Translate this page into:
Erysacleuxins C and D, new isoflavones from the twigs of Erythrina sacleuxii Hua and their cytotoxic activity
⁎Corresponding author. jeffombito@gmail.com (Japheth O. Ombito)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Two previously undescribed isoflavones, erysacleuxin C (1) and erysacleuxin D (2), together with seven known compounds (3–9), were isolated and identified from the EtOAc extract of the twigs of Erythrina sacleuxii Hua (Leguminosae). The structures of the isolated compounds were determined on the basis of their spectroscopic and spectrometric data. Evaluation of their cytotoxicity against the human cancer HeLa-S3 cell lines indicated IC50 values of 130.4, 54.9 and 73.9 µM for erysacleuxin C (1), erysacleuxin D (2) and butin (9), respectively.
Keywords
Erythrina sacleuxii
Leguminosae
Isoflavone
Erysacleuxin C
Erysacleuxin D
Cytotoxicity
1 Introduction
The genus Erythrina (Leguminosae) comprises more than 110 species of red or orange flowered trees and shrubs, distributed throughout the tropical and subtropical regions of Africa, America and Asia (Kone et al., 2011). Plants from the genus Erythrina are used in traditional medicine for the treatment of asthma, cancer, insomnia, inflammations, malaria fever, microbial infections, and toothache (Mitscher et al., 1987; Kokwaro, 1993). Erythrina sacleuxii Hua is a red-flowered 9–24 m tall tree endemic to Kenya and Tanzania (Gillett et al., 1971). Previous studies on the roots and stem barks of Kenyan specimens of E. sacleuxii yielded flavones, isoflavones, isoflavanones, pterocarpans, and isoflav-3-enes (Yenesew et al., 1998a, 1998b; Yenesew et al., 2000; Andayi et al., 2006; Ombito et al., 2018). Herein, the isolation and structural elucidation of two new isoflavones, together with seven known compounds from the twigs of the Kenyan specimens of E. sacleuxii is reported. The compounds were assayed for cytotoxicity against human cancer HeLa-S3 cell lines.
2 Results and discussion
The powder of air-dried twigs of E. sacleuxii was extracted with CH2Cl2-CH3OH (1:1) at room temperature. The crude extract was then suspended in water and successively extracted with CH2Cl2 and EtOAc. Chromatographic separation and purification of the EtOAc extract led to the isolation of erysacleuxin C (1), erysacleuxin D (2), vanillin (3) (Harish et al., 2005), 26-hydroxyhexacosyl-(E)-ferulate (4) (Ali et al., 2010), genistein (5), liquiritigenin (6) (Jahromi et al., 1993), 5′-formylpratensein (7) (Yenesew et al., 1998a), calycosin (8) (Markham et al., 1968) and butin (9) (Roux and Paulus, 1961) (Fig. 1). The chemical structures of the known compounds were established by comparison of their spectroscopic and spectrometric data with the literature data.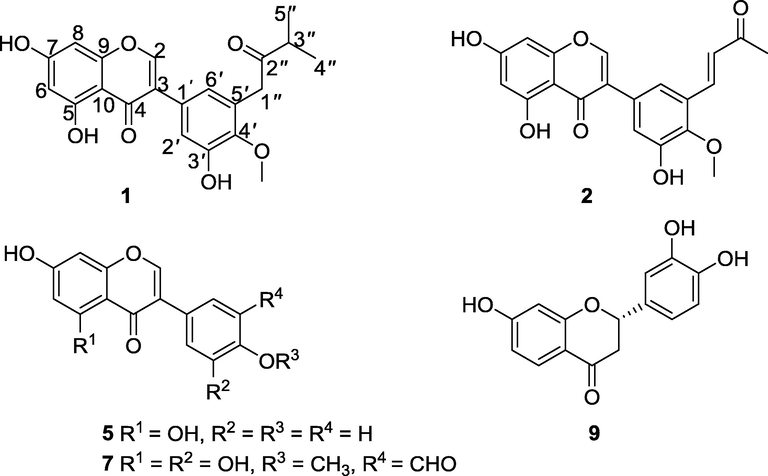
Chemical structures of the isolated compounds.
Compound 1 was obtained as a brown solid and the molecular formula C21H20O7 was assigned based on the ESI-HRMS ([M+Na]+ m/z 407.1109, calcd. 407.1101) and NMR analyses (Table 1). The UV spectrum of compound 1 in acetonitrile displayed two distinct absorption maxima, one at λmax 262 nm for band II and one at 290 nm for band I, typical for an isoflavone skeleton (Mabry et al., 1970) while its IR spectrum showed absorption bands for chelated hydroxyl (3326 cm−1) and conjugated carbonyl (1651 cm−1). It is worth mentioning that although the IR spectrum of compound 1 should have displayed an extra strong absorption band in the carbonyl region for the unconjugated ketone function (>1700 cm−1), the only band observed was for the conjugated carbonyl (1651 cm−1). This is not the first time that this kind of anomaly has been reported (Yu et al., 2012). The possible reason for this anomaly is not known to us. Its NMR spectra were in agreement with an isoflavone backbone with a singlet signal at δH 8.18 typical for H-2 proton of isoflavones (Harborne et al., 1975). The 5,7-dioxygenation pattern of ring-A suggested by the meta-coupling (J = 2.1 Hz) of protons H-6 (δH 6.28) and H-8 (δH 6.42) is in line with the biosynthetically viable substitution pattern of ring-A (Nkengfack et al., 1989; Dewick, 2002). This substitution pattern was further supported by the HMBC cross-peaks (Table 1) of 5-OH (δH 13.03) with C-5 (δC 163.9), C-6 (δC 99.9) and C-10 (δC 106.2), H-6 with C-5 (δC 163.9), C-8 (δC 94.6) and C-10 (δC 106.2), and those of H-8 with C-6 (δC 99.9), C-7 (δC 166.1), C-9 (δC 159.0) and C-10 (δC 106.2). The substitution pattern of ring B was revealed by meta-coupled (J = 2.1 Hz) H-2′ (δH 7.11) and H-6′ (δH 6.87) protons and the HMBC cross-peaks of H-2′ with C-3 (δC 123.7), C-3′ (δC 150.5), C-4′ (δC 147.1) and C-6′ (δC 123.6), and those of H-6′ with C-3 (δC 123.7), C-1′ (δC 127.6), C-2′ (δC 117.6) and C-4′ (δC 147.1). The C-4′ position of the methoxy substituent (δH 3.77, δC 60.4) was suggested the HMBC cross-peaks of 4′-OCH3 (δH 3.77) with C-4′ (δC 147.1). Additionally, the 1H NMR spectrum of compound 1 displayed a singlet signal at δH 3.83 (2H) for methylene protons (H-1″), a septet at δH 2.82 (1H) for a methine proton (H-3″) and a doublet at δH 1.11 (6H, d, H-4″ & H-5″) in the non-aromatic region, which were deduced to constitute a 3-methyl-2-oxobutyl unit. The attachment of this oxoprenyl unit at C-5′ was suggested by the strong long-range HMBC correlations between H-1″ and C-1′ (δC 127.6), C-4′ (δC 147.1), C-5′ (δC 130.4) and C-6′ (δC 123.6). This new compound (1), erysacleuxin C, was, therefore, characterized as 5,7,3′-trihydroxy-4′-methoxy-5′-(3-methyl-2-oxobutyl)isoflavone.
Position
1
2
δH, mult. (J in Hz)
δC
HMBC
δH, mult. (J in Hz)
δC
HMBC
2
8.18 s
154.9
C-3, C-4, C-9
8.32 s
155.3
C-3, C-4, C-9
3
123.7
122.8
4
181.4
181.1
5
163.9
163.6
6
6.28 d (1.9)
99.9
C-5, C-8, C-10
6.30 d (2.1)
100.1
C-5, C-8
7
166.1
163.9
8
6.42 d (1.9)
94.6
C-6, C-7, C-9, C-10
6.44 d (2.1)
94.7
C-6, C-7, C-9
9
159.0
159.1
10
106.2
105.9
1′
127.6
128.7
2′
7.11 d (2.1)
117.6
C-3, C-3′, C-4′, C-6′
7.31 d (2.0)
120.3
C-3, C-3′, C-4′, C-6′
3′
150.5
151.0
4′
147.1
147.9
5′
130.4
118.9
6′
6.87 d (2.1)
123.6
C-3, C-1′, C-2′, C-4′
7.44 d (2.0)
119.6
C-3, C-1′, C-2′, C-4′
1″
3.83 s
42.9
C-1′, C-4′, C-5′, C-6′
7.84 d (16.5)
137.8
C-4′, C-5′, C-2″, C-3″
2″
211.4
6.86 d (16.5)
129.3
3″
2.82 sept (7.0)
40.7
198.1
4″
1.11 d (6.9)
18.7
2.35 s
27.7
C-3″
5″
1.11 d (6.9)
18.7
4′-OMe
3.77 s
60.4
C-4′
3.89 s
61.8
C-4′
5-OH
13.03 s
C-5, C-6, C-10
12.94 s
C-5, C-6, C-10
Compound 2 was isolated as a white powder. The ESI-HRMS analysis in the positive mode revealed an [M+Na]+ ion at m/z 391.0790, corresponding to the molecular formula C20H16O7 (calcd. for C20H16O7Na, 391.0788). The UV spectrum of compound 2 in acetonitrile revealed two distinct absorption maxima, one at 255 nm for band II and one at 292 nm for band I, typical for an isoflavone nucleus (Mabry et al., 1970) while its IR spectrum displayed absorption bands at 3260 cm−1 (OH) and 1650 cm−1 (conjugated carbonyl). The 1H NMR and 13C NMR spectra (Table 1) of 2 closely resembled those of compound 1, except that the signals for the 3-methyl-2-oxobutyl moiety at δH 3.83 (2H, s, H-1″), δH 2.82 (1H, sept, H-3″), δH 1.11 (6H, d, H-4″ & H-5″) in compound 1 were replaced by the signals for a 3-oxobut-1-en-1-yl unit at δH 7.84 (1H, d, J = 16.5 Hz, H-1″), δH 6.86 (1H, d, J = 16.5 Hz, H-2″) and at δH 2.35 (3H, s, H-4″) in compound 2. The trans-geometry of the double bond of the 3-oxobut-1-en-1-yl unit was deduced from the coupling constant (J = 16.5 Hz) of the two olefinic protons. The HMBC cross-peaks (Table 1) of H-1″ with C-4′ (δC 147.9), C-5′ (δC 118.9), C-2″ (δC 129.3) and C-3″ (δC 198.1) suggested the attachment of the 3-oxobut-1-en-1-yl unit at C-5′ of ring B. Therefore, compound 2, was characterized as (E)-5,7,3′-trihydroxy-4′-methoxy-5′-(3-oxobut-1-en-1-yl)isoflavone trivially named erysacleuxin D.
From the biosynthetic point of view, the proposed biosynthetic pathway of compounds 1, 2 and 7 from compound 5 is through biological hydroxylation, epoxidation and ring opening, methylation, oxidation, dehydration, oxidative cleavage of double bonds, and prenylation as illustrated in Scheme 17 (Supplementary data).
As part of our ongoing investigation of African Erythrina species in search for novel cytotoxic natural products, the isolated compounds were assayed for in vitro activity against the human cancer cell lines, HeLa-S3 using the Giesma staining assay. Compounds 1, 2 and 9 showed weak cytotoxicity with IC50 values of 130.4, 54.9 and 73.9 µM, respectively. The positive control, campthotecin had an IC50 of 14.6 µM. Butin (9) has previously shown cytotoxicity activity against HL60 cells with IC50 value of 8.3 μΜ (Fotso et al., 2017). With regard to the structure-activity relationships of compounds 1 and 2, some features that might influence their cytotoxic activity can be drawn when comparing their chemical structures. It was observed that erysacleuxin D (2) with an α,β-unsaturated carbonyl group on the side chain was more potent than erysacleuxin C (1) with a saturated double bond at C-1″. The possible logical explanation is that the strong Michael acceptors in compound 2 can induce cell damage and cause cytotoxicity (Amslinger, 2010).
3 Experimental section
3.1 General experimental procedures
Melting points were determined by a Stuart melting point apparatus SMP1 (UK) and are uncorrected. An Evolution 201 UV–visible spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA) was used to record UV spectra. A Bruker Tensor 27 IR spectrometer equipped with a diamond ATR unit was used to record IR spectra. The NMR spectra were recorded on a Bruker Avance-III spectrometer (Bruker, Karlsruhe, Germany) at 600 MHz and 150 MHz (1H and 13C, respectively) equipped with a 5 mm TCI cryoprobe and Avance-III spectrometer (Bruker, Karlsruhe, Germany) at 400 and 100 MHz (1H and 13C, respectively) equipped with a 5 mm probe head. ESI-HRMS was obtained with a Q-ToF ULTIMA-III quadrupole TOF mass spectrometer (Waters, Eschborn, Germany). Silica gel 60 Å (0.035–0.070 mm) was used for Column chromatography and preparative TLC performed on glass plates measuring 20 × 20 cm precoated with silica gel 60 PF254+366 having 0.75 mm thickness. The spots and bands were visualized under a UV lamp (254 and 366 nm). Reversed-phase preparative HPLC separation was carried out on a Knauer System equipped with a binary pump and integrated diode array detector and an ACE C18-PFP column (5 µm, 150 mm × 30 mm, 20 °C) at a flow rate of 37.5 mL/min.
3.2 Plant material
The twigs of E. sacleuxii were collected from Mutsengo, Kilifi County along the Kenyan coast in December 2016. Identification of the plant species was done by Prof. S.T. Kariuki of Biological Sciences, Egerton University. A voucher specimen was deposited at the Department of Biological Sciences, Egerton University (no. 0136EUH).
3.3 Extraction and isolation
The air-dried and ground twigs of E. sacleuxii (3.1 kg) were extracted by percolation with CH2Cl2-CH3OH (1:1) (3 × 7.5 L) at room temperature, yielding 180.3 g of crude extract after evaporation under reduced pressure. The dried crude extract was suspended in water (800 mL) and partitioned between CH2Cl2 and EtOAc. The EtOAc fraction (43.9 g) was adsorbed on silica gel (silica gel 60, 0.035–0.070 mm) and subjected to Column chromatography eluting with increasing amounts of EtOAc in cyclohexane to give a total of ninety two fractions, 200 mL each. On the basis of TLC profiles, the fractions were combined to give eight fractions (A-H). Fraction B (1.3 g) was purified over silica gel, eluting with 10% EtOAc in petroleum ether, yielding two sub-fractions (B1 and B2). Sub-fraction B2 was further purified with preparative TLC with a mobile phase of CH2Cl2-CH3OH (20: 0.1) to yield vanillin (3, 12.3 mg, Rf = 0.62). Fraction F (1.23 g) was subjected to column chromatography, eluting with 4% CH3OH in CH2Cl2 to yield three sub-fractions (F1, F2 and F3). Sub-fraction F1 was purified with preparative TLC using 5% CH3OH in CH2Cl2 to give 26-hydroxyhexacosyl-(E)-ferulate (4, 5.6 mg, Rf = 0.33), while genistein (5, 6.2 mg, Rf = 0.32) was obtained from sub-fraction F3, after purification on preparative TLC with 6% CH3OH in CH2Cl2. Column chromatographic purification of fraction G (3 g) eluting with 4% CH3OH in CH2Cl2 gave sub-fractions G1, G2 and G3. Sub-fraction G2 was separated on reversed-phase preparative HPLC with MeCN-H2O (30:70) isocratic, to afford liquiritigenin (6, 4 mg) at tR = 5.69 min, 5′-formylpratensein (7, 2 mg) at tR = 10.53 min and erysacleuxin C (1, 2.2 mg) at tR = 12.21 min. Fraction H (4.2 g), eluting with 60% CH2Cl2 in EtOAc, was subjected to column chromatography to give sub-fractions H1 and H2. Further separation of H2 by column chromatography with 5% CH3OH in CH2Cl2 as eluent yielded four sub-fractions (H2.1, H2.2, H2.3, and H2.4). Sub-fraction H2.1 was separated by reversed-phase HPLC with a mobile phase of MeCN-H2O (40:60) to afford calycosin (8, 4 mg) at tR = 3.07 min and erysacleuxin D (2, 2.1 mg) at tR = 5.62 min. Purification of H2.4 by preparative TLC using 8% CH3OH in CH2Cl2 yielded butin (9, 15 mg, Rf = 0.57).
The CH2Cl2 extract (8.6 g) was subjected to column chromatography (silica gel, CH2Cl2: EtOAc 100:0–0:100) to produce four fractions. Fraction 2 was repeatedly purified by column chromatography eluting with 60% CH2Cl2 in EtOAc to give an additional quantity of 3 (12.3 mg). Fraction 4 was subjected to preparative TLC developed with n-hexane-EtOAc (70:30) to afford additional quantity compound 4 (7.2 mg).
3.3.1 Erysacleuxin C (1)
Pale yellow solid; mp 180–182 °C; UV (MeCN) λmax (log ε) 262 (4.26), 290 (3.22) nm; IR (KBr) νmax 3326, 2972, 2936, 1699, 1651, 1506, 1440, 1365, 1311, 1196, 1165, 1051 and 962 cm−1; 1H (600 MHz) and 13C NMR (150 MHz) spectroscopic data in acetone‑d6, see Table 1; ESI-HRMS (positive) m/z 407.1109 [M+Na]+ (calcd. for C21H20O7Na, 407.1101).
3.3.2 Erysacleuxin D (2)
White powder; mp 174–176 °C ; UV (MeCN) λmax (log ε) 255 (4.30), 292 (3.30) nm; IR (KBr) νmax 3260, 1650, 1576, 1442, 1365, 1282, 1196 and 834 cm−1; 1H (600 MHz) and 13C NMR (150 MHz) spectroscopic data in acetone‑d6, see Table 1; ESI-HRMS (positive) m/z 391.0790 [M+Na]+ (calcd. for C20H16O7Na, 391.0788).
3.4 Cytotoxicity assays
Cytotoxic activities were carried out according to Gruhn et al. (2007) with minor modifications: The cell culture medium was removed from the cells and the cells were washed with PBS, MeOH was added for fixation for 10 min, cell layer was dried and stained for 5 min with Giemsa solution (Giemsa's Azure Eosin Methylene Blue Solution; 1:10 diluted with 0.9% NaCl in H2O bidest.). The stained cell layer was then washed with water and the stain was extracted with 0.1 M HCl while shaking. The stained solution was transferred to a new 96 well plate and measured at 600 nm. All tests were done in triplicate.
4 Conclusions
In conclusion, two new isoflavones, erysacleuxin C (1) and erysacleuxin D (2), along with seven known compounds (3–9) were isolated from twigs of E. sacleuxii. Erysacleuxin C (1), erysacleuxin D (2) and butin (9), showed weak cytotoxicity against human cancer cell lines, HeLa-S3, with IC50 values of 130.4, 54.9 and 73.9 µM, respectively.
Acknowledgments
The authors are grateful to Dr. J. C. Liermann and Dr. C. Kampf (both from University of Mainz) for recording NMR and mass spectra and to Prof. S.T. Kariuki of Biological Sciences, Egerton University, for plant identification. A Ph.D. scholarship by the German Academic Exchange Service (DAAD) through the Natural Products Research Network for Eastern and Central Africa (NAPRECA) to J.O.O is gratefully acknowledged. This work was supported by the Rhineland Palatibate Center for Natural Products Research.
Declaration of Competing Interest
No potential conflict of interest was reported by authors.
References
- Formadienoate-A and B: two new long chained feruloyl esters from Clerodendrum formicarum (Lamiaceae) of Cameroon. Nat. Prod. Commun.. 2010;5:919-922.
- [Google Scholar]
- The tunable functionality of α, β-unsaturated carbonyl compounds enables their differential application in biological systems. ChemMedChem.. 2010;5:351-356.
- [Google Scholar]
- Medicinal Natural Products: A Biosynthetic Approach (second ed.). West Sussex: John Wiley and Sons; 2002.
- Secondary metabolites with antiproliferative effects from Albizia glaberrima var glabrescens Oliv. (Mimosoideae) Nat. Prod. Res.. 2017;31:1981-1987.
- [Google Scholar]
- Gillett, J.B, Polhill, R.M., Verdcourt, B., 1971. Flora of tropical East Africa. Leguminosae (Part 3) Subfamily Papilionoideae, p. 541.
- Biologically active metabolites from the basidiomycete Limacella illinita (Fr.) Murr. Z Naturforsch. C. 2007;62:808-812.
- [Google Scholar]
- Harborne J.B., Mabry T.J., Mabry H., eds. The Flavonoids. London: Chapman & Hall; 1975.
- Isolation of antioxidant compounds from the methanolic extract of the roots of Decalepis hamiltonii (Wight and Arn.) J. Agric. Food Chem.. 2005;53:7709-7714.
- [Google Scholar]
- Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J. Nat. Prod.. 1993;56:989-994.
- [Google Scholar]
- Medicinal plants of East Africa (second ed.). Nairobi: Kenya Literature Bureau; 1993.
- Assessing sub-Saharan Erythrina for efficacy: traditional uses, biological activities and phytochemistry. Pak. J. Biol. Sci.. 2011;14:560-571.
- [Google Scholar]
- The ultraviolet spectra of isoflavones, flavanones and dihydroflavonols. In: Mabry T.J., ed. The Systematic Identification of Flavonoids. Berlin: Springer; 1970. p. :165-226.
- [Google Scholar]
- New isoflavones from the genus Baptisia (Leguminosae) Phytochemistry. 1968;7:803-808.
- [Google Scholar]
- A modern look at folkloric use of anti-infective agents. J. Nat. Prod.. 1987;50:1025-1040.
- [Google Scholar]
- Prenylated isoflavones from the stem bark of Erythrina sacleuxii. Phytochem. Lett.. 2018;26:110-114.
- [Google Scholar]
- Condensed tannins. 10. Isolation of (-)-butin and butein from wattle heartwoods. Biochem. J.. 1961;80:62.
- [Google Scholar]
- Four isoflavones from the stem bark of Erythrina sacleuxii. Phytochemistry. 1998;49:247-249.
- [Google Scholar]
- Two isoflavanones from the stem bark of Erythrina sacleuxii. Phytochemistry. 2000;55:457-459.
- [Google Scholar]
- Two prenylated flavanones from stem bark of Erythrina burttii. Phytochemistry. 1998;48:1439-1443.
- [Google Scholar]
- New isoprenylated flavones and stilbene derivative from Artocarpus hypargyreus. Chem. Biodivers.. 2012;9:394-402.
- [Google Scholar]
Appendix A
Supplementary material
NMR Spectra, ESI-HRMS spectra, UV Spectra, and IR Spectra. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.05.007.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







