Translate this page into:
Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities
⁎Corresponding author at: Institut National de Recherche et d’Analyse Physico-chimique (INRAP), Sidi Thabet 2020, Tunisia. karim_hosni1972@yahoo.fr (Karim Hosni) karim.hosni@inrap.rnrt.tn (Karim Hosni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
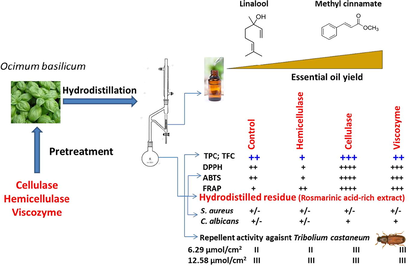
Enzyme pretreatement enhanced extraction of essential oils from basil leaves. Phenylpropanoids are dominants in cellulase and viscozyme-treated leaves. Hydrodistilled residue-by-products is a good source of phenolics. Rosmarinic acid-rich extract had strong antioxidant activity. Hydrodistilled residue-by-products had repellent activity against Tribolium castaneum.
Abstract
In the present study, the effect of enzyme pretreatment on essential oil recoveries from sweet basil (Ocimum basilicum L.) leaves was evaluated. Moreover, the consideration on the use of hydrodistilled residue by-products as a source of bioactive phytochemicals with antioxidant, antimicrobial, and repellent effects against the stored-grain pest Tribolium castaneum was examined. Results showed that the enzymatic pretreatment increased the extraction yield of essential oil by 400, 417, and 478% in hemicellulase-, cellulase-, and viscozyme-treated samples, respectively. Phenylpropanoids including methyl cinnamate, methyl eugenol, eugenol and estragol were found as the main components, and were particularly abundant in cellulase-treated samples. From the hydrodistilled residue of enzyme-treated samples, better recoveries of total phenols (TPC) (258.3–470.9 mg GAE/g extract) and flavonoids (TFC) (59.4–94.3 mg QE/g extract) were observed. Using the DPPH, ABTS, and FRAP assays, a strong antioxidant activity of the rosmarinic-rich extract was observed. Such an activity which was mediated through electron transfer mechanism was highly correlated with the TPC, TFC and rosmarinic acid content. The in vitro bioassay showed that methanol extract (6.29 and 12.58 µL/cm2) had repellent activity against the stored-grain pest Tribolium castaneum. These results suggest the potential of enzyme pretreatment to promote the use of hydrodistilled residue by-products as a valuable source of natural antioxidants and repellents ingredients.
Keywords
Enzyme
Sweet basil
Hydrodistilled residue by-products
Phenylpropanoids
Rosmarinic acid
Bioactivity
1 Introduction
The genus Ocimum (Lamiaceae) encompasses more than 160 species mainly distributed throughout temperate, tropical and subtropical regions of the word (Chowdhury et al., 2017). The market value of Ocimum species is predominately based on their essential oil content and ornamental characters. Among Ocimum species, O. basilicum (basil; sweet basil; common basil) has attracted particular attention due to its culinary, medicinal, cosmetic, pharmaceutical, and food uses (Politeo et al., 2007).
This multipurpose medicinal herb is known for its antioxidant, antimicrobial, anticancer, anti-inflammatory, antinociceptive, antiulcer, analgesic, cardiovascular, immunomodulatory, repellent, and insecticidal activities, among others (Pandey et al., 2014). These biological activities were mainly attributed to their linalool-rich essential oils (Filip et al., 2016) and phenolic compounds (Izadiyan and Hemmateenejad, 2016).
Basil essential oils are traditionally recovered by hydrodistillation, steam distillation and solvent extraction (Lee et al., 2005). Carbon dioxide in its supercritical (Supercritical CO2) state (Filip et al., 2016) and solvent-free microwave extraction (Chenni et al., 2016) have also been used for the extraction of basil essential oil. However, the main disadvantage of these methods is the low extraction yield. Hence, pretreatments (assistant treatments) could be effectively applied to maximize the extraction efficiency of essential oils. Among these pretreatments, enzyme-assisted extraction (EAE) has proved to be effective and highly yielding methodology due to their ability to disrupt cell walls enabling thereby, better release of volatile compounds without modification of the original chemical composition (Boulila et al., 2015).
By using EAE, improved essential oil recovery with concomitant up-graded biological activities was observed in many essential oil bearing plants such as thyme, rosemary (Hosni et al., 2013), laurel (Boulila et al., 2015) and coriander (Abbassi et al., 2018). So far, no detailed study on the effect of cell wall degrading enzyme pretreatment on essential oil yield, composition, and bioactivities of sweet basil has been performed.
Therefore, the aim of this work was to inspect the feasibility of using enzymes to extract essential oils from basil leaves. The tested enzymes include cellulase, hemicellulase, and viscozyme, a multi-enzyme complex containing cellulase, arabanase, β-glucanase, hemicellulase, and xylanase. Phytochemical analysis including the determination of total phenolics content (TPC), total flavonoid content (TFC), profiling of phenolic compounds by UHPLC-DAD of the residual hydrodistilled leaves (by-product of the distillation process) were performed. The antioxidant, antimicrobial and repellent activity against the red flour beetle, Tribolium castaneum of the methanol extracts from the residue by-products were evaluated.
2 Material and methods
2.1 Chemical and reagents
Cellulase (8.9 U/mg), hemicellulase (13.8 U/mg), viscozyme (greater than100 U/g), folin-Ciocalteu reagent, gallic acid, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis-(3-ethylbenzothiozoline-6-sulphonic acid di-ammonium salt) (ABTS), ferric chloride, 2,4,6-tripyridyl-s-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), were purchased from Sigma-Aldrich (Steinheim, Germany). Anhydrous sulphate (Na2SO4) and n-alkanes (C7-C42) were obtained from Fluka Chemicals (Seelze, Germany). Solvents of analytical and HPLC-grade were purchased from Carlo Erba Reactif-CDS (Val de Reuil, France).
2.2 Plant material and enzyme pretreatment
Dried basil leaves were bought from a local market (Souk el Blat, Tunis, Tunisia). For enzyme pretreatment, leaves (50 g) were mixed with 250 mL distilled water containing 5 mg of enzyme (cellulase, hemicellulase, and viscozyme), and stirred for 1 h at 40 °C prior to essential oil extraction (Boulila et al., 2015).
2.3 Extraction and analysis of essential oils
Enzyme-pretreated and control non-treated leaves were subjected to hydrodistillation for 2 h using a Clevenger-type apparatus. The obtained essential oils were recovered, weighed, dried over anhydrous Na2SO4, and stored in amber and airtight vials at −20 °C until analyzed. The solid residue remained after the distillation process (called hydrodistilled residue by-product) was recovered, oven dried at 40 °C, ground to a fine powder, and then stored at −20 °C for further uses (Abbassi et al., 2018).
2.4 Essential oil analysis
For essential oil analysis, a 50 µL aliquot was dissolved in hexane to a final volume of 1.5 mL and then chromatographed using an HP 6890 gas-chromatograph (GC) equipped with flame ionization detector (FID) (Agilent Technologies, Palo Alto, CA, USA). Separation of individual components were performed on an HP-5 capillary column (60 m × 0.25 mm i.d., 0.25 mm film thickness) (Supelco, Bellefonte, PA, USA) using the following conditions: temperature program: isotherm at 50 °C for 1 min, rise to 280 °C at a rate of 2 °C/min, and then kept isotherm for 4 min; nitrogen at a flow rate of 1.2 mL/min was used as carrier gas; injector and FID detector were kept at 250 and 300 °C, respectively.
GC–MS analyses were performed on a gas chromatograph HP 6890 (II) interfaced with an HP 5975 mass selective detector (Agilent Technologies, Palo Alto, CA, USA) and equipped with an HP-5MS capillary column (60 m × 0.25 mm i.d., 0.25 mm film thickness) from the same company. The column temperature was programmed to rise from 40 to 280 °C at a rate of 5 °C/min. The carrier gas helium was used at a flow rate of 2 mL/min. The injector port and ion source were kept at 270 and 230 °C, respectively. Positive electron impacts were recorded at 70 eV in the range m/z 50–550. Volatile compounds of essential oils were identified by comparison of their retention indices (RI) relatives to a series of straight chain n-alkanes (C7–C42) (Adams, 2001), and their mass spectra with those of pure commercial standards when available, as well as those from the Wiley 275. L library.
2.5 Scanning electron microscopy (SEM)
To evaluate morphological alterations, basil leaf surface of untreated control and enzyme-treated samples were subjected to scanning electron microscopy examination. Leaf samples were fixed on adhesive tape, sputtered with gold, and scanned using a SEM type Quanta-200 (FEI Co., Hillsboro, OR, USA).
2.6 Preparation of methanol extracts from the hydrodistilled residue by-products
Powdered hydrodistilled residues (1 g) from untreated control and enzyme-treated leaf samples were mixed with 20 mL methanol, and the suspension was sonicated for 30 min using a Branson 1510 ultrasonic cleaning bath (Branson Ultrasonics Corp., Danbury, CT, USA) operating at 42 kHz frequency and 80 W, and then left at room temperature with gentle stirring (150 rpm) for 48 h. After filtration through Whatman # 1 filter paper (Bärenstain, Germany), supernatants were concentrated in a rotary evaporator under reduced pressure, and the resulting methanol extracts were recovered, weighed, and stored at −20 °C until used.
2.7 Determination of total phenolic content (TPC) and total flavonoid content (TFC)
Total phenolic content (TPC) was determined using the colorimetric Folin-Ciocalteu (FC) method (Singleton and Rossi, 1965). Briefly, a 500 µL aliquot of each sample extract (1 mg/mL) or the standard gallic acid (GA) was mixed with 2.5 mL of 10-fold diluted FC reagent followed by incubation for 4 min at room temperature (22 ± 2 °C). Then, 2 mL of 7.5% NaCO3 was added, followed by incubation for 2 h at room temperature. Finally, the absorbance was measured at 760 nm using a Jasco V-630 UV–visible spectrophotometer (Jasco Corp., Tokyo, Japan). The TPC were expressed as mg GAE/g extract.
For total flavonoid content (TFC), the colorimetric method with AlCl3 was used with modifications (Chang et al., 2002). Briefly, A 500 µL aliquot of each sample extract (1 mg/mL) or the standard quercetin (Q) was mixed with 4 mL distilled water, 600 µL 10% AlCl3, 100 µL of 5% NaNO2, and 2 mL of NaOH (1 M). After 6 min incubation at room temperature, the absorbance was measured at 510 nm and the results were expressed as mg QE/g extract.
2.8 Identification of phenolic compounds by UPLC-DAD
Identification of phenolic compounds was conducted using an UPLC Perkin Elmer series 275 equipped with a series 275 binary micro pump and a diode array detector (DAD) series 275 (Waltham, MA, USA), and a 20 µL injection loop. Separation of individual phenolic compounds was performed on an Hypersil Gold RP C18 (50 mm × 3 mm (i.d); 1.3 particle size) at 30 °C. The mobile phase consisted of solvent A (0.1% acetic acid) and solvent B (0.1% acetic acid in acetonitrile) with a constant flow rate of 0.8 mL/min over 60 min. The linear gradient was used as follows: hold at 92% A, 0–2 min 90% A, 2–26 min 70% A, 26–49 min 10% A, 49–55 min 0% A, 55–60 min 92% A. Both samples and mobile phase were ultrasonically degassed prior to UPLC analysis. Detection wavelengths were set at 265 and 325 nm. Phenolic compounds were identified by comparison of their elution time with those of reference compounds.
2.9 In vitro biological activities
2.9.1 Antioxidant activity
The antioxidant activity of different extracts was determined using 3 complementary in vitro assays: DPPH-free radical scavenging, ABTṠ+ radical cation scavenging, and ferric reducing antioxidant power (FRAP) assay.
For the DPPH-free radical scavenging activity, the method of Binsan et al. (2008) was used. Briefly, 1 mL of sample extract at various concentrations (from 0.01 to 2 mg mL−1) was added to 2 mL of 0.1 mM DPPH methanolic solution. The mixture was Vortexed (Stuart SA8, Thermo Fisher Scientific Inc., Bordeaux, France) vigorously and allowed to stand for 30 min in the dark, and then the absorbance was measured at 517 nm.
The ability to neutralize the radical ABTṠ+ was evaluated using the method of Re et al. (1999). Briefly, the radical ABTṠ+ was prepared by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate (K2S2O8). The mixture was allowed to stand for 12–16 h in the dark at room temperature before use. The concentration of the ABTṠ+ solution was adjusted with methanol to an absorbance of 0.7 at 734 nm. Sample extract (150 µL) was mixed with 1850 µL ABTṠ+ solution. After 15 min of incubation at room temperature, the absorbance of the mixture was measured at 734 nm. In both assays, the results were expressed as EC50 values which represent the effective concentration providing 50% radical DPPH or ABTṠ+ scavenging activity.
The ferric reducing antioxidant power (FRAP) was evaluated as the capacity to reduce Fe3+ to Fe2+ (Benzie and Strain, 1996). Briefly, the working FRAP solution was freshly prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM FeCl3 solution in a ratio of 10:1:1. The working solution was pre-warmed at 37 °C before use. Sample extract (150 µL) was mixed with 2850 µL FRAP solution and the reaction mixture was incubated at 37 °C for 30 min, and then the absorbance was measured at 593 nm. Trolox was used as a positive control and the results were expressed as µmol TE/g extract.
2.9.2 Antimicrobial activity
For the evaluation of the antimicrobial activity of different extract, 5 microorganisms including 2 g-positive strains Staphylococcus aureus ATCC 6538 and Enterococcus faecium ATCC19434, 2 g-negative bacteria Escherichia coli ATCC8739 and Salmonella typhimurium ATCC14028, and the yeast Candida albicans ATCC10231 were used. The antimicrobial activity screening was achieved using the disc diffusion method described by the National Committee for Clinical Laboratory Standards (NCCLS, 1997). The results were expressed by the diameter of the inhibition zone produced around the disks after 24 h and 72 h of incubation for the bacteria and the yeast, respectively.
2.9.3 Repellent activity against red flour beetle Tribolium castaneum (Herbst)
Trilobium castaneum specimens were reared on whole wheat flour at 30 °C, in the dark. Two to three old adults were selected for bioassays. The repellent effect of methanol extracts at two different concentrations (0.5 and 1 mg/mL) was evaluated using the area preference method as described by McDonald et al. (1970). Positive repellent activity was considered when the insect moves to the area free of extract. Briefly, a 9 cm diameter filter paper was equally divided into two areas, and 400 µL of methanol extracts were applied uniformly to half of the filter paper. The other half was treated with methanol and served as negative control. After complete evaporation of solvent, both halves were joined with clear adhesive tape and then placed into Petri dish. Thereafter, twenty adult beetles of T. castaeum were released at the center of each filter paper disc, and then the Petri dish was covered and incubated at 30 °C in the dark. The number of insect present in each area was recorded after 1, 2, 3, and 4 h exposure, respectively. Repellent activity (RA), expressed in terms of percent repellency was calculated according to the formula: where Nc is the number of insect present in the control area, and Nt is the number of insect present in the treated area.
The mean repellency values were calculated and categorized according to the following repellency classes: Class 0 (RA < 0.1%), Class I (0.1 ≤ RA ≤ 20%), Class II (20.1 ≤ RA ≤ 40%), Class III (40.1 ≤ RA ≤ 60%), Class IV (60.1 ≤ RA ≤ 80), and Class V (80.1 ≤ RA ≤ 100%).
3 Statistical analysis
All experiments were performed in triplicate and the results were expressed as mean ± SD. Difference between means were tested using one way analysis of variance (ANOVA) followed by Tukey’s post hoc test at p < 0.05. To determine the relationships between different antioxidant tests and phytochemical parameters, Pearson coefficient correlation were calculated. All analyses were performed using SPSS v. 18 (SPSS Inc., Chicago, IL, USA).
4 Results and discussion
4.1 1. Effect of enzymatic pretreatment on yield and essential oil composition
From basil leaves, pale yellowish oil was obtained with a yield of 0.23% (Fig. 1). Enzyme pretreatment resulted in 4-fold increase in the essential oil content (4, 417, and 480% for hemicellulase, cellulase, and viscozyme, respectively) with respect to the non-treated control samples. In spite of the rather small differences between oil yields, treatment with the viscozyme was virtually the most effective in extracting basil essential oil. These results indicate a cooperative action between the cell wall degrading enzymes contained in the viscozyme preparation which include cellulase, arabanase, β-glucanase, hemicellulase, and xylanase, improving thereby the release of essential oils from their site of biosynthesis represented by glandular trichomes. To confirm such hypothesis, scanning electron micrography (SEM) of the control untreated and enzyme-treated basil leaves was performed (Fig. 2). As shown, enzyme treatment was associated with increased number of deflated peltate glandular trichome. The effect was however more pronounced in the leaves pretreated with the viscozyme (Fig. 2D) and cellulase (Fig. 2B). These morphological changes in glandular trichomes could explain the increase in the essential oil yield in enzyme-treated leaves. The positive effect of enzyme pretreatment on extraction yield has already been described in numerous plant species including garlic (Sowbhagya et al., 2009), celery (Sowbhagya et al., 2010), cumin (Sowbhagya et al., 2011), thyme and rosemary (Hosni et al., 2013), laurel (Boulila et al., 2015), and coriander (Abbassi et al., 2018).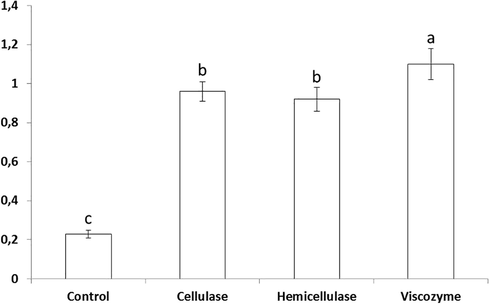
Effect of enzyme pretreatment on essential oils yields in basil leaves. Bars marked with different superscript letters (a-c) are significantly different at p < 0.05.
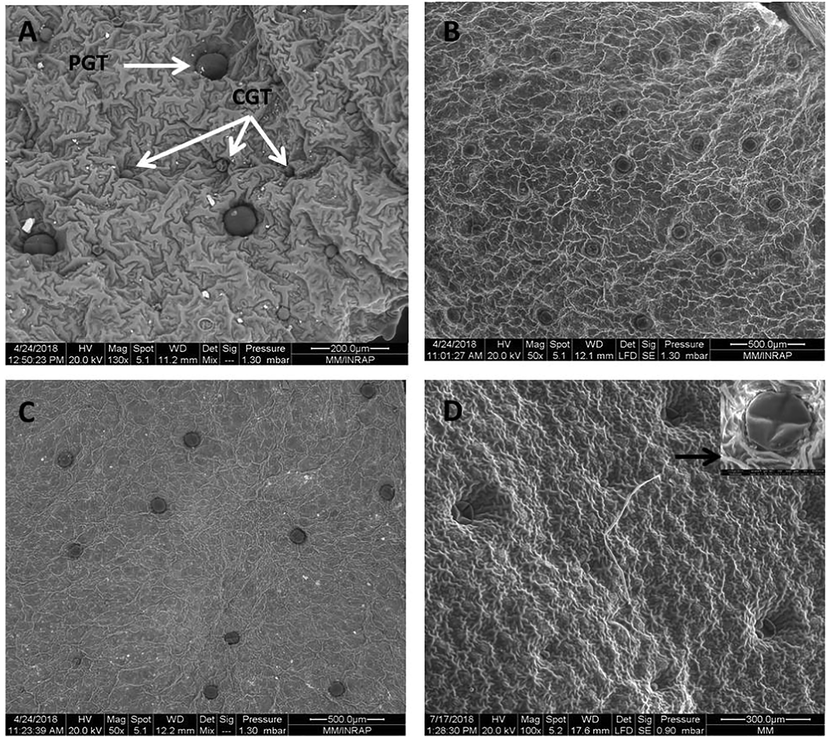
SEM micrographs showing the glandular trichomes of control untreated and enzyme-treated leaves of Ocimum basilicum: A: control; B: Cellulase-treated leaves; C: Hemicellulase-treated leaves; D: Viscoszyme-treated leaves. PGT: Peltate glandular trichome, CGT: Capitate glandular trichome, Black arrow: deflated PGT.
4.2 Chemical composition of essential oils
As shown in Table 1, the chromatographic profiles of the essential oils were resolved into 9 compounds, representing more than 99% of total areas. Identified components include 4 phenylpropanoids, 2 oxygenated monoterpenes, 2 sesquiterpene hydrocarbons and 1 monoterpene hydrocarbon. Phenylpropanoids with methyl cinnamate (42.05–63.23%), methyl eugenol (4.09–11.48%), eugenol (2.64–8.04%), and estragol (2.48–4.71%) were found as the most plentiful components. Although that enzyme pretreatment did not induce perceptible chemical changes in the oil composition, the highest amounts of phenylpropanoids coincided with the treatment with cellulase and viscozyme. At this point, it seems that the release of these phenylpropanoids was associated with the increased number of deflated peltate glandular trichomes. These results confirm earlier molecular studies showing that phenylpropanoids were specifically biosynthesized and stored in peltate glandular trichomes of basil leaves (Kapteyn et al., 2007; Deschamps and Simon, 2010). Because of their economical relevance as natural feedstock for the flavor, fragrance, cosmetic, pharmaceutic, food, and nutraceutical industries, the recovery of phenylpropanoids by using cellulase-based enzyme preparations is encouraged. In contrast to phenylpropanoids, the release of the oxygenated monoterpene linalool was hampered by enzyme pretreatment. This suggests that linalool is a glucosidically bound volatile, and that the enzymes used herein were inefficient in extracting the linalool aglycone. Linalool is considered common in the aglycone fraction of Lamiaceae (Politeo et al., 2007), and its limited liberation by using cellulase- or hemicellulase-based enzyme preparations has already been described in thyme, rosemary (Hosni et al., 2013), laurel (Boulila et al., 2015), and coriander (Abbassi et al., 2018). However, in the aforementioned studies, a remarkable increase in the content of oxygenated monoterpene has been observed. Such increase was likely attributed to increased oxidation rate following cell disruption (Charoensiddhi and Anprung, 2010). Another possible explanation for these results is that the commercial enzyme preparations contained oxidases that catalyze the oxidation of volatile compounds.
Compound
RI*
Control
Cellulase
Hemicellulase
Viscosyme
β-Pinene
980
0.32 ± 0.03a
0.18 ± 0.02b
1.8-Cineole
1031
2.72 ± 0.23b
2.76 ± 0.12b
4.07 ± 0.36a
2.68 ± 0.34b
Linalool
1101
37.91 ± 2.24a
15.07 ± 1.82c
26.18 ± 1.86b
16.85 ± 1.44c
Estragol
1195
2.48 ± 0.34c
3.96 ± 0.31b
4.71 ± 053a
4 ± 0.36b
Eugenol
1356
2.64 ± 0.24c
5.06 ± 0.67b
8.04 ± 0.92a
5.39 ± 0.45b
Methyl cinnamate
1379
42.7 ± 2.44b
62.73 ± 3.23a
42.05 ± 3.86b
63.23 ± 2.49a
Methyl eugenol
1407
6.95 ± 0.71b
5.84 ± 0.43c
11.48 ± 1.81a
4.09 ± 0.35d
α-Bergamotene
1436
3.18 ± 0.41a
2.36 ± 0.28b
1.57 ± 0.14c
2.28 ± 0.34b
Germacrene-D
1485
1.34 ± 0.05b
1.37 ± 0.14b
1.78 ± 0.12a
1.25 ± 0.07b
Group components
Monoterpene hydrocarbons
–
0.32 ± 0.04a
–
0.18 ± 0.02b
Oxygenated monoterpenes
40.63 ± 3.48a
17.83 ± 1.67c
30.25 ± 3.28b
19.53 ± 2.07c
Phenylpropanoids
54.77 ± 2.69c
77.59 ± 5.44a
66.28 ± 4.35b
76.71 ± 5.19a
Sesquiterpene hydrocarbons
4.52 ± 0.39a
3.73 ± 0.22b
3.35 ± 0.42b
3.53 ± 0.23b
Total identified
99.92
99.47
99.88
99.95
In contrast, the application of cellulase and viscozyme to cumin seeds increased the content of hydrocarbons in the resulting essential oils (Sowbhagya et al., 2011). One year later, Chandran et al (2012) showed that the pretreatment of black pepper with lumicellulase increased significantly the content of hydrocarbons, while it decreased the content of oxygenated compounds. In the present study, the effect of enzyme pretreatment on hydrocarbons namely represented by the sesquiterpene α-bergamotene and germacrene-D was less evident. These results indicate that the effectiveness of enzyme-assisted extraction procedure in extracting hydrocarbons or oxygenated compounds is somewhat dependent on plant species, enzyme used, and duration of treatment (Boulila et al., 2015).
In general, enzyme pretreatment increased the recovery of basil essential oils without affecting its chemical attributes. It also enhanced the content of some components especially phenylpropanoids, to which numerous biological activities including antioxidant, antimicrobial, anti-inflammatory, anticancer, cardiovascular, and insecticidal activities are attributed (Pandey et al., 2014). In order to maximize the benefits of enzymatic approach, and looking for the integral exploitation of the matrix through the recovery of the remaining bioactive phytochemicals namely phenolics, it will be of interest to determine these phytochemicals (TPC, TFC and UPLC profiling) in the hydrodistilled residue by-products, and evaluate their antioxidant, antimicrobial and insecticidal activity.
4.3 Phytochemical analysis of hydrodistilled residue by-products
4.3.1 Yield of extraction, TPC and TFC
Data presented in Table 2 showed that the yield of extraction ranged between 6.1 in non-treated control samples to 10.4% in cellulase-treated ones, suggesting that the hydrodistilled residue raw materials could represent a potential source of bioactive compounds. The highest yield was obtained in enzyme-treated samples, while the lowest was found in non-treated control samples. These results could be attributed to the joint extraction of compounds other than phenolics such as carbohydrates and proteins contributing thereby to increased extraction yield. Similar results have recently been reported in coriander seeds (Abbassi et al., 2018). *Values are means ± SD (n = 3). Different superscripts (a-d) within columns are significantly different at p < 0.05.
Extract yield (%)
TPC(mg GAE/g extract)
TFC(mg QE/g extract)
DPPH(IC50 µg/mL)
ABTS(IC50 µg/mL)
FRAP(µM TE/g extract)
Control
6.1 ± 0.18a
372.9 ± 23.44b
27.1 ± 3.12b
80.05 ± 6.42b
512.37 ± 24.22b
2.61 ± 0.08b
Cellulase
10.4 ± 0.69a
470.9 ± 28.16a
42.3 ± 2.89a
59.36 ± 4.67d
379 ± 16.81d
2.98 ± 0.18a
Hemicellulase
9.1 ± 0.58b
258.3 ± 16.79c
18.3 ± 1.28c
94.26 ± 3.36a
615.75 ± 31.64a
2.11 ± 0.06c
Viscozyme
9.2 ± 0.32b
342.9 ± 24.37b
26.9 ± 2.32b
68.95 ± 4.24c
447.17 ± 26.82c
2.62 ± 0.24b
The total phenol content (TPC) ranged from 258.3 to 470.9 mg GAE/g extract in hemicellulase- and cellulase-treated samples, respectively (Table 2). The total flavonoid content (TFC) showed similar trend to that of TPC with the highest value (42.3 mg QE/g extract) being observed in cellulase-treated samples, while the lowest (18.3 mg QE/g extract) were found in hemicellulase-treated samples. These results are on a par with our earlier observation on coriander seeds (Abbassi et al., 2018). The increased amount of TPC and TFC in enzyme-treated samples was likely due to the enhanced degradation of cell-wall structures, especially glycosidic/bound linkage between phenolic compounds and cell wall polysaccharides. A fact which was intensified by the increased temperature during hydrodistillation and subsequently the pressure, and the acidity of the bulk medium, leading ultimately to enhanced destructuration of cell wall components, which leads in turn to a better release of phenolic components (Barba et al., 2016; Ennigrou et al., 2017). When compared with literature, our TPC values are markedly higher than those reported (15.12–27 mg GAE/g DW) for different basil cultivars (Flanigan and Niemeyer, 2014), species (7.15–107.43 mg GAE/g DW) (Moghaddam and Mehdizadeh, 2015), or different solvent (15–150 mg GAE/g FW) extracts (Złotek et al., 2016). The TFC content reported herein was however slightly higher than that recorded in different Iranian Ocimum species and accessions (Moghaddam and Mehdizadeh, 2015). In general, discrepancies between results are likely attributed to differences in species/cultivars, agronomic practices, harvest and storage conditions, extraction procedures, and analytical methods.
4.3.2 Identification of phenolic compounds of the hydrodistilled residual extracts by UHPLC-DAD
The identification of phenolic compounds using UHPLC-DAD was based on comparison of their retention times with those of standard compounds, and their order of elution through RP-C18 column (Fig. S1). A total of 9 compounds including 5 phenolic acids, 3 flavonol-glycosides, and 1 flavan-3-ol were positively identified (Table 3). The main phenolic acids were rosmarinic (4.92–26.88%), syringic (0.87–15.89%), vanillic (4.17–21.49%), p-coumaric (1.12–16.18%), and caffeic (3.24–10.09%) acids. The main flavonol-glycosides were identified as quercetin-3-O-galactoside (0.17–15.89%), quercetin-3-O-glucoside (0.13–4.21%), and kaempferol-3-O-glucoside (0.42–5.3%). Catechin was found as the only flavan-3-ol, and was particularly abundant (4.36%) in non-treated samples. In general, cellulase was more efficient in extracting rosmarinic acid, whereas hemicellulase was efficient towards the extraction of vanillic acid. The recovery of syringic and caffeic acids was better with viscozyme. *Values are means ± SD (n = 3). Different superscripts (a-d) within lines are significantly different at p < 0.05. Q-3-O-galactoside: Quercetin-3-O-galactoside; Q-3-O-glucoside: Quercetin-3-O-glucoside; K-3-O-glucoside: Kaempferol-3-O-glucoside. -: not detected.
Phenolic classes
Compounds
Control
Cellulase
Hemicellulase
Viscoszyme
Phenolic acids
Rosmarinic acid
20.22 ± 0.62b
26.88 ± 1.87a
4.92 ± 0.22d
16.86 ± 1.64c
Syringic acid
9.13 ± 0.34b
9.44 ± 0.61b
0.87 ± 0.06c
15.89 ± 1.18a
Vanillic acid
5.61 ± 0.44c
21.49 ± 0.88a
4.17 ± 0.42d
7.52 ± 0.54b
p-Coumaric acid
16.18 ± 1.12a
9.41 ± 0.73b
1.12 ± 0.14d
6.8 ± 0.39c
Caffeic acid
5.11 ± 0.29c
8.32 ± 0.53b
3.24 ± 0.09d
10.09 ± 0.82a
Flavonols
Q-3-O-galactoside
7.17 ± 0.42b
0.17 ± 0.02d
0.45 ± 0.02c
15.89 ± 1.32a
Q-3-O-glucoside
–
0.13 ± 0.01b
4.21 ± 0.05a
–
K-3-O-glucoside
0.55 ± 0.05c
2.64 ± 0.12b
0.42 ± 0.02d
5.3 ± 0.04a
Flavan-3-ols
Catechin
4.36 ± 0.28a
2.48 ± 0.16c
0.38 ± 0.04d
3.72 ± 0.12b
As the biological activities of an extract is dependent on the TPC, TFC and individual phenolic compounds, the antioxidant, antimicrobial and insecticidal activities of different extracts were evaluated.
4.4 Antioxidant activity
The antioxidant activity of basil methanol extracts was evaluated through three complementary in vitro assays: DPPH, ABTS and FRAP.
Overall, all extracts showed strong free radical scavenging activity against DPPH and ABTS radicals. Methanol extract from cellulase-treated samples was found as the most effective radical scavengers having IC50 values of 59.36 and 379 µg/mL in DPPH and ABTS assays, respectively. Similar trend was observed in the FRAP assay (Table 2). The strong antioxidant activity was in turn associated with high TPC and TFC contents, suggesting that phenolic compounds are the main contributors to the antioxidant activity. On the other hand, it can be proposed that TPC and TFC represent reliable indicators of the antioxidant activity in basil hydrodistilled residual by-products. Confirmation of such assumption was provided by analysis of linear correlations. Results of Pearson’s correlation (Table 4) revealed that the antioxidant activity was highly correlated with TPC (R2 = 0.79–0.95), and TFC (R2 = 0.83–0.90). High correlation DPPH vis ABTS (R2 = 0.998), DPPH vis FRAP (R2 = 0.915), and ABTS vis FRAP (0.935) were also found. These observations suggest that not only the same components are involved in the antioxidant activity, but also, the antioxidant activity was mediated through electron transfer mechanism. To identify the putative phenolic compounds that could be implied in the antioxidant activity, additional analyses were performed (Table 4). They showed high correlation (R2 = 0.801–0.971) between rosmarinic acid and all antioxidant assays suggesting that the radical scavenging activity and the ferric reducing power of basil hydrodistilled residue by-products was mainly due to rosmarinic acid. Caffeic acid was associated with DPPH (R2 = 0.750) and ABTS (R2 = 0.727) radical scavenging activity, while quercetin-3-O-glucoside was related to the FRAP assay (R2 = 0.743). The antioxidant activity of the remaining components was less evident (Table 4). To the best of our knowledge, the antioxidant activity of basil hydrodistilled residue by-products has not been described yet. However, our results are congruent with those of Nguyen et al. (2010) and Flanigan and Niemeyer (2014) who showed a strong positive correlation between TPC, DPPH, and FRAP. Regarding individual compounds, strong antioxidant activities of rosmarinic acid and its metabolite caffeic acid have been described in different in vitro and in vivo systems (Fadel et al., 2011; Adomako-Bonsu et al., 2017; Alagawany et al., 2017). Correlation statistically significant at *) p < 0.05 ; **) p < 0.01 ; ***) p < 0.001. R.A: Rosmarinic acid; S.A; Syringic acid; V.A: vanillic acid; p-C.A: p-Coumaric acid; C.A: Caffeic acid; Q-3-O-galactoside: Quercetin-3-O-galactoside; Q-3-O-glucoside: Quercetin-3-O-glucoside; K-3-O-glucoside: Kaempferol-3-O-glucoside.
TPC
TFC
DPPH
ABTS
FRAP
TPC
0.981***
0.7973*
0.8236*
0.953**
TFC
0.981***
0.8323*
0.8466*
0.9093**
DPPH
0.7973*
0.8323*
0.9983***
0.915***
ABTS
0.8236*
0.8466*
0.9983***
0.9359**
FRAP
0.953**
0.9093**
0.915***
0.9359***
R.A
0.978**
0.926*
0.801**
0.8324**
0.9713***
S.A
0.469
0.423
0.541
0.537
0.4197
V.A
0.955**
0.883
0.689
0.688
0.689
p-C.A
0.596
0.427
0.144
0.1728
0.3555
C.A
0.589
0.577
0.7505*
0.7265*
0.5086
Q-3-O-gal
0.121
0.186
0.004
0.04
0.008
Q-3-O-glu
0.764
0.67
0.659
0.684
0.743
K-3-O-glu
0.258
0.315
0.4401
0.409
0.1964
Catechin
0.467
0.0317
0.2455
0.2667
0.3379
In general, the potent antioxidant activity of hydrodistilled residue by-products paves the way for developing new bio-based antioxidants from residual raw materials, giving thereby a value-added to vegetable wastes.
4.5 Antimicrobial activity
The antimicrobial activity of different extracts of O. basilicum was qualitatively evaluated using disc diffusion method (Table 5). The results showed that all extracts were ineffective against E. coli, S. typhimurium, and E. faecium, whereas they exhibited low activity against the Gram+ S. aureus (30% inhibition compared to the control ampicillin) and the yeast C. albicans (48.6% inhibition compared to the control nystatin). These results were expected because of the abundance of phenolic acids, recognized for their low antimicrobial activity (Daglia 2012). When compared with the few studies concerned with the antimicrobial activity of basil extracts, our results are consistent with those of Adigüzel et al. (2005) who found that methanol extracts of Turkish O. basilicum had a very low antimicrobial activity being effective against 6% of the 146 tested microorganisms. Later, slight to moderate antifungal activity (10–65% inhibition) has been reported in methanol extract from Pakistani O. basilicum specimen (Ahmad et al., 2016). These results seem to be confirmed for the hydrodistilled residue by-products. *Values are means ± SD (n = 3). NA: Not active.
Microbial strain
Diameter of inhibition zone (mm)
Control
Cellulase
Hemicellulase
Viscozyme
Ampicilline
Nystatine
E. coli
NA
NA
NA
NA
11.75 ± 0.3
–
S. typhimurium
NA
NA
NA
NA
13.75 ± 1
–
S. aureus
9.5 ± 0.7
9.5 ± 0.5
10 ± 0.7
10.75 ± 0.3
35.5 ± 0.7
–
E. faecium
NA
NA
NA
NA
37.5 ± 1
–
C. albicans
15.5 ± 0.7
14 ± 1.4
17.5 ± 0.7
17.25 ± 0.3
–
36 ± 0.3
4.6 Repellent activity
Results of repellency assay of methanol extracts from basil hydrodistilled residue by-products are given in Table 6. All extract exhibited low (Class II) to moderate repellent (Class III) activity with an average mean repellency ranged from 33.33 to 58.33% for the hemicellulase-treated samples and non-treated control samples, respectively. The highest protective efficacy (86.66%) was observed after 4 h exposure for extract derived from cellulase-treated (12.58 µL/cm2). The same extract at a concentration of 6.29 µL/cm2 has the ability to repel 80% of T. castaneum. In general, the repellent activity was found to be concentration-dependent for all extracts. Despite that the insect repellency of Ocimum secondary metabolites (essential oils and phenolic compounds) have been exhaustively studied, information about the bioactivities of hydrodistilled residue by-products are not available. Nevertheless, similar results on repellent activity of Ocimum extracts against Aedes egypti (Kiplang’at and Mwangi, 2013), Culex pipiens (Hassan et al., 2015), Spodoptera litura (Summarwar and Pandey, 2015), Sitophilis zeamais (Ouko et al., 2017), and Anopheles gambiae (Afolabi et al., 2018) have been reported. The repellency of O. basilicum leaf powder against the grain pest T. castaneum has also been proved by using the stimuli-enriched bioassay (Utono and Gibson, 2015). Regarding individual compounds, it seems logical to suppose that the high repellent activity of the extract derived from cellulase-treated samples might be associated with its high amount of rosmarinic acid, to which the insecticidal activity is attributed (Khan et al., 2019). *Values are means ± SD (n = 3). Superscripts uppercase letters (A-D) are significantly different at p < 0.05 for a dose of 6.29 µL/cm2; Superscripts lowercase letters (a-d) are significantly different at p < 0.05 for a dose of 12.58 µL/cm2.
Samples
ConcentrationµL/cm2
60 min
120 min
180 min
240 min
Mean repellency (%)
Repellency class
Control
6.29
13.66 ± 1.22D
46.66 ± 4.87B
33.33 ± 2.64B
66.66 ± 7.12B
40.08 ± 3.88B
II
12.58
26.66 ± 2.44c
80 ± 6.34a
46.66 ± 3.47c
80 ± 8.28a
58.33 ± 4.69a
III
Cellulase
6.29
20 ± 2C
46.66 ± 5.22B
20 ± 1.84C
80 ± 6.71A
41.67 ± 4.44B
III
12.58
46.66 ± 3.21a
26.66 ± 1.88c
33.33 ± 3.48d
86.66 ± 9.18a
48.33 ± 5.07b
III
Hemicellulase
6.29 ± 0.44
60 ± 5.34A
20 ± 1.62C
13.33 ± 1.27D
40 ± 4.82C
33.33 ± 3.22C
II
12.58 ± 1.78
46.66 ± 4.14a
66.66 ± 6.43b
60 ± 6.22a
53.33 ± 5.67b
56.66 ± 6.34a
III
Viscosyme
6.29 ± 0.78
40 ± 4.28B
53.33 ± 4.12A
73.33 ± 8.34A
26.66 ± 2.39D
48.33 ± 3.34A
III
12.58 ± 1.46
33.33 ± 3.18b
6.66 ± 0.72d
53.33 ± 5.19b
80 ± 7.67a
43.33 ± 4.76b
III
In general, results of the present study suggest that the extracts from the hydrodistilled residue by-products of O. basilicum leaves have the potential to be used as a promising alternative for the control of T. castaneum and/or the development of new bio-based products with repellent activity against stored-grain pests.
5 Conclusions
Results of this study proved that enzyme pretreatment could be used as an efficient procedure to improve the recovery of essential oils without affecting their original chemical composition. It also enhanced the release of bioactive phytochemicals from O. basilicum leaves and their hydrodistilled residue by-products, respectively. Viscozyme was more effective in extracting essential oils, whereas cellulase was better in extracting phenolic compounds from the hydrodistilled residue-by products, exhibiting thereby the strongest antioxidant activity as revealed by the DPPH, ABTS and FRAP assays. From practical stand point, these results are of particular interest since they combined the integrated waste and pest management approaches through the use of hydrodistilled residue by-products as an unusual residual matrix for the extraction of bioactive ingredients with good repellent activity against stored-grain pests.
Acknowledgments
We gratefully acknowledge the financial support of the Tunisian Ministry of Higher Education and Scientific Research (Grant N°: LR19INRAP02).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Enzyme-aided release of bioactive compounds from coriander (Coriandrum sativum L.) and their residue by-products and evaluation of their antioxidant activity. J. Food Sci. Technol.. 2018;55:3065-3076.
- [Google Scholar]
- Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Streampp: Allured Publishing Corporation; 2001. p. :455.
- Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turkish J. Biol.. 2005;29:155-160.
- [Google Scholar]
- Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: importance of physico-chemical characteristics. Toxicol. In Vitro. 2017;40:248-255.
- [Google Scholar]
- Adulticidal and repellent activities of some botanical oils against malaria mosquito: Anopheles gambiae (Diptera: Culicidae) Beni-Suef Univ. J. Basic Appl. Sci.. 2018;7:135-138.
- [Google Scholar]
- Antifungal, phytotoxic and hemagglutination activity of methanolic extracts of Ocimum basilicum. J. Tradit. Chin. Med.. 2016;36(6):794-798.
- [Google Scholar]
- Rosmarinic acid: modes of action, medicinal values and health benefits. Animal Health Res. Rev.. 2017;18(2):167-176.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem.. 2008;106:185-193.
- [Google Scholar]
- Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci. Technol.. 2016;49:96-109.
- [Google Scholar]
- Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.) Ind. Crops Prod.. 2015;74:485-493.
- [Google Scholar]
- Effect of enzyme-assisted extraction on quality and yield of volatile oil from black pepper and cardamom. Food Sci. Biotechnol.. 2012;21:1611-1617.
- [Google Scholar]
- Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal.. 2002;10:178-182.
- [Google Scholar]
- Characterization of bael fruit (Aegle marmelos (L.) Correa) hydrolysate as affected by enzyme treatment. J. Food Biochem.. 2010;34:1249-1267.
- [Google Scholar]
- Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules. 2016;21:113.
- [CrossRef] [Google Scholar]
- Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC-MS) analysis. J. Genet. Eng. Biotechnol.. 2017;15:275-285.
- [Google Scholar]
- Phenylpropanoid biosynthesis in leaves and glandular trichomes of basil (Ocimum basilicum L.). Methods Mol Biol.. 2010;643:263-273.
- [CrossRef] [Google Scholar]
- Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. S. Afr. J. Bot.. 2017;108:407-415.
- [Google Scholar]
- The phenolic compounds of Ceratonia siliqua pulps and seeds. J. Mater. Environ. Sci.. 2011;2(3):285-292.
- [Google Scholar]
- Chemical composition and antioxidant properties of Ocimum basilicum L. extracts obtained by supercritical carbon dioxide extraction: Drug exhausting method. J. Supercrit. Fluids. 2016;109:20-25.
- [Google Scholar]
- Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.) Food Chem.. 2014;164:518-526.
- [Google Scholar]
- Repellent effect of Ocimum basilicum and glycyrrhiza glabra extracts against the mosquito vector, culex pipiens (diptera: culicidae) J. Egypt. Soc. Parasitol.. 2015;45(2):239-246.
- [Google Scholar]
- Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): impact on yield chemical composition and antimicrobial activity. Ind. Crops Prod.. 2013;47:291-299.
- [Google Scholar]
- Multi-response optimization of factors affecting ultrasonic assisted extraction from Iranian basil using central composite design. Food Chem.. 2016;190:864-870.
- [Google Scholar]
- Khan, S., Taning, C.N.T., Bonneure, E., Mangelinckx, S., Smagghe, G., Ahmad, R., Fatima, N., Asif, M., Shah, M.M., 2019. Bioactivity-guided isolation of rosmarinic acid as the principle bioactive compounds from the butanol extract of isodon rugosus against the pea aphid, Acyrthosiphon pisum. PlosOne, doi: 10.1371/journal.prone.0215048.
- Evolution if cinnamate/p-coumarate carboxyl methyltransferase and their role in the biosynthesis of methyl cinnamate. Plant Cell. 2007;19:3212-3229.
- [CrossRef] [Google Scholar]
- Repellent activities of Ocimum basilicum, Azadirachta indica and Eucalyptus citriodora extracts on rabbit skin against Aedes aegypti. J. Entomology Zoology Stud.. 2013;1(5):84-91.
- [Google Scholar]
- Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem.. 2005;91:131-137.
- [Google Scholar]
- McDonald, L.L., Guy, R.H., Speirs, R.D., 1970. Preliminary evaluation of new candidate materials as toxicants, repellents and attractants against stored-product insects. Agricultural Research Service, US Department of Agriculture, Washington DC.: Marketing Research Report No. 882.
- Variability of total phenolic, flavonoid and rosmarinic acid content among Iranian basil accessions. LWT – Food Sci. Technol.. 2015;63:535-540.
- [Google Scholar]
- Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chem.. 2010;123:1235-1241.
- [Google Scholar]
- Bioefficacy of organic extracts of Ocimum basilicum against Sitophilus zea mais. Entomology, Ornithology & Herpetology. 2017;6:1.
- [CrossRef] [Google Scholar]
- Chemistry and bioactivities of essential oils of some Ocimum species: A, overview. Asian Pacific J. Tropical Biomed.. 2014;4(9):682-694.
- [Google Scholar]
- Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem.. 2007;101:379-385.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Antifeedant activity of leaf extracts of catharanthus roseus and ocimum sanctum against fourth instar larvae of spodoptera litura. Int. J. Pure Appl. Zoology. 2015;3(3):259-262.
- [Google Scholar]
- Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem.. 2009;113:1234-1238.
- [Google Scholar]
- Effect of enzymes on extraction of volatiles from celery seeds. Food Chem.. 2010;120:230-234.
- [Google Scholar]
- Enzyme-assisted extraction of volatiles from cumin (Cuminum cyminum L.) seeds. Food Chem.. 2011;127:1856-1861.
- [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enology Viticulture. 1965;16:144-158.
- [Google Scholar]
- New ‘stimuli-enriched’ laboratory bioassay used to identify improved botanical repellent treatment, lem-ocimum, to control the stored-grain pest Tribolium castaneum. J. Stored Prod. Res.. 2015;64:27-35.
- [CrossRef] [Google Scholar]
- The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci.. 2016;23:628-633.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.06.003.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







