Translate this page into:
Evaluation of the similarity of dispensing granules of Banxia Houpo Tang with traditional decoction by chemical analysis and spasmolytic activity
⁎Corresponding authors at: Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Dong Nei Nan Xiao Jie 16, Beijing 100700, China (L. Tang). lytang@icmm.ac.cn (Liying Tang), whw9905012@163.com (Hongwei Wu), hongjun0420@vip.sina.com (Hongjun Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dispensing granule, an innovative preparations of traditional Chinese medicine (TCM) decoction. The convenience of carrying and using makes this innovative herbal product more and more attractive in clinical practice. However, the consistency of quality and efficacy of such granules after constituting into a decoction compared with traditional decoctions is the subject of controversy. In the present study, BXHPT (Banxia Houpo Tang) was evaluated as a typical TCM. Consistency of BXHPT dispensing granules compared with a traditional decoction was performed by chemical analysis and assessment of biological activity. A novel high performance liquid chromatography (HPLC) protocol in which four quality control markers were simultaneously detected, including rosmarinic acid, 6-gingerol, magnolol, and honokiol, was developed to rapidly evaluate the chemical similarity between BXHPT as a decoction and granules. The ability of a decoction and granules of BXHPT to reduce the contraction of duodenal strips evoked by ACh were compared, in addition to 4 quality control markers. The BXHPT decoction, dispensing granules, and 4 quality control markers all displayed spasmolytic activity. However, there were differences in the concentrations of the quality control markers and the spasmolytic activity of BXHPT dispensing granules compared with decoction, differences related to the process of preparation of the dispensing granules. The present study provides new methodology for the chemical analysis and evaluation of spasmolytic activity of BXHPT and analysis of the similarity of the prescription as a dispensing granule compared to a traditional decoction.
Keywords
Banxia Houpo Tang
Dispensing granules
Quality consistency evaluation
Spasmolytic activity
1 Introduction
Traditional Chinese Medicine (TCM) prescriptions and preparations have been developed and used over thousands of years in China. Decoction is the most common preparation for prescriptions in clinic. Traditional decoctions are usually prepared by combining a prepared mixture of crude drugs and boiling them in water. However, as the pace of life has accelerated, traditional decoctions are often considered inconvenient due to the soaking, decocting, filtration, and concentration required. In recent years, innovative preparations of TCM as dispensing granules, also known as decoction-free granules, have been developed and prescribed by practitioners of traditional medicine in several countries, including China, Japan, and Korea (Zhou., 2016). Dispensing granules are extracted from the raw materials of individual Chinese herbal medicines and then concentrated, dried, and granulated. Patients and practitioners can easily mix and dissolve different granules with hot water to prepare a decoction. The convenience of this form of innovative herbal product is increasingly attractive for those in clinical practice. However, the consistency of quality and efficacy of such granules after constituting into a decoction compared with traditional decoctions is the subject of controversy.
Banxia Houpo Tang (BXHPT), a well-known and classical TCM prescription, is an effective prescription commonly used in clinical practice. It was first recorded in “Jin Gui Yao Lue” during the Han Dynasty (202BC-220AD). The recipe comprises five herbal medicines, Pinellia ternata (Thunb.) Breit., dried tuber; Magnolia officinalis Rehd. et Wils., dried cortex; Poria cocos (Schw.) Wolf., dried sclerotium of its fungus; Zingiber officinale Rose., fresh rhizomes; and Perilla frutescens(L.) Britt., dried leaves. In TCM, this formula causes a descending counterflow of qi, dissipates mass, and resolves phlegm (Li et al., 2020). In clinic, BXHPT is usually used for the treatment of digestive system diseases and psychiatric disorders such as functional dyspepsia (Oikawa et al., 2009; Oikawa et al., 2005; Xiao et al., 2013), bowel ileus (Hirano et al., 2020), peptic ulcer (Satoh et al., 2001), chronic pharyngitis (Xu et al., 2020), or depression (Guo et al., 2004; Luo et al., 2000; Ma et al., 2013; Sato et al., 2005; Yoshinaga et al., 2020). It also improves the swallowing reflex and deglutition disorder (Iwasaki et al., 2007; Iwasaki et al., 2000; Iwasaki et al., 1999; Sugaya et al., 1983), reducing the sensation of a lump in the throat and throat dysesthesia (Kagohashi et al., 2016), and improves sleep choking syndrome (Hisanaga et al., 2002).
In the present study, BXHPT was evaluated as a typical TCM. Consistency of BXHPT dispensing granules compared with a traditional decoction was performed by chemical analysis and assessment of biological activity. A number of reports have previously compared dispending granules with traditional decoction using different TCM formulations. Even by combining studies in a systematic review and performing calculations with a meta-analysis prevented definite conclusions from being drawn because of the low quality of the randomized controlled trials (RCTs) and conflicting results (Qiu et al., 2018). It was apparent that the raw materials used by pharmaceutical companies to produce the granules were of a different quality to those used in traditional decoction in some reports, and so some of the comparisons were of limited value (Zhang et al., 2021).
Therefore, in the present study, the dispensing granules and traditional decoction of BXHPT were prepared using raw materials that were homogeneous. A quality control process using HPLC with 4 compounds, including rosmarinic acid, 6-gingerol, magnolol, and honokiol, was developed to rapidly evaluate the chemical similarity of BXHPT by decoction and in granules. Furthermore, based on the clinical efficacy of BXHPT for gastrointestinal disorders, the spasmolytic activity on gut smooth muscle was tested to evaluate and compare the differences between dispensing granules and traditional decoction of BXHPT. In an attempt to evaluate quality consistency of dispensing granule and traditional decoction of BXHPT, the integrated quality-based strategy based on chemical analysis and spasmolytic activity which was briefly delivered in Fig. 1.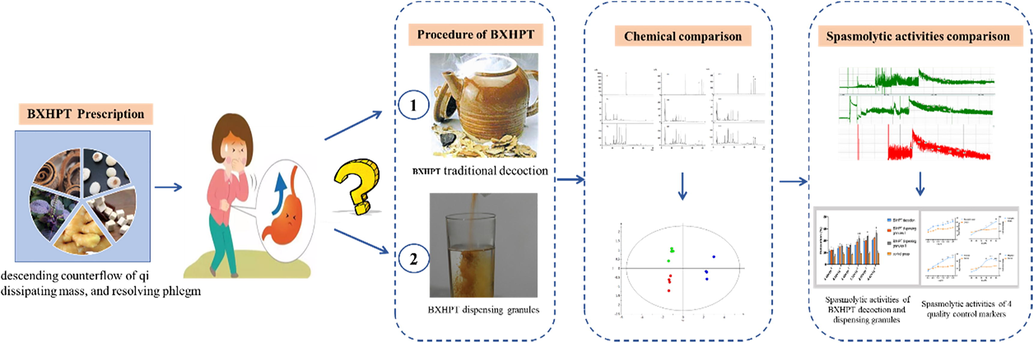
The totality-of-the-evidence approach for consistency evaluation between dispensing granule and traditional decoction of BXHPT.
2 Materials and methods
2.1 Chemicals, reagents, and animals
Reference standards of honokiol, magnolol, 6-gingerol, and rosmarinic acid were purchased from the National Institutes for Food and Drug Control (Beijing, China). The purity of each standard was greater than 98%. The prepared slice of raw herbal materials, including Pinellia ternate, Magnolia officinalis, Poria cocos, Zingiber officinale, and Perilla frutescens were obtained from Beijing Nengji Chinese Herbal Pieces Co., Ltd. (Beijing, China), and authenticated by Prof. Hongwei Wu. HPLC-grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). Acetylcholine chloride (ACh), dimethyl sulfoxide (DMSO) (Sigma Chemicals Co, St. Louis, USA). CaCl2 (Merck, Darmstadt, Germany), NaCl, KCl, MgSO4, NaHCO3 and glucose were purchased from Beijing Chemical Work (Beijing, China).
Sprague-Dawley (SD) rats (250 ± 20 g) were obtained from the Animal Breeding Centre of Beijing Vital River Laboratories Company (Beijing, China). All experimental animal procedures were approved by the China Academy of Chinese Medical Sciences’ Administrative Panel on Laboratory Animal Care and were performed in accordance with the institutional guidelines and ethics of the committee as a procedure of the China Academy of Chinese Medical Sciences.
2.2 BXHPT decoction procedure
To reduce experimental error, the quantity of crude herbs used in BXHPT decoction was just twice the daily dosage. A total of 108 g mixed prepared sliced crude herbs consisting of Pinellia ternate (24 g), Magnolia officinalis (18 g), Poria cocos (24 g), Zingiber officinale (30 g), and Perilla frutescens (12 g), were extracted twice, in 864 mL (8 fold greater than the total weight of raw material) then 540 mL (5 fold greater than the total weight of raw material) of water with refluxing, for 0.5 h and 0.3 h, respectively. Each decoction was filtered using a 200 mesh filter, then combined. BXHPT decoction was then obtained after direct lyophilization. Four batches of BXHPT decoction were prepared in parallel. Additional blank samples of the BXHPT formulation decocted without one of the herbal medicines were also prepared as described above, for validation of the HPLC method.
2.3 Preparation of BXHPT dispensing granules
Two different methods were used to prepare BXHPT dispensing granules. The only difference between the two preparations was the quantity of water added to the individual crude herbs.
2.3.1 BXHPT dispensing granules I
To prepare BXHPT dispensing granules I, individually prepared sliced crude herbs of Pinellia ternate (24 g), Magnolia officinalis (18 g), Poria cocos (24 g), Zingiber officinale (30 g), and Perilla frutescens (12 g) were extracted in water. For the first decoction, the weight of water was 8-fold that of each crude drug (i.e., 1 g prepared herb in 8 mL water). For the second decoction, the quantity of water was 5-fold greater than that of each crude drug (i.e., 1 g prepared herb in 5 mL water). The ratio of water to crude drug was the same as that used for the BXHPT decoction, as were other parameters, such as duration of decoction. The water extracts of herbs were then lyophilized and the freeze-dried powders of each individual crude herb were combined and mixed, defined as BXHPT dispensing granules I. Four batches of BXHPT dispensing granules I were prepared in parallel.
2.3.2 BXHPT dispensing granules II
The individually prepared sliced crude herbs Pinellia ternate (24 g), Magnolia officinalis (18 g), Poria cocos (24 g), Zingiber officinale (30 g), and Perilla frutescens (12 g) were each extracted using a fixed quantity of added water, namely 864 mL water in the first decoction and 540 mL in the second. The volume of water added matched that used in the preparation of the BXHPT decoction, as were the other parameters. Each aqueous extract of individual crude drug was lyophilized after which the freeze-dried powders were combined and mixed, defined as BXHPT dispensing granules II. Four batches were prepared in parallel.
2.4 Development and validation of the HPLC protocol for the evaluation of quality of BXHPT decoction and BXHPT granules
2.4.1 Sample and reference solution preparation
Solutions of standards were prepared: magnolol (8.83 mg), honokiol (8.64 mg), 6-gingerol (7.98 mg), and rosmarinic acid (8.81 mg). Each was accurately weighed and transferred into 10 mL volumetric flasks then dissolved in methanol, each flask topped up to the calibration mark to obtain standard solutions for each analyte. An appropriate volume of stock solution of each single component was added to another 25 mL volumetric flask, which was then diluted to the mark with methanol to obtain a mixed reference stock solution. The mixed reference stock solution was diluted into a series of mixed reference solutions of different concentrations.
To prepare a sample solution, a total of 0.5 g freeze-dried powder (BXHPT decoction, BXHPT dispensing granules I, or BXHPT dispensing granules II) were accurately weighed and placed into a 100 mL flask and dissolved in precisely 25 mL 20% methanol. The vial was weighed and recorded, after which the sealed flask was extracted for 40 min by ultrasonication at room temperature. After cooling, 20% methanol was added to restore it to its initial weight. The samples were then finally filtered through a 0.22 μm membrane filter prior to injection into the HPLC system.
2.4.2 Chromatographic conditions
HPLC analysis was conducted using a Shimadzu LC-20A HPLC system (LC solution software) equipped with a quaternary pump and degasser, thermostated column compartment, diode array detector (DAD), and an auto sample injector (Shimadzu, Tokyo, Japan). Chromatographic analysis was conducted using a Diamonsil Plus C18 column (250 mm*4.6 mm, particle size: 5 μm) maintained at 30 °C. Detection wavelengths were 329 nm for rosmarinic acid, 280 nm for 6-gingerol, and 294 nm for both honokiol and magnolol. The mobile phase consisted of acetonitrile (A) and 0.2% (v/v) aqueous phosphoric acid (B) with the following gradient: 10–30% (A) from 0 to 25 min, 30–40% (A) from 25 to 30 min, 40–100% (A) from 30 to 50 min. The flow rate was 1 mL·min−1. The injected volume was 10 μL.
2.4.3 Validation of methodology
The method was validated for specificity, precision, linearity, limit of quantification (LOQ), limit of detection (LOD), stability, repeatability, and accuracy. The specificity of this method was evaluated by comparing the chromatograms of the study samples, sample blank, and standards. Inter-day and intra-day precision were determined by analysis of the same sample solution six times within one day and on three different days, respectively. Linearity was verified using correlation of determination after analyzing five concentrations of mixed standard solution in triplicate. The LOQ and LOD were respectively estimated at signal-to-noise ratios of 10 and 3 by injecting a series of dilute solutions at known concentration. Sample stability was determined by analyzing a single sample solution that was stored at room temperature for 0, 4, 9, 12, 24, and 48 h. Repeatability was determined by analyzing six separate samples from the same source. The accuracy of the assay method was evaluated by recovery of standards from samples.
2.5 Spasmolytic activity experiments for BXHPT decoction and BXHPT dispensing granules.
Four male SD rats weighing 250 ± 20 g were fasted for 24 h, then anesthetized with 1% sodium pentobarbital (40 mg/kg) via intraperitoneal injection. Segments (10 cm) of the duodenum close to the pylorus were removed quickly and maintained in ice-cold modified Krebs-Henseleit (K-H) buffer (NaCl: 119.9 mmol/L; KCl: 4.8 mmol/L; KH2PO4: 2.8 mmol/L; MgSO4: 1.2 mmol/L; NaHCO3: 14.5 mmol/L; CalCl2: 2.5 mmol/L; and glucose: 12.5 mmol/L). The mucosa and submucosa were removed gently with fine tweezers. The strips of duodenum were cut into approximately 1 cm segments in length (tubes) to test for contractility of the intestinal smooth muscle. The strips were incubated in separate chambers (20 mL) containing K-H buffer at 37 °C in an atmosphere of 95% O2 and 5% CO2, equilibrated for 30 min after which the contents of the bath solution were replaced every 15 min.
Forces generated by the duodenal strips were measured isometrically with a BiopacMP 150 multipurpose polygraph recorder connected to a force displacement transducer, after which samples were equilibrated for 30 min at a resting tension of 1 g. Smooth muscle agonists were dissolved in deionized water. The intestinal smooth muscle was stimulated using acetylcholine (10 μM) until the plateau phase was reached, the tension recorded as Emax. Changes in force were recorded in the strips after the addition of BXHPT decoction, BXHPT dispensing granules dissolved in deionized water, or chemical compounds dissolved in 0.5% DMSO. Solutions of samples were added to the system, respectively, in a cumulative manner. Following intervention with the final concentration of each drug in the tissue chamber for 6 min, the mean value of change in force was calculated, prior to the subsequent intervention. Experimental results were performed in at least triplicate. All experiments were conducted on isolated rat duodenal strips in isotonic conditions.
2.6 Data processing and statistical analysis
Spasmolytic activity data are presented as means ± standard deviation (SD). Statistical significance was determined by a one-way ANOVA followed by a Tukey’s multiple comparison test or Student’s t-test. P-values < 0.05 were considered statistically significant. Principal component analysis was performed using SIMCA-P 12.0 software.
3 Results and discussion
3.1 Comparison of the extraction yield between BXHPT decoction, BXHPT dispensing granules I, and BXHPT dispensing granules II
Extraction yields for BXHPT decoction, BXHPT dispensing granules I, and BXHPT dispensing granules II were calculated from the ratio of the quantity of freeze-dried powder obtained to the total quantity of decocted crude herbs. In accordance with the process of preparation, as described in Sections 2.2 and 2.3, the total quantity of crude drug in the three preparations was the same. As shown in Fig. 2, there were no apparent differences in extraction yield between BXHPT decoction and BXHPT granules I. However, the extraction yield of BXHPT granules II was significantly higher than the other two. The results suggest that the quantity of extract received orally is probably different in clinic if dispensing granules are used compared with traditional decoction even if based on the same prescription. Moreover the differences probably affect the clinical efficacy. The results of the extraction yield showed the quality of BXHPT dispensing granules is closely related to the extraction process of the crude drug. In order to ensure the consistency of quality, the production process of dispensing granules produced by different pharmaceutical companies should be firstly consistent. Then it is necessary to establish an unified quality standards for traditional decoctions, so that the quality of dispensing granules can be consistent with the traditional decoction.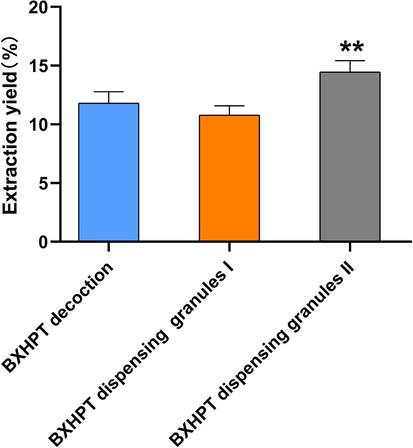
Comparison of extraction yield between BXHPT decoction, BXHPT dispensing granules I, and BXHPT dispensing granules II (means ± SD, n = 4; **P < 0.01, BXHPT dispensing granules II versus BXHPT decoction).
3.2 Optimization of chromatographic conditions and pretreatment procedure
The four compounds, 6-gingerol from Zingiber officinale Rose., rosmarinic acid from Perilla frutescens(L.) Britt, and honokiol and magnolol from Magnolia officinalis Rehd. et Wils. were selected as quality control markers for the BXHPT decoction and BXHPT dispensing granules. All four are recorded as quality control markers for the raw crude drug in the Chinese Pharmacopoeia, 2020 edition (State Pharmacopoeia Commission., 2020). The structures of all compounds are displayed in Fig. 3.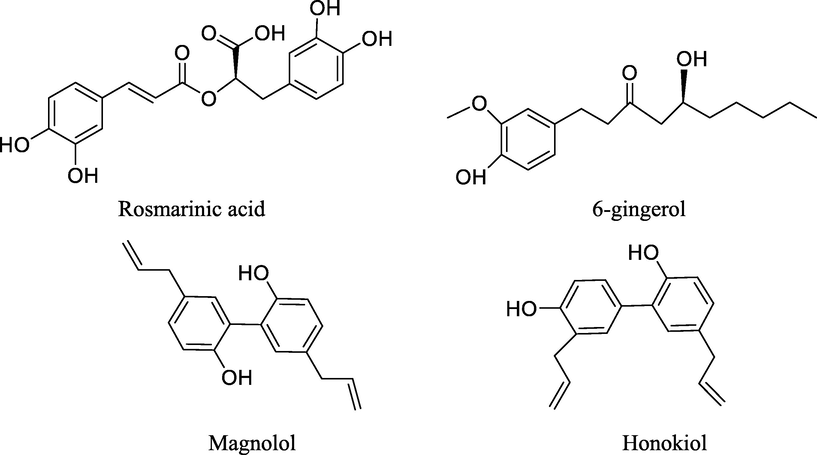
Chemical structure of quality control markers measured by HPLC for BXHPT decoction and BXHPT dispensing granules.
A procedure using HPLC to simultaneously determine the 4 compounds within the freeze-dried powder of a BXHPT decoction was established by investigating different sample pretreatment procedures. Reflux and ultrasonic extraction were conducted using different concentrations of methanol (0%, 20%, 40%, 80%, and 100%) and compared. Ultimately, the four components were extracted ultrasonically with 20% methanol, representing the greatest extraction efficiency for each.
To establish the wavelengths providing the greatest sensitivity for the four compounds, the wavelength at the maximum absorption was measured using a diode array detector (DAD) (rosmarinic acid: 329 nm; 6-gingerol: 280 nm; honokiol and magnolol: 294 nm). Chromatographic modifiers were used and the gradient program optimized to enhance the shape of the peaks of the four components. Methanol-water and acetonitrile–water were tested as mobile phase systems. Acetonitrile-water displayed superior signal intensity than that of methanol–water. Furthermore, the signal intensity and peak shape of the four detected compounds improved significantly after the addition of 0.1% formic acid. Thus, acetonitrile–water with 0.1% formic acid was finally selected as the elution solvent.
3.3 HPLC method validation
3.3.1 Specificity
In the present study, a protocol using HPLC was developed for the simultaneous quantification of the four standard compounds. Typical HPLC chromatograms for each detected compound are depicted in Fig. 4. Specificity was evaluated by comparison of chromatograms of the study samples, sample blank, and standards. In the sample blank, no other components interfered with the detection of target compounds. As shown in Fig. 4, the four compounds displayed ideal chromatographic separation, achieving a good shape of peak and symmetry factor for the chromatographic conditions described in Section 2.4.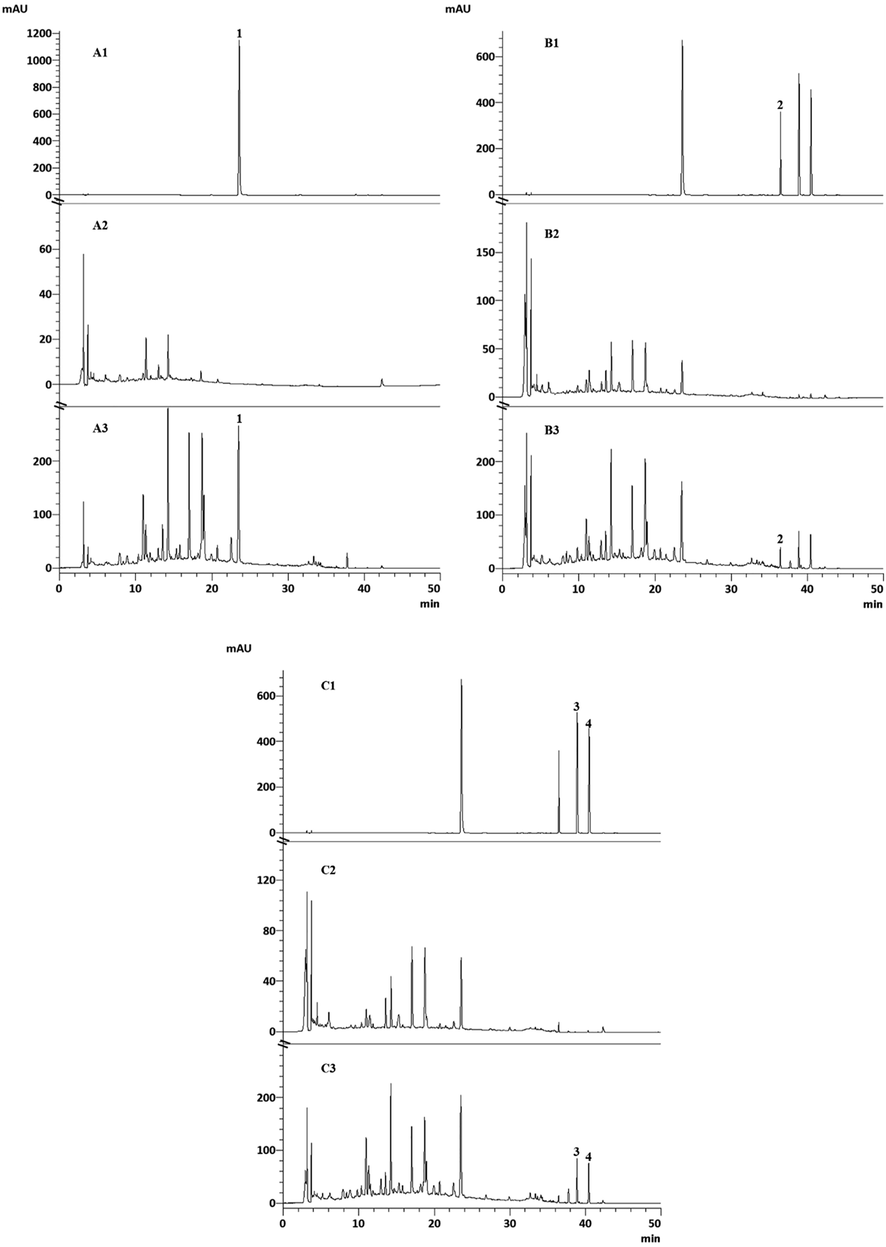
Typical HPLC chromatograms of the mixed standards, sample blank, and BXHPT decoction (1: rosmarinic acid; 2: 6-gingerol; 3: honokiol; 4: magnolol). A: Chromatogram at 329 nm for rosmarinic acid (A1: mixed standards, A2: Perilla frutescens (L.) Britt blank samples, A3: BXHPT decoction); B: Chromatogram at 280 nm for 6-gingerol (B1: mixed standards, B2: Zingiber officinale Rose. blank samples, B3: BXHPT decoction); C: Chromatogram at 294 nm for honokiol and magnolol (C1: mixed standards, C2: Magnolia officinalis Rehd. et Wils. blank samples, C3: BXHPT decoction);
3.3.2 Linearity and limit of quantitation
For the range of study sample concentrations determined from the pre-assessment, the stock solution of mixed standards was diluted to concentrations that were suitable, which were then analyzed by HPLC-DAD. A calibration curve was generated using area of the peaks for each quantitative ion (Y value) and the corresponding mass concentration (X value, μg/mL). A linear equation and correlation coefficient were then calculated. Good linear correlations for each compound were recorded, r values from 0.9998 to 0.9999 obtained in the selected ranges. Characteristic parameters for the regression equations and correlation coefficients are displayed in Table 1. The limits of quantification (LOQ) were determined using a corresponding standard solution at a signal-to-noise (S/N) ratio of 10 for each component. The results, as displayed in Table 1, indicate that the LOQs for each compound satisfied the requirements for detection with suitable levels of sensitivity. RSD: relative standard deviation; r: Correlation coefficient.
Rosmarinic acid
6-Gingerol
Honokiol
Magnolol
Linearity range (µg/mL)
0.7–173.3
0.8–199.5
0.6–141
0.9–220.8
Regression equation
Y = 2643465.58X + 5698.11 (r = 0.9998)
Y = 494168.26X + 2477.65 (r = 0.9999)
Y = 1450388.53X + 5413.90 (r = 0.9999)
Y = 1348645.92X + 7731.36 (r = 0.9999)
LOQ (µg/mL)
0.19
0.079
0.16
0.88
LOD(ug/mL)
0.06
0.04
0.05
0.22
Inter-day precision RSD (%)
(n = 9)2.25
2.12
1.93
2.52
Intra-day precision RSD (%)
(n = 6)0.50
0.53
1.49
2.20
Repeatability RSD (%)
(n = 6)1.75
1.49
2.17
1.43
Stability RSD (%)
(n = 6)2.22
3.11
1.72
2.36
Mean recovery (%)
(n = 6)98.0
100.2
96.7
103.2
3.3.3 Precision, repeatability and stability
Six consecutive injections of the same solution of samples were performed on the same day. The relative standard deviation (RSD) values of the areas of the peaks for each compound were between 0.5% and 2.20%, indicating good intra-day precision. Furthermore, the inter-day variation of the RSD values was <3% in each case.
To verify repeatability, six samples prepared in parallel from the same source material of freeze-dried powders were analyzed. The RSD of the concentrations for the four compounds was 1.43–2.17%, indicating that the method was repeatable.
To examine stability of the samples, a single solution of each sample, stored at room temperature (25 ± 3 °C), was analyzed 0, 4, 9, 12, 24, and 48 h after preparation. The RSDs of the areas under the peaks for each compound were between 1.72% and 3.11%, indicating that solutions of the samples were stable within a 48-hour period.
3.3.4 Accuracy
The accuracy of the HPLC method was determined by spiking a known quantity of mixed standards into a known quantity of freeze-dried BXHPT in sextuplicate, at a level 100% greater than the specified limit. The fortified samples were then prepared and analyzed with the procedure, as described above. Recovery was estimated by the formula: Recovery = (measured quantity − original quantity) / quantity spiked. The recovery values of the four analytes were calculated, as displayed in Table 1. The calculated recovery of the standard components ranged from 96.7% to 103.2% with RSD values all<5.0%, demonstrating that the method had good reliability and accuracy.
3.4 Chemical comparison between BXHPT decoction, BXHPT dispensing granules I, and BXHPT dispensing granules II
The HPLC protocol developed here was used to simultaneously quantify the four quality control markers in the BXHPT decoction, and BXHPT dispensing granules I and II samples. To compare in parallel, the relative concentration (expressed in percentage) of the four compounds in the freeze-dried powders were converted to an absolute quantity (expressed in grams) obtained from the raw materials (108 g of the prepared herbs) of BXHPT decoction and dispensing granules.
The global variation among the three groups was first investigated using principal component analysis (PCA), based on absolute quantities of the four compounds, using SIMCA software (version 13.0). PCA is an unsupervised pattern recognition method that can identify the overall difference based on all imported indices. As shown in the PCA score plot (Fig. 5A), an overview of all samples in the data exhibited clear groupings (R2X: 0.989; Q2: 0.938) between traditional decoction, dispensing granules I, and dispensing granules II. This indicates that the BXHPT decoction group was closer in its characteristics to the dispensing granules I group and distant from the dispensing granules II group. In particular, as displayed in Fig. 5B, compared with traditional decoction, the quantity of rosmarinic acid was clearly greater and 6-gingerol clearly lower in the dispensing granules I samples, while there were no significant differences in the quantity of honokiol and magnolol. For dispensing granules II, the quantity of 6-gingerol, rosmarinic acid, honokiol, and magnolol were significantly higher compared to traditional decoction, in each case.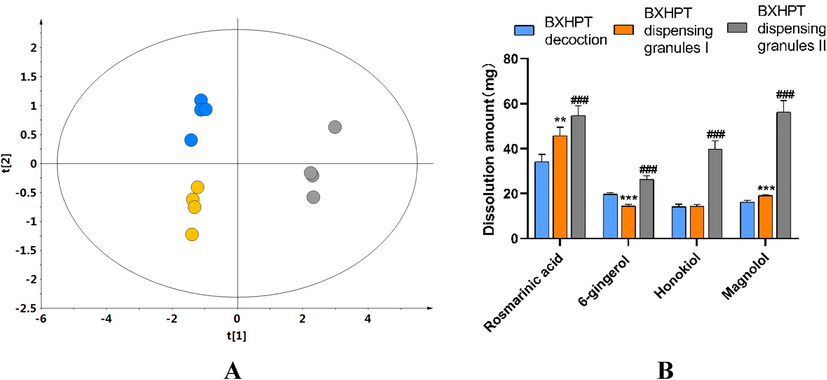
Comparison of constituents of BXHPT decoction compared with BXHPT dispensing granules I and II. A: PCA score scatter plot based on the absolute quantities of the four quality control markers (blue: BXHPT decoction, Orange: BXHPT dispensing granules I, gray: BXHPT dispensing granules II); B: Comparison of the absolute quantities obtained from the raw materials (108 g of the prepared herbs) of BXHPT decoction, BXHPT dispensing granules I and BXHPT dispensing granules II (∗p < 0 05, ∗∗p < 0 01, and ∗∗∗ p < 0 001 for BXHPT dispensing granules I versus BXHPT decoction; #p < 0 05, ##p < 0 01, and ###p < 0 001 for BXHPT dispensing granules II versus BXHPT decoction).
3.5 Comparison of spasmolytic activity between BXHPT decoction, BXHPT dispensing granules I, and BXHPT dispensing granules II
The differences in spasmolytic activity between BXHPT dispensing granules and BXHPT decoction were compared in samples from the three groups by evaluating the ability of BXHPT to reduce contractions evoked by acetylcholine (ACh). Relaxation (%) was calculated using the relationship: (Emax-E)/Emax, where Emax represents the contraction caused by ACh and E represents the contraction recorded after treatment with the test sample. Greater relaxation (%) values indicate a stronger inhibition of contraction of the bowel caused by ACh. In Fig. 6, the Y-axis represents the degree of relaxation while the X-axis represents the final projected concentration of raw material in the bath. Compared with the control group, all samples displayed clear inhibitory properties over the concentration range 4.42 × 10−4 ∼ 4.42 × 10−2 g (raw material)/mL. At the same concentrations of 1.33 × 10−2, 2.65 × 10−2, and 4.42 × 10−2 g raw material/mL, dispensing granules II displayed significantly greater inhibition than traditional decoction (P < 0.05), highlighting the differences in bioactivity between granules and traditional decoction. These observations were consistent with the results of chemical analysis.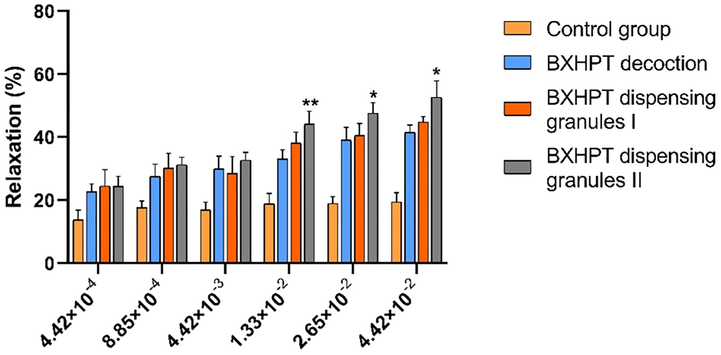
Comparison of spasmolytic activity of BXHPT decoction, BXHPT dispensing granules I and BXHPT dispensing granules II. The results represent means ± SE (n = 3 duodenal strips per tested concentration). ∗ p < 0 05, ∗∗p < 0 01, and ∗∗∗ p < 0 001 for BXHPT dispensing granules II versus BXHPT decoction.
In addition, the inhibitory capability of the four quality control markers toward duodenum precontracted with ACh was also evaluated. As displayed in Fig. 7, rosmarinic acid, 6-gingerol, magnolol, and honokiol all displayed inhibitory effects, providing a rational basis for their selection as quality control markers. All four compounds displayed the capability to inhibit the contractions evoked by ACh in a dose-dependent manner. However, the relaxation effect due to any of the four compounds was a maximum of only approximately 50%, even where a concentration that was greater was administered.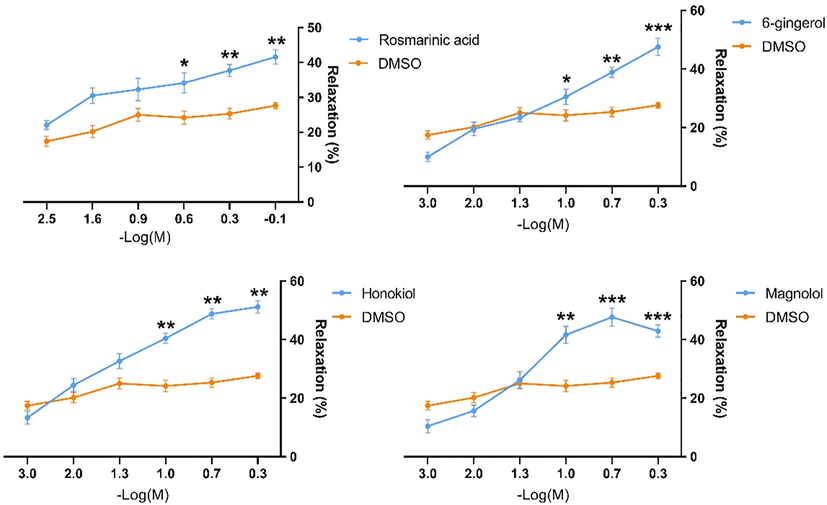
Spasmolytic activity of the 4 quality control markers against Ach-induced contraction in duodenal strips. The results represent means ± SE (n = 3 duodenal strips per tested concentration). ∗ p < 0 05, ∗∗p < 0 01, and ∗∗∗ p < 0 001 for rosmarinic acid, 6-gingerol, magnolol, and honokiol versus the reagent blank.
4 Conclusions
The usage of dispensing granules in TCM has increased dramatically in clinics over the past few decades, although the consistency in quality and efficacy compared with traditional decoction has caused controversy. In the present study, dispensing granules and traditional decoction of BXHPT were prepared using a homogeneous preparation of raw materials. To adequately compare BXHPT decoction with BXHPT granules, dispensing granules were prepared using two different methods. A novel HPLC protocol was established to simultaneously detect four quality control markers, namely rosmarinic acid, 6-gingerol, magnolol, and honokiol, thereby allowing rapid evaluation of the similarity between BXHPT that was decocted and the preparation with dispensing granules. Furthermore, the clinical efficacy of the different preparations of BXHPT was assessed by measuring the spasmolytic activity on gut smooth muscle. The spasmolytic activity of the four quality control markers was also evaluated.
A comparison of the extraction yield indicated that the quantity of herbal compounds received orally probably differs when prescribed as dispensing granules rather than as a traditional decoction. Chemical analysis and spasmolytic activity of the BXHPT decoction were clearly different from that of BXHPT dispensing granules especially that of BXHPT granules II. The differences were closely related to the process of preparation of the granules. A limitation of this study was that biological similarity was only evaluated in vitro. Whether a difference can be observed in clinical efficacy remains to be further studied. The present study provides a methodology applicable to the chemical analysis and evaluation of spasmolytic activity of BXHPT dispensing granules compared with traditional decoction.
Acknowledgments
This study was financially supported by Autonomic Project of China Academy of Chinese Medical Sciences (No. ZZ13-019) and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2019ZX09201005; 2019ZX09721001-005-002).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Guo, Y., Kong, L., Wang, Y., Huang, Z., 2004. Antidepressant evaluation of polysaccharides from a Chinese herbal medicine Banxia-houpu decoction. Phytotherapy Res.: PTR 18, 204–207. https://doi.org/10.1002/ptr.1394.
- Hirano, Y., Isai, H., Onuki, A., Watanabe, K., 2020. Integrative treatment of paralytic small intestine following acute cervical cord injury: A case report. Surgical neurology international 11, 80. https://doi.org/10.25259/sni_62_2020.
- Hisanaga, A., Itoh, T., Hasegawa, Y., Emori, K., Kita, T., Okabe, A., Kurachi, M., 2002. A case of sleep choking syndrome improved by the Kampo extract of Hange-koboku-to. Psychiatry Clinical Neurosci., 56, 325–327. https://doi.org/10.1046/j.1440-1819.2002.01001.x.
- A pilot study of banxia houpu tang, a traditional Chinese medicine, for reducing pneumonia risk in older adults with dementia. J. Am. Geriatr. Soc.. 2007;55:2035-2040.
- [CrossRef] [Google Scholar]
- Iwasaki, K., Wang, Q., Nakagawa, T., Suzuki, T., Sasaki, H., 1999. The traditional Chinese medicine banxia houpo tang improves swallowing reflex. Phytomedicine: Int. J. Phytotherapy Phytopharmacology 6, 103–106. https://doi.org/10.1016/s0944-7113(99)80043-9.
- The effects of the traditional chinese medicine, “Banxia Houpo Tang (Hange-Koboku To)” on the swallowing reflex in Parkinson's disease. Phytomed.: Int. J. Phytotherapy Phytopharmacology. 2000;7(4):259-263.
- [CrossRef] [Google Scholar]
- Effect of a traditional herbal medicine, hangekobokuto, on the sensation of a lump in the throat in patients with respiratory diseases. Biomed. Rep.. 2016;4:384-386.
- [CrossRef] [Google Scholar]
- Li, Y., Chen, R., Li, l., Xue, H., Lu, Y., Cheng jian ming, 2020. CHENG Jian-ming. Analysis of Banxia Houpo Tang Based on Ancient Literatures. Chinese Journal of Experimental Traditional Medical Formulae.
- Antidepressant effects of Banxia Houpu decoction, a traditional Chinese medicinal empirical formula. J. Ethnopharmacol.. 2000;73(1-2):277-281.
- [CrossRef] [Google Scholar]
- Metabonomic study on the antidepressant-like effects of banxia houpu decoction and its action mechanism. Evidence-based Complementary Alternative Medicine : eCAM. 2013;2013:1-9.
- [CrossRef] [Google Scholar]
- Hangekobokuto (Banxia-houpo-tang), a Kampo medicine that treats functional dyspepsia. Evidence-based Complementary and Alternative Medicine : eCAM. 2009;6(3):375-378.
- [CrossRef] [Google Scholar]
- Prokinetic effect of a Kampo medicine, Hange-koboku-to (Banxia-houpo-tang), on patients with functional dyspepsia. Phytomed.: Int. J. Phytotherapy Phytopharmacology. 2005;12(10):730-734.
- [CrossRef] [Google Scholar]
- Qiu, R., Zhang, X., Zhao, C., Li, M., Shang, H., 2018. Comparison of the efficacy of dispensing granules with traditional decoction: a systematic review and meta-analysis. Ann. Translational Med. 6, 38. https://doi.org/10.21037/atm.2017.10.22.
- Effects of some kampo medicines on plasma levels of neuropeptide Y under venipuncture stress. Biol. Pharm. Bull.. 2005;28(9):1757-1761.
- [CrossRef] [Google Scholar]
- The effects of kampo-formulation and the constituting crude drugs, prescribed for the treatment of peptic ulcer on H, K-ATPase activity胃疾患に繁用される漢方方剤及び構成生薬エキス, 生薬含有成分のH,K-ATPase活性に及ぼす影響. Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan. 2001;121(2):173-178.
- [CrossRef] [Google Scholar]
- Chinese Pharmacopoeia. China Medical Science Press; 2020.
- Effect of Chinese herbal medicine “Hange-Koboku-To” on laryngeal reflex of cats and in other pharmacological tests. Planta Med.. 1983;47(01):59-62.
- [CrossRef] [Google Scholar]
- Randomized controlled trial of modified banxia houpo decoction in treating functional dyspepsia patients with psychological factors. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chin. J. Integrated Traditional and Western Med.. 2013;33:298-302.
- [Google Scholar]
- The efficacy and safety of Banxia-Houpo-Tang for chronic pharyngitis: A protocol for systematic review and meta analysis. Medicine. 2020;99:e19922
- [CrossRef] [Google Scholar]
- Discontinuation or reduction in benzodiazepine use by treatment with the traditional herbal medicine Hangekobokuto, case reports. J. General Family Med.. 2020;21(4):143-145.
- [CrossRef] [Google Scholar]
- Consistency evaluation between dispensing granule and traditional decoction from Coptidis Rhizoma by using an integrated quality-based strategy. Phytochem. Analysis: PCA. 2021;32(2):153-164.
- [CrossRef] [Google Scholar]
- Historic review and future prospect of TCM formula granules. Modern Chin. Med.. 2016;18(9):1093-1096.
- [Google Scholar]







