Translate this page into:
Enhancing flame retardant and wrinkle-resistant performances of silk fabric with bio-based Maillard reaction products between glucose and poly(glutamic acid)
⁎Corresponding authors. zhangwen@wtu.edu.cn (Wen Zhang), liqing@wtu.edu.cn (Qing Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Bio-based materials have garnered considerable attention in the flame retardant field due to their inherent safety and environmental benefits. This study introduces a novel and eco-friendly flame retardant prepared through the Maillard reaction between glucose and poly(glutamic acid) for treating silk fabric. The study elucidates the synthesis of Maillard reaction products (MRPs) and verifies their deposition onto the silk fabric surface by observing changes in surface morphology, functional groups, and charged characteristics. The flammability tests demonstrate that MRPs treated silk fabrics had a high limiting oxygen index of over 27 % and a charred length of less than 12 cm, indicating effective flame retardancy. Moreover, the introduction of MRPs led to a significant decrease in smoke release when silk fabric underwent combustion. This observation can be attributed to the enhanced char formation and increased thermal degradation temperature of MRPs treated silk fabric. The electrostatic interaction between silk fiber and MRPs contributed to the fabric's resistance to repeated washing. Moreover, MRPs treated silk fabric retained their tensile properties and showed enhanced wrinkle-resistant performance. Generally, this research opens up a new path for the green preparation of halogen-free and phosphorus-free flame retardant protein fibers.
Keywords
Bio-based materials
Maillard reaction products
Green flame retardant
Thermal degradation
Silk fabric
Wrinkle-resistant performance
1 Introduction
Fire is indispensable in daily life as it is involved in diverse activities such as cooking, heating buildings, powering engines, and providing illumination. However, the potential hazards of fire raise people's concerns. The majority of deaths resulting from house fires is attributed to textiles, according to Khandual (Khandual, 2016). Additionally, during fires, the substantial smoke generated, comprising organic carbon, black carbon, and polycyclic aromatic hydrocarbons (Kurwadkar et al., 2023), poses a significant threat to human health and safety. Flame retardant textiles offer a promising solution by not just limiting fire spread and delaying critical moments but also reducing the release of smoke. These are benefiting human safety and minimizing property damage caused by house fires. Halogen-, phosphorus-, and nitrogen-containing organic compounds, as well as metal complexes are commonly used as flame retardant agents in the textile industry (Horrocks et al., 2005). The usage of halogen-containing flame retardant agents, such as polybrominated biphenyls, polybrominated diphenyl ethers, etc., has obviously decreased due to their toxic and bioaccumulative properties (Chen and Wang, 2010). Phosphorus-containing flame retardant agents such as Pyrovatex CP and ammonium polyphosphate are being used to replace halogenated ones because of their superior flame retardancy (Levchik and Weil, 2016). However, recent research has shown that phosphorus-containing flame retardants pose environmental risks owing to the fact that discharging of phosphorus-containing wastewater can lead to the overgrowth of algae and depletion of oxygen in bodies of water (Malucelli et al., 2014). The flame retardant treatment of protein fibers often involves the use of “Zippro” metal complexes (Benisek et al., 1979), which consists of hexafluorozirconate and hexafluorotitanate. However, the application of these complexes results in the release of metal ions that can have harmful effects on the soil and water ecosystems (Taib et al., 205 (2022)). In recent years, the development of eco-friendly and non-toxic flame retardants has been attracting scholarly attention due to their low toxicity, abundance, and cost-effectiveness (Khandual, 2016; Bhakare et al., 2023). Cheng and colleagues (Cheng et al., 2016) prepared flame-retardant wool fabric using phytic acid extracted from soybean through dipping method. Nevertheless, the significant water solubility and limited reactivity of phytic acid resulted in only electrostatic interactions with wool fibers, leading to suboptimal durability of the flame retardant characteristics. Carosio et al. (Carosio et al., 2014) and Bosco et al. (Bosco et al., 2017) respectively reported the preparation methods of flame retardant polyester-cotton fabric and raw cotton fabric by coating their surface with caseins and nucleic acids. Coating with caseins and nucleic acids can enhance the char formation ability and slow the flame spread rate visibly on the textile surface. Nonetheless, the treated textiles do not possess sufficient flame retardancy, with a limiting oxygen index (LOI) of less than 27 %. Xia et al. (Xia et al., 2018) synthesized a cost-effective flame retardant agent known as tannic acid terephthalate using tannic acid and terephthalyl chloride. The purpose of this research was to impart good char formation and flame retardance to Nylon 66 fabric. However, the inclusion of terephthalyl chloride had a negative impact on the environmental friendliness of the entire system.
Silk is a highly sought-after textile fabric due to its soft touch and elegant appearance. Unfortunately, silk fabrics tend to wrinkle easily during home laundering or when damp, which can be inconvenient for users. The poor wet resilience of silk is due to the low crystallinity of the silk structure and the lack of intermolecular cross-linking, causing the silk fabric to wrinkle. In the early days, urea–formaldehyde resins represented by dimethylol dihydroxyethylene urea (DMDHEU) were used for the anti-wrinkle finishing of silk (Liu et al., 2012), which had a significant anti-wrinkle effect. However, the finished fabric releases formaldehyde during storage or wear, which poses a great health hazard to people. Polycarboxylic acids (Reddy et al., 2011), such as BTCA and citric acid, are non-formaldehyde substitutes for urea–formaldehyde resins. They have achieved satisfactory resilience, whiteness, and washability, but they have a significant impact on their breaking strength. In addition, there are reports of non-formaldehyde anti-wrinkle agents such as epoxy compounds, siloxanes, and glutaraldehyde (Liu et al., 2012). Recently, natural macromolecules have been found to be used as anti-wrinkle agents for silk, such as poly(amino acid), chitosan, and cyclodextrin (Xing et al., 2015; Wang et al., 2014; Zare, 2022). The use of these macromolecules can reduce the use of synthetic chemical products, providing a viable solution for the green manufacturing and sustainable development of textiles.
Maillard reaction is a sort of carbonyl–amine reactions between carbonyl compounds (reducing sugars) and amino-containing molecules (amino acids and proteins), which involves sugar-amine condensation, Schiff base reaction, Amadori rearrange-ment, amino acid degradation, and so on (Ames, 1998). The Maillard reaction is a non-enzymatic browning reaction that imparts foods with their unique flavor, aroma, and color (Wang et al., 2011). The Maillard reaction advances through several stages, resulting in the formation of numerous intermediate and end products. Among these, melanoidins are the principal products of the Maillard reaction (Bork et al., 2022; Xu et al., 2019). These are brown, high molecular weight compounds that are responsible for the dark color of foods such as bread crusts, coffee, and roasted meat (Pérez-Burillo et al., 2020). Maillard reaction products (MRPs) are generally high nitrogen- and carbon-containing, heterogeneous, anionic polymers with different molecular weights (Chandra et al., 2008). MRPs have been widely studied in food, medicine, and biochemistry fields, owing to their excellent antibacterial and antioxidant properties (Ames, 1998; Feng et al., 2020). Jia et al. (Jia et al., 2023) reported that MRPs can be used as antioxidant and emulsifying agent to counteract lipid oxidation for achieving food products with high oxidative stability. Yang et al. (Yang et al., 2022) used MRPs to ameliorate dextran sulfate sodium-induced colitis. Liang et al. (Liang et al., 2014) designed a biological antibacterial agent through MRPs between polylysine and chitosan. In the field of textile industry, MRPs were employed to enhance the functional property and color of polyamide fabrics. Ohe et al. (Ohe and Yoshimura, 2013) proved that the MRPs obtained from the in-site reaction between sugars and polyamide fibers could be used to prepare coloristic and antibacterial textiles. In our previous work (Zhang and Tang, 2018), the MRPs prepared using glucose (Glc) and poly(glutamic acid) (PGA) was employed as a multi-functional dyestuff to impart silk fabric with deep color, antibacterial and antioxidant properties.
Reducing sugars and amino compounds are both crucial reactants in the Maillard reaction. Moreover, these two compounds are often employed as key ingredients in flame retardants (Ma et al., 2023; Liu et al., 2022; Kundu et al., 2021). This suggests that MRPs have potential in the field of flame retardancy. However, up to the present, there has been limited research on this topic. The present study focuses on the MRPs prepared using Glc and PGA, which were successfully used for the preparation of flame retardant silk without the employment of phosphorus- and halogen-containing compounds. The synthetic route of MRPs prepared with Glc and PGA was proposed. The chemical structures and groups of MRPs treated silk fabrics were characterized by attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS). The surface charged characteristics of MRPs treated silk fabrics was measured by Zeta potential analyses. The flammability of MRPs treated silk fabrics was evaluated via LOI and vertical burning tests. The washing durability was evaluated by testing the LOI and damaged length of flame retardant silk fabrics after repeated washing cycles. The smoke release capacity of MRPs treated silk fabric was measured. The thermal degradation behaviors of MRPs treated silk fabrics in nitrogen and air were measured by thermogravimetric (TG) analysis and differential thermal analysis (DTA). The morphological and chemical structures of charred products of silk fabric from vertical burning test were characterized by scanning electron microscope (SEM) and Fourier transform infrared spectroscopy (FT-IR). Finally, the tensile and wrinkle-resistant properties of MRPs treated silk fabric were measured.
2 Materials and methods
2.1 Materials
The crepe de Chine silk fabric was obtained from Suzhou Jiaduoli Silk Apparel Co. Ltd. in Suzhou, China. The silk fabric had a warp and weft count of 23.3 dtex/2, a warp density of 42 threads/cm, a weft density of 60 threads/cm, and a weight of 52 g/m2. Before use, it was scoured. The cosmetic grade polyglutamicacid (PGA) with a molecular weight ranging from 70 to 100 kDa (Nanjing Shineking Biotech Co. Ltd., Nanjing, China) and glucose (Glc) (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) were used. The necessary reagents, such as sodium hydroxide and sulfuric acid, were of analytical reagent grade. Lastly, the commercial detergents for wool and silk were purchased from a local department store.
2.2 Preparation of flame retardant solution
The same concentration of PGA and Glc was added in water bath, and the Maillard reaction between PGA and Glc was conducted in an alkaline medium whose initial pH was adjusted to 12 using diluted sodium hydroxide. The PGA and Glc concentration varied from 0 to 60 g/L. The reaction mixture was heated at a constant rate of 5 °C/min, from an initial temperature of 20 °C to a final temperature of 90 °C, and then held at this temperature for 120 min. Finally, the obtained MRPs solution was adjusted to pH 3 using diluted hydrochloric acid for the flame retardant treatment of silk fabric.
2.3 Flame retardant treatment of silk fabric
The treatment of silk using MRPs solution was conducted at constant temperatures in sealed and conical flasks placed in the XW-ZDR low-noise oscillated dyeing machine (Jingjiang Xinwang Dyeing and Finishing Machinery Factory, Jingjiang, China). Silk fabrics were soaked for 120 min in different concentrations (0–60 g/L) of MRPs at a 50:1 liquor-to-fabric ratio, with a temperature of 90 °C and a pH of 3. The specimens were then rinsed and left to air dry. In the subsequent sections, the silk fabric treated with 60 g/L MRPs was designated as MRPs treated silk fabric and was used for instrument characterization and flammability testing.
2.4 Measurements
The Nicolet 5700 FT-IR spectrometer was used to analyze silk samples and record their ATR-FTIR spectra. The measurements were performed using a scanning rate of 4 cm−1 in transmission mode spanning the spectral range from 400 to 4000 cm−1 (Patidar et al., 2023). KBr pellets were employed to measure the charred products of burned silk fabrics using the same instrument. The Axis Ultra DLD spectrometer was used to obtain the XPS spectra of silk samples. The monochromatized X-ray source using the 1486.6 eV aluminum Kα was employed for the measurement. The SurPASS electrokinetic analyzer was used to assess the Zeta potentials of silk samples. The limiting oxygen index (LOI) of the samples (158 mm × 58 mm) was measured as per GB/T 5454–1997, which is equivalent to the ASTM Standard Method D2863, using the FTT0080 oxygen index apparatus. Three measurements were taken, and the average was calculated. The damaged length of the samples (300 mm × 80 mm) was tested using the YG815B automatic vertical flammability cabinet based on GB/T 5455–2014, which is equivalent to ASTM Standard Method D6413. In this test, the propane flame was used to ignite the bottom of each sample for 12 s, and five measurements were taken, and the average was calculated. The thermal and thermal-oxidative degradation of silk fabrics treated with MRPs were studied using a thermal analyzer from the Diamond TG/DTA SⅡ series. Samples of approximately 5 mg were placed in pans made of alumina and tested using nitrogen and air flow rate of 20 mL/min at a heating rate of 10 °C/min. Smoke suppression properties were evaluated in accordance with ISO 5659–2 using a smoke density box from the FTT0064 model, exposing samples (80 mm × 80 mm, 3 layers) to a temperature of 550 °C for a duration of 700 s at an incident heat flux of 25 kW/m2. The optical density of smoke is the measure of the degree of opacity of smoke, taken as the negative common logarithm of the relative transmission of light. The specific optical density is that optical density multiplied by a factor which is calculated by dividing the volume of the test chamber by the product of the exposed area of the specimen and the path the light beam. The specific optical density is obtained from the output of the instrument. Silk fabrics and their charred products were coated with a 15 nm gold layer using sputter-coating, and their surface texture was analyzed with a Hitachi TM3030 scanning electron microscope. The wash-resistance of FR capability was determined by assessing the flammability of the samples that have undergone multiple washings. The washing tests were conducted using the WashTec–P fastness tester with a 2 g/L commercial detergent solution developed for silk and wool garments and a 50:1 liquor ratio at 40 °C for 30 min. The fabric was removed, gently squeezed, and rinsed with tap water after each washing. The washing was repeated 5, 10, 15, and 20 times to evaluate the washing durability of flame retardant silk fabric. The tensile properties of the silk fabric samples were assessed by means of an Instron 3365 tester. The samples were climatized in a room with constant temperature and humidity conditions (65 % relative humidity and 21 °C), and the average of 5 tests was calculated. The crease recovery angle of the silk fabric samples was measured according to the method specified by GB/T 3819–1997, and the average of 5 tests was calculated.
3 Results
3.1 Schematic synthesis of MRPs
Fig. 1 illustrates the Maillard reaction mechanism occurring between Glc and amino-containing compounds, along with the process for preparing flame retardant silk fabric. As shown in Fig. 1a, in the initial step, the carbonyl group of Glc conjugates with the amino group of amino-containing compounds, forming an N-substituted glycosylamine compound (Ames, 1998). Subsequently, the glycosylamine compound undergoes dehydration, leading to the formation of an imine, commonly known as the Schiff base (Wang et al., 2011). The Schiff base compound is thermodynamically unstable and spontaneously undergoes rearrangement, forming a 1,2-enaminol, which is further converted into a more stable aminoketose through Amadori rearrangement. The Amadori product can subsequently undergo enolization, yielding either 1,2-enaminol or 2,3-enaminol, and forming deoxyosones. These dicarbonyl compounds are highly reactive substances that promptly react with amino-containing compounds. Subsequently, melanoidins are generated after several steps, such as dehydration, Strecker degradation, polymeration. In this work, equal concentrations of Glc and PGA underwent the Maillard reaction in an alkaline aqueous solution. The resulting MRPs solutions were then adjusted to pH 3 for flame retardant treatment of silk fabric. Melanoidins are usually regarded as the principal products of the Maillard reaction and generally manifest as nitrogen-containing and anionic polymers with different molecular weights (Shang et al., 2020). Besides, Maillard reaction intermediates, such as reductones and aldehydes, are also present in the products of the Maillard reaction. The process of treating silk fabric with the resulting MRPs solution is shown in Fig. 1b. During the treatment, silk fabric and the MRPs may occur three possible interactions: the ionic bond between the negatively charged melanoidins and the protonated amino groups of silk fabrics; the hydrogen bond between the amino groups of silk fabrics and the hydroxyl groups of melanoidins; the Schiff base reaction between the amino groups of silk fabrics and the reductones and aldehydes in MRPs. These interactions were responsible for the deposition of MRPs on the silk fabric surface. Finally, the flame retardant silk fabric was obtained after the treatment of MRPs.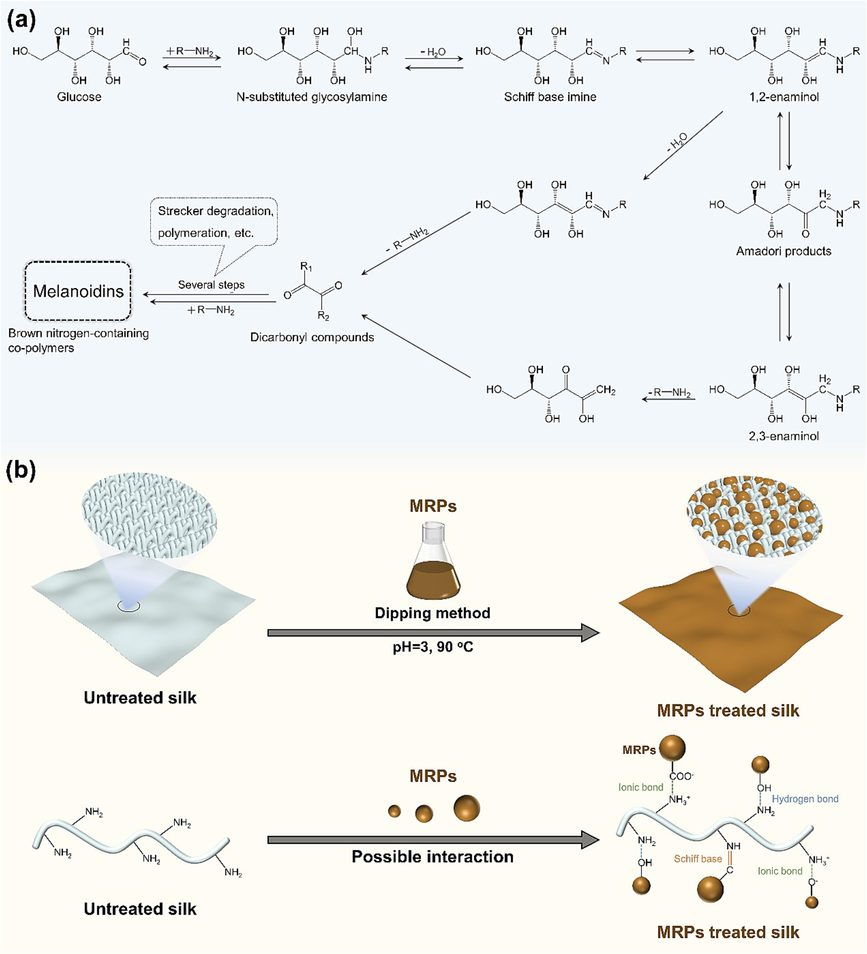
Illustration of (a) Maillard reaction between Glc and amino-containing compounds and (b) the preparation process of MRPs treated silk fabric.
3.2 Characterization of MRPs treated silk fabric
In our previous work (Zhang and Tang, 2018), the FT-IR spectrum of MRPs prepared with PGA and Glc displayed the bands corresponding to N—H (3430 cm−1), C⚌O (1720 cm−1), C—O in Amide I (1635 cm−1), C⚌N (1550 cm−1), and C—O (1130 cm−1). The appearance of C⚌O and C⚌N stretching vibration confirmed the formation of carbonyl compounds and Schiff base compounds, respectively. The pronounced intensity peak at 1130 cm−1 (C—O stretching vibration) provides evidences of the presence of melanoidin structures (Zhang and Tang, 2018). The surface functional groups of untreated and MRPs treated silk fabrics were analyzed by ATR-FTIR, as shown in Fig. 2a. The spectrum of untreated silk fabric had C⚌O in carbonyl group (1699 cm−1), C⚌O in Amide I (1625 cm−1), and C—O in hydroxyl group (1232 cm−1), accompanied by the bending vibration of N—H in Amide I (1517 cm−1) (Gore et al., 2019). In comparison with untreated silk fabric, the spectrum of MRPs treated silk showed enhanced absorption intensity at 1699, 1517, and 1232 cm−1, indicating an increase of carbonyl, amide, and hydroxyl groups on the fabric surface. This phenomenon is attributed to the deposition of MRPs on silk fabric surface.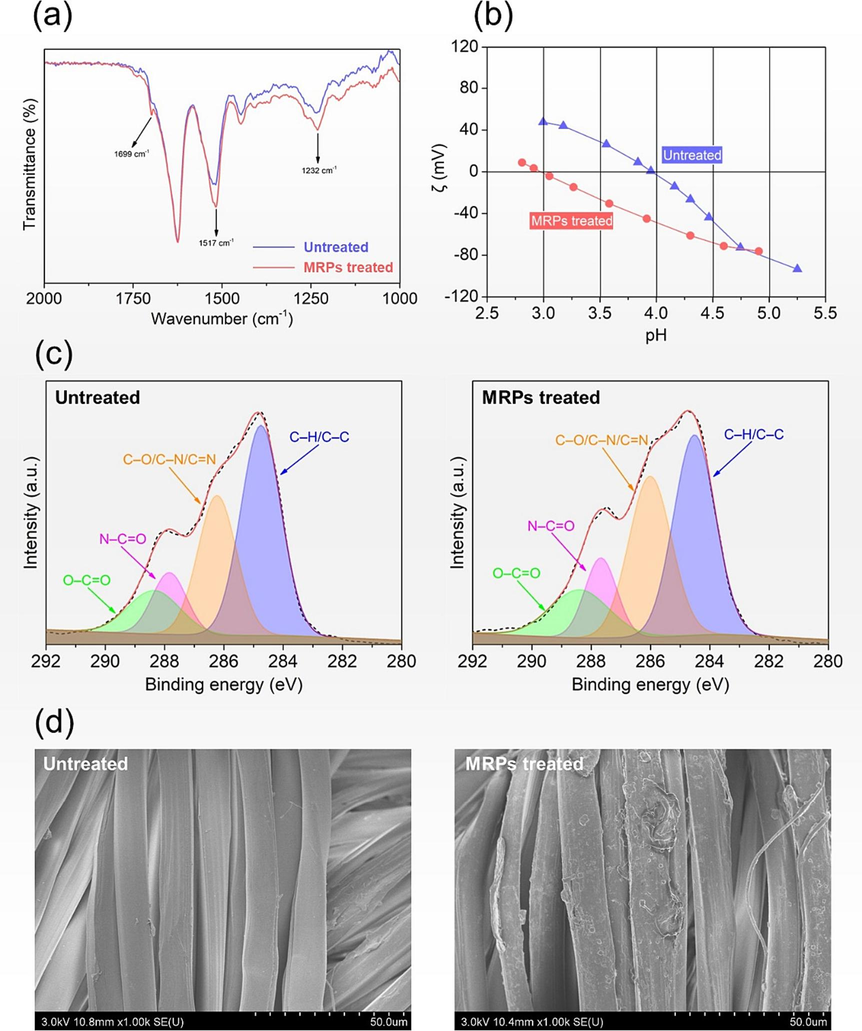
ATR-FTIR spectra (a), Zeta potential (b), XPS C1s spectra (c), and SEM micrographs (d) of the untreated and MRPs treated silk fabrics.
The Zeta potential provides a comprehensive insight into the distribution of acidic and basic groups on the surface of silk fabric. Fig. 2b reveals the Zeta potentials of the untreated and MRPs treated silk fabrics at varying pH conditions. Both untreated and MRPs treated silk fabrics exhibited clear amphoteric features (Zhang et al., 2020). As the pH value increased, their Zeta potential transitioned from positive to negative at isoelectric points (when the number of negative charges equaled that of positive charges). The isoelectric point of silk fabric shifted from 4.2 to 3.3 in response to MRPs treatment, providing evidence that MRPs derived from the reaction of PGA and Glc were anionic compounds due to the presence of abundant hydroxyl and carboxyl groups. The reduction in the isoelectric point provides supplementary evidence that MRPs were introduced onto the surface of silk fabric. The results of ATR-FTIR, XPS, and Zeta potential consistently support the successful introduction of MRPs prepared using PGA and Glc on the surface of silk fabric.
The alterations in the chemical components of silk fiber, resulting from the inclusion of MRPs, were assessed through XPS analyses. The high-resolution C1s spectrum of untreated silk fabric was deconvoluted into four peaks, which included C—C/C—H, C—N/C⚌N/C—O, O⚌C—N, and O⚌C—O bonds, with their respective binding energies of 284.7, 286.2, 287.7, and 288.4 eV (Kondyurin et al., 2011; Wang et al., 2023), as indicated in Fig. 2c. MRPs treatment resulted in some noticeable differences in the C1s peaks of silk fabric, with detailed results provided in Table 1. The MRPs treatment on silk fabric resulted in a significant increase in the percentage of C—N/C⚌N/C—O, which rose from 29.7 % in untreated silk fabric to 33.2 %. This increase can be attributed to the formation of glycosylamine and Schiff base imines and the introduction of hydroxy groups of Glc. Furthermore, the augmented percentages of O⚌C—N and O⚌C—O bonds were associated with the introduction of PGA molecules which are rich in carbonyl and amide groups.
Sample
Chemical component percentages
C—C/C—H
C—N/C⚌N/C—O
O⚌C—N
O⚌C—O
Untreated
46.3
29.7
11.6
12.4
MRPs treated
40.2
33.2
12.4
14.2
The SEM micrographs depict the changes in silk fabrics before and after treatment with MRPs, as illustrated in Fig. 2d. It can be observed that the untreated silk fabric had a pristine and clean surface. By comparison, MRPs treated silk fabric had a texturally rougher surface as a result of the deposition of MRPs on the surface of the silk fibers. MRPs coated the surface of silk fibers rather than diffused into the fibers, primarily due to their high molecular weight. The introduction of MRPs induced the variation of silk fabric surface in the chemical structure, charge effect, and morphological structure.
3.3 Flammability of MRPs treated silk fabric
In our study, the flammability of silk fabric was evaluated by LOI and vertical burning tests, and the relevant data was listed in Table S1. Fig. 3a shows the weight gain, LOI value, and washing durability of untreated and MRPs treated silk fabrics. The untreated silk fabric had an LOI value of 24.4 %. In the case of silk fabrics treated with either PGA or Glc individually (60 g/L), there was only a marginal enhancement observed in their LOI value. In other words, a single PGA or Glc cannot significantly affect the flammability of silk fabrics. Interestingly, as the concentration of MRPs increased, the LOI value of the silk fabric distinctly increased, which implies the flame retardancy of MRPs for silk fabrics. In particular, silk fabric treated with 60 g/L MRPs revealed an LOI value of over 27.0 %. The silk fabric treated with 60 g/L MRPs was subjected to repeated washing to evaluate its durability. After repeated washing, MRPs treated silk fabric showed a downward trend in the weight gain and LOI value with the increment of washing times. However, despite this decrease, the LOI value consistently exceeded 26 % after enduring 10 washing cycles, indicating that the treatment sustained the fabric's fire-retardant properties throughout multiple wash cycles. According to afore-mentioned results, it was inferable that the introduction of MRPs enhanced the flame retardancy of silk fabrics.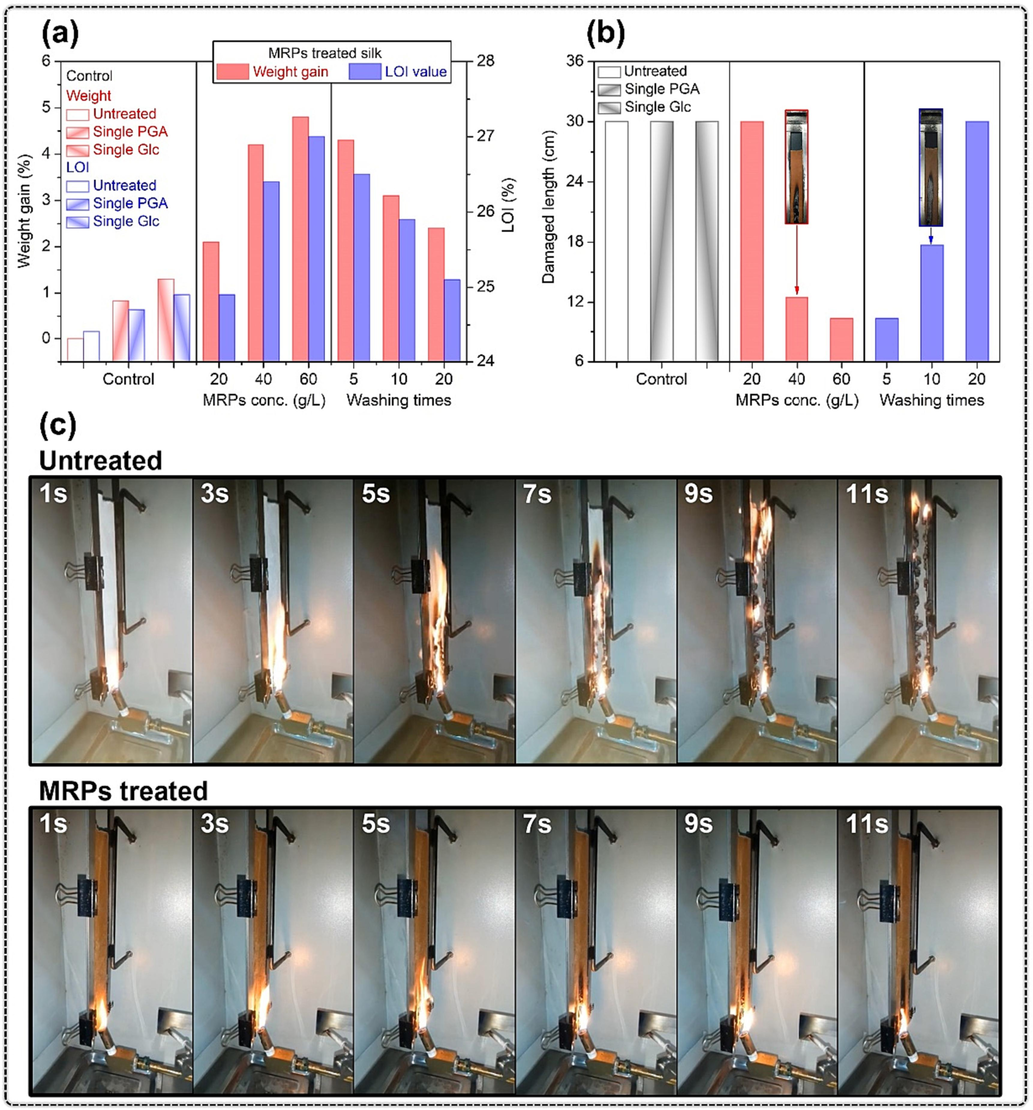
(a) Weight gain, LOI value, and washing durability of silk fabrics; (b) damaged length and washing durability of silk fabrics; (c) vertical burning results of silk fabrics.
The vertical burning test of textiles can intuitively evaluate the flame retardancy of textiles. As per the Chinese Standard GB/T 17591–2006 (Gb, t 17591–2006, 2006), flame retardant furnishing fabrics can be classified as either B1 or B2 grade fabrics, depending on the length of damage incurred during the test. For B1 grade fabric, the damaged length must be less than 15 cm, while the requirement for B2 grade fabric is that the damaged length is less than 20 cm. Fig. 3b illustrates the damage length of untreated and MRPs treated silk fabrics, as well as the durability of flame retardant fabrics after washing. During vertical burning test, the untreated, single PGA treated, and single Glc treated silk fabric were completely burned after being set alight. The measured damaged length for these fabrics was over 30 cm, with no self-extinguishing phenomenon observed during the vertical burning test, indicating poor flame retardancy. On the other hand, MRPs treatment reduced the damaged length of silk fabric to 11.7 cm, with a dosage of 60 g/L required for optimal results. During the vertical burning test of MRPs treated silk fabric, self-extinguishing occurred after 7 s (as displayed in Fig. 3c) and the damaged length was 10.4 cm, meeting the standard for B1 grade fabric. Even after being washed 10 times, the MRPs treated silk fabric still had a damaged length of 17.7 cm, allowing it to be evaluated for B2 grade fabric. However, after the 20th washing cycle, the MRPs treated silk fabric was burned out, exemplifying the resistance of the fabric to only 10 washing times. MRPs treated silk fabrics maintaining flame retardant properties offered prolonged fire safety in places like hospitals or industries with high hygiene standards, potentially reducing replacement needs despite washing, ensuring both safety and cost-effectiveness.
The high weight gain and semi-durability of MRPs treated silk fabric demonstrate a strong bond between MRPs and silk fiber. The possible interactions between MRPs and silk fiber include: protonated amino groups of silk fiber form an electrovalent bond with negatively charged carboxyl and hydroxyl groups of MRPs through ionic interactions; MRPs and silk fiber form a hydrogen bond; the reducing MRPs (such as reductones and aldehydes) formed a covalent bond with the amino groups of silk fabric through a Schiff base reaction.
3.4 Smoke release capability of MRPs treated silk fabric
The flammable materials release hazardous smoke during a fire that contributes significantly to fatalities caused by poisoning or suffocation. Additionally, smokescreen effect caused by smoke can hinder evacuation making people trapped in the fire zone (He et al., 2022). Reducing the smoke release capability of flammable materials is essential to reduce the risk of fires. The effect of MRPs on the smoke release capability of silk fabric was investigated, and the results are revealed in Fig. 4. The specific optical density of untreated silk fabric increased rapidly in 135 s, and it remained steady between 135 and 600 s, peaking at 41.8. By contrast, MRPs treated silk fabric experienced a rapid increase in specific optical density at 105 s and a gradual increase between 105 and 600 s, peaking at 34.9. Overall, MRPs treated silk fabric demonstrated lower smoke release capability when compared to untreated silk fabric, implying lower fire hazard.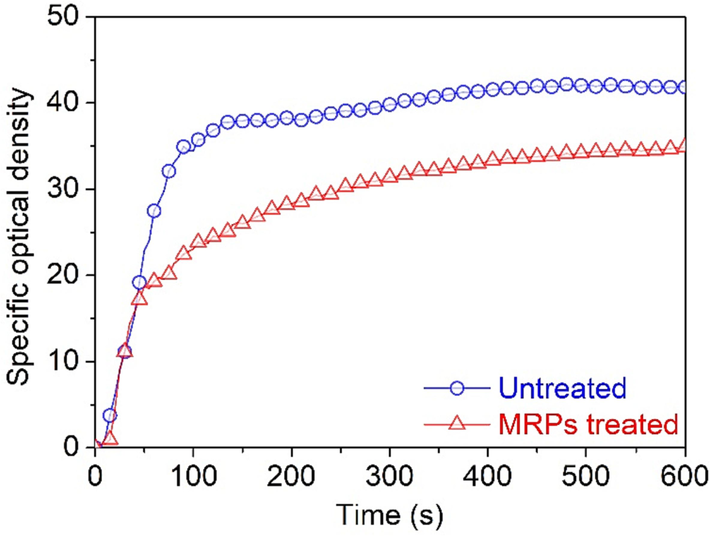
Smoke release capabilities of the untreated and MRPs treated silk fabrics.
3.5 Flame retardant mechanism of MRPs treated silk fabric
The TG analysis was performed to investigate the thermal degradation of silk fabric. Fig. 5a display the residual weight of the silk fabrics that were untreated and treated with MRPs, in the temperature range of 100–600 °C under nitrogen and air atmospheres. Under nitrogen atmosphere, untreated silk fabric showed three degradation stages, including water evaporation (30–150 °C), pyrolysis of protein (220–360 °C), and further degradation of pyrolysis products of protein (450–550 °C), as identified by Beck, Gordon, and Ingham (Beck et al., 2016). Under air atmosphere, silk fabric underwent a fourth degradation stage (550–700 °C), involving thermal oxidation decomposition of residual char. The DTG curves of samples are shown in Figure S1. Relative to untreated silk fabric, the MRPs treated silk fabric exhibited variations only in the pyrolysis stage of protein under both nitrogen and air atmospheres. Table 2 lists the TG parameters of untreated and MRPs treated silk fabrics under nitrogen and air atmospheres. T10% and T40% denote the temperature at which the weight loss was 10 % and 40 %, respectively. Tmax was the temperature at which maximum weight loss rate occurred. Char residue signifies the weight retention of silk fabric at 600 °C. The T10% value of untreated silk fabric was higher compared to MRPs treated silk fabric. However, the T40% and Tmax values of untreated silk fabric were lower than those of MRPs treated silk fabric. These findings suggest that MRPs treated silk fabric had a higher degradation temperature at high temperature, potentially due to the high char residue of pure MRPs.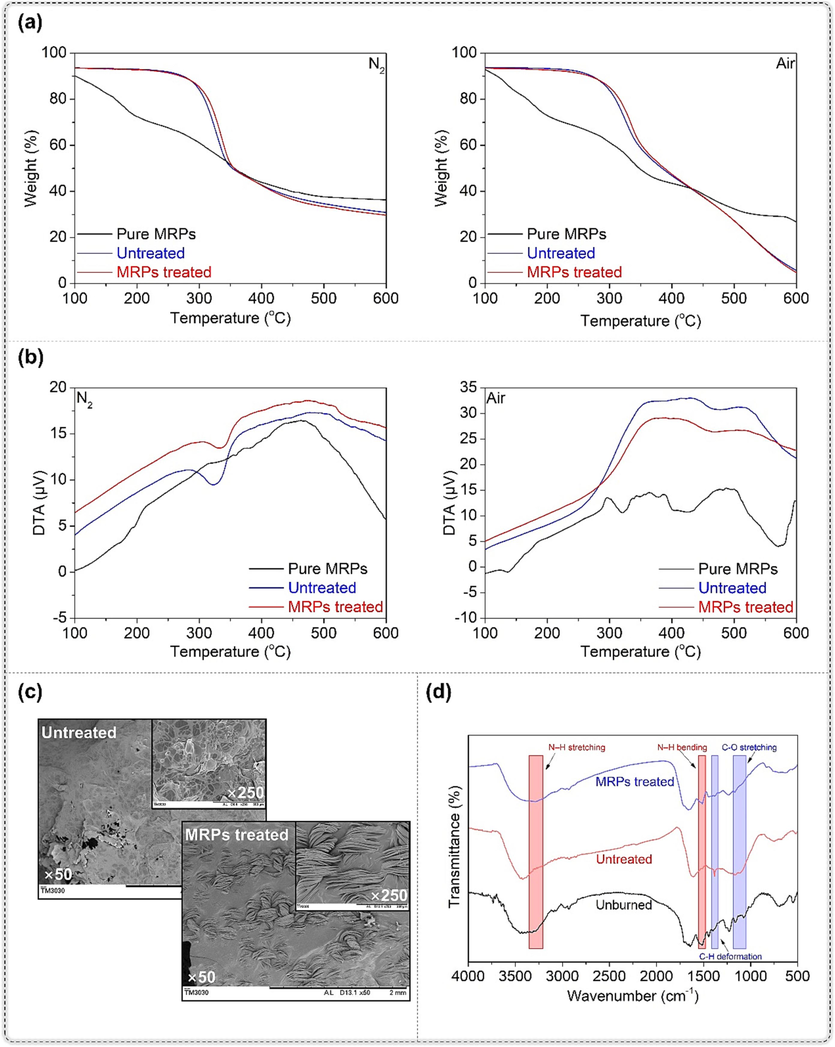
(a) TG curves of silk fabrics under nitrogen and air; (b) DTA curves of silk fabrics under nitrogen and air; (c) morphological structures and (d) FT-IR spectra of charred products of silk fabrics from vertical burning test.
Sample
T10% (oC)
T40% (oC)
Tmax (oC)
Char residue (%)
Nitrogen
Pure MRPs
101.6
306.1
348.6
36.3
Untreated
273.8
333.6
324.6
30.8
MRPs treated
270.3
339.5
336.6
29.7
Air
Pure MRPs
119.7
307.0
333.6
26.7
Untreated
271.9
346.5
325.2
5.7
MRPs treated
268.4
351.6
334.3
4.7
DTA analysis was utilized to provide insight into the thermal decomposition of untreated and MRPs treated silk fabrics. Fig. 5b illustrate the DTA curves of the untreated and MRPs treated silk fabrics under nitrogen and air atmospheres. Under nitrogen, it is found that the untreated silk fabric exhibited an endothermic peak around 324 °C, which corresponded with the pyrolysis of silk fiber (Yasukawa et al., 2021). As for the MRPs treatment, the endothermic peak of the silk fabric shifted to roughly 338 °C, indicating a higher pyrolysis temperature. Nevertheless, under air, both untreated and MRPs treated silk fabrics demonstrated exothermic peaks within the 300–550 °C temperature range. These peaks corresponded with the thermal oxidation of silk fiber. Additionally, the thermal oxidation temperature of the MRPs treated silk fabric was higher than that of the untreated silk fabric. The DTA analysis supported the results of TG analysis which indicates that the presence of MRPs can enhance silk fabric's thermal degradation temperature.
The surface morphology of the charred products of silk fabrics acquired from vertical burning test was observed using SEM, and the results are shown in Fig. 5c. During the vertical burning test, the untreated silk fabric lost its fibrous structure and formed bubbled and fusional charred products, which is a result of full combustion and incomplete char-formation. In contrast, MRPs treated silk fabric exhibited a compact residual char with a partial fibrous structure, suggesting better char formation and thermal stability. However, these findings are inconsistent with the result of char residue from TG analysis, which may be attributed to the disparity in heating rates between burning test and TG analysis (Alongi et al., 2015).
The chemical structures of charred products from the vertical burning test were measured by means of FT-IR. As demonstrated in Fig. 5d, the FT-IR spectra of charred products of untreated silk fabric showed the absence of peaks at 3290 and 1508 cm−1, which correspond to N—H stretching and the N—H bending vibration (Amide II) (Jose et al., 2022), respectively, in comparison to untreated silk fabric. This illustrates that the charred products of MRPs treated silk fabric possessed higher content of nitrogen than that of untreated silk fabric (Yang et al., 2018). Furthermore, the charred products of untreated silk fabric showed higher extent of oxidation and carbonization when compared to that of MRPs treated silk fabric, which is proved by the stronger intensity of peaks at 1382 and 1112 cm−1 corresponding to C⚌H deformation and C⚌O stretching vibration. In can also be observed that the charred products of MRPs treated silk fabric clearly revealed all the absorption bands of the protein chain of silk fibers. This finding suggests that the chemical structure of MRPs treated silk fabric was well-retained during burning, as opposed to the charred products of untreated silk fabric. This is consistent with the results of the SEM analysis.
The introduction of MRPs had a discernible effect on the thermal degradation processes of silk fabric, as indicated by the results of TG, SEM, and FT-IR analyses. The improved thermal degradation temperature of MRPs treated silk fabric at high temperatures led to the formation of high-quality charred products during burning, while simultaneously decreasing smoke production. The resulting fibrous charred products served as a barrier, blocking energy and fuel transmission and preventing the continued burning of substrates throughout condensed phases. The aforementioned phenomenon is shown in Fig. 6. Consequently, silk fabrics treated with MRPs exhibited displayed satisfactory flame retardancy and aided in smoke suppression, both of which reflect positively on safety concerns.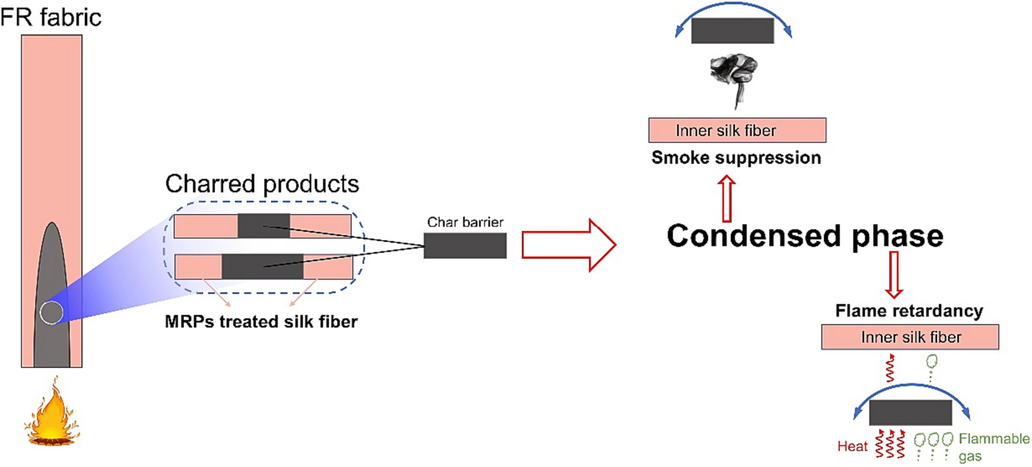
Flame retardant and smoke suppression mechanism of MRPs treated silk fabric.
3.6 Mechanical properties of MRPs treated silk fabric
The influence of MRPs on the mechanical properties of silk fabric was investigated. Table 3 presents the tensile properties of untreated and MRPs treated silk fabrics. The untreated silk fabric had a tensile strength of 300 N in the warp direction and 424 N in the weft direction. The silk fabric showed a 7.3 % decrease in tensile strength in the warp direction but a 10.1 % increase in the weft direction after MRPs treatment. The untreated silk fabric had a breaking elongation of 30 % in the warp direction and 38 % in the weft direction. After MRPs treatment, the breaking elongation of the silk fabric showed a 3.3 % decrease in the warp direction and a 7.9 % increase in the weft direction. Also, Table 3 lists the anti-wrinkle properties of untreated and MRPs treated silk fabric. The untreated silk fabric had a wrinkle recovery angle of 169° in the warp direction and 164° in the weft direction. In the case of MRPs treatment, the silk fabric showed a wrinkle recovery angle of 176° in the warp direction and 171° in the weft direction, indicating a 7° increase in both directions. This result might be attributed to the crosslinking of protein chains of silk through interaction between the carbonyl groups of MRPs and the amino groups of the protein (Wang et al., 2023). Overall, the results show that MRPs treatment has a negligible effect on the tensile properties of silk fabric but enhances the anti-wrinkle properties of the silk fabric.
Mechanical properties
Untreated
MRPs treated
Warp
Weft
Warp
Weft
Tensile strength (N)
300
424
278
467
Breaking elongation (%)
30
38
29
41
Wrinkle recovery angle (o)
169
164
176
171
3.7 Flammability of MRPs treatment on other fabrics
The amount of MRPs deposited on the surface of wool, polyamide, and cotton fabrics had great influences on their flammability. Table S2 lists the weight gain and flammability of the three fabrics treated with MRPs. Wool and polyamide fabrics can be positively charged under acidic conditions and can absorb a large number of negatively charged MRPs through hydrogen bond, electrostatic interaction, etc. As a result, they had a significant increment in the LOI value, indicating elevated flame retardancy. In addition, wool fabric showed a self-extinguishing phenomenon during the vertical burning test because its LOI value was higher than 27 %. However, negatively charged MRPs did not deposit extensively on cotton fabric due to their electrostatic repulsion. Thus, MRPs treatment had less effect on the flammability of cotton fabric.
4 Conclusions
Our study proposes a novel and green route to prepare flame retardant silk fabric without the employment of phosphorus- and halogen-containing compounds. The Maillard reaction products between PGA and Glc were deposited on the surface of silk fabric by dipping method to endow it to flame retardant and wrinkle resistant properties. The MRPs between PGA and Glc were generated via a series of reactions, including sugar-amine condensation, Schiff base reaction, and Amadori rearrangement. The elevated content of carbonyl and hydroxyl groups on fiber surface was observed with the deposition of MRPs, which led to the decrease of the isoelectric point of the fabric. Silk fabric treated with 60 g/L MRPs revealed an LOI value of over 27 % and a damaged length of less than 12 cm. Besides, MRPs treated silk fabric also showed lower smoke release capacity than untreated silk fabric. After 10 washing times, the MRPs treated silk fabric can be still evaluated for B2 grade fabric, indicating semi-durability to washing. Moreover, the TG and DTA analyses suggested that the thermal degradation temperature of the silk fabric increases in the presence of MRPs. The SEM and FT-IR analyses of charred products supported the enhanced char formation ability of MRPs treated silk fabric. In light of these, it is inferred that MRPs are involved in altering the thermal degradation of silk fabric, resulting in the formation of high-quality fibrous charred products. These charred products serve as barriers, impeding the transfer of energy and fuel, preventing the continuous combustion of the substrate throughout the entire condensation phase, and concurrently reducing the production of smoke. The MRPs treatment had less negative affect on the mechanical behavior of silk fabric, and increased its wrinkle resistant property. In summary, the MRPs of PGA and Glc hold significant promise as halogen-free and phosphate-free flame retardants for silk fabric.
CRediT authorship contribution statement
Huiyu Jiang: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – review & editing. Ying Chen: Investigation, Data curation, Writing – original draft. Wen Zhang: Conceptualization, Formal analysis, Writing – review & editing, Supervision. Qing Li: Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing, Supervision.
Acknowledgements
This research was funded by the Key Laboratory of Flame Retardancy Finishing of Textile Materials, CNTAC (Q811580522), Hubei Key Laboratory of Biomass Fibers & Eco-Dyeing & Finishing (Wuhan Textile University), No. STRZ202230 and STRZ202303, Key Research and Development Plan of Hubei Provincial Department of Science and Technology (Assisting Enterprises in Poverty Alleviation and Package Insurance, 2022BAD012), and the National Natural Science Foundation of China (52303062).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- How much the fabric grammage may affect cotton combustion? Cellulose. 2015;22:3477-3489.
- [CrossRef] [Google Scholar]
- Applications of the Maillard reaction in the food industry. Food Chem.. 1998;62:431-439.
- [CrossRef] [Google Scholar]
- Thermogravimetric analysis of flame–retardant–treated wools. Text. Res. J.. 2016;46:478-483.
- [CrossRef] [Google Scholar]
- Protective clothing evaluation of zirpro wool and other fabrics. Fire Mater.. 1979;3:156-166.
- [CrossRef] [Google Scholar]
- Dual functions of bioinspired, water-based, reusable composite as a highly efficient flame retardant and strong adhesive. Chem. Eng. J.. 2023;454:140421
- [CrossRef] [Google Scholar]
- Structural characterization of polar melanoidins deriving from Maillard reaction intermediates–A model approach. Food Chem.. 2022;395:133592
- [CrossRef] [Google Scholar]
- Nucleic acids from agro–industrial wastes: a green recovery method for fire retardant applications. Ind. Crop. Prod.. 2017;108:208-218.
- [CrossRef] [Google Scholar]
- Flame retardancy of polyester and polyester–cotton blends treated with caseins. Ind. Eng. Chem. Res.. 2014;53:3917-3923.
- [CrossRef] [Google Scholar]
- Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Bioresour. Technol.. 2008;99:4648-4660.
- [CrossRef] [Google Scholar]
- A review on flame retardant technology in China. Part I: development of flame retardants. Polym. Adv. Technol.. 2010;21:1-26.
- [CrossRef] [Google Scholar]
- Adsorption and flame retardant properties of bio–based phytic acid on wool fabric. Polymers.. 2016;8:1-18.
- [CrossRef] [Google Scholar]
- A novel drug delivery system obtained from hydrophobic modified amphiphilic polymers by Maillard reaction. Int. J. Biol. Macromol.. 2020;157:146-150.
- [CrossRef] [Google Scholar]
- GB/T 17591-2006, 2006. Flame Retardant Fabrics. China’s General Administration of Quality Supervision, Inspection and Quarantine and Standardization Administration of China, Beijing, China.
- Progress in silk materials for integrated water treatments: fabrication, modification and applications. Chem. Eng. J.. 2019;374:437-470.
- [CrossRef] [Google Scholar]
- Smart fire alarm systems for rapid early fire warning: advances and challenges. Chem. Eng. J.. 2022;450:137927
- [CrossRef] [Google Scholar]
- Developments in flame retardant textiles-a review. Polym. Degrad. Stab.. 2005;88:3-12.
- [CrossRef] [Google Scholar]
- Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem.. 2023;404:134541
- [CrossRef] [Google Scholar]
- Physico-mechanical, thermal, morphological, and aging characteristics of green hybrid composites prepared from wool-Sisal and Wool-Palf with Natural Rubber. Polymers. 2022;14:4882.
- [CrossRef] [Google Scholar]
- Khandual, A., 2016. Green Flame Retardants for Textiles. In: Green Fashion, 2nd ed., S.S. Muthu, M.A. Gardetti, Eds. Publisher: Springer Singapore, Singapore, pp. 154–196. https://doi.org/10.1007/978–981–10–0245–8_6.
- Surface attachment of horseradish peroxidase to nylon modified by plasma–immersion ion implantation. J. Appl. Polym. Sci.. 2011;120:2891-2903.
- [CrossRef] [Google Scholar]
- Sucrose derivative as a cross-linking agent in enhancing coating stability and flame retardancy of polyamide 66 textiles. Prog. Org. Coat.. 2021;159:106438
- [CrossRef] [Google Scholar]
- Emissions of black carbon and polycyclic aromatic hydrocarbons: potential implications of cultural practices during the Covid-19 pandemic. Gondwana Res.. 2023;114:4-14.
- [CrossRef] [Google Scholar]
- A review of recent progress in phosphorus–based flame retardants. J. Fire Sci.. 2016;24:345-364.
- [CrossRef] [Google Scholar]
- Structure and antimicrobial mechanism of ɛ-polylysine–chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol.. 2014;70:427-434.
- [CrossRef] [Google Scholar]
- A multi-element flame retardant on the basis of silicon-phosphorus-nitrogen for combustibility suppressing of epoxy. Polym. Test.. 2022;111:107582
- [CrossRef] [Google Scholar]
- Study on silk anti-crease finishing with polycarboxyl-terminated trichlorotriazine derivatives by surface analysis methods. Appl. Surf. Sci.. 2012;261:255-261.
- [CrossRef] [Google Scholar]
- Construction of green versatile coating integrating fireproof, electric-conduction and self-cleaning performance for cotton fabrics. Appl. Surf. Sci.. 2023;637:157862
- [CrossRef] [Google Scholar]
- Biomacromolecules as novel green flame retardant systems for textiles: an overview. RSC Adv.. 2014;4:46024-46039.
- [CrossRef] [Google Scholar]
- Coloration of polyamide fibers in an aqueous solution by Maillard reaction. Text. Res. J.. 2013;84:539-545.
- [CrossRef] [Google Scholar]
- Microplastics as heavy metal vectors in the freshwater environment: distribution, variations, sources and health risk. Phys. Chem. Earth. 2023;131:103448
- [CrossRef] [Google Scholar]
- Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem.. 2020;316:126309
- [CrossRef] [Google Scholar]
- Low-temperature wet-cross-linking of silk with citric acid. Ind. Eng. Chem. Res.. 2011;50:4458-4463.
- [CrossRef] [Google Scholar]
- Structure and physiochemical characteristics of whey protein isolate conjugated with xylose through Maillard reaction at different degrees. Arab. J. Chem.. 2020;13:8051-8059.
- [CrossRef] [Google Scholar]
- Current progress of biopolymer-based flame retardant. Polym. Degrad. Stab.. 2022;205:110153
- [CrossRef] [Google Scholar]
- A review on the status of formaldehyde-free anti-wrinkle cross-linking agents for cotton fabrics: Mechanisms and applications. Ind. Crop. Prod.. 2023;200:116831
- [CrossRef] [Google Scholar]
- Melanoidins produced by the Maillard reaction: structure and biological activity. Food Chem.. 2011;128:573-584.
- [CrossRef] [Google Scholar]
- Constructing recyclable photocatalytic BiOBr/Ag nanowires/cotton fabric for efficient dye degradation under visible light. Arab. J. Chem.. 2023;16(4):104624
- [CrossRef] [Google Scholar]
- Modification of B ombyx mori silk fabrics by tyrosinase-catalyzed grafting of chitosan. Eng. Life Sci.. 2014;14:211-217.
- [CrossRef] [Google Scholar]
- Fire resistant polyphenols based on chemical modification of bio-derived tannic acid. Polym. Degrad. Stab.. 2018;153:227-243.
- [CrossRef] [Google Scholar]
- Preparation and properties of silk fabric grafted with ɛ-polylysine by tyrosinase. Text. Res. J.. 2015;85:1743-1748.
- [CrossRef] [Google Scholar]
- Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol.. 2019;131:601-607.
- [CrossRef] [Google Scholar]
- Instrumental characterization and functional assessment of the two-color silk fabric coated by the extract from Dioscorea cirrhosa tuber and mordanted by iron salt-containing mud. Ind. Crop. Prod.. 2018;111:117-125.
- [CrossRef] [Google Scholar]
- Ovalbumin and its Maillard reaction products ameliorate dextran sulfate sodium-induced colitis by mitigating the imbalance of gut microbiota and metabolites. Int. J. Biol. Macromol.. 2022;222:715-724.
- [CrossRef] [Google Scholar]
- Dyeing silk and cotton fabrics using Fuji apple peel and the properties of the dyed fabrics. Text. Res. J.. 2021;91:2669-2681.
- [CrossRef] [Google Scholar]
- Citric acid as environment friendly crease-resistance finishing agent for silk fabric combined by ß-cyclodextrin. Res. J. Text. Appar.. 2022;26:238-254.
- [CrossRef] [Google Scholar]
- Application of the products from the Maillard reaction of polyglutamic acid and glucose to prepare colored and bioactive silk. Polymers. 2018;10:648.
- [CrossRef] [Google Scholar]
- Hydrophilic and antibacterial surface functionalization of polyamide fabric by coating with polylysine biomolecule. Prog. Org. Coat.. 2020;142:105571
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105497.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
The Supplementary material contains the flammability data of silk farbics, the DTG curves of silk farbics, and the flammability data of other fabrics.







