Translate this page into:
Ethnopharmacological and phytochemical attributes of Indian Tinospora species: A comprehensive review
⁎Corresponding author. bsingh@jpr.amity.edu (Bharat Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Total 34 species of Tinospora genus are found in Africa, Australia, Asia, Madagascar and Pacific regions of the World. Nine species of Tinospora are naturalized in the different states of India. In traditional medicine, different parts of Tinospora are used in the treatment of syphilis, ulcers, bronchitis, jaundice, urinary disease, piles, skin and liver diseases. The information of traditional uses, phytochemical and pharmacological properties of Indian Tinospora species was collected from the published books, MSc/MTech, PhD dissertations, PubMed, Wiley, and Elsevier. Moreover, the reference books, the relevant reviews, and the digital records were critically examined to present a complete overview of Indian Tinospora species. Indian Tinospora species possess various pharmacological attributes such as antioxidant, hepatopotective, radioprotective, neuroprotective, antidiabetic, anthelmintic, antmicrobial, analgesic, anti-fertility, antiarthritic, anti-tumor, antistress, anti-inflammatory, immunomodulatory, wound healing, and antiulcer activities. These biological activities of Indian Tinospora species can be attributed to the presence of a wide range of phytoconstituents including alkaloids (tinoscorsides A-B, palmatine, tembetarine, jatrorrhizine, magnoflorine, berberine, isocolumbin), clerodane furano diterpene glucosides (amritosides A-D, tinoscorside C, borapetoside B and F, and cordifolide C), flavonoids (diosmetin, genkwanin, genkwanin 7-glucoside, and rutin), lignans (Secoisolariciresinol, syringaresinol, makisterone C), and sterols (campesterol, β-sitosterol, stigmasterol). This review describes the detailed botany, ethnomedicinal uses, phytochemistry, pharmacological attributes of 9 Indian Tinospora species. Moreover, we also included the clinical importance and toxicological effects of Indian Tinospora species with the aim to investigating its potential uses as ingredients for pharmaceutical industry.
Keywords
Tinospora species
Ethnomedicinal properties
Phytochemistry
Pharmacology
Clinical and toxicological studies
- DPPH
-
2,2-Diphenyl-1-picrylhydrazyl
- ABTS
-
2,2-Azinobis-(3-ethylbenzothiazoline-6-sulfonate)
- DMSO
-
Dimethylsulfoxide
- NO
-
Nitric oxide
- SOD
-
Superoxide dismutase
- BSA
-
Bovine serum albumin
- MTT assay
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- CFU assay
-
Colony forming unit
- CAT
-
Catalase
- CCl4
-
Carbon tetrachloride
- TNF-α
-
Tumor necrosis factor
Abbreviations
1 Introduction
Since the inception of human civilization, plants have been used as a source of natural medicines. Currently, researchers are showing a great interest in the identification of bioactive compounds from medicinal plants. India with its rich plant biodiversity and enormous knowledge of Ayurvedic, Himeopathic, Siddha, Unani system of medicines, provide a strong base for the utilization of plants in general healthcare and common complaints of the people (Bharathi et al., 2018; Nazar et al., 2020). This curing capacity of plants can be substantiated by many scientific studies (Banerjee et al., 2011; Anwar et al., 2016). In response to growing challenges in health care system, researchers are concentrating their attention on plants to isolate and identify the bioactive principles (Russell and Duthie, 2011; Muhammad et al., 2016). The dependability on phytomedicines for treatment of various diseases is higher in present times than never before. In Indian traditional medicines, more than 25,000 plant-derived drug formulations have been recorded (Ashraf et al., 2016). Indian Tinospora species are used in the treatment of jaundice, fever, urinary diseases, asthma, gout, diabetes, diarrhoea, skin disease, and snakebites (Gupta and Kumar, 2002; Jakhar et al., 2004). About 34 species of Tinospora genus are found in Africa, Australia, Asia, Madagascar and Pacific regions of the World. Nine species of Tinospora are naturalized in the different states of India (Udayan and Pradeep, 2009).
T. baenzigeri Forman is a woody climber and distributed in Telyababa forest range, Burhanpur district, Madhya Pradesh state of India (Mishra et al., 2020). It has been used as a traditional medicine in the treatment of headache, cold, fever, diarrhea, ulcer, digestive disorder, and rheumatoid arthritis (Tuntiwachwuttikul and Taylor, 2001). T. cordifolia (Willd.) Miers [syn. Menispermum cordifolium Willd., T. gibbericaulis Handel-Mazzetti, T. mastersii Diels, T. rumphii Boerlage, T. thorelii Gagnepain] is a medicinally and commercially important plant species, commonly known as “Guduchi” or “Amrita”, and distributed in several states of India (Chadha, 1948; Sinha et al., 2004; Sharma et al., 2010; Singh and Chaudhuri, 2017). The leaf decoction is used to enhance body resistance as well as in boosting of immune system of humans (Saha and Ghosh, 2012; Bhattacharyya, 2013; Biswas et al., 2015). The whole plant extracts are widely used in the treatment of jaundice, and rheumatism (Sharma and Pandey, 2010a; Gupta and Sharma, 2011; Sharma et al., 2019), intermittent fever, eye and liver ailments (Choudhary et al., 2013). The alkaloids [jatrorrhizine, palmatine, magnoflorine (Chintalwar et al., 2003; Sarma et al., 2009; Kiem et al., 2010)], clerodane furano diterpene glucosides [cordifoliside D, cordifoliside D tetraacetate, cordifoliside E, cordifoliside E tetraacetate (Gangan et al., 1994, 1995; Sharma et al., 2019)], daucane-type sesquiterpenes [tinocordifolin, tinocordifolioside and tinocordifolioside tetraacetate (Maurya et al., 1997] and sesquiterpenoids (Maurya and Handa, 1998) have been isolated and identified from T. cordifolia. T. cordifolia possesses antioxidant (Polu et al., 2017), antiarthritic (Ramya and Maheswari, 2016), antidiabetic (Khedekar et al., 2015), anti-inflammatory (Sannegowda et al., 2015), antiulcer (Antonisamy et al., 2014), and anticancer properties (Bhadane et al., 2018). Whole plant is useful in the treatment of debility, dyspepsia, fever, inflammation, bronchitis, jaundice, urinary, skin and liver related diseases (Anonymous, 1976).
T. crispa (L.) Hook f. and Thomson is an herbaceous climber, found in tropical and subtropical areas of northeast region of India (Pathak et al., 1995; Patel et al., 2013a). In traditional medicines, it has been used in the treatment of jaundice, rheumatism, urinary disorders, fever, malaria, diabetes, fracture, scabies, hypertension, reducing thirst, increasing appetite (Najib Nik a Rahman et al., 1999; Dweck and Cavin, 2006). T. crispa showed the presence of alkaloids (Fukuda et al., 1983; Choudhary et al., 2010a), flavonoids (Umi Kalsom and Noor, 1995; Lin, 2009), diterpenes and diterpene glycosides, cis clerodane-type furanoditerpenoids, lignans (Fukuda et al., 1985, 1986; Chung, 2011). Different parts T. crispa possesses anti-inflammatory (Abood et al., 2014), antimicrobial (Zakaria et al., 2006), antioxidant (Amom et al., 2008; Zulkhairi et al., 2009), antimalarial (Najib Nik a Rahman et al., 1999) activities.
T. formanii Udayan & Pradeep is a woody dioecious vine and naturalized in the Western Ghats of Thrissur district, Kerala, India (Sheema Dharmapal et al., 2017). T. glabra (F. Burm.) Merr. (syn. M. glabrum Burm.f..) is a deciduous woody climber, and naturalized in the tropics of Asia, Africa and Australia. T. glabra widely occurs in the northeast parts and the Andaman and Nicobar Islands of India (Merrill, 1938; Nayampalli et al., 1982). The water extract is used in the treatment of jaundice, rheumatism, urinary problems, intermittent fever, eye and liver complaints. As per Ayurvedic medicine, plant species possesses adaptogenic and immuno-modulatory properties (Gupta et al., 1967; Rishikesan et al., 2016). T. maqsoodiana Mujaffar, Moinuddin and Mustakim is a wood climber and naturalized in Madhya Pradesh state of India (Mujaffar et al., 2014).
T. sinensis (Lour.) Merr. [syn. Menispermum malabaricum Lam., syn. T. malabarica Miers. (Miers.), M. tomentosum (Colebr.) Roxb.] is found in different states of India. Its stem is used in Ayurvedic formulations for the treatment of jaundice and diarrhea (Udayan et al., 2004; Ahmed et al., 2006). Its stem paste is used to heal ankylosis, fracture, lumbar disc herniation, rheumatic complaints and in the treatment of knee joint osteoarthritis (Sahu, 2002; Srinivasan et al., 2008; Chi et al., 2016; Jiang et al., 2017). The Chinese preparation “Qutan Tongluo Tang” (from T. sinensis) is useful in the treatment of irregular heartbeat, high fever and diabetes (Liu, 2004; Wang et al., 2005; Chen et al., 2007; Zhang et al., 2010). Phytochemical analysis of different parts of T. sinensis revealed the presence of lignans (Maurya et al., 2009; Lam et al., 2018), phenylpropanoid glycosides (Xu et al., 2017a; Jiang et al., 2018), and alkaloids (Srinivasan et al., 2008; Maurya et al., 2009). T. sinensis also demonstrated antidiabetic (Sandhyarani and Kumar, 2014a), antistress (Sharma et al., 2007), anticancer (Punitha et al., 2012) activities. T. smilacina Benth is a semi deciduous, woody, creeping climber and distributed on Maruthamalai hills, Western Ghats of Tamil Nadu state of India and northern New South Wales and Central Northern Australia (Barr et al., 1988; Parthipan et al., 2016). T. subcordata (Miq.) Diels is a small woody vine and found in Khandwa district, Madhya Pradesh state of India (Mishra and Mishra, 2020). As per clinical studies, T. cordifolia stem powder (capsules) demonstrated antidiabetic (Mishra et al., 2015b), anti-HIV (Kalikar et al., 2008), wound healing properties in post-operative patients (Shrestha et al., 2017). Similarly, T. crispa stem dry extract also exhibited anti-diabetic activity in patients (Sangsuwan et al., 2004). The toxicological effects of T. cordifolia stem powder (Sharma and Dabur, 2016; Ghatpande et al., 2019), T. crispa stem powder (Langrand et al., 2014) and T. sinensis stem powder (Khayum et al., 2009; Nagarkar et al., 2013) have been documented.
So far, no comprehensive review has been systematized to provide botany, traditional uses, phytochemistry, pharmacological attributes of 9 Indian Tinospora species in recent years in order to bridge the knowledge gap among scientists. The review will engage and help ethnobotanists, phytochemists, and pharmacologists to know about bioactive potential and pharmaceutical applications of Indian Tinospora species.
2 Botany and ethnomedicinal uses of Indian Tinospora species
T. baenzigeri is a woody climber, grows up to 15 m high, and stem with scattered pustular-lenticels. Leaves are broadly ovate to orbicular, apex usually long-acuminate, base shallowly to deeply cordate. Male inflorescence appears from the older, leafless stems, pseudoracemose, long (7–20 cm), flowers in 1–3 flowered clusters; sepals pale green, outer 3 ovate and inner 3 obovate; stamens 6, long (2 mm). Fruits are drupes, yellow and orange, radiating from subglobose carpophore, pericarp very thin and endocarp thinly bony (Mishra et al., 2020). In traditional medicine, it is useful in curing of headache, cold, fever, diarrhoea, ulcer, indigestion, and rheumatoid arthritis (Tuntiwachwuttikul and Taylor, 2001; Table 1; Fig. 1).
Species
Diseases/ complaints
Mode/parts of application
References
T. baenzigeri
Diarrhoea, cold, fever and ulcers
It is used in the treatment of headache, cold, fever, diarrhoea, ulcer, digestive disorder, and rheumatoid arthritis
Tuntiwachwuttikul and Taylor (2001)
T. cordifolia
Fever
Pills are prepared from the paste of stem of the T. cordifolia and the roots of Bhatkatiaya (Solanum surattense); Decoction of stem is administered orally also
Chopra (1994), Sharma and Kumar (2013), Upadhyay et al. (2010)
Sharma and Kumar (2013), Upadhyay et al. (2010)
Cough
The warm juice of root of T. cordifolia is taken orally
Chopra (1994), Sharma and Kumar (2013)
Jaundice, chronic diarrhoea, periodic fever
The whole plant is used
Tripathi (2006b), Upadhyay et al. (2010)
Cancer, dysentery, diarrhoea and periodic fever
Powdered root and steam bark of T. cordifolia with milk for cancer; root decoction for dysentery and diarrhoea; old stem decoction for periodic fever
Chopra (1994), Tripathi (2006a, b)
Balashosha (emaciation in children), daha (burning)
Dyed shirt soaked in juice of Guduchi worn by children for Balashosha; paste or juice of Amrita (T. cordifolia) leaves and Sarsapa beeja churna (seed powder of Brassica campestris) are used for daha
Sahu (2002), Tripathi (2006a)
Bone fracture
The whole plant paste is used in bone fractures
Sharma et al. (2018)
General debility
Decoction of stem with cold and hot water (about 3–4 g) in morning in an empty stomach, used as a tonic
Sharma and Kumar (2013), Tripathi (2006b)
Kasa (cough)
Powder of Terminalia chebula (Haritiki), Tinospora cordifolia (Amrita) and Trachyspermum ammi (Ajwain) in equal quantity is administered orally once daily early morning with a little salt
Rathore (2002), Saini (2009)
Karna Shula (pain in the ear)
Two drops of juice of leaves of allied species or guduchi (T. sinensis) are dropped in the affected ear
Chopra (1994), Tripathi (2006a, b)
Asthma
Juice of stem orally given with honey
Sharma and Kumar (2013), Upadhyay et al. (2010)
Twak-roga (skin disease).
A decoction of the stem is administered orally
Sahu (2002)
T. crispa
Pyorrhoea, diabetes, hypertension, injury
In Thai medicine, stem water decoction is used in the treatment of internal inflammations, pyorrhoea, appetite, and in thirst and body temperature management
Kongsaktrakoon et al. (1984), Dweck and Cavin (2006)
Bruises, septicemia, fever, bone fracture, and scabies
In Chinese medicine, it is used to treat bruises, septicemia, fever, bone fracture, scabies, and tropical ulcer-related disorders
Li et al. (2006)
Parasitic worm’s disease; aching eyes and syphilitic sores; wounds
Stem infusion taken as vermifuge; stem decoction use to wash eyes and sores; the crushed leaves applied in wound healing
Dweck and Cavin (2006)
T. formanii
–
No report is available in literature on traditional uses
–
T. glabra
–
No traditional values reported in literature
–
T. maqsoodiana
–
No report is available in literature
–
T. sinensis
Knee joint osteoarthritis
Decoction is of stem is used
Qin et al. (2006), Hegde and Jayaraj (2016)
Lumbar disc herniation
Paste of stem extract of T. sinensis
Singh (1998), Tripathi (2006a)
Ankylosis (acute and chronic arthritis, rheumatic arthritis)
Whole plant paste applied topically to reduce swelling and relieve pain in the joints and muscles
Zhang et al. (2010), Bhardwaj (2011)
Bone fractures
Decoction of aerial parts used in healing of fractures
Wang et al. (2005), Srivastava (2003)
Visceral leishmaniasis
Decoction of stem is used
Yadav (2007), Singh et al. (2008)
T. smilacina
Wound injury, rheumatism
In Australian medicine, the leaf, stem and root are used in the treatment pain, wounds, swelling, rheumatism, severe colds, infections and snakebite
Cribb and Cribb (1981), Hungerford et al. (1998)
T. subcordata
–
No report is available in literature on its uses in traditional medicine
–

Indian Tinospora species reported from different states of India.

Indian Tinospora species reported from different states of India.
T. cordifolia is a large deciduous climber, grows on wide range of hedges and trees (Kirtikar and Basu, 1918; Anonymous, 1976). Its stem is green, and contains succulent bark. The pendulous fleshy roots hang from branches, and its roots have pale shining or glabrous bark. On dryness, stem shrinks and bark separates from the wood. Leaves are membranous, round, cordate or heart shaped; contain 2.5–7.0 cm long petiole. The flowers bloom in the summer season. Inflorescence is of raceme type. Male flowers are small, yellow or green in colour, and occur in clusters in the axils of small subulate bracts. Sepals are 6 (3 + 3, outer and inner whorl), membranous, broadly elliptical, concave, yellow. The petals are 6, equal and spathulate, stamen pistillode. Female flowers are solitary, sepals green, margins not reflexed, staminode short; ovary has 1–3 carpels, widely separated on the short fleshy gynophores, and dorsally convexed (Hooker, 1875; Kirtikar and Basu, 2005). T. cordifolia stem is used in the stimulation of gastric activity, bile secretions, enrichment of blood composition and to treat skin diseases, spleen enlargement, vaginal and urethral discharges (Sahu, 2002). Stem decoction is used for washing of eyes and syphilitic sores, acts as an antidote to treat snakebites and scorpion stings (Trivedi, 2006; Spandana et al., 2013), and in the treatment of pyorrhoea, malaria, chronic diarrhea, asthma, dysentery, urinary, skin diseases and respiratory complaints (Trivedi, 2009; Pandey et al., 2012; Ramadevi et al., 2018; Sharmila et al., 2018). The aqueous extract of roots is used as an emetic and analgesic agent, and also useful in the treatment visceral pain (Stanely et al., 2000). The crushed leaves are mixed with honey and used to cure ulcers, gout and bacterial skin infections. Decoction of leaves is useful in malaria and enhancement of women’s fertility (Agarwal et al., 2002; Singh et al., 2003a, b, Singh and Chaudhuri, 2017). Dried fruit powder is mixed with ghee or honey and used as a tonic as well as in the treatment of jaundice and rheumatic complaints. The combination of ripened fruit juice and honey is recommended daily (for 3–5 days) for treatment of cold in children (Sahu, 2002). Whole plant extract is useful in diarrhoea, stomach complaints, and anemia (Sujatha and Mariya, 2015; Alsuhaibani and Khan, 2017). The stem is a rich source of copper, calcium, phosphorus, iron, zinc, manganese hence, used in the treatment of metabolic disorders of humans (Upadhyay et al., 2010; Dhama et al., 2017; Table 1; Fig. 1).
T. crispa is an herbaceous vine, with brownish and fleshy stem. The leaves are large (6–12 cm long and 7–12 cm wide), both surfaces glabrous and heart shaped. Flowers are 2–3, small, and yellowish or green in colour. Male inflorescence is slender, longer (5–10 cm), flowers six green, sepals in two whorls (outer three ovate and inner three obovate); female inflorescence long (2–6 cm), one flower per node. Fruits are drupe and long (7–8 mm). As per Vietnamese and Indian traditional medicine system, it is useful in malaria, cough, poor digestion, colitis, and diabetes [Ahmad et al., 2016; Guo et al., 1999) and also used in the treatment of jaundice, rheumatism, bone fractures, scabies, and hypertension (Cowan, 1999; Ahmad et al., 2016). It is taken in the management of thirst (Guo et al., 1999), hunger resistance and heat clearing (Pham and Nguyen, 2020), and used in curing of diabetes (Thomas et al. 2016; Table 1; Fig. 1).
T. formanii is a woody dioecious vine, with thick stem (4 cm), clear concentric rings, brown, smooth papery bark, peeling off into scales. Leaves are alternate, large (6–14 × 4–8 cm), ovate to elliptic-lanceolate, coriaceous, glabrous, acuminate at apex, cordate at base; lateral nerves 3–4 pairs, reticulate venation on lower surface; petioles slender, and long (5–12 cm). Male inflorescences and flowers unknown but, female inflorescence a compound elongated pseudoraceme, long (5–18 cm); sepals 6, triangular ovate, long (2 mm), greenish-yellow. Fruits are drupes, globose, across (2–3 cm), red on maturity; pericarp thin; endocarp broadly elliptic to subrotund in outline. Seeds are oblong, subellipsoid and dorsally convex (Udayan and Pradeep, 2009; Sheema Dharmapal et al., 2017; Table 1; Fig. 1).
T. glabra is a climber, seriate stem, with thin papery bark; leaves oblong-ovate or narrowly to broadly ovate, base cordate to truncate, apex acuminate; male inflorescence axillary, pseudo-racemose, 10–20 cm, flower solitary, bract 1 mm long, female inflorescence similar to male; fruit drupe red, radiating from unbranched short to columnar carpophore, endocarp thinly bony, keeled at apex (Merrill, 1938; Table 1; Fig. 1). T. maqsoodiana is similar to C. cordifolia but different in its young stems, papillose-glandular, leaf shape triangular-ovate, broad and long, ovate deltoid, elliptic, basal lobes slightly lobed, lamina base cordate to cuneate, endocarp with papillose surface (Mujaffar et al., 2014; Table 1; Fig. 1).
T. sinensis is a large fleshy deciduous climber with shiny stem and papery bark. Its young parts are covered with densely matted woolly hairs. The leaves are broadly ovate to suborbicular, membranous, sparingly pubescent above, pilose beneath with glandular patches at basal nerve axils, abruptly truncate or cuneate or subcordate at base; petioles 5–12 cm long, puberulous, and thickened at base. Flowers are greenish-yellow. Inflorescence is of raceme type, panicles 3–12 cm long, and slender. Pedicels of male flowers are 2–5 mm long, sepals yellowish-green, and glabrous, 6 (3 + 3, outer three ovate, 1–1.5 mm long and inner three broadly elliptic, 3–5 × 2–5 mm); petals six, obliquely rhomboid-ovate (size 3–4 × 1–3 mm); stamens 1.5–2 mm long, anthers dehisce longitudinally. Female flowers are tricarpellary, 2-lobed stigma, gynophore 1 mm long. Fruit is drupe, globose (1–3, 10–13 mm across), red, scarlet or orange red; carpophores 2–3 mm long on 8–10 mm long peduncles, dorsally keeled and ventrally concave, 7–9 × 5–6 mm, tuberculate, flowering occurs in December–February (Matthew, 1981–1984, 1991; Udayan et al., 2004). Leaf and stem juice are used in the treatment of chronic rheumatism, ulcerated wounds and piles (Rajgopal et al., 2013). Aqueous extract of whole plant is useful in debility, dyspepsia, fever, inflammation, syphilis, ulcers, bronchitis, jaundice, urinary disease, skin disease and liver disease (Anonymous, 1976). The juice or powder of T. sinensis stem is useful in the treatment of diabetes and gastritis (Balami, 2004). T. sinensis possesses adaptogenic and immunomodulatory (Kirtikar and Basu, 1993; Rege et al., 1999; Manjrekar et al., 2000), anti-inflammatory (Li et al., 2004a), and anti-diabetic activities (Yonemitsu et al., 1993; Table 1; Fig. 1).
T. smilacina Benth is a semi-deciduous herb, with woody climber stem (7 cm diameter). Leaves are alternate, size 4–5-12 × 4–11 cm, long petioles (2.5–9 cm), five veins, including the midrib, radiate from the base of the leaf blade. Inflorescence slender, male flower sepals arrange in two whorls of three; female flowers also arrange in two whorls of three, outer sepals about 2 mm long, inner sepals petaloid. Fruit is drupe, globular to ellipsoid (6–10 × 6–9 mm) in shape. Seed surface is coarsely rugose (6–7 × 4–5 mm; Barr et al., 1988). As per Australian traditional medicine, whole plant is used in the treatment of pain, wounds, swelling, rheumatism, severe colds, infections and snakebites (Cribb and Cribb, 1981). T. subcordata is a small woody climber, glabrous, stem with lenticels; leaves with petioles, long (2.5–8 cm), lamina triangular to broadly triangular, base broadly cordate to truncate with rounded; male inflorescence axillary, long (5–14 cm), pseudoracemose, long, flowers arrange on slender pedicels, sepals white, outer 3 ovate, inner 3 elliptic, petals 6, obovate-cuneate; female inflorescence peudoracemose, flowers arrange on pedicels, sepals and petals similar to male but slightly smaller and petals thin; fruit drupe, red, endocarp bony, elliptic in outline (Mishra and Mishra, 2020; Table 1; Fig. 1).
3 Phytochemistry
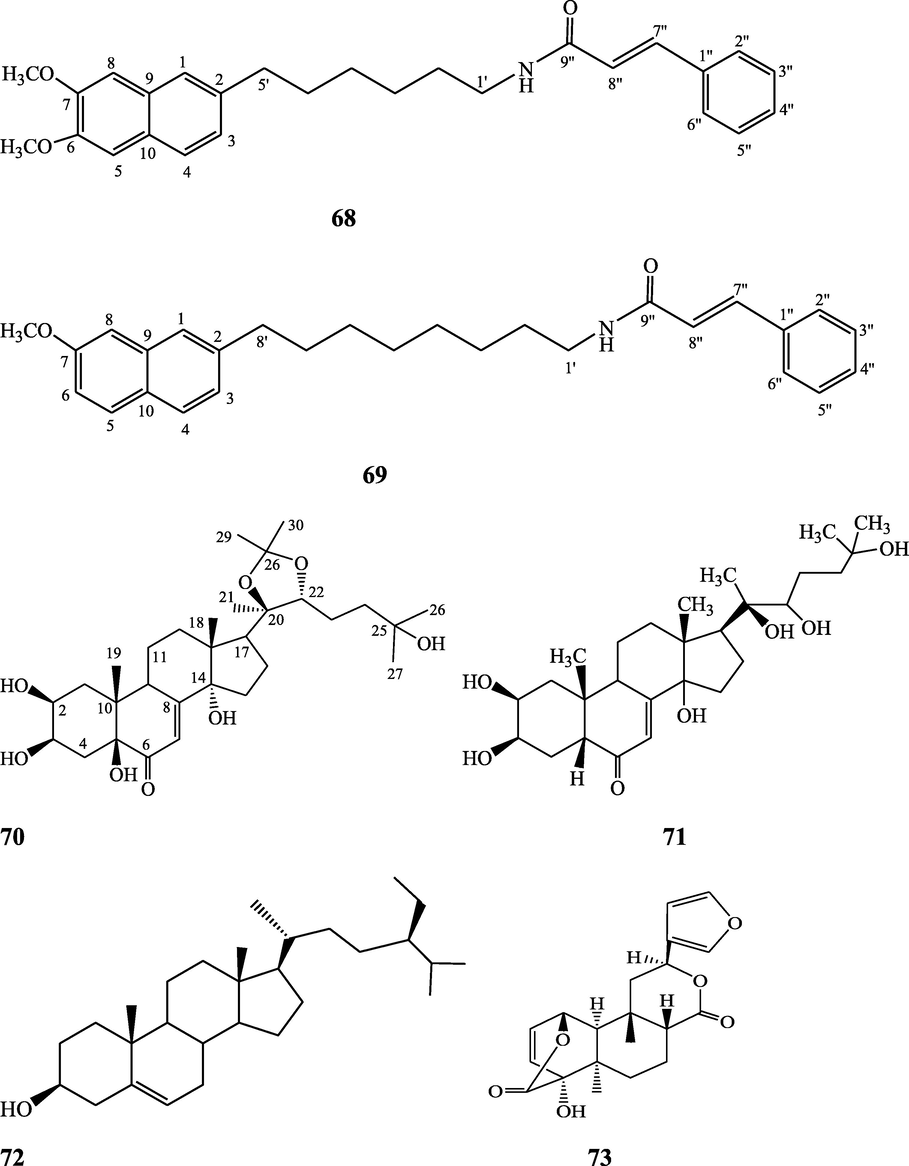
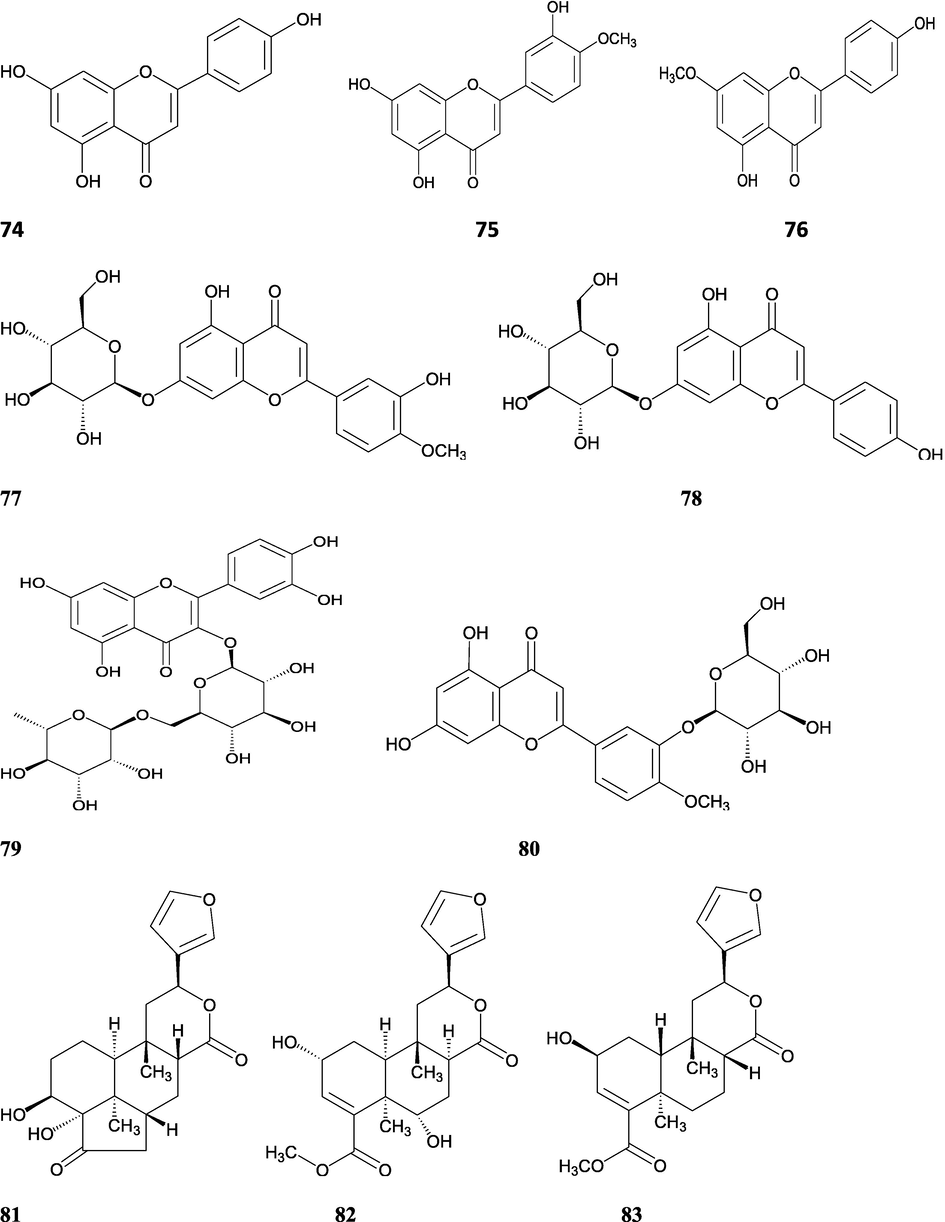
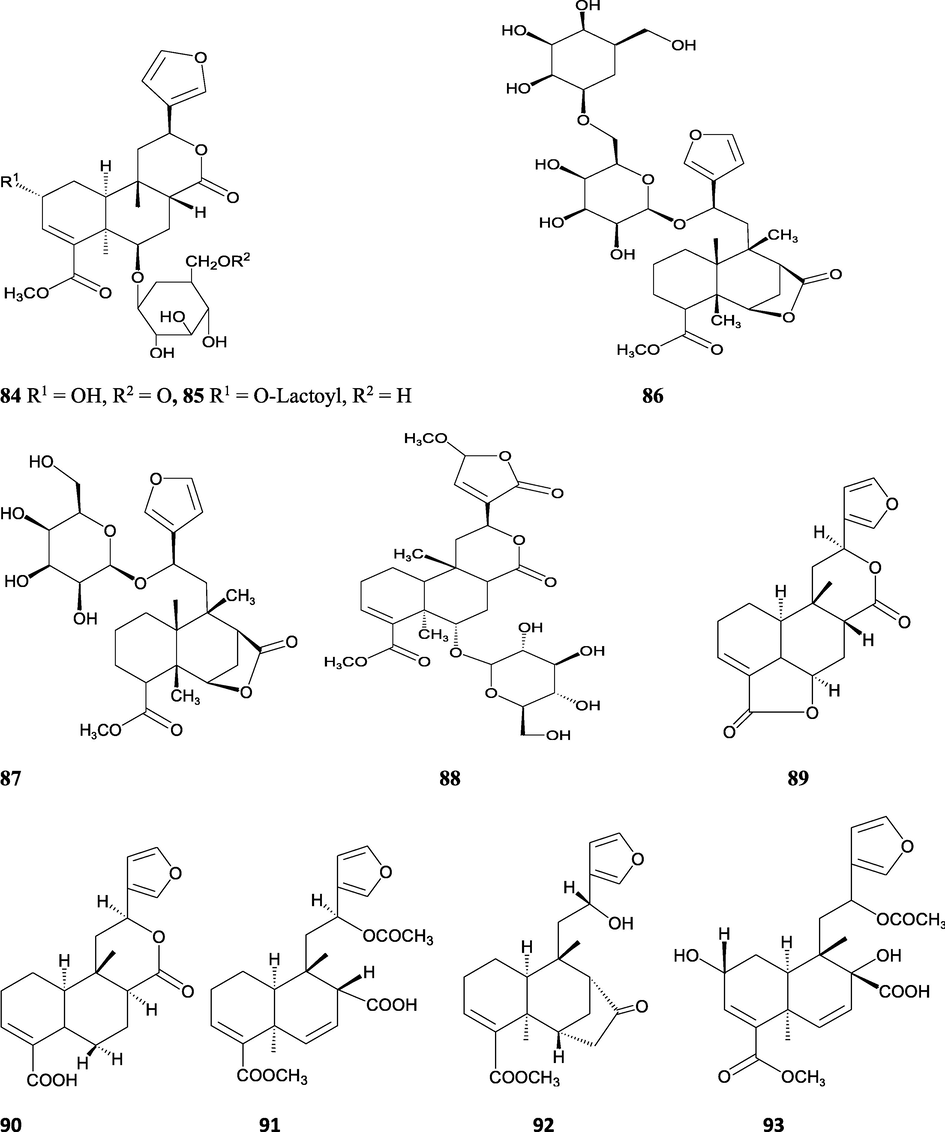
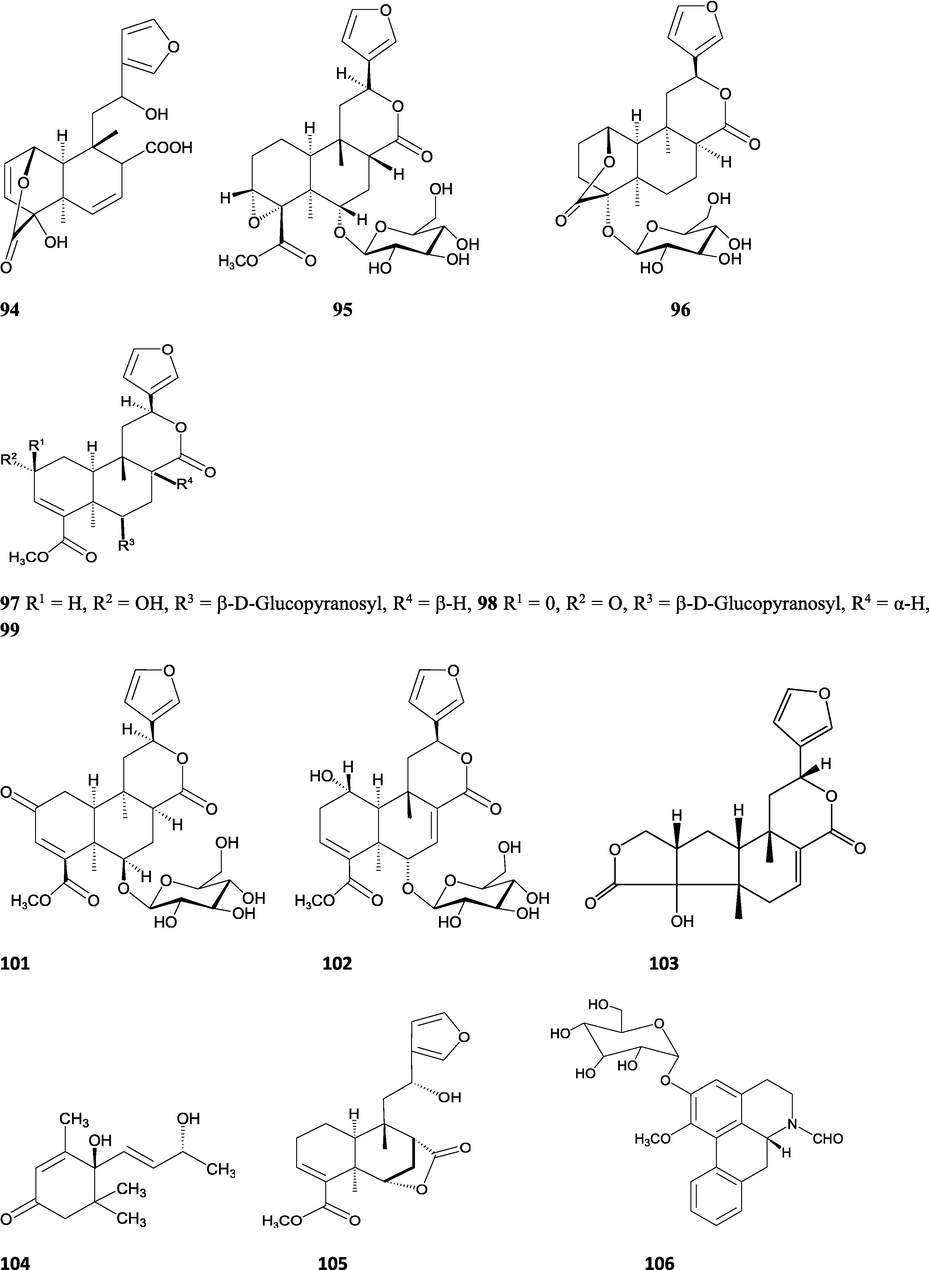
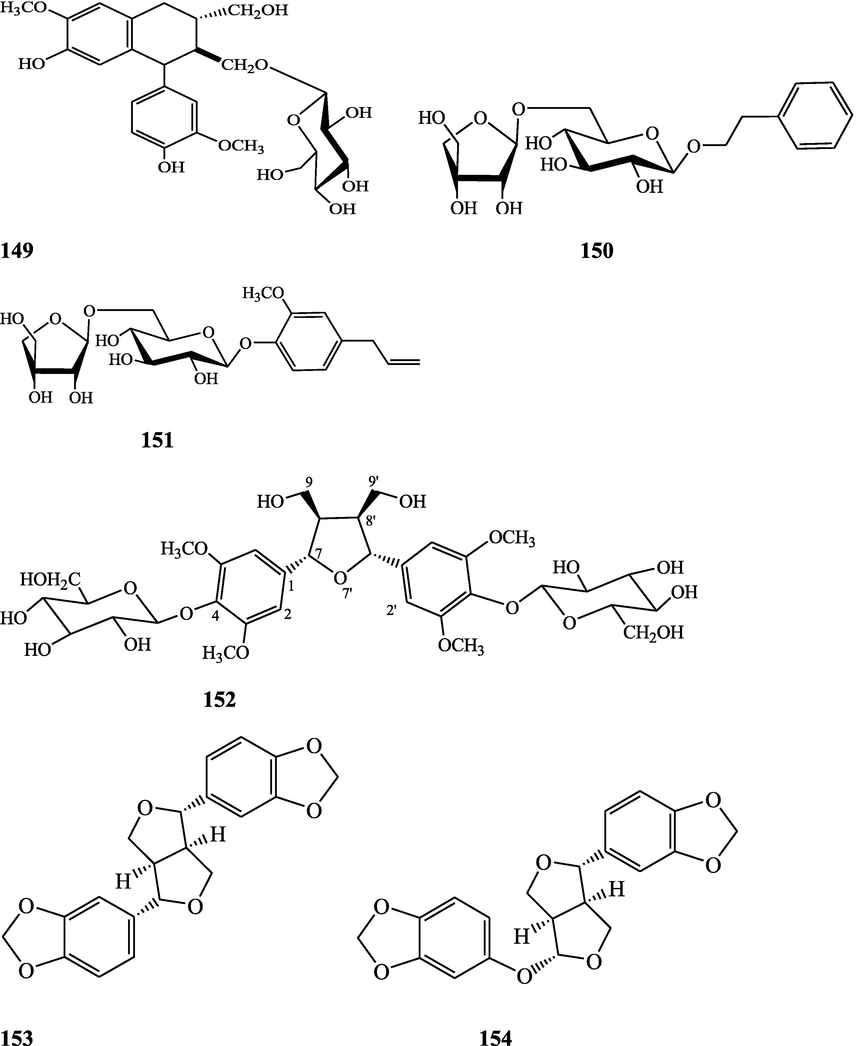
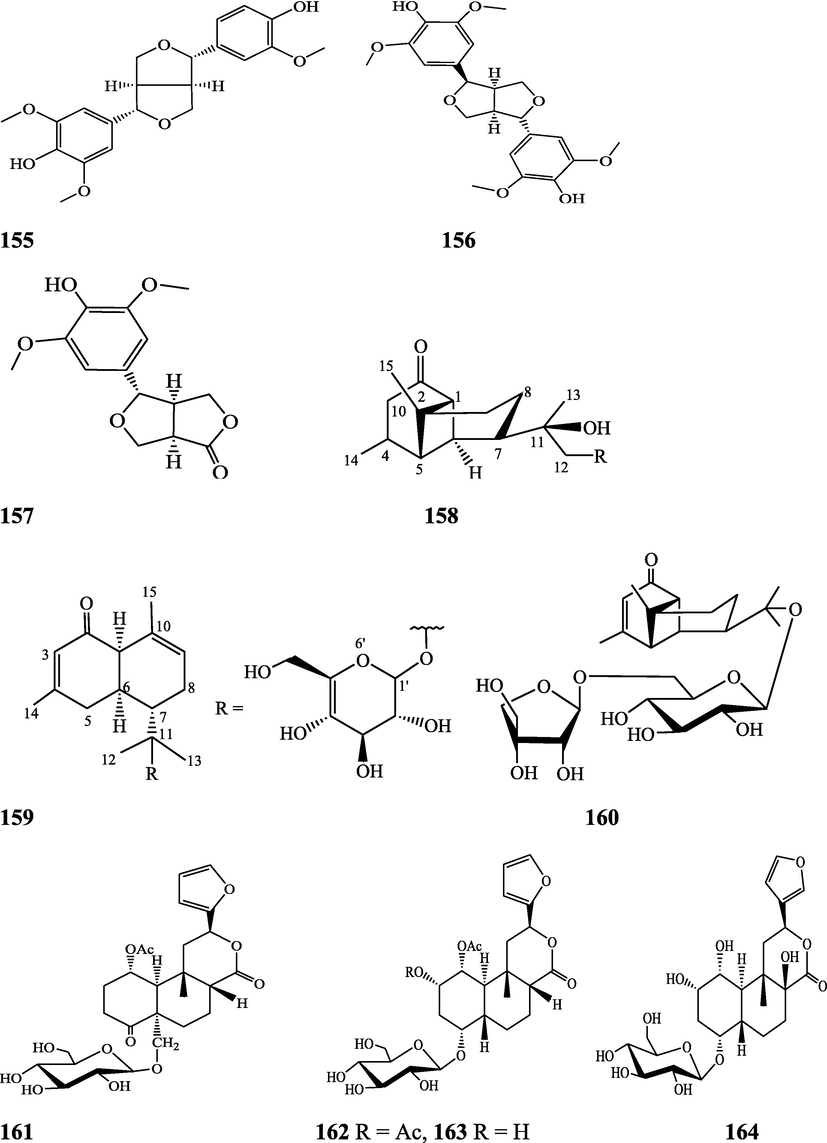
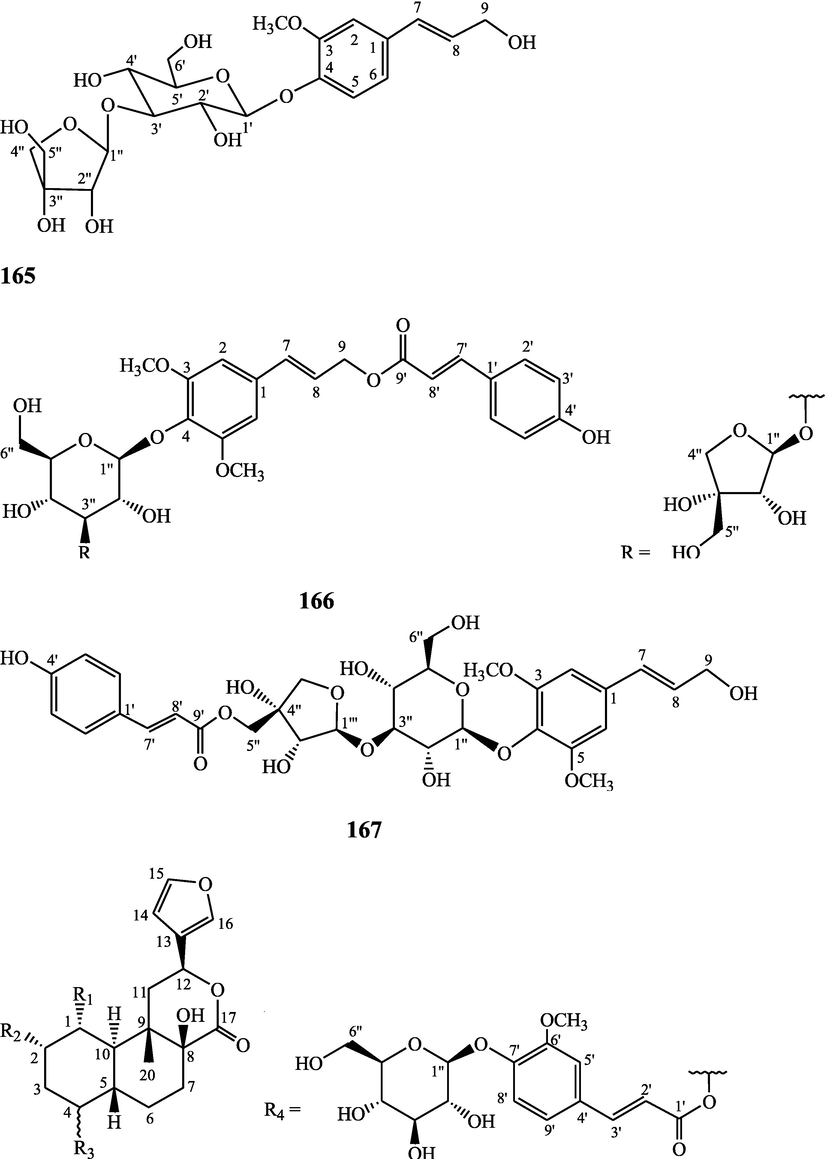
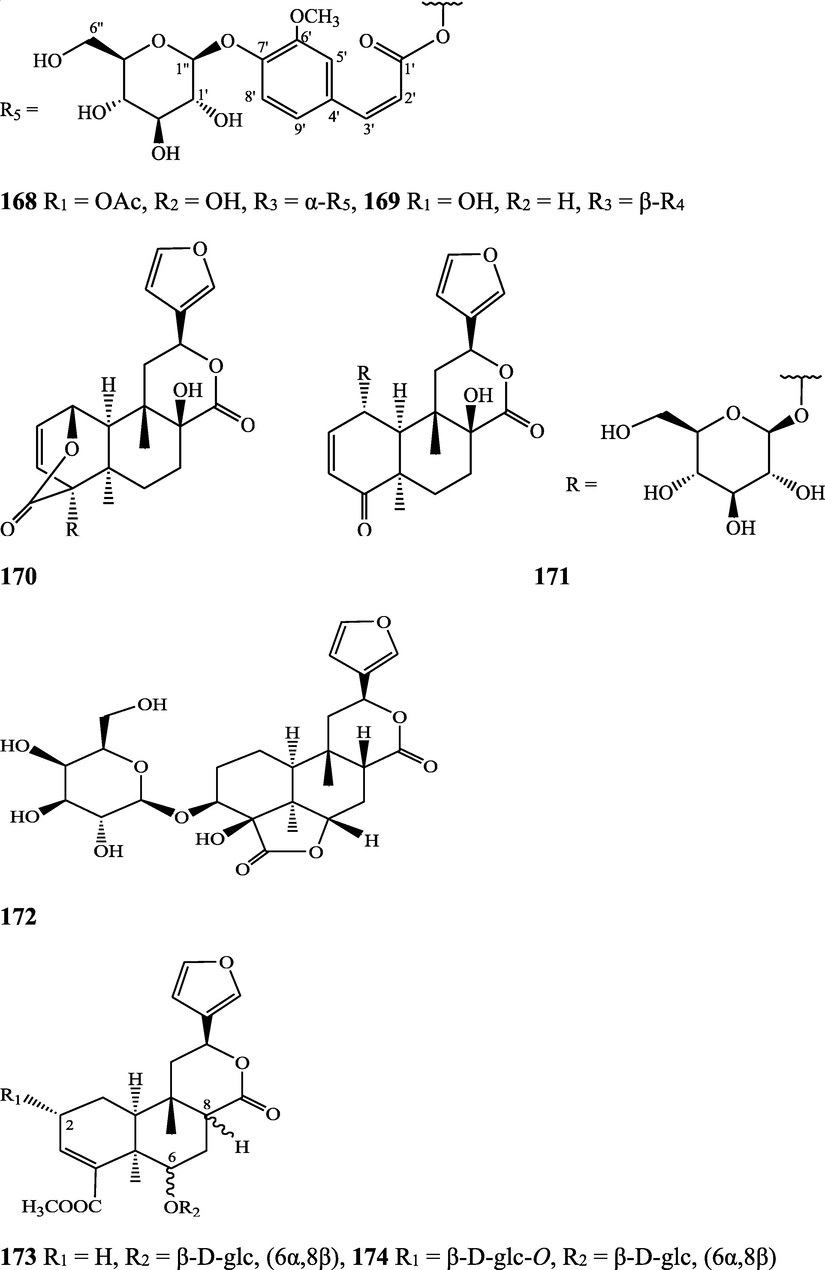
The alkaloids, glycosides, diterpenoid lactones, flavonoids, steroids and sesquiterpenoids have been isolated and identified from 4 species of Indian Tinospora while other 5 species have not been investigated for the presence of phytochemicals. The state-wise distribution, extract types, parts used, identified compounds and their structures have been mentioned in Table 2 and Fig. 2. No compound is reported from 5 Indian Tinospora species (T. formanii, T. glabra, T. maqsoodiana, T. smilacina and T. subcordata). The isolated and identified compounds from 4 Indian Tinospora species (T. baenzigeri, T. cordifolia, T. crispa and T. sinensis) are summarized below –
Plant species
Distribution (states of India)
Extract type
Plant parts
Isolated Compounds
References
T. baenzigeri
Telyababa forest range, Burhanpur district, Madhya Pradesh, India
Ethanolic
Stem and leaves
Clerodane diterpenoids - Baenzigeride A (1), baenzigeroside A (2), Baenzigeride B (3), , baenzigeroside B (4), tinobaenzigeride (5), tinobaenzigeroside (6), 4-epi-baenzigeride A (7), 4-epi-baenzigeride A glucoside (8), tinobaenzin A (9), tinobaenzin B (10), 4,12-di-epi-baenzigeride A (11), caruilignan D (12)
Tuntiwachwuttikul et al. (1999), Tuntiwachwuttikul and Taylor (2001), Pudhom et al. (2019) Hanthanong et al. (2019, 2021)
T. cordifolia
Rajasthan, Uttar Pradesh, Bihar, West Bengal, Gujarat, Punjab, Tamil Nadu, Kerala and Karnataka
Methanolic and ethanolic
Aerial parts and stem
Alkaloids - Tinoscorside A (13), tinoscorside B (14), palmatine (15), tembetarine (16), jatrorrhizine (17), magnoflorine (18), berberine (19), isocolumbin (20)
Srinivasan et al. (2008), Kiem et al. (2010), Gupta and Sharma (2011), Patel and Mishra (2012), Bala et al. (2015a)
Ethanolic
Roots
Alkaloids – Tetrahydropalmatin (21), jatrorrhizine (17), magnoflorine (18)
Sarma et al. (2009)
Methanolic
Cell cultures
Alkaloids – Berberine (7) and jatrorrhizine (5)
Chintalwar et al. (2003)
Methanolic
Aerial parts
Phenylpropene disaccharides - Angelicoidenol 2-O-β-D-apiofuranosyl-(1→6)- β-D-glucopyranoside (22), secoisolariciresinol-9’-O-β-D-glucopyranoside (23)
Kiem et al. (2010)
Methanolic
Stem
Sulfur-containing clerodane diterpene glycoside - Cordifolide A (24)
Pan et al. (2012)
Aqueous-methanolic
Stem
Clerodane furano diterpene glycoside - 2β,3β:15,16-Diepoxy- 4α, 6β-dihydroxy-13(16),14-clerodadiene-17,12:18,1-diolide (25)
Sharma et al. (2018)
Methanolic
Stem and stem bark
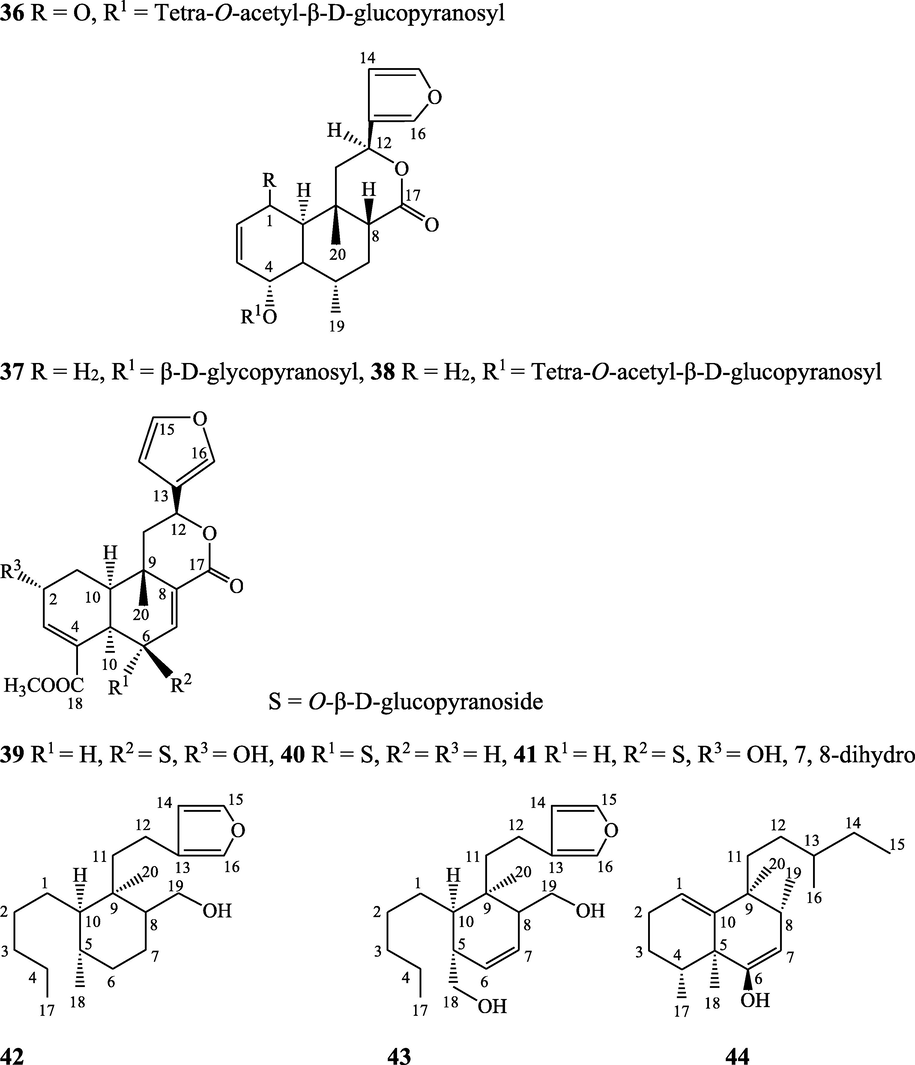
Clerodane furano diterpene glucosides - Amritoside A (26), amritoside A pentaacetate (27), amritoside B (28), amritoside B pentaacetate (29), amritoside C (30), amritoside C pentaacetate (31), amritoside D (32), amritoside D tetraacetate (33), tinosponone (34), tinosporaside (35), tinosporaside tetraacetate (36), tinocordioside (37), tinocordioside tetraacetate (38), tinoscorside C (39), borapetoside F (40), borapetoside B (41), tinosporafuranol (42), tinosporaclerodanol (43), tinosporafuradiol (44), tinosporaclerodanoid (45), cordifolide B (46), cordifolide C (47)
Maurya et al. (1995, 2004), Ahmad et al. (2010), Kiem et al. (2010), Pan et al. (2012)
Methanolic
Stem
Daucane-type sesquiterpene – Tinocordifolin (48), tinocordifolioside (49), tinocordifolioside tetraacetate (50)
Maurya et al. (1997), Maurya and Handa (1998)
Butanolic and methanolic
Stem
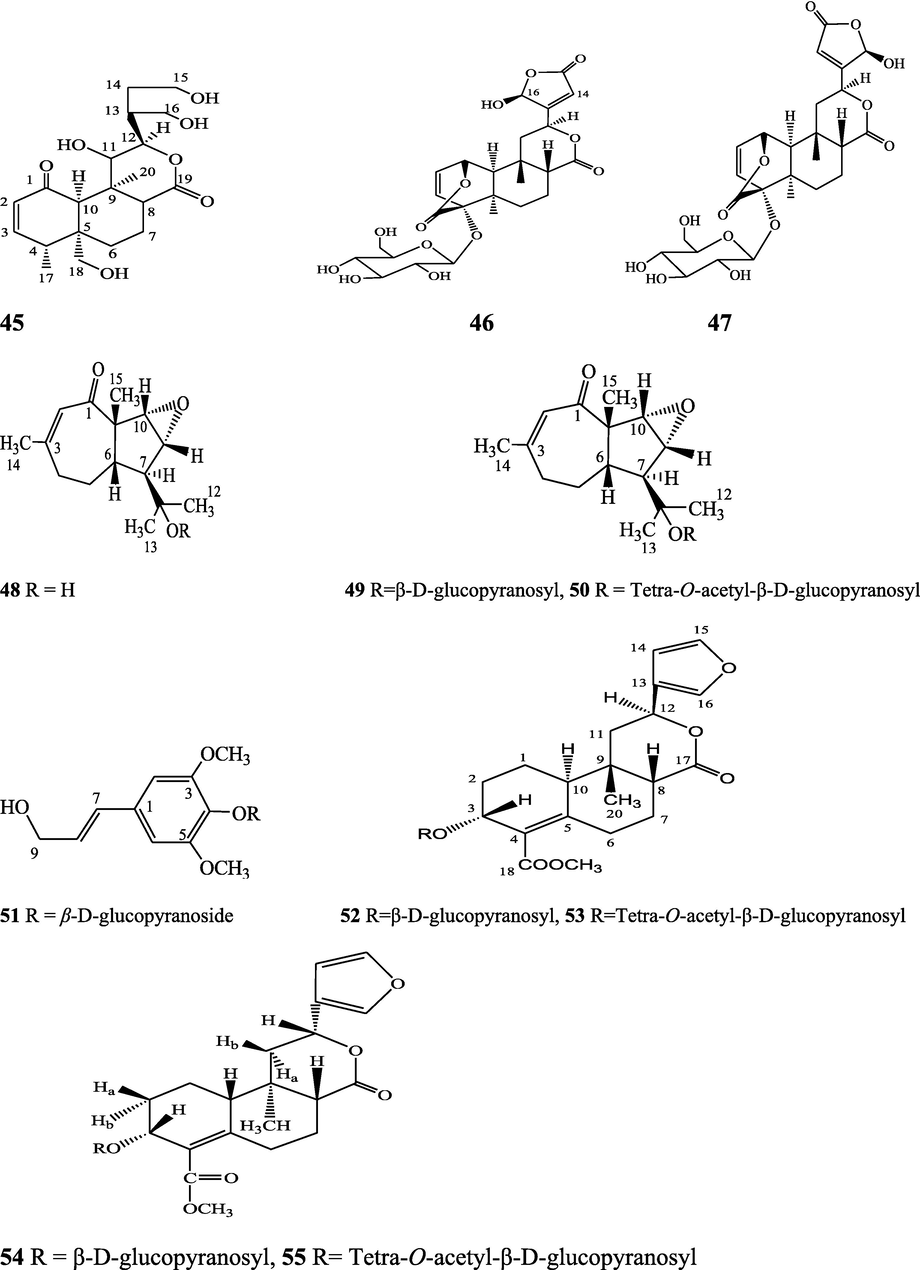
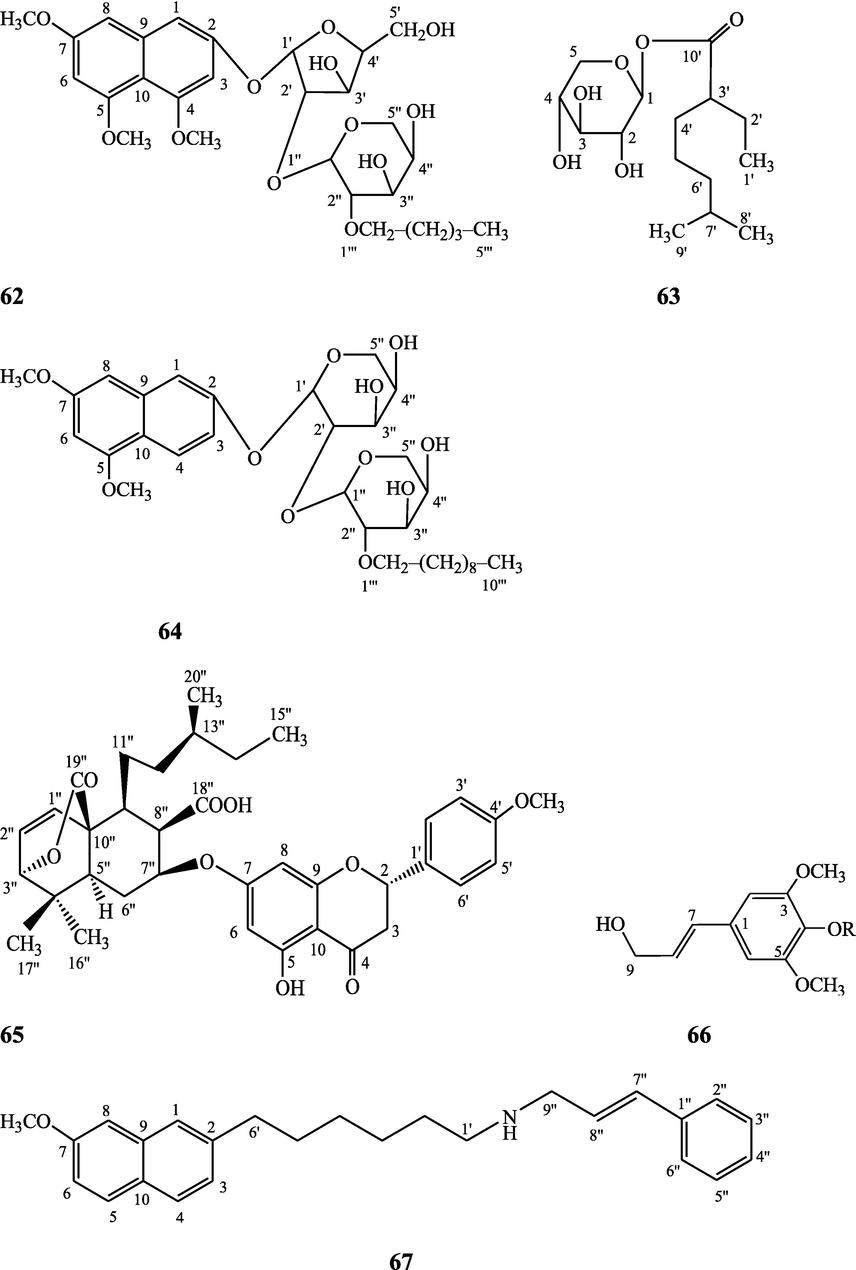
Norditerpene furan glycosides – Syringin (51), cordifoliside A (52), cordifoliside A tetraacetate (53), cordifoliside B (54), cordifoliside B tetraacetate (55), cordifoliside C (56), cordifoliside C tetraacetate (57), cordifoliside D (58), cordifoliside D tetraacetate (59), cordifoliside E (60), cordifoliside E tetraacetate (61), 4,5,7-trimethoxy-2-naphthol-2-O-α-L-arabinofuranosyl-(2′→1′′)-O-α-L-arabinopyranosyl-2′′-O-pentane (62), β-D-arabinosyl-O-geranilan-10′-oate (63), 5,7-dimethoxy-2-naphthol-2-O-α-L-arabino pyranosyl-(2′→1′′)-α-L-arabinopyranosyl-2′′-O-decane (64)
Gangan et al. (1994, 1995), Kiem et al. (2010) Sultana et al. (2017)
Methanolic
Stem
Diterpenic flavanone and phenylpropanoid - 5-hydroxy-4'-methoxy-7-flavanoxy-(7→7'')-β-O-labdan-1-en-3''α,19''-olide-18''-oic acid (65), tinoscorside D (66)
Kiem et al. 2010; Sultana et al. (2017)
Methanolic
Stem
Aromatic amides - Trans-cinnamoyl-2-n-hexanyl-7-methoxynaphthyl amide (67), trans-cinnamoyl-2-n-pentanyl-6,7-dimethoxynaphthyl amide (68), trans-cinnamoyl-2-n-octanyl-7-methoxynaphthyl amide (69)
Sultana et al. (2017)
Methanolic and aqueous-methanolic
Aerial parts
Ecdysteroids and steroids - Polypodine B 20, 22-acetonide (70), 20-p-hydroxyecdysone (71), β-sitosterol (72)
Pathak et al. (1995), Kiem et al. (2010), Sharma et al. (2018)
Chloroform
Whole plant
Diterpenoid furanolactone – Columbin (73)
Swaminathan et al. (1989)
T. crispa
Tropical and sub-tropical regions of India
Methanolic
Stem
Flavonoids – apigenin (74), diosmetin (75), genkwanin (76), luteolin 4′-methyl ether 7- glucoside (77), genkwanin 7-glucoside (78), rutin (79), and luteolin 4′-methyl ether 3′ -glucoside (80)
Umi Kalsom and Noor (1995), Harwoko and Warsinah (2020).
Methanolic
Whole plant
Diterpene and diterpene glucosides - borapetol A (81) and borapetol B (82)
Chung (2011)
Ethanolic
Stem
Diterpene and diterpene glucosides - Tinocrispol A (83), 6′-O-lactoylborapetoside B (84), 2-O-lactoylborapetoside B (85), columbin (73), borapetoside B (41), borapetoside D (86), borapetoside E (87), rumphioside B (88), syringin (51), crispene A (89), crispene B (90), crispene C (91), crispene D (92), crispene F (93) and crispene G (94)
Cavin et al. (1998), Choudhary et al. (2010b), Chung (2011) Lam et al. (2012) Hossen et al. (2016), Al Noman et al. (2018)
Ethanolic
Aerial parts and stem
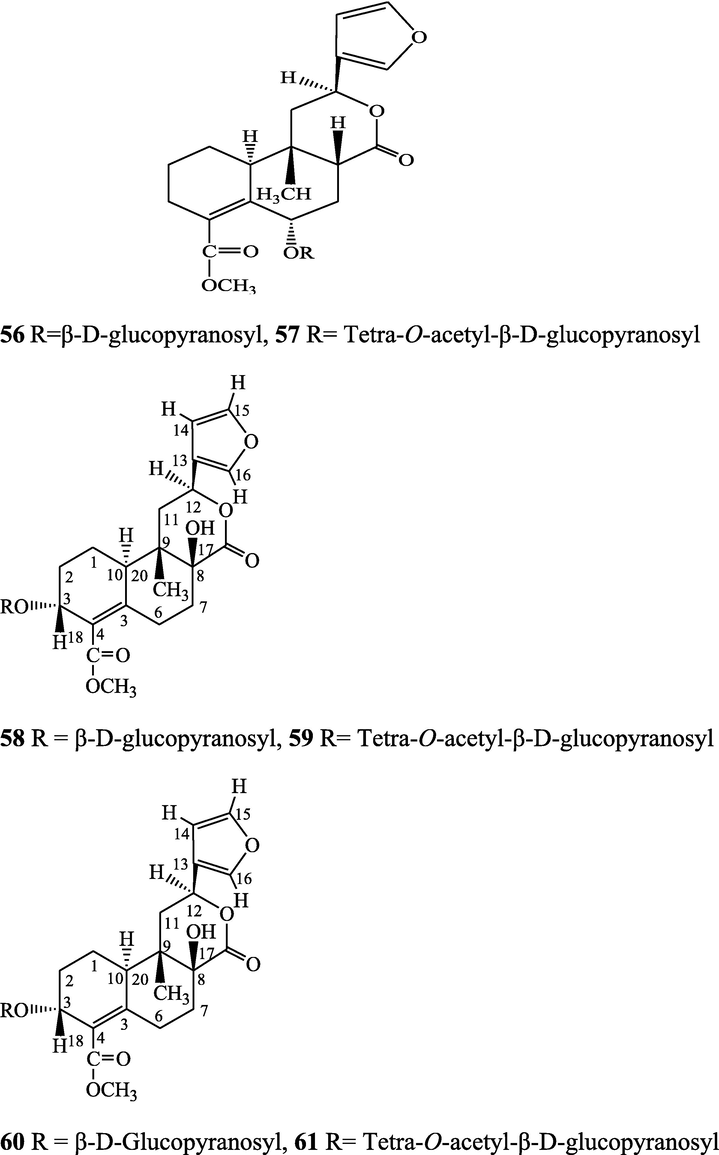
cis-Clerodane-type furanoditerpenoids - (3R,4R,5R,6S,8R,9S,10S,12S)-15,16-Epoxy-3,4-epoxy-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (95), (1R,4S,5R,8S,9R,10S,12S)-15,16-epoxy-4-O-(β-D-glucopyranosyl)-cleroda-2,13(16),14-triene-17(12),18(1)-diolide (96), (2R,5R,6R,8R,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (97), (5R,6R,8S,9R,10R,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (98), (2R,5R,6R,8S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-{β-D-glucopyranosyl-(1-6)α-D-xylopyranosyl}-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (99), (5R,6R,8S,9R,10S,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (100), (5R,6S,9S,10S,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,7,13(16),14-tetraen-17,12- olid-18-oic acid methyl ester (101), (2R,5R,6S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl)-cleroda-3,7,13(16),14-tetraen-17,12-olid-18-oic acid methyl ester (102), tinocrispide (103), baenzigeride A (1), (6S, 9 R)-vomifoliol (104), rumphiol E (105)
Choudhary et al. (2010b), Parveen et al. (2021)
Ethanolic/chloroform
Stem
Alkaloids - N-formylasimilobine 2-O-β-D-glucopyranoside (106), N-cis-feruloyltyramine (107), N-trans-feruloyltyramine (108), paprazine (109), N-trans-caffeoyltyramine (110), N-demethyl-N-formyldehydronornuciferine (111), N-formylasimilobine 2-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside (112), 4,13-dihydroxy-2,8,9-trimethoxydibenzo[a,g]quinolizinium (113), columbamine (114), dihydrodiscretamin (115), magnoflorine (18), N-formylnornuciferine (116), N-acetylnornuciferine (117), N-formylanonaine (118), N-acetylanonaine (119), tyramine (120), higenamine (121), salsolinol (122). (−)-litcubinine (123), and steponine (124)
Pachaly et al. (1992), Lin (2009), Choudhary et al. (2010a), Chung (2011), Praman et al. (2012), Yusoff et al. (2014),Parveen et al. (2021)
Ethanolic
Stem
Lignans, and sterols – Secoisolariciresinol (125), syringaresinol (126), campesterol (127), β-sitosterol (72), stigmasterol (128), makisterone C (129), gorgost-5-en-3-ol, (3β)- (130), lathosterol (131), ergost-7-en-3-ol (132), cholest-5-en-3-ol (3β)- (133), cholesta-5,22-dien-3-ol, (3β)-(134), ergosta-5,24(28)-dien-3-ol, (3β)- (135), desmosterol (136), 5,6-dihydroergosterol (137), lupeol (138), lup-20(29)-en-3-ol, acetate, (3β)-(139), 25-hydroxycholesterol, 3-methyl ether (140), and betulin (141)
Lin (2009), Chung (2011), Praman et al. (2012), Rakib et al. (2020c).
T. formanii
Western Ghats of Thrissur district, Kerala, India
-
-
-
-
T. glabra
Northeast region, Andaman and Nicobar Islands of India
-
-
-
-
T. maqsoodiana
Madhya Pradesh state of India
-
-
-
-
T. sinensis
Assam, Bihar, Orissa, Maharashtra, Andhra Pradesh, Karnataka, Kerala, and Tamil Nadu
Ethanolic
Stem
Lignans and its glucosides - Lirioresino-β-dimethyl ether (142), tinosposide A (143), tinosposide B (144), tanegoside (145), (+)-pinoresinol O-β-D-glucopyranoside (146), (+)-pinoresinol monomethyl ether O-β-D-glucopyranoside (147), (+)-syringaresinol O-β-D-glucopyranoside (148), (-)-isolariciresinol 3α-O-β-D-glucopyranoside (149), icariside D1 (150), 4-allyl-2-methoxyphenyl 6-O-β-D-apiofuranosyl (1→6)-β-D-glucopyranoside (151), tinosporide A (152), sesamin (153), sesamolin (154), medioresinol (155), syringaresinol (126), (+)-epi-syringaresinol (156), (+)-glaberide I (157)
Li et al., (2004a, 2004b), Maurya et al. (2009), Lam et al. (2018)
Methanolic
Stem
Sesquiterpene glucosides - Tinosinenoside G (158), tinosinenoside H (159), tinosinenside (160)
Li et al. (2007), Jiang et al. (2018)
Ethanolic
Stem
Clerodane diterpene glycosides - Tinosposinenside A (161), tinosposinenside B (162), tinosposinenside C (163), 1-deacetyltinosposide A (164)
Li et al. (2007), Dong et al. (2010)
Methanolic
Stem
Phenolic glycoside – Tinosinen (165)
Yonemitsu et al. (1993)
Ethanolic
Stem
Phenylpropanoid glycosides - Tinosinenoside I (166), tinosinenoside J (167)
Diterpenoid glucosides - Tinosinenoside K (168), 4-epi-2-deacetoxytinosinenoside D (169), tinosinenoside A (170), tinosinenoside B (171), borapetoside A (172), borapetoside B (41), borapetoside C (173), borapetoside H (174), borapetoside G (175), rumphioside A (176), rumphioside D (177), rumphioside F (178), rumphioside I (179), cordifolide A (24), tinocrisposide (180), 6’-O-lactoylborapetoside B (181), sagittatayunnanoside A (182), sagittatayunnanoside B (183), sagittatayunnanoside C (184), tinosponone (34), tinotufolin C (185), tinotufolin D (186)
Xu et al. (2017a, 2017b), Jiang et al. (2017) Xu et al. (2017a, 2017b), Jiang et al. (2018)
Methanolic
Stem
Alkaloids – Berberine (19), 3-hydroxy-2,9,11-trimethoxy-5,6-dihydro isoquino[3,2-a]isoquinolinylium (187), palmatine (15), jatrorrhizine (17), palmatrubin (188), tinosporin A (189), methyl 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoate (190), methyl 4-[formyl5-(methoxymethyl)-1H-pyrrol-1-yl] butanoate (191), 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid (192), 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]butanoic acid (193)
Srinivasan et al. (2008), Maurya et al. (2009), Lam et al. (2018)
Methanolic
Stem
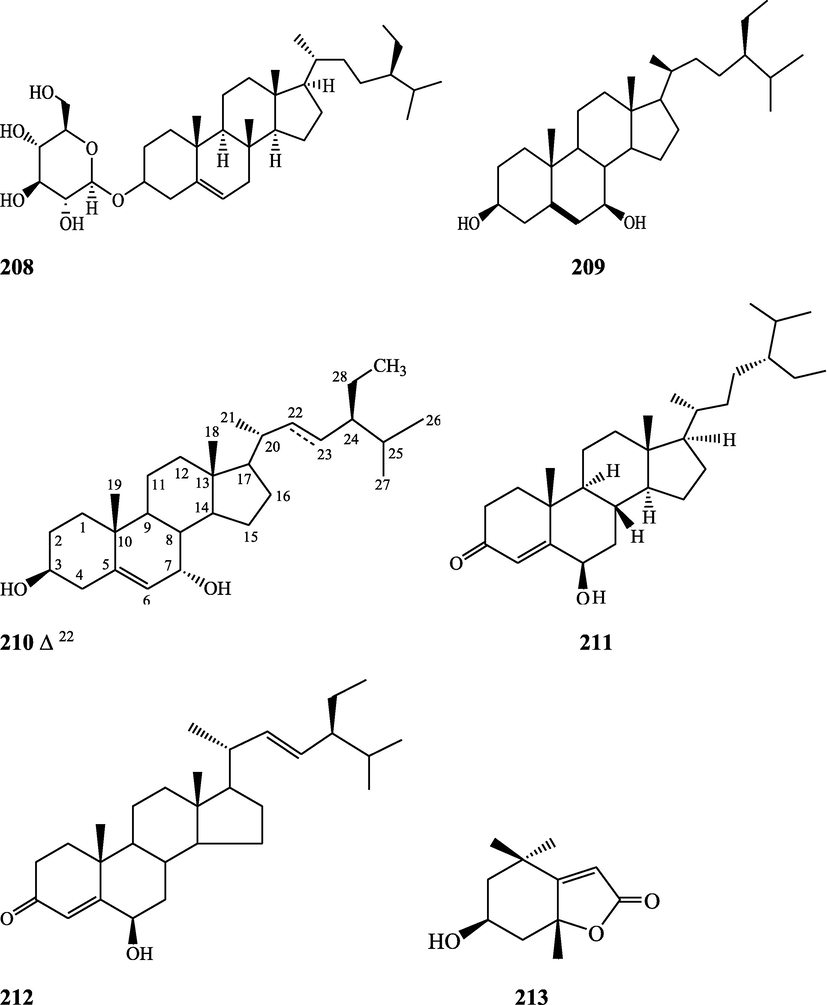
Benzoids – Rhodiolate (194), tinosporin B (195), methyl ferulate (196), β-hydroxypropiovanillone (197), tachioside (198), icariside D2 (199), salidroside (200), vanillic acid (201), syringic acid (202), p-hydroxybenzoic acid (203), 4-(2-hydroxyethyl) benzoic acid (204), isovanillic acid (205), cordifolioside A (206)
Lam et al. (2018)
Ethanolic
Stem
Unsaturated carboxylic acid ester - 4-methyl-heptadec-6-enoic acid ethyl ester (207)
Maurya et al. (2009)
Ethanolic and methanolic
Stem
Steroids - β-sitosterol (72), daucosterol (208), 7α-hydroxysitosterol (209), 7α-hydroxystigmasterol (210), 6β-hydroxystigmast-4-en-3-one (211), 6β-hydroxystigmasta-4,22-dien-3-one (212)
Maurya et al. (2009), Dong et al. (2010), Lam et al. (2018)
Methanolic
Stem
Terpenes – Loliolide (213), abscisic acid (214), 3-O-acetyloleanolic acid (215), lupeol (138), cycloeucalenol (216), cycloartane-3β,25-diol (217), cycloart-22-ene-3β,25-diol (218), malabarolide (219)
Lam et al. (2018)
Methanolic
Stem
Amides - N-cis-feruloyltyramine (107), N-trans-feruloyltyramine (108), N-trans-feruloyldopamine (220)
Lam et al. (2018)
Methanolic
Stem
Coumarin – Scopoletin (221)
Lam et al. (2018)
Methanolic
Stem
Xanthone – Lichexanthone (222)
Lam et al. (2018)
T. smilacina
Western Ghats of Tamil Nadu, India
-
-
-
-
T. subcordata
Khandwa district, Madhya Pradesh state of India
-
-
-
-
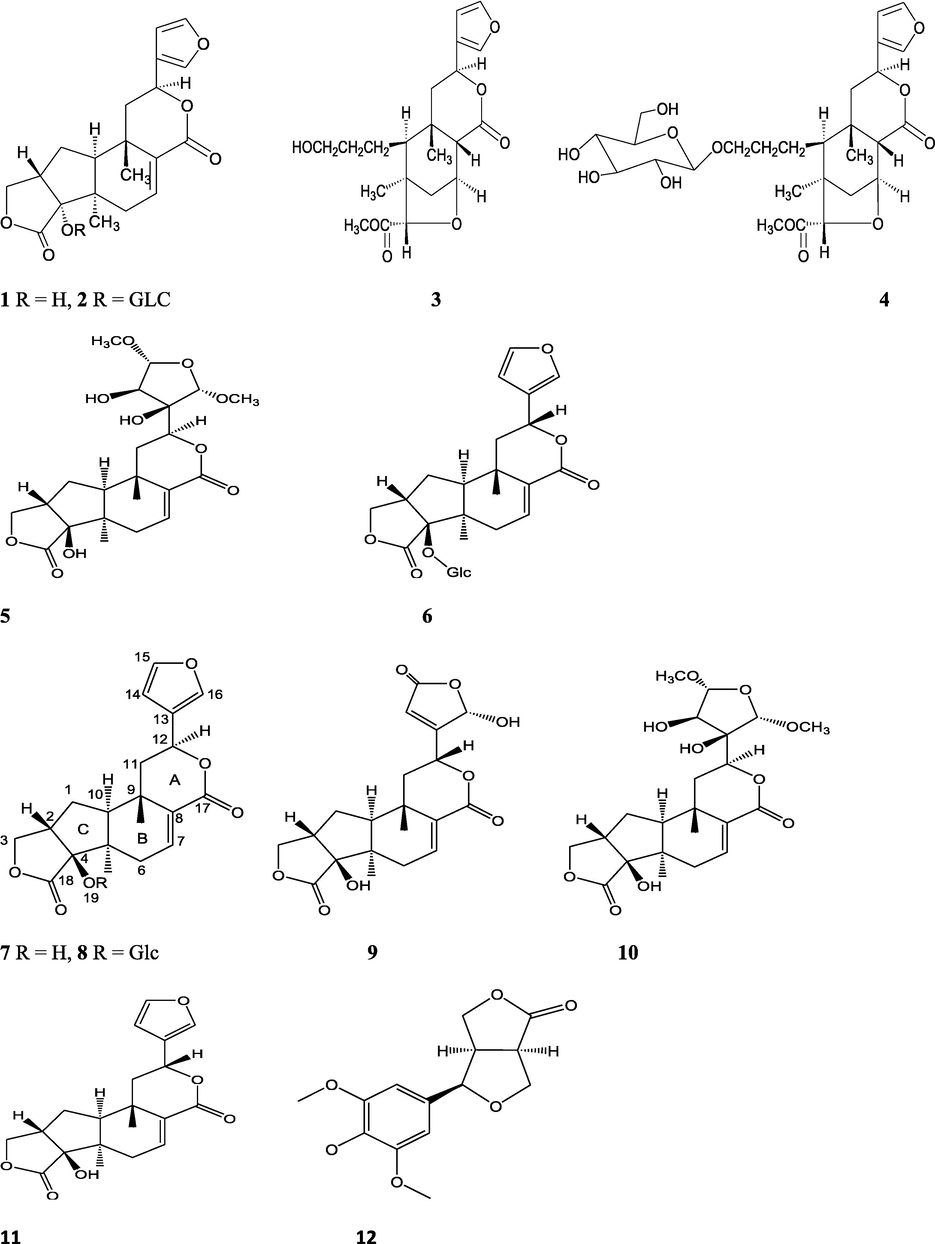
Structures of isolated compounds from Indian Tinospora species.
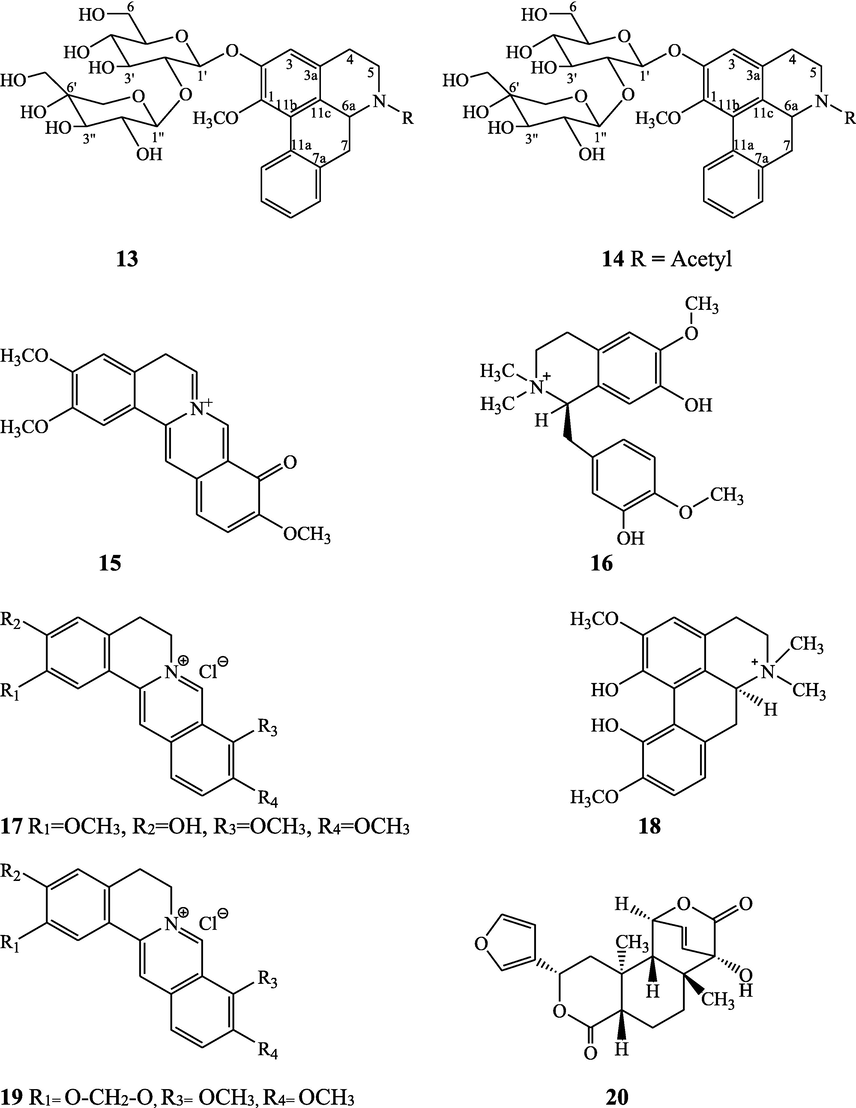
Structures of isolated compounds from Indian Tinospora species.
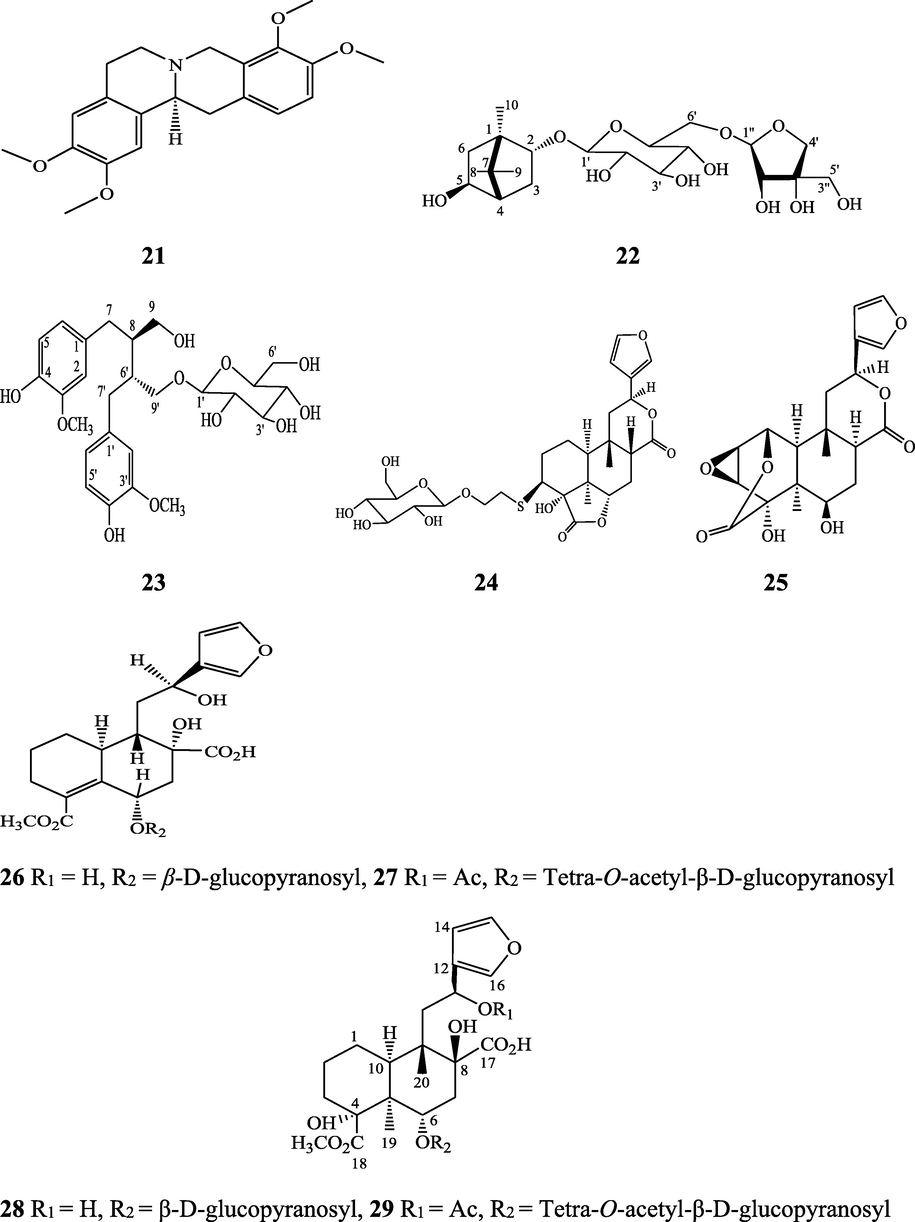
Structures of isolated compounds from Indian Tinospora species.
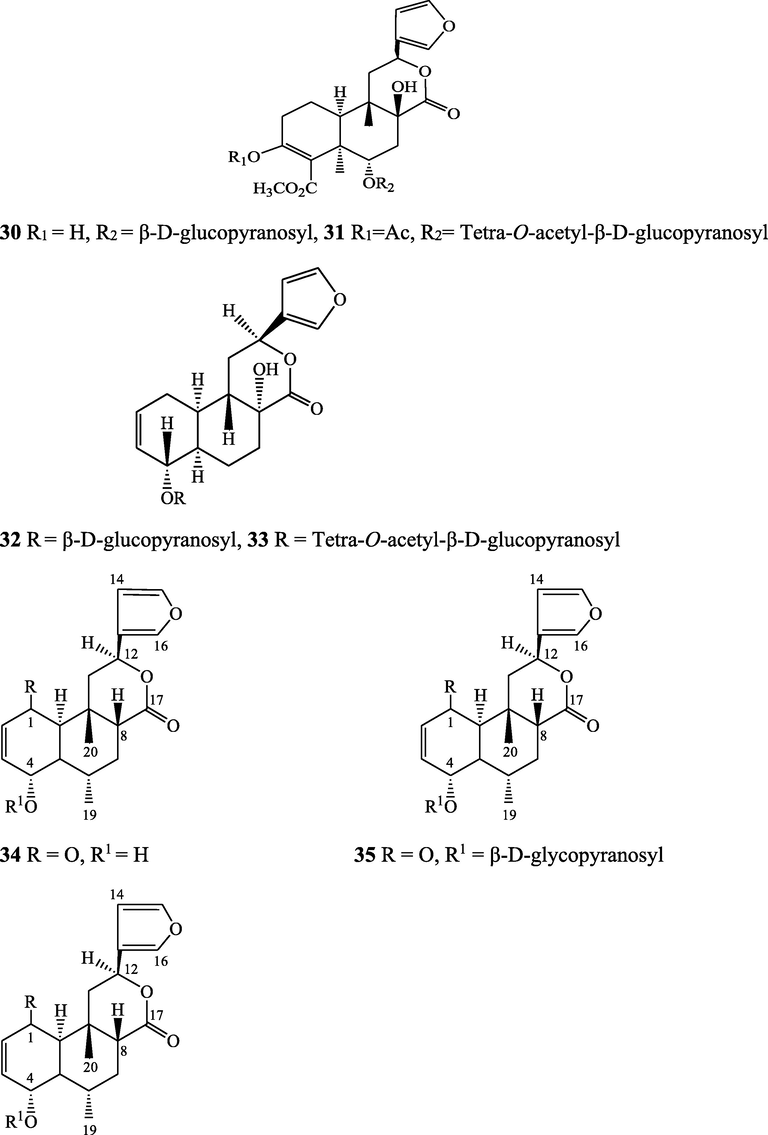
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
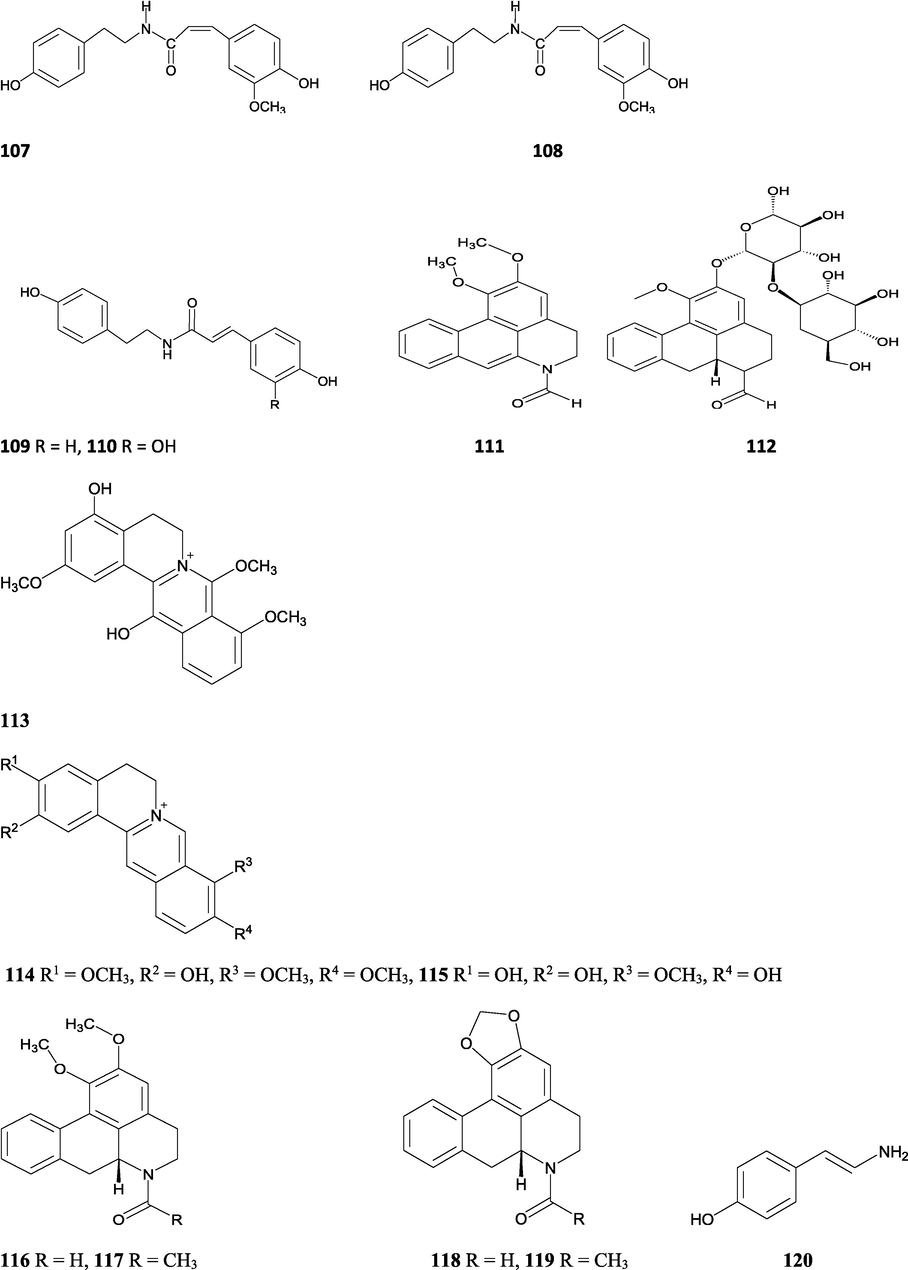
Structures of isolated compounds from Indian Tinospora species.
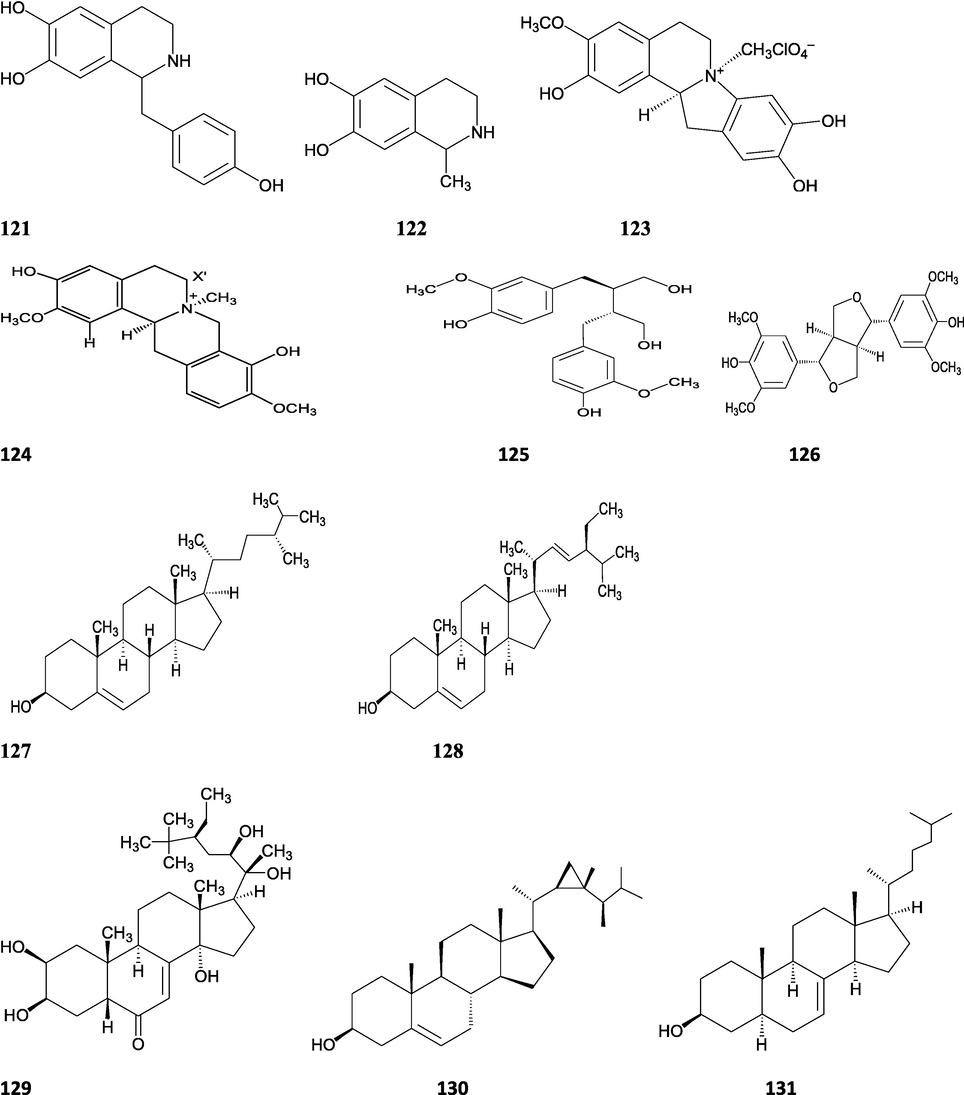
Structures of isolated compounds from Indian Tinospora species.
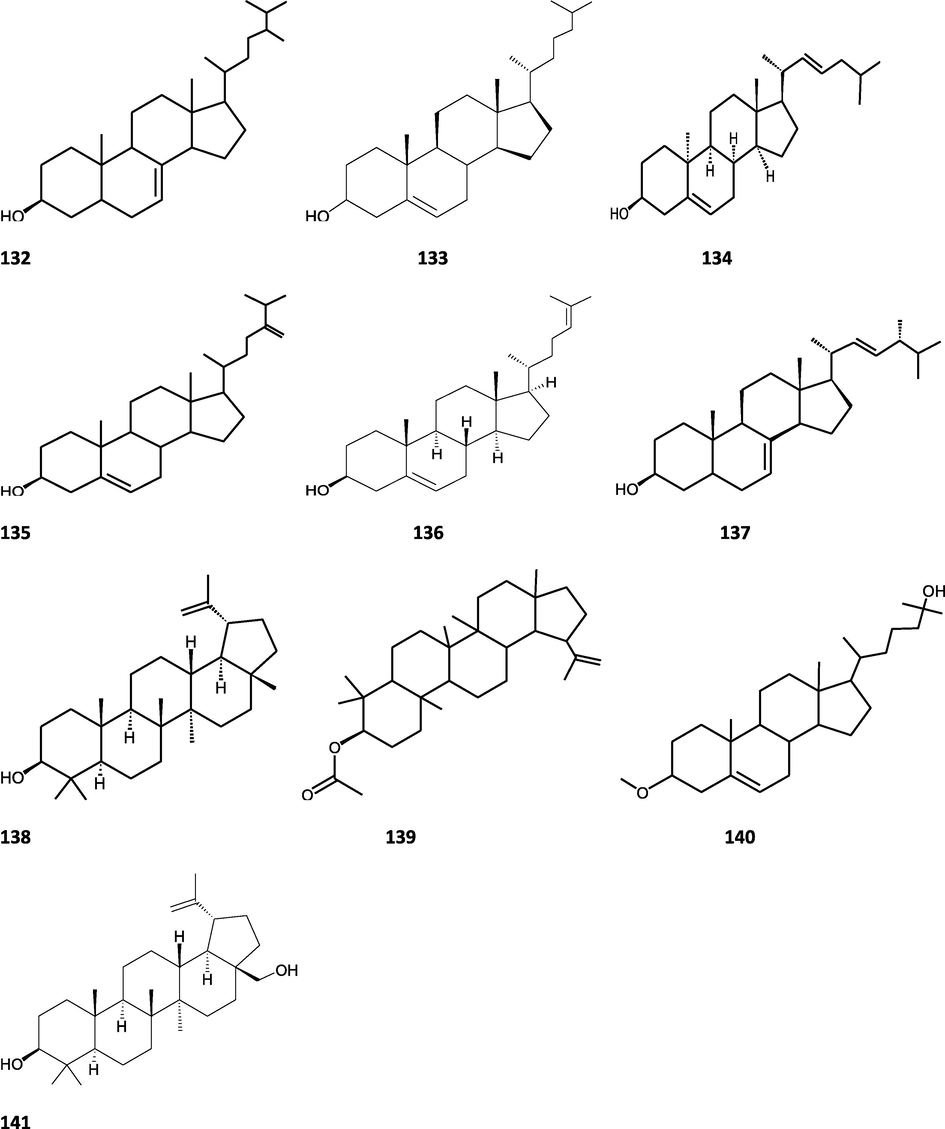
Structures of isolated compounds from Indian Tinospora species.
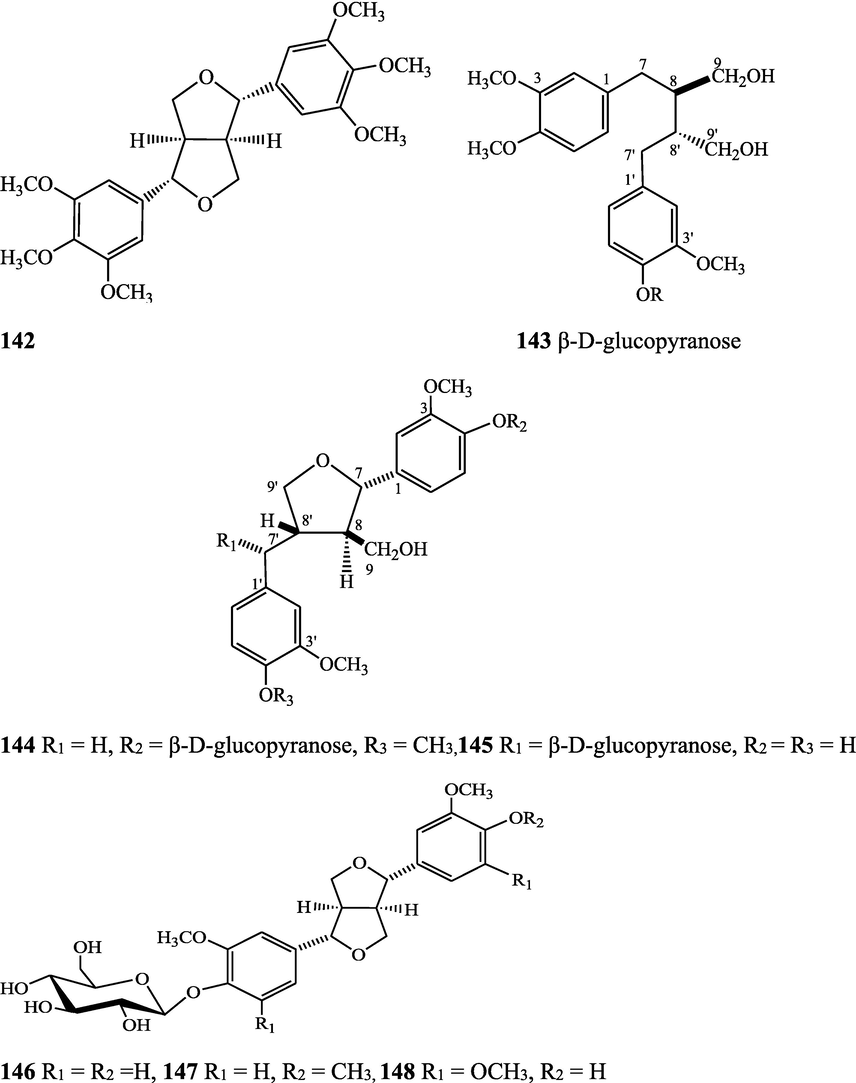
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
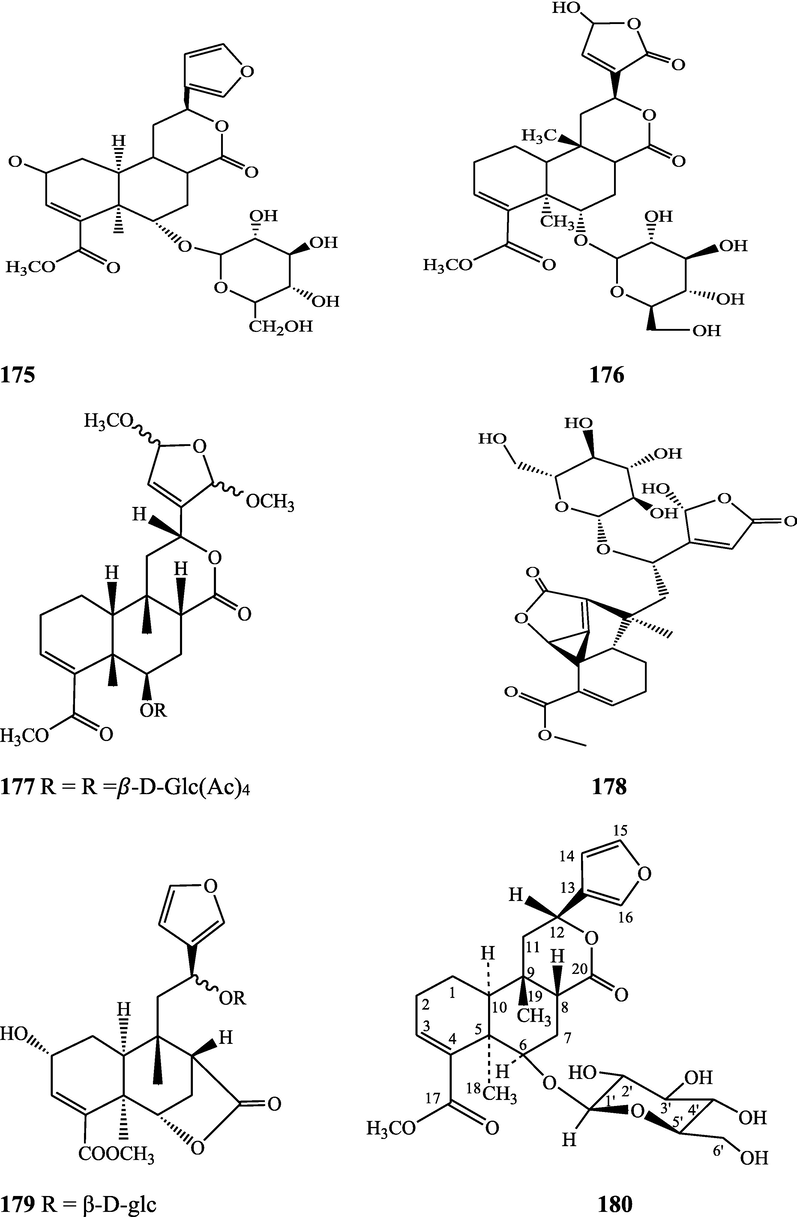
Structures of isolated compounds from Indian Tinospora species.
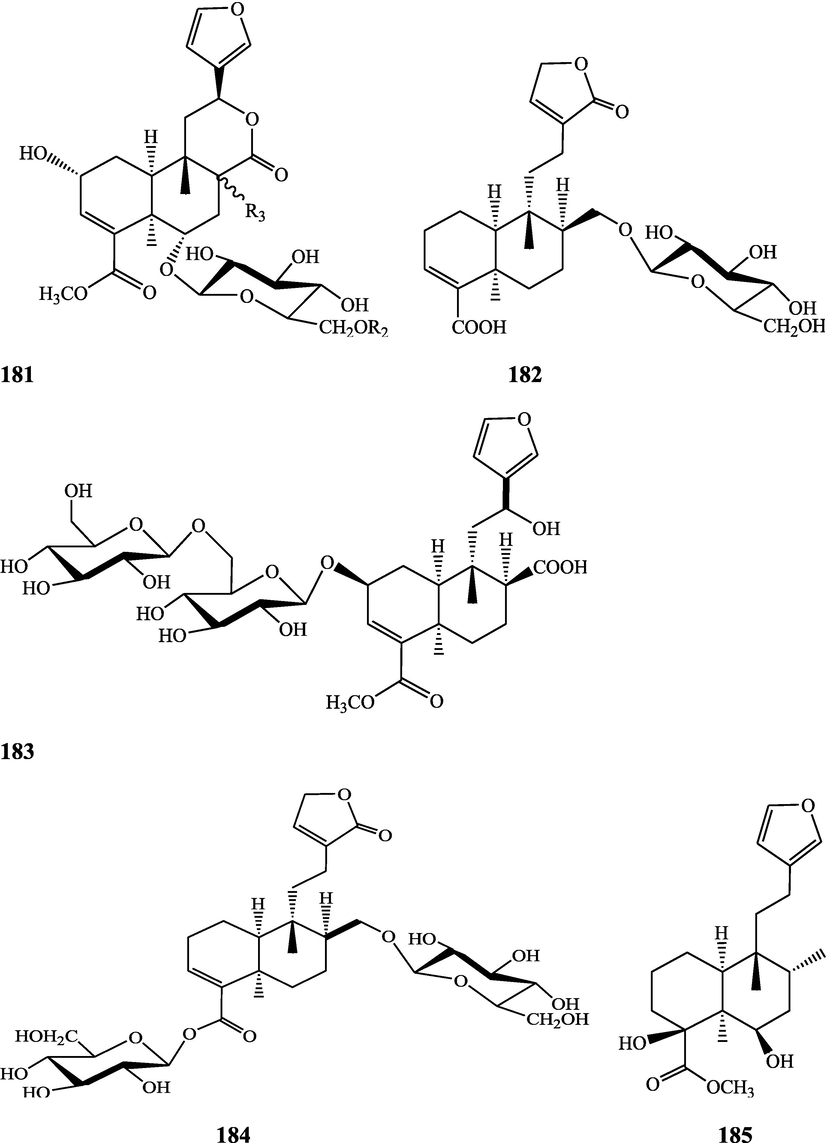
Structures of isolated compounds from Indian Tinospora species.
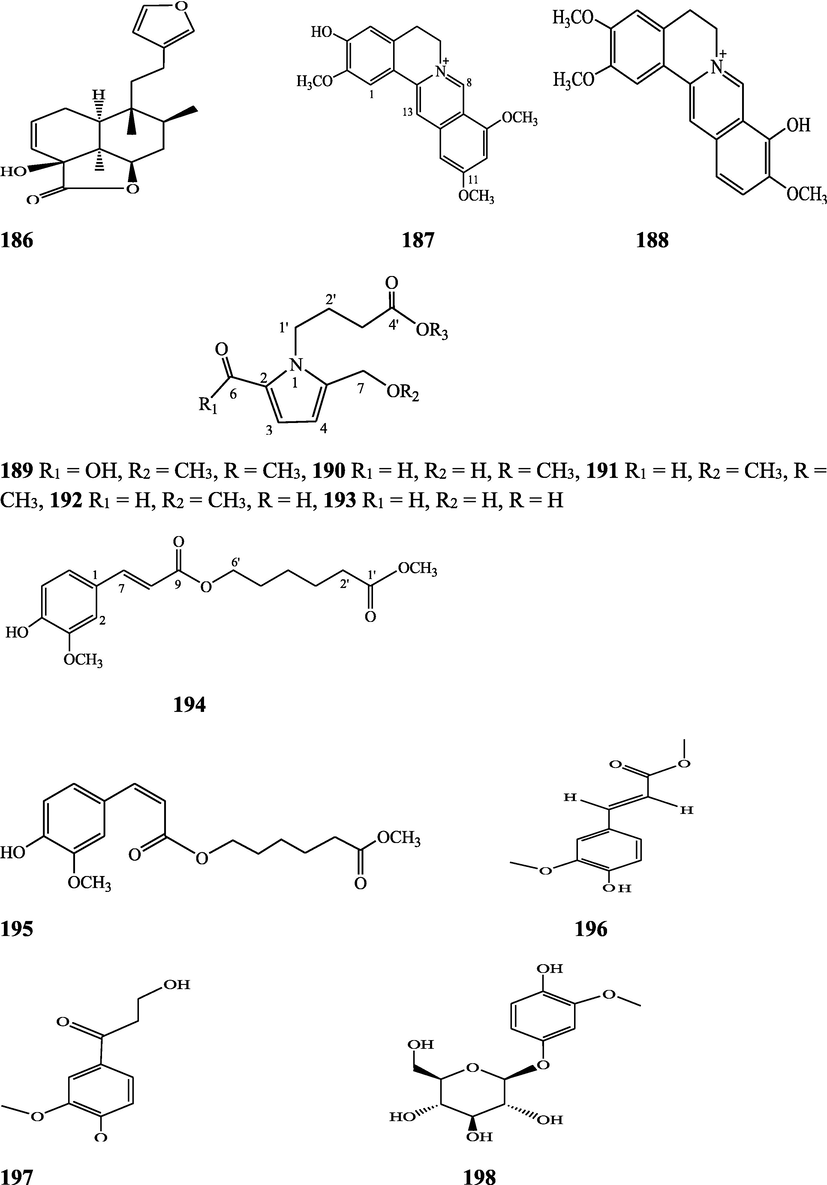
Structures of isolated compounds from Indian Tinospora species.
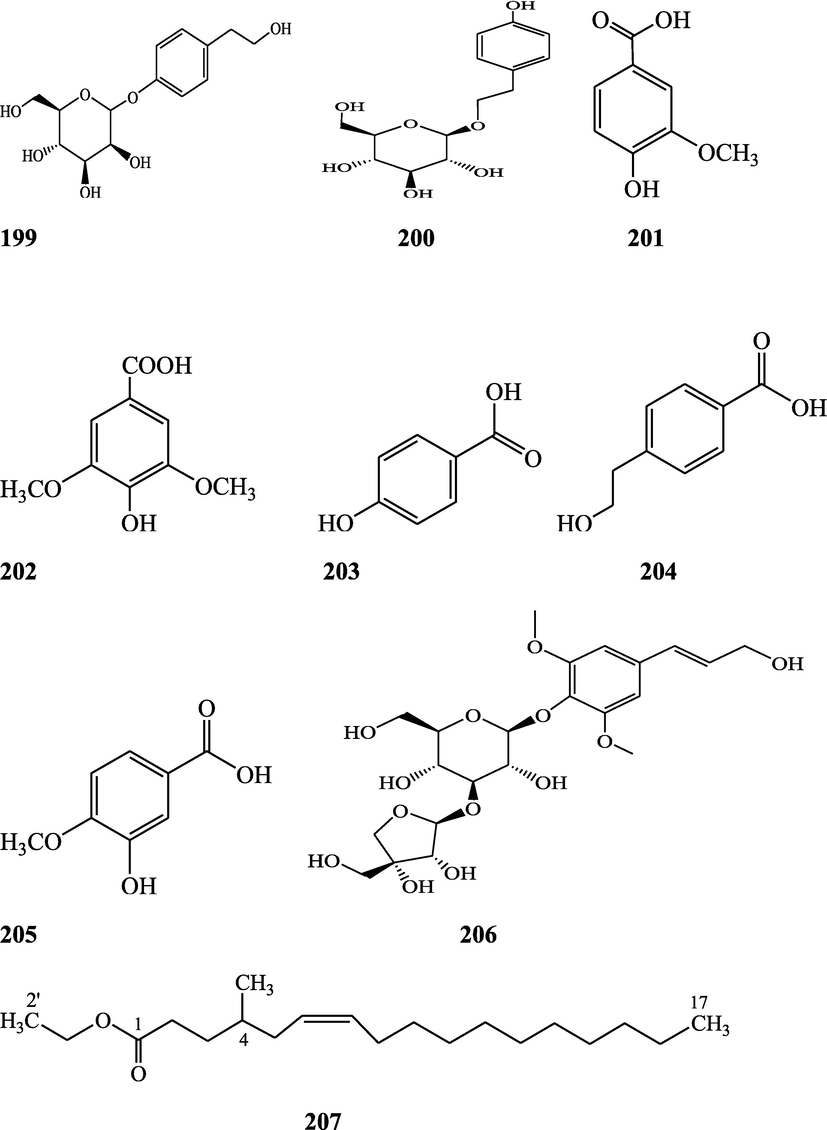
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Structures of isolated compounds from Indian Tinospora species.
Clerodane diterpenes are a large group of plant-derived secondary products found in thousands of plant species from many plant families. During the last few decades, more than 1300 diterpenoids and nor-diterpenoids with the clerodane carbon skeleton have been identified from different plant species (Li et al., 2016). Some clerodane diterpenes possess anti-inflammatory, antiparasitic, antifungal, antibacterial, antitumor, opioid receptor agonist, nerve growth factor-potentiating, anti-ulcer, cytotoxic, and antimicrobial activities (Hagiwara, 2019). The Baenzigeride A, and baenzigeroside A (Tuntiwachwuttikul et al., 1999), baenzigeride B and baenzigeroside B (Tuntiwachwuttikul and Taylor, 2001), tinobaenzigeride and tinobaenzigeroside A have been identified from T. baenzigeri stem. The isolated compounds (baenzigeride B and baenzigeroside B) showed cytotoxicity against Hep-G2 and MCF-7 cancer cells (IC50 25 Μm; Pudhom et al., 2019; Hanthanong et al., 2019, 2021). The amritoside A, amritoside A pentaacetate, amritoside B, amritoside B pentaacetate, amritoside C, amritoside C pentaacetate, amritoside D, amritoside D tetraacetate (Maurya et al., 1995, 2004), tinosponone, tinosporaside, tinosporaside tetraacetate, tinocordioside, tinocordioside tetraacetate (Iqbal et al., 2005; Puratchimani and Jha, 2007), tinoscorside C, borapetoside F, borapetoside B, cordifolide A (Kiem et al., 2010; Pan et al., 2012), tinosporafuranol, tinosporaclerodanol, tinosporafuradiol, tinosporaclerodanoid (Ahmad et al., 2010; Phan et al., 2010), cordioside (Wazir et al., 1995) from stem and stem bark (Kumar et al., 2019), syringin (Kiem et al., 2010), cordifoliside A, cordifoliside A tetraacetate, cordifoliside B, cordifoliside B tetraacetate, cordifoliside C, cordifoliside C tetraacetate, cordifoliside D, cordifoliside D tetraacetate, cordifoliside E, cordifoliside E tetraacetate; (Gangan et al., 1994, 1995; Sharma et al., 2018, 2019), 4,5,7-trimethoxy-2-naphthol-2-O-α-L-arabinofuranosyl-(2′→1′′)-O-α-L-arabinopyranosyl-2′′-O-pentane, β-D-arabinosyl-O-geranilan-10′-oate, 5,7-dimethoxy-2-naphthol-2-O-α-L-arabinopyranosyl-(2′→1′′)-α-L-arabinopyranosyl-2′′-O-decane (Sultana et al., 2017) have been isolated and characterized from T. cordifolia stem (Kattupalli et al., 2019). The tinocrispol A, 6′-O-lactoylborapetoside B, columbin, 2-O-lactoylborapetoside B (Lam et al., 2012) and borapetosides A-E (Chung, 2011) and borapetoside G and H (Choudhary et al., 2010b; Lam et al., 2012), rumphiosides A and B (Chung, 2011), syringin (Cavin et al., 1998; Chung, 2011), crispene A, B, C, and D (Hossen et al., 2016), crispenes F and G from stem (Al Noman et al., 2018), borapetol A and B were reported from T. crispa whole plant (Chung, 2011). Several clerodane-type furanoditerpenoids {(3R,4R,5R,6S,8R,9S,10S,12S)-15,16-Epoxy-3,4-epoxy-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester, rumphiol E, (5R,6R,8S,9R,10S,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester, (5R,6S,9S,10S,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,7,13(16),14-tetraen-17,12- olid-18-oic acid methyl ester, (2R,5R,6S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl)-cleroda-3,7,13(16),14-tetraen-17,12-olid-18-oic acid methyl ester} from aerial parts (Choudhary et al., 2010b), baenzigeride A, (6S, 9 R)-vomifoliol and steponine were reported from stem of T. crispa (Parveen et al., 2021).
Diterpenoids are secondary products containing 20 atoms of carbon derived from the condensation of four isoprenyl units, widely found in the plant kingdom, and biosynthetically derived from geranylgeranyl diphosphate. They are found in several forms (Sandjo and Kuete, 2013) such as acyclic (phytanes), bicyclic (labdanes, halimanes, clerodanes), tricyclic (pimaranes, abietanes, cassanes, rosanes, vouacapanes, podocarpanes), tetracyclic (trachylobanes, kauranes, aphidicolanes, stemodanes, stemaranes, atisanes, gibberellanes), and macrocyclic diterpenes (taxanes, cembranes, daphnanes, tiglianes, ingenanes). Clerodane diterpenoids have been found in several families of plants (Verbenaceae and Lamiaceae) and possess potent anti-inflammatory and analgesic activities (Gonzalez-Coloma, 2010; Marrero et al., 2010). The tinosinenoside K, 4-epi-2-deacetoxytinosinenoside D, tinosinenoside A, tinosinenoside B, borapetoside A, borapetoside B, borapetoside C, borapetoside G, borapetoside H, rumphioside A, rumphioside D, rumphioside F, rumphioside I, cordifolide A, tinocrisposide, 6′-O-lactoylborapetoside B, sagittatayunnanoside A, sagittatayunnanoside B, sagittatayunnanoside C, tinosponone were isolated and characterized from T. sinensis stem (Jiang et al., 2017, 2018; Xu et al., 2017a, 2017b).
Sesquiterpenes consist of three isoprene units (C15H24) with acyclic rings. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids (Davis and Croteau, 2000). Cyclic sesquiterpenes are more common than cyclic monoterpenes because of the increased chain length and additional double bond in the sesquiterpene precursors (Chizzola, 2013). The plant-derived sesquiterpenoids possess antitumor, antiinflammatory, antibacterial and antiviral activities (Salazar-Gómez et al., 2020; Jiang et al., 2021). The tinocordifolin, tinocordifolioside, and tinocordifolioside tetraacetate were isolated and identified from the stem of this plant species (Maurya et al., 1997; Maurya and Handa, 1998). The trans-cinnamoyl-2-n-hexanyl-7-methoxynaphthyl amide, trans-cinnamoyl-2-n-pentanyl-6,7-dimethoxynaphthyl amide, trans-cinnamoyl-2-n-octanyl-7-methoxynaphthyl amide were identified from T. cordifolia stem (Sultana et al., 2017). More than 300 benzoid compounds have been identified from plants which include methylbenzoate, methylsalicylate, phenylacetaldehyde, phenylethyl acetate, benzyl acetate, phenylethanol, eugenol, and isoeugenol (Knudsen and Gershenzon, 2006). The rhodiolate, methyl ferulate, β-hydroxypropiovanillone, 2-methyl-4,5-dimethoxybenzoic acid, vanillic acid, p-hydroxyl phenethanol, tachioside, icariside D2, salidroside, syringin, cordifolioside A, p-hydroxybenzoic acid, 4-(2-hydroxyethyl) benzoic acid, syringic acid-4-O-α-L-rhamnoside, isovanillic acid, syringic acid have been characterized from T. sinensis (Lam et al., 2018). The loliolide, abscisic acid, 3(17)-phytene 1,2-diol, malabarolide, lupeol, 3-O-acetyloleanolic acid, cycloeucalenol, cycloabyssinone, cycloartane-3β,25-diol, cycloart-22-ene-3β,25-diol, β-sitosterol, stigmasterol, 7α-hydroxysitosterol, 7α-hydroxystigmasterol, 6β-hydroxystigmast-4-en-3-one, 6β-hydroxystigmasta-4,22-dien-3-one, and 7-ketosterol have been isolated from the aerial parts of T. sinensis (Lam et al., 2018).
Alkaloids are a large group of naturally occurring secondary products which contain nitrogen atom(s) in their structures. These nitrogen atoms are usually situated in cyclic ring system (Kurek, 2019). Based on the cyclic ring system, alkaloids can be grouped into several classes such as indoles, acridines, quinolines, isoquinolines, pyrrolidines, pyridines, pyrrolizidines, quinazolines and tropanes. Alkaloids are usually bitter, colorless, coloured (sanguinarine, berberine), odorless crystalline solids, but sometimes they can be yellowish liquids (nicotine). Nearly more than 3000 alkaloids have been investigated in different 4000 plant species (Frédérich et al., 2002; Ge et al., 2015; Gaziano et al., 2016). Several alkaloids (catharanthine, indicine-N-oxide, vincamine, vincristine, ajmalicine, vinblastine, strychnine, quinine, and ajmaline) possess anticancer, antimalarial, anti-inflammatory, and antimicrobial activities (Thawabteh et al., 2019). The tinoscorside A and B (Kiem et al. 2010), jatrorrhizine, palmatine, magnoflorine (Chintalwar et al., 2003; Sarma et al., 2009; Bala et al., 2015b), berberine, isocolumbin, tembetarine (Srinivasan et al., 2008; Reddy and Reddy, 2015) have been reported from aerial parts and stem of this plant species. The angelicoidenol 2-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranoside, secoisolariciresinol-9ʹ-O-β-D-glucopyranoside, cordifoliosides A and B have been identified from T. cordifolia aerial parts (Maurya et al., 1996; Kiem et al., 2010). The N-formylasimilobine 2-O-β-D-glucopyranoside, N-trans-feruloyltyramine, paprazine, N-demethyl-N-formyldehydronornuciferine, N-formylasimilobine 2-O-β-D-glucopyranosyl-(1 → 2)-β-D-glucopyranoside, 4,13-dihydroxy-2,8,9-trimethoxydibenzo[a,g]quinolizinium, columbamine, magnoflorine, dihydrodiscretamin (Choudhary et al., 2010a; Yusoff et al., 2014), N-formylnornuciferine, N-acetylnornuciferine, N-cis-feruloyltyramine (Pachaly et al., 1992; Chung, 2011), N-trans-caffeoyltyramine, N-formylanonaine, N-acetylanonaine (Lin 2009), tyramine, higenamine, salsolinol and (−)-litcubinine from have been isolated and characterized from T. crispa stem (Praman et al., 2012).
Pyrrole alkaloids are heterocyclic organic compounds synthesized by plants and found in Asteraceae, Boraginaceae, Heliotropiaceae, Apocynaceae, Orchidaceae and the Fabaceae families. Approximately 100 pyrrole alkaloids have been recognized and possess toxic hepatotoxic effects (Schramm et al., 2019). The 5-(hydroxymethyl)-1H-pyrrole-2-carbaldehyde, methyl 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoate, methyl 4-[formyl 5-(methoxymethyl)-1H-pyrrol-1yl]butanoate, 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid, and 4-[formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoic acid have been obtained from T. sinensis (Lam et al., 2018).
Flavonoids are medicinally important secondary metabolites and are synthesized via the phenylpropanoid pathway, converting phenylalanine into 4-coumaroylCoA, which gets in the flavonoid biosynthesis pathway (Falcone Ferreyra et al., 2012). These are hydroxylated phenolic compounds and are known to be produced by the plants in response to microbial infection (Kumar et al., 2013a, 2013b). Activities of human protective enzymes are induced by the plant-derived flavonoids. Various studies have suggested the protective effects of flavonoids against bacterial and viral infections and also against cardiovascular diseases, cancers, and other age-related disorders (Rice-Evans et al. 1995; Cook and Samman, 1996; Kumar et al., 2013). Total six compounds (apigenin, diosmetin, genkwanin, luteolin 4′-methyl ether 7- glucoside, genkwanin 7-lucoside, and luteolin 4′-methyl ether 3′ -glucoside (Umi Kalsom and Noor, 1995), and rutin have been identified from T. crsipa stem (Harwoko and Warsinah, 2020).
Phytoecdysteroids are natural polyhydroxylated constituents that contain a four-ringed skeleton, usually made up of either 27 carbon atoms or 28–29 carbon atoms (Dini, 2020). These compounds are derived from S‐squalene‐2,3‐epoxide via acetate‐mevalonate pathway and possess hypocholesterolemic activity (Tarkowská, 2019). Phytoecdysteroid-like compounds are found in various families of angiosperms. These compounds accumulate in various plant organs, viz, fruits, seeds, flowers, anthers, leaves, and roots. Phytoecdysteroids (Ajuga decumbens) demonstrated significant inhibitory effects on early induction and potent antitumor-promoting activities of Epstein–Barr virus on a mouse skin. Besides anticancer properties, phytoecdysteroids are also used as nutraceutical additives in food products (Bajguz et al., 2015; Saleem and Nazir, 2015). Phytoecdysteroid-like compounds possess antidiabetic, growth-promoting, hepatoprotective, immunoprotective, antioxidant, hypoglycemic, performance-enhancing, anti-osteoporotic, wound healing properties (Adki et al., 2020; Laddha et al., 2020). The polypodine B 20, 22-acetonide, 20-p-hydroxyecdysone, β-sitosterol, stigmasterol and campesterol were isolated and identified from aerial parts of T. cordifolia (Pathak et al., 1995; Kiem et al., 2010).
Lignans are a class of diphenolic constituents found in the bran layer of grain of cereals. Wheat bran contains a secoisolariciresinol diglucoside (a major compound); it is converted by intestinal microflora to enterodiol and enterolactone. Lignan are considered as a potent antioxidants and free radical scavengers, leading to decrease in risk of cancer development (Higuchi, 2014). On the basis of cyclization patterns and the way in which oxygen is incorporated into the skeleton, the lignans are divided into eight sub-groups - furofuran, furan, dibenzylbutane, dibenzylbutyrolactone, aryltetralin, arylnaphthalene, dibenzocyclooctadiene, and dibenzylbutyrolactol (Tsopmo et al., 2013). Secoisolariciresinol, syringaresinol from stem (Chung, 2011), adenosine, uridine and adenine from stem (Praman et al., 2012), benzeneethanamine, camphenol, strophanthidin, retinal, trans-geranylgeraniol, 3,4-dihydroxymandelic acid, imidazolidin-4-one, 2-imino-1-(4-methoxy-6-dimethylamino-1,3,5-triazin-2-yl), cholest-22-ene-21-ol, 3,5-dehydro-6-methoxy, δ-mannitol, 1-O-(16-hydroxyhexadecyl)-, heneicosanoic acid, methyl ester, gorgost-5-en-3-ol, (3β)-, TMS derivative, retinol, octacosanol, α-santalol, santalol, E-cis,epi-β, spiro[4,5]dec-6-en-1-ol, 2,6,10,10-tetramethyl were separated and identified from T. crispa whole plant (Lin, 2009; Rakib et al., 2020c). The lirioresino-β-dimethyl ether, tinosposide A, tinosposide B, tanegoside, (+)-pinoresinol O-β-D-glucopyranoside (Maurya et al., 2009), (+)-pinoresinol, syringaresinol, medioresinol, (+)-epi-syringaresinol, (+)-pinoresinol monomethyl ether, (+)-glaberide I, sesamin, and sesamolin have been identified from the T. sinensis (Li et al., 2004a; Lam et al., 2018). The tinosposinensides A, tinosposinensides B, tinosposinensides C, 1-deacetyltinosposide A (Li et al., 2007), 1-deacetyltinosposide have been reported from ethanolic extract of T. sinensis stem (Dong et al., 2010).
4 Pharmacological attributes of Tinospora species
Ethanolic extract of T. cordifolia is extensively used in the formulation of 'Septilin' syrup, recommended as remedy in the treatment of bronchitis and earache (Spelman, 2001; Singh et al., 2003a, b). The isolated compounds berberine and jatrorrhizine showed antimicrobial, anti-inflammatory (Patgiri et al., 2014), anthelminthic (Pawar et al., 2014), antineoplastic (Jagetia and Rao, 2006b), antidiarrheal, antiulcer (Kumar et al., 2014) and anti-diabetic activities (Agarwal et al., 2002; Sinha et al., 2004; Sangeetha et al., 2013). The pharmacological activities of 9 Indian Tinospora species are presented in Table 3.
Pharmacological activity
Plant species
Plant parts used
Extract/compounds tested
Concentration/dose tested
Model tested/mode of administration
Study outcomes
References
Antioxidant activity
T. cordifolia
Stem
Methanolic
500 mg/kg body weight (b.w.)/ given for 40 days
Lipid peroxidase/catalase/ alloxan-induced diabetic rats/p.o.
Extract increased lipid peroxidase and catalase activities but decreased the superoxide dismutase, glutathione peroxidase activities significantly (P<0.01)
Sivakumar and Rajan (2010)
Ethanolic
300 mg/kg b. w./ given for 30 days
Catalase and superoxide dismutase/cancer bearing rats/p.o.
Extract significantly reduced catalase and superoxide dismutase (57.05±5.67 and 6.69±0.19) activities in treated animals (P<0.01)
Jayaprakash et al. (2015)
n-Butanolic
200 μg/mL
DPPH, ABTS, nitric oxide scavenging activity, iron chelating activity/ in vitro assays
IC50 14.81 ± 0.53, 29.48 ± 2.23, 58.20 ± 0.70 and 21.17 ± 1.19 μg/mL values reported in DPPH, ABTS, nitric oxide and iron chelating assays
Polu et al. (2017)
T. sinensis
Stem
Ethanolic
50-150 µg/mL
DPPH, ABTS, DMSO, NO radical scavenging activity, SOD scavenging and lipid peroxidation in vitro assays
Extract showed significant antioxidant activity against selected in vitro assays (DPPH – IC50 = 94.66± 0.049 μg/mL; ABST – IC50 = 90.44 ± 0.36 μg/mL; DMSO – IC50 = 97.99±0.15 μg/mL; NO – IC50 = 87.25± 2.72 μg/mL; SOD – IC50 = 93.72± 0.91 μg/mL; lipid peroxidation – IC50 = 92.14 ± 0.91 μg/mL)
Jain et al. (2010a)
T. crispa
Stem
aqueous, methanol and chloroform
10–100 µg/mL
DPPH free radical scavenging assay
Methanol extract significantly increased radical scavenging activity (IC50 12 µg/mL) and radical activity (100%) similar to vitamin
Ibahim et al. (2011)
0.1–0.5 mg/kg
DPPH free radical scavenging assay
Methanolic extract displayed significant inhibition in DPPH (IC50 0.118 mg/mL) assay
Zulkefli et al. (2013)
0.0625–1 mg/mL
Metal chelating assay
Methanol extract displayed significant inhibition to metal chelating assays (81.97%)
0.0625–1 mg/mL
Reducing power assay
Methanol extract demonstrated antioxidant effect by reducing ferric ion (Fe3+) to ferrous ion (Fe2+)
Ethanolic
80 µg/mL
DPPH free radical scavenging assay
Extract showed maximum activity (IC50 44.92 ppm)
Warsinah et al. (2020)
Anti-diabetic activity
T. cordifolia
Aerial parts
Ethanolic
20 mL/kg b. w./given for 30 days (twice a day) before half an hour of feeding
Antidiabetic effects/alloxan-induced diabetic rats/p.o.
The significant reduction (P<0.001) in levels of blood sugar was observed in treated animals
Kinkar and Patil (2015)
Leaf
Magnoflorine, jatrorrhizine, palmatine, and berberine
100 mg/kg b. w.
Wistar rats/ streptozotocin-induced diabetic rats and blood glucose levels/ aldose reductase inhibition assay/ p.o.
Treatment with magnoflorine decreased the serum glucose to normal level similar to that of the standard drug metformin; significant inhibition of aldose reductase activity was also displayed by magnoflorine
Cherku et al. (2019)
Bark
Methanolic
250 mg/kg b. w./given daily for 100 days
Streptozotocin- induced diabetic rats/male albino Wistar rats/p.o.
Extract significantly decreased the glycosylated hemoglobin level as compared with diabetic control (P<0.001); it also reduced the glucokinase levels but it increased the glucose-6-phoaphatase activity
Rajalakshmi et al. (2009)
Stem
Aqueous
200 and 400 mg/kg b. w./ given for 30 days
Streptozotocin-induced diabetic albino rats/p.o.
Extract showed significant (P<0.05) anti-diabetic activity in diabetic animals but, did not cause any increase in serum insulin levels or regeneration of pancreatic β cells; it increased hepatic glycogen synthase but decreased glycogen phosphorylase activity
Puranik et al. (2008)
Root
Aqueous
5.0 g/kg b. w./ given for 6 weeks
Alloxan-induced diabetic albino rats/p.o.
Extract showed maximum hypolipidaemic effect; better than glibenclamide (standard drug)
Prince et al. (1999)
Ethanolic
5.0 g/kg b. w./ given for 6 weeks
Alloxan-induced diabetic rats/Swiss albino rats/p.o.
Extract resulted in a significant reduction in blood and urine glucose levels of serum and tissues; extract also prevented a decrease in body weight
Prince and Menon (2003)
Root, shoot and leaf
Palmatine, jatrorrhizine and magnoflorine
40 mg/kg b. w.
Insulin-mimicking and insulin-releasing effect in vitro (rat pancreatic β-cell line, RINm5F) and in vivo/p.o.
Palmatine, jatrorrhizine and magnoflorine significantly reduced serum glucose levels and contained the increase in blood glucose levels
Patel and Mishra (2011)
T. sinensis
Flowers
Ethanolic
200 mg/kg b. w./given per day
Male wistar rats/ hydrogenated groundnut oil induced hypercholesterolemia/p.o.
Extract altered the improper metabolic profile and it was leads to hypolipidemia significantly (P<0.05)
Sandhyarani and Kumar (2014a)
Aqueous
100 mg/kg b. w./given per day
Male Wistar rats/ hydrogenated groundnut oil induced hyper cholesterolemia/ p.o.
Treatment with extract altered the rearranged metabolic profile and was effective in producing hypolipidemia (P<0.05)
Kumar and Gandhimathi (2014)
Leaf
Ethyl acetate
200 mg/kg b. w.
Wistar rats of either sex/alloxan induced diabetic rats/p.o.
Extract showed significant (P<0.001) antidiabetic activity (149±0.66)
Pimpriker et al. (2009)
T. crispa
Stem
Aqueous extract
4 g/L extract dissolved in drinking water
Alloxan-diabetic male Wistar albino rats model
The extract reduced the levels of fasting blood glucose and higher serum insulin
Noor et al. (1989)
4 g/L extract dissolved in drinking water
Alloxan-diabetic rats; acute intravenous treatment with the extract (50 mg/kg)
Showed improvement in glucose tolerance; acute treatment caused an enhancement in plasma insulin levels
Noor and Ashcroft (1989)
1 g/kg suspended in 10% Tween 80
Diabetic rats; p.o.
Significantly reduced the levels of glucose in diabetic rats
Hassani et al. (2016)
9.5% ethanolic extract
250, and 500 mg/kg bw
Normoglycemic and alloxan-diabetic male Sprague-dawley rats model; p.o.
The blood sugar level of diabetic rats decreased after receiving the extract (15.42%)
Anulukanapakorn et al. (2012)
borapetosides A and C from ethanolic extract
5 mg/kg, ip
Type 1 and Type 2 diabetic induced ICR mice. Type 1 induced by streptozotocin and type 2 induced by fat-rich chow and 20% fructose-sweetened water
Borapetosides A and C reduced the levels of plasma glucose in normal and streptozotocin-induced type 1 diabetic mice. Borapetoside C increased glucose utilization in peripheral tissues but, reduced hepatic gluconeogenesis
Lam et al. (2012)
Borapetoside A
10 mg/kg
Streptozotocin-induced type 1 diabetes mellitus, diet-induced type 2 diabetes mellitus
Increased the levels of insulin but decreased the levels of blood glucose significantly (P<0.05).
Ruan et al. (2013)
Borapetoside C
5 mg/kg
Type 2 diabetes mellitus; twice daily a for 7 days
Attenuated the increased plasma glucose induced by oral glucose in normal and type 2 diabetes mellitus mice
Ruan et al. (2012)
Borapetoside E
10 mg/kg
High fat diet-induced obese mice
Compound significantly improved hyperglycemia, insulin resistance, hepatic steatosis, hyperlipidemia, and oxygen consumption in obese mice
Xu et al. (2017b)
Borapetol B
10 µg/100 g b. w.
Blood glucose and plasma insulin in normoglycemic Wistar and type 2 diabetic Goto-Kakizaki rats were determined/ glucose tolerance test; p.o.
Compound reduced the levels of blood glucose significantly (P<0.001) but enhanced the levels of insulin in treated normoglycemic Wistar and type 2 diabetic Goto-Kakizaki rats
Lokman et al. (2013)
Anti-arthritic activity
T. cordifolia
Aerial parts
Methanolic
1 g/kg/2 mL volume
Male Lewis rats/ adjuvant arthritis/p.o.
Extract suppressed arthritic inflammation and damage in bone and cartilage significantly (P<0.05); extract also altered the levels of mediators of bone remodeling which favors of anti-osteoclastic activity (P<0.05)
Sannegowda et al. (2015)
Stem bark
Methanol extract
500 μg/mL
In vitro, BSA denaturation essay
Extract showed significant minimum protein denaturation (43%); similarly, the diclofenac sodium showed significant inhibition (88.8±3.4%) at 500 µg/mL concentration
Ramya and Maheswari (2016)
Anti-stress activity
T. cordifolia
Aerial parts
Aqueous
200 mg/kg b. w.
male Sprague Dawley rats/ cold water swim stress method/p.o.
Extract decreased the stress levels by inducing the levels of lipid peroxide, serum glucose, and triglycerides significantly (P<0.005)
Biswas and Saha (2015)
Stem
Ethanolic
100 mg/kg b. w.
Sprague Dawley male rats/cold water swim stress method/p.o.
Extract of both species (T. cordifolia and Centella asiatica) in combination demonstrated antistress activity
Sarma et al. (1996)
Fresh leaves
-
50 mg/kg b.w. and 10 mg/kg
Swiss albino mice/ forced swim test/ tail suspension test/
Fresh leaves and imipramine showed significant decrease in the immobility period (forced swim and tail suspension tests)
Kalabharathi et al. (2014)
T. sinensis
Stem
Aqueous
1000 mg/kg b. w.
Adult swiss albino mice; anoxia stress tolerance, swimming endurance test, cold resistant stress/p.o.
Extract increased the anoxia stress tolerance at the end of 1st, 2nd and 3rd weeks of treatment significantly (P<0.01); it also reduced the elevated levels of serum biochemical parameters, blood cell count, and prevented alterations in the weight of the liver, adrenal gland
Sharma et al. (2007)
Anticancer/antitumor activity
T. cordifolia
Aerial parts
Palmatine
200 mg/kg b. w./given daily for 16 weeks
Swiss albino mice; 7,12-dimethylbenz(a)anthracene-induced skin cancer model in mice/p.o.
Palmatine reduced the tumor size significantly (P<0.05); compound also increased the levels of glutathione, superoxide dismutase, and catalase in the skin of treated animals (P<0.05)
Ali and Dixit (2013)
Stem
Methanolic
200 mg/kg b. w./given daily for 5 days
BALB/c mice/antitumor assay/i.p.
Extract increased total white blood cell count, bone marrow cells (18.16×106/femur) and α-esterase positive cells (1423/4000 cells) significantly (P<0.001)
Mathew and Kuttan (1999)
Tinocordiside and yangambin
100 μg concentration
KB and CHOK-1 cells/in vitro assay
Tinocordiside demonstrated moderate activity against KB and CHOK-1 cells while yangambin found active against KB cells only
Bala et al. (2015a)
Methanolic
100 μg/mL
MB-231 human breast cancer cell line/trypan blue dye exclusion assay/methyl tetrazolium in vitro assay
Extract showed significant anticancer activity against MDA-MB-231 human breast cancer cell line (IC50 59±4.05 μg/mL in 0.25% DMSO)
Ahmad et al. (2015)
Dichloromethane
100 mg/kg b. w./given once a daily for 9 days
Female Swiss albino mice/ Ehrlich ascites carcinoma implant assay/p.o.
Maximum anticancer activity was reported at III phase (long-term survivors were 17%)
Jagetia and Rao (2006a)
Clerodane furano diterpene glycoside (TC-2)
1, 10, 30 and 50 µM for 24 h
HCT116 cells (colon cancer)/in vitro assay
Compound showed significant anticancer activity against cancer cells [IC50 8 µM (HCT116)]
Sharma et al. (2018)
T. sinensis
Aerial parts
Ethanolic
1000 μg/mL
Human melanoma cancer cell line (A 375) and skin cancer cell line (A 431)/microculture tetrazolium assay/in vitro
Extract showed significant cytotoxicity (IC50 49.87 μg/mL and 112.54 µg/mL) against A375 and A 431 cancer cells, respectively
Punitha et al. (2012)
T. crispa
Stem
Aqueous
100 µg/mL
MCF-7 breast cancer cell lines; MTT assay
Cell viability significantly decreased (IC50 42.75 µg/mL)
Ibahim et al. (2010)
200 µg/mL
Human cancer cell lines: MCF-7; HeLa; Caov-3; HepG2
IC50 values - MCF-7: 107 µg/mL; HeLa: 165 µg/mL; Caov-3: 100 µg/mL; HepG2: 165 µg/mL
Amom et al. (2008)
Tinocrisposide
100 µg/mL
H1299 and MCF-7 cell lines; MTT assay
H1299 cell line viability decreased significantly (IC50 70.9 µg/mL); MCF-7 cell line (IC50 >100 µg/mL)
Adnan et al. (2016).
Methanolic
0.03- 1 mg/mL
HL-60 leukemic cells, HepG2 hepatoma cells and Hep3B hepatoma cells
Inhibition with IC50 as follows: HL-60: 0.12 mg/mL; HepG2: 1.03 mg/mL; Hep3B: 0.16 mg/mL
Sinchaikul et al. (2007)
Whole plant
Ethanolic
50.0 mg/mL
Head and neck squamous cell carcinoma cell lines (HN22 and HSC-3 cells); MTT assay
Extract significantly reduced the cell viability (50%) in HN22 cells and (60%) in HSC-3 cells
Phienwej et al. (2015)
Aqueous, methanol and chloroform
10–100 µg/mL (each extract)
MCF-7, MDA-MB-231, HeLa, and 3T3 fibroblast cells
All extracts showed dose-dependent antiproliferative activity
Ibahim et al. (2011)
Radioprotective activity
T. cordifolia
Stem
n-butanol fraction
120 mg/kg b.w./given up to 15th day
Wistar albino mice/endogenous spleen CFU assay /MN assay/i.p.
Extract (120 mg/kg) produced significant protection against radiation in terms of increased survival rate, body weight retention, hematological parameters (P<0.01) but, decreased MN expression (P<0.01)
Patel et al. (2013b)
Aqueous
10 mg/kg b. w./given per day
Swiss albino mice/ radiation (8 Gy) exposure/p.o.
Extract did not show mortality until day 13 and 50% of the animals survived until day 30
Pahadiya and Sharma (2003)
Hydroalcoholic
200 mg/kg b. w.
Swiss albino strain ‘A’ male mice/endogenous spleen CFU assay/ micronucleus assay/i.p.
Extract rendered significantly higher (P<0.05) CFU counts in comparison to the corresponding irradiated groups
Goel et al. (2004)
Polysaccharide preparation
4.5 mg/mL
Saccharomyces cerevisiae X2180 strain as the in vivo test model/ catalase and superoxide dismutase assay
The preparation did not enhance the expression of the protective enzymes, catalase and superoxide dismutase in the yeast cells
Subramanian et al. (2003)
Aerial root
Hydroalcoholic
2000 mg/kgb. w./given once a day for 5 consecutive days
Swiss albino mice (BALB “c” strain)/ lipid peroxidation and glutathione assays/p.o.
The levels of lipid peroxidase were higher in irradiated controls as compared to experimental group (21.42% higher than normal); steady decreasing pattern was followed by ovarian glutathione level up to day 7 in both irradiated control and experimental group
Sharma (2015)
Root
Aqueous
75 mg/kg b. w./given once a daily for 5 consecutive days
7.5 Gy gamma radiation exposure/ Swiss albino mice/p.o.
Extract administration before irradiation significantly ameliorated radiation induced elevation in lipid peroxidation and decline in glutathione concentration in testes
Sharma et al. (2011)
Hepatoprotective activity
T. cordifolia
Stem
Aqueous
2 mL/100 g/ given twice daily for 10, 20 and 30 days
Swiss albino Wistar rats/CCl4 induced hepatotoxicity/p.o.
Extract administration for 20 days showed significant (P<0.05) limitation of serum bilirubin rise
Kumar et al. (2013)
Stem bark
Aqueous
250 mg/kg b. w.
Wistar albino male rats/CCl4 (2:5, v/v in paraffin oil) induced acute liver damage/i.p.
Extract increased superoxide dismutase, catalase, peroxidase levels but decreased lipid peroxidation levels
Singh et al. (2010)
Stem and leaves
Aqueous
400 mg/kg b. w./given once a daily for 30 days
Male Swiss albino mice/ lead-treated / red blood corpuscles, packed cell volume and hemoglobin value/p.o.
The effects of lead were prevented by concurrent daily administration extract; protected against lead intoxication
Sharma and Pandey (2010a)
Ethanolic
400 mg/kg b. w./given once a daily for 30 days
Male Swiss albino mice/SOD and CAT assays/lead nitrate induced damage/p.o.
Aqueous extract of stem and leaves along with the lead nitrate increased the activities of catalase but decreased the levels of aspartate aminotransferase, and alanine aminotransferase enzymes
Sharma and Pandey (2010b)
Root
Aqueous
100 mg/kg b. w./given once a daily for 45 days
Wistar rats/streptozotocin induced liver injury in diabetic rats /p.o.
Significant elevations (P<0.001) in serum alanine aminotransferase and aspartate aminotransferase were reported in the diabetic control rats and after treatment with extract, their serum levels were close to those in the normal control rats
Dhanush et al. (2013)
T. sinensis
Root
Ethanol
300 mg/kg b. w./ given for 8 days
Wistar albino rats/ CCl4 induced Hepatotoxicity/p.o.
Extract reduced sinusoidal dilation along with mild inflammagens. Significant reduction in (P<0.001) in serum enzyme levels was reported in treated animals
Naik et al. (2013)
Neuroprotective and neuroregenerative activity
T. cordifolia
Stem
Butanol extract
20 μg/mL concentration
Neuronal markers (MAP-2, GAP-43, NF200) and anti-apoptotic marker (Bcl-xL); in vitro assay/wound scratch and gelatin zymogram assay
Extract showed neuroprotective and neuroregenerative potential against catastrophic consequences of glutamate-mediated excitotoxicity
Sharma and Kaur (2018)
Aerial parts
Ethanolic
400 mg/kg b. w./ given once a daily for 30 days
Adult male Wistar rats/6-hydroxy dopamine lesion rat model of Parkinson's disease/p.o.
Extract exhibited significant neuroprotection by increasing the dopamine levels (1.96 ± 0.20 and 2.45 ± 0.40 ng/mg of protein) and complex I activity (77.14 ± 0.89 and 78.50 ± 0.96 nmol/min/mg of protein); iron asymmetry ratio was also significantly attenuated by extract (1.11 ± 0.15)
Kosaraju et al. (2014)
Flower
Ethanolic
400 mg/kg b.w./ given once a daily (in the morning) for 14 days
Adult male Wistar rat/lipopolysaccharide-induced neuroinflammation/p.o.
Extract significantly (P<0.001) reduced the body weight, locomotor activity, latency period in passive avoidance test; also decreased the anti-oxidant levels of glutathione, SOD and CAT
Prakash et al. (2017)
Anti-inflammatory activity
T. cordifolia
Stem
Chloroform
2000 mg/kg b. w.
RAW264.7 macrophages/in vitro/Swiss albino rats/carrageenan-induced paw model/p.o.
Extract suppressed the lipopolysaccharide-induced upregulation of pro-inflammatory biomarkers activity without hampering of cyclooxygenase 1 activity; the extract showed significant decrease in volume of paw oedema (P<0.05)
Philip et al. (2018)
Aqueous
120 mg/kg b. w.
Male Swiss albino rats/carrageenan-induced paw edema model/p.o.
Extract showed significant reduction in paw volume (P<0.05)
Deepika et al. (2016)
Leaf
Methanolic
400 μg/mL
In vitro study - lipoxygenase inhibition and protein denaturation assay
Extract inhibited the lipoxygenase activity (IC50 389.3 µg/ml); maximum inhibition of heat induced protein denaturation (75%; IC50 237.6 μg/ml)
Shwetha et al. (2016)
T. sinensis
Whole plant
Diosgenin
400 µg/kg b. w.
Adult albino rats/ carrageenan-induced paw oedema/p.o.
Diosgenin showed maximum anti-inflammatory activity (82.25%) as compared to the indomethacin (82.01%); significantly (P<0.01) reduced the mean paw edema volume at 3 h of carrageenan injection
Punitha et al. (2013)
T. crispa
Stem
Aqueous and methanolic
600 µg/mL
TNF-α stimulated inflammation in human umbilical vein endothelial cells
Both extracts displayed significant suppression of signaling molecules ICAM-1, VCAM-1, MCP-1, M-CSF
Kamarazaman et al. (2012a)
Aqueous and methanolic
600 µg/mL
H2O2 and tumor necrosis factor–α-induced inflammation in human umbilical vein endothelial cells; MTT assay
Aqueous extract showed an inhibition on TNF-α induced release of ICAM-1, VCAM-1 signaling molecule whereas nitric oxide secretion was enhanced
Kamarazaman et al. (2012b)
Methanolic
300 mg/kg
Carrageenan induced inflammation in Sprague-dawley rats; i.p.
Extract significantly suppressed the development of edema
Hipol et al. (2012)
Tinocrisposide and freeze-dried aqueous extract
1000 μg/mL
In vitro hemolytic and anti-inflammatory test; membrane stabilization assay
Tinocrisposide and freeze-dried aqueous extract increased the membrane stability of lysosome cell that was equal to physiological property with erythrocytes and it did not show hemolytic activity
Adnan et al. (2019)
Ethanolic
300 mg/kg bw
Carrageenan-induced inflammation in Sprague-Dawley rats
Extract significantly (P<0.05) inhibited the development of paw edema
Sulaiman et al. (2008)
Immunomodulatory activity
T. cordifolia
Stem
Methanolic and aqueous extract
100 mg/kg b. w.
BALB/c mice/ murine macrophage cell line J774/p.o.
Aqueous extract produced 86.67±13.48 pg/mL of IFN-γ, respectively, whereas methanol extract produced 144.0 ± 11.02 pg/mL of IFN-γ
Alsuhaibani and Khan (2017)
Aqueous
100 mg/kg b. w./given for 10 days
Swiss albino mice/ agar colony assay/ p.o.
Extract increased the higher number of total white cell count in treated mice (14237±1236); 82% increase in number was statistically found significant (P<0.01)
Thatte et al. (1994)
80 μg/mL
Macrophage J774A.1 cell line/ viability, phagocytosis and pinocytosis and red blood cell lysis assays
Extract increased phagocytosis and pinocytosis in vitro; macrophages demonstrated an increase in phagocytosis to non-infective microbes and live Escherichia coli
More and Pai (2017)
50 mg/kg
Charles Foster strain albino rats/ haemagglutination antibody titre method for humoral immunity and footpad swelling method for cell mediated immunity/p.o.
Increase in antibody titre (22.34%), weight of body (31.79%), spleen (21.86%) and thymus (16.06%) observed in treated animals; significant (P<0.05) decrease in paw edema observed in treated group
Umretia et al. (2013)
Syringin and cordiol
500 mg/kg b. w.
Guinea pig/in vitro/ immunohaemolysis/p.o.
Both compounds significantly (P<0.01) increased the number of IgG antibodies in serum of treated pigs
Kapil and Sharma (1997)
Cordifolioside A
0.1–2.5 μg/mL
Human neutrophil cells/NBT, NO and chemiluminescence assays/in vitro
The compound showed significant enhancement in phagocytic activity and increase in nitric oxide and reactive oxygen species generation
Sharma et al. (2012)
Guduchi ImP (25 kDa protein)
60 μg/mL
Female BALB/c mice/intranasal administration on days 1 till day 42/ mitogenic activity
Guduchi ImP administration displayed a significant increase in anti-guduchi ImP IgG (5-7-fold) on day 50
Aranha and Venkatesh (2020)
Guduchi Satwa (Neem –Guduchi; Azadirachta indica-T. cordifolia) extract
300 mg/kg b. w.
Male Wistar rats/ neutrophil adhesion test/p.o.
Guduchi Satwa showed potent immunomodulatory effects in treated animals (P<0.01) than control
Narkhede et al. (2014)
Aqueous extract
100 mg/kg b.w.
Mice/ cyclophosphamide-induced leukopenia/p.o.
Extract exhibited 60% survival rate; extract also rejuvenate the weakened immune system and eliminate systemic candidiasis
Alrumaihi et al. (2019)
Ethanolic extract
100 mg/kg b.w.
Male Wister rats/ cyclophosphamide-induced thiobabituric acid assay/p.o.
Extract increased the level of liver mitochondrial enzymes like GSH, CAT and SOD but decreased the level of LPO in liver in treated animals; also enhanced the concentration of melatonin in pineal gland and the level of cytokines (IL-2, IL-10 and TNF-α)
Aher and Wahi (2012)
T. sinensis
Stem
Aqueous
100 mg/kg (in 0.1% w/v CMC)
Male Wistar rats/ cyclophosphamide-induced anemia/p.o.
Extract showed significant effectivity
Manjrekar et al. (2000), Haque et al. (2017)
T. crispa
Ethanolic and fractions
1000 µg/mL
Intracellular cytokine determination in LPS-stimulated murine macrophage cell line RAW264.7; MTT proliferation assay
Extract and fractions induced RAW264.7 cell viability and intracellular expressions of cytokine, INF-γ, IL-6, and IL-8
Abood et al. (2014)
Ethanolic extract and magnoflorine and syringin
12.5 µg/mL
Chemotaxis, phagocytosis, production of inflammatory mediators ROS, NO, PGE2 and pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and MCP-1) on RAW 264.7 macrophages
Ethanolic extract and magnoflorine showed significant stimulatory effects on the chemotaxis, phagocytic activity, ROS and NO productions and the secretions of IL-1β, TNF-α, IL6, PGE2 and MCP-1
Ahmad et al. (2018)
Ethanolic (80%) and syringin and magnoflorine
4.68 to 75 μg/mL
Immunomodulatory effect in lipopolysaccharide (LPS)-induced U937 human macrophages
Extract stimulated the MyD88-dependent signaling pathways by upregulating the various immune inflammatory related parameters
Haque et al. (2020)
Wound healing activity
T. cordifolia
Root
Ointment (10% ointment -10 g methanolic extract incorporated with 100 g petroleum jelly B.P.)
Extract ointment applied once a daily
Albino mice/ excision and incision wound/ topical administration
Ointment showed significant (P<0.01) wound healing activity in albino mice
Nema et al. (2012)
Leaf
Ethanolic
300 mg/kg b. w.
Wistar strain albino rats/ excision and incision wound study/granuloma study/p.o.
Extract showed significant increase (8.917 ±0.13 μg) in levels of hydroxyproline in treated animals (6.2 ± 0.10 μg)
Ramachandra et al. (2011)
T. crispa
Stem
Ethanolic and topical ointment
-
Alloxan monohydrate-induced diabetic albino mice; wound incision model; i.p.
Extract significantly reduced the blood glucose level by almost fifty percent after fourteen days; epithelialization time was increased
Arcueno et al. (2015)
Methanolic extract and chloroform fraction ointment
100 mg/kg per day
Albino rats; wound excision model;
Treated animals showed high percentage of wound closure area which was similar to the values of standard drug treated group
Vijendren et al. (2017)
Antimicrobial activity
T. cordifolia
Stem
Aqueous
50 mg/mL per hole
Enterococcus faecalis, Salmonella typhi, S. pneumoniae, E. coli/agar well diffusion assay/in vitro
Extract exhibited maximum zone of inhibition against E. faecalis (23 mm) and S. pneumoniae (24 mm)
Nageswari et al. (2016)
Ethanolic
0.2 mL/disc
E. coli, Proteus vulgaris, E. faecalis, S. typhi, S. aureus and Serratia marcescens/ agar disc diffusion assay
Extract exhibited significant antibacterial activity against P. vulgaris, E. coli and moderate activity against E. faecalis
Jeyachandran et al. (2003)
Methanolic
1 mg/10 mL
S. aureus, E. coli, P. aeruginosa, S. typhimurium and C. Albicans/agar well diffusion assay
Extract found active as follows: S. aureus (20 mm) E. coli (19 mm), S. typhimurium (19 mm), P. aeruginosa (17 mm) and C. albicans (19 mm)
Singh and Saxena (2016)
Chloroform
400 µg/mL
E. coli, K. pneumoniae, P. vulgaris, P. aeruginosa/ agar well diffusion assay
Extract showed maximum zone of inhibition against P. aeruginosa and moderate inhibition exhibited against K. pneumoniae and E. coli
Shanthi and Nelson (2013)
Leaf
Methanolic
30µL/disc
E. coli/agar disc diffusion assay
Extract showed significant inhibition to E. coli (0.8 mm)
Prajwala et al. (2018)
Aerial parts
Ethanolic
40 μL at 2% concentration
S. mutans/agar diffusion assay
Maximum inhibition zone (19 mm) recorded against bacteria
Agarwal et al. (2019)
T. crispa
Roots
Ethanolic, methanolic, aqueous and chloroform
50 µg/disc
S. pneumoniae, E. coli, and C. albicans; disc diffusion assay
Ethanolic extract showed maximum inhibition (3.6±0.2 mm) against E. coli
Mohammed et al. (2012)
Stem
Chloroform, methanol and aqueous
1 mg/disc; 50 μL for MIC
Bacteria - Bacillus cereus, B. subtilis, S. aureus, E. coli, P. aeruginosa and S. typhimurium; fungi – A. niger, C. albicans and S. cerevisiae; disc diffusion method; MIC and MBC assays
Methanol extract showed maximum inhibition (4.0±0.5 mm for B. cereus and 8.3±0.6 mm for S. aureus) to bacterial species; also displayed significant MIC and MBC
Awang et al. (2020)
Antileishmanial activity
T. sinensis
Stem
Ethanolic
500 mg/kg b. w./day x 5 oral dose
Hamsters/IC50 values analysis assay; p.o.
Extract exhibited an appreciable activity against promastigotes (IC50 37.6±6.2 µg/mL) and intracellular amastigotes (IC50 29.8±3.4 µg/mL); in hamsters, it showed 76.2±9.2% inhibition
Singh et al. (2008)
3-hydroxy-2,9,11-trimethoxy-5,6-dihydro isoquino[3,2-a]isoquinolinylium
100 mg/mL
Visceral leishmaniasis/in vitro antileishmanial activity
Compound resulted in 96.6% inhibition of promastigotes and amastigotes (86.6%)
Maurya et al. (2009)
Analgesic activity
T. cordifolia
Stem
“Guduchi” capsules (from the Himalaya Drug Co., Bangalore, India)
300 mg/kg b. w.
Swiss albino rats (male and female)/ hot plate and abdominal writhing methods/p.o.
Extract produced significant analgesia (P<0.001 after 120 minutes
Goel et al. (2014)
Water and methanol (30/70).
300 mg/kg b. w.
Male and female Swiss mice/acetic acid-induced writhing and thermal tests (hot plate and tail-flick tests)/p.o.
Extract significantly inhibited the number of writhes in dose-dependent manner; aqueous -methanolic extract showed better results in hot plate-induced analgesia test
Hussain et al. (2015)
Aqueous
600 mg/kg b. w.
Adult Wistar rats/ hot plate and tail immersion method, acetic acid-induced writhing/p.o.
Extract showed significant (P<0.05) analgesic effects when compared with control group
Gupta et al. (2013)
Leaf
Aqueous
200 mg/kg b. w.
Swiss albino mice and Wistar rats/ Eddy’s hot plate method/p.o.
Extract showed significant increase in the reaction time (pain threshold; P<0.001)
Siddalinga et al. (2011)
T. sinensis
Leaf
Ethanolic
500 mg/kg b. w.
Adult male Wister rats/tail flick and acetic acid induced writhing tests/p.o.
Extract showed significant (P<0.001) increase in latency time
Sandhyarani, and Kumar (2014b)
T. crispa
Stem
Methanolic extract, petroleum ether and chloroform fraction
400 mg/kg b. w.
Swiss-albino mice; acetic acid induce writing method; p.o.
Petroleum ether fraction displayed significant analgesic effect (51.94%) when compared with standard (65.12%)
Islam et al. (2014)
Whole plant
Methanolic, n-hexane and chloroform
400 mg/kg b. w.
Swiss albino mice; acetic acid-induced writhing and formalin-induced paw licking tests; p.o.
Hexane fraction showed significant antinociceptive effect (56.28%; P<0.01) in acetic acid-induced writhing test; chloroform fraction displayed significant inhibition in the late phase (64.36%) of formalin-induced paw licking test
Rakib et al. (2020b)
Anti-psychotic/ antiepileptic activity
T. cordifolia
Stem
Aqueous ethanol (50:50)
500 mg/kg b. w.
Female Wistar albino mice/amphetamine induced hyperactivity /p.o.
Extract did not show significant antipsychotic activity
Jain et al. (2010b)
Stem
Methanolic
2000 mg/kg b. w.
Wistar albino rats/ haloperidol induced hyperprolactinemia/sulpiride induced hyperprolactinemia/p.o.
Significant (P<0.05) increase in serum prolactin level and decrease in dopamine level reported in the extract treated animals
Tiwari et al. (2019)
Antiulcer activity
T. cordifolia
Stem
Aqueous
400 mg/kg b. w.
Albino Wistar rats/ aspirin induced and ethanol induced gastric ulcer/p.o.
In pylorus ligated rats, the extract demonstrated significant (P<0.01) reduction in gastric volume, total acidity and ulcer index as compared to control
Chandan et al. (2013)
Ethanolic
600 mg/kg b. w.
Albino Wistar rats/ aspirin and ethanol-induced ulcer/p.o.
Extract showed significant (P<0.05) protection in ethanol-induced gastric lesions
Khan et al. (2015)
Epoxy clerodane diterpene
50 mg/kg b. w.
Albino Wistar rats/ indomethacin-induced gastric ulcer/p.o.
Compound exerted its antiulcer activity by reinforcement of defensive elements and diminishing the offensive elements
Antonisamy et al. (2014)
Stem bark
Ethanolic
500 mg/kg b. w.
Albino Wistar rats/ pylorus ligation-induced ulcer and ethanol-induced ulcer/p.o.
Extracts showed reduction in ulcer index, gastric volume, total acidity but, displayed an increase in pH of gastric content in both the models significantly (P<0.01)
Kaur et al. (2014)
Whole plant
Ethanolic
400 mg/kg b. w.
Male Wistar rats/ pylorus-, stress-, ibuprofen-induced ulcers/p.o.
Extract significantly (P<0.05) reduced the ulcer index; its effect was significantly (P<0.05) lesser than that of famotidine
Bairy et al. (2001)
T. sinensis
Stem
Aqueous and ethanolic
500 mg/kg b. w. (each)
Adult Swiss albino mice and Wistar rats/pylorus ligation induced gastric ulcers and water immersion stress induced ulcer/p. o.
Extract reduced ulcer index (P<0.001) significantly in all the models
Khayum et al. (2009)
Leaf
Ethanolic
300 mg/kg b. w.
Adult Swiss albino rats/pylorus ligation induced ulcer and ethanol induced gastric ulcer/p.o.
Extract showed significant (P<0.05) reduction in gastric volume, free acidity and ulcer index as compared to control
Avanapu et al. (2014)
Antifertility activity
T. cordifolia
Stem
Ethanolic
100 mg/rat/day given for 60 days
Male albino Wistar rats/sperm motility and density/hormonal assay/p.o.
Extract reduced the formation of round spermatids (73.12%); Leydig cell nuclear area and mature Leydig cell numbers in treated rats significantly (P<0.001)
Gupta and Sharma (2003)
Aqueous
100 mg/kg b. w./given for 60 days
Adult Wistar albino rats/ antifertility and antiandrogenic activity, sperm motility/p.o.
Extract caused a significant reduction in average litter size, sperm count, and number of viable and motile sperm
Ittiavirah and Habeeb Rahman (2013)
Roots
Methanolic
100 mg/kg b. w./given for 5 days
Fertile female albino rats/fertility test/p.o.
Results revealed that extract exhibit antifertility activity
Fatima Rose et al. (2010)
Antimalarial activity
T. crispa
Stem
Methanolic
200 mg/kg b. w.
Female ICR mice; chloroquine-sensitive Plasmodium berghei ANKA (PbANKA) strain; p.o.
Extract showed significant inhibitory effect on the parasite (50%; P<0.01)
Niljan et al. (2014)
Methanolic extract and fractions (F1 – F8)
100 mg/kg b. w.
Male ICR mice; chloroquine-sensitive P. berghei ANKA (PbANKA) strain; p.o.
Methanol extract and fraction F5 showed the maximum parasitemia inhibitory effects
Lee et al. (2020)
Methanolic
2.0 mg/mL
P. falciparum 3D7 strain
Extract showed antiplasmodium activity (IC50 0.29 mg/mL) with reducing parasitemia degree (56.83%) after 72 h of incubation
Ihwan et al. (2014)
Aqueous
500 mg/kg
Female ICR mice; chloroquine-sensitive P. berghei strain ANKA (PbANKA)
Extract decreased parasitemia significantly (P<0.01) in infected mice
Somsak et al. (2015)
Ethanolic
80 mg/kg
Female ICR mice; P. yoeli 17X (lethal) strain - infected erythrocytes
Extract decreased parasitemia significantly in infected mice on day 20; extract showed dose-dependent antimalarial activity
Rungruang and Boonmars, (2009)
T. baenzigeri
Aqueous
500 mg/kg b. w.
Female ICR mice; chloroquine-sensitive P. berghei ANKA strain (PbANKA); p.o.
Extract significantly (P<0.01) suppressed parasitemia (74.95% inhibition); extract did not show any toxicity at 2,000 mg/kg; extract showed markedly prolonged mean survival time similar to the chloroquine-treated group
Ounjaijean et al. (2019)
4.1 Antioxidant activity
The oxidative stress is considered as a main cause for the development of various diseases. The food materials promote antioxidative defences in human body to combat non-desirable effects of reactive oxygen species. Plants are able to biosynthesize a wide range of non-enzymatic antioxidative molecules capable of attenuating the reactive oxygen species-induced oxidative destruction (Kasote et al., 2015). Ethanolic extract of T. cordifolia stem (300 mg/kg b.w., p.o., for 30 days) significantly (P < 0.01) reduced the levels of catalase and superoxide dismutase (57.05 ± 5.67 and 6.69 ± 0.19) in cancer bearing animals but, it did not show any change in the animals of control group (Jayaprakash et al., 2015; Polu et al., 2017). The n-butanolic extract of T. cordifolia stem (200 µg/mL) showed significant antioxidant activity in DPPH, ABTS, nitric oxide scavenging and iron chelating assays (Polu et al., 2017). The methanol extract of T. crispa displayed maximum antioxidant activity which was evaluated by measuring total flavonoid content (9.53 ± 0.50 mg quercetin equivalent/ g sample), total phenolic content (255.33 ± 10.79 mg gallic acid equivalent/g sample), and DPPH free radical scavenging activity (IC50 12 µg/mL). The activity could be associated with the presence of phenolic compounds (Ibahim et al., 2011).
4.2 Anti-diabetic activity
Diabetes mellitus type 2 (an endocrine metabolic disorder) is the main cause (microvascular and macrovascular complications) of morbidity and mortality in human population (Patel et al., 2011a, b). There are about 69.2 million people affected by diabetes and are anticipated to cross 123.5 million by 2040 in India. Furthermore, about 193 million diabetics remain unfamiliar leading them to the occurrence of several long-term health problems of untreated chronic diabetes (Chawla et al., 2016). The methanolic extract of T. cordifolia stem was administered (p.o.) for 24 weeks to diabetic rats and levels of blood glucose were monitored in treated and non-treated groups. Methanolic extract lowered the blood glucose levels in treated animals significantly (P < 0.001) when compared with normal rats (Agrawal et al., 2012). Ethanolic extract (100 and 200 mg/kg body weight) of T. cordifolia stem suppressed 6-phosphatase and fructose 1, 6-diphosphatase activities significantly (P < 0.001) but stimulated the formation of glycogen contents in liver (P < 0.005; Sangeetha et al., 2011). Ethanol extract increased the body weight, total haemoglobin and hepatic hexokinase activity but, decreased the levels of hepatic glucose-6-phosphatase, serum acid phosphatase, alkaline phosphatase, and lactate dehydrogenase activities in diabetic rats significantly (P < 0.001; Stanely et al., 2000). The formulation of water extract (T. cordifolia stem) + honey suspension reduced the levels of blood glucose and glycated haemoglobin in streptozotocin treated Wistar albino rats (Khedekar et al., 2015). Magnoflorine isolated from T. cordifolia leaf (100 mg/kg b.w.) significantly reduced the levels of serum glucose in streptozotocin-induced diabetic Wistar rats (Cherku et al., 2019). Dry powder of T. crispa stem (given 250 mg, twice a daily) showed significant hypoglycemic effect on patients with metabolic syndrome. The dry powder significantly reduced fasting blood glucose levels from the baseline (Sriyapai et al., 2009). Borapetol B isolated from T. crispa stem (100 µg/ 100 g b.w.) reduced the levels of blood glucose significantly (P < 0.001) but enhanced the levels of insulin in treated normoglycemic Wistar and type 2 diabetic Goto-Kakizaki rats (Lokman et al., 2013). T. crispa extract increased glucose uptake in L6 myotubes which was linked to the enhanced levels of GLUT1 transporter, AMPKα, and PPARγ transcript (Noipha and Ninla-Aesong, 2011). Ethanolic extract of T. sinensis flowers (200 mg/kg b. w./day) altered the improper metabolic profile and it was led to hypolipidemia significantly (P < 0.05; Sandhyarani and Kumar, 2014a). Similarly, the ethyl acetate extract of leaves demonstrated significant (P < 0.001) antidiabetic activity (149 ± 0.66) at 200 mg/kg dose (Pimpriker et al., 2009).
4.3 Anti-arthritic activity
Rheumatoid arthritis is a chronic systemic inflammatory disease that causes joint inflammation, synovial proliferation, and destruction of articular cartilage. The condition gets worse over the time unless the inflammation is stopped or slowed. It is the most common cause of physical disability in developed countries, and its prevalence is found between 0.3% − 1.50% in human population (Lin et al., 2006). The in vitro results were not found optimistic but, T. cordifolia stem extract (p.o.) showed significant results (50 mg/kg b. w./day; given for 21 days followed by treatment for 12 weeks) in ovariectomy-induced bone loss (Abiramasundari et al., 2017). The minimum protein denaturation (23%, 36%, and 43%) was reported in animals treated with methanolic extract of T. cordifolia stem bark (100 μg, 250 μg and 500 μg/mL). Methanolic extract of T. cordifolia stem significantly inhibited the denaturation of proteins when compared with diclofenac sodium in treated animals (Ramya and Maheswari, 2016).
4.4 Anti-stress activity
Stress is known to stimulate alterations in various physiological responses even dominating to pathological stress (Chrousos, 1998). The chronic stress exposure is known as causative factor of free radical formation and reactive oxygen species increase in the body (Wang et al., 2007). Normally stress-induced alterations are self limiting events that override the ‘threshold’ limits become irreversible and pathological (Seyle, 1973). Stress is involved in the development of various diseases such as hypertension, peptic ulcer, Alzheimer's disease, and depression (Piato et al., 2008). Ethanolic extract of T. cordifolia fresh leaves (50 mg/kg) showed significant decrease in the immobility period (force swim and tail immersion tests (Kalabharath et al., 2014). In patients of chronic mental stress, the levels of serum glucose, triglyceride, cholesterol, anxiety, and depression were reported higher than healthy men. The patients were administered with T. cordifolia (3 g twice daily) powder and advised to do yoga daily. The experimental study was followed for 60 days and results revealed that T. cordifolia with continuous yoga practice in patients showed significant antistress activity when compared with diazepam (P < 0.001; Biswas et al., 2015). Pretreatment with aqueous and alcoholic extracts of T. sinensis had increased the anoxia stress tolerance at the end of 1st, 2nd and 3rd weeks of treatment with both doses i.e., 500 mg/kg (P < 0.05) and 1000 mg/kg (P < 0.01). Aqueous and alcoholic extracts of T. sinensis stem reduced the elevated levels of serum biochemical parameters, and blood cell count. Aqueous extract prevented alterations in the weight of the liver, adrenal gland but increased the weight of spleen in treated animals (Sharma et al., 2007).
4.5 Anticancer/antitumor activity
Cancer is one of the most dreaded disease and is a leading cause of death worldwide. It is the third leading cause of death worldwide following cardiovascular and infectious diseases (Kelloff, 2008). Breast cancer is the most common type of cancer in women. The maximum prevalence of breast cancer is found in South-Central Asian countries (Soliman et al., 2006). It has been proven that several diseases occur in humans due to development of oxidative stress. The oxidative stress developed by free radicals, seek stabilization through electron pairing with proteins and DNA in healthy cells of human, cause DNA and protein damage. These alterations contribute to the development of cancer, cardiovascular problems, and ageing disorders (Maxwell, 1995; Braca et al., 2002). Due to lack of preventive measures, high cost of chemo- and radiotherapies, and the adverse effects of anticancer agents, attempts are being made to search for novel and effective plant-derived molecules that could suppress cancer development (Babior, 2000; Bhadane et al., 2018). Methanolic extract (20 mg/kg) of T. cordifolia stem significantly suppressed tumour directed capillary formation (B16F10 melanoma cell-induced capillary) in rats. Extract inhibited the levels of IL-1β, IL-6, TNF-α, and granulocyte monocyte-colony stimulating factor in treated animals (Leyon and Kuttan, 2004a, b; Jagetia et al., 1998). Hexane fraction of ethanolic extract of T. cordifolia stem induced the formation of apoptotic bodies, nuclear condensation, and activation of caspase-3 activity, but suppressed the growth of Ehrlich ascites tumor in mice (Thippeswamy and Salimath, 2007). Palmatine isolated from T. cordifolia aerial parts reduced the tumor size significantly (P < 0.05) and also increased the levels of glutathione, superoxide dismutase and catalase in the skin of treated animals (P < 0.05; Ali and Dixit, 2013). Methanol extract of T. crispa stem inhibited the growth of HL-60 (human promyelocytic leukemia cells), HepG2 and virus infected Hep3B human cancer cell lines in dose-dependent manner (Sinchaikul et al., 2007). Ethanolic extract of T. sinensis aerial parts showed significant cytotoxicity (IC50 49.87 μg/mL and 112.54 µg/mL) against A375 and A 431 cancer cells, respectively (Punitha et al., 2012).
4.6 Radioprotective activity
The radiation exposure has been increased during last 100 years with the development and use of X-rays and radio-isotopes for therapeutic purposes, and also by testing of nuclear weapons (Donya et al., 2014). The radiation exposure can develop mutational changes, immune system alterations, and cancer development in humans (Mamedov et al., 2011). Amifostine (aminothiols) significantly reduced the mortality rate of radio-exposed patients but showed adverse effects. Unfortunately, no safe synthetic radioprotective compounds are available to date; therefore, the investigation of plants has been ongoing for several decades to treat radio exposed patients (Bhandari, 2013). Aqueous-ethanolic extract (1:1, 200 mg/kg) of T. cordifolia stem improved the several parameters in treated animals e.g., the spleen weight (49% in irradiated control while 93% in treated), apoptosis (from 19% to 2.8%), DNA fragmentation (from 43% to 20.4%), macrophage adherence (75% in irradiated control while 120% in treated) and macrophage spread size (from 8 μm to 15 μm). Extract also increased the levels of IL-1β, GM-CSF [from 56 pg/mL and 53 pg/mL in irradiated group (control) to 59 pg/mL and 63 pg/mL (treated)] in experimental animals (Singh et al., 2007). Ethanol extract (200 mg/kg b.w.) displayed significant recovery of spleen weight (from 49% to 93%), apoptosis (from 19% to 2.8%) and DNA fragmentation (from 43% to 20.4%). Aqueous extract of T. cordifolia showed radioprotective effects in Co-60 gamma irradiated Swiss albino mice. The control group of mice showed sickness, ruffled hair, and diarrhoea hence, died on 14th day. The oral administration of aqueous extract induced more survivability rate in irradiated rats (Pahadiya and Sharma, 2003).
4.7 Hepatoprotective activity
Liver is known as an important organ of human body and has roles in the maintenance, functioning and regulation of homeostasis in all the biochemical pathways of growth, defense mechanisms, digestion, energy supply and reproduction. Hence, it is needed to maintain healthy liver for healthy lifestyles. But sometimes healthy liver is exposed to various exogenous compounds like environmental pollutants, toxic drugs and alcohol which can eventually led to various liver diseases such as hepatocellular, cholestatic, or mixed type of liver disorders (Dange, 2010). The hepatoprotective effect of petroleum ether, ethanolic and aqueous extracts of the leaf, stem and root of T. cordifolia were evaluated in carbon tetrachloride-induced liver damage in Wistar albino rats. Out of these tested extracts, the ethanolic extract exhibited significant (P < 0.01) hepatoprotective activity in treated animals. Ethanol extract showed significant decrease in the levels of serum bilirubin in treated animals (Kavitha et al., 2011). T. cordifolia ethanol extract (100 mg/kg/b. w. for 15 days) showed significant liver hepatoprotective effect in CCl4 intoxicated rats. A significant decrease in the levels of serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transminase, alkaline phosphatase, bilirubin was reported in treated animals (Bishayi et al., 2002). Ethanolic extract of T. sinensis roots reduced the sinusoidal dilation along with mild inflammagens in treated animals. This was evident from significant reduction (P < 0.01) in serum enzyme levels (Naik et al., 2013).
4.8 Neuroprotective and neuroregenerative activity
Neurodegenerative diseases are caused due to disruptions in functions of the neurons of central nervous system and often resulted in the deterioration of cognitive functions in humans. The deterioration in cognitive functions includes gradual loss in memory, and motor coordination (Adewusi et al., 2010; Nieoullon, 2011). Various plants and plant-derived products have been used in traditional medicine for neuroprotective, memory enhancement, and antiageing purposes (Iriti et al., 2010; Elufioye et al., 2017). Excitotoxicity is connected with the pathological processes of several neurodegenerative diseases viz, trauma, brain injury and memory loss. Butanol extract (20 μg/mL) of T. cordifolia stem showed significant (P < 0.01) neuroprotective activity against catastrophic glutamate-induced excitotoxicity (Sharma and Kaur, 2018). Ethanolic extract (400 mg/kg) of T. cordifolia aerial parts exhibited significant neuroprotective effects by enhancing the levels of dopamine against 6-hydroxy dopamine lesion rat model of Parkinson's disease (Kosaraju et al., 2014).
4.9 Anti-inflammatory activity
Inflammation is the immune system's response to pathogens, damaged cells, toxic constituents, or irradiation, and acts by eliminating harmful stimuli and inducing the healing process (Chen et al., 2018). Non-steroidal drugs are normally used for the treatment of inflammations; however, their prolonged use has adverse side effects (Bhadane et al., 2018). The anti-inflammatory effects of Guduchi Ghana (market product) and aqueous extract of T. cordifolia (50 mg/kg, p.o.) were compared in carrageenan-induced paw edema in albino rats. The Guduchi Ghana showed 3-fold higher reduction in paw volume (33.06%) than aqueous extract (11.71%; Patgiri et al., 2014). Similarly, in other study, ethanolic extract (500 mg/kg b. w.) exhibited maximum inhibition of edema (83.21%) in treated male wistar rats. T. cordifolia stem extract demonstrated anti-inflammatory effects in both acute (Wesley et al., 2008) and sub-acute inflammations (Ghatpande et al. 2019). Methanolic extract (1 g/kg in a 2 mL volume) of the aerial parts of T. cordifolia displayed anti-inflammatory activity against adjuvant arthritis model and bone damage in experimental animals. The antiarthritic activity difference was found significant (P < 0.05) in between extract-treated and control groups from day 17 to day 29. Methanolic extract also reduced the activity of IL-1β, TNF-α, IL-6, and IL-17 producing T cells as well as production of chemokines significantly (P < 0.05). No major change was reported in IFN-γ levels in animals of control group (Li et al., 2003; Sannegowda et al., 2015). Aqueous extract of T. cordifolia protects dopaminergic neurons by inhibiting neuroinflammation in 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine-induced Parkinsonian mouse model. Expression of tumor necrosis factor-α, interleukin-12, pro-inflammatory cytokine genes, and interleukin-1β were reported to be upregulated in 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine-intoxicated mice, while aqueous extract recovered their levels. Moreover, interleukin-10 gene was reported to be downregulated in 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine-intoxicated mice which were significantly recovered by the aqueous extract. The expression of tyrosine hydroxylase was decreased in 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine-intoxicated mice but, its expression was significantly enhanced in aqueous extract treated animals (Birla et al., 2019). Ethanol extract and ethyl acetate fraction of T. crispa stem increased the intracellular expressions of cytokine, INF-γ, IL-6, and IL-8 significantly (P ≤ 0.05) in RAW264.7 macrophages (Abood et al., 2014). Tinocrisposide isolated from T. crispa stem (1000 µg/mL) increased the membrane stability of lysosome cell that was equal to physiological property with erythrocytes and it did not show hemolytic activity (Adnan et al., 2019). Diosgenin isolated from T. sinenesis whole plant (400 µg/kg) showed maximum anti-inflammatory activity (82.25%-diosgenin; 82.01%-indomethacin; P < 0.01) against carrageenan-induced paw edema in animals (Punitha et al., 2013). The ethanol extract of T. smilacina stem and root displayed significant inhibitory effect on COX-1, COX-2, 5-LO and PA2 (IC50 63.5, 81.2, 92.1 and 30.5 µg/mL). Extracts did not show cytotoxicity on cell lines of skin fibroblasts, human Caucasian hepatocyte carcinoma and human Caucasian promyelocytic leukaemia cells (HL-60; Li et al., 2004b).
4.10 Immunomodulatory activity
Immune system is the most complex defense system of body and it helps in protection of human body from various types of infections. Modulation in immune system of body by stimulation or suppression mechanisms helps in maintaining the disease-free lifestyles (Nfambi et al., 2015). Guduchi (formulation of T. cordifolia; Himalaya Drug Company, India) showed maximum viability (94 ± 0.89%) in J774A.1 macrophage cells at dose of 80 µg/ml (More and Pai, 2011). Similarly, the aqueous extract of T. cordifolia stem (80 µg/mL) increased phagocytosis and pinocytosis (in vitro) property of macrophage J774A.1 cell line. Moreover, macrophages demonstrated an increase in phagocytosis to non-infective microbes and live Escherichia coli (More and Pai, 2017). The cordifolioside A isolated from T. cordifolia stem (0.1–2.5 µg/mL) showed significant enhancement in phagocytic activity and an increase in formation of nitric oxide and reactive oxygen species (Sharma et al., 2012). Guduchi ImP protein (10 μg/mL) demonstrated significant mitogenic activity (∼3-fold higher than control) against murine splenocytes. Due to the presence of immunomodulatory protein (Guduchi ImP protein) in T. cordifolia stem, the guduchi may be used in Ayurvedic preparations for immunomodilatory actions (Aranha et al., 2012). The aqueous extract of T. cordifolia stem (1.56 µg/ml) induced the higher production of IL-6 in splenocytes when compared with un-stimulated cells (Upadhyay et al., 2011; Kumar et al., 2011). Ethanolic extract (8 mg/kg) significantly (P < 0.001) increased the neutrophil activity in Oreochromis mossambicus (Sudhakaran et al., 2006). Magnoflorine, syringin, N-formylannonain, N-formylnornuciferine, lysicamine, and 1-octacosanol were isolated from the ethanolic extract of T. crispa stem. The isolated compound magnoflorine showed significant stimulatory effects on the chemotaxis, phagocytic activity, reactive oxygen species and nitric oxide productions as well as on secretions of IL-1β, TNF-α, IL-6, PGE2 and MCP-1 from RAW 264.7 macrophages (Ahmad et al., 2018). Aqueous extract of T. sinensis stem showed greater immunomodulatory effects than T. cordifolia in cyclophosphamide-induced anemia in male Wistar rats (Haque et al., 2017).
4.11 Wound healing activity
The healing of wounds is a complex process of repairing of injury of the skin and soft tissues. During the process of healing, an inflammation occurs and the below layers of the skin induce the formation of collagen layer. In the later stage of development, the epithelial tissue is regenerated in skin. Wound healing can be classified into four stages - inflammation, coagulation, proliferation, and remodelling at vicinity of the injury (Mutsaers et al., 1997; Garg and Paliwal, 2011). It is often considered as a major problem in clinical practices (Kokane et al., 2009). The plant-derived extracts and pure compounds are gaining importance in wound healing (Ambika and Nair, 2019). T. cordifolia cream (1 g dried powder mixed with 99 g of cold cream) was applied topically over the surface of wound of treated animals and healing was compared with untreated group (control). In treated animals, the process of epithelialisation of wound (20.333 ± 1.633%, percentage of original area in mm2) was rapid than the animals of control group (24.500 ± 2.167, percentage of original area in mm2). The results were found significant (P < 0.05) in treated animals than control group (Girish and Kamdod, 2012). Methanolic extract of T. cordifolia stem (250 mg/kg b. w.) enhanced wound healing in resutured incision, dead space, and excision wound methods significantly (P < 0.05) in male Wistar rats. The closure of wound excision in treated animals was not significantly different from that of control group on days 4, 8 but, on 16th (94.60 ± 0.40) and 18th day (99.40 ± 0.40), the significant (P < 0.0001) results were obtained. The breaking strength of 10 day old resutured incision in treated animals was significantly (P < 0.0001) higher (543.0 ± 34.63 g) than that of control (Hashilkar et al., 2016). Ethanolic extract significantly (P < 0.05) induced the healing process in resutured incision and dead space wound models (Girish and Priyadarshini, 2012; Singh et al., 2017). Methanolic extract and chloroform fraction ointment of T. crispa stem (100 mg/kg/day) showed high percentage of wound closure area and which was similar to that of standard drug (Vijendren et al., 2017).
4.12 Antimicrobial activity
Since last few decades, bacterial and fungal infections, multidrug resistance, have been the immense challenges, which threaten the health of human population. The microbial infections are causing the death of millions of people in different countries of the world. The development of multidrug resistance in microbes has caused the antimicrobial molecules to become less efficacious or even ineffective (Gupta et al., 2019; Khameneh et al., 2019). However, synthetic antibacterial and antifungal agents have been already used in various countries yet, usage of plant-derived secondary products attracts the attention of researchers (Gyawali and Ibrahim, 2014; Moloney, 2016). The plant-derived secondary metabolites have demonstrated promising results in overcoming the multidrug resistance in microbial pathogens (Rossiter et al., 2017). Plants and their products have been used as antimicrobial agents in traditional system of medicine. In developing countries, 70% population are treated with the plant-derived drugs (Mittal and Sharma, 2014; Ojiako, 2015). On first day, Escherichia coli (1x10 viable) cells were injected intraperitoneally for the evaluation of antibacterial activity of T. cordifolia aqueous extract in the adult Swiss albino mice. The aqueous extract (100 mg/kg/day by intragastric tube) of stem was administered to the albino mice. The mice mortality rate in control group was 100% while in the extract treated group, mortality rate was reported as 17.8%. In gentamicin treated animals, mortality was reported as 11.1% (P < 0.001; Thatte et al., 1992). Ethanolic extract of T. cordifolia leaf showed maximum inhibitory effects against Klebsiella pneumoniae (inhibition zone 12.0 mm) and Pseudomonas aeruginosa (inhibition zone 9.0 mm) at 400 μg/mL concentration. Similarly, the ethanol extract of stem also exhibited potent activity to K. pneumoniae (inhibition zone 15.0 mm) but displayed moderate effect against P. aeruginosa (inhibition zone 12.0 mm; 400 μg/mL; Jeyachandran et al., 2003; Shanthi and Nelson, 2013; Agarwal et al., 2019). Ethanol extract (95%) of T. cordifolia showed significant antipyretic activity in Himalayan rabbits (50 mg/mL for 10 days; Vedavathy and Rao, 1991; Prasad and Chauhan, 2019). Ethanolic extract of T. crispa roots (50 µg/disc) demonstrated maximum antibacterial activity against E. coli (inhibition zone 3.6 ± 0.2 mm; Mahammed et al. 2012).
4.13 Antileishmanial activity
Leishmania donovani is a protozoan animal, infects hyraxes, canidae, rodents, and humans and currently affecting more than 12 million people in different countries of the world. Leishmaniasis (caused by Leishmania species) is a parasitic disease found in 98 countries of the world (Alvar et al., 2012). It is considered as a serious health issue worldwide so, researchers are searching novel plant-derived agents that could be used for the treatment of leishmaniasis (Chiheb et al., 1999; El-Aasri et al., 2016). Ethanolic extract of T. sinensis stem showed significant antileishmanial activity against promastigotes (IC50 37.6 ± 6.2 μg/mL) and intracellular amastigotes (IC50 29.8 ± 3.4 μg /mL). Therefore, T. sinensis may be used in the development of alternative medicine against visceral leishmaniasis (Singh et al., 2008). The 3-hydroxy-2,9,11-trimethoxy-5,6-dihydro isoquino[3,2-a] isoquinolinylium isolated from T. sinensis demonstrated significant in vitro antileishmanial activity against Leishmania donovani (96.6% inhibition of promastigotes and 86.6% inhibition of amastigotes; Maurya et al., 2009).
4.14 Analgesic activity
Pain is an unpleasant and sensory feeling associated with potential tissue damage and its control is the most important therapeutic priority for human beings (Rang et al., 2003; Hassan et al., 2015). The prostaglandins, and bradykinins (biochemical mediators) act on the nociceptors causing the sensation discharged by tissue damage and are known as the immediate cause of pain. Rapid start and short duration of pain that last for hours are known as acute pain whereas chronic pain is distinguished by continuous pain over a long period of time (Millan, 1999; Banerjee et al., 2012). Many pharmaceutical drugs have been developed by pharmaceutical industries but they are associated with serious adverse effects such as ulceration, gastrointestinal bleeding, addictive potential, respiratory distress, drowsiness, nausea (Laurence et al., 1997; Mate et al., 2008; Raquibul et al., 2010) hence, opportunities are available for researchers in searching of plant-derived molecules for the treatment of pain. Guduchi capsules (300 mg/kg; p.o.) showed statistically significant analgesia (60 min P < 0.01; 90 min P < 0.01; 120 min P < 0.05) in treated animals (Goel et al., 2014). Aqueous-methanol (30:70) extract of T. cordifolia stem (300 mg/kg) inhibited the number of writhes in dose-dependent manner (Hussain et al., 2015). Aqueous extract of T. cordifolia leaves (200 mg/kg) showed significant (P < 0.001) increase in the reaction time (pain threshold) in treated Swiss albino mice and Wistar rats (Siddalingappa et al. 2011). Ethanol extract of T. sinensis leaves (500 mg/kg) displayed maximum (P < 0.001) increase in latency time in treated rats (Sandhyarani and Kumar, 2014b). The aqueous extract of T. crispa stem (666 mL) exhibited promising central analgesic activity (Almeida et al., 2001).
4.15 Anti-psychotic/antiepileptic activity
Due to the ambitious lifestyle, urbanization, and stressful environment, people are suffering by mental disorders. Psychosis is a one of the severe mental condition in which a sufferer experiences a distortion or loss of contact with reality and clouding of consciousness (Yadav et al., 2015). It is characterised by depression, delusion, hallucination, anxiety, sleep disturbance, thought disorder, social isolation and impaired role functioning (McNamara, 1996). Methanol extract of T. cordifolia stem showed significant increase (P < 0.05) in serum prolactin while decrease in dopamine, superoxide dismutase and catalase levels in haloperidol and sulpiride treated animals (P < 0.05; Tiwari et al., 2019). Aqueous-ethanolic extract T. cordifolia stem (500 mg/kg b.w.) did not show any antipsychotic activity in amphetamine-induced hyperactivity in mice when compared with standard compound (amphetamine). Extract (500 mg/kg b.w.) displayed a decrease in locomotor activity when compared to the control (Jain et al., 2010b).
4.16 Antiulcer activity
Ulcers are characterized by an open sore of skin or inflamed break in dead tissue of mucous membrane (Chan and Graham, 2004). There are several types of ulcers found in human beings viz, mouth ulcer, esophagus ulcer, peptic ulcer, and genital ulcer (Paguigan et al., 2014). Out of these, peptic ulcer is commonly seen among many people, and characterized by erosion of lining of stomach or the duodenum (Debjit et al., 2010; Vimala and Shoba, 2014). Aqueous extract of T. cordifolia stem (400 mg/kg) showed significant (P < 0.01) decrease in gastric volume, total acidity and ulcer index in treated animals (Chandan et al., 2013). Ethanol extract of stem (600 mg/kg) demonstrated statistically significant (P < 0.05) better protection in aspirin and ethanol-induced ulcers in animals (Khan et al., 2015). Etahnol extract also showed significant protective activity against an 8 h restraint stress induced ulcerization (Sarma et al., 1995). Aqueous extract of T. sinensis stem decreased ulcer index (P < 0.001) and ulcerogenic effects higher (85.65%) than standard drug (71.52%; Khayum et al., 2009).
4.17 Antifertility activity
Increasing human population throughout the world has detrimental effects on health care, food security, employment, education, housing, and environment hence, it suggests that fertility control is a problem but, in my opinion, it is an issue that needs to be tackled by public health (Gupta and Sharma, 2006; Mamatha et al., 2012; Devi et al., 2015). Ethanolic extract of T. cordifolia stem interrupted the process of spermatogenesis and formation of round spermatids (73.12%) in treated animals. It also reduced the serum testosterone levels and surface area of Sertoli cells when compared to controls (P < 0.001). Methanolic (70%) extract (100 mg/kg, given for 5 days) of its roots decreased the fertility index in fertile female albino rats (Fatima Rose et al., 2010).
4.18 Antimalarial activity
Malaria is considered as an important global health issue affecting large human population of the world. As per World Health Organization report, there are about 216 million people suffered in developing countries by malaria in year 2016 (WHO, 2017). Effective drug discovery is still our one of the main efforts to control malaria. During the last few decades, various antimalarial compounds have been reported from plants, and many of these compounds demonstrate potent activity against Plasmodium falciparum in vitro (Pan et al., 2018). As plant-derived secondary products are still considered as an important source for discovery and development of therapeutic agents. The aqueous extract of T. cordifolia stem in combination with chloroquine showed regression of spleen by 37–50% after six weeks and 45–69% after six months from the start of treatment (Singh, 2005). Methanol extract of T. crispa whole plant (2.5 mg/mL) showed significant antimalarial activity against P. falciparum after 72 h (Najib Nik a Rahman et al., 1999; Ihwan and Fitri, 2014). The aqueous extract of T. baenzigeri stem (500 mg/kg b. w.) significantly (P < 0.01) suppressed parasitemia in a dose-dependent manner (74.95% inhibition). Aqueous extract did not show any sign of toxicity up to the dose of 2000 mg/kg in treated rats (Ounjaijean et al., 2019).
5 Discussion
Several Tinospora species have been considered for their therapeutic properies owing to their strong antidiabetic and antiarthritic activities attributable to the presence of high contents of alkaloids, clerodane furano diterpenes and glucosides, phenolics and flavonoids (Sarma et al., 2009; Ahmad et al., 2021). Secondary metabolites have been used since long time for the treatment of human diseases and as a result, a large number of current drugs in modern medicine have been formulated from secondary metabolites. Now, various researchers are searching biologically active natural products and are trying to establish their possible therapeutic roles in disease control mechanisms (Newman et al., 2015). Such new natural products can often serve as chemical templates for the design and the synthesis of novel pharmaceutical drugs (Newman and Cragg, 2016). Indian Tinospora species possesses immunomodulatory, hypoglycaemic, antiallergic and anti-inflammatory properties (Bhalerao et al., 2016; Lade et al., 2018). T. cordifolia dried fruits are useful in jaundice and rheumatism, while the leaves are used in curing diabetes. Its roots possess antistress, antioxidant, antiulcer, and hypoglycemic properties, as well as for the treatment of visceral obstructions (Noreen et al., 1992; Prince et al., 1999).
Alkaloids are nitrogenous and basic compounds present in plant tissues as water soluble salts of organic acids (oxalic, citric, malic, and lactic acids), esters (atropine, scopolamine, cocaine, aconitine), combined with tannins (Cinchona bark), or sugars (glycoalkaloids of Solanum species) rather than as free bases. Majority of alkaloids are colourless and bitter but orange-yellow (berberine and colchicines), red (betaine), brick red (sanguinarine), or the orange-colored (canadine) alkaloids are also found in plants (Wiart, 2014; Kukula-Koch and Widelski, 2017). Magnoflorine, a quaternary alkaloid isolated from T. cordifolia has been reported to have potent anticancer and antidiabetic properties (Guo et al. 2018; Cherku et al., 2019). Recent studies have shown that magnoflorine has a certain anti-inflammatory effect (Li and Wang, 2014). Similarly, jatrorrhizine an isoquinoline alkaloid isolated from T. cordifolia has various bioactivities, such as antioxidant, low host toxicity and highly potent antimicrobial activity (Slobodnikova et al., 2004; Rackova et al., 2004).
Furanoditerpenoids are a special group of diterpenoids consisting of one or more furan rings, which are found in members of Euphorbiaceae, Fabaceae, Lamiaceae, Asteraceae, Codoniaceae, Dioscoreaceae, Fossombroniaceae, Jamesoniellaceae, Meliaceae, Menispermaceae, Olacaceae, Psathyrellaceae, Sapindaceae and Scapaniaceae families. There are seven types of furanoditerpenoids have been reported from plants: clerodane-, labdane-, cassane-, abietane-, spongian-, prenylbisabolane- and miscellaneous types (Afiyatullov et al., 2007; Bao et al., 2017; Parveen et al., 2021). The columbin-rich ethanol extract of T. cordifolia was assessed for its protective effects against acute cold stress in male Sprague-Dawley rats. The study showed significant biochemical alterations in urinary metabolites. The tricarboxylic acid cycle, renal function, gut microbiota, catecholamines and muscle metabolism were altered by cold stress and the columbin-rich extract restored them to normal level. This study forms the basis of future studies to develop potential biomarkers for cold stress in humans and lay down the optimum dosage of T. cordifolia to be given for providing immunity to the body as prophylactic and mitigating agent against cold stress (Gandhi et al., 2012).
Lignans are commonly characterized as phytoestrogens, or fiber-associated compounds found in plants of several families (Pedaliaceae, Asteraceae, Graminae) and used as common foods including grains, nuts, seeds, vegetables, and drinks such as tea, coffee. The dietary (secoisolariciresinol diglucoside) and non-dietary lignans (sesamin, matairesinol, pinoresinol and lariciresinol) possess anti-inflammatory and antioxidative properties (Yoder et al., 2015). Lignans (entroldiol and enterolactone) are involved in cytostatic activity against colon cancer cell lines (Wcislo and Szarlej-Wcislo, 2014). Sesamin and sesamolin found in T. cordifolia and T. sinensis have been demonstrated to contain several biological properties useful for human health. Excess formation of nitric oxide in lipopolysaccharide-stimulated rat primary microglia cells was significantly reduced when they were administered with sesamin or sesamolin. The neuroprotective effect of sesamin and sesamolin were also reported (in vivo) in focal cerebral ischemia stimulated by occlusion of the right common carotid artery and the right middle cerebral artery of animals (Cheng et al., 2006).
Triterpenes are composed of three terpene (C30H48) units or consist of six isoprene units, found in various plant families (Solanaceae, Araliaceae, Ranunculaceae, Leguminosae, Caryophyllaceae, Cucurbitaceae, and Umbelliferae). Triterpenes exist in a large variety of structures with nearly 200 different skeletons identified from plant kingdom (Xu et al., 2004; Rakib et al., 2020a). Based on their chemical structures, triterpenes can be divided into linear, monocyclic, dicyclic, tricyclic or up to pentacyclic compounds (Laszczyk, 2009; Connolly and Hill, 2010). Triterpenoids containing fruits (apple, grape berry, olive, tomato, and mango) possess cardio-protective and antioxidant activities. Several clinical studies have been conducted on humans assessing the potential role of triterpenoid usage in the prevention of chronic diseases, and the possible mechanisms responsible for the observed therapeutic roles (Battineni et al., 2018). Ativiral activity of betulin was widely examined for various types of viruses including human immunodeficiency virus: HIV-1NL4-3 and HIV1JRCSF (X4-HIV-1 and R5-HIV-1, respectively; IC50 0.04 µg/ml; Theo et al. 2009). Betulin also demonstrated anti-herpes symplex virus-1 activity (EC50 84.37 µg/mL; Shamsabadipour et al. 2013). Diosgenin (identified from T. sinensis) significantly (P < 0.01) reduced the mean paw edema volume at 3 h of carrageenan injection (Punitha et al., 2013).
Flavonoids are considered as the largest class of secondary metabolites containing of a broad class of polyphenolic compounds with low molecular weight that share a common skeleton of phenyl-benzo-g-pyran (C6-C3-C6), also known as the flavan nucleus, composed of two phenyl rings (A and B) connected through a pyran ring (C). Flavonoids include flavonols, flavones, flavonoids, flavanones, anthocyanidins, and isoflavones and are widely distributed the leaves, seeds, bark and flowers of plants (Queiroz Ferreira et al., 2015). Flavonoids also play a significant role on the stability, sensory, and health properties of foods (Santos-Buelga and Feliciano, 2017). Plant-derived flavonoids possess anti-viral/bacterial, anti-inflammatory, cardioprotective, anti-diabetic, anti-cancer, anti-aging, properties (Ragab et al., 2014; Tian et al., 2014; Zhang et al., 2015). Apigenin is a normal dietary flavonoid that is widely present in many fruits, vegetables and flowers and possesses anti-inflammatory, antioxidant, antibacterial and antiviral activities and antidiabetic activities (Papay et al., 2017; Wang and Huang, 2013; Zhu et al., 2016). Diosmetin demonstrated anti-inflammatory and hepatoprotective activities by inhibiting malonaldehyde, inducible nitric oxide synthase, prostaglandin E2, and cyclooxygenase-2 expression, NF-κB signaling pathway activity, hepatocyte apoptosis, and the activities of alanine aminotransferase and aspartate aminotransferase in different models (Yang et al., 2017; Chung et al., 2020).
Diabetes mellitus is an insulin-dependent metabolic disease characterized by prolonged hyperglycemia. This disease has been primarily treated by several synthetic drugs that improve the changed glycemic status in diabetic patients. However, synthetic drugs are capable, they have remarkable adverse effects together with their beneficial effects. Medicinal plants have been used to treat diabetes and related complaints in various healthcare systems around the world. The medicinal plants have been used since long time as primary health care demand, they have not been fully employed as acceptable drugs in the treatment of diabetes because of unavailability of knowledge of their chemical characterization, preparation procedure, possible adverse effects and administration doses. The large number of medicinal plants have been attributed to possess anti-diabetic activity in preliminary examinations, but most of them do not reach the clinical trial level due to lack of proper information on the above-mentioned parameters (Ahmed et al., 2021; Naveen et al., 2021). T. cordifolia one tablet (500 mg) was given three times a day for 6 months to diabetic patients as add-on therapy. After 6 months of treatment, the fasting blood glucose levels reduced significantly in treated patients (Mishr et al., 2015a).
6 Clinical studies
The use of medicinal plants and their products has been increased tremendously over the past few decades but incidents of adverse effects from usage of herbal medicines are being reported recently (WHO, 2004, 2005). The adverse effects are attributable to several factors including wrong idenfication of plant species, adulteration of herbal products, and contamination with toxic compounds, improper use of herbal medicines by healthcare providers and use of herbal medicines concomitantly with other medicines therefore, rigorous clinical trials of medicinal plants and their products are to be conducted (Singh and Sharma, 2020). One tablet of T. cordifolia (500 mg) given to healthy volunteers with morning breakfast. As per clinical studies, no healthy volunteer complained of any negative effects on clinical chemistry and hematological measurements, blood pressure, heart rate, or body weight during and after the period of drug intake at the given dose and duration (Karkal and Bairy, 2007; Table 4). T. cordifolia extract (one tablet; 500 mg) reduced the fasting blood glucose levels in the type-2 diabetic patients and also restored the altered functions of liver of patients (Mishr et al., 2015a, b). T. cordifolia pills demonstrated positive effects on surgical outcome (92% survival in treated patients and 40% in non-treated patients) of patients of obstructive jaundice (Rege et al., 1993). Capsules (formed from stem extract) improved the physical performance as well as suppressed the activation of sympathetic nervous system (Salve et al., 2015). Aqueous extract of T. cordifolia was found effective in relieving the clinical symptoms in patients of allergic rhinitis, cold and fever (Geeta et al., 2017). The aqueous extract showed statistically significant increase in total leucocyte count (P < 0.001), absolute lymphocyte count (P < 0.001) and lymphocyte percentage (P < 0.001) when compared with placebo (Sharma and Sharma, 2015). A randomized double-blind placebo-controlled trial was conducted to validate the efficacy and safety of T. crispa in the treatment of type 2 diabetes mellitus patients which of those refused insulin injection and did not respond to oral hypoglycemic drugs (Sangsuwan et al., 2004). Similarly, T. crsipa capsules (250 mg stem powder) were also used in clinical study of diabetic patients. Total twenty participants (10 diabetic and 10 healthy) were recruited for this study. Total 10 participants with diabetes mellitus type 2 (7 women and 3 men; age 32 to 64 years) were given 250 mg stem powder daily. The participants with diabetes mellitus 2 responded to oral hypoglycemic drugs and discontinued oral hypoglycemic drugs at least for 1 month during this study. Serum samples from 10 healthy and 10 diabetic participants, who had fasted overnight, were collected every 30–60 min during the 3 h of regular fasting and during the 3 h after consumption of 75 g of glucose with or without consumption of T. crispa dry powder capsule (250 mg). The mean serum glucose (areas under the curve) in both healthy and diabetic participants was not significantly different between with and without T. crispa. In diabetic patients, the mean serum glucose (areas under the curve) was slightly lower when T. crispa (250 mg dry extract) was consumed, but did not reach on levels of statistical significance (478 and 444 mg min/mL, respectively, P = 0.57). The results suggest that T. crispa consumption cannot affect the levels of serum glucose and insulin in healthy or diabetic patients (Klangjareonchai and Roongpisuthipong, 2012). T. crispa dry powder capsule (250 mg twice a day) was given for 2 months to the patients of metabolic syndrome and showed reduced levels of fasting blood glucose significantly when compared with baseline (6.29 ± 10.47 mg/dL, P < 0.01; Sriyapai et al., 2009).
Study
Age (years)
Treatment
Doses
Recommended time (in days)
Useful outcomes
References
T. cordifolia
A double-blind randomized placebo-controlled study
30 healthy volunteers (22 males and 8 females), age 18–30 years
One tablet of T. cordifolia
500 mg taken orally once a daily in the morning with breakfast
21
No volunteer complained of any adverse effects on clinical chemistry and hematological measurements, urinalysis, electrocardiogram, blood pressure, heart rate, or body weight during study
Karkal and Bairy (2007)
Add-on therapy in patients with type-2 diabetes
30 (both male and female); diabetic patients type-2; age 30–60 years
One tablet of T. cordifolia (group A was given anti-diabetic medications, T. cordifolia was given to group B patients)
500 mg was given three times daily before meals
After 6 months, the fasting blood glucose values were 121.76 ± 29.57 mg/dl in group A and 119.99 ± 34.36 mg/dl in B group; Decrease in the post prandial blood glucose levels was recorded both groups
Mishra et al. (2015a)
Add-on therapy on the blood glucose levels
100 patients (type 2 diabetic); age 30–60 years
One tablet (T. cordifolia)
500 mg was given three times daily along with meals
180
Decrease in the fasting, postprandial, and glycosylated hemoglobin levels of the patients was found statistically significant (P < 0.005) in treated patients
Mishra et al. (2015b)
Randomized controlled trial
30 (16 men and 14 women) patients of obstructive jaundice; age 17–70 years
T. cordifolia pills
65 mg/kg/day dried aqueous extract
21
Post drainage bactobilia observed in 8 patients in group I and 7 in group II but clinical evidence of septicemia was observed in 50% patients in group I as against none in group II; Pills demonstrated positive effects on surgical outcome (92% survival in treated patients and 40% in non-treated patients)
Rege et al. (1993)
Open-label,
randomized, placebo-controlled trial
30 participants; blood pressure patients; age 18–45 years
T. cordifolia capsule (TC)
Maize starch capsule (placebo), 300 mg/kg/day
28
Capsule (on day 28) showed a significant decrease in mean systolic blood pressure on fixed workload exercise compared to placebo; improved physical performance and suppressed over activation of the sympathetic nervous system
Salve et al. (2015)
Open label-controlled trial
30 (15 male and 15 female) healthy persons as control, 60 (male-30, female-30) type- 2 diabetic patients; age 47–57 years
Stem powder
50 mg/kg b. w./day/ stem powder macerated with water; p.o.
15
Stem powder increased the levels of fasting blood sugar (57%), glycosylated hemoglobin (40%); also caused reversal in the levels (fasting blood sugar 9%, glycosylated hemoglobin 14%) in treated patients
Kumar et al. (2016)
Open label-controlled trial
30 clinically diagnosed patients of Sthaulya Roga (obesity); age 16–60 years
Guduchi Sattva Kalpana (group I) and Guduchi Kwatha. Kalpana (group II)
250–500 mg (Guduchi Sattva) 50–100 mL (Guduchi Kwatha) administered 1 h after meal
30
Group A— Patients were treated with Guduchi Sattva Kalpana; group B—Patients were treated with Guduchi Kwatha Kalpana; Guduchi Kwatha is more effective than Guduchi Sattva; Kwatha gets absorbed more easily and quickly than the Sattva which has large amount of starch in it
Kapoor et al. (2013)
Diabetic patients from out patients department (OPD) and inpatient departments (IPD)
50 patients (diabetes mellitus type II)
Group A-treated with Guduchi sattava along with diet and exercise
Group B-treated with diet and exercise only; age 30–70 years
Guduchi sattava
1 g (twice daily) was given orally
90
Guduchi sattava was found very effective in the treatment of diabetes; initial mean value of serum cholesterol level of group A patients was 150 and group B patients was 148
Kundu et al. (2016)
–
25 patients (acute conditions like fever, cold, allergy and rhinitis)
T. cordifolia extract in capsule form (from the Himalaya Drug Company)
250 mg/thrice a day
15
Capsule significantly changed the neutrophil, and eosinophil counts (P < 0.05); capsules were found effective in relieving the clinical symptoms in cases of allergic rhinitis, cold and fever
Geeta et al. (2017)
Double blind, randomized and placebo controlled
Thirty healthy volunteers (male-22 and female-8), visual, logical and verbal memories; age 18–30 years
Stem aqueous extract
One tablet (500 mg) placebo was taken once daily (p.o.) in the morning
21
Extract enhanced verbal, logical and learning memories (of immediate and short-term type) in compare to placebo in healthy volunteers
Bairy et al. (2004)
Open labelled randomized controlled trial
Thirty patients (type-2 diabetic); age 50–60 years
Encapsulated mature stem
250 mg pre-meal twice a day along with prescribed oral hypoglycemic agents and statin and control group (n = 30) only on OHAs and statin
60
Intervention led to significant reductions in total cholesterol, low density lipoprotein, triglycerides and very low-density lipoprotein; no toxicity was observed in patients
Roy et al. (2015)
Comparative open clinical trial
30 patients (14 men, 16 women) with premature aging due to stress (without any other acute or serious systemic disorders); age 25–60 years
Capsules prepared from the drugs Guduchi (T. cordifolia)
Group A − 500 mg soft gelatin capsules of Guduchi were given for 3 months; group B –500 mg soft gelatin capsules of Amalaki were given for 3 months; group C- 500 mg/day capsule of Guduchi and 500 mg/day capsule Amalaki were given for 3 months, once daily in the form of soft gelatin capsule
90
Guduchi and Amalaki were found effective in delaying premature ageing due to stress, but considering the small size of the trial population, the trial should further be extended to larger sample size and for longer trial duration to draw more conclusive evidence
Pal et al. (2017)
An open-labelled, placebo-controlled, randomized controlled trial
400 children (status of immunity in children); children with genetic disorders, severe or chronic illnesses, congenital diseases, mentally challenged; age 1–15 years
Stem and leaf powder
100 mg/kg b. w. twice a daily after food with honey
60
Stem and leaf powder showed significant increase in total leucocyte count (P < 0.001), absolute lymphocyte count (P < 0.001) and lymphocyte percentage (P < 0.001) as compared to placebo; powder significantly improved immunity in children and can be used as an adjuvant to vaccination
Sharma and Sharma (2015)
A randomized, double-blind, placebo-controlled
250 patients (dengue); age 18–75 years
Leaf extract, 5 mL twice daily or placebo for five consecutive days
5 mL dosage
5
Extract showed significant increase (P < 0.05) in platelet count; can be used to treat thrombocytopenia
Babu et al. (2017)
A randomized double-blind placebo controlled
Seventy-five patients of allergic rhinitis
Stem aqueous extract
–
56
Extract showed 100% relief from sneezing in 83% patients, in 69% from nasal discharge, in 61% from nasal obstruction and in 71% from nasal pruritus
Badar et al. (2005)
A single blind, randomized controlled trial
66 pediatric patients diagnosed with scabies; age 2–22 years
T. cordifolia lotion (T. cordifolia extract was mixed with 0.15 g methyl paraben and 39.70 g water to form the water paste)
This was done daily for three consecutive days per week for two weeks
14
Lotion exhibited anti-scabies activity comparable with permethrin; its incorporation as therapeutic reagent in Sarcoptes scabiei infections is highly recommended
Castillo et al. (2013)
Double-blind, split-mouth study
Forty-eight patients (diagnosed suffering from periodontitis with or without dental fluorosis); age 18–50 years
Guduchi gel [Guduchi stem powder (1500 g); the guduchi gel was prepared in Bapuji Pharmacy College]
6.25%
21
Guduchi gel was delivered into each periodontal pocket using sterile Dispo‑Van™ single-use bent needle till the gel overflow the site; guduchi gel (6.25%) showed clinically significant anti-inflammatory and antimicrobial effects along with scaling and root planning
Ghosh et al. (2017)
Controlled clinical trial
60 patients (post-operative and traumatic fresh wounds were selected from outpatient department and inpatient department of Shalya Tantra)
Guduchi (stem fiber) as suturing material for skin suturing in post-operative and traumatic wounds
–
7
Guduchi (stem fibers) sutures can be used as a safe alternative for cotton thread in skin closure
Shrestha et al. (2017)
An open label, non-comparative, interventional, and exploratory clinical trial
30 children (19 male and 11 female); growth of children; age 6–8 years
Stem juice
20 mL/day (empty stomach morning)
90
Mean score of skin luster was 0.43 ± 0.5 and it was significantly increased to 1.17 ± 0.37; diet intake was increased significantly; can be used in herbal formulation for the growth of children
Patil (2012)
A prospective double-blind randomized controlled study
Forty-five patients [23 study group (17 males and 6 females) + 22 control group (19 males and 3 females)]; chronic diabetic patients with wounds; age 56.9 years
Stem extract
–
537
Stem extract showed significant improvement in wound healing; also reduced debridements and improved phagocytosis, indicating beneficial effects of immunomodulation for ulcer healing
Purandare, and Supe (2007)
Randomized double-blind placebo-controlled trial
68 patients HIV positive; age 18 – 50 years
Stem aqueous extract (Tinofend, Pharmanza India Ltd, Gujarat)
300 mg
–
Extract caused significant reduction in eosinophil count and hemoglobin percentage; some of common complaints reported in patients (anorexia, nausea, vomiting, and weakness)
Kalikar et al. (2008)
T. crispa
Randomized double-blind placebo-controlled trial
20 patients with type 2 diabetes mellitus
Stem powder
1 g in capsule given thrice a day
180
Changes in fasting plasma glucose, glycosylated hemoglobin and insulin levels were observed in treated patients; the patients receiving stem powder may have an increased risk of hepatic dysfunction
Sangsuwan et al. (2004)
7 Toxicological effects
Most of the medicinal plants are non-toxic but, some are poisonous to humans causing damage to certain organs in the body (Okoye et al., 2014). Global use of medicinal plants is growing rapidly and many more new plant-derived products are introduced into the market so, proper attention is needed in safe use of medicinal plants in the treatment of diseases (Ekor, 2014). In alcoholics patients, the higher levels of γ-glutamyl transferase, aspartate transaminase, alanine transaminase, triglycerides, cholesterol, high density lipoproteins and low-density lipoproteins (P < 0.05) were reported but aqueous extract of T. cordifolia stem decreased their levels in treated patients (Sharma and Dabur, 2016). Acute toxicity result showed that drug (T. cordifolia aqueous extract) did not show any signs and symptoms of toxicity or mortality up to an oral dose of 2000 mg/kg in rats. Although the drug (aqueous extract) demonstrated mild to moderate adverse changes in kidney, liver, intestine, and stomach of patients at therapeutic equivalent dose (×10 dose level) in a clinical trial study (Gokarn et al., 2017). No acute toxicity was reported in male albino mice treated (p.o.) with aqueous extract (Sengupta et al., 2011). Embryo-toxic effect of aqueous extracts of T. cordifolia leaf and bark was dependent on dose and time of exposure. Leaf extract (10%) showed maximum mortality (100%) while bark extract (10%) exhibited 33.33% mortality in treated animals (Zebrafish embryos; Romagosa et al., 2016). T. cordifolia stem is used in the management of depression, treatment of Alzheimer's disease and attention deficit hyperactivity problems. No evidence is available in the literature of serious toxicity of T. cordifolia in depression management, Alzheimer's disease and attention deficit hyperactivity disorder (Mutalik, 2011). The high doses of T. crispa extract (the patients who received 10 pellets of T. crispa per day) showed a complaint of dark urine and pale stools, linked with asthenia and right hypochondrial pain which led to jaundice. The histopathological observations also confirmed a toxic reaction in treated patients (Langrand et al., 2014). Acute toxicity results of aqueous extract of T. crispa stem displayed LD50 values of about 20–24 g/kg and found safe during study (Kongkathip et al., 2006). Satwa (T. sinensis formulation) reduced the elevated levels of serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and total bilirubin in paracetamol-induced hepatic toxicity of experimental animals (Nagarkar et al., 2013). Aqueous, alcoholic (up to a dose of 5000 mg/kg b. w.) and petroleum ether extracts (2000 mg/kg) of T. sinensis stem did not show any adverse effects in treated Swiss albino mice (pylorus ligation induced gastric ulcers; Khayum et al., 2009). Short-term uses of T. cordifolia aqueous extract confirm its safety but long-term use may cause constipation, decrease in blood sugar levels, overstimulation of immune system, complications in pregnant women (Bhardwaj, 2003; Table 5).
Plant species
Plant part
Extract/compound
Dose
Type of toxicity
Model/ symptoms
Reference
T. cordifolia
Fresh stem
Aqueous extract
100 mL extract (3.0 g solid extract) given early in the morning with an empty stomach for 14 days
Chronic liver disease in humans
Extract showed significant decrease in the levels of γ-glutamyl transferase, aspartate transaminase, alanine transaminase, triglyceride, cholesterol, high density lipoproteins and low-density lipoprotein while displayed increase in intestinal absorption and retaining power of liver (that regulated by alcohol-induced multivitamin deficiency) in treated patients
Sharma and Dabur (2016)
Solidified aqueous extract
2000 mg/kg given for 90 days
Oral acute toxicity in humans
In clinical trials, the acute toxicity result showed that drug did not produce any signs and symptoms of toxicity or mortality up to an oral dose of 2000 mg/kg in rats; although the extract demonstrated mild to moderate adverse changes (in kidney, liver, intestine and stomach) at therapeutic equivalent dose × 10 dose level
Gokarn et al. (2017)
Aqueous extract (from Chaitanya Pharmaceuticals Ltd, India)
100, 200 or 400 mg/kg b. w.
Acute oral toxicity in rats
Reported results indicate that the extract therapy was able to maintain circulating iron through reduction of inflammatory cytokines and expression of hepcidin in rats
Ghatpande et al. (2019)
Stem parts
Aqueous extract
150 mg/kg b. w.
Acute toxicity in albino mice; p.o.
No acute toxicity was reported in treated male albino mice
Sengupta et al. (2011)
Stem
Aqueous extract
300 mg/patient
Patients of HIV positive
Some of common complaints viz. anorexia, nausea, vomiting, and weakness reported in treated patients
Kalikar et al. (2008)
Leaf and stem bark
Aqueous extract of leaf and stem bark
Embryos were exposed to the different concentrations (10%, 5%, 1%, 0.5%, 0.1%, 0.05%, 0.01%) of each extract
Embryo-toxic and teratogenic effects in Zebrafish (Danio rerio) embryos
Maximum mortality (100%) displayed by leaf extract while bark extract showed mortality of 11.11% and 33.33% at 5% and 10% concentrations
Romagosa et al. (2016)
T. crispa
Stem
Aqueous extract
Chronic use of high doses (10 pellets per day) was taken
49-Year-old male with chronic low back pain
T. crispa induced toxic hepatitis; problem of dark urine and pale stools, linked with asthenia and right hypochondrial pain which led to jaundice; recovery completed after discontinuation
Langrand et al. (2014)
Ethanolic
100 and 200 mg/kg b. w. for 8 weeks
Thioacetamide-induced hepatotoxicity in Sprague Dawley rats
Ethanol extract contained certain hepatotoxins which may be responsible for this effect
Kadir et al. (2011)
4.0 g/kg b. w. per day for 6 months
Bile duct proliferation and focal liver cell hyperplasia
Extract did not cause any signs of toxicity or animal death at a dose of 4.0 g/kg of body weight (g/kg b. w.) but at a dose of 9.26 g/kg/b. w./day to rats caused hepatic and renal toxicities
Chavalittumrong et al. (1997)
Herbal preparation
400 mg daily
37 malaria patients
A human hepatotoxicity case was reported due to chronic over use of herbal preparation as a prophylactic agent against malaria
Denis et al. (2007)
T. sinensis
Fresh stem
Aqueous extract
200 and 400 mg/kg b. w. (p.o.; male Wistar rats)
Paracetamol induced hepatic toxicity
Extract significantly reduced the paracetamol induced elevated levels of serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and total bilirubin in experimental animals
Nagarkar et al. (2013)
Stem
Aqueous, ethanolic and petroleum ether extracts
5000 mg/kg b. w. (adult Swiss albino mice and Wistar rats)
Pylorus ligation induced gastric ulcers and water immersion stress induced ulcer
Aqueous and alcoholic extracts up to a dose of 5000 mg/kg and petroleum ether extract up to a dose of 2000 mg/kg were found safe in treated animals
Khayum et al. (2009)
8 Tinospora formulations
Some Tinospora formulations are used in preclinical and clinical preparations with hepatoprotective, antioxidant, pancreatic islet superoxide dismutase, antiatherogenic, antiarthritis, immunomodulatory, antihyperglycemic, antihyperlipidemic, antidepressant, antiamoebic, and antistress properties (Tasaduq et al., 2003; Deole et al., 2011; Sohni et al., 1995; Nasreen, 2011; Bhattacharya et al. 1997; Mary et al., 2003; Chavali and Forse, 1997). T. cordifolia capsules are used in the treatment of diabetes. The capsule is filled with 500 mg of T. cordifolia ethanol extract, 2% (w/w) tannins, bitters 10 mg per capsule, as well as 75 mg polysaccharides. Similalrly, tinosporine capsule is filled with water soluble extractive 81.40% (w/w), alcohol soluble extractive 3.85% (w/w), tannins 14.37 (mg/capsule), bitters 12.08 (mg/capsule), and polysaccharides 122.40 (mg/capsule; Rane et al., 2017). T. cordifolia displays protective effect by immunomodulation of polymorpho nuclear cells, phagocytes and on macrophage function. The capsule contains a polysaccharide which is responsible for immune-stimulatory effects. It acts by inducing the phagocytic activity of macrophages, formation of reactive oxygen species and nitric oxide in human neutrophil cells (Khare and Katiyar, 2012; Salkar et al. 2014). Rasna Saptak Kwatha is an Ayurvedic polyherbal decoction prescribed as medicine for the treatment of arthritis. Rasna Saptak Kwatha has been traditionally used by Ayurvedic practitioners, to treat various painful afflictions and body joints swelling (Sharma, 2011). Its formulation contains medicinal plants 4 viz. Pluchea lanceolata, Tribulus terrestris, Tinospora cardifolia, Boerhavia diffusa, Ricinus communis, Cedrus deodara, Cassia fistula and Zingiber officinale (Pandey and Chaudhary, 2017). Crude ethanolic extract of T. cordifolia stem incorporated in gel base (10%) showed significant enhancement in tensile strength (P < 0.05) of the wound when compared with Curiosin gel and reduction in mean paw size (P < 0.001) of the Sprague-Dawley rats when compared with Voltaren as standard drug, respectively. The gel demonstrated negligible irritant activity therefore, T. cordifolia gel can be used safely as topical formulations to treat wounds and inflammation (Bagon et al., 2016).
The Hemoliv and HP-1 formulations displayed protective effects against carbon tetrachloride-induced hepatic injury in rats (Panchabhai et al., 2008). The Caps HT2, a polyherbal formulation, containing T. cordifolia methanol extract presented anticoagulant, platelet antiaggregatory, lipoprotein lipase releasing, and hypolipidemic properties (Mary et al., 2003). The Diasulin and Dihar (two polyherbal formulations) containing ethanolic extract of T. cordifolia roots displayed antihyperlipidemic, antiperoxidative and antioxidant properties (Patel et al., 2009; Saravanan and Pari, 2005). The therapeutic uses of T. cordifolia in Ayurvedic formulations with different doses have been validated by practitioners (Panchabhai et al., 2008).
9 Conclusions and future prospects
Different parts of Indian Tinospora species are used in the treatment of fever, urinary diseases, asthma, gout, diabetes, diarrhoea, and skin infections. The presence of various bioactive compounds provides the plant for its antidiabetic, antioxidant, anticancer, antimicrobial, antifertility, hepatoprotective, radioprotective, antiarthritic antioxidant, hypolipidemic, and anti-inflammatory activities. The clerodane furano diterpene glycosides showed promising anticancer and isoquinoline alkaloids exhibited antidiabetic activities in different models. T. cordifolia stem has been used by Indian people in the treatment of diabetes. Antidiabetic efficacy and safety of T. cordifolia stem capsules (dry powder) in diabetic patients has proven in clinical trial as well as in Indian people. This attribute of T. cordifolia may be exploited by commercialization of these plants for the identification of potential compounds for the treatment of diabetes. T. cordifolia capsules (300 mg/kg/day) showed a significant decrease in mean systolic blood pressure on fixed workload exercise when compared to placebo and also improved the physical performance. Capsules significantly changed the neutrophil, and eosinophil counts (P < 0.05) and were found effective in relieving the clinical symptoms in cases of allergic rhinitis, cold and fever. Although some studies have done to reveal the antidiabetic activities of T. cordifolia, its true potential is yet to be acknowledged. But there is still a need to conduct further scientific-based study to explore the chemical characterizations and pharmacological evaluations of 5 Indian Tinospora species (T. formanii, T. glabra, T. maqsoodiana, T. smilacina, and T. subcordata). More scientific studies are to be conducted on therapeutic and toxicological properties of Tinospora that will help in the development of new therapeutic products in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ethnomedicine based evaluation of osteoprotective properties of Tinospora cordifolia on in vitro and in vivo model systems. Biomed. Pharmacother.. 2017;87:341-354.
- [Google Scholar]
- Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-γ, IL-6 and IL-8. BMC Complement. Alter. Med.. 2014;14:205.
- [Google Scholar]
- Medicinal plants with cholinesterase inhibitory activity: A review. African J. Biotechnol.. 2010;9:8257-8276.
- [Google Scholar]
- Potential role of seeds from India in diabetes. In: Preedy V.R., Watson R.R., eds. Nuts and seeds in health and disease prevention. New York: Academic Press; 2020. p. :365-391.
- [Google Scholar]
- In vitro anti-inflammatory activity test of tinocrisposide and freeze-dried aqueous extract of Tinospora crispa stems on human red blood cell by increasing membrane stability experiment. Asian J. Pharm. Clin. Res.. 2019;12:125-129.
- [Google Scholar]
- Cytotoxic activity assay of tinocrisposide from Tinospora crispa on human cancer cells. Der Pharmacia Lett.. 2016;8:102-106.
- [Google Scholar]
- Isolation and structures of erylosides from the Carribean sponge Erylus goffrilleri. J. Nat. Prod.. 2007;70:1871-1877.
- [Google Scholar]
- Effect of Tinospora cordifolia on learning and memory in normal and memory deficit rats. Indian J. Pharmacol.. 2002;34:339-349.
- [Google Scholar]
- Assessment of antimicrobial activity of different concentrations of Tinospora cordifolia against Streptococcus mutans: An in vitro study. Dent. Res. J. (Isfahan). 2019;16:24-28.
- [Google Scholar]
- Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms. Food Chem. Toxicol.. 2012;50:3126-3132.
- [Google Scholar]
- Biotechnological approach to evaluate the immunomodulatory activity of ethanolic extract of Tinospora cordifolia stem (Mango Plant Climber) Iran J. Pharm. Res.. 2012;11:863-872.
- [Google Scholar]
- New phytoconstituents from the stem bark of Tinospora cordifolia Miers. Nat. Prod. Res.. 2010;24:926-934.
- [Google Scholar]
- Evaluation of clinical trials for natural products used in diabetes: An evidence-based systemic literature review. Medicine. 2021;100:e25641
- [Google Scholar]
- Evaluation of in vitro anticancer activity of stem of Tinospora cordifolia against human breast cancer and Vero cell lines. J. Med. Plants Res.. 2015;3:33-37.
- [Google Scholar]
- Ahmad, W., Jantan, I., Bukhari, S.N.A., 2016. Tinospora crispa (L.) Hook. f. & Thomson: A review of its ethnobotanical, phytochemical, and pharmacological aspects. Front. Pharmacol. 7, 59.
- Immunomodulatory effects of Tinospora crispa extract and its major compounds on the immune functions of RAW 264.7 macrophages. Int. Immunopharmacol.. 2018;60:141-151.
- [Google Scholar]
- Molecular phylogeny in Indian Tinospora species by DNA based molecular markers. Plant Syst. Evol.. 2006;256:75-87.
- [Google Scholar]
- Crispenes F and G, cis-clerodane furanoditerpenoids from Tinospora crispa, inhibit STAT3 dimerization. J. Nat. Prod.. 2018;81:236-242.
- [Google Scholar]
- Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid palmatine. Sci. World J.. 2013;2013:376216
- [Google Scholar]
- Plants with central analgesic activity. Phytomed.. 2001;8:310-322.
- Tinospora cordifolia aqueous extract alleviates cyclophosphamide- induced immune suppression, toxicity and systemic candidiasis in immunosuppressed mice: In vivo study in comparison to antifungal drug fluconazole. Curr. Pharm. Biotechnol.. 2019;20:1055-1063.
- [Google Scholar]
- Immune-stimulatory and therapeutic activity of Tinospora cordifolia: Double-edged sword against salmonellosis. J. Immunol. Res.. 2017;2017:1787803.
- [Google Scholar]
- Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671
- [Google Scholar]
- Wound healing activity of plants from the Convolvulaceae family. Adv. Wound Care (New Rochelle). 2019;8:28-37.
- [Google Scholar]
- Wealth of India: Raw materials. Vol Vol X. New Delhi: CSIR; 1976.
- Gastroprotective effect of epoxy clerodane diterpene isolated from Tinospora cordifolia Miers (Guduchi) on indomethacin-induced gastric ulcer in rats. Phytomed.. 2014;21:966-969.
- [Google Scholar]
- Hypoglycemic effect of Tinospora crispa (Linn.) Mier ex Hook F. & Thorns (Menispermaceae) in Rats. Bull. Depart. Med. Sci.. 2012;41:231-243.
- [Google Scholar]
- Capparis spinosa L.: A plant with high potential for development of functional foods and nutraceuticals/pharmaceuticals. Int. J. Pharmacol.. 2016;12:201-219.
- [Google Scholar]
- Humoral immune and adjuvant responses of mucosally-administered Tinospora cordifolia immunomodulatory protein in BALB/c mice. J. Ayurveda Integr. Med.. 2020;11:140-146.
- [Google Scholar]
- Immunostimulatory properties of the major protein from the stem of the Ayurvedic medicinal herb, guduchi (Tinospora cordifolia) J. Ethnopharmacol.. 2012;139:366-372.
- [Google Scholar]
- Wound healing potential of Tinospora crispa (Willd.) Miers [Menispermaceae] stem on diabetic mice. J. Med. Plants Stud.. 2015;3:106-109.
- [Google Scholar]
- Cydonia oblonga M., a medicinal plant rich in phytonutrients for pharmaceuticals. Front. Pharmacol.. 2016;7:163.
- [Google Scholar]
- Evaluation of antiulcer activity of Tinospora sinensis leaves. Singapore J. Pharm. Res.. 2014;1:8-11.
- [Google Scholar]
- Awang, N., Nasir, N.N.M., Kamaludin, N.F., Nur Faizah Abu Bakar, N.F.A., 2020. Evaluation of the antimicrobial activities of crude extracts of Tinospora crispa stem. J. Biol. Sci. 20, 94 – 99.
- Babu, R., Kubavat, S., Poulose, K.P., Srinivasa Kumar, K.P., Handae, S., Satyanarayan, Dhamodaran, V., Elangovan, B., 2017. A randomized, double-blind, placebo-controlled, proof of concept study to assess the safety and efficacy of Carica papaya and Tinospora cordifolia leaf extract (thrombobliss) in subjects undergoing chemotherapy treatment and subjects with systemic microbial infection and subsequent reduction in platelet count. Int. J. Clin. Trials 4, 116 – 121.
- Efficacy of Tinospora cordifolia in allergic rhinitis. J. Ethnopharmacol.. 2005;96:445-449.
- [Google Scholar]
- Gel trial formulation of the crude ethanolic extract of Tinospora cordifolia (willd.) miers. stem and evaluation of its anti-inflammatory, wound-healing and skin irritation activities. Planta Med.. 2016;82:PB16.
- [Google Scholar]
- Efficacy of Tinospora cordifolia on learning and memory in healthy volunteers: A double-blind, randomized, placebo controlled study. Iranian J. Pharmacol. Ther.. 2004;3:57-60.
- [Google Scholar]
- Bajguz, A., Bąkała, I., Talarek, M., 2015. Ecdysteroids in plants and their pharmacological effects in vertebrates and humans. In: Atta-ur-Rahman (Ed), Studies in natural products chemistry. Elsevier, Amsterdam 45, 121 – 145.
- Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol.. 2015;175:131-137.
- [Google Scholar]
- Chemical prospection of important Ayurvedic plant Tinospora cordifolia by UPLC-DAD-ESI-QTOF-MS/MS and NMR. Nat. Prod. Commun.. 2015;10:43-48.
- [Google Scholar]
- Ethnomedicinal uses of plants among the Newar community of pharping village of Kathmandu District. Nepal. Tribhuvan Univ. J.. 2004;24:13-19.
- [Google Scholar]
- Alkaline peroxide pretreatment of corn stover: effects of biomass, peroxide, and enzyme loading and composition on yields of glucose and xylose. Biotechnol. Biofuels.. 2011;4:16-30.
- [Google Scholar]
- Evaluation of analgesic activities of methanolic extract of medicinal plant Juniperus communis Linn. Int. J. Pharm. Pharm. Sci.. 2012;4:550-555.
- [Google Scholar]
- Naturally occurring furanoditerpenoids: Distribution, chemistry and their pharmacological activities. Phytochem.. 2017;16:235-270.
- [Google Scholar]
- Traditional bush medicine, an aboriginal pharmacopoeia. Victoria, Australia: Greenhouse Publications; 1988.
- Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: A review. Phytotherapy Res.. 2018;32:1181-1210.
- [Google Scholar]
- Tinospora cordifolia (Thunb.) Miers (Guduchi) – An overview. Int. J. Green Herbal Chem.. 2016;5:1-12.
- [Google Scholar]
- A review on medicinal properties of Tinospora cordifolia. Int. J. Sci. Res. Rev.. 2018;7:585-598.
- [Google Scholar]
- Bhardwaj, K., 2011. Ethnomedicinal properties of Bharatpur district (Rajasthan) plants. MSc Dissertation, MSJ (PG) College, Bharatpur, India.
- Ethnobotanical investigation of Rajasthani plants for antidiabetic property. In: Department of Botany. Jaipur, India: University of Rajasthan; 2003.
- [Google Scholar]
- Effect of Trasina, an Ayurvedic herbal formulation, on pancreatic islet superoxide dismutase activity in hyperglycemic rats. Indian J. Exp. Biol.. 1997;35:297-299.
- [Google Scholar]
- Therapeutic potential of Giloe Tinospora cordifolia: The magical herb of Ayurveda. Int. J. Pharm. Biol. Archiv.. 2013;4:558-584.
- [Google Scholar]
- Tinospora cordifolia suppresses neuroinflammation in Parkinsonian mouse model. Neuromolecular Med.. 2019;21:42-53.
- [Google Scholar]
- Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J. Toxicol. Sci.. 2002;27:139-146.
- [Google Scholar]
- Evaluation of antistress activity of extract of Tinospora cordifolia & Asparagus racemosus in rats. Int. J. Ayurveda Pharma. Res.. 2015;3:35-39.
- [Google Scholar]
- Antistress activity of Tinospora cordifolia with application of Yoga. Int. J. Ayurv. Med.. 2015;6:220-224.
- [Google Scholar]
- Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol.. 2002;79:379-381.
- [Google Scholar]
- Efficacy and safety of Tinospora cordifolia lotion in Sarcoptes scabiei var hominis-infected pediatric patients: A single blind, randomized controlled trial. J. Pharmacol. Pharmacother.. 2013;4:39-46.
- [Google Scholar]
- Antioxidant and lipophilic constituents of Tinospora crispa. Planta Med.. 1998;64:393-396.
- [Google Scholar]
- The wealth of India. CSIR, New Delhi: PID; 1948.
- Review article: Prevention of non-steroidal anti-inflammatory drug gastrointestinal complications—review and recommendations based on risk assessment. Aliment Pharmacol. Ther.. 2004;19:1051-1061.
- [Google Scholar]
- Evaluation of antiulcer activity of Tinospora cordifolia in albino rats. Int. J. Pharm. Biosci.. 2013;4:78-85.
- [Google Scholar]
- Chavali, S.R., Forse, R.A., 1997. Formulation for alleviating symptoms associated with arthritis. Appl No. 691865, US005683698.
- Toxicological study of crude extract of Tinospora crispa Mier ex Hook F. & Thoms. Thai J. Pharm. Sci.. 1997;21:199-210.
- [Google Scholar]
- Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian. J. Endocrinol. Metab.. 2016;20:546-551.
- [Google Scholar]
- Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204-7218.
- [Google Scholar]
- Treatment for supine and prone external ratationed ankle fracture. J. Guangzhou Univ. Trad. Chinese Med.. 2007;24:456-458.
- [Google Scholar]
- Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int J Biomed Sci.. 2006;2:284-288.
- [Google Scholar]
- Inhibitory activity of leaf extract of Tinospora cordifolia and magnoflorine on aldose reductase for control of diabetes. Int. J. Green Pharm.. 2019;13:186-192.
- [Google Scholar]
- Genus Tinospora: Ethnopharmacology, phytochemistry, and pharmacology. Evid. Based Complement. Alter. Med.. 2016;2016:9232593.
- [Google Scholar]
- Leishmania tropica cutaneous leishmaniasis in an emerging focus in North Morocco: New clinical forms. Ann Dermatol. Venereol.. 1999;126:419-422.
- [Google Scholar]
- Protoberberine alkaloids from callus and cell suspension cultures of Tinospora cordifolia. Pharm. Biol.. 2003;41:81-86.
- [Google Scholar]
- Regular monoterpenes and sesquiterpenes (essential oils). Berlin, Heidelberg: Springer; 2013.
- Chopra's indigenous drugs of India. Calcutta: BK Dhur Acad. Publ; 1994.
- cis-Clerodane-type furanoditerpenoids from Tinospora crispa. J. Nat. Prod.. 2010;73:541-547.
- [Google Scholar]
- Tinospora cordifolia: Ethnobotany, phytopharmacology and phytochemistry aspects. Int. J. Pharm. Sci. Res.. 2013;4:891-899.
- [Google Scholar]
- Stress as a medical and scientific idea and its implications. Adv. Pharmacol.. 1998;42:552-556.
- [Google Scholar]
- Comparative study of the effects of diosmin and diosmetin on fat accumulation, dyslipidemia, and glucose intolerance in mice fed a high-fat high-sucrose diet. Food Sci. Nutr.. 2020;8:5976-5984.
- [Google Scholar]
- Studies on the constituents of the dry stem of Tinospora crispa (Lour.). Taichung, Taiwan: China Medical University; 2011. Merr. Masters Dissertation
- Cook, N.C., and S. Samman, S., 1996. Review: flavonoids-chemistry, metabolism, cardioprotective effects and dietary sources. J. Nutr. Biochem. 7, 66 – 76.
- Wild medicine in Australia. Sydney: William Collins; 1981.
- Liv. 52 in the prevention of hepatotoxicity in patients receiving antitubercular drugs: A meta-analysis. Indian J. Clin. Practice. 2010;21:81-86.
- [Google Scholar]
- Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Topics Curr. Chem.. 2000;209:53-95.
- [Google Scholar]
- Recent trends of treatment and medication peptic ulcerative disorder. Int. J. PharmTech Res.. 2010;2:970-980.
- [Google Scholar]
- Deepika, L.P., Priyambada, J.S., Sowmya., 2016 Analgesic, anti-inflammatory activity of Tinospora cordifolia (Guduchi) and Valeriana wallichi (Tagara) in albino rats. J. Pharm. Biol. Sci. 11, 18 – 22.
- Malarial prophylaxis with medicinal plants: Toxic hepatitis due to Tinospora crispa. Therapie. 2007;62:271-272.
- [Google Scholar]
- Evaluation of anti-depressant and anxiolytic activity of Rasayana Ghana tablet (A compound Ayurvedic formulation) in albino mice. Ayu. 2011;32:375-379.
- [Google Scholar]
- Antifertility activity of medicinal plants on male and female reproduction. Int. J. Pharm. Sci. Res.. 2015;6:988-1001.
- [Google Scholar]
- Medicinal and beneficial health applications of Tinospora cordifolia (Guduchi): A miraculous herb countering various diseases/disorders and its immunomodulatory effects. Recent Patents Endocrine Metabolic Immune Drug Discovery. 2017;10:96-111.
- [Google Scholar]
- Hepatoprotective effects of Tinospora cordifolia extyract in streptozotocin induced damage in diabetic rats. Indian J. Vet. Pathol.. 2013;37:153-158.
- [Google Scholar]
- Kancolla seeds: High nutritional foods with nutraceutical properties. In: Preedy V.R., Watson R.R., eds. Nuts and seeds in health and disease prevention. New York: Academic Press; 2020. p. :211-227.
- [Google Scholar]
- A new dinorclerone diterpenoid glycoside from Tinospora sinensis. Nat. Prod. Res.. 2010;24:13-17.
- [Google Scholar]
- Radiation in medicine: Origins, risks and aspirations. Glob. Cardiol. Sci. Pract.. 2014;2014:437-448.
- [Google Scholar]
- The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol.. 2014;4:177.
- [Google Scholar]
- Epidemiology of cutaneous leishmaniasis in Sidi Kacem province, Northwestern Morocco (2006–2014) Asian Pac. J. Trop. Dis.. 2016;6:783-786.
- [Google Scholar]
- Plants-derived neuroprotective agents: Cutting the cycle of cell death through multiple mechanisms. Evidence-Based Complement. Alter. Med.. 2017;2017:3574012.
- [Google Scholar]
- Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci.. 2012;3:1-15.
- [Google Scholar]
- Anti-implantation activity of the methanolic root extract of Tinospora cordifolia (Willd.) in female rats. Pharmacologyonline. 2010;1:872-876.
- [Google Scholar]
- Antiplasmodial activity of alkaloids from various Strychnos species. J. Nat. Prod.. 2002;65:1381-1386.
- [Google Scholar]
- Studies on the constituents of the stems of Tinospora tuberculata Beumee. I. N-trans-and N-cis-feruloyl tyramine, and a new phenolic glucoside, tinotuberide. Chem. Pharm. Bull.. 1983;31:156-161.
- [Google Scholar]
- Studies on the constituents of the stems of Tinospora tuberculata Beumee, III: new diterpenoids, borapetoside B and borapetol B. Chem. Pharm. Bull.. 1986;34:2868-2872.
- [Google Scholar]
- Studies on the constituents of the stems of Tinospora tuberculata Beumee. II. New diterpenoids, borapetoside A and borapetol A. Chem. Pharm. Bull.. 1985;33:4438-4444.
- [Google Scholar]
- Metabolic regulatory variations in rats due to acute cold stress & Tinospora cordifolia intervention: High resolution 1H NMR approach. Metabolomics. 2012;8:444-453.
- [Google Scholar]
- Cordifolisides A, B, C: Norditerpene furan glycosides from Tinospora cordifolia. Phytochem.. 1994;37:781-786.
- [Google Scholar]
- Norditerpene furan glycosides from Tinospora cordifolia. Phytochem.. 1995;39:1139-1142.
- [Google Scholar]
- Wound-healing activity of ethanolic and aqueous extracts of Ficus benghalensis. J. Adv. Pharm. Technol. Res.. 2011;2:110-114.
- [Google Scholar]
- Antitumor effects of the benzophenanthridine alkaloid sanguinarine: Evidence and perspectives. World J. Gastrointest. Oncol.. 2016;15:30-39.
- [Google Scholar]
- Insecticidal constituents and activity of alkaloids from Cynanchum mongolicum. Molecules. 2015;20:17483-17492.
- [Google Scholar]
- A clinical analysis of evaluating the usefulness and efficacy of the ayurvedic drug Tinospora cordifolia in humans. Adv. Sci. Lett.. 2017;23:2007-2008.
- [Google Scholar]
- Tinospora cordifolia protects against inflammation associated anemia by modulating inflammatory cytokines and hepcidin expression in male Wistar rats. Scientific Rep.. 2019;9:10969.
- [Google Scholar]
- Tinospora cordifolia in the treatment of chronic and aggressive periodontitis patients with and without dental fluorosis: A clinical, microbiological, and biochemical study. Int. J. Oral Health Sci.. 2017;7:16-23.
- [Google Scholar]
- Effect of topical Tinospora cordifolia on excisional wound in albino rats. Int. J. Pharm. Biol. Sci.. 2012;2:25-29.
- [Google Scholar]
- The influence of Tinospora cordifolia on wound healing in albino rats. Int. J. Pharm. Biol. Sci.. 2012;3:379-384.
- [Google Scholar]
- Goel, B., Pathak, N., Nim, D.K., Singh, S.K., Dixit, R.K., Chaurasia, R., 2014. Clinical evaluation of analgesic activity of guduchi (Tinospora cordifolia) using animal model. J. Clin. Diagnostic Res. 8, HC01 - HC04.
- Radioprotective potential of an herbal extract of Tinospora cordifolia. J. Radiat. Res.. 2004;45:61-68.
- [Google Scholar]
- Toxicological studies of Rasasindura, an Ayurvedic formulation. Indian J. Pharm. Sci.. 2017;79:633-640.
- [Google Scholar]
- Gonzalez-Coloma, A., Reina, M., Diaz, C.E., Fraga, B.M., 2010. Natural product-based biopesticides for insect control. In: (Ben) Liu, H.W., Mander, L., (Eds), Comprehensive natural products II: Chemistry and biology. Elsevier, Amsterdam, pp. 237 – 268.
- Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front. Pharmacol.. 2018;9:982.
- [Google Scholar]
- A new N-methyltetrahydroprotoberberine alkaloid from Tinospora hainanensis. Chem. Pharm. Bull.. 1999;47:287-289.
- [Google Scholar]
- Studies of anti-inflammatory, antipyretic and analgesic effects of aqueous extract of traditional herbal drug on rodents. J. Pharmacog. Phytother.. 2013;5:106-113.
- [Google Scholar]
- Comparative potential of Simvastatin, Rosuvastatin and Fluvastatin against bacterial infection: An in silico and in vitro study. Oriental Pharm. Exp. Med.. 2019;19:259-275.
- [Google Scholar]
- Ethnobotanical and Ayurvedic applications of methi-Trigonella foenum-graceum Linn. Int. J. Mendal. 2002;19:124-127.
- [Google Scholar]
- Ameliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by aflatoxin-b(1) in mice kidney. Toxicol. Int.. 2011;18:94-98.
- [Google Scholar]
- Antifertility effect of Tinospora cordifolia (Willd.) stem extract in male rats. Indian J. Exp. Biol.. 2003;41:885-889.
- [Google Scholar]
- A review on medicinal plants exhibiting antifertility activity in males. Nat. Prod. Radiance. 2006;5:389-410.
- [Google Scholar]
- Antidiabetic effect of Tinospora cordifolia. Effect on fasting blood sugar level, glucose tolerance and adrenaline induced hyperglycemia. Indian J. Med. Res.. 1967;55:733-745.
- [Google Scholar]
- Total syntheses of clerodane diterpenoids—A review. Nat. Prod. Commun.. 2019;2019:1-17.
- [Google Scholar]
- Two new rearranged clerodane diterpenes from Thai Tinospora baenzigeri. J. Nat. Med.. 2021;75:201-206.
- [Google Scholar]
- Rearranged clerodane diterpenoids from the stems of Tinospora baenzigeri. J. Nat. Prod.. 2019;82:1405-1411.
- [Google Scholar]
- Tinospora species: An overview of their modulating effects on the immune system. J. Ethnopharmacol.. 2017;207:67-85.
- [Google Scholar]
- Standardized ethanol extract of Tinospora crispa upregulates pro-inflammatory mediators release in LPS-primed U937 human macrophages through stimulation of MAPK, NF-κB and PI3K-Akt signalling networks. BMC Complement. Med. Ther.. 2020;20:245.
- [Google Scholar]
- Phytochemical analysis and evaluation of purified extract of Tinospora crispa stem for in vivo antihyperuricemic effect. J. Rep. Pharm. Sci.. 2020;9:46-51.
- [Google Scholar]
- Influence of Tinospora cordifolia on wound healing in wistar rats. Int. J. Basic Clin. Pharmacol.. 2016;5:923-928.
- [Google Scholar]
- Analgesic, antiinflammatory and antipyretic activities of the methanol leaf extract of Dalbergia saxatilis Hook.F in rats and mice. J. Ethnopharmacol.. 2015;166:74-78.
- [Google Scholar]
- Preliminary investigation of normoglycemic, anti-hyperglycemic and dyslipidemic activities of different extracts of Tinospora crispa on diabetic rat. Acta Pol. Pharm.. 2016;73:129-134.
- [Google Scholar]
- A review of the medicinal properties, phytochemical and biological active compounds of Tinospora sinensis (Lour.) Merr. J. Biol. Active Prod. Nat.. 2016;6:84-94.
- [Google Scholar]
- Antioxidant properties of wheat bran against oxidative stress. In: Watson R.R., Zibadi S., eds. Wheat and rice in disease prevention and health. New York: Academic Press; 2014. p. :181-199.
- [Google Scholar]
- Anti- inflammatory activities of the aqueous extract of the stem of Tinospora crispa (Family Menispermaceae) J. Nat. Stud.. 2012;11:88-95.
- [Google Scholar]
- Flora of British India. Vol Vol I. London: L Reeve & Co; 1875.
- Crispene A, B, C and D, four new clerodane type furanoid diterpenes from Tinospora crispa (L.) Pharmacog. Mag.. 2016;12(Suppl 1):S37-S41.
- [Google Scholar]
- Isolation and structure of some constituents of the Australian medicinal plant Tinospora smilacina (‘snakevine’) Aust. J. Chem.. 1998;51:1103-1111.
- [Google Scholar]
- The analgesic, anti-inflammatory and anti-pyretic activities of Tinospora cordifolia. Adv. Clin. Exp. Med.. 2015;24:957-964.
- [Google Scholar]
- Ibahim, M., I’zzah, W.N.W., Narimah, A., Asyikin, N.Z., Shafinas, S.-N.S., Froemming, G., 2011 Anti-proliperative and antioxidant effects of Tinospora crispa (Batawali). Biomed. Res. India 22, 57 – 62.
- Ibahim, M.J., Wan-Nor I'zzah, W.W.Z., Froemming, G.A.R., 2010. Effect of Tinospora crispa on MCF-7 cell line viability and morphology. Malaysian J. Microscopy 6, 36 – 42.
- Antiplasmodial test of Tinospora crispa stem extract against Plasmodium falciparum 3D7 strain in vitro. J. Kedokteran Brawijaya. 2014;28:91-96.
- [Google Scholar]
- Sensitized photooxygenation of tinosponone, a clerodane diterpene from Tinospora cordifolia. Acta Chim. Slov.. 2005;52:455.
- [Google Scholar]
- Neuroprotective herbs and foods from different traditional medicines and diets. Molecules. 2010;15:3517-3555.
- [Google Scholar]
- Evaluation of analgesic and antimicrobial activity of different fractions of crude methanol extract of Tinospora crispa stem. Int. J. Pharm. Sci. Res.. 2014;5:16-21.
- [Google Scholar]
- Evaluation of spermicidal and antiandrogenic activities of aqueous extract of Tinospora cordifolia (Willd.) stem. African J. Pharm. Pharmacol.. 2013;7:2392-2396.
- [Google Scholar]
- Evaluation of cytotoxic effects of dichloromethane extract of guduchi (Tinospora cordifolia Miers ex Hook F & THOMS) on cultured HeLa cells. Evid-Based Complement. Alter. Med.. 2006;3:267-272.
- [Google Scholar]
- Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in cultured HeLa cells. Cancer Lett.. 1998;127:71-82.
- [Google Scholar]
- Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biol. Pharm. Bull.. 2006;29:460-466.
- [Google Scholar]
- Antipsychotic activity of aqueous ethanolic extract of Tinospora cordifolia in amphetamine challenged mice model. J. Adv. Pharm. Technol. Res.. 2010;1:30-33.
- [Google Scholar]
- Evaluation of antioxidant potential of Tinospora cordifolia (Willd.) Miers. and Tinospora sinensis. Int. J. Pharm. Sci. Res.. 2010;1:122-128.
- [Google Scholar]
- Enhancing the export potential of medicinal plants through biodiversity conservation and development under multi-diversity environment. In: Trivedi P.C., ed. Medicinal plants utilization and conservation. Jaipur, India: Avishkar publishers; 2004. p. :36-78.
- [Google Scholar]
- Antioxidant activity of ethanolic extract of Tinospora cordifolia on N-nitrosodiethylamine (diethylnitrosamine) induced liver cancer in male Wister albino rats. J. Pharm. Bioallied Sci.. 2015;7:40.
- [Google Scholar]
- Antibacterial activity of stem extracts of Tinospora cordifolia (Willd) Hook. f & Thomson. Ancient Sci. Life. 2003;23:40-43.
- [Google Scholar]
- New terpenoid and phenylpropanoid glycosides from Tinospora sinensis. Fitoterapia. 2018;131:127-133.
- [Google Scholar]
- Clerodane diterpenoid glucosides from the stems of Tinospora sinensis. J. Nat. Prod.. 2017;80:975-982.
- [Google Scholar]
- Chemistry and pharmacological activity of sesquiterpenoids from the Chrysanthemum genus. Molecules. 2021;26:3038.
- [Google Scholar]
- Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J. Pharmacol.. 2011;43:64-68.
- [Google Scholar]
- Anti depressant activity of fresh leaves of Tinospora cordifolia miers (Amruthaballi) in albino mice. Int. J. Pharm. Sci. Res.. 2014;5:5420-5424.
- [Google Scholar]
- Immunomodulatory effect of Tinospora cordifolia in human immune-deficiency virus positive patients. Indian J. Pharmacol.. 2008;40:107-110.
- [Google Scholar]
- Inhibitory properties of Tinospora crispa extracts on TNF-α induced inflammation on human umbilical vein endothelial cells (HUVECS) Int. J. Trop. Med.. 2012;7:24-29.
- [Google Scholar]
- Protective effects of Tinospora crispa extracts on H2O2 induced oxidative stress and TNF-α-induced inflammation on human umbilical vein endothelial cells (HUVECs) J. Med. Plants Res.. 2012;6:3013-3021.
- [Google Scholar]
- Immunopotentiating compounds from Tinospora cordifolia. J. Ethnopharmacol.. 1997;58:89-95.
- [Google Scholar]
- A clinical study of guduchi (Tinospora cordifolia Willd. Miers ex Hook. f. Thoms) in antihyperlipidemic effect w.s.r. to sthaulya. Int. J. Pharm. Biol. Archiv.. 2013;4:472-475.
- [Google Scholar]
- Safety of aqueous extract of Tinospora cordifolia (Tc) in healthy volunteers: a double-blind randomised placebo-controlled study. Iranian J. Pharmacol. Ther.. 2007;6:59-61.
- [Google Scholar]
- Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci.. 2015;11:982-991.
- [Google Scholar]
- The multi-activity herbaceous vine - Tinospora cordifolia. Asian J. Pharm. Clin. Res.. 2019;12:23-26.
- [Google Scholar]
- Comparative antidiarrheal and antiulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats. J. Adv. Pharm. Technol. Res.. 2014;5:122-128.
- [Google Scholar]
- Phytochemical analysis and hepatoprotective properties of Tinospora cordifolia against carbon tetrachloride-induced hepatic damage in rats. J. Basic Clin. Pharm.. 2011;2:139-142.
- [Google Scholar]
- Perspectives on cancer chemoprevention research and drug development. Adv. Cancer Res.. 2008;78:199-334.
- [Google Scholar]
- Review on plant antimicrobials: A mechanistic viewpoint. Antimicrobial Resistance Infection Control. 2019;8:118.
- [Google Scholar]
- Evaluation of anti – ulcer activity of Tinospora cordifolia against experimentally induced gastric ulcers in rats. Int. J. Phytopharmacol.. 2015;6:57-61.
- [Google Scholar]
- The modern Ayurveda – milestones beyond the classical age. Boca Raton, London: CRC Press; 2012.
- Antiulcer activity of stem extracts of Tinospora malabarica Miers. (Lamk.) Pharmacologyonline. 2009;1:885-890.
- [Google Scholar]
- Anti-diabetic activity of dried extract of Tionspora cordifolia (Guduchi ghana) and honey in streptozotacin induced diabetic rats. Int. J. Green Pharm.. 2015;9:S31-S38.
- [Google Scholar]
- Aporphine alkaloids, clerodane diterpenes, and other constituents from Tinospora cordifolia. Fitoterapia. 2010;81:485-489.
- [Google Scholar]
- Antidiabetic activity of Tinospora cordifolia (Fam: Menispermaceae) in alloxan treated albino rats. Appl. Res. J.. 2015;1:316-319.
- [Google Scholar]
- Indian medicinal plants. Bahadurganj, India: Sudhindra Nath Basu; 1918.
- Kirtikar, K.R., Basu, B.D., 1993 Indian Medicinal Plants. Vol I-IV, Lalit Mohan Basu, Allahabad, India.
- Indian medicinal plants. International Book Distributors, New Delhi: Vol-I; 2005.
- Klangjareonchai, T., Roongpisuthipong, C., 2012. The effect of Tinospora crispa on serum glucose and insulin levels in patients with type 2 diabetes mellitus. J Biomed Biotechnol 2012, Article ID 808762.
- The chemical diversity of floral scent. In: Dudareva N., Pichersky E., eds. Biology of floral scent. Florida: CRC Press; 2006. p. :27-52.
- [Google Scholar]
- Evaluation of wound healing activity of root of Mimosa pudica. J. Ethnopharmacol.. 2009;124:311-315.
- [Google Scholar]
- Extraction and purification of hypoglycemic agent from Tinospora Crispa. Final Report, Deptt: Chem., Fac. Sci, Kasetsart Univ., Bangkok; 2006.
- The antipyretic effect of Tinospora crispa Mier ex Hook. f. & Thoms. Mahidol Univ. J. Pharm. Sci.. 1984;21:1-6.
- [Google Scholar]
- Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced Parkinsonism. Indian J. Pharmacol.. 2014;46:176-180.
- [Google Scholar]
- Alkaloids. In: Badal S., Rupika Delgoda R., eds. Pharmacognosy fundamentals, applications and strategies. New York: Academic Press; 2017. p. :163-198.
- [Google Scholar]
- Review on pharmacological profile of medicinal vine: Tinospora cordifolia. Curr. J. Appl. Sci. Technol.. 2019;35:1-11.
- [Google Scholar]
- Kumar, M., Sing,h A., Kaur, B., 2014. Comparative antidiarrheal and antiulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats. J. Adv. Pharm. Technol. Res. 5, 122 – 128.
- Kumar, S., Gupta, A., A. K. Pandey, A.K., 2013. Calotropis procera root extract has capability to combat free radical mediated damage. ISRN Pharmacol. 2013, Article ID 691372.
- Kumar, S., Pandey, A.K., 2013. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, Article ID 162750.
- Hypolipidaemic activity of Tinospora sinensis flowers on hydrogenated groundnut oil induced hypercholesterolemic rats. Singapore J. Pharm. Res.. 2014;1:19-22.
- [Google Scholar]
- Immunomodulatory effects of some traditional medicinal plants. J. Chem. Pharm. Res.. 2011;3:675-684.
- [Google Scholar]
- A clinical trial to assess the antidiabetic, antidyslipidemic and antioxidant activities of Tinospora cordifolia in management of type – 2 diabetes mellitus. Int. J. Pharm. Sci. Res.. 2016;7:57-64.
- [Google Scholar]
- Exploration of hepatoprotective activity of aqueous extract of Tinospora cordifolia - An experimental study. Asian J. Pharm. Clin. Res.. 2013;6:87-91.
- [Google Scholar]
- A clinical study on guduchi sattava (starch obtain from stem of Tinospora cordifolia) in madhumehaW.S.R. to diabetes mellitus type II. Int. J. Ayu. Pharma. Res.. 2016;4:32-37.
- [Google Scholar]
- Alkaloids - their importance in nature and for human life. In: Kurek J., ed. Alkaloids - their importance in nature and human life. London: IntechOpen Limited; 2019. p. :1-7.
- [Google Scholar]
- Beneficial effects of nuts from india in cardiovascular disorders. In: Nuts and seeds in health and disease prevention. New York: Academic Press; 2020. p. :453-469.
- [Google Scholar]
- Diversity in a widely distributed dioecious medicinal plant, Tinospora cordifolia (Willd.) Miers. ex. Hook F. and Thomas. Curr. Sci.. 2018;114:1520-1526.
- [Google Scholar]
- Chemical constituents from the stems of Tinospora sinensis and their bioactivity. Molecules. 2018;23:2541.
- [Google Scholar]
- Toxic hepatitis induced by a herbal medicine: Tinospora crispa. Phytomed.. 2014;21:1120-1123.
- [Google Scholar]
- Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med.. 2009;75:1549-1560.
- [Google Scholar]
- Clinical pharmacology (8th Edn). Edinburgh: Church Hill Livingstone; 1997.
- In vivo antimalarial potential of Tinospora crispa miers in mice and identification of the bioactive compound. Pharmacog. Mag.. 2020;16:76-82.
- [Google Scholar]
- Inhibitory effect of a polysaccharide from Tinospora cordifolia on experimental metastasis. J. Ethnopharmacol.. 2004;90:233-237.
- [Google Scholar]
- Effect of Tinospora cordifolia on the cytokine profile of angiogenesis-induced animals. Int. Immunopharmacol.. 2004;4:1569-1575.
- [Google Scholar]
- Potential biological activities of magnoflorine: A compound from Aristolochia debilis sieb. et Zucc. Korean J. Plant Res.. 2014;27:223-228.
- [Google Scholar]
- Anti-inflammatory activity, cytotoxicity and active compounds of Tinospora smilacina Benth. Phytotherapy Res.. 2004;18:78-83.
- [Google Scholar]
- Anti-inflammatory activity of Chinese medicinal vine plants. J. Ethnopharmacol.. 2003;85:61-67.
- [Google Scholar]
- New lignan glucosides from the stems of Tinospora sinensis. Chem. Pharm. Bull.. 2004;52:638-640.
- [Google Scholar]
- Structure and absolute configuration of clerodane diterpene glycosides and a rearranged cadinane sesquiterpene glycoside from the stems of Tinospora sinensis. J. Nat. Prod.. 2007;70:1971-1976.
- [Google Scholar]
- Clerodane diterpenes: sources, structures, and biological activities. Nat. Prod. Rep.. 2016;33:1166-1226.
- [Google Scholar]
- Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J. Pharmacol. Exp. Ther.. 2006;319:1096-1103.
- [Google Scholar]
- Studies on the chemical constituents of Tinospora crispa and synthesis of the analogous of penta-O-galloyl-D-glucopyranose. Taichung, Taiwan: China Medical University; 2009. Masters Dissertation
- Antidiabetic effect of oral borapetol B compound, isolated from the plant Tinospora crispa, by stimulating insulin release. Evid-Based Complement. Alter. Med.. 2013;2013:727602
- [Google Scholar]
- Radioprotective activity of some medicinal plant extracts. Acta Horticult.. 2011;925–46:315-320.
- [Google Scholar]
- Comparative studies of the immunomodulatory activity of Tinospora cordifolia and Tinospora sinensis. Fitoterapia. 2000;71:254-257.
- [Google Scholar]
- Marrero, J., Rodríguez, I.I., Rodríguez, A.D., 2010. The natural products chemistry of the Gorgonian genus Pseudopterogorgia (Octocorallia: Gorgoniidae). In: (Ben) Liu, H.-W., Mander, I., (Eds), Comprehensive natural products II: Chemistry and biology. Elsevier, Amsterdam, pp. 363 – 428.
- Antiatherogenic effect of Caps HT2, a herbal Ayurvedic medicine formulation. Phytomed.. 2003;10:474-482.
- [Google Scholar]
- Evaluation of anti-nociceptive activity of Cissus quadrangularis on albino mice. Int. J. Green Pharm.. 2008;2:118-121.
- [Google Scholar]
- Immunomodulatory and antitumour activities of Tinospora cordifolia. Fitoterapia. 1999;70:35-43.
- [Google Scholar]
- Matthew, K.M., 1981-1984. The flora of Tamilnadu Carnatic. Vol IIII, The Rapinat Herbarium, St. Joseph’s College, Thiruchirapalli.
- An Excursion flora of Central Tamil Nadu. India: The Rapinat Herbarium, St. Joseph College, Thiruchirapalli; 1991.
- Constituents of Tinospora sinensis and their antileishmanial activity against Leishmania donovani. Nat. Prod. Res.. 2009;23:1134-1143.
- [Google Scholar]
- Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochem.. 1998;49:1343-1345.
- [Google Scholar]
- Amritosides A, B, C and D: clerodane furano diterpene glucosides from Tinospora cordifolia. Phytochem.. 2004;65:2051-2055.
- [Google Scholar]
- Cordifolioside A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat. Prod. Lett.. 1996;8:7-10.
- [Google Scholar]
- Drugs effective in the therapy of the epilepsies. In: Hardman J.G., Limbird J.E., eds. Goodman and Gilman's: The pharmacological basis of therapeutics (9th Ed). New York: McGraw-Hill Med Publ Div; 1996. p. :521-548.
- [Google Scholar]
- A critical consideration of Houttuyn's new genera and new species of plants, 1773–1783. J. Arnold Arbor.. 1938;19:340.
- [Google Scholar]
- . Efficacy and safety of Tinospora cordifolia (Tc) as an add-on therapy in patients with type-2 diabetes. Int. J. Res. Med. Sci. 3, 1109–1113.
- Occurrence of a Tinospora subcordata (Menispermaceae): A new record to flora of India, from Khandwa District. India. Biosci. Discovery. 2020;11:85-89.
- [Google Scholar]
- Tinospora baenzigeri (Menispermaceae): A new distribution record for the flora of India, from Burhanpur district, Madhya Pradesh. India. Biosci. Discovery. 2020;11:137-141.
- [Google Scholar]
- Effect of Tinospora cordifolia as an add - on therapy on the blood glucose levels of patients with Type 2 diabetes. Int. J. Basic Clin. Pharmacol.. 2015;4:537-541.
- [Google Scholar]
- Tinospora cordifolia: A multipurpose medicinal plant-A review. J. Med. Plant Studies. 2014;2:32-47.
- [Google Scholar]
- Antimicrobial activity of Tinospora crispa root extracts. Int. J. Res. Ayurveda Pharm.. 2012;3:417-419.
- [Google Scholar]
- Natural products as a source for novel antibiotics. Trends Pharmacol. Sci.. 2016;37:689-701.
- [Google Scholar]
- Immunomodulatory effects of Tinospora cordifolia (Guduchi) on macrophage activation. Biol. Med.. 2011;3:134-140.
- [Google Scholar]
- Effect of Tinospora cordifolia (Guduchi) on the phagocytic and pinocytic activity of murine macrophages in vitro. Indian J. Exp. Biol.. 2017;55:21-26.
- [Google Scholar]
- Mimosa pudica L., a high-value medicinal plant as a source of bioactives for pharmaceuticals. Compr. Rev. Food Sci. Food Saf.. 2016;15:303-315.
- [Google Scholar]
- Tinospora maqsoodiana (menispermaceae), a new species from Madhya Pradesh, India. India Forester. 2014;140:528-530.
- [Google Scholar]
- Tinospora cordifolia: Role in depression, cognition, and memory. Aust. J. Med. Herbal. 2011;23:168-173.
- [Google Scholar]
- Mechanisms of tissue repair: From wound healing to fibrosis. Int. J. Biochem. Cell Biol.. 1997;29:5-17.
- [Google Scholar]
- Comparative hepatoprotective potential of Tinospora cordifolia, Tinospora sinensis and Neem-guduchi. British J. Pharm. Res.. 2013;3:906-916.
- [Google Scholar]
- Antibacterial activity of Tinopsora cordifolia extracts on clinical isolates from HIV-infected patients. Int. J. Curr. Res.. 2016;8:33072-33077.
- [Google Scholar]
- Naik, N.D., Salma, F.S., Durga1, K., Ashwani, K., Elumalai, A., Malothu, R., 2013 Evaluation of hepato protective activity of ethanolic root extract of Tinospora sinensis. Int. J. Biol. Pharm. Res. 4, 1065 – 1069.
- Najib Nik a Rahman, N., Furuta, T., Takane, K., Ali Mohd, M., 1999. Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 64, 249 – 254.
- Comparative immunomodulation potential of Tinospora cordifolia (Willd.) Miers ex Hook. F., Tinospora sinensis (Lour.) Merrill and Tinospora cordifolia growing on Azadirachta indica A. Juss. Indian J. Exp. Biol.. 2014;52:808-813.
- [Google Scholar]
- Evaluation of preformulation and formulation parameters of antistress herbal capsule. Int. J. Pharm Bio Sci. 2011;2:867-877.
- [Google Scholar]
- A review on medicinal plants evaluated for anti-diabetic potential in clinical trials: Present status and future perspective. J. Herbal Med.. 2021;28:100436
- [Google Scholar]
- Anti-inflammatory and antiallergic properties of Tinospora cordifolia. Indian J. Pharmacol.. 1982;14:64-66.
- [Google Scholar]
- Capparis decidua Edgew (Forssk.): A comprehensive review of its traditional uses, phytochemistry, pharmacology and nutrapharmaceutical potential. Arab. J. Chem.. 2020;13:1901-1916.
- [Google Scholar]
- Evaluation of wound healing activity of Tinospora cordifolia Willd. Der Pharm. Sin.. 2012;3:126-130.
- [Google Scholar]
- Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.. 2016;79:629-661.
- [Google Scholar]
- Natural products as pharmaceuticals and sources for lead structures. In: Wermuth C.G., Aldous D., Raboisson P., Rognan D., eds. The practice of medicinal chemistry (4th Ed). Amsterdam: Elsevier; 2015. p. :101-139.
- [Google Scholar]
- Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J. Basic Clin. Physiol. Pharmacol.. 2015;26:603-611.
- [Google Scholar]
- Neurodegenerative diseases and neuroprotection: Current views and prospects. J. Appl. Biomed.. 2011;9:173-183.
- [Google Scholar]
- Antimalarial activity of stem extract of Tinospora crispa against Plasmodium berghei infection in mice. J. Health Res.. 2014;28:1-6.
- [Google Scholar]
- The activation of GLUT1, AMPK alpha and PPAR gamma by Tinospora crispa in L6 myotubes. Spatula DD. 2011;1:245-249.
- [Google Scholar]
- Antidiabetic effects of Tinospora crispa in rats. J. Ethnopharmacol.. 1989;27:149-161.
- [Google Scholar]
- The hypoglycaemic and insulinotropic activity of Tinospora crispa: Studies with human and rat islets and HIT-T15 B cells. Diabetologia. 1989;32:354-359.
- [Google Scholar]
- Effect of Tinospora cordifolia on blood glucose and total lipid levels of normal and diabetic rabbits. Planta Med.. 1992;58:131-136.
- [Google Scholar]
- Immunomodulatory effects of Stachytarpheta cayennensis leaf extract and its synergistic effect with artesunate. BMC Complement. Alter. Med.. 2014;14:376.
- [Google Scholar]
- Ounjaijean, S., Kotepui, M., Somsak, V., 2019. Antimalarial activity of Tinospora baenzigeri against Plasmodium berghei-infected mice. J. Trop. Med. 2019, Article ID 5464519.
- NMR-assignments of N-acylaporphine alkaloids from Tinospora crispa. Planta Med.. 1992;58:184-187.
- [Google Scholar]
- Alteration of lethal effects of gamma rays in Swiss albino mice by Tinospora cordifolia. Phytotherapy Res.. 2003;17:552-554.
- [Google Scholar]
- Healing role of Guduchi [Tinospora cordifolia (Willd.) Miers] and Amalaki (Emblica officinalis Gaertn.) capsules in premature aging due to stress: A comparative open clinical trial. European J. Med. Plants. 2017;21:1-13.
- [Google Scholar]
- Cordifolide A, a sulfur-containing clerodane diterpene glycoside from Tinospora cordifolia. Org. Lett.. 2012;14:2118-2121.
- [Google Scholar]
- Protective effect of Tinospora cordifolia, Phyllanthus emblica and their combination against antitubercular drug induced hepatic damage: An experimental study. Phytotherapy Res.. 2008;22:646-650.
- [Google Scholar]
- Tinospora cordifolia: A climbing shrub in health care management. Int. J. Pharm. Biol. Sci.. 2012;3:612-628.
- [Google Scholar]
- A review on rasna saptak kwath: An ayurvedic polyherbal formulation for arthritis. Int. J. Res. Ayurveda Pharm.. 2017;8(Suppl 1):4-11.
- [Google Scholar]
- Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J. Aerosol Med. Pulm. Drug Deliv.. 2017;30:274-288.
- [Google Scholar]
- Occurrence of an Australian species Tinospora smilacina Benth. (Menispermaceae) in India. Res. Rev. J. Bot.. 2016;5:16-18.
- [Google Scholar]
- Rearranged clerodane diterpenoid from Tinospora crispa. Nat. Prod. Res.. 2021;35:369-376.
- [Google Scholar]
- Radioprotective and cytoprotective activity of Tinospora cordifolia stem enriched extract containing cordifolioside-A. Indian J. Pharmacol.. 2013;45:237-243.
- [Google Scholar]
- Pedalium murex Linn (Pedaliaceae) fruits: a comparative antioxidant activity of its different fractions. Asian Pac. J. Trop. Biomed.. 2011;1:395-400.
- [Google Scholar]
- Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed.. 2011;1:316-322.
- [Google Scholar]
- Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomed.. 2011;18:1045-1052.
- [Google Scholar]
- Isoquinoline alkaloids from Tinospora cordifolia inhibit rat lens aldose reductase. Phytotherapy Res.. 2012;26:1342-1347.
- [Google Scholar]
- Indian Tinospora species: Natural immunomodulators and therapeutic agents. Int. J. Pharm. Biol. Chem. Sci.. 2013;2:1-9.
- [Google Scholar]
- Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar; a polyherbal Ayurvedic formulation in streptozotocin induced diabetic rats. Indian J. Exp. Biol.. 2009;47:564-570.
- [Google Scholar]
- Anti-inflammatory activity of Guduchi Ghana (aqueous extract of Tinospora Cordifolia Miers.) Int. Quarterly J. Res. Ayur.. 2014;35:108-110.
- [Google Scholar]
- NMR studies of 20β-hydroxyecdysone, a steroid; isolated from Tinospora cordifolia. Indian J. Chem.. 1995;34B:674-676.
- [Google Scholar]
- An open label clinical study to evaluate effect of juice of Tinospora cordifolia Linn. on growth of children. Int. J. Res. Ayur. Pharm.. 2012;3:77-79.
- [Google Scholar]
- Study of anthelmintic activity of chloroform extract of Tinospora cordifolia. World J. Pharm. Pharm. Sci.. 2014;3:2253-2268.
- [Google Scholar]
- Pham, T.N., Nguyen, T.V., 2020. Evaluation of antibacterial activity of fractions from stem extract of Tinospora crispa (L.) Hook. f. & Thomson. IOP Conf Ser: Materials Sci. Eng. 991, 012058.
- Aporphine alkaloids, clerodane diterpenes, and other constituents from Tinospora cordifolia. Fitoterapia. 2010;81:485-489.
- [Google Scholar]
- Tinospora crispa extract inhibits MMP-13 and migration of head and neck squamous cell carcinoma cell lines. Asian Pac. J. Trop. Biomed.. 2015;5:738-743.
- [Google Scholar]
- Evaluation of the anti-inflammatory activity of Tinospora cordifolia (Willd.) Miers chloroform extract - a preclinical study. J. Pharm. Pharmacol.. 2018;70:1113-1125.
- [Google Scholar]
- Effects of Marapuama in the chronic mild stress model: Further indication of antidepressant properties. J. Ethnopharmacol.. 2008;118:300-304.
- [Google Scholar]
- Hypoglycemic activity of Tinospora sinensis (Linn) leaves. J. Pharm. Res.. 2009;2:729-730.
- [Google Scholar]
- Assessment of free radical scavenging and anti-proliferative activities of Tinospora cordifolia Miers (Willd) BMC Complement. Alter. Med.. 2017;17:457.
- [Google Scholar]
- In vitro anti-bacterial activity of Tinospora cordifolia leaf extract and its phytochemical screening. J. Biomed. Sci.. 2018;5:10-17.
- [Google Scholar]
- Neuroprotective activity of ethanolic extract of Tinospora cordifolia on LPS induced neuroinflammation. Transl. Biomed.. 2017;8:135.
- [Google Scholar]
- Hypotensive and cardio-chronotropic constituents of Tinospora crispa and mechanisms of action on the cardiovascular system in anesthetized rats. J. Ethnopharmacol.. 2012;140:166-178.
- [Google Scholar]
- Anti-oxidant and antimicrobial studies of Tinospora cordifolia (guduchi/giloy) stems and roots under in vitro condition. Int. J. Adv. Microbiol. Health Res.. 2019;3:1-10.
- [Google Scholar]
- Hypoglycaemic and hypolipidaemic action of alcohol extract of Tinospora cordifolia roots in chemical induced diabetes in rats. Phytotherapy Res.. 2003;17:410-413.
- [Google Scholar]
- Hypolipidaemic action of Tinospora cordifolia roots in alloxan diabetic rats. J. Ethnopharmacol.. 1999;64:53-57.
- [Google Scholar]
- Rearranged clerodanes from the stems of Tinospora baenzigeri. Planta Med.. 2019;85:1502.
- [Google Scholar]
- Inhibition of cancer cell growth by crude ethanolic extract of Tinospora malabarica Miers. (Miers.) Ann: An endangered medicinal plant. American J. Pharmtech. Res.. 2012;2:545-550.
- [Google Scholar]
- Anti-inflammatory activity of characterized compound diosgenin isolated from Tinospora malabarica Miers. Miers in Ann. (Menispermaceae) in animal model. Int. J. Herbal Med.. 2013;1:76-78.
- [Google Scholar]
- Immunomodulatory role of Tinospora cordifolia as an adjuvant in surgical treatment of diabetic foot ulcers: A prospective randomized controlled study. Indian J. Med. Sci.. 2007;61:347-355.
- [Google Scholar]
- Efficacy of Tinospora cordifolia (Willd.) extracts on blood lipid profile in streptozotocin diabetic rats. Is it beneficial to the heart? Biomed Res.. 2008;19:92-96.
- [Google Scholar]
- HPTLC standardization of Tinospora cordifolia using tinosporaside. Indian J. Pharm. Sci.. 2007;69:578-581.
- [Google Scholar]
- The observation of curative effect on knee osteoarthritis through intra-articular injection of traditional Chinese drug ion-introduction coupled with triamcinolone acetonide about 78 cases. New J. Trad. Chinese Med.. 2006;38:36-37.
- [Google Scholar]
- Electrochemical quantification of the structure/antioxidant activity relationship of flavonoids. Electrochim. Acta. 2015;163:161-166.
- [Google Scholar]
- Rackova, L., Majekova, M., Kost’alova, D., Stefek, M. 2004. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 12, 4709 – 4715.
- Design, synthesis and structure–activity relationship of novel semi-synthetic flavonoids as antiproliferative agents. European J. Med. Chem.. 2014;82:506-520.
- [Google Scholar]
- Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin-induced diabetic rats. African J. Pharm. Pharmacol.. 2009;3:171-180.
- [Google Scholar]
- Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci. Nutr.. 2020;8:547-556.
- [Google Scholar]
- Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phytotherapy Res.. 2020;34:2978-2984.
- [Google Scholar]
- Biochemical and computational approach of selected phytocompounds from Tinospora crispa in the management of COVID-19. Molecules. 2020;25:3936.
- [Google Scholar]
- Investigation of wound healing activity of extracts from Tinospora cordifolia. Deccan J. Pharmacol.. 2011;2:43-53.
- [Google Scholar]
- Phytochemical screening and biological studies on the leaves of Tinospora cordifolia. Int. J. Pharm. Sci. Res.. 2018;9:2573-2578.
- [Google Scholar]
- Anti arthritic activity of Tinospora cordifolia leaves by denaturation studies. Int. J. Innovative Res. Med. Sci.. 2016;1:10-23.
- [Google Scholar]
- Analytical evaluation of Tinospora cordifolia extract and capsule. Int. J. Herbal Med.. 2017;5:53-57.
- [Google Scholar]
- Pharmacology (5th Edn). New Delhi: Elsevier Science Ltd; 2003.
- Analgesic activity of the different fractions of the aerial parts of Commenila benghalensis Linn. Int. J. Pharmacol.. 2010;6:63-67.
- [Google Scholar]
- Ethnomedicinal properties of plants of Rajasthan. Ph D Dissertation, Modi Institute of. Rajasthan: Technology (Deemed University), Lakshamangarh; 2002.
- Tinospora cordifolia chemical constituents and medicinal properties: A review. Sci Acad. J. Pharm.. 2015;4:364-369.
- [Google Scholar]
- Immunotherapy with Tinospora cordifolia: A new lead in the management of obstructive jaundice. Indian J. Gastroenterol.. 1993;12:5-8.
- [Google Scholar]
- Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytotherapy Res.. 1999;13:275-291.
- [Google Scholar]
- The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Res.. 1995;22:375-383.
- [Google Scholar]
- Isolation and characterisation of clerodane diterpenoids from the traditional medicinal plant -Tinospora glabra (Burm. f.) Merrill. Der Pharm. Lett.. 2016;8:280-287.
- [Google Scholar]
- Embryo-toxic and teratogenic effects of Tinospora cordifolia leaves and bark extracts in Zebrafish (Danio rerio) embryos. Asian J. Plant Sci. Res.. 2016;6:37-41.
- [Google Scholar]
- Natural products as platforms to overcome antibiotic resistance. Chem Rev.. 2017;117:12415-12474.
- [Google Scholar]
- Tinospora cordifolia stem supplementation in diabetic dyslipidemia: An open labelled randomized controlled trial. Funct. Foods Health Dis.. 2015;5:265-274.
- [Google Scholar]
- Borapetoside C from Tinospora crispa improves insulin sensitivity in diabetic mice. Phytomed.. 2012;19:719-724.
- [Google Scholar]
- Hypoglycemic action of borapetoside A from the plant Tinospora crispa in mice. Phytomed.. 2013;20:667-675.
- [Google Scholar]
- In vivo antiparasitic activity of the thai traditional medicine plant-Tinospora crispa-against Plasmodium yoelii. Southeast Asian. J. Trop. Med. Public Health. 2009;40:898-900.
- [Google Scholar]
- Plant secondary metabolites and gut health: The case for phenolic acids. Proc. Nutr. Soc.. 2011;70:389-396.
- [Google Scholar]
- Scope of arid zone plants for their medicinal properties. Jaipur), Jaipur, Rajasthan: Poddar PG College (Affiliated to University of Rajasthan; 2002. Masters Dissertation
- Saini, A.K., 2009. Antimicrobial activity of Sariska Tiger Researve plants (Rajasthan). Masters Dissertation, RR PG (Autonomous) College, Alwar, Rajasthan.
- The potential role of sesquiterpene lactones isolated from medicinal plants in the treatment of the metabolic syndrome—A review. South African J. Bot.. 2020;135:240-251.
- [Google Scholar]
- Saleem, M., Nazir, M., 2015. Bioactive natural products from marine-derived fungi: An update. In: Atta-ur-Rehman (Ed), Studies in natural products chemistry. Elsevier, Amsterdam, 45, 297 – 361.
- Study of Immunomodulatory activity of Tinospora cordifolia extract. Int. J. Adv. Pharm. Biol. Chem.. 2014;3:880-883.
- [Google Scholar]
- Effect of Tinospora cordifolia on physical and cardiovascular performance induced by physical stress in healthy human volunteers. Ayu. 2015;36:265-270.
- [Google Scholar]
- Anti-hyperlipidaemic effect of Tinospora sinensis flowers on hydrogenated groundnut oil induced hypercholesterolemia in albino rats. Int. J. Exp. Pharmacol.. 2014;4:101-104.
- [Google Scholar]
- Evaluation of analgesic activity of ethanolic extract of Tinospora sinensis leaves in rats. Int. J. Preclin. Pharm. Res.. 2014;5:34-37.
- [Google Scholar]
- Diterpenoids from the medicinal plants of Africa. In: Kuete V., ed. Medicinal plant research in Africa: Pharmacology and chemistry. Amsterdam: Elsevier; 2013. p. :105-133.
- [Google Scholar]
- Tinospora cordifolia attenuates oxidative stress and distorted carbohydrate metabolism in experimentally induced type 2 diabetes in rats. J. Nat. Med.. 2011;65:544-550.
- [Google Scholar]
- Anti-diabetic property of Tinospora cordifolia and its active compound is mediated through the expression of Glut-4 in L6 myotubes. Phytomed.. 2013;20:246-248.
- [Google Scholar]
- Randomized controlled trial of Tinospora crispa for additional therapy in patients with type 2 diabetes mellitus. J. Med. Assoc. Thai. 2004;87:543-546.
- [Google Scholar]
- Tinospora cordifolia inhibits autoimmune arthritis by regulating key immune mediators of inflammation and bone damage. Int. J. Immunopathol. Pharm.. 2015;28:521-531.
- [Google Scholar]
- Antihyperlipidemic and antiperoxidative effect of Diasulin, a polyherbal formulation in alloxan induced hyperglycemic rats. BMC Complement. Alter. Med.. 2005;5:14.
- [Google Scholar]
- Antiulcer activity of Tinospora cordifolia Miers and Centella asiatica Linn extracts. Phytotherapy Res.. 1995;9:589-590.
- [Google Scholar]
- Antistress activity of Tinospora cordifolia and Centella asiatica extracts. Phytotherapy Res.. 1996;10:181-183.
- [Google Scholar]
- Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules. 2019;24:498.
- [Google Scholar]
- Effect of aqueous extract of Tinospora cordifolia on functions of peritoneal macrophages isolated from CCl4 intoxicated male albino mice. BMC Complement. Alter. Med.. 2011;11:102.
- [Google Scholar]
- Triterpenes and steroids from Euphorbia denticulata Lam. With anti-herpes symplex virus activity. Iran J. Pharm. Res.. 2013;12:759-767.
- [Google Scholar]
- Antibacterial activity of Tinospora cordifolia (Willd) Hook.F.Thoms on urinary tract pathogens. Int. J. Curr. Microbiol. Appl. Sci.. 2013;2:190-194.
- [Google Scholar]
- Tinospora cordifolia (Willd) Hook.F.Thomson- a plant with immense economic potential. J. Chem. Pharm. Res.. 2010;2:327-333.
- [Google Scholar]
- Tinospora cordifolia as a potential neuroregenerative candidate against glutamate induced excitotoxicity: an in vitro perspective. BMC Complement. Alter. Med.. 2018;18:268.
- [Google Scholar]
- Protective effects of Tinospora cordifolia on hepatic and gastrointestinal toxicity induced by chronic and moderate alcoholism. Alcohol and Alcoholism. 2016;51:1-10.
- [Google Scholar]
- A study on adaptogenic activity of stem extracts of Tinospora malabarica Miers. (Lamk) Pharmacologyonline. 2007;1:349-358.
- [Google Scholar]
- Tinospora cordifolia enhances Vyadhikshamatwa (immunity) in children. The J. Phytopharmacol.. 2015;4:227-230.
- [Google Scholar]
- Traditional medicinal plants of Rajasthan used in tribal medicine: A review. Int. J. Life Sci. Pharm. Res.. 2013;3:L38-L42.
- [Google Scholar]
- A new clerodane furano diterpene glycoside from Tinospora cordifolia triggers autophagy and apoptosis in HCT-116 colon cancer cells. J. Ethnopharmacol.. 2018;211:295-310.
- [Google Scholar]
- The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon. 2019;5:e02437
- [Google Scholar]
- Radiation-induced testicular injury and its amelioration by Tinospora cordifolia (an indian medicinal plant) extract. Evidence-Based Complement. Alter. Med.. 2011;2011:643847
- [Google Scholar]
- Agnivesh. Charak Samhita: Chaukhambha Orientalia Publishers, Varanasi; 2011.
- In vivo delivery of Tinospora cordifolia root extract preventing radiation-induced dystrophies in mice ovaries. Evidence-Based Complement. Alter. Med.. 2015;2015:346427
- [Google Scholar]
- Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol.. 2012;141:918-926.
- [Google Scholar]
- Beneficial effects of Tinospora cordifolia on blood profiles in male mice exposed to lead. Toxicol. Int.. 2010;17:8-11.
- [Google Scholar]
- Protective role of Tinospora cordifolia against lead-induced hepatotoxicity. Toxicol. Int.. 2010;17:12-17.
- [Google Scholar]
- Survey of medicinal plants in Vellalar College for Women Campus, Erode, Tamil Nadu. India. Int. J. Pharm. Sci. Rev. Res.. 2018;53:4-13.
- [Google Scholar]
- In vitro conservation of Tinospora formanii Udayan and Pradeep-A rare endemic plant from Western Ghats. IOSR J. Biotechnol. Biochem.. 2017;3:65-69.
- [Google Scholar]
- Guduchi fibres (Tinospora cordifolia Linn.) as a skin suturing material - A controlled clinical trial. Ayurline: IJ-RIM. 2017;1:71-76.
- [Google Scholar]
- Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J. Chem. Biol. Phys. Sci.. 2016;6:497-512.
- [Google Scholar]
- Evaluation of analgesic and anti-inflammatory activities of Tinospora cordifolia in rodents. Int. J. Basic Med. Sci.. 2011;2:306-311.
- [Google Scholar]
- Tumor cell selective antiproliferative effect of the extract from Tinospora crispa (borapet) Bull. Health Sci. Technol.. 2007;7:75-84.
- [Google Scholar]
- Comparative evaluation of the wound healing potential of Tinospora cordifolia and its combination with local insulin therapy in diabetic rabbits. J. Pharmacog. Phytochem.. 2017;6:1812-1817.
- [Google Scholar]
- Chemistry and pharmacology of Tinospora cordifolia. Nat. Prod. Commun.. 2017;12:299-308.
- [Google Scholar]
- Evaluation of antimicrobial activity of Tinospora cordifolia and Hymenocallis littoralis medicinal plants by using different solvents extract. Int. Res. J. Eng. Technol.. 2016;3:928-931.
- [Google Scholar]
- Singh, H.M., 1998. Medicinal plants of Churu and Bikaner district of Rajasthan. Masters Dissertation, Govt Dungar College, Bikaner (Affiliated to Bikaner University, Bikaner), Rajasthan.
- Hepatoprotective activity of herbal extracts in carbon tetrachloride intoxicated albino rats by measuring anti-oxidant enzymes. Int. J. PharmTech. Res.. 2010;2:2112-2115.
- [Google Scholar]
- Traditional uses of Tinospora cordifolia (Guduchi) J. Med. Aromat Plant Sci.. 2003;25:748-751.
- [Google Scholar]
- Effect of Tinospora cordifolia on gamma ray-induced perturbations in macrophages and splenocytes. J. Radiat. Res.. 2007;48:305-315.
- [Google Scholar]
- Evaluation of antileishmanial potential of Tinospora sinensis against experimental visceral leishmaniasis. Parasitol. Res.. 2008;102:561-565.
- [Google Scholar]
- Tinospora cordifolia as an adjuvant drug in the treatment of hyper-reactive malarious splenomegaly–case reports. J. Vector Borne Dis.. 2005;42:36-38.
- [Google Scholar]
- Chemistry and medicinal properties of Tinospora cordifolia (Guduchi) Indian J. Pharmacol.. 2003;35:83-91.
- [Google Scholar]
- Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic applications: A review. Indian J. Trad. Knowledge. 2004;3:257-270.
- [Google Scholar]
- Antioxidant effect of Tinospora cordifolia extract in alloxan-induced diabetic rats. Indian J. Pharm. Sci.. 2010;72:795-798.
- [Google Scholar]
- Slobodnikova, L., Kost’alova, D., Labudova, D., Kotulova, D., Kettmann, V. 2004. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother Res. 18, 674 – 676.
- The antiamoebic effect of a crude drug formulation of herbal extract against Entamoeba histolytica in vitro and in vivo. J. Ethnopharmacol. 1995;45:43-52.
- [Google Scholar]
- Brief continuing medical education (CME) module raises knowledge of developing country physicians international electronic. J. Health Educ.. 2006;9:31-41.
- [Google Scholar]
- Effect of aqueous crude extract of Tinospora crispa on Plasmodium berghei induced liver damage in mice. Malar Chemoth. Cont. Elimination. 2015;4:127.
- [Google Scholar]
- Traditional and clinical uses of Tinospora cordifolia, guduchi. Aust. J. Med. Herbalism. 2001;13:49-57.
- [Google Scholar]
- HPLC estimation of berberine in Tinospora cordifolia and Tinospora sinensis. Indian J. Pharm. Sci.. 2008;70:96-99.
- [Google Scholar]
- Ethnopharmacological properties and active compounds from plants of Rajasthan. Jaipur, Rajasthan: University of Rajasthan; 2003. PhD Thesis
- Hypoglycemic effect of Tinospora crispa dry powder in outpatients with metabolic syndrome at king chulalongkorn memorial hospital. J. Health Res.. 2009;23:125-133.
- [Google Scholar]
- Hypoglycaemic and other related actions of Tinospora cordifolia roots in alloxan-induced diabetic rats. J. Ethnopharmacol.. 2000;70:9-15.
- [Google Scholar]
- Radioprotective property of polysaccharide in Tinospora cordifolia. Indian J. Biochem. Biophys.. 2003;40:22-26.
- [Google Scholar]
- Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J. Exp. Biol.. 2006;44:726-732.
- [Google Scholar]
- Ethnomedicinal survey of flora used by Chettaipatti inhabitants. Manapparai. Asian J. Biochem. Pharm. Res.. 2015;4:16-31.
- [Google Scholar]
- Antinociceptive and anti-inflammatory activities of Tinospora crispa in various animal models. Int. J. Trop. Med.. 2008;3:66-69.
- [Google Scholar]
- Phytochemical investigation and isolation of new compounds from the stems of Tinospora cordifolia Miers. Trends Phytochem. Res.. 2017;1:83-92.
- [Google Scholar]
- Structure of columbin, a diterpenoid furanolactone from Tinospora cordifolia Miers. Acta Crystallogr. C. 1989;45(Pt 2):300-303.
- [Google Scholar]
- Plants are capable of synthesizing animal steroid hormones. Molecules. 2019;24:2585.
- [Google Scholar]
- Hepatocurative and antioxidant profile of HP-1, a polyherbal phytomedicine. Human Exp. Toxicol.. 2003;22:639-645.
- [Google Scholar]
- Immunotherapeutic modification of Escherichia coli peritonitis and bacteremia by Tinospora cordifolia. J. Postgrad. Med.. 1992;38:13-15.
- [Google Scholar]
- Tinospora cordifolia induces colony stimulating activity in serum. J. Postgrad. Med.. 1994;40:202-203.
- [Google Scholar]
- The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins (Basel). 2019;11:656.
- [Google Scholar]
- Peltophorum africanum, a traditional South African medicinal plant, contains an anti HIV-1 constituent, betulinic acid. Tohoku J. Exp. Med.. 2009;217:93-99.
- [Google Scholar]
- Induction of caspase-3 activated DNase mediated apoptosis by hexane fraction of Tinospora cordifolia in EAT cells. Environ. Toxicol. Pharmacol.. 2007;23:212-220.
- [Google Scholar]
- The significance of Tinospora crispa in treatment of diabetes mellitus. Phytotherapy Res.. 2016;30:357-366.
- [Google Scholar]
- Flavonoids from the leaves of Carya cathayensis Sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia. 2014;92:34-40.
- [Google Scholar]
- Tinospora cordifolia attenuates antipsychotic drug induced hyperprolactinemia in Wistar rats. Asian Pac. J. Reprod.. 2019;8:132-140.
- [Google Scholar]
- Arkaprakash: Tritiya shatak. Varanasi: Chaukhambha Krishnadas Academy; 2006.
- Astanga samgraha (sutrasthana). Varanasi: Chaukhambha Sanskrit Pratisthan; 2006.
- Medicinal plants: Traditional knowledge. New Delhi: IK International Publishing House Pvt Ltd; 2006.
- Medicinal plants: Utilisation & conservation. Jaipur, Rajasthan: Aaviskar Poblishers & Distributors; 2009.
- Lignans and stilbenes from African medicinal plants. In: Kuete V., ed. Medicinal plant research in Africa: Pharmacology and chemistry. Amsterdam: Elsevier; 2013. p. :435-478.
- [Google Scholar]
- Rearranged clerodane diterpenes from Tinospora baenzigeri. Phytochem.. 1999;52:1335-1340.
- [Google Scholar]
- New rearranged clerodane diterpenes from Tinospora baenzigeri. Chem. Pharm. Bull.. 2001;49:854-857.
- [Google Scholar]
- Tinospora sinensis (Lour.) Merr. from Sickupara, Kolli Hills Forest, Namakkal District. Tamil Nadu. Zoos’ Print J.. 2004;19:1622-1623.
- [Google Scholar]
- A new species of Tinospora (Menispermaceae) from South India. Edinb. J. Bot.. 2009;66:77-80.
- [Google Scholar]
- Immunomodulatory activity of guduchi Ghana (aqueous extract of Tinospora cordifolia Miers) Natl. J. Integr. Res. Med.. 2013;4:90-96.
- [Google Scholar]
- Tinospora cordifolia (Willd.) Hook.f. and Thomas. (Guduchi)-validation of the ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res.. 2010;1:112-121.
- [Google Scholar]
- Assessment of the multi-faceted immunomodulatory potential of the aqueous extract of Tinospora cordifolia. Res. J. Chem. Sci.. 2011;1:71-79.
- [Google Scholar]
- Antipyretic activity of six indigenous medicinal plants of Tirumala Hills, Andhra Pradesh. Indian. J. Ethnopharmacol.. 1991;33:193-196.
- [Google Scholar]
- Assessment of in-vitro wound healing activity of the Tinospora crispa extracts. Int. J. Pharmacol. Pharm. Technol.. 2017;1:1-5.
- [Google Scholar]
- A review on antiulcer activity of few Indian medicinal plants. Int. J. Microbiol.. 2014;2014:519590
- [Google Scholar]
- Psychological stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evid-based Complement. Alter. Med.. 2007;4:195-202.
- [Google Scholar]
- The observation of curative effect of adult humeral shaft fracture through integrated traditional Chinese and western medicine treatment. New J. Trad. Chinese Med.. 2005;37:59-60.
- [Google Scholar]
- In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol.. 2013;53:376-383.
- [Google Scholar]
- Phytochemical analysis and antioxidant activity of brotowali (Tinospora crispa L. Mier) stem. Molekul. 2020;15:73-78.
- [Google Scholar]
- Cordioside, a clerodane furano diterpene glucoside from Tinospora cordifolia. Phytochem.. 1995;38:447-449.
- [Google Scholar]
- colorectal cancer prevention by wheat consumption: A three-valued logic – true, false, or otherwise? In: Watson R.R., Zibadi S., eds. Wheat and rice in disease prevention and health. New York: Academic Press; 2014. p. :91-111.
- [Google Scholar]
- Effect of alcoholic extract of Tinospora cordifolia on acute and subacute inflammation. Pharmacologyonline. 2008;3:683-687.
- [Google Scholar]
- WHO., 2004. WHO Guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. World Health Organization, Geneva, Switzerland.
- WHO., 2005. National policy on traditional medicine and regulation of herbal medicines. World Health Organization, Geneva, Switzerland.
- WHO., 2017. World Malaria Report 2017. WHO Press, Geneva, Switzerland.
- Alkaloids. In: Wiart C., ed. Lead compounds from medicinal plants for the treatment of neurodegenerative diseases. New York: Academic Press; 2014. p. :1-188.
- [Google Scholar]
- Rapid screening and identification of diterpenoids in Tinospora sinensis based on high-performance liquid chromatography coupled with linear ion Trap-Orbitrap mass spectrometry. Molecules. 2017;22:912.
- [Google Scholar]
- Borapetoside E, a clerodane diterpenoid extracted from Tinospora crispa, improves hyperglycemia and hyperlipidemia in high-fat-diet-induced type 2 diabetes mice. J. Nat. Prod.. 2017;80:2319-2327.
- [Google Scholar]
- A review on psychosis and anti-psychotic plants. Asian J. Pharm. Clin. Res.. 2015;8:24-28.
- [Google Scholar]
- Yadav, S., 2007. Medicinal plants of Keoladeo Bird Sanctuary, Bharatpur (Rajasthan). Masters Dissertation, MSJ (PG) College, Bharatpur, Rajasthan.
- Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget. 2017;8:30723-30733.
- [Google Scholar]
- Gut microbial metabolism of plant lignans: influence on human health. In: Tuohy K., Del Rio D., eds. Diet-microbe interactions in the gut effects on human health and disease. New York: Academic Press; 2015. p. :103-117.
- [Google Scholar]
- Studies on the constituents of Tinospora sinensis; I. Separation and structure of the new phenolic glycoside tinosinen. Planta Med.. 1993;59:552-553.
- [Google Scholar]
- Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules. 2014;19:1201-1211.
- [Google Scholar]
- The in vitro antibacterial activity of Tinospora crispa extracts. J. Biol. Sci.. 2006;6:398-401.
- [Google Scholar]
- The observation of curative effect of knee ankylosis after injury auxiliary through huoxue Shujin Xunxi recipe. Shaanxi J. Trad. Chinese Med.. 2010;31:997-998.
- [Google Scholar]
- Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods. 2015;14:144-153.
- [Google Scholar]
- Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lalpha in rats. Food Funct.. 2016;7:1992-1998.
- [Google Scholar]
- Nutritional composition, antioxidant ability and flavonoid content of Tinospora crispa stem. Adv. Nat. Appl. Sci.. 2009;3:88-94.
- [Google Scholar]
- Zulkefli, H.N., Mohama, J., Abidin, N.Z., 2013. Antioxidant activity of methanol extract of Tinospora crispa and Tabernaemontana corymbosa. Sains Malays 42, 697–706.







