the preparation of carbon nanofiber (CNF) membrane, which was prepared according to a published work in our group (Sun et al., 2021). In detail, 0.8 g of Zn(Ac)2·2H2O was completely dissolved in 20.0 g of DMF with magnetic stirring at room temperature. Then, 2.2 g of PAN was thoroughly mixed with the above solution by magnetically stirring overnight at 40 °C to prepare a clear and homogeneous electrospinning precursor solution. Afterwards, the appropriate amount of precursor solution was used to produce the polymer membrane via electrospinning technology. The parameters in the electrospinning process were set as follows: the feeding speed was 0.9 mL/h, the spinning voltage was 15 kV, the receiving distance in the middle of the stainless metal needle and the drum receiver was kept at 16 cm, the drum receiver was about 150 rpm, the spinning time was 10 h, the humidity was controlled at 25 %-35 %, and the ambient temperature was set at 25 °C. The PAN/Zn(Ac)2 polymer membrane collected on the tin foil was placed in a draught drying cabinet at 60 °C overnight. Finally, the CNF membrane was obtained by heat treatment of the PAN/Zn(Ac)2 polymer membrane, including pre-oxidization at 280 °C for 2 h in the atmosphere of air and subsequently carbonization at 1000 °C for 2 h under the protection of an inert atmosphere of Ar.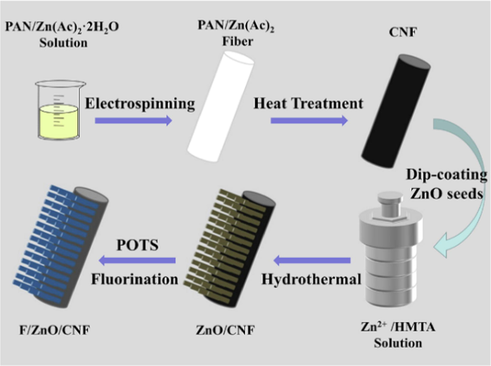
Schematic demonstrating the fabrication procedure of the superhydrophobic F/ZnO/CNF.
Second step was the in-situ growth of ZnO nanorods on the CNF surface to construct the roughness of fiber membrane by dip coating and hydrothermal treatment. Typically, the CNF membranes with a size of 25 mm × 25 mm were dipped in an ethanol solution containing 1 mg/mL Zn(Ac)2·2H2O, and the solution was heated in water bath at 50℃ for 2 h. Then the membrane was dried for 2 h at 150 °C. The above step was repeated five times to obtain the CNF coated with the ZnO seed layer. Subsequently, the dip-coated CNF membranes were immersed in 50 mL deionized water containing Zn(NO3)2·6H2O (0.45 g) and HMTA (0.225 g) and transferred to a high pressure vessel seal for hydrothermal reaction at 90 °C. The membranes obtained with different reaction time (1 h, 2 h, 3 h, 4 h) were recorded as ZnO/CNF-1, ZnO/CNF-2, ZnO/CNF-3, ZnO/CNF-4. As a comparison, the membrane prepared without dip coating and hydrothermal treatment was recorded as ZnO/CNF-0. The obtained ZnO/CNF-x membranes were repeatedly rinsed five times with deionized water and ethanol and heat-treated at overnight 60 °C. The mechanism of hydrothermal growth of ZnO nanorods is presented in Table S1 in the Supporting Information.
Third step was the fluorinated treatment of ZnO/CNF-x membranes to reduce the surface energy. Detailedly, the ZnO/CNF-x membrane and 50 μL of 2 % fluorinated solution (POTS/ethanol: 1 g/50 g) were moved to a sealed container, which was treated at 120 °C for 2 h. Then, the membrane was took out and heated at 150 °C for 1 h to evaporate the ungrafted POTS. The finally obtained membrane was denoted as F/ZnO/CNF-x.
2.3 Characterization
The micro-/nanostructure and morphology of the F/ZnO/CNF-x membranes were determined with a field-emission scanning electron microscopy (FE-SEM, Sigma 300, Zeiss) in a vacuum environment. The element distribution of F/ZnO/CNF-3 was tested by energy dispersive spectrometer (EDS, Quanta 250, FEI). The functional groups and crystal structure of F/ZnO/CNF-x membranes were revealed by Fourier transform infrared spectroscopy (FT-IR, Nicolet iS10, Thermo) and X-ray diffraction (XRD, Dmax2500, Rigaku), respectively. The water contact angle (WCA) and oil contact angle (OCA) of five different sites on the surface of the F/ZnO/CNF-x membranes were measured on the SDC 320 instrument (SINDIN, China) to appraise the wettability of the sample at room temperature. The mechanical properties of the membrane were tested by a computer tension machine (XK-508). The water content of the filtrate after separation was analyzed with a Carl Fischer water titrator (C30, Mettler Toledo). The density and porosity of membrane are calculated from Text 1 in the Supporting Information.
2.4 Oil-water separation experiment
The separation property of oil–water mixture was evaluated using a glass filtration device, and the filtration experiment was conducted under gravity drive. Carbon tetrachloride, toluene, petroleum ether, ethyl acetate and dichloromethane were selected as the oil phase, and the volume ratio of oil phase to water was 1:1. Water and oil phases were colored successively with methylene blue and oil red O. The separation efficiency (Sf) of the F/ZnO/CNF-x membranes was determined according to the following formula:
where M1 (g) and M2 (g) were the mass of water before and after filtering the oil–water mixture (Feng et al., 2019).
Details of emulsion separation experiments (Text 2) are in the support information.
The permeation flux (P) was used to calculate the following formula:
where V (L), S (m2), and t (h) were the volume of oil collected, the separation area of the membrane, and the time required for filtration, respectively (Jiang et al., 2017).
3 Results and discussion
3.1 Characterization of F/ZnO/CNF membranes
The morphology and structure of F/ZnO/CNF-x membranes were observed by FE-SEM (Fig. 2). Compared with the relatively smooth pristine carbon fibers (Fig. 2a), the surface of the F/ZnO/CNF-x membranes are uniformly deposited by ZnO crystals (Fig. 2b-e), which enhance the surface roughness of the carbon nanofibers. It is obviously observed that the ZnO nanorods structure of the F/ZnO/CNF membranes gradually increases with the increased hydrothermal growth time. In addition, the ZnO nanorods are uniformly dispersed on the surface of F/ZnO/CNF-x membranes. In addition, the element mappings also show that the Zn, F and Si are uniformly distributed on F/ZnO/CNF-3 surface (Fig. S2), indicating that the F/ZnO/CNF-3 is successfully functionalized.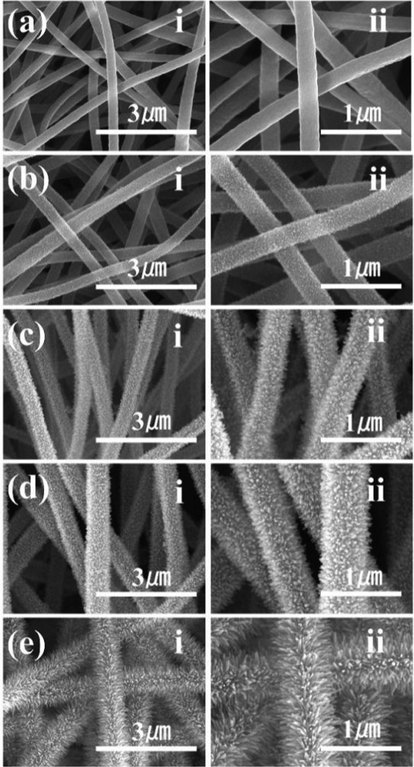
FE-SEM micrographs of CNF (a), F/ZnO/CNF-1 (b), F/ZnO/CNF-2 (c), F/ZnO/CNF-3 (d), and F/ZnO/CNF-4 (e).
From Fig. 3, the XRD spectra of all samples exhibit a relatively broad diffraction peak between 20°-30°, which indicates the typical amorphous structure of carbon. Meanwhile, the ZnO/CNF-3 and F/ZnO/CNF-3 show a series of sharp peaks at 31.8°, 34.4°, 36.3°, 47.6°, 56.6°, 62.9°, 66.4°, 68.0°, 69.1°, which belong to the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1) crystal planes of hexagonal wurtzite structure of ZnO, respectively (PDF#36–1451) (Yang et al., 2016). These evidences indicate that the fluoridation treatment of ZnO/CNF did not change its hexagonal wurtzite structure.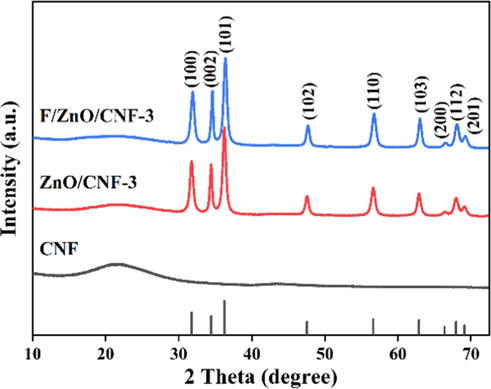
XRD spectra of CNF, ZnO/CNF-3 and F/ZnO/CNF-3.
Fig. 4 is the FTIR spectra of CNF, ZnO/CNF-3 and F/ZnO/CNF-3 membranes. The FT-IR spectrum of CNF membrane has only two broad peaks around 1241 cm−1 and 1579 cm−1, which are assigned to the C⚌C stretching and bending vibrations. For ZnO/CNF membrane, the peak at 500 cm−1 is related to Zn-O bond of ZnO (Li et al., 2015). After fluoridation of ZnO/CNF membrane, five new and different absorption peaks centered at 1365 cm−1, 1317 cm−1, 1207 cm−1, 1144 cm−1 and 1117 cm−1 are observed from F/ZnO/CNF-3, mainly attributing to the stretching vibration of C-F in the -C-F2- and -C-F3 groups of the POTS (Song et al., 2012). The peak bands located at 810 cm−1 and 1063 cm−1 are assigned to the asymmetric stretching vibration and deformation of the Si-O-Si bonds in POTS (Guo et al., 2016). This indicates that POTS are successfully loaded on the F/ZnO/CNF-3 membrane. Moreover, compared with ZnO/CNF-3, the broad peak at 3430 cm−1 corresponding to the –OH disappear for F/ZnO/CNF-3, because the –OH groups on ZnO/CNF-3 are used as a “bridge” for the grafting of POTS (He et al., 2019). These results reveal that POTS is stably bound on the ZnO/CNF-3 surface by chemical bond (Fig. S1). In addition, CNF and ZnO can interact with each other through van der Waals forces (Song et al., 2016).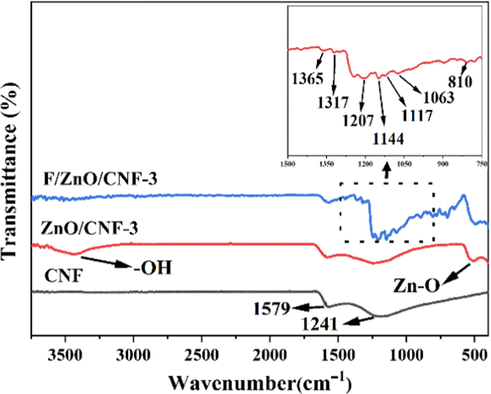
FTIR spectra of CNF, ZnO/CNF-3 and F/ZnO/CNF-3.
The wettability of F/ZnO/CNF-x membranes were assessed and the results are shown in Fig. 5. The F/ZnO/CNF-3 membrane exhibits the good resistance to water adhesion (Fig. 5a) and efficient oil permeability (Fig. 5b). The WCA of the original CNF membrane after fluorination treatment is 143.8° (Fig. 5c). With the growth of ZnO nanorods on nanofiber surface before modification by POTS, the F/ZnO/CNF-x membrane exhibits superhydrophobic property (WCA > 150°). Interestingly, the WCA of the as-prepared F/ZnO/CNF-3 reaches 159.7°, which is obviously improved compared with the F/CNF membrane. The growth of ZnO nanorods on CNF surface remarkably increases the roughness of membrane surface. Meanwhile, the subsequent fluorination decreases the surface energy of ZnO/CNF membrane. These two processes were combined to improve the hydrophobicity of F/ZnO/CNF-x membranes. The wettability mechanism of F/ZnO/CNF-x membrane to water may be described by Fig. 5d. The grooves between the ZnO nanorods contain limited air, which can form air pockets after contact with water droplets to avoid the water droplets from penetrating into the nanostructures (Cassie-Baxter state) (Khranovskyy et al., 2012). With the growth time of ZnO is prolonged, the WCA of F/ZnO/CNF increases from 153.2° to 159.7°. When the hydrothermal growth time is up to 4 h, the WCA decreases to 157.4°. This phenomenon mainly stems from the fact that the change of ZnO nanorods length and packing density affect the surface morphology of carbon nanofibers, resulting in increased wetted area of water droplets (Khan et al., 2020; Pauporté et al., 2010).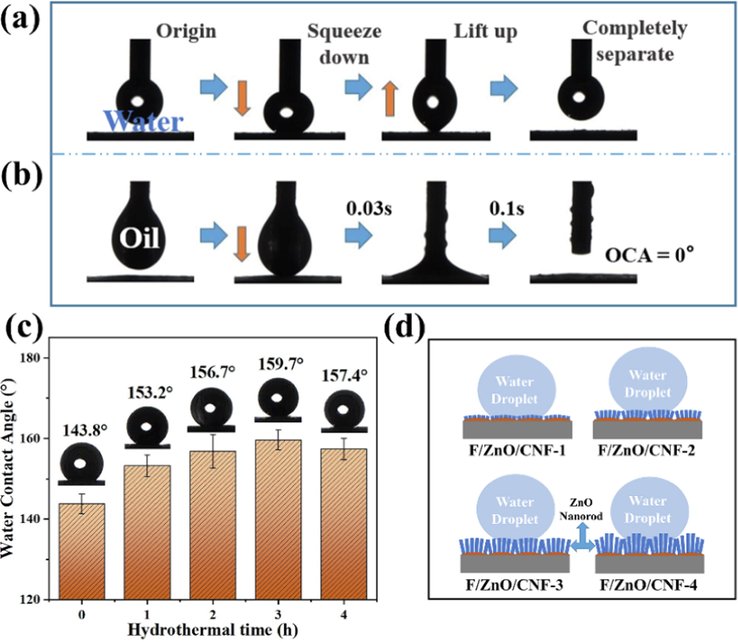
Photograph of the dynamic water (15 μL) adhesion measurement on F/ZnO/CNF-3 membrane surface (a), photograph of oil (dichloromethane 15 μL) permeation behavior on F/ZnO/CNF-3 membrane (b), WCA of prepared F/ZnO/CNF-x membranes (c), schematic diagram of water droplets on F/ZnO/CNF membrane (d).
In addition, with the increase of hydrothermal time, the tensile strength of the membrane remains at about 1 MPa, and the Young's modulus increases significantly (Fig. S3). This indicates that ZnO nanorods on the surface of fiber can improve the Young's modulus of the membrane, and have little effect on the tensile strength.
3.2 Oil/water separation performance
The excellent superhydrophobicity and superoleophilicity of the F/ZnO/CNF-3 membrane prompt us to further evaluate its separation performance for oil–water mixtures on a gravity-driven glass apparatus. The filtration device and process as illustrated in Fig. 6a, the water in the oil–water mixture would retain in the upper container due to the superhydrophobicity of the membrane, and the oil phase would penetrate into the bottom glass bottle. Taking water-in-carbon tetrachloride emulsion as example, the optical microscope images and digital photographs of the emulsion and filtrate are shown in Fig. S4. The emulsion is milky with a large number of water droplets. The filtrate shows a clear state without obvious water droplets. In addition, Fig. 6b and Fig. S4b show that the separation efficiencies of F/ZnO/CNF-x membranes for oil–water mixture and emulsion are all above 97 %. Meanwhile, the permeate fluxes of the membrane gradually decrease with hydrothermal reaction time, which may be attributed to increased size of ZnO nanorods and decreased porosity of the membrane (Table S2). Compared with other membrane materials reported in the literature, the F/ZnO/CNF-3 shows better separation performance (Table S3). The cyclic separation results of the carbon tetrachloride-water mixture with the F/ZnO/CNF-3 membrane is demonstrated in Fig. 6c. The filtration efficiency remains higher than 99 % even after 15 cycles, and the separation flux keeps stable. For the emulsion, the separation flux decreases slightly as the number of cycles increases. And the separation efficiency remains at about 98.5 % after 15 cycles (Fig. S4c). These results indicate that the F/ZnO/CNF-3 membrane has good recyclability and stability. The fluxes of F/ZnO/CNF-3 membranes for the different oils are all>1000 L/(m2·h) and the permeation flux of dichloromethane can reach 1667 L/(m2·h) (Fig. 6d).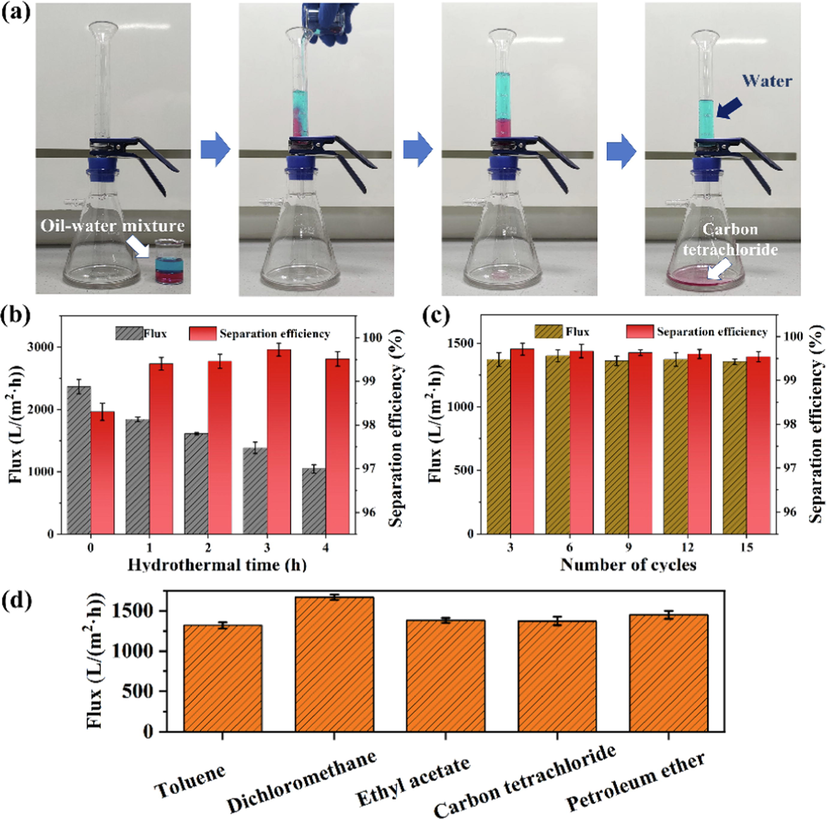
Photograph of separation of oil–water mixture (a), oil permeation flux and separation efficiency of F/ZnO/CNF-x membranes (b), cycle separation performance of F/ZnO/CNF-3 membrane for oil–water mixtures (c) and separation flux of F/ZnO/CNF-3 membrane for different oils (d).
3.3 Environmental stability
In practical applications, superhydrophobic membranes with excellent environmental stability are extremely important, especially in severe environments such as low and high temperature, and immersion in various organic solvents. The stability of F/ZnO/CNF-3 membrane in various solvent environments was investigated (Fig. 7). On one hand, the F/ZnO/CNF-3 membrane exhibits excellent stability in deionized water at low or high temperature, and the sample remains superhydrophobic with the slightly reduced WCA after immersion for 12 h. On the other hand, when the F/ZnO/CNF-3 membrane is soaked in cyclohexane, acetone, DMF and dichloromethane for 7 days at room temperature (24 °C), the separation fluxes of F/ZnO/CNF-3 membranes are all>1500 L/(m2·h)), and the separation performance remains above 99.5 % (Fig. 7b). Meanwhile, after immersion in organic solvent, little change of WCA of the F/ZnO/CNF-3 can be observed, and the WCA of the membranes are still above 156° (Fig. 7c-f). The stability of F/ZnO/CNF-3 membrane in different pH solutions was further studied. As shown in Fig. S5, after soaking in different pH solutions for 2 h, the WCA of F/ZnO/CNF-3 does not decrease significantly and still remains superhydrophobic. Then the separation efficiency of F/ZnO/CNF-3 for oil–water mixtures is still maintains at a high level (>99 %). This is mainly because the POTS grafted can protect ZnO nanorods and reduce their erosion in hydrochloric acid or sodium hydroxide. Therefore, the F/ZnO/CNF-3 membrane displays good environmental stability in both acidic or alkaline environments. The above results indicate that the superhydrophobic F/ZnO/CNF-3 membrane exhibits wonderful environmental stability in harsh environments.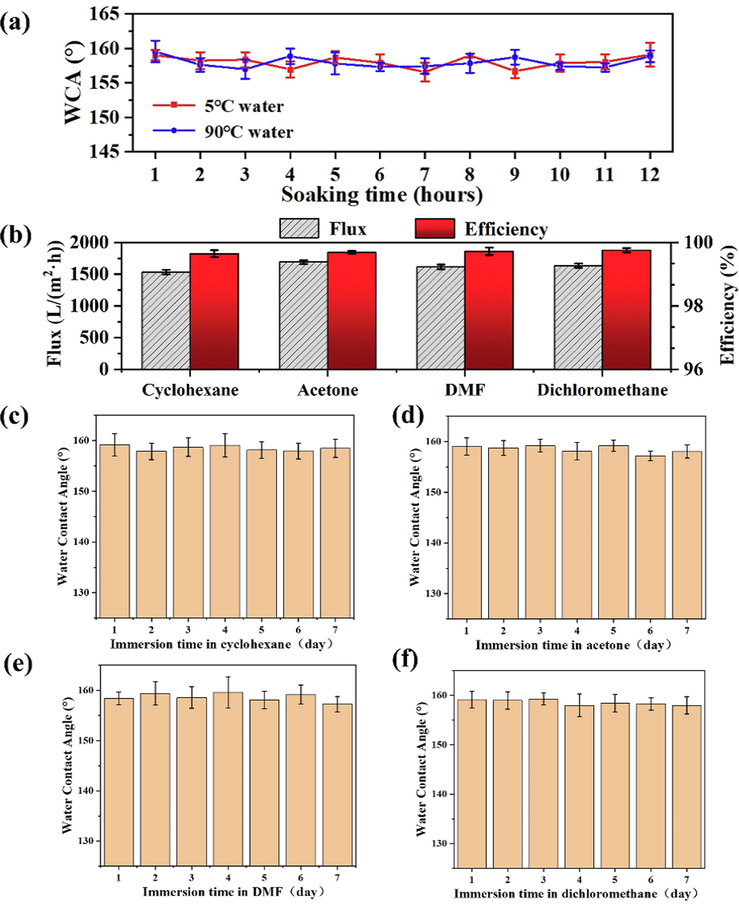
WCA of F/ZnO/CNF-3 membrane after immersion in water at 5 °C and 90 °C with different time (a), the flux and separation efficiency of F/ZnO/CNF-3 after 7 days of immersion in four different organic solvents (cyclohexane, acetone, DMF, dichloromethane) (b) and the WCA of F/ZnO/CNF-3 after socking in various organic solvents with different times (c-f).
4 Conclusions
A simple hydrothermal synthesis was utilized to successfully prepare needle-like ZnO nanorods deposited on surface of the electrospun CNF membrane to achieve a rough surface. Then, the fluorination treatment was performed to achieve low surface energy to significantly strengthen the hydrophobicity of membrane. The prepared F/ZnO/CNF-3 membrane shows a high WCA of 159.7°. Under gravity drive only, the F/ZnO/CNF-3 demonstrates excellent permeation flux (1667 L/(m2·h)) and the illustrious separation capability of oil–water mixture reaches 99.5 %. Additionally, benefiting from its excellent structural stability in harsh environments, the as-prepared F/ZnO/CNF-3 membrane exhibits desirable low and/or high temperature resistance, and improved resistance to various organic solvents.
Acknowledgements
This work was supported by the Shandong Provincial Natural Science Foundation (Grant No. ZR2020ME051), the Innovation Research Fund of Zhaoyuan Institute of Industrial Technology (Grant No. 220192), the Key Research Project of Shandong Province (No. 2019JZZY010806), and the Young Taishan Scholars Program of Shandong Province.
References
- Recent developments of carbon based nanomaterials and membranes for oily wastewater treatment. RSC Adv.. 2017;7(34):20981-20994.
- [CrossRef] [Google Scholar]
- Rosin acid and SiO2 modified cotton fabric to prepare fluorine-free durable superhydrophobic coating for oil-water separation. J. Hazard. Mater.. 2022;440:129797
- [CrossRef] [Google Scholar]
- Mineral-coated polymer membranes with superhydrophilicity and underwater superoleophobicity for effective oil/water separation. Sci. Rep.. 2013;3(1):2776.
- [CrossRef] [Google Scholar]
- Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed.. 2015;54(8):2328-2338.
- [CrossRef] [Google Scholar]
- Efficient oil/water separation by a durable underwater superoleophobic mesh membrane with TiO2 coating via biomineralization. Sep. Purif. Technol.. 2019;222:35-44.
- [CrossRef] [Google Scholar]
- Wettability behavior of special microscale ZnO nail-coated mesh films for oil–water separation. J. Colloid Interface Sci.. 2015;458:79-86.
- [CrossRef] [Google Scholar]
- Development of highly hydrophobic and superoleophilic fluoro organothiol-coated carbonized melamine sponge/rGO composite absorbent material for the efficient and selective absorption of oily substances from aqueous environments. J. Environ. Chem. Eng.. 2021;9(2):105093
- [CrossRef] [Google Scholar]
- Highly hydrophobic and superoleophilic agar/PVA aerogels for selective removal of oily substances from water. Carbohydr. Polym.. 2022;286:119275
- [CrossRef] [Google Scholar]
- Duman, O., Uğurlu, H., Diker, Diker, C.Ö., Tunç, S., 2022b. Fabrication of highly hydrophobic or superhydrophobic electrospun PVA and agar/PVA membrane materials for efficient and selective oil/water separation. J. Environ. Chem. Eng. 10(3), 107405. https://doi.org/10.1016/j.jece.2022.107405.
- Preparation and property of extremely stable superhydrophobic carbon fibers with core-shell structure. Carbon. 2019;150:284-291.
- [CrossRef] [Google Scholar]
- Rational design of materials interface at nanoscale towards intelligent oil-water separation. Nanoscale Horiz.. 2018;3(3):235-260.
- [CrossRef] [Google Scholar]
- Rapid fabrication and characterization of superhydrophobic tri-dimensional Ni/Al coatings. Appl. Surf. Sci.. 2016;387:8-15.
- [CrossRef] [Google Scholar]
- Gravity-driven and high flux super-hydrophobic/super-oleophilic poly(arylene ether nitrile) nanofibrous composite membranes for efficient water-in-oil emulsions separation in harsh environments. Compos. B. Eng.. 2019;177:107439
- [CrossRef] [Google Scholar]
- Design of stable super-hydrophobic/super-oleophilic 3D carbon fiber felt decorated with Fe3O4 nanoparticles: facial strategy, magnetic drive and continuous oil/water separation in harsh environments. Appl. Surf. Sci.. 2019;494:1072-1082.
- [CrossRef] [Google Scholar]
- A review of lanthanide-based fluorescent nanofiber membranes by electrospinning and their applications. J. Mater. Sci.. 2022;57:3892-3922.
- [CrossRef] [Google Scholar]
- Switchable oil/water separation with efficient and robust Janus nanofiber membranes. Carbon. 2017;115:477-485.
- [CrossRef] [Google Scholar]
- Growth of ZnO nanorods on cotton fabrics via microwave hydrothermal method: effect of size and shape of nanorods on superhydrophobic and UV-blocking properties. Cellulose. 2020;27(17):10519-10539.
- [CrossRef] [Google Scholar]
- Surface morphology effects on the light-controlled wettability of ZnO nanostructures. Appl. Surf. Sci.. 2012;258(20):8146-8152.
- [CrossRef] [Google Scholar]
- Recent advances in carbon nanotube sponge–based sorption technologies for mitigation of marine oil spills. J. Colloid Interface Sci.. 2020;570:411-422.
- [CrossRef] [Google Scholar]
- Investigation of water separation from water-in-oil emulsion using electric field. J. Ind. Eng. Chem.. 2010;16(5):684-687.
- [CrossRef] [Google Scholar]
- Reversibly light-switchable wettability between superhydrophobicity and superhydrophilicity of hybrid ZnO/bamboo surfaces via alternation of UV irradiation and dark storage. Prog. Org. Coat.. 2015;87:155-160.
- [CrossRef] [Google Scholar]
- Progress in electrospun polymeric nanofibrous membranes for water treatment: fabrication, modification and applications. Prog. Polym. Sci.. 2018;77:69-94.
- [CrossRef] [Google Scholar]
- Facile fabrication of natural superhydrophobic eleostearic acid-SiO2@cotton fabric for efficient separation of oil/water mixtures and emulsions. Sustainable Mater. Technol.. 2022;32:e00418.
- [Google Scholar]
- Flexible macroporous carbon nanofiber film with high oil adsorption capacity. J. Mater. Chem. A. 2014;2(10):3557-3562.
- [CrossRef] [Google Scholar]
- Clam's shell inspired high-energy inorganic coatings with underwater low adhesive superoleophobicity. Adv. Mater.. 2012;24(25):3401-3405.
- [CrossRef] [Google Scholar]
- Recent development of advanced materials with special wettability for selective oil/water separation. Small. 2016;12(16):2186-2202.
- [CrossRef] [Google Scholar]
- Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules. 2005;38(23):9742-9748.
- [CrossRef] [Google Scholar]
- Durable superhydrophobic and superoleophilic electrospun nanofibrous membrane for oil-water emulsion separation. J. Colloid Interface Sci.. 2018;532:12-23.
- [CrossRef] [Google Scholar]
- Membrane technology enhancement in oil–water separation. A review. Desalination. 2015;357:197-207.
- [CrossRef] [Google Scholar]
- Well-Aligned ZnO Nanowire Arrays Prepared by Seed-Layer-Free Electrodeposition and Their Cassie−Wenzel Transition after Hydrophobization. J. Phys. Chem. C. 2010;114(1):194-202.
- [Google Scholar]
- Electrospun nanofibers as effective superhydrophobic surfaces: a brief review. Surf. Interfaces. 2021;24:101140
- [CrossRef] [Google Scholar]
- Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des.. 2021;204:109599
- [CrossRef] [Google Scholar]
- An overview of oil–water separation using gas flotation systems. Chemosphere. 2016;144:671-680.
- [CrossRef] [Google Scholar]
- Structure–property relationships of nanosheeted 3D hierarchical roughness MgAl–layered double hydroxide branched to an electrospun porous nanomembrane: a superior oil-removing nanofabric. ACS Appl. Mater. Interfaces. 2016;8(42):28964-28973.
- [CrossRef] [Google Scholar]
- Bioinspired designs of superhydrophobic and superhydrophilic materials. ACS Cent. Sci.. 2018;4(9):1102-1112.
- [CrossRef] [Google Scholar]
- A novel coating method using zinc oxide nanorods to improve the interfacial shear strength between carbon fiber and a thermoplastic matrix. Compos. Sci. Technol.. 2016;134:106-114.
- [CrossRef] [Google Scholar]
- Ultrafast fabrication of rough structures required by superhydrophobic surfaces on Al substrates using an immersion method. Chem. Eng. J.. 2012;211–212:143-152.
- [CrossRef] [Google Scholar]
- Robust preparation of flexibly super-hydrophobic carbon fiber membrane by electrospinning for efficient oil-water separation in harsh environments. Carbon. 2021;182:11-22.
- [CrossRef] [Google Scholar]
- Highly efficient and flexible electrospun carbon–silica nanofibrous membrane for ultrafast gravity-driven oil–water separation. ACS Appl. Mater. Interfaces. 2014;6(12):9393-9401.
- [CrossRef] [Google Scholar]
- Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature. Chem. Soc. Rev.. 2015;44(1):336-361.
- [CrossRef] [Google Scholar]
- Sensitive Cu2+ detection by reversible on-off fluorescence using Eu3+ complexes in SiO2, in chitosan/polyethylene oxide nanofibers. Mater. Des.. 2021;205:109708
- [CrossRef] [Google Scholar]
- Polyacrylonitrile fluorescent nanofibers for selective and reversible copper detection in aqueous solutions. Appl. Surf. Sci.. 2022;602:154302
- [CrossRef] [Google Scholar]
- Coagulation/flocculation in dewatering of sludge: A review. Water Research. 2018;143:608-631.
- [CrossRef] [Google Scholar]
- Specially wettable membranes for oil–water separation. Adv. Mater. Interfaces. 2018;5(23):1800576.
- [CrossRef] [Google Scholar]
- Hierarchically tunable structure of polystyrene-based microfiber membranes for separation and selective adsorption of oil-water. Appl. Surf. Sci.. 2020;532:147400
- [CrossRef] [Google Scholar]
- Fabrication of superhydrophobic stainless-steel mesh for oil-water separation by jet electrodeposition. Colloid Surf. A. 2022;649:129434
- [CrossRef] [Google Scholar]
- Surface modification with hierarchical CuO arrays toward a flexible, durable superhydrophobic and self-cleaning material. Chem. Eng. J.. 2017;313:1328-1334.
- [CrossRef] [Google Scholar]
- Electrospinning nanofibers and nanomembranes for oil/water separation. J. Mater. Chem. A. 2021;9(38):21659-21684.
- [CrossRef] [Google Scholar]
- Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil-water separation. Colloid Surf. A. 2019;570:172-181.
- [CrossRef] [Google Scholar]
- Hydrothermal growth of ZnO nanowires scaffolds within mesoporous TiO2 photoanodes for dye-sensitized solar cells with enhanced efficiency. Electrochim. Acta. 2016;196:348-356.
- [CrossRef] [Google Scholar]
- Functional nanocomposite aerogels based on nanocrystalline cellulose for selective oil/water separation and antibacterial applications. Chem. Eng. J.. 2019;371:306-313.
- [CrossRef] [Google Scholar]
- Superhydrophobic and superoleophilic PH-CNT membrane for emulsified oil-water separation. Desalination. 2022;526:115536
- [CrossRef] [Google Scholar]
- Facile preparation of superhydrophobic porous wood for continuous oil-water separation. J. Water Process Eng.. 2020;36:101279
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104523.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







